User login
Conflicts of interest unreported in five of six oncology guidelines in Japan
, according to results from a study published in JAMA Network Open.
Hiroaki Saito, MD, of Tottori University in Yonago, Japan, and colleagues retrospectively analyzed 2016-2017 payment data from 78 pharmaceutical companies in regard to 326 oncology guideline authors in Japan. Data collected included clinician demographic information, the amount of payments received, types of payments, and information related to disclosure methods.
The team reviewed oncology guidelines for gastric, breast, hepatocellular, pancreatic, lung, and colorectal cancers. Subsequently, they confirmed whether the amount of payment received was in accordance with each guideline’s policy of conflict of interest (COI) disclosure.
“Because no unified and ready-made database encompassing all the companies was available, we obtained each company’s data individually and organized the data into a unified database,” the researchers wrote.
The researchers found that among 326 guideline authors, 255 (78.2%) received compensation from pharmaceutical companies in 2016, with 25.8% receiving over $10,000. In addition, they reported that only the breast cancer guidelines included the authors’ COI disclosures in a detectable matter.
“Guidelines for lung, colorectal, pancreatic, and hepatocellular carcinomas disclosed the financial relationships between the authors and companies anonymously; and the gastric carcinoma [guidelines] did not have a COI disclosure section,” Dr. Saito and his colleagues wrote.
The researchers acknowledged that a key limitation of the study could be measurement error as the findings were dependent on the accuracy of information entered into the database.
The study was funded by the Medical Governance Research Institute and the Waseda Chronicle. The authors reported financial affiliations with Taiho Pharmaceutical and Medical Network Systems.
SOURCE: Saito H et al. JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2834.
One question that remains from the study by Hiroaki Saito, MD, and colleagues is the significance of the underreporting of clinical practice guidelines authors’ financial conflicts of interest.
The recommendations provided in clinical practice guidelines have major financial implications for numerous stakeholder groups, including clinicians, patients, drug manufacturers, and society. As Japan is the third largest pharmaceutical market worldwide, the quality of conflict of interest disclosures is important, the researchers wrote.
In 2011, the U.S. Institute of Medicine [now the National Academy of Medicine] published recommendations on the development of clinical practice guidelines, which have now become the international standards. Their recommendations include mandatory reporting of financial conflicts of interests for all members of the development group, minimizing authors with financial conflict of interests, and selecting chairpersons without any financial conflicts.
Recent studies have suggested the importance of author financial affiliations as certain drugs endorsed in clinical practice guidelines were found to be correlated with authors’ conflicts of interest. Leaders and stakeholders must address concerns related to underreporting of conflicts of interest to uphold public trust.
Philip B. Mitchell, AM, MBBS, MD, is associated with the School of Psychiatry at the University of New South Wales in Sydney. No conflicts of interest were reported. These comments are adapted from his editorial (JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2840 ).
One question that remains from the study by Hiroaki Saito, MD, and colleagues is the significance of the underreporting of clinical practice guidelines authors’ financial conflicts of interest.
The recommendations provided in clinical practice guidelines have major financial implications for numerous stakeholder groups, including clinicians, patients, drug manufacturers, and society. As Japan is the third largest pharmaceutical market worldwide, the quality of conflict of interest disclosures is important, the researchers wrote.
In 2011, the U.S. Institute of Medicine [now the National Academy of Medicine] published recommendations on the development of clinical practice guidelines, which have now become the international standards. Their recommendations include mandatory reporting of financial conflicts of interests for all members of the development group, minimizing authors with financial conflict of interests, and selecting chairpersons without any financial conflicts.
Recent studies have suggested the importance of author financial affiliations as certain drugs endorsed in clinical practice guidelines were found to be correlated with authors’ conflicts of interest. Leaders and stakeholders must address concerns related to underreporting of conflicts of interest to uphold public trust.
Philip B. Mitchell, AM, MBBS, MD, is associated with the School of Psychiatry at the University of New South Wales in Sydney. No conflicts of interest were reported. These comments are adapted from his editorial (JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2840 ).
One question that remains from the study by Hiroaki Saito, MD, and colleagues is the significance of the underreporting of clinical practice guidelines authors’ financial conflicts of interest.
The recommendations provided in clinical practice guidelines have major financial implications for numerous stakeholder groups, including clinicians, patients, drug manufacturers, and society. As Japan is the third largest pharmaceutical market worldwide, the quality of conflict of interest disclosures is important, the researchers wrote.
In 2011, the U.S. Institute of Medicine [now the National Academy of Medicine] published recommendations on the development of clinical practice guidelines, which have now become the international standards. Their recommendations include mandatory reporting of financial conflicts of interests for all members of the development group, minimizing authors with financial conflict of interests, and selecting chairpersons without any financial conflicts.
Recent studies have suggested the importance of author financial affiliations as certain drugs endorsed in clinical practice guidelines were found to be correlated with authors’ conflicts of interest. Leaders and stakeholders must address concerns related to underreporting of conflicts of interest to uphold public trust.
Philip B. Mitchell, AM, MBBS, MD, is associated with the School of Psychiatry at the University of New South Wales in Sydney. No conflicts of interest were reported. These comments are adapted from his editorial (JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2840 ).
, according to results from a study published in JAMA Network Open.
Hiroaki Saito, MD, of Tottori University in Yonago, Japan, and colleagues retrospectively analyzed 2016-2017 payment data from 78 pharmaceutical companies in regard to 326 oncology guideline authors in Japan. Data collected included clinician demographic information, the amount of payments received, types of payments, and information related to disclosure methods.
The team reviewed oncology guidelines for gastric, breast, hepatocellular, pancreatic, lung, and colorectal cancers. Subsequently, they confirmed whether the amount of payment received was in accordance with each guideline’s policy of conflict of interest (COI) disclosure.
“Because no unified and ready-made database encompassing all the companies was available, we obtained each company’s data individually and organized the data into a unified database,” the researchers wrote.
The researchers found that among 326 guideline authors, 255 (78.2%) received compensation from pharmaceutical companies in 2016, with 25.8% receiving over $10,000. In addition, they reported that only the breast cancer guidelines included the authors’ COI disclosures in a detectable matter.
“Guidelines for lung, colorectal, pancreatic, and hepatocellular carcinomas disclosed the financial relationships between the authors and companies anonymously; and the gastric carcinoma [guidelines] did not have a COI disclosure section,” Dr. Saito and his colleagues wrote.
The researchers acknowledged that a key limitation of the study could be measurement error as the findings were dependent on the accuracy of information entered into the database.
The study was funded by the Medical Governance Research Institute and the Waseda Chronicle. The authors reported financial affiliations with Taiho Pharmaceutical and Medical Network Systems.
SOURCE: Saito H et al. JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2834.
, according to results from a study published in JAMA Network Open.
Hiroaki Saito, MD, of Tottori University in Yonago, Japan, and colleagues retrospectively analyzed 2016-2017 payment data from 78 pharmaceutical companies in regard to 326 oncology guideline authors in Japan. Data collected included clinician demographic information, the amount of payments received, types of payments, and information related to disclosure methods.
The team reviewed oncology guidelines for gastric, breast, hepatocellular, pancreatic, lung, and colorectal cancers. Subsequently, they confirmed whether the amount of payment received was in accordance with each guideline’s policy of conflict of interest (COI) disclosure.
“Because no unified and ready-made database encompassing all the companies was available, we obtained each company’s data individually and organized the data into a unified database,” the researchers wrote.
The researchers found that among 326 guideline authors, 255 (78.2%) received compensation from pharmaceutical companies in 2016, with 25.8% receiving over $10,000. In addition, they reported that only the breast cancer guidelines included the authors’ COI disclosures in a detectable matter.
“Guidelines for lung, colorectal, pancreatic, and hepatocellular carcinomas disclosed the financial relationships between the authors and companies anonymously; and the gastric carcinoma [guidelines] did not have a COI disclosure section,” Dr. Saito and his colleagues wrote.
The researchers acknowledged that a key limitation of the study could be measurement error as the findings were dependent on the accuracy of information entered into the database.
The study was funded by the Medical Governance Research Institute and the Waseda Chronicle. The authors reported financial affiliations with Taiho Pharmaceutical and Medical Network Systems.
SOURCE: Saito H et al. JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2834.
FROM JAMA NETWORK OPEN
Methotrexate pneumonitis called ‘super rare’
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2019
Acute-Onset Alopecia
The Diagnosis: Thallium-Induced Alopecia
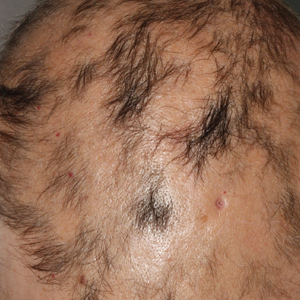
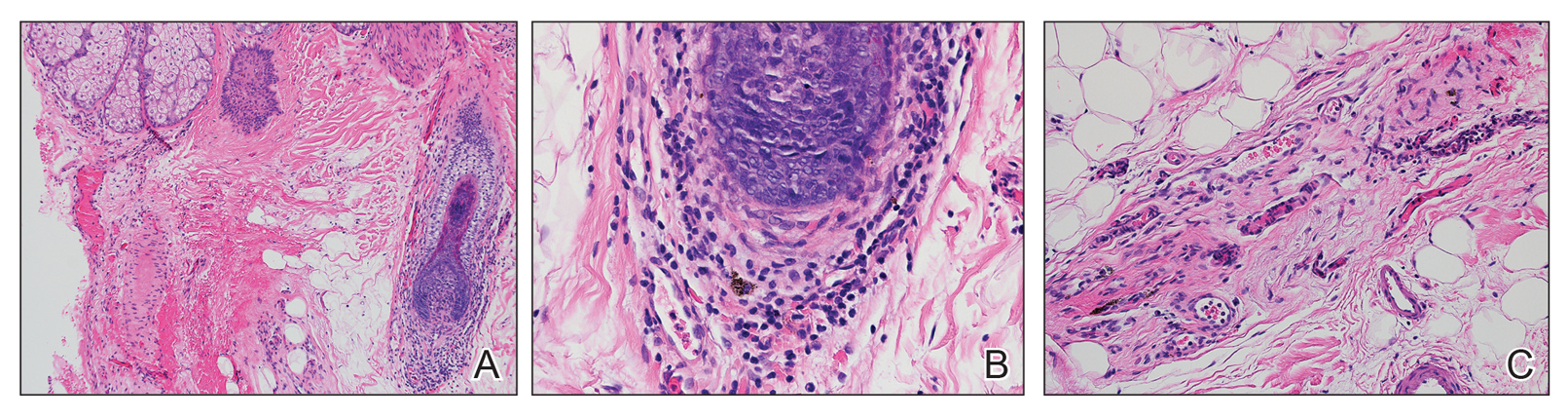
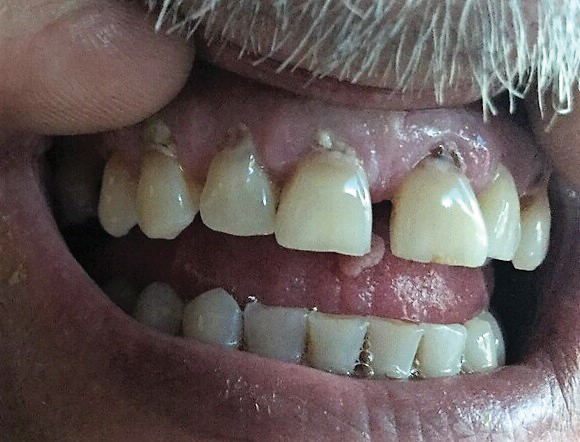
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).
Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).


Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).


Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.

A previously healthy 45-year-old man presented to the dermatology department with abrupt onset of patchy, progressively worsening alopecia of the scalp as well as nausea with emesis and blurry vision of a few weeks' duration. All symptoms were temporally associated with a new demolition job the patient had started at an industrial site. He reported 10 other contractors were similarly affected. The patient denied paresthesia or other skin changes. On physical examination, large patches of smooth alopecia without erythema, scale, scarring, tenderness, or edema that coalesced to involve the majority of the scalp, eyebrows, and eyelashes (inset) were noted.
Tailoring the Mediterranean diet for NAFLD
Adults with nonalcoholic fatty liver disease (NAFLD) were more likely to implement the Mediterranean diet when they had greater nutritional knowledge and skills, family support, nutritional care, and positive reinforcement in the media, according to an in-depth study of 19 patients.
Barriers to adopting the diet included “an obesogenic environment, life stressors, and demand for convenience. Poor understanding of the causes and significance of NAFLD adversely affected readiness to change dietary habits,” wrote Laura Haigh of Newcastle University in Newcastle Upon Tyne, England, and associates. The study, which included both standard quantitative methods and semistructured interviews, was published in Clinical Gastroenterology and Hepatology.
The Mediterranean diet emphasizes vegetables, legumes, fish, fruits, whole grains, nuts, and olive oil in lieu of processed foods, sweets, saturated fats, and red meat. This diet has been definitively shown to improve insulin sensitivity and steatosis, even when patients do not lose weight. This has sparked interest in its use for NAFLD disease, but keys to its successful adoption in Northern Europe are not well understood.
Therefore, the researchers recruited 19 NAFLD patients from a tertiary care center in the United Kingdom for a 12-week Mediterranean diet intervention. Most were female, white, in their late 50s, obese, and had type 2 diabetes. “Participants were taught behavioral strategies through the provision of shopping lists, meal planners, and recipes. No advice was given on calorie allowances or physical activities,” the investigators noted.
By using a 14-point assessment tool, they found that dietary adherence rose significantly at 12 weeks, compared with baseline (P = .006). In all, 79% of patients lost weight (mean, 2.4 kg; P = .001 versus baseline), and 72% significantly increased their serum level of HDL cholesterol. Interviews linked successful adoption of the diet with diverse factors, such as believing that NAFLD is lifestyle associated, realizing that healthier nutrition can improve health outcomes, and having access to transportation and budget grocery stories. Patients generally saw the Mediterranean diet as flexible and affordable, but they struggled to adopt it if they worked irregular hours, experienced substantial life stress or were very busy, or tended to eat for self-reward or self-comfort.
Other cited barriers included “diet saboteurs” (including spouses), the plethora of unhealthy foods available in patients’ environments, low nutritional or medical knowledge, and cultural, social, or taste incompatibility, the researchers reported. Taken together, the findings underscore “the futility of a one-size-fits-all approach” when implementing the Mediterranean diet in this population, they concluded. Instead, their patients valued a collaborative, tailored approach – ideally one that incorporated in-person and group-based treatment, as well as online support.
Funders included the North East of England hub of the Allied Health Professions Research Network, the Elucidating Pathways of Steatohepatitis consortium, the Horizon 2020 Framework Program of the European Union, and the Newcastle NIHR Biomedical Research Centre. The researchers reported having no conflicts of interest.
SOURCE: Haigh L et al. Clin Gastroenterol Hepatol. 2018 Oct 31. doi: 10.1016/j.cgh.2018.10.044.
Adults with nonalcoholic fatty liver disease (NAFLD) were more likely to implement the Mediterranean diet when they had greater nutritional knowledge and skills, family support, nutritional care, and positive reinforcement in the media, according to an in-depth study of 19 patients.
Barriers to adopting the diet included “an obesogenic environment, life stressors, and demand for convenience. Poor understanding of the causes and significance of NAFLD adversely affected readiness to change dietary habits,” wrote Laura Haigh of Newcastle University in Newcastle Upon Tyne, England, and associates. The study, which included both standard quantitative methods and semistructured interviews, was published in Clinical Gastroenterology and Hepatology.
The Mediterranean diet emphasizes vegetables, legumes, fish, fruits, whole grains, nuts, and olive oil in lieu of processed foods, sweets, saturated fats, and red meat. This diet has been definitively shown to improve insulin sensitivity and steatosis, even when patients do not lose weight. This has sparked interest in its use for NAFLD disease, but keys to its successful adoption in Northern Europe are not well understood.
Therefore, the researchers recruited 19 NAFLD patients from a tertiary care center in the United Kingdom for a 12-week Mediterranean diet intervention. Most were female, white, in their late 50s, obese, and had type 2 diabetes. “Participants were taught behavioral strategies through the provision of shopping lists, meal planners, and recipes. No advice was given on calorie allowances or physical activities,” the investigators noted.
By using a 14-point assessment tool, they found that dietary adherence rose significantly at 12 weeks, compared with baseline (P = .006). In all, 79% of patients lost weight (mean, 2.4 kg; P = .001 versus baseline), and 72% significantly increased their serum level of HDL cholesterol. Interviews linked successful adoption of the diet with diverse factors, such as believing that NAFLD is lifestyle associated, realizing that healthier nutrition can improve health outcomes, and having access to transportation and budget grocery stories. Patients generally saw the Mediterranean diet as flexible and affordable, but they struggled to adopt it if they worked irregular hours, experienced substantial life stress or were very busy, or tended to eat for self-reward or self-comfort.
Other cited barriers included “diet saboteurs” (including spouses), the plethora of unhealthy foods available in patients’ environments, low nutritional or medical knowledge, and cultural, social, or taste incompatibility, the researchers reported. Taken together, the findings underscore “the futility of a one-size-fits-all approach” when implementing the Mediterranean diet in this population, they concluded. Instead, their patients valued a collaborative, tailored approach – ideally one that incorporated in-person and group-based treatment, as well as online support.
Funders included the North East of England hub of the Allied Health Professions Research Network, the Elucidating Pathways of Steatohepatitis consortium, the Horizon 2020 Framework Program of the European Union, and the Newcastle NIHR Biomedical Research Centre. The researchers reported having no conflicts of interest.
SOURCE: Haigh L et al. Clin Gastroenterol Hepatol. 2018 Oct 31. doi: 10.1016/j.cgh.2018.10.044.
Adults with nonalcoholic fatty liver disease (NAFLD) were more likely to implement the Mediterranean diet when they had greater nutritional knowledge and skills, family support, nutritional care, and positive reinforcement in the media, according to an in-depth study of 19 patients.
Barriers to adopting the diet included “an obesogenic environment, life stressors, and demand for convenience. Poor understanding of the causes and significance of NAFLD adversely affected readiness to change dietary habits,” wrote Laura Haigh of Newcastle University in Newcastle Upon Tyne, England, and associates. The study, which included both standard quantitative methods and semistructured interviews, was published in Clinical Gastroenterology and Hepatology.
The Mediterranean diet emphasizes vegetables, legumes, fish, fruits, whole grains, nuts, and olive oil in lieu of processed foods, sweets, saturated fats, and red meat. This diet has been definitively shown to improve insulin sensitivity and steatosis, even when patients do not lose weight. This has sparked interest in its use for NAFLD disease, but keys to its successful adoption in Northern Europe are not well understood.
Therefore, the researchers recruited 19 NAFLD patients from a tertiary care center in the United Kingdom for a 12-week Mediterranean diet intervention. Most were female, white, in their late 50s, obese, and had type 2 diabetes. “Participants were taught behavioral strategies through the provision of shopping lists, meal planners, and recipes. No advice was given on calorie allowances or physical activities,” the investigators noted.
By using a 14-point assessment tool, they found that dietary adherence rose significantly at 12 weeks, compared with baseline (P = .006). In all, 79% of patients lost weight (mean, 2.4 kg; P = .001 versus baseline), and 72% significantly increased their serum level of HDL cholesterol. Interviews linked successful adoption of the diet with diverse factors, such as believing that NAFLD is lifestyle associated, realizing that healthier nutrition can improve health outcomes, and having access to transportation and budget grocery stories. Patients generally saw the Mediterranean diet as flexible and affordable, but they struggled to adopt it if they worked irregular hours, experienced substantial life stress or were very busy, or tended to eat for self-reward or self-comfort.
Other cited barriers included “diet saboteurs” (including spouses), the plethora of unhealthy foods available in patients’ environments, low nutritional or medical knowledge, and cultural, social, or taste incompatibility, the researchers reported. Taken together, the findings underscore “the futility of a one-size-fits-all approach” when implementing the Mediterranean diet in this population, they concluded. Instead, their patients valued a collaborative, tailored approach – ideally one that incorporated in-person and group-based treatment, as well as online support.
Funders included the North East of England hub of the Allied Health Professions Research Network, the Elucidating Pathways of Steatohepatitis consortium, the Horizon 2020 Framework Program of the European Union, and the Newcastle NIHR Biomedical Research Centre. The researchers reported having no conflicts of interest.
SOURCE: Haigh L et al. Clin Gastroenterol Hepatol. 2018 Oct 31. doi: 10.1016/j.cgh.2018.10.044.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
PsA patients had durable responses after 1 year of IV golimumab treatment
, according to follow-up results of a randomized clinical trial.
The improvements in joint disease, skin disease, and health-related quality of life seen at 24 weeks in the phase 3 GO-VIBRANT study were maintained at this 52-week follow-up, according to M. Elaine Husni, MD, of the Cleveland Clinic, and coinvestigators.
Patients who crossed over to golimumab treatment after 24 weeks of placebo had similar rates of clinical response at 52 weeks, while patients receiving concomitant methotrexate had similar ACR response rates, compared with patients on golimumab monotherapy, Dr. Husni and colleagues reported.
Many patients who were not ACR20 responders at week 52 nevertheless had improvements in skin disease, enthesitis, and dactylitis, an exploratory analysis showed.
“These factors may have contributed to these patients remaining in the trial and continuing golimumab therapy despite not achieving an ACR20 response,” wrote Dr. Husni and coauthors. The report is in Arthritis Care & Research.
The Food and Drug Administration approved a once-monthly subcutaneous formulation of golimumab (Simponi) in 2009 for treatment of moderate to severe active psoriatic arthritis, rheumatoid arthritis, and active ankylosing spondylitis. The intravenous formulation of this TNF inhibitor (Simponi Aria) received a psoriatic arthritis indication in 2017 based on GO-VIBRANT data. Published results at the time showed that compared with placebo, intravenous golimumab given as a 2-mg/kg infusion at weeks 0, 4, and then every 8 weeks produced greater improvements in psoriatic arthritis signs and symptoms and less radiographic progression through week 24 of the study, and had adverse events consistent with other TNF inhibitors, according to investigators.
The follow-up report includes efficacy and safety data for golimumab-treated patients beyond 24 weeks, as well as data for patients on the placebo arm, who crossed over to receive golimumab at week 24, week 28, and then every 8 weeks thereafter.
The results show ACR response rates were maintained from week 24 to 52 in golimumab-treated patients, and were similar in the placebo crossover patients. The ACR20, ACR50, and ACR70 response rates in the golimumab group were 76.8%, 58.1%, and 38.6%, respectively, while in the crossover group, they were 77.0%, 53.6%, and 33.9%, respectively.
Radiographic progression was measured using van der Heijde-Sharp (vdH-S) score with modifications for psoriatic arthritis. The mean change in vdH-S score at 24 weeks was –0.4 and 2.0 in the golimumab and placebo groups, respectively; by week 52, the mean change was –0.5 and 0.8 for golimumab and placebo crossover.
Infection was the most common adverse event throughout 60 weeks of safety evaluation, occurring in 22.8% of all golimumab-treated patients, investigators said. Four infusion reactions occurred following golimumab administration, though none were considered serious or severe.
The GO-VIBRANT study, which comprised 480 adults, had limited follow-up and was not powered to identify rare safety events, investigators said.
“However, the totality of results through 1 year of the GO-VIBRANT study show a durable response to IV golimumab 2 mg/kg across several clinical efficacy, HRQoL, and radiographic endpoints with no new safety signals,” they concluded.
Study authors reported disclosures with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Horizon, Janssen, Novartis, Pfizer, Sanofi, and UCB. Several study authors reported current or former employment with Janssen Research & Development and stock or stock options in Johnson & Johnson.
SOURCE: Husni ME et al. Arthritis Care Res. 2019 Apr 12. doi: 10.1002/acr.23905.
, according to follow-up results of a randomized clinical trial.
The improvements in joint disease, skin disease, and health-related quality of life seen at 24 weeks in the phase 3 GO-VIBRANT study were maintained at this 52-week follow-up, according to M. Elaine Husni, MD, of the Cleveland Clinic, and coinvestigators.
Patients who crossed over to golimumab treatment after 24 weeks of placebo had similar rates of clinical response at 52 weeks, while patients receiving concomitant methotrexate had similar ACR response rates, compared with patients on golimumab monotherapy, Dr. Husni and colleagues reported.
Many patients who were not ACR20 responders at week 52 nevertheless had improvements in skin disease, enthesitis, and dactylitis, an exploratory analysis showed.
“These factors may have contributed to these patients remaining in the trial and continuing golimumab therapy despite not achieving an ACR20 response,” wrote Dr. Husni and coauthors. The report is in Arthritis Care & Research.
The Food and Drug Administration approved a once-monthly subcutaneous formulation of golimumab (Simponi) in 2009 for treatment of moderate to severe active psoriatic arthritis, rheumatoid arthritis, and active ankylosing spondylitis. The intravenous formulation of this TNF inhibitor (Simponi Aria) received a psoriatic arthritis indication in 2017 based on GO-VIBRANT data. Published results at the time showed that compared with placebo, intravenous golimumab given as a 2-mg/kg infusion at weeks 0, 4, and then every 8 weeks produced greater improvements in psoriatic arthritis signs and symptoms and less radiographic progression through week 24 of the study, and had adverse events consistent with other TNF inhibitors, according to investigators.
The follow-up report includes efficacy and safety data for golimumab-treated patients beyond 24 weeks, as well as data for patients on the placebo arm, who crossed over to receive golimumab at week 24, week 28, and then every 8 weeks thereafter.
The results show ACR response rates were maintained from week 24 to 52 in golimumab-treated patients, and were similar in the placebo crossover patients. The ACR20, ACR50, and ACR70 response rates in the golimumab group were 76.8%, 58.1%, and 38.6%, respectively, while in the crossover group, they were 77.0%, 53.6%, and 33.9%, respectively.
Radiographic progression was measured using van der Heijde-Sharp (vdH-S) score with modifications for psoriatic arthritis. The mean change in vdH-S score at 24 weeks was –0.4 and 2.0 in the golimumab and placebo groups, respectively; by week 52, the mean change was –0.5 and 0.8 for golimumab and placebo crossover.
Infection was the most common adverse event throughout 60 weeks of safety evaluation, occurring in 22.8% of all golimumab-treated patients, investigators said. Four infusion reactions occurred following golimumab administration, though none were considered serious or severe.
The GO-VIBRANT study, which comprised 480 adults, had limited follow-up and was not powered to identify rare safety events, investigators said.
“However, the totality of results through 1 year of the GO-VIBRANT study show a durable response to IV golimumab 2 mg/kg across several clinical efficacy, HRQoL, and radiographic endpoints with no new safety signals,” they concluded.
Study authors reported disclosures with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Horizon, Janssen, Novartis, Pfizer, Sanofi, and UCB. Several study authors reported current or former employment with Janssen Research & Development and stock or stock options in Johnson & Johnson.
SOURCE: Husni ME et al. Arthritis Care Res. 2019 Apr 12. doi: 10.1002/acr.23905.
, according to follow-up results of a randomized clinical trial.
The improvements in joint disease, skin disease, and health-related quality of life seen at 24 weeks in the phase 3 GO-VIBRANT study were maintained at this 52-week follow-up, according to M. Elaine Husni, MD, of the Cleveland Clinic, and coinvestigators.
Patients who crossed over to golimumab treatment after 24 weeks of placebo had similar rates of clinical response at 52 weeks, while patients receiving concomitant methotrexate had similar ACR response rates, compared with patients on golimumab monotherapy, Dr. Husni and colleagues reported.
Many patients who were not ACR20 responders at week 52 nevertheless had improvements in skin disease, enthesitis, and dactylitis, an exploratory analysis showed.
“These factors may have contributed to these patients remaining in the trial and continuing golimumab therapy despite not achieving an ACR20 response,” wrote Dr. Husni and coauthors. The report is in Arthritis Care & Research.
The Food and Drug Administration approved a once-monthly subcutaneous formulation of golimumab (Simponi) in 2009 for treatment of moderate to severe active psoriatic arthritis, rheumatoid arthritis, and active ankylosing spondylitis. The intravenous formulation of this TNF inhibitor (Simponi Aria) received a psoriatic arthritis indication in 2017 based on GO-VIBRANT data. Published results at the time showed that compared with placebo, intravenous golimumab given as a 2-mg/kg infusion at weeks 0, 4, and then every 8 weeks produced greater improvements in psoriatic arthritis signs and symptoms and less radiographic progression through week 24 of the study, and had adverse events consistent with other TNF inhibitors, according to investigators.
The follow-up report includes efficacy and safety data for golimumab-treated patients beyond 24 weeks, as well as data for patients on the placebo arm, who crossed over to receive golimumab at week 24, week 28, and then every 8 weeks thereafter.
The results show ACR response rates were maintained from week 24 to 52 in golimumab-treated patients, and were similar in the placebo crossover patients. The ACR20, ACR50, and ACR70 response rates in the golimumab group were 76.8%, 58.1%, and 38.6%, respectively, while in the crossover group, they were 77.0%, 53.6%, and 33.9%, respectively.
Radiographic progression was measured using van der Heijde-Sharp (vdH-S) score with modifications for psoriatic arthritis. The mean change in vdH-S score at 24 weeks was –0.4 and 2.0 in the golimumab and placebo groups, respectively; by week 52, the mean change was –0.5 and 0.8 for golimumab and placebo crossover.
Infection was the most common adverse event throughout 60 weeks of safety evaluation, occurring in 22.8% of all golimumab-treated patients, investigators said. Four infusion reactions occurred following golimumab administration, though none were considered serious or severe.
The GO-VIBRANT study, which comprised 480 adults, had limited follow-up and was not powered to identify rare safety events, investigators said.
“However, the totality of results through 1 year of the GO-VIBRANT study show a durable response to IV golimumab 2 mg/kg across several clinical efficacy, HRQoL, and radiographic endpoints with no new safety signals,” they concluded.
Study authors reported disclosures with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Horizon, Janssen, Novartis, Pfizer, Sanofi, and UCB. Several study authors reported current or former employment with Janssen Research & Development and stock or stock options in Johnson & Johnson.
SOURCE: Husni ME et al. Arthritis Care Res. 2019 Apr 12. doi: 10.1002/acr.23905.
FROM ARTHRITIS CARE & RESEARCH
Zika knowledge, preparedness low among U.S. pediatricians
BALTIMORE – U.S. pediatricians feel comfortable providing patients with preventive information and travel advice related to Zika, but few feel prepared when it comes to testing and management of infants exposed prenatally to Zika infections, a study found.
“Areas where pediatricians were less likely to report preparedness included recommending testing, providing data to the Centers for Disease Control and Prevention’s Zika Pregnancy Registry, managing infants exposed to Zika prenatally, and informing parents of social services for Zika-infected infants,” senior author Amy J. Houtrow, MD, MPH, PhD, and colleagues reported at the Pediatric Academic Societies annual meeting.
“Results indicate that additional education efforts are needed to grow the overall Zika knowledge of pediatricians and boost preparedness, particularly around recommending Zika testing and providing data to CDC,” they concluded.
But these findings are not surprising given how rare congenital Zika virus syndrome is, explained Dr. Houtrow, an associate professor of physical medicine and rehabilitation and pediatrics at the University of Pittsburgh.
“For most rare conditions, pediatricians report better general than specific knowledge,” Dr. Houtrow said in an interview. “We expect pediatricians have a broad range of knowledge for a multitude of conditions and to be well versed in the care of infants and children with common conditions, coupled with the ability to access knowledge and expertise about rarer conditions such as congenital Zika syndrome.”
Dr. Houtrow and associates drew their findings from the 2018 AAP Periodic Survey of Fellows, which includes both primary care physicians and neonatologists. The survey’s response rate was 42%, with 672 of 1,599 surveys returned, but the researchers limited their analysis to 576 postresidency respondents who were providing direct patient care.
Overall, 39% of physicians reported being knowledgeable about Zika virus, and 47% said they wanted to learn more. More than half of responding doctors (57%) reported feeling moderately or very prepared when it came to informing patients of preventive measures to reduce risk of Zika infection, and nearly half (49%) felt confident about giving patients travel advice.
However, physicians’ preparedness gradually dropped for clinical situations requiring more direct experience with Zika. For example, 37% felt moderately or very prepared to provide clinical referrals for infant patients with an infection, and 33% felt prepared to talk with pregnant women about the risks of birth defects from Zika infection.
Just one in five physicians (22%) felt prepared for recommending Zika virus testing, and 16% felt prepared about providing data to the CDC’s U.S. Zika Pregnancy Registry or managing infants who had been prenatally exposed to Zika infection. Only 15% felt they had the preparedness to tell parents about social services for Zika-affected infants.
Preparedness did not differ by gender, specialty, practice setting, hours worked per week, or population density (urban, rural and suburban). However, differences did appear based on respondents’ age and U.S. region.
Older doctors reported greater knowledge about Zika than younger doctors. Compared with those aged 39 years or younger, those aged 40-49 and 50-59 reported feeling more knowledgeable (adjusted odds ratio, 1.74 and 1.72, respectively; P less than .05). The odds of feeling more knowledgeable was nearly triple among those aged at least 60 years, compared with those under 40 (aOR, 2.92; P less than .001).
Those practicing in the Northeast United States (aOR, 2.19; P less than .01) and in the South (aOR, 1.74; P less than .05) also reported feeling more knowledgeable than those in the West or Midwest.
“This makes sense because infants with a history of prenatal exposure to the Zika Virus are more likely to be seen in practices with more immigrants from the Caribbean and Latin America,” Dr. Houtrow said in an interview.
“ but the urgency of the need for education about Zika virus has diminished because the rates of new congenital Zika syndrome have dropped,” she continued.
Study limitations include the inability to generalize the findings beyond U.S. members of the AAP and the possibility that nonrespondents differed from respondents in terms of Zika knowledge and preparedness.
The research was funded by the AAP and CDC.
BALTIMORE – U.S. pediatricians feel comfortable providing patients with preventive information and travel advice related to Zika, but few feel prepared when it comes to testing and management of infants exposed prenatally to Zika infections, a study found.
“Areas where pediatricians were less likely to report preparedness included recommending testing, providing data to the Centers for Disease Control and Prevention’s Zika Pregnancy Registry, managing infants exposed to Zika prenatally, and informing parents of social services for Zika-infected infants,” senior author Amy J. Houtrow, MD, MPH, PhD, and colleagues reported at the Pediatric Academic Societies annual meeting.
“Results indicate that additional education efforts are needed to grow the overall Zika knowledge of pediatricians and boost preparedness, particularly around recommending Zika testing and providing data to CDC,” they concluded.
But these findings are not surprising given how rare congenital Zika virus syndrome is, explained Dr. Houtrow, an associate professor of physical medicine and rehabilitation and pediatrics at the University of Pittsburgh.
“For most rare conditions, pediatricians report better general than specific knowledge,” Dr. Houtrow said in an interview. “We expect pediatricians have a broad range of knowledge for a multitude of conditions and to be well versed in the care of infants and children with common conditions, coupled with the ability to access knowledge and expertise about rarer conditions such as congenital Zika syndrome.”
Dr. Houtrow and associates drew their findings from the 2018 AAP Periodic Survey of Fellows, which includes both primary care physicians and neonatologists. The survey’s response rate was 42%, with 672 of 1,599 surveys returned, but the researchers limited their analysis to 576 postresidency respondents who were providing direct patient care.
Overall, 39% of physicians reported being knowledgeable about Zika virus, and 47% said they wanted to learn more. More than half of responding doctors (57%) reported feeling moderately or very prepared when it came to informing patients of preventive measures to reduce risk of Zika infection, and nearly half (49%) felt confident about giving patients travel advice.
However, physicians’ preparedness gradually dropped for clinical situations requiring more direct experience with Zika. For example, 37% felt moderately or very prepared to provide clinical referrals for infant patients with an infection, and 33% felt prepared to talk with pregnant women about the risks of birth defects from Zika infection.
Just one in five physicians (22%) felt prepared for recommending Zika virus testing, and 16% felt prepared about providing data to the CDC’s U.S. Zika Pregnancy Registry or managing infants who had been prenatally exposed to Zika infection. Only 15% felt they had the preparedness to tell parents about social services for Zika-affected infants.
Preparedness did not differ by gender, specialty, practice setting, hours worked per week, or population density (urban, rural and suburban). However, differences did appear based on respondents’ age and U.S. region.
Older doctors reported greater knowledge about Zika than younger doctors. Compared with those aged 39 years or younger, those aged 40-49 and 50-59 reported feeling more knowledgeable (adjusted odds ratio, 1.74 and 1.72, respectively; P less than .05). The odds of feeling more knowledgeable was nearly triple among those aged at least 60 years, compared with those under 40 (aOR, 2.92; P less than .001).
Those practicing in the Northeast United States (aOR, 2.19; P less than .01) and in the South (aOR, 1.74; P less than .05) also reported feeling more knowledgeable than those in the West or Midwest.
“This makes sense because infants with a history of prenatal exposure to the Zika Virus are more likely to be seen in practices with more immigrants from the Caribbean and Latin America,” Dr. Houtrow said in an interview.
“ but the urgency of the need for education about Zika virus has diminished because the rates of new congenital Zika syndrome have dropped,” she continued.
Study limitations include the inability to generalize the findings beyond U.S. members of the AAP and the possibility that nonrespondents differed from respondents in terms of Zika knowledge and preparedness.
The research was funded by the AAP and CDC.
BALTIMORE – U.S. pediatricians feel comfortable providing patients with preventive information and travel advice related to Zika, but few feel prepared when it comes to testing and management of infants exposed prenatally to Zika infections, a study found.
“Areas where pediatricians were less likely to report preparedness included recommending testing, providing data to the Centers for Disease Control and Prevention’s Zika Pregnancy Registry, managing infants exposed to Zika prenatally, and informing parents of social services for Zika-infected infants,” senior author Amy J. Houtrow, MD, MPH, PhD, and colleagues reported at the Pediatric Academic Societies annual meeting.
“Results indicate that additional education efforts are needed to grow the overall Zika knowledge of pediatricians and boost preparedness, particularly around recommending Zika testing and providing data to CDC,” they concluded.
But these findings are not surprising given how rare congenital Zika virus syndrome is, explained Dr. Houtrow, an associate professor of physical medicine and rehabilitation and pediatrics at the University of Pittsburgh.
“For most rare conditions, pediatricians report better general than specific knowledge,” Dr. Houtrow said in an interview. “We expect pediatricians have a broad range of knowledge for a multitude of conditions and to be well versed in the care of infants and children with common conditions, coupled with the ability to access knowledge and expertise about rarer conditions such as congenital Zika syndrome.”
Dr. Houtrow and associates drew their findings from the 2018 AAP Periodic Survey of Fellows, which includes both primary care physicians and neonatologists. The survey’s response rate was 42%, with 672 of 1,599 surveys returned, but the researchers limited their analysis to 576 postresidency respondents who were providing direct patient care.
Overall, 39% of physicians reported being knowledgeable about Zika virus, and 47% said they wanted to learn more. More than half of responding doctors (57%) reported feeling moderately or very prepared when it came to informing patients of preventive measures to reduce risk of Zika infection, and nearly half (49%) felt confident about giving patients travel advice.
However, physicians’ preparedness gradually dropped for clinical situations requiring more direct experience with Zika. For example, 37% felt moderately or very prepared to provide clinical referrals for infant patients with an infection, and 33% felt prepared to talk with pregnant women about the risks of birth defects from Zika infection.
Just one in five physicians (22%) felt prepared for recommending Zika virus testing, and 16% felt prepared about providing data to the CDC’s U.S. Zika Pregnancy Registry or managing infants who had been prenatally exposed to Zika infection. Only 15% felt they had the preparedness to tell parents about social services for Zika-affected infants.
Preparedness did not differ by gender, specialty, practice setting, hours worked per week, or population density (urban, rural and suburban). However, differences did appear based on respondents’ age and U.S. region.
Older doctors reported greater knowledge about Zika than younger doctors. Compared with those aged 39 years or younger, those aged 40-49 and 50-59 reported feeling more knowledgeable (adjusted odds ratio, 1.74 and 1.72, respectively; P less than .05). The odds of feeling more knowledgeable was nearly triple among those aged at least 60 years, compared with those under 40 (aOR, 2.92; P less than .001).
Those practicing in the Northeast United States (aOR, 2.19; P less than .01) and in the South (aOR, 1.74; P less than .05) also reported feeling more knowledgeable than those in the West or Midwest.
“This makes sense because infants with a history of prenatal exposure to the Zika Virus are more likely to be seen in practices with more immigrants from the Caribbean and Latin America,” Dr. Houtrow said in an interview.
“ but the urgency of the need for education about Zika virus has diminished because the rates of new congenital Zika syndrome have dropped,” she continued.
Study limitations include the inability to generalize the findings beyond U.S. members of the AAP and the possibility that nonrespondents differed from respondents in terms of Zika knowledge and preparedness.
The research was funded by the AAP and CDC.
REPORTING FROM PAS 2019
Fecal microbiota transplant shows promise for hepatic encephalopathy
VIENNA –
The oral fecal microbiota transplant (FMT), modeled on guideline-directed treatment for Clostridium difficile (Clin Infect Dis. 2018 April 1;66[7]:e1-48), was linked with a cut in hospitalizations and serious adverse events, as well as a clinically meaningful improvement in a cognitive measure specific for hepatic encephalopathy, Jasmohan S. Bajaj, MD, said at the meeting sponsored by the European Association for the Study of the Liver. Given the preliminary scope of the study, the next step is to assess the treatment in more patients and to evaluate delivery of the FMT specifically to the upper or lower gastrointestinal tract, said Dr. Bajaj, a hepatologist at Virginia Commonwealth University and McGuire VA Medical Center, both in Richmond.
The study included 20 patients with recurrent hepatic encephalopathy (RHE) and a history of at least two encephalopathy episodes despite treatment with lactulose and rifaximin (Xifaxan). After a baseline assessment, 10 patients received a single, oral dose of FMT contained in 15 capsules and composed of fecal material from the OpenBiome collection, and 10 patients received placebo capsules. All of the FMT material came from a single donor and contained a high level of beneficial microbial types, specifically Lachnospiraceae and Ruminococcaceae species. Patients averaged 64 years of age.
During 5 months of follow-up, 6 of the 10 placebo patients had a serious adverse event versus 1 of the 10 patients treated with an active FMT; altogether, there were 11 serious adverse events among the placebo patients versus only 1 event among the FMT patients, Dr. Bajaj reported. Three patients in the control arm had a total of seven hepatic encephalopathy events, compared with a single patient with one event in the intervention arm.
Enrolled patients also underwent two cognitive tests at baseline and during follow-up. Using a Stroop smartphone app (EncephalApp) designed to assess patients with RHE (Hepatology. 2013 Sept;58[3]:1122-32), the researchers found an average 51-second improvement in OffTime+OnTime, a statistically significant and clinically meaningful improvement in the patients treated with FMT, whereas the control patients showed no statistically significant change in this parameter. The second cognitive measure was the average performance by patients using the Psychometric Hepatic Encephalopathy Score (Curr Gastroenterol Rep. 2014 Jan;16[1]:362), which showed no significant change after treatment in either study arm. The actively treated patients also showed favorable changes in the microbial composition of their stool and mucosa, as well as an enhanced small intestinal barrier, following treatment, Dr. Bajaj said.
SOURCE: Bajaj JS et al. J Hepatol. 2019 April;70[1]:e55.
VIENNA –
The oral fecal microbiota transplant (FMT), modeled on guideline-directed treatment for Clostridium difficile (Clin Infect Dis. 2018 April 1;66[7]:e1-48), was linked with a cut in hospitalizations and serious adverse events, as well as a clinically meaningful improvement in a cognitive measure specific for hepatic encephalopathy, Jasmohan S. Bajaj, MD, said at the meeting sponsored by the European Association for the Study of the Liver. Given the preliminary scope of the study, the next step is to assess the treatment in more patients and to evaluate delivery of the FMT specifically to the upper or lower gastrointestinal tract, said Dr. Bajaj, a hepatologist at Virginia Commonwealth University and McGuire VA Medical Center, both in Richmond.
The study included 20 patients with recurrent hepatic encephalopathy (RHE) and a history of at least two encephalopathy episodes despite treatment with lactulose and rifaximin (Xifaxan). After a baseline assessment, 10 patients received a single, oral dose of FMT contained in 15 capsules and composed of fecal material from the OpenBiome collection, and 10 patients received placebo capsules. All of the FMT material came from a single donor and contained a high level of beneficial microbial types, specifically Lachnospiraceae and Ruminococcaceae species. Patients averaged 64 years of age.
During 5 months of follow-up, 6 of the 10 placebo patients had a serious adverse event versus 1 of the 10 patients treated with an active FMT; altogether, there were 11 serious adverse events among the placebo patients versus only 1 event among the FMT patients, Dr. Bajaj reported. Three patients in the control arm had a total of seven hepatic encephalopathy events, compared with a single patient with one event in the intervention arm.
Enrolled patients also underwent two cognitive tests at baseline and during follow-up. Using a Stroop smartphone app (EncephalApp) designed to assess patients with RHE (Hepatology. 2013 Sept;58[3]:1122-32), the researchers found an average 51-second improvement in OffTime+OnTime, a statistically significant and clinically meaningful improvement in the patients treated with FMT, whereas the control patients showed no statistically significant change in this parameter. The second cognitive measure was the average performance by patients using the Psychometric Hepatic Encephalopathy Score (Curr Gastroenterol Rep. 2014 Jan;16[1]:362), which showed no significant change after treatment in either study arm. The actively treated patients also showed favorable changes in the microbial composition of their stool and mucosa, as well as an enhanced small intestinal barrier, following treatment, Dr. Bajaj said.
SOURCE: Bajaj JS et al. J Hepatol. 2019 April;70[1]:e55.
VIENNA –
The oral fecal microbiota transplant (FMT), modeled on guideline-directed treatment for Clostridium difficile (Clin Infect Dis. 2018 April 1;66[7]:e1-48), was linked with a cut in hospitalizations and serious adverse events, as well as a clinically meaningful improvement in a cognitive measure specific for hepatic encephalopathy, Jasmohan S. Bajaj, MD, said at the meeting sponsored by the European Association for the Study of the Liver. Given the preliminary scope of the study, the next step is to assess the treatment in more patients and to evaluate delivery of the FMT specifically to the upper or lower gastrointestinal tract, said Dr. Bajaj, a hepatologist at Virginia Commonwealth University and McGuire VA Medical Center, both in Richmond.
The study included 20 patients with recurrent hepatic encephalopathy (RHE) and a history of at least two encephalopathy episodes despite treatment with lactulose and rifaximin (Xifaxan). After a baseline assessment, 10 patients received a single, oral dose of FMT contained in 15 capsules and composed of fecal material from the OpenBiome collection, and 10 patients received placebo capsules. All of the FMT material came from a single donor and contained a high level of beneficial microbial types, specifically Lachnospiraceae and Ruminococcaceae species. Patients averaged 64 years of age.
During 5 months of follow-up, 6 of the 10 placebo patients had a serious adverse event versus 1 of the 10 patients treated with an active FMT; altogether, there were 11 serious adverse events among the placebo patients versus only 1 event among the FMT patients, Dr. Bajaj reported. Three patients in the control arm had a total of seven hepatic encephalopathy events, compared with a single patient with one event in the intervention arm.
Enrolled patients also underwent two cognitive tests at baseline and during follow-up. Using a Stroop smartphone app (EncephalApp) designed to assess patients with RHE (Hepatology. 2013 Sept;58[3]:1122-32), the researchers found an average 51-second improvement in OffTime+OnTime, a statistically significant and clinically meaningful improvement in the patients treated with FMT, whereas the control patients showed no statistically significant change in this parameter. The second cognitive measure was the average performance by patients using the Psychometric Hepatic Encephalopathy Score (Curr Gastroenterol Rep. 2014 Jan;16[1]:362), which showed no significant change after treatment in either study arm. The actively treated patients also showed favorable changes in the microbial composition of their stool and mucosa, as well as an enhanced small intestinal barrier, following treatment, Dr. Bajaj said.
SOURCE: Bajaj JS et al. J Hepatol. 2019 April;70[1]:e55.
REPORTING FROM ILC 2019
Vaginal brachytherapy more toxic than pelvic RT in endometrial carcinoma
, according to results from a phase 3 trial.
The GOG-0249 study comprised 601 patients with high-intermediate– and high-risk early-stage endometrial carcinoma who were randomized in a 1:1 fashion to receive vaginal cuff brachytherapy plus paclitaxel and carboplatin or pelvic RT every 21 days for a total of three cycles.
Those in the brachytherapy group received treatment at both high and low-dosing rates. Paclitaxel was given at a dose of 175 mg/m2 infused over 3 hours, succeeded by carboplatin (area under the curve, 6) infused over 45 minutes. Pelvic RT was provided at a dose of 45 to 50.4 Gy over a period of 5-6 weeks.
“The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy increases recurrence-free survival (RFS) compared with pelvic RT,” wrote Marcus E. Randall, MD, of the University of Kentucky, Lexington, and colleagues. The report is in the Journal of Clinical Oncology.
Additional outcomes measured were overall survival and acute toxicity.
After analysis, the researchers found that vaginal brachytherapy plus chemotherapy did not show superiority over pelvic RT in terms of 60-month RFS (hazard ratio, 0.92; 90% confidence limit, 0.69-1.23). Also, there was no significant difference for overall survival (HR, 1.04; 90% confidence limit, 0.71-1.52).
With respect to safety, acute adverse events were more common and severe in the vaginal brachytherapy plus chemotherapy group. However, “differences in late toxicity were minimal,” Dr. Randall and colleagues reported.
“Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma,” they wrote. “Novel combinations, dose intensification, and additional translational research represent potential paths forward.”
The study was supported by grant funding from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center. The authors reported financial affiliations with AstraZeneca, Genentech, Genmab, Janssen, Johnson & Johnson, Tesaro, and several others.
SOURCE: Randall ME et al. J Clin Oncol. 2019 Apr 17. doi: 10.1200/JCO.18.01575.
, according to results from a phase 3 trial.
The GOG-0249 study comprised 601 patients with high-intermediate– and high-risk early-stage endometrial carcinoma who were randomized in a 1:1 fashion to receive vaginal cuff brachytherapy plus paclitaxel and carboplatin or pelvic RT every 21 days for a total of three cycles.
Those in the brachytherapy group received treatment at both high and low-dosing rates. Paclitaxel was given at a dose of 175 mg/m2 infused over 3 hours, succeeded by carboplatin (area under the curve, 6) infused over 45 minutes. Pelvic RT was provided at a dose of 45 to 50.4 Gy over a period of 5-6 weeks.
“The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy increases recurrence-free survival (RFS) compared with pelvic RT,” wrote Marcus E. Randall, MD, of the University of Kentucky, Lexington, and colleagues. The report is in the Journal of Clinical Oncology.
Additional outcomes measured were overall survival and acute toxicity.
After analysis, the researchers found that vaginal brachytherapy plus chemotherapy did not show superiority over pelvic RT in terms of 60-month RFS (hazard ratio, 0.92; 90% confidence limit, 0.69-1.23). Also, there was no significant difference for overall survival (HR, 1.04; 90% confidence limit, 0.71-1.52).
With respect to safety, acute adverse events were more common and severe in the vaginal brachytherapy plus chemotherapy group. However, “differences in late toxicity were minimal,” Dr. Randall and colleagues reported.
“Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma,” they wrote. “Novel combinations, dose intensification, and additional translational research represent potential paths forward.”
The study was supported by grant funding from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center. The authors reported financial affiliations with AstraZeneca, Genentech, Genmab, Janssen, Johnson & Johnson, Tesaro, and several others.
SOURCE: Randall ME et al. J Clin Oncol. 2019 Apr 17. doi: 10.1200/JCO.18.01575.
, according to results from a phase 3 trial.
The GOG-0249 study comprised 601 patients with high-intermediate– and high-risk early-stage endometrial carcinoma who were randomized in a 1:1 fashion to receive vaginal cuff brachytherapy plus paclitaxel and carboplatin or pelvic RT every 21 days for a total of three cycles.
Those in the brachytherapy group received treatment at both high and low-dosing rates. Paclitaxel was given at a dose of 175 mg/m2 infused over 3 hours, succeeded by carboplatin (area under the curve, 6) infused over 45 minutes. Pelvic RT was provided at a dose of 45 to 50.4 Gy over a period of 5-6 weeks.
“The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy increases recurrence-free survival (RFS) compared with pelvic RT,” wrote Marcus E. Randall, MD, of the University of Kentucky, Lexington, and colleagues. The report is in the Journal of Clinical Oncology.
Additional outcomes measured were overall survival and acute toxicity.
After analysis, the researchers found that vaginal brachytherapy plus chemotherapy did not show superiority over pelvic RT in terms of 60-month RFS (hazard ratio, 0.92; 90% confidence limit, 0.69-1.23). Also, there was no significant difference for overall survival (HR, 1.04; 90% confidence limit, 0.71-1.52).
With respect to safety, acute adverse events were more common and severe in the vaginal brachytherapy plus chemotherapy group. However, “differences in late toxicity were minimal,” Dr. Randall and colleagues reported.
“Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma,” they wrote. “Novel combinations, dose intensification, and additional translational research represent potential paths forward.”
The study was supported by grant funding from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center. The authors reported financial affiliations with AstraZeneca, Genentech, Genmab, Janssen, Johnson & Johnson, Tesaro, and several others.
SOURCE: Randall ME et al. J Clin Oncol. 2019 Apr 17. doi: 10.1200/JCO.18.01575.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Damage of the Lateral Geniculate Nucleus in MS
Key clinical point: Lateral geniculate nucleus (LGN) volume loss in multiple sclerosis (MS) indicates structural damage with potential functional relevance.
Major finding: LGN volume was reduced in patients with relapsing-remitting MS vs healthy controls and was associated with ganglion cell-inner plexiform layer (GC-IPL) thickness and correlated with optic radiation (OR) lesion volume.
Study details: A cross-sectional study of 34 patients with relapsing-remitting MS and 33 matched healthy controls.
Disclosures: The lead author received funding for speaker or travel honoraria from Sanofi-Genzyme, Bayer AG, Teva, UCB-Pharma AG, and Hoffmann-La Roche.
Citation: Papadopoulou, et al. Neurology. doi:10.1212/WNL.0000000000007450.
Key clinical point: Lateral geniculate nucleus (LGN) volume loss in multiple sclerosis (MS) indicates structural damage with potential functional relevance.
Major finding: LGN volume was reduced in patients with relapsing-remitting MS vs healthy controls and was associated with ganglion cell-inner plexiform layer (GC-IPL) thickness and correlated with optic radiation (OR) lesion volume.
Study details: A cross-sectional study of 34 patients with relapsing-remitting MS and 33 matched healthy controls.
Disclosures: The lead author received funding for speaker or travel honoraria from Sanofi-Genzyme, Bayer AG, Teva, UCB-Pharma AG, and Hoffmann-La Roche.
Citation: Papadopoulou, et al. Neurology. doi:10.1212/WNL.0000000000007450.
Key clinical point: Lateral geniculate nucleus (LGN) volume loss in multiple sclerosis (MS) indicates structural damage with potential functional relevance.
Major finding: LGN volume was reduced in patients with relapsing-remitting MS vs healthy controls and was associated with ganglion cell-inner plexiform layer (GC-IPL) thickness and correlated with optic radiation (OR) lesion volume.
Study details: A cross-sectional study of 34 patients with relapsing-remitting MS and 33 matched healthy controls.
Disclosures: The lead author received funding for speaker or travel honoraria from Sanofi-Genzyme, Bayer AG, Teva, UCB-Pharma AG, and Hoffmann-La Roche.
Citation: Papadopoulou, et al. Neurology. doi:10.1212/WNL.0000000000007450.
Survey of MS Patients Reveals Pregnancy-Related Concerns
Key clinical point: Patients with multiple sclerosis report a wide range of concerns about family planning and pregnancy.
Major finding: Of the 137 respondents who did not become pregnant following diagnosis, 22 (16%) indicated that their decision was driven by multiple sclerosis–related concerns, including MS worsening with pregnancy (64%).
Study details: A survey of 174 women with confirmed MS diagnosis who received care at the University of Virginia Medical Center.
Disclosures: The study was supported by the ziMS Foundation.
Citation: Engel CE et al. ACTRIMS Forum 2019, Poster 307.
Key clinical point: Patients with multiple sclerosis report a wide range of concerns about family planning and pregnancy.
Major finding: Of the 137 respondents who did not become pregnant following diagnosis, 22 (16%) indicated that their decision was driven by multiple sclerosis–related concerns, including MS worsening with pregnancy (64%).
Study details: A survey of 174 women with confirmed MS diagnosis who received care at the University of Virginia Medical Center.
Disclosures: The study was supported by the ziMS Foundation.
Citation: Engel CE et al. ACTRIMS Forum 2019, Poster 307.
Key clinical point: Patients with multiple sclerosis report a wide range of concerns about family planning and pregnancy.
Major finding: Of the 137 respondents who did not become pregnant following diagnosis, 22 (16%) indicated that their decision was driven by multiple sclerosis–related concerns, including MS worsening with pregnancy (64%).
Study details: A survey of 174 women with confirmed MS diagnosis who received care at the University of Virginia Medical Center.
Disclosures: The study was supported by the ziMS Foundation.
Citation: Engel CE et al. ACTRIMS Forum 2019, Poster 307.