User login
Student Hospitalist Scholars: Discovering a passion for research
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
When I decided to leave the business world to pursue a career in medicine, I envisioned myself in a clinic or an operating room helping the people in my community with the knowledge and skills acquired in my medical training. The thought of becoming a researcher had never even crossed my mind.
I grew up in Scottsdale, Arizona, a city which has no major academic medical centers. Prior to entering medical school, I was enrolled in a postbaccalaureate program at Johns Hopkins University, where I took the basic science classes necessary to apply. I was quite surprised to learn that, even at this level of education, I was required to participate in a research project. This experience changed the way I envisioned my entire career as a physician.
I am now a fourth year medical student and a pioneer of the “new curriculum” at Weill Cornell Medical College. In contrast to the traditional medical school curriculum, Cornell carved out 6 months of protected research time for all medical students by condensing the preclinical curriculum from 2 years to 1.5 years. I learned how much I enjoyed research at Johns Hopkins, which is one of the main reasons I applied here.
Despite my interest in research, I still struggled with the ultimate career question: What kind of doctor do I want to be?
After completing my medicine clerkship, I remember feeling intellectually stimulated in a way I hadn’t experienced in the previous years. While this may have had to do with the subject matter, I attribute much of this feeling to my clerkship director whose passion for medicine and teaching was contagious. I ultimately chose Ernie Esquivel, MD, to be my research mentor because of how much he impacted my education.
Together we came up with a project to study the utility of bone biopsies in the management of osteomyelitis. We are doing this by analyzing changes from empiric to final antibiotics after bone biopsy results become available to determine how clinicians use this information to guide their management of the disease. We were also interested in analyzing predictors of positive bone cultures in this population. The success of this project will mostly be based on our ability to perform these analyses, regardless of what the results may be. We hypothesize that, in fact, bone biopsy results are not likely to have a significant impact on antibiotic management of osteomyelitis in nonvertebral bones.
I was one of the lucky few to be awarded a grant from the Society of Hospital Medicine, which will be instrumental in the success of the project. This grant will not only support my ongoing research efforts but will also afford me the opportunity to attend the annual SHM conference and become integrated into the medical community in a way that would otherwise never be possible.
Cole Hirschfeld is originally from Phoenix, Ariz. He received undergraduate degrees in finance and entrepreneurship from the University of Arizona and went on to work in the finance industry for 2 years before deciding to change careers and attend medical school. He is now a fourth year medical student at Weill Cornell Medical College and plans to apply for residency in internal medicine.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
When I decided to leave the business world to pursue a career in medicine, I envisioned myself in a clinic or an operating room helping the people in my community with the knowledge and skills acquired in my medical training. The thought of becoming a researcher had never even crossed my mind.
I grew up in Scottsdale, Arizona, a city which has no major academic medical centers. Prior to entering medical school, I was enrolled in a postbaccalaureate program at Johns Hopkins University, where I took the basic science classes necessary to apply. I was quite surprised to learn that, even at this level of education, I was required to participate in a research project. This experience changed the way I envisioned my entire career as a physician.
I am now a fourth year medical student and a pioneer of the “new curriculum” at Weill Cornell Medical College. In contrast to the traditional medical school curriculum, Cornell carved out 6 months of protected research time for all medical students by condensing the preclinical curriculum from 2 years to 1.5 years. I learned how much I enjoyed research at Johns Hopkins, which is one of the main reasons I applied here.
Despite my interest in research, I still struggled with the ultimate career question: What kind of doctor do I want to be?
After completing my medicine clerkship, I remember feeling intellectually stimulated in a way I hadn’t experienced in the previous years. While this may have had to do with the subject matter, I attribute much of this feeling to my clerkship director whose passion for medicine and teaching was contagious. I ultimately chose Ernie Esquivel, MD, to be my research mentor because of how much he impacted my education.
Together we came up with a project to study the utility of bone biopsies in the management of osteomyelitis. We are doing this by analyzing changes from empiric to final antibiotics after bone biopsy results become available to determine how clinicians use this information to guide their management of the disease. We were also interested in analyzing predictors of positive bone cultures in this population. The success of this project will mostly be based on our ability to perform these analyses, regardless of what the results may be. We hypothesize that, in fact, bone biopsy results are not likely to have a significant impact on antibiotic management of osteomyelitis in nonvertebral bones.
I was one of the lucky few to be awarded a grant from the Society of Hospital Medicine, which will be instrumental in the success of the project. This grant will not only support my ongoing research efforts but will also afford me the opportunity to attend the annual SHM conference and become integrated into the medical community in a way that would otherwise never be possible.
Cole Hirschfeld is originally from Phoenix, Ariz. He received undergraduate degrees in finance and entrepreneurship from the University of Arizona and went on to work in the finance industry for 2 years before deciding to change careers and attend medical school. He is now a fourth year medical student at Weill Cornell Medical College and plans to apply for residency in internal medicine.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
When I decided to leave the business world to pursue a career in medicine, I envisioned myself in a clinic or an operating room helping the people in my community with the knowledge and skills acquired in my medical training. The thought of becoming a researcher had never even crossed my mind.
I grew up in Scottsdale, Arizona, a city which has no major academic medical centers. Prior to entering medical school, I was enrolled in a postbaccalaureate program at Johns Hopkins University, where I took the basic science classes necessary to apply. I was quite surprised to learn that, even at this level of education, I was required to participate in a research project. This experience changed the way I envisioned my entire career as a physician.
I am now a fourth year medical student and a pioneer of the “new curriculum” at Weill Cornell Medical College. In contrast to the traditional medical school curriculum, Cornell carved out 6 months of protected research time for all medical students by condensing the preclinical curriculum from 2 years to 1.5 years. I learned how much I enjoyed research at Johns Hopkins, which is one of the main reasons I applied here.
Despite my interest in research, I still struggled with the ultimate career question: What kind of doctor do I want to be?
After completing my medicine clerkship, I remember feeling intellectually stimulated in a way I hadn’t experienced in the previous years. While this may have had to do with the subject matter, I attribute much of this feeling to my clerkship director whose passion for medicine and teaching was contagious. I ultimately chose Ernie Esquivel, MD, to be my research mentor because of how much he impacted my education.
Together we came up with a project to study the utility of bone biopsies in the management of osteomyelitis. We are doing this by analyzing changes from empiric to final antibiotics after bone biopsy results become available to determine how clinicians use this information to guide their management of the disease. We were also interested in analyzing predictors of positive bone cultures in this population. The success of this project will mostly be based on our ability to perform these analyses, regardless of what the results may be. We hypothesize that, in fact, bone biopsy results are not likely to have a significant impact on antibiotic management of osteomyelitis in nonvertebral bones.
I was one of the lucky few to be awarded a grant from the Society of Hospital Medicine, which will be instrumental in the success of the project. This grant will not only support my ongoing research efforts but will also afford me the opportunity to attend the annual SHM conference and become integrated into the medical community in a way that would otherwise never be possible.
Cole Hirschfeld is originally from Phoenix, Ariz. He received undergraduate degrees in finance and entrepreneurship from the University of Arizona and went on to work in the finance industry for 2 years before deciding to change careers and attend medical school. He is now a fourth year medical student at Weill Cornell Medical College and plans to apply for residency in internal medicine.
Less lenalidomide may be more in frail elderly multiple myeloma patients
In frail elderly patients with multiple myeloma, starting lenalidomide at low doses was associated with fewer adverse events and less treatment discontinuation, and did not compromise overall response rates, in a single-center, retrospective study conducted in Japan.
Although most of the 56 study patients received 5-10 mg/day of lenalidomide, not the recommended 25-mg/day dose, their overall response rate was 73% (complete response in 17% of patients, very good partial response in 19%, and partial response in 37%), Aya Nakaya, MD, PhD, of Kansai Medical University, Hirakata, and colleagues wrote (Acta Haematol. 2017;138:55-60). In addition, 23% of patients had stable disease and 4% had disease progression. Nine patients developed other types of malignancies during treatment with lenalidomide.
Starting patients on a reduced dose and increasing it gradually while monitoring carefully for adverse events meant that patients did not have to stop treatment, the researchers said. Continuous treatment may improve survival, and “treatment with lenalidomide for long periods of time, even in small doses, may yield favorable outcomes.”
The 30 men and 26 women, mean age 76.5 years, were consecutively diagnosed as transplant-ineligible patients with relapsed/refractory multiple myeloma; 34%, 32%, and 34% had stages I-III disease, respectively. The M-protein consisted of IgG in 52% of patients and IgA in 30%, with Bence-Jones protein detected in 14%.
They were treated with lenalidomide plus dexamethasone at a starting dose determined by the treating physician; 73% were treated with lenalidomide as a second-line regimen and 14% as a third-line regimen. During each 28-day treatment cycle, patients received lenalidomide on days 1-21 and dexamethasone (20 or 40 mg) on days 1, 8, 15, and 22.
The most common starting lenalidomide dose was 10 mg/day (45%), followed by 5 mg/day (21%), 15 mg/day (20%), 20 mg/day (4%), and 25 mg/day (10%). The treatment dose used for the longest period was 10 mg/day (46% of patients), followed by 5 mg/day (25%), 15 mg/day (16%), 20 mg/day (4%), and 25 mg/day (9%).
The most frequent reasons for dose reduction were renal dysfunction (54%), fatigue (20%), hematologic disorder (14%), and rash (9%).
The median treatment period was 9 months (range 1-60 months) and the median follow-up period was 16 months.
The median time to disease progression was 11.8 months (range 8.4-21.9), and the median overall survival was 39.2 months. For those who took 5-10 mg of lenalidomide, the median time to progression was 14.5 months; for those who took lenalidomide at a dose of more than 10 mg, the median time to progression was 8.9 months. The median overall survival of the patients who received a 5- to 10-mg dose of lenalidomide was 38.9 months; the median overall survival of the patients given a dose of more than 10 mg was not available.
The authors declared no competing financial interests in relation to this work.
[email protected]
On Twitter @maryjodales
In frail elderly patients with multiple myeloma, starting lenalidomide at low doses was associated with fewer adverse events and less treatment discontinuation, and did not compromise overall response rates, in a single-center, retrospective study conducted in Japan.
Although most of the 56 study patients received 5-10 mg/day of lenalidomide, not the recommended 25-mg/day dose, their overall response rate was 73% (complete response in 17% of patients, very good partial response in 19%, and partial response in 37%), Aya Nakaya, MD, PhD, of Kansai Medical University, Hirakata, and colleagues wrote (Acta Haematol. 2017;138:55-60). In addition, 23% of patients had stable disease and 4% had disease progression. Nine patients developed other types of malignancies during treatment with lenalidomide.
Starting patients on a reduced dose and increasing it gradually while monitoring carefully for adverse events meant that patients did not have to stop treatment, the researchers said. Continuous treatment may improve survival, and “treatment with lenalidomide for long periods of time, even in small doses, may yield favorable outcomes.”
The 30 men and 26 women, mean age 76.5 years, were consecutively diagnosed as transplant-ineligible patients with relapsed/refractory multiple myeloma; 34%, 32%, and 34% had stages I-III disease, respectively. The M-protein consisted of IgG in 52% of patients and IgA in 30%, with Bence-Jones protein detected in 14%.
They were treated with lenalidomide plus dexamethasone at a starting dose determined by the treating physician; 73% were treated with lenalidomide as a second-line regimen and 14% as a third-line regimen. During each 28-day treatment cycle, patients received lenalidomide on days 1-21 and dexamethasone (20 or 40 mg) on days 1, 8, 15, and 22.
The most common starting lenalidomide dose was 10 mg/day (45%), followed by 5 mg/day (21%), 15 mg/day (20%), 20 mg/day (4%), and 25 mg/day (10%). The treatment dose used for the longest period was 10 mg/day (46% of patients), followed by 5 mg/day (25%), 15 mg/day (16%), 20 mg/day (4%), and 25 mg/day (9%).
The most frequent reasons for dose reduction were renal dysfunction (54%), fatigue (20%), hematologic disorder (14%), and rash (9%).
The median treatment period was 9 months (range 1-60 months) and the median follow-up period was 16 months.
The median time to disease progression was 11.8 months (range 8.4-21.9), and the median overall survival was 39.2 months. For those who took 5-10 mg of lenalidomide, the median time to progression was 14.5 months; for those who took lenalidomide at a dose of more than 10 mg, the median time to progression was 8.9 months. The median overall survival of the patients who received a 5- to 10-mg dose of lenalidomide was 38.9 months; the median overall survival of the patients given a dose of more than 10 mg was not available.
The authors declared no competing financial interests in relation to this work.
[email protected]
On Twitter @maryjodales
In frail elderly patients with multiple myeloma, starting lenalidomide at low doses was associated with fewer adverse events and less treatment discontinuation, and did not compromise overall response rates, in a single-center, retrospective study conducted in Japan.
Although most of the 56 study patients received 5-10 mg/day of lenalidomide, not the recommended 25-mg/day dose, their overall response rate was 73% (complete response in 17% of patients, very good partial response in 19%, and partial response in 37%), Aya Nakaya, MD, PhD, of Kansai Medical University, Hirakata, and colleagues wrote (Acta Haematol. 2017;138:55-60). In addition, 23% of patients had stable disease and 4% had disease progression. Nine patients developed other types of malignancies during treatment with lenalidomide.
Starting patients on a reduced dose and increasing it gradually while monitoring carefully for adverse events meant that patients did not have to stop treatment, the researchers said. Continuous treatment may improve survival, and “treatment with lenalidomide for long periods of time, even in small doses, may yield favorable outcomes.”
The 30 men and 26 women, mean age 76.5 years, were consecutively diagnosed as transplant-ineligible patients with relapsed/refractory multiple myeloma; 34%, 32%, and 34% had stages I-III disease, respectively. The M-protein consisted of IgG in 52% of patients and IgA in 30%, with Bence-Jones protein detected in 14%.
They were treated with lenalidomide plus dexamethasone at a starting dose determined by the treating physician; 73% were treated with lenalidomide as a second-line regimen and 14% as a third-line regimen. During each 28-day treatment cycle, patients received lenalidomide on days 1-21 and dexamethasone (20 or 40 mg) on days 1, 8, 15, and 22.
The most common starting lenalidomide dose was 10 mg/day (45%), followed by 5 mg/day (21%), 15 mg/day (20%), 20 mg/day (4%), and 25 mg/day (10%). The treatment dose used for the longest period was 10 mg/day (46% of patients), followed by 5 mg/day (25%), 15 mg/day (16%), 20 mg/day (4%), and 25 mg/day (9%).
The most frequent reasons for dose reduction were renal dysfunction (54%), fatigue (20%), hematologic disorder (14%), and rash (9%).
The median treatment period was 9 months (range 1-60 months) and the median follow-up period was 16 months.
The median time to disease progression was 11.8 months (range 8.4-21.9), and the median overall survival was 39.2 months. For those who took 5-10 mg of lenalidomide, the median time to progression was 14.5 months; for those who took lenalidomide at a dose of more than 10 mg, the median time to progression was 8.9 months. The median overall survival of the patients who received a 5- to 10-mg dose of lenalidomide was 38.9 months; the median overall survival of the patients given a dose of more than 10 mg was not available.
The authors declared no competing financial interests in relation to this work.
[email protected]
On Twitter @maryjodales
FROM ACTA HAEMATOLOGICA
Key clinical point:
Major finding: Although most of the 56 study patients received 5-10 mg/day of lenalidomide, not the recommended 25-mg/day dose, their overall response rate was 73%.
Data source: A single-center, retrospective study of 56 consecutively diagnosed transplant-ineligible patients in Japan.
Disclosures: The authors declared no competing financial interests in relation to this work.
Eye spy: Name that IUD
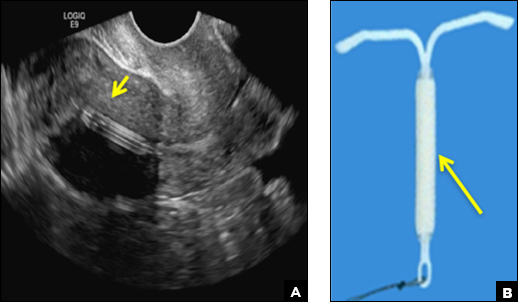
A) Mirena or Liletta (52 mg LNG-IUD) CORRECT
Mirena (Bayer) and Liletta (Allergan) are progestin-releasing intrauterine devices (IUDs) of similar size and shape. On ultrasonography, both the arms and the distal tip are echogenic. The progestin-containing plastic sleeve surrounding the stem in the middle demonstrates a laminated acoustic shadowing with distinctive parallel lines.1–4
B) Small-framed LNG-IUDs: Skyla (13.5 mg) or Kyleena (19.5 mg) INCORRECT
Skyla (Bayer) and Kyleena (Bayer) are small-framed LNG-IUDs. The ultrasound appearance of Skyla (LNG 13.5 mg) is similar to that of Mirena but has a markedly echogenic silver ring superiorly just below the crossbar, best seen with 2D (sagittal) views but also imaged with 3D ultrasound.1,2,5
The Kyleena device (LNG 19.5 mg) uses the same smaller T-shaped frame and metal ring, but the plastic sleeve is longer to accommodate the greater quantity of progestin.6

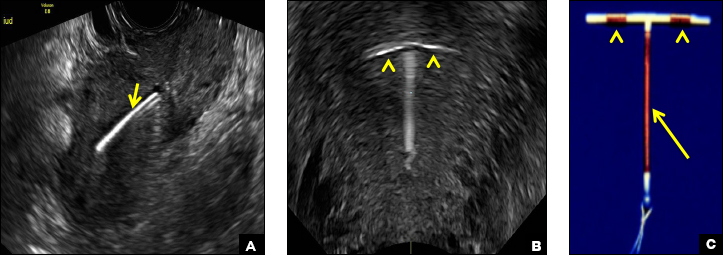
C) Paragard (intrauterine copper contraceptive) INCORRECT
Paragard (Teva Women’s Health) is a nonhormonal IUD containing copper wire wrapped around its stem and solid copper bands on each crossbar. On ultrasonography, the stem is uniformly and markedly echogenic due to the copper wire.1,2,7
- Stalnaker ML, Kaunitz AM. How to identify and localize IUDs on ultrasound. OBG Manag. 2014;26(8):38,40–41,44.
- Boortz HE, Margolis DJ, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352.
- Mirena [package insert]. Whippany, NJ: Bayer; 2000.
- Liletta [package insert]. Parsippany, NJ: Allergan; 2015.
- Skyla [package insert]. Whippany, NJ: Bayer; 2000.
- Kyleena [package insert]. Whippany, NJ: Bayer; 2000.
- Paragard [package insert]. North Wales, PA: Teva Women’s Health, Inc; 2014.
A) Mirena or Liletta (52 mg LNG-IUD) CORRECT
Mirena (Bayer) and Liletta (Allergan) are progestin-releasing intrauterine devices (IUDs) of similar size and shape. On ultrasonography, both the arms and the distal tip are echogenic. The progestin-containing plastic sleeve surrounding the stem in the middle demonstrates a laminated acoustic shadowing with distinctive parallel lines.1–4

B) Small-framed LNG-IUDs: Skyla (13.5 mg) or Kyleena (19.5 mg) INCORRECT
Skyla (Bayer) and Kyleena (Bayer) are small-framed LNG-IUDs. The ultrasound appearance of Skyla (LNG 13.5 mg) is similar to that of Mirena but has a markedly echogenic silver ring superiorly just below the crossbar, best seen with 2D (sagittal) views but also imaged with 3D ultrasound.1,2,5
The Kyleena device (LNG 19.5 mg) uses the same smaller T-shaped frame and metal ring, but the plastic sleeve is longer to accommodate the greater quantity of progestin.6

C) Paragard (intrauterine copper contraceptive) INCORRECT
Paragard (Teva Women’s Health) is a nonhormonal IUD containing copper wire wrapped around its stem and solid copper bands on each crossbar. On ultrasonography, the stem is uniformly and markedly echogenic due to the copper wire.1,2,7

A) Mirena or Liletta (52 mg LNG-IUD) CORRECT
Mirena (Bayer) and Liletta (Allergan) are progestin-releasing intrauterine devices (IUDs) of similar size and shape. On ultrasonography, both the arms and the distal tip are echogenic. The progestin-containing plastic sleeve surrounding the stem in the middle demonstrates a laminated acoustic shadowing with distinctive parallel lines.1–4

B) Small-framed LNG-IUDs: Skyla (13.5 mg) or Kyleena (19.5 mg) INCORRECT
Skyla (Bayer) and Kyleena (Bayer) are small-framed LNG-IUDs. The ultrasound appearance of Skyla (LNG 13.5 mg) is similar to that of Mirena but has a markedly echogenic silver ring superiorly just below the crossbar, best seen with 2D (sagittal) views but also imaged with 3D ultrasound.1,2,5
The Kyleena device (LNG 19.5 mg) uses the same smaller T-shaped frame and metal ring, but the plastic sleeve is longer to accommodate the greater quantity of progestin.6

C) Paragard (intrauterine copper contraceptive) INCORRECT
Paragard (Teva Women’s Health) is a nonhormonal IUD containing copper wire wrapped around its stem and solid copper bands on each crossbar. On ultrasonography, the stem is uniformly and markedly echogenic due to the copper wire.1,2,7

- Stalnaker ML, Kaunitz AM. How to identify and localize IUDs on ultrasound. OBG Manag. 2014;26(8):38,40–41,44.
- Boortz HE, Margolis DJ, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352.
- Mirena [package insert]. Whippany, NJ: Bayer; 2000.
- Liletta [package insert]. Parsippany, NJ: Allergan; 2015.
- Skyla [package insert]. Whippany, NJ: Bayer; 2000.
- Kyleena [package insert]. Whippany, NJ: Bayer; 2000.
- Paragard [package insert]. North Wales, PA: Teva Women’s Health, Inc; 2014.
- Stalnaker ML, Kaunitz AM. How to identify and localize IUDs on ultrasound. OBG Manag. 2014;26(8):38,40–41,44.
- Boortz HE, Margolis DJ, Ragavendra N, Patel MK, Kadell BM. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics. 2012;32(2):335–352.
- Mirena [package insert]. Whippany, NJ: Bayer; 2000.
- Liletta [package insert]. Parsippany, NJ: Allergan; 2015.
- Skyla [package insert]. Whippany, NJ: Bayer; 2000.
- Kyleena [package insert]. Whippany, NJ: Bayer; 2000.
- Paragard [package insert]. North Wales, PA: Teva Women’s Health, Inc; 2014.
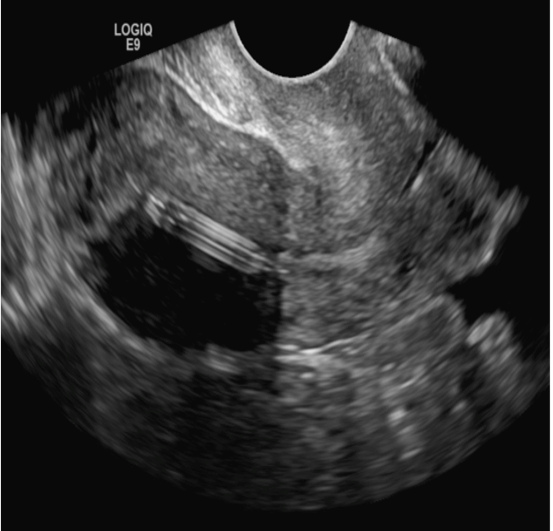
A 25-year-old woman using an IUD presented to her ObGyn’s office for follow-up care of a 4- to 5-cm left simple ovarian cyst. A pelvic ultrasound was performed. The ovarian cyst had resolved and a fundally-positioned IUD was imaged.
Tips and tricks for appealing an audit
CHICAGO – The question is not if a physician will face a Medicare or Medicaid billing audit, but when, according to Abby Pendleton, a New York–based health law attorney. That’s why it pays to know how to handle an audit before one probe disrupts your practice. At a recent American Bar Association meeting, Ms. Pendleton and H. Rusty Comley, a Jackson, Mississippi–based health law attorney, offered answers to top audit questions and provided guidance on how physicians can successfully appeal an audit.
When should you appeal?
There are a number of factors to consider when deciding whether to appeal audit findings. For starters, consider the cost of the payback amount and the basis of the findings.
“In other words, the provider would spend more money and time to appeal the audit than to pay the audit, and the issue or mistake is not likely repeated in past or future claims,” it might make sense to just pay, he said.
If the basis of the findings stem from an interpretation of a local coverage decision that the physician disagrees with, he or she may also want to appeal, Ms. Pendleton added.
“If you don’t fight it, there’s an argument that, ‘Well, guess what? You had that issue going back 6 years for all these other claims, and now we get into the [Medicare] 60 day overpayment identification [rule],’ ” she said at the meeting. “If a physician is [not] aware of payments they’re not entitled to, even if they think they were right on the front end, but they later become aware, they have 60 days to refund it or its a false claim. Those are considerations that really need to be looked at.”
What should you expect from an appeal?
Expect to go through more than one appeals process step to succeed. There are five stages to the appeals process .
“At the redetermination stage, I don’t see a whole lot of movement in terms of great success at that first stage,” Ms. Pendleton said. “So, don’t think, ‘If we get to that first level of appeal, we’re expecting to win.’ If you look at the statistics, it’s not really that realistic.”
Although a provider has 120 days to file an appeal, it’s smarter to file within the first 30 days, Ms. Pendleton advised. If an appeal is filed within 30 days, the government cannot recoup it’s demand from a doctor’s current Medicare payments.
Expect a lengthy time frame for a final outcome. Under federal law, once an appeal gets to the administrative law judge (ALJ) stage (the third stage), the appellant should receive a hearing decision within 90 days. However, because of heavy case backlogs, physicians typically don’t get a hearing for 3 years, Ms. Pendleton said.
“The problem is, your MAC [Medicare administrative contractor] can start taking your money after the [second] stage,” she said. “If it’s a huge dollar amount, you’re probably going to have to enter into a payment plan [with the government]. You will eventually get your money back, if you win, three to four years later.”
Note that physicians generally experience a higher degree of success at the ALJ stage, so it may be worth continuing the appeal through this stage, she noted.
Overall, more than one-third of audit findings are reversed in providers’ favor during the appeals process. Of 170,482 Medicare appeal decisions in 2015, 37% were made in favor of the health provider, an increase from 23% in 2014, according to 2015 Medicare data and 2014 reports.
The cost to appeal varies significantly between Medicaid and Medicare and depends largely on the complexity of the audit, Mr. Comley said. A Medicaid audit appeal, through an ALJ hearing and written appeal to a court, may cost between $20,000 to $60,000 depending on the circumstances, he said. By contrast, a Medicare appeal resolved in the first stage of appeal may only cost a few thousand dollars for a relatively simple audit.
“Of course, the costs will rise at each level of the Medicare appeal process, especially in the third stage involving the ALJ telephonic hearing, but, in most cases, the Medicare appeal costs will still be below a similar Medicaid appeal,” he said.
What strategies can help you win?
Consider reaching out to your congressional representative or senators, Mr. Comley advised. Particularly if the issue involves a medical treatment decision or a medically necessary determination, it may be helpful to copy “your favorite Congressman or senator’s office” on correspondence with the MAC. Clearly state your argument against the findings and how/why the medical decision was made. Legislators will often get involved and could help your appeal, Mr. Comley said.
Further, don’t just review the claims that auditors denied. Also evaluate the claims they have approved in the past, he added.
“In almost every case I’ve been involved in, they’ll approve claims that, on the other hand, they deny,” Mr. Comley said. “Under most legal standards, that’s a good way to win – it’s called arbitrary and capricious.”
Find the best experts to back your case, Ms. Pendleton advised. Consider including expert opinions in written responses to the government that support the services provided and/or have medical experts ready to testify during hearings. If the government based its findings on statistics or cited statistics in its review, involve a statistical expert who can argue against the government’s conclusion.
If the case is significant enough, consider skipping steps in the appeals process to get the case before a federal court sooner. Appellants can escalate their appeal through the process at nearly every stage if the government fails to respond within a timely manner. At the second stage, for example, if the qualified independent contractor does not issue a decision within 60 days, an appellant generally has the right to escalate the case to an administrative law judge. If the ALJ does not issue a decision within 90 days, the appeal can generally be escalated to the Appeals Council level, and, if the council does not issue a decision within 90 days, appellants can seek judicial review.
It may be worth it to have your day in court sooner, Ms. Pendleton said.
“It might be an option for providers if you have a large audit with a lot at stake,” she said. “Escalate it through. Get it to federal court and argue it.”
The 5 steps of the Medicare appeals process
There are five stages of the Medicare audit appeals process, according to the Centers for Medicare & Medicaid Services. They include:
1. Redetermination by the Fiscal Intermediary. A redetermination is an examination of a claim by a Medicare administrative contractor (MAC) separate from the personnel who made the initial claim determination. The appellant has 120 days from the date of initial claim determination receipt to file an appeal.
2. Reconsideration by a Qualified Independent Contractor (QIC). A QIC is an independent contractor who didn’t take part in the level 1 decision. The QIC will review the request for a reconsideration and make a decision. An appellant must file a request for reconsideration within 180 days of Medicare redetermination notice or remittance advice receipt.
3. Administrative Law Judge (ALJ) hearing. Appellants present their case to an ALJ who will review the facts of the appeal and listen to testimony before making a decision. An ALJ hearing is usually held by phone or video conference. Appellants can ask the ALJ to make a decision without a hearing. The ALJ may also issue a decision without holding a hearing if evidence in the record supports a decision that’s fully in the appellant’s favor.
4. Medicare Appeals Council review. If you disagree with the ALJ decision or wish to escalate the appeal because the ALJ ruling time frame has passed, a request for a Medicare Appeals Council review can be made. A request for a Medicare Appeals Council review must be made within 60 days of receipt of the ALJ’s decision or after the ALJ ruling time frame expires.
5. Judicial review in U.S. District Court. A party may file an action in federal district court within 60 calendar days after the date receiving notice of the Medicare Appeals Council’s decision or after a council notice that it is not able to reach a decision. To get a judicial review in federal district court, the case amount must meet a minimum dollar amount ($1,560 in 2017).
Each state has its own Medicaid appeals process. Contact your state’s Medicaid office to find out how to appeal a Medicaid audit finding.
[email protected]
On Twitter @legal_med
CHICAGO – The question is not if a physician will face a Medicare or Medicaid billing audit, but when, according to Abby Pendleton, a New York–based health law attorney. That’s why it pays to know how to handle an audit before one probe disrupts your practice. At a recent American Bar Association meeting, Ms. Pendleton and H. Rusty Comley, a Jackson, Mississippi–based health law attorney, offered answers to top audit questions and provided guidance on how physicians can successfully appeal an audit.
When should you appeal?
There are a number of factors to consider when deciding whether to appeal audit findings. For starters, consider the cost of the payback amount and the basis of the findings.
“In other words, the provider would spend more money and time to appeal the audit than to pay the audit, and the issue or mistake is not likely repeated in past or future claims,” it might make sense to just pay, he said.
If the basis of the findings stem from an interpretation of a local coverage decision that the physician disagrees with, he or she may also want to appeal, Ms. Pendleton added.
“If you don’t fight it, there’s an argument that, ‘Well, guess what? You had that issue going back 6 years for all these other claims, and now we get into the [Medicare] 60 day overpayment identification [rule],’ ” she said at the meeting. “If a physician is [not] aware of payments they’re not entitled to, even if they think they were right on the front end, but they later become aware, they have 60 days to refund it or its a false claim. Those are considerations that really need to be looked at.”
What should you expect from an appeal?
Expect to go through more than one appeals process step to succeed. There are five stages to the appeals process .
“At the redetermination stage, I don’t see a whole lot of movement in terms of great success at that first stage,” Ms. Pendleton said. “So, don’t think, ‘If we get to that first level of appeal, we’re expecting to win.’ If you look at the statistics, it’s not really that realistic.”
Although a provider has 120 days to file an appeal, it’s smarter to file within the first 30 days, Ms. Pendleton advised. If an appeal is filed within 30 days, the government cannot recoup it’s demand from a doctor’s current Medicare payments.
Expect a lengthy time frame for a final outcome. Under federal law, once an appeal gets to the administrative law judge (ALJ) stage (the third stage), the appellant should receive a hearing decision within 90 days. However, because of heavy case backlogs, physicians typically don’t get a hearing for 3 years, Ms. Pendleton said.
“The problem is, your MAC [Medicare administrative contractor] can start taking your money after the [second] stage,” she said. “If it’s a huge dollar amount, you’re probably going to have to enter into a payment plan [with the government]. You will eventually get your money back, if you win, three to four years later.”
Note that physicians generally experience a higher degree of success at the ALJ stage, so it may be worth continuing the appeal through this stage, she noted.
Overall, more than one-third of audit findings are reversed in providers’ favor during the appeals process. Of 170,482 Medicare appeal decisions in 2015, 37% were made in favor of the health provider, an increase from 23% in 2014, according to 2015 Medicare data and 2014 reports.
The cost to appeal varies significantly between Medicaid and Medicare and depends largely on the complexity of the audit, Mr. Comley said. A Medicaid audit appeal, through an ALJ hearing and written appeal to a court, may cost between $20,000 to $60,000 depending on the circumstances, he said. By contrast, a Medicare appeal resolved in the first stage of appeal may only cost a few thousand dollars for a relatively simple audit.
“Of course, the costs will rise at each level of the Medicare appeal process, especially in the third stage involving the ALJ telephonic hearing, but, in most cases, the Medicare appeal costs will still be below a similar Medicaid appeal,” he said.
What strategies can help you win?
Consider reaching out to your congressional representative or senators, Mr. Comley advised. Particularly if the issue involves a medical treatment decision or a medically necessary determination, it may be helpful to copy “your favorite Congressman or senator’s office” on correspondence with the MAC. Clearly state your argument against the findings and how/why the medical decision was made. Legislators will often get involved and could help your appeal, Mr. Comley said.
Further, don’t just review the claims that auditors denied. Also evaluate the claims they have approved in the past, he added.
“In almost every case I’ve been involved in, they’ll approve claims that, on the other hand, they deny,” Mr. Comley said. “Under most legal standards, that’s a good way to win – it’s called arbitrary and capricious.”
Find the best experts to back your case, Ms. Pendleton advised. Consider including expert opinions in written responses to the government that support the services provided and/or have medical experts ready to testify during hearings. If the government based its findings on statistics or cited statistics in its review, involve a statistical expert who can argue against the government’s conclusion.
If the case is significant enough, consider skipping steps in the appeals process to get the case before a federal court sooner. Appellants can escalate their appeal through the process at nearly every stage if the government fails to respond within a timely manner. At the second stage, for example, if the qualified independent contractor does not issue a decision within 60 days, an appellant generally has the right to escalate the case to an administrative law judge. If the ALJ does not issue a decision within 90 days, the appeal can generally be escalated to the Appeals Council level, and, if the council does not issue a decision within 90 days, appellants can seek judicial review.
It may be worth it to have your day in court sooner, Ms. Pendleton said.
“It might be an option for providers if you have a large audit with a lot at stake,” she said. “Escalate it through. Get it to federal court and argue it.”
The 5 steps of the Medicare appeals process
There are five stages of the Medicare audit appeals process, according to the Centers for Medicare & Medicaid Services. They include:
1. Redetermination by the Fiscal Intermediary. A redetermination is an examination of a claim by a Medicare administrative contractor (MAC) separate from the personnel who made the initial claim determination. The appellant has 120 days from the date of initial claim determination receipt to file an appeal.
2. Reconsideration by a Qualified Independent Contractor (QIC). A QIC is an independent contractor who didn’t take part in the level 1 decision. The QIC will review the request for a reconsideration and make a decision. An appellant must file a request for reconsideration within 180 days of Medicare redetermination notice or remittance advice receipt.
3. Administrative Law Judge (ALJ) hearing. Appellants present their case to an ALJ who will review the facts of the appeal and listen to testimony before making a decision. An ALJ hearing is usually held by phone or video conference. Appellants can ask the ALJ to make a decision without a hearing. The ALJ may also issue a decision without holding a hearing if evidence in the record supports a decision that’s fully in the appellant’s favor.
4. Medicare Appeals Council review. If you disagree with the ALJ decision or wish to escalate the appeal because the ALJ ruling time frame has passed, a request for a Medicare Appeals Council review can be made. A request for a Medicare Appeals Council review must be made within 60 days of receipt of the ALJ’s decision or after the ALJ ruling time frame expires.
5. Judicial review in U.S. District Court. A party may file an action in federal district court within 60 calendar days after the date receiving notice of the Medicare Appeals Council’s decision or after a council notice that it is not able to reach a decision. To get a judicial review in federal district court, the case amount must meet a minimum dollar amount ($1,560 in 2017).
Each state has its own Medicaid appeals process. Contact your state’s Medicaid office to find out how to appeal a Medicaid audit finding.
[email protected]
On Twitter @legal_med
CHICAGO – The question is not if a physician will face a Medicare or Medicaid billing audit, but when, according to Abby Pendleton, a New York–based health law attorney. That’s why it pays to know how to handle an audit before one probe disrupts your practice. At a recent American Bar Association meeting, Ms. Pendleton and H. Rusty Comley, a Jackson, Mississippi–based health law attorney, offered answers to top audit questions and provided guidance on how physicians can successfully appeal an audit.
When should you appeal?
There are a number of factors to consider when deciding whether to appeal audit findings. For starters, consider the cost of the payback amount and the basis of the findings.
“In other words, the provider would spend more money and time to appeal the audit than to pay the audit, and the issue or mistake is not likely repeated in past or future claims,” it might make sense to just pay, he said.
If the basis of the findings stem from an interpretation of a local coverage decision that the physician disagrees with, he or she may also want to appeal, Ms. Pendleton added.
“If you don’t fight it, there’s an argument that, ‘Well, guess what? You had that issue going back 6 years for all these other claims, and now we get into the [Medicare] 60 day overpayment identification [rule],’ ” she said at the meeting. “If a physician is [not] aware of payments they’re not entitled to, even if they think they were right on the front end, but they later become aware, they have 60 days to refund it or its a false claim. Those are considerations that really need to be looked at.”
What should you expect from an appeal?
Expect to go through more than one appeals process step to succeed. There are five stages to the appeals process .
“At the redetermination stage, I don’t see a whole lot of movement in terms of great success at that first stage,” Ms. Pendleton said. “So, don’t think, ‘If we get to that first level of appeal, we’re expecting to win.’ If you look at the statistics, it’s not really that realistic.”
Although a provider has 120 days to file an appeal, it’s smarter to file within the first 30 days, Ms. Pendleton advised. If an appeal is filed within 30 days, the government cannot recoup it’s demand from a doctor’s current Medicare payments.
Expect a lengthy time frame for a final outcome. Under federal law, once an appeal gets to the administrative law judge (ALJ) stage (the third stage), the appellant should receive a hearing decision within 90 days. However, because of heavy case backlogs, physicians typically don’t get a hearing for 3 years, Ms. Pendleton said.
“The problem is, your MAC [Medicare administrative contractor] can start taking your money after the [second] stage,” she said. “If it’s a huge dollar amount, you’re probably going to have to enter into a payment plan [with the government]. You will eventually get your money back, if you win, three to four years later.”
Note that physicians generally experience a higher degree of success at the ALJ stage, so it may be worth continuing the appeal through this stage, she noted.
Overall, more than one-third of audit findings are reversed in providers’ favor during the appeals process. Of 170,482 Medicare appeal decisions in 2015, 37% were made in favor of the health provider, an increase from 23% in 2014, according to 2015 Medicare data and 2014 reports.
The cost to appeal varies significantly between Medicaid and Medicare and depends largely on the complexity of the audit, Mr. Comley said. A Medicaid audit appeal, through an ALJ hearing and written appeal to a court, may cost between $20,000 to $60,000 depending on the circumstances, he said. By contrast, a Medicare appeal resolved in the first stage of appeal may only cost a few thousand dollars for a relatively simple audit.
“Of course, the costs will rise at each level of the Medicare appeal process, especially in the third stage involving the ALJ telephonic hearing, but, in most cases, the Medicare appeal costs will still be below a similar Medicaid appeal,” he said.
What strategies can help you win?
Consider reaching out to your congressional representative or senators, Mr. Comley advised. Particularly if the issue involves a medical treatment decision or a medically necessary determination, it may be helpful to copy “your favorite Congressman or senator’s office” on correspondence with the MAC. Clearly state your argument against the findings and how/why the medical decision was made. Legislators will often get involved and could help your appeal, Mr. Comley said.
Further, don’t just review the claims that auditors denied. Also evaluate the claims they have approved in the past, he added.
“In almost every case I’ve been involved in, they’ll approve claims that, on the other hand, they deny,” Mr. Comley said. “Under most legal standards, that’s a good way to win – it’s called arbitrary and capricious.”
Find the best experts to back your case, Ms. Pendleton advised. Consider including expert opinions in written responses to the government that support the services provided and/or have medical experts ready to testify during hearings. If the government based its findings on statistics or cited statistics in its review, involve a statistical expert who can argue against the government’s conclusion.
If the case is significant enough, consider skipping steps in the appeals process to get the case before a federal court sooner. Appellants can escalate their appeal through the process at nearly every stage if the government fails to respond within a timely manner. At the second stage, for example, if the qualified independent contractor does not issue a decision within 60 days, an appellant generally has the right to escalate the case to an administrative law judge. If the ALJ does not issue a decision within 90 days, the appeal can generally be escalated to the Appeals Council level, and, if the council does not issue a decision within 90 days, appellants can seek judicial review.
It may be worth it to have your day in court sooner, Ms. Pendleton said.
“It might be an option for providers if you have a large audit with a lot at stake,” she said. “Escalate it through. Get it to federal court and argue it.”
The 5 steps of the Medicare appeals process
There are five stages of the Medicare audit appeals process, according to the Centers for Medicare & Medicaid Services. They include:
1. Redetermination by the Fiscal Intermediary. A redetermination is an examination of a claim by a Medicare administrative contractor (MAC) separate from the personnel who made the initial claim determination. The appellant has 120 days from the date of initial claim determination receipt to file an appeal.
2. Reconsideration by a Qualified Independent Contractor (QIC). A QIC is an independent contractor who didn’t take part in the level 1 decision. The QIC will review the request for a reconsideration and make a decision. An appellant must file a request for reconsideration within 180 days of Medicare redetermination notice or remittance advice receipt.
3. Administrative Law Judge (ALJ) hearing. Appellants present their case to an ALJ who will review the facts of the appeal and listen to testimony before making a decision. An ALJ hearing is usually held by phone or video conference. Appellants can ask the ALJ to make a decision without a hearing. The ALJ may also issue a decision without holding a hearing if evidence in the record supports a decision that’s fully in the appellant’s favor.
4. Medicare Appeals Council review. If you disagree with the ALJ decision or wish to escalate the appeal because the ALJ ruling time frame has passed, a request for a Medicare Appeals Council review can be made. A request for a Medicare Appeals Council review must be made within 60 days of receipt of the ALJ’s decision or after the ALJ ruling time frame expires.
5. Judicial review in U.S. District Court. A party may file an action in federal district court within 60 calendar days after the date receiving notice of the Medicare Appeals Council’s decision or after a council notice that it is not able to reach a decision. To get a judicial review in federal district court, the case amount must meet a minimum dollar amount ($1,560 in 2017).
Each state has its own Medicaid appeals process. Contact your state’s Medicaid office to find out how to appeal a Medicaid audit finding.
[email protected]
On Twitter @legal_med
AT THE PHYSICIAN LEGAL ISSUES CONFERENCE
Lasers still play a role in treatment of dermatologic conditions in children
CHICAGO – Multiple laser and light options are available to treat children with infantile hemangiomas, port wine birthmarks, and angiofibromas, according to Kristen M. Kelly, MD.
“Combination treatments with procedures and medications can improve treatment in many cases,” Dr. Kelly said at the World Congress of Pediatric Dermatology.
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, said that the use of lasers and other light sources for infantile hemangiomas has dramatically decreased since propranolol, timolol, and other beta-blockers have become available. Most children are candidates for beta-blocker therapy, she said, but for those who are not, the pulsed dye laser (PDL) may be a good option. She also considers using the PDL for ulcerated lesions. “Of course concern comes up, because lasers can sometimes cause ulcerations, so you have to be aware of that,” she said.
“For the more proliferative phase of infantile hemangiomas, I’ll use a larger spot size: 10-12 mm, and short pulse durations: 0.45 to 1.5 milliseconds, and low energies,” Dr. Kelly said. “I would start with an energy of 5 or 5.5 J/cm2. I may creep that up a little with time, but I don’t feel that you need to use very high energies. For lesions that are starting to involute, you could consider higher energies.”
Consider the combination of PDL and propranolol for patients who have a superficial component, for ulcerated lesions, or for rapidly progressing lesions that are not responding to your treatment. “You can also use the combination of PDL and timolol,” she said. “Starting treatment can avoid the need for reconstruction later.”
Dr. Kelly then discussed her approach to treating port wine birthmarks. She almost exclusively uses the PDL and the 755-nm Alexandrite lasers for these lesions. “For some of the resistant lesions, I’ll consider some of the combined treatments, like the combined 1064/532 nm system,” she said. “If I have really young patients, I use the PDL almost exclusively. I find the Alexandrite laser useful when I have thicker lesions that have hypertrophied.”
For optimal effect, she recommends treating lesions as early as possible and increasing chromophore target by placing patients with facial lesions in the Trendelenburg position during treatment sessions. Her preferred PDL parameters are a wavelength of 585 nm or 595 nm with a pulse duration of 0.45 to 1.5 milliseconds for the vast majority of lesions. “I try to vary the pulse duration over time, so if I’m getting a great result with 0.45 milliseconds, I’ll do that a couple of times,” Dr. Kelly said. “Once I feel I’ve reached a plateau, I might change to 1.5 milliseconds, or consider doing a second pass.”
Whenever possible she uses larger spot sizes and chooses the level of energy based on the type of lesion she’s treating. “I think it’s important to look for an endpoint,” she said. “I like to see deep purpura but I don’t like to see gray, because I feel that’s where you’re going to get epidermal injury or [there is] the chance for scarring and dyspigmentation, which can be permanent in some patients.”
Patients with port wine birthmarks require 3-15 treatments or more, typically 4 weeks apart. “Some people do 2- or 3-week intervals; that’s something to consider,” Dr. Kelly said. “In a darker-skinned patient with hyperpigmentation, I will use longer intervals, especially on an extremity that may take a little longer to heal.”
Alternative treatments are being studied, including the use of lasers in combination with antiangiogenic agents. “Rapamycin has been looked at most extensively, and it’s been shown to have a significant benefit,” she said.
According to Dr. Kelly, a new device for treating port wine birthmarks is being developed that combines pulse dye laser, Nd:YAG, and radiofrequency. “The potential advantage of this is that when we use the PDL alone, we probably cannot get very deep into those vessels,” she said. “The combination of the PDL and radiofrequency may allow us to more completely coagulate these vessels and get better response.”
Dr. Kelly closed her presentation by discussing angiofibromas, disfiguring skin lesions that are associated with tuberous sclerosis and have a fairly rapid recurrence. Topical and/or oral rapamycin are treatment options, but so are laser and light sources. She cited approaches published by Roy Geronemus MD, of the Laser and Skin Surgery Center of New York, and his associates, which included PDL treatment with a 10-mm spot size delivered at 7.5 J/cm2 with a pulse duration of 1.5 ms, and dynamic cooling spray duration of 30 ms (Lasers Surg Med 2013;45:555-7). This was followed by ablative fractional resurfacing with a 15-mm spot size at 70 mJ per pulse and 40% coverage. Other treatment options for angiofibromas include pinpoint electrosurgery to papular, fibrotic lesions and topical rapamycin ointment twice a day.
Dr. Kelly disclosed having drugs or devices donated by Light Sciences Oncology, Solta Medical, Cynosure, Syneron Candela, and Novartis. She is a consultant for MundiPharma, Allergan, and Syneron Candela, and has received research funding from the American Society of Laser Medicine and Surgery, the National Institutes of Health, the Sturge-Weber Foundation, and the UC Irvine Institute of Clinical and Translational Science.
CHICAGO – Multiple laser and light options are available to treat children with infantile hemangiomas, port wine birthmarks, and angiofibromas, according to Kristen M. Kelly, MD.
“Combination treatments with procedures and medications can improve treatment in many cases,” Dr. Kelly said at the World Congress of Pediatric Dermatology.
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, said that the use of lasers and other light sources for infantile hemangiomas has dramatically decreased since propranolol, timolol, and other beta-blockers have become available. Most children are candidates for beta-blocker therapy, she said, but for those who are not, the pulsed dye laser (PDL) may be a good option. She also considers using the PDL for ulcerated lesions. “Of course concern comes up, because lasers can sometimes cause ulcerations, so you have to be aware of that,” she said.
“For the more proliferative phase of infantile hemangiomas, I’ll use a larger spot size: 10-12 mm, and short pulse durations: 0.45 to 1.5 milliseconds, and low energies,” Dr. Kelly said. “I would start with an energy of 5 or 5.5 J/cm2. I may creep that up a little with time, but I don’t feel that you need to use very high energies. For lesions that are starting to involute, you could consider higher energies.”
Consider the combination of PDL and propranolol for patients who have a superficial component, for ulcerated lesions, or for rapidly progressing lesions that are not responding to your treatment. “You can also use the combination of PDL and timolol,” she said. “Starting treatment can avoid the need for reconstruction later.”
Dr. Kelly then discussed her approach to treating port wine birthmarks. She almost exclusively uses the PDL and the 755-nm Alexandrite lasers for these lesions. “For some of the resistant lesions, I’ll consider some of the combined treatments, like the combined 1064/532 nm system,” she said. “If I have really young patients, I use the PDL almost exclusively. I find the Alexandrite laser useful when I have thicker lesions that have hypertrophied.”
For optimal effect, she recommends treating lesions as early as possible and increasing chromophore target by placing patients with facial lesions in the Trendelenburg position during treatment sessions. Her preferred PDL parameters are a wavelength of 585 nm or 595 nm with a pulse duration of 0.45 to 1.5 milliseconds for the vast majority of lesions. “I try to vary the pulse duration over time, so if I’m getting a great result with 0.45 milliseconds, I’ll do that a couple of times,” Dr. Kelly said. “Once I feel I’ve reached a plateau, I might change to 1.5 milliseconds, or consider doing a second pass.”
Whenever possible she uses larger spot sizes and chooses the level of energy based on the type of lesion she’s treating. “I think it’s important to look for an endpoint,” she said. “I like to see deep purpura but I don’t like to see gray, because I feel that’s where you’re going to get epidermal injury or [there is] the chance for scarring and dyspigmentation, which can be permanent in some patients.”
Patients with port wine birthmarks require 3-15 treatments or more, typically 4 weeks apart. “Some people do 2- or 3-week intervals; that’s something to consider,” Dr. Kelly said. “In a darker-skinned patient with hyperpigmentation, I will use longer intervals, especially on an extremity that may take a little longer to heal.”
Alternative treatments are being studied, including the use of lasers in combination with antiangiogenic agents. “Rapamycin has been looked at most extensively, and it’s been shown to have a significant benefit,” she said.
According to Dr. Kelly, a new device for treating port wine birthmarks is being developed that combines pulse dye laser, Nd:YAG, and radiofrequency. “The potential advantage of this is that when we use the PDL alone, we probably cannot get very deep into those vessels,” she said. “The combination of the PDL and radiofrequency may allow us to more completely coagulate these vessels and get better response.”
Dr. Kelly closed her presentation by discussing angiofibromas, disfiguring skin lesions that are associated with tuberous sclerosis and have a fairly rapid recurrence. Topical and/or oral rapamycin are treatment options, but so are laser and light sources. She cited approaches published by Roy Geronemus MD, of the Laser and Skin Surgery Center of New York, and his associates, which included PDL treatment with a 10-mm spot size delivered at 7.5 J/cm2 with a pulse duration of 1.5 ms, and dynamic cooling spray duration of 30 ms (Lasers Surg Med 2013;45:555-7). This was followed by ablative fractional resurfacing with a 15-mm spot size at 70 mJ per pulse and 40% coverage. Other treatment options for angiofibromas include pinpoint electrosurgery to papular, fibrotic lesions and topical rapamycin ointment twice a day.
Dr. Kelly disclosed having drugs or devices donated by Light Sciences Oncology, Solta Medical, Cynosure, Syneron Candela, and Novartis. She is a consultant for MundiPharma, Allergan, and Syneron Candela, and has received research funding from the American Society of Laser Medicine and Surgery, the National Institutes of Health, the Sturge-Weber Foundation, and the UC Irvine Institute of Clinical and Translational Science.
CHICAGO – Multiple laser and light options are available to treat children with infantile hemangiomas, port wine birthmarks, and angiofibromas, according to Kristen M. Kelly, MD.
“Combination treatments with procedures and medications can improve treatment in many cases,” Dr. Kelly said at the World Congress of Pediatric Dermatology.
Dr. Kelly, professor of dermatology and surgery at the University of California, Irvine, said that the use of lasers and other light sources for infantile hemangiomas has dramatically decreased since propranolol, timolol, and other beta-blockers have become available. Most children are candidates for beta-blocker therapy, she said, but for those who are not, the pulsed dye laser (PDL) may be a good option. She also considers using the PDL for ulcerated lesions. “Of course concern comes up, because lasers can sometimes cause ulcerations, so you have to be aware of that,” she said.
“For the more proliferative phase of infantile hemangiomas, I’ll use a larger spot size: 10-12 mm, and short pulse durations: 0.45 to 1.5 milliseconds, and low energies,” Dr. Kelly said. “I would start with an energy of 5 or 5.5 J/cm2. I may creep that up a little with time, but I don’t feel that you need to use very high energies. For lesions that are starting to involute, you could consider higher energies.”
Consider the combination of PDL and propranolol for patients who have a superficial component, for ulcerated lesions, or for rapidly progressing lesions that are not responding to your treatment. “You can also use the combination of PDL and timolol,” she said. “Starting treatment can avoid the need for reconstruction later.”
Dr. Kelly then discussed her approach to treating port wine birthmarks. She almost exclusively uses the PDL and the 755-nm Alexandrite lasers for these lesions. “For some of the resistant lesions, I’ll consider some of the combined treatments, like the combined 1064/532 nm system,” she said. “If I have really young patients, I use the PDL almost exclusively. I find the Alexandrite laser useful when I have thicker lesions that have hypertrophied.”
For optimal effect, she recommends treating lesions as early as possible and increasing chromophore target by placing patients with facial lesions in the Trendelenburg position during treatment sessions. Her preferred PDL parameters are a wavelength of 585 nm or 595 nm with a pulse duration of 0.45 to 1.5 milliseconds for the vast majority of lesions. “I try to vary the pulse duration over time, so if I’m getting a great result with 0.45 milliseconds, I’ll do that a couple of times,” Dr. Kelly said. “Once I feel I’ve reached a plateau, I might change to 1.5 milliseconds, or consider doing a second pass.”
Whenever possible she uses larger spot sizes and chooses the level of energy based on the type of lesion she’s treating. “I think it’s important to look for an endpoint,” she said. “I like to see deep purpura but I don’t like to see gray, because I feel that’s where you’re going to get epidermal injury or [there is] the chance for scarring and dyspigmentation, which can be permanent in some patients.”
Patients with port wine birthmarks require 3-15 treatments or more, typically 4 weeks apart. “Some people do 2- or 3-week intervals; that’s something to consider,” Dr. Kelly said. “In a darker-skinned patient with hyperpigmentation, I will use longer intervals, especially on an extremity that may take a little longer to heal.”
Alternative treatments are being studied, including the use of lasers in combination with antiangiogenic agents. “Rapamycin has been looked at most extensively, and it’s been shown to have a significant benefit,” she said.
According to Dr. Kelly, a new device for treating port wine birthmarks is being developed that combines pulse dye laser, Nd:YAG, and radiofrequency. “The potential advantage of this is that when we use the PDL alone, we probably cannot get very deep into those vessels,” she said. “The combination of the PDL and radiofrequency may allow us to more completely coagulate these vessels and get better response.”
Dr. Kelly closed her presentation by discussing angiofibromas, disfiguring skin lesions that are associated with tuberous sclerosis and have a fairly rapid recurrence. Topical and/or oral rapamycin are treatment options, but so are laser and light sources. She cited approaches published by Roy Geronemus MD, of the Laser and Skin Surgery Center of New York, and his associates, which included PDL treatment with a 10-mm spot size delivered at 7.5 J/cm2 with a pulse duration of 1.5 ms, and dynamic cooling spray duration of 30 ms (Lasers Surg Med 2013;45:555-7). This was followed by ablative fractional resurfacing with a 15-mm spot size at 70 mJ per pulse and 40% coverage. Other treatment options for angiofibromas include pinpoint electrosurgery to papular, fibrotic lesions and topical rapamycin ointment twice a day.
Dr. Kelly disclosed having drugs or devices donated by Light Sciences Oncology, Solta Medical, Cynosure, Syneron Candela, and Novartis. She is a consultant for MundiPharma, Allergan, and Syneron Candela, and has received research funding from the American Society of Laser Medicine and Surgery, the National Institutes of Health, the Sturge-Weber Foundation, and the UC Irvine Institute of Clinical and Translational Science.
AT WCPD 2017
CT scoring system may improve sacroiliitis treatment in IBD
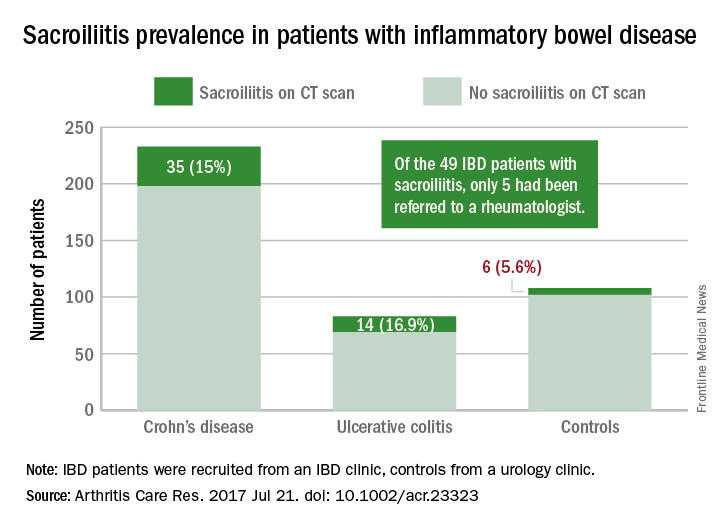
A standardized scoring system to identify sacroiliitis could enable patients with inflammatory bowel disease to get earlier rheumatology referrals and improve treatment, according to an analysis of IBD patients with pre-existing abdominal CT scans.
Of the 316 patients recruited from an IBD clinic in Toronto, the validated CT scan scoring system identified 49 with sacroiliitis, of whom only 5 had been referred to an outpatient rheumatology clinic in the city. Rates of sacroiliitis were similar between the 233 patients with Crohn’s disease (15%) and the 83 with ulcerative colitis (16.9%). The scoring system indicated sacroiliitis in 6 (5.6%) of the 108 control subjects, who were recruited from a urology clinic and had no prior history of chronic back pain, reported Jonathan Chan, MD, of the University of Toronto and his associates (Arthritis Care Res. 2017 Jul 21. doi: 10.1002/acr.23323).“Previous studies using CT scan to detect sacroiliitis have relied upon an adaptation of the [modified New York] criteria or a radiologist’s gestalt. Such an adaptation may not be appropriate since changes suggestive of sacroiliitis can be found in the healthy population due to the increased sensitivity of CT scans,” the investigators said. They developed a CT-scan scoring system in which the sacroiliac joints are divided into left/right and iliac/sacral segments. The slice with the greatest number of erosions in each of the four segments contributes that value to the total erosion score, with a score of 3 or greater identifying the presence of sacroiliitis.
By demonstrating that sacroiliitis is three times more prevalent among IBD patients and can be reliably detected in CT scans performed in the clinical care of those patients, this study suggests that “more timely referral for rheumatology assessment” could avoid unnecessary treatment with biologics, Dr. Chan and his associates wrote.
The study was supported in part by a fellowship grant from the Assessment of Spondyloarthritis International Society and in part by Janssen. The investigators reported having no conflicts of interest for the study.
A standardized scoring system to identify sacroiliitis could enable patients with inflammatory bowel disease to get earlier rheumatology referrals and improve treatment, according to an analysis of IBD patients with pre-existing abdominal CT scans.
Of the 316 patients recruited from an IBD clinic in Toronto, the validated CT scan scoring system identified 49 with sacroiliitis, of whom only 5 had been referred to an outpatient rheumatology clinic in the city. Rates of sacroiliitis were similar between the 233 patients with Crohn’s disease (15%) and the 83 with ulcerative colitis (16.9%). The scoring system indicated sacroiliitis in 6 (5.6%) of the 108 control subjects, who were recruited from a urology clinic and had no prior history of chronic back pain, reported Jonathan Chan, MD, of the University of Toronto and his associates (Arthritis Care Res. 2017 Jul 21. doi: 10.1002/acr.23323).“Previous studies using CT scan to detect sacroiliitis have relied upon an adaptation of the [modified New York] criteria or a radiologist’s gestalt. Such an adaptation may not be appropriate since changes suggestive of sacroiliitis can be found in the healthy population due to the increased sensitivity of CT scans,” the investigators said. They developed a CT-scan scoring system in which the sacroiliac joints are divided into left/right and iliac/sacral segments. The slice with the greatest number of erosions in each of the four segments contributes that value to the total erosion score, with a score of 3 or greater identifying the presence of sacroiliitis.
By demonstrating that sacroiliitis is three times more prevalent among IBD patients and can be reliably detected in CT scans performed in the clinical care of those patients, this study suggests that “more timely referral for rheumatology assessment” could avoid unnecessary treatment with biologics, Dr. Chan and his associates wrote.
The study was supported in part by a fellowship grant from the Assessment of Spondyloarthritis International Society and in part by Janssen. The investigators reported having no conflicts of interest for the study.
A standardized scoring system to identify sacroiliitis could enable patients with inflammatory bowel disease to get earlier rheumatology referrals and improve treatment, according to an analysis of IBD patients with pre-existing abdominal CT scans.
Of the 316 patients recruited from an IBD clinic in Toronto, the validated CT scan scoring system identified 49 with sacroiliitis, of whom only 5 had been referred to an outpatient rheumatology clinic in the city. Rates of sacroiliitis were similar between the 233 patients with Crohn’s disease (15%) and the 83 with ulcerative colitis (16.9%). The scoring system indicated sacroiliitis in 6 (5.6%) of the 108 control subjects, who were recruited from a urology clinic and had no prior history of chronic back pain, reported Jonathan Chan, MD, of the University of Toronto and his associates (Arthritis Care Res. 2017 Jul 21. doi: 10.1002/acr.23323).“Previous studies using CT scan to detect sacroiliitis have relied upon an adaptation of the [modified New York] criteria or a radiologist’s gestalt. Such an adaptation may not be appropriate since changes suggestive of sacroiliitis can be found in the healthy population due to the increased sensitivity of CT scans,” the investigators said. They developed a CT-scan scoring system in which the sacroiliac joints are divided into left/right and iliac/sacral segments. The slice with the greatest number of erosions in each of the four segments contributes that value to the total erosion score, with a score of 3 or greater identifying the presence of sacroiliitis.
By demonstrating that sacroiliitis is three times more prevalent among IBD patients and can be reliably detected in CT scans performed in the clinical care of those patients, this study suggests that “more timely referral for rheumatology assessment” could avoid unnecessary treatment with biologics, Dr. Chan and his associates wrote.
The study was supported in part by a fellowship grant from the Assessment of Spondyloarthritis International Society and in part by Janssen. The investigators reported having no conflicts of interest for the study.
FROM ARTHRITIS CARE & RESEARCH
Potential new role for FFR
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
EXPERT ANALYSIS FROM EuroPCR
It's Not Appealing, But It Is A-Peeling
A 6-year-old boy is referred to dermatology for evaluation of his dry, thin skin. Since birth, it has frequently torn, and it burns with the application of almost any product or soap. Neither OTC nor prescription products have helped.
The boy is reportedly in good health otherwise and is not atopic. Nonetheless, two of his siblings are similarly affected, and there is a strong family history of similar dermatologic problems on his father’s side. He and his family are from Mexico and have type IV skin.
EXAMINATION
The patient is in no distress but does complain about his skin problems. His skin is quite thin and dry, and fine scaling covers his palms, face, legs, trunk, and scalp. In short, none of his skin looks normal. The skin on his legs is especially scaly and has a pronounced reticulated appearance.
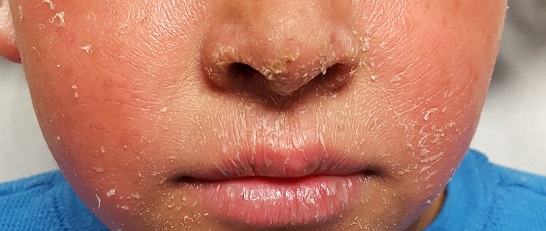
His fingernails are dystrophic, with transverse ridging and a loss of connection between the cuticles and nail plates.
What is the diagnosis?
Ichthyosis vulgaris (IV) is one of a family of disorders that cause a breakdown of normal filamentous structures (filaggrin) that hold the layers of skin together. This breakdown can lead to excessive water loss, as well as vulnerability of skin to penetration by allergens and other noxious substances.
IV is by far the most common variation, comprising at least 95% of all ichthyosiform dermatoses. It results from an inherited abnormality of epidermal differentiation or metabolism; affected patients have a higher incidence of eye problems (eg, keratitis, cataracts) in addition to their skin problems.
A total of 28 types of ichthyosis have been described, many of which are part of larger syndromes (such as keratitis-ichthyosis-deafness syndrome or Netherton syndrome). Another uncommon type, X-linked recessive ichthyosis, manifests in about 1 in 5,000 births; these patients improve dramatically in the summertime with additional sun exposure.
Ichthyosis manifests with varying degrees of severity. While this case is fairly severe, the worst cases (Harlequin and lamellar forms) begin at birth with a nearly absent stratum corneum.
This patient and his family were advised on the use of emollients and avoidance of excessive drying of skin. They were also strongly encouraged to seek genetic counseling.
TAKE-HOME LEARNING POINTS
- Ichthyosis is a family of disorders that cause a breakdown of normal filamentous structures (filaggrin) that hold the layers of skin together.
- The resultant water loss can leave skin vulnerable to penetration by allergens and other noxious substances.
- Ichthyosis vulgaris is by far the most common member of this family of disorders, comprising more than 95% of cases.
- Heavy emollients and avoidance of drying constitute the bulk of treatment efforts.
A 6-year-old boy is referred to dermatology for evaluation of his dry, thin skin. Since birth, it has frequently torn, and it burns with the application of almost any product or soap. Neither OTC nor prescription products have helped.
The boy is reportedly in good health otherwise and is not atopic. Nonetheless, two of his siblings are similarly affected, and there is a strong family history of similar dermatologic problems on his father’s side. He and his family are from Mexico and have type IV skin.
EXAMINATION
The patient is in no distress but does complain about his skin problems. His skin is quite thin and dry, and fine scaling covers his palms, face, legs, trunk, and scalp. In short, none of his skin looks normal. The skin on his legs is especially scaly and has a pronounced reticulated appearance.

His fingernails are dystrophic, with transverse ridging and a loss of connection between the cuticles and nail plates.
What is the diagnosis?
Ichthyosis vulgaris (IV) is one of a family of disorders that cause a breakdown of normal filamentous structures (filaggrin) that hold the layers of skin together. This breakdown can lead to excessive water loss, as well as vulnerability of skin to penetration by allergens and other noxious substances.
IV is by far the most common variation, comprising at least 95% of all ichthyosiform dermatoses. It results from an inherited abnormality of epidermal differentiation or metabolism; affected patients have a higher incidence of eye problems (eg, keratitis, cataracts) in addition to their skin problems.
A total of 28 types of ichthyosis have been described, many of which are part of larger syndromes (such as keratitis-ichthyosis-deafness syndrome or Netherton syndrome). Another uncommon type, X-linked recessive ichthyosis, manifests in about 1 in 5,000 births; these patients improve dramatically in the summertime with additional sun exposure.
Ichthyosis manifests with varying degrees of severity. While this case is fairly severe, the worst cases (Harlequin and lamellar forms) begin at birth with a nearly absent stratum corneum.
This patient and his family were advised on the use of emollients and avoidance of excessive drying of skin. They were also strongly encouraged to seek genetic counseling.
TAKE-HOME LEARNING POINTS
- Ichthyosis is a family of disorders that cause a breakdown of normal filamentous structures (filaggrin) that hold the layers of skin together.
- The resultant water loss can leave skin vulnerable to penetration by allergens and other noxious substances.
- Ichthyosis vulgaris is by far the most common member of this family of disorders, comprising more than 95% of cases.
- Heavy emollients and avoidance of drying constitute the bulk of treatment efforts.
A 6-year-old boy is referred to dermatology for evaluation of his dry, thin skin. Since birth, it has frequently torn, and it burns with the application of almost any product or soap. Neither OTC nor prescription products have helped.
The boy is reportedly in good health otherwise and is not atopic. Nonetheless, two of his siblings are similarly affected, and there is a strong family history of similar dermatologic problems on his father’s side. He and his family are from Mexico and have type IV skin.
EXAMINATION
The patient is in no distress but does complain about his skin problems. His skin is quite thin and dry, and fine scaling covers his palms, face, legs, trunk, and scalp. In short, none of his skin looks normal. The skin on his legs is especially scaly and has a pronounced reticulated appearance.

His fingernails are dystrophic, with transverse ridging and a loss of connection between the cuticles and nail plates.
What is the diagnosis?
Ichthyosis vulgaris (IV) is one of a family of disorders that cause a breakdown of normal filamentous structures (filaggrin) that hold the layers of skin together. This breakdown can lead to excessive water loss, as well as vulnerability of skin to penetration by allergens and other noxious substances.
IV is by far the most common variation, comprising at least 95% of all ichthyosiform dermatoses. It results from an inherited abnormality of epidermal differentiation or metabolism; affected patients have a higher incidence of eye problems (eg, keratitis, cataracts) in addition to their skin problems.
A total of 28 types of ichthyosis have been described, many of which are part of larger syndromes (such as keratitis-ichthyosis-deafness syndrome or Netherton syndrome). Another uncommon type, X-linked recessive ichthyosis, manifests in about 1 in 5,000 births; these patients improve dramatically in the summertime with additional sun exposure.
Ichthyosis manifests with varying degrees of severity. While this case is fairly severe, the worst cases (Harlequin and lamellar forms) begin at birth with a nearly absent stratum corneum.
This patient and his family were advised on the use of emollients and avoidance of excessive drying of skin. They were also strongly encouraged to seek genetic counseling.
TAKE-HOME LEARNING POINTS
- Ichthyosis is a family of disorders that cause a breakdown of normal filamentous structures (filaggrin) that hold the layers of skin together.
- The resultant water loss can leave skin vulnerable to penetration by allergens and other noxious substances.
- Ichthyosis vulgaris is by far the most common member of this family of disorders, comprising more than 95% of cases.
- Heavy emollients and avoidance of drying constitute the bulk of treatment efforts.
Decreasing RBC transfusions saves center $1 million
Researchers have reported that interventions designed to decrease the need for red blood cell (RBC) transfusions proved effective when implemented at an academic medical center, and they resulted in an annual cost savings of more than $1 million.
The interventions included educational tools, a “best practice advisory,” and changes to the computerized provider order entry system.
They enabled the medical center to reduce multi-unit transfusions by 67.1% and transfusions for patients with hemoglobin (Hb) values of at least 7 g/dL by 47.4%.
Ian Jenkins, MD, of University of California San Diego (UCSD) Health, and his colleagues reported these results in The Joint Commission Journal on Quality and Patient Safety.
This work began with a multidisciplinary team at UCSD Health reviewing the transfusion literature on clinical trials, meta-analyses, guidelines, and improvement efforts.
The team used the information gleaned from this review and implemented the following interventions in an attempt to reduce unnecessary RBC transfusions:
- Educational tools (handouts, a video, PowerPoint presentations, etc)
- Enhancements to the health system’s computerized provider order entry system (eg, changing default RBC dose from 2 units to 1 unit)
- A “best practice advisory” intended to reduce unnecessary blood product use and costs by using real-time clinical decision support, a process for providing information at the point of care to help inform decisions about a patient’s care.
To assess the impact of their interventions, the researchers evaluated data on most non-infant, inpatient RBC transfusions given at UCSD Health between January 1, 2014, and September 30, 2016.
They excluded units given to patients with gastrointestinal bleeding and units given within 12 hours of a surgical procedure. The team said they excluded these transfusions because of the higher probability of unstable blood volume or special circumstances that might justify off-protocol transfusion.
The researchers then calculated the rate of inpatient RBC units transfused per 1000 patient-days (without exclusions), the percentage of inpatient RBC units transfused for patients with Hb ≥ 7 g/dL, and the percentage of multi-unit RBC transfusions, divided into 3 periods:
- Pre-intervention or baseline (January 1, 2014–September 30, 2014)
- During the intervention (October 1, 2014–April 30, 2015)
- Post-intervention (May 1, 2015–September 30, 2016).
There were 36,386 RBC units administered during the study period, which was 464,424 patient days.
Multi-unit transfusions decreased from 59.9% at baseline to 41.7% during the intervention period and 19.7% during the post-intervention period (P<0.0001).
Transfusions in patients with Hb values ≥ 7 g/dL decreased from 72.3% at baseline to 57.8% during the intervention period and 38.0% during the post-intervention period (P<0.0001).
The total RBC transfusion rate (units per 1000 patient-days) decreased from 89.8 at baseline to 78.1 during the intervention period and 72.7 during the post-intervention period (P<0.0001).
The estimated savings, based on a cost of $250 per RBC unit, was about $1,050,750 per year. ![]()
Researchers have reported that interventions designed to decrease the need for red blood cell (RBC) transfusions proved effective when implemented at an academic medical center, and they resulted in an annual cost savings of more than $1 million.
The interventions included educational tools, a “best practice advisory,” and changes to the computerized provider order entry system.
They enabled the medical center to reduce multi-unit transfusions by 67.1% and transfusions for patients with hemoglobin (Hb) values of at least 7 g/dL by 47.4%.
Ian Jenkins, MD, of University of California San Diego (UCSD) Health, and his colleagues reported these results in The Joint Commission Journal on Quality and Patient Safety.
This work began with a multidisciplinary team at UCSD Health reviewing the transfusion literature on clinical trials, meta-analyses, guidelines, and improvement efforts.
The team used the information gleaned from this review and implemented the following interventions in an attempt to reduce unnecessary RBC transfusions:
- Educational tools (handouts, a video, PowerPoint presentations, etc)
- Enhancements to the health system’s computerized provider order entry system (eg, changing default RBC dose from 2 units to 1 unit)
- A “best practice advisory” intended to reduce unnecessary blood product use and costs by using real-time clinical decision support, a process for providing information at the point of care to help inform decisions about a patient’s care.
To assess the impact of their interventions, the researchers evaluated data on most non-infant, inpatient RBC transfusions given at UCSD Health between January 1, 2014, and September 30, 2016.
They excluded units given to patients with gastrointestinal bleeding and units given within 12 hours of a surgical procedure. The team said they excluded these transfusions because of the higher probability of unstable blood volume or special circumstances that might justify off-protocol transfusion.
The researchers then calculated the rate of inpatient RBC units transfused per 1000 patient-days (without exclusions), the percentage of inpatient RBC units transfused for patients with Hb ≥ 7 g/dL, and the percentage of multi-unit RBC transfusions, divided into 3 periods:
- Pre-intervention or baseline (January 1, 2014–September 30, 2014)
- During the intervention (October 1, 2014–April 30, 2015)
- Post-intervention (May 1, 2015–September 30, 2016).
There were 36,386 RBC units administered during the study period, which was 464,424 patient days.
Multi-unit transfusions decreased from 59.9% at baseline to 41.7% during the intervention period and 19.7% during the post-intervention period (P<0.0001).
Transfusions in patients with Hb values ≥ 7 g/dL decreased from 72.3% at baseline to 57.8% during the intervention period and 38.0% during the post-intervention period (P<0.0001).
The total RBC transfusion rate (units per 1000 patient-days) decreased from 89.8 at baseline to 78.1 during the intervention period and 72.7 during the post-intervention period (P<0.0001).
The estimated savings, based on a cost of $250 per RBC unit, was about $1,050,750 per year. ![]()
Researchers have reported that interventions designed to decrease the need for red blood cell (RBC) transfusions proved effective when implemented at an academic medical center, and they resulted in an annual cost savings of more than $1 million.
The interventions included educational tools, a “best practice advisory,” and changes to the computerized provider order entry system.
They enabled the medical center to reduce multi-unit transfusions by 67.1% and transfusions for patients with hemoglobin (Hb) values of at least 7 g/dL by 47.4%.
Ian Jenkins, MD, of University of California San Diego (UCSD) Health, and his colleagues reported these results in The Joint Commission Journal on Quality and Patient Safety.
This work began with a multidisciplinary team at UCSD Health reviewing the transfusion literature on clinical trials, meta-analyses, guidelines, and improvement efforts.
The team used the information gleaned from this review and implemented the following interventions in an attempt to reduce unnecessary RBC transfusions:
- Educational tools (handouts, a video, PowerPoint presentations, etc)
- Enhancements to the health system’s computerized provider order entry system (eg, changing default RBC dose from 2 units to 1 unit)
- A “best practice advisory” intended to reduce unnecessary blood product use and costs by using real-time clinical decision support, a process for providing information at the point of care to help inform decisions about a patient’s care.
To assess the impact of their interventions, the researchers evaluated data on most non-infant, inpatient RBC transfusions given at UCSD Health between January 1, 2014, and September 30, 2016.
They excluded units given to patients with gastrointestinal bleeding and units given within 12 hours of a surgical procedure. The team said they excluded these transfusions because of the higher probability of unstable blood volume or special circumstances that might justify off-protocol transfusion.
The researchers then calculated the rate of inpatient RBC units transfused per 1000 patient-days (without exclusions), the percentage of inpatient RBC units transfused for patients with Hb ≥ 7 g/dL, and the percentage of multi-unit RBC transfusions, divided into 3 periods:
- Pre-intervention or baseline (January 1, 2014–September 30, 2014)
- During the intervention (October 1, 2014–April 30, 2015)
- Post-intervention (May 1, 2015–September 30, 2016).
There were 36,386 RBC units administered during the study period, which was 464,424 patient days.
Multi-unit transfusions decreased from 59.9% at baseline to 41.7% during the intervention period and 19.7% during the post-intervention period (P<0.0001).
Transfusions in patients with Hb values ≥ 7 g/dL decreased from 72.3% at baseline to 57.8% during the intervention period and 38.0% during the post-intervention period (P<0.0001).
The total RBC transfusion rate (units per 1000 patient-days) decreased from 89.8 at baseline to 78.1 during the intervention period and 72.7 during the post-intervention period (P<0.0001).
The estimated savings, based on a cost of $250 per RBC unit, was about $1,050,750 per year. ![]()
GEP classifier predicts risk in MM
A gene expression profiling (GEP) classifier can accurately identify high- and low-risk patients with multiple myeloma (MM), according to research published in Clinical Lymphoma, Myeloma and Leukemia.
The GEP classifier is known as SKY92, and researchers found they could accurately identify high-risk MM patients using SKY92 alone.
By combining SKY92 with the International Staging System (ISS), the team was able to identify low-risk MM patients as well.
“We have demonstrated the prognostic value of SKY92 not only as a marker of high risk, but also as a marker of low risk when combined with ISS,” said study author Erik H. van Beers, PhD, vice president of genomics at SkylineDx, the company that developed SKY92.
“Both validated markers may serve as the basis for the discovery of improved individualized therapies for patients with multiple myeloma.”
Dr van Beers and his colleagues compared 8 risk-assessment platforms to analyze gene expression data from 91 newly diagnosed MM patients. The patients were included in an independent dataset amassed by the Multiple Myeloma Research Foundation and the Multiple Myeloma Genomics Initiative (MMRF/MMGI).
The researchers used the GEP classifiers SKY92, UAMS70, UAMS80, IFM15, HM19, Cancer Testis Antigen, Centrosome Index, and Proliferation Index to identify high-risk patients.
Of the 8 classifiers, SKY92 identified the largest proportion (21%) of high-risk cases and also attained the highest Cox proportional hazard ratio (8.2) for overall survival (OS).
Additionally, SKY92 predicted 9 of the 13 (60%) deaths that occurred at 2 years and 16 of the 31 (52%) deaths at 5 years, a predictive rate that was higher than any of the other classifiers.
Dr van Beers and his colleagues also combined the SKY92 standard-risk classifier with the ISS to identify low-risk patients in the MMRF/MMGI cohort. This combination was recently identified as a marker for detecting low-risk MM.*
The SKY92/ISS marker identified 42% of patients as low risk, with their median OS not reached at 96 months. The low-risk classification was strongly supported by the achieved hazard ratio of 10.
“The MMRF/MMGI dataset was not part of our previous discovery*, which demonstrated that the combination of SKY92 and ISS identifies the lowest-risk patients with high accuracy and leaves the smallest proportion of patients identified as intermediate risk,” Dr van Beers said. “Thus, the dataset remained available for independent validation—an important factor in the adoption of gene expression profiling tools.”
“We are pleased to have validated the SKY92/ISS low-risk marker by applying it to the well-characterized MMRF/MMGI cohort,” added study author Rafael Fonseca, MD, of the Mayo Clinic in Scottsdale, Arizona. “Our findings further strengthen the prognostic utility of the combination marker.” ![]()
*Kuiper R, van Duin M, van Vliet, MH, et al. Prediction of high- and low-risk multiple myeloma based on gene expression and the International Staging System. Blood. 2015;126(17):1996-2004.
A gene expression profiling (GEP) classifier can accurately identify high- and low-risk patients with multiple myeloma (MM), according to research published in Clinical Lymphoma, Myeloma and Leukemia.
The GEP classifier is known as SKY92, and researchers found they could accurately identify high-risk MM patients using SKY92 alone.
By combining SKY92 with the International Staging System (ISS), the team was able to identify low-risk MM patients as well.
“We have demonstrated the prognostic value of SKY92 not only as a marker of high risk, but also as a marker of low risk when combined with ISS,” said study author Erik H. van Beers, PhD, vice president of genomics at SkylineDx, the company that developed SKY92.
“Both validated markers may serve as the basis for the discovery of improved individualized therapies for patients with multiple myeloma.”
Dr van Beers and his colleagues compared 8 risk-assessment platforms to analyze gene expression data from 91 newly diagnosed MM patients. The patients were included in an independent dataset amassed by the Multiple Myeloma Research Foundation and the Multiple Myeloma Genomics Initiative (MMRF/MMGI).
The researchers used the GEP classifiers SKY92, UAMS70, UAMS80, IFM15, HM19, Cancer Testis Antigen, Centrosome Index, and Proliferation Index to identify high-risk patients.
Of the 8 classifiers, SKY92 identified the largest proportion (21%) of high-risk cases and also attained the highest Cox proportional hazard ratio (8.2) for overall survival (OS).
Additionally, SKY92 predicted 9 of the 13 (60%) deaths that occurred at 2 years and 16 of the 31 (52%) deaths at 5 years, a predictive rate that was higher than any of the other classifiers.
Dr van Beers and his colleagues also combined the SKY92 standard-risk classifier with the ISS to identify low-risk patients in the MMRF/MMGI cohort. This combination was recently identified as a marker for detecting low-risk MM.*
The SKY92/ISS marker identified 42% of patients as low risk, with their median OS not reached at 96 months. The low-risk classification was strongly supported by the achieved hazard ratio of 10.
“The MMRF/MMGI dataset was not part of our previous discovery*, which demonstrated that the combination of SKY92 and ISS identifies the lowest-risk patients with high accuracy and leaves the smallest proportion of patients identified as intermediate risk,” Dr van Beers said. “Thus, the dataset remained available for independent validation—an important factor in the adoption of gene expression profiling tools.”
“We are pleased to have validated the SKY92/ISS low-risk marker by applying it to the well-characterized MMRF/MMGI cohort,” added study author Rafael Fonseca, MD, of the Mayo Clinic in Scottsdale, Arizona. “Our findings further strengthen the prognostic utility of the combination marker.” ![]()
*Kuiper R, van Duin M, van Vliet, MH, et al. Prediction of high- and low-risk multiple myeloma based on gene expression and the International Staging System. Blood. 2015;126(17):1996-2004.
A gene expression profiling (GEP) classifier can accurately identify high- and low-risk patients with multiple myeloma (MM), according to research published in Clinical Lymphoma, Myeloma and Leukemia.
The GEP classifier is known as SKY92, and researchers found they could accurately identify high-risk MM patients using SKY92 alone.
By combining SKY92 with the International Staging System (ISS), the team was able to identify low-risk MM patients as well.
“We have demonstrated the prognostic value of SKY92 not only as a marker of high risk, but also as a marker of low risk when combined with ISS,” said study author Erik H. van Beers, PhD, vice president of genomics at SkylineDx, the company that developed SKY92.
“Both validated markers may serve as the basis for the discovery of improved individualized therapies for patients with multiple myeloma.”
Dr van Beers and his colleagues compared 8 risk-assessment platforms to analyze gene expression data from 91 newly diagnosed MM patients. The patients were included in an independent dataset amassed by the Multiple Myeloma Research Foundation and the Multiple Myeloma Genomics Initiative (MMRF/MMGI).
The researchers used the GEP classifiers SKY92, UAMS70, UAMS80, IFM15, HM19, Cancer Testis Antigen, Centrosome Index, and Proliferation Index to identify high-risk patients.
Of the 8 classifiers, SKY92 identified the largest proportion (21%) of high-risk cases and also attained the highest Cox proportional hazard ratio (8.2) for overall survival (OS).
Additionally, SKY92 predicted 9 of the 13 (60%) deaths that occurred at 2 years and 16 of the 31 (52%) deaths at 5 years, a predictive rate that was higher than any of the other classifiers.
Dr van Beers and his colleagues also combined the SKY92 standard-risk classifier with the ISS to identify low-risk patients in the MMRF/MMGI cohort. This combination was recently identified as a marker for detecting low-risk MM.*
The SKY92/ISS marker identified 42% of patients as low risk, with their median OS not reached at 96 months. The low-risk classification was strongly supported by the achieved hazard ratio of 10.
“The MMRF/MMGI dataset was not part of our previous discovery*, which demonstrated that the combination of SKY92 and ISS identifies the lowest-risk patients with high accuracy and leaves the smallest proportion of patients identified as intermediate risk,” Dr van Beers said. “Thus, the dataset remained available for independent validation—an important factor in the adoption of gene expression profiling tools.”
“We are pleased to have validated the SKY92/ISS low-risk marker by applying it to the well-characterized MMRF/MMGI cohort,” added study author Rafael Fonseca, MD, of the Mayo Clinic in Scottsdale, Arizona. “Our findings further strengthen the prognostic utility of the combination marker.” ![]()
*Kuiper R, van Duin M, van Vliet, MH, et al. Prediction of high- and low-risk multiple myeloma based on gene expression and the International Staging System. Blood. 2015;126(17):1996-2004.