User login
New guidelines highlight rapid, interdisciplinary treatment of PANS/PANDAS
Prompt, symptomatic, multidisciplinary treatment is the best way to curtail the symptoms of pediatric acute-onset neuropsychiatric syndrome (PANS) and pediatric autoimmune neuropsychiatric syndrome associated with streptococcal infection (PANDAS), according to new guidelines.
Clinicians should tailor treatment to symptoms, wrote Margo Thienemann, MD, of Stanford (Calif.) University and her coauthors in the clinical management section of the guidelines. For example, children with mild psychiatric symptoms might only need cognitive-behavioral therapy (CBT), while more severe symptoms can merit a slow up-titration of psychoactive medications.
Clinical management of PANS/PANDAS includes psychoeducational, psychotherapeutic, behavioral, family- and school-based, and pharmacologic interventions, Dr. Thienemann and her associates wrote. Starting CBT (exposure-response prevention) has the best chance of halting OCD behaviors. Acutely ill children might not be ready for CBT, but parents still can learn to “hold the line” to avoid accommodating and worsening irrational fears.
Options for psychoactive medications include benzodiazepines for anxiety; aripiprazole, risperidone, olanzapine, haloperidol, or quetiapine for psychosis; and SSRIs, such as fluoxetine, sertraline, and fluvoxamine for depression and OCD. Severe depression merits both psychotherapy and an SSRI. Antipsychotics are not indicated for OCD unless children are incapacitated and only if their QTc interval does not exceed 450 milliseconds. Because PANS/PANDAS patients can react severely to psychotropics, clinicians should “start low” at about a quarter of a typical dose and “go slow,” gradually titrating up.
It’s best to rule out other medical disorders first when patients refuse or restrict food or fluids. Next, clinicians should assess medical stability and support nutrition and hydration while treating underlying brain inflammation. “Feeding tubes may be necessary, at least during the acute phases of the illness,” the authors wrote. Chronic symptoms can warrant treatments for eating disorders.
Bouts of aggression or irritability are classic and can be especially challenging for families. Parents can refocus the child with toys or by dancing, singing, or acting silly but should also make a safety plan, such as calling 911, if aggressive behaviors are endangering the patient or family members. Pharmacologic options for aggression include diphenhydramine, benzodiazepines, and antipsychotics.
For tics, options include comprehensive behavioral intervention for tics, habit reversal training, and cautiously monitored pharmacotherapy with alpha-2 adrenergic agonists, clonidine, guanfacine, or short-course antipsychotics. Symptoms of attention-deficit/hyperactivity disorder merit classroom accommodations; methylphenidate compounds can be added if needed. For children with sleep disturbances, the best strategy is to focus on sleep hygiene before considering low-dose diphenhydramine, melatonin, cyproheptadine, clonidine, trazodone, or zolpidem. Pain, however, needs early treatment to stop it from becoming refractory. Pain and stiffness after awakening can signal arthritis, enthesitis, or inflammatory back pain and warrant physical therapy and evaluation by a pediatric rheumatologist or pain specialist.
Part II of the guidelines covers immunomodulators. As in other severe brain disorders, early treatment improves outcomes and helps prevent relapses, wrote Jennifer Frankovich, MD, also of Stanford University, and her associates. Clinicians should start second-line therapies if first-line treatment fails. Acute impairment can remit with NSAIDs or a short course of oral corticosteroids, but chronic symptoms often need more aggressive and prolonged immunotherapy. Children with moderate to severe impairment should receive intravenous immunoglobulins, and those with severe, chronic impairment may need bursts of high-dose corticosteroids or longer-term corticosteroids with taper. Patients with extreme or life-threatening impairment should receive first-line therapeutic plasma exchange alone or with intravenous immunoglobulins, high-dose intravenous corticosteroids, and rituximab.
Part III of the guidelines covers infections. Most cases involve group A streptococci (GAS), but other culprits include Mycoplasma pneumoniae and viruses, such as influenza, wrote Michael S. Cooperstock, MD, MPH, of the University of Missouri-Columbia and his associates. They recommend antistreptococcal treatment for “essentially all” newly diagnosed cases. They also suggest secondary antistreptococcal prophylaxis for severe neuropsychiatric symptoms or intermittent exacerbations associated with GAS. “For all other [cases], vigilance for GAS infection in both the patient and close contacts is recommended,” they wrote. “Since any intercurrent infection may induce a symptom flare, close observation with appropriate therapy for any treatable intercurrent infection is warranted.” They also recommend standard childhood immunizations and monitoring vitamin D levels.
The National Institutes of Health supported the research summarized in the guidelines. Dr. Thienemann disclosed grants from Auspex, Shire, Pfizer, F. Hoffmann-La Roche, AstraZeneca, Sunovion, Neurocrine Biosciences, Psyadon, and the PANDAS Network, as well as personal fees from the International OCD Foundation and the Tourette Syndrome Association. Dr. Frankovich and Dr. Cooperstock had no relevant disclosures.
Prompt, symptomatic, multidisciplinary treatment is the best way to curtail the symptoms of pediatric acute-onset neuropsychiatric syndrome (PANS) and pediatric autoimmune neuropsychiatric syndrome associated with streptococcal infection (PANDAS), according to new guidelines.
Clinicians should tailor treatment to symptoms, wrote Margo Thienemann, MD, of Stanford (Calif.) University and her coauthors in the clinical management section of the guidelines. For example, children with mild psychiatric symptoms might only need cognitive-behavioral therapy (CBT), while more severe symptoms can merit a slow up-titration of psychoactive medications.
Clinical management of PANS/PANDAS includes psychoeducational, psychotherapeutic, behavioral, family- and school-based, and pharmacologic interventions, Dr. Thienemann and her associates wrote. Starting CBT (exposure-response prevention) has the best chance of halting OCD behaviors. Acutely ill children might not be ready for CBT, but parents still can learn to “hold the line” to avoid accommodating and worsening irrational fears.
Options for psychoactive medications include benzodiazepines for anxiety; aripiprazole, risperidone, olanzapine, haloperidol, or quetiapine for psychosis; and SSRIs, such as fluoxetine, sertraline, and fluvoxamine for depression and OCD. Severe depression merits both psychotherapy and an SSRI. Antipsychotics are not indicated for OCD unless children are incapacitated and only if their QTc interval does not exceed 450 milliseconds. Because PANS/PANDAS patients can react severely to psychotropics, clinicians should “start low” at about a quarter of a typical dose and “go slow,” gradually titrating up.
It’s best to rule out other medical disorders first when patients refuse or restrict food or fluids. Next, clinicians should assess medical stability and support nutrition and hydration while treating underlying brain inflammation. “Feeding tubes may be necessary, at least during the acute phases of the illness,” the authors wrote. Chronic symptoms can warrant treatments for eating disorders.
Bouts of aggression or irritability are classic and can be especially challenging for families. Parents can refocus the child with toys or by dancing, singing, or acting silly but should also make a safety plan, such as calling 911, if aggressive behaviors are endangering the patient or family members. Pharmacologic options for aggression include diphenhydramine, benzodiazepines, and antipsychotics.
For tics, options include comprehensive behavioral intervention for tics, habit reversal training, and cautiously monitored pharmacotherapy with alpha-2 adrenergic agonists, clonidine, guanfacine, or short-course antipsychotics. Symptoms of attention-deficit/hyperactivity disorder merit classroom accommodations; methylphenidate compounds can be added if needed. For children with sleep disturbances, the best strategy is to focus on sleep hygiene before considering low-dose diphenhydramine, melatonin, cyproheptadine, clonidine, trazodone, or zolpidem. Pain, however, needs early treatment to stop it from becoming refractory. Pain and stiffness after awakening can signal arthritis, enthesitis, or inflammatory back pain and warrant physical therapy and evaluation by a pediatric rheumatologist or pain specialist.
Part II of the guidelines covers immunomodulators. As in other severe brain disorders, early treatment improves outcomes and helps prevent relapses, wrote Jennifer Frankovich, MD, also of Stanford University, and her associates. Clinicians should start second-line therapies if first-line treatment fails. Acute impairment can remit with NSAIDs or a short course of oral corticosteroids, but chronic symptoms often need more aggressive and prolonged immunotherapy. Children with moderate to severe impairment should receive intravenous immunoglobulins, and those with severe, chronic impairment may need bursts of high-dose corticosteroids or longer-term corticosteroids with taper. Patients with extreme or life-threatening impairment should receive first-line therapeutic plasma exchange alone or with intravenous immunoglobulins, high-dose intravenous corticosteroids, and rituximab.
Part III of the guidelines covers infections. Most cases involve group A streptococci (GAS), but other culprits include Mycoplasma pneumoniae and viruses, such as influenza, wrote Michael S. Cooperstock, MD, MPH, of the University of Missouri-Columbia and his associates. They recommend antistreptococcal treatment for “essentially all” newly diagnosed cases. They also suggest secondary antistreptococcal prophylaxis for severe neuropsychiatric symptoms or intermittent exacerbations associated with GAS. “For all other [cases], vigilance for GAS infection in both the patient and close contacts is recommended,” they wrote. “Since any intercurrent infection may induce a symptom flare, close observation with appropriate therapy for any treatable intercurrent infection is warranted.” They also recommend standard childhood immunizations and monitoring vitamin D levels.
The National Institutes of Health supported the research summarized in the guidelines. Dr. Thienemann disclosed grants from Auspex, Shire, Pfizer, F. Hoffmann-La Roche, AstraZeneca, Sunovion, Neurocrine Biosciences, Psyadon, and the PANDAS Network, as well as personal fees from the International OCD Foundation and the Tourette Syndrome Association. Dr. Frankovich and Dr. Cooperstock had no relevant disclosures.
Prompt, symptomatic, multidisciplinary treatment is the best way to curtail the symptoms of pediatric acute-onset neuropsychiatric syndrome (PANS) and pediatric autoimmune neuropsychiatric syndrome associated with streptococcal infection (PANDAS), according to new guidelines.
Clinicians should tailor treatment to symptoms, wrote Margo Thienemann, MD, of Stanford (Calif.) University and her coauthors in the clinical management section of the guidelines. For example, children with mild psychiatric symptoms might only need cognitive-behavioral therapy (CBT), while more severe symptoms can merit a slow up-titration of psychoactive medications.
Clinical management of PANS/PANDAS includes psychoeducational, psychotherapeutic, behavioral, family- and school-based, and pharmacologic interventions, Dr. Thienemann and her associates wrote. Starting CBT (exposure-response prevention) has the best chance of halting OCD behaviors. Acutely ill children might not be ready for CBT, but parents still can learn to “hold the line” to avoid accommodating and worsening irrational fears.
Options for psychoactive medications include benzodiazepines for anxiety; aripiprazole, risperidone, olanzapine, haloperidol, or quetiapine for psychosis; and SSRIs, such as fluoxetine, sertraline, and fluvoxamine for depression and OCD. Severe depression merits both psychotherapy and an SSRI. Antipsychotics are not indicated for OCD unless children are incapacitated and only if their QTc interval does not exceed 450 milliseconds. Because PANS/PANDAS patients can react severely to psychotropics, clinicians should “start low” at about a quarter of a typical dose and “go slow,” gradually titrating up.
It’s best to rule out other medical disorders first when patients refuse or restrict food or fluids. Next, clinicians should assess medical stability and support nutrition and hydration while treating underlying brain inflammation. “Feeding tubes may be necessary, at least during the acute phases of the illness,” the authors wrote. Chronic symptoms can warrant treatments for eating disorders.
Bouts of aggression or irritability are classic and can be especially challenging for families. Parents can refocus the child with toys or by dancing, singing, or acting silly but should also make a safety plan, such as calling 911, if aggressive behaviors are endangering the patient or family members. Pharmacologic options for aggression include diphenhydramine, benzodiazepines, and antipsychotics.
For tics, options include comprehensive behavioral intervention for tics, habit reversal training, and cautiously monitored pharmacotherapy with alpha-2 adrenergic agonists, clonidine, guanfacine, or short-course antipsychotics. Symptoms of attention-deficit/hyperactivity disorder merit classroom accommodations; methylphenidate compounds can be added if needed. For children with sleep disturbances, the best strategy is to focus on sleep hygiene before considering low-dose diphenhydramine, melatonin, cyproheptadine, clonidine, trazodone, or zolpidem. Pain, however, needs early treatment to stop it from becoming refractory. Pain and stiffness after awakening can signal arthritis, enthesitis, or inflammatory back pain and warrant physical therapy and evaluation by a pediatric rheumatologist or pain specialist.
Part II of the guidelines covers immunomodulators. As in other severe brain disorders, early treatment improves outcomes and helps prevent relapses, wrote Jennifer Frankovich, MD, also of Stanford University, and her associates. Clinicians should start second-line therapies if first-line treatment fails. Acute impairment can remit with NSAIDs or a short course of oral corticosteroids, but chronic symptoms often need more aggressive and prolonged immunotherapy. Children with moderate to severe impairment should receive intravenous immunoglobulins, and those with severe, chronic impairment may need bursts of high-dose corticosteroids or longer-term corticosteroids with taper. Patients with extreme or life-threatening impairment should receive first-line therapeutic plasma exchange alone or with intravenous immunoglobulins, high-dose intravenous corticosteroids, and rituximab.
Part III of the guidelines covers infections. Most cases involve group A streptococci (GAS), but other culprits include Mycoplasma pneumoniae and viruses, such as influenza, wrote Michael S. Cooperstock, MD, MPH, of the University of Missouri-Columbia and his associates. They recommend antistreptococcal treatment for “essentially all” newly diagnosed cases. They also suggest secondary antistreptococcal prophylaxis for severe neuropsychiatric symptoms or intermittent exacerbations associated with GAS. “For all other [cases], vigilance for GAS infection in both the patient and close contacts is recommended,” they wrote. “Since any intercurrent infection may induce a symptom flare, close observation with appropriate therapy for any treatable intercurrent infection is warranted.” They also recommend standard childhood immunizations and monitoring vitamin D levels.
The National Institutes of Health supported the research summarized in the guidelines. Dr. Thienemann disclosed grants from Auspex, Shire, Pfizer, F. Hoffmann-La Roche, AstraZeneca, Sunovion, Neurocrine Biosciences, Psyadon, and the PANDAS Network, as well as personal fees from the International OCD Foundation and the Tourette Syndrome Association. Dr. Frankovich and Dr. Cooperstock had no relevant disclosures.
FROM JOURNAL OF CHILD AND ADOLESCENT PSYCHOPHARMACOLOGY
We Want to Know: Eliciting Hospitalized Patients’ Perspectives on Breakdowns in Care
There is growing recognition that patients and family members have critical insights into healthcare experiences. As consumers of healthcare, patient experience is the definitive gauge of whether healthcare is patient centered. In addition, patients may know things about their healthcare that the care team does not. Several studies have demonstrated that patients have knowledge of adverse events and medical errors that are not detected by other methods.1-5 For these reasons, systems designed to elicit patient perspectives of care and detect patient-perceived breakdowns in care could be used to improve healthcare safety and quality, including the patient experience.
Historically, hospitals have relied on patient-initiated reporting via complaints or legal action as the main source of information regarding patient-perceived breakdowns in care. However, many patients are hesitant to speak up about problems or uncertain about how to report concerns.6-8 As a result, healthcare systems often only learn of the most severe breakdowns in care from a subset of activated patients, thus underestimating how widespread patient-perceived breakdowns are.
To overcome these limitations of patient-initiated reporting, hospitals could conduct outreach to patients to actively identify and learn about patient-perceived breakdowns in care. Potential benefits of outreach to patients include more reliable detection of patient-perceived breakdowns in care, identification of a broader range of types of breakdowns commonly experienced by patients, and recognition of problems in real-time when there is more opportunity for redress. Indeed, some hospitals have adopted active outreach programs such as structured nurse manager rounding or postdischarge phone calls.9
It is possible that outreach will not overcome patients’ reluctance to speak up, or patients may not share serious or actionable breakdowns. The manner in which outreach is conducted is likely to influence the information patients are willing to share. Prior studies examining patient perspectives of healthcare have primarily taken a structured approach with close-ended questions or a focus on specific aspects of care.1,10,11 Limited data collected using an open-ended approach suggest patient-perceived breakdowns in care may be very common.2,12,13 However, the impact of such breakdowns on patients has not been well characterized.
In order to design systems that can optimally detect patient-perceived breakdowns in care, additional information is needed to understand whether patients will report breakdowns in response to outreach programs, what types of problems they will report, and how these problems impact them. Understanding such issues will allow healthcare systems to respond to calls by federal health agencies to develop mechanisms for patients to report concerns about breakdowns in care, thereby providing truly patient-centered care.14 Therefore, we undertook this study with the overall goal of describing what may be learned from an open-ended outreach approach that directly asks patients about problems they have encountered during hospitalization. Specifically, we aim to (1) describe the types of problems reported by patients in response to this outreach approach and (2) characterize patients’ perceptions of the impact of these events.
METHODS
Setting
We conducted this study in 2 hospitals between June 2014 and February 2015. One participating hospital is a large, urban, tertiary care medical center serving a predominantly white (78%) patient population in Baltimore, Maryland. The second hospital is a large, inner city, tertiary care medical center serving a predominantly African-American (71%) patient population in Washington, DC.
Three medical-surgical units (MSUs) at each hospital participated. We selected MSUs because MSU patients interact with a variety of clinicians, often have long stays, and are at risk for adverse events. Hospitalists were part of the clinical care team in each of the participating units, serving either as the attending of record or by comanaging patients.
Patient Eligibility
Patients were potentially eligible if they were at least 18 years old, able to speak English or Spanish, and admitted to the hospital for more than 24 hours. Ineligibility criteria included the following: imminent discharge, observation (noninpatient) status, on hospice, on infection precautions (for inpatient interviews only), psychiatric or violence concerns, prisoner status, significant confusion, or inability to provide informed consent.
Eligible patients in each unit were randomized. Interviewers consecutively approached patients according to their random assignment. If a patient was not available, the interviewer proceeded to the next room. Interviewers returned to rooms of missed patients when possible. Recruitment in the unit ended when the recruitment target for that unit was achieved.
Interviewers
Five interviewers conducted interviews. One author (KS) provided interviewer training that included didactic instruction, observation, feedback, and modeling. Interviewers participated in weekly debriefing sessions. One interviewer speaks Spanish fluently and was able to conduct interviews in Spanish. Translator services were available for the other interviewers.
Interview Process
Interviews were conducted in person while the patients were hospitalized or via telephone 7 - 30 days postdischarge. A patient who had completed an interview while hospitalized was not eligible for a postdischarge telephone interview. Family members or friends present at the time of the interviews could also participate in addition to or in lieu of the patients with the patients’ assent. Interviewers obtained verbal, informed consent at the start of each interview.
The interview domains and sample questions were developed specifically for the current study and are listed in Table 1. The goal of the interview was to elicit the patient’s (or family member’s) perception of their care experiences and their perceptions of the consequences of any problems with their care. The interviewer sought to obtain sufficient detail to understand the patient’s concerns and to determine what, if any, action might be needed to remediate problems reported by patients. Interviewers captured patient responses by taking detailed notes on a case report form or by directly entering patient responses using a computer or iPad at the time of interview at the discretion of the interviewer.
We defined a patient-perceived breakdown as something that went wrong during the hospitalization according to the patient. If a patient-perceived breakdown in care was identified, the interviewer attempted to resolve the concern. Some breakdowns had occurred in the past, making further resolution impossible (eg, a long wait in the emergency department). Other breakdowns were active and addressable, such as the patient having clinical questions that had not been answered. In such cases, the interviewer attempted to address the patient’s concerns, typically by working with unit nursing staff. For patients interviewed postdischarge, the interviewer worked to resolve ongoing patient concerns with the assistance of the patient safety, quality, and compliance teams as needed. The interviewer provided a brief narrative summary of all interviews to unit nursing leadership within 24 hours. Positive comments were sent to leadership but not captured systematically for research purposes. Further details of the process of responding to patients’ concerns will be reported elsewhere. All data were entered into REDCap to facilitate data management and reporting.15
The MedStar Health Research Institute Institutional Review Board reviewed and approved this study.
Categorizing Patients’ Responses: The Patient Experience Coding Tool
Using directed content analysis,16 we deductively created the Patient Experience Coding Tool (PECT) in order to summarize the narrative information captured during the interviews and categorize patient-perceived breakdowns in care. First, we referred to our prior interviews of patients’ views on breakdowns in cancer care6 and surrogate decision-makers’ views on breakdowns in intensive care units13 to create the initial categories. We then applied the resultant framework to the interviews in the present study and refined the categories. This involved applying the categorization to an initial set of interviews to check the sufficiency of the coding categories. We clarified the scope of each category (ie, what types of events fit under each category) and created additional categories (eg, medication-related problems) to capture patient experiences that were not included in the initial framework.
We then coded each interview using the PECT. A minimum of 2 readers reviewed the narrative notes for each interview. The first reader provided an initial categorization; the second reader reviewed the narrative and confirmed or questioned the initial categorization to improve coding accuracy. If a reader was uncertain about the correct categorization, it was discussed by three readers until an agreement was achieved. Because facilities-related problems (eg, food or parking) fall outside the realm of provider-based hospital care, such comments were not the focus of the outreach efforts and were not consistently recorded. Therefore, they were not included in the PECT and are not reported here.
Analyses
We computed simple, descriptive statistics including the number and percentage of patients identifying at least one breakdown, as well as the number and percent reporting each type of breakdown. We also computed the number and percentage of patients reporting any harm and each type of harm. We computed the percentage of patients reporting at least 1 breakdown by hospital, type of interview (postdischarge vs inpatient), selected patient demographic characteristics (eg, gender, age, education, race), and interviewee (patient vs someone other than the patient interviewed or present during the interview) using the chi-square statistic to test the statistical significance of the resulting differences. All statistical analyses were performed using SPSS version 22.
RESULTS
A total of 979 outreach interviews were conducted. Of these, 349 were conducted via telephone postdischarge, and 630 were conducted in person during hospitalization. The average interview duration was 8.5 minutes for telephone interviews and 12.2 minutes for in-person interviews. Of the patients approached to participate, 67% completed an interview (61% in person, 83% via telephone). Patient characteristics are summarized in Table 2.
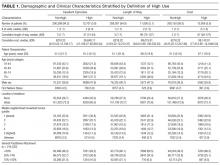
Overall, 386 of 979 interviewees (39.4%) believed they had experienced at least one breakdown in care. The types of patient-perceived breakdowns reported were categorized using the PECT and are summarized in Table 3 and the Figure. The most common concern involved information exchange. Approximately 1 in 10 patients (n = 105, 10.7%) felt that they had not received the information they needed when they needed it. Medication-related concerns were reported by 12.3% (n = 120) of interviewees and predominantly included concerns about what medications were being administered (5.7%) and inadequately treated pain (5.6%). Many of the patients expressing concerns about what medications were administered questioned why they were not receiving their outpatient medications or did not understand why a different medication was being administered, suggesting that many of these instances were related to breakdowns in communication as well. Other relatively common concerns were delays in the admissions process (reported by 9.2% of interviewees), poor team communication (reported by 6.6% of interviewees), healthcare providers with a rude or uncaring manner (reported by 6.3% of interviewees), and problems related to discharge (reported by 5.7% of interviewees).
Of the 386 interviewees who perceived a breakdown in care, 140 (36.3%) perceived harm associated with the event (Table 3). The most common harms were physical (eg, pain; n = 66) and emotional (eg, distress, worry; n = 60). In addition, patients reported instances of damage to relationships with providers (n = 28) resulting in a loss of trust, with participants citing breakdowns as a reason for not coming back to a particular hospital or provider. In other cases, patients believed that breakdowns in care resulted in the need for additional care or a prolonged hospital stay.
We found no difference between the 2 hospitals where the study was conducted in the percentage of interviewees reporting at least 1 breakdown (39.1% vs 39.9%, P = 0.80). We also found no difference between interview method, (ie, in person vs telephone; 40.6% vs 37.2%, respectively, P = 0.30), patient gender (40.6% and 38.8% for men and women, respectively, P = 0.57), race (41.0% and 36.8% for white and black or African-American, respectively, P = 0.20) or between interviewers (P = 0.37). We did identify differences in rates of reporting at least 1 breakdown in care related to age (45.4% of patients aged 60 years or younger vs 34.5% of patients older than 60 years, P < 0.001) and education (32.7% of patients with a high school education or less vs 46.8% of those with at least some college education, P < 0.001). Patients interviewed alone reported fewer breakdowns than if another person was present during the interview or was interviewed in lieu of the patient (37.8% vs 53.4%, P = 0.002). The rate of reporting breakdowns for patients interviewed alone in the hospital is very similar to the rates of those interviewed via telephone (37.8% vs 37.2%). For most types of breakdowns, there were no differences in reporting for in-person vs postdischarge interviews. Discharge-related problems were more frequently reported among patients interviewed postdischarge (8.9% postdischarge vs 4.0% in person, P = 0.002). Patients interviewed in person were more likely to report problems with information exchange compared to patients interviewed postdischarge (17.6% vs 13.5%, respectively; P = 0.09), although this did not reach statistical significance.
DISCUSSION
Through interviews with nearly 1000 patients, we have found that approximately 4 in 10 hospitalized patients believed they experienced a breakdown in care. Not only are patient-perceived breakdowns in care distressingly common, more than one-third of these events resulted in harm according to the patient. Patients reported a diverse range of breakdowns, including problems related to patient experience, as well as breakdowns in technical aspects of medical care. Collectively, these findings illustrate a striking need to identify and address these frequent and potentially harmful breakdowns.
Our findings are consistent with prior studies in which 20% to 50% of patients identified a problem during hospitalization. For example, Weingart et al. interviewed patients in a single general medical unit and found that 20% experienced an adverse event, near miss, or medical error, while nearly 40% experienced what was defined as a service quality incident.2,12 Of note, both our study and the study by Weingart et al. systematically elicited patients’ perspectives of breakdowns in care with explicit questions about problems or breakdowns in care.2,12 Because patients are often reluctant to speak up about problems in care,without such efforts to actively identify problems, providers and leaders are likely to be unaware of the majority of these concerns.6-8 These findings suggest that hospital-based providers should at least consider routinely asking patients about breakdowns in care to identify and respond to patients’ concerns.
Not only are patient-perceived breakdowns common, more than one-third of patients who experienced a breakdown considered it to be harmful. This suggests that our outreach approach identified predominantly nontrivial concerns. We adopted a broad definition of harm that includes emotional distress, damage to the relationship with providers, and life disruption. This differs from other studies examining patient reports of breakdowns in care, in which harm was restricted to physical injury.1,2 We consider this inclusive definition of harm to be a strength of the present study as it provides the most complete picture of the impact of such events on patients. This approach is supported by other studies demonstrating that patients place great emphasis on the psychological consequences of adverse events.17-19 Thus, it is clear from our work and other studies that nonphysical harm is important and warrants concerted efforts to address.
Patients in our study reported a variety of breakdowns, including breakdowns related to patient experience (eg, communication, relationship with providers) and technical aspects of healthcare delivery (eg, diagnosis, treatment). This is consistent with other studies examining patient perspectives of breakdowns in care. Weingart et al.found that hospitalized patients reported a broad range of problems, including adverse events, medical errors, communication breakdowns, and problems with food.2,12 This variety of events suggests a need for integration between the various hospital groups that handle patient-perceived breakdowns, including bedside providers, risk management, patient relations, patient advocates, and quality and safety groups, in order to provide a coordinated and effective response to the full spectrum of patient-perceived breakdowns in care.
Patients in our study were more likely to report breakdowns related to communication and relationships with providers than those related to technical aspects of care. Similarly, Kuzel et al.found that the most common problems cited by patients in the primary care setting were breakdowns in the clinician-patient relationship and access-related problems.17This is not surprising, as patients are likely to have more direct knowledge about communication and interpersonal relationships than about technical aspects of care.
We identified several factors associated with the likelihood of reporting a breakdown in care. Most strikingly, involving a friend or family member in the interview was strongly associated with reporting a breakdown. Other work has also suggested that patients’ friends and family members are an important source of information about safety concerns.20,21 In addition, several patient characteristics were associated with an increased likelihood of reporting a breakdown, including being younger and better educated. These findings highlight the importance of engaging patients’ friends and families in efforts to elicit patient concerns about healthcare, and they confirm recommendations for patients to bring a friend or family member to healthcare encounters.22 In addition, they illustrate the need to better understand how patient characteristics affect perceptions of breakdowns in care and their willingness to speak up, as this could inform efforts to target outreach to especially vulnerable patients.
A strength of this study is the number of interviews completed (almost 1000), which provides a diverse range of patient views and experiences, as evidenced by the demographic characteristics of participants. Interviews were conducted at two hospitals that differ substantially with regard to populations served, further enhancing the generalizability of our findings. Despite the large number of interviews and diverse patient characteristics, patients were drawn from only 3 units at 2 hospitals, which may limit generalizability.
We did not conduct medical record reviews to validate patients’ reports of problems, which may be viewed as a limitation. While such a comparison would be informative, the intent of the current study was to elicit patients’ perceptions of care, including aspects of care that are not typically captured in the medical record, such as communication. Other studies have demonstrated that patients’ reports of medical errors and adverse events tend to differ from providers’ reports of the same subjects.19,23 Therefore, we considered the patients’ perceptions of care to be a useful endpoint in and of itself. We did not determine the extent to which providers were already aware of patients’ concerns or whether they considered patients’ concerns valid. A related limitation is our inability to determine whether the differences we identified in the rates of breakdown reporting based on patient characteristics reflect differences in willingness to report or differences in experiences. Because we included patients in an MSU, it is possible that breakdowns were related to medical care, surgical care, or both. We did not follow patients longitudinally and therefore only captured harm perceived by a patient at the time of the interview. It is possible that patients may have experienced harm later in their hospitalization or following discharge that was not measured. Lastly, we did not measure interrater reliability of the interview coding and therefore do not present the PECT as a validated instrument. These important questions should be targeted for future study.
CONCLUSION
When directly asked about their experiences, almost 4 out of 10 hospitalized patients reported a breakdown in their care, many of which were perceived to be harmful. Not all hospitals will have the resources to implement the intensive approach used in this study to elicit patient-perceived breakdowns. Therefore, further work is needed to develop sustainable methods to overcome patients’ reluctance to report breakdowns in care. Engaging patients’ families and friends may be a particularly fruitful strategy. We offer the PECT as a tool that hospitals could use to summarize a variety of sources of patient feedback such as complaints, responses to surveys, and consumer reviews. Hospitals that effectively encourage patients and their family members to speak up about perceived breakdowns will identify many opportunities to address patient concerns, potentially leading to improved patient safety and experience.
1. Weissman JS, Schneider EC, Weingart SN, et al. Comparing patient-reported hospital adverse events with medical record review: Do patients know something that hospitals do not? Ann Intern Med. 2008;149(2):100-108. PubMed
2. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. PubMed
3. Wetzels R, Wolters R, van Weel C, Wensing M. Mix of methods is needed to identify adverse events in general practice: A prospective observational study. BMC Fam Pract. 2008;9:35. PubMed
4. Friedman SM, Provan D, Moore S, Hanneman K. Errors, near misses and adverse events in the emergency department: What can patients tell us? CJEM. 2008;10(5):421-427. PubMed
5. Iedema R, Allen S, Britton K, Gallagher TH. What do patients and relatives know about problems and failures in care? BMJ Qual Saf. 2012;21(3):198-205. PubMed
6. Mazor KM, Roblin DW, Greene SM, et al. Toward patient-centered cancer care: Patient perceptions of problematic events, impact, and response. J Clin Oncol. 2012;30(15):1784-1790. PubMed
7. Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled ‘difficult’ among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. PubMed
8. Entwistle VA, McCaughan D, Watt IS, et al. Speaking up about safety concerns: Multi-setting qualitative study of patients’ views and experiences. Qual Saf Health Care. 2010;19(6):e33. PubMed
9. Tan M, Lang D. Effectiveness of nurse leader rounding and post-discharge telephone calls in patient satisfaction: A systematic review. JBI database of systematic reviews and implementation reports. 2015;13(7):154-176. PubMed
10. Garbutt J, Bose D, McCawley BA, Burroughs T, Medoff G. Soliciting patient complaints to improve performance. Jt Comm J Qual Saf. 2003;29(3):103-112. PubMed
11. Agoritsas T, Bovier PA, Perneger TV. Patient reports of undesirable events during hospitalization. J Gen Intern Med. 2005;20(10):922-928. PubMed
12. Weingart SN, Pagovich O, Sands DZ, et al. Patient-reported service quality on a medicine unit. Int J Qual Health Care. 2006;18(2):95-101. PubMed
13. Fisher KA, Ahmad S, Jackson M, Mazor KM. Surrogate decision makers’ perspectives on preventable breakdowns in care among critically ill patients: A qualitative study. Patient Educ Couns. 2016;99(10):1685-1693. PubMed
14. Halpern MT, Roussel AE, Treiman K, Nerz PA, Hatlie MJ, Sheridan S. Designing consumer reporting systems for patient safety events. Final Report (Prepared by RTI International and Consumers Advancing Patient Safety under Contract No. 290-06-00001-5). AHRQ Publication No. 11-0060-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed
16. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. PubMed
17. Kuzel AJ, Woolf SH, Gilchrist VJ, et al. Patient reports of preventable problems and harms in primary health care. Ann Fam Med. 2004;2(4):333-340. PubMed
18. Sokol-Hessner L, Folcarelli PH, Sands KE. Emotional harm from disrespect: The neglected preventable harm. BMJ Qual Saf. 2015;24(9):550-553. PubMed
19. Masso Guijarro P, Aranaz Andres JM, Mira JJ, Perdiguero E, Aibar C. Adverse events in hospitals: The patient’s point of view. Qual Saf Health Care. 2010;19(2):144-147. PubMed
20. Bardach NS, Lyndon A, Asteria-Penaloza R, Goldman LE, Lin GA, Dudley RA. From the closest observers of patient care: A thematic analysis of online narrative reviews of hospitals. BMJ Qual Saf. 2015. PubMed
21. Schneider EC, Ridgely MS, Quigley DD, et al. Developing and testing the health care safety hotline: A prototype consumer reporting system for patient safety events. Final Report (Prepared by RAND Corporation under contract HHSA2902010000171). Rockvelle, MD: Agency for Healthcare Research and Quality; May 2016.
22. Shekelle PG, Pronovost PJ, Wachter RM, et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158(5 Pt 2):365-368. PubMed
23. Lawton R, O’Hara JK, Sheard L, et al. Can staff and patient perspectives on hospital safety predict harm-free care? an analysis of staff and patient survey data and routinely collected outcomes. BMJ Qual Saf. 2015;24(6):369-376. PubMed
There is growing recognition that patients and family members have critical insights into healthcare experiences. As consumers of healthcare, patient experience is the definitive gauge of whether healthcare is patient centered. In addition, patients may know things about their healthcare that the care team does not. Several studies have demonstrated that patients have knowledge of adverse events and medical errors that are not detected by other methods.1-5 For these reasons, systems designed to elicit patient perspectives of care and detect patient-perceived breakdowns in care could be used to improve healthcare safety and quality, including the patient experience.
Historically, hospitals have relied on patient-initiated reporting via complaints or legal action as the main source of information regarding patient-perceived breakdowns in care. However, many patients are hesitant to speak up about problems or uncertain about how to report concerns.6-8 As a result, healthcare systems often only learn of the most severe breakdowns in care from a subset of activated patients, thus underestimating how widespread patient-perceived breakdowns are.
To overcome these limitations of patient-initiated reporting, hospitals could conduct outreach to patients to actively identify and learn about patient-perceived breakdowns in care. Potential benefits of outreach to patients include more reliable detection of patient-perceived breakdowns in care, identification of a broader range of types of breakdowns commonly experienced by patients, and recognition of problems in real-time when there is more opportunity for redress. Indeed, some hospitals have adopted active outreach programs such as structured nurse manager rounding or postdischarge phone calls.9
It is possible that outreach will not overcome patients’ reluctance to speak up, or patients may not share serious or actionable breakdowns. The manner in which outreach is conducted is likely to influence the information patients are willing to share. Prior studies examining patient perspectives of healthcare have primarily taken a structured approach with close-ended questions or a focus on specific aspects of care.1,10,11 Limited data collected using an open-ended approach suggest patient-perceived breakdowns in care may be very common.2,12,13 However, the impact of such breakdowns on patients has not been well characterized.
In order to design systems that can optimally detect patient-perceived breakdowns in care, additional information is needed to understand whether patients will report breakdowns in response to outreach programs, what types of problems they will report, and how these problems impact them. Understanding such issues will allow healthcare systems to respond to calls by federal health agencies to develop mechanisms for patients to report concerns about breakdowns in care, thereby providing truly patient-centered care.14 Therefore, we undertook this study with the overall goal of describing what may be learned from an open-ended outreach approach that directly asks patients about problems they have encountered during hospitalization. Specifically, we aim to (1) describe the types of problems reported by patients in response to this outreach approach and (2) characterize patients’ perceptions of the impact of these events.
METHODS
Setting
We conducted this study in 2 hospitals between June 2014 and February 2015. One participating hospital is a large, urban, tertiary care medical center serving a predominantly white (78%) patient population in Baltimore, Maryland. The second hospital is a large, inner city, tertiary care medical center serving a predominantly African-American (71%) patient population in Washington, DC.
Three medical-surgical units (MSUs) at each hospital participated. We selected MSUs because MSU patients interact with a variety of clinicians, often have long stays, and are at risk for adverse events. Hospitalists were part of the clinical care team in each of the participating units, serving either as the attending of record or by comanaging patients.
Patient Eligibility
Patients were potentially eligible if they were at least 18 years old, able to speak English or Spanish, and admitted to the hospital for more than 24 hours. Ineligibility criteria included the following: imminent discharge, observation (noninpatient) status, on hospice, on infection precautions (for inpatient interviews only), psychiatric or violence concerns, prisoner status, significant confusion, or inability to provide informed consent.
Eligible patients in each unit were randomized. Interviewers consecutively approached patients according to their random assignment. If a patient was not available, the interviewer proceeded to the next room. Interviewers returned to rooms of missed patients when possible. Recruitment in the unit ended when the recruitment target for that unit was achieved.
Interviewers
Five interviewers conducted interviews. One author (KS) provided interviewer training that included didactic instruction, observation, feedback, and modeling. Interviewers participated in weekly debriefing sessions. One interviewer speaks Spanish fluently and was able to conduct interviews in Spanish. Translator services were available for the other interviewers.
Interview Process
Interviews were conducted in person while the patients were hospitalized or via telephone 7 - 30 days postdischarge. A patient who had completed an interview while hospitalized was not eligible for a postdischarge telephone interview. Family members or friends present at the time of the interviews could also participate in addition to or in lieu of the patients with the patients’ assent. Interviewers obtained verbal, informed consent at the start of each interview.
The interview domains and sample questions were developed specifically for the current study and are listed in Table 1. The goal of the interview was to elicit the patient’s (or family member’s) perception of their care experiences and their perceptions of the consequences of any problems with their care. The interviewer sought to obtain sufficient detail to understand the patient’s concerns and to determine what, if any, action might be needed to remediate problems reported by patients. Interviewers captured patient responses by taking detailed notes on a case report form or by directly entering patient responses using a computer or iPad at the time of interview at the discretion of the interviewer.
We defined a patient-perceived breakdown as something that went wrong during the hospitalization according to the patient. If a patient-perceived breakdown in care was identified, the interviewer attempted to resolve the concern. Some breakdowns had occurred in the past, making further resolution impossible (eg, a long wait in the emergency department). Other breakdowns were active and addressable, such as the patient having clinical questions that had not been answered. In such cases, the interviewer attempted to address the patient’s concerns, typically by working with unit nursing staff. For patients interviewed postdischarge, the interviewer worked to resolve ongoing patient concerns with the assistance of the patient safety, quality, and compliance teams as needed. The interviewer provided a brief narrative summary of all interviews to unit nursing leadership within 24 hours. Positive comments were sent to leadership but not captured systematically for research purposes. Further details of the process of responding to patients’ concerns will be reported elsewhere. All data were entered into REDCap to facilitate data management and reporting.15
The MedStar Health Research Institute Institutional Review Board reviewed and approved this study.
Categorizing Patients’ Responses: The Patient Experience Coding Tool
Using directed content analysis,16 we deductively created the Patient Experience Coding Tool (PECT) in order to summarize the narrative information captured during the interviews and categorize patient-perceived breakdowns in care. First, we referred to our prior interviews of patients’ views on breakdowns in cancer care6 and surrogate decision-makers’ views on breakdowns in intensive care units13 to create the initial categories. We then applied the resultant framework to the interviews in the present study and refined the categories. This involved applying the categorization to an initial set of interviews to check the sufficiency of the coding categories. We clarified the scope of each category (ie, what types of events fit under each category) and created additional categories (eg, medication-related problems) to capture patient experiences that were not included in the initial framework.
We then coded each interview using the PECT. A minimum of 2 readers reviewed the narrative notes for each interview. The first reader provided an initial categorization; the second reader reviewed the narrative and confirmed or questioned the initial categorization to improve coding accuracy. If a reader was uncertain about the correct categorization, it was discussed by three readers until an agreement was achieved. Because facilities-related problems (eg, food or parking) fall outside the realm of provider-based hospital care, such comments were not the focus of the outreach efforts and were not consistently recorded. Therefore, they were not included in the PECT and are not reported here.
Analyses
We computed simple, descriptive statistics including the number and percentage of patients identifying at least one breakdown, as well as the number and percent reporting each type of breakdown. We also computed the number and percentage of patients reporting any harm and each type of harm. We computed the percentage of patients reporting at least 1 breakdown by hospital, type of interview (postdischarge vs inpatient), selected patient demographic characteristics (eg, gender, age, education, race), and interviewee (patient vs someone other than the patient interviewed or present during the interview) using the chi-square statistic to test the statistical significance of the resulting differences. All statistical analyses were performed using SPSS version 22.
RESULTS
A total of 979 outreach interviews were conducted. Of these, 349 were conducted via telephone postdischarge, and 630 were conducted in person during hospitalization. The average interview duration was 8.5 minutes for telephone interviews and 12.2 minutes for in-person interviews. Of the patients approached to participate, 67% completed an interview (61% in person, 83% via telephone). Patient characteristics are summarized in Table 2.
Overall, 386 of 979 interviewees (39.4%) believed they had experienced at least one breakdown in care. The types of patient-perceived breakdowns reported were categorized using the PECT and are summarized in Table 3 and the Figure. The most common concern involved information exchange. Approximately 1 in 10 patients (n = 105, 10.7%) felt that they had not received the information they needed when they needed it. Medication-related concerns were reported by 12.3% (n = 120) of interviewees and predominantly included concerns about what medications were being administered (5.7%) and inadequately treated pain (5.6%). Many of the patients expressing concerns about what medications were administered questioned why they were not receiving their outpatient medications or did not understand why a different medication was being administered, suggesting that many of these instances were related to breakdowns in communication as well. Other relatively common concerns were delays in the admissions process (reported by 9.2% of interviewees), poor team communication (reported by 6.6% of interviewees), healthcare providers with a rude or uncaring manner (reported by 6.3% of interviewees), and problems related to discharge (reported by 5.7% of interviewees).
Of the 386 interviewees who perceived a breakdown in care, 140 (36.3%) perceived harm associated with the event (Table 3). The most common harms were physical (eg, pain; n = 66) and emotional (eg, distress, worry; n = 60). In addition, patients reported instances of damage to relationships with providers (n = 28) resulting in a loss of trust, with participants citing breakdowns as a reason for not coming back to a particular hospital or provider. In other cases, patients believed that breakdowns in care resulted in the need for additional care or a prolonged hospital stay.
We found no difference between the 2 hospitals where the study was conducted in the percentage of interviewees reporting at least 1 breakdown (39.1% vs 39.9%, P = 0.80). We also found no difference between interview method, (ie, in person vs telephone; 40.6% vs 37.2%, respectively, P = 0.30), patient gender (40.6% and 38.8% for men and women, respectively, P = 0.57), race (41.0% and 36.8% for white and black or African-American, respectively, P = 0.20) or between interviewers (P = 0.37). We did identify differences in rates of reporting at least 1 breakdown in care related to age (45.4% of patients aged 60 years or younger vs 34.5% of patients older than 60 years, P < 0.001) and education (32.7% of patients with a high school education or less vs 46.8% of those with at least some college education, P < 0.001). Patients interviewed alone reported fewer breakdowns than if another person was present during the interview or was interviewed in lieu of the patient (37.8% vs 53.4%, P = 0.002). The rate of reporting breakdowns for patients interviewed alone in the hospital is very similar to the rates of those interviewed via telephone (37.8% vs 37.2%). For most types of breakdowns, there were no differences in reporting for in-person vs postdischarge interviews. Discharge-related problems were more frequently reported among patients interviewed postdischarge (8.9% postdischarge vs 4.0% in person, P = 0.002). Patients interviewed in person were more likely to report problems with information exchange compared to patients interviewed postdischarge (17.6% vs 13.5%, respectively; P = 0.09), although this did not reach statistical significance.
DISCUSSION
Through interviews with nearly 1000 patients, we have found that approximately 4 in 10 hospitalized patients believed they experienced a breakdown in care. Not only are patient-perceived breakdowns in care distressingly common, more than one-third of these events resulted in harm according to the patient. Patients reported a diverse range of breakdowns, including problems related to patient experience, as well as breakdowns in technical aspects of medical care. Collectively, these findings illustrate a striking need to identify and address these frequent and potentially harmful breakdowns.
Our findings are consistent with prior studies in which 20% to 50% of patients identified a problem during hospitalization. For example, Weingart et al. interviewed patients in a single general medical unit and found that 20% experienced an adverse event, near miss, or medical error, while nearly 40% experienced what was defined as a service quality incident.2,12 Of note, both our study and the study by Weingart et al. systematically elicited patients’ perspectives of breakdowns in care with explicit questions about problems or breakdowns in care.2,12 Because patients are often reluctant to speak up about problems in care,without such efforts to actively identify problems, providers and leaders are likely to be unaware of the majority of these concerns.6-8 These findings suggest that hospital-based providers should at least consider routinely asking patients about breakdowns in care to identify and respond to patients’ concerns.
Not only are patient-perceived breakdowns common, more than one-third of patients who experienced a breakdown considered it to be harmful. This suggests that our outreach approach identified predominantly nontrivial concerns. We adopted a broad definition of harm that includes emotional distress, damage to the relationship with providers, and life disruption. This differs from other studies examining patient reports of breakdowns in care, in which harm was restricted to physical injury.1,2 We consider this inclusive definition of harm to be a strength of the present study as it provides the most complete picture of the impact of such events on patients. This approach is supported by other studies demonstrating that patients place great emphasis on the psychological consequences of adverse events.17-19 Thus, it is clear from our work and other studies that nonphysical harm is important and warrants concerted efforts to address.
Patients in our study reported a variety of breakdowns, including breakdowns related to patient experience (eg, communication, relationship with providers) and technical aspects of healthcare delivery (eg, diagnosis, treatment). This is consistent with other studies examining patient perspectives of breakdowns in care. Weingart et al.found that hospitalized patients reported a broad range of problems, including adverse events, medical errors, communication breakdowns, and problems with food.2,12 This variety of events suggests a need for integration between the various hospital groups that handle patient-perceived breakdowns, including bedside providers, risk management, patient relations, patient advocates, and quality and safety groups, in order to provide a coordinated and effective response to the full spectrum of patient-perceived breakdowns in care.
Patients in our study were more likely to report breakdowns related to communication and relationships with providers than those related to technical aspects of care. Similarly, Kuzel et al.found that the most common problems cited by patients in the primary care setting were breakdowns in the clinician-patient relationship and access-related problems.17This is not surprising, as patients are likely to have more direct knowledge about communication and interpersonal relationships than about technical aspects of care.
We identified several factors associated with the likelihood of reporting a breakdown in care. Most strikingly, involving a friend or family member in the interview was strongly associated with reporting a breakdown. Other work has also suggested that patients’ friends and family members are an important source of information about safety concerns.20,21 In addition, several patient characteristics were associated with an increased likelihood of reporting a breakdown, including being younger and better educated. These findings highlight the importance of engaging patients’ friends and families in efforts to elicit patient concerns about healthcare, and they confirm recommendations for patients to bring a friend or family member to healthcare encounters.22 In addition, they illustrate the need to better understand how patient characteristics affect perceptions of breakdowns in care and their willingness to speak up, as this could inform efforts to target outreach to especially vulnerable patients.
A strength of this study is the number of interviews completed (almost 1000), which provides a diverse range of patient views and experiences, as evidenced by the demographic characteristics of participants. Interviews were conducted at two hospitals that differ substantially with regard to populations served, further enhancing the generalizability of our findings. Despite the large number of interviews and diverse patient characteristics, patients were drawn from only 3 units at 2 hospitals, which may limit generalizability.
We did not conduct medical record reviews to validate patients’ reports of problems, which may be viewed as a limitation. While such a comparison would be informative, the intent of the current study was to elicit patients’ perceptions of care, including aspects of care that are not typically captured in the medical record, such as communication. Other studies have demonstrated that patients’ reports of medical errors and adverse events tend to differ from providers’ reports of the same subjects.19,23 Therefore, we considered the patients’ perceptions of care to be a useful endpoint in and of itself. We did not determine the extent to which providers were already aware of patients’ concerns or whether they considered patients’ concerns valid. A related limitation is our inability to determine whether the differences we identified in the rates of breakdown reporting based on patient characteristics reflect differences in willingness to report or differences in experiences. Because we included patients in an MSU, it is possible that breakdowns were related to medical care, surgical care, or both. We did not follow patients longitudinally and therefore only captured harm perceived by a patient at the time of the interview. It is possible that patients may have experienced harm later in their hospitalization or following discharge that was not measured. Lastly, we did not measure interrater reliability of the interview coding and therefore do not present the PECT as a validated instrument. These important questions should be targeted for future study.
CONCLUSION
When directly asked about their experiences, almost 4 out of 10 hospitalized patients reported a breakdown in their care, many of which were perceived to be harmful. Not all hospitals will have the resources to implement the intensive approach used in this study to elicit patient-perceived breakdowns. Therefore, further work is needed to develop sustainable methods to overcome patients’ reluctance to report breakdowns in care. Engaging patients’ families and friends may be a particularly fruitful strategy. We offer the PECT as a tool that hospitals could use to summarize a variety of sources of patient feedback such as complaints, responses to surveys, and consumer reviews. Hospitals that effectively encourage patients and their family members to speak up about perceived breakdowns will identify many opportunities to address patient concerns, potentially leading to improved patient safety and experience.
There is growing recognition that patients and family members have critical insights into healthcare experiences. As consumers of healthcare, patient experience is the definitive gauge of whether healthcare is patient centered. In addition, patients may know things about their healthcare that the care team does not. Several studies have demonstrated that patients have knowledge of adverse events and medical errors that are not detected by other methods.1-5 For these reasons, systems designed to elicit patient perspectives of care and detect patient-perceived breakdowns in care could be used to improve healthcare safety and quality, including the patient experience.
Historically, hospitals have relied on patient-initiated reporting via complaints or legal action as the main source of information regarding patient-perceived breakdowns in care. However, many patients are hesitant to speak up about problems or uncertain about how to report concerns.6-8 As a result, healthcare systems often only learn of the most severe breakdowns in care from a subset of activated patients, thus underestimating how widespread patient-perceived breakdowns are.
To overcome these limitations of patient-initiated reporting, hospitals could conduct outreach to patients to actively identify and learn about patient-perceived breakdowns in care. Potential benefits of outreach to patients include more reliable detection of patient-perceived breakdowns in care, identification of a broader range of types of breakdowns commonly experienced by patients, and recognition of problems in real-time when there is more opportunity for redress. Indeed, some hospitals have adopted active outreach programs such as structured nurse manager rounding or postdischarge phone calls.9
It is possible that outreach will not overcome patients’ reluctance to speak up, or patients may not share serious or actionable breakdowns. The manner in which outreach is conducted is likely to influence the information patients are willing to share. Prior studies examining patient perspectives of healthcare have primarily taken a structured approach with close-ended questions or a focus on specific aspects of care.1,10,11 Limited data collected using an open-ended approach suggest patient-perceived breakdowns in care may be very common.2,12,13 However, the impact of such breakdowns on patients has not been well characterized.
In order to design systems that can optimally detect patient-perceived breakdowns in care, additional information is needed to understand whether patients will report breakdowns in response to outreach programs, what types of problems they will report, and how these problems impact them. Understanding such issues will allow healthcare systems to respond to calls by federal health agencies to develop mechanisms for patients to report concerns about breakdowns in care, thereby providing truly patient-centered care.14 Therefore, we undertook this study with the overall goal of describing what may be learned from an open-ended outreach approach that directly asks patients about problems they have encountered during hospitalization. Specifically, we aim to (1) describe the types of problems reported by patients in response to this outreach approach and (2) characterize patients’ perceptions of the impact of these events.
METHODS
Setting
We conducted this study in 2 hospitals between June 2014 and February 2015. One participating hospital is a large, urban, tertiary care medical center serving a predominantly white (78%) patient population in Baltimore, Maryland. The second hospital is a large, inner city, tertiary care medical center serving a predominantly African-American (71%) patient population in Washington, DC.
Three medical-surgical units (MSUs) at each hospital participated. We selected MSUs because MSU patients interact with a variety of clinicians, often have long stays, and are at risk for adverse events. Hospitalists were part of the clinical care team in each of the participating units, serving either as the attending of record or by comanaging patients.
Patient Eligibility
Patients were potentially eligible if they were at least 18 years old, able to speak English or Spanish, and admitted to the hospital for more than 24 hours. Ineligibility criteria included the following: imminent discharge, observation (noninpatient) status, on hospice, on infection precautions (for inpatient interviews only), psychiatric or violence concerns, prisoner status, significant confusion, or inability to provide informed consent.
Eligible patients in each unit were randomized. Interviewers consecutively approached patients according to their random assignment. If a patient was not available, the interviewer proceeded to the next room. Interviewers returned to rooms of missed patients when possible. Recruitment in the unit ended when the recruitment target for that unit was achieved.
Interviewers
Five interviewers conducted interviews. One author (KS) provided interviewer training that included didactic instruction, observation, feedback, and modeling. Interviewers participated in weekly debriefing sessions. One interviewer speaks Spanish fluently and was able to conduct interviews in Spanish. Translator services were available for the other interviewers.
Interview Process
Interviews were conducted in person while the patients were hospitalized or via telephone 7 - 30 days postdischarge. A patient who had completed an interview while hospitalized was not eligible for a postdischarge telephone interview. Family members or friends present at the time of the interviews could also participate in addition to or in lieu of the patients with the patients’ assent. Interviewers obtained verbal, informed consent at the start of each interview.
The interview domains and sample questions were developed specifically for the current study and are listed in Table 1. The goal of the interview was to elicit the patient’s (or family member’s) perception of their care experiences and their perceptions of the consequences of any problems with their care. The interviewer sought to obtain sufficient detail to understand the patient’s concerns and to determine what, if any, action might be needed to remediate problems reported by patients. Interviewers captured patient responses by taking detailed notes on a case report form or by directly entering patient responses using a computer or iPad at the time of interview at the discretion of the interviewer.
We defined a patient-perceived breakdown as something that went wrong during the hospitalization according to the patient. If a patient-perceived breakdown in care was identified, the interviewer attempted to resolve the concern. Some breakdowns had occurred in the past, making further resolution impossible (eg, a long wait in the emergency department). Other breakdowns were active and addressable, such as the patient having clinical questions that had not been answered. In such cases, the interviewer attempted to address the patient’s concerns, typically by working with unit nursing staff. For patients interviewed postdischarge, the interviewer worked to resolve ongoing patient concerns with the assistance of the patient safety, quality, and compliance teams as needed. The interviewer provided a brief narrative summary of all interviews to unit nursing leadership within 24 hours. Positive comments were sent to leadership but not captured systematically for research purposes. Further details of the process of responding to patients’ concerns will be reported elsewhere. All data were entered into REDCap to facilitate data management and reporting.15
The MedStar Health Research Institute Institutional Review Board reviewed and approved this study.
Categorizing Patients’ Responses: The Patient Experience Coding Tool
Using directed content analysis,16 we deductively created the Patient Experience Coding Tool (PECT) in order to summarize the narrative information captured during the interviews and categorize patient-perceived breakdowns in care. First, we referred to our prior interviews of patients’ views on breakdowns in cancer care6 and surrogate decision-makers’ views on breakdowns in intensive care units13 to create the initial categories. We then applied the resultant framework to the interviews in the present study and refined the categories. This involved applying the categorization to an initial set of interviews to check the sufficiency of the coding categories. We clarified the scope of each category (ie, what types of events fit under each category) and created additional categories (eg, medication-related problems) to capture patient experiences that were not included in the initial framework.
We then coded each interview using the PECT. A minimum of 2 readers reviewed the narrative notes for each interview. The first reader provided an initial categorization; the second reader reviewed the narrative and confirmed or questioned the initial categorization to improve coding accuracy. If a reader was uncertain about the correct categorization, it was discussed by three readers until an agreement was achieved. Because facilities-related problems (eg, food or parking) fall outside the realm of provider-based hospital care, such comments were not the focus of the outreach efforts and were not consistently recorded. Therefore, they were not included in the PECT and are not reported here.
Analyses
We computed simple, descriptive statistics including the number and percentage of patients identifying at least one breakdown, as well as the number and percent reporting each type of breakdown. We also computed the number and percentage of patients reporting any harm and each type of harm. We computed the percentage of patients reporting at least 1 breakdown by hospital, type of interview (postdischarge vs inpatient), selected patient demographic characteristics (eg, gender, age, education, race), and interviewee (patient vs someone other than the patient interviewed or present during the interview) using the chi-square statistic to test the statistical significance of the resulting differences. All statistical analyses were performed using SPSS version 22.
RESULTS
A total of 979 outreach interviews were conducted. Of these, 349 were conducted via telephone postdischarge, and 630 were conducted in person during hospitalization. The average interview duration was 8.5 minutes for telephone interviews and 12.2 minutes for in-person interviews. Of the patients approached to participate, 67% completed an interview (61% in person, 83% via telephone). Patient characteristics are summarized in Table 2.
Overall, 386 of 979 interviewees (39.4%) believed they had experienced at least one breakdown in care. The types of patient-perceived breakdowns reported were categorized using the PECT and are summarized in Table 3 and the Figure. The most common concern involved information exchange. Approximately 1 in 10 patients (n = 105, 10.7%) felt that they had not received the information they needed when they needed it. Medication-related concerns were reported by 12.3% (n = 120) of interviewees and predominantly included concerns about what medications were being administered (5.7%) and inadequately treated pain (5.6%). Many of the patients expressing concerns about what medications were administered questioned why they were not receiving their outpatient medications or did not understand why a different medication was being administered, suggesting that many of these instances were related to breakdowns in communication as well. Other relatively common concerns were delays in the admissions process (reported by 9.2% of interviewees), poor team communication (reported by 6.6% of interviewees), healthcare providers with a rude or uncaring manner (reported by 6.3% of interviewees), and problems related to discharge (reported by 5.7% of interviewees).
Of the 386 interviewees who perceived a breakdown in care, 140 (36.3%) perceived harm associated with the event (Table 3). The most common harms were physical (eg, pain; n = 66) and emotional (eg, distress, worry; n = 60). In addition, patients reported instances of damage to relationships with providers (n = 28) resulting in a loss of trust, with participants citing breakdowns as a reason for not coming back to a particular hospital or provider. In other cases, patients believed that breakdowns in care resulted in the need for additional care or a prolonged hospital stay.
We found no difference between the 2 hospitals where the study was conducted in the percentage of interviewees reporting at least 1 breakdown (39.1% vs 39.9%, P = 0.80). We also found no difference between interview method, (ie, in person vs telephone; 40.6% vs 37.2%, respectively, P = 0.30), patient gender (40.6% and 38.8% for men and women, respectively, P = 0.57), race (41.0% and 36.8% for white and black or African-American, respectively, P = 0.20) or between interviewers (P = 0.37). We did identify differences in rates of reporting at least 1 breakdown in care related to age (45.4% of patients aged 60 years or younger vs 34.5% of patients older than 60 years, P < 0.001) and education (32.7% of patients with a high school education or less vs 46.8% of those with at least some college education, P < 0.001). Patients interviewed alone reported fewer breakdowns than if another person was present during the interview or was interviewed in lieu of the patient (37.8% vs 53.4%, P = 0.002). The rate of reporting breakdowns for patients interviewed alone in the hospital is very similar to the rates of those interviewed via telephone (37.8% vs 37.2%). For most types of breakdowns, there were no differences in reporting for in-person vs postdischarge interviews. Discharge-related problems were more frequently reported among patients interviewed postdischarge (8.9% postdischarge vs 4.0% in person, P = 0.002). Patients interviewed in person were more likely to report problems with information exchange compared to patients interviewed postdischarge (17.6% vs 13.5%, respectively; P = 0.09), although this did not reach statistical significance.
DISCUSSION
Through interviews with nearly 1000 patients, we have found that approximately 4 in 10 hospitalized patients believed they experienced a breakdown in care. Not only are patient-perceived breakdowns in care distressingly common, more than one-third of these events resulted in harm according to the patient. Patients reported a diverse range of breakdowns, including problems related to patient experience, as well as breakdowns in technical aspects of medical care. Collectively, these findings illustrate a striking need to identify and address these frequent and potentially harmful breakdowns.
Our findings are consistent with prior studies in which 20% to 50% of patients identified a problem during hospitalization. For example, Weingart et al. interviewed patients in a single general medical unit and found that 20% experienced an adverse event, near miss, or medical error, while nearly 40% experienced what was defined as a service quality incident.2,12 Of note, both our study and the study by Weingart et al. systematically elicited patients’ perspectives of breakdowns in care with explicit questions about problems or breakdowns in care.2,12 Because patients are often reluctant to speak up about problems in care,without such efforts to actively identify problems, providers and leaders are likely to be unaware of the majority of these concerns.6-8 These findings suggest that hospital-based providers should at least consider routinely asking patients about breakdowns in care to identify and respond to patients’ concerns.
Not only are patient-perceived breakdowns common, more than one-third of patients who experienced a breakdown considered it to be harmful. This suggests that our outreach approach identified predominantly nontrivial concerns. We adopted a broad definition of harm that includes emotional distress, damage to the relationship with providers, and life disruption. This differs from other studies examining patient reports of breakdowns in care, in which harm was restricted to physical injury.1,2 We consider this inclusive definition of harm to be a strength of the present study as it provides the most complete picture of the impact of such events on patients. This approach is supported by other studies demonstrating that patients place great emphasis on the psychological consequences of adverse events.17-19 Thus, it is clear from our work and other studies that nonphysical harm is important and warrants concerted efforts to address.
Patients in our study reported a variety of breakdowns, including breakdowns related to patient experience (eg, communication, relationship with providers) and technical aspects of healthcare delivery (eg, diagnosis, treatment). This is consistent with other studies examining patient perspectives of breakdowns in care. Weingart et al.found that hospitalized patients reported a broad range of problems, including adverse events, medical errors, communication breakdowns, and problems with food.2,12 This variety of events suggests a need for integration between the various hospital groups that handle patient-perceived breakdowns, including bedside providers, risk management, patient relations, patient advocates, and quality and safety groups, in order to provide a coordinated and effective response to the full spectrum of patient-perceived breakdowns in care.
Patients in our study were more likely to report breakdowns related to communication and relationships with providers than those related to technical aspects of care. Similarly, Kuzel et al.found that the most common problems cited by patients in the primary care setting were breakdowns in the clinician-patient relationship and access-related problems.17This is not surprising, as patients are likely to have more direct knowledge about communication and interpersonal relationships than about technical aspects of care.
We identified several factors associated with the likelihood of reporting a breakdown in care. Most strikingly, involving a friend or family member in the interview was strongly associated with reporting a breakdown. Other work has also suggested that patients’ friends and family members are an important source of information about safety concerns.20,21 In addition, several patient characteristics were associated with an increased likelihood of reporting a breakdown, including being younger and better educated. These findings highlight the importance of engaging patients’ friends and families in efforts to elicit patient concerns about healthcare, and they confirm recommendations for patients to bring a friend or family member to healthcare encounters.22 In addition, they illustrate the need to better understand how patient characteristics affect perceptions of breakdowns in care and their willingness to speak up, as this could inform efforts to target outreach to especially vulnerable patients.
A strength of this study is the number of interviews completed (almost 1000), which provides a diverse range of patient views and experiences, as evidenced by the demographic characteristics of participants. Interviews were conducted at two hospitals that differ substantially with regard to populations served, further enhancing the generalizability of our findings. Despite the large number of interviews and diverse patient characteristics, patients were drawn from only 3 units at 2 hospitals, which may limit generalizability.
We did not conduct medical record reviews to validate patients’ reports of problems, which may be viewed as a limitation. While such a comparison would be informative, the intent of the current study was to elicit patients’ perceptions of care, including aspects of care that are not typically captured in the medical record, such as communication. Other studies have demonstrated that patients’ reports of medical errors and adverse events tend to differ from providers’ reports of the same subjects.19,23 Therefore, we considered the patients’ perceptions of care to be a useful endpoint in and of itself. We did not determine the extent to which providers were already aware of patients’ concerns or whether they considered patients’ concerns valid. A related limitation is our inability to determine whether the differences we identified in the rates of breakdown reporting based on patient characteristics reflect differences in willingness to report or differences in experiences. Because we included patients in an MSU, it is possible that breakdowns were related to medical care, surgical care, or both. We did not follow patients longitudinally and therefore only captured harm perceived by a patient at the time of the interview. It is possible that patients may have experienced harm later in their hospitalization or following discharge that was not measured. Lastly, we did not measure interrater reliability of the interview coding and therefore do not present the PECT as a validated instrument. These important questions should be targeted for future study.
CONCLUSION
When directly asked about their experiences, almost 4 out of 10 hospitalized patients reported a breakdown in their care, many of which were perceived to be harmful. Not all hospitals will have the resources to implement the intensive approach used in this study to elicit patient-perceived breakdowns. Therefore, further work is needed to develop sustainable methods to overcome patients’ reluctance to report breakdowns in care. Engaging patients’ families and friends may be a particularly fruitful strategy. We offer the PECT as a tool that hospitals could use to summarize a variety of sources of patient feedback such as complaints, responses to surveys, and consumer reviews. Hospitals that effectively encourage patients and their family members to speak up about perceived breakdowns will identify many opportunities to address patient concerns, potentially leading to improved patient safety and experience.
1. Weissman JS, Schneider EC, Weingart SN, et al. Comparing patient-reported hospital adverse events with medical record review: Do patients know something that hospitals do not? Ann Intern Med. 2008;149(2):100-108. PubMed
2. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. PubMed
3. Wetzels R, Wolters R, van Weel C, Wensing M. Mix of methods is needed to identify adverse events in general practice: A prospective observational study. BMC Fam Pract. 2008;9:35. PubMed
4. Friedman SM, Provan D, Moore S, Hanneman K. Errors, near misses and adverse events in the emergency department: What can patients tell us? CJEM. 2008;10(5):421-427. PubMed
5. Iedema R, Allen S, Britton K, Gallagher TH. What do patients and relatives know about problems and failures in care? BMJ Qual Saf. 2012;21(3):198-205. PubMed
6. Mazor KM, Roblin DW, Greene SM, et al. Toward patient-centered cancer care: Patient perceptions of problematic events, impact, and response. J Clin Oncol. 2012;30(15):1784-1790. PubMed
7. Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled ‘difficult’ among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. PubMed
8. Entwistle VA, McCaughan D, Watt IS, et al. Speaking up about safety concerns: Multi-setting qualitative study of patients’ views and experiences. Qual Saf Health Care. 2010;19(6):e33. PubMed
9. Tan M, Lang D. Effectiveness of nurse leader rounding and post-discharge telephone calls in patient satisfaction: A systematic review. JBI database of systematic reviews and implementation reports. 2015;13(7):154-176. PubMed
10. Garbutt J, Bose D, McCawley BA, Burroughs T, Medoff G. Soliciting patient complaints to improve performance. Jt Comm J Qual Saf. 2003;29(3):103-112. PubMed
11. Agoritsas T, Bovier PA, Perneger TV. Patient reports of undesirable events during hospitalization. J Gen Intern Med. 2005;20(10):922-928. PubMed
12. Weingart SN, Pagovich O, Sands DZ, et al. Patient-reported service quality on a medicine unit. Int J Qual Health Care. 2006;18(2):95-101. PubMed
13. Fisher KA, Ahmad S, Jackson M, Mazor KM. Surrogate decision makers’ perspectives on preventable breakdowns in care among critically ill patients: A qualitative study. Patient Educ Couns. 2016;99(10):1685-1693. PubMed
14. Halpern MT, Roussel AE, Treiman K, Nerz PA, Hatlie MJ, Sheridan S. Designing consumer reporting systems for patient safety events. Final Report (Prepared by RTI International and Consumers Advancing Patient Safety under Contract No. 290-06-00001-5). AHRQ Publication No. 11-0060-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed
16. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. PubMed
17. Kuzel AJ, Woolf SH, Gilchrist VJ, et al. Patient reports of preventable problems and harms in primary health care. Ann Fam Med. 2004;2(4):333-340. PubMed
18. Sokol-Hessner L, Folcarelli PH, Sands KE. Emotional harm from disrespect: The neglected preventable harm. BMJ Qual Saf. 2015;24(9):550-553. PubMed
19. Masso Guijarro P, Aranaz Andres JM, Mira JJ, Perdiguero E, Aibar C. Adverse events in hospitals: The patient’s point of view. Qual Saf Health Care. 2010;19(2):144-147. PubMed
20. Bardach NS, Lyndon A, Asteria-Penaloza R, Goldman LE, Lin GA, Dudley RA. From the closest observers of patient care: A thematic analysis of online narrative reviews of hospitals. BMJ Qual Saf. 2015. PubMed
21. Schneider EC, Ridgely MS, Quigley DD, et al. Developing and testing the health care safety hotline: A prototype consumer reporting system for patient safety events. Final Report (Prepared by RAND Corporation under contract HHSA2902010000171). Rockvelle, MD: Agency for Healthcare Research and Quality; May 2016.
22. Shekelle PG, Pronovost PJ, Wachter RM, et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158(5 Pt 2):365-368. PubMed
23. Lawton R, O’Hara JK, Sheard L, et al. Can staff and patient perspectives on hospital safety predict harm-free care? an analysis of staff and patient survey data and routinely collected outcomes. BMJ Qual Saf. 2015;24(6):369-376. PubMed
1. Weissman JS, Schneider EC, Weingart SN, et al. Comparing patient-reported hospital adverse events with medical record review: Do patients know something that hospitals do not? Ann Intern Med. 2008;149(2):100-108. PubMed
2. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. PubMed
3. Wetzels R, Wolters R, van Weel C, Wensing M. Mix of methods is needed to identify adverse events in general practice: A prospective observational study. BMC Fam Pract. 2008;9:35. PubMed
4. Friedman SM, Provan D, Moore S, Hanneman K. Errors, near misses and adverse events in the emergency department: What can patients tell us? CJEM. 2008;10(5):421-427. PubMed
5. Iedema R, Allen S, Britton K, Gallagher TH. What do patients and relatives know about problems and failures in care? BMJ Qual Saf. 2012;21(3):198-205. PubMed
6. Mazor KM, Roblin DW, Greene SM, et al. Toward patient-centered cancer care: Patient perceptions of problematic events, impact, and response. J Clin Oncol. 2012;30(15):1784-1790. PubMed
7. Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled ‘difficult’ among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. PubMed
8. Entwistle VA, McCaughan D, Watt IS, et al. Speaking up about safety concerns: Multi-setting qualitative study of patients’ views and experiences. Qual Saf Health Care. 2010;19(6):e33. PubMed
9. Tan M, Lang D. Effectiveness of nurse leader rounding and post-discharge telephone calls in patient satisfaction: A systematic review. JBI database of systematic reviews and implementation reports. 2015;13(7):154-176. PubMed
10. Garbutt J, Bose D, McCawley BA, Burroughs T, Medoff G. Soliciting patient complaints to improve performance. Jt Comm J Qual Saf. 2003;29(3):103-112. PubMed
11. Agoritsas T, Bovier PA, Perneger TV. Patient reports of undesirable events during hospitalization. J Gen Intern Med. 2005;20(10):922-928. PubMed
12. Weingart SN, Pagovich O, Sands DZ, et al. Patient-reported service quality on a medicine unit. Int J Qual Health Care. 2006;18(2):95-101. PubMed
13. Fisher KA, Ahmad S, Jackson M, Mazor KM. Surrogate decision makers’ perspectives on preventable breakdowns in care among critically ill patients: A qualitative study. Patient Educ Couns. 2016;99(10):1685-1693. PubMed
14. Halpern MT, Roussel AE, Treiman K, Nerz PA, Hatlie MJ, Sheridan S. Designing consumer reporting systems for patient safety events. Final Report (Prepared by RTI International and Consumers Advancing Patient Safety under Contract No. 290-06-00001-5). AHRQ Publication No. 11-0060-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed
16. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. PubMed
17. Kuzel AJ, Woolf SH, Gilchrist VJ, et al. Patient reports of preventable problems and harms in primary health care. Ann Fam Med. 2004;2(4):333-340. PubMed
18. Sokol-Hessner L, Folcarelli PH, Sands KE. Emotional harm from disrespect: The neglected preventable harm. BMJ Qual Saf. 2015;24(9):550-553. PubMed
19. Masso Guijarro P, Aranaz Andres JM, Mira JJ, Perdiguero E, Aibar C. Adverse events in hospitals: The patient’s point of view. Qual Saf Health Care. 2010;19(2):144-147. PubMed
20. Bardach NS, Lyndon A, Asteria-Penaloza R, Goldman LE, Lin GA, Dudley RA. From the closest observers of patient care: A thematic analysis of online narrative reviews of hospitals. BMJ Qual Saf. 2015. PubMed
21. Schneider EC, Ridgely MS, Quigley DD, et al. Developing and testing the health care safety hotline: A prototype consumer reporting system for patient safety events. Final Report (Prepared by RAND Corporation under contract HHSA2902010000171). Rockvelle, MD: Agency for Healthcare Research and Quality; May 2016.
22. Shekelle PG, Pronovost PJ, Wachter RM, et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158(5 Pt 2):365-368. PubMed
23. Lawton R, O’Hara JK, Sheard L, et al. Can staff and patient perspectives on hospital safety predict harm-free care? an analysis of staff and patient survey data and routinely collected outcomes. BMJ Qual Saf. 2015;24(6):369-376. PubMed
© 2017 Society of Hospital Medicine
First interchangeability study for an adalimumab biosimilar has begun
The VOLTAIRE-X study of a biosimilar candidate for adalimumab (Humira) for chronic plaque psoriasis has enrolled its first patient, announced Boehringer Ingelheim, the biosimilar’s developer, on July 27.
This is the first study in the United States to investigate whether a biosimilar candidate should be granted an interchangeability designation with adalimumab. The candidate, BI 695501, is up against adalimumab’s 40-mg injection.
In VOLTAIRE-X, some patients will alternate between adalimumab and BI 695501, and others will take adalimumab continuously. The study will compare the pharmacokinetics, clinical outcomes, safety, immunogenicity, and efficacy between the two groups of patients. The estimated enrollment of adult patients with moderate to severe chronic plaque psoriasis is 240, and the study is expected to conclude in July 2019.
A phase 3 study of BI 695501’s performance for rheumatoid arthritis patients, completed in 2016, demonstrated similar efficacy, safety, and immunogenicity.
The VOLTAIRE-X study of a biosimilar candidate for adalimumab (Humira) for chronic plaque psoriasis has enrolled its first patient, announced Boehringer Ingelheim, the biosimilar’s developer, on July 27.
This is the first study in the United States to investigate whether a biosimilar candidate should be granted an interchangeability designation with adalimumab. The candidate, BI 695501, is up against adalimumab’s 40-mg injection.
In VOLTAIRE-X, some patients will alternate between adalimumab and BI 695501, and others will take adalimumab continuously. The study will compare the pharmacokinetics, clinical outcomes, safety, immunogenicity, and efficacy between the two groups of patients. The estimated enrollment of adult patients with moderate to severe chronic plaque psoriasis is 240, and the study is expected to conclude in July 2019.
A phase 3 study of BI 695501’s performance for rheumatoid arthritis patients, completed in 2016, demonstrated similar efficacy, safety, and immunogenicity.
The VOLTAIRE-X study of a biosimilar candidate for adalimumab (Humira) for chronic plaque psoriasis has enrolled its first patient, announced Boehringer Ingelheim, the biosimilar’s developer, on July 27.
This is the first study in the United States to investigate whether a biosimilar candidate should be granted an interchangeability designation with adalimumab. The candidate, BI 695501, is up against adalimumab’s 40-mg injection.
In VOLTAIRE-X, some patients will alternate between adalimumab and BI 695501, and others will take adalimumab continuously. The study will compare the pharmacokinetics, clinical outcomes, safety, immunogenicity, and efficacy between the two groups of patients. The estimated enrollment of adult patients with moderate to severe chronic plaque psoriasis is 240, and the study is expected to conclude in July 2019.
A phase 3 study of BI 695501’s performance for rheumatoid arthritis patients, completed in 2016, demonstrated similar efficacy, safety, and immunogenicity.
Is that thyroid nodule malignant?
ESTES PARK, COLO. – Before ordering any tests at all, an astute clinician can have a pretty good idea as to whether a palpable thyroid nodule is at higher or lower risk for being malignant based upon the patient’s history and physical examination, Michael T. McDermott, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“The possibility of cancer is what scares people, and that’s what people want to know,” observed Dr. McDermott, professor of medicine and director of endocrinology and diabetes practice at University of Colorado Hospital.
This is a common clinical problem.
“If you felt the neck of every patient that you saw, 4%-7% would have a palpable thyroid nodule,” the endocrinologist said.
Moreover, upon imaging the thyroid by ultrasound, CT, or MRI, the prevalence of a thyroid nodule is close to 40%. And because the prevalence increases with age, thyroid nodules are found at autopsy in 50%-60% of individuals.
Ninety percent of thyroid nodules are benign. And even when they are malignant, for the most part the prognosis is very good. Ten-year survival is in excess of 90% in patients with papillary, follicular, or Hurthle-cell thyroid cancer and greater than 60% in the 2%-5% of thyroid cancers classified as medullary. The one bad actor is anaplastic thyroid cancer, with a 1-year survival rate of less than 5%; however, only 2%-5% of thyroid cancers are of the anaplastic type.
The cancer risk of a palpable thyroid nodule is increased if it is 3 cm or larger in size, of a firm and fixed consistency, or cervical lymph nodes are palpable. Clues of increased risk from the patient history include a family history of papillary or medullary thyroid cancer, compressive symptoms such as a mass sensation or difficulty swallowing, or radiation therapy to the head and neck before age 20 years for treatment of acne, ringworm, or for tonsil/adenoid shrinkage, a fairly common practice in the 1950s and 1960s.
The first test to order in evaluating a thyroid nodule is always a serum TSH. If the level is low or undetectable, that’s a relief: It is not cancer. A mildly elevated free T4 and total T3 in conjunction with the low TSH indicate an autonomously functioning nodule.
In this situation, the next test to order is a radioactive iodine uptake and scan.
“Every nuclear medicine department in the country does these,” Dr. McDermott observed.
A normal radioactive iodine uptake value is 20%-40%; however, in the setting of suppressed serum TSH, there is no physiologic stimulation for uptake, so any uptake would be considered inappropriately high and constitutes confirmation that this is a patient with hyperthyroidism.
If the serum TSH is normal or high, there is no need to order a free T4 and total T3. Instead, the next test to order is a neck ultrasound; the size and features of the nodule upon imaging will determine the need for fine needle aspiration as a next step in the evaluation. Ultrasound findings suggestive of malignancy are a dark, hypoechoic nodule with irregular margins, microcalcifications, and/or increased blood flow upon Doppler imaging.
“Patterns and combinations of findings are more informative than any single finding,” he said.
Fine needle aspiration pathology results are reported as benign, malignant, or indeterminate. If the report is benign, there is still about a 2.5% chance of malignancy due to sampling error.
Molecular markers have a role in the 30% or more of fine needle aspiration results labeled indeterminate based upon a finding of atypia of unknown significance, a follicular lesion of unknown significance, or a follicular neoplasm. The Afirma gene expression classifier (Veracyte) has a high negative predictive value because it identifies patterns of mRNA expression consistent with benign tumors. The ThyroSeq oncogene panel (Interspace Diagnostics) has a moderate negative predictive value but a high positive predictive value. The tests each run about $1,200, but they spare patients with indeterminate aspiration biopsy findings from additional invasive diagnostic procedures – and the tests are covered by insurers.
“The gene expression classifier is a good test when you’re trying to avoid surgery, and the oncogene panel tells you when you really need surgery,” Dr. McDermott explained.
He recommends as a thyroid screen palpating the thyroid as part of a routine annual physical exam and ordering a serum TSH every 5 years starting at age 50. He is opposed to proactive ultrasound imaging of the thyroid as a means of screening for thyroid cancer because that practice picks up a huge number of nonharmful nodules.
Dr. McDermott cited the current American Thyroid Association guidelines on the evaluation and management of thyroid nodules as “one of the most important recent developments in the field.” The guidelines include detailed evidence-based recommendations regarding initial evaluation, criteria for fine-needle aspiration biopsy, interpretation of the biopsy results, use of molecular markers, and management of benign thyroid nodules. The guidelines also address the initial management of thyroid cancer and surveillance for recurrent disease.
Dr. McDermott reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – Before ordering any tests at all, an astute clinician can have a pretty good idea as to whether a palpable thyroid nodule is at higher or lower risk for being malignant based upon the patient’s history and physical examination, Michael T. McDermott, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“The possibility of cancer is what scares people, and that’s what people want to know,” observed Dr. McDermott, professor of medicine and director of endocrinology and diabetes practice at University of Colorado Hospital.
This is a common clinical problem.
“If you felt the neck of every patient that you saw, 4%-7% would have a palpable thyroid nodule,” the endocrinologist said.
Moreover, upon imaging the thyroid by ultrasound, CT, or MRI, the prevalence of a thyroid nodule is close to 40%. And because the prevalence increases with age, thyroid nodules are found at autopsy in 50%-60% of individuals.
Ninety percent of thyroid nodules are benign. And even when they are malignant, for the most part the prognosis is very good. Ten-year survival is in excess of 90% in patients with papillary, follicular, or Hurthle-cell thyroid cancer and greater than 60% in the 2%-5% of thyroid cancers classified as medullary. The one bad actor is anaplastic thyroid cancer, with a 1-year survival rate of less than 5%; however, only 2%-5% of thyroid cancers are of the anaplastic type.
The cancer risk of a palpable thyroid nodule is increased if it is 3 cm or larger in size, of a firm and fixed consistency, or cervical lymph nodes are palpable. Clues of increased risk from the patient history include a family history of papillary or medullary thyroid cancer, compressive symptoms such as a mass sensation or difficulty swallowing, or radiation therapy to the head and neck before age 20 years for treatment of acne, ringworm, or for tonsil/adenoid shrinkage, a fairly common practice in the 1950s and 1960s.
The first test to order in evaluating a thyroid nodule is always a serum TSH. If the level is low or undetectable, that’s a relief: It is not cancer. A mildly elevated free T4 and total T3 in conjunction with the low TSH indicate an autonomously functioning nodule.
In this situation, the next test to order is a radioactive iodine uptake and scan.
“Every nuclear medicine department in the country does these,” Dr. McDermott observed.
A normal radioactive iodine uptake value is 20%-40%; however, in the setting of suppressed serum TSH, there is no physiologic stimulation for uptake, so any uptake would be considered inappropriately high and constitutes confirmation that this is a patient with hyperthyroidism.
If the serum TSH is normal or high, there is no need to order a free T4 and total T3. Instead, the next test to order is a neck ultrasound; the size and features of the nodule upon imaging will determine the need for fine needle aspiration as a next step in the evaluation. Ultrasound findings suggestive of malignancy are a dark, hypoechoic nodule with irregular margins, microcalcifications, and/or increased blood flow upon Doppler imaging.
“Patterns and combinations of findings are more informative than any single finding,” he said.
Fine needle aspiration pathology results are reported as benign, malignant, or indeterminate. If the report is benign, there is still about a 2.5% chance of malignancy due to sampling error.
Molecular markers have a role in the 30% or more of fine needle aspiration results labeled indeterminate based upon a finding of atypia of unknown significance, a follicular lesion of unknown significance, or a follicular neoplasm. The Afirma gene expression classifier (Veracyte) has a high negative predictive value because it identifies patterns of mRNA expression consistent with benign tumors. The ThyroSeq oncogene panel (Interspace Diagnostics) has a moderate negative predictive value but a high positive predictive value. The tests each run about $1,200, but they spare patients with indeterminate aspiration biopsy findings from additional invasive diagnostic procedures – and the tests are covered by insurers.
“The gene expression classifier is a good test when you’re trying to avoid surgery, and the oncogene panel tells you when you really need surgery,” Dr. McDermott explained.
He recommends as a thyroid screen palpating the thyroid as part of a routine annual physical exam and ordering a serum TSH every 5 years starting at age 50. He is opposed to proactive ultrasound imaging of the thyroid as a means of screening for thyroid cancer because that practice picks up a huge number of nonharmful nodules.
Dr. McDermott cited the current American Thyroid Association guidelines on the evaluation and management of thyroid nodules as “one of the most important recent developments in the field.” The guidelines include detailed evidence-based recommendations regarding initial evaluation, criteria for fine-needle aspiration biopsy, interpretation of the biopsy results, use of molecular markers, and management of benign thyroid nodules. The guidelines also address the initial management of thyroid cancer and surveillance for recurrent disease.
Dr. McDermott reported having no financial conflicts regarding his presentation.
ESTES PARK, COLO. – Before ordering any tests at all, an astute clinician can have a pretty good idea as to whether a palpable thyroid nodule is at higher or lower risk for being malignant based upon the patient’s history and physical examination, Michael T. McDermott, MD, said at a conference on internal medicine sponsored by the University of Colorado.
“The possibility of cancer is what scares people, and that’s what people want to know,” observed Dr. McDermott, professor of medicine and director of endocrinology and diabetes practice at University of Colorado Hospital.
This is a common clinical problem.
“If you felt the neck of every patient that you saw, 4%-7% would have a palpable thyroid nodule,” the endocrinologist said.
Moreover, upon imaging the thyroid by ultrasound, CT, or MRI, the prevalence of a thyroid nodule is close to 40%. And because the prevalence increases with age, thyroid nodules are found at autopsy in 50%-60% of individuals.
Ninety percent of thyroid nodules are benign. And even when they are malignant, for the most part the prognosis is very good. Ten-year survival is in excess of 90% in patients with papillary, follicular, or Hurthle-cell thyroid cancer and greater than 60% in the 2%-5% of thyroid cancers classified as medullary. The one bad actor is anaplastic thyroid cancer, with a 1-year survival rate of less than 5%; however, only 2%-5% of thyroid cancers are of the anaplastic type.
The cancer risk of a palpable thyroid nodule is increased if it is 3 cm or larger in size, of a firm and fixed consistency, or cervical lymph nodes are palpable. Clues of increased risk from the patient history include a family history of papillary or medullary thyroid cancer, compressive symptoms such as a mass sensation or difficulty swallowing, or radiation therapy to the head and neck before age 20 years for treatment of acne, ringworm, or for tonsil/adenoid shrinkage, a fairly common practice in the 1950s and 1960s.
The first test to order in evaluating a thyroid nodule is always a serum TSH. If the level is low or undetectable, that’s a relief: It is not cancer. A mildly elevated free T4 and total T3 in conjunction with the low TSH indicate an autonomously functioning nodule.
In this situation, the next test to order is a radioactive iodine uptake and scan.
“Every nuclear medicine department in the country does these,” Dr. McDermott observed.
A normal radioactive iodine uptake value is 20%-40%; however, in the setting of suppressed serum TSH, there is no physiologic stimulation for uptake, so any uptake would be considered inappropriately high and constitutes confirmation that this is a patient with hyperthyroidism.
If the serum TSH is normal or high, there is no need to order a free T4 and total T3. Instead, the next test to order is a neck ultrasound; the size and features of the nodule upon imaging will determine the need for fine needle aspiration as a next step in the evaluation. Ultrasound findings suggestive of malignancy are a dark, hypoechoic nodule with irregular margins, microcalcifications, and/or increased blood flow upon Doppler imaging.
“Patterns and combinations of findings are more informative than any single finding,” he said.
Fine needle aspiration pathology results are reported as benign, malignant, or indeterminate. If the report is benign, there is still about a 2.5% chance of malignancy due to sampling error.
Molecular markers have a role in the 30% or more of fine needle aspiration results labeled indeterminate based upon a finding of atypia of unknown significance, a follicular lesion of unknown significance, or a follicular neoplasm. The Afirma gene expression classifier (Veracyte) has a high negative predictive value because it identifies patterns of mRNA expression consistent with benign tumors. The ThyroSeq oncogene panel (Interspace Diagnostics) has a moderate negative predictive value but a high positive predictive value. The tests each run about $1,200, but they spare patients with indeterminate aspiration biopsy findings from additional invasive diagnostic procedures – and the tests are covered by insurers.
“The gene expression classifier is a good test when you’re trying to avoid surgery, and the oncogene panel tells you when you really need surgery,” Dr. McDermott explained.
He recommends as a thyroid screen palpating the thyroid as part of a routine annual physical exam and ordering a serum TSH every 5 years starting at age 50. He is opposed to proactive ultrasound imaging of the thyroid as a means of screening for thyroid cancer because that practice picks up a huge number of nonharmful nodules.
Dr. McDermott cited the current American Thyroid Association guidelines on the evaluation and management of thyroid nodules as “one of the most important recent developments in the field.” The guidelines include detailed evidence-based recommendations regarding initial evaluation, criteria for fine-needle aspiration biopsy, interpretation of the biopsy results, use of molecular markers, and management of benign thyroid nodules. The guidelines also address the initial management of thyroid cancer and surveillance for recurrent disease.
Dr. McDermott reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE ANNUAL INTERNAL MEDICINE PROGRAM
Product Update: Hologic Aptima HSV Assay, Cianna SAVI SCOUT, Olympus Hystero-Resectoscope, and Clarius Ultrasound Scanners
DIAGNOSE HERPES SIMPLEX VIRUS
According to the CDC, infections with HSV-2 affect more than 24 million Americans. Patients with HSV-2 strain are at increased risk for contracting and transmitting HIV. Pregnant women infected with HSV-2 are at risk of transmitting the virus to their babies, with increased risk for neurologic complications in the child.
FOR MORE INFORMATION, VISIT: http://www.hologic.com/search/site/aptima%20hsv
PRECISELY TARGET TISSUE DURING LUMPECTOMY OR BIOPSY
Cianna Medical reported recent data showing that, when compared with wire localization, the SCOUT reduces breast surgery operating room (OR) delay times by 72.5%, resulted in an average 29- minute reduction in OR waiting time, and significantly improved workflow efficiency.
FOR MORE INFORMATION, VISIT: https://www.ciannamedical.com/savi-scout/
PLASMA HYSTEROSCOPIC RESECTION
During gynecologic procedures, the Olympus 8.5-mm hystero-resectoscope uses a combination of radio frequency, energy, and saline to create plasma, an electrically conductive gas cloud of vapor and charged particles. Due to its conductivity, plasma allows energy to cross into targeted tissue at lower energy levels than with more traditional approaches. This effect leads to lower operating temperatures and therefore less thermal spread.
FOR MORE INFORMATION, VISIT: http://olympusmedical.com.sg
APP-BASED HANDHELD ULTRASOUND
High-resolution images can be saved, reviewed, and managed on the secure Clarius Cloud. Built with a durable magnesium shell, each device has an IPX7 immersion rating so it can be sterilized. Power is obtained from a rechargeable battery that will last for more than 45 minutes of scanning; 2 batteries come with each Clarius device.
FOR MORE INFORMATION, VISIT: https://www.clarius.me/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DIAGNOSE HERPES SIMPLEX VIRUS
According to the CDC, infections with HSV-2 affect more than 24 million Americans. Patients with HSV-2 strain are at increased risk for contracting and transmitting HIV. Pregnant women infected with HSV-2 are at risk of transmitting the virus to their babies, with increased risk for neurologic complications in the child.
FOR MORE INFORMATION, VISIT: http://www.hologic.com/search/site/aptima%20hsv
PRECISELY TARGET TISSUE DURING LUMPECTOMY OR BIOPSY
Cianna Medical reported recent data showing that, when compared with wire localization, the SCOUT reduces breast surgery operating room (OR) delay times by 72.5%, resulted in an average 29- minute reduction in OR waiting time, and significantly improved workflow efficiency.
FOR MORE INFORMATION, VISIT: https://www.ciannamedical.com/savi-scout/
PLASMA HYSTEROSCOPIC RESECTION
During gynecologic procedures, the Olympus 8.5-mm hystero-resectoscope uses a combination of radio frequency, energy, and saline to create plasma, an electrically conductive gas cloud of vapor and charged particles. Due to its conductivity, plasma allows energy to cross into targeted tissue at lower energy levels than with more traditional approaches. This effect leads to lower operating temperatures and therefore less thermal spread.
FOR MORE INFORMATION, VISIT: http://olympusmedical.com.sg
APP-BASED HANDHELD ULTRASOUND
High-resolution images can be saved, reviewed, and managed on the secure Clarius Cloud. Built with a durable magnesium shell, each device has an IPX7 immersion rating so it can be sterilized. Power is obtained from a rechargeable battery that will last for more than 45 minutes of scanning; 2 batteries come with each Clarius device.
FOR MORE INFORMATION, VISIT: https://www.clarius.me/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DIAGNOSE HERPES SIMPLEX VIRUS
According to the CDC, infections with HSV-2 affect more than 24 million Americans. Patients with HSV-2 strain are at increased risk for contracting and transmitting HIV. Pregnant women infected with HSV-2 are at risk of transmitting the virus to their babies, with increased risk for neurologic complications in the child.
FOR MORE INFORMATION, VISIT: http://www.hologic.com/search/site/aptima%20hsv
PRECISELY TARGET TISSUE DURING LUMPECTOMY OR BIOPSY
Cianna Medical reported recent data showing that, when compared with wire localization, the SCOUT reduces breast surgery operating room (OR) delay times by 72.5%, resulted in an average 29- minute reduction in OR waiting time, and significantly improved workflow efficiency.
FOR MORE INFORMATION, VISIT: https://www.ciannamedical.com/savi-scout/
PLASMA HYSTEROSCOPIC RESECTION
During gynecologic procedures, the Olympus 8.5-mm hystero-resectoscope uses a combination of radio frequency, energy, and saline to create plasma, an electrically conductive gas cloud of vapor and charged particles. Due to its conductivity, plasma allows energy to cross into targeted tissue at lower energy levels than with more traditional approaches. This effect leads to lower operating temperatures and therefore less thermal spread.
FOR MORE INFORMATION, VISIT: http://olympusmedical.com.sg
APP-BASED HANDHELD ULTRASOUND
High-resolution images can be saved, reviewed, and managed on the secure Clarius Cloud. Built with a durable magnesium shell, each device has an IPX7 immersion rating so it can be sterilized. Power is obtained from a rechargeable battery that will last for more than 45 minutes of scanning; 2 batteries come with each Clarius device.
FOR MORE INFORMATION, VISIT: https://www.clarius.me/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Comparison of Methods to Define High Use of Inpatient Services Using Population-Based Data
As healthcare system use and costs continue to rise, increased importance has been placed on identifying the small subgroup of patients that drive this trend.1 It is estimated that 5% of healthcare users account for over 60% of healthcare spending.2-6 Furthermore, care for these “high users” is expensive due to an over-reliance on inpatient services. Approximately 40% of all health spending is for inpatient care, the largest single category of health spending, which is similarly skewed toward high users.1,3,5 Improving our understanding of this population may provide an opportunity to direct improvement efforts to a select group of patients with a potentially high benefit, as well as move care away from the costly inpatient setting.
However, the development of effective interventions to improve patient experience and outcomes while decreasing costs (referred to as the “Triple Aim” by the Institute for Health Improvement) for high users of inpatient services hinges on the methodology used to identify this high-risk population.7 There is substantial variability in definitions of high users; the most common definitions are based on the number of hospital encounters, days spent in the hospital, and hospital costs.8-15 Definitions have intrinsic differences in their implications around appropriateness, efficiency, and financial sustainability of inpatient resource use. Though the constructs underlying these definitions are highly variable, direct comparisons of differences in patient capture are limited.
A recent study from a single US center explored the clinical characteristics of hospital patients based on definitions of use vs cost and observed important differences in patients’ profiles and outcomes.12 While this suggests that the choice of definition may have major implications for whom to target (and the efficacy of any proposed interventions), this concept has not been explored at the population level. Therefore, we used population-based administrative data from a single-payer healthcare system to compare 3 common definitions of high inpatient service use and their influence on patient capture, health outcomes, and inpatient system burden.
METHODS
Data Sources and Study Population
We conducted a retrospective population-based study using administrative and clinical data for the province of Alberta, including the discharge abstracts database, physician claims, ambulatory care records, population health registry file, and aggregated data from the Canadian census.16 We identified all adults who had 1 or more hospitalizations with a discharge date between April 1, 2012, and March 31, 2013, though the admission date could be prior to April 1, 2012.
Definition of High-Inpatient Use
High-inpatient use was defined using 3 metrics: number of inpatient episodes, length of stay, and cost. As in prior studies, for each definition, individuals in the upper5th percentile of the relevant distribution were designated “high users,”2,15 while patients in the lower 95th percentile were considered “nonhigh users.” Patients could be defined as a high user in more than 1 definition.
Patients with 3 or more hospital episodes were defined as high users for the “number of inpatient episodes” definition. A hospital episode of care was defined as an event that resulted in discharge (or death) from an inpatient facility. If an individual was admitted to a hospital and transferred to another facility within 1 day of discharge, the hospitalizations were considered part of the same episode of care.
The “length of stay” definition refers to the cumulative number of days spent in an inpatient facility for all eligible episodes of care. Patients with 56 or more days in hospital during the study period were considered high users. Day of admission and discharge were considered full inpatient days, regardless of the time of admission and discharge.
The “cost” definition considered the cumulative estimated cost of every eligible episode of care. We estimated costs for each hospitalization using resource intensity weights (RIW). This is a relative weighted value for the average inpatient case after taking factors such as age, comorbidity, and procedures into account. The RIW for each episode was multiplied by the national average inpatient cost.17 Based on this definition, patients with a cumulative hospital cost of ≥ $63,597 were deemed high users. All costs were calculated in Canadian Dollars (CAD, $) and adjusted to 2013 dollars based on Statistics Canada’s Consumer Price Index.18
Demographic, Clinical, and Encounter Characteristics
Individual characteristics were measured using a combination of provincial administrative data sources. All measures were recorded as of the admission date of the first eligible hospitalization. Demographic characteristics included age, sex, First Nations status, urban/rural status (based on the individual’s residential postal code), and median neighborhood income quintile. Clinical characteristics included 28 comorbid conditions defined based on separate validated International Statistical Classification of Disease and Health Related Problems, Tenth Revision, Canada (ICD-10-CA) coding algorithms reported individually and cumulatively (categorized as 0, 1, 2–3, and 4+).19 Primary care attachment was defined as the percentage of all outpatient primary care visits made to a single practitioner in the 2-year period prior to their first hospitalization (among those with ≥3 visits). Attachment was categorized as 75%-100% (good attachment), 50%-74% (moderate attachment), or <50% (low attachment).20,21
We also identified hospital encounter-level characteristics. These included the most responsible diagnosis, admission category (elective or urgent/emergent), and discharge disposition for each hospital episode. Reported health outcomes included the proportion of patients with in-hospital mortality and those with at least one 30-day, all-cause readmission to hospital.
Analysis
Patient characteristics were described using proportions and means (standard deviation) as appropriate for high users and nonhigh users within and across each definition. Encounter characteristics were also described and stratified by age category (18-64 or 65+ years). Comparison of patient capture was then analyzed among patients who were high use by at least 1 definition. The overlap and agreement of the 3 definitions were compared using a Venn diagram and kappa statistic. The 10 most responsible diagnoses (based on frequency) were also compared across definitions and stratified by age.
Finally, the percentage of system burden accounted for by each measure was calculated as the amount used by high users divided by the total amount used by the entire study population (x 100). To assess the potential modifying effect of age, results were stratified by age category for each definition.
All analyses were conducted using Stata 11.2 (StataCorp LP, College Station, TX).22 The Conjoint Health Research Ethics Board of the University of Calgary approved this study and granted waiver of patient consent. This manuscript is written in accordance with reporting guidelines for studies conducted using observational routinely collected health data (RECORD statement).23
RESULTS
Comparison of Patient and Encounter-level Characterist
ics
A total of 219,106 adults had 283,204 inpatient episodes of care within the study timeframe. There were 12,707 (5.8%), 11,095 (5.1%), and 10,956 (5.0%) patients defined as high users based on number of inpatient episodes, length of stay, and cost, respectively (supplementary Figure 1). Regardless of definition, when compared to their non–high use counterparts, patients classified as high use were more likely to be male, older, in a lower median neighborhood income quintile, and have a higher level of comorbidity. Comparing across definitions of high use, those defined by number of inpatient episodes were more likely to be younger, live in rural areas, have better primary care attachment, and have fewer comorbidities, compared to the other definitions. High users by length of stay were more likely to be older and had a higher proportion of mental health–related comorbidities, including dementia and depression, as compared with the other definitions. Results were largely similar for those defined by cost (Table 1).
Encounter-level analyses
Comparison of Patient Capture and Inpatient Burden
Of the 22,691 individuals who were defined as high use by at least 1 definition, 2,331 (10.3%) were consistently high use across all 3 definitions (kappa = 0.38; Figure 1). Of the 13,682 individuals classified as high use by at least 1 of length of stay or cost, 8369 (61.2%) were defined as high use by both definitions (kappa = 0.75). However, of the 12,707 defined as high use by the number of inpatient episodes, only 3698 (29.1%) were also defined as high use by another definition. Exploration of the most responsible diagnoses across definitions showed that congestive heart failure (2.8%-3.5%), chronic obstructive pulmonary disease (1.6%-3.2%), and dementia (0.6%-2.2%) were the most frequent. Acute medical conditions (eg, pneumonia [1.8%] or gastroenteritis [0.7%]) that may result in multiple shorter hospitalizations were observed at higher frequencies among high users defined by inpatient episodes, while conditions commonly requiring rehabilitation (eg, fracture [1.8%] and stroke [1.7%]) were more common among high users defined by length of stay and cost (supplementary Table 2). Stratification by age showed marked differences in the diagnoses across high-use definitions. Among hi
When assessing inpatient system burden, high users by number of inpatient episodes accounted for 47,044 (16.6%) of the 283,204 episodes. High users defined by length of stay accounted for 1,286,539 (46.4%) days of 2,773,561 total days, while high users defined by cost accumulated $1.4 billion (38.9%) of the estimated $3.7 billion in inpatient expenditures. High users defined by cost and length of stay each accounted for comparatively few episode
DISCUSSION
Using a large population-based cohort of all adults with at least 1 hospitalization in the province of Alberta, Canada, within a 12-month period, we compared 3 commonly used definitions of high inpatient use. The choice of definition had a substantial influence on the types of patients categorized as high use, as well as the proportion of total inpatient utilization that was associated with high users. The definition based on number of inpatient episodes captured a distinct population of high users, while the populations identified using cumulative length of stay or cost were similar.
Differences within and between definitions were especially apparent in age-stratified analyses: Greater length of stay or higher cost among patients aged 18-64 years identifies a large proportion of psychological conditions, while a greater number of inpatient episodes identifies acute conditions and childbirth or labor-related complications. Conversely, definitions based on length of stay and cost in the elderly (65+) identified groups with chronic conditions that result in progressive functional decline (often requiring increasing supportive services upon discharge) or conditions that require significant rehabilitation prior to discharge. Regarding inpatient system burden, high users defined by number of inpatient episodes accounted for a small proportion of total inpatient episodes, while high users defined by length of stay and cost accounted for nearly half of the accumulated hospital days and cost for each. These findings highlight the need for careful consideration of how high use is defined when studying high-user populations and implications for targeting subpopulations for intervention.
Our results add to those from previous studies. A US-based, single-center study of 2566 individuals compared definitions of high inpatient use based on cost and frequency of admission and found that patients defined by cost were predominantly hospitalized for surgical conditions, while those fulfilling the episode-based definition were often hospitalized for medical conditions.12 The most responsible diagnoses for patient hospitalizations in our study reflect this. We extended this comparison to consider the impact of age on outcomes and inpatient system burden and found that older age was also linked to poorer outcomes and increased burden. We also considered a third definition (cumulative length of stay), which provided another opportunity for comparison. The presence of chronic conditions requiring rehabilitation and possible alternate level of care days within our cohort highlights the utility of this length of stay-based approach when considering definitions. Although there were similarities between patients defined by length of stay and cost, partly due to cost being largely a function of length of stay, there were also important differences in their patient profiles. Those defined by cost tended to have conditions requiring surgical procedures not requiring extended in-hospital rehabilitation. Furthermore, the higher proportion of in-hospital mortality among those defined by cost may also reflect the fact that patients tend to accrue the majority of their healthcare expenditures during the final 120 days of life.24
Each definition of high use identified complex patients; however, the differences between the various types of high users identified by these definitions suggest that they are not interchangeable. Arguably, selection of the most appropriate definition should depend on the objective of measuring high users, particularly if an intervention is planned. Interventions for high users are complex, requiring both medical and nonmedical components. The current literature in this area has often focused on case management programs, collaboration with community-based social support programs, and improving coordination and transitions of care.25-27 While many of these approaches require considerable involvement outside of the inpatient setting, these interventions can be informed by defining who high users of inpatient services are. Our findings show several possible subgroups of high users, which could be targeted for intervention. For example, an inpatient episode-based definition, which identifies patients with frequent encounters for acute conditions (eg, pneumonia and urinary tract infections), would be informative if an intervention targeted reductions in inpatient use and readmission rates. Alternatively, an intervention designed to improve community-based mental health programs would best be informed by a definition based on length of stay in which high users with underlying mental health conditions were prevalent. Such interventions are rarely mutually exclusive and require multiple perspectives to inform their objectives. A well-designed intervention will not only address the medical characteristics of high users but also the social determinants of health that place patients at risk of high inpatient use.
Our study should be interpreted in light of its limitations. First, measures of disease severity were not available to further characterize similarities and differences across high-use groups. Furthermore, we were unable to account for other social determinants of health that may be relevant to inpatient system usage. Second, direct cost of hospitalizations was estimated based on RIW and is thus reflective of expected rather than actual costs. However, this will have minimal impact on capture, as patients defined by this metric require substantial costs to be included in the top fifth percentile, and thus deviations in individual hospitalization costs will have minimal influence on the cumulative cost. Finally, while inpatient spending makes up a large proportion of healthcare spending, there is likely a number of different high-use profiles found outside of the acute care setting. Despite these limitations, our study includes several key strengths. The use of population-level data allows for analysis that is robust and more generalizable than studies from single centers. Additionally, the comparison of 3 independent definitions allows for a greater comparison of the nuances of each definition. Our study also considers the important impact of age as an effect modifier of inpatient use in the general population and identifies distinct patient profiles that exist across each definition.
CONCLUSIONS
Definitions of high use of inpatient services based on number of inpatient episodes, days spent in hospital, and total hospital costs identify patient populations with different characteristics and differ substantially in their impact on health outcomes and inpatient burden. These results highlight the need for careful consideration of the context of the study or intervention and the implications of selecting a specific definition of high inpatient use at study conception. Ultimately, the performance of an intervention in high-use populations is likely to be conditional on the fit of the patient population generated by the chosen definition of high inpatient use to the objectives of a study.
Acknowledgments
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
Disclosure
Dr. Hemmelgarn is supported by the Roy and Vi Baay Chair in Kidney Research. Dr. Manns is supported by the Svare Professorship in Health Economics and by a Health Scholar Award by Alberta Innovates Health Solutions (AIHS). Dr. Tonelli is supported by the David Freeze chair in Health Services Research. The Interdisciplinary Chronic Disease Collaboration is funded by AIHS—Collaborative Research and Innovation Opportunity (CRIO) Team Grants Program.
1. National Health Expenditure Trends, 1975 to 2015. Canadian Institute for Health Information. 2015. https://secure.cihi.ca/free_products/nhex_trends_narrative_report_2015_en.pdf. Accessed on June 23, 2016.
2. Berk ML, Monheit AC. The concentration of health care expenditures, revisited. Health Aff (Millwood). 2001;20:9-18. PubMed
3. Wodchis WP, Austin PC, Henry DA. A 3-year study of high-cost users of health care. CMAJ. 2016;188(3):182-188. PubMed
4. Forget EL, Roos LL, Deber RB, Wald R. Variations in Lifetime Healthcare Costs across a Population. Healthc Policy. 2008;4:e148-e167. PubMed
5. Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572-2578. PubMed
6. Riley GF. Long-term trends in the concentration of Medicare spending. Health Aff (Millwood). 2007;26:808-816. PubMed
7. IHI Triple Aim Initiative. Institute for Healthcare Improvement. 2015. http://www.ihi.org/engage/initiatives/TripleAim/Pages/default.aspx. Accessed on June 17, 2016.
8. Johansen H, Nair C, Bond J. Who goes to the hospital? An investigation of high users of hospital days. Health Reports. 1994;6(2):253-277. PubMed
9. Conwell LJ, Cohen JW. Characteristics of persons with high medical expenditures in the US civilian noninstitutionalized population. MEPS Statistical Brief# 73. 2002.
10. Lemstra M, Mackenbach J, Neudorf C, Nannapaneni U. High health care utilization and costs associated with lower socio-economic status: Results from a linked dataset. CJPH. 2009;100(3):180-183. PubMed
11. Macnee CL, McCabe S, Clarke PN, Fiske M, Campbell S. Typology of high users of health services among a rural medicaid population. Pub Health Nurs. 2009;26(5):396-404. PubMed
12. Nguyen OK, Tang N, Hillman JM, Gonzales R. What’s cost got to do with it? Association between hospital costs and frequency of admissions among “high users” of hospital care. J. Hosp Med. 2013;8(12):665-671. PubMed
13. Rosella LC, Fitzpatrick T, Wodchis WP, Calzavara A, Manson H, Goel V. High-cost health care users in Ontario, Canada: Demographic, socio-economic, and health status characteristics. BMC Health Serv Res. 2014;14(1):532. PubMed
14. Cohen SB. The Concentration of Health Care Expenditures and Related Expenses for Costly Medical Conditions, 2009. Agency for Healthcare Research and Quality Statistical Brief #359; 2012.
15. Ronksley PE, McKay JA, Kobewka DM, Mulpuru S, Forster AJ. Patterns of health care use in a high-cost inpatient population in Ottawa, Ontario: A retrospective observational study. CMAJ Open. 2015; 3:E111-E118. PubMed
16. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. PubMed
17. DAD Resource Intensity Weights and Expected Length of Stay. Canadian Institute for Health Information. 2016. https://www.cihi.ca/en/data-and-standards/standards/case-mix/resource-indicators-dad-resource-intensity-weights-and. Accessed on June 24, 2016.
18. Statistics Canada. The Canadian Consumer Price Index Reference Paper, Statistics Canada Catalogue no. 62-553-X.
19. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak. 2015;17:15(1):1. PubMed
20. Jaakkimainen RL, Klein-Geltink J, Guttmann A, Barnsley J, Jagorski B, Kopp A. Indicators of primary care based on administrative data. In Primary Care in Ontario: ICES Atlas. Toronto, Ontario: Institute for Clinical Evaluative Sciences; 2006.
21. Jee SH, Cabana MD. Indices for continuity of care: A systematic review of the literature. Med Care Res Rev. 2006;63:158-188. PubMed
22. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP. 2009.
23. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. PubMed
24. Tanuseputro P, Wodchis WP, Fowler R, et al. The health care cost of dying: A population-based retrospective cohort study of the last year of life in ontario, canada. PLoS One. 2015;10(3):e0121759. PubMed
25. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: What makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19. PubMed
26. Birnbaum M, Halper DE. Rethinking service delivery for high-cost Medicaid patients. Medicaid Institute. 2009. http://shnny.org/research/rethinking-service-delivery-for-high-cost-medicaid-patients/. Accessed on Jan 11, 2017.
27. Pan-Canadian forum on high users of health care. Canadian Institute for Health Information. 2014. https://secure.cihi.ca/free_products/highusers_summary_report_revised_EN_web.pdf. Accessed on Jan 11, 2017.
As healthcare system use and costs continue to rise, increased importance has been placed on identifying the small subgroup of patients that drive this trend.1 It is estimated that 5% of healthcare users account for over 60% of healthcare spending.2-6 Furthermore, care for these “high users” is expensive due to an over-reliance on inpatient services. Approximately 40% of all health spending is for inpatient care, the largest single category of health spending, which is similarly skewed toward high users.1,3,5 Improving our understanding of this population may provide an opportunity to direct improvement efforts to a select group of patients with a potentially high benefit, as well as move care away from the costly inpatient setting.
However, the development of effective interventions to improve patient experience and outcomes while decreasing costs (referred to as the “Triple Aim” by the Institute for Health Improvement) for high users of inpatient services hinges on the methodology used to identify this high-risk population.7 There is substantial variability in definitions of high users; the most common definitions are based on the number of hospital encounters, days spent in the hospital, and hospital costs.8-15 Definitions have intrinsic differences in their implications around appropriateness, efficiency, and financial sustainability of inpatient resource use. Though the constructs underlying these definitions are highly variable, direct comparisons of differences in patient capture are limited.
A recent study from a single US center explored the clinical characteristics of hospital patients based on definitions of use vs cost and observed important differences in patients’ profiles and outcomes.12 While this suggests that the choice of definition may have major implications for whom to target (and the efficacy of any proposed interventions), this concept has not been explored at the population level. Therefore, we used population-based administrative data from a single-payer healthcare system to compare 3 common definitions of high inpatient service use and their influence on patient capture, health outcomes, and inpatient system burden.
METHODS
Data Sources and Study Population
We conducted a retrospective population-based study using administrative and clinical data for the province of Alberta, including the discharge abstracts database, physician claims, ambulatory care records, population health registry file, and aggregated data from the Canadian census.16 We identified all adults who had 1 or more hospitalizations with a discharge date between April 1, 2012, and March 31, 2013, though the admission date could be prior to April 1, 2012.
Definition of High-Inpatient Use
High-inpatient use was defined using 3 metrics: number of inpatient episodes, length of stay, and cost. As in prior studies, for each definition, individuals in the upper5th percentile of the relevant distribution were designated “high users,”2,15 while patients in the lower 95th percentile were considered “nonhigh users.” Patients could be defined as a high user in more than 1 definition.
Patients with 3 or more hospital episodes were defined as high users for the “number of inpatient episodes” definition. A hospital episode of care was defined as an event that resulted in discharge (or death) from an inpatient facility. If an individual was admitted to a hospital and transferred to another facility within 1 day of discharge, the hospitalizations were considered part of the same episode of care.
The “length of stay” definition refers to the cumulative number of days spent in an inpatient facility for all eligible episodes of care. Patients with 56 or more days in hospital during the study period were considered high users. Day of admission and discharge were considered full inpatient days, regardless of the time of admission and discharge.
The “cost” definition considered the cumulative estimated cost of every eligible episode of care. We estimated costs for each hospitalization using resource intensity weights (RIW). This is a relative weighted value for the average inpatient case after taking factors such as age, comorbidity, and procedures into account. The RIW for each episode was multiplied by the national average inpatient cost.17 Based on this definition, patients with a cumulative hospital cost of ≥ $63,597 were deemed high users. All costs were calculated in Canadian Dollars (CAD, $) and adjusted to 2013 dollars based on Statistics Canada’s Consumer Price Index.18
Demographic, Clinical, and Encounter Characteristics
Individual characteristics were measured using a combination of provincial administrative data sources. All measures were recorded as of the admission date of the first eligible hospitalization. Demographic characteristics included age, sex, First Nations status, urban/rural status (based on the individual’s residential postal code), and median neighborhood income quintile. Clinical characteristics included 28 comorbid conditions defined based on separate validated International Statistical Classification of Disease and Health Related Problems, Tenth Revision, Canada (ICD-10-CA) coding algorithms reported individually and cumulatively (categorized as 0, 1, 2–3, and 4+).19 Primary care attachment was defined as the percentage of all outpatient primary care visits made to a single practitioner in the 2-year period prior to their first hospitalization (among those with ≥3 visits). Attachment was categorized as 75%-100% (good attachment), 50%-74% (moderate attachment), or <50% (low attachment).20,21
We also identified hospital encounter-level characteristics. These included the most responsible diagnosis, admission category (elective or urgent/emergent), and discharge disposition for each hospital episode. Reported health outcomes included the proportion of patients with in-hospital mortality and those with at least one 30-day, all-cause readmission to hospital.
Analysis
Patient characteristics were described using proportions and means (standard deviation) as appropriate for high users and nonhigh users within and across each definition. Encounter characteristics were also described and stratified by age category (18-64 or 65+ years). Comparison of patient capture was then analyzed among patients who were high use by at least 1 definition. The overlap and agreement of the 3 definitions were compared using a Venn diagram and kappa statistic. The 10 most responsible diagnoses (based on frequency) were also compared across definitions and stratified by age.
Finally, the percentage of system burden accounted for by each measure was calculated as the amount used by high users divided by the total amount used by the entire study population (x 100). To assess the potential modifying effect of age, results were stratified by age category for each definition.
All analyses were conducted using Stata 11.2 (StataCorp LP, College Station, TX).22 The Conjoint Health Research Ethics Board of the University of Calgary approved this study and granted waiver of patient consent. This manuscript is written in accordance with reporting guidelines for studies conducted using observational routinely collected health data (RECORD statement).23
RESULTS
Comparison of Patient and Encounter-level Characterist
ics
A total of 219,106 adults had 283,204 inpatient episodes of care within the study timeframe. There were 12,707 (5.8%), 11,095 (5.1%), and 10,956 (5.0%) patients defined as high users based on number of inpatient episodes, length of stay, and cost, respectively (supplementary Figure 1). Regardless of definition, when compared to their non–high use counterparts, patients classified as high use were more likely to be male, older, in a lower median neighborhood income quintile, and have a higher level of comorbidity. Comparing across definitions of high use, those defined by number of inpatient episodes were more likely to be younger, live in rural areas, have better primary care attachment, and have fewer comorbidities, compared to the other definitions. High users by length of stay were more likely to be older and had a higher proportion of mental health–related comorbidities, including dementia and depression, as compared with the other definitions. Results were largely similar for those defined by cost (Table 1).
Encounter-level analyses
Comparison of Patient Capture and Inpatient Burden
Of the 22,691 individuals who were defined as high use by at least 1 definition, 2,331 (10.3%) were consistently high use across all 3 definitions (kappa = 0.38; Figure 1). Of the 13,682 individuals classified as high use by at least 1 of length of stay or cost, 8369 (61.2%) were defined as high use by both definitions (kappa = 0.75). However, of the 12,707 defined as high use by the number of inpatient episodes, only 3698 (29.1%) were also defined as high use by another definition. Exploration of the most responsible diagnoses across definitions showed that congestive heart failure (2.8%-3.5%), chronic obstructive pulmonary disease (1.6%-3.2%), and dementia (0.6%-2.2%) were the most frequent. Acute medical conditions (eg, pneumonia [1.8%] or gastroenteritis [0.7%]) that may result in multiple shorter hospitalizations were observed at higher frequencies among high users defined by inpatient episodes, while conditions commonly requiring rehabilitation (eg, fracture [1.8%] and stroke [1.7%]) were more common among high users defined by length of stay and cost (supplementary Table 2). Stratification by age showed marked differences in the diagnoses across high-use definitions. Among hi
When assessing inpatient system burden, high users by number of inpatient episodes accounted for 47,044 (16.6%) of the 283,204 episodes. High users defined by length of stay accounted for 1,286,539 (46.4%) days of 2,773,561 total days, while high users defined by cost accumulated $1.4 billion (38.9%) of the estimated $3.7 billion in inpatient expenditures. High users defined by cost and length of stay each accounted for comparatively few episode
DISCUSSION
Using a large population-based cohort of all adults with at least 1 hospitalization in the province of Alberta, Canada, within a 12-month period, we compared 3 commonly used definitions of high inpatient use. The choice of definition had a substantial influence on the types of patients categorized as high use, as well as the proportion of total inpatient utilization that was associated with high users. The definition based on number of inpatient episodes captured a distinct population of high users, while the populations identified using cumulative length of stay or cost were similar.
Differences within and between definitions were especially apparent in age-stratified analyses: Greater length of stay or higher cost among patients aged 18-64 years identifies a large proportion of psychological conditions, while a greater number of inpatient episodes identifies acute conditions and childbirth or labor-related complications. Conversely, definitions based on length of stay and cost in the elderly (65+) identified groups with chronic conditions that result in progressive functional decline (often requiring increasing supportive services upon discharge) or conditions that require significant rehabilitation prior to discharge. Regarding inpatient system burden, high users defined by number of inpatient episodes accounted for a small proportion of total inpatient episodes, while high users defined by length of stay and cost accounted for nearly half of the accumulated hospital days and cost for each. These findings highlight the need for careful consideration of how high use is defined when studying high-user populations and implications for targeting subpopulations for intervention.
Our results add to those from previous studies. A US-based, single-center study of 2566 individuals compared definitions of high inpatient use based on cost and frequency of admission and found that patients defined by cost were predominantly hospitalized for surgical conditions, while those fulfilling the episode-based definition were often hospitalized for medical conditions.12 The most responsible diagnoses for patient hospitalizations in our study reflect this. We extended this comparison to consider the impact of age on outcomes and inpatient system burden and found that older age was also linked to poorer outcomes and increased burden. We also considered a third definition (cumulative length of stay), which provided another opportunity for comparison. The presence of chronic conditions requiring rehabilitation and possible alternate level of care days within our cohort highlights the utility of this length of stay-based approach when considering definitions. Although there were similarities between patients defined by length of stay and cost, partly due to cost being largely a function of length of stay, there were also important differences in their patient profiles. Those defined by cost tended to have conditions requiring surgical procedures not requiring extended in-hospital rehabilitation. Furthermore, the higher proportion of in-hospital mortality among those defined by cost may also reflect the fact that patients tend to accrue the majority of their healthcare expenditures during the final 120 days of life.24
Each definition of high use identified complex patients; however, the differences between the various types of high users identified by these definitions suggest that they are not interchangeable. Arguably, selection of the most appropriate definition should depend on the objective of measuring high users, particularly if an intervention is planned. Interventions for high users are complex, requiring both medical and nonmedical components. The current literature in this area has often focused on case management programs, collaboration with community-based social support programs, and improving coordination and transitions of care.25-27 While many of these approaches require considerable involvement outside of the inpatient setting, these interventions can be informed by defining who high users of inpatient services are. Our findings show several possible subgroups of high users, which could be targeted for intervention. For example, an inpatient episode-based definition, which identifies patients with frequent encounters for acute conditions (eg, pneumonia and urinary tract infections), would be informative if an intervention targeted reductions in inpatient use and readmission rates. Alternatively, an intervention designed to improve community-based mental health programs would best be informed by a definition based on length of stay in which high users with underlying mental health conditions were prevalent. Such interventions are rarely mutually exclusive and require multiple perspectives to inform their objectives. A well-designed intervention will not only address the medical characteristics of high users but also the social determinants of health that place patients at risk of high inpatient use.
Our study should be interpreted in light of its limitations. First, measures of disease severity were not available to further characterize similarities and differences across high-use groups. Furthermore, we were unable to account for other social determinants of health that may be relevant to inpatient system usage. Second, direct cost of hospitalizations was estimated based on RIW and is thus reflective of expected rather than actual costs. However, this will have minimal impact on capture, as patients defined by this metric require substantial costs to be included in the top fifth percentile, and thus deviations in individual hospitalization costs will have minimal influence on the cumulative cost. Finally, while inpatient spending makes up a large proportion of healthcare spending, there is likely a number of different high-use profiles found outside of the acute care setting. Despite these limitations, our study includes several key strengths. The use of population-level data allows for analysis that is robust and more generalizable than studies from single centers. Additionally, the comparison of 3 independent definitions allows for a greater comparison of the nuances of each definition. Our study also considers the important impact of age as an effect modifier of inpatient use in the general population and identifies distinct patient profiles that exist across each definition.
CONCLUSIONS
Definitions of high use of inpatient services based on number of inpatient episodes, days spent in hospital, and total hospital costs identify patient populations with different characteristics and differ substantially in their impact on health outcomes and inpatient burden. These results highlight the need for careful consideration of the context of the study or intervention and the implications of selecting a specific definition of high inpatient use at study conception. Ultimately, the performance of an intervention in high-use populations is likely to be conditional on the fit of the patient population generated by the chosen definition of high inpatient use to the objectives of a study.
Acknowledgments
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
Disclosure
Dr. Hemmelgarn is supported by the Roy and Vi Baay Chair in Kidney Research. Dr. Manns is supported by the Svare Professorship in Health Economics and by a Health Scholar Award by Alberta Innovates Health Solutions (AIHS). Dr. Tonelli is supported by the David Freeze chair in Health Services Research. The Interdisciplinary Chronic Disease Collaboration is funded by AIHS—Collaborative Research and Innovation Opportunity (CRIO) Team Grants Program.
As healthcare system use and costs continue to rise, increased importance has been placed on identifying the small subgroup of patients that drive this trend.1 It is estimated that 5% of healthcare users account for over 60% of healthcare spending.2-6 Furthermore, care for these “high users” is expensive due to an over-reliance on inpatient services. Approximately 40% of all health spending is for inpatient care, the largest single category of health spending, which is similarly skewed toward high users.1,3,5 Improving our understanding of this population may provide an opportunity to direct improvement efforts to a select group of patients with a potentially high benefit, as well as move care away from the costly inpatient setting.
However, the development of effective interventions to improve patient experience and outcomes while decreasing costs (referred to as the “Triple Aim” by the Institute for Health Improvement) for high users of inpatient services hinges on the methodology used to identify this high-risk population.7 There is substantial variability in definitions of high users; the most common definitions are based on the number of hospital encounters, days spent in the hospital, and hospital costs.8-15 Definitions have intrinsic differences in their implications around appropriateness, efficiency, and financial sustainability of inpatient resource use. Though the constructs underlying these definitions are highly variable, direct comparisons of differences in patient capture are limited.
A recent study from a single US center explored the clinical characteristics of hospital patients based on definitions of use vs cost and observed important differences in patients’ profiles and outcomes.12 While this suggests that the choice of definition may have major implications for whom to target (and the efficacy of any proposed interventions), this concept has not been explored at the population level. Therefore, we used population-based administrative data from a single-payer healthcare system to compare 3 common definitions of high inpatient service use and their influence on patient capture, health outcomes, and inpatient system burden.
METHODS
Data Sources and Study Population
We conducted a retrospective population-based study using administrative and clinical data for the province of Alberta, including the discharge abstracts database, physician claims, ambulatory care records, population health registry file, and aggregated data from the Canadian census.16 We identified all adults who had 1 or more hospitalizations with a discharge date between April 1, 2012, and March 31, 2013, though the admission date could be prior to April 1, 2012.
Definition of High-Inpatient Use
High-inpatient use was defined using 3 metrics: number of inpatient episodes, length of stay, and cost. As in prior studies, for each definition, individuals in the upper5th percentile of the relevant distribution were designated “high users,”2,15 while patients in the lower 95th percentile were considered “nonhigh users.” Patients could be defined as a high user in more than 1 definition.
Patients with 3 or more hospital episodes were defined as high users for the “number of inpatient episodes” definition. A hospital episode of care was defined as an event that resulted in discharge (or death) from an inpatient facility. If an individual was admitted to a hospital and transferred to another facility within 1 day of discharge, the hospitalizations were considered part of the same episode of care.
The “length of stay” definition refers to the cumulative number of days spent in an inpatient facility for all eligible episodes of care. Patients with 56 or more days in hospital during the study period were considered high users. Day of admission and discharge were considered full inpatient days, regardless of the time of admission and discharge.
The “cost” definition considered the cumulative estimated cost of every eligible episode of care. We estimated costs for each hospitalization using resource intensity weights (RIW). This is a relative weighted value for the average inpatient case after taking factors such as age, comorbidity, and procedures into account. The RIW for each episode was multiplied by the national average inpatient cost.17 Based on this definition, patients with a cumulative hospital cost of ≥ $63,597 were deemed high users. All costs were calculated in Canadian Dollars (CAD, $) and adjusted to 2013 dollars based on Statistics Canada’s Consumer Price Index.18
Demographic, Clinical, and Encounter Characteristics
Individual characteristics were measured using a combination of provincial administrative data sources. All measures were recorded as of the admission date of the first eligible hospitalization. Demographic characteristics included age, sex, First Nations status, urban/rural status (based on the individual’s residential postal code), and median neighborhood income quintile. Clinical characteristics included 28 comorbid conditions defined based on separate validated International Statistical Classification of Disease and Health Related Problems, Tenth Revision, Canada (ICD-10-CA) coding algorithms reported individually and cumulatively (categorized as 0, 1, 2–3, and 4+).19 Primary care attachment was defined as the percentage of all outpatient primary care visits made to a single practitioner in the 2-year period prior to their first hospitalization (among those with ≥3 visits). Attachment was categorized as 75%-100% (good attachment), 50%-74% (moderate attachment), or <50% (low attachment).20,21
We also identified hospital encounter-level characteristics. These included the most responsible diagnosis, admission category (elective or urgent/emergent), and discharge disposition for each hospital episode. Reported health outcomes included the proportion of patients with in-hospital mortality and those with at least one 30-day, all-cause readmission to hospital.
Analysis
Patient characteristics were described using proportions and means (standard deviation) as appropriate for high users and nonhigh users within and across each definition. Encounter characteristics were also described and stratified by age category (18-64 or 65+ years). Comparison of patient capture was then analyzed among patients who were high use by at least 1 definition. The overlap and agreement of the 3 definitions were compared using a Venn diagram and kappa statistic. The 10 most responsible diagnoses (based on frequency) were also compared across definitions and stratified by age.
Finally, the percentage of system burden accounted for by each measure was calculated as the amount used by high users divided by the total amount used by the entire study population (x 100). To assess the potential modifying effect of age, results were stratified by age category for each definition.
All analyses were conducted using Stata 11.2 (StataCorp LP, College Station, TX).22 The Conjoint Health Research Ethics Board of the University of Calgary approved this study and granted waiver of patient consent. This manuscript is written in accordance with reporting guidelines for studies conducted using observational routinely collected health data (RECORD statement).23
RESULTS
Comparison of Patient and Encounter-level Characterist
ics
A total of 219,106 adults had 283,204 inpatient episodes of care within the study timeframe. There were 12,707 (5.8%), 11,095 (5.1%), and 10,956 (5.0%) patients defined as high users based on number of inpatient episodes, length of stay, and cost, respectively (supplementary Figure 1). Regardless of definition, when compared to their non–high use counterparts, patients classified as high use were more likely to be male, older, in a lower median neighborhood income quintile, and have a higher level of comorbidity. Comparing across definitions of high use, those defined by number of inpatient episodes were more likely to be younger, live in rural areas, have better primary care attachment, and have fewer comorbidities, compared to the other definitions. High users by length of stay were more likely to be older and had a higher proportion of mental health–related comorbidities, including dementia and depression, as compared with the other definitions. Results were largely similar for those defined by cost (Table 1).
Encounter-level analyses
Comparison of Patient Capture and Inpatient Burden
Of the 22,691 individuals who were defined as high use by at least 1 definition, 2,331 (10.3%) were consistently high use across all 3 definitions (kappa = 0.38; Figure 1). Of the 13,682 individuals classified as high use by at least 1 of length of stay or cost, 8369 (61.2%) were defined as high use by both definitions (kappa = 0.75). However, of the 12,707 defined as high use by the number of inpatient episodes, only 3698 (29.1%) were also defined as high use by another definition. Exploration of the most responsible diagnoses across definitions showed that congestive heart failure (2.8%-3.5%), chronic obstructive pulmonary disease (1.6%-3.2%), and dementia (0.6%-2.2%) were the most frequent. Acute medical conditions (eg, pneumonia [1.8%] or gastroenteritis [0.7%]) that may result in multiple shorter hospitalizations were observed at higher frequencies among high users defined by inpatient episodes, while conditions commonly requiring rehabilitation (eg, fracture [1.8%] and stroke [1.7%]) were more common among high users defined by length of stay and cost (supplementary Table 2). Stratification by age showed marked differences in the diagnoses across high-use definitions. Among hi
When assessing inpatient system burden, high users by number of inpatient episodes accounted for 47,044 (16.6%) of the 283,204 episodes. High users defined by length of stay accounted for 1,286,539 (46.4%) days of 2,773,561 total days, while high users defined by cost accumulated $1.4 billion (38.9%) of the estimated $3.7 billion in inpatient expenditures. High users defined by cost and length of stay each accounted for comparatively few episode
DISCUSSION
Using a large population-based cohort of all adults with at least 1 hospitalization in the province of Alberta, Canada, within a 12-month period, we compared 3 commonly used definitions of high inpatient use. The choice of definition had a substantial influence on the types of patients categorized as high use, as well as the proportion of total inpatient utilization that was associated with high users. The definition based on number of inpatient episodes captured a distinct population of high users, while the populations identified using cumulative length of stay or cost were similar.
Differences within and between definitions were especially apparent in age-stratified analyses: Greater length of stay or higher cost among patients aged 18-64 years identifies a large proportion of psychological conditions, while a greater number of inpatient episodes identifies acute conditions and childbirth or labor-related complications. Conversely, definitions based on length of stay and cost in the elderly (65+) identified groups with chronic conditions that result in progressive functional decline (often requiring increasing supportive services upon discharge) or conditions that require significant rehabilitation prior to discharge. Regarding inpatient system burden, high users defined by number of inpatient episodes accounted for a small proportion of total inpatient episodes, while high users defined by length of stay and cost accounted for nearly half of the accumulated hospital days and cost for each. These findings highlight the need for careful consideration of how high use is defined when studying high-user populations and implications for targeting subpopulations for intervention.
Our results add to those from previous studies. A US-based, single-center study of 2566 individuals compared definitions of high inpatient use based on cost and frequency of admission and found that patients defined by cost were predominantly hospitalized for surgical conditions, while those fulfilling the episode-based definition were often hospitalized for medical conditions.12 The most responsible diagnoses for patient hospitalizations in our study reflect this. We extended this comparison to consider the impact of age on outcomes and inpatient system burden and found that older age was also linked to poorer outcomes and increased burden. We also considered a third definition (cumulative length of stay), which provided another opportunity for comparison. The presence of chronic conditions requiring rehabilitation and possible alternate level of care days within our cohort highlights the utility of this length of stay-based approach when considering definitions. Although there were similarities between patients defined by length of stay and cost, partly due to cost being largely a function of length of stay, there were also important differences in their patient profiles. Those defined by cost tended to have conditions requiring surgical procedures not requiring extended in-hospital rehabilitation. Furthermore, the higher proportion of in-hospital mortality among those defined by cost may also reflect the fact that patients tend to accrue the majority of their healthcare expenditures during the final 120 days of life.24
Each definition of high use identified complex patients; however, the differences between the various types of high users identified by these definitions suggest that they are not interchangeable. Arguably, selection of the most appropriate definition should depend on the objective of measuring high users, particularly if an intervention is planned. Interventions for high users are complex, requiring both medical and nonmedical components. The current literature in this area has often focused on case management programs, collaboration with community-based social support programs, and improving coordination and transitions of care.25-27 While many of these approaches require considerable involvement outside of the inpatient setting, these interventions can be informed by defining who high users of inpatient services are. Our findings show several possible subgroups of high users, which could be targeted for intervention. For example, an inpatient episode-based definition, which identifies patients with frequent encounters for acute conditions (eg, pneumonia and urinary tract infections), would be informative if an intervention targeted reductions in inpatient use and readmission rates. Alternatively, an intervention designed to improve community-based mental health programs would best be informed by a definition based on length of stay in which high users with underlying mental health conditions were prevalent. Such interventions are rarely mutually exclusive and require multiple perspectives to inform their objectives. A well-designed intervention will not only address the medical characteristics of high users but also the social determinants of health that place patients at risk of high inpatient use.
Our study should be interpreted in light of its limitations. First, measures of disease severity were not available to further characterize similarities and differences across high-use groups. Furthermore, we were unable to account for other social determinants of health that may be relevant to inpatient system usage. Second, direct cost of hospitalizations was estimated based on RIW and is thus reflective of expected rather than actual costs. However, this will have minimal impact on capture, as patients defined by this metric require substantial costs to be included in the top fifth percentile, and thus deviations in individual hospitalization costs will have minimal influence on the cumulative cost. Finally, while inpatient spending makes up a large proportion of healthcare spending, there is likely a number of different high-use profiles found outside of the acute care setting. Despite these limitations, our study includes several key strengths. The use of population-level data allows for analysis that is robust and more generalizable than studies from single centers. Additionally, the comparison of 3 independent definitions allows for a greater comparison of the nuances of each definition. Our study also considers the important impact of age as an effect modifier of inpatient use in the general population and identifies distinct patient profiles that exist across each definition.
CONCLUSIONS
Definitions of high use of inpatient services based on number of inpatient episodes, days spent in hospital, and total hospital costs identify patient populations with different characteristics and differ substantially in their impact on health outcomes and inpatient burden. These results highlight the need for careful consideration of the context of the study or intervention and the implications of selecting a specific definition of high inpatient use at study conception. Ultimately, the performance of an intervention in high-use populations is likely to be conditional on the fit of the patient population generated by the chosen definition of high inpatient use to the objectives of a study.
Acknowledgments
This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
Disclosure
Dr. Hemmelgarn is supported by the Roy and Vi Baay Chair in Kidney Research. Dr. Manns is supported by the Svare Professorship in Health Economics and by a Health Scholar Award by Alberta Innovates Health Solutions (AIHS). Dr. Tonelli is supported by the David Freeze chair in Health Services Research. The Interdisciplinary Chronic Disease Collaboration is funded by AIHS—Collaborative Research and Innovation Opportunity (CRIO) Team Grants Program.
1. National Health Expenditure Trends, 1975 to 2015. Canadian Institute for Health Information. 2015. https://secure.cihi.ca/free_products/nhex_trends_narrative_report_2015_en.pdf. Accessed on June 23, 2016.
2. Berk ML, Monheit AC. The concentration of health care expenditures, revisited. Health Aff (Millwood). 2001;20:9-18. PubMed
3. Wodchis WP, Austin PC, Henry DA. A 3-year study of high-cost users of health care. CMAJ. 2016;188(3):182-188. PubMed
4. Forget EL, Roos LL, Deber RB, Wald R. Variations in Lifetime Healthcare Costs across a Population. Healthc Policy. 2008;4:e148-e167. PubMed
5. Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572-2578. PubMed
6. Riley GF. Long-term trends in the concentration of Medicare spending. Health Aff (Millwood). 2007;26:808-816. PubMed
7. IHI Triple Aim Initiative. Institute for Healthcare Improvement. 2015. http://www.ihi.org/engage/initiatives/TripleAim/Pages/default.aspx. Accessed on June 17, 2016.
8. Johansen H, Nair C, Bond J. Who goes to the hospital? An investigation of high users of hospital days. Health Reports. 1994;6(2):253-277. PubMed
9. Conwell LJ, Cohen JW. Characteristics of persons with high medical expenditures in the US civilian noninstitutionalized population. MEPS Statistical Brief# 73. 2002.
10. Lemstra M, Mackenbach J, Neudorf C, Nannapaneni U. High health care utilization and costs associated with lower socio-economic status: Results from a linked dataset. CJPH. 2009;100(3):180-183. PubMed
11. Macnee CL, McCabe S, Clarke PN, Fiske M, Campbell S. Typology of high users of health services among a rural medicaid population. Pub Health Nurs. 2009;26(5):396-404. PubMed
12. Nguyen OK, Tang N, Hillman JM, Gonzales R. What’s cost got to do with it? Association between hospital costs and frequency of admissions among “high users” of hospital care. J. Hosp Med. 2013;8(12):665-671. PubMed
13. Rosella LC, Fitzpatrick T, Wodchis WP, Calzavara A, Manson H, Goel V. High-cost health care users in Ontario, Canada: Demographic, socio-economic, and health status characteristics. BMC Health Serv Res. 2014;14(1):532. PubMed
14. Cohen SB. The Concentration of Health Care Expenditures and Related Expenses for Costly Medical Conditions, 2009. Agency for Healthcare Research and Quality Statistical Brief #359; 2012.
15. Ronksley PE, McKay JA, Kobewka DM, Mulpuru S, Forster AJ. Patterns of health care use in a high-cost inpatient population in Ottawa, Ontario: A retrospective observational study. CMAJ Open. 2015; 3:E111-E118. PubMed
16. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. PubMed
17. DAD Resource Intensity Weights and Expected Length of Stay. Canadian Institute for Health Information. 2016. https://www.cihi.ca/en/data-and-standards/standards/case-mix/resource-indicators-dad-resource-intensity-weights-and. Accessed on June 24, 2016.
18. Statistics Canada. The Canadian Consumer Price Index Reference Paper, Statistics Canada Catalogue no. 62-553-X.
19. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak. 2015;17:15(1):1. PubMed
20. Jaakkimainen RL, Klein-Geltink J, Guttmann A, Barnsley J, Jagorski B, Kopp A. Indicators of primary care based on administrative data. In Primary Care in Ontario: ICES Atlas. Toronto, Ontario: Institute for Clinical Evaluative Sciences; 2006.
21. Jee SH, Cabana MD. Indices for continuity of care: A systematic review of the literature. Med Care Res Rev. 2006;63:158-188. PubMed
22. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP. 2009.
23. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. PubMed
24. Tanuseputro P, Wodchis WP, Fowler R, et al. The health care cost of dying: A population-based retrospective cohort study of the last year of life in ontario, canada. PLoS One. 2015;10(3):e0121759. PubMed
25. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: What makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19. PubMed
26. Birnbaum M, Halper DE. Rethinking service delivery for high-cost Medicaid patients. Medicaid Institute. 2009. http://shnny.org/research/rethinking-service-delivery-for-high-cost-medicaid-patients/. Accessed on Jan 11, 2017.
27. Pan-Canadian forum on high users of health care. Canadian Institute for Health Information. 2014. https://secure.cihi.ca/free_products/highusers_summary_report_revised_EN_web.pdf. Accessed on Jan 11, 2017.
1. National Health Expenditure Trends, 1975 to 2015. Canadian Institute for Health Information. 2015. https://secure.cihi.ca/free_products/nhex_trends_narrative_report_2015_en.pdf. Accessed on June 23, 2016.
2. Berk ML, Monheit AC. The concentration of health care expenditures, revisited. Health Aff (Millwood). 2001;20:9-18. PubMed
3. Wodchis WP, Austin PC, Henry DA. A 3-year study of high-cost users of health care. CMAJ. 2016;188(3):182-188. PubMed
4. Forget EL, Roos LL, Deber RB, Wald R. Variations in Lifetime Healthcare Costs across a Population. Healthc Policy. 2008;4:e148-e167. PubMed
5. Joynt KE, Gawande AA, Orav EJ, Jha AK. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572-2578. PubMed
6. Riley GF. Long-term trends in the concentration of Medicare spending. Health Aff (Millwood). 2007;26:808-816. PubMed
7. IHI Triple Aim Initiative. Institute for Healthcare Improvement. 2015. http://www.ihi.org/engage/initiatives/TripleAim/Pages/default.aspx. Accessed on June 17, 2016.
8. Johansen H, Nair C, Bond J. Who goes to the hospital? An investigation of high users of hospital days. Health Reports. 1994;6(2):253-277. PubMed
9. Conwell LJ, Cohen JW. Characteristics of persons with high medical expenditures in the US civilian noninstitutionalized population. MEPS Statistical Brief# 73. 2002.
10. Lemstra M, Mackenbach J, Neudorf C, Nannapaneni U. High health care utilization and costs associated with lower socio-economic status: Results from a linked dataset. CJPH. 2009;100(3):180-183. PubMed
11. Macnee CL, McCabe S, Clarke PN, Fiske M, Campbell S. Typology of high users of health services among a rural medicaid population. Pub Health Nurs. 2009;26(5):396-404. PubMed
12. Nguyen OK, Tang N, Hillman JM, Gonzales R. What’s cost got to do with it? Association between hospital costs and frequency of admissions among “high users” of hospital care. J. Hosp Med. 2013;8(12):665-671. PubMed
13. Rosella LC, Fitzpatrick T, Wodchis WP, Calzavara A, Manson H, Goel V. High-cost health care users in Ontario, Canada: Demographic, socio-economic, and health status characteristics. BMC Health Serv Res. 2014;14(1):532. PubMed
14. Cohen SB. The Concentration of Health Care Expenditures and Related Expenses for Costly Medical Conditions, 2009. Agency for Healthcare Research and Quality Statistical Brief #359; 2012.
15. Ronksley PE, McKay JA, Kobewka DM, Mulpuru S, Forster AJ. Patterns of health care use in a high-cost inpatient population in Ottawa, Ontario: A retrospective observational study. CMAJ Open. 2015; 3:E111-E118. PubMed
16. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. PubMed
17. DAD Resource Intensity Weights and Expected Length of Stay. Canadian Institute for Health Information. 2016. https://www.cihi.ca/en/data-and-standards/standards/case-mix/resource-indicators-dad-resource-intensity-weights-and. Accessed on June 24, 2016.
18. Statistics Canada. The Canadian Consumer Price Index Reference Paper, Statistics Canada Catalogue no. 62-553-X.
19. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak. 2015;17:15(1):1. PubMed
20. Jaakkimainen RL, Klein-Geltink J, Guttmann A, Barnsley J, Jagorski B, Kopp A. Indicators of primary care based on administrative data. In Primary Care in Ontario: ICES Atlas. Toronto, Ontario: Institute for Clinical Evaluative Sciences; 2006.
21. Jee SH, Cabana MD. Indices for continuity of care: A systematic review of the literature. Med Care Res Rev. 2006;63:158-188. PubMed
22. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP. 2009.
23. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. PubMed
24. Tanuseputro P, Wodchis WP, Fowler R, et al. The health care cost of dying: A population-based retrospective cohort study of the last year of life in ontario, canada. PLoS One. 2015;10(3):e0121759. PubMed
25. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: What makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19. PubMed
26. Birnbaum M, Halper DE. Rethinking service delivery for high-cost Medicaid patients. Medicaid Institute. 2009. http://shnny.org/research/rethinking-service-delivery-for-high-cost-medicaid-patients/. Accessed on Jan 11, 2017.
27. Pan-Canadian forum on high users of health care. Canadian Institute for Health Information. 2014. https://secure.cihi.ca/free_products/highusers_summary_report_revised_EN_web.pdf. Accessed on Jan 11, 2017.
© 2017 Society of Hospital Medicine
Trans-Scaphoid Transcapitate Perilunate Fracture-Dislocation
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Models provide new understanding of sickle cell disease
Computer models have revealed new details of what happens inside a red blood cell affected by sickle cell disease (SCD), according to research published in Biophysical Journal.
In patients with SCD, mutated hemoglobin can polymerize, assembling into long fibers that push against the membranes of red blood cells and force them out of shape.
“The goal of our work is to model both how these sickle hemoglobin fibers form as well as the mechanical properties of those fibers,” said study author Lu Lu, a PhD student at Brown University in Providence, Rhode Island.
“There had been separate models for each of these things individually developed by us, but this brings those together into one comprehensive model.”
The model uses detailed biomechanical data on how sickle hemoglobin molecules behave and bind with each other to simulate the assembly of a polymer fiber.
Prior to this work, the problem had been that, as the fiber grows, so does the amount of data the model must crunch. Modeling an entire polymer fiber at a cellular scale using the details of each molecule was simply too computationally expensive.
“Even the world’s fastest supercomputers wouldn’t be able to handle it,” said study author George Karniadakis, PhD, of Brown University.
“There’s just too much happening and no way to capture it all computationally. That’s what we were able to overcome with this work.”
The researchers’ solution was to apply what they call a mesoscopic adaptive resolution scheme (MARS).
The MARS model calculates the detailed dynamics of each individual hemoglobin molecule only at the end of polymer fibers, where new molecules are being recruited into the fiber.
Once 4 layers of a fiber have been established, the model automatically dials back the resolution at which it represents that section. The model retains the important information about how the fiber behaves mechanically but glosses over the fine details of each constituent molecule.
“By eliminating the fine details where we don’t need them, we develop a model that can simulate this whole process and its effects on a red blood cell,” Dr Karniadakis said.
Using the new MARS simulations, the researchers were able to show how different configurations of growing polymer fibers are able to produce cells with different shapes.
“We are able to produce a polymerization profile for each of the cell types associated with the disease,” Dr Karniadakis said. “Now, the goal is to use these models to look for ways of preventing the disease onset.”
Using these new models, Dr Karniadakis and his colleagues can run simulations that include fetal hemoglobin. Those simulations could be used to confirm the theory that fetal hemoglobin disrupts polymerization, as well as determine how much fetal hemoglobin is necessary.
That could help in establishing better dosage guidelines for hydroxyurea or in developing new and more effective drugs for SCD, according to the researchers.
“The models give us a way to do preliminary testing on new approaches to stopping this disease,” Dr Karniadakis said. “Now that we can simulate the entire polymerization process, we think the models will be much more useful.” ![]()
Computer models have revealed new details of what happens inside a red blood cell affected by sickle cell disease (SCD), according to research published in Biophysical Journal.
In patients with SCD, mutated hemoglobin can polymerize, assembling into long fibers that push against the membranes of red blood cells and force them out of shape.
“The goal of our work is to model both how these sickle hemoglobin fibers form as well as the mechanical properties of those fibers,” said study author Lu Lu, a PhD student at Brown University in Providence, Rhode Island.
“There had been separate models for each of these things individually developed by us, but this brings those together into one comprehensive model.”
The model uses detailed biomechanical data on how sickle hemoglobin molecules behave and bind with each other to simulate the assembly of a polymer fiber.
Prior to this work, the problem had been that, as the fiber grows, so does the amount of data the model must crunch. Modeling an entire polymer fiber at a cellular scale using the details of each molecule was simply too computationally expensive.
“Even the world’s fastest supercomputers wouldn’t be able to handle it,” said study author George Karniadakis, PhD, of Brown University.
“There’s just too much happening and no way to capture it all computationally. That’s what we were able to overcome with this work.”
The researchers’ solution was to apply what they call a mesoscopic adaptive resolution scheme (MARS).
The MARS model calculates the detailed dynamics of each individual hemoglobin molecule only at the end of polymer fibers, where new molecules are being recruited into the fiber.
Once 4 layers of a fiber have been established, the model automatically dials back the resolution at which it represents that section. The model retains the important information about how the fiber behaves mechanically but glosses over the fine details of each constituent molecule.
“By eliminating the fine details where we don’t need them, we develop a model that can simulate this whole process and its effects on a red blood cell,” Dr Karniadakis said.
Using the new MARS simulations, the researchers were able to show how different configurations of growing polymer fibers are able to produce cells with different shapes.
“We are able to produce a polymerization profile for each of the cell types associated with the disease,” Dr Karniadakis said. “Now, the goal is to use these models to look for ways of preventing the disease onset.”
Using these new models, Dr Karniadakis and his colleagues can run simulations that include fetal hemoglobin. Those simulations could be used to confirm the theory that fetal hemoglobin disrupts polymerization, as well as determine how much fetal hemoglobin is necessary.
That could help in establishing better dosage guidelines for hydroxyurea or in developing new and more effective drugs for SCD, according to the researchers.
“The models give us a way to do preliminary testing on new approaches to stopping this disease,” Dr Karniadakis said. “Now that we can simulate the entire polymerization process, we think the models will be much more useful.” ![]()
Computer models have revealed new details of what happens inside a red blood cell affected by sickle cell disease (SCD), according to research published in Biophysical Journal.
In patients with SCD, mutated hemoglobin can polymerize, assembling into long fibers that push against the membranes of red blood cells and force them out of shape.
“The goal of our work is to model both how these sickle hemoglobin fibers form as well as the mechanical properties of those fibers,” said study author Lu Lu, a PhD student at Brown University in Providence, Rhode Island.
“There had been separate models for each of these things individually developed by us, but this brings those together into one comprehensive model.”
The model uses detailed biomechanical data on how sickle hemoglobin molecules behave and bind with each other to simulate the assembly of a polymer fiber.
Prior to this work, the problem had been that, as the fiber grows, so does the amount of data the model must crunch. Modeling an entire polymer fiber at a cellular scale using the details of each molecule was simply too computationally expensive.
“Even the world’s fastest supercomputers wouldn’t be able to handle it,” said study author George Karniadakis, PhD, of Brown University.
“There’s just too much happening and no way to capture it all computationally. That’s what we were able to overcome with this work.”
The researchers’ solution was to apply what they call a mesoscopic adaptive resolution scheme (MARS).
The MARS model calculates the detailed dynamics of each individual hemoglobin molecule only at the end of polymer fibers, where new molecules are being recruited into the fiber.
Once 4 layers of a fiber have been established, the model automatically dials back the resolution at which it represents that section. The model retains the important information about how the fiber behaves mechanically but glosses over the fine details of each constituent molecule.
“By eliminating the fine details where we don’t need them, we develop a model that can simulate this whole process and its effects on a red blood cell,” Dr Karniadakis said.
Using the new MARS simulations, the researchers were able to show how different configurations of growing polymer fibers are able to produce cells with different shapes.
“We are able to produce a polymerization profile for each of the cell types associated with the disease,” Dr Karniadakis said. “Now, the goal is to use these models to look for ways of preventing the disease onset.”
Using these new models, Dr Karniadakis and his colleagues can run simulations that include fetal hemoglobin. Those simulations could be used to confirm the theory that fetal hemoglobin disrupts polymerization, as well as determine how much fetal hemoglobin is necessary.
That could help in establishing better dosage guidelines for hydroxyurea or in developing new and more effective drugs for SCD, according to the researchers.
“The models give us a way to do preliminary testing on new approaches to stopping this disease,” Dr Karniadakis said. “Now that we can simulate the entire polymerization process, we think the models will be much more useful.” ![]()
FDA grants drug breakthrough designation for AML
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to venetoclax (Venclexta®).
The designation is for venetoclax in combination with low-dose cytarabine to treat elderly patients with previously untreated acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy.
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About venetoclax
Venetoclax is a small molecule designed to selectively bind and inhibit the BCL-2 protein. The drug is being developed by AbbVie and Roche.
Last year, the FDA granted venetoclax accelerated approval to treat patients with chronic lymphocytic leukemia who have 17p deletion and have received at least one prior therapy. Continued approval of venetoclax for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA granted venetoclax breakthrough therapy designation for the AML indication based on data from an ongoing phase 1/2 study. Preliminary data from the study were presented at the 22nd European Hematology Association (EHA) Annual Congress.
The presentation included data on 61 elderly patients (older than 65) with previously untreated AML who were ineligible for intensive chemotherapy.
They received venetoclax in combination with low-dose cytarabine (as well as prophylaxis for tumor lysis syndrome). The patients’ median time on treatment was 6 months (range, <1 to 19 months), and 72% of patients discontinued treatment.
The overall response rate was 65%. Twenty-five percent of patients achieved a complete response, 38% had a complete response with incomplete blood count recovery, and 2% had a partial response.
The 30-day death rate was 3%, the 60-day death rate was 15%, and the median overall survival was approximately 12 months.
The most common adverse events of any grade (occurring in at least 30% of patients) were nausea (74%), hypokalemia (46%), diarrhea (46%), fatigue (44%), decreased appetite (41%), constipation (34%), hypomagnesemia (34%), vomiting (31%), thrombocytopenia (44%), febrile neutropenia (38%), and neutropenia (33%).
Grade 3/4 adverse events (occurring in at least 10% of patients) included thrombocytopenia (44%), febrile neutropenia (36%), neutropenia (33%), anemia (28%), hypokalemia (16%), hypophosphatemia (13%), and hypertension (12%). ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to venetoclax (Venclexta®).
The designation is for venetoclax in combination with low-dose cytarabine to treat elderly patients with previously untreated acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy.
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About venetoclax
Venetoclax is a small molecule designed to selectively bind and inhibit the BCL-2 protein. The drug is being developed by AbbVie and Roche.
Last year, the FDA granted venetoclax accelerated approval to treat patients with chronic lymphocytic leukemia who have 17p deletion and have received at least one prior therapy. Continued approval of venetoclax for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA granted venetoclax breakthrough therapy designation for the AML indication based on data from an ongoing phase 1/2 study. Preliminary data from the study were presented at the 22nd European Hematology Association (EHA) Annual Congress.
The presentation included data on 61 elderly patients (older than 65) with previously untreated AML who were ineligible for intensive chemotherapy.
They received venetoclax in combination with low-dose cytarabine (as well as prophylaxis for tumor lysis syndrome). The patients’ median time on treatment was 6 months (range, <1 to 19 months), and 72% of patients discontinued treatment.
The overall response rate was 65%. Twenty-five percent of patients achieved a complete response, 38% had a complete response with incomplete blood count recovery, and 2% had a partial response.
The 30-day death rate was 3%, the 60-day death rate was 15%, and the median overall survival was approximately 12 months.
The most common adverse events of any grade (occurring in at least 30% of patients) were nausea (74%), hypokalemia (46%), diarrhea (46%), fatigue (44%), decreased appetite (41%), constipation (34%), hypomagnesemia (34%), vomiting (31%), thrombocytopenia (44%), febrile neutropenia (38%), and neutropenia (33%).
Grade 3/4 adverse events (occurring in at least 10% of patients) included thrombocytopenia (44%), febrile neutropenia (36%), neutropenia (33%), anemia (28%), hypokalemia (16%), hypophosphatemia (13%), and hypertension (12%). ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to venetoclax (Venclexta®).
The designation is for venetoclax in combination with low-dose cytarabine to treat elderly patients with previously untreated acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy.
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
About venetoclax
Venetoclax is a small molecule designed to selectively bind and inhibit the BCL-2 protein. The drug is being developed by AbbVie and Roche.
Last year, the FDA granted venetoclax accelerated approval to treat patients with chronic lymphocytic leukemia who have 17p deletion and have received at least one prior therapy. Continued approval of venetoclax for this indication may be contingent upon verification of clinical benefit in confirmatory trials.
The FDA granted venetoclax breakthrough therapy designation for the AML indication based on data from an ongoing phase 1/2 study. Preliminary data from the study were presented at the 22nd European Hematology Association (EHA) Annual Congress.
The presentation included data on 61 elderly patients (older than 65) with previously untreated AML who were ineligible for intensive chemotherapy.
They received venetoclax in combination with low-dose cytarabine (as well as prophylaxis for tumor lysis syndrome). The patients’ median time on treatment was 6 months (range, <1 to 19 months), and 72% of patients discontinued treatment.
The overall response rate was 65%. Twenty-five percent of patients achieved a complete response, 38% had a complete response with incomplete blood count recovery, and 2% had a partial response.
The 30-day death rate was 3%, the 60-day death rate was 15%, and the median overall survival was approximately 12 months.
The most common adverse events of any grade (occurring in at least 30% of patients) were nausea (74%), hypokalemia (46%), diarrhea (46%), fatigue (44%), decreased appetite (41%), constipation (34%), hypomagnesemia (34%), vomiting (31%), thrombocytopenia (44%), febrile neutropenia (38%), and neutropenia (33%).
Grade 3/4 adverse events (occurring in at least 10% of patients) included thrombocytopenia (44%), febrile neutropenia (36%), neutropenia (33%), anemia (28%), hypokalemia (16%), hypophosphatemia (13%), and hypertension (12%). ![]()
FDA clears new plasmapheresis system
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300. ![]()