User login
CHMP recommends generic deferiprone
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for Deferiprone Lipomed to treat iron overload in patients with thalassemia major.
Deferiprone Lipomed is a generic version of the iron chelating agent Ferriprox, which has been authorized in the European Union since August 1999.
According to the CHMP, studies have shown that Deferiprone Lipomed is of satisfactory quality and bioequivalent to Ferriprox.
The CHMP’s recommendation for Deferiprone Lipomed will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Deferiprone Lipomed will be available as 500-mg film-coated tablets.
The drug will be authorized for the following uses:
- As monotherapy to treat iron overload in patients with thalassemia major when current chelation therapy is contraindicated or inadequate
- In combination with another chelator in patients with thalassemia major when monotherapy with any iron chelator is ineffective or when prevention or treatment of life-threatening consequences of iron overload justifies rapid or intensive correction.
According to the prescribing information for Ferriprox, the combination of iron chelators should be considered on a case-by-case basis, and patients should be monitored for response and adverse events.
Fatalities and life-threatening situations (caused by agranulocytosis) have been reported with the combination of deferiprone and deferoxamine.
Combination therapy is not recommended when monotherapy with either chelator is adequate or when serum ferritin falls below 500 μg/l. Additionally, there are limited data on the combined use of Ferriprox and deferasirox.
The applicant for Deferiprone Lipomed is Lipomed GmbH.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for Deferiprone Lipomed to treat iron overload in patients with thalassemia major.
Deferiprone Lipomed is a generic version of the iron chelating agent Ferriprox, which has been authorized in the European Union since August 1999.
According to the CHMP, studies have shown that Deferiprone Lipomed is of satisfactory quality and bioequivalent to Ferriprox.
The CHMP’s recommendation for Deferiprone Lipomed will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Deferiprone Lipomed will be available as 500-mg film-coated tablets.
The drug will be authorized for the following uses:
- As monotherapy to treat iron overload in patients with thalassemia major when current chelation therapy is contraindicated or inadequate
- In combination with another chelator in patients with thalassemia major when monotherapy with any iron chelator is ineffective or when prevention or treatment of life-threatening consequences of iron overload justifies rapid or intensive correction.
According to the prescribing information for Ferriprox, the combination of iron chelators should be considered on a case-by-case basis, and patients should be monitored for response and adverse events.
Fatalities and life-threatening situations (caused by agranulocytosis) have been reported with the combination of deferiprone and deferoxamine.
Combination therapy is not recommended when monotherapy with either chelator is adequate or when serum ferritin falls below 500 μg/l. Additionally, there are limited data on the combined use of Ferriprox and deferasirox.
The applicant for Deferiprone Lipomed is Lipomed GmbH.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for Deferiprone Lipomed to treat iron overload in patients with thalassemia major.
Deferiprone Lipomed is a generic version of the iron chelating agent Ferriprox, which has been authorized in the European Union since August 1999.
According to the CHMP, studies have shown that Deferiprone Lipomed is of satisfactory quality and bioequivalent to Ferriprox.
The CHMP’s recommendation for Deferiprone Lipomed will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Deferiprone Lipomed will be available as 500-mg film-coated tablets.
The drug will be authorized for the following uses:
- As monotherapy to treat iron overload in patients with thalassemia major when current chelation therapy is contraindicated or inadequate
- In combination with another chelator in patients with thalassemia major when monotherapy with any iron chelator is ineffective or when prevention or treatment of life-threatening consequences of iron overload justifies rapid or intensive correction.
According to the prescribing information for Ferriprox, the combination of iron chelators should be considered on a case-by-case basis, and patients should be monitored for response and adverse events.
Fatalities and life-threatening situations (caused by agranulocytosis) have been reported with the combination of deferiprone and deferoxamine.
Combination therapy is not recommended when monotherapy with either chelator is adequate or when serum ferritin falls below 500 μg/l. Additionally, there are limited data on the combined use of Ferriprox and deferasirox.
The applicant for Deferiprone Lipomed is Lipomed GmbH.
CHMP backs generic lenalidomide
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for Lenalidomide Accord as a treatment for multiple myeloma (MM).
Lenalidomide Accord is a generic version of the immunomodulatory agent Revlimid, which has been authorized in the European Union since June 2007.
The CHMP said studies have demonstrated the satisfactory quality of Lenalidomide Accord and its bioequivalence to Revlimid.
The CHMP’s recommendation for Lenalidomide Accord will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Lenalidomide Accord will be available as capsules (2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, and 25 mg) and authorized for the following uses:
- As monotherapy for the maintenance treatment of adults with newly diagnosed MM who have undergone autologous stem cell transplant
- In combination with melphalan and prednisone followed by lenalidomide maintenance in adults with previously untreated MM who are not eligible for transplant
- In combination with dexamethasone to treat MM in adults who have received at least 1 prior therapy.
The applicant for Lenalidomide Accord is Accord Healthcare Limited.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for Lenalidomide Accord as a treatment for multiple myeloma (MM).
Lenalidomide Accord is a generic version of the immunomodulatory agent Revlimid, which has been authorized in the European Union since June 2007.
The CHMP said studies have demonstrated the satisfactory quality of Lenalidomide Accord and its bioequivalence to Revlimid.
The CHMP’s recommendation for Lenalidomide Accord will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Lenalidomide Accord will be available as capsules (2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, and 25 mg) and authorized for the following uses:
- As monotherapy for the maintenance treatment of adults with newly diagnosed MM who have undergone autologous stem cell transplant
- In combination with melphalan and prednisone followed by lenalidomide maintenance in adults with previously untreated MM who are not eligible for transplant
- In combination with dexamethasone to treat MM in adults who have received at least 1 prior therapy.
The applicant for Lenalidomide Accord is Accord Healthcare Limited.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for Lenalidomide Accord as a treatment for multiple myeloma (MM).
Lenalidomide Accord is a generic version of the immunomodulatory agent Revlimid, which has been authorized in the European Union since June 2007.
The CHMP said studies have demonstrated the satisfactory quality of Lenalidomide Accord and its bioequivalence to Revlimid.
The CHMP’s recommendation for Lenalidomide Accord will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Lenalidomide Accord will be available as capsules (2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg, and 25 mg) and authorized for the following uses:
- As monotherapy for the maintenance treatment of adults with newly diagnosed MM who have undergone autologous stem cell transplant
- In combination with melphalan and prednisone followed by lenalidomide maintenance in adults with previously untreated MM who are not eligible for transplant
- In combination with dexamethasone to treat MM in adults who have received at least 1 prior therapy.
The applicant for Lenalidomide Accord is Accord Healthcare Limited.
Catatonia: How to identify and treat it
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
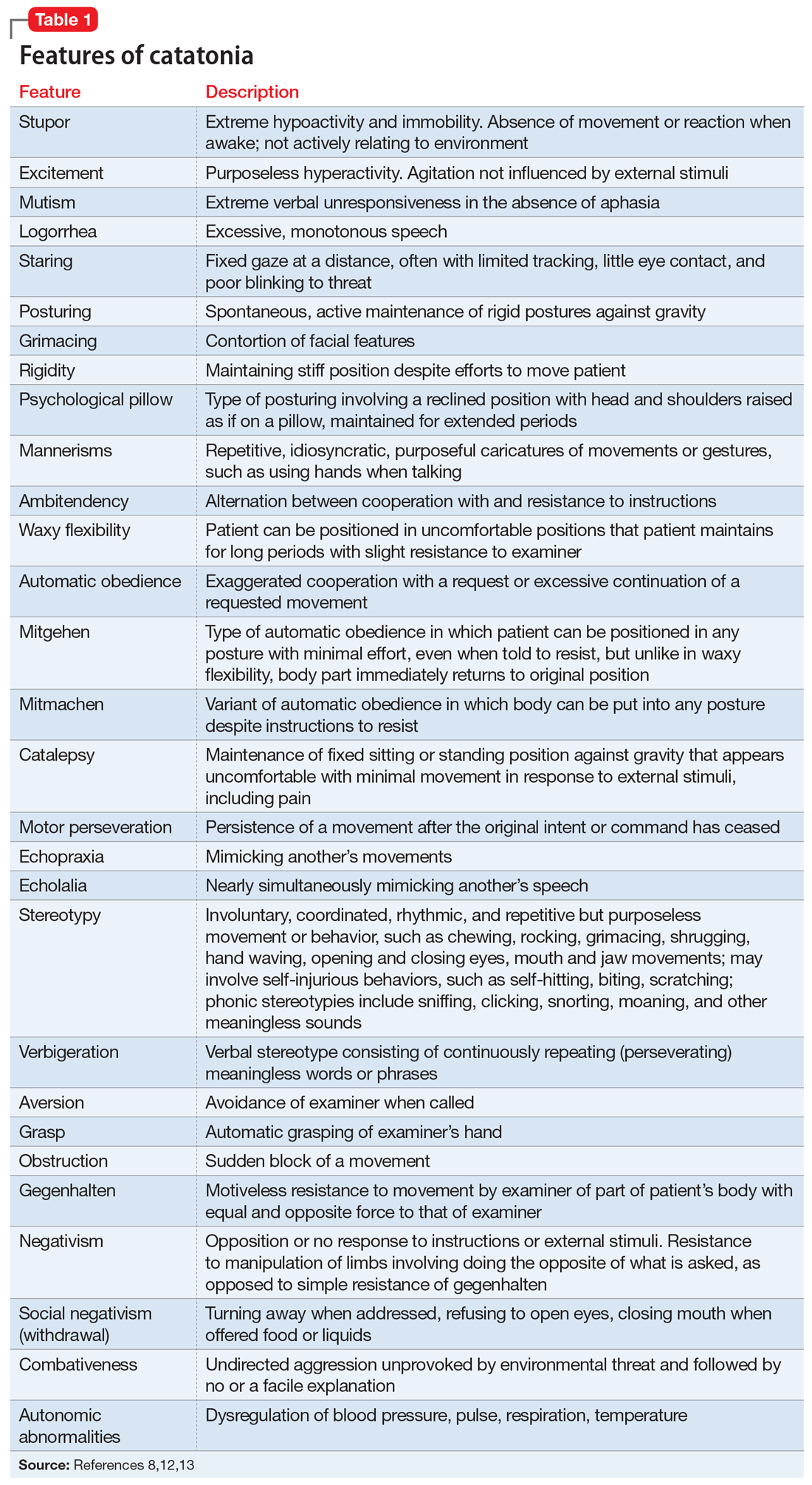
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14
Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
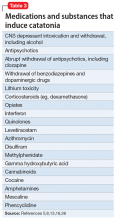
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
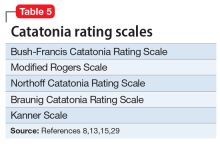
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
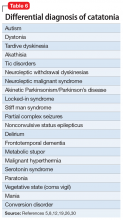
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
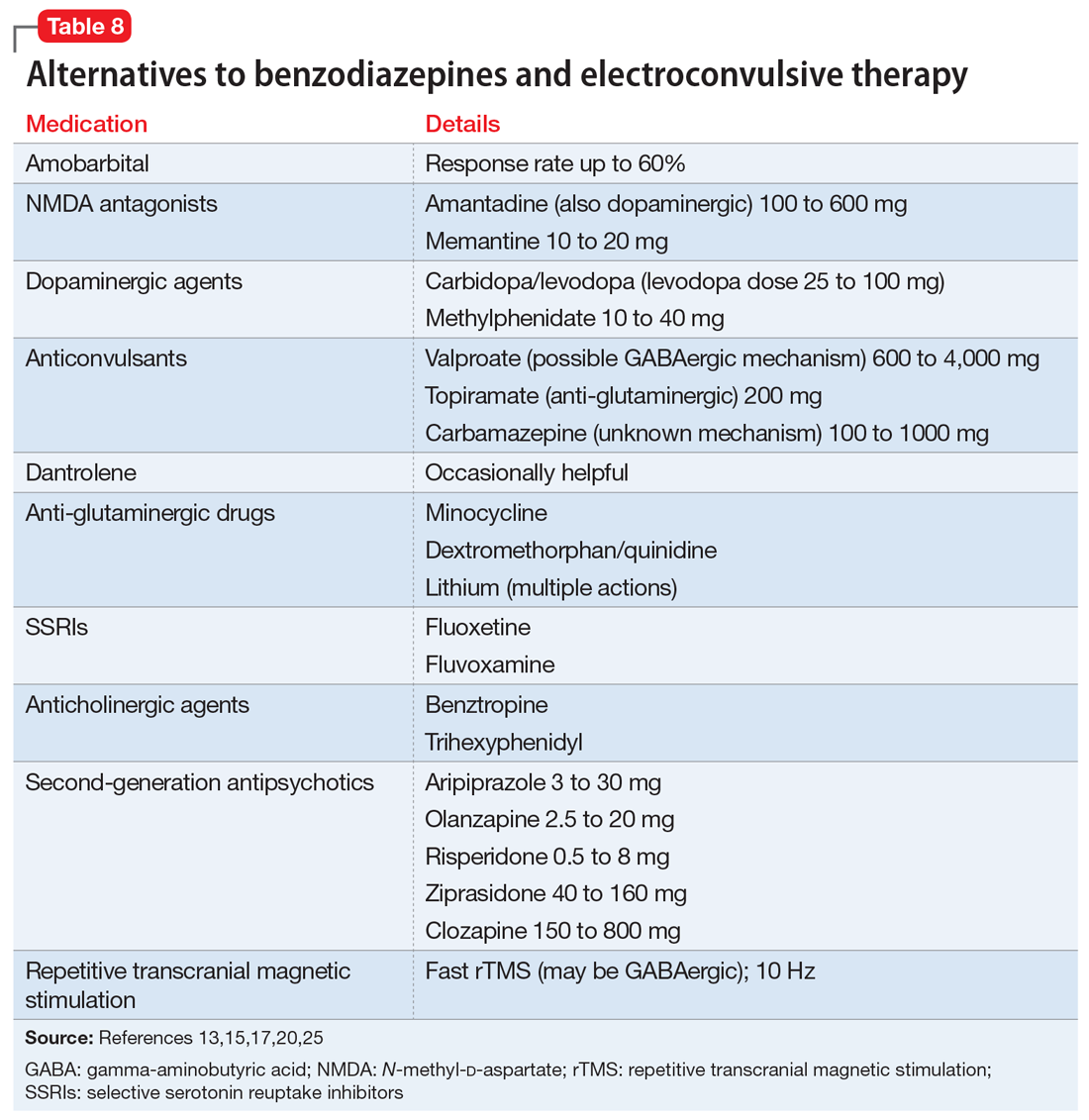
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.
Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14

Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.

Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14

Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.

Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Pertussis vaccination: We can do better
Resources
Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44.
Centers for Disease Control and Prevention. Pertussis (whooping cough). Available at: https://www.cdc.gov/pertussis/index.html. Accessed July 6, 2018.
Resources
Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44.
Centers for Disease Control and Prevention. Pertussis (whooping cough). Available at: https://www.cdc.gov/pertussis/index.html. Accessed July 6, 2018.
Resources
Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44.
Centers for Disease Control and Prevention. Pertussis (whooping cough). Available at: https://www.cdc.gov/pertussis/index.html. Accessed July 6, 2018.
A bright—not bleak—future for family medicine
Recently, a medical consulting group published, “The disruption of primary care: How customer-obsessed companies are changing everything.”1 The essay paints a not-too-rosy picture for the future of traditional family medicine in our Internet-dominated, immediate-gratification-seeking society. They contend:
“The future of primary care extends far beyond the physician’s office to pharmacies, supermarkets and retail clinics including CVS, Walgreens, Target and CityMD, as well as virtual care companies such as MDLive and Amwell. Increasingly, Internet and technology companies like Amazon, Google and Apple are showing signs of getting into the healthcare services and information arena. … These formidable customer-centric companies are primed to become preferred alternative providers of health information and low-acuity services, while lowering the price point of primary care services.”
While it is an interesting piece, I remain bullish on family medicine and believe the future remains bright for those who practice high-quality primary care. Why?
1. Cost efficacy. For common medical conditions, family physicians (FPs) are much more cost-effective than specialty or emergency department care. For example, a young man recently hit his thumb and had a subungual hematoma. He visited an orthopedic physician’s office, where the physician ordered an unnecessary x-ray and sent him home without draining the hematoma. The cost was more than $300. The patient was referred to our office where, later that day, we drained the hematoma with a hypodermic needle at a cost of
2. Immediate care. Many family medicine groups have responded to the demand for immediate care with extended hours, assigning a doctor of the day, and/or having an open-access schedule that allows for a sufficient number of same-day appointments. Many FPs are now available for “virtual visits,” since Web portals for electronic medical records have been become easy to use for secure communication. In addition, many FPs have developed e-consult services to streamline specialist consultations. At the Cleveland Clinic, an FP leads the primary care telemedicine program.
3. A future that is not mutually exclusive. The authors contend that the future will be a matrix of health care services available via the Internet like the Amazon model. I see that model as fully compatible with excellent family medicine. In such a model, a skilled FP and staff provide timely acute care and chronic disease management; they connect patients to other health-related services and high-quality health care information; and they guide patients through our increasingly complex medical system. Isn’t that what we’re already doing?
1. McCain M, Werner M, Bailey C, et al. The disruption of primary care: How customer-obsessed companies are changing everything. The Chartis Group. Available at: https://www.chartisforum.com/wp-content/uploads/2018/06/WP_The-Disruption-of-Primary-Care_Final.pdf. Accessed July 11, 2018.
Recently, a medical consulting group published, “The disruption of primary care: How customer-obsessed companies are changing everything.”1 The essay paints a not-too-rosy picture for the future of traditional family medicine in our Internet-dominated, immediate-gratification-seeking society. They contend:
“The future of primary care extends far beyond the physician’s office to pharmacies, supermarkets and retail clinics including CVS, Walgreens, Target and CityMD, as well as virtual care companies such as MDLive and Amwell. Increasingly, Internet and technology companies like Amazon, Google and Apple are showing signs of getting into the healthcare services and information arena. … These formidable customer-centric companies are primed to become preferred alternative providers of health information and low-acuity services, while lowering the price point of primary care services.”
While it is an interesting piece, I remain bullish on family medicine and believe the future remains bright for those who practice high-quality primary care. Why?
1. Cost efficacy. For common medical conditions, family physicians (FPs) are much more cost-effective than specialty or emergency department care. For example, a young man recently hit his thumb and had a subungual hematoma. He visited an orthopedic physician’s office, where the physician ordered an unnecessary x-ray and sent him home without draining the hematoma. The cost was more than $300. The patient was referred to our office where, later that day, we drained the hematoma with a hypodermic needle at a cost of
2. Immediate care. Many family medicine groups have responded to the demand for immediate care with extended hours, assigning a doctor of the day, and/or having an open-access schedule that allows for a sufficient number of same-day appointments. Many FPs are now available for “virtual visits,” since Web portals for electronic medical records have been become easy to use for secure communication. In addition, many FPs have developed e-consult services to streamline specialist consultations. At the Cleveland Clinic, an FP leads the primary care telemedicine program.
3. A future that is not mutually exclusive. The authors contend that the future will be a matrix of health care services available via the Internet like the Amazon model. I see that model as fully compatible with excellent family medicine. In such a model, a skilled FP and staff provide timely acute care and chronic disease management; they connect patients to other health-related services and high-quality health care information; and they guide patients through our increasingly complex medical system. Isn’t that what we’re already doing?
Recently, a medical consulting group published, “The disruption of primary care: How customer-obsessed companies are changing everything.”1 The essay paints a not-too-rosy picture for the future of traditional family medicine in our Internet-dominated, immediate-gratification-seeking society. They contend:
“The future of primary care extends far beyond the physician’s office to pharmacies, supermarkets and retail clinics including CVS, Walgreens, Target and CityMD, as well as virtual care companies such as MDLive and Amwell. Increasingly, Internet and technology companies like Amazon, Google and Apple are showing signs of getting into the healthcare services and information arena. … These formidable customer-centric companies are primed to become preferred alternative providers of health information and low-acuity services, while lowering the price point of primary care services.”
While it is an interesting piece, I remain bullish on family medicine and believe the future remains bright for those who practice high-quality primary care. Why?
1. Cost efficacy. For common medical conditions, family physicians (FPs) are much more cost-effective than specialty or emergency department care. For example, a young man recently hit his thumb and had a subungual hematoma. He visited an orthopedic physician’s office, where the physician ordered an unnecessary x-ray and sent him home without draining the hematoma. The cost was more than $300. The patient was referred to our office where, later that day, we drained the hematoma with a hypodermic needle at a cost of
2. Immediate care. Many family medicine groups have responded to the demand for immediate care with extended hours, assigning a doctor of the day, and/or having an open-access schedule that allows for a sufficient number of same-day appointments. Many FPs are now available for “virtual visits,” since Web portals for electronic medical records have been become easy to use for secure communication. In addition, many FPs have developed e-consult services to streamline specialist consultations. At the Cleveland Clinic, an FP leads the primary care telemedicine program.
3. A future that is not mutually exclusive. The authors contend that the future will be a matrix of health care services available via the Internet like the Amazon model. I see that model as fully compatible with excellent family medicine. In such a model, a skilled FP and staff provide timely acute care and chronic disease management; they connect patients to other health-related services and high-quality health care information; and they guide patients through our increasingly complex medical system. Isn’t that what we’re already doing?
1. McCain M, Werner M, Bailey C, et al. The disruption of primary care: How customer-obsessed companies are changing everything. The Chartis Group. Available at: https://www.chartisforum.com/wp-content/uploads/2018/06/WP_The-Disruption-of-Primary-Care_Final.pdf. Accessed July 11, 2018.
1. McCain M, Werner M, Bailey C, et al. The disruption of primary care: How customer-obsessed companies are changing everything. The Chartis Group. Available at: https://www.chartisforum.com/wp-content/uploads/2018/06/WP_The-Disruption-of-Primary-Care_Final.pdf. Accessed July 11, 2018.
What’s the best secondary treatment for patients who fail initial triple therapy for H pylori?
EVIDENCE SUMMARY
A meta-analysis of RCTs evaluating levofloxacin-based triple therapy as a secondary treatment regimen for patients with H pylori infection who had failed initial clarithromycin-based triple therapy found cure rates averaging 76% (TABLE).1 Most of the regimens comprised levofloxacin (500 mg), amoxicillin (1 g), and a PPI (40 mg), all twice daily for 7 to 10 days. Ten-day regimens produced better cure rates than 7-day regimens (84% vs 69%; comparison statistic not supplied).
The meta-analysis also included RCTs evaluating bismuth-based quadruple therapy as secondary treatment, which found cure rates averaging 78%.1 The regimens varied, comprising bismuth salts (120-600 mg, 2-4 times daily), metronidazole (250-500 mg, 2-4 times daily), tetracycline (250-500 mg, 2-4 times daily), and a PPI (40 mg twice daily). Longer duration of therapy produced higher cure rates (7 days=76%; 95% confidence interval [CI], 0.72-0.80 in 29 RCTs with 2097 patients; 10 days=77%; 95% CI, 0.60-0.93 in 2 RCTs with 142 patients; 14 days=82%; 95% CI, 0.76-0.88 in 12 RCTs with 831 patients).
Repeating the original clarithromycin-based triple therapy (8 RCTs, 265 patients) produced low cure rates (46%).1
Metronidazole-based therapy has high cure rate in a homogeneous population
A meta-analysis of 24 RCTs (1611 patients) that evaluated metronidazole-based triple therapy (mostly composed of amoxicillin 750 mg, metronidazole 250 mg, and any of a number of PPIs, all dosed at 40 mg) twice daily for 7 days found cure rates averaging 87% in an exclusively Japanese study population.1
Comparable cure rates for levofloxacin- and bismuth-based therapy
Six RCTs with a total of 1057 patients compared cure rates for levofloxacin-based triple therapy with bismuth-based quadruple therapy and found no difference.1
Two earlier meta-analyses not included in the previously described study, comprising 8 RCTs with a total of 613 patients, produced conflicting results. The larger study (15 RCTs, 1462 patients) found no difference in cure rates.2 The smaller study (7 RCTs, 787 patients) favored quadruple therapy.3
Continue to: Two secondary antibiotic regimens show similar cure rates
Two secondary antibiotic regimens show similar cure rates
A meta-analysis of 4 RCTs (total 460 patients) that compared susceptibility-guided antibiotic secondary treatment (SGT) with empiric antibiotic secondary treatment found no difference in cure rates, although the largest single RCT (172 patients) favored SGT.4
RECOMMENDATIONS
The Maastricht IV/Florence Consensus Report (a periodically updated European study group evaluating Helicobacter management) includes expert opinion-based guidelines for H pylori treatment that recommend using antibiotic susceptibility to select treatment regimens in the event of 2 treatment failures.5 The report also notes that bismuth-based quadruple therapy may not be available in all countries and has a more complex dosing regimen, and that local resistance to levofloxacin must be taken into account when prescribing levofloxacin-based triple therapy.
1. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861.
2. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastro. 2012;18:5669-5678.
3. Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138.
4. Lopez-Gongora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447-2455.
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
EVIDENCE SUMMARY
A meta-analysis of RCTs evaluating levofloxacin-based triple therapy as a secondary treatment regimen for patients with H pylori infection who had failed initial clarithromycin-based triple therapy found cure rates averaging 76% (TABLE).1 Most of the regimens comprised levofloxacin (500 mg), amoxicillin (1 g), and a PPI (40 mg), all twice daily for 7 to 10 days. Ten-day regimens produced better cure rates than 7-day regimens (84% vs 69%; comparison statistic not supplied).

The meta-analysis also included RCTs evaluating bismuth-based quadruple therapy as secondary treatment, which found cure rates averaging 78%.1 The regimens varied, comprising bismuth salts (120-600 mg, 2-4 times daily), metronidazole (250-500 mg, 2-4 times daily), tetracycline (250-500 mg, 2-4 times daily), and a PPI (40 mg twice daily). Longer duration of therapy produced higher cure rates (7 days=76%; 95% confidence interval [CI], 0.72-0.80 in 29 RCTs with 2097 patients; 10 days=77%; 95% CI, 0.60-0.93 in 2 RCTs with 142 patients; 14 days=82%; 95% CI, 0.76-0.88 in 12 RCTs with 831 patients).
Repeating the original clarithromycin-based triple therapy (8 RCTs, 265 patients) produced low cure rates (46%).1
Metronidazole-based therapy has high cure rate in a homogeneous population
A meta-analysis of 24 RCTs (1611 patients) that evaluated metronidazole-based triple therapy (mostly composed of amoxicillin 750 mg, metronidazole 250 mg, and any of a number of PPIs, all dosed at 40 mg) twice daily for 7 days found cure rates averaging 87% in an exclusively Japanese study population.1
Comparable cure rates for levofloxacin- and bismuth-based therapy
Six RCTs with a total of 1057 patients compared cure rates for levofloxacin-based triple therapy with bismuth-based quadruple therapy and found no difference.1
Two earlier meta-analyses not included in the previously described study, comprising 8 RCTs with a total of 613 patients, produced conflicting results. The larger study (15 RCTs, 1462 patients) found no difference in cure rates.2 The smaller study (7 RCTs, 787 patients) favored quadruple therapy.3
Continue to: Two secondary antibiotic regimens show similar cure rates
Two secondary antibiotic regimens show similar cure rates
A meta-analysis of 4 RCTs (total 460 patients) that compared susceptibility-guided antibiotic secondary treatment (SGT) with empiric antibiotic secondary treatment found no difference in cure rates, although the largest single RCT (172 patients) favored SGT.4
RECOMMENDATIONS
The Maastricht IV/Florence Consensus Report (a periodically updated European study group evaluating Helicobacter management) includes expert opinion-based guidelines for H pylori treatment that recommend using antibiotic susceptibility to select treatment regimens in the event of 2 treatment failures.5 The report also notes that bismuth-based quadruple therapy may not be available in all countries and has a more complex dosing regimen, and that local resistance to levofloxacin must be taken into account when prescribing levofloxacin-based triple therapy.
EVIDENCE SUMMARY
A meta-analysis of RCTs evaluating levofloxacin-based triple therapy as a secondary treatment regimen for patients with H pylori infection who had failed initial clarithromycin-based triple therapy found cure rates averaging 76% (TABLE).1 Most of the regimens comprised levofloxacin (500 mg), amoxicillin (1 g), and a PPI (40 mg), all twice daily for 7 to 10 days. Ten-day regimens produced better cure rates than 7-day regimens (84% vs 69%; comparison statistic not supplied).

The meta-analysis also included RCTs evaluating bismuth-based quadruple therapy as secondary treatment, which found cure rates averaging 78%.1 The regimens varied, comprising bismuth salts (120-600 mg, 2-4 times daily), metronidazole (250-500 mg, 2-4 times daily), tetracycline (250-500 mg, 2-4 times daily), and a PPI (40 mg twice daily). Longer duration of therapy produced higher cure rates (7 days=76%; 95% confidence interval [CI], 0.72-0.80 in 29 RCTs with 2097 patients; 10 days=77%; 95% CI, 0.60-0.93 in 2 RCTs with 142 patients; 14 days=82%; 95% CI, 0.76-0.88 in 12 RCTs with 831 patients).
Repeating the original clarithromycin-based triple therapy (8 RCTs, 265 patients) produced low cure rates (46%).1
Metronidazole-based therapy has high cure rate in a homogeneous population
A meta-analysis of 24 RCTs (1611 patients) that evaluated metronidazole-based triple therapy (mostly composed of amoxicillin 750 mg, metronidazole 250 mg, and any of a number of PPIs, all dosed at 40 mg) twice daily for 7 days found cure rates averaging 87% in an exclusively Japanese study population.1
Comparable cure rates for levofloxacin- and bismuth-based therapy
Six RCTs with a total of 1057 patients compared cure rates for levofloxacin-based triple therapy with bismuth-based quadruple therapy and found no difference.1
Two earlier meta-analyses not included in the previously described study, comprising 8 RCTs with a total of 613 patients, produced conflicting results. The larger study (15 RCTs, 1462 patients) found no difference in cure rates.2 The smaller study (7 RCTs, 787 patients) favored quadruple therapy.3
Continue to: Two secondary antibiotic regimens show similar cure rates
Two secondary antibiotic regimens show similar cure rates
A meta-analysis of 4 RCTs (total 460 patients) that compared susceptibility-guided antibiotic secondary treatment (SGT) with empiric antibiotic secondary treatment found no difference in cure rates, although the largest single RCT (172 patients) favored SGT.4
RECOMMENDATIONS
The Maastricht IV/Florence Consensus Report (a periodically updated European study group evaluating Helicobacter management) includes expert opinion-based guidelines for H pylori treatment that recommend using antibiotic susceptibility to select treatment regimens in the event of 2 treatment failures.5 The report also notes that bismuth-based quadruple therapy may not be available in all countries and has a more complex dosing regimen, and that local resistance to levofloxacin must be taken into account when prescribing levofloxacin-based triple therapy.
1. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861.
2. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastro. 2012;18:5669-5678.
3. Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138.
4. Lopez-Gongora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447-2455.
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
1. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861.
2. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastro. 2012;18:5669-5678.
3. Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138.
4. Lopez-Gongora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447-2455.
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
EVIDENCE-BASED ANSWER:
Treating patients with Helicobacter pylori infection who have failed clarithromycin-based triple therapy with either levofloxacin-based triple therapy (with amoxicillin and a proton pump inhibitor [PPI]) or a bismuth-based quadruple therapy produces cure rates of 75% to 81%. Ten-day regimens produce higher cure rates than 7-day regimens. Repeating the initial clarithromycin-based triple therapy cures fewer than half of patients (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Treating with a metronidazole-based triple therapy (with amoxicillin and a PPI) also produces high (87%) cure rates (SOR: A, meta-analyses of RCTs in exclusively Japanese populations).
Selecting a secondary treatment regimen based on H pylori antibiotic susceptibility testing probably doesn’t improve cure rates over empiric antibiotic treatment (SOR: B, meta-analyses of RCTs with conflicting results). However, after 2 treatment failures it may be necessary (SOR: C, expert opinion-based guidelines).
Bismuth-based quadruple therapy has a more complex dosing regimen, and bismuth isn’t available in some countries. Rising rates of H pylori resistance to levofloxacin in certain areas could make levofloxacin-based triple therapy less effective in the future (SOR: C, expert opinion-based guidelines).
13 weeks' gestation • heart palpitations • chest tightness • Dx?
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).
Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)
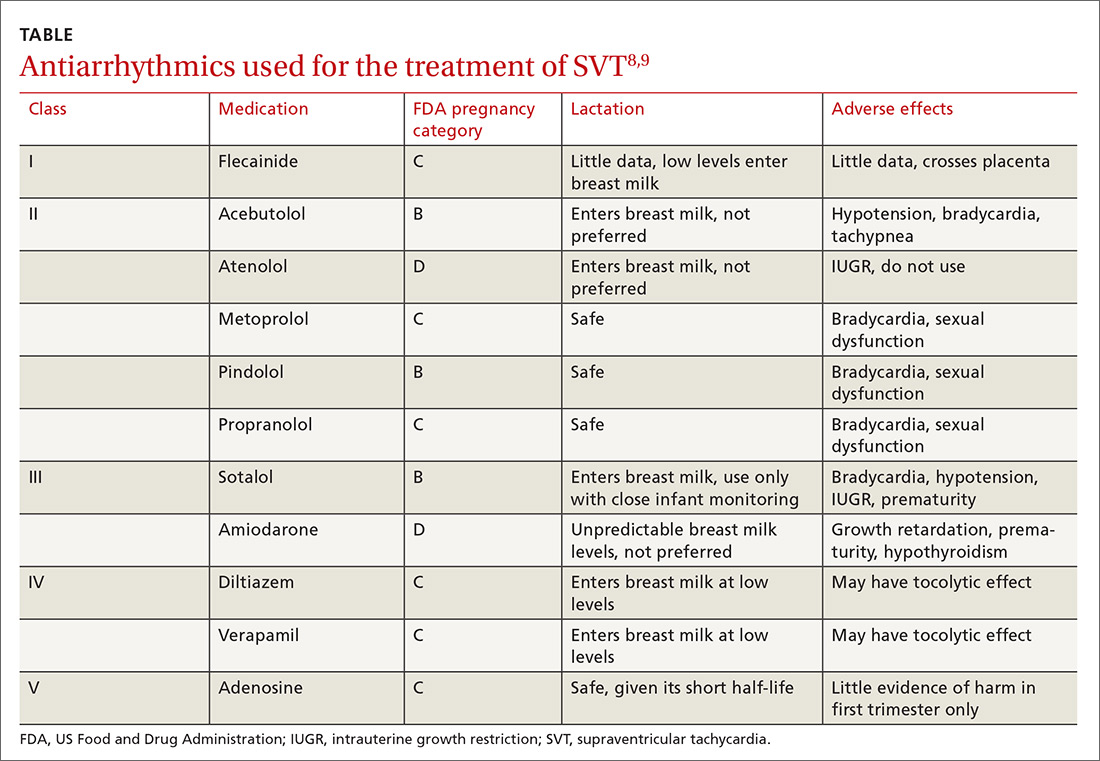
Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).

Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)

Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).

Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)

Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
Painful blisters on fingertips and toes
A 52-year-old woman presented to the emergency department (ED) with a 4-month history of recurrent painful blisters on her fingertips and the tips of her toes (FIGURE 1), arthralgias, painful discoloration of her distal toes and fingers when exposed to cold, and painful nodules on her forearms. She was started on prednisone and was sent to our clinic for follow-up.
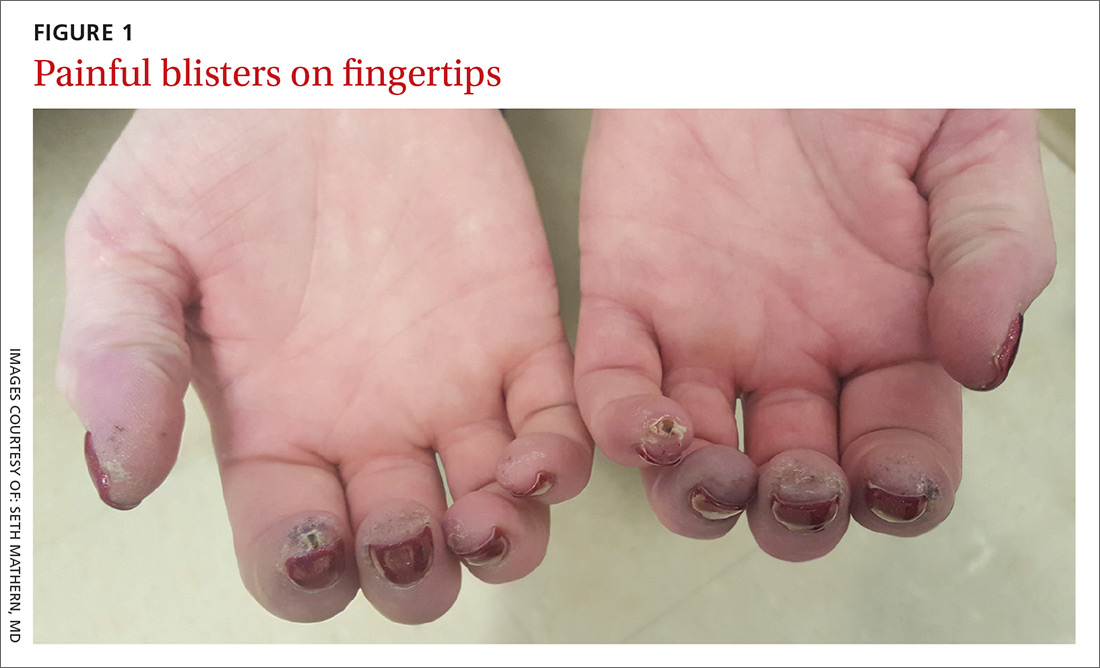
At her initial visit to our office, she was continued on prednisone and referred to Rheumatology and Interventional Cardiology, where a work-up for rheumatoid arthritis, systemic lupus erythematosus, and other vasculitides was negative. The patient had normal arterial pressures and a normal echocardiogram. An angiogram revealed segmental occlusions of the distal vessels in her arms and legs. The patient denied chest pain, syncope, dyspnea on exertion, or fever. She reported a >30 pack-year history of cigarette smoking.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Thromboangiitis obliterans
Thromboangiitis obliterans (TAO), or Buerger’s disease, is a rare nonatherosclerotic disease that affects the medium and small arteries. The disease has a male predominance, primarily occurs in those younger than 45 years of age, and is most common in people from the Middle and Far East.1 Its distinctive features include ulcerations of the distal extremities and symptoms of claudication and pain at rest. More than 40% of affected patients develop Raynaud’s phenomenon.1 Superficial thrombophlebitis in the form of painful nodules has also been described.2
The etiology of TAO is likely due to disordered inflammation of endothelial cells, which has a strong association with smoking.3 The exact pathogenesis is unknown, but genetics and autoimmunity are suspected contributing factors.
The diagnosis is based on exclusion of other causes
The differential diagnosis includes diabetic angiopathy, embolic disease, atherosclerosis, hypercoagulability/thrombophilia, vasculitis or connective tissue diseases, and drug-associated (eg, cocaine) vasculitis.4
The diagnosis of TAO is based on the exclusion of other causes, although several diagnostic criteria have been proposed, including:
- age <45 years
- current or recent history of tobacco use
- distal extremity involvement (ulcers, claudication, or pain at rest)
- exclusion of diabetes, peripheral artery disease, thrombophilia, or embolic disease
- typical arteriographic findings on imaging, including distal small to medium vessel involvement, segmental occlusions, and “corkscrew-shaped” collaterals.1,2,5,6
Continue to: Lab tests
Lab tests. There are no specific laboratory markers for TAO. The initial evaluation should include an erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete metabolic panel (CMP), and urinalysis (UA). Tests to exclude other autoimmune diseases include rheumatoid factor, antinuclear antibody, anticentromere antibody and Scl-70 to exclude CREST syndrome and scleroderma, antiphospholipid antibodies to exclude disorders of hypercoagulability, and drug testing and history-taking to evaluate for drug-related (eg, cocaine) etiologies. Further studies should be performed based on clinical suspicion.
Imaging. Patients with suspected TAO should undergo an arteriogram of the affected extremities and large arteries. Other imaging modalities include computed tomographic angiography and magnetic resonance angiography. Biopsy is rarely indicated, unless there are atypical findings, such as large artery involvement or arterial nodules. Interestingly, a positive Allen test in a young smoker can be highly suggestive of TAO.1 (For a demonstration of the Allen test, see https://www.youtube.com/watch?v=D1tJO0RW9UM.)
Our patient tested negative for rheumatoid arthritis, CREST, and scleroderma and had a normal UA and CMP. She did have a slightly elevated anticardiolipin antibody test, but a negative lupus anticoagulant test, the significance of which is uncertain. Her CRP and ESR were elevated.
Complete smoking cessation is essential for treatment
Several treatments have been proposed, including prostanoids and surgery (surgical revascularization or endovascular therapy).1,4 In severe cases, amputation may be required to remove the affected extremity. However, the most important and most effective treatment for TAO is smoking cessation.1 Of note, several case reports have found that replacing smoking with other nicotine-containing products (eg, chewing tobacco) may not prevent limb loss.7-9
Our patient was tapered off prednisone and was continued on amlodipine 5 mg/d for vasospasm. She was started on varenicline 0.5 mg/d, which was increased to twice daily by Day 4 to aid with smoking cessation. Two months later, the patient’s pain and ulcerations had almost completely resolved (FIGURE 2). She experienced occasional relapses with smoking, during which her ulcerations and Raynaud’s would return. This case reinforces the age-old aphorism of “no tobacco, no Buerger’s disease.”4
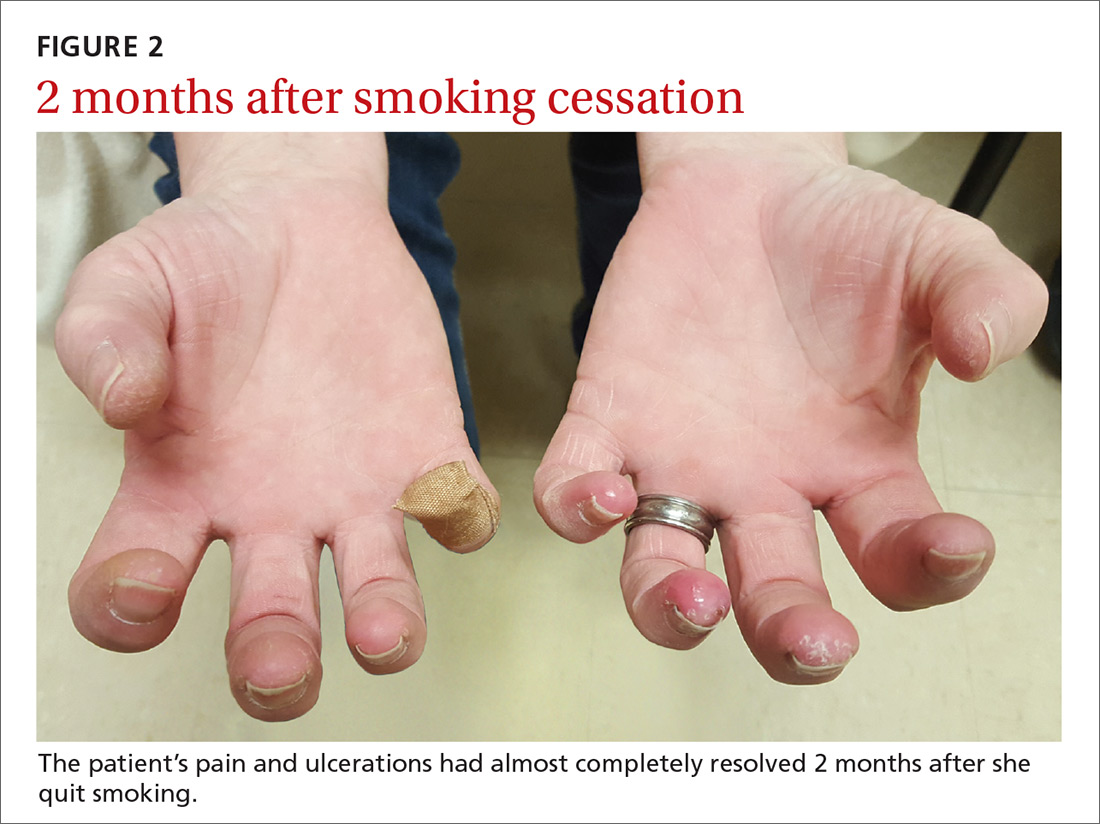
CORRESPONDENCE
Seth Mathern, MD, 14300 Orchard Parkway, Westminster, CO 80023; [email protected].
1. Olin JW. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864-869.
2. Piazza G, Creager MA. Thromboangiitis obliterans. Circulation. 2010;121:1858-1861.
3. Azizi M, Boutouyrie P, Bura-Rivière A, et al. Thromboangiitis obliterans and endothelial function. Eur J Clin Invest. 2010;40:518-526.
4. Klein-Weigel PF, Richter JG. Thromboangiitis obliterans (Buerger’s disease). Vasa. 2014;43:337-346.
5. Papa MZ, Rabi I, Adar R. A point scoring system for the clinical diagnosis of Buerger’s disease. Eur J Vasc Endovasc Surg. 1996;11:335-339.
6. Mills JL, Porter JM. Buerger’s disease: a review and update. Semin Vasc Surg. 1993;6:14-23.
7. Lie JT. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1988;31:812-813.
8. O’Dell JR, Linder J, Markin RS, et al. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1987;30:1054-1056.
9. Lawrence PF, Lund OI, Jimenez JC, et al. Substitution of smokeless tobacco for cigarettes in Buerger’s disease does not prevent limb loss. J Vasc Surg. 2008;48:210-212.
A 52-year-old woman presented to the emergency department (ED) with a 4-month history of recurrent painful blisters on her fingertips and the tips of her toes (FIGURE 1), arthralgias, painful discoloration of her distal toes and fingers when exposed to cold, and painful nodules on her forearms. She was started on prednisone and was sent to our clinic for follow-up.

At her initial visit to our office, she was continued on prednisone and referred to Rheumatology and Interventional Cardiology, where a work-up for rheumatoid arthritis, systemic lupus erythematosus, and other vasculitides was negative. The patient had normal arterial pressures and a normal echocardiogram. An angiogram revealed segmental occlusions of the distal vessels in her arms and legs. The patient denied chest pain, syncope, dyspnea on exertion, or fever. She reported a >30 pack-year history of cigarette smoking.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Thromboangiitis obliterans
Thromboangiitis obliterans (TAO), or Buerger’s disease, is a rare nonatherosclerotic disease that affects the medium and small arteries. The disease has a male predominance, primarily occurs in those younger than 45 years of age, and is most common in people from the Middle and Far East.1 Its distinctive features include ulcerations of the distal extremities and symptoms of claudication and pain at rest. More than 40% of affected patients develop Raynaud’s phenomenon.1 Superficial thrombophlebitis in the form of painful nodules has also been described.2
The etiology of TAO is likely due to disordered inflammation of endothelial cells, which has a strong association with smoking.3 The exact pathogenesis is unknown, but genetics and autoimmunity are suspected contributing factors.
The diagnosis is based on exclusion of other causes
The differential diagnosis includes diabetic angiopathy, embolic disease, atherosclerosis, hypercoagulability/thrombophilia, vasculitis or connective tissue diseases, and drug-associated (eg, cocaine) vasculitis.4
The diagnosis of TAO is based on the exclusion of other causes, although several diagnostic criteria have been proposed, including:
- age <45 years
- current or recent history of tobacco use
- distal extremity involvement (ulcers, claudication, or pain at rest)
- exclusion of diabetes, peripheral artery disease, thrombophilia, or embolic disease
- typical arteriographic findings on imaging, including distal small to medium vessel involvement, segmental occlusions, and “corkscrew-shaped” collaterals.1,2,5,6
Continue to: Lab tests
Lab tests. There are no specific laboratory markers for TAO. The initial evaluation should include an erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete metabolic panel (CMP), and urinalysis (UA). Tests to exclude other autoimmune diseases include rheumatoid factor, antinuclear antibody, anticentromere antibody and Scl-70 to exclude CREST syndrome and scleroderma, antiphospholipid antibodies to exclude disorders of hypercoagulability, and drug testing and history-taking to evaluate for drug-related (eg, cocaine) etiologies. Further studies should be performed based on clinical suspicion.
Imaging. Patients with suspected TAO should undergo an arteriogram of the affected extremities and large arteries. Other imaging modalities include computed tomographic angiography and magnetic resonance angiography. Biopsy is rarely indicated, unless there are atypical findings, such as large artery involvement or arterial nodules. Interestingly, a positive Allen test in a young smoker can be highly suggestive of TAO.1 (For a demonstration of the Allen test, see https://www.youtube.com/watch?v=D1tJO0RW9UM.)
Our patient tested negative for rheumatoid arthritis, CREST, and scleroderma and had a normal UA and CMP. She did have a slightly elevated anticardiolipin antibody test, but a negative lupus anticoagulant test, the significance of which is uncertain. Her CRP and ESR were elevated.
Complete smoking cessation is essential for treatment
Several treatments have been proposed, including prostanoids and surgery (surgical revascularization or endovascular therapy).1,4 In severe cases, amputation may be required to remove the affected extremity. However, the most important and most effective treatment for TAO is smoking cessation.1 Of note, several case reports have found that replacing smoking with other nicotine-containing products (eg, chewing tobacco) may not prevent limb loss.7-9
Our patient was tapered off prednisone and was continued on amlodipine 5 mg/d for vasospasm. She was started on varenicline 0.5 mg/d, which was increased to twice daily by Day 4 to aid with smoking cessation. Two months later, the patient’s pain and ulcerations had almost completely resolved (FIGURE 2). She experienced occasional relapses with smoking, during which her ulcerations and Raynaud’s would return. This case reinforces the age-old aphorism of “no tobacco, no Buerger’s disease.”4

CORRESPONDENCE
Seth Mathern, MD, 14300 Orchard Parkway, Westminster, CO 80023; [email protected].
A 52-year-old woman presented to the emergency department (ED) with a 4-month history of recurrent painful blisters on her fingertips and the tips of her toes (FIGURE 1), arthralgias, painful discoloration of her distal toes and fingers when exposed to cold, and painful nodules on her forearms. She was started on prednisone and was sent to our clinic for follow-up.

At her initial visit to our office, she was continued on prednisone and referred to Rheumatology and Interventional Cardiology, where a work-up for rheumatoid arthritis, systemic lupus erythematosus, and other vasculitides was negative. The patient had normal arterial pressures and a normal echocardiogram. An angiogram revealed segmental occlusions of the distal vessels in her arms and legs. The patient denied chest pain, syncope, dyspnea on exertion, or fever. She reported a >30 pack-year history of cigarette smoking.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Thromboangiitis obliterans
Thromboangiitis obliterans (TAO), or Buerger’s disease, is a rare nonatherosclerotic disease that affects the medium and small arteries. The disease has a male predominance, primarily occurs in those younger than 45 years of age, and is most common in people from the Middle and Far East.1 Its distinctive features include ulcerations of the distal extremities and symptoms of claudication and pain at rest. More than 40% of affected patients develop Raynaud’s phenomenon.1 Superficial thrombophlebitis in the form of painful nodules has also been described.2
The etiology of TAO is likely due to disordered inflammation of endothelial cells, which has a strong association with smoking.3 The exact pathogenesis is unknown, but genetics and autoimmunity are suspected contributing factors.
The diagnosis is based on exclusion of other causes
The differential diagnosis includes diabetic angiopathy, embolic disease, atherosclerosis, hypercoagulability/thrombophilia, vasculitis or connective tissue diseases, and drug-associated (eg, cocaine) vasculitis.4
The diagnosis of TAO is based on the exclusion of other causes, although several diagnostic criteria have been proposed, including:
- age <45 years
- current or recent history of tobacco use
- distal extremity involvement (ulcers, claudication, or pain at rest)
- exclusion of diabetes, peripheral artery disease, thrombophilia, or embolic disease
- typical arteriographic findings on imaging, including distal small to medium vessel involvement, segmental occlusions, and “corkscrew-shaped” collaterals.1,2,5,6
Continue to: Lab tests
Lab tests. There are no specific laboratory markers for TAO. The initial evaluation should include an erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete metabolic panel (CMP), and urinalysis (UA). Tests to exclude other autoimmune diseases include rheumatoid factor, antinuclear antibody, anticentromere antibody and Scl-70 to exclude CREST syndrome and scleroderma, antiphospholipid antibodies to exclude disorders of hypercoagulability, and drug testing and history-taking to evaluate for drug-related (eg, cocaine) etiologies. Further studies should be performed based on clinical suspicion.
Imaging. Patients with suspected TAO should undergo an arteriogram of the affected extremities and large arteries. Other imaging modalities include computed tomographic angiography and magnetic resonance angiography. Biopsy is rarely indicated, unless there are atypical findings, such as large artery involvement or arterial nodules. Interestingly, a positive Allen test in a young smoker can be highly suggestive of TAO.1 (For a demonstration of the Allen test, see https://www.youtube.com/watch?v=D1tJO0RW9UM.)
Our patient tested negative for rheumatoid arthritis, CREST, and scleroderma and had a normal UA and CMP. She did have a slightly elevated anticardiolipin antibody test, but a negative lupus anticoagulant test, the significance of which is uncertain. Her CRP and ESR were elevated.
Complete smoking cessation is essential for treatment
Several treatments have been proposed, including prostanoids and surgery (surgical revascularization or endovascular therapy).1,4 In severe cases, amputation may be required to remove the affected extremity. However, the most important and most effective treatment for TAO is smoking cessation.1 Of note, several case reports have found that replacing smoking with other nicotine-containing products (eg, chewing tobacco) may not prevent limb loss.7-9
Our patient was tapered off prednisone and was continued on amlodipine 5 mg/d for vasospasm. She was started on varenicline 0.5 mg/d, which was increased to twice daily by Day 4 to aid with smoking cessation. Two months later, the patient’s pain and ulcerations had almost completely resolved (FIGURE 2). She experienced occasional relapses with smoking, during which her ulcerations and Raynaud’s would return. This case reinforces the age-old aphorism of “no tobacco, no Buerger’s disease.”4

CORRESPONDENCE
Seth Mathern, MD, 14300 Orchard Parkway, Westminster, CO 80023; [email protected].
1. Olin JW. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864-869.
2. Piazza G, Creager MA. Thromboangiitis obliterans. Circulation. 2010;121:1858-1861.
3. Azizi M, Boutouyrie P, Bura-Rivière A, et al. Thromboangiitis obliterans and endothelial function. Eur J Clin Invest. 2010;40:518-526.
4. Klein-Weigel PF, Richter JG. Thromboangiitis obliterans (Buerger’s disease). Vasa. 2014;43:337-346.
5. Papa MZ, Rabi I, Adar R. A point scoring system for the clinical diagnosis of Buerger’s disease. Eur J Vasc Endovasc Surg. 1996;11:335-339.
6. Mills JL, Porter JM. Buerger’s disease: a review and update. Semin Vasc Surg. 1993;6:14-23.
7. Lie JT. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1988;31:812-813.
8. O’Dell JR, Linder J, Markin RS, et al. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1987;30:1054-1056.
9. Lawrence PF, Lund OI, Jimenez JC, et al. Substitution of smokeless tobacco for cigarettes in Buerger’s disease does not prevent limb loss. J Vasc Surg. 2008;48:210-212.
1. Olin JW. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864-869.
2. Piazza G, Creager MA. Thromboangiitis obliterans. Circulation. 2010;121:1858-1861.
3. Azizi M, Boutouyrie P, Bura-Rivière A, et al. Thromboangiitis obliterans and endothelial function. Eur J Clin Invest. 2010;40:518-526.
4. Klein-Weigel PF, Richter JG. Thromboangiitis obliterans (Buerger’s disease). Vasa. 2014;43:337-346.
5. Papa MZ, Rabi I, Adar R. A point scoring system for the clinical diagnosis of Buerger’s disease. Eur J Vasc Endovasc Surg. 1996;11:335-339.
6. Mills JL, Porter JM. Buerger’s disease: a review and update. Semin Vasc Surg. 1993;6:14-23.
7. Lie JT. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1988;31:812-813.
8. O’Dell JR, Linder J, Markin RS, et al. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1987;30:1054-1056.
9. Lawrence PF, Lund OI, Jimenez JC, et al. Substitution of smokeless tobacco for cigarettes in Buerger’s disease does not prevent limb loss. J Vasc Surg. 2008;48:210-212.
Time to switch to nonsterile gloves for these procedures?
ILLUSTRATIVE CASE
Your practice manager comes to you to discuss ways that you can reduce expenses. He asks whether the practice could reduce the amount of money spent on gloves for procedures. How do you reply?
A decision involving a small difference, spread over a larger number of events, can have a sizable effect. An example is whether to use sterile vs nonsterile gloves for minor procedures. The cost difference between a box of sterile gloves and a box of nonsterile gloves is relatively small, and certainly worth the difference if the more expensive sterile gloves reduce the number of surgical site infections (SSIs).
However, if there is no difference in the number of SSIs, there may be no value to the extra cost, which, given the number of such procedures, becomes a large unnecessary expense. The choice to use sterile gloves often stems from habit, product availability, or the perceived benefit of fewer SSIs.2 While some evidence exists comparing glove choice, there is wide variability in physicians’ choice of gloves.3-5 This large systematic review compared rates of SSIs using sterile vs nonsterile gloves.
STUDY SUMMARY
RCTs/observational studies find sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 randomized controlled trials (RCTs) and observational (prospective or retrospective) studies compared infection rates using sterile vs nonsterile gloves in 11,071 unique patients undergoing cutaneous surgery, including Mohs microsurgery or outpatient dental procedures. The methods used in the review followed the Cochrane collaboration guidelines.6 The inclusion criteria were that the studies had to be either RCTs or observational studies. Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. The authors of the analysis reviewed a total of 512 publications for inclusion; of these, 14 met the inclusion criteria. One study was later removed due to incomplete data, leaving a total of 13 trials for the analysis. Of the 11,071 patients included in the final analysis, 1360 patients were randomly assigned to treatment with sterile gloves, while 1381 patients were assigned to treatment with nonsterile gloves as the intervention in a clinical trial. The remaining patients participated in either prospective or retrospective observational trials; 4680 patients were treated with sterile gloves, and 3650 patients were treated with nonsterile gloves. Heterogeneity was low for the included studies. Of note, the researchers performed a subgroup analysis on 9 total studies (4 RCTs and 5 observational studies) involving cutaneous surgeries only. These represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile vs nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR]=1.06; 95% confidence interval [CI], 0.81-1.39). There was also no difference in infection rates in the subgroup analysis of 9 trials limited to cutaneous surgery (2.2% vs 2.2%, respectively; RR=1.02; 95% CI, 0.78-1.34) or when the analysis was limited to only RCTs.
[polldaddy:10063798]
Continue to: WHAT'S NEW
WHAT’S NEW
Highest-quality evidence shows no difference in SSIs
This systematic review found no difference in SSI rates when using sterile vs nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
CAVEATS
A risk of bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk of bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Physicians may worry about potential medicolegal ramifications from this change.1 Lastly, some physicians may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204:976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21:148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. Available at: http://methods.cochrane.org/. Accessed July 15, 2018.
ILLUSTRATIVE CASE
Your practice manager comes to you to discuss ways that you can reduce expenses. He asks whether the practice could reduce the amount of money spent on gloves for procedures. How do you reply?
A decision involving a small difference, spread over a larger number of events, can have a sizable effect. An example is whether to use sterile vs nonsterile gloves for minor procedures. The cost difference between a box of sterile gloves and a box of nonsterile gloves is relatively small, and certainly worth the difference if the more expensive sterile gloves reduce the number of surgical site infections (SSIs).
However, if there is no difference in the number of SSIs, there may be no value to the extra cost, which, given the number of such procedures, becomes a large unnecessary expense. The choice to use sterile gloves often stems from habit, product availability, or the perceived benefit of fewer SSIs.2 While some evidence exists comparing glove choice, there is wide variability in physicians’ choice of gloves.3-5 This large systematic review compared rates of SSIs using sterile vs nonsterile gloves.
STUDY SUMMARY
RCTs/observational studies find sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 randomized controlled trials (RCTs) and observational (prospective or retrospective) studies compared infection rates using sterile vs nonsterile gloves in 11,071 unique patients undergoing cutaneous surgery, including Mohs microsurgery or outpatient dental procedures. The methods used in the review followed the Cochrane collaboration guidelines.6 The inclusion criteria were that the studies had to be either RCTs or observational studies. Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. The authors of the analysis reviewed a total of 512 publications for inclusion; of these, 14 met the inclusion criteria. One study was later removed due to incomplete data, leaving a total of 13 trials for the analysis. Of the 11,071 patients included in the final analysis, 1360 patients were randomly assigned to treatment with sterile gloves, while 1381 patients were assigned to treatment with nonsterile gloves as the intervention in a clinical trial. The remaining patients participated in either prospective or retrospective observational trials; 4680 patients were treated with sterile gloves, and 3650 patients were treated with nonsterile gloves. Heterogeneity was low for the included studies. Of note, the researchers performed a subgroup analysis on 9 total studies (4 RCTs and 5 observational studies) involving cutaneous surgeries only. These represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile vs nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR]=1.06; 95% confidence interval [CI], 0.81-1.39). There was also no difference in infection rates in the subgroup analysis of 9 trials limited to cutaneous surgery (2.2% vs 2.2%, respectively; RR=1.02; 95% CI, 0.78-1.34) or when the analysis was limited to only RCTs.
[polldaddy:10063798]
Continue to: WHAT'S NEW
WHAT’S NEW
Highest-quality evidence shows no difference in SSIs
This systematic review found no difference in SSI rates when using sterile vs nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
CAVEATS
A risk of bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk of bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Physicians may worry about potential medicolegal ramifications from this change.1 Lastly, some physicians may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Your practice manager comes to you to discuss ways that you can reduce expenses. He asks whether the practice could reduce the amount of money spent on gloves for procedures. How do you reply?
A decision involving a small difference, spread over a larger number of events, can have a sizable effect. An example is whether to use sterile vs nonsterile gloves for minor procedures. The cost difference between a box of sterile gloves and a box of nonsterile gloves is relatively small, and certainly worth the difference if the more expensive sterile gloves reduce the number of surgical site infections (SSIs).
However, if there is no difference in the number of SSIs, there may be no value to the extra cost, which, given the number of such procedures, becomes a large unnecessary expense. The choice to use sterile gloves often stems from habit, product availability, or the perceived benefit of fewer SSIs.2 While some evidence exists comparing glove choice, there is wide variability in physicians’ choice of gloves.3-5 This large systematic review compared rates of SSIs using sterile vs nonsterile gloves.
STUDY SUMMARY
RCTs/observational studies find sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 randomized controlled trials (RCTs) and observational (prospective or retrospective) studies compared infection rates using sterile vs nonsterile gloves in 11,071 unique patients undergoing cutaneous surgery, including Mohs microsurgery or outpatient dental procedures. The methods used in the review followed the Cochrane collaboration guidelines.6 The inclusion criteria were that the studies had to be either RCTs or observational studies. Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. The authors of the analysis reviewed a total of 512 publications for inclusion; of these, 14 met the inclusion criteria. One study was later removed due to incomplete data, leaving a total of 13 trials for the analysis. Of the 11,071 patients included in the final analysis, 1360 patients were randomly assigned to treatment with sterile gloves, while 1381 patients were assigned to treatment with nonsterile gloves as the intervention in a clinical trial. The remaining patients participated in either prospective or retrospective observational trials; 4680 patients were treated with sterile gloves, and 3650 patients were treated with nonsterile gloves. Heterogeneity was low for the included studies. Of note, the researchers performed a subgroup analysis on 9 total studies (4 RCTs and 5 observational studies) involving cutaneous surgeries only. These represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile vs nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR]=1.06; 95% confidence interval [CI], 0.81-1.39). There was also no difference in infection rates in the subgroup analysis of 9 trials limited to cutaneous surgery (2.2% vs 2.2%, respectively; RR=1.02; 95% CI, 0.78-1.34) or when the analysis was limited to only RCTs.
[polldaddy:10063798]
Continue to: WHAT'S NEW
WHAT’S NEW
Highest-quality evidence shows no difference in SSIs
This systematic review found no difference in SSI rates when using sterile vs nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
CAVEATS
A risk of bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk of bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Physicians may worry about potential medicolegal ramifications from this change.1 Lastly, some physicians may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204:976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21:148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. Available at: http://methods.cochrane.org/. Accessed July 15, 2018.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204:976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21:148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. Available at: http://methods.cochrane.org/. Accessed July 15, 2018.
PRACTICE CHANGER
Using nonsterile gloves for common primary care skin procedures causes no more infections than using sterile gloves.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 13 randomized controlled trials.
Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
Anterolateral hip pain • no specific injury • Dx?
THE CASE
A 22-year-old man presented to our family medicine clinic with hip pain of 2 weeks’ duration. The patient played hockey around the time of onset, but denied any specific injury. The pain, which affected the anterolateral aspect of the patient’s right hip, first started when he stood up after eating a meal. He rated the pain as an 8/10 on average and said that it was worse with movement. The patient had not shown improvement with conservative therapy (rest, ice, and ibuprofen). His medical and surgi
The physical exam revealed pain on active flexion and abduction of the hip. Passive range of motion (ROM) was negative for pain. The right hip was grossly normal with no pain on palpation or crepitus. There was no associated muscle tenderness. The patient was advised to continue to rest and ice the hip, as well as to take ibuprofen for pain relief. He was referred to Physical Therapy.
He returned to our clinic 4 weeks later with no improvement in his symptoms despite several sessions of physical therapy. We ordered radiographic images and magnetic resonance imaging (MRI) of the right hip.
THE DIAGNOSIS
Plain films (FIGURE 1A) showed bilateral avascular necrosis (AVN) of the femoral heads, which was worse on the right side than the left. An MRI (FIGURE 1B) further supported this diagnosis, revealing changes in the femoral neck consistent with a stress reaction and no significant collapse of the femoral head.
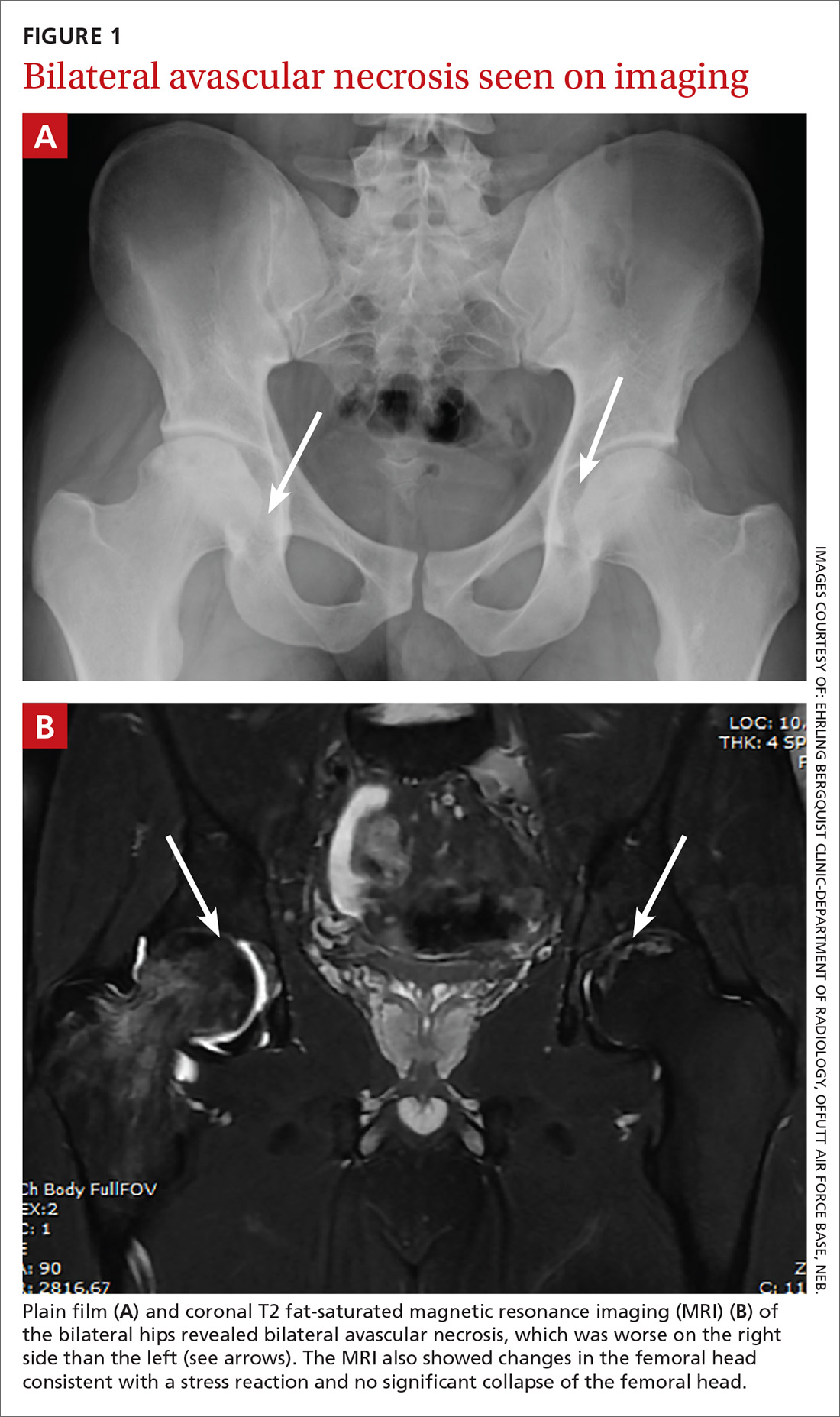
DISCUSSION
AVN of the hip has an incidence ranging from 10,000 to 20,000 new cases annually.1,2 It has many possible causes, including trauma, systemic lupus erythematosus, glucocorticoid use, and chronic excessive alcohol use. Although the underlying pathophysiology varies, experts hypothesize that most cases are caused by a disruption of the blood supply, which leads to hyperemia and cortical destruction and collapse.1,2
Certain medications can cause AVN
A more thorough history-taking at this patient’s initial visit would have prompted imaging at that time and ensured that the standard of care was met. Upon further investigation at his follow-up appointment, it was discovered that he had been diagnosed with acute pre-B cell lymphoblastic leukemia (ALL) 2 years earlier and had undergone chemotherapy with cytarabine, vincristine, L-asparaginase, daunorubicin, methotrexate, and glucocorticoids. This discovery, along with the lack of symptom improvement, prompted the ordering of his imaging studies. Long-term glucocorticoid therapy is the second leading cause of AVN, following traumatic events.3 High daily dosages (>40 mg/d) and high cumulative dosages of glucocorticoids are associated with a significantly increased risk for AVN.4,5
The other chemotherapy agents with which our patient had been treated (cytarabine, vincristine, L-asparaginase, daunorubicin, and methotrexate) have no reported links to AVN. When mentioned in the literature, however, they are usually coupled with the use of dexamethasone or prednisone.
Continue to: One case report described a patient with...
One case report described a patient with acute promyelocytic leukemia who was treated with all-transretinoic acid, daunomycin, cytarabine, and a short course of dexamethasone, and was diagnosed with AVN 2 years after the cessation of chemotherapy.6 This demonstrates that steroid use does not need to be recent to have a contributory effect.
Did leukemic burden play a role?
We also considered whether the patient’s leukemic burden contributed to his osteonecrosis. Leukemia and its therapy regimens have been reported to cause cerebrovascular complications,7 so it would be logical to postulate that they might also pose a risk to the vasculature of the femoral head. One case report describes hip pain and AVN as the initial manifestation of chronic myeloid leukemia (CML).8 But CML is more often associated with a severely increased white blood cell (WBC) count than is ALL, and our patient’s WBC count was in the expected range for a patient in the maintenance phase of chemotherapy, making leukemic burden a less likely culprit.
Know your patient’s history
Our patient had received an initial dose of approximately 120 mg/d prednisone alone during the first 28 days of his induction therapy for ALL. In addition, he received dexamethasone maintenance therapy, which can accumulate to >140 mg/m2 over the course of therapy.9 This information was ultimately integral to his diagnosis and treatment.
Our patient was referred to Orthopedics. He underwent therapy with alendronate and did not require surgical intervention.
THE TAKEAWAY
This case illustrates the importance of obtaining a thorough medical history, including previous drug exposures, as a means to raise or lower one’s index of suspicion appropriately.
CORRESPONDENCE
Patrick Basile, 7124 Bristol Boulevard, Edina, MN 55435; [email protected].
1. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
2. Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons;1997:43-49.
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-190.
4. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford). 2011;50:2023-2028.
5. Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J. 2013;95-B:1708-1713.
6. Abhyankar D, Nair R, Menon H, et al. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk Lymphoma. 2000;37:635-637.
7. Muñiz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42:651-654.
8. Gupta D, Gaiha M, Siddaraju N, et al. Chronic myeloid leukemia presenting with avascular necrosis of femur head. J Assoc Physicians, India. 2003;51:214-215.
9. Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963.
THE CASE
A 22-year-old man presented to our family medicine clinic with hip pain of 2 weeks’ duration. The patient played hockey around the time of onset, but denied any specific injury. The pain, which affected the anterolateral aspect of the patient’s right hip, first started when he stood up after eating a meal. He rated the pain as an 8/10 on average and said that it was worse with movement. The patient had not shown improvement with conservative therapy (rest, ice, and ibuprofen). His medical and surgi
The physical exam revealed pain on active flexion and abduction of the hip. Passive range of motion (ROM) was negative for pain. The right hip was grossly normal with no pain on palpation or crepitus. There was no associated muscle tenderness. The patient was advised to continue to rest and ice the hip, as well as to take ibuprofen for pain relief. He was referred to Physical Therapy.
He returned to our clinic 4 weeks later with no improvement in his symptoms despite several sessions of physical therapy. We ordered radiographic images and magnetic resonance imaging (MRI) of the right hip.
THE DIAGNOSIS
Plain films (FIGURE 1A) showed bilateral avascular necrosis (AVN) of the femoral heads, which was worse on the right side than the left. An MRI (FIGURE 1B) further supported this diagnosis, revealing changes in the femoral neck consistent with a stress reaction and no significant collapse of the femoral head.

DISCUSSION
AVN of the hip has an incidence ranging from 10,000 to 20,000 new cases annually.1,2 It has many possible causes, including trauma, systemic lupus erythematosus, glucocorticoid use, and chronic excessive alcohol use. Although the underlying pathophysiology varies, experts hypothesize that most cases are caused by a disruption of the blood supply, which leads to hyperemia and cortical destruction and collapse.1,2
Certain medications can cause AVN
A more thorough history-taking at this patient’s initial visit would have prompted imaging at that time and ensured that the standard of care was met. Upon further investigation at his follow-up appointment, it was discovered that he had been diagnosed with acute pre-B cell lymphoblastic leukemia (ALL) 2 years earlier and had undergone chemotherapy with cytarabine, vincristine, L-asparaginase, daunorubicin, methotrexate, and glucocorticoids. This discovery, along with the lack of symptom improvement, prompted the ordering of his imaging studies. Long-term glucocorticoid therapy is the second leading cause of AVN, following traumatic events.3 High daily dosages (>40 mg/d) and high cumulative dosages of glucocorticoids are associated with a significantly increased risk for AVN.4,5
The other chemotherapy agents with which our patient had been treated (cytarabine, vincristine, L-asparaginase, daunorubicin, and methotrexate) have no reported links to AVN. When mentioned in the literature, however, they are usually coupled with the use of dexamethasone or prednisone.
Continue to: One case report described a patient with...
One case report described a patient with acute promyelocytic leukemia who was treated with all-transretinoic acid, daunomycin, cytarabine, and a short course of dexamethasone, and was diagnosed with AVN 2 years after the cessation of chemotherapy.6 This demonstrates that steroid use does not need to be recent to have a contributory effect.
Did leukemic burden play a role?
We also considered whether the patient’s leukemic burden contributed to his osteonecrosis. Leukemia and its therapy regimens have been reported to cause cerebrovascular complications,7 so it would be logical to postulate that they might also pose a risk to the vasculature of the femoral head. One case report describes hip pain and AVN as the initial manifestation of chronic myeloid leukemia (CML).8 But CML is more often associated with a severely increased white blood cell (WBC) count than is ALL, and our patient’s WBC count was in the expected range for a patient in the maintenance phase of chemotherapy, making leukemic burden a less likely culprit.
Know your patient’s history
Our patient had received an initial dose of approximately 120 mg/d prednisone alone during the first 28 days of his induction therapy for ALL. In addition, he received dexamethasone maintenance therapy, which can accumulate to >140 mg/m2 over the course of therapy.9 This information was ultimately integral to his diagnosis and treatment.
Our patient was referred to Orthopedics. He underwent therapy with alendronate and did not require surgical intervention.
THE TAKEAWAY
This case illustrates the importance of obtaining a thorough medical history, including previous drug exposures, as a means to raise or lower one’s index of suspicion appropriately.
CORRESPONDENCE
Patrick Basile, 7124 Bristol Boulevard, Edina, MN 55435; [email protected].
THE CASE
A 22-year-old man presented to our family medicine clinic with hip pain of 2 weeks’ duration. The patient played hockey around the time of onset, but denied any specific injury. The pain, which affected the anterolateral aspect of the patient’s right hip, first started when he stood up after eating a meal. He rated the pain as an 8/10 on average and said that it was worse with movement. The patient had not shown improvement with conservative therapy (rest, ice, and ibuprofen). His medical and surgi
The physical exam revealed pain on active flexion and abduction of the hip. Passive range of motion (ROM) was negative for pain. The right hip was grossly normal with no pain on palpation or crepitus. There was no associated muscle tenderness. The patient was advised to continue to rest and ice the hip, as well as to take ibuprofen for pain relief. He was referred to Physical Therapy.
He returned to our clinic 4 weeks later with no improvement in his symptoms despite several sessions of physical therapy. We ordered radiographic images and magnetic resonance imaging (MRI) of the right hip.
THE DIAGNOSIS
Plain films (FIGURE 1A) showed bilateral avascular necrosis (AVN) of the femoral heads, which was worse on the right side than the left. An MRI (FIGURE 1B) further supported this diagnosis, revealing changes in the femoral neck consistent with a stress reaction and no significant collapse of the femoral head.

DISCUSSION
AVN of the hip has an incidence ranging from 10,000 to 20,000 new cases annually.1,2 It has many possible causes, including trauma, systemic lupus erythematosus, glucocorticoid use, and chronic excessive alcohol use. Although the underlying pathophysiology varies, experts hypothesize that most cases are caused by a disruption of the blood supply, which leads to hyperemia and cortical destruction and collapse.1,2
Certain medications can cause AVN
A more thorough history-taking at this patient’s initial visit would have prompted imaging at that time and ensured that the standard of care was met. Upon further investigation at his follow-up appointment, it was discovered that he had been diagnosed with acute pre-B cell lymphoblastic leukemia (ALL) 2 years earlier and had undergone chemotherapy with cytarabine, vincristine, L-asparaginase, daunorubicin, methotrexate, and glucocorticoids. This discovery, along with the lack of symptom improvement, prompted the ordering of his imaging studies. Long-term glucocorticoid therapy is the second leading cause of AVN, following traumatic events.3 High daily dosages (>40 mg/d) and high cumulative dosages of glucocorticoids are associated with a significantly increased risk for AVN.4,5
The other chemotherapy agents with which our patient had been treated (cytarabine, vincristine, L-asparaginase, daunorubicin, and methotrexate) have no reported links to AVN. When mentioned in the literature, however, they are usually coupled with the use of dexamethasone or prednisone.
Continue to: One case report described a patient with...
One case report described a patient with acute promyelocytic leukemia who was treated with all-transretinoic acid, daunomycin, cytarabine, and a short course of dexamethasone, and was diagnosed with AVN 2 years after the cessation of chemotherapy.6 This demonstrates that steroid use does not need to be recent to have a contributory effect.
Did leukemic burden play a role?
We also considered whether the patient’s leukemic burden contributed to his osteonecrosis. Leukemia and its therapy regimens have been reported to cause cerebrovascular complications,7 so it would be logical to postulate that they might also pose a risk to the vasculature of the femoral head. One case report describes hip pain and AVN as the initial manifestation of chronic myeloid leukemia (CML).8 But CML is more often associated with a severely increased white blood cell (WBC) count than is ALL, and our patient’s WBC count was in the expected range for a patient in the maintenance phase of chemotherapy, making leukemic burden a less likely culprit.
Know your patient’s history
Our patient had received an initial dose of approximately 120 mg/d prednisone alone during the first 28 days of his induction therapy for ALL. In addition, he received dexamethasone maintenance therapy, which can accumulate to >140 mg/m2 over the course of therapy.9 This information was ultimately integral to his diagnosis and treatment.
Our patient was referred to Orthopedics. He underwent therapy with alendronate and did not require surgical intervention.
THE TAKEAWAY
This case illustrates the importance of obtaining a thorough medical history, including previous drug exposures, as a means to raise or lower one’s index of suspicion appropriately.
CORRESPONDENCE
Patrick Basile, 7124 Bristol Boulevard, Edina, MN 55435; [email protected].
1. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
2. Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons;1997:43-49.
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-190.
4. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford). 2011;50:2023-2028.
5. Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J. 2013;95-B:1708-1713.
6. Abhyankar D, Nair R, Menon H, et al. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk Lymphoma. 2000;37:635-637.
7. Muñiz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42:651-654.
8. Gupta D, Gaiha M, Siddaraju N, et al. Chronic myeloid leukemia presenting with avascular necrosis of femur head. J Assoc Physicians, India. 2003;51:214-215.
9. Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963.
1. Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7:250-261.
2. Vail TP, Covington DB. The incidence of osteonecrosis. In: Urbaniak JR, Jones JR, eds. Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons;1997:43-49.
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-190.
4. Shigemura T, Nakamura J, Kishida S, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology (Oxford). 2011;50:2023-2028.
5. Salem KH, Brockert AK, Mertens R, et al. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Joint J. 2013;95-B:1708-1713.
6. Abhyankar D, Nair R, Menon H, et al. Avascular necrosis of head of femur in a patient with acute promyelocytic leukemia. Leuk Lymphoma. 2000;37:635-637.
7. Muñiz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42:651-654.
8. Gupta D, Gaiha M, Siddaraju N, et al. Chronic myeloid leukemia presenting with avascular necrosis of femur head. J Assoc Physicians, India. 2003;51:214-215.
9. Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963.