User login
Outcomes of neoadjuvant therapy vary by subtype in bladder cancer
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer.
Major finding: Luminal tumors had the best overall survival independent of NAC and basal tumors the best response to NAC.
Data source: Experimental study that evaluated the efficacy of NAC in the molecular subtypes of muscle invasive bladder cancer.
Disclosures: The funding source is not disclosed. Dr Seiler has no disclosures. Several of his coauthors report relationships with multiple pharmaceutical companies. Dr Rosenberg reports financial ties to multiple pharmaceutical companies.
Does Patient History Affect the Treatment of Pediatric Refractory Status Epilepticus?
Children with refractory status epilepticus do not receive more timely treatment if they have a prior diagnosis of epilepsy, according to an investigation published January 24 in Neurology. A history of status epilepticus, however, is associated with more timely administration of abortive medication.
Most episodes of pediatric status epilepticus occur in children with no history of seizures. Investigators had not examined whether having a history of seizures or status epilepticus leads to more timely treatment, including escalation of therapy when indicated, and better outcomes.
Iván Sánchez Fernández, MD, Clinical Fellow in Neurology at Boston Children’s Hospital, and colleagues conducted a prospective observational study to compare the management and outcomes of refractory status epilepticus in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus. Eligible patients were between ages one month and 21 years and had convulsive seizures at onset that continued after administration of at least two antiepileptic drugs (AEDs).
The investigators enrolled 189 participants (53% male) with a median age of 4.2 years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of status epilepticus.
The time to first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 minutes vs 16.5 minutes). Patients with a diagnosis of epilepsy received their first nonbenzodiazepine AED later (93 minutes vs 50.5 minutes) and were less likely to receive at least one continuous infusion (39.3% vs 57%). The time to the first continuous infusion, however, was similar in patients with and without a diagnosis of epilepsy (258.5 vs 149 minutes)
Patients with a diagnosis of epilepsy were less likely to be intubated (66.3% vs 83%), had a longer duration of status epilepticus (174 minutes vs 120 minutes), and had a shorter ICU stay (3.9 days vs 5 days). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (75% vs 65%) and had lower mortality (0% vs 7%).
Time to the first benzodiazepine was shorter for patients with a history of status epilepticus, compared with patients without (8 minutes vs 20 minutes). The time to first nonbenzodiazepine AED was similar in patients with and without a history of status epilepticus (76.5 minutes vs 65 minutes). Patients with and without a history of status epilepticus were as likely to receive continuous infusions (52.9% vs 47.7%), had a similar time to the first continuous infusion (182 vs 180 minutes), and were as likely to be intubated (82.4% vs 73.5%). Patients with a history of status epilepticus had a longer duration of status epilepticus (150 minutes vs 124.5 minutes) and a shorter ICU stay (5 vs 4.3 days).
At hospital discharge, return to baseline function was similar between patients with and without a history of status epilepticus (76.5% vs 68.4%). The difference between groups in mortality rate was not statistically significant (0% vs 4.5%). The more timely administration of the first benzodiazepine in patients with a history of status epilepticus mainly resulted from cases with onset outside a hospital, while management of status epilepticus with in-hospital onset was similar in patients with and without a history of status epilepticus.
—Erik Greb
Suggested Reading
Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386-394.
Children with refractory status epilepticus do not receive more timely treatment if they have a prior diagnosis of epilepsy, according to an investigation published January 24 in Neurology. A history of status epilepticus, however, is associated with more timely administration of abortive medication.
Most episodes of pediatric status epilepticus occur in children with no history of seizures. Investigators had not examined whether having a history of seizures or status epilepticus leads to more timely treatment, including escalation of therapy when indicated, and better outcomes.
Iván Sánchez Fernández, MD, Clinical Fellow in Neurology at Boston Children’s Hospital, and colleagues conducted a prospective observational study to compare the management and outcomes of refractory status epilepticus in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus. Eligible patients were between ages one month and 21 years and had convulsive seizures at onset that continued after administration of at least two antiepileptic drugs (AEDs).
The investigators enrolled 189 participants (53% male) with a median age of 4.2 years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of status epilepticus.
The time to first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 minutes vs 16.5 minutes). Patients with a diagnosis of epilepsy received their first nonbenzodiazepine AED later (93 minutes vs 50.5 minutes) and were less likely to receive at least one continuous infusion (39.3% vs 57%). The time to the first continuous infusion, however, was similar in patients with and without a diagnosis of epilepsy (258.5 vs 149 minutes)
Patients with a diagnosis of epilepsy were less likely to be intubated (66.3% vs 83%), had a longer duration of status epilepticus (174 minutes vs 120 minutes), and had a shorter ICU stay (3.9 days vs 5 days). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (75% vs 65%) and had lower mortality (0% vs 7%).
Time to the first benzodiazepine was shorter for patients with a history of status epilepticus, compared with patients without (8 minutes vs 20 minutes). The time to first nonbenzodiazepine AED was similar in patients with and without a history of status epilepticus (76.5 minutes vs 65 minutes). Patients with and without a history of status epilepticus were as likely to receive continuous infusions (52.9% vs 47.7%), had a similar time to the first continuous infusion (182 vs 180 minutes), and were as likely to be intubated (82.4% vs 73.5%). Patients with a history of status epilepticus had a longer duration of status epilepticus (150 minutes vs 124.5 minutes) and a shorter ICU stay (5 vs 4.3 days).
At hospital discharge, return to baseline function was similar between patients with and without a history of status epilepticus (76.5% vs 68.4%). The difference between groups in mortality rate was not statistically significant (0% vs 4.5%). The more timely administration of the first benzodiazepine in patients with a history of status epilepticus mainly resulted from cases with onset outside a hospital, while management of status epilepticus with in-hospital onset was similar in patients with and without a history of status epilepticus.
—Erik Greb
Suggested Reading
Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386-394.
Children with refractory status epilepticus do not receive more timely treatment if they have a prior diagnosis of epilepsy, according to an investigation published January 24 in Neurology. A history of status epilepticus, however, is associated with more timely administration of abortive medication.
Most episodes of pediatric status epilepticus occur in children with no history of seizures. Investigators had not examined whether having a history of seizures or status epilepticus leads to more timely treatment, including escalation of therapy when indicated, and better outcomes.
Iván Sánchez Fernández, MD, Clinical Fellow in Neurology at Boston Children’s Hospital, and colleagues conducted a prospective observational study to compare the management and outcomes of refractory status epilepticus in children with and without a prior diagnosis of epilepsy and with and without a history of status epilepticus. Eligible patients were between ages one month and 21 years and had convulsive seizures at onset that continued after administration of at least two antiepileptic drugs (AEDs).
The investigators enrolled 189 participants (53% male) with a median age of 4.2 years. Eighty-nine (47%) patients had a prior diagnosis of epilepsy. Thirty-four (18%) patients had a history of status epilepticus.
The time to first benzodiazepine was similar in participants with and without a diagnosis of epilepsy (15 minutes vs 16.5 minutes). Patients with a diagnosis of epilepsy received their first nonbenzodiazepine AED later (93 minutes vs 50.5 minutes) and were less likely to receive at least one continuous infusion (39.3% vs 57%). The time to the first continuous infusion, however, was similar in patients with and without a diagnosis of epilepsy (258.5 vs 149 minutes)
Patients with a diagnosis of epilepsy were less likely to be intubated (66.3% vs 83%), had a longer duration of status epilepticus (174 minutes vs 120 minutes), and had a shorter ICU stay (3.9 days vs 5 days). At hospital discharge, patients with a diagnosis of epilepsy were more likely to return to baseline (75% vs 65%) and had lower mortality (0% vs 7%).
Time to the first benzodiazepine was shorter for patients with a history of status epilepticus, compared with patients without (8 minutes vs 20 minutes). The time to first nonbenzodiazepine AED was similar in patients with and without a history of status epilepticus (76.5 minutes vs 65 minutes). Patients with and without a history of status epilepticus were as likely to receive continuous infusions (52.9% vs 47.7%), had a similar time to the first continuous infusion (182 vs 180 minutes), and were as likely to be intubated (82.4% vs 73.5%). Patients with a history of status epilepticus had a longer duration of status epilepticus (150 minutes vs 124.5 minutes) and a shorter ICU stay (5 vs 4.3 days).
At hospital discharge, return to baseline function was similar between patients with and without a history of status epilepticus (76.5% vs 68.4%). The difference between groups in mortality rate was not statistically significant (0% vs 4.5%). The more timely administration of the first benzodiazepine in patients with a history of status epilepticus mainly resulted from cases with onset outside a hospital, while management of status epilepticus with in-hospital onset was similar in patients with and without a history of status epilepticus.
—Erik Greb
Suggested Reading
Sánchez Fernández I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386-394.
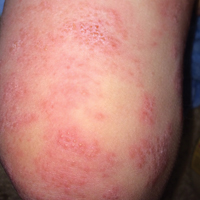
When atopic dermatitis is really contact dermatitis
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
ATLANTA – When patients present with atopic dermatitis that worsens, changes distribution, fails to improve, or immediately rebounds, think contact dermatitis, Luz Fonacier, MD, advised at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Clinical signs of contact dermatitis include lesions with an atypical distribution/pattern, such as head, eyelid, or cheilitis/perioral predominance, or lesions on the hand or foot. Also elevate your suspicion in patients with therapy-resistant hand eczema, adult- or childhood-onset atopic dermatitis without childhood eczema, as well as in cases of severe or widespread dermatitis prior to initiating a systemic immunosuppressant. The list of potential allergens to consider includes metal (especially nickel, cobalt, and potassium dichromate), fragrances such as formaldehyde and balsam of Peru, preservatives, as well as topical emollients, corticosteroids, antibiotics, and antiseptics.
Dr. Fonacier, professor of medicine at the State University of New York at Stony Brook and section head of allergy at Winthrop University Hospital, Mineola, N.Y., recommends loading acrylates, fragrances, and allergens in an aqueous vehicle immediately before application. She noted that delayed patch test readings are common to metals, topical antibiotics, and topical corticosteroids, and that positive reactions to gold are often not clinically relevant. “The patch test positivity of gold can be as high as 30% in adults and a little bit less in children, but results from two large studies show clinical relevance in only 10%-15% of cases,” she said. A trial of gold avoidance may be warranted in patients with suspected jewelry allergy, facial or eyelid dermatitis, or exposure through gold dental restorations.
She went on to share tips for reading skin patch tests. The first reading should be done after 48 hours, while the second should be done 3, 4, or 7 days after application. “The second reading helps distinguish irritant from allergic responses,” she said. “Thirty percent of negative tests at 48 hours may be positive on delayed readings.” Most true allergic reactions occur between 72 and 96 hours. Allergens that may peak early include thiuram mix, carba mix, and balsam of Peru. Those that disappear after 5 days include balsam of Peru, benzoic acid, disperse blue #124, fragrance mix, mercury, methyldibromo glutaronitrile, phenoxyethanol, and octyl gallate. Delayed patch test reactions after five days include metals (gold potassium dichromate, nickel, and cobalt), topical antibiotics (neomycin and bacitracin) as well as topic corticosteroids.
Resources she recommended to attendees include the American Contact Dermatitis Society and the Contact Dermatitis Institute. Health and safety information about household products can be found here.
Dr. Fonacier disclosed that she has received research and educational grants from Baxter and Genentech. She is also a consultant to Church and Dwight and Regeneron.
EXPERT ANALYSIS AT THE 2017 AAAAI ANNUAL MEETING
Online Algorithm Identifies People at Risk of Parkinson’s Disease
The online PREDICT-PD algorithm effectively identifies people at increased risk of Parkinson’s disease, according to research published online ahead of print January 16 in Movement Disorders. The risk scores that the algorithm assigns are significantly associated with intermediate markers of Parkinson’s disease. Higher PREDICT-PD risk score at baseline also is associated with an increased rate of incident Parkinson’s disease.
Previous research with the goal of identifying patients at risk of Parkinson’s disease mainly has focused on people with a family history of the disease, asymptomatic carriers of genes associated with the disease, or people with imaging abnormalities associated with the disease (eg, hyperechogenicity on transcranial sonography). The rarity, the representativeness, and the feasibility of identifying these factors have limited these studies, however.
Internet-Based Testing
To overcome these limitations, Anette-Eleonore Schrag, PhD, Professor of Clinical Neurosciences at University College London, and colleagues developed an online, evidence-based algorithm to identify risk indicators of Parkinson’s disease in the UK population. The investigators used a study website to recruit participants between ages 60 and 80 who did not have Parkinson’s disease, any other movement disorder, or stroke. Participants were prompted to return to the website yearly and complete tests.
Among the tests was a survey that included demographic questions and validated questionnaires about early nonmotor features of and risk factors for Parkinson’s disease (eg, the Hospital Anxiety Depression Scale and the REM sleep behavior disorder Screening Questionnaire [RBDSQ]). Participants also took the Bradykinesia Akinesia Incoordination (BRAIN) test, an online keyboard-tapping task. At baseline and at year three, participants took the University of Pennsylvania Smell Identification Test (UPSIT). The annual survey included a question about whether the participants had received any new diagnoses, including Parkinson’s disease or movement disorder. At year three, participants also gave saliva samples that were genotyped for mutations in exons 8 to 11 of glucocerebrosidase (GBA) and exon 41 in the LRRK2 gene.
The investigators used participants’ early features and risk factors to calculate their risk scores and rank participants according to their risk. Dr. Schrag and colleagues also tested support for enrichment of the population at risk of Parkinson’s disease by examining associations between risk scores and the following intermediate markers of Parkinson’s disease: reduced sense of smell, presence of subjective RBD, and slowing of finger tapping speed.
Risk Scores Were Associated With Incident Disease
The researchers recruited 1,323 eligible volunteers. At baseline, their mean age was 66, and approximately 61% of the population was female. In all, 1,040 of the participants completed follow-up testing at year 1, 939 completed year 2 follow-up, and 846 completed year 3 follow-up. A total of 223 participants completed the baseline assessment only.
Baseline risk scores were associated with significantly higher rates of all intermediate markers of Parkinson’s disease during each year of follow-up. The group of patients at higher risk (ie, those above the 15th percentile) had significantly worse UPSIT score, RBDSQ score, and finger tapping in all years, compared with patients at lower risk (ie, those below the 85th percentile).
Risk scores in the entire sample were strongly associated with intermediate markers of Parkinson’s disease each year. Higher- and lower-risk groups differed significantly in median UPSIT score, RBDSQ score, and mean finger tapping speed in all years of follow-up.
At year 1, three patients had been newly diagnosed with Parkinson’s disease. An additional participant received the diagnosis in year 2, and three other participants received the diagnosis in year 3. All of the participants with newly diagnosed Parkinson’s disease at year 1 were in the higher risk group, and two had also been in the higher risk group at baseline. The participant diagnosed at year 2 was in the higher risk group at baseline, year 1, and year 2. All of the three participants diagnosed by year 3 were in the middle risk group at baseline. “There was substantial heterogeneity in the occurrence of intermediate markers in these individuals,” said the researchers.
The incidence of independently diagnosed Parkinson’s disease in participants who had been in the higher risk group during three years of follow-up was 1.6% per year and 0.2% across the whole population. Exploratory Cox regression analysis using incident Parkinson’s disease over three years as the outcome indicated an association with baseline risk estimate. In participants for whom GBA and LRRK2 status was known, the association between baseline risk and incident Parkinson’s disease was weaker. But the addition of GBA and LRRK2 variants in the algorithm improved the strength of association between baseline risk and incident Parkinson’s disease. Among the intermediate markers of disease, baseline finger tapping was associated with incident Parkinson’s disease at three years.
Results Could Reflect Selection Bias
“Although only a small number of individuals have been independently diagnosed with Parkinson’s disease during follow-up so far, the overall incidence of 0.2% is consistent with the expected incidence rate in the age group of 60 to 80 from the general population (one to three per 1,000 per year) and supports the representativeness of our sample,” said Dr. Schrag.
The recruitment of participants may have introduced selection bias into the study, she acknowledged. “However, we did not use family history as an entry criterion, and the proportion with a positive family history was lower than in other landmark studies.” The fact that 17% of participants only completed the baseline assessment also may have introduced selection bias, “although there were no differences between those with or without follow-up, meaning that bias as a result of loss to follow-up was less likely.
“This Internet-based approach may be useful for population screening because it can easily be scaled upward,” added Dr. Schrag. “It will allow larger numbers of Parkinson’s disease cases that represent the spectrum of the disease to be identified, rather than what would be possible from cohorts of carriers of specific risk factors.”
—Erik Greb
Suggested Readings
Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Jan 16 [Epub ahead of print].
The online PREDICT-PD algorithm effectively identifies people at increased risk of Parkinson’s disease, according to research published online ahead of print January 16 in Movement Disorders. The risk scores that the algorithm assigns are significantly associated with intermediate markers of Parkinson’s disease. Higher PREDICT-PD risk score at baseline also is associated with an increased rate of incident Parkinson’s disease.
Previous research with the goal of identifying patients at risk of Parkinson’s disease mainly has focused on people with a family history of the disease, asymptomatic carriers of genes associated with the disease, or people with imaging abnormalities associated with the disease (eg, hyperechogenicity on transcranial sonography). The rarity, the representativeness, and the feasibility of identifying these factors have limited these studies, however.
Internet-Based Testing
To overcome these limitations, Anette-Eleonore Schrag, PhD, Professor of Clinical Neurosciences at University College London, and colleagues developed an online, evidence-based algorithm to identify risk indicators of Parkinson’s disease in the UK population. The investigators used a study website to recruit participants between ages 60 and 80 who did not have Parkinson’s disease, any other movement disorder, or stroke. Participants were prompted to return to the website yearly and complete tests.
Among the tests was a survey that included demographic questions and validated questionnaires about early nonmotor features of and risk factors for Parkinson’s disease (eg, the Hospital Anxiety Depression Scale and the REM sleep behavior disorder Screening Questionnaire [RBDSQ]). Participants also took the Bradykinesia Akinesia Incoordination (BRAIN) test, an online keyboard-tapping task. At baseline and at year three, participants took the University of Pennsylvania Smell Identification Test (UPSIT). The annual survey included a question about whether the participants had received any new diagnoses, including Parkinson’s disease or movement disorder. At year three, participants also gave saliva samples that were genotyped for mutations in exons 8 to 11 of glucocerebrosidase (GBA) and exon 41 in the LRRK2 gene.
The investigators used participants’ early features and risk factors to calculate their risk scores and rank participants according to their risk. Dr. Schrag and colleagues also tested support for enrichment of the population at risk of Parkinson’s disease by examining associations between risk scores and the following intermediate markers of Parkinson’s disease: reduced sense of smell, presence of subjective RBD, and slowing of finger tapping speed.
Risk Scores Were Associated With Incident Disease
The researchers recruited 1,323 eligible volunteers. At baseline, their mean age was 66, and approximately 61% of the population was female. In all, 1,040 of the participants completed follow-up testing at year 1, 939 completed year 2 follow-up, and 846 completed year 3 follow-up. A total of 223 participants completed the baseline assessment only.
Baseline risk scores were associated with significantly higher rates of all intermediate markers of Parkinson’s disease during each year of follow-up. The group of patients at higher risk (ie, those above the 15th percentile) had significantly worse UPSIT score, RBDSQ score, and finger tapping in all years, compared with patients at lower risk (ie, those below the 85th percentile).
Risk scores in the entire sample were strongly associated with intermediate markers of Parkinson’s disease each year. Higher- and lower-risk groups differed significantly in median UPSIT score, RBDSQ score, and mean finger tapping speed in all years of follow-up.
At year 1, three patients had been newly diagnosed with Parkinson’s disease. An additional participant received the diagnosis in year 2, and three other participants received the diagnosis in year 3. All of the participants with newly diagnosed Parkinson’s disease at year 1 were in the higher risk group, and two had also been in the higher risk group at baseline. The participant diagnosed at year 2 was in the higher risk group at baseline, year 1, and year 2. All of the three participants diagnosed by year 3 were in the middle risk group at baseline. “There was substantial heterogeneity in the occurrence of intermediate markers in these individuals,” said the researchers.
The incidence of independently diagnosed Parkinson’s disease in participants who had been in the higher risk group during three years of follow-up was 1.6% per year and 0.2% across the whole population. Exploratory Cox regression analysis using incident Parkinson’s disease over three years as the outcome indicated an association with baseline risk estimate. In participants for whom GBA and LRRK2 status was known, the association between baseline risk and incident Parkinson’s disease was weaker. But the addition of GBA and LRRK2 variants in the algorithm improved the strength of association between baseline risk and incident Parkinson’s disease. Among the intermediate markers of disease, baseline finger tapping was associated with incident Parkinson’s disease at three years.
Results Could Reflect Selection Bias
“Although only a small number of individuals have been independently diagnosed with Parkinson’s disease during follow-up so far, the overall incidence of 0.2% is consistent with the expected incidence rate in the age group of 60 to 80 from the general population (one to three per 1,000 per year) and supports the representativeness of our sample,” said Dr. Schrag.
The recruitment of participants may have introduced selection bias into the study, she acknowledged. “However, we did not use family history as an entry criterion, and the proportion with a positive family history was lower than in other landmark studies.” The fact that 17% of participants only completed the baseline assessment also may have introduced selection bias, “although there were no differences between those with or without follow-up, meaning that bias as a result of loss to follow-up was less likely.
“This Internet-based approach may be useful for population screening because it can easily be scaled upward,” added Dr. Schrag. “It will allow larger numbers of Parkinson’s disease cases that represent the spectrum of the disease to be identified, rather than what would be possible from cohorts of carriers of specific risk factors.”
—Erik Greb
Suggested Readings
Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Jan 16 [Epub ahead of print].
The online PREDICT-PD algorithm effectively identifies people at increased risk of Parkinson’s disease, according to research published online ahead of print January 16 in Movement Disorders. The risk scores that the algorithm assigns are significantly associated with intermediate markers of Parkinson’s disease. Higher PREDICT-PD risk score at baseline also is associated with an increased rate of incident Parkinson’s disease.
Previous research with the goal of identifying patients at risk of Parkinson’s disease mainly has focused on people with a family history of the disease, asymptomatic carriers of genes associated with the disease, or people with imaging abnormalities associated with the disease (eg, hyperechogenicity on transcranial sonography). The rarity, the representativeness, and the feasibility of identifying these factors have limited these studies, however.
Internet-Based Testing
To overcome these limitations, Anette-Eleonore Schrag, PhD, Professor of Clinical Neurosciences at University College London, and colleagues developed an online, evidence-based algorithm to identify risk indicators of Parkinson’s disease in the UK population. The investigators used a study website to recruit participants between ages 60 and 80 who did not have Parkinson’s disease, any other movement disorder, or stroke. Participants were prompted to return to the website yearly and complete tests.
Among the tests was a survey that included demographic questions and validated questionnaires about early nonmotor features of and risk factors for Parkinson’s disease (eg, the Hospital Anxiety Depression Scale and the REM sleep behavior disorder Screening Questionnaire [RBDSQ]). Participants also took the Bradykinesia Akinesia Incoordination (BRAIN) test, an online keyboard-tapping task. At baseline and at year three, participants took the University of Pennsylvania Smell Identification Test (UPSIT). The annual survey included a question about whether the participants had received any new diagnoses, including Parkinson’s disease or movement disorder. At year three, participants also gave saliva samples that were genotyped for mutations in exons 8 to 11 of glucocerebrosidase (GBA) and exon 41 in the LRRK2 gene.
The investigators used participants’ early features and risk factors to calculate their risk scores and rank participants according to their risk. Dr. Schrag and colleagues also tested support for enrichment of the population at risk of Parkinson’s disease by examining associations between risk scores and the following intermediate markers of Parkinson’s disease: reduced sense of smell, presence of subjective RBD, and slowing of finger tapping speed.
Risk Scores Were Associated With Incident Disease
The researchers recruited 1,323 eligible volunteers. At baseline, their mean age was 66, and approximately 61% of the population was female. In all, 1,040 of the participants completed follow-up testing at year 1, 939 completed year 2 follow-up, and 846 completed year 3 follow-up. A total of 223 participants completed the baseline assessment only.
Baseline risk scores were associated with significantly higher rates of all intermediate markers of Parkinson’s disease during each year of follow-up. The group of patients at higher risk (ie, those above the 15th percentile) had significantly worse UPSIT score, RBDSQ score, and finger tapping in all years, compared with patients at lower risk (ie, those below the 85th percentile).
Risk scores in the entire sample were strongly associated with intermediate markers of Parkinson’s disease each year. Higher- and lower-risk groups differed significantly in median UPSIT score, RBDSQ score, and mean finger tapping speed in all years of follow-up.
At year 1, three patients had been newly diagnosed with Parkinson’s disease. An additional participant received the diagnosis in year 2, and three other participants received the diagnosis in year 3. All of the participants with newly diagnosed Parkinson’s disease at year 1 were in the higher risk group, and two had also been in the higher risk group at baseline. The participant diagnosed at year 2 was in the higher risk group at baseline, year 1, and year 2. All of the three participants diagnosed by year 3 were in the middle risk group at baseline. “There was substantial heterogeneity in the occurrence of intermediate markers in these individuals,” said the researchers.
The incidence of independently diagnosed Parkinson’s disease in participants who had been in the higher risk group during three years of follow-up was 1.6% per year and 0.2% across the whole population. Exploratory Cox regression analysis using incident Parkinson’s disease over three years as the outcome indicated an association with baseline risk estimate. In participants for whom GBA and LRRK2 status was known, the association between baseline risk and incident Parkinson’s disease was weaker. But the addition of GBA and LRRK2 variants in the algorithm improved the strength of association between baseline risk and incident Parkinson’s disease. Among the intermediate markers of disease, baseline finger tapping was associated with incident Parkinson’s disease at three years.
Results Could Reflect Selection Bias
“Although only a small number of individuals have been independently diagnosed with Parkinson’s disease during follow-up so far, the overall incidence of 0.2% is consistent with the expected incidence rate in the age group of 60 to 80 from the general population (one to three per 1,000 per year) and supports the representativeness of our sample,” said Dr. Schrag.
The recruitment of participants may have introduced selection bias into the study, she acknowledged. “However, we did not use family history as an entry criterion, and the proportion with a positive family history was lower than in other landmark studies.” The fact that 17% of participants only completed the baseline assessment also may have introduced selection bias, “although there were no differences between those with or without follow-up, meaning that bias as a result of loss to follow-up was less likely.
“This Internet-based approach may be useful for population screening because it can easily be scaled upward,” added Dr. Schrag. “It will allow larger numbers of Parkinson’s disease cases that represent the spectrum of the disease to be identified, rather than what would be possible from cohorts of carriers of specific risk factors.”
—Erik Greb
Suggested Readings
Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017 Jan 16 [Epub ahead of print].
Pulmonary embolism common in patients with AE-COPD
About 16% of patients with unexplained chronic obstructive pulmonary disease (COPD) acute exacerbations (AE-COPD) had an accompanying pulmonary embolism (PE), usually in regions that could be targeted with anticoagulants, according to a new systematic review and meta-analysis.
About 70% of the time an AE is a response to infection, but about 30% of the time, an AE has no clear cause, the authors said in a report on their research (CHEST. 2017 March;151[3]:544-54). There is a known biological link between inflammation and coagulation, which suggests that patients experiencing AE-COPD may be at increased risk of PE.
The researchers reviewed and analyzed seven studies, comprising 880 patients. Among the authors’ reasons for conducting this research was to update the pooled prevalence of PE in AE-COPD from a previous systematic review published in CHEST in 2009.
The meta-analysis revealed that 16.1% of patients with AE-COPD were also diagnosed with PE (95% confidence interval 8.3%-25.8%). There was a wide range of variation between individual studies (prevalence 3.3%-29.1%). In six studies that reported on deep vein thrombosis, the pooled prevalence of DVT was 10.5% (95% CI 4.3%-19.0%).
Five of the studies identified the PE location. An analysis of those studies showed that 35.0% were in the main pulmonary artery, and 31.7% were in the lobar and inter-lobar arteries. Such findings “[suggest] that the majority of these embolisms have important clinical consequences,” the authors wrote.
The researchers also looked at clinical markers that accompanied AE-COPD and found a potential signal with respect to pleuritic chest pain. One study found a strong association between pleuritic chest pain and AE-COPD patients with PE (81.0% versus 40.0% in those without PE). A second study showed a similar association (24.0% in PE versus 11.5% in non-PE patients), and a third study found no significant difference.
The presence of PE was also linked to hypotension, syncope, and acute right failure on ultrasonography, suggesting that PE may be associated with heart failure.
Patients with PE were less likely to have symptoms consistent with a respiratory tract infection. They also tended to have higher mortality rates and longer hospitalization rates compared with those without PE.
The meta-analysis had some limitations, including the heterogeneity of findings in the included studies, as well as the potential for publication bias, since reports showing unusually low or high rates may be more likely to be published, the researchers noted. There was also a high proportion of male subjects in the included studies.
Overall, the researchers concluded that PE is more likely in patients with pleuritic chest pain and signs of heart failure, and less likely in patients with signs of a respiratory infection. That information “might add to the clinical decision-making in patients with an AE-COPD, because it would be undesirable to perform [computed tomography pulmonary angiography] in every patient with an AE-COPD,” the researchers wrote.
“Early identification of these noninfectious events is important as standard antiexacerbation therapies including systemic corticosteroids and antibiotics are unlikely to be clinically useful for these etiologies and, importantly, may result in delays in the diagnosis and treatment of noninfectious causes of exacerbation such as acute coronary syndromes or congestive heart failure, leading to poor clinical outcomes.
“There is a clear and compelling need for more high quality evidence to determine the value of detecting PEs in patients with acute COPD exacerbations. There is an urgent need to understand the risks as well as the benefits of using CTPA [computed tomography pulmonary angiography] in the evaluation of acute COPD exacerbations. A Spanish group is currently conducting a randomized clinical trial to examine the clinical benefits and the safety of “routinely” deploying CTPA in the evaluation of hospitalized COPD patients with acute exacerbations (NCT02238639).
“What should clinicians do until high quality data from these and other studies are available? We suggest that in patients with typical infectious symptoms (e.g. increased cough, change in sputum volume or colour), CTPA is probably not required. CTPA may be considered for those who present with ‘atypical’ exacerbation symptoms (e.g. pleuritic chest pain, signs of cardiac failure, no clear identification of infectious origin) and in those with a prior history of thromboembolic disease. While we agree with Aleva and colleagues that the prevalence of PE is common (approximately 20%-25%) in unexplained COPD exacerbations, we remain unconvinced that all of these events require active treatment with anticoagulant therapy. Until compelling data from well-conducted randomized controlled trials are available, we suggest a conservative [first, no harm] approach to the management of acute exacerbations of COPD and [using] CTPA judiciously.”
Seung Won Ra, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver, as well as Ulsan (South Korea) University Hospital, University of Ulsan College of Medicine. Don D. Sin, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver. They had no relevant disclosures and made these remarks in an editorial (Chest. 2017;151[3]:523-4) that accompanied the published study.
“Early identification of these noninfectious events is important as standard antiexacerbation therapies including systemic corticosteroids and antibiotics are unlikely to be clinically useful for these etiologies and, importantly, may result in delays in the diagnosis and treatment of noninfectious causes of exacerbation such as acute coronary syndromes or congestive heart failure, leading to poor clinical outcomes.
“There is a clear and compelling need for more high quality evidence to determine the value of detecting PEs in patients with acute COPD exacerbations. There is an urgent need to understand the risks as well as the benefits of using CTPA [computed tomography pulmonary angiography] in the evaluation of acute COPD exacerbations. A Spanish group is currently conducting a randomized clinical trial to examine the clinical benefits and the safety of “routinely” deploying CTPA in the evaluation of hospitalized COPD patients with acute exacerbations (NCT02238639).
“What should clinicians do until high quality data from these and other studies are available? We suggest that in patients with typical infectious symptoms (e.g. increased cough, change in sputum volume or colour), CTPA is probably not required. CTPA may be considered for those who present with ‘atypical’ exacerbation symptoms (e.g. pleuritic chest pain, signs of cardiac failure, no clear identification of infectious origin) and in those with a prior history of thromboembolic disease. While we agree with Aleva and colleagues that the prevalence of PE is common (approximately 20%-25%) in unexplained COPD exacerbations, we remain unconvinced that all of these events require active treatment with anticoagulant therapy. Until compelling data from well-conducted randomized controlled trials are available, we suggest a conservative [first, no harm] approach to the management of acute exacerbations of COPD and [using] CTPA judiciously.”
Seung Won Ra, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver, as well as Ulsan (South Korea) University Hospital, University of Ulsan College of Medicine. Don D. Sin, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver. They had no relevant disclosures and made these remarks in an editorial (Chest. 2017;151[3]:523-4) that accompanied the published study.
“Early identification of these noninfectious events is important as standard antiexacerbation therapies including systemic corticosteroids and antibiotics are unlikely to be clinically useful for these etiologies and, importantly, may result in delays in the diagnosis and treatment of noninfectious causes of exacerbation such as acute coronary syndromes or congestive heart failure, leading to poor clinical outcomes.
“There is a clear and compelling need for more high quality evidence to determine the value of detecting PEs in patients with acute COPD exacerbations. There is an urgent need to understand the risks as well as the benefits of using CTPA [computed tomography pulmonary angiography] in the evaluation of acute COPD exacerbations. A Spanish group is currently conducting a randomized clinical trial to examine the clinical benefits and the safety of “routinely” deploying CTPA in the evaluation of hospitalized COPD patients with acute exacerbations (NCT02238639).
“What should clinicians do until high quality data from these and other studies are available? We suggest that in patients with typical infectious symptoms (e.g. increased cough, change in sputum volume or colour), CTPA is probably not required. CTPA may be considered for those who present with ‘atypical’ exacerbation symptoms (e.g. pleuritic chest pain, signs of cardiac failure, no clear identification of infectious origin) and in those with a prior history of thromboembolic disease. While we agree with Aleva and colleagues that the prevalence of PE is common (approximately 20%-25%) in unexplained COPD exacerbations, we remain unconvinced that all of these events require active treatment with anticoagulant therapy. Until compelling data from well-conducted randomized controlled trials are available, we suggest a conservative [first, no harm] approach to the management of acute exacerbations of COPD and [using] CTPA judiciously.”
Seung Won Ra, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver, as well as Ulsan (South Korea) University Hospital, University of Ulsan College of Medicine. Don D. Sin, MD, PhD is with the Centre for Heart Lung Innovation, St. Paul’s Hospital and the department of medicine (respiratory division) at the University of British Columbia, Vancouver. They had no relevant disclosures and made these remarks in an editorial (Chest. 2017;151[3]:523-4) that accompanied the published study.
About 16% of patients with unexplained chronic obstructive pulmonary disease (COPD) acute exacerbations (AE-COPD) had an accompanying pulmonary embolism (PE), usually in regions that could be targeted with anticoagulants, according to a new systematic review and meta-analysis.
About 70% of the time an AE is a response to infection, but about 30% of the time, an AE has no clear cause, the authors said in a report on their research (CHEST. 2017 March;151[3]:544-54). There is a known biological link between inflammation and coagulation, which suggests that patients experiencing AE-COPD may be at increased risk of PE.
The researchers reviewed and analyzed seven studies, comprising 880 patients. Among the authors’ reasons for conducting this research was to update the pooled prevalence of PE in AE-COPD from a previous systematic review published in CHEST in 2009.
The meta-analysis revealed that 16.1% of patients with AE-COPD were also diagnosed with PE (95% confidence interval 8.3%-25.8%). There was a wide range of variation between individual studies (prevalence 3.3%-29.1%). In six studies that reported on deep vein thrombosis, the pooled prevalence of DVT was 10.5% (95% CI 4.3%-19.0%).
Five of the studies identified the PE location. An analysis of those studies showed that 35.0% were in the main pulmonary artery, and 31.7% were in the lobar and inter-lobar arteries. Such findings “[suggest] that the majority of these embolisms have important clinical consequences,” the authors wrote.
The researchers also looked at clinical markers that accompanied AE-COPD and found a potential signal with respect to pleuritic chest pain. One study found a strong association between pleuritic chest pain and AE-COPD patients with PE (81.0% versus 40.0% in those without PE). A second study showed a similar association (24.0% in PE versus 11.5% in non-PE patients), and a third study found no significant difference.
The presence of PE was also linked to hypotension, syncope, and acute right failure on ultrasonography, suggesting that PE may be associated with heart failure.
Patients with PE were less likely to have symptoms consistent with a respiratory tract infection. They also tended to have higher mortality rates and longer hospitalization rates compared with those without PE.
The meta-analysis had some limitations, including the heterogeneity of findings in the included studies, as well as the potential for publication bias, since reports showing unusually low or high rates may be more likely to be published, the researchers noted. There was also a high proportion of male subjects in the included studies.
Overall, the researchers concluded that PE is more likely in patients with pleuritic chest pain and signs of heart failure, and less likely in patients with signs of a respiratory infection. That information “might add to the clinical decision-making in patients with an AE-COPD, because it would be undesirable to perform [computed tomography pulmonary angiography] in every patient with an AE-COPD,” the researchers wrote.
About 16% of patients with unexplained chronic obstructive pulmonary disease (COPD) acute exacerbations (AE-COPD) had an accompanying pulmonary embolism (PE), usually in regions that could be targeted with anticoagulants, according to a new systematic review and meta-analysis.
About 70% of the time an AE is a response to infection, but about 30% of the time, an AE has no clear cause, the authors said in a report on their research (CHEST. 2017 March;151[3]:544-54). There is a known biological link between inflammation and coagulation, which suggests that patients experiencing AE-COPD may be at increased risk of PE.
The researchers reviewed and analyzed seven studies, comprising 880 patients. Among the authors’ reasons for conducting this research was to update the pooled prevalence of PE in AE-COPD from a previous systematic review published in CHEST in 2009.
The meta-analysis revealed that 16.1% of patients with AE-COPD were also diagnosed with PE (95% confidence interval 8.3%-25.8%). There was a wide range of variation between individual studies (prevalence 3.3%-29.1%). In six studies that reported on deep vein thrombosis, the pooled prevalence of DVT was 10.5% (95% CI 4.3%-19.0%).
Five of the studies identified the PE location. An analysis of those studies showed that 35.0% were in the main pulmonary artery, and 31.7% were in the lobar and inter-lobar arteries. Such findings “[suggest] that the majority of these embolisms have important clinical consequences,” the authors wrote.
The researchers also looked at clinical markers that accompanied AE-COPD and found a potential signal with respect to pleuritic chest pain. One study found a strong association between pleuritic chest pain and AE-COPD patients with PE (81.0% versus 40.0% in those without PE). A second study showed a similar association (24.0% in PE versus 11.5% in non-PE patients), and a third study found no significant difference.
The presence of PE was also linked to hypotension, syncope, and acute right failure on ultrasonography, suggesting that PE may be associated with heart failure.
Patients with PE were less likely to have symptoms consistent with a respiratory tract infection. They also tended to have higher mortality rates and longer hospitalization rates compared with those without PE.
The meta-analysis had some limitations, including the heterogeneity of findings in the included studies, as well as the potential for publication bias, since reports showing unusually low or high rates may be more likely to be published, the researchers noted. There was also a high proportion of male subjects in the included studies.
Overall, the researchers concluded that PE is more likely in patients with pleuritic chest pain and signs of heart failure, and less likely in patients with signs of a respiratory infection. That information “might add to the clinical decision-making in patients with an AE-COPD, because it would be undesirable to perform [computed tomography pulmonary angiography] in every patient with an AE-COPD,” the researchers wrote.
FROM CHEST
Key clinical point: Pulmonary embolisms are often present in unexplained acute exacerbations of COPD.
Major finding: About 16% of unexplained exacerbations occurred in patients who had a pulmonary embolism.
Data source: Systematic review and meta-analysis of seven studies (880 patients).
Disclosures: The study received no funding. The authors reported having no financial disclosures.
When to Suspect a CSF Leak in Patients With Headache
OJAI, CA—Spontaneous CSF leaks are treatable, often misdiagnosed, and can cause a neurologic syndrome that may include headache, nausea, and tinnitus. Spinal fluid leaks also can lead to serious complications, including seizures. Patients may have a CSF leak for years or decades before it is diagnosed.
Although CSF leaks may not be readily apparent on imaging, a suspected CSF leak is important to consider because it is fixable, said Dr. Carroll, Assistant Professor of Anesthesiology and Perioperative and Pain Medicine at Stanford University Medical Center in California and a member of the Stanford CSF leak program.
Postdural Puncture Headache Versus Spontaneous CSF Leaks
A spontaneous CSF leak and the clinical syndrome that it causes may be confused with a postdural puncture headache.
With a postdural puncture headache, a patient usually has a single leak site in the dorsal dura. “There is up to a 90% response to a single epidural blood patch. Its natural history is generally well understood and benign. It is rarely mysterious, and it is ultimately fixable,” said Dr. Carroll. In contrast, “a spontaneous CSF leak is often mysterious in terms of the onset, cause, and diagnosis.”
The natural history of CSF leaks is poorly understood. The percentage of patients whose spontaneous leaks seal on their own or whose leaks cause a catastrophe (eg, coma, seizures, or hematomas) is not known. Between 30% and 40% of patients with spontaneous leaks have leaks from multiple sites at diagnosis. “With a spontaneous leak a dural rent is more likely in the ventral dura, anterior to the spinal cord or at the nerve root, making the dura less amenable to patching. A single patch at the correct spinal level will fix the problem only 30% of the time. With multiple patches, the success rate can approach 65% to 75%.” If the first epidural blood patch fails, it should be repeated. Directed epidural blood patches, placement of fibrin sealant, and surgical treatment are other treatment options.
Headaches
Most patients with post-puncture CSF leaks have classic orthostatic headaches. The orthostatic headaches from spontaneous leaks are often atypical, however, in that patients may not feel better immediately when they lie down and worse when they stand, Dr. Carroll said. Headaches may occur late in the day after prolonged upright time or with exertion. In addition, patients may “go from having an orthostatic headache to having a terrible headache all the time, regardless of what position they are in.”
Nausea and Vomiting
Nausea and vomiting can be the main symptom of a CSF leak. Dr. Carroll described a patient with complex regional pain syndrome who underwent a spinal cord stimulation trial. Afterward, she had a postdural puncture headache and received an epidural blood patch. “After that, she developed vomiting up to nine times a day.” A CSF leak was not visible on the first CT myelogram but was apparent on the second. The leak was “so small it was missed by the slice thickness” of the first CT myelogram, said Dr. Carroll. She ultimately required surgery to fix the leak. The patient improved, although she continued vomiting three times per day.
Tinnitus
CSF leaks may cause tinnitus. “You can get ringing in the ears when you have migraine,” Dr. Carroll said. But if patients have tinnitus when they are not having headaches, “you should be thinking that there is something else going on.” Data suggest that CSF fluid is connected to inner ear fluid so a change of pressure in CSF changes inner ear pressure, and patients with high or low CSF pressure may get tinnitus.
Other symptoms may include neck pain and fatigue. “I have had the parents of patients tell me that the most remarkable thing that they see when we patch their sons or daughters is how they are bouncing around the house,” he said. Many patients complain of difficulty with concentrating, task persistence, and other nondescript, nonfocal neurologic symptoms.
Imaging Limitations
Imaging of patients with CSF leaks often initially is read as normal, and MRI is not an adequate evaluation in the high clinical suspicion of a leak, Dr. Carroll said. “It is a good place to start, because if you see a leak on your MRI, maybe you do not have to get a CT myelogram,” he said. “But if you have a clinical suspicion of a leak … you should pursue that in the face of your radiologist telling you that there is nothing.”
Schievink et al in 2007 looked at several years of data from an emergency department to assess how often imaging findings consistent with CSF leaks were missed. They reviewed MRIs of patients with headache to look for evidence of intracranial hypotension, and then compared the number of CSF leaks with the number of subarachnoid hemorrhages seen during the same time. They found that for every subarachnoid hemorrhage, there were approximately 0.5 CSF leaks (23 subarachnoid hemorrhages and 11 CSF leaks). The results suggested that spontaneous intracranial hypotension is more common than previously thought and its diagnosis in emergency departments is problematic. The 11 people with MRI evidence of intracranial hypotension subsequently were diagnosed with CSF leaks and treated. None were diagnosed at the time of the MRI while in the emergency department.
Causes of Leaks
The four main causes of CSF leaks are medical procedures; whiplash; bony, sharp calcifications penetrating the dura; and genetic disorders of connective tissue.
Webb et al conducted a study to evaluate headaches in patients who had a known wet tap (ie, unintentional dural puncture) after a labor epidural. The researchers reviewed quality assurance data in an obstetrics anesthesia division and identified 40 patients who had known wet taps and 40 controls who had received an epidural without a wet tap during the same week and were matched for age and weight. Investigators contacted patients between 12 and 24 months later (mean, 18 months) and asked them about the incidence of chronic headache. The incidence of chronic headache in controls was 5% versus nearly 30% in patients who had had a wet tap. The investigators then compared patients who were managed conservatively (ie, they did not receive an epidural blood patch) versus patients who were managed with a blood patch. “If you got a blood patch, your risk of having a chronic headache 18 months later was only half as much as if you did not get a blood patch,” he said.
Connective Tissue Disorders and Calcifications
Because connective tissue disorders are associated with CSF leaks, headache physicians should determine patients’ Beighton Hypermobility Scores, Dr. Carroll said. The score is derived from a simple test that assesses joint hypermobility. For instance, patients receive a point if they can touch their thumb to their wrist or straighten their elbow more than 10° beyond 180°. A score between 3 and 5 raises suspicion that the patient might have a hereditary disorder of connective tissue. Cataracts at an early age, being unusually tall or short, degenerative disc disease, and history of aneurysm also are associated with an increased risk of CSF leaks.
With regard to calcifications, Dr. Carroll described a patient whose main complaint was confusion upon standing too long. The patient also had neck pain. They determined that he had a calcified bone spur that was puncturing the spinal cord, causing a leak.
Whiplash
Trauma and whiplash can cause leaks. Researchers in Japan studied 66 patients with chronic whiplash-associated disorders (ie, they had a whiplash accident and complained of neck and head pain, as well as difficulty with fatigue or memory). The patients were given a radionuclide cisternogram. Thirty-seven of the 66 patients had imaging that was positive for a CSF leak. “After being patched, roughly half the people who were found to have a leak went back to work, whereas they had not been working before,” he said. Another study found that 10% of people with brachial plexus injuries have spinal fluid leaks.
Overlap With POTS
The fact that Ehlers-Danlos also is associated with postural orthostatic tachycardia syndrome (POTS) raises the possibility that patients with CSF leaks may be misdiagnosed as having POTS.
“Why should a hereditary disorder of connective tissue be associated with the only two known conditions that are associated with feeling worse when you are up?” Dr. Carroll asked. Among patients with POTS, 60% have headaches, and many have dizziness and nausea. Dr. Carroll asked the POTS clinics at Stanford to refer patients with POTS, headache, and Ehlers-Danlos syndrome to him. The first referred patient’s history was consistent with a CSF leak. She had been passing out and had severe headaches for more than 10 years. Although her initial imaging was read as negative for CSF leaks, and an MRI showed no signs of intracranial hypotension, “when we patched her, in fact, she got better.” Subsequently, more patients diagnosed with POTS have been referred to the CSF leak program.
Patients initially may be diagnosed as having chronic fatigue syndrome, fibromyalgia, or irritable bowel syndrome when a CSF leak is causing their symptoms. It is a tragedy when patients “have a fixable leak and … nothing is done to treat that underlying problem,” Dr. Carroll said.
—Jake Remaly
Suggested Reading
Ishikawa S, Yokoyama M, Mizobuchi S, et al. Epidural blood patch therapy for chronic whiplash-associated disorder. Anesth Analg. 2007;105(3):809-814.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296.
Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328.
Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115(1):124-132.
OJAI, CA—Spontaneous CSF leaks are treatable, often misdiagnosed, and can cause a neurologic syndrome that may include headache, nausea, and tinnitus. Spinal fluid leaks also can lead to serious complications, including seizures. Patients may have a CSF leak for years or decades before it is diagnosed.
Although CSF leaks may not be readily apparent on imaging, a suspected CSF leak is important to consider because it is fixable, said Dr. Carroll, Assistant Professor of Anesthesiology and Perioperative and Pain Medicine at Stanford University Medical Center in California and a member of the Stanford CSF leak program.
Postdural Puncture Headache Versus Spontaneous CSF Leaks
A spontaneous CSF leak and the clinical syndrome that it causes may be confused with a postdural puncture headache.
With a postdural puncture headache, a patient usually has a single leak site in the dorsal dura. “There is up to a 90% response to a single epidural blood patch. Its natural history is generally well understood and benign. It is rarely mysterious, and it is ultimately fixable,” said Dr. Carroll. In contrast, “a spontaneous CSF leak is often mysterious in terms of the onset, cause, and diagnosis.”
The natural history of CSF leaks is poorly understood. The percentage of patients whose spontaneous leaks seal on their own or whose leaks cause a catastrophe (eg, coma, seizures, or hematomas) is not known. Between 30% and 40% of patients with spontaneous leaks have leaks from multiple sites at diagnosis. “With a spontaneous leak a dural rent is more likely in the ventral dura, anterior to the spinal cord or at the nerve root, making the dura less amenable to patching. A single patch at the correct spinal level will fix the problem only 30% of the time. With multiple patches, the success rate can approach 65% to 75%.” If the first epidural blood patch fails, it should be repeated. Directed epidural blood patches, placement of fibrin sealant, and surgical treatment are other treatment options.
Headaches
Most patients with post-puncture CSF leaks have classic orthostatic headaches. The orthostatic headaches from spontaneous leaks are often atypical, however, in that patients may not feel better immediately when they lie down and worse when they stand, Dr. Carroll said. Headaches may occur late in the day after prolonged upright time or with exertion. In addition, patients may “go from having an orthostatic headache to having a terrible headache all the time, regardless of what position they are in.”
Nausea and Vomiting
Nausea and vomiting can be the main symptom of a CSF leak. Dr. Carroll described a patient with complex regional pain syndrome who underwent a spinal cord stimulation trial. Afterward, she had a postdural puncture headache and received an epidural blood patch. “After that, she developed vomiting up to nine times a day.” A CSF leak was not visible on the first CT myelogram but was apparent on the second. The leak was “so small it was missed by the slice thickness” of the first CT myelogram, said Dr. Carroll. She ultimately required surgery to fix the leak. The patient improved, although she continued vomiting three times per day.
Tinnitus
CSF leaks may cause tinnitus. “You can get ringing in the ears when you have migraine,” Dr. Carroll said. But if patients have tinnitus when they are not having headaches, “you should be thinking that there is something else going on.” Data suggest that CSF fluid is connected to inner ear fluid so a change of pressure in CSF changes inner ear pressure, and patients with high or low CSF pressure may get tinnitus.
Other symptoms may include neck pain and fatigue. “I have had the parents of patients tell me that the most remarkable thing that they see when we patch their sons or daughters is how they are bouncing around the house,” he said. Many patients complain of difficulty with concentrating, task persistence, and other nondescript, nonfocal neurologic symptoms.
Imaging Limitations
Imaging of patients with CSF leaks often initially is read as normal, and MRI is not an adequate evaluation in the high clinical suspicion of a leak, Dr. Carroll said. “It is a good place to start, because if you see a leak on your MRI, maybe you do not have to get a CT myelogram,” he said. “But if you have a clinical suspicion of a leak … you should pursue that in the face of your radiologist telling you that there is nothing.”
Schievink et al in 2007 looked at several years of data from an emergency department to assess how often imaging findings consistent with CSF leaks were missed. They reviewed MRIs of patients with headache to look for evidence of intracranial hypotension, and then compared the number of CSF leaks with the number of subarachnoid hemorrhages seen during the same time. They found that for every subarachnoid hemorrhage, there were approximately 0.5 CSF leaks (23 subarachnoid hemorrhages and 11 CSF leaks). The results suggested that spontaneous intracranial hypotension is more common than previously thought and its diagnosis in emergency departments is problematic. The 11 people with MRI evidence of intracranial hypotension subsequently were diagnosed with CSF leaks and treated. None were diagnosed at the time of the MRI while in the emergency department.
Causes of Leaks
The four main causes of CSF leaks are medical procedures; whiplash; bony, sharp calcifications penetrating the dura; and genetic disorders of connective tissue.
Webb et al conducted a study to evaluate headaches in patients who had a known wet tap (ie, unintentional dural puncture) after a labor epidural. The researchers reviewed quality assurance data in an obstetrics anesthesia division and identified 40 patients who had known wet taps and 40 controls who had received an epidural without a wet tap during the same week and were matched for age and weight. Investigators contacted patients between 12 and 24 months later (mean, 18 months) and asked them about the incidence of chronic headache. The incidence of chronic headache in controls was 5% versus nearly 30% in patients who had had a wet tap. The investigators then compared patients who were managed conservatively (ie, they did not receive an epidural blood patch) versus patients who were managed with a blood patch. “If you got a blood patch, your risk of having a chronic headache 18 months later was only half as much as if you did not get a blood patch,” he said.
Connective Tissue Disorders and Calcifications
Because connective tissue disorders are associated with CSF leaks, headache physicians should determine patients’ Beighton Hypermobility Scores, Dr. Carroll said. The score is derived from a simple test that assesses joint hypermobility. For instance, patients receive a point if they can touch their thumb to their wrist or straighten their elbow more than 10° beyond 180°. A score between 3 and 5 raises suspicion that the patient might have a hereditary disorder of connective tissue. Cataracts at an early age, being unusually tall or short, degenerative disc disease, and history of aneurysm also are associated with an increased risk of CSF leaks.
With regard to calcifications, Dr. Carroll described a patient whose main complaint was confusion upon standing too long. The patient also had neck pain. They determined that he had a calcified bone spur that was puncturing the spinal cord, causing a leak.
Whiplash
Trauma and whiplash can cause leaks. Researchers in Japan studied 66 patients with chronic whiplash-associated disorders (ie, they had a whiplash accident and complained of neck and head pain, as well as difficulty with fatigue or memory). The patients were given a radionuclide cisternogram. Thirty-seven of the 66 patients had imaging that was positive for a CSF leak. “After being patched, roughly half the people who were found to have a leak went back to work, whereas they had not been working before,” he said. Another study found that 10% of people with brachial plexus injuries have spinal fluid leaks.
Overlap With POTS
The fact that Ehlers-Danlos also is associated with postural orthostatic tachycardia syndrome (POTS) raises the possibility that patients with CSF leaks may be misdiagnosed as having POTS.
“Why should a hereditary disorder of connective tissue be associated with the only two known conditions that are associated with feeling worse when you are up?” Dr. Carroll asked. Among patients with POTS, 60% have headaches, and many have dizziness and nausea. Dr. Carroll asked the POTS clinics at Stanford to refer patients with POTS, headache, and Ehlers-Danlos syndrome to him. The first referred patient’s history was consistent with a CSF leak. She had been passing out and had severe headaches for more than 10 years. Although her initial imaging was read as negative for CSF leaks, and an MRI showed no signs of intracranial hypotension, “when we patched her, in fact, she got better.” Subsequently, more patients diagnosed with POTS have been referred to the CSF leak program.
Patients initially may be diagnosed as having chronic fatigue syndrome, fibromyalgia, or irritable bowel syndrome when a CSF leak is causing their symptoms. It is a tragedy when patients “have a fixable leak and … nothing is done to treat that underlying problem,” Dr. Carroll said.
—Jake Remaly
Suggested Reading
Ishikawa S, Yokoyama M, Mizobuchi S, et al. Epidural blood patch therapy for chronic whiplash-associated disorder. Anesth Analg. 2007;105(3):809-814.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296.
Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328.
Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115(1):124-132.
OJAI, CA—Spontaneous CSF leaks are treatable, often misdiagnosed, and can cause a neurologic syndrome that may include headache, nausea, and tinnitus. Spinal fluid leaks also can lead to serious complications, including seizures. Patients may have a CSF leak for years or decades before it is diagnosed.
Although CSF leaks may not be readily apparent on imaging, a suspected CSF leak is important to consider because it is fixable, said Dr. Carroll, Assistant Professor of Anesthesiology and Perioperative and Pain Medicine at Stanford University Medical Center in California and a member of the Stanford CSF leak program.
Postdural Puncture Headache Versus Spontaneous CSF Leaks
A spontaneous CSF leak and the clinical syndrome that it causes may be confused with a postdural puncture headache.
With a postdural puncture headache, a patient usually has a single leak site in the dorsal dura. “There is up to a 90% response to a single epidural blood patch. Its natural history is generally well understood and benign. It is rarely mysterious, and it is ultimately fixable,” said Dr. Carroll. In contrast, “a spontaneous CSF leak is often mysterious in terms of the onset, cause, and diagnosis.”
The natural history of CSF leaks is poorly understood. The percentage of patients whose spontaneous leaks seal on their own or whose leaks cause a catastrophe (eg, coma, seizures, or hematomas) is not known. Between 30% and 40% of patients with spontaneous leaks have leaks from multiple sites at diagnosis. “With a spontaneous leak a dural rent is more likely in the ventral dura, anterior to the spinal cord or at the nerve root, making the dura less amenable to patching. A single patch at the correct spinal level will fix the problem only 30% of the time. With multiple patches, the success rate can approach 65% to 75%.” If the first epidural blood patch fails, it should be repeated. Directed epidural blood patches, placement of fibrin sealant, and surgical treatment are other treatment options.
Headaches
Most patients with post-puncture CSF leaks have classic orthostatic headaches. The orthostatic headaches from spontaneous leaks are often atypical, however, in that patients may not feel better immediately when they lie down and worse when they stand, Dr. Carroll said. Headaches may occur late in the day after prolonged upright time or with exertion. In addition, patients may “go from having an orthostatic headache to having a terrible headache all the time, regardless of what position they are in.”
Nausea and Vomiting
Nausea and vomiting can be the main symptom of a CSF leak. Dr. Carroll described a patient with complex regional pain syndrome who underwent a spinal cord stimulation trial. Afterward, she had a postdural puncture headache and received an epidural blood patch. “After that, she developed vomiting up to nine times a day.” A CSF leak was not visible on the first CT myelogram but was apparent on the second. The leak was “so small it was missed by the slice thickness” of the first CT myelogram, said Dr. Carroll. She ultimately required surgery to fix the leak. The patient improved, although she continued vomiting three times per day.
Tinnitus
CSF leaks may cause tinnitus. “You can get ringing in the ears when you have migraine,” Dr. Carroll said. But if patients have tinnitus when they are not having headaches, “you should be thinking that there is something else going on.” Data suggest that CSF fluid is connected to inner ear fluid so a change of pressure in CSF changes inner ear pressure, and patients with high or low CSF pressure may get tinnitus.
Other symptoms may include neck pain and fatigue. “I have had the parents of patients tell me that the most remarkable thing that they see when we patch their sons or daughters is how they are bouncing around the house,” he said. Many patients complain of difficulty with concentrating, task persistence, and other nondescript, nonfocal neurologic symptoms.
Imaging Limitations
Imaging of patients with CSF leaks often initially is read as normal, and MRI is not an adequate evaluation in the high clinical suspicion of a leak, Dr. Carroll said. “It is a good place to start, because if you see a leak on your MRI, maybe you do not have to get a CT myelogram,” he said. “But if you have a clinical suspicion of a leak … you should pursue that in the face of your radiologist telling you that there is nothing.”
Schievink et al in 2007 looked at several years of data from an emergency department to assess how often imaging findings consistent with CSF leaks were missed. They reviewed MRIs of patients with headache to look for evidence of intracranial hypotension, and then compared the number of CSF leaks with the number of subarachnoid hemorrhages seen during the same time. They found that for every subarachnoid hemorrhage, there were approximately 0.5 CSF leaks (23 subarachnoid hemorrhages and 11 CSF leaks). The results suggested that spontaneous intracranial hypotension is more common than previously thought and its diagnosis in emergency departments is problematic. The 11 people with MRI evidence of intracranial hypotension subsequently were diagnosed with CSF leaks and treated. None were diagnosed at the time of the MRI while in the emergency department.
Causes of Leaks
The four main causes of CSF leaks are medical procedures; whiplash; bony, sharp calcifications penetrating the dura; and genetic disorders of connective tissue.
Webb et al conducted a study to evaluate headaches in patients who had a known wet tap (ie, unintentional dural puncture) after a labor epidural. The researchers reviewed quality assurance data in an obstetrics anesthesia division and identified 40 patients who had known wet taps and 40 controls who had received an epidural without a wet tap during the same week and were matched for age and weight. Investigators contacted patients between 12 and 24 months later (mean, 18 months) and asked them about the incidence of chronic headache. The incidence of chronic headache in controls was 5% versus nearly 30% in patients who had had a wet tap. The investigators then compared patients who were managed conservatively (ie, they did not receive an epidural blood patch) versus patients who were managed with a blood patch. “If you got a blood patch, your risk of having a chronic headache 18 months later was only half as much as if you did not get a blood patch,” he said.
Connective Tissue Disorders and Calcifications
Because connective tissue disorders are associated with CSF leaks, headache physicians should determine patients’ Beighton Hypermobility Scores, Dr. Carroll said. The score is derived from a simple test that assesses joint hypermobility. For instance, patients receive a point if they can touch their thumb to their wrist or straighten their elbow more than 10° beyond 180°. A score between 3 and 5 raises suspicion that the patient might have a hereditary disorder of connective tissue. Cataracts at an early age, being unusually tall or short, degenerative disc disease, and history of aneurysm also are associated with an increased risk of CSF leaks.
With regard to calcifications, Dr. Carroll described a patient whose main complaint was confusion upon standing too long. The patient also had neck pain. They determined that he had a calcified bone spur that was puncturing the spinal cord, causing a leak.
Whiplash
Trauma and whiplash can cause leaks. Researchers in Japan studied 66 patients with chronic whiplash-associated disorders (ie, they had a whiplash accident and complained of neck and head pain, as well as difficulty with fatigue or memory). The patients were given a radionuclide cisternogram. Thirty-seven of the 66 patients had imaging that was positive for a CSF leak. “After being patched, roughly half the people who were found to have a leak went back to work, whereas they had not been working before,” he said. Another study found that 10% of people with brachial plexus injuries have spinal fluid leaks.
Overlap With POTS
The fact that Ehlers-Danlos also is associated with postural orthostatic tachycardia syndrome (POTS) raises the possibility that patients with CSF leaks may be misdiagnosed as having POTS.
“Why should a hereditary disorder of connective tissue be associated with the only two known conditions that are associated with feeling worse when you are up?” Dr. Carroll asked. Among patients with POTS, 60% have headaches, and many have dizziness and nausea. Dr. Carroll asked the POTS clinics at Stanford to refer patients with POTS, headache, and Ehlers-Danlos syndrome to him. The first referred patient’s history was consistent with a CSF leak. She had been passing out and had severe headaches for more than 10 years. Although her initial imaging was read as negative for CSF leaks, and an MRI showed no signs of intracranial hypotension, “when we patched her, in fact, she got better.” Subsequently, more patients diagnosed with POTS have been referred to the CSF leak program.
Patients initially may be diagnosed as having chronic fatigue syndrome, fibromyalgia, or irritable bowel syndrome when a CSF leak is causing their symptoms. It is a tragedy when patients “have a fixable leak and … nothing is done to treat that underlying problem,” Dr. Carroll said.
—Jake Remaly
Suggested Reading
Ishikawa S, Yokoyama M, Mizobuchi S, et al. Epidural blood patch therapy for chronic whiplash-associated disorder. Anesth Analg. 2007;105(3):809-814.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296.
Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328.
Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115(1):124-132.
Friable Warty Plaque on the Heel
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.
Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.

Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.

Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
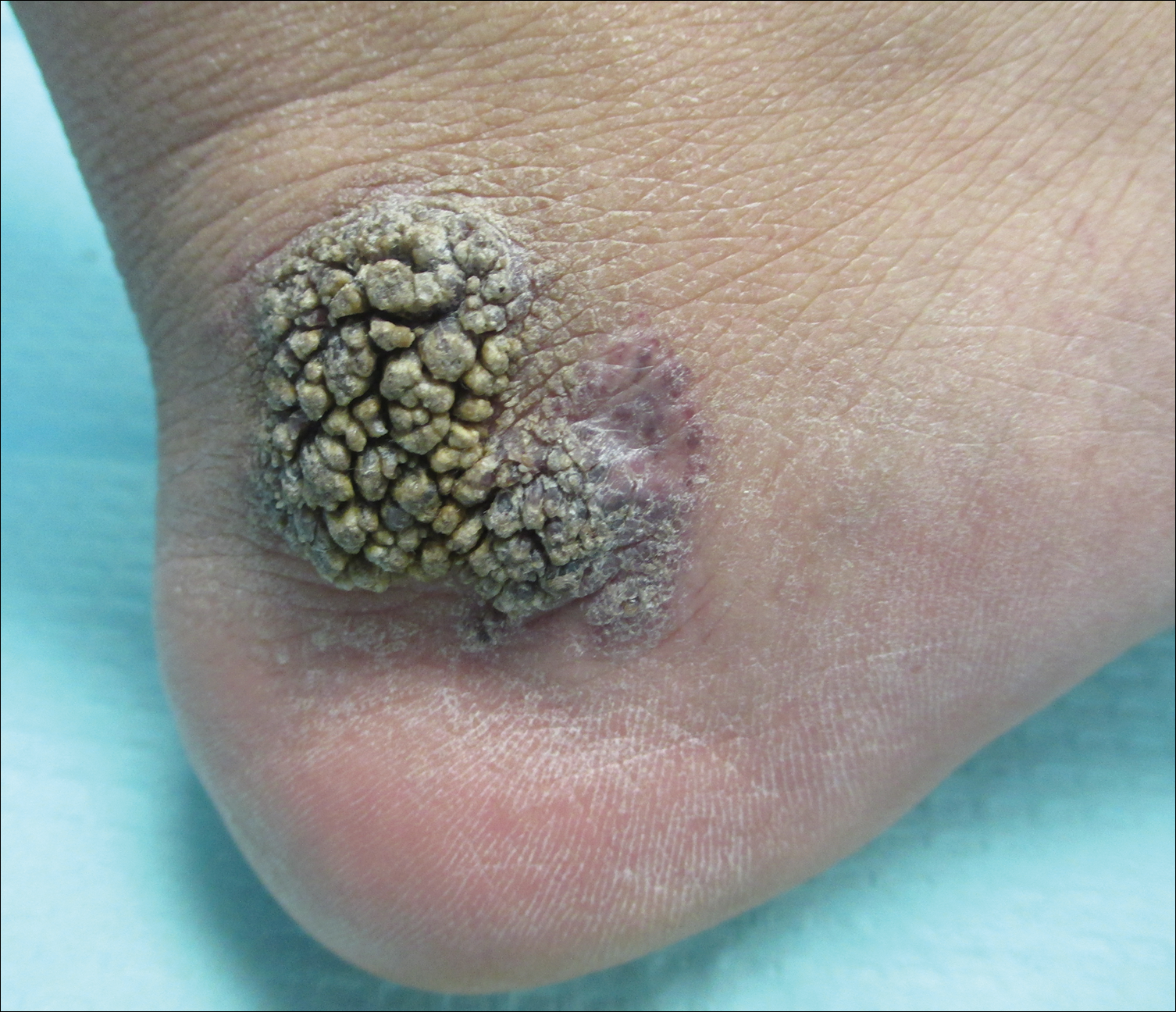
A 31-year-old man presented with a large friable and warty plaque on the left heel. He recalled that the lesion had been present since birth as a flat red birthmark that grew proportionally with him. Throughout his adolescence its surface became increasingly rough and bumpy. The patient described receiving laser treatment twice in his early 20s without notable improvement. He wanted the lesion removed because it was easily traumatized, resulting in bleeding, pain, and infection. The patient reported being otherwise healthy.
Willingness to Take Weight Loss Medication Among Obese Primary Care Patients
From Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA.
Abstracts
- Objective: To identify patient factors associated with willingness to take daily weight loss medication and weight loss expectations using these medications.
- Methods: A random sample of 331 primary care patients aged 18–65 years with a BMI ≥ 35 kg/m2 were recruited from 4 diverse primary care practices in Boston, MA. We conducted telephone interviews and chart reviews to assess patients’ willingness to take a weight loss medication and their expectations for weight loss. We used sequential logistic regression models to identify demographic, clinical, and quality of life (QOL) factors associated with this willingness.
- Results: Of 331 subjects, 69% were women, 35% were white, 35% were black, and 25% were Hispanic; 249 (75%) of patients were willing to take a daily weight loss medication if recommended by their doctor but required a median weight loss of 15% to 24%; only 17% of patients were willing to take a medication for ≤ 10% weight loss. Men were significantly more willing than women (1.2 [95% CI 1.0–1.4]). Diabetes was the only comorbidity associated with willingness to consider pharmacotherapy (1.2 [1.0–1.3]) but only modestly improved model performance (C-statistic increased from 0.59 to 0.60). In contrast, lower QOL, especially low self-esteem and sex life, were stronger correlates (C-statistic 0.72).
- Conclusion: A majority of obese primary care patients were willing to take a daily weight loss pill; however, most required more than 10% weight loss to consider pharmacotherapy worthwhile. Poor QOL, especially low self-esteem and poor sex life, were stronger correlates than having diabetes.
Key words: obesity; primary care; weight loss medication.
In the United States, obesity continues to be unrelentingly prevalent, affecting more than one-third of adults (34.9%) [1]. This statistic has ominous implications when considering that obesity is a risk factor for numerous chronic diseases, such as coronary heart disease, diabetes, sleep apnea, osteoarthritis, and some types of cancers [2]. Moreover, it is associated with increased risk of all-cause and cardiovascular disease mortality. Promisingly, an initial 5% to 10% weight loss over 6 months has been associated with improvement in LDL, HDL, triglycerides, glucose, hemoglobin A1C, diabetes risk, blood pressure, and medication use [2]. Therefore, although patients may not be able to achieve their ideal body weight or normal BMI, modest weight loss can still have beneficial health effects.
Weight loss medications are effective adjunctive therapies in helping patients lose up to 10% of their body weight on average when combined with diet and exercise [3–5]. There are currently 5 medications approved by the Food and Drug Administration for long-term use for weight loss: orlistat, lorcaserin, phentermine-topiramate, bupropion-naltrexone, and liraglutide. Despite their proven efficacy, there are barriers to initiating a long-term weight loss medication. Insurance reimbursement is limited for these medications, thus resulting in high out-of-pocket cost for patients that they may be unable or unwilling to pay [6]. There may also be safety concerns given that several weight loss medications, including fenfluramine, sibutramine, and rimonabant, have been withdrawn from the market because of adverse effects [7]. Thus, in deciding whether to initiate a pharmacologic weight loss regimen, patients must believe that the weight loss benefits will exceed the potential risks.
Little is known, however, about patients’ willingness to take weight loss medications or the minimum weight loss they expect to lose to make pharmacotherapy worthwhile. Only a few studies have investigated patient willingness to adopt pharmacotherapy as part of a weight loss regimen, and only one investigated obese patients in the United States [8]. In this context, we surveyed a sociodemographically diverse group of primary care patients with moderate to severe obesity to examine patient characteristics associated with willingness to pursue weight loss pharmacotherapy. We also aimed to evaluate how much weight patients expected to lose in order to make taking a daily medication worth the effort. Characterizing patients seen in primary care who are willing to adopt pharmacotherapy to lose weight may guide weight loss counseling in the primary care setting. Furthermore, determining whether patients have realistic weight loss expectations can help clinicians better counsel their patients on weight loss goals.
Methods
Study Sample
We recruited 337 subjects from 4 diverse primary care practices in Boston, Massachusetts: a large hospital-based academic practice, a community practice in a working-class suburb, a community practice in an affluent suburb, and a health center serving a predominantly socially disadvantaged population. The primary goal of the parent study was to understand the preferences of patients for weight loss treatment, especially bariatric surgery. Therefore, to be included, patients needed to have a BMI ≥ 35 kg/m2 at the time of recruitment, been seen in clinic within the past year, be aged 18–65 years, and be English or Spanish speaking. By design, African-American and Hispanic patients were oversampled from an electronic list of potentially eligible patient groups so that we could examine for racial differences in treatment preferences. Study details have been previously described [9].
Data Collection and Measures
Trained interviewers conducted a 45- to 60-minute telephone interview with each participant in either English or Spanish. To assess willingness to use a daily weight loss medication, subjects were asked, “If your doctor recommended it, would you be willing to take a pill or medication every day in order to lose weight?” Those who answered affirmatively were then asked the minimum amount of weight they would have to lose to make taking a pill everyday worthwhile.
Subjects were also asked about demographic information (age, race, education, marital status) and comorbid health conditions commonly associated with obesity (diabetes mellitus, hypertension, asthma, obstructive sleep apnea, GERD, depression, anxiety, back pain, and cardiovascular problems). We assessed quality of life (QOL) using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite), a 31-item instrument designed specifically to assess the impact of obesity on QOL capturing 5 domains (physical function, self-esteem, sexual life, public distress, and work). Subjects were asked to rate a series of statements beginning with “Because of my weight…” as “always true,” “usually true,” “sometimes true,” “rarely true,” or “never true.” Global and domain scores ranged from 0 to 100; higher scores reflected better QOL [10].
Data Analysis
We used descriptive statistics to characterize the proportion of subjects willing to use a daily weight loss medication and the weight loss required for patients to be willing to consider pharmacotherapy. We used a stepwise logistic model to examine demographic, QOL, and clinical factors associated with the willingness to take a weight loss medication as the outcome, with an entry criteria of P value of 0.1 and an exit criteria of 0.05. Log-Poisson distribution using the sandwich estimator was used to obtain relative risks for each significant variable. Adjusted models included age, BMI, sex, and race and any significant comorbidities. We added overall QOL score and individual QOL scores in subsequent models to examine the relative influence of overall vs. domain-specific QOL. Statistical analyses were conducted with SAS (SAS Institute, Cary, NC). We considered the change in model C-statistic when specific variables were added to the model to determine the importance of these factors in contributing to patients’ willingness to consider pharmacotherapy; larger changes in model C-statistic signifies a greater contribution.
Results
Table 2 displays sequentially adjusted models examining various demographic, clinical, and QOL factors associated with willingness to take a weight loss medication.
Discussion
In our study, we found that a large proportion (75%) of primary care patients with at least moderate obesity were willing to take a daily weight loss medication if their doctor recommended it. After full adjustment, men, those with lower quality of life (QOL), and patients with diabetes were more likely to pursue weight loss pharmacotherapy than their counterparts. Moreover, QOL appeared more important than comorbid diagnoses in contributing to whether patients would consider taking a weight loss medication. Most patients expected to lose more than 10% of their weight to make taking a daily medication worthwhile.
Few studies have examined patients’ willingness to take a medication to lose weight. Tan et al [11] found that only about half of their surveyed outpatients were likely to take a medication to lose weight; however, approximately a quarter of the patients in that study were of normal BMI. In contrast, our study interviewed patients with at least a BMI of 35 kg/m2 and the majority of these patients reported a willingness to take a weight loss medication. Nevertheless, patients appear to have unrealistic expectations of the weight loss potential of pharmacotherapy. Only a minority of patients in our study would be willing to take a weight loss medication if the weight loss was no more than 10%, a level that is more consistent with the outcomes achievable in most clinical trials of weight loss medications [12]. Prior studies have also shown that patients often have unrealistic weight loss expectations and are unable to achieve their ideal body weight using diet, exercise, or pharmacotherapy [13,14]. Doyle et al found that percentage of weight loss was the most important treatment attribute when considering weight loss pharmacotherapy when compared to cost, health improvements, side effects, diet and exercise requirements, and method of medication administration [8]. Thus it is important to educate patients on realistic goal setting and the benefits of modest weight loss when considering pharmacotherapy. The weight loss preferences expressed in our study may also influence the weight loss outcomes targets pursued in pharmaceutical development. Interestingly, after full adjustment, BMI did not correlate with willingness to take a weight loss medication. Given that all patients in our study had a BMI of ≥ 35 kg/m2, this may imply that variations beyond this BMI threshold did not significantly affect a patient’s willingness to use pharmacotherapy. In contrast, weight-related QOL was an important correlate.
Men were slightly more likely than women to be willing to take a weight loss medication, which is interesting since men have been shown to be less likely to participate in behavioral weight loss programs and diets [15]. One reason may be that many weight loss programs are delivered in group settings which may deter men from participating. Whether this hypothetical willingness to undergo pharmacotherapy would translate to actual use is unclear, especially since there are barriers to pharmacotherapy including out-of-pocket costs. In a prior study in the United Kingdom, women were more likely to have reported prior weight loss medication use than men [16].
Our study did not find differences in willingness to pursue weight loss medication by race or educational attainment. This is consistent with our prior work demonstrating that racial and ethnic minorities were no less likely to consider bariatric surgery if the treatment were recommended by their doctor [9]. However, our other work did suggest that clinicians may be less likely to recommend bariatric surgery to their medically eligible minority patients as compared to their Caucasian patients. Whether this may be the case for pharmacotherapy is unclear since this was not explicitly queried in our current study [9].
Our study also found that patients with diabetes but not other comorbidities were more likely to consider weight loss medication after adjusting for QOL. This may reflect a stronger link between diabetes and obesity perceived by patients. Our result is consistent with our earlier data showing that diabetes but not other comorbid conditions was associated with a higher likelihood of considering weight loss surgery [9]. Nevertheless, having diabetes contributed only modestly to the variation in patient preferences regarding pharmacotherapy as reflected by the trivial change in model C-statistic when diabetes status was added to the model.
In contrast, lower QOL scores, especially in the domains of self-esteem and sex life, were associated with increased willingness to take a weight loss medication and appeared to be a stronger predictor than individual comorbidities. This is consistent with other studies showing that patients seeking treatment for obesity tend to have lower health-related QOL [9,17]. Our findings are also consistent with our previous research demonstrating that impairments in specific QOL domains are often more important to patients and stronger drivers of diminished well-being than measures of overall QOL [18]. Hence, given their importance to patients, clinicians need to consider QOL benefits when counseling patients about the risks and benefits of various obesity treatments.
This study is the first to our knowledge to systematically characterize demographic factors associated with the likelihood of primary care patients with obesity considering weight loss pharmacotherapy. This information may aid outpatient weight loss counseling by increasing awareness of gender and patient specific preferences. The fact that many patients with obesity appear to be interested in pursuing weight loss medication may also support public health initiatives in providing equitable access to weight loss pharmacotherapy. As our study characterizes patients who are willing to pursue weight loss medications, future studies may include retrospective analyses on actual use of weight loss medications among various demographic groups. Further investigation on specific reasons why patients choose whether or not to use weight loss medication may also be helpful.
This study has important limitations. The sample size was modest and potentially underpowered to detect small differences across different subgroups. Our sample was also limited to practices in Boston, which limits generalizability; although, by design, we oversampled racial and ethnic subjects to ensure diverse representation. Finally, our study examined patients’ hypothetical willingness to take weight loss medications rather than their actual adherence to treatment if offered.
Conclusion
In this sample of obese primary care patients, we found that the majority of patients were willing to take a daily medication to lose weight; however, patients had expectations for weight loss that far exceeded the level achievable by patients in pharmaceutical trials of these agents. Men and patients with diabetes were more likely to be willing to pursue weight loss medication; however, lower weight-related QOL, especially low self-esteem and impaired sexual function, appeared to be a stronger correlate of willingness to consider pharmacotherapy than comorbid diagnoses.
Corresponding author: Christina C. Wee, MD, MPH, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215, [email protected].
Funding/support: This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK073302, PI Wee). Dr. Wee is also supported by an NIH midcareer mentorship award (K24DK087932). The sponsor had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures: None reported.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;(131):1–8.
2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023.
3. Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
4. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med 2015;26:89–94.
5. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
6. Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol 2006;2:357–77.
7. Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–81.
8. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obesity (Silver Spring) 2012;20:2019–26.
9. Wee CC HK, Bolcic-Jankovic D, Colten ME, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
10. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11.
11. Tan DZN, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
12. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
13. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85.
14. Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–45.
15. Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:1–424.
16. Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes Relat Metab Disord 2000;24:164–70.
17. Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res 2002;10:748–56.
18. Wee C, Davis R, Chiodi S, et al. Sex, race, and the adverse effects of social stigma vs. other quality of life factors among primary care patients with moderate to severe obesity. J Gen Intern Med 2015;30:229–35.
From Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA.
Abstracts
- Objective: To identify patient factors associated with willingness to take daily weight loss medication and weight loss expectations using these medications.
- Methods: A random sample of 331 primary care patients aged 18–65 years with a BMI ≥ 35 kg/m2 were recruited from 4 diverse primary care practices in Boston, MA. We conducted telephone interviews and chart reviews to assess patients’ willingness to take a weight loss medication and their expectations for weight loss. We used sequential logistic regression models to identify demographic, clinical, and quality of life (QOL) factors associated with this willingness.
- Results: Of 331 subjects, 69% were women, 35% were white, 35% were black, and 25% were Hispanic; 249 (75%) of patients were willing to take a daily weight loss medication if recommended by their doctor but required a median weight loss of 15% to 24%; only 17% of patients were willing to take a medication for ≤ 10% weight loss. Men were significantly more willing than women (1.2 [95% CI 1.0–1.4]). Diabetes was the only comorbidity associated with willingness to consider pharmacotherapy (1.2 [1.0–1.3]) but only modestly improved model performance (C-statistic increased from 0.59 to 0.60). In contrast, lower QOL, especially low self-esteem and sex life, were stronger correlates (C-statistic 0.72).
- Conclusion: A majority of obese primary care patients were willing to take a daily weight loss pill; however, most required more than 10% weight loss to consider pharmacotherapy worthwhile. Poor QOL, especially low self-esteem and poor sex life, were stronger correlates than having diabetes.
Key words: obesity; primary care; weight loss medication.
In the United States, obesity continues to be unrelentingly prevalent, affecting more than one-third of adults (34.9%) [1]. This statistic has ominous implications when considering that obesity is a risk factor for numerous chronic diseases, such as coronary heart disease, diabetes, sleep apnea, osteoarthritis, and some types of cancers [2]. Moreover, it is associated with increased risk of all-cause and cardiovascular disease mortality. Promisingly, an initial 5% to 10% weight loss over 6 months has been associated with improvement in LDL, HDL, triglycerides, glucose, hemoglobin A1C, diabetes risk, blood pressure, and medication use [2]. Therefore, although patients may not be able to achieve their ideal body weight or normal BMI, modest weight loss can still have beneficial health effects.
Weight loss medications are effective adjunctive therapies in helping patients lose up to 10% of their body weight on average when combined with diet and exercise [3–5]. There are currently 5 medications approved by the Food and Drug Administration for long-term use for weight loss: orlistat, lorcaserin, phentermine-topiramate, bupropion-naltrexone, and liraglutide. Despite their proven efficacy, there are barriers to initiating a long-term weight loss medication. Insurance reimbursement is limited for these medications, thus resulting in high out-of-pocket cost for patients that they may be unable or unwilling to pay [6]. There may also be safety concerns given that several weight loss medications, including fenfluramine, sibutramine, and rimonabant, have been withdrawn from the market because of adverse effects [7]. Thus, in deciding whether to initiate a pharmacologic weight loss regimen, patients must believe that the weight loss benefits will exceed the potential risks.
Little is known, however, about patients’ willingness to take weight loss medications or the minimum weight loss they expect to lose to make pharmacotherapy worthwhile. Only a few studies have investigated patient willingness to adopt pharmacotherapy as part of a weight loss regimen, and only one investigated obese patients in the United States [8]. In this context, we surveyed a sociodemographically diverse group of primary care patients with moderate to severe obesity to examine patient characteristics associated with willingness to pursue weight loss pharmacotherapy. We also aimed to evaluate how much weight patients expected to lose in order to make taking a daily medication worth the effort. Characterizing patients seen in primary care who are willing to adopt pharmacotherapy to lose weight may guide weight loss counseling in the primary care setting. Furthermore, determining whether patients have realistic weight loss expectations can help clinicians better counsel their patients on weight loss goals.
Methods
Study Sample
We recruited 337 subjects from 4 diverse primary care practices in Boston, Massachusetts: a large hospital-based academic practice, a community practice in a working-class suburb, a community practice in an affluent suburb, and a health center serving a predominantly socially disadvantaged population. The primary goal of the parent study was to understand the preferences of patients for weight loss treatment, especially bariatric surgery. Therefore, to be included, patients needed to have a BMI ≥ 35 kg/m2 at the time of recruitment, been seen in clinic within the past year, be aged 18–65 years, and be English or Spanish speaking. By design, African-American and Hispanic patients were oversampled from an electronic list of potentially eligible patient groups so that we could examine for racial differences in treatment preferences. Study details have been previously described [9].
Data Collection and Measures
Trained interviewers conducted a 45- to 60-minute telephone interview with each participant in either English or Spanish. To assess willingness to use a daily weight loss medication, subjects were asked, “If your doctor recommended it, would you be willing to take a pill or medication every day in order to lose weight?” Those who answered affirmatively were then asked the minimum amount of weight they would have to lose to make taking a pill everyday worthwhile.
Subjects were also asked about demographic information (age, race, education, marital status) and comorbid health conditions commonly associated with obesity (diabetes mellitus, hypertension, asthma, obstructive sleep apnea, GERD, depression, anxiety, back pain, and cardiovascular problems). We assessed quality of life (QOL) using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite), a 31-item instrument designed specifically to assess the impact of obesity on QOL capturing 5 domains (physical function, self-esteem, sexual life, public distress, and work). Subjects were asked to rate a series of statements beginning with “Because of my weight…” as “always true,” “usually true,” “sometimes true,” “rarely true,” or “never true.” Global and domain scores ranged from 0 to 100; higher scores reflected better QOL [10].
Data Analysis
We used descriptive statistics to characterize the proportion of subjects willing to use a daily weight loss medication and the weight loss required for patients to be willing to consider pharmacotherapy. We used a stepwise logistic model to examine demographic, QOL, and clinical factors associated with the willingness to take a weight loss medication as the outcome, with an entry criteria of P value of 0.1 and an exit criteria of 0.05. Log-Poisson distribution using the sandwich estimator was used to obtain relative risks for each significant variable. Adjusted models included age, BMI, sex, and race and any significant comorbidities. We added overall QOL score and individual QOL scores in subsequent models to examine the relative influence of overall vs. domain-specific QOL. Statistical analyses were conducted with SAS (SAS Institute, Cary, NC). We considered the change in model C-statistic when specific variables were added to the model to determine the importance of these factors in contributing to patients’ willingness to consider pharmacotherapy; larger changes in model C-statistic signifies a greater contribution.
Results
Table 2 displays sequentially adjusted models examining various demographic, clinical, and QOL factors associated with willingness to take a weight loss medication.
Discussion
In our study, we found that a large proportion (75%) of primary care patients with at least moderate obesity were willing to take a daily weight loss medication if their doctor recommended it. After full adjustment, men, those with lower quality of life (QOL), and patients with diabetes were more likely to pursue weight loss pharmacotherapy than their counterparts. Moreover, QOL appeared more important than comorbid diagnoses in contributing to whether patients would consider taking a weight loss medication. Most patients expected to lose more than 10% of their weight to make taking a daily medication worthwhile.
Few studies have examined patients’ willingness to take a medication to lose weight. Tan et al [11] found that only about half of their surveyed outpatients were likely to take a medication to lose weight; however, approximately a quarter of the patients in that study were of normal BMI. In contrast, our study interviewed patients with at least a BMI of 35 kg/m2 and the majority of these patients reported a willingness to take a weight loss medication. Nevertheless, patients appear to have unrealistic expectations of the weight loss potential of pharmacotherapy. Only a minority of patients in our study would be willing to take a weight loss medication if the weight loss was no more than 10%, a level that is more consistent with the outcomes achievable in most clinical trials of weight loss medications [12]. Prior studies have also shown that patients often have unrealistic weight loss expectations and are unable to achieve their ideal body weight using diet, exercise, or pharmacotherapy [13,14]. Doyle et al found that percentage of weight loss was the most important treatment attribute when considering weight loss pharmacotherapy when compared to cost, health improvements, side effects, diet and exercise requirements, and method of medication administration [8]. Thus it is important to educate patients on realistic goal setting and the benefits of modest weight loss when considering pharmacotherapy. The weight loss preferences expressed in our study may also influence the weight loss outcomes targets pursued in pharmaceutical development. Interestingly, after full adjustment, BMI did not correlate with willingness to take a weight loss medication. Given that all patients in our study had a BMI of ≥ 35 kg/m2, this may imply that variations beyond this BMI threshold did not significantly affect a patient’s willingness to use pharmacotherapy. In contrast, weight-related QOL was an important correlate.
Men were slightly more likely than women to be willing to take a weight loss medication, which is interesting since men have been shown to be less likely to participate in behavioral weight loss programs and diets [15]. One reason may be that many weight loss programs are delivered in group settings which may deter men from participating. Whether this hypothetical willingness to undergo pharmacotherapy would translate to actual use is unclear, especially since there are barriers to pharmacotherapy including out-of-pocket costs. In a prior study in the United Kingdom, women were more likely to have reported prior weight loss medication use than men [16].
Our study did not find differences in willingness to pursue weight loss medication by race or educational attainment. This is consistent with our prior work demonstrating that racial and ethnic minorities were no less likely to consider bariatric surgery if the treatment were recommended by their doctor [9]. However, our other work did suggest that clinicians may be less likely to recommend bariatric surgery to their medically eligible minority patients as compared to their Caucasian patients. Whether this may be the case for pharmacotherapy is unclear since this was not explicitly queried in our current study [9].
Our study also found that patients with diabetes but not other comorbidities were more likely to consider weight loss medication after adjusting for QOL. This may reflect a stronger link between diabetes and obesity perceived by patients. Our result is consistent with our earlier data showing that diabetes but not other comorbid conditions was associated with a higher likelihood of considering weight loss surgery [9]. Nevertheless, having diabetes contributed only modestly to the variation in patient preferences regarding pharmacotherapy as reflected by the trivial change in model C-statistic when diabetes status was added to the model.
In contrast, lower QOL scores, especially in the domains of self-esteem and sex life, were associated with increased willingness to take a weight loss medication and appeared to be a stronger predictor than individual comorbidities. This is consistent with other studies showing that patients seeking treatment for obesity tend to have lower health-related QOL [9,17]. Our findings are also consistent with our previous research demonstrating that impairments in specific QOL domains are often more important to patients and stronger drivers of diminished well-being than measures of overall QOL [18]. Hence, given their importance to patients, clinicians need to consider QOL benefits when counseling patients about the risks and benefits of various obesity treatments.
This study is the first to our knowledge to systematically characterize demographic factors associated with the likelihood of primary care patients with obesity considering weight loss pharmacotherapy. This information may aid outpatient weight loss counseling by increasing awareness of gender and patient specific preferences. The fact that many patients with obesity appear to be interested in pursuing weight loss medication may also support public health initiatives in providing equitable access to weight loss pharmacotherapy. As our study characterizes patients who are willing to pursue weight loss medications, future studies may include retrospective analyses on actual use of weight loss medications among various demographic groups. Further investigation on specific reasons why patients choose whether or not to use weight loss medication may also be helpful.
This study has important limitations. The sample size was modest and potentially underpowered to detect small differences across different subgroups. Our sample was also limited to practices in Boston, which limits generalizability; although, by design, we oversampled racial and ethnic subjects to ensure diverse representation. Finally, our study examined patients’ hypothetical willingness to take weight loss medications rather than their actual adherence to treatment if offered.
Conclusion
In this sample of obese primary care patients, we found that the majority of patients were willing to take a daily medication to lose weight; however, patients had expectations for weight loss that far exceeded the level achievable by patients in pharmaceutical trials of these agents. Men and patients with diabetes were more likely to be willing to pursue weight loss medication; however, lower weight-related QOL, especially low self-esteem and impaired sexual function, appeared to be a stronger correlate of willingness to consider pharmacotherapy than comorbid diagnoses.
Corresponding author: Christina C. Wee, MD, MPH, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215, [email protected].
Funding/support: This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK073302, PI Wee). Dr. Wee is also supported by an NIH midcareer mentorship award (K24DK087932). The sponsor had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures: None reported.
From Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA.
Abstracts
- Objective: To identify patient factors associated with willingness to take daily weight loss medication and weight loss expectations using these medications.
- Methods: A random sample of 331 primary care patients aged 18–65 years with a BMI ≥ 35 kg/m2 were recruited from 4 diverse primary care practices in Boston, MA. We conducted telephone interviews and chart reviews to assess patients’ willingness to take a weight loss medication and their expectations for weight loss. We used sequential logistic regression models to identify demographic, clinical, and quality of life (QOL) factors associated with this willingness.
- Results: Of 331 subjects, 69% were women, 35% were white, 35% were black, and 25% were Hispanic; 249 (75%) of patients were willing to take a daily weight loss medication if recommended by their doctor but required a median weight loss of 15% to 24%; only 17% of patients were willing to take a medication for ≤ 10% weight loss. Men were significantly more willing than women (1.2 [95% CI 1.0–1.4]). Diabetes was the only comorbidity associated with willingness to consider pharmacotherapy (1.2 [1.0–1.3]) but only modestly improved model performance (C-statistic increased from 0.59 to 0.60). In contrast, lower QOL, especially low self-esteem and sex life, were stronger correlates (C-statistic 0.72).
- Conclusion: A majority of obese primary care patients were willing to take a daily weight loss pill; however, most required more than 10% weight loss to consider pharmacotherapy worthwhile. Poor QOL, especially low self-esteem and poor sex life, were stronger correlates than having diabetes.
Key words: obesity; primary care; weight loss medication.
In the United States, obesity continues to be unrelentingly prevalent, affecting more than one-third of adults (34.9%) [1]. This statistic has ominous implications when considering that obesity is a risk factor for numerous chronic diseases, such as coronary heart disease, diabetes, sleep apnea, osteoarthritis, and some types of cancers [2]. Moreover, it is associated with increased risk of all-cause and cardiovascular disease mortality. Promisingly, an initial 5% to 10% weight loss over 6 months has been associated with improvement in LDL, HDL, triglycerides, glucose, hemoglobin A1C, diabetes risk, blood pressure, and medication use [2]. Therefore, although patients may not be able to achieve their ideal body weight or normal BMI, modest weight loss can still have beneficial health effects.
Weight loss medications are effective adjunctive therapies in helping patients lose up to 10% of their body weight on average when combined with diet and exercise [3–5]. There are currently 5 medications approved by the Food and Drug Administration for long-term use for weight loss: orlistat, lorcaserin, phentermine-topiramate, bupropion-naltrexone, and liraglutide. Despite their proven efficacy, there are barriers to initiating a long-term weight loss medication. Insurance reimbursement is limited for these medications, thus resulting in high out-of-pocket cost for patients that they may be unable or unwilling to pay [6]. There may also be safety concerns given that several weight loss medications, including fenfluramine, sibutramine, and rimonabant, have been withdrawn from the market because of adverse effects [7]. Thus, in deciding whether to initiate a pharmacologic weight loss regimen, patients must believe that the weight loss benefits will exceed the potential risks.
Little is known, however, about patients’ willingness to take weight loss medications or the minimum weight loss they expect to lose to make pharmacotherapy worthwhile. Only a few studies have investigated patient willingness to adopt pharmacotherapy as part of a weight loss regimen, and only one investigated obese patients in the United States [8]. In this context, we surveyed a sociodemographically diverse group of primary care patients with moderate to severe obesity to examine patient characteristics associated with willingness to pursue weight loss pharmacotherapy. We also aimed to evaluate how much weight patients expected to lose in order to make taking a daily medication worth the effort. Characterizing patients seen in primary care who are willing to adopt pharmacotherapy to lose weight may guide weight loss counseling in the primary care setting. Furthermore, determining whether patients have realistic weight loss expectations can help clinicians better counsel their patients on weight loss goals.
Methods
Study Sample
We recruited 337 subjects from 4 diverse primary care practices in Boston, Massachusetts: a large hospital-based academic practice, a community practice in a working-class suburb, a community practice in an affluent suburb, and a health center serving a predominantly socially disadvantaged population. The primary goal of the parent study was to understand the preferences of patients for weight loss treatment, especially bariatric surgery. Therefore, to be included, patients needed to have a BMI ≥ 35 kg/m2 at the time of recruitment, been seen in clinic within the past year, be aged 18–65 years, and be English or Spanish speaking. By design, African-American and Hispanic patients were oversampled from an electronic list of potentially eligible patient groups so that we could examine for racial differences in treatment preferences. Study details have been previously described [9].
Data Collection and Measures
Trained interviewers conducted a 45- to 60-minute telephone interview with each participant in either English or Spanish. To assess willingness to use a daily weight loss medication, subjects were asked, “If your doctor recommended it, would you be willing to take a pill or medication every day in order to lose weight?” Those who answered affirmatively were then asked the minimum amount of weight they would have to lose to make taking a pill everyday worthwhile.
Subjects were also asked about demographic information (age, race, education, marital status) and comorbid health conditions commonly associated with obesity (diabetes mellitus, hypertension, asthma, obstructive sleep apnea, GERD, depression, anxiety, back pain, and cardiovascular problems). We assessed quality of life (QOL) using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite), a 31-item instrument designed specifically to assess the impact of obesity on QOL capturing 5 domains (physical function, self-esteem, sexual life, public distress, and work). Subjects were asked to rate a series of statements beginning with “Because of my weight…” as “always true,” “usually true,” “sometimes true,” “rarely true,” or “never true.” Global and domain scores ranged from 0 to 100; higher scores reflected better QOL [10].
Data Analysis
We used descriptive statistics to characterize the proportion of subjects willing to use a daily weight loss medication and the weight loss required for patients to be willing to consider pharmacotherapy. We used a stepwise logistic model to examine demographic, QOL, and clinical factors associated with the willingness to take a weight loss medication as the outcome, with an entry criteria of P value of 0.1 and an exit criteria of 0.05. Log-Poisson distribution using the sandwich estimator was used to obtain relative risks for each significant variable. Adjusted models included age, BMI, sex, and race and any significant comorbidities. We added overall QOL score and individual QOL scores in subsequent models to examine the relative influence of overall vs. domain-specific QOL. Statistical analyses were conducted with SAS (SAS Institute, Cary, NC). We considered the change in model C-statistic when specific variables were added to the model to determine the importance of these factors in contributing to patients’ willingness to consider pharmacotherapy; larger changes in model C-statistic signifies a greater contribution.
Results
Table 2 displays sequentially adjusted models examining various demographic, clinical, and QOL factors associated with willingness to take a weight loss medication.
Discussion
In our study, we found that a large proportion (75%) of primary care patients with at least moderate obesity were willing to take a daily weight loss medication if their doctor recommended it. After full adjustment, men, those with lower quality of life (QOL), and patients with diabetes were more likely to pursue weight loss pharmacotherapy than their counterparts. Moreover, QOL appeared more important than comorbid diagnoses in contributing to whether patients would consider taking a weight loss medication. Most patients expected to lose more than 10% of their weight to make taking a daily medication worthwhile.
Few studies have examined patients’ willingness to take a medication to lose weight. Tan et al [11] found that only about half of their surveyed outpatients were likely to take a medication to lose weight; however, approximately a quarter of the patients in that study were of normal BMI. In contrast, our study interviewed patients with at least a BMI of 35 kg/m2 and the majority of these patients reported a willingness to take a weight loss medication. Nevertheless, patients appear to have unrealistic expectations of the weight loss potential of pharmacotherapy. Only a minority of patients in our study would be willing to take a weight loss medication if the weight loss was no more than 10%, a level that is more consistent with the outcomes achievable in most clinical trials of weight loss medications [12]. Prior studies have also shown that patients often have unrealistic weight loss expectations and are unable to achieve their ideal body weight using diet, exercise, or pharmacotherapy [13,14]. Doyle et al found that percentage of weight loss was the most important treatment attribute when considering weight loss pharmacotherapy when compared to cost, health improvements, side effects, diet and exercise requirements, and method of medication administration [8]. Thus it is important to educate patients on realistic goal setting and the benefits of modest weight loss when considering pharmacotherapy. The weight loss preferences expressed in our study may also influence the weight loss outcomes targets pursued in pharmaceutical development. Interestingly, after full adjustment, BMI did not correlate with willingness to take a weight loss medication. Given that all patients in our study had a BMI of ≥ 35 kg/m2, this may imply that variations beyond this BMI threshold did not significantly affect a patient’s willingness to use pharmacotherapy. In contrast, weight-related QOL was an important correlate.
Men were slightly more likely than women to be willing to take a weight loss medication, which is interesting since men have been shown to be less likely to participate in behavioral weight loss programs and diets [15]. One reason may be that many weight loss programs are delivered in group settings which may deter men from participating. Whether this hypothetical willingness to undergo pharmacotherapy would translate to actual use is unclear, especially since there are barriers to pharmacotherapy including out-of-pocket costs. In a prior study in the United Kingdom, women were more likely to have reported prior weight loss medication use than men [16].
Our study did not find differences in willingness to pursue weight loss medication by race or educational attainment. This is consistent with our prior work demonstrating that racial and ethnic minorities were no less likely to consider bariatric surgery if the treatment were recommended by their doctor [9]. However, our other work did suggest that clinicians may be less likely to recommend bariatric surgery to their medically eligible minority patients as compared to their Caucasian patients. Whether this may be the case for pharmacotherapy is unclear since this was not explicitly queried in our current study [9].
Our study also found that patients with diabetes but not other comorbidities were more likely to consider weight loss medication after adjusting for QOL. This may reflect a stronger link between diabetes and obesity perceived by patients. Our result is consistent with our earlier data showing that diabetes but not other comorbid conditions was associated with a higher likelihood of considering weight loss surgery [9]. Nevertheless, having diabetes contributed only modestly to the variation in patient preferences regarding pharmacotherapy as reflected by the trivial change in model C-statistic when diabetes status was added to the model.
In contrast, lower QOL scores, especially in the domains of self-esteem and sex life, were associated with increased willingness to take a weight loss medication and appeared to be a stronger predictor than individual comorbidities. This is consistent with other studies showing that patients seeking treatment for obesity tend to have lower health-related QOL [9,17]. Our findings are also consistent with our previous research demonstrating that impairments in specific QOL domains are often more important to patients and stronger drivers of diminished well-being than measures of overall QOL [18]. Hence, given their importance to patients, clinicians need to consider QOL benefits when counseling patients about the risks and benefits of various obesity treatments.
This study is the first to our knowledge to systematically characterize demographic factors associated with the likelihood of primary care patients with obesity considering weight loss pharmacotherapy. This information may aid outpatient weight loss counseling by increasing awareness of gender and patient specific preferences. The fact that many patients with obesity appear to be interested in pursuing weight loss medication may also support public health initiatives in providing equitable access to weight loss pharmacotherapy. As our study characterizes patients who are willing to pursue weight loss medications, future studies may include retrospective analyses on actual use of weight loss medications among various demographic groups. Further investigation on specific reasons why patients choose whether or not to use weight loss medication may also be helpful.
This study has important limitations. The sample size was modest and potentially underpowered to detect small differences across different subgroups. Our sample was also limited to practices in Boston, which limits generalizability; although, by design, we oversampled racial and ethnic subjects to ensure diverse representation. Finally, our study examined patients’ hypothetical willingness to take weight loss medications rather than their actual adherence to treatment if offered.
Conclusion
In this sample of obese primary care patients, we found that the majority of patients were willing to take a daily medication to lose weight; however, patients had expectations for weight loss that far exceeded the level achievable by patients in pharmaceutical trials of these agents. Men and patients with diabetes were more likely to be willing to pursue weight loss medication; however, lower weight-related QOL, especially low self-esteem and impaired sexual function, appeared to be a stronger correlate of willingness to consider pharmacotherapy than comorbid diagnoses.
Corresponding author: Christina C. Wee, MD, MPH, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215, [email protected].
Funding/support: This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases (R01 DK073302, PI Wee). Dr. Wee is also supported by an NIH midcareer mentorship award (K24DK087932). The sponsor had no role in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial disclosures: None reported.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;(131):1–8.
2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023.
3. Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
4. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med 2015;26:89–94.
5. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
6. Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol 2006;2:357–77.
7. Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–81.
8. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obesity (Silver Spring) 2012;20:2019–26.
9. Wee CC HK, Bolcic-Jankovic D, Colten ME, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
10. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11.
11. Tan DZN, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
12. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
13. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85.
14. Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–45.
15. Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:1–424.
16. Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes Relat Metab Disord 2000;24:164–70.
17. Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res 2002;10:748–56.
18. Wee C, Davis R, Chiodi S, et al. Sex, race, and the adverse effects of social stigma vs. other quality of life factors among primary care patients with moderate to severe obesity. J Gen Intern Med 2015;30:229–35.
1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013;(131):1–8.
2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023.
3. Hauptman J, Lucas C, Boldrin MN, et al. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
4. Kakkar AK, Dahiya N. Drug treatment of obesity: current status and future prospects. Eur J Intern Med 2015;26:89–94.
5. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42.
6. Fabricatore AN, Wadden TA. Obesity. Annu Rev Clin Psychol 2006;2:357–77.
7. Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–81.
8. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obesity (Silver Spring) 2012;20:2019–26.
9. Wee CC HK, Bolcic-Jankovic D, Colten ME, et al. Sex, race, and consideration of bariatric surgery among primary care patients with moderate to severe obesity. J Gen Intern Med 2014;29:68–75.
10. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11.
11. Tan DZN, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
12. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86.
13. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 1997;65:79–85.
14. Fabricatore AN, Wadden TA, Womble LG, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–45.
15. Robertson C, Archibald D, Avenell A, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18:1–424.
16. Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes Relat Metab Disord 2000;24:164–70.
17. Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res 2002;10:748–56.
18. Wee C, Davis R, Chiodi S, et al. Sex, race, and the adverse effects of social stigma vs. other quality of life factors among primary care patients with moderate to severe obesity. J Gen Intern Med 2015;30:229–35.
How to Treat Pediatric MS
VANCOUVER—Pediatric multiple sclerosis (MS) presents unique concerns, making appropriate treatment especially important, according to an overview presented at the 45th Annual Meeting of the Child Neurology Society. Currently, disease-modifying therapies with FDA approval in adult MS have not been approved for the treatment of pediatric MS. In addition, brain growth and cognition are adversely affected in pediatric MS. Furthermore, children with MS tend to become disabled at a younger age, compared with adults.
Diagnosis and Initiation of Therapy
Neurologists should be prepared to engage with children and their families to ensure the most efficacious treatment. “Whenever you are having that conversation or you are considering starting the disease-modifying therapy, you need to balance the need to treat the disease with the idea that you are going to be starting a therapy for a lifetime,” said Dr. Lotze.
Disease-modifying therapies target the inflammatory aspects of pediatric-onset MS and should be initiated in children with a confirmed diagnosis, said Dr. Lotze. Children with clinically isolated syndrome who appear to be at high risk for MS and children with positive oligoclonal bands or elevated IgG index may also need to begin a disease-modifying therapy. Distinguishing between MS, acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica (NMO) poses challenges, especially in children under age 10. Certain interferons can exacerbate NMO and may be harmful in children with ADEM or multiphasic ADEM.
Setting Goals
Neurologists are encouraged to counsel patients about the purpose and side effects of treatment, as well as to establish reasonable expectations for treatment. Additionally, children and families should be aware that another attack may occur during treatment and should be informed about what to do if it does.
No therapy is 100% efficacious in pediatric or adult MS, said Dr. Lotze. Research suggests that 50% of adults have no evidence of disease activity (NEDA) at two years of the disease. After seven years, however, 7% of these adults meet NEDA criteria. As a result, minimal disease progression (defined as less than one attack per year, fewer than three new lesions on a yearly MRI, and no progression in disability) may be a more attainable goal. “We would like to see a drug in a single individual or patient that is effective in achieving NEDA. If that is not possible … you might find that ultimately what you can go for is minimal disease progression,” said Dr. Lotze. He added that there appears to be no clear difference in terms of long-term outcome between children with MS who achieve NEDA and those who achieve minimal disease progression.
First-Line Therapies and Follow-Up Appointments
First-line agents for pediatric MS are the injectable drugs known as platform therapies. The International Pediatric MS Society Group (IPMSSG) recommends that all patients start first-line therapy (ie, interferon β or glatiramer acetate) soon after diagnosis.
Positive results from phase IV observational studies suggest that interferon β and glatiramer acetate are safe and efficacious treatments in pediatric MS. When studied in populations ranging in age from 12 to 17, these therapies decreased relapse rate, stabilized disability, and reduced accrual of new lesions. However, injectable treatments are associated with flu-like symptoms and injection-site reactions. Neurologists should start pediatric patients on interferon β with 25% to 50% of full dosing and titrate to a full adult dosing over four to six weeks. When initiating glatiramer acetate, neurologists should start children with a full adult dosing, 20 mg subcutaneous daily or 40 mg subcutaneous three times per week.
Follow-up appointments should occur every three to six months to assess adherence and determine efficacy of treatment. Neurologists are advised to ask patients whether they are comfortable with taking shots. Patients with needle anxiety, a common problem in pediatric MS, may need assistance from a child psychologist or child life specialist. Asking patients how often the patient or family forgets to take the disease-modifying therapy is also necessary, said Dr. Lotze. In addition, the transition from pediatric to adult care should be addressed in follow-up discussions. Teens must understand that certain disease-modifying therapies are contraindicated for pregnant patients. They also should be aware of how alcohol and other drugs interact with treatment.
Treatment Failure
Research indicates that approximately 30% of patients with pediatric-onset MS will not respond to the first-line therapies, and numerous variables may explain why. Age and disease duration can influence treatment efficacy, as can the number of relapses and the level of disease activity before treatment initiation. Patients who are nonadherent because of side effects and who continue to struggle with needle anxiety may experience treatment failure. The IPMSSG defines treatment failure as adherence to treatment for at least six months with no reduction in relapse rate or in new MRI T2 or contrast-enhancing lesions, or with two or more relapses within a 12-month period.
Second-Line Therapies
Trials of several oral agents are currently under way. The IPMSSG, however, urges neurologists to use extreme caution when considering nonplatform therapies for pediatric patients.
Fingolimod is a second-line drug for pediatric MS that blocks the egress of lymphocytes from the lymph nodes. A small percentage of patients taking this oral agent may develop bradycardia. Monitoring is required for the first six hours of treatment to ensure that the patient has no side effects. Some adverse effects associated with the drug include: first dose bradycardia, macular edema, and herpetic infections.
Dimethyl fumarate is an Nrf2 antioxidant pathway modulator that is associated with adverse effects such as flushing and gastrointestinal upset, said Dr. Lotze. Low-dose aspirin may help with flushing, and a proton pump inhibitor can help to manage the gastrointestinal upset. This treatment requires patients to undergo monitoring for blood count and liver function, as does fingolimod.
Teriflunomide, a pyrimidine synthesis inhibitor, is a Pregnancy Category X drug because it increases the risk of birth defects. Rituximab, an anti-CD20 chimeric monoclonal antibody, is gaining popularity for treating MS. Studies suggest that ocrelizumab may be well tolerated in pediatric MS. Natlizumamb, cladribine, and alemtuzumab are typically used to treat more aggressive forms of MS.
Neurologists rarely prescribe cyclophosphamide or mitoxantrone in pediatric MS. Cyclophosphamide has no formal FDA approval for adult MS or pediatric-onset MS and is associated with increased risks of bladder cancer, secondary leukemia, and infertility. Mitoxantrone is FDA-approved for adults with aggressive relapsing-remitting MS and secondary progressive MS. It is associated with increased cancer risk, however, and is highly cardiotoxic.
“After you have initiated a second-line agent, you need to continue to monitor aspects of disease control, including relapse rate, disability, MRI changes, and other adverse events. If you continue to see breakthrough disease, then you may need to consider … changing to another agent or moving to a more aggressive therapy such as rituximab or natalizumab,” said Dr. Lotze.
—Erica Tricarico
Suggested Reading
Chitnis T, Ghezzi A, Bajer-Korneck B, et al. Pediatric multiple sclerosis: Escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S10 3-S109.
Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: Current concepts and treatment options. Neurol Ther. 2016:5(2):131-1
VANCOUVER—Pediatric multiple sclerosis (MS) presents unique concerns, making appropriate treatment especially important, according to an overview presented at the 45th Annual Meeting of the Child Neurology Society. Currently, disease-modifying therapies with FDA approval in adult MS have not been approved for the treatment of pediatric MS. In addition, brain growth and cognition are adversely affected in pediatric MS. Furthermore, children with MS tend to become disabled at a younger age, compared with adults.
Diagnosis and Initiation of Therapy
Neurologists should be prepared to engage with children and their families to ensure the most efficacious treatment. “Whenever you are having that conversation or you are considering starting the disease-modifying therapy, you need to balance the need to treat the disease with the idea that you are going to be starting a therapy for a lifetime,” said Dr. Lotze.
Disease-modifying therapies target the inflammatory aspects of pediatric-onset MS and should be initiated in children with a confirmed diagnosis, said Dr. Lotze. Children with clinically isolated syndrome who appear to be at high risk for MS and children with positive oligoclonal bands or elevated IgG index may also need to begin a disease-modifying therapy. Distinguishing between MS, acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica (NMO) poses challenges, especially in children under age 10. Certain interferons can exacerbate NMO and may be harmful in children with ADEM or multiphasic ADEM.
Setting Goals
Neurologists are encouraged to counsel patients about the purpose and side effects of treatment, as well as to establish reasonable expectations for treatment. Additionally, children and families should be aware that another attack may occur during treatment and should be informed about what to do if it does.
No therapy is 100% efficacious in pediatric or adult MS, said Dr. Lotze. Research suggests that 50% of adults have no evidence of disease activity (NEDA) at two years of the disease. After seven years, however, 7% of these adults meet NEDA criteria. As a result, minimal disease progression (defined as less than one attack per year, fewer than three new lesions on a yearly MRI, and no progression in disability) may be a more attainable goal. “We would like to see a drug in a single individual or patient that is effective in achieving NEDA. If that is not possible … you might find that ultimately what you can go for is minimal disease progression,” said Dr. Lotze. He added that there appears to be no clear difference in terms of long-term outcome between children with MS who achieve NEDA and those who achieve minimal disease progression.
First-Line Therapies and Follow-Up Appointments
First-line agents for pediatric MS are the injectable drugs known as platform therapies. The International Pediatric MS Society Group (IPMSSG) recommends that all patients start first-line therapy (ie, interferon β or glatiramer acetate) soon after diagnosis.
Positive results from phase IV observational studies suggest that interferon β and glatiramer acetate are safe and efficacious treatments in pediatric MS. When studied in populations ranging in age from 12 to 17, these therapies decreased relapse rate, stabilized disability, and reduced accrual of new lesions. However, injectable treatments are associated with flu-like symptoms and injection-site reactions. Neurologists should start pediatric patients on interferon β with 25% to 50% of full dosing and titrate to a full adult dosing over four to six weeks. When initiating glatiramer acetate, neurologists should start children with a full adult dosing, 20 mg subcutaneous daily or 40 mg subcutaneous three times per week.
Follow-up appointments should occur every three to six months to assess adherence and determine efficacy of treatment. Neurologists are advised to ask patients whether they are comfortable with taking shots. Patients with needle anxiety, a common problem in pediatric MS, may need assistance from a child psychologist or child life specialist. Asking patients how often the patient or family forgets to take the disease-modifying therapy is also necessary, said Dr. Lotze. In addition, the transition from pediatric to adult care should be addressed in follow-up discussions. Teens must understand that certain disease-modifying therapies are contraindicated for pregnant patients. They also should be aware of how alcohol and other drugs interact with treatment.
Treatment Failure
Research indicates that approximately 30% of patients with pediatric-onset MS will not respond to the first-line therapies, and numerous variables may explain why. Age and disease duration can influence treatment efficacy, as can the number of relapses and the level of disease activity before treatment initiation. Patients who are nonadherent because of side effects and who continue to struggle with needle anxiety may experience treatment failure. The IPMSSG defines treatment failure as adherence to treatment for at least six months with no reduction in relapse rate or in new MRI T2 or contrast-enhancing lesions, or with two or more relapses within a 12-month period.
Second-Line Therapies
Trials of several oral agents are currently under way. The IPMSSG, however, urges neurologists to use extreme caution when considering nonplatform therapies for pediatric patients.
Fingolimod is a second-line drug for pediatric MS that blocks the egress of lymphocytes from the lymph nodes. A small percentage of patients taking this oral agent may develop bradycardia. Monitoring is required for the first six hours of treatment to ensure that the patient has no side effects. Some adverse effects associated with the drug include: first dose bradycardia, macular edema, and herpetic infections.
Dimethyl fumarate is an Nrf2 antioxidant pathway modulator that is associated with adverse effects such as flushing and gastrointestinal upset, said Dr. Lotze. Low-dose aspirin may help with flushing, and a proton pump inhibitor can help to manage the gastrointestinal upset. This treatment requires patients to undergo monitoring for blood count and liver function, as does fingolimod.
Teriflunomide, a pyrimidine synthesis inhibitor, is a Pregnancy Category X drug because it increases the risk of birth defects. Rituximab, an anti-CD20 chimeric monoclonal antibody, is gaining popularity for treating MS. Studies suggest that ocrelizumab may be well tolerated in pediatric MS. Natlizumamb, cladribine, and alemtuzumab are typically used to treat more aggressive forms of MS.
Neurologists rarely prescribe cyclophosphamide or mitoxantrone in pediatric MS. Cyclophosphamide has no formal FDA approval for adult MS or pediatric-onset MS and is associated with increased risks of bladder cancer, secondary leukemia, and infertility. Mitoxantrone is FDA-approved for adults with aggressive relapsing-remitting MS and secondary progressive MS. It is associated with increased cancer risk, however, and is highly cardiotoxic.
“After you have initiated a second-line agent, you need to continue to monitor aspects of disease control, including relapse rate, disability, MRI changes, and other adverse events. If you continue to see breakthrough disease, then you may need to consider … changing to another agent or moving to a more aggressive therapy such as rituximab or natalizumab,” said Dr. Lotze.
—Erica Tricarico
Suggested Reading
Chitnis T, Ghezzi A, Bajer-Korneck B, et al. Pediatric multiple sclerosis: Escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S10 3-S109.
Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: Current concepts and treatment options. Neurol Ther. 2016:5(2):131-1
VANCOUVER—Pediatric multiple sclerosis (MS) presents unique concerns, making appropriate treatment especially important, according to an overview presented at the 45th Annual Meeting of the Child Neurology Society. Currently, disease-modifying therapies with FDA approval in adult MS have not been approved for the treatment of pediatric MS. In addition, brain growth and cognition are adversely affected in pediatric MS. Furthermore, children with MS tend to become disabled at a younger age, compared with adults.
Diagnosis and Initiation of Therapy
Neurologists should be prepared to engage with children and their families to ensure the most efficacious treatment. “Whenever you are having that conversation or you are considering starting the disease-modifying therapy, you need to balance the need to treat the disease with the idea that you are going to be starting a therapy for a lifetime,” said Dr. Lotze.
Disease-modifying therapies target the inflammatory aspects of pediatric-onset MS and should be initiated in children with a confirmed diagnosis, said Dr. Lotze. Children with clinically isolated syndrome who appear to be at high risk for MS and children with positive oligoclonal bands or elevated IgG index may also need to begin a disease-modifying therapy. Distinguishing between MS, acute disseminated encephalomyelitis (ADEM), and neuromyelitis optica (NMO) poses challenges, especially in children under age 10. Certain interferons can exacerbate NMO and may be harmful in children with ADEM or multiphasic ADEM.
Setting Goals
Neurologists are encouraged to counsel patients about the purpose and side effects of treatment, as well as to establish reasonable expectations for treatment. Additionally, children and families should be aware that another attack may occur during treatment and should be informed about what to do if it does.
No therapy is 100% efficacious in pediatric or adult MS, said Dr. Lotze. Research suggests that 50% of adults have no evidence of disease activity (NEDA) at two years of the disease. After seven years, however, 7% of these adults meet NEDA criteria. As a result, minimal disease progression (defined as less than one attack per year, fewer than three new lesions on a yearly MRI, and no progression in disability) may be a more attainable goal. “We would like to see a drug in a single individual or patient that is effective in achieving NEDA. If that is not possible … you might find that ultimately what you can go for is minimal disease progression,” said Dr. Lotze. He added that there appears to be no clear difference in terms of long-term outcome between children with MS who achieve NEDA and those who achieve minimal disease progression.
First-Line Therapies and Follow-Up Appointments
First-line agents for pediatric MS are the injectable drugs known as platform therapies. The International Pediatric MS Society Group (IPMSSG) recommends that all patients start first-line therapy (ie, interferon β or glatiramer acetate) soon after diagnosis.
Positive results from phase IV observational studies suggest that interferon β and glatiramer acetate are safe and efficacious treatments in pediatric MS. When studied in populations ranging in age from 12 to 17, these therapies decreased relapse rate, stabilized disability, and reduced accrual of new lesions. However, injectable treatments are associated with flu-like symptoms and injection-site reactions. Neurologists should start pediatric patients on interferon β with 25% to 50% of full dosing and titrate to a full adult dosing over four to six weeks. When initiating glatiramer acetate, neurologists should start children with a full adult dosing, 20 mg subcutaneous daily or 40 mg subcutaneous three times per week.
Follow-up appointments should occur every three to six months to assess adherence and determine efficacy of treatment. Neurologists are advised to ask patients whether they are comfortable with taking shots. Patients with needle anxiety, a common problem in pediatric MS, may need assistance from a child psychologist or child life specialist. Asking patients how often the patient or family forgets to take the disease-modifying therapy is also necessary, said Dr. Lotze. In addition, the transition from pediatric to adult care should be addressed in follow-up discussions. Teens must understand that certain disease-modifying therapies are contraindicated for pregnant patients. They also should be aware of how alcohol and other drugs interact with treatment.
Treatment Failure
Research indicates that approximately 30% of patients with pediatric-onset MS will not respond to the first-line therapies, and numerous variables may explain why. Age and disease duration can influence treatment efficacy, as can the number of relapses and the level of disease activity before treatment initiation. Patients who are nonadherent because of side effects and who continue to struggle with needle anxiety may experience treatment failure. The IPMSSG defines treatment failure as adherence to treatment for at least six months with no reduction in relapse rate or in new MRI T2 or contrast-enhancing lesions, or with two or more relapses within a 12-month period.
Second-Line Therapies
Trials of several oral agents are currently under way. The IPMSSG, however, urges neurologists to use extreme caution when considering nonplatform therapies for pediatric patients.
Fingolimod is a second-line drug for pediatric MS that blocks the egress of lymphocytes from the lymph nodes. A small percentage of patients taking this oral agent may develop bradycardia. Monitoring is required for the first six hours of treatment to ensure that the patient has no side effects. Some adverse effects associated with the drug include: first dose bradycardia, macular edema, and herpetic infections.
Dimethyl fumarate is an Nrf2 antioxidant pathway modulator that is associated with adverse effects such as flushing and gastrointestinal upset, said Dr. Lotze. Low-dose aspirin may help with flushing, and a proton pump inhibitor can help to manage the gastrointestinal upset. This treatment requires patients to undergo monitoring for blood count and liver function, as does fingolimod.
Teriflunomide, a pyrimidine synthesis inhibitor, is a Pregnancy Category X drug because it increases the risk of birth defects. Rituximab, an anti-CD20 chimeric monoclonal antibody, is gaining popularity for treating MS. Studies suggest that ocrelizumab may be well tolerated in pediatric MS. Natlizumamb, cladribine, and alemtuzumab are typically used to treat more aggressive forms of MS.
Neurologists rarely prescribe cyclophosphamide or mitoxantrone in pediatric MS. Cyclophosphamide has no formal FDA approval for adult MS or pediatric-onset MS and is associated with increased risks of bladder cancer, secondary leukemia, and infertility. Mitoxantrone is FDA-approved for adults with aggressive relapsing-remitting MS and secondary progressive MS. It is associated with increased cancer risk, however, and is highly cardiotoxic.
“After you have initiated a second-line agent, you need to continue to monitor aspects of disease control, including relapse rate, disability, MRI changes, and other adverse events. If you continue to see breakthrough disease, then you may need to consider … changing to another agent or moving to a more aggressive therapy such as rituximab or natalizumab,” said Dr. Lotze.
—Erica Tricarico
Suggested Reading
Chitnis T, Ghezzi A, Bajer-Korneck B, et al. Pediatric multiple sclerosis: Escalation and emerging treatments. Neurology. 2016;87(9 Suppl 2):S10 3-S109.
Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: Current concepts and treatment options. Neurol Ther. 2016:5(2):131-1
Herpes Zoster Following Varicella Vaccination in Children
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.
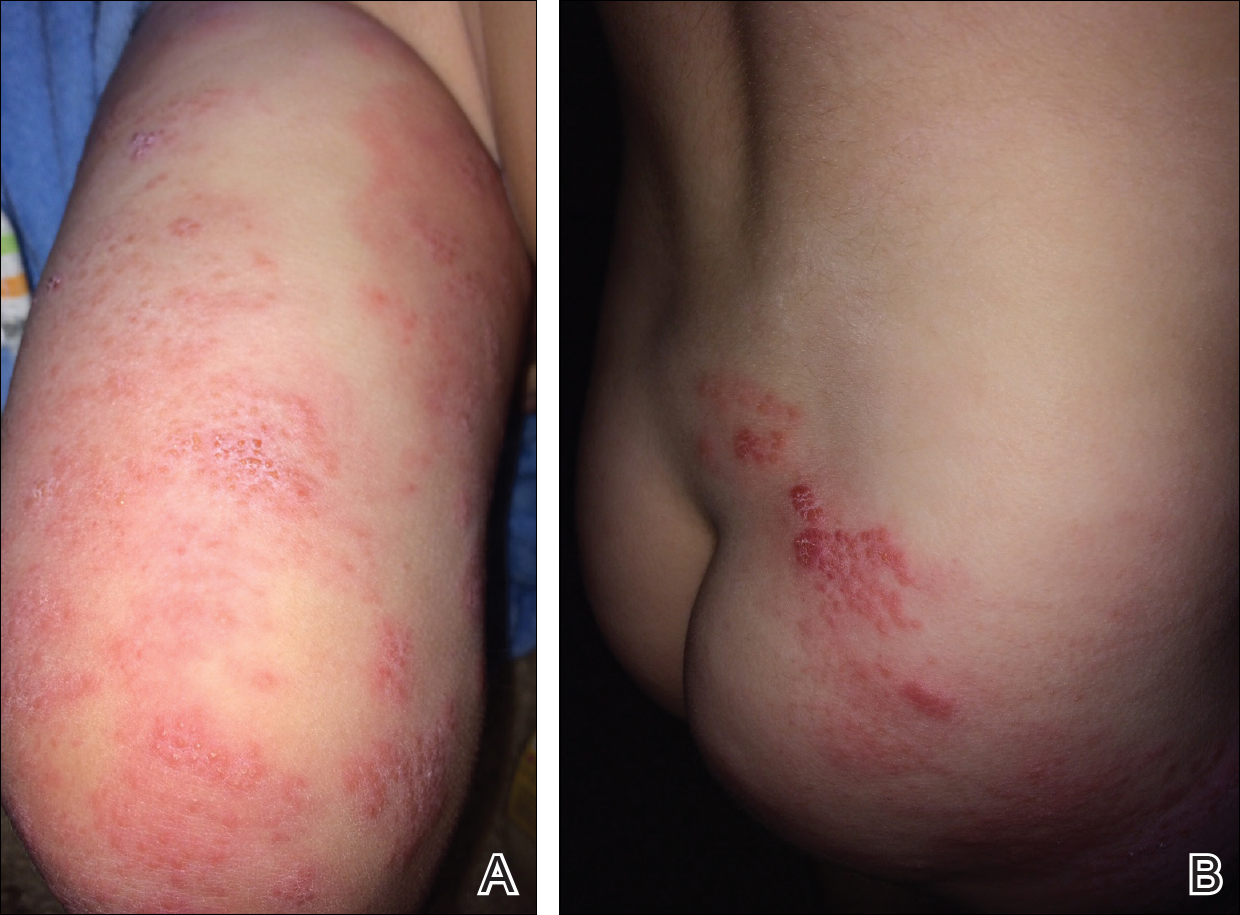
Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.
The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Practice Points
- Most children with herpes zoster are immunocompromised, have a history of varicella, or were exposed to varicella in utero.
- Herpes zoster has been reported in immunocompetent children due to either wild-type or vaccine-strain varicella-zoster virus.