User login
Oleander extract for COVID-19? That’s a hard ‘no’ experts say
“Though renowned for its beauty and use in landscaping, this Mediterranean shrub is responsible for cases of accidental poisoning across the globe. All parts of the plant are poisonous,” Cassandra Quave, PhD, ethnobotanist and herbarium curator at Emory University, Atlanta, cautioned in an article in The Conversation, an independent, not-for-profit publication.
Oleandrin has properties similar to digoxin; the onset of toxicity occurs several hours after consumption.
The first symptoms of oleandrin poisoning may be gastrointestinal, such as nausea, vomiting, abdominal pain, diarrhea (which may contain blood), and loss of appetite.
After these first symptoms, the heart may be affected by tachyarrhythmia, bradyarrhythmia, premature ventricular contractions, or atrioventricular blockage. Xanthopsia (yellow vision), a burning sensation in the eyes, paralysis of the gastrointestinal tract, and respiratory symptoms may also occur.
Oleandrin poisoning may affect the central nervous system, as evidenced by drowsiness, tremors, seizures, collapse, and coma leading to death. When applied to the skin, oleander sap can cause skin irritations and allergic reactions characterized by dermatitis.
Diagnosis of oleandrin poisoning is mainly made on the basis of a description of the plant, how much of it was ingested, how much time has elapsed since ingestion, and symptoms. Confirmation of oleandrin in blood involves fluorescence polarization immunoassay, digoxin immunoassay, or liquid chromatography-electrospray tandem mass spectrometry.
Neither oleander nor oleandrin is approved by regulatory agencies as a prescription drug or dietary supplement.
In vitro study
Oleandrin for COVID-19 made headlines after President Trump met in the Oval Office with Andrew Whitney, vice chairman and director of Phoenix Biotechnology, along with Housing and Urban Development Secretary Ben Carson, MD, and MyPillow founder/CEO Mike Lindell, a strong supporter of Trump and an investor in the biotech company, to learn about oleandrin, which Whitney called a “cure” for COVID-19, Axios reported.
In an in vitro study, researchers from Phoenix Biotechnology and the University of Texas Medical Branch, Galveston, tested oleandrin against SARS-CoV-2 in cultured Vero cells.
“When administered both before and after virus infection, nanogram doses of oleandrin significantly inhibited replication by 45 to 3000-fold,” the researchers said in an article posted on bioRxiv, a free online archive and distribution service for unpublished preprints in the life sciences. The study has not been peer reviewed.
On the basis of these in vitro findings, the researchers said the plant extract has “potential to prevent disease and virus spread in persons recently exposed to SARS-CoV-2, as well as to prevent severe disease in persons at high risk.”
But it’s a far cry from test tube to human, one expert cautioned.
“This is an understatement: Care must be taken when inferring potential therapeutic benefits from in vitro antiviral effects,” Harlan Krumholz, MD, cardiologist and director, Yale New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, Connecticut, told Medscape Medical News.
“There is a chasm between a single in vitro study and any use in humans outside of a protocol. People should be cautioned about that distance and the need [to] avoid such remedies unless part of a credible research project,” said Krumholz.
Yet Lindell told Axios that, in the Oval Office meeting, Trump expressed enthusiasm for the Food and Drug Administration to allow oleandrin to be marketed as a dietary supplement or approved for COVID-19.
“This is really just nonsense and a distraction,” Jonathan Reiner, MD, of George Washington University Medical Center, Washington, DC, said on CNN.
This article first appeared on Medscape.com.
“Though renowned for its beauty and use in landscaping, this Mediterranean shrub is responsible for cases of accidental poisoning across the globe. All parts of the plant are poisonous,” Cassandra Quave, PhD, ethnobotanist and herbarium curator at Emory University, Atlanta, cautioned in an article in The Conversation, an independent, not-for-profit publication.
Oleandrin has properties similar to digoxin; the onset of toxicity occurs several hours after consumption.
The first symptoms of oleandrin poisoning may be gastrointestinal, such as nausea, vomiting, abdominal pain, diarrhea (which may contain blood), and loss of appetite.
After these first symptoms, the heart may be affected by tachyarrhythmia, bradyarrhythmia, premature ventricular contractions, or atrioventricular blockage. Xanthopsia (yellow vision), a burning sensation in the eyes, paralysis of the gastrointestinal tract, and respiratory symptoms may also occur.
Oleandrin poisoning may affect the central nervous system, as evidenced by drowsiness, tremors, seizures, collapse, and coma leading to death. When applied to the skin, oleander sap can cause skin irritations and allergic reactions characterized by dermatitis.
Diagnosis of oleandrin poisoning is mainly made on the basis of a description of the plant, how much of it was ingested, how much time has elapsed since ingestion, and symptoms. Confirmation of oleandrin in blood involves fluorescence polarization immunoassay, digoxin immunoassay, or liquid chromatography-electrospray tandem mass spectrometry.
Neither oleander nor oleandrin is approved by regulatory agencies as a prescription drug or dietary supplement.
In vitro study
Oleandrin for COVID-19 made headlines after President Trump met in the Oval Office with Andrew Whitney, vice chairman and director of Phoenix Biotechnology, along with Housing and Urban Development Secretary Ben Carson, MD, and MyPillow founder/CEO Mike Lindell, a strong supporter of Trump and an investor in the biotech company, to learn about oleandrin, which Whitney called a “cure” for COVID-19, Axios reported.
In an in vitro study, researchers from Phoenix Biotechnology and the University of Texas Medical Branch, Galveston, tested oleandrin against SARS-CoV-2 in cultured Vero cells.
“When administered both before and after virus infection, nanogram doses of oleandrin significantly inhibited replication by 45 to 3000-fold,” the researchers said in an article posted on bioRxiv, a free online archive and distribution service for unpublished preprints in the life sciences. The study has not been peer reviewed.
On the basis of these in vitro findings, the researchers said the plant extract has “potential to prevent disease and virus spread in persons recently exposed to SARS-CoV-2, as well as to prevent severe disease in persons at high risk.”
But it’s a far cry from test tube to human, one expert cautioned.
“This is an understatement: Care must be taken when inferring potential therapeutic benefits from in vitro antiviral effects,” Harlan Krumholz, MD, cardiologist and director, Yale New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, Connecticut, told Medscape Medical News.
“There is a chasm between a single in vitro study and any use in humans outside of a protocol. People should be cautioned about that distance and the need [to] avoid such remedies unless part of a credible research project,” said Krumholz.
Yet Lindell told Axios that, in the Oval Office meeting, Trump expressed enthusiasm for the Food and Drug Administration to allow oleandrin to be marketed as a dietary supplement or approved for COVID-19.
“This is really just nonsense and a distraction,” Jonathan Reiner, MD, of George Washington University Medical Center, Washington, DC, said on CNN.
This article first appeared on Medscape.com.
“Though renowned for its beauty and use in landscaping, this Mediterranean shrub is responsible for cases of accidental poisoning across the globe. All parts of the plant are poisonous,” Cassandra Quave, PhD, ethnobotanist and herbarium curator at Emory University, Atlanta, cautioned in an article in The Conversation, an independent, not-for-profit publication.
Oleandrin has properties similar to digoxin; the onset of toxicity occurs several hours after consumption.
The first symptoms of oleandrin poisoning may be gastrointestinal, such as nausea, vomiting, abdominal pain, diarrhea (which may contain blood), and loss of appetite.
After these first symptoms, the heart may be affected by tachyarrhythmia, bradyarrhythmia, premature ventricular contractions, or atrioventricular blockage. Xanthopsia (yellow vision), a burning sensation in the eyes, paralysis of the gastrointestinal tract, and respiratory symptoms may also occur.
Oleandrin poisoning may affect the central nervous system, as evidenced by drowsiness, tremors, seizures, collapse, and coma leading to death. When applied to the skin, oleander sap can cause skin irritations and allergic reactions characterized by dermatitis.
Diagnosis of oleandrin poisoning is mainly made on the basis of a description of the plant, how much of it was ingested, how much time has elapsed since ingestion, and symptoms. Confirmation of oleandrin in blood involves fluorescence polarization immunoassay, digoxin immunoassay, or liquid chromatography-electrospray tandem mass spectrometry.
Neither oleander nor oleandrin is approved by regulatory agencies as a prescription drug or dietary supplement.
In vitro study
Oleandrin for COVID-19 made headlines after President Trump met in the Oval Office with Andrew Whitney, vice chairman and director of Phoenix Biotechnology, along with Housing and Urban Development Secretary Ben Carson, MD, and MyPillow founder/CEO Mike Lindell, a strong supporter of Trump and an investor in the biotech company, to learn about oleandrin, which Whitney called a “cure” for COVID-19, Axios reported.
In an in vitro study, researchers from Phoenix Biotechnology and the University of Texas Medical Branch, Galveston, tested oleandrin against SARS-CoV-2 in cultured Vero cells.
“When administered both before and after virus infection, nanogram doses of oleandrin significantly inhibited replication by 45 to 3000-fold,” the researchers said in an article posted on bioRxiv, a free online archive and distribution service for unpublished preprints in the life sciences. The study has not been peer reviewed.
On the basis of these in vitro findings, the researchers said the plant extract has “potential to prevent disease and virus spread in persons recently exposed to SARS-CoV-2, as well as to prevent severe disease in persons at high risk.”
But it’s a far cry from test tube to human, one expert cautioned.
“This is an understatement: Care must be taken when inferring potential therapeutic benefits from in vitro antiviral effects,” Harlan Krumholz, MD, cardiologist and director, Yale New Haven Hospital Center for Outcomes Research and Evaluation, New Haven, Connecticut, told Medscape Medical News.
“There is a chasm between a single in vitro study and any use in humans outside of a protocol. People should be cautioned about that distance and the need [to] avoid such remedies unless part of a credible research project,” said Krumholz.
Yet Lindell told Axios that, in the Oval Office meeting, Trump expressed enthusiasm for the Food and Drug Administration to allow oleandrin to be marketed as a dietary supplement or approved for COVID-19.
“This is really just nonsense and a distraction,” Jonathan Reiner, MD, of George Washington University Medical Center, Washington, DC, said on CNN.
This article first appeared on Medscape.com.
Pulmonary artery denervation eases PAH after endarterectomy
Pulmonary artery denervation (PADN) provides persistent and clinically significant hemodynamic improvements in patients with persistent chronic thromboembolic hypertension (CTEPH) after pulmonary endarterectomy (PEA), according to a randomized, sham-controlled trial.
“PADN in patients with CTEPH after PEA was safe and effective,” according to an investigating team led by Alexander Romanov, MD, PhD.
The mean reduction in pulmonary vascular resistance (PVR) was 258 dyn/sec per cm–5 for those randomized to PADN versus 149 dyn/sec per cm–5 (P = .001) for those randomized to the sham procedure, according to the newly published findings.
For the 6-minute walk test (6MWT), the mean distance was 470 m for the experimental group versus 399 m (P = .03) for the controls.
Several secondary endpoints measuring hemodynamics also favored PADN relative to the sham procedure at 12 months. This included the relative increase in tricuspid annular systolic excursion (P = .03) and the increase in the right ventricular fraction area (P < .001).
A total of 50 patients with residual CTEPH for at least 6 months after PEA despite medical therapy were enrolled and randomized. Entry criteria included a mean pulmonary artery pressure (PAP) of 25 mm Hg or greater or PVR greater than 400 dyn/sec per cm–5 on right heart catheterization. Patients with comorbidities associated with a life expectancy of less than 1 year were excluded.
Those randomized to the sham group were treated with riociguat over the course of follow-up. This therapy was not offered to patients in the PADN group, but all patients were blinded to the procedure and told that riociguat might or might not be administered.
Following the procedure, participating clinicians, who were also blinded to the procedure, were instructed to provide standard therapies for heart failure, such beta-blockers, diuretics, or digoxin, as needed. All patients were placed on an oral anticoagulant.
At 12 months the mean PAP (26 vs. 35 mm Hg; P < .001) and the mean systolic PAP (46 vs. 54 mm Hg; P = .01) were significantly lower in the PADN group versus those who underwent a sham procedure.
About 52% of the PADN group versus 12% of the sham group were classified as responders by the definition of a PVR reduction of at least 150 dyn/sec per cm–5 and 6MWT improvement of at least 20%, compared with baseline, reported Dr. Romanov, of the E. Meshalkin National Medical Research Center, ministry of health, Novosibirsk, Russia, and coinvestigators.
Of the three deaths caused by heart failure over the course of follow-up, two occurred in the sham group. Of the eight hospitalizations for heart failure, seven (29% of the sham group) occurred among controls versus one in those treated with PADN (4% of this group; P = .049).
There was one groin hematoma at the puncture site in each group. Both resolved without any consequences prior to hospital discharge. There were no other significant procedure-related complications in either group.
Larger multicenter trials are needed to confirm these findings, according to both the trial investigators and Marius M. Hoeper, MD, who is charge of the pulmonary hypertension program at the Hannover (Germany) Medical School.
In an editorial that accompanied publication of these findings, Dr. Hoeper identified the small sample size of this study as one of its limitations, but he said the results are consistent with several other small studies associating pulmonary artery denervation with benefit in pulmonary hypertension.
“It appears as if we are currently witnessing the emergence of a new treatment option for various forms of pulmonary hypertension,” Dr. Hoeper wrote. In his critique of the study, he suggested that it would have been “more informative” if both groups were on background riociguat, but the data from this and other studies so far indicates that ablation to achieve denervation “is safe and feasible.”
The PADN technique used in this study might be relevant to the results. Dr. Hoeper noted that the investigators employed catheter tip–based electroanatomic mapping with a novel remote navigation system with three-dimensional imaging of the right ventricle and central pulmonary arteries.
“Apparently, this approach minimizes radiation exposure and provides precise location of ablation sites,” Dr. Hoeper observed. However, he called for direct comparisons of this tool to the guidance systems used in other studies.
In an interview, Dr. Hoeper acknowledged that it is not yet clear that a large-scale trial of pulmonary artery denervation for the indication evaluated in this study is coming. He noted several strategies in CTEPH are widely used without trials confirming a reduction in clinical events.
“Balloon pulmonary angioplasty for CTEPH has become an established treatment around the world without any randomized, controlled trial and without demonstration of improved outcomes. A couple of well-conducted observational trials might be sufficient to convince physicians to introduce PADN as well,” he said. If such studies associated PADN with “improvements in hemodynamics, exercise capacity, and patient-reported outcomes, it might be sufficient.”
Currently, Dr. Hoeper is most concerned about obtaining further evidence of safety, which he characterized as a “major issue.”
If a multicenter trial is conducted “the primary endpoint should be focused on clinical events,” according to Dr. Romanov, who was asked to comment on the next steps in validating PADN for the treatment of CTEPH-associated pulmonary hypertension persisting after endarterectomy.
“The mortality rate during 1-year long-term follow-up is not so high, but heart failure progression is a problem. So in my view, the primary endpoint should be a composite of death and heart failure hospitalization,” he said. He called for follow-up duration of 2-3 years.
Jonathan Steinberg, MD, director of cardiac clinical trials and education, Summit Medical Group, Montclair, N.J., also called a trial with hard endpoints, such as death, the ideal.
In the meantime, hemodynamic and functional measures “are still quite valuable and move the ball forward for this intervention,” he said in an interview. Senior author of this trial and principle investigator of the recent ERADICATE-AF trial, which evaluated renal denervation in preventing recurrence of atrial fibrillation (JAMA. 2020;323:248-55), Dr. Steinberg predicted, “I do indeed suspect we will see trials that are more accomplishable [than a large-scale, randomized, controlled trial] in the not too distant future.”
Dr. Romanov received funding from Biosense Webster. Dr. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, Janssen, Merck Sharp & Dohme, and Pfizer.
SOURCE: Romanov A et al. J Am Coll Cardiol. 2020 Aug 17;76:916-26.
Pulmonary artery denervation (PADN) provides persistent and clinically significant hemodynamic improvements in patients with persistent chronic thromboembolic hypertension (CTEPH) after pulmonary endarterectomy (PEA), according to a randomized, sham-controlled trial.
“PADN in patients with CTEPH after PEA was safe and effective,” according to an investigating team led by Alexander Romanov, MD, PhD.
The mean reduction in pulmonary vascular resistance (PVR) was 258 dyn/sec per cm–5 for those randomized to PADN versus 149 dyn/sec per cm–5 (P = .001) for those randomized to the sham procedure, according to the newly published findings.
For the 6-minute walk test (6MWT), the mean distance was 470 m for the experimental group versus 399 m (P = .03) for the controls.
Several secondary endpoints measuring hemodynamics also favored PADN relative to the sham procedure at 12 months. This included the relative increase in tricuspid annular systolic excursion (P = .03) and the increase in the right ventricular fraction area (P < .001).
A total of 50 patients with residual CTEPH for at least 6 months after PEA despite medical therapy were enrolled and randomized. Entry criteria included a mean pulmonary artery pressure (PAP) of 25 mm Hg or greater or PVR greater than 400 dyn/sec per cm–5 on right heart catheterization. Patients with comorbidities associated with a life expectancy of less than 1 year were excluded.
Those randomized to the sham group were treated with riociguat over the course of follow-up. This therapy was not offered to patients in the PADN group, but all patients were blinded to the procedure and told that riociguat might or might not be administered.
Following the procedure, participating clinicians, who were also blinded to the procedure, were instructed to provide standard therapies for heart failure, such beta-blockers, diuretics, or digoxin, as needed. All patients were placed on an oral anticoagulant.
At 12 months the mean PAP (26 vs. 35 mm Hg; P < .001) and the mean systolic PAP (46 vs. 54 mm Hg; P = .01) were significantly lower in the PADN group versus those who underwent a sham procedure.
About 52% of the PADN group versus 12% of the sham group were classified as responders by the definition of a PVR reduction of at least 150 dyn/sec per cm–5 and 6MWT improvement of at least 20%, compared with baseline, reported Dr. Romanov, of the E. Meshalkin National Medical Research Center, ministry of health, Novosibirsk, Russia, and coinvestigators.
Of the three deaths caused by heart failure over the course of follow-up, two occurred in the sham group. Of the eight hospitalizations for heart failure, seven (29% of the sham group) occurred among controls versus one in those treated with PADN (4% of this group; P = .049).
There was one groin hematoma at the puncture site in each group. Both resolved without any consequences prior to hospital discharge. There were no other significant procedure-related complications in either group.
Larger multicenter trials are needed to confirm these findings, according to both the trial investigators and Marius M. Hoeper, MD, who is charge of the pulmonary hypertension program at the Hannover (Germany) Medical School.
In an editorial that accompanied publication of these findings, Dr. Hoeper identified the small sample size of this study as one of its limitations, but he said the results are consistent with several other small studies associating pulmonary artery denervation with benefit in pulmonary hypertension.
“It appears as if we are currently witnessing the emergence of a new treatment option for various forms of pulmonary hypertension,” Dr. Hoeper wrote. In his critique of the study, he suggested that it would have been “more informative” if both groups were on background riociguat, but the data from this and other studies so far indicates that ablation to achieve denervation “is safe and feasible.”
The PADN technique used in this study might be relevant to the results. Dr. Hoeper noted that the investigators employed catheter tip–based electroanatomic mapping with a novel remote navigation system with three-dimensional imaging of the right ventricle and central pulmonary arteries.
“Apparently, this approach minimizes radiation exposure and provides precise location of ablation sites,” Dr. Hoeper observed. However, he called for direct comparisons of this tool to the guidance systems used in other studies.
In an interview, Dr. Hoeper acknowledged that it is not yet clear that a large-scale trial of pulmonary artery denervation for the indication evaluated in this study is coming. He noted several strategies in CTEPH are widely used without trials confirming a reduction in clinical events.
“Balloon pulmonary angioplasty for CTEPH has become an established treatment around the world without any randomized, controlled trial and without demonstration of improved outcomes. A couple of well-conducted observational trials might be sufficient to convince physicians to introduce PADN as well,” he said. If such studies associated PADN with “improvements in hemodynamics, exercise capacity, and patient-reported outcomes, it might be sufficient.”
Currently, Dr. Hoeper is most concerned about obtaining further evidence of safety, which he characterized as a “major issue.”
If a multicenter trial is conducted “the primary endpoint should be focused on clinical events,” according to Dr. Romanov, who was asked to comment on the next steps in validating PADN for the treatment of CTEPH-associated pulmonary hypertension persisting after endarterectomy.
“The mortality rate during 1-year long-term follow-up is not so high, but heart failure progression is a problem. So in my view, the primary endpoint should be a composite of death and heart failure hospitalization,” he said. He called for follow-up duration of 2-3 years.
Jonathan Steinberg, MD, director of cardiac clinical trials and education, Summit Medical Group, Montclair, N.J., also called a trial with hard endpoints, such as death, the ideal.
In the meantime, hemodynamic and functional measures “are still quite valuable and move the ball forward for this intervention,” he said in an interview. Senior author of this trial and principle investigator of the recent ERADICATE-AF trial, which evaluated renal denervation in preventing recurrence of atrial fibrillation (JAMA. 2020;323:248-55), Dr. Steinberg predicted, “I do indeed suspect we will see trials that are more accomplishable [than a large-scale, randomized, controlled trial] in the not too distant future.”
Dr. Romanov received funding from Biosense Webster. Dr. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, Janssen, Merck Sharp & Dohme, and Pfizer.
SOURCE: Romanov A et al. J Am Coll Cardiol. 2020 Aug 17;76:916-26.
Pulmonary artery denervation (PADN) provides persistent and clinically significant hemodynamic improvements in patients with persistent chronic thromboembolic hypertension (CTEPH) after pulmonary endarterectomy (PEA), according to a randomized, sham-controlled trial.
“PADN in patients with CTEPH after PEA was safe and effective,” according to an investigating team led by Alexander Romanov, MD, PhD.
The mean reduction in pulmonary vascular resistance (PVR) was 258 dyn/sec per cm–5 for those randomized to PADN versus 149 dyn/sec per cm–5 (P = .001) for those randomized to the sham procedure, according to the newly published findings.
For the 6-minute walk test (6MWT), the mean distance was 470 m for the experimental group versus 399 m (P = .03) for the controls.
Several secondary endpoints measuring hemodynamics also favored PADN relative to the sham procedure at 12 months. This included the relative increase in tricuspid annular systolic excursion (P = .03) and the increase in the right ventricular fraction area (P < .001).
A total of 50 patients with residual CTEPH for at least 6 months after PEA despite medical therapy were enrolled and randomized. Entry criteria included a mean pulmonary artery pressure (PAP) of 25 mm Hg or greater or PVR greater than 400 dyn/sec per cm–5 on right heart catheterization. Patients with comorbidities associated with a life expectancy of less than 1 year were excluded.
Those randomized to the sham group were treated with riociguat over the course of follow-up. This therapy was not offered to patients in the PADN group, but all patients were blinded to the procedure and told that riociguat might or might not be administered.
Following the procedure, participating clinicians, who were also blinded to the procedure, were instructed to provide standard therapies for heart failure, such beta-blockers, diuretics, or digoxin, as needed. All patients were placed on an oral anticoagulant.
At 12 months the mean PAP (26 vs. 35 mm Hg; P < .001) and the mean systolic PAP (46 vs. 54 mm Hg; P = .01) were significantly lower in the PADN group versus those who underwent a sham procedure.
About 52% of the PADN group versus 12% of the sham group were classified as responders by the definition of a PVR reduction of at least 150 dyn/sec per cm–5 and 6MWT improvement of at least 20%, compared with baseline, reported Dr. Romanov, of the E. Meshalkin National Medical Research Center, ministry of health, Novosibirsk, Russia, and coinvestigators.
Of the three deaths caused by heart failure over the course of follow-up, two occurred in the sham group. Of the eight hospitalizations for heart failure, seven (29% of the sham group) occurred among controls versus one in those treated with PADN (4% of this group; P = .049).
There was one groin hematoma at the puncture site in each group. Both resolved without any consequences prior to hospital discharge. There were no other significant procedure-related complications in either group.
Larger multicenter trials are needed to confirm these findings, according to both the trial investigators and Marius M. Hoeper, MD, who is charge of the pulmonary hypertension program at the Hannover (Germany) Medical School.
In an editorial that accompanied publication of these findings, Dr. Hoeper identified the small sample size of this study as one of its limitations, but he said the results are consistent with several other small studies associating pulmonary artery denervation with benefit in pulmonary hypertension.
“It appears as if we are currently witnessing the emergence of a new treatment option for various forms of pulmonary hypertension,” Dr. Hoeper wrote. In his critique of the study, he suggested that it would have been “more informative” if both groups were on background riociguat, but the data from this and other studies so far indicates that ablation to achieve denervation “is safe and feasible.”
The PADN technique used in this study might be relevant to the results. Dr. Hoeper noted that the investigators employed catheter tip–based electroanatomic mapping with a novel remote navigation system with three-dimensional imaging of the right ventricle and central pulmonary arteries.
“Apparently, this approach minimizes radiation exposure and provides precise location of ablation sites,” Dr. Hoeper observed. However, he called for direct comparisons of this tool to the guidance systems used in other studies.
In an interview, Dr. Hoeper acknowledged that it is not yet clear that a large-scale trial of pulmonary artery denervation for the indication evaluated in this study is coming. He noted several strategies in CTEPH are widely used without trials confirming a reduction in clinical events.
“Balloon pulmonary angioplasty for CTEPH has become an established treatment around the world without any randomized, controlled trial and without demonstration of improved outcomes. A couple of well-conducted observational trials might be sufficient to convince physicians to introduce PADN as well,” he said. If such studies associated PADN with “improvements in hemodynamics, exercise capacity, and patient-reported outcomes, it might be sufficient.”
Currently, Dr. Hoeper is most concerned about obtaining further evidence of safety, which he characterized as a “major issue.”
If a multicenter trial is conducted “the primary endpoint should be focused on clinical events,” according to Dr. Romanov, who was asked to comment on the next steps in validating PADN for the treatment of CTEPH-associated pulmonary hypertension persisting after endarterectomy.
“The mortality rate during 1-year long-term follow-up is not so high, but heart failure progression is a problem. So in my view, the primary endpoint should be a composite of death and heart failure hospitalization,” he said. He called for follow-up duration of 2-3 years.
Jonathan Steinberg, MD, director of cardiac clinical trials and education, Summit Medical Group, Montclair, N.J., also called a trial with hard endpoints, such as death, the ideal.
In the meantime, hemodynamic and functional measures “are still quite valuable and move the ball forward for this intervention,” he said in an interview. Senior author of this trial and principle investigator of the recent ERADICATE-AF trial, which evaluated renal denervation in preventing recurrence of atrial fibrillation (JAMA. 2020;323:248-55), Dr. Steinberg predicted, “I do indeed suspect we will see trials that are more accomplishable [than a large-scale, randomized, controlled trial] in the not too distant future.”
Dr. Romanov received funding from Biosense Webster. Dr. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, Janssen, Merck Sharp & Dohme, and Pfizer.
SOURCE: Romanov A et al. J Am Coll Cardiol. 2020 Aug 17;76:916-26.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Machine learning shows ability to predict diastolic dysfunction with ECG
A machine-learning model that uses readily available clinical and electrocardiography data may have the potential to identify left ventricular (LV) diastolic dysfunction, a key biomarker in predicting heart failure, without echocardiography, but a workable clinical platform is still far off, a team of North American researchers reported.
“This cost-effective strategy may be a valuable first clinical step for assessing the presence of LV dysfunction and may potentially aid in the early diagnosis and management of heart failure patients,” Nobuyuki Kagiyama, MD, PhD, of West Virginia University, Morgantown, and colleagues, wrote in the Journal of the American Academy of Cardiology.
The researchers reported on a multicenter, prospective study that evaluated 1,202 patients from three centers in the United States and one in Canada. To develop machine-learning models, the study pooled 814 patients from the U.S. institutions as an internal cohort. They were then randomly divided into a training set and an internal test set on an 80:20 basis (651 and 163). The 388 Canadian patients were reserved as an external set to test the model.
All patients had 12-lead ECG and simultaneous body surface signal-processed ECG (spECG) along with comprehensive two-dimensional Doppler ECG on the same day.
How the model works
The machine-learning model estimated echocardiographic LV relaxation velocities (e’) values using traditional ECG and spECG features. The model also took into account 10 basic clinical features: age; sex; systolic and diastolic blood pressure; and comorbid conditions such as cerebrovascular and cardiovascular disease, diabetes, hypertension, dyslipidemia, and chronic kidney disease.
Patient characteristics were starkly different between the internal (United States) and external (Canadian) cohorts, with the latter being 10 years older on average (65 vs. 44; P < .001), predominantly male (58.2% vs. 47.3%; P < .001) and with significantly lower rates of coronary artery disease (1.8% vs. 21.1%; P < .001), although average blood pressure was similar between the two groups.
The study used area under the curve (AUC) to calculate the predictability of the machine-learning estimated e’ values versus the guideline-based reduced e’, finding close correlation between the internal (AUC, 0.83; sensitivity, 78%; specificity, 77%; negative predictive value, 73%; and positive predictive value, 82%) and external test sets (AUC, 0.84; sensitivity, 90%; specificity, 61%; NPV, 81%; and PPV, 77%).
Similar variations between the two cohorts were reported for global LV diastolic dysfunction and reduced LV ejection fraction.
The final model used 18 features in all, including 3 clinical features (age, dyslipidemia, and hypertension), 7 scores from spECG features, and 8 from traditional ECG features.
Interpreting the results
Dr. Kagiyama and colleagues noted that, because impaired myocardial relaxation is an early sign of cardiac tissue deterioration, screening for it can aid in early detection of subclinical LVDD and earlier treatment for hypertension and diabetes. But they acknowledged that further studies are needed.
In an invited editorial, Khurram Nasir, MD, MPH, MSc, of Houston Methodist DeBakey Heart and Vascular Center and Rohan Khera, MD, MS, of Yale University, New Haven, Conn., wrote that the machine-learning model has a way to go.
They noted that the 73%-77% accuracy of the model in identifying diastolic dysfunction impedes its imminent use. “Although we are excited about the prospects of such developments, we hold out for better evidence for their actual use,” they wrote, adding that the algorithms have limited use in the clinic because most patients already get “definitive testing” if they need it.
Developing a machine-learning model that obviates the need for ECG for evaluating LV diastolic dysfunction seems dubious at this time, said Luigi Di Biase, MD, PhD, section head of electrophysiology and director of arrhythmia services at Montefiore Medical Center and professor at Albert Einstein College of Medicine, both in New York. “The echo is not a difficult test. It’s the most proven usable tool that we have in cardiology because it’s easy to reproduce, low cost, and noninvasive – so we have all that we want in medicine.”
But machine learning does have potential, added Dr. Di Biase, who’s also a member of the American College of Cardiology’s Electrophysiology Section Leadership Council. “If this application could predict the people that would develop diastolic dysfunction that leads to heart failure – because an echo at that time may be negative but there may be other features that tell me this patient will develop disease – then it would have a much different clinical impact.”
The National Science Foundation provided funding for the study. Heart Test Laboratories, doing business as Heart Sciences, provided funding and spECG devices. Dr. Kagiyama reported receiving a research grant from Hitachi Healthcare. A coauthor disclosed financial relationships with Heart Sciences, Ultronics, and Kencor Health.
Dr. Nasir, Dr. Khera, and Dr. Di Biase have no relevant financial relationships to disclose.
SOURCE: Kagiyama N et al. J Am Coll Cardiol. 2020;76:930-41.
A machine-learning model that uses readily available clinical and electrocardiography data may have the potential to identify left ventricular (LV) diastolic dysfunction, a key biomarker in predicting heart failure, without echocardiography, but a workable clinical platform is still far off, a team of North American researchers reported.
“This cost-effective strategy may be a valuable first clinical step for assessing the presence of LV dysfunction and may potentially aid in the early diagnosis and management of heart failure patients,” Nobuyuki Kagiyama, MD, PhD, of West Virginia University, Morgantown, and colleagues, wrote in the Journal of the American Academy of Cardiology.
The researchers reported on a multicenter, prospective study that evaluated 1,202 patients from three centers in the United States and one in Canada. To develop machine-learning models, the study pooled 814 patients from the U.S. institutions as an internal cohort. They were then randomly divided into a training set and an internal test set on an 80:20 basis (651 and 163). The 388 Canadian patients were reserved as an external set to test the model.
All patients had 12-lead ECG and simultaneous body surface signal-processed ECG (spECG) along with comprehensive two-dimensional Doppler ECG on the same day.
How the model works
The machine-learning model estimated echocardiographic LV relaxation velocities (e’) values using traditional ECG and spECG features. The model also took into account 10 basic clinical features: age; sex; systolic and diastolic blood pressure; and comorbid conditions such as cerebrovascular and cardiovascular disease, diabetes, hypertension, dyslipidemia, and chronic kidney disease.
Patient characteristics were starkly different between the internal (United States) and external (Canadian) cohorts, with the latter being 10 years older on average (65 vs. 44; P < .001), predominantly male (58.2% vs. 47.3%; P < .001) and with significantly lower rates of coronary artery disease (1.8% vs. 21.1%; P < .001), although average blood pressure was similar between the two groups.
The study used area under the curve (AUC) to calculate the predictability of the machine-learning estimated e’ values versus the guideline-based reduced e’, finding close correlation between the internal (AUC, 0.83; sensitivity, 78%; specificity, 77%; negative predictive value, 73%; and positive predictive value, 82%) and external test sets (AUC, 0.84; sensitivity, 90%; specificity, 61%; NPV, 81%; and PPV, 77%).
Similar variations between the two cohorts were reported for global LV diastolic dysfunction and reduced LV ejection fraction.
The final model used 18 features in all, including 3 clinical features (age, dyslipidemia, and hypertension), 7 scores from spECG features, and 8 from traditional ECG features.
Interpreting the results
Dr. Kagiyama and colleagues noted that, because impaired myocardial relaxation is an early sign of cardiac tissue deterioration, screening for it can aid in early detection of subclinical LVDD and earlier treatment for hypertension and diabetes. But they acknowledged that further studies are needed.
In an invited editorial, Khurram Nasir, MD, MPH, MSc, of Houston Methodist DeBakey Heart and Vascular Center and Rohan Khera, MD, MS, of Yale University, New Haven, Conn., wrote that the machine-learning model has a way to go.
They noted that the 73%-77% accuracy of the model in identifying diastolic dysfunction impedes its imminent use. “Although we are excited about the prospects of such developments, we hold out for better evidence for their actual use,” they wrote, adding that the algorithms have limited use in the clinic because most patients already get “definitive testing” if they need it.
Developing a machine-learning model that obviates the need for ECG for evaluating LV diastolic dysfunction seems dubious at this time, said Luigi Di Biase, MD, PhD, section head of electrophysiology and director of arrhythmia services at Montefiore Medical Center and professor at Albert Einstein College of Medicine, both in New York. “The echo is not a difficult test. It’s the most proven usable tool that we have in cardiology because it’s easy to reproduce, low cost, and noninvasive – so we have all that we want in medicine.”
But machine learning does have potential, added Dr. Di Biase, who’s also a member of the American College of Cardiology’s Electrophysiology Section Leadership Council. “If this application could predict the people that would develop diastolic dysfunction that leads to heart failure – because an echo at that time may be negative but there may be other features that tell me this patient will develop disease – then it would have a much different clinical impact.”
The National Science Foundation provided funding for the study. Heart Test Laboratories, doing business as Heart Sciences, provided funding and spECG devices. Dr. Kagiyama reported receiving a research grant from Hitachi Healthcare. A coauthor disclosed financial relationships with Heart Sciences, Ultronics, and Kencor Health.
Dr. Nasir, Dr. Khera, and Dr. Di Biase have no relevant financial relationships to disclose.
SOURCE: Kagiyama N et al. J Am Coll Cardiol. 2020;76:930-41.
A machine-learning model that uses readily available clinical and electrocardiography data may have the potential to identify left ventricular (LV) diastolic dysfunction, a key biomarker in predicting heart failure, without echocardiography, but a workable clinical platform is still far off, a team of North American researchers reported.
“This cost-effective strategy may be a valuable first clinical step for assessing the presence of LV dysfunction and may potentially aid in the early diagnosis and management of heart failure patients,” Nobuyuki Kagiyama, MD, PhD, of West Virginia University, Morgantown, and colleagues, wrote in the Journal of the American Academy of Cardiology.
The researchers reported on a multicenter, prospective study that evaluated 1,202 patients from three centers in the United States and one in Canada. To develop machine-learning models, the study pooled 814 patients from the U.S. institutions as an internal cohort. They were then randomly divided into a training set and an internal test set on an 80:20 basis (651 and 163). The 388 Canadian patients were reserved as an external set to test the model.
All patients had 12-lead ECG and simultaneous body surface signal-processed ECG (spECG) along with comprehensive two-dimensional Doppler ECG on the same day.
How the model works
The machine-learning model estimated echocardiographic LV relaxation velocities (e’) values using traditional ECG and spECG features. The model also took into account 10 basic clinical features: age; sex; systolic and diastolic blood pressure; and comorbid conditions such as cerebrovascular and cardiovascular disease, diabetes, hypertension, dyslipidemia, and chronic kidney disease.
Patient characteristics were starkly different between the internal (United States) and external (Canadian) cohorts, with the latter being 10 years older on average (65 vs. 44; P < .001), predominantly male (58.2% vs. 47.3%; P < .001) and with significantly lower rates of coronary artery disease (1.8% vs. 21.1%; P < .001), although average blood pressure was similar between the two groups.
The study used area under the curve (AUC) to calculate the predictability of the machine-learning estimated e’ values versus the guideline-based reduced e’, finding close correlation between the internal (AUC, 0.83; sensitivity, 78%; specificity, 77%; negative predictive value, 73%; and positive predictive value, 82%) and external test sets (AUC, 0.84; sensitivity, 90%; specificity, 61%; NPV, 81%; and PPV, 77%).
Similar variations between the two cohorts were reported for global LV diastolic dysfunction and reduced LV ejection fraction.
The final model used 18 features in all, including 3 clinical features (age, dyslipidemia, and hypertension), 7 scores from spECG features, and 8 from traditional ECG features.
Interpreting the results
Dr. Kagiyama and colleagues noted that, because impaired myocardial relaxation is an early sign of cardiac tissue deterioration, screening for it can aid in early detection of subclinical LVDD and earlier treatment for hypertension and diabetes. But they acknowledged that further studies are needed.
In an invited editorial, Khurram Nasir, MD, MPH, MSc, of Houston Methodist DeBakey Heart and Vascular Center and Rohan Khera, MD, MS, of Yale University, New Haven, Conn., wrote that the machine-learning model has a way to go.
They noted that the 73%-77% accuracy of the model in identifying diastolic dysfunction impedes its imminent use. “Although we are excited about the prospects of such developments, we hold out for better evidence for their actual use,” they wrote, adding that the algorithms have limited use in the clinic because most patients already get “definitive testing” if they need it.
Developing a machine-learning model that obviates the need for ECG for evaluating LV diastolic dysfunction seems dubious at this time, said Luigi Di Biase, MD, PhD, section head of electrophysiology and director of arrhythmia services at Montefiore Medical Center and professor at Albert Einstein College of Medicine, both in New York. “The echo is not a difficult test. It’s the most proven usable tool that we have in cardiology because it’s easy to reproduce, low cost, and noninvasive – so we have all that we want in medicine.”
But machine learning does have potential, added Dr. Di Biase, who’s also a member of the American College of Cardiology’s Electrophysiology Section Leadership Council. “If this application could predict the people that would develop diastolic dysfunction that leads to heart failure – because an echo at that time may be negative but there may be other features that tell me this patient will develop disease – then it would have a much different clinical impact.”
The National Science Foundation provided funding for the study. Heart Test Laboratories, doing business as Heart Sciences, provided funding and spECG devices. Dr. Kagiyama reported receiving a research grant from Hitachi Healthcare. A coauthor disclosed financial relationships with Heart Sciences, Ultronics, and Kencor Health.
Dr. Nasir, Dr. Khera, and Dr. Di Biase have no relevant financial relationships to disclose.
SOURCE: Kagiyama N et al. J Am Coll Cardiol. 2020;76:930-41.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Ulcerated hand lesion
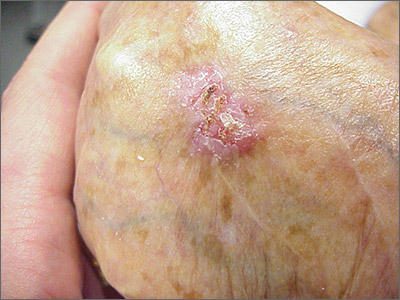
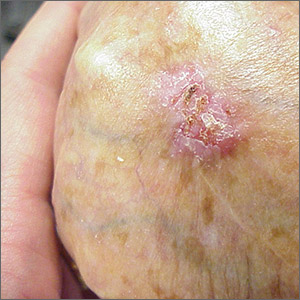
While the eroded scabbed area in a raised lesion with pearly borders raised the suspicion of basal cell carcinoma (BCC), a superficial shave biopsy revealed that this was sclerosing basosquamous carcinoma.
Basosquamous carcinoma is an uncommon form of BCC. It features nests/clusters of basaloid cells arising from the basal layer of the epidermis (a hallmark of BCC), as well as keratinization, which is seen in squamous cell carcinoma (SCC). Basosquamous carcinoma occurs in the same areas as more common types of nonmelanoma skin cancers (NMSCs)—that is, sun-exposed areas. Not surprisingly, then, the highest risk areas for basosquamous carcinoma are the face, hands, arms, scalp (in those without hair), and back. People with occupational or intentional sun exposure through tanning have even higher rates of NMSC than the average population; patients using tanning booths exposing their entire bodies warrant more extensive physical exams.
Basosquamous carcinoma is one of the subtypes of BCC that is associated with a higher risk of local invasion, recurrence, and metastasis. Treatment with Mohs micrographic surgery (MMS) reduces the risk of recurrence to 4% (from 12% to 51%). Even with MMS, basosquamous carcinoma can be particularly challenging. It may require additional excision stages, as well as a larger than anticipated excision size at the time of surgery.
In this case, the patient was referred for MMS due to the high-risk nature of this lesion.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J Am Acad Dermatol. 2009;60:137-143.

While the eroded scabbed area in a raised lesion with pearly borders raised the suspicion of basal cell carcinoma (BCC), a superficial shave biopsy revealed that this was sclerosing basosquamous carcinoma.
Basosquamous carcinoma is an uncommon form of BCC. It features nests/clusters of basaloid cells arising from the basal layer of the epidermis (a hallmark of BCC), as well as keratinization, which is seen in squamous cell carcinoma (SCC). Basosquamous carcinoma occurs in the same areas as more common types of nonmelanoma skin cancers (NMSCs)—that is, sun-exposed areas. Not surprisingly, then, the highest risk areas for basosquamous carcinoma are the face, hands, arms, scalp (in those without hair), and back. People with occupational or intentional sun exposure through tanning have even higher rates of NMSC than the average population; patients using tanning booths exposing their entire bodies warrant more extensive physical exams.
Basosquamous carcinoma is one of the subtypes of BCC that is associated with a higher risk of local invasion, recurrence, and metastasis. Treatment with Mohs micrographic surgery (MMS) reduces the risk of recurrence to 4% (from 12% to 51%). Even with MMS, basosquamous carcinoma can be particularly challenging. It may require additional excision stages, as well as a larger than anticipated excision size at the time of surgery.
In this case, the patient was referred for MMS due to the high-risk nature of this lesion.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

While the eroded scabbed area in a raised lesion with pearly borders raised the suspicion of basal cell carcinoma (BCC), a superficial shave biopsy revealed that this was sclerosing basosquamous carcinoma.
Basosquamous carcinoma is an uncommon form of BCC. It features nests/clusters of basaloid cells arising from the basal layer of the epidermis (a hallmark of BCC), as well as keratinization, which is seen in squamous cell carcinoma (SCC). Basosquamous carcinoma occurs in the same areas as more common types of nonmelanoma skin cancers (NMSCs)—that is, sun-exposed areas. Not surprisingly, then, the highest risk areas for basosquamous carcinoma are the face, hands, arms, scalp (in those without hair), and back. People with occupational or intentional sun exposure through tanning have even higher rates of NMSC than the average population; patients using tanning booths exposing their entire bodies warrant more extensive physical exams.
Basosquamous carcinoma is one of the subtypes of BCC that is associated with a higher risk of local invasion, recurrence, and metastasis. Treatment with Mohs micrographic surgery (MMS) reduces the risk of recurrence to 4% (from 12% to 51%). Even with MMS, basosquamous carcinoma can be particularly challenging. It may require additional excision stages, as well as a larger than anticipated excision size at the time of surgery.
In this case, the patient was referred for MMS due to the high-risk nature of this lesion.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J Am Acad Dermatol. 2009;60:137-143.
Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J Am Acad Dermatol. 2009;60:137-143.
Canakinumab (Ilaris) tapering tested in systemic JIA trial
A trial that tested two ways of tapering canakinumab (Ilaris) monotherapy in children with systemic juvenile idiopathic arthritis (sJIA) showed that both approaches might be feasible, but the lack of a control arm means that more data are needed before putting either into routine clinical practice.
Pierre Quartier, MD, and associates reported in Arthritis & Rheumatology. That’s with the proviso that children are taking canakinumab at the recommended dose of 4 mg/kg every 4 weeks and achieve clinical remission before any tapering is started.
The researchers found that 70%-80% of children who were in complete clinical remission (CR) maintained this for 6 months after the first dose-tapering step had been taken. However, at least one-fifth of study participants experienced a disease flare during treatment withdrawal, and only one-third were able to discontinue treatment altogether, which suggests continued treatment is needed.
“The results are a step in the right direction,” commented Athimaleipet Ramanan, MBBS, a consultant pediatric rheumatologist who was not involved in the study. They “offer the first vision of whether we can actually taper a medication” in sJIA because “until now we have not had any evidence for this,” he added.
“What we really need to know, which the study doesn’t tell you, is: Is decreasing the dose better than stopping?” said Dr. Ramanan, of the Bristol (England) Royal Hospital for Children.
Another thing that is important to know is: Does reducing the dose lead to the development of anti-drug antibodies? he said.
“There were some concerns that, when you give less of a monoclonal antibody, you might get more neutralizing anti-drug antibodies or you might make a drug more immunogenic,” he said. These data perhaps suggest that this isn’t the case because only one child on one occasion had detectable non-neutralizing anti-drug antibodies during the entire study, and that was 11 weeks after the last dose of canakinumab had been given.
Study results and design
Canakinumab is a monoclonal antibody that inhibits interleukin-1 (IL-1) that has been approved in the United States and Europe for the treatment of sJIA since 2013. Reducing a child’s exposure to canakinumab once their disease is under control is an attractive proposition given the treat-to-target approach used increasingly throughout modern rheumatologic practice. It could also help reduce the cost of what is an expensive treatment, compared with other available options for sJIA such as the interleukin-6 inhibitor tocilizumab (Actemra) or another IL-1 inhibitor anakinra (Kineret), which is not FDA-approved for use in sJIA and requires weekly injections.
“We don’t want to the disease to reappear, to flare, but we also don’t want to overuse treatments in these patients,” explained Dr. Quartier of Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris in an interview.
The study he coconducted, given the acronym B-SPECIFIC-4 Patients, was an open-label study that consisted of two parts. In part 1, 182 children were given subcutaneous canakinumab 4 mg/kg every 4 weeks. In part 2, 76 children who were in complete CR after canakinumab treatment were randomized to one of two tapering strategies. In one arm, the dose of canakinumab was reduced from 4 mg/kg to 2 mg/kg given every 4 weeks before eventually discontinuing treatment, and in other arm the duration between dosing was increased by 4-week intervals, from 4 to 8 weeks, then 12 weeks, then discontinuation.
If children were taking glucocorticoids or methotrexate, the treating physicians were encouraged to stop these medications if possible, with 34%-39% and 42%-59% of children, respectively, being able to do so. The rate depended on whether children had received canakinumab before entering the study because some had been recruited from a long-term extension study while others were naive to the biologic; all had inactive disease at study entry.
This was the first study to evaluate the effectiveness of canakinumab in enabling the discontinuation of methotrexate, but the primary objective was to assess whether more than 40% of randomized patients in either discontinuation arm remained in CR for 24 weeks after the first step of discontinuation. This was achieved in 71% of children who were in the dose-reduction arm and in 84% of children in the dose-prolongation arm (P ≤ .0001 for each arm vs. 40%).
Prior exposure to canakinumab did not seem to affect the maintenance of CR, but it was also found that more children who maintained CR at their second but not their first attempt at tapering were in CR than those who were still in CR at the first step (76% and 89% in the dose-reduction and dose-prolongation arms, respectively).
Among the two dosing regimens, failure occurred in 18% of children in the dose-reduction arm at the first step, 10% at the second, and 8% at the third, whereas 2.7% of children in the dose-prolongation arm experienced regimen failure at the first step, 6.1% at the second, and 15% at the third.
No substantial difference between the two tapering approaches was observed because the study was not powered to look at this. There was also no control arm, such as a group continuing treatment while the other groups tapered, or as Dr. Ramanan had pointed out, stopping canakinumab altogether.
“As long as the treatment was continued, even at very low dosage, most patients remained in inactive disease,” Dr. Quartier said. He added, however, that only a minority of patients could stop treatment completely. Treatment should not be stopped abruptly, he advised, because this was associated with a substantial number of disease flares that needed treatment to be reinstated.
These findings suggest that “a certain level of sustained inhibition of the IL-1 pathway seems important for the maintenance of CR in most sJIA patients,” Dr. Quartier and coinvestigators wrote in their article.
They added: “We believe that these results are relevant for clinical practice, particularly for designing personalized tapering strategies that can allow an adequate control of disease while minimizing the side effects of certain medications, notably glucocorticoids.”
The study was funded by Novartis in collaboration with the Pediatric Rheumatology International Trials Organization and the Pediatric Rheumatology Collaborative Study Group. Dr. Quartier was an investigator for the trial and has received research and consultancy fees from Novartis, among other pharmaceutical companies. Two of his coauthors are employees of Novartis. Dr. Ramanan has acted as an investigator in prior canakinumab trials and has received consultancy fees from Novartis and multiple other companies.
SOURCE: Quartier P et al. Arthritis Rheumatol. 2020 Aug 11. doi: 10.1002/art.41488.
A trial that tested two ways of tapering canakinumab (Ilaris) monotherapy in children with systemic juvenile idiopathic arthritis (sJIA) showed that both approaches might be feasible, but the lack of a control arm means that more data are needed before putting either into routine clinical practice.
Pierre Quartier, MD, and associates reported in Arthritis & Rheumatology. That’s with the proviso that children are taking canakinumab at the recommended dose of 4 mg/kg every 4 weeks and achieve clinical remission before any tapering is started.
The researchers found that 70%-80% of children who were in complete clinical remission (CR) maintained this for 6 months after the first dose-tapering step had been taken. However, at least one-fifth of study participants experienced a disease flare during treatment withdrawal, and only one-third were able to discontinue treatment altogether, which suggests continued treatment is needed.
“The results are a step in the right direction,” commented Athimaleipet Ramanan, MBBS, a consultant pediatric rheumatologist who was not involved in the study. They “offer the first vision of whether we can actually taper a medication” in sJIA because “until now we have not had any evidence for this,” he added.
“What we really need to know, which the study doesn’t tell you, is: Is decreasing the dose better than stopping?” said Dr. Ramanan, of the Bristol (England) Royal Hospital for Children.
Another thing that is important to know is: Does reducing the dose lead to the development of anti-drug antibodies? he said.
“There were some concerns that, when you give less of a monoclonal antibody, you might get more neutralizing anti-drug antibodies or you might make a drug more immunogenic,” he said. These data perhaps suggest that this isn’t the case because only one child on one occasion had detectable non-neutralizing anti-drug antibodies during the entire study, and that was 11 weeks after the last dose of canakinumab had been given.
Study results and design
Canakinumab is a monoclonal antibody that inhibits interleukin-1 (IL-1) that has been approved in the United States and Europe for the treatment of sJIA since 2013. Reducing a child’s exposure to canakinumab once their disease is under control is an attractive proposition given the treat-to-target approach used increasingly throughout modern rheumatologic practice. It could also help reduce the cost of what is an expensive treatment, compared with other available options for sJIA such as the interleukin-6 inhibitor tocilizumab (Actemra) or another IL-1 inhibitor anakinra (Kineret), which is not FDA-approved for use in sJIA and requires weekly injections.
“We don’t want to the disease to reappear, to flare, but we also don’t want to overuse treatments in these patients,” explained Dr. Quartier of Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris in an interview.
The study he coconducted, given the acronym B-SPECIFIC-4 Patients, was an open-label study that consisted of two parts. In part 1, 182 children were given subcutaneous canakinumab 4 mg/kg every 4 weeks. In part 2, 76 children who were in complete CR after canakinumab treatment were randomized to one of two tapering strategies. In one arm, the dose of canakinumab was reduced from 4 mg/kg to 2 mg/kg given every 4 weeks before eventually discontinuing treatment, and in other arm the duration between dosing was increased by 4-week intervals, from 4 to 8 weeks, then 12 weeks, then discontinuation.
If children were taking glucocorticoids or methotrexate, the treating physicians were encouraged to stop these medications if possible, with 34%-39% and 42%-59% of children, respectively, being able to do so. The rate depended on whether children had received canakinumab before entering the study because some had been recruited from a long-term extension study while others were naive to the biologic; all had inactive disease at study entry.
This was the first study to evaluate the effectiveness of canakinumab in enabling the discontinuation of methotrexate, but the primary objective was to assess whether more than 40% of randomized patients in either discontinuation arm remained in CR for 24 weeks after the first step of discontinuation. This was achieved in 71% of children who were in the dose-reduction arm and in 84% of children in the dose-prolongation arm (P ≤ .0001 for each arm vs. 40%).
Prior exposure to canakinumab did not seem to affect the maintenance of CR, but it was also found that more children who maintained CR at their second but not their first attempt at tapering were in CR than those who were still in CR at the first step (76% and 89% in the dose-reduction and dose-prolongation arms, respectively).
Among the two dosing regimens, failure occurred in 18% of children in the dose-reduction arm at the first step, 10% at the second, and 8% at the third, whereas 2.7% of children in the dose-prolongation arm experienced regimen failure at the first step, 6.1% at the second, and 15% at the third.
No substantial difference between the two tapering approaches was observed because the study was not powered to look at this. There was also no control arm, such as a group continuing treatment while the other groups tapered, or as Dr. Ramanan had pointed out, stopping canakinumab altogether.
“As long as the treatment was continued, even at very low dosage, most patients remained in inactive disease,” Dr. Quartier said. He added, however, that only a minority of patients could stop treatment completely. Treatment should not be stopped abruptly, he advised, because this was associated with a substantial number of disease flares that needed treatment to be reinstated.
These findings suggest that “a certain level of sustained inhibition of the IL-1 pathway seems important for the maintenance of CR in most sJIA patients,” Dr. Quartier and coinvestigators wrote in their article.
They added: “We believe that these results are relevant for clinical practice, particularly for designing personalized tapering strategies that can allow an adequate control of disease while minimizing the side effects of certain medications, notably glucocorticoids.”
The study was funded by Novartis in collaboration with the Pediatric Rheumatology International Trials Organization and the Pediatric Rheumatology Collaborative Study Group. Dr. Quartier was an investigator for the trial and has received research and consultancy fees from Novartis, among other pharmaceutical companies. Two of his coauthors are employees of Novartis. Dr. Ramanan has acted as an investigator in prior canakinumab trials and has received consultancy fees from Novartis and multiple other companies.
SOURCE: Quartier P et al. Arthritis Rheumatol. 2020 Aug 11. doi: 10.1002/art.41488.
A trial that tested two ways of tapering canakinumab (Ilaris) monotherapy in children with systemic juvenile idiopathic arthritis (sJIA) showed that both approaches might be feasible, but the lack of a control arm means that more data are needed before putting either into routine clinical practice.
Pierre Quartier, MD, and associates reported in Arthritis & Rheumatology. That’s with the proviso that children are taking canakinumab at the recommended dose of 4 mg/kg every 4 weeks and achieve clinical remission before any tapering is started.
The researchers found that 70%-80% of children who were in complete clinical remission (CR) maintained this for 6 months after the first dose-tapering step had been taken. However, at least one-fifth of study participants experienced a disease flare during treatment withdrawal, and only one-third were able to discontinue treatment altogether, which suggests continued treatment is needed.
“The results are a step in the right direction,” commented Athimaleipet Ramanan, MBBS, a consultant pediatric rheumatologist who was not involved in the study. They “offer the first vision of whether we can actually taper a medication” in sJIA because “until now we have not had any evidence for this,” he added.
“What we really need to know, which the study doesn’t tell you, is: Is decreasing the dose better than stopping?” said Dr. Ramanan, of the Bristol (England) Royal Hospital for Children.
Another thing that is important to know is: Does reducing the dose lead to the development of anti-drug antibodies? he said.
“There were some concerns that, when you give less of a monoclonal antibody, you might get more neutralizing anti-drug antibodies or you might make a drug more immunogenic,” he said. These data perhaps suggest that this isn’t the case because only one child on one occasion had detectable non-neutralizing anti-drug antibodies during the entire study, and that was 11 weeks after the last dose of canakinumab had been given.
Study results and design
Canakinumab is a monoclonal antibody that inhibits interleukin-1 (IL-1) that has been approved in the United States and Europe for the treatment of sJIA since 2013. Reducing a child’s exposure to canakinumab once their disease is under control is an attractive proposition given the treat-to-target approach used increasingly throughout modern rheumatologic practice. It could also help reduce the cost of what is an expensive treatment, compared with other available options for sJIA such as the interleukin-6 inhibitor tocilizumab (Actemra) or another IL-1 inhibitor anakinra (Kineret), which is not FDA-approved for use in sJIA and requires weekly injections.
“We don’t want to the disease to reappear, to flare, but we also don’t want to overuse treatments in these patients,” explained Dr. Quartier of Hôpital Necker-Enfants Malades, Assistance Publique-Hôpitaux de Paris in an interview.
The study he coconducted, given the acronym B-SPECIFIC-4 Patients, was an open-label study that consisted of two parts. In part 1, 182 children were given subcutaneous canakinumab 4 mg/kg every 4 weeks. In part 2, 76 children who were in complete CR after canakinumab treatment were randomized to one of two tapering strategies. In one arm, the dose of canakinumab was reduced from 4 mg/kg to 2 mg/kg given every 4 weeks before eventually discontinuing treatment, and in other arm the duration between dosing was increased by 4-week intervals, from 4 to 8 weeks, then 12 weeks, then discontinuation.
If children were taking glucocorticoids or methotrexate, the treating physicians were encouraged to stop these medications if possible, with 34%-39% and 42%-59% of children, respectively, being able to do so. The rate depended on whether children had received canakinumab before entering the study because some had been recruited from a long-term extension study while others were naive to the biologic; all had inactive disease at study entry.
This was the first study to evaluate the effectiveness of canakinumab in enabling the discontinuation of methotrexate, but the primary objective was to assess whether more than 40% of randomized patients in either discontinuation arm remained in CR for 24 weeks after the first step of discontinuation. This was achieved in 71% of children who were in the dose-reduction arm and in 84% of children in the dose-prolongation arm (P ≤ .0001 for each arm vs. 40%).
Prior exposure to canakinumab did not seem to affect the maintenance of CR, but it was also found that more children who maintained CR at their second but not their first attempt at tapering were in CR than those who were still in CR at the first step (76% and 89% in the dose-reduction and dose-prolongation arms, respectively).
Among the two dosing regimens, failure occurred in 18% of children in the dose-reduction arm at the first step, 10% at the second, and 8% at the third, whereas 2.7% of children in the dose-prolongation arm experienced regimen failure at the first step, 6.1% at the second, and 15% at the third.
No substantial difference between the two tapering approaches was observed because the study was not powered to look at this. There was also no control arm, such as a group continuing treatment while the other groups tapered, or as Dr. Ramanan had pointed out, stopping canakinumab altogether.
“As long as the treatment was continued, even at very low dosage, most patients remained in inactive disease,” Dr. Quartier said. He added, however, that only a minority of patients could stop treatment completely. Treatment should not be stopped abruptly, he advised, because this was associated with a substantial number of disease flares that needed treatment to be reinstated.
These findings suggest that “a certain level of sustained inhibition of the IL-1 pathway seems important for the maintenance of CR in most sJIA patients,” Dr. Quartier and coinvestigators wrote in their article.
They added: “We believe that these results are relevant for clinical practice, particularly for designing personalized tapering strategies that can allow an adequate control of disease while minimizing the side effects of certain medications, notably glucocorticoids.”
The study was funded by Novartis in collaboration with the Pediatric Rheumatology International Trials Organization and the Pediatric Rheumatology Collaborative Study Group. Dr. Quartier was an investigator for the trial and has received research and consultancy fees from Novartis, among other pharmaceutical companies. Two of his coauthors are employees of Novartis. Dr. Ramanan has acted as an investigator in prior canakinumab trials and has received consultancy fees from Novartis and multiple other companies.
SOURCE: Quartier P et al. Arthritis Rheumatol. 2020 Aug 11. doi: 10.1002/art.41488.
FROM ARTHRITIS & RHEUMATOLOGY
Novel botulinum toxin type A earns high marks for forehead lines
, Jeremy B. Green, MD, said at the virtual annual meeting of the American Academy of Dermatology.
When the study is completed, conclusions can be reached about the investigational product’s durability of benefit for treatment of dynamic forehead lines, which are notoriously challenging to treat. However, much is already known about the product’s durability for treatment of glabellar lines, as demonstrated in SAKURA 1 and SAKURA 2, two pivotal, phase 3, multicenter, randomized, double-blind, placebo-controlled trials totaling 609 patients.
In SAKURA 1 and 2, glabellar line severity didn’t return to baseline until a median of 28 and 26 weeks after injection. In contrast, as the study authors noted, the majority of patients whose glabellar lines are treated with the currently available botulinum toxin type A products are no longer responders by 3-4 months after treatment. Since surveys indicate most patients receive repeated injections every 5-6 months, that means they’re walking around with suboptimal results for the last 2-3 months before their next treatment session (Plast Reconstr Surg. 2020 Jan;145[1]:45-58).
This investigational neuromodulator, known as DaxibotulinumtoxinA for Injection, or DAXI, is composed of a highly purified 150-KDa botulinum toxin type A coupled with a proprietary stabilizing peptide. The product is formulated without human serum albumin and, once reconstituted, is stable at room temperature.
Dr. Green, a dermatologist in private practice in Coral Gables, Fla., reported on 61 participants in the phase 2 study, all with moderate or severe forehead lines and glabellar lines as assessed by both investigators and patients on structured scales. The patients’ glabellar lines were treated with 40 U of DAXI at baseline. Then 2 weeks later, their dynamic forehead lines were treated with either 12 U, 18 U, 24 U, or 30 U of DAXI. This sequential treatment recapitulates the approach widely used in clinical practice, he noted.
At baseline, two-thirds of patients had severe forehead lines at maximum eyebrow elevation as determined by Investigator Global Assessment – Forehead Wrinkle Severity and Patient Forehead Wrinkle Severity. The other third of participants had moderate forehead lines.
The primary endpoint was the presence of no or mild forehead lines by investigator assessment 4 weeks after treatment. This was achieved in 86% of patients who received 12 U of DAXI, 87% who recieved 18 U, 94% who received 24 U, and 100% of those who received 30 U.
“There appears to be a dose-dependent response, but this hasn’t yet been statistically analyzed,” Dr. Green said.
By patient assessment, there were no or only mild forehead lines at 4 weeks in 57% of those who received the lowest dose of DAXI, with rates of 80%, 100%, and 93% in those who received 18 U, 24 U, and 30 U.
At week 4, 57% of patients who got 12 U of DAXI pronounced themselves “satisfied” or “very satisfied” with DAXI therapy, as did 73%, 100%, and 93% of those who got the higher doses.
The treatment-related adverse events consisted of a smattering of cases of edema, erythema, or headache, similar to what’s described in the product labeling of all the neuromodulators.
Revance Therapeutics has applied to the Food and Drug Administration for marketing approval of DAXI for the treatment of glabellar lines. A regulatory decision is expected in late November. The company is also developing DAXI for the treatment of variety of neurologic and musculoskeletal conditions, including poststroke upper limb spasticity.
In an interview, Dr. Green said he was favorably impressed with DAXI’s durability for amelioration of forehead lines in the patients he personally treated in the ongoing phase 2 study, although there was no head-to-head comparison with other neuromodulators in the trial. He’s not aware of any planned phase 3 trial aimed at obtaining a forehead line indication.
“Of course, all four of the neuromodulators currently approved in the U.S. have glabellar line indications, but all are also used off-label in other locations, so I would imagine that DAXI will be used similarly if and when it is FDA-approved,” the dermatologist added.
He reported serving as a paid investigator for Revance.
, Jeremy B. Green, MD, said at the virtual annual meeting of the American Academy of Dermatology.
When the study is completed, conclusions can be reached about the investigational product’s durability of benefit for treatment of dynamic forehead lines, which are notoriously challenging to treat. However, much is already known about the product’s durability for treatment of glabellar lines, as demonstrated in SAKURA 1 and SAKURA 2, two pivotal, phase 3, multicenter, randomized, double-blind, placebo-controlled trials totaling 609 patients.
In SAKURA 1 and 2, glabellar line severity didn’t return to baseline until a median of 28 and 26 weeks after injection. In contrast, as the study authors noted, the majority of patients whose glabellar lines are treated with the currently available botulinum toxin type A products are no longer responders by 3-4 months after treatment. Since surveys indicate most patients receive repeated injections every 5-6 months, that means they’re walking around with suboptimal results for the last 2-3 months before their next treatment session (Plast Reconstr Surg. 2020 Jan;145[1]:45-58).
This investigational neuromodulator, known as DaxibotulinumtoxinA for Injection, or DAXI, is composed of a highly purified 150-KDa botulinum toxin type A coupled with a proprietary stabilizing peptide. The product is formulated without human serum albumin and, once reconstituted, is stable at room temperature.
Dr. Green, a dermatologist in private practice in Coral Gables, Fla., reported on 61 participants in the phase 2 study, all with moderate or severe forehead lines and glabellar lines as assessed by both investigators and patients on structured scales. The patients’ glabellar lines were treated with 40 U of DAXI at baseline. Then 2 weeks later, their dynamic forehead lines were treated with either 12 U, 18 U, 24 U, or 30 U of DAXI. This sequential treatment recapitulates the approach widely used in clinical practice, he noted.
At baseline, two-thirds of patients had severe forehead lines at maximum eyebrow elevation as determined by Investigator Global Assessment – Forehead Wrinkle Severity and Patient Forehead Wrinkle Severity. The other third of participants had moderate forehead lines.
The primary endpoint was the presence of no or mild forehead lines by investigator assessment 4 weeks after treatment. This was achieved in 86% of patients who received 12 U of DAXI, 87% who recieved 18 U, 94% who received 24 U, and 100% of those who received 30 U.
“There appears to be a dose-dependent response, but this hasn’t yet been statistically analyzed,” Dr. Green said.
By patient assessment, there were no or only mild forehead lines at 4 weeks in 57% of those who received the lowest dose of DAXI, with rates of 80%, 100%, and 93% in those who received 18 U, 24 U, and 30 U.
At week 4, 57% of patients who got 12 U of DAXI pronounced themselves “satisfied” or “very satisfied” with DAXI therapy, as did 73%, 100%, and 93% of those who got the higher doses.
The treatment-related adverse events consisted of a smattering of cases of edema, erythema, or headache, similar to what’s described in the product labeling of all the neuromodulators.
Revance Therapeutics has applied to the Food and Drug Administration for marketing approval of DAXI for the treatment of glabellar lines. A regulatory decision is expected in late November. The company is also developing DAXI for the treatment of variety of neurologic and musculoskeletal conditions, including poststroke upper limb spasticity.
In an interview, Dr. Green said he was favorably impressed with DAXI’s durability for amelioration of forehead lines in the patients he personally treated in the ongoing phase 2 study, although there was no head-to-head comparison with other neuromodulators in the trial. He’s not aware of any planned phase 3 trial aimed at obtaining a forehead line indication.
“Of course, all four of the neuromodulators currently approved in the U.S. have glabellar line indications, but all are also used off-label in other locations, so I would imagine that DAXI will be used similarly if and when it is FDA-approved,” the dermatologist added.
He reported serving as a paid investigator for Revance.
, Jeremy B. Green, MD, said at the virtual annual meeting of the American Academy of Dermatology.
When the study is completed, conclusions can be reached about the investigational product’s durability of benefit for treatment of dynamic forehead lines, which are notoriously challenging to treat. However, much is already known about the product’s durability for treatment of glabellar lines, as demonstrated in SAKURA 1 and SAKURA 2, two pivotal, phase 3, multicenter, randomized, double-blind, placebo-controlled trials totaling 609 patients.
In SAKURA 1 and 2, glabellar line severity didn’t return to baseline until a median of 28 and 26 weeks after injection. In contrast, as the study authors noted, the majority of patients whose glabellar lines are treated with the currently available botulinum toxin type A products are no longer responders by 3-4 months after treatment. Since surveys indicate most patients receive repeated injections every 5-6 months, that means they’re walking around with suboptimal results for the last 2-3 months before their next treatment session (Plast Reconstr Surg. 2020 Jan;145[1]:45-58).
This investigational neuromodulator, known as DaxibotulinumtoxinA for Injection, or DAXI, is composed of a highly purified 150-KDa botulinum toxin type A coupled with a proprietary stabilizing peptide. The product is formulated without human serum albumin and, once reconstituted, is stable at room temperature.
Dr. Green, a dermatologist in private practice in Coral Gables, Fla., reported on 61 participants in the phase 2 study, all with moderate or severe forehead lines and glabellar lines as assessed by both investigators and patients on structured scales. The patients’ glabellar lines were treated with 40 U of DAXI at baseline. Then 2 weeks later, their dynamic forehead lines were treated with either 12 U, 18 U, 24 U, or 30 U of DAXI. This sequential treatment recapitulates the approach widely used in clinical practice, he noted.
At baseline, two-thirds of patients had severe forehead lines at maximum eyebrow elevation as determined by Investigator Global Assessment – Forehead Wrinkle Severity and Patient Forehead Wrinkle Severity. The other third of participants had moderate forehead lines.
The primary endpoint was the presence of no or mild forehead lines by investigator assessment 4 weeks after treatment. This was achieved in 86% of patients who received 12 U of DAXI, 87% who recieved 18 U, 94% who received 24 U, and 100% of those who received 30 U.
“There appears to be a dose-dependent response, but this hasn’t yet been statistically analyzed,” Dr. Green said.
By patient assessment, there were no or only mild forehead lines at 4 weeks in 57% of those who received the lowest dose of DAXI, with rates of 80%, 100%, and 93% in those who received 18 U, 24 U, and 30 U.
At week 4, 57% of patients who got 12 U of DAXI pronounced themselves “satisfied” or “very satisfied” with DAXI therapy, as did 73%, 100%, and 93% of those who got the higher doses.
The treatment-related adverse events consisted of a smattering of cases of edema, erythema, or headache, similar to what’s described in the product labeling of all the neuromodulators.
Revance Therapeutics has applied to the Food and Drug Administration for marketing approval of DAXI for the treatment of glabellar lines. A regulatory decision is expected in late November. The company is also developing DAXI for the treatment of variety of neurologic and musculoskeletal conditions, including poststroke upper limb spasticity.
In an interview, Dr. Green said he was favorably impressed with DAXI’s durability for amelioration of forehead lines in the patients he personally treated in the ongoing phase 2 study, although there was no head-to-head comparison with other neuromodulators in the trial. He’s not aware of any planned phase 3 trial aimed at obtaining a forehead line indication.
“Of course, all four of the neuromodulators currently approved in the U.S. have glabellar line indications, but all are also used off-label in other locations, so I would imagine that DAXI will be used similarly if and when it is FDA-approved,” the dermatologist added.
He reported serving as a paid investigator for Revance.
FROM AAD 20
Maternal depression may derail children’s school readiness
based on data from a cohort study of more than 50,000 children.
Previous research supports a link between maternal depression and poor cognitive development in children, but the effect of children’s age when exposed to depression on their development has not been well studied, wrote Elizabeth Wall-Wieler, PhD, of Stanford (Calif.) University, and colleagues.
In a population-based cohort study published in Pediatrics, the researchers examined data from 52,103 children born in Manitoba, Canada who completed the Early Development Instrument (EDI) between 2005 and 2016. The EDI is a 103-item questionnaire given to kindergarteners in a classroom setting during the second half of the school year. The EDI is designed to assess five domains: physical health and well-being, social competence, emotional maturity, language and cognitive development, and communication skills and general knowledge. The researchers assessed maternal depression using a combination of physician visits, hospitalizations, and pharmaceutical data; 19% of the children had a mother diagnosed with depression during the study.
Difficulties emerged in social functioning, emotion regulation
Children whose mothers had a diagnosis of depression before they reached age 5 years had a 1.17 times increased risk of having a problem in one or more of the developmental domains at school entry, compared with those whose mothers had no such diagnosis. Overall, exposure to maternal depression was significantly associated with problems in the areas of social competence (adjusted risk ratio 1.28), physical health and well-being (aRR 1.28), and emotional maturity (aRR 1.27) in kindergarten. For these three domains, children exposed to maternal depression between the ages of 4 and 5 years showed the greatest risk of developmental vulnerability, the researchers said.
“It is noteworthy that these difficulties were reported by the children’s teachers, avoiding negative biases inherent in having mothers with depression serve as informants,” Dr. Wall-Wieler and associates wrote.
The study findings were limited by several factors related to the observational design, including incomplete data on maternal depression prevalence and severity, the researchers said. In addition, the study did not account for confounding variables related to parenting style and information on the presence and psychiatric profiles of fathers.
The results support previous studies by identifying the impact of exposure to maternal depression on particular developmental domains, the researchers concluded. However, more research is needed to extend their findings.
“In particular, investigators should work to elucidate the mechanisms that underlie this intergenerational transmission to young children of mothers with depression at risk for social and emotional difficulties, focusing on aspects of the caregiving environments and behaviors to which these children are exposed,” Dr. Wall-Wieler and associates concluded.
Approximately 70% of the children exposed to maternal depression did not test as vulnerable in any of the developmental domains. Factors that promoted resilience in the children exposed to maternal depression who did not experience developmental vulnerability should be identified, they noted.
Maternal depression is a public health crisis
“The widespread prevalence of maternal depression in the United States and worldwide, with estimates ranging from 3% to 60%, constitutes a public health crisis, particularly one affecting low-income women and their children,” Stephanie Klees Goeglein, MD, and Yvette E. Yatchmink, MD, PhD, of Brown University, Providence, R.I., wrote in an accompanying editorial (Pediatrics. 2020 Aug 17. doi: 10.1542/peds.2020-010413). The study adds to the research and understanding of the negative impact of maternal depression on child development, but also raises questions because some key confounders were not addressed, mainly “the presence of prenatal depression, severity and chronicity of maternal depression, comorbidities, and parenting practices including the involvement of fathers or partners,” they noted.
Don’t discount the impact of prenatal depression, the editorialists stressed. “Surprising to some, prenatal depression is more common than postpartum depression and may be a major, unexplored contributor to the developmental vulnerability of the children in the sample,” Dr. Goeglein and Dr. Yatchmink said.
However, the fact that approximately 70% of the children exposed to maternal depression did not have developmental vulnerability in any domain is encouraging, and speaks to the resiliency of children to overcome adverse conditions, they added.
Pediatricians can play a role in reducing maternal depression by screening mothers starting at a prenatal visit and continuing through post partum, infancy, and beyond. In addition, actions to improve access to mental health care for mothers and to develop culturally-sensitive positive parenting programs may help reduce the effects of depression on mothers and children, Dr. Goeglein and Dr. Yatchmink emphasized.
Empower depressed mothers to seek assistance
The results were not surprising, but a study that “validates that which we feel we know is clinically significant,” Lillian M. Beard, MD, of Children’s National in Washington, said in an interview.
“This study restates why it is so important for us to evaluate and recognize maternal depression,” she said. “As a busy pediatrician, once I recognize a problem, I struggle with how to provide viable professional and valuable community resources for these mothers.
“My take-home message from the study is that we must screen for maternal depression. When we suspect maternal depression, it is usually present,” Dr. Beard said. “While we cannot change all the factors that contribute to this condition, to help the children we serve to be physically and emotionally sound, we must help empower their mothers to seek assistance, when needed, to be emotionally healthy,” she emphasized.
“More research is needed to recognize the efficacy and value of community-based assistance for maternal and family depression,” said Dr. Beard, who was not involved with the study and was asked to comment on the findings.
The study was supported by the Canadian Institutes of Health Research, the National Institute of Mental Health, and the National Institutes of Health. The researchers and editorialists had no financial conflicts to disclose. Dr. Beard, a member of the Pediatric News editorial advisory board, had no relevant financial disclosures.
SOURCE: Wall-Wieler E et al. Pediatrics. 2020 Aug 17. doi: 10.1542/peds.2020-0794.
based on data from a cohort study of more than 50,000 children.
Previous research supports a link between maternal depression and poor cognitive development in children, but the effect of children’s age when exposed to depression on their development has not been well studied, wrote Elizabeth Wall-Wieler, PhD, of Stanford (Calif.) University, and colleagues.
In a population-based cohort study published in Pediatrics, the researchers examined data from 52,103 children born in Manitoba, Canada who completed the Early Development Instrument (EDI) between 2005 and 2016. The EDI is a 103-item questionnaire given to kindergarteners in a classroom setting during the second half of the school year. The EDI is designed to assess five domains: physical health and well-being, social competence, emotional maturity, language and cognitive development, and communication skills and general knowledge. The researchers assessed maternal depression using a combination of physician visits, hospitalizations, and pharmaceutical data; 19% of the children had a mother diagnosed with depression during the study.
Difficulties emerged in social functioning, emotion regulation
Children whose mothers had a diagnosis of depression before they reached age 5 years had a 1.17 times increased risk of having a problem in one or more of the developmental domains at school entry, compared with those whose mothers had no such diagnosis. Overall, exposure to maternal depression was significantly associated with problems in the areas of social competence (adjusted risk ratio 1.28), physical health and well-being (aRR 1.28), and emotional maturity (aRR 1.27) in kindergarten. For these three domains, children exposed to maternal depression between the ages of 4 and 5 years showed the greatest risk of developmental vulnerability, the researchers said.
“It is noteworthy that these difficulties were reported by the children’s teachers, avoiding negative biases inherent in having mothers with depression serve as informants,” Dr. Wall-Wieler and associates wrote.
The study findings were limited by several factors related to the observational design, including incomplete data on maternal depression prevalence and severity, the researchers said. In addition, the study did not account for confounding variables related to parenting style and information on the presence and psychiatric profiles of fathers.
The results support previous studies by identifying the impact of exposure to maternal depression on particular developmental domains, the researchers concluded. However, more research is needed to extend their findings.
“In particular, investigators should work to elucidate the mechanisms that underlie this intergenerational transmission to young children of mothers with depression at risk for social and emotional difficulties, focusing on aspects of the caregiving environments and behaviors to which these children are exposed,” Dr. Wall-Wieler and associates concluded.
Approximately 70% of the children exposed to maternal depression did not test as vulnerable in any of the developmental domains. Factors that promoted resilience in the children exposed to maternal depression who did not experience developmental vulnerability should be identified, they noted.
Maternal depression is a public health crisis
“The widespread prevalence of maternal depression in the United States and worldwide, with estimates ranging from 3% to 60%, constitutes a public health crisis, particularly one affecting low-income women and their children,” Stephanie Klees Goeglein, MD, and Yvette E. Yatchmink, MD, PhD, of Brown University, Providence, R.I., wrote in an accompanying editorial (Pediatrics. 2020 Aug 17. doi: 10.1542/peds.2020-010413). The study adds to the research and understanding of the negative impact of maternal depression on child development, but also raises questions because some key confounders were not addressed, mainly “the presence of prenatal depression, severity and chronicity of maternal depression, comorbidities, and parenting practices including the involvement of fathers or partners,” they noted.
Don’t discount the impact of prenatal depression, the editorialists stressed. “Surprising to some, prenatal depression is more common than postpartum depression and may be a major, unexplored contributor to the developmental vulnerability of the children in the sample,” Dr. Goeglein and Dr. Yatchmink said.
However, the fact that approximately 70% of the children exposed to maternal depression did not have developmental vulnerability in any domain is encouraging, and speaks to the resiliency of children to overcome adverse conditions, they added.
Pediatricians can play a role in reducing maternal depression by screening mothers starting at a prenatal visit and continuing through post partum, infancy, and beyond. In addition, actions to improve access to mental health care for mothers and to develop culturally-sensitive positive parenting programs may help reduce the effects of depression on mothers and children, Dr. Goeglein and Dr. Yatchmink emphasized.
Empower depressed mothers to seek assistance
The results were not surprising, but a study that “validates that which we feel we know is clinically significant,” Lillian M. Beard, MD, of Children’s National in Washington, said in an interview.
“This study restates why it is so important for us to evaluate and recognize maternal depression,” she said. “As a busy pediatrician, once I recognize a problem, I struggle with how to provide viable professional and valuable community resources for these mothers.
“My take-home message from the study is that we must screen for maternal depression. When we suspect maternal depression, it is usually present,” Dr. Beard said. “While we cannot change all the factors that contribute to this condition, to help the children we serve to be physically and emotionally sound, we must help empower their mothers to seek assistance, when needed, to be emotionally healthy,” she emphasized.
“More research is needed to recognize the efficacy and value of community-based assistance for maternal and family depression,” said Dr. Beard, who was not involved with the study and was asked to comment on the findings.
The study was supported by the Canadian Institutes of Health Research, the National Institute of Mental Health, and the National Institutes of Health. The researchers and editorialists had no financial conflicts to disclose. Dr. Beard, a member of the Pediatric News editorial advisory board, had no relevant financial disclosures.
SOURCE: Wall-Wieler E et al. Pediatrics. 2020 Aug 17. doi: 10.1542/peds.2020-0794.
based on data from a cohort study of more than 50,000 children.
Previous research supports a link between maternal depression and poor cognitive development in children, but the effect of children’s age when exposed to depression on their development has not been well studied, wrote Elizabeth Wall-Wieler, PhD, of Stanford (Calif.) University, and colleagues.
In a population-based cohort study published in Pediatrics, the researchers examined data from 52,103 children born in Manitoba, Canada who completed the Early Development Instrument (EDI) between 2005 and 2016. The EDI is a 103-item questionnaire given to kindergarteners in a classroom setting during the second half of the school year. The EDI is designed to assess five domains: physical health and well-being, social competence, emotional maturity, language and cognitive development, and communication skills and general knowledge. The researchers assessed maternal depression using a combination of physician visits, hospitalizations, and pharmaceutical data; 19% of the children had a mother diagnosed with depression during the study.
Difficulties emerged in social functioning, emotion regulation
Children whose mothers had a diagnosis of depression before they reached age 5 years had a 1.17 times increased risk of having a problem in one or more of the developmental domains at school entry, compared with those whose mothers had no such diagnosis. Overall, exposure to maternal depression was significantly associated with problems in the areas of social competence (adjusted risk ratio 1.28), physical health and well-being (aRR 1.28), and emotional maturity (aRR 1.27) in kindergarten. For these three domains, children exposed to maternal depression between the ages of 4 and 5 years showed the greatest risk of developmental vulnerability, the researchers said.
“It is noteworthy that these difficulties were reported by the children’s teachers, avoiding negative biases inherent in having mothers with depression serve as informants,” Dr. Wall-Wieler and associates wrote.
The study findings were limited by several factors related to the observational design, including incomplete data on maternal depression prevalence and severity, the researchers said. In addition, the study did not account for confounding variables related to parenting style and information on the presence and psychiatric profiles of fathers.
The results support previous studies by identifying the impact of exposure to maternal depression on particular developmental domains, the researchers concluded. However, more research is needed to extend their findings.
“In particular, investigators should work to elucidate the mechanisms that underlie this intergenerational transmission to young children of mothers with depression at risk for social and emotional difficulties, focusing on aspects of the caregiving environments and behaviors to which these children are exposed,” Dr. Wall-Wieler and associates concluded.
Approximately 70% of the children exposed to maternal depression did not test as vulnerable in any of the developmental domains. Factors that promoted resilience in the children exposed to maternal depression who did not experience developmental vulnerability should be identified, they noted.
Maternal depression is a public health crisis
“The widespread prevalence of maternal depression in the United States and worldwide, with estimates ranging from 3% to 60%, constitutes a public health crisis, particularly one affecting low-income women and their children,” Stephanie Klees Goeglein, MD, and Yvette E. Yatchmink, MD, PhD, of Brown University, Providence, R.I., wrote in an accompanying editorial (Pediatrics. 2020 Aug 17. doi: 10.1542/peds.2020-010413). The study adds to the research and understanding of the negative impact of maternal depression on child development, but also raises questions because some key confounders were not addressed, mainly “the presence of prenatal depression, severity and chronicity of maternal depression, comorbidities, and parenting practices including the involvement of fathers or partners,” they noted.
Don’t discount the impact of prenatal depression, the editorialists stressed. “Surprising to some, prenatal depression is more common than postpartum depression and may be a major, unexplored contributor to the developmental vulnerability of the children in the sample,” Dr. Goeglein and Dr. Yatchmink said.
However, the fact that approximately 70% of the children exposed to maternal depression did not have developmental vulnerability in any domain is encouraging, and speaks to the resiliency of children to overcome adverse conditions, they added.
Pediatricians can play a role in reducing maternal depression by screening mothers starting at a prenatal visit and continuing through post partum, infancy, and beyond. In addition, actions to improve access to mental health care for mothers and to develop culturally-sensitive positive parenting programs may help reduce the effects of depression on mothers and children, Dr. Goeglein and Dr. Yatchmink emphasized.
Empower depressed mothers to seek assistance
The results were not surprising, but a study that “validates that which we feel we know is clinically significant,” Lillian M. Beard, MD, of Children’s National in Washington, said in an interview.
“This study restates why it is so important for us to evaluate and recognize maternal depression,” she said. “As a busy pediatrician, once I recognize a problem, I struggle with how to provide viable professional and valuable community resources for these mothers.
“My take-home message from the study is that we must screen for maternal depression. When we suspect maternal depression, it is usually present,” Dr. Beard said. “While we cannot change all the factors that contribute to this condition, to help the children we serve to be physically and emotionally sound, we must help empower their mothers to seek assistance, when needed, to be emotionally healthy,” she emphasized.
“More research is needed to recognize the efficacy and value of community-based assistance for maternal and family depression,” said Dr. Beard, who was not involved with the study and was asked to comment on the findings.
The study was supported by the Canadian Institutes of Health Research, the National Institute of Mental Health, and the National Institutes of Health. The researchers and editorialists had no financial conflicts to disclose. Dr. Beard, a member of the Pediatric News editorial advisory board, had no relevant financial disclosures.
SOURCE: Wall-Wieler E et al. Pediatrics. 2020 Aug 17. doi: 10.1542/peds.2020-0794.
FROM PEDIATRICS
Compression therapy cuts cellulitis risk in chronic leg edema
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The effect was so striking that the randomized controlled trial was stopped early and all patients in the study were given the therapy.
“In a climate of increasing antibiotic resistance, we are delighted to have discovered a nondrug management strategy that has such a dramatic impact on the risk of cellulitis,” senior author Bernie Bissett, PhD, from the Discipline of Physiotherapy, Faculty of Health, the University of Canberra, Australia, said in an interview.
“We hope this leads to a shift in preventative medical strategy for patients with chronic edema and cellulitis around the world,” she said.
Lead author Elizabeth Webb, MPH, from the Physiotherapy Department at Calvary Public Hospital Bruce, in Bruce, Australia, and colleagues report their findings in an article published online August 12 in The New England Journal of Medicine.
Dr. Bisset explained that Webb is a “leading lymphedema physiotherapist” and a PhD candidate at the University of Canberra. She added that this is the first study to show that “compression therapy dramatically reduces the risk of cellulitis for patients with chronic edema.”
Penicillin is often given preventively; some research suggests effectiveness wanes after the antibiotic is stopped.
For the current trial, Ms. Webb and colleagues enrolled 84 adults with chronic edema of the leg and recurrent cellulitis. They randomly assigned patients in a 1:1 ratio to receive leg compression therapy plus education about preventing cellulitis (compression group; n = 41) or education only (control group; n = 43).
Compression therapy consisted of wearing knee-high stockings that applied maximum compression at the ankles. The compression gradually decreased up the legs. In addition, 26 patients were treated with “therapist-applied compression bandaging” for 3 to 5 days before receiving the stockings.
Participants underwent follow-up assessments every 6 months for a maximum of 3 years or until 45 episodes of cellulitis, the primary outcome, occurred. Those in the control group crossed over to the compression group once they experienced cellulitis.
The trial was stopped early for reasons of efficacy. “The statistical analysis plan prespecified that after 23 episodes of cellulitis had occurred, an independent data monitoring committee would review the results of the interim analysis and recommend whether the trial should stop early,” the authors write.
At the time of the monitoring committee’s review, six patients (15%) who wore compression stockings and 17 (40%) in the control group had experienced a cellulitis episode (hazard ratio, 0.23; P = .002; relative risk [post hoc analysis], 0.37; P = .02). On the basis of those findings, the researchers stopped the study, and patients in the control group were started on compression therapy.
“Clinicians should definitely consider referring their patients to a skilled lymphedema therapist who can individually prescribe and fit compression garments,” Dr. Bissett said. “In our study, these were well tolerated and reduced the risk of another episode of cellulitis by a huge 77%,” she added.
Secondary outcomes included hospitalization related to cellulitis and quality-of-life assessments.
Three patients (7%) in the compression group and six (14%) in the control group were admitted to the hospital for cellulitis (hazard ratio, 0.38). There were no differences in quality of life outcomes between the treatment groups.
The authors say compression therapy has the potential to decrease cellulitis risk by reducing edema, boosting immune response and skin integrity, and protecting the skin.
“Patients with a history of leg swelling (chronic edema) and previous episodes of cellulitis are ideal candidates for this compression therapy,” Dr. Bissett said.
“Given the lack of side effects of the therapy in our study and the potential to reduce other skin problems in these patients, compression therapy is an ideal prophylactic strategy,” she said.
The authors note several study limitations, including a lack of blinding. In addition, patients in the study had to have access to lymphedema specialists, who might be unavailable to patients outside the study. This could have influenced adherence and limit generalizability. Difficulty putting on and taking off compression garments often leads patients to be less adherent to compression therapy, but 88% of patients in this study wore them at least 4 days per week.
Dr. Bissett said compression therapy would be useful for primary care physicians to consider for patients with chronic edema.
“Primary care physicians are highly likely to encounter patients with chronic edema in their day-to-day practice. We can now confidently say that referral to lymphedema therapists for compression therapy should be a first line of defense against future episodes of cellulitis in this vulnerable patient group,” she explained.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Anxiety disorders begin earlier in life, differ by gender
Anxiety disorders start very early in life and may manifest themselves first as other conditions like social anxiety disorder, according to Jeffrey R. Strawn, MD.
An adolescent presenting to a mental health clinician with anxiety at 16 years old, for example has likely struggled with her anxiety for years before visiting a clinic. “That child may have been someone who had separation anxiety earlier in life and who as, even an infant, had behavioral inhibitions, that reluctance or timidness to explore new things, that tendency to retreat from novel stimuli,” Dr. Strawn, associate professor of psychiatry, pediatrics and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists. “Anxiety disorders are enduring and persistent, and they begin very early in life.”
Social anxiety disorder is one of the first anxiety disorders that appear in childhood or adolescents, which rises during puberty and during a time in a child’s life when they are dealing with new social pressures and challenges, such as graduating from elementary to middle school, Dr. Strawn noted. Generalized anxiety disorder is usually the next to emerge, followed by panic disorder. On the other hand, agoraphobia, another anxiety disorder that begins in childhood, “often represents behavioral avoidance as opposed to agoraphobia as we classically think about it as adult psychiatrists.”
Onset of anxiety disorders also differ by gender. “In terms of the emergence of these anxiety disorders, another thing that’s important to know is that the onset seems to be a bit different with regard to girls and boys. We see that break there emerging really around the time of puberty or as people are moving into late puberty, at least for girls,” Dr. Strawn said at the meeting presented by Global Academy for Medical Education. .
A shift occurs in amygdala prefrontal circuitry as children age, Dr. Strawn explained. Younger children do not have the ability to modulate the amygdala with their prefrontal cortex, but this amygdala–medial prefrontal cortex functional connectivity will change as children grow. A study by Dylan G. Gee, PhD, and colleagues found positive amygdala–medial prefrontal cortex functional connectivity at younger than 10 years old, and a “steady decline in amygdala activity” from 10-13 years to adulthood at 22 years old (J Neurosci. 2013 Mar 6;33[10]:4584-93).
“In essence, what we’re seeing is that there’s improvement or more effectiveness in terms of that connection between the prefrontal cortex, the amygdala, and that ability to amplify the brake to the amygdala,” Dr. Strawn said.
SSRIs, SNRIs for pediatric patients
Selective serotonin reuptake inhibitors can be effective for pediatric patients with anxiety disorders. Results from the Child/Adolescent Anxiety Multimodal Study (CAMS) show that patients with generalized separation or social anxiety disorder treated with sertraline or cognitive-behavioral therapy (CBT) for 3 months responded better to treatment than placebo. A combination of sertraline and CBT performing best, compared with either intervention alone (N Engl J Med. 2008;359:2753-66).
When examining treatment response in 76 patients from CAMS, the researchers saw improvement at 4 weeks from baseline in patients with anxiety symptoms receiving CBT, but no significant change in improvement after 4 weeks up to 12 weeks (J Child Adolesc Psychopharm. 2017 Aug 1. doi: 10.1089/cap.2016.0198).
“What that actually means is that your improvement at week 4 is better than your improvement at baseline, and your improvement at week 8 is greater than your improvement at week 4. Similarly, in your improvement, week 12 is greater than your improvement at week 8,” Dr. Strawn said.
However, “that’s not the case for aggressively titrated sertraline,” which had no statistically significant difference in improvement at 8 weeks and 12 weeks, he explained. “What this actually means is that, if I have not had improvement by week 8, there is a three-to-one odds against improvement over those next 4 weeks. The take-home message here is really that an adequate trial for an SSRI in pediatric anxiety disorders is probably about 8 weeks – not 12, not longer.”
Serotonin norepinephrine reuptake inhibitors (SNRIs) are also effective in pediatric patients with anxiety disorders.
“Both SNRIs as well as SSRIs have certainly demonstrated efficacy in terms of treating pediatric patients with anxiety, but there is a very important difference here with regard to the trajectory of improvement and also the magnitude of improvement,” Dr. Strawn said. SNRIs like atomoxetine, duloxetine, or venlafaxine “do not improve as rapidly and do not improve to the same extent as kids who are treated with an SSRI.”
Dose is another factor that affects symptom improvement in patients with pediatric anxiety disorders. In a 2018 meta-analysis, Dr. Strawn and colleagues found that patients treated with a higher dose of SSRIs demonstrated more rapid improvement at 2 weeks, compared with patients who received SNRIs (P = .002), but there was no significant difference in overall response trajectory (J Am Acad Child Adolesc Psychiatry. 2018 Apr;57[4]:235-44.E2).
Response to SSRIs can depend a patient’s genotype, Dr. Strawn said. The serotonin transporter promotor polymorphism has received “considerable attention in adults with depressive disorders primarily” but also might play a role in anxiety disorder response in pediatric patients. One study presented by his group at the 2019 annual meeting of the American Academy of Child & Adolescent Psychiatry showed that patients with a short-short copy of the serotonin transporter promoter polymorphism instead of a long copy had “shallower and less improvement over the course of treatment” when taking escitalopram.
“This is something that doesn’t necessarily compel us to use an SNRI over an SSRI, but it’s something that does give us some important information in terms of the trajectory of improvement,” he said.
When it comes to side effects of SNRIs and SSRIs, the profile is “pretty consistent with what we know to be the side effect profile in adults with depressive and anxiety disorders,” Dr. Strawn noted. “SNRIs tend to be a little bit better tolerated, both in terms of adverse event–related discontinuation and also in terms of their likelihood of producing activation.”
Patient and caregiver expectations can further affect response to treatment. “I think this has implications in terms of how we actively manage expectations and discussions about the evidence for interventions with our patients in the clinic.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Edgemont Pharmaceuticals, Eli Lilly, Forest Research Laboratories, Lundbeck, the National Institutes of Health, Neuronetics, and Shire. He also reported receiving royalties from Springer Publishing, and is a consultant for and receives material support from Assurex/Genesight.
Anxiety disorders start very early in life and may manifest themselves first as other conditions like social anxiety disorder, according to Jeffrey R. Strawn, MD.
An adolescent presenting to a mental health clinician with anxiety at 16 years old, for example has likely struggled with her anxiety for years before visiting a clinic. “That child may have been someone who had separation anxiety earlier in life and who as, even an infant, had behavioral inhibitions, that reluctance or timidness to explore new things, that tendency to retreat from novel stimuli,” Dr. Strawn, associate professor of psychiatry, pediatrics and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists. “Anxiety disorders are enduring and persistent, and they begin very early in life.”
Social anxiety disorder is one of the first anxiety disorders that appear in childhood or adolescents, which rises during puberty and during a time in a child’s life when they are dealing with new social pressures and challenges, such as graduating from elementary to middle school, Dr. Strawn noted. Generalized anxiety disorder is usually the next to emerge, followed by panic disorder. On the other hand, agoraphobia, another anxiety disorder that begins in childhood, “often represents behavioral avoidance as opposed to agoraphobia as we classically think about it as adult psychiatrists.”
Onset of anxiety disorders also differ by gender. “In terms of the emergence of these anxiety disorders, another thing that’s important to know is that the onset seems to be a bit different with regard to girls and boys. We see that break there emerging really around the time of puberty or as people are moving into late puberty, at least for girls,” Dr. Strawn said at the meeting presented by Global Academy for Medical Education. .
A shift occurs in amygdala prefrontal circuitry as children age, Dr. Strawn explained. Younger children do not have the ability to modulate the amygdala with their prefrontal cortex, but this amygdala–medial prefrontal cortex functional connectivity will change as children grow. A study by Dylan G. Gee, PhD, and colleagues found positive amygdala–medial prefrontal cortex functional connectivity at younger than 10 years old, and a “steady decline in amygdala activity” from 10-13 years to adulthood at 22 years old (J Neurosci. 2013 Mar 6;33[10]:4584-93).
“In essence, what we’re seeing is that there’s improvement or more effectiveness in terms of that connection between the prefrontal cortex, the amygdala, and that ability to amplify the brake to the amygdala,” Dr. Strawn said.
SSRIs, SNRIs for pediatric patients
Selective serotonin reuptake inhibitors can be effective for pediatric patients with anxiety disorders. Results from the Child/Adolescent Anxiety Multimodal Study (CAMS) show that patients with generalized separation or social anxiety disorder treated with sertraline or cognitive-behavioral therapy (CBT) for 3 months responded better to treatment than placebo. A combination of sertraline and CBT performing best, compared with either intervention alone (N Engl J Med. 2008;359:2753-66).
When examining treatment response in 76 patients from CAMS, the researchers saw improvement at 4 weeks from baseline in patients with anxiety symptoms receiving CBT, but no significant change in improvement after 4 weeks up to 12 weeks (J Child Adolesc Psychopharm. 2017 Aug 1. doi: 10.1089/cap.2016.0198).
“What that actually means is that your improvement at week 4 is better than your improvement at baseline, and your improvement at week 8 is greater than your improvement at week 4. Similarly, in your improvement, week 12 is greater than your improvement at week 8,” Dr. Strawn said.
However, “that’s not the case for aggressively titrated sertraline,” which had no statistically significant difference in improvement at 8 weeks and 12 weeks, he explained. “What this actually means is that, if I have not had improvement by week 8, there is a three-to-one odds against improvement over those next 4 weeks. The take-home message here is really that an adequate trial for an SSRI in pediatric anxiety disorders is probably about 8 weeks – not 12, not longer.”
Serotonin norepinephrine reuptake inhibitors (SNRIs) are also effective in pediatric patients with anxiety disorders.
“Both SNRIs as well as SSRIs have certainly demonstrated efficacy in terms of treating pediatric patients with anxiety, but there is a very important difference here with regard to the trajectory of improvement and also the magnitude of improvement,” Dr. Strawn said. SNRIs like atomoxetine, duloxetine, or venlafaxine “do not improve as rapidly and do not improve to the same extent as kids who are treated with an SSRI.”
Dose is another factor that affects symptom improvement in patients with pediatric anxiety disorders. In a 2018 meta-analysis, Dr. Strawn and colleagues found that patients treated with a higher dose of SSRIs demonstrated more rapid improvement at 2 weeks, compared with patients who received SNRIs (P = .002), but there was no significant difference in overall response trajectory (J Am Acad Child Adolesc Psychiatry. 2018 Apr;57[4]:235-44.E2).
Response to SSRIs can depend a patient’s genotype, Dr. Strawn said. The serotonin transporter promotor polymorphism has received “considerable attention in adults with depressive disorders primarily” but also might play a role in anxiety disorder response in pediatric patients. One study presented by his group at the 2019 annual meeting of the American Academy of Child & Adolescent Psychiatry showed that patients with a short-short copy of the serotonin transporter promoter polymorphism instead of a long copy had “shallower and less improvement over the course of treatment” when taking escitalopram.
“This is something that doesn’t necessarily compel us to use an SNRI over an SSRI, but it’s something that does give us some important information in terms of the trajectory of improvement,” he said.
When it comes to side effects of SNRIs and SSRIs, the profile is “pretty consistent with what we know to be the side effect profile in adults with depressive and anxiety disorders,” Dr. Strawn noted. “SNRIs tend to be a little bit better tolerated, both in terms of adverse event–related discontinuation and also in terms of their likelihood of producing activation.”
Patient and caregiver expectations can further affect response to treatment. “I think this has implications in terms of how we actively manage expectations and discussions about the evidence for interventions with our patients in the clinic.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Edgemont Pharmaceuticals, Eli Lilly, Forest Research Laboratories, Lundbeck, the National Institutes of Health, Neuronetics, and Shire. He also reported receiving royalties from Springer Publishing, and is a consultant for and receives material support from Assurex/Genesight.
Anxiety disorders start very early in life and may manifest themselves first as other conditions like social anxiety disorder, according to Jeffrey R. Strawn, MD.
An adolescent presenting to a mental health clinician with anxiety at 16 years old, for example has likely struggled with her anxiety for years before visiting a clinic. “That child may have been someone who had separation anxiety earlier in life and who as, even an infant, had behavioral inhibitions, that reluctance or timidness to explore new things, that tendency to retreat from novel stimuli,” Dr. Strawn, associate professor of psychiatry, pediatrics and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists. “Anxiety disorders are enduring and persistent, and they begin very early in life.”
Social anxiety disorder is one of the first anxiety disorders that appear in childhood or adolescents, which rises during puberty and during a time in a child’s life when they are dealing with new social pressures and challenges, such as graduating from elementary to middle school, Dr. Strawn noted. Generalized anxiety disorder is usually the next to emerge, followed by panic disorder. On the other hand, agoraphobia, another anxiety disorder that begins in childhood, “often represents behavioral avoidance as opposed to agoraphobia as we classically think about it as adult psychiatrists.”
Onset of anxiety disorders also differ by gender. “In terms of the emergence of these anxiety disorders, another thing that’s important to know is that the onset seems to be a bit different with regard to girls and boys. We see that break there emerging really around the time of puberty or as people are moving into late puberty, at least for girls,” Dr. Strawn said at the meeting presented by Global Academy for Medical Education. .
A shift occurs in amygdala prefrontal circuitry as children age, Dr. Strawn explained. Younger children do not have the ability to modulate the amygdala with their prefrontal cortex, but this amygdala–medial prefrontal cortex functional connectivity will change as children grow. A study by Dylan G. Gee, PhD, and colleagues found positive amygdala–medial prefrontal cortex functional connectivity at younger than 10 years old, and a “steady decline in amygdala activity” from 10-13 years to adulthood at 22 years old (J Neurosci. 2013 Mar 6;33[10]:4584-93).
“In essence, what we’re seeing is that there’s improvement or more effectiveness in terms of that connection between the prefrontal cortex, the amygdala, and that ability to amplify the brake to the amygdala,” Dr. Strawn said.
SSRIs, SNRIs for pediatric patients
Selective serotonin reuptake inhibitors can be effective for pediatric patients with anxiety disorders. Results from the Child/Adolescent Anxiety Multimodal Study (CAMS) show that patients with generalized separation or social anxiety disorder treated with sertraline or cognitive-behavioral therapy (CBT) for 3 months responded better to treatment than placebo. A combination of sertraline and CBT performing best, compared with either intervention alone (N Engl J Med. 2008;359:2753-66).
When examining treatment response in 76 patients from CAMS, the researchers saw improvement at 4 weeks from baseline in patients with anxiety symptoms receiving CBT, but no significant change in improvement after 4 weeks up to 12 weeks (J Child Adolesc Psychopharm. 2017 Aug 1. doi: 10.1089/cap.2016.0198).
“What that actually means is that your improvement at week 4 is better than your improvement at baseline, and your improvement at week 8 is greater than your improvement at week 4. Similarly, in your improvement, week 12 is greater than your improvement at week 8,” Dr. Strawn said.
However, “that’s not the case for aggressively titrated sertraline,” which had no statistically significant difference in improvement at 8 weeks and 12 weeks, he explained. “What this actually means is that, if I have not had improvement by week 8, there is a three-to-one odds against improvement over those next 4 weeks. The take-home message here is really that an adequate trial for an SSRI in pediatric anxiety disorders is probably about 8 weeks – not 12, not longer.”
Serotonin norepinephrine reuptake inhibitors (SNRIs) are also effective in pediatric patients with anxiety disorders.
“Both SNRIs as well as SSRIs have certainly demonstrated efficacy in terms of treating pediatric patients with anxiety, but there is a very important difference here with regard to the trajectory of improvement and also the magnitude of improvement,” Dr. Strawn said. SNRIs like atomoxetine, duloxetine, or venlafaxine “do not improve as rapidly and do not improve to the same extent as kids who are treated with an SSRI.”
Dose is another factor that affects symptom improvement in patients with pediatric anxiety disorders. In a 2018 meta-analysis, Dr. Strawn and colleagues found that patients treated with a higher dose of SSRIs demonstrated more rapid improvement at 2 weeks, compared with patients who received SNRIs (P = .002), but there was no significant difference in overall response trajectory (J Am Acad Child Adolesc Psychiatry. 2018 Apr;57[4]:235-44.E2).
Response to SSRIs can depend a patient’s genotype, Dr. Strawn said. The serotonin transporter promotor polymorphism has received “considerable attention in adults with depressive disorders primarily” but also might play a role in anxiety disorder response in pediatric patients. One study presented by his group at the 2019 annual meeting of the American Academy of Child & Adolescent Psychiatry showed that patients with a short-short copy of the serotonin transporter promoter polymorphism instead of a long copy had “shallower and less improvement over the course of treatment” when taking escitalopram.
“This is something that doesn’t necessarily compel us to use an SNRI over an SSRI, but it’s something that does give us some important information in terms of the trajectory of improvement,” he said.
When it comes to side effects of SNRIs and SSRIs, the profile is “pretty consistent with what we know to be the side effect profile in adults with depressive and anxiety disorders,” Dr. Strawn noted. “SNRIs tend to be a little bit better tolerated, both in terms of adverse event–related discontinuation and also in terms of their likelihood of producing activation.”
Patient and caregiver expectations can further affect response to treatment. “I think this has implications in terms of how we actively manage expectations and discussions about the evidence for interventions with our patients in the clinic.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Edgemont Pharmaceuticals, Eli Lilly, Forest Research Laboratories, Lundbeck, the National Institutes of Health, Neuronetics, and Shire. He also reported receiving royalties from Springer Publishing, and is a consultant for and receives material support from Assurex/Genesight.
FROM Focus on Neuropsychiatry 2020
Pulmonary rehab reduces COPD readmissions
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pulmonary rehabilitation reduces the likelihood that patients with chronic obstructive pulmonary disease (COPD) will be readmitted to the hospital in the year after discharge by 33%, new research shows, but few patients participate in those programs.
In fact, in a retrospective cohort of 197,376 patients from 4446 hospitals, only 1.5% of patients initiated pulmonary rehabilitation in the 90 days after hospital discharge.
“This is a striking finding,” said Mihaela Stefan, PhD, from the University of Massachusetts Medical School–Baystate in Springfield. “Our study demonstrates that we need to increase access to rehabilitation to reduce the risk of readmissions.”
Not enough patients are initiating rehabilitation, but the onus is not only on them; the system is failing them. “We wanted to understand how much pulmonary rehabilitation lowers the readmission rate,” Stefan told Medscape Medical News.
So she and her colleagues examined the records of patients who were hospitalized for COPD in 2014 to see whether they had begun rehabilitation in the 90 days after discharge and whether they were readmitted to the hospital in the subsequent 12 months.
Patients who were unlikely to initiate pulmonary rehabilitation — such as those with dementia or metastatic cancer and those discharged to hospice care or a nursing home — were excluded from the analysis, Stefan said during her presentation at the study results at the virtual American Thoracic Society (ATS) 2020 International Conference.
The risk analysis was complex because many patients died before the year was out, and “a patient who dies has no risk of being readmitted,” she explained. Selection bias was also a factor because patients who do pulmonary rehab tend to be in better shape.
The researchers used propensity score matching and Anderson–Gill models of cumulative rehospitalizations or death at 1 year with time-varying exposure to pulmonary rehabilitation to account for clustering of individual events and adjust for covariates. “It was a complicated risk analysis,” she said.
In the year after discharge, 130,660 patients (66%) were readmitted to the hospital. The rate of rehospitalization was lower for those who initiated rehabilitation than for those who did not (59% vs 66%), as was the mean number of readmissions per patient (1.4 vs 1.8).
Rehabilitation was associated with a lower risk for readmission or death (hazard ratio, 0.67; 95% CI, 0.66 - 0.69).
“We know the referral rates are low and that pulmonary rehabilitation is effective in clinical trials,” said Stefan, and now “we see that pulmonary rehabilitation is effective when you look at patients in real life.”
From a provider perspective, “we need to make sure that hospitals get more money for pulmonary rehabilitation. Cardiac rehabilitation is paid for,” she explained. "But pulmonary rehab is not a lucrative business. I don›t know why the CMS pays more for cardiac."
A rehabilitation program generally consists of 36 sessions, held two or three times a week, and many patients can’t afford that on their own, she noted. Transportation is another huge issue.
A recent study in which semi-structured interviews were conducted with 15 COPD patients showed that the main barriers to enrollment in a pulmonary rehabilitation program are lack of awareness, family obligations, transportation, and lack of motivation, said Stefan, who was involved in that research.
Telehealth rehabilitation programs might become more available in the near future, given the COVID pandemic. But “currently, Medicare doesn’t pay for telerehab,” she said. Virtual sessions might attract more patients, but lack of computer access and training could present another barrier for some.
PAH rehab
Uptake for pulmonary rehabilitation is as low for patients with pulmonary arterial hypertension (PAH) as it is for those with COPD, according to another study presented at the virtual ATS meeting.
An examination of the electronic health records of 111,356 veterans who experienced incident PAH from 2010 to 2016 showed that only 1,737 (1.6%) followed through on pulmonary rehabilitation.
“Exercise therapy is safe and effective at improving outcomes,” lead author Thomas Cascino, MD, from the University of Michigan in Ann Arbor, said in an ATS press release. “Recognizing that it is being underutilized is a necessary first step in working toward increasing patient access to rehab.
His group is currently working on a trial for home-based rehabilitation “using wearable technology as a means to expand access for people unable to come to center-based rehab for a variety of reasons,” he explained.
“The goal of all our treatments is to help people feel better and live longer,” Cascino added.
Stefan and Cascino have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.