User login
Rowell Syndrome: Targeting a True Definition
Case Report
A 37-year-old woman was admitted to the intensive care unit secondary to the acute development of an erythematous rash with tissue sloughing that involved acral sites and mucosal surfaces. Her medical history was notable for anti-Ro/Sjögren syndrome antigen A (SS-A)–positive lupus erythematosus (LE) with a morphologic semblance to subacute cutaneous LE (SCLE). Prior treatment had included oral corticosteroids. In addition, she reported a concurrent history of acral and mucosal lesions that appeared to flare with her lupus. The nature of these lesions was not clear to the patient or her physicians. Before this particular episode, her primary care physician had attempted to wean her off of the corticosteroids. As she dropped below 20 mg of prednisone daily, new lesions developed. The patient stated that her social situation was poor and that these lesions did seem to develop more frequently during times of physical and emotional stress. She recounted her first episode developing during her second pregnancy. Oral prednisone and over-the-counter calcium with vitamin D were her only reported medications. She denied the use of any other medications, including nonsteroidal anti-inflammatory drugs, acetaminophen, and recent antibiotic therapy.
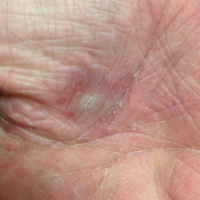
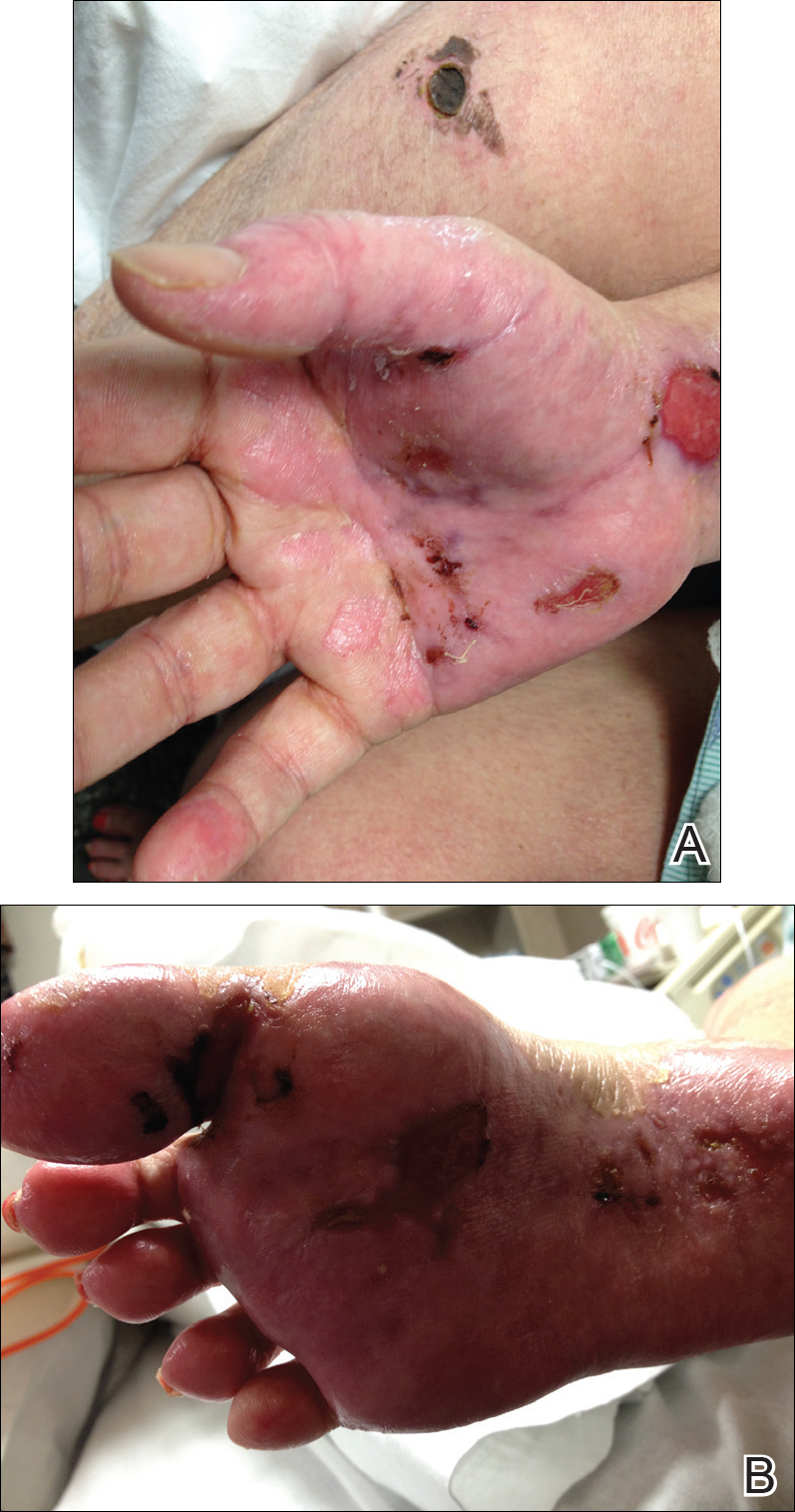
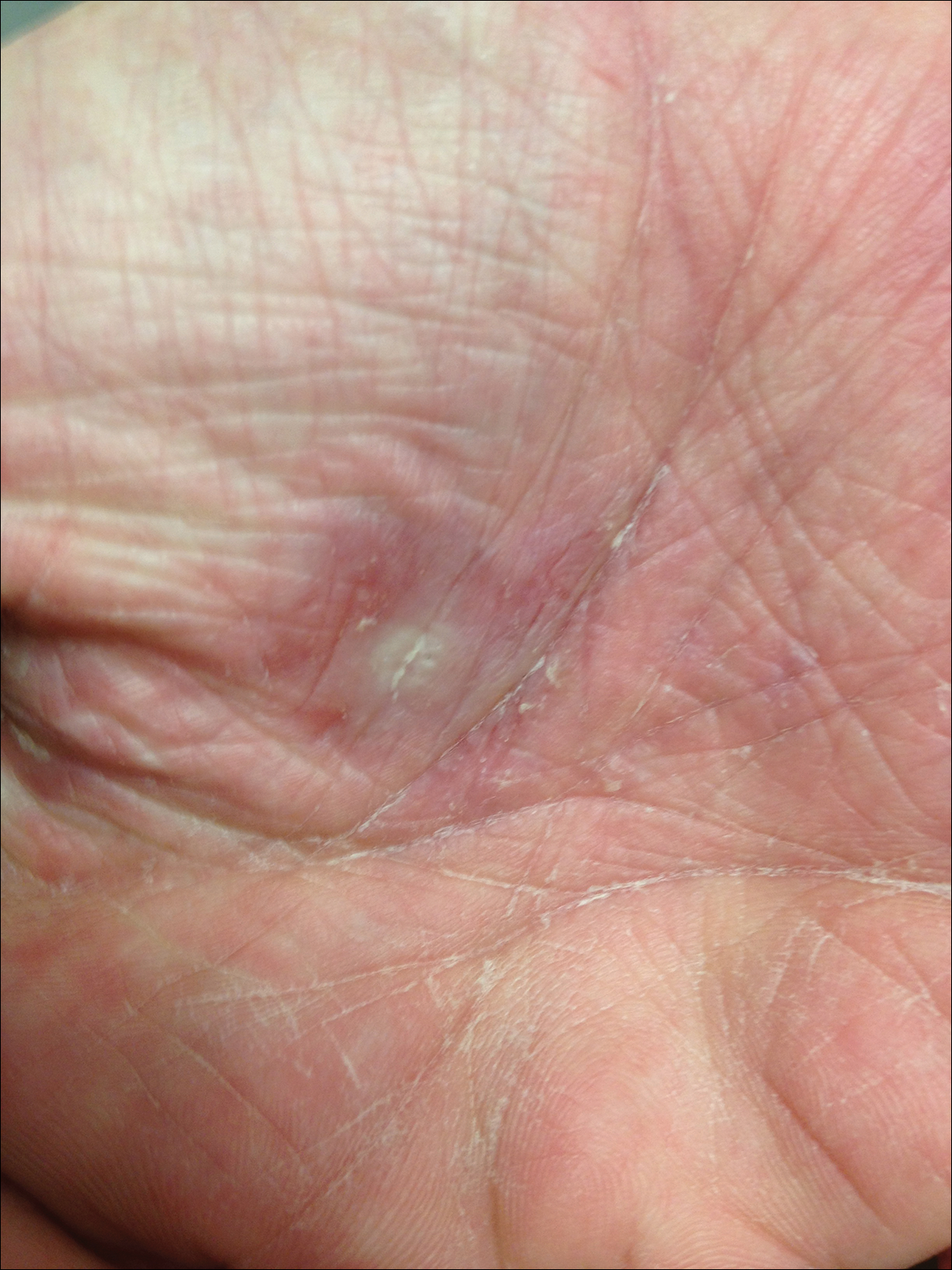
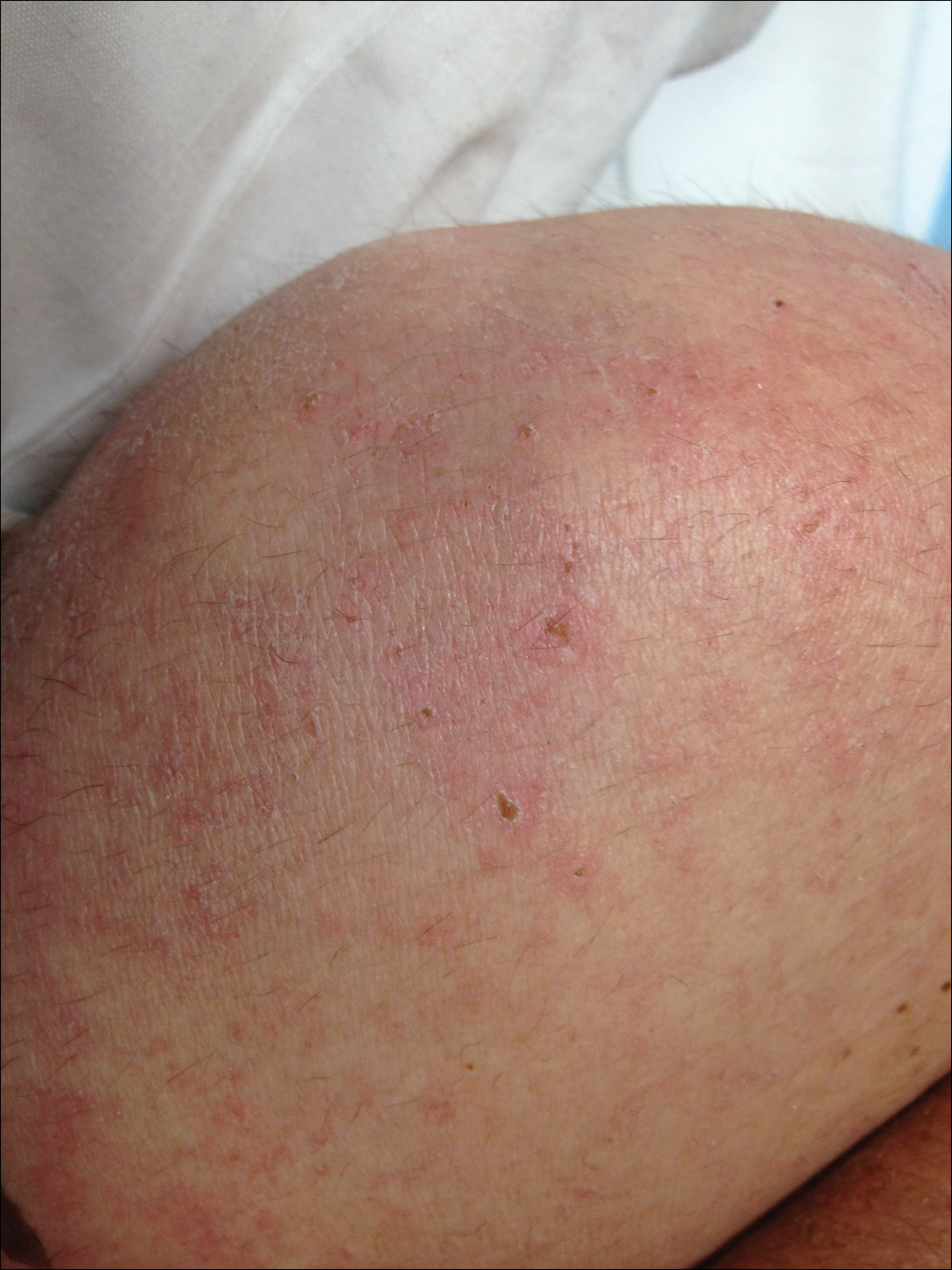
Dermatology was called in for consultation, and physical examination revealed areas of epidermal sloughing on the hands and feet. Complete clinical exposure of the underlying dermis was noted with remarkable tenderness. These lesions were noted to be in various stages of healing (Figure 1). Figure 2 displays a lesion in early development. The mucosal surfaces of the lips and eyes demonstrated hemorrhagic crusting, and some tissue sloughing was noted on the ears. A widespread erythematous exanthema with fine scaling was noted on the face, neck, chest, back, abdomen, arms, and legs (Figure 3).
Laboratory evaluation revealed positive antinuclear antibodies (ANAs), anti-Ro/SS-A antibodies, anti-La/Sjögren syndrome antigen B (SS-B) antibodies, and anti–double-stranded DNA. The hemoglobin level was 9.4 g/dL (reference range, 12–15 g/dL) and hematocrit was 28.8% (reference range, 36%–47%). The mean corpuscular hemoglobin level was 32 pg/cell (reference range, 27–31 pg/cell), and the mean corpuscular hemoglobin concentration was 32.5 g/dL (reference range, 30–35 g/dL). Rheumatoid factor (RF) and herpes simplex virus types 1 and 2 IgM were all found to be negative.
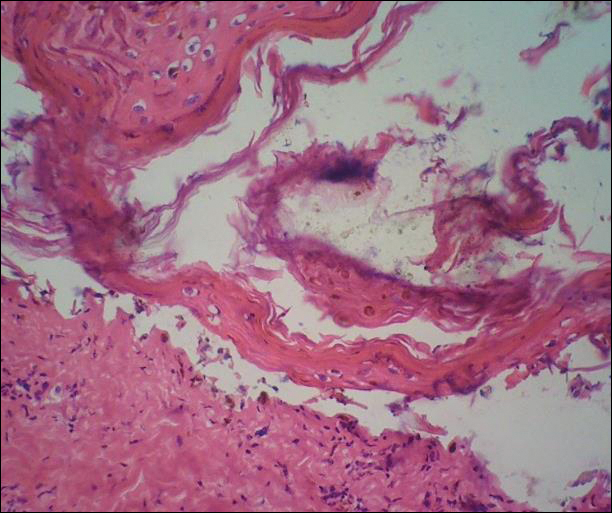
A deep shave biopsy obtained from the patient’s right knee revealed an atrophic interface dermatitis associated with a lymphocytic eccrine hidradenitis accompanied by abundant mesenchymal mucin deposition (Figure 4). Direct immunofluorescence (DIF) from the same area demonstrated IgG and IgM along the dermoepidermal junction with some granular deposition. Frozen sections performed on acral lesions demonstrated epidermal necrosis (Figure 5). Direct immunofluorescence of acral lesions was negative. In light of these findings, a diagnosis of Rowell syndrome (RS) was suspected to be the most likely explanation for the presentation.
Intravenous corticosteroids and antibiotics were administered, and over a 2-week hospitalization, the lesions on the feet and hands slowly reepithelialized. Physical therapy was required to aid in ambulation. The patient was discharged on a tapering course of oral prednisone and hydroxychloroquine. After 6 months of therapy with hydroxychloroquine 200 mg twice daily, the patient continued to experience recurrent bouts of acral lesions, and pulse doses of oral prednisone were required. The lesions currently are controlled with azathioprine 50 mg twice daily and prednisone 10 mg by mouth daily.

Comment
The 4 prototypical patients identified by Rowell et al1 in 1963 in the first account of the eponymous syndrome were all females with discoid lupus erythematosus (DLE) and perniosis. In addition, they all displayed positive RF and saline extract of human tissue antibodies (analogous to anti-Ro/SS-A and anti-La/SS-B).2 Since then, at least 132 patients with clinical symptoms suspicious of RS have been identified with variations on these original criteria.3 The reported permutations of the lupus component of the disease include cutaneous LE (CLE), bullous systemic LE, necrotic lesions associated with antiphospholipid syndrome, annular/polycyclic SCLE, systemic LE (SLE) without CLE, SLE with lupus nephritis, SLE with pericarditis, SLE with systemic vasculitis, Sjögren syndrome, rheumatoid arthritis, and necrotizing lymphadenitis.2 In addition, variations of the erythema multiforme (EM)–like lesions found in reported cases include changes to their gross appearance (flat vs raised), location (acral or mucosal involvement), and resemblance to other conditions (Stevens-Johnson syndrome or toxic epidermal necrolysis).2,3 From this information alone, it is clear that, as further cases have been chronicled, defining exact criteria for the disease has been challenging.
The essential question concerning the existence of RS hinges on the strength of its distinctiveness: Is it a unique disorder or merely another variant of lupus? Antiga et al2 concluded that it should be characterized as a variant of SCLE. Lee at al4 agreed, stating that “[i]n view of the lack of specific features that distinguish RS from LE, Kuhn et al5 suggested that [RS] is probably not a distinct entity and is now widely considered to be a variant of SCLE.” One of the primary contributors to this conclusion is that the laboratory findings of reported patients with SCLE have more closely mirrored the original cases from Rowell et al’s1 report than those of typical LE. Patients with SCLE have demonstrated positive ANA antibodies in 60% to 80% of cases, positive anti-Ro/SS-A antibodies in 40% to 100% of cases, positive anti-La/SS-B antibodies in 12% to 42% of cases, positive anti–double-stranded DNA in 1.2% to 10% of cases, and positive RF antibodies in 33% of cases.2 An argument could certainly be made to ascribe our patient’s condition to an SCLE variant, as 4 of 5 preceding laboratory findings were found to be positive; however, the majority of reported cases of SCLE have been linked to drugs (ie, hydrochlorothiazide, angiotensin-converting enzyme inhibitors, calcium channel blockers, terbinafine),2 which has not commonly been the attributable etiology of other cases of RS, including the 4 cases reported by Rowell et al.1
In a review of the literature on RS since 2010 in addition to their report of 132 new cases, Torchia et al3 outlined a set of diagnostic standards for the condition consisting of major and minor criteria. According to the authors, if all 4 major and 1 minor criteria are met, the patient meets the standards for true RS. The major criteria include the following: (1) presence of chronic CLE [DLE and/or chilblain]; (2) presence of EM-like lesions [typical or atypical targets]; (3) at least 1 positivity among speckled ANA, anti-Ro/SS-A, and anti-La/SS-B antibodies; and (4) negative DIF on lesional EM-like targetoid lesions. The minor criteria include the following: (1) absence of infectious or pharmacologic triggers; (2) absence of typical EM location (acral and mucosal); and (3) presence of at least 1 additional American College of Rheumatology criterion for diagnosis of SLE8 besides discoid rash and positive ANA antibodies and excluding photosensitivity, malar rash, and oral ulcers. Using these criteria, the patient in our case met the standards for diagnosis of RS.
One area of disagreement that has been encountered in the literature is the exact histologic determination of true RS, specifically related to the microscopic findings of the EM-like lesions. Two cases presented by Modi et al6 were interpreted under the stipulation that true RS must contain histologic LE and histologic EM. Because the EM-appearing lesions revealed LE histology, the cases were concluded to be variants of LE. These cases are similar to our case in that the EM-like lesions in our patient demonstrated LE pathology. Torchia et al,3 as demonstrated in the above criteria, seemed to be less concerned about the histology of the EM-like lesions, only requiring them to show negative DIF.
Conclusion
In the search for answers concerning RS, many unanswered questions remain: Where should the line be drawn in the inclusion of so many variations of both the LE and EM components of the condition? Also, should these elements even be approached as distinct components in the first place? Viewing the majority of RS cases as simply simultaneous LE and EM, Shteyngarts et al7 concluded that “the concomitant occurrence of EM with LE did not change the course, therapy, or prognosis of either disease. SLE and DLE can coexist with EM, but the coexistence does not impart any unusual characteristic to either illness. Rowell’s syndrome is not reproducible, and the immunologic disturbances in such patients are probably coincidental.”
If the condition is a genuine pathological individuality, should we not view the seemingly separate LE and EM as the product of a single underlying biochemical process? These questions and others in the search for a true definition of the disease should continue to be debated. It is clear that further investigation is warranted in the understanding of the underlying mechanism of the pathology.
- Rowell NR, Beck JS, Anderson JR. Lupus erythematosus and erythema multiforme-like lesions: a syndrome with characteristic immunological abnormalities. Arch Dermatol. 1963;88:176-180.
- Antiga E, Caproni M, Bonciani D, et al. The last word on the so-called ‘Rowell’s syndrome’? Lupus. 2012;21:577-585.
- Torchia D, Romanelli P, Kerdel FA. Erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis associated with lupus erythematosus. J Am Acad Dermatol. 2012;67:417-421.
- Lee A, Batra P, Furer V, et al. Rowell syndrome (systemic lupus erythematosus + erythema multiforme). Dermatol Online J. 2009;15:1.
- Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124-1140.
- Modi GM, Shen A, Mazloom A, et al. Lupus erythematosus masquerading as erythema multiforme: does Rowell syndrome really exist? Dermatol Online J. 2009;15:5.
- Shteyngarts AR, Warner MR, Camisa C. Lupus erythematosus associated with erythema multiforme: does Rowell’s syndrome exist? J Am Acad Dermatol. 1999;40(5 pt 1):773-777.
- Lupus diagnosis. Lupus Research Alliance website. http://lupusresearchinstitute.org/lupus-facts/lupus-diagnosis. Accessed July 11, 2017.
Case Report
A 37-year-old woman was admitted to the intensive care unit secondary to the acute development of an erythematous rash with tissue sloughing that involved acral sites and mucosal surfaces. Her medical history was notable for anti-Ro/Sjögren syndrome antigen A (SS-A)–positive lupus erythematosus (LE) with a morphologic semblance to subacute cutaneous LE (SCLE). Prior treatment had included oral corticosteroids. In addition, she reported a concurrent history of acral and mucosal lesions that appeared to flare with her lupus. The nature of these lesions was not clear to the patient or her physicians. Before this particular episode, her primary care physician had attempted to wean her off of the corticosteroids. As she dropped below 20 mg of prednisone daily, new lesions developed. The patient stated that her social situation was poor and that these lesions did seem to develop more frequently during times of physical and emotional stress. She recounted her first episode developing during her second pregnancy. Oral prednisone and over-the-counter calcium with vitamin D were her only reported medications. She denied the use of any other medications, including nonsteroidal anti-inflammatory drugs, acetaminophen, and recent antibiotic therapy.
Dermatology was called in for consultation, and physical examination revealed areas of epidermal sloughing on the hands and feet. Complete clinical exposure of the underlying dermis was noted with remarkable tenderness. These lesions were noted to be in various stages of healing (Figure 1). Figure 2 displays a lesion in early development. The mucosal surfaces of the lips and eyes demonstrated hemorrhagic crusting, and some tissue sloughing was noted on the ears. A widespread erythematous exanthema with fine scaling was noted on the face, neck, chest, back, abdomen, arms, and legs (Figure 3).



Laboratory evaluation revealed positive antinuclear antibodies (ANAs), anti-Ro/SS-A antibodies, anti-La/Sjögren syndrome antigen B (SS-B) antibodies, and anti–double-stranded DNA. The hemoglobin level was 9.4 g/dL (reference range, 12–15 g/dL) and hematocrit was 28.8% (reference range, 36%–47%). The mean corpuscular hemoglobin level was 32 pg/cell (reference range, 27–31 pg/cell), and the mean corpuscular hemoglobin concentration was 32.5 g/dL (reference range, 30–35 g/dL). Rheumatoid factor (RF) and herpes simplex virus types 1 and 2 IgM were all found to be negative.
A deep shave biopsy obtained from the patient’s right knee revealed an atrophic interface dermatitis associated with a lymphocytic eccrine hidradenitis accompanied by abundant mesenchymal mucin deposition (Figure 4). Direct immunofluorescence (DIF) from the same area demonstrated IgG and IgM along the dermoepidermal junction with some granular deposition. Frozen sections performed on acral lesions demonstrated epidermal necrosis (Figure 5). Direct immunofluorescence of acral lesions was negative. In light of these findings, a diagnosis of Rowell syndrome (RS) was suspected to be the most likely explanation for the presentation.
Intravenous corticosteroids and antibiotics were administered, and over a 2-week hospitalization, the lesions on the feet and hands slowly reepithelialized. Physical therapy was required to aid in ambulation. The patient was discharged on a tapering course of oral prednisone and hydroxychloroquine. After 6 months of therapy with hydroxychloroquine 200 mg twice daily, the patient continued to experience recurrent bouts of acral lesions, and pulse doses of oral prednisone were required. The lesions currently are controlled with azathioprine 50 mg twice daily and prednisone 10 mg by mouth daily.


Comment
The 4 prototypical patients identified by Rowell et al1 in 1963 in the first account of the eponymous syndrome were all females with discoid lupus erythematosus (DLE) and perniosis. In addition, they all displayed positive RF and saline extract of human tissue antibodies (analogous to anti-Ro/SS-A and anti-La/SS-B).2 Since then, at least 132 patients with clinical symptoms suspicious of RS have been identified with variations on these original criteria.3 The reported permutations of the lupus component of the disease include cutaneous LE (CLE), bullous systemic LE, necrotic lesions associated with antiphospholipid syndrome, annular/polycyclic SCLE, systemic LE (SLE) without CLE, SLE with lupus nephritis, SLE with pericarditis, SLE with systemic vasculitis, Sjögren syndrome, rheumatoid arthritis, and necrotizing lymphadenitis.2 In addition, variations of the erythema multiforme (EM)–like lesions found in reported cases include changes to their gross appearance (flat vs raised), location (acral or mucosal involvement), and resemblance to other conditions (Stevens-Johnson syndrome or toxic epidermal necrolysis).2,3 From this information alone, it is clear that, as further cases have been chronicled, defining exact criteria for the disease has been challenging.
The essential question concerning the existence of RS hinges on the strength of its distinctiveness: Is it a unique disorder or merely another variant of lupus? Antiga et al2 concluded that it should be characterized as a variant of SCLE. Lee at al4 agreed, stating that “[i]n view of the lack of specific features that distinguish RS from LE, Kuhn et al5 suggested that [RS] is probably not a distinct entity and is now widely considered to be a variant of SCLE.” One of the primary contributors to this conclusion is that the laboratory findings of reported patients with SCLE have more closely mirrored the original cases from Rowell et al’s1 report than those of typical LE. Patients with SCLE have demonstrated positive ANA antibodies in 60% to 80% of cases, positive anti-Ro/SS-A antibodies in 40% to 100% of cases, positive anti-La/SS-B antibodies in 12% to 42% of cases, positive anti–double-stranded DNA in 1.2% to 10% of cases, and positive RF antibodies in 33% of cases.2 An argument could certainly be made to ascribe our patient’s condition to an SCLE variant, as 4 of 5 preceding laboratory findings were found to be positive; however, the majority of reported cases of SCLE have been linked to drugs (ie, hydrochlorothiazide, angiotensin-converting enzyme inhibitors, calcium channel blockers, terbinafine),2 which has not commonly been the attributable etiology of other cases of RS, including the 4 cases reported by Rowell et al.1
In a review of the literature on RS since 2010 in addition to their report of 132 new cases, Torchia et al3 outlined a set of diagnostic standards for the condition consisting of major and minor criteria. According to the authors, if all 4 major and 1 minor criteria are met, the patient meets the standards for true RS. The major criteria include the following: (1) presence of chronic CLE [DLE and/or chilblain]; (2) presence of EM-like lesions [typical or atypical targets]; (3) at least 1 positivity among speckled ANA, anti-Ro/SS-A, and anti-La/SS-B antibodies; and (4) negative DIF on lesional EM-like targetoid lesions. The minor criteria include the following: (1) absence of infectious or pharmacologic triggers; (2) absence of typical EM location (acral and mucosal); and (3) presence of at least 1 additional American College of Rheumatology criterion for diagnosis of SLE8 besides discoid rash and positive ANA antibodies and excluding photosensitivity, malar rash, and oral ulcers. Using these criteria, the patient in our case met the standards for diagnosis of RS.
One area of disagreement that has been encountered in the literature is the exact histologic determination of true RS, specifically related to the microscopic findings of the EM-like lesions. Two cases presented by Modi et al6 were interpreted under the stipulation that true RS must contain histologic LE and histologic EM. Because the EM-appearing lesions revealed LE histology, the cases were concluded to be variants of LE. These cases are similar to our case in that the EM-like lesions in our patient demonstrated LE pathology. Torchia et al,3 as demonstrated in the above criteria, seemed to be less concerned about the histology of the EM-like lesions, only requiring them to show negative DIF.
Conclusion
In the search for answers concerning RS, many unanswered questions remain: Where should the line be drawn in the inclusion of so many variations of both the LE and EM components of the condition? Also, should these elements even be approached as distinct components in the first place? Viewing the majority of RS cases as simply simultaneous LE and EM, Shteyngarts et al7 concluded that “the concomitant occurrence of EM with LE did not change the course, therapy, or prognosis of either disease. SLE and DLE can coexist with EM, but the coexistence does not impart any unusual characteristic to either illness. Rowell’s syndrome is not reproducible, and the immunologic disturbances in such patients are probably coincidental.”
If the condition is a genuine pathological individuality, should we not view the seemingly separate LE and EM as the product of a single underlying biochemical process? These questions and others in the search for a true definition of the disease should continue to be debated. It is clear that further investigation is warranted in the understanding of the underlying mechanism of the pathology.
Case Report
A 37-year-old woman was admitted to the intensive care unit secondary to the acute development of an erythematous rash with tissue sloughing that involved acral sites and mucosal surfaces. Her medical history was notable for anti-Ro/Sjögren syndrome antigen A (SS-A)–positive lupus erythematosus (LE) with a morphologic semblance to subacute cutaneous LE (SCLE). Prior treatment had included oral corticosteroids. In addition, she reported a concurrent history of acral and mucosal lesions that appeared to flare with her lupus. The nature of these lesions was not clear to the patient or her physicians. Before this particular episode, her primary care physician had attempted to wean her off of the corticosteroids. As she dropped below 20 mg of prednisone daily, new lesions developed. The patient stated that her social situation was poor and that these lesions did seem to develop more frequently during times of physical and emotional stress. She recounted her first episode developing during her second pregnancy. Oral prednisone and over-the-counter calcium with vitamin D were her only reported medications. She denied the use of any other medications, including nonsteroidal anti-inflammatory drugs, acetaminophen, and recent antibiotic therapy.
Dermatology was called in for consultation, and physical examination revealed areas of epidermal sloughing on the hands and feet. Complete clinical exposure of the underlying dermis was noted with remarkable tenderness. These lesions were noted to be in various stages of healing (Figure 1). Figure 2 displays a lesion in early development. The mucosal surfaces of the lips and eyes demonstrated hemorrhagic crusting, and some tissue sloughing was noted on the ears. A widespread erythematous exanthema with fine scaling was noted on the face, neck, chest, back, abdomen, arms, and legs (Figure 3).



Laboratory evaluation revealed positive antinuclear antibodies (ANAs), anti-Ro/SS-A antibodies, anti-La/Sjögren syndrome antigen B (SS-B) antibodies, and anti–double-stranded DNA. The hemoglobin level was 9.4 g/dL (reference range, 12–15 g/dL) and hematocrit was 28.8% (reference range, 36%–47%). The mean corpuscular hemoglobin level was 32 pg/cell (reference range, 27–31 pg/cell), and the mean corpuscular hemoglobin concentration was 32.5 g/dL (reference range, 30–35 g/dL). Rheumatoid factor (RF) and herpes simplex virus types 1 and 2 IgM were all found to be negative.
A deep shave biopsy obtained from the patient’s right knee revealed an atrophic interface dermatitis associated with a lymphocytic eccrine hidradenitis accompanied by abundant mesenchymal mucin deposition (Figure 4). Direct immunofluorescence (DIF) from the same area demonstrated IgG and IgM along the dermoepidermal junction with some granular deposition. Frozen sections performed on acral lesions demonstrated epidermal necrosis (Figure 5). Direct immunofluorescence of acral lesions was negative. In light of these findings, a diagnosis of Rowell syndrome (RS) was suspected to be the most likely explanation for the presentation.
Intravenous corticosteroids and antibiotics were administered, and over a 2-week hospitalization, the lesions on the feet and hands slowly reepithelialized. Physical therapy was required to aid in ambulation. The patient was discharged on a tapering course of oral prednisone and hydroxychloroquine. After 6 months of therapy with hydroxychloroquine 200 mg twice daily, the patient continued to experience recurrent bouts of acral lesions, and pulse doses of oral prednisone were required. The lesions currently are controlled with azathioprine 50 mg twice daily and prednisone 10 mg by mouth daily.


Comment
The 4 prototypical patients identified by Rowell et al1 in 1963 in the first account of the eponymous syndrome were all females with discoid lupus erythematosus (DLE) and perniosis. In addition, they all displayed positive RF and saline extract of human tissue antibodies (analogous to anti-Ro/SS-A and anti-La/SS-B).2 Since then, at least 132 patients with clinical symptoms suspicious of RS have been identified with variations on these original criteria.3 The reported permutations of the lupus component of the disease include cutaneous LE (CLE), bullous systemic LE, necrotic lesions associated with antiphospholipid syndrome, annular/polycyclic SCLE, systemic LE (SLE) without CLE, SLE with lupus nephritis, SLE with pericarditis, SLE with systemic vasculitis, Sjögren syndrome, rheumatoid arthritis, and necrotizing lymphadenitis.2 In addition, variations of the erythema multiforme (EM)–like lesions found in reported cases include changes to their gross appearance (flat vs raised), location (acral or mucosal involvement), and resemblance to other conditions (Stevens-Johnson syndrome or toxic epidermal necrolysis).2,3 From this information alone, it is clear that, as further cases have been chronicled, defining exact criteria for the disease has been challenging.
The essential question concerning the existence of RS hinges on the strength of its distinctiveness: Is it a unique disorder or merely another variant of lupus? Antiga et al2 concluded that it should be characterized as a variant of SCLE. Lee at al4 agreed, stating that “[i]n view of the lack of specific features that distinguish RS from LE, Kuhn et al5 suggested that [RS] is probably not a distinct entity and is now widely considered to be a variant of SCLE.” One of the primary contributors to this conclusion is that the laboratory findings of reported patients with SCLE have more closely mirrored the original cases from Rowell et al’s1 report than those of typical LE. Patients with SCLE have demonstrated positive ANA antibodies in 60% to 80% of cases, positive anti-Ro/SS-A antibodies in 40% to 100% of cases, positive anti-La/SS-B antibodies in 12% to 42% of cases, positive anti–double-stranded DNA in 1.2% to 10% of cases, and positive RF antibodies in 33% of cases.2 An argument could certainly be made to ascribe our patient’s condition to an SCLE variant, as 4 of 5 preceding laboratory findings were found to be positive; however, the majority of reported cases of SCLE have been linked to drugs (ie, hydrochlorothiazide, angiotensin-converting enzyme inhibitors, calcium channel blockers, terbinafine),2 which has not commonly been the attributable etiology of other cases of RS, including the 4 cases reported by Rowell et al.1
In a review of the literature on RS since 2010 in addition to their report of 132 new cases, Torchia et al3 outlined a set of diagnostic standards for the condition consisting of major and minor criteria. According to the authors, if all 4 major and 1 minor criteria are met, the patient meets the standards for true RS. The major criteria include the following: (1) presence of chronic CLE [DLE and/or chilblain]; (2) presence of EM-like lesions [typical or atypical targets]; (3) at least 1 positivity among speckled ANA, anti-Ro/SS-A, and anti-La/SS-B antibodies; and (4) negative DIF on lesional EM-like targetoid lesions. The minor criteria include the following: (1) absence of infectious or pharmacologic triggers; (2) absence of typical EM location (acral and mucosal); and (3) presence of at least 1 additional American College of Rheumatology criterion for diagnosis of SLE8 besides discoid rash and positive ANA antibodies and excluding photosensitivity, malar rash, and oral ulcers. Using these criteria, the patient in our case met the standards for diagnosis of RS.
One area of disagreement that has been encountered in the literature is the exact histologic determination of true RS, specifically related to the microscopic findings of the EM-like lesions. Two cases presented by Modi et al6 were interpreted under the stipulation that true RS must contain histologic LE and histologic EM. Because the EM-appearing lesions revealed LE histology, the cases were concluded to be variants of LE. These cases are similar to our case in that the EM-like lesions in our patient demonstrated LE pathology. Torchia et al,3 as demonstrated in the above criteria, seemed to be less concerned about the histology of the EM-like lesions, only requiring them to show negative DIF.
Conclusion
In the search for answers concerning RS, many unanswered questions remain: Where should the line be drawn in the inclusion of so many variations of both the LE and EM components of the condition? Also, should these elements even be approached as distinct components in the first place? Viewing the majority of RS cases as simply simultaneous LE and EM, Shteyngarts et al7 concluded that “the concomitant occurrence of EM with LE did not change the course, therapy, or prognosis of either disease. SLE and DLE can coexist with EM, but the coexistence does not impart any unusual characteristic to either illness. Rowell’s syndrome is not reproducible, and the immunologic disturbances in such patients are probably coincidental.”
If the condition is a genuine pathological individuality, should we not view the seemingly separate LE and EM as the product of a single underlying biochemical process? These questions and others in the search for a true definition of the disease should continue to be debated. It is clear that further investigation is warranted in the understanding of the underlying mechanism of the pathology.
- Rowell NR, Beck JS, Anderson JR. Lupus erythematosus and erythema multiforme-like lesions: a syndrome with characteristic immunological abnormalities. Arch Dermatol. 1963;88:176-180.
- Antiga E, Caproni M, Bonciani D, et al. The last word on the so-called ‘Rowell’s syndrome’? Lupus. 2012;21:577-585.
- Torchia D, Romanelli P, Kerdel FA. Erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis associated with lupus erythematosus. J Am Acad Dermatol. 2012;67:417-421.
- Lee A, Batra P, Furer V, et al. Rowell syndrome (systemic lupus erythematosus + erythema multiforme). Dermatol Online J. 2009;15:1.
- Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124-1140.
- Modi GM, Shen A, Mazloom A, et al. Lupus erythematosus masquerading as erythema multiforme: does Rowell syndrome really exist? Dermatol Online J. 2009;15:5.
- Shteyngarts AR, Warner MR, Camisa C. Lupus erythematosus associated with erythema multiforme: does Rowell’s syndrome exist? J Am Acad Dermatol. 1999;40(5 pt 1):773-777.
- Lupus diagnosis. Lupus Research Alliance website. http://lupusresearchinstitute.org/lupus-facts/lupus-diagnosis. Accessed July 11, 2017.
- Rowell NR, Beck JS, Anderson JR. Lupus erythematosus and erythema multiforme-like lesions: a syndrome with characteristic immunological abnormalities. Arch Dermatol. 1963;88:176-180.
- Antiga E, Caproni M, Bonciani D, et al. The last word on the so-called ‘Rowell’s syndrome’? Lupus. 2012;21:577-585.
- Torchia D, Romanelli P, Kerdel FA. Erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis associated with lupus erythematosus. J Am Acad Dermatol. 2012;67:417-421.
- Lee A, Batra P, Furer V, et al. Rowell syndrome (systemic lupus erythematosus + erythema multiforme). Dermatol Online J. 2009;15:1.
- Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124-1140.
- Modi GM, Shen A, Mazloom A, et al. Lupus erythematosus masquerading as erythema multiforme: does Rowell syndrome really exist? Dermatol Online J. 2009;15:5.
- Shteyngarts AR, Warner MR, Camisa C. Lupus erythematosus associated with erythema multiforme: does Rowell’s syndrome exist? J Am Acad Dermatol. 1999;40(5 pt 1):773-777.
- Lupus diagnosis. Lupus Research Alliance website. http://lupusresearchinstitute.org/lupus-facts/lupus-diagnosis. Accessed July 11, 2017.
Practice Points
- Rowell syndrome (RS) is an often unrecognized unique presentation of lupus erythematosus.
- There have been a variety of historical criteria that have sought to characterize RS.
Chronic Diffuse Erythematous Papulonodules
The Diagnosis: Lymphomatoid Papulosis
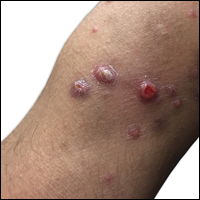
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
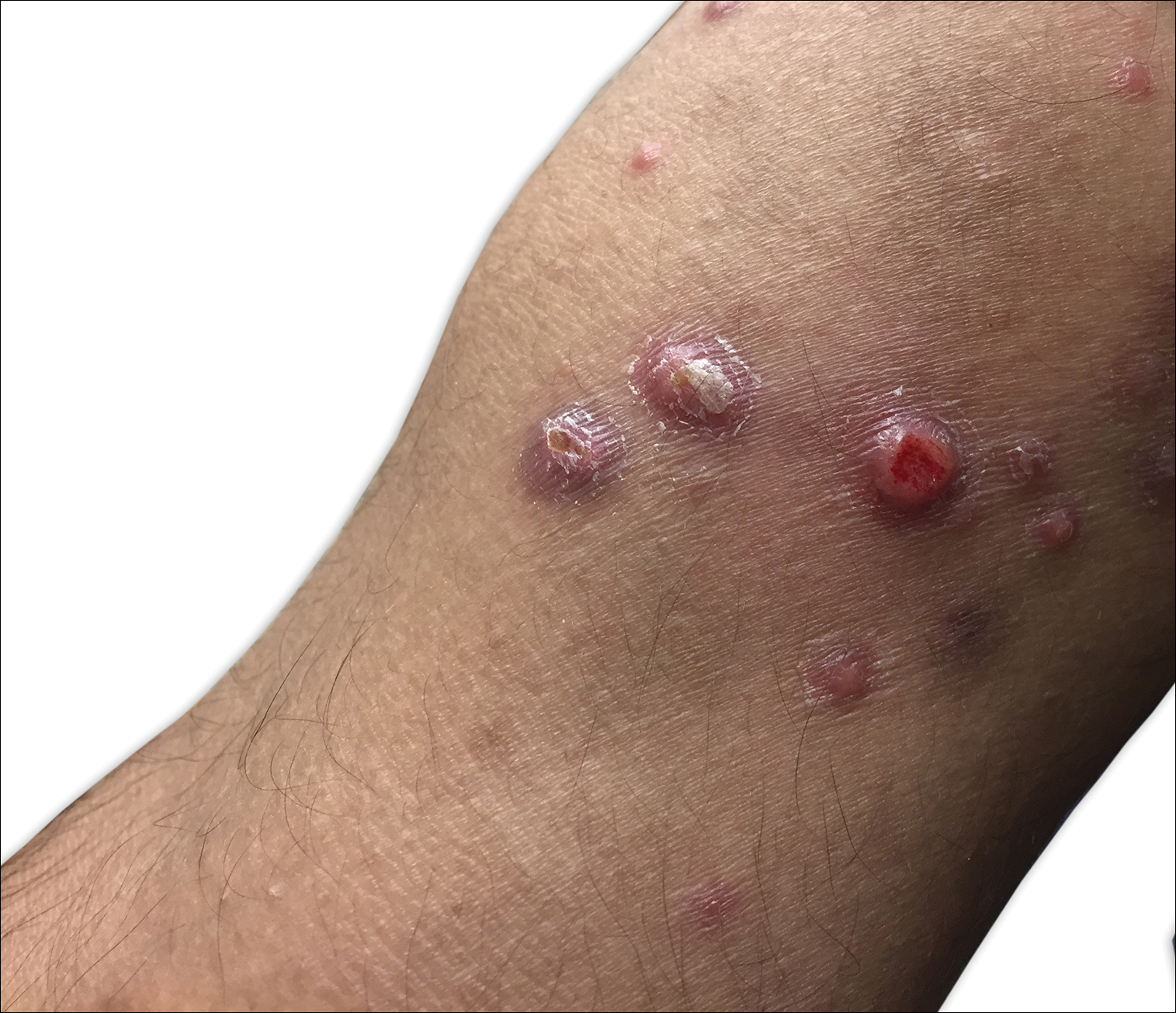
A 29-year-old man from Saudi Arabia presented with slightly tender skin lesions occurring in crops every few months over the last 7 years. The lesions typically would occur on the inguinal area, lower abdomen, buttocks, thighs, or arms, resolving within a few weeks despite no treatment. The patient denied having systemic symptoms such as fevers, chills, sweats, chest pain, shortness of breath, or unexpected weight loss. Physical examination revealed multiple erythematous papulonodules, some ulcerated with a superficial crust, grouped predominantly on the medial aspect of the right upper arm and left lower inguinal region. Isolated lesions also were present on the forearms, dorsal aspects of the hands, abdomen, and thighs. The grouped papulonodules were intermixed with faint hyperpigmented macules indicative of prior lesions. No oral lesions were noted, and there was no marked axillary or inguinal lymphadenopathy.
Genetic predisposition to hypercalcemia linked to CAD, MI
in a large mendelian randomization study published online July 25 in JAMA.
Each 0.5-mg rise in genetically predicted serum calcium concentration increased the odds of coronary artery disease (CAD) and myocardial infarction by about 25%, reported Susanna C. Larsson, Ph.D., of Karolinska Institutet in Stockholm, Sweden, and her associates. It remains unclear whether short- or medium-term calcium supplementation also increases the risk of these outcomes, they added.
Observational studies have linked high serum calcium with cardiovascular disease, but such studies are subject to confounding, the researchers noted. Randomized trials indicate that calcium supplementation might contribute to MI, but the trials are not designed to quantify long-term risks. Therefore, the investigators evaluated a proxy for lifelong hypercalcemia – six single nucleotide polymorphisms (SNPs) that have been linked to high serum calcium, but not to other CAD risk factors such as type 2 diabetes, fasting glucose and insulin levels, body mass index, waist-to-hip ratio, major lipids, or hypertension (JAMA. 2017 Jul 25;318[4]:371-80. doi: 10.1001/jama.2017.8981).
To examine how these SNPs affect the risk of CAD and MI, the researchers analyzed summary statistics for 184,305 individuals from a meta-analysis of CAD genome-wide association studies (Nat Genet. 2015;47:1121-30), including 60,801 cases (of whom about 70% also had MI) and 123,504 controls.
Together, these six SNPs explained about 0.8% of variations in serum calcium levels. Each 0.5-mg/dL (about one standard deviation) increase in genetically predicted serum calcium level significantly increased the risk of CAD (odds ratio, 1.25; 95% confidence interval, 1.08-1.45; P = .003) and MI (OR, 1.24; 95% CI, 1.05-1.46; P = .009). The genetic variant rs1801725 exerted the greatest effect on serum calcium levels, the investigators noted. This SNP affects the CASR gene, which encodes a calcium-sensing receptor that “plays a key role in calcium homeostasis.” However, four of the other five variants also had odds ratios above 1.0, and three had odds ratios above 1.25. A sensitivity analysis that excluded the CASR variant generated an identical odds ratio, although the confidence interval was wider. Studies of other risk factors for CAD have yielded odds ratios between 1.3 (triglyceride levels) and 1.7 (LDL cholesterol levels), the researchers noted.
A link between calcium supplementation and MI remains debatable. However, supplementation can lead to hypercalcemia and greater formation of insoluble calciprotein particles, the investigators said. Coronary artery disease might result from downstream effects on vascular calcification, vascular cells, blood coagulation pathways, or gene expression, but such mechanisms need more study, they added.
This analysis included men and women from the United States, Canada, the United Kingdom, Germany, Sweden, Ireland, the Netherlands, Finland, Iceland, Italy, Estonia, Lebanon, China, Korea, India, Pakistan and Greece. Participants tended to be men in their 50s and 60s, but more than half of studies lacked data on age and sex. Nearly all participants were of white European ancestry.
Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
in a large mendelian randomization study published online July 25 in JAMA.
Each 0.5-mg rise in genetically predicted serum calcium concentration increased the odds of coronary artery disease (CAD) and myocardial infarction by about 25%, reported Susanna C. Larsson, Ph.D., of Karolinska Institutet in Stockholm, Sweden, and her associates. It remains unclear whether short- or medium-term calcium supplementation also increases the risk of these outcomes, they added.
Observational studies have linked high serum calcium with cardiovascular disease, but such studies are subject to confounding, the researchers noted. Randomized trials indicate that calcium supplementation might contribute to MI, but the trials are not designed to quantify long-term risks. Therefore, the investigators evaluated a proxy for lifelong hypercalcemia – six single nucleotide polymorphisms (SNPs) that have been linked to high serum calcium, but not to other CAD risk factors such as type 2 diabetes, fasting glucose and insulin levels, body mass index, waist-to-hip ratio, major lipids, or hypertension (JAMA. 2017 Jul 25;318[4]:371-80. doi: 10.1001/jama.2017.8981).
To examine how these SNPs affect the risk of CAD and MI, the researchers analyzed summary statistics for 184,305 individuals from a meta-analysis of CAD genome-wide association studies (Nat Genet. 2015;47:1121-30), including 60,801 cases (of whom about 70% also had MI) and 123,504 controls.
Together, these six SNPs explained about 0.8% of variations in serum calcium levels. Each 0.5-mg/dL (about one standard deviation) increase in genetically predicted serum calcium level significantly increased the risk of CAD (odds ratio, 1.25; 95% confidence interval, 1.08-1.45; P = .003) and MI (OR, 1.24; 95% CI, 1.05-1.46; P = .009). The genetic variant rs1801725 exerted the greatest effect on serum calcium levels, the investigators noted. This SNP affects the CASR gene, which encodes a calcium-sensing receptor that “plays a key role in calcium homeostasis.” However, four of the other five variants also had odds ratios above 1.0, and three had odds ratios above 1.25. A sensitivity analysis that excluded the CASR variant generated an identical odds ratio, although the confidence interval was wider. Studies of other risk factors for CAD have yielded odds ratios between 1.3 (triglyceride levels) and 1.7 (LDL cholesterol levels), the researchers noted.
A link between calcium supplementation and MI remains debatable. However, supplementation can lead to hypercalcemia and greater formation of insoluble calciprotein particles, the investigators said. Coronary artery disease might result from downstream effects on vascular calcification, vascular cells, blood coagulation pathways, or gene expression, but such mechanisms need more study, they added.
This analysis included men and women from the United States, Canada, the United Kingdom, Germany, Sweden, Ireland, the Netherlands, Finland, Iceland, Italy, Estonia, Lebanon, China, Korea, India, Pakistan and Greece. Participants tended to be men in their 50s and 60s, but more than half of studies lacked data on age and sex. Nearly all participants were of white European ancestry.
Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
in a large mendelian randomization study published online July 25 in JAMA.
Each 0.5-mg rise in genetically predicted serum calcium concentration increased the odds of coronary artery disease (CAD) and myocardial infarction by about 25%, reported Susanna C. Larsson, Ph.D., of Karolinska Institutet in Stockholm, Sweden, and her associates. It remains unclear whether short- or medium-term calcium supplementation also increases the risk of these outcomes, they added.
Observational studies have linked high serum calcium with cardiovascular disease, but such studies are subject to confounding, the researchers noted. Randomized trials indicate that calcium supplementation might contribute to MI, but the trials are not designed to quantify long-term risks. Therefore, the investigators evaluated a proxy for lifelong hypercalcemia – six single nucleotide polymorphisms (SNPs) that have been linked to high serum calcium, but not to other CAD risk factors such as type 2 diabetes, fasting glucose and insulin levels, body mass index, waist-to-hip ratio, major lipids, or hypertension (JAMA. 2017 Jul 25;318[4]:371-80. doi: 10.1001/jama.2017.8981).
To examine how these SNPs affect the risk of CAD and MI, the researchers analyzed summary statistics for 184,305 individuals from a meta-analysis of CAD genome-wide association studies (Nat Genet. 2015;47:1121-30), including 60,801 cases (of whom about 70% also had MI) and 123,504 controls.
Together, these six SNPs explained about 0.8% of variations in serum calcium levels. Each 0.5-mg/dL (about one standard deviation) increase in genetically predicted serum calcium level significantly increased the risk of CAD (odds ratio, 1.25; 95% confidence interval, 1.08-1.45; P = .003) and MI (OR, 1.24; 95% CI, 1.05-1.46; P = .009). The genetic variant rs1801725 exerted the greatest effect on serum calcium levels, the investigators noted. This SNP affects the CASR gene, which encodes a calcium-sensing receptor that “plays a key role in calcium homeostasis.” However, four of the other five variants also had odds ratios above 1.0, and three had odds ratios above 1.25. A sensitivity analysis that excluded the CASR variant generated an identical odds ratio, although the confidence interval was wider. Studies of other risk factors for CAD have yielded odds ratios between 1.3 (triglyceride levels) and 1.7 (LDL cholesterol levels), the researchers noted.
A link between calcium supplementation and MI remains debatable. However, supplementation can lead to hypercalcemia and greater formation of insoluble calciprotein particles, the investigators said. Coronary artery disease might result from downstream effects on vascular calcification, vascular cells, blood coagulation pathways, or gene expression, but such mechanisms need more study, they added.
This analysis included men and women from the United States, Canada, the United Kingdom, Germany, Sweden, Ireland, the Netherlands, Finland, Iceland, Italy, Estonia, Lebanon, China, Korea, India, Pakistan and Greece. Participants tended to be men in their 50s and 60s, but more than half of studies lacked data on age and sex. Nearly all participants were of white European ancestry.
Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
FROM JAMA
Key clinical point: Genetic predisposition to higher serum calcium levels was significantly associated with coronary artery disease and myocardial infarction.
Major finding: Each 0.5-mg per dL rise in serum calcium increased the odds of these outcomes by about 25% (odds ratios, 1.25 and 1.24, respectively).
Data source: A mendelian randomization study of 60,801 cases of coronary artery disease, 123,504 controls, and six single nucleotide polymorphisms linked to serum calcium but not to other risk factors for coronary artery disease.
Disclosures: Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
Hereditary Hypotrichosis Simplex of the Scalp
To the Editor:
Hereditary hypotrichosis simplex (HHS)(Online Mendelian Inheritance in Man [OMIM] 146520) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.1 Most patients are unaffected at birth and otherwise healthy without abnormalities of the nails, teeth, or perspiration. Examination of the scalp reveals normal follicular ostia and absence of scale and erythema; however, decreased follicular density may be noted.1 The histopathologic findings of HHS reveal velluslike hair follicles without associated fibrosis or inflammation.2 Examination of hair follicles with light microscopy is unremarkable.3,4 Historically, this condition has been largely regarded as autosomal dominant, with variable severity also described within families. Herein, we report a case of this rare disease in a child, with 2 family members displaying a less severe phenotype.
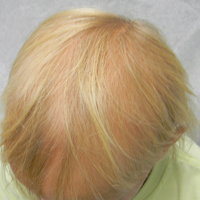
A 7-year-old girl presented with gradual thinning of the scalp hair of 3 to 4 years’ duration. Her mother reported the patient had normal hair density at birth. Over the next several years, she was noted to have an inability to grow lengthy hair. At approximately 3 years of age, thinning of scalp hair was identified. There was no prior history of increased shedding, hypohidrosis, or tooth or nail abnormalities. Family history revealed fine hair in her older sister and fine thin hair at the frontal scalp in her mother. Her mother reported similar inability to grow lengthy hair. Physical examination of the patient demonstrated short blonde hair with diffuse thinning of the crown (Figure 1). The longest hair was approximately 10 cm in length. Follicular ostia were without erythema or scale but notably fewer in number on the crown. Eyebrows, eyelashes, teeth, and fingernails were without abnormalities. A hair pull test was negative and hair mount revealed normal bulb and shaft. Microscopy of hair shafts under polarized light was unremarkable.
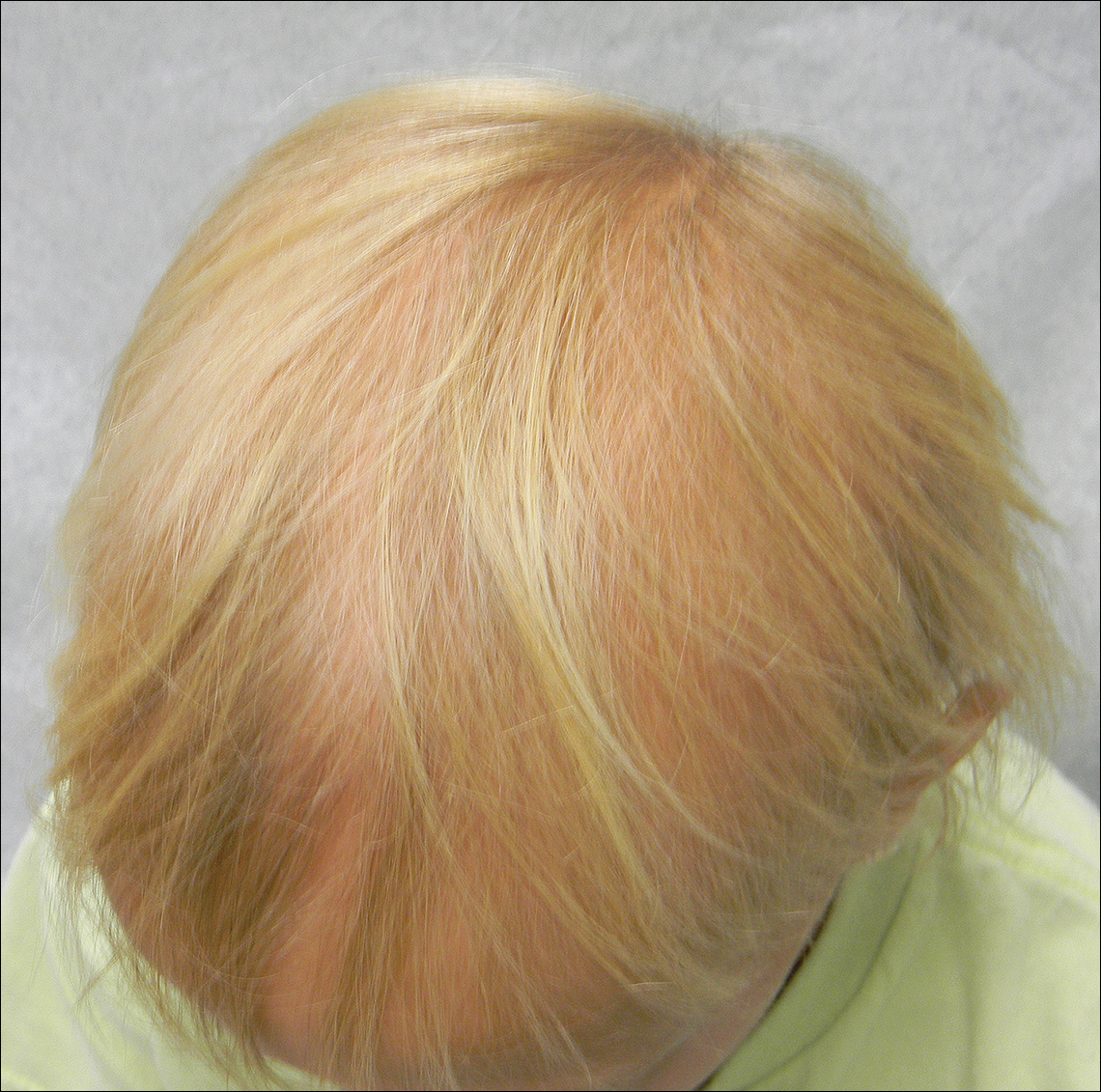
Two punch biopsies were obtained and submitted for vertical and horizontal sectioning. Sections demonstrated an intact epidermis, decreased follicle number, and small follicles with hypoplastic velluslike appearance (Figure 2). Fibrosis and inflammation were not seen; there was no increase in catagen or telogen hairs. Clinical and histopathological findings were consistent with HHS.
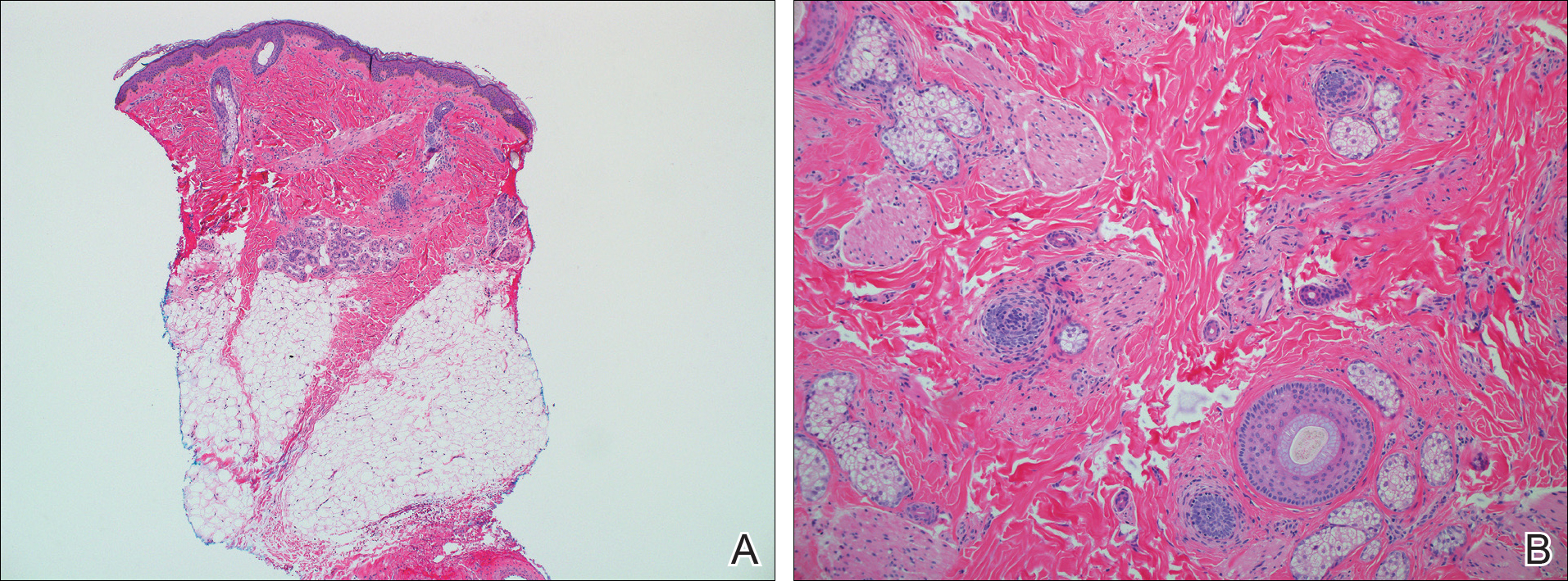
Hereditary hypotrichosis localized to the scalp was first described by Toribio and Quinones5 in 1974 in a large Spanish family presenting with normal scalp hair at birth followed by gradual diffuse hair loss. Hair loss that usually began in school-aged children with subsequent few fine hairs remaining on the scalp by the third decade of life was identified in these individuals.Eyelashes, eyebrows, pubic, axillary, and other truncal hairs were normal.5 Several similar cases of HHS localized to the scalp have since been reported.2,6 Hereditary hypotrichosis simplex is inherited in an autosomal-dominant fashion, with the exception of 1 reported sporadic case.3
Research on HHS has primarily focused on genetic analyses of several affected families. Betz et al7 mapped the gene for HHS to band 6p21.3 in 2 families of Danish origin and in the Spanish family initially described by Toribio and Quinones.5 Three years later, a nonsense mutation in the CDSN gene encoding corneodesmosin was described.8 Despite these genetic advances, the pathogenesis of HHS and the role that corneodesmosin may play remain unclear.
Generalized forms of hypotrichosis (OMIM #605389) have long been reported and described as loss of scalp hair with involvement of eyebrows, eyelashes, and other body hair.9 Genetic studies have allowed for genome-wide linkage analysis, linking 3 families with this more generalized HHS phenotype to chromosome 18; specifically, an Italian family with sparse scalp and body hair but normal eyelashes and eyebrows,4 and 2 Pakistani families with thinning scalp hair and sparse truncal hair.10 A mutation in the APC downregulated 1 gene, APCDD1, also has been identified in these families.10 These genetic findings indicate that the generalized form of HHS is a distinct syndrome.
The differential diagnosis of HHS includes Marie-Unna hereditary hypotrichosis, loose anagen hair syndrome, trichothiodystrophy, and androgenetic alopecia. Marie-Unna hereditary hypotrichosis usually presents as near-complete absence of scalp hair at birth, development of wiry twisted hair in childhood, and progressive alopecia.3 Loose anagen hair syndrome usually demonstrates a ruffled cuticle on hair pull test and remits in late childhood. Polarization of the hair shaft can identify patients with trichothiodystrophy. Follicular miniaturization may lead one to consider early-onset androgenetic alopecia in some patients.
There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.2,3,5 As with other hair loss disorders, wigs or additional over-the-counter cosmetic options may be considered.3 Currently, there are no known patient resources specific for HHS. Therefore, our patient’s family was referred to the National Alopecia Areata Foundation website (https://naaf.org/) for resources on discussing alopecia with school-aged children. The psychological impact of alopecia should not be overlooked and psychiatric referral should be provided, if needed. Examination of family members along with clinical monitoring are recommended. Genetic counseling also may be offered.3
- Rodríguez Díaz E, Fernández Blasco G, Martín Pascual A, et al. Heredity hypotrichosis simplex of the scalp. Dermatology. 1995;191:139-141.
- Ibsen HH, Clemmensen OJ, Brandrup F. Familial hypotrichosis of the scalp. autosomal dominant inheritance in four generations. Acta Derm Venereol. 1991;71:349-351.
- Cambiaghi S, Barbareschi M. A sporadic case of congenital hypotrichosis simplex of the scalp: difficulties in diagnosis and classification. Pediatr Dermatol. 1999;16:301-304.
- Baumer A, Belli S, Trueb RM, et al. An autosomal dominant form of hereditary hypotrichosis simple maps to 18p11.32-p11.23 in an Italian family. Eur J Hum Genet. 2000;8:443-448.
- Toribio J, Quinones PA. Heredity hypotrichosis simplex of the scalp. evidence for autosomal dominant inheritance. Br J Dermatol. 1974;91:687-696.
- Kohn G, Metzker A. Hereditary hypotrichosis simplex of the scalp. Clin Genet. 1987;32:120-124.
- Betz RC, Lee YA, Bygum A, et al. A gene for hypotrichosis simplex of the scalp maps to chromosome 6p21.3. Am J Hum Genet. 2000;66:1979-1983.
- Levy-Nissenbaum E, Betz R, Frydman M, et al. Hypotrichosis of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151-153.
- Just M, Ribera M, Fuente MJ, et al. Hereditary hypotrichosis simplex. Dermatology. 1998;196:339-342.
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2011;44:1043-1047.
To the Editor:
Hereditary hypotrichosis simplex (HHS)(Online Mendelian Inheritance in Man [OMIM] 146520) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.1 Most patients are unaffected at birth and otherwise healthy without abnormalities of the nails, teeth, or perspiration. Examination of the scalp reveals normal follicular ostia and absence of scale and erythema; however, decreased follicular density may be noted.1 The histopathologic findings of HHS reveal velluslike hair follicles without associated fibrosis or inflammation.2 Examination of hair follicles with light microscopy is unremarkable.3,4 Historically, this condition has been largely regarded as autosomal dominant, with variable severity also described within families. Herein, we report a case of this rare disease in a child, with 2 family members displaying a less severe phenotype.
A 7-year-old girl presented with gradual thinning of the scalp hair of 3 to 4 years’ duration. Her mother reported the patient had normal hair density at birth. Over the next several years, she was noted to have an inability to grow lengthy hair. At approximately 3 years of age, thinning of scalp hair was identified. There was no prior history of increased shedding, hypohidrosis, or tooth or nail abnormalities. Family history revealed fine hair in her older sister and fine thin hair at the frontal scalp in her mother. Her mother reported similar inability to grow lengthy hair. Physical examination of the patient demonstrated short blonde hair with diffuse thinning of the crown (Figure 1). The longest hair was approximately 10 cm in length. Follicular ostia were without erythema or scale but notably fewer in number on the crown. Eyebrows, eyelashes, teeth, and fingernails were without abnormalities. A hair pull test was negative and hair mount revealed normal bulb and shaft. Microscopy of hair shafts under polarized light was unremarkable.

Two punch biopsies were obtained and submitted for vertical and horizontal sectioning. Sections demonstrated an intact epidermis, decreased follicle number, and small follicles with hypoplastic velluslike appearance (Figure 2). Fibrosis and inflammation were not seen; there was no increase in catagen or telogen hairs. Clinical and histopathological findings were consistent with HHS.

Hereditary hypotrichosis localized to the scalp was first described by Toribio and Quinones5 in 1974 in a large Spanish family presenting with normal scalp hair at birth followed by gradual diffuse hair loss. Hair loss that usually began in school-aged children with subsequent few fine hairs remaining on the scalp by the third decade of life was identified in these individuals.Eyelashes, eyebrows, pubic, axillary, and other truncal hairs were normal.5 Several similar cases of HHS localized to the scalp have since been reported.2,6 Hereditary hypotrichosis simplex is inherited in an autosomal-dominant fashion, with the exception of 1 reported sporadic case.3
Research on HHS has primarily focused on genetic analyses of several affected families. Betz et al7 mapped the gene for HHS to band 6p21.3 in 2 families of Danish origin and in the Spanish family initially described by Toribio and Quinones.5 Three years later, a nonsense mutation in the CDSN gene encoding corneodesmosin was described.8 Despite these genetic advances, the pathogenesis of HHS and the role that corneodesmosin may play remain unclear.
Generalized forms of hypotrichosis (OMIM #605389) have long been reported and described as loss of scalp hair with involvement of eyebrows, eyelashes, and other body hair.9 Genetic studies have allowed for genome-wide linkage analysis, linking 3 families with this more generalized HHS phenotype to chromosome 18; specifically, an Italian family with sparse scalp and body hair but normal eyelashes and eyebrows,4 and 2 Pakistani families with thinning scalp hair and sparse truncal hair.10 A mutation in the APC downregulated 1 gene, APCDD1, also has been identified in these families.10 These genetic findings indicate that the generalized form of HHS is a distinct syndrome.
The differential diagnosis of HHS includes Marie-Unna hereditary hypotrichosis, loose anagen hair syndrome, trichothiodystrophy, and androgenetic alopecia. Marie-Unna hereditary hypotrichosis usually presents as near-complete absence of scalp hair at birth, development of wiry twisted hair in childhood, and progressive alopecia.3 Loose anagen hair syndrome usually demonstrates a ruffled cuticle on hair pull test and remits in late childhood. Polarization of the hair shaft can identify patients with trichothiodystrophy. Follicular miniaturization may lead one to consider early-onset androgenetic alopecia in some patients.
There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.2,3,5 As with other hair loss disorders, wigs or additional over-the-counter cosmetic options may be considered.3 Currently, there are no known patient resources specific for HHS. Therefore, our patient’s family was referred to the National Alopecia Areata Foundation website (https://naaf.org/) for resources on discussing alopecia with school-aged children. The psychological impact of alopecia should not be overlooked and psychiatric referral should be provided, if needed. Examination of family members along with clinical monitoring are recommended. Genetic counseling also may be offered.3
To the Editor:
Hereditary hypotrichosis simplex (HHS)(Online Mendelian Inheritance in Man [OMIM] 146520) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.1 Most patients are unaffected at birth and otherwise healthy without abnormalities of the nails, teeth, or perspiration. Examination of the scalp reveals normal follicular ostia and absence of scale and erythema; however, decreased follicular density may be noted.1 The histopathologic findings of HHS reveal velluslike hair follicles without associated fibrosis or inflammation.2 Examination of hair follicles with light microscopy is unremarkable.3,4 Historically, this condition has been largely regarded as autosomal dominant, with variable severity also described within families. Herein, we report a case of this rare disease in a child, with 2 family members displaying a less severe phenotype.
A 7-year-old girl presented with gradual thinning of the scalp hair of 3 to 4 years’ duration. Her mother reported the patient had normal hair density at birth. Over the next several years, she was noted to have an inability to grow lengthy hair. At approximately 3 years of age, thinning of scalp hair was identified. There was no prior history of increased shedding, hypohidrosis, or tooth or nail abnormalities. Family history revealed fine hair in her older sister and fine thin hair at the frontal scalp in her mother. Her mother reported similar inability to grow lengthy hair. Physical examination of the patient demonstrated short blonde hair with diffuse thinning of the crown (Figure 1). The longest hair was approximately 10 cm in length. Follicular ostia were without erythema or scale but notably fewer in number on the crown. Eyebrows, eyelashes, teeth, and fingernails were without abnormalities. A hair pull test was negative and hair mount revealed normal bulb and shaft. Microscopy of hair shafts under polarized light was unremarkable.

Two punch biopsies were obtained and submitted for vertical and horizontal sectioning. Sections demonstrated an intact epidermis, decreased follicle number, and small follicles with hypoplastic velluslike appearance (Figure 2). Fibrosis and inflammation were not seen; there was no increase in catagen or telogen hairs. Clinical and histopathological findings were consistent with HHS.

Hereditary hypotrichosis localized to the scalp was first described by Toribio and Quinones5 in 1974 in a large Spanish family presenting with normal scalp hair at birth followed by gradual diffuse hair loss. Hair loss that usually began in school-aged children with subsequent few fine hairs remaining on the scalp by the third decade of life was identified in these individuals.Eyelashes, eyebrows, pubic, axillary, and other truncal hairs were normal.5 Several similar cases of HHS localized to the scalp have since been reported.2,6 Hereditary hypotrichosis simplex is inherited in an autosomal-dominant fashion, with the exception of 1 reported sporadic case.3
Research on HHS has primarily focused on genetic analyses of several affected families. Betz et al7 mapped the gene for HHS to band 6p21.3 in 2 families of Danish origin and in the Spanish family initially described by Toribio and Quinones.5 Three years later, a nonsense mutation in the CDSN gene encoding corneodesmosin was described.8 Despite these genetic advances, the pathogenesis of HHS and the role that corneodesmosin may play remain unclear.
Generalized forms of hypotrichosis (OMIM #605389) have long been reported and described as loss of scalp hair with involvement of eyebrows, eyelashes, and other body hair.9 Genetic studies have allowed for genome-wide linkage analysis, linking 3 families with this more generalized HHS phenotype to chromosome 18; specifically, an Italian family with sparse scalp and body hair but normal eyelashes and eyebrows,4 and 2 Pakistani families with thinning scalp hair and sparse truncal hair.10 A mutation in the APC downregulated 1 gene, APCDD1, also has been identified in these families.10 These genetic findings indicate that the generalized form of HHS is a distinct syndrome.
The differential diagnosis of HHS includes Marie-Unna hereditary hypotrichosis, loose anagen hair syndrome, trichothiodystrophy, and androgenetic alopecia. Marie-Unna hereditary hypotrichosis usually presents as near-complete absence of scalp hair at birth, development of wiry twisted hair in childhood, and progressive alopecia.3 Loose anagen hair syndrome usually demonstrates a ruffled cuticle on hair pull test and remits in late childhood. Polarization of the hair shaft can identify patients with trichothiodystrophy. Follicular miniaturization may lead one to consider early-onset androgenetic alopecia in some patients.
There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.2,3,5 As with other hair loss disorders, wigs or additional over-the-counter cosmetic options may be considered.3 Currently, there are no known patient resources specific for HHS. Therefore, our patient’s family was referred to the National Alopecia Areata Foundation website (https://naaf.org/) for resources on discussing alopecia with school-aged children. The psychological impact of alopecia should not be overlooked and psychiatric referral should be provided, if needed. Examination of family members along with clinical monitoring are recommended. Genetic counseling also may be offered.3
- Rodríguez Díaz E, Fernández Blasco G, Martín Pascual A, et al. Heredity hypotrichosis simplex of the scalp. Dermatology. 1995;191:139-141.
- Ibsen HH, Clemmensen OJ, Brandrup F. Familial hypotrichosis of the scalp. autosomal dominant inheritance in four generations. Acta Derm Venereol. 1991;71:349-351.
- Cambiaghi S, Barbareschi M. A sporadic case of congenital hypotrichosis simplex of the scalp: difficulties in diagnosis and classification. Pediatr Dermatol. 1999;16:301-304.
- Baumer A, Belli S, Trueb RM, et al. An autosomal dominant form of hereditary hypotrichosis simple maps to 18p11.32-p11.23 in an Italian family. Eur J Hum Genet. 2000;8:443-448.
- Toribio J, Quinones PA. Heredity hypotrichosis simplex of the scalp. evidence for autosomal dominant inheritance. Br J Dermatol. 1974;91:687-696.
- Kohn G, Metzker A. Hereditary hypotrichosis simplex of the scalp. Clin Genet. 1987;32:120-124.
- Betz RC, Lee YA, Bygum A, et al. A gene for hypotrichosis simplex of the scalp maps to chromosome 6p21.3. Am J Hum Genet. 2000;66:1979-1983.
- Levy-Nissenbaum E, Betz R, Frydman M, et al. Hypotrichosis of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151-153.
- Just M, Ribera M, Fuente MJ, et al. Hereditary hypotrichosis simplex. Dermatology. 1998;196:339-342.
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2011;44:1043-1047.
- Rodríguez Díaz E, Fernández Blasco G, Martín Pascual A, et al. Heredity hypotrichosis simplex of the scalp. Dermatology. 1995;191:139-141.
- Ibsen HH, Clemmensen OJ, Brandrup F. Familial hypotrichosis of the scalp. autosomal dominant inheritance in four generations. Acta Derm Venereol. 1991;71:349-351.
- Cambiaghi S, Barbareschi M. A sporadic case of congenital hypotrichosis simplex of the scalp: difficulties in diagnosis and classification. Pediatr Dermatol. 1999;16:301-304.
- Baumer A, Belli S, Trueb RM, et al. An autosomal dominant form of hereditary hypotrichosis simple maps to 18p11.32-p11.23 in an Italian family. Eur J Hum Genet. 2000;8:443-448.
- Toribio J, Quinones PA. Heredity hypotrichosis simplex of the scalp. evidence for autosomal dominant inheritance. Br J Dermatol. 1974;91:687-696.
- Kohn G, Metzker A. Hereditary hypotrichosis simplex of the scalp. Clin Genet. 1987;32:120-124.
- Betz RC, Lee YA, Bygum A, et al. A gene for hypotrichosis simplex of the scalp maps to chromosome 6p21.3. Am J Hum Genet. 2000;66:1979-1983.
- Levy-Nissenbaum E, Betz R, Frydman M, et al. Hypotrichosis of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151-153.
- Just M, Ribera M, Fuente MJ, et al. Hereditary hypotrichosis simplex. Dermatology. 1998;196:339-342.
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2011;44:1043-1047.
Practice Points
- Hereditary hypotrichosis simplex (HHS) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.
- Historically, HHS has been largely regarded as autosomal dominant, with variable severity also described within families.
- There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.
Exercise, CBT linked to higher drop in depression in type 2 diabetes
SAN DIEGO – The odds of full remission from clinically diagnosed major depression greatly improved in patients with type 2 diabetes who took part in 12-week supervised exercise and cognitive behavioral therapy (CBT). By the end of the study, 96% of the CBT participants no longer met diagnostic criteria for major depression, compared with just 65% of those on usual care, judging from the findings of a new study.
Another approach – a combination treatment of both exercise and CBT therapies – did not show a statistically significant effect on full remission rates but showed improvement in some other areas.
Dr. De Groot and her colleagues recruited 140 adults – mean age 57 years, 77% female, 71% white, 52% married – who had a diagnosis of both type 2 diabetes and diagnosed clinical depression. They came from three states and had various levels of income and educational background.
The researchers randomly assigned the participants to usual care, 12 weeks of exercise with a personal trainer, 10 individual CBT sessions, or a combination of both exercise and CBT therapies. There were 34-36 participants in each group.
The researchers found improvements in depressive symptoms (P less than .05); negative automatic thoughts (P less than .03), and diabetes distress (P less than .01) and physical quality of life (P less than .03 for all except P greater than 0.1 for CBT) for all three intervention groups compared with usual care. Diabetes-specific quality of life improved in the exercise and combination groups only (P less than .01).
The researchers calculated odds ratios of full or partial remission as 12.4 (CBT) and 5.8 (exercise) compared to usual care (P less than .03), controlling for changes in antidepressant drugs. However, the odds ratio for combination therapy was 2.3 and not deemed statistically significant (P = .218).
The researchers also examined results in subjects with a baseline hemoglobin A1c of 7% or higher and found evidence linking the exercise therapy to clinically meaningful 0.7% improvements in HbA1c (P less than .04).
It’s also not clear whether the interventions will hold up over the long term.
The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. De Groot reported no relevant disclosures.
SAN DIEGO – The odds of full remission from clinically diagnosed major depression greatly improved in patients with type 2 diabetes who took part in 12-week supervised exercise and cognitive behavioral therapy (CBT). By the end of the study, 96% of the CBT participants no longer met diagnostic criteria for major depression, compared with just 65% of those on usual care, judging from the findings of a new study.
Another approach – a combination treatment of both exercise and CBT therapies – did not show a statistically significant effect on full remission rates but showed improvement in some other areas.
Dr. De Groot and her colleagues recruited 140 adults – mean age 57 years, 77% female, 71% white, 52% married – who had a diagnosis of both type 2 diabetes and diagnosed clinical depression. They came from three states and had various levels of income and educational background.
The researchers randomly assigned the participants to usual care, 12 weeks of exercise with a personal trainer, 10 individual CBT sessions, or a combination of both exercise and CBT therapies. There were 34-36 participants in each group.
The researchers found improvements in depressive symptoms (P less than .05); negative automatic thoughts (P less than .03), and diabetes distress (P less than .01) and physical quality of life (P less than .03 for all except P greater than 0.1 for CBT) for all three intervention groups compared with usual care. Diabetes-specific quality of life improved in the exercise and combination groups only (P less than .01).
The researchers calculated odds ratios of full or partial remission as 12.4 (CBT) and 5.8 (exercise) compared to usual care (P less than .03), controlling for changes in antidepressant drugs. However, the odds ratio for combination therapy was 2.3 and not deemed statistically significant (P = .218).
The researchers also examined results in subjects with a baseline hemoglobin A1c of 7% or higher and found evidence linking the exercise therapy to clinically meaningful 0.7% improvements in HbA1c (P less than .04).
It’s also not clear whether the interventions will hold up over the long term.
The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. De Groot reported no relevant disclosures.
SAN DIEGO – The odds of full remission from clinically diagnosed major depression greatly improved in patients with type 2 diabetes who took part in 12-week supervised exercise and cognitive behavioral therapy (CBT). By the end of the study, 96% of the CBT participants no longer met diagnostic criteria for major depression, compared with just 65% of those on usual care, judging from the findings of a new study.
Another approach – a combination treatment of both exercise and CBT therapies – did not show a statistically significant effect on full remission rates but showed improvement in some other areas.
Dr. De Groot and her colleagues recruited 140 adults – mean age 57 years, 77% female, 71% white, 52% married – who had a diagnosis of both type 2 diabetes and diagnosed clinical depression. They came from three states and had various levels of income and educational background.
The researchers randomly assigned the participants to usual care, 12 weeks of exercise with a personal trainer, 10 individual CBT sessions, or a combination of both exercise and CBT therapies. There were 34-36 participants in each group.
The researchers found improvements in depressive symptoms (P less than .05); negative automatic thoughts (P less than .03), and diabetes distress (P less than .01) and physical quality of life (P less than .03 for all except P greater than 0.1 for CBT) for all three intervention groups compared with usual care. Diabetes-specific quality of life improved in the exercise and combination groups only (P less than .01).
The researchers calculated odds ratios of full or partial remission as 12.4 (CBT) and 5.8 (exercise) compared to usual care (P less than .03), controlling for changes in antidepressant drugs. However, the odds ratio for combination therapy was 2.3 and not deemed statistically significant (P = .218).
The researchers also examined results in subjects with a baseline hemoglobin A1c of 7% or higher and found evidence linking the exercise therapy to clinically meaningful 0.7% improvements in HbA1c (P less than .04).
It’s also not clear whether the interventions will hold up over the long term.
The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. De Groot reported no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Supervised exercise and cognitive behavioral therapies are linked to higher rates of recovery from major depression at 12 weeks in patients with type 2 diabetes.
Major finding: Full or partial remission was more likely in CBT and exercise groups compared with usual care after researchers controlled for changes in antidepressant drugs.
Data source: Prospective study of 140 adults with type 2 diabetes randomly assigned to 12 weeks of exercise with a physical trainer, 10 individual CBT sessions, a combination of the two therapies, or usual care.
Disclosures: The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study.
Student Hospitalist Scholars: Preventing unplanned PICU transfers
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experiences on a biweekly basis.
I’m a rising second year medical student working this summer on a project to determine predictors for pediatric clinical deterioration and unplanned transfers to the pediatric ICU.
We’re hoping to identify characteristics of the pediatric population that is more prone to these unplanned transfers, as well as determine what clinical signs serve as reliable warnings so that an intervention can be designed to prevent these emergency transfers.
So far, I have been searching the literature for what current interventions exist to prevent pediatric clinical deterioration. I have been reading about rapid response teams and their effectiveness in preventing codes, as well as what measures are used to evaluate the condition of a pediatric patient who is at risk for clinical deterioration. It is clear that more investigation is needed to identify reliable predictors that indicate a possible ICU transfer for the child patient.
I was interested in this project, and in quality improvement, because of its power to directly improve patient care and safety. It is vital to identify and fix problems that are preventable. It is directly related to the work of the physician, and the interprofessional collaboration aspect is key to improve communication that directly affects the patients’ outcomes.
I was introduced to the field during the past year in medical school, and this prompted me to start looking for research projects in the hospital medicine department at Cincinnati Children’s Hospital. I was connected with Patrick Brady, MD, an attending physician in the division of hospital medicine at Cincinnati Children’s, whose work involves studying patient safety. His goals of investigating how to prevent clinical deterioration in pediatric patients aligned with what I wanted to learn during my research experience.
After partnering with my primary mentor, Dr. Brady, we discussed how the Student Hospitalist Scholar Grant would be a good fit for me, so I decided to apply.
I am excited to continue this experience this summer, as I believe it would not only educate me about applying interventions to better patient care but also about medicine in general. I plan to carry on and apply these lessons learned during my third year of medical school for rotations.
Farah Hussain is a second year medical student at the University of Cincinnati and student researcher at Cincinnati Children’s Hospital Medical Center. Her research interests involve bettering patient care in vulnerable populations.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experiences on a biweekly basis.
I’m a rising second year medical student working this summer on a project to determine predictors for pediatric clinical deterioration and unplanned transfers to the pediatric ICU.
We’re hoping to identify characteristics of the pediatric population that is more prone to these unplanned transfers, as well as determine what clinical signs serve as reliable warnings so that an intervention can be designed to prevent these emergency transfers.
So far, I have been searching the literature for what current interventions exist to prevent pediatric clinical deterioration. I have been reading about rapid response teams and their effectiveness in preventing codes, as well as what measures are used to evaluate the condition of a pediatric patient who is at risk for clinical deterioration. It is clear that more investigation is needed to identify reliable predictors that indicate a possible ICU transfer for the child patient.
I was interested in this project, and in quality improvement, because of its power to directly improve patient care and safety. It is vital to identify and fix problems that are preventable. It is directly related to the work of the physician, and the interprofessional collaboration aspect is key to improve communication that directly affects the patients’ outcomes.
I was introduced to the field during the past year in medical school, and this prompted me to start looking for research projects in the hospital medicine department at Cincinnati Children’s Hospital. I was connected with Patrick Brady, MD, an attending physician in the division of hospital medicine at Cincinnati Children’s, whose work involves studying patient safety. His goals of investigating how to prevent clinical deterioration in pediatric patients aligned with what I wanted to learn during my research experience.
After partnering with my primary mentor, Dr. Brady, we discussed how the Student Hospitalist Scholar Grant would be a good fit for me, so I decided to apply.
I am excited to continue this experience this summer, as I believe it would not only educate me about applying interventions to better patient care but also about medicine in general. I plan to carry on and apply these lessons learned during my third year of medical school for rotations.
Farah Hussain is a second year medical student at the University of Cincinnati and student researcher at Cincinnati Children’s Hospital Medical Center. Her research interests involve bettering patient care in vulnerable populations.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experiences on a biweekly basis.
I’m a rising second year medical student working this summer on a project to determine predictors for pediatric clinical deterioration and unplanned transfers to the pediatric ICU.
We’re hoping to identify characteristics of the pediatric population that is more prone to these unplanned transfers, as well as determine what clinical signs serve as reliable warnings so that an intervention can be designed to prevent these emergency transfers.
So far, I have been searching the literature for what current interventions exist to prevent pediatric clinical deterioration. I have been reading about rapid response teams and their effectiveness in preventing codes, as well as what measures are used to evaluate the condition of a pediatric patient who is at risk for clinical deterioration. It is clear that more investigation is needed to identify reliable predictors that indicate a possible ICU transfer for the child patient.
I was interested in this project, and in quality improvement, because of its power to directly improve patient care and safety. It is vital to identify and fix problems that are preventable. It is directly related to the work of the physician, and the interprofessional collaboration aspect is key to improve communication that directly affects the patients’ outcomes.
I was introduced to the field during the past year in medical school, and this prompted me to start looking for research projects in the hospital medicine department at Cincinnati Children’s Hospital. I was connected with Patrick Brady, MD, an attending physician in the division of hospital medicine at Cincinnati Children’s, whose work involves studying patient safety. His goals of investigating how to prevent clinical deterioration in pediatric patients aligned with what I wanted to learn during my research experience.
After partnering with my primary mentor, Dr. Brady, we discussed how the Student Hospitalist Scholar Grant would be a good fit for me, so I decided to apply.
I am excited to continue this experience this summer, as I believe it would not only educate me about applying interventions to better patient care but also about medicine in general. I plan to carry on and apply these lessons learned during my third year of medical school for rotations.
Farah Hussain is a second year medical student at the University of Cincinnati and student researcher at Cincinnati Children’s Hospital Medical Center. Her research interests involve bettering patient care in vulnerable populations.
The Prognostic Value of Circulating Plasma Cells in Multiple Myeloma
Some studies have suggested that circulating plasma cells (CPCs) might have prognostic value in multiple myeloma (MM), but the findings remain controversial, say researchers from Zhengzhou University in the People’s Republic of China. However, the development of highly sensitive and specific diagnostic methods, such as polymerase chain reaction (PCR) and flow cytometry (FCM), the researchers say, make it possible to explore whether CPCs can serve as a biomarker in MM. To that end, they conducted the first meta-analysis to provide better insight into the prognostic value of CPCs in MM.
The researchers examined findings from 11 studies involving 2,943 patients in 5 countries. Peripheral blood samples were analyzed using FCM, PCR, slide-based immunofluorescence assay (IF), and conventional morphology (CM).
Circulating plasma cell status reflected aggressive disease more than tumor burden, the researchers say. Patients in the CPC-positive groups had more aggressive disease and a worse overall survival (OS) rate compared with patients in the CPC-negative groups. The presence of CPCs was “strikingly” associated with elevated the International Staging System score but not the Durie-Salm staging system (DS) score. This difference may be associated, the researchers suggest, with the fact that the DS stage predominantly reflects tumor burden, which is significantly reduced now by newer therapies.
In subgroup analyses, the patients in the FCM and CM groups had worse prognosis for both disease progression and OS. The PCR subgroup showed prognostic significance for disease progression but not OS, and the IF subgroup for OS but not disease progression.
One question the researchers were also interested in answering was whether it mattered when the sample was taken. However, pooled hazard ratios for OS and disease progression were “fairly stable,” they say, and not influenced by sampling time. Regardless of whether CPCs are detected in an early stage or in relapse patients, the researchers add, CPC status may serve as a useful tool to guide treatment and prognosis.
Source:
Li J, Wang N, Tesfaluul N, Gao X, Liu S, Yue B. PLoS One. 2017;12(7):e0181447.
doi: 10.1371/journal.pone.0181447.
Some studies have suggested that circulating plasma cells (CPCs) might have prognostic value in multiple myeloma (MM), but the findings remain controversial, say researchers from Zhengzhou University in the People’s Republic of China. However, the development of highly sensitive and specific diagnostic methods, such as polymerase chain reaction (PCR) and flow cytometry (FCM), the researchers say, make it possible to explore whether CPCs can serve as a biomarker in MM. To that end, they conducted the first meta-analysis to provide better insight into the prognostic value of CPCs in MM.
The researchers examined findings from 11 studies involving 2,943 patients in 5 countries. Peripheral blood samples were analyzed using FCM, PCR, slide-based immunofluorescence assay (IF), and conventional morphology (CM).
Circulating plasma cell status reflected aggressive disease more than tumor burden, the researchers say. Patients in the CPC-positive groups had more aggressive disease and a worse overall survival (OS) rate compared with patients in the CPC-negative groups. The presence of CPCs was “strikingly” associated with elevated the International Staging System score but not the Durie-Salm staging system (DS) score. This difference may be associated, the researchers suggest, with the fact that the DS stage predominantly reflects tumor burden, which is significantly reduced now by newer therapies.
In subgroup analyses, the patients in the FCM and CM groups had worse prognosis for both disease progression and OS. The PCR subgroup showed prognostic significance for disease progression but not OS, and the IF subgroup for OS but not disease progression.
One question the researchers were also interested in answering was whether it mattered when the sample was taken. However, pooled hazard ratios for OS and disease progression were “fairly stable,” they say, and not influenced by sampling time. Regardless of whether CPCs are detected in an early stage or in relapse patients, the researchers add, CPC status may serve as a useful tool to guide treatment and prognosis.
Source:
Li J, Wang N, Tesfaluul N, Gao X, Liu S, Yue B. PLoS One. 2017;12(7):e0181447.
doi: 10.1371/journal.pone.0181447.
Some studies have suggested that circulating plasma cells (CPCs) might have prognostic value in multiple myeloma (MM), but the findings remain controversial, say researchers from Zhengzhou University in the People’s Republic of China. However, the development of highly sensitive and specific diagnostic methods, such as polymerase chain reaction (PCR) and flow cytometry (FCM), the researchers say, make it possible to explore whether CPCs can serve as a biomarker in MM. To that end, they conducted the first meta-analysis to provide better insight into the prognostic value of CPCs in MM.
The researchers examined findings from 11 studies involving 2,943 patients in 5 countries. Peripheral blood samples were analyzed using FCM, PCR, slide-based immunofluorescence assay (IF), and conventional morphology (CM).
Circulating plasma cell status reflected aggressive disease more than tumor burden, the researchers say. Patients in the CPC-positive groups had more aggressive disease and a worse overall survival (OS) rate compared with patients in the CPC-negative groups. The presence of CPCs was “strikingly” associated with elevated the International Staging System score but not the Durie-Salm staging system (DS) score. This difference may be associated, the researchers suggest, with the fact that the DS stage predominantly reflects tumor burden, which is significantly reduced now by newer therapies.
In subgroup analyses, the patients in the FCM and CM groups had worse prognosis for both disease progression and OS. The PCR subgroup showed prognostic significance for disease progression but not OS, and the IF subgroup for OS but not disease progression.
One question the researchers were also interested in answering was whether it mattered when the sample was taken. However, pooled hazard ratios for OS and disease progression were “fairly stable,” they say, and not influenced by sampling time. Regardless of whether CPCs are detected in an early stage or in relapse patients, the researchers add, CPC status may serve as a useful tool to guide treatment and prognosis.
Source:
Li J, Wang N, Tesfaluul N, Gao X, Liu S, Yue B. PLoS One. 2017;12(7):e0181447.
doi: 10.1371/journal.pone.0181447.
Pancreatitis associated with newer classes of antineoplastic therapies
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
Women Living Longer With Metastatic Breast Cancer
More women are living longer with distant metastatic breast cancer (MBC), according to a National Cancer Institute study. Between 1992-1994 and 2005-2012, 5-year relative survival among women who were diagnosed with MBC at ages 15 to 49 doubled, from 18% to 36%.
Researchers also found that relative survival time increased from 22.3 months to 38.7 months for women diagnosed aged 15 -49 years, and from 19.1 months to 29.7 months for those aged 50 – 64 years.
Moreover, a “small but meaningful” number of women are living years after an initial diagnosis of MBC, the study found. More than 11% of women diagnosed between 2000-2004 aged < 64 years survived ≥ 10 years. Although nearly half of women with MBC have had it for ≤ 2 , one third have lived with it for ≥ 5 years.
The study findings “make clear that the majority of MBC patients, those who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented,” said Angela Mariotto, PhD, chief of the NCI Data Analytics Branch of the Division of Cancer Control and Population Sciences. By including women with recurrence, the study provides a more accurate number of women in the U.S. living with MBC, which can help with health care planning.
More women are living longer with distant metastatic breast cancer (MBC), according to a National Cancer Institute study. Between 1992-1994 and 2005-2012, 5-year relative survival among women who were diagnosed with MBC at ages 15 to 49 doubled, from 18% to 36%.
Researchers also found that relative survival time increased from 22.3 months to 38.7 months for women diagnosed aged 15 -49 years, and from 19.1 months to 29.7 months for those aged 50 – 64 years.
Moreover, a “small but meaningful” number of women are living years after an initial diagnosis of MBC, the study found. More than 11% of women diagnosed between 2000-2004 aged < 64 years survived ≥ 10 years. Although nearly half of women with MBC have had it for ≤ 2 , one third have lived with it for ≥ 5 years.
The study findings “make clear that the majority of MBC patients, those who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented,” said Angela Mariotto, PhD, chief of the NCI Data Analytics Branch of the Division of Cancer Control and Population Sciences. By including women with recurrence, the study provides a more accurate number of women in the U.S. living with MBC, which can help with health care planning.
More women are living longer with distant metastatic breast cancer (MBC), according to a National Cancer Institute study. Between 1992-1994 and 2005-2012, 5-year relative survival among women who were diagnosed with MBC at ages 15 to 49 doubled, from 18% to 36%.
Researchers also found that relative survival time increased from 22.3 months to 38.7 months for women diagnosed aged 15 -49 years, and from 19.1 months to 29.7 months for those aged 50 – 64 years.
Moreover, a “small but meaningful” number of women are living years after an initial diagnosis of MBC, the study found. More than 11% of women diagnosed between 2000-2004 aged < 64 years survived ≥ 10 years. Although nearly half of women with MBC have had it for ≤ 2 , one third have lived with it for ≥ 5 years.
The study findings “make clear that the majority of MBC patients, those who are diagnosed with non-metastatic cancer but progress to distant disease, have never been properly documented,” said Angela Mariotto, PhD, chief of the NCI Data Analytics Branch of the Division of Cancer Control and Population Sciences. By including women with recurrence, the study provides a more accurate number of women in the U.S. living with MBC, which can help with health care planning.
England, Scotland to shorten deferral for high-risk blood donors
England and Scotland are changing some of their policies regarding blood donation, shortening the deferral periods for donors who engage in “high-risk” sexual behavior.
Wales is considering making the same changes to its blood donation policies but has not yet made a commitment to do so.
The policy changes will mean that men who have sex with men (MSM), commercial sex workers, and people with sexual partners who have a high risk of sexually transmitted infections (including those who have been sexually active in areas where HIV is common) will be able to donate blood after 3 months have passed since their last sexual activity.
At present, MSMs and individuals with high-risk sexual partners can only donate blood after 12 months have passed since their last sexual activity, and commercial sex workers are permanently banned from giving blood.
The change in deferral periods will begin to take effect in England in early 2018 and in Scotland in November 2017. Until then, the existing rules still apply.
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), which advises UK ministers and health departments, recommended the aforementioned policy changes following a review of blood donor criteria and risk assessment of certain behaviors.
SaBTO also recommended shortening the deferral period for potential donors who have undergone acupuncture, piercing, tattooing, and endoscopy, as well as those with a history of non-prescribed injectable drug use.
However, this change requires changing UK legislation, in addition to a derogation from or amendments to current European Union legislation.
“We welcome the review by SaBTO and the recommendations,” said Moira Carter, Associate Director of Donor Services and Transport for the Scottish National Blood Transfusion Service.
“The updates for donor eligibility will allow more people the opportunity to give blood. The changes take into account the latest available medical and scientific evidence about the risk of acquiring infections that can be passed on in blood, along with evidence supporting the reliability of the blood screening tests we use.”
“We’re pleased that the lifetime ban on former and current sex workers has been lifted, and the deferral period is now in line with other deferrals based on sexual behavior,” said Alex Phillips, Blood Donations Policy Lead at Terrence Higgins Trust in London.
“We know from our research that the majority of sex workers take great care of their sexual health, with 98% of sex workers we asked rating their sexual health as very important, 76% having a sexual health check up every 3 months, and 98% knowing their HIV status.”
“Medical evidence is, of course, constantly and quickly being updated, so it’s important that the deferral periods are regularly reviewed in line with the latest evidence. We therefore hope that today’s changes will pave the way for more progress as further evidence becomes available.” ![]()
England and Scotland are changing some of their policies regarding blood donation, shortening the deferral periods for donors who engage in “high-risk” sexual behavior.
Wales is considering making the same changes to its blood donation policies but has not yet made a commitment to do so.
The policy changes will mean that men who have sex with men (MSM), commercial sex workers, and people with sexual partners who have a high risk of sexually transmitted infections (including those who have been sexually active in areas where HIV is common) will be able to donate blood after 3 months have passed since their last sexual activity.
At present, MSMs and individuals with high-risk sexual partners can only donate blood after 12 months have passed since their last sexual activity, and commercial sex workers are permanently banned from giving blood.
The change in deferral periods will begin to take effect in England in early 2018 and in Scotland in November 2017. Until then, the existing rules still apply.
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), which advises UK ministers and health departments, recommended the aforementioned policy changes following a review of blood donor criteria and risk assessment of certain behaviors.
SaBTO also recommended shortening the deferral period for potential donors who have undergone acupuncture, piercing, tattooing, and endoscopy, as well as those with a history of non-prescribed injectable drug use.
However, this change requires changing UK legislation, in addition to a derogation from or amendments to current European Union legislation.
“We welcome the review by SaBTO and the recommendations,” said Moira Carter, Associate Director of Donor Services and Transport for the Scottish National Blood Transfusion Service.
“The updates for donor eligibility will allow more people the opportunity to give blood. The changes take into account the latest available medical and scientific evidence about the risk of acquiring infections that can be passed on in blood, along with evidence supporting the reliability of the blood screening tests we use.”
“We’re pleased that the lifetime ban on former and current sex workers has been lifted, and the deferral period is now in line with other deferrals based on sexual behavior,” said Alex Phillips, Blood Donations Policy Lead at Terrence Higgins Trust in London.
“We know from our research that the majority of sex workers take great care of their sexual health, with 98% of sex workers we asked rating their sexual health as very important, 76% having a sexual health check up every 3 months, and 98% knowing their HIV status.”
“Medical evidence is, of course, constantly and quickly being updated, so it’s important that the deferral periods are regularly reviewed in line with the latest evidence. We therefore hope that today’s changes will pave the way for more progress as further evidence becomes available.” ![]()
England and Scotland are changing some of their policies regarding blood donation, shortening the deferral periods for donors who engage in “high-risk” sexual behavior.
Wales is considering making the same changes to its blood donation policies but has not yet made a commitment to do so.
The policy changes will mean that men who have sex with men (MSM), commercial sex workers, and people with sexual partners who have a high risk of sexually transmitted infections (including those who have been sexually active in areas where HIV is common) will be able to donate blood after 3 months have passed since their last sexual activity.
At present, MSMs and individuals with high-risk sexual partners can only donate blood after 12 months have passed since their last sexual activity, and commercial sex workers are permanently banned from giving blood.
The change in deferral periods will begin to take effect in England in early 2018 and in Scotland in November 2017. Until then, the existing rules still apply.
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), which advises UK ministers and health departments, recommended the aforementioned policy changes following a review of blood donor criteria and risk assessment of certain behaviors.
SaBTO also recommended shortening the deferral period for potential donors who have undergone acupuncture, piercing, tattooing, and endoscopy, as well as those with a history of non-prescribed injectable drug use.
However, this change requires changing UK legislation, in addition to a derogation from or amendments to current European Union legislation.
“We welcome the review by SaBTO and the recommendations,” said Moira Carter, Associate Director of Donor Services and Transport for the Scottish National Blood Transfusion Service.
“The updates for donor eligibility will allow more people the opportunity to give blood. The changes take into account the latest available medical and scientific evidence about the risk of acquiring infections that can be passed on in blood, along with evidence supporting the reliability of the blood screening tests we use.”
“We’re pleased that the lifetime ban on former and current sex workers has been lifted, and the deferral period is now in line with other deferrals based on sexual behavior,” said Alex Phillips, Blood Donations Policy Lead at Terrence Higgins Trust in London.
“We know from our research that the majority of sex workers take great care of their sexual health, with 98% of sex workers we asked rating their sexual health as very important, 76% having a sexual health check up every 3 months, and 98% knowing their HIV status.”
“Medical evidence is, of course, constantly and quickly being updated, so it’s important that the deferral periods are regularly reviewed in line with the latest evidence. We therefore hope that today’s changes will pave the way for more progress as further evidence becomes available.” ![]()