User login
Things We Do For No Reason: Failing to Question a Penicillin Allergy History
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
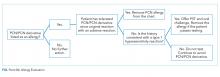
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
©2019 Society of Hospital Medicine
Genetic signature helps ID MS risk
Also today, the CDC has a plan to cut undiagnosed and untreated HIV, which patients who have diabetes benefit the most from long-term metformin, and why amlodopine may be the best choice for lowering blood pressure in black patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, the CDC has a plan to cut undiagnosed and untreated HIV, which patients who have diabetes benefit the most from long-term metformin, and why amlodopine may be the best choice for lowering blood pressure in black patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, the CDC has a plan to cut undiagnosed and untreated HIV, which patients who have diabetes benefit the most from long-term metformin, and why amlodopine may be the best choice for lowering blood pressure in black patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Study Provides Insight Into Alcohol’s Effects on the Brain
The findings could lead the way to understanding the brain’s intake and output of energy in good health and bad and the part that alcohol plays.
In previous studies, the researchers have shown that alcohol significantly affects brain glucose metabolism, a measure of energy use, as well as regional brain activity, assessed through changes in blood oxygenation. But regional differences in glucose metabolism are hard to interpret, they say. In a study with healthy volunteers, they used brain imaging techniques to help quantify “match and mismatch” in energy consumption and expenditure across the brain—what they termed power and cost.
The researchers assessed power by observing to what extent brain regions are active and use energy, and cost by observing how brain regions expended energy. They found that different brain regions that serve distinct functions have “notably different power and different cost.”
Next, they tested a group of light drinkers and heavy drinkers and found both acute and chronic exposure to alcohol affected power and cost. In heavy drinkers, the researchers say, they saw less regional power, for example, in the thalamus, the sensory gateway, and frontal cortex. The researchers interpreted the decreases in power as reflecting the toxic effects of long-term exposure to alcohol on the brain cells.
They also found power dropped in the visual regions during acute alcohol exposure, which was related to disruption of visual processing. Visual regions also had the most significant drops in cost of activity during intoxication. That is consistent with the reliance of those regions on alternative energy sources, such as acetate (a byproduct of alcohol metabolism), the researchers say.
Their approach for characterizing brain energetic patterns related to alcohol use could be useful in other ways, the researchers say. “Studying energetic signatures of brain regions in different neuropsychiatric diseases is an important future direction,” said co-lead investigator Dr. Ehsan Schokri-Kojori. “The measures of power and cost may provide new multimodal biomarkers.”
The findings could lead the way to understanding the brain’s intake and output of energy in good health and bad and the part that alcohol plays.
In previous studies, the researchers have shown that alcohol significantly affects brain glucose metabolism, a measure of energy use, as well as regional brain activity, assessed through changes in blood oxygenation. But regional differences in glucose metabolism are hard to interpret, they say. In a study with healthy volunteers, they used brain imaging techniques to help quantify “match and mismatch” in energy consumption and expenditure across the brain—what they termed power and cost.
The researchers assessed power by observing to what extent brain regions are active and use energy, and cost by observing how brain regions expended energy. They found that different brain regions that serve distinct functions have “notably different power and different cost.”
Next, they tested a group of light drinkers and heavy drinkers and found both acute and chronic exposure to alcohol affected power and cost. In heavy drinkers, the researchers say, they saw less regional power, for example, in the thalamus, the sensory gateway, and frontal cortex. The researchers interpreted the decreases in power as reflecting the toxic effects of long-term exposure to alcohol on the brain cells.
They also found power dropped in the visual regions during acute alcohol exposure, which was related to disruption of visual processing. Visual regions also had the most significant drops in cost of activity during intoxication. That is consistent with the reliance of those regions on alternative energy sources, such as acetate (a byproduct of alcohol metabolism), the researchers say.
Their approach for characterizing brain energetic patterns related to alcohol use could be useful in other ways, the researchers say. “Studying energetic signatures of brain regions in different neuropsychiatric diseases is an important future direction,” said co-lead investigator Dr. Ehsan Schokri-Kojori. “The measures of power and cost may provide new multimodal biomarkers.”
The findings could lead the way to understanding the brain’s intake and output of energy in good health and bad and the part that alcohol plays.
In previous studies, the researchers have shown that alcohol significantly affects brain glucose metabolism, a measure of energy use, as well as regional brain activity, assessed through changes in blood oxygenation. But regional differences in glucose metabolism are hard to interpret, they say. In a study with healthy volunteers, they used brain imaging techniques to help quantify “match and mismatch” in energy consumption and expenditure across the brain—what they termed power and cost.
The researchers assessed power by observing to what extent brain regions are active and use energy, and cost by observing how brain regions expended energy. They found that different brain regions that serve distinct functions have “notably different power and different cost.”
Next, they tested a group of light drinkers and heavy drinkers and found both acute and chronic exposure to alcohol affected power and cost. In heavy drinkers, the researchers say, they saw less regional power, for example, in the thalamus, the sensory gateway, and frontal cortex. The researchers interpreted the decreases in power as reflecting the toxic effects of long-term exposure to alcohol on the brain cells.
They also found power dropped in the visual regions during acute alcohol exposure, which was related to disruption of visual processing. Visual regions also had the most significant drops in cost of activity during intoxication. That is consistent with the reliance of those regions on alternative energy sources, such as acetate (a byproduct of alcohol metabolism), the researchers say.
Their approach for characterizing brain energetic patterns related to alcohol use could be useful in other ways, the researchers say. “Studying energetic signatures of brain regions in different neuropsychiatric diseases is an important future direction,” said co-lead investigator Dr. Ehsan Schokri-Kojori. “The measures of power and cost may provide new multimodal biomarkers.”
Sjögren’s syndrome risk increases with infections
Patients with a history of infection have nearly double the risk of developing Sjögren’s syndrome when compared with the general population (odds ratio, 1.9; 95% confidence interval, 1.6-2.3), according to new findings reported online March 20 in the Journal of Internal Medicine (doi: 10.1111/joim.12888).
The risk is almost three times higher among patients with a history of infection plus Ro/SSA and La/SSB antibodies (OR, 2.7; 95% CI, 2.0-3.5). The study included 945 Swedish patients with primary Sjögren’s syndrome and compared their data with those from 9,048 matched controls from the general population.
We previously covered results from this study when they were presented at the International Symposium on Sjögren’s Syndrome in Washington. Read our previous story at the link above.
Patients with a history of infection have nearly double the risk of developing Sjögren’s syndrome when compared with the general population (odds ratio, 1.9; 95% confidence interval, 1.6-2.3), according to new findings reported online March 20 in the Journal of Internal Medicine (doi: 10.1111/joim.12888).
The risk is almost three times higher among patients with a history of infection plus Ro/SSA and La/SSB antibodies (OR, 2.7; 95% CI, 2.0-3.5). The study included 945 Swedish patients with primary Sjögren’s syndrome and compared their data with those from 9,048 matched controls from the general population.
We previously covered results from this study when they were presented at the International Symposium on Sjögren’s Syndrome in Washington. Read our previous story at the link above.
Patients with a history of infection have nearly double the risk of developing Sjögren’s syndrome when compared with the general population (odds ratio, 1.9; 95% confidence interval, 1.6-2.3), according to new findings reported online March 20 in the Journal of Internal Medicine (doi: 10.1111/joim.12888).
The risk is almost three times higher among patients with a history of infection plus Ro/SSA and La/SSB antibodies (OR, 2.7; 95% CI, 2.0-3.5). The study included 945 Swedish patients with primary Sjögren’s syndrome and compared their data with those from 9,048 matched controls from the general population.
We previously covered results from this study when they were presented at the International Symposium on Sjögren’s Syndrome in Washington. Read our previous story at the link above.
FROM THE JOURNAL OF INTERNAL MEDICINE
Disease burden in OA worse than RA 6 months post presentation
Patients with osteoarthritis (OA) have RAPID3 scores at their initial visit (16.0) similar to patients with rheumatoid arthritis (RA) and either prior use of disease-modifying antirheumatic drugs (DMARDs) or no exposure to DMARDs (15.6 and 15.5, respectively). After 6 months of treatment, the RAPID3 (Routine Assessment of Patient Index Data 3) score fell by just 1.7 points for patients with OA, compared with 5.7 points in RA patients naive to DMARDs and 4.3 points in those with prior DMARD exposure. These findings were published March 20 in Arthritis & Rheumatology (doi: 10.1002/art.40869).
We reported this story at the 2018 World Congress on Osteoarthritis before it was published in the journal. Read the story at the link above.
Patients with osteoarthritis (OA) have RAPID3 scores at their initial visit (16.0) similar to patients with rheumatoid arthritis (RA) and either prior use of disease-modifying antirheumatic drugs (DMARDs) or no exposure to DMARDs (15.6 and 15.5, respectively). After 6 months of treatment, the RAPID3 (Routine Assessment of Patient Index Data 3) score fell by just 1.7 points for patients with OA, compared with 5.7 points in RA patients naive to DMARDs and 4.3 points in those with prior DMARD exposure. These findings were published March 20 in Arthritis & Rheumatology (doi: 10.1002/art.40869).
We reported this story at the 2018 World Congress on Osteoarthritis before it was published in the journal. Read the story at the link above.
Patients with osteoarthritis (OA) have RAPID3 scores at their initial visit (16.0) similar to patients with rheumatoid arthritis (RA) and either prior use of disease-modifying antirheumatic drugs (DMARDs) or no exposure to DMARDs (15.6 and 15.5, respectively). After 6 months of treatment, the RAPID3 (Routine Assessment of Patient Index Data 3) score fell by just 1.7 points for patients with OA, compared with 5.7 points in RA patients naive to DMARDs and 4.3 points in those with prior DMARD exposure. These findings were published March 20 in Arthritis & Rheumatology (doi: 10.1002/art.40869).
We reported this story at the 2018 World Congress on Osteoarthritis before it was published in the journal. Read the story at the link above.
FROM ARTHRITIS & RHEUMATOLOGY
Opportunistic salpingectomy appears to reduce risk of ovarian cancer
Women at high risk of ovarian cancer secondary to genetic predisposition (BRCA gene mutation, Lynch syndrome) still are recommended to undergo bilateral salpingo-oophorectomy after completion of child bearing or by age 40-45 years depending on the specific mutation and family history. For a woman not at risk of hereditary-related ovarian cancer, opportunistic salpingectomy would appear to reduce the risk of ovarian cancer.
Unlike bilateral tubal ligation, which has a greater protective risk of endometrioid and clear-cell carcinoma of the ovary, as well. A Swedish population-based cohort study involving over a quarter of a million women undergoing benign surgery noted a statistically significant decrease in ovarian cancer risk with salpingectomy. The degree of risk reduction was greater when bilateral salpingectomy was performed.1 Moreover, a Danish case-control study of over 13,000 women with ovarian cancer demonstrated a 42% decrease in epithelial carcinoma risk following bilateral salpingectomy.2
Bilateral salpingectomy does not appear to decrease ovarian function. A study by Venturella et al. that compared 91 women undergoing bilateral salpingectomy with 95 women with mesosalpinx removal within the tubes during salpingectomy observed no significant difference in change of ovarian reserve.3 Moreover, Kotlyar et al. performed a literature review and noted similar findings.4 Finally, in another study by Venturella et al. no effects were noted 3-5 years following prophylactic bilateral salpingectomy on ovarian reserve in women undergoing total laparoscopic hysterectomy in their late reproductive years, compared with healthy women with intact uterus and adnexa.5
Introduction of opportunistic salpingectomy secondary to potential ovarian cancer reduction has seen increased adoption over the years. A U.S. study of 400,000 hysterectomies performed for benign indications from 1998 to 2011 showed an increased annual rate of bilateral salpingectomy of 8% (1998-2008) and a 24% annual increase (2008-2011).6 A retrospective study of 12,143 hysterectomies performed within a large U.S. health care system reported an increased rate of salpingectomy from 15% in 2011 to 45% in 2012 to 73% in 2014.7
Given the fact that the American College of Obstetricians and Gynecologists and the AAGL recommend vaginal hysterectomy as the approach of choice when feasible, tips and tricks on opportunistic salpingectomy form an important topic.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rosanne M. Kho, MD. Dr. Kho’s academic and clinical work focuses on advancing vaginal and minimally invasive surgery. Dr. Kho is a strong advocate of the vaginal approach for benign hysterectomy and is recognized for her passion for bringing vaginal surgery back into the armamentarium of the gynecologic surgeon. Dr. Kho is published in the field of gynecologic surgery, having authored many peer-reviewed manuscripts and book chapters. She is currently an associate editor for the Journal of Minimally Invasive Gynecology (JMIG).
It is truly a pleasure to welcome Dr. Kho to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Natl Cancer Inst. 2015 Jan 27. doi: 10.1093/jnci/dju410.
2. Acta Obstet Gynecol Scand. 2015 Jan;94(1):86-94.
3. Fertil Steril. 2015 Nov;104(5):1332-9.
4. J Minim Invasive Gynecol. 2017 May-Jun;24(4):563-78.
5. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
6. Am J Obstet Gynecol. 2015 Nov;213(5):713.e1-13.
7. Obstet Gynecol. 2016 Aug;128(2):277-83.
Women at high risk of ovarian cancer secondary to genetic predisposition (BRCA gene mutation, Lynch syndrome) still are recommended to undergo bilateral salpingo-oophorectomy after completion of child bearing or by age 40-45 years depending on the specific mutation and family history. For a woman not at risk of hereditary-related ovarian cancer, opportunistic salpingectomy would appear to reduce the risk of ovarian cancer.
Unlike bilateral tubal ligation, which has a greater protective risk of endometrioid and clear-cell carcinoma of the ovary, as well. A Swedish population-based cohort study involving over a quarter of a million women undergoing benign surgery noted a statistically significant decrease in ovarian cancer risk with salpingectomy. The degree of risk reduction was greater when bilateral salpingectomy was performed.1 Moreover, a Danish case-control study of over 13,000 women with ovarian cancer demonstrated a 42% decrease in epithelial carcinoma risk following bilateral salpingectomy.2
Bilateral salpingectomy does not appear to decrease ovarian function. A study by Venturella et al. that compared 91 women undergoing bilateral salpingectomy with 95 women with mesosalpinx removal within the tubes during salpingectomy observed no significant difference in change of ovarian reserve.3 Moreover, Kotlyar et al. performed a literature review and noted similar findings.4 Finally, in another study by Venturella et al. no effects were noted 3-5 years following prophylactic bilateral salpingectomy on ovarian reserve in women undergoing total laparoscopic hysterectomy in their late reproductive years, compared with healthy women with intact uterus and adnexa.5
Introduction of opportunistic salpingectomy secondary to potential ovarian cancer reduction has seen increased adoption over the years. A U.S. study of 400,000 hysterectomies performed for benign indications from 1998 to 2011 showed an increased annual rate of bilateral salpingectomy of 8% (1998-2008) and a 24% annual increase (2008-2011).6 A retrospective study of 12,143 hysterectomies performed within a large U.S. health care system reported an increased rate of salpingectomy from 15% in 2011 to 45% in 2012 to 73% in 2014.7
Given the fact that the American College of Obstetricians and Gynecologists and the AAGL recommend vaginal hysterectomy as the approach of choice when feasible, tips and tricks on opportunistic salpingectomy form an important topic.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rosanne M. Kho, MD. Dr. Kho’s academic and clinical work focuses on advancing vaginal and minimally invasive surgery. Dr. Kho is a strong advocate of the vaginal approach for benign hysterectomy and is recognized for her passion for bringing vaginal surgery back into the armamentarium of the gynecologic surgeon. Dr. Kho is published in the field of gynecologic surgery, having authored many peer-reviewed manuscripts and book chapters. She is currently an associate editor for the Journal of Minimally Invasive Gynecology (JMIG).
It is truly a pleasure to welcome Dr. Kho to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Natl Cancer Inst. 2015 Jan 27. doi: 10.1093/jnci/dju410.
2. Acta Obstet Gynecol Scand. 2015 Jan;94(1):86-94.
3. Fertil Steril. 2015 Nov;104(5):1332-9.
4. J Minim Invasive Gynecol. 2017 May-Jun;24(4):563-78.
5. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
6. Am J Obstet Gynecol. 2015 Nov;213(5):713.e1-13.
7. Obstet Gynecol. 2016 Aug;128(2):277-83.
Women at high risk of ovarian cancer secondary to genetic predisposition (BRCA gene mutation, Lynch syndrome) still are recommended to undergo bilateral salpingo-oophorectomy after completion of child bearing or by age 40-45 years depending on the specific mutation and family history. For a woman not at risk of hereditary-related ovarian cancer, opportunistic salpingectomy would appear to reduce the risk of ovarian cancer.
Unlike bilateral tubal ligation, which has a greater protective risk of endometrioid and clear-cell carcinoma of the ovary, as well. A Swedish population-based cohort study involving over a quarter of a million women undergoing benign surgery noted a statistically significant decrease in ovarian cancer risk with salpingectomy. The degree of risk reduction was greater when bilateral salpingectomy was performed.1 Moreover, a Danish case-control study of over 13,000 women with ovarian cancer demonstrated a 42% decrease in epithelial carcinoma risk following bilateral salpingectomy.2
Bilateral salpingectomy does not appear to decrease ovarian function. A study by Venturella et al. that compared 91 women undergoing bilateral salpingectomy with 95 women with mesosalpinx removal within the tubes during salpingectomy observed no significant difference in change of ovarian reserve.3 Moreover, Kotlyar et al. performed a literature review and noted similar findings.4 Finally, in another study by Venturella et al. no effects were noted 3-5 years following prophylactic bilateral salpingectomy on ovarian reserve in women undergoing total laparoscopic hysterectomy in their late reproductive years, compared with healthy women with intact uterus and adnexa.5
Introduction of opportunistic salpingectomy secondary to potential ovarian cancer reduction has seen increased adoption over the years. A U.S. study of 400,000 hysterectomies performed for benign indications from 1998 to 2011 showed an increased annual rate of bilateral salpingectomy of 8% (1998-2008) and a 24% annual increase (2008-2011).6 A retrospective study of 12,143 hysterectomies performed within a large U.S. health care system reported an increased rate of salpingectomy from 15% in 2011 to 45% in 2012 to 73% in 2014.7
Given the fact that the American College of Obstetricians and Gynecologists and the AAGL recommend vaginal hysterectomy as the approach of choice when feasible, tips and tricks on opportunistic salpingectomy form an important topic.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Rosanne M. Kho, MD. Dr. Kho’s academic and clinical work focuses on advancing vaginal and minimally invasive surgery. Dr. Kho is a strong advocate of the vaginal approach for benign hysterectomy and is recognized for her passion for bringing vaginal surgery back into the armamentarium of the gynecologic surgeon. Dr. Kho is published in the field of gynecologic surgery, having authored many peer-reviewed manuscripts and book chapters. She is currently an associate editor for the Journal of Minimally Invasive Gynecology (JMIG).
It is truly a pleasure to welcome Dr. Kho to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Natl Cancer Inst. 2015 Jan 27. doi: 10.1093/jnci/dju410.
2. Acta Obstet Gynecol Scand. 2015 Jan;94(1):86-94.
3. Fertil Steril. 2015 Nov;104(5):1332-9.
4. J Minim Invasive Gynecol. 2017 May-Jun;24(4):563-78.
5. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
6. Am J Obstet Gynecol. 2015 Nov;213(5):713.e1-13.
7. Obstet Gynecol. 2016 Aug;128(2):277-83.
Can prophylactic salpingectomies be achieved with the vaginal approach?
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique
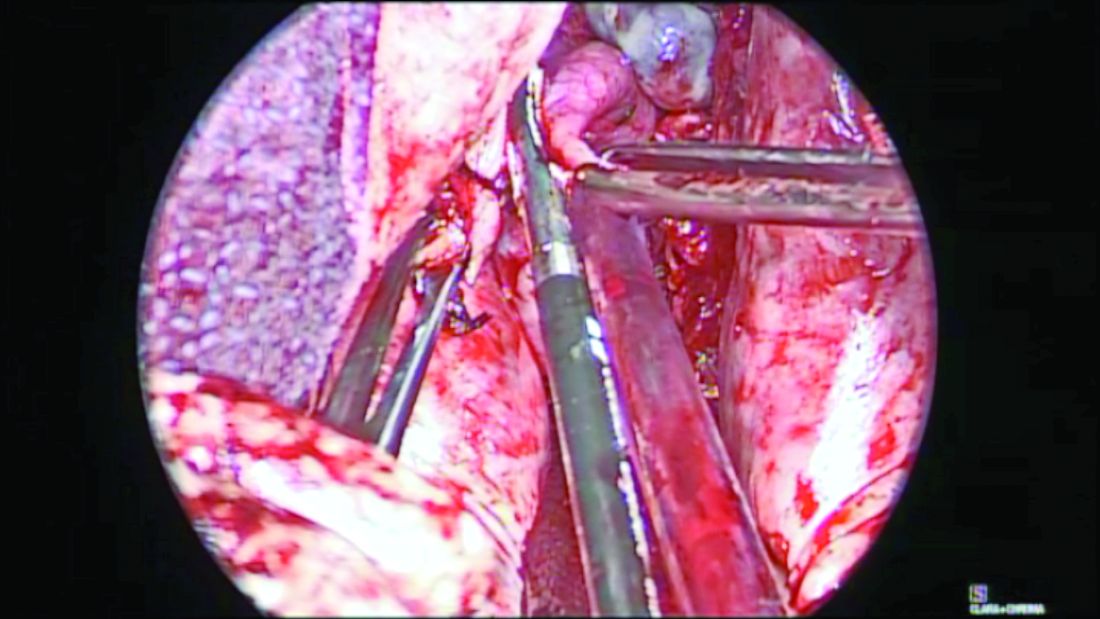
Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique
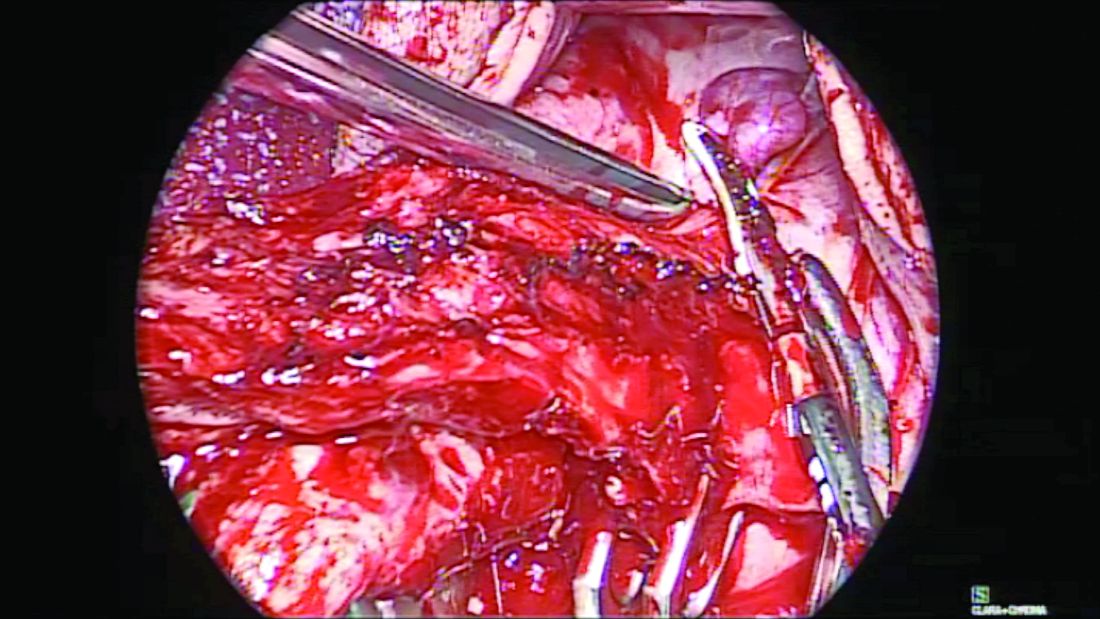
When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
Cannabis use, potency linked to psychotic disorder risk
Daily cannabis use, particularly high-potency cannabis, might be a significant contributor to the incidence of psychotic disorder, results of a multicenter, case-control study suggest.
“We provide the first direct evidence that cannabis use has an effect on variation in the incidence of psychotic disorders,” Marta Di Forti, PhD, and her coauthors wrote in the Lancet.
In the study, Dr. Di Forti and her coauthors looked at cannabis use in 901 patients presenting with a first psychotic episode to one of 11 sites across Europe and Brazil, compared with 1,237 population controls from the same locations. They found that daily cannabis users had more than threefold higher odds of psychotic disorder, compared with individuals who had never used cannabis (odds ratio, 3.2; P less than .0001), even after adjusting for sociodemographic factors and use of tobacco, stimulants, ketamine, and hallucinogenics.
Those who reported using high-potency cannabis (delta 9-tetrahydrocannabinol greater than or equal to 10%) – also showed a significant 60% increase in the odds of psychotic disorder, compared with never-users, which decreased slightly to 50% after controlling for daily use.
“The large sample size and the different types of cannabis available across Europe have allowed us to report that the dose-response relationship characterizing the association between cannabis use and psychosis reflects not only the use of high-potency cannabis but also the daily use of types with an amount of THC consistent with more traditional varieties,” wrote Dr. Di Forti, of the Social, Genetic, and Developmental Psychiatry Centre at King’s College London, and her coauthors.
When the authors looked at the population-attributable fractions, they calculated that 12.2% of cases of first-episode psychosis would be avoided if high-potency cannabis were not available.
(P = .0122), while those who started using high-potency cannabis at that age had more than a doubling of risk (OR, 2.3).
Similarly, those who used high-potency cannabis on a daily basis had nearly fivefold higher odds of psychotic disorder, compared with never-users, while daily users of low-potency had a 2.2-fold increase in risk.
Researchers also examined patterns of cannabis use and psychotic disorder across the 11 sites, which included Amsterdam, London; Cambridge, England; Madrid; Palermo, Italy; Paris; and Ribeirão Preto, Brazil.
They noted that there were significant variations in the incidence of psychotic disorder across the study sites, and that those variations correlated with the prevalence of daily cannabis use.
London and Amsterdam, where daily use was the most common, had the highest adjusted incidence rates of psychotic disorder (45.7 cases per 100,000 person-years in London and 37.9 per 100,000 person-years in Amsterdam). In contrast, the incidence in Bologna, Italy – where daily use was less frequent – was half that of London.
They estimated that 43% of new cases of psychotic disorder in Amsterdam were attributable to daily use of cannabis, and 50.3% were attributable to high-potency cannabis, compared with 1.2% and 2.3% of cases in Puy de Dôme in France.
“Use of high-potency cannabis was a strong predictor of psychotic disorder in Amsterdam, London, and Paris, where high-potency cannabis was widely available, by contrast with sites such as Palermo where this type was not yet available,” the authors wrote. “Our results show that, in areas where daily use and use of high-potency cannabis are more prevalent in the general population, there is an excess of cases of psychotic disorder.”
The authors did point out that the study relied on self-reported cannabis use, rather than biological sampling measures. But previous studies have shown self-reported use to be a reliable measure, they said.
“Education is needed to inform the public about the mental health hazards of regular use of high-potency cannabis, which is becoming increasingly available worldwide,” they wrote.
The study was supported by the Medical Research Council, the European Community’s Seventh Framework Program, the São Paulo Research Foundation, the National Institute for Health Research Biomedical Research Centre, and the Wellcome Trust. Five authors declared personal fees and grants from the pharmaceutical industry. No other conflicts of interest were declared.
SOURCE: Di Forti M et al. Lancet. 2019 Mar 19. doi: 10.1016/S2215-0366(19)30048-3.
Epidemiologic and experimental studies generally have established a link between heavy cannabis use and psychosis. However, a long-running issue has been that, while cannabis use has increased in some populations, the rates of psychosis have not necessarily done the same. The results of this study go against that, suggesting that differing rates and intensity of cannabis use across Europe appear to correlate with differing rates of psychosis.
This does not necessarily imply causality. For example, genetic studies suggest that individuals predisposed to psychosis also may have a predisposition to use cannabis. Another possibility is that subclinical mental health issues existed in those participants before the start of cannabis use. The challenge, therefore, remains to identify which individuals are most at risk from psychosis related to cannabis use, and to develop strategies aimed at mitigating this risk.
Suzanne H. Gage, PhD, is affiliated with the department of psychological sciences at the University of Liverpool in England. These comments are adapted from an accompanying editorial (Lancet. 2019 Mar 19. doi: 10.1016/ S2215-0366[19]30086-0). No conflicts of interest were declared.
Epidemiologic and experimental studies generally have established a link between heavy cannabis use and psychosis. However, a long-running issue has been that, while cannabis use has increased in some populations, the rates of psychosis have not necessarily done the same. The results of this study go against that, suggesting that differing rates and intensity of cannabis use across Europe appear to correlate with differing rates of psychosis.
This does not necessarily imply causality. For example, genetic studies suggest that individuals predisposed to psychosis also may have a predisposition to use cannabis. Another possibility is that subclinical mental health issues existed in those participants before the start of cannabis use. The challenge, therefore, remains to identify which individuals are most at risk from psychosis related to cannabis use, and to develop strategies aimed at mitigating this risk.
Suzanne H. Gage, PhD, is affiliated with the department of psychological sciences at the University of Liverpool in England. These comments are adapted from an accompanying editorial (Lancet. 2019 Mar 19. doi: 10.1016/ S2215-0366[19]30086-0). No conflicts of interest were declared.
Epidemiologic and experimental studies generally have established a link between heavy cannabis use and psychosis. However, a long-running issue has been that, while cannabis use has increased in some populations, the rates of psychosis have not necessarily done the same. The results of this study go against that, suggesting that differing rates and intensity of cannabis use across Europe appear to correlate with differing rates of psychosis.
This does not necessarily imply causality. For example, genetic studies suggest that individuals predisposed to psychosis also may have a predisposition to use cannabis. Another possibility is that subclinical mental health issues existed in those participants before the start of cannabis use. The challenge, therefore, remains to identify which individuals are most at risk from psychosis related to cannabis use, and to develop strategies aimed at mitigating this risk.
Suzanne H. Gage, PhD, is affiliated with the department of psychological sciences at the University of Liverpool in England. These comments are adapted from an accompanying editorial (Lancet. 2019 Mar 19. doi: 10.1016/ S2215-0366[19]30086-0). No conflicts of interest were declared.
Daily cannabis use, particularly high-potency cannabis, might be a significant contributor to the incidence of psychotic disorder, results of a multicenter, case-control study suggest.
“We provide the first direct evidence that cannabis use has an effect on variation in the incidence of psychotic disorders,” Marta Di Forti, PhD, and her coauthors wrote in the Lancet.
In the study, Dr. Di Forti and her coauthors looked at cannabis use in 901 patients presenting with a first psychotic episode to one of 11 sites across Europe and Brazil, compared with 1,237 population controls from the same locations. They found that daily cannabis users had more than threefold higher odds of psychotic disorder, compared with individuals who had never used cannabis (odds ratio, 3.2; P less than .0001), even after adjusting for sociodemographic factors and use of tobacco, stimulants, ketamine, and hallucinogenics.
Those who reported using high-potency cannabis (delta 9-tetrahydrocannabinol greater than or equal to 10%) – also showed a significant 60% increase in the odds of psychotic disorder, compared with never-users, which decreased slightly to 50% after controlling for daily use.
“The large sample size and the different types of cannabis available across Europe have allowed us to report that the dose-response relationship characterizing the association between cannabis use and psychosis reflects not only the use of high-potency cannabis but also the daily use of types with an amount of THC consistent with more traditional varieties,” wrote Dr. Di Forti, of the Social, Genetic, and Developmental Psychiatry Centre at King’s College London, and her coauthors.
When the authors looked at the population-attributable fractions, they calculated that 12.2% of cases of first-episode psychosis would be avoided if high-potency cannabis were not available.
(P = .0122), while those who started using high-potency cannabis at that age had more than a doubling of risk (OR, 2.3).
Similarly, those who used high-potency cannabis on a daily basis had nearly fivefold higher odds of psychotic disorder, compared with never-users, while daily users of low-potency had a 2.2-fold increase in risk.
Researchers also examined patterns of cannabis use and psychotic disorder across the 11 sites, which included Amsterdam, London; Cambridge, England; Madrid; Palermo, Italy; Paris; and Ribeirão Preto, Brazil.
They noted that there were significant variations in the incidence of psychotic disorder across the study sites, and that those variations correlated with the prevalence of daily cannabis use.
London and Amsterdam, where daily use was the most common, had the highest adjusted incidence rates of psychotic disorder (45.7 cases per 100,000 person-years in London and 37.9 per 100,000 person-years in Amsterdam). In contrast, the incidence in Bologna, Italy – where daily use was less frequent – was half that of London.
They estimated that 43% of new cases of psychotic disorder in Amsterdam were attributable to daily use of cannabis, and 50.3% were attributable to high-potency cannabis, compared with 1.2% and 2.3% of cases in Puy de Dôme in France.
“Use of high-potency cannabis was a strong predictor of psychotic disorder in Amsterdam, London, and Paris, where high-potency cannabis was widely available, by contrast with sites such as Palermo where this type was not yet available,” the authors wrote. “Our results show that, in areas where daily use and use of high-potency cannabis are more prevalent in the general population, there is an excess of cases of psychotic disorder.”
The authors did point out that the study relied on self-reported cannabis use, rather than biological sampling measures. But previous studies have shown self-reported use to be a reliable measure, they said.
“Education is needed to inform the public about the mental health hazards of regular use of high-potency cannabis, which is becoming increasingly available worldwide,” they wrote.
The study was supported by the Medical Research Council, the European Community’s Seventh Framework Program, the São Paulo Research Foundation, the National Institute for Health Research Biomedical Research Centre, and the Wellcome Trust. Five authors declared personal fees and grants from the pharmaceutical industry. No other conflicts of interest were declared.
SOURCE: Di Forti M et al. Lancet. 2019 Mar 19. doi: 10.1016/S2215-0366(19)30048-3.
Daily cannabis use, particularly high-potency cannabis, might be a significant contributor to the incidence of psychotic disorder, results of a multicenter, case-control study suggest.
“We provide the first direct evidence that cannabis use has an effect on variation in the incidence of psychotic disorders,” Marta Di Forti, PhD, and her coauthors wrote in the Lancet.
In the study, Dr. Di Forti and her coauthors looked at cannabis use in 901 patients presenting with a first psychotic episode to one of 11 sites across Europe and Brazil, compared with 1,237 population controls from the same locations. They found that daily cannabis users had more than threefold higher odds of psychotic disorder, compared with individuals who had never used cannabis (odds ratio, 3.2; P less than .0001), even after adjusting for sociodemographic factors and use of tobacco, stimulants, ketamine, and hallucinogenics.
Those who reported using high-potency cannabis (delta 9-tetrahydrocannabinol greater than or equal to 10%) – also showed a significant 60% increase in the odds of psychotic disorder, compared with never-users, which decreased slightly to 50% after controlling for daily use.
“The large sample size and the different types of cannabis available across Europe have allowed us to report that the dose-response relationship characterizing the association between cannabis use and psychosis reflects not only the use of high-potency cannabis but also the daily use of types with an amount of THC consistent with more traditional varieties,” wrote Dr. Di Forti, of the Social, Genetic, and Developmental Psychiatry Centre at King’s College London, and her coauthors.
When the authors looked at the population-attributable fractions, they calculated that 12.2% of cases of first-episode psychosis would be avoided if high-potency cannabis were not available.
(P = .0122), while those who started using high-potency cannabis at that age had more than a doubling of risk (OR, 2.3).
Similarly, those who used high-potency cannabis on a daily basis had nearly fivefold higher odds of psychotic disorder, compared with never-users, while daily users of low-potency had a 2.2-fold increase in risk.
Researchers also examined patterns of cannabis use and psychotic disorder across the 11 sites, which included Amsterdam, London; Cambridge, England; Madrid; Palermo, Italy; Paris; and Ribeirão Preto, Brazil.
They noted that there were significant variations in the incidence of psychotic disorder across the study sites, and that those variations correlated with the prevalence of daily cannabis use.
London and Amsterdam, where daily use was the most common, had the highest adjusted incidence rates of psychotic disorder (45.7 cases per 100,000 person-years in London and 37.9 per 100,000 person-years in Amsterdam). In contrast, the incidence in Bologna, Italy – where daily use was less frequent – was half that of London.
They estimated that 43% of new cases of psychotic disorder in Amsterdam were attributable to daily use of cannabis, and 50.3% were attributable to high-potency cannabis, compared with 1.2% and 2.3% of cases in Puy de Dôme in France.
“Use of high-potency cannabis was a strong predictor of psychotic disorder in Amsterdam, London, and Paris, where high-potency cannabis was widely available, by contrast with sites such as Palermo where this type was not yet available,” the authors wrote. “Our results show that, in areas where daily use and use of high-potency cannabis are more prevalent in the general population, there is an excess of cases of psychotic disorder.”
The authors did point out that the study relied on self-reported cannabis use, rather than biological sampling measures. But previous studies have shown self-reported use to be a reliable measure, they said.
“Education is needed to inform the public about the mental health hazards of regular use of high-potency cannabis, which is becoming increasingly available worldwide,” they wrote.
The study was supported by the Medical Research Council, the European Community’s Seventh Framework Program, the São Paulo Research Foundation, the National Institute for Health Research Biomedical Research Centre, and the Wellcome Trust. Five authors declared personal fees and grants from the pharmaceutical industry. No other conflicts of interest were declared.
SOURCE: Di Forti M et al. Lancet. 2019 Mar 19. doi: 10.1016/S2215-0366(19)30048-3.
FROM THE LANCET
FDA approves brexanolone for postpartum depression
The Food and Drug Administration on March 19 approved the first medication specifically for the treatment of postpartum depression.
The drug, brexanolone (Zulresso), is to be administered as a single continuous 60-hour infusion for each episode of postpartum depression. . By binding to GABA A receptors, brexanolone increases receptor functionality. The recommended maximum dose of brexanolone is 90 µg/kg/h, and the infusion includes three dosing phases.
Brexanolone provides “an important new treatment option,” said Tiffany Farchione, MD, acting director of the division of psychiatry products in the FDA’s Center for Drug Evaluation and Research, in a press release. “Because of concerns about serious risks, including excessive sedation or sudden loss of consciousness during administration, Zulresso has been approved with a Risk Evaluation and Mitigation Strategy (REMS) and is only available to patients through a restricted distribution program at certified health care facilities where the health care provider can carefully monitor the patient.”
The approval was based on results of three phase 3 trials, which were double-blind, randomized, and placebo-controlled studies in which the primary efficacy endpoint was a change in baseline 60 hours after the start of the infusion on the Hamilton Depression Rating Scale (HAM-D). In all two of the trials, known as Hummingbird 202B and 202C, brexanolone’s impact on the patients’ HAM-D scores was greater than that of placebo, the FDA reported in briefing document released late last year. In addition, the impact of brexanolone on postpartum depression proved both rapid and durable.
Side effects observed in about 3% of the brexanolone patients included dizziness, dry mouth, fatigue, headache, infusion site pain, somnolence, and loss of consciousness. The FDA’s concern about loss of consciousness led the agency to recommend a REMS protocol before a hearing of its Psychopharmacologic Drugs Advisory and Drug Safety and Risk Management Advisory panels late last year. The Zulresso REMS Program will require that the drug be administered by a clinician in a health care facility that is certified. Patients will have to be monitored for excessive sedation and “sudden loss of consciousness and have continuous pulse oximetry monitoring (monitors oxygen levels in the blood),” the FDA said. Another requirement is that patients who receive the infusion will have to be accompanied while interacting with their children. Patients will be advised not to drive, operate machinery or engage in other dangerous activities until they feel totally alert. Those requirements will be addressed in a boxed warning.
The drug should be either adjusted or discontinued for patients whose postpartum depression becomes worse or for those experience suicidal thoughts and behaviors after taking brexanolone, the agency said.
Some physicians use antidepressants to treat postpartum depression, but their effectiveness is limited, according to the FDA. Interventions such as electroconvulsive therapy and psychotherapy also are used, but getting results can several weeks.
The symptoms of postpartum depression are indistinguishable from major depressive disorder, but “the timing of its onset has led to its recognition as potentially unique illness,” the FDA said. Postpartum depression in the United States affects up to 12% of births. In the developed world, suicide is the most common cause of maternal death after childbirth. This suicide risk makes postpartum depression a condition that is life-threatening. In addition, the condition has “profound negative effects on the maternal-infant bond and later infant development,” the FDA said.
SAGE Therapeutics, developer of brexanolone, secured the approval through the FDA’s breakthrough therapy designation process.
Heidi Splete contributed to this article.
The Food and Drug Administration on March 19 approved the first medication specifically for the treatment of postpartum depression.
The drug, brexanolone (Zulresso), is to be administered as a single continuous 60-hour infusion for each episode of postpartum depression. . By binding to GABA A receptors, brexanolone increases receptor functionality. The recommended maximum dose of brexanolone is 90 µg/kg/h, and the infusion includes three dosing phases.
Brexanolone provides “an important new treatment option,” said Tiffany Farchione, MD, acting director of the division of psychiatry products in the FDA’s Center for Drug Evaluation and Research, in a press release. “Because of concerns about serious risks, including excessive sedation or sudden loss of consciousness during administration, Zulresso has been approved with a Risk Evaluation and Mitigation Strategy (REMS) and is only available to patients through a restricted distribution program at certified health care facilities where the health care provider can carefully monitor the patient.”
The approval was based on results of three phase 3 trials, which were double-blind, randomized, and placebo-controlled studies in which the primary efficacy endpoint was a change in baseline 60 hours after the start of the infusion on the Hamilton Depression Rating Scale (HAM-D). In all two of the trials, known as Hummingbird 202B and 202C, brexanolone’s impact on the patients’ HAM-D scores was greater than that of placebo, the FDA reported in briefing document released late last year. In addition, the impact of brexanolone on postpartum depression proved both rapid and durable.
Side effects observed in about 3% of the brexanolone patients included dizziness, dry mouth, fatigue, headache, infusion site pain, somnolence, and loss of consciousness. The FDA’s concern about loss of consciousness led the agency to recommend a REMS protocol before a hearing of its Psychopharmacologic Drugs Advisory and Drug Safety and Risk Management Advisory panels late last year. The Zulresso REMS Program will require that the drug be administered by a clinician in a health care facility that is certified. Patients will have to be monitored for excessive sedation and “sudden loss of consciousness and have continuous pulse oximetry monitoring (monitors oxygen levels in the blood),” the FDA said. Another requirement is that patients who receive the infusion will have to be accompanied while interacting with their children. Patients will be advised not to drive, operate machinery or engage in other dangerous activities until they feel totally alert. Those requirements will be addressed in a boxed warning.
The drug should be either adjusted or discontinued for patients whose postpartum depression becomes worse or for those experience suicidal thoughts and behaviors after taking brexanolone, the agency said.
Some physicians use antidepressants to treat postpartum depression, but their effectiveness is limited, according to the FDA. Interventions such as electroconvulsive therapy and psychotherapy also are used, but getting results can several weeks.
The symptoms of postpartum depression are indistinguishable from major depressive disorder, but “the timing of its onset has led to its recognition as potentially unique illness,” the FDA said. Postpartum depression in the United States affects up to 12% of births. In the developed world, suicide is the most common cause of maternal death after childbirth. This suicide risk makes postpartum depression a condition that is life-threatening. In addition, the condition has “profound negative effects on the maternal-infant bond and later infant development,” the FDA said.
SAGE Therapeutics, developer of brexanolone, secured the approval through the FDA’s breakthrough therapy designation process.
Heidi Splete contributed to this article.
The Food and Drug Administration on March 19 approved the first medication specifically for the treatment of postpartum depression.
The drug, brexanolone (Zulresso), is to be administered as a single continuous 60-hour infusion for each episode of postpartum depression. . By binding to GABA A receptors, brexanolone increases receptor functionality. The recommended maximum dose of brexanolone is 90 µg/kg/h, and the infusion includes three dosing phases.
Brexanolone provides “an important new treatment option,” said Tiffany Farchione, MD, acting director of the division of psychiatry products in the FDA’s Center for Drug Evaluation and Research, in a press release. “Because of concerns about serious risks, including excessive sedation or sudden loss of consciousness during administration, Zulresso has been approved with a Risk Evaluation and Mitigation Strategy (REMS) and is only available to patients through a restricted distribution program at certified health care facilities where the health care provider can carefully monitor the patient.”
The approval was based on results of three phase 3 trials, which were double-blind, randomized, and placebo-controlled studies in which the primary efficacy endpoint was a change in baseline 60 hours after the start of the infusion on the Hamilton Depression Rating Scale (HAM-D). In all two of the trials, known as Hummingbird 202B and 202C, brexanolone’s impact on the patients’ HAM-D scores was greater than that of placebo, the FDA reported in briefing document released late last year. In addition, the impact of brexanolone on postpartum depression proved both rapid and durable.
Side effects observed in about 3% of the brexanolone patients included dizziness, dry mouth, fatigue, headache, infusion site pain, somnolence, and loss of consciousness. The FDA’s concern about loss of consciousness led the agency to recommend a REMS protocol before a hearing of its Psychopharmacologic Drugs Advisory and Drug Safety and Risk Management Advisory panels late last year. The Zulresso REMS Program will require that the drug be administered by a clinician in a health care facility that is certified. Patients will have to be monitored for excessive sedation and “sudden loss of consciousness and have continuous pulse oximetry monitoring (monitors oxygen levels in the blood),” the FDA said. Another requirement is that patients who receive the infusion will have to be accompanied while interacting with their children. Patients will be advised not to drive, operate machinery or engage in other dangerous activities until they feel totally alert. Those requirements will be addressed in a boxed warning.
The drug should be either adjusted or discontinued for patients whose postpartum depression becomes worse or for those experience suicidal thoughts and behaviors after taking brexanolone, the agency said.
Some physicians use antidepressants to treat postpartum depression, but their effectiveness is limited, according to the FDA. Interventions such as electroconvulsive therapy and psychotherapy also are used, but getting results can several weeks.
The symptoms of postpartum depression are indistinguishable from major depressive disorder, but “the timing of its onset has led to its recognition as potentially unique illness,” the FDA said. Postpartum depression in the United States affects up to 12% of births. In the developed world, suicide is the most common cause of maternal death after childbirth. This suicide risk makes postpartum depression a condition that is life-threatening. In addition, the condition has “profound negative effects on the maternal-infant bond and later infant development,” the FDA said.
SAGE Therapeutics, developer of brexanolone, secured the approval through the FDA’s breakthrough therapy designation process.
Heidi Splete contributed to this article.
Will patient rewards for lower-cost choices impact physicians?
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
FROM HEALTH AFFAIRS