User login
Looking back to reflect on how far we’ve come
During the holiday break I took some time to organize a lot of old family pictures: deleting duplicates, merging those I pulled off my dad’s computer when he died (which was over 5 years ago), importing ones I took with old digital cameras that were in separate folders ... a bunch of stuff. Some were even childhood pics of me that had been scanned into digital formats. Lots of gigabytes. Lots of time spent watching the little “importing” wheel spin.
As I scrolled through them – literally 5,891 pics and 679 videos – I watched as it became more than a bunch of photos. I watched myself grow up, go through medical school, get married, raise a family. My hair went from brown to gray and receding. My kids went from toddlers to young adults about to leave for college.
It was the story of my life. Without meaning to, it’s what the pictures had become.
It was late at night, but I kept scrolling back and forth. My parents, wife, and others aged in front of me.
Looking in the mirror, or seeing others each day, we never notice the slow changes that time brings. You don’t really see it just thumbing through old photos, either.
But here, in the photos app (something entirely undreamed of in my childhood), I was watching it like it was a movie. Even childhood pictures of my parents. Them dating and getting married. Holding me after bringing me home from the hospital.
I’m certainly not the first to have these thoughts, nor will I be the last. We all go through life in a somewhat organized yet haphazard way, and only when looking backward do we really see how far we’ve come ... often realizing we’re past the halfway point.
Not that this is a bad thing. I mean, that’s life on Earth. It has its good and bad, and aging is part of the rules for all of us.
I suppose you could look at this in terms of our profession. We all (or at least most of us) start out as hospital patients. As we get older and become doctors, hopefully we need to see our own kind less often while at the same time seeing others as patients. As time goes by, most of us start to need to see doctors again, and as we retire and stop practicing medicine, we move back toward being patients ourselves.
For me, the pictures bring back memories and strike emotions in the way hearing or reading stories never can. They give new life to long-forgotten thoughts. Happy and sad, but overall a feeling of contentment that, so far, I feel like I’ve done more good than bad, more right than wrong.
I hope I always feel that way.
I hope everyone else does, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
During the holiday break I took some time to organize a lot of old family pictures: deleting duplicates, merging those I pulled off my dad’s computer when he died (which was over 5 years ago), importing ones I took with old digital cameras that were in separate folders ... a bunch of stuff. Some were even childhood pics of me that had been scanned into digital formats. Lots of gigabytes. Lots of time spent watching the little “importing” wheel spin.
As I scrolled through them – literally 5,891 pics and 679 videos – I watched as it became more than a bunch of photos. I watched myself grow up, go through medical school, get married, raise a family. My hair went from brown to gray and receding. My kids went from toddlers to young adults about to leave for college.
It was the story of my life. Without meaning to, it’s what the pictures had become.
It was late at night, but I kept scrolling back and forth. My parents, wife, and others aged in front of me.
Looking in the mirror, or seeing others each day, we never notice the slow changes that time brings. You don’t really see it just thumbing through old photos, either.
But here, in the photos app (something entirely undreamed of in my childhood), I was watching it like it was a movie. Even childhood pictures of my parents. Them dating and getting married. Holding me after bringing me home from the hospital.
I’m certainly not the first to have these thoughts, nor will I be the last. We all go through life in a somewhat organized yet haphazard way, and only when looking backward do we really see how far we’ve come ... often realizing we’re past the halfway point.
Not that this is a bad thing. I mean, that’s life on Earth. It has its good and bad, and aging is part of the rules for all of us.
I suppose you could look at this in terms of our profession. We all (or at least most of us) start out as hospital patients. As we get older and become doctors, hopefully we need to see our own kind less often while at the same time seeing others as patients. As time goes by, most of us start to need to see doctors again, and as we retire and stop practicing medicine, we move back toward being patients ourselves.
For me, the pictures bring back memories and strike emotions in the way hearing or reading stories never can. They give new life to long-forgotten thoughts. Happy and sad, but overall a feeling of contentment that, so far, I feel like I’ve done more good than bad, more right than wrong.
I hope I always feel that way.
I hope everyone else does, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
During the holiday break I took some time to organize a lot of old family pictures: deleting duplicates, merging those I pulled off my dad’s computer when he died (which was over 5 years ago), importing ones I took with old digital cameras that were in separate folders ... a bunch of stuff. Some were even childhood pics of me that had been scanned into digital formats. Lots of gigabytes. Lots of time spent watching the little “importing” wheel spin.
As I scrolled through them – literally 5,891 pics and 679 videos – I watched as it became more than a bunch of photos. I watched myself grow up, go through medical school, get married, raise a family. My hair went from brown to gray and receding. My kids went from toddlers to young adults about to leave for college.
It was the story of my life. Without meaning to, it’s what the pictures had become.
It was late at night, but I kept scrolling back and forth. My parents, wife, and others aged in front of me.
Looking in the mirror, or seeing others each day, we never notice the slow changes that time brings. You don’t really see it just thumbing through old photos, either.
But here, in the photos app (something entirely undreamed of in my childhood), I was watching it like it was a movie. Even childhood pictures of my parents. Them dating and getting married. Holding me after bringing me home from the hospital.
I’m certainly not the first to have these thoughts, nor will I be the last. We all go through life in a somewhat organized yet haphazard way, and only when looking backward do we really see how far we’ve come ... often realizing we’re past the halfway point.
Not that this is a bad thing. I mean, that’s life on Earth. It has its good and bad, and aging is part of the rules for all of us.
I suppose you could look at this in terms of our profession. We all (or at least most of us) start out as hospital patients. As we get older and become doctors, hopefully we need to see our own kind less often while at the same time seeing others as patients. As time goes by, most of us start to need to see doctors again, and as we retire and stop practicing medicine, we move back toward being patients ourselves.
For me, the pictures bring back memories and strike emotions in the way hearing or reading stories never can. They give new life to long-forgotten thoughts. Happy and sad, but overall a feeling of contentment that, so far, I feel like I’ve done more good than bad, more right than wrong.
I hope I always feel that way.
I hope everyone else does, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
End Tidal Capnography in the Emergency Department
Capnography is the measurement of the partial pressure of carbon dioxide (CO2) in exhaled air.1 It provides real-time information on ventilation (elimination of CO2), perfusion (CO2 transportation in vasculature), and metabolism (production of CO2 via cellular metabolism).2 The technology was originally developed in the 1970s to monitor general anesthesia patients; however, its reach has since broadened, with numerous applications currently in use and in development for the emergency provider (EP).3
Capnography exists in two configurations: a mainstream device that attaches directly to the hub of an endotracheal tube (ETT) and a side-stream device that measure levels via nasal or nasal-oral cannula.1,3
Qualitative monitors use a colorimetric device that monitors the end-tidal CO2 (EtCO2) in exhaled gas and changes color depending on the amount of CO2 present.2,4 Expired CO2 and H20 form carbonic acid, causing the specially treated litmus paper inside the device to change from purple to yellow.2,4 Quantitative monitors display a capnogram, the waveform of expired CO2 as a function of time; as well as the capnometer, which depicts the numerical EtCO2 for each breath.4 In this overview, we will discuss the general interpretation of capnography and its specific uses in the ED.
The Capnogram
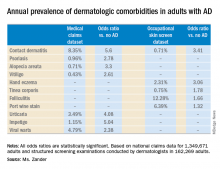
Just like the various stages of an electrocardiogram represent different phases of the cardiac cycle, different phases of a capnogram correspond to different phases of the respiratory cycle. Knowing how to analyze and interpret each phase will contribute to the utility of capnography. While there has been considerable ambiguity in the terminology related to the capnogram,5-7 the most frequently referenced capnogram terminology consists of the following phases (Figure 1):
Phase I: represents beginning of exhalation, where the dead space is cleared from the upper airway.2 This should be zero unless the patient is rebreathing CO2-laden expired gas from either artificially increased dead space or hypoventilation.2,8 A precipitous rise in both the baseline and EtCO2 may indicate contamination of the sensor, such as with secretions or water vapor.2,6
Phase II: rapid rise in exhaled as the CO2 from the alveoli reaches the sensor.4 This rise should be steep, particularly when ventilation to perfusion (V/Q) is well matched. More V/Q heterogeneity, such as with COPD or asthma, leads to a more gradual slope.9 A more gradual phase 2 slope may also indicate a delay in CO2 delivery to the sampling site, such as with bronchospasm or ETT kinking.2
Phase III: the expiratory plateau, which represents the CO2 concentration approaching equilibrium from alveoli to nose. The plateau should be nearly horizontal.2 If all alveoli had the same pCO2, this plateau would be perfectly flat, but spatial and temporal mismatch in alveolar V/Q ratios result in variable exhaled CO2. When there is substantial V/Q heterogeneity, the slope of the plateau will increase.1,2,6
Phase IV: the initiation of inspiration, which should be a nearly vertical drop to a baseline. If prolonged or bleeding into the expiratory phase, consider a leak in the expiratory portion of the circuit, such as an ETT tube cuff leak.2
Phase 0: the inspiratory segment
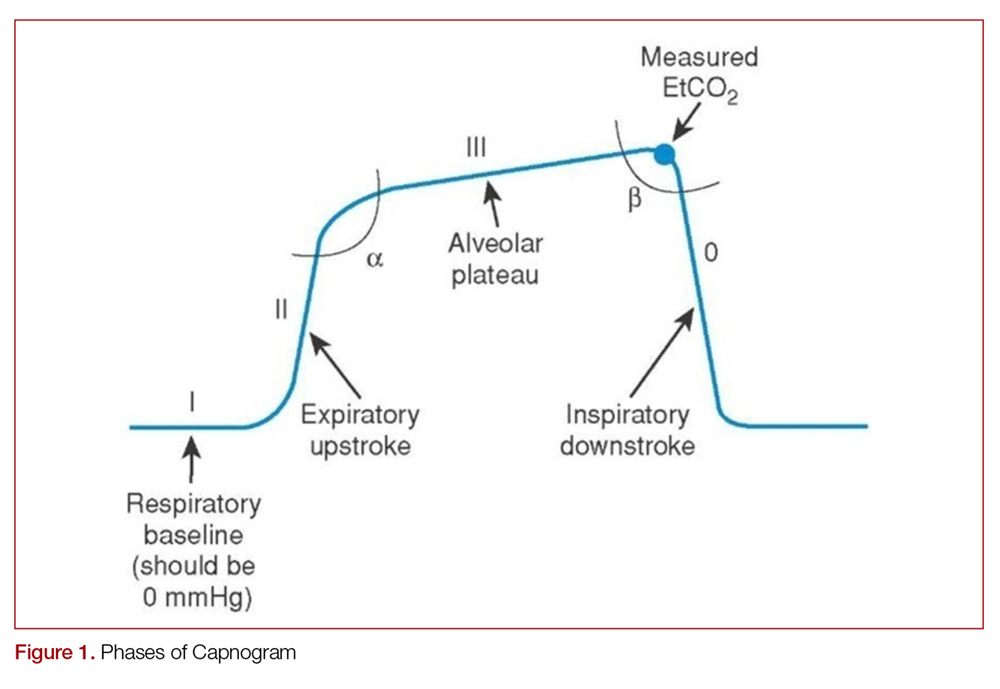
Another important part of the capnogram is the alpha angle. This is the angle of transition between Phase II and Phase III. The combination of a prolonged phase II and steeper phase III leads to a more obtuse alpha angle and will have a “shark-fin” appearance to the capnogram. This suggests an obstructive process, such as asthma or COPD (Figure 2).1,2,6
Standard Uses
Intubation
Capnography, along with visualizing ETT placement through the vocal cords, is the standard of care for confirming correct placement during intubation.4,10,11 Alternative signs of endotracheal intubation, such as chest wall movement, auscultation, condensation of water vapor in the tube lumen, or pulse oximetry, are less accurate.12
While not ideal, correct ETT placement can be confirmed qualitatively using a colorimetric device.13 Upon correct placement, the resultant exhalation of CO2 will change the paper color from purple to yellow (indicating EtCO2 values > 15 mm Hg).2,4 Without this color change, tube placement should be verified to rule out esophageal intubation. Unfortunately, qualitative capnography has false positives and negatives that limit its utility in the ED, and this method should be avoided if quantitative capnography is available.
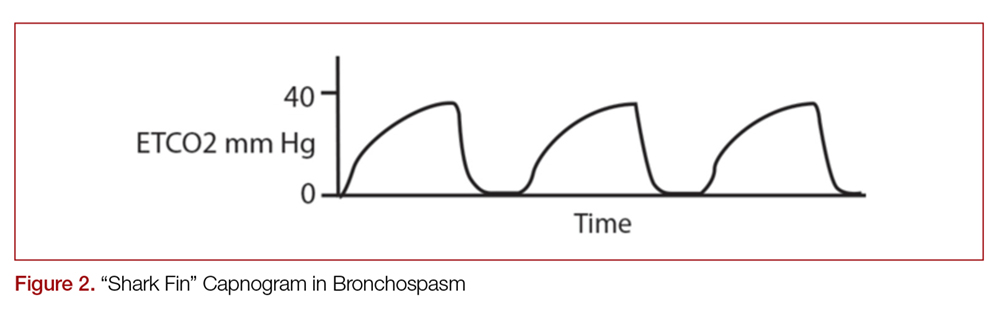
With quantitative capnography, obtaining the typical box-waveform on the capnogram reflects endotracheal intubation. In comparison, a flat capnogram is more indicative of an esophageal intubation (Figure 3).10 While other things may cause this waveform, such as technical malfunction or complete airway obstruction distal to the tube, tube placement confirmation to rule out esophageal intubation would be the first step to troubleshooting this waveform. In addition, if the ETT is placed in the hypopharynx above the vocal cords, the waveform may initially appear appropriate but will likely become erratic appearing over time.10
Quantitative capnography does have some limitations. For example, a main-stem bronchus intubation would still likely demonstrate normal-appearing capnography, so secondary strategies and a confirmatory chest x-ray are still indicated. False-negative ETCO2 readings can occur in low CO2 elimination states, such as cardiac arrest, pulmonary embolus, or pulmonary edema, while false-positives can theoretically occur after ingestion of large amounts of carbonated liquids or contamination of the sensor with stomach contents or acidic drugs.10 However, many of these misleading results can be caught by simply checking for an appropriate waveform.
Cardiac Arrest
Capnography has numerous uses in the monitoring, management, and prognostication of intubated patients in cardiac arrest.1,3,4,10,14 Under normal conditions, EtCO2 is 35-40 mm Hg. While the body still makes CO2 during cardiac arrest, it will not reach the alveoli without circulating blood.10 Without CPR, CO2 accumulates peripherally and won’t reach the lungs, causing EtCO2 to approach zero. This means that EtCO2 correlates directly with cardiac output during CPR, as long as ventilation remains constant.
This means the effectiveness of cardiac chest compression can be assessed in intubated patients using EtCO2, with higher values during CPR correlated with increased return of spontaneous circulation (ROSC) and survival.14-18 Using EtCO2 monitoring during cardiac arrest may improve outcomes,19 and the American Heart Association (AHA) recommends monitoring capnography during cardiac arrest to assess compression efficacy.10,20 EtCO2 >20 mm Hg is considered optimal, while EtCO2 <10-15 mm Hg is considered suboptimal.4,10,16 In a recent meta-analysis, the average EtCO2 was 13.1 mm Hg in those who did not obtain ROSC, compared to 25.8 mm Hg in those who did.21 As such, goal EtCO2 for effective compressions may be even higher in future recommendations. If EtCO2 is low, either compression technique should be improved or a different operator should do compressions. Every 1 cm increase in depth will increase EtCO2 by approximately 1.4 mm Hg.16 Interestingly, compression rate is not a significant predictor of EtCO2 over the dynamic range of chest compression delivery.16
An abrupt increase in EtCO2 is an early indicator of ROSC.10,14-16,22,23 A return of a perfusing rhythm will increase cardiac output. This allows for accumulated peripheral CO2 to reach the lungs, subsequently causing a rapid rise in EtCO2.24 It is important to note that when it comes to evaluating for ROSC, the actual numbers are less important than the change from pre- to post-ROSC. Providers should look for a jump of at least 10 mm Hg on capnometry.4 Nevertheless, an abrupt rise in EtCO2 is a non-sensitive marker for ROSC (33%, 95% CI 22-47% in one multicenter cross-sectional study), meaning that the lack of an abrupt rise of EtCO2 may not necessarily mean a lack of ROSC.23
The EtCO2 level may help guide decision-making in assessing whether continued resuscitation in cardiac arrest is futile. Values <10 mm Hg after 20 minutes of active resuscitation have consistently demonstrated minimal chance of survival.17,25,26 In one study, an EtCO2 of <10 mm Hg at 20 minutes had a sensitivity, specificity, PPV, and NPV of 100% for death in PEA arrest.17 However, determination of the specific EtCO2 cutoff and the timing is still an area of research with a final consensus pending.17,18,25-30 One recent study suggested that even 3 min with EtCO2 <10 mm Hg could be an appropriate cutoff to cease resuscitation efforts.27
Unfortunately, there is a large amount of heterogeneity in the available literature using capnography to assess for ROSC and in guiding resuscitation efforts. EtCO2 should not be used as the only factor in the determination to cease resuscitation. In addition, the AHA recommends that EtCO2 for prognostication should be limited to intubated patients only.20
It is important to note that while cardiac output is the largest factor for EtCO2 in arrest, other physiologic and iatrogenic causes may affect EtCO2 during resuscitation. For example, there is considerable variation in EtCO2 with changes in ventilation rate.4 Measured CO2 may be significantly lower with manual instead of mechanical ventilation, likely due to over-ventilation that not only reduces alveolar CO2 but also causes excess intra-thoracic pressure, reducing venous return.21 For these reasons, use caution when using EtCO2 during manual ventilation of an intubated patient in cardiac arrest. In addition, administration of epinephrine may cause a small decrease in EtCO2, although the effect may vary for each individual.10,31 Sodium bicarbonate can also cause a transient increase in CO2 due to its conversion into CO2 and H2O.10
Procedural Sedation
Capnography is being used with increasing frequency to monitor patients during procedural sedation; it is now considered standard of care in many settings.32 Although rare, hypoventilation is a risk of procedural sedation.33 Typically, respiratory depression during procedural sedation is diagnosed with non-invasive pulse oximetry and visual inspection.34 However, capnography has been shown to identify respiratory depression, airway obstruction, apnea, and laryngospasm earlier than pulse oximetry, allowing the provider to intervene quicker.34,35 Unlike pulse oximetry, the capnogram also remains stable during patient motion and is reliable in low-perfusion states.36
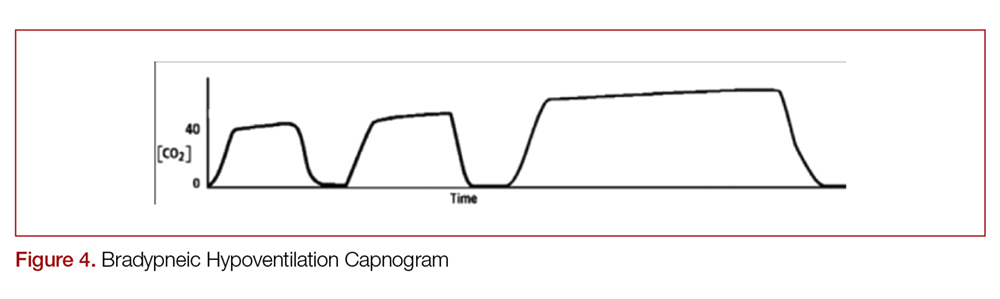
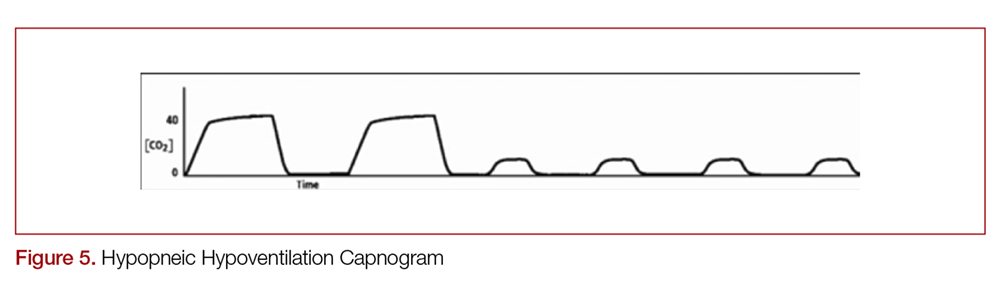
There are two distinct types of hypoventilation detected by capnography. Bradypneic hypoventilation (type 1), which is characterized by a decreased respiratory rate, results in a decreased expiratory time and a subsequent rise in EtCO2.36 This is depicted on capnography by a high EtCO2 and longer waveform, and is commonly observed after oversedation with opioids (Figure 4).36 In contrast, hypopneic hypoventilation (type 2) occurs with low tidal volumes but a normal respiratory rate.36 Type 2 is graphically represented by a suddenly lower ETCO2 with otherwise normal waveform and occurs most commonly with sedative-hypnotic drugs (Figure 5).36 Seeing either type during procedural sedation should alert the clinician to assess for airway obstruction, consider supplemental oxygen, cease drug administration or reduce dosing, and consider reversal if appropriate.36
There is some debate as to the utility of capnography for procedural sedation. While it is clear that capnography decreases the incidence of hypoxia, some studies suggest that it may not reduce patient-centered outcomes such as adverse respiratory events, neurologic injury, aspiration, or death compared to standard monitoring.35,37,38 However, pulse oximetry alone can suffer response delay, while EtCO2 can rapidly detect hypoventilation.39
Potential Uses/Applications
Respiratory Distress
Capnography can provide dynamic monitoring in patients with acute respiratory distress. Measuring EtCO2 with each breath provides instantaneous feedback on the clinical status of the patient and has numerous specific uses.1,3,4
Determining the etiology of respiratory distress in either the obtunded patient or those with multiple comorbidities can be a challenge. Vital sign abnormalities and physical exam findings can overlap in numerous conditions, which may only further obscure the diagnosis. Since different etiologies for respiratory disease require different management modalities, anything that can help clue in to the specific cause can be beneficial. As discussed above, obstructive diseases such as COPD or asthma demonstrate a “shark-fin appearance” on capnogram due to both V/Q heterogeneity and a prolonged expiratory phase due to airway constriction, which will contrast to the typical box-waveform in other conditions (Figure 2).1,2,6 Some studies have been able differentiate COPD from congestive heart failure (CHF) by waveform analysis alone, though this was primarily done via computer algorithms.40 Seeing the shark-fin (or the lack thereof) can help guide management of respiratory distress in conjunction with the remainder of the initial assessment.
Monitoring capnography can help with management and disposition in those with COPD or asthma. During exacerbations, EtCO2 levels may initially drop as the patient hyperventilates to compensate.1 It is not until ventilation becomes less effective that EtCO2 levels begin to rise. This may occur before hypoxia sets in and can prompt the clinician to escalate ventilation strategies. In addition, the normalization of the “shark-fin” obstructive pattern towards the more typical box-form wave may indicate effective treatment, though more data is needed before it can be recommended.41 One of the advantages of this technique would be that it is independent of patient effort, unlike peak-flow monitoring.
EtCO2 can be beneficial even before patients get to the ED. In one study, prehospital patients presenting with asthma or COPD who were found to have EtCO2 of >50 mm Hg or <28 mm Hg, representing the upper and lower limits in the study, had greater rates of intubation, critical care admission, and mortality.42 The patients in this cohort with higher EtCO2 were likely tiring after prolonged hyperventilation and therefore would be more likely to need ventilatory support. Those on the lower end were likely hyperventilating and had not yet tired out. It is important to note that while arrival EtCO2 levels may aid in determining the more critically ill, post-treatment levels were not found to have a statistical difference in determining disposition in patients with asthma or COPD.43
Caution is advised when attempting to use EtCO2 to approximate an arterial blood gas CO2 (PaCO2). While EtCO2 can correlate with PaCO2 within 5 mm Hg in greater than 80% of patients with dyspnea,44 large discrepancies are common depending on the disease state.45 In general, the EtCO2 should always be lower than the PaCO2 due to the contribution to the ETCO2 from dead space, which has a low CO2 content due to lack of perfusion.
Sepsis
EtCO2 may help identify septic patients given its inverse relationship with lactate levels.46-49 In conditions of poor tissue perfusion, lactate builds up. This begins to make the blood acidotic in the form of newly acquired anions, with a resultant anion gap metabolic acidosis. The body then tries to acutely compensate for this by hyperventilating, resulting in the observed lowering of EtCO2. Since lactate is a predictor of mortality in sepsis,50 and monitoring lactate clearance to evaluate resuscitation efforts in sepsis is recommended,51 EtCO2 could play a similar role. One group in particular has demonstrated that, when used with SIRS criteria, abnormally low prehospital EtCO2 levels is predictive of sepsis and inhospital mortality, and is more predictive than SIRS criteria alone.48,50 That said, EtCO2 was not associated with lactate temporally at 3 and 6 hours,51 so it should not be used to guide resuscitation like a lactate clearance. It appears that EtCO2 may be helpful for triage in sepsis, but more study is needed to determine the exact role particularly given most of the available research involves multiple studies from one group.47,48,52
Diabetic Ketoacidosis
Initial bicarbonate levels and venous pH are associated with low EtCO2 readings in diabetic ketoacidosis (DKA).54,55 This could have many practical uses, in particular for patients presenting with hyperglycemia to rule out DKA. One study demonstrated that a blood glucose >250 mg/dL and capnography of >24.5 mm Hg had 90% sensitivity for excluding DKA.55 A value of 35 mm Hg or greater demonstrated 100% sensitivity for excluding DKA in patients with initial glucose >550 mg/dL,56 though this blood glucose is not practical, as this excludes many patients the EP would seek to rule out DKA (recall that blood glucose only has to be >250 mg/dL for the diagnosis). Smaller studies focused on the pediatric population found a 100% sensitivity marker for DKA varied from >30 to >36 mm Hg.57,58 Clearly a role exists, but no study has demonstrated sufficient sensitivity for ruling out DKA with EtCO2 and blood glucose alone within the framework of clinically relevant values.
Trauma
As described above, low EtCO2 is inversely correlated with lactate.46 Because of this, it could theoretically be a marker of hypoperfusion in trauma. Initial EtCO2 values <25 mm Hg have been associated with mortality and hemorrhage in intubated trauma patients,59 as well as mortality prior to discharge in nonintubated trauma patients.60 However, it did not demonstrate added clinical utility when combined with Glasgow Coma Scale (GCS) score, systolic blood pressure, and age in predicting severe injury.61
Pulmonary Embolism
A pulmonary embolism (PE) causes a blockage in blood flow to alveoli, which results in a decrease in CO2 transportation to the alveoli and thus lower EtCO2, while also widening the gradient between PaCO2 and EtCO2.37 Because of this, it has a theoretical role in the diagnosis of PE, though numerous studies have demonstrated that EtCO2 alone is not sensitive nor specific enough for this role.62-66 In a recent meta-analysis, a pretest probability of 10% could lead to a posttest probability of 3% using capnography.62 While further study is needed before recommendation, this indicates that capnography could obviate the need for imaging in low to intermediate risk patients either after a positive D-Dimer or instead of obtaining a D-dimer.62-64
Triage
Simply measuring an initial EtCO2 as a triage vital sign may have added benefit to the EP, and consideration could be made for making this a policy in your ED. One study demonstrated that abnormal initial EtCO2 (outside of 35-45 mm Hg) was predictive of admisison (RR 2.5, 95% CI 1.5-4.0).67 An abnormal EtCO2 (outside of 31-41 mm Hg for this study) was 93% sensitive (95% CI 79-98%), with expectedly low specificity of 44% (95% CI 41-48%) for mortality prior to discharge.47 This potential vital sign may be treated similarly to tachycardia; while an abnormal heart rate should increase a clinician’s concern for a pathological condition, it needs to be taken in context of the situation to accurately interpret it.
Summary
Capnography has numerous uses in the ED in both intubated and spontaneously breathing patients. Quantitative capnography is the standard of care for confirming endotracheal intubation. It is recommended as an aide in maximizing chest compressions during cardiac arrest and can assist in prognostication. It rapidly identifies hypoventilation during procedural sedation. It also has many more potential applications that continue to be explored in areas such as respiratory distress, sepsis, trauma, DKA, and PE. Ultimately, capnography should always be used in association with the remainder of the clinical assessment.
- Manifold CA, Davids B, Villers LC, Wampler DA. Capnography for the nonintubated patient in the emergency setting. J Emerg Med. 2013;45(4):626-632.
- Ward K, Yealy DM. End-tidal carbon dioxide monitoring in emergency medicine, part 1: basic principles. Acad Emerg Med. 1998;5(6):628-636.
- Krauss B, Falk JL. Carbon dioxide monitoring (capnography). UpToDate. Waltham MA: UpToDate Inc. www.uptodate.com.
- Long B, Koyfman A, Michael AV. Capnography in the emergency department: a review of uses, waveforms, and limitations. J Emerg Med. 2017;(53)6:829-842.
- Shankar Kodali B. Capnography: A Comprehensive Educational Website. Boston, MA. www.capnography.com.
- Kodali B. Capnography outside the operating room. Anesthesiology. 2013;118:192-201.
- Bhavani, S. Defining segments and phases of a time capnogram. Anesth Analg. 2000;91(4):973-977.
- Petersson J, Glenny R. Gas exchange and ventilation-perfusion relationships in the lung. Eur Resp J. 2014;44(4):1023-1041.
- Nassar B, Schmidt GA. Capnography during critical illness. Chest. 2016;149(2):576-585.
- Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S729-S767.
- Burns SM, Carpenter R, Blevins C, Bragg S, Marshall M, Browne L, et al. Detection of inadvertent airway intubation during gastric tube insertion: capnography versus a colorimetric carbon dioxide detector. Am J Crit Care. 2006:15(2):188-195.
- Goldberg JS, Rawle PR, Zehnder JL, Sladen RN. Colorimetric end-tidal carbon dioxide monitoring for tracheal intubation. Anesthesia and analgesia. 1990:70(2):191-194.
- O'Flaherty D, Adams AP. The end-tidal carbon dioxide detector: assessment of a new method to distinguish oesophageal from tracheal intubation. Anaesthesia. 1990:45(8):653-655.
- Garnett AR, Ornato JP, Gonzalez ER, Johnson EB. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512-515.
- Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318(10):607-611.
- Sheak KR, Wiebe DJ, Leary M, Babaeizadeh S, Yuen TC, Zive D, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149-154.
- Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301-306.
- Touma O, Davies M. The prognostic value of end tidal carbon dioxide during cardiac arrest: a systematic review. Resuscitation. 2013;84(11):1470-1479.
- Chen JJ, Lee YK, Hou SW, et al. End-tidal carbon dioxide monitoring may be associated with a higher possibility of return of spontaneous circulation during out-of-hospital cardiac arrest: a population-based study. Scan J Trauma Resusc Emerg Med. 2015;23:104.
- Neumar RW, Shuster M, Callaway CW et al. Part 7: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(suppl 2):S315-S367.
- Hartmann SW, Farris RW, Di Gennaro JL, Roberts JS. Systematic review and meta-analysis of end-tidal carbon dioxide values associated with return of spontaneous circulation during cardiopulmonary resuscitation. J Intensive Care Med. 2015;(30):426-435.
- Eckstein, M, Hatch, L, Malleck, J et al. EtCO2 as a predictor of survival in out-of hospital cardiac arrest. Prehosp Disaster Med. 2016;104:53-58.
- Lui CT, Poon KM, Tsui KL. Abrupt rise of end tidal carbon dioxide was a specific but non-sensitive marker of return of spontaneous circulation with out-of-hospital cardiac arrest. Resuscitation. 2016;104:53-58.
- Pokorna M, Necas E, Kratochvil J et al. A sudden increase in partial pressure end-tidal carbon dioxide at the moment of return of spontaneous circulation. J Emerg Med. 2010;38:614-621.
- Sanders A, Kern K, Otto C, et al. End-tidal carbon dixoide monitoring during cardiopulmonary resuscitation: a prognostic indicator for survival. JAMA. 1989;262:1347-1351.
- Wayne M, Levine R, And Miller C. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann Emerg Med. 1995;25(6):762-767.
- Poon KM, Lui CT, Tsui KL. Prognostication of out-of-hopsital cardiac arrest patients by 3-min end-tidal capnometry level in emergency department. Resuscitation. 2016;102:80-84.
- Einav S, Bromiker R, Weiniger C, Matot I. Mathematical modeling for prediction of survival from resuscitation based on computerized continuous capnography: proof of concept. Acad Emerg Med. 2011;18:468-475.
- Pearce A, Davis D, Minokadeh A, Sell R. Initial end-tidal carbon dioxide as a prognostic indicator for inpatient PEA arrest. Resuscitation. 2015;92:77-81.
- Akinci, E, Ramadan H, Yuzbasioglu Y, Coksun F. Comparison of end-tidal carbon dioxide levels with cardiopulmonary resuscitation success presented to emergency department with cardiopulmonary arrest. Pak J Med Sci. 2014;30(1):16-21.
- Callaham M, Barton C, Matthay M. Effect of epinephrine on the ability of end-tidal carbon dioxide readings to predict initial resuscitation from cardiac arrest. Crit Care Med. 1992; 20:337-343.
- Wall BF, Magee K, Campbell SG, Zed PJ. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database of Systematic Reviews. 2017(3).
- Langhan ML, Shabanova V, Li FY, Bernstein SL, Shapiro ED. A randomized controlled trial of capnography during sedation in a pediatric emergency setting. Am J Emerg Med. 2015;33(1):25-30.
- Campbell SG, Magee KD, Zed PJ, Froese P, Etsell G, LaPierre A et al. End-tidal capnometry during emergency department procedural sedation and analgesia: a randomized, controlled study. World J Emerg Med. 2016;7(1):13.
- Waugh JB, Epps CA, Khodneva YA. Capnography enhances surveillance of respiratory events during procedural sedation: a meta-analysis. J Clin Anesth. 2011;23(3):189-196.
- Krauss B, Hess DR. Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;50(2):172-181.
- Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55(3):258-264.
- Godwin SA, Caro DA, Wolf SJ, Jagoda AS, Charles R, Marett BE, Moore J. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45(2):177-196.
- Hamber EA, Bailey PI, James SW et al. Delays in the detection of hypoxemia due to site of pulse oximetry pulse placement. J Clin Anesth. 1999;11:113-118.
- Mieloszyk RJ, Vergehese GC, Deitch K, et al. Automated quantitative analysis of capnogram shape for COPD-normal and COPD-CHF classification. IEEE Trans Biomed Eng. 2014;61:2882-2890.
- Howe TA, Jaalam K, R. Ahmad, Sheng CK, Ab Rahman NHN. The use of end-tidal capnography to monitor non-intubated patients presenting with acute exacerbation of asthma in the emergency department. J Emerg Med. 2011:41:581-589.
- Nagurka R, Bechmann S, Gluckman W et al. Utility of initial prehospital end-tidal carbon dioxide measurements to predict poor outcomes in adult asthmatic patients. Prehospital Emerg Care. 2014;18:180-184.
- Doğan NÖ, Şener A, Günaydın GP, İçme F, Çelik GK, Kavaklı HŞ, Temrel TA. The accuracy of mainstream end-tidal carbon dioxide levels to predict the severity of chronic obstructive pulmonary disease exacerbations presented to the ED. Am J Emerg Med. 2014;32(5):408-411.
- Cinar O, Acar YA, Arziman I, et al. Can mainstream end-tidal carbon dioxide measurement accurately predict the arterial carbon dioxide levels of patients with acute dyspnea in ED. Am J Emerg Med. 2012;30:358-361.
- Nassar BS, Schmidt GA. Capnography during critical illness. Chest. 2016:149(2):576-585.
- Caputo ND, Fraser RM, Paliga A et al. Nasal cannula end-tidal CO2 correlates with serum lactate levels and odds of operative intervention in penetrating trauma patients: a prospective cohort study. J Trauma Acute Care Surg. 2012;73:1202-1207.
- Hunter CL, Silvestri S, Ralls G, Bright S, Papa L. The sixth vital sign: prehospital end-tidal carbon dioxide predicts in-hospital mortality and metabolic disturbances. Am J Emerg Med. 2014;32(2):160-165.
- Hunter CL, Silvestri S, Dean M, Falk JL, Papa L. End-tidal carbon dioxide is associated with mortality and lactate in patients with suspected sepsis. Am J Emerg Med. 2013;31(1):64-71.
- McGillicuddy DC, Tang A, Cataldo L, et al. Evaluation of end-tidal carbon dioxide role in predicting elevated SOFA and lactic acidosis. Intern Emerg Med. 2009;4:41-44.
- Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524-528.
- Levy, MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46:997-1000.
- Hunter CL, Silvestri S, Ralls G et al. A prehospital screening tool utilizing end-tidal carbon dioxide predicts sepsis and severe sepsis. Am J Emerg Med. 2016;34:813-819.
- Guirgis FW, Williams DJ, Kalynych CJ, Hardy ME, Jones AE, Dodani S, Wears RL. End-tidal carbon dioxide as a goal of early sepsis therapy. Am J Emerg Med. 2014;32(11):1351-1356.
- Kartal M, Eray O, Rinnert S, Gosku E, Bektas F, Eken C. ETCO2: a predictive tool for excluding metabolic disturbances in nonintubated patients. Am J Emerg Med. 2011;29: 65-69.
- Solmeinpur H, Taghizadieh A, Niafar M, Rahmani F, Golzari SE, Esfanjani RM. Predictive value of capnography for diagnosis in patients with suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. 2013;14:590-594.
- Bou Chebl R, Madden B, Belsky J, Harmouche E, Yessayan L. Diagnostic value of end tidal capnography in patients with hyperglycemia in the emergency department. BMC Emerg Med. 2016;16:7.
- Fearon DM, Steele DW. End-tidal carbon dioxide predicts the presence and severity of acidosis in children with diabetes. Acad Emerg Med. 2002;9:1373-1378.
- Gilhotra Y, Porter P. Predicting diabetic ketoacidosis in children by measuring end-tidal CO2 via non-invasive nasal capnography. J Paediatr Child Health. 2007;43:677-680.
- Dunham CM, Chirichella TJ, Gruber BS, et al. In emergently ventilated trauma patients, low end-tidal CO2 and low cardiac output are associated and correlate with hemodynamic instability, hemorrhage, abnormal pupils, and death. BMC Anesthesiol. 2013;13-20.
- Deakin CD, Sado DM, Coats TJ, Davies G. Prehospital end-tidal carbon dioxide concentration and outcome in major trauma. J Trauma.2004;57:65-68.
- Williams DJ, Guirgis FW, Morrissey TK, Wilkerson J, Wears RL, Kalynych C, Kerwin AJ, Godwin SA. End-tidal carbon dioxide and occult injury in trauma patients: ETCO2 does not rule out severe injury. Am J Emerg Med. 2016;34(11):2146-2149.
- Manara A, D’hoore W, Thys F. Capnography as a diagnostic tool for pulmonary embolism: a meta-analysis. Ann Emerg Med. 2013;52:584-591.
- Yoon YH, Lee SW, Jung DM et al. The additional use of end-tidal alveolar dead space fraction following D-dimer test to improve diagnostic accuracy for pulmonary embolism in the emergency department. Emerg Med J. 2010;27:663-667.
- Hemnes AR, Newman AL, Rosenbaum B, et al. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Resp J. 2010;35:735-741.
- Rias I Jacob B. Pulmonary embolism in Bradford, UK: role of end-tidal CO2 as a screening tool. Clin Med (Lond). 2014;14:128-133.
- Yuksel M, Pekdemir M, Yilmaz S, et al. Diagnostic accuracy of noninvasive end-tidal carbon dioxide measurement in emergency department patients with suspected pulmonary embolism. Turk J Med Sci. 2016;46:84–90.
- Williams D, Morrissey T, Caro D, Wears R, Kalynyc C. Side-stream qunatitative end-tidal carbon dioxide measurement as a triage tool in emergency medicine. Ann Emerg Med. 2011;58:S212-S213.
Capnography is the measurement of the partial pressure of carbon dioxide (CO2) in exhaled air.1 It provides real-time information on ventilation (elimination of CO2), perfusion (CO2 transportation in vasculature), and metabolism (production of CO2 via cellular metabolism).2 The technology was originally developed in the 1970s to monitor general anesthesia patients; however, its reach has since broadened, with numerous applications currently in use and in development for the emergency provider (EP).3
Capnography exists in two configurations: a mainstream device that attaches directly to the hub of an endotracheal tube (ETT) and a side-stream device that measure levels via nasal or nasal-oral cannula.1,3
Qualitative monitors use a colorimetric device that monitors the end-tidal CO2 (EtCO2) in exhaled gas and changes color depending on the amount of CO2 present.2,4 Expired CO2 and H20 form carbonic acid, causing the specially treated litmus paper inside the device to change from purple to yellow.2,4 Quantitative monitors display a capnogram, the waveform of expired CO2 as a function of time; as well as the capnometer, which depicts the numerical EtCO2 for each breath.4 In this overview, we will discuss the general interpretation of capnography and its specific uses in the ED.
The Capnogram
Just like the various stages of an electrocardiogram represent different phases of the cardiac cycle, different phases of a capnogram correspond to different phases of the respiratory cycle. Knowing how to analyze and interpret each phase will contribute to the utility of capnography. While there has been considerable ambiguity in the terminology related to the capnogram,5-7 the most frequently referenced capnogram terminology consists of the following phases (Figure 1):
Phase I: represents beginning of exhalation, where the dead space is cleared from the upper airway.2 This should be zero unless the patient is rebreathing CO2-laden expired gas from either artificially increased dead space or hypoventilation.2,8 A precipitous rise in both the baseline and EtCO2 may indicate contamination of the sensor, such as with secretions or water vapor.2,6
Phase II: rapid rise in exhaled as the CO2 from the alveoli reaches the sensor.4 This rise should be steep, particularly when ventilation to perfusion (V/Q) is well matched. More V/Q heterogeneity, such as with COPD or asthma, leads to a more gradual slope.9 A more gradual phase 2 slope may also indicate a delay in CO2 delivery to the sampling site, such as with bronchospasm or ETT kinking.2
Phase III: the expiratory plateau, which represents the CO2 concentration approaching equilibrium from alveoli to nose. The plateau should be nearly horizontal.2 If all alveoli had the same pCO2, this plateau would be perfectly flat, but spatial and temporal mismatch in alveolar V/Q ratios result in variable exhaled CO2. When there is substantial V/Q heterogeneity, the slope of the plateau will increase.1,2,6
Phase IV: the initiation of inspiration, which should be a nearly vertical drop to a baseline. If prolonged or bleeding into the expiratory phase, consider a leak in the expiratory portion of the circuit, such as an ETT tube cuff leak.2
Phase 0: the inspiratory segment

Another important part of the capnogram is the alpha angle. This is the angle of transition between Phase II and Phase III. The combination of a prolonged phase II and steeper phase III leads to a more obtuse alpha angle and will have a “shark-fin” appearance to the capnogram. This suggests an obstructive process, such as asthma or COPD (Figure 2).1,2,6

Standard Uses
Intubation
Capnography, along with visualizing ETT placement through the vocal cords, is the standard of care for confirming correct placement during intubation.4,10,11 Alternative signs of endotracheal intubation, such as chest wall movement, auscultation, condensation of water vapor in the tube lumen, or pulse oximetry, are less accurate.12
While not ideal, correct ETT placement can be confirmed qualitatively using a colorimetric device.13 Upon correct placement, the resultant exhalation of CO2 will change the paper color from purple to yellow (indicating EtCO2 values > 15 mm Hg).2,4 Without this color change, tube placement should be verified to rule out esophageal intubation. Unfortunately, qualitative capnography has false positives and negatives that limit its utility in the ED, and this method should be avoided if quantitative capnography is available.
With quantitative capnography, obtaining the typical box-waveform on the capnogram reflects endotracheal intubation. In comparison, a flat capnogram is more indicative of an esophageal intubation (Figure 3).10 While other things may cause this waveform, such as technical malfunction or complete airway obstruction distal to the tube, tube placement confirmation to rule out esophageal intubation would be the first step to troubleshooting this waveform. In addition, if the ETT is placed in the hypopharynx above the vocal cords, the waveform may initially appear appropriate but will likely become erratic appearing over time.10

Quantitative capnography does have some limitations. For example, a main-stem bronchus intubation would still likely demonstrate normal-appearing capnography, so secondary strategies and a confirmatory chest x-ray are still indicated. False-negative ETCO2 readings can occur in low CO2 elimination states, such as cardiac arrest, pulmonary embolus, or pulmonary edema, while false-positives can theoretically occur after ingestion of large amounts of carbonated liquids or contamination of the sensor with stomach contents or acidic drugs.10 However, many of these misleading results can be caught by simply checking for an appropriate waveform.
Cardiac Arrest
Capnography has numerous uses in the monitoring, management, and prognostication of intubated patients in cardiac arrest.1,3,4,10,14 Under normal conditions, EtCO2 is 35-40 mm Hg. While the body still makes CO2 during cardiac arrest, it will not reach the alveoli without circulating blood.10 Without CPR, CO2 accumulates peripherally and won’t reach the lungs, causing EtCO2 to approach zero. This means that EtCO2 correlates directly with cardiac output during CPR, as long as ventilation remains constant.
This means the effectiveness of cardiac chest compression can be assessed in intubated patients using EtCO2, with higher values during CPR correlated with increased return of spontaneous circulation (ROSC) and survival.14-18 Using EtCO2 monitoring during cardiac arrest may improve outcomes,19 and the American Heart Association (AHA) recommends monitoring capnography during cardiac arrest to assess compression efficacy.10,20 EtCO2 >20 mm Hg is considered optimal, while EtCO2 <10-15 mm Hg is considered suboptimal.4,10,16 In a recent meta-analysis, the average EtCO2 was 13.1 mm Hg in those who did not obtain ROSC, compared to 25.8 mm Hg in those who did.21 As such, goal EtCO2 for effective compressions may be even higher in future recommendations. If EtCO2 is low, either compression technique should be improved or a different operator should do compressions. Every 1 cm increase in depth will increase EtCO2 by approximately 1.4 mm Hg.16 Interestingly, compression rate is not a significant predictor of EtCO2 over the dynamic range of chest compression delivery.16
An abrupt increase in EtCO2 is an early indicator of ROSC.10,14-16,22,23 A return of a perfusing rhythm will increase cardiac output. This allows for accumulated peripheral CO2 to reach the lungs, subsequently causing a rapid rise in EtCO2.24 It is important to note that when it comes to evaluating for ROSC, the actual numbers are less important than the change from pre- to post-ROSC. Providers should look for a jump of at least 10 mm Hg on capnometry.4 Nevertheless, an abrupt rise in EtCO2 is a non-sensitive marker for ROSC (33%, 95% CI 22-47% in one multicenter cross-sectional study), meaning that the lack of an abrupt rise of EtCO2 may not necessarily mean a lack of ROSC.23
The EtCO2 level may help guide decision-making in assessing whether continued resuscitation in cardiac arrest is futile. Values <10 mm Hg after 20 minutes of active resuscitation have consistently demonstrated minimal chance of survival.17,25,26 In one study, an EtCO2 of <10 mm Hg at 20 minutes had a sensitivity, specificity, PPV, and NPV of 100% for death in PEA arrest.17 However, determination of the specific EtCO2 cutoff and the timing is still an area of research with a final consensus pending.17,18,25-30 One recent study suggested that even 3 min with EtCO2 <10 mm Hg could be an appropriate cutoff to cease resuscitation efforts.27
Unfortunately, there is a large amount of heterogeneity in the available literature using capnography to assess for ROSC and in guiding resuscitation efforts. EtCO2 should not be used as the only factor in the determination to cease resuscitation. In addition, the AHA recommends that EtCO2 for prognostication should be limited to intubated patients only.20
It is important to note that while cardiac output is the largest factor for EtCO2 in arrest, other physiologic and iatrogenic causes may affect EtCO2 during resuscitation. For example, there is considerable variation in EtCO2 with changes in ventilation rate.4 Measured CO2 may be significantly lower with manual instead of mechanical ventilation, likely due to over-ventilation that not only reduces alveolar CO2 but also causes excess intra-thoracic pressure, reducing venous return.21 For these reasons, use caution when using EtCO2 during manual ventilation of an intubated patient in cardiac arrest. In addition, administration of epinephrine may cause a small decrease in EtCO2, although the effect may vary for each individual.10,31 Sodium bicarbonate can also cause a transient increase in CO2 due to its conversion into CO2 and H2O.10
Procedural Sedation
Capnography is being used with increasing frequency to monitor patients during procedural sedation; it is now considered standard of care in many settings.32 Although rare, hypoventilation is a risk of procedural sedation.33 Typically, respiratory depression during procedural sedation is diagnosed with non-invasive pulse oximetry and visual inspection.34 However, capnography has been shown to identify respiratory depression, airway obstruction, apnea, and laryngospasm earlier than pulse oximetry, allowing the provider to intervene quicker.34,35 Unlike pulse oximetry, the capnogram also remains stable during patient motion and is reliable in low-perfusion states.36

There are two distinct types of hypoventilation detected by capnography. Bradypneic hypoventilation (type 1), which is characterized by a decreased respiratory rate, results in a decreased expiratory time and a subsequent rise in EtCO2.36 This is depicted on capnography by a high EtCO2 and longer waveform, and is commonly observed after oversedation with opioids (Figure 4).36 In contrast, hypopneic hypoventilation (type 2) occurs with low tidal volumes but a normal respiratory rate.36 Type 2 is graphically represented by a suddenly lower ETCO2 with otherwise normal waveform and occurs most commonly with sedative-hypnotic drugs (Figure 5).36 Seeing either type during procedural sedation should alert the clinician to assess for airway obstruction, consider supplemental oxygen, cease drug administration or reduce dosing, and consider reversal if appropriate.36

There is some debate as to the utility of capnography for procedural sedation. While it is clear that capnography decreases the incidence of hypoxia, some studies suggest that it may not reduce patient-centered outcomes such as adverse respiratory events, neurologic injury, aspiration, or death compared to standard monitoring.35,37,38 However, pulse oximetry alone can suffer response delay, while EtCO2 can rapidly detect hypoventilation.39
Potential Uses/Applications
Respiratory Distress
Capnography can provide dynamic monitoring in patients with acute respiratory distress. Measuring EtCO2 with each breath provides instantaneous feedback on the clinical status of the patient and has numerous specific uses.1,3,4
Determining the etiology of respiratory distress in either the obtunded patient or those with multiple comorbidities can be a challenge. Vital sign abnormalities and physical exam findings can overlap in numerous conditions, which may only further obscure the diagnosis. Since different etiologies for respiratory disease require different management modalities, anything that can help clue in to the specific cause can be beneficial. As discussed above, obstructive diseases such as COPD or asthma demonstrate a “shark-fin appearance” on capnogram due to both V/Q heterogeneity and a prolonged expiratory phase due to airway constriction, which will contrast to the typical box-waveform in other conditions (Figure 2).1,2,6 Some studies have been able differentiate COPD from congestive heart failure (CHF) by waveform analysis alone, though this was primarily done via computer algorithms.40 Seeing the shark-fin (or the lack thereof) can help guide management of respiratory distress in conjunction with the remainder of the initial assessment.
Monitoring capnography can help with management and disposition in those with COPD or asthma. During exacerbations, EtCO2 levels may initially drop as the patient hyperventilates to compensate.1 It is not until ventilation becomes less effective that EtCO2 levels begin to rise. This may occur before hypoxia sets in and can prompt the clinician to escalate ventilation strategies. In addition, the normalization of the “shark-fin” obstructive pattern towards the more typical box-form wave may indicate effective treatment, though more data is needed before it can be recommended.41 One of the advantages of this technique would be that it is independent of patient effort, unlike peak-flow monitoring.
EtCO2 can be beneficial even before patients get to the ED. In one study, prehospital patients presenting with asthma or COPD who were found to have EtCO2 of >50 mm Hg or <28 mm Hg, representing the upper and lower limits in the study, had greater rates of intubation, critical care admission, and mortality.42 The patients in this cohort with higher EtCO2 were likely tiring after prolonged hyperventilation and therefore would be more likely to need ventilatory support. Those on the lower end were likely hyperventilating and had not yet tired out. It is important to note that while arrival EtCO2 levels may aid in determining the more critically ill, post-treatment levels were not found to have a statistical difference in determining disposition in patients with asthma or COPD.43
Caution is advised when attempting to use EtCO2 to approximate an arterial blood gas CO2 (PaCO2). While EtCO2 can correlate with PaCO2 within 5 mm Hg in greater than 80% of patients with dyspnea,44 large discrepancies are common depending on the disease state.45 In general, the EtCO2 should always be lower than the PaCO2 due to the contribution to the ETCO2 from dead space, which has a low CO2 content due to lack of perfusion.
Sepsis
EtCO2 may help identify septic patients given its inverse relationship with lactate levels.46-49 In conditions of poor tissue perfusion, lactate builds up. This begins to make the blood acidotic in the form of newly acquired anions, with a resultant anion gap metabolic acidosis. The body then tries to acutely compensate for this by hyperventilating, resulting in the observed lowering of EtCO2. Since lactate is a predictor of mortality in sepsis,50 and monitoring lactate clearance to evaluate resuscitation efforts in sepsis is recommended,51 EtCO2 could play a similar role. One group in particular has demonstrated that, when used with SIRS criteria, abnormally low prehospital EtCO2 levels is predictive of sepsis and inhospital mortality, and is more predictive than SIRS criteria alone.48,50 That said, EtCO2 was not associated with lactate temporally at 3 and 6 hours,51 so it should not be used to guide resuscitation like a lactate clearance. It appears that EtCO2 may be helpful for triage in sepsis, but more study is needed to determine the exact role particularly given most of the available research involves multiple studies from one group.47,48,52
Diabetic Ketoacidosis
Initial bicarbonate levels and venous pH are associated with low EtCO2 readings in diabetic ketoacidosis (DKA).54,55 This could have many practical uses, in particular for patients presenting with hyperglycemia to rule out DKA. One study demonstrated that a blood glucose >250 mg/dL and capnography of >24.5 mm Hg had 90% sensitivity for excluding DKA.55 A value of 35 mm Hg or greater demonstrated 100% sensitivity for excluding DKA in patients with initial glucose >550 mg/dL,56 though this blood glucose is not practical, as this excludes many patients the EP would seek to rule out DKA (recall that blood glucose only has to be >250 mg/dL for the diagnosis). Smaller studies focused on the pediatric population found a 100% sensitivity marker for DKA varied from >30 to >36 mm Hg.57,58 Clearly a role exists, but no study has demonstrated sufficient sensitivity for ruling out DKA with EtCO2 and blood glucose alone within the framework of clinically relevant values.
Trauma
As described above, low EtCO2 is inversely correlated with lactate.46 Because of this, it could theoretically be a marker of hypoperfusion in trauma. Initial EtCO2 values <25 mm Hg have been associated with mortality and hemorrhage in intubated trauma patients,59 as well as mortality prior to discharge in nonintubated trauma patients.60 However, it did not demonstrate added clinical utility when combined with Glasgow Coma Scale (GCS) score, systolic blood pressure, and age in predicting severe injury.61
Pulmonary Embolism
A pulmonary embolism (PE) causes a blockage in blood flow to alveoli, which results in a decrease in CO2 transportation to the alveoli and thus lower EtCO2, while also widening the gradient between PaCO2 and EtCO2.37 Because of this, it has a theoretical role in the diagnosis of PE, though numerous studies have demonstrated that EtCO2 alone is not sensitive nor specific enough for this role.62-66 In a recent meta-analysis, a pretest probability of 10% could lead to a posttest probability of 3% using capnography.62 While further study is needed before recommendation, this indicates that capnography could obviate the need for imaging in low to intermediate risk patients either after a positive D-Dimer or instead of obtaining a D-dimer.62-64
Triage
Simply measuring an initial EtCO2 as a triage vital sign may have added benefit to the EP, and consideration could be made for making this a policy in your ED. One study demonstrated that abnormal initial EtCO2 (outside of 35-45 mm Hg) was predictive of admisison (RR 2.5, 95% CI 1.5-4.0).67 An abnormal EtCO2 (outside of 31-41 mm Hg for this study) was 93% sensitive (95% CI 79-98%), with expectedly low specificity of 44% (95% CI 41-48%) for mortality prior to discharge.47 This potential vital sign may be treated similarly to tachycardia; while an abnormal heart rate should increase a clinician’s concern for a pathological condition, it needs to be taken in context of the situation to accurately interpret it.
Summary
Capnography has numerous uses in the ED in both intubated and spontaneously breathing patients. Quantitative capnography is the standard of care for confirming endotracheal intubation. It is recommended as an aide in maximizing chest compressions during cardiac arrest and can assist in prognostication. It rapidly identifies hypoventilation during procedural sedation. It also has many more potential applications that continue to be explored in areas such as respiratory distress, sepsis, trauma, DKA, and PE. Ultimately, capnography should always be used in association with the remainder of the clinical assessment.
Capnography is the measurement of the partial pressure of carbon dioxide (CO2) in exhaled air.1 It provides real-time information on ventilation (elimination of CO2), perfusion (CO2 transportation in vasculature), and metabolism (production of CO2 via cellular metabolism).2 The technology was originally developed in the 1970s to monitor general anesthesia patients; however, its reach has since broadened, with numerous applications currently in use and in development for the emergency provider (EP).3
Capnography exists in two configurations: a mainstream device that attaches directly to the hub of an endotracheal tube (ETT) and a side-stream device that measure levels via nasal or nasal-oral cannula.1,3
Qualitative monitors use a colorimetric device that monitors the end-tidal CO2 (EtCO2) in exhaled gas and changes color depending on the amount of CO2 present.2,4 Expired CO2 and H20 form carbonic acid, causing the specially treated litmus paper inside the device to change from purple to yellow.2,4 Quantitative monitors display a capnogram, the waveform of expired CO2 as a function of time; as well as the capnometer, which depicts the numerical EtCO2 for each breath.4 In this overview, we will discuss the general interpretation of capnography and its specific uses in the ED.
The Capnogram
Just like the various stages of an electrocardiogram represent different phases of the cardiac cycle, different phases of a capnogram correspond to different phases of the respiratory cycle. Knowing how to analyze and interpret each phase will contribute to the utility of capnography. While there has been considerable ambiguity in the terminology related to the capnogram,5-7 the most frequently referenced capnogram terminology consists of the following phases (Figure 1):
Phase I: represents beginning of exhalation, where the dead space is cleared from the upper airway.2 This should be zero unless the patient is rebreathing CO2-laden expired gas from either artificially increased dead space or hypoventilation.2,8 A precipitous rise in both the baseline and EtCO2 may indicate contamination of the sensor, such as with secretions or water vapor.2,6
Phase II: rapid rise in exhaled as the CO2 from the alveoli reaches the sensor.4 This rise should be steep, particularly when ventilation to perfusion (V/Q) is well matched. More V/Q heterogeneity, such as with COPD or asthma, leads to a more gradual slope.9 A more gradual phase 2 slope may also indicate a delay in CO2 delivery to the sampling site, such as with bronchospasm or ETT kinking.2
Phase III: the expiratory plateau, which represents the CO2 concentration approaching equilibrium from alveoli to nose. The plateau should be nearly horizontal.2 If all alveoli had the same pCO2, this plateau would be perfectly flat, but spatial and temporal mismatch in alveolar V/Q ratios result in variable exhaled CO2. When there is substantial V/Q heterogeneity, the slope of the plateau will increase.1,2,6
Phase IV: the initiation of inspiration, which should be a nearly vertical drop to a baseline. If prolonged or bleeding into the expiratory phase, consider a leak in the expiratory portion of the circuit, such as an ETT tube cuff leak.2
Phase 0: the inspiratory segment

Another important part of the capnogram is the alpha angle. This is the angle of transition between Phase II and Phase III. The combination of a prolonged phase II and steeper phase III leads to a more obtuse alpha angle and will have a “shark-fin” appearance to the capnogram. This suggests an obstructive process, such as asthma or COPD (Figure 2).1,2,6

Standard Uses
Intubation
Capnography, along with visualizing ETT placement through the vocal cords, is the standard of care for confirming correct placement during intubation.4,10,11 Alternative signs of endotracheal intubation, such as chest wall movement, auscultation, condensation of water vapor in the tube lumen, or pulse oximetry, are less accurate.12
While not ideal, correct ETT placement can be confirmed qualitatively using a colorimetric device.13 Upon correct placement, the resultant exhalation of CO2 will change the paper color from purple to yellow (indicating EtCO2 values > 15 mm Hg).2,4 Without this color change, tube placement should be verified to rule out esophageal intubation. Unfortunately, qualitative capnography has false positives and negatives that limit its utility in the ED, and this method should be avoided if quantitative capnography is available.
With quantitative capnography, obtaining the typical box-waveform on the capnogram reflects endotracheal intubation. In comparison, a flat capnogram is more indicative of an esophageal intubation (Figure 3).10 While other things may cause this waveform, such as technical malfunction or complete airway obstruction distal to the tube, tube placement confirmation to rule out esophageal intubation would be the first step to troubleshooting this waveform. In addition, if the ETT is placed in the hypopharynx above the vocal cords, the waveform may initially appear appropriate but will likely become erratic appearing over time.10

Quantitative capnography does have some limitations. For example, a main-stem bronchus intubation would still likely demonstrate normal-appearing capnography, so secondary strategies and a confirmatory chest x-ray are still indicated. False-negative ETCO2 readings can occur in low CO2 elimination states, such as cardiac arrest, pulmonary embolus, or pulmonary edema, while false-positives can theoretically occur after ingestion of large amounts of carbonated liquids or contamination of the sensor with stomach contents or acidic drugs.10 However, many of these misleading results can be caught by simply checking for an appropriate waveform.
Cardiac Arrest
Capnography has numerous uses in the monitoring, management, and prognostication of intubated patients in cardiac arrest.1,3,4,10,14 Under normal conditions, EtCO2 is 35-40 mm Hg. While the body still makes CO2 during cardiac arrest, it will not reach the alveoli without circulating blood.10 Without CPR, CO2 accumulates peripherally and won’t reach the lungs, causing EtCO2 to approach zero. This means that EtCO2 correlates directly with cardiac output during CPR, as long as ventilation remains constant.
This means the effectiveness of cardiac chest compression can be assessed in intubated patients using EtCO2, with higher values during CPR correlated with increased return of spontaneous circulation (ROSC) and survival.14-18 Using EtCO2 monitoring during cardiac arrest may improve outcomes,19 and the American Heart Association (AHA) recommends monitoring capnography during cardiac arrest to assess compression efficacy.10,20 EtCO2 >20 mm Hg is considered optimal, while EtCO2 <10-15 mm Hg is considered suboptimal.4,10,16 In a recent meta-analysis, the average EtCO2 was 13.1 mm Hg in those who did not obtain ROSC, compared to 25.8 mm Hg in those who did.21 As such, goal EtCO2 for effective compressions may be even higher in future recommendations. If EtCO2 is low, either compression technique should be improved or a different operator should do compressions. Every 1 cm increase in depth will increase EtCO2 by approximately 1.4 mm Hg.16 Interestingly, compression rate is not a significant predictor of EtCO2 over the dynamic range of chest compression delivery.16
An abrupt increase in EtCO2 is an early indicator of ROSC.10,14-16,22,23 A return of a perfusing rhythm will increase cardiac output. This allows for accumulated peripheral CO2 to reach the lungs, subsequently causing a rapid rise in EtCO2.24 It is important to note that when it comes to evaluating for ROSC, the actual numbers are less important than the change from pre- to post-ROSC. Providers should look for a jump of at least 10 mm Hg on capnometry.4 Nevertheless, an abrupt rise in EtCO2 is a non-sensitive marker for ROSC (33%, 95% CI 22-47% in one multicenter cross-sectional study), meaning that the lack of an abrupt rise of EtCO2 may not necessarily mean a lack of ROSC.23
The EtCO2 level may help guide decision-making in assessing whether continued resuscitation in cardiac arrest is futile. Values <10 mm Hg after 20 minutes of active resuscitation have consistently demonstrated minimal chance of survival.17,25,26 In one study, an EtCO2 of <10 mm Hg at 20 minutes had a sensitivity, specificity, PPV, and NPV of 100% for death in PEA arrest.17 However, determination of the specific EtCO2 cutoff and the timing is still an area of research with a final consensus pending.17,18,25-30 One recent study suggested that even 3 min with EtCO2 <10 mm Hg could be an appropriate cutoff to cease resuscitation efforts.27
Unfortunately, there is a large amount of heterogeneity in the available literature using capnography to assess for ROSC and in guiding resuscitation efforts. EtCO2 should not be used as the only factor in the determination to cease resuscitation. In addition, the AHA recommends that EtCO2 for prognostication should be limited to intubated patients only.20
It is important to note that while cardiac output is the largest factor for EtCO2 in arrest, other physiologic and iatrogenic causes may affect EtCO2 during resuscitation. For example, there is considerable variation in EtCO2 with changes in ventilation rate.4 Measured CO2 may be significantly lower with manual instead of mechanical ventilation, likely due to over-ventilation that not only reduces alveolar CO2 but also causes excess intra-thoracic pressure, reducing venous return.21 For these reasons, use caution when using EtCO2 during manual ventilation of an intubated patient in cardiac arrest. In addition, administration of epinephrine may cause a small decrease in EtCO2, although the effect may vary for each individual.10,31 Sodium bicarbonate can also cause a transient increase in CO2 due to its conversion into CO2 and H2O.10
Procedural Sedation
Capnography is being used with increasing frequency to monitor patients during procedural sedation; it is now considered standard of care in many settings.32 Although rare, hypoventilation is a risk of procedural sedation.33 Typically, respiratory depression during procedural sedation is diagnosed with non-invasive pulse oximetry and visual inspection.34 However, capnography has been shown to identify respiratory depression, airway obstruction, apnea, and laryngospasm earlier than pulse oximetry, allowing the provider to intervene quicker.34,35 Unlike pulse oximetry, the capnogram also remains stable during patient motion and is reliable in low-perfusion states.36

There are two distinct types of hypoventilation detected by capnography. Bradypneic hypoventilation (type 1), which is characterized by a decreased respiratory rate, results in a decreased expiratory time and a subsequent rise in EtCO2.36 This is depicted on capnography by a high EtCO2 and longer waveform, and is commonly observed after oversedation with opioids (Figure 4).36 In contrast, hypopneic hypoventilation (type 2) occurs with low tidal volumes but a normal respiratory rate.36 Type 2 is graphically represented by a suddenly lower ETCO2 with otherwise normal waveform and occurs most commonly with sedative-hypnotic drugs (Figure 5).36 Seeing either type during procedural sedation should alert the clinician to assess for airway obstruction, consider supplemental oxygen, cease drug administration or reduce dosing, and consider reversal if appropriate.36

There is some debate as to the utility of capnography for procedural sedation. While it is clear that capnography decreases the incidence of hypoxia, some studies suggest that it may not reduce patient-centered outcomes such as adverse respiratory events, neurologic injury, aspiration, or death compared to standard monitoring.35,37,38 However, pulse oximetry alone can suffer response delay, while EtCO2 can rapidly detect hypoventilation.39
Potential Uses/Applications
Respiratory Distress
Capnography can provide dynamic monitoring in patients with acute respiratory distress. Measuring EtCO2 with each breath provides instantaneous feedback on the clinical status of the patient and has numerous specific uses.1,3,4
Determining the etiology of respiratory distress in either the obtunded patient or those with multiple comorbidities can be a challenge. Vital sign abnormalities and physical exam findings can overlap in numerous conditions, which may only further obscure the diagnosis. Since different etiologies for respiratory disease require different management modalities, anything that can help clue in to the specific cause can be beneficial. As discussed above, obstructive diseases such as COPD or asthma demonstrate a “shark-fin appearance” on capnogram due to both V/Q heterogeneity and a prolonged expiratory phase due to airway constriction, which will contrast to the typical box-waveform in other conditions (Figure 2).1,2,6 Some studies have been able differentiate COPD from congestive heart failure (CHF) by waveform analysis alone, though this was primarily done via computer algorithms.40 Seeing the shark-fin (or the lack thereof) can help guide management of respiratory distress in conjunction with the remainder of the initial assessment.
Monitoring capnography can help with management and disposition in those with COPD or asthma. During exacerbations, EtCO2 levels may initially drop as the patient hyperventilates to compensate.1 It is not until ventilation becomes less effective that EtCO2 levels begin to rise. This may occur before hypoxia sets in and can prompt the clinician to escalate ventilation strategies. In addition, the normalization of the “shark-fin” obstructive pattern towards the more typical box-form wave may indicate effective treatment, though more data is needed before it can be recommended.41 One of the advantages of this technique would be that it is independent of patient effort, unlike peak-flow monitoring.
EtCO2 can be beneficial even before patients get to the ED. In one study, prehospital patients presenting with asthma or COPD who were found to have EtCO2 of >50 mm Hg or <28 mm Hg, representing the upper and lower limits in the study, had greater rates of intubation, critical care admission, and mortality.42 The patients in this cohort with higher EtCO2 were likely tiring after prolonged hyperventilation and therefore would be more likely to need ventilatory support. Those on the lower end were likely hyperventilating and had not yet tired out. It is important to note that while arrival EtCO2 levels may aid in determining the more critically ill, post-treatment levels were not found to have a statistical difference in determining disposition in patients with asthma or COPD.43
Caution is advised when attempting to use EtCO2 to approximate an arterial blood gas CO2 (PaCO2). While EtCO2 can correlate with PaCO2 within 5 mm Hg in greater than 80% of patients with dyspnea,44 large discrepancies are common depending on the disease state.45 In general, the EtCO2 should always be lower than the PaCO2 due to the contribution to the ETCO2 from dead space, which has a low CO2 content due to lack of perfusion.
Sepsis
EtCO2 may help identify septic patients given its inverse relationship with lactate levels.46-49 In conditions of poor tissue perfusion, lactate builds up. This begins to make the blood acidotic in the form of newly acquired anions, with a resultant anion gap metabolic acidosis. The body then tries to acutely compensate for this by hyperventilating, resulting in the observed lowering of EtCO2. Since lactate is a predictor of mortality in sepsis,50 and monitoring lactate clearance to evaluate resuscitation efforts in sepsis is recommended,51 EtCO2 could play a similar role. One group in particular has demonstrated that, when used with SIRS criteria, abnormally low prehospital EtCO2 levels is predictive of sepsis and inhospital mortality, and is more predictive than SIRS criteria alone.48,50 That said, EtCO2 was not associated with lactate temporally at 3 and 6 hours,51 so it should not be used to guide resuscitation like a lactate clearance. It appears that EtCO2 may be helpful for triage in sepsis, but more study is needed to determine the exact role particularly given most of the available research involves multiple studies from one group.47,48,52
Diabetic Ketoacidosis
Initial bicarbonate levels and venous pH are associated with low EtCO2 readings in diabetic ketoacidosis (DKA).54,55 This could have many practical uses, in particular for patients presenting with hyperglycemia to rule out DKA. One study demonstrated that a blood glucose >250 mg/dL and capnography of >24.5 mm Hg had 90% sensitivity for excluding DKA.55 A value of 35 mm Hg or greater demonstrated 100% sensitivity for excluding DKA in patients with initial glucose >550 mg/dL,56 though this blood glucose is not practical, as this excludes many patients the EP would seek to rule out DKA (recall that blood glucose only has to be >250 mg/dL for the diagnosis). Smaller studies focused on the pediatric population found a 100% sensitivity marker for DKA varied from >30 to >36 mm Hg.57,58 Clearly a role exists, but no study has demonstrated sufficient sensitivity for ruling out DKA with EtCO2 and blood glucose alone within the framework of clinically relevant values.
Trauma
As described above, low EtCO2 is inversely correlated with lactate.46 Because of this, it could theoretically be a marker of hypoperfusion in trauma. Initial EtCO2 values <25 mm Hg have been associated with mortality and hemorrhage in intubated trauma patients,59 as well as mortality prior to discharge in nonintubated trauma patients.60 However, it did not demonstrate added clinical utility when combined with Glasgow Coma Scale (GCS) score, systolic blood pressure, and age in predicting severe injury.61
Pulmonary Embolism
A pulmonary embolism (PE) causes a blockage in blood flow to alveoli, which results in a decrease in CO2 transportation to the alveoli and thus lower EtCO2, while also widening the gradient between PaCO2 and EtCO2.37 Because of this, it has a theoretical role in the diagnosis of PE, though numerous studies have demonstrated that EtCO2 alone is not sensitive nor specific enough for this role.62-66 In a recent meta-analysis, a pretest probability of 10% could lead to a posttest probability of 3% using capnography.62 While further study is needed before recommendation, this indicates that capnography could obviate the need for imaging in low to intermediate risk patients either after a positive D-Dimer or instead of obtaining a D-dimer.62-64
Triage
Simply measuring an initial EtCO2 as a triage vital sign may have added benefit to the EP, and consideration could be made for making this a policy in your ED. One study demonstrated that abnormal initial EtCO2 (outside of 35-45 mm Hg) was predictive of admisison (RR 2.5, 95% CI 1.5-4.0).67 An abnormal EtCO2 (outside of 31-41 mm Hg for this study) was 93% sensitive (95% CI 79-98%), with expectedly low specificity of 44% (95% CI 41-48%) for mortality prior to discharge.47 This potential vital sign may be treated similarly to tachycardia; while an abnormal heart rate should increase a clinician’s concern for a pathological condition, it needs to be taken in context of the situation to accurately interpret it.
Summary
Capnography has numerous uses in the ED in both intubated and spontaneously breathing patients. Quantitative capnography is the standard of care for confirming endotracheal intubation. It is recommended as an aide in maximizing chest compressions during cardiac arrest and can assist in prognostication. It rapidly identifies hypoventilation during procedural sedation. It also has many more potential applications that continue to be explored in areas such as respiratory distress, sepsis, trauma, DKA, and PE. Ultimately, capnography should always be used in association with the remainder of the clinical assessment.
- Manifold CA, Davids B, Villers LC, Wampler DA. Capnography for the nonintubated patient in the emergency setting. J Emerg Med. 2013;45(4):626-632.
- Ward K, Yealy DM. End-tidal carbon dioxide monitoring in emergency medicine, part 1: basic principles. Acad Emerg Med. 1998;5(6):628-636.
- Krauss B, Falk JL. Carbon dioxide monitoring (capnography). UpToDate. Waltham MA: UpToDate Inc. www.uptodate.com.
- Long B, Koyfman A, Michael AV. Capnography in the emergency department: a review of uses, waveforms, and limitations. J Emerg Med. 2017;(53)6:829-842.
- Shankar Kodali B. Capnography: A Comprehensive Educational Website. Boston, MA. www.capnography.com.
- Kodali B. Capnography outside the operating room. Anesthesiology. 2013;118:192-201.
- Bhavani, S. Defining segments and phases of a time capnogram. Anesth Analg. 2000;91(4):973-977.
- Petersson J, Glenny R. Gas exchange and ventilation-perfusion relationships in the lung. Eur Resp J. 2014;44(4):1023-1041.
- Nassar B, Schmidt GA. Capnography during critical illness. Chest. 2016;149(2):576-585.
- Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S729-S767.
- Burns SM, Carpenter R, Blevins C, Bragg S, Marshall M, Browne L, et al. Detection of inadvertent airway intubation during gastric tube insertion: capnography versus a colorimetric carbon dioxide detector. Am J Crit Care. 2006:15(2):188-195.
- Goldberg JS, Rawle PR, Zehnder JL, Sladen RN. Colorimetric end-tidal carbon dioxide monitoring for tracheal intubation. Anesthesia and analgesia. 1990:70(2):191-194.
- O'Flaherty D, Adams AP. The end-tidal carbon dioxide detector: assessment of a new method to distinguish oesophageal from tracheal intubation. Anaesthesia. 1990:45(8):653-655.
- Garnett AR, Ornato JP, Gonzalez ER, Johnson EB. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512-515.
- Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318(10):607-611.
- Sheak KR, Wiebe DJ, Leary M, Babaeizadeh S, Yuen TC, Zive D, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149-154.
- Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301-306.
- Touma O, Davies M. The prognostic value of end tidal carbon dioxide during cardiac arrest: a systematic review. Resuscitation. 2013;84(11):1470-1479.
- Chen JJ, Lee YK, Hou SW, et al. End-tidal carbon dioxide monitoring may be associated with a higher possibility of return of spontaneous circulation during out-of-hospital cardiac arrest: a population-based study. Scan J Trauma Resusc Emerg Med. 2015;23:104.
- Neumar RW, Shuster M, Callaway CW et al. Part 7: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(suppl 2):S315-S367.
- Hartmann SW, Farris RW, Di Gennaro JL, Roberts JS. Systematic review and meta-analysis of end-tidal carbon dioxide values associated with return of spontaneous circulation during cardiopulmonary resuscitation. J Intensive Care Med. 2015;(30):426-435.
- Eckstein, M, Hatch, L, Malleck, J et al. EtCO2 as a predictor of survival in out-of hospital cardiac arrest. Prehosp Disaster Med. 2016;104:53-58.
- Lui CT, Poon KM, Tsui KL. Abrupt rise of end tidal carbon dioxide was a specific but non-sensitive marker of return of spontaneous circulation with out-of-hospital cardiac arrest. Resuscitation. 2016;104:53-58.
- Pokorna M, Necas E, Kratochvil J et al. A sudden increase in partial pressure end-tidal carbon dioxide at the moment of return of spontaneous circulation. J Emerg Med. 2010;38:614-621.
- Sanders A, Kern K, Otto C, et al. End-tidal carbon dixoide monitoring during cardiopulmonary resuscitation: a prognostic indicator for survival. JAMA. 1989;262:1347-1351.
- Wayne M, Levine R, And Miller C. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann Emerg Med. 1995;25(6):762-767.
- Poon KM, Lui CT, Tsui KL. Prognostication of out-of-hopsital cardiac arrest patients by 3-min end-tidal capnometry level in emergency department. Resuscitation. 2016;102:80-84.
- Einav S, Bromiker R, Weiniger C, Matot I. Mathematical modeling for prediction of survival from resuscitation based on computerized continuous capnography: proof of concept. Acad Emerg Med. 2011;18:468-475.
- Pearce A, Davis D, Minokadeh A, Sell R. Initial end-tidal carbon dioxide as a prognostic indicator for inpatient PEA arrest. Resuscitation. 2015;92:77-81.
- Akinci, E, Ramadan H, Yuzbasioglu Y, Coksun F. Comparison of end-tidal carbon dioxide levels with cardiopulmonary resuscitation success presented to emergency department with cardiopulmonary arrest. Pak J Med Sci. 2014;30(1):16-21.
- Callaham M, Barton C, Matthay M. Effect of epinephrine on the ability of end-tidal carbon dioxide readings to predict initial resuscitation from cardiac arrest. Crit Care Med. 1992; 20:337-343.
- Wall BF, Magee K, Campbell SG, Zed PJ. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database of Systematic Reviews. 2017(3).
- Langhan ML, Shabanova V, Li FY, Bernstein SL, Shapiro ED. A randomized controlled trial of capnography during sedation in a pediatric emergency setting. Am J Emerg Med. 2015;33(1):25-30.
- Campbell SG, Magee KD, Zed PJ, Froese P, Etsell G, LaPierre A et al. End-tidal capnometry during emergency department procedural sedation and analgesia: a randomized, controlled study. World J Emerg Med. 2016;7(1):13.
- Waugh JB, Epps CA, Khodneva YA. Capnography enhances surveillance of respiratory events during procedural sedation: a meta-analysis. J Clin Anesth. 2011;23(3):189-196.
- Krauss B, Hess DR. Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;50(2):172-181.
- Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55(3):258-264.
- Godwin SA, Caro DA, Wolf SJ, Jagoda AS, Charles R, Marett BE, Moore J. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45(2):177-196.
- Hamber EA, Bailey PI, James SW et al. Delays in the detection of hypoxemia due to site of pulse oximetry pulse placement. J Clin Anesth. 1999;11:113-118.
- Mieloszyk RJ, Vergehese GC, Deitch K, et al. Automated quantitative analysis of capnogram shape for COPD-normal and COPD-CHF classification. IEEE Trans Biomed Eng. 2014;61:2882-2890.
- Howe TA, Jaalam K, R. Ahmad, Sheng CK, Ab Rahman NHN. The use of end-tidal capnography to monitor non-intubated patients presenting with acute exacerbation of asthma in the emergency department. J Emerg Med. 2011:41:581-589.
- Nagurka R, Bechmann S, Gluckman W et al. Utility of initial prehospital end-tidal carbon dioxide measurements to predict poor outcomes in adult asthmatic patients. Prehospital Emerg Care. 2014;18:180-184.
- Doğan NÖ, Şener A, Günaydın GP, İçme F, Çelik GK, Kavaklı HŞ, Temrel TA. The accuracy of mainstream end-tidal carbon dioxide levels to predict the severity of chronic obstructive pulmonary disease exacerbations presented to the ED. Am J Emerg Med. 2014;32(5):408-411.
- Cinar O, Acar YA, Arziman I, et al. Can mainstream end-tidal carbon dioxide measurement accurately predict the arterial carbon dioxide levels of patients with acute dyspnea in ED. Am J Emerg Med. 2012;30:358-361.
- Nassar BS, Schmidt GA. Capnography during critical illness. Chest. 2016:149(2):576-585.
- Caputo ND, Fraser RM, Paliga A et al. Nasal cannula end-tidal CO2 correlates with serum lactate levels and odds of operative intervention in penetrating trauma patients: a prospective cohort study. J Trauma Acute Care Surg. 2012;73:1202-1207.
- Hunter CL, Silvestri S, Ralls G, Bright S, Papa L. The sixth vital sign: prehospital end-tidal carbon dioxide predicts in-hospital mortality and metabolic disturbances. Am J Emerg Med. 2014;32(2):160-165.
- Hunter CL, Silvestri S, Dean M, Falk JL, Papa L. End-tidal carbon dioxide is associated with mortality and lactate in patients with suspected sepsis. Am J Emerg Med. 2013;31(1):64-71.
- McGillicuddy DC, Tang A, Cataldo L, et al. Evaluation of end-tidal carbon dioxide role in predicting elevated SOFA and lactic acidosis. Intern Emerg Med. 2009;4:41-44.
- Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524-528.
- Levy, MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46:997-1000.
- Hunter CL, Silvestri S, Ralls G et al. A prehospital screening tool utilizing end-tidal carbon dioxide predicts sepsis and severe sepsis. Am J Emerg Med. 2016;34:813-819.
- Guirgis FW, Williams DJ, Kalynych CJ, Hardy ME, Jones AE, Dodani S, Wears RL. End-tidal carbon dioxide as a goal of early sepsis therapy. Am J Emerg Med. 2014;32(11):1351-1356.
- Kartal M, Eray O, Rinnert S, Gosku E, Bektas F, Eken C. ETCO2: a predictive tool for excluding metabolic disturbances in nonintubated patients. Am J Emerg Med. 2011;29: 65-69.
- Solmeinpur H, Taghizadieh A, Niafar M, Rahmani F, Golzari SE, Esfanjani RM. Predictive value of capnography for diagnosis in patients with suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. 2013;14:590-594.
- Bou Chebl R, Madden B, Belsky J, Harmouche E, Yessayan L. Diagnostic value of end tidal capnography in patients with hyperglycemia in the emergency department. BMC Emerg Med. 2016;16:7.
- Fearon DM, Steele DW. End-tidal carbon dioxide predicts the presence and severity of acidosis in children with diabetes. Acad Emerg Med. 2002;9:1373-1378.
- Gilhotra Y, Porter P. Predicting diabetic ketoacidosis in children by measuring end-tidal CO2 via non-invasive nasal capnography. J Paediatr Child Health. 2007;43:677-680.
- Dunham CM, Chirichella TJ, Gruber BS, et al. In emergently ventilated trauma patients, low end-tidal CO2 and low cardiac output are associated and correlate with hemodynamic instability, hemorrhage, abnormal pupils, and death. BMC Anesthesiol. 2013;13-20.
- Deakin CD, Sado DM, Coats TJ, Davies G. Prehospital end-tidal carbon dioxide concentration and outcome in major trauma. J Trauma.2004;57:65-68.
- Williams DJ, Guirgis FW, Morrissey TK, Wilkerson J, Wears RL, Kalynych C, Kerwin AJ, Godwin SA. End-tidal carbon dioxide and occult injury in trauma patients: ETCO2 does not rule out severe injury. Am J Emerg Med. 2016;34(11):2146-2149.
- Manara A, D’hoore W, Thys F. Capnography as a diagnostic tool for pulmonary embolism: a meta-analysis. Ann Emerg Med. 2013;52:584-591.
- Yoon YH, Lee SW, Jung DM et al. The additional use of end-tidal alveolar dead space fraction following D-dimer test to improve diagnostic accuracy for pulmonary embolism in the emergency department. Emerg Med J. 2010;27:663-667.
- Hemnes AR, Newman AL, Rosenbaum B, et al. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Resp J. 2010;35:735-741.
- Rias I Jacob B. Pulmonary embolism in Bradford, UK: role of end-tidal CO2 as a screening tool. Clin Med (Lond). 2014;14:128-133.
- Yuksel M, Pekdemir M, Yilmaz S, et al. Diagnostic accuracy of noninvasive end-tidal carbon dioxide measurement in emergency department patients with suspected pulmonary embolism. Turk J Med Sci. 2016;46:84–90.
- Williams D, Morrissey T, Caro D, Wears R, Kalynyc C. Side-stream qunatitative end-tidal carbon dioxide measurement as a triage tool in emergency medicine. Ann Emerg Med. 2011;58:S212-S213.
- Manifold CA, Davids B, Villers LC, Wampler DA. Capnography for the nonintubated patient in the emergency setting. J Emerg Med. 2013;45(4):626-632.
- Ward K, Yealy DM. End-tidal carbon dioxide monitoring in emergency medicine, part 1: basic principles. Acad Emerg Med. 1998;5(6):628-636.
- Krauss B, Falk JL. Carbon dioxide monitoring (capnography). UpToDate. Waltham MA: UpToDate Inc. www.uptodate.com.
- Long B, Koyfman A, Michael AV. Capnography in the emergency department: a review of uses, waveforms, and limitations. J Emerg Med. 2017;(53)6:829-842.
- Shankar Kodali B. Capnography: A Comprehensive Educational Website. Boston, MA. www.capnography.com.
- Kodali B. Capnography outside the operating room. Anesthesiology. 2013;118:192-201.
- Bhavani, S. Defining segments and phases of a time capnogram. Anesth Analg. 2000;91(4):973-977.
- Petersson J, Glenny R. Gas exchange and ventilation-perfusion relationships in the lung. Eur Resp J. 2014;44(4):1023-1041.
- Nassar B, Schmidt GA. Capnography during critical illness. Chest. 2016;149(2):576-585.
- Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S729-S767.
- Burns SM, Carpenter R, Blevins C, Bragg S, Marshall M, Browne L, et al. Detection of inadvertent airway intubation during gastric tube insertion: capnography versus a colorimetric carbon dioxide detector. Am J Crit Care. 2006:15(2):188-195.
- Goldberg JS, Rawle PR, Zehnder JL, Sladen RN. Colorimetric end-tidal carbon dioxide monitoring for tracheal intubation. Anesthesia and analgesia. 1990:70(2):191-194.
- O'Flaherty D, Adams AP. The end-tidal carbon dioxide detector: assessment of a new method to distinguish oesophageal from tracheal intubation. Anaesthesia. 1990:45(8):653-655.
- Garnett AR, Ornato JP, Gonzalez ER, Johnson EB. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512-515.
- Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318(10):607-611.
- Sheak KR, Wiebe DJ, Leary M, Babaeizadeh S, Yuen TC, Zive D, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149-154.
- Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301-306.
- Touma O, Davies M. The prognostic value of end tidal carbon dioxide during cardiac arrest: a systematic review. Resuscitation. 2013;84(11):1470-1479.
- Chen JJ, Lee YK, Hou SW, et al. End-tidal carbon dioxide monitoring may be associated with a higher possibility of return of spontaneous circulation during out-of-hospital cardiac arrest: a population-based study. Scan J Trauma Resusc Emerg Med. 2015;23:104.
- Neumar RW, Shuster M, Callaway CW et al. Part 7: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(suppl 2):S315-S367.
- Hartmann SW, Farris RW, Di Gennaro JL, Roberts JS. Systematic review and meta-analysis of end-tidal carbon dioxide values associated with return of spontaneous circulation during cardiopulmonary resuscitation. J Intensive Care Med. 2015;(30):426-435.
- Eckstein, M, Hatch, L, Malleck, J et al. EtCO2 as a predictor of survival in out-of hospital cardiac arrest. Prehosp Disaster Med. 2016;104:53-58.
- Lui CT, Poon KM, Tsui KL. Abrupt rise of end tidal carbon dioxide was a specific but non-sensitive marker of return of spontaneous circulation with out-of-hospital cardiac arrest. Resuscitation. 2016;104:53-58.
- Pokorna M, Necas E, Kratochvil J et al. A sudden increase in partial pressure end-tidal carbon dioxide at the moment of return of spontaneous circulation. J Emerg Med. 2010;38:614-621.
- Sanders A, Kern K, Otto C, et al. End-tidal carbon dixoide monitoring during cardiopulmonary resuscitation: a prognostic indicator for survival. JAMA. 1989;262:1347-1351.
- Wayne M, Levine R, And Miller C. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann Emerg Med. 1995;25(6):762-767.
- Poon KM, Lui CT, Tsui KL. Prognostication of out-of-hopsital cardiac arrest patients by 3-min end-tidal capnometry level in emergency department. Resuscitation. 2016;102:80-84.
- Einav S, Bromiker R, Weiniger C, Matot I. Mathematical modeling for prediction of survival from resuscitation based on computerized continuous capnography: proof of concept. Acad Emerg Med. 2011;18:468-475.
- Pearce A, Davis D, Minokadeh A, Sell R. Initial end-tidal carbon dioxide as a prognostic indicator for inpatient PEA arrest. Resuscitation. 2015;92:77-81.
- Akinci, E, Ramadan H, Yuzbasioglu Y, Coksun F. Comparison of end-tidal carbon dioxide levels with cardiopulmonary resuscitation success presented to emergency department with cardiopulmonary arrest. Pak J Med Sci. 2014;30(1):16-21.
- Callaham M, Barton C, Matthay M. Effect of epinephrine on the ability of end-tidal carbon dioxide readings to predict initial resuscitation from cardiac arrest. Crit Care Med. 1992; 20:337-343.
- Wall BF, Magee K, Campbell SG, Zed PJ. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database of Systematic Reviews. 2017(3).
- Langhan ML, Shabanova V, Li FY, Bernstein SL, Shapiro ED. A randomized controlled trial of capnography during sedation in a pediatric emergency setting. Am J Emerg Med. 2015;33(1):25-30.
- Campbell SG, Magee KD, Zed PJ, Froese P, Etsell G, LaPierre A et al. End-tidal capnometry during emergency department procedural sedation and analgesia: a randomized, controlled study. World J Emerg Med. 2016;7(1):13.
- Waugh JB, Epps CA, Khodneva YA. Capnography enhances surveillance of respiratory events during procedural sedation: a meta-analysis. J Clin Anesth. 2011;23(3):189-196.
- Krauss B, Hess DR. Capnography for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;50(2):172-181.
- Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55(3):258-264.
- Godwin SA, Caro DA, Wolf SJ, Jagoda AS, Charles R, Marett BE, Moore J. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2005;45(2):177-196.
- Hamber EA, Bailey PI, James SW et al. Delays in the detection of hypoxemia due to site of pulse oximetry pulse placement. J Clin Anesth. 1999;11:113-118.
- Mieloszyk RJ, Vergehese GC, Deitch K, et al. Automated quantitative analysis of capnogram shape for COPD-normal and COPD-CHF classification. IEEE Trans Biomed Eng. 2014;61:2882-2890.
- Howe TA, Jaalam K, R. Ahmad, Sheng CK, Ab Rahman NHN. The use of end-tidal capnography to monitor non-intubated patients presenting with acute exacerbation of asthma in the emergency department. J Emerg Med. 2011:41:581-589.
- Nagurka R, Bechmann S, Gluckman W et al. Utility of initial prehospital end-tidal carbon dioxide measurements to predict poor outcomes in adult asthmatic patients. Prehospital Emerg Care. 2014;18:180-184.
- Doğan NÖ, Şener A, Günaydın GP, İçme F, Çelik GK, Kavaklı HŞ, Temrel TA. The accuracy of mainstream end-tidal carbon dioxide levels to predict the severity of chronic obstructive pulmonary disease exacerbations presented to the ED. Am J Emerg Med. 2014;32(5):408-411.
- Cinar O, Acar YA, Arziman I, et al. Can mainstream end-tidal carbon dioxide measurement accurately predict the arterial carbon dioxide levels of patients with acute dyspnea in ED. Am J Emerg Med. 2012;30:358-361.
- Nassar BS, Schmidt GA. Capnography during critical illness. Chest. 2016:149(2):576-585.
- Caputo ND, Fraser RM, Paliga A et al. Nasal cannula end-tidal CO2 correlates with serum lactate levels and odds of operative intervention in penetrating trauma patients: a prospective cohort study. J Trauma Acute Care Surg. 2012;73:1202-1207.
- Hunter CL, Silvestri S, Ralls G, Bright S, Papa L. The sixth vital sign: prehospital end-tidal carbon dioxide predicts in-hospital mortality and metabolic disturbances. Am J Emerg Med. 2014;32(2):160-165.
- Hunter CL, Silvestri S, Dean M, Falk JL, Papa L. End-tidal carbon dioxide is associated with mortality and lactate in patients with suspected sepsis. Am J Emerg Med. 2013;31(1):64-71.
- McGillicuddy DC, Tang A, Cataldo L, et al. Evaluation of end-tidal carbon dioxide role in predicting elevated SOFA and lactic acidosis. Intern Emerg Med. 2009;4:41-44.
- Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524-528.
- Levy, MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46:997-1000.
- Hunter CL, Silvestri S, Ralls G et al. A prehospital screening tool utilizing end-tidal carbon dioxide predicts sepsis and severe sepsis. Am J Emerg Med. 2016;34:813-819.
- Guirgis FW, Williams DJ, Kalynych CJ, Hardy ME, Jones AE, Dodani S, Wears RL. End-tidal carbon dioxide as a goal of early sepsis therapy. Am J Emerg Med. 2014;32(11):1351-1356.
- Kartal M, Eray O, Rinnert S, Gosku E, Bektas F, Eken C. ETCO2: a predictive tool for excluding metabolic disturbances in nonintubated patients. Am J Emerg Med. 2011;29: 65-69.
- Solmeinpur H, Taghizadieh A, Niafar M, Rahmani F, Golzari SE, Esfanjani RM. Predictive value of capnography for diagnosis in patients with suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. 2013;14:590-594.
- Bou Chebl R, Madden B, Belsky J, Harmouche E, Yessayan L. Diagnostic value of end tidal capnography in patients with hyperglycemia in the emergency department. BMC Emerg Med. 2016;16:7.
- Fearon DM, Steele DW. End-tidal carbon dioxide predicts the presence and severity of acidosis in children with diabetes. Acad Emerg Med. 2002;9:1373-1378.
- Gilhotra Y, Porter P. Predicting diabetic ketoacidosis in children by measuring end-tidal CO2 via non-invasive nasal capnography. J Paediatr Child Health. 2007;43:677-680.
- Dunham CM, Chirichella TJ, Gruber BS, et al. In emergently ventilated trauma patients, low end-tidal CO2 and low cardiac output are associated and correlate with hemodynamic instability, hemorrhage, abnormal pupils, and death. BMC Anesthesiol. 2013;13-20.
- Deakin CD, Sado DM, Coats TJ, Davies G. Prehospital end-tidal carbon dioxide concentration and outcome in major trauma. J Trauma.2004;57:65-68.
- Williams DJ, Guirgis FW, Morrissey TK, Wilkerson J, Wears RL, Kalynych C, Kerwin AJ, Godwin SA. End-tidal carbon dioxide and occult injury in trauma patients: ETCO2 does not rule out severe injury. Am J Emerg Med. 2016;34(11):2146-2149.
- Manara A, D’hoore W, Thys F. Capnography as a diagnostic tool for pulmonary embolism: a meta-analysis. Ann Emerg Med. 2013;52:584-591.
- Yoon YH, Lee SW, Jung DM et al. The additional use of end-tidal alveolar dead space fraction following D-dimer test to improve diagnostic accuracy for pulmonary embolism in the emergency department. Emerg Med J. 2010;27:663-667.
- Hemnes AR, Newman AL, Rosenbaum B, et al. Bedside end-tidal CO2 tension as a screening tool to exclude pulmonary embolism. Eur Resp J. 2010;35:735-741.
- Rias I Jacob B. Pulmonary embolism in Bradford, UK: role of end-tidal CO2 as a screening tool. Clin Med (Lond). 2014;14:128-133.
- Yuksel M, Pekdemir M, Yilmaz S, et al. Diagnostic accuracy of noninvasive end-tidal carbon dioxide measurement in emergency department patients with suspected pulmonary embolism. Turk J Med Sci. 2016;46:84–90.
- Williams D, Morrissey T, Caro D, Wears R, Kalynyc C. Side-stream qunatitative end-tidal carbon dioxide measurement as a triage tool in emergency medicine. Ann Emerg Med. 2011;58:S212-S213.
The risks of intensive parenting
“Parenthood in the United States has become much more demanding than it used to be.” It is hard to argue with this opening sentence in Clair Cain Miller’s op-ed piece titled “The Relentlessness of Modern Parenting,” published in the Dec. 25, 2018, electronic edition of the New York Times. But just in case you don’t agree with her premise, she lays out her case with evidence that parents in this country are investing more time, attention, and money into raising their children than was the norm several decades ago. She goes on to describe how this “intensive parenting” is taking its toll on parents on both sides of our nation’s widening economic divide. I’m sure you have seen it in your office in the tired faces and stooped shoulders of your patients’ parents. You may even be struggling yourself to find the time and energy to be the parent you believe your children need and deserve.
While there is debate on whether “parent” is inherently a verb or a noun (“Parent is a Noun, Not a Verb,” Cliff Price, the Australian Family Association; “Parent is a Verb – and we All do it,” Zaeli Kane, mother.ly), it is clear that “parenting” used as a verb has become one of the hot topics in pediatrics over the last quarter century and with it an epidemic of parental anxiety. What are the driving forces behind this shift in attitude? How has a relatively relaxed nature-will-take-its-course philosophy become an anxiety-provoking, stress-inducing phenomenon that will inevitably result in a disturbed and disappointed adult without a parent’s relentless attention to creating a nurturing and optimally stimulating environment?
Of course, parents have always worried about the health of their children and hope that they will be successful, regardless of how one defines success. But this natural parental concern seems to have gotten out of hand.
Is it because North Americans are having fewer children? Is it because in smaller families children become adults with little or no practical experience with hands-on child rearing? Are parents reacting to the predictions that the next generation may not be able to earn enough to match their parents’ lifestyle?
How much blame should fall on those of us who market ourselves as child health experts? Have we failed to put the research supporting the importance of early life experiences in the proper perspective? Are our recommendations creating unrealistic goals for parents? The American Academy of Pediatrics advice on breastfeeding duration and room sharing come to mind immediately. How realistic is it for parents to coview the majority of television shows their children are watching?
On one hand, we are beginning to realize that free play is important, but for years pediatricians have been one of the loudest voices supporting playground and toy safety. These two initiatives can certainly coexist, but I fear that at times we have begun to sound a bit like that annoying parent who is constantly warning his or her child, “Don’t do that, you’ll hurt yourself?”
Have we become the worry merchants? As a marketing strategy it seems to be working well. If we generate enough advice that supports an intensive parenting style, we can fill our waiting rooms with families struggling to meet the expectations we have been promoting.
A child can thrive without intensive parenting as long as he feels loved and he has been provided an environment with sensible limits to keep him safe.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“Parenthood in the United States has become much more demanding than it used to be.” It is hard to argue with this opening sentence in Clair Cain Miller’s op-ed piece titled “The Relentlessness of Modern Parenting,” published in the Dec. 25, 2018, electronic edition of the New York Times. But just in case you don’t agree with her premise, she lays out her case with evidence that parents in this country are investing more time, attention, and money into raising their children than was the norm several decades ago. She goes on to describe how this “intensive parenting” is taking its toll on parents on both sides of our nation’s widening economic divide. I’m sure you have seen it in your office in the tired faces and stooped shoulders of your patients’ parents. You may even be struggling yourself to find the time and energy to be the parent you believe your children need and deserve.
While there is debate on whether “parent” is inherently a verb or a noun (“Parent is a Noun, Not a Verb,” Cliff Price, the Australian Family Association; “Parent is a Verb – and we All do it,” Zaeli Kane, mother.ly), it is clear that “parenting” used as a verb has become one of the hot topics in pediatrics over the last quarter century and with it an epidemic of parental anxiety. What are the driving forces behind this shift in attitude? How has a relatively relaxed nature-will-take-its-course philosophy become an anxiety-provoking, stress-inducing phenomenon that will inevitably result in a disturbed and disappointed adult without a parent’s relentless attention to creating a nurturing and optimally stimulating environment?
Of course, parents have always worried about the health of their children and hope that they will be successful, regardless of how one defines success. But this natural parental concern seems to have gotten out of hand.
Is it because North Americans are having fewer children? Is it because in smaller families children become adults with little or no practical experience with hands-on child rearing? Are parents reacting to the predictions that the next generation may not be able to earn enough to match their parents’ lifestyle?
How much blame should fall on those of us who market ourselves as child health experts? Have we failed to put the research supporting the importance of early life experiences in the proper perspective? Are our recommendations creating unrealistic goals for parents? The American Academy of Pediatrics advice on breastfeeding duration and room sharing come to mind immediately. How realistic is it for parents to coview the majority of television shows their children are watching?
On one hand, we are beginning to realize that free play is important, but for years pediatricians have been one of the loudest voices supporting playground and toy safety. These two initiatives can certainly coexist, but I fear that at times we have begun to sound a bit like that annoying parent who is constantly warning his or her child, “Don’t do that, you’ll hurt yourself?”
Have we become the worry merchants? As a marketing strategy it seems to be working well. If we generate enough advice that supports an intensive parenting style, we can fill our waiting rooms with families struggling to meet the expectations we have been promoting.
A child can thrive without intensive parenting as long as he feels loved and he has been provided an environment with sensible limits to keep him safe.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“Parenthood in the United States has become much more demanding than it used to be.” It is hard to argue with this opening sentence in Clair Cain Miller’s op-ed piece titled “The Relentlessness of Modern Parenting,” published in the Dec. 25, 2018, electronic edition of the New York Times. But just in case you don’t agree with her premise, she lays out her case with evidence that parents in this country are investing more time, attention, and money into raising their children than was the norm several decades ago. She goes on to describe how this “intensive parenting” is taking its toll on parents on both sides of our nation’s widening economic divide. I’m sure you have seen it in your office in the tired faces and stooped shoulders of your patients’ parents. You may even be struggling yourself to find the time and energy to be the parent you believe your children need and deserve.
While there is debate on whether “parent” is inherently a verb or a noun (“Parent is a Noun, Not a Verb,” Cliff Price, the Australian Family Association; “Parent is a Verb – and we All do it,” Zaeli Kane, mother.ly), it is clear that “parenting” used as a verb has become one of the hot topics in pediatrics over the last quarter century and with it an epidemic of parental anxiety. What are the driving forces behind this shift in attitude? How has a relatively relaxed nature-will-take-its-course philosophy become an anxiety-provoking, stress-inducing phenomenon that will inevitably result in a disturbed and disappointed adult without a parent’s relentless attention to creating a nurturing and optimally stimulating environment?
Of course, parents have always worried about the health of their children and hope that they will be successful, regardless of how one defines success. But this natural parental concern seems to have gotten out of hand.
Is it because North Americans are having fewer children? Is it because in smaller families children become adults with little or no practical experience with hands-on child rearing? Are parents reacting to the predictions that the next generation may not be able to earn enough to match their parents’ lifestyle?
How much blame should fall on those of us who market ourselves as child health experts? Have we failed to put the research supporting the importance of early life experiences in the proper perspective? Are our recommendations creating unrealistic goals for parents? The American Academy of Pediatrics advice on breastfeeding duration and room sharing come to mind immediately. How realistic is it for parents to coview the majority of television shows their children are watching?
On one hand, we are beginning to realize that free play is important, but for years pediatricians have been one of the loudest voices supporting playground and toy safety. These two initiatives can certainly coexist, but I fear that at times we have begun to sound a bit like that annoying parent who is constantly warning his or her child, “Don’t do that, you’ll hurt yourself?”
Have we become the worry merchants? As a marketing strategy it seems to be working well. If we generate enough advice that supports an intensive parenting style, we can fill our waiting rooms with families struggling to meet the expectations we have been promoting.
A child can thrive without intensive parenting as long as he feels loved and he has been provided an environment with sensible limits to keep him safe.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Adult atopic dermatitis is fraught with dermatologic comorbidities
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Adult atopic dermatitis patients have higher prevalence of psoriasis, alopecia areata, vitiligo, and other dermatologic disorders.
Major finding: The risk of contact dermatitis is increased 3.4- to 5.6-fold.
Study details: This German population-based study used both a medical claims database including more than 1.3 million adults and dermatologist-conducted occupational skin screening in more than 162,000 workers.
Disclosures: The study presenter reported having no financial conflicts regarding her study, conducted without commercial support.
Ray Barfield Part II: Philosophy and Medicine
In part I of the conversation, Dr. Barfield and MDedge host Nick Andrews discussed physician burnout and Dr. Barfield’s journey back to medicine. In this episode, Dr. Barfield and Nick discuss philosophy and science.
You can listen to part I of this conversation here:
Apple Podcasts
Google Podcasts
Spotify
In part I of the conversation, Dr. Barfield and MDedge host Nick Andrews discussed physician burnout and Dr. Barfield’s journey back to medicine. In this episode, Dr. Barfield and Nick discuss philosophy and science.
You can listen to part I of this conversation here:
Apple Podcasts
Google Podcasts
Spotify
In part I of the conversation, Dr. Barfield and MDedge host Nick Andrews discussed physician burnout and Dr. Barfield’s journey back to medicine. In this episode, Dr. Barfield and Nick discuss philosophy and science.
You can listen to part I of this conversation here:
Apple Podcasts
Google Podcasts
Spotify
Deep sleep decreases, Alzheimer’s increases
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Adding umbralisib to ibrutinib produced responses in MCL, CLL
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Of CLL patients, 90% achieved an overall response.
Study details: Phase 1-1b trial of umbralisib and ibrutinib in patients with relapsed or refractory MCL or CLL.
Disclosures: The study was supported by TG Therapeutics and the Leukemia and Lymphoma Therapy Accelerator Program. Dr. Davids and his coauthors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
Source: Davids MS et al. Lancet Haematol. 2019;6:e38-47.
Benralizumab maintains effectiveness in severe asthma at 2 years
out to 2 years, according findings of the BORA trial, an extension study of the phase 3 SIROCCO and CALIMA trials. The study follows up and reinforces previously reported 1-year data and was reported by William W. Busse, MD, of University of Wisconsin, Madison, and his colleagues in the Lancet Respiratory Medicine.
Benralizumab is a monoclonal antibody that targets interleukin-5 receptor alpha. It causes rapid deletion of eosinophils through cell-mediated cytotoxicity. A 30-mg dose of benralizumab every 8 weeks is approved for severe asthma treatment in Canada, Europe, Japan, the United States, and other countries.
In the second year of treatment, there were no new adverse events associated with depleted eosinophils, and the frequency of opportunistic infections was similar to that observed in the first year.
Eosinophilic inflammation occurs in about half of asthma cases and is associated with greater severity.
The 48-week SIROCCO trial, the 56-week CALIMA trial, and the 28-week ZONDA trial tested the effect of benralizumab 30 mg given every 4 weeks or 8 weeks, combined with high-dosage inhaled steroids and long-acting beta2-agonists. The 8-week dose of the drug reduced annual exacerbations by 51%, compared with placebo in the SIROCCO trial and by 28% in the CALIMA trial. In the ZONDA trial, benralizumab reduced oral glucocorticoid use by 75%, compared with placebo, and by 25% from baseline.
The BORA extension trial included participants in the previous three trials. In the current report, researchers presented results from the analysis from BORA participants recruited from the SIROCCO and CALIMA trials. Data from participants from all three trials will be reported in the future.
The analysis included 1,576 patients who continued to receive benralizumab after being assigned to the treatment arm in SIROCCO or CALIMA, or who had received placebo were randomized to benralizumab on the 4-week (n = 783; 265 from placebo) or 8-week dose (n = 793; 281 from placebo) schedule.
A total of 166 patients, or about 10% in each group, discontinued treatment. The frequency of any serious adverse event (SAE) ranged between 10% and 11% in all groups. SAEs associated with infections ranged from 1% to 3%, indicating that there were no significant differences in SAE frequencies between those who were originally assigned to placebo and those who originally received benralizumab. That suggests no safety differences between receiving the drug for 1 year or 2 years.
A total of 1,046 subjects had blood eosinophil counts of 300 cells per mcL or greater at baseline; 72% of these patients had no asthma exacerbations during the BORA study. This was true for 74% of patients in the 8-week treatment arm.
The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm, compared with placebo (95% confidence interval, 0.42-0.56) and 0.46 in the 8-week arm (95% CI, 0.39-0.53). For patients who started out on placebo, the crude exacerbation rate during BORA was 0.53 in the 4-week group (95% CI, 0.43-0.65) and 0.57 in the 8-week group (95% CI, 0.47-0.68).
Patients who started on benralizumab had similar exacerbation frequencies during year 1 and year 2.
AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
SOURCE: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
out to 2 years, according findings of the BORA trial, an extension study of the phase 3 SIROCCO and CALIMA trials. The study follows up and reinforces previously reported 1-year data and was reported by William W. Busse, MD, of University of Wisconsin, Madison, and his colleagues in the Lancet Respiratory Medicine.
Benralizumab is a monoclonal antibody that targets interleukin-5 receptor alpha. It causes rapid deletion of eosinophils through cell-mediated cytotoxicity. A 30-mg dose of benralizumab every 8 weeks is approved for severe asthma treatment in Canada, Europe, Japan, the United States, and other countries.
In the second year of treatment, there were no new adverse events associated with depleted eosinophils, and the frequency of opportunistic infections was similar to that observed in the first year.
Eosinophilic inflammation occurs in about half of asthma cases and is associated with greater severity.
The 48-week SIROCCO trial, the 56-week CALIMA trial, and the 28-week ZONDA trial tested the effect of benralizumab 30 mg given every 4 weeks or 8 weeks, combined with high-dosage inhaled steroids and long-acting beta2-agonists. The 8-week dose of the drug reduced annual exacerbations by 51%, compared with placebo in the SIROCCO trial and by 28% in the CALIMA trial. In the ZONDA trial, benralizumab reduced oral glucocorticoid use by 75%, compared with placebo, and by 25% from baseline.
The BORA extension trial included participants in the previous three trials. In the current report, researchers presented results from the analysis from BORA participants recruited from the SIROCCO and CALIMA trials. Data from participants from all three trials will be reported in the future.
The analysis included 1,576 patients who continued to receive benralizumab after being assigned to the treatment arm in SIROCCO or CALIMA, or who had received placebo were randomized to benralizumab on the 4-week (n = 783; 265 from placebo) or 8-week dose (n = 793; 281 from placebo) schedule.
A total of 166 patients, or about 10% in each group, discontinued treatment. The frequency of any serious adverse event (SAE) ranged between 10% and 11% in all groups. SAEs associated with infections ranged from 1% to 3%, indicating that there were no significant differences in SAE frequencies between those who were originally assigned to placebo and those who originally received benralizumab. That suggests no safety differences between receiving the drug for 1 year or 2 years.
A total of 1,046 subjects had blood eosinophil counts of 300 cells per mcL or greater at baseline; 72% of these patients had no asthma exacerbations during the BORA study. This was true for 74% of patients in the 8-week treatment arm.
The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm, compared with placebo (95% confidence interval, 0.42-0.56) and 0.46 in the 8-week arm (95% CI, 0.39-0.53). For patients who started out on placebo, the crude exacerbation rate during BORA was 0.53 in the 4-week group (95% CI, 0.43-0.65) and 0.57 in the 8-week group (95% CI, 0.47-0.68).
Patients who started on benralizumab had similar exacerbation frequencies during year 1 and year 2.
AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
SOURCE: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
out to 2 years, according findings of the BORA trial, an extension study of the phase 3 SIROCCO and CALIMA trials. The study follows up and reinforces previously reported 1-year data and was reported by William W. Busse, MD, of University of Wisconsin, Madison, and his colleagues in the Lancet Respiratory Medicine.
Benralizumab is a monoclonal antibody that targets interleukin-5 receptor alpha. It causes rapid deletion of eosinophils through cell-mediated cytotoxicity. A 30-mg dose of benralizumab every 8 weeks is approved for severe asthma treatment in Canada, Europe, Japan, the United States, and other countries.
In the second year of treatment, there were no new adverse events associated with depleted eosinophils, and the frequency of opportunistic infections was similar to that observed in the first year.
Eosinophilic inflammation occurs in about half of asthma cases and is associated with greater severity.
The 48-week SIROCCO trial, the 56-week CALIMA trial, and the 28-week ZONDA trial tested the effect of benralizumab 30 mg given every 4 weeks or 8 weeks, combined with high-dosage inhaled steroids and long-acting beta2-agonists. The 8-week dose of the drug reduced annual exacerbations by 51%, compared with placebo in the SIROCCO trial and by 28% in the CALIMA trial. In the ZONDA trial, benralizumab reduced oral glucocorticoid use by 75%, compared with placebo, and by 25% from baseline.
The BORA extension trial included participants in the previous three trials. In the current report, researchers presented results from the analysis from BORA participants recruited from the SIROCCO and CALIMA trials. Data from participants from all three trials will be reported in the future.
The analysis included 1,576 patients who continued to receive benralizumab after being assigned to the treatment arm in SIROCCO or CALIMA, or who had received placebo were randomized to benralizumab on the 4-week (n = 783; 265 from placebo) or 8-week dose (n = 793; 281 from placebo) schedule.
A total of 166 patients, or about 10% in each group, discontinued treatment. The frequency of any serious adverse event (SAE) ranged between 10% and 11% in all groups. SAEs associated with infections ranged from 1% to 3%, indicating that there were no significant differences in SAE frequencies between those who were originally assigned to placebo and those who originally received benralizumab. That suggests no safety differences between receiving the drug for 1 year or 2 years.
A total of 1,046 subjects had blood eosinophil counts of 300 cells per mcL or greater at baseline; 72% of these patients had no asthma exacerbations during the BORA study. This was true for 74% of patients in the 8-week treatment arm.
The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm, compared with placebo (95% confidence interval, 0.42-0.56) and 0.46 in the 8-week arm (95% CI, 0.39-0.53). For patients who started out on placebo, the crude exacerbation rate during BORA was 0.53 in the 4-week group (95% CI, 0.43-0.65) and 0.57 in the 8-week group (95% CI, 0.47-0.68).
Patients who started on benralizumab had similar exacerbation frequencies during year 1 and year 2.
AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
SOURCE: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: The antibody had similar safety, efficacy in year 2 as in year 1.
Major finding: The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm and 0.46 in the 8-week arm; the crude exacerbation rate during BORA was 0.53 in the 4-week group and 0.57 in the 8-week group.
Study details: Extension of randomized, clinical trial (n = 1,576).
Disclosures: AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
Source: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
Biodegradable stent polymer offers no long-term protection
This week from MDedge Cardiology, a biodegradable polymer shows no long-term benefit in heart stents, appropriate use criteria for imaging in nonvalvular heart disease are released, ACOG updates guidance on hypertension in pregnancy, and more losartan lots are recalled.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon AlexaApple Podcasts
This week from MDedge Cardiology, a biodegradable polymer shows no long-term benefit in heart stents, appropriate use criteria for imaging in nonvalvular heart disease are released, ACOG updates guidance on hypertension in pregnancy, and more losartan lots are recalled.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon AlexaApple Podcasts
This week from MDedge Cardiology, a biodegradable polymer shows no long-term benefit in heart stents, appropriate use criteria for imaging in nonvalvular heart disease are released, ACOG updates guidance on hypertension in pregnancy, and more losartan lots are recalled.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon AlexaApple Podcasts
Annular Elastolytic Giant Cell Granuloma: Mysterious Enlarging Scarring Lesions
To the Editor:
A 52-year-old woman with a medical history of migraines and cervicalgia presented with lesions on the right arm, back, and right calf. The patient stated that the lesions began as small papules that had grown over 13 months, with the largest papule on the right forearm. She reported no itching, bleeding, pain, discharge, or other symptoms associated with the lesions. She had a multiple-year history of similar lesions that did not respond to treatment with antifungals, moderate-potency steroids, and other over-the-counter creams. The lesions would resolve spontaneously with scarring and subsequently recur. Prior skin biopsies were inconclusive. The patient did not report any systemic symptoms or a personal or family history of connective tissue diseases.
Physical examination revealed a 4-cm asymmetric, annular, erythematous plaque with central clearing on the right dorsal forearm with defined margins except over the distal aspect (Figure 1). She also had several 1- to 2-cm erythematous, nummular, asymmetric plaques on the right upper arm with well-defined margins. She had several lesions over the central and left sides of the upper back that were similar to the lesions on the upper arm.
Two 4-mm punch biopsies of the right dorsal forearm and left side of the upper back revealed similar histologic features with a predominantly unremarkable epidermis. The dermis revealed a lymphohistiocytic infiltrate with prominent multinucleated giant cells organized into foreign body–type granulomas that extended into the deep dermis and subcutaneous tissue (Figure 2). In the granulomatous areas, there was a near-complete loss of elastic fibers with focal elastophagocytosis highlighted with Verhoeff-van Gieson (elastin) stain (Figure 3). Grocott-Gomori methenamine-silver and Fite stains for microorganisms were negative, and there was an absence of necrobiosis, lipids, and mucin.
The histologic findings of a granulomatous dermatitis with loss of elastic fibers and elastophagocytosis in addition to the patient’s clinical presentation and history were consistent with the diagnosis of annular elastolytic giant cell granuloma (AEGCG). Infectious and other granulomatous diseases including sarcoidosis were ruled out via clinical history, unremarkable laboratory analysis (ie, complete blood cell count, chemistry panel, antinuclear antibody, urinalysis), and a normal chest radiograph. The histologic findings via the various stains were instrumental to the diagnosis. The patient was treated with fluocinonide and subsequently lost to follow-up.
Annular elastolytic giant cell granuloma is an uncommon cutaneous disease that presents with recurring annular plaques with raised erythematous borders and subsequent residual scarring.1 O’Brien2 originally described this condition in 1975 as an actinic granuloma due to similar histologic findings in areas of the patient’s sun-exposed skin. Ragaz and Ackerman3 disputed O’Brien’s2 description, claiming granulomatous inflammation was a primary pathologic process and not a consequence to damaged elastotic material. In 1979, Hanke et al4 termed the lesions as AEGCG because he did not find a correlation to the sun-exposed areas of the patients and did not see solar elastosis.
Although AEGCG has an unclear pathogenesis, cellular immunologic reactions induced by modified function of elastic fibers’ antigenicity contribute to AEGCG formation.5 Therefore, environmental and host factors may play a role in its etiopathogenesis. In one study, 37% of 38 Japanese patients with AEGCG were found to have definitive or latent diabetes mellitus, raising the possible role of diabetes in the structural damage of the elastic fibers.6
Patients typically are middle-aged women who present clinically with red or atrophic plaques that have slightly elevated borders. They have centripetal spread with a resulting atrophic center.7 Clinically, the differential diagnosis of this condition includes actinic granuloma, granuloma annulare, and granuloma multiforme.8
Histologically, AEGCG has a granulomatous component with multinucleated giant cells in the upper and mid dermis. This component typically is distributed peripherally to a central zone that lacks elastic tissue. Elastophagocytosis, a classic finding in AEGCG, is the phagocytosis of elastic fibers that can microscopically be seen in the cytoplasm of histiocytes and multinucleated giant cells. There also is an absence of necrobiosis, lipids, mucin, and a palisading arrangement of the granulomas. These findings distinguish AEGCG from granuloma annulare and necrobiosis lipoidica, the primary histologic differential diagnoses.9 In addition, consideration of entities consistently exhibiting elastophagocytosis such as mid-dermal elastolysis, papillary dermal elastolysis, actinic granuloma, and granulomatous slack skin should be considered.5,10,11
Therapy for AEGCG is broad and includes topical, intralesional, and systemic corticosteroids. Hydroxychloroquine, isotretinoin, clofazimine, dapsone, photochemotherapy, and cyclosporine also have been utilized with varying results. Other reports show improvement with surgical excision, cryotherapy, or cauterization of small lesions.12-15
1. Tock CL, Cohen PR. Annular elastolytic giant cell granuloma. Cutis. 1998;62:181-187.
2. O’Brien JP. Actinic granuloma: an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
3. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
4. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
5. El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934-44.
6. Aso Y, Izaki Y, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919.
7. Pestoni C, Pereiro M Jr, Toribio J. Annular elastolytic giant cell granuloma produced on an old burn scar and spreading after a mechanical trauma. Acta Derm Venereol. 2003;83:312-313.
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40:220-222.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. McGrae JD Jr. Actinic granuloma: a clinical, histopathologic, and immunocytochemical study. Arch Dermatol. 1986;122:43-47.
11. Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
12. Chou WT, Tsai TF, Hung CM, et al. Multiple annular erythematous plaques on the back. Annular elastolytic giant cell granuloma (AEGCG). Indian J Dermatol Venereol Leprol. 2011;77:727-728.
13. Pérez-Pérez L, Garcia-Gavin J, Alleque F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralen-ultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266.
14. Babuna G, Buyukbabani N, Yazganoglu KD, et al. Effective treatment with hydroxychloroquine in a case of annular elastolytic giant cell granuloma. Indian J Dermatol Venereol Leprol. 2011;77:110-111.
15. Can B, Kavala M, Türkoglu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxylchloroquine. Int J Dermatol. 2013;52:509-511.
To the Editor:
A 52-year-old woman with a medical history of migraines and cervicalgia presented with lesions on the right arm, back, and right calf. The patient stated that the lesions began as small papules that had grown over 13 months, with the largest papule on the right forearm. She reported no itching, bleeding, pain, discharge, or other symptoms associated with the lesions. She had a multiple-year history of similar lesions that did not respond to treatment with antifungals, moderate-potency steroids, and other over-the-counter creams. The lesions would resolve spontaneously with scarring and subsequently recur. Prior skin biopsies were inconclusive. The patient did not report any systemic symptoms or a personal or family history of connective tissue diseases.
Physical examination revealed a 4-cm asymmetric, annular, erythematous plaque with central clearing on the right dorsal forearm with defined margins except over the distal aspect (Figure 1). She also had several 1- to 2-cm erythematous, nummular, asymmetric plaques on the right upper arm with well-defined margins. She had several lesions over the central and left sides of the upper back that were similar to the lesions on the upper arm.

Two 4-mm punch biopsies of the right dorsal forearm and left side of the upper back revealed similar histologic features with a predominantly unremarkable epidermis. The dermis revealed a lymphohistiocytic infiltrate with prominent multinucleated giant cells organized into foreign body–type granulomas that extended into the deep dermis and subcutaneous tissue (Figure 2). In the granulomatous areas, there was a near-complete loss of elastic fibers with focal elastophagocytosis highlighted with Verhoeff-van Gieson (elastin) stain (Figure 3). Grocott-Gomori methenamine-silver and Fite stains for microorganisms were negative, and there was an absence of necrobiosis, lipids, and mucin.


The histologic findings of a granulomatous dermatitis with loss of elastic fibers and elastophagocytosis in addition to the patient’s clinical presentation and history were consistent with the diagnosis of annular elastolytic giant cell granuloma (AEGCG). Infectious and other granulomatous diseases including sarcoidosis were ruled out via clinical history, unremarkable laboratory analysis (ie, complete blood cell count, chemistry panel, antinuclear antibody, urinalysis), and a normal chest radiograph. The histologic findings via the various stains were instrumental to the diagnosis. The patient was treated with fluocinonide and subsequently lost to follow-up.
Annular elastolytic giant cell granuloma is an uncommon cutaneous disease that presents with recurring annular plaques with raised erythematous borders and subsequent residual scarring.1 O’Brien2 originally described this condition in 1975 as an actinic granuloma due to similar histologic findings in areas of the patient’s sun-exposed skin. Ragaz and Ackerman3 disputed O’Brien’s2 description, claiming granulomatous inflammation was a primary pathologic process and not a consequence to damaged elastotic material. In 1979, Hanke et al4 termed the lesions as AEGCG because he did not find a correlation to the sun-exposed areas of the patients and did not see solar elastosis.
Although AEGCG has an unclear pathogenesis, cellular immunologic reactions induced by modified function of elastic fibers’ antigenicity contribute to AEGCG formation.5 Therefore, environmental and host factors may play a role in its etiopathogenesis. In one study, 37% of 38 Japanese patients with AEGCG were found to have definitive or latent diabetes mellitus, raising the possible role of diabetes in the structural damage of the elastic fibers.6
Patients typically are middle-aged women who present clinically with red or atrophic plaques that have slightly elevated borders. They have centripetal spread with a resulting atrophic center.7 Clinically, the differential diagnosis of this condition includes actinic granuloma, granuloma annulare, and granuloma multiforme.8
Histologically, AEGCG has a granulomatous component with multinucleated giant cells in the upper and mid dermis. This component typically is distributed peripherally to a central zone that lacks elastic tissue. Elastophagocytosis, a classic finding in AEGCG, is the phagocytosis of elastic fibers that can microscopically be seen in the cytoplasm of histiocytes and multinucleated giant cells. There also is an absence of necrobiosis, lipids, mucin, and a palisading arrangement of the granulomas. These findings distinguish AEGCG from granuloma annulare and necrobiosis lipoidica, the primary histologic differential diagnoses.9 In addition, consideration of entities consistently exhibiting elastophagocytosis such as mid-dermal elastolysis, papillary dermal elastolysis, actinic granuloma, and granulomatous slack skin should be considered.5,10,11
Therapy for AEGCG is broad and includes topical, intralesional, and systemic corticosteroids. Hydroxychloroquine, isotretinoin, clofazimine, dapsone, photochemotherapy, and cyclosporine also have been utilized with varying results. Other reports show improvement with surgical excision, cryotherapy, or cauterization of small lesions.12-15
To the Editor:
A 52-year-old woman with a medical history of migraines and cervicalgia presented with lesions on the right arm, back, and right calf. The patient stated that the lesions began as small papules that had grown over 13 months, with the largest papule on the right forearm. She reported no itching, bleeding, pain, discharge, or other symptoms associated with the lesions. She had a multiple-year history of similar lesions that did not respond to treatment with antifungals, moderate-potency steroids, and other over-the-counter creams. The lesions would resolve spontaneously with scarring and subsequently recur. Prior skin biopsies were inconclusive. The patient did not report any systemic symptoms or a personal or family history of connective tissue diseases.
Physical examination revealed a 4-cm asymmetric, annular, erythematous plaque with central clearing on the right dorsal forearm with defined margins except over the distal aspect (Figure 1). She also had several 1- to 2-cm erythematous, nummular, asymmetric plaques on the right upper arm with well-defined margins. She had several lesions over the central and left sides of the upper back that were similar to the lesions on the upper arm.

Two 4-mm punch biopsies of the right dorsal forearm and left side of the upper back revealed similar histologic features with a predominantly unremarkable epidermis. The dermis revealed a lymphohistiocytic infiltrate with prominent multinucleated giant cells organized into foreign body–type granulomas that extended into the deep dermis and subcutaneous tissue (Figure 2). In the granulomatous areas, there was a near-complete loss of elastic fibers with focal elastophagocytosis highlighted with Verhoeff-van Gieson (elastin) stain (Figure 3). Grocott-Gomori methenamine-silver and Fite stains for microorganisms were negative, and there was an absence of necrobiosis, lipids, and mucin.


The histologic findings of a granulomatous dermatitis with loss of elastic fibers and elastophagocytosis in addition to the patient’s clinical presentation and history were consistent with the diagnosis of annular elastolytic giant cell granuloma (AEGCG). Infectious and other granulomatous diseases including sarcoidosis were ruled out via clinical history, unremarkable laboratory analysis (ie, complete blood cell count, chemistry panel, antinuclear antibody, urinalysis), and a normal chest radiograph. The histologic findings via the various stains were instrumental to the diagnosis. The patient was treated with fluocinonide and subsequently lost to follow-up.
Annular elastolytic giant cell granuloma is an uncommon cutaneous disease that presents with recurring annular plaques with raised erythematous borders and subsequent residual scarring.1 O’Brien2 originally described this condition in 1975 as an actinic granuloma due to similar histologic findings in areas of the patient’s sun-exposed skin. Ragaz and Ackerman3 disputed O’Brien’s2 description, claiming granulomatous inflammation was a primary pathologic process and not a consequence to damaged elastotic material. In 1979, Hanke et al4 termed the lesions as AEGCG because he did not find a correlation to the sun-exposed areas of the patients and did not see solar elastosis.
Although AEGCG has an unclear pathogenesis, cellular immunologic reactions induced by modified function of elastic fibers’ antigenicity contribute to AEGCG formation.5 Therefore, environmental and host factors may play a role in its etiopathogenesis. In one study, 37% of 38 Japanese patients with AEGCG were found to have definitive or latent diabetes mellitus, raising the possible role of diabetes in the structural damage of the elastic fibers.6
Patients typically are middle-aged women who present clinically with red or atrophic plaques that have slightly elevated borders. They have centripetal spread with a resulting atrophic center.7 Clinically, the differential diagnosis of this condition includes actinic granuloma, granuloma annulare, and granuloma multiforme.8
Histologically, AEGCG has a granulomatous component with multinucleated giant cells in the upper and mid dermis. This component typically is distributed peripherally to a central zone that lacks elastic tissue. Elastophagocytosis, a classic finding in AEGCG, is the phagocytosis of elastic fibers that can microscopically be seen in the cytoplasm of histiocytes and multinucleated giant cells. There also is an absence of necrobiosis, lipids, mucin, and a palisading arrangement of the granulomas. These findings distinguish AEGCG from granuloma annulare and necrobiosis lipoidica, the primary histologic differential diagnoses.9 In addition, consideration of entities consistently exhibiting elastophagocytosis such as mid-dermal elastolysis, papillary dermal elastolysis, actinic granuloma, and granulomatous slack skin should be considered.5,10,11
Therapy for AEGCG is broad and includes topical, intralesional, and systemic corticosteroids. Hydroxychloroquine, isotretinoin, clofazimine, dapsone, photochemotherapy, and cyclosporine also have been utilized with varying results. Other reports show improvement with surgical excision, cryotherapy, or cauterization of small lesions.12-15
1. Tock CL, Cohen PR. Annular elastolytic giant cell granuloma. Cutis. 1998;62:181-187.
2. O’Brien JP. Actinic granuloma: an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
3. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
4. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
5. El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934-44.
6. Aso Y, Izaki Y, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919.
7. Pestoni C, Pereiro M Jr, Toribio J. Annular elastolytic giant cell granuloma produced on an old burn scar and spreading after a mechanical trauma. Acta Derm Venereol. 2003;83:312-313.
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40:220-222.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. McGrae JD Jr. Actinic granuloma: a clinical, histopathologic, and immunocytochemical study. Arch Dermatol. 1986;122:43-47.
11. Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
12. Chou WT, Tsai TF, Hung CM, et al. Multiple annular erythematous plaques on the back. Annular elastolytic giant cell granuloma (AEGCG). Indian J Dermatol Venereol Leprol. 2011;77:727-728.
13. Pérez-Pérez L, Garcia-Gavin J, Alleque F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralen-ultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266.
14. Babuna G, Buyukbabani N, Yazganoglu KD, et al. Effective treatment with hydroxychloroquine in a case of annular elastolytic giant cell granuloma. Indian J Dermatol Venereol Leprol. 2011;77:110-111.
15. Can B, Kavala M, Türkoglu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxylchloroquine. Int J Dermatol. 2013;52:509-511.
1. Tock CL, Cohen PR. Annular elastolytic giant cell granuloma. Cutis. 1998;62:181-187.
2. O’Brien JP. Actinic granuloma: an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
3. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
4. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
5. El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934-44.
6. Aso Y, Izaki Y, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919.
7. Pestoni C, Pereiro M Jr, Toribio J. Annular elastolytic giant cell granuloma produced on an old burn scar and spreading after a mechanical trauma. Acta Derm Venereol. 2003;83:312-313.
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40:220-222.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. McGrae JD Jr. Actinic granuloma: a clinical, histopathologic, and immunocytochemical study. Arch Dermatol. 1986;122:43-47.
11. Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
12. Chou WT, Tsai TF, Hung CM, et al. Multiple annular erythematous plaques on the back. Annular elastolytic giant cell granuloma (AEGCG). Indian J Dermatol Venereol Leprol. 2011;77:727-728.
13. Pérez-Pérez L, Garcia-Gavin J, Alleque F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralen-ultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266.
14. Babuna G, Buyukbabani N, Yazganoglu KD, et al. Effective treatment with hydroxychloroquine in a case of annular elastolytic giant cell granuloma. Indian J Dermatol Venereol Leprol. 2011;77:110-111.
15. Can B, Kavala M, Türkoglu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxylchloroquine. Int J Dermatol. 2013;52:509-511.
Practice Points
- Annular elastolytic giant cell granuloma (AEGCG) should be kept in the differential diagnosis when assessing a middle-aged woman with recurring annular plaques with a raised border and an atrophic center on both sun-exposed and sun-protected areas of the body.
- Histologically, AEGCG classically has a granulomatous component in the dermis that lacks elastic tissue and has no necrobiosis, lipids, or mucin. Staining with elastin may be necessary to highlight these areas as well as demonstrate elastophagocytosis.