User login
This Month in CHEST: Editor’s Picks
E
GOLD 2017: A New Report
By Dr. P. J. Barnes
Original Research
Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. By Dr. S. Sawada, et al.
ICU Telemedicine Program Financial Outcomes. By Dr. C. M. Lilly et al.
Accuracy of Lung Ultrasonography in the Diagnosis of Pneumonia in Adults: Systematic Review and Meta-Analysis. By Dr. A. M. Llamas-Álvarez, et al.
Evidence-based Medicine
Cough in the Athlete: CHEST Guideline and Expert Panel Report. By Dr. L-P Boulet, et al, on behalf of the CHEST Expert Cough Panel.
E
GOLD 2017: A New Report
By Dr. P. J. Barnes
Original Research
Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. By Dr. S. Sawada, et al.
ICU Telemedicine Program Financial Outcomes. By Dr. C. M. Lilly et al.
Accuracy of Lung Ultrasonography in the Diagnosis of Pneumonia in Adults: Systematic Review and Meta-Analysis. By Dr. A. M. Llamas-Álvarez, et al.
Evidence-based Medicine
Cough in the Athlete: CHEST Guideline and Expert Panel Report. By Dr. L-P Boulet, et al, on behalf of the CHEST Expert Cough Panel.
E
GOLD 2017: A New Report
By Dr. P. J. Barnes
Original Research
Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. By Dr. S. Sawada, et al.
ICU Telemedicine Program Financial Outcomes. By Dr. C. M. Lilly et al.
Accuracy of Lung Ultrasonography in the Diagnosis of Pneumonia in Adults: Systematic Review and Meta-Analysis. By Dr. A. M. Llamas-Álvarez, et al.
Evidence-based Medicine
Cough in the Athlete: CHEST Guideline and Expert Panel Report. By Dr. L-P Boulet, et al, on behalf of the CHEST Expert Cough Panel.
Meet the CHEST President-Designate
Clayton T. Cowl, MD, FCCP, is the CHEST President-Designate and sits as a member of the Board of Regents. Dr. Cowl’s presidential term will be 2018-2019. He currently is the Chair of the Division of Preventive, Occupational, and Aerospace Medicine with a joint appointment in the Division of Pulmonary and Critical Care Medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Cowl is triple board-certified in Pulmonary and Critical Care Medicine, Occupational Medicine, and Internal Medicine, with an interest in airway disorders, occupational-related respiratory health, toxicology, altitude physiology, and transportation medicine.
His research focus has included projects in altitude physiology at Mayo Clinic’s altitude chamber and testing for the emergency oxygen passenger mask in the Boeing 787 airliner. He has also published in the areas of occupational asthma and toxic inhalations.
He is currently the President of the Civil Aviation Medical Association and is a Senior Aviation Medical Examiner designated by the Federal Aviation Administration.
Dr. Cowl has been a recipient of the Innovation in Education Award from the Mayo School of Continuous Professional Development, and the Laureate Award in the Mayo Clinic Department of Medicine.
Clayton T. Cowl, MD, FCCP, is the CHEST President-Designate and sits as a member of the Board of Regents. Dr. Cowl’s presidential term will be 2018-2019. He currently is the Chair of the Division of Preventive, Occupational, and Aerospace Medicine with a joint appointment in the Division of Pulmonary and Critical Care Medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Cowl is triple board-certified in Pulmonary and Critical Care Medicine, Occupational Medicine, and Internal Medicine, with an interest in airway disorders, occupational-related respiratory health, toxicology, altitude physiology, and transportation medicine.
His research focus has included projects in altitude physiology at Mayo Clinic’s altitude chamber and testing for the emergency oxygen passenger mask in the Boeing 787 airliner. He has also published in the areas of occupational asthma and toxic inhalations.
He is currently the President of the Civil Aviation Medical Association and is a Senior Aviation Medical Examiner designated by the Federal Aviation Administration.
Dr. Cowl has been a recipient of the Innovation in Education Award from the Mayo School of Continuous Professional Development, and the Laureate Award in the Mayo Clinic Department of Medicine.
Clayton T. Cowl, MD, FCCP, is the CHEST President-Designate and sits as a member of the Board of Regents. Dr. Cowl’s presidential term will be 2018-2019. He currently is the Chair of the Division of Preventive, Occupational, and Aerospace Medicine with a joint appointment in the Division of Pulmonary and Critical Care Medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Cowl is triple board-certified in Pulmonary and Critical Care Medicine, Occupational Medicine, and Internal Medicine, with an interest in airway disorders, occupational-related respiratory health, toxicology, altitude physiology, and transportation medicine.
His research focus has included projects in altitude physiology at Mayo Clinic’s altitude chamber and testing for the emergency oxygen passenger mask in the Boeing 787 airliner. He has also published in the areas of occupational asthma and toxic inhalations.
He is currently the President of the Civil Aviation Medical Association and is a Senior Aviation Medical Examiner designated by the Federal Aviation Administration.
Dr. Cowl has been a recipient of the Innovation in Education Award from the Mayo School of Continuous Professional Development, and the Laureate Award in the Mayo Clinic Department of Medicine.
Transgender Patients: Providing Sensitive Care
Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
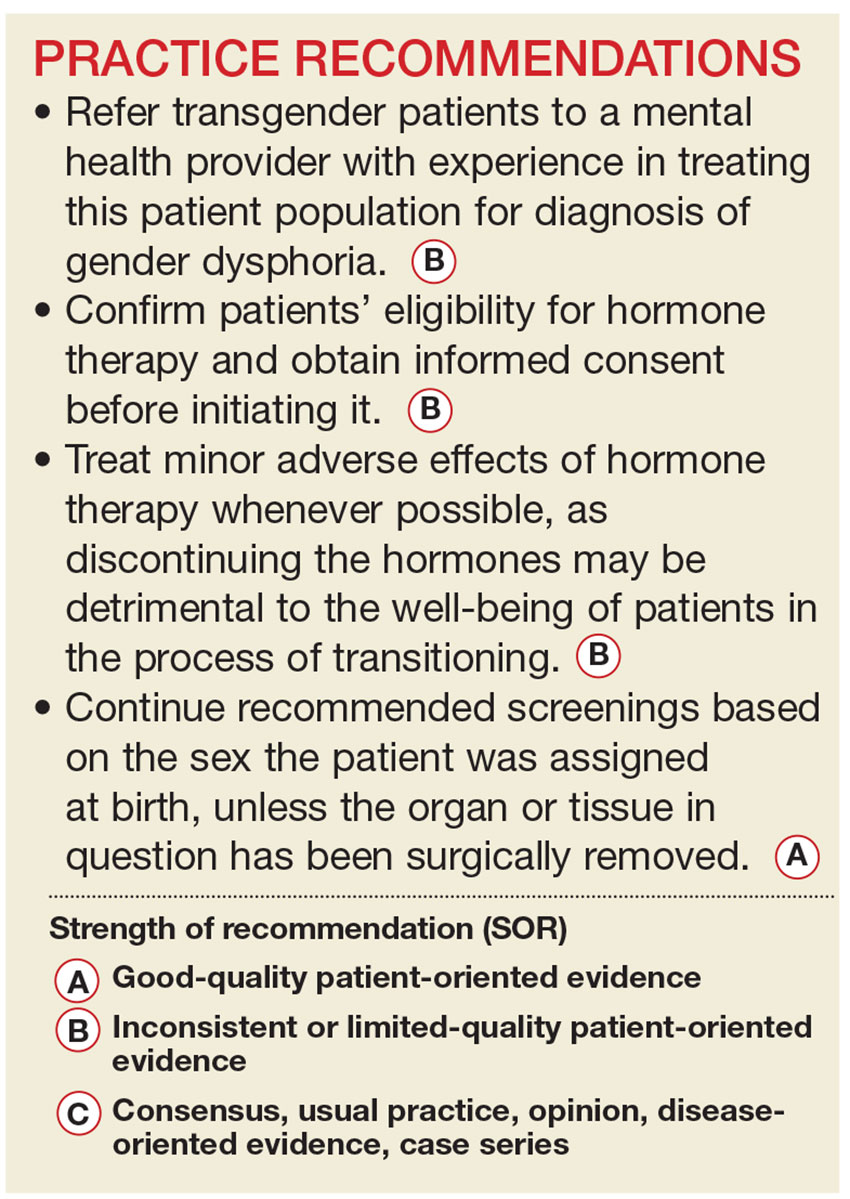
Primary care providers are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (see Table 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.
UNDERSTANDING AND DIAGNOSING GENDER DYSPHORIA
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed (DSM-5)8 and the International Classification of Diseases, 10th Rev (ICD-10).9
ICD-10 criteria use the term gender identity disorder; DSM-5 refers to gender dysphoria instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet six or more of the following for at least six months
- A repeatedly stated desire to be, or insistence that he or she is, of the other gender
- In boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- Strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- A strong rejection of toys/games typically associated with the child’s sex
- Intense desire to participate in stereotypical games and pastimes of the other gender
- Strong preference for playmates of the other gender
- A strong dislike of one’s sexual anatomy
- A strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet two or more of the following for at least six months
- A noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- An intense need to do away with (or prevent) his or her primary or secondary sex features
- An intense desire to have the primary and/or secondary sex features of the other gender
- A deep desire to transform into another gender
- A profound need for society to treat them as someone of the other gender
- A powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care provider to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not an MHP confirms a diagnosis of GD, it is still up to the treating provider to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented real-life experience, defined as having fully adopted the new gender role in everyday life for at least three months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated (see “How to Help Transgender Teens”).11
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
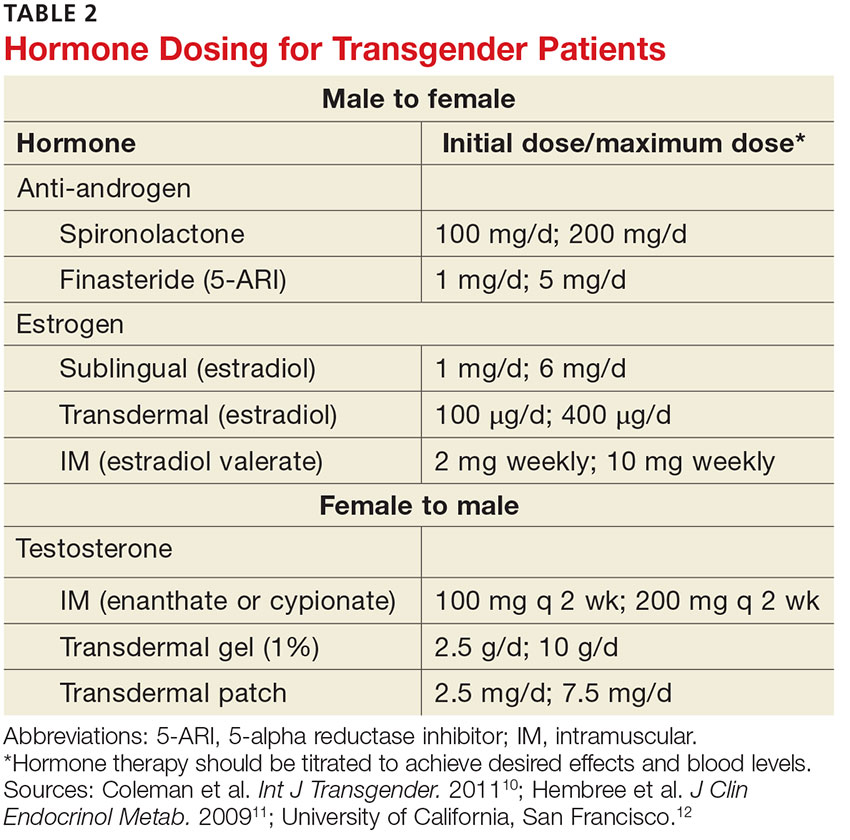
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychologic more congruent. Hormone treatment (see Table 2) is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body.10,11,21 Psychologic conditions, such as depression, tend to improve as the transitioning process gets underway.22
FEMALE-TO-MALE TRANSITION
CASE 1 Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care provider for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the past several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.
Physical changes induced by masculinizing hormone therapy have an expected onset of one to six months and achieve maximum effect in approximately two to five years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk for pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk for cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks/benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit > 55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every three months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for > 6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with an MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice, and greater muscle mass, among others—and review the likely time frame.
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of an MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
MALE-TO-FEMALE TRANSITION
CASE 2 Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history but reports that she has been taking spironolactone and estrogen for the past three years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between three months and five years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-α reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-α-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (< 200 pg/mL) and the level of testosterone should be in the normal female range (< 55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk for adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients taking feminizing hormones (transwomen) should be seen every two to three months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every three months; serum electrolytes, specifically potassium, should be monitored every two to three months in the first year until stable.
CASE 2 You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in six months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at age 40 to 50 (as it should for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available, as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed January 13, 2017.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed January 13, 2017.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed January 13, 2017.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed January 13, 2017.
6. Istar A. How queer! The development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013: 451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011; 13:165-232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endo Metabol. 2009;94:3132-3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed January 13, 2017.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S36.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed January 13, 2017.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed January 13, 2017.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed January 13, 2017.
18. TransYouth Family Allies. Empowering transgender youth & families. www.imatyfa.org/. Accessed January 13, 2017.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed January 13, 2017.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. www.glbthotline.org. Accessed January 13, 2017.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed January 13, 2017.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008; 5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of Thermablate Endometrial Ablation System as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed January 13, 2017.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995; 82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed January 13, 2017.

Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Primary care providers are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (see Table 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.

UNDERSTANDING AND DIAGNOSING GENDER DYSPHORIA
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed (DSM-5)8 and the International Classification of Diseases, 10th Rev (ICD-10).9
ICD-10 criteria use the term gender identity disorder; DSM-5 refers to gender dysphoria instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet six or more of the following for at least six months
- A repeatedly stated desire to be, or insistence that he or she is, of the other gender
- In boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- Strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- A strong rejection of toys/games typically associated with the child’s sex
- Intense desire to participate in stereotypical games and pastimes of the other gender
- Strong preference for playmates of the other gender
- A strong dislike of one’s sexual anatomy
- A strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet two or more of the following for at least six months
- A noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- An intense need to do away with (or prevent) his or her primary or secondary sex features
- An intense desire to have the primary and/or secondary sex features of the other gender
- A deep desire to transform into another gender
- A profound need for society to treat them as someone of the other gender
- A powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care provider to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not an MHP confirms a diagnosis of GD, it is still up to the treating provider to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented real-life experience, defined as having fully adopted the new gender role in everyday life for at least three months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated (see “How to Help Transgender Teens”).11
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychologic more congruent. Hormone treatment (see Table 2) is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body.10,11,21 Psychologic conditions, such as depression, tend to improve as the transitioning process gets underway.22

FEMALE-TO-MALE TRANSITION
CASE 1 Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care provider for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the past several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.
Physical changes induced by masculinizing hormone therapy have an expected onset of one to six months and achieve maximum effect in approximately two to five years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk for pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk for cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks/benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit > 55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every three months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for > 6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with an MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice, and greater muscle mass, among others—and review the likely time frame.
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of an MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
MALE-TO-FEMALE TRANSITION
CASE 2 Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history but reports that she has been taking spironolactone and estrogen for the past three years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between three months and five years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-α reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-α-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (< 200 pg/mL) and the level of testosterone should be in the normal female range (< 55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk for adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients taking feminizing hormones (transwomen) should be seen every two to three months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every three months; serum electrolytes, specifically potassium, should be monitored every two to three months in the first year until stable.
CASE 2 You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in six months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at age 40 to 50 (as it should for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available, as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.

Civil rights for the lesbian, gay, bisexual, and transgender population have advanced markedly in the past decade, and the medical community has gradually begun to address more of their health concerns. More recently, media attention to transgender individuals has encouraged many more to openly seek care.1,2
It is estimated that anywhere from 0.3% to 5% of the US population identifies as transgender.1-3 While awareness of this population has slowly increased, there is a paucity of research on the hormone treatment that is often essential to patients’ well-being. Studies of surgical options for transgender patients have been minimal, as well.
Primary care providers are uniquely positioned to coordinate medical services and ensure continuity of care for transgender patients as they strive to become their authentic selves. Our goal in writing this article is to equip you with the tools to provide this patient population with sensitive, high-quality care (see Table 1).4-7 Our focus is on the diagnosis of gender dysphoria (GD) and its medical and hormonal management—the realm of primary care providers. We briefly discuss surgical management of GD, as well.

UNDERSTANDING AND DIAGNOSING GENDER DYSPHORIA
Two classification systems are used for diagnoses related to GD: the Diagnostic and Statistical Manual of Mental Disorders, Fifth Ed (DSM-5)8 and the International Classification of Diseases, 10th Rev (ICD-10).9
ICD-10 criteria use the term gender identity disorder; DSM-5 refers to gender dysphoria instead. It is important to emphasize that these classification systems represent an attempt to categorize a group of signs and symptoms that lead to distress for the patient and are not meant to suggest that being transgender is pathological. In fact, in DSM-5—released in 2013—the American Psychiatric Association revised the terminology to emphasize that such individuals are not “disordered” by the nature of their identity, but rather by the distress that being transgender causes.8
For a diagnosis of GD in children, DSM-5 criteria include characteristics perceived to be incongruent between the child’s sex at birth and the self-identified gender based on preferred activities or dislike of his or her own sexual anatomy. The child must meet six or more of the following for at least six months
- A repeatedly stated desire to be, or insistence that he or she is, of the other gender
- In boys, a preference for cross-dressing or simulating female attire; in girls, insistence on wearing only stereotypical masculine clothing
- Strong and persistent preferences for cross-gender roles in make-believe play or fantasy
- A strong rejection of toys/games typically associated with the child’s sex
- Intense desire to participate in stereotypical games and pastimes of the other gender
- Strong preference for playmates of the other gender
- A strong dislike of one’s sexual anatomy
- A strong desire for the primary (eg, penis or vagina) or secondary (eg, menstruation) sex characteristics of the other gender.8
Adolescents and adults must meet two or more of the following for at least six months
- A noticeable incongruence between the gender that the patient sees themselves as and their sex characteristics
- An intense need to do away with (or prevent) his or her primary or secondary sex features
- An intense desire to have the primary and/or secondary sex features of the other gender
- A deep desire to transform into another gender
- A profound need for society to treat them as someone of the other gender
- A powerful assurance of having the characteristic feelings and responses of the other gender.8
For children as well as adolescents and adults, the condition should cause the patient significant distress or significantly affect him or her socially, at work or school, and in other important areas of life.8
Is the patient a candidate for hormone therapy?
Two primary sources—Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7, issued by the World Professional Association for Transgender Health (WPATH)10 and Endocrine Treatment of Transsexual Persons11 by the Endocrine Society—offer clinical practice guidance based on evidence and expert opinion.
WPATH recommends that a mental health professional (MHP) experienced in transgender care diagnose GD to ensure that it is not mistaken for a psychiatric condition manifesting as altered gender identity. However, if no one with such experience is available or accessible in the region, it is reasonable for a primary care provider to make the diagnosis and consider initiating hormone therapy without a mental health referral,12 as the expected benefits outweigh the risks of nontreatment.13
Whether or not an MHP confirms a diagnosis of GD, it is still up to the treating provider to confirm the patient’s eligibility and readiness for hormone therapy: He or she should meet DSM-5 or ICD-10 criteria for GD, have no psychiatric comorbidity (eg, schizophrenia, body dysmorphic disorder, or uncontrolled bipolar disorder) likely to interfere with treatment, understand the expected outcomes and the social benefits and risks, and have indicated a willingness to take the hormones responsibly.
Historically, patients were required to have a documented real-life experience, defined as having fully adopted the new gender role in everyday life for at least three months.10,11 This model has fallen out of favor, however, as it is unsupported by evidence and may place transgender individuals at physical and emotional risk. Instead, readiness is confirmed by obtaining informed consent.12
Puberty may be suppressed with a gonadotropin-releasing hormone (GnRH) agonist in adolescents who have a GD diagnosis and are at Tanner stage 2 to 3 of puberty until age 16. At that point, hormone therapy consistent with their gender identification may be initiated (see “How to Help Transgender Teens”).11
Beginning the transition
The transitioning process is a complex and individualized journey that can include inward or outward change, or both.
For patients interested in medical interventions, possible therapies include cross-sex hormone administration and gender-affirming surgery. Both are aimed at making the physical and the psychologic more congruent. Hormone treatment (see Table 2) is often essential to reduce the distress of individuals with GD and to help them feel comfortable in their own body.10,11,21 Psychologic conditions, such as depression, tend to improve as the transitioning process gets underway.22

FEMALE-TO-MALE TRANSITION
CASE 1 Jennie R, a 55-year-old postmenopausal patient, comes to your office for an annual exam. Although you’ve been her primary care provider for several years, she confides for the first time that she has never been comfortable as a woman. “I’ve always felt that my body didn’t belong to me,” the patient admits, and goes on to say that for the past several years she has been living as a man. Jennie R says she is ready to start hormone therapy to assist with the gender transition and asks about the process, the benefits and risks, and how quickly she can expect to achieve the desired results.
If Jennie R were your patient, how would you respond?
Masculinizing hormone treatment
As you would explain to a patient like Jennie R, the goal of hormone therapy is to suppress the effects of the sex assigned at birth and replace them with those of the desired gender. In the case of a female transitioning to a male (known as a transman), masculinizing hormones would promote growth of facial and body hair, cessation of menses, increased muscle mass, deepening of the voice, and clitoral enlargement.
Physical changes induced by masculinizing hormone therapy have an expected onset of one to six months and achieve maximum effect in approximately two to five years.10,11 Although there have been no controlled clinical trials evaluating the safety or efficacy of any transitional hormone regimen, WPATH and the Center of Excellence for Transgender Health at the University of California, San Francisco, suggest initiating intramuscular or transdermal testosterone at increasing doses until normal physiologic male testosterone levels between 350 and 700 ng/dL are achieved, or until cessation of menses.13,25-28 The dose at which either, or both, occur should be continued as long-term maintenance therapy. Medroxyprogesterone can be added, if necessary for menstrual cessation, and a GnRH agonist or endometrial ablation can be used for refractory uterine bleeding.29,30
Testosterone is not a contraceptive. It is important to emphasize to transmen like Jennie that they remain at risk for pregnancy if they are having sex with fertile males. Caution patients not to assume that the possibility of pregnancy ends when menses stop.
Treat minor adverse effects. Adverse effects of masculinizing hormones include vaginal atrophy, fat redistribution and weight gain, polycythemia, acne, scalp hair loss, sleep apnea, elevated liver enzymes, hyperlipidemia, cardiovascular disease, diabetes, and bone density loss. Increased risk for cancer of the female organs has not been proven.10,11 It is reasonable to treat minor adverse effects after reviewing the risks/benefits of doing so, as discontinuing hormone therapy could be detrimental to the well-being of transitioning patients.11
There are absolute contraindications to masculinizing hormone therapy, however, including pregnancy, unstable coronary artery disease, and untreated polycythemia with a hematocrit > 55%.10
Monitoring is essential. Patients receiving masculinizing hormone therapy should be monitored every three months during the first year and once or twice a year thereafter, with a focused history (including mood symptoms), physical exam (including weight and blood pressure), and labs (including complete blood count, liver function, renal function, and lipids) at each visit.11,23 Some clinicians also check estradiol levels until they fall below 50 pg/mL,23,27 while others take the cessation of uterine bleeding for > 6 months as an indicator of estrogen suppression.
Preventive health measures continue. Routine screening should continue, based on the patient’s assigned sex at birth. Thus, a transman who has not had a hysterectomy still needs Pap smears, mammograms if the patient has not had a double mastectomy, and bone mineral density (BMD) testing to screen for osteoporosis.31,32 Some experts recommend starting to test BMD at age 50 for patients receiving masculinizing hormones, given the unknown effect of testosterone on bone density.11,31,32
CASE 1 The first question for a transgender patient is about his or her current gender identity, but Jennie R has already reported living as a man. So you start by asking “What name do you prefer to use?” and “Do you prefer to be referred to with male or female pronouns?”
The patient tells you that he sees himself as a man, he wants to be called Jeff, and he prefers male pronouns. You explain that you believe he has gender dysphoria and would benefit from hormone therapy, but it is important to confirm this diagnosis with an MHP. You explain that testosterone can be prescribed for masculinizing effects, and describe the expected effects—more facial and body hair, a deeper voice, and greater muscle mass, among others—and review the likely time frame.
You also discuss the risks of masculinizing hormones (hyperlipidemia, cardiovascular disease, diabetes, and loss of bone density) that will need to be monitored. Before he leaves, you give him the name of an MHP who is experienced in transgender care and tell him to make a follow-up appointment with you after he has seen her. At the conclusion of the visit, you make a note of the patient’s name and gender identity in the chart and inform the staff of the changes.
MALE-TO-FEMALE TRANSITION
CASE 2 Before heading into your office to talk to a new patient named Carl S, you glance at his chart and see that he is a healthy 21-year-old who has come in for a routine physical. When you enter the room, you find Carl wearing a dress, heels, and make-up. After confirming that you have the right patient, you ask, “What is your current gender identity?” “Female,” says Carl, who indicates that she now goes by Carol. The patient has no medical problems, surgical history, or significant family history but reports that she has been taking spironolactone and estrogen for the past three years. Carol also says she has a new female partner and is having unprotected sexual activity.
Feminizing hormone treatment
The desired effects of feminizing hormones include voice change, decreased hair growth, breast growth, body fat redistribution, decreased muscle mass, skin softening, decreased oiliness of skin and hair, and a decrease in spontaneous erections, testicular volume, and sperm production.10,11 The onset of feminizing effects ranges from one month to one year and the expected maximum effect occurs anywhere between three months and five years.10,11 Regimens usually include anti-androgen agents and estrogen.13,26-28
The medications that have been most studied with anti-androgenic effects include spironolactone and 5-α reductase inhibitors (5-ARIs) such as finasteride. Spironolactone inhibits testosterone secretion and inhibits androgen binding to androgen receptors; 5-ARIs block the conversion of testosterone to 5-α-dihydrotestosterone, the more active form.
Estrogen can be administered via oral, sublingual, transdermal, or intramuscular route, but parenteral formulations are preferred to avoid first-pass metabolism. The serum estradiol target is similar to the mean daily level of premenopausal women (< 200 pg/mL) and the level of testosterone should be in the normal female range (< 55 ng/dL).13,26-28
The selection of medications should be individualized for each patient. Comorbidities must be considered, as well as the risk for adverse effects, which include venous thromboembolism, elevated liver enzymes, breast cancer, cardiovascular disease, diabetes, hyperprolactinemia, weight gain, gallstones, cerebrovascular disease, and severe migraine headaches.10,11 Estrogen therapy is not reported to induce hypertrophy or premalignant changes in the prostate.33 As is the case for masculinizing hormones, feminizing hormone therapy should be continued indefinitely for long-term effects.
Frequent monitoring is recommended. Patients taking feminizing hormones (transwomen) should be seen every two to three months in the first year and monitored once or twice a year thereafter. Serum testosterone and estradiol levels should initially be monitored every three months; serum electrolytes, specifically potassium, should be monitored every two to three months in the first year until stable.
CASE 2 You recommend that Carol S be screened annually for sexually transmitted diseases, as you would for any 21-year-old patient. You point out, too, that while estrogen and androgen-suppressing therapy decrease sperm production, there is a possibility that the patient could impregnate a female partner and recommend that contraception be used if the couple is not trying to conceive.
You also discuss the risks and benefits of hormone therapy and reasonable expectations of continued treatment. You ask Carol to schedule a follow-up visit in six months, as her hormone regimen is stable. Finally, if the patient remains on hormone therapy, you mention that the only screening unique to men transitioning to women is for breast cancer, which should begin at age 40 to 50 (as it should for all women).
Gender-affirming surgical options
Surgical management of transgender patients is not within the scope of family medicine. But it is essential to know what procedures are available, as you may have occasion to advocate for patients during the surgical referral process and possibly to provide postoperative care.
For transmen, surgical options include chest reconstruction, hysterectomy/oophorectomy, metoidioplasty (using the clitoris to surgically approximate a penis), phalloplasty, scrotoplasty, urethroplasty, and vaginectomy.10,34 The surgeries available for transwomen are orchiectomy, vaginoplasty, penectomy, breast augmentation, thyroid chondroplasty and voice surgery, and facial feminization.10,34 Keep in mind that not all transgender individuals desire surgery as part of the transitioning process.
The authors would like to acknowledge the assistance of Michelle Forcier, MD, MPH, and Karen S. Bernstein, MD, MPH, in the preparation of this manuscript.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed January 13, 2017.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed January 13, 2017.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed January 13, 2017.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed January 13, 2017.
6. Istar A. How queer! The development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013: 451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011; 13:165-232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endo Metabol. 2009;94:3132-3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed January 13, 2017.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S36.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed January 13, 2017.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed January 13, 2017.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed January 13, 2017.
18. TransYouth Family Allies. Empowering transgender youth & families. www.imatyfa.org/. Accessed January 13, 2017.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed January 13, 2017.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. www.glbthotline.org. Accessed January 13, 2017.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed January 13, 2017.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008; 5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of Thermablate Endometrial Ablation System as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed January 13, 2017.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995; 82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed January 13, 2017.
1. Pew Research Center. A survey of LGBT Americans: attitudes, experiences and values in changing times. www.pewsocialtrends.org/2013/06/13/a-survey-of-lgbt-americans. Accessed January 13, 2017.
2. Gates GJ. How many people are lesbian, gay, bisexual and transgender? http://williamsinstitute.law.ucla.edu/wp-content/uploads/Gates-How-Many-People-LGBT-Apr-2011.pdf. Accessed January 13, 2017.
3. van Kesteren PJ, Gooren LJ, Megens JA. An epidemiological and demographic study of transsexuals in The Netherlands. Arch Sex Behav. 1996;25:589-600.
4. Bhola S. An ally’s guide to terminology: talking about LGBT people & equality. www.glaad.org/2011/07/28/an-allys-guide-to-terminology-talking-about-lgbt-people-equality. Accessed January 13, 2017.
5. University of California, San Francisco. Transgender terminology. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-terminology. Accessed January 13, 2017.
6. Istar A. How queer! The development of gender identity and sexual orientation in LGBTQ-headed families. Fam Process. 2010;49:268-290.
7. Goins ES, Pye D. Check the box that best describes you: reflexively managing theory and praxis in LGBTQ health communication research. Health Commun. 2013;28:397-407.
8. American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013: 451-459.
9. World Health Organization. The International Classification of Diseases, 10th rev. Classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. 1992; Geneva.
10. Coleman E, Bockting W, Botzer M, et al; World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgender. 2011; 13:165-232.
11. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endo Metabol. 2009;94:3132-3154.
12. University of California, San Francisco. Assessing readiness for hormones. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/tcoe?page=protocol-hormone-ready. Accessed January 13, 2017.
13. Gooren L. Hormone treatment of the adult transsexual patient. Horm Res. 2005;64(suppl 2):S31-S36.
14. Hembree WC. Guidelines for pubertal suspension and gender reassignment for transgender adolescents. Child Adolesc Psychiatr Clin N Am. 2011;20:725-732.
15. Gay, Lesbian, and Straight Education Network (GLSEN). Harsh realities. The experiences of transgender youth in our nation’s schools. www.glsen.org/sites/default/files/Harsh%20Realities.pdf. Accessed January 13, 2017.
16. Berman M, Balingit M. Eleven states sue Obama administration over bathroom guidance for transgender students. May 25, 2016. Washington Post. www.washingtonpost.com/news/post-nation/wp/2016/05/25/texas-governor-says-state-will-sue-obama-administration-over-bathroom-directive/. Accessed January 13, 2017.
17. de Vries AL, Cohen-Kettenis PT, Delemarre-van de Waal H. Clinical management of gender dysphoria in adolescents. 2006. Vancouver Coastal Health - Transgender Health Program. www.amsa.org/wp-content/uploads/2015/04/CaringForTransgenderAdolescents.pdf. Accessed January 13, 2017.
18. TransYouth Family Allies. Empowering transgender youth & families. www.imatyfa.org/. Accessed January 13, 2017.
19. Human Rights Campaign. On our own: a survival guide for independent LGBTQ youth. www.hrc.org/resources/on-our-own-a-survival-guide-for-independent-lgbtq-youth. Accessed January 13, 2017.
20. Gay, Lesbian, Bisexual, and Transgender National Help Center. www.glbthotline.org. Accessed January 13, 2017.
21. University of California, San Francisco. Hormone administration. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-hormones. Accessed January 13, 2017.
22. Gorin-Lazard A, Baumstarck K, Boyer L, et al. Hormonal therapy is associated with better self-esteem, mood, and quality of life in transsexuals. J Nerv Ment Dis. 2013;201:996-1000.
23. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010.
24. Boloña ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20-28.
25. Gooren LJ, Giltay EJ. Review of studies of androgen treatment of female-to-male transsexuals: effects and risks of administration of androgens to females. J Sex Med. 2008; 5:765-776.
26. Levy A, Crown A, Reid R. Endocrine intervention for transsexuals. Clin Endocrinol (Oxf). 2003;59:409-418.
27. Moore E, Wisniewski A, Dobs A. Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects. J Clin Endocrinol Metab. 2003;88:3467-3473.
28. Tangpricha V, Ducharme SH, Barber TW, et al. Endocrinologic treatment of gender identity disorders. Endocr Pract. 2003;9:12-21.
29. Dickersin K, Munro MG, Clark M, et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2007;110:1279-1289.
30. Prasad P, Powell MC. Prospective observational study of Thermablate Endometrial Ablation System as an outpatient procedure. J Minim Invasive Gynecol. 2008;15:476-479.
31. University of California, San Francisco. General prevention and screening. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-screening. Accessed January 13, 2017.
32. Ganly I, Taylor EW. Breast cancer in a trans-sexual man receiving hormone replacement therapy. Br J Surg. 1995; 82:341.
33. Meriggiola MC, Gava G. Endocrine care of transpeople part II: a review of cross-sex hormonal treatments, outcomes and adverse effects in transwomen. Clin Endocrinol (Oxf). 2015;83:607-615.
34. University of California, San Francisco. Surgical options. UCSF Center of Excellence for Transgender Health. http://transhealth.ucsf.edu/trans?page=protocol-surgery. Accessed January 13, 2017.
Critical Care Commentary: Highlights from the 2016 hospital-acquired and ventilator-associated pneumonia guideline
The 2016 hospital-acquired and ventilator-associated pneumonia guidelines, sponsored by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS), and endorsed by the American College of Chest Physicians (CHEST), Society of Critical Care Medicine (SCCM), and the Society for Healthcare Epidemiology, was published recently (Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63[5]:575-82).
This Critical Care Commentary aims to provide the highlights of the new guideline and to motivate readers to read the complete report that best represents the primary intent of the guideline panelists.
The new guideline was written using the Grading of Recommendations Assessment, Development, and Evaluation methodology. This was the framework to address all clinical questions referred to as PICOs (patient; intervention; comparator; outcome), which can be explicitly seen in the published guideline. For every PICO question, the wording “we suggest” was used for a weak recommendation (lack of high confidence; further evidence could change it), and “we recommend” was used for a strong recommendation (high confidence; further evidence is unlikely to change it). Also, part of the panel framework was the requirement to disclose any actual, potential, or perceived conflicts of interest for each panelist to be accepted to participate, as well as to remain in the panel for the duration of the process. The cochairs remained free of any financial conflicts during the entire process.
Choosing an empiric antibiotic regimen for patients with HAP and VAP requires balancing the potentially competing goals of ensuring that likely infecting pathogens are covered while avoiding excess antibiotic use. In order to guide clinicians on empiric antibiotic therapy, the panel performed a comprehensive review of the potential risk factors for HAP and VAP. For VAP, three factors associated with disease severity (septic shock at time of VAP, ARDS preceding VAP, and acute renal replacement prior to VAP onset) and two epidemiologic factors (prior use of IV antibiotic use within 90 days, and 5 or more days of hospitalization prior to the occurrence of VAP) made the final risk factors list. For HAP, only the prior use of IV antibiotics within 90 days was associated with risk for MDR. However, because of the limitations and small number of studies on HAP only, the panel decided to add risk factors for mortality (ventilator support for HAP and septic shock) as surrogates for MDR risk factors in patients with HAP, as these factors presumably increase the risk of poor outcomes if there is initial inadequate empiric therapy.
In conjunction with the bedside evaluation of risk factors for MDR, the guideline recommends the use of local antibiograms not only to guide empiric therapy but also to decide if antibiotic coverage for MDR is needed. Ideally, the antibiogram should be based on the specific ICU, but if this is not feasible, or the hospital is of small size, an institutional antibiogram can also be helpful. The first benefit of local antibiograms is derived from the knowledge gained regarding the prevalence of each microorganism; for example, if only 3% of all VAP or HAP in a given unit or hospital is caused by Pseudomonas aeruginosa, it is likely that an empiric coverage for this microorganism will neither be necessary nor appropriate for most patients. The second benefit is derived from the knowledge concerning the frequency of MDR microorganisms within the unit or hospital: for example, patients with VAP in units where 10%-20% of Staphylococcus aureus isolates are resistant to methicillin, or greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy, should receive antibiotics for MDR infections. With these two critical pieces of information, the clinician will have a higher probability of starting the correct empiric antibiotics, and, consequently, improve the survival outcomes of patients with HAP and VAP.
The choice of the empirical treatment of VAP and HAP becomes a natural derivation of the three main factors discussed above: (1) epidemiologic history of antibiotics’ use and prior hospitalization length, (2) local antibiogram for the prevalence and resistance of microorganisms, and (3) disease severity and risk of mortality by the identification of septic shock, ARDS, and acute renal replacement therapy. For example, if 17% of all VAPs in your unit is from P aeruginosa (which is the national prevalence in patients with VAP), and 8% of these strains are resistant to an agent being considered for gram-negative monotherapy, not prescribing double coverage for P aeruginosa would still result in initial appropriate therapy in 98.6% (derived from 1-[0.17 x 0.08]) of cases. The reason why the panelists chose the threshold of 10% for P aeruginosa, and 10%-20% for S aureus, was based on the national prevalence rates reported by the Centers for Disease Control and Prevention, with the goal of limiting the initial inappropriate antibiotic therapy decision to less than 5% of all cases. We strongly believe that this “epidemiologic/antibiogram/disease severity” approach to select the empiric therapy is both clinically intuitive and essential to improve patients’ outcomes. Further, this approach will substantially reduce the unnecessary use of double antibiotic therapy in patients with VAP or HAP.
This guideline suggests that the use of inhaled antibiotic therapy in conjunction with IV antibiotics may benefit patients with VAP or HAP from MDR microorganisms that are sensitive to only polymyxins or aminoglycosides. The panel also suggested that the use of pharmacokinetic and pharmacodynamics should be used to optimize the administration of antibiotic therapy for all patients with HAP or VAP.
Last, after an extensive review and multiple analyses of all available evidence, the panel concluded that the majority of patients with HAP or VAP should be treated with 7 days of therapy, independent of the microorganism causing the pneumonia. In several meta-analyses performed by the panelists to evaluate all patients with VAP, as well as only patients with VAP caused by nonfermenting gram-negative organisms such as Pseudomonas species, Stenotrophomonas species, and Acinetobacter species, the panel did not find differences between short and long courses of antibiotics regarding mortality, clinical cure, pneumonia recurrence, and mechanical ventilation duration. In recognition of the individual needs of each patient, we made a remark that shorter or longer duration of antibiotics may be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters. Several adjunctive methods of deescalation were assessed, but only procalcitonin was suggested to aid health care providers to shorten the course of antibiotic therapy.
In conclusion, the authors of this 2016 HAP/VAP IDSA/ATS guideline hope to achieve the ultimate goal of improving the treatment and outcomes of patients with HAP and VAP and reducing unnecessary antibiotic use.
Dr. Kalil is with the department of internal medicine, division of infectious diseases, University of Nebraska Medical Center, Omaha; Dr. Metersky is with the division of pulmonary and critical care medicine, University of Connecticut, Farmington.
The 2016 hospital-acquired and ventilator-associated pneumonia guidelines, sponsored by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS), and endorsed by the American College of Chest Physicians (CHEST), Society of Critical Care Medicine (SCCM), and the Society for Healthcare Epidemiology, was published recently (Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63[5]:575-82).
This Critical Care Commentary aims to provide the highlights of the new guideline and to motivate readers to read the complete report that best represents the primary intent of the guideline panelists.
The new guideline was written using the Grading of Recommendations Assessment, Development, and Evaluation methodology. This was the framework to address all clinical questions referred to as PICOs (patient; intervention; comparator; outcome), which can be explicitly seen in the published guideline. For every PICO question, the wording “we suggest” was used for a weak recommendation (lack of high confidence; further evidence could change it), and “we recommend” was used for a strong recommendation (high confidence; further evidence is unlikely to change it). Also, part of the panel framework was the requirement to disclose any actual, potential, or perceived conflicts of interest for each panelist to be accepted to participate, as well as to remain in the panel for the duration of the process. The cochairs remained free of any financial conflicts during the entire process.
Choosing an empiric antibiotic regimen for patients with HAP and VAP requires balancing the potentially competing goals of ensuring that likely infecting pathogens are covered while avoiding excess antibiotic use. In order to guide clinicians on empiric antibiotic therapy, the panel performed a comprehensive review of the potential risk factors for HAP and VAP. For VAP, three factors associated with disease severity (septic shock at time of VAP, ARDS preceding VAP, and acute renal replacement prior to VAP onset) and two epidemiologic factors (prior use of IV antibiotic use within 90 days, and 5 or more days of hospitalization prior to the occurrence of VAP) made the final risk factors list. For HAP, only the prior use of IV antibiotics within 90 days was associated with risk for MDR. However, because of the limitations and small number of studies on HAP only, the panel decided to add risk factors for mortality (ventilator support for HAP and septic shock) as surrogates for MDR risk factors in patients with HAP, as these factors presumably increase the risk of poor outcomes if there is initial inadequate empiric therapy.
In conjunction with the bedside evaluation of risk factors for MDR, the guideline recommends the use of local antibiograms not only to guide empiric therapy but also to decide if antibiotic coverage for MDR is needed. Ideally, the antibiogram should be based on the specific ICU, but if this is not feasible, or the hospital is of small size, an institutional antibiogram can also be helpful. The first benefit of local antibiograms is derived from the knowledge gained regarding the prevalence of each microorganism; for example, if only 3% of all VAP or HAP in a given unit or hospital is caused by Pseudomonas aeruginosa, it is likely that an empiric coverage for this microorganism will neither be necessary nor appropriate for most patients. The second benefit is derived from the knowledge concerning the frequency of MDR microorganisms within the unit or hospital: for example, patients with VAP in units where 10%-20% of Staphylococcus aureus isolates are resistant to methicillin, or greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy, should receive antibiotics for MDR infections. With these two critical pieces of information, the clinician will have a higher probability of starting the correct empiric antibiotics, and, consequently, improve the survival outcomes of patients with HAP and VAP.
The choice of the empirical treatment of VAP and HAP becomes a natural derivation of the three main factors discussed above: (1) epidemiologic history of antibiotics’ use and prior hospitalization length, (2) local antibiogram for the prevalence and resistance of microorganisms, and (3) disease severity and risk of mortality by the identification of septic shock, ARDS, and acute renal replacement therapy. For example, if 17% of all VAPs in your unit is from P aeruginosa (which is the national prevalence in patients with VAP), and 8% of these strains are resistant to an agent being considered for gram-negative monotherapy, not prescribing double coverage for P aeruginosa would still result in initial appropriate therapy in 98.6% (derived from 1-[0.17 x 0.08]) of cases. The reason why the panelists chose the threshold of 10% for P aeruginosa, and 10%-20% for S aureus, was based on the national prevalence rates reported by the Centers for Disease Control and Prevention, with the goal of limiting the initial inappropriate antibiotic therapy decision to less than 5% of all cases. We strongly believe that this “epidemiologic/antibiogram/disease severity” approach to select the empiric therapy is both clinically intuitive and essential to improve patients’ outcomes. Further, this approach will substantially reduce the unnecessary use of double antibiotic therapy in patients with VAP or HAP.
This guideline suggests that the use of inhaled antibiotic therapy in conjunction with IV antibiotics may benefit patients with VAP or HAP from MDR microorganisms that are sensitive to only polymyxins or aminoglycosides. The panel also suggested that the use of pharmacokinetic and pharmacodynamics should be used to optimize the administration of antibiotic therapy for all patients with HAP or VAP.
Last, after an extensive review and multiple analyses of all available evidence, the panel concluded that the majority of patients with HAP or VAP should be treated with 7 days of therapy, independent of the microorganism causing the pneumonia. In several meta-analyses performed by the panelists to evaluate all patients with VAP, as well as only patients with VAP caused by nonfermenting gram-negative organisms such as Pseudomonas species, Stenotrophomonas species, and Acinetobacter species, the panel did not find differences between short and long courses of antibiotics regarding mortality, clinical cure, pneumonia recurrence, and mechanical ventilation duration. In recognition of the individual needs of each patient, we made a remark that shorter or longer duration of antibiotics may be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters. Several adjunctive methods of deescalation were assessed, but only procalcitonin was suggested to aid health care providers to shorten the course of antibiotic therapy.
In conclusion, the authors of this 2016 HAP/VAP IDSA/ATS guideline hope to achieve the ultimate goal of improving the treatment and outcomes of patients with HAP and VAP and reducing unnecessary antibiotic use.
Dr. Kalil is with the department of internal medicine, division of infectious diseases, University of Nebraska Medical Center, Omaha; Dr. Metersky is with the division of pulmonary and critical care medicine, University of Connecticut, Farmington.
The 2016 hospital-acquired and ventilator-associated pneumonia guidelines, sponsored by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS), and endorsed by the American College of Chest Physicians (CHEST), Society of Critical Care Medicine (SCCM), and the Society for Healthcare Epidemiology, was published recently (Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63[5]:575-82).
This Critical Care Commentary aims to provide the highlights of the new guideline and to motivate readers to read the complete report that best represents the primary intent of the guideline panelists.
The new guideline was written using the Grading of Recommendations Assessment, Development, and Evaluation methodology. This was the framework to address all clinical questions referred to as PICOs (patient; intervention; comparator; outcome), which can be explicitly seen in the published guideline. For every PICO question, the wording “we suggest” was used for a weak recommendation (lack of high confidence; further evidence could change it), and “we recommend” was used for a strong recommendation (high confidence; further evidence is unlikely to change it). Also, part of the panel framework was the requirement to disclose any actual, potential, or perceived conflicts of interest for each panelist to be accepted to participate, as well as to remain in the panel for the duration of the process. The cochairs remained free of any financial conflicts during the entire process.
Choosing an empiric antibiotic regimen for patients with HAP and VAP requires balancing the potentially competing goals of ensuring that likely infecting pathogens are covered while avoiding excess antibiotic use. In order to guide clinicians on empiric antibiotic therapy, the panel performed a comprehensive review of the potential risk factors for HAP and VAP. For VAP, three factors associated with disease severity (septic shock at time of VAP, ARDS preceding VAP, and acute renal replacement prior to VAP onset) and two epidemiologic factors (prior use of IV antibiotic use within 90 days, and 5 or more days of hospitalization prior to the occurrence of VAP) made the final risk factors list. For HAP, only the prior use of IV antibiotics within 90 days was associated with risk for MDR. However, because of the limitations and small number of studies on HAP only, the panel decided to add risk factors for mortality (ventilator support for HAP and septic shock) as surrogates for MDR risk factors in patients with HAP, as these factors presumably increase the risk of poor outcomes if there is initial inadequate empiric therapy.
In conjunction with the bedside evaluation of risk factors for MDR, the guideline recommends the use of local antibiograms not only to guide empiric therapy but also to decide if antibiotic coverage for MDR is needed. Ideally, the antibiogram should be based on the specific ICU, but if this is not feasible, or the hospital is of small size, an institutional antibiogram can also be helpful. The first benefit of local antibiograms is derived from the knowledge gained regarding the prevalence of each microorganism; for example, if only 3% of all VAP or HAP in a given unit or hospital is caused by Pseudomonas aeruginosa, it is likely that an empiric coverage for this microorganism will neither be necessary nor appropriate for most patients. The second benefit is derived from the knowledge concerning the frequency of MDR microorganisms within the unit or hospital: for example, patients with VAP in units where 10%-20% of Staphylococcus aureus isolates are resistant to methicillin, or greater than 10% of gram-negative isolates are resistant to an agent being considered for monotherapy, should receive antibiotics for MDR infections. With these two critical pieces of information, the clinician will have a higher probability of starting the correct empiric antibiotics, and, consequently, improve the survival outcomes of patients with HAP and VAP.
The choice of the empirical treatment of VAP and HAP becomes a natural derivation of the three main factors discussed above: (1) epidemiologic history of antibiotics’ use and prior hospitalization length, (2) local antibiogram for the prevalence and resistance of microorganisms, and (3) disease severity and risk of mortality by the identification of septic shock, ARDS, and acute renal replacement therapy. For example, if 17% of all VAPs in your unit is from P aeruginosa (which is the national prevalence in patients with VAP), and 8% of these strains are resistant to an agent being considered for gram-negative monotherapy, not prescribing double coverage for P aeruginosa would still result in initial appropriate therapy in 98.6% (derived from 1-[0.17 x 0.08]) of cases. The reason why the panelists chose the threshold of 10% for P aeruginosa, and 10%-20% for S aureus, was based on the national prevalence rates reported by the Centers for Disease Control and Prevention, with the goal of limiting the initial inappropriate antibiotic therapy decision to less than 5% of all cases. We strongly believe that this “epidemiologic/antibiogram/disease severity” approach to select the empiric therapy is both clinically intuitive and essential to improve patients’ outcomes. Further, this approach will substantially reduce the unnecessary use of double antibiotic therapy in patients with VAP or HAP.
This guideline suggests that the use of inhaled antibiotic therapy in conjunction with IV antibiotics may benefit patients with VAP or HAP from MDR microorganisms that are sensitive to only polymyxins or aminoglycosides. The panel also suggested that the use of pharmacokinetic and pharmacodynamics should be used to optimize the administration of antibiotic therapy for all patients with HAP or VAP.
Last, after an extensive review and multiple analyses of all available evidence, the panel concluded that the majority of patients with HAP or VAP should be treated with 7 days of therapy, independent of the microorganism causing the pneumonia. In several meta-analyses performed by the panelists to evaluate all patients with VAP, as well as only patients with VAP caused by nonfermenting gram-negative organisms such as Pseudomonas species, Stenotrophomonas species, and Acinetobacter species, the panel did not find differences between short and long courses of antibiotics regarding mortality, clinical cure, pneumonia recurrence, and mechanical ventilation duration. In recognition of the individual needs of each patient, we made a remark that shorter or longer duration of antibiotics may be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters. Several adjunctive methods of deescalation were assessed, but only procalcitonin was suggested to aid health care providers to shorten the course of antibiotic therapy.
In conclusion, the authors of this 2016 HAP/VAP IDSA/ATS guideline hope to achieve the ultimate goal of improving the treatment and outcomes of patients with HAP and VAP and reducing unnecessary antibiotic use.
Dr. Kalil is with the department of internal medicine, division of infectious diseases, University of Nebraska Medical Center, Omaha; Dr. Metersky is with the division of pulmonary and critical care medicine, University of Connecticut, Farmington.
Optimal adjuvant endocrine therapy use in breast cancer remains elusive
Appropriate use of adjuvant endocrine therapy for breast cancer improved over a 10-year period, but optimal use has not been achieved, according to findings from a retrospective review of more than 980,000 women with stages I-III breast cancer.
As a result, an estimated 14,630 lives were unnecessarily lost during the study period, according to Bobby M. Daly, MD, of Memorial Sloan Kettering Cancer Center in New York, and his colleagues.
Of the 981,729 women in the National Cancer Database from Jan. 1, 2004, to Dec. 31, 2013, who received all or part of their care at the reporting institution and who met eligibility criteria, 818,435 had hormone receptor positive (HR+) disease and 163,294 had hormone receptor negative (HR-) disease.
The percentage of HR+ patients receiving adjuvant endocrine therapy (AET) increased from 69.8% in 2004 to 82.4% in 2013 (annual percentage change, 1.51%), and the percentage of HR- patients decreased from 5.2% to 3.4% during the same time period (annual percentage change, -0.17%), the authors reported online Feb. 2 in JAMA Oncology (2017 Feb 2. doi: 10.1001jamaoncol.2016.6380).
Notably, receipt of AET varied significantly by age, race, geographic location, and receptor status. For example, more than 80% of those aged 50-69 years received AET, compared with 79.1% of those younger than age 40 years and 60.5% of those 80 years or older. African American and Hispanic patients were less likely than non-Hispanic white patients to receive AET (76.4% and 75.9% vs. 79.0%, respectively). The latter finding could be an important contributing factor to the racial disparity in breast cancer survival, the authors noted.
Facility factors also played a role in AET receipt; the rate of receipt varied substantially by facility volume and geographic location.
“We found that facilities in west south central states and low-volume institutions were more likely to misuse and underuse AET,” the investigators wrote, noting that these deficits could affect breast cancer mortality, as geographic differences in such mortality are well documented.
AET receipt also varied based on hormone receptor status, tumor size, and local treatment. Surgery and radiotherapy were the factors most significantly associated with appropriate AET receipt, with only 45% of those who underwent lumpectomy without radiotherapy receiving AET, compared with 90.1% of those receiving postmastectomy radiotherapy, 85.5% in those with postlumpectomy radiotherapy, and 73.2% of those with mastectomy alone.
Women included in this study were age 18 years or older (mean age, 60.8 years). Those undergoing surgery but receiving no neoadjuvant systemic treatment were eligible. Those with prior cancer diagnoses or with missing hormone receptor or AET status were excluded.
Based on recent trial results, the American Society of Clinical Oncology updated treatment guidelines in 2016 to recommend ovarian suppression for 5 years in combination with AET for high-risk premenopausal women, and AET alone both for women with stage I breast cancers that don’t warrant chemotherapy and for node-negative cancers of 1.0 cm or less.
“Similarly, the National Quality Forum (cancer measure 0220) endorsed tamoxifen or a third-generation aromatase inhibitor (considered or administered) within 1 year of diagnosis as a marker of quality care for patients with HR+ American Joint Committee on Cancer stage T1cN0M0, II, or III disease,” the researchers wrote. Studies demonstrated benefit with AET even in those with node-negative cancers of 1.0 cm or less, they added.
Improving adherence to these guidelines has lifesaving potential. In the current cohort, receipt of AET was associated with a 29% relative risk reduction in mortality, after adjusting for numerous patient, disease, and facility-related factors. This suggests that if all women with HR+ disease received AET in concordance with guidelines, 14,630 more lives would have been saved over the 10-year study period, the investigators said.
As for approaches that could help improve the appropriate use of AET, the findings of this cohort study support those from previous studies suggesting that a team-based approach is of benefit.
The finding that local treatments are key factors associated with appropriate AET use suggests that patients who undergo radiotherapy may be more likely to receive standard-of-care therapy in general, the authors explained, adding that “with more physicians involved in a patient’s care, these patients would be more likely to be recommended for guideline concordant care.
“Facilitation of multidisciplinary team-based care may help optimize guideline-concordant treatment by ensuring patients are not lost to follow-up and are recommended for evidence-based care,” the study authors concluded.
Their hope is that with the coming launch of the Medicare Access and Chip Reauthorization Act and value-based reimbursement, efforts will be made to close the quality gap affecting patients in certain age groups, racial minority groups, and geographic regions, and to thereby prevent the loss of lives.
MACRA may boost outcomes
In an interview, Dr. Daly said he believes the coming changes with respect to value-based reimbursement will indeed have an important impact on outcomes.
“The oncology care model, for example, mandates that physicians document that they are providing guideline-concordant care,” said Dr. Daly, assistant attending physician at Memorial Sloan Kettering Cancer Center. “There are also new technologies such as oncology clinical pathways ... that also try to ensure that all patients are receiving care according to guidelines.”
Further, the increasing use of team-based approaches to care and the incorporation of “tumor boards” might explain the growth in optimal AET usage seen in this cohort.
Dr. Daly said he was surprised to find that certain patient groups were being left behind, such as those with estrogen receptor-negative/progesterone receptor-positive disease, African American patients, and younger and older patients, who were less likely to receive AET.
“I think that helps us also focus on patient populations we can target to make sure they are receiving optimal care,” he said, stressing that the findings have important policy implications for figuring out why those patients are being left behind, and raising the standard of care for all patients.
“We are making great strides to providing appropriate guideline-concordant care for breast cancer patients, but there’s still room to improve,” he said.
Dr. Daly serves as a director of and receives compensation from Quadrant Holdings. Frontline Medical News is a subsidiary of Quadrant Holdings. Dr. Daly also reported financial relationships with CVS Health, Johnson & Johnson, McKesson, and Walgreens Boots Alliance.
Appropriate use of adjuvant endocrine therapy for breast cancer improved over a 10-year period, but optimal use has not been achieved, according to findings from a retrospective review of more than 980,000 women with stages I-III breast cancer.
As a result, an estimated 14,630 lives were unnecessarily lost during the study period, according to Bobby M. Daly, MD, of Memorial Sloan Kettering Cancer Center in New York, and his colleagues.
Of the 981,729 women in the National Cancer Database from Jan. 1, 2004, to Dec. 31, 2013, who received all or part of their care at the reporting institution and who met eligibility criteria, 818,435 had hormone receptor positive (HR+) disease and 163,294 had hormone receptor negative (HR-) disease.
The percentage of HR+ patients receiving adjuvant endocrine therapy (AET) increased from 69.8% in 2004 to 82.4% in 2013 (annual percentage change, 1.51%), and the percentage of HR- patients decreased from 5.2% to 3.4% during the same time period (annual percentage change, -0.17%), the authors reported online Feb. 2 in JAMA Oncology (2017 Feb 2. doi: 10.1001jamaoncol.2016.6380).
Notably, receipt of AET varied significantly by age, race, geographic location, and receptor status. For example, more than 80% of those aged 50-69 years received AET, compared with 79.1% of those younger than age 40 years and 60.5% of those 80 years or older. African American and Hispanic patients were less likely than non-Hispanic white patients to receive AET (76.4% and 75.9% vs. 79.0%, respectively). The latter finding could be an important contributing factor to the racial disparity in breast cancer survival, the authors noted.
Facility factors also played a role in AET receipt; the rate of receipt varied substantially by facility volume and geographic location.
“We found that facilities in west south central states and low-volume institutions were more likely to misuse and underuse AET,” the investigators wrote, noting that these deficits could affect breast cancer mortality, as geographic differences in such mortality are well documented.
AET receipt also varied based on hormone receptor status, tumor size, and local treatment. Surgery and radiotherapy were the factors most significantly associated with appropriate AET receipt, with only 45% of those who underwent lumpectomy without radiotherapy receiving AET, compared with 90.1% of those receiving postmastectomy radiotherapy, 85.5% in those with postlumpectomy radiotherapy, and 73.2% of those with mastectomy alone.
Women included in this study were age 18 years or older (mean age, 60.8 years). Those undergoing surgery but receiving no neoadjuvant systemic treatment were eligible. Those with prior cancer diagnoses or with missing hormone receptor or AET status were excluded.
Based on recent trial results, the American Society of Clinical Oncology updated treatment guidelines in 2016 to recommend ovarian suppression for 5 years in combination with AET for high-risk premenopausal women, and AET alone both for women with stage I breast cancers that don’t warrant chemotherapy and for node-negative cancers of 1.0 cm or less.
“Similarly, the National Quality Forum (cancer measure 0220) endorsed tamoxifen or a third-generation aromatase inhibitor (considered or administered) within 1 year of diagnosis as a marker of quality care for patients with HR+ American Joint Committee on Cancer stage T1cN0M0, II, or III disease,” the researchers wrote. Studies demonstrated benefit with AET even in those with node-negative cancers of 1.0 cm or less, they added.
Improving adherence to these guidelines has lifesaving potential. In the current cohort, receipt of AET was associated with a 29% relative risk reduction in mortality, after adjusting for numerous patient, disease, and facility-related factors. This suggests that if all women with HR+ disease received AET in concordance with guidelines, 14,630 more lives would have been saved over the 10-year study period, the investigators said.
As for approaches that could help improve the appropriate use of AET, the findings of this cohort study support those from previous studies suggesting that a team-based approach is of benefit.
The finding that local treatments are key factors associated with appropriate AET use suggests that patients who undergo radiotherapy may be more likely to receive standard-of-care therapy in general, the authors explained, adding that “with more physicians involved in a patient’s care, these patients would be more likely to be recommended for guideline concordant care.
“Facilitation of multidisciplinary team-based care may help optimize guideline-concordant treatment by ensuring patients are not lost to follow-up and are recommended for evidence-based care,” the study authors concluded.
Their hope is that with the coming launch of the Medicare Access and Chip Reauthorization Act and value-based reimbursement, efforts will be made to close the quality gap affecting patients in certain age groups, racial minority groups, and geographic regions, and to thereby prevent the loss of lives.
MACRA may boost outcomes
In an interview, Dr. Daly said he believes the coming changes with respect to value-based reimbursement will indeed have an important impact on outcomes.
“The oncology care model, for example, mandates that physicians document that they are providing guideline-concordant care,” said Dr. Daly, assistant attending physician at Memorial Sloan Kettering Cancer Center. “There are also new technologies such as oncology clinical pathways ... that also try to ensure that all patients are receiving care according to guidelines.”
Further, the increasing use of team-based approaches to care and the incorporation of “tumor boards” might explain the growth in optimal AET usage seen in this cohort.
Dr. Daly said he was surprised to find that certain patient groups were being left behind, such as those with estrogen receptor-negative/progesterone receptor-positive disease, African American patients, and younger and older patients, who were less likely to receive AET.
“I think that helps us also focus on patient populations we can target to make sure they are receiving optimal care,” he said, stressing that the findings have important policy implications for figuring out why those patients are being left behind, and raising the standard of care for all patients.
“We are making great strides to providing appropriate guideline-concordant care for breast cancer patients, but there’s still room to improve,” he said.
Dr. Daly serves as a director of and receives compensation from Quadrant Holdings. Frontline Medical News is a subsidiary of Quadrant Holdings. Dr. Daly also reported financial relationships with CVS Health, Johnson & Johnson, McKesson, and Walgreens Boots Alliance.
Appropriate use of adjuvant endocrine therapy for breast cancer improved over a 10-year period, but optimal use has not been achieved, according to findings from a retrospective review of more than 980,000 women with stages I-III breast cancer.
As a result, an estimated 14,630 lives were unnecessarily lost during the study period, according to Bobby M. Daly, MD, of Memorial Sloan Kettering Cancer Center in New York, and his colleagues.
Of the 981,729 women in the National Cancer Database from Jan. 1, 2004, to Dec. 31, 2013, who received all or part of their care at the reporting institution and who met eligibility criteria, 818,435 had hormone receptor positive (HR+) disease and 163,294 had hormone receptor negative (HR-) disease.
The percentage of HR+ patients receiving adjuvant endocrine therapy (AET) increased from 69.8% in 2004 to 82.4% in 2013 (annual percentage change, 1.51%), and the percentage of HR- patients decreased from 5.2% to 3.4% during the same time period (annual percentage change, -0.17%), the authors reported online Feb. 2 in JAMA Oncology (2017 Feb 2. doi: 10.1001jamaoncol.2016.6380).
Notably, receipt of AET varied significantly by age, race, geographic location, and receptor status. For example, more than 80% of those aged 50-69 years received AET, compared with 79.1% of those younger than age 40 years and 60.5% of those 80 years or older. African American and Hispanic patients were less likely than non-Hispanic white patients to receive AET (76.4% and 75.9% vs. 79.0%, respectively). The latter finding could be an important contributing factor to the racial disparity in breast cancer survival, the authors noted.
Facility factors also played a role in AET receipt; the rate of receipt varied substantially by facility volume and geographic location.
“We found that facilities in west south central states and low-volume institutions were more likely to misuse and underuse AET,” the investigators wrote, noting that these deficits could affect breast cancer mortality, as geographic differences in such mortality are well documented.
AET receipt also varied based on hormone receptor status, tumor size, and local treatment. Surgery and radiotherapy were the factors most significantly associated with appropriate AET receipt, with only 45% of those who underwent lumpectomy without radiotherapy receiving AET, compared with 90.1% of those receiving postmastectomy radiotherapy, 85.5% in those with postlumpectomy radiotherapy, and 73.2% of those with mastectomy alone.
Women included in this study were age 18 years or older (mean age, 60.8 years). Those undergoing surgery but receiving no neoadjuvant systemic treatment were eligible. Those with prior cancer diagnoses or with missing hormone receptor or AET status were excluded.
Based on recent trial results, the American Society of Clinical Oncology updated treatment guidelines in 2016 to recommend ovarian suppression for 5 years in combination with AET for high-risk premenopausal women, and AET alone both for women with stage I breast cancers that don’t warrant chemotherapy and for node-negative cancers of 1.0 cm or less.
“Similarly, the National Quality Forum (cancer measure 0220) endorsed tamoxifen or a third-generation aromatase inhibitor (considered or administered) within 1 year of diagnosis as a marker of quality care for patients with HR+ American Joint Committee on Cancer stage T1cN0M0, II, or III disease,” the researchers wrote. Studies demonstrated benefit with AET even in those with node-negative cancers of 1.0 cm or less, they added.
Improving adherence to these guidelines has lifesaving potential. In the current cohort, receipt of AET was associated with a 29% relative risk reduction in mortality, after adjusting for numerous patient, disease, and facility-related factors. This suggests that if all women with HR+ disease received AET in concordance with guidelines, 14,630 more lives would have been saved over the 10-year study period, the investigators said.
As for approaches that could help improve the appropriate use of AET, the findings of this cohort study support those from previous studies suggesting that a team-based approach is of benefit.
The finding that local treatments are key factors associated with appropriate AET use suggests that patients who undergo radiotherapy may be more likely to receive standard-of-care therapy in general, the authors explained, adding that “with more physicians involved in a patient’s care, these patients would be more likely to be recommended for guideline concordant care.
“Facilitation of multidisciplinary team-based care may help optimize guideline-concordant treatment by ensuring patients are not lost to follow-up and are recommended for evidence-based care,” the study authors concluded.
Their hope is that with the coming launch of the Medicare Access and Chip Reauthorization Act and value-based reimbursement, efforts will be made to close the quality gap affecting patients in certain age groups, racial minority groups, and geographic regions, and to thereby prevent the loss of lives.
MACRA may boost outcomes
In an interview, Dr. Daly said he believes the coming changes with respect to value-based reimbursement will indeed have an important impact on outcomes.
“The oncology care model, for example, mandates that physicians document that they are providing guideline-concordant care,” said Dr. Daly, assistant attending physician at Memorial Sloan Kettering Cancer Center. “There are also new technologies such as oncology clinical pathways ... that also try to ensure that all patients are receiving care according to guidelines.”
Further, the increasing use of team-based approaches to care and the incorporation of “tumor boards” might explain the growth in optimal AET usage seen in this cohort.
Dr. Daly said he was surprised to find that certain patient groups were being left behind, such as those with estrogen receptor-negative/progesterone receptor-positive disease, African American patients, and younger and older patients, who were less likely to receive AET.
“I think that helps us also focus on patient populations we can target to make sure they are receiving optimal care,” he said, stressing that the findings have important policy implications for figuring out why those patients are being left behind, and raising the standard of care for all patients.
“We are making great strides to providing appropriate guideline-concordant care for breast cancer patients, but there’s still room to improve,” he said.
Dr. Daly serves as a director of and receives compensation from Quadrant Holdings. Frontline Medical News is a subsidiary of Quadrant Holdings. Dr. Daly also reported financial relationships with CVS Health, Johnson & Johnson, McKesson, and Walgreens Boots Alliance.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Receipt of AET was associated with a 29% relative risk reduction in mortality; use of AET in concordance with guidelines would have saved an estimated 14,630 additional lives over 10 years.
Data source: A retrospective cohort study of 981,729 women with breast cancer.
Disclosures: Dr. Daly serves as a director of and receives compensation from Quadrant Holdings. Frontline Medical News is a subsidiary of Quadrant Holdings. Dr. Daly also reported financial relationships with CVS Health, Johnson & Johnson, McKesson, and Walgreens Boots Alliance.
Neuropathic pain puts cancer survivors out of work
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
Key clinical point: Chronic neuropathic pain is a barrier to employment for many cancer survivors.
Major finding: Cancer survivors with chronic neuropathic pain were twice as likely to be unemployed 5 years after diagnosis as patients with no pain.
Data source: Longitudinal study of French cancer survivors.
Disclosures: The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
Toddlers’ neurodevelopmental deficits linked with maternal diabetes
LAS VEGAS – Children born to obese women with insulin resistance during pregnancy showed significantly impaired neurodevelopment at 2 years of age, compared with children born to obese mothers without insulin resistance in a prospective observational study with 75 pregnant women.
The neurodevelopmental deficits were specific for the domains of motor function and attention, and the deficits correlated with several markers of abnormal glucose and fat metabolism in the insulin-resistant women, Alison G. Cahill, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The differences in neurodevelopment appear to not be global but instead specifically affect domains of motor development and attention,” said Dr. Cahill, chief of maternal fetal medicine at Washington University in St. Louis. “These findings are consistent with results from animal studies that suggest certain brain regions are more sensitive than others to metabolic abnormalities” while in utero.
“These are among the first data in humans to characterize the impact of metabolic abnormalities on brain development,” she added.
Dr. Cahill said that results from the lean mothers uniformly matched those from the obese mothers without insulin resistance, and so for brevity she only reported results from the obese control group.
Average gestational age at birth was 37 weeks in the insulin-resistant mothers and 38.7 weeks among the obese mothers without insulin resistance, a significant difference. Birth weight averaged 3,617 g in the mothers with insulin resistance and 3,373 g in the mothers without insulin resistance, a difference that was not statistically significant.
Dr. Cahill and her associates assessed the 2-year-olds with a battery of behavioral and functional assessments. They measured motor function, cognition, and language with the Bayley Scales of Infant and Toddler Development, prespecified as the study’s primary endpoint. They also applied the Modified Checklist for Autism in Toddlers (M-CHAT), as well as the Infant-Toddler Social and Emotional Assessment (ITSEA) to assess competence, externalizing, internalizing, and dysregulation.
The results of these analyses showed statistically significant deficits for the motor composite score on the Bayley assessment and for the competence component of the ITSEA assessment, Dr. Cahill reported. The average composite Bayley motor score was 88 in children from mothers with insulin resistance and 98 in the control children, a statistically significant difference.
Further analyses showed that the motor deficit was primarily in fine motor function, and that motor scores were depressed throughout the entire cohort of children born to mothers with insulin resistance.
Depressed competence scores on the ITSEA assessment reflect attention abnormalities, she explained.
A final analysis examined the correlation between the motor deficits identified and various metabolic tests of fat, glucose, and protein metabolism run on the enrolled mothers during the last weeks of gestation. This showed significant links between depressed motor development and maternal lipolytic rate, plasma free fatty acids, and hepatic glucose output.
This finding “suggests an association between abnormal lipid and glucose metabolism in mothers and aspects of neurodevelopment” in their children, Dr. Cahill said.
Dr. Cahill had no disclosures.
[email protected] On Twitter @mitchelzoler
LAS VEGAS – Children born to obese women with insulin resistance during pregnancy showed significantly impaired neurodevelopment at 2 years of age, compared with children born to obese mothers without insulin resistance in a prospective observational study with 75 pregnant women.
The neurodevelopmental deficits were specific for the domains of motor function and attention, and the deficits correlated with several markers of abnormal glucose and fat metabolism in the insulin-resistant women, Alison G. Cahill, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The differences in neurodevelopment appear to not be global but instead specifically affect domains of motor development and attention,” said Dr. Cahill, chief of maternal fetal medicine at Washington University in St. Louis. “These findings are consistent with results from animal studies that suggest certain brain regions are more sensitive than others to metabolic abnormalities” while in utero.
“These are among the first data in humans to characterize the impact of metabolic abnormalities on brain development,” she added.
Dr. Cahill said that results from the lean mothers uniformly matched those from the obese mothers without insulin resistance, and so for brevity she only reported results from the obese control group.
Average gestational age at birth was 37 weeks in the insulin-resistant mothers and 38.7 weeks among the obese mothers without insulin resistance, a significant difference. Birth weight averaged 3,617 g in the mothers with insulin resistance and 3,373 g in the mothers without insulin resistance, a difference that was not statistically significant.
Dr. Cahill and her associates assessed the 2-year-olds with a battery of behavioral and functional assessments. They measured motor function, cognition, and language with the Bayley Scales of Infant and Toddler Development, prespecified as the study’s primary endpoint. They also applied the Modified Checklist for Autism in Toddlers (M-CHAT), as well as the Infant-Toddler Social and Emotional Assessment (ITSEA) to assess competence, externalizing, internalizing, and dysregulation.
The results of these analyses showed statistically significant deficits for the motor composite score on the Bayley assessment and for the competence component of the ITSEA assessment, Dr. Cahill reported. The average composite Bayley motor score was 88 in children from mothers with insulin resistance and 98 in the control children, a statistically significant difference.
Further analyses showed that the motor deficit was primarily in fine motor function, and that motor scores were depressed throughout the entire cohort of children born to mothers with insulin resistance.
Depressed competence scores on the ITSEA assessment reflect attention abnormalities, she explained.
A final analysis examined the correlation between the motor deficits identified and various metabolic tests of fat, glucose, and protein metabolism run on the enrolled mothers during the last weeks of gestation. This showed significant links between depressed motor development and maternal lipolytic rate, plasma free fatty acids, and hepatic glucose output.
This finding “suggests an association between abnormal lipid and glucose metabolism in mothers and aspects of neurodevelopment” in their children, Dr. Cahill said.
Dr. Cahill had no disclosures.
[email protected] On Twitter @mitchelzoler
LAS VEGAS – Children born to obese women with insulin resistance during pregnancy showed significantly impaired neurodevelopment at 2 years of age, compared with children born to obese mothers without insulin resistance in a prospective observational study with 75 pregnant women.
The neurodevelopmental deficits were specific for the domains of motor function and attention, and the deficits correlated with several markers of abnormal glucose and fat metabolism in the insulin-resistant women, Alison G. Cahill, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“The differences in neurodevelopment appear to not be global but instead specifically affect domains of motor development and attention,” said Dr. Cahill, chief of maternal fetal medicine at Washington University in St. Louis. “These findings are consistent with results from animal studies that suggest certain brain regions are more sensitive than others to metabolic abnormalities” while in utero.
“These are among the first data in humans to characterize the impact of metabolic abnormalities on brain development,” she added.
Dr. Cahill said that results from the lean mothers uniformly matched those from the obese mothers without insulin resistance, and so for brevity she only reported results from the obese control group.
Average gestational age at birth was 37 weeks in the insulin-resistant mothers and 38.7 weeks among the obese mothers without insulin resistance, a significant difference. Birth weight averaged 3,617 g in the mothers with insulin resistance and 3,373 g in the mothers without insulin resistance, a difference that was not statistically significant.
Dr. Cahill and her associates assessed the 2-year-olds with a battery of behavioral and functional assessments. They measured motor function, cognition, and language with the Bayley Scales of Infant and Toddler Development, prespecified as the study’s primary endpoint. They also applied the Modified Checklist for Autism in Toddlers (M-CHAT), as well as the Infant-Toddler Social and Emotional Assessment (ITSEA) to assess competence, externalizing, internalizing, and dysregulation.
The results of these analyses showed statistically significant deficits for the motor composite score on the Bayley assessment and for the competence component of the ITSEA assessment, Dr. Cahill reported. The average composite Bayley motor score was 88 in children from mothers with insulin resistance and 98 in the control children, a statistically significant difference.
Further analyses showed that the motor deficit was primarily in fine motor function, and that motor scores were depressed throughout the entire cohort of children born to mothers with insulin resistance.
Depressed competence scores on the ITSEA assessment reflect attention abnormalities, she explained.
A final analysis examined the correlation between the motor deficits identified and various metabolic tests of fat, glucose, and protein metabolism run on the enrolled mothers during the last weeks of gestation. This showed significant links between depressed motor development and maternal lipolytic rate, plasma free fatty acids, and hepatic glucose output.
This finding “suggests an association between abnormal lipid and glucose metabolism in mothers and aspects of neurodevelopment” in their children, Dr. Cahill said.
Dr. Cahill had no disclosures.
[email protected] On Twitter @mitchelzoler
Key clinical point:
Major finding: The Bayley motor scale score averaged 88 in children from insulin-resistant mothers and 98 when no insulin resistance existed.
Data source: Prospective, single-center observational study with 75 pregnant women.
Disclosures: Dr. Cahill had no disclosures.
Study finds community-hospital lung cancer screening feasible
HOUSTON – Implementing a lung cancer screening program in a community hospital setting is feasible and saves lives, but implementation comes with its share of challenges, results from a single-center analysis demonstrated.
“Deaths from lung cancer surpass mortality of all other malignancies,” Simran Randhawa, MD, said during a press briefing at the annual meeting of the Society of Thoracic Surgeons. “The majority of lung cancers are found at an advanced stage, and the 5-year overall survival has only marginally improved over the past 40 years, and is approximately 17% according to recent data.
“Most people who die from lung cancer are former smokers,” said Dr. Randhawa, of the Einstein Healthcare Network, Philadelphia. “Former smokers cannot benefit from primary prevention, so this is where lung cancer screening comes into action.”
In 2011, the National Lung Screening Trial (NLST) showed a 20% relative reduction in lung cancer death with annual low-dose CT of the chest in high-risk patients. The reduction in mortality by any cause was by 6.7%.
“The number needed to be screened to prevent one death is 320, which may seem like a lot, but it is a very competitive number when compared to mammography or colonoscopy,” Dr. Randhawa said. “Lung cancer screening is also cost effective. It costs about $73,000 per quality adjusted life year, which can be further improved if offered with smoking cessation intervention.”
In October 2013, Einstein Healthcare Network offered a free lung cancer screening program. The purpose of the current study, which was led by Doraid Jarrar, MD, at the University of Pennsylvania, Philadelphia, was to evaluate the feasibility of establishing a lung cancer screening program in a community hospital system, to identify barriers to adoption, and to benchmark their experience with NLST results.
They promoted the screening program through flyers, radio programs, face-to-face information sessions, and a multidisciplinary lung symposium, prospectively collected data over 12 months, and decided patient eligibility based on NLST criteria. Results were reported using the Lung CT Screening Reporting and Data System (Lung-RADS).
Dr. Randhawa reported results from a total of 278 patients. Their average age was 64 years, 62% were female, 65% were African American, and the average number of pack years was 43.
Most (88%) were diagnosed with Lung-RADS 1 or 2 (negative or benign appearance), 7% were Lung-RADS 3 (probably benign but requiring close follow-up with CT scan), and 5% were Lung-RADS 4 (suspicious with a chance of malignancy).
Of the 11 patients who were diagnosed with Lung-RADS 4, 4 underwent lifesaving lung resection surgery for stage 1 disease, 1 patient was diagnosed with stage 4 disease, and the rest were either benign on follow-up or lost to follow-up.
“On further investigation, we found that 60% of the patients who showed up for the lung cancer screening were referred to us by their primary care physician, 22% had heard about our program through flyers as well as radio advertisements, 2% via Web search, and 1% through newsletters,” Dr. Randhawa said.
At the end of the study period, the researchers distributed a survey to all primary care and referring physicians. About 42% said they referred more than 10 patients in the last year, while 16% made no such referrals. When asked if they knew that lung cancer screening is recommended by the U.S. Preventive Services Task Force on par with mammography and colonoscopy, 74% said that they knew, but 26% indicated that they were not aware of that fact.
“When asked about any barriers they may have encountered, one physician commented on the lack of time to counsel his patients,” Dr. Randhawa said. “Most of the physicians expressed concerns about precertification [delays] and requirement for prior authorization for lung cancer screening.”
She acknowledged certain limitations of the study, including its small sample size and the fact that the researchers interviewed primary care physicians catering to an underserved population in the community, “which is not comparable to the NLST population,” she said.
“However, [our results] could be potentially more generalizable,” Dr. Randhawa noted. “In the future, we hope that there is evolving technology used for CT screening with reduction in radiation dose, and more accurate biomarkers will be developed to identify patients at highest risk for lung cancer. We aim to save lives through early detection of lung cancer with responsible CT lung screening.”
One of the study authors, Tracy Kane, MD, disclosed being a member of the speakers bureau for AstraZeneca and receiving honoraria from the company. The other researchers reported having no financial disclosures.
HOUSTON – Implementing a lung cancer screening program in a community hospital setting is feasible and saves lives, but implementation comes with its share of challenges, results from a single-center analysis demonstrated.
“Deaths from lung cancer surpass mortality of all other malignancies,” Simran Randhawa, MD, said during a press briefing at the annual meeting of the Society of Thoracic Surgeons. “The majority of lung cancers are found at an advanced stage, and the 5-year overall survival has only marginally improved over the past 40 years, and is approximately 17% according to recent data.
“Most people who die from lung cancer are former smokers,” said Dr. Randhawa, of the Einstein Healthcare Network, Philadelphia. “Former smokers cannot benefit from primary prevention, so this is where lung cancer screening comes into action.”
In 2011, the National Lung Screening Trial (NLST) showed a 20% relative reduction in lung cancer death with annual low-dose CT of the chest in high-risk patients. The reduction in mortality by any cause was by 6.7%.
“The number needed to be screened to prevent one death is 320, which may seem like a lot, but it is a very competitive number when compared to mammography or colonoscopy,” Dr. Randhawa said. “Lung cancer screening is also cost effective. It costs about $73,000 per quality adjusted life year, which can be further improved if offered with smoking cessation intervention.”
In October 2013, Einstein Healthcare Network offered a free lung cancer screening program. The purpose of the current study, which was led by Doraid Jarrar, MD, at the University of Pennsylvania, Philadelphia, was to evaluate the feasibility of establishing a lung cancer screening program in a community hospital system, to identify barriers to adoption, and to benchmark their experience with NLST results.
They promoted the screening program through flyers, radio programs, face-to-face information sessions, and a multidisciplinary lung symposium, prospectively collected data over 12 months, and decided patient eligibility based on NLST criteria. Results were reported using the Lung CT Screening Reporting and Data System (Lung-RADS).
Dr. Randhawa reported results from a total of 278 patients. Their average age was 64 years, 62% were female, 65% were African American, and the average number of pack years was 43.
Most (88%) were diagnosed with Lung-RADS 1 or 2 (negative or benign appearance), 7% were Lung-RADS 3 (probably benign but requiring close follow-up with CT scan), and 5% were Lung-RADS 4 (suspicious with a chance of malignancy).
Of the 11 patients who were diagnosed with Lung-RADS 4, 4 underwent lifesaving lung resection surgery for stage 1 disease, 1 patient was diagnosed with stage 4 disease, and the rest were either benign on follow-up or lost to follow-up.
“On further investigation, we found that 60% of the patients who showed up for the lung cancer screening were referred to us by their primary care physician, 22% had heard about our program through flyers as well as radio advertisements, 2% via Web search, and 1% through newsletters,” Dr. Randhawa said.
At the end of the study period, the researchers distributed a survey to all primary care and referring physicians. About 42% said they referred more than 10 patients in the last year, while 16% made no such referrals. When asked if they knew that lung cancer screening is recommended by the U.S. Preventive Services Task Force on par with mammography and colonoscopy, 74% said that they knew, but 26% indicated that they were not aware of that fact.
“When asked about any barriers they may have encountered, one physician commented on the lack of time to counsel his patients,” Dr. Randhawa said. “Most of the physicians expressed concerns about precertification [delays] and requirement for prior authorization for lung cancer screening.”
She acknowledged certain limitations of the study, including its small sample size and the fact that the researchers interviewed primary care physicians catering to an underserved population in the community, “which is not comparable to the NLST population,” she said.
“However, [our results] could be potentially more generalizable,” Dr. Randhawa noted. “In the future, we hope that there is evolving technology used for CT screening with reduction in radiation dose, and more accurate biomarkers will be developed to identify patients at highest risk for lung cancer. We aim to save lives through early detection of lung cancer with responsible CT lung screening.”
One of the study authors, Tracy Kane, MD, disclosed being a member of the speakers bureau for AstraZeneca and receiving honoraria from the company. The other researchers reported having no financial disclosures.
HOUSTON – Implementing a lung cancer screening program in a community hospital setting is feasible and saves lives, but implementation comes with its share of challenges, results from a single-center analysis demonstrated.
“Deaths from lung cancer surpass mortality of all other malignancies,” Simran Randhawa, MD, said during a press briefing at the annual meeting of the Society of Thoracic Surgeons. “The majority of lung cancers are found at an advanced stage, and the 5-year overall survival has only marginally improved over the past 40 years, and is approximately 17% according to recent data.
“Most people who die from lung cancer are former smokers,” said Dr. Randhawa, of the Einstein Healthcare Network, Philadelphia. “Former smokers cannot benefit from primary prevention, so this is where lung cancer screening comes into action.”
In 2011, the National Lung Screening Trial (NLST) showed a 20% relative reduction in lung cancer death with annual low-dose CT of the chest in high-risk patients. The reduction in mortality by any cause was by 6.7%.
“The number needed to be screened to prevent one death is 320, which may seem like a lot, but it is a very competitive number when compared to mammography or colonoscopy,” Dr. Randhawa said. “Lung cancer screening is also cost effective. It costs about $73,000 per quality adjusted life year, which can be further improved if offered with smoking cessation intervention.”
In October 2013, Einstein Healthcare Network offered a free lung cancer screening program. The purpose of the current study, which was led by Doraid Jarrar, MD, at the University of Pennsylvania, Philadelphia, was to evaluate the feasibility of establishing a lung cancer screening program in a community hospital system, to identify barriers to adoption, and to benchmark their experience with NLST results.
They promoted the screening program through flyers, radio programs, face-to-face information sessions, and a multidisciplinary lung symposium, prospectively collected data over 12 months, and decided patient eligibility based on NLST criteria. Results were reported using the Lung CT Screening Reporting and Data System (Lung-RADS).
Dr. Randhawa reported results from a total of 278 patients. Their average age was 64 years, 62% were female, 65% were African American, and the average number of pack years was 43.
Most (88%) were diagnosed with Lung-RADS 1 or 2 (negative or benign appearance), 7% were Lung-RADS 3 (probably benign but requiring close follow-up with CT scan), and 5% were Lung-RADS 4 (suspicious with a chance of malignancy).
Of the 11 patients who were diagnosed with Lung-RADS 4, 4 underwent lifesaving lung resection surgery for stage 1 disease, 1 patient was diagnosed with stage 4 disease, and the rest were either benign on follow-up or lost to follow-up.
“On further investigation, we found that 60% of the patients who showed up for the lung cancer screening were referred to us by their primary care physician, 22% had heard about our program through flyers as well as radio advertisements, 2% via Web search, and 1% through newsletters,” Dr. Randhawa said.
At the end of the study period, the researchers distributed a survey to all primary care and referring physicians. About 42% said they referred more than 10 patients in the last year, while 16% made no such referrals. When asked if they knew that lung cancer screening is recommended by the U.S. Preventive Services Task Force on par with mammography and colonoscopy, 74% said that they knew, but 26% indicated that they were not aware of that fact.
“When asked about any barriers they may have encountered, one physician commented on the lack of time to counsel his patients,” Dr. Randhawa said. “Most of the physicians expressed concerns about precertification [delays] and requirement for prior authorization for lung cancer screening.”
She acknowledged certain limitations of the study, including its small sample size and the fact that the researchers interviewed primary care physicians catering to an underserved population in the community, “which is not comparable to the NLST population,” she said.
“However, [our results] could be potentially more generalizable,” Dr. Randhawa noted. “In the future, we hope that there is evolving technology used for CT screening with reduction in radiation dose, and more accurate biomarkers will be developed to identify patients at highest risk for lung cancer. We aim to save lives through early detection of lung cancer with responsible CT lung screening.”
One of the study authors, Tracy Kane, MD, disclosed being a member of the speakers bureau for AstraZeneca and receiving honoraria from the company. The other researchers reported having no financial disclosures.
Key clinical point:
Major finding: Of patients who participated in a community screening program over the course of 1 year, 88% were diagnosed with Lung-RADS 1 or 2, 7% were Lung-RADS 3, and 5% were Lung-RADS 4.
Data source: A 12-month prospective study of 278 patients at risk for lung cancer.
Disclosures: One of the study authors, Tracy Kane, MD, disclosed being a member of the speakers bureau for AstraZeneca and receiving honoraria from the company. The other researchers reported having no financial disclosures.
VIDEO: Public reporting of congenital heart disease outcomes should be easily understood
HOUSTON – Survival statistics, surgeon-specific experience, and complication rates are the types of information most sought by parents of children with congenital heart disease, results from a large survey suggest.
Future efforts in public reporting for congenital heart surgery outcomes should have better methods for presenting the data in a valid, easily interpreted format, explained study investigator Mallory L. Irons, MD, an integrated cardiac surgery resident at the Hospital of the University of Pennsylvania, Philadelphia.
“We’re doing a good job of public reporting currently, but what we’re doing is not meeting the needs of all of our stakeholders – in this case, the parents of children with congenital heart disease,” Dr. Irons said in an interview at the annual meeting of the Society of Thoracic Surgeons. “The optimal public reporting scheme still has yet to be determined.”
Dr. Irons reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON – Survival statistics, surgeon-specific experience, and complication rates are the types of information most sought by parents of children with congenital heart disease, results from a large survey suggest.
Future efforts in public reporting for congenital heart surgery outcomes should have better methods for presenting the data in a valid, easily interpreted format, explained study investigator Mallory L. Irons, MD, an integrated cardiac surgery resident at the Hospital of the University of Pennsylvania, Philadelphia.
“We’re doing a good job of public reporting currently, but what we’re doing is not meeting the needs of all of our stakeholders – in this case, the parents of children with congenital heart disease,” Dr. Irons said in an interview at the annual meeting of the Society of Thoracic Surgeons. “The optimal public reporting scheme still has yet to be determined.”
Dr. Irons reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON – Survival statistics, surgeon-specific experience, and complication rates are the types of information most sought by parents of children with congenital heart disease, results from a large survey suggest.
Future efforts in public reporting for congenital heart surgery outcomes should have better methods for presenting the data in a valid, easily interpreted format, explained study investigator Mallory L. Irons, MD, an integrated cardiac surgery resident at the Hospital of the University of Pennsylvania, Philadelphia.
“We’re doing a good job of public reporting currently, but what we’re doing is not meeting the needs of all of our stakeholders – in this case, the parents of children with congenital heart disease,” Dr. Irons said in an interview at the annual meeting of the Society of Thoracic Surgeons. “The optimal public reporting scheme still has yet to be determined.”
Dr. Irons reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE STS ANNUAL MEETING HOUSTON
Our new year’s resolutions
Be at war with your vices, at peace with your neighbors, and let every new year find you a better person.
– Benjamin Franklin
Traditionally, the new year is a time for reflection, looking back to review what could have been done better, and looking forward to the opportunity to rectify those inadequacies over the coming year. We thought we would take this opportunity to look at our use of the electronic health record and think about the things we might do over the next year to make our lives easier and our charting better.
Top of our list is a renewed commitment to finish our notes by the end of each session. Too many physicians we know rush through patient hours and then are left with 10-20 notes to finish at the end of the day. Realistically, this is when we least feel like completing notes. Such work encroaches on personal and family time, likely contributes to the burnout that has been increasing among physicians, and is much less likely to accurately represent the encounter than notes completed in real time.
As we have spoken with many of our colleagues, it has become clear to us that many clinicians have learned how to be “just proficient enough” in their use of their EHR; they are not pulling out their hair every 5 minutes in frustration, but they have not taken the extra time and effort that are needed to optimize their productivity. To efficiently use an EHR requires some time spent designing templates and macros to make it easy to repetitively carry out common tasks.
A lot of physicians – particularly physicians over 40 years of age – are still typing their notes with the ol’ two-finger hunt-and-peck technique. This is incredibly time consuming, inefficient, and frustrating.
While many solutions have been proposed, including having a scribe walk around with the doctor, the simplest and easiest to implement is voice transcription. Even though medical transcription software is expensive, the return on investment is large for those who do not type well. After a short period of training on the software, notes are generally of higher quality and are finished considerably faster than when typing. The technology also has the ability to learn the names of frequently used consultants, medications, and procedures, so users don’t even have to type uncommon names or words.
Another area in which we hope to advance over the next year is working more effectively as a team to share the documentation burden. Nurses and medical assistants – within the boundaries of their licensing – can be empowered to document in predefined areas of the chart as much as possible.
For example, given the fact that the prevalence of depression is about twice as high in patients with diabetes as it is in the general population, our medical assistants now screen our diabetes patients with a PHQ-2 depression screen and record the results in the chart. This has been good for our patients, satisfying for our medical assistants, and has offloaded this task from the doctors.
We need to think of more areas where we can facilitate team care and really make everyone – physicians, nurses, front staff, and patients – more satisfied with the care that is being given.
Most EHRs have a reminder function – the ability to prompt a user to follow up on an abnormal x-ray or lab results in case a patient does not come back into the office as recommended. Our sense is that most of us are not using this function. It is worth finding out how to use it and giving it a try.
Patient portals have gained a lot of traction over the past few years. For a little while, we were really making an effort to have patients register, so that currently many (but by far not most) of our patients have signed up. We want to make better use of this fantastic resource.
We say “fantastic” because when we talk to patients (or friends or family) who use the portal, they have shared that it really makes their lives easier. They are able to see their labs, ponder the meaning of their results (perhaps of a slightly high glucose or an LDL cholesterol level), and if they have questions, they can correspond electronically with their care providers. It enhances care and allows us to spend less time on the phone, while giving patients better access to information.
New year’s resolutions are an opportunity for reflection and optimism. As we look back on the past year, we should learn from our experience and approach the year in front of us with greater enthusiasm, in the hope that through that enthusiasm we can continue to grow, be better and healthier, and simply be more like the people we want to be.
The electronic health record affects all of our interactions with patients and colleagues, and, when not used optimally, encroaches into our personal and family lives. It is a perfect place to focus during the new year to enable us to have more productive, effective, and happier times both in the office and at home.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Be at war with your vices, at peace with your neighbors, and let every new year find you a better person.
– Benjamin Franklin
Traditionally, the new year is a time for reflection, looking back to review what could have been done better, and looking forward to the opportunity to rectify those inadequacies over the coming year. We thought we would take this opportunity to look at our use of the electronic health record and think about the things we might do over the next year to make our lives easier and our charting better.
Top of our list is a renewed commitment to finish our notes by the end of each session. Too many physicians we know rush through patient hours and then are left with 10-20 notes to finish at the end of the day. Realistically, this is when we least feel like completing notes. Such work encroaches on personal and family time, likely contributes to the burnout that has been increasing among physicians, and is much less likely to accurately represent the encounter than notes completed in real time.
As we have spoken with many of our colleagues, it has become clear to us that many clinicians have learned how to be “just proficient enough” in their use of their EHR; they are not pulling out their hair every 5 minutes in frustration, but they have not taken the extra time and effort that are needed to optimize their productivity. To efficiently use an EHR requires some time spent designing templates and macros to make it easy to repetitively carry out common tasks.
A lot of physicians – particularly physicians over 40 years of age – are still typing their notes with the ol’ two-finger hunt-and-peck technique. This is incredibly time consuming, inefficient, and frustrating.
While many solutions have been proposed, including having a scribe walk around with the doctor, the simplest and easiest to implement is voice transcription. Even though medical transcription software is expensive, the return on investment is large for those who do not type well. After a short period of training on the software, notes are generally of higher quality and are finished considerably faster than when typing. The technology also has the ability to learn the names of frequently used consultants, medications, and procedures, so users don’t even have to type uncommon names or words.
Another area in which we hope to advance over the next year is working more effectively as a team to share the documentation burden. Nurses and medical assistants – within the boundaries of their licensing – can be empowered to document in predefined areas of the chart as much as possible.
For example, given the fact that the prevalence of depression is about twice as high in patients with diabetes as it is in the general population, our medical assistants now screen our diabetes patients with a PHQ-2 depression screen and record the results in the chart. This has been good for our patients, satisfying for our medical assistants, and has offloaded this task from the doctors.
We need to think of more areas where we can facilitate team care and really make everyone – physicians, nurses, front staff, and patients – more satisfied with the care that is being given.
Most EHRs have a reminder function – the ability to prompt a user to follow up on an abnormal x-ray or lab results in case a patient does not come back into the office as recommended. Our sense is that most of us are not using this function. It is worth finding out how to use it and giving it a try.
Patient portals have gained a lot of traction over the past few years. For a little while, we were really making an effort to have patients register, so that currently many (but by far not most) of our patients have signed up. We want to make better use of this fantastic resource.
We say “fantastic” because when we talk to patients (or friends or family) who use the portal, they have shared that it really makes their lives easier. They are able to see their labs, ponder the meaning of their results (perhaps of a slightly high glucose or an LDL cholesterol level), and if they have questions, they can correspond electronically with their care providers. It enhances care and allows us to spend less time on the phone, while giving patients better access to information.
New year’s resolutions are an opportunity for reflection and optimism. As we look back on the past year, we should learn from our experience and approach the year in front of us with greater enthusiasm, in the hope that through that enthusiasm we can continue to grow, be better and healthier, and simply be more like the people we want to be.
The electronic health record affects all of our interactions with patients and colleagues, and, when not used optimally, encroaches into our personal and family lives. It is a perfect place to focus during the new year to enable us to have more productive, effective, and happier times both in the office and at home.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Be at war with your vices, at peace with your neighbors, and let every new year find you a better person.
– Benjamin Franklin
Traditionally, the new year is a time for reflection, looking back to review what could have been done better, and looking forward to the opportunity to rectify those inadequacies over the coming year. We thought we would take this opportunity to look at our use of the electronic health record and think about the things we might do over the next year to make our lives easier and our charting better.
Top of our list is a renewed commitment to finish our notes by the end of each session. Too many physicians we know rush through patient hours and then are left with 10-20 notes to finish at the end of the day. Realistically, this is when we least feel like completing notes. Such work encroaches on personal and family time, likely contributes to the burnout that has been increasing among physicians, and is much less likely to accurately represent the encounter than notes completed in real time.
As we have spoken with many of our colleagues, it has become clear to us that many clinicians have learned how to be “just proficient enough” in their use of their EHR; they are not pulling out their hair every 5 minutes in frustration, but they have not taken the extra time and effort that are needed to optimize their productivity. To efficiently use an EHR requires some time spent designing templates and macros to make it easy to repetitively carry out common tasks.
A lot of physicians – particularly physicians over 40 years of age – are still typing their notes with the ol’ two-finger hunt-and-peck technique. This is incredibly time consuming, inefficient, and frustrating.
While many solutions have been proposed, including having a scribe walk around with the doctor, the simplest and easiest to implement is voice transcription. Even though medical transcription software is expensive, the return on investment is large for those who do not type well. After a short period of training on the software, notes are generally of higher quality and are finished considerably faster than when typing. The technology also has the ability to learn the names of frequently used consultants, medications, and procedures, so users don’t even have to type uncommon names or words.
Another area in which we hope to advance over the next year is working more effectively as a team to share the documentation burden. Nurses and medical assistants – within the boundaries of their licensing – can be empowered to document in predefined areas of the chart as much as possible.
For example, given the fact that the prevalence of depression is about twice as high in patients with diabetes as it is in the general population, our medical assistants now screen our diabetes patients with a PHQ-2 depression screen and record the results in the chart. This has been good for our patients, satisfying for our medical assistants, and has offloaded this task from the doctors.
We need to think of more areas where we can facilitate team care and really make everyone – physicians, nurses, front staff, and patients – more satisfied with the care that is being given.
Most EHRs have a reminder function – the ability to prompt a user to follow up on an abnormal x-ray or lab results in case a patient does not come back into the office as recommended. Our sense is that most of us are not using this function. It is worth finding out how to use it and giving it a try.
Patient portals have gained a lot of traction over the past few years. For a little while, we were really making an effort to have patients register, so that currently many (but by far not most) of our patients have signed up. We want to make better use of this fantastic resource.
We say “fantastic” because when we talk to patients (or friends or family) who use the portal, they have shared that it really makes their lives easier. They are able to see their labs, ponder the meaning of their results (perhaps of a slightly high glucose or an LDL cholesterol level), and if they have questions, they can correspond electronically with their care providers. It enhances care and allows us to spend less time on the phone, while giving patients better access to information.
New year’s resolutions are an opportunity for reflection and optimism. As we look back on the past year, we should learn from our experience and approach the year in front of us with greater enthusiasm, in the hope that through that enthusiasm we can continue to grow, be better and healthier, and simply be more like the people we want to be.
The electronic health record affects all of our interactions with patients and colleagues, and, when not used optimally, encroaches into our personal and family lives. It is a perfect place to focus during the new year to enable us to have more productive, effective, and happier times both in the office and at home.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.














