User login
Pink Papules on the Cheek
The Diagnosis: Cutaneous Rosai-Dorfman Disease
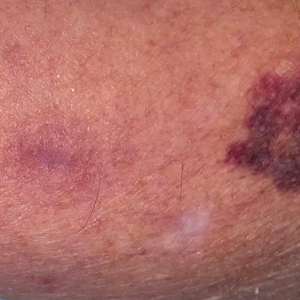
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
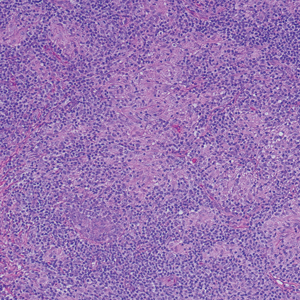
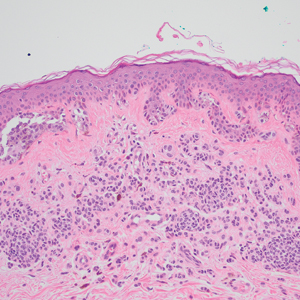
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
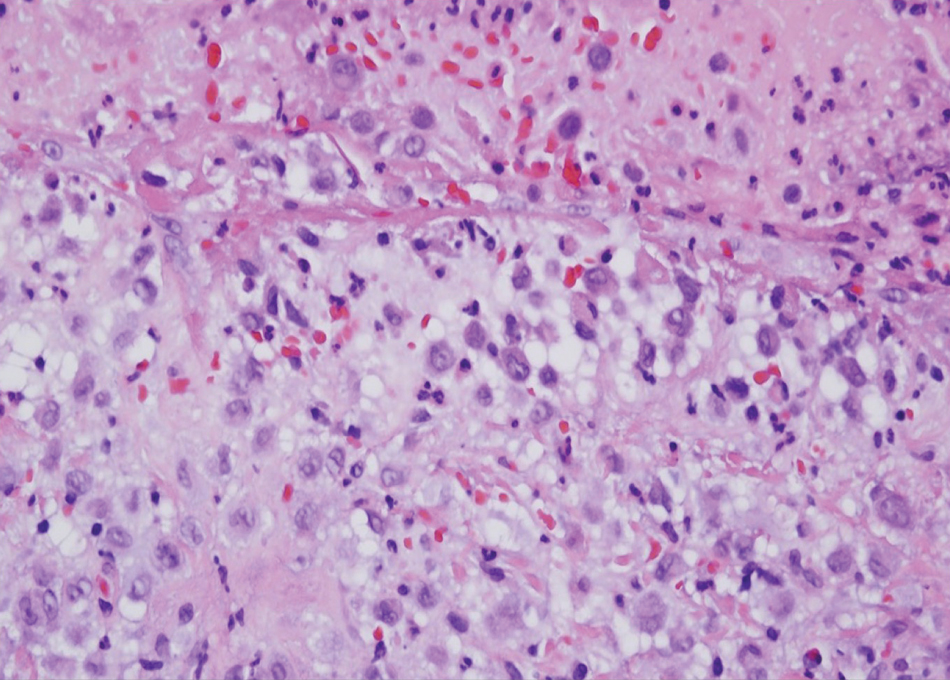
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7
A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9
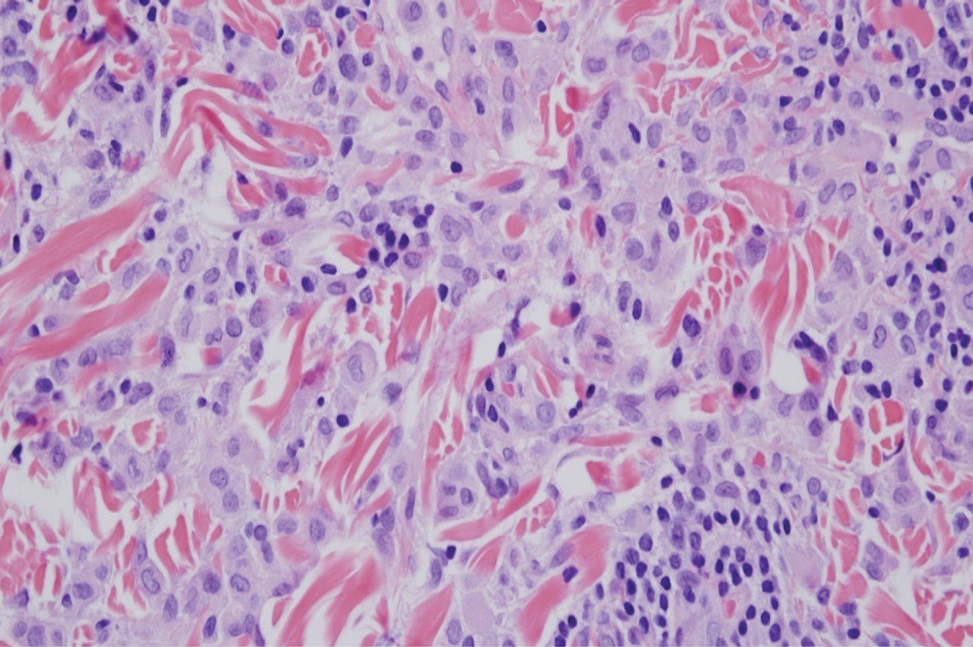
Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12
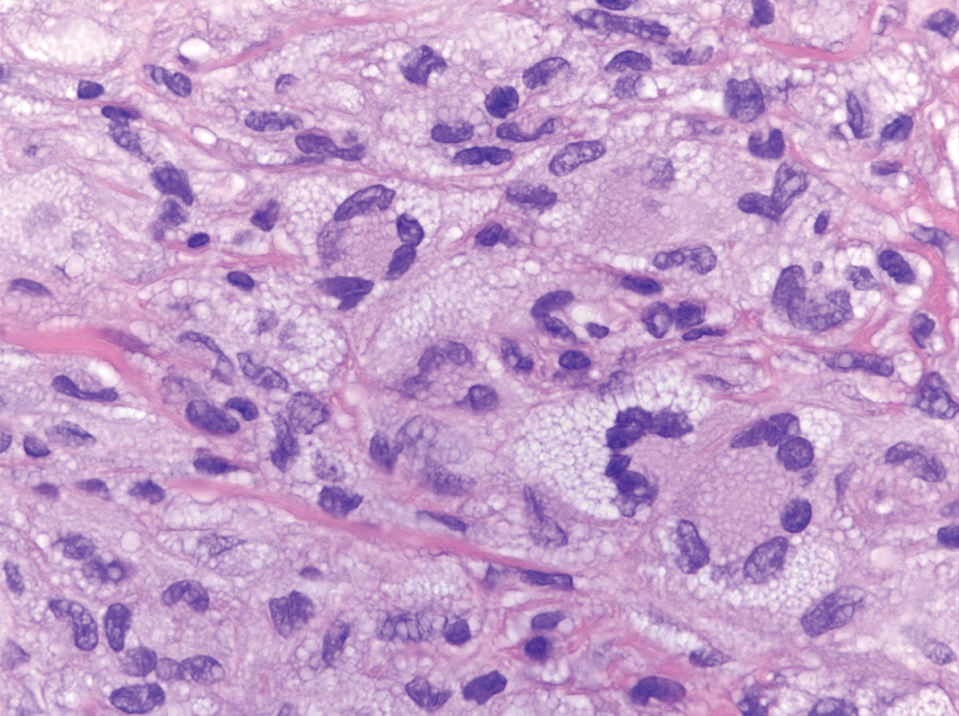
Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7

A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9

Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12

Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.

The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7

A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9

Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12

Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.

- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
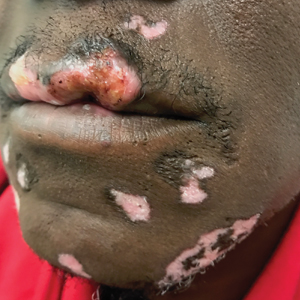
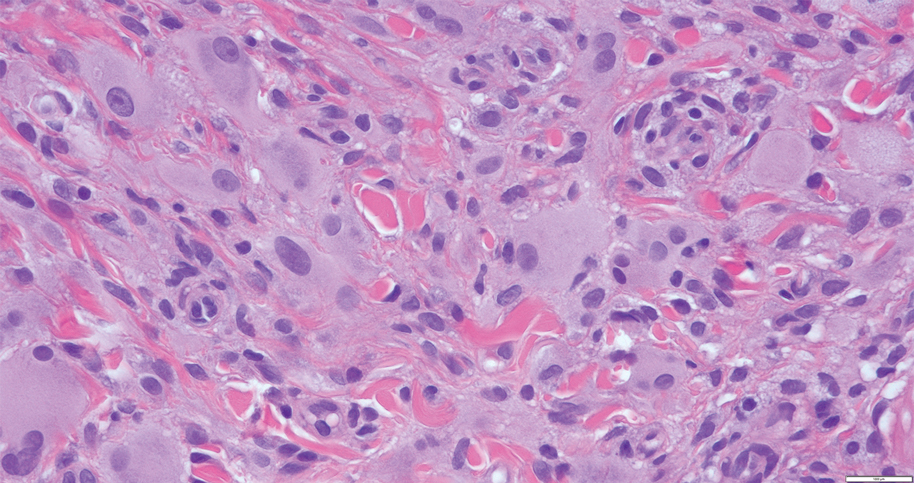
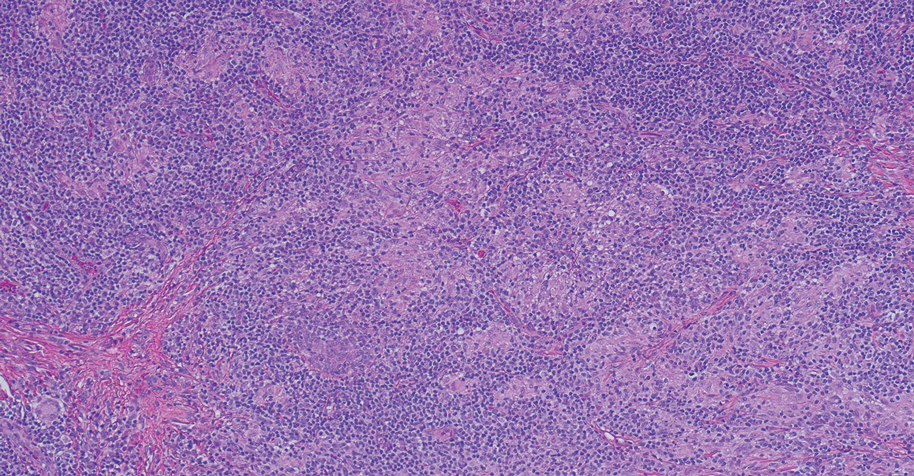
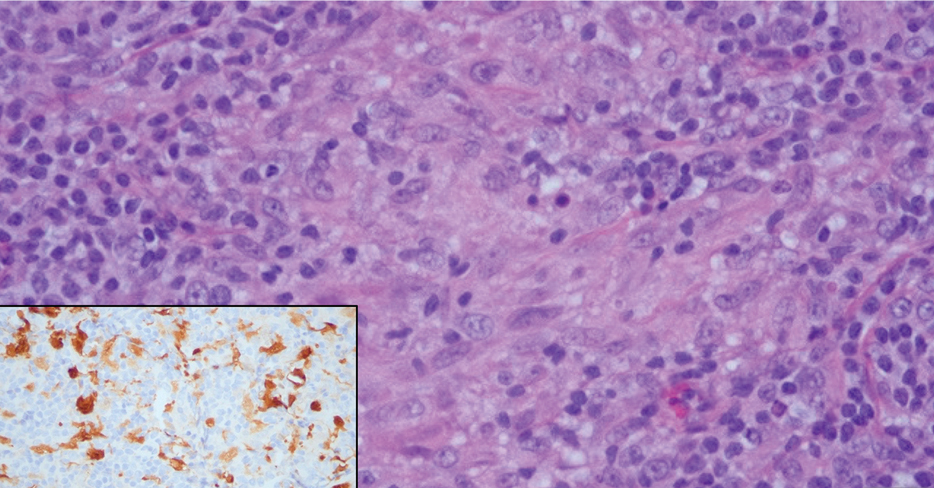
A 31-year-old woman presented with a slow-growing, tender, pruritic lesion on the right cheek of 4 to 5 months’ duration. She had been applying petroleum jelly and hydrocortisone cream 2.5% without any improvement. Physical examination revealed a 1×5-mm, pearly pink, erythematous, crusted papule with arborizing vessels surrounded by scattered pink papules with white dots within. No cervical lymphadenopathy was appreciated on physical examination, and the patient denied any other systemic symptoms. Shave and punch biopsies of the lesion were performed; stains for microorganisms were negative. The biopsy showed a dense reticular mixed inflammatory cell infiltrate comprised of a mixture of histiocytes (top), lymphocytes, neutrophils, and plasma cells that assumed a diffuse growth pattern within the dermis. The histiocytes exhibited abundant watery cytoplasms with ill-defined cytoplasmic membranes; intact leukocytes were found within the cytoplasms. The histiocytes demonstrated a unique phenotype characterized by S-100 (bottom) and CD68 positivity.
Androgenetic Alopecia: What Works?
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.
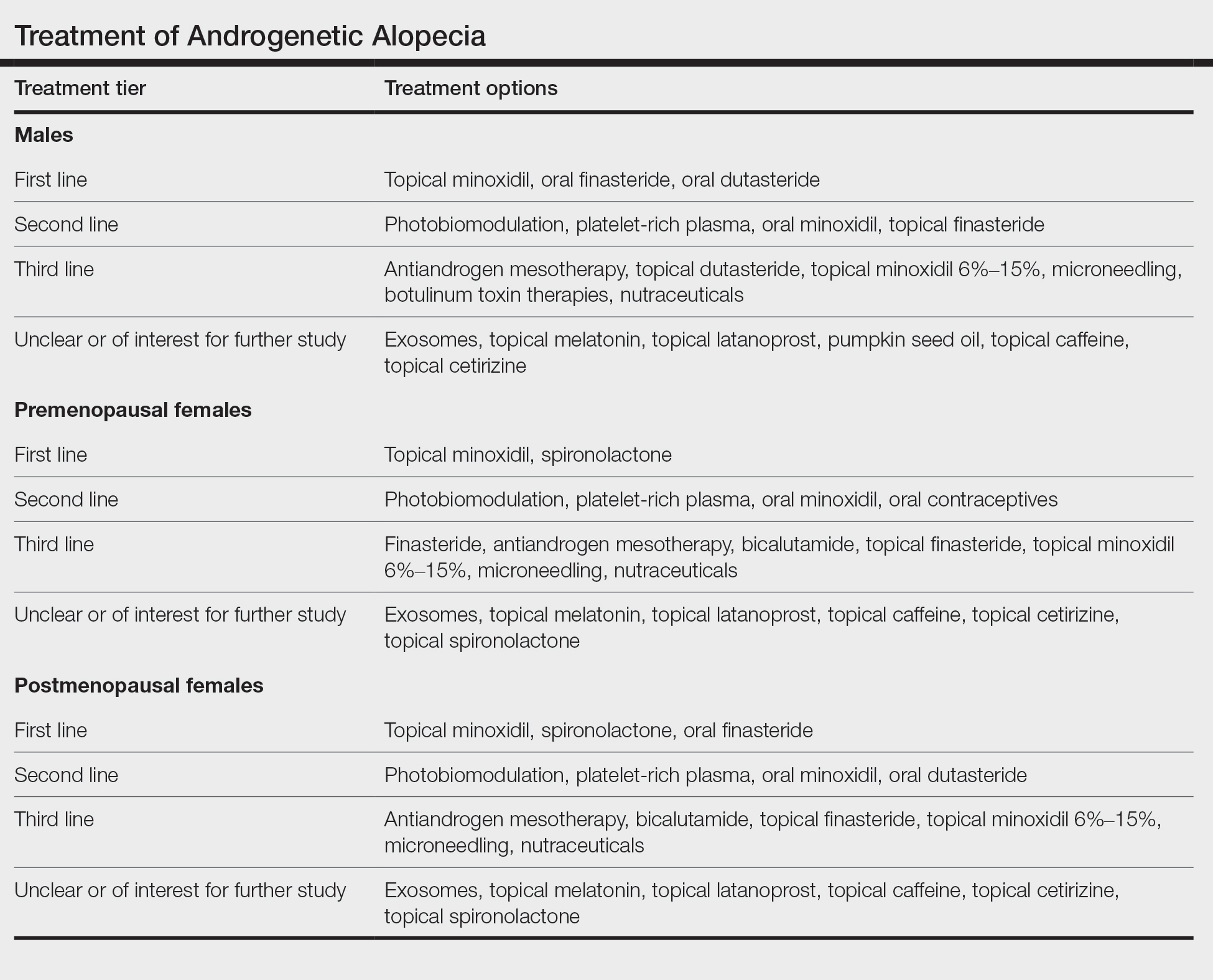
When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
The Struggle to Provide Gender-Affirming Care to Youth
Pediatrician Michelle Collins-Ogle, MD, already has a busy practice helping young people address questions about their gender identity. She has treated more than 230 patients over the past 2 years at Children’s Hospital at Montefiore in the Bronx, New York.
Dr. Collins-Ogle specializes in adolescent medicine in New York, a state without the restrictions on such care that have been enacted in roughly half the country.
On December 13, 2023, Ohio lawmakers passed a bill banning gender-affirming medical care to minors which Gov. Mike DeWine vetoed on December 29. Another 26 states have similar restrictions in place, according to a tally provided to this news organization by the Human Rights Campaign, which tracks this issue.
Clinicians like Dr. Collins-Ogle are feeling the impact. In her practice, Dr. Collins-Ogle met a couple that moved from Texas to New York to allow their child to access gender-affirming medical care.
“They wanted their child to be able to receive medical care, but they also were afraid for their own safety, of having their child taken from them, and being locked up,” Dr. Collins-Ogle told this news organization.
With patients have also come protestors and harassment. In fact, many physicians are reluctant to speak on this topic amid a recent spate of threats. Psychiatric News reported that conservative pundits and high-profile social media accounts have targeted physicians who provide gender-affirming medical care, spurring harassment campaigns against clinics in cities such as Akron, Boston, and Nashville. “The attackers asserted that the clinics were mutilating children and giving them ‘chemical castration drugs,’ among other claims,” the Psychiatric News reported.
This news organization contacted more than a half dozen organizations that provide gender-affirming care for adolescents and teens seeking interviews about the effects of these restrictions.
All but Montefiore’s Dr. Collins-Ogle turned down the request.
“If my kids are brave enough to come see me, I can’t cower,” Dr. Collins-Ogle said.
But Dr. Collins-Ogle emphasized she understands why many fellow physicians are concerned about speaking publicly about gender-affirming medical care.
Dissenters Spread Misinformation and Threats
Recent years have seen increasing politicization of this issue, often due to inaccurate depictions of gender-affirming medical care circulating on social media.
In 2022, the American Medical Association (AMA), the American Academy of Pediatrics (AAP), and the Children’s Hospital Association asked the Justice Department to investigate what they called “increasing threats of violence against physicians, hospitals, and families of children for providing and seeking evidence-based gender-affirming care.”
The three organizations also called on X (formerly known as Twitter), TikTok, and Meta, which owns Facebook and Instagram, to do more to address coordinated campaigns of disinformation.
“We cannot stand by as threats of violence against our members and their patients proliferate with little consequence,” said Moira Szilagyi, MD, PhD, then AAP president in a statement.
Medical Groups Defend Care to Prevent Suicide
The AAP, AMA, and other influential medical associations are banding together to fight new legal restrictions on gender-affirming medical care for teens and adolescents. (These briefs do not discuss surgeries typically available for adults.)
Since 2022, these medical organizations have filed amicus briefs in cases challenging new restrictions put in place in Arkansas, Alabama, Florida, Georgia, Idaho, Indiana, Kentucky, North Dakota, Oklahoma, Tennessee, and Texas.
Other signers to the amicus briefs:
- Academic Pediatric Association
- American Academy of Child & Adolescent Psychiatry
- American Academy of Family Physicians
- American Academy of Nursing
- GLMA: Health Professionals Advancing LGBTQ+ Equality
- American College of Obstetricians and Gynecologists
- American College of Osteopathic Pediatricians
- The American College of Physicians
- American Pediatric Society
- Association of Medical School Pediatric Department Chairs, Inc.
- Endocrine Society
- National Association of Pediatric Nurse Practitioners
- The Pediatric Endocrine Society, Societies for Pediatric Urology
- Society for Adolescent Health and Medicine
- Society for Pediatric Research
- The Society of Pediatric Nurses
- World Professional Association for Transgender Health
In these amicus briefs, the medical groups argue that evidence-based guidelines support the use of medication in treating gender dysphoria. The amicus briefs in particular cite an Endocrine Society guideline and the standards of care developed by the World Professional Association for Transgender Health (WPATH).
Research shows that adolescents with gender dysphoria who receive puberty blockers and other medications experience less depression, anxiety, and suicidal ideation, the groups have said.
“In light of this evidence supporting the connection between lack of access to gender-affirming care and lifetime suicide risk, banning such care can put patients’ lives at risk,” the AAP and other groups said.
Debate Over Source of Gender Identity Concerns
Having doubts and concerns about one’s gender remains a relatively rare phenomena, although it appears more common among younger people.
Among US adults, 0.5% or about 1.3 million people identify as transgender whereas about 1.4% or about 300,000 people in the 13-17–year-old group do so, according to a report issued in 2022 by the Williams Institute of the UCLA School of Law.
Questionable Diagnosis Drives Bans on Care
The term “rapid-onset gender dysphoria,” referring to young people who suddenly question their gender as part of peer group dynamics, persists in political debates. The conservative Heritage Foundation has used the term as well as “social contagion” in its effort to seek restrictions on gender-affirming care for young people.
Ohio Rep. Gary Click, a Republican, said at an April 2023 hearing that his Save Adolescents from Experimentation (SAFE) bill would prevent teens from being harmed due to “social contagion” or “ rapid-onset gender dysphoria.”
The bill, which the Ohio legislature cleared in December, would block physicians from starting new patients on puberty blockers. (It also bars surgeries as part of gender-affirming medical care, although hospital officials and physicians told lawmakers these are not done in Ohio.)
Among the groups opposing Click’s bill were the Ohio chapter of the AAP, the Ohio State Medical Association and several hospitals and hospital groups as well as physicians speaking independently.
Gender-Affirming Care ‘Buys Time’ to Avoid Impulsive Decisions
Kate Krueck, MD, a pediatrician with a practice in the Columbus area, testified about her experience as the mother of a transgender child who once attempted suicide.
“It wasn’t always easy to reconstruct my vision of a baby with a vagina into the adolescent before me with a new name and changed pronouns, but they were still the same incredible person,” Krueck said.
She urged lawmakers to understand how puberty blockers can “buy time” for teens to cope with a body at odds with their vision of themselves, noting that many of the effects of these medications are largely reversible. The side effects that are not reversible, such as facial hair growth and the growth of Adam’s Apple, are certainly outweighed by the risks of withholding treatment, she said.
Bad Patient Experience Drives Detractor Activist
Arguing against that point was Chloe Cole, a detransitioner activist who had returned to a female identity. At the Ohio legislative hearings, Ms. Cole spoke of her experience in California as a teen treated for gender dysphoria.
“I was fast-tracked by medical butchers starting at 13 when I was given cross sex hormones, and they took my breasts away from me at 15 years old,” she said.
Ms. Cole appears frequently to testify in favor of bans on gender-affirming medical care. In 2022, she told the Ohio lawmakers about her experience of attending a class with about a dozen other young people in the midst of female-to-male transitions. She now sees that class as having inadvertently helped reinforce her decision to have her breasts removed.
“Despite all these consultations and classes, I don’t feel like I understood all the ramifications that came with any of the medical decisions I was making,” Ms. Cole said. “I didn’t realize how traumatic the recovery would be, and it wasn’t until I was almost a year post-op that I realized I may want to breastfeed my future children; I will never be able to do that.”
Ms. Cole also spoke in July before the US House subcommittee on the Constitution and Limited Government.
“I look in the mirror sometimes, and I feel like a monster,” Ms. Cole said at the House hearing, which was titled “ The Dangers and Due Process Violations of ‘Gender-Affirming Care’.”
During the hearing, Shannon Minter, legal director of the National Center for Lesbian Rights (NCLR), who also made a gender transition, thanked Ms. Cole but noted that her case is an exception.
A 2022 Lancet Child and Adolescent Health article reported that 704 (98%) people in the Netherlands who had started gender-affirming medical treatment in adolescence continued to use gender-affirming hormones at follow-up. Ms. Minter credits this high rate of continuation to clinicians taking their duties to adolescents seriously.
State legislatures and medical boards oversee the regulation of medical practice in the US. But a few Republicans in both chambers of the US Congress have shown an interest in enacting a federal ban restricting physicians’ ability to provide gender-affirming medical care.
They include Rep. Mike Johnson of Louisiana, who in October 2023 became Speaker of the House. He chaired the July hearing at which Ms. Cole spoke. He’s also a sponsor of a House bill introduced by Rep. Marjorie Taylor Greene (R-GA).
This measure, which has the support of 45 House Republicans, would make it a felony to perform any gender-affirming care on a minor, and it permits a minor on whom such care is performed to bring a civil action against each individual who provided the care. Sen. JD Vance (R-OH) introduced the companion Senate measure.
Reality of Gender-Affirming Care
The drive to pass laws like those in Ohio and Arkansas stem from a lack of knowledge about gender-affirming treatments, including a false idea that doctors prescribe medications at teens’ requests, Montefiore’s Dr. Collins-Ogle said.
“There’s a misperception that young people will say ‘I’m transgender’ and that those of us who provide care are just giving them hormones or whatever they want. It’s not true, and it doesn’t happen that way,” Dr. Collins-Ogle said.
At the Children’s Hospital at Montefiore, Dr. Collins-Ogle said her work with patients wrestling with gender identity issues begins with questions.
“What’s your understanding of dysphoria? Where’s the incongruence between the gender you were assigned at birth and what you’re feeling now? You have to be able to verbalize that” before the treatment proceeds, she said.
Sometimes teens leave after an initial conversation and then return later when they have a more clearly defined sense of what dysphoria means.
“There are other kids who clearly, clearly understand that the gender they were assigned at birth is not who they are,” she said.
Children now wrestle with added concerns that their parents could be put at risk for trying to help them, she said.
“These kids go through so much. And we have these people in powerful positions telling them that they don’t matter and telling them, ‘We’re going to cut off your access to healthcare, Medicaid; if your parents tried to seek out this care for you, we’re going to put them in jail,’” she said.
“It’s the biggest factor in fear mongering,” she said.
Dr. Collins-Ogle said she wonders why legislators who lack medical training are trying to dictate how physicians can practice.
“I took a Hippocratic oath to do no harm. I have a medical board that I answer to,” she said. “I don’t understand how legislators can get away with legislating about something they know nothing about.”
A version of this article appeared on Medscape.com.
Pediatrician Michelle Collins-Ogle, MD, already has a busy practice helping young people address questions about their gender identity. She has treated more than 230 patients over the past 2 years at Children’s Hospital at Montefiore in the Bronx, New York.
Dr. Collins-Ogle specializes in adolescent medicine in New York, a state without the restrictions on such care that have been enacted in roughly half the country.
On December 13, 2023, Ohio lawmakers passed a bill banning gender-affirming medical care to minors which Gov. Mike DeWine vetoed on December 29. Another 26 states have similar restrictions in place, according to a tally provided to this news organization by the Human Rights Campaign, which tracks this issue.
Clinicians like Dr. Collins-Ogle are feeling the impact. In her practice, Dr. Collins-Ogle met a couple that moved from Texas to New York to allow their child to access gender-affirming medical care.
“They wanted their child to be able to receive medical care, but they also were afraid for their own safety, of having their child taken from them, and being locked up,” Dr. Collins-Ogle told this news organization.
With patients have also come protestors and harassment. In fact, many physicians are reluctant to speak on this topic amid a recent spate of threats. Psychiatric News reported that conservative pundits and high-profile social media accounts have targeted physicians who provide gender-affirming medical care, spurring harassment campaigns against clinics in cities such as Akron, Boston, and Nashville. “The attackers asserted that the clinics were mutilating children and giving them ‘chemical castration drugs,’ among other claims,” the Psychiatric News reported.
This news organization contacted more than a half dozen organizations that provide gender-affirming care for adolescents and teens seeking interviews about the effects of these restrictions.
All but Montefiore’s Dr. Collins-Ogle turned down the request.
“If my kids are brave enough to come see me, I can’t cower,” Dr. Collins-Ogle said.
But Dr. Collins-Ogle emphasized she understands why many fellow physicians are concerned about speaking publicly about gender-affirming medical care.
Dissenters Spread Misinformation and Threats
Recent years have seen increasing politicization of this issue, often due to inaccurate depictions of gender-affirming medical care circulating on social media.
In 2022, the American Medical Association (AMA), the American Academy of Pediatrics (AAP), and the Children’s Hospital Association asked the Justice Department to investigate what they called “increasing threats of violence against physicians, hospitals, and families of children for providing and seeking evidence-based gender-affirming care.”
The three organizations also called on X (formerly known as Twitter), TikTok, and Meta, which owns Facebook and Instagram, to do more to address coordinated campaigns of disinformation.
“We cannot stand by as threats of violence against our members and their patients proliferate with little consequence,” said Moira Szilagyi, MD, PhD, then AAP president in a statement.
Medical Groups Defend Care to Prevent Suicide
The AAP, AMA, and other influential medical associations are banding together to fight new legal restrictions on gender-affirming medical care for teens and adolescents. (These briefs do not discuss surgeries typically available for adults.)
Since 2022, these medical organizations have filed amicus briefs in cases challenging new restrictions put in place in Arkansas, Alabama, Florida, Georgia, Idaho, Indiana, Kentucky, North Dakota, Oklahoma, Tennessee, and Texas.
Other signers to the amicus briefs:
- Academic Pediatric Association
- American Academy of Child & Adolescent Psychiatry
- American Academy of Family Physicians
- American Academy of Nursing
- GLMA: Health Professionals Advancing LGBTQ+ Equality
- American College of Obstetricians and Gynecologists
- American College of Osteopathic Pediatricians
- The American College of Physicians
- American Pediatric Society
- Association of Medical School Pediatric Department Chairs, Inc.
- Endocrine Society
- National Association of Pediatric Nurse Practitioners
- The Pediatric Endocrine Society, Societies for Pediatric Urology
- Society for Adolescent Health and Medicine
- Society for Pediatric Research
- The Society of Pediatric Nurses
- World Professional Association for Transgender Health
In these amicus briefs, the medical groups argue that evidence-based guidelines support the use of medication in treating gender dysphoria. The amicus briefs in particular cite an Endocrine Society guideline and the standards of care developed by the World Professional Association for Transgender Health (WPATH).
Research shows that adolescents with gender dysphoria who receive puberty blockers and other medications experience less depression, anxiety, and suicidal ideation, the groups have said.
“In light of this evidence supporting the connection between lack of access to gender-affirming care and lifetime suicide risk, banning such care can put patients’ lives at risk,” the AAP and other groups said.
Debate Over Source of Gender Identity Concerns
Having doubts and concerns about one’s gender remains a relatively rare phenomena, although it appears more common among younger people.
Among US adults, 0.5% or about 1.3 million people identify as transgender whereas about 1.4% or about 300,000 people in the 13-17–year-old group do so, according to a report issued in 2022 by the Williams Institute of the UCLA School of Law.
Questionable Diagnosis Drives Bans on Care
The term “rapid-onset gender dysphoria,” referring to young people who suddenly question their gender as part of peer group dynamics, persists in political debates. The conservative Heritage Foundation has used the term as well as “social contagion” in its effort to seek restrictions on gender-affirming care for young people.
Ohio Rep. Gary Click, a Republican, said at an April 2023 hearing that his Save Adolescents from Experimentation (SAFE) bill would prevent teens from being harmed due to “social contagion” or “ rapid-onset gender dysphoria.”
The bill, which the Ohio legislature cleared in December, would block physicians from starting new patients on puberty blockers. (It also bars surgeries as part of gender-affirming medical care, although hospital officials and physicians told lawmakers these are not done in Ohio.)
Among the groups opposing Click’s bill were the Ohio chapter of the AAP, the Ohio State Medical Association and several hospitals and hospital groups as well as physicians speaking independently.
Gender-Affirming Care ‘Buys Time’ to Avoid Impulsive Decisions
Kate Krueck, MD, a pediatrician with a practice in the Columbus area, testified about her experience as the mother of a transgender child who once attempted suicide.
“It wasn’t always easy to reconstruct my vision of a baby with a vagina into the adolescent before me with a new name and changed pronouns, but they were still the same incredible person,” Krueck said.
She urged lawmakers to understand how puberty blockers can “buy time” for teens to cope with a body at odds with their vision of themselves, noting that many of the effects of these medications are largely reversible. The side effects that are not reversible, such as facial hair growth and the growth of Adam’s Apple, are certainly outweighed by the risks of withholding treatment, she said.
Bad Patient Experience Drives Detractor Activist
Arguing against that point was Chloe Cole, a detransitioner activist who had returned to a female identity. At the Ohio legislative hearings, Ms. Cole spoke of her experience in California as a teen treated for gender dysphoria.
“I was fast-tracked by medical butchers starting at 13 when I was given cross sex hormones, and they took my breasts away from me at 15 years old,” she said.
Ms. Cole appears frequently to testify in favor of bans on gender-affirming medical care. In 2022, she told the Ohio lawmakers about her experience of attending a class with about a dozen other young people in the midst of female-to-male transitions. She now sees that class as having inadvertently helped reinforce her decision to have her breasts removed.
“Despite all these consultations and classes, I don’t feel like I understood all the ramifications that came with any of the medical decisions I was making,” Ms. Cole said. “I didn’t realize how traumatic the recovery would be, and it wasn’t until I was almost a year post-op that I realized I may want to breastfeed my future children; I will never be able to do that.”
Ms. Cole also spoke in July before the US House subcommittee on the Constitution and Limited Government.
“I look in the mirror sometimes, and I feel like a monster,” Ms. Cole said at the House hearing, which was titled “ The Dangers and Due Process Violations of ‘Gender-Affirming Care’.”
During the hearing, Shannon Minter, legal director of the National Center for Lesbian Rights (NCLR), who also made a gender transition, thanked Ms. Cole but noted that her case is an exception.
A 2022 Lancet Child and Adolescent Health article reported that 704 (98%) people in the Netherlands who had started gender-affirming medical treatment in adolescence continued to use gender-affirming hormones at follow-up. Ms. Minter credits this high rate of continuation to clinicians taking their duties to adolescents seriously.
State legislatures and medical boards oversee the regulation of medical practice in the US. But a few Republicans in both chambers of the US Congress have shown an interest in enacting a federal ban restricting physicians’ ability to provide gender-affirming medical care.
They include Rep. Mike Johnson of Louisiana, who in October 2023 became Speaker of the House. He chaired the July hearing at which Ms. Cole spoke. He’s also a sponsor of a House bill introduced by Rep. Marjorie Taylor Greene (R-GA).
This measure, which has the support of 45 House Republicans, would make it a felony to perform any gender-affirming care on a minor, and it permits a minor on whom such care is performed to bring a civil action against each individual who provided the care. Sen. JD Vance (R-OH) introduced the companion Senate measure.
Reality of Gender-Affirming Care
The drive to pass laws like those in Ohio and Arkansas stem from a lack of knowledge about gender-affirming treatments, including a false idea that doctors prescribe medications at teens’ requests, Montefiore’s Dr. Collins-Ogle said.
“There’s a misperception that young people will say ‘I’m transgender’ and that those of us who provide care are just giving them hormones or whatever they want. It’s not true, and it doesn’t happen that way,” Dr. Collins-Ogle said.
At the Children’s Hospital at Montefiore, Dr. Collins-Ogle said her work with patients wrestling with gender identity issues begins with questions.
“What’s your understanding of dysphoria? Where’s the incongruence between the gender you were assigned at birth and what you’re feeling now? You have to be able to verbalize that” before the treatment proceeds, she said.
Sometimes teens leave after an initial conversation and then return later when they have a more clearly defined sense of what dysphoria means.
“There are other kids who clearly, clearly understand that the gender they were assigned at birth is not who they are,” she said.
Children now wrestle with added concerns that their parents could be put at risk for trying to help them, she said.
“These kids go through so much. And we have these people in powerful positions telling them that they don’t matter and telling them, ‘We’re going to cut off your access to healthcare, Medicaid; if your parents tried to seek out this care for you, we’re going to put them in jail,’” she said.
“It’s the biggest factor in fear mongering,” she said.
Dr. Collins-Ogle said she wonders why legislators who lack medical training are trying to dictate how physicians can practice.
“I took a Hippocratic oath to do no harm. I have a medical board that I answer to,” she said. “I don’t understand how legislators can get away with legislating about something they know nothing about.”
A version of this article appeared on Medscape.com.
Pediatrician Michelle Collins-Ogle, MD, already has a busy practice helping young people address questions about their gender identity. She has treated more than 230 patients over the past 2 years at Children’s Hospital at Montefiore in the Bronx, New York.
Dr. Collins-Ogle specializes in adolescent medicine in New York, a state without the restrictions on such care that have been enacted in roughly half the country.
On December 13, 2023, Ohio lawmakers passed a bill banning gender-affirming medical care to minors which Gov. Mike DeWine vetoed on December 29. Another 26 states have similar restrictions in place, according to a tally provided to this news organization by the Human Rights Campaign, which tracks this issue.
Clinicians like Dr. Collins-Ogle are feeling the impact. In her practice, Dr. Collins-Ogle met a couple that moved from Texas to New York to allow their child to access gender-affirming medical care.
“They wanted their child to be able to receive medical care, but they also were afraid for their own safety, of having their child taken from them, and being locked up,” Dr. Collins-Ogle told this news organization.
With patients have also come protestors and harassment. In fact, many physicians are reluctant to speak on this topic amid a recent spate of threats. Psychiatric News reported that conservative pundits and high-profile social media accounts have targeted physicians who provide gender-affirming medical care, spurring harassment campaigns against clinics in cities such as Akron, Boston, and Nashville. “The attackers asserted that the clinics were mutilating children and giving them ‘chemical castration drugs,’ among other claims,” the Psychiatric News reported.
This news organization contacted more than a half dozen organizations that provide gender-affirming care for adolescents and teens seeking interviews about the effects of these restrictions.
All but Montefiore’s Dr. Collins-Ogle turned down the request.
“If my kids are brave enough to come see me, I can’t cower,” Dr. Collins-Ogle said.
But Dr. Collins-Ogle emphasized she understands why many fellow physicians are concerned about speaking publicly about gender-affirming medical care.
Dissenters Spread Misinformation and Threats
Recent years have seen increasing politicization of this issue, often due to inaccurate depictions of gender-affirming medical care circulating on social media.
In 2022, the American Medical Association (AMA), the American Academy of Pediatrics (AAP), and the Children’s Hospital Association asked the Justice Department to investigate what they called “increasing threats of violence against physicians, hospitals, and families of children for providing and seeking evidence-based gender-affirming care.”
The three organizations also called on X (formerly known as Twitter), TikTok, and Meta, which owns Facebook and Instagram, to do more to address coordinated campaigns of disinformation.
“We cannot stand by as threats of violence against our members and their patients proliferate with little consequence,” said Moira Szilagyi, MD, PhD, then AAP president in a statement.
Medical Groups Defend Care to Prevent Suicide
The AAP, AMA, and other influential medical associations are banding together to fight new legal restrictions on gender-affirming medical care for teens and adolescents. (These briefs do not discuss surgeries typically available for adults.)
Since 2022, these medical organizations have filed amicus briefs in cases challenging new restrictions put in place in Arkansas, Alabama, Florida, Georgia, Idaho, Indiana, Kentucky, North Dakota, Oklahoma, Tennessee, and Texas.
Other signers to the amicus briefs:
- Academic Pediatric Association
- American Academy of Child & Adolescent Psychiatry
- American Academy of Family Physicians
- American Academy of Nursing
- GLMA: Health Professionals Advancing LGBTQ+ Equality
- American College of Obstetricians and Gynecologists
- American College of Osteopathic Pediatricians
- The American College of Physicians
- American Pediatric Society
- Association of Medical School Pediatric Department Chairs, Inc.
- Endocrine Society
- National Association of Pediatric Nurse Practitioners
- The Pediatric Endocrine Society, Societies for Pediatric Urology
- Society for Adolescent Health and Medicine
- Society for Pediatric Research
- The Society of Pediatric Nurses
- World Professional Association for Transgender Health
In these amicus briefs, the medical groups argue that evidence-based guidelines support the use of medication in treating gender dysphoria. The amicus briefs in particular cite an Endocrine Society guideline and the standards of care developed by the World Professional Association for Transgender Health (WPATH).
Research shows that adolescents with gender dysphoria who receive puberty blockers and other medications experience less depression, anxiety, and suicidal ideation, the groups have said.
“In light of this evidence supporting the connection between lack of access to gender-affirming care and lifetime suicide risk, banning such care can put patients’ lives at risk,” the AAP and other groups said.
Debate Over Source of Gender Identity Concerns
Having doubts and concerns about one’s gender remains a relatively rare phenomena, although it appears more common among younger people.
Among US adults, 0.5% or about 1.3 million people identify as transgender whereas about 1.4% or about 300,000 people in the 13-17–year-old group do so, according to a report issued in 2022 by the Williams Institute of the UCLA School of Law.
Questionable Diagnosis Drives Bans on Care
The term “rapid-onset gender dysphoria,” referring to young people who suddenly question their gender as part of peer group dynamics, persists in political debates. The conservative Heritage Foundation has used the term as well as “social contagion” in its effort to seek restrictions on gender-affirming care for young people.
Ohio Rep. Gary Click, a Republican, said at an April 2023 hearing that his Save Adolescents from Experimentation (SAFE) bill would prevent teens from being harmed due to “social contagion” or “ rapid-onset gender dysphoria.”
The bill, which the Ohio legislature cleared in December, would block physicians from starting new patients on puberty blockers. (It also bars surgeries as part of gender-affirming medical care, although hospital officials and physicians told lawmakers these are not done in Ohio.)
Among the groups opposing Click’s bill were the Ohio chapter of the AAP, the Ohio State Medical Association and several hospitals and hospital groups as well as physicians speaking independently.
Gender-Affirming Care ‘Buys Time’ to Avoid Impulsive Decisions
Kate Krueck, MD, a pediatrician with a practice in the Columbus area, testified about her experience as the mother of a transgender child who once attempted suicide.
“It wasn’t always easy to reconstruct my vision of a baby with a vagina into the adolescent before me with a new name and changed pronouns, but they were still the same incredible person,” Krueck said.
She urged lawmakers to understand how puberty blockers can “buy time” for teens to cope with a body at odds with their vision of themselves, noting that many of the effects of these medications are largely reversible. The side effects that are not reversible, such as facial hair growth and the growth of Adam’s Apple, are certainly outweighed by the risks of withholding treatment, she said.
Bad Patient Experience Drives Detractor Activist
Arguing against that point was Chloe Cole, a detransitioner activist who had returned to a female identity. At the Ohio legislative hearings, Ms. Cole spoke of her experience in California as a teen treated for gender dysphoria.
“I was fast-tracked by medical butchers starting at 13 when I was given cross sex hormones, and they took my breasts away from me at 15 years old,” she said.
Ms. Cole appears frequently to testify in favor of bans on gender-affirming medical care. In 2022, she told the Ohio lawmakers about her experience of attending a class with about a dozen other young people in the midst of female-to-male transitions. She now sees that class as having inadvertently helped reinforce her decision to have her breasts removed.
“Despite all these consultations and classes, I don’t feel like I understood all the ramifications that came with any of the medical decisions I was making,” Ms. Cole said. “I didn’t realize how traumatic the recovery would be, and it wasn’t until I was almost a year post-op that I realized I may want to breastfeed my future children; I will never be able to do that.”
Ms. Cole also spoke in July before the US House subcommittee on the Constitution and Limited Government.
“I look in the mirror sometimes, and I feel like a monster,” Ms. Cole said at the House hearing, which was titled “ The Dangers and Due Process Violations of ‘Gender-Affirming Care’.”
During the hearing, Shannon Minter, legal director of the National Center for Lesbian Rights (NCLR), who also made a gender transition, thanked Ms. Cole but noted that her case is an exception.
A 2022 Lancet Child and Adolescent Health article reported that 704 (98%) people in the Netherlands who had started gender-affirming medical treatment in adolescence continued to use gender-affirming hormones at follow-up. Ms. Minter credits this high rate of continuation to clinicians taking their duties to adolescents seriously.
State legislatures and medical boards oversee the regulation of medical practice in the US. But a few Republicans in both chambers of the US Congress have shown an interest in enacting a federal ban restricting physicians’ ability to provide gender-affirming medical care.
They include Rep. Mike Johnson of Louisiana, who in October 2023 became Speaker of the House. He chaired the July hearing at which Ms. Cole spoke. He’s also a sponsor of a House bill introduced by Rep. Marjorie Taylor Greene (R-GA).
This measure, which has the support of 45 House Republicans, would make it a felony to perform any gender-affirming care on a minor, and it permits a minor on whom such care is performed to bring a civil action against each individual who provided the care. Sen. JD Vance (R-OH) introduced the companion Senate measure.
Reality of Gender-Affirming Care
The drive to pass laws like those in Ohio and Arkansas stem from a lack of knowledge about gender-affirming treatments, including a false idea that doctors prescribe medications at teens’ requests, Montefiore’s Dr. Collins-Ogle said.
“There’s a misperception that young people will say ‘I’m transgender’ and that those of us who provide care are just giving them hormones or whatever they want. It’s not true, and it doesn’t happen that way,” Dr. Collins-Ogle said.
At the Children’s Hospital at Montefiore, Dr. Collins-Ogle said her work with patients wrestling with gender identity issues begins with questions.
“What’s your understanding of dysphoria? Where’s the incongruence between the gender you were assigned at birth and what you’re feeling now? You have to be able to verbalize that” before the treatment proceeds, she said.
Sometimes teens leave after an initial conversation and then return later when they have a more clearly defined sense of what dysphoria means.
“There are other kids who clearly, clearly understand that the gender they were assigned at birth is not who they are,” she said.
Children now wrestle with added concerns that their parents could be put at risk for trying to help them, she said.
“These kids go through so much. And we have these people in powerful positions telling them that they don’t matter and telling them, ‘We’re going to cut off your access to healthcare, Medicaid; if your parents tried to seek out this care for you, we’re going to put them in jail,’” she said.
“It’s the biggest factor in fear mongering,” she said.
Dr. Collins-Ogle said she wonders why legislators who lack medical training are trying to dictate how physicians can practice.
“I took a Hippocratic oath to do no harm. I have a medical board that I answer to,” she said. “I don’t understand how legislators can get away with legislating about something they know nothing about.”
A version of this article appeared on Medscape.com.
The Many Uses of the Humble Alcohol Swab
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).
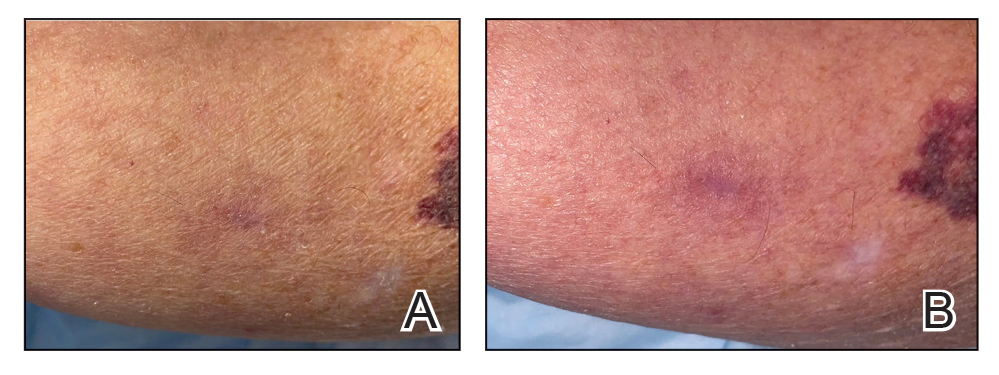
Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).

Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).

Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
AAAAI/ACAAI Joint Task Force Issues Updated ‘Practice-Changing’ Guidelines to Manage AD
and new methodological standards for making recommendations. The new guidelines update 2012 recommendations.
The JTFPP AD guidelines represent “an evolution” in trustworthy allergy guidelines and provide systematic reviews of the evidence with multidisciplinary panelist engagement, adherence to a rigorous guideline development process, the involvement of the patient and caregiver voice from start to finish, clear translation of evidence to clinically actionable and contextual recommendations, and novel approaches to facilitate knowledge translation, task force cochair Derek K. Chu, MD, PhD, said in an interview. Dr. Chu, director of the Evidence in Allergy research group at McMaster University, Hamilton, Ontario, Canada, cochaired the task force with Lynda Schneider, MD, section chief of the allergy and asthma program at Boston Children’s Hospital.
The new guidelines were published online on December 17, 2023, in Annals of Allergy, Asthma, & Immunology. They include 25 recommendations and address optimal use of topical treatments, such as topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors, topical crisaborole, and topical antimicrobials; dilute bleach baths; dietary elimination; allergen immunotherapy by subcutaneous (SCIT) and sublingual (SLIT) routes; and systemic treatments with dupilumab and tralokinumab, cyclosporine, azathioprine, methotrexate, mycophenolate, oral JAK inhibitors, systemic corticosteroids; and phototherapy.
“There’s something in here for all clinicians — from primary care to AD experts— and patients may benefit as well, so the key individual recommendations will vary,” Dr. Chu told this news organization.
“Throughout the guideline, we emphasize shared decision-making, key factors to consider for each recommendation, and the specific evidence behind each recommendation,” he said. “There is a major focus on addressing equity, diversity, inclusiveness; and addressing health disparities, and key gaps to address in future research.”
Among the changes to the 2012 JTFPP guidelines, the 2023 update suggests using dilute bleach baths for patients with AD with moderate to severe disease as an additive therapy and suggests using allergen immunotherapy (AIT) for moderate to severe AD.
In other changes, the 2023 update suggests against using elimination diets for AD; recommends against very low dose baricitinib (1 mg); suggests against azathioprine, methotrexate, and mycophenolate mofetil; and suggests against adding topical JAK inhibitors, such as ruxolitinib, for patients with mild to moderate AD refractory to moisturization alone.
The 38-page guidelines include an infographic that summarizes comparative effects of systemic treatments on patient-important outcomes for AD that are important to patients, and includes other key summary tables that can be used at the point of care.
In addition to addressing evidence underlying each recommendation, the guideline’s eAppendix contains 1- to 2-page handouts that address practical issues for each treatment and can be used to facilitate shared decision making.
Dr. Chu said that the updated guidelines “provide important changes to almost all aspects of AD care — my own and my colleagues’ — and I strongly recommend all clinicians treating AD to read the full guidelines and use them in clinical practice. We’re grateful to all our contributors, especially our patient and caregiver partners, for helping make these guidelines. We will continue to periodically update the guidelines as part of maintaining them as living guidelines.”
The guidelines incorporate the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence.
The work was funded by the AAAAI/ACAAI JTFPP. Dr. Chu disclosed that he has received a faculty development award from the AAAAI Foundation and research grants to McMaster from the Canadian Institutes of Health Research, the Ontario Ministry of Health, and the Ontario Medical Association.
and new methodological standards for making recommendations. The new guidelines update 2012 recommendations.
The JTFPP AD guidelines represent “an evolution” in trustworthy allergy guidelines and provide systematic reviews of the evidence with multidisciplinary panelist engagement, adherence to a rigorous guideline development process, the involvement of the patient and caregiver voice from start to finish, clear translation of evidence to clinically actionable and contextual recommendations, and novel approaches to facilitate knowledge translation, task force cochair Derek K. Chu, MD, PhD, said in an interview. Dr. Chu, director of the Evidence in Allergy research group at McMaster University, Hamilton, Ontario, Canada, cochaired the task force with Lynda Schneider, MD, section chief of the allergy and asthma program at Boston Children’s Hospital.
The new guidelines were published online on December 17, 2023, in Annals of Allergy, Asthma, & Immunology. They include 25 recommendations and address optimal use of topical treatments, such as topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors, topical crisaborole, and topical antimicrobials; dilute bleach baths; dietary elimination; allergen immunotherapy by subcutaneous (SCIT) and sublingual (SLIT) routes; and systemic treatments with dupilumab and tralokinumab, cyclosporine, azathioprine, methotrexate, mycophenolate, oral JAK inhibitors, systemic corticosteroids; and phototherapy.
“There’s something in here for all clinicians — from primary care to AD experts— and patients may benefit as well, so the key individual recommendations will vary,” Dr. Chu told this news organization.
“Throughout the guideline, we emphasize shared decision-making, key factors to consider for each recommendation, and the specific evidence behind each recommendation,” he said. “There is a major focus on addressing equity, diversity, inclusiveness; and addressing health disparities, and key gaps to address in future research.”
Among the changes to the 2012 JTFPP guidelines, the 2023 update suggests using dilute bleach baths for patients with AD with moderate to severe disease as an additive therapy and suggests using allergen immunotherapy (AIT) for moderate to severe AD.
In other changes, the 2023 update suggests against using elimination diets for AD; recommends against very low dose baricitinib (1 mg); suggests against azathioprine, methotrexate, and mycophenolate mofetil; and suggests against adding topical JAK inhibitors, such as ruxolitinib, for patients with mild to moderate AD refractory to moisturization alone.
The 38-page guidelines include an infographic that summarizes comparative effects of systemic treatments on patient-important outcomes for AD that are important to patients, and includes other key summary tables that can be used at the point of care.
In addition to addressing evidence underlying each recommendation, the guideline’s eAppendix contains 1- to 2-page handouts that address practical issues for each treatment and can be used to facilitate shared decision making.
Dr. Chu said that the updated guidelines “provide important changes to almost all aspects of AD care — my own and my colleagues’ — and I strongly recommend all clinicians treating AD to read the full guidelines and use them in clinical practice. We’re grateful to all our contributors, especially our patient and caregiver partners, for helping make these guidelines. We will continue to periodically update the guidelines as part of maintaining them as living guidelines.”
The guidelines incorporate the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence.
The work was funded by the AAAAI/ACAAI JTFPP. Dr. Chu disclosed that he has received a faculty development award from the AAAAI Foundation and research grants to McMaster from the Canadian Institutes of Health Research, the Ontario Ministry of Health, and the Ontario Medical Association.
and new methodological standards for making recommendations. The new guidelines update 2012 recommendations.
The JTFPP AD guidelines represent “an evolution” in trustworthy allergy guidelines and provide systematic reviews of the evidence with multidisciplinary panelist engagement, adherence to a rigorous guideline development process, the involvement of the patient and caregiver voice from start to finish, clear translation of evidence to clinically actionable and contextual recommendations, and novel approaches to facilitate knowledge translation, task force cochair Derek K. Chu, MD, PhD, said in an interview. Dr. Chu, director of the Evidence in Allergy research group at McMaster University, Hamilton, Ontario, Canada, cochaired the task force with Lynda Schneider, MD, section chief of the allergy and asthma program at Boston Children’s Hospital.
The new guidelines were published online on December 17, 2023, in Annals of Allergy, Asthma, & Immunology. They include 25 recommendations and address optimal use of topical treatments, such as topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors, topical crisaborole, and topical antimicrobials; dilute bleach baths; dietary elimination; allergen immunotherapy by subcutaneous (SCIT) and sublingual (SLIT) routes; and systemic treatments with dupilumab and tralokinumab, cyclosporine, azathioprine, methotrexate, mycophenolate, oral JAK inhibitors, systemic corticosteroids; and phototherapy.
“There’s something in here for all clinicians — from primary care to AD experts— and patients may benefit as well, so the key individual recommendations will vary,” Dr. Chu told this news organization.
“Throughout the guideline, we emphasize shared decision-making, key factors to consider for each recommendation, and the specific evidence behind each recommendation,” he said. “There is a major focus on addressing equity, diversity, inclusiveness; and addressing health disparities, and key gaps to address in future research.”
Among the changes to the 2012 JTFPP guidelines, the 2023 update suggests using dilute bleach baths for patients with AD with moderate to severe disease as an additive therapy and suggests using allergen immunotherapy (AIT) for moderate to severe AD.
In other changes, the 2023 update suggests against using elimination diets for AD; recommends against very low dose baricitinib (1 mg); suggests against azathioprine, methotrexate, and mycophenolate mofetil; and suggests against adding topical JAK inhibitors, such as ruxolitinib, for patients with mild to moderate AD refractory to moisturization alone.
The 38-page guidelines include an infographic that summarizes comparative effects of systemic treatments on patient-important outcomes for AD that are important to patients, and includes other key summary tables that can be used at the point of care.
In addition to addressing evidence underlying each recommendation, the guideline’s eAppendix contains 1- to 2-page handouts that address practical issues for each treatment and can be used to facilitate shared decision making.
Dr. Chu said that the updated guidelines “provide important changes to almost all aspects of AD care — my own and my colleagues’ — and I strongly recommend all clinicians treating AD to read the full guidelines and use them in clinical practice. We’re grateful to all our contributors, especially our patient and caregiver partners, for helping make these guidelines. We will continue to periodically update the guidelines as part of maintaining them as living guidelines.”
The guidelines incorporate the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence.
The work was funded by the AAAAI/ACAAI JTFPP. Dr. Chu disclosed that he has received a faculty development award from the AAAAI Foundation and research grants to McMaster from the Canadian Institutes of Health Research, the Ontario Ministry of Health, and the Ontario Medical Association.
FROM ANNALS OF ALLERGY, ASTHMA, AND IMMUNOLOGY
PRAME Expression in Melanocytic Proliferations in Special Sites
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.
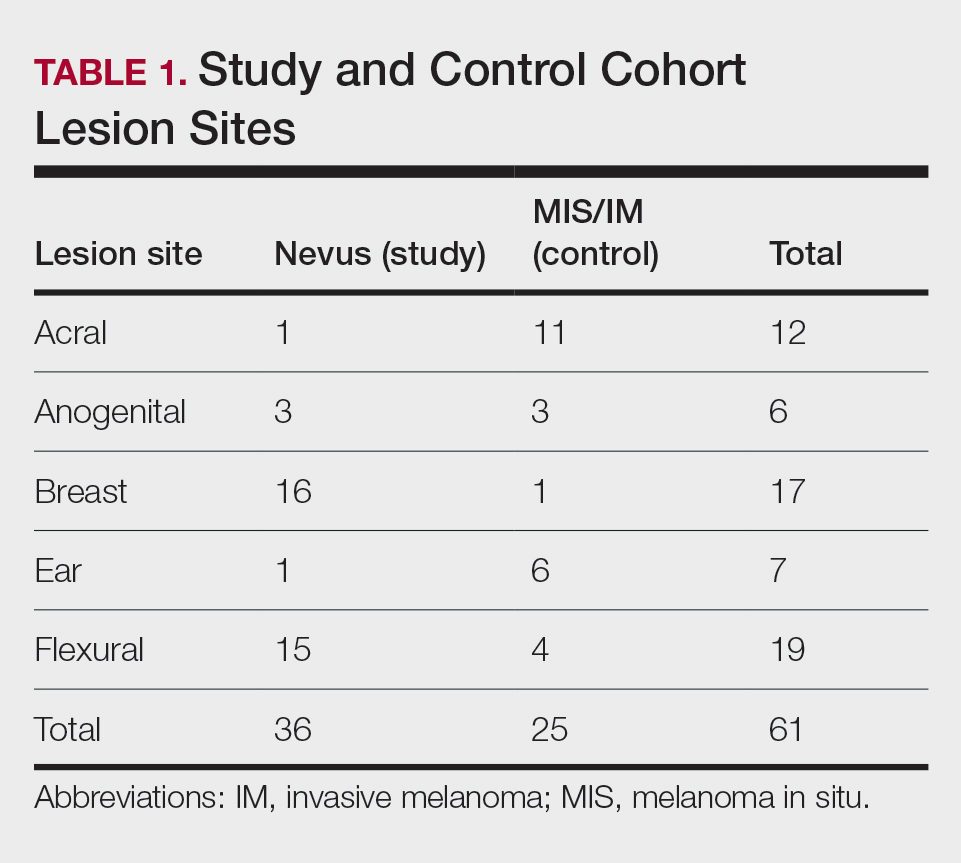
Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).
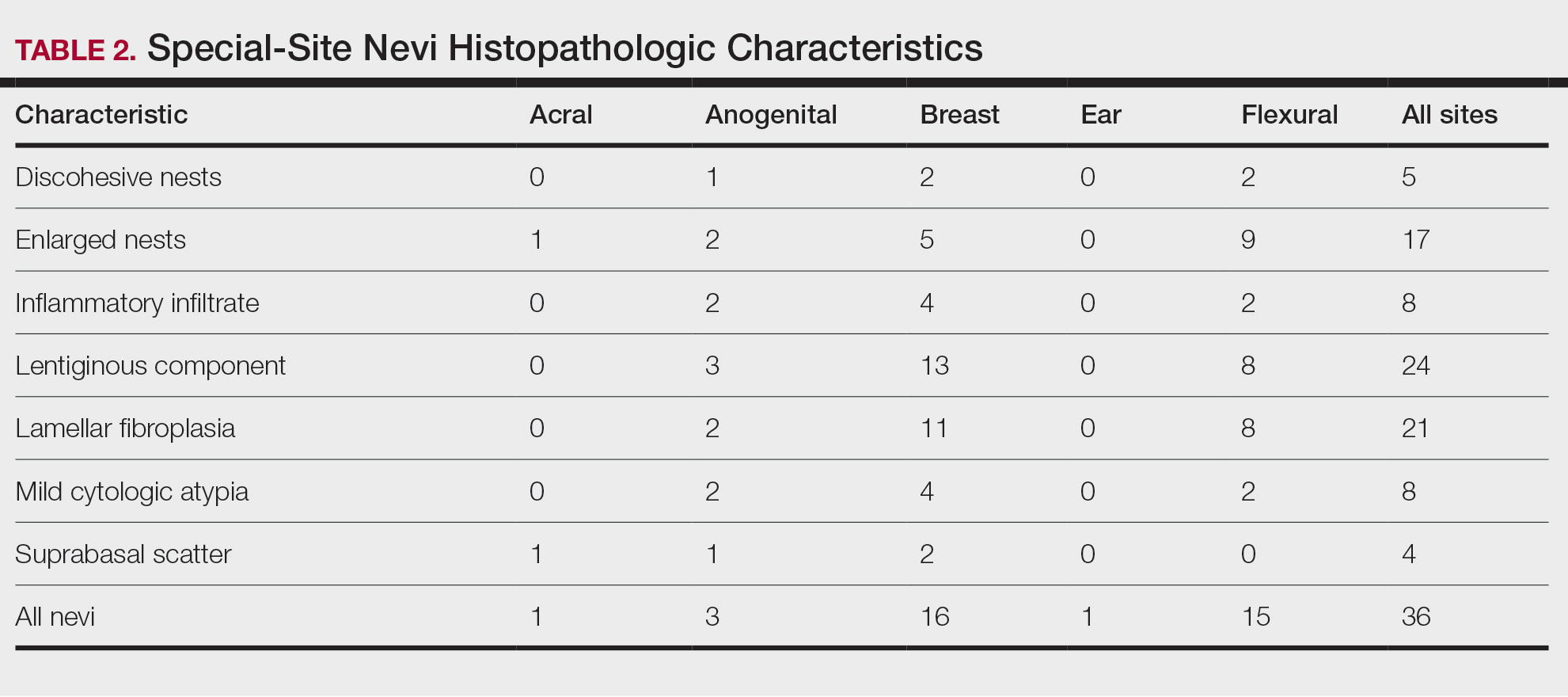
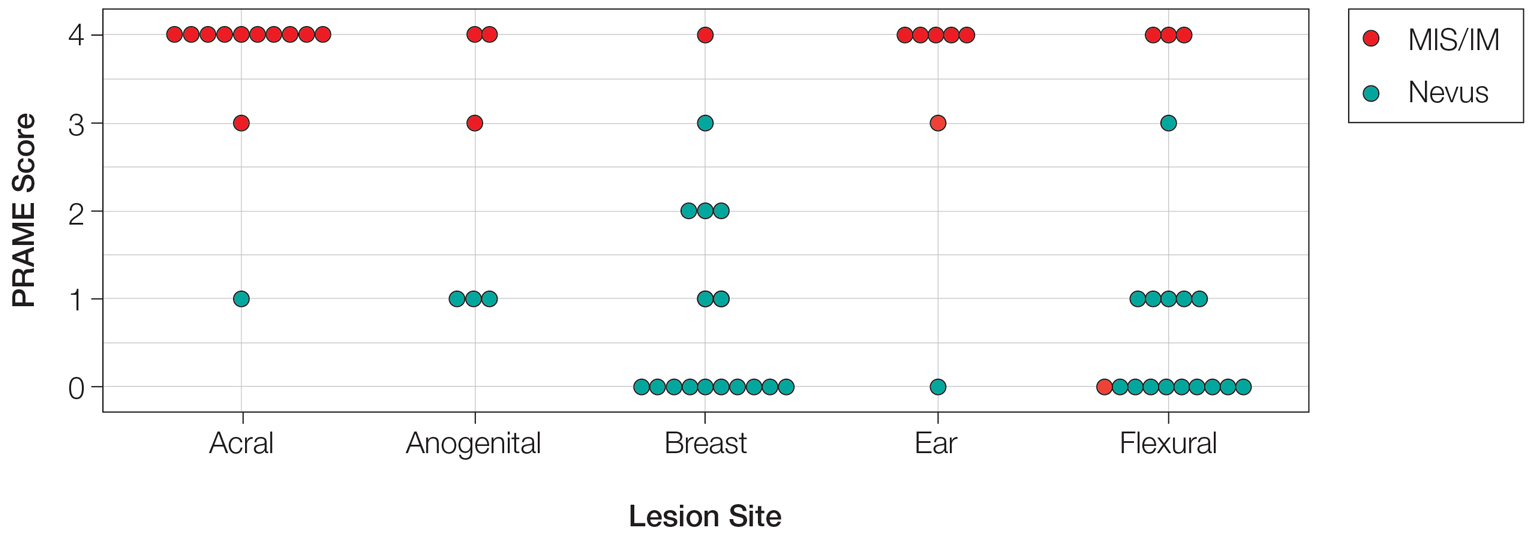
Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.
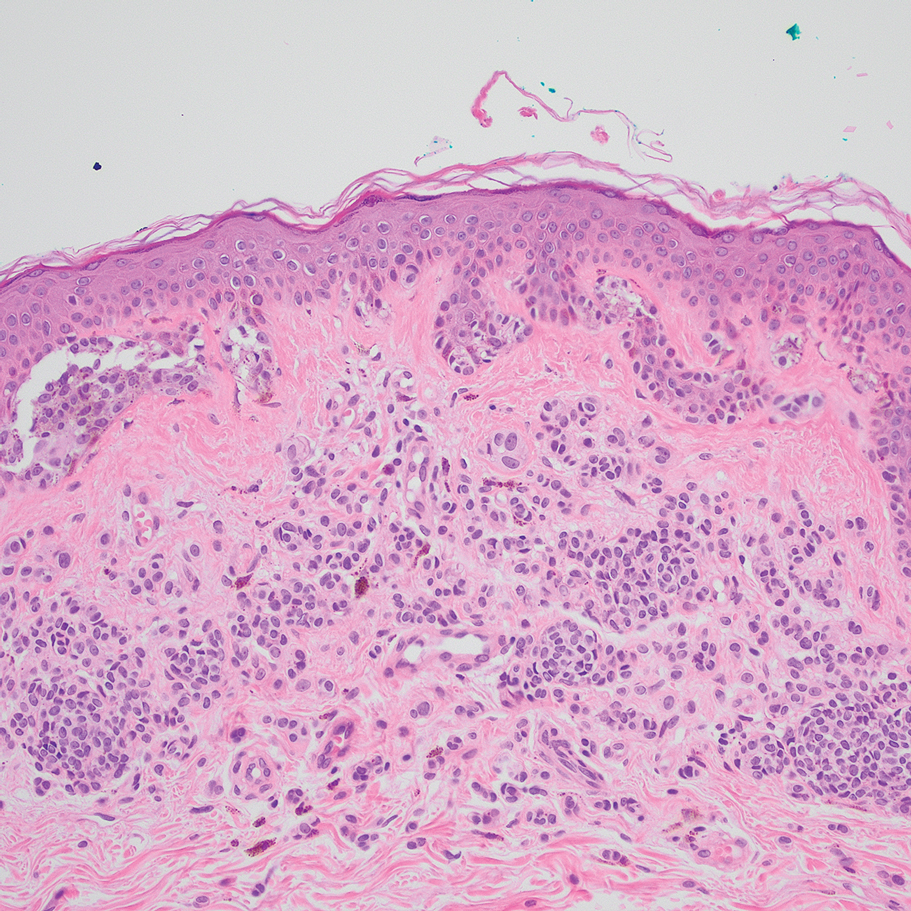
Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.
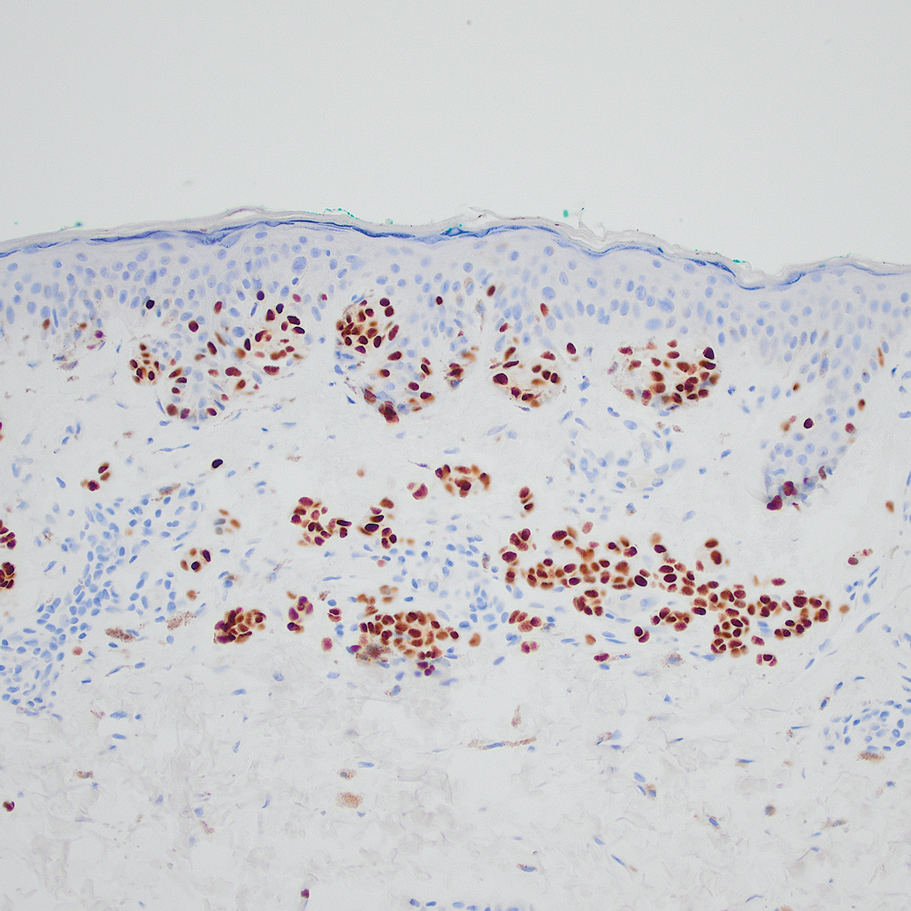
Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.
Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.
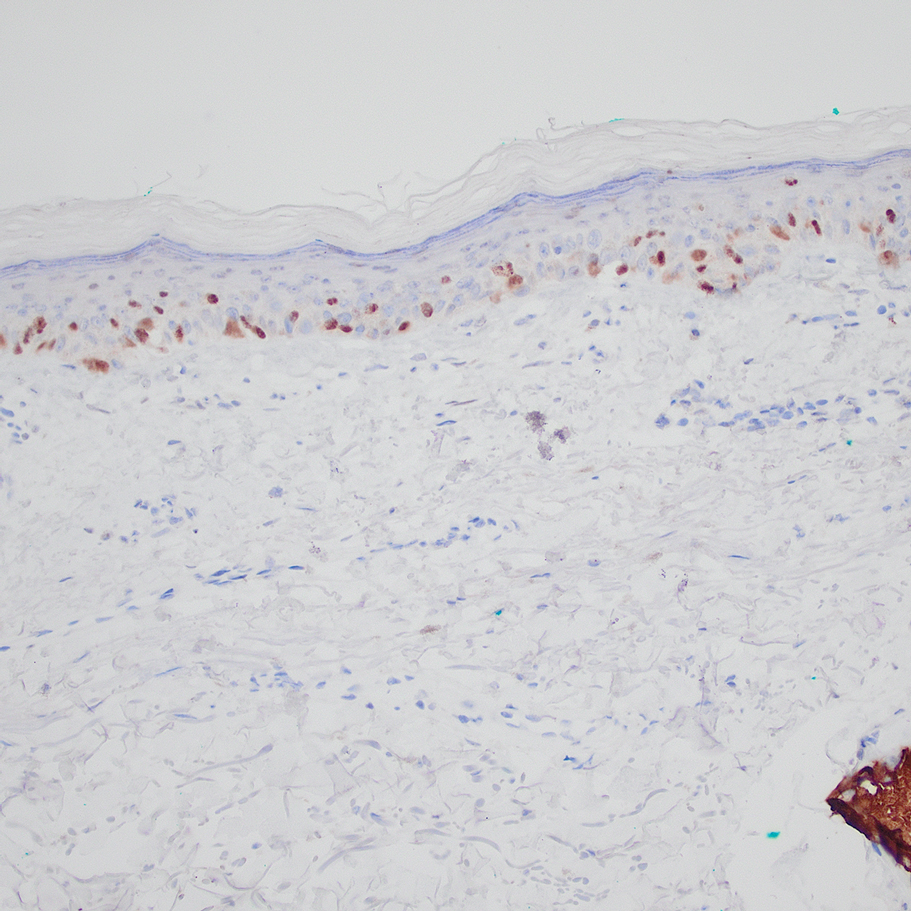
Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
Practice Points
- Special-site nevi are benign melanocytic proliferations at special anatomic sites. Although cytologic atypia and architectural distortion may be present, they are centrally located and should not be present at the borders of the lesion.
- Strong expression of the preferentially expressed antigen in melanoma (PRAME) via immunohistochemistry provides a reliable indicator for benignity in differentiating a special-site nevus from a malignant melanoma occurring at a special site.
Axillary Contact Dermatitis: An Update on Potential Allergens and Management
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34
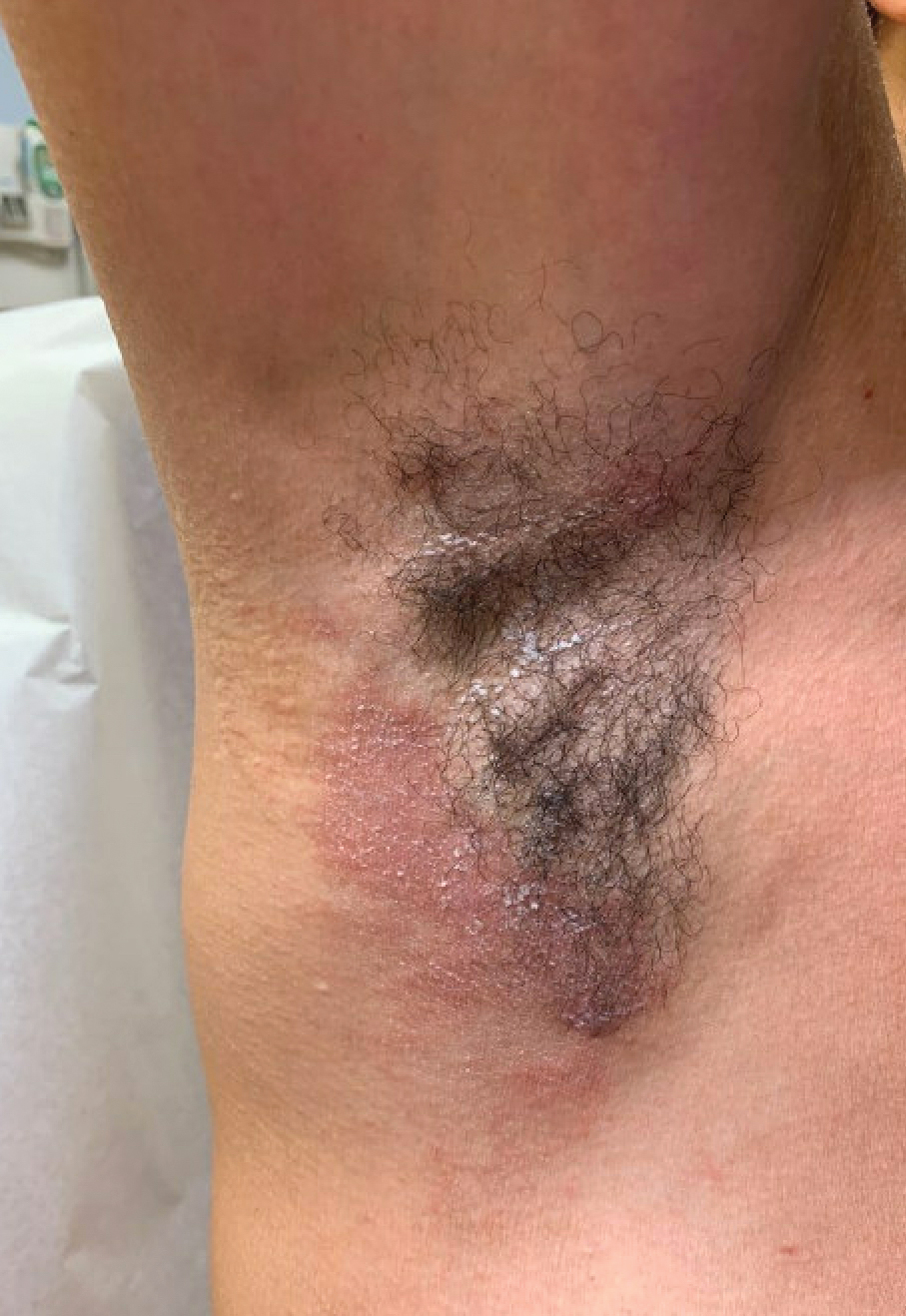
Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58
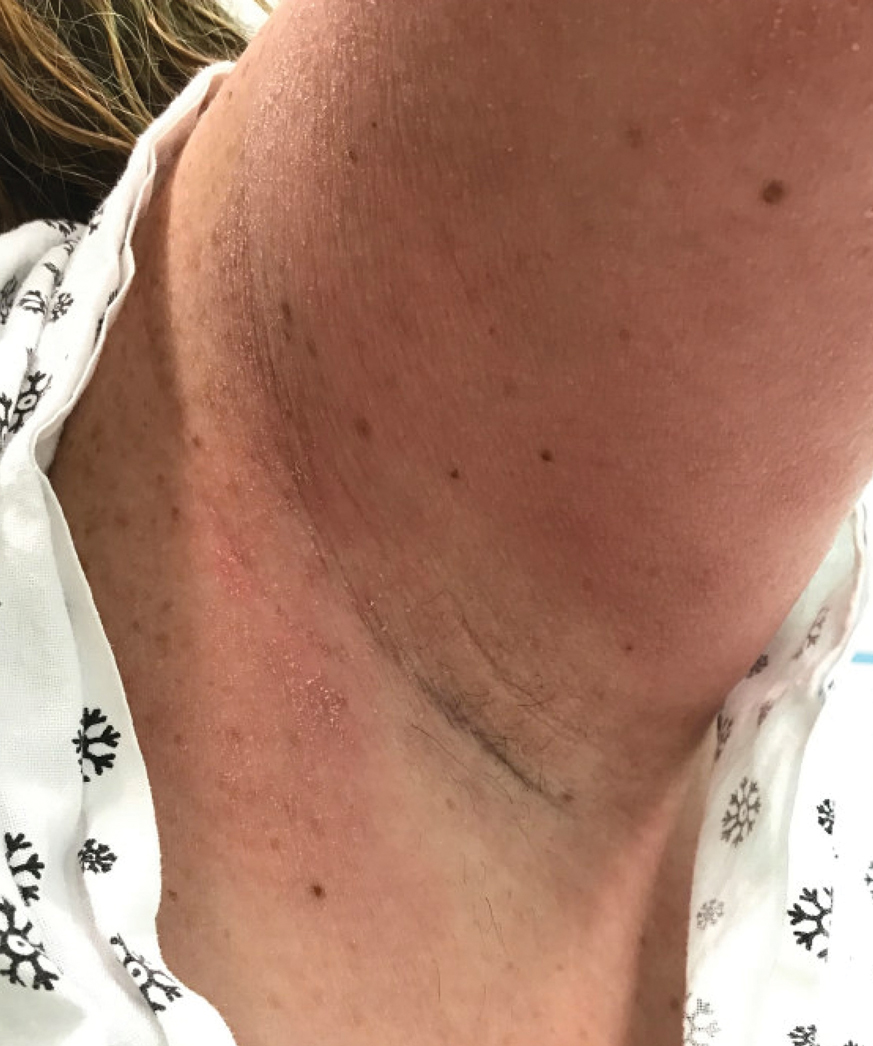
Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65
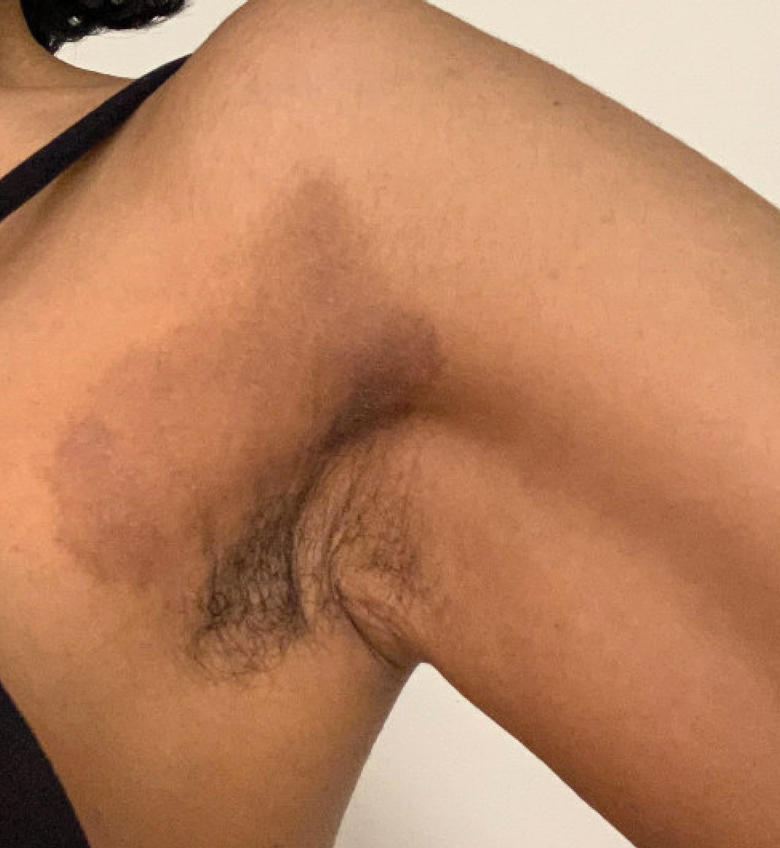
Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92
Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Practice Points
- The differential diagnosis of axillary dermatitis is broad. Contact dermatitis—both irritant and allergic—represents common etiologies.
- Understanding the clinical features and range of potential sources in axillary contact dermatitis allows for efficient recognition and elimination of causative exposure.
- For cases of suspected allergic contact dermatitis, patch testing and subsequent allergen avoidance are paramount in the management of axillary eruptions.
Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10
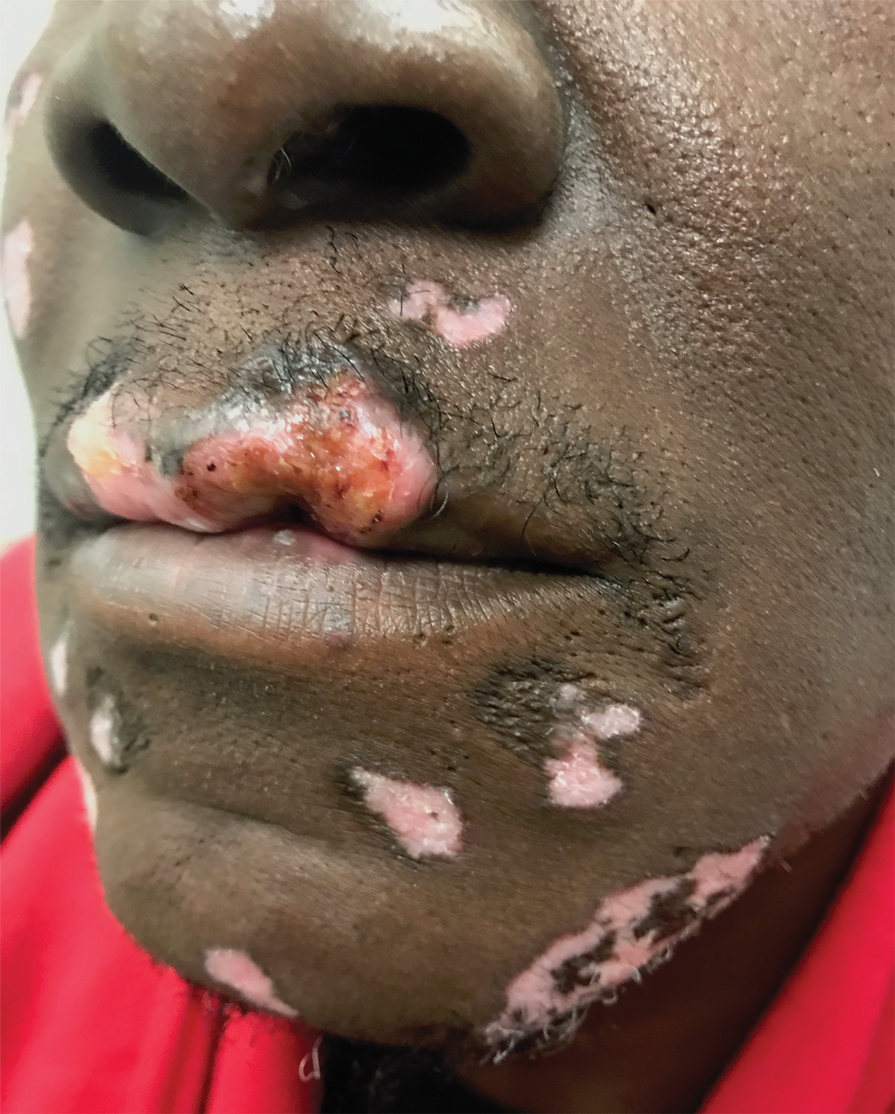
Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28
In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
PRACTICE POINTS
- Squamous cell carcinoma can develop within chronic inflammatory dermatoses.
- Orolabial discoid lupus erythematosus (DLE), oral lichen planus, and lichen sclerosus can lead to relatively rapid tumorigenesis. Squamous cell carcinoma arising in cutaneous DLE, hidradenitis suppurativa (HS), necrobiosis lipoidica, chronic wounds, and hypertrophic lichen planus tends to appear after decades of inflammation.
- Be especially mindful of new orolabial DLE cases and chronic cases of HS and Marjolin ulcer because malignancies in these settings are particularly aggressive.
Meeting the Critical Need for More Native American Physicians
America was already facing a critical health care workforce shortage before the COVID-19 pandemic exacerbated the problem. The American Medical Association (AMA) projects that there will be a national shortage of up to 48,000 primary care physicians and 77,100 non-primary care physicians by 2034.
The dearth is particularly striking among physicians who practice in rural areas and those who are Native American. As of 2021, fewer than 3000 physicians—of 841,322—identified as American Indian or Alaska Native, according to the latest statistics from the Physician Specialty Data Report, published by the Association of American Medical Colleges (AAMC).
The lack of Native American physicians is “nothing new, it’s been going on for decades,” says Mary Owen (Tlingit), MD, director of the Center of American Indian and Minority Health and associate dean of Native American Health at the University of Minnesota Medical School, speaking in a Native America Calling podcast in October.
“These numbers are… actually lessening—and we had paltry numbers to begin with,” said Owen. “It doesn’t take a genius to look back and figure out where it’s from. We don’t have enough students coming through the pathways in the first place. For instance, our high school graduation rate in this country is easily 10 points below that of non-Natives. In Duluth, Minnesota, the high school graduation rate is only 43%… We have to recognize that this is an area we have to work on.”
Senators Tim Kaine (D-VA) and Alex Padilla (D-CA) have introduced the Expanding Medical Education Act, legislation to get more students from underrepresented groups into the physician pipeline. The bill would provide grants through the Health Resources and Services Administration (HRSA) for colleges and universities to establish or expand allopathic (MD-granting) or osteopathic (DO-granting) medical schools in underserved areas or at institutions for underrepresented populations, including Historically Black Colleges and Universities (HBCUs).
Addressing Rural Needs
However, projections on the growth of health care professions show that supply will not meet demand over the next 10 years. The shortage is more dire in rural areas. According to the US Department of Health and Human Services (HHS), since 2010, more than 150 rural hospitals have either closed their doors entirely or stopped providing inpatient hospital services. Often, rural communities have fewer local HCPs available.
More than half (54%) of American Indian or Alaska Native people live in rural and small-town areas, and 68% live on or near their tribal homelands, according to the nonprofit First Nations Development Institute. Many live far—even hours—away from the nearest health care facility. But according to Population Health in Rural America in 2020: Proceedings of a Workshop, only 10% of primary care practitioners and < 7% of specialty care practitioners live in rural areas. About 5% of rural counties do not have any family physicians. What’s more, language and culture differ among the nearly 600 tribes across the country. The Indian Health Council, for instance, counts 9 individual reservations and tribes within a 5-mile radius in San Diego County, “all of which have their own unique customs,” which contribute to the “level of care they deem appropriate.”
“If you’re a rural impoverished community, it’s hard to recruit doctors. We’re more likely to return to our communities,” said Donald K. Warne (Oglala Lakota), MD, MPH, Associate Dean for Diversity, Equity, and Inclusion at the University of North Dakota School of Medicine and Health Sciences, during the 2019 American Indian or Alaska Native Physicians Summit, which was cosponsored by the AMA, Association of American Indian Physicians (AAIP), and the AAMC.
“Communities of color and those living in rural and underserved areas have long faced significant barriers to health care, including a lack of providers that look like them or practice close by,” said Senator Kaine in a statement. “Since research shows that physicians are more likely to practice in the areas they’re from, supporting medical schools at minority-serving institutions and HBCUs in underserved areas can help improve care in those communities.”
Where Are the Native Medical Students?
Only 9% of medical schools have more than 4 American Indian or Alaska Native students; 43% have none, says Siobhan M. Wescott, MD, MPH, chair of the AMA Minority Affairs Section (MAS), and an assistant professor at the University of North Dakota. Dr. Wescott, who hosted the AMA co-sponsored summit on behalf of the AMA-MAS, is an Alaska Native and 1 of only 3 physicians from her tribe. The AAMC has also found that less than half of MD-granting medical schools in the US have enrolled more than 5 Native students.
Among other things, the Expanding Medical Education Act would prioritize grants to institutions of higher education that propose to use the funds to establish a medical school or branch campus in an area in which no other such school is based and is a medically underserved community or “health professional shortage” area. Eligible uses for the grants include hiring diverse faculty and other staff, and recruiting students from underrepresented racial and ethnic minorities, students from rural and underserved areas, low-income students, and first-generation college students.
The legislation has been endorsed by the AAMC, American Association of Colleges of Osteopathic Medicine, Association of American Indian Physicians, Association of Clinicians for the Underserved, National Hispanic Medical Association, Society for Advancement of Chicanos/Hispanics and Native Americans in Science, and Ochsner Health.
Funding Is Key
Federal agencies are investing in funding and training. Medicare is allocating 1000 new training slots for medical residents, prioritizing rural and underserved areas. Centers for Medicare and Medicaid Services (CMS) is offering another 200 slots, at least 100 of which are specifically for psychiatry residencies in 2026. HHS awarded more than $11 million through the Rural Residency Planning and Development Program (RRPD) to help establish new rural residency programs. Accredited RRPD-funded programs are already training more than 300 resident physicians in family medicine, internal medicine, psychiatry, and general surgery. HRSA published an opportunity for $5 million in FY 2024 to develop and implement clinical rotations for physician assistant students in rural areas that will integrate behavioral health with primary care services.
The Biden-Harris Administration has already taken several steps to improve access to health care for the more than 60 million people who live in rural areas, including: building on the Affordable Care Act and Inflation Reduction Act to increase access to affordable health coverage and care for those living in rural communities; keeping more rural hospitals open to provide critical services in their communities; and bolstering the rural health workforce, including for primary care and behavioral HCPs.
The administration also has funded small rural hospitals and Medicare-certified Rural Health Clinics. Critical access hospitals and small hospitals in rural areas have a new option: to convert to a Rural Emergency Hospital (REH), a new Medicare provider type. CMS has changed the payment method for Tribal and Indian Health Services–operated REHs, to address certain barriers that may have discouraged Tribal and Indian Health Service (IHS)–operated hospitals from converting to REHs. Beginning in FY 2022, HHS, through HRSA, dedicated $5 million to provide technical assistance to rural hospitals that are considering converting to the REH designation.
HHS also has several grant opportunities to support rural communities, including $28 million to provide direct health services and expand infrastructure and $16 million to provide technical assistance to rural hospitals facing financial distress. This year, 60 rural hospitals will receive technical assistance to maintain financial viability and ensure continued access to care.
The HRSA National Health Service Corps Rural Community Loan Repayment Program has invested $80 million to support substance use disorder treatment, assist in recovery, and prevent overdose deaths. Medicare will also cover opioid use disorder treatment services delivered by mobile units of registered opioid treatment programs, which can now be accessed via telehealth or audio-only communications.
Curricula Also Lack Native Diversity
As of 2017, only 11% of MD-granting schools in the US say they have included Native American health content in their curricula. Dr. Owen notes some of the challenges indigenous students face: They are in a crowd that is primarily non-Native, far from their own family and community; unlike White students, they usually do not have mentors; they may not have the wherewithal to continue school and graduate.
A 2022 study of the association of sociodemographic characteristics with US medical student attrition, published in JAMA Internal Medicine, found that American Indian, Alaska Native, Native Hawaiian, and Pacific Islander students were more than 4 times as likely to drop out compared with White students. More than 10% of Indigenous medical students don’t graduate—the highest of any group the researchers examined.
In 1973 the University of North Dakota, for instance, launched Indians Into Medicine (INMED), a program that has since recruited, supported, and trained 250 American Indian doctors, and, in 2019, the country’s first PhD program in indigenous health. Dr. Warne, the director of INMED, calls it “by far, the most successful indigenous medical training program in the world,” having helped 228 American Indians and Alaska Natives graduate since its inception. A new cohort of 6 students has just enrolled.
Oregon Health & Science University (OHSU) received $800,000 in federal funding for its Future Leaders in Indigenous Health (FLIGHT) project, managed through OHSU’s Northwest Native American Center of Excellence (NNACoE). In 2012, just 8 Native students were enrolled in the OHSU School of Medicine; a decade later, there were 29. In 2022, the newest medical class included 12 American Indian or Alaska Native students. According to the school, it is believed to be the largest group of Natives in any single US medical school MD class in history. The number of Native faculty in the OHSU School of Medicine grew from 7 in 2014 to 13 in 2022.
America was already facing a critical health care workforce shortage before the COVID-19 pandemic exacerbated the problem. The American Medical Association (AMA) projects that there will be a national shortage of up to 48,000 primary care physicians and 77,100 non-primary care physicians by 2034.
The dearth is particularly striking among physicians who practice in rural areas and those who are Native American. As of 2021, fewer than 3000 physicians—of 841,322—identified as American Indian or Alaska Native, according to the latest statistics from the Physician Specialty Data Report, published by the Association of American Medical Colleges (AAMC).
The lack of Native American physicians is “nothing new, it’s been going on for decades,” says Mary Owen (Tlingit), MD, director of the Center of American Indian and Minority Health and associate dean of Native American Health at the University of Minnesota Medical School, speaking in a Native America Calling podcast in October.
“These numbers are… actually lessening—and we had paltry numbers to begin with,” said Owen. “It doesn’t take a genius to look back and figure out where it’s from. We don’t have enough students coming through the pathways in the first place. For instance, our high school graduation rate in this country is easily 10 points below that of non-Natives. In Duluth, Minnesota, the high school graduation rate is only 43%… We have to recognize that this is an area we have to work on.”
Senators Tim Kaine (D-VA) and Alex Padilla (D-CA) have introduced the Expanding Medical Education Act, legislation to get more students from underrepresented groups into the physician pipeline. The bill would provide grants through the Health Resources and Services Administration (HRSA) for colleges and universities to establish or expand allopathic (MD-granting) or osteopathic (DO-granting) medical schools in underserved areas or at institutions for underrepresented populations, including Historically Black Colleges and Universities (HBCUs).
Addressing Rural Needs
However, projections on the growth of health care professions show that supply will not meet demand over the next 10 years. The shortage is more dire in rural areas. According to the US Department of Health and Human Services (HHS), since 2010, more than 150 rural hospitals have either closed their doors entirely or stopped providing inpatient hospital services. Often, rural communities have fewer local HCPs available.
More than half (54%) of American Indian or Alaska Native people live in rural and small-town areas, and 68% live on or near their tribal homelands, according to the nonprofit First Nations Development Institute. Many live far—even hours—away from the nearest health care facility. But according to Population Health in Rural America in 2020: Proceedings of a Workshop, only 10% of primary care practitioners and < 7% of specialty care practitioners live in rural areas. About 5% of rural counties do not have any family physicians. What’s more, language and culture differ among the nearly 600 tribes across the country. The Indian Health Council, for instance, counts 9 individual reservations and tribes within a 5-mile radius in San Diego County, “all of which have their own unique customs,” which contribute to the “level of care they deem appropriate.”
“If you’re a rural impoverished community, it’s hard to recruit doctors. We’re more likely to return to our communities,” said Donald K. Warne (Oglala Lakota), MD, MPH, Associate Dean for Diversity, Equity, and Inclusion at the University of North Dakota School of Medicine and Health Sciences, during the 2019 American Indian or Alaska Native Physicians Summit, which was cosponsored by the AMA, Association of American Indian Physicians (AAIP), and the AAMC.
“Communities of color and those living in rural and underserved areas have long faced significant barriers to health care, including a lack of providers that look like them or practice close by,” said Senator Kaine in a statement. “Since research shows that physicians are more likely to practice in the areas they’re from, supporting medical schools at minority-serving institutions and HBCUs in underserved areas can help improve care in those communities.”
Where Are the Native Medical Students?
Only 9% of medical schools have more than 4 American Indian or Alaska Native students; 43% have none, says Siobhan M. Wescott, MD, MPH, chair of the AMA Minority Affairs Section (MAS), and an assistant professor at the University of North Dakota. Dr. Wescott, who hosted the AMA co-sponsored summit on behalf of the AMA-MAS, is an Alaska Native and 1 of only 3 physicians from her tribe. The AAMC has also found that less than half of MD-granting medical schools in the US have enrolled more than 5 Native students.
Among other things, the Expanding Medical Education Act would prioritize grants to institutions of higher education that propose to use the funds to establish a medical school or branch campus in an area in which no other such school is based and is a medically underserved community or “health professional shortage” area. Eligible uses for the grants include hiring diverse faculty and other staff, and recruiting students from underrepresented racial and ethnic minorities, students from rural and underserved areas, low-income students, and first-generation college students.
The legislation has been endorsed by the AAMC, American Association of Colleges of Osteopathic Medicine, Association of American Indian Physicians, Association of Clinicians for the Underserved, National Hispanic Medical Association, Society for Advancement of Chicanos/Hispanics and Native Americans in Science, and Ochsner Health.
Funding Is Key
Federal agencies are investing in funding and training. Medicare is allocating 1000 new training slots for medical residents, prioritizing rural and underserved areas. Centers for Medicare and Medicaid Services (CMS) is offering another 200 slots, at least 100 of which are specifically for psychiatry residencies in 2026. HHS awarded more than $11 million through the Rural Residency Planning and Development Program (RRPD) to help establish new rural residency programs. Accredited RRPD-funded programs are already training more than 300 resident physicians in family medicine, internal medicine, psychiatry, and general surgery. HRSA published an opportunity for $5 million in FY 2024 to develop and implement clinical rotations for physician assistant students in rural areas that will integrate behavioral health with primary care services.
The Biden-Harris Administration has already taken several steps to improve access to health care for the more than 60 million people who live in rural areas, including: building on the Affordable Care Act and Inflation Reduction Act to increase access to affordable health coverage and care for those living in rural communities; keeping more rural hospitals open to provide critical services in their communities; and bolstering the rural health workforce, including for primary care and behavioral HCPs.
The administration also has funded small rural hospitals and Medicare-certified Rural Health Clinics. Critical access hospitals and small hospitals in rural areas have a new option: to convert to a Rural Emergency Hospital (REH), a new Medicare provider type. CMS has changed the payment method for Tribal and Indian Health Services–operated REHs, to address certain barriers that may have discouraged Tribal and Indian Health Service (IHS)–operated hospitals from converting to REHs. Beginning in FY 2022, HHS, through HRSA, dedicated $5 million to provide technical assistance to rural hospitals that are considering converting to the REH designation.
HHS also has several grant opportunities to support rural communities, including $28 million to provide direct health services and expand infrastructure and $16 million to provide technical assistance to rural hospitals facing financial distress. This year, 60 rural hospitals will receive technical assistance to maintain financial viability and ensure continued access to care.
The HRSA National Health Service Corps Rural Community Loan Repayment Program has invested $80 million to support substance use disorder treatment, assist in recovery, and prevent overdose deaths. Medicare will also cover opioid use disorder treatment services delivered by mobile units of registered opioid treatment programs, which can now be accessed via telehealth or audio-only communications.
Curricula Also Lack Native Diversity
As of 2017, only 11% of MD-granting schools in the US say they have included Native American health content in their curricula. Dr. Owen notes some of the challenges indigenous students face: They are in a crowd that is primarily non-Native, far from their own family and community; unlike White students, they usually do not have mentors; they may not have the wherewithal to continue school and graduate.
A 2022 study of the association of sociodemographic characteristics with US medical student attrition, published in JAMA Internal Medicine, found that American Indian, Alaska Native, Native Hawaiian, and Pacific Islander students were more than 4 times as likely to drop out compared with White students. More than 10% of Indigenous medical students don’t graduate—the highest of any group the researchers examined.
In 1973 the University of North Dakota, for instance, launched Indians Into Medicine (INMED), a program that has since recruited, supported, and trained 250 American Indian doctors, and, in 2019, the country’s first PhD program in indigenous health. Dr. Warne, the director of INMED, calls it “by far, the most successful indigenous medical training program in the world,” having helped 228 American Indians and Alaska Natives graduate since its inception. A new cohort of 6 students has just enrolled.
Oregon Health & Science University (OHSU) received $800,000 in federal funding for its Future Leaders in Indigenous Health (FLIGHT) project, managed through OHSU’s Northwest Native American Center of Excellence (NNACoE). In 2012, just 8 Native students were enrolled in the OHSU School of Medicine; a decade later, there were 29. In 2022, the newest medical class included 12 American Indian or Alaska Native students. According to the school, it is believed to be the largest group of Natives in any single US medical school MD class in history. The number of Native faculty in the OHSU School of Medicine grew from 7 in 2014 to 13 in 2022.
America was already facing a critical health care workforce shortage before the COVID-19 pandemic exacerbated the problem. The American Medical Association (AMA) projects that there will be a national shortage of up to 48,000 primary care physicians and 77,100 non-primary care physicians by 2034.
The dearth is particularly striking among physicians who practice in rural areas and those who are Native American. As of 2021, fewer than 3000 physicians—of 841,322—identified as American Indian or Alaska Native, according to the latest statistics from the Physician Specialty Data Report, published by the Association of American Medical Colleges (AAMC).
The lack of Native American physicians is “nothing new, it’s been going on for decades,” says Mary Owen (Tlingit), MD, director of the Center of American Indian and Minority Health and associate dean of Native American Health at the University of Minnesota Medical School, speaking in a Native America Calling podcast in October.
“These numbers are… actually lessening—and we had paltry numbers to begin with,” said Owen. “It doesn’t take a genius to look back and figure out where it’s from. We don’t have enough students coming through the pathways in the first place. For instance, our high school graduation rate in this country is easily 10 points below that of non-Natives. In Duluth, Minnesota, the high school graduation rate is only 43%… We have to recognize that this is an area we have to work on.”
Senators Tim Kaine (D-VA) and Alex Padilla (D-CA) have introduced the Expanding Medical Education Act, legislation to get more students from underrepresented groups into the physician pipeline. The bill would provide grants through the Health Resources and Services Administration (HRSA) for colleges and universities to establish or expand allopathic (MD-granting) or osteopathic (DO-granting) medical schools in underserved areas or at institutions for underrepresented populations, including Historically Black Colleges and Universities (HBCUs).
Addressing Rural Needs
However, projections on the growth of health care professions show that supply will not meet demand over the next 10 years. The shortage is more dire in rural areas. According to the US Department of Health and Human Services (HHS), since 2010, more than 150 rural hospitals have either closed their doors entirely or stopped providing inpatient hospital services. Often, rural communities have fewer local HCPs available.
More than half (54%) of American Indian or Alaska Native people live in rural and small-town areas, and 68% live on or near their tribal homelands, according to the nonprofit First Nations Development Institute. Many live far—even hours—away from the nearest health care facility. But according to Population Health in Rural America in 2020: Proceedings of a Workshop, only 10% of primary care practitioners and < 7% of specialty care practitioners live in rural areas. About 5% of rural counties do not have any family physicians. What’s more, language and culture differ among the nearly 600 tribes across the country. The Indian Health Council, for instance, counts 9 individual reservations and tribes within a 5-mile radius in San Diego County, “all of which have their own unique customs,” which contribute to the “level of care they deem appropriate.”
“If you’re a rural impoverished community, it’s hard to recruit doctors. We’re more likely to return to our communities,” said Donald K. Warne (Oglala Lakota), MD, MPH, Associate Dean for Diversity, Equity, and Inclusion at the University of North Dakota School of Medicine and Health Sciences, during the 2019 American Indian or Alaska Native Physicians Summit, which was cosponsored by the AMA, Association of American Indian Physicians (AAIP), and the AAMC.
“Communities of color and those living in rural and underserved areas have long faced significant barriers to health care, including a lack of providers that look like them or practice close by,” said Senator Kaine in a statement. “Since research shows that physicians are more likely to practice in the areas they’re from, supporting medical schools at minority-serving institutions and HBCUs in underserved areas can help improve care in those communities.”
Where Are the Native Medical Students?
Only 9% of medical schools have more than 4 American Indian or Alaska Native students; 43% have none, says Siobhan M. Wescott, MD, MPH, chair of the AMA Minority Affairs Section (MAS), and an assistant professor at the University of North Dakota. Dr. Wescott, who hosted the AMA co-sponsored summit on behalf of the AMA-MAS, is an Alaska Native and 1 of only 3 physicians from her tribe. The AAMC has also found that less than half of MD-granting medical schools in the US have enrolled more than 5 Native students.
Among other things, the Expanding Medical Education Act would prioritize grants to institutions of higher education that propose to use the funds to establish a medical school or branch campus in an area in which no other such school is based and is a medically underserved community or “health professional shortage” area. Eligible uses for the grants include hiring diverse faculty and other staff, and recruiting students from underrepresented racial and ethnic minorities, students from rural and underserved areas, low-income students, and first-generation college students.
The legislation has been endorsed by the AAMC, American Association of Colleges of Osteopathic Medicine, Association of American Indian Physicians, Association of Clinicians for the Underserved, National Hispanic Medical Association, Society for Advancement of Chicanos/Hispanics and Native Americans in Science, and Ochsner Health.
Funding Is Key
Federal agencies are investing in funding and training. Medicare is allocating 1000 new training slots for medical residents, prioritizing rural and underserved areas. Centers for Medicare and Medicaid Services (CMS) is offering another 200 slots, at least 100 of which are specifically for psychiatry residencies in 2026. HHS awarded more than $11 million through the Rural Residency Planning and Development Program (RRPD) to help establish new rural residency programs. Accredited RRPD-funded programs are already training more than 300 resident physicians in family medicine, internal medicine, psychiatry, and general surgery. HRSA published an opportunity for $5 million in FY 2024 to develop and implement clinical rotations for physician assistant students in rural areas that will integrate behavioral health with primary care services.
The Biden-Harris Administration has already taken several steps to improve access to health care for the more than 60 million people who live in rural areas, including: building on the Affordable Care Act and Inflation Reduction Act to increase access to affordable health coverage and care for those living in rural communities; keeping more rural hospitals open to provide critical services in their communities; and bolstering the rural health workforce, including for primary care and behavioral HCPs.
The administration also has funded small rural hospitals and Medicare-certified Rural Health Clinics. Critical access hospitals and small hospitals in rural areas have a new option: to convert to a Rural Emergency Hospital (REH), a new Medicare provider type. CMS has changed the payment method for Tribal and Indian Health Services–operated REHs, to address certain barriers that may have discouraged Tribal and Indian Health Service (IHS)–operated hospitals from converting to REHs. Beginning in FY 2022, HHS, through HRSA, dedicated $5 million to provide technical assistance to rural hospitals that are considering converting to the REH designation.
HHS also has several grant opportunities to support rural communities, including $28 million to provide direct health services and expand infrastructure and $16 million to provide technical assistance to rural hospitals facing financial distress. This year, 60 rural hospitals will receive technical assistance to maintain financial viability and ensure continued access to care.
The HRSA National Health Service Corps Rural Community Loan Repayment Program has invested $80 million to support substance use disorder treatment, assist in recovery, and prevent overdose deaths. Medicare will also cover opioid use disorder treatment services delivered by mobile units of registered opioid treatment programs, which can now be accessed via telehealth or audio-only communications.
Curricula Also Lack Native Diversity
As of 2017, only 11% of MD-granting schools in the US say they have included Native American health content in their curricula. Dr. Owen notes some of the challenges indigenous students face: They are in a crowd that is primarily non-Native, far from their own family and community; unlike White students, they usually do not have mentors; they may not have the wherewithal to continue school and graduate.
A 2022 study of the association of sociodemographic characteristics with US medical student attrition, published in JAMA Internal Medicine, found that American Indian, Alaska Native, Native Hawaiian, and Pacific Islander students were more than 4 times as likely to drop out compared with White students. More than 10% of Indigenous medical students don’t graduate—the highest of any group the researchers examined.
In 1973 the University of North Dakota, for instance, launched Indians Into Medicine (INMED), a program that has since recruited, supported, and trained 250 American Indian doctors, and, in 2019, the country’s first PhD program in indigenous health. Dr. Warne, the director of INMED, calls it “by far, the most successful indigenous medical training program in the world,” having helped 228 American Indians and Alaska Natives graduate since its inception. A new cohort of 6 students has just enrolled.
Oregon Health & Science University (OHSU) received $800,000 in federal funding for its Future Leaders in Indigenous Health (FLIGHT) project, managed through OHSU’s Northwest Native American Center of Excellence (NNACoE). In 2012, just 8 Native students were enrolled in the OHSU School of Medicine; a decade later, there were 29. In 2022, the newest medical class included 12 American Indian or Alaska Native students. According to the school, it is believed to be the largest group of Natives in any single US medical school MD class in history. The number of Native faculty in the OHSU School of Medicine grew from 7 in 2014 to 13 in 2022.
ALL: ASH Draws Up Tx Guidelines For Patients 15-39
At the crux of the matter is the unusual nature of ALL, said University of Chicago leukemia specialist Wendy Stock, MD, in a presentation at the annual meeting of the American Society of Hematology in December 2023. The disease is both rare and unique since it spans the entire lifetime from infancy to old age, she said.
The guidelines will focus on adolescents and young adults, which the National Cancer Institute defines as those aged 15-39. For these patients, “treatment is administered by the whole gamut of practitioners in the world of hematology, from pediatricians to adult hematologist/oncologists, which provides unique challenges in terms of understanding and access to care,” Dr. Stock said.
As she explained, ALL “is the bread and butter of pediatric oncology, but in the world of adult hematology-oncology, many patients are treated in small-practice settings where there have been very few uniform approaches available to the treating practitioners,” she said. “There’s not going to ever be the ability to get every — or even the majority — of adults into those big academic centers.”
Meanwhile, research from around the world has highlighted major mortality gaps between pediatric and adult care in ALL. “This has been our huge challenge: Is it the treatment approach? Is it the disease biology, the patient biology, the doctors who treat these diseases? Is it the geographic location where they’re treated? Well, we now know that, of course, it’s probably all of the above, and a lot more than that.”
In light of the need for guidance in ALL treatment, it will be crucial to disseminate data and recommendations via the guidelines, she said.
In 2021, ASH members approved the development of new clinical practice guidelines for this population. The process so far has been difficult, said pediatric oncologist Sumit Gupta, MD, PhD, of the Hospital for Sick Children in Toronto, Ontario, at the ASH presentation.
“At one point,” Dr. Gupta recalled, “someone on our methodology team said this was the most challenging systematic review and guideline creation that they’d ever worked on, which is not what you want to hear as a co-chair.”
One major challenge for the guideline drafters is to balance ALL research findings that cover only certain ages, Dr. Gupta said. A study, for example, may only include patients up to age 21 or over age 35, making it difficult to decide how it fits into a larger evidence base for adolescents and young adults.
“We don’t always have perfect evidence. But we’re trying to take all of that and translate it into a formalized systematic review,” he said. “This is tricky for any guideline. But ALL poses a particular challenge because of how the evidence base is spread out.”
Another challenge is figuring out how to review psychosocial interventions in ALL. They are obviously crucial, he said. But should guidelines only take into account strategies that were tested in ALL? Or should they look at a wider perspective and encompass research into non–ALL-specific approaches?
In terms of guidance about frontline treatment, the guideline developers are focusing on several topics, said University of Rochester hematologist/oncologist Kristen O’Dwyer, MD, at the ASH presentation. These include: Should adolescents and young adults receive pediatric or adult regimens? Where do targeted therapy, immunotherapy, steroids, allogeneic stem cell transplants, and central nervous system (CNS) prophylaxis fit in?
“Finally, there are a series of questions that are addressing the toxicity prevention and management that go along with these intensive chemotherapy regimens,” she said.
On one front, there’s a “knowledge gap” about the value of stem cell transplant vs pediatric-inspired chemotherapy as postremission therapies, Dr. O’Dwyer said, because there are no direct comparisons. What to do? “There are retrospective comparisons that are emerging along with population-level analysis, single-arm observational studies that suggest that a pediatric-based chemotherapy approach is superior with similar relapse rates and less treatment-related mortality,” she said.
ASH expects to release a draft of its ALL guidelines for adolescents and young adults later this year and publish final recommendations in late 2024 or early 2025.
Dr. Stock, Dr. Gupta, and Dr. O’Dwyer have no disclosures.
At the crux of the matter is the unusual nature of ALL, said University of Chicago leukemia specialist Wendy Stock, MD, in a presentation at the annual meeting of the American Society of Hematology in December 2023. The disease is both rare and unique since it spans the entire lifetime from infancy to old age, she said.
The guidelines will focus on adolescents and young adults, which the National Cancer Institute defines as those aged 15-39. For these patients, “treatment is administered by the whole gamut of practitioners in the world of hematology, from pediatricians to adult hematologist/oncologists, which provides unique challenges in terms of understanding and access to care,” Dr. Stock said.
As she explained, ALL “is the bread and butter of pediatric oncology, but in the world of adult hematology-oncology, many patients are treated in small-practice settings where there have been very few uniform approaches available to the treating practitioners,” she said. “There’s not going to ever be the ability to get every — or even the majority — of adults into those big academic centers.”
Meanwhile, research from around the world has highlighted major mortality gaps between pediatric and adult care in ALL. “This has been our huge challenge: Is it the treatment approach? Is it the disease biology, the patient biology, the doctors who treat these diseases? Is it the geographic location where they’re treated? Well, we now know that, of course, it’s probably all of the above, and a lot more than that.”
In light of the need for guidance in ALL treatment, it will be crucial to disseminate data and recommendations via the guidelines, she said.
In 2021, ASH members approved the development of new clinical practice guidelines for this population. The process so far has been difficult, said pediatric oncologist Sumit Gupta, MD, PhD, of the Hospital for Sick Children in Toronto, Ontario, at the ASH presentation.
“At one point,” Dr. Gupta recalled, “someone on our methodology team said this was the most challenging systematic review and guideline creation that they’d ever worked on, which is not what you want to hear as a co-chair.”
One major challenge for the guideline drafters is to balance ALL research findings that cover only certain ages, Dr. Gupta said. A study, for example, may only include patients up to age 21 or over age 35, making it difficult to decide how it fits into a larger evidence base for adolescents and young adults.
“We don’t always have perfect evidence. But we’re trying to take all of that and translate it into a formalized systematic review,” he said. “This is tricky for any guideline. But ALL poses a particular challenge because of how the evidence base is spread out.”
Another challenge is figuring out how to review psychosocial interventions in ALL. They are obviously crucial, he said. But should guidelines only take into account strategies that were tested in ALL? Or should they look at a wider perspective and encompass research into non–ALL-specific approaches?
In terms of guidance about frontline treatment, the guideline developers are focusing on several topics, said University of Rochester hematologist/oncologist Kristen O’Dwyer, MD, at the ASH presentation. These include: Should adolescents and young adults receive pediatric or adult regimens? Where do targeted therapy, immunotherapy, steroids, allogeneic stem cell transplants, and central nervous system (CNS) prophylaxis fit in?
“Finally, there are a series of questions that are addressing the toxicity prevention and management that go along with these intensive chemotherapy regimens,” she said.
On one front, there’s a “knowledge gap” about the value of stem cell transplant vs pediatric-inspired chemotherapy as postremission therapies, Dr. O’Dwyer said, because there are no direct comparisons. What to do? “There are retrospective comparisons that are emerging along with population-level analysis, single-arm observational studies that suggest that a pediatric-based chemotherapy approach is superior with similar relapse rates and less treatment-related mortality,” she said.
ASH expects to release a draft of its ALL guidelines for adolescents and young adults later this year and publish final recommendations in late 2024 or early 2025.
Dr. Stock, Dr. Gupta, and Dr. O’Dwyer have no disclosures.
At the crux of the matter is the unusual nature of ALL, said University of Chicago leukemia specialist Wendy Stock, MD, in a presentation at the annual meeting of the American Society of Hematology in December 2023. The disease is both rare and unique since it spans the entire lifetime from infancy to old age, she said.
The guidelines will focus on adolescents and young adults, which the National Cancer Institute defines as those aged 15-39. For these patients, “treatment is administered by the whole gamut of practitioners in the world of hematology, from pediatricians to adult hematologist/oncologists, which provides unique challenges in terms of understanding and access to care,” Dr. Stock said.
As she explained, ALL “is the bread and butter of pediatric oncology, but in the world of adult hematology-oncology, many patients are treated in small-practice settings where there have been very few uniform approaches available to the treating practitioners,” she said. “There’s not going to ever be the ability to get every — or even the majority — of adults into those big academic centers.”
Meanwhile, research from around the world has highlighted major mortality gaps between pediatric and adult care in ALL. “This has been our huge challenge: Is it the treatment approach? Is it the disease biology, the patient biology, the doctors who treat these diseases? Is it the geographic location where they’re treated? Well, we now know that, of course, it’s probably all of the above, and a lot more than that.”
In light of the need for guidance in ALL treatment, it will be crucial to disseminate data and recommendations via the guidelines, she said.
In 2021, ASH members approved the development of new clinical practice guidelines for this population. The process so far has been difficult, said pediatric oncologist Sumit Gupta, MD, PhD, of the Hospital for Sick Children in Toronto, Ontario, at the ASH presentation.
“At one point,” Dr. Gupta recalled, “someone on our methodology team said this was the most challenging systematic review and guideline creation that they’d ever worked on, which is not what you want to hear as a co-chair.”
One major challenge for the guideline drafters is to balance ALL research findings that cover only certain ages, Dr. Gupta said. A study, for example, may only include patients up to age 21 or over age 35, making it difficult to decide how it fits into a larger evidence base for adolescents and young adults.
“We don’t always have perfect evidence. But we’re trying to take all of that and translate it into a formalized systematic review,” he said. “This is tricky for any guideline. But ALL poses a particular challenge because of how the evidence base is spread out.”
Another challenge is figuring out how to review psychosocial interventions in ALL. They are obviously crucial, he said. But should guidelines only take into account strategies that were tested in ALL? Or should they look at a wider perspective and encompass research into non–ALL-specific approaches?
In terms of guidance about frontline treatment, the guideline developers are focusing on several topics, said University of Rochester hematologist/oncologist Kristen O’Dwyer, MD, at the ASH presentation. These include: Should adolescents and young adults receive pediatric or adult regimens? Where do targeted therapy, immunotherapy, steroids, allogeneic stem cell transplants, and central nervous system (CNS) prophylaxis fit in?
“Finally, there are a series of questions that are addressing the toxicity prevention and management that go along with these intensive chemotherapy regimens,” she said.
On one front, there’s a “knowledge gap” about the value of stem cell transplant vs pediatric-inspired chemotherapy as postremission therapies, Dr. O’Dwyer said, because there are no direct comparisons. What to do? “There are retrospective comparisons that are emerging along with population-level analysis, single-arm observational studies that suggest that a pediatric-based chemotherapy approach is superior with similar relapse rates and less treatment-related mortality,” she said.
ASH expects to release a draft of its ALL guidelines for adolescents and young adults later this year and publish final recommendations in late 2024 or early 2025.
Dr. Stock, Dr. Gupta, and Dr. O’Dwyer have no disclosures.
FROM ASH 2023