User login
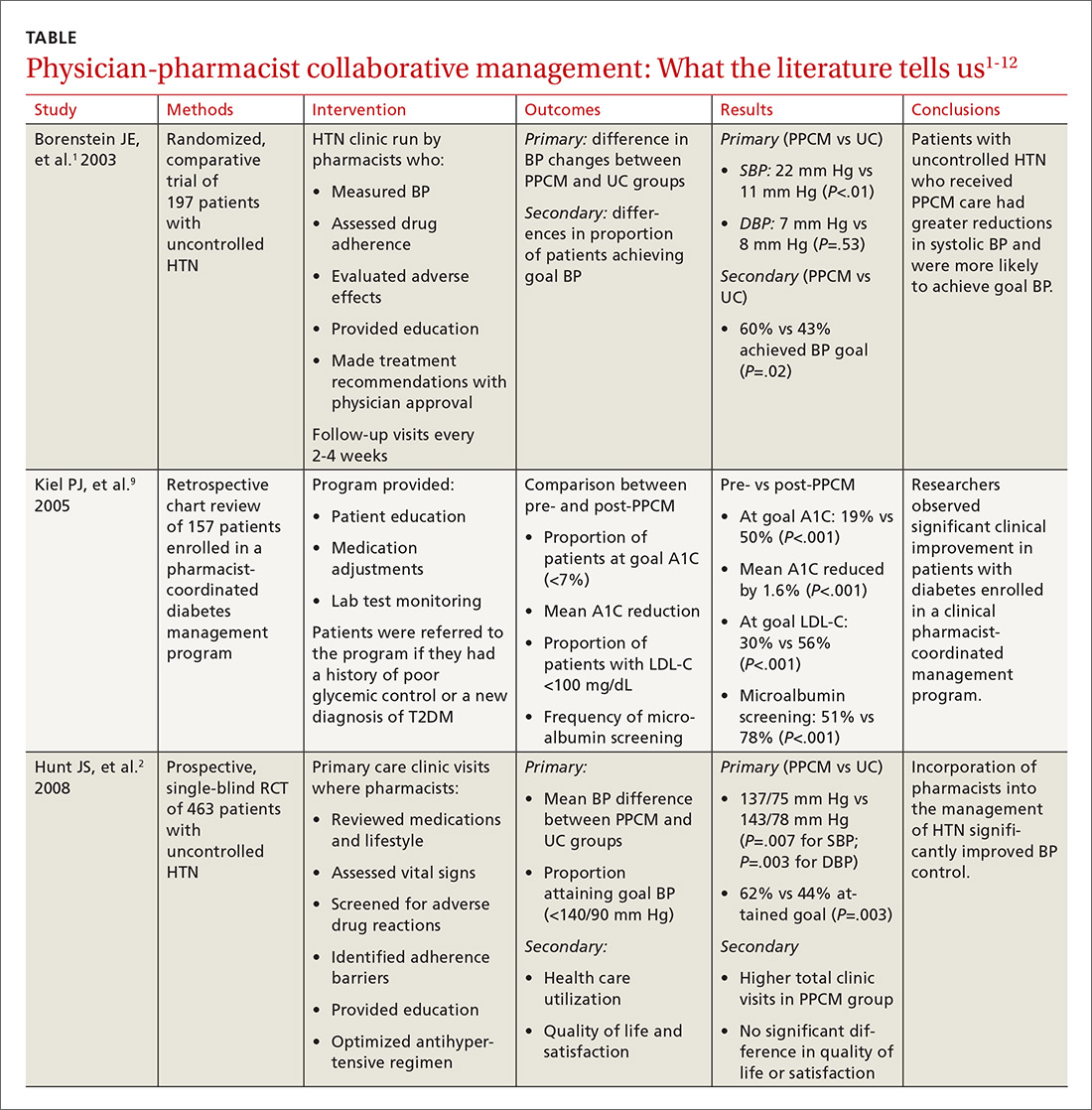
Does azithromycin have a role in cesarean sections?
ILLUSTRATIVE CASE
A 26-year-old G1P0 at 40w1d presents in spontaneous labor and is dilated to 4 cm. The patient reached complete cervical dilation after artificial rupture of membranes and oxytocin augmentation. After 4 hours of pushing, there has been minimal descent of the fetal vertex beyond +1 station with significant caput succedaneum. Her physician decides to proceed with cesarean delivery.2,3 What antibiotics should be administered prior to incision to reduce postoperative infection?
The Centers for Disease Control and Prevention (CDC) reports that nearly 1.3 million cesarean deliveries were performed in the United States in 2015, which represents about a third of all births.4 C-section is the most common major surgical procedure performed in this country and is associated with an infection rate 5 to 10 times that of vaginal delivery.5,6 Pregnancy-associated infection, particularly during delivery, is a significant risk and the fourth most common cause of maternal death in the United States.5
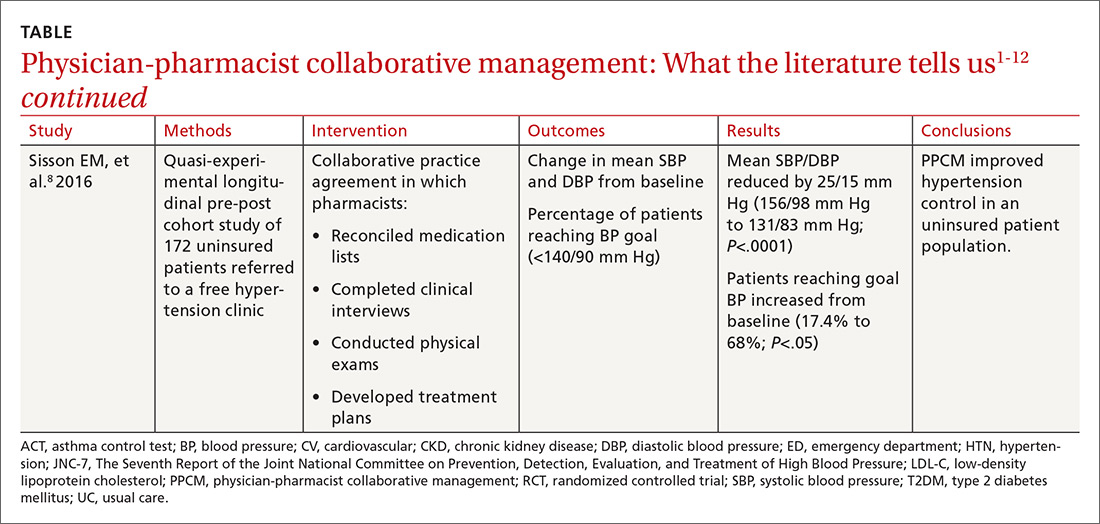
The current standard of care in cesarean delivery is antibiotic prophylaxis (often a first-generation cephalosporin) prior to skin incision.7 The majority of c-sections performed are nonelective, and of these, postoperative infections occur in 12% of women who receive standard prophylaxis.8,9 A small, single-center design trial suggested azithromycin adjunctive therapy expands antibiotic coverage to Ureaplasma species, resulting in a lower risk of postoperative infection.10
This study evaluated the use of azithromycin adjunctive therapy, in addition to standard antibiotic prophylaxis, to reduce the risk of postoperative infections in women receiving nonelective c-sections.
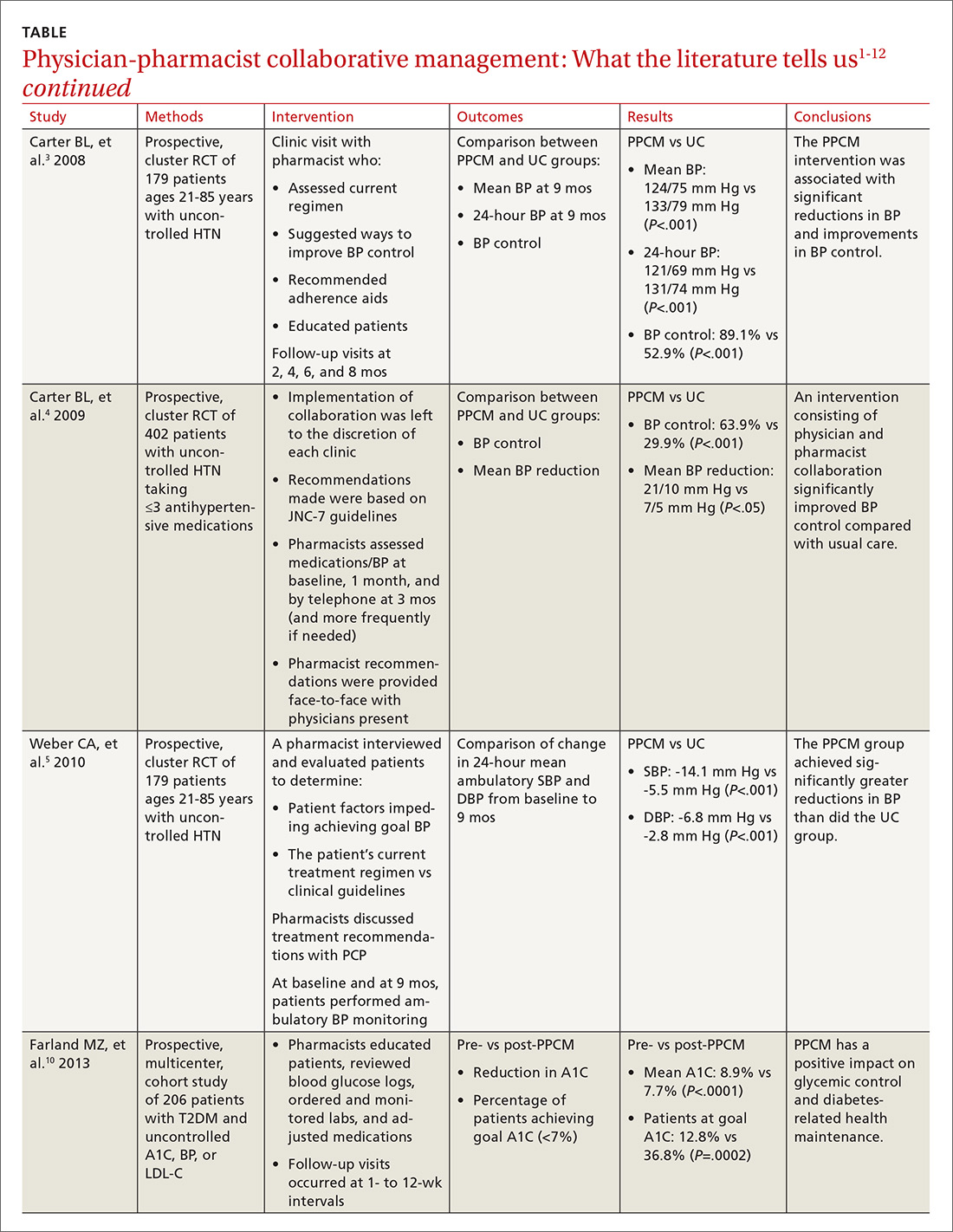
STUDY SUMMARY
Azithromycin reduced maternal infections up to 6 weeks post–c-section
A multicenter, randomized double-blind trial conducted in 14 hospitals in the United States evaluated the effect of a one-time dose of 500 mg intravenous (IV) azithromycin on post-cesarean infections. Women with a singleton pregnancy of at least 24 weeks’ gestation were eligible for inclusion if they required nonelective cesarean delivery during labor or at least 4 hours after membrane rupture. Patients were excluded if they had a known azithromycin allergy, subsequent vaginal delivery, azithromycin use within the week prior to randomization, extensive hepatic or renal dysfunction, a known history of prolonged QT interval, or substantial electrolyte abnormalities. Patients were eligible even if they were receiving other antibiotics for a positive group B Streptococcus screening.1
All patients (N=2013) were treated with standard antibiotic prophylaxis, most often cefazolin, according to individual institution protocols. The women were randomized to receive either an azithromycin 500 mg/250 mL IV infusion (n=1019) or an identical placebo IV infusion (n=994) within one hour of the procedure. The primary outcome was a composite endpoint of endometritis, wound infection, or other infections occurring up to 6 weeks after the c-section. Secondary outcomes included neonatal death, sepsis, and other neonatal and maternal complications.1
Patients in the placebo group had a higher rate of smoking during pregnancy; the researchers found no other significant differences.1
Results. The primary composite outcome occurred less frequently in the azithromycin group than in the placebo group (6.1% vs 12.1%; relative risk [RR]=0.51; 95% confidence interval [CI], 0.38-0.68; number needed to treat [NNT]=17). When the researchers looked at the individual elements of the primary composite outcome, 2 had significant reductions vs placebo.
Endometritis (3.8% vs 6.1%; RR=0.62; 95% CI, 0.42-0.92; NNT=44) and wound infections (2.4% vs 6.6%; RR=0.35; 95% CI, 0.22-0.56; NNT=24) occurred significantly less frequently, but there was no difference for other infections (0.3% vs 0.6%; RR=0.49; 95% CI, 0.12-1.94). Serious maternal adverse events were also lower with treatment than in the control group (1.5% vs 2.9%; RR=0.5; 95% CI, 0.27-0.94; NNT=71). There was no difference in composite secondary neonatal outcomes including death and serious complications (14.3% vs 13.6%; RR=1.05; 95% CI, 0.85-1.31).1
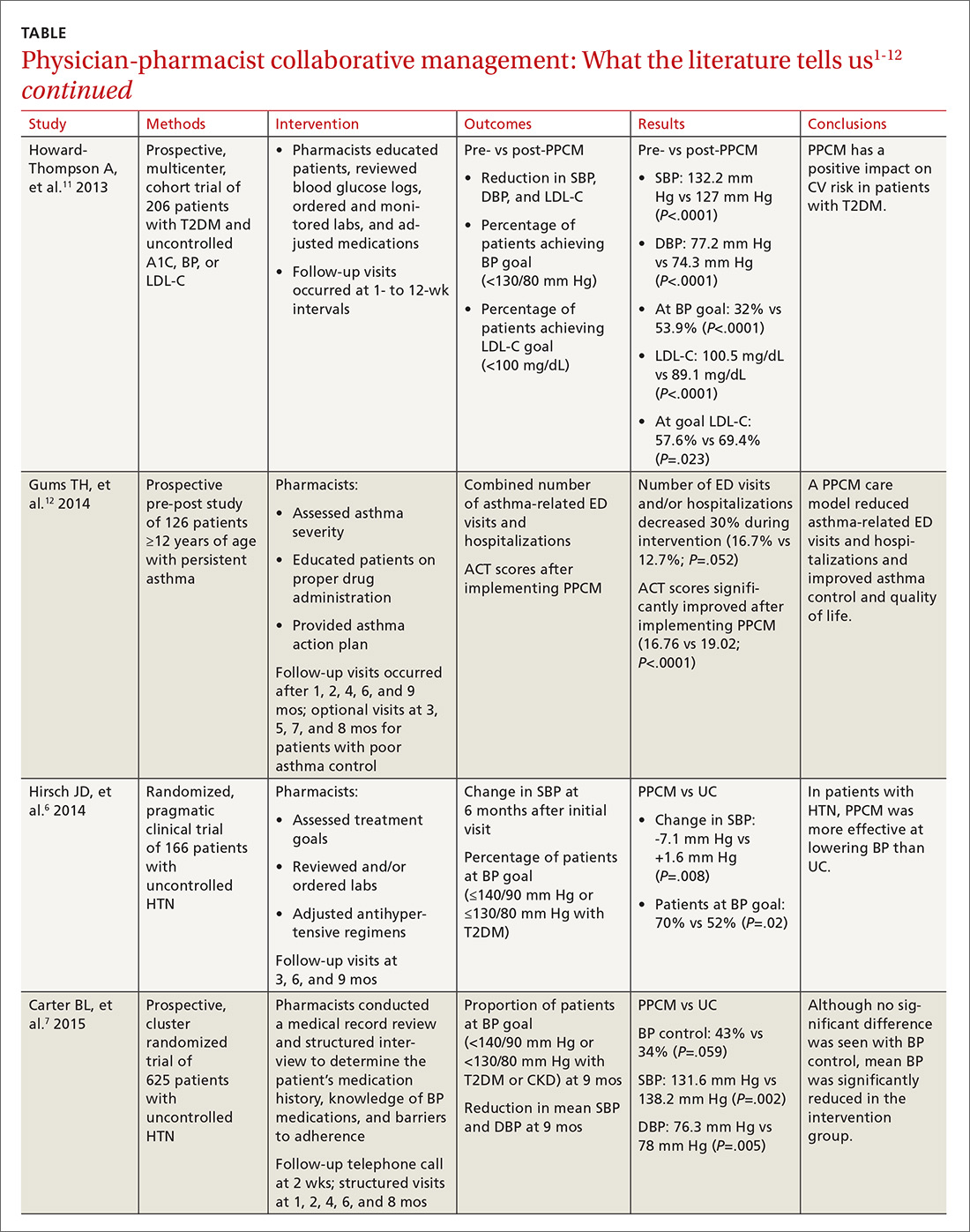
WHAT’S NEW
Azithromycin reduces infections without increasing adverse events
This study showed that adding azithromycin to standard antibiotic prophylaxis within one hour of a c-section reduces post-cesarean delivery infection rates without increasing the risk of maternal or neonatal adverse events.
CAVEATS
Proceed with caution in those with prolonged QT intervals
While azithromycin was efficacious and well tolerated in the study, not every patient can take it. Patients with a previous drug reaction or allergy should avoid it, and experts advise prescribing it with caution for patients who have (or are at increased risk for) a prolonged QT interval, including those on other QT-prolonging medications.
Of note, women with scheduled c-sections and those with chorioamnionitis or another infection requiring postpartum antibiotics were excluded from this study. Thus, it is unknown if azithromycin use decreases complications in these patients.
CHALLENGES TO IMPLEMENTATION
Speed of procedure is often paramount, so drug availability is key
Nonelective c-sections occur based on many factors that include a non-reassuring fetal heart rate. In many of these cases, speed of cesarean delivery may mean the difference between positive and negative outcomes. Availability of azithromycin on labor and delivery floors for timely administration within one hour of the procedure is important.
Additionally, azithromycin has known QT prolongation risks.11 While the baseline QT interval is not known for many healthy, young women, this should be considered when azithromycin is utilized in combination with other medications that may prolong the QT interval.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
2. Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:693-711.
3. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357. e1-e7.
4. National Vital Statistics Reports. Centers for Disease Control and Prevention: Births, Mode of Delivery. Available at: https://www.cdc.gov/nchs/fastats/delivery.htm. Updated January 5, 2017. Accessed August 4, 2017.
5. Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196-203.
6. DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:1-209.
7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472-1483.
8. Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005;192:1864-1868.
9. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199:301. e1-e6.
10. Andrews WW, Hauth JC, Cliver SP, et al. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183-1189.
11. Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551.
ILLUSTRATIVE CASE
A 26-year-old G1P0 at 40w1d presents in spontaneous labor and is dilated to 4 cm. The patient reached complete cervical dilation after artificial rupture of membranes and oxytocin augmentation. After 4 hours of pushing, there has been minimal descent of the fetal vertex beyond +1 station with significant caput succedaneum. Her physician decides to proceed with cesarean delivery.2,3 What antibiotics should be administered prior to incision to reduce postoperative infection?
The Centers for Disease Control and Prevention (CDC) reports that nearly 1.3 million cesarean deliveries were performed in the United States in 2015, which represents about a third of all births.4 C-section is the most common major surgical procedure performed in this country and is associated with an infection rate 5 to 10 times that of vaginal delivery.5,6 Pregnancy-associated infection, particularly during delivery, is a significant risk and the fourth most common cause of maternal death in the United States.5
The current standard of care in cesarean delivery is antibiotic prophylaxis (often a first-generation cephalosporin) prior to skin incision.7 The majority of c-sections performed are nonelective, and of these, postoperative infections occur in 12% of women who receive standard prophylaxis.8,9 A small, single-center design trial suggested azithromycin adjunctive therapy expands antibiotic coverage to Ureaplasma species, resulting in a lower risk of postoperative infection.10
This study evaluated the use of azithromycin adjunctive therapy, in addition to standard antibiotic prophylaxis, to reduce the risk of postoperative infections in women receiving nonelective c-sections.
STUDY SUMMARY
Azithromycin reduced maternal infections up to 6 weeks post–c-section
A multicenter, randomized double-blind trial conducted in 14 hospitals in the United States evaluated the effect of a one-time dose of 500 mg intravenous (IV) azithromycin on post-cesarean infections. Women with a singleton pregnancy of at least 24 weeks’ gestation were eligible for inclusion if they required nonelective cesarean delivery during labor or at least 4 hours after membrane rupture. Patients were excluded if they had a known azithromycin allergy, subsequent vaginal delivery, azithromycin use within the week prior to randomization, extensive hepatic or renal dysfunction, a known history of prolonged QT interval, or substantial electrolyte abnormalities. Patients were eligible even if they were receiving other antibiotics for a positive group B Streptococcus screening.1
All patients (N=2013) were treated with standard antibiotic prophylaxis, most often cefazolin, according to individual institution protocols. The women were randomized to receive either an azithromycin 500 mg/250 mL IV infusion (n=1019) or an identical placebo IV infusion (n=994) within one hour of the procedure. The primary outcome was a composite endpoint of endometritis, wound infection, or other infections occurring up to 6 weeks after the c-section. Secondary outcomes included neonatal death, sepsis, and other neonatal and maternal complications.1
Patients in the placebo group had a higher rate of smoking during pregnancy; the researchers found no other significant differences.1
Results. The primary composite outcome occurred less frequently in the azithromycin group than in the placebo group (6.1% vs 12.1%; relative risk [RR]=0.51; 95% confidence interval [CI], 0.38-0.68; number needed to treat [NNT]=17). When the researchers looked at the individual elements of the primary composite outcome, 2 had significant reductions vs placebo.
Endometritis (3.8% vs 6.1%; RR=0.62; 95% CI, 0.42-0.92; NNT=44) and wound infections (2.4% vs 6.6%; RR=0.35; 95% CI, 0.22-0.56; NNT=24) occurred significantly less frequently, but there was no difference for other infections (0.3% vs 0.6%; RR=0.49; 95% CI, 0.12-1.94). Serious maternal adverse events were also lower with treatment than in the control group (1.5% vs 2.9%; RR=0.5; 95% CI, 0.27-0.94; NNT=71). There was no difference in composite secondary neonatal outcomes including death and serious complications (14.3% vs 13.6%; RR=1.05; 95% CI, 0.85-1.31).1
WHAT’S NEW
Azithromycin reduces infections without increasing adverse events
This study showed that adding azithromycin to standard antibiotic prophylaxis within one hour of a c-section reduces post-cesarean delivery infection rates without increasing the risk of maternal or neonatal adverse events.
CAVEATS
Proceed with caution in those with prolonged QT intervals
While azithromycin was efficacious and well tolerated in the study, not every patient can take it. Patients with a previous drug reaction or allergy should avoid it, and experts advise prescribing it with caution for patients who have (or are at increased risk for) a prolonged QT interval, including those on other QT-prolonging medications.
Of note, women with scheduled c-sections and those with chorioamnionitis or another infection requiring postpartum antibiotics were excluded from this study. Thus, it is unknown if azithromycin use decreases complications in these patients.
CHALLENGES TO IMPLEMENTATION
Speed of procedure is often paramount, so drug availability is key
Nonelective c-sections occur based on many factors that include a non-reassuring fetal heart rate. In many of these cases, speed of cesarean delivery may mean the difference between positive and negative outcomes. Availability of azithromycin on labor and delivery floors for timely administration within one hour of the procedure is important.
Additionally, azithromycin has known QT prolongation risks.11 While the baseline QT interval is not known for many healthy, young women, this should be considered when azithromycin is utilized in combination with other medications that may prolong the QT interval.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 26-year-old G1P0 at 40w1d presents in spontaneous labor and is dilated to 4 cm. The patient reached complete cervical dilation after artificial rupture of membranes and oxytocin augmentation. After 4 hours of pushing, there has been minimal descent of the fetal vertex beyond +1 station with significant caput succedaneum. Her physician decides to proceed with cesarean delivery.2,3 What antibiotics should be administered prior to incision to reduce postoperative infection?
The Centers for Disease Control and Prevention (CDC) reports that nearly 1.3 million cesarean deliveries were performed in the United States in 2015, which represents about a third of all births.4 C-section is the most common major surgical procedure performed in this country and is associated with an infection rate 5 to 10 times that of vaginal delivery.5,6 Pregnancy-associated infection, particularly during delivery, is a significant risk and the fourth most common cause of maternal death in the United States.5
The current standard of care in cesarean delivery is antibiotic prophylaxis (often a first-generation cephalosporin) prior to skin incision.7 The majority of c-sections performed are nonelective, and of these, postoperative infections occur in 12% of women who receive standard prophylaxis.8,9 A small, single-center design trial suggested azithromycin adjunctive therapy expands antibiotic coverage to Ureaplasma species, resulting in a lower risk of postoperative infection.10
This study evaluated the use of azithromycin adjunctive therapy, in addition to standard antibiotic prophylaxis, to reduce the risk of postoperative infections in women receiving nonelective c-sections.
STUDY SUMMARY
Azithromycin reduced maternal infections up to 6 weeks post–c-section
A multicenter, randomized double-blind trial conducted in 14 hospitals in the United States evaluated the effect of a one-time dose of 500 mg intravenous (IV) azithromycin on post-cesarean infections. Women with a singleton pregnancy of at least 24 weeks’ gestation were eligible for inclusion if they required nonelective cesarean delivery during labor or at least 4 hours after membrane rupture. Patients were excluded if they had a known azithromycin allergy, subsequent vaginal delivery, azithromycin use within the week prior to randomization, extensive hepatic or renal dysfunction, a known history of prolonged QT interval, or substantial electrolyte abnormalities. Patients were eligible even if they were receiving other antibiotics for a positive group B Streptococcus screening.1
All patients (N=2013) were treated with standard antibiotic prophylaxis, most often cefazolin, according to individual institution protocols. The women were randomized to receive either an azithromycin 500 mg/250 mL IV infusion (n=1019) or an identical placebo IV infusion (n=994) within one hour of the procedure. The primary outcome was a composite endpoint of endometritis, wound infection, or other infections occurring up to 6 weeks after the c-section. Secondary outcomes included neonatal death, sepsis, and other neonatal and maternal complications.1
Patients in the placebo group had a higher rate of smoking during pregnancy; the researchers found no other significant differences.1
Results. The primary composite outcome occurred less frequently in the azithromycin group than in the placebo group (6.1% vs 12.1%; relative risk [RR]=0.51; 95% confidence interval [CI], 0.38-0.68; number needed to treat [NNT]=17). When the researchers looked at the individual elements of the primary composite outcome, 2 had significant reductions vs placebo.
Endometritis (3.8% vs 6.1%; RR=0.62; 95% CI, 0.42-0.92; NNT=44) and wound infections (2.4% vs 6.6%; RR=0.35; 95% CI, 0.22-0.56; NNT=24) occurred significantly less frequently, but there was no difference for other infections (0.3% vs 0.6%; RR=0.49; 95% CI, 0.12-1.94). Serious maternal adverse events were also lower with treatment than in the control group (1.5% vs 2.9%; RR=0.5; 95% CI, 0.27-0.94; NNT=71). There was no difference in composite secondary neonatal outcomes including death and serious complications (14.3% vs 13.6%; RR=1.05; 95% CI, 0.85-1.31).1
WHAT’S NEW
Azithromycin reduces infections without increasing adverse events
This study showed that adding azithromycin to standard antibiotic prophylaxis within one hour of a c-section reduces post-cesarean delivery infection rates without increasing the risk of maternal or neonatal adverse events.
CAVEATS
Proceed with caution in those with prolonged QT intervals
While azithromycin was efficacious and well tolerated in the study, not every patient can take it. Patients with a previous drug reaction or allergy should avoid it, and experts advise prescribing it with caution for patients who have (or are at increased risk for) a prolonged QT interval, including those on other QT-prolonging medications.
Of note, women with scheduled c-sections and those with chorioamnionitis or another infection requiring postpartum antibiotics were excluded from this study. Thus, it is unknown if azithromycin use decreases complications in these patients.
CHALLENGES TO IMPLEMENTATION
Speed of procedure is often paramount, so drug availability is key
Nonelective c-sections occur based on many factors that include a non-reassuring fetal heart rate. In many of these cases, speed of cesarean delivery may mean the difference between positive and negative outcomes. Availability of azithromycin on labor and delivery floors for timely administration within one hour of the procedure is important.
Additionally, azithromycin has known QT prolongation risks.11 While the baseline QT interval is not known for many healthy, young women, this should be considered when azithromycin is utilized in combination with other medications that may prolong the QT interval.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
2. Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:693-711.
3. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357. e1-e7.
4. National Vital Statistics Reports. Centers for Disease Control and Prevention: Births, Mode of Delivery. Available at: https://www.cdc.gov/nchs/fastats/delivery.htm. Updated January 5, 2017. Accessed August 4, 2017.
5. Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196-203.
6. DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:1-209.
7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472-1483.
8. Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005;192:1864-1868.
9. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199:301. e1-e6.
10. Andrews WW, Hauth JC, Cliver SP, et al. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183-1189.
11. Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551.
1. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
2. Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2014;123:693-711.
3. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357. e1-e7.
4. National Vital Statistics Reports. Centers for Disease Control and Prevention: Births, Mode of Delivery. Available at: https://www.cdc.gov/nchs/fastats/delivery.htm. Updated January 5, 2017. Accessed August 4, 2017.
5. Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196-203.
6. DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:1-209.
7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472-1483.
8. Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005;192:1864-1868.
9. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199:301. e1-e6.
10. Andrews WW, Hauth JC, Cliver SP, et al. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183-1189.
11. Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe a one-time dose of azithromycin 500 mg intravenously, along with standard antibiotic prophylaxis, at the time of cesarean delivery to prevent postoperative infections.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality, randomized controlled trial.
Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
Erythematous, friable nipple with loss of protrusion • history of breastfeeding • Dx?
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.
THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).
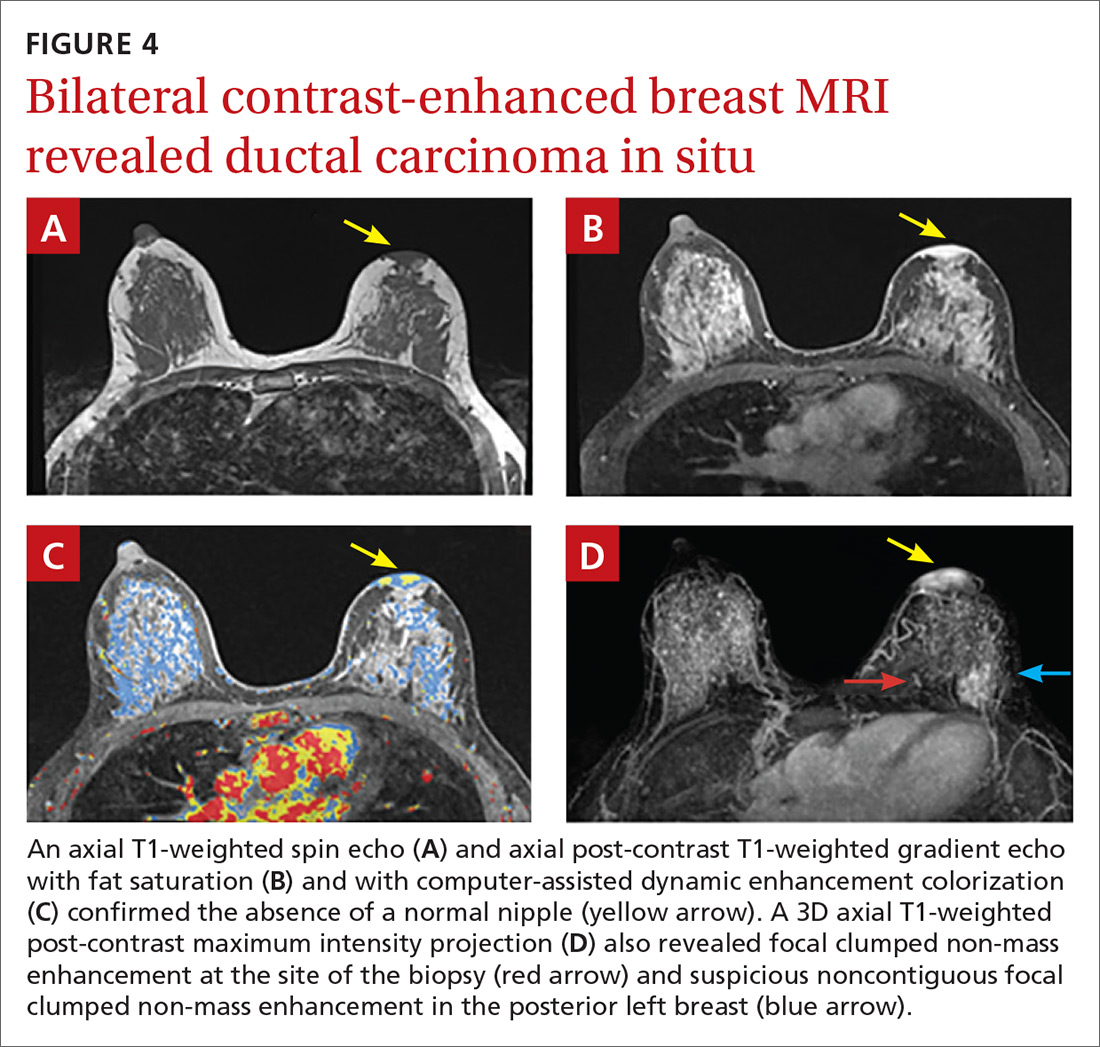
Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.
DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.
THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).
Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.
DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
THE CASE
A 34-year-old healthy woman presented to the breast surgical oncology clinic with skin changes to her left nipple after being referred by her primary care provider. She attributed the skin changes to shearing from breastfeeding her third child 5 years earlier. Physical examination revealed an erythematous and friable nipple with loss of protrusion (FIGURE 1). The patient reported routine bleeding from her nipple, but said the skin changes had remained stable and denied any breast masses. The patient’s last mammogram was 2.5 years earlier and had only been remarkable for bilateral benign calcifications.
THE DIAGNOSIS
A screening mammogram showed flattening and retraction of the left nipple, as well as suspicious left breast calcifications (BIRADS [Breast Imaging Reporting and Data System] 4 classification, FIGURE 2). A subsequent diagnostic mammogram showed a cluster of fine pleomorphic calcifications in the upper inner quadrant of the left breast (FIGURE 3). A stereotactic core needle biopsy was performed, and results confirmed a diagnosis of high-grade, estrogen receptor-negative, ductal carcinoma in situ (DCIS).
Subsequent work-up included a staging magnetic resonance imaging (MRI) and a left areola punch biopsy. MRI revealed an absence of a normal left nipple and extensive focal clumped non-mass enhancement in the area of the known DCIS (FIGURE 4). Biopsy results revealed enlarged atypical single cells within the epidermis. The cells stained positive for mucicarmine and cytokeratin 7 and negative for carcinoembryonic antigen and S-100 protein. This ruled out a pagetoid spread of melanoma and confirmed a diagnosis of Paget’s disease (PD) of the breast.
DISCUSSION
PD of the breast is a rare disorder (accounting for 0.5%-5% of all breast cancers) that is clinically characterized by erythematous, eczematous changes of the nipple-areolar complex (NAC).1-7 PD is almost always unilateral and symptoms include pain, burning, and itching of the nipple, often with bloody nipple discharge.1,3-8
PD can be mistaken for benign skin changes and diagnosed as dermatitis or eczema.3,5 Because such changes often resolve temporarily with the use of topical corticosteroids or no treatment at all,2 diagnosis is often delayed. PD of the breast is associated with underlying ductal carcinoma in 90% to 100% of cases,1,2,5,8 so any skin pathology involving the nipple should be assumed to be PD until proven otherwise.
When no palpable mass is noted on physical exam, DCIS is usually found centrally behind the nipple.1 In addition, lymph node involvement is noted in about 60% of cases.1
Confirm the diagnosis with these tests
Diagnosis of PD of the breast is primarily clinical, with pathologic confirmation. All patients with clinically suspected PD should be evaluated using the following tests to determine the need for biopsy.
Mammography with magnification views of the NAC will show thickening, retraction, or flattening of the nipple, microcalcifications of the retroareolar region, and/or a subareolar mass.3 However, because breast tissue appears normal on mammography in 22% to 71% of patients,1,5 the use of ultrasound and potentially MRI to delineate the extent of disease is warranted.
Ultrasound. While there are no characteristic findings on ultrasound, it can be used to detect dilation of the subareolar ducts, calcification, or a mass.4
MRI has a higher sensitivity for detection of occult disease.2,5 MRI is also useful in the evaluation of axillary node asymmetry, which may indicate nodal involvement.2
Treatment is variable and has not been widely studied
Due to the rarity of PD, there are no randomized studies to point toward optimal treatment strategies.7 Treatment for PD is typically surgical and often involves mastectomy, with or without axillary node dissection.1 Retrospective analyses have demonstrated that central lumpectomy (complete resection of the NAC and underlying disease) with radiation therapy has outcomes similar to mastectomy;2 however, the cosmetic result is sometimes unfavorable.
In cases where there is no palpable mass nor mammographic findings of disease, breast conserving therapy may be considered. If chemotherapy is considered, it should be chosen based on the receptor profile of the disease and subsequent oncotype scoring.
The prognosis for patients with PD who are adequately treated and remain disease free after 5 years is excellent. These patients are likely to have achieved cure.2
Our patient underwent left simple mastectomy with sentinel node biopsy and tissue expander placement. Her postoperative course was uncomplicated, and she was discharged home on postoperative Day 1. On final pathology, the 2 sentinel nodes were disease free. The left mastectomy specimen was found to have high-grade DCIS with clear surgical margins. The area of involvement was found to be 3.5 cm × 3 cm in size and had clear skin margins. At follow-up one year later, the patient was doing well with no evidence of disease. She subsequently underwent implant insertion.
THE TAKEAWAY
This case highlights the unique progression of undiagnosed PD of the breast. It also highlights the importance of ruling out PD when skin changes involving the nipple are present, despite other possible explanations for those changes. This case in particular was complicated by a proximal history of breastfeeding, which erroneously provided an explanation and false reassurance for the primary care provider and patient.
Due to the common association of PD of the breast with underlying DCIS or invasive cancer, the most important aspect of care is early diagnostic work-up and appropriate referral. Primary care physicians have a unique role in obtaining appropriate early diagnostic tests (including mammogram and ultrasound) and making the necessary referral to a breast specialist in the presence of an abnormal physical exam involving the NAC, even in the absence of a palpable mass. In our patient’s case, punch biopsy of the NAC would have been appropriate at the first signs of friable, erythematous changes.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
1. Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg. 1998;187:171-177.
2. Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
3. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141:1-12.
4. Soler T, Lerin A, Serrano T, et al. Pigmented paget disease of the breast nipple with underlying infiltrating carcinoma: a case report and review of the literature. Am J Dermatopathol. 2011;33:e54-e57.
5. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2:137-142.
6. Sakorafas GH, Blanchard DK, Sarr MG, et al. Paget’s disease of the breast: a clinical perspective. Langenbecks Arch Surg. 2001;386;444-450.
7. Durkan B, Bresee C, Bose S, et al. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79:1009-1012.
8. Ward KA, Burton JL. Dermatologic diseases of the breast in young women. Clin Dermatol. 1997;15:45-52.
Screening for tuberculosis: Updated recommendations
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
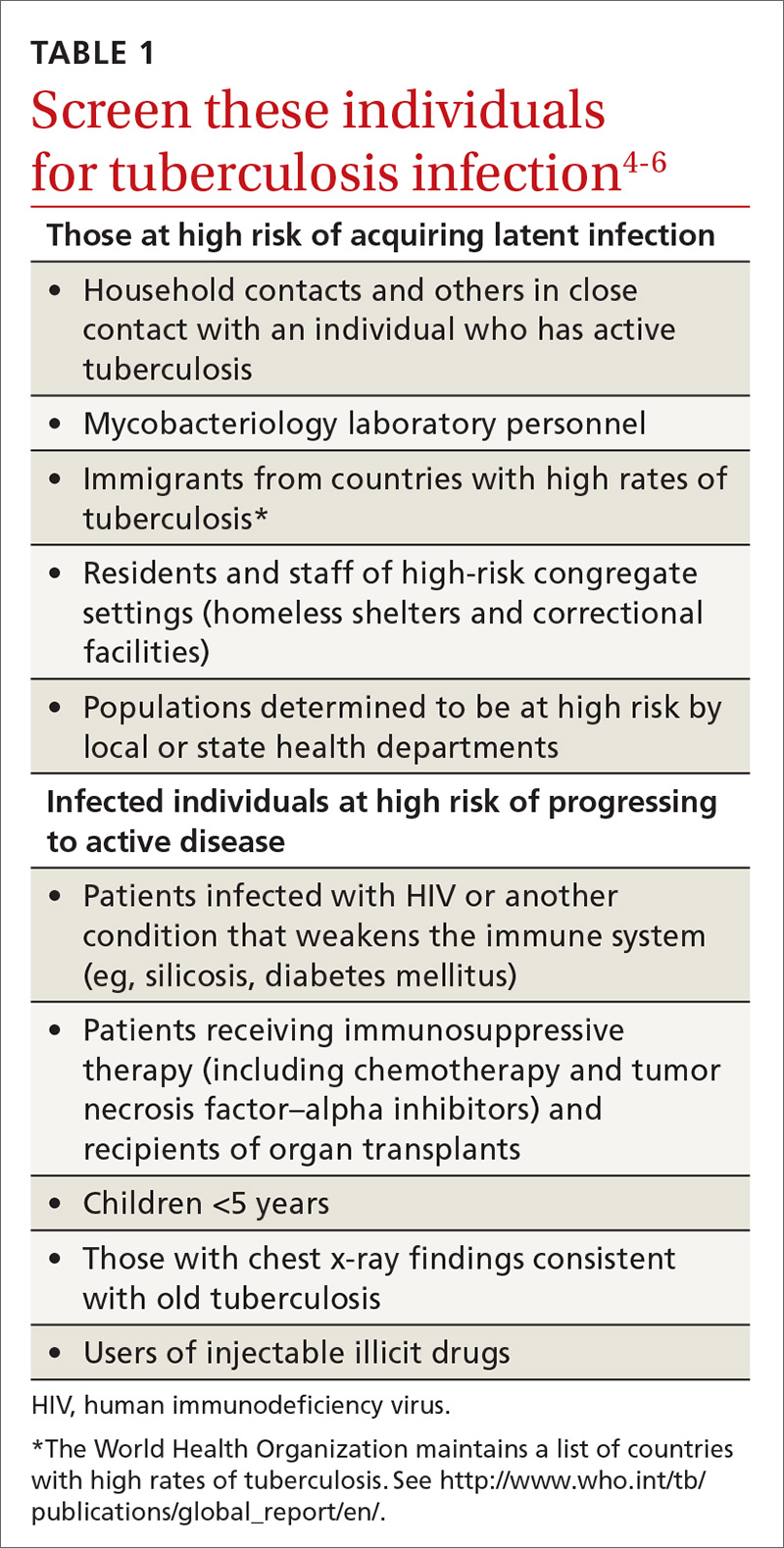
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
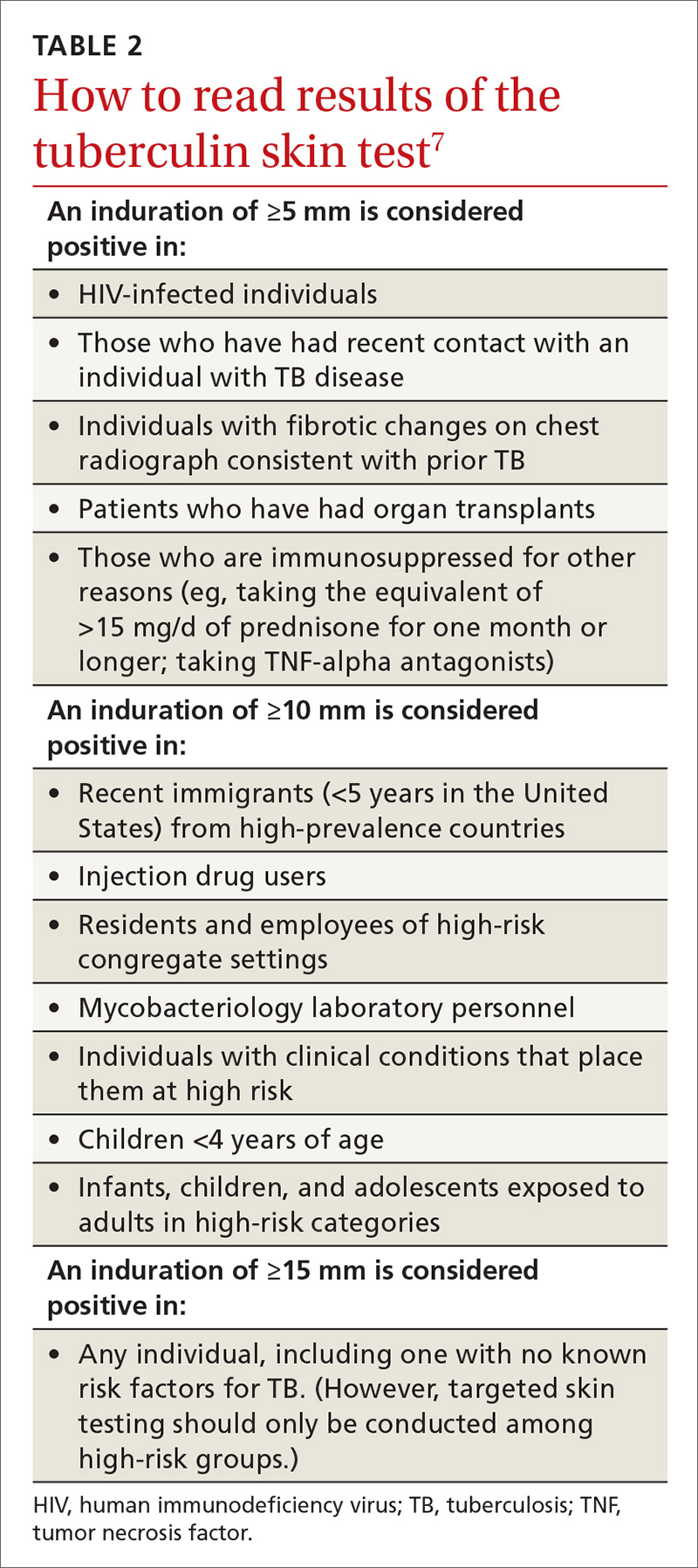
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
From The Journal of Family Practice | 2017;66(12):755-757.
Ensuring prompt recognition and treatment of panic disorder
THE CASE
Lorna D* was seen by her primary care physician (PCP) as follow-up to a visit she made to the emergency department (ED). The 37 year old had gone to the ED 4 times in the previous year. Each time she presented with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she was having a heart attack. In spite of negative work-ups at each visit (electrocardiogram, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Ms. D was terrified that the ED doctors were missing something. She was still “rattled” by the chest pain and shortness of breath she had experienced. Mild symptoms were persisting and she was worried that she would have a heart attack and die without the treatment she believed she needed.
How would you proceed with this patient?
*The patient’s name has been changed to protect her privacy.
MANY PANIC ATTACKS PROMPT AN ED VISIT
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks, and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least 4 of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5):1
- palpitations, pounding heart, or accelerated heart rate
- sweating
- trembling or shaking
- sensations of shortness of breath or smothering
- feeling of choking
- chest pain or discomfort
- nausea or abdominal distress
- feeling dizzy, unsteady, lightheaded, or faint
- de-realization (feelings of unreality) or depersonalization (being detached from oneself)
- fear of losing control or going crazy
- fear of dying
- paresthesia (numbness or tingling sensations)
- chills or hot flushes.
Lifetime incidence rates of panic disorder are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for panic disorder.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary physician.5 Patients with panic disorder tend to use health care resources at a disproportionately high rate.6
An international review of panic disorder research suggests the average age of onset for PD is 32 years.7 Triggers can vary widely and no single
Differential goes far beyond myocardial infarction
Many medical conditions can mimic panic disorder symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants such as cocaine, amphetamines); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with panic-like symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with panic-like symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and D-dimer assay to exclude pulmonary embolism).
If organic causes are ruled out, focus on a psychiatric assessment:9
- history of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- psychiatric history
- history of substance use
- family history of psychiatric disorders (especially anxiety disorders)
- social history (life events, including those preceding onset of panic; history of child abuse)
- medications
- mental status examination
- safety (panic disorder is associated with higher risk of suicidal ideation).
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (FIGURE9), although there is not enough evidence to recommend one vs the other or combination therapy vs monotherapy.9
CASE
For Ms. D, all medical test results came back negative, and the psychiatric assessment revealed that she met the DSM-5 criteria for panic disorder. Counting on the strength of their relationship, her physician talked to her about PD and discussed treatment options, which included counseling, medication, or both. Ms. D agreed to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her physician’s primary care clinic and to begin taking medication. Her PCP started her on sertraline 25 mg/d.
In CBT, Ms. D’s psychologist taught her about “fight or flight” and explained that it was a normal physiologic response that could lead to panic. Ms. D. learned to approach her physical symptoms in a different way, and how to breathe in a way that slowed her panic reaction.
Consider SSRIs and SNRIs
First-line medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors. MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although many clinicians start lower, at 5 mg/d), to avoid hypersensitivity reactions. Gradually titrate the dose upward within 3 to 7 days after initiation until a therapeutic dose is reached over 2 to 6 weeks. Schedule follow-up visits for every one to 2 weeks at the beginning of treatment and every 2 to 4 weeks until the therapeutic dose is reached. Assess safety/suicidality at each visit.
Keep in mind that the onset of therapeutic effect is between 2 and 4 weeks, but that clinical response can take up to 8 to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only 4 to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid)9 may be increased 3 to 5 days following initiation.
The evidence supports the use of CBT for panic disorder
CBT is an evidenced-based treatment for panic disorder.10-13 Up to 75% of patients treated with CBT are panic free within 4 months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psycho-education, exposure, and imagery.14
Treatment with medications and CBT either combined or used individually is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or 2 adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
CASE
Ten days after Ms. D started the sertraline 25 mg/d, she called the PCP to report daily diarrhea. She stopped the sertraline on her own and asked for another medication. She also expressed her frustration with the severity of the symptoms. She was having 3 to 5 panic attacks daily and had been missing many days from work.
On the day of her follow-up PCP appointment, Ms. D also saw the psychologist. She reported that she’d been practicing relaxation breathing, tracking her panic attacks, limiting caffeine intake, and exercising regularly. But the attacks were still occurring.
The PCP switched her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribed clonazepam 0.5 mg bid. Two weeks later, Ms. D reported that she was feeling a little better, had returned to work, and was hopeful that she would be her “normal self again.” The PCP planned to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and to slowly taper the clonazepam toward discontinuation in the near future.
CORRESPONDENCE
Eric H. Berko, PhD, Case Western Reserve University School of Medicine, Department of Family Medicine, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109-7878; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing: Arlington, VA; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33:1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. Practice guideline for the treatment of patients with panic disorder, 2nd ed. 2010. American Psychiatric Association; Washington DC. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed October 26, 2017.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Available at: https://www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed October 26, 2017.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993;61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE., Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595–606
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. Available at: https://www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed October 26, 2017.
THE CASE
Lorna D* was seen by her primary care physician (PCP) as follow-up to a visit she made to the emergency department (ED). The 37 year old had gone to the ED 4 times in the previous year. Each time she presented with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she was having a heart attack. In spite of negative work-ups at each visit (electrocardiogram, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Ms. D was terrified that the ED doctors were missing something. She was still “rattled” by the chest pain and shortness of breath she had experienced. Mild symptoms were persisting and she was worried that she would have a heart attack and die without the treatment she believed she needed.
How would you proceed with this patient?
*The patient’s name has been changed to protect her privacy.
MANY PANIC ATTACKS PROMPT AN ED VISIT
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks, and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least 4 of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5):1
- palpitations, pounding heart, or accelerated heart rate
- sweating
- trembling or shaking
- sensations of shortness of breath or smothering
- feeling of choking
- chest pain or discomfort
- nausea or abdominal distress
- feeling dizzy, unsteady, lightheaded, or faint
- de-realization (feelings of unreality) or depersonalization (being detached from oneself)
- fear of losing control or going crazy
- fear of dying
- paresthesia (numbness or tingling sensations)
- chills or hot flushes.
Lifetime incidence rates of panic disorder are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for panic disorder.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary physician.5 Patients with panic disorder tend to use health care resources at a disproportionately high rate.6
An international review of panic disorder research suggests the average age of onset for PD is 32 years.7 Triggers can vary widely and no single
Differential goes far beyond myocardial infarction
Many medical conditions can mimic panic disorder symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants such as cocaine, amphetamines); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with panic-like symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with panic-like symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and D-dimer assay to exclude pulmonary embolism).
If organic causes are ruled out, focus on a psychiatric assessment:9
- history of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- psychiatric history
- history of substance use
- family history of psychiatric disorders (especially anxiety disorders)
- social history (life events, including those preceding onset of panic; history of child abuse)
- medications
- mental status examination
- safety (panic disorder is associated with higher risk of suicidal ideation).
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (FIGURE9), although there is not enough evidence to recommend one vs the other or combination therapy vs monotherapy.9
CASE
For Ms. D, all medical test results came back negative, and the psychiatric assessment revealed that she met the DSM-5 criteria for panic disorder. Counting on the strength of their relationship, her physician talked to her about PD and discussed treatment options, which included counseling, medication, or both. Ms. D agreed to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her physician’s primary care clinic and to begin taking medication. Her PCP started her on sertraline 25 mg/d.
In CBT, Ms. D’s psychologist taught her about “fight or flight” and explained that it was a normal physiologic response that could lead to panic. Ms. D. learned to approach her physical symptoms in a different way, and how to breathe in a way that slowed her panic reaction.
Consider SSRIs and SNRIs
First-line medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors. MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although many clinicians start lower, at 5 mg/d), to avoid hypersensitivity reactions. Gradually titrate the dose upward within 3 to 7 days after initiation until a therapeutic dose is reached over 2 to 6 weeks. Schedule follow-up visits for every one to 2 weeks at the beginning of treatment and every 2 to 4 weeks until the therapeutic dose is reached. Assess safety/suicidality at each visit.
Keep in mind that the onset of therapeutic effect is between 2 and 4 weeks, but that clinical response can take up to 8 to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only 4 to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid)9 may be increased 3 to 5 days following initiation.
The evidence supports the use of CBT for panic disorder
CBT is an evidenced-based treatment for panic disorder.10-13 Up to 75% of patients treated with CBT are panic free within 4 months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psycho-education, exposure, and imagery.14
Treatment with medications and CBT either combined or used individually is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or 2 adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
CASE
Ten days after Ms. D started the sertraline 25 mg/d, she called the PCP to report daily diarrhea. She stopped the sertraline on her own and asked for another medication. She also expressed her frustration with the severity of the symptoms. She was having 3 to 5 panic attacks daily and had been missing many days from work.
On the day of her follow-up PCP appointment, Ms. D also saw the psychologist. She reported that she’d been practicing relaxation breathing, tracking her panic attacks, limiting caffeine intake, and exercising regularly. But the attacks were still occurring.
The PCP switched her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribed clonazepam 0.5 mg bid. Two weeks later, Ms. D reported that she was feeling a little better, had returned to work, and was hopeful that she would be her “normal self again.” The PCP planned to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and to slowly taper the clonazepam toward discontinuation in the near future.
CORRESPONDENCE
Eric H. Berko, PhD, Case Western Reserve University School of Medicine, Department of Family Medicine, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109-7878; [email protected].
THE CASE
Lorna D* was seen by her primary care physician (PCP) as follow-up to a visit she made to the emergency department (ED). The 37 year old had gone to the ED 4 times in the previous year. Each time she presented with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she was having a heart attack. In spite of negative work-ups at each visit (electrocardiogram, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Ms. D was terrified that the ED doctors were missing something. She was still “rattled” by the chest pain and shortness of breath she had experienced. Mild symptoms were persisting and she was worried that she would have a heart attack and die without the treatment she believed she needed.
How would you proceed with this patient?
*The patient’s name has been changed to protect her privacy.
MANY PANIC ATTACKS PROMPT AN ED VISIT
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks, and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least 4 of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5):1
- palpitations, pounding heart, or accelerated heart rate
- sweating
- trembling or shaking
- sensations of shortness of breath or smothering
- feeling of choking
- chest pain or discomfort
- nausea or abdominal distress
- feeling dizzy, unsteady, lightheaded, or faint
- de-realization (feelings of unreality) or depersonalization (being detached from oneself)
- fear of losing control or going crazy
- fear of dying
- paresthesia (numbness or tingling sensations)
- chills or hot flushes.
Lifetime incidence rates of panic disorder are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for panic disorder.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary physician.5 Patients with panic disorder tend to use health care resources at a disproportionately high rate.6
An international review of panic disorder research suggests the average age of onset for PD is 32 years.7 Triggers can vary widely and no single
Differential goes far beyond myocardial infarction
Many medical conditions can mimic panic disorder symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants such as cocaine, amphetamines); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with panic-like symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with panic-like symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and D-dimer assay to exclude pulmonary embolism).
If organic causes are ruled out, focus on a psychiatric assessment:9
- history of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- psychiatric history
- history of substance use
- family history of psychiatric disorders (especially anxiety disorders)
- social history (life events, including those preceding onset of panic; history of child abuse)
- medications
- mental status examination
- safety (panic disorder is associated with higher risk of suicidal ideation).
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (FIGURE9), although there is not enough evidence to recommend one vs the other or combination therapy vs monotherapy.9
CASE
For Ms. D, all medical test results came back negative, and the psychiatric assessment revealed that she met the DSM-5 criteria for panic disorder. Counting on the strength of their relationship, her physician talked to her about PD and discussed treatment options, which included counseling, medication, or both. Ms. D agreed to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her physician’s primary care clinic and to begin taking medication. Her PCP started her on sertraline 25 mg/d.
In CBT, Ms. D’s psychologist taught her about “fight or flight” and explained that it was a normal physiologic response that could lead to panic. Ms. D. learned to approach her physical symptoms in a different way, and how to breathe in a way that slowed her panic reaction.
Consider SSRIs and SNRIs
First-line medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors. MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although many clinicians start lower, at 5 mg/d), to avoid hypersensitivity reactions. Gradually titrate the dose upward within 3 to 7 days after initiation until a therapeutic dose is reached over 2 to 6 weeks. Schedule follow-up visits for every one to 2 weeks at the beginning of treatment and every 2 to 4 weeks until the therapeutic dose is reached. Assess safety/suicidality at each visit.
Keep in mind that the onset of therapeutic effect is between 2 and 4 weeks, but that clinical response can take up to 8 to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only 4 to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid)9 may be increased 3 to 5 days following initiation.
The evidence supports the use of CBT for panic disorder
CBT is an evidenced-based treatment for panic disorder.10-13 Up to 75% of patients treated with CBT are panic free within 4 months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psycho-education, exposure, and imagery.14
Treatment with medications and CBT either combined or used individually is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or 2 adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
CASE
Ten days after Ms. D started the sertraline 25 mg/d, she called the PCP to report daily diarrhea. She stopped the sertraline on her own and asked for another medication. She also expressed her frustration with the severity of the symptoms. She was having 3 to 5 panic attacks daily and had been missing many days from work.
On the day of her follow-up PCP appointment, Ms. D also saw the psychologist. She reported that she’d been practicing relaxation breathing, tracking her panic attacks, limiting caffeine intake, and exercising regularly. But the attacks were still occurring.
The PCP switched her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribed clonazepam 0.5 mg bid. Two weeks later, Ms. D reported that she was feeling a little better, had returned to work, and was hopeful that she would be her “normal self again.” The PCP planned to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and to slowly taper the clonazepam toward discontinuation in the near future.
CORRESPONDENCE
Eric H. Berko, PhD, Case Western Reserve University School of Medicine, Department of Family Medicine, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109-7878; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing: Arlington, VA; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33:1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. Practice guideline for the treatment of patients with panic disorder, 2nd ed. 2010. American Psychiatric Association; Washington DC. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed October 26, 2017.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Available at: https://www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed October 26, 2017.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993;61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE., Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595–606
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. Available at: https://www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed October 26, 2017.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing: Arlington, VA; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33:1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. Practice guideline for the treatment of patients with panic disorder, 2nd ed. 2010. American Psychiatric Association; Washington DC. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed October 26, 2017.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Available at: https://www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed October 26, 2017.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993;61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE., Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595–606
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. Available at: https://www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed October 26, 2017.
Let’s get physical!
We see many patients in our office with acute or chronic musculoskeletal complaints. Most acute musculoskeletal injuries resolve with rest and a short course of a narcotic or over-the-counter pain medication.
But when pain lingers beyond a few weeks, patients get nervous and so do we. This is the critical time period during which patients may request more narcotic pain medication and/or develop chronic pain syndromes, which are enormously difficult to treat. What treatments can we suggest at this point that have good evidence of effectiveness?
Reassurance. The simplest effective intervention is reassurance—especially from a physician. Patients who are reassured are more likely to recover from low back pain.1 Patients often have unrealistic goals, expecting to be pain free in a couple of weeks, whereas the natural healing of soft tissue injuries takes 8 to 12 weeks (and sometimes longer) for more severe injuries.
Physical modalities. Nearly all of the other non-medicinal, effective interventions for subacute and chronic musculoskeletal pain are physical in nature. The article by Slattengren et al provides an evidence-based review of osteopathic manipulation techniques (OMT) for pain and other conditions, as well. The evidence for the effectiveness of OMT for low back pain is the strongest.
There is evidence for the effectiveness of other types of physical techniques for low back pain, too. A recent meta-analysis of spinal manipulation for acute low back pain concluded that it is moderately effective in reducing pain and increasing function.2 And although the evidence is not considered strong, the American College of Physicians included massage, tai chi, yoga, acupuncture, motor control exercises, and progressive relaxation in their recent recommendations for the treatment of acute, subacute, and chronic low back pain.3
My personal favorite treatment for chronic low back pain is walking. In a clever randomized trial, Irish researchers randomized patients with chronic low back pain to 3 groups: standard physical therapy, weekly exercise classes designed for people with low back pain, and a tailored, gradually increasing walking program.4 Participants in the last group were instructed to walk at least 4 days a week, starting with 10 minutes/day and working up to 30 minutes of brisk walking 5 days/week. The improvement in pain and disability after 2 months, although modest, was as good in the walking group as in the other 2 treatment groups.
So let’s try relying more on physical activity to help our patients manage their aches and pains. It may produce benefits for other health problems, too, and start many patients down a road to healthier living.
1. Traeger AC, Hübscher M, Henschke N, et al. Effect of primary care-based education on reassurance in patients with acute low back pain: systematic review and meta-analysis. JAMA Intern Med. 2015;175:733-743.
2. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
3. Qaseem A, Wilt TJ, McLean RM, et al. Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
4. Hurley DA, Tully MA, Lonsdale C, et al. Supervised walking in comparison with fitness training for chronic back pain in physiotherapy: results of the SWIFT single-blinded randomized controlled trial (ISRCTN17592092). Pain. 2015;156:131-147.
We see many patients in our office with acute or chronic musculoskeletal complaints. Most acute musculoskeletal injuries resolve with rest and a short course of a narcotic or over-the-counter pain medication.
But when pain lingers beyond a few weeks, patients get nervous and so do we. This is the critical time period during which patients may request more narcotic pain medication and/or develop chronic pain syndromes, which are enormously difficult to treat. What treatments can we suggest at this point that have good evidence of effectiveness?
Reassurance. The simplest effective intervention is reassurance—especially from a physician. Patients who are reassured are more likely to recover from low back pain.1 Patients often have unrealistic goals, expecting to be pain free in a couple of weeks, whereas the natural healing of soft tissue injuries takes 8 to 12 weeks (and sometimes longer) for more severe injuries.
Physical modalities. Nearly all of the other non-medicinal, effective interventions for subacute and chronic musculoskeletal pain are physical in nature. The article by Slattengren et al provides an evidence-based review of osteopathic manipulation techniques (OMT) for pain and other conditions, as well. The evidence for the effectiveness of OMT for low back pain is the strongest.
There is evidence for the effectiveness of other types of physical techniques for low back pain, too. A recent meta-analysis of spinal manipulation for acute low back pain concluded that it is moderately effective in reducing pain and increasing function.2 And although the evidence is not considered strong, the American College of Physicians included massage, tai chi, yoga, acupuncture, motor control exercises, and progressive relaxation in their recent recommendations for the treatment of acute, subacute, and chronic low back pain.3
My personal favorite treatment for chronic low back pain is walking. In a clever randomized trial, Irish researchers randomized patients with chronic low back pain to 3 groups: standard physical therapy, weekly exercise classes designed for people with low back pain, and a tailored, gradually increasing walking program.4 Participants in the last group were instructed to walk at least 4 days a week, starting with 10 minutes/day and working up to 30 minutes of brisk walking 5 days/week. The improvement in pain and disability after 2 months, although modest, was as good in the walking group as in the other 2 treatment groups.
So let’s try relying more on physical activity to help our patients manage their aches and pains. It may produce benefits for other health problems, too, and start many patients down a road to healthier living.
We see many patients in our office with acute or chronic musculoskeletal complaints. Most acute musculoskeletal injuries resolve with rest and a short course of a narcotic or over-the-counter pain medication.
But when pain lingers beyond a few weeks, patients get nervous and so do we. This is the critical time period during which patients may request more narcotic pain medication and/or develop chronic pain syndromes, which are enormously difficult to treat. What treatments can we suggest at this point that have good evidence of effectiveness?
Reassurance. The simplest effective intervention is reassurance—especially from a physician. Patients who are reassured are more likely to recover from low back pain.1 Patients often have unrealistic goals, expecting to be pain free in a couple of weeks, whereas the natural healing of soft tissue injuries takes 8 to 12 weeks (and sometimes longer) for more severe injuries.
Physical modalities. Nearly all of the other non-medicinal, effective interventions for subacute and chronic musculoskeletal pain are physical in nature. The article by Slattengren et al provides an evidence-based review of osteopathic manipulation techniques (OMT) for pain and other conditions, as well. The evidence for the effectiveness of OMT for low back pain is the strongest.
There is evidence for the effectiveness of other types of physical techniques for low back pain, too. A recent meta-analysis of spinal manipulation for acute low back pain concluded that it is moderately effective in reducing pain and increasing function.2 And although the evidence is not considered strong, the American College of Physicians included massage, tai chi, yoga, acupuncture, motor control exercises, and progressive relaxation in their recent recommendations for the treatment of acute, subacute, and chronic low back pain.3
My personal favorite treatment for chronic low back pain is walking. In a clever randomized trial, Irish researchers randomized patients with chronic low back pain to 3 groups: standard physical therapy, weekly exercise classes designed for people with low back pain, and a tailored, gradually increasing walking program.4 Participants in the last group were instructed to walk at least 4 days a week, starting with 10 minutes/day and working up to 30 minutes of brisk walking 5 days/week. The improvement in pain and disability after 2 months, although modest, was as good in the walking group as in the other 2 treatment groups.
So let’s try relying more on physical activity to help our patients manage their aches and pains. It may produce benefits for other health problems, too, and start many patients down a road to healthier living.
1. Traeger AC, Hübscher M, Henschke N, et al. Effect of primary care-based education on reassurance in patients with acute low back pain: systematic review and meta-analysis. JAMA Intern Med. 2015;175:733-743.
2. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
3. Qaseem A, Wilt TJ, McLean RM, et al. Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
4. Hurley DA, Tully MA, Lonsdale C, et al. Supervised walking in comparison with fitness training for chronic back pain in physiotherapy: results of the SWIFT single-blinded randomized controlled trial (ISRCTN17592092). Pain. 2015;156:131-147.
1. Traeger AC, Hübscher M, Henschke N, et al. Effect of primary care-based education on reassurance in patients with acute low back pain: systematic review and meta-analysis. JAMA Intern Med. 2015;175:733-743.
2. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
3. Qaseem A, Wilt TJ, McLean RM, et al. Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
4. Hurley DA, Tully MA, Lonsdale C, et al. Supervised walking in comparison with fitness training for chronic back pain in physiotherapy: results of the SWIFT single-blinded randomized controlled trial (ISRCTN17592092). Pain. 2015;156:131-147.
The benefits of physician-pharmacist collaboration
Over the past decade, physician-pharmacist collaborative practices have gained traction in primary care as a way to implement team-based-care models. And there is evidence pointing to the effectiveness of this multidisciplinary heath care team approach, in which pharmacists are typically responsible for such things as obtaining medication histories, identifying barriers to adherence, and adjusting medication regimens.
Several studies have shown the significant impact that physician-pharmacist collaborative management (PPCM) can have on blood pressure (BP) control among patients with hypertension (HTN).1-8 Additionally, PPCM may have positive effects on HbA1c reduction and diabetes control,9-11 suggesting that benefits may extend to other chronic diseases, too.
In the review that follows, we’ll detail the impact that PPCM can have on patient care, health-care utilization, and cost effectiveness. (For a look at PPCM “in action,” see the sidebar below.) We’ll also review the challenges of implementing this model that, at present, is mostly found in academically-affiliated clinics and large health systems.
SIDEBAR
The physician-pharmacist collaborative care model in actionFor patients with chronic diseases such as hypertension and diabetes, pharmacists can be invaluable members of multidisciplinary health care teams by providing direct consultation to optimize pharmacotherapy. Although their particular role and responsibilities can vary widely from one primary care setting to the next, the following describes the general workflow of a physician-pharmacist collaborative care model in action.
The patient, 60-year-old Isabel B, arrives for an appointment for pharmacotherapy management of her hypertension. After checking in, a registered nurse (RN), medical assistant (MA), or the pharmacist obtains her vital signs, height, and weight prior to rooming. Additionally, any necessary point-of-care lab tests are obtained at this time.
Once the patient is roomed, the pharmacist collects a thorough medication history from Ms. B, verifying and updating her current medication list, confirming the dose and frequency of each medication, and gathering information regarding adverse effects and barriers to adherence. The pharmacist may also review current laboratory results and vital signs to assess the appropriateness and therapeutic efficacy of the current drug therapy regimen.
Depending upon the collaborative practice plan in place, one of the following steps may occur:
A. The pharmacist makes a change to Ms. B's medication regimen and orders any necessary laboratory tests for monitoring. A progress note is forwarded to Ms. B's primary care provider (PCP) to inform him/her of the changes made to the regimen and the follow-up interval.
B. The pharmacist presents pharmacotherapy recommendations to the attending physician or Ms. B's PCP. The therapeutic and monitoring plans are discussed and approved as a team at the time of Ms. B's visit.
C. The pharmacist sends a message to Ms. B's PCP regarding information discovered during the interview and provides recommendations for a treatment plan based on the visit. The PCP reviews the recommendations, and can either 1) send approval to the pharmacist through a message or 2) implement the appropriate drug therapy changes at Ms. B's next visit.
In Cases A and B, the pharmacist then reviews the final pharmacotherapy plan with Ms. B, discusses the medication and monitoring parameters, answers any questions related to the new treatment regimen, and schedules a follow-up visit. In Case C, the pharmacist may still provide medication counseling and answer questions related to drug therapy during the visit; however, review of the final pharmacotherapy plan may be done over the telephone after approval by the PCP. Alternatively, a follow-up appointment with Ms. B's PCP can be scheduled shortly after the visit with the pharmacist to discuss any recommended drug therapy changes.
PPCM impacts chronic diseases
The current literature is rife with studies investigating the impact of PPCM on chronic diseases in the primary care setting.1-12 Although no specific guidelines on implementing PPCM exist, these studies utilized similar interventions that provided pharmacists with the ability to manage medication therapy under the supervision of a physician. A number of these studies incorporated collaborative practice plans to delineate the specific duties performed by physicians and pharmacists.2,6,8,10,11 Responsibilities for pharmacists often included assessing vital signs, reviewing laboratory parameters and ordering appropriate tests, providing patient education, screening for drug interactions, identifying barriers to medication adherence, and adjusting medication regimens. The TABLE1-12 provides a summary of studies investigating the impact of PPCM in the primary care setting.
PPCM leads to greater BP reductions, improved BP control
The majority of research surrounding PPCM has focused on uncontrolled HTN.1-8 Patients in many of these studies saw a pharmacist in a specialized HTN clinic, where the multidisciplinary staff performed a thorough evaluation of the patient’s current hypertensive management. The pharmacists in these PPCM programs closely monitored patients and made adjustments to antihypertensive regimens as necessary. Systolic and diastolic BP reductions in the intervention groups ranged from 14 to 36 mm Hg and 7 to 15 mm Hg, respectively.1-5,7,8 The percentage of patients with BP control at the end of the studies ranged from 43% to 89%.1,3,4,6,7
In a prospective, cluster-randomized trial performed at 32 primary care offices in 15 states, researchers assigned 625 patients with uncontrolled HTN to receive physician-pharmacist collaborative care or usual care with primary care provider management.7 As part of the PPCM intervention, clinical pharmacists conducted a thorough medical record review and a structured interview of the patients. During the interview, the clinical pharmacists reviewed the patient’s medication history, assessed the patient’s knowledge of BP medications, and addressed any barriers to adherence. In collaboration with the physician, the pharmacists developed a care plan with recommendations for optimizing the drug regimen. After the baseline visit, the pharmacists conducted structured face-to-face interviews with patients at 1, 2, 4, 6, and 8 months, with additional visits scheduled if BP was still uncontrolled.
At 9 months, patients in the PPCM group had significantly greater reductions in BP than those in the control group, and BP control was achieved in 43% of the PPCM group vs 34% of the control group. This study corroborates results from previous (similar) studies investigating the impact of PPCM on patients with uncontrolled HTN.1-6
PPCM helps patients reduce their HbA1c levels
Researchers have also studied the impact of PPCM strategies on the management of diabetes mellitus.9-11 In one retrospective study of 157 patients, implementation of a pharmacy-coordinated diabetes (any type) management program significantly improved HbA1c and increased the percentage of patients reaching their HbA1c goal.9 Furthermore, researchers observed improvements in low-density lipoprotein cholesterol (LDL-C) levels and an increased number of patients obtaining a microalbumin screening after initiation of the program.
A more recent prospective, multicenter cohort study of 206 patients with uncontrolled type 2 diabetes had similar results.10 In collaboration with the primary care physician (PCP), clinical pharmacists provided medication therapy management through adjustment of antihyperglycemic, antihypertensive, or lipid-lowering medications. Additional interventions provided by the pharmacists included reviewing blood glucose logs, ordering and monitoring laboratory tests, performing sensory foot examinations, and providing patient education.
Implementation of PPCM reduced the average HbA1c by 1.2% and increased the percentage of patients achieving an HbA1c <7% by about 24%. The researchers also observed improvements in BP and LDL-C levels in this patient population.11
Asthma and beyond
Future studies may well show that the benefits of PPCM extend to the management of other chronic diseases. One prospective, pre-post study of 126 patients with asthma found that the number of emergency department (ED) visits and/or hospitalizations decreased 30% during 9 months with a PPCM intervention and then returned to levels similar to baseline once the intervention ceased.12 Other potential disease areas that have been studied, or are being studied, include chronic obstructive pulmonary disease, chronic kidney disease, dyslipidemia, and congestive heart failure.13
Benefits derive from altered health care utilization
Researchers attribute much of the benefit observed with PPCM to the increased—albeit different—health-care utilization among the patients in the intervention groups. In general, patients participating in PPCM have an increased total number of visits, but more of those visits are with pharmacists and fewer are with physicians; they also are prescribed more medications, but don’t necessarily take more pills per day.1,2,5 In the end, patients have been found to achieve significantly better disease control without compromising quality of life or satisfaction.2
Some studies have found that continued pharmacist involvement may be necessary to sustain the benefits achieved.6 However, other studies have suggested that the benefits are maintained even after discontinuation of the pharmacist intervention.14,15 Thus, further research is necessary to determine which patients may benefit most from ongoing involvement with a pharmacist.
How cost-effective is the PPCM model?
Implementing a PPCM model in a primary care setting often hinges upon whether the intervention will be cost-effective. Several studies have reported the cost-effectiveness of clinical pharmacists in the management of HTN.1,16,17
Borenstein and colleagues found significantly lower provider visit costs per patient in the PPCM group ($160) compared with the usual care group ($195), a difference that the authors attributed to a decreased number of visits to PCPs and an increased number of lower cost visits with pharmacists in the PPCM group.1 However, the difference could have been affected by the arbitrary measurement of physician-pharmacist collaboration time in the study.
Overcoming implementation challenges
Implementation of pharmacist collaboration within primary care medicine may pose a challenge, as the requirements and resources vary widely among primary care settings. Health-system administrators, for example, may need to reorganize the clinic structure and budget resources in order to overcome some of the obstacles to implementing a PPCM model.
Experts have reported several strategies that help in establishing PPCM within primary care clinics,18 including proactively identifying patients who may benefit from pharmacist intervention, requiring appropriate training and credentialing of pharmacists, and establishing a set schedule for pharmacists to interview patients. Clinics would also be well served to model interventions outlined in the studies mentioned in this article and provide adequate time for pharmacists to perform structured activities, including review of medication history, assessment of current disease state control, and adjustment of medication therapy regimens. And, of course, given the diversity of primary care settings, administrators will need to identify the specific PPCM strategies that best complement their respective collaborative practice plans and environments.
The lack of well-defined reimbursement models for pharmacy services has presented a challenge for generating revenue and effectively implementing PPCM within many primary care settings. Currently, the Centers for Medicare and Medicaid Services and third-party payers do not recognize pharmacists as independent providers, creating a barrier for obtaining reimbursement for clinical pharmacy services. Typically, pharmacists have charged for clinic visits under a consultant physician through the “incident to” billing model, with the option to bill at higher levels if the patient was seen jointly with the physician.
Can this model benefit the underserved?
A prospective, cluster-randomized clinic study has shown pharmacist intervention to reduce racial and socioeconomic disparities in the treatment of elevated BP.19 This study is the first to show that a team-care model can overcome inequalities arising from low income, low patient education status, and little or no insurance to produce the same health care benefit as in those with higher socioeconomic and educational status. This type of collaborative care model may be particularly beneficial when incorporated within a PCMH catering to underserved populations.20
However, sparse data currently exist regarding the benefits of the PPCM model within a PCMH, despite the fact that integration of this type of collaborative model is expected to contribute positively to patient care.21
Physician acceptance of pharmacist involvement is mixed
While physician acceptance of pharmacist recommendations is generally high, at least one study indicated that some health-care professionals in patient-care teams are reluctant to incorporate pharmacists into a PCMH. Reasons include difficulty in coordination of care with pharmacy services and limited knowledge by other professionals of pharmacists’ training.22
Centralization can combat a lack of resources
As noted earlier, primary care offices that implement PPCM models are mostly academically affiliated or are part of large health systems. Many private primary care offices lack the resources to employ a pharmacist in their office. As an alternative, prospective clinical trials are looking at a centralized, Web-based cardiovascular risk service managed by pharmacists.23,24 This service’s primary objective is to improve adherence to metric-based outcomes developed as part of The Guideline Advantage quality improvement program put forth by the American Cancer Society, American Diabetes Association, and the American Heart and Stroke Associations. (See http://www.guidelineadvantage.org/TGA/ for more information.)
Researchers hope to prove that a centralized, pharmacist-run, clinical service can meet metric-driven outcomes that many primary care offices are now being required to meet in order to receive compensation from insurance companies. One of these studies is specifically looking at rural private offices that lack many of the resources that many large academic offices possess.23 The study is ongoing and results are expected sometime in 2018.
CORRESPONDENCE
John G. Gums, PharmD, College of Pharmacy, University of Florida, 1225 Center Drive, HPNP 4332, Gainesville, FL 32601; [email protected].
1. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209-216.
2. Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966-1972.
3. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260-271.
4. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996-2002.
5. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634-1639.
6. Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36:1244-1254.
7. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243.
8. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
9. Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828-1832.
10. Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47:781-789.
11. Howard-Thompson A, Farland MZ, Byrd DC, et al. Pharmacist-physician collaboration for diabetes care: cardiovascular outcomes. Ann Pharmacother. 2013;47:1471-1477.
12. Gums TH, Carter BL, Milavetz G, et al. Physician-pharmacist collaborative management of asthma in primary care. Pharmacotherapy. 2014;34:1033-1042.
13. Greer N, Bolduc J, Geurkink E, et al. VA Evidence-based Synthesis Program Reports. Pharmacist-Led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared to Usual Care. Washington (DC): Department of Veterans Affairs (US); 2015.
14. Wentzlaff DM, Carter BL, Ardery G, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention. J Clin Hypertens (Greenwich). 2011;13:431-437.
15. Carter BL, Vander Weg MW, Parker CP, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention for veterans. J Clin Hypertens (Greenwich). 2015;17:701-708.
16. Kulchaitanaroaj P, Brooks JM, Ardery G, et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
17. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145-1151.
18. Carter BL. Primary care physician-pharmacist collaborative care model: strategies for implementation. Pharmacotherapy. 2016;36:363-373.
19. Anderegg MD, Gums TH, Uribe L, et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
20. Moczygemba LR, Goode JV, Gatewood SBS, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:167-172.
21. Scott MA, Hitch B, Ray L, et al. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:161-166.
22. Patterson BJ, Solimeo SL, Stewart KR, et al. Perceptions of pharmacists’ integration into patient-centered medical home teams. Res Social Adm Pharm. 2015;11:85-95.
23. Carter BL, Levy BT, Gryzlak B, et al. A centralized cardiovascular risk service to improve guideline adherence in private primary care offices. Contemp Clin Trials. 2015;43:25-32.
24. Carter BL, Coffey CS, Chrischilles EA, et al. A cluster-randomized trial of a centralized clinical pharmacy cardiovascular risk service to improve guideline adherence. Pharmacotherapy. 2015;35:653-662.
Over the past decade, physician-pharmacist collaborative practices have gained traction in primary care as a way to implement team-based-care models. And there is evidence pointing to the effectiveness of this multidisciplinary heath care team approach, in which pharmacists are typically responsible for such things as obtaining medication histories, identifying barriers to adherence, and adjusting medication regimens.
Several studies have shown the significant impact that physician-pharmacist collaborative management (PPCM) can have on blood pressure (BP) control among patients with hypertension (HTN).1-8 Additionally, PPCM may have positive effects on HbA1c reduction and diabetes control,9-11 suggesting that benefits may extend to other chronic diseases, too.
In the review that follows, we’ll detail the impact that PPCM can have on patient care, health-care utilization, and cost effectiveness. (For a look at PPCM “in action,” see the sidebar below.) We’ll also review the challenges of implementing this model that, at present, is mostly found in academically-affiliated clinics and large health systems.
SIDEBAR
The physician-pharmacist collaborative care model in actionFor patients with chronic diseases such as hypertension and diabetes, pharmacists can be invaluable members of multidisciplinary health care teams by providing direct consultation to optimize pharmacotherapy. Although their particular role and responsibilities can vary widely from one primary care setting to the next, the following describes the general workflow of a physician-pharmacist collaborative care model in action.
The patient, 60-year-old Isabel B, arrives for an appointment for pharmacotherapy management of her hypertension. After checking in, a registered nurse (RN), medical assistant (MA), or the pharmacist obtains her vital signs, height, and weight prior to rooming. Additionally, any necessary point-of-care lab tests are obtained at this time.
Once the patient is roomed, the pharmacist collects a thorough medication history from Ms. B, verifying and updating her current medication list, confirming the dose and frequency of each medication, and gathering information regarding adverse effects and barriers to adherence. The pharmacist may also review current laboratory results and vital signs to assess the appropriateness and therapeutic efficacy of the current drug therapy regimen.
Depending upon the collaborative practice plan in place, one of the following steps may occur:
A. The pharmacist makes a change to Ms. B's medication regimen and orders any necessary laboratory tests for monitoring. A progress note is forwarded to Ms. B's primary care provider (PCP) to inform him/her of the changes made to the regimen and the follow-up interval.
B. The pharmacist presents pharmacotherapy recommendations to the attending physician or Ms. B's PCP. The therapeutic and monitoring plans are discussed and approved as a team at the time of Ms. B's visit.
C. The pharmacist sends a message to Ms. B's PCP regarding information discovered during the interview and provides recommendations for a treatment plan based on the visit. The PCP reviews the recommendations, and can either 1) send approval to the pharmacist through a message or 2) implement the appropriate drug therapy changes at Ms. B's next visit.
In Cases A and B, the pharmacist then reviews the final pharmacotherapy plan with Ms. B, discusses the medication and monitoring parameters, answers any questions related to the new treatment regimen, and schedules a follow-up visit. In Case C, the pharmacist may still provide medication counseling and answer questions related to drug therapy during the visit; however, review of the final pharmacotherapy plan may be done over the telephone after approval by the PCP. Alternatively, a follow-up appointment with Ms. B's PCP can be scheduled shortly after the visit with the pharmacist to discuss any recommended drug therapy changes.
PPCM impacts chronic diseases
The current literature is rife with studies investigating the impact of PPCM on chronic diseases in the primary care setting.1-12 Although no specific guidelines on implementing PPCM exist, these studies utilized similar interventions that provided pharmacists with the ability to manage medication therapy under the supervision of a physician. A number of these studies incorporated collaborative practice plans to delineate the specific duties performed by physicians and pharmacists.2,6,8,10,11 Responsibilities for pharmacists often included assessing vital signs, reviewing laboratory parameters and ordering appropriate tests, providing patient education, screening for drug interactions, identifying barriers to medication adherence, and adjusting medication regimens. The TABLE1-12 provides a summary of studies investigating the impact of PPCM in the primary care setting.
PPCM leads to greater BP reductions, improved BP control
The majority of research surrounding PPCM has focused on uncontrolled HTN.1-8 Patients in many of these studies saw a pharmacist in a specialized HTN clinic, where the multidisciplinary staff performed a thorough evaluation of the patient’s current hypertensive management. The pharmacists in these PPCM programs closely monitored patients and made adjustments to antihypertensive regimens as necessary. Systolic and diastolic BP reductions in the intervention groups ranged from 14 to 36 mm Hg and 7 to 15 mm Hg, respectively.1-5,7,8 The percentage of patients with BP control at the end of the studies ranged from 43% to 89%.1,3,4,6,7
In a prospective, cluster-randomized trial performed at 32 primary care offices in 15 states, researchers assigned 625 patients with uncontrolled HTN to receive physician-pharmacist collaborative care or usual care with primary care provider management.7 As part of the PPCM intervention, clinical pharmacists conducted a thorough medical record review and a structured interview of the patients. During the interview, the clinical pharmacists reviewed the patient’s medication history, assessed the patient’s knowledge of BP medications, and addressed any barriers to adherence. In collaboration with the physician, the pharmacists developed a care plan with recommendations for optimizing the drug regimen. After the baseline visit, the pharmacists conducted structured face-to-face interviews with patients at 1, 2, 4, 6, and 8 months, with additional visits scheduled if BP was still uncontrolled.
At 9 months, patients in the PPCM group had significantly greater reductions in BP than those in the control group, and BP control was achieved in 43% of the PPCM group vs 34% of the control group. This study corroborates results from previous (similar) studies investigating the impact of PPCM on patients with uncontrolled HTN.1-6
PPCM helps patients reduce their HbA1c levels
Researchers have also studied the impact of PPCM strategies on the management of diabetes mellitus.9-11 In one retrospective study of 157 patients, implementation of a pharmacy-coordinated diabetes (any type) management program significantly improved HbA1c and increased the percentage of patients reaching their HbA1c goal.9 Furthermore, researchers observed improvements in low-density lipoprotein cholesterol (LDL-C) levels and an increased number of patients obtaining a microalbumin screening after initiation of the program.
A more recent prospective, multicenter cohort study of 206 patients with uncontrolled type 2 diabetes had similar results.10 In collaboration with the primary care physician (PCP), clinical pharmacists provided medication therapy management through adjustment of antihyperglycemic, antihypertensive, or lipid-lowering medications. Additional interventions provided by the pharmacists included reviewing blood glucose logs, ordering and monitoring laboratory tests, performing sensory foot examinations, and providing patient education.
Implementation of PPCM reduced the average HbA1c by 1.2% and increased the percentage of patients achieving an HbA1c <7% by about 24%. The researchers also observed improvements in BP and LDL-C levels in this patient population.11
Asthma and beyond
Future studies may well show that the benefits of PPCM extend to the management of other chronic diseases. One prospective, pre-post study of 126 patients with asthma found that the number of emergency department (ED) visits and/or hospitalizations decreased 30% during 9 months with a PPCM intervention and then returned to levels similar to baseline once the intervention ceased.12 Other potential disease areas that have been studied, or are being studied, include chronic obstructive pulmonary disease, chronic kidney disease, dyslipidemia, and congestive heart failure.13
Benefits derive from altered health care utilization
Researchers attribute much of the benefit observed with PPCM to the increased—albeit different—health-care utilization among the patients in the intervention groups. In general, patients participating in PPCM have an increased total number of visits, but more of those visits are with pharmacists and fewer are with physicians; they also are prescribed more medications, but don’t necessarily take more pills per day.1,2,5 In the end, patients have been found to achieve significantly better disease control without compromising quality of life or satisfaction.2
Some studies have found that continued pharmacist involvement may be necessary to sustain the benefits achieved.6 However, other studies have suggested that the benefits are maintained even after discontinuation of the pharmacist intervention.14,15 Thus, further research is necessary to determine which patients may benefit most from ongoing involvement with a pharmacist.
How cost-effective is the PPCM model?
Implementing a PPCM model in a primary care setting often hinges upon whether the intervention will be cost-effective. Several studies have reported the cost-effectiveness of clinical pharmacists in the management of HTN.1,16,17
Borenstein and colleagues found significantly lower provider visit costs per patient in the PPCM group ($160) compared with the usual care group ($195), a difference that the authors attributed to a decreased number of visits to PCPs and an increased number of lower cost visits with pharmacists in the PPCM group.1 However, the difference could have been affected by the arbitrary measurement of physician-pharmacist collaboration time in the study.
Overcoming implementation challenges
Implementation of pharmacist collaboration within primary care medicine may pose a challenge, as the requirements and resources vary widely among primary care settings. Health-system administrators, for example, may need to reorganize the clinic structure and budget resources in order to overcome some of the obstacles to implementing a PPCM model.
Experts have reported several strategies that help in establishing PPCM within primary care clinics,18 including proactively identifying patients who may benefit from pharmacist intervention, requiring appropriate training and credentialing of pharmacists, and establishing a set schedule for pharmacists to interview patients. Clinics would also be well served to model interventions outlined in the studies mentioned in this article and provide adequate time for pharmacists to perform structured activities, including review of medication history, assessment of current disease state control, and adjustment of medication therapy regimens. And, of course, given the diversity of primary care settings, administrators will need to identify the specific PPCM strategies that best complement their respective collaborative practice plans and environments.
The lack of well-defined reimbursement models for pharmacy services has presented a challenge for generating revenue and effectively implementing PPCM within many primary care settings. Currently, the Centers for Medicare and Medicaid Services and third-party payers do not recognize pharmacists as independent providers, creating a barrier for obtaining reimbursement for clinical pharmacy services. Typically, pharmacists have charged for clinic visits under a consultant physician through the “incident to” billing model, with the option to bill at higher levels if the patient was seen jointly with the physician.
Can this model benefit the underserved?
A prospective, cluster-randomized clinic study has shown pharmacist intervention to reduce racial and socioeconomic disparities in the treatment of elevated BP.19 This study is the first to show that a team-care model can overcome inequalities arising from low income, low patient education status, and little or no insurance to produce the same health care benefit as in those with higher socioeconomic and educational status. This type of collaborative care model may be particularly beneficial when incorporated within a PCMH catering to underserved populations.20
However, sparse data currently exist regarding the benefits of the PPCM model within a PCMH, despite the fact that integration of this type of collaborative model is expected to contribute positively to patient care.21
Physician acceptance of pharmacist involvement is mixed
While physician acceptance of pharmacist recommendations is generally high, at least one study indicated that some health-care professionals in patient-care teams are reluctant to incorporate pharmacists into a PCMH. Reasons include difficulty in coordination of care with pharmacy services and limited knowledge by other professionals of pharmacists’ training.22
Centralization can combat a lack of resources
As noted earlier, primary care offices that implement PPCM models are mostly academically affiliated or are part of large health systems. Many private primary care offices lack the resources to employ a pharmacist in their office. As an alternative, prospective clinical trials are looking at a centralized, Web-based cardiovascular risk service managed by pharmacists.23,24 This service’s primary objective is to improve adherence to metric-based outcomes developed as part of The Guideline Advantage quality improvement program put forth by the American Cancer Society, American Diabetes Association, and the American Heart and Stroke Associations. (See http://www.guidelineadvantage.org/TGA/ for more information.)
Researchers hope to prove that a centralized, pharmacist-run, clinical service can meet metric-driven outcomes that many primary care offices are now being required to meet in order to receive compensation from insurance companies. One of these studies is specifically looking at rural private offices that lack many of the resources that many large academic offices possess.23 The study is ongoing and results are expected sometime in 2018.
CORRESPONDENCE
John G. Gums, PharmD, College of Pharmacy, University of Florida, 1225 Center Drive, HPNP 4332, Gainesville, FL 32601; [email protected].
Over the past decade, physician-pharmacist collaborative practices have gained traction in primary care as a way to implement team-based-care models. And there is evidence pointing to the effectiveness of this multidisciplinary heath care team approach, in which pharmacists are typically responsible for such things as obtaining medication histories, identifying barriers to adherence, and adjusting medication regimens.
Several studies have shown the significant impact that physician-pharmacist collaborative management (PPCM) can have on blood pressure (BP) control among patients with hypertension (HTN).1-8 Additionally, PPCM may have positive effects on HbA1c reduction and diabetes control,9-11 suggesting that benefits may extend to other chronic diseases, too.
In the review that follows, we’ll detail the impact that PPCM can have on patient care, health-care utilization, and cost effectiveness. (For a look at PPCM “in action,” see the sidebar below.) We’ll also review the challenges of implementing this model that, at present, is mostly found in academically-affiliated clinics and large health systems.
SIDEBAR
The physician-pharmacist collaborative care model in actionFor patients with chronic diseases such as hypertension and diabetes, pharmacists can be invaluable members of multidisciplinary health care teams by providing direct consultation to optimize pharmacotherapy. Although their particular role and responsibilities can vary widely from one primary care setting to the next, the following describes the general workflow of a physician-pharmacist collaborative care model in action.
The patient, 60-year-old Isabel B, arrives for an appointment for pharmacotherapy management of her hypertension. After checking in, a registered nurse (RN), medical assistant (MA), or the pharmacist obtains her vital signs, height, and weight prior to rooming. Additionally, any necessary point-of-care lab tests are obtained at this time.
Once the patient is roomed, the pharmacist collects a thorough medication history from Ms. B, verifying and updating her current medication list, confirming the dose and frequency of each medication, and gathering information regarding adverse effects and barriers to adherence. The pharmacist may also review current laboratory results and vital signs to assess the appropriateness and therapeutic efficacy of the current drug therapy regimen.
Depending upon the collaborative practice plan in place, one of the following steps may occur:
A. The pharmacist makes a change to Ms. B's medication regimen and orders any necessary laboratory tests for monitoring. A progress note is forwarded to Ms. B's primary care provider (PCP) to inform him/her of the changes made to the regimen and the follow-up interval.
B. The pharmacist presents pharmacotherapy recommendations to the attending physician or Ms. B's PCP. The therapeutic and monitoring plans are discussed and approved as a team at the time of Ms. B's visit.
C. The pharmacist sends a message to Ms. B's PCP regarding information discovered during the interview and provides recommendations for a treatment plan based on the visit. The PCP reviews the recommendations, and can either 1) send approval to the pharmacist through a message or 2) implement the appropriate drug therapy changes at Ms. B's next visit.
In Cases A and B, the pharmacist then reviews the final pharmacotherapy plan with Ms. B, discusses the medication and monitoring parameters, answers any questions related to the new treatment regimen, and schedules a follow-up visit. In Case C, the pharmacist may still provide medication counseling and answer questions related to drug therapy during the visit; however, review of the final pharmacotherapy plan may be done over the telephone after approval by the PCP. Alternatively, a follow-up appointment with Ms. B's PCP can be scheduled shortly after the visit with the pharmacist to discuss any recommended drug therapy changes.
PPCM impacts chronic diseases
The current literature is rife with studies investigating the impact of PPCM on chronic diseases in the primary care setting.1-12 Although no specific guidelines on implementing PPCM exist, these studies utilized similar interventions that provided pharmacists with the ability to manage medication therapy under the supervision of a physician. A number of these studies incorporated collaborative practice plans to delineate the specific duties performed by physicians and pharmacists.2,6,8,10,11 Responsibilities for pharmacists often included assessing vital signs, reviewing laboratory parameters and ordering appropriate tests, providing patient education, screening for drug interactions, identifying barriers to medication adherence, and adjusting medication regimens. The TABLE1-12 provides a summary of studies investigating the impact of PPCM in the primary care setting.
PPCM leads to greater BP reductions, improved BP control
The majority of research surrounding PPCM has focused on uncontrolled HTN.1-8 Patients in many of these studies saw a pharmacist in a specialized HTN clinic, where the multidisciplinary staff performed a thorough evaluation of the patient’s current hypertensive management. The pharmacists in these PPCM programs closely monitored patients and made adjustments to antihypertensive regimens as necessary. Systolic and diastolic BP reductions in the intervention groups ranged from 14 to 36 mm Hg and 7 to 15 mm Hg, respectively.1-5,7,8 The percentage of patients with BP control at the end of the studies ranged from 43% to 89%.1,3,4,6,7
In a prospective, cluster-randomized trial performed at 32 primary care offices in 15 states, researchers assigned 625 patients with uncontrolled HTN to receive physician-pharmacist collaborative care or usual care with primary care provider management.7 As part of the PPCM intervention, clinical pharmacists conducted a thorough medical record review and a structured interview of the patients. During the interview, the clinical pharmacists reviewed the patient’s medication history, assessed the patient’s knowledge of BP medications, and addressed any barriers to adherence. In collaboration with the physician, the pharmacists developed a care plan with recommendations for optimizing the drug regimen. After the baseline visit, the pharmacists conducted structured face-to-face interviews with patients at 1, 2, 4, 6, and 8 months, with additional visits scheduled if BP was still uncontrolled.
At 9 months, patients in the PPCM group had significantly greater reductions in BP than those in the control group, and BP control was achieved in 43% of the PPCM group vs 34% of the control group. This study corroborates results from previous (similar) studies investigating the impact of PPCM on patients with uncontrolled HTN.1-6
PPCM helps patients reduce their HbA1c levels
Researchers have also studied the impact of PPCM strategies on the management of diabetes mellitus.9-11 In one retrospective study of 157 patients, implementation of a pharmacy-coordinated diabetes (any type) management program significantly improved HbA1c and increased the percentage of patients reaching their HbA1c goal.9 Furthermore, researchers observed improvements in low-density lipoprotein cholesterol (LDL-C) levels and an increased number of patients obtaining a microalbumin screening after initiation of the program.
A more recent prospective, multicenter cohort study of 206 patients with uncontrolled type 2 diabetes had similar results.10 In collaboration with the primary care physician (PCP), clinical pharmacists provided medication therapy management through adjustment of antihyperglycemic, antihypertensive, or lipid-lowering medications. Additional interventions provided by the pharmacists included reviewing blood glucose logs, ordering and monitoring laboratory tests, performing sensory foot examinations, and providing patient education.
Implementation of PPCM reduced the average HbA1c by 1.2% and increased the percentage of patients achieving an HbA1c <7% by about 24%. The researchers also observed improvements in BP and LDL-C levels in this patient population.11
Asthma and beyond
Future studies may well show that the benefits of PPCM extend to the management of other chronic diseases. One prospective, pre-post study of 126 patients with asthma found that the number of emergency department (ED) visits and/or hospitalizations decreased 30% during 9 months with a PPCM intervention and then returned to levels similar to baseline once the intervention ceased.12 Other potential disease areas that have been studied, or are being studied, include chronic obstructive pulmonary disease, chronic kidney disease, dyslipidemia, and congestive heart failure.13
Benefits derive from altered health care utilization
Researchers attribute much of the benefit observed with PPCM to the increased—albeit different—health-care utilization among the patients in the intervention groups. In general, patients participating in PPCM have an increased total number of visits, but more of those visits are with pharmacists and fewer are with physicians; they also are prescribed more medications, but don’t necessarily take more pills per day.1,2,5 In the end, patients have been found to achieve significantly better disease control without compromising quality of life or satisfaction.2
Some studies have found that continued pharmacist involvement may be necessary to sustain the benefits achieved.6 However, other studies have suggested that the benefits are maintained even after discontinuation of the pharmacist intervention.14,15 Thus, further research is necessary to determine which patients may benefit most from ongoing involvement with a pharmacist.
How cost-effective is the PPCM model?
Implementing a PPCM model in a primary care setting often hinges upon whether the intervention will be cost-effective. Several studies have reported the cost-effectiveness of clinical pharmacists in the management of HTN.1,16,17
Borenstein and colleagues found significantly lower provider visit costs per patient in the PPCM group ($160) compared with the usual care group ($195), a difference that the authors attributed to a decreased number of visits to PCPs and an increased number of lower cost visits with pharmacists in the PPCM group.1 However, the difference could have been affected by the arbitrary measurement of physician-pharmacist collaboration time in the study.
Overcoming implementation challenges
Implementation of pharmacist collaboration within primary care medicine may pose a challenge, as the requirements and resources vary widely among primary care settings. Health-system administrators, for example, may need to reorganize the clinic structure and budget resources in order to overcome some of the obstacles to implementing a PPCM model.
Experts have reported several strategies that help in establishing PPCM within primary care clinics,18 including proactively identifying patients who may benefit from pharmacist intervention, requiring appropriate training and credentialing of pharmacists, and establishing a set schedule for pharmacists to interview patients. Clinics would also be well served to model interventions outlined in the studies mentioned in this article and provide adequate time for pharmacists to perform structured activities, including review of medication history, assessment of current disease state control, and adjustment of medication therapy regimens. And, of course, given the diversity of primary care settings, administrators will need to identify the specific PPCM strategies that best complement their respective collaborative practice plans and environments.
The lack of well-defined reimbursement models for pharmacy services has presented a challenge for generating revenue and effectively implementing PPCM within many primary care settings. Currently, the Centers for Medicare and Medicaid Services and third-party payers do not recognize pharmacists as independent providers, creating a barrier for obtaining reimbursement for clinical pharmacy services. Typically, pharmacists have charged for clinic visits under a consultant physician through the “incident to” billing model, with the option to bill at higher levels if the patient was seen jointly with the physician.
Can this model benefit the underserved?
A prospective, cluster-randomized clinic study has shown pharmacist intervention to reduce racial and socioeconomic disparities in the treatment of elevated BP.19 This study is the first to show that a team-care model can overcome inequalities arising from low income, low patient education status, and little or no insurance to produce the same health care benefit as in those with higher socioeconomic and educational status. This type of collaborative care model may be particularly beneficial when incorporated within a PCMH catering to underserved populations.20
However, sparse data currently exist regarding the benefits of the PPCM model within a PCMH, despite the fact that integration of this type of collaborative model is expected to contribute positively to patient care.21
Physician acceptance of pharmacist involvement is mixed
While physician acceptance of pharmacist recommendations is generally high, at least one study indicated that some health-care professionals in patient-care teams are reluctant to incorporate pharmacists into a PCMH. Reasons include difficulty in coordination of care with pharmacy services and limited knowledge by other professionals of pharmacists’ training.22
Centralization can combat a lack of resources
As noted earlier, primary care offices that implement PPCM models are mostly academically affiliated or are part of large health systems. Many private primary care offices lack the resources to employ a pharmacist in their office. As an alternative, prospective clinical trials are looking at a centralized, Web-based cardiovascular risk service managed by pharmacists.23,24 This service’s primary objective is to improve adherence to metric-based outcomes developed as part of The Guideline Advantage quality improvement program put forth by the American Cancer Society, American Diabetes Association, and the American Heart and Stroke Associations. (See http://www.guidelineadvantage.org/TGA/ for more information.)
Researchers hope to prove that a centralized, pharmacist-run, clinical service can meet metric-driven outcomes that many primary care offices are now being required to meet in order to receive compensation from insurance companies. One of these studies is specifically looking at rural private offices that lack many of the resources that many large academic offices possess.23 The study is ongoing and results are expected sometime in 2018.
CORRESPONDENCE
John G. Gums, PharmD, College of Pharmacy, University of Florida, 1225 Center Drive, HPNP 4332, Gainesville, FL 32601; [email protected].
1. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209-216.
2. Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966-1972.
3. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260-271.
4. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996-2002.
5. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634-1639.
6. Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36:1244-1254.
7. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243.
8. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
9. Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828-1832.
10. Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47:781-789.
11. Howard-Thompson A, Farland MZ, Byrd DC, et al. Pharmacist-physician collaboration for diabetes care: cardiovascular outcomes. Ann Pharmacother. 2013;47:1471-1477.
12. Gums TH, Carter BL, Milavetz G, et al. Physician-pharmacist collaborative management of asthma in primary care. Pharmacotherapy. 2014;34:1033-1042.
13. Greer N, Bolduc J, Geurkink E, et al. VA Evidence-based Synthesis Program Reports. Pharmacist-Led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared to Usual Care. Washington (DC): Department of Veterans Affairs (US); 2015.
14. Wentzlaff DM, Carter BL, Ardery G, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention. J Clin Hypertens (Greenwich). 2011;13:431-437.
15. Carter BL, Vander Weg MW, Parker CP, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention for veterans. J Clin Hypertens (Greenwich). 2015;17:701-708.
16. Kulchaitanaroaj P, Brooks JM, Ardery G, et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
17. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145-1151.
18. Carter BL. Primary care physician-pharmacist collaborative care model: strategies for implementation. Pharmacotherapy. 2016;36:363-373.
19. Anderegg MD, Gums TH, Uribe L, et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
20. Moczygemba LR, Goode JV, Gatewood SBS, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:167-172.
21. Scott MA, Hitch B, Ray L, et al. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:161-166.
22. Patterson BJ, Solimeo SL, Stewart KR, et al. Perceptions of pharmacists’ integration into patient-centered medical home teams. Res Social Adm Pharm. 2015;11:85-95.
23. Carter BL, Levy BT, Gryzlak B, et al. A centralized cardiovascular risk service to improve guideline adherence in private primary care offices. Contemp Clin Trials. 2015;43:25-32.
24. Carter BL, Coffey CS, Chrischilles EA, et al. A cluster-randomized trial of a centralized clinical pharmacy cardiovascular risk service to improve guideline adherence. Pharmacotherapy. 2015;35:653-662.
1. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209-216.
2. Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966-1972.
3. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260-271.
4. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996-2002.
5. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634-1639.
6. Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36:1244-1254.
7. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243.
8. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
9. Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828-1832.
10. Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47:781-789.
11. Howard-Thompson A, Farland MZ, Byrd DC, et al. Pharmacist-physician collaboration for diabetes care: cardiovascular outcomes. Ann Pharmacother. 2013;47:1471-1477.
12. Gums TH, Carter BL, Milavetz G, et al. Physician-pharmacist collaborative management of asthma in primary care. Pharmacotherapy. 2014;34:1033-1042.
13. Greer N, Bolduc J, Geurkink E, et al. VA Evidence-based Synthesis Program Reports. Pharmacist-Led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared to Usual Care. Washington (DC): Department of Veterans Affairs (US); 2015.
14. Wentzlaff DM, Carter BL, Ardery G, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention. J Clin Hypertens (Greenwich). 2011;13:431-437.
15. Carter BL, Vander Weg MW, Parker CP, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention for veterans. J Clin Hypertens (Greenwich). 2015;17:701-708.
16. Kulchaitanaroaj P, Brooks JM, Ardery G, et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
17. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145-1151.
18. Carter BL. Primary care physician-pharmacist collaborative care model: strategies for implementation. Pharmacotherapy. 2016;36:363-373.
19. Anderegg MD, Gums TH, Uribe L, et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
20. Moczygemba LR, Goode JV, Gatewood SBS, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:167-172.
21. Scott MA, Hitch B, Ray L, et al. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:161-166.
22. Patterson BJ, Solimeo SL, Stewart KR, et al. Perceptions of pharmacists’ integration into patient-centered medical home teams. Res Social Adm Pharm. 2015;11:85-95.
23. Carter BL, Levy BT, Gryzlak B, et al. A centralized cardiovascular risk service to improve guideline adherence in private primary care offices. Contemp Clin Trials. 2015;43:25-32.
24. Carter BL, Coffey CS, Chrischilles EA, et al. A cluster-randomized trial of a centralized clinical pharmacy cardiovascular risk service to improve guideline adherence. Pharmacotherapy. 2015;35:653-662.
PRACTICE RECOMMENDATIONS
› Consider physician-pharmacist collaboration as a way by which to improve the management of your patients with hypertension and diabetes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Best uses of osteopathic manipulation
Interest in osteopathy continues to rise in this country. Currently, more than 20% of medical students in the United States are training to be osteopathic physicians.1 In addition, the 2007 National Health Interview Survey found that spinal manipulation was among the most common complementary and alternative medicine (CAM) therapies used; with 8.6% of US adults reporting that they used it within the previous 12 months.2
With the growing number of DOs and the high utilization of osteopathic manipulative treatment (OMT), it is important for all physicians to understand the role OMT can play in the treatment of conditions ranging from low back pain to irritable bowel syndrome so that patients may be offered, or referred for, the treatment when appropriate.
To clarify when OMT may be most beneficial, we performed a literature review. Our findings are summarized here. But first, a word about osteopathic medicine and what OMT entails.
Osteopathic physicians view the body as a whole
According to the American Osteopathic Association, “the osteopathic philosophy of medicine sees an interrelated unity in all systems of the body, with each working with the other to heal in times of illness."3 This “whole-person approach to medicine” focuses on looking beyond symptoms alone to understand how lifestyle and environmental factors impact well-being.
As part of their education, DOs receive special training in the musculoskeletal system and in OMT. OMT is the process by which DOs use their hands to diagnose illness and injury and then mobilize a patient’s joints and soft tissues using techniques that include muscle activation, stretching, joint articulation, and gentle pressure to encourage the body’s natural tendency to heal itself.
These patients with low back pain will likely benefit
In the past, studies with small sample sizes, blinding issues, differing controls, and subjective outcome measurements have marred research efforts to demonstrate the effectiveness of OMT. More recently, researchers have attempted to minimize these issues, particularly when evaluating the efficacy of OMT for low back pain.
In addition to increasing sample size, studies have compared OMT to usual care, to sham manipulation, and more recently to other manual modalities including ultrasound to equalize the subjective effects of interventions.4 With improved study designs, there has been increased awareness of the effectiveness of spinal manipulation by organizations that develop guidelines for the care of patients with low back pain. The most recent clinical practice guideline from the American College of Physicians includes spinal manipulation as a treatment modality that should be considered by clinicians for patients who have acute, subacute, or chronic low back pain.5
Chronic nonspecific low back pain. Looking at OMT vs other interventions for chronic nonspecific low back pain, a 2014 meta-analysis found moderate quality evidence for clinically relevant effects of OMT on low back pain and function. In 6 studies that evaluated 769 patients with chronic nonspecific low back pain, there was a significant difference in pain—equivalent to a 1.5-point improvement (mean difference [MD]= -14.93; 95% confidence interval [CI], -25.18
Acute and chronic nonspecific low back pain. Similarly, in the same 2014 meta-analysis, 1141 participants with acute and chronic nonspecific low back pain in 10 studies had the equivalent of 1.3 points more pain relief with OMT compared with controls (MD= -12.91; 95% CI, -20.00 to -5.82). The authors used the standardized mean difference (SMD), which is the difference in means divided by the standard deviation, to interpret the magnitude of difference in function between participants who received OMT and those in the control groups. Further, 1046 participants with acute and chronic nonspecific low back pain in 9 studies had a small improvement in functional status using the Roland-Morris Disability Questionnaire (RMDQ) or Oswestry-Disability Index (SMD= -0.36; 95% CI, -0.58 to -0.14).6
A 2005 meta-analysis that evaluated 6 randomized controlled trials (RCTs) involving 549 patients with low back pain found that 318 patients who received OMT had significantly less low back pain compared with 231 controls (effect size= -0.30; 95% CI, -0.47 to -0.13; P=.001).7 Although significant, an effect size of this magnitude is characterized as small.8
Other benefits of OMT include increased patient satisfaction, fewer meds
A randomized double-blind, sham-controlled study involving 455 patients with chronic low back pain compared outcomes of OMT to sham OMT applied in 6 treatment sessions over 8 weeks.9 Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at Week 12 (≥30% and ≥50% pain reductions from baseline, respectively). Based on the Cochrane Back Review Group criteria for effect sizes, response ratios were calculated to determine if the differences seen were considered clinically relevant.10
Patients receiving OMT were more likely to achieve moderate (response ratio=1.38; 95% CI, 1.16-1.64; P<.001) and substantial (response ratio=1.41; 95% CI, 1.13-1.76; P=.002) improvements in low back pain at Week 12. The calculated number needed to treat (NNT) for moderate and significant improvement in pain at 12 weeks was 6 and 7, respectively. In addition, patients in the OMT group were more likely to be very satisfied with their care (P<.001) with an NNT of 5, and used fewer medications than did patients in the sham group during the 12 weeks of the study (use ratio=0.66; 95% CI, 0.43-1.00; P=.048; NNT=15).9
Pregnant women may benefit from OMT in the third trimester
A 2013 RCT involving 144 patients randomized to OMT, sham ultrasound, or usual obstetric care found that 68 patients (47%) experienced back-specific dysfunction during their third trimester of pregnancy (defined by a ≥2-point increase in the RMDQ).11
OMT reduced the risk of back-specific dysfunction by 40% vs the ultrasound group (relative risk [RR]=0.6; 95% CI, 0.3-1; P=.046) and 60% vs the usual obstetric care group (RR=0.4; 95% CI, 0.2-0.7; P<.001). The corresponding NNTs were 5.1 (95% CI, 2.7-282.2) for the OMT group vs the ultrasound group and 2.5 (95% CI, 1.8-4.9) vs the usual care group. The outcomes of this study were not conclusive because the initial RMDQ score was 1.8 points worse for the OMT group than for the usual care group.11
Subsequently, the PROMOTE (Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects) study involving 400 patients demonstrated that a standard OMT protocol was effective for decreasing pain and function deterioration compared with usual obstetric care.12 However, results from the OMT group did not differ significantly from those of the ultrasound group, which were labeled as subtherapeutic in the study.12
The most recent Cochrane Review on low back pain in pregnancy noted that there was moderate quality evidence (due to study design limitations or imprecision) that OMT significantly reduced low back pain and function disability.13
OMT for other conditions? The evidence is limited
To date, studies on conditions other than low back pain have not demonstrated the same robust improvements in design as have those concerning low back pain (ie, larger sample sizes, comparisons to usual care and other treatments, etc.), and available data are not sufficiently significant to compel a change in clinical practice. Despite this, patients seek out, and receive, OMT as an alternative or adjunctive treatment for many conditions other than low back pain,2 and family physicians should be aware of the current evidence for OMT in those conditions.
OMT for acute neck pain: A comparison with ketorolac
Researchers randomized 58 patients presenting to 3 emergency departments with neck pain of less than 3 weeks’ duration to receive either OMT or 30 mg IM ketorolac.14 OMT techniques were provided at the discretion of the physician based on patient needs. Patients rated their pain intensity on an 11-point numerical scale at the time of presentation and one hour after treatment. Patients receiving ketorolac or OMT had significant reductions in pain intensity with improvements of 1.7 +/- 1.6 (95% CI, 1.1-2.3; P<.001) and 2.8 +/- 1.7 (95% CI, 2.1-3.4; P<.001), respectively.
Although the pain reduction changes were statistically significant in both groups, the improvements were small enough to question if they were functionally significant. Compared to those receiving ketorolac, those receiving OMT reported a significantly greater decrease in their pain intensity (2.8 vs 1.7; 95% CI, 0.2-1.9; P=.02), but it’s worth noting that the dose of ketorolac was half the recommended dose for moderate or severe pain.14
Patients may have more headache-free days with OMT
To assess the use of OMT to treat chronic migraine, researchers conducted a prospective, single-blind RCT in which 105 chronic migraine sufferers (average of 22.5 migraine days/month) were split into 3 treatment groups: OMT plus medications, sham OMT plus medications, and medications alone.15
OMT led to fewer days with migraines compared with the medication group (MD= -21.06; 95% CI, -23.19 to -18.92; P<.001) and sham OMT group (MD= -17.43; 95% CI, -19.57 to -15.29; P<.001), resulting in less functional disability (P<.001).15 Caution should be taken in interpreting the results of this small trial, however, as an effect of this size has not been replicated in other studies.
A small (N=29) single-blind RCT looked at progressive muscular relaxation with and without OMT for the treatment of tension headache. Patients who completed relaxation exercises plus 3 sessions of OMT experienced significantly more headache-free days (1.79 vs 0.21; P=.016).16 Despite this finding, headache intensity and headache diary ratings were not different between the 2 groups in this study.
Postoperative OMT may decrease length of stay
In a retrospective study evaluating the effect of OMT on postoperative outcomes in 55 patients who underwent gastrointestinal surgery, a total of 17 patients who received a single OMT session within 48 hours of surgery had a mean time to flatus of 3.1 days compared with 4.7 days in the usual care control group (P=.035).17 The mean length of stay was 6.1 days in the OMT group and 11.5 days in the non-OMT group (P=.006).
Major limitations of this study include that it was retrospective in design and that only 17 of 55 patients had OMT performed, indicating a possible selection bias.
Pneumonia: OMT may reduce LOS and duration of antibiotic usage
The Multicenter Osteopathic Pneumonia Study in the Elderly (MOPSE), a double-blind RCT, looked at 406 patients ≥50 years hospitalized with pneumonia. Researchers randomized the group to receive either conventional care (CC; antibiotic treatment only), OMT and antibiotic therapy, or light-touch sham therapy with antibiotics.18 The researchers found no significant differences between the groups for any outcomes in the intention-to-treat analysis.
In results obtained from the per protocol analysis, however, the median length of stay for those in the OMT group was 3.5 days, compared with 4.5 days for those in the CC group (95% CI, 3.2-4.0; P=.01). Multiple comparisons also indicated a reduction in mean duration of intravenous antibiotic use of 3 days in the OMT group (95% CI, 2.7-3.5) vs 3.5 days in the CC group (95% CI, 3.2-3.9). The treatment end-points of either death or respiratory failure occurred significantly less frequently in the OMT group compared with the CC group (P=.006).18
A Cochrane review of RCTs assessing the efficacy of adjunctive techniques compared with conventional therapy for patients with pneumonia revealed a reduction in hospital stay of 2 days (95% CI, -3.5 to -0.6) for patients who received OMT and positive expiratory pressure vs those who received neither intervention.19 Additionally, the duration of IV antibiotics and total duration of all (IV and oral) antibiotic treatment required in those treated adjunctively with OMT was shorter (MD for IV antibiotics= -2.1 days; 95% CI, -3.4 to -0.9 and MD for all antibiotics= -1.9 days; 95% CI, -3.1 to -0.7).19 The review was notable for a small sample size, with only 79 patients assessed.
OMT may improve IBS symptoms
A crossover study of 31 patients that compared visceral manipulation and sacral articulation OMT with sham therapy for the treatment of irritable bowel syndrome (IBS) demonstrated that OMT significantly decreased self-reported diarrhea (P=.016), abdominal distention (P=.043), abdominal pain (P=.013), and rectal sensitivity (P<.001), but did not significantly affect constipation.20
In another study, researchers randomized 30 patients with IBS in a 2:1 distribution to OMT vs sham treatment.21 OMT included abdominal visceral techniques and direct and indirect spine techniques. All of the patients received 2 treatment sessions, and the researchers evaluated them at 7 and 28 days. At 7 days, both groups demonstrated a significant reduction in IBS symptoms, although the OMT group had significantly greater improvement (P=.01). At 28 days, however, neither group showed a significant reduction in symptoms.21
The lack of a control group (in the first study due to the crossover design), small sample sizes, and self-reported symptoms are major limitations to applying these studies to IBS treatment recommendations.
CORRESPONDENCE
Andrew H. Slattengren, DO, Broadway Family Medicine Clinic, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
1. American Association of Colleges of Osteopathic Medicine. What is osteopathic medicine? Available at: https://www.aacom.org/become-a-doctor/about-om. Accessed July 10, 2017.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19361005. Accessed November 10, 2015.
3. American Osteopathic Association. What is osteopathic medicine? Available at: http://www.osteopathic.org/osteopathic-health/Pages/what-is-osteopathic-medicine.aspx. Accessed November 17, 2017.
4. Licciardone JC, Russo DP. Blinding Protocols, Treatment Credibility, and Expectancy: Methodologic Issues in Clinical Trials of Osteopathic Manipulative Treatment. J Am Osteopath Assoc. 2006;106:457-463.
5. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
6. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286.
7. Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43.
8. Cohen J. Statistical Power Analysis for the Behavioral Sciences.82nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988.
9. Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11:122-129.
10. Furlan AD, Pennick V, Bombardier C, et al, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929-1941.
11. Licciardone JC, Aryal S. Prevention of progressive back-specific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113:728-736.
12. Hensel KL, Buchanan S, Brown SK, et al. Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9.
13. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139.
14. McReynolds TM, Sheridan BJ. Intramuscular ketorolac versus osteopathic manipulative treatment in the management of acute neck pain in the emergency department: a randomized clinical trial. J Am Osteopath Assoc. 2005;105:57-68.
15. Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149-156.
16. Anderson RE, Seniscal C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache. 2006;46:1273-1280.
17. Baltazar GA, Betler MP, Akella K, et al. Effect of osteopathic manipulative treatment on incidence of postoperative ileus and hospital length of stay in general surgical patients. J Am Osteopath Assoc. 2013;113:204-209.
18. Noll DR, Degenhardt BF, Morley TF, et al. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2.
19. Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev. 2013;2:CD006338.
20. Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis. 2013;14:654-661.
21. Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol. 2012;24:944-949.
Interest in osteopathy continues to rise in this country. Currently, more than 20% of medical students in the United States are training to be osteopathic physicians.1 In addition, the 2007 National Health Interview Survey found that spinal manipulation was among the most common complementary and alternative medicine (CAM) therapies used; with 8.6% of US adults reporting that they used it within the previous 12 months.2
With the growing number of DOs and the high utilization of osteopathic manipulative treatment (OMT), it is important for all physicians to understand the role OMT can play in the treatment of conditions ranging from low back pain to irritable bowel syndrome so that patients may be offered, or referred for, the treatment when appropriate.
To clarify when OMT may be most beneficial, we performed a literature review. Our findings are summarized here. But first, a word about osteopathic medicine and what OMT entails.
Osteopathic physicians view the body as a whole
According to the American Osteopathic Association, “the osteopathic philosophy of medicine sees an interrelated unity in all systems of the body, with each working with the other to heal in times of illness."3 This “whole-person approach to medicine” focuses on looking beyond symptoms alone to understand how lifestyle and environmental factors impact well-being.
As part of their education, DOs receive special training in the musculoskeletal system and in OMT. OMT is the process by which DOs use their hands to diagnose illness and injury and then mobilize a patient’s joints and soft tissues using techniques that include muscle activation, stretching, joint articulation, and gentle pressure to encourage the body’s natural tendency to heal itself.
These patients with low back pain will likely benefit
In the past, studies with small sample sizes, blinding issues, differing controls, and subjective outcome measurements have marred research efforts to demonstrate the effectiveness of OMT. More recently, researchers have attempted to minimize these issues, particularly when evaluating the efficacy of OMT for low back pain.
In addition to increasing sample size, studies have compared OMT to usual care, to sham manipulation, and more recently to other manual modalities including ultrasound to equalize the subjective effects of interventions.4 With improved study designs, there has been increased awareness of the effectiveness of spinal manipulation by organizations that develop guidelines for the care of patients with low back pain. The most recent clinical practice guideline from the American College of Physicians includes spinal manipulation as a treatment modality that should be considered by clinicians for patients who have acute, subacute, or chronic low back pain.5
Chronic nonspecific low back pain. Looking at OMT vs other interventions for chronic nonspecific low back pain, a 2014 meta-analysis found moderate quality evidence for clinically relevant effects of OMT on low back pain and function. In 6 studies that evaluated 769 patients with chronic nonspecific low back pain, there was a significant difference in pain—equivalent to a 1.5-point improvement (mean difference [MD]= -14.93; 95% confidence interval [CI], -25.18
Acute and chronic nonspecific low back pain. Similarly, in the same 2014 meta-analysis, 1141 participants with acute and chronic nonspecific low back pain in 10 studies had the equivalent of 1.3 points more pain relief with OMT compared with controls (MD= -12.91; 95% CI, -20.00 to -5.82). The authors used the standardized mean difference (SMD), which is the difference in means divided by the standard deviation, to interpret the magnitude of difference in function between participants who received OMT and those in the control groups. Further, 1046 participants with acute and chronic nonspecific low back pain in 9 studies had a small improvement in functional status using the Roland-Morris Disability Questionnaire (RMDQ) or Oswestry-Disability Index (SMD= -0.36; 95% CI, -0.58 to -0.14).6
A 2005 meta-analysis that evaluated 6 randomized controlled trials (RCTs) involving 549 patients with low back pain found that 318 patients who received OMT had significantly less low back pain compared with 231 controls (effect size= -0.30; 95% CI, -0.47 to -0.13; P=.001).7 Although significant, an effect size of this magnitude is characterized as small.8
Other benefits of OMT include increased patient satisfaction, fewer meds
A randomized double-blind, sham-controlled study involving 455 patients with chronic low back pain compared outcomes of OMT to sham OMT applied in 6 treatment sessions over 8 weeks.9 Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at Week 12 (≥30% and ≥50% pain reductions from baseline, respectively). Based on the Cochrane Back Review Group criteria for effect sizes, response ratios were calculated to determine if the differences seen were considered clinically relevant.10
Patients receiving OMT were more likely to achieve moderate (response ratio=1.38; 95% CI, 1.16-1.64; P<.001) and substantial (response ratio=1.41; 95% CI, 1.13-1.76; P=.002) improvements in low back pain at Week 12. The calculated number needed to treat (NNT) for moderate and significant improvement in pain at 12 weeks was 6 and 7, respectively. In addition, patients in the OMT group were more likely to be very satisfied with their care (P<.001) with an NNT of 5, and used fewer medications than did patients in the sham group during the 12 weeks of the study (use ratio=0.66; 95% CI, 0.43-1.00; P=.048; NNT=15).9
Pregnant women may benefit from OMT in the third trimester
A 2013 RCT involving 144 patients randomized to OMT, sham ultrasound, or usual obstetric care found that 68 patients (47%) experienced back-specific dysfunction during their third trimester of pregnancy (defined by a ≥2-point increase in the RMDQ).11
OMT reduced the risk of back-specific dysfunction by 40% vs the ultrasound group (relative risk [RR]=0.6; 95% CI, 0.3-1; P=.046) and 60% vs the usual obstetric care group (RR=0.4; 95% CI, 0.2-0.7; P<.001). The corresponding NNTs were 5.1 (95% CI, 2.7-282.2) for the OMT group vs the ultrasound group and 2.5 (95% CI, 1.8-4.9) vs the usual care group. The outcomes of this study were not conclusive because the initial RMDQ score was 1.8 points worse for the OMT group than for the usual care group.11
Subsequently, the PROMOTE (Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects) study involving 400 patients demonstrated that a standard OMT protocol was effective for decreasing pain and function deterioration compared with usual obstetric care.12 However, results from the OMT group did not differ significantly from those of the ultrasound group, which were labeled as subtherapeutic in the study.12
The most recent Cochrane Review on low back pain in pregnancy noted that there was moderate quality evidence (due to study design limitations or imprecision) that OMT significantly reduced low back pain and function disability.13
OMT for other conditions? The evidence is limited
To date, studies on conditions other than low back pain have not demonstrated the same robust improvements in design as have those concerning low back pain (ie, larger sample sizes, comparisons to usual care and other treatments, etc.), and available data are not sufficiently significant to compel a change in clinical practice. Despite this, patients seek out, and receive, OMT as an alternative or adjunctive treatment for many conditions other than low back pain,2 and family physicians should be aware of the current evidence for OMT in those conditions.
OMT for acute neck pain: A comparison with ketorolac
Researchers randomized 58 patients presenting to 3 emergency departments with neck pain of less than 3 weeks’ duration to receive either OMT or 30 mg IM ketorolac.14 OMT techniques were provided at the discretion of the physician based on patient needs. Patients rated their pain intensity on an 11-point numerical scale at the time of presentation and one hour after treatment. Patients receiving ketorolac or OMT had significant reductions in pain intensity with improvements of 1.7 +/- 1.6 (95% CI, 1.1-2.3; P<.001) and 2.8 +/- 1.7 (95% CI, 2.1-3.4; P<.001), respectively.
Although the pain reduction changes were statistically significant in both groups, the improvements were small enough to question if they were functionally significant. Compared to those receiving ketorolac, those receiving OMT reported a significantly greater decrease in their pain intensity (2.8 vs 1.7; 95% CI, 0.2-1.9; P=.02), but it’s worth noting that the dose of ketorolac was half the recommended dose for moderate or severe pain.14
Patients may have more headache-free days with OMT
To assess the use of OMT to treat chronic migraine, researchers conducted a prospective, single-blind RCT in which 105 chronic migraine sufferers (average of 22.5 migraine days/month) were split into 3 treatment groups: OMT plus medications, sham OMT plus medications, and medications alone.15
OMT led to fewer days with migraines compared with the medication group (MD= -21.06; 95% CI, -23.19 to -18.92; P<.001) and sham OMT group (MD= -17.43; 95% CI, -19.57 to -15.29; P<.001), resulting in less functional disability (P<.001).15 Caution should be taken in interpreting the results of this small trial, however, as an effect of this size has not been replicated in other studies.
A small (N=29) single-blind RCT looked at progressive muscular relaxation with and without OMT for the treatment of tension headache. Patients who completed relaxation exercises plus 3 sessions of OMT experienced significantly more headache-free days (1.79 vs 0.21; P=.016).16 Despite this finding, headache intensity and headache diary ratings were not different between the 2 groups in this study.
Postoperative OMT may decrease length of stay
In a retrospective study evaluating the effect of OMT on postoperative outcomes in 55 patients who underwent gastrointestinal surgery, a total of 17 patients who received a single OMT session within 48 hours of surgery had a mean time to flatus of 3.1 days compared with 4.7 days in the usual care control group (P=.035).17 The mean length of stay was 6.1 days in the OMT group and 11.5 days in the non-OMT group (P=.006).
Major limitations of this study include that it was retrospective in design and that only 17 of 55 patients had OMT performed, indicating a possible selection bias.
Pneumonia: OMT may reduce LOS and duration of antibiotic usage
The Multicenter Osteopathic Pneumonia Study in the Elderly (MOPSE), a double-blind RCT, looked at 406 patients ≥50 years hospitalized with pneumonia. Researchers randomized the group to receive either conventional care (CC; antibiotic treatment only), OMT and antibiotic therapy, or light-touch sham therapy with antibiotics.18 The researchers found no significant differences between the groups for any outcomes in the intention-to-treat analysis.
In results obtained from the per protocol analysis, however, the median length of stay for those in the OMT group was 3.5 days, compared with 4.5 days for those in the CC group (95% CI, 3.2-4.0; P=.01). Multiple comparisons also indicated a reduction in mean duration of intravenous antibiotic use of 3 days in the OMT group (95% CI, 2.7-3.5) vs 3.5 days in the CC group (95% CI, 3.2-3.9). The treatment end-points of either death or respiratory failure occurred significantly less frequently in the OMT group compared with the CC group (P=.006).18
A Cochrane review of RCTs assessing the efficacy of adjunctive techniques compared with conventional therapy for patients with pneumonia revealed a reduction in hospital stay of 2 days (95% CI, -3.5 to -0.6) for patients who received OMT and positive expiratory pressure vs those who received neither intervention.19 Additionally, the duration of IV antibiotics and total duration of all (IV and oral) antibiotic treatment required in those treated adjunctively with OMT was shorter (MD for IV antibiotics= -2.1 days; 95% CI, -3.4 to -0.9 and MD for all antibiotics= -1.9 days; 95% CI, -3.1 to -0.7).19 The review was notable for a small sample size, with only 79 patients assessed.
OMT may improve IBS symptoms
A crossover study of 31 patients that compared visceral manipulation and sacral articulation OMT with sham therapy for the treatment of irritable bowel syndrome (IBS) demonstrated that OMT significantly decreased self-reported diarrhea (P=.016), abdominal distention (P=.043), abdominal pain (P=.013), and rectal sensitivity (P<.001), but did not significantly affect constipation.20
In another study, researchers randomized 30 patients with IBS in a 2:1 distribution to OMT vs sham treatment.21 OMT included abdominal visceral techniques and direct and indirect spine techniques. All of the patients received 2 treatment sessions, and the researchers evaluated them at 7 and 28 days. At 7 days, both groups demonstrated a significant reduction in IBS symptoms, although the OMT group had significantly greater improvement (P=.01). At 28 days, however, neither group showed a significant reduction in symptoms.21
The lack of a control group (in the first study due to the crossover design), small sample sizes, and self-reported symptoms are major limitations to applying these studies to IBS treatment recommendations.
CORRESPONDENCE
Andrew H. Slattengren, DO, Broadway Family Medicine Clinic, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
Interest in osteopathy continues to rise in this country. Currently, more than 20% of medical students in the United States are training to be osteopathic physicians.1 In addition, the 2007 National Health Interview Survey found that spinal manipulation was among the most common complementary and alternative medicine (CAM) therapies used; with 8.6% of US adults reporting that they used it within the previous 12 months.2
With the growing number of DOs and the high utilization of osteopathic manipulative treatment (OMT), it is important for all physicians to understand the role OMT can play in the treatment of conditions ranging from low back pain to irritable bowel syndrome so that patients may be offered, or referred for, the treatment when appropriate.
To clarify when OMT may be most beneficial, we performed a literature review. Our findings are summarized here. But first, a word about osteopathic medicine and what OMT entails.
Osteopathic physicians view the body as a whole
According to the American Osteopathic Association, “the osteopathic philosophy of medicine sees an interrelated unity in all systems of the body, with each working with the other to heal in times of illness."3 This “whole-person approach to medicine” focuses on looking beyond symptoms alone to understand how lifestyle and environmental factors impact well-being.
As part of their education, DOs receive special training in the musculoskeletal system and in OMT. OMT is the process by which DOs use their hands to diagnose illness and injury and then mobilize a patient’s joints and soft tissues using techniques that include muscle activation, stretching, joint articulation, and gentle pressure to encourage the body’s natural tendency to heal itself.
These patients with low back pain will likely benefit
In the past, studies with small sample sizes, blinding issues, differing controls, and subjective outcome measurements have marred research efforts to demonstrate the effectiveness of OMT. More recently, researchers have attempted to minimize these issues, particularly when evaluating the efficacy of OMT for low back pain.
In addition to increasing sample size, studies have compared OMT to usual care, to sham manipulation, and more recently to other manual modalities including ultrasound to equalize the subjective effects of interventions.4 With improved study designs, there has been increased awareness of the effectiveness of spinal manipulation by organizations that develop guidelines for the care of patients with low back pain. The most recent clinical practice guideline from the American College of Physicians includes spinal manipulation as a treatment modality that should be considered by clinicians for patients who have acute, subacute, or chronic low back pain.5
Chronic nonspecific low back pain. Looking at OMT vs other interventions for chronic nonspecific low back pain, a 2014 meta-analysis found moderate quality evidence for clinically relevant effects of OMT on low back pain and function. In 6 studies that evaluated 769 patients with chronic nonspecific low back pain, there was a significant difference in pain—equivalent to a 1.5-point improvement (mean difference [MD]= -14.93; 95% confidence interval [CI], -25.18
Acute and chronic nonspecific low back pain. Similarly, in the same 2014 meta-analysis, 1141 participants with acute and chronic nonspecific low back pain in 10 studies had the equivalent of 1.3 points more pain relief with OMT compared with controls (MD= -12.91; 95% CI, -20.00 to -5.82). The authors used the standardized mean difference (SMD), which is the difference in means divided by the standard deviation, to interpret the magnitude of difference in function between participants who received OMT and those in the control groups. Further, 1046 participants with acute and chronic nonspecific low back pain in 9 studies had a small improvement in functional status using the Roland-Morris Disability Questionnaire (RMDQ) or Oswestry-Disability Index (SMD= -0.36; 95% CI, -0.58 to -0.14).6
A 2005 meta-analysis that evaluated 6 randomized controlled trials (RCTs) involving 549 patients with low back pain found that 318 patients who received OMT had significantly less low back pain compared with 231 controls (effect size= -0.30; 95% CI, -0.47 to -0.13; P=.001).7 Although significant, an effect size of this magnitude is characterized as small.8
Other benefits of OMT include increased patient satisfaction, fewer meds
A randomized double-blind, sham-controlled study involving 455 patients with chronic low back pain compared outcomes of OMT to sham OMT applied in 6 treatment sessions over 8 weeks.9 Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at Week 12 (≥30% and ≥50% pain reductions from baseline, respectively). Based on the Cochrane Back Review Group criteria for effect sizes, response ratios were calculated to determine if the differences seen were considered clinically relevant.10
Patients receiving OMT were more likely to achieve moderate (response ratio=1.38; 95% CI, 1.16-1.64; P<.001) and substantial (response ratio=1.41; 95% CI, 1.13-1.76; P=.002) improvements in low back pain at Week 12. The calculated number needed to treat (NNT) for moderate and significant improvement in pain at 12 weeks was 6 and 7, respectively. In addition, patients in the OMT group were more likely to be very satisfied with their care (P<.001) with an NNT of 5, and used fewer medications than did patients in the sham group during the 12 weeks of the study (use ratio=0.66; 95% CI, 0.43-1.00; P=.048; NNT=15).9
Pregnant women may benefit from OMT in the third trimester
A 2013 RCT involving 144 patients randomized to OMT, sham ultrasound, or usual obstetric care found that 68 patients (47%) experienced back-specific dysfunction during their third trimester of pregnancy (defined by a ≥2-point increase in the RMDQ).11
OMT reduced the risk of back-specific dysfunction by 40% vs the ultrasound group (relative risk [RR]=0.6; 95% CI, 0.3-1; P=.046) and 60% vs the usual obstetric care group (RR=0.4; 95% CI, 0.2-0.7; P<.001). The corresponding NNTs were 5.1 (95% CI, 2.7-282.2) for the OMT group vs the ultrasound group and 2.5 (95% CI, 1.8-4.9) vs the usual care group. The outcomes of this study were not conclusive because the initial RMDQ score was 1.8 points worse for the OMT group than for the usual care group.11
Subsequently, the PROMOTE (Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects) study involving 400 patients demonstrated that a standard OMT protocol was effective for decreasing pain and function deterioration compared with usual obstetric care.12 However, results from the OMT group did not differ significantly from those of the ultrasound group, which were labeled as subtherapeutic in the study.12
The most recent Cochrane Review on low back pain in pregnancy noted that there was moderate quality evidence (due to study design limitations or imprecision) that OMT significantly reduced low back pain and function disability.13
OMT for other conditions? The evidence is limited
To date, studies on conditions other than low back pain have not demonstrated the same robust improvements in design as have those concerning low back pain (ie, larger sample sizes, comparisons to usual care and other treatments, etc.), and available data are not sufficiently significant to compel a change in clinical practice. Despite this, patients seek out, and receive, OMT as an alternative or adjunctive treatment for many conditions other than low back pain,2 and family physicians should be aware of the current evidence for OMT in those conditions.
OMT for acute neck pain: A comparison with ketorolac
Researchers randomized 58 patients presenting to 3 emergency departments with neck pain of less than 3 weeks’ duration to receive either OMT or 30 mg IM ketorolac.14 OMT techniques were provided at the discretion of the physician based on patient needs. Patients rated their pain intensity on an 11-point numerical scale at the time of presentation and one hour after treatment. Patients receiving ketorolac or OMT had significant reductions in pain intensity with improvements of 1.7 +/- 1.6 (95% CI, 1.1-2.3; P<.001) and 2.8 +/- 1.7 (95% CI, 2.1-3.4; P<.001), respectively.
Although the pain reduction changes were statistically significant in both groups, the improvements were small enough to question if they were functionally significant. Compared to those receiving ketorolac, those receiving OMT reported a significantly greater decrease in their pain intensity (2.8 vs 1.7; 95% CI, 0.2-1.9; P=.02), but it’s worth noting that the dose of ketorolac was half the recommended dose for moderate or severe pain.14
Patients may have more headache-free days with OMT
To assess the use of OMT to treat chronic migraine, researchers conducted a prospective, single-blind RCT in which 105 chronic migraine sufferers (average of 22.5 migraine days/month) were split into 3 treatment groups: OMT plus medications, sham OMT plus medications, and medications alone.15
OMT led to fewer days with migraines compared with the medication group (MD= -21.06; 95% CI, -23.19 to -18.92; P<.001) and sham OMT group (MD= -17.43; 95% CI, -19.57 to -15.29; P<.001), resulting in less functional disability (P<.001).15 Caution should be taken in interpreting the results of this small trial, however, as an effect of this size has not been replicated in other studies.
A small (N=29) single-blind RCT looked at progressive muscular relaxation with and without OMT for the treatment of tension headache. Patients who completed relaxation exercises plus 3 sessions of OMT experienced significantly more headache-free days (1.79 vs 0.21; P=.016).16 Despite this finding, headache intensity and headache diary ratings were not different between the 2 groups in this study.
Postoperative OMT may decrease length of stay
In a retrospective study evaluating the effect of OMT on postoperative outcomes in 55 patients who underwent gastrointestinal surgery, a total of 17 patients who received a single OMT session within 48 hours of surgery had a mean time to flatus of 3.1 days compared with 4.7 days in the usual care control group (P=.035).17 The mean length of stay was 6.1 days in the OMT group and 11.5 days in the non-OMT group (P=.006).
Major limitations of this study include that it was retrospective in design and that only 17 of 55 patients had OMT performed, indicating a possible selection bias.
Pneumonia: OMT may reduce LOS and duration of antibiotic usage
The Multicenter Osteopathic Pneumonia Study in the Elderly (MOPSE), a double-blind RCT, looked at 406 patients ≥50 years hospitalized with pneumonia. Researchers randomized the group to receive either conventional care (CC; antibiotic treatment only), OMT and antibiotic therapy, or light-touch sham therapy with antibiotics.18 The researchers found no significant differences between the groups for any outcomes in the intention-to-treat analysis.
In results obtained from the per protocol analysis, however, the median length of stay for those in the OMT group was 3.5 days, compared with 4.5 days for those in the CC group (95% CI, 3.2-4.0; P=.01). Multiple comparisons also indicated a reduction in mean duration of intravenous antibiotic use of 3 days in the OMT group (95% CI, 2.7-3.5) vs 3.5 days in the CC group (95% CI, 3.2-3.9). The treatment end-points of either death or respiratory failure occurred significantly less frequently in the OMT group compared with the CC group (P=.006).18
A Cochrane review of RCTs assessing the efficacy of adjunctive techniques compared with conventional therapy for patients with pneumonia revealed a reduction in hospital stay of 2 days (95% CI, -3.5 to -0.6) for patients who received OMT and positive expiratory pressure vs those who received neither intervention.19 Additionally, the duration of IV antibiotics and total duration of all (IV and oral) antibiotic treatment required in those treated adjunctively with OMT was shorter (MD for IV antibiotics= -2.1 days; 95% CI, -3.4 to -0.9 and MD for all antibiotics= -1.9 days; 95% CI, -3.1 to -0.7).19 The review was notable for a small sample size, with only 79 patients assessed.
OMT may improve IBS symptoms
A crossover study of 31 patients that compared visceral manipulation and sacral articulation OMT with sham therapy for the treatment of irritable bowel syndrome (IBS) demonstrated that OMT significantly decreased self-reported diarrhea (P=.016), abdominal distention (P=.043), abdominal pain (P=.013), and rectal sensitivity (P<.001), but did not significantly affect constipation.20
In another study, researchers randomized 30 patients with IBS in a 2:1 distribution to OMT vs sham treatment.21 OMT included abdominal visceral techniques and direct and indirect spine techniques. All of the patients received 2 treatment sessions, and the researchers evaluated them at 7 and 28 days. At 7 days, both groups demonstrated a significant reduction in IBS symptoms, although the OMT group had significantly greater improvement (P=.01). At 28 days, however, neither group showed a significant reduction in symptoms.21
The lack of a control group (in the first study due to the crossover design), small sample sizes, and self-reported symptoms are major limitations to applying these studies to IBS treatment recommendations.
CORRESPONDENCE
Andrew H. Slattengren, DO, Broadway Family Medicine Clinic, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
1. American Association of Colleges of Osteopathic Medicine. What is osteopathic medicine? Available at: https://www.aacom.org/become-a-doctor/about-om. Accessed July 10, 2017.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19361005. Accessed November 10, 2015.
3. American Osteopathic Association. What is osteopathic medicine? Available at: http://www.osteopathic.org/osteopathic-health/Pages/what-is-osteopathic-medicine.aspx. Accessed November 17, 2017.
4. Licciardone JC, Russo DP. Blinding Protocols, Treatment Credibility, and Expectancy: Methodologic Issues in Clinical Trials of Osteopathic Manipulative Treatment. J Am Osteopath Assoc. 2006;106:457-463.
5. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
6. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286.
7. Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43.
8. Cohen J. Statistical Power Analysis for the Behavioral Sciences.82nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988.
9. Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11:122-129.
10. Furlan AD, Pennick V, Bombardier C, et al, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929-1941.
11. Licciardone JC, Aryal S. Prevention of progressive back-specific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113:728-736.
12. Hensel KL, Buchanan S, Brown SK, et al. Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9.
13. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139.
14. McReynolds TM, Sheridan BJ. Intramuscular ketorolac versus osteopathic manipulative treatment in the management of acute neck pain in the emergency department: a randomized clinical trial. J Am Osteopath Assoc. 2005;105:57-68.
15. Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149-156.
16. Anderson RE, Seniscal C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache. 2006;46:1273-1280.
17. Baltazar GA, Betler MP, Akella K, et al. Effect of osteopathic manipulative treatment on incidence of postoperative ileus and hospital length of stay in general surgical patients. J Am Osteopath Assoc. 2013;113:204-209.
18. Noll DR, Degenhardt BF, Morley TF, et al. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2.
19. Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev. 2013;2:CD006338.
20. Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis. 2013;14:654-661.
21. Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol. 2012;24:944-949.
1. American Association of Colleges of Osteopathic Medicine. What is osteopathic medicine? Available at: https://www.aacom.org/become-a-doctor/about-om. Accessed July 10, 2017.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19361005. Accessed November 10, 2015.
3. American Osteopathic Association. What is osteopathic medicine? Available at: http://www.osteopathic.org/osteopathic-health/Pages/what-is-osteopathic-medicine.aspx. Accessed November 17, 2017.
4. Licciardone JC, Russo DP. Blinding Protocols, Treatment Credibility, and Expectancy: Methodologic Issues in Clinical Trials of Osteopathic Manipulative Treatment. J Am Osteopath Assoc. 2006;106:457-463.
5. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
6. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286.
7. Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43.
8. Cohen J. Statistical Power Analysis for the Behavioral Sciences.82nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988.
9. Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11:122-129.
10. Furlan AD, Pennick V, Bombardier C, et al, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929-1941.
11. Licciardone JC, Aryal S. Prevention of progressive back-specific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113:728-736.
12. Hensel KL, Buchanan S, Brown SK, et al. Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9.
13. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139.
14. McReynolds TM, Sheridan BJ. Intramuscular ketorolac versus osteopathic manipulative treatment in the management of acute neck pain in the emergency department: a randomized clinical trial. J Am Osteopath Assoc. 2005;105:57-68.
15. Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149-156.
16. Anderson RE, Seniscal C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache. 2006;46:1273-1280.
17. Baltazar GA, Betler MP, Akella K, et al. Effect of osteopathic manipulative treatment on incidence of postoperative ileus and hospital length of stay in general surgical patients. J Am Osteopath Assoc. 2013;113:204-209.
18. Noll DR, Degenhardt BF, Morley TF, et al. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2.
19. Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev. 2013;2:CD006338.
20. Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis. 2013;14:654-661.
21. Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol. 2012;24:944-949.
PRACTICE RECOMMENDATIONS
› Recommend osteopathic manipulative treatment to your patients with low back pain, as those who receive OMT have decreased pain, improved function, and use less medication. B
› Consider OMT as an adjunctive modality to decrease back-specific dysfunction in the third trimester of pregnancy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The evaluation and management of female sexual dysfunction
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11
Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11
Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11
Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
PRACTICE RECOMMENDATIONS
› Obtain a detailed history and evaluate obstetric, gynecologic, sexually transmitted disease, sexual abuse, urinary and bowel complaint, and surgical history in women of all ages. B
› Consider a variety of lifestyle and pharmacologic approaches, as well as biofeedback in combination with pelvic floor physical therapy, to address your female patient’s sexual dysfunction. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
December 2017: Click for Credit
Here are 5 articles in the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. When Is It Really Recurrent Strep Throat?
To take the posttest, go to: http://bit.ly/2lHFh8i
Expires September 21, 2018
2. Revised Bethesda System Resets Thyroid Malignancy Risks
To take the posttest, go to: http://bit.ly/2iSLOvM
Expires August 10, 2018
3. Tips for Avoiding Potentially Dangerous Patients
To take the posttest, go to: http://bit.ly/2lH1Fi7
Expires August 10, 2018
4. Study Findings Support Uncapping MELD Score
To take the posttest, go to: http://bit.ly/2xOA7sI
Expires September 12, 2018
5. 'Motivational Pharmacotherapy' Engages Latino Patients With Depression
To take the posttest, go to: http://bit.ly/2zs2ly4
Expires August 14, 2018
Here are 5 articles in the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. When Is It Really Recurrent Strep Throat?
To take the posttest, go to: http://bit.ly/2lHFh8i
Expires September 21, 2018
2. Revised Bethesda System Resets Thyroid Malignancy Risks
To take the posttest, go to: http://bit.ly/2iSLOvM
Expires August 10, 2018
3. Tips for Avoiding Potentially Dangerous Patients
To take the posttest, go to: http://bit.ly/2lH1Fi7
Expires August 10, 2018
4. Study Findings Support Uncapping MELD Score
To take the posttest, go to: http://bit.ly/2xOA7sI
Expires September 12, 2018
5. 'Motivational Pharmacotherapy' Engages Latino Patients With Depression
To take the posttest, go to: http://bit.ly/2zs2ly4
Expires August 14, 2018
Here are 5 articles in the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. When Is It Really Recurrent Strep Throat?
To take the posttest, go to: http://bit.ly/2lHFh8i
Expires September 21, 2018
2. Revised Bethesda System Resets Thyroid Malignancy Risks
To take the posttest, go to: http://bit.ly/2iSLOvM
Expires August 10, 2018
3. Tips for Avoiding Potentially Dangerous Patients
To take the posttest, go to: http://bit.ly/2lH1Fi7
Expires August 10, 2018
4. Study Findings Support Uncapping MELD Score
To take the posttest, go to: http://bit.ly/2xOA7sI
Expires September 12, 2018
5. 'Motivational Pharmacotherapy' Engages Latino Patients With Depression
To take the posttest, go to: http://bit.ly/2zs2ly4
Expires August 14, 2018
Letter from the Editor
This month we learned of the passing of Dr. Marv Sleisenger. There are few physicians who have had a greater impact on our field than Dr. Sleisenger. He was a consummate gentleman, enthusiastic teacher, great mentor, authored hundreds of research papers, and edited the most famous textbook of gastroenterology. Our thoughts and hearts are with his family and friends.
I would like to highlight our liver coverage. AASLD had their annual meeting in Washington in November. My colleague at University of Michigan (Anna Lok, MD) is the current president and helped spearhead a meeting that was packed with research and clinical information. We will be covering AASLD in greater depth in the months to come.
And while initial efforts to repeal the ACA have stalled, several key parts of the ACA continue to be modified or repealed either by Executive Orders or as part of the current tax reform efforts. We continue to view these efforts through the lens of our patients’ access to care.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This month we learned of the passing of Dr. Marv Sleisenger. There are few physicians who have had a greater impact on our field than Dr. Sleisenger. He was a consummate gentleman, enthusiastic teacher, great mentor, authored hundreds of research papers, and edited the most famous textbook of gastroenterology. Our thoughts and hearts are with his family and friends.
I would like to highlight our liver coverage. AASLD had their annual meeting in Washington in November. My colleague at University of Michigan (Anna Lok, MD) is the current president and helped spearhead a meeting that was packed with research and clinical information. We will be covering AASLD in greater depth in the months to come.
And while initial efforts to repeal the ACA have stalled, several key parts of the ACA continue to be modified or repealed either by Executive Orders or as part of the current tax reform efforts. We continue to view these efforts through the lens of our patients’ access to care.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This month we learned of the passing of Dr. Marv Sleisenger. There are few physicians who have had a greater impact on our field than Dr. Sleisenger. He was a consummate gentleman, enthusiastic teacher, great mentor, authored hundreds of research papers, and edited the most famous textbook of gastroenterology. Our thoughts and hearts are with his family and friends.
I would like to highlight our liver coverage. AASLD had their annual meeting in Washington in November. My colleague at University of Michigan (Anna Lok, MD) is the current president and helped spearhead a meeting that was packed with research and clinical information. We will be covering AASLD in greater depth in the months to come.
And while initial efforts to repeal the ACA have stalled, several key parts of the ACA continue to be modified or repealed either by Executive Orders or as part of the current tax reform efforts. We continue to view these efforts through the lens of our patients’ access to care.
John I. Allen, MD, MBA, AGAF
Editor in Chief