User login
Implementation of a Communication Training Program Is Associated with Reduction of Antipsychotic Medication Use in Nursing Homes
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
Study Overview
Objective. To evaluate the effectiveness of OASIS, a large-scale, statewide communication training program, on the reduction of antipsychotic use in nursing homes (NHs).
Design. Quasi-experimental longitudinal study with external controls.
Setting and participants. The participants were residents living in NHs between 1 March 2011 and 31 August 2013. The intervention group consisted of NHs in Massachusetts that were enrolled in the OASIS intervention and the control group consisted of NHs in Massachusetts and New York. The Centers for Medicare & Medicaid Services Minimum Data Set (MDS) 3.0 data was analyzed to determine medication use and behavior of residents of NHs. Residents of these NHs were excluded if they had a US Food and Drug Administration (FDA)-approved indication for antipsychotic use (eg, schizophrenia); were short-term residents (length of stay < 90 days); or had missing data on psychopharmacological medication use or behavior.
Intervention. The OASIS is an educational program that targeted both direct care and non-direct care staff in NHs to assist them in meeting the needs and challenges of caring for long-term care residents. Utilizing a train-the-trainer model, OASIS program coordinators and champions from each intervention NH participated in an 8-hour in-person training session that focused on enhancing communication skills between NH staff and residents with cognitive impairment. These trainers subsequently instructed the OASIS program to staff at their respective NHs using a team-based care approach. Addi-tional support of the OASIS educational program, such as telephone support, 12 webinars, 2 regional seminars, and 2 booster sessions, were provided to participating NHs.
Main outcome measures. The main outcome measure was facility-level prevalence of antipsychotic use in long-term NH residents captured by MDS in the 7 days preceding the MDS assessment. The secondary outcome measures were facility-level quarterly prevalence of psychotropic medications that may have been substituted for antipsychotic medications (ie, anxiolytics, antidepressants, and hypnotics) and behavioral disturbances (ie, physically abusive behavior, verbally abusive behavior, and rejecting care). All secondary outcomes were dichotomized in the 7 days preceding the MDS assessment and aggregated at the facility level for each quarter.
The analysis utilized an interrupted time series model of facility-level prevalence of antipsychotic medication use, other psychotropic medication use, and behavioral disturbances to evaluate the OASIS intervention’s effectiveness in participating facilities compared with control NHs. This methodology allowed the assessment of changes in the trend of antipsychotic use after the OASIS intervention controlling for historical trends. Data from the 18-month pre-intervention (baseline) period was compared with that of a 3-month training phase, a 6-month implementation phase, and a 3-month maintenance phase.
Main results. 93 NHs received OASIS intervention (27 with high prevalence of antipsychotic use) while 831 NHs did not (non-intervention control). The intervention NHs had a higher prevalence of antipsychotic use before OASIS training (baseline period) than the control NHs (34.1% vs. 22.7%, P < 0.001). The intervention NHs compared to controls were smaller in size (122 beds [interquartile range {IQR}, 88–152 beds] vs. 140 beds; [IQR, 104–200 beds]; P < 0.001), more likely to be for profit (77.4% vs. 62.0%, P = 0.009), had corporate ownership (93.5% vs. 74.6%, P < 0.001), and provided resident-only councils (78.5% vs. 52.9%, P < 0.001). The intervention NHs had higher registered nurse (RN) staffing hours per resident (0.8 vs. 0.7; P = 0.01) but lower certified nursing assistant (CNA) hours per resident (2.3 vs. 2.4; P = 0.04) than control NHs. There was no difference in licensed practical nurse hours per resident between groups.
All 93 intervention NHs completed the 8-hour in-person training session and attended an average of 6.5 (range, 0–12) subsequent support webinars. Thirteen NHs (14.0%) attended no regional seminars, 32 (34.4%) attended one, and 48 (51.6%) attended both. Four NHs (4.3%) attended one booster session, and 13 (14.0%) attended both. The NH staff most often trained in the OASIS training program were the directors of nursing, RNs, CNAs, and activities personnel. Support staff including housekeeping and dietary were trained in about half of the reporting intervention NHs, while physicians and nurse practitioners participated infrequently. Concurrent training programs in dementia care (Hand-in-Hand, Alzheimer Association training, MassPRO dementia care training) were implemented in 67.2% of intervention NHs.
In the intervention NHs, the prevalence of antipsych-otic prescribing decreased from 34.1% at baseline to 26.5% at the study end (7.6% absolute reduction, 22.3% relative reduction). In comparison, the prevalence of antipsychotic prescribing in control NHs decreased from 22.7% to 18.8% over the same period (3.9% absolute reduction, 17.2% relative reduction). During the OASIS implementation phase, the intervention NHs had a reduc-tion in prevalence of antipsychotic use (–1.20% [95% confidence interval {CI}, –1.85% to –0.09% per quarter]) greater than that of the control NHs (–0.23% [95% CI, –0.47% to 0.01% per quarter]), resulting in a net OASIS influence of –0.97% (95% CI, –1.85% to –0.09% per quarter; P = 0.03). The antipsychotic use reduction observed in the implementation phase was not sustained in the maintenance phase (difference of 0.93%; 95% CI, –0.66% to 2.54%; P = 0.48). No increases in other psychotropic medication use (anxiolytics, antidepressants, hypnotics) or behavioral disturbances (physically abusive behavior, verbally abusive behavior, and rejecting care) were observed during the OASIS training and implementation phases.
Conclusion. The OASIS communication training program reduced the prevalence of antipsychotic use in NHs during its implementation phase, but its effect was not sustained in the subsequent maintenance phase. The use of other psychotropic medications and behavior disturbances did not increase during the implementation of OASIS program. The findings from this study provided further support for utilizing nonpharmacologic programs to treat behavioral and psychological symptoms of dementia in older adults who reside in NHs.
Commentary
The use of both conventional and atypical antipsychotic medications is associated with a dose-related, approximately 2-fold increased risk of sudden cardiac death in older adults [1,2]. In 2006, the FDA issued a public health advisory stating that both conventional and atypical anti-psychotic medications are associated with an increased risk of mortality in elderly patients treated for dementia-related psychosis. Despite this black box warning and growing recognition that antipsychotic medications are not indicated for the treatment of dementia-related psychosis, the off-label use of antipsychotic medications to treat behavioral and psychological symptoms of dementia in older adults remains a common practice in nursing homes [3]. Thus, there is an urgent need to assess and develop effective interventions that reduce the practice of antipsychotic medication prescribing in long-term care. To that effect, the study reported by Tjia et al appropriately investigated the impact of the OASIS communication training program, a nonpharmacologic intervention, on the reduction of antipsychotic use in NHs.
This study was well designed and had a number of strengths. It utilized an interrupted time series model, one of the strongest quasi-experimental approaches due to its robustness to threats of internal validity, for evaluating longitudinal effects of an intervention intended to improve the quality of medication use. Moreover, this study included a large sample size and comparison facilities from the same geographical areas (NHs in Massachusetts and New York State) that served as external controls. Several potential weaknesses of the study were identified. Because facility-level aggregate data from NHs were used for analysis, individual level (long-term care resident) characteristics were not accounted for in the analysis. In addition, while the post-OASIS intervention questionnaire response rate was 65.6% (61 of 93 intervention NHs), a higher response rate would provide better characterization of NH staff that participated in OASIS program training, program completion rate, and a more complete representation of competing dementia care training programs concurrently implemented in these NHs.
Several studies, most utilizing various provider education methods, had explored whether these interventions could curb antipsychotic use in NHs with limited success. The largest successful intervention was reported by Meador et al [4], where a focused provider education program facilitated a relative reduction in antipsychotic medication use of 23% compared to control NHs. However, the implementation of this specific program was time- and resource-intensive, requiring geropsychiatry evaluation to all physicians (45 to 60 min), nurse-educator in-service programs for NH staff (5 to 6 one-hr sessions), management specialist consultation to NH administrators (4 hr), and evening meeting for the families of NH residents. The current study by Tjia et al, the largest study to date conducted in the context of competing dementia care training programs and increased awareness of the danger of antipsychotic use in the elderly, similarly showed a meaningful reduction in antipsychotic medication use in NHs that received the OASIS communication training program. The OASIS program appears to be less resource-intensive than the provider education program modeled by Meador et al, and its train-the-trainer model is likely more adaptable to meet the limitations (eg, low staffing and staff turnover) inherent in NHs. The beneficial effect of the OASIS program on reduction of antipsychotic medication prescribing was observed despite low participation by prescribers (11.5% of physicians and 11.5% of nurse practitioners). Although it is unclear why this was observed, this finding is intriguing in that a communication training program that reframes challenging behavior of NH residents with cognitive impairment as (1) communication of unmet needs, (2) train staff to anticipate resident needs, and (3) integrate resident strengths into daily care plans can alter provider prescription behavior. The implication of this is that provider practice in managing behavioral and psychological symptoms of dementia can be improved by optimizing communication training in NH staff. Taken together, this study adds to evidence in favor of utilizing nonpharmacologic interventions to reduce antipsychotic use in long-term care.
Applications for Clinical Practice
OASIS, a communication training program for NH staff, reduces antipsychotic medication use in NHs during its implementation phase. Future studies need to investigate pragmatic methods to sustain the beneficial effect of OASIS after its implementation phase.
—Fred Ko, MD, MS, Icahn School of Medicine at Mount Sinai, New York, NY
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
1. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–35.
2. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–41.
3. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010;170:89–95.
4. Meador KG, Taylor JA, Thapa PB, et al. Predictors of anti-
psychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc 1997;45:207–10.
Nocturnal Seizures Linked to Severe Hypoxemia
Patients who have seizures while asleep are more likely to experience severe and longer episodes of hypoxemia compared with seizures while awake according to an examination of 48 recorded seizures from 20 adults with epilepsy. The analysis also suggested that an increased risk of sudden death may be caused by hypoxemia from nocturnal seizures.
Latreille V, Abdennadher M, Dworetzky BA et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression [published online ahead of print July 17, 2017]. Epilepsia. 2017;doi: 10.1111/epi.13841.
Patients who have seizures while asleep are more likely to experience severe and longer episodes of hypoxemia compared with seizures while awake according to an examination of 48 recorded seizures from 20 adults with epilepsy. The analysis also suggested that an increased risk of sudden death may be caused by hypoxemia from nocturnal seizures.
Latreille V, Abdennadher M, Dworetzky BA et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression [published online ahead of print July 17, 2017]. Epilepsia. 2017;doi: 10.1111/epi.13841.
Patients who have seizures while asleep are more likely to experience severe and longer episodes of hypoxemia compared with seizures while awake according to an examination of 48 recorded seizures from 20 adults with epilepsy. The analysis also suggested that an increased risk of sudden death may be caused by hypoxemia from nocturnal seizures.
Latreille V, Abdennadher M, Dworetzky BA et al. Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression [published online ahead of print July 17, 2017]. Epilepsia. 2017;doi: 10.1111/epi.13841.
Finding a Noninvasive Approach to Epilepsy Diagnosis
A combination of metabolites and genomic indicators hold promise as a noninvasive biomarker for epilepsy suggests a recent study published in Epilepsia. Wu et al found that a metabolomic-genomic signature that included reduced lactate and increased creatine plus phosphocreatine and choline was able to predict the existence of an altered metabolic state in epileptic brain regions, suggesting it may eventually be an alternative to surgically invasive approaches to diagnosis.
Wu HC, Dachet F, Ghoddoussi F, et al. Altered metabolomic-genomic signature: a potential noninvasive biomarker of epilepsy [published online ahead of print July 17, 2017]. Epilepsia. 2017; doi: 10.1111/epi.13848.
A combination of metabolites and genomic indicators hold promise as a noninvasive biomarker for epilepsy suggests a recent study published in Epilepsia. Wu et al found that a metabolomic-genomic signature that included reduced lactate and increased creatine plus phosphocreatine and choline was able to predict the existence of an altered metabolic state in epileptic brain regions, suggesting it may eventually be an alternative to surgically invasive approaches to diagnosis.
Wu HC, Dachet F, Ghoddoussi F, et al. Altered metabolomic-genomic signature: a potential noninvasive biomarker of epilepsy [published online ahead of print July 17, 2017]. Epilepsia. 2017; doi: 10.1111/epi.13848.
A combination of metabolites and genomic indicators hold promise as a noninvasive biomarker for epilepsy suggests a recent study published in Epilepsia. Wu et al found that a metabolomic-genomic signature that included reduced lactate and increased creatine plus phosphocreatine and choline was able to predict the existence of an altered metabolic state in epileptic brain regions, suggesting it may eventually be an alternative to surgically invasive approaches to diagnosis.
Wu HC, Dachet F, Ghoddoussi F, et al. Altered metabolomic-genomic signature: a potential noninvasive biomarker of epilepsy [published online ahead of print July 17, 2017]. Epilepsia. 2017; doi: 10.1111/epi.13848.
Deep Brain Stimulation Offers Benefit for Intractable Epilepsy
Intracranial deep brain stimulation (DBS) may offer some benefit to patients with epilepsy who do not respond to more conservative therapy, but most of the evidence comes from short-term randomized controlled trials, according to a recent review from the Cochrane Library. Experts concluded that anterior thalamic DBS, responsive ictal onset zone stimulation, and hippocampal DBS can reduce the frequency of seizures in patients with refractory epilepsy.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017 Jul 18;7:CD008497.
Intracranial deep brain stimulation (DBS) may offer some benefit to patients with epilepsy who do not respond to more conservative therapy, but most of the evidence comes from short-term randomized controlled trials, according to a recent review from the Cochrane Library. Experts concluded that anterior thalamic DBS, responsive ictal onset zone stimulation, and hippocampal DBS can reduce the frequency of seizures in patients with refractory epilepsy.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017 Jul 18;7:CD008497.
Intracranial deep brain stimulation (DBS) may offer some benefit to patients with epilepsy who do not respond to more conservative therapy, but most of the evidence comes from short-term randomized controlled trials, according to a recent review from the Cochrane Library. Experts concluded that anterior thalamic DBS, responsive ictal onset zone stimulation, and hippocampal DBS can reduce the frequency of seizures in patients with refractory epilepsy.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev. 2017 Jul 18;7:CD008497.
Managing psychiatric illness during pregnancy and breastfeeding: Tools for decision making
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.
Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
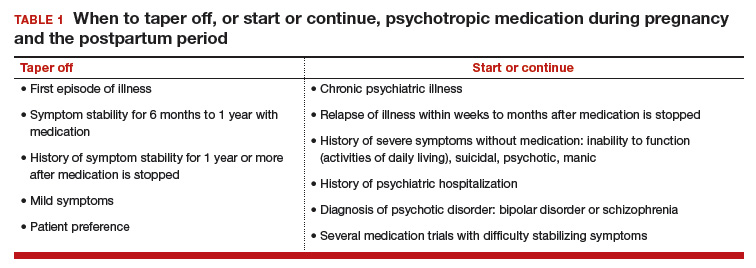
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).
In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
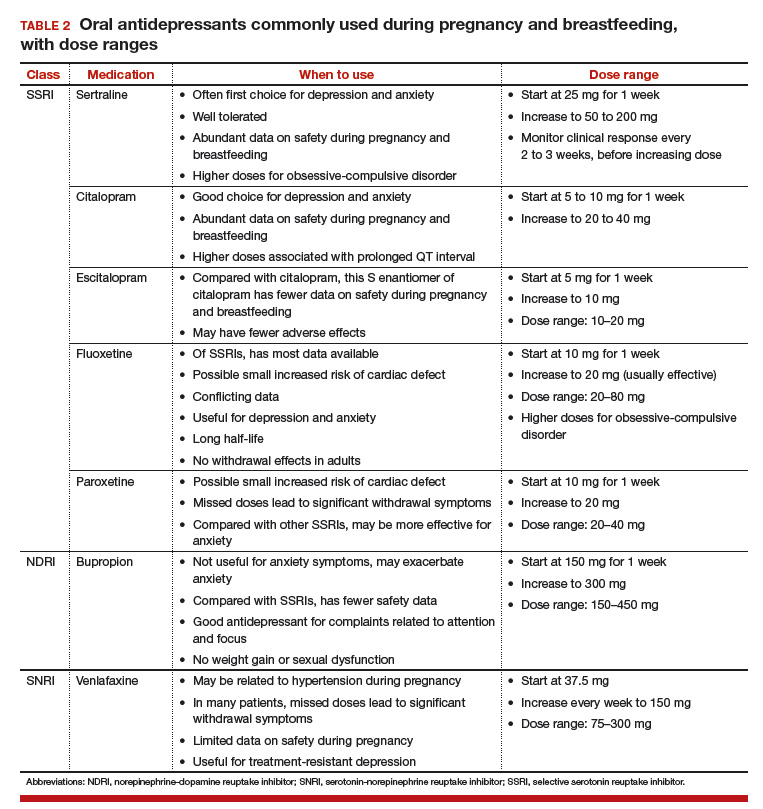
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29
One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.

Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).

In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29

One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Increasingly, women with psychiatric illness are undergoing pharmacologic treatment during pregnancy. In the United States, an estimated 8% of pregnant women are prescribed antidepressants, and the number of such cases has risen over the past 15 years.1 Women with a psychiatric diagnosis were once instructed either to discontinue all medication immediately on learning they were pregnant, or to forgo motherhood because their illness might have a negative effect on a child or because avoiding medication during pregnancy might lead to a relapse.
Fortunately, women with depression, anxiety, bipolar disorder, or schizophrenia no longer are being told that they cannot become mothers. For many women, however, stopping medication is not an option. Furthermore, psychiatric illness sometimes is diagnosed initially during pregnancy and requires treatment.

Pregnant women and their physicians need accurate information about when to taper off medication, when to start or continue, and which medications are safest. Even for clinicians with a solid knowledge base, counseling a woman who needs or may need psychotropic medication during pregnancy and breastfeeding is a daunting task. Some clinicians still recommend no drug treatment as the safest and best option, given the potential risks to the fetus.
In this review we offer a methodologic approach for decision making about pharmacologic treatment during pregnancy. As the scientific literature is constantly being updated, it is imperative to have the most current information on psychotropics and to know how to individualize that information when counseling a pregnant woman and her family. Using this framework for analyzing the risks and benefits for both mother and fetus, clinicians can avoid the unanswerable question of which medication is the “safest.”
A patient’s mental health care provider is a useful resource for information about a woman’s mental health history and current stability, but he or she may not be expert or comfortable in recommending treatment for a pregnant patient. During pregnancy, a woman’s obstetrician often becomes the “expert” for all treatment decisions.
Antidepressants. Previous studies may have overestimated the association between prenatal use of antidepressants and attention deficit/hyperactivity disorder (ADHD) in children because they did not control for shared family factors, according to investigators who say that their recent study findings raise the possibility that "confounding by indication" might partially explain the observed association.1
In a population-based cohort study in Hong Kong, Man and colleagues analyzed the records of 190,618 maternal-child pairs.1 A total of 1,252 children were exposed to maternal antidepressant use during pregnancy. Medications included selective serotonin reuptake inhibitors (SSRIs), non-SSRIs, and antipsychotics as monotherapy or in various combination regimens. Overall, 5,659 of the cohort children (3%) were diagnosed with or received treatment for ADHD.
When gestational medication users were compared with nongestational users, the crude hazard ratio (HR) of antidepressant use during pregnancy and ADHD was 2.26 (P<.01). After adjusting for potential confounding factors (such as maternal psychiatric disorders and use of other psychotropic drugs), this reduced to 1.39 (95% confidence interval [CI], 1.07-1.82; P = .01). Children of mothers with psychiatric disorders had a higher risk of ADHD than did children of mothers without psychiatric disorders (HR, 1.84; 95% CI, 1.54-2.18; P<.01), even if the mothers had never used antidepressants.
While acknowledging the potential for type 2 error in the study analysis, the investigators proposed that the results "further strengthen our hypothesis that confounding by indication may play a major role in the observed positive association between gestational use of antidepressants and ADHD in offspring."
Lithium. Similarly, investigators of another recently published study found that the magnitude of the association between prenatal lithium use and increased risk of cardiac malformations in infants was smaller than previously shown.2 This finding may be important clinically because lithium is a first-line treatment for many US women of reproductive age with bipolar disorder.
Most earlier data were derived from a database registry, case reports, and small studies that often had conflicting results. However, Patorno and colleagues conducted a large retrospective cohort study that involved data on 1,325,563 pregnancies in women enrolled in Medicaid.2 Exposure to lithium was defined as at least 1 filled prescription during the first trimester, and the primary reference group included women with no lithium or lamotrigine (another mood stabilizer not associated with congenital malformations) dispensing during the 3 months before the start of pregnancy or during the first trimester.
A total of 663 pregnancies (0.05%) were exposed to lithium and 1,945 (0.15%) were exposed to lamotrigine during the first trimester. The adjusted risk ratios for cardiac malformations among infants exposed to lithium were 1.65 (95% CI, 1.02-2.68) as compared with nonexposed infants and 2.25 (95% CI, 1.17-4.34) as compared with lamotrigine-exposed infants. Notably, all right ventricular outflow tract obstruction defects identified in the infants exposed to lithium occurred with a daily dose of more than 600 mg.
Although the study results suggest an increased risk of cardiac malformations--of approximately 1 additional case per 100 live births--associated with lithium use in early pregnancy, the magnitude of risk is much lower than originally proposed based on early lithium registry data.
-- Kathy Christie, Senior Editor
References
- Man KC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350.
- Patorno E, Huybrechts KR, Bateman BT, et al. Lithium use in pregnancy and risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
Analyze risks and benefits of medication versus no medication
The US Food and Drug Administration (FDA) has not approved any psychotropic medication for use during pregnancy. While a clinical study would provide more scientifically rigorous safety data, conducting a double-blinded, placebo-controlled trial in pregnant women with a psychiatric disorder is unethical. Thus, the literature consists mostly of reports on case series, retrospective chart reviews, prospective naturalistic studies, and analyses of large registry databases. Each has benefits and limitations. It is important to understand the limitations when making treatment decisions.
In 1979, the FDA developed a 5-lettersystem (A, B, C, D, X) for classifying the relative safety of medications used during pregnancy.2 Many clinicians and pregnant women relied on this system to decide which medications were safe. Unfortunately, the information in the system was inadequate for making informed decisions. For example, although a class B medication might have appeared safer than one in class C, the studies of risk in humans might not have been adequate to permit comparisons. Drug safety classifications were seldom changed, despite the availability of additional data.
In June 2015, the FDA changed the requirements for the Pregnancy and Lactation subsections of the labeling for human prescription drugs and biologic products. Drug manufacturers must now include in each subsection a risk summary, clinical considerations supporting patient care decisions and counseling, and detailed data. These subsections provide information on available human and animal studies, known or potential maternal or fetal adverse reactions, and dose adjustments needed during pregnancy and the postpartum period. In addition, the FDA added a subsection: Females and Males of Reproductive Potential.3
These changes acknowledge there is no list of “safe” medications. The safest medication generally is the one that works for a particular patient at the lowest effective dose. As each woman’s history of illness and effective treatment is different, the best medication may differ as well, even among women with the same illness. Therefore, medication should be individualized to the patient. A risk–benefit analysis comparing psychotropic medication treatment with no medication treatment must be performed for each patient according to her personal history and the best available data.
Read about the risks of untreated illness during pregnancy
What is the risk of untreated illness during pregnancy?
During pregnancy, women are treated for many medical disorders, including psychiatric illness. One general guideline is that, if a pregnant woman does not need a medication—whether it be for an allergy, hypertension, or another disorder—she should not take it. Conversely, if a medication is required for a patient’s well-being, her physician should continue it or switch to a safer one. This general guideline is the same for women with depression, anxiety, or a psychotic disorder.
Managing hypertension during pregnancy is an example of choosing treatment when the risk of the illness to the mother and the infant outweighs the likely small risk associated with taking a medication. Blood pressure is monitored, and, when it reaches a threshold, an antihypertensive is started promptly to avoid morbidity and mortality.
Psychiatric illness carries risks for both mother and fetus as well, but no data show a clear threshold for initiating pharmacologic treatment. Therefore, in prescribing medication the most important steps are to take a complete history and perform a thorough evaluation. Important information includes the number and severity of previous episodes, prior history of hospitalization or suicidal thoughts or attempts, and any history of psychotic or manic status.
Whether to continue or discontinue medication is often decided after inquiring about other times a medication was discontinued. A patient who in the past stayed well for several years after stopping a medication may be able to taper off a medication and conceive during a window of wellness. Some women who have experienced only one episode of illness and have been stable for at least a year may be able to taper off a medication before conceiving (TABLE 1).

In the risk–benefit analysis, assess the need for pharmacologic treatment by considering the risk that untreated illness poses for both mother and fetus, the benefits of treatment for both, and the risk of medication exposure for the fetus.4
Mother: Risk of untreated illness versus benefit of treatment
A complete history and a current symptom evaluation are needed to assess the risk that nonpharmacologic treatment poses for the mother. Women with functional impairment, including inability to work, to perform activities of daily living, or to take care of other children, likely require treatment. Studies have found that women who discontinue treatment for a psychiatric illness around the time of conception are likely to experience a recurrence of illness during pregnancy, often in the first trimester, and must restart medication.5,6 For some diagnoses, particularly bipolar disorder, symptoms during a relapse can be more severe and more difficult to treat, and they carry a risk for both mother and fetus.7 A longitudinal study of pregnant women who stopped medication for bipolar disorder found a 71% rate of relapse.7 In cases in which there is a history of hospitalization, suicide attempt, or psychosis, discontinuing treatment is not an option; instead, the physician must determine which medication is safest for the particular patient.
Related article:
Does PTSD during pregnancy increase the likelihood of preterm birth?
Fetus: Risk of untreated illness versus benefit of treatment
Mothers with untreated psychiatric illness are at higher risk for poor prenatal care, substance abuse, and inadequate nutrition, all of which increase the risk of negative obstetric and neonatal outcomes.8 Evidence indicates that untreated maternal depression increases the risk of preterm delivery and low birth weight.9 Children born to mothers with depression have more behavioral problems, more psychiatric illness, more visits to pediatricians, lower IQ scores, and attachment issues.10 Some of the long-term negative effects of intrauterine stress, which include hypertension, coronary heart disease, and autoimmune disorders, persist into adulthood.11
Fetus: Risk of medication exposure
With any pharmacologic treatment, the timing of fetal exposure affects resultant risks and therefore must be considered in the management plan.
Before conception. Is there any effect on ovulation or fertilization?
Implantation. Does the exposure impair the blastocyst’s ability to implant in the uterine lining?
First trimester. This is the period of organogenesis. Regardless of drug exposure, there is a 2% to 4% baseline risk of a major malformation during any pregnancy. The risk of a particular malformation must be weighed against this baseline risk.
According to limited data, selective serotonin reuptake inhibitors (SSRIs) may increase the risk of early miscarriage.12 SSRIs also have been implicated in increasing the risk of cardiovascular malformations, although the data are conflicting.13,14
Antiepileptics such as valproate and carbamazepine are used as mood stabilizers in the treatment of bipolar disorder.15 Extensive data have shown an association with teratogenicity. Pregnant women who require either of these medications also should be prescribed folic acid 4 or 5 mg/day. Given the high risk of birth defects and cognitive delay, valproate no longer is recommended for women of reproductive potential.16
Lithium, one of the safest medications used in the treatment of bipolar disorder, is associated with a very small risk of Ebstein anomaly.17
Lamotrigine is used to treat bipolar depression and appears to have a good safety profile, along with a possible small increased risk of oral clefts.18,19
Atypical antipsychotics (such as aripiprazole, olanzapine, quetiapine, and risperidone) are often used first-line in the treatment of psychotic disorders and bipolar disorder in women who are not pregnant. Although the safety data on use of these drugs during pregnancy are limited, a recent analysis of pregnant Medicaid enrollees found no increased risk of birth defects after controlling for potential confounding factors.20 Common practice is to avoid these newer agents, given their limited data and the time needed for rare malformations to emerge (adequate numbers require many exposures during pregnancy).
Read additional fetal risks of medication exposure
Second trimester. This is a period of growth and neural development. A 2006 study suggested that SSRI exposure after pregnancy week 20 increases the risk of persistent pulmonary hypertension of the newborn (PPHN).21 In 2011, however, the FDA removed the PPHN warning label for SSRIs, citing inconsistent data. Whether the PPHN risk is increased with SSRI use is unclear, but the risk is presumed to be smaller than previously suggested.22 Stopping SSRIs before week 20 puts the mother at risk for relapse during pregnancy and increases her risk of developing postpartum depression. If we follow the recommendation to prescribe medication only for women who need it most, then stopping the medication at any time during pregnancy is not an option.
Third trimester. This is a period of continued growth and lung maturation.
Delivery. Is there a potential for impairment in parturition?
Neonatal adaptation. Newborns are active mainly in adapting to extrauterine life: They regulate their temperature and muscle tone and learn to coordinate sucking, swallowing, and breathing. Does medication exposure impair adaptation, or are signs or symptoms of withdrawal or toxicity present? The evidence that in utero SSRI exposure increases the risk of neonatal adaptation syndrome is consistent, but symptoms are mild and self-limited.23 Tapering off SSRIs before delivery currently is not recommended, as doing so increases the mother’s risk for postpartum depression and, according to one study, does not prevent symptoms of neonatal adaptation syndrome from developing.24
Behavioral teratogenicity. What are the long-term developmental outcomes for the child? Are there any differences in IQ, speech and language, or psychiatric illness? One study found an increased risk of autism with in utero exposure to sertraline, but the study had many methodologic flaws and its findings have not been replicated.25 Most studies have not found consistent differences in speech, IQ, or behavior between infants exposed and infants not exposed to antidepressants.26,27 By contrast, in utero exposure to anticonvulsants, particularly valproate, has led to significant developmental problems in children.28 The data on atypical antipsychotics are limited.
Related article:
Do antidepressants really cause autism?
None of the medications used to treat depression, bipolar disorder, anxiety, or schizophrenia is considered first-line or safest therapy for the pregnant woman. For any woman who is doing well on a certain medication, but particularly for a pregnant woman, there is no compelling, data-supported reason to switch to another agent. For depression, options include all of the SSRIs, with the possible exception of paroxetine (TABLE 2). In conflicting studies, paroxetine was no different from any other SSRI in not being associated with cardiovascular defects.29

One goal in treatment is to use a medication that previously was effective in the remission of symptoms and to use it at the lowest dose possible. Treating simply to maintain a low dose of drug, however, and not to effect symptom remission, exposes the fetus to both the drug and the illness. Again, the lowest effective dose is the best choice.
Read about treatment during breastfeeding
Treatment during breastfeeding
Women are encouraged to breastfeed for physical and psychological health benefits, for both themselves and their babies. Many medications are compatible with breastfeeding.30 The amount of drug an infant receives through breast milk is considerably less than the amount received during the mother’s pregnancy. Breastfeeding generally is allowed if the calculated infant dose is less than 10% of the weight-adjusted maternal dose.31
The amount of drug transferred from maternal plasma into milk is highest for drugs with low protein binding and high lipid solubility.32 Drug clearance in infants must be considered as well. Renal clearance is decreased in newborns and does not reach adult levels until 5 or 6 months of age. In addition, liver metabolism is impaired in neonates and even more so in premature infants.33 Drugs that require extensive first-pass metabolism may have higher bioavailability, and this factor should be considered.
Some clinicians recommend pumping and discarding breast milk when the drug in it is at its peak level; although the drug is not eliminated, the infant ingests less of it.34 Most women who are anxious about breastfeeding while on medication “pump and dump” until they are more comfortable nursing and the infants are doing well. Except in cases of mother preference, most physicians with expertise in reproductive mental health generally recommend against pumping and discarding milk.
Through breast milk, infants ingest drugs in varying amounts. The amount depends on the qualities of the medication, the timing and duration of breastfeeding, and the characteristics of the infant. Few psychotropic drugs have significant effects on breastfed infants. Even lithium, previously contraindicated, is successfully used, with infant monitoring, during breastfeeding.35 Given breastfeeding’s benefits for both mother and child, many more women on psychotropic medications are choosing to breastfeed.
Related article:
USPSTF Recommendations to Support Breastfeeding
Balance the pros and cons
Deciding to use medication during pregnancy and breastfeeding involves considering the risk of untreated illness versus the benefit of treatment for both mother and fetus, and the risk of medication exposure for the fetus. Mother and fetus are inseparable, and neither can be isolated from the other in treatment decisions. Avoiding psychotropic medication during pregnancy is not always the safest option for mother or fetus. The patient and her clinician and support system must make an informed decision that is based on the best available data and that takes into account the mother’s history of illness and effective treatment. Many women with psychiatric illness no longer have to choose between mental health and starting a family, and their babies will be healthy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–e5.
- Hecht A. Drug safety labeling for doctors. FDA Consum. 1979;13(8):12–13.
- Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: retirement of risk categories. Pharmacotherapy. 2014;34(4):389–395.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507.
- O’Brien L, Laporte A, Koren G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can J Psychiatry. 2009;54(6):399–408.
- Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824.
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735.
- Straub H, Adams M, Kim JJ, Silver RK. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 2012;207(4):329.e1–e4.
- Hayes LJ, Goodman SH, Carlson E. Maternal antenatal depression and infant disorganized attachment at 12 months. Attach Hum Dev. 2013;15(2):133–153.
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14.
- Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion—a population-based study. PLoS One. 2013;8(8):e72095.
- Nordeng H, van Gelder MM, Spigset O, Koren G, Einarson A, Eberhard-Gran M. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186–194.
- Källén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301–308.
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803–813.
- Balon R, Riba M. Should women of childbearing potential be prescribed valproate? A call to action. J Clin Psychiatry. 2016;77(4):525–526.
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Design. 2006;12(12):1531–1541.
- Nguyen HT, Sharma V, McIntyre RS. Teratogenesis associated with antibipolar agents. Adv Ther. 2009;26(3):281–294.
- Campbell E, Kennedy F, Irwin B, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy. J Neurol Neurosurg Psychiatry. 2013;84(11):e2.
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry. 2016;73(9):938–946.
- Chambers CD, Hernández-Díaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587.
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34(3):293–297.
- Oberlander TF, Misri S, Fitzgerald CE, Kostaras X, Rurak D, Riggs W. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry. 2004;65(2):230–237.
- Warburton W, Hertzman C, Oberlander TF. A register study of the impact of stopping third trimester selective serotonin reuptake inhibitor exposure on neonatal health. Acta Psychiatr Scand. 2010;121(6):471–479.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68(11):1104–1112.
- Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol. 2013;30(4):297–301.
- Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr. 2013;102(11):1054–1059.
- Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–643.
- Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749–752.
- Davanzo R, Copertino M, De Cunto A, Minen F, Amaddeo A. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6(2):89–98.
- Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–126.
- Suri RA, Altshuler LL, Burt VK, Hendrick VC. Managing psychiatric medications in the breast-feeding woman. Medscape Womens Health. 1998;3(1):1.
- Milsap RL, Jusko WJ. Pharmacokinetics in the infant. Environ Health Perspect. 1994;102(suppl 11):107–110.
- Newport DJ, Hostetter A, Arnold A, Stowe ZN. The treatment of postpartum depression: minimizing infant exposures. J Clin Psychiatry. 2002;63(suppl 7):31–44.
- Viguera AC, Newport DJ, Ritchie J, et al. Lithium in breast milk and nursing infants: clinical implications. Am J Psychiatry. 2007;164(2):342–345.
Association between concurrent use of prescription opiates and benzos
Title: Short periods of concurrent benzodiazepine and opioid use increase overdose risk
Clinical Question: What is the impact of concurrent benzodiazepine use in chronic versus intermittent opioid use on risk for opioid overdose?
Study Design: Retrospective study.
Setting: Private insurance administrative claims in the United States.
Synopsis: In 315,428 privately insured adults younger than 65 without malignancy who filled at least one opioid prescription between 2001 and 2013, concurrent benzodiazepine use doubled (increasing from 9% to 17%) and was associated with an increased risk for hospitalization for opioid overdose (1.16% versus 2.42%, odds ratio = 2.14, P less than .001). The risk was increased in both chronic opioid users versus nonusers (5.36% versus 3.13%, odds ratio = 1.8, CI, 1.67-1.96, P less than .001) and in intermittent opioid users as compared to nonusers (1.45% versus 1.02%, odds ratio = 1.42, CI, 1.33-1.51, P less than .001).
These results are similar to prior studies performed in other patient populations, but add to those by including short periods of co-prescription between opioid and benzodiazepine prescriptions (including a single day of overlap). Limitations of this study include that it included only patients who were seen in the ED or hospital.
Bottom Line: There may be no safe duration of opioid use in patients who are also taking benzodiazepines.
Citation: Sun EC, Dixit, A, Humphreys K, et. al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356(760):1-7.
Dr. Barrett is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: Short periods of concurrent benzodiazepine and opioid use increase overdose risk
Clinical Question: What is the impact of concurrent benzodiazepine use in chronic versus intermittent opioid use on risk for opioid overdose?
Study Design: Retrospective study.
Setting: Private insurance administrative claims in the United States.
Synopsis: In 315,428 privately insured adults younger than 65 without malignancy who filled at least one opioid prescription between 2001 and 2013, concurrent benzodiazepine use doubled (increasing from 9% to 17%) and was associated with an increased risk for hospitalization for opioid overdose (1.16% versus 2.42%, odds ratio = 2.14, P less than .001). The risk was increased in both chronic opioid users versus nonusers (5.36% versus 3.13%, odds ratio = 1.8, CI, 1.67-1.96, P less than .001) and in intermittent opioid users as compared to nonusers (1.45% versus 1.02%, odds ratio = 1.42, CI, 1.33-1.51, P less than .001).
These results are similar to prior studies performed in other patient populations, but add to those by including short periods of co-prescription between opioid and benzodiazepine prescriptions (including a single day of overlap). Limitations of this study include that it included only patients who were seen in the ED or hospital.
Bottom Line: There may be no safe duration of opioid use in patients who are also taking benzodiazepines.
Citation: Sun EC, Dixit, A, Humphreys K, et. al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356(760):1-7.
Dr. Barrett is assistant professor in the division of hospital medicine at the University of New Mexico.
Title: Short periods of concurrent benzodiazepine and opioid use increase overdose risk
Clinical Question: What is the impact of concurrent benzodiazepine use in chronic versus intermittent opioid use on risk for opioid overdose?
Study Design: Retrospective study.
Setting: Private insurance administrative claims in the United States.
Synopsis: In 315,428 privately insured adults younger than 65 without malignancy who filled at least one opioid prescription between 2001 and 2013, concurrent benzodiazepine use doubled (increasing from 9% to 17%) and was associated with an increased risk for hospitalization for opioid overdose (1.16% versus 2.42%, odds ratio = 2.14, P less than .001). The risk was increased in both chronic opioid users versus nonusers (5.36% versus 3.13%, odds ratio = 1.8, CI, 1.67-1.96, P less than .001) and in intermittent opioid users as compared to nonusers (1.45% versus 1.02%, odds ratio = 1.42, CI, 1.33-1.51, P less than .001).
These results are similar to prior studies performed in other patient populations, but add to those by including short periods of co-prescription between opioid and benzodiazepine prescriptions (including a single day of overlap). Limitations of this study include that it included only patients who were seen in the ED or hospital.
Bottom Line: There may be no safe duration of opioid use in patients who are also taking benzodiazepines.
Citation: Sun EC, Dixit, A, Humphreys K, et. al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356(760):1-7.
Dr. Barrett is assistant professor in the division of hospital medicine at the University of New Mexico.
First trimester antibiotics may increase birth defect risk
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Exposure to clindamycin and doxycycline during the first trimester may be associated with an increased risk of organ-specific malformations such as ventricular/atrial septal defect, new research suggests.
In a population-based cohort study in 139,938 liveborn singletons, there was an 81% increased risk of ventricular/septal defect (adjusted odds ratio, 1.81) associated with clindamycin exposure during the first trimester, compared with no exposure. Similarly, there was a 67% greater risk of musculoskeletal system malformations (aOR, 1.67) associated with clindamycin exposure (Br J Clin Pharmacol. 2017 Jul 19. doi: 10.1111/bcp.13364).
Doxycycline exposure was associated with a greater than threefold increased risk of ventricular/atrial septal defect (aOR, 3.19), and greater than twofold increase in the risk of circulatory system malformation (aOR, 2.38) and cardiac malformations (aOR, 2.46).
The study also found a 46% higher risk of digestive system malformations associated with macrolide exposure (aOR, 1.46), while quinolone exposure was associated with an 89% higher risk of urinary system malformations (aOR, 1.89).
“There is currently a debate on a possible association between macrolide use and infantile pyloric stenosis,” the researchers wrote. “Though evidence suggested that late pregnancy and early infancy were the time windows of interest for this malformation, little attention has been paid to the first trimester of pregnancy.”
Phenoxymethylpenicillin exposure was associated with a 85% increased risk of nervous system malformations (aOR, 1.85), and erythromycin exposure doubled the risk of urinary system malformations (aOR, 2.12). Moxifloxacin exposure was associated with a fivefold increased risk of respiratory system malformations (aOR, 5.48), but the authors noted that there were just two exposed cases.
However, there was no increased risk for major congenital malformation seen with amoxicillin, cephalosporins, and nitrofurantoin.
Overall, 11% of pregnancies in the study recorded exposure to antibiotics during the first trimester, and 9.9% of the study population were diagnosed with a major congenital malformation in the first year of life.
“Though the absolute risks for specific birth defects was small, physicians should consider prescribing safer antibiotics for the treatment of maternal infections when possible until more data are available,” the researchers wrote.
The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
FROM THE BRITISH JOURNAL OF CLINICAL PHARMACOLOGY
Key clinical point:
Major finding: Clindamycin exposure during the first trimester is associated with an 81% increased risk of ventricular/septal defect and 67% greater risk of musculoskeletal system malformations.
Data source: Population-based cohort study in 139,938 liveborn singletons.
Disclosures: The study was supported by the Réseau Québécois. One author reported being a consultant on litigation involving antidepressants and birth defects. No other conflicts of interest were declared.
Reducing Readmissions or Length of Stay—Which Is More Important?
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
Whether robbing banks or reducing healthcare spending, it makes sense to go where the money is. In the case of healthcare, 32% of spending goes to inpatient care, so hospitals represent a logical target for cost-reduction efforts. Because most hospital costs are fixed, there are basically 2 approaches to reducing spending—shorten length of stay or keep patients out of the hospital altogether. The government has tried both, using the power of financial incentives to spur adoption.
Faced with soaring hospital costs in the 1980s, Medicare introduced its prospective payment system, offering hospitals a fixed payment for each specific Diagnosis-Related Group. Hospitals responded by discharging patients sooner, with a resultant rise in admissions to skilled nursing facilities (SNFs) and rapid growth of the home care industry. Length of stay fell dramatically, dropping 9% in 1984 alone.1 It continued to decline through the 1990s, falling by almost 20% between 1993 and 2000. In the following decade, despite the rise of hospital medicine, the rate of decrease slowed to 0.2% per year.2
Attention then turned to readmissions. In 2008, the Medicare Payment Advisory Committee proposed that hospitals with high risk-adjusted readmission rates receive lower payments, arguing that readmissions accounted for $15 billion in Medicare spending and that many were preventable. Thus the Hospital Readmissions Reduction Program was born, introducing readmission penalties in 2012.
Numerous interventions emerged from government and nongovernment parties to reduce readmissions. Many used intensive transitional care programs focusing on early follow-up or medication safety, and some even went as far as providing transitional housing.3 Shortly after passage of the Affordable Care Act, readmission rates fell rapidly. Within a few years, however, the rate of decline slowed dramatically and may have reached a plateau.4 Many have argued that only a small proportion of readmissions are preventable and that there are more direct ways to promote improved discharge planning without diverting resources from other areas.5 It seems that readmissions may not be feasibly reduced much further.
With the advent of accountable care organizations, health systems are now turning their focus to the small population of patients who consume a disproportionate share of healthcare dollars. Because the top 1% of patients—so-called super-utilizers—account for 21% of spending, efforts to reduce their utilization could produce outsized returns.6 Initial anecdotal reports described patients with complex physical, behavior, and social needs receiving fragmented care resulting in myriad expensive admissions. The response comprised teams of social workers and community health workers coupled with robust primary care, formulating individualized solutions. However, data supporting the effectiveness of this common-sense approach are lacking. In addition, our understanding of high-cost patients is evolving. For one thing, being a super-utilizer is often temporary, as just over one-quarter are still in that category a year later.7 Moreover, not all high-cost patients are frequently admitted.8
In this issue of The Journal of Hospital Medicine, Wick et al.9 provide additional insight into high utilizers of hospital services. The authors compare definitions of high utilizers based on cost, number of admissions, or cumulative length of stay over one year. Only 10 percent of high utilizers met all 3 definitions. The overlap between high utilizers by cost and length of stay was twice the overlap between high utilizers by number of admissions and either group. This finding is not surprising because hospitals have high fixed costs, so total cost tends to mirror length of stay.
The study was performed in Canada, and the overlap among these groups may be different in the US. The Canadian patients were hospitalized less frequently than their American counterparts, perhaps reflecting better access to primary care in the Canadian system. Regardless, Wick et al.9 add to the growing literature suggesting that the terms “high utilization” and “high cost” do not always describe the same population. This finding is important because strategies aimed at patients who are frequently admitted may not be effective for those who generate the highest costs. In trying to reduce overall costs, it may be time to revisit length of stay.
Given the long history of prospective payment in the US and the stagnation in length of stay over the past decade, it is reasonable to wonder whether further reductions are possible. In the study by Wick et al.,9 patients with longer lengths of stay were discharged to long-term care facilities. This observation is consistent with others’ reports. Studies of delays in care show that at least 10% of all hospital days can be attributed to delays in discharge, especially to SNFs. In the most recent study, 11% of hospital days were deemed unnecessary by hospitalists, with one-third of those delays due to lack of availability at an extended care facility.10 Six years earlier, Carey et al. found that 13.5% of inpatient days were unnecessary, with more than 60% of delays attributable to waiting for discharge to a SNF.11
How, then, might we curtail unnecessary waiting, and whose job is it to solve the problem? The prospective payment system should reward hospitals for eliminating waiting—particularly those hospitals operating at capacity, for which the opportunity costs of occupied beds are most acute. Hospitalists, per se, have no incentive to discharge patients who are waiting; these patients are easy to round on, rarely have emergencies, and generate daily bills. Even when hospitalists are employed by the hospital and incentives for both are aligned, hospitalists may still be powerless to discharge waiting patients, summon busy consultants, or create extra slots in the endoscopy suite.
The move to value at the system level may offer hope. As health systems become responsible for the total cost of care, their focus must shift from the individual areas where care is provided to the transitions of care between treatment areas. It is in these transitions that US healthcare has failed most spectacularly, and consequently, it is where the greatest opportunity lies.
To date, most discharge interventions have focused on communication, with a goal of improving patient safety and, to a lesser extent, preventing readmissions. Partnering with SNFs can reduce the rate of readmissions,12 but for the most part, the incentives for hospitals and post-acute care facilities remain misaligned. Because post-acute care facilities are paid per diem, they have little incentive to reduce patients’ stays or to admit new patients, who are more expensive to care for than existing ones. Physicians round on SNF patients infrequently and have no incentive to discharge patients, exacerbating the problem. Because post-acute care represents a growing proportion of costs for both medical and surgical patients, health systems will need to either have their own facilities or enter into contracts that align the incentives.
What can hospitalists do? As the predominant coordinators of hospitalized patients’ care both for medical and surgical teams, hospitalists meaningfully impact readmissions and lengths of stay through the care they provide.13 More important, as their roles in optimizing hospital throughput14 continue to expand, hospitalists are perhaps best positioned to observe a diverse range of inefficiencies and inadequacies in inpatient practice and translate those observations into new systems of care. Through thoughtful participation in hospital operations, administration, and health services research, hospitalists hold the key to improving the value of care we provide.
Disclosure
Nothing to report.
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
1. Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117-131. PubMed
2. Healthcare Cost and Utilization Project (HCUP). Statistical Brief #180. Overview of Hospital Stays in the United States, 2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed July 17, 2017.
3. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11(3):221-230. PubMed
4. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. PubMed
5. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
6. Stanton MW, Rutherford MK. Research in Action: The high concentration of U.S. health care expenditures. Agency for Healthcare Research and Quality. Available at: https://meps.ahrq.gov/data_files/publications/ra19/ra19.pdf. Accessed July 17, 2017.
7. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff (Millwood). 2015;34(8):1312-1319. PubMed
8. Lee NS, Whitman N, Vakharia N, PhD GB, Rothberg MB. High-cost patients: hot-spotters don’t explain the half of it. J Gen Intern Med. 2017;32(1):28-34. PubMed
9. Wick JP, Hemmelgarn BR,Manns BJ, et al. Comparison of methods to define high use of inpatient services using population-based data. J Hosp Med. 2017;12(8):596-602. PubMed
10. Kim CS, Hart AL, Paretti RF, et al. Excess hospitalization days in an academic medical center: perceptions of hospitalists and discharge planners. Am J Manag Care. 2011;17(2):e34-42. PubMed
11. Carey MR, Sheth H, Braithwaite RS. A prospective study of reasons for prolonged hospitalizations on a general medicine teaching service. J Gen Intern Med. 2005;20(2):108-115. PubMed
12. Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med. 2017;12(4):238-244. PubMed
13. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. PubMed
14. Chadaga SR, Maher MP, Maller N, et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649-654. PubMed
© 2017 Society of Hospital Medicine
Continued Learning in Supporting Value-Based Decision Making
Physicians, researchers, and policymakers aspire to improve the value of healthcare, with reduced overall costs of care and improved outcomes. An important component of increasing healthcare costs in the United States is the rising cost of prescription medications, accounting for an estimated 17% of all spending in healthcare services.1 One potentially modifiable driver of low-value prescribing is poor awareness of medication cost.2 While displaying price to the ordering physician has reduced laboratory order volume and associated testing costs,3,4 applying cost transparency to medication ordering has produced variable results, perhaps reflecting conceptual differences in decision making regarding diagnosis and treatment.4-6
In this issue of the Journal of Hospital Medicine, Conway et al.7 performed a retrospective analysis applying interrupted times series models to measure the impact of passive cost display on the ordering frequency of 9 high-cost intravenous (IV) or inhaled medications that were identified as likely overused. For 7 of the IV medications, lower-cost oral alternatives were available; 2 study medications had no clear therapeutic alternatives. It was expected that lower-cost oral alternatives would have a concomitant increase in ordering rate as the order rate of the study medications decreased (eg, oral linezolid use would increase as IV linezolid use decreased). Order rate was the primary outcome, reported each week as treatment orders per 10,000 patient days, and was compared for both the pre- and postimplementation time periods. The particular methodology of segmented regressions allowed the research team to control for preintervention trends in medication ordering, as well as to analyze both immediate and delayed effects of the cost-display intervention. The research team framed the cost display as a passive approach. The intervention displayed average wholesale cost data and lower-cost oral alternatives on the ordering screen, which did not significantly reduce the ordering rate. Over the course of the study, outside influences led to 2 more active approaches to higher-cost medications, and Conway et al. wisely measured their effect as well. Specifically, the IV pantoprazole ordering rate decreased after restrictions secondary to a national medication shortage, and the oral voriconazole ordering rate decreased following an oncology order set change from oral voriconazole to oral posaconazole. These ordering-rate decreases were not temporally related to the implementation of the cost display intervention.
It is important to note several limitations of this study, some of which the authors discuss in the manuscript. Because 2 of the medications studied (eculizumab and calcitonin) do not have direct therapeutic alternatives, it is not surprising that price display alone would have no effect. The ordering providers who received this cost information had a more complex decision to make than they would in a scenario with a lower-cost alternative, essentially requiring them to ask “Does this patient need this class of medications at all?” rather than simply, “Is a lower-cost alternative appropriate?” Similarly, choosing medication alternatives that would require different routes of administration (ie, IV and oral) may have limited the effectiveness of a price intervention, given that factors such as illness severity also may influence the decision between IV and oral agents. Thus, the lack of an effect for the price display intervention for these specific medications may not be generalizable to all other medication decisions. Additionally, this manuscript offers limited data on the context in which the intervention was implemented and what adaptations, if any, were made based on early findings. The results may have varied greatly based on the visual design and how the cost display was presented within the electronic medical record. The wider organizational context may also have affected the intervention’s impact. A cost-display intervention appearing in isolation could understandably have a different impact, compared with an intervention within the context of a broader cost/value curriculum directed at house staff and faculty.
In summary, Conway et al. found that just displaying cost data did little to change prescribing patterns, but that more active approaches were quite efficacious. So where does this leave value-minded hospitalists looking to reduce overuse? Relatedly, what are the next steps for research and improvement science? We think there are 3 key strategic areas on which to focus. First, behavioral economics offers a critically important middle ground between the passive approaches studied here and more heavy-handed approaches that may limit provider autonomy, such as restricting drug use at the formulary.8 An improved choice architecture that presents the preferred higher-value option as the default selection may result in improved adoption of the high-value choice while also preserving provider autonomy and expertise required when clinical circumstances make the higher-cost drug the better choice.9,10 The second consideration is to minimize ethical tensions between cost displays that discourage use and a provider’s belief that a treatment is beneficial. Using available ethical frameworks for high-value care that engage both patient and societal concerns may help us choose and design interventions with more successful outcomes.11 Finally, research has shown that providers have poor knowledge of both cost and the relative benefits and harms of treatments and testing.12 Thus, the third opportunity for improvement is to provide appropriate clinical information (ie, relative therapeutic equivalency or adverse effects in alternative therapies) to support decision making at the point of order entry. Encouraging data already exists regarding how drug facts boxes can help patients understand benefits and side effects.13 A similar approach may aid physicians and may prove an easier task than improving patient understanding, given physicians’ substantial existing knowledge. These strategies may help guide providers to make a more informed value determination and obviate some ethical concerns related to clinical decisions based on cost alone. Despite their negative results, Conway et al.7 provided additional evidence that influencing complex decision making is not easy. However, we believe that continuing research into the factors that lead to successful value interventions has incredible potential for supporting high-value decision making in the future.
Disclosure
Nothing to report.
1. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. PubMed
2. Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. PubMed
3. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
4. Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. PubMed
5. Guterman JJ, Chernof BA, Mares B, Gross-Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low-tech approach to pharmaceutical ordering. J Gen Intern Med. 2002;17(10):792-796. PubMed
6. Goetz C, Rotman SR, Hartoularos G, Bishop TF. The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835-842. PubMed
7. Conway SJ, Brotman DJ, Merola D, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. PubMed
8. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press: 2008.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. PubMed
10. Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965-970. PubMed
11. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. PubMed
12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. PubMed
13. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516-527. PubMed
Physicians, researchers, and policymakers aspire to improve the value of healthcare, with reduced overall costs of care and improved outcomes. An important component of increasing healthcare costs in the United States is the rising cost of prescription medications, accounting for an estimated 17% of all spending in healthcare services.1 One potentially modifiable driver of low-value prescribing is poor awareness of medication cost.2 While displaying price to the ordering physician has reduced laboratory order volume and associated testing costs,3,4 applying cost transparency to medication ordering has produced variable results, perhaps reflecting conceptual differences in decision making regarding diagnosis and treatment.4-6
In this issue of the Journal of Hospital Medicine, Conway et al.7 performed a retrospective analysis applying interrupted times series models to measure the impact of passive cost display on the ordering frequency of 9 high-cost intravenous (IV) or inhaled medications that were identified as likely overused. For 7 of the IV medications, lower-cost oral alternatives were available; 2 study medications had no clear therapeutic alternatives. It was expected that lower-cost oral alternatives would have a concomitant increase in ordering rate as the order rate of the study medications decreased (eg, oral linezolid use would increase as IV linezolid use decreased). Order rate was the primary outcome, reported each week as treatment orders per 10,000 patient days, and was compared for both the pre- and postimplementation time periods. The particular methodology of segmented regressions allowed the research team to control for preintervention trends in medication ordering, as well as to analyze both immediate and delayed effects of the cost-display intervention. The research team framed the cost display as a passive approach. The intervention displayed average wholesale cost data and lower-cost oral alternatives on the ordering screen, which did not significantly reduce the ordering rate. Over the course of the study, outside influences led to 2 more active approaches to higher-cost medications, and Conway et al. wisely measured their effect as well. Specifically, the IV pantoprazole ordering rate decreased after restrictions secondary to a national medication shortage, and the oral voriconazole ordering rate decreased following an oncology order set change from oral voriconazole to oral posaconazole. These ordering-rate decreases were not temporally related to the implementation of the cost display intervention.
It is important to note several limitations of this study, some of which the authors discuss in the manuscript. Because 2 of the medications studied (eculizumab and calcitonin) do not have direct therapeutic alternatives, it is not surprising that price display alone would have no effect. The ordering providers who received this cost information had a more complex decision to make than they would in a scenario with a lower-cost alternative, essentially requiring them to ask “Does this patient need this class of medications at all?” rather than simply, “Is a lower-cost alternative appropriate?” Similarly, choosing medication alternatives that would require different routes of administration (ie, IV and oral) may have limited the effectiveness of a price intervention, given that factors such as illness severity also may influence the decision between IV and oral agents. Thus, the lack of an effect for the price display intervention for these specific medications may not be generalizable to all other medication decisions. Additionally, this manuscript offers limited data on the context in which the intervention was implemented and what adaptations, if any, were made based on early findings. The results may have varied greatly based on the visual design and how the cost display was presented within the electronic medical record. The wider organizational context may also have affected the intervention’s impact. A cost-display intervention appearing in isolation could understandably have a different impact, compared with an intervention within the context of a broader cost/value curriculum directed at house staff and faculty.
In summary, Conway et al. found that just displaying cost data did little to change prescribing patterns, but that more active approaches were quite efficacious. So where does this leave value-minded hospitalists looking to reduce overuse? Relatedly, what are the next steps for research and improvement science? We think there are 3 key strategic areas on which to focus. First, behavioral economics offers a critically important middle ground between the passive approaches studied here and more heavy-handed approaches that may limit provider autonomy, such as restricting drug use at the formulary.8 An improved choice architecture that presents the preferred higher-value option as the default selection may result in improved adoption of the high-value choice while also preserving provider autonomy and expertise required when clinical circumstances make the higher-cost drug the better choice.9,10 The second consideration is to minimize ethical tensions between cost displays that discourage use and a provider’s belief that a treatment is beneficial. Using available ethical frameworks for high-value care that engage both patient and societal concerns may help us choose and design interventions with more successful outcomes.11 Finally, research has shown that providers have poor knowledge of both cost and the relative benefits and harms of treatments and testing.12 Thus, the third opportunity for improvement is to provide appropriate clinical information (ie, relative therapeutic equivalency or adverse effects in alternative therapies) to support decision making at the point of order entry. Encouraging data already exists regarding how drug facts boxes can help patients understand benefits and side effects.13 A similar approach may aid physicians and may prove an easier task than improving patient understanding, given physicians’ substantial existing knowledge. These strategies may help guide providers to make a more informed value determination and obviate some ethical concerns related to clinical decisions based on cost alone. Despite their negative results, Conway et al.7 provided additional evidence that influencing complex decision making is not easy. However, we believe that continuing research into the factors that lead to successful value interventions has incredible potential for supporting high-value decision making in the future.
Disclosure
Nothing to report.
Physicians, researchers, and policymakers aspire to improve the value of healthcare, with reduced overall costs of care and improved outcomes. An important component of increasing healthcare costs in the United States is the rising cost of prescription medications, accounting for an estimated 17% of all spending in healthcare services.1 One potentially modifiable driver of low-value prescribing is poor awareness of medication cost.2 While displaying price to the ordering physician has reduced laboratory order volume and associated testing costs,3,4 applying cost transparency to medication ordering has produced variable results, perhaps reflecting conceptual differences in decision making regarding diagnosis and treatment.4-6
In this issue of the Journal of Hospital Medicine, Conway et al.7 performed a retrospective analysis applying interrupted times series models to measure the impact of passive cost display on the ordering frequency of 9 high-cost intravenous (IV) or inhaled medications that were identified as likely overused. For 7 of the IV medications, lower-cost oral alternatives were available; 2 study medications had no clear therapeutic alternatives. It was expected that lower-cost oral alternatives would have a concomitant increase in ordering rate as the order rate of the study medications decreased (eg, oral linezolid use would increase as IV linezolid use decreased). Order rate was the primary outcome, reported each week as treatment orders per 10,000 patient days, and was compared for both the pre- and postimplementation time periods. The particular methodology of segmented regressions allowed the research team to control for preintervention trends in medication ordering, as well as to analyze both immediate and delayed effects of the cost-display intervention. The research team framed the cost display as a passive approach. The intervention displayed average wholesale cost data and lower-cost oral alternatives on the ordering screen, which did not significantly reduce the ordering rate. Over the course of the study, outside influences led to 2 more active approaches to higher-cost medications, and Conway et al. wisely measured their effect as well. Specifically, the IV pantoprazole ordering rate decreased after restrictions secondary to a national medication shortage, and the oral voriconazole ordering rate decreased following an oncology order set change from oral voriconazole to oral posaconazole. These ordering-rate decreases were not temporally related to the implementation of the cost display intervention.
It is important to note several limitations of this study, some of which the authors discuss in the manuscript. Because 2 of the medications studied (eculizumab and calcitonin) do not have direct therapeutic alternatives, it is not surprising that price display alone would have no effect. The ordering providers who received this cost information had a more complex decision to make than they would in a scenario with a lower-cost alternative, essentially requiring them to ask “Does this patient need this class of medications at all?” rather than simply, “Is a lower-cost alternative appropriate?” Similarly, choosing medication alternatives that would require different routes of administration (ie, IV and oral) may have limited the effectiveness of a price intervention, given that factors such as illness severity also may influence the decision between IV and oral agents. Thus, the lack of an effect for the price display intervention for these specific medications may not be generalizable to all other medication decisions. Additionally, this manuscript offers limited data on the context in which the intervention was implemented and what adaptations, if any, were made based on early findings. The results may have varied greatly based on the visual design and how the cost display was presented within the electronic medical record. The wider organizational context may also have affected the intervention’s impact. A cost-display intervention appearing in isolation could understandably have a different impact, compared with an intervention within the context of a broader cost/value curriculum directed at house staff and faculty.
In summary, Conway et al. found that just displaying cost data did little to change prescribing patterns, but that more active approaches were quite efficacious. So where does this leave value-minded hospitalists looking to reduce overuse? Relatedly, what are the next steps for research and improvement science? We think there are 3 key strategic areas on which to focus. First, behavioral economics offers a critically important middle ground between the passive approaches studied here and more heavy-handed approaches that may limit provider autonomy, such as restricting drug use at the formulary.8 An improved choice architecture that presents the preferred higher-value option as the default selection may result in improved adoption of the high-value choice while also preserving provider autonomy and expertise required when clinical circumstances make the higher-cost drug the better choice.9,10 The second consideration is to minimize ethical tensions between cost displays that discourage use and a provider’s belief that a treatment is beneficial. Using available ethical frameworks for high-value care that engage both patient and societal concerns may help us choose and design interventions with more successful outcomes.11 Finally, research has shown that providers have poor knowledge of both cost and the relative benefits and harms of treatments and testing.12 Thus, the third opportunity for improvement is to provide appropriate clinical information (ie, relative therapeutic equivalency or adverse effects in alternative therapies) to support decision making at the point of order entry. Encouraging data already exists regarding how drug facts boxes can help patients understand benefits and side effects.13 A similar approach may aid physicians and may prove an easier task than improving patient understanding, given physicians’ substantial existing knowledge. These strategies may help guide providers to make a more informed value determination and obviate some ethical concerns related to clinical decisions based on cost alone. Despite their negative results, Conway et al.7 provided additional evidence that influencing complex decision making is not easy. However, we believe that continuing research into the factors that lead to successful value interventions has incredible potential for supporting high-value decision making in the future.
Disclosure
Nothing to report.
1. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. PubMed
2. Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. PubMed
3. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
4. Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. PubMed
5. Guterman JJ, Chernof BA, Mares B, Gross-Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low-tech approach to pharmaceutical ordering. J Gen Intern Med. 2002;17(10):792-796. PubMed
6. Goetz C, Rotman SR, Hartoularos G, Bishop TF. The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835-842. PubMed
7. Conway SJ, Brotman DJ, Merola D, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. PubMed
8. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press: 2008.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. PubMed
10. Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965-970. PubMed
11. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. PubMed
12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. PubMed
13. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516-527. PubMed
1. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858-871. PubMed
2. Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: a systematic review. PLoS Med. 2007;4(9):e283. PubMed
3. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903-908. PubMed
4. Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. PubMed
5. Guterman JJ, Chernof BA, Mares B, Gross-Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low-tech approach to pharmaceutical ordering. J Gen Intern Med. 2002;17(10):792-796. PubMed
6. Goetz C, Rotman SR, Hartoularos G, Bishop TF. The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835-842. PubMed
7. Conway SJ, Brotman DJ, Merola D, et al. Impact of displaying inpatient pharmaceutical costs at the time of order entry: lessons from a tertiary care center. J Hosp Med. 2017;12(8):639-645. PubMed
8. Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. New Haven: Yale University Press: 2008.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. PubMed
10. Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001;345(13):965-970. PubMed
11. DeCamp M, Tilburt JC. Ethics and high-value care. J Med Ethics. 2017;43(5):307-309. PubMed
12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. PubMed
13. Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Ann Intern Med. 2009;150(8):516-527. PubMed
© 2017 Society of Hospital Medicine
The Impact of Checklists on Inpatient Safety Outcomes: A Systematic Review of Randomized Controlled Trials
In response to widely publicized reports highlighting the challenges of suboptimal quality of healthcare, improving patient safety has been a leading healthcare initiative for more than 10 years.1-4 Numerous strategies to improve patient safety have been proposed,5-9 but improvements have been limited, which raises questions about whether the right approaches are being employed.10,11
Checklists have served as a foundation for the standardization and safety of aviation and nuclear power12,13 and are advocated as simple and effective instruments for ensuring safe care.7,14,15 Systematic reviews of observational studies suggest that checklists can reduce medical errors and adverse events,15-19 but these reviews are at risk of bias due to the limitations of observational methods. Furthermore, discordant results of recent high-profile evaluations of the World Health Organization (WHO) Surgical Safety Checklist highlight the need for checklist evaluations using rigorous study designs.20-22 Therefore, we sought to conduct a systematic review of RCTs (randomized controlled trials) to determine whether checklists, as a type of decision-support tool, are effective at improving patient safety outcomes in hospitalized patients.
METHODS
The study protocol was registered with the PROSPERO Register of Systematic Reviews (registration number: CRD42016037441) and developed according to the Preferred Reporting Items in Systematic Reviews and Meta-analyses (PRISMA) statement.23
Search Strategy
On December 8, 2016, we systematically searched Ovid MEDLINE, Ovid EMBASE, PubMed, and the Cochrane Central Register of Controlled Trials. The search was performed using no language or publication date restrictions and included 2 groups of terms (key words with similar characteristics): ‘checklists’ and ‘patient outcomes assessment’. We restricted our search to patient outcomes because these are more patient-oriented than the proximal processes of care that may not translate into outcomes. The search was restricted to RCTs using the Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials from the Cochrane Collaborative.24 The MEDLINE search strategy is depicted in Appendix I (Supplementary File 1). Reference lists of included articles were manually searched for additional publications. The search strategy was designed with the help of an information scientist (DL). EndNote X7 (Thomas Reuters, Philadelphia, PA, USA) was the reference software used for the management of citations.
Eligibility Criteria
We selected all studies reporting patient safety outcomes of a checklist intervention, using the following inclusion criteria: 1) acute care hospital inpatient population, 2) checklist intervention, 3) contain a control group (ie, no checklist), 4) report one or more patient safety outcome, as defined by the authors (eg, medical errors, adverse events, mortality), and 5) RCT design. We restricted our focus to inpatient populations given the heterogeneity of illness and patient care between acute and community settings. We defined a checklist as a tool that details the essential steps of a task, requiring the target provider to indicate whether an item was completed or not.1,7 Tools that included only 1 item (eg, electronic prompts) or did not require acknowledgement of the items (eg, guidelines) were excluded. We defined patient safety outcomes as the authors’ definition of patient safety (eg, medical error, adverse event, provider compliance with safety regulations).
Study Selection
Two reviewers (JMB, GW) independently, and in duplicate, reviewed the titles and abstracts of the retrieved citations against the eligibility criteria. The same 2 reviewers subsequently reviewed the full text of relevant articles for inclusion. Eligibility disagreements were resolved by consensus. A Kappa statistic was calculated for reviewer agreement of full-text screening.25 Reviewers were not blinded to author or journal names.26
Data Extraction
The structured data extraction form was calibrated using the first 2 articles. The 2 reviewers (JMB, GW) independently, and in duplicate, extracted data from included studies on the study characteristics, setting, study population, sample size, intervention used, outcomes examined, analytic method, and study quality. The data extraction form is depicted in Appendix II (Supplementary File 2). Coding discrepancies were resolved by consensus.
Quality Assessment
The 2 reviewers (JMB, GW) extracted data on study quality independently and in duplicate using 2 approaches. First, reviewers assessed study quality using a component method derived from the Cochrane Collaboration criteria.24 For each included study, the reviewers documented if the authors had adequately described inclusion/exclusion criteria, randomization, allocation concealment, blinding of participants/outcome assessors, attrition, cross over, baseline characteristics, and power calculation. Second, the reviewers calculated and reported the Jadad score for each included study, a validated assessment scale that assigns points (1 to 5) based on randomization, blinding, and attrition.27
Analysis
Owing to the heterogeneity of the data and the small number of studies that satisfied the inclusion criteria, the data were analyzed using guidelines for the narrative synthesis of a systematic review.28 Descriptive statistical findings from each included study were reported. The DerSimonian and Laird method for random-effects models was used to calculate a pooled estimate of 30-day all-cause mortality from the raw data available from a subset of studies (number of events, study population).29 Stata SE version 13.1 (Stata Corp, LP, College Station, TX) was used to perform the statistical analyses.
RESULTS
The literature search identified 11,225 unique citations from which 83 abstracts were eligible for full-text review. We identified 9 full-text articles for inclusion in the review (Figure 1 [Supplementary File 3]). The main reasons for citation exclusion during the full-text review were that the study design was not an RCT (39%) or there was no checklist intervention (34%
Study Characteristics
Characteristics of the included studies are summarized in Tables 1 and 2. Six of the studies were conducted in at least 1teaching hospital.30-35 The studies varied in target populations for both the checklist user and patients. The outcomes reported varied; 3 studies examined 30-day mortality,21,30,36 4 studies examined hospital length of stay,21,30,33,36 and 2 studies reported user compliance with the checklist.21,31 Five of the studies reported patient outcomes,21,30,33,35,36 and 5 studies reported provider-level outcomes related to patient safety (eg, compliance with checklist items such as communication of medications, isolation precautions, etc.).31-34,37
Description of Checklists
Supplementary File 4 (Table 3) provides a detailed breakdown of the checklists’ purpose and components. Six of the checklists were designed to directly reduce patient safety events,21,30,33,35-37 whereas 3 of the checklists were designed to indirectly reduce patient safety events by increasing compliance with processes of care.31,32,34 Six checklists were constructed and pilot tested by the research team conducting the RCT30-35 and the 3 remaining studies used modified versions of previously validated checklists.21,36,37 The number of items included in the checklist ranged from 2 to 54.
Impact of the Checklist
Table 4 summarizes the adverse events, medical errors, resource utilization and/or compliance reported for each checklist. Chaudhary et al. reported significant decreases in Grade III (requiring intervention)38 and IV (life-threatening)38 postoperative complications (23% v. 33%, P = 0.04) and 30-day mortality (5.7% vs 10.0%, P = 0.04) for patients assigned to the Modified WHO Surgical Safety Checklist compared to controls.21 Conversely, Haugen et al. reported a nonsignificant reduction in 30-day mortality between the WHO Surgical Safety Checklist group and controls (1.0% vs 1.6%, P = 0.151).36 Bassor et al. reported no significant difference in 30-day hospital readmission for decompensated heart failure for the heart failure discharge checklist group when compared to controls (6% vs. 4%, P = NS); however, an exploratory analysis that excluded patients who died during the follow-up period found a significant difference in 30-day readmission rates (2% vs. 20%, P = 0.02).30 Gentili et al. reported a higher proportion of patients with pain control in the checklist group compared to the controls (67.6% vs. 54.8%), as well as fewer incidents of analgesic therapy–related uncontrolled adverse events (25.9% vs. 49.9%); however, the statistical significance of these differences were not reported.35 The Writing Group for CHECKLIST-ICU reported no significant difference for in-hospital mortality between the checklist and control groups (adjusted odds ratio [AOR] 1.02, 95% CI, 0.82-1.26, P = 0.88), nor for the secondary clinical outcomes examined (Table 4).33 However, there was a significant difference between the checklist group and control group for 3 of the 7 outcomes related to processes of patient care, including a reduction in the use of both urinary catheters (adjusted rate ratio [ARR] 0.86, 95% CI, 0.80-0.93, P < 0.001) and central venous catheters (ARR 0.90, 95% CI 0.83-0.98, P = 0.02). Masson et al. reported that when using the FASTHUG-MAIDENS checklist, more drug-related problems were identified by pharmacy residents (in relation to the number identified by the ICU pharmacist) both per patient encounter (P = 0.008) and overall (P < 0.001).37 Ong et al. reported higher rates of compliance with isolation precautions for infectious diseases in the checklist group (71% vs. 38%, P < 0.01); however, compliance with the checklist was low (40%) and qualitative analyses found participants were dissatisfied with the checklist.31 Salzwedel et al. reported the number of items handed over by anesthesia residents postoperatively to be higher in the checklist group than the control group (48.7% vs. 32.4%, P < 0.001).32 In a more recent study, Salzwedel et al. reported that proportion of items deemed by the attending anesthesiologist as “must be handed over” were more often actually handed over by the anesthesia residents assigned to the checklist group when compared to controls (87.1% vs. 75.0%, P = 0.005).34
30-day Mortality
A random-effects model pooling data from the 3 studies that reported data for 30-day all cause mortality suggested a significant reduction with use of a checklist (OR 0.60, 95% CI, 0.41-0.89; P = 0.01, I2 = 0.0%, P = 0.573).
Study Quality
Supplementary File 5 (Table 5) summarizes the quality assessment of the 9 studies. The clarity of description for each intervention varied. All studies reported inclusion/exclusion criteria and randomization procedures. Three studies indicated that outcome assessors were blinded to intervention allocation;32,34,36 while this was unclear in 2 studies.21,30 Three studies reported baseline characteristics.21,30,36 Two studies reported power calculations;33,37 however, one study had a sample size that was less than that required to achieve the target power.37 The Jadad scores ranged from 1to 5.
DISCUSSION
This systematic review identified 9 RCTs that examined the impact of a checklist on patient safety outcomes in hospitalized patients. The studies employed checklists with different purposes and elements and measured different patient safety outcomes. The methodological quality of the included studies was moderate. In aggregate, the results suggest that checklists may be effective at improving patient safety outcomes, but the small number of moderate quality studies and the heterogeneity of interventions and outcome measures suggests that there is an urgent need for further evaluation.
The most important observation from our systematic review is the paucity of high quality evidence evaluating checklists’ impact on patient safety outcomes in acute inpatient care. The implementation of checklists is increasingly common as they are relatively low cost to develop and implement, and intuitively make sense. This is particularly true in an era of increasing efforts to standardize care as a means for improving quality and minimizing cost (ie, previous systematic reviews cite 38 unique studies).39 However, implementation of an inadequately tested checklist risks unintended consequences (eg, inefficient resource utilization).18 The small number of RCTs identified might be owing to quality improvement efforts traditionally focusing on ‘real life’ applicability over rigorous research methodology.40 The translation of evidence into clinical practice is known to be slow;41 however, these more rigorous methodologies reduce the risk of biases and generate high-quality evidence, which help to fulfill the necessity to identify best practices while avoiding these unintended consequences.
The studies varied both in the approaches used to develop checklists and in the number of items included (ranging from 2 to 54). What is the optimal method for developing a checklist and how does this impact their effectiveness?42 The answers to these questions are not known. However, this review highlights some important issues to consider when developing a checklist. As the number of items or complexity of a task increases, our ability to efficiently perform the task without aid decreases.43-45 As such, a well-designed checklist should detail explicit instructions on the what, where, when, and how of a given task in a fashion that ensures a consistent accuracy for completing the work.5 It is recommended that construction of a checklist follow the principles of human factors engineering: engage stakeholders and human factors experts in the design; are developed based on user needs and realities; list items in order of importance; are concise and subgroup sections of checklists by task or chronological order; ensure usability and evaluate potential negative consequences (eg time to complete); are pilot tested and validated before implementation; are updated as needed based the on generation of new findings or changes in operational procedures.46 These general principles of human factors engineering46 provide a practical approach for the development and evaluation of a checklist. In addition, standardization of operational definitions (ie, process, outcome, compliance) is important for study replication and robust meta-analyses.
Checklists used in aviation are perhaps best known12 and the evidence of their effectiveness is derived from the attribution of aviation errors to incomplete checklists.12 Although more recently implemented in medicine, checklists have the potential to guide the successful completion of complex tasks in healthcare.7 Systematic reviews of observational studies have been conducted for specific checklists (eg, WHO Surgical Safety Checklist) and for select patient populations (eg, surgical patients), and the number of included studies ranges from 7-27 (n = 38 unique studies).15,16,18,19 For example, Gillespie et al. in a systematic review and meta-analysis reported the implementation of Surgical Safety Checklists to be associated with a reduction in postoperative complications (relative risk [RR] 0.63, 95% CI, 0.58-0.72, P = < 0.001), but not mortality (RR 1.03, 95% CI, 0.73-1.4, P = 0.857).19 Similarly, Treadwell et al. reported in a systematic review of Surgical Safety Checklists that while data are promising, more evaluation of their impact on clinical outcomes is needed.18 These recommendations are nicely illustrated by Urbach et al.’s20 and O’Leary et al.’s47 evaluations of the mandatory adoption of Surgical Safety Checklists across all hospitals in Ontario, Canada, which respectively demonstrated no significant reductions in 30-day perioperatively conplications for both adult (OR 0.97, 95% CI, 0.90-1.03, P = 0.29) and pediatric (AOR 1.01, 95% CI, 0.90-1.14, P = 0.9) patients. These data not only highlight the need for further evaluation of checklists but are also a reminder that checklists and their associated implementation strategies are complex interventions for which there may be important differences between the efficacy reported in clinical trials and the effectiveness reported in implementation studies.48 This all suggests that if checklists are to be effective in improving patient safety, process evaluations of implementation49 and realist reviews of published studies50 may be important to determine optimal approaches for implementation. We believe that, based on the limited currently available evidence, there is urgency for further robust evaluations of checklists before their widespread implementation. If effective, they should be widely implemented. If ineffective, they should be abandoned to minimize unintended consequences and inefficient use of resources.
There are 4 primary limitations to this review that should be considered when interpreting the findings. First, the RCT design is not the study design employed by most quality improvement initiatives.40 While some quality improvement experts may argue that an RCT design is insufficiently flexible for applied settings, it does minimize the risk of biased assessments of intervention effectiveness. Second, our search strategy included an RCT filter. The filter helped restrict the number of citations to be reviewed (n = 11,225) but could have resulted in improperly indexed studies being excluded. To guard against this risk, we used the validated Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials,24 reviewed reference lists of citations included in the review, and solicited suggestions for missing studies from quality improvement experts. Third, our review was restricted to hospitalized patients. Although the studies evaluated commonly reported safety outcomes across patients with diverse clinical conditions, care settings, and providers that broadly reflect hospital-based care, evaluations of checklists in additional patient and provider groups are needed (eg, hospitalists). Furthermore, the effectiveness of checklists for improving patient safety outcomes in outpatients is important; however, the organizational and patient characteristics of these 2 settings (hospitalized vs outpatient) are sufficiently different to warrant separate systematic reviews. Finally, owing to the heterogeneity of the checklists used and outcomes measured, we were unable to perform a robust meta-analysis. Heterogeneity, combined with the small number of studies identified in our search, prevented us from applying statistical methods to assess for publication bias. This limitation of our systematic review highlights an important gap in the literature and emphasizes the importance of additional primary research to evaluate checklists.
In summary, we identified few RCTs that examined checklists designed to improve patient safety outcomes. The small number of existing studies suggests that checklists may improve patient safety outcomes; however, these observations were not reported for all outcomes examined and the studies were heterogeneous and of limited methodological quality. There is an urgent need for high-quality evaluations of the effectiveness of patient safety checklists in inpatient healthcare settings to substantiate their perceived benefits.
Acknowledgments
We would like to thank Diane Lorenzetti for her help with the development of the search strategy.
Disclosure: The authors have no known conflicts of interest to declare.
Jamie Boyd was supported by a W21C – Alberta Innovates-Health Solutions (AIHS) Collaborative Research and Innovation Opportunities (CRIO) Health Services Research graduate studentship. Guosong Wu was supported by a Western Regional Training Centre (WRTC) for Health Services Research graduate studentship. Dr. Stelfox was supported by a Population Health Investigator Award from Alberta Innovates Health Solutions.
Authors’ Contributions
HTS was responsible for the study’s conception. All 3 authors contributed to the study’s design and interpretation. JB and GW were responsible for searching the literature, reviewing abstracts, selecting full-text articles and critically appraising them. All 3 authors performed the analyses. JB drafted the manuscript and all 3 authors assisted in the successive revisions of the final manuscript. All authors have read and approved the final manuscript.
1. World Health Organization. Patient safety. Available at: http://www.who.int/patientsafety/about/en/. Accessed June 21, 2016.
2. Institute of Medicine. To err is human: Building a safer health system. In: Kohn L, Corrigan J, Donaldson M, eds. Institute of Medicine-Committee on Quality of Health Care in America. Washington DC: National Academy Press; 1999:86-101. PubMed
3. Institute of Medicine Committee on the Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington DC: National Academy Press; 2001. PubMed
4. Stelfox HT, Palmisani S, Scurlock C, Orav EJ, Bates DW. The “to err is human” report and the patient safety literature. Qual Saf Health Care. 2006; 15(3):174-178. PubMed
5. Winters BD, Gurses AP, Lehmann H, Sexton JB, Rampersad CJ, Pronovost, PJ. Clinical review: Checklists - translating evidence into practice. Crit Care. 2009; 13(6):210. PubMed
6. Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999; 159(2):439-446. PubMed
7. Gawande A. The checklist manifesto: How to get things right. Great Britain: Profile Books LTD; 2010.
8. Pronovost P, Vohr E. Safe patients, smart hospitals. New York, NY: Hudson Street Press; 2010.
9. Hughes RG. Advances in patient safety: Tools and strategies for quality improvement and patient safety. In: Hughes RG, ed. Patient safety and quality: An evidence-based handbook for nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. PubMed
10. Henriksen K, Oppenheimer C, Leape LL, et al. Envisioning patient safety in the year 2025: Eight perspectives. In: Henriksen K, Battles JB, Keyes MA, et al., eds. Advances in patient safety: New directions and alternative approaches. Rockville, MD: Agency for Healthcare Research and Quality; 2008. PubMed
11. Gaba DM, Howard SK. Patient safety: Fatigue among clinicians and the safety of patients. N Engl J Med. 2002; 347(16):1249-1255. PubMed
12. Degani A, Wiener EL. Cockpit checklists: Concepts, design, and use. Human Factors: The Journal of the Human Factors and Ergonomics Society 1993; 35(2):345-359.
13. Swain AD, Guttmann HE. Handbook of human reliability analysis with emphasis on nuclear power plant applications: Final report. Washington, DC: U.S. Nuclear Regulatroy Commission; 1983.
14. de Vries EN, Prins HA, Crolla RM, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010; 363(20):1928-1937. PubMed
15. Bergs J, Hellings J, Cleemput I, et al. Systematic review and meta-analysis of the effect of the world health organization surgical safety checklist on postoperative complications. Br J Surg. 2014; 101(3):150-158. PubMed
16. Pucher PH, Johnston MJ, Aggarwal R, Arora S, Darzi A. Effectiveness of interventions to improve patient handover in surgery: A systematic review. Surgery. 2015; 158(1):85-95. PubMed
17. Bergs J, Lambrechts F, Simons P, et al. Barriers and facilitators related to the implementation of surgical safety checklists: A systematic review of the qualitative evidence. BMJ Qual Saf. 2015; 23(12):776-786. PubMed
18. Treadwell JR, Lucas S, Tsou AY. Surgical checklists: A systematic review of impacts and implementation. BMJ Qual Saf. 2014; 23(4):299-318. PubMed
19. Gillespie BM, Chaboyer W, Thalib L, John M, Fairweather N, Slater K. Effect of using a safety checklist on patient complications after surgery: A systematic review and meta-analysis. Anesthesiology. 2014; 120(6):1380-1389. PubMed
20. Reames BN, Krell RW, Campbell DA Jr, Dimick JB. A checklist-based intervention to improve surgical outcomes in michigan: Evaluation of the keystone surgery program. JAMA Surg. 2015; 150(3):208-215. PubMed
21. Chaudhary N, Varma V, Kapoor S, Mehta N, Kumaran V, Nundy S. Implementation of a surgical safety checklist and postoperative outcomes: A prospective randomized controlled study. J Gastrointest Surg. 2015; 19(5):935-942. PubMed
22. Reames BN, Krell RW, Campbell DA, Jr., Dimick JB. A checklist-based intervention to improve surgical outcomes in Michigan: Evaluation of the Keystone Surgery program. JAMA surgery. 2015; 150(3):208-215. PubMed
23. Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009; 151(4):W65-94. PubMed
24. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Oxford, UK: The Cochrane Collaboration, 2011.
25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159-174. PubMed
26. Berlin JA. Does blinding of readers affect the results of meta-analyses? University of pennsylvania meta-analysis blinding study group. Lancet 1997;350(9072):185-186. PubMed
27. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17(1):1-12. PubMed
28. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews: A product form the esrc methods programme. Available at: https://www.researchgate.net/profile/Mark_Rodgers4/publication/233866356_Guidance_on_the_conduct_of_narrative_synthesis_in_systematic_reviews_A_product_from_the_ESRC_Methods_Programme/links/02e7e5231e8f3a6183000000.pdf. Accessed June 17, 2016.
29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177-188. PubMed
30. Basoor A, Doshi NC, Cotant JF, et al. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013; 19(4):200-206. PubMed
31. Ong MS, Magrabi F, Post J, et al. Communication interventions to improve adherence to infection control precautions: A randomised crossover trial. BMC Infect Dis. 2013; 13:72. PubMed
32. Salzwedel C, Bartz HJ, Kuhnelt I, et al. The effect of a checklist on the quality of post-anaesthesia patient handover: A randomized controlled trial. Int J Qual Health Care. 2013; 25(2):176-181.
2012; 7: PubMed
33. Implement Sci. 50. Rycroft-Malone J, McCormack B, Hutchinson AM, et al. Realist synthesis: Illustrating the method for implementation research. 2008; 337.BMJ. PubMed
49. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: The new medical research council guidance. 2014; 9(9):e108585.PloS one. PubMed
48. Gagliardi AR, Straus SE, Shojania KG, Urbach DR. Multiple interacting factors influence adherence, and outcomes associated with surgical safety checklists: A qualitative study.
2016; 188(9):E191-E198.CMAJ. PubMed
47. O’Leary JD, Wijeysundera DN, Crawford MW. Effect of surgical safety checklists on pediatric surgical complications in Ontario. Rockville, MD: Agency for Healthcare Research and Quality; 2013.Human factors and ergonomics. Making health care safer ii: An updated critical analysis of the evidence for patient safety practices. PubMed
46. Carayon P, Xie A, Kianfar SH. 2005; 16(1):70-76.Psychol Sci. 2004; 30(4):689-707.
45. Halford GS, Baker R, McCredden JE, Bain JD. How many variables can humans process? J Exp Psychol Hum Percept Perform. PubMed
44. Oberauer K, Kliegl R. Simultaneous cognitive operations in working memory after dual-task practice. 1956; 63(2):81-97.Psychol Rev. PubMed
43. Miller GA. The magical number seven plus or minus two: Some limits on our capacity for processing information. 2008; 20(1):22-30.Int J Qual Health Care. PubMed
42. Hales B, Terblanche M, Fowler R, Sibbald W. Development of medical checklists for improved quality of patient care. 2011; 104(12):510-520.J R Soc Med. PubMed
41. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. 2015; 24(5):325-336.BMJ Qual Saf. PubMed
40. Portela MC, Pronovost PJ, Woodcock T, Carter P, Dixon-Woods M. How to study improvement interventions: A brief overview of possible study types. Washington (DC): National Academies Press (US); 2013.Best care at lower cost: The path to continuously learning health care in america. PubMed
39. Institute of Medicine. Committee on the learning health care system in America. In: Smith M, Saunders R, Stuckhardt L, et al., eds. 2004; 240(2):205-213.Ann Surg. PubMed
38. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. 2013; 66(3):157-162.Can J Hosp Pharm. PubMed
37. Masson SC, Mabasa VH, Malyuk DL, Perrott JL. Validity evidence for fasthug-maidens, a mnemonic for identifying drug-related problems in the intensive care unit. 2015; 261(5):821-828.Ann Surg. PubMed
36. Haugen AS, Softeland E, Almeland SK, et al. Effect of the world health organization checklist on patient outcomes: A stepped wedge cluster randomized controlled trial.
2016; 12(2):199-205.Future Oncol. PubMed
35. Gentili M, Clerico M, Spizzichino M, Fanelli G. Use of a checklist to improve pain control in hospitalized cancer patients: the 38Checkpain project. 2016; 32:170-174.J Crit Care. PubMed
34. Salzwedel C, Mai V, Punke MA, Kluge S, Reuter DA. The effect of a checklist on the quality of patient handover from the operating room to the intensive care unit: A randomized controlled trial. 2016; 315(14):1480-1490.JAMA. PubMed
33. The Writing Group for CHECKLIST-ICU, Cavalcanti AB, Bozza FA, et al. Effect of a Quality Improvement Intervention With Daily Round Checklists, Goal Setting, and Clinician Prompting on Mortality of Critically Ill Patients: A Randomized Clinical Trial. PubMed
In response to widely publicized reports highlighting the challenges of suboptimal quality of healthcare, improving patient safety has been a leading healthcare initiative for more than 10 years.1-4 Numerous strategies to improve patient safety have been proposed,5-9 but improvements have been limited, which raises questions about whether the right approaches are being employed.10,11
Checklists have served as a foundation for the standardization and safety of aviation and nuclear power12,13 and are advocated as simple and effective instruments for ensuring safe care.7,14,15 Systematic reviews of observational studies suggest that checklists can reduce medical errors and adverse events,15-19 but these reviews are at risk of bias due to the limitations of observational methods. Furthermore, discordant results of recent high-profile evaluations of the World Health Organization (WHO) Surgical Safety Checklist highlight the need for checklist evaluations using rigorous study designs.20-22 Therefore, we sought to conduct a systematic review of RCTs (randomized controlled trials) to determine whether checklists, as a type of decision-support tool, are effective at improving patient safety outcomes in hospitalized patients.
METHODS
The study protocol was registered with the PROSPERO Register of Systematic Reviews (registration number: CRD42016037441) and developed according to the Preferred Reporting Items in Systematic Reviews and Meta-analyses (PRISMA) statement.23
Search Strategy
On December 8, 2016, we systematically searched Ovid MEDLINE, Ovid EMBASE, PubMed, and the Cochrane Central Register of Controlled Trials. The search was performed using no language or publication date restrictions and included 2 groups of terms (key words with similar characteristics): ‘checklists’ and ‘patient outcomes assessment’. We restricted our search to patient outcomes because these are more patient-oriented than the proximal processes of care that may not translate into outcomes. The search was restricted to RCTs using the Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials from the Cochrane Collaborative.24 The MEDLINE search strategy is depicted in Appendix I (Supplementary File 1). Reference lists of included articles were manually searched for additional publications. The search strategy was designed with the help of an information scientist (DL). EndNote X7 (Thomas Reuters, Philadelphia, PA, USA) was the reference software used for the management of citations.
Eligibility Criteria
We selected all studies reporting patient safety outcomes of a checklist intervention, using the following inclusion criteria: 1) acute care hospital inpatient population, 2) checklist intervention, 3) contain a control group (ie, no checklist), 4) report one or more patient safety outcome, as defined by the authors (eg, medical errors, adverse events, mortality), and 5) RCT design. We restricted our focus to inpatient populations given the heterogeneity of illness and patient care between acute and community settings. We defined a checklist as a tool that details the essential steps of a task, requiring the target provider to indicate whether an item was completed or not.1,7 Tools that included only 1 item (eg, electronic prompts) or did not require acknowledgement of the items (eg, guidelines) were excluded. We defined patient safety outcomes as the authors’ definition of patient safety (eg, medical error, adverse event, provider compliance with safety regulations).
Study Selection
Two reviewers (JMB, GW) independently, and in duplicate, reviewed the titles and abstracts of the retrieved citations against the eligibility criteria. The same 2 reviewers subsequently reviewed the full text of relevant articles for inclusion. Eligibility disagreements were resolved by consensus. A Kappa statistic was calculated for reviewer agreement of full-text screening.25 Reviewers were not blinded to author or journal names.26
Data Extraction
The structured data extraction form was calibrated using the first 2 articles. The 2 reviewers (JMB, GW) independently, and in duplicate, extracted data from included studies on the study characteristics, setting, study population, sample size, intervention used, outcomes examined, analytic method, and study quality. The data extraction form is depicted in Appendix II (Supplementary File 2). Coding discrepancies were resolved by consensus.
Quality Assessment
The 2 reviewers (JMB, GW) extracted data on study quality independently and in duplicate using 2 approaches. First, reviewers assessed study quality using a component method derived from the Cochrane Collaboration criteria.24 For each included study, the reviewers documented if the authors had adequately described inclusion/exclusion criteria, randomization, allocation concealment, blinding of participants/outcome assessors, attrition, cross over, baseline characteristics, and power calculation. Second, the reviewers calculated and reported the Jadad score for each included study, a validated assessment scale that assigns points (1 to 5) based on randomization, blinding, and attrition.27
Analysis
Owing to the heterogeneity of the data and the small number of studies that satisfied the inclusion criteria, the data were analyzed using guidelines for the narrative synthesis of a systematic review.28 Descriptive statistical findings from each included study were reported. The DerSimonian and Laird method for random-effects models was used to calculate a pooled estimate of 30-day all-cause mortality from the raw data available from a subset of studies (number of events, study population).29 Stata SE version 13.1 (Stata Corp, LP, College Station, TX) was used to perform the statistical analyses.
RESULTS
The literature search identified 11,225 unique citations from which 83 abstracts were eligible for full-text review. We identified 9 full-text articles for inclusion in the review (Figure 1 [Supplementary File 3]). The main reasons for citation exclusion during the full-text review were that the study design was not an RCT (39%) or there was no checklist intervention (34%
Study Characteristics
Characteristics of the included studies are summarized in Tables 1 and 2. Six of the studies were conducted in at least 1teaching hospital.30-35 The studies varied in target populations for both the checklist user and patients. The outcomes reported varied; 3 studies examined 30-day mortality,21,30,36 4 studies examined hospital length of stay,21,30,33,36 and 2 studies reported user compliance with the checklist.21,31 Five of the studies reported patient outcomes,21,30,33,35,36 and 5 studies reported provider-level outcomes related to patient safety (eg, compliance with checklist items such as communication of medications, isolation precautions, etc.).31-34,37
Description of Checklists
Supplementary File 4 (Table 3) provides a detailed breakdown of the checklists’ purpose and components. Six of the checklists were designed to directly reduce patient safety events,21,30,33,35-37 whereas 3 of the checklists were designed to indirectly reduce patient safety events by increasing compliance with processes of care.31,32,34 Six checklists were constructed and pilot tested by the research team conducting the RCT30-35 and the 3 remaining studies used modified versions of previously validated checklists.21,36,37 The number of items included in the checklist ranged from 2 to 54.
Impact of the Checklist
Table 4 summarizes the adverse events, medical errors, resource utilization and/or compliance reported for each checklist. Chaudhary et al. reported significant decreases in Grade III (requiring intervention)38 and IV (life-threatening)38 postoperative complications (23% v. 33%, P = 0.04) and 30-day mortality (5.7% vs 10.0%, P = 0.04) for patients assigned to the Modified WHO Surgical Safety Checklist compared to controls.21 Conversely, Haugen et al. reported a nonsignificant reduction in 30-day mortality between the WHO Surgical Safety Checklist group and controls (1.0% vs 1.6%, P = 0.151).36 Bassor et al. reported no significant difference in 30-day hospital readmission for decompensated heart failure for the heart failure discharge checklist group when compared to controls (6% vs. 4%, P = NS); however, an exploratory analysis that excluded patients who died during the follow-up period found a significant difference in 30-day readmission rates (2% vs. 20%, P = 0.02).30 Gentili et al. reported a higher proportion of patients with pain control in the checklist group compared to the controls (67.6% vs. 54.8%), as well as fewer incidents of analgesic therapy–related uncontrolled adverse events (25.9% vs. 49.9%); however, the statistical significance of these differences were not reported.35 The Writing Group for CHECKLIST-ICU reported no significant difference for in-hospital mortality between the checklist and control groups (adjusted odds ratio [AOR] 1.02, 95% CI, 0.82-1.26, P = 0.88), nor for the secondary clinical outcomes examined (Table 4).33 However, there was a significant difference between the checklist group and control group for 3 of the 7 outcomes related to processes of patient care, including a reduction in the use of both urinary catheters (adjusted rate ratio [ARR] 0.86, 95% CI, 0.80-0.93, P < 0.001) and central venous catheters (ARR 0.90, 95% CI 0.83-0.98, P = 0.02). Masson et al. reported that when using the FASTHUG-MAIDENS checklist, more drug-related problems were identified by pharmacy residents (in relation to the number identified by the ICU pharmacist) both per patient encounter (P = 0.008) and overall (P < 0.001).37 Ong et al. reported higher rates of compliance with isolation precautions for infectious diseases in the checklist group (71% vs. 38%, P < 0.01); however, compliance with the checklist was low (40%) and qualitative analyses found participants were dissatisfied with the checklist.31 Salzwedel et al. reported the number of items handed over by anesthesia residents postoperatively to be higher in the checklist group than the control group (48.7% vs. 32.4%, P < 0.001).32 In a more recent study, Salzwedel et al. reported that proportion of items deemed by the attending anesthesiologist as “must be handed over” were more often actually handed over by the anesthesia residents assigned to the checklist group when compared to controls (87.1% vs. 75.0%, P = 0.005).34
30-day Mortality
A random-effects model pooling data from the 3 studies that reported data for 30-day all cause mortality suggested a significant reduction with use of a checklist (OR 0.60, 95% CI, 0.41-0.89; P = 0.01, I2 = 0.0%, P = 0.573).
Study Quality
Supplementary File 5 (Table 5) summarizes the quality assessment of the 9 studies. The clarity of description for each intervention varied. All studies reported inclusion/exclusion criteria and randomization procedures. Three studies indicated that outcome assessors were blinded to intervention allocation;32,34,36 while this was unclear in 2 studies.21,30 Three studies reported baseline characteristics.21,30,36 Two studies reported power calculations;33,37 however, one study had a sample size that was less than that required to achieve the target power.37 The Jadad scores ranged from 1to 5.
DISCUSSION
This systematic review identified 9 RCTs that examined the impact of a checklist on patient safety outcomes in hospitalized patients. The studies employed checklists with different purposes and elements and measured different patient safety outcomes. The methodological quality of the included studies was moderate. In aggregate, the results suggest that checklists may be effective at improving patient safety outcomes, but the small number of moderate quality studies and the heterogeneity of interventions and outcome measures suggests that there is an urgent need for further evaluation.
The most important observation from our systematic review is the paucity of high quality evidence evaluating checklists’ impact on patient safety outcomes in acute inpatient care. The implementation of checklists is increasingly common as they are relatively low cost to develop and implement, and intuitively make sense. This is particularly true in an era of increasing efforts to standardize care as a means for improving quality and minimizing cost (ie, previous systematic reviews cite 38 unique studies).39 However, implementation of an inadequately tested checklist risks unintended consequences (eg, inefficient resource utilization).18 The small number of RCTs identified might be owing to quality improvement efforts traditionally focusing on ‘real life’ applicability over rigorous research methodology.40 The translation of evidence into clinical practice is known to be slow;41 however, these more rigorous methodologies reduce the risk of biases and generate high-quality evidence, which help to fulfill the necessity to identify best practices while avoiding these unintended consequences.
The studies varied both in the approaches used to develop checklists and in the number of items included (ranging from 2 to 54). What is the optimal method for developing a checklist and how does this impact their effectiveness?42 The answers to these questions are not known. However, this review highlights some important issues to consider when developing a checklist. As the number of items or complexity of a task increases, our ability to efficiently perform the task without aid decreases.43-45 As such, a well-designed checklist should detail explicit instructions on the what, where, when, and how of a given task in a fashion that ensures a consistent accuracy for completing the work.5 It is recommended that construction of a checklist follow the principles of human factors engineering: engage stakeholders and human factors experts in the design; are developed based on user needs and realities; list items in order of importance; are concise and subgroup sections of checklists by task or chronological order; ensure usability and evaluate potential negative consequences (eg time to complete); are pilot tested and validated before implementation; are updated as needed based the on generation of new findings or changes in operational procedures.46 These general principles of human factors engineering46 provide a practical approach for the development and evaluation of a checklist. In addition, standardization of operational definitions (ie, process, outcome, compliance) is important for study replication and robust meta-analyses.
Checklists used in aviation are perhaps best known12 and the evidence of their effectiveness is derived from the attribution of aviation errors to incomplete checklists.12 Although more recently implemented in medicine, checklists have the potential to guide the successful completion of complex tasks in healthcare.7 Systematic reviews of observational studies have been conducted for specific checklists (eg, WHO Surgical Safety Checklist) and for select patient populations (eg, surgical patients), and the number of included studies ranges from 7-27 (n = 38 unique studies).15,16,18,19 For example, Gillespie et al. in a systematic review and meta-analysis reported the implementation of Surgical Safety Checklists to be associated with a reduction in postoperative complications (relative risk [RR] 0.63, 95% CI, 0.58-0.72, P = < 0.001), but not mortality (RR 1.03, 95% CI, 0.73-1.4, P = 0.857).19 Similarly, Treadwell et al. reported in a systematic review of Surgical Safety Checklists that while data are promising, more evaluation of their impact on clinical outcomes is needed.18 These recommendations are nicely illustrated by Urbach et al.’s20 and O’Leary et al.’s47 evaluations of the mandatory adoption of Surgical Safety Checklists across all hospitals in Ontario, Canada, which respectively demonstrated no significant reductions in 30-day perioperatively conplications for both adult (OR 0.97, 95% CI, 0.90-1.03, P = 0.29) and pediatric (AOR 1.01, 95% CI, 0.90-1.14, P = 0.9) patients. These data not only highlight the need for further evaluation of checklists but are also a reminder that checklists and their associated implementation strategies are complex interventions for which there may be important differences between the efficacy reported in clinical trials and the effectiveness reported in implementation studies.48 This all suggests that if checklists are to be effective in improving patient safety, process evaluations of implementation49 and realist reviews of published studies50 may be important to determine optimal approaches for implementation. We believe that, based on the limited currently available evidence, there is urgency for further robust evaluations of checklists before their widespread implementation. If effective, they should be widely implemented. If ineffective, they should be abandoned to minimize unintended consequences and inefficient use of resources.
There are 4 primary limitations to this review that should be considered when interpreting the findings. First, the RCT design is not the study design employed by most quality improvement initiatives.40 While some quality improvement experts may argue that an RCT design is insufficiently flexible for applied settings, it does minimize the risk of biased assessments of intervention effectiveness. Second, our search strategy included an RCT filter. The filter helped restrict the number of citations to be reviewed (n = 11,225) but could have resulted in improperly indexed studies being excluded. To guard against this risk, we used the validated Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials,24 reviewed reference lists of citations included in the review, and solicited suggestions for missing studies from quality improvement experts. Third, our review was restricted to hospitalized patients. Although the studies evaluated commonly reported safety outcomes across patients with diverse clinical conditions, care settings, and providers that broadly reflect hospital-based care, evaluations of checklists in additional patient and provider groups are needed (eg, hospitalists). Furthermore, the effectiveness of checklists for improving patient safety outcomes in outpatients is important; however, the organizational and patient characteristics of these 2 settings (hospitalized vs outpatient) are sufficiently different to warrant separate systematic reviews. Finally, owing to the heterogeneity of the checklists used and outcomes measured, we were unable to perform a robust meta-analysis. Heterogeneity, combined with the small number of studies identified in our search, prevented us from applying statistical methods to assess for publication bias. This limitation of our systematic review highlights an important gap in the literature and emphasizes the importance of additional primary research to evaluate checklists.
In summary, we identified few RCTs that examined checklists designed to improve patient safety outcomes. The small number of existing studies suggests that checklists may improve patient safety outcomes; however, these observations were not reported for all outcomes examined and the studies were heterogeneous and of limited methodological quality. There is an urgent need for high-quality evaluations of the effectiveness of patient safety checklists in inpatient healthcare settings to substantiate their perceived benefits.
Acknowledgments
We would like to thank Diane Lorenzetti for her help with the development of the search strategy.
Disclosure: The authors have no known conflicts of interest to declare.
Jamie Boyd was supported by a W21C – Alberta Innovates-Health Solutions (AIHS) Collaborative Research and Innovation Opportunities (CRIO) Health Services Research graduate studentship. Guosong Wu was supported by a Western Regional Training Centre (WRTC) for Health Services Research graduate studentship. Dr. Stelfox was supported by a Population Health Investigator Award from Alberta Innovates Health Solutions.
Authors’ Contributions
HTS was responsible for the study’s conception. All 3 authors contributed to the study’s design and interpretation. JB and GW were responsible for searching the literature, reviewing abstracts, selecting full-text articles and critically appraising them. All 3 authors performed the analyses. JB drafted the manuscript and all 3 authors assisted in the successive revisions of the final manuscript. All authors have read and approved the final manuscript.
In response to widely publicized reports highlighting the challenges of suboptimal quality of healthcare, improving patient safety has been a leading healthcare initiative for more than 10 years.1-4 Numerous strategies to improve patient safety have been proposed,5-9 but improvements have been limited, which raises questions about whether the right approaches are being employed.10,11
Checklists have served as a foundation for the standardization and safety of aviation and nuclear power12,13 and are advocated as simple and effective instruments for ensuring safe care.7,14,15 Systematic reviews of observational studies suggest that checklists can reduce medical errors and adverse events,15-19 but these reviews are at risk of bias due to the limitations of observational methods. Furthermore, discordant results of recent high-profile evaluations of the World Health Organization (WHO) Surgical Safety Checklist highlight the need for checklist evaluations using rigorous study designs.20-22 Therefore, we sought to conduct a systematic review of RCTs (randomized controlled trials) to determine whether checklists, as a type of decision-support tool, are effective at improving patient safety outcomes in hospitalized patients.
METHODS
The study protocol was registered with the PROSPERO Register of Systematic Reviews (registration number: CRD42016037441) and developed according to the Preferred Reporting Items in Systematic Reviews and Meta-analyses (PRISMA) statement.23
Search Strategy
On December 8, 2016, we systematically searched Ovid MEDLINE, Ovid EMBASE, PubMed, and the Cochrane Central Register of Controlled Trials. The search was performed using no language or publication date restrictions and included 2 groups of terms (key words with similar characteristics): ‘checklists’ and ‘patient outcomes assessment’. We restricted our search to patient outcomes because these are more patient-oriented than the proximal processes of care that may not translate into outcomes. The search was restricted to RCTs using the Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials from the Cochrane Collaborative.24 The MEDLINE search strategy is depicted in Appendix I (Supplementary File 1). Reference lists of included articles were manually searched for additional publications. The search strategy was designed with the help of an information scientist (DL). EndNote X7 (Thomas Reuters, Philadelphia, PA, USA) was the reference software used for the management of citations.
Eligibility Criteria
We selected all studies reporting patient safety outcomes of a checklist intervention, using the following inclusion criteria: 1) acute care hospital inpatient population, 2) checklist intervention, 3) contain a control group (ie, no checklist), 4) report one or more patient safety outcome, as defined by the authors (eg, medical errors, adverse events, mortality), and 5) RCT design. We restricted our focus to inpatient populations given the heterogeneity of illness and patient care between acute and community settings. We defined a checklist as a tool that details the essential steps of a task, requiring the target provider to indicate whether an item was completed or not.1,7 Tools that included only 1 item (eg, electronic prompts) or did not require acknowledgement of the items (eg, guidelines) were excluded. We defined patient safety outcomes as the authors’ definition of patient safety (eg, medical error, adverse event, provider compliance with safety regulations).
Study Selection
Two reviewers (JMB, GW) independently, and in duplicate, reviewed the titles and abstracts of the retrieved citations against the eligibility criteria. The same 2 reviewers subsequently reviewed the full text of relevant articles for inclusion. Eligibility disagreements were resolved by consensus. A Kappa statistic was calculated for reviewer agreement of full-text screening.25 Reviewers were not blinded to author or journal names.26
Data Extraction
The structured data extraction form was calibrated using the first 2 articles. The 2 reviewers (JMB, GW) independently, and in duplicate, extracted data from included studies on the study characteristics, setting, study population, sample size, intervention used, outcomes examined, analytic method, and study quality. The data extraction form is depicted in Appendix II (Supplementary File 2). Coding discrepancies were resolved by consensus.
Quality Assessment
The 2 reviewers (JMB, GW) extracted data on study quality independently and in duplicate using 2 approaches. First, reviewers assessed study quality using a component method derived from the Cochrane Collaboration criteria.24 For each included study, the reviewers documented if the authors had adequately described inclusion/exclusion criteria, randomization, allocation concealment, blinding of participants/outcome assessors, attrition, cross over, baseline characteristics, and power calculation. Second, the reviewers calculated and reported the Jadad score for each included study, a validated assessment scale that assigns points (1 to 5) based on randomization, blinding, and attrition.27
Analysis
Owing to the heterogeneity of the data and the small number of studies that satisfied the inclusion criteria, the data were analyzed using guidelines for the narrative synthesis of a systematic review.28 Descriptive statistical findings from each included study were reported. The DerSimonian and Laird method for random-effects models was used to calculate a pooled estimate of 30-day all-cause mortality from the raw data available from a subset of studies (number of events, study population).29 Stata SE version 13.1 (Stata Corp, LP, College Station, TX) was used to perform the statistical analyses.
RESULTS
The literature search identified 11,225 unique citations from which 83 abstracts were eligible for full-text review. We identified 9 full-text articles for inclusion in the review (Figure 1 [Supplementary File 3]). The main reasons for citation exclusion during the full-text review were that the study design was not an RCT (39%) or there was no checklist intervention (34%
Study Characteristics
Characteristics of the included studies are summarized in Tables 1 and 2. Six of the studies were conducted in at least 1teaching hospital.30-35 The studies varied in target populations for both the checklist user and patients. The outcomes reported varied; 3 studies examined 30-day mortality,21,30,36 4 studies examined hospital length of stay,21,30,33,36 and 2 studies reported user compliance with the checklist.21,31 Five of the studies reported patient outcomes,21,30,33,35,36 and 5 studies reported provider-level outcomes related to patient safety (eg, compliance with checklist items such as communication of medications, isolation precautions, etc.).31-34,37
Description of Checklists
Supplementary File 4 (Table 3) provides a detailed breakdown of the checklists’ purpose and components. Six of the checklists were designed to directly reduce patient safety events,21,30,33,35-37 whereas 3 of the checklists were designed to indirectly reduce patient safety events by increasing compliance with processes of care.31,32,34 Six checklists were constructed and pilot tested by the research team conducting the RCT30-35 and the 3 remaining studies used modified versions of previously validated checklists.21,36,37 The number of items included in the checklist ranged from 2 to 54.
Impact of the Checklist
Table 4 summarizes the adverse events, medical errors, resource utilization and/or compliance reported for each checklist. Chaudhary et al. reported significant decreases in Grade III (requiring intervention)38 and IV (life-threatening)38 postoperative complications (23% v. 33%, P = 0.04) and 30-day mortality (5.7% vs 10.0%, P = 0.04) for patients assigned to the Modified WHO Surgical Safety Checklist compared to controls.21 Conversely, Haugen et al. reported a nonsignificant reduction in 30-day mortality between the WHO Surgical Safety Checklist group and controls (1.0% vs 1.6%, P = 0.151).36 Bassor et al. reported no significant difference in 30-day hospital readmission for decompensated heart failure for the heart failure discharge checklist group when compared to controls (6% vs. 4%, P = NS); however, an exploratory analysis that excluded patients who died during the follow-up period found a significant difference in 30-day readmission rates (2% vs. 20%, P = 0.02).30 Gentili et al. reported a higher proportion of patients with pain control in the checklist group compared to the controls (67.6% vs. 54.8%), as well as fewer incidents of analgesic therapy–related uncontrolled adverse events (25.9% vs. 49.9%); however, the statistical significance of these differences were not reported.35 The Writing Group for CHECKLIST-ICU reported no significant difference for in-hospital mortality between the checklist and control groups (adjusted odds ratio [AOR] 1.02, 95% CI, 0.82-1.26, P = 0.88), nor for the secondary clinical outcomes examined (Table 4).33 However, there was a significant difference between the checklist group and control group for 3 of the 7 outcomes related to processes of patient care, including a reduction in the use of both urinary catheters (adjusted rate ratio [ARR] 0.86, 95% CI, 0.80-0.93, P < 0.001) and central venous catheters (ARR 0.90, 95% CI 0.83-0.98, P = 0.02). Masson et al. reported that when using the FASTHUG-MAIDENS checklist, more drug-related problems were identified by pharmacy residents (in relation to the number identified by the ICU pharmacist) both per patient encounter (P = 0.008) and overall (P < 0.001).37 Ong et al. reported higher rates of compliance with isolation precautions for infectious diseases in the checklist group (71% vs. 38%, P < 0.01); however, compliance with the checklist was low (40%) and qualitative analyses found participants were dissatisfied with the checklist.31 Salzwedel et al. reported the number of items handed over by anesthesia residents postoperatively to be higher in the checklist group than the control group (48.7% vs. 32.4%, P < 0.001).32 In a more recent study, Salzwedel et al. reported that proportion of items deemed by the attending anesthesiologist as “must be handed over” were more often actually handed over by the anesthesia residents assigned to the checklist group when compared to controls (87.1% vs. 75.0%, P = 0.005).34
30-day Mortality
A random-effects model pooling data from the 3 studies that reported data for 30-day all cause mortality suggested a significant reduction with use of a checklist (OR 0.60, 95% CI, 0.41-0.89; P = 0.01, I2 = 0.0%, P = 0.573).
Study Quality
Supplementary File 5 (Table 5) summarizes the quality assessment of the 9 studies. The clarity of description for each intervention varied. All studies reported inclusion/exclusion criteria and randomization procedures. Three studies indicated that outcome assessors were blinded to intervention allocation;32,34,36 while this was unclear in 2 studies.21,30 Three studies reported baseline characteristics.21,30,36 Two studies reported power calculations;33,37 however, one study had a sample size that was less than that required to achieve the target power.37 The Jadad scores ranged from 1to 5.
DISCUSSION
This systematic review identified 9 RCTs that examined the impact of a checklist on patient safety outcomes in hospitalized patients. The studies employed checklists with different purposes and elements and measured different patient safety outcomes. The methodological quality of the included studies was moderate. In aggregate, the results suggest that checklists may be effective at improving patient safety outcomes, but the small number of moderate quality studies and the heterogeneity of interventions and outcome measures suggests that there is an urgent need for further evaluation.
The most important observation from our systematic review is the paucity of high quality evidence evaluating checklists’ impact on patient safety outcomes in acute inpatient care. The implementation of checklists is increasingly common as they are relatively low cost to develop and implement, and intuitively make sense. This is particularly true in an era of increasing efforts to standardize care as a means for improving quality and minimizing cost (ie, previous systematic reviews cite 38 unique studies).39 However, implementation of an inadequately tested checklist risks unintended consequences (eg, inefficient resource utilization).18 The small number of RCTs identified might be owing to quality improvement efforts traditionally focusing on ‘real life’ applicability over rigorous research methodology.40 The translation of evidence into clinical practice is known to be slow;41 however, these more rigorous methodologies reduce the risk of biases and generate high-quality evidence, which help to fulfill the necessity to identify best practices while avoiding these unintended consequences.
The studies varied both in the approaches used to develop checklists and in the number of items included (ranging from 2 to 54). What is the optimal method for developing a checklist and how does this impact their effectiveness?42 The answers to these questions are not known. However, this review highlights some important issues to consider when developing a checklist. As the number of items or complexity of a task increases, our ability to efficiently perform the task without aid decreases.43-45 As such, a well-designed checklist should detail explicit instructions on the what, where, when, and how of a given task in a fashion that ensures a consistent accuracy for completing the work.5 It is recommended that construction of a checklist follow the principles of human factors engineering: engage stakeholders and human factors experts in the design; are developed based on user needs and realities; list items in order of importance; are concise and subgroup sections of checklists by task or chronological order; ensure usability and evaluate potential negative consequences (eg time to complete); are pilot tested and validated before implementation; are updated as needed based the on generation of new findings or changes in operational procedures.46 These general principles of human factors engineering46 provide a practical approach for the development and evaluation of a checklist. In addition, standardization of operational definitions (ie, process, outcome, compliance) is important for study replication and robust meta-analyses.
Checklists used in aviation are perhaps best known12 and the evidence of their effectiveness is derived from the attribution of aviation errors to incomplete checklists.12 Although more recently implemented in medicine, checklists have the potential to guide the successful completion of complex tasks in healthcare.7 Systematic reviews of observational studies have been conducted for specific checklists (eg, WHO Surgical Safety Checklist) and for select patient populations (eg, surgical patients), and the number of included studies ranges from 7-27 (n = 38 unique studies).15,16,18,19 For example, Gillespie et al. in a systematic review and meta-analysis reported the implementation of Surgical Safety Checklists to be associated with a reduction in postoperative complications (relative risk [RR] 0.63, 95% CI, 0.58-0.72, P = < 0.001), but not mortality (RR 1.03, 95% CI, 0.73-1.4, P = 0.857).19 Similarly, Treadwell et al. reported in a systematic review of Surgical Safety Checklists that while data are promising, more evaluation of their impact on clinical outcomes is needed.18 These recommendations are nicely illustrated by Urbach et al.’s20 and O’Leary et al.’s47 evaluations of the mandatory adoption of Surgical Safety Checklists across all hospitals in Ontario, Canada, which respectively demonstrated no significant reductions in 30-day perioperatively conplications for both adult (OR 0.97, 95% CI, 0.90-1.03, P = 0.29) and pediatric (AOR 1.01, 95% CI, 0.90-1.14, P = 0.9) patients. These data not only highlight the need for further evaluation of checklists but are also a reminder that checklists and their associated implementation strategies are complex interventions for which there may be important differences between the efficacy reported in clinical trials and the effectiveness reported in implementation studies.48 This all suggests that if checklists are to be effective in improving patient safety, process evaluations of implementation49 and realist reviews of published studies50 may be important to determine optimal approaches for implementation. We believe that, based on the limited currently available evidence, there is urgency for further robust evaluations of checklists before their widespread implementation. If effective, they should be widely implemented. If ineffective, they should be abandoned to minimize unintended consequences and inefficient use of resources.
There are 4 primary limitations to this review that should be considered when interpreting the findings. First, the RCT design is not the study design employed by most quality improvement initiatives.40 While some quality improvement experts may argue that an RCT design is insufficiently flexible for applied settings, it does minimize the risk of biased assessments of intervention effectiveness. Second, our search strategy included an RCT filter. The filter helped restrict the number of citations to be reviewed (n = 11,225) but could have resulted in improperly indexed studies being excluded. To guard against this risk, we used the validated Cochrane Highly Sensitive Search Strategy for Identifying Randomized Trials,24 reviewed reference lists of citations included in the review, and solicited suggestions for missing studies from quality improvement experts. Third, our review was restricted to hospitalized patients. Although the studies evaluated commonly reported safety outcomes across patients with diverse clinical conditions, care settings, and providers that broadly reflect hospital-based care, evaluations of checklists in additional patient and provider groups are needed (eg, hospitalists). Furthermore, the effectiveness of checklists for improving patient safety outcomes in outpatients is important; however, the organizational and patient characteristics of these 2 settings (hospitalized vs outpatient) are sufficiently different to warrant separate systematic reviews. Finally, owing to the heterogeneity of the checklists used and outcomes measured, we were unable to perform a robust meta-analysis. Heterogeneity, combined with the small number of studies identified in our search, prevented us from applying statistical methods to assess for publication bias. This limitation of our systematic review highlights an important gap in the literature and emphasizes the importance of additional primary research to evaluate checklists.
In summary, we identified few RCTs that examined checklists designed to improve patient safety outcomes. The small number of existing studies suggests that checklists may improve patient safety outcomes; however, these observations were not reported for all outcomes examined and the studies were heterogeneous and of limited methodological quality. There is an urgent need for high-quality evaluations of the effectiveness of patient safety checklists in inpatient healthcare settings to substantiate their perceived benefits.
Acknowledgments
We would like to thank Diane Lorenzetti for her help with the development of the search strategy.
Disclosure: The authors have no known conflicts of interest to declare.
Jamie Boyd was supported by a W21C – Alberta Innovates-Health Solutions (AIHS) Collaborative Research and Innovation Opportunities (CRIO) Health Services Research graduate studentship. Guosong Wu was supported by a Western Regional Training Centre (WRTC) for Health Services Research graduate studentship. Dr. Stelfox was supported by a Population Health Investigator Award from Alberta Innovates Health Solutions.
Authors’ Contributions
HTS was responsible for the study’s conception. All 3 authors contributed to the study’s design and interpretation. JB and GW were responsible for searching the literature, reviewing abstracts, selecting full-text articles and critically appraising them. All 3 authors performed the analyses. JB drafted the manuscript and all 3 authors assisted in the successive revisions of the final manuscript. All authors have read and approved the final manuscript.
1. World Health Organization. Patient safety. Available at: http://www.who.int/patientsafety/about/en/. Accessed June 21, 2016.
2. Institute of Medicine. To err is human: Building a safer health system. In: Kohn L, Corrigan J, Donaldson M, eds. Institute of Medicine-Committee on Quality of Health Care in America. Washington DC: National Academy Press; 1999:86-101. PubMed
3. Institute of Medicine Committee on the Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington DC: National Academy Press; 2001. PubMed
4. Stelfox HT, Palmisani S, Scurlock C, Orav EJ, Bates DW. The “to err is human” report and the patient safety literature. Qual Saf Health Care. 2006; 15(3):174-178. PubMed
5. Winters BD, Gurses AP, Lehmann H, Sexton JB, Rampersad CJ, Pronovost, PJ. Clinical review: Checklists - translating evidence into practice. Crit Care. 2009; 13(6):210. PubMed
6. Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999; 159(2):439-446. PubMed
7. Gawande A. The checklist manifesto: How to get things right. Great Britain: Profile Books LTD; 2010.
8. Pronovost P, Vohr E. Safe patients, smart hospitals. New York, NY: Hudson Street Press; 2010.
9. Hughes RG. Advances in patient safety: Tools and strategies for quality improvement and patient safety. In: Hughes RG, ed. Patient safety and quality: An evidence-based handbook for nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. PubMed
10. Henriksen K, Oppenheimer C, Leape LL, et al. Envisioning patient safety in the year 2025: Eight perspectives. In: Henriksen K, Battles JB, Keyes MA, et al., eds. Advances in patient safety: New directions and alternative approaches. Rockville, MD: Agency for Healthcare Research and Quality; 2008. PubMed
11. Gaba DM, Howard SK. Patient safety: Fatigue among clinicians and the safety of patients. N Engl J Med. 2002; 347(16):1249-1255. PubMed
12. Degani A, Wiener EL. Cockpit checklists: Concepts, design, and use. Human Factors: The Journal of the Human Factors and Ergonomics Society 1993; 35(2):345-359.
13. Swain AD, Guttmann HE. Handbook of human reliability analysis with emphasis on nuclear power plant applications: Final report. Washington, DC: U.S. Nuclear Regulatroy Commission; 1983.
14. de Vries EN, Prins HA, Crolla RM, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010; 363(20):1928-1937. PubMed
15. Bergs J, Hellings J, Cleemput I, et al. Systematic review and meta-analysis of the effect of the world health organization surgical safety checklist on postoperative complications. Br J Surg. 2014; 101(3):150-158. PubMed
16. Pucher PH, Johnston MJ, Aggarwal R, Arora S, Darzi A. Effectiveness of interventions to improve patient handover in surgery: A systematic review. Surgery. 2015; 158(1):85-95. PubMed
17. Bergs J, Lambrechts F, Simons P, et al. Barriers and facilitators related to the implementation of surgical safety checklists: A systematic review of the qualitative evidence. BMJ Qual Saf. 2015; 23(12):776-786. PubMed
18. Treadwell JR, Lucas S, Tsou AY. Surgical checklists: A systematic review of impacts and implementation. BMJ Qual Saf. 2014; 23(4):299-318. PubMed
19. Gillespie BM, Chaboyer W, Thalib L, John M, Fairweather N, Slater K. Effect of using a safety checklist on patient complications after surgery: A systematic review and meta-analysis. Anesthesiology. 2014; 120(6):1380-1389. PubMed
20. Reames BN, Krell RW, Campbell DA Jr, Dimick JB. A checklist-based intervention to improve surgical outcomes in michigan: Evaluation of the keystone surgery program. JAMA Surg. 2015; 150(3):208-215. PubMed
21. Chaudhary N, Varma V, Kapoor S, Mehta N, Kumaran V, Nundy S. Implementation of a surgical safety checklist and postoperative outcomes: A prospective randomized controlled study. J Gastrointest Surg. 2015; 19(5):935-942. PubMed
22. Reames BN, Krell RW, Campbell DA, Jr., Dimick JB. A checklist-based intervention to improve surgical outcomes in Michigan: Evaluation of the Keystone Surgery program. JAMA surgery. 2015; 150(3):208-215. PubMed
23. Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009; 151(4):W65-94. PubMed
24. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Oxford, UK: The Cochrane Collaboration, 2011.
25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159-174. PubMed
26. Berlin JA. Does blinding of readers affect the results of meta-analyses? University of pennsylvania meta-analysis blinding study group. Lancet 1997;350(9072):185-186. PubMed
27. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17(1):1-12. PubMed
28. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews: A product form the esrc methods programme. Available at: https://www.researchgate.net/profile/Mark_Rodgers4/publication/233866356_Guidance_on_the_conduct_of_narrative_synthesis_in_systematic_reviews_A_product_from_the_ESRC_Methods_Programme/links/02e7e5231e8f3a6183000000.pdf. Accessed June 17, 2016.
29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177-188. PubMed
30. Basoor A, Doshi NC, Cotant JF, et al. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013; 19(4):200-206. PubMed
31. Ong MS, Magrabi F, Post J, et al. Communication interventions to improve adherence to infection control precautions: A randomised crossover trial. BMC Infect Dis. 2013; 13:72. PubMed
32. Salzwedel C, Bartz HJ, Kuhnelt I, et al. The effect of a checklist on the quality of post-anaesthesia patient handover: A randomized controlled trial. Int J Qual Health Care. 2013; 25(2):176-181.
2012; 7: PubMed
33. Implement Sci. 50. Rycroft-Malone J, McCormack B, Hutchinson AM, et al. Realist synthesis: Illustrating the method for implementation research. 2008; 337.BMJ. PubMed
49. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: The new medical research council guidance. 2014; 9(9):e108585.PloS one. PubMed
48. Gagliardi AR, Straus SE, Shojania KG, Urbach DR. Multiple interacting factors influence adherence, and outcomes associated with surgical safety checklists: A qualitative study.
2016; 188(9):E191-E198.CMAJ. PubMed
47. O’Leary JD, Wijeysundera DN, Crawford MW. Effect of surgical safety checklists on pediatric surgical complications in Ontario. Rockville, MD: Agency for Healthcare Research and Quality; 2013.Human factors and ergonomics. Making health care safer ii: An updated critical analysis of the evidence for patient safety practices. PubMed
46. Carayon P, Xie A, Kianfar SH. 2005; 16(1):70-76.Psychol Sci. 2004; 30(4):689-707.
45. Halford GS, Baker R, McCredden JE, Bain JD. How many variables can humans process? J Exp Psychol Hum Percept Perform. PubMed
44. Oberauer K, Kliegl R. Simultaneous cognitive operations in working memory after dual-task practice. 1956; 63(2):81-97.Psychol Rev. PubMed
43. Miller GA. The magical number seven plus or minus two: Some limits on our capacity for processing information. 2008; 20(1):22-30.Int J Qual Health Care. PubMed
42. Hales B, Terblanche M, Fowler R, Sibbald W. Development of medical checklists for improved quality of patient care. 2011; 104(12):510-520.J R Soc Med. PubMed
41. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. 2015; 24(5):325-336.BMJ Qual Saf. PubMed
40. Portela MC, Pronovost PJ, Woodcock T, Carter P, Dixon-Woods M. How to study improvement interventions: A brief overview of possible study types. Washington (DC): National Academies Press (US); 2013.Best care at lower cost: The path to continuously learning health care in america. PubMed
39. Institute of Medicine. Committee on the learning health care system in America. In: Smith M, Saunders R, Stuckhardt L, et al., eds. 2004; 240(2):205-213.Ann Surg. PubMed
38. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. 2013; 66(3):157-162.Can J Hosp Pharm. PubMed
37. Masson SC, Mabasa VH, Malyuk DL, Perrott JL. Validity evidence for fasthug-maidens, a mnemonic for identifying drug-related problems in the intensive care unit. 2015; 261(5):821-828.Ann Surg. PubMed
36. Haugen AS, Softeland E, Almeland SK, et al. Effect of the world health organization checklist on patient outcomes: A stepped wedge cluster randomized controlled trial.
2016; 12(2):199-205.Future Oncol. PubMed
35. Gentili M, Clerico M, Spizzichino M, Fanelli G. Use of a checklist to improve pain control in hospitalized cancer patients: the 38Checkpain project. 2016; 32:170-174.J Crit Care. PubMed
34. Salzwedel C, Mai V, Punke MA, Kluge S, Reuter DA. The effect of a checklist on the quality of patient handover from the operating room to the intensive care unit: A randomized controlled trial. 2016; 315(14):1480-1490.JAMA. PubMed
33. The Writing Group for CHECKLIST-ICU, Cavalcanti AB, Bozza FA, et al. Effect of a Quality Improvement Intervention With Daily Round Checklists, Goal Setting, and Clinician Prompting on Mortality of Critically Ill Patients: A Randomized Clinical Trial. PubMed
1. World Health Organization. Patient safety. Available at: http://www.who.int/patientsafety/about/en/. Accessed June 21, 2016.
2. Institute of Medicine. To err is human: Building a safer health system. In: Kohn L, Corrigan J, Donaldson M, eds. Institute of Medicine-Committee on Quality of Health Care in America. Washington DC: National Academy Press; 1999:86-101. PubMed
3. Institute of Medicine Committee on the Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington DC: National Academy Press; 2001. PubMed
4. Stelfox HT, Palmisani S, Scurlock C, Orav EJ, Bates DW. The “to err is human” report and the patient safety literature. Qual Saf Health Care. 2006; 15(3):174-178. PubMed
5. Winters BD, Gurses AP, Lehmann H, Sexton JB, Rampersad CJ, Pronovost, PJ. Clinical review: Checklists - translating evidence into practice. Crit Care. 2009; 13(6):210. PubMed
6. Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999; 159(2):439-446. PubMed
7. Gawande A. The checklist manifesto: How to get things right. Great Britain: Profile Books LTD; 2010.
8. Pronovost P, Vohr E. Safe patients, smart hospitals. New York, NY: Hudson Street Press; 2010.
9. Hughes RG. Advances in patient safety: Tools and strategies for quality improvement and patient safety. In: Hughes RG, ed. Patient safety and quality: An evidence-based handbook for nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. PubMed
10. Henriksen K, Oppenheimer C, Leape LL, et al. Envisioning patient safety in the year 2025: Eight perspectives. In: Henriksen K, Battles JB, Keyes MA, et al., eds. Advances in patient safety: New directions and alternative approaches. Rockville, MD: Agency for Healthcare Research and Quality; 2008. PubMed
11. Gaba DM, Howard SK. Patient safety: Fatigue among clinicians and the safety of patients. N Engl J Med. 2002; 347(16):1249-1255. PubMed
12. Degani A, Wiener EL. Cockpit checklists: Concepts, design, and use. Human Factors: The Journal of the Human Factors and Ergonomics Society 1993; 35(2):345-359.
13. Swain AD, Guttmann HE. Handbook of human reliability analysis with emphasis on nuclear power plant applications: Final report. Washington, DC: U.S. Nuclear Regulatroy Commission; 1983.
14. de Vries EN, Prins HA, Crolla RM, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010; 363(20):1928-1937. PubMed
15. Bergs J, Hellings J, Cleemput I, et al. Systematic review and meta-analysis of the effect of the world health organization surgical safety checklist on postoperative complications. Br J Surg. 2014; 101(3):150-158. PubMed
16. Pucher PH, Johnston MJ, Aggarwal R, Arora S, Darzi A. Effectiveness of interventions to improve patient handover in surgery: A systematic review. Surgery. 2015; 158(1):85-95. PubMed
17. Bergs J, Lambrechts F, Simons P, et al. Barriers and facilitators related to the implementation of surgical safety checklists: A systematic review of the qualitative evidence. BMJ Qual Saf. 2015; 23(12):776-786. PubMed
18. Treadwell JR, Lucas S, Tsou AY. Surgical checklists: A systematic review of impacts and implementation. BMJ Qual Saf. 2014; 23(4):299-318. PubMed
19. Gillespie BM, Chaboyer W, Thalib L, John M, Fairweather N, Slater K. Effect of using a safety checklist on patient complications after surgery: A systematic review and meta-analysis. Anesthesiology. 2014; 120(6):1380-1389. PubMed
20. Reames BN, Krell RW, Campbell DA Jr, Dimick JB. A checklist-based intervention to improve surgical outcomes in michigan: Evaluation of the keystone surgery program. JAMA Surg. 2015; 150(3):208-215. PubMed
21. Chaudhary N, Varma V, Kapoor S, Mehta N, Kumaran V, Nundy S. Implementation of a surgical safety checklist and postoperative outcomes: A prospective randomized controlled study. J Gastrointest Surg. 2015; 19(5):935-942. PubMed
22. Reames BN, Krell RW, Campbell DA, Jr., Dimick JB. A checklist-based intervention to improve surgical outcomes in Michigan: Evaluation of the Keystone Surgery program. JAMA surgery. 2015; 150(3):208-215. PubMed
23. Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009; 151(4):W65-94. PubMed
24. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Oxford, UK: The Cochrane Collaboration, 2011.
25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159-174. PubMed
26. Berlin JA. Does blinding of readers affect the results of meta-analyses? University of pennsylvania meta-analysis blinding study group. Lancet 1997;350(9072):185-186. PubMed
27. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17(1):1-12. PubMed
28. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews: A product form the esrc methods programme. Available at: https://www.researchgate.net/profile/Mark_Rodgers4/publication/233866356_Guidance_on_the_conduct_of_narrative_synthesis_in_systematic_reviews_A_product_from_the_ESRC_Methods_Programme/links/02e7e5231e8f3a6183000000.pdf. Accessed June 17, 2016.
29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177-188. PubMed
30. Basoor A, Doshi NC, Cotant JF, et al. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013; 19(4):200-206. PubMed
31. Ong MS, Magrabi F, Post J, et al. Communication interventions to improve adherence to infection control precautions: A randomised crossover trial. BMC Infect Dis. 2013; 13:72. PubMed
32. Salzwedel C, Bartz HJ, Kuhnelt I, et al. The effect of a checklist on the quality of post-anaesthesia patient handover: A randomized controlled trial. Int J Qual Health Care. 2013; 25(2):176-181.
2012; 7: PubMed
33. Implement Sci. 50. Rycroft-Malone J, McCormack B, Hutchinson AM, et al. Realist synthesis: Illustrating the method for implementation research. 2008; 337.BMJ. PubMed
49. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: The new medical research council guidance. 2014; 9(9):e108585.PloS one. PubMed
48. Gagliardi AR, Straus SE, Shojania KG, Urbach DR. Multiple interacting factors influence adherence, and outcomes associated with surgical safety checklists: A qualitative study.
2016; 188(9):E191-E198.CMAJ. PubMed
47. O’Leary JD, Wijeysundera DN, Crawford MW. Effect of surgical safety checklists on pediatric surgical complications in Ontario. Rockville, MD: Agency for Healthcare Research and Quality; 2013.Human factors and ergonomics. Making health care safer ii: An updated critical analysis of the evidence for patient safety practices. PubMed
46. Carayon P, Xie A, Kianfar SH. 2005; 16(1):70-76.Psychol Sci. 2004; 30(4):689-707.
45. Halford GS, Baker R, McCredden JE, Bain JD. How many variables can humans process? J Exp Psychol Hum Percept Perform. PubMed
44. Oberauer K, Kliegl R. Simultaneous cognitive operations in working memory after dual-task practice. 1956; 63(2):81-97.Psychol Rev. PubMed
43. Miller GA. The magical number seven plus or minus two: Some limits on our capacity for processing information. 2008; 20(1):22-30.Int J Qual Health Care. PubMed
42. Hales B, Terblanche M, Fowler R, Sibbald W. Development of medical checklists for improved quality of patient care. 2011; 104(12):510-520.J R Soc Med. PubMed
41. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. 2015; 24(5):325-336.BMJ Qual Saf. PubMed
40. Portela MC, Pronovost PJ, Woodcock T, Carter P, Dixon-Woods M. How to study improvement interventions: A brief overview of possible study types. Washington (DC): National Academies Press (US); 2013.Best care at lower cost: The path to continuously learning health care in america. PubMed
39. Institute of Medicine. Committee on the learning health care system in America. In: Smith M, Saunders R, Stuckhardt L, et al., eds. 2004; 240(2):205-213.Ann Surg. PubMed
38. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. 2013; 66(3):157-162.Can J Hosp Pharm. PubMed
37. Masson SC, Mabasa VH, Malyuk DL, Perrott JL. Validity evidence for fasthug-maidens, a mnemonic for identifying drug-related problems in the intensive care unit. 2015; 261(5):821-828.Ann Surg. PubMed
36. Haugen AS, Softeland E, Almeland SK, et al. Effect of the world health organization checklist on patient outcomes: A stepped wedge cluster randomized controlled trial.
2016; 12(2):199-205.Future Oncol. PubMed
35. Gentili M, Clerico M, Spizzichino M, Fanelli G. Use of a checklist to improve pain control in hospitalized cancer patients: the 38Checkpain project. 2016; 32:170-174.J Crit Care. PubMed
34. Salzwedel C, Mai V, Punke MA, Kluge S, Reuter DA. The effect of a checklist on the quality of patient handover from the operating room to the intensive care unit: A randomized controlled trial. 2016; 315(14):1480-1490.JAMA. PubMed
33. The Writing Group for CHECKLIST-ICU, Cavalcanti AB, Bozza FA, et al. Effect of a Quality Improvement Intervention With Daily Round Checklists, Goal Setting, and Clinician Prompting on Mortality of Critically Ill Patients: A Randomized Clinical Trial. PubMed
© 2017 Society of Hospital Medicine