User login
What’s Eating You? Lone Star Tick (Amblyomma americanum)
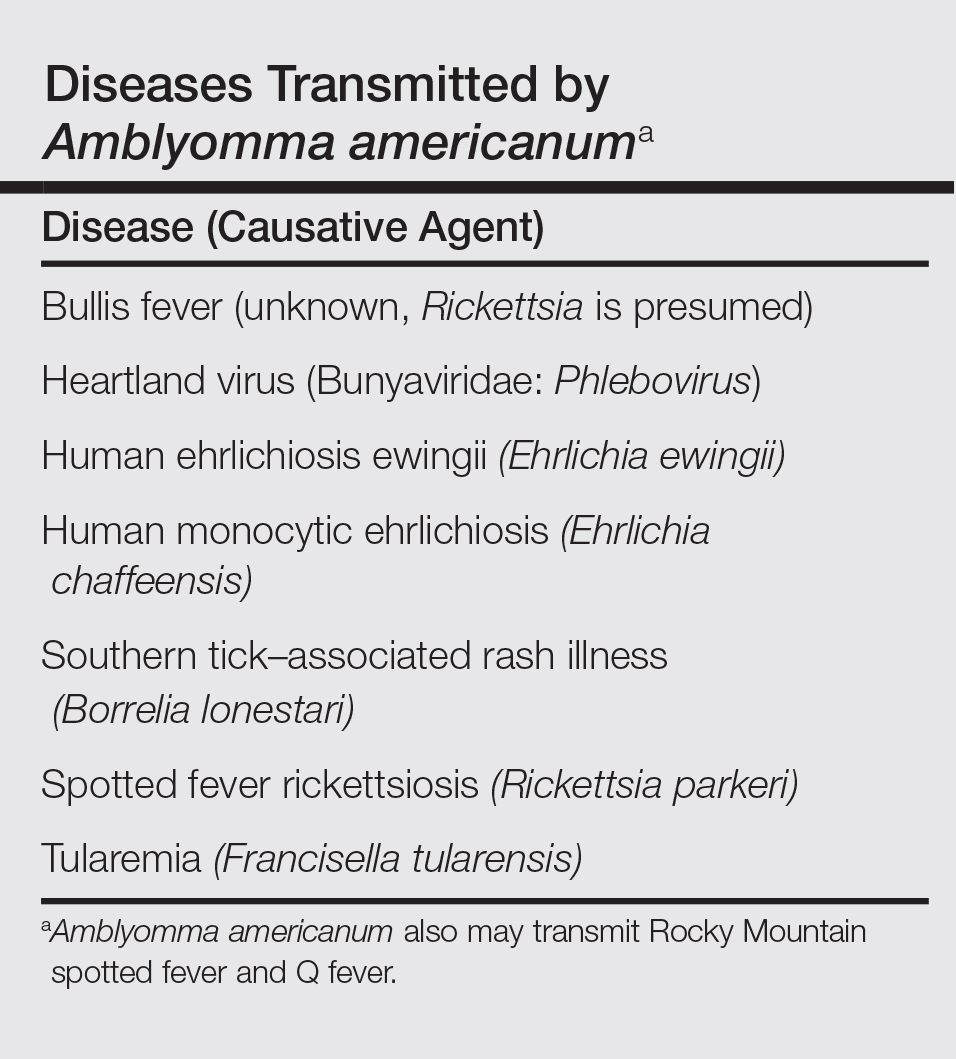
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
Tick Characteristics
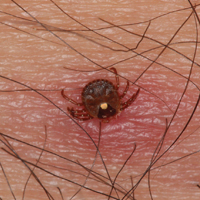
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.
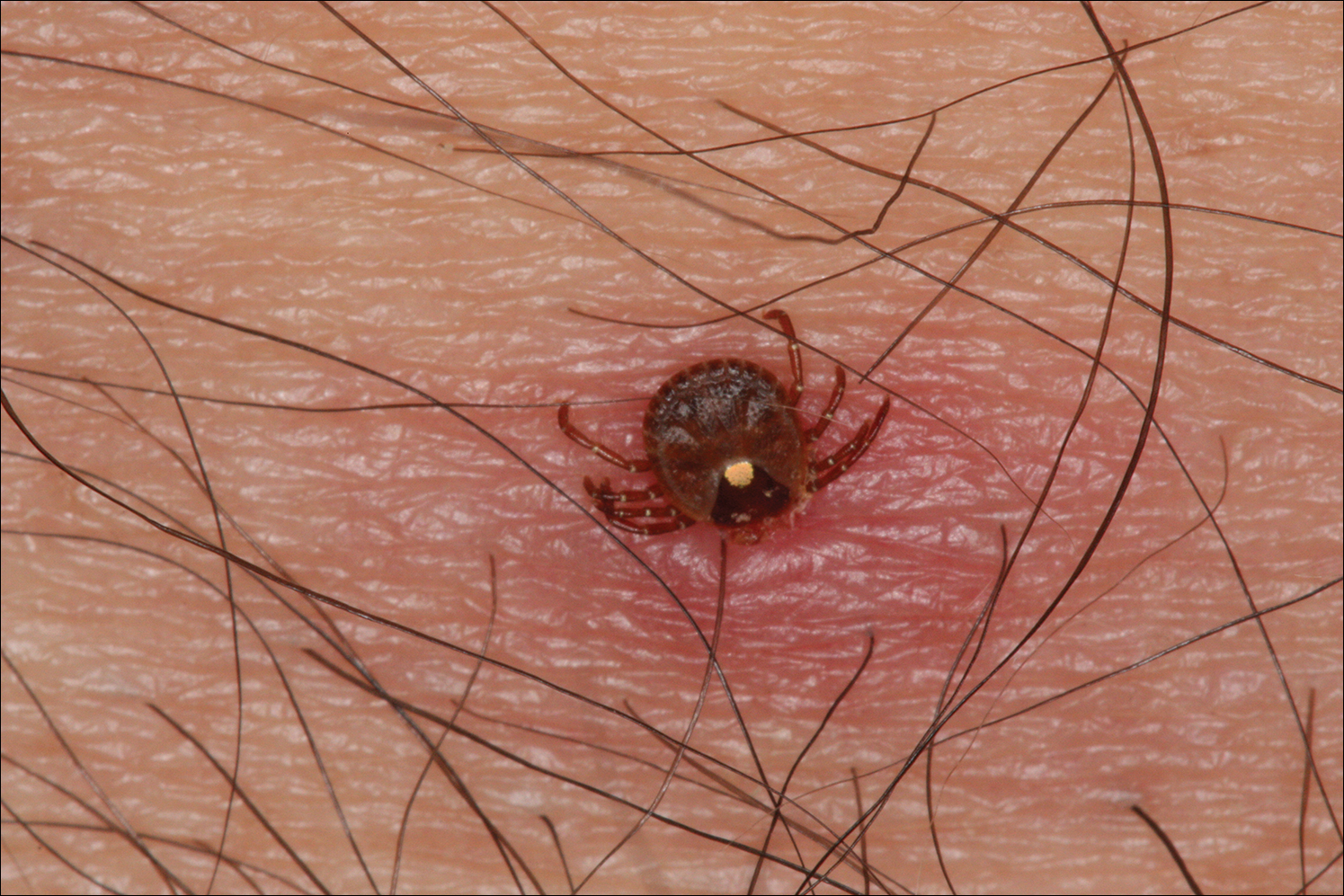
Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
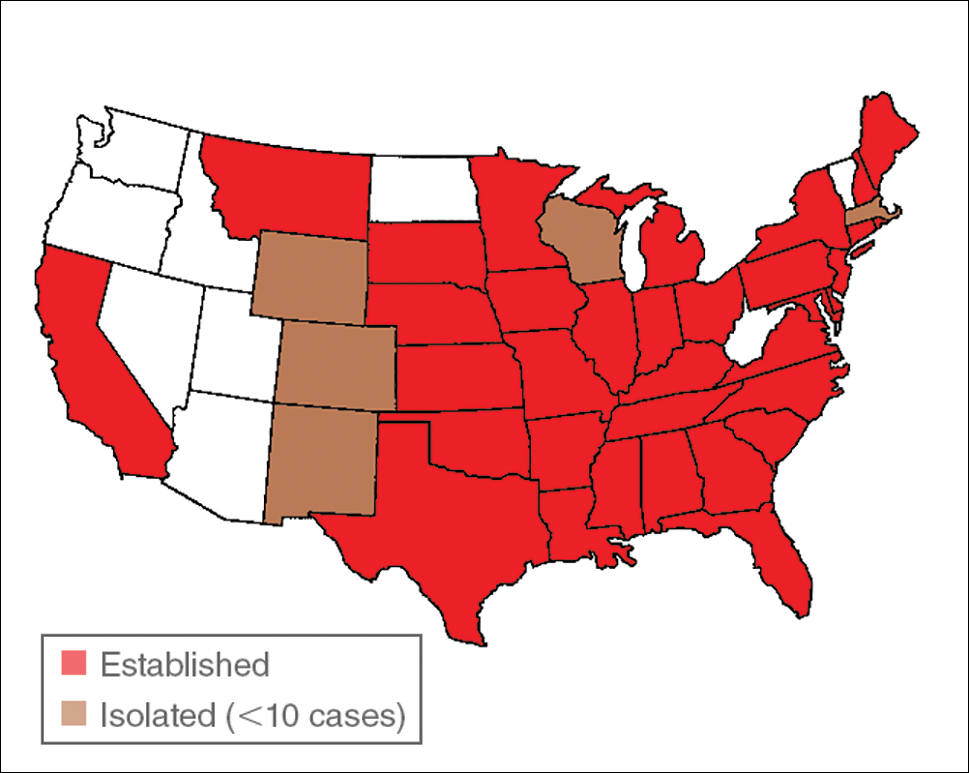
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.
Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
Practice Points
- Amblyomma americanum (lone star tick) is widely distributed throughout the United States and is an important cause of several tick-borne illnesses.
- Prompt diagnosis and treatment of tick-borne disease improves patient outcomes.
- In some cases, tick bites may cause the human host to develop certain IgE antibodies that result in a delayed-onset anaphylaxis after ingestion of red meat.
Biosimilars in Psoriasis: The Future or Not?
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19
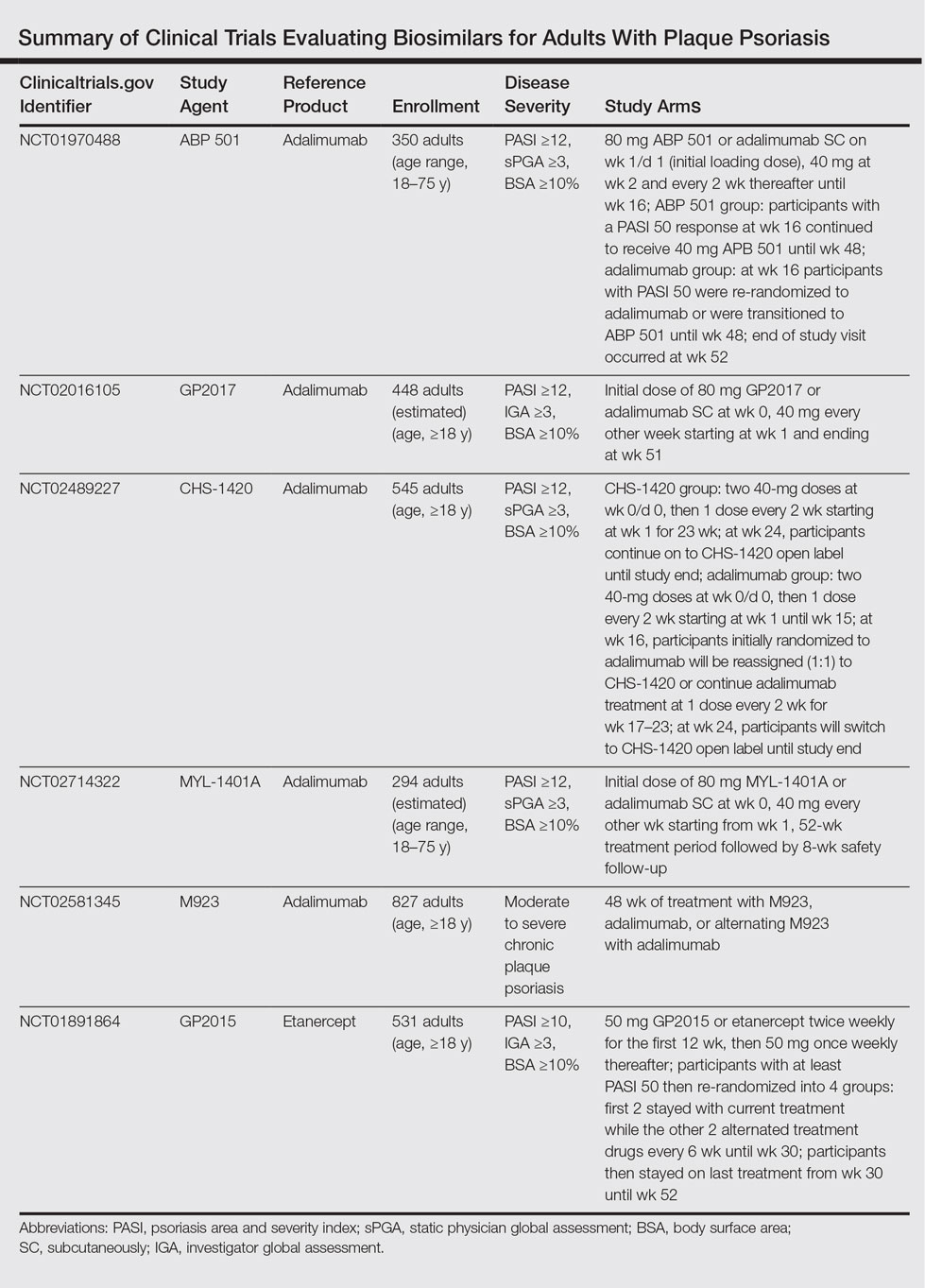
A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19

A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19

A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
Practice Points
- Three biosimilars have been approved by the US Food and Drug Administration to treat adult patients with plaque psoriasis and psoriatic arthritis.
- By virtue of their production, biosimilars are not identical to their reference products, and we must ensure that their safety is comparable.
Microneedling With Platelet-Rich Plasma



Therapy demonstrates safety, efficacy in kids with hemophilia B

Photo courtesy of Biogen
A recombinant factor IX Fc fusion protein (rFIXFc) has produced favorable results in children with severe hemophilia B, researchers have reported.
The team said rFIXFc was generally well-tolerated in the phase 3 Kids B-LONG study, and none of the patients on this trial developed inhibitors.
In addition, the annualized bleeding rate was low, and most patients were able to maintain once-weekly dosing.
“To date, Kids B-LONG is the largest study to evaluate the safety and efficacy of extended half-life factor IX therapy in children with hemophilia B, and the study’s results align with those in studies of [rFIXFc] in adults and adolescents,” said Roshni Kulkarni, MD, of Michigan State University in East Lansing.
Dr Kulkarni and her colleagues reported results of Kids B-LONG in The Lancet Haematology. The research was funded by Biogen and Sobi, the companies marketing rFIXFc (also known as eftrenonacog alfa) as Alprolix.
The study included 30 males under the age of 12 with previously treated, severe hemophilia B.
All patients initially received rFIXFc prophylaxis (50-60 IU/kg) once per week. Doses were adjusted as needed (≤100 IU/kg per infusion, up to 2 times per week).
Twenty-seven patients (90%) completed the study. The median time spent on study was 49.4 weeks, and 24 patients received rFIXFc injections on at least 50 separate days.
None of the patients developed inhibitors to rFIXFc. There were no reports of anaphylaxis or serious hypersensitivity reactions, no vascular thrombotic events, and no deaths.
The most common adverse events were common cold (n=7, 23%) and fall (n=6, 20%). Four patients experienced serious adverse events while on study, all of which were considered unrelated to rFIXFc.
The median annualized bleeding rate was 2.0 overall, and there were no spontaneous joint bleeds.
Of all 30 patients treated, 10 (33%) experienced no bleeding episodes, and 19 (63%) reported no joint bleeding on-study.
Overall, 92% of bleeding episodes were controlled by 1 or 2 injections of rFIXFc.
Following a switch to rFIXFc, 80% of children who completed the study (22/27) extended their dosing interval compared to previous treatment.
One patient had his dosing interval reduced to 5 days, but all other patients remained on once-weekly prophylactic dosing throughout the study. ![]()

Photo courtesy of Biogen
A recombinant factor IX Fc fusion protein (rFIXFc) has produced favorable results in children with severe hemophilia B, researchers have reported.
The team said rFIXFc was generally well-tolerated in the phase 3 Kids B-LONG study, and none of the patients on this trial developed inhibitors.
In addition, the annualized bleeding rate was low, and most patients were able to maintain once-weekly dosing.
“To date, Kids B-LONG is the largest study to evaluate the safety and efficacy of extended half-life factor IX therapy in children with hemophilia B, and the study’s results align with those in studies of [rFIXFc] in adults and adolescents,” said Roshni Kulkarni, MD, of Michigan State University in East Lansing.
Dr Kulkarni and her colleagues reported results of Kids B-LONG in The Lancet Haematology. The research was funded by Biogen and Sobi, the companies marketing rFIXFc (also known as eftrenonacog alfa) as Alprolix.
The study included 30 males under the age of 12 with previously treated, severe hemophilia B.
All patients initially received rFIXFc prophylaxis (50-60 IU/kg) once per week. Doses were adjusted as needed (≤100 IU/kg per infusion, up to 2 times per week).
Twenty-seven patients (90%) completed the study. The median time spent on study was 49.4 weeks, and 24 patients received rFIXFc injections on at least 50 separate days.
None of the patients developed inhibitors to rFIXFc. There were no reports of anaphylaxis or serious hypersensitivity reactions, no vascular thrombotic events, and no deaths.
The most common adverse events were common cold (n=7, 23%) and fall (n=6, 20%). Four patients experienced serious adverse events while on study, all of which were considered unrelated to rFIXFc.
The median annualized bleeding rate was 2.0 overall, and there were no spontaneous joint bleeds.
Of all 30 patients treated, 10 (33%) experienced no bleeding episodes, and 19 (63%) reported no joint bleeding on-study.
Overall, 92% of bleeding episodes were controlled by 1 or 2 injections of rFIXFc.
Following a switch to rFIXFc, 80% of children who completed the study (22/27) extended their dosing interval compared to previous treatment.
One patient had his dosing interval reduced to 5 days, but all other patients remained on once-weekly prophylactic dosing throughout the study. ![]()

Photo courtesy of Biogen
A recombinant factor IX Fc fusion protein (rFIXFc) has produced favorable results in children with severe hemophilia B, researchers have reported.
The team said rFIXFc was generally well-tolerated in the phase 3 Kids B-LONG study, and none of the patients on this trial developed inhibitors.
In addition, the annualized bleeding rate was low, and most patients were able to maintain once-weekly dosing.
“To date, Kids B-LONG is the largest study to evaluate the safety and efficacy of extended half-life factor IX therapy in children with hemophilia B, and the study’s results align with those in studies of [rFIXFc] in adults and adolescents,” said Roshni Kulkarni, MD, of Michigan State University in East Lansing.
Dr Kulkarni and her colleagues reported results of Kids B-LONG in The Lancet Haematology. The research was funded by Biogen and Sobi, the companies marketing rFIXFc (also known as eftrenonacog alfa) as Alprolix.
The study included 30 males under the age of 12 with previously treated, severe hemophilia B.
All patients initially received rFIXFc prophylaxis (50-60 IU/kg) once per week. Doses were adjusted as needed (≤100 IU/kg per infusion, up to 2 times per week).
Twenty-seven patients (90%) completed the study. The median time spent on study was 49.4 weeks, and 24 patients received rFIXFc injections on at least 50 separate days.
None of the patients developed inhibitors to rFIXFc. There were no reports of anaphylaxis or serious hypersensitivity reactions, no vascular thrombotic events, and no deaths.
The most common adverse events were common cold (n=7, 23%) and fall (n=6, 20%). Four patients experienced serious adverse events while on study, all of which were considered unrelated to rFIXFc.
The median annualized bleeding rate was 2.0 overall, and there were no spontaneous joint bleeds.
Of all 30 patients treated, 10 (33%) experienced no bleeding episodes, and 19 (63%) reported no joint bleeding on-study.
Overall, 92% of bleeding episodes were controlled by 1 or 2 injections of rFIXFc.
Following a switch to rFIXFc, 80% of children who completed the study (22/27) extended their dosing interval compared to previous treatment.
One patient had his dosing interval reduced to 5 days, but all other patients remained on once-weekly prophylactic dosing throughout the study. ![]()
Pulmonary Perspectives: High levels of air pollution in Delhi and adverse health effects
“Nature’s condition, rightly interpreted, reveals a society’s culture and traditions as directly as does a novel or a newspaper or a code of laws.”
– Roderick F. Nash
Adverse effects of air pollution on human health have been known ever since the “Great London Smog” in 1952. Mankind is paying for rapid industrialization by adversely affecting the air that we breathe. The developed world has been able to improve the environmental standards by following stringent norms and practices regarding engines, fuels, and industrial safety. However, the same cannot be said about developing countries. Delhi, the capital of India, has seen high levels of air pollution for the last several decades.
The number of registered vehicles in Delhi has doubled over the last 10 years. This, along with rapidly increasing numbers of small scale industries and inconsistently regulated construction work, has led to ever-increasing levels of air pollution in Delhi. The city has witnessed smog for the last few years.
Smog causing disruption of daily life and health hazards has been reported from Los Angeles, Beijing, and many other major cities around the world. The London Smog of 1952 caused approximately 4,000 deaths within 4 days (Davis D, et al. Environ Health Perspectives. 2002;110[12]:A734) and caused another 8,000 deaths over next few weeks to months (Bell ML, et al. Environ Health Perspectives. 2004;112[1]:6).
Common sources and pollutants with reference to Delhi
As in most cities around the world, rapid industrialization and increases in vehicles using fossil fuels are important contributors to ambient air pollution in Delhi. Additional sources of air pollution unique to Delhi include dust generation during building construction, ash generation from thermal power plants, crop residue burning in neighboring states, and burning of fossil fuels for domestic, as well as small scale, industrial use. Major pollutants include particulate matter (both PM2.5 and PM10), nitrogen oxides (NOx), carbon monoxide (CO), sulfur dioxide (SO2), and ozone (O3).
Delhi is distinct in its geographic location adjoining the Great Indian Desert (Thar) in the west and cool hilly regions in the north and east. This accounts for great seasonal variations in temperature, humidity, and wind speed. Also, being a landlocked territory, there are no moderating effects of sea breeze available to other metropolitan cities (like Mumbai and Chennai).
Dust storms during the summer from the neighboring state of Rajasthan cause an increase in suspended particulate matter (SPM). All these contribute to seasonal and climatic variations in air quality. In addition, the use of fire crackers during the festival of Diwali leads to dangerous levels of air pollution also.
Adverse health effects as witnessed in clinics and community
Many adults, without any prior history of respiratory illness, attended our outpatient department (OPD) with breathlessness, chest congestion, and wheezing requiring inhaled bronchodilators. A significant proportion of patients with previously diagnosed respiratory diseases (including COPD, bronchial asthma, or interstitial lung disease) reported to OPDs or emergency services with worsening cough, wheezing, and breathlessness. A few patients coming from outside Delhi for routine follow-up had exacerbation of COPD after coming to Delhi (personal observations).
We have previously reported increases in asthma, COPD, and acute coronary events (by 21.30%, 24.90%, and 24.30%, respectively) due to higher than acceptable levels of air pollutants in Delhi (Pande JN, et al. Indian J Chest Dis Allied Sci. 2002;44[1]:13). Another concerning development has been the increase in the number of persons being diagnosed with bronchial asthma in middle age, probably related to worsening air quality. Persons at extremes of age (young children and elderly) are particularly affected.
Studies in Delhi assessing ambient air pollution–related morbidity and mortality
Studies have used risk of mortality/morbidity due to air pollution model (Ri–MAP) to assess health impact of various air pollutants in Delhi. According to their estimates, there were 18,229 excess deaths in Delhi in the year 2010, more than 50% of which were due to cardiovascular or respiratory causes. Also, 26,525 excess hospital admissions due to COPD exacerbation could be attributed to ambient air pollution (Nagpure A, et al. Atmospheric Pollution Res. 2014;5[3]:1309).
Interventions: Work in progress
The Central Pollution Control Board convened an Expert Committee (Dr. Khilnani as a member) for formulation and implementation of Air Quality Index (AQI) in major Indian cities (http://cpcb.nic.in/FINAL-REPORT_AQI_.pdf).
Currently reported AQI is calculated by using the following parameters: sulfur dioxide (SO2), nitrogen dioxide (NO2), particulate matter (PM10,PM2.5) averaged over 24 hours, along with ozone (O3) and carbon monoxide (CO), averaged over 1-8 hours. AQI is classified as good (0-50), satisfactory (51-100), moderate (101-200), poor (201-300), very poor (301-400), and severe (greater than 401).
AQI is reported daily in leading newspapers along with public and private news channels. Thanks to the mainstream and social media, smog has become a commonly understood word. Air pollution is a hot topic of discussion among people of all socioeconomic and demographic strata.
Children of almost all schools in Delhi pledged not to use firecrackers this Diwali. People are increasingly sharing taxis or carpooling. Utilization of public transport is gradually increasing.
The Delhi government ordered temporarily shutting off the only working thermal power plant in the megacity (source of 10%-15% of ambient air pollution). The government is also working on an action plan based on air quality, which includes both preventive and prohibitive measures.
Delhi Transport Corporation operates one of the world’s largest fleets of compressed natural gas–operated buses. Delhi Metro Corporation has been lauded by the United Nations for its efforts in reducing the carbon footprint and air pollution.
Yet, a lot needs to be done to improve the air quality in Delhi. Last mile connectivity remains a big hurdle; improving this will go a long way in promoting use of public transport. Implementation of methods to reduce particulate matter generation at construction sites, promoting use of vehicles using electricity or compressed natural gas, increasing parking charges for vehicles, banning the use of diesel-driven heavy vehicles in the city, road cleaning with vacuum cleaners to reduce PM 10 generation, increasing green areas, and promoting carpooling or taxi sharing are some other initiatives that need to be implemented on priority. Delhi and surrounding states need to strengthen awareness drives and norms to discourage crop residue burning on a priority basis.
Conclusion
Delhi’s poor air quality during this winter has indeed affected the respiratory health of the population. Healthy people, as well as those with preexisting respiratory diseases, are adversely affected. A series of actions at the personal and institutional level is required to control this menace.
Dr. Khilnani is Professor, and Dr. Tiwari is Research Officer, Department of Pulmonary Medicine & Sleep Disorders, All India Institute of Medical Sciences, New Delhi, India.
“Nature’s condition, rightly interpreted, reveals a society’s culture and traditions as directly as does a novel or a newspaper or a code of laws.”
– Roderick F. Nash
Adverse effects of air pollution on human health have been known ever since the “Great London Smog” in 1952. Mankind is paying for rapid industrialization by adversely affecting the air that we breathe. The developed world has been able to improve the environmental standards by following stringent norms and practices regarding engines, fuels, and industrial safety. However, the same cannot be said about developing countries. Delhi, the capital of India, has seen high levels of air pollution for the last several decades.
The number of registered vehicles in Delhi has doubled over the last 10 years. This, along with rapidly increasing numbers of small scale industries and inconsistently regulated construction work, has led to ever-increasing levels of air pollution in Delhi. The city has witnessed smog for the last few years.
Smog causing disruption of daily life and health hazards has been reported from Los Angeles, Beijing, and many other major cities around the world. The London Smog of 1952 caused approximately 4,000 deaths within 4 days (Davis D, et al. Environ Health Perspectives. 2002;110[12]:A734) and caused another 8,000 deaths over next few weeks to months (Bell ML, et al. Environ Health Perspectives. 2004;112[1]:6).
Common sources and pollutants with reference to Delhi
As in most cities around the world, rapid industrialization and increases in vehicles using fossil fuels are important contributors to ambient air pollution in Delhi. Additional sources of air pollution unique to Delhi include dust generation during building construction, ash generation from thermal power plants, crop residue burning in neighboring states, and burning of fossil fuels for domestic, as well as small scale, industrial use. Major pollutants include particulate matter (both PM2.5 and PM10), nitrogen oxides (NOx), carbon monoxide (CO), sulfur dioxide (SO2), and ozone (O3).
Delhi is distinct in its geographic location adjoining the Great Indian Desert (Thar) in the west and cool hilly regions in the north and east. This accounts for great seasonal variations in temperature, humidity, and wind speed. Also, being a landlocked territory, there are no moderating effects of sea breeze available to other metropolitan cities (like Mumbai and Chennai).
Dust storms during the summer from the neighboring state of Rajasthan cause an increase in suspended particulate matter (SPM). All these contribute to seasonal and climatic variations in air quality. In addition, the use of fire crackers during the festival of Diwali leads to dangerous levels of air pollution also.
Adverse health effects as witnessed in clinics and community
Many adults, without any prior history of respiratory illness, attended our outpatient department (OPD) with breathlessness, chest congestion, and wheezing requiring inhaled bronchodilators. A significant proportion of patients with previously diagnosed respiratory diseases (including COPD, bronchial asthma, or interstitial lung disease) reported to OPDs or emergency services with worsening cough, wheezing, and breathlessness. A few patients coming from outside Delhi for routine follow-up had exacerbation of COPD after coming to Delhi (personal observations).
We have previously reported increases in asthma, COPD, and acute coronary events (by 21.30%, 24.90%, and 24.30%, respectively) due to higher than acceptable levels of air pollutants in Delhi (Pande JN, et al. Indian J Chest Dis Allied Sci. 2002;44[1]:13). Another concerning development has been the increase in the number of persons being diagnosed with bronchial asthma in middle age, probably related to worsening air quality. Persons at extremes of age (young children and elderly) are particularly affected.
Studies in Delhi assessing ambient air pollution–related morbidity and mortality
Studies have used risk of mortality/morbidity due to air pollution model (Ri–MAP) to assess health impact of various air pollutants in Delhi. According to their estimates, there were 18,229 excess deaths in Delhi in the year 2010, more than 50% of which were due to cardiovascular or respiratory causes. Also, 26,525 excess hospital admissions due to COPD exacerbation could be attributed to ambient air pollution (Nagpure A, et al. Atmospheric Pollution Res. 2014;5[3]:1309).
Interventions: Work in progress
The Central Pollution Control Board convened an Expert Committee (Dr. Khilnani as a member) for formulation and implementation of Air Quality Index (AQI) in major Indian cities (http://cpcb.nic.in/FINAL-REPORT_AQI_.pdf).
Currently reported AQI is calculated by using the following parameters: sulfur dioxide (SO2), nitrogen dioxide (NO2), particulate matter (PM10,PM2.5) averaged over 24 hours, along with ozone (O3) and carbon monoxide (CO), averaged over 1-8 hours. AQI is classified as good (0-50), satisfactory (51-100), moderate (101-200), poor (201-300), very poor (301-400), and severe (greater than 401).
AQI is reported daily in leading newspapers along with public and private news channels. Thanks to the mainstream and social media, smog has become a commonly understood word. Air pollution is a hot topic of discussion among people of all socioeconomic and demographic strata.
Children of almost all schools in Delhi pledged not to use firecrackers this Diwali. People are increasingly sharing taxis or carpooling. Utilization of public transport is gradually increasing.
The Delhi government ordered temporarily shutting off the only working thermal power plant in the megacity (source of 10%-15% of ambient air pollution). The government is also working on an action plan based on air quality, which includes both preventive and prohibitive measures.
Delhi Transport Corporation operates one of the world’s largest fleets of compressed natural gas–operated buses. Delhi Metro Corporation has been lauded by the United Nations for its efforts in reducing the carbon footprint and air pollution.
Yet, a lot needs to be done to improve the air quality in Delhi. Last mile connectivity remains a big hurdle; improving this will go a long way in promoting use of public transport. Implementation of methods to reduce particulate matter generation at construction sites, promoting use of vehicles using electricity or compressed natural gas, increasing parking charges for vehicles, banning the use of diesel-driven heavy vehicles in the city, road cleaning with vacuum cleaners to reduce PM 10 generation, increasing green areas, and promoting carpooling or taxi sharing are some other initiatives that need to be implemented on priority. Delhi and surrounding states need to strengthen awareness drives and norms to discourage crop residue burning on a priority basis.
Conclusion
Delhi’s poor air quality during this winter has indeed affected the respiratory health of the population. Healthy people, as well as those with preexisting respiratory diseases, are adversely affected. A series of actions at the personal and institutional level is required to control this menace.
Dr. Khilnani is Professor, and Dr. Tiwari is Research Officer, Department of Pulmonary Medicine & Sleep Disorders, All India Institute of Medical Sciences, New Delhi, India.
“Nature’s condition, rightly interpreted, reveals a society’s culture and traditions as directly as does a novel or a newspaper or a code of laws.”
– Roderick F. Nash
Adverse effects of air pollution on human health have been known ever since the “Great London Smog” in 1952. Mankind is paying for rapid industrialization by adversely affecting the air that we breathe. The developed world has been able to improve the environmental standards by following stringent norms and practices regarding engines, fuels, and industrial safety. However, the same cannot be said about developing countries. Delhi, the capital of India, has seen high levels of air pollution for the last several decades.
The number of registered vehicles in Delhi has doubled over the last 10 years. This, along with rapidly increasing numbers of small scale industries and inconsistently regulated construction work, has led to ever-increasing levels of air pollution in Delhi. The city has witnessed smog for the last few years.
Smog causing disruption of daily life and health hazards has been reported from Los Angeles, Beijing, and many other major cities around the world. The London Smog of 1952 caused approximately 4,000 deaths within 4 days (Davis D, et al. Environ Health Perspectives. 2002;110[12]:A734) and caused another 8,000 deaths over next few weeks to months (Bell ML, et al. Environ Health Perspectives. 2004;112[1]:6).
Common sources and pollutants with reference to Delhi
As in most cities around the world, rapid industrialization and increases in vehicles using fossil fuels are important contributors to ambient air pollution in Delhi. Additional sources of air pollution unique to Delhi include dust generation during building construction, ash generation from thermal power plants, crop residue burning in neighboring states, and burning of fossil fuels for domestic, as well as small scale, industrial use. Major pollutants include particulate matter (both PM2.5 and PM10), nitrogen oxides (NOx), carbon monoxide (CO), sulfur dioxide (SO2), and ozone (O3).
Delhi is distinct in its geographic location adjoining the Great Indian Desert (Thar) in the west and cool hilly regions in the north and east. This accounts for great seasonal variations in temperature, humidity, and wind speed. Also, being a landlocked territory, there are no moderating effects of sea breeze available to other metropolitan cities (like Mumbai and Chennai).
Dust storms during the summer from the neighboring state of Rajasthan cause an increase in suspended particulate matter (SPM). All these contribute to seasonal and climatic variations in air quality. In addition, the use of fire crackers during the festival of Diwali leads to dangerous levels of air pollution also.
Adverse health effects as witnessed in clinics and community
Many adults, without any prior history of respiratory illness, attended our outpatient department (OPD) with breathlessness, chest congestion, and wheezing requiring inhaled bronchodilators. A significant proportion of patients with previously diagnosed respiratory diseases (including COPD, bronchial asthma, or interstitial lung disease) reported to OPDs or emergency services with worsening cough, wheezing, and breathlessness. A few patients coming from outside Delhi for routine follow-up had exacerbation of COPD after coming to Delhi (personal observations).
We have previously reported increases in asthma, COPD, and acute coronary events (by 21.30%, 24.90%, and 24.30%, respectively) due to higher than acceptable levels of air pollutants in Delhi (Pande JN, et al. Indian J Chest Dis Allied Sci. 2002;44[1]:13). Another concerning development has been the increase in the number of persons being diagnosed with bronchial asthma in middle age, probably related to worsening air quality. Persons at extremes of age (young children and elderly) are particularly affected.
Studies in Delhi assessing ambient air pollution–related morbidity and mortality
Studies have used risk of mortality/morbidity due to air pollution model (Ri–MAP) to assess health impact of various air pollutants in Delhi. According to their estimates, there were 18,229 excess deaths in Delhi in the year 2010, more than 50% of which were due to cardiovascular or respiratory causes. Also, 26,525 excess hospital admissions due to COPD exacerbation could be attributed to ambient air pollution (Nagpure A, et al. Atmospheric Pollution Res. 2014;5[3]:1309).
Interventions: Work in progress
The Central Pollution Control Board convened an Expert Committee (Dr. Khilnani as a member) for formulation and implementation of Air Quality Index (AQI) in major Indian cities (http://cpcb.nic.in/FINAL-REPORT_AQI_.pdf).
Currently reported AQI is calculated by using the following parameters: sulfur dioxide (SO2), nitrogen dioxide (NO2), particulate matter (PM10,PM2.5) averaged over 24 hours, along with ozone (O3) and carbon monoxide (CO), averaged over 1-8 hours. AQI is classified as good (0-50), satisfactory (51-100), moderate (101-200), poor (201-300), very poor (301-400), and severe (greater than 401).
AQI is reported daily in leading newspapers along with public and private news channels. Thanks to the mainstream and social media, smog has become a commonly understood word. Air pollution is a hot topic of discussion among people of all socioeconomic and demographic strata.
Children of almost all schools in Delhi pledged not to use firecrackers this Diwali. People are increasingly sharing taxis or carpooling. Utilization of public transport is gradually increasing.
The Delhi government ordered temporarily shutting off the only working thermal power plant in the megacity (source of 10%-15% of ambient air pollution). The government is also working on an action plan based on air quality, which includes both preventive and prohibitive measures.
Delhi Transport Corporation operates one of the world’s largest fleets of compressed natural gas–operated buses. Delhi Metro Corporation has been lauded by the United Nations for its efforts in reducing the carbon footprint and air pollution.
Yet, a lot needs to be done to improve the air quality in Delhi. Last mile connectivity remains a big hurdle; improving this will go a long way in promoting use of public transport. Implementation of methods to reduce particulate matter generation at construction sites, promoting use of vehicles using electricity or compressed natural gas, increasing parking charges for vehicles, banning the use of diesel-driven heavy vehicles in the city, road cleaning with vacuum cleaners to reduce PM 10 generation, increasing green areas, and promoting carpooling or taxi sharing are some other initiatives that need to be implemented on priority. Delhi and surrounding states need to strengthen awareness drives and norms to discourage crop residue burning on a priority basis.
Conclusion
Delhi’s poor air quality during this winter has indeed affected the respiratory health of the population. Healthy people, as well as those with preexisting respiratory diseases, are adversely affected. A series of actions at the personal and institutional level is required to control this menace.
Dr. Khilnani is Professor, and Dr. Tiwari is Research Officer, Department of Pulmonary Medicine & Sleep Disorders, All India Institute of Medical Sciences, New Delhi, India.
NetWorks: SEALs help physicians, blood storage questions, more. . .
Clinical Research
The unrecognized battlefield in our hospitals: Lessons from the US Navy SEALs
Burnout syndrome (BOS) is a psychological state resulting from prolonged exposure to job stressors. It is characterized by a vicious cycle of emotional exhaustion, detachment from others, and a feeling of decreased accomplishment. Severe BOS is seen in up to 45% of physicians and 33% of nurses who work in ICUs.1
BOS has far-reaching consequences, being associated with an alarmingly high prevalence of posttraumatic stress disorder (PTSD) and substance abuse, almost equivalent to that experienced by veterans returning from war.2 BOS also is associated with self-reported suboptimal patient care practices.3This crisis has long been underrecognized, but now that we have identified the problem, where does that leave us? There are currently no quality studies evaluating how to best treat and prevent BOS/PTSD in health-care professionals. Previous studies have focused on addressing organizational factors to alleviate job stressors, but the psychosocial characteristics of the individual have been largely ignored.
Our medical education has historically focused on an individual’s intelligence quotient (IQ), but developing an individual’s emotional quotient (EQ) is just as valuable. It has long been known that Navy SEALs have the lowest prevalence of PTSD among combat veterans due partially to their specific training in emotional resilience and adaptive psychosocial coping mechanisms.
For this reason, the research team at the University of Texas Health Science Center at San Antonio is collaborating with the US Navy SEAL team to design and validate a tool that teaches critical care staff resilience training similar to what their combat trainees undergo. The goal is to curb these alarming trends in BOS and create a paradigm shift in medical education within medical and nursing schools.
Bravein Amalakuhan, MD
Fellow-in-Training Member
References
1. Embriaco N, Azoulay E, Barrau K, et al. Am J Respir Crit Care Med. 2007;175(7):686.
2. Mealer ML, Shelton A, Berg B, et al. Am J Respir Crit Care Med. 2007;175(7):693.
3. Shanafelt TD, Bradley KA, Wipf JE, et al. Ann Intern Med. 2002;136(5):358.
Critical Care
End of the era for age of blood concerns?
Blood transfusions are common in critically ill patients, with two in five adults admitted to an ICU receiving a transfusion.1,2 Recently, randomized trials have found that more restrictive thresholds for transfusions are associated with improved outcomes.3,4 One theorized explanation for this somewhat counterintuitive association is that the prolonged storage time (i.e., the age of the blood being transfused) might affect outcomes.
There have been three recent publications that help to shed some more light on this. First, Lacroix et al.5 performed a multicenter randomized blinded trial in over 2,400 critically ill patients in 64 centers comparing new blood (mean storage (±SD) of 6.1±4.9 days) vs old blood with storage of 22.0±8.4 days (P less than .001). There was no statistically significant difference in 90-day mortality.5
The second study is a meta-analysis by Alexander et al.6 The investigators looked at 12 trials and 5,229 patients and compared “fresh blood” or blood stored for 3-10 days to “older blood” stored for longer durations. They found that there was no difference in mortality and no difference in adverse events, such as acute transfusion reactions, when comparing the two groups.
Lastly, Heddle et al.7 conducted a randomized trial that compared outcomes in 20,858 hospitalized patients transfused with fresh blood (mean storage time 13.0±7.6 days) to older blood (mean storage time 23.6±8.9 days). They found no differences in mortality when comparing those transfused with fresh vs. old blood (8.7% vs. 9.1%). In addition, there was no difference when examining the predetermined subgroups, including those undergoing cardiovascular surgery, those with cancer, and those admitted to the ICU.
So, is this the end of an era for health-care provider concern about how long blood can be stored to be safe for ICU patients? Possibly.
There may still be high-risk populations (such as patients receiving massive transfusions) for which age of the blood does matter. In addition, it is still unclear based on the present data as to whether blood stored between 35 and 42 days has any significant inherent risk.
However, these publications among others suggest that the age of transfused blood may not matter, even in critically ill patients. Therefore, the present storage practices of many blood banks around the United States and beyond are validated by the present publications regarding the scarce resource of blood.
Christopher L. Carroll, MD, MS, FCCP
Steering Committee Member
Steven Greenberg, MD, FCCP
Steering Committee Member
References
1. Corwin HL, Gettinger A, Pearl RG, et al. Crit Care Med. 2004;32(1):39.
2. Vincent JL, Baron JF, Reinhart K, et al.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. JAMA. 2002;288(12):1499.
3. Holst LB, Haase N, Wetterslev J, et al.; TRISS Trial Group; Scandinavian Critical Care Trials Group. N Engl J Med. 2014;371(15):1381.
4. Lacroix J, Hebert PC, Hutchison JS, et al.; TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. N Engl J Med. 2007;356(16):1609.
5. Lacroix J, Hebert P, Fergusson DA, et al. N Engl J Med. 2015;372:1410.
6. Alexander PE, Barty R, Fei Y, et al. Blood. 2016;127(4):400.
7. Heddle NM, Cook RJ, Arnold DM, et al. N Engl J Med. 2016;375(2):1937.
Airways Disorders
Inhaled corticosteroids in COPD: When to hold and when to fold
The 2017 GOLD guidelines reiterated that inhaled corticosteroids (ICS) be reserved for COPD patients with continued symptoms and exacerbations, despite use of long-acting beta-agonists (LABAs) and long-acting muscarinic agents (LAMAs). ICS are appropriate in approximately 40% of patients; however, prescribing rates can exceed 80% (Yawn et al. 2016; Primary Care Respir J. 26:16068).
Recent literature has begun to define the appropriate use of ICS in COPD. ICS/LABA combinations improve outcomes in patients with moderate to very severe COPD with frequent exacerbations. However, ICS/LABA may not further diminish exacerbation risk compared with those treated with a LABA/LAMA combination (Wedzicha et al., N Engl J Med. 2016;374:2222).
While the addition of LAMA to an ICS/LABA combination (triple therapy) improved lung function and decreased exacerbation risk, the addition of ICS to LABA/LAMA combination did not decrease exacerbations (GOLD Guidelines 2017). It has been suggested that those with asthma-COPD overlap identified by sputum eosinophilia represent ideal candidates for ICS therapy (GINA Guideline 2016).
ICS use in COPD increases pneumonia risk. The risk was highest in the very group for which guidelines recommend its use – those with a FEV1 less than 50% of predicted or with prior COPD exacerbation (Ernst et al. Eur Respir J. 2015;45:525).
ICS may be safely withdrawn in low-risk patients (FEV1 less than 50% predicted and no exacerbations in the previous year [Yawn et al.]).
In a trial comparing patients with severe COPD (FEV1 less than 50%) on continued LAMA/LABA/ICS triple therapy vs LAMA/LABA with ICS withdrawal, the risk of moderate or severe exacerbations at 52 weeks was not increased (Magnussen et al. N Engl J Med. 2014;371:1285).
Conclusions
Based on the 2017 GOLD guidelines:
• Monotherapy with ICS is not recommended in COPD.
• In patients with continued respiratory-related symptoms without exacerbations (GOLD group B), LAMA or LABA or LAMA/LABA combination is recommended. There is no recommendation for ICS in this group.
• In patients with frequent exacerbations (GOLD groups C and D), LAMA/LABA combinations are preferred to LABA/ICS because of superior effectiveness (especially in Group D) and the increased pneumonia risk with ICS. Escalation to triple therapy can be considered if there are continued exacerbations.
Allen Blaivas, DO, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Advances in neuromuscular disease
Spinal muscular atrophy (SMA) type 1 is the most deadly inherited disease among infants, with most infants dying by 1 to 2 years of age without supportive therapies, such as assisted ventilation. It is caused by homozygous deletions or mutations in the survival motor neuron 1 (SMN1) gene. Disease severity varies in part depending on the number of backup SMN2 gene copies that can produce some functional SMN protein (Arnold et al. Muscle Nerve. 2015;51[2]:157).
Recent developments of disease-modifying agents are giving hope to individuals with SMA and their families. Nusinersen (an antisense oligonucleotide) is an intrathecal medication that increases the production of functional SMN protein by increasing SMN2 exon 7 transcription (Chiriboga et al. Neurology. 2016;86[10]:890).
A recent open-label clinical trial by Finkel et al. (Lancet. 2017;388[10063]:3017) showed a “promising clinical response” that altered the natural history of disease progression. Most infants treated with multiple intrathecal doses of nusinersen had incremental improvement in their motor milestones and motor function (P = .008), as well as improved survival and/or avoidance of ventilation (P = .0014).
Moreover, the study found significant uptake of nusinersen by the motor neuron throughout the spinal cord and other neurons throughout the CNS. It appeared to be well tolerated. Disease-modifying medications may soon become “game changers” in many neuromuscular conditions.
However, a significant concern is the expected prohibitive cost both of a rare-disease-modifying therapy and of administrating intrathecal medications to fragile infants. As such, those obstacles will need to be overcome as neuromuscular clinics, hospitals, and payers start planning for the coming advances that our patients will be expecting.
Ahlam Mazi, MBBS
Fellow-in-Training Member
Interstitial and Diffuse Lung Disease
New advancements in predictive risk factors of IPF
In the last few years, many predictive risk factors were studied in clinical trials monitoring idiopathic pulmonary fibrosis (IPF), such as forced vital capacity and diffuse lung capacity for carbon monoxide (King TE Jr, et al. ASCEND Study Group. N Engl J Med. 2014;18;371[12]:1172; Richeldi L, et al. INPULSIS Trial Investigators. N Engl J Med. 2015;20;373[8]:782; Ley B, et al. Am J Respir Crit Care Med. 2016;15;194[6]:711).
Recent data that have not yet been published by Carbone et al evaluate the prognostic value of the New York Heart Association index (NYHA) compared with high resolution CT scan, somatostatin receptor scintigraphy (octreoscan), and echocardiography in a study population of 128 patients suffering from IPF (61% male subjects), nonspecific interstitial pneumonia, and granulomatous lung diseases (alveolitis, sarcoidosis, granulomatosis with polyangiitis). All patients were confirmed histologically.
The NYHA came out as a reliable prognostic factor in each setting. In fact, the log-rank test showed significant differences among NYHA categories, as cases included with disease showed the worst survival rate while no death cases were observed when NYHA was negative.
Moreover, the prognostic value of NYHA was confirmed by multivariate analysis, where the survival rate results were significantly different among patients with level 7 after adjustment for other variables included in the model.
Furthermore, the prognostic value of the NYHA index was once again confirmed when the analysis was limited to cases with the histological pattern of IPF (usual interstitial pneumonia).
The authors, therefore, strongly recommend utilization of the NYHA index as a prognostic factor of IPF as well as granulomatous lung diseases.
Roberto Carbone, MD, FCCP
Steering Committee Member
A. Monselise, MD, PhD
NetWork Nonmember
Clinical Research
The unrecognized battlefield in our hospitals: Lessons from the US Navy SEALs
Burnout syndrome (BOS) is a psychological state resulting from prolonged exposure to job stressors. It is characterized by a vicious cycle of emotional exhaustion, detachment from others, and a feeling of decreased accomplishment. Severe BOS is seen in up to 45% of physicians and 33% of nurses who work in ICUs.1
BOS has far-reaching consequences, being associated with an alarmingly high prevalence of posttraumatic stress disorder (PTSD) and substance abuse, almost equivalent to that experienced by veterans returning from war.2 BOS also is associated with self-reported suboptimal patient care practices.3This crisis has long been underrecognized, but now that we have identified the problem, where does that leave us? There are currently no quality studies evaluating how to best treat and prevent BOS/PTSD in health-care professionals. Previous studies have focused on addressing organizational factors to alleviate job stressors, but the psychosocial characteristics of the individual have been largely ignored.
Our medical education has historically focused on an individual’s intelligence quotient (IQ), but developing an individual’s emotional quotient (EQ) is just as valuable. It has long been known that Navy SEALs have the lowest prevalence of PTSD among combat veterans due partially to their specific training in emotional resilience and adaptive psychosocial coping mechanisms.
For this reason, the research team at the University of Texas Health Science Center at San Antonio is collaborating with the US Navy SEAL team to design and validate a tool that teaches critical care staff resilience training similar to what their combat trainees undergo. The goal is to curb these alarming trends in BOS and create a paradigm shift in medical education within medical and nursing schools.
Bravein Amalakuhan, MD
Fellow-in-Training Member
References
1. Embriaco N, Azoulay E, Barrau K, et al. Am J Respir Crit Care Med. 2007;175(7):686.
2. Mealer ML, Shelton A, Berg B, et al. Am J Respir Crit Care Med. 2007;175(7):693.
3. Shanafelt TD, Bradley KA, Wipf JE, et al. Ann Intern Med. 2002;136(5):358.
Critical Care
End of the era for age of blood concerns?
Blood transfusions are common in critically ill patients, with two in five adults admitted to an ICU receiving a transfusion.1,2 Recently, randomized trials have found that more restrictive thresholds for transfusions are associated with improved outcomes.3,4 One theorized explanation for this somewhat counterintuitive association is that the prolonged storage time (i.e., the age of the blood being transfused) might affect outcomes.
There have been three recent publications that help to shed some more light on this. First, Lacroix et al.5 performed a multicenter randomized blinded trial in over 2,400 critically ill patients in 64 centers comparing new blood (mean storage (±SD) of 6.1±4.9 days) vs old blood with storage of 22.0±8.4 days (P less than .001). There was no statistically significant difference in 90-day mortality.5
The second study is a meta-analysis by Alexander et al.6 The investigators looked at 12 trials and 5,229 patients and compared “fresh blood” or blood stored for 3-10 days to “older blood” stored for longer durations. They found that there was no difference in mortality and no difference in adverse events, such as acute transfusion reactions, when comparing the two groups.
Lastly, Heddle et al.7 conducted a randomized trial that compared outcomes in 20,858 hospitalized patients transfused with fresh blood (mean storage time 13.0±7.6 days) to older blood (mean storage time 23.6±8.9 days). They found no differences in mortality when comparing those transfused with fresh vs. old blood (8.7% vs. 9.1%). In addition, there was no difference when examining the predetermined subgroups, including those undergoing cardiovascular surgery, those with cancer, and those admitted to the ICU.
So, is this the end of an era for health-care provider concern about how long blood can be stored to be safe for ICU patients? Possibly.
There may still be high-risk populations (such as patients receiving massive transfusions) for which age of the blood does matter. In addition, it is still unclear based on the present data as to whether blood stored between 35 and 42 days has any significant inherent risk.
However, these publications among others suggest that the age of transfused blood may not matter, even in critically ill patients. Therefore, the present storage practices of many blood banks around the United States and beyond are validated by the present publications regarding the scarce resource of blood.
Christopher L. Carroll, MD, MS, FCCP
Steering Committee Member
Steven Greenberg, MD, FCCP
Steering Committee Member
References
1. Corwin HL, Gettinger A, Pearl RG, et al. Crit Care Med. 2004;32(1):39.
2. Vincent JL, Baron JF, Reinhart K, et al.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. JAMA. 2002;288(12):1499.
3. Holst LB, Haase N, Wetterslev J, et al.; TRISS Trial Group; Scandinavian Critical Care Trials Group. N Engl J Med. 2014;371(15):1381.
4. Lacroix J, Hebert PC, Hutchison JS, et al.; TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. N Engl J Med. 2007;356(16):1609.
5. Lacroix J, Hebert P, Fergusson DA, et al. N Engl J Med. 2015;372:1410.
6. Alexander PE, Barty R, Fei Y, et al. Blood. 2016;127(4):400.
7. Heddle NM, Cook RJ, Arnold DM, et al. N Engl J Med. 2016;375(2):1937.
Airways Disorders
Inhaled corticosteroids in COPD: When to hold and when to fold
The 2017 GOLD guidelines reiterated that inhaled corticosteroids (ICS) be reserved for COPD patients with continued symptoms and exacerbations, despite use of long-acting beta-agonists (LABAs) and long-acting muscarinic agents (LAMAs). ICS are appropriate in approximately 40% of patients; however, prescribing rates can exceed 80% (Yawn et al. 2016; Primary Care Respir J. 26:16068).
Recent literature has begun to define the appropriate use of ICS in COPD. ICS/LABA combinations improve outcomes in patients with moderate to very severe COPD with frequent exacerbations. However, ICS/LABA may not further diminish exacerbation risk compared with those treated with a LABA/LAMA combination (Wedzicha et al., N Engl J Med. 2016;374:2222).
While the addition of LAMA to an ICS/LABA combination (triple therapy) improved lung function and decreased exacerbation risk, the addition of ICS to LABA/LAMA combination did not decrease exacerbations (GOLD Guidelines 2017). It has been suggested that those with asthma-COPD overlap identified by sputum eosinophilia represent ideal candidates for ICS therapy (GINA Guideline 2016).
ICS use in COPD increases pneumonia risk. The risk was highest in the very group for which guidelines recommend its use – those with a FEV1 less than 50% of predicted or with prior COPD exacerbation (Ernst et al. Eur Respir J. 2015;45:525).
ICS may be safely withdrawn in low-risk patients (FEV1 less than 50% predicted and no exacerbations in the previous year [Yawn et al.]).
In a trial comparing patients with severe COPD (FEV1 less than 50%) on continued LAMA/LABA/ICS triple therapy vs LAMA/LABA with ICS withdrawal, the risk of moderate or severe exacerbations at 52 weeks was not increased (Magnussen et al. N Engl J Med. 2014;371:1285).
Conclusions
Based on the 2017 GOLD guidelines:
• Monotherapy with ICS is not recommended in COPD.
• In patients with continued respiratory-related symptoms without exacerbations (GOLD group B), LAMA or LABA or LAMA/LABA combination is recommended. There is no recommendation for ICS in this group.
• In patients with frequent exacerbations (GOLD groups C and D), LAMA/LABA combinations are preferred to LABA/ICS because of superior effectiveness (especially in Group D) and the increased pneumonia risk with ICS. Escalation to triple therapy can be considered if there are continued exacerbations.
Allen Blaivas, DO, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Advances in neuromuscular disease
Spinal muscular atrophy (SMA) type 1 is the most deadly inherited disease among infants, with most infants dying by 1 to 2 years of age without supportive therapies, such as assisted ventilation. It is caused by homozygous deletions or mutations in the survival motor neuron 1 (SMN1) gene. Disease severity varies in part depending on the number of backup SMN2 gene copies that can produce some functional SMN protein (Arnold et al. Muscle Nerve. 2015;51[2]:157).
Recent developments of disease-modifying agents are giving hope to individuals with SMA and their families. Nusinersen (an antisense oligonucleotide) is an intrathecal medication that increases the production of functional SMN protein by increasing SMN2 exon 7 transcription (Chiriboga et al. Neurology. 2016;86[10]:890).
A recent open-label clinical trial by Finkel et al. (Lancet. 2017;388[10063]:3017) showed a “promising clinical response” that altered the natural history of disease progression. Most infants treated with multiple intrathecal doses of nusinersen had incremental improvement in their motor milestones and motor function (P = .008), as well as improved survival and/or avoidance of ventilation (P = .0014).
Moreover, the study found significant uptake of nusinersen by the motor neuron throughout the spinal cord and other neurons throughout the CNS. It appeared to be well tolerated. Disease-modifying medications may soon become “game changers” in many neuromuscular conditions.
However, a significant concern is the expected prohibitive cost both of a rare-disease-modifying therapy and of administrating intrathecal medications to fragile infants. As such, those obstacles will need to be overcome as neuromuscular clinics, hospitals, and payers start planning for the coming advances that our patients will be expecting.
Ahlam Mazi, MBBS
Fellow-in-Training Member
Interstitial and Diffuse Lung Disease
New advancements in predictive risk factors of IPF
In the last few years, many predictive risk factors were studied in clinical trials monitoring idiopathic pulmonary fibrosis (IPF), such as forced vital capacity and diffuse lung capacity for carbon monoxide (King TE Jr, et al. ASCEND Study Group. N Engl J Med. 2014;18;371[12]:1172; Richeldi L, et al. INPULSIS Trial Investigators. N Engl J Med. 2015;20;373[8]:782; Ley B, et al. Am J Respir Crit Care Med. 2016;15;194[6]:711).
Recent data that have not yet been published by Carbone et al evaluate the prognostic value of the New York Heart Association index (NYHA) compared with high resolution CT scan, somatostatin receptor scintigraphy (octreoscan), and echocardiography in a study population of 128 patients suffering from IPF (61% male subjects), nonspecific interstitial pneumonia, and granulomatous lung diseases (alveolitis, sarcoidosis, granulomatosis with polyangiitis). All patients were confirmed histologically.
The NYHA came out as a reliable prognostic factor in each setting. In fact, the log-rank test showed significant differences among NYHA categories, as cases included with disease showed the worst survival rate while no death cases were observed when NYHA was negative.
Moreover, the prognostic value of NYHA was confirmed by multivariate analysis, where the survival rate results were significantly different among patients with level 7 after adjustment for other variables included in the model.
Furthermore, the prognostic value of the NYHA index was once again confirmed when the analysis was limited to cases with the histological pattern of IPF (usual interstitial pneumonia).
The authors, therefore, strongly recommend utilization of the NYHA index as a prognostic factor of IPF as well as granulomatous lung diseases.
Roberto Carbone, MD, FCCP
Steering Committee Member
A. Monselise, MD, PhD
NetWork Nonmember
Clinical Research
The unrecognized battlefield in our hospitals: Lessons from the US Navy SEALs
Burnout syndrome (BOS) is a psychological state resulting from prolonged exposure to job stressors. It is characterized by a vicious cycle of emotional exhaustion, detachment from others, and a feeling of decreased accomplishment. Severe BOS is seen in up to 45% of physicians and 33% of nurses who work in ICUs.1
BOS has far-reaching consequences, being associated with an alarmingly high prevalence of posttraumatic stress disorder (PTSD) and substance abuse, almost equivalent to that experienced by veterans returning from war.2 BOS also is associated with self-reported suboptimal patient care practices.3This crisis has long been underrecognized, but now that we have identified the problem, where does that leave us? There are currently no quality studies evaluating how to best treat and prevent BOS/PTSD in health-care professionals. Previous studies have focused on addressing organizational factors to alleviate job stressors, but the psychosocial characteristics of the individual have been largely ignored.
Our medical education has historically focused on an individual’s intelligence quotient (IQ), but developing an individual’s emotional quotient (EQ) is just as valuable. It has long been known that Navy SEALs have the lowest prevalence of PTSD among combat veterans due partially to their specific training in emotional resilience and adaptive psychosocial coping mechanisms.
For this reason, the research team at the University of Texas Health Science Center at San Antonio is collaborating with the US Navy SEAL team to design and validate a tool that teaches critical care staff resilience training similar to what their combat trainees undergo. The goal is to curb these alarming trends in BOS and create a paradigm shift in medical education within medical and nursing schools.
Bravein Amalakuhan, MD
Fellow-in-Training Member
References
1. Embriaco N, Azoulay E, Barrau K, et al. Am J Respir Crit Care Med. 2007;175(7):686.
2. Mealer ML, Shelton A, Berg B, et al. Am J Respir Crit Care Med. 2007;175(7):693.
3. Shanafelt TD, Bradley KA, Wipf JE, et al. Ann Intern Med. 2002;136(5):358.
Critical Care
End of the era for age of blood concerns?
Blood transfusions are common in critically ill patients, with two in five adults admitted to an ICU receiving a transfusion.1,2 Recently, randomized trials have found that more restrictive thresholds for transfusions are associated with improved outcomes.3,4 One theorized explanation for this somewhat counterintuitive association is that the prolonged storage time (i.e., the age of the blood being transfused) might affect outcomes.
There have been three recent publications that help to shed some more light on this. First, Lacroix et al.5 performed a multicenter randomized blinded trial in over 2,400 critically ill patients in 64 centers comparing new blood (mean storage (±SD) of 6.1±4.9 days) vs old blood with storage of 22.0±8.4 days (P less than .001). There was no statistically significant difference in 90-day mortality.5
The second study is a meta-analysis by Alexander et al.6 The investigators looked at 12 trials and 5,229 patients and compared “fresh blood” or blood stored for 3-10 days to “older blood” stored for longer durations. They found that there was no difference in mortality and no difference in adverse events, such as acute transfusion reactions, when comparing the two groups.
Lastly, Heddle et al.7 conducted a randomized trial that compared outcomes in 20,858 hospitalized patients transfused with fresh blood (mean storage time 13.0±7.6 days) to older blood (mean storage time 23.6±8.9 days). They found no differences in mortality when comparing those transfused with fresh vs. old blood (8.7% vs. 9.1%). In addition, there was no difference when examining the predetermined subgroups, including those undergoing cardiovascular surgery, those with cancer, and those admitted to the ICU.
So, is this the end of an era for health-care provider concern about how long blood can be stored to be safe for ICU patients? Possibly.
There may still be high-risk populations (such as patients receiving massive transfusions) for which age of the blood does matter. In addition, it is still unclear based on the present data as to whether blood stored between 35 and 42 days has any significant inherent risk.
However, these publications among others suggest that the age of transfused blood may not matter, even in critically ill patients. Therefore, the present storage practices of many blood banks around the United States and beyond are validated by the present publications regarding the scarce resource of blood.
Christopher L. Carroll, MD, MS, FCCP
Steering Committee Member
Steven Greenberg, MD, FCCP
Steering Committee Member
References
1. Corwin HL, Gettinger A, Pearl RG, et al. Crit Care Med. 2004;32(1):39.
2. Vincent JL, Baron JF, Reinhart K, et al.; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. JAMA. 2002;288(12):1499.
3. Holst LB, Haase N, Wetterslev J, et al.; TRISS Trial Group; Scandinavian Critical Care Trials Group. N Engl J Med. 2014;371(15):1381.
4. Lacroix J, Hebert PC, Hutchison JS, et al.; TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. N Engl J Med. 2007;356(16):1609.
5. Lacroix J, Hebert P, Fergusson DA, et al. N Engl J Med. 2015;372:1410.
6. Alexander PE, Barty R, Fei Y, et al. Blood. 2016;127(4):400.
7. Heddle NM, Cook RJ, Arnold DM, et al. N Engl J Med. 2016;375(2):1937.
Airways Disorders
Inhaled corticosteroids in COPD: When to hold and when to fold
The 2017 GOLD guidelines reiterated that inhaled corticosteroids (ICS) be reserved for COPD patients with continued symptoms and exacerbations, despite use of long-acting beta-agonists (LABAs) and long-acting muscarinic agents (LAMAs). ICS are appropriate in approximately 40% of patients; however, prescribing rates can exceed 80% (Yawn et al. 2016; Primary Care Respir J. 26:16068).
Recent literature has begun to define the appropriate use of ICS in COPD. ICS/LABA combinations improve outcomes in patients with moderate to very severe COPD with frequent exacerbations. However, ICS/LABA may not further diminish exacerbation risk compared with those treated with a LABA/LAMA combination (Wedzicha et al., N Engl J Med. 2016;374:2222).
While the addition of LAMA to an ICS/LABA combination (triple therapy) improved lung function and decreased exacerbation risk, the addition of ICS to LABA/LAMA combination did not decrease exacerbations (GOLD Guidelines 2017). It has been suggested that those with asthma-COPD overlap identified by sputum eosinophilia represent ideal candidates for ICS therapy (GINA Guideline 2016).
ICS use in COPD increases pneumonia risk. The risk was highest in the very group for which guidelines recommend its use – those with a FEV1 less than 50% of predicted or with prior COPD exacerbation (Ernst et al. Eur Respir J. 2015;45:525).
ICS may be safely withdrawn in low-risk patients (FEV1 less than 50% predicted and no exacerbations in the previous year [Yawn et al.]).
In a trial comparing patients with severe COPD (FEV1 less than 50%) on continued LAMA/LABA/ICS triple therapy vs LAMA/LABA with ICS withdrawal, the risk of moderate or severe exacerbations at 52 weeks was not increased (Magnussen et al. N Engl J Med. 2014;371:1285).
Conclusions
Based on the 2017 GOLD guidelines:
• Monotherapy with ICS is not recommended in COPD.
• In patients with continued respiratory-related symptoms without exacerbations (GOLD group B), LAMA or LABA or LAMA/LABA combination is recommended. There is no recommendation for ICS in this group.
• In patients with frequent exacerbations (GOLD groups C and D), LAMA/LABA combinations are preferred to LABA/ICS because of superior effectiveness (especially in Group D) and the increased pneumonia risk with ICS. Escalation to triple therapy can be considered if there are continued exacerbations.
Allen Blaivas, DO, FCCP
Steering Committee Member
Navitha Ramesh, MD, MBBS
Fellow-in-Training Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Advances in neuromuscular disease
Spinal muscular atrophy (SMA) type 1 is the most deadly inherited disease among infants, with most infants dying by 1 to 2 years of age without supportive therapies, such as assisted ventilation. It is caused by homozygous deletions or mutations in the survival motor neuron 1 (SMN1) gene. Disease severity varies in part depending on the number of backup SMN2 gene copies that can produce some functional SMN protein (Arnold et al. Muscle Nerve. 2015;51[2]:157).
Recent developments of disease-modifying agents are giving hope to individuals with SMA and their families. Nusinersen (an antisense oligonucleotide) is an intrathecal medication that increases the production of functional SMN protein by increasing SMN2 exon 7 transcription (Chiriboga et al. Neurology. 2016;86[10]:890).
A recent open-label clinical trial by Finkel et al. (Lancet. 2017;388[10063]:3017) showed a “promising clinical response” that altered the natural history of disease progression. Most infants treated with multiple intrathecal doses of nusinersen had incremental improvement in their motor milestones and motor function (P = .008), as well as improved survival and/or avoidance of ventilation (P = .0014).
Moreover, the study found significant uptake of nusinersen by the motor neuron throughout the spinal cord and other neurons throughout the CNS. It appeared to be well tolerated. Disease-modifying medications may soon become “game changers” in many neuromuscular conditions.
However, a significant concern is the expected prohibitive cost both of a rare-disease-modifying therapy and of administrating intrathecal medications to fragile infants. As such, those obstacles will need to be overcome as neuromuscular clinics, hospitals, and payers start planning for the coming advances that our patients will be expecting.
Ahlam Mazi, MBBS
Fellow-in-Training Member
Interstitial and Diffuse Lung Disease
New advancements in predictive risk factors of IPF
In the last few years, many predictive risk factors were studied in clinical trials monitoring idiopathic pulmonary fibrosis (IPF), such as forced vital capacity and diffuse lung capacity for carbon monoxide (King TE Jr, et al. ASCEND Study Group. N Engl J Med. 2014;18;371[12]:1172; Richeldi L, et al. INPULSIS Trial Investigators. N Engl J Med. 2015;20;373[8]:782; Ley B, et al. Am J Respir Crit Care Med. 2016;15;194[6]:711).
Recent data that have not yet been published by Carbone et al evaluate the prognostic value of the New York Heart Association index (NYHA) compared with high resolution CT scan, somatostatin receptor scintigraphy (octreoscan), and echocardiography in a study population of 128 patients suffering from IPF (61% male subjects), nonspecific interstitial pneumonia, and granulomatous lung diseases (alveolitis, sarcoidosis, granulomatosis with polyangiitis). All patients were confirmed histologically.
The NYHA came out as a reliable prognostic factor in each setting. In fact, the log-rank test showed significant differences among NYHA categories, as cases included with disease showed the worst survival rate while no death cases were observed when NYHA was negative.
Moreover, the prognostic value of NYHA was confirmed by multivariate analysis, where the survival rate results were significantly different among patients with level 7 after adjustment for other variables included in the model.
Furthermore, the prognostic value of the NYHA index was once again confirmed when the analysis was limited to cases with the histological pattern of IPF (usual interstitial pneumonia).
The authors, therefore, strongly recommend utilization of the NYHA index as a prognostic factor of IPF as well as granulomatous lung diseases.
Roberto Carbone, MD, FCCP
Steering Committee Member
A. Monselise, MD, PhD
NetWork Nonmember
Join Us for COPD: Current Excellence and Future Development May 7-9, 2017 Amsterdam, the Netherlands
The global burden of COPD is increasing and is one of the leading causes of disability worldwide. Attend COPD: Current Excellence and Future Development and join clinicians, experts, and specialists as they convene in Amsterdam to discuss best practices and future directions in diagnosis, treatment, and therapeutic innovations. Plan to discuss the latest cutting-edge findings in COPD with like-minded clinicians.
The conference, taking place at the NH Grand Hotel Krasnapolsky, in the center of Amsterdam, will include these session themes:
- History and burden of COPD.
- Polymorbidity in COPD.
- Infections and exacerbations in COPD.
- Current treatment of COPD.
- The future of COPD.
Don’t Miss These Speakers
- Dirkje Postma (Keynote speaker) – Professor of Pulmonary Medicine at the University of Groningen and the University Medical Center of Groningen. Professor Postma will give a keynote session “From Past to Present, Circle With COPD.”
- David M. Mannino (Conference chair) – Professor and Chair in the Department of Preventive Medicine and Environmental Health at the University of Kentucky (Lexington) College of Public Health. Dr. Mannino’s session topic is “The Natural History of COPD.”
- John Hurst, (Co-chair and speaker) – Senior Lecturer at University College, London, UK, Dr. Hurst’s session topic is “The Importance of Acute Exacerbations.”
- Alberto Papi (Co-chair and speaker) – Professor of Respiratory Medicine and Vice President of the School of Medicine at the University of Ferrara, Italy, and Director of the Respiratory Unit of the Department of Emergency Medicine, S. Anna University Hospital, Ferrara. Professor Papi’s talk will explore “The Role of Infections.”
- Peter J. Barnes (Conference speaker) – Margaret-Turner Warwick Professor of Medicine at the National Heart and Lung Institute, Head of Respiratory Medicine at Imperial College and Honorary Consultant Physician at Royal Brompton Hospital, London. Professor Barnes’ presentation will focus on “Future Novel Therapies.”
- Sally Singh (Conference speaker) - Professor of Pulmonary and Cardiac Rehabilitation at the University Hospitals of Leicester (one of the largest rehabilitation programs in the UK). Professor Singh’s session is on “Pulmonary Rehabilitation.”
- Nicholas Hopkinson (Conference speaker) – Dr. Hopkinson is a Reader in Respiratory Medicine & Honorary Consultant Physician at the National Heart and Lung Institute of Imperial College and the Royal Brompton Hospital. His session focuses on “Cigarette Smoking.”
- Joan Soriano (Conference speaker) - Since 2007, Dr. Soriano has been an Associate Editor of the European Respiratory Journal and since 2013 of the Lancet Respiratory Medicine. His session focuses on “Asthma-COPD Overlap.”
Learn more about what the conference has to offer and how to register at chestcopdconference.com.
The global burden of COPD is increasing and is one of the leading causes of disability worldwide. Attend COPD: Current Excellence and Future Development and join clinicians, experts, and specialists as they convene in Amsterdam to discuss best practices and future directions in diagnosis, treatment, and therapeutic innovations. Plan to discuss the latest cutting-edge findings in COPD with like-minded clinicians.
The conference, taking place at the NH Grand Hotel Krasnapolsky, in the center of Amsterdam, will include these session themes:
- History and burden of COPD.
- Polymorbidity in COPD.
- Infections and exacerbations in COPD.
- Current treatment of COPD.
- The future of COPD.
Don’t Miss These Speakers
- Dirkje Postma (Keynote speaker) – Professor of Pulmonary Medicine at the University of Groningen and the University Medical Center of Groningen. Professor Postma will give a keynote session “From Past to Present, Circle With COPD.”
- David M. Mannino (Conference chair) – Professor and Chair in the Department of Preventive Medicine and Environmental Health at the University of Kentucky (Lexington) College of Public Health. Dr. Mannino’s session topic is “The Natural History of COPD.”
- John Hurst, (Co-chair and speaker) – Senior Lecturer at University College, London, UK, Dr. Hurst’s session topic is “The Importance of Acute Exacerbations.”
- Alberto Papi (Co-chair and speaker) – Professor of Respiratory Medicine and Vice President of the School of Medicine at the University of Ferrara, Italy, and Director of the Respiratory Unit of the Department of Emergency Medicine, S. Anna University Hospital, Ferrara. Professor Papi’s talk will explore “The Role of Infections.”
- Peter J. Barnes (Conference speaker) – Margaret-Turner Warwick Professor of Medicine at the National Heart and Lung Institute, Head of Respiratory Medicine at Imperial College and Honorary Consultant Physician at Royal Brompton Hospital, London. Professor Barnes’ presentation will focus on “Future Novel Therapies.”
- Sally Singh (Conference speaker) - Professor of Pulmonary and Cardiac Rehabilitation at the University Hospitals of Leicester (one of the largest rehabilitation programs in the UK). Professor Singh’s session is on “Pulmonary Rehabilitation.”
- Nicholas Hopkinson (Conference speaker) – Dr. Hopkinson is a Reader in Respiratory Medicine & Honorary Consultant Physician at the National Heart and Lung Institute of Imperial College and the Royal Brompton Hospital. His session focuses on “Cigarette Smoking.”
- Joan Soriano (Conference speaker) - Since 2007, Dr. Soriano has been an Associate Editor of the European Respiratory Journal and since 2013 of the Lancet Respiratory Medicine. His session focuses on “Asthma-COPD Overlap.”
Learn more about what the conference has to offer and how to register at chestcopdconference.com.
The global burden of COPD is increasing and is one of the leading causes of disability worldwide. Attend COPD: Current Excellence and Future Development and join clinicians, experts, and specialists as they convene in Amsterdam to discuss best practices and future directions in diagnosis, treatment, and therapeutic innovations. Plan to discuss the latest cutting-edge findings in COPD with like-minded clinicians.
The conference, taking place at the NH Grand Hotel Krasnapolsky, in the center of Amsterdam, will include these session themes:
- History and burden of COPD.
- Polymorbidity in COPD.
- Infections and exacerbations in COPD.
- Current treatment of COPD.
- The future of COPD.
Don’t Miss These Speakers
- Dirkje Postma (Keynote speaker) – Professor of Pulmonary Medicine at the University of Groningen and the University Medical Center of Groningen. Professor Postma will give a keynote session “From Past to Present, Circle With COPD.”
- David M. Mannino (Conference chair) – Professor and Chair in the Department of Preventive Medicine and Environmental Health at the University of Kentucky (Lexington) College of Public Health. Dr. Mannino’s session topic is “The Natural History of COPD.”
- John Hurst, (Co-chair and speaker) – Senior Lecturer at University College, London, UK, Dr. Hurst’s session topic is “The Importance of Acute Exacerbations.”
- Alberto Papi (Co-chair and speaker) – Professor of Respiratory Medicine and Vice President of the School of Medicine at the University of Ferrara, Italy, and Director of the Respiratory Unit of the Department of Emergency Medicine, S. Anna University Hospital, Ferrara. Professor Papi’s talk will explore “The Role of Infections.”
- Peter J. Barnes (Conference speaker) – Margaret-Turner Warwick Professor of Medicine at the National Heart and Lung Institute, Head of Respiratory Medicine at Imperial College and Honorary Consultant Physician at Royal Brompton Hospital, London. Professor Barnes’ presentation will focus on “Future Novel Therapies.”
- Sally Singh (Conference speaker) - Professor of Pulmonary and Cardiac Rehabilitation at the University Hospitals of Leicester (one of the largest rehabilitation programs in the UK). Professor Singh’s session is on “Pulmonary Rehabilitation.”
- Nicholas Hopkinson (Conference speaker) – Dr. Hopkinson is a Reader in Respiratory Medicine & Honorary Consultant Physician at the National Heart and Lung Institute of Imperial College and the Royal Brompton Hospital. His session focuses on “Cigarette Smoking.”
- Joan Soriano (Conference speaker) - Since 2007, Dr. Soriano has been an Associate Editor of the European Respiratory Journal and since 2013 of the Lancet Respiratory Medicine. His session focuses on “Asthma-COPD Overlap.”
Learn more about what the conference has to offer and how to register at chestcopdconference.com.
PCCM endorsed as pilot subspecialty by the Chinese National Health and Family Planning Commission
On Dec. 23, 2016, the Chinese National Health and Family Planning Commission officially endorsed Pulmonary and Critical Care Medicine (PCCM) as a pilot subspecialty within China. PCCM is one of three subspecialties (together with neurosurgery and cardiology) to pioneer fellowship training education in China. With the official endorsement of PCCM, local efforts will progress within China to administer programs and extend the standards of training throughout medical education in China. PCCM certification will now become a requirement for appointment of pulmonary department chairs and for promotion within the subspecialty.
Since 2012, CHEST has worked closely with partners, such as the Chinese Thoracic Society, the Chinese Association of Chest Physicians, and the Chinese Medical Doctor Association, on the development of China’s first fellowship program offering standardized training in PCCM for Chinese physicians. As a result of these collective efforts, PCCM has now officially earned endorsement as a medical subspecialty – the first of its kind in a country where medical training typically ends after a physician completes residency training. Only a decade ago, physicians in China went directly into practice following medical school. The development of a PCCM subspecialty in China – made possible through the engagement of CHEST’s expert faculty and administration – parallels what has occurred over the past 3 decades in the United States, during which the fields of pulmonary and critical care medicine evolved into the combined subspecialty of PCCM.
The China-CHEST PCCM Fellowship Program was officially launched in 2013 with 12 participating Chinese institutions starting their PCCM training programs. By the end of 2017, 30 programs with 300 fellows and 60 faculty will be participating at institutions throughout China, with the potential to impact the care of thousands of patients. The China-PCCM Fellowship Program proudly graduated its first class of fellows in September 2016.
China-CHEST leaders, including Renli Qiao, MD, PhD, FCCP; Chen Wang, MD, PhD, FCCP; and Jack Buckley, MD, MPH, FCCP; with Steve Welch, CHEST Executive Vice President, recently participated in local site visits to provide ongoing education and support to Chinese PCCM fellowship programs. They also participated in the November 2016 Mingdao Forum in Beijing to highlight the history and achievements of the China-CHEST PCCM program.
The vast reach and clinical exposure of this program highlights how an international professional medical association like CHEST, through innovative education and strategic collaborative partnerships, is able to impact medical training both within and beyond its specialty on a global scale.
Darcy Marciniuk, MD, FCCP
Chair, China–CHEST PCCM Steering Committee
Professor of Medicine, University of Saskatchewan,
Saskatoon, SK, Canada
Renli Qiao, MD, PhD, FCCP
Medical Director, China–CHEST PCCM Program
Professor of Clinical Medicine,
Keck School of Medicine of USC,
Los Angeles, California
Robb Rabito, CHCP
Director, Education Operations CHEST
Glenview, Illinois
On Dec. 23, 2016, the Chinese National Health and Family Planning Commission officially endorsed Pulmonary and Critical Care Medicine (PCCM) as a pilot subspecialty within China. PCCM is one of three subspecialties (together with neurosurgery and cardiology) to pioneer fellowship training education in China. With the official endorsement of PCCM, local efforts will progress within China to administer programs and extend the standards of training throughout medical education in China. PCCM certification will now become a requirement for appointment of pulmonary department chairs and for promotion within the subspecialty.
Since 2012, CHEST has worked closely with partners, such as the Chinese Thoracic Society, the Chinese Association of Chest Physicians, and the Chinese Medical Doctor Association, on the development of China’s first fellowship program offering standardized training in PCCM for Chinese physicians. As a result of these collective efforts, PCCM has now officially earned endorsement as a medical subspecialty – the first of its kind in a country where medical training typically ends after a physician completes residency training. Only a decade ago, physicians in China went directly into practice following medical school. The development of a PCCM subspecialty in China – made possible through the engagement of CHEST’s expert faculty and administration – parallels what has occurred over the past 3 decades in the United States, during which the fields of pulmonary and critical care medicine evolved into the combined subspecialty of PCCM.
The China-CHEST PCCM Fellowship Program was officially launched in 2013 with 12 participating Chinese institutions starting their PCCM training programs. By the end of 2017, 30 programs with 300 fellows and 60 faculty will be participating at institutions throughout China, with the potential to impact the care of thousands of patients. The China-PCCM Fellowship Program proudly graduated its first class of fellows in September 2016.
China-CHEST leaders, including Renli Qiao, MD, PhD, FCCP; Chen Wang, MD, PhD, FCCP; and Jack Buckley, MD, MPH, FCCP; with Steve Welch, CHEST Executive Vice President, recently participated in local site visits to provide ongoing education and support to Chinese PCCM fellowship programs. They also participated in the November 2016 Mingdao Forum in Beijing to highlight the history and achievements of the China-CHEST PCCM program.
The vast reach and clinical exposure of this program highlights how an international professional medical association like CHEST, through innovative education and strategic collaborative partnerships, is able to impact medical training both within and beyond its specialty on a global scale.
Darcy Marciniuk, MD, FCCP
Chair, China–CHEST PCCM Steering Committee
Professor of Medicine, University of Saskatchewan,
Saskatoon, SK, Canada
Renli Qiao, MD, PhD, FCCP
Medical Director, China–CHEST PCCM Program
Professor of Clinical Medicine,
Keck School of Medicine of USC,
Los Angeles, California
Robb Rabito, CHCP
Director, Education Operations CHEST
Glenview, Illinois
On Dec. 23, 2016, the Chinese National Health and Family Planning Commission officially endorsed Pulmonary and Critical Care Medicine (PCCM) as a pilot subspecialty within China. PCCM is one of three subspecialties (together with neurosurgery and cardiology) to pioneer fellowship training education in China. With the official endorsement of PCCM, local efforts will progress within China to administer programs and extend the standards of training throughout medical education in China. PCCM certification will now become a requirement for appointment of pulmonary department chairs and for promotion within the subspecialty.
Since 2012, CHEST has worked closely with partners, such as the Chinese Thoracic Society, the Chinese Association of Chest Physicians, and the Chinese Medical Doctor Association, on the development of China’s first fellowship program offering standardized training in PCCM for Chinese physicians. As a result of these collective efforts, PCCM has now officially earned endorsement as a medical subspecialty – the first of its kind in a country where medical training typically ends after a physician completes residency training. Only a decade ago, physicians in China went directly into practice following medical school. The development of a PCCM subspecialty in China – made possible through the engagement of CHEST’s expert faculty and administration – parallels what has occurred over the past 3 decades in the United States, during which the fields of pulmonary and critical care medicine evolved into the combined subspecialty of PCCM.
The China-CHEST PCCM Fellowship Program was officially launched in 2013 with 12 participating Chinese institutions starting their PCCM training programs. By the end of 2017, 30 programs with 300 fellows and 60 faculty will be participating at institutions throughout China, with the potential to impact the care of thousands of patients. The China-PCCM Fellowship Program proudly graduated its first class of fellows in September 2016.
China-CHEST leaders, including Renli Qiao, MD, PhD, FCCP; Chen Wang, MD, PhD, FCCP; and Jack Buckley, MD, MPH, FCCP; with Steve Welch, CHEST Executive Vice President, recently participated in local site visits to provide ongoing education and support to Chinese PCCM fellowship programs. They also participated in the November 2016 Mingdao Forum in Beijing to highlight the history and achievements of the China-CHEST PCCM program.
The vast reach and clinical exposure of this program highlights how an international professional medical association like CHEST, through innovative education and strategic collaborative partnerships, is able to impact medical training both within and beyond its specialty on a global scale.
Darcy Marciniuk, MD, FCCP
Chair, China–CHEST PCCM Steering Committee
Professor of Medicine, University of Saskatchewan,
Saskatoon, SK, Canada
Renli Qiao, MD, PhD, FCCP
Medical Director, China–CHEST PCCM Program
Professor of Clinical Medicine,
Keck School of Medicine of USC,
Los Angeles, California
Robb Rabito, CHCP
Director, Education Operations CHEST
Glenview, Illinois
CCSC issues five Choosing Wisely recommendations
Overutilization of tests, treatments, and procedures is an important example of low-value care that adds to the high cost of health care and provides little to no benefit for patients. To combat this problem, the American Board of Internal Medicine Foundation developed the Choosing Wisely Campaign, tasking professional societies to develop lists of the top five medical services that patients should question.
The Critical Care Societies Collaborative (CCSC), which comprises the four major U.S. professional and scientific societies – the American Association of Critical-Care Nurses, the American College of Chest Physicians, the American Thoracic Society, and the Society of Critical Care Medicine – participated by creating a task force that addressed this task to focus on critical care delivery.
Five CCSC recommendations were formulated:
1. Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.
2. Don’t transfuse red blood cells in hemodynamically stable, nonbleeding patients with a hemoglobin concentration greater than 7 mg/dL.
3. Don’t use parenteral nutrition in adequately nourished critically ill patients within the first 7 days of an ICU stay.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort.
The CCSC is tracking use/implementation of the Choosing Wisely recommendations among its four member organizations. Please complete this short survey at https://redcap.rush.edu/redcap/surveys/?s. Please click submit when finished.
Overutilization of tests, treatments, and procedures is an important example of low-value care that adds to the high cost of health care and provides little to no benefit for patients. To combat this problem, the American Board of Internal Medicine Foundation developed the Choosing Wisely Campaign, tasking professional societies to develop lists of the top five medical services that patients should question.
The Critical Care Societies Collaborative (CCSC), which comprises the four major U.S. professional and scientific societies – the American Association of Critical-Care Nurses, the American College of Chest Physicians, the American Thoracic Society, and the Society of Critical Care Medicine – participated by creating a task force that addressed this task to focus on critical care delivery.
Five CCSC recommendations were formulated:
1. Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.
2. Don’t transfuse red blood cells in hemodynamically stable, nonbleeding patients with a hemoglobin concentration greater than 7 mg/dL.
3. Don’t use parenteral nutrition in adequately nourished critically ill patients within the first 7 days of an ICU stay.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort.
The CCSC is tracking use/implementation of the Choosing Wisely recommendations among its four member organizations. Please complete this short survey at https://redcap.rush.edu/redcap/surveys/?s. Please click submit when finished.
Overutilization of tests, treatments, and procedures is an important example of low-value care that adds to the high cost of health care and provides little to no benefit for patients. To combat this problem, the American Board of Internal Medicine Foundation developed the Choosing Wisely Campaign, tasking professional societies to develop lists of the top five medical services that patients should question.
The Critical Care Societies Collaborative (CCSC), which comprises the four major U.S. professional and scientific societies – the American Association of Critical-Care Nurses, the American College of Chest Physicians, the American Thoracic Society, and the Society of Critical Care Medicine – participated by creating a task force that addressed this task to focus on critical care delivery.
Five CCSC recommendations were formulated:
1. Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.
2. Don’t transfuse red blood cells in hemodynamically stable, nonbleeding patients with a hemoglobin concentration greater than 7 mg/dL.
3. Don’t use parenteral nutrition in adequately nourished critically ill patients within the first 7 days of an ICU stay.
4. Don’t deeply sedate mechanically ventilated patients without a specific indication and without daily attempts to lighten sedation.
5. Don’t continue life support for patients at high risk for death or severely impaired functional recovery without offering patients and their families the alternative of care focused entirely on comfort.
The CCSC is tracking use/implementation of the Choosing Wisely recommendations among its four member organizations. Please complete this short survey at https://redcap.rush.edu/redcap/surveys/?s. Please click submit when finished.
CHEST Foundation can give more than $500,000 in grants
Every year, the CHEST Foundation awards more than a half-million dollars in grants to the next generation of lung health champions. February 2017 marks the start of the foundation’s next grant cycle, and we are excited to announce a new clinical research grant in Cystic Fibrosis, among many other disease-state topics. In 2016, the foundation awarded 11 CHEST members for their innovative and inspiring research proposals and community service programs.
“I am very proud to have been awarded a CHEST Foundation grant and pleased that clinical research and real-world evidence are a priority to the foundation,” stated Alice Turner, MBChB, PhD. Dr. Turner was awarded the 2016 CHEST Foundation and the Alpha-1 Foundation Clinical Research Grant in Alpha-1 Antitrypsin Deficiency. “This award means that my patients can now see publicly the efforts that are being made to reduce inequities in care and ensure that the best treatments are made available in the UK.”

The award will allow Dr. Turner to compare patients who are being treated in the United States with those who are untreated in the United Kingdom and then analyze the effects on mortality, hospitalization, and quality of life to make inferences about whether or not the treatment should be implemented in the United Kingdom. Currently, the type of treatment used to treat patients with alpha-1 antitrypsin deficiency in the United States is not available in the United Kingdom, and the results of this study will be provided to the National Health Service in England to help overcome the barriers of legalizing the treatment in the United Kingdom.
Sydney Montesi, MD, was awarded the CHEST Foundation Research Grant in Pulmonary Fibrosis for her work on using noninvasive lung imaging to see how contrast agents can be used to measure disease activity and progression.
“As a provider, it can be very difficult when we first meet a patient to know what disease course they will take, but if we had this information, it would help us in determining earlier lung transplant referrals, choosing the best therapies and treatments, and ultimately lowering the mortality rate of idiopathic pulmonary fibrosis,” Dr. Montesi said of her research. “Receiving this grant is essential because it will allow us to test our hypothesis that vascular leakage is increased in patients with pulmonary fibrosis, and we will also be able to look more in depth at the comparison of patients with stable disease and those with progressive disease.”
These grants help advance the work of young investigators all over the globe. Over the last 20 years, thousands of researchers and community service volunteers have received more than $10 million in funding.
Beginning in February 2017, the Foundation will have more than a half-million dollars available in funding toward the next generation of lung health champions.
Learn more about the CHEST Foundation grant application process at chestnet.org/grants or e-mail the foundation at [email protected].
Every year, the CHEST Foundation awards more than a half-million dollars in grants to the next generation of lung health champions. February 2017 marks the start of the foundation’s next grant cycle, and we are excited to announce a new clinical research grant in Cystic Fibrosis, among many other disease-state topics. In 2016, the foundation awarded 11 CHEST members for their innovative and inspiring research proposals and community service programs.
“I am very proud to have been awarded a CHEST Foundation grant and pleased that clinical research and real-world evidence are a priority to the foundation,” stated Alice Turner, MBChB, PhD. Dr. Turner was awarded the 2016 CHEST Foundation and the Alpha-1 Foundation Clinical Research Grant in Alpha-1 Antitrypsin Deficiency. “This award means that my patients can now see publicly the efforts that are being made to reduce inequities in care and ensure that the best treatments are made available in the UK.”

The award will allow Dr. Turner to compare patients who are being treated in the United States with those who are untreated in the United Kingdom and then analyze the effects on mortality, hospitalization, and quality of life to make inferences about whether or not the treatment should be implemented in the United Kingdom. Currently, the type of treatment used to treat patients with alpha-1 antitrypsin deficiency in the United States is not available in the United Kingdom, and the results of this study will be provided to the National Health Service in England to help overcome the barriers of legalizing the treatment in the United Kingdom.
Sydney Montesi, MD, was awarded the CHEST Foundation Research Grant in Pulmonary Fibrosis for her work on using noninvasive lung imaging to see how contrast agents can be used to measure disease activity and progression.
“As a provider, it can be very difficult when we first meet a patient to know what disease course they will take, but if we had this information, it would help us in determining earlier lung transplant referrals, choosing the best therapies and treatments, and ultimately lowering the mortality rate of idiopathic pulmonary fibrosis,” Dr. Montesi said of her research. “Receiving this grant is essential because it will allow us to test our hypothesis that vascular leakage is increased in patients with pulmonary fibrosis, and we will also be able to look more in depth at the comparison of patients with stable disease and those with progressive disease.”
These grants help advance the work of young investigators all over the globe. Over the last 20 years, thousands of researchers and community service volunteers have received more than $10 million in funding.
Beginning in February 2017, the Foundation will have more than a half-million dollars available in funding toward the next generation of lung health champions.
Learn more about the CHEST Foundation grant application process at chestnet.org/grants or e-mail the foundation at [email protected].
Every year, the CHEST Foundation awards more than a half-million dollars in grants to the next generation of lung health champions. February 2017 marks the start of the foundation’s next grant cycle, and we are excited to announce a new clinical research grant in Cystic Fibrosis, among many other disease-state topics. In 2016, the foundation awarded 11 CHEST members for their innovative and inspiring research proposals and community service programs.
“I am very proud to have been awarded a CHEST Foundation grant and pleased that clinical research and real-world evidence are a priority to the foundation,” stated Alice Turner, MBChB, PhD. Dr. Turner was awarded the 2016 CHEST Foundation and the Alpha-1 Foundation Clinical Research Grant in Alpha-1 Antitrypsin Deficiency. “This award means that my patients can now see publicly the efforts that are being made to reduce inequities in care and ensure that the best treatments are made available in the UK.”

The award will allow Dr. Turner to compare patients who are being treated in the United States with those who are untreated in the United Kingdom and then analyze the effects on mortality, hospitalization, and quality of life to make inferences about whether or not the treatment should be implemented in the United Kingdom. Currently, the type of treatment used to treat patients with alpha-1 antitrypsin deficiency in the United States is not available in the United Kingdom, and the results of this study will be provided to the National Health Service in England to help overcome the barriers of legalizing the treatment in the United Kingdom.
Sydney Montesi, MD, was awarded the CHEST Foundation Research Grant in Pulmonary Fibrosis for her work on using noninvasive lung imaging to see how contrast agents can be used to measure disease activity and progression.
“As a provider, it can be very difficult when we first meet a patient to know what disease course they will take, but if we had this information, it would help us in determining earlier lung transplant referrals, choosing the best therapies and treatments, and ultimately lowering the mortality rate of idiopathic pulmonary fibrosis,” Dr. Montesi said of her research. “Receiving this grant is essential because it will allow us to test our hypothesis that vascular leakage is increased in patients with pulmonary fibrosis, and we will also be able to look more in depth at the comparison of patients with stable disease and those with progressive disease.”
These grants help advance the work of young investigators all over the globe. Over the last 20 years, thousands of researchers and community service volunteers have received more than $10 million in funding.
Beginning in February 2017, the Foundation will have more than a half-million dollars available in funding toward the next generation of lung health champions.
Learn more about the CHEST Foundation grant application process at chestnet.org/grants or e-mail the foundation at [email protected].