User login
Unilateral Papules on the Face
The Diagnosis: Mosaic Tuberous Sclerosis
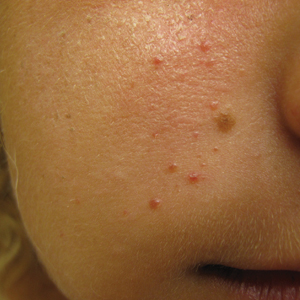
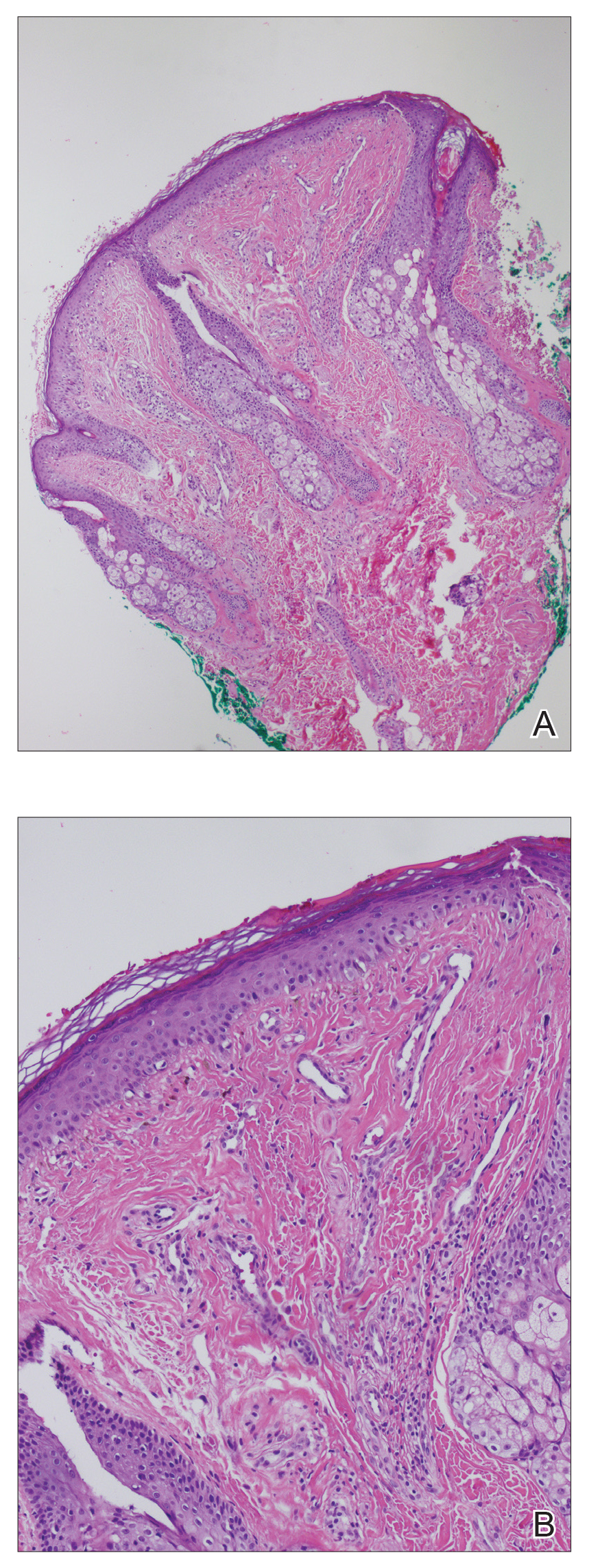
A punch biopsy of the facial lesion was stained with hematoxylin and eosin, which demonstrated spindled and stellate fibroblasts with dilated blood vessels (Figure), consistent with an angiofibroma. Given the clinical presentation and histologic findings, there was concern for a diagnosis of tuberous sclerosis (TSC). The patient was referred for genetic workup but tested negative for mutations of the TSC genes in the blood. Because the patient had only unilateral facial lesions, a possible cortical tuber, no other symptoms, and tested negative for TSC gene mutations, mosaic TSC was considered a likely diagnosis. Her facial lesions were treated with pulsed dye vascular laser therapy.
Tuberous sclerosis is an autosomal-dominant neurocutaneous disorder caused by inactivation of the genes TSC1 (encoding hamartin) and TSC2 (encoding tuberin). Mutation results in overactivation of the downstream mTOR (mammalian target of rapamycin) pathway, resulting in abnormal cellular proliferation and hamartomas. These benign tumors can be found in nearly every organ, most often in the central nervous system and skin, and they provide for a highly variable presentation of the disease.1
Tuberous sclerosis affects 1 in 6000 to 10,000 live births and has a prevalence of 1 in 20,000 individuals. Of these individuals, 75% carry sporadic mutations, and 75% to 90% eventually test positive for a TSC gene mutation.2 Genetic mosaicism has been reported in 28% of cases affected by large deletions1 and as few as 1% of cases involving small mutations.3
The dermatologic manifestation of mosaic TSC most often includes unilateral angiofibromas, whereas in nonmosaic cases, angiofibromas cover both cheeks, the forehead, and the eyelids. The other skin lesions of TSC--shagreen patches, forehead plaques, hypomelanotic macules, and ungual fibromas--are seen less frequently.4-6 Additionally, neurologic disease in mosaic patients is notably milder, with 57% of mosaic patients found to have epilepsy compared to 91% of nonmosaic patients.7 Our patient had both unilateral facial angiofibromas and a cortical lesion suspicious for a tuber, prompting a suspected diagnosis of mosaic TSC.
The methods of diagnosis outlined by the International Tuberous Sclerosis Complex Consensus Group pose a challenge in diagnosing mosaic TSC. The clinical criteria require 2 major (eg, multiple angiofibromas, angiomyolipomas, a shagreen patch) and 1 minor feature (eg, dental enamel pit, renal cyst).2 However, case reports detailing unilateral facial angiofibromas have described patients with isolated dermatologic findings.5,6 Further, it has been demonstrated that genetic studies in mosaic TSC can be unreliable depending on the tissue sampled.8 Thus, for patients who have mosaic TSC, establishing a definitive diagnosis is not always possible and may rely solely on the clinical picture.
Considering the differential diagnosis, benign cephalic histiocytosis usually would present with small red-brown macules and papules symmetrically located on the head and neck. The lesions occur at a younger age, usually in the first year or two of life. Fibrofolliculomas present as multiple whitish, slightly larger papules found on the central face. They are a marker for Birt-Hogg-Dubé syndrome, which also is associated with spontaneous pneumothorax.
Agminated means clustering or grouping of lesions. Agminated melanocytic nevi and agminated Spitz nevi are clusters of nevi. These lesions can vary in size and color. They may be elevated or flat. Melanocytic nevi usually are tan-brown or black. Spitz nevi may be pink or pigmented brown or black. To definitively differentiate between these 2 diagnoses and this patient's diagnosis of angiofibroma, a biopsy is needed.
- Curatolo P, Moavero R, Roberto D, et al. Genotype/phenotype correlations in tuberous sclerosis complex. Semin Pediatr Neurol. 2015;22:259-273.
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
- Kwiatkowski DJ, Whittemore VH, Thiele EA. Tuberous Sclerosis Complex: Genes, Clinical Features and Therapeutics. Weinham, Germany: Wiley-Blackwell; 2011.
- Alshaiji JM, Spock CR, Connelly EA, et al. Facial angiofibromas in a mosaic pattern tuberous sclerosis: a case report. Dermatol Online J. 2012;18:8.
- Gutte R, Khopkar U. Unilateral multiple facial angiofibromas: a case report with brief review of literature. Indian J Dermatol. 2013;58:159.
- Silvestre JF, Bañuls J, Ramón R, et al. Unilateral multiple facial angiofibromas: a mosaic form of TSC. J Am Acad Dermatol. 2000;43(1, pt 1):127-129.
- Kozlowski P, Roberts P, Dabora S, et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum Genet. 2007;121:389-400.
- Kwiatkowska J, Wigowska-Sowinska J, Napierala D, et al. Mosaicism in TSC as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999;340:703-707.
The Diagnosis: Mosaic Tuberous Sclerosis
A punch biopsy of the facial lesion was stained with hematoxylin and eosin, which demonstrated spindled and stellate fibroblasts with dilated blood vessels (Figure), consistent with an angiofibroma. Given the clinical presentation and histologic findings, there was concern for a diagnosis of tuberous sclerosis (TSC). The patient was referred for genetic workup but tested negative for mutations of the TSC genes in the blood. Because the patient had only unilateral facial lesions, a possible cortical tuber, no other symptoms, and tested negative for TSC gene mutations, mosaic TSC was considered a likely diagnosis. Her facial lesions were treated with pulsed dye vascular laser therapy.

Tuberous sclerosis is an autosomal-dominant neurocutaneous disorder caused by inactivation of the genes TSC1 (encoding hamartin) and TSC2 (encoding tuberin). Mutation results in overactivation of the downstream mTOR (mammalian target of rapamycin) pathway, resulting in abnormal cellular proliferation and hamartomas. These benign tumors can be found in nearly every organ, most often in the central nervous system and skin, and they provide for a highly variable presentation of the disease.1
Tuberous sclerosis affects 1 in 6000 to 10,000 live births and has a prevalence of 1 in 20,000 individuals. Of these individuals, 75% carry sporadic mutations, and 75% to 90% eventually test positive for a TSC gene mutation.2 Genetic mosaicism has been reported in 28% of cases affected by large deletions1 and as few as 1% of cases involving small mutations.3
The dermatologic manifestation of mosaic TSC most often includes unilateral angiofibromas, whereas in nonmosaic cases, angiofibromas cover both cheeks, the forehead, and the eyelids. The other skin lesions of TSC--shagreen patches, forehead plaques, hypomelanotic macules, and ungual fibromas--are seen less frequently.4-6 Additionally, neurologic disease in mosaic patients is notably milder, with 57% of mosaic patients found to have epilepsy compared to 91% of nonmosaic patients.7 Our patient had both unilateral facial angiofibromas and a cortical lesion suspicious for a tuber, prompting a suspected diagnosis of mosaic TSC.
The methods of diagnosis outlined by the International Tuberous Sclerosis Complex Consensus Group pose a challenge in diagnosing mosaic TSC. The clinical criteria require 2 major (eg, multiple angiofibromas, angiomyolipomas, a shagreen patch) and 1 minor feature (eg, dental enamel pit, renal cyst).2 However, case reports detailing unilateral facial angiofibromas have described patients with isolated dermatologic findings.5,6 Further, it has been demonstrated that genetic studies in mosaic TSC can be unreliable depending on the tissue sampled.8 Thus, for patients who have mosaic TSC, establishing a definitive diagnosis is not always possible and may rely solely on the clinical picture.
Considering the differential diagnosis, benign cephalic histiocytosis usually would present with small red-brown macules and papules symmetrically located on the head and neck. The lesions occur at a younger age, usually in the first year or two of life. Fibrofolliculomas present as multiple whitish, slightly larger papules found on the central face. They are a marker for Birt-Hogg-Dubé syndrome, which also is associated with spontaneous pneumothorax.
Agminated means clustering or grouping of lesions. Agminated melanocytic nevi and agminated Spitz nevi are clusters of nevi. These lesions can vary in size and color. They may be elevated or flat. Melanocytic nevi usually are tan-brown or black. Spitz nevi may be pink or pigmented brown or black. To definitively differentiate between these 2 diagnoses and this patient's diagnosis of angiofibroma, a biopsy is needed.
The Diagnosis: Mosaic Tuberous Sclerosis
A punch biopsy of the facial lesion was stained with hematoxylin and eosin, which demonstrated spindled and stellate fibroblasts with dilated blood vessels (Figure), consistent with an angiofibroma. Given the clinical presentation and histologic findings, there was concern for a diagnosis of tuberous sclerosis (TSC). The patient was referred for genetic workup but tested negative for mutations of the TSC genes in the blood. Because the patient had only unilateral facial lesions, a possible cortical tuber, no other symptoms, and tested negative for TSC gene mutations, mosaic TSC was considered a likely diagnosis. Her facial lesions were treated with pulsed dye vascular laser therapy.

Tuberous sclerosis is an autosomal-dominant neurocutaneous disorder caused by inactivation of the genes TSC1 (encoding hamartin) and TSC2 (encoding tuberin). Mutation results in overactivation of the downstream mTOR (mammalian target of rapamycin) pathway, resulting in abnormal cellular proliferation and hamartomas. These benign tumors can be found in nearly every organ, most often in the central nervous system and skin, and they provide for a highly variable presentation of the disease.1
Tuberous sclerosis affects 1 in 6000 to 10,000 live births and has a prevalence of 1 in 20,000 individuals. Of these individuals, 75% carry sporadic mutations, and 75% to 90% eventually test positive for a TSC gene mutation.2 Genetic mosaicism has been reported in 28% of cases affected by large deletions1 and as few as 1% of cases involving small mutations.3
The dermatologic manifestation of mosaic TSC most often includes unilateral angiofibromas, whereas in nonmosaic cases, angiofibromas cover both cheeks, the forehead, and the eyelids. The other skin lesions of TSC--shagreen patches, forehead plaques, hypomelanotic macules, and ungual fibromas--are seen less frequently.4-6 Additionally, neurologic disease in mosaic patients is notably milder, with 57% of mosaic patients found to have epilepsy compared to 91% of nonmosaic patients.7 Our patient had both unilateral facial angiofibromas and a cortical lesion suspicious for a tuber, prompting a suspected diagnosis of mosaic TSC.
The methods of diagnosis outlined by the International Tuberous Sclerosis Complex Consensus Group pose a challenge in diagnosing mosaic TSC. The clinical criteria require 2 major (eg, multiple angiofibromas, angiomyolipomas, a shagreen patch) and 1 minor feature (eg, dental enamel pit, renal cyst).2 However, case reports detailing unilateral facial angiofibromas have described patients with isolated dermatologic findings.5,6 Further, it has been demonstrated that genetic studies in mosaic TSC can be unreliable depending on the tissue sampled.8 Thus, for patients who have mosaic TSC, establishing a definitive diagnosis is not always possible and may rely solely on the clinical picture.
Considering the differential diagnosis, benign cephalic histiocytosis usually would present with small red-brown macules and papules symmetrically located on the head and neck. The lesions occur at a younger age, usually in the first year or two of life. Fibrofolliculomas present as multiple whitish, slightly larger papules found on the central face. They are a marker for Birt-Hogg-Dubé syndrome, which also is associated with spontaneous pneumothorax.
Agminated means clustering or grouping of lesions. Agminated melanocytic nevi and agminated Spitz nevi are clusters of nevi. These lesions can vary in size and color. They may be elevated or flat. Melanocytic nevi usually are tan-brown or black. Spitz nevi may be pink or pigmented brown or black. To definitively differentiate between these 2 diagnoses and this patient's diagnosis of angiofibroma, a biopsy is needed.
- Curatolo P, Moavero R, Roberto D, et al. Genotype/phenotype correlations in tuberous sclerosis complex. Semin Pediatr Neurol. 2015;22:259-273.
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
- Kwiatkowski DJ, Whittemore VH, Thiele EA. Tuberous Sclerosis Complex: Genes, Clinical Features and Therapeutics. Weinham, Germany: Wiley-Blackwell; 2011.
- Alshaiji JM, Spock CR, Connelly EA, et al. Facial angiofibromas in a mosaic pattern tuberous sclerosis: a case report. Dermatol Online J. 2012;18:8.
- Gutte R, Khopkar U. Unilateral multiple facial angiofibromas: a case report with brief review of literature. Indian J Dermatol. 2013;58:159.
- Silvestre JF, Bañuls J, Ramón R, et al. Unilateral multiple facial angiofibromas: a mosaic form of TSC. J Am Acad Dermatol. 2000;43(1, pt 1):127-129.
- Kozlowski P, Roberts P, Dabora S, et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum Genet. 2007;121:389-400.
- Kwiatkowska J, Wigowska-Sowinska J, Napierala D, et al. Mosaicism in TSC as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999;340:703-707.
- Curatolo P, Moavero R, Roberto D, et al. Genotype/phenotype correlations in tuberous sclerosis complex. Semin Pediatr Neurol. 2015;22:259-273.
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
- Kwiatkowski DJ, Whittemore VH, Thiele EA. Tuberous Sclerosis Complex: Genes, Clinical Features and Therapeutics. Weinham, Germany: Wiley-Blackwell; 2011.
- Alshaiji JM, Spock CR, Connelly EA, et al. Facial angiofibromas in a mosaic pattern tuberous sclerosis: a case report. Dermatol Online J. 2012;18:8.
- Gutte R, Khopkar U. Unilateral multiple facial angiofibromas: a case report with brief review of literature. Indian J Dermatol. 2013;58:159.
- Silvestre JF, Bañuls J, Ramón R, et al. Unilateral multiple facial angiofibromas: a mosaic form of TSC. J Am Acad Dermatol. 2000;43(1, pt 1):127-129.
- Kozlowski P, Roberts P, Dabora S, et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum Genet. 2007;121:389-400.
- Kwiatkowska J, Wigowska-Sowinska J, Napierala D, et al. Mosaicism in TSC as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999;340:703-707.
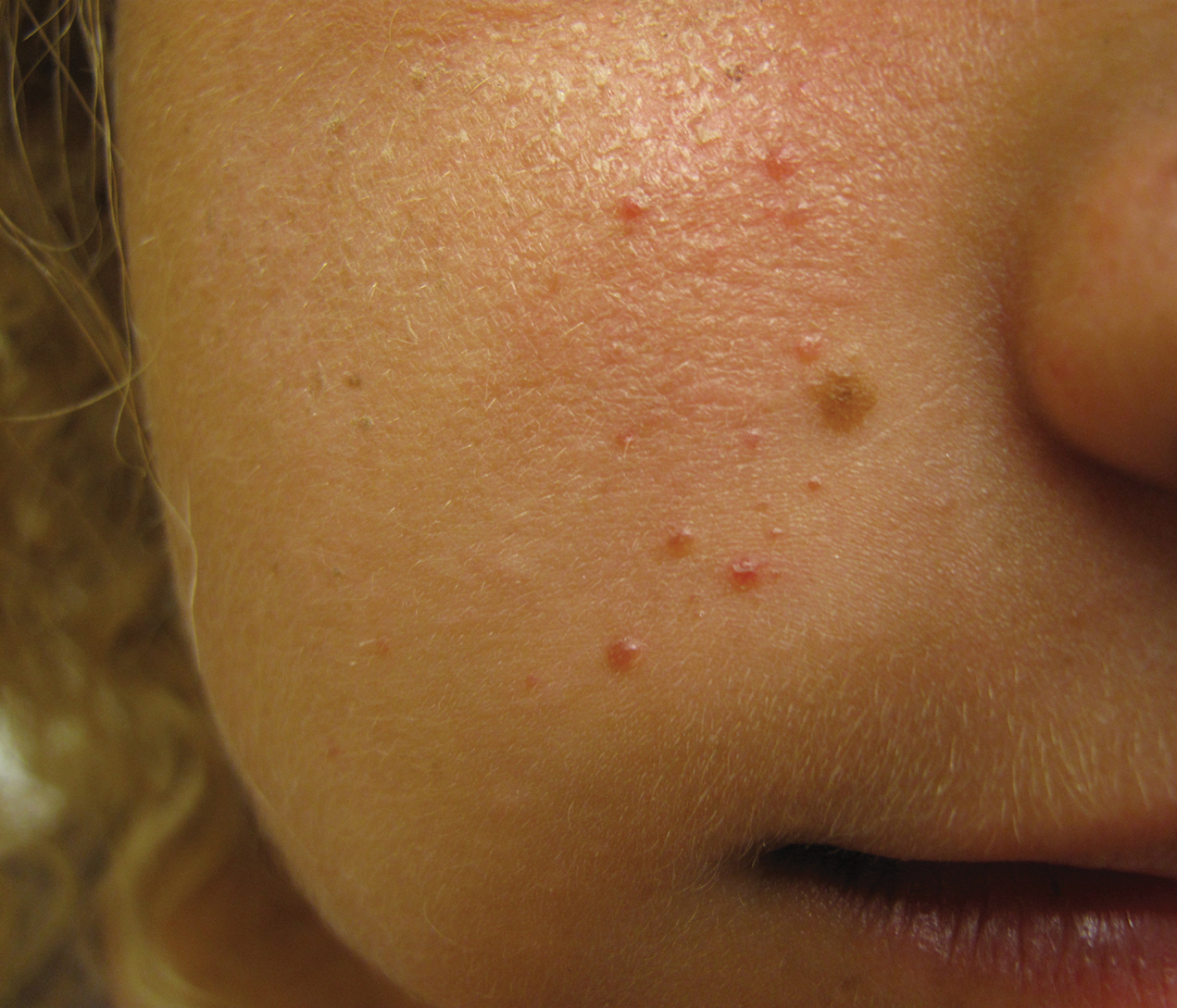
An 18-year-old woman presented with a progressive appearance of firm, red-brown, asymptomatic, 1- to 3-mm, dome-shaped papules on the right cheek that developed over the course of 2 years. She had 10 lesions that covered a 2.2 ×4-cm area on the right medial cheek. No similar-appearing lesions were detectable on a full-body skin examination, and no periungual tumors, café au lait macules, or shagreen patches were noted. A full-body skin examination using a Wood lamp revealed 1 small hypopigmented macule on the right second finger. The patient had a history of treatment-refractory migraines; magnetic resonance imaging 5 years prior to the current presentation revealed a nonspecific lesion in the left parietal gyrus. There was no personal or family history of seizures, cognitive delay, kidney disease, or ocular disease. Punch biopsy of a facial lesion was performed for histopathologic correlation.
Don’t let a foodborne illness dampen the holiday season
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.
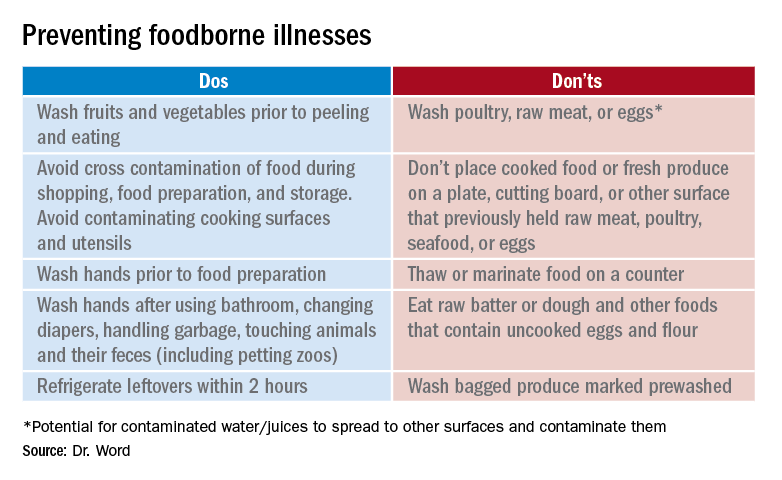
Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
According to the Centers for Disease Control and Prevention, a foodborne disease occurs in one in six persons (48 million), resulting in 128,000 hospitalizations and 3,000 deaths annually in the United States. The Foodborne Active Surveillance Network (FoodNet) of the CDC’s Emerging Infections Program monitors cases of eight laboratory diagnosed infections from 10 U.S. sites (covering 15% of the U.S. population). Monitored organisms include Campylobacter, Cyclospora, Listeria, Salmonella, Shiga toxin–producing Escherichia coli (STEC), Shigella, Vibrio, and Yersinia. In 2018, FoodNet identified 25,606 cases of infection, 5,893 hospitalizations, and 120 deaths. The incidence of infection (cases/100,000) was highest for Campylobacter (20), Salmonella (18), STEC (6), Shigella (5), Vibrio (1), Yersinia (0.9), Cyclospora (0.7), and Listeria (0.3). How might these pathogens affect your patients? First, a quick review about the four more common infections. Treatment is beyond the scope of our discussion and you are referred to the 2018-2021 Red Book for assistance. The goal of this column is to prevent your patients from becoming a statistic this holiday season.

Campylobacter
It has been the most common infection reported in FoodNet since 2013. Clinically, patients present with fever, abdominal pain, and nonbloody diarrhea. However, bloody diarrhea maybe the only symptom in neonates and young infants. Abdominal pain can mimic acute appendicitis or intussusception. Bacteremia is rare but has been reported in the elderly and in some patients with underlying conditions. During convalescence, immunoreactive complications including Guillain-Barré syndrome, reactive arthritis, and erythema nodosum may occur. In patients with diarrhea, Campylobacter jejuni and C. coli are the most frequently isolated species.
Campylobacter is present in the intestinal tract of both domestic and wild birds and animals. Transmission is via consumption of contaminated food or water. Undercooked poultry, untreated water, and unpasteurized milk are the three main vehicles of transmission. Campylobacter can be isolated in stool and blood, however isolation from stool requires special media. Rehydration is the primary therapy. Use of azithromycin or erythromycin can shorten both the duration of symptoms and bacterial shedding.
Salmonella
Nontyphoidal salmonella (NTS) are responsible for a variety of infections including asymptomatic carriage, gastroenteritis, bacteremia, and serious focal infections. Gastroenteritis is the most common illness and is manifested as diarrhea, abdominal pain, and fever. If bacteremia occurs, up to 10% of patients will develop focal infections. Invasive disease occurs most frequently in infants, persons with hemoglobinopathies, immunosuppressive disorders, and malignancies. The genus Salmonella is divided into two species, S. enterica and S. bongori with S. enterica subspecies accounting for about half of culture-confirmed Salmonella isolates reported by public health laboratories.
Although infections are more common in the summer, infections can occur year-round. In 2018, the CDC investigated at least 15 food-related NTS outbreaks and 6 have been investigated so far in 2019. In industrialized countries, acquisition usually occurs from ingestion of poultry, eggs, and milk products. Infection also has been reported after animal contact and consumption of fresh produce, meats, and contaminated water. Ground beef is the source of the November 2019 outbreak of S. dublin. Diarrhea develops within 12-72 hours. Salmonella can be isolated from stool, blood, and urine. Treatment usually is not indicated for uncomplicated gastroenteritis. While benefit has not been proven, it is recommended for those at increased risk for developing invasive disease.
Shigella
Shigella is the classic cause of colonic or dysenteric diarrhea. Humans are the primary hosts but other primates can be infected. Transmission occurs through direct person-to-person spread, from ingestion of contaminated food and water, and contact with contaminated inanimate objects. Bacteria can survive up to 6 months in food and 30 days in water. As few as 10 organisms can initiate disease. Typically mucoid or bloody diarrhea with abdominal cramps and fever occurs 1-7 days following exposure. Isolation is from stool. Bacteremia is unusual. Therapy is recommended for severe disease.
Shiga toxin–producing Escherichia coli (STEC)
STEC causes hemorrhagic colitis, which can be complicated by hemolytic uremic syndrome. While E. coli O157:H7 is the serotype most often implicated, other serotypes can cause disease. STEC is shed in feces of cattle and other animals. Infection most often is associated with ingestion of undercooked ground beef, but outbreaks also have confirmed that contaminated leafy vegetables, drinking water, peanut butter, and unpasteurized milk have been the source. Symptoms usually develop 3 to 4 days after exposure. Stools initially may be nonbloody. Abdominal pain and bloody diarrhea occur over the next 2-3 days. Fever often is absent or low grade. Stools should be sent for culture and Shiga toxin for diagnosis. Antimicrobial treatment generally is not warranted if STEC is suspected or diagnosed.

Prevention
It seems so simple. Here are the basic guidelines:
- Clean. Wash hands and surfaces frequently.
- Separate. Separate raw meats and eggs from other foods.
- Cook. Cook all meats to the right temperature.
- Chill. Refrigerate food properly.
Finally, two comments about food poisoning:
Abrupt onset of nausea, vomiting and abdominal cramping due to staphylococcal food poisoning begins 30 minutes to 6 hours after ingestion of food contaminated by enterotoxigenic strains of Staphylococcus aureus which is usually introduced by a food preparer with a purulent lesion. Food left at room temperature allows bacteria to multiply and produce a heat stable toxin. Individuals with purulent lesions of the hands, face, eyes, or nose should not be involved with food preparation.
Clostridium perfringens is the second most common bacterial cause of food poisoning. Symptoms (watery diarrhea and cramping) begin 6-24 hours after ingestion of C. perfringens spores not killed during cooking, which now have multiplied in food left at room temperature that was inadequately reheated. Illness is caused by the production of enterotoxin in the intestine. Outbreaks occur most often in November and December.
This article was updated on 11/12/19.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Information sources
1. foodsafety.gov
2. cdc.gov/foodsafety
3. The United States Department of Agriculture Meat and Poultry Hotline: 888-674-6854
4. Appendix VII: Clinical syndromes associated with foodborne diseases, Red Book online, 31st ed. (Washington DC: Red Book online, 2018, pp. 1086-92).
5. Foodkeeper App available at the App store. Provides appropriate food storage information; food recalls also are available.
Smokers with PE have higher rate of hospital readmission
NEW ORLEANS – , according to a retrospective study.
The rate of readmission was significantly higher among patients with tobacco dependence, and tobacco dependence was independently associated with an increased risk of readmission.
“This is the first study to quantify the increased rate of hospital readmission due to smoking,” said study investigator Kam Sing Ho, MD, of Mount Sinai St. Luke’s and Mount Sinai West, New York.
Dr. Ho and colleagues described this study and its results in a poster presented at the annual meeting of the American College of Chest Physicians.
The researchers analyzed data on 168,891 hospital admissions of adults with PE, 34.2% of whom had tobacco dependence. Patients with and without tobacco dependence were propensity matched for baseline characteristics (n = 24,262 in each group).
The 30-day readmission rate was significantly higher in patients with tobacco dependence than in those without it – 11.0% and 8.9%, respectively (P less than .001). The most common reason for readmission in both groups was PE.
Dr. Ho said the higher readmission rate among patients with tobacco dependence might be explained by the fact that smokers have a higher level of fibrinogen, which may affect blood viscosity and contribute to thrombus formation (Proc Am Thorac Soc. 2005;2[1]:71-7).
The investigators also found that tobacco dependence was an independent predictor of readmission (hazard ratio, 1.43; P less than .001). And the mortality rate was significantly higher after readmission than after index admission – 6.27% and 3.15%, respectively (P less than .001).
The increased risk of readmission and death among smokers highlights the importance of smoking cessation services. Dr. Ho cited previous research suggesting these services are underused in the hospital setting (BMJ Qual Improv Rep. 2014;3[1]:u204964.w2110).
“Given that smoking is a common phenomenon among patients admitted with pulmonary embolism, we suggest that more rigorous smoking cessation services are implemented prior to discharge for all active smokers,” Dr. Ho said. “[P]atients have the right to be informed on the benefits of smoking cessation and the autonomy to choose. Future research will focus on implementing inpatient smoking cessation at our hospital and its effect on local readmission rate, health resources utilization, and mortality.”
Dr. Ho has no relevant relationships to disclose.
SOURCE: Ho KS et al. CHEST 2019 October. doi: 10.1016/j.chest.2019.08.1551.
NEW ORLEANS – , according to a retrospective study.
The rate of readmission was significantly higher among patients with tobacco dependence, and tobacco dependence was independently associated with an increased risk of readmission.
“This is the first study to quantify the increased rate of hospital readmission due to smoking,” said study investigator Kam Sing Ho, MD, of Mount Sinai St. Luke’s and Mount Sinai West, New York.
Dr. Ho and colleagues described this study and its results in a poster presented at the annual meeting of the American College of Chest Physicians.
The researchers analyzed data on 168,891 hospital admissions of adults with PE, 34.2% of whom had tobacco dependence. Patients with and without tobacco dependence were propensity matched for baseline characteristics (n = 24,262 in each group).
The 30-day readmission rate was significantly higher in patients with tobacco dependence than in those without it – 11.0% and 8.9%, respectively (P less than .001). The most common reason for readmission in both groups was PE.
Dr. Ho said the higher readmission rate among patients with tobacco dependence might be explained by the fact that smokers have a higher level of fibrinogen, which may affect blood viscosity and contribute to thrombus formation (Proc Am Thorac Soc. 2005;2[1]:71-7).
The investigators also found that tobacco dependence was an independent predictor of readmission (hazard ratio, 1.43; P less than .001). And the mortality rate was significantly higher after readmission than after index admission – 6.27% and 3.15%, respectively (P less than .001).
The increased risk of readmission and death among smokers highlights the importance of smoking cessation services. Dr. Ho cited previous research suggesting these services are underused in the hospital setting (BMJ Qual Improv Rep. 2014;3[1]:u204964.w2110).
“Given that smoking is a common phenomenon among patients admitted with pulmonary embolism, we suggest that more rigorous smoking cessation services are implemented prior to discharge for all active smokers,” Dr. Ho said. “[P]atients have the right to be informed on the benefits of smoking cessation and the autonomy to choose. Future research will focus on implementing inpatient smoking cessation at our hospital and its effect on local readmission rate, health resources utilization, and mortality.”
Dr. Ho has no relevant relationships to disclose.
SOURCE: Ho KS et al. CHEST 2019 October. doi: 10.1016/j.chest.2019.08.1551.
NEW ORLEANS – , according to a retrospective study.
The rate of readmission was significantly higher among patients with tobacco dependence, and tobacco dependence was independently associated with an increased risk of readmission.
“This is the first study to quantify the increased rate of hospital readmission due to smoking,” said study investigator Kam Sing Ho, MD, of Mount Sinai St. Luke’s and Mount Sinai West, New York.
Dr. Ho and colleagues described this study and its results in a poster presented at the annual meeting of the American College of Chest Physicians.
The researchers analyzed data on 168,891 hospital admissions of adults with PE, 34.2% of whom had tobacco dependence. Patients with and without tobacco dependence were propensity matched for baseline characteristics (n = 24,262 in each group).
The 30-day readmission rate was significantly higher in patients with tobacco dependence than in those without it – 11.0% and 8.9%, respectively (P less than .001). The most common reason for readmission in both groups was PE.
Dr. Ho said the higher readmission rate among patients with tobacco dependence might be explained by the fact that smokers have a higher level of fibrinogen, which may affect blood viscosity and contribute to thrombus formation (Proc Am Thorac Soc. 2005;2[1]:71-7).
The investigators also found that tobacco dependence was an independent predictor of readmission (hazard ratio, 1.43; P less than .001). And the mortality rate was significantly higher after readmission than after index admission – 6.27% and 3.15%, respectively (P less than .001).
The increased risk of readmission and death among smokers highlights the importance of smoking cessation services. Dr. Ho cited previous research suggesting these services are underused in the hospital setting (BMJ Qual Improv Rep. 2014;3[1]:u204964.w2110).
“Given that smoking is a common phenomenon among patients admitted with pulmonary embolism, we suggest that more rigorous smoking cessation services are implemented prior to discharge for all active smokers,” Dr. Ho said. “[P]atients have the right to be informed on the benefits of smoking cessation and the autonomy to choose. Future research will focus on implementing inpatient smoking cessation at our hospital and its effect on local readmission rate, health resources utilization, and mortality.”
Dr. Ho has no relevant relationships to disclose.
SOURCE: Ho KS et al. CHEST 2019 October. doi: 10.1016/j.chest.2019.08.1551.
REPORTING FROM CHEST 2019
Does tranexamic acid reduce mortality in women with postpartum hemorrhage?
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
EVIDENCE-BASED ANSWER:
Yes. When used in conjunction with the standard of care, 1 g intravenous (IV) tranexamic acid given 1 to 3 hours after delivery is associated with a significant reduction in maternal mortality from postpartum hemorrhage (PPH) (strength of recommendation: A, randomized controlled trial [RCT] and Cochrane review).
No known significant risks are associated with the use of tranexamic acid to treat PPH.
5 Key Points on Dietary Counseling of Acne Patients



Molecule exhibits activity in heavily pretreated, HER2-positive solid tumors
NATIONAL HARBOR, MD – PRS-343, a 4-1BB/HER2 bispecific molecule, has demonstrated safety and antitumor activity in patients with heavily pretreated, HER2-positive solid tumors, an investigator reported at the annual meeting of the Society for Immunotherapy of Cancer.
In a phase 1 trial of 18 evaluable patients, PRS-343 produced partial responses in 2 patients and enabled 8 patients to maintain stable disease. PRS-343 was considered well tolerated at all doses and schedules tested.
“PRS-343 is a bispecific construct targeting HER2 as well as 4-1BB,” said Geoffrey Y. Ku, MD, of Memorial Sloan Kettering Cancer Center in New York. “The HER2 component of the molecule localizes it into the tumor microenvironment of any HER2-positive cells. If the density of the HER2 protein is high enough, that facilitates cross-linkage of 4-1BB.
“4-1BB is an immune agonist that’s present in activated T cells, and cross-linkage helps to improve T-cell exhaustion and is also critical for T-cell expansion. The idea is that, by localizing 4-1BB activation to the tumor microenvironment, we can avoid some of the systemic toxicities associated with naked 4-1BB antibodies,” Dr. Ku added.
The ongoing, phase 1 trial of PRS-343 (NCT03330561) has enrolled 53 patients with a range of HER2-positive malignancies. To be eligible, patients must have progressed on standard therapy or have a tumor for which no standard therapy is available.
The most common diagnosis among enrolled patients is gastroesophageal cancer (n = 19), followed by breast cancer (n = 14), gynecologic cancers (n = 6), colorectal cancer (n = 5), and other malignancies.
The patients’ median age at baseline was 61 years (range, 29-92 years), and a majority were female (62%). Most patients (79%) had received three or more prior lines of therapy, including anti-HER2 treatments. Breast cancer patients had received a median of four anti-HER2 treatments, and gastric cancer patients had received a median of two.
The patients have been treated with PRS-343 at 11 dose levels, ranging from 0.0005 mg/kg to 8 mg/kg, given every 3 weeks. The highest dose, 8 mg/kg, was also given every 2 weeks.
Treatment-related adverse events (TRAEs) included infusion-related reactions (9%), fatigue (9%), chills (6%), flushing (6%), nausea (6%), diarrhea (6%), vomiting (5%), and noncardiac chest pain (4%).
“This was an extremely well-tolerated drug,” Dr. Ku said. “Out of 111 TRAEs, only a tiny proportion were grade 3, and toxicities mostly clustered around infusion-related reactions, constitutional symptoms, as well as gastrointestinal symptoms.”
Grade 3 TRAEs included infusion-related reactions (2%), fatigue (1%), flushing (3%), and noncardiac chest pain (1%). There were no grade 4-5 TRAEs.
At the data cutoff (Oct. 23, 2019), 18 patients were evaluable for a response at active dose levels (2.5 mg/kg, 5 mg/kg, and 8 mg/kg).
Two patients achieved a partial response, and eight had stable disease. “This translates to a response rate of 11% and a disease control rate of 55%,” Dr. Ku noted.
Both responders received PRS-343 at 8 mg/kg every 2 weeks. One of these patients had stage 4 gastric adenocarcinoma, and one had stage 4 gynecologic carcinoma.
Of the eight patients with stable disease, three received PRS-343 at 8 mg/kg every 2 weeks, two received 8 mg/kg every 3 weeks, one received the 5 mg/kg dose, and two received the 2.5 mg/kg dose.
Dr. Ku noted that the average time on treatment significantly increased in patients who received PRS-343 at 8 mg/kg every 2 weeks. Additionally, both responders and patients with stable disease had a “clear increase” in CD8+ T cells.
“[PRS-343] has demonstrated antitumor activity in heavily pretreated patients across multiple tumor types, and the treatment history, specifically the receipt of prior anti-HER2 therapy, indicates this is a 4-1BB-driven mechanism of action,” Dr. Ku said. “Based on these results, future studies are planned for continued development in defined HER2-positive indications.”
The current study is sponsored by Pieris Pharmaceuticals. Dr. Ku disclosed relationships with Arog Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Zymeworks, and Pieris Pharmaceuticals.
SOURCE: Ku GY et al. SITC 2019, Abstract O82.
NATIONAL HARBOR, MD – PRS-343, a 4-1BB/HER2 bispecific molecule, has demonstrated safety and antitumor activity in patients with heavily pretreated, HER2-positive solid tumors, an investigator reported at the annual meeting of the Society for Immunotherapy of Cancer.
In a phase 1 trial of 18 evaluable patients, PRS-343 produced partial responses in 2 patients and enabled 8 patients to maintain stable disease. PRS-343 was considered well tolerated at all doses and schedules tested.
“PRS-343 is a bispecific construct targeting HER2 as well as 4-1BB,” said Geoffrey Y. Ku, MD, of Memorial Sloan Kettering Cancer Center in New York. “The HER2 component of the molecule localizes it into the tumor microenvironment of any HER2-positive cells. If the density of the HER2 protein is high enough, that facilitates cross-linkage of 4-1BB.
“4-1BB is an immune agonist that’s present in activated T cells, and cross-linkage helps to improve T-cell exhaustion and is also critical for T-cell expansion. The idea is that, by localizing 4-1BB activation to the tumor microenvironment, we can avoid some of the systemic toxicities associated with naked 4-1BB antibodies,” Dr. Ku added.
The ongoing, phase 1 trial of PRS-343 (NCT03330561) has enrolled 53 patients with a range of HER2-positive malignancies. To be eligible, patients must have progressed on standard therapy or have a tumor for which no standard therapy is available.
The most common diagnosis among enrolled patients is gastroesophageal cancer (n = 19), followed by breast cancer (n = 14), gynecologic cancers (n = 6), colorectal cancer (n = 5), and other malignancies.
The patients’ median age at baseline was 61 years (range, 29-92 years), and a majority were female (62%). Most patients (79%) had received three or more prior lines of therapy, including anti-HER2 treatments. Breast cancer patients had received a median of four anti-HER2 treatments, and gastric cancer patients had received a median of two.
The patients have been treated with PRS-343 at 11 dose levels, ranging from 0.0005 mg/kg to 8 mg/kg, given every 3 weeks. The highest dose, 8 mg/kg, was also given every 2 weeks.
Treatment-related adverse events (TRAEs) included infusion-related reactions (9%), fatigue (9%), chills (6%), flushing (6%), nausea (6%), diarrhea (6%), vomiting (5%), and noncardiac chest pain (4%).
“This was an extremely well-tolerated drug,” Dr. Ku said. “Out of 111 TRAEs, only a tiny proportion were grade 3, and toxicities mostly clustered around infusion-related reactions, constitutional symptoms, as well as gastrointestinal symptoms.”
Grade 3 TRAEs included infusion-related reactions (2%), fatigue (1%), flushing (3%), and noncardiac chest pain (1%). There were no grade 4-5 TRAEs.
At the data cutoff (Oct. 23, 2019), 18 patients were evaluable for a response at active dose levels (2.5 mg/kg, 5 mg/kg, and 8 mg/kg).
Two patients achieved a partial response, and eight had stable disease. “This translates to a response rate of 11% and a disease control rate of 55%,” Dr. Ku noted.
Both responders received PRS-343 at 8 mg/kg every 2 weeks. One of these patients had stage 4 gastric adenocarcinoma, and one had stage 4 gynecologic carcinoma.
Of the eight patients with stable disease, three received PRS-343 at 8 mg/kg every 2 weeks, two received 8 mg/kg every 3 weeks, one received the 5 mg/kg dose, and two received the 2.5 mg/kg dose.
Dr. Ku noted that the average time on treatment significantly increased in patients who received PRS-343 at 8 mg/kg every 2 weeks. Additionally, both responders and patients with stable disease had a “clear increase” in CD8+ T cells.
“[PRS-343] has demonstrated antitumor activity in heavily pretreated patients across multiple tumor types, and the treatment history, specifically the receipt of prior anti-HER2 therapy, indicates this is a 4-1BB-driven mechanism of action,” Dr. Ku said. “Based on these results, future studies are planned for continued development in defined HER2-positive indications.”
The current study is sponsored by Pieris Pharmaceuticals. Dr. Ku disclosed relationships with Arog Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Zymeworks, and Pieris Pharmaceuticals.
SOURCE: Ku GY et al. SITC 2019, Abstract O82.
NATIONAL HARBOR, MD – PRS-343, a 4-1BB/HER2 bispecific molecule, has demonstrated safety and antitumor activity in patients with heavily pretreated, HER2-positive solid tumors, an investigator reported at the annual meeting of the Society for Immunotherapy of Cancer.
In a phase 1 trial of 18 evaluable patients, PRS-343 produced partial responses in 2 patients and enabled 8 patients to maintain stable disease. PRS-343 was considered well tolerated at all doses and schedules tested.
“PRS-343 is a bispecific construct targeting HER2 as well as 4-1BB,” said Geoffrey Y. Ku, MD, of Memorial Sloan Kettering Cancer Center in New York. “The HER2 component of the molecule localizes it into the tumor microenvironment of any HER2-positive cells. If the density of the HER2 protein is high enough, that facilitates cross-linkage of 4-1BB.
“4-1BB is an immune agonist that’s present in activated T cells, and cross-linkage helps to improve T-cell exhaustion and is also critical for T-cell expansion. The idea is that, by localizing 4-1BB activation to the tumor microenvironment, we can avoid some of the systemic toxicities associated with naked 4-1BB antibodies,” Dr. Ku added.
The ongoing, phase 1 trial of PRS-343 (NCT03330561) has enrolled 53 patients with a range of HER2-positive malignancies. To be eligible, patients must have progressed on standard therapy or have a tumor for which no standard therapy is available.
The most common diagnosis among enrolled patients is gastroesophageal cancer (n = 19), followed by breast cancer (n = 14), gynecologic cancers (n = 6), colorectal cancer (n = 5), and other malignancies.
The patients’ median age at baseline was 61 years (range, 29-92 years), and a majority were female (62%). Most patients (79%) had received three or more prior lines of therapy, including anti-HER2 treatments. Breast cancer patients had received a median of four anti-HER2 treatments, and gastric cancer patients had received a median of two.
The patients have been treated with PRS-343 at 11 dose levels, ranging from 0.0005 mg/kg to 8 mg/kg, given every 3 weeks. The highest dose, 8 mg/kg, was also given every 2 weeks.
Treatment-related adverse events (TRAEs) included infusion-related reactions (9%), fatigue (9%), chills (6%), flushing (6%), nausea (6%), diarrhea (6%), vomiting (5%), and noncardiac chest pain (4%).
“This was an extremely well-tolerated drug,” Dr. Ku said. “Out of 111 TRAEs, only a tiny proportion were grade 3, and toxicities mostly clustered around infusion-related reactions, constitutional symptoms, as well as gastrointestinal symptoms.”
Grade 3 TRAEs included infusion-related reactions (2%), fatigue (1%), flushing (3%), and noncardiac chest pain (1%). There were no grade 4-5 TRAEs.
At the data cutoff (Oct. 23, 2019), 18 patients were evaluable for a response at active dose levels (2.5 mg/kg, 5 mg/kg, and 8 mg/kg).
Two patients achieved a partial response, and eight had stable disease. “This translates to a response rate of 11% and a disease control rate of 55%,” Dr. Ku noted.
Both responders received PRS-343 at 8 mg/kg every 2 weeks. One of these patients had stage 4 gastric adenocarcinoma, and one had stage 4 gynecologic carcinoma.
Of the eight patients with stable disease, three received PRS-343 at 8 mg/kg every 2 weeks, two received 8 mg/kg every 3 weeks, one received the 5 mg/kg dose, and two received the 2.5 mg/kg dose.
Dr. Ku noted that the average time on treatment significantly increased in patients who received PRS-343 at 8 mg/kg every 2 weeks. Additionally, both responders and patients with stable disease had a “clear increase” in CD8+ T cells.
“[PRS-343] has demonstrated antitumor activity in heavily pretreated patients across multiple tumor types, and the treatment history, specifically the receipt of prior anti-HER2 therapy, indicates this is a 4-1BB-driven mechanism of action,” Dr. Ku said. “Based on these results, future studies are planned for continued development in defined HER2-positive indications.”
The current study is sponsored by Pieris Pharmaceuticals. Dr. Ku disclosed relationships with Arog Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Zymeworks, and Pieris Pharmaceuticals.
SOURCE: Ku GY et al. SITC 2019, Abstract O82.
REPORTING FROM SITC 2019
Statins cut long-term CVD risk in kids with familial hypercholesterolemia
Statins started in childhood for patients with familial hypercholesterolemia reduced progression of carotid thickening and cut the risk of cardiovascular disease 20 years later, according to authors of a long-term follow-up study.
There were no deaths and one cardiovascular event by the age of 39 years for patients in the observational study who had, as children, participated in a placebo-controlled, 2-year safety and efficacy study of pravastatin.
These positive effects on disease and a disease marker (carotid intima-media thickness) were observed even though only 20% of patients met LDL cholesterol goals, according to study senior authors John J.P. Kastelein, MD, PhD, and Barbara A. Hutten, PhD, of Amsterdam University Medical Centers in the Netherlands, and their colleagues.
“If corroborated, such findings would underscore the current pediatric guidelines, which recommend starting treatment from the age of 8 years or 10 years, with less-stringent targets than those for adults,” the investigators wrote in the report, which was published in the New England Journal of Medicine.
The study was based in part on follow-up visits with 184 of the original cohort of 214 patients with familial hypercholesterolemia in the 2-year pravastatin study, along with 77 of 95 unaffected siblings who had served as a control group in that study.
At the time of the 20-year follow-up, 79% of the familial hypercholesterolemia patients said they were using lipid-lowering medication, the investigators wrote.
The mean LDL cholesterol level at follow-up was 160.7 mg/dL for those with familial hypercholesterolemia, a drop of 32% when compared with the mean LDL cholesterol level at baseline in the original study, according to the investigators. By contrast, LDL cholesterol in the unaffected siblings was 121.9 mg/dL, up 24% from baseline.
Just 37 patients with familial hypercholesterolemia, or about 20%, reached the LDL cholesterol treatment target of less than 100 mg/dL, the investigators wrote.
At the start of the original trial, carotid intima-media thickness was greater in patients with familial hypercholesterolemia, compared with their unaffected siblings, with a mean difference of 0.012 mm (95% confidence interval, 0.002-0.021) after adjustment for age and sex.
Some 20 years later, the mean difference in thickness for patients with familial hypercholesterolemia and unaffected siblings, was 0.555 mm and 0.551 mm, respectively, for a mean difference of just 0.008 mm (95% CI, –0.009 to 0.026) after adjustments.
Data on cardiovascular events and deaths for affected parents was collected, as each child in the study had a parent with confirmed familial hypercholesterolemia, the investigators wrote.
A total of 7% of affected parents had died of MI before the age of 40 years, whereas there were no deaths from cardiovascular causes in all 214 patients with familial hypercholesterolemia from the original study.
Similarly, about a quarter of affected parents had a cardiovascular event before age 40, whereas there was only one event recorded in the patients in the study. That event, angina pectoris resulting in percutaneous coronary intervention, occurred in a patient who stopped taking the drug at the end of the trial, the investigators wrote.
The study was supported by a grant from the AMC Foundation. The study authors reported disclosures related to numerous pharmaceutical companies and government, nonprofit, or academic entities.
SOURCE: Kastelein JJP et al. N Engl J Med. 2019;381:1547-56.
Statins started in childhood for patients with familial hypercholesterolemia reduced progression of carotid thickening and cut the risk of cardiovascular disease 20 years later, according to authors of a long-term follow-up study.
There were no deaths and one cardiovascular event by the age of 39 years for patients in the observational study who had, as children, participated in a placebo-controlled, 2-year safety and efficacy study of pravastatin.
These positive effects on disease and a disease marker (carotid intima-media thickness) were observed even though only 20% of patients met LDL cholesterol goals, according to study senior authors John J.P. Kastelein, MD, PhD, and Barbara A. Hutten, PhD, of Amsterdam University Medical Centers in the Netherlands, and their colleagues.
“If corroborated, such findings would underscore the current pediatric guidelines, which recommend starting treatment from the age of 8 years or 10 years, with less-stringent targets than those for adults,” the investigators wrote in the report, which was published in the New England Journal of Medicine.
The study was based in part on follow-up visits with 184 of the original cohort of 214 patients with familial hypercholesterolemia in the 2-year pravastatin study, along with 77 of 95 unaffected siblings who had served as a control group in that study.
At the time of the 20-year follow-up, 79% of the familial hypercholesterolemia patients said they were using lipid-lowering medication, the investigators wrote.
The mean LDL cholesterol level at follow-up was 160.7 mg/dL for those with familial hypercholesterolemia, a drop of 32% when compared with the mean LDL cholesterol level at baseline in the original study, according to the investigators. By contrast, LDL cholesterol in the unaffected siblings was 121.9 mg/dL, up 24% from baseline.
Just 37 patients with familial hypercholesterolemia, or about 20%, reached the LDL cholesterol treatment target of less than 100 mg/dL, the investigators wrote.
At the start of the original trial, carotid intima-media thickness was greater in patients with familial hypercholesterolemia, compared with their unaffected siblings, with a mean difference of 0.012 mm (95% confidence interval, 0.002-0.021) after adjustment for age and sex.
Some 20 years later, the mean difference in thickness for patients with familial hypercholesterolemia and unaffected siblings, was 0.555 mm and 0.551 mm, respectively, for a mean difference of just 0.008 mm (95% CI, –0.009 to 0.026) after adjustments.
Data on cardiovascular events and deaths for affected parents was collected, as each child in the study had a parent with confirmed familial hypercholesterolemia, the investigators wrote.
A total of 7% of affected parents had died of MI before the age of 40 years, whereas there were no deaths from cardiovascular causes in all 214 patients with familial hypercholesterolemia from the original study.
Similarly, about a quarter of affected parents had a cardiovascular event before age 40, whereas there was only one event recorded in the patients in the study. That event, angina pectoris resulting in percutaneous coronary intervention, occurred in a patient who stopped taking the drug at the end of the trial, the investigators wrote.
The study was supported by a grant from the AMC Foundation. The study authors reported disclosures related to numerous pharmaceutical companies and government, nonprofit, or academic entities.
SOURCE: Kastelein JJP et al. N Engl J Med. 2019;381:1547-56.
Statins started in childhood for patients with familial hypercholesterolemia reduced progression of carotid thickening and cut the risk of cardiovascular disease 20 years later, according to authors of a long-term follow-up study.
There were no deaths and one cardiovascular event by the age of 39 years for patients in the observational study who had, as children, participated in a placebo-controlled, 2-year safety and efficacy study of pravastatin.
These positive effects on disease and a disease marker (carotid intima-media thickness) were observed even though only 20% of patients met LDL cholesterol goals, according to study senior authors John J.P. Kastelein, MD, PhD, and Barbara A. Hutten, PhD, of Amsterdam University Medical Centers in the Netherlands, and their colleagues.
“If corroborated, such findings would underscore the current pediatric guidelines, which recommend starting treatment from the age of 8 years or 10 years, with less-stringent targets than those for adults,” the investigators wrote in the report, which was published in the New England Journal of Medicine.
The study was based in part on follow-up visits with 184 of the original cohort of 214 patients with familial hypercholesterolemia in the 2-year pravastatin study, along with 77 of 95 unaffected siblings who had served as a control group in that study.
At the time of the 20-year follow-up, 79% of the familial hypercholesterolemia patients said they were using lipid-lowering medication, the investigators wrote.
The mean LDL cholesterol level at follow-up was 160.7 mg/dL for those with familial hypercholesterolemia, a drop of 32% when compared with the mean LDL cholesterol level at baseline in the original study, according to the investigators. By contrast, LDL cholesterol in the unaffected siblings was 121.9 mg/dL, up 24% from baseline.
Just 37 patients with familial hypercholesterolemia, or about 20%, reached the LDL cholesterol treatment target of less than 100 mg/dL, the investigators wrote.
At the start of the original trial, carotid intima-media thickness was greater in patients with familial hypercholesterolemia, compared with their unaffected siblings, with a mean difference of 0.012 mm (95% confidence interval, 0.002-0.021) after adjustment for age and sex.
Some 20 years later, the mean difference in thickness for patients with familial hypercholesterolemia and unaffected siblings, was 0.555 mm and 0.551 mm, respectively, for a mean difference of just 0.008 mm (95% CI, –0.009 to 0.026) after adjustments.
Data on cardiovascular events and deaths for affected parents was collected, as each child in the study had a parent with confirmed familial hypercholesterolemia, the investigators wrote.
A total of 7% of affected parents had died of MI before the age of 40 years, whereas there were no deaths from cardiovascular causes in all 214 patients with familial hypercholesterolemia from the original study.
Similarly, about a quarter of affected parents had a cardiovascular event before age 40, whereas there was only one event recorded in the patients in the study. That event, angina pectoris resulting in percutaneous coronary intervention, occurred in a patient who stopped taking the drug at the end of the trial, the investigators wrote.
The study was supported by a grant from the AMC Foundation. The study authors reported disclosures related to numerous pharmaceutical companies and government, nonprofit, or academic entities.
SOURCE: Kastelein JJP et al. N Engl J Med. 2019;381:1547-56.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Chondrodermatitis Nodularis Helicis in an Adolescent Boy: Not Just for Old Men
Chondrodermatitis nodularis helicis (CNH) is a chronic painful or crusted, 4- to 6-mm, solitary nodule, primarily on the upper part of the ear (most commonly on the right side). The presence of pain, which increases the likelihood that a person will seek treatment, clinically distinguishes CNH from other cutaneous tumors in the differential diagnosis that produce painless ulceration.
It is roughly 5 times more prevalent in males (72.9%),1 with an average age of onset of 65 years.2 However, CNH has been reported in females3 and rarely in individuals younger than 20 years. According to a PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms chrondodermatitis nodularis helices child, only 6 cases of CNH have been reported in the pediatric population.4-8 The youngest reported case was a 9-month-old infant.8 Including the present case, males and females in the pediatric population are equally affected; 4 patients had an underlying dermatomyositis,7 rheumatoid nodule,8 or systemic disease, including systemic lupus erythematosus and Beckwith-Wiedemann syndrome.5,9 Chronic intermittent pressure from headwear was the etiologic agent in the remaining cases.4 Recognizing that CNH can occur in young patients and can be associated with underlying autoimmune disease helps direct management and avoid overly invasive treatment.
Case Report
A 17-year-old adolescent boy presented with a painful ulcerated papule on the right upper helix of 3 months’ duration (Figure 1). The patient habitually slept on the right side, pressed a cell phone to that ear, and wore a tight-fitting visor while lifeguarding, which, along with solar damage, all may have contributed to the disease process. He was otherwise in good health, without a history of underlying systemic disease. Given the patient’s extensive occupational sun exposure, biopsy of the lesion was taken under the impression of CNH vs squamous cell carcinoma or basal cell carcinoma.

Histopathologic analysis revealed a central area of ulceration with edematous degenerated dermal collagen and overlying inflammatory crust, characteristic of CNH (Figure 2A). Biopsy in this patient demonstrated classic histopathologic findings of CNH, including a central area of epidermal ulceration capped by an inflammatory crust and an underlying edematous degenerated dermal collagen (Figure 2B).
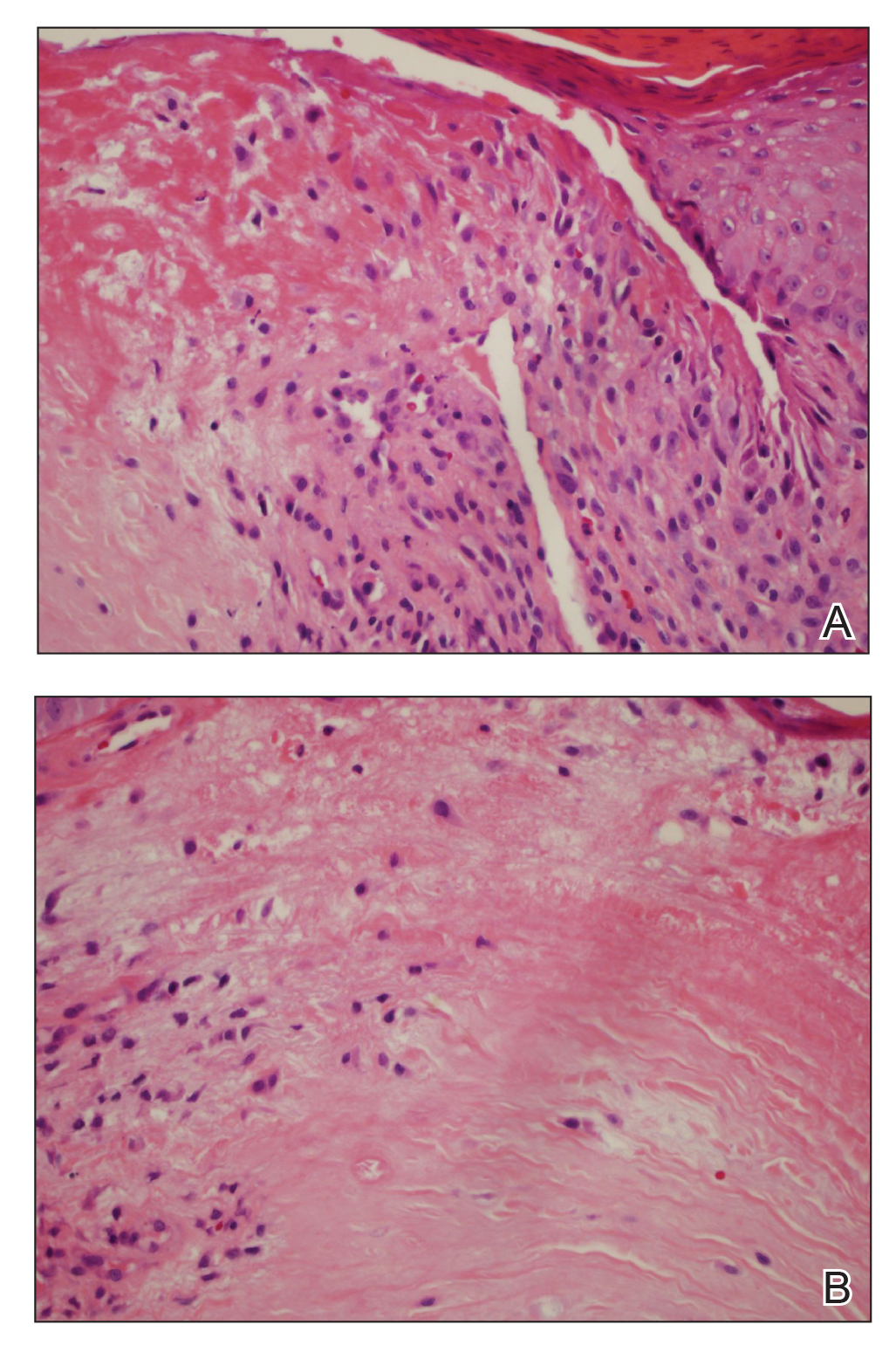
Following biopsy, the patient was advised of this diagnosis and recommended to avoid applying pressure to the area with cell phones or hats or when sleeping to prevent recurrence. At 3-month follow-up, no residual lesion remained.
Comment
Pathogenesis
The exact cause of CNH is unknown but is probably the result of prolonged and excessive pressure on the ear that leads to ischemic injury to cartilage and skin. The external location of CNH, lack of bony support, and exquisitely thin padding or insulation in the form of subcutaneous tissue make the small dermal blood vessels supplying the outer ear vulnerable to compression. Dermal inflammation; edema; and necrosis from trauma, cold, or actinic damage also can help initiate CNH. This disruption of blood perfusion to the external ear also inhibits the ear’s ability to heal. A cycle of pressure from objects such as a pillow or cell phone, followed by inadequate healing, leads to secondary perichondritis and remodeling of perichondrial arterioles, which is demonstrated histologically by the presence of perichondrial fibrous thickening, mild chronic inflammation, collagen degeneration, hyalinization, and rarely necrosis or calcification. Healed lesions often show dermal fibrosis overlying perichondrium.
Repeated pressure can lead to vascular changes, but underlying vascular disease also can predispose a person to CNH at a younger age. A striking case of bilateral CNH was reported in an 8-year-old girl with a known history of dermatomyositis.7 Furthermore, in 24 patients with CNH (mean age, 43 years), Magro et al9 observed an association between CNH and collagen vascular disease, scleroderma, hypertension, thyroid disease, and heart disease, with a higher incidence of any of these medical problems in younger patients. Therefore, screening all patients presenting with CNH, particularly those younger than their fourth decade, for underlying vasculopathy and an autoimmune connective tissue disorder is advised.9
Other findings of CNH reported in the literature include loss of elastic fibers in the central area of degenerated dermal collagen and nerve hyperplasia, which might account for pain.6 Many of the biopsies in cases of CNH reported in the literature also demonstrate perichondrial fibrous thickening, mild chronic inflammation, and degenerative changes in collagen, including hyalinization and rarely necrosis and calcification. Skin at the periphery of the lesion usually contains granulation tissue, with a mild to moderate inflammatory infiltrate and dilated vessels extending beyond the lesion.2
Genetics might play a role in the disorder, which is suggested by the occurrence of CNH in monozygotic twins10 and in an otherwise healthy 16-year-old adolescent girl with CNH of the right ear who screened negative for underlying connective tissue disease—serologic tests included antinuclear antibody, anti-Sm, anti-SCL-70, anti-Ro, anti-La, and rheumatoid factor—but who had a family history of a maternal grandmother with CNH, also on the right side.6
In the present case, there was no family history or signs and symptoms of underlying systemic disease at the time of diagnosis. The social history revealed excessive occupational sun exposure, habitually wearing a tight visor, and frequent cell phone use, all of which might have contributed to CNH.
Management
Medical management is geared toward relieving pressure at the site of the lesion, which was accomplished by use of an off-loading, ring-shaped, foam pillow at night in a 9-month-old girl with CNH, in which the smaller of her 2 left-sided lesions completely resolved by 6-month follow-up.8 However, it often is difficult to achieve adequate relief of pressure because of the patient’s preference for holding a cell phone to a particular ear or unconscious sleeping habits that perpetuate lesions. There are many creative physical interventions to offload aggravating pressure from the area during sleep. A prosthesis can be fashioned by cutting a hole from the center of a bath sponge and securing it with a headband,11 or a crescentic or rectangular piece of self-adhering foam sponge can be applied to the non–hair-bearing postauricular scalp during sleep.12 Topical antibiotics might relieve pain caused by secondary infection.
Surgical intervention, with or without placement of a full-thickness skin graft, is the mainstay of therapy. Excision was performed in 3 previously reported pediatric cases, with no recurrence reported at 6- to 24-month follow-up. Other treatments employed to varying effect include topical and intralesional steroids, collagen injection, cryotherapy, nitroglycerin paste 2% twice daily,13 and electrodesiccation and curettage.14 In adults, if full resolution is desired, multiple surgeries might be required to remove underlying protuberant cartilage; however, this strategy is not without risk of complication, including formation of adjacent cartilaginous nodules that can become site(s) of CNH recurrence due to a change in pressure points.
Conclusion
Although uncommon, CNH can present on the ears of young patients. A causal link between underlying vasculopathy and CNH has yet to be determined, but the association discovered by Magro et al9 merits obtaining a more detailed rheumatologic history and examination, followed by serologic testing (if indicated). Once the diagnosis of CNH is determined, patient education is paramount to prevent recurrence. Increased awareness of habits that inflict persistent repetitive trauma or pressure to the site—from sleeping patterns to cell phone use—will help to extinguish the behavior and therefore the lesion.
- Rex J, Rivera M, Bielsa I, et al. Narrow elliptical skin excision and cartilage shaving for treatment of chondrodermatitis nodularis. Dermatol Surg. 2006;32:400-404.
- Wettlé C, Keller F, Will F, et al. Chondrodermatitis nodularis chronical helicis: a descriptive study of 99 patients [in French]. Ann Dermatol Venereol. 2013;140:687-692.
- Oelzner S, Elsner P. Bilateral chondrodermatitis nodularis chronica helicis on the free border of the helix in a woman. J Am Acad Dermatol. 2003;49:720-722.
- Grigoryants V, Qureshi H, Patterson J, et al. Pediatric chondrodermatitis nodularis helicis. J Craniofac Surg. 2007;18:228-231.
- Fix WC, Cornejo C, Duffy KA, et al. Pediatric chondrodermatitis nodularis helicis (CNH) in a child with Beckwith-Wiedemann syndrome (BWS). Pediatr Dermatol. 2019;36:388-390.
- Rogers NE, Farris PK, Wang AR. Juvenile chondrodermatitis nodularis helicis: case report and literature review. Pediatr Dermatol. 2003;20:488-490.
- Sasaki T, Nishizawa H, Sugita Y. Chondrodermatitis nodularis helicis in childhood dermatomyositis. Br J Dermatol. 1999;141:363-365.
- Tsai TH, Lin YC, Chen HC. Infantile chondrodermatitis nodularis. Pediatr Dermatol. 2007;24:337-339.
- Magro CM, Frambach GE, Crowson AN. Chondrodermatitis nodularis helicis as a marker of internal disease associated with microvascular injury. J Cutan Pathol. 2005;32:329-333.
- Chan HP, Neuhaus IM, Maibach HI. Chondrodermatitis nodularis chronica helicis in monozygotic twins. Clin Exp Dermatol. 2009;34:358-359.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894.
- Travelute CR. Self-adhering foam: a simple method for pressure relief during sleep in patients with chondrodermatitis nodularis helicis. Dermatol Surg. 2013;39:317-319.
- Flynn V, Chisholm C, Grimwood R. Topical nitroglycerin: a promising treatment option for chondrodermatitis nodularis helicis. J Am Acad Dermatol. 2011;65:531-536.
- Kromann N, Høyer H, Reymann F. Chondrodermatitis nodularis chronica helicis treated with curettage and electrocauterization: follow-up of a 15-year material. Acta Derm Venereol. 1983;63:85-87.
Chondrodermatitis nodularis helicis (CNH) is a chronic painful or crusted, 4- to 6-mm, solitary nodule, primarily on the upper part of the ear (most commonly on the right side). The presence of pain, which increases the likelihood that a person will seek treatment, clinically distinguishes CNH from other cutaneous tumors in the differential diagnosis that produce painless ulceration.
It is roughly 5 times more prevalent in males (72.9%),1 with an average age of onset of 65 years.2 However, CNH has been reported in females3 and rarely in individuals younger than 20 years. According to a PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms chrondodermatitis nodularis helices child, only 6 cases of CNH have been reported in the pediatric population.4-8 The youngest reported case was a 9-month-old infant.8 Including the present case, males and females in the pediatric population are equally affected; 4 patients had an underlying dermatomyositis,7 rheumatoid nodule,8 or systemic disease, including systemic lupus erythematosus and Beckwith-Wiedemann syndrome.5,9 Chronic intermittent pressure from headwear was the etiologic agent in the remaining cases.4 Recognizing that CNH can occur in young patients and can be associated with underlying autoimmune disease helps direct management and avoid overly invasive treatment.
Case Report
A 17-year-old adolescent boy presented with a painful ulcerated papule on the right upper helix of 3 months’ duration (Figure 1). The patient habitually slept on the right side, pressed a cell phone to that ear, and wore a tight-fitting visor while lifeguarding, which, along with solar damage, all may have contributed to the disease process. He was otherwise in good health, without a history of underlying systemic disease. Given the patient’s extensive occupational sun exposure, biopsy of the lesion was taken under the impression of CNH vs squamous cell carcinoma or basal cell carcinoma.

Histopathologic analysis revealed a central area of ulceration with edematous degenerated dermal collagen and overlying inflammatory crust, characteristic of CNH (Figure 2A). Biopsy in this patient demonstrated classic histopathologic findings of CNH, including a central area of epidermal ulceration capped by an inflammatory crust and an underlying edematous degenerated dermal collagen (Figure 2B).

Following biopsy, the patient was advised of this diagnosis and recommended to avoid applying pressure to the area with cell phones or hats or when sleeping to prevent recurrence. At 3-month follow-up, no residual lesion remained.
Comment
Pathogenesis
The exact cause of CNH is unknown but is probably the result of prolonged and excessive pressure on the ear that leads to ischemic injury to cartilage and skin. The external location of CNH, lack of bony support, and exquisitely thin padding or insulation in the form of subcutaneous tissue make the small dermal blood vessels supplying the outer ear vulnerable to compression. Dermal inflammation; edema; and necrosis from trauma, cold, or actinic damage also can help initiate CNH. This disruption of blood perfusion to the external ear also inhibits the ear’s ability to heal. A cycle of pressure from objects such as a pillow or cell phone, followed by inadequate healing, leads to secondary perichondritis and remodeling of perichondrial arterioles, which is demonstrated histologically by the presence of perichondrial fibrous thickening, mild chronic inflammation, collagen degeneration, hyalinization, and rarely necrosis or calcification. Healed lesions often show dermal fibrosis overlying perichondrium.
Repeated pressure can lead to vascular changes, but underlying vascular disease also can predispose a person to CNH at a younger age. A striking case of bilateral CNH was reported in an 8-year-old girl with a known history of dermatomyositis.7 Furthermore, in 24 patients with CNH (mean age, 43 years), Magro et al9 observed an association between CNH and collagen vascular disease, scleroderma, hypertension, thyroid disease, and heart disease, with a higher incidence of any of these medical problems in younger patients. Therefore, screening all patients presenting with CNH, particularly those younger than their fourth decade, for underlying vasculopathy and an autoimmune connective tissue disorder is advised.9
Other findings of CNH reported in the literature include loss of elastic fibers in the central area of degenerated dermal collagen and nerve hyperplasia, which might account for pain.6 Many of the biopsies in cases of CNH reported in the literature also demonstrate perichondrial fibrous thickening, mild chronic inflammation, and degenerative changes in collagen, including hyalinization and rarely necrosis and calcification. Skin at the periphery of the lesion usually contains granulation tissue, with a mild to moderate inflammatory infiltrate and dilated vessels extending beyond the lesion.2
Genetics might play a role in the disorder, which is suggested by the occurrence of CNH in monozygotic twins10 and in an otherwise healthy 16-year-old adolescent girl with CNH of the right ear who screened negative for underlying connective tissue disease—serologic tests included antinuclear antibody, anti-Sm, anti-SCL-70, anti-Ro, anti-La, and rheumatoid factor—but who had a family history of a maternal grandmother with CNH, also on the right side.6
In the present case, there was no family history or signs and symptoms of underlying systemic disease at the time of diagnosis. The social history revealed excessive occupational sun exposure, habitually wearing a tight visor, and frequent cell phone use, all of which might have contributed to CNH.
Management
Medical management is geared toward relieving pressure at the site of the lesion, which was accomplished by use of an off-loading, ring-shaped, foam pillow at night in a 9-month-old girl with CNH, in which the smaller of her 2 left-sided lesions completely resolved by 6-month follow-up.8 However, it often is difficult to achieve adequate relief of pressure because of the patient’s preference for holding a cell phone to a particular ear or unconscious sleeping habits that perpetuate lesions. There are many creative physical interventions to offload aggravating pressure from the area during sleep. A prosthesis can be fashioned by cutting a hole from the center of a bath sponge and securing it with a headband,11 or a crescentic or rectangular piece of self-adhering foam sponge can be applied to the non–hair-bearing postauricular scalp during sleep.12 Topical antibiotics might relieve pain caused by secondary infection.
Surgical intervention, with or without placement of a full-thickness skin graft, is the mainstay of therapy. Excision was performed in 3 previously reported pediatric cases, with no recurrence reported at 6- to 24-month follow-up. Other treatments employed to varying effect include topical and intralesional steroids, collagen injection, cryotherapy, nitroglycerin paste 2% twice daily,13 and electrodesiccation and curettage.14 In adults, if full resolution is desired, multiple surgeries might be required to remove underlying protuberant cartilage; however, this strategy is not without risk of complication, including formation of adjacent cartilaginous nodules that can become site(s) of CNH recurrence due to a change in pressure points.
Conclusion
Although uncommon, CNH can present on the ears of young patients. A causal link between underlying vasculopathy and CNH has yet to be determined, but the association discovered by Magro et al9 merits obtaining a more detailed rheumatologic history and examination, followed by serologic testing (if indicated). Once the diagnosis of CNH is determined, patient education is paramount to prevent recurrence. Increased awareness of habits that inflict persistent repetitive trauma or pressure to the site—from sleeping patterns to cell phone use—will help to extinguish the behavior and therefore the lesion.
Chondrodermatitis nodularis helicis (CNH) is a chronic painful or crusted, 4- to 6-mm, solitary nodule, primarily on the upper part of the ear (most commonly on the right side). The presence of pain, which increases the likelihood that a person will seek treatment, clinically distinguishes CNH from other cutaneous tumors in the differential diagnosis that produce painless ulceration.
It is roughly 5 times more prevalent in males (72.9%),1 with an average age of onset of 65 years.2 However, CNH has been reported in females3 and rarely in individuals younger than 20 years. According to a PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms chrondodermatitis nodularis helices child, only 6 cases of CNH have been reported in the pediatric population.4-8 The youngest reported case was a 9-month-old infant.8 Including the present case, males and females in the pediatric population are equally affected; 4 patients had an underlying dermatomyositis,7 rheumatoid nodule,8 or systemic disease, including systemic lupus erythematosus and Beckwith-Wiedemann syndrome.5,9 Chronic intermittent pressure from headwear was the etiologic agent in the remaining cases.4 Recognizing that CNH can occur in young patients and can be associated with underlying autoimmune disease helps direct management and avoid overly invasive treatment.
Case Report
A 17-year-old adolescent boy presented with a painful ulcerated papule on the right upper helix of 3 months’ duration (Figure 1). The patient habitually slept on the right side, pressed a cell phone to that ear, and wore a tight-fitting visor while lifeguarding, which, along with solar damage, all may have contributed to the disease process. He was otherwise in good health, without a history of underlying systemic disease. Given the patient’s extensive occupational sun exposure, biopsy of the lesion was taken under the impression of CNH vs squamous cell carcinoma or basal cell carcinoma.

Histopathologic analysis revealed a central area of ulceration with edematous degenerated dermal collagen and overlying inflammatory crust, characteristic of CNH (Figure 2A). Biopsy in this patient demonstrated classic histopathologic findings of CNH, including a central area of epidermal ulceration capped by an inflammatory crust and an underlying edematous degenerated dermal collagen (Figure 2B).

Following biopsy, the patient was advised of this diagnosis and recommended to avoid applying pressure to the area with cell phones or hats or when sleeping to prevent recurrence. At 3-month follow-up, no residual lesion remained.
Comment
Pathogenesis
The exact cause of CNH is unknown but is probably the result of prolonged and excessive pressure on the ear that leads to ischemic injury to cartilage and skin. The external location of CNH, lack of bony support, and exquisitely thin padding or insulation in the form of subcutaneous tissue make the small dermal blood vessels supplying the outer ear vulnerable to compression. Dermal inflammation; edema; and necrosis from trauma, cold, or actinic damage also can help initiate CNH. This disruption of blood perfusion to the external ear also inhibits the ear’s ability to heal. A cycle of pressure from objects such as a pillow or cell phone, followed by inadequate healing, leads to secondary perichondritis and remodeling of perichondrial arterioles, which is demonstrated histologically by the presence of perichondrial fibrous thickening, mild chronic inflammation, collagen degeneration, hyalinization, and rarely necrosis or calcification. Healed lesions often show dermal fibrosis overlying perichondrium.
Repeated pressure can lead to vascular changes, but underlying vascular disease also can predispose a person to CNH at a younger age. A striking case of bilateral CNH was reported in an 8-year-old girl with a known history of dermatomyositis.7 Furthermore, in 24 patients with CNH (mean age, 43 years), Magro et al9 observed an association between CNH and collagen vascular disease, scleroderma, hypertension, thyroid disease, and heart disease, with a higher incidence of any of these medical problems in younger patients. Therefore, screening all patients presenting with CNH, particularly those younger than their fourth decade, for underlying vasculopathy and an autoimmune connective tissue disorder is advised.9
Other findings of CNH reported in the literature include loss of elastic fibers in the central area of degenerated dermal collagen and nerve hyperplasia, which might account for pain.6 Many of the biopsies in cases of CNH reported in the literature also demonstrate perichondrial fibrous thickening, mild chronic inflammation, and degenerative changes in collagen, including hyalinization and rarely necrosis and calcification. Skin at the periphery of the lesion usually contains granulation tissue, with a mild to moderate inflammatory infiltrate and dilated vessels extending beyond the lesion.2
Genetics might play a role in the disorder, which is suggested by the occurrence of CNH in monozygotic twins10 and in an otherwise healthy 16-year-old adolescent girl with CNH of the right ear who screened negative for underlying connective tissue disease—serologic tests included antinuclear antibody, anti-Sm, anti-SCL-70, anti-Ro, anti-La, and rheumatoid factor—but who had a family history of a maternal grandmother with CNH, also on the right side.6
In the present case, there was no family history or signs and symptoms of underlying systemic disease at the time of diagnosis. The social history revealed excessive occupational sun exposure, habitually wearing a tight visor, and frequent cell phone use, all of which might have contributed to CNH.
Management
Medical management is geared toward relieving pressure at the site of the lesion, which was accomplished by use of an off-loading, ring-shaped, foam pillow at night in a 9-month-old girl with CNH, in which the smaller of her 2 left-sided lesions completely resolved by 6-month follow-up.8 However, it often is difficult to achieve adequate relief of pressure because of the patient’s preference for holding a cell phone to a particular ear or unconscious sleeping habits that perpetuate lesions. There are many creative physical interventions to offload aggravating pressure from the area during sleep. A prosthesis can be fashioned by cutting a hole from the center of a bath sponge and securing it with a headband,11 or a crescentic or rectangular piece of self-adhering foam sponge can be applied to the non–hair-bearing postauricular scalp during sleep.12 Topical antibiotics might relieve pain caused by secondary infection.
Surgical intervention, with or without placement of a full-thickness skin graft, is the mainstay of therapy. Excision was performed in 3 previously reported pediatric cases, with no recurrence reported at 6- to 24-month follow-up. Other treatments employed to varying effect include topical and intralesional steroids, collagen injection, cryotherapy, nitroglycerin paste 2% twice daily,13 and electrodesiccation and curettage.14 In adults, if full resolution is desired, multiple surgeries might be required to remove underlying protuberant cartilage; however, this strategy is not without risk of complication, including formation of adjacent cartilaginous nodules that can become site(s) of CNH recurrence due to a change in pressure points.
Conclusion
Although uncommon, CNH can present on the ears of young patients. A causal link between underlying vasculopathy and CNH has yet to be determined, but the association discovered by Magro et al9 merits obtaining a more detailed rheumatologic history and examination, followed by serologic testing (if indicated). Once the diagnosis of CNH is determined, patient education is paramount to prevent recurrence. Increased awareness of habits that inflict persistent repetitive trauma or pressure to the site—from sleeping patterns to cell phone use—will help to extinguish the behavior and therefore the lesion.
- Rex J, Rivera M, Bielsa I, et al. Narrow elliptical skin excision and cartilage shaving for treatment of chondrodermatitis nodularis. Dermatol Surg. 2006;32:400-404.
- Wettlé C, Keller F, Will F, et al. Chondrodermatitis nodularis chronical helicis: a descriptive study of 99 patients [in French]. Ann Dermatol Venereol. 2013;140:687-692.
- Oelzner S, Elsner P. Bilateral chondrodermatitis nodularis chronica helicis on the free border of the helix in a woman. J Am Acad Dermatol. 2003;49:720-722.
- Grigoryants V, Qureshi H, Patterson J, et al. Pediatric chondrodermatitis nodularis helicis. J Craniofac Surg. 2007;18:228-231.
- Fix WC, Cornejo C, Duffy KA, et al. Pediatric chondrodermatitis nodularis helicis (CNH) in a child with Beckwith-Wiedemann syndrome (BWS). Pediatr Dermatol. 2019;36:388-390.
- Rogers NE, Farris PK, Wang AR. Juvenile chondrodermatitis nodularis helicis: case report and literature review. Pediatr Dermatol. 2003;20:488-490.
- Sasaki T, Nishizawa H, Sugita Y. Chondrodermatitis nodularis helicis in childhood dermatomyositis. Br J Dermatol. 1999;141:363-365.
- Tsai TH, Lin YC, Chen HC. Infantile chondrodermatitis nodularis. Pediatr Dermatol. 2007;24:337-339.
- Magro CM, Frambach GE, Crowson AN. Chondrodermatitis nodularis helicis as a marker of internal disease associated with microvascular injury. J Cutan Pathol. 2005;32:329-333.
- Chan HP, Neuhaus IM, Maibach HI. Chondrodermatitis nodularis chronica helicis in monozygotic twins. Clin Exp Dermatol. 2009;34:358-359.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894.
- Travelute CR. Self-adhering foam: a simple method for pressure relief during sleep in patients with chondrodermatitis nodularis helicis. Dermatol Surg. 2013;39:317-319.
- Flynn V, Chisholm C, Grimwood R. Topical nitroglycerin: a promising treatment option for chondrodermatitis nodularis helicis. J Am Acad Dermatol. 2011;65:531-536.
- Kromann N, Høyer H, Reymann F. Chondrodermatitis nodularis chronica helicis treated with curettage and electrocauterization: follow-up of a 15-year material. Acta Derm Venereol. 1983;63:85-87.
- Rex J, Rivera M, Bielsa I, et al. Narrow elliptical skin excision and cartilage shaving for treatment of chondrodermatitis nodularis. Dermatol Surg. 2006;32:400-404.
- Wettlé C, Keller F, Will F, et al. Chondrodermatitis nodularis chronical helicis: a descriptive study of 99 patients [in French]. Ann Dermatol Venereol. 2013;140:687-692.
- Oelzner S, Elsner P. Bilateral chondrodermatitis nodularis chronica helicis on the free border of the helix in a woman. J Am Acad Dermatol. 2003;49:720-722.
- Grigoryants V, Qureshi H, Patterson J, et al. Pediatric chondrodermatitis nodularis helicis. J Craniofac Surg. 2007;18:228-231.
- Fix WC, Cornejo C, Duffy KA, et al. Pediatric chondrodermatitis nodularis helicis (CNH) in a child with Beckwith-Wiedemann syndrome (BWS). Pediatr Dermatol. 2019;36:388-390.
- Rogers NE, Farris PK, Wang AR. Juvenile chondrodermatitis nodularis helicis: case report and literature review. Pediatr Dermatol. 2003;20:488-490.
- Sasaki T, Nishizawa H, Sugita Y. Chondrodermatitis nodularis helicis in childhood dermatomyositis. Br J Dermatol. 1999;141:363-365.
- Tsai TH, Lin YC, Chen HC. Infantile chondrodermatitis nodularis. Pediatr Dermatol. 2007;24:337-339.
- Magro CM, Frambach GE, Crowson AN. Chondrodermatitis nodularis helicis as a marker of internal disease associated with microvascular injury. J Cutan Pathol. 2005;32:329-333.
- Chan HP, Neuhaus IM, Maibach HI. Chondrodermatitis nodularis chronica helicis in monozygotic twins. Clin Exp Dermatol. 2009;34:358-359.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894.
- Travelute CR. Self-adhering foam: a simple method for pressure relief during sleep in patients with chondrodermatitis nodularis helicis. Dermatol Surg. 2013;39:317-319.
- Flynn V, Chisholm C, Grimwood R. Topical nitroglycerin: a promising treatment option for chondrodermatitis nodularis helicis. J Am Acad Dermatol. 2011;65:531-536.
- Kromann N, Høyer H, Reymann F. Chondrodermatitis nodularis chronica helicis treated with curettage and electrocauterization: follow-up of a 15-year material. Acta Derm Venereol. 1983;63:85-87.
Practice Points
- Chondrodermatitis nodularis helicis should be in the differential for nodular lesions on the ears of adolescents, as societal shifts in behavior have altered the epidemiology of this condition such that it is no longer exclusive to the geriatric population.
- Make sure to get a thorough history of potential pressure triggers when evaluating nodules on the ears of adolescents.
Cancer pain management inadequate in opioid-saturated areas
Patients with cancer who live in regions with high levels of opioid misuse may be undertreated for pain, according to investigators who studied opioid prescription patterns and cancer incidence in rural southwest Virginia.
Among 4,324 patients with cancer, only 22.16% were prescribed a Controlled Schedule II (C-II) prescription opioid medication at least 3 times in 1 year, from prescribers likely to be treating cancer pain. More than 60% of patients never received a C-II opioid prescription, reported Virginia T. LeBaron, PhD, of the University of Virginia School of Nursing in Charlottesville, and colleagues.
“A clearer view of geographic patterns and predictors of both POM [prescription opioid medication] prescribing and potential harms can inform targeted interventions and policy initiatives that achieve a balanced approach to POMs – ensuring access for patients in need while reducing risk to both patients and communities. Our research makes an important contribution by exploring how the current ‘opioid epidemic’ relates to rural patients with cancer,” they wrote. Their report is in Journal of Oncology Practice.
The investigators studied the confluence of disproportionately high cancer mortality rates and opioid fatality rates in rural southwest Virginia, in the heart of Appalachia.
They conducted a longitudinal, exploratory secondary analysis of data from the Commonwealth of Virginia All Payer Claims database to look at opioid prescribing patterns and explore whether concerns about opioid misuse could result in undertreatment of pain in cancer patients.
They looked at prescribing patterns at the patient, provider, and insurance claim levels, predictors of opioid prescription frequency, opioid-related harms and patterns related to opioid prescribing, cancer incidence, and fatalities.
They identified 4,324 patients with cancer, 958 of whom (22.16%) received a C-II opioid at least three times in any study year. The majority of patients were in the 45-64 age range, and approximately 88% were diagnosed with solid malignancies, with breast cancer and lung cancer being the most frequent diagnoses.
As noted, more than 60% of patients never received a C-II prescription.
“The large percentages of cancer patients never prescribed a C-II are concerning for a number of reasons, especially when we consider the results per year,” the investigators wrote. “First, the ‘no C-II’ patients remain over 80% of the total sample, each year, even after accounting for the upscheduling (from C-III to C-II) of commonly-prescribed hydrocodone products in 2014. Second, anecdotal data and emerging empirical evidence demonstrate that patients with legitimate pain needs, including patients with cancer, experience significant difficulty accessing POMs.”
They noted that regulations regarding opioid prescriptions have become increasingly strict since the end date of their analysis in 2015, suggesting that the number of patients with cancer who are not receiving C-II opioids today may be even higher.
They also pointed to evidence of prescription practices suggesting suboptimal pain management or potential patient harm, such as frequent prescription of opioid-acetaminophen combinations that are dose-limited due to acetaminophen toxicity; coprescription of opioids and benzodiazepines, which is not recommended under current prescribing guidelines; and infrequent use of deterrent formulations of C-II opioids such as crush-resistant tablets.
The study was supported by the University of Virginia Cancer Center, Cancer Control & Population Health Division and the Virginia Tobacco Region Revitalization Commission. The authors reported having no disclaimers or conflicts of interest.
SOURCE: LeBaron VT et al. J Oncol Pract. 2019 Nov. 4. doi: 10.1200/JOP.19.00149.
Patients with cancer who live in regions with high levels of opioid misuse may be undertreated for pain, according to investigators who studied opioid prescription patterns and cancer incidence in rural southwest Virginia.
Among 4,324 patients with cancer, only 22.16% were prescribed a Controlled Schedule II (C-II) prescription opioid medication at least 3 times in 1 year, from prescribers likely to be treating cancer pain. More than 60% of patients never received a C-II opioid prescription, reported Virginia T. LeBaron, PhD, of the University of Virginia School of Nursing in Charlottesville, and colleagues.
“A clearer view of geographic patterns and predictors of both POM [prescription opioid medication] prescribing and potential harms can inform targeted interventions and policy initiatives that achieve a balanced approach to POMs – ensuring access for patients in need while reducing risk to both patients and communities. Our research makes an important contribution by exploring how the current ‘opioid epidemic’ relates to rural patients with cancer,” they wrote. Their report is in Journal of Oncology Practice.
The investigators studied the confluence of disproportionately high cancer mortality rates and opioid fatality rates in rural southwest Virginia, in the heart of Appalachia.
They conducted a longitudinal, exploratory secondary analysis of data from the Commonwealth of Virginia All Payer Claims database to look at opioid prescribing patterns and explore whether concerns about opioid misuse could result in undertreatment of pain in cancer patients.
They looked at prescribing patterns at the patient, provider, and insurance claim levels, predictors of opioid prescription frequency, opioid-related harms and patterns related to opioid prescribing, cancer incidence, and fatalities.
They identified 4,324 patients with cancer, 958 of whom (22.16%) received a C-II opioid at least three times in any study year. The majority of patients were in the 45-64 age range, and approximately 88% were diagnosed with solid malignancies, with breast cancer and lung cancer being the most frequent diagnoses.
As noted, more than 60% of patients never received a C-II prescription.
“The large percentages of cancer patients never prescribed a C-II are concerning for a number of reasons, especially when we consider the results per year,” the investigators wrote. “First, the ‘no C-II’ patients remain over 80% of the total sample, each year, even after accounting for the upscheduling (from C-III to C-II) of commonly-prescribed hydrocodone products in 2014. Second, anecdotal data and emerging empirical evidence demonstrate that patients with legitimate pain needs, including patients with cancer, experience significant difficulty accessing POMs.”
They noted that regulations regarding opioid prescriptions have become increasingly strict since the end date of their analysis in 2015, suggesting that the number of patients with cancer who are not receiving C-II opioids today may be even higher.
They also pointed to evidence of prescription practices suggesting suboptimal pain management or potential patient harm, such as frequent prescription of opioid-acetaminophen combinations that are dose-limited due to acetaminophen toxicity; coprescription of opioids and benzodiazepines, which is not recommended under current prescribing guidelines; and infrequent use of deterrent formulations of C-II opioids such as crush-resistant tablets.
The study was supported by the University of Virginia Cancer Center, Cancer Control & Population Health Division and the Virginia Tobacco Region Revitalization Commission. The authors reported having no disclaimers or conflicts of interest.
SOURCE: LeBaron VT et al. J Oncol Pract. 2019 Nov. 4. doi: 10.1200/JOP.19.00149.
Patients with cancer who live in regions with high levels of opioid misuse may be undertreated for pain, according to investigators who studied opioid prescription patterns and cancer incidence in rural southwest Virginia.
Among 4,324 patients with cancer, only 22.16% were prescribed a Controlled Schedule II (C-II) prescription opioid medication at least 3 times in 1 year, from prescribers likely to be treating cancer pain. More than 60% of patients never received a C-II opioid prescription, reported Virginia T. LeBaron, PhD, of the University of Virginia School of Nursing in Charlottesville, and colleagues.
“A clearer view of geographic patterns and predictors of both POM [prescription opioid medication] prescribing and potential harms can inform targeted interventions and policy initiatives that achieve a balanced approach to POMs – ensuring access for patients in need while reducing risk to both patients and communities. Our research makes an important contribution by exploring how the current ‘opioid epidemic’ relates to rural patients with cancer,” they wrote. Their report is in Journal of Oncology Practice.
The investigators studied the confluence of disproportionately high cancer mortality rates and opioid fatality rates in rural southwest Virginia, in the heart of Appalachia.
They conducted a longitudinal, exploratory secondary analysis of data from the Commonwealth of Virginia All Payer Claims database to look at opioid prescribing patterns and explore whether concerns about opioid misuse could result in undertreatment of pain in cancer patients.
They looked at prescribing patterns at the patient, provider, and insurance claim levels, predictors of opioid prescription frequency, opioid-related harms and patterns related to opioid prescribing, cancer incidence, and fatalities.
They identified 4,324 patients with cancer, 958 of whom (22.16%) received a C-II opioid at least three times in any study year. The majority of patients were in the 45-64 age range, and approximately 88% were diagnosed with solid malignancies, with breast cancer and lung cancer being the most frequent diagnoses.
As noted, more than 60% of patients never received a C-II prescription.
“The large percentages of cancer patients never prescribed a C-II are concerning for a number of reasons, especially when we consider the results per year,” the investigators wrote. “First, the ‘no C-II’ patients remain over 80% of the total sample, each year, even after accounting for the upscheduling (from C-III to C-II) of commonly-prescribed hydrocodone products in 2014. Second, anecdotal data and emerging empirical evidence demonstrate that patients with legitimate pain needs, including patients with cancer, experience significant difficulty accessing POMs.”
They noted that regulations regarding opioid prescriptions have become increasingly strict since the end date of their analysis in 2015, suggesting that the number of patients with cancer who are not receiving C-II opioids today may be even higher.
They also pointed to evidence of prescription practices suggesting suboptimal pain management or potential patient harm, such as frequent prescription of opioid-acetaminophen combinations that are dose-limited due to acetaminophen toxicity; coprescription of opioids and benzodiazepines, which is not recommended under current prescribing guidelines; and infrequent use of deterrent formulations of C-II opioids such as crush-resistant tablets.
The study was supported by the University of Virginia Cancer Center, Cancer Control & Population Health Division and the Virginia Tobacco Region Revitalization Commission. The authors reported having no disclaimers or conflicts of interest.
SOURCE: LeBaron VT et al. J Oncol Pract. 2019 Nov. 4. doi: 10.1200/JOP.19.00149.
FROM JOURNAL OF ONCOLOGY PRACTICE
One in five chest tube placements/removals goes awry
SAN FRANCISCO – , according to a prospective observational study conducted at 14 adult trauma centers.
“The sad part is, I don’t know if it was surprisingly high, but I’m glad somebody has taken the time to document it,” said Robert Sawyer, MD, professor of surgery at Western Michigan University, Kalamazoo, Mich., who comoderated the session at the annual clinical congress of the American College of Surgeons, where the study was presented.
The researchers examined error rates in both insertions and removals, and compared some of the practices and characteristics of trauma centers with unusually good or poor records. The work could begin to inform quality improvement initiatives. “That’s very parallel to where we were 20 or 25 years ago with central venous catheters. We used to put them in and thought it was never a problem, and then we started taking a close look at it and found out, yeah, there was a problem. We systematically made our procedures more consistent and had better outcomes. I think chest tubes is going to be ripe for that,” Dr. Sawyer said in an interview.
“In some ways we have been lying to ourselves. We acknowledge that trainees have a high rate of complications in chest tube insertion and removal, but we haven’t fixed it as a systematic problem. We’re behind in our work to reduce complications for this bedside procedure,” echoed the session’s other comoderator, Tam Pham, MD, professor of surgery at the University of Washington, Seattle, in an interview.
The researchers defined chest tube errors as anything that resulted in a need to manipulate, replace, or revise an existing tube; a worsening of the condition that the tube was intended to address; or complications that resulted in additional length of stay or interventions. A total of 381 chest tubes were placed in 273 patients over a 3-month period, about 55% by residents and about 28% by trauma attending physicians. Around 80% were traditional chest tubes, and most of the rest were Pigtail, with a very small fraction of Trocar chest tubes, according to a pie chart displayed by Michaela West, MD, a trauma surgeon at North Memorial Health, Robbinsdale, Minn., who presented the research.
Dr. West reported a wide range of complication rates among the 14 institutions, ranging from under 10% to nearly 60%, and some centers reported far more complications with removal or insertion, while some had closer to an even split. The overall average rate of insertion complications was 18.7%, and the average for removal was 17.7%.
When the researchers looked at some of the best and worst performing centers, they identified some trends. A total of 98.6% of chest tubes were tunneled in the best-performing centers, while 14.3% were tunneled in the worst. An initial air leak was more common in the best performing centers (52.5% versus 21.7%). Higher performing centers had a greater percentage of patients with gunshot wounds (24.3% versus 13%), and had a longer duration of stay (5.3 days versus 3.4 days; P less than .05 for all).
In the single highest performing center, all chest tubes were removed by midlevel individuals, and the other two best performing centers relied on an attending physician or resident. The worst performing centers often had postgraduate year 1 and 2 residents removing the chest tubes.
Dr. West, Dr. Pham, and Dr. Sawyer have no relevant financial disclosures.
SOURCE: West M et al. Clinical Congress 2019 Abstract.
SAN FRANCISCO – , according to a prospective observational study conducted at 14 adult trauma centers.
“The sad part is, I don’t know if it was surprisingly high, but I’m glad somebody has taken the time to document it,” said Robert Sawyer, MD, professor of surgery at Western Michigan University, Kalamazoo, Mich., who comoderated the session at the annual clinical congress of the American College of Surgeons, where the study was presented.
The researchers examined error rates in both insertions and removals, and compared some of the practices and characteristics of trauma centers with unusually good or poor records. The work could begin to inform quality improvement initiatives. “That’s very parallel to where we were 20 or 25 years ago with central venous catheters. We used to put them in and thought it was never a problem, and then we started taking a close look at it and found out, yeah, there was a problem. We systematically made our procedures more consistent and had better outcomes. I think chest tubes is going to be ripe for that,” Dr. Sawyer said in an interview.
“In some ways we have been lying to ourselves. We acknowledge that trainees have a high rate of complications in chest tube insertion and removal, but we haven’t fixed it as a systematic problem. We’re behind in our work to reduce complications for this bedside procedure,” echoed the session’s other comoderator, Tam Pham, MD, professor of surgery at the University of Washington, Seattle, in an interview.
The researchers defined chest tube errors as anything that resulted in a need to manipulate, replace, or revise an existing tube; a worsening of the condition that the tube was intended to address; or complications that resulted in additional length of stay or interventions. A total of 381 chest tubes were placed in 273 patients over a 3-month period, about 55% by residents and about 28% by trauma attending physicians. Around 80% were traditional chest tubes, and most of the rest were Pigtail, with a very small fraction of Trocar chest tubes, according to a pie chart displayed by Michaela West, MD, a trauma surgeon at North Memorial Health, Robbinsdale, Minn., who presented the research.
Dr. West reported a wide range of complication rates among the 14 institutions, ranging from under 10% to nearly 60%, and some centers reported far more complications with removal or insertion, while some had closer to an even split. The overall average rate of insertion complications was 18.7%, and the average for removal was 17.7%.
When the researchers looked at some of the best and worst performing centers, they identified some trends. A total of 98.6% of chest tubes were tunneled in the best-performing centers, while 14.3% were tunneled in the worst. An initial air leak was more common in the best performing centers (52.5% versus 21.7%). Higher performing centers had a greater percentage of patients with gunshot wounds (24.3% versus 13%), and had a longer duration of stay (5.3 days versus 3.4 days; P less than .05 for all).
In the single highest performing center, all chest tubes were removed by midlevel individuals, and the other two best performing centers relied on an attending physician or resident. The worst performing centers often had postgraduate year 1 and 2 residents removing the chest tubes.
Dr. West, Dr. Pham, and Dr. Sawyer have no relevant financial disclosures.
SOURCE: West M et al. Clinical Congress 2019 Abstract.
SAN FRANCISCO – , according to a prospective observational study conducted at 14 adult trauma centers.
“The sad part is, I don’t know if it was surprisingly high, but I’m glad somebody has taken the time to document it,” said Robert Sawyer, MD, professor of surgery at Western Michigan University, Kalamazoo, Mich., who comoderated the session at the annual clinical congress of the American College of Surgeons, where the study was presented.
The researchers examined error rates in both insertions and removals, and compared some of the practices and characteristics of trauma centers with unusually good or poor records. The work could begin to inform quality improvement initiatives. “That’s very parallel to where we were 20 or 25 years ago with central venous catheters. We used to put them in and thought it was never a problem, and then we started taking a close look at it and found out, yeah, there was a problem. We systematically made our procedures more consistent and had better outcomes. I think chest tubes is going to be ripe for that,” Dr. Sawyer said in an interview.
“In some ways we have been lying to ourselves. We acknowledge that trainees have a high rate of complications in chest tube insertion and removal, but we haven’t fixed it as a systematic problem. We’re behind in our work to reduce complications for this bedside procedure,” echoed the session’s other comoderator, Tam Pham, MD, professor of surgery at the University of Washington, Seattle, in an interview.
The researchers defined chest tube errors as anything that resulted in a need to manipulate, replace, or revise an existing tube; a worsening of the condition that the tube was intended to address; or complications that resulted in additional length of stay or interventions. A total of 381 chest tubes were placed in 273 patients over a 3-month period, about 55% by residents and about 28% by trauma attending physicians. Around 80% were traditional chest tubes, and most of the rest were Pigtail, with a very small fraction of Trocar chest tubes, according to a pie chart displayed by Michaela West, MD, a trauma surgeon at North Memorial Health, Robbinsdale, Minn., who presented the research.
Dr. West reported a wide range of complication rates among the 14 institutions, ranging from under 10% to nearly 60%, and some centers reported far more complications with removal or insertion, while some had closer to an even split. The overall average rate of insertion complications was 18.7%, and the average for removal was 17.7%.
When the researchers looked at some of the best and worst performing centers, they identified some trends. A total of 98.6% of chest tubes were tunneled in the best-performing centers, while 14.3% were tunneled in the worst. An initial air leak was more common in the best performing centers (52.5% versus 21.7%). Higher performing centers had a greater percentage of patients with gunshot wounds (24.3% versus 13%), and had a longer duration of stay (5.3 days versus 3.4 days; P less than .05 for all).
In the single highest performing center, all chest tubes were removed by midlevel individuals, and the other two best performing centers relied on an attending physician or resident. The worst performing centers often had postgraduate year 1 and 2 residents removing the chest tubes.
Dr. West, Dr. Pham, and Dr. Sawyer have no relevant financial disclosures.
SOURCE: West M et al. Clinical Congress 2019 Abstract.
REPORTING FROM CLINICAL CONGRESS 2019