User login
Treatment of HCV in special populations
MAUI, HAWAII – Treatment of acute rather than chronic hepatitis C infection is well worth considering in selected circumstances, Norah Terrault, MD, asserted at the Gastroenterology Updates, IBD, Liver Disease meeting.
This is not at present guideline-recommended therapy. Current American Association for the Study of Liver Disease/Infectious Diseases Society of America guidance states that while there is emerging data to support treatment of acute hepatitis C, the evidence isn’t yet sufficiently robust to support a particular regimen or duration. The guidelines currently recommend waiting 6 months to see if the acute infection resolves spontaneously, as happens in a minority of cases, or becomes chronic, at which point it becomes guideline-directed treatment time. But Dr. Terrault believes persuasive evidence to back treatment of acute hepatitis C infection (HCV) is forthcoming, and she noted that the guidelines leave the door ajar by stating, “There are instances wherein a clinician may decide that the benefits of early treatment outweigh waiting for possible spontaneous clearance.”
Treatment of acute HCV
Dr. Terrault deems treatment of acute HCV warranted in circumstances in which there is significant danger of transmission from the acutely infected individual to others. For example, health care providers with a needlestick HCV infection, injecting drug users, and men with acute HCV/HIV coinfection. She also treats acute HCV in patients with underlying chronic liver disease.
“Clearly, I wouldn’t want those individuals to have any worsening of their liver function, so I would treat them acutely,” explained Dr. Terrault, professor of medicine and director of the Viral Hepatitis Center at the University of California, San Francisco.
She cited as particularly impressive the results of the SWIFT-C trial presented by Suzanna Naggie, MD, of Duke University, Durham, N.C., at the 2017 AASLD annual meeting. In this modest-size, National Institutes of Health–sponsored, multicenter study of HIV-infected men with acute HCV coinfection, the sustained viral response (SVR) rate with 8 weeks of ledipasvir/sofosbuvir (Harvoni) was 100%, regardless of their baseline HCV RNA level.
“I think this is remarkable. They cleared virus quite late and yet they went on to achieve HCV eradication. It highlights how little we really know about the treatment of individuals in this phase and that relying on HCV RNA levels may not tell the whole story. I think this is important data to suggest maybe when we treat acute hepatitis C we can use a shorter duration of treatment for that population. There are also other small studies testing 8 weeks of treatment in non–HIV-infected individuals with acute hepatitis C in which they also showed very high SVR rates,” the hepatologist said.
Copanelist Steven L. Flamm, MD, said that when he encounters a patient with acute HCV he, too, is prepared to offer treatment – he finds the available supporting evidence sufficiently compelling – but he often encounters a problem.
“Sometimes I’m blocked by insurance companies because this isn’t officially approved,” noted Dr. Flamm, professor of medicine and chief of the hepatology program at Northwestern University, Chicago.
“You’re right,” Dr. Terrault commented, “we have to make a pretty compelling argument to the insurer as to why we’re treating. But ‘treat to prevent transmission to others’ usually is successful in our hands.”
HCV in patients with end-stage renal disease
The product labeling for sofosbuvir (Sovaldi) says the drug’s safety and efficacy haven’t been established in patients with severe renal impairment or end-stage renal disease. However, a small multicenter study presented at the 2017 AASLD meeting demonstrated that 12 weeks of ledipasvir/sofosbuvir achieved a 100% SVR rate in patients with genotype 1 HCV and severe renal impairment, including some on dialysis, with no clinically meaningful change in estimated glomerular filtration rate or any signal of cardiac arrhythmia.
“The serum drug levels went up significantly, but reassuringly they saw no meaningful safety signals,” according to Dr. Terrault. “This, I think, is initial reassuring information that we were all very much waiting for.”
Still, as the AASLD/IDSA guidelines point out, ledipasvir/sofosbuvir is not a recommended option for HCV treatment in end-stage renal disease.
“In general, I think glecapravir/pibrentasvir [Mavyret] has become the go-to drug for patients who have renal dysfunction because it’s a pangenic regimen, it doesn’t require use of sofosbuvir, and there’s no dose adjustment. But I would say you could encounter situations where you might want to use sofosbuvir, and for me that situation is typically those direct-acting, antiviral-experienced patients who have failed other therapies and you really need to use sofosbuvir/velpatasvir/voxilaprevir [Vosevi] as your last or rescue therapy,” the hepatologist continued.
HCV in liver transplant recipients
“In the years before the direct-acting antivirals, treating transplant patients was always very challenging,” Dr. Terrault recalled. “They had very low response rates to therapy. That’s all gone away. Now we can say that liver transplant recipients who require treatment have response rates that are the same as in individuals who have not had a transplant. These patients are now being treated earlier and earlier after their transplant because you can do it safely.”
She pointed to a study presented at the 2017 AASLD meeting by Kosh Agarwal, MD, of Kings College London. It involved 79 adults with recurrent genotypes 1-4 HCV infection post–liver transplant who were treated with sofosbuvir/velpatasvir (Epclusa) for 12 weeks with a total SVR rate of 96%.
“The nice thing about sofosbuvir/velpatasvir is there are no drug-drug interactions with immunosuppressive drugs. Now it’s very easy to take care of these patients. The SVR rates are excellent,” Dr. Terrault observed.
The other combination that’s been studied specifically in liver transplant recipients, and in kidney transplant recipients as well, is glecapravir/pibrentasvir. In the MAGELLAN-2 study of 100 such patients with genotypes 1-6 HCV, the SVR rate was 99% with no drug-related adverse events leading to discontinuation.
Persons who inject drugs
The Centers for Disease Control and Prevention and the World Health Organization want HCV eradicated by 2030. If that’s going to happen, physicians will have to become more comfortable treating the disease in injectable drug users, a population with a high prevalence of HCV. Several studies have now shown that very high SVR rates can be achieved with direct-acting antiviral regimens as short as 8 weeks in these individuals, even if they are concurrently injecting drugs.
“There is increasing evidence that we should be doing more treatment in persons who inject drugs. Many of these individuals have very early disease and their response rates are excellent,” according to Dr. Terrault.
Moreover, their reinfection rates “are not outrageous,” she said: 1% or less in individuals who stopped injecting drugs decades prior to anti-HCV treatment, 5%-10% over the course of 3-5 years in those who continue injecting drugs after achieving SVR, and about 2% in those on methadone substitution therapy.
“These are very acceptable levels of reinfection if our goal is to move toward elimination of hepatitis C in this population,” she said.
She reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – Treatment of acute rather than chronic hepatitis C infection is well worth considering in selected circumstances, Norah Terrault, MD, asserted at the Gastroenterology Updates, IBD, Liver Disease meeting.
This is not at present guideline-recommended therapy. Current American Association for the Study of Liver Disease/Infectious Diseases Society of America guidance states that while there is emerging data to support treatment of acute hepatitis C, the evidence isn’t yet sufficiently robust to support a particular regimen or duration. The guidelines currently recommend waiting 6 months to see if the acute infection resolves spontaneously, as happens in a minority of cases, or becomes chronic, at which point it becomes guideline-directed treatment time. But Dr. Terrault believes persuasive evidence to back treatment of acute hepatitis C infection (HCV) is forthcoming, and she noted that the guidelines leave the door ajar by stating, “There are instances wherein a clinician may decide that the benefits of early treatment outweigh waiting for possible spontaneous clearance.”
Treatment of acute HCV
Dr. Terrault deems treatment of acute HCV warranted in circumstances in which there is significant danger of transmission from the acutely infected individual to others. For example, health care providers with a needlestick HCV infection, injecting drug users, and men with acute HCV/HIV coinfection. She also treats acute HCV in patients with underlying chronic liver disease.
“Clearly, I wouldn’t want those individuals to have any worsening of their liver function, so I would treat them acutely,” explained Dr. Terrault, professor of medicine and director of the Viral Hepatitis Center at the University of California, San Francisco.
She cited as particularly impressive the results of the SWIFT-C trial presented by Suzanna Naggie, MD, of Duke University, Durham, N.C., at the 2017 AASLD annual meeting. In this modest-size, National Institutes of Health–sponsored, multicenter study of HIV-infected men with acute HCV coinfection, the sustained viral response (SVR) rate with 8 weeks of ledipasvir/sofosbuvir (Harvoni) was 100%, regardless of their baseline HCV RNA level.
“I think this is remarkable. They cleared virus quite late and yet they went on to achieve HCV eradication. It highlights how little we really know about the treatment of individuals in this phase and that relying on HCV RNA levels may not tell the whole story. I think this is important data to suggest maybe when we treat acute hepatitis C we can use a shorter duration of treatment for that population. There are also other small studies testing 8 weeks of treatment in non–HIV-infected individuals with acute hepatitis C in which they also showed very high SVR rates,” the hepatologist said.
Copanelist Steven L. Flamm, MD, said that when he encounters a patient with acute HCV he, too, is prepared to offer treatment – he finds the available supporting evidence sufficiently compelling – but he often encounters a problem.
“Sometimes I’m blocked by insurance companies because this isn’t officially approved,” noted Dr. Flamm, professor of medicine and chief of the hepatology program at Northwestern University, Chicago.
“You’re right,” Dr. Terrault commented, “we have to make a pretty compelling argument to the insurer as to why we’re treating. But ‘treat to prevent transmission to others’ usually is successful in our hands.”
HCV in patients with end-stage renal disease
The product labeling for sofosbuvir (Sovaldi) says the drug’s safety and efficacy haven’t been established in patients with severe renal impairment or end-stage renal disease. However, a small multicenter study presented at the 2017 AASLD meeting demonstrated that 12 weeks of ledipasvir/sofosbuvir achieved a 100% SVR rate in patients with genotype 1 HCV and severe renal impairment, including some on dialysis, with no clinically meaningful change in estimated glomerular filtration rate or any signal of cardiac arrhythmia.
“The serum drug levels went up significantly, but reassuringly they saw no meaningful safety signals,” according to Dr. Terrault. “This, I think, is initial reassuring information that we were all very much waiting for.”
Still, as the AASLD/IDSA guidelines point out, ledipasvir/sofosbuvir is not a recommended option for HCV treatment in end-stage renal disease.
“In general, I think glecapravir/pibrentasvir [Mavyret] has become the go-to drug for patients who have renal dysfunction because it’s a pangenic regimen, it doesn’t require use of sofosbuvir, and there’s no dose adjustment. But I would say you could encounter situations where you might want to use sofosbuvir, and for me that situation is typically those direct-acting, antiviral-experienced patients who have failed other therapies and you really need to use sofosbuvir/velpatasvir/voxilaprevir [Vosevi] as your last or rescue therapy,” the hepatologist continued.
HCV in liver transplant recipients
“In the years before the direct-acting antivirals, treating transplant patients was always very challenging,” Dr. Terrault recalled. “They had very low response rates to therapy. That’s all gone away. Now we can say that liver transplant recipients who require treatment have response rates that are the same as in individuals who have not had a transplant. These patients are now being treated earlier and earlier after their transplant because you can do it safely.”
She pointed to a study presented at the 2017 AASLD meeting by Kosh Agarwal, MD, of Kings College London. It involved 79 adults with recurrent genotypes 1-4 HCV infection post–liver transplant who were treated with sofosbuvir/velpatasvir (Epclusa) for 12 weeks with a total SVR rate of 96%.
“The nice thing about sofosbuvir/velpatasvir is there are no drug-drug interactions with immunosuppressive drugs. Now it’s very easy to take care of these patients. The SVR rates are excellent,” Dr. Terrault observed.
The other combination that’s been studied specifically in liver transplant recipients, and in kidney transplant recipients as well, is glecapravir/pibrentasvir. In the MAGELLAN-2 study of 100 such patients with genotypes 1-6 HCV, the SVR rate was 99% with no drug-related adverse events leading to discontinuation.
Persons who inject drugs
The Centers for Disease Control and Prevention and the World Health Organization want HCV eradicated by 2030. If that’s going to happen, physicians will have to become more comfortable treating the disease in injectable drug users, a population with a high prevalence of HCV. Several studies have now shown that very high SVR rates can be achieved with direct-acting antiviral regimens as short as 8 weeks in these individuals, even if they are concurrently injecting drugs.
“There is increasing evidence that we should be doing more treatment in persons who inject drugs. Many of these individuals have very early disease and their response rates are excellent,” according to Dr. Terrault.
Moreover, their reinfection rates “are not outrageous,” she said: 1% or less in individuals who stopped injecting drugs decades prior to anti-HCV treatment, 5%-10% over the course of 3-5 years in those who continue injecting drugs after achieving SVR, and about 2% in those on methadone substitution therapy.
“These are very acceptable levels of reinfection if our goal is to move toward elimination of hepatitis C in this population,” she said.
She reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – Treatment of acute rather than chronic hepatitis C infection is well worth considering in selected circumstances, Norah Terrault, MD, asserted at the Gastroenterology Updates, IBD, Liver Disease meeting.
This is not at present guideline-recommended therapy. Current American Association for the Study of Liver Disease/Infectious Diseases Society of America guidance states that while there is emerging data to support treatment of acute hepatitis C, the evidence isn’t yet sufficiently robust to support a particular regimen or duration. The guidelines currently recommend waiting 6 months to see if the acute infection resolves spontaneously, as happens in a minority of cases, or becomes chronic, at which point it becomes guideline-directed treatment time. But Dr. Terrault believes persuasive evidence to back treatment of acute hepatitis C infection (HCV) is forthcoming, and she noted that the guidelines leave the door ajar by stating, “There are instances wherein a clinician may decide that the benefits of early treatment outweigh waiting for possible spontaneous clearance.”
Treatment of acute HCV
Dr. Terrault deems treatment of acute HCV warranted in circumstances in which there is significant danger of transmission from the acutely infected individual to others. For example, health care providers with a needlestick HCV infection, injecting drug users, and men with acute HCV/HIV coinfection. She also treats acute HCV in patients with underlying chronic liver disease.
“Clearly, I wouldn’t want those individuals to have any worsening of their liver function, so I would treat them acutely,” explained Dr. Terrault, professor of medicine and director of the Viral Hepatitis Center at the University of California, San Francisco.
She cited as particularly impressive the results of the SWIFT-C trial presented by Suzanna Naggie, MD, of Duke University, Durham, N.C., at the 2017 AASLD annual meeting. In this modest-size, National Institutes of Health–sponsored, multicenter study of HIV-infected men with acute HCV coinfection, the sustained viral response (SVR) rate with 8 weeks of ledipasvir/sofosbuvir (Harvoni) was 100%, regardless of their baseline HCV RNA level.
“I think this is remarkable. They cleared virus quite late and yet they went on to achieve HCV eradication. It highlights how little we really know about the treatment of individuals in this phase and that relying on HCV RNA levels may not tell the whole story. I think this is important data to suggest maybe when we treat acute hepatitis C we can use a shorter duration of treatment for that population. There are also other small studies testing 8 weeks of treatment in non–HIV-infected individuals with acute hepatitis C in which they also showed very high SVR rates,” the hepatologist said.
Copanelist Steven L. Flamm, MD, said that when he encounters a patient with acute HCV he, too, is prepared to offer treatment – he finds the available supporting evidence sufficiently compelling – but he often encounters a problem.
“Sometimes I’m blocked by insurance companies because this isn’t officially approved,” noted Dr. Flamm, professor of medicine and chief of the hepatology program at Northwestern University, Chicago.
“You’re right,” Dr. Terrault commented, “we have to make a pretty compelling argument to the insurer as to why we’re treating. But ‘treat to prevent transmission to others’ usually is successful in our hands.”
HCV in patients with end-stage renal disease
The product labeling for sofosbuvir (Sovaldi) says the drug’s safety and efficacy haven’t been established in patients with severe renal impairment or end-stage renal disease. However, a small multicenter study presented at the 2017 AASLD meeting demonstrated that 12 weeks of ledipasvir/sofosbuvir achieved a 100% SVR rate in patients with genotype 1 HCV and severe renal impairment, including some on dialysis, with no clinically meaningful change in estimated glomerular filtration rate or any signal of cardiac arrhythmia.
“The serum drug levels went up significantly, but reassuringly they saw no meaningful safety signals,” according to Dr. Terrault. “This, I think, is initial reassuring information that we were all very much waiting for.”
Still, as the AASLD/IDSA guidelines point out, ledipasvir/sofosbuvir is not a recommended option for HCV treatment in end-stage renal disease.
“In general, I think glecapravir/pibrentasvir [Mavyret] has become the go-to drug for patients who have renal dysfunction because it’s a pangenic regimen, it doesn’t require use of sofosbuvir, and there’s no dose adjustment. But I would say you could encounter situations where you might want to use sofosbuvir, and for me that situation is typically those direct-acting, antiviral-experienced patients who have failed other therapies and you really need to use sofosbuvir/velpatasvir/voxilaprevir [Vosevi] as your last or rescue therapy,” the hepatologist continued.
HCV in liver transplant recipients
“In the years before the direct-acting antivirals, treating transplant patients was always very challenging,” Dr. Terrault recalled. “They had very low response rates to therapy. That’s all gone away. Now we can say that liver transplant recipients who require treatment have response rates that are the same as in individuals who have not had a transplant. These patients are now being treated earlier and earlier after their transplant because you can do it safely.”
She pointed to a study presented at the 2017 AASLD meeting by Kosh Agarwal, MD, of Kings College London. It involved 79 adults with recurrent genotypes 1-4 HCV infection post–liver transplant who were treated with sofosbuvir/velpatasvir (Epclusa) for 12 weeks with a total SVR rate of 96%.
“The nice thing about sofosbuvir/velpatasvir is there are no drug-drug interactions with immunosuppressive drugs. Now it’s very easy to take care of these patients. The SVR rates are excellent,” Dr. Terrault observed.
The other combination that’s been studied specifically in liver transplant recipients, and in kidney transplant recipients as well, is glecapravir/pibrentasvir. In the MAGELLAN-2 study of 100 such patients with genotypes 1-6 HCV, the SVR rate was 99% with no drug-related adverse events leading to discontinuation.
Persons who inject drugs
The Centers for Disease Control and Prevention and the World Health Organization want HCV eradicated by 2030. If that’s going to happen, physicians will have to become more comfortable treating the disease in injectable drug users, a population with a high prevalence of HCV. Several studies have now shown that very high SVR rates can be achieved with direct-acting antiviral regimens as short as 8 weeks in these individuals, even if they are concurrently injecting drugs.
“There is increasing evidence that we should be doing more treatment in persons who inject drugs. Many of these individuals have very early disease and their response rates are excellent,” according to Dr. Terrault.
Moreover, their reinfection rates “are not outrageous,” she said: 1% or less in individuals who stopped injecting drugs decades prior to anti-HCV treatment, 5%-10% over the course of 3-5 years in those who continue injecting drugs after achieving SVR, and about 2% in those on methadone substitution therapy.
“These are very acceptable levels of reinfection if our goal is to move toward elimination of hepatitis C in this population,” she said.
She reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM GUILD 2018
Inhaled nitrous oxide for labor analgesia: Pearls from clinical experience
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
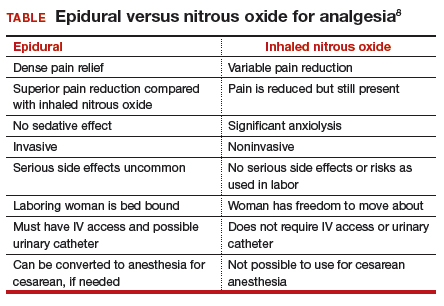
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.
Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
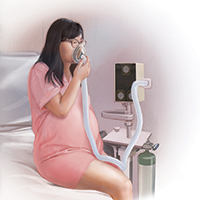
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.

Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.

Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
What’s Eating You? Ixodes Tick and Related Diseases, Part 3: Coinfection and Tick-Bite Prevention
Tick-borne diseases are increasing in prevalence, likely due to climate change in combination with human movement into tick habitats.1-3 The Ixodes genus of hard ticks is a common vector for the transmission of pathogenic viruses, bacteria, parasites, and toxins. Among these, Lyme disease, which is caused by Borrelia burgdorferi, is the most prevalent, followed by babesiosis and human granulocytic anaplasmosis (HGA), respectively.4 In Europe, tick-borne encephalitis is commonly encountered. More recently identified diseases transmitted by Ixodes ticks include Powassan virus and Borrelia miyamotoi infection; however, these diseases are less frequently encountered than other tick-borne diseases.5,6
As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing.7 Therefore, it is important for physicians who practice in endemic areas to be aware of the possibility of coinfection, which can alter clinical presentation, disease severity, and treatment response in tick-borne diseases. Additionally, public education on tick-bite prevention and prompt tick removal is necessary to combat the rising prevalence of these diseases.
Coinfection
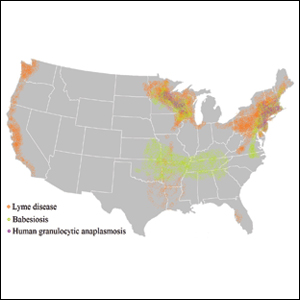
Risk of coinfection with more than one tick-borne disease is contingent on the geographic distribution of the tick species as well as the particular pathogen’s prevalence within reservoir hosts in a given area (Figure). Most coinfections occur with B. burgdorferi and an additional pathogen, usually Anaplasma phagocytophilum (which causes human granulocytic anaplasmosis [HGA]) or Babesia microti (which causes babesiosis). In Europe, coinfection with tick-borne encephalitis virus may occur. There is limited evidence of human coinfection with B miyamotoi or Powassan virus, as isolated infection with either of these pathogens is rare.
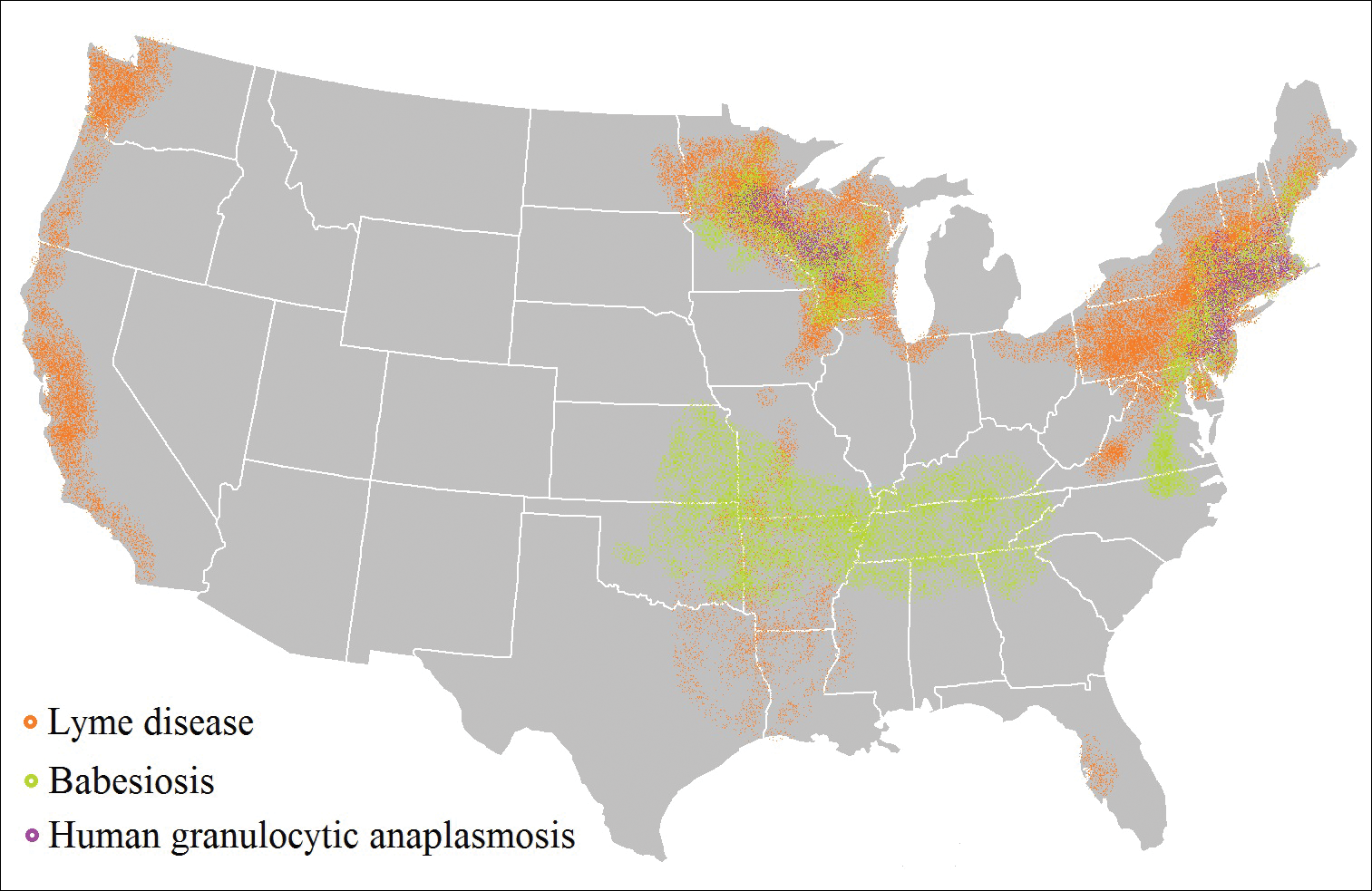
In patients with Lyme disease, as many as 35% may have concurrent babesiosis, and as many as 12% may have concurrent HGA in endemic areas (eg, northeast and northern central United States).7-9 Concurrent HGA and babesiosis in the absence of Lyme disease also has been documented.7-9 Coinfection generally increases the diversity of presenting symptoms, often obscuring the primary diagnosis. In addition, these patients may have more severe and prolonged illness.8,10,11
In endemic areas, coinfection with B burgdorferi and an additional pathogen should be suspected if a patient presents with typical symptoms of early Lyme disease, especially erythema migrans, along with (1) combination of fever, chills, and headache; (2) prolonged viral-like illness, particularly 48 hours after appropriate antibiotic treatment; and (3) unexplained blood dyscrasia.7,11,12 When a patient presents with erythema migrans, it is unnecessary to test for HGA, as treatment of Lyme disease with doxycycline also is adequate for treating HGA; however, if systemic symptoms persist despite treatment, testing for babesiosis and other tick-borne illnesses should be considered, as babesiosis requires treatment with atovaquone plus azithromycin or clindamycin plus quinine.13
A complete blood count and peripheral blood smear can aid in the diagnosis of coinfection. The complete blood count may reveal leukopenia, anemia, or thrombocytopenia associated with HGA or babesiosis. The peripheral blood smear can reveal inclusions of intra-erythrocytic ring forms and tetrads (the “Maltese cross” appearance) in babesiosis and intragranulocytic morulae in HGA.12 The most sensitive diagnostic tests for tick-borne diseases are organism-specific IgM and IgG serology for Lyme disease, babesiosis, and HGA and polymerase chain reaction for babesiosis and HGA.7
Prevention Strategies
The most effective means of controlling tick-borne disease is avoiding tick bites altogether. One method is to avoid spending time in high-risk areas that may be infested with ticks, particularly low-lying brush, where ticks are likely to hide.14 For individuals traveling in environments with a high risk of tick exposure, behavioral methods of avoidance are indicated, including wearing long pants and a shirt with long sleeves, tucking the shirt into the pants, and wearing closed-toe shoes. Wearing light-colored clothing may aid in tick identification and prompt removal prior to attachment. Permethrin-impregnated clothing has been proven to decrease the likelihood of tick bites in adults working outdoors.15-17
Topical repellents also play a role in the prevention of tick-borne diseases. The most effective and safe synthetic repellents are N,N-diethyl-meta-toluamide (DEET); picaridin; p-menthane-3,8-diol; and insect repellent 3535 (IR3535)(ethyl butylacetylaminopropionate).16-19 Plant-based repellents also are available, but their efficacy is strongly influenced by the surrounding environment (eg, temperature, humidity, organic matter).20-22 Individuals also may be exposed to ticks following contact with domesticated animals and pets.23,24 Tick prevention in pets with the use of ectoparasiticides should be directed by a qualified veterinarian.25
Tick Removal
Following a bite, the tick should be removed promptly to avoid transmission of pathogens. Numerous commercial and in-home methods of tick removal are available, but not all are equally effective. Detachment techniques include removal with a card or commercially available radiofrequency device, lassoing, or freezing.26,27 However, the most effective method is simple removal with tweezers. The tick should be grasped close to the skin surface and pulled upward with an even pressure. Commercially available tick-removal devices have not been shown to produce better outcomes than removal of the tick with tweezers.28
Conclusion
When patients do not respond to therapy for presumed tick-borne infection, the diagnosis should be reconsidered. One important consideration is coinfection with a second organism. Prompt identification and removal of ticks can prevent disease transmission.
- McMichael C, Barnett J, McMichael AJ. An ill wind? climate change, migration, and health. Environ Health Perspect. 2012;120:646-654.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140051.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134(pt 2):209-227.
- Tickborne diseases of the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/ticks/diseases/index.html. Updated July 25, 2017. Accessed April 10, 2018.
- Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733-740.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Krause PJ, McKay K, Thompson CA, et al; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184-1191.
- Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472-1477.
- Sweeny CJ, Ghassemi M, Agger WA, et al. Coinfection with Babesia microti and Borrelia burgdorferi in a western Wisconsin resident. Mayo Clin Proc.1998;73:338-341.
- Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27-30.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
- Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473-480.
- Miller NJ, Rainone EE, Dyer MC, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327-333.
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453-462.
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93.
- Büchel K, Bendin J, Gharbi A, et al. Repellent efficacy of DEET, icaridin, and EBAAP against Ixodes ricinus and Ixodes scapularis nymphs (Acari, Ixodidae). Ticks Tick Borne Dis. 2015;6:494-498.
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors. 2008;8:1-8.
- Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239-250.
- Feaster JE, Scialdone MA, Todd RG, et al. Dihydronepetalactones deter feeding activity by mosquitoes, stable flies, and deer ticks. J Med Entomol. 2009;46:832-840.
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasit Vectors. 2013;6:358.
- Rand PW, Smith RP Jr, Lacombe EH. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am J Public Health. 1991;81:1331-1334.
- Baneth G, Bourdeau P, Bourdoiseau G, et al; CVBD World Forum. Vector-borne diseases—constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. 2012;5:55.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393-397.
- Ashique KT, Kaliyadan F. Radiofrequency device for tick removal. J Am Acad Dermatol. 2015;72:155-156.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
Tick-borne diseases are increasing in prevalence, likely due to climate change in combination with human movement into tick habitats.1-3 The Ixodes genus of hard ticks is a common vector for the transmission of pathogenic viruses, bacteria, parasites, and toxins. Among these, Lyme disease, which is caused by Borrelia burgdorferi, is the most prevalent, followed by babesiosis and human granulocytic anaplasmosis (HGA), respectively.4 In Europe, tick-borne encephalitis is commonly encountered. More recently identified diseases transmitted by Ixodes ticks include Powassan virus and Borrelia miyamotoi infection; however, these diseases are less frequently encountered than other tick-borne diseases.5,6
As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing.7 Therefore, it is important for physicians who practice in endemic areas to be aware of the possibility of coinfection, which can alter clinical presentation, disease severity, and treatment response in tick-borne diseases. Additionally, public education on tick-bite prevention and prompt tick removal is necessary to combat the rising prevalence of these diseases.
Coinfection
Risk of coinfection with more than one tick-borne disease is contingent on the geographic distribution of the tick species as well as the particular pathogen’s prevalence within reservoir hosts in a given area (Figure). Most coinfections occur with B. burgdorferi and an additional pathogen, usually Anaplasma phagocytophilum (which causes human granulocytic anaplasmosis [HGA]) or Babesia microti (which causes babesiosis). In Europe, coinfection with tick-borne encephalitis virus may occur. There is limited evidence of human coinfection with B miyamotoi or Powassan virus, as isolated infection with either of these pathogens is rare.

In patients with Lyme disease, as many as 35% may have concurrent babesiosis, and as many as 12% may have concurrent HGA in endemic areas (eg, northeast and northern central United States).7-9 Concurrent HGA and babesiosis in the absence of Lyme disease also has been documented.7-9 Coinfection generally increases the diversity of presenting symptoms, often obscuring the primary diagnosis. In addition, these patients may have more severe and prolonged illness.8,10,11
In endemic areas, coinfection with B burgdorferi and an additional pathogen should be suspected if a patient presents with typical symptoms of early Lyme disease, especially erythema migrans, along with (1) combination of fever, chills, and headache; (2) prolonged viral-like illness, particularly 48 hours after appropriate antibiotic treatment; and (3) unexplained blood dyscrasia.7,11,12 When a patient presents with erythema migrans, it is unnecessary to test for HGA, as treatment of Lyme disease with doxycycline also is adequate for treating HGA; however, if systemic symptoms persist despite treatment, testing for babesiosis and other tick-borne illnesses should be considered, as babesiosis requires treatment with atovaquone plus azithromycin or clindamycin plus quinine.13
A complete blood count and peripheral blood smear can aid in the diagnosis of coinfection. The complete blood count may reveal leukopenia, anemia, or thrombocytopenia associated with HGA or babesiosis. The peripheral blood smear can reveal inclusions of intra-erythrocytic ring forms and tetrads (the “Maltese cross” appearance) in babesiosis and intragranulocytic morulae in HGA.12 The most sensitive diagnostic tests for tick-borne diseases are organism-specific IgM and IgG serology for Lyme disease, babesiosis, and HGA and polymerase chain reaction for babesiosis and HGA.7
Prevention Strategies
The most effective means of controlling tick-borne disease is avoiding tick bites altogether. One method is to avoid spending time in high-risk areas that may be infested with ticks, particularly low-lying brush, where ticks are likely to hide.14 For individuals traveling in environments with a high risk of tick exposure, behavioral methods of avoidance are indicated, including wearing long pants and a shirt with long sleeves, tucking the shirt into the pants, and wearing closed-toe shoes. Wearing light-colored clothing may aid in tick identification and prompt removal prior to attachment. Permethrin-impregnated clothing has been proven to decrease the likelihood of tick bites in adults working outdoors.15-17
Topical repellents also play a role in the prevention of tick-borne diseases. The most effective and safe synthetic repellents are N,N-diethyl-meta-toluamide (DEET); picaridin; p-menthane-3,8-diol; and insect repellent 3535 (IR3535)(ethyl butylacetylaminopropionate).16-19 Plant-based repellents also are available, but their efficacy is strongly influenced by the surrounding environment (eg, temperature, humidity, organic matter).20-22 Individuals also may be exposed to ticks following contact with domesticated animals and pets.23,24 Tick prevention in pets with the use of ectoparasiticides should be directed by a qualified veterinarian.25
Tick Removal
Following a bite, the tick should be removed promptly to avoid transmission of pathogens. Numerous commercial and in-home methods of tick removal are available, but not all are equally effective. Detachment techniques include removal with a card or commercially available radiofrequency device, lassoing, or freezing.26,27 However, the most effective method is simple removal with tweezers. The tick should be grasped close to the skin surface and pulled upward with an even pressure. Commercially available tick-removal devices have not been shown to produce better outcomes than removal of the tick with tweezers.28
Conclusion
When patients do not respond to therapy for presumed tick-borne infection, the diagnosis should be reconsidered. One important consideration is coinfection with a second organism. Prompt identification and removal of ticks can prevent disease transmission.
Tick-borne diseases are increasing in prevalence, likely due to climate change in combination with human movement into tick habitats.1-3 The Ixodes genus of hard ticks is a common vector for the transmission of pathogenic viruses, bacteria, parasites, and toxins. Among these, Lyme disease, which is caused by Borrelia burgdorferi, is the most prevalent, followed by babesiosis and human granulocytic anaplasmosis (HGA), respectively.4 In Europe, tick-borne encephalitis is commonly encountered. More recently identified diseases transmitted by Ixodes ticks include Powassan virus and Borrelia miyamotoi infection; however, these diseases are less frequently encountered than other tick-borne diseases.5,6
As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing.7 Therefore, it is important for physicians who practice in endemic areas to be aware of the possibility of coinfection, which can alter clinical presentation, disease severity, and treatment response in tick-borne diseases. Additionally, public education on tick-bite prevention and prompt tick removal is necessary to combat the rising prevalence of these diseases.
Coinfection
Risk of coinfection with more than one tick-borne disease is contingent on the geographic distribution of the tick species as well as the particular pathogen’s prevalence within reservoir hosts in a given area (Figure). Most coinfections occur with B. burgdorferi and an additional pathogen, usually Anaplasma phagocytophilum (which causes human granulocytic anaplasmosis [HGA]) or Babesia microti (which causes babesiosis). In Europe, coinfection with tick-borne encephalitis virus may occur. There is limited evidence of human coinfection with B miyamotoi or Powassan virus, as isolated infection with either of these pathogens is rare.

In patients with Lyme disease, as many as 35% may have concurrent babesiosis, and as many as 12% may have concurrent HGA in endemic areas (eg, northeast and northern central United States).7-9 Concurrent HGA and babesiosis in the absence of Lyme disease also has been documented.7-9 Coinfection generally increases the diversity of presenting symptoms, often obscuring the primary diagnosis. In addition, these patients may have more severe and prolonged illness.8,10,11
In endemic areas, coinfection with B burgdorferi and an additional pathogen should be suspected if a patient presents with typical symptoms of early Lyme disease, especially erythema migrans, along with (1) combination of fever, chills, and headache; (2) prolonged viral-like illness, particularly 48 hours after appropriate antibiotic treatment; and (3) unexplained blood dyscrasia.7,11,12 When a patient presents with erythema migrans, it is unnecessary to test for HGA, as treatment of Lyme disease with doxycycline also is adequate for treating HGA; however, if systemic symptoms persist despite treatment, testing for babesiosis and other tick-borne illnesses should be considered, as babesiosis requires treatment with atovaquone plus azithromycin or clindamycin plus quinine.13
A complete blood count and peripheral blood smear can aid in the diagnosis of coinfection. The complete blood count may reveal leukopenia, anemia, or thrombocytopenia associated with HGA or babesiosis. The peripheral blood smear can reveal inclusions of intra-erythrocytic ring forms and tetrads (the “Maltese cross” appearance) in babesiosis and intragranulocytic morulae in HGA.12 The most sensitive diagnostic tests for tick-borne diseases are organism-specific IgM and IgG serology for Lyme disease, babesiosis, and HGA and polymerase chain reaction for babesiosis and HGA.7
Prevention Strategies
The most effective means of controlling tick-borne disease is avoiding tick bites altogether. One method is to avoid spending time in high-risk areas that may be infested with ticks, particularly low-lying brush, where ticks are likely to hide.14 For individuals traveling in environments with a high risk of tick exposure, behavioral methods of avoidance are indicated, including wearing long pants and a shirt with long sleeves, tucking the shirt into the pants, and wearing closed-toe shoes. Wearing light-colored clothing may aid in tick identification and prompt removal prior to attachment. Permethrin-impregnated clothing has been proven to decrease the likelihood of tick bites in adults working outdoors.15-17
Topical repellents also play a role in the prevention of tick-borne diseases. The most effective and safe synthetic repellents are N,N-diethyl-meta-toluamide (DEET); picaridin; p-menthane-3,8-diol; and insect repellent 3535 (IR3535)(ethyl butylacetylaminopropionate).16-19 Plant-based repellents also are available, but their efficacy is strongly influenced by the surrounding environment (eg, temperature, humidity, organic matter).20-22 Individuals also may be exposed to ticks following contact with domesticated animals and pets.23,24 Tick prevention in pets with the use of ectoparasiticides should be directed by a qualified veterinarian.25
Tick Removal
Following a bite, the tick should be removed promptly to avoid transmission of pathogens. Numerous commercial and in-home methods of tick removal are available, but not all are equally effective. Detachment techniques include removal with a card or commercially available radiofrequency device, lassoing, or freezing.26,27 However, the most effective method is simple removal with tweezers. The tick should be grasped close to the skin surface and pulled upward with an even pressure. Commercially available tick-removal devices have not been shown to produce better outcomes than removal of the tick with tweezers.28
Conclusion
When patients do not respond to therapy for presumed tick-borne infection, the diagnosis should be reconsidered. One important consideration is coinfection with a second organism. Prompt identification and removal of ticks can prevent disease transmission.
- McMichael C, Barnett J, McMichael AJ. An ill wind? climate change, migration, and health. Environ Health Perspect. 2012;120:646-654.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140051.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134(pt 2):209-227.
- Tickborne diseases of the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/ticks/diseases/index.html. Updated July 25, 2017. Accessed April 10, 2018.
- Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733-740.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Krause PJ, McKay K, Thompson CA, et al; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184-1191.
- Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472-1477.
- Sweeny CJ, Ghassemi M, Agger WA, et al. Coinfection with Babesia microti and Borrelia burgdorferi in a western Wisconsin resident. Mayo Clin Proc.1998;73:338-341.
- Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27-30.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
- Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473-480.
- Miller NJ, Rainone EE, Dyer MC, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327-333.
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453-462.
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93.
- Büchel K, Bendin J, Gharbi A, et al. Repellent efficacy of DEET, icaridin, and EBAAP against Ixodes ricinus and Ixodes scapularis nymphs (Acari, Ixodidae). Ticks Tick Borne Dis. 2015;6:494-498.
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors. 2008;8:1-8.
- Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239-250.
- Feaster JE, Scialdone MA, Todd RG, et al. Dihydronepetalactones deter feeding activity by mosquitoes, stable flies, and deer ticks. J Med Entomol. 2009;46:832-840.
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasit Vectors. 2013;6:358.
- Rand PW, Smith RP Jr, Lacombe EH. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am J Public Health. 1991;81:1331-1334.
- Baneth G, Bourdeau P, Bourdoiseau G, et al; CVBD World Forum. Vector-borne diseases—constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. 2012;5:55.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393-397.
- Ashique KT, Kaliyadan F. Radiofrequency device for tick removal. J Am Acad Dermatol. 2015;72:155-156.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
- McMichael C, Barnett J, McMichael AJ. An ill wind? climate change, migration, and health. Environ Health Perspect. 2012;120:646-654.
- Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140051.
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007;134(pt 2):209-227.
- Tickborne diseases of the United States. Centers for Disease Control and Prevention website. http://www.cdc.gov/ticks/diseases/index.html. Updated July 25, 2017. Accessed April 10, 2018.
- Hinten SR, Beckett GA, Gensheimer KF, et al. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733-740.
- Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816-1823.
- Krause PJ, McKay K, Thompson CA, et al; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184-1191.
- Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. evidence for increased severity and duration of illness. JAMA. 1996;275:1657-1660.
- Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472-1477.
- Sweeny CJ, Ghassemi M, Agger WA, et al. Coinfection with Babesia microti and Borrelia burgdorferi in a western Wisconsin resident. Mayo Clin Proc.1998;73:338-341.
- Nadelman RB, Horowitz HW, Hsieh TC, et al. Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27-30.
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
- Swanson SJ, Neitzel D, Reed DK, et al. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708-727.
- Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
- Vaughn MF, Funkhouser SW, Lin FC, et al. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014;46:473-480.
- Miller NJ, Rainone EE, Dyer MC, et al. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011;48:327-333.
- Richards SL, Balanay JAG, Harris JW. Effectiveness of permethrin-treated clothing to prevent tick exposure in foresters in the central Appalachian region of the USA. Int J Environ Health Res. 2015;25:453-462.
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93.
- Büchel K, Bendin J, Gharbi A, et al. Repellent efficacy of DEET, icaridin, and EBAAP against Ixodes ricinus and Ixodes scapularis nymphs (Acari, Ixodidae). Ticks Tick Borne Dis. 2015;6:494-498.
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors. 2008;8:1-8.
- Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239-250.
- Feaster JE, Scialdone MA, Todd RG, et al. Dihydronepetalactones deter feeding activity by mosquitoes, stable flies, and deer ticks. J Med Entomol. 2009;46:832-840.
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasit Vectors. 2013;6:358.
- Rand PW, Smith RP Jr, Lacombe EH. Canine seroprevalence and the distribution of Ixodes dammini in an area of emerging Lyme disease. Am J Public Health. 1991;81:1331-1334.
- Baneth G, Bourdeau P, Bourdoiseau G, et al; CVBD World Forum. Vector-borne diseases—constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. 2012;5:55.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393-397.
- Ashique KT, Kaliyadan F. Radiofrequency device for tick removal. J Am Acad Dermatol. 2015;72:155-156.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
Practice Points
- As tick-borne diseases become more prevalent, the likelihood of coinfection with more than one Ixodes-transmitted pathogen is increasing, particularly in endemic areas.
- Coinfection generally increases the diversity of presenting symptoms, obscuring the primary diagnosis. The disease course also may be prolonged and more severe.
- Prevention of tick attachment and prompt tick removal are critical to combating the rising prevalence of tick-borne diseases.
Painful Mouth Ulcers
The Diagnosis: Paraneoplastic Pemphigus
A workup for infectious organisms and vasculitis was negative. The patient reported unintentional weight loss despite taking oral steroids prescribed by her pulmonologist for severe obstructive lung disease that appeared to develop around the same time as the mouth ulcers.
Computed tomography of the abdomen revealed an 8.1-cm pelvic mass that a subsequent biopsy revealed to be a follicular dendritic cell sarcoma. Biopsies of the mouth ulcers showed a mildly hyperplastic mucosa with acantholysis and interface change with dyskeratosis. Direct immunofluorescence of the perilesional mucosa showed IgG and complement C3 in an intercellular distribution (Figure 1). The pathologic findings were consistent with a diagnosis of paraneoplastic pemphigus (PNP). Serologic testing via enzyme-linked immunosorbent assay, immunoblotting, and indirect immunofluorescence were not performed. The patient died within a few months after the initial presentation from bronchiolitis obliterans, a potentially fatal complication of PNP.
Paraneoplastic pemphigus is an autoimmune blistering disease associated with neoplasia, particularly lymphoproliferative disorders and thymoma.1 Oral mucosal erosions and crusting along the lips commonly is seen along with cutaneous involvement. The main histologic features are interface changes with dyskeratosis and a lichenoid infiltrate and variable acantholysis.2
Direct immunofluorescence of perilesional skin classically shows IgG and complement C3 in an intercellular distribution, usually in a granular or linear distribution along the basement membrane. This same pattern of direct immunofluorescence is seen in pemphigus erythematosus; however, pemphigus erythematosus is clinically distinct from PNP, lacking mucosal involvement and affecting the face and/or seborrheic areas with an appearance more similar to seborrheic dermatitis or lupus erythematosus, depending on the patient.3 Indirect immunofluorescence with rat bladder epithelium typically is positive in PNP and can be a helpful feature in distinguishing PNP from other autoimmune blistering diseases (eg, pemphigus erythematosus, pemphigus vulgaris, pemphigus foliaceus).2
Immunoblotting assays via serology often detect numerous antigens in patients with PNP, including but not limited to plectin, desmoplakin, bullous pemphigoid antigens, envoplakin, desmoplakin II, and desmogleins 1 and 3.4 Some of these autoantibodies have been identified in tumors associated with paraneoplastic pemphigus, particularly Castleman disease and follicular dendritic cell sarcoma.
Acute graft-versus-host disease (GVHD) can have a similar histologic appearance to PNP with prominent dyskeratosis and characteristically shows satellite cell necrosis consisting of dyskeratosis with surrounding lymphocytes (Figure 2). Unlike PNP, acantholysis is not a feature of GVHD. Direct immunofluorescence typically is negative; however, nonspecific IgM and complement C3 deposition at the dermoepidermal junction and around the superficial vasculature has been reported in 39% of cases.5 Early chronic GVHD often shows retained lichenoid interface change, but late chronic GVHD has a sclerodermoid morphology that is easily distinguished histologically from PNP. Patients also have a history of either a bone marrow or solid organ transplant.6

Lichen planus also shows interface change with dyskeratosis and a lichenoid infiltrate; however, acantholysis typically is not seen and, there often is prominent hypergranulosis (Figure 3). Mucosal lesions often show more subtle features with decreased hyperkeratosis, more subtle hypergranulosis, and decreased interface change with plasma cells in the inflammatory infiltrate.6 Additionally, direct immunofluorescence is either negative or shows IgM-positive colloid bodies and/or an irregular band of fibrinogen at the dermoepidermal junction. The characteristic intercellular and granular/linear IgG positivity at the dermoepidermal junction of PNP is not seen.
Lupus erythematosus is an interface dermatitis with histologic features that can overlap with PNP, in addition to positive direct immunofluorescence, which has been seen in 50% to 94% of cases and can vary depending on previous steroid treatment and timing of the biopsy in the disease process.7 Unlike PNP, lupus erythematosus has a full-house pattern on direct immunofluorescence with IgG, IgM, IgA, and complement C3 deposition in a granular pattern at the dermoepidermal junction. While PNP also typically shows granular deposition of IgG and complement C3 at the dermoepidermal junction, there also is intercellular positivity without a full-house pattern. While both conditions show interface change, histologic features that distinguish lupus erythematosus from PNP are a superficial and deep perivascular lymphocytic infiltrate, basement membrane thickening, follicular plugging, and increased dermal mucin (Figure 4). Subacute lupus erythematosus and discoid lupus erythematosus can have similar histologic features, and definitive distinction on biopsy is not always possible; however, subacute lupus erythematosus shows milder follicular plugging and milder to absent basement membrane thickening, and the inflammatory infiltrate typically is sparser than in discoid lupus erythematosus.7 Subacute lupus erythematosus also can show anti-Ro/Sjögren syndrome antigen A antibodies, which typically are not seen in discoid lupus eythematosus.8
Stevens-Johnson syndrome (SJS) is on a spectrum with toxic epidermal necrolysis, with SJS involving less than 10% and toxic epidermal necrolysis involving 30% or more of the body surface area.5 Erythema multiforme also is on the histologic spectrum of SJS and toxic epidermal necrolysis; however, erythema multiforme typically is more inflammatory than SJS and toxic epidermal necrolysis. Stevens-Johnson syndrome typically affects older adults and shows both cutaneous and mucosal involvement; however, isolated mucosal involvement can be seen in children.5 Drugs, particularly sulfonamide antibiotics, usually are implicated as causative agents, but infections from Mycoplasma and other pathogens also may be the cause. There is notable clinical (with a combination of mucosal and cutaneous lesions) as well as histologic overlap between SJS and PNP. The density of the lichenoid infiltrate is variable, with dyskeratosis, basal cell hydropic degeneration, and occasional formation of subepidermal clefts (Figure 5). Unlike PNP, acantholysis is not a characteristic feature of SJS, and direct immunofluorescence generally is negative.
- Camisa C, Helm TN. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch Dermatol. 1993;129:883-886.
- Joly P, Richard C, Gilbert D, et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43:619-626.
- Calonje E, Brenn T, Lazar A. Acantholytic disorders. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:151-179.
- Billet ES, Grando AS, Pittelkow MR. Paraneoplastic autoimmune multiorgan syndrome: review of the literature and support for a cytotoxic role in pathogenesis. Autoimmunity. 2006;36:617-630.
- Calonje E, Brenn T, Lazar A. Lichenoid and interface dermatitis. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:219-255.
- Billings SD, Cotton J. Inflammatory Dermatopathology: A Pathologist's Survival Guide. 2nd ed. Switzerland: Springer International Publishing; 2016.
- Calonje E, Brenn T, Lazar A. Idiopathic connective tissue disorders. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:711-757.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
The Diagnosis: Paraneoplastic Pemphigus
A workup for infectious organisms and vasculitis was negative. The patient reported unintentional weight loss despite taking oral steroids prescribed by her pulmonologist for severe obstructive lung disease that appeared to develop around the same time as the mouth ulcers.
Computed tomography of the abdomen revealed an 8.1-cm pelvic mass that a subsequent biopsy revealed to be a follicular dendritic cell sarcoma. Biopsies of the mouth ulcers showed a mildly hyperplastic mucosa with acantholysis and interface change with dyskeratosis. Direct immunofluorescence of the perilesional mucosa showed IgG and complement C3 in an intercellular distribution (Figure 1). The pathologic findings were consistent with a diagnosis of paraneoplastic pemphigus (PNP). Serologic testing via enzyme-linked immunosorbent assay, immunoblotting, and indirect immunofluorescence were not performed. The patient died within a few months after the initial presentation from bronchiolitis obliterans, a potentially fatal complication of PNP.

Paraneoplastic pemphigus is an autoimmune blistering disease associated with neoplasia, particularly lymphoproliferative disorders and thymoma.1 Oral mucosal erosions and crusting along the lips commonly is seen along with cutaneous involvement. The main histologic features are interface changes with dyskeratosis and a lichenoid infiltrate and variable acantholysis.2
Direct immunofluorescence of perilesional skin classically shows IgG and complement C3 in an intercellular distribution, usually in a granular or linear distribution along the basement membrane. This same pattern of direct immunofluorescence is seen in pemphigus erythematosus; however, pemphigus erythematosus is clinically distinct from PNP, lacking mucosal involvement and affecting the face and/or seborrheic areas with an appearance more similar to seborrheic dermatitis or lupus erythematosus, depending on the patient.3 Indirect immunofluorescence with rat bladder epithelium typically is positive in PNP and can be a helpful feature in distinguishing PNP from other autoimmune blistering diseases (eg, pemphigus erythematosus, pemphigus vulgaris, pemphigus foliaceus).2
Immunoblotting assays via serology often detect numerous antigens in patients with PNP, including but not limited to plectin, desmoplakin, bullous pemphigoid antigens, envoplakin, desmoplakin II, and desmogleins 1 and 3.4 Some of these autoantibodies have been identified in tumors associated with paraneoplastic pemphigus, particularly Castleman disease and follicular dendritic cell sarcoma.
Acute graft-versus-host disease (GVHD) can have a similar histologic appearance to PNP with prominent dyskeratosis and characteristically shows satellite cell necrosis consisting of dyskeratosis with surrounding lymphocytes (Figure 2). Unlike PNP, acantholysis is not a feature of GVHD. Direct immunofluorescence typically is negative; however, nonspecific IgM and complement C3 deposition at the dermoepidermal junction and around the superficial vasculature has been reported in 39% of cases.5 Early chronic GVHD often shows retained lichenoid interface change, but late chronic GVHD has a sclerodermoid morphology that is easily distinguished histologically from PNP. Patients also have a history of either a bone marrow or solid organ transplant.6

Lichen planus also shows interface change with dyskeratosis and a lichenoid infiltrate; however, acantholysis typically is not seen and, there often is prominent hypergranulosis (Figure 3). Mucosal lesions often show more subtle features with decreased hyperkeratosis, more subtle hypergranulosis, and decreased interface change with plasma cells in the inflammatory infiltrate.6 Additionally, direct immunofluorescence is either negative or shows IgM-positive colloid bodies and/or an irregular band of fibrinogen at the dermoepidermal junction. The characteristic intercellular and granular/linear IgG positivity at the dermoepidermal junction of PNP is not seen.

Lupus erythematosus is an interface dermatitis with histologic features that can overlap with PNP, in addition to positive direct immunofluorescence, which has been seen in 50% to 94% of cases and can vary depending on previous steroid treatment and timing of the biopsy in the disease process.7 Unlike PNP, lupus erythematosus has a full-house pattern on direct immunofluorescence with IgG, IgM, IgA, and complement C3 deposition in a granular pattern at the dermoepidermal junction. While PNP also typically shows granular deposition of IgG and complement C3 at the dermoepidermal junction, there also is intercellular positivity without a full-house pattern. While both conditions show interface change, histologic features that distinguish lupus erythematosus from PNP are a superficial and deep perivascular lymphocytic infiltrate, basement membrane thickening, follicular plugging, and increased dermal mucin (Figure 4). Subacute lupus erythematosus and discoid lupus erythematosus can have similar histologic features, and definitive distinction on biopsy is not always possible; however, subacute lupus erythematosus shows milder follicular plugging and milder to absent basement membrane thickening, and the inflammatory infiltrate typically is sparser than in discoid lupus erythematosus.7 Subacute lupus erythematosus also can show anti-Ro/Sjögren syndrome antigen A antibodies, which typically are not seen in discoid lupus eythematosus.8

Stevens-Johnson syndrome (SJS) is on a spectrum with toxic epidermal necrolysis, with SJS involving less than 10% and toxic epidermal necrolysis involving 30% or more of the body surface area.5 Erythema multiforme also is on the histologic spectrum of SJS and toxic epidermal necrolysis; however, erythema multiforme typically is more inflammatory than SJS and toxic epidermal necrolysis. Stevens-Johnson syndrome typically affects older adults and shows both cutaneous and mucosal involvement; however, isolated mucosal involvement can be seen in children.5 Drugs, particularly sulfonamide antibiotics, usually are implicated as causative agents, but infections from Mycoplasma and other pathogens also may be the cause. There is notable clinical (with a combination of mucosal and cutaneous lesions) as well as histologic overlap between SJS and PNP. The density of the lichenoid infiltrate is variable, with dyskeratosis, basal cell hydropic degeneration, and occasional formation of subepidermal clefts (Figure 5). Unlike PNP, acantholysis is not a characteristic feature of SJS, and direct immunofluorescence generally is negative.

The Diagnosis: Paraneoplastic Pemphigus
A workup for infectious organisms and vasculitis was negative. The patient reported unintentional weight loss despite taking oral steroids prescribed by her pulmonologist for severe obstructive lung disease that appeared to develop around the same time as the mouth ulcers.
Computed tomography of the abdomen revealed an 8.1-cm pelvic mass that a subsequent biopsy revealed to be a follicular dendritic cell sarcoma. Biopsies of the mouth ulcers showed a mildly hyperplastic mucosa with acantholysis and interface change with dyskeratosis. Direct immunofluorescence of the perilesional mucosa showed IgG and complement C3 in an intercellular distribution (Figure 1). The pathologic findings were consistent with a diagnosis of paraneoplastic pemphigus (PNP). Serologic testing via enzyme-linked immunosorbent assay, immunoblotting, and indirect immunofluorescence were not performed. The patient died within a few months after the initial presentation from bronchiolitis obliterans, a potentially fatal complication of PNP.

Paraneoplastic pemphigus is an autoimmune blistering disease associated with neoplasia, particularly lymphoproliferative disorders and thymoma.1 Oral mucosal erosions and crusting along the lips commonly is seen along with cutaneous involvement. The main histologic features are interface changes with dyskeratosis and a lichenoid infiltrate and variable acantholysis.2
Direct immunofluorescence of perilesional skin classically shows IgG and complement C3 in an intercellular distribution, usually in a granular or linear distribution along the basement membrane. This same pattern of direct immunofluorescence is seen in pemphigus erythematosus; however, pemphigus erythematosus is clinically distinct from PNP, lacking mucosal involvement and affecting the face and/or seborrheic areas with an appearance more similar to seborrheic dermatitis or lupus erythematosus, depending on the patient.3 Indirect immunofluorescence with rat bladder epithelium typically is positive in PNP and can be a helpful feature in distinguishing PNP from other autoimmune blistering diseases (eg, pemphigus erythematosus, pemphigus vulgaris, pemphigus foliaceus).2
Immunoblotting assays via serology often detect numerous antigens in patients with PNP, including but not limited to plectin, desmoplakin, bullous pemphigoid antigens, envoplakin, desmoplakin II, and desmogleins 1 and 3.4 Some of these autoantibodies have been identified in tumors associated with paraneoplastic pemphigus, particularly Castleman disease and follicular dendritic cell sarcoma.
Acute graft-versus-host disease (GVHD) can have a similar histologic appearance to PNP with prominent dyskeratosis and characteristically shows satellite cell necrosis consisting of dyskeratosis with surrounding lymphocytes (Figure 2). Unlike PNP, acantholysis is not a feature of GVHD. Direct immunofluorescence typically is negative; however, nonspecific IgM and complement C3 deposition at the dermoepidermal junction and around the superficial vasculature has been reported in 39% of cases.5 Early chronic GVHD often shows retained lichenoid interface change, but late chronic GVHD has a sclerodermoid morphology that is easily distinguished histologically from PNP. Patients also have a history of either a bone marrow or solid organ transplant.6

Lichen planus also shows interface change with dyskeratosis and a lichenoid infiltrate; however, acantholysis typically is not seen and, there often is prominent hypergranulosis (Figure 3). Mucosal lesions often show more subtle features with decreased hyperkeratosis, more subtle hypergranulosis, and decreased interface change with plasma cells in the inflammatory infiltrate.6 Additionally, direct immunofluorescence is either negative or shows IgM-positive colloid bodies and/or an irregular band of fibrinogen at the dermoepidermal junction. The characteristic intercellular and granular/linear IgG positivity at the dermoepidermal junction of PNP is not seen.

Lupus erythematosus is an interface dermatitis with histologic features that can overlap with PNP, in addition to positive direct immunofluorescence, which has been seen in 50% to 94% of cases and can vary depending on previous steroid treatment and timing of the biopsy in the disease process.7 Unlike PNP, lupus erythematosus has a full-house pattern on direct immunofluorescence with IgG, IgM, IgA, and complement C3 deposition in a granular pattern at the dermoepidermal junction. While PNP also typically shows granular deposition of IgG and complement C3 at the dermoepidermal junction, there also is intercellular positivity without a full-house pattern. While both conditions show interface change, histologic features that distinguish lupus erythematosus from PNP are a superficial and deep perivascular lymphocytic infiltrate, basement membrane thickening, follicular plugging, and increased dermal mucin (Figure 4). Subacute lupus erythematosus and discoid lupus erythematosus can have similar histologic features, and definitive distinction on biopsy is not always possible; however, subacute lupus erythematosus shows milder follicular plugging and milder to absent basement membrane thickening, and the inflammatory infiltrate typically is sparser than in discoid lupus erythematosus.7 Subacute lupus erythematosus also can show anti-Ro/Sjögren syndrome antigen A antibodies, which typically are not seen in discoid lupus eythematosus.8

Stevens-Johnson syndrome (SJS) is on a spectrum with toxic epidermal necrolysis, with SJS involving less than 10% and toxic epidermal necrolysis involving 30% or more of the body surface area.5 Erythema multiforme also is on the histologic spectrum of SJS and toxic epidermal necrolysis; however, erythema multiforme typically is more inflammatory than SJS and toxic epidermal necrolysis. Stevens-Johnson syndrome typically affects older adults and shows both cutaneous and mucosal involvement; however, isolated mucosal involvement can be seen in children.5 Drugs, particularly sulfonamide antibiotics, usually are implicated as causative agents, but infections from Mycoplasma and other pathogens also may be the cause. There is notable clinical (with a combination of mucosal and cutaneous lesions) as well as histologic overlap between SJS and PNP. The density of the lichenoid infiltrate is variable, with dyskeratosis, basal cell hydropic degeneration, and occasional formation of subepidermal clefts (Figure 5). Unlike PNP, acantholysis is not a characteristic feature of SJS, and direct immunofluorescence generally is negative.

- Camisa C, Helm TN. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch Dermatol. 1993;129:883-886.
- Joly P, Richard C, Gilbert D, et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43:619-626.
- Calonje E, Brenn T, Lazar A. Acantholytic disorders. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:151-179.
- Billet ES, Grando AS, Pittelkow MR. Paraneoplastic autoimmune multiorgan syndrome: review of the literature and support for a cytotoxic role in pathogenesis. Autoimmunity. 2006;36:617-630.
- Calonje E, Brenn T, Lazar A. Lichenoid and interface dermatitis. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:219-255.
- Billings SD, Cotton J. Inflammatory Dermatopathology: A Pathologist's Survival Guide. 2nd ed. Switzerland: Springer International Publishing; 2016.
- Calonje E, Brenn T, Lazar A. Idiopathic connective tissue disorders. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:711-757.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Camisa C, Helm TN. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch Dermatol. 1993;129:883-886.
- Joly P, Richard C, Gilbert D, et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43:619-626.
- Calonje E, Brenn T, Lazar A. Acantholytic disorders. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:151-179.
- Billet ES, Grando AS, Pittelkow MR. Paraneoplastic autoimmune multiorgan syndrome: review of the literature and support for a cytotoxic role in pathogenesis. Autoimmunity. 2006;36:617-630.
- Calonje E, Brenn T, Lazar A. Lichenoid and interface dermatitis. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:219-255.
- Billings SD, Cotton J. Inflammatory Dermatopathology: A Pathologist's Survival Guide. 2nd ed. Switzerland: Springer International Publishing; 2016.
- Calonje E, Brenn T, Lazar A. Idiopathic connective tissue disorders. McKee's Pathology of the Skin With Clinical Correlations. 4th ed. Philadelphia, PA: Elsevier; 2011:711-757.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
A 41-year-old woman presented with painful ulcers on the oral mucosa of 2 months' duration that were unresponsive to treatment with acyclovir. She had been diagnosed with a pelvic tumor a few weeks prior to the development of the mouth ulcers. Direct immunofluorescence of the perilesional mucosa showed positive IgG and complement C3 with an intercellular distribution. A biopsy of an oral lesion was performed.
Reticular Hyperpigmented Patches With Indurated Subcutaneous Plaques
The Diagnosis: Superficial Migratory Thrombophlebitis
On initial presentation, the differential diagnosis included livedoid vasculopathy, cutaneous polyarteritis nodosa, erythema ab igne, cholesterol embolism, and livedo reticularis. Laboratory investigation included antiphospholipid antibody syndrome (APS), antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, serum protein electrophoresis, and coagulation tests. Pertinent findings included transient low total complement activity but normal complement protein C2, C3, and C5 levels and negative cryoglobulins. Additional laboratory testing revealed elevated antiphosphatidylserine IgG, which remained elevated 12 weeks later.
New lesions continued to appear over the next several months as painful, erythematous, linear, pruritic nodules that resolved as hyperpigmented linear patches, which intersected to form a livedo reticularis-like pattern that covered the lower legs. Biopsy of an erythematous nodule on the right leg revealed fibrin occlusion of a medium-sized vein in the subcutaneous fat. Direct immunofluorescence was not specific. Venous duplex ultrasonography demonstrated chronic superficial thrombophlebitis and was crucial to the diagnosis. Ultimately, the patient's history, clinical presentation, laboratory results, venous studies, and histopathologic analysis were consistent with a diagnosis of superficial migratory thrombophlebitis (SMT) with resultant postinflammatory hyperpigmentation presenting in a reticular pattern that mimicked livedoid vasculopathy, livedo reticularis, or erythema ab igne.
Superficial migratory thrombophlebitis, also known as thrombophlebitis migrans, is defined as the recurrent formation of thrombi within superficial veins.1 The presence of a thrombus in a superficial vein evokes an inflammatory response, resulting in swelling, tenderness, erythema, and warmth in the affected area. Superficial migratory thrombophlebitis has been associated with several etiologies, including pregnancy, oral contraceptive use, APS, vasculitic disorders, and malignancies (eg, pancreas, lung, breast), as well as infections such as secondary syphilis.1
When SMT is associated with an occult malignancy, it is known as Trousseau syndrome. Common malignancies found in association with Trousseau syndrome include pancreatic, lung, and breast cancers.2 A systematic review from 2008 evaluated the utility of extensive cancer screening strategies in patients with newly diagnosed, unprovoked venous thromboembolic events.3 Using a wide screening strategy that included computed tomography of the abdomen and pelvis, the investigators detected a considerable number of formerly undiagnosed cancers, increasing detection rates from 49.4% to 69.7%. After the diagnosis of SMT was made in our patient, computed tomography of the chest, abdomen, and pelvis was performed, but the findings were unremarkable.
Because occult malignancy was excluded in our patient, the likely etiology of SMT was APS, an acquired autoimmune condition diagnosed based on the presence of a vascular thrombosis and/or pregnancy failure in women as well as elevation of at least one antiphospholipid antibody laboratory marker (eg, lupus anticoagulant, anticardiolipin antibody, and anti-β2 glycoprotein I antibody) on 2 or more occasions at least 12 weeks apart.4 Other antibodies such as those directed against negatively charged phospholipids (eg, antiphosphatidylserine [which was elevated in our patient], phosphatidylinositol, phosphatidic acid) have been reported in patients with APS, although their diagnostic use is controversial.5 For example, the presence of antiphosphatidylserine antibodies has been considered common but not specific in patients with APS.4 However, a recent observational study demonstrated that antiphosphatidylserine antibodies are highly specific (87%) and useful in diagnosing clinical APS cases in the presence of other negative markers.6
In our patient, diagnosis of SMT with resultant postinflammatory hyperpigmentation in a reticular pattern was based on the patient's medical history, clinical examination, and histopathologic findings, as well as laboratory results and venous studies. However, it is important to note that a livedo reticularis-like pattern also is a very common finding in APS and must be included in the differential diagnosis of a reticular network on the skin.7 Moreover, differentiating livedo reticularis from SMT has prognostic importance since SMT may be associated with underlying malignancies while livedo reticularis may be associated with Sneddon syndrome, a disorder in which neurologic vascular events (eg, cerebrovascular accidents) are present.8 While this distinction is important, there are no pathognomonic histologic findings seen in livedo reticularis, and consideration of the clinical picture and additional testing is critical.4,8
Livedo vasculopathy was excluded in our patient due to the lack of diagnostic histopathologic findings, such as fibrin deposition and thrombus formation involving the upper- and mid-dermal capillaries.9 Furthermore, characteristic direct immunofluorescence findings of a homogenous or granular deposition in the vessel wall consisting of immune complexes, complement, and fibrin were absent in our patient.9 Our patient also lacked common clinical findings found in livedo vasculopathy such as small ulcerations or atrophic, porcelain-white scars on the lower legs. Erythema ab igne also was excluded in our patient due to the absence of heat exposure and presence of fibrin occlusion in the superficial leg veins. Physiologic livedo reticularis, defined as a livedoid pattern due to physiologic changes in the skin in response to cold exposure,10 also was excluded, as our patient's cutaneous changes included an alteration in pigmentation with a brown reticular pattern and no blanching, erythematous or violaceous hue, warmth, or tenderness.
In conclusion, SMT is a disorder with multiple associations that may clinically mimic livedo reticularis and livedoid vasculopathy when postinflammatory hyperpigmentation has a lacelike or livedoid pattern. While nontraditional antibodies may be useful in diagnosis in patients suspected of having APS with otherwise negative markers, standardized assays and further studies are needed to determine the specificity and value of these antibodies, particularly when used in isolation. Our patient's elevated antiphosphatidylserine IgG may have been the cause of her hypercoagulable state causing the SMT. A livedoid pattern is a common finding in APS and also was seen in our patient with SMT, but the differentiation of the brown pigmentary change and more active erythema was critical to the appropriate clinical workup of our patient.
- Samlaska CP, James WD. Superficial thrombophlebitis. II. secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1-18.
- Rigdon EE. Trousseau's syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214-218.
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323-333.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Bertolaccini ML, Amengual O, Atsumi T, et al. 'Non-criteria' aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191-205.
- Khogeer H, Alfattani A, Al Kaff M, et al. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. 2015;24:186-190.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6 pt 1):970-982.
- Francès C, Papo T, Wechsler B, et al. Sneddon syndrome with or without antiphospholipid antibodies. a comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-488.
- James WD, Berger TG, Elston DM. Andrews' Diseases Of The Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2006.
The Diagnosis: Superficial Migratory Thrombophlebitis
On initial presentation, the differential diagnosis included livedoid vasculopathy, cutaneous polyarteritis nodosa, erythema ab igne, cholesterol embolism, and livedo reticularis. Laboratory investigation included antiphospholipid antibody syndrome (APS), antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, serum protein electrophoresis, and coagulation tests. Pertinent findings included transient low total complement activity but normal complement protein C2, C3, and C5 levels and negative cryoglobulins. Additional laboratory testing revealed elevated antiphosphatidylserine IgG, which remained elevated 12 weeks later.
New lesions continued to appear over the next several months as painful, erythematous, linear, pruritic nodules that resolved as hyperpigmented linear patches, which intersected to form a livedo reticularis-like pattern that covered the lower legs. Biopsy of an erythematous nodule on the right leg revealed fibrin occlusion of a medium-sized vein in the subcutaneous fat. Direct immunofluorescence was not specific. Venous duplex ultrasonography demonstrated chronic superficial thrombophlebitis and was crucial to the diagnosis. Ultimately, the patient's history, clinical presentation, laboratory results, venous studies, and histopathologic analysis were consistent with a diagnosis of superficial migratory thrombophlebitis (SMT) with resultant postinflammatory hyperpigmentation presenting in a reticular pattern that mimicked livedoid vasculopathy, livedo reticularis, or erythema ab igne.
Superficial migratory thrombophlebitis, also known as thrombophlebitis migrans, is defined as the recurrent formation of thrombi within superficial veins.1 The presence of a thrombus in a superficial vein evokes an inflammatory response, resulting in swelling, tenderness, erythema, and warmth in the affected area. Superficial migratory thrombophlebitis has been associated with several etiologies, including pregnancy, oral contraceptive use, APS, vasculitic disorders, and malignancies (eg, pancreas, lung, breast), as well as infections such as secondary syphilis.1
When SMT is associated with an occult malignancy, it is known as Trousseau syndrome. Common malignancies found in association with Trousseau syndrome include pancreatic, lung, and breast cancers.2 A systematic review from 2008 evaluated the utility of extensive cancer screening strategies in patients with newly diagnosed, unprovoked venous thromboembolic events.3 Using a wide screening strategy that included computed tomography of the abdomen and pelvis, the investigators detected a considerable number of formerly undiagnosed cancers, increasing detection rates from 49.4% to 69.7%. After the diagnosis of SMT was made in our patient, computed tomography of the chest, abdomen, and pelvis was performed, but the findings were unremarkable.
Because occult malignancy was excluded in our patient, the likely etiology of SMT was APS, an acquired autoimmune condition diagnosed based on the presence of a vascular thrombosis and/or pregnancy failure in women as well as elevation of at least one antiphospholipid antibody laboratory marker (eg, lupus anticoagulant, anticardiolipin antibody, and anti-β2 glycoprotein I antibody) on 2 or more occasions at least 12 weeks apart.4 Other antibodies such as those directed against negatively charged phospholipids (eg, antiphosphatidylserine [which was elevated in our patient], phosphatidylinositol, phosphatidic acid) have been reported in patients with APS, although their diagnostic use is controversial.5 For example, the presence of antiphosphatidylserine antibodies has been considered common but not specific in patients with APS.4 However, a recent observational study demonstrated that antiphosphatidylserine antibodies are highly specific (87%) and useful in diagnosing clinical APS cases in the presence of other negative markers.6
In our patient, diagnosis of SMT with resultant postinflammatory hyperpigmentation in a reticular pattern was based on the patient's medical history, clinical examination, and histopathologic findings, as well as laboratory results and venous studies. However, it is important to note that a livedo reticularis-like pattern also is a very common finding in APS and must be included in the differential diagnosis of a reticular network on the skin.7 Moreover, differentiating livedo reticularis from SMT has prognostic importance since SMT may be associated with underlying malignancies while livedo reticularis may be associated with Sneddon syndrome, a disorder in which neurologic vascular events (eg, cerebrovascular accidents) are present.8 While this distinction is important, there are no pathognomonic histologic findings seen in livedo reticularis, and consideration of the clinical picture and additional testing is critical.4,8
Livedo vasculopathy was excluded in our patient due to the lack of diagnostic histopathologic findings, such as fibrin deposition and thrombus formation involving the upper- and mid-dermal capillaries.9 Furthermore, characteristic direct immunofluorescence findings of a homogenous or granular deposition in the vessel wall consisting of immune complexes, complement, and fibrin were absent in our patient.9 Our patient also lacked common clinical findings found in livedo vasculopathy such as small ulcerations or atrophic, porcelain-white scars on the lower legs. Erythema ab igne also was excluded in our patient due to the absence of heat exposure and presence of fibrin occlusion in the superficial leg veins. Physiologic livedo reticularis, defined as a livedoid pattern due to physiologic changes in the skin in response to cold exposure,10 also was excluded, as our patient's cutaneous changes included an alteration in pigmentation with a brown reticular pattern and no blanching, erythematous or violaceous hue, warmth, or tenderness.
In conclusion, SMT is a disorder with multiple associations that may clinically mimic livedo reticularis and livedoid vasculopathy when postinflammatory hyperpigmentation has a lacelike or livedoid pattern. While nontraditional antibodies may be useful in diagnosis in patients suspected of having APS with otherwise negative markers, standardized assays and further studies are needed to determine the specificity and value of these antibodies, particularly when used in isolation. Our patient's elevated antiphosphatidylserine IgG may have been the cause of her hypercoagulable state causing the SMT. A livedoid pattern is a common finding in APS and also was seen in our patient with SMT, but the differentiation of the brown pigmentary change and more active erythema was critical to the appropriate clinical workup of our patient.
The Diagnosis: Superficial Migratory Thrombophlebitis
On initial presentation, the differential diagnosis included livedoid vasculopathy, cutaneous polyarteritis nodosa, erythema ab igne, cholesterol embolism, and livedo reticularis. Laboratory investigation included antiphospholipid antibody syndrome (APS), antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, serum protein electrophoresis, and coagulation tests. Pertinent findings included transient low total complement activity but normal complement protein C2, C3, and C5 levels and negative cryoglobulins. Additional laboratory testing revealed elevated antiphosphatidylserine IgG, which remained elevated 12 weeks later.
New lesions continued to appear over the next several months as painful, erythematous, linear, pruritic nodules that resolved as hyperpigmented linear patches, which intersected to form a livedo reticularis-like pattern that covered the lower legs. Biopsy of an erythematous nodule on the right leg revealed fibrin occlusion of a medium-sized vein in the subcutaneous fat. Direct immunofluorescence was not specific. Venous duplex ultrasonography demonstrated chronic superficial thrombophlebitis and was crucial to the diagnosis. Ultimately, the patient's history, clinical presentation, laboratory results, venous studies, and histopathologic analysis were consistent with a diagnosis of superficial migratory thrombophlebitis (SMT) with resultant postinflammatory hyperpigmentation presenting in a reticular pattern that mimicked livedoid vasculopathy, livedo reticularis, or erythema ab igne.
Superficial migratory thrombophlebitis, also known as thrombophlebitis migrans, is defined as the recurrent formation of thrombi within superficial veins.1 The presence of a thrombus in a superficial vein evokes an inflammatory response, resulting in swelling, tenderness, erythema, and warmth in the affected area. Superficial migratory thrombophlebitis has been associated with several etiologies, including pregnancy, oral contraceptive use, APS, vasculitic disorders, and malignancies (eg, pancreas, lung, breast), as well as infections such as secondary syphilis.1
When SMT is associated with an occult malignancy, it is known as Trousseau syndrome. Common malignancies found in association with Trousseau syndrome include pancreatic, lung, and breast cancers.2 A systematic review from 2008 evaluated the utility of extensive cancer screening strategies in patients with newly diagnosed, unprovoked venous thromboembolic events.3 Using a wide screening strategy that included computed tomography of the abdomen and pelvis, the investigators detected a considerable number of formerly undiagnosed cancers, increasing detection rates from 49.4% to 69.7%. After the diagnosis of SMT was made in our patient, computed tomography of the chest, abdomen, and pelvis was performed, but the findings were unremarkable.
Because occult malignancy was excluded in our patient, the likely etiology of SMT was APS, an acquired autoimmune condition diagnosed based on the presence of a vascular thrombosis and/or pregnancy failure in women as well as elevation of at least one antiphospholipid antibody laboratory marker (eg, lupus anticoagulant, anticardiolipin antibody, and anti-β2 glycoprotein I antibody) on 2 or more occasions at least 12 weeks apart.4 Other antibodies such as those directed against negatively charged phospholipids (eg, antiphosphatidylserine [which was elevated in our patient], phosphatidylinositol, phosphatidic acid) have been reported in patients with APS, although their diagnostic use is controversial.5 For example, the presence of antiphosphatidylserine antibodies has been considered common but not specific in patients with APS.4 However, a recent observational study demonstrated that antiphosphatidylserine antibodies are highly specific (87%) and useful in diagnosing clinical APS cases in the presence of other negative markers.6
In our patient, diagnosis of SMT with resultant postinflammatory hyperpigmentation in a reticular pattern was based on the patient's medical history, clinical examination, and histopathologic findings, as well as laboratory results and venous studies. However, it is important to note that a livedo reticularis-like pattern also is a very common finding in APS and must be included in the differential diagnosis of a reticular network on the skin.7 Moreover, differentiating livedo reticularis from SMT has prognostic importance since SMT may be associated with underlying malignancies while livedo reticularis may be associated with Sneddon syndrome, a disorder in which neurologic vascular events (eg, cerebrovascular accidents) are present.8 While this distinction is important, there are no pathognomonic histologic findings seen in livedo reticularis, and consideration of the clinical picture and additional testing is critical.4,8
Livedo vasculopathy was excluded in our patient due to the lack of diagnostic histopathologic findings, such as fibrin deposition and thrombus formation involving the upper- and mid-dermal capillaries.9 Furthermore, characteristic direct immunofluorescence findings of a homogenous or granular deposition in the vessel wall consisting of immune complexes, complement, and fibrin were absent in our patient.9 Our patient also lacked common clinical findings found in livedo vasculopathy such as small ulcerations or atrophic, porcelain-white scars on the lower legs. Erythema ab igne also was excluded in our patient due to the absence of heat exposure and presence of fibrin occlusion in the superficial leg veins. Physiologic livedo reticularis, defined as a livedoid pattern due to physiologic changes in the skin in response to cold exposure,10 also was excluded, as our patient's cutaneous changes included an alteration in pigmentation with a brown reticular pattern and no blanching, erythematous or violaceous hue, warmth, or tenderness.
In conclusion, SMT is a disorder with multiple associations that may clinically mimic livedo reticularis and livedoid vasculopathy when postinflammatory hyperpigmentation has a lacelike or livedoid pattern. While nontraditional antibodies may be useful in diagnosis in patients suspected of having APS with otherwise negative markers, standardized assays and further studies are needed to determine the specificity and value of these antibodies, particularly when used in isolation. Our patient's elevated antiphosphatidylserine IgG may have been the cause of her hypercoagulable state causing the SMT. A livedoid pattern is a common finding in APS and also was seen in our patient with SMT, but the differentiation of the brown pigmentary change and more active erythema was critical to the appropriate clinical workup of our patient.
- Samlaska CP, James WD. Superficial thrombophlebitis. II. secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1-18.
- Rigdon EE. Trousseau's syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214-218.
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323-333.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Bertolaccini ML, Amengual O, Atsumi T, et al. 'Non-criteria' aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191-205.
- Khogeer H, Alfattani A, Al Kaff M, et al. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. 2015;24:186-190.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6 pt 1):970-982.
- Francès C, Papo T, Wechsler B, et al. Sneddon syndrome with or without antiphospholipid antibodies. a comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-488.
- James WD, Berger TG, Elston DM. Andrews' Diseases Of The Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2006.
- Samlaska CP, James WD. Superficial thrombophlebitis. II. secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1-18.
- Rigdon EE. Trousseau's syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214-218.
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323-333.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Bertolaccini ML, Amengual O, Atsumi T, et al. 'Non-criteria' aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191-205.
- Khogeer H, Alfattani A, Al Kaff M, et al. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. 2015;24:186-190.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6 pt 1):970-982.
- Francès C, Papo T, Wechsler B, et al. Sneddon syndrome with or without antiphospholipid antibodies. a comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-488.
- James WD, Berger TG, Elston DM. Andrews' Diseases Of The Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2006.

A 32-year-old woman presented for evaluation of small, tender, erythematous nodules on the lower legs of 1 year's duration that had started to spread to the thighs over several months prior to presentation. The patient reported no history of ulceration or other cutaneous findings. On physical examination, a hyperpigmented, linear to reticular pattern was noted on the lower legs with a few 1-cm, erythematous, mildly indurated and tender subcutaneous nodules. The patient denied any recent medical procedures, history of malignancy or cardiovascular disease, use of tobacco or illicit drugs, prolonged contact with a heat source, recent unintentional weight loss, fevers, or night sweats. Her medical history was notable for asthma and migraines, which were treated with albuterol, fluticasone, and topiramate.
Colorectal surgeons see barriers to optimal palliative care
While to its optimal implementation at the patient, family, and clinician level, results of a recent survey suggest.
“We found that surgeons reported the most important barriers to be their own,” said Pasithorn A. Suwanabol, MD, division of colorectal surgery, University of Michigan, Ann Arbor, and coauthors of a report on the survey.
More than three-quarters of surgeons said they had no formal education in palliative care, and a substantial number specifically noted inadequate training in both communication and techniques to forgo life-sustaining measures, according to the report, published in the Journal of Palliative Medicine.
Dr. Suwanabol and her colleagues sought surgeon perspectives on palliative care for stage IV colorectal cancer in part because palliative care is often not integrated with cancer treatment in these potentially incurable patients.
“Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care,” they said in the report.
They conducted a mixed methods study including members of a surgical society (American Society of Colon and Rectal Surgeons) who were asked to submit Internet responses to a validated survey. This mixed methods study is believed to be the first to characterize surgeons’ perceived barriers to optimal palliative and end-of life care for patients with advanced colorectal cancer, Dr. Suwanabol and her colleagues noted.
A total of 131 surgeons responded to the survey, for a response rate of 16.5%. The majority of surgeons responding (76.1%) said they did not have any formal palliative care training, while 42.7% said they lacked specific education in communication and 37.9% lacked training in techniques to forgo life-sustaining measures.
Many patients with stage IV colorectal cancer are candidates for palliative care. “Specialty palliative care should be offered to patients who have more complex issues such as refractory pain, depression and anxiety, and existential distress. In addition, specialty palliative care may be helpful in those with challenging family dynamics and/or conflict between family members and/or members of the care team. Patients with stage IV colon cancer may not be candidates for curative therapies and a palliative approach allows the surgeon and patient to carefully weigh the risk and benefits of each therapy in the context of the overall goals of the patient,” Dr. Suwanabol said in an interview.
Among the survey responders, 61.8% said discussion of palliation was limited because of patients and families who had unrealistic expectations and demanded aggressive interventions.
One such recollection in the report reads as follows: ‘‘Patient with poorly responding stage IV colon cancer in multisystem organ failure getting same chemo that already failed ... family wanted everything done…’’
The report includes a number of other reflections from surgeons that suggest a level of anxiety, frustration, disappointment, or uncertainty regarding palliative care.
“I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity,” one surgeon said in his survey response. “(He) went to another major center where they reoperated … I still have doubts about whether or not (a) I didn’t do enough for the patient or (b) the other center did too much.’’
Another surgeon reflected on the uncertainty of prognostication in some cases of advanced colon cancer. “I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive. Days later he is awake and alert, off pressors, on trach collar, and fully communicative. Sometimes even experienced clinicians cannot predict when a patient will die or recover.”
Communication barriers remain. “A number of surgeons reported not knowing how to discuss this with families knowing that there is a stigma associated with palliative care – how to convey that they may pursue continued treatment in conjunction with palliative care,” said Dr. Suwanabol.
Surgeons adept at incorporating palliative care into their treatment plans continue to see the patients. “Even if a patient is not pursuing curative treatment, we do not want the patient to lose hope or feel abandoned by us, and I continue seeing my patients in follow-up until they feel overburdened by their clinic visits and choose not to come,” said Dr. Suwanabol.
In the absence of required palliative care training in medical schools or residencies, alternative approaches to achieving competency could include the American College of Surgeons Palliative Surgical Care Course, mentorships or collaborations with local palliative care specialists, or structured curricula implemented by experienced faculty, they added.
Dr. Suwanabol and her coauthors reported that they had no competing financial interests related to their study.
SOURCE: Suwanabol PA, et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
While to its optimal implementation at the patient, family, and clinician level, results of a recent survey suggest.
“We found that surgeons reported the most important barriers to be their own,” said Pasithorn A. Suwanabol, MD, division of colorectal surgery, University of Michigan, Ann Arbor, and coauthors of a report on the survey.
More than three-quarters of surgeons said they had no formal education in palliative care, and a substantial number specifically noted inadequate training in both communication and techniques to forgo life-sustaining measures, according to the report, published in the Journal of Palliative Medicine.
Dr. Suwanabol and her colleagues sought surgeon perspectives on palliative care for stage IV colorectal cancer in part because palliative care is often not integrated with cancer treatment in these potentially incurable patients.
“Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care,” they said in the report.
They conducted a mixed methods study including members of a surgical society (American Society of Colon and Rectal Surgeons) who were asked to submit Internet responses to a validated survey. This mixed methods study is believed to be the first to characterize surgeons’ perceived barriers to optimal palliative and end-of life care for patients with advanced colorectal cancer, Dr. Suwanabol and her colleagues noted.
A total of 131 surgeons responded to the survey, for a response rate of 16.5%. The majority of surgeons responding (76.1%) said they did not have any formal palliative care training, while 42.7% said they lacked specific education in communication and 37.9% lacked training in techniques to forgo life-sustaining measures.
Many patients with stage IV colorectal cancer are candidates for palliative care. “Specialty palliative care should be offered to patients who have more complex issues such as refractory pain, depression and anxiety, and existential distress. In addition, specialty palliative care may be helpful in those with challenging family dynamics and/or conflict between family members and/or members of the care team. Patients with stage IV colon cancer may not be candidates for curative therapies and a palliative approach allows the surgeon and patient to carefully weigh the risk and benefits of each therapy in the context of the overall goals of the patient,” Dr. Suwanabol said in an interview.
Among the survey responders, 61.8% said discussion of palliation was limited because of patients and families who had unrealistic expectations and demanded aggressive interventions.
One such recollection in the report reads as follows: ‘‘Patient with poorly responding stage IV colon cancer in multisystem organ failure getting same chemo that already failed ... family wanted everything done…’’
The report includes a number of other reflections from surgeons that suggest a level of anxiety, frustration, disappointment, or uncertainty regarding palliative care.
“I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity,” one surgeon said in his survey response. “(He) went to another major center where they reoperated … I still have doubts about whether or not (a) I didn’t do enough for the patient or (b) the other center did too much.’’
Another surgeon reflected on the uncertainty of prognostication in some cases of advanced colon cancer. “I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive. Days later he is awake and alert, off pressors, on trach collar, and fully communicative. Sometimes even experienced clinicians cannot predict when a patient will die or recover.”
Communication barriers remain. “A number of surgeons reported not knowing how to discuss this with families knowing that there is a stigma associated with palliative care – how to convey that they may pursue continued treatment in conjunction with palliative care,” said Dr. Suwanabol.
Surgeons adept at incorporating palliative care into their treatment plans continue to see the patients. “Even if a patient is not pursuing curative treatment, we do not want the patient to lose hope or feel abandoned by us, and I continue seeing my patients in follow-up until they feel overburdened by their clinic visits and choose not to come,” said Dr. Suwanabol.
In the absence of required palliative care training in medical schools or residencies, alternative approaches to achieving competency could include the American College of Surgeons Palliative Surgical Care Course, mentorships or collaborations with local palliative care specialists, or structured curricula implemented by experienced faculty, they added.
Dr. Suwanabol and her coauthors reported that they had no competing financial interests related to their study.
SOURCE: Suwanabol PA, et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
While to its optimal implementation at the patient, family, and clinician level, results of a recent survey suggest.
“We found that surgeons reported the most important barriers to be their own,” said Pasithorn A. Suwanabol, MD, division of colorectal surgery, University of Michigan, Ann Arbor, and coauthors of a report on the survey.
More than three-quarters of surgeons said they had no formal education in palliative care, and a substantial number specifically noted inadequate training in both communication and techniques to forgo life-sustaining measures, according to the report, published in the Journal of Palliative Medicine.
Dr. Suwanabol and her colleagues sought surgeon perspectives on palliative care for stage IV colorectal cancer in part because palliative care is often not integrated with cancer treatment in these potentially incurable patients.
“Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care,” they said in the report.
They conducted a mixed methods study including members of a surgical society (American Society of Colon and Rectal Surgeons) who were asked to submit Internet responses to a validated survey. This mixed methods study is believed to be the first to characterize surgeons’ perceived barriers to optimal palliative and end-of life care for patients with advanced colorectal cancer, Dr. Suwanabol and her colleagues noted.
A total of 131 surgeons responded to the survey, for a response rate of 16.5%. The majority of surgeons responding (76.1%) said they did not have any formal palliative care training, while 42.7% said they lacked specific education in communication and 37.9% lacked training in techniques to forgo life-sustaining measures.
Many patients with stage IV colorectal cancer are candidates for palliative care. “Specialty palliative care should be offered to patients who have more complex issues such as refractory pain, depression and anxiety, and existential distress. In addition, specialty palliative care may be helpful in those with challenging family dynamics and/or conflict between family members and/or members of the care team. Patients with stage IV colon cancer may not be candidates for curative therapies and a palliative approach allows the surgeon and patient to carefully weigh the risk and benefits of each therapy in the context of the overall goals of the patient,” Dr. Suwanabol said in an interview.
Among the survey responders, 61.8% said discussion of palliation was limited because of patients and families who had unrealistic expectations and demanded aggressive interventions.
One such recollection in the report reads as follows: ‘‘Patient with poorly responding stage IV colon cancer in multisystem organ failure getting same chemo that already failed ... family wanted everything done…’’
The report includes a number of other reflections from surgeons that suggest a level of anxiety, frustration, disappointment, or uncertainty regarding palliative care.
“I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity,” one surgeon said in his survey response. “(He) went to another major center where they reoperated … I still have doubts about whether or not (a) I didn’t do enough for the patient or (b) the other center did too much.’’
Another surgeon reflected on the uncertainty of prognostication in some cases of advanced colon cancer. “I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive. Days later he is awake and alert, off pressors, on trach collar, and fully communicative. Sometimes even experienced clinicians cannot predict when a patient will die or recover.”
Communication barriers remain. “A number of surgeons reported not knowing how to discuss this with families knowing that there is a stigma associated with palliative care – how to convey that they may pursue continued treatment in conjunction with palliative care,” said Dr. Suwanabol.
Surgeons adept at incorporating palliative care into their treatment plans continue to see the patients. “Even if a patient is not pursuing curative treatment, we do not want the patient to lose hope or feel abandoned by us, and I continue seeing my patients in follow-up until they feel overburdened by their clinic visits and choose not to come,” said Dr. Suwanabol.
In the absence of required palliative care training in medical schools or residencies, alternative approaches to achieving competency could include the American College of Surgeons Palliative Surgical Care Course, mentorships or collaborations with local palliative care specialists, or structured curricula implemented by experienced faculty, they added.
Dr. Suwanabol and her coauthors reported that they had no competing financial interests related to their study.
SOURCE: Suwanabol PA, et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
FROM THE JOURNAL OF PALLIATIVE MEDICINE
Key clinical point: Surgeons valued palliative and end-of-life care for patients with stage IV colorectal cancer, but reported multiple barriers to implementation.
Major finding: More than three-quarters of surgeons (76.1%) reported no formal education in palliative care, and 61.8% said patients and families had unrealistic expectations.
Study details: A mixed methods study including 131 members of a surgical society (American Society of Colon and Rectal Surgeons) who submitted Internet responses to a validated survey.
Disclosures: The authors declared that no competing interests relative to this report exist.
Source: Suwanabol PA et al. J Palliat Med. 2018 Mar 13. doi: 10.1089/jpm.2017.0470.
New Guidelines of Care for the Management of Nonmelanoma Skin Cancer
In January 2018, the American Academy of Dermatology (AAD) released its first guidelines of care for the management of nonmelanoma skin cancer (NMSC), which established official recommendations for the treatment of basal cell carcinoma (BCC)1 and cutaneous squamous cell carcinoma (cSCC).2 The guidelines will help dermatologists address the growing health concern of skin cancer, which remains the most common of any type of cancer in the United States.3 Affecting more than 3 million Americans every year, NMSC is the most common type of skin cancer, and its incidence has continued to increase every year over the past few decades.3,4 During the past 30 years, the incidence of both BCC and cSCC has more than doubled.5
Commonly used guidelines for the management of NMSC are available from the National Comprehensive Cancer Network (NCCN).6,7 Although the NCCN aimed to develop multidisciplinary guidelines, the new AAD guidelines were established primarily by dermatologists for dermatologists. The NCCN guidelines frequently are referenced throughout the new AAD guidelines, which also recognize the importance of multidisciplinary care. The authors of the AAD guidelines noted that, although many of the NCCN recommendations reiterated prevailing knowledge or current practice, some recommendations highlighted alternative tenets that were not as widely considered or were supported by insufficient evidence.
The AAD guidelines address the complete management of NMSC, which includes biopsy technique, staging, treatment, follow-up, metastatic disease, and prevention.1,2 Also included are evidence tables evaluating the current literature and available recommendations.
BCC Guidelines
For suspected BCCs, the recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy, all of which can detect the most aggressive histology subtypes.1 Rebiopsy is recommended if the initial specimen is inadequate. The pathology report should include histologic subtype, invasion beyond the reticular dermis, and perineural involvement. The AAD guidelines do not include a formal staging system for risk stratification but rather refer to the NCCN guidelines, which take both clinical and pathologic parameters into account. The AAD treatment recommendations are based on this stratification.1
Treatment of BCC includes a broad range of therapeutic modalities. Recurrence rate, preservation of function, patient expectations, and potential adverse effects should be considered in the treatment plan.1 Curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm margins is recommended for low-risk primary tumors. For high-risk BCC, Mohs micrographic surgery is recommended, although standard excision along with attention to margin control may also be considered. Nonsurgical treatments also may be considered when more effective surgical therapies are contraindicated or impractical. If surgical therapy is not feasible or preferred, other treatment options for low-risk BCCs include cryotherapy, topical
Multidisciplinary consultation is recommended in patients with metastatic BCCs along with first-line treatment with a smoothened inhibitor.1 Alternative treatment options include platinum-based chemotherapy and/or supportive care. For locally advanced disease, surgery and radiation therapy remain the initial treatments, but smoothened inhibitors and supportive care are suitable alternative treatments.1
The AAD guidelines also offer recommendations for follow-up and reducing future risk of skin cancer. After the first diagnosis of BCC, a skin cancer screening should be performed at least annually, and patients should be counseled about self-examinations and sun protection.1 Topical and oral retinoids are not recommended for the prevention of additional skin cancers, nor is dietary supplementation with selenium or beta-carotene. There also is insufficient evidence regarding the use of oral nicotinamide, celecoxib, or α-difluoromethylornithine for chemoprevention of disease.1
cSCC Guidelines
For suspected cSCCs, no single optimal biopsy technique is recommended, but repeat biopsy may be considered if the initial biopsy is insufficient for diagnosis.2 The guidelines further recommend an extensive list of elements to be included in the final pathology report (eg, lesion size, immunosuppression, depth of invasion, degree of differentiation). There is no universally recognized stratification for localized cSCC; therefore, the AAD guidelines refer to the framework provided by the NCCN. Also mentioned is the recent release of the American Joint Committee on Cancer’s staging manual,8 which includes the management of cSCC in conjunction with all SCCs of the head and neck. The Brigham and Women’s system9 was considered as an alternative classification system; however, the NCCN guidelines were chosen because they primarily provide clinical guidance for treatment of cSCC rather than provide accurate prognostication or outcome assessment.
Considerations for surgical treatment of cSCC are similar to those for BCC.2 In low-risk tumors, surgical excision with 4- to 6-mm margins to the midsubcutaneous fat or curettage with electrodessication may be considered. Mohs micrographic surgery or standard excision with attention to margin control may be considered for high-risk tumors. Nonsurgical therapies generally are not recommended as a first-line treatment, particularly in cSCC, due to possible recurrence and metastasis. When nonsurgical therapies are preferred, options may include cryosurgery or radiation therapy, with the understanding that cure rates may be lower than with surgical options. Topical therapy with imiquimod or 5-fluorouracil as well as photodynamic or laser therapy are not recommended for cSCCs.2
For patients with metastatic cSCC or locally advanced disease, multidisciplinary consultation is recommended.2 In cSCCs with regional lymph node metastases, the recommended approach includes surgical resection with possible adjuvant radiation therapy and/or systemic therapy. For inoperable disease, combination chemoradiation may be considered. Epidermal growth factor inhibitors and cisplatin may be considered in metastatic disease, although there are limited data to support their efficacy. As with BCC, all patients with cSCCs should receive supportive and palliative care to optimize quality of life.2
Recommendations for follow-up after the first diagnosis of cSCC are the same as those for BCC.2 Additionally, acitretin is the only therapy that may be beneficial in the reduction of recurrent skin cancer in patients who are solid-organ transplant recipients.
Final Thoughts
A comprehensive understanding of the management of NMSC and the evidence on which recommendations are based is critically important for optimal patient care. These guidelines are an efficient way for dermatologists and their colleagues to understand the latest evidence and recommendations. The AAD guidelines provide support for clinical decision making with standardized approaches to the diagnosis, care, and prevention of NMSC that are consistent with established practice patterns.
With few exceptions, surgical therapy is the most effective approach for the treatment of BCC and cSCC; however, the AAD guidelines include an important review on nonsurgical management options.1,2 The AAD guidelines help to highlight where data on evidence-based outcomes exist and reveal where data remain insufficient. This is illustrated by the guideline recommendations for providing additional histopathologic characteristics in the pathology reports, which will likely produce future data to enhance the prognosis and eventual treatment of patients with NMSC.1,2 Future guidelines also may include newer technologies (eg, gene expression profiling).
The guidelines do not cover the management of premalignant and in situ lesions, nor do they provide details on the management of metastatic or locally advanced disease. These topics certainly will require a similar critical review and may be addressed separately. The guidelines are identifying unanswered questions about patient care and are concurrently establishing the collection of appropriate data to answer these questions in the future.
Official guidelines often become the primary source for the measured standard of both treatment and outcomes in patient care; therefore, it is critical that dermatologists and the AAD take the lead in creating these guidelines so that we can provide our patients with the best evidenced-based comprehensive care.
The AAD guidelines emphasize the importance of considering the patient perspective in determining how to treat BCCs and cSCCs.1,2 It is important for patients to understand the available treatment options and participate in their own medical care. The AAD work group for these guidelines included patient advocates to ensure that the guidelines would promote further dialogue between physicians and their patients.
The AAD guidelines for the management of NMSC were developed by board-certified dermatologists and other experts in the field. They allow dermatologists to work with patients diagnosed with NMSC to determine the treatment option that is best for each individual patient.
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Burden of skin disease. American Academy of Dermatology website. https://www.aad.org/about/burden-of-skin-disease. Accessed April 17, 2018.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081-1086.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmstead County, Minnnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Basal Cell Skin Cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Published September 18, 2017. Accessed April 17, 2018.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous Cell Skin Cancer. National Comprehensive Cancer Network website. Published October 5, 2017. Accessed April 17, 2018.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
In January 2018, the American Academy of Dermatology (AAD) released its first guidelines of care for the management of nonmelanoma skin cancer (NMSC), which established official recommendations for the treatment of basal cell carcinoma (BCC)1 and cutaneous squamous cell carcinoma (cSCC).2 The guidelines will help dermatologists address the growing health concern of skin cancer, which remains the most common of any type of cancer in the United States.3 Affecting more than 3 million Americans every year, NMSC is the most common type of skin cancer, and its incidence has continued to increase every year over the past few decades.3,4 During the past 30 years, the incidence of both BCC and cSCC has more than doubled.5
Commonly used guidelines for the management of NMSC are available from the National Comprehensive Cancer Network (NCCN).6,7 Although the NCCN aimed to develop multidisciplinary guidelines, the new AAD guidelines were established primarily by dermatologists for dermatologists. The NCCN guidelines frequently are referenced throughout the new AAD guidelines, which also recognize the importance of multidisciplinary care. The authors of the AAD guidelines noted that, although many of the NCCN recommendations reiterated prevailing knowledge or current practice, some recommendations highlighted alternative tenets that were not as widely considered or were supported by insufficient evidence.
The AAD guidelines address the complete management of NMSC, which includes biopsy technique, staging, treatment, follow-up, metastatic disease, and prevention.1,2 Also included are evidence tables evaluating the current literature and available recommendations.
BCC Guidelines
For suspected BCCs, the recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy, all of which can detect the most aggressive histology subtypes.1 Rebiopsy is recommended if the initial specimen is inadequate. The pathology report should include histologic subtype, invasion beyond the reticular dermis, and perineural involvement. The AAD guidelines do not include a formal staging system for risk stratification but rather refer to the NCCN guidelines, which take both clinical and pathologic parameters into account. The AAD treatment recommendations are based on this stratification.1
Treatment of BCC includes a broad range of therapeutic modalities. Recurrence rate, preservation of function, patient expectations, and potential adverse effects should be considered in the treatment plan.1 Curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm margins is recommended for low-risk primary tumors. For high-risk BCC, Mohs micrographic surgery is recommended, although standard excision along with attention to margin control may also be considered. Nonsurgical treatments also may be considered when more effective surgical therapies are contraindicated or impractical. If surgical therapy is not feasible or preferred, other treatment options for low-risk BCCs include cryotherapy, topical
Multidisciplinary consultation is recommended in patients with metastatic BCCs along with first-line treatment with a smoothened inhibitor.1 Alternative treatment options include platinum-based chemotherapy and/or supportive care. For locally advanced disease, surgery and radiation therapy remain the initial treatments, but smoothened inhibitors and supportive care are suitable alternative treatments.1
The AAD guidelines also offer recommendations for follow-up and reducing future risk of skin cancer. After the first diagnosis of BCC, a skin cancer screening should be performed at least annually, and patients should be counseled about self-examinations and sun protection.1 Topical and oral retinoids are not recommended for the prevention of additional skin cancers, nor is dietary supplementation with selenium or beta-carotene. There also is insufficient evidence regarding the use of oral nicotinamide, celecoxib, or α-difluoromethylornithine for chemoprevention of disease.1
cSCC Guidelines
For suspected cSCCs, no single optimal biopsy technique is recommended, but repeat biopsy may be considered if the initial biopsy is insufficient for diagnosis.2 The guidelines further recommend an extensive list of elements to be included in the final pathology report (eg, lesion size, immunosuppression, depth of invasion, degree of differentiation). There is no universally recognized stratification for localized cSCC; therefore, the AAD guidelines refer to the framework provided by the NCCN. Also mentioned is the recent release of the American Joint Committee on Cancer’s staging manual,8 which includes the management of cSCC in conjunction with all SCCs of the head and neck. The Brigham and Women’s system9 was considered as an alternative classification system; however, the NCCN guidelines were chosen because they primarily provide clinical guidance for treatment of cSCC rather than provide accurate prognostication or outcome assessment.
Considerations for surgical treatment of cSCC are similar to those for BCC.2 In low-risk tumors, surgical excision with 4- to 6-mm margins to the midsubcutaneous fat or curettage with electrodessication may be considered. Mohs micrographic surgery or standard excision with attention to margin control may be considered for high-risk tumors. Nonsurgical therapies generally are not recommended as a first-line treatment, particularly in cSCC, due to possible recurrence and metastasis. When nonsurgical therapies are preferred, options may include cryosurgery or radiation therapy, with the understanding that cure rates may be lower than with surgical options. Topical therapy with imiquimod or 5-fluorouracil as well as photodynamic or laser therapy are not recommended for cSCCs.2
For patients with metastatic cSCC or locally advanced disease, multidisciplinary consultation is recommended.2 In cSCCs with regional lymph node metastases, the recommended approach includes surgical resection with possible adjuvant radiation therapy and/or systemic therapy. For inoperable disease, combination chemoradiation may be considered. Epidermal growth factor inhibitors and cisplatin may be considered in metastatic disease, although there are limited data to support their efficacy. As with BCC, all patients with cSCCs should receive supportive and palliative care to optimize quality of life.2
Recommendations for follow-up after the first diagnosis of cSCC are the same as those for BCC.2 Additionally, acitretin is the only therapy that may be beneficial in the reduction of recurrent skin cancer in patients who are solid-organ transplant recipients.
Final Thoughts
A comprehensive understanding of the management of NMSC and the evidence on which recommendations are based is critically important for optimal patient care. These guidelines are an efficient way for dermatologists and their colleagues to understand the latest evidence and recommendations. The AAD guidelines provide support for clinical decision making with standardized approaches to the diagnosis, care, and prevention of NMSC that are consistent with established practice patterns.
With few exceptions, surgical therapy is the most effective approach for the treatment of BCC and cSCC; however, the AAD guidelines include an important review on nonsurgical management options.1,2 The AAD guidelines help to highlight where data on evidence-based outcomes exist and reveal where data remain insufficient. This is illustrated by the guideline recommendations for providing additional histopathologic characteristics in the pathology reports, which will likely produce future data to enhance the prognosis and eventual treatment of patients with NMSC.1,2 Future guidelines also may include newer technologies (eg, gene expression profiling).
The guidelines do not cover the management of premalignant and in situ lesions, nor do they provide details on the management of metastatic or locally advanced disease. These topics certainly will require a similar critical review and may be addressed separately. The guidelines are identifying unanswered questions about patient care and are concurrently establishing the collection of appropriate data to answer these questions in the future.
Official guidelines often become the primary source for the measured standard of both treatment and outcomes in patient care; therefore, it is critical that dermatologists and the AAD take the lead in creating these guidelines so that we can provide our patients with the best evidenced-based comprehensive care.
The AAD guidelines emphasize the importance of considering the patient perspective in determining how to treat BCCs and cSCCs.1,2 It is important for patients to understand the available treatment options and participate in their own medical care. The AAD work group for these guidelines included patient advocates to ensure that the guidelines would promote further dialogue between physicians and their patients.
The AAD guidelines for the management of NMSC were developed by board-certified dermatologists and other experts in the field. They allow dermatologists to work with patients diagnosed with NMSC to determine the treatment option that is best for each individual patient.
In January 2018, the American Academy of Dermatology (AAD) released its first guidelines of care for the management of nonmelanoma skin cancer (NMSC), which established official recommendations for the treatment of basal cell carcinoma (BCC)1 and cutaneous squamous cell carcinoma (cSCC).2 The guidelines will help dermatologists address the growing health concern of skin cancer, which remains the most common of any type of cancer in the United States.3 Affecting more than 3 million Americans every year, NMSC is the most common type of skin cancer, and its incidence has continued to increase every year over the past few decades.3,4 During the past 30 years, the incidence of both BCC and cSCC has more than doubled.5
Commonly used guidelines for the management of NMSC are available from the National Comprehensive Cancer Network (NCCN).6,7 Although the NCCN aimed to develop multidisciplinary guidelines, the new AAD guidelines were established primarily by dermatologists for dermatologists. The NCCN guidelines frequently are referenced throughout the new AAD guidelines, which also recognize the importance of multidisciplinary care. The authors of the AAD guidelines noted that, although many of the NCCN recommendations reiterated prevailing knowledge or current practice, some recommendations highlighted alternative tenets that were not as widely considered or were supported by insufficient evidence.
The AAD guidelines address the complete management of NMSC, which includes biopsy technique, staging, treatment, follow-up, metastatic disease, and prevention.1,2 Also included are evidence tables evaluating the current literature and available recommendations.
BCC Guidelines
For suspected BCCs, the recommended biopsy techniques are punch biopsy, shave biopsy, and excisional biopsy, all of which can detect the most aggressive histology subtypes.1 Rebiopsy is recommended if the initial specimen is inadequate. The pathology report should include histologic subtype, invasion beyond the reticular dermis, and perineural involvement. The AAD guidelines do not include a formal staging system for risk stratification but rather refer to the NCCN guidelines, which take both clinical and pathologic parameters into account. The AAD treatment recommendations are based on this stratification.1
Treatment of BCC includes a broad range of therapeutic modalities. Recurrence rate, preservation of function, patient expectations, and potential adverse effects should be considered in the treatment plan.1 Curettage and electrodessication may be considered for low-risk tumors in nonterminal hair-bearing locations. Surgical excision with 4-mm margins is recommended for low-risk primary tumors. For high-risk BCC, Mohs micrographic surgery is recommended, although standard excision along with attention to margin control may also be considered. Nonsurgical treatments also may be considered when more effective surgical therapies are contraindicated or impractical. If surgical therapy is not feasible or preferred, other treatment options for low-risk BCCs include cryotherapy, topical
Multidisciplinary consultation is recommended in patients with metastatic BCCs along with first-line treatment with a smoothened inhibitor.1 Alternative treatment options include platinum-based chemotherapy and/or supportive care. For locally advanced disease, surgery and radiation therapy remain the initial treatments, but smoothened inhibitors and supportive care are suitable alternative treatments.1
The AAD guidelines also offer recommendations for follow-up and reducing future risk of skin cancer. After the first diagnosis of BCC, a skin cancer screening should be performed at least annually, and patients should be counseled about self-examinations and sun protection.1 Topical and oral retinoids are not recommended for the prevention of additional skin cancers, nor is dietary supplementation with selenium or beta-carotene. There also is insufficient evidence regarding the use of oral nicotinamide, celecoxib, or α-difluoromethylornithine for chemoprevention of disease.1
cSCC Guidelines
For suspected cSCCs, no single optimal biopsy technique is recommended, but repeat biopsy may be considered if the initial biopsy is insufficient for diagnosis.2 The guidelines further recommend an extensive list of elements to be included in the final pathology report (eg, lesion size, immunosuppression, depth of invasion, degree of differentiation). There is no universally recognized stratification for localized cSCC; therefore, the AAD guidelines refer to the framework provided by the NCCN. Also mentioned is the recent release of the American Joint Committee on Cancer’s staging manual,8 which includes the management of cSCC in conjunction with all SCCs of the head and neck. The Brigham and Women’s system9 was considered as an alternative classification system; however, the NCCN guidelines were chosen because they primarily provide clinical guidance for treatment of cSCC rather than provide accurate prognostication or outcome assessment.
Considerations for surgical treatment of cSCC are similar to those for BCC.2 In low-risk tumors, surgical excision with 4- to 6-mm margins to the midsubcutaneous fat or curettage with electrodessication may be considered. Mohs micrographic surgery or standard excision with attention to margin control may be considered for high-risk tumors. Nonsurgical therapies generally are not recommended as a first-line treatment, particularly in cSCC, due to possible recurrence and metastasis. When nonsurgical therapies are preferred, options may include cryosurgery or radiation therapy, with the understanding that cure rates may be lower than with surgical options. Topical therapy with imiquimod or 5-fluorouracil as well as photodynamic or laser therapy are not recommended for cSCCs.2
For patients with metastatic cSCC or locally advanced disease, multidisciplinary consultation is recommended.2 In cSCCs with regional lymph node metastases, the recommended approach includes surgical resection with possible adjuvant radiation therapy and/or systemic therapy. For inoperable disease, combination chemoradiation may be considered. Epidermal growth factor inhibitors and cisplatin may be considered in metastatic disease, although there are limited data to support their efficacy. As with BCC, all patients with cSCCs should receive supportive and palliative care to optimize quality of life.2
Recommendations for follow-up after the first diagnosis of cSCC are the same as those for BCC.2 Additionally, acitretin is the only therapy that may be beneficial in the reduction of recurrent skin cancer in patients who are solid-organ transplant recipients.
Final Thoughts
A comprehensive understanding of the management of NMSC and the evidence on which recommendations are based is critically important for optimal patient care. These guidelines are an efficient way for dermatologists and their colleagues to understand the latest evidence and recommendations. The AAD guidelines provide support for clinical decision making with standardized approaches to the diagnosis, care, and prevention of NMSC that are consistent with established practice patterns.
With few exceptions, surgical therapy is the most effective approach for the treatment of BCC and cSCC; however, the AAD guidelines include an important review on nonsurgical management options.1,2 The AAD guidelines help to highlight where data on evidence-based outcomes exist and reveal where data remain insufficient. This is illustrated by the guideline recommendations for providing additional histopathologic characteristics in the pathology reports, which will likely produce future data to enhance the prognosis and eventual treatment of patients with NMSC.1,2 Future guidelines also may include newer technologies (eg, gene expression profiling).
The guidelines do not cover the management of premalignant and in situ lesions, nor do they provide details on the management of metastatic or locally advanced disease. These topics certainly will require a similar critical review and may be addressed separately. The guidelines are identifying unanswered questions about patient care and are concurrently establishing the collection of appropriate data to answer these questions in the future.
Official guidelines often become the primary source for the measured standard of both treatment and outcomes in patient care; therefore, it is critical that dermatologists and the AAD take the lead in creating these guidelines so that we can provide our patients with the best evidenced-based comprehensive care.
The AAD guidelines emphasize the importance of considering the patient perspective in determining how to treat BCCs and cSCCs.1,2 It is important for patients to understand the available treatment options and participate in their own medical care. The AAD work group for these guidelines included patient advocates to ensure that the guidelines would promote further dialogue between physicians and their patients.
The AAD guidelines for the management of NMSC were developed by board-certified dermatologists and other experts in the field. They allow dermatologists to work with patients diagnosed with NMSC to determine the treatment option that is best for each individual patient.
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Burden of skin disease. American Academy of Dermatology website. https://www.aad.org/about/burden-of-skin-disease. Accessed April 17, 2018.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081-1086.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmstead County, Minnnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Basal Cell Skin Cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Published September 18, 2017. Accessed April 17, 2018.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous Cell Skin Cancer. National Comprehensive Cancer Network website. Published October 5, 2017. Accessed April 17, 2018.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Burden of skin disease. American Academy of Dermatology website. https://www.aad.org/about/burden-of-skin-disease. Accessed April 17, 2018.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population. JAMA Dermatol. 2015;151:1081-1086.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmstead County, Minnnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Basal Cell Skin Cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Published September 18, 2017. Accessed April 17, 2018.
- Bichakjian CK, Olencki T, Aasi SZ, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous Cell Skin Cancer. National Comprehensive Cancer Network website. Published October 5, 2017. Accessed April 17, 2018.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
‘You’re not going to tell my parents about this are you?’
You are on the front lines of the prevention, screening, and treatment decisions for adolescent substance use disorders. You often must choose whether to disclose information about substance use to parents and other concerned adults.
The risk of developing a substance use disorder increases dramatically the earlier an individual begins using a given substance.1 The neurobiology behind this risk is becoming increasingly clear. Young brains are undergoing crucial developmental processes, including synaptic pruning and myelination. The brain increasingly becomes more efficient in a staggered pattern, with limbic regions preceding frontal and executive regions, so we see adolescents with “more gas than brakes.” This has wisely been identified as developmentally appropriate, and even beneficial, rather than evidence that adolescents are somehow broken.2
Age-appropriate screening for substance use should occur as early as the preteen years and continue throughout adolescence. The most widely studied screening tools include the CRAFFT screening instrument and the Screening, Brief Intervention, and Referral to Treatment (SBIRT) approach.3,4 During formal and informal screening, you should lead with genuine concern for the well-being of the adolescent. Beginning a discussion with open-ended questions about substance use in the school and home is a way to build understanding of an adolescent’s environment prior to asking about personal use. While screening, consider well known risk factors including family history of substance use disorders, poor parental supervision, childhood maltreatment or abuse, low academic achievement, and untreated psychiatric disorders such as ADHD, depression, or anxiety, which may contribute to a higher likelihood or more rapid progression of a substance use disorder. Adolescents are more likely to disclose substance use when screening is done in private, rather than in the presence of a parent.5
Discussing the limits of confidentiality (generally when there is substantial risk of harm to self or others) with an adolescent shows respect and can be an expression of genuine care and concern. Once substance use or other risk-associated behaviors and choices are disclosed, you often may be asked not to share the information with parents. In some instances, privacy cannot be broken without consent. Be aware of your state laws governing parental and adolescent rights related to confidentiality.
You should strongly consider discussing substance use with the concerned adults when there are these red flags: daily use of any substance, any intravenous substance use, a score of 2 or higher on the CRAFFT, prescription medication misuse, or any change in medical status resulting from substance use, such as alcohol-related blackouts.
In most cases, adolescents should be informed of a decision to disclose substance use to their parents. Inviting adolescents to discuss how this will be done, including if the adolescent will be present, and whether you or the adolescent will disclose the use can be an opportunity to discuss their concerns. You should seek to understand if an adolescent has specific fears related to such a disclosure including careful consideration of any history of domestic violence or abuse.
Although adolescents increasingly identify with the opinions and values of their peers, it is a mistake to assume that they therefore do not value the opinions of their parents and the concerned adults in their lives. Parents play an integral role in preventing and treating adolescent substance use disorders. Except in rare instances of severe parent-child relationship problems or abuse, parents can and should be engaged as invaluable participants
Being aware of the legal and ethical obligations in treatment of adolescents presenting with any level of substance use, you can improve outcomes by thoughtfully inviting the participation of parents and other concerned adults into the prevention, screening, and treatment of adolescent substance use disorders.
Dr. Jackson is a child and adolescent psychiatrist at the University of Vermont, Burlington.
References
1. “Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings.” NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. (Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013).
2. “The Amazing Teen Brain,” Jay N. Geidd, Scientific American, May 2016.
3. Pediatrics 2011 Oct. doi: 10.1542/peds.2011-1754.
4. Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide.
5. Pediatrics. 2016 Jul 1. doi: 10.1542/peds.2016-1211.
6. J Fam Commun. 2014 Jan 1:14(4):328-51.
7. J Clin Child Adolesc. Psychol. 2008;37(1):236-59.
8. J Child Adolesc Subst Abuse. 2015 May 4;24(3):155-65.
9. Arch Pediatr Adolesc Med. 2012;166(12):1132-9.
You are on the front lines of the prevention, screening, and treatment decisions for adolescent substance use disorders. You often must choose whether to disclose information about substance use to parents and other concerned adults.
The risk of developing a substance use disorder increases dramatically the earlier an individual begins using a given substance.1 The neurobiology behind this risk is becoming increasingly clear. Young brains are undergoing crucial developmental processes, including synaptic pruning and myelination. The brain increasingly becomes more efficient in a staggered pattern, with limbic regions preceding frontal and executive regions, so we see adolescents with “more gas than brakes.” This has wisely been identified as developmentally appropriate, and even beneficial, rather than evidence that adolescents are somehow broken.2
Age-appropriate screening for substance use should occur as early as the preteen years and continue throughout adolescence. The most widely studied screening tools include the CRAFFT screening instrument and the Screening, Brief Intervention, and Referral to Treatment (SBIRT) approach.3,4 During formal and informal screening, you should lead with genuine concern for the well-being of the adolescent. Beginning a discussion with open-ended questions about substance use in the school and home is a way to build understanding of an adolescent’s environment prior to asking about personal use. While screening, consider well known risk factors including family history of substance use disorders, poor parental supervision, childhood maltreatment or abuse, low academic achievement, and untreated psychiatric disorders such as ADHD, depression, or anxiety, which may contribute to a higher likelihood or more rapid progression of a substance use disorder. Adolescents are more likely to disclose substance use when screening is done in private, rather than in the presence of a parent.5
Discussing the limits of confidentiality (generally when there is substantial risk of harm to self or others) with an adolescent shows respect and can be an expression of genuine care and concern. Once substance use or other risk-associated behaviors and choices are disclosed, you often may be asked not to share the information with parents. In some instances, privacy cannot be broken without consent. Be aware of your state laws governing parental and adolescent rights related to confidentiality.
You should strongly consider discussing substance use with the concerned adults when there are these red flags: daily use of any substance, any intravenous substance use, a score of 2 or higher on the CRAFFT, prescription medication misuse, or any change in medical status resulting from substance use, such as alcohol-related blackouts.
In most cases, adolescents should be informed of a decision to disclose substance use to their parents. Inviting adolescents to discuss how this will be done, including if the adolescent will be present, and whether you or the adolescent will disclose the use can be an opportunity to discuss their concerns. You should seek to understand if an adolescent has specific fears related to such a disclosure including careful consideration of any history of domestic violence or abuse.
Although adolescents increasingly identify with the opinions and values of their peers, it is a mistake to assume that they therefore do not value the opinions of their parents and the concerned adults in their lives. Parents play an integral role in preventing and treating adolescent substance use disorders. Except in rare instances of severe parent-child relationship problems or abuse, parents can and should be engaged as invaluable participants
Being aware of the legal and ethical obligations in treatment of adolescents presenting with any level of substance use, you can improve outcomes by thoughtfully inviting the participation of parents and other concerned adults into the prevention, screening, and treatment of adolescent substance use disorders.
Dr. Jackson is a child and adolescent psychiatrist at the University of Vermont, Burlington.
References
1. “Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings.” NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. (Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013).
2. “The Amazing Teen Brain,” Jay N. Geidd, Scientific American, May 2016.
3. Pediatrics 2011 Oct. doi: 10.1542/peds.2011-1754.
4. Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide.
5. Pediatrics. 2016 Jul 1. doi: 10.1542/peds.2016-1211.
6. J Fam Commun. 2014 Jan 1:14(4):328-51.
7. J Clin Child Adolesc. Psychol. 2008;37(1):236-59.
8. J Child Adolesc Subst Abuse. 2015 May 4;24(3):155-65.
9. Arch Pediatr Adolesc Med. 2012;166(12):1132-9.
You are on the front lines of the prevention, screening, and treatment decisions for adolescent substance use disorders. You often must choose whether to disclose information about substance use to parents and other concerned adults.
The risk of developing a substance use disorder increases dramatically the earlier an individual begins using a given substance.1 The neurobiology behind this risk is becoming increasingly clear. Young brains are undergoing crucial developmental processes, including synaptic pruning and myelination. The brain increasingly becomes more efficient in a staggered pattern, with limbic regions preceding frontal and executive regions, so we see adolescents with “more gas than brakes.” This has wisely been identified as developmentally appropriate, and even beneficial, rather than evidence that adolescents are somehow broken.2
Age-appropriate screening for substance use should occur as early as the preteen years and continue throughout adolescence. The most widely studied screening tools include the CRAFFT screening instrument and the Screening, Brief Intervention, and Referral to Treatment (SBIRT) approach.3,4 During formal and informal screening, you should lead with genuine concern for the well-being of the adolescent. Beginning a discussion with open-ended questions about substance use in the school and home is a way to build understanding of an adolescent’s environment prior to asking about personal use. While screening, consider well known risk factors including family history of substance use disorders, poor parental supervision, childhood maltreatment or abuse, low academic achievement, and untreated psychiatric disorders such as ADHD, depression, or anxiety, which may contribute to a higher likelihood or more rapid progression of a substance use disorder. Adolescents are more likely to disclose substance use when screening is done in private, rather than in the presence of a parent.5
Discussing the limits of confidentiality (generally when there is substantial risk of harm to self or others) with an adolescent shows respect and can be an expression of genuine care and concern. Once substance use or other risk-associated behaviors and choices are disclosed, you often may be asked not to share the information with parents. In some instances, privacy cannot be broken without consent. Be aware of your state laws governing parental and adolescent rights related to confidentiality.
You should strongly consider discussing substance use with the concerned adults when there are these red flags: daily use of any substance, any intravenous substance use, a score of 2 or higher on the CRAFFT, prescription medication misuse, or any change in medical status resulting from substance use, such as alcohol-related blackouts.
In most cases, adolescents should be informed of a decision to disclose substance use to their parents. Inviting adolescents to discuss how this will be done, including if the adolescent will be present, and whether you or the adolescent will disclose the use can be an opportunity to discuss their concerns. You should seek to understand if an adolescent has specific fears related to such a disclosure including careful consideration of any history of domestic violence or abuse.
Although adolescents increasingly identify with the opinions and values of their peers, it is a mistake to assume that they therefore do not value the opinions of their parents and the concerned adults in their lives. Parents play an integral role in preventing and treating adolescent substance use disorders. Except in rare instances of severe parent-child relationship problems or abuse, parents can and should be engaged as invaluable participants
Being aware of the legal and ethical obligations in treatment of adolescents presenting with any level of substance use, you can improve outcomes by thoughtfully inviting the participation of parents and other concerned adults into the prevention, screening, and treatment of adolescent substance use disorders.
Dr. Jackson is a child and adolescent psychiatrist at the University of Vermont, Burlington.
References
1. “Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings.” NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. (Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013).
2. “The Amazing Teen Brain,” Jay N. Geidd, Scientific American, May 2016.
3. Pediatrics 2011 Oct. doi: 10.1542/peds.2011-1754.
4. Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide.
5. Pediatrics. 2016 Jul 1. doi: 10.1542/peds.2016-1211.
6. J Fam Commun. 2014 Jan 1:14(4):328-51.
7. J Clin Child Adolesc. Psychol. 2008;37(1):236-59.
8. J Child Adolesc Subst Abuse. 2015 May 4;24(3):155-65.
9. Arch Pediatr Adolesc Med. 2012;166(12):1132-9.
Update on AGA-Medtronic Research and Development Pilot Award in Technology
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research and Development Pilot Award in Technology, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and functioning. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington, D.C., as a poster of distinction.
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiologic mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using the Wireless Motility Capsule (WMC, SmartPill) to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO – just the sort of innovative technology that the AGA Center for GI Innovation and Technology (CGIT) has fostered. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with IBS and SIBO have increased expression of pro-inflammatory markers compared to those with only IBS; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries, without understanding the etiology for this syndrome,” said Dr. Roland, director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
In data to be presented in the DDW poster, Dr. Roland’s team used the SmartPill to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared to patients with IBS without evidence of SIBO. “Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as TNF (tumor necrosis factor)-alpha and IL (interleukin)-6, to patients with IBS alone. They will use their data to apply for future funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research and Development Pilot Award in Technology, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and functioning. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington, D.C., as a poster of distinction.
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiologic mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using the Wireless Motility Capsule (WMC, SmartPill) to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO – just the sort of innovative technology that the AGA Center for GI Innovation and Technology (CGIT) has fostered. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with IBS and SIBO have increased expression of pro-inflammatory markers compared to those with only IBS; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries, without understanding the etiology for this syndrome,” said Dr. Roland, director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
In data to be presented in the DDW poster, Dr. Roland’s team used the SmartPill to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared to patients with IBS without evidence of SIBO. “Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as TNF (tumor necrosis factor)-alpha and IL (interleukin)-6, to patients with IBS alone. They will use their data to apply for future funding.
BOSTON – It’s been just a year since Bani Chander Roland, MD, FACG, was awarded the 2017 AGA-Medtronic Research and Development Pilot Award in Technology, and her team already has recruited 30 patients with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO) for a study of the gut microbiome and functioning. Interim data from her grant will be presented at Digestive Disease Week® 2018 in June in Washington, D.C., as a poster of distinction.
Dr. Roland and her team are testing the hypothesis that IBS and SIBO result from several distinct pathophysiologic mechanisms, each of which are associated with their own distinct microbial and inflammatory profile. For the study, they are using the Wireless Motility Capsule (WMC, SmartPill) to assess alterations in gastrointestinal pathophysiology in patients with suspected IBS and SIBO – just the sort of innovative technology that the AGA Center for GI Innovation and Technology (CGIT) has fostered. They also are obtaining microflora from oropharyngeal, gastric, small bowel, and fecal samples for DNA sequencing. In addition, the team is beginning to study serum samples to test the hypothesis that patients with IBS and SIBO have increased expression of pro-inflammatory markers compared to those with only IBS; they are attempting to correlate the inflammatory markers to specific bacteria.
“IBS is a very common gastrointestinal disorder, and we’re continuing to see an increase in prevalence in Western countries, without understanding the etiology for this syndrome,” said Dr. Roland, director of gastrointestinal motility at Lenox Hill Hospital and Northwell Health System in New York. “Unfortunately, we don’t have any specific or targeted therapies for this patient population because the underlying physiological mechanisms that cause IBS are not very well understood. When we treat these patients with antibiotics, often their symptoms come right back. If we can target the causes of disease in subsets of these patients, we may be able to successfully treat them.”
“We’re very excited to see what changes in the microbiome exist in this patient population, to determine if the microbiome may be another potential area that we can target for treatment,” she added.
In data to be presented in the DDW poster, Dr. Roland’s team used the SmartPill to measure the gastrointestinal transit times, pH, and ileocecal junction pressures of patients with IBS and SIBO as compared to patients with IBS without evidence of SIBO. “Interestingly, patients who had IBS and SIBO had significantly higher contraction frequency in the stomach and small bowel compared to patients with IBS alone,” Dr. Roland said. Those with both conditions also had lower ileocecal junction pressures. “These are physiological mechanisms that have not been well understood before,” Dr. Roland said. “We have been able to begin delineating some of the underlying physiological mechanisms in this challenging patient population for the first time, using a noninvasive, wireless motility capsule.”
Dr. Roland’s team is now partnering with the hospital’s endocrinology division to compare the circulating inflammatory markers in patients with IBS and SIBO, such as TNF (tumor necrosis factor)-alpha and IL (interleukin)-6, to patients with IBS alone. They will use their data to apply for future funding.
2018 AGA TECH SUMMIT
Variants in One Gene May Account for 7% of Juvenile Myoclonic Epilepsy Cases
Rare genetic variants that affect the maturation, migration, and death of neurons appear to be responsible for about 7% of cases of juvenile myoclonic epilepsy.
Variants of the intestinal-cell kinase gene (ICK) occurred in 12 members of a family affected by the disorder and were confirmed in 22 of 310 additional patients, Julia N. Bailey, PhD, of the University of California, Los Angeles (UCLA), and her colleagues reported in the March 15 issue of the New England Journal of Medicine.
But among these 34 patients, the variants manifested as different epileptic phenotypes, suggesting genetic pleiotropism, the investigators said.
Clinical Heterogeneity
“We report striking variation with respect to epilepsy phenotypes both within and among families,” the researchers said. “Of 34 affected nonproband family members, five (15%) had juvenile myoclonic epilepsy, 10 (29%) had myoclonic-tonic-clonic seizures, four (12%) had pyknoleptic petit mal seizures alone or with myoclonic-tonic-clonic seizures, four (12%) had febrile seizures alone or with absence seizures or myoclonias, and 11 (32%) were clinically asymptomatic but had polyspikes or focal spikes on EEG. These results strongly suggest that ICK is pleiotropic ... and that epistatic loci with different genes are present in affected family members and interact with ICK and contribute to pleiotropism and clinical heterogeneity.”
ICK “is expressed in all tissues,” said senior study author Antonio Delgado-Escueta, MD, Professor of Neurology at UCLA. The subtle brain dysplasia, or microdysgenesis, that occurs in patients with juvenile myoclonic epilepsy is “diagnosed mainly microscopically, and has neuronal cells that migrated from periventricular zones to the wrong places in wrong layers of the cortical gray matter and even the white matter of the brain,” he said. “The cells can also be abnormally large and bunch up as a thicker gray matter. On voxel-based brain MRI ... focal thickenings of these abnormally migrated cells can also be partly explained by decreased pruning of cells and circuits (apoptosis).”
The gene encoding for ICK is located close to EFHC1 on chromosome 6p12. EFHC1, which encodes for a calcium-binding protein, has been implicated in juvenile myoclonic epilepsy. Dr. Bailey and her colleagues examined whether several genes in close proximity to EFHC1 also influenced that risk.
An Epilepsy Database
The investigators drew data from the GENESS (Genetic Epilepsies Studies) consortium, which has study sites in the United States, Mexico, Honduras, Brazil, and Japan. The current study analyzed information from 334 families with genetic generalized epilepsies. Among these families, 310 patients had adolescent-onset myoclonic seizures and polyspike waves or had a diagnosis of juvenile myoclonic epilepsy.
The investigators first performed an exome-wide analysis of four affected members of a large family with genetic juvenile myoclonic epilepsy. They observed the same variants in all four patients, then ran the screen in all 37 family members. Next, they screened these candidate genes in all 334 of the GENESS families and calculated risk scores for juvenile myoclonic epilepsy.
A linkage analysis confirmed two candidate genes on chromosome 6p12.2. Further analyses pinpointed a single variant: K305T on the ICK gene. This trait was present in each of the 12 affected members and three unaffected members of the initial family examined. Of those affected, three had juvenile myoclonic epilepsy, two had myoclonic-tonic-clonic convulsions only, two had febrile convulsions plus childhood absence seizures or neonatal myoclonus, one had febrile convulsions only, and four had polyspikes on EEG and were clinically asymptomatic.
“These results genetically implicated K305T as an autosomal dominant, possibly disease-causing trait,” the authors noted.
ICK variants were also present in 24 of the 310 database patients who had juvenile myoclonic epilepsy (8%). Of these, nine belonged to families with other affected members. The researchers tested 24 ICK variants for pathogenicity and determined that 13 exerted significant juvenile myoclonic epilepsy risk, with odds ratios exceeding 5.0.
When the researchers looked for these variants in the Genome Aggregation Database (gnomAD), they found that 12 of the variants were present but extremely rare, and eight were absent. They also found an additional ICK variant in a Mexican patient who was in gnomAD. That variant was a benign polymorphism in Africans.
Dr. Bailey and her colleagues thus concluded that 21 ICK variants in 22 patients with juvenile myoclonic epilepsy accounted for 7% of the juvenile myoclonic epilepsy among the 310 cases examined.
Experiments in Mice
The team also conducted a series of in vitro and in vivo mouse experiments. They determined that ICK variants impaired the migration of neuronal progenitor cells and lowered their mitotic index. ICK transgenic mice during light sleep displayed muscle movements similar to human myoclonic seizures. These mice also displayed diffuse polyspike brain waves on EEG recordings.
“The data we obtained through the use of electroporated slices of mouse brain support the conclusion that pathogenic variants in ICK cause 7% of cases of juvenile myoclonic epilepsy by disrupting mitosis, neuroblast migration, and apoptosis,” they concluded.
The study was funded by private and public grants. Several authors are coholders of patents on EFHC1-based diagnostics and therapeutics that have been licensed to Athena Diagnostics.
—Michele G. Sullivan
Suggested Reading
Bailey JN, de Nijs L, Bai D, et al. Variant intestinal-cell kinase in juvenile myoclonic epilepsy. N Engl J Med. 2018; 378(11): 1018-1028.
Rare genetic variants that affect the maturation, migration, and death of neurons appear to be responsible for about 7% of cases of juvenile myoclonic epilepsy.
Variants of the intestinal-cell kinase gene (ICK) occurred in 12 members of a family affected by the disorder and were confirmed in 22 of 310 additional patients, Julia N. Bailey, PhD, of the University of California, Los Angeles (UCLA), and her colleagues reported in the March 15 issue of the New England Journal of Medicine.
But among these 34 patients, the variants manifested as different epileptic phenotypes, suggesting genetic pleiotropism, the investigators said.
Clinical Heterogeneity
“We report striking variation with respect to epilepsy phenotypes both within and among families,” the researchers said. “Of 34 affected nonproband family members, five (15%) had juvenile myoclonic epilepsy, 10 (29%) had myoclonic-tonic-clonic seizures, four (12%) had pyknoleptic petit mal seizures alone or with myoclonic-tonic-clonic seizures, four (12%) had febrile seizures alone or with absence seizures or myoclonias, and 11 (32%) were clinically asymptomatic but had polyspikes or focal spikes on EEG. These results strongly suggest that ICK is pleiotropic ... and that epistatic loci with different genes are present in affected family members and interact with ICK and contribute to pleiotropism and clinical heterogeneity.”
ICK “is expressed in all tissues,” said senior study author Antonio Delgado-Escueta, MD, Professor of Neurology at UCLA. The subtle brain dysplasia, or microdysgenesis, that occurs in patients with juvenile myoclonic epilepsy is “diagnosed mainly microscopically, and has neuronal cells that migrated from periventricular zones to the wrong places in wrong layers of the cortical gray matter and even the white matter of the brain,” he said. “The cells can also be abnormally large and bunch up as a thicker gray matter. On voxel-based brain MRI ... focal thickenings of these abnormally migrated cells can also be partly explained by decreased pruning of cells and circuits (apoptosis).”
The gene encoding for ICK is located close to EFHC1 on chromosome 6p12. EFHC1, which encodes for a calcium-binding protein, has been implicated in juvenile myoclonic epilepsy. Dr. Bailey and her colleagues examined whether several genes in close proximity to EFHC1 also influenced that risk.
An Epilepsy Database
The investigators drew data from the GENESS (Genetic Epilepsies Studies) consortium, which has study sites in the United States, Mexico, Honduras, Brazil, and Japan. The current study analyzed information from 334 families with genetic generalized epilepsies. Among these families, 310 patients had adolescent-onset myoclonic seizures and polyspike waves or had a diagnosis of juvenile myoclonic epilepsy.
The investigators first performed an exome-wide analysis of four affected members of a large family with genetic juvenile myoclonic epilepsy. They observed the same variants in all four patients, then ran the screen in all 37 family members. Next, they screened these candidate genes in all 334 of the GENESS families and calculated risk scores for juvenile myoclonic epilepsy.
A linkage analysis confirmed two candidate genes on chromosome 6p12.2. Further analyses pinpointed a single variant: K305T on the ICK gene. This trait was present in each of the 12 affected members and three unaffected members of the initial family examined. Of those affected, three had juvenile myoclonic epilepsy, two had myoclonic-tonic-clonic convulsions only, two had febrile convulsions plus childhood absence seizures or neonatal myoclonus, one had febrile convulsions only, and four had polyspikes on EEG and were clinically asymptomatic.
“These results genetically implicated K305T as an autosomal dominant, possibly disease-causing trait,” the authors noted.
ICK variants were also present in 24 of the 310 database patients who had juvenile myoclonic epilepsy (8%). Of these, nine belonged to families with other affected members. The researchers tested 24 ICK variants for pathogenicity and determined that 13 exerted significant juvenile myoclonic epilepsy risk, with odds ratios exceeding 5.0.
When the researchers looked for these variants in the Genome Aggregation Database (gnomAD), they found that 12 of the variants were present but extremely rare, and eight were absent. They also found an additional ICK variant in a Mexican patient who was in gnomAD. That variant was a benign polymorphism in Africans.
Dr. Bailey and her colleagues thus concluded that 21 ICK variants in 22 patients with juvenile myoclonic epilepsy accounted for 7% of the juvenile myoclonic epilepsy among the 310 cases examined.
Experiments in Mice
The team also conducted a series of in vitro and in vivo mouse experiments. They determined that ICK variants impaired the migration of neuronal progenitor cells and lowered their mitotic index. ICK transgenic mice during light sleep displayed muscle movements similar to human myoclonic seizures. These mice also displayed diffuse polyspike brain waves on EEG recordings.
“The data we obtained through the use of electroporated slices of mouse brain support the conclusion that pathogenic variants in ICK cause 7% of cases of juvenile myoclonic epilepsy by disrupting mitosis, neuroblast migration, and apoptosis,” they concluded.
The study was funded by private and public grants. Several authors are coholders of patents on EFHC1-based diagnostics and therapeutics that have been licensed to Athena Diagnostics.
—Michele G. Sullivan
Suggested Reading
Bailey JN, de Nijs L, Bai D, et al. Variant intestinal-cell kinase in juvenile myoclonic epilepsy. N Engl J Med. 2018; 378(11): 1018-1028.
Rare genetic variants that affect the maturation, migration, and death of neurons appear to be responsible for about 7% of cases of juvenile myoclonic epilepsy.
Variants of the intestinal-cell kinase gene (ICK) occurred in 12 members of a family affected by the disorder and were confirmed in 22 of 310 additional patients, Julia N. Bailey, PhD, of the University of California, Los Angeles (UCLA), and her colleagues reported in the March 15 issue of the New England Journal of Medicine.
But among these 34 patients, the variants manifested as different epileptic phenotypes, suggesting genetic pleiotropism, the investigators said.
Clinical Heterogeneity
“We report striking variation with respect to epilepsy phenotypes both within and among families,” the researchers said. “Of 34 affected nonproband family members, five (15%) had juvenile myoclonic epilepsy, 10 (29%) had myoclonic-tonic-clonic seizures, four (12%) had pyknoleptic petit mal seizures alone or with myoclonic-tonic-clonic seizures, four (12%) had febrile seizures alone or with absence seizures or myoclonias, and 11 (32%) were clinically asymptomatic but had polyspikes or focal spikes on EEG. These results strongly suggest that ICK is pleiotropic ... and that epistatic loci with different genes are present in affected family members and interact with ICK and contribute to pleiotropism and clinical heterogeneity.”
ICK “is expressed in all tissues,” said senior study author Antonio Delgado-Escueta, MD, Professor of Neurology at UCLA. The subtle brain dysplasia, or microdysgenesis, that occurs in patients with juvenile myoclonic epilepsy is “diagnosed mainly microscopically, and has neuronal cells that migrated from periventricular zones to the wrong places in wrong layers of the cortical gray matter and even the white matter of the brain,” he said. “The cells can also be abnormally large and bunch up as a thicker gray matter. On voxel-based brain MRI ... focal thickenings of these abnormally migrated cells can also be partly explained by decreased pruning of cells and circuits (apoptosis).”
The gene encoding for ICK is located close to EFHC1 on chromosome 6p12. EFHC1, which encodes for a calcium-binding protein, has been implicated in juvenile myoclonic epilepsy. Dr. Bailey and her colleagues examined whether several genes in close proximity to EFHC1 also influenced that risk.
An Epilepsy Database
The investigators drew data from the GENESS (Genetic Epilepsies Studies) consortium, which has study sites in the United States, Mexico, Honduras, Brazil, and Japan. The current study analyzed information from 334 families with genetic generalized epilepsies. Among these families, 310 patients had adolescent-onset myoclonic seizures and polyspike waves or had a diagnosis of juvenile myoclonic epilepsy.
The investigators first performed an exome-wide analysis of four affected members of a large family with genetic juvenile myoclonic epilepsy. They observed the same variants in all four patients, then ran the screen in all 37 family members. Next, they screened these candidate genes in all 334 of the GENESS families and calculated risk scores for juvenile myoclonic epilepsy.
A linkage analysis confirmed two candidate genes on chromosome 6p12.2. Further analyses pinpointed a single variant: K305T on the ICK gene. This trait was present in each of the 12 affected members and three unaffected members of the initial family examined. Of those affected, three had juvenile myoclonic epilepsy, two had myoclonic-tonic-clonic convulsions only, two had febrile convulsions plus childhood absence seizures or neonatal myoclonus, one had febrile convulsions only, and four had polyspikes on EEG and were clinically asymptomatic.
“These results genetically implicated K305T as an autosomal dominant, possibly disease-causing trait,” the authors noted.
ICK variants were also present in 24 of the 310 database patients who had juvenile myoclonic epilepsy (8%). Of these, nine belonged to families with other affected members. The researchers tested 24 ICK variants for pathogenicity and determined that 13 exerted significant juvenile myoclonic epilepsy risk, with odds ratios exceeding 5.0.
When the researchers looked for these variants in the Genome Aggregation Database (gnomAD), they found that 12 of the variants were present but extremely rare, and eight were absent. They also found an additional ICK variant in a Mexican patient who was in gnomAD. That variant was a benign polymorphism in Africans.
Dr. Bailey and her colleagues thus concluded that 21 ICK variants in 22 patients with juvenile myoclonic epilepsy accounted for 7% of the juvenile myoclonic epilepsy among the 310 cases examined.
Experiments in Mice
The team also conducted a series of in vitro and in vivo mouse experiments. They determined that ICK variants impaired the migration of neuronal progenitor cells and lowered their mitotic index. ICK transgenic mice during light sleep displayed muscle movements similar to human myoclonic seizures. These mice also displayed diffuse polyspike brain waves on EEG recordings.
“The data we obtained through the use of electroporated slices of mouse brain support the conclusion that pathogenic variants in ICK cause 7% of cases of juvenile myoclonic epilepsy by disrupting mitosis, neuroblast migration, and apoptosis,” they concluded.
The study was funded by private and public grants. Several authors are coholders of patents on EFHC1-based diagnostics and therapeutics that have been licensed to Athena Diagnostics.
—Michele G. Sullivan
Suggested Reading
Bailey JN, de Nijs L, Bai D, et al. Variant intestinal-cell kinase in juvenile myoclonic epilepsy. N Engl J Med. 2018; 378(11): 1018-1028.