User login
Five-day treatment of ear infections
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?
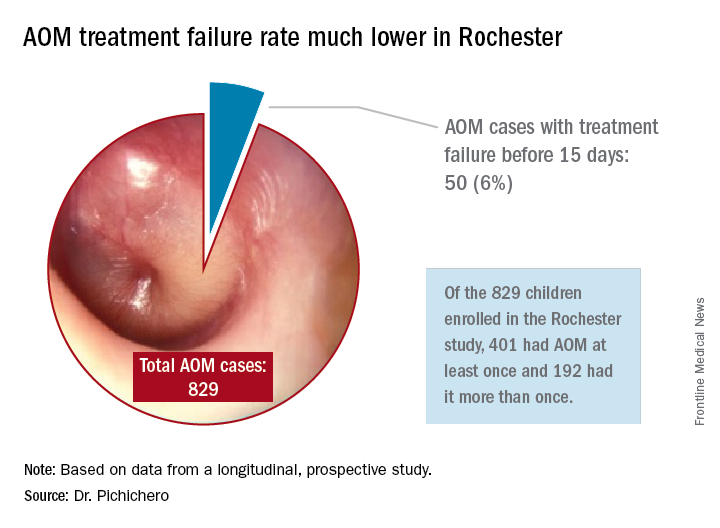
Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?

Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?

Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Ticagrelor improves platelet reactivity, but not clinical outcomes, in Chinese stroke patients
HOUSTON – The combination of ticagrelor and aspirin reduced the incidence of high on-treatment platelet reactivity compared with clopidogrel plus aspirin in an interim analysis of a trial of patients with minor acute ischemic stroke or high-risk transient ischemic attack, but was associated with more treatment-limiting side effects.
Significantly more patients taking the ticagrelor combination dropped out because of dyspnea and minor bleeding, Yilong Wang, MD, said at the International Stroke Conference, sponsored by the American Heart Association. Although the ticagrelor combination also prevented a few more recurrent strokes than did clopidogrel plus aspirin, the difference was not statistically significant.
Dr. Wang of Beijing Tiantan Hospital reported the results as part of an interim safety and efficacy analysis of the Platelet Reactivity in Acute Stroke or Transient Ischemic Attack (PRINCE) trial.
“Ticagrelor has been shown to be more effective in acute coronary syndromes than clopidogrel, regardless of genotype, as it is primarily metabolized by the cytochrome P3A4 enzyme,” Dr. Wang said. “In the SOCRATES Asian substudy, we saw a trend of better efficacy in reducing the risk of subsequent vascular events in the ticagrelor group. But there are limited data on the safety and efficacy of ticagrelor, compared with clopidogrel, over background aspirin in stroke patients.”
PRINCE sought to determine the safety of ticagrelor plus aspirin and their effect on platelet reactivity and clinical outcomes in Asian patients who had experienced a minor stroke or high-risk transient ischemic attack (TIA). The 90-day trial is being conducted in 26 centers in China. Its primary endpoints are P2Y12 reaction units (PRU) and the number of patients with high on-treatment platelet reactivity. Secondary outcomes were the incidence of stroke at 90 days, and a composite vascular outcome of any stroke, heart attack, or vascular death within 90 days.
The primary safety outcomes were major and minor bleeding, intracerebral hemorrhage, and total mortality. The study’s researchers hope to enroll 952 patients. The interim analysis was conducted in 476 who have completed the treatment and follow-up period.
The ticagrelor group (237) received an initial loading dose of 180 mg with 100-300 mg aspirin on day 1, followed by 180 mg ticagrelor plus 100 mg aspirin for 21 days. Thereafter, they discontinued the aspirin and continued with 180 mg ticagrelor.
The clopidogrel group (239) received a 300-mg clopidogrel loading dose plus 100-300 mg aspirin on day 1, followed by 75 mg clopidogrel and 100 mg aspirin daily for 21 days. Thereafter they discontinued the aspirin and took 75 mg clopidogrel daily.
Patients were a median of 60 years old. Most (75%) were male. The median blood pressure was 152/90; 60% were hypertensive. About a quarter had diabetes. The qualifying stroke was a TIA in 22%; the rest had a minor ischemic stroke. The median National Institutes of Health Stroke Scale score was 2.
Platelet function was measured at baseline and 2 hours after the initial loading dose, and then again at 24 hours, and at 7, 21, and 90 days.
The baseline PRU was about 250 in each group. Two hours after the loading dose, it dropped significantly more among those taking ticagrelor than in those on clopidogrel (45.7 vs. 222.32). It remained significantly lower at every time point. At 90 days, the PRU favored ticagrelor (69 vs. 175).
Ticagrelor was also associated with significantly less high on-treatment platelet reactivity at every time point. The final separation at 90 days significantly favored ticagrelor (13% vs. 30%).
Clinical endpoints numerically favored ticagrelor, although none of the findings were statistically significant. Any stroke occurred in 4.6% of the ticagrelor group and 7.5% of the clopidogrel group. The rate of ischemic stroke was 4.2% and 6.7%, respectively.
There were three deaths: two in the ticagrelor and one in the clopidogrel group, and three major bleeds in each group. There was no significant difference in minor bleeding or intracerebral hemorrhage. However, ticagrelor was associated with significantly more incidents of minimal bleeding (17% vs. 7%). This difference drove the final, statistically significant doubling of bleeding risk associated with ticagrelor (hazard ratio, 2.26).
There were 49 adverse events leading to dropouts. Most of these were due to bleeding, which was significantly higher in those taking ticagrelor (12 vs. 5 events). Dyspnea was the next-leading cause of study dropout (5 vs. 1 case). The remainder of the dropouts were due to study noncompliance and patient decisions.
The study will continue, Dr. Wang noted.
PRINCE is being sponsored by the Chinese government. Dr. Wang had no financial disclosures. AstraZeneca is providing the study drug at no charge, and is not otherwise involved in the trial.
[email protected]
On Twitter @alz_gal
HOUSTON – The combination of ticagrelor and aspirin reduced the incidence of high on-treatment platelet reactivity compared with clopidogrel plus aspirin in an interim analysis of a trial of patients with minor acute ischemic stroke or high-risk transient ischemic attack, but was associated with more treatment-limiting side effects.
Significantly more patients taking the ticagrelor combination dropped out because of dyspnea and minor bleeding, Yilong Wang, MD, said at the International Stroke Conference, sponsored by the American Heart Association. Although the ticagrelor combination also prevented a few more recurrent strokes than did clopidogrel plus aspirin, the difference was not statistically significant.
Dr. Wang of Beijing Tiantan Hospital reported the results as part of an interim safety and efficacy analysis of the Platelet Reactivity in Acute Stroke or Transient Ischemic Attack (PRINCE) trial.
“Ticagrelor has been shown to be more effective in acute coronary syndromes than clopidogrel, regardless of genotype, as it is primarily metabolized by the cytochrome P3A4 enzyme,” Dr. Wang said. “In the SOCRATES Asian substudy, we saw a trend of better efficacy in reducing the risk of subsequent vascular events in the ticagrelor group. But there are limited data on the safety and efficacy of ticagrelor, compared with clopidogrel, over background aspirin in stroke patients.”
PRINCE sought to determine the safety of ticagrelor plus aspirin and their effect on platelet reactivity and clinical outcomes in Asian patients who had experienced a minor stroke or high-risk transient ischemic attack (TIA). The 90-day trial is being conducted in 26 centers in China. Its primary endpoints are P2Y12 reaction units (PRU) and the number of patients with high on-treatment platelet reactivity. Secondary outcomes were the incidence of stroke at 90 days, and a composite vascular outcome of any stroke, heart attack, or vascular death within 90 days.
The primary safety outcomes were major and minor bleeding, intracerebral hemorrhage, and total mortality. The study’s researchers hope to enroll 952 patients. The interim analysis was conducted in 476 who have completed the treatment and follow-up period.
The ticagrelor group (237) received an initial loading dose of 180 mg with 100-300 mg aspirin on day 1, followed by 180 mg ticagrelor plus 100 mg aspirin for 21 days. Thereafter, they discontinued the aspirin and continued with 180 mg ticagrelor.
The clopidogrel group (239) received a 300-mg clopidogrel loading dose plus 100-300 mg aspirin on day 1, followed by 75 mg clopidogrel and 100 mg aspirin daily for 21 days. Thereafter they discontinued the aspirin and took 75 mg clopidogrel daily.
Patients were a median of 60 years old. Most (75%) were male. The median blood pressure was 152/90; 60% were hypertensive. About a quarter had diabetes. The qualifying stroke was a TIA in 22%; the rest had a minor ischemic stroke. The median National Institutes of Health Stroke Scale score was 2.
Platelet function was measured at baseline and 2 hours after the initial loading dose, and then again at 24 hours, and at 7, 21, and 90 days.
The baseline PRU was about 250 in each group. Two hours after the loading dose, it dropped significantly more among those taking ticagrelor than in those on clopidogrel (45.7 vs. 222.32). It remained significantly lower at every time point. At 90 days, the PRU favored ticagrelor (69 vs. 175).
Ticagrelor was also associated with significantly less high on-treatment platelet reactivity at every time point. The final separation at 90 days significantly favored ticagrelor (13% vs. 30%).
Clinical endpoints numerically favored ticagrelor, although none of the findings were statistically significant. Any stroke occurred in 4.6% of the ticagrelor group and 7.5% of the clopidogrel group. The rate of ischemic stroke was 4.2% and 6.7%, respectively.
There were three deaths: two in the ticagrelor and one in the clopidogrel group, and three major bleeds in each group. There was no significant difference in minor bleeding or intracerebral hemorrhage. However, ticagrelor was associated with significantly more incidents of minimal bleeding (17% vs. 7%). This difference drove the final, statistically significant doubling of bleeding risk associated with ticagrelor (hazard ratio, 2.26).
There were 49 adverse events leading to dropouts. Most of these were due to bleeding, which was significantly higher in those taking ticagrelor (12 vs. 5 events). Dyspnea was the next-leading cause of study dropout (5 vs. 1 case). The remainder of the dropouts were due to study noncompliance and patient decisions.
The study will continue, Dr. Wang noted.
PRINCE is being sponsored by the Chinese government. Dr. Wang had no financial disclosures. AstraZeneca is providing the study drug at no charge, and is not otherwise involved in the trial.
[email protected]
On Twitter @alz_gal
HOUSTON – The combination of ticagrelor and aspirin reduced the incidence of high on-treatment platelet reactivity compared with clopidogrel plus aspirin in an interim analysis of a trial of patients with minor acute ischemic stroke or high-risk transient ischemic attack, but was associated with more treatment-limiting side effects.
Significantly more patients taking the ticagrelor combination dropped out because of dyspnea and minor bleeding, Yilong Wang, MD, said at the International Stroke Conference, sponsored by the American Heart Association. Although the ticagrelor combination also prevented a few more recurrent strokes than did clopidogrel plus aspirin, the difference was not statistically significant.
Dr. Wang of Beijing Tiantan Hospital reported the results as part of an interim safety and efficacy analysis of the Platelet Reactivity in Acute Stroke or Transient Ischemic Attack (PRINCE) trial.
“Ticagrelor has been shown to be more effective in acute coronary syndromes than clopidogrel, regardless of genotype, as it is primarily metabolized by the cytochrome P3A4 enzyme,” Dr. Wang said. “In the SOCRATES Asian substudy, we saw a trend of better efficacy in reducing the risk of subsequent vascular events in the ticagrelor group. But there are limited data on the safety and efficacy of ticagrelor, compared with clopidogrel, over background aspirin in stroke patients.”
PRINCE sought to determine the safety of ticagrelor plus aspirin and their effect on platelet reactivity and clinical outcomes in Asian patients who had experienced a minor stroke or high-risk transient ischemic attack (TIA). The 90-day trial is being conducted in 26 centers in China. Its primary endpoints are P2Y12 reaction units (PRU) and the number of patients with high on-treatment platelet reactivity. Secondary outcomes were the incidence of stroke at 90 days, and a composite vascular outcome of any stroke, heart attack, or vascular death within 90 days.
The primary safety outcomes were major and minor bleeding, intracerebral hemorrhage, and total mortality. The study’s researchers hope to enroll 952 patients. The interim analysis was conducted in 476 who have completed the treatment and follow-up period.
The ticagrelor group (237) received an initial loading dose of 180 mg with 100-300 mg aspirin on day 1, followed by 180 mg ticagrelor plus 100 mg aspirin for 21 days. Thereafter, they discontinued the aspirin and continued with 180 mg ticagrelor.
The clopidogrel group (239) received a 300-mg clopidogrel loading dose plus 100-300 mg aspirin on day 1, followed by 75 mg clopidogrel and 100 mg aspirin daily for 21 days. Thereafter they discontinued the aspirin and took 75 mg clopidogrel daily.
Patients were a median of 60 years old. Most (75%) were male. The median blood pressure was 152/90; 60% were hypertensive. About a quarter had diabetes. The qualifying stroke was a TIA in 22%; the rest had a minor ischemic stroke. The median National Institutes of Health Stroke Scale score was 2.
Platelet function was measured at baseline and 2 hours after the initial loading dose, and then again at 24 hours, and at 7, 21, and 90 days.
The baseline PRU was about 250 in each group. Two hours after the loading dose, it dropped significantly more among those taking ticagrelor than in those on clopidogrel (45.7 vs. 222.32). It remained significantly lower at every time point. At 90 days, the PRU favored ticagrelor (69 vs. 175).
Ticagrelor was also associated with significantly less high on-treatment platelet reactivity at every time point. The final separation at 90 days significantly favored ticagrelor (13% vs. 30%).
Clinical endpoints numerically favored ticagrelor, although none of the findings were statistically significant. Any stroke occurred in 4.6% of the ticagrelor group and 7.5% of the clopidogrel group. The rate of ischemic stroke was 4.2% and 6.7%, respectively.
There were three deaths: two in the ticagrelor and one in the clopidogrel group, and three major bleeds in each group. There was no significant difference in minor bleeding or intracerebral hemorrhage. However, ticagrelor was associated with significantly more incidents of minimal bleeding (17% vs. 7%). This difference drove the final, statistically significant doubling of bleeding risk associated with ticagrelor (hazard ratio, 2.26).
There were 49 adverse events leading to dropouts. Most of these were due to bleeding, which was significantly higher in those taking ticagrelor (12 vs. 5 events). Dyspnea was the next-leading cause of study dropout (5 vs. 1 case). The remainder of the dropouts were due to study noncompliance and patient decisions.
The study will continue, Dr. Wang noted.
PRINCE is being sponsored by the Chinese government. Dr. Wang had no financial disclosures. AstraZeneca is providing the study drug at no charge, and is not otherwise involved in the trial.
[email protected]
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: At 90 days, P2Y12 reaction units favored ticagrelor (69 vs. 175).
Data source: The interim analysis of the PRINCE trial comprised 476 patients with minor acute ischemic stroke or high-risk transient ischemic attack.
Disclosures: PRINCE is being sponsored by the Chinese government. Dr. Wang had no financial disclosures. AstraZeneca is providing the study drug at no charge, and is not otherwise involved in the trial.
President’s report Strategic planning, travel ban, CHEST 2017
Dear Colleagues,
It doesn’t seem possible, but I have just completed the first quarter of my term as your 79th President and recently returned from chairing my first board meeting – a scary experience to be sure. All in all, it went well. We officially offered Steve Welch the position of Executive Vice President, thereby ushering in one of our own to lead the organization. Steve has successfully served as CHEST’s interim EVP/CEO since May 2016, after 22 years of service with this organization, most recently as Senior Vice President of Publications and Digital Content. I am utterly and completely confident in our choice and want you to know he has the full backing of the board, the Past Presidents, and nearly every doctor he has come in contact with.
One of our strategic areas of focus for the past 5 years is how we serve our international members. CHEST is now truly a global organization. Our international membership continues to grow, and that impacts all areas of the College. In 2016, we provided education for more than 4,300 international members through our national meeting and courses provided all around the globe. In addition, the College has, in partnership with Chinese CHEST leadership and ministry of health officials, led the effort to begin the first pulmonary and critical care fellowship training programs in China. This was an amazing undertaking. The first four graduates were introduced and honored at CHEST 2016, and 20 more are scheduled to graduate next year. An additional 25 more fellowship training programs are to start this next year, and the Chinese National Health and Family Planning Commission recently approved the program as one of only three official fellowship training programs in China. I firmly believe we will look back on this endeavor as one of the greatest accomplishments in our organization’s long and storied history. Countless lives of patients with pulmonary diseases and critical illness are likely to be saved or extended in that country because of this work.
This brings me to CHEST’s position on the travel ban recently imposed and currently on hold in the United States. We, along with 11 other medical societies, sent a letter to the Secretary of Homeland Security underscoring our concern for such a ban, as it could most definitely adversely affect health-care delivery worldwide in ways not previously contemplated. For example, international medical graduates reportedly make up 25% of our physician workforce and provide a disproportionate amount of care to underserved communities. Should we not allow them to come and train here, we could be putting patients in those areas at risk. The ban could result in patients who need specialized health care being denied entrance to the country. We worry that our global physician colleagues will be unable to travel to the United States for educational programs meant to provide them with the tools they need to care for their patients back home. I encourage you to read the full letter if you are interested.
On a brighter note, the program committee is busy planning CHEST 2017, which will be held in Toronto, Oct 28 to Nov 1. Our theme is Team-Based: Patient-Centered. Our advanced practice providers, critical care nurses, and respiratory therapists, among others, will participate in the planning and help shape different aspects of the program. We encourage our physician members to invite a friend, and come and enjoy the meeting. The traditional CHEST program with simulation and interactive, interdisciplinary symposia will be back by popular demand. There will be something in this meeting for everyone. I would be remiss if I didn’t mention that we are working closely with the American Board of Internal Medicine on Maintenance of Certification (MOC) and getting credit by using CHEST products, such as CHEST SEEK, e-learning modules, and live learning opportunities. In fact, CHEST 2016 made getting MOC points easy. Much of the program this year will qualify for MOC, and I would encourage you to take advantage of it. For those who I have had the pleasure of working with and hearing from this year, I thank you for your comments, welcome all opinions, and hope to hear from any member who has something CHEST-related on their mind.
Gerard A. Silvestri, MD, MS, FCCP
President
Dear Colleagues,
It doesn’t seem possible, but I have just completed the first quarter of my term as your 79th President and recently returned from chairing my first board meeting – a scary experience to be sure. All in all, it went well. We officially offered Steve Welch the position of Executive Vice President, thereby ushering in one of our own to lead the organization. Steve has successfully served as CHEST’s interim EVP/CEO since May 2016, after 22 years of service with this organization, most recently as Senior Vice President of Publications and Digital Content. I am utterly and completely confident in our choice and want you to know he has the full backing of the board, the Past Presidents, and nearly every doctor he has come in contact with.
One of our strategic areas of focus for the past 5 years is how we serve our international members. CHEST is now truly a global organization. Our international membership continues to grow, and that impacts all areas of the College. In 2016, we provided education for more than 4,300 international members through our national meeting and courses provided all around the globe. In addition, the College has, in partnership with Chinese CHEST leadership and ministry of health officials, led the effort to begin the first pulmonary and critical care fellowship training programs in China. This was an amazing undertaking. The first four graduates were introduced and honored at CHEST 2016, and 20 more are scheduled to graduate next year. An additional 25 more fellowship training programs are to start this next year, and the Chinese National Health and Family Planning Commission recently approved the program as one of only three official fellowship training programs in China. I firmly believe we will look back on this endeavor as one of the greatest accomplishments in our organization’s long and storied history. Countless lives of patients with pulmonary diseases and critical illness are likely to be saved or extended in that country because of this work.
This brings me to CHEST’s position on the travel ban recently imposed and currently on hold in the United States. We, along with 11 other medical societies, sent a letter to the Secretary of Homeland Security underscoring our concern for such a ban, as it could most definitely adversely affect health-care delivery worldwide in ways not previously contemplated. For example, international medical graduates reportedly make up 25% of our physician workforce and provide a disproportionate amount of care to underserved communities. Should we not allow them to come and train here, we could be putting patients in those areas at risk. The ban could result in patients who need specialized health care being denied entrance to the country. We worry that our global physician colleagues will be unable to travel to the United States for educational programs meant to provide them with the tools they need to care for their patients back home. I encourage you to read the full letter if you are interested.
On a brighter note, the program committee is busy planning CHEST 2017, which will be held in Toronto, Oct 28 to Nov 1. Our theme is Team-Based: Patient-Centered. Our advanced practice providers, critical care nurses, and respiratory therapists, among others, will participate in the planning and help shape different aspects of the program. We encourage our physician members to invite a friend, and come and enjoy the meeting. The traditional CHEST program with simulation and interactive, interdisciplinary symposia will be back by popular demand. There will be something in this meeting for everyone. I would be remiss if I didn’t mention that we are working closely with the American Board of Internal Medicine on Maintenance of Certification (MOC) and getting credit by using CHEST products, such as CHEST SEEK, e-learning modules, and live learning opportunities. In fact, CHEST 2016 made getting MOC points easy. Much of the program this year will qualify for MOC, and I would encourage you to take advantage of it. For those who I have had the pleasure of working with and hearing from this year, I thank you for your comments, welcome all opinions, and hope to hear from any member who has something CHEST-related on their mind.
Gerard A. Silvestri, MD, MS, FCCP
President
Dear Colleagues,
It doesn’t seem possible, but I have just completed the first quarter of my term as your 79th President and recently returned from chairing my first board meeting – a scary experience to be sure. All in all, it went well. We officially offered Steve Welch the position of Executive Vice President, thereby ushering in one of our own to lead the organization. Steve has successfully served as CHEST’s interim EVP/CEO since May 2016, after 22 years of service with this organization, most recently as Senior Vice President of Publications and Digital Content. I am utterly and completely confident in our choice and want you to know he has the full backing of the board, the Past Presidents, and nearly every doctor he has come in contact with.
One of our strategic areas of focus for the past 5 years is how we serve our international members. CHEST is now truly a global organization. Our international membership continues to grow, and that impacts all areas of the College. In 2016, we provided education for more than 4,300 international members through our national meeting and courses provided all around the globe. In addition, the College has, in partnership with Chinese CHEST leadership and ministry of health officials, led the effort to begin the first pulmonary and critical care fellowship training programs in China. This was an amazing undertaking. The first four graduates were introduced and honored at CHEST 2016, and 20 more are scheduled to graduate next year. An additional 25 more fellowship training programs are to start this next year, and the Chinese National Health and Family Planning Commission recently approved the program as one of only three official fellowship training programs in China. I firmly believe we will look back on this endeavor as one of the greatest accomplishments in our organization’s long and storied history. Countless lives of patients with pulmonary diseases and critical illness are likely to be saved or extended in that country because of this work.
This brings me to CHEST’s position on the travel ban recently imposed and currently on hold in the United States. We, along with 11 other medical societies, sent a letter to the Secretary of Homeland Security underscoring our concern for such a ban, as it could most definitely adversely affect health-care delivery worldwide in ways not previously contemplated. For example, international medical graduates reportedly make up 25% of our physician workforce and provide a disproportionate amount of care to underserved communities. Should we not allow them to come and train here, we could be putting patients in those areas at risk. The ban could result in patients who need specialized health care being denied entrance to the country. We worry that our global physician colleagues will be unable to travel to the United States for educational programs meant to provide them with the tools they need to care for their patients back home. I encourage you to read the full letter if you are interested.
On a brighter note, the program committee is busy planning CHEST 2017, which will be held in Toronto, Oct 28 to Nov 1. Our theme is Team-Based: Patient-Centered. Our advanced practice providers, critical care nurses, and respiratory therapists, among others, will participate in the planning and help shape different aspects of the program. We encourage our physician members to invite a friend, and come and enjoy the meeting. The traditional CHEST program with simulation and interactive, interdisciplinary symposia will be back by popular demand. There will be something in this meeting for everyone. I would be remiss if I didn’t mention that we are working closely with the American Board of Internal Medicine on Maintenance of Certification (MOC) and getting credit by using CHEST products, such as CHEST SEEK, e-learning modules, and live learning opportunities. In fact, CHEST 2016 made getting MOC points easy. Much of the program this year will qualify for MOC, and I would encourage you to take advantage of it. For those who I have had the pleasure of working with and hearing from this year, I thank you for your comments, welcome all opinions, and hope to hear from any member who has something CHEST-related on their mind.
Gerard A. Silvestri, MD, MS, FCCP
President
Cosmetic Treatments for Skin of Color: Report From the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Update on Confocal Microscopy and Skin Cancer Imaging: Report from the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Lung cancer pathways reduce cost of care without compromising outcomes
ORLANDO – Implementation of clinical pathways aimed at improving appropriate, evidence-based care for patients with metastatic non–small-cell lung cancer (NSCLC) reduces costs without negatively affecting survival, the Dana-Farber Cancer Institute’s experience suggests.
“At Dana-Farber ... we have looked toward pathways as a potential tool to help manage complexity and resource utilization,” senior author David M. Jackman, MD, explained at a symposium on quality care sponsored by the American Society of Clinical Oncology. “We see pathways as a patient-centered platform that provides real-time decision-making support across the continuum of cancer care. We think that these should be based on preemptive decision making, reflect current standards of care, incorporate feedback from which we can learn from our practice patterns, and support clinical research.”
After the customized Dana-Farber Lung Pathways were implemented in 2014, the cost of outpatient care per patient in the first year after diagnosis fell by about $17,000, or 25%, primarily driven by reduced use of antineoplastic agents, according to data reported at the symposium and simultaneously published (J Oncol Pract. 2017 Mar 4. doi: 10.1200/JOP.2017.021741). Meanwhile, median survival remained at about 11 months, even trending slightly upward.
“Frankly, I’d like to think that we were delivering reasonable and expert care prior to 2014, so I did not anticipate that we were going to see a major change in terms of improvement in survival. But it is important for us to make sure that as we implemented Pathways, there was certainly no decrease in such care,”said Dr. Jackman, medical director of Clinical Pathways at Dana-Farber and an assistant professor of medicine, Harvard Medical School, Boston.
He and his colleagues plan to expand Pathways to cover the full spectrum of cancer care at their center, encompassing medical, radiation, and surgical oncology, he said.
“We also think that pathways can have a major impact on things like symptom management and survivorship care,” he added. “And as we work to embed all of our trials within our Pathways system, and as we push to have our trials in our satellites and in our network affiliates, we hope that this combination of activity can help move us from being not just a good care network, but also a research network.”
The pathways will still have to address some of the thornier issues related to the value of care, Dr. Jackman acknowledged “It’s incredibly easy for us to look at two equivalent therapies in terms of toxicity and efficacy and pick the cheaper one. The harder conversations are to come, that is, what if something is x dollars more expensive and only improves things by a small number of months, is it really worth it?
“Finally, we hope that pathways can be an area for innovation, not used solely to manage costs and to make decisions based on yesteryear, but also to help us move forward and to be the watering hole where everybody comes, as we build out our system that is looking granularly at genomics in order to help match patients with trial opportunities, and for researchers, to help them find specific patients for their trials,” he said. “Pathways can potentially be the nexus where everyone comes and where doctors are informed in real time about opportunities for their patients.”
More evidence of benefit
The Dana-Farber study adds to others showing that the benefits of pathways are real and reproducible, according to invited discussant Thomas J. Smith, MD, professor of oncology and palliative medicine at Johns Hopkins Medicine in Baltimore.
“More importantly, I think, for patients, who are getting hit with these bills and might have a 20% copay, it’s going to reduce their copays and for all the right reasons,” Dr. Smith concluded.
Pathways development
In developing the pathways, Dana-Farber began with lung cancer in part because the center sees a high volume of patients with the disease. In addition, decision making for this malignancy is complex, and there was considerable variation in oncologists’ practices.
“Our platform exists as an independent web-based system that currently lives outside of our EMR. Physicians can access this in real time, in the clinic room with the patient if they so choose,” Dr. Jackman explained. “From our EMR, we are flagged every time a provider orders a new start [of therapy], whether it’s IV chemo, oral chemo, or hormonal therapy. From our vendor, we receive granular treatment decision information made within the pathways system – information about the provider and site, information about the patients, their disease, and the line of therapy, as well as other important factors that drive decision making. Finally, from our clinical trials system interface, we can confirm trial enrollment data.”
Oncologists are free to leave the suggested pathway if their clinical judgment favors an alternate course, according to Dr. Jackman.
“We always want our physicians to feel comfortable treating the patients in front of them however they see best fit. If that means an off-pathway therapy, we want them to have the freedom to do that,” he said. “But we think one of the major tools of the pathways is to help capture the reasons why. So if they think it’s warranted and appropriate, go ahead, go off pathway, but tell us why you are doing it so we can learn from it.”
Using Pathways has not proved burdensome, according to Dr. Jackman. Navigating through the system requires about a minute or two, and use is required only when a patient is starting a new therapy, which typically occurs less than once per half-day clinic session.
Study details
In the study, he and colleagues compared costs of care in the first year after diagnosis of stage IV NSCLC between 160 patients treated at Dana-Farber in 2012 (before Pathways implementation) and 210 patients treated there in 2014 (after Pathways implementation).
“It should be noted that because we are a free-standing outpatient cancer center, all of the costs that we were able to gather are intramural and therefore related only to outpatient activities,” he pointed out.
The total annual costs of care per patient, adjusted for potential confounders (age, sex, race, distance to the institute, clinical trial enrollment, and EGFR and ALK status) fell by $17,085 after implementation of Pathways, from $69,122 to $52,037 (P = .01), he reported.
The largest source of cost savings by far, accounting for 73% of the total, was reduced use of antineoplastic agents (chemotherapy, biologics, and other anticancer agents). Cost for this component fell from $44,237 per patient to $31,846 (P less than .01).
“The majority of this savings came through a reduction in the use of what we considered unwarranted use of combination chemotherapy,” Dr. Jackman said. “In the first-line setting, we specifically went after the regimen of carboplatin, pemetrexed, and bevacizumab; based on our interpretation of the PointBreak study, we felt that that regimen did not bring additional efficacy but did essentially double drug costs. In going after that, we reduced not only use of that but also the subsequent use of pemetrexed plus bevacizumab maintenance. In the second-line setting, with the implementation of Pathways, we saw a decrease in the use of inappropriate platinum-based doublet therapy in those patients who had previously progressed on a platinum-based doublet.”
Median overall survival did not decrease and in fact increased slightly, from 10.7 months before Pathways implementation to 11.2 months afterward (P = .08). Corresponding 1-year rates of survival were 52% and 64%.
“We stand on the shoulders of those who came before us, who have also shown savings associated with implementation of pathways,” concluded Dr. Jackman. “But we hope that we add our voice and our data to this argument that pathways, I think, are a reasonable tool as we try to manage complexity and resource utilization. In addition, we do so without impinging upon clinical outcomes.”
The study was limited by its inclusion of only outpatient costs at Dana-Farber, he acknowledged. “You and we would be very interested in being able to know whether our Pathways implementation affected ED [emergency department] visits or hospitalizations. To that end, we are working with some of our regional payers to try to transparently share data around outcomes, costs, and usage, so that we can learn more in this regard.”
Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
ORLANDO – Implementation of clinical pathways aimed at improving appropriate, evidence-based care for patients with metastatic non–small-cell lung cancer (NSCLC) reduces costs without negatively affecting survival, the Dana-Farber Cancer Institute’s experience suggests.
“At Dana-Farber ... we have looked toward pathways as a potential tool to help manage complexity and resource utilization,” senior author David M. Jackman, MD, explained at a symposium on quality care sponsored by the American Society of Clinical Oncology. “We see pathways as a patient-centered platform that provides real-time decision-making support across the continuum of cancer care. We think that these should be based on preemptive decision making, reflect current standards of care, incorporate feedback from which we can learn from our practice patterns, and support clinical research.”
After the customized Dana-Farber Lung Pathways were implemented in 2014, the cost of outpatient care per patient in the first year after diagnosis fell by about $17,000, or 25%, primarily driven by reduced use of antineoplastic agents, according to data reported at the symposium and simultaneously published (J Oncol Pract. 2017 Mar 4. doi: 10.1200/JOP.2017.021741). Meanwhile, median survival remained at about 11 months, even trending slightly upward.
“Frankly, I’d like to think that we were delivering reasonable and expert care prior to 2014, so I did not anticipate that we were going to see a major change in terms of improvement in survival. But it is important for us to make sure that as we implemented Pathways, there was certainly no decrease in such care,”said Dr. Jackman, medical director of Clinical Pathways at Dana-Farber and an assistant professor of medicine, Harvard Medical School, Boston.
He and his colleagues plan to expand Pathways to cover the full spectrum of cancer care at their center, encompassing medical, radiation, and surgical oncology, he said.
“We also think that pathways can have a major impact on things like symptom management and survivorship care,” he added. “And as we work to embed all of our trials within our Pathways system, and as we push to have our trials in our satellites and in our network affiliates, we hope that this combination of activity can help move us from being not just a good care network, but also a research network.”
The pathways will still have to address some of the thornier issues related to the value of care, Dr. Jackman acknowledged “It’s incredibly easy for us to look at two equivalent therapies in terms of toxicity and efficacy and pick the cheaper one. The harder conversations are to come, that is, what if something is x dollars more expensive and only improves things by a small number of months, is it really worth it?
“Finally, we hope that pathways can be an area for innovation, not used solely to manage costs and to make decisions based on yesteryear, but also to help us move forward and to be the watering hole where everybody comes, as we build out our system that is looking granularly at genomics in order to help match patients with trial opportunities, and for researchers, to help them find specific patients for their trials,” he said. “Pathways can potentially be the nexus where everyone comes and where doctors are informed in real time about opportunities for their patients.”
More evidence of benefit
The Dana-Farber study adds to others showing that the benefits of pathways are real and reproducible, according to invited discussant Thomas J. Smith, MD, professor of oncology and palliative medicine at Johns Hopkins Medicine in Baltimore.
“More importantly, I think, for patients, who are getting hit with these bills and might have a 20% copay, it’s going to reduce their copays and for all the right reasons,” Dr. Smith concluded.
Pathways development
In developing the pathways, Dana-Farber began with lung cancer in part because the center sees a high volume of patients with the disease. In addition, decision making for this malignancy is complex, and there was considerable variation in oncologists’ practices.
“Our platform exists as an independent web-based system that currently lives outside of our EMR. Physicians can access this in real time, in the clinic room with the patient if they so choose,” Dr. Jackman explained. “From our EMR, we are flagged every time a provider orders a new start [of therapy], whether it’s IV chemo, oral chemo, or hormonal therapy. From our vendor, we receive granular treatment decision information made within the pathways system – information about the provider and site, information about the patients, their disease, and the line of therapy, as well as other important factors that drive decision making. Finally, from our clinical trials system interface, we can confirm trial enrollment data.”
Oncologists are free to leave the suggested pathway if their clinical judgment favors an alternate course, according to Dr. Jackman.
“We always want our physicians to feel comfortable treating the patients in front of them however they see best fit. If that means an off-pathway therapy, we want them to have the freedom to do that,” he said. “But we think one of the major tools of the pathways is to help capture the reasons why. So if they think it’s warranted and appropriate, go ahead, go off pathway, but tell us why you are doing it so we can learn from it.”
Using Pathways has not proved burdensome, according to Dr. Jackman. Navigating through the system requires about a minute or two, and use is required only when a patient is starting a new therapy, which typically occurs less than once per half-day clinic session.
Study details
In the study, he and colleagues compared costs of care in the first year after diagnosis of stage IV NSCLC between 160 patients treated at Dana-Farber in 2012 (before Pathways implementation) and 210 patients treated there in 2014 (after Pathways implementation).
“It should be noted that because we are a free-standing outpatient cancer center, all of the costs that we were able to gather are intramural and therefore related only to outpatient activities,” he pointed out.
The total annual costs of care per patient, adjusted for potential confounders (age, sex, race, distance to the institute, clinical trial enrollment, and EGFR and ALK status) fell by $17,085 after implementation of Pathways, from $69,122 to $52,037 (P = .01), he reported.
The largest source of cost savings by far, accounting for 73% of the total, was reduced use of antineoplastic agents (chemotherapy, biologics, and other anticancer agents). Cost for this component fell from $44,237 per patient to $31,846 (P less than .01).
“The majority of this savings came through a reduction in the use of what we considered unwarranted use of combination chemotherapy,” Dr. Jackman said. “In the first-line setting, we specifically went after the regimen of carboplatin, pemetrexed, and bevacizumab; based on our interpretation of the PointBreak study, we felt that that regimen did not bring additional efficacy but did essentially double drug costs. In going after that, we reduced not only use of that but also the subsequent use of pemetrexed plus bevacizumab maintenance. In the second-line setting, with the implementation of Pathways, we saw a decrease in the use of inappropriate platinum-based doublet therapy in those patients who had previously progressed on a platinum-based doublet.”
Median overall survival did not decrease and in fact increased slightly, from 10.7 months before Pathways implementation to 11.2 months afterward (P = .08). Corresponding 1-year rates of survival were 52% and 64%.
“We stand on the shoulders of those who came before us, who have also shown savings associated with implementation of pathways,” concluded Dr. Jackman. “But we hope that we add our voice and our data to this argument that pathways, I think, are a reasonable tool as we try to manage complexity and resource utilization. In addition, we do so without impinging upon clinical outcomes.”
The study was limited by its inclusion of only outpatient costs at Dana-Farber, he acknowledged. “You and we would be very interested in being able to know whether our Pathways implementation affected ED [emergency department] visits or hospitalizations. To that end, we are working with some of our regional payers to try to transparently share data around outcomes, costs, and usage, so that we can learn more in this regard.”
Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
ORLANDO – Implementation of clinical pathways aimed at improving appropriate, evidence-based care for patients with metastatic non–small-cell lung cancer (NSCLC) reduces costs without negatively affecting survival, the Dana-Farber Cancer Institute’s experience suggests.
“At Dana-Farber ... we have looked toward pathways as a potential tool to help manage complexity and resource utilization,” senior author David M. Jackman, MD, explained at a symposium on quality care sponsored by the American Society of Clinical Oncology. “We see pathways as a patient-centered platform that provides real-time decision-making support across the continuum of cancer care. We think that these should be based on preemptive decision making, reflect current standards of care, incorporate feedback from which we can learn from our practice patterns, and support clinical research.”
After the customized Dana-Farber Lung Pathways were implemented in 2014, the cost of outpatient care per patient in the first year after diagnosis fell by about $17,000, or 25%, primarily driven by reduced use of antineoplastic agents, according to data reported at the symposium and simultaneously published (J Oncol Pract. 2017 Mar 4. doi: 10.1200/JOP.2017.021741). Meanwhile, median survival remained at about 11 months, even trending slightly upward.
“Frankly, I’d like to think that we were delivering reasonable and expert care prior to 2014, so I did not anticipate that we were going to see a major change in terms of improvement in survival. But it is important for us to make sure that as we implemented Pathways, there was certainly no decrease in such care,”said Dr. Jackman, medical director of Clinical Pathways at Dana-Farber and an assistant professor of medicine, Harvard Medical School, Boston.
He and his colleagues plan to expand Pathways to cover the full spectrum of cancer care at their center, encompassing medical, radiation, and surgical oncology, he said.
“We also think that pathways can have a major impact on things like symptom management and survivorship care,” he added. “And as we work to embed all of our trials within our Pathways system, and as we push to have our trials in our satellites and in our network affiliates, we hope that this combination of activity can help move us from being not just a good care network, but also a research network.”
The pathways will still have to address some of the thornier issues related to the value of care, Dr. Jackman acknowledged “It’s incredibly easy for us to look at two equivalent therapies in terms of toxicity and efficacy and pick the cheaper one. The harder conversations are to come, that is, what if something is x dollars more expensive and only improves things by a small number of months, is it really worth it?
“Finally, we hope that pathways can be an area for innovation, not used solely to manage costs and to make decisions based on yesteryear, but also to help us move forward and to be the watering hole where everybody comes, as we build out our system that is looking granularly at genomics in order to help match patients with trial opportunities, and for researchers, to help them find specific patients for their trials,” he said. “Pathways can potentially be the nexus where everyone comes and where doctors are informed in real time about opportunities for their patients.”
More evidence of benefit
The Dana-Farber study adds to others showing that the benefits of pathways are real and reproducible, according to invited discussant Thomas J. Smith, MD, professor of oncology and palliative medicine at Johns Hopkins Medicine in Baltimore.
“More importantly, I think, for patients, who are getting hit with these bills and might have a 20% copay, it’s going to reduce their copays and for all the right reasons,” Dr. Smith concluded.
Pathways development
In developing the pathways, Dana-Farber began with lung cancer in part because the center sees a high volume of patients with the disease. In addition, decision making for this malignancy is complex, and there was considerable variation in oncologists’ practices.
“Our platform exists as an independent web-based system that currently lives outside of our EMR. Physicians can access this in real time, in the clinic room with the patient if they so choose,” Dr. Jackman explained. “From our EMR, we are flagged every time a provider orders a new start [of therapy], whether it’s IV chemo, oral chemo, or hormonal therapy. From our vendor, we receive granular treatment decision information made within the pathways system – information about the provider and site, information about the patients, their disease, and the line of therapy, as well as other important factors that drive decision making. Finally, from our clinical trials system interface, we can confirm trial enrollment data.”
Oncologists are free to leave the suggested pathway if their clinical judgment favors an alternate course, according to Dr. Jackman.
“We always want our physicians to feel comfortable treating the patients in front of them however they see best fit. If that means an off-pathway therapy, we want them to have the freedom to do that,” he said. “But we think one of the major tools of the pathways is to help capture the reasons why. So if they think it’s warranted and appropriate, go ahead, go off pathway, but tell us why you are doing it so we can learn from it.”
Using Pathways has not proved burdensome, according to Dr. Jackman. Navigating through the system requires about a minute or two, and use is required only when a patient is starting a new therapy, which typically occurs less than once per half-day clinic session.
Study details
In the study, he and colleagues compared costs of care in the first year after diagnosis of stage IV NSCLC between 160 patients treated at Dana-Farber in 2012 (before Pathways implementation) and 210 patients treated there in 2014 (after Pathways implementation).
“It should be noted that because we are a free-standing outpatient cancer center, all of the costs that we were able to gather are intramural and therefore related only to outpatient activities,” he pointed out.
The total annual costs of care per patient, adjusted for potential confounders (age, sex, race, distance to the institute, clinical trial enrollment, and EGFR and ALK status) fell by $17,085 after implementation of Pathways, from $69,122 to $52,037 (P = .01), he reported.
The largest source of cost savings by far, accounting for 73% of the total, was reduced use of antineoplastic agents (chemotherapy, biologics, and other anticancer agents). Cost for this component fell from $44,237 per patient to $31,846 (P less than .01).
“The majority of this savings came through a reduction in the use of what we considered unwarranted use of combination chemotherapy,” Dr. Jackman said. “In the first-line setting, we specifically went after the regimen of carboplatin, pemetrexed, and bevacizumab; based on our interpretation of the PointBreak study, we felt that that regimen did not bring additional efficacy but did essentially double drug costs. In going after that, we reduced not only use of that but also the subsequent use of pemetrexed plus bevacizumab maintenance. In the second-line setting, with the implementation of Pathways, we saw a decrease in the use of inappropriate platinum-based doublet therapy in those patients who had previously progressed on a platinum-based doublet.”
Median overall survival did not decrease and in fact increased slightly, from 10.7 months before Pathways implementation to 11.2 months afterward (P = .08). Corresponding 1-year rates of survival were 52% and 64%.
“We stand on the shoulders of those who came before us, who have also shown savings associated with implementation of pathways,” concluded Dr. Jackman. “But we hope that we add our voice and our data to this argument that pathways, I think, are a reasonable tool as we try to manage complexity and resource utilization. In addition, we do so without impinging upon clinical outcomes.”
The study was limited by its inclusion of only outpatient costs at Dana-Farber, he acknowledged. “You and we would be very interested in being able to know whether our Pathways implementation affected ED [emergency department] visits or hospitalizations. To that end, we are working with some of our regional payers to try to transparently share data around outcomes, costs, and usage, so that we can learn more in this regard.”
Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: The annual cost of outpatient care per patient fell by $17,085, mainly because of reduced use of antineoplastic agents, whereas median survival remained at about 11 months.
Data source: A cohort study among patients with newly diagnosed metastatic NSCLC, comparing 160 treated before and 210 treated after pathways implementation.
Disclosures: Dr. Jackman disclosed that he is an adviser or consultant to Bayer, Celgene, CVS Caremark, Genentech, and Lilly.
Book Review: Psychiatrist rejects ‘physician as cog’ model of care
The title of the book, “Passion for Patients,” by Lee H. Beecher, MD, DLFAPA, FASAM, with writer Dave Racer, MLitt (St. Paul, Minn., Alethos Press, 2017), clearly represents Dr. Beecher’s approach to his professional life: His focal interest has been his patients ever since he went to medical school and started a very long and successful practice.
Dr. Beecher’s years of practice encompass many of the changes that the practice of medicine has seen in the last 50 years.
He attended medical school when an office was the place where a physician and his patients would get together to exchange thoughts, feelings, ideas, and plans so that they would eventually work together directly and unencumbered on the same concepts that they share and that they considered crucial to their relationship.
Shortly after Dr. Beecher graduated, Medicaid and Medicare came into medical practice, together with progressive limitations, threats, and a great many unwelcome interlopers, whose mission drastically changed the doctor-patient relationship. No matter how one examines the actions of the numerous new participants – be they auditors, insurance companies, employers, or money managers – one of their main missions was to modify, qualify, re-identify, and limit the interaction between the doctor and the patient.
“What is amazing – and contrary to truth – about the current evolution of medical care reform is its manifold references to safeguarding the best interests of the patient,” Dr. Beecher wrote. “On the contrary, the medical care reformers in current vogue see the physician as but one cog in the production of a specified medical care outcome – a cog that must be greased by evidence-based medicine and managed by analytical applications derived from data, cured in the crucible of number crunching, and controlled by payment systems.”
We live in an age when forces other than medical thinking and practice are trying to define what psychiatrists do, how we do it, and whether our effort is worth being paid for. This has created lack of satisfaction in the exercise of psychiatry, early retirements, and lack of growth in many quarters. When one considers that practically all psychiatric endeavors can be traced to the efforts of devoted practitioners interested in improving the profession, one can see that the future might look bleak because people other than psychiatrists define, quantify, and evaluate the practice of our specialty.
“I escaped from managed care into the practice model that had served so well for decades prior to HMOs, [preferred provider organizations], and other externally controlled practice models,” he wrote. “My patients paid me directly.”
Dr. Beecher is a witness and protester, as well as a thinking innovator, coming to defend patients and physicians at a time when they are under attack from precisely the same forces that were supposed to help and support them.
Throughout his book, Dr. Beecher tells us the story of his many points of disagreement with the intruders and his many arguments in favor of patients and doctors, going back to the beginning of the forces that are controlling and destroying their relationship at this time and advocating principled resistance and a careful search for independence. The reader easily accompanies the author to the points when independence blends with excellence – accepting that neither one exists without the other.
Dr. Muñoz, a former president of the American Psychiatric Association, has written eight books and more than 200 articles about various aspects of psychiatry. He is a professor of psychiatry at the University of California, San Diego, and has a private practice. Dr. Muñoz and Dr. Beecher serve on the Editorial Advisory Board of Clinical Psychiatry News.
The title of the book, “Passion for Patients,” by Lee H. Beecher, MD, DLFAPA, FASAM, with writer Dave Racer, MLitt (St. Paul, Minn., Alethos Press, 2017), clearly represents Dr. Beecher’s approach to his professional life: His focal interest has been his patients ever since he went to medical school and started a very long and successful practice.
Dr. Beecher’s years of practice encompass many of the changes that the practice of medicine has seen in the last 50 years.
He attended medical school when an office was the place where a physician and his patients would get together to exchange thoughts, feelings, ideas, and plans so that they would eventually work together directly and unencumbered on the same concepts that they share and that they considered crucial to their relationship.
Shortly after Dr. Beecher graduated, Medicaid and Medicare came into medical practice, together with progressive limitations, threats, and a great many unwelcome interlopers, whose mission drastically changed the doctor-patient relationship. No matter how one examines the actions of the numerous new participants – be they auditors, insurance companies, employers, or money managers – one of their main missions was to modify, qualify, re-identify, and limit the interaction between the doctor and the patient.
“What is amazing – and contrary to truth – about the current evolution of medical care reform is its manifold references to safeguarding the best interests of the patient,” Dr. Beecher wrote. “On the contrary, the medical care reformers in current vogue see the physician as but one cog in the production of a specified medical care outcome – a cog that must be greased by evidence-based medicine and managed by analytical applications derived from data, cured in the crucible of number crunching, and controlled by payment systems.”
We live in an age when forces other than medical thinking and practice are trying to define what psychiatrists do, how we do it, and whether our effort is worth being paid for. This has created lack of satisfaction in the exercise of psychiatry, early retirements, and lack of growth in many quarters. When one considers that practically all psychiatric endeavors can be traced to the efforts of devoted practitioners interested in improving the profession, one can see that the future might look bleak because people other than psychiatrists define, quantify, and evaluate the practice of our specialty.
“I escaped from managed care into the practice model that had served so well for decades prior to HMOs, [preferred provider organizations], and other externally controlled practice models,” he wrote. “My patients paid me directly.”
Dr. Beecher is a witness and protester, as well as a thinking innovator, coming to defend patients and physicians at a time when they are under attack from precisely the same forces that were supposed to help and support them.
Throughout his book, Dr. Beecher tells us the story of his many points of disagreement with the intruders and his many arguments in favor of patients and doctors, going back to the beginning of the forces that are controlling and destroying their relationship at this time and advocating principled resistance and a careful search for independence. The reader easily accompanies the author to the points when independence blends with excellence – accepting that neither one exists without the other.
Dr. Muñoz, a former president of the American Psychiatric Association, has written eight books and more than 200 articles about various aspects of psychiatry. He is a professor of psychiatry at the University of California, San Diego, and has a private practice. Dr. Muñoz and Dr. Beecher serve on the Editorial Advisory Board of Clinical Psychiatry News.
The title of the book, “Passion for Patients,” by Lee H. Beecher, MD, DLFAPA, FASAM, with writer Dave Racer, MLitt (St. Paul, Minn., Alethos Press, 2017), clearly represents Dr. Beecher’s approach to his professional life: His focal interest has been his patients ever since he went to medical school and started a very long and successful practice.
Dr. Beecher’s years of practice encompass many of the changes that the practice of medicine has seen in the last 50 years.
He attended medical school when an office was the place where a physician and his patients would get together to exchange thoughts, feelings, ideas, and plans so that they would eventually work together directly and unencumbered on the same concepts that they share and that they considered crucial to their relationship.
Shortly after Dr. Beecher graduated, Medicaid and Medicare came into medical practice, together with progressive limitations, threats, and a great many unwelcome interlopers, whose mission drastically changed the doctor-patient relationship. No matter how one examines the actions of the numerous new participants – be they auditors, insurance companies, employers, or money managers – one of their main missions was to modify, qualify, re-identify, and limit the interaction between the doctor and the patient.
“What is amazing – and contrary to truth – about the current evolution of medical care reform is its manifold references to safeguarding the best interests of the patient,” Dr. Beecher wrote. “On the contrary, the medical care reformers in current vogue see the physician as but one cog in the production of a specified medical care outcome – a cog that must be greased by evidence-based medicine and managed by analytical applications derived from data, cured in the crucible of number crunching, and controlled by payment systems.”
We live in an age when forces other than medical thinking and practice are trying to define what psychiatrists do, how we do it, and whether our effort is worth being paid for. This has created lack of satisfaction in the exercise of psychiatry, early retirements, and lack of growth in many quarters. When one considers that practically all psychiatric endeavors can be traced to the efforts of devoted practitioners interested in improving the profession, one can see that the future might look bleak because people other than psychiatrists define, quantify, and evaluate the practice of our specialty.
“I escaped from managed care into the practice model that had served so well for decades prior to HMOs, [preferred provider organizations], and other externally controlled practice models,” he wrote. “My patients paid me directly.”
Dr. Beecher is a witness and protester, as well as a thinking innovator, coming to defend patients and physicians at a time when they are under attack from precisely the same forces that were supposed to help and support them.
Throughout his book, Dr. Beecher tells us the story of his many points of disagreement with the intruders and his many arguments in favor of patients and doctors, going back to the beginning of the forces that are controlling and destroying their relationship at this time and advocating principled resistance and a careful search for independence. The reader easily accompanies the author to the points when independence blends with excellence – accepting that neither one exists without the other.
Dr. Muñoz, a former president of the American Psychiatric Association, has written eight books and more than 200 articles about various aspects of psychiatry. He is a professor of psychiatry at the University of California, San Diego, and has a private practice. Dr. Muñoz and Dr. Beecher serve on the Editorial Advisory Board of Clinical Psychiatry News.
VIDEO: Consider alopecia ‘camouflage kits’ to boost patients’ self-esteem
ORLANDO – The hair loss encounter – which can be challenging for both physicians and patients – should address the negative psychological effects of hair loss, including ways to camouflage hair loss, advised Adriana N. Schmidt, MD, a dermatologist in Santa Monica, Calif.
Dermatologists may spend so much time on the work-up – reviewing history regarding medication, lab values, and hair care practices – that they do not spend time to simply say to patients, “I want to help you feel better about yourself, and here’s how,” she said in a video interview at the annual meeting of the American Academy of Dermatology.
“What we can do is offer them a way to camouflage the hair loss,” Dr. Schmidt said. She shared tips that include creating a kit to keep in the office filled with lists of reputable hairpiece vendors and tattoo specialists in the community, as well as sample wigs, cosmetic powders, and other items to show to patients during hair loss consultations. She also offers thoughts on working with new hair styles and stylists to help improve the self-esteem of alopecia patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Schmidt had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
ORLANDO – The hair loss encounter – which can be challenging for both physicians and patients – should address the negative psychological effects of hair loss, including ways to camouflage hair loss, advised Adriana N. Schmidt, MD, a dermatologist in Santa Monica, Calif.
Dermatologists may spend so much time on the work-up – reviewing history regarding medication, lab values, and hair care practices – that they do not spend time to simply say to patients, “I want to help you feel better about yourself, and here’s how,” she said in a video interview at the annual meeting of the American Academy of Dermatology.
“What we can do is offer them a way to camouflage the hair loss,” Dr. Schmidt said. She shared tips that include creating a kit to keep in the office filled with lists of reputable hairpiece vendors and tattoo specialists in the community, as well as sample wigs, cosmetic powders, and other items to show to patients during hair loss consultations. She also offers thoughts on working with new hair styles and stylists to help improve the self-esteem of alopecia patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Schmidt had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
ORLANDO – The hair loss encounter – which can be challenging for both physicians and patients – should address the negative psychological effects of hair loss, including ways to camouflage hair loss, advised Adriana N. Schmidt, MD, a dermatologist in Santa Monica, Calif.
Dermatologists may spend so much time on the work-up – reviewing history regarding medication, lab values, and hair care practices – that they do not spend time to simply say to patients, “I want to help you feel better about yourself, and here’s how,” she said in a video interview at the annual meeting of the American Academy of Dermatology.
“What we can do is offer them a way to camouflage the hair loss,” Dr. Schmidt said. She shared tips that include creating a kit to keep in the office filled with lists of reputable hairpiece vendors and tattoo specialists in the community, as well as sample wigs, cosmetic powders, and other items to show to patients during hair loss consultations. She also offers thoughts on working with new hair styles and stylists to help improve the self-esteem of alopecia patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Schmidt had no relevant disclosures.
[email protected]
On Twitter @whitneymcknight
AT AAD 2017
Improving Caregiver Knowledge of Support Resources
In 2012, 1.3 million veterans who were wounded in action had a severe service-connected disability—nearly triple the number in 2001 (482,000).1 Given the increased number of wounded veterans, the need for caregivers also increased.
The act of caring for an individual with a chronic disability can be a daunting task for the caregiver. However, what is not so commonly recognized is the need for caregiver awareness of the available support resources. Caregivers who do not receive necessary support experience physical and emotional consequences that interfere with their ability to care for veterans with disabilities. Therefore, there is a significant need to provide adequate support for the caregiver to maintain optimum care of the veteran.2
Increased caregiver strain among family caregivers of veterans with long-term disabilities and their lack of knowledge of support resources is a clinical concern. A comprehensive review of the literature provided evidence that access and use of caregiver support resources improved caregiver quality of life.
The purpose of this project was to provide an educational intervention of caregiver resources that were available at the Durham VA Health Care System in North Carolina and in the surrounding community. The desired outcomes included (a) increasing the caregiver’s knowledge of resources available at the VA and within the community to decrease caregiver burden; and (b) assisting the caregiver in determining the best resources for the caregiver and patient. This project was deemed to be a quality improvement project and did not require institutional review board (IRB) approval.
Background
The term strain is used to describe the burden, trouble, or burnout that a caregiver encounters when caring for a person with a long-term illness or disability.3 Caregivers of veterans remain in their role longer and have a heavier burden of care than that of all other caregivers: 65% are in a high-burden caregiving situation compared with 31% nationally.4 The consequence of providing care without assistance has all the features of chronic stress.2 Moreover, the decline of the caregiver’s health can significantly compromise the ability to provide care.3
Empirical observations of the negative health effects of caregiving noted over the past 2 decades have helped convince policymakers that supporting caregivers is an important public health issue.2 To this end, Congress mandated legislation that required the VA to provide a support program for veteran caregivers. In May 2010, President Obama signed the Caregivers and Veterans Omnibus Health Services Act of 2010 into law.1
Supporting Literature
The VA caregiver resource program offers a variety of support resources.1 A better understanding of caregiver needs is necessary to provide the right support resources, improve the health and well-being of caregivers, and make decisions regarding individual caregiving situations.5 For example, respite care offers temporary or periodic relief from caregiving, allowing caregivers to attend to personal tasks, such as shopping, running errands, relaxing, and socializing. This service can increase the physical and mental well-being of the caregiver.6 Studies show that early use of support services is paramount in order for caregivers to receive the greatest positive impact.5
Chen and colleaguesconducted a study of 164 caregivers. The study showed that caregivers who received assistance with accessing the correct support resource exhibited considerably higher satisfaction with the services they received.7 Determining which support therapy was best for the caregiver and the patient for whom they were caring was seen as the initial step. Providing a tool that supplies all the information caregivers need as well as assisting them with accessing services more efficiently is beneficial.
The National Alliance for Caregiving conducted a study to evaluate the needs of caregivers of veterans of various conflicts.4 In the study, caregivers reported that a resource guide would be beneficial. Some of the services they wanted to include in the directory were VA disability benefits, respite care, home health care, hospice services, assisted living, rehabilitation therapies, caregiver support group information, and community resources.
Based on the literature, the author believed that better knowledge of support resources was needed for caregivers. The literature included detailed descriptions of how knowledge of support resources improved caregiver’s well-being by increasing his or her ability to cope with stress related to providing care. However, the literature could have provided a more elaborate discussion on this topic. That was the only weakness identified in each of the studies. Nonetheless, it was clearly noted that resource knowledge yielded a positive effect.
Methods
The project took place at the Durham VA Health Care System and was implemented from August 2015 to October 2015. The participants targeted were caregivers of veterans with disabilities who were considered the veteran’s primary caregiver. Participation was voluntary.
During the veteran’s clinic appointment, the caregiver was given an implied consent letter, pre- and postquestionnaire forms, and a caregiver resource manual. The manual included information on caregiver support resources at the Durham VA Health Care System and in the community (eg, adult day care centers; home-based primary care, hospice care, skilled care, and telehealth; homemaker and home health aide programs; respite care). Other information was provided, such as the Caregiver Support Program application process, contact names, numbers, and helpful websites. Before reading the manual, participants completed the prequestionnaire form and returned it the day of the veteran’s visit. After reading the manual, the caregiver was instructed to complete the postquestionnaire form.
The project coordinator (PC) collaborated with the Veteran Health Education coordinator in developing the caregiver resource manual and questionnaires to ensure that the material met the requirements set forth by the educational program within the Durham VA Health Care System. The PC also collaborated with the Caregiver Support Program subject experts, the chief and acting assistant chief of social work when formulating the contents of the manual and questionnaires. The questionnaires were used to assess the effectiveness of the manual.
The 3 questions on the prequestionnaire and 3 questions on the postquestionnaire were geared to measure the caregiver’s knowledge. There also were 4 questions on the postquestionnaire that were used to address manual revisions.
On the prequestionnaire form, the following questions were asked: (1) If you needed to find caregiver support resources, how much knowledge do you have finding the resources that fit your needs as well as the veteran’s needs? (2) Rate how aware you are with knowing what caregiver support resources are available at the VA and within the community; and (3) Would knowing which caregiver support resources to choose from at the VA and within the community decrease your stress level and give you “peace of mind?”
The same questions were asked postintervention, and the participants were asked to rate their knowledge after reviewing the manual. The participant’s responses on the questionnaires were measured using a 5-point Likert scale.
Participant Demographics
Demographic information was obtained from the cover letter distributed to each participant. The demographic information included age, gender, relationship to the veteran, and number of years to date in the current caregiving role. Participants eligible for inclusion in this project were primary caregivers of veterans with disabilities from all eras of conflict, aged ≥ 18 years.
Fifteen caregivers participated by returning the cover letter containing the implied consent, reading the manual, and completing the pre- and postquestionnaires. There was a wide age range of caregivers who participated, from 29 to 77 years. Of those who responded, there also was a wide range in time in their current caregiving role, ranging from 1 to 41 years. The mean number of years in the current caregiving role was 7 years.
Of the 15 participants, most were female spouses. There were no husbands who participated. The relative’s category included a cousin, a son, and a daughter. The “other” category included a son-in-law and a fiancé.
Data Analysis
Both outcomes were measured using the responses from questions 1 through 3 with the use of running a descriptive statistical analysis. In addition, a t test was used to determine statistical significance, set at α level < .05 of knowledge increase from pre- to postintervention data. Based on the facility, educational benchmarks were set at 80% with the 80% equal to 4 on the Likert scale. Therefore, 80% was the identified benchmark for this project. The goal was that > 70% of the participants would score 80% or better on the postquestionnaire.
Results
Both outcomes were met: (1) increasing the caregiver’s knowledge regarding resources available at the VA and within the community to decrease caregiver burden; and (2) assisting the caregiver in deciding which caregiver resources located in the manual were the right fit for the caregiver and the veteran for whom they were caring. The percentage of participants who scored 80% or better on the prequestionnaire was 54% (n = 8). The postquestionnaire outcomes were considered an improvement based on caregiver’s knowledge of support resources as well as whether the information in the manual decreased their stress level and gave them peace of mind. The intended outcome for the postquestionnaire was that > 70% of the participants would score ≥ 80% after the intervention. This goal was met as final results revealed 73% (n = 11) of the participants scored > 80% on the postquestionnaire.
The postresults supported that caregivers’ knowledge increased, they had peace of mind, and stress levels were decreased with the use of an educational intervention, a comprehensive Caregiver Resource Manual. The postquestionnaire revealed that all of the participants found the Caregiver Resource Manual easy to navigate, and 93% of participants found the Caregiver Resource Manual useful. Out of 15 participants, 8 provided comments. Seven provided positive comments, reporting that the information in the manual was interesting, the manual was simple/easy to read, and the outside resources listed were helpful.
The participant who provided a negative comment was one of the caregivers who did not meet the benchmark of 80% on the pre- or postquestionnaire. The participant was a 33-year-old wife of a veteran with disabilities who had been in the current caregiving role for 9 years. This participant reported that the Caregiver Resource Manual was not geared to younger caregivers, so she would not benefit from using the manual. This caregiver also was the only participant who reported that the Caregiver Resource Manual neither gave her peace of mind nor decreased her stress level.
Comments or suggestions would have been helpful from the other 8 individuals. Because it was not written in the IRB proposal to contact the participants other than to follow-up with telephone calls regarding unreturned questionnaires, no further contact was made with the participants.
Discussion
The preliminary success of this project suggests that there is a significant need for an educational conduit to ensure sufficient caregiver knowledge. Interprofessional collaborative efforts along with using information systems/technology to deliver the Caregiver Resource Manual electronically are important future consideration for improvement of the overall outcomes for a wide range of caregivers, veterans, and health care providers. Health care policy changes on the organizational level, systems level, and national level could further support caregivers of disabled veterans by enabling easy access to caregiver resources as a mandated practice.
Limitations
Limitations centered on the recruitment process. There were a total of 15 caregivers who participated. Although the participation did not meet the PC’s expectation, the final number of participants was adequate in obtaining data regarding evaluating the impact of this project.
Conclusion
The results of this project provided evidence that the Caregiver Resource Manual was effective. Caregivers gained a sense of knowledge and empowerment regarding available resources within the VA and the community. Providing the caregiver with peace of mind and improving the overall health and well-being of the caregiver and veteran were essential.
Moreover, just as the veterans who fought for freedom were equipped with full body armor to help protect them from the potential negative consequences of combat, caregivers who care for these brave soldiers are now equipped with a resource tool and a “full armor of knowledge” to care for their loved ones…our nation’s heroes…our veterans.
1. VA Health Care. Actions needed to address higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/665928.pdf. Published September 18, 2014. Accessed January 9, 2017.
2. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(suppl 9):23-27.
3. Centers for Disease Control; Kimberly-Clark Corporation. Assuring healthy caregivers, a public health approach to translating research into practice: the RE-AIM framework. https://www.cdc.gov /aging/pdf/caregiving_monograph.pdf. Published 2008. Accessed January 9, 2017.
4. National Alliance for Caregiving. Caregivers of veterans—serving on the home front report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB _FINAL.pdf. Published 2010. Accessed January 9, 2017.
5. Whittier S, Coon D, Aaker J. Caregiving support interventions. http://cssr.berkeley.edu/pdfs/famcare_04.pdf. Updated 2001. Accessed January 9, 2017.
6. Alzheimer’s Association. Respite care. http://www .alz.org/care/alzheimers-dementia-caregiver-respite.asp. Updated 2016. Accessed January 9, 2017.
7. Chen YM, Hedrick SC, Young HM. A pilot evaluation of the family caregiver support program. Eval Program Plann. 2010;33(2):113-119.
In 2012, 1.3 million veterans who were wounded in action had a severe service-connected disability—nearly triple the number in 2001 (482,000).1 Given the increased number of wounded veterans, the need for caregivers also increased.
The act of caring for an individual with a chronic disability can be a daunting task for the caregiver. However, what is not so commonly recognized is the need for caregiver awareness of the available support resources. Caregivers who do not receive necessary support experience physical and emotional consequences that interfere with their ability to care for veterans with disabilities. Therefore, there is a significant need to provide adequate support for the caregiver to maintain optimum care of the veteran.2
Increased caregiver strain among family caregivers of veterans with long-term disabilities and their lack of knowledge of support resources is a clinical concern. A comprehensive review of the literature provided evidence that access and use of caregiver support resources improved caregiver quality of life.
The purpose of this project was to provide an educational intervention of caregiver resources that were available at the Durham VA Health Care System in North Carolina and in the surrounding community. The desired outcomes included (a) increasing the caregiver’s knowledge of resources available at the VA and within the community to decrease caregiver burden; and (b) assisting the caregiver in determining the best resources for the caregiver and patient. This project was deemed to be a quality improvement project and did not require institutional review board (IRB) approval.
Background
The term strain is used to describe the burden, trouble, or burnout that a caregiver encounters when caring for a person with a long-term illness or disability.3 Caregivers of veterans remain in their role longer and have a heavier burden of care than that of all other caregivers: 65% are in a high-burden caregiving situation compared with 31% nationally.4 The consequence of providing care without assistance has all the features of chronic stress.2 Moreover, the decline of the caregiver’s health can significantly compromise the ability to provide care.3
Empirical observations of the negative health effects of caregiving noted over the past 2 decades have helped convince policymakers that supporting caregivers is an important public health issue.2 To this end, Congress mandated legislation that required the VA to provide a support program for veteran caregivers. In May 2010, President Obama signed the Caregivers and Veterans Omnibus Health Services Act of 2010 into law.1
Supporting Literature
The VA caregiver resource program offers a variety of support resources.1 A better understanding of caregiver needs is necessary to provide the right support resources, improve the health and well-being of caregivers, and make decisions regarding individual caregiving situations.5 For example, respite care offers temporary or periodic relief from caregiving, allowing caregivers to attend to personal tasks, such as shopping, running errands, relaxing, and socializing. This service can increase the physical and mental well-being of the caregiver.6 Studies show that early use of support services is paramount in order for caregivers to receive the greatest positive impact.5
Chen and colleaguesconducted a study of 164 caregivers. The study showed that caregivers who received assistance with accessing the correct support resource exhibited considerably higher satisfaction with the services they received.7 Determining which support therapy was best for the caregiver and the patient for whom they were caring was seen as the initial step. Providing a tool that supplies all the information caregivers need as well as assisting them with accessing services more efficiently is beneficial.
The National Alliance for Caregiving conducted a study to evaluate the needs of caregivers of veterans of various conflicts.4 In the study, caregivers reported that a resource guide would be beneficial. Some of the services they wanted to include in the directory were VA disability benefits, respite care, home health care, hospice services, assisted living, rehabilitation therapies, caregiver support group information, and community resources.
Based on the literature, the author believed that better knowledge of support resources was needed for caregivers. The literature included detailed descriptions of how knowledge of support resources improved caregiver’s well-being by increasing his or her ability to cope with stress related to providing care. However, the literature could have provided a more elaborate discussion on this topic. That was the only weakness identified in each of the studies. Nonetheless, it was clearly noted that resource knowledge yielded a positive effect.
Methods
The project took place at the Durham VA Health Care System and was implemented from August 2015 to October 2015. The participants targeted were caregivers of veterans with disabilities who were considered the veteran’s primary caregiver. Participation was voluntary.
During the veteran’s clinic appointment, the caregiver was given an implied consent letter, pre- and postquestionnaire forms, and a caregiver resource manual. The manual included information on caregiver support resources at the Durham VA Health Care System and in the community (eg, adult day care centers; home-based primary care, hospice care, skilled care, and telehealth; homemaker and home health aide programs; respite care). Other information was provided, such as the Caregiver Support Program application process, contact names, numbers, and helpful websites. Before reading the manual, participants completed the prequestionnaire form and returned it the day of the veteran’s visit. After reading the manual, the caregiver was instructed to complete the postquestionnaire form.
The project coordinator (PC) collaborated with the Veteran Health Education coordinator in developing the caregiver resource manual and questionnaires to ensure that the material met the requirements set forth by the educational program within the Durham VA Health Care System. The PC also collaborated with the Caregiver Support Program subject experts, the chief and acting assistant chief of social work when formulating the contents of the manual and questionnaires. The questionnaires were used to assess the effectiveness of the manual.
The 3 questions on the prequestionnaire and 3 questions on the postquestionnaire were geared to measure the caregiver’s knowledge. There also were 4 questions on the postquestionnaire that were used to address manual revisions.
On the prequestionnaire form, the following questions were asked: (1) If you needed to find caregiver support resources, how much knowledge do you have finding the resources that fit your needs as well as the veteran’s needs? (2) Rate how aware you are with knowing what caregiver support resources are available at the VA and within the community; and (3) Would knowing which caregiver support resources to choose from at the VA and within the community decrease your stress level and give you “peace of mind?”
The same questions were asked postintervention, and the participants were asked to rate their knowledge after reviewing the manual. The participant’s responses on the questionnaires were measured using a 5-point Likert scale.
Participant Demographics
Demographic information was obtained from the cover letter distributed to each participant. The demographic information included age, gender, relationship to the veteran, and number of years to date in the current caregiving role. Participants eligible for inclusion in this project were primary caregivers of veterans with disabilities from all eras of conflict, aged ≥ 18 years.
Fifteen caregivers participated by returning the cover letter containing the implied consent, reading the manual, and completing the pre- and postquestionnaires. There was a wide age range of caregivers who participated, from 29 to 77 years. Of those who responded, there also was a wide range in time in their current caregiving role, ranging from 1 to 41 years. The mean number of years in the current caregiving role was 7 years.
Of the 15 participants, most were female spouses. There were no husbands who participated. The relative’s category included a cousin, a son, and a daughter. The “other” category included a son-in-law and a fiancé.
Data Analysis
Both outcomes were measured using the responses from questions 1 through 3 with the use of running a descriptive statistical analysis. In addition, a t test was used to determine statistical significance, set at α level < .05 of knowledge increase from pre- to postintervention data. Based on the facility, educational benchmarks were set at 80% with the 80% equal to 4 on the Likert scale. Therefore, 80% was the identified benchmark for this project. The goal was that > 70% of the participants would score 80% or better on the postquestionnaire.
Results
Both outcomes were met: (1) increasing the caregiver’s knowledge regarding resources available at the VA and within the community to decrease caregiver burden; and (2) assisting the caregiver in deciding which caregiver resources located in the manual were the right fit for the caregiver and the veteran for whom they were caring. The percentage of participants who scored 80% or better on the prequestionnaire was 54% (n = 8). The postquestionnaire outcomes were considered an improvement based on caregiver’s knowledge of support resources as well as whether the information in the manual decreased their stress level and gave them peace of mind. The intended outcome for the postquestionnaire was that > 70% of the participants would score ≥ 80% after the intervention. This goal was met as final results revealed 73% (n = 11) of the participants scored > 80% on the postquestionnaire.
The postresults supported that caregivers’ knowledge increased, they had peace of mind, and stress levels were decreased with the use of an educational intervention, a comprehensive Caregiver Resource Manual. The postquestionnaire revealed that all of the participants found the Caregiver Resource Manual easy to navigate, and 93% of participants found the Caregiver Resource Manual useful. Out of 15 participants, 8 provided comments. Seven provided positive comments, reporting that the information in the manual was interesting, the manual was simple/easy to read, and the outside resources listed were helpful.
The participant who provided a negative comment was one of the caregivers who did not meet the benchmark of 80% on the pre- or postquestionnaire. The participant was a 33-year-old wife of a veteran with disabilities who had been in the current caregiving role for 9 years. This participant reported that the Caregiver Resource Manual was not geared to younger caregivers, so she would not benefit from using the manual. This caregiver also was the only participant who reported that the Caregiver Resource Manual neither gave her peace of mind nor decreased her stress level.
Comments or suggestions would have been helpful from the other 8 individuals. Because it was not written in the IRB proposal to contact the participants other than to follow-up with telephone calls regarding unreturned questionnaires, no further contact was made with the participants.
Discussion
The preliminary success of this project suggests that there is a significant need for an educational conduit to ensure sufficient caregiver knowledge. Interprofessional collaborative efforts along with using information systems/technology to deliver the Caregiver Resource Manual electronically are important future consideration for improvement of the overall outcomes for a wide range of caregivers, veterans, and health care providers. Health care policy changes on the organizational level, systems level, and national level could further support caregivers of disabled veterans by enabling easy access to caregiver resources as a mandated practice.
Limitations
Limitations centered on the recruitment process. There were a total of 15 caregivers who participated. Although the participation did not meet the PC’s expectation, the final number of participants was adequate in obtaining data regarding evaluating the impact of this project.
Conclusion
The results of this project provided evidence that the Caregiver Resource Manual was effective. Caregivers gained a sense of knowledge and empowerment regarding available resources within the VA and the community. Providing the caregiver with peace of mind and improving the overall health and well-being of the caregiver and veteran were essential.
Moreover, just as the veterans who fought for freedom were equipped with full body armor to help protect them from the potential negative consequences of combat, caregivers who care for these brave soldiers are now equipped with a resource tool and a “full armor of knowledge” to care for their loved ones…our nation’s heroes…our veterans.
In 2012, 1.3 million veterans who were wounded in action had a severe service-connected disability—nearly triple the number in 2001 (482,000).1 Given the increased number of wounded veterans, the need for caregivers also increased.
The act of caring for an individual with a chronic disability can be a daunting task for the caregiver. However, what is not so commonly recognized is the need for caregiver awareness of the available support resources. Caregivers who do not receive necessary support experience physical and emotional consequences that interfere with their ability to care for veterans with disabilities. Therefore, there is a significant need to provide adequate support for the caregiver to maintain optimum care of the veteran.2
Increased caregiver strain among family caregivers of veterans with long-term disabilities and their lack of knowledge of support resources is a clinical concern. A comprehensive review of the literature provided evidence that access and use of caregiver support resources improved caregiver quality of life.
The purpose of this project was to provide an educational intervention of caregiver resources that were available at the Durham VA Health Care System in North Carolina and in the surrounding community. The desired outcomes included (a) increasing the caregiver’s knowledge of resources available at the VA and within the community to decrease caregiver burden; and (b) assisting the caregiver in determining the best resources for the caregiver and patient. This project was deemed to be a quality improvement project and did not require institutional review board (IRB) approval.
Background
The term strain is used to describe the burden, trouble, or burnout that a caregiver encounters when caring for a person with a long-term illness or disability.3 Caregivers of veterans remain in their role longer and have a heavier burden of care than that of all other caregivers: 65% are in a high-burden caregiving situation compared with 31% nationally.4 The consequence of providing care without assistance has all the features of chronic stress.2 Moreover, the decline of the caregiver’s health can significantly compromise the ability to provide care.3
Empirical observations of the negative health effects of caregiving noted over the past 2 decades have helped convince policymakers that supporting caregivers is an important public health issue.2 To this end, Congress mandated legislation that required the VA to provide a support program for veteran caregivers. In May 2010, President Obama signed the Caregivers and Veterans Omnibus Health Services Act of 2010 into law.1
Supporting Literature
The VA caregiver resource program offers a variety of support resources.1 A better understanding of caregiver needs is necessary to provide the right support resources, improve the health and well-being of caregivers, and make decisions regarding individual caregiving situations.5 For example, respite care offers temporary or periodic relief from caregiving, allowing caregivers to attend to personal tasks, such as shopping, running errands, relaxing, and socializing. This service can increase the physical and mental well-being of the caregiver.6 Studies show that early use of support services is paramount in order for caregivers to receive the greatest positive impact.5
Chen and colleaguesconducted a study of 164 caregivers. The study showed that caregivers who received assistance with accessing the correct support resource exhibited considerably higher satisfaction with the services they received.7 Determining which support therapy was best for the caregiver and the patient for whom they were caring was seen as the initial step. Providing a tool that supplies all the information caregivers need as well as assisting them with accessing services more efficiently is beneficial.
The National Alliance for Caregiving conducted a study to evaluate the needs of caregivers of veterans of various conflicts.4 In the study, caregivers reported that a resource guide would be beneficial. Some of the services they wanted to include in the directory were VA disability benefits, respite care, home health care, hospice services, assisted living, rehabilitation therapies, caregiver support group information, and community resources.
Based on the literature, the author believed that better knowledge of support resources was needed for caregivers. The literature included detailed descriptions of how knowledge of support resources improved caregiver’s well-being by increasing his or her ability to cope with stress related to providing care. However, the literature could have provided a more elaborate discussion on this topic. That was the only weakness identified in each of the studies. Nonetheless, it was clearly noted that resource knowledge yielded a positive effect.
Methods
The project took place at the Durham VA Health Care System and was implemented from August 2015 to October 2015. The participants targeted were caregivers of veterans with disabilities who were considered the veteran’s primary caregiver. Participation was voluntary.
During the veteran’s clinic appointment, the caregiver was given an implied consent letter, pre- and postquestionnaire forms, and a caregiver resource manual. The manual included information on caregiver support resources at the Durham VA Health Care System and in the community (eg, adult day care centers; home-based primary care, hospice care, skilled care, and telehealth; homemaker and home health aide programs; respite care). Other information was provided, such as the Caregiver Support Program application process, contact names, numbers, and helpful websites. Before reading the manual, participants completed the prequestionnaire form and returned it the day of the veteran’s visit. After reading the manual, the caregiver was instructed to complete the postquestionnaire form.
The project coordinator (PC) collaborated with the Veteran Health Education coordinator in developing the caregiver resource manual and questionnaires to ensure that the material met the requirements set forth by the educational program within the Durham VA Health Care System. The PC also collaborated with the Caregiver Support Program subject experts, the chief and acting assistant chief of social work when formulating the contents of the manual and questionnaires. The questionnaires were used to assess the effectiveness of the manual.
The 3 questions on the prequestionnaire and 3 questions on the postquestionnaire were geared to measure the caregiver’s knowledge. There also were 4 questions on the postquestionnaire that were used to address manual revisions.
On the prequestionnaire form, the following questions were asked: (1) If you needed to find caregiver support resources, how much knowledge do you have finding the resources that fit your needs as well as the veteran’s needs? (2) Rate how aware you are with knowing what caregiver support resources are available at the VA and within the community; and (3) Would knowing which caregiver support resources to choose from at the VA and within the community decrease your stress level and give you “peace of mind?”
The same questions were asked postintervention, and the participants were asked to rate their knowledge after reviewing the manual. The participant’s responses on the questionnaires were measured using a 5-point Likert scale.
Participant Demographics
Demographic information was obtained from the cover letter distributed to each participant. The demographic information included age, gender, relationship to the veteran, and number of years to date in the current caregiving role. Participants eligible for inclusion in this project were primary caregivers of veterans with disabilities from all eras of conflict, aged ≥ 18 years.
Fifteen caregivers participated by returning the cover letter containing the implied consent, reading the manual, and completing the pre- and postquestionnaires. There was a wide age range of caregivers who participated, from 29 to 77 years. Of those who responded, there also was a wide range in time in their current caregiving role, ranging from 1 to 41 years. The mean number of years in the current caregiving role was 7 years.
Of the 15 participants, most were female spouses. There were no husbands who participated. The relative’s category included a cousin, a son, and a daughter. The “other” category included a son-in-law and a fiancé.
Data Analysis
Both outcomes were measured using the responses from questions 1 through 3 with the use of running a descriptive statistical analysis. In addition, a t test was used to determine statistical significance, set at α level < .05 of knowledge increase from pre- to postintervention data. Based on the facility, educational benchmarks were set at 80% with the 80% equal to 4 on the Likert scale. Therefore, 80% was the identified benchmark for this project. The goal was that > 70% of the participants would score 80% or better on the postquestionnaire.
Results
Both outcomes were met: (1) increasing the caregiver’s knowledge regarding resources available at the VA and within the community to decrease caregiver burden; and (2) assisting the caregiver in deciding which caregiver resources located in the manual were the right fit for the caregiver and the veteran for whom they were caring. The percentage of participants who scored 80% or better on the prequestionnaire was 54% (n = 8). The postquestionnaire outcomes were considered an improvement based on caregiver’s knowledge of support resources as well as whether the information in the manual decreased their stress level and gave them peace of mind. The intended outcome for the postquestionnaire was that > 70% of the participants would score ≥ 80% after the intervention. This goal was met as final results revealed 73% (n = 11) of the participants scored > 80% on the postquestionnaire.
The postresults supported that caregivers’ knowledge increased, they had peace of mind, and stress levels were decreased with the use of an educational intervention, a comprehensive Caregiver Resource Manual. The postquestionnaire revealed that all of the participants found the Caregiver Resource Manual easy to navigate, and 93% of participants found the Caregiver Resource Manual useful. Out of 15 participants, 8 provided comments. Seven provided positive comments, reporting that the information in the manual was interesting, the manual was simple/easy to read, and the outside resources listed were helpful.
The participant who provided a negative comment was one of the caregivers who did not meet the benchmark of 80% on the pre- or postquestionnaire. The participant was a 33-year-old wife of a veteran with disabilities who had been in the current caregiving role for 9 years. This participant reported that the Caregiver Resource Manual was not geared to younger caregivers, so she would not benefit from using the manual. This caregiver also was the only participant who reported that the Caregiver Resource Manual neither gave her peace of mind nor decreased her stress level.
Comments or suggestions would have been helpful from the other 8 individuals. Because it was not written in the IRB proposal to contact the participants other than to follow-up with telephone calls regarding unreturned questionnaires, no further contact was made with the participants.
Discussion
The preliminary success of this project suggests that there is a significant need for an educational conduit to ensure sufficient caregiver knowledge. Interprofessional collaborative efforts along with using information systems/technology to deliver the Caregiver Resource Manual electronically are important future consideration for improvement of the overall outcomes for a wide range of caregivers, veterans, and health care providers. Health care policy changes on the organizational level, systems level, and national level could further support caregivers of disabled veterans by enabling easy access to caregiver resources as a mandated practice.
Limitations
Limitations centered on the recruitment process. There were a total of 15 caregivers who participated. Although the participation did not meet the PC’s expectation, the final number of participants was adequate in obtaining data regarding evaluating the impact of this project.
Conclusion
The results of this project provided evidence that the Caregiver Resource Manual was effective. Caregivers gained a sense of knowledge and empowerment regarding available resources within the VA and the community. Providing the caregiver with peace of mind and improving the overall health and well-being of the caregiver and veteran were essential.
Moreover, just as the veterans who fought for freedom were equipped with full body armor to help protect them from the potential negative consequences of combat, caregivers who care for these brave soldiers are now equipped with a resource tool and a “full armor of knowledge” to care for their loved ones…our nation’s heroes…our veterans.
1. VA Health Care. Actions needed to address higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/665928.pdf. Published September 18, 2014. Accessed January 9, 2017.
2. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(suppl 9):23-27.
3. Centers for Disease Control; Kimberly-Clark Corporation. Assuring healthy caregivers, a public health approach to translating research into practice: the RE-AIM framework. https://www.cdc.gov /aging/pdf/caregiving_monograph.pdf. Published 2008. Accessed January 9, 2017.
4. National Alliance for Caregiving. Caregivers of veterans—serving on the home front report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB _FINAL.pdf. Published 2010. Accessed January 9, 2017.
5. Whittier S, Coon D, Aaker J. Caregiving support interventions. http://cssr.berkeley.edu/pdfs/famcare_04.pdf. Updated 2001. Accessed January 9, 2017.
6. Alzheimer’s Association. Respite care. http://www .alz.org/care/alzheimers-dementia-caregiver-respite.asp. Updated 2016. Accessed January 9, 2017.
7. Chen YM, Hedrick SC, Young HM. A pilot evaluation of the family caregiver support program. Eval Program Plann. 2010;33(2):113-119.
1. VA Health Care. Actions needed to address higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/665928.pdf. Published September 18, 2014. Accessed January 9, 2017.
2. Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(suppl 9):23-27.
3. Centers for Disease Control; Kimberly-Clark Corporation. Assuring healthy caregivers, a public health approach to translating research into practice: the RE-AIM framework. https://www.cdc.gov /aging/pdf/caregiving_monograph.pdf. Published 2008. Accessed January 9, 2017.
4. National Alliance for Caregiving. Caregivers of veterans—serving on the home front report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB _FINAL.pdf. Published 2010. Accessed January 9, 2017.
5. Whittier S, Coon D, Aaker J. Caregiving support interventions. http://cssr.berkeley.edu/pdfs/famcare_04.pdf. Updated 2001. Accessed January 9, 2017.
6. Alzheimer’s Association. Respite care. http://www .alz.org/care/alzheimers-dementia-caregiver-respite.asp. Updated 2016. Accessed January 9, 2017.
7. Chen YM, Hedrick SC, Young HM. A pilot evaluation of the family caregiver support program. Eval Program Plann. 2010;33(2):113-119.
Oxybutynin Treatment for Hyperhidrosis in Spinal Cord Injury Patients
Hyperhidrosis (HH) is sweating beyond that which is required for thermoregulation.1 Secondary HH, which is usually caused by an underlying medical condition or drug, may be seen in patients with spinal cord injury (SCI) and can negatively impact psychosocial well-being and quality of life (QOL) if not treated.1
Information on the current prevalence of HH is lacking. In 2004, one study projected the prevalence of HH in the U.S. to be 2.8%.2 A previous study found that about 27% of the 154 patients with SCI reported experiencing HH that was annoying, with 28 (14.6%) of those reporting no contributing somatic causes.3 Somatic causes of HH include autonomic dysreflexia, posttraumatic syringomyelia, or orthostatic hypotension.4
Autonomic dysreflexia is a syndrome that describes a dramatic increase in blood pressure (BP) in patients with spinal cord lesion at or above T6 and is characterized by exaggerated autonomic responses to stimuli that are innocuous to individuals without SCI.5,6 Noxious stimuli that may trigger autonomic dysreflexia include bowel or bladder distension or obstruction, urinary infection, catheter insertion, suprapubic palpation, or skin irritation.6 Syringomyelia, another somatic cause of HH, is a cystic lesion on the spinal cord that may develop secondary to congenital anomalies or SCI.6-8
The following case reports describe 2 patients with SCI with different diagnoses and presentations of HH. Both were treated with oxybutynin for HH.
Case 1 Presentation
Mr. J is a 49-year-old with C6-C7 SCI attributed to a motor vehicle accident 26 years before. He presented to the primary care clinic for a routine visit in a self-propelled wheelchair. His diagnosis included tetraplegia, muscle spasms, osteoporosis, chronic pain syndrome, benign prosthetic hypertrophy, neurogenic bladder, and neurogenic bowel. He was noted to have a bath towel around his neck to wipe sweat from his face and neck. He did not recall when this condition started; however, he reported a prior trial of diazepam 5 mg as needed in 2006 in the mornings and before transfers when sweating was usually worst. He continued to use diazepam because it helped with his muscle spasms but not with sweating. His other medications included oral baclofen, alendronate, ibuprofen, docusate sodium, tamsulosin, calcium/vitamin C supplement, and bisacodyl suppository.
The patient’s surgical history was significant for anterior discectomy with C6-C7 fusion, sphincterotomy, transurethral resection of the prostate, and right urethral stent placement for hydronephrosis in 2004. His cystoscopy and renal sonogram were within normal limits. On physical examination, his vital signs were within normal limits. However, his long-sleeved shirt was wet on the front, and his neck, chest, and arms also were moist from excessive sweating. During his transfer to and from his wheelchair, he was noted to have chattering of his teeth. The remainder of the physical examination was negative for any other acute findings.
Mr. J was prescribed a 30-day trial of oxybutynin 5 mg 1 tablet by mouth per day for HH. During a 3-week follow-up telephone call, Mr. J reported that the oxybutynin was working well; the sweating on his chest had improved and had resolved on his face. Except for mild dryness of mouth, he was tolerating the medication well. There were no changes in his neurogenic bladder, which was managed with an external urinary device.
Six months later, Mr. J reported that oxybutynin continued to work well, and he no longer had to travel with a towel. He was able to go to a football game, social activities were more enjoyable, and he was not embarrassed because of excessive sweating.
Case 2 Presentation
Mr. B is a 48-year-old with T12 paraplegia secondary to a motor vehicle accident in 1994. He called the primary clinic for a visit because he was concerned about cold clammy hands for the past 6 to 7 months when he woke up in the morning and sometimes throughout the day. He was preparing to start his first semester in college. His diagnosis included neurogenic bowel and bladder and muscle spasms. There was no history of posttraumatic syringomyelia, and his medications included baclofen, dantrolene, diazepam, and multivitamins.
Mr. B took tolterodine 4 mg/d for several years, and for unknown reasons, about 6 years previously the prescription was changed to oxybutynin 15-mg extended release for his neurogenic bladder. He continued to have urinary leakage between the every 4-hour intermittent catherization, and oxybutynin was increased to 10-mg tablet twice per day.
About 7 months prior to this appointment, Mr. B independently stopped the oxybutynin as he felt that it was not making a difference in the management of his neurogenic bladder. It was noted that his cold clammy hands started about the same time that he discontinued the oxybutynin. He could not recall whether he had this symptom prior to initiation of any medication. It was mutually decided to restart the oxybutynin at a lower dose, this time not for his bladder but for the HH. He was ordered a 30-day trial of a 5-mg tablet once per day. About 3 weeks later, he sent a secure message to report oxybutynin’s effectiveness and to request a refill.
Discussion
Sweating is a physiological process that involves the active secretion of water by specialized sweat glands in the skin.9-11 There are 2 types of sweat glands in the skin; apocrine and eccrine.9 Collectively the 3 million eccrine sweat glands of the average person approximately equal the mass of a kidney and exceed the secretory rate of exocrine glands.9 They function in evaporative cooling in response to thermal or physiologic stimuli and are widely distributed over the body, especially on the forehead, back, palms, and soles.10
Sympathetic cholinergic nerves are mainly responsible for sweat secretion by the release of acetylcholine to activate muscarinic receptors on the gland.11 Postganglionic fibers from sympathetic nerve cells innervate sweat glands that release cholinergics.6 Postganglionic cholinergic receptors that are activated by muscarinic drugs are termed muscarinic receptors and are readily accessible to antimuscarinic drugs.6,12 Anticholinergic/antimuscarinic agents antagonize muscarinic receptors and suppress premature detrusor contractions to enhance bladder storage.13 They include oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine.13 Oxybutynin was used in both cases because it is on VA formulary. It was effective in treating HH, although the etiology is unclear and the presentations were different.
One retrospective study that analyzed 20 patients who received oxybutynin for primary HH at uncommon sites, such as the back and groin, found that QOL improved in 85% of the subjects after 6 weeks.14 Randomized placebo-controlled trials also have found oxybutynin effective for treatment of palmar and axillary HH and generalized HH.15,16
Syringomyelia was ruled out in both cases based on history and radiologic studies, specifically magnetic resonance imaging. Autonomic dysreflexia was ruled out as the HH was not an acute finding and BP was within normal limits. Orthostatic hypotension is a common finding in SCI, mainly in tetraplegic patients, and could be suspected in both cases. Sweating was usually worse in the mornings in both cases and during transfers, as noted in the first case.17 However, chronic autoregulation allows for chronic adaption to tissue hypoperfusion over time.16
Hyperhidrosis or other disorders of eccrine sweating can occur for various reasons, including changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons; dysfunction of the thermoregulatory centers in the brain’s central autonomic network; or changes in the muscarinic cholinergic synapse on sweat glands.18
Conclusion
Patients with SCI may have an acute or chronic presentation of HH. Removal of the inciting cause in the case of autonomic dysreflexia and/or the administration of a pharmaceutical agent is the usual treatment.
Regardless of the etiology of HH that persists, effective treatment should be a goal, especially in those patients whose QOL is affected by this condition. The outcome of treatment with oxybutynin in these case reports is consistent with the findings of the limited retrospective study and randomized placebo-controlled studies that show oxybutynin is effective for treating bothersome HH.14-16
The results of these case reports are not generalizable to patients with SCI and HH, nor are the results of the limited retrospective study and randomized placebo-controlled studies, as their sample sizes were small.14,16,17 However, information on the use of oxybutynin for the effective treatment of HH in the SCI population is promising. Research studies on the prevalence of HH and randomized placebo-controlled trials with a larger SCI population are considerations for future studies.
1. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241-248.
2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64(4):690-695.
3. Andersen LS, Biering-Sørensen F, Müller PG, Jensen IL, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;(30):184-191.
4. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5, pt 1):713-726.
5. Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59(1):1-21.
6. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
7. Milhort TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1.
8. National Institute of Neurological Disorders and Stroke. Syringomyelia fact sheet. https://www.ninds .nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Syringomyelia-Fact-Sheet. Accessed February 13, 2017.
9. Meschner AL. Junqueira’s Basic Histology. 14th ed. New York, NY: McGraw-Hill; 2016:371-392.
10. Mauro TM. Biology of eccrine and apocrine glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
11. Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016:261-272.
12. Kellogg DL Jr. Thermoregulation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
13. Rovner ES, Wyman J, Lam S. Urinary Incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1377-1396.
14. Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics (Sao Paulo). 2014;69(9):608-614.
15. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized-placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696-1700.
16. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis. Br J Dermatol. 2015;173(5):1163-1168.
17. Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):1-7.
18. Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012:chap 84.
Hyperhidrosis (HH) is sweating beyond that which is required for thermoregulation.1 Secondary HH, which is usually caused by an underlying medical condition or drug, may be seen in patients with spinal cord injury (SCI) and can negatively impact psychosocial well-being and quality of life (QOL) if not treated.1
Information on the current prevalence of HH is lacking. In 2004, one study projected the prevalence of HH in the U.S. to be 2.8%.2 A previous study found that about 27% of the 154 patients with SCI reported experiencing HH that was annoying, with 28 (14.6%) of those reporting no contributing somatic causes.3 Somatic causes of HH include autonomic dysreflexia, posttraumatic syringomyelia, or orthostatic hypotension.4
Autonomic dysreflexia is a syndrome that describes a dramatic increase in blood pressure (BP) in patients with spinal cord lesion at or above T6 and is characterized by exaggerated autonomic responses to stimuli that are innocuous to individuals without SCI.5,6 Noxious stimuli that may trigger autonomic dysreflexia include bowel or bladder distension or obstruction, urinary infection, catheter insertion, suprapubic palpation, or skin irritation.6 Syringomyelia, another somatic cause of HH, is a cystic lesion on the spinal cord that may develop secondary to congenital anomalies or SCI.6-8
The following case reports describe 2 patients with SCI with different diagnoses and presentations of HH. Both were treated with oxybutynin for HH.
Case 1 Presentation
Mr. J is a 49-year-old with C6-C7 SCI attributed to a motor vehicle accident 26 years before. He presented to the primary care clinic for a routine visit in a self-propelled wheelchair. His diagnosis included tetraplegia, muscle spasms, osteoporosis, chronic pain syndrome, benign prosthetic hypertrophy, neurogenic bladder, and neurogenic bowel. He was noted to have a bath towel around his neck to wipe sweat from his face and neck. He did not recall when this condition started; however, he reported a prior trial of diazepam 5 mg as needed in 2006 in the mornings and before transfers when sweating was usually worst. He continued to use diazepam because it helped with his muscle spasms but not with sweating. His other medications included oral baclofen, alendronate, ibuprofen, docusate sodium, tamsulosin, calcium/vitamin C supplement, and bisacodyl suppository.
The patient’s surgical history was significant for anterior discectomy with C6-C7 fusion, sphincterotomy, transurethral resection of the prostate, and right urethral stent placement for hydronephrosis in 2004. His cystoscopy and renal sonogram were within normal limits. On physical examination, his vital signs were within normal limits. However, his long-sleeved shirt was wet on the front, and his neck, chest, and arms also were moist from excessive sweating. During his transfer to and from his wheelchair, he was noted to have chattering of his teeth. The remainder of the physical examination was negative for any other acute findings.
Mr. J was prescribed a 30-day trial of oxybutynin 5 mg 1 tablet by mouth per day for HH. During a 3-week follow-up telephone call, Mr. J reported that the oxybutynin was working well; the sweating on his chest had improved and had resolved on his face. Except for mild dryness of mouth, he was tolerating the medication well. There were no changes in his neurogenic bladder, which was managed with an external urinary device.
Six months later, Mr. J reported that oxybutynin continued to work well, and he no longer had to travel with a towel. He was able to go to a football game, social activities were more enjoyable, and he was not embarrassed because of excessive sweating.
Case 2 Presentation
Mr. B is a 48-year-old with T12 paraplegia secondary to a motor vehicle accident in 1994. He called the primary clinic for a visit because he was concerned about cold clammy hands for the past 6 to 7 months when he woke up in the morning and sometimes throughout the day. He was preparing to start his first semester in college. His diagnosis included neurogenic bowel and bladder and muscle spasms. There was no history of posttraumatic syringomyelia, and his medications included baclofen, dantrolene, diazepam, and multivitamins.
Mr. B took tolterodine 4 mg/d for several years, and for unknown reasons, about 6 years previously the prescription was changed to oxybutynin 15-mg extended release for his neurogenic bladder. He continued to have urinary leakage between the every 4-hour intermittent catherization, and oxybutynin was increased to 10-mg tablet twice per day.
About 7 months prior to this appointment, Mr. B independently stopped the oxybutynin as he felt that it was not making a difference in the management of his neurogenic bladder. It was noted that his cold clammy hands started about the same time that he discontinued the oxybutynin. He could not recall whether he had this symptom prior to initiation of any medication. It was mutually decided to restart the oxybutynin at a lower dose, this time not for his bladder but for the HH. He was ordered a 30-day trial of a 5-mg tablet once per day. About 3 weeks later, he sent a secure message to report oxybutynin’s effectiveness and to request a refill.
Discussion
Sweating is a physiological process that involves the active secretion of water by specialized sweat glands in the skin.9-11 There are 2 types of sweat glands in the skin; apocrine and eccrine.9 Collectively the 3 million eccrine sweat glands of the average person approximately equal the mass of a kidney and exceed the secretory rate of exocrine glands.9 They function in evaporative cooling in response to thermal or physiologic stimuli and are widely distributed over the body, especially on the forehead, back, palms, and soles.10
Sympathetic cholinergic nerves are mainly responsible for sweat secretion by the release of acetylcholine to activate muscarinic receptors on the gland.11 Postganglionic fibers from sympathetic nerve cells innervate sweat glands that release cholinergics.6 Postganglionic cholinergic receptors that are activated by muscarinic drugs are termed muscarinic receptors and are readily accessible to antimuscarinic drugs.6,12 Anticholinergic/antimuscarinic agents antagonize muscarinic receptors and suppress premature detrusor contractions to enhance bladder storage.13 They include oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine.13 Oxybutynin was used in both cases because it is on VA formulary. It was effective in treating HH, although the etiology is unclear and the presentations were different.
One retrospective study that analyzed 20 patients who received oxybutynin for primary HH at uncommon sites, such as the back and groin, found that QOL improved in 85% of the subjects after 6 weeks.14 Randomized placebo-controlled trials also have found oxybutynin effective for treatment of palmar and axillary HH and generalized HH.15,16
Syringomyelia was ruled out in both cases based on history and radiologic studies, specifically magnetic resonance imaging. Autonomic dysreflexia was ruled out as the HH was not an acute finding and BP was within normal limits. Orthostatic hypotension is a common finding in SCI, mainly in tetraplegic patients, and could be suspected in both cases. Sweating was usually worse in the mornings in both cases and during transfers, as noted in the first case.17 However, chronic autoregulation allows for chronic adaption to tissue hypoperfusion over time.16
Hyperhidrosis or other disorders of eccrine sweating can occur for various reasons, including changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons; dysfunction of the thermoregulatory centers in the brain’s central autonomic network; or changes in the muscarinic cholinergic synapse on sweat glands.18
Conclusion
Patients with SCI may have an acute or chronic presentation of HH. Removal of the inciting cause in the case of autonomic dysreflexia and/or the administration of a pharmaceutical agent is the usual treatment.
Regardless of the etiology of HH that persists, effective treatment should be a goal, especially in those patients whose QOL is affected by this condition. The outcome of treatment with oxybutynin in these case reports is consistent with the findings of the limited retrospective study and randomized placebo-controlled studies that show oxybutynin is effective for treating bothersome HH.14-16
The results of these case reports are not generalizable to patients with SCI and HH, nor are the results of the limited retrospective study and randomized placebo-controlled studies, as their sample sizes were small.14,16,17 However, information on the use of oxybutynin for the effective treatment of HH in the SCI population is promising. Research studies on the prevalence of HH and randomized placebo-controlled trials with a larger SCI population are considerations for future studies.
Hyperhidrosis (HH) is sweating beyond that which is required for thermoregulation.1 Secondary HH, which is usually caused by an underlying medical condition or drug, may be seen in patients with spinal cord injury (SCI) and can negatively impact psychosocial well-being and quality of life (QOL) if not treated.1
Information on the current prevalence of HH is lacking. In 2004, one study projected the prevalence of HH in the U.S. to be 2.8%.2 A previous study found that about 27% of the 154 patients with SCI reported experiencing HH that was annoying, with 28 (14.6%) of those reporting no contributing somatic causes.3 Somatic causes of HH include autonomic dysreflexia, posttraumatic syringomyelia, or orthostatic hypotension.4
Autonomic dysreflexia is a syndrome that describes a dramatic increase in blood pressure (BP) in patients with spinal cord lesion at or above T6 and is characterized by exaggerated autonomic responses to stimuli that are innocuous to individuals without SCI.5,6 Noxious stimuli that may trigger autonomic dysreflexia include bowel or bladder distension or obstruction, urinary infection, catheter insertion, suprapubic palpation, or skin irritation.6 Syringomyelia, another somatic cause of HH, is a cystic lesion on the spinal cord that may develop secondary to congenital anomalies or SCI.6-8
The following case reports describe 2 patients with SCI with different diagnoses and presentations of HH. Both were treated with oxybutynin for HH.
Case 1 Presentation
Mr. J is a 49-year-old with C6-C7 SCI attributed to a motor vehicle accident 26 years before. He presented to the primary care clinic for a routine visit in a self-propelled wheelchair. His diagnosis included tetraplegia, muscle spasms, osteoporosis, chronic pain syndrome, benign prosthetic hypertrophy, neurogenic bladder, and neurogenic bowel. He was noted to have a bath towel around his neck to wipe sweat from his face and neck. He did not recall when this condition started; however, he reported a prior trial of diazepam 5 mg as needed in 2006 in the mornings and before transfers when sweating was usually worst. He continued to use diazepam because it helped with his muscle spasms but not with sweating. His other medications included oral baclofen, alendronate, ibuprofen, docusate sodium, tamsulosin, calcium/vitamin C supplement, and bisacodyl suppository.
The patient’s surgical history was significant for anterior discectomy with C6-C7 fusion, sphincterotomy, transurethral resection of the prostate, and right urethral stent placement for hydronephrosis in 2004. His cystoscopy and renal sonogram were within normal limits. On physical examination, his vital signs were within normal limits. However, his long-sleeved shirt was wet on the front, and his neck, chest, and arms also were moist from excessive sweating. During his transfer to and from his wheelchair, he was noted to have chattering of his teeth. The remainder of the physical examination was negative for any other acute findings.
Mr. J was prescribed a 30-day trial of oxybutynin 5 mg 1 tablet by mouth per day for HH. During a 3-week follow-up telephone call, Mr. J reported that the oxybutynin was working well; the sweating on his chest had improved and had resolved on his face. Except for mild dryness of mouth, he was tolerating the medication well. There were no changes in his neurogenic bladder, which was managed with an external urinary device.
Six months later, Mr. J reported that oxybutynin continued to work well, and he no longer had to travel with a towel. He was able to go to a football game, social activities were more enjoyable, and he was not embarrassed because of excessive sweating.
Case 2 Presentation
Mr. B is a 48-year-old with T12 paraplegia secondary to a motor vehicle accident in 1994. He called the primary clinic for a visit because he was concerned about cold clammy hands for the past 6 to 7 months when he woke up in the morning and sometimes throughout the day. He was preparing to start his first semester in college. His diagnosis included neurogenic bowel and bladder and muscle spasms. There was no history of posttraumatic syringomyelia, and his medications included baclofen, dantrolene, diazepam, and multivitamins.
Mr. B took tolterodine 4 mg/d for several years, and for unknown reasons, about 6 years previously the prescription was changed to oxybutynin 15-mg extended release for his neurogenic bladder. He continued to have urinary leakage between the every 4-hour intermittent catherization, and oxybutynin was increased to 10-mg tablet twice per day.
About 7 months prior to this appointment, Mr. B independently stopped the oxybutynin as he felt that it was not making a difference in the management of his neurogenic bladder. It was noted that his cold clammy hands started about the same time that he discontinued the oxybutynin. He could not recall whether he had this symptom prior to initiation of any medication. It was mutually decided to restart the oxybutynin at a lower dose, this time not for his bladder but for the HH. He was ordered a 30-day trial of a 5-mg tablet once per day. About 3 weeks later, he sent a secure message to report oxybutynin’s effectiveness and to request a refill.
Discussion
Sweating is a physiological process that involves the active secretion of water by specialized sweat glands in the skin.9-11 There are 2 types of sweat glands in the skin; apocrine and eccrine.9 Collectively the 3 million eccrine sweat glands of the average person approximately equal the mass of a kidney and exceed the secretory rate of exocrine glands.9 They function in evaporative cooling in response to thermal or physiologic stimuli and are widely distributed over the body, especially on the forehead, back, palms, and soles.10
Sympathetic cholinergic nerves are mainly responsible for sweat secretion by the release of acetylcholine to activate muscarinic receptors on the gland.11 Postganglionic fibers from sympathetic nerve cells innervate sweat glands that release cholinergics.6 Postganglionic cholinergic receptors that are activated by muscarinic drugs are termed muscarinic receptors and are readily accessible to antimuscarinic drugs.6,12 Anticholinergic/antimuscarinic agents antagonize muscarinic receptors and suppress premature detrusor contractions to enhance bladder storage.13 They include oxybutynin, tolterodine, trospium, solifenacin, darifenacin, and fesoterodine.13 Oxybutynin was used in both cases because it is on VA formulary. It was effective in treating HH, although the etiology is unclear and the presentations were different.
One retrospective study that analyzed 20 patients who received oxybutynin for primary HH at uncommon sites, such as the back and groin, found that QOL improved in 85% of the subjects after 6 weeks.14 Randomized placebo-controlled trials also have found oxybutynin effective for treatment of palmar and axillary HH and generalized HH.15,16
Syringomyelia was ruled out in both cases based on history and radiologic studies, specifically magnetic resonance imaging. Autonomic dysreflexia was ruled out as the HH was not an acute finding and BP was within normal limits. Orthostatic hypotension is a common finding in SCI, mainly in tetraplegic patients, and could be suspected in both cases. Sweating was usually worse in the mornings in both cases and during transfers, as noted in the first case.17 However, chronic autoregulation allows for chronic adaption to tissue hypoperfusion over time.16
Hyperhidrosis or other disorders of eccrine sweating can occur for various reasons, including changes in the spinal sympathetic preganglionic, ganglionic, or postganglionic neurons; dysfunction of the thermoregulatory centers in the brain’s central autonomic network; or changes in the muscarinic cholinergic synapse on sweat glands.18
Conclusion
Patients with SCI may have an acute or chronic presentation of HH. Removal of the inciting cause in the case of autonomic dysreflexia and/or the administration of a pharmaceutical agent is the usual treatment.
Regardless of the etiology of HH that persists, effective treatment should be a goal, especially in those patients whose QOL is affected by this condition. The outcome of treatment with oxybutynin in these case reports is consistent with the findings of the limited retrospective study and randomized placebo-controlled studies that show oxybutynin is effective for treating bothersome HH.14-16
The results of these case reports are not generalizable to patients with SCI and HH, nor are the results of the limited retrospective study and randomized placebo-controlled studies, as their sample sizes were small.14,16,17 However, information on the use of oxybutynin for the effective treatment of HH in the SCI population is promising. Research studies on the prevalence of HH and randomized placebo-controlled trials with a larger SCI population are considerations for future studies.
1. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241-248.
2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64(4):690-695.
3. Andersen LS, Biering-Sørensen F, Müller PG, Jensen IL, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;(30):184-191.
4. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5, pt 1):713-726.
5. Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59(1):1-21.
6. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
7. Milhort TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1.
8. National Institute of Neurological Disorders and Stroke. Syringomyelia fact sheet. https://www.ninds .nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Syringomyelia-Fact-Sheet. Accessed February 13, 2017.
9. Meschner AL. Junqueira’s Basic Histology. 14th ed. New York, NY: McGraw-Hill; 2016:371-392.
10. Mauro TM. Biology of eccrine and apocrine glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
11. Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016:261-272.
12. Kellogg DL Jr. Thermoregulation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
13. Rovner ES, Wyman J, Lam S. Urinary Incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1377-1396.
14. Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics (Sao Paulo). 2014;69(9):608-614.
15. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized-placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696-1700.
16. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis. Br J Dermatol. 2015;173(5):1163-1168.
17. Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):1-7.
18. Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012:chap 84.
1. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51(2):241-248.
2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol. 2011;64(4):690-695.
3. Andersen LS, Biering-Sørensen F, Müller PG, Jensen IL, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;(30):184-191.
4. Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5, pt 1):713-726.
5. Kewalramani LS. Autonomic dysreflexia in traumatic myelopathy. Am J Phys Med. 1980;59(1):1-21.
6. Low PA, Engstrom JW. Disorders of the autonomic nervous system. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
7. Milhort TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1.
8. National Institute of Neurological Disorders and Stroke. Syringomyelia fact sheet. https://www.ninds .nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Syringomyelia-Fact-Sheet. Accessed February 13, 2017.
9. Meschner AL. Junqueira’s Basic Histology. 14th ed. New York, NY: McGraw-Hill; 2016:371-392.
10. Mauro TM. Biology of eccrine and apocrine glands. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
11. Barrett KE, Barman SM, Boitano S, Brooks HL. Ganong’s Review of Medical Physiology. 25th ed. New York, NY: McGraw-Hill; 2016:261-272.
12. Kellogg DL Jr. Thermoregulation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
13. Rovner ES, Wyman J, Lam S. Urinary Incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014:1377-1396.
14. Teivelis MP, Wolosker N, Krutman M, Kauffman P, Campos JR, Puech-Leão P. Treatment of uncommon sites of focal primary hyperhidrosis: experience with pharmacological therapy using oxybutynin. Clinics (Sao Paulo). 2014;69(9):608-614.
15. Wolosker N, de Campos JR, Kauffman P, Puech-Leão P. A randomized-placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55(6):1696-1700.
16. Schollhammer M, Brenaut E, Menard-Andivot N, et al. Oxybutynin as a treatment for generalized hyperhidrosis. Br J Dermatol. 2015;173(5):1163-1168.
17. Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):1-7.
18. Fealey RD, Hebert AA. Disorders of the eccrine sweat glands and sweating. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012:chap 84.














