User login
Benefit of thrombectomy may be universal
The location of the arterial occlusive lesion and the imaging technique used to select patients for the procedure also do not influence the therapy’s benefits, the researchers said. Although the proportional benefit of thrombectomy plus medical management is uniform across subgroups, compared with medical management alone, patients may have different amounts of absolute benefit.
The results of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial, which were published in 2018, indicated that endovascular thrombectomy provided clinical benefits for patients with acute ischemic stroke if administered at 6-16 hours after stroke onset. As part of the trial’s prespecified analyses, Maarten G. Lansberg, MD, PhD, associate professor of neurology and neurological sciences at Stanford (Calif.) University Medical Center in California, and his colleagues sought to determine whether thrombectomy had uniform benefit among various patient subgroups (e.g., elderly people, patients with mild symptoms, and those who present late after onset).
A total of 296 patients were enrolled in the randomized, open-label, blinded-endpoint DEFUSE 3 trial at 38 sites in the United States. Eligible participants had acute ischemic stroke resulting from an occlusion of the internal carotid artery or middle cerebral artery and evidence of salvageable tissue on perfusion CT or MRI. In all, 182 patients met these criteria and were randomized and included in the intention-to-treat analysis. The researchers stopped DEFUSE 3 early because of efficacy.
The study’s primary endpoint was functional outcome at day 90, as measured with the modified Rankin Scale. Dr. Lansberg and his colleagues performed multivariate ordinal logistic regression to calculate the adjusted proportional association between endovascular treatment and clinical outcome among participants of various ages, baseline stroke severities, periods between onset and treatment, locations of the arterial occlusion, and imaging modalities, such as CT or MRI, used to identify salvageable tissue.
The population’s median age was 70 years, and 51% of participants were women. The median National Institutes of Health Stroke Scale score was 16. When the researchers considered the whole sample, they found that younger age, lower baseline NIHSS score, and lower serum glucose level independently predicted better functional outcome. The common odds ratio for improved functional outcome with endovascular therapy, adjusted for these variables, was 3.1. Age, NIHSS score, time to randomization, imaging modality, and location of the arterial occlusion did not interact significantly with treatment effect.
“Our results indicate that advanced age, up to 90 years, should not be considered a contraindication to thrombectomy, provided that the patient is fully independent prior to stroke onset,” said the researchers. “Although age did not modify the treatment effect, it was a strong independent predictor of 90-day disability, which is consistent with prior studies of both tissue plasminogen activator and endovascular therapy.”
The trial’s small sample size may have allowed small differences between groups to pass unnoticed, said the reseachers. Other trials of late-window thrombectomy will be required to validate these results, they concluded.
The National Institute for Neurological Disorders and Stroke supported the study through grants. Several investigators received consulting fees from and hold shares in iSchemaView, which manufactures the software that the investigators used for postprocessing of CT and MRI perfusion studies. Other authors received consulting fees from various pharmaceutical and medical device companies, including Genentech, Medtronic, Pfizer, and Stryker Neurovascular.
SOURCE: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
The location of the arterial occlusive lesion and the imaging technique used to select patients for the procedure also do not influence the therapy’s benefits, the researchers said. Although the proportional benefit of thrombectomy plus medical management is uniform across subgroups, compared with medical management alone, patients may have different amounts of absolute benefit.
The results of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial, which were published in 2018, indicated that endovascular thrombectomy provided clinical benefits for patients with acute ischemic stroke if administered at 6-16 hours after stroke onset. As part of the trial’s prespecified analyses, Maarten G. Lansberg, MD, PhD, associate professor of neurology and neurological sciences at Stanford (Calif.) University Medical Center in California, and his colleagues sought to determine whether thrombectomy had uniform benefit among various patient subgroups (e.g., elderly people, patients with mild symptoms, and those who present late after onset).
A total of 296 patients were enrolled in the randomized, open-label, blinded-endpoint DEFUSE 3 trial at 38 sites in the United States. Eligible participants had acute ischemic stroke resulting from an occlusion of the internal carotid artery or middle cerebral artery and evidence of salvageable tissue on perfusion CT or MRI. In all, 182 patients met these criteria and were randomized and included in the intention-to-treat analysis. The researchers stopped DEFUSE 3 early because of efficacy.
The study’s primary endpoint was functional outcome at day 90, as measured with the modified Rankin Scale. Dr. Lansberg and his colleagues performed multivariate ordinal logistic regression to calculate the adjusted proportional association between endovascular treatment and clinical outcome among participants of various ages, baseline stroke severities, periods between onset and treatment, locations of the arterial occlusion, and imaging modalities, such as CT or MRI, used to identify salvageable tissue.
The population’s median age was 70 years, and 51% of participants were women. The median National Institutes of Health Stroke Scale score was 16. When the researchers considered the whole sample, they found that younger age, lower baseline NIHSS score, and lower serum glucose level independently predicted better functional outcome. The common odds ratio for improved functional outcome with endovascular therapy, adjusted for these variables, was 3.1. Age, NIHSS score, time to randomization, imaging modality, and location of the arterial occlusion did not interact significantly with treatment effect.
“Our results indicate that advanced age, up to 90 years, should not be considered a contraindication to thrombectomy, provided that the patient is fully independent prior to stroke onset,” said the researchers. “Although age did not modify the treatment effect, it was a strong independent predictor of 90-day disability, which is consistent with prior studies of both tissue plasminogen activator and endovascular therapy.”
The trial’s small sample size may have allowed small differences between groups to pass unnoticed, said the reseachers. Other trials of late-window thrombectomy will be required to validate these results, they concluded.
The National Institute for Neurological Disorders and Stroke supported the study through grants. Several investigators received consulting fees from and hold shares in iSchemaView, which manufactures the software that the investigators used for postprocessing of CT and MRI perfusion studies. Other authors received consulting fees from various pharmaceutical and medical device companies, including Genentech, Medtronic, Pfizer, and Stryker Neurovascular.
SOURCE: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
The location of the arterial occlusive lesion and the imaging technique used to select patients for the procedure also do not influence the therapy’s benefits, the researchers said. Although the proportional benefit of thrombectomy plus medical management is uniform across subgroups, compared with medical management alone, patients may have different amounts of absolute benefit.
The results of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial, which were published in 2018, indicated that endovascular thrombectomy provided clinical benefits for patients with acute ischemic stroke if administered at 6-16 hours after stroke onset. As part of the trial’s prespecified analyses, Maarten G. Lansberg, MD, PhD, associate professor of neurology and neurological sciences at Stanford (Calif.) University Medical Center in California, and his colleagues sought to determine whether thrombectomy had uniform benefit among various patient subgroups (e.g., elderly people, patients with mild symptoms, and those who present late after onset).
A total of 296 patients were enrolled in the randomized, open-label, blinded-endpoint DEFUSE 3 trial at 38 sites in the United States. Eligible participants had acute ischemic stroke resulting from an occlusion of the internal carotid artery or middle cerebral artery and evidence of salvageable tissue on perfusion CT or MRI. In all, 182 patients met these criteria and were randomized and included in the intention-to-treat analysis. The researchers stopped DEFUSE 3 early because of efficacy.
The study’s primary endpoint was functional outcome at day 90, as measured with the modified Rankin Scale. Dr. Lansberg and his colleagues performed multivariate ordinal logistic regression to calculate the adjusted proportional association between endovascular treatment and clinical outcome among participants of various ages, baseline stroke severities, periods between onset and treatment, locations of the arterial occlusion, and imaging modalities, such as CT or MRI, used to identify salvageable tissue.
The population’s median age was 70 years, and 51% of participants were women. The median National Institutes of Health Stroke Scale score was 16. When the researchers considered the whole sample, they found that younger age, lower baseline NIHSS score, and lower serum glucose level independently predicted better functional outcome. The common odds ratio for improved functional outcome with endovascular therapy, adjusted for these variables, was 3.1. Age, NIHSS score, time to randomization, imaging modality, and location of the arterial occlusion did not interact significantly with treatment effect.
“Our results indicate that advanced age, up to 90 years, should not be considered a contraindication to thrombectomy, provided that the patient is fully independent prior to stroke onset,” said the researchers. “Although age did not modify the treatment effect, it was a strong independent predictor of 90-day disability, which is consistent with prior studies of both tissue plasminogen activator and endovascular therapy.”
The trial’s small sample size may have allowed small differences between groups to pass unnoticed, said the reseachers. Other trials of late-window thrombectomy will be required to validate these results, they concluded.
The National Institute for Neurological Disorders and Stroke supported the study through grants. Several investigators received consulting fees from and hold shares in iSchemaView, which manufactures the software that the investigators used for postprocessing of CT and MRI perfusion studies. Other authors received consulting fees from various pharmaceutical and medical device companies, including Genentech, Medtronic, Pfizer, and Stryker Neurovascular.
SOURCE: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
FROM JAMA NEUROLOGY
Key clinical point: Age, symptom severity, and serum glucose do not influence the benefit of thrombectomy for acute ischemic stroke.
Major finding: The adjusted common odds ratio for improved functional outcome with endovascular therapy was 3.1.
Study details: The randomized, open-label, blinded-end-point DEFUSE 3 trial included 182 patients.
Disclosures: The National Institute for Neurological Disorders and Stroke funded the study through grants.
Source: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
Anxiety, depression, burnout higher in physician mothers caring for others at home
Physicians who are also mothers have a higher risk of burnout and mood and anxiety disorders if they are also caring for someone with a serious illness or disability outside of work, according to a cross-sectional survey reported in a letter in JAMA Internal Medicine.
“Our findings highlight the additional caregiving responsibilities of some women physicians and the potential consequences of these additional responsibilities for their behavioral health and careers,” wrote Veronica Yank, MD, of the department of medicine at the University of California, San Francisco, and her colleagues.
“To reduce burnout and improve workforce retention, health care systems should develop new approaches to identify and address the needs of these physician mothers,” they wrote.
The researchers used data from a June-July 2016 online survey of respondents from the Physicians Moms Group online community. Approximately 16,059 members saw the posting for the survey, and 5,613 United States–based mothers participated.
Among the questions was one on non–work related caregiving responsibilities that asked whether the respondent provided “regular care or assistance to a friend or family member with a serious health problem, long-term illness or disability” during the last year. Other questions assessed alcohol and drug use, history of a mood or anxiety disorder, career satisfaction and burnout.
Among the 16.4% of respondents who had additional caregiving responsibilities outside of work for someone chronically or seriously ill or disabled, nearly half (48.3%) said they cared for ill parents, 16.9% for children or infants, 7.7% for a partner, and 28.6% for another relative. In addition, 16.7% of respondents had such caregiving responsibilities for more than one person.
The women with these extra caregiving responsibilities were 21% more likely to have a mood or anxiety disorder (adjusted relative risk, 1.21; P = .02) and 25% more likely to report burnout (aRR, 1.25; P = .007), compared with those who did not have such extra responsibilities.
There were no significant differences, however, on rates of career satisfaction, risky drinking behaviors, or substance abuse between physician mothers who did have additional caregiving responsibilities and those who did not.
Among the study’s limitations were its cross-sectional nature, use of a convenience sample that may not be generalizable or representative, and lack of data on fathers or non-parent physicians for comparison.
SOURCE: Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
Physicians who are also mothers have a higher risk of burnout and mood and anxiety disorders if they are also caring for someone with a serious illness or disability outside of work, according to a cross-sectional survey reported in a letter in JAMA Internal Medicine.
“Our findings highlight the additional caregiving responsibilities of some women physicians and the potential consequences of these additional responsibilities for their behavioral health and careers,” wrote Veronica Yank, MD, of the department of medicine at the University of California, San Francisco, and her colleagues.
“To reduce burnout and improve workforce retention, health care systems should develop new approaches to identify and address the needs of these physician mothers,” they wrote.
The researchers used data from a June-July 2016 online survey of respondents from the Physicians Moms Group online community. Approximately 16,059 members saw the posting for the survey, and 5,613 United States–based mothers participated.
Among the questions was one on non–work related caregiving responsibilities that asked whether the respondent provided “regular care or assistance to a friend or family member with a serious health problem, long-term illness or disability” during the last year. Other questions assessed alcohol and drug use, history of a mood or anxiety disorder, career satisfaction and burnout.
Among the 16.4% of respondents who had additional caregiving responsibilities outside of work for someone chronically or seriously ill or disabled, nearly half (48.3%) said they cared for ill parents, 16.9% for children or infants, 7.7% for a partner, and 28.6% for another relative. In addition, 16.7% of respondents had such caregiving responsibilities for more than one person.
The women with these extra caregiving responsibilities were 21% more likely to have a mood or anxiety disorder (adjusted relative risk, 1.21; P = .02) and 25% more likely to report burnout (aRR, 1.25; P = .007), compared with those who did not have such extra responsibilities.
There were no significant differences, however, on rates of career satisfaction, risky drinking behaviors, or substance abuse between physician mothers who did have additional caregiving responsibilities and those who did not.
Among the study’s limitations were its cross-sectional nature, use of a convenience sample that may not be generalizable or representative, and lack of data on fathers or non-parent physicians for comparison.
SOURCE: Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
Physicians who are also mothers have a higher risk of burnout and mood and anxiety disorders if they are also caring for someone with a serious illness or disability outside of work, according to a cross-sectional survey reported in a letter in JAMA Internal Medicine.
“Our findings highlight the additional caregiving responsibilities of some women physicians and the potential consequences of these additional responsibilities for their behavioral health and careers,” wrote Veronica Yank, MD, of the department of medicine at the University of California, San Francisco, and her colleagues.
“To reduce burnout and improve workforce retention, health care systems should develop new approaches to identify and address the needs of these physician mothers,” they wrote.
The researchers used data from a June-July 2016 online survey of respondents from the Physicians Moms Group online community. Approximately 16,059 members saw the posting for the survey, and 5,613 United States–based mothers participated.
Among the questions was one on non–work related caregiving responsibilities that asked whether the respondent provided “regular care or assistance to a friend or family member with a serious health problem, long-term illness or disability” during the last year. Other questions assessed alcohol and drug use, history of a mood or anxiety disorder, career satisfaction and burnout.
Among the 16.4% of respondents who had additional caregiving responsibilities outside of work for someone chronically or seriously ill or disabled, nearly half (48.3%) said they cared for ill parents, 16.9% for children or infants, 7.7% for a partner, and 28.6% for another relative. In addition, 16.7% of respondents had such caregiving responsibilities for more than one person.
The women with these extra caregiving responsibilities were 21% more likely to have a mood or anxiety disorder (adjusted relative risk, 1.21; P = .02) and 25% more likely to report burnout (aRR, 1.25; P = .007), compared with those who did not have such extra responsibilities.
There were no significant differences, however, on rates of career satisfaction, risky drinking behaviors, or substance abuse between physician mothers who did have additional caregiving responsibilities and those who did not.
Among the study’s limitations were its cross-sectional nature, use of a convenience sample that may not be generalizable or representative, and lack of data on fathers or non-parent physicians for comparison.
SOURCE: Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Risk of anxiety and mood disorders is 21% higher and burnout is 25% higher among physician mothers with extra caregiving at home.
Study details: The findings are based on an online cross-sectional survey of 5,613 United States–based physician mothers conducted from June to July 2016.
Disclosures: No single entity directly funded the study, but the authors were supported by a variety of grants from foundations and the National Institutes of Health at the time it was completed. One coauthor is founder of Equity Quotient, a company that provides gender equity culture analytics for institutions, and another has consulted for Amgen and Vizient and receives stock options as an Equity Quotient advisory board member.
Source: Yank V et al. JAMA Internal Medicine. 2018 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
Routine clinical data may predict psychiatric adverse effects from levetiracetam
Among patients with epilepsy, a simple model that incorporates factors such as a patient’s sex and history of depression, anxiety, and recreational drug use may help predict the risk of a psychiatric adverse effect from levetiracetam, according to a study published in JAMA Neurology.
“This study derived 2 simple models that predict the risk of a psychiatric adverse effect from levetiracetam” and can “guide prescription in clinical practice,” said Colin B. Josephson, MD, of the department of clinical neurosciences at the University of Calgary (Canada) and his research colleagues.
Levetiracetam is a commonly used first-line treatment for epilepsy because of its ease of use, broad spectrum of action, and safety profile, the researchers said. Still, psychiatric adverse reactions occur in as many as 16% of patients and frequently require treatment discontinuation.
To evaluate whether routine clinical data can predict which patients with epilepsy will experience a psychiatric adverse event from levetiracetam, the investigators analyzed data from The Health Improvement Network (THIN) database, which includes anonymized patient records from general practices in the United Kingdom. They assessed 21 variables for possible inclusion in prediction models. They identified these variables by searching the literature and weighing input from a panel of experts.
Their analysis included data from Jan. 1, 2000–May 31, 2012. Among the more than 11 million patients in THIN, the researchers identified 7,300 incident cases of epilepsy. The researchers examined when patients received a first prescription for levetiracetam and whether patients experienced a psychiatric symptom or disorder within 2 years of the prescription.
Among 1,173 patients with epilepsy receiving levetiracetam, the median age was 39 years; about half were women. In all, 14.1% experienced a psychiatric symptom or disorder within 2 years of prescription. Women were more likely to report a psychiatric symptom (odds ratio, 1.41), as were patients with a history of social deprivation (OR, 1.15), anxiety (OR, 1.74), recreational drug use (OR, 2.02), or depression (OR, 2.20).
The final model included female sex, history of depression, history of anxiety, and history of recreational drug use. Low socioeconomic status was not included because “it would be challenging to assign this score in clinic,” the authors said.
“There was a gradient in risk probabilities increasing from 8% for 0 risk factors to 11%-17% for 1, 17% to 31% for 2, 30%-42% for 3, and 49% when all risk factors were present,” Dr. Josephson and his colleagues indicated. “The discovered incremental probability of reporting a psychiatric sign can help generate an index of suspicion to counsel patients.”
Using the example of a woman patient with depression, the model “suggests she would be at risk,” with a 22% chance of a psychiatric adverse event in the 2 years after receiving a levetiracetam prescription.
The researchers created a second prediction algorithm based on data from patients without documentation of a mental health sign, symptom, or disorder prior to their levetiracetam prescription. This model incorporated age, sex, recreational drug use, and levetiracetam daily dose; it performed comparably well and might be used to determine safety of prescription, according to Dr. Josephson and his colleagues.
The authors noted that the study was limited by an inability to evaluate medication adherence and seizure type and frequency. One advantage of the study’s design is that it may have circumvented expectation bias because general practitioners were not prone to anticipating psychiatric adverse events or to have a lower threshold for diagnosing them.
The authors disclosed research fellowships and support from foundations and federal agencies.
SOURCE: Josephson CB et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4561.
Among patients with epilepsy, a simple model that incorporates factors such as a patient’s sex and history of depression, anxiety, and recreational drug use may help predict the risk of a psychiatric adverse effect from levetiracetam, according to a study published in JAMA Neurology.
“This study derived 2 simple models that predict the risk of a psychiatric adverse effect from levetiracetam” and can “guide prescription in clinical practice,” said Colin B. Josephson, MD, of the department of clinical neurosciences at the University of Calgary (Canada) and his research colleagues.
Levetiracetam is a commonly used first-line treatment for epilepsy because of its ease of use, broad spectrum of action, and safety profile, the researchers said. Still, psychiatric adverse reactions occur in as many as 16% of patients and frequently require treatment discontinuation.
To evaluate whether routine clinical data can predict which patients with epilepsy will experience a psychiatric adverse event from levetiracetam, the investigators analyzed data from The Health Improvement Network (THIN) database, which includes anonymized patient records from general practices in the United Kingdom. They assessed 21 variables for possible inclusion in prediction models. They identified these variables by searching the literature and weighing input from a panel of experts.
Their analysis included data from Jan. 1, 2000–May 31, 2012. Among the more than 11 million patients in THIN, the researchers identified 7,300 incident cases of epilepsy. The researchers examined when patients received a first prescription for levetiracetam and whether patients experienced a psychiatric symptom or disorder within 2 years of the prescription.
Among 1,173 patients with epilepsy receiving levetiracetam, the median age was 39 years; about half were women. In all, 14.1% experienced a psychiatric symptom or disorder within 2 years of prescription. Women were more likely to report a psychiatric symptom (odds ratio, 1.41), as were patients with a history of social deprivation (OR, 1.15), anxiety (OR, 1.74), recreational drug use (OR, 2.02), or depression (OR, 2.20).
The final model included female sex, history of depression, history of anxiety, and history of recreational drug use. Low socioeconomic status was not included because “it would be challenging to assign this score in clinic,” the authors said.
“There was a gradient in risk probabilities increasing from 8% for 0 risk factors to 11%-17% for 1, 17% to 31% for 2, 30%-42% for 3, and 49% when all risk factors were present,” Dr. Josephson and his colleagues indicated. “The discovered incremental probability of reporting a psychiatric sign can help generate an index of suspicion to counsel patients.”
Using the example of a woman patient with depression, the model “suggests she would be at risk,” with a 22% chance of a psychiatric adverse event in the 2 years after receiving a levetiracetam prescription.
The researchers created a second prediction algorithm based on data from patients without documentation of a mental health sign, symptom, or disorder prior to their levetiracetam prescription. This model incorporated age, sex, recreational drug use, and levetiracetam daily dose; it performed comparably well and might be used to determine safety of prescription, according to Dr. Josephson and his colleagues.
The authors noted that the study was limited by an inability to evaluate medication adherence and seizure type and frequency. One advantage of the study’s design is that it may have circumvented expectation bias because general practitioners were not prone to anticipating psychiatric adverse events or to have a lower threshold for diagnosing them.
The authors disclosed research fellowships and support from foundations and federal agencies.
SOURCE: Josephson CB et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4561.
Among patients with epilepsy, a simple model that incorporates factors such as a patient’s sex and history of depression, anxiety, and recreational drug use may help predict the risk of a psychiatric adverse effect from levetiracetam, according to a study published in JAMA Neurology.
“This study derived 2 simple models that predict the risk of a psychiatric adverse effect from levetiracetam” and can “guide prescription in clinical practice,” said Colin B. Josephson, MD, of the department of clinical neurosciences at the University of Calgary (Canada) and his research colleagues.
Levetiracetam is a commonly used first-line treatment for epilepsy because of its ease of use, broad spectrum of action, and safety profile, the researchers said. Still, psychiatric adverse reactions occur in as many as 16% of patients and frequently require treatment discontinuation.
To evaluate whether routine clinical data can predict which patients with epilepsy will experience a psychiatric adverse event from levetiracetam, the investigators analyzed data from The Health Improvement Network (THIN) database, which includes anonymized patient records from general practices in the United Kingdom. They assessed 21 variables for possible inclusion in prediction models. They identified these variables by searching the literature and weighing input from a panel of experts.
Their analysis included data from Jan. 1, 2000–May 31, 2012. Among the more than 11 million patients in THIN, the researchers identified 7,300 incident cases of epilepsy. The researchers examined when patients received a first prescription for levetiracetam and whether patients experienced a psychiatric symptom or disorder within 2 years of the prescription.
Among 1,173 patients with epilepsy receiving levetiracetam, the median age was 39 years; about half were women. In all, 14.1% experienced a psychiatric symptom or disorder within 2 years of prescription. Women were more likely to report a psychiatric symptom (odds ratio, 1.41), as were patients with a history of social deprivation (OR, 1.15), anxiety (OR, 1.74), recreational drug use (OR, 2.02), or depression (OR, 2.20).
The final model included female sex, history of depression, history of anxiety, and history of recreational drug use. Low socioeconomic status was not included because “it would be challenging to assign this score in clinic,” the authors said.
“There was a gradient in risk probabilities increasing from 8% for 0 risk factors to 11%-17% for 1, 17% to 31% for 2, 30%-42% for 3, and 49% when all risk factors were present,” Dr. Josephson and his colleagues indicated. “The discovered incremental probability of reporting a psychiatric sign can help generate an index of suspicion to counsel patients.”
Using the example of a woman patient with depression, the model “suggests she would be at risk,” with a 22% chance of a psychiatric adverse event in the 2 years after receiving a levetiracetam prescription.
The researchers created a second prediction algorithm based on data from patients without documentation of a mental health sign, symptom, or disorder prior to their levetiracetam prescription. This model incorporated age, sex, recreational drug use, and levetiracetam daily dose; it performed comparably well and might be used to determine safety of prescription, according to Dr. Josephson and his colleagues.
The authors noted that the study was limited by an inability to evaluate medication adherence and seizure type and frequency. One advantage of the study’s design is that it may have circumvented expectation bias because general practitioners were not prone to anticipating psychiatric adverse events or to have a lower threshold for diagnosing them.
The authors disclosed research fellowships and support from foundations and federal agencies.
SOURCE: Josephson CB et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4561.
FROM JAMA NEUROLOGY
Key clinical point: Among patients with epilepsy, a simple model may help predict the risk of a psychiatric adverse effect from levetiracetam.
Major finding: The likelihood of a psychiatric adverse event increases from 8% for patients with no risk factors to 49% with all risk factors present.
Study details: A retrospective open cohort study of 1,173 patients with epilepsy receiving levetiracetam in the United Kingdom.
Disclosures: The authors disclosed research fellowships and support from foundations and federal agencies.
Source: Josephson CB et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4561
Polysomnography beats Fitbit
Also today, there is no evidence for the disease-modifying effect of levodopa in Parkinson’s Disease, and ablation surpassed drugs for raising quality of life for atrial fibrillation.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, there is no evidence for the disease-modifying effect of levodopa in Parkinson’s Disease, and ablation surpassed drugs for raising quality of life for atrial fibrillation.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, there is no evidence for the disease-modifying effect of levodopa in Parkinson’s Disease, and ablation surpassed drugs for raising quality of life for atrial fibrillation.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Have you paid your 2019 SVS membership dues yet?
To those members who have not yet paid their 2019 dues -- are you planning to attend the Vascular Annual Meeting? Do you find your Journal of Vascular Surgery publications invaluable? Those are just two of the member benefits that will be lost to those who do not pay their dues soon. Others include use of trademarked professional designations and access to the new online communications forum, SVSConnect. Renew today to continue to receive these and all other SVS member benefits.
To those members who have not yet paid their 2019 dues -- are you planning to attend the Vascular Annual Meeting? Do you find your Journal of Vascular Surgery publications invaluable? Those are just two of the member benefits that will be lost to those who do not pay their dues soon. Others include use of trademarked professional designations and access to the new online communications forum, SVSConnect. Renew today to continue to receive these and all other SVS member benefits.
To those members who have not yet paid their 2019 dues -- are you planning to attend the Vascular Annual Meeting? Do you find your Journal of Vascular Surgery publications invaluable? Those are just two of the member benefits that will be lost to those who do not pay their dues soon. Others include use of trademarked professional designations and access to the new online communications forum, SVSConnect. Renew today to continue to receive these and all other SVS member benefits.
Women’s Leadership Training Grant
Because leadership skills are integral to success but are not typically taught in medical school, the SVS Leadership Development and Diversity Committee offers the Women's Leadership Training Grant to help women sharpen their leadership skills. The $5,000 awards (offered at three levels of a woman's career) defray costs to attend leadership courses and activities. Applications are due March 1.
Because leadership skills are integral to success but are not typically taught in medical school, the SVS Leadership Development and Diversity Committee offers the Women's Leadership Training Grant to help women sharpen their leadership skills. The $5,000 awards (offered at three levels of a woman's career) defray costs to attend leadership courses and activities. Applications are due March 1.
Because leadership skills are integral to success but are not typically taught in medical school, the SVS Leadership Development and Diversity Committee offers the Women's Leadership Training Grant to help women sharpen their leadership skills. The $5,000 awards (offered at three levels of a woman's career) defray costs to attend leadership courses and activities. Applications are due March 1.
FDA permits marketing of first M. genitalium diagnostic test
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
Tamsulosin not effective in promoting stone expulsion in symptomatic patients
Clinical question: Does tamsulosin provide benefit in ureteral stone expulsion for patients who present with a symptomatic stone less than 9 mm?
Background: Treatment of urinary stone disease often includes the use of alpha-blockers such as tamsulosin to promote stone passage, and between 15% and 55% of patients presenting to EDs for renal colic are prescribed alpha-blockers. Current treatment guidelines support the use of tamsulosin, with recent evidence suggesting that this treatment is more effective for larger stones (5-10 mm). However, other prospective trials have called these guidelines into question.
Study design: Double-blind, placebo-controlled study.
Setting: Six emergency departments at U.S. tertiary-care hospitals.
Synopsis: 512 participants with symptomatic ureteral stones were randomized to either tamsulosin or placebo. At the end of a 28-day treatment period, the rate of urinary stone passage was 49.6% in the tamsulosin group vs. 47.3% in the placebo group (95.8% confidence interval, 0.87-1.27; P = .60). The time to stone passage also was not different between treatment groups (P = .92). A second phase of the trial also evaluated stone passage by CT scan at 28 days, with stone passage rates of 83.6% in the tamsulosin group and 77.6% in the placebo group (95% CI, 0.95-1.22; P = .24). This study is the largest of its kind in the United States, with findings similar to those of two recent international multisite trials, increasing the evidence that tamsulosin is not beneficial for larger stone passage.
Bottom line: For patients presenting to the ED for renal colic from ureteral stones smaller than 9 mm, tamsulosin does not appear to promote stone passage.
Citation: Meltzer AC et al. Effect of tamsulosin on passage of symptomatic ureteral stones: A randomized clinical trial. JAMA Intern Med. 2018;178(8):1051-7. Published online June 18, 2018.
Dr. Breviu is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Clinical question: Does tamsulosin provide benefit in ureteral stone expulsion for patients who present with a symptomatic stone less than 9 mm?
Background: Treatment of urinary stone disease often includes the use of alpha-blockers such as tamsulosin to promote stone passage, and between 15% and 55% of patients presenting to EDs for renal colic are prescribed alpha-blockers. Current treatment guidelines support the use of tamsulosin, with recent evidence suggesting that this treatment is more effective for larger stones (5-10 mm). However, other prospective trials have called these guidelines into question.
Study design: Double-blind, placebo-controlled study.
Setting: Six emergency departments at U.S. tertiary-care hospitals.
Synopsis: 512 participants with symptomatic ureteral stones were randomized to either tamsulosin or placebo. At the end of a 28-day treatment period, the rate of urinary stone passage was 49.6% in the tamsulosin group vs. 47.3% in the placebo group (95.8% confidence interval, 0.87-1.27; P = .60). The time to stone passage also was not different between treatment groups (P = .92). A second phase of the trial also evaluated stone passage by CT scan at 28 days, with stone passage rates of 83.6% in the tamsulosin group and 77.6% in the placebo group (95% CI, 0.95-1.22; P = .24). This study is the largest of its kind in the United States, with findings similar to those of two recent international multisite trials, increasing the evidence that tamsulosin is not beneficial for larger stone passage.
Bottom line: For patients presenting to the ED for renal colic from ureteral stones smaller than 9 mm, tamsulosin does not appear to promote stone passage.
Citation: Meltzer AC et al. Effect of tamsulosin on passage of symptomatic ureteral stones: A randomized clinical trial. JAMA Intern Med. 2018;178(8):1051-7. Published online June 18, 2018.
Dr. Breviu is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Clinical question: Does tamsulosin provide benefit in ureteral stone expulsion for patients who present with a symptomatic stone less than 9 mm?
Background: Treatment of urinary stone disease often includes the use of alpha-blockers such as tamsulosin to promote stone passage, and between 15% and 55% of patients presenting to EDs for renal colic are prescribed alpha-blockers. Current treatment guidelines support the use of tamsulosin, with recent evidence suggesting that this treatment is more effective for larger stones (5-10 mm). However, other prospective trials have called these guidelines into question.
Study design: Double-blind, placebo-controlled study.
Setting: Six emergency departments at U.S. tertiary-care hospitals.
Synopsis: 512 participants with symptomatic ureteral stones were randomized to either tamsulosin or placebo. At the end of a 28-day treatment period, the rate of urinary stone passage was 49.6% in the tamsulosin group vs. 47.3% in the placebo group (95.8% confidence interval, 0.87-1.27; P = .60). The time to stone passage also was not different between treatment groups (P = .92). A second phase of the trial also evaluated stone passage by CT scan at 28 days, with stone passage rates of 83.6% in the tamsulosin group and 77.6% in the placebo group (95% CI, 0.95-1.22; P = .24). This study is the largest of its kind in the United States, with findings similar to those of two recent international multisite trials, increasing the evidence that tamsulosin is not beneficial for larger stone passage.
Bottom line: For patients presenting to the ED for renal colic from ureteral stones smaller than 9 mm, tamsulosin does not appear to promote stone passage.
Citation: Meltzer AC et al. Effect of tamsulosin on passage of symptomatic ureteral stones: A randomized clinical trial. JAMA Intern Med. 2018;178(8):1051-7. Published online June 18, 2018.
Dr. Breviu is assistant professor of medicine and an academic hospitalist, University of Utah, Salt Lake City.
Before you refer for AF ablation
SNOWMASS, COLO. – Appropriate in the way of benefit, along with instilling awareness of the warning signals heralding serious late complications, Samuel J. Asirvatham, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Who to steer toward ablation? You have to have a symptomatic patient – that’s a given. For the ones who are paroxysmal, the ones with a relatively normal heart, there’s a much better chance that you’ll help manage their symptoms with ablation than if they have persistent or permanent A-fib. Notice I do not use the word ‘cure’ for A-fib. We talk about controlling symptoms and decreasing frequency, because the longer follow-up you have with intensive monitoring, the more you realize that patients still tend to have some A-fib,” explained Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
The rationale for early atrial fibrillation (AF) ablation in younger patients with troublesome symptoms of paroxysmal AF despite pharmacologic attempts at rate or rhythm control is that it will arrest the progression from an atrial arrhythmia that has just a few triggers readily neutralized by pulmonary vein isolation to persistent AF with a diseased heart and a multitude of arrhythmia trigger points coming from many directions.
A solid candidate for ablation of paroxysmal AF has about a 75% likelihood of having a successful first ablation procedure, with substantial improvement in symptoms and no need for medication. Another 9%-10% will achieve marked reduction in symptom burden upon addition of antiarrhythmic agents that weren’t effective before ablation.
Late complications can be deceptive
Periprocedural stroke/transient ischemic attack, tamponade, or bleeding on the table are infrequent complications readily recognized by the interventionalist. More problematic are several late complications which are often misinterpreted, with the resultant delay causing major harm.
- Pulmonary vein stenosis. This complication of inadvertent ablation inside the pulmonary vein manifests as shortness of breath, typically beginning about 4 weeks post ablation.
“This is very different from the shortness of breath they had with atrial fibrillation. They almost always have a cough that they didn’t have before, and they may have hemoptysis. It’s very important to recognize this promptly, because before it closes completely we can do an angioplasty and stent the vein with good results. But once it closes completely, it becomes an extremely complicated procedure to try to reopen that vein,” according to Dr. Asirvatham.
Very often the patient’s general cardiologist, chest physician, or primary care physician fails to recognize what’s happening. He cited an example: He recently had a patient with a cough who was first referred to an infectious disease specialist, who ordered a bronchoalveolar lavage. The specimen grew atypical actinomycetes. That prompted a referral to thoracic surgery for an open-lung biopsy. But that procedure required cardiac clearance beforehand. It was a cardiologist who said, ‘Wait – all this started after you had an ablation?’
“That patient had pulmonary vein stenosis. And, unfortunately, that complication has not gone away. Being a referral center for pulmonary vein isolation, we see just as many cases of pulmonary vein stenosis today as we did a few years ago,” he said.
- Atrial esophageal fistula. The hallmark of this complication is onset of a plethora of what Dr. Asirvatham called “funny symptoms” more than a month post ablation. These include fever, transient ischemic attacks (TIAs), sepsislike symptoms, discomfort in swallowing, and in some cases hemoptysis.
“The predominant picture is endocarditis/TIA/stroke. If you see this, and the patient has had ablation, immediately refer to surgery to have the fistula between the esophagus and heart fixed. This is not a patient where you say, ‘Nothing by mouth, give some antibiotics, and see what happens.’ I can tell you what will happen: The patient will die,” the cardiologist said.
- Atrial stiffness. This typically occurs about a month after a second or third ablation procedure, when the patient develops shortness of breath that keeps worsening.
“You think ‘pulmonary vein stenosis,’ but the CT scan shows the veins are wide open. Many of these patients will get misdiagnosed as having heart failure with preserved ejection fraction even though they never had it before. The problem here is the atrium has become too stiff from the ablation, and this stiff atrium causes increased pressure, resulting in the shortness of breath. Sometimes patients feel better over time, but sometimes it’s very difficult to treat. But it’s important to recognize atrial stiffness and exclude other causes like pulmonary vein stenosis,” Dr. Asirvatham continued.
- Gastroparesis. This occurs because of injury to the vagus nerve branches located at the top of the esophagus, with resultant delayed gastric emptying.
“It’s an uncomfortable feeling of fullness all the time. The patient will say, ‘It seems like I just ate, even though I ate 8 hours ago,” the electrophysiologist said. “Most of these patients will recover in about 6 months. They may feel better on a gastric motility agent, like a macrolide antibiotic. I personally have not seen a patient who did not feel better within 6-8 months.”
Novel treatment approaches: “A-fib may be an autonomic epilepsy of the heart”
“Patients sometimes will ask you, ‘What is this ablation? What does that mean?’ You have to be truthful and tell them that it’s just a fancy word for burning,” the electrophysiologist said.
Achievement of AF ablation without radiofrequency or cryoablation, instead utilizing nonthermal direct-current pulsed electrical fields, is “the hottest topic in the field of electrophysiology,” according to Dr. Asirvatham.
These electrical fields result in irreversible electroporation of targeted myocardial cell membranes, leading to cell death. It is a tissue-specific intervention, so it’s much less likely than conventional ablation to cause collateral damage to the esophagus and other structures.
“Direct current electroporation has transitioned from proof-of-concept studies to three relatively large patient trials. This is potentially an important breakthrough because if we don’t heat, a lot of the complications of A-fib ablation will probably decrease,” he explained.
Two other promising outside-the-box approaches to the treatment of AF are autonomic nervous system modulation at sites distant from the heart and particle beam ablation without need for cardiac catheters.
“If you put electrodes everywhere in the body to see where A-fib starts, it’s not in the atrium, not in the pulmonary veins, it’s in the nerves behind the pulmonary veins, and before those nerves it’s in some other area of the autonomic nervous system. This has given rise to the notion that A-fib may be an autonomic epilepsy of the heart,” according to the electrophysiologist.
This concept has given rise to a completely different approach to treatment of AF through neurostimulation. That’s how acupuncture works. Also, headphones have been used successfully to terminate and prevent AF by stimulating autonomic nerve centers near the ears. Low-level electrical stimulation of the vagus nerve in order to reduce stellate ganglion activity is under study. So is the application of botulinum toxin at key points in the autonomic nervous system.
“Catheters, drugs, and devices that target these areas, maybe without any ablation in the heart itself, is an exciting area of future management of A-fib,” he said.
Another promising approach is borrowed from radiation oncology: particulate ablation using beams of carbon atoms, protons, or photons.
“The first patients have now been treated for ventricular tachycardia and A-fib. It really is quite amazing how precise the lesion formation is. And with no catheters in the heart, clot can’t form on catheters,” he observed.
Dr. Asirvatham reported having no financial conflicts regarding his presentation, although he serves as a consultant to a handful of medical startup companies and holds patents on intellectual property, the royalties for which go directly to the Mayo Clinic.
SNOWMASS, COLO. – Appropriate in the way of benefit, along with instilling awareness of the warning signals heralding serious late complications, Samuel J. Asirvatham, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Who to steer toward ablation? You have to have a symptomatic patient – that’s a given. For the ones who are paroxysmal, the ones with a relatively normal heart, there’s a much better chance that you’ll help manage their symptoms with ablation than if they have persistent or permanent A-fib. Notice I do not use the word ‘cure’ for A-fib. We talk about controlling symptoms and decreasing frequency, because the longer follow-up you have with intensive monitoring, the more you realize that patients still tend to have some A-fib,” explained Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
The rationale for early atrial fibrillation (AF) ablation in younger patients with troublesome symptoms of paroxysmal AF despite pharmacologic attempts at rate or rhythm control is that it will arrest the progression from an atrial arrhythmia that has just a few triggers readily neutralized by pulmonary vein isolation to persistent AF with a diseased heart and a multitude of arrhythmia trigger points coming from many directions.
A solid candidate for ablation of paroxysmal AF has about a 75% likelihood of having a successful first ablation procedure, with substantial improvement in symptoms and no need for medication. Another 9%-10% will achieve marked reduction in symptom burden upon addition of antiarrhythmic agents that weren’t effective before ablation.
Late complications can be deceptive
Periprocedural stroke/transient ischemic attack, tamponade, or bleeding on the table are infrequent complications readily recognized by the interventionalist. More problematic are several late complications which are often misinterpreted, with the resultant delay causing major harm.
- Pulmonary vein stenosis. This complication of inadvertent ablation inside the pulmonary vein manifests as shortness of breath, typically beginning about 4 weeks post ablation.
“This is very different from the shortness of breath they had with atrial fibrillation. They almost always have a cough that they didn’t have before, and they may have hemoptysis. It’s very important to recognize this promptly, because before it closes completely we can do an angioplasty and stent the vein with good results. But once it closes completely, it becomes an extremely complicated procedure to try to reopen that vein,” according to Dr. Asirvatham.
Very often the patient’s general cardiologist, chest physician, or primary care physician fails to recognize what’s happening. He cited an example: He recently had a patient with a cough who was first referred to an infectious disease specialist, who ordered a bronchoalveolar lavage. The specimen grew atypical actinomycetes. That prompted a referral to thoracic surgery for an open-lung biopsy. But that procedure required cardiac clearance beforehand. It was a cardiologist who said, ‘Wait – all this started after you had an ablation?’
“That patient had pulmonary vein stenosis. And, unfortunately, that complication has not gone away. Being a referral center for pulmonary vein isolation, we see just as many cases of pulmonary vein stenosis today as we did a few years ago,” he said.
- Atrial esophageal fistula. The hallmark of this complication is onset of a plethora of what Dr. Asirvatham called “funny symptoms” more than a month post ablation. These include fever, transient ischemic attacks (TIAs), sepsislike symptoms, discomfort in swallowing, and in some cases hemoptysis.
“The predominant picture is endocarditis/TIA/stroke. If you see this, and the patient has had ablation, immediately refer to surgery to have the fistula between the esophagus and heart fixed. This is not a patient where you say, ‘Nothing by mouth, give some antibiotics, and see what happens.’ I can tell you what will happen: The patient will die,” the cardiologist said.
- Atrial stiffness. This typically occurs about a month after a second or third ablation procedure, when the patient develops shortness of breath that keeps worsening.
“You think ‘pulmonary vein stenosis,’ but the CT scan shows the veins are wide open. Many of these patients will get misdiagnosed as having heart failure with preserved ejection fraction even though they never had it before. The problem here is the atrium has become too stiff from the ablation, and this stiff atrium causes increased pressure, resulting in the shortness of breath. Sometimes patients feel better over time, but sometimes it’s very difficult to treat. But it’s important to recognize atrial stiffness and exclude other causes like pulmonary vein stenosis,” Dr. Asirvatham continued.
- Gastroparesis. This occurs because of injury to the vagus nerve branches located at the top of the esophagus, with resultant delayed gastric emptying.
“It’s an uncomfortable feeling of fullness all the time. The patient will say, ‘It seems like I just ate, even though I ate 8 hours ago,” the electrophysiologist said. “Most of these patients will recover in about 6 months. They may feel better on a gastric motility agent, like a macrolide antibiotic. I personally have not seen a patient who did not feel better within 6-8 months.”
Novel treatment approaches: “A-fib may be an autonomic epilepsy of the heart”
“Patients sometimes will ask you, ‘What is this ablation? What does that mean?’ You have to be truthful and tell them that it’s just a fancy word for burning,” the electrophysiologist said.
Achievement of AF ablation without radiofrequency or cryoablation, instead utilizing nonthermal direct-current pulsed electrical fields, is “the hottest topic in the field of electrophysiology,” according to Dr. Asirvatham.
These electrical fields result in irreversible electroporation of targeted myocardial cell membranes, leading to cell death. It is a tissue-specific intervention, so it’s much less likely than conventional ablation to cause collateral damage to the esophagus and other structures.
“Direct current electroporation has transitioned from proof-of-concept studies to three relatively large patient trials. This is potentially an important breakthrough because if we don’t heat, a lot of the complications of A-fib ablation will probably decrease,” he explained.
Two other promising outside-the-box approaches to the treatment of AF are autonomic nervous system modulation at sites distant from the heart and particle beam ablation without need for cardiac catheters.
“If you put electrodes everywhere in the body to see where A-fib starts, it’s not in the atrium, not in the pulmonary veins, it’s in the nerves behind the pulmonary veins, and before those nerves it’s in some other area of the autonomic nervous system. This has given rise to the notion that A-fib may be an autonomic epilepsy of the heart,” according to the electrophysiologist.
This concept has given rise to a completely different approach to treatment of AF through neurostimulation. That’s how acupuncture works. Also, headphones have been used successfully to terminate and prevent AF by stimulating autonomic nerve centers near the ears. Low-level electrical stimulation of the vagus nerve in order to reduce stellate ganglion activity is under study. So is the application of botulinum toxin at key points in the autonomic nervous system.
“Catheters, drugs, and devices that target these areas, maybe without any ablation in the heart itself, is an exciting area of future management of A-fib,” he said.
Another promising approach is borrowed from radiation oncology: particulate ablation using beams of carbon atoms, protons, or photons.
“The first patients have now been treated for ventricular tachycardia and A-fib. It really is quite amazing how precise the lesion formation is. And with no catheters in the heart, clot can’t form on catheters,” he observed.
Dr. Asirvatham reported having no financial conflicts regarding his presentation, although he serves as a consultant to a handful of medical startup companies and holds patents on intellectual property, the royalties for which go directly to the Mayo Clinic.
SNOWMASS, COLO. – Appropriate in the way of benefit, along with instilling awareness of the warning signals heralding serious late complications, Samuel J. Asirvatham, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Who to steer toward ablation? You have to have a symptomatic patient – that’s a given. For the ones who are paroxysmal, the ones with a relatively normal heart, there’s a much better chance that you’ll help manage their symptoms with ablation than if they have persistent or permanent A-fib. Notice I do not use the word ‘cure’ for A-fib. We talk about controlling symptoms and decreasing frequency, because the longer follow-up you have with intensive monitoring, the more you realize that patients still tend to have some A-fib,” explained Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
The rationale for early atrial fibrillation (AF) ablation in younger patients with troublesome symptoms of paroxysmal AF despite pharmacologic attempts at rate or rhythm control is that it will arrest the progression from an atrial arrhythmia that has just a few triggers readily neutralized by pulmonary vein isolation to persistent AF with a diseased heart and a multitude of arrhythmia trigger points coming from many directions.
A solid candidate for ablation of paroxysmal AF has about a 75% likelihood of having a successful first ablation procedure, with substantial improvement in symptoms and no need for medication. Another 9%-10% will achieve marked reduction in symptom burden upon addition of antiarrhythmic agents that weren’t effective before ablation.
Late complications can be deceptive
Periprocedural stroke/transient ischemic attack, tamponade, or bleeding on the table are infrequent complications readily recognized by the interventionalist. More problematic are several late complications which are often misinterpreted, with the resultant delay causing major harm.
- Pulmonary vein stenosis. This complication of inadvertent ablation inside the pulmonary vein manifests as shortness of breath, typically beginning about 4 weeks post ablation.
“This is very different from the shortness of breath they had with atrial fibrillation. They almost always have a cough that they didn’t have before, and they may have hemoptysis. It’s very important to recognize this promptly, because before it closes completely we can do an angioplasty and stent the vein with good results. But once it closes completely, it becomes an extremely complicated procedure to try to reopen that vein,” according to Dr. Asirvatham.
Very often the patient’s general cardiologist, chest physician, or primary care physician fails to recognize what’s happening. He cited an example: He recently had a patient with a cough who was first referred to an infectious disease specialist, who ordered a bronchoalveolar lavage. The specimen grew atypical actinomycetes. That prompted a referral to thoracic surgery for an open-lung biopsy. But that procedure required cardiac clearance beforehand. It was a cardiologist who said, ‘Wait – all this started after you had an ablation?’
“That patient had pulmonary vein stenosis. And, unfortunately, that complication has not gone away. Being a referral center for pulmonary vein isolation, we see just as many cases of pulmonary vein stenosis today as we did a few years ago,” he said.
- Atrial esophageal fistula. The hallmark of this complication is onset of a plethora of what Dr. Asirvatham called “funny symptoms” more than a month post ablation. These include fever, transient ischemic attacks (TIAs), sepsislike symptoms, discomfort in swallowing, and in some cases hemoptysis.
“The predominant picture is endocarditis/TIA/stroke. If you see this, and the patient has had ablation, immediately refer to surgery to have the fistula between the esophagus and heart fixed. This is not a patient where you say, ‘Nothing by mouth, give some antibiotics, and see what happens.’ I can tell you what will happen: The patient will die,” the cardiologist said.
- Atrial stiffness. This typically occurs about a month after a second or third ablation procedure, when the patient develops shortness of breath that keeps worsening.
“You think ‘pulmonary vein stenosis,’ but the CT scan shows the veins are wide open. Many of these patients will get misdiagnosed as having heart failure with preserved ejection fraction even though they never had it before. The problem here is the atrium has become too stiff from the ablation, and this stiff atrium causes increased pressure, resulting in the shortness of breath. Sometimes patients feel better over time, but sometimes it’s very difficult to treat. But it’s important to recognize atrial stiffness and exclude other causes like pulmonary vein stenosis,” Dr. Asirvatham continued.
- Gastroparesis. This occurs because of injury to the vagus nerve branches located at the top of the esophagus, with resultant delayed gastric emptying.
“It’s an uncomfortable feeling of fullness all the time. The patient will say, ‘It seems like I just ate, even though I ate 8 hours ago,” the electrophysiologist said. “Most of these patients will recover in about 6 months. They may feel better on a gastric motility agent, like a macrolide antibiotic. I personally have not seen a patient who did not feel better within 6-8 months.”
Novel treatment approaches: “A-fib may be an autonomic epilepsy of the heart”
“Patients sometimes will ask you, ‘What is this ablation? What does that mean?’ You have to be truthful and tell them that it’s just a fancy word for burning,” the electrophysiologist said.
Achievement of AF ablation without radiofrequency or cryoablation, instead utilizing nonthermal direct-current pulsed electrical fields, is “the hottest topic in the field of electrophysiology,” according to Dr. Asirvatham.
These electrical fields result in irreversible electroporation of targeted myocardial cell membranes, leading to cell death. It is a tissue-specific intervention, so it’s much less likely than conventional ablation to cause collateral damage to the esophagus and other structures.
“Direct current electroporation has transitioned from proof-of-concept studies to three relatively large patient trials. This is potentially an important breakthrough because if we don’t heat, a lot of the complications of A-fib ablation will probably decrease,” he explained.
Two other promising outside-the-box approaches to the treatment of AF are autonomic nervous system modulation at sites distant from the heart and particle beam ablation without need for cardiac catheters.
“If you put electrodes everywhere in the body to see where A-fib starts, it’s not in the atrium, not in the pulmonary veins, it’s in the nerves behind the pulmonary veins, and before those nerves it’s in some other area of the autonomic nervous system. This has given rise to the notion that A-fib may be an autonomic epilepsy of the heart,” according to the electrophysiologist.
This concept has given rise to a completely different approach to treatment of AF through neurostimulation. That’s how acupuncture works. Also, headphones have been used successfully to terminate and prevent AF by stimulating autonomic nerve centers near the ears. Low-level electrical stimulation of the vagus nerve in order to reduce stellate ganglion activity is under study. So is the application of botulinum toxin at key points in the autonomic nervous system.
“Catheters, drugs, and devices that target these areas, maybe without any ablation in the heart itself, is an exciting area of future management of A-fib,” he said.
Another promising approach is borrowed from radiation oncology: particulate ablation using beams of carbon atoms, protons, or photons.
“The first patients have now been treated for ventricular tachycardia and A-fib. It really is quite amazing how precise the lesion formation is. And with no catheters in the heart, clot can’t form on catheters,” he observed.
Dr. Asirvatham reported having no financial conflicts regarding his presentation, although he serves as a consultant to a handful of medical startup companies and holds patents on intellectual property, the royalties for which go directly to the Mayo Clinic.
REPORTING FROM ACC SNOWMASS 2019
A theory of relativity for rosacea patients
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.
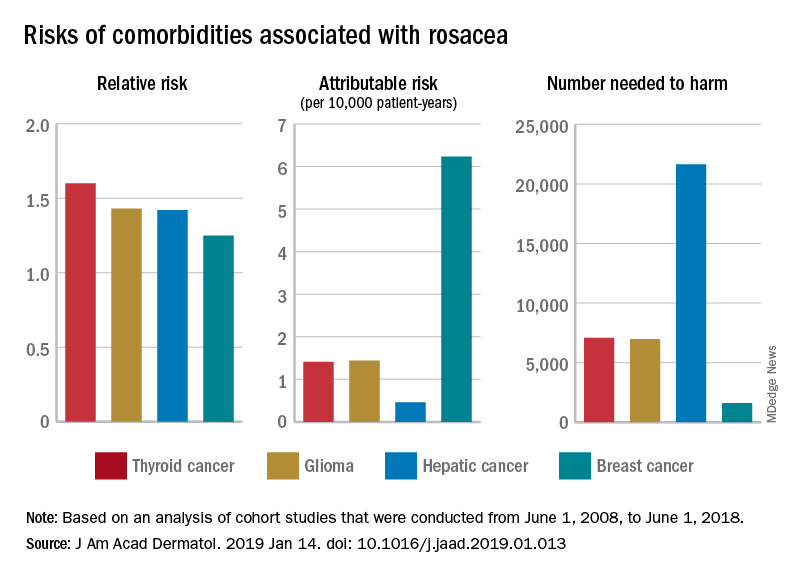
For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.

For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.

For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY








