User login
A case of cold burn reported with whole-body cryotherapy
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
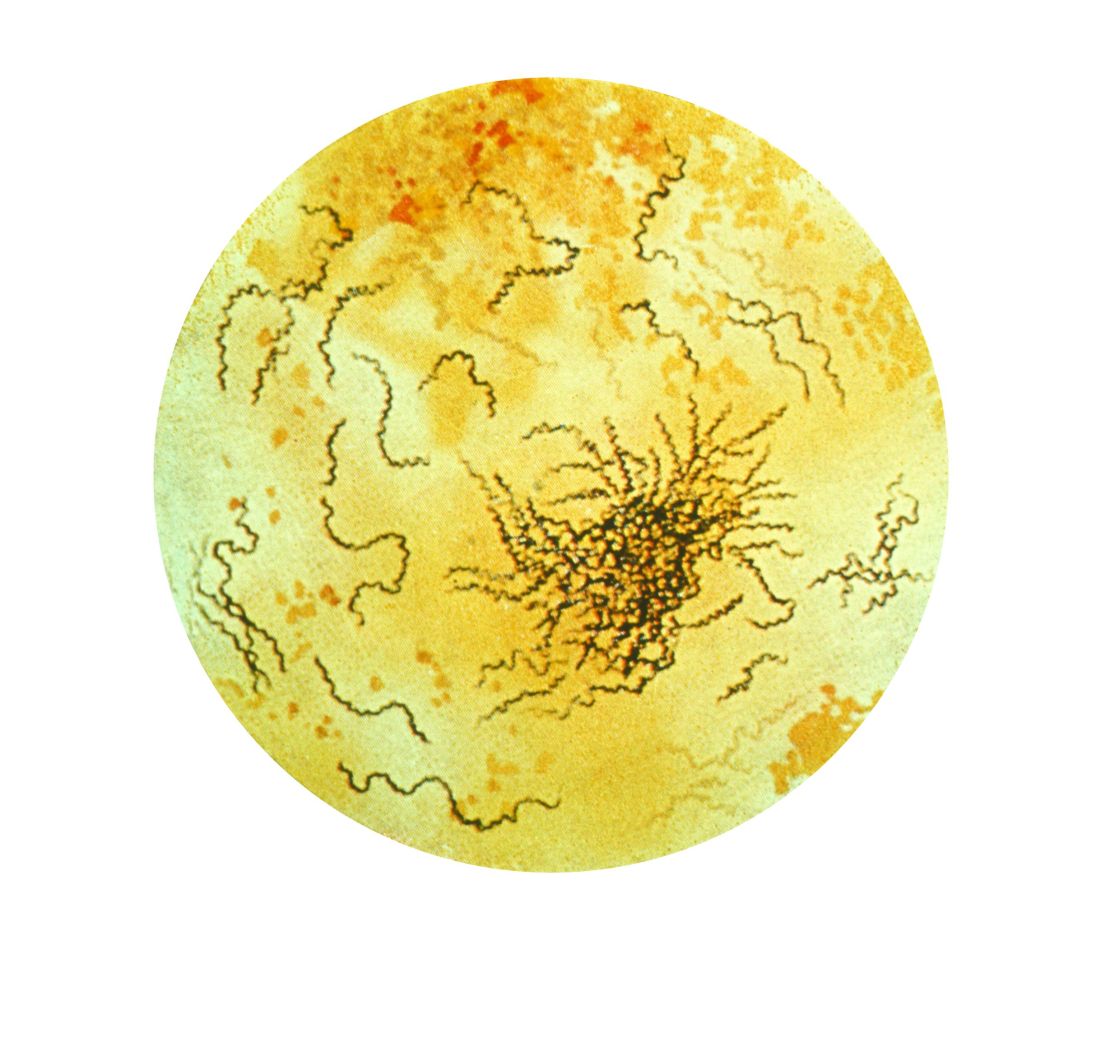
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
by Mackenzie O’Connor and her colleagues in the department of dermatology and cutaneous biology at Thomas Jefferson University, Philadelphia.
In the report, they describe the case of a 71-year-old man who presented with a cold burn injury a day after a WBC session. These treatments typically involve sessions of 2-5 minutes, in a chamber that is cooled down to –100°C to –140°C.
The likely cause in this case was a nozzle malfunction that caused liquid nitrogen to come in direct contact with the patient’s skin for a prolonged period of time (less than 1 minute), causing stinging and pain, followed by redness and blistering of the skin. The patient had received four WBC treatments previously for arthritis and back pain, with no adverse effects. In addition to ibuprofen, he was treated with systemic steroids, topical corticosteroids, and silver sulfadiazine cream.
Despite claims that WBC can aid muscle recovery and alleviate joint pain, and can improve skin health, and is increasingly available in spas and other sites, the Food and Drug Administration has not approved the procedure for treatment of any medical conditions, the researchers noted (JAAD Case Rep. 2019;5[1]:29-30). They also referred to a 2015 Cochrane review, which found insufficient evidence that WBC treatment is beneficial for muscle recovery in active young adult men.
FROM JAAD CASE REPORTS
Check for neuromyelitis optica spectrum disorder in suspect HIV patients
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
FROM MULTIPLE SCLEROSIS AND RELATED DISORDERS
Algorithm proposes approach for managing TNF inhibitor–induced psoriasis
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
FROM JOURNAL OF PSORIASIS AND PSORIATIC ARTHRITIS
Duodenoscopes contain more bacteria than expected
Reprocessed duodenoscopes are more contaminated than expected, with up to 3% of samples testing positive for disease-causing bacteria including Escherichia coli and Staphylococcus aureus, according to an updated safety communication issued by the Food and Drug Administration on December 10.
“Because of the higher-than-expected contamination rates and to help protect patients from bacterial infections associated with the use of duodenoscopes, we have included in today’s safety communication updated recommendations regarding steps that health care providers can take to enhance duodenoscope reprocessing,” Jeff Shuren, MD, director of the Center for Devices and Radiological Health, wrote in the statement.
The FDA advised clinicians to follow additional cleaning measures including microbiological culturing, sterilization, use of a liquid chemical sterilant processing system, and repeated high-level disinfection beyond what is recommended by duodenoscope manufacturers.
The interim data cited in the safety communication come from postmarket surveillance studies conducted by duodenoscope manufacturers at the FDA’s request as part of the agency’s ongoing efforts to prevent patient infections caused by contaminated duodenoscopes. In addition to the positive tests for disease-causing bacteria, up to 3% of properly collected samples contained more than 100 colony-forming units of other organisms unlikely to cause infection. However, the presence of such organisms further highlights the failure of the current reprocessing protocol to adequately clean the devices, according to the FDA.
Dr. Shuren emphasized that the risk of infection from a duodenoscope for an individual patient remains low and that infection rates have declined in recent years in response to the FDA’s enhanced safety measures and stated that the agency remains “committed to enhancing the safety margin of procedures with reprocessed medical devices.”
Read the full safety communication here: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm628020.htm.
Reprocessed duodenoscopes are more contaminated than expected, with up to 3% of samples testing positive for disease-causing bacteria including Escherichia coli and Staphylococcus aureus, according to an updated safety communication issued by the Food and Drug Administration on December 10.
“Because of the higher-than-expected contamination rates and to help protect patients from bacterial infections associated with the use of duodenoscopes, we have included in today’s safety communication updated recommendations regarding steps that health care providers can take to enhance duodenoscope reprocessing,” Jeff Shuren, MD, director of the Center for Devices and Radiological Health, wrote in the statement.
The FDA advised clinicians to follow additional cleaning measures including microbiological culturing, sterilization, use of a liquid chemical sterilant processing system, and repeated high-level disinfection beyond what is recommended by duodenoscope manufacturers.
The interim data cited in the safety communication come from postmarket surveillance studies conducted by duodenoscope manufacturers at the FDA’s request as part of the agency’s ongoing efforts to prevent patient infections caused by contaminated duodenoscopes. In addition to the positive tests for disease-causing bacteria, up to 3% of properly collected samples contained more than 100 colony-forming units of other organisms unlikely to cause infection. However, the presence of such organisms further highlights the failure of the current reprocessing protocol to adequately clean the devices, according to the FDA.
Dr. Shuren emphasized that the risk of infection from a duodenoscope for an individual patient remains low and that infection rates have declined in recent years in response to the FDA’s enhanced safety measures and stated that the agency remains “committed to enhancing the safety margin of procedures with reprocessed medical devices.”
Read the full safety communication here: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm628020.htm.
Reprocessed duodenoscopes are more contaminated than expected, with up to 3% of samples testing positive for disease-causing bacteria including Escherichia coli and Staphylococcus aureus, according to an updated safety communication issued by the Food and Drug Administration on December 10.
“Because of the higher-than-expected contamination rates and to help protect patients from bacterial infections associated with the use of duodenoscopes, we have included in today’s safety communication updated recommendations regarding steps that health care providers can take to enhance duodenoscope reprocessing,” Jeff Shuren, MD, director of the Center for Devices and Radiological Health, wrote in the statement.
The FDA advised clinicians to follow additional cleaning measures including microbiological culturing, sterilization, use of a liquid chemical sterilant processing system, and repeated high-level disinfection beyond what is recommended by duodenoscope manufacturers.
The interim data cited in the safety communication come from postmarket surveillance studies conducted by duodenoscope manufacturers at the FDA’s request as part of the agency’s ongoing efforts to prevent patient infections caused by contaminated duodenoscopes. In addition to the positive tests for disease-causing bacteria, up to 3% of properly collected samples contained more than 100 colony-forming units of other organisms unlikely to cause infection. However, the presence of such organisms further highlights the failure of the current reprocessing protocol to adequately clean the devices, according to the FDA.
Dr. Shuren emphasized that the risk of infection from a duodenoscope for an individual patient remains low and that infection rates have declined in recent years in response to the FDA’s enhanced safety measures and stated that the agency remains “committed to enhancing the safety margin of procedures with reprocessed medical devices.”
Read the full safety communication here: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm628020.htm.
Designing a better EHR
Hospitals can create a more effective system
It’s well known that overuse is an enormous problem in medicine, and when it comes to antibiotics, the problem is even more striking.
“Half of all inpatient antibiotic use is inappropriate,” says Valerie Vaughn, MD, MSc, a hospitalist at the University of Michigan, Ann Arbor, and coauthor of a BMJ editorial about EHRs and antibiotic overuse.
“This has led to an increase in antibiotic-related adverse events (~20% of all hospitalized patients on antibiotics), Clostridium difficile infections (half a million infections and 29,000 deaths in U.S. annually), and resistant bacteria (which now account for nearly 12% of all bacterial infections, costing $2.2 billion annually).”
EHRs can be a tool to combat that trend – if they are well designed. Clinicians are influenced by the design of their electronic health record, Dr. Vaughn said. “Rather than leave its influence to chance, we should capitalize on what is known about design to promote appropriate testing and treatment through the EHR.” Hospitalists – integral to quality improvement – can have a role in making these changes.
“These improvements will be the most effective if behavioral economics and nudging are considered while designing,” Dr. Vaughn said. “For example, when creating order sets, list recommended options first and when possible make them the default,” she said. “This little change will greatly improve appropriate use.”
For every hour physicians spend on direct patient care, they spend another two with the EHR, Dr. Vaughn wrote. “Given this degree of attention, it is not surprising that the EHR influences physician behavior, especially the overuse of low-value medical care. … Displaying brand-name instead of generic options leads to more expensive prescribing. Allowing labs to be ordered recurrently increases unnecessary phlebotomy. Even individually listing inappropriate antibiotics (rather than grouping them) can make them more noticeable, resulting in more broad-spectrum use.”
“All hospitalists – and humans – are affected by knee-jerk responses. One of the most common in medicine is the urge to treat a positive culture or any positive test. Recognize this urge and resist!” she said. “Antibiotics may be the correct response, but clinicians should first think about whether treatment is necessary based on that patient’s symptoms and comorbidities. Resist the knee-jerk urge to give antibiotics for every positive culture.”
Reference
Vaughn VM et al. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 24 Mar 2018. doi: 10.1136/bmjqs-2017-007578.
Hospitals can create a more effective system
Hospitals can create a more effective system
It’s well known that overuse is an enormous problem in medicine, and when it comes to antibiotics, the problem is even more striking.
“Half of all inpatient antibiotic use is inappropriate,” says Valerie Vaughn, MD, MSc, a hospitalist at the University of Michigan, Ann Arbor, and coauthor of a BMJ editorial about EHRs and antibiotic overuse.
“This has led to an increase in antibiotic-related adverse events (~20% of all hospitalized patients on antibiotics), Clostridium difficile infections (half a million infections and 29,000 deaths in U.S. annually), and resistant bacteria (which now account for nearly 12% of all bacterial infections, costing $2.2 billion annually).”
EHRs can be a tool to combat that trend – if they are well designed. Clinicians are influenced by the design of their electronic health record, Dr. Vaughn said. “Rather than leave its influence to chance, we should capitalize on what is known about design to promote appropriate testing and treatment through the EHR.” Hospitalists – integral to quality improvement – can have a role in making these changes.
“These improvements will be the most effective if behavioral economics and nudging are considered while designing,” Dr. Vaughn said. “For example, when creating order sets, list recommended options first and when possible make them the default,” she said. “This little change will greatly improve appropriate use.”
For every hour physicians spend on direct patient care, they spend another two with the EHR, Dr. Vaughn wrote. “Given this degree of attention, it is not surprising that the EHR influences physician behavior, especially the overuse of low-value medical care. … Displaying brand-name instead of generic options leads to more expensive prescribing. Allowing labs to be ordered recurrently increases unnecessary phlebotomy. Even individually listing inappropriate antibiotics (rather than grouping them) can make them more noticeable, resulting in more broad-spectrum use.”
“All hospitalists – and humans – are affected by knee-jerk responses. One of the most common in medicine is the urge to treat a positive culture or any positive test. Recognize this urge and resist!” she said. “Antibiotics may be the correct response, but clinicians should first think about whether treatment is necessary based on that patient’s symptoms and comorbidities. Resist the knee-jerk urge to give antibiotics for every positive culture.”
Reference
Vaughn VM et al. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 24 Mar 2018. doi: 10.1136/bmjqs-2017-007578.
It’s well known that overuse is an enormous problem in medicine, and when it comes to antibiotics, the problem is even more striking.
“Half of all inpatient antibiotic use is inappropriate,” says Valerie Vaughn, MD, MSc, a hospitalist at the University of Michigan, Ann Arbor, and coauthor of a BMJ editorial about EHRs and antibiotic overuse.
“This has led to an increase in antibiotic-related adverse events (~20% of all hospitalized patients on antibiotics), Clostridium difficile infections (half a million infections and 29,000 deaths in U.S. annually), and resistant bacteria (which now account for nearly 12% of all bacterial infections, costing $2.2 billion annually).”
EHRs can be a tool to combat that trend – if they are well designed. Clinicians are influenced by the design of their electronic health record, Dr. Vaughn said. “Rather than leave its influence to chance, we should capitalize on what is known about design to promote appropriate testing and treatment through the EHR.” Hospitalists – integral to quality improvement – can have a role in making these changes.
“These improvements will be the most effective if behavioral economics and nudging are considered while designing,” Dr. Vaughn said. “For example, when creating order sets, list recommended options first and when possible make them the default,” she said. “This little change will greatly improve appropriate use.”
For every hour physicians spend on direct patient care, they spend another two with the EHR, Dr. Vaughn wrote. “Given this degree of attention, it is not surprising that the EHR influences physician behavior, especially the overuse of low-value medical care. … Displaying brand-name instead of generic options leads to more expensive prescribing. Allowing labs to be ordered recurrently increases unnecessary phlebotomy. Even individually listing inappropriate antibiotics (rather than grouping them) can make them more noticeable, resulting in more broad-spectrum use.”
“All hospitalists – and humans – are affected by knee-jerk responses. One of the most common in medicine is the urge to treat a positive culture or any positive test. Recognize this urge and resist!” she said. “Antibiotics may be the correct response, but clinicians should first think about whether treatment is necessary based on that patient’s symptoms and comorbidities. Resist the knee-jerk urge to give antibiotics for every positive culture.”
Reference
Vaughn VM et al. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 24 Mar 2018. doi: 10.1136/bmjqs-2017-007578.
Biomarker algorithm may offer noninvasive look at liver fibrosis
Serum biomarkers may enable a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), according to results from a recent study.
An algorithm created by the investigators distinguished NAFLD patients with advanced liver fibrosis from those with mild to moderate fibrosis, reported lead author Rohit Loomba, MD, of the University of California at San Diego and his colleagues.
“Liver biopsy is currently the gold standard for diagnosing NASH [nonalcoholic steatohepatitis] and staging liver fibrosis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, it is a costly and invasive procedure with an all-cause mortality risk of approximately 0.2%. Liver biopsy typically samples only 1/50,000th of the organ, and it is liable to sampling error with an error rate of 25% for diagnosis of hepatic fibrosis.”
Existing serum-based tests are reliable for diagnosing nonfibrotic NAFLD, but they may misdiagnosis patients with advanced fibrosis. Although imaging-based techniques may provide better diagnostic accuracy, some are available only for subgroups of patients, while others come with a high financial burden. Diagnostic shortcomings may have a major effect on patient outcomes, particularly when risk groups are considered.
“Fibrosis stages F3 and F4 (advanced fibrosis) are primary predictors of liver-related morbidity and mortality, with 11%-22% of NASH patients reported to have advanced fibrosis,” the investigators noted.
The investigators therefore aimed to distinguish such high-risk NAFLD patients from those with mild or moderate liver fibrosis. Three biomarkers were included: hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and alpha2-macroglobulin (A2M). Each biomarker has documented associations with liver fibrosis. For instance, higher A2M concentrations inhibit fibrinolysis, HA is associated with excessive extracellular matrix and fibrotic tissue, and TIMP-1 is a known liver fibrosis marker and inhibitor of extracellular matrix degradation. The relative strengths of each in detecting advanced liver fibrosis was determined through an algorithm.
The investigators relied on archived serum samples from Duke University, Durham, N.C., (n = 792) and University of California at San Diego (n = 244) that were collected within 11 days of liver biopsy. Biopsies were performed with 15- to 16-gauge needles using at least eight portal tracts, and these samples were used to diagnose NAFLD. Patients with alcoholic liver disease or hepatitis C virus were excluded.
Algorithm training was based on serum measurements from 396 patients treated at Duke University. Samples were divided into mild to moderate (F0-F2) or advanced (F3-F4) fibrosis and split into 10 subsets. The logical regression model was trained on nine subsets and tested on the 10th, with iterations 10 times through this sequence until all 10 samples were tested. This process was repeated 10,000 times. Using the median coefficients from 100,000 logistical regression models, the samples were scored using the algorithm from 0 to 100, with higher numbers representing more advanced fibrosis, and the relative weights of each biomarker measurement were determined.
A noninferiority protocol was used to validate the algorithm, through which the area under the receiver operating characteristic (AUROC) curve was calculated. The AUROC curve of the validation samples was 0.856, with 0.5 being the score for a random algorithm. The algorithm correctly classified 90.0% of F0 cases, 75.0% of F1 cases, 53.8% of F2 cases, 77.4% of F3 cases, and 94.4% of F4 cases. The sensitivity was 79.7% and the specificity was 75.7%.
The algorithm was superior to Fibrosis-4 (FIB-4) and NAFLD Fibrosis Score (NFS) in two validation cohorts. In a combination of validation cohorts, the algorithm correctly identified 79.5% of F3-F4 patients, compared with rates of 25.8% and 28.0% from FIB-4 and NFS, respectively. The investigators noted that the algorithm was unaffected by sex or age. In contrast, FIB-4 is biased toward females, and both FIB-4 and NFS are less accurate with patients aged 35 years or younger.
“Performance of the training and validation sets was robust and well matched, enabling the reliable differentiation of NAFLD patients with and without advanced fibrosis,” the investigators concluded.
The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
SOURCE: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
Serum biomarkers may enable a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), according to results from a recent study.
An algorithm created by the investigators distinguished NAFLD patients with advanced liver fibrosis from those with mild to moderate fibrosis, reported lead author Rohit Loomba, MD, of the University of California at San Diego and his colleagues.
“Liver biopsy is currently the gold standard for diagnosing NASH [nonalcoholic steatohepatitis] and staging liver fibrosis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, it is a costly and invasive procedure with an all-cause mortality risk of approximately 0.2%. Liver biopsy typically samples only 1/50,000th of the organ, and it is liable to sampling error with an error rate of 25% for diagnosis of hepatic fibrosis.”
Existing serum-based tests are reliable for diagnosing nonfibrotic NAFLD, but they may misdiagnosis patients with advanced fibrosis. Although imaging-based techniques may provide better diagnostic accuracy, some are available only for subgroups of patients, while others come with a high financial burden. Diagnostic shortcomings may have a major effect on patient outcomes, particularly when risk groups are considered.
“Fibrosis stages F3 and F4 (advanced fibrosis) are primary predictors of liver-related morbidity and mortality, with 11%-22% of NASH patients reported to have advanced fibrosis,” the investigators noted.
The investigators therefore aimed to distinguish such high-risk NAFLD patients from those with mild or moderate liver fibrosis. Three biomarkers were included: hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and alpha2-macroglobulin (A2M). Each biomarker has documented associations with liver fibrosis. For instance, higher A2M concentrations inhibit fibrinolysis, HA is associated with excessive extracellular matrix and fibrotic tissue, and TIMP-1 is a known liver fibrosis marker and inhibitor of extracellular matrix degradation. The relative strengths of each in detecting advanced liver fibrosis was determined through an algorithm.
The investigators relied on archived serum samples from Duke University, Durham, N.C., (n = 792) and University of California at San Diego (n = 244) that were collected within 11 days of liver biopsy. Biopsies were performed with 15- to 16-gauge needles using at least eight portal tracts, and these samples were used to diagnose NAFLD. Patients with alcoholic liver disease or hepatitis C virus were excluded.
Algorithm training was based on serum measurements from 396 patients treated at Duke University. Samples were divided into mild to moderate (F0-F2) or advanced (F3-F4) fibrosis and split into 10 subsets. The logical regression model was trained on nine subsets and tested on the 10th, with iterations 10 times through this sequence until all 10 samples were tested. This process was repeated 10,000 times. Using the median coefficients from 100,000 logistical regression models, the samples were scored using the algorithm from 0 to 100, with higher numbers representing more advanced fibrosis, and the relative weights of each biomarker measurement were determined.
A noninferiority protocol was used to validate the algorithm, through which the area under the receiver operating characteristic (AUROC) curve was calculated. The AUROC curve of the validation samples was 0.856, with 0.5 being the score for a random algorithm. The algorithm correctly classified 90.0% of F0 cases, 75.0% of F1 cases, 53.8% of F2 cases, 77.4% of F3 cases, and 94.4% of F4 cases. The sensitivity was 79.7% and the specificity was 75.7%.
The algorithm was superior to Fibrosis-4 (FIB-4) and NAFLD Fibrosis Score (NFS) in two validation cohorts. In a combination of validation cohorts, the algorithm correctly identified 79.5% of F3-F4 patients, compared with rates of 25.8% and 28.0% from FIB-4 and NFS, respectively. The investigators noted that the algorithm was unaffected by sex or age. In contrast, FIB-4 is biased toward females, and both FIB-4 and NFS are less accurate with patients aged 35 years or younger.
“Performance of the training and validation sets was robust and well matched, enabling the reliable differentiation of NAFLD patients with and without advanced fibrosis,” the investigators concluded.
The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
SOURCE: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
Serum biomarkers may enable a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD), according to results from a recent study.
An algorithm created by the investigators distinguished NAFLD patients with advanced liver fibrosis from those with mild to moderate fibrosis, reported lead author Rohit Loomba, MD, of the University of California at San Diego and his colleagues.
“Liver biopsy is currently the gold standard for diagnosing NASH [nonalcoholic steatohepatitis] and staging liver fibrosis,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, it is a costly and invasive procedure with an all-cause mortality risk of approximately 0.2%. Liver biopsy typically samples only 1/50,000th of the organ, and it is liable to sampling error with an error rate of 25% for diagnosis of hepatic fibrosis.”
Existing serum-based tests are reliable for diagnosing nonfibrotic NAFLD, but they may misdiagnosis patients with advanced fibrosis. Although imaging-based techniques may provide better diagnostic accuracy, some are available only for subgroups of patients, while others come with a high financial burden. Diagnostic shortcomings may have a major effect on patient outcomes, particularly when risk groups are considered.
“Fibrosis stages F3 and F4 (advanced fibrosis) are primary predictors of liver-related morbidity and mortality, with 11%-22% of NASH patients reported to have advanced fibrosis,” the investigators noted.
The investigators therefore aimed to distinguish such high-risk NAFLD patients from those with mild or moderate liver fibrosis. Three biomarkers were included: hyaluronic acid (HA), TIMP metallopeptidase inhibitor 1 (TIMP-1), and alpha2-macroglobulin (A2M). Each biomarker has documented associations with liver fibrosis. For instance, higher A2M concentrations inhibit fibrinolysis, HA is associated with excessive extracellular matrix and fibrotic tissue, and TIMP-1 is a known liver fibrosis marker and inhibitor of extracellular matrix degradation. The relative strengths of each in detecting advanced liver fibrosis was determined through an algorithm.
The investigators relied on archived serum samples from Duke University, Durham, N.C., (n = 792) and University of California at San Diego (n = 244) that were collected within 11 days of liver biopsy. Biopsies were performed with 15- to 16-gauge needles using at least eight portal tracts, and these samples were used to diagnose NAFLD. Patients with alcoholic liver disease or hepatitis C virus were excluded.
Algorithm training was based on serum measurements from 396 patients treated at Duke University. Samples were divided into mild to moderate (F0-F2) or advanced (F3-F4) fibrosis and split into 10 subsets. The logical regression model was trained on nine subsets and tested on the 10th, with iterations 10 times through this sequence until all 10 samples were tested. This process was repeated 10,000 times. Using the median coefficients from 100,000 logistical regression models, the samples were scored using the algorithm from 0 to 100, with higher numbers representing more advanced fibrosis, and the relative weights of each biomarker measurement were determined.
A noninferiority protocol was used to validate the algorithm, through which the area under the receiver operating characteristic (AUROC) curve was calculated. The AUROC curve of the validation samples was 0.856, with 0.5 being the score for a random algorithm. The algorithm correctly classified 90.0% of F0 cases, 75.0% of F1 cases, 53.8% of F2 cases, 77.4% of F3 cases, and 94.4% of F4 cases. The sensitivity was 79.7% and the specificity was 75.7%.
The algorithm was superior to Fibrosis-4 (FIB-4) and NAFLD Fibrosis Score (NFS) in two validation cohorts. In a combination of validation cohorts, the algorithm correctly identified 79.5% of F3-F4 patients, compared with rates of 25.8% and 28.0% from FIB-4 and NFS, respectively. The investigators noted that the algorithm was unaffected by sex or age. In contrast, FIB-4 is biased toward females, and both FIB-4 and NFS are less accurate with patients aged 35 years or younger.
“Performance of the training and validation sets was robust and well matched, enabling the reliable differentiation of NAFLD patients with and without advanced fibrosis,” the investigators concluded.
The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
SOURCE: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: A serum biomarker–based algorithm may provide a noninvasive method of detecting advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease (NAFLD).
Major finding: The area under the receiver operator characteristic (AUROC) curve for a combination of validation samples was 0.856.
Study details: A retrospective study of liver fibrosis serum markers and clinical data from 396 patients with NAFLD and various stages of fibrosis.
Disclosures: The study was supported by Prometheus Laboratories. Authors not employed by Prometheus Laboratories were employed by Duke University or the University of California, San Diego; each institution received funding from Prometheus Laboratories.
Source: Loomba R et al. Clin Gastroenterol Hepatol. 2018 Nov 15. doi: 10.1016/j.cgh.2018.11.004.
AHA statement on statin risks, app that diagnoses STEMI, and more
This week, the American Heart Association says that statins’ benefits far outweigh the risks, a smartphone app is nearly as good as an ECG at diagnosing STEMI, stroke thrombolysis appears safe in patients prone to GI bleeding, and a new strategy for pausing DOAC therapy works in A-fib patients.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
This week, the American Heart Association says that statins’ benefits far outweigh the risks, a smartphone app is nearly as good as an ECG at diagnosing STEMI, stroke thrombolysis appears safe in patients prone to GI bleeding, and a new strategy for pausing DOAC therapy works in A-fib patients.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
This week, the American Heart Association says that statins’ benefits far outweigh the risks, a smartphone app is nearly as good as an ECG at diagnosing STEMI, stroke thrombolysis appears safe in patients prone to GI bleeding, and a new strategy for pausing DOAC therapy works in A-fib patients.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
New syphilis cases for pregnant women rose 61% over 5 years
Syphilis cases increased by 61% between 2012 and 2016 among pregnant women, and the proportion of syphilis cases was higher for women who were non-Hispanic black race and Hispanic ethnicity, according to research in Obstetrics & Gynecology.
“These findings support current recommendations for universal syphilis screening at the first prenatal visit and indicate that it may be necessary to include population context when determining whether to implement repeat screening during pregnancy,” Shivika Trivedi, MD, MSc, of the CDC Foundation and the Division of STD Prevention at the Centers for Disease Control and Prevention and colleagues wrote.
Dr. Trivedi and colleagues identified 9,883 pregnant women with reported syphilis in the CDC National Notifiable Diseases Surveillance System during 2012-2016. During that time, there was an increase in the number of female syphilis cases from 9,551 cases in 2012 to 14,838 cases in 2016 (55%), while there was an increase in the number of syphilis cases for pregnant women from 1,561 cases in 2012 to 2,508 cases in 2016 (61%). Of the risk factors reported for syphilis, 49% reported no risk factors within 12 priors before diagnosis, 43% said they had had at least one sexually transmitted disease, and 30% reported more than one sexual partner within the last year.
The greatest prevalence for syphilis was among women who were in their 20s (59%), located in the South (56%), and were non-Hispanic black (49%) or Hispanic (28%). However, researchers noted the rates of syphilis increased among all women between 18 years and 45 years and in every race and ethnicity group between 2012 and 2016. Early syphilis cases increased from 35% in 2012 to 58% in 2016, while late latent cases decreased from 65% in 2012 to 42% in 2016.
Researchers noted several limitations in the study, including case-based surveillance data, which potentially underreported the rates of syphilis, and a lack of pregnancy outcomes for pregnant women with syphilitic infections. However, they noted the data do show a trend of syphilis infections in pregnant women because the live birth rate “was relatively stable and did not fluctuate more than” 1.5% between 2012 and 2016.
“Through an increased awareness of the rising syphilis cases among pregnant women as well as these trend data, health care providers can be better informed to ensure they are following national guidelines and state policies for syphilis screening in pregnancy,” Dr. Trivedi and colleagues concluded.
The authors reported no relevant conflicts of interest.
SOURCE: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
I think this is an important topic of which pregnant women and their providers should be aware. It is possible the rising incidence is a result of increased screening and awareness; however, regardless of whether this is the case, it is important to identify the cases of congenital syphilis as preventable.
It is important for providers to be aware of their local syphilis prevalence and regulations on prenatal syphilis screening because given the effects of congenital syphilis and the ease of treatment.
Martina L. Badell, MD, is an assistant professor in the department of gynecology and obstetrics and maternal-fetal medicine at Emory University in Atlanta. She reported no relevant conflicts of interest.
I think this is an important topic of which pregnant women and their providers should be aware. It is possible the rising incidence is a result of increased screening and awareness; however, regardless of whether this is the case, it is important to identify the cases of congenital syphilis as preventable.
It is important for providers to be aware of their local syphilis prevalence and regulations on prenatal syphilis screening because given the effects of congenital syphilis and the ease of treatment.
Martina L. Badell, MD, is an assistant professor in the department of gynecology and obstetrics and maternal-fetal medicine at Emory University in Atlanta. She reported no relevant conflicts of interest.
I think this is an important topic of which pregnant women and their providers should be aware. It is possible the rising incidence is a result of increased screening and awareness; however, regardless of whether this is the case, it is important to identify the cases of congenital syphilis as preventable.
It is important for providers to be aware of their local syphilis prevalence and regulations on prenatal syphilis screening because given the effects of congenital syphilis and the ease of treatment.
Martina L. Badell, MD, is an assistant professor in the department of gynecology and obstetrics and maternal-fetal medicine at Emory University in Atlanta. She reported no relevant conflicts of interest.
Syphilis cases increased by 61% between 2012 and 2016 among pregnant women, and the proportion of syphilis cases was higher for women who were non-Hispanic black race and Hispanic ethnicity, according to research in Obstetrics & Gynecology.
“These findings support current recommendations for universal syphilis screening at the first prenatal visit and indicate that it may be necessary to include population context when determining whether to implement repeat screening during pregnancy,” Shivika Trivedi, MD, MSc, of the CDC Foundation and the Division of STD Prevention at the Centers for Disease Control and Prevention and colleagues wrote.
Dr. Trivedi and colleagues identified 9,883 pregnant women with reported syphilis in the CDC National Notifiable Diseases Surveillance System during 2012-2016. During that time, there was an increase in the number of female syphilis cases from 9,551 cases in 2012 to 14,838 cases in 2016 (55%), while there was an increase in the number of syphilis cases for pregnant women from 1,561 cases in 2012 to 2,508 cases in 2016 (61%). Of the risk factors reported for syphilis, 49% reported no risk factors within 12 priors before diagnosis, 43% said they had had at least one sexually transmitted disease, and 30% reported more than one sexual partner within the last year.
The greatest prevalence for syphilis was among women who were in their 20s (59%), located in the South (56%), and were non-Hispanic black (49%) or Hispanic (28%). However, researchers noted the rates of syphilis increased among all women between 18 years and 45 years and in every race and ethnicity group between 2012 and 2016. Early syphilis cases increased from 35% in 2012 to 58% in 2016, while late latent cases decreased from 65% in 2012 to 42% in 2016.
Researchers noted several limitations in the study, including case-based surveillance data, which potentially underreported the rates of syphilis, and a lack of pregnancy outcomes for pregnant women with syphilitic infections. However, they noted the data do show a trend of syphilis infections in pregnant women because the live birth rate “was relatively stable and did not fluctuate more than” 1.5% between 2012 and 2016.
“Through an increased awareness of the rising syphilis cases among pregnant women as well as these trend data, health care providers can be better informed to ensure they are following national guidelines and state policies for syphilis screening in pregnancy,” Dr. Trivedi and colleagues concluded.
The authors reported no relevant conflicts of interest.
SOURCE: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
Syphilis cases increased by 61% between 2012 and 2016 among pregnant women, and the proportion of syphilis cases was higher for women who were non-Hispanic black race and Hispanic ethnicity, according to research in Obstetrics & Gynecology.
“These findings support current recommendations for universal syphilis screening at the first prenatal visit and indicate that it may be necessary to include population context when determining whether to implement repeat screening during pregnancy,” Shivika Trivedi, MD, MSc, of the CDC Foundation and the Division of STD Prevention at the Centers for Disease Control and Prevention and colleagues wrote.
Dr. Trivedi and colleagues identified 9,883 pregnant women with reported syphilis in the CDC National Notifiable Diseases Surveillance System during 2012-2016. During that time, there was an increase in the number of female syphilis cases from 9,551 cases in 2012 to 14,838 cases in 2016 (55%), while there was an increase in the number of syphilis cases for pregnant women from 1,561 cases in 2012 to 2,508 cases in 2016 (61%). Of the risk factors reported for syphilis, 49% reported no risk factors within 12 priors before diagnosis, 43% said they had had at least one sexually transmitted disease, and 30% reported more than one sexual partner within the last year.
The greatest prevalence for syphilis was among women who were in their 20s (59%), located in the South (56%), and were non-Hispanic black (49%) or Hispanic (28%). However, researchers noted the rates of syphilis increased among all women between 18 years and 45 years and in every race and ethnicity group between 2012 and 2016. Early syphilis cases increased from 35% in 2012 to 58% in 2016, while late latent cases decreased from 65% in 2012 to 42% in 2016.
Researchers noted several limitations in the study, including case-based surveillance data, which potentially underreported the rates of syphilis, and a lack of pregnancy outcomes for pregnant women with syphilitic infections. However, they noted the data do show a trend of syphilis infections in pregnant women because the live birth rate “was relatively stable and did not fluctuate more than” 1.5% between 2012 and 2016.
“Through an increased awareness of the rising syphilis cases among pregnant women as well as these trend data, health care providers can be better informed to ensure they are following national guidelines and state policies for syphilis screening in pregnancy,” Dr. Trivedi and colleagues concluded.
The authors reported no relevant conflicts of interest.
SOURCE: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Syphilis rates rose more in pregnant women between 2012 and 2016, compared with women in the general population.
Major finding: There was an increase of syphilis cases by 61% among pregnant women, compared with a 55% increase among women overall.
Study details: A study of national case report data from 9,883 pregnant women with reported syphilis during 2012-2016.
Disclosures: The authors reported no relevant conflicts of interest.
Source: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
Drug test results ‘should not dictate treatment’
BONITA SPRINGS, FLA. – Urine drug screening is a vital part of clinical care, but many clinicians say they do not know enough about how the tests work, an expert said at the annual meeting of the American Academy of Addiction Psychiatry.
Rebecca Ann Payne, MD, said clinicians, including residents, tend to cite little training as a reason for their uncertainty about how to interpret urine drug screen results. Also, primary care clinicians say they need more education on implementing and interpreting the screens.
The good news is that medicine and pediatric residents said they felt that a 30-minute educational program significantly boosted their knowledge base and comfort in interpreting urine drug screens, said Dr. Payne, assistant professor of neuropsychiatry and behavioral science at Palmetto Health–University of South Carolina Medical Group. She offered several points that addiction psychiatrists should be aware of:
“A positive test doesn’t necessarily mean there’s a substance use disorder,” she said. “You still need to walk through those criteria with your patients. And a positive test doesn’t mean they’re physically dependent upon it.” She said she sometimes hears from patients who say that they’d been on a certain treatment – then failed a test given by their clinician – and had their treatment stripped away.
“Drug testing is meant to be a source of information and should not dictate treatment,” she said. “I have found it’s not unusual to hear from the community that decisions are being made solely on the results of these tests, which can be problematic.”
- Point-of-care tests, which sometimes can be bought in drug stores, she said, are “much less than perfect.”
The false-positive rate for benzodiazepines has been found to be 61%; and for methadone, it is 46%; for opioids, 22%; and for amphetamines, 21%.
- Know what your lab is actually testing for, because “it’s not universal.”
She emphasized knowing the particulars of opiate testing.
“A lot of times in a hospital setting, your lab is really only testing those opiates that are directly derived from the poppy – we’re talking about things like codeine, heroin, morphine. They’re not testing for things like your semi-synthetics or your full-synthetic opiates.”
- Know the answer to the question: “Can you get a positive result on a marijuana drug screen just from passive inhalation?”
Physicians often will be confronted by patients who insist they were only in the car or in the same room with someone who was smoking marijuana. How likely is it that their test could be positive?
“Possible,” she said, “but not probable.”
Dr. Payne’s key interest areas include teaching medical students and residents, treating substance use and psychiatric disorders that are comorbid, and conducting research in addiction psychiatry.
BONITA SPRINGS, FLA. – Urine drug screening is a vital part of clinical care, but many clinicians say they do not know enough about how the tests work, an expert said at the annual meeting of the American Academy of Addiction Psychiatry.
Rebecca Ann Payne, MD, said clinicians, including residents, tend to cite little training as a reason for their uncertainty about how to interpret urine drug screen results. Also, primary care clinicians say they need more education on implementing and interpreting the screens.
The good news is that medicine and pediatric residents said they felt that a 30-minute educational program significantly boosted their knowledge base and comfort in interpreting urine drug screens, said Dr. Payne, assistant professor of neuropsychiatry and behavioral science at Palmetto Health–University of South Carolina Medical Group. She offered several points that addiction psychiatrists should be aware of:
“A positive test doesn’t necessarily mean there’s a substance use disorder,” she said. “You still need to walk through those criteria with your patients. And a positive test doesn’t mean they’re physically dependent upon it.” She said she sometimes hears from patients who say that they’d been on a certain treatment – then failed a test given by their clinician – and had their treatment stripped away.
“Drug testing is meant to be a source of information and should not dictate treatment,” she said. “I have found it’s not unusual to hear from the community that decisions are being made solely on the results of these tests, which can be problematic.”
- Point-of-care tests, which sometimes can be bought in drug stores, she said, are “much less than perfect.”
The false-positive rate for benzodiazepines has been found to be 61%; and for methadone, it is 46%; for opioids, 22%; and for amphetamines, 21%.
- Know what your lab is actually testing for, because “it’s not universal.”
She emphasized knowing the particulars of opiate testing.
“A lot of times in a hospital setting, your lab is really only testing those opiates that are directly derived from the poppy – we’re talking about things like codeine, heroin, morphine. They’re not testing for things like your semi-synthetics or your full-synthetic opiates.”
- Know the answer to the question: “Can you get a positive result on a marijuana drug screen just from passive inhalation?”
Physicians often will be confronted by patients who insist they were only in the car or in the same room with someone who was smoking marijuana. How likely is it that their test could be positive?
“Possible,” she said, “but not probable.”
Dr. Payne’s key interest areas include teaching medical students and residents, treating substance use and psychiatric disorders that are comorbid, and conducting research in addiction psychiatry.
BONITA SPRINGS, FLA. – Urine drug screening is a vital part of clinical care, but many clinicians say they do not know enough about how the tests work, an expert said at the annual meeting of the American Academy of Addiction Psychiatry.
Rebecca Ann Payne, MD, said clinicians, including residents, tend to cite little training as a reason for their uncertainty about how to interpret urine drug screen results. Also, primary care clinicians say they need more education on implementing and interpreting the screens.
The good news is that medicine and pediatric residents said they felt that a 30-minute educational program significantly boosted their knowledge base and comfort in interpreting urine drug screens, said Dr. Payne, assistant professor of neuropsychiatry and behavioral science at Palmetto Health–University of South Carolina Medical Group. She offered several points that addiction psychiatrists should be aware of:
“A positive test doesn’t necessarily mean there’s a substance use disorder,” she said. “You still need to walk through those criteria with your patients. And a positive test doesn’t mean they’re physically dependent upon it.” She said she sometimes hears from patients who say that they’d been on a certain treatment – then failed a test given by their clinician – and had their treatment stripped away.
“Drug testing is meant to be a source of information and should not dictate treatment,” she said. “I have found it’s not unusual to hear from the community that decisions are being made solely on the results of these tests, which can be problematic.”
- Point-of-care tests, which sometimes can be bought in drug stores, she said, are “much less than perfect.”
The false-positive rate for benzodiazepines has been found to be 61%; and for methadone, it is 46%; for opioids, 22%; and for amphetamines, 21%.
- Know what your lab is actually testing for, because “it’s not universal.”
She emphasized knowing the particulars of opiate testing.
“A lot of times in a hospital setting, your lab is really only testing those opiates that are directly derived from the poppy – we’re talking about things like codeine, heroin, morphine. They’re not testing for things like your semi-synthetics or your full-synthetic opiates.”
- Know the answer to the question: “Can you get a positive result on a marijuana drug screen just from passive inhalation?”
Physicians often will be confronted by patients who insist they were only in the car or in the same room with someone who was smoking marijuana. How likely is it that their test could be positive?
“Possible,” she said, “but not probable.”
Dr. Payne’s key interest areas include teaching medical students and residents, treating substance use and psychiatric disorders that are comorbid, and conducting research in addiction psychiatry.
REPORTING FROM AAAP 2018
HCC screening linked with improved tumor detection
“Our study’s aim was to characterize utilization of HCC screening receipt and its association with early tumor detection and improved survival in a nationally representative cohort of patients in the United States,” wrote Debra T. Choi, PhD, MPH, of Baylor College of Medicine, Houston, and her colleagues.
The researchers retrospectively studied a cohort of 13,174 patients with HCC from 2003 to 2013 included in the Surveillance, Epidemiology, and End Results Program–Medicare database. They examined the acquisition of HCC in the 3 years leading up to HCC diagnosis using three separate categories: consistent, inconsistent, or no screening. Dr. Choi and her colleagues studied the associations between receiving HCC screening and subsequent effects on overall survival.
“HCC prognosis depends on tumor stage at the time of diagnosis, with curative treatment options only available for patients diagnosed at an early stage,” the researchers wrote. “Patients with early-stage HCC can achieve 5-year survival rates of 70% if they undergo surgical resection or liver transplantation, compared to a median survival of 1 year for patients with advanced HCC,” they added.
After multivariable analysis, the investigators found that 51.1% of patients with cirrhosis did not receive screening in the 3 years leading up to HCC diagnosis. In addition, they went on to report that only 6.8% of patients were consistently screened on an annual basis.
“HCC screening receipt was associated with early tumor detection and potentially improved overall survival, with attenuated benefits in those with inconsistent screening compared to those who had received consistent screening,” they explained.
In terms of efficacy, consistent screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98, 95% confidence interval, 1.68-2.33) and decreased risk of death (hazard ratio, 0.76, 95% CI, 0.70-0.83) after adjustment for lead time bias. Given these results, Dr. Choi and her colleagues said that early HCC screening may help with hepatic tumor detection at later disease stages.
“Given the demonstrated benefits of HCC screening, [it] is an important step to reverse the high rates of late stage diagnosis and poor survival,” they added.
The investigators noted that several patient-specific factors may be driving these associations. In particular, they found a link between female sex and receiving HCC screening. Dr. Choi and her colleagues suggested this association is not related to the perceived benefits from screening.
“Studies have suggested females may be more likely to adhere to screening recommendations; however, patient adherence is not a common barrier to HCC screening completion and therefore it is unclear if this is the sole driver of this association,” they acknowledged.
Moving forward, the researchers highlighted the importance of educating primary care providers about the benefits of screening. Moreover, they said that screening receipt is currently on the rise, which has shown positive effects on overall survival.
The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
SOURCE: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
“Our study’s aim was to characterize utilization of HCC screening receipt and its association with early tumor detection and improved survival in a nationally representative cohort of patients in the United States,” wrote Debra T. Choi, PhD, MPH, of Baylor College of Medicine, Houston, and her colleagues.
The researchers retrospectively studied a cohort of 13,174 patients with HCC from 2003 to 2013 included in the Surveillance, Epidemiology, and End Results Program–Medicare database. They examined the acquisition of HCC in the 3 years leading up to HCC diagnosis using three separate categories: consistent, inconsistent, or no screening. Dr. Choi and her colleagues studied the associations between receiving HCC screening and subsequent effects on overall survival.
“HCC prognosis depends on tumor stage at the time of diagnosis, with curative treatment options only available for patients diagnosed at an early stage,” the researchers wrote. “Patients with early-stage HCC can achieve 5-year survival rates of 70% if they undergo surgical resection or liver transplantation, compared to a median survival of 1 year for patients with advanced HCC,” they added.
After multivariable analysis, the investigators found that 51.1% of patients with cirrhosis did not receive screening in the 3 years leading up to HCC diagnosis. In addition, they went on to report that only 6.8% of patients were consistently screened on an annual basis.
“HCC screening receipt was associated with early tumor detection and potentially improved overall survival, with attenuated benefits in those with inconsistent screening compared to those who had received consistent screening,” they explained.
In terms of efficacy, consistent screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98, 95% confidence interval, 1.68-2.33) and decreased risk of death (hazard ratio, 0.76, 95% CI, 0.70-0.83) after adjustment for lead time bias. Given these results, Dr. Choi and her colleagues said that early HCC screening may help with hepatic tumor detection at later disease stages.
“Given the demonstrated benefits of HCC screening, [it] is an important step to reverse the high rates of late stage diagnosis and poor survival,” they added.
The investigators noted that several patient-specific factors may be driving these associations. In particular, they found a link between female sex and receiving HCC screening. Dr. Choi and her colleagues suggested this association is not related to the perceived benefits from screening.
“Studies have suggested females may be more likely to adhere to screening recommendations; however, patient adherence is not a common barrier to HCC screening completion and therefore it is unclear if this is the sole driver of this association,” they acknowledged.
Moving forward, the researchers highlighted the importance of educating primary care providers about the benefits of screening. Moreover, they said that screening receipt is currently on the rise, which has shown positive effects on overall survival.
The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
SOURCE: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
“Our study’s aim was to characterize utilization of HCC screening receipt and its association with early tumor detection and improved survival in a nationally representative cohort of patients in the United States,” wrote Debra T. Choi, PhD, MPH, of Baylor College of Medicine, Houston, and her colleagues.
The researchers retrospectively studied a cohort of 13,174 patients with HCC from 2003 to 2013 included in the Surveillance, Epidemiology, and End Results Program–Medicare database. They examined the acquisition of HCC in the 3 years leading up to HCC diagnosis using three separate categories: consistent, inconsistent, or no screening. Dr. Choi and her colleagues studied the associations between receiving HCC screening and subsequent effects on overall survival.
“HCC prognosis depends on tumor stage at the time of diagnosis, with curative treatment options only available for patients diagnosed at an early stage,” the researchers wrote. “Patients with early-stage HCC can achieve 5-year survival rates of 70% if they undergo surgical resection or liver transplantation, compared to a median survival of 1 year for patients with advanced HCC,” they added.
After multivariable analysis, the investigators found that 51.1% of patients with cirrhosis did not receive screening in the 3 years leading up to HCC diagnosis. In addition, they went on to report that only 6.8% of patients were consistently screened on an annual basis.
“HCC screening receipt was associated with early tumor detection and potentially improved overall survival, with attenuated benefits in those with inconsistent screening compared to those who had received consistent screening,” they explained.
In terms of efficacy, consistent screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98, 95% confidence interval, 1.68-2.33) and decreased risk of death (hazard ratio, 0.76, 95% CI, 0.70-0.83) after adjustment for lead time bias. Given these results, Dr. Choi and her colleagues said that early HCC screening may help with hepatic tumor detection at later disease stages.
“Given the demonstrated benefits of HCC screening, [it] is an important step to reverse the high rates of late stage diagnosis and poor survival,” they added.
The investigators noted that several patient-specific factors may be driving these associations. In particular, they found a link between female sex and receiving HCC screening. Dr. Choi and her colleagues suggested this association is not related to the perceived benefits from screening.
“Studies have suggested females may be more likely to adhere to screening recommendations; however, patient adherence is not a common barrier to HCC screening completion and therefore it is unclear if this is the sole driver of this association,” they acknowledged.
Moving forward, the researchers highlighted the importance of educating primary care providers about the benefits of screening. Moreover, they said that screening receipt is currently on the rise, which has shown positive effects on overall survival.
The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
SOURCE: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Hepatocellular carcinoma (HCC) screening for patients with cirrhosis remains an underutilized technique to detect early-stage malignancy.
Major finding: Regular HCC screening was associated with an increased rate of early-stage tumor detection (odds ratio, 1.98).
Study details: Retrospective analysis of 13,174 patients with HCC who were screened 3 years prior to cancer diagnosis.
Disclosures: The Center for Innovations in Quality, Effectiveness, and Safety funded the study. Additional support was provided by the Texas A&M Health Science Center Engineering Experiment Station big data seed grant program. The authors reported no conflicts of interest.
Source: Choi DT et al. Clin Gastroenterol Hepatol. 2018 Oct 25. doi: 10.1016/j.cgh.2018.10.031.