User login
Drug spending driving up Part B premiums and deductibles
Medicare beneficiaries charged the standard premium for Medicare Part B coverage will be paying $144.60 each month in 2020, up $9.10 from 2019.
Deductibles also will increase to $198 next year, up $13 from the current year.
The Centers for Medicare & Medicaid Services said in a statement announcing the hikes that the increases are “largely due to rising spending on physician administered drugs. These higher costs have a ripple effect and result in higher Part B premiums and deductibles.”
The formal details on the premium and deductible increases have been posted online and are scheduled for publication in the Federal Register on Nov. 13.
The CMS and Congress are looking into a number of options to help contain the spending on drugs, including the use of an international pricing index to put U.S. spending more in line with the lower prices offered in foreign countries, automatic rebates when drug prices rise faster than the rate of inflation, and a modern take on the failed competitive acquisition program.
The agency also announced increases in the inpatient hospital deductible that will be paid under Medicare Part A when beneficiaries are admitted into a hospital in 2020. The deductible increases to $1,408 next year, up from $1,364 this year. The daily coinsurance for the 61st-90th day increases to $352 from $341, while the daily coinsurance for lifetime reserve days increases to $704 from $682.
Skilled nursing facility coinsurance also rises during this same time period to $176 from $170.50.
More information on Part A deductibles can be found here, while information on Part A premiums can be found here.
Medicare beneficiaries charged the standard premium for Medicare Part B coverage will be paying $144.60 each month in 2020, up $9.10 from 2019.
Deductibles also will increase to $198 next year, up $13 from the current year.
The Centers for Medicare & Medicaid Services said in a statement announcing the hikes that the increases are “largely due to rising spending on physician administered drugs. These higher costs have a ripple effect and result in higher Part B premiums and deductibles.”
The formal details on the premium and deductible increases have been posted online and are scheduled for publication in the Federal Register on Nov. 13.
The CMS and Congress are looking into a number of options to help contain the spending on drugs, including the use of an international pricing index to put U.S. spending more in line with the lower prices offered in foreign countries, automatic rebates when drug prices rise faster than the rate of inflation, and a modern take on the failed competitive acquisition program.
The agency also announced increases in the inpatient hospital deductible that will be paid under Medicare Part A when beneficiaries are admitted into a hospital in 2020. The deductible increases to $1,408 next year, up from $1,364 this year. The daily coinsurance for the 61st-90th day increases to $352 from $341, while the daily coinsurance for lifetime reserve days increases to $704 from $682.
Skilled nursing facility coinsurance also rises during this same time period to $176 from $170.50.
More information on Part A deductibles can be found here, while information on Part A premiums can be found here.
Medicare beneficiaries charged the standard premium for Medicare Part B coverage will be paying $144.60 each month in 2020, up $9.10 from 2019.
Deductibles also will increase to $198 next year, up $13 from the current year.
The Centers for Medicare & Medicaid Services said in a statement announcing the hikes that the increases are “largely due to rising spending on physician administered drugs. These higher costs have a ripple effect and result in higher Part B premiums and deductibles.”
The formal details on the premium and deductible increases have been posted online and are scheduled for publication in the Federal Register on Nov. 13.
The CMS and Congress are looking into a number of options to help contain the spending on drugs, including the use of an international pricing index to put U.S. spending more in line with the lower prices offered in foreign countries, automatic rebates when drug prices rise faster than the rate of inflation, and a modern take on the failed competitive acquisition program.
The agency also announced increases in the inpatient hospital deductible that will be paid under Medicare Part A when beneficiaries are admitted into a hospital in 2020. The deductible increases to $1,408 next year, up from $1,364 this year. The daily coinsurance for the 61st-90th day increases to $352 from $341, while the daily coinsurance for lifetime reserve days increases to $704 from $682.
Skilled nursing facility coinsurance also rises during this same time period to $176 from $170.50.
More information on Part A deductibles can be found here, while information on Part A premiums can be found here.
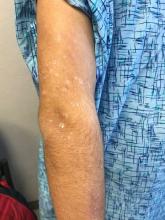
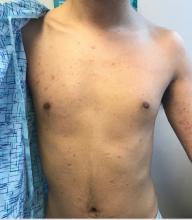
Marked increase in psoriasis seen with TNFi use in pediatric inflammatory diseases
ATLANTA – in a review of 4,111 patients at the Children’s Hospital of Philadelphia.
The finding confirms a clinical suspicion that biologics can cause psoriasis in children, just as has been shown in adults, said lead investigator Lisa Buckley, MD, a rheumatology fellow at the hospital when she conducted the study, but now a pediatric rheumatologist at Vanderbilt University, Nashville, Tenn. The study was recently published in Arthritis Care & Research.
“I don’t think this will change my prescribing habits” because tumor necrosis factor inhibitors (TNFi) are so useful, but “what this will change is how much information I give to families about the risk of psoriasis, especially in kids with a family history,” which also predisposed children in the study to psoriasis, Dr. Buckley said . “Anecdotally, psoriasis has not been part of the traditional risk-benefit conversation with families. This has added that to my” discussion, he added.
TNFi “psoriasis can be a really big deal for these children, especially in adolescence. They don’t want to go to school and things like that. Children and parents often prioritize [it] over the underlying disease,” she said.
For now, how to best manage TNFi psoriasis is uncertain. Children often are in remission when it starts, and a decision has to be made whether to discontinue treatment, reduce the dose, or add something for the psoriasis. There are no clear answers at the moment. “This is the beginning of the beginning of studies looking at this. It just proves that there actually is a problem,” Dr. Buckley said at the annual meeting of the American College of Rheumatology.
About three-quarters of the children had inflammatory bowel disease, and most of the rest had juvenile idiopathic arthritis. Just 2% had chronic nonbacterial osteomyelitis. Billing codes were used to confirm diagnosis, new-onset psoriasis, and incident TNFi exposure, defined as at least one prescription for adalimumab, etanercept, or infliximab.
Overall, 1,614 children (39%) were treated with a TNFi and 2,497 (61%) were not. There were 58 cases of psoriasis in the TNFi group for an incidence ratio of 12.3 cases per 1,000 person-years, and a standard IR – observed psoriasis cases over expected cases in the general pediatrics population – of 30.
There were 25 cases among children not treated with a TNFi, for an IR of 3.8 per 1,000 person-years and SIR of 9.3.
In the end, TNFi exposure was associated with a marked increase in psoriasis risk (hazard ratio, 3.84; 95% confidence interval, 2.28-6.47; P less than .001). Family history was positive in 8% of subjects and was a known risk factor; it was the only other independent predictor (HR, 3.11; 95% CI, 1.79-5.41; P less than .001).
Obesity, which was linked in previous studies to psoriasis and was higher in the non-TNFI group, did not influence risk, nor did methotrexate, which was also used more commonly in the TNFi group. “We thought that including patients on methotrexate, which is a treatment for psoriasis, might have altered the outcomes, but it didn’t seem to make any difference in developing psoriasis,” Dr. Buckley said.
It’s possible that children on a TNFi had higher disease activity, and that in itself increased the risk of psoriasis, but there isn’t an association in the literature between high disease activity and psoriasis, she said. In past reports, TNFi-induced psoriasis was most likely to occur in adults who were in disease remission.
Subjects were aged about 11 years on average and about equally split between boys and girls; three-quarters were white. Children previously diagnosed with psoriasis were excluded. Average follow up was about 2 years.
The National Institutes of Health funded the work. The investigators didn’t report any relevant disclosures.
SOURCE: Buckley L et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1816.
ATLANTA – in a review of 4,111 patients at the Children’s Hospital of Philadelphia.
The finding confirms a clinical suspicion that biologics can cause psoriasis in children, just as has been shown in adults, said lead investigator Lisa Buckley, MD, a rheumatology fellow at the hospital when she conducted the study, but now a pediatric rheumatologist at Vanderbilt University, Nashville, Tenn. The study was recently published in Arthritis Care & Research.
“I don’t think this will change my prescribing habits” because tumor necrosis factor inhibitors (TNFi) are so useful, but “what this will change is how much information I give to families about the risk of psoriasis, especially in kids with a family history,” which also predisposed children in the study to psoriasis, Dr. Buckley said . “Anecdotally, psoriasis has not been part of the traditional risk-benefit conversation with families. This has added that to my” discussion, he added.
TNFi “psoriasis can be a really big deal for these children, especially in adolescence. They don’t want to go to school and things like that. Children and parents often prioritize [it] over the underlying disease,” she said.
For now, how to best manage TNFi psoriasis is uncertain. Children often are in remission when it starts, and a decision has to be made whether to discontinue treatment, reduce the dose, or add something for the psoriasis. There are no clear answers at the moment. “This is the beginning of the beginning of studies looking at this. It just proves that there actually is a problem,” Dr. Buckley said at the annual meeting of the American College of Rheumatology.
About three-quarters of the children had inflammatory bowel disease, and most of the rest had juvenile idiopathic arthritis. Just 2% had chronic nonbacterial osteomyelitis. Billing codes were used to confirm diagnosis, new-onset psoriasis, and incident TNFi exposure, defined as at least one prescription for adalimumab, etanercept, or infliximab.
Overall, 1,614 children (39%) were treated with a TNFi and 2,497 (61%) were not. There were 58 cases of psoriasis in the TNFi group for an incidence ratio of 12.3 cases per 1,000 person-years, and a standard IR – observed psoriasis cases over expected cases in the general pediatrics population – of 30.
There were 25 cases among children not treated with a TNFi, for an IR of 3.8 per 1,000 person-years and SIR of 9.3.
In the end, TNFi exposure was associated with a marked increase in psoriasis risk (hazard ratio, 3.84; 95% confidence interval, 2.28-6.47; P less than .001). Family history was positive in 8% of subjects and was a known risk factor; it was the only other independent predictor (HR, 3.11; 95% CI, 1.79-5.41; P less than .001).
Obesity, which was linked in previous studies to psoriasis and was higher in the non-TNFI group, did not influence risk, nor did methotrexate, which was also used more commonly in the TNFi group. “We thought that including patients on methotrexate, which is a treatment for psoriasis, might have altered the outcomes, but it didn’t seem to make any difference in developing psoriasis,” Dr. Buckley said.
It’s possible that children on a TNFi had higher disease activity, and that in itself increased the risk of psoriasis, but there isn’t an association in the literature between high disease activity and psoriasis, she said. In past reports, TNFi-induced psoriasis was most likely to occur in adults who were in disease remission.
Subjects were aged about 11 years on average and about equally split between boys and girls; three-quarters were white. Children previously diagnosed with psoriasis were excluded. Average follow up was about 2 years.
The National Institutes of Health funded the work. The investigators didn’t report any relevant disclosures.
SOURCE: Buckley L et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1816.
ATLANTA – in a review of 4,111 patients at the Children’s Hospital of Philadelphia.
The finding confirms a clinical suspicion that biologics can cause psoriasis in children, just as has been shown in adults, said lead investigator Lisa Buckley, MD, a rheumatology fellow at the hospital when she conducted the study, but now a pediatric rheumatologist at Vanderbilt University, Nashville, Tenn. The study was recently published in Arthritis Care & Research.
“I don’t think this will change my prescribing habits” because tumor necrosis factor inhibitors (TNFi) are so useful, but “what this will change is how much information I give to families about the risk of psoriasis, especially in kids with a family history,” which also predisposed children in the study to psoriasis, Dr. Buckley said . “Anecdotally, psoriasis has not been part of the traditional risk-benefit conversation with families. This has added that to my” discussion, he added.
TNFi “psoriasis can be a really big deal for these children, especially in adolescence. They don’t want to go to school and things like that. Children and parents often prioritize [it] over the underlying disease,” she said.
For now, how to best manage TNFi psoriasis is uncertain. Children often are in remission when it starts, and a decision has to be made whether to discontinue treatment, reduce the dose, or add something for the psoriasis. There are no clear answers at the moment. “This is the beginning of the beginning of studies looking at this. It just proves that there actually is a problem,” Dr. Buckley said at the annual meeting of the American College of Rheumatology.
About three-quarters of the children had inflammatory bowel disease, and most of the rest had juvenile idiopathic arthritis. Just 2% had chronic nonbacterial osteomyelitis. Billing codes were used to confirm diagnosis, new-onset psoriasis, and incident TNFi exposure, defined as at least one prescription for adalimumab, etanercept, or infliximab.
Overall, 1,614 children (39%) were treated with a TNFi and 2,497 (61%) were not. There were 58 cases of psoriasis in the TNFi group for an incidence ratio of 12.3 cases per 1,000 person-years, and a standard IR – observed psoriasis cases over expected cases in the general pediatrics population – of 30.
There were 25 cases among children not treated with a TNFi, for an IR of 3.8 per 1,000 person-years and SIR of 9.3.
In the end, TNFi exposure was associated with a marked increase in psoriasis risk (hazard ratio, 3.84; 95% confidence interval, 2.28-6.47; P less than .001). Family history was positive in 8% of subjects and was a known risk factor; it was the only other independent predictor (HR, 3.11; 95% CI, 1.79-5.41; P less than .001).
Obesity, which was linked in previous studies to psoriasis and was higher in the non-TNFI group, did not influence risk, nor did methotrexate, which was also used more commonly in the TNFi group. “We thought that including patients on methotrexate, which is a treatment for psoriasis, might have altered the outcomes, but it didn’t seem to make any difference in developing psoriasis,” Dr. Buckley said.
It’s possible that children on a TNFi had higher disease activity, and that in itself increased the risk of psoriasis, but there isn’t an association in the literature between high disease activity and psoriasis, she said. In past reports, TNFi-induced psoriasis was most likely to occur in adults who were in disease remission.
Subjects were aged about 11 years on average and about equally split between boys and girls; three-quarters were white. Children previously diagnosed with psoriasis were excluded. Average follow up was about 2 years.
The National Institutes of Health funded the work. The investigators didn’t report any relevant disclosures.
SOURCE: Buckley L et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1816.
REPORTING FROM ACR 2019
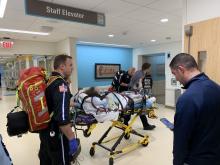
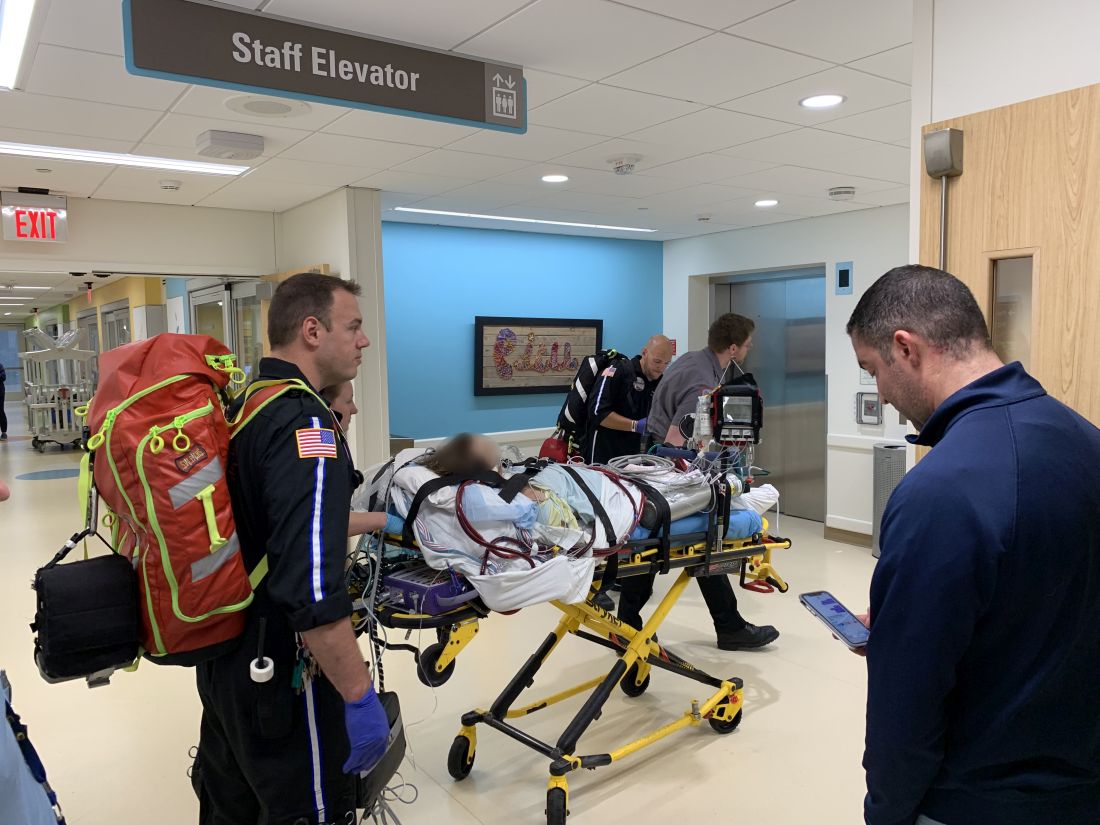
Teen survives double lung transplant after vaping injury
A Michigan teenager, described as an athlete and otherwise healthy, has survived a double lung transplant following lung damage attributed to vaping.
“On the 15th of October, the transplant team performed what we believe is the first double lung transplant done in the nation for a vaping-injury victim, who is a teenager,” Hassan Nemeh, MD, cardiothoracic surgeon with the Henry Ford Health System in Detroit, said during a Nov. 12, 2019, press conference to discuss the surgery.
“What I saw in his lungs is nothing that I have ever seen before and I have been doing lung transplants for 20 years,” Dr. Nemeh said. “There was an enormous amount of inflammation and scarring, in addition to multiple spots of dead tissue. The lung itself was so firm and scarred, we had to deliver it out of the chest. This is an evil that I haven’t faced before.”
He noted that the patient, now 17 years old but 16 when the surgical procedure occurred, is doing well in his recovery, and although the patient and the family are not yet ready to be identified, the health system made the decision to tell the story of the surgery as a cautionary tale.
“The reason we wanted to bring this case to public attention is because of the epidemic of e-cigarettes and vaping-induced lung injury that we are witnessing in the country,” including more than 2,000 cases of injury and 39 deaths that have been confirmed from lung failure related to e-cigarettes and vaping that have been reported to the Centers for Disease Control and Prevention, he said.
“Our teenage patient would have faced certain death if it weren’t for the lung transplant happening,” Dr. Nemeh said, adding that, while vaping and e-cigarettes are being presented as a benign habit, there are potentially very deadly consequences that Henry Ford Hospital System wanted to highlight. He described the patient’s lungs as essentially being nonfunctional with very little air being able to be passed into them, with the destruction to his native lung from pneumonia and dead tissue almost completely covering his lungs.
This story began with a morning call on Oct. 1 from the Children’s Hospital of Michigan alerting the Henry Ford Health System that they had a patient on life support because of complete lung failure who was not showing signs of healing and asking if the Henry Ford Health System could possibly handle a lung transplant for this patient.
Dr. Nemeh said that the patient was on a nontransportable extracorporeal membrane oxygenation (ECMO) machine at Children’s. Dr. Nemeh and the team at Henry Ford determined that the situation for the patient was so dire that they put a portable ECMO machine into the trunk of Dr. Nemeh’s car and delivered it to Children’s in order to facilitate the transfer of the patient for transplantation surgery.
Victor Coba, MD, a critical care specialist and medical director of the ECMO program at Henry Ford, said: “We evaluated the irreversible lung damage that had occurred associated with vaping. Working closely with the lung transplant team and noting that his lungs would not recover, we worked to get him on the lung transplant list.”
Lisa Allenspach, MD, pulmonologist and medical director of the lung transplant program at Henry Ford, reiterated the need for caution when it comes to vaping and e-cigarette use.
“Vaping-related injuries are all too common these days and, actually, our adolescents are faced with a crisis,” she said. “I believe we are just beginning to see the tip of the iceberg. Making sure that our teens understand the danger of vaping is of paramount importance.”
She did not disclose specific details about the teen’s use of vaping/e-cigarette products, so it is unknown whether the injury was caused by standard off-the-shelf products or if it was related to vaping cartridges containing tetrahydrocannabinol.
“We are here today to beg the public to pay special attention to the steps that were taken in this case,” said Nicholas Yeldo, MD, anesthesiology and critical care specialist with Henry Ford. “Without the heroic measures that were taken in this case, this young patient would have died. There is no doubt about it. ... This was not just an unlucky one. This is happening way, way too much.”
Dr. Allenspach was positive that the young patient could live a long life, noting that there are those who have received lung transplants have survived for 15-20 years and second transplants are possible.
A Michigan teenager, described as an athlete and otherwise healthy, has survived a double lung transplant following lung damage attributed to vaping.
“On the 15th of October, the transplant team performed what we believe is the first double lung transplant done in the nation for a vaping-injury victim, who is a teenager,” Hassan Nemeh, MD, cardiothoracic surgeon with the Henry Ford Health System in Detroit, said during a Nov. 12, 2019, press conference to discuss the surgery.
“What I saw in his lungs is nothing that I have ever seen before and I have been doing lung transplants for 20 years,” Dr. Nemeh said. “There was an enormous amount of inflammation and scarring, in addition to multiple spots of dead tissue. The lung itself was so firm and scarred, we had to deliver it out of the chest. This is an evil that I haven’t faced before.”
He noted that the patient, now 17 years old but 16 when the surgical procedure occurred, is doing well in his recovery, and although the patient and the family are not yet ready to be identified, the health system made the decision to tell the story of the surgery as a cautionary tale.
“The reason we wanted to bring this case to public attention is because of the epidemic of e-cigarettes and vaping-induced lung injury that we are witnessing in the country,” including more than 2,000 cases of injury and 39 deaths that have been confirmed from lung failure related to e-cigarettes and vaping that have been reported to the Centers for Disease Control and Prevention, he said.
“Our teenage patient would have faced certain death if it weren’t for the lung transplant happening,” Dr. Nemeh said, adding that, while vaping and e-cigarettes are being presented as a benign habit, there are potentially very deadly consequences that Henry Ford Hospital System wanted to highlight. He described the patient’s lungs as essentially being nonfunctional with very little air being able to be passed into them, with the destruction to his native lung from pneumonia and dead tissue almost completely covering his lungs.
This story began with a morning call on Oct. 1 from the Children’s Hospital of Michigan alerting the Henry Ford Health System that they had a patient on life support because of complete lung failure who was not showing signs of healing and asking if the Henry Ford Health System could possibly handle a lung transplant for this patient.
Dr. Nemeh said that the patient was on a nontransportable extracorporeal membrane oxygenation (ECMO) machine at Children’s. Dr. Nemeh and the team at Henry Ford determined that the situation for the patient was so dire that they put a portable ECMO machine into the trunk of Dr. Nemeh’s car and delivered it to Children’s in order to facilitate the transfer of the patient for transplantation surgery.
Victor Coba, MD, a critical care specialist and medical director of the ECMO program at Henry Ford, said: “We evaluated the irreversible lung damage that had occurred associated with vaping. Working closely with the lung transplant team and noting that his lungs would not recover, we worked to get him on the lung transplant list.”
Lisa Allenspach, MD, pulmonologist and medical director of the lung transplant program at Henry Ford, reiterated the need for caution when it comes to vaping and e-cigarette use.
“Vaping-related injuries are all too common these days and, actually, our adolescents are faced with a crisis,” she said. “I believe we are just beginning to see the tip of the iceberg. Making sure that our teens understand the danger of vaping is of paramount importance.”
She did not disclose specific details about the teen’s use of vaping/e-cigarette products, so it is unknown whether the injury was caused by standard off-the-shelf products or if it was related to vaping cartridges containing tetrahydrocannabinol.
“We are here today to beg the public to pay special attention to the steps that were taken in this case,” said Nicholas Yeldo, MD, anesthesiology and critical care specialist with Henry Ford. “Without the heroic measures that were taken in this case, this young patient would have died. There is no doubt about it. ... This was not just an unlucky one. This is happening way, way too much.”
Dr. Allenspach was positive that the young patient could live a long life, noting that there are those who have received lung transplants have survived for 15-20 years and second transplants are possible.
A Michigan teenager, described as an athlete and otherwise healthy, has survived a double lung transplant following lung damage attributed to vaping.
“On the 15th of October, the transplant team performed what we believe is the first double lung transplant done in the nation for a vaping-injury victim, who is a teenager,” Hassan Nemeh, MD, cardiothoracic surgeon with the Henry Ford Health System in Detroit, said during a Nov. 12, 2019, press conference to discuss the surgery.
“What I saw in his lungs is nothing that I have ever seen before and I have been doing lung transplants for 20 years,” Dr. Nemeh said. “There was an enormous amount of inflammation and scarring, in addition to multiple spots of dead tissue. The lung itself was so firm and scarred, we had to deliver it out of the chest. This is an evil that I haven’t faced before.”
He noted that the patient, now 17 years old but 16 when the surgical procedure occurred, is doing well in his recovery, and although the patient and the family are not yet ready to be identified, the health system made the decision to tell the story of the surgery as a cautionary tale.
“The reason we wanted to bring this case to public attention is because of the epidemic of e-cigarettes and vaping-induced lung injury that we are witnessing in the country,” including more than 2,000 cases of injury and 39 deaths that have been confirmed from lung failure related to e-cigarettes and vaping that have been reported to the Centers for Disease Control and Prevention, he said.
“Our teenage patient would have faced certain death if it weren’t for the lung transplant happening,” Dr. Nemeh said, adding that, while vaping and e-cigarettes are being presented as a benign habit, there are potentially very deadly consequences that Henry Ford Hospital System wanted to highlight. He described the patient’s lungs as essentially being nonfunctional with very little air being able to be passed into them, with the destruction to his native lung from pneumonia and dead tissue almost completely covering his lungs.
This story began with a morning call on Oct. 1 from the Children’s Hospital of Michigan alerting the Henry Ford Health System that they had a patient on life support because of complete lung failure who was not showing signs of healing and asking if the Henry Ford Health System could possibly handle a lung transplant for this patient.
Dr. Nemeh said that the patient was on a nontransportable extracorporeal membrane oxygenation (ECMO) machine at Children’s. Dr. Nemeh and the team at Henry Ford determined that the situation for the patient was so dire that they put a portable ECMO machine into the trunk of Dr. Nemeh’s car and delivered it to Children’s in order to facilitate the transfer of the patient for transplantation surgery.
Victor Coba, MD, a critical care specialist and medical director of the ECMO program at Henry Ford, said: “We evaluated the irreversible lung damage that had occurred associated with vaping. Working closely with the lung transplant team and noting that his lungs would not recover, we worked to get him on the lung transplant list.”
Lisa Allenspach, MD, pulmonologist and medical director of the lung transplant program at Henry Ford, reiterated the need for caution when it comes to vaping and e-cigarette use.
“Vaping-related injuries are all too common these days and, actually, our adolescents are faced with a crisis,” she said. “I believe we are just beginning to see the tip of the iceberg. Making sure that our teens understand the danger of vaping is of paramount importance.”
She did not disclose specific details about the teen’s use of vaping/e-cigarette products, so it is unknown whether the injury was caused by standard off-the-shelf products or if it was related to vaping cartridges containing tetrahydrocannabinol.
“We are here today to beg the public to pay special attention to the steps that were taken in this case,” said Nicholas Yeldo, MD, anesthesiology and critical care specialist with Henry Ford. “Without the heroic measures that were taken in this case, this young patient would have died. There is no doubt about it. ... This was not just an unlucky one. This is happening way, way too much.”
Dr. Allenspach was positive that the young patient could live a long life, noting that there are those who have received lung transplants have survived for 15-20 years and second transplants are possible.
DACA lands before Supreme Court
Whether the Trump administration can rescind a safe-haven program for the children of first-generation immigrants will soon be decided by the U.S. Supreme Court.
Justices on Nov. 12, 2019, heard oral arguments in the case of Regents of the University of California v. Department of Homeland Security, which centers on the legality of the Deferred Action for Childhood Arrivals (DACA) policy.
The DACA case before the court, which consolidates three legal challenges, revolves around two primary legal arguments, said Austin, Tex., attorney Faye Kolly, who coauthored a brief in support of DACA. The plaintiffs argue that the decision to rescind the DACA program was arbitrary and capricious under the Administrative Procedure Act (APA) because there was no justification to end the policy. The DHS’s rescission memorandum did not acknowledge, nor weigh, the profound interests and devastating consequences that would be caused by the rescission to hundreds of thousands of DACA recipients and countless stakeholders who rely on the program, attorneys for the University of California wrote in its brief to the Supreme Court.
The DHS contends the DACA policy itself is unlawful because the Obama administration lacked the statutory authority to launch such a program. The government’s decision to revoke the program was based on the legal and practical implications of maintaining DACA in light of concerns about its legality and ongoing litigation challenging similar programs, attorneys for the DHS wrote in a brief to the Supreme Court.
With a conservative majority on the court, analysts say the Trump administration may have an upper hand in the case. However, Ms. Kolly noted that Chief Justice John G. Roberts Jr. is the wild card to watch.
Although Chief Justice Roberts tends to lean more conservatively, he recently sided with the court’s liberal justices last term in a case involving citizenship, said Ms. Kolly. In that case, Department of Commerce v. New York, Chief Justice Roberts voted to block the Department of Commerce from reinstating a citizenship question on the 2020 Census. In the majority ruling, Justice Roberts wrote that the department’s reasons for reinstating the citizenship question were “incongruent with what the record reveals about the agency’s priorities and decision making.”
Considering that the government’s rationale to end DACA is a key piece to the DACA dispute, it’s possible that Chief Justice Roberts could apply a similar line of reasoning to the case, Ms. Kolly said.
“I think the court will look very closely [at whether] the arbitrary and capricious standard has been violated by the government,” she said in an interview. “I do think they will split along [ideological] lines, but the surprise vote may be Roberts.”
A number of physician and health care organizations have weighed in on the DACA case, including the American Medical Association, the Association of American Medical Colleges, and the American College of Obstetricians and Gynecologists. In a joint brief to the Supreme Court, the organizations wrote that an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States, including nearly 200 medical students, residents, and physicians. If these physicians and trainees retain their work eligibility, each will care for an average of 1,533-4,600 patients a year, according to the brief.
“Together, over the course of their careers, they will touch the lives of 1.7 [million] to 5.1 million U.S. patients,” the groups wrote. “If DACA is rescinded, however, almost none of these people will be able to serve the American public in their chosen fields. This action would therefore nullify the substantial and long-term investments that DACA recipients, educational institutions, and the public have made in educating and training those recipients to provide needed health care services to the nation.”
More than 25 other individuals and organizations have also submitted briefs – either in support or in opposition – regarding the case. In their brief, attorneys for the Immigration Law Reform Institute, wrote that the DACA policy is void because it was issued in violation of APA notice-and-comment requirements “by virtue of its creating rights and cabining discretion in a sufficiently binding manner to exceed its mere enforcement-discretion justification.”
“DACA also violates the [Immigration and Naturalization Act] on both substantive and procedural grounds, and either sort of violation renders DACA a nullity,” the brief states.
In a Nov. 12 tweet, President Trump indicated that, if the Supreme Court strikes down DACA, he will consider working with Democrats on a legislative remedy that would protect current DACA recipients from deportation.
“Many of the people in DACA, no longer very young, are far from ‘angels,’ ” President Trump tweeted. “Some are very tough, hardened criminals, President Obama said he had no legal right to sign order, but would anyway. If Supreme Court remedies with overturn, a deal will be made with Dems for them to stay!”
Whether the Trump administration can rescind a safe-haven program for the children of first-generation immigrants will soon be decided by the U.S. Supreme Court.
Justices on Nov. 12, 2019, heard oral arguments in the case of Regents of the University of California v. Department of Homeland Security, which centers on the legality of the Deferred Action for Childhood Arrivals (DACA) policy.
The DACA case before the court, which consolidates three legal challenges, revolves around two primary legal arguments, said Austin, Tex., attorney Faye Kolly, who coauthored a brief in support of DACA. The plaintiffs argue that the decision to rescind the DACA program was arbitrary and capricious under the Administrative Procedure Act (APA) because there was no justification to end the policy. The DHS’s rescission memorandum did not acknowledge, nor weigh, the profound interests and devastating consequences that would be caused by the rescission to hundreds of thousands of DACA recipients and countless stakeholders who rely on the program, attorneys for the University of California wrote in its brief to the Supreme Court.
The DHS contends the DACA policy itself is unlawful because the Obama administration lacked the statutory authority to launch such a program. The government’s decision to revoke the program was based on the legal and practical implications of maintaining DACA in light of concerns about its legality and ongoing litigation challenging similar programs, attorneys for the DHS wrote in a brief to the Supreme Court.
With a conservative majority on the court, analysts say the Trump administration may have an upper hand in the case. However, Ms. Kolly noted that Chief Justice John G. Roberts Jr. is the wild card to watch.
Although Chief Justice Roberts tends to lean more conservatively, he recently sided with the court’s liberal justices last term in a case involving citizenship, said Ms. Kolly. In that case, Department of Commerce v. New York, Chief Justice Roberts voted to block the Department of Commerce from reinstating a citizenship question on the 2020 Census. In the majority ruling, Justice Roberts wrote that the department’s reasons for reinstating the citizenship question were “incongruent with what the record reveals about the agency’s priorities and decision making.”
Considering that the government’s rationale to end DACA is a key piece to the DACA dispute, it’s possible that Chief Justice Roberts could apply a similar line of reasoning to the case, Ms. Kolly said.
“I think the court will look very closely [at whether] the arbitrary and capricious standard has been violated by the government,” she said in an interview. “I do think they will split along [ideological] lines, but the surprise vote may be Roberts.”
A number of physician and health care organizations have weighed in on the DACA case, including the American Medical Association, the Association of American Medical Colleges, and the American College of Obstetricians and Gynecologists. In a joint brief to the Supreme Court, the organizations wrote that an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States, including nearly 200 medical students, residents, and physicians. If these physicians and trainees retain their work eligibility, each will care for an average of 1,533-4,600 patients a year, according to the brief.
“Together, over the course of their careers, they will touch the lives of 1.7 [million] to 5.1 million U.S. patients,” the groups wrote. “If DACA is rescinded, however, almost none of these people will be able to serve the American public in their chosen fields. This action would therefore nullify the substantial and long-term investments that DACA recipients, educational institutions, and the public have made in educating and training those recipients to provide needed health care services to the nation.”
More than 25 other individuals and organizations have also submitted briefs – either in support or in opposition – regarding the case. In their brief, attorneys for the Immigration Law Reform Institute, wrote that the DACA policy is void because it was issued in violation of APA notice-and-comment requirements “by virtue of its creating rights and cabining discretion in a sufficiently binding manner to exceed its mere enforcement-discretion justification.”
“DACA also violates the [Immigration and Naturalization Act] on both substantive and procedural grounds, and either sort of violation renders DACA a nullity,” the brief states.
In a Nov. 12 tweet, President Trump indicated that, if the Supreme Court strikes down DACA, he will consider working with Democrats on a legislative remedy that would protect current DACA recipients from deportation.
“Many of the people in DACA, no longer very young, are far from ‘angels,’ ” President Trump tweeted. “Some are very tough, hardened criminals, President Obama said he had no legal right to sign order, but would anyway. If Supreme Court remedies with overturn, a deal will be made with Dems for them to stay!”
Whether the Trump administration can rescind a safe-haven program for the children of first-generation immigrants will soon be decided by the U.S. Supreme Court.
Justices on Nov. 12, 2019, heard oral arguments in the case of Regents of the University of California v. Department of Homeland Security, which centers on the legality of the Deferred Action for Childhood Arrivals (DACA) policy.
The DACA case before the court, which consolidates three legal challenges, revolves around two primary legal arguments, said Austin, Tex., attorney Faye Kolly, who coauthored a brief in support of DACA. The plaintiffs argue that the decision to rescind the DACA program was arbitrary and capricious under the Administrative Procedure Act (APA) because there was no justification to end the policy. The DHS’s rescission memorandum did not acknowledge, nor weigh, the profound interests and devastating consequences that would be caused by the rescission to hundreds of thousands of DACA recipients and countless stakeholders who rely on the program, attorneys for the University of California wrote in its brief to the Supreme Court.
The DHS contends the DACA policy itself is unlawful because the Obama administration lacked the statutory authority to launch such a program. The government’s decision to revoke the program was based on the legal and practical implications of maintaining DACA in light of concerns about its legality and ongoing litigation challenging similar programs, attorneys for the DHS wrote in a brief to the Supreme Court.
With a conservative majority on the court, analysts say the Trump administration may have an upper hand in the case. However, Ms. Kolly noted that Chief Justice John G. Roberts Jr. is the wild card to watch.
Although Chief Justice Roberts tends to lean more conservatively, he recently sided with the court’s liberal justices last term in a case involving citizenship, said Ms. Kolly. In that case, Department of Commerce v. New York, Chief Justice Roberts voted to block the Department of Commerce from reinstating a citizenship question on the 2020 Census. In the majority ruling, Justice Roberts wrote that the department’s reasons for reinstating the citizenship question were “incongruent with what the record reveals about the agency’s priorities and decision making.”
Considering that the government’s rationale to end DACA is a key piece to the DACA dispute, it’s possible that Chief Justice Roberts could apply a similar line of reasoning to the case, Ms. Kolly said.
“I think the court will look very closely [at whether] the arbitrary and capricious standard has been violated by the government,” she said in an interview. “I do think they will split along [ideological] lines, but the surprise vote may be Roberts.”
A number of physician and health care organizations have weighed in on the DACA case, including the American Medical Association, the Association of American Medical Colleges, and the American College of Obstetricians and Gynecologists. In a joint brief to the Supreme Court, the organizations wrote that an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States, including nearly 200 medical students, residents, and physicians. If these physicians and trainees retain their work eligibility, each will care for an average of 1,533-4,600 patients a year, according to the brief.
“Together, over the course of their careers, they will touch the lives of 1.7 [million] to 5.1 million U.S. patients,” the groups wrote. “If DACA is rescinded, however, almost none of these people will be able to serve the American public in their chosen fields. This action would therefore nullify the substantial and long-term investments that DACA recipients, educational institutions, and the public have made in educating and training those recipients to provide needed health care services to the nation.”
More than 25 other individuals and organizations have also submitted briefs – either in support or in opposition – regarding the case. In their brief, attorneys for the Immigration Law Reform Institute, wrote that the DACA policy is void because it was issued in violation of APA notice-and-comment requirements “by virtue of its creating rights and cabining discretion in a sufficiently binding manner to exceed its mere enforcement-discretion justification.”
“DACA also violates the [Immigration and Naturalization Act] on both substantive and procedural grounds, and either sort of violation renders DACA a nullity,” the brief states.
In a Nov. 12 tweet, President Trump indicated that, if the Supreme Court strikes down DACA, he will consider working with Democrats on a legislative remedy that would protect current DACA recipients from deportation.
“Many of the people in DACA, no longer very young, are far from ‘angels,’ ” President Trump tweeted. “Some are very tough, hardened criminals, President Obama said he had no legal right to sign order, but would anyway. If Supreme Court remedies with overturn, a deal will be made with Dems for them to stay!”
Hypoglycemia Safety Initiative: Working With PACT Clinical Pharmacy Specialists to Individualize HbA1c Goals (FULL)
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
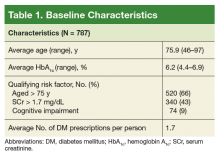
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
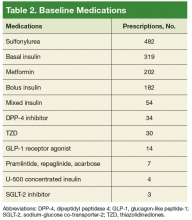
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
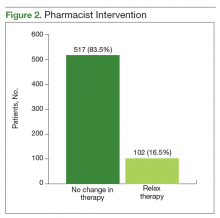
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
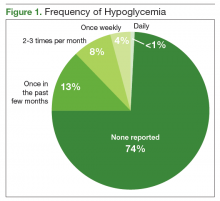
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
Polypharmacy in an Aging HIV Population
PORTLAND—“We are now working with an HIV population that is aging, and along with aging comes a lot of other diseases, such as heart and lung disease, that often result in the use of medications,” began Jennifer Cocohoba, PharmD, AAHIP, Professor of Clinical Pharmacy, UCSF School of Pharmacy. She explained that “the growing problem of having too many medications applies to all, but is particularly important among those living with HIV.”
Polypharmacy, as often defined in research studies, is the taking of at least 5 prescription medications. About 40% of US adults fall into this category, “and it’s not much different for people with HIV,” Cocohoba said. “Persons living with HIV experience polypharmacy at about the same rate.” But people living with HIV may reach that number faster, given that 2 to 3 of the 5 drugs may be for HIV. In addition, those living with HIV are potentially at greater risk for drug interactions.
Quality, not just quantity
Cocohoba explained that it’s important to pay attention to the number of medications a patient is taking because the greater the number, the greater the likelihood of interactions and adverse effects and the more difficult it is for patients to adhere to their medication regimens.
“But we should be looking also at appropriateness of using those medications in a particular person,” she continued, which moves into the realm of quality. She reviewed some criteria that can be used to evaluate the quality of prescribing.
The BEERS Criteria, published by the American Geriatric Society, present classes and specific medications that may be inappropriate in certain elderly persons. Benzodiazepines, for example, commonly used to treat anxiety, are not metabolized as efficiently in the elderly as they are in younger patients. As a result, older adults may experience sedation, falls, or other potentially harmful sequelae. “If a patient age 65 or older is taking a benzodiazepine, that’s a red flag for a clinician to look at that medication and see if it’s really the best choice or whether alternatives might be more appropriate,” explained Cocohoba.
START/STOPP Criteria. Other criteria include the Screening Tool To Alert To Right Treatment (START) and the Screening Tool Of Older People's Prescriptions (STOPP). START focuses on looking for medications that would be appropriate for a particular patient but that are missing from the patient’s medication list, while STOPP focuses on looking for medications that are on the list that might be inappropriate, much like the BEERS Criteria.
The Good Palliative Geriatric Practice Algorithm is a clinical decision-making tool that aids clinicians in thinking through whether a medication is appropriate or inappropriate and whether to maintain, alter, or discontinue the therapy. Cocohoba said it’s often used as a partner to BEERS.
Continue to: Reducing polypharmacy in HIV treatment
Reducing polypharmacy in HIV treatment
Cocohoba explained that there are essentially 2 frameworks for reducing polypharmacy that can be applied to HIV treatment regimens.
Consolidation. With consolidation, the focus is on reducing pill burden—but not by omitting medications. “It’s about looking into simpler dosage forms or combination medications to improve adherence and make life easier,” Cocohoba explained. “Same regimen, fewer pills.”
Simplification, on the other hand, is removing, and thus reducing the number of, agents a patient is taking. The question clinicians should be asking is, according to Cocohoba, “In what situations is it safe to strip down therapy to the bare essentials for the purposes of exposing people to fewer medications, reducing adverse effects, and keeping treatment as manageable as possible to optimize adherence?”
Simplification may be applied to either treatment-naïve or treatment-experienced patients. With the former, clinicians consider, for example, whether patients can be started on HIV treatment consisting of 2 rather than 3 medications. In the latter, “Clinicians may be dealing with patients who are fully virally suppressed on certain regimens and have been for a while; here we see if we can subtract medications, reducing say from triple to double therapy, while maintaining suppression,” explained Cocohoba.
“We want to offer people robust HIV treatment that is going to maintain viral suppression and prevent sequelae of HIV disease,” Cocohoba said, “but we need to balance that with safety, tolerability, and adherence.”
Continue to: An ongoing discussion and multidisciplinary effort
An ongoing discussion and multidisciplinary effort
“Medications can easily pile [up],” Cocohoba said, “so it’s important for clinicians to regularly review medication lists with patients to see if maybe they aren’t using certain agents anymore.” Another reason for clinicians to periodically review medication lists is to make certain they are aware of agents being prescribed by the patient’s other health care providers.
Polypharmacy is the responsibility of everyone on the health care team, both prescribers and nonprescribers, such as social workers and case managers, explained Cocohoba. “If ever there was a health care problem that could use an interdisciplinary approach, polypharmacy is it.”
PORTLAND—“We are now working with an HIV population that is aging, and along with aging comes a lot of other diseases, such as heart and lung disease, that often result in the use of medications,” began Jennifer Cocohoba, PharmD, AAHIP, Professor of Clinical Pharmacy, UCSF School of Pharmacy. She explained that “the growing problem of having too many medications applies to all, but is particularly important among those living with HIV.”
Polypharmacy, as often defined in research studies, is the taking of at least 5 prescription medications. About 40% of US adults fall into this category, “and it’s not much different for people with HIV,” Cocohoba said. “Persons living with HIV experience polypharmacy at about the same rate.” But people living with HIV may reach that number faster, given that 2 to 3 of the 5 drugs may be for HIV. In addition, those living with HIV are potentially at greater risk for drug interactions.
Quality, not just quantity
Cocohoba explained that it’s important to pay attention to the number of medications a patient is taking because the greater the number, the greater the likelihood of interactions and adverse effects and the more difficult it is for patients to adhere to their medication regimens.
“But we should be looking also at appropriateness of using those medications in a particular person,” she continued, which moves into the realm of quality. She reviewed some criteria that can be used to evaluate the quality of prescribing.
The BEERS Criteria, published by the American Geriatric Society, present classes and specific medications that may be inappropriate in certain elderly persons. Benzodiazepines, for example, commonly used to treat anxiety, are not metabolized as efficiently in the elderly as they are in younger patients. As a result, older adults may experience sedation, falls, or other potentially harmful sequelae. “If a patient age 65 or older is taking a benzodiazepine, that’s a red flag for a clinician to look at that medication and see if it’s really the best choice or whether alternatives might be more appropriate,” explained Cocohoba.
START/STOPP Criteria. Other criteria include the Screening Tool To Alert To Right Treatment (START) and the Screening Tool Of Older People's Prescriptions (STOPP). START focuses on looking for medications that would be appropriate for a particular patient but that are missing from the patient’s medication list, while STOPP focuses on looking for medications that are on the list that might be inappropriate, much like the BEERS Criteria.
The Good Palliative Geriatric Practice Algorithm is a clinical decision-making tool that aids clinicians in thinking through whether a medication is appropriate or inappropriate and whether to maintain, alter, or discontinue the therapy. Cocohoba said it’s often used as a partner to BEERS.
Continue to: Reducing polypharmacy in HIV treatment
Reducing polypharmacy in HIV treatment
Cocohoba explained that there are essentially 2 frameworks for reducing polypharmacy that can be applied to HIV treatment regimens.
Consolidation. With consolidation, the focus is on reducing pill burden—but not by omitting medications. “It’s about looking into simpler dosage forms or combination medications to improve adherence and make life easier,” Cocohoba explained. “Same regimen, fewer pills.”
Simplification, on the other hand, is removing, and thus reducing the number of, agents a patient is taking. The question clinicians should be asking is, according to Cocohoba, “In what situations is it safe to strip down therapy to the bare essentials for the purposes of exposing people to fewer medications, reducing adverse effects, and keeping treatment as manageable as possible to optimize adherence?”
Simplification may be applied to either treatment-naïve or treatment-experienced patients. With the former, clinicians consider, for example, whether patients can be started on HIV treatment consisting of 2 rather than 3 medications. In the latter, “Clinicians may be dealing with patients who are fully virally suppressed on certain regimens and have been for a while; here we see if we can subtract medications, reducing say from triple to double therapy, while maintaining suppression,” explained Cocohoba.
“We want to offer people robust HIV treatment that is going to maintain viral suppression and prevent sequelae of HIV disease,” Cocohoba said, “but we need to balance that with safety, tolerability, and adherence.”
Continue to: An ongoing discussion and multidisciplinary effort
An ongoing discussion and multidisciplinary effort
“Medications can easily pile [up],” Cocohoba said, “so it’s important for clinicians to regularly review medication lists with patients to see if maybe they aren’t using certain agents anymore.” Another reason for clinicians to periodically review medication lists is to make certain they are aware of agents being prescribed by the patient’s other health care providers.
Polypharmacy is the responsibility of everyone on the health care team, both prescribers and nonprescribers, such as social workers and case managers, explained Cocohoba. “If ever there was a health care problem that could use an interdisciplinary approach, polypharmacy is it.”
PORTLAND—“We are now working with an HIV population that is aging, and along with aging comes a lot of other diseases, such as heart and lung disease, that often result in the use of medications,” began Jennifer Cocohoba, PharmD, AAHIP, Professor of Clinical Pharmacy, UCSF School of Pharmacy. She explained that “the growing problem of having too many medications applies to all, but is particularly important among those living with HIV.”
Polypharmacy, as often defined in research studies, is the taking of at least 5 prescription medications. About 40% of US adults fall into this category, “and it’s not much different for people with HIV,” Cocohoba said. “Persons living with HIV experience polypharmacy at about the same rate.” But people living with HIV may reach that number faster, given that 2 to 3 of the 5 drugs may be for HIV. In addition, those living with HIV are potentially at greater risk for drug interactions.
Quality, not just quantity
Cocohoba explained that it’s important to pay attention to the number of medications a patient is taking because the greater the number, the greater the likelihood of interactions and adverse effects and the more difficult it is for patients to adhere to their medication regimens.
“But we should be looking also at appropriateness of using those medications in a particular person,” she continued, which moves into the realm of quality. She reviewed some criteria that can be used to evaluate the quality of prescribing.
The BEERS Criteria, published by the American Geriatric Society, present classes and specific medications that may be inappropriate in certain elderly persons. Benzodiazepines, for example, commonly used to treat anxiety, are not metabolized as efficiently in the elderly as they are in younger patients. As a result, older adults may experience sedation, falls, or other potentially harmful sequelae. “If a patient age 65 or older is taking a benzodiazepine, that’s a red flag for a clinician to look at that medication and see if it’s really the best choice or whether alternatives might be more appropriate,” explained Cocohoba.
START/STOPP Criteria. Other criteria include the Screening Tool To Alert To Right Treatment (START) and the Screening Tool Of Older People's Prescriptions (STOPP). START focuses on looking for medications that would be appropriate for a particular patient but that are missing from the patient’s medication list, while STOPP focuses on looking for medications that are on the list that might be inappropriate, much like the BEERS Criteria.
The Good Palliative Geriatric Practice Algorithm is a clinical decision-making tool that aids clinicians in thinking through whether a medication is appropriate or inappropriate and whether to maintain, alter, or discontinue the therapy. Cocohoba said it’s often used as a partner to BEERS.
Continue to: Reducing polypharmacy in HIV treatment
Reducing polypharmacy in HIV treatment
Cocohoba explained that there are essentially 2 frameworks for reducing polypharmacy that can be applied to HIV treatment regimens.
Consolidation. With consolidation, the focus is on reducing pill burden—but not by omitting medications. “It’s about looking into simpler dosage forms or combination medications to improve adherence and make life easier,” Cocohoba explained. “Same regimen, fewer pills.”
Simplification, on the other hand, is removing, and thus reducing the number of, agents a patient is taking. The question clinicians should be asking is, according to Cocohoba, “In what situations is it safe to strip down therapy to the bare essentials for the purposes of exposing people to fewer medications, reducing adverse effects, and keeping treatment as manageable as possible to optimize adherence?”
Simplification may be applied to either treatment-naïve or treatment-experienced patients. With the former, clinicians consider, for example, whether patients can be started on HIV treatment consisting of 2 rather than 3 medications. In the latter, “Clinicians may be dealing with patients who are fully virally suppressed on certain regimens and have been for a while; here we see if we can subtract medications, reducing say from triple to double therapy, while maintaining suppression,” explained Cocohoba.
“We want to offer people robust HIV treatment that is going to maintain viral suppression and prevent sequelae of HIV disease,” Cocohoba said, “but we need to balance that with safety, tolerability, and adherence.”
Continue to: An ongoing discussion and multidisciplinary effort
An ongoing discussion and multidisciplinary effort
“Medications can easily pile [up],” Cocohoba said, “so it’s important for clinicians to regularly review medication lists with patients to see if maybe they aren’t using certain agents anymore.” Another reason for clinicians to periodically review medication lists is to make certain they are aware of agents being prescribed by the patient’s other health care providers.
Polypharmacy is the responsibility of everyone on the health care team, both prescribers and nonprescribers, such as social workers and case managers, explained Cocohoba. “If ever there was a health care problem that could use an interdisciplinary approach, polypharmacy is it.”
Association of Nurses in AIDS Care 2019
Physical Therapists as Pain Specialists in HIV Care
PORTLAND—A lot of health care providers involved in the care of patients with HIV/AIDS think physical therapists (PTs) have a very narrow role to play in chronic pain when in actuality, "we have a very big footprint," says Michael DeArman, DPT, Fellow of the American Association of Orthopedic Manual Therapists.
In his session, "Physical therapy alternatives for people living with HIV and persistent pain," he reviewed how his role in the care of patients with HIV has evolved over the years. He explained that the emphasis in physical therapy used to be on exercises that would help to maintain lean body mass, because it was a predictor of survivability and helped patients avoid opportunistic infections.
After that, the role of the PT focused largely on helping patients deal with the adverse effects of medications. DeArman explained that back in the days of azathioprine (AZT), for example, many patients developed peripheral neuropathy, lipodystrophy, and low cervical fat deposition leading to neck pain. PTs also helped patients deal with the complications of surviving with HIV, including the effects of meningitis and stroke. "But we don't see much of that anymore because the medications are so much better,” DeArman said. “That's not the profile for HIV anymore."
But now patients are dealing with chronic pain
DeArman reported that 80% to 90% of people with HIV/AIDS have chronic pain for at least 2 months, if not much longer. He said, "PT can be an alternative to opioids," and explained that his efforts are twofold. "Graded activity to slowly build peoples' ability to do aerobic exercise is a really good way to manage pain,” he said, “but the other half of it is a lot of counseling and education, talking about how pain works and doesn't work, and how what you're told and what you believe and experience and your expectations can affect the experience of pain and the level of pain at any given moment."
He remarked that now about 60% of this professional time is devoted to the counseling and educational aspects of pain and 40% to movement and exercise: "It's not really the kind of therapist I trained to be, and it's not what most people think of as physical therapy work, but it's falling in our lap."
He said that as a society, we rely heavily on more formal counseling by psychotherapists and counselors, for example, to manage depression and anxiety, but that few others in the medical realm are picking up the slack when it comes to pain education for patients with HIV.
Tools for talking
DeArman discussed some of the tools and techniques he uses when talking to patients about their pain, such as motivational interviewing and assessing readiness for change. He uses the Fear-Avoidance Beliefs Questionnaire (FABQ) to uncover how a patient's fear-avoidance beliefs about physical activity may be affecting their pain. He also assesses levels of catastrophization, or how seriously a patient interprets a physical symptom; a high level correlates with greater pain states. Similarly, those with low self-efficacy—feelings about how well you can control your destiny and situations—are more likely to experience chronic pain states than those with high self-efficacy.
Continue to: He said that using...
He said that using these tools has made a huge difference in the way he and others in his field approach pain and its outcomes. He says that not only are they helping people get better, but "we are giving patients a way to manage their pain before they go down the road of pharmacologic solutions."
DeArman said his main message is "to think about physical therapy for your patients with chronic pain." He pointed out that there's a new generation of PTs coming out of school that have a much better understanding of how to manage chronic pain, so "expect [this type of treatment] to be available from those PTs to whom you refer patients. Just realize it may still take a little looking to find it."
PORTLAND—A lot of health care providers involved in the care of patients with HIV/AIDS think physical therapists (PTs) have a very narrow role to play in chronic pain when in actuality, "we have a very big footprint," says Michael DeArman, DPT, Fellow of the American Association of Orthopedic Manual Therapists.
In his session, "Physical therapy alternatives for people living with HIV and persistent pain," he reviewed how his role in the care of patients with HIV has evolved over the years. He explained that the emphasis in physical therapy used to be on exercises that would help to maintain lean body mass, because it was a predictor of survivability and helped patients avoid opportunistic infections.
After that, the role of the PT focused largely on helping patients deal with the adverse effects of medications. DeArman explained that back in the days of azathioprine (AZT), for example, many patients developed peripheral neuropathy, lipodystrophy, and low cervical fat deposition leading to neck pain. PTs also helped patients deal with the complications of surviving with HIV, including the effects of meningitis and stroke. "But we don't see much of that anymore because the medications are so much better,” DeArman said. “That's not the profile for HIV anymore."
But now patients are dealing with chronic pain
DeArman reported that 80% to 90% of people with HIV/AIDS have chronic pain for at least 2 months, if not much longer. He said, "PT can be an alternative to opioids," and explained that his efforts are twofold. "Graded activity to slowly build peoples' ability to do aerobic exercise is a really good way to manage pain,” he said, “but the other half of it is a lot of counseling and education, talking about how pain works and doesn't work, and how what you're told and what you believe and experience and your expectations can affect the experience of pain and the level of pain at any given moment."
He remarked that now about 60% of this professional time is devoted to the counseling and educational aspects of pain and 40% to movement and exercise: "It's not really the kind of therapist I trained to be, and it's not what most people think of as physical therapy work, but it's falling in our lap."
He said that as a society, we rely heavily on more formal counseling by psychotherapists and counselors, for example, to manage depression and anxiety, but that few others in the medical realm are picking up the slack when it comes to pain education for patients with HIV.
Tools for talking
DeArman discussed some of the tools and techniques he uses when talking to patients about their pain, such as motivational interviewing and assessing readiness for change. He uses the Fear-Avoidance Beliefs Questionnaire (FABQ) to uncover how a patient's fear-avoidance beliefs about physical activity may be affecting their pain. He also assesses levels of catastrophization, or how seriously a patient interprets a physical symptom; a high level correlates with greater pain states. Similarly, those with low self-efficacy—feelings about how well you can control your destiny and situations—are more likely to experience chronic pain states than those with high self-efficacy.
Continue to: He said that using...
He said that using these tools has made a huge difference in the way he and others in his field approach pain and its outcomes. He says that not only are they helping people get better, but "we are giving patients a way to manage their pain before they go down the road of pharmacologic solutions."
DeArman said his main message is "to think about physical therapy for your patients with chronic pain." He pointed out that there's a new generation of PTs coming out of school that have a much better understanding of how to manage chronic pain, so "expect [this type of treatment] to be available from those PTs to whom you refer patients. Just realize it may still take a little looking to find it."
PORTLAND—A lot of health care providers involved in the care of patients with HIV/AIDS think physical therapists (PTs) have a very narrow role to play in chronic pain when in actuality, "we have a very big footprint," says Michael DeArman, DPT, Fellow of the American Association of Orthopedic Manual Therapists.
In his session, "Physical therapy alternatives for people living with HIV and persistent pain," he reviewed how his role in the care of patients with HIV has evolved over the years. He explained that the emphasis in physical therapy used to be on exercises that would help to maintain lean body mass, because it was a predictor of survivability and helped patients avoid opportunistic infections.
After that, the role of the PT focused largely on helping patients deal with the adverse effects of medications. DeArman explained that back in the days of azathioprine (AZT), for example, many patients developed peripheral neuropathy, lipodystrophy, and low cervical fat deposition leading to neck pain. PTs also helped patients deal with the complications of surviving with HIV, including the effects of meningitis and stroke. "But we don't see much of that anymore because the medications are so much better,” DeArman said. “That's not the profile for HIV anymore."
But now patients are dealing with chronic pain
DeArman reported that 80% to 90% of people with HIV/AIDS have chronic pain for at least 2 months, if not much longer. He said, "PT can be an alternative to opioids," and explained that his efforts are twofold. "Graded activity to slowly build peoples' ability to do aerobic exercise is a really good way to manage pain,” he said, “but the other half of it is a lot of counseling and education, talking about how pain works and doesn't work, and how what you're told and what you believe and experience and your expectations can affect the experience of pain and the level of pain at any given moment."
He remarked that now about 60% of this professional time is devoted to the counseling and educational aspects of pain and 40% to movement and exercise: "It's not really the kind of therapist I trained to be, and it's not what most people think of as physical therapy work, but it's falling in our lap."
He said that as a society, we rely heavily on more formal counseling by psychotherapists and counselors, for example, to manage depression and anxiety, but that few others in the medical realm are picking up the slack when it comes to pain education for patients with HIV.
Tools for talking
DeArman discussed some of the tools and techniques he uses when talking to patients about their pain, such as motivational interviewing and assessing readiness for change. He uses the Fear-Avoidance Beliefs Questionnaire (FABQ) to uncover how a patient's fear-avoidance beliefs about physical activity may be affecting their pain. He also assesses levels of catastrophization, or how seriously a patient interprets a physical symptom; a high level correlates with greater pain states. Similarly, those with low self-efficacy—feelings about how well you can control your destiny and situations—are more likely to experience chronic pain states than those with high self-efficacy.
Continue to: He said that using...
He said that using these tools has made a huge difference in the way he and others in his field approach pain and its outcomes. He says that not only are they helping people get better, but "we are giving patients a way to manage their pain before they go down the road of pharmacologic solutions."
DeArman said his main message is "to think about physical therapy for your patients with chronic pain." He pointed out that there's a new generation of PTs coming out of school that have a much better understanding of how to manage chronic pain, so "expect [this type of treatment] to be available from those PTs to whom you refer patients. Just realize it may still take a little looking to find it."
Association of Nurses in AIDS Care 2019
ARV Therapy: Current Issues and Controversies
PORTLAND—The investigational drug islatravir “could be a game changer in the field of HIV," said David H. Spach, MD, Professor of Medicine, Division of Infectious Diseases, University of Washington, Seattle, in a session called, "Antiretroviral therapy 2019 update: Mechanism of action, new medications, current guidelines, and controversies."
Dr. Spach reported that islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), is highly potent, is well tolerated, has a high genetic barrier to resistance, and has an extremely long half-life, according to the findings of preliminary studies. Its long half-life enables subdermal implantation and maintenance of therapeutic levels even after a year in place. Researchers are studying the compound in combination with other agents for treatment and independently for preexposure prophylaxis.
Another noteworthy investigational agent, according to Dr. Spach, is cabotegravir, an integrase strand transfer inhibitor (INSTI) with the potential for intramuscular administration every 4 to 8 weeks. Dr. Spach explained that researchers are studying the agent in 3 different clinical situations: oral cabotegravir in combination with rilpivirine for lead-in therapy; an extended-release injectable of cabotegravir and rilpivirine for maintenance antiretroviral (ARV) treatment every 4 weeks; and an extended-release cabotegravir injectable for preexposure prophylaxis every 8 weeks. The agent is highly potent, with a high genetic barrier to resistance.
ARV: The current state of affairs
"We are in an era now where everyone who is living with HIV ideally should be receiving fully suppressive antiretroviral therapy," remarked Dr. Spach. He explained that not only does this benefit the person living with HIV by reducing the onset and progression of chronic inflammatory disease states that occur along with HIV, such as cardiovascular disease, stroke, and cancer, but also "fully suppressive ARV therapy virtually eliminates sexual transmission of HIV to another person."
Spach explained that the most recent (2018) Health and Human Services ARV therapy guidelines have greatly simplified the choices for ARV therapy. The current recommendations for initial ARV therapy for most people are to use a 2-drug backbone regimen, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), combined with a single anchor drug, which should be an INSTI. The INSTI should have the highest potency and highest genetic barrier to resistance available, which effectively means using a regimen that contains either dolutegravir or bictegravir. The latter is available only in combination with emtricitabine and tenofovir alafenamide as a single-tablet regimen.
Spach also explained that "the guidelines have moved away from using boosted regimens for initial therapy, so elvitegravir boosted with cobicistat and protease inhibitors (PIs) boosted with ritonavir or cobicistat are no longer recommended as preferred initial therapy, although boosted PIs are still very useful as second- or third-line therapies."
Newer medications
Doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), was approved by the FDA in 2018. "It probably won't have a big impact on initial therapy, but it is likely to have a significant effect on second- and third-line therapy," Dr. Spach said. "Because of its high potency and high genetic barrier to resistance, those taking etravirine twice a day, for example, may be able to simplify to once-a-day doravirine." In addition, "Doravirine may have advantages over rilpivirine in that it has no restrictions when used with acid-suppressing agents such as H2 blockers or proton pump inhibitors."
Continue to: Ibalizumab
Ibalizumab. Another newer agent “worth mentioning,” according to Dr. Spach, is ibalizumab. This monoclonal antibody has a unique mechanism of action. It is 1 of only 2 drugs used for HIV treatment that targets human receptors (all of the others work by inhibiting either an HIV enzyme or binding to the HIV virus itself). Ibalizumab targets the D2 region of the CD4 receptor. It is an injectable (intravenous) compound administered with an initial loading dose and then every 2 weeks thereafter. Dr. Spach reported that the data surrounding ibalizumab show that it is effective as an add-on medication in more advanced resistant settings. Also, it provides an option for people who can't take oral drugs, such as those who have had major surgery or trauma.
Remaining questions
Dr. Spach explained that 1 of the questions that remains is whether to prescribe ARV therapy for patients who are viremic controllers (those who inherently control HIV through their own immunologic response to a level < 200 copies) or elite controllers (those whose own immunologic response controls the virus to a level < 50 copies, which is in the undetectable range). Both of these groups still have a higher risk for hospitalization and for HIV-related inflammatory conditions such as heart disease, according to Dr. Spach. Current thinking among most experts is to initiate and maintain therapy as long as it is tolerated well.
3 drugs to 2? Another question that remains is whether to switch patients who are doing well on 3-drug maintenance therapy to 2-drug maintenance therapy. According to Dr Spach, studies involving the combination dolutegravir/rilpivirine indicate that patients who have suppressed HIV RNA levels for at least 6 months on a 3-drug maintenance regimen do well after switching to the 2-drug combination dolutegravir/rilpivirine, as long as they do not have baseline resistance to either dolutegravir or rilpivirine. But he questioned the need for the change if the individual is tolerating a 3-drug regimen well, saying that given the safety of current regimens, the only broader motivating force may be cost savings. For now, he said, if patients are without complaints or tolerability issues on 3 drugs, leave them alone.
INSTIs and weight gain. The last issue is weight gain with the use of INSTIs. Preliminary data suggest disproportionate weight gain with these drugs (on the order of about 6 kg over a year and half, which may be 2-3 kg greater than that which occurs with PI-based and NNRTI-based regimens). At this point, experts do not recommend avoiding these agents, mainly because of the tremendous benefits that have been observed with INSTIs. Dr. Spach concluded, "Although we will continue to use INSTIs widely in clinical practice, there may be a subset of individuals taking an INSTI who have pronounced weight gain and may need to switch to another regimen that does not contain an INSTI.”
PORTLAND—The investigational drug islatravir “could be a game changer in the field of HIV," said David H. Spach, MD, Professor of Medicine, Division of Infectious Diseases, University of Washington, Seattle, in a session called, "Antiretroviral therapy 2019 update: Mechanism of action, new medications, current guidelines, and controversies."
Dr. Spach reported that islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), is highly potent, is well tolerated, has a high genetic barrier to resistance, and has an extremely long half-life, according to the findings of preliminary studies. Its long half-life enables subdermal implantation and maintenance of therapeutic levels even after a year in place. Researchers are studying the compound in combination with other agents for treatment and independently for preexposure prophylaxis.
Another noteworthy investigational agent, according to Dr. Spach, is cabotegravir, an integrase strand transfer inhibitor (INSTI) with the potential for intramuscular administration every 4 to 8 weeks. Dr. Spach explained that researchers are studying the agent in 3 different clinical situations: oral cabotegravir in combination with rilpivirine for lead-in therapy; an extended-release injectable of cabotegravir and rilpivirine for maintenance antiretroviral (ARV) treatment every 4 weeks; and an extended-release cabotegravir injectable for preexposure prophylaxis every 8 weeks. The agent is highly potent, with a high genetic barrier to resistance.
ARV: The current state of affairs
"We are in an era now where everyone who is living with HIV ideally should be receiving fully suppressive antiretroviral therapy," remarked Dr. Spach. He explained that not only does this benefit the person living with HIV by reducing the onset and progression of chronic inflammatory disease states that occur along with HIV, such as cardiovascular disease, stroke, and cancer, but also "fully suppressive ARV therapy virtually eliminates sexual transmission of HIV to another person."
Spach explained that the most recent (2018) Health and Human Services ARV therapy guidelines have greatly simplified the choices for ARV therapy. The current recommendations for initial ARV therapy for most people are to use a 2-drug backbone regimen, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), combined with a single anchor drug, which should be an INSTI. The INSTI should have the highest potency and highest genetic barrier to resistance available, which effectively means using a regimen that contains either dolutegravir or bictegravir. The latter is available only in combination with emtricitabine and tenofovir alafenamide as a single-tablet regimen.
Spach also explained that "the guidelines have moved away from using boosted regimens for initial therapy, so elvitegravir boosted with cobicistat and protease inhibitors (PIs) boosted with ritonavir or cobicistat are no longer recommended as preferred initial therapy, although boosted PIs are still very useful as second- or third-line therapies."
Newer medications
Doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), was approved by the FDA in 2018. "It probably won't have a big impact on initial therapy, but it is likely to have a significant effect on second- and third-line therapy," Dr. Spach said. "Because of its high potency and high genetic barrier to resistance, those taking etravirine twice a day, for example, may be able to simplify to once-a-day doravirine." In addition, "Doravirine may have advantages over rilpivirine in that it has no restrictions when used with acid-suppressing agents such as H2 blockers or proton pump inhibitors."
Continue to: Ibalizumab
Ibalizumab. Another newer agent “worth mentioning,” according to Dr. Spach, is ibalizumab. This monoclonal antibody has a unique mechanism of action. It is 1 of only 2 drugs used for HIV treatment that targets human receptors (all of the others work by inhibiting either an HIV enzyme or binding to the HIV virus itself). Ibalizumab targets the D2 region of the CD4 receptor. It is an injectable (intravenous) compound administered with an initial loading dose and then every 2 weeks thereafter. Dr. Spach reported that the data surrounding ibalizumab show that it is effective as an add-on medication in more advanced resistant settings. Also, it provides an option for people who can't take oral drugs, such as those who have had major surgery or trauma.
Remaining questions
Dr. Spach explained that 1 of the questions that remains is whether to prescribe ARV therapy for patients who are viremic controllers (those who inherently control HIV through their own immunologic response to a level < 200 copies) or elite controllers (those whose own immunologic response controls the virus to a level < 50 copies, which is in the undetectable range). Both of these groups still have a higher risk for hospitalization and for HIV-related inflammatory conditions such as heart disease, according to Dr. Spach. Current thinking among most experts is to initiate and maintain therapy as long as it is tolerated well.
3 drugs to 2? Another question that remains is whether to switch patients who are doing well on 3-drug maintenance therapy to 2-drug maintenance therapy. According to Dr Spach, studies involving the combination dolutegravir/rilpivirine indicate that patients who have suppressed HIV RNA levels for at least 6 months on a 3-drug maintenance regimen do well after switching to the 2-drug combination dolutegravir/rilpivirine, as long as they do not have baseline resistance to either dolutegravir or rilpivirine. But he questioned the need for the change if the individual is tolerating a 3-drug regimen well, saying that given the safety of current regimens, the only broader motivating force may be cost savings. For now, he said, if patients are without complaints or tolerability issues on 3 drugs, leave them alone.
INSTIs and weight gain. The last issue is weight gain with the use of INSTIs. Preliminary data suggest disproportionate weight gain with these drugs (on the order of about 6 kg over a year and half, which may be 2-3 kg greater than that which occurs with PI-based and NNRTI-based regimens). At this point, experts do not recommend avoiding these agents, mainly because of the tremendous benefits that have been observed with INSTIs. Dr. Spach concluded, "Although we will continue to use INSTIs widely in clinical practice, there may be a subset of individuals taking an INSTI who have pronounced weight gain and may need to switch to another regimen that does not contain an INSTI.”
PORTLAND—The investigational drug islatravir “could be a game changer in the field of HIV," said David H. Spach, MD, Professor of Medicine, Division of Infectious Diseases, University of Washington, Seattle, in a session called, "Antiretroviral therapy 2019 update: Mechanism of action, new medications, current guidelines, and controversies."
Dr. Spach reported that islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), is highly potent, is well tolerated, has a high genetic barrier to resistance, and has an extremely long half-life, according to the findings of preliminary studies. Its long half-life enables subdermal implantation and maintenance of therapeutic levels even after a year in place. Researchers are studying the compound in combination with other agents for treatment and independently for preexposure prophylaxis.
Another noteworthy investigational agent, according to Dr. Spach, is cabotegravir, an integrase strand transfer inhibitor (INSTI) with the potential for intramuscular administration every 4 to 8 weeks. Dr. Spach explained that researchers are studying the agent in 3 different clinical situations: oral cabotegravir in combination with rilpivirine for lead-in therapy; an extended-release injectable of cabotegravir and rilpivirine for maintenance antiretroviral (ARV) treatment every 4 weeks; and an extended-release cabotegravir injectable for preexposure prophylaxis every 8 weeks. The agent is highly potent, with a high genetic barrier to resistance.
ARV: The current state of affairs
"We are in an era now where everyone who is living with HIV ideally should be receiving fully suppressive antiretroviral therapy," remarked Dr. Spach. He explained that not only does this benefit the person living with HIV by reducing the onset and progression of chronic inflammatory disease states that occur along with HIV, such as cardiovascular disease, stroke, and cancer, but also "fully suppressive ARV therapy virtually eliminates sexual transmission of HIV to another person."
Spach explained that the most recent (2018) Health and Human Services ARV therapy guidelines have greatly simplified the choices for ARV therapy. The current recommendations for initial ARV therapy for most people are to use a 2-drug backbone regimen, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs), combined with a single anchor drug, which should be an INSTI. The INSTI should have the highest potency and highest genetic barrier to resistance available, which effectively means using a regimen that contains either dolutegravir or bictegravir. The latter is available only in combination with emtricitabine and tenofovir alafenamide as a single-tablet regimen.
Spach also explained that "the guidelines have moved away from using boosted regimens for initial therapy, so elvitegravir boosted with cobicistat and protease inhibitors (PIs) boosted with ritonavir or cobicistat are no longer recommended as preferred initial therapy, although boosted PIs are still very useful as second- or third-line therapies."
Newer medications
Doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), was approved by the FDA in 2018. "It probably won't have a big impact on initial therapy, but it is likely to have a significant effect on second- and third-line therapy," Dr. Spach said. "Because of its high potency and high genetic barrier to resistance, those taking etravirine twice a day, for example, may be able to simplify to once-a-day doravirine." In addition, "Doravirine may have advantages over rilpivirine in that it has no restrictions when used with acid-suppressing agents such as H2 blockers or proton pump inhibitors."
Continue to: Ibalizumab
Ibalizumab. Another newer agent “worth mentioning,” according to Dr. Spach, is ibalizumab. This monoclonal antibody has a unique mechanism of action. It is 1 of only 2 drugs used for HIV treatment that targets human receptors (all of the others work by inhibiting either an HIV enzyme or binding to the HIV virus itself). Ibalizumab targets the D2 region of the CD4 receptor. It is an injectable (intravenous) compound administered with an initial loading dose and then every 2 weeks thereafter. Dr. Spach reported that the data surrounding ibalizumab show that it is effective as an add-on medication in more advanced resistant settings. Also, it provides an option for people who can't take oral drugs, such as those who have had major surgery or trauma.
Remaining questions
Dr. Spach explained that 1 of the questions that remains is whether to prescribe ARV therapy for patients who are viremic controllers (those who inherently control HIV through their own immunologic response to a level < 200 copies) or elite controllers (those whose own immunologic response controls the virus to a level < 50 copies, which is in the undetectable range). Both of these groups still have a higher risk for hospitalization and for HIV-related inflammatory conditions such as heart disease, according to Dr. Spach. Current thinking among most experts is to initiate and maintain therapy as long as it is tolerated well.
3 drugs to 2? Another question that remains is whether to switch patients who are doing well on 3-drug maintenance therapy to 2-drug maintenance therapy. According to Dr Spach, studies involving the combination dolutegravir/rilpivirine indicate that patients who have suppressed HIV RNA levels for at least 6 months on a 3-drug maintenance regimen do well after switching to the 2-drug combination dolutegravir/rilpivirine, as long as they do not have baseline resistance to either dolutegravir or rilpivirine. But he questioned the need for the change if the individual is tolerating a 3-drug regimen well, saying that given the safety of current regimens, the only broader motivating force may be cost savings. For now, he said, if patients are without complaints or tolerability issues on 3 drugs, leave them alone.
INSTIs and weight gain. The last issue is weight gain with the use of INSTIs. Preliminary data suggest disproportionate weight gain with these drugs (on the order of about 6 kg over a year and half, which may be 2-3 kg greater than that which occurs with PI-based and NNRTI-based regimens). At this point, experts do not recommend avoiding these agents, mainly because of the tremendous benefits that have been observed with INSTIs. Dr. Spach concluded, "Although we will continue to use INSTIs widely in clinical practice, there may be a subset of individuals taking an INSTI who have pronounced weight gain and may need to switch to another regimen that does not contain an INSTI.”
Association of Nurses in AIDS Care 2019
Reappraising standard treatment of comorbid insomnia/depression
COPENHAGEN – The traditional treatment paradigm for patients with comorbid depression and insomnia has been to focus on the depression in expectation that the sleep problems will fade away with the depressive symptoms.
Big mistake, Kerstin Blom, PhD, said during the annual congress of the European College of Neuropsychopharmacology.
That treatment strategy is insufficient, because untreated insomnia seldom improves. It hinders recovery from depression, increases the risk of new depressive episodes, and causes continued suffering because of poor sleep, asserted Dr. Blom, a clinical psychologist and researcher at the Internet Psychiatry Clinic at the Karolinska Institute in Stockholm.
She presented highlights of a series of three randomized, controlled trials for which she was first author. The take-home message: Insomnia with comorbid depression is not merely a symptom of depression; it requires specific treatment.
“Insomnia needs to be treated according to guidelines – that is, with cognitive-behavioral therapy – when it’s comorbid with depression,” she declared. “Insomnia therapy also treats comorbid depression, but it’s not so much the other way around. There are some effects on insomnia when you treat depression, but they’re not very large.”
The first study in her series included 43 adults with psychiatrist-diagnosed comorbid insomnia and major depression who were randomized to an 8-week course of psychologist-guided, Internet-delivered cognitive-behavioral therapy (ICBT) for one disorder or the other. At 6- and 12-month follow-up, patients who received ICBT for insomnia had significantly greater improvement in their insomnia as measured by the self-rated Insomnia Severity Index than did those who got ICBT for depression, while both forms of treatment were similarly effective in reducing depression severity as reflected in Montgomery-Åsberg Depression Rating Scale (MADRS) scores (Sleep. 2015 Feb 1;38[2]:267-77).
At 3-year follow-up, the beneficial impact of ICBT for insomnia remained strong, with recipients reporting less need for additional sleep treatment and less use of sleep medication than did the patients who got ICBT for depression. Both groups were left with mild depression, pointing to the need to develop a combined form of CBT that would simultaneously address both disorders in patients with comorbid depression and insomnia (Sleep. 2017 Aug 1;40[8]. doi. 10.1093/sleep/zsx108).
The Swedish investigators went on to create a 9-week course of psychologist-guided combination ICBT for both insomnia and depression. Then they randomized 126 dual-diagnosis patients to that treatment program or to therapist-guided ICBT for depression plus a placebo sleep intervention, which included education about sleep hygiene, stress management, and use of a sleep diary. At 6 months of follow-up, the dual-target ICBT group had a significantly greater reduction in Insomnia Severity Index scores than those who got ICBT for depression plus placebo. No between-group difference were found in the reduction in MADRS scores.
Dr. Blom observed. Follow-up out to 36 months is ongoing.
The third study included 148 nondepressed adults with insomnia who were randomized to the 8-week ICBT insomnia intervention or an active control treatment, which again included patient education, stress management, and a sleep diary. At 6 months, the active CBT-insomnia group had significantly lower Insomnia Severity Index scores than controls. However, at 12 and 36 months, the control group caught up, and there was no longer a between-group difference, with 74% of participants no longer meeting diagnostic criteria for insomnia at 36 months. The explanation for the catch-up? The control group used significantly more hypnotic sleep medications and more frequently sought additional insomnia treatments, including yoga and mindfulness, outside of the study setting during follow-up (Sleep. 2016 Jun 1;39[6]:1267-74).
It was Dr. Blom’s intent to also use this randomized, controlled trials to test the hypothesis that improving insomnia in nondepressed patients prevents future episodes of depression. She was thwarted in this attempt.
“After they got our low-intensity control version of a sleep intervention, they went out and got more treatment and that seems to have helped them, which is great,” Dr. Blom said. “But it sort of ruined our prediction study.”
However, in a post hoc analysis, study participants who were poor sleepers at 12 months had significantly more depressive symptoms at 36 months than did those with improved sleep at 12 months. The effect size was quite large, with a between-group 5.5-point difference in MADRS scores at 36 months in a study population that was nondepressed at baseline.
“So improved sleep may prevent depression long term,” Dr. Blom said. “The jury is still out on that one.”
She reported having no financial conflicts regarding her studies, which were supported by government research funding.
COPENHAGEN – The traditional treatment paradigm for patients with comorbid depression and insomnia has been to focus on the depression in expectation that the sleep problems will fade away with the depressive symptoms.
Big mistake, Kerstin Blom, PhD, said during the annual congress of the European College of Neuropsychopharmacology.
That treatment strategy is insufficient, because untreated insomnia seldom improves. It hinders recovery from depression, increases the risk of new depressive episodes, and causes continued suffering because of poor sleep, asserted Dr. Blom, a clinical psychologist and researcher at the Internet Psychiatry Clinic at the Karolinska Institute in Stockholm.
She presented highlights of a series of three randomized, controlled trials for which she was first author. The take-home message: Insomnia with comorbid depression is not merely a symptom of depression; it requires specific treatment.
“Insomnia needs to be treated according to guidelines – that is, with cognitive-behavioral therapy – when it’s comorbid with depression,” she declared. “Insomnia therapy also treats comorbid depression, but it’s not so much the other way around. There are some effects on insomnia when you treat depression, but they’re not very large.”
The first study in her series included 43 adults with psychiatrist-diagnosed comorbid insomnia and major depression who were randomized to an 8-week course of psychologist-guided, Internet-delivered cognitive-behavioral therapy (ICBT) for one disorder or the other. At 6- and 12-month follow-up, patients who received ICBT for insomnia had significantly greater improvement in their insomnia as measured by the self-rated Insomnia Severity Index than did those who got ICBT for depression, while both forms of treatment were similarly effective in reducing depression severity as reflected in Montgomery-Åsberg Depression Rating Scale (MADRS) scores (Sleep. 2015 Feb 1;38[2]:267-77).
At 3-year follow-up, the beneficial impact of ICBT for insomnia remained strong, with recipients reporting less need for additional sleep treatment and less use of sleep medication than did the patients who got ICBT for depression. Both groups were left with mild depression, pointing to the need to develop a combined form of CBT that would simultaneously address both disorders in patients with comorbid depression and insomnia (Sleep. 2017 Aug 1;40[8]. doi. 10.1093/sleep/zsx108).
The Swedish investigators went on to create a 9-week course of psychologist-guided combination ICBT for both insomnia and depression. Then they randomized 126 dual-diagnosis patients to that treatment program or to therapist-guided ICBT for depression plus a placebo sleep intervention, which included education about sleep hygiene, stress management, and use of a sleep diary. At 6 months of follow-up, the dual-target ICBT group had a significantly greater reduction in Insomnia Severity Index scores than those who got ICBT for depression plus placebo. No between-group difference were found in the reduction in MADRS scores.
Dr. Blom observed. Follow-up out to 36 months is ongoing.
The third study included 148 nondepressed adults with insomnia who were randomized to the 8-week ICBT insomnia intervention or an active control treatment, which again included patient education, stress management, and a sleep diary. At 6 months, the active CBT-insomnia group had significantly lower Insomnia Severity Index scores than controls. However, at 12 and 36 months, the control group caught up, and there was no longer a between-group difference, with 74% of participants no longer meeting diagnostic criteria for insomnia at 36 months. The explanation for the catch-up? The control group used significantly more hypnotic sleep medications and more frequently sought additional insomnia treatments, including yoga and mindfulness, outside of the study setting during follow-up (Sleep. 2016 Jun 1;39[6]:1267-74).
It was Dr. Blom’s intent to also use this randomized, controlled trials to test the hypothesis that improving insomnia in nondepressed patients prevents future episodes of depression. She was thwarted in this attempt.
“After they got our low-intensity control version of a sleep intervention, they went out and got more treatment and that seems to have helped them, which is great,” Dr. Blom said. “But it sort of ruined our prediction study.”
However, in a post hoc analysis, study participants who were poor sleepers at 12 months had significantly more depressive symptoms at 36 months than did those with improved sleep at 12 months. The effect size was quite large, with a between-group 5.5-point difference in MADRS scores at 36 months in a study population that was nondepressed at baseline.
“So improved sleep may prevent depression long term,” Dr. Blom said. “The jury is still out on that one.”
She reported having no financial conflicts regarding her studies, which were supported by government research funding.
COPENHAGEN – The traditional treatment paradigm for patients with comorbid depression and insomnia has been to focus on the depression in expectation that the sleep problems will fade away with the depressive symptoms.
Big mistake, Kerstin Blom, PhD, said during the annual congress of the European College of Neuropsychopharmacology.
That treatment strategy is insufficient, because untreated insomnia seldom improves. It hinders recovery from depression, increases the risk of new depressive episodes, and causes continued suffering because of poor sleep, asserted Dr. Blom, a clinical psychologist and researcher at the Internet Psychiatry Clinic at the Karolinska Institute in Stockholm.
She presented highlights of a series of three randomized, controlled trials for which she was first author. The take-home message: Insomnia with comorbid depression is not merely a symptom of depression; it requires specific treatment.
“Insomnia needs to be treated according to guidelines – that is, with cognitive-behavioral therapy – when it’s comorbid with depression,” she declared. “Insomnia therapy also treats comorbid depression, but it’s not so much the other way around. There are some effects on insomnia when you treat depression, but they’re not very large.”
The first study in her series included 43 adults with psychiatrist-diagnosed comorbid insomnia and major depression who were randomized to an 8-week course of psychologist-guided, Internet-delivered cognitive-behavioral therapy (ICBT) for one disorder or the other. At 6- and 12-month follow-up, patients who received ICBT for insomnia had significantly greater improvement in their insomnia as measured by the self-rated Insomnia Severity Index than did those who got ICBT for depression, while both forms of treatment were similarly effective in reducing depression severity as reflected in Montgomery-Åsberg Depression Rating Scale (MADRS) scores (Sleep. 2015 Feb 1;38[2]:267-77).
At 3-year follow-up, the beneficial impact of ICBT for insomnia remained strong, with recipients reporting less need for additional sleep treatment and less use of sleep medication than did the patients who got ICBT for depression. Both groups were left with mild depression, pointing to the need to develop a combined form of CBT that would simultaneously address both disorders in patients with comorbid depression and insomnia (Sleep. 2017 Aug 1;40[8]. doi. 10.1093/sleep/zsx108).
The Swedish investigators went on to create a 9-week course of psychologist-guided combination ICBT for both insomnia and depression. Then they randomized 126 dual-diagnosis patients to that treatment program or to therapist-guided ICBT for depression plus a placebo sleep intervention, which included education about sleep hygiene, stress management, and use of a sleep diary. At 6 months of follow-up, the dual-target ICBT group had a significantly greater reduction in Insomnia Severity Index scores than those who got ICBT for depression plus placebo. No between-group difference were found in the reduction in MADRS scores.
Dr. Blom observed. Follow-up out to 36 months is ongoing.
The third study included 148 nondepressed adults with insomnia who were randomized to the 8-week ICBT insomnia intervention or an active control treatment, which again included patient education, stress management, and a sleep diary. At 6 months, the active CBT-insomnia group had significantly lower Insomnia Severity Index scores than controls. However, at 12 and 36 months, the control group caught up, and there was no longer a between-group difference, with 74% of participants no longer meeting diagnostic criteria for insomnia at 36 months. The explanation for the catch-up? The control group used significantly more hypnotic sleep medications and more frequently sought additional insomnia treatments, including yoga and mindfulness, outside of the study setting during follow-up (Sleep. 2016 Jun 1;39[6]:1267-74).
It was Dr. Blom’s intent to also use this randomized, controlled trials to test the hypothesis that improving insomnia in nondepressed patients prevents future episodes of depression. She was thwarted in this attempt.
“After they got our low-intensity control version of a sleep intervention, they went out and got more treatment and that seems to have helped them, which is great,” Dr. Blom said. “But it sort of ruined our prediction study.”
However, in a post hoc analysis, study participants who were poor sleepers at 12 months had significantly more depressive symptoms at 36 months than did those with improved sleep at 12 months. The effect size was quite large, with a between-group 5.5-point difference in MADRS scores at 36 months in a study population that was nondepressed at baseline.
“So improved sleep may prevent depression long term,” Dr. Blom said. “The jury is still out on that one.”
She reported having no financial conflicts regarding her studies, which were supported by government research funding.
REPORTING FROM ECNP 2019
Pink scaly rash on torso and extremities
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
A 17-year-old male with a history of Crohn's disease, well controlled on infliximab, is seen for evaluation of a pink scaly rash on the torso, upper extremities, and lower extremities. The rash began 5 months previously and has been mostly asymptomatic. The patient denies pruritus or pain at the affected areas. There is no fever or drainage from any of the sites. The patient has not undergone any treatments. He does not have a personal or family history of chronic skin conditions.
On physical exam, he is noted to have numerous pink papules and plaques with overlying scale on his trunk, as well as the dorsal aspects of bilateral hands and the plantar surfaces of bilateral feet. His skin is otherwise unremarkable.