User login
Pediatric Dermatology Consult - January 2017
Scabies
BY JENNA BOROK AND LAWRENCE EICHENFIELD, MD
Frontline Medical News
The scabies mite or the “itch mite” was discovered in 1687 as the first identifiable microorganism that caused human disease.1 Scabies is an infection of the epidermis with the mite, Sarcoptes scabiei variety hominis (S. scabiei) affecting approximately 300 million people worldwide.2 It is a common disease, especially among school-aged children and is particularly rampant in areas of poor sanitation and overcrowding.2,3
S. scabiei live in and on human skin where the impregnated female mite burrows into the stratum corneum and lays two to three eggs daily for as long as 30 days.3,4 The egg becomes a larva, which leaves the burrow and molts into a nymph, and it continues to molt into a mature mite.3 Mating then occurs during the mite stage. After mating, the male mite dies and the female completes the life cycle by burrowing back into the stratum corneum; this process takes about 2 weeks.3,4
Diagnosis
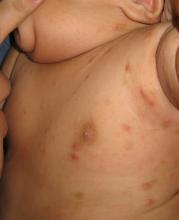
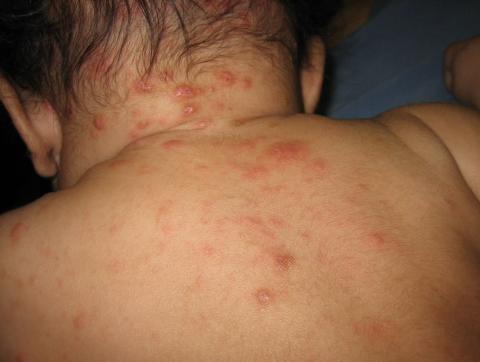
The diagnosis is usually based on strong clinical suspicion. The chief complaint is often a generalized itching rash that is often worse at nighttime.3,6 Small inflammatory papules are the main physical exam finding, and they usually are widely distributed, the favored locations including the finger webs, wrists, elbows, axillae, girdle area, and feet.3 In addition, male genitalia oftentimes are involved, and small itching papules on the penis should be considered scabies until proven otherwise.3 The head almost always is spared except in infants, who may present with pustules on the palms and soles of the feet and vesicles or lesions on the neck and face.7
The pathognomonic exam finding is the mite’s burrow, which appears as a 2-5 mm white, superficial, threadlike line. Upon close inspection, a tiny black speck often can be seen at the end of the burrow, which represents the adult mite.3 The presence of mites, eggs, or feces also is diagnostic and is accomplished by a skin scraping with a No. 15 blade.3 A positive scraping is diagnostic, although a negative scraping does not rule out the condition. (We explain this to our patients by saying “If you call someone’s home phone and they answer the phone, you know they are home. If you call and there is no answer, it could be that they aren’t home, or they are home and just not answering.”) A biopsy usually is not required, but may provide a diagnosis when scabies is unsuspected.3
The differential for red papules that itch in children includes atopic dermatitis, impetigo, papular urticaria, contact dermatitis, and other infestations, including bites from mosquitoes, fleas, and bed bugs. Atopic dermatitis (AD) can present as eczematous, erythematous papular lesions with oozing in flexural areas similar to areas affected by scabies. However, there are no burrows seen in AD, and the lesions are not in the interdigital web spaces. Impetigo has erythematous vesiculopustular lesions that form honey-colored crusts. Papular urticaria may be a hypersensitivity reaction to another insect, and the urticarial lesions are usually on the exposed parts of extremities. Unlike scabies, there are no burrows and usually no symptomatic family members. Contact dermatitis can present with vesicular, bullous, and sometimes papular erythematous lesions. It occurs after exposure to an allergen and often has well-demarcated borders with geometric shapes.
Treatment
Few randomized control or head-to-head trials exist on scabies treatment.2 First-line treatment is permethrin 5% cream, which is applied to the entire body surface from the neck down in children, but includes the face and scalp in infants.8,9 It is applied at bedtime and is washed off in the morning, and a second application is recommended after 7 days.3,8 It can be used in infants 2 months of age and older, and in pregnant females.3 Household contacts should be treated too, and those who are asymptomatic only require one application of permethrin.3 Permethrin is effective and more cost effective than ivermectin. Oral ivermectin has been used to treat scabies with a dose of 0.2 mg/kg and then repeated 2 weeks later, but the safety in children under 15 kg has not been determined.9 Lindane is an alternative option and is not recommended as first-line therapy because of its toxicity. It only should be used if the patient cannot tolerate or failed the previously mentioned therapies, with particular concern in children less than 50 kg.8,9 Infants and young children should be treated with permethrin and not with lindane.8 Clothes and bed linens can be decontaminated by machine-washing at a hot temperature.3,8 Topical corticosteroids and oral antihistamines can be helpful post-treatment to minimize pruritus and secondary eczematous changes.9
References
1. Int J Dermatol. 1998 Aug;37(8):625-30.
3. Lookingbill and Marks’ Principles of Dermatology, 5th edition (Philadelphia: Elsevier, 2013).
4. BMJ. 2005 Sep 17;331(7517):619-22.
5. Br Med J. 1941 Sep 20;2(4211):405-6.
6. Lancet. 2006 May 27;367(9524):1767-74.
7. Am J Clin Dermatol. 2002;3(1):9-18.
8. MMWR June 5, 2015 / 64(RR3);1-137.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Borok is a medical student at the University of California, Los Angeles. Dr. Eichenfield and Ms. Borok said they had no relevant financial disclosures. Email them at [email protected].
Scabies
BY JENNA BOROK AND LAWRENCE EICHENFIELD, MD
Frontline Medical News
The scabies mite or the “itch mite” was discovered in 1687 as the first identifiable microorganism that caused human disease.1 Scabies is an infection of the epidermis with the mite, Sarcoptes scabiei variety hominis (S. scabiei) affecting approximately 300 million people worldwide.2 It is a common disease, especially among school-aged children and is particularly rampant in areas of poor sanitation and overcrowding.2,3
S. scabiei live in and on human skin where the impregnated female mite burrows into the stratum corneum and lays two to three eggs daily for as long as 30 days.3,4 The egg becomes a larva, which leaves the burrow and molts into a nymph, and it continues to molt into a mature mite.3 Mating then occurs during the mite stage. After mating, the male mite dies and the female completes the life cycle by burrowing back into the stratum corneum; this process takes about 2 weeks.3,4
Diagnosis
The diagnosis is usually based on strong clinical suspicion. The chief complaint is often a generalized itching rash that is often worse at nighttime.3,6 Small inflammatory papules are the main physical exam finding, and they usually are widely distributed, the favored locations including the finger webs, wrists, elbows, axillae, girdle area, and feet.3 In addition, male genitalia oftentimes are involved, and small itching papules on the penis should be considered scabies until proven otherwise.3 The head almost always is spared except in infants, who may present with pustules on the palms and soles of the feet and vesicles or lesions on the neck and face.7
The pathognomonic exam finding is the mite’s burrow, which appears as a 2-5 mm white, superficial, threadlike line. Upon close inspection, a tiny black speck often can be seen at the end of the burrow, which represents the adult mite.3 The presence of mites, eggs, or feces also is diagnostic and is accomplished by a skin scraping with a No. 15 blade.3 A positive scraping is diagnostic, although a negative scraping does not rule out the condition. (We explain this to our patients by saying “If you call someone’s home phone and they answer the phone, you know they are home. If you call and there is no answer, it could be that they aren’t home, or they are home and just not answering.”) A biopsy usually is not required, but may provide a diagnosis when scabies is unsuspected.3
The differential for red papules that itch in children includes atopic dermatitis, impetigo, papular urticaria, contact dermatitis, and other infestations, including bites from mosquitoes, fleas, and bed bugs. Atopic dermatitis (AD) can present as eczematous, erythematous papular lesions with oozing in flexural areas similar to areas affected by scabies. However, there are no burrows seen in AD, and the lesions are not in the interdigital web spaces. Impetigo has erythematous vesiculopustular lesions that form honey-colored crusts. Papular urticaria may be a hypersensitivity reaction to another insect, and the urticarial lesions are usually on the exposed parts of extremities. Unlike scabies, there are no burrows and usually no symptomatic family members. Contact dermatitis can present with vesicular, bullous, and sometimes papular erythematous lesions. It occurs after exposure to an allergen and often has well-demarcated borders with geometric shapes.
Treatment
Few randomized control or head-to-head trials exist on scabies treatment.2 First-line treatment is permethrin 5% cream, which is applied to the entire body surface from the neck down in children, but includes the face and scalp in infants.8,9 It is applied at bedtime and is washed off in the morning, and a second application is recommended after 7 days.3,8 It can be used in infants 2 months of age and older, and in pregnant females.3 Household contacts should be treated too, and those who are asymptomatic only require one application of permethrin.3 Permethrin is effective and more cost effective than ivermectin. Oral ivermectin has been used to treat scabies with a dose of 0.2 mg/kg and then repeated 2 weeks later, but the safety in children under 15 kg has not been determined.9 Lindane is an alternative option and is not recommended as first-line therapy because of its toxicity. It only should be used if the patient cannot tolerate or failed the previously mentioned therapies, with particular concern in children less than 50 kg.8,9 Infants and young children should be treated with permethrin and not with lindane.8 Clothes and bed linens can be decontaminated by machine-washing at a hot temperature.3,8 Topical corticosteroids and oral antihistamines can be helpful post-treatment to minimize pruritus and secondary eczematous changes.9
References
1. Int J Dermatol. 1998 Aug;37(8):625-30.
3. Lookingbill and Marks’ Principles of Dermatology, 5th edition (Philadelphia: Elsevier, 2013).
4. BMJ. 2005 Sep 17;331(7517):619-22.
5. Br Med J. 1941 Sep 20;2(4211):405-6.
6. Lancet. 2006 May 27;367(9524):1767-74.
7. Am J Clin Dermatol. 2002;3(1):9-18.
8. MMWR June 5, 2015 / 64(RR3);1-137.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Borok is a medical student at the University of California, Los Angeles. Dr. Eichenfield and Ms. Borok said they had no relevant financial disclosures. Email them at [email protected].
Scabies
BY JENNA BOROK AND LAWRENCE EICHENFIELD, MD
Frontline Medical News
The scabies mite or the “itch mite” was discovered in 1687 as the first identifiable microorganism that caused human disease.1 Scabies is an infection of the epidermis with the mite, Sarcoptes scabiei variety hominis (S. scabiei) affecting approximately 300 million people worldwide.2 It is a common disease, especially among school-aged children and is particularly rampant in areas of poor sanitation and overcrowding.2,3
S. scabiei live in and on human skin where the impregnated female mite burrows into the stratum corneum and lays two to three eggs daily for as long as 30 days.3,4 The egg becomes a larva, which leaves the burrow and molts into a nymph, and it continues to molt into a mature mite.3 Mating then occurs during the mite stage. After mating, the male mite dies and the female completes the life cycle by burrowing back into the stratum corneum; this process takes about 2 weeks.3,4
Diagnosis
The diagnosis is usually based on strong clinical suspicion. The chief complaint is often a generalized itching rash that is often worse at nighttime.3,6 Small inflammatory papules are the main physical exam finding, and they usually are widely distributed, the favored locations including the finger webs, wrists, elbows, axillae, girdle area, and feet.3 In addition, male genitalia oftentimes are involved, and small itching papules on the penis should be considered scabies until proven otherwise.3 The head almost always is spared except in infants, who may present with pustules on the palms and soles of the feet and vesicles or lesions on the neck and face.7
The pathognomonic exam finding is the mite’s burrow, which appears as a 2-5 mm white, superficial, threadlike line. Upon close inspection, a tiny black speck often can be seen at the end of the burrow, which represents the adult mite.3 The presence of mites, eggs, or feces also is diagnostic and is accomplished by a skin scraping with a No. 15 blade.3 A positive scraping is diagnostic, although a negative scraping does not rule out the condition. (We explain this to our patients by saying “If you call someone’s home phone and they answer the phone, you know they are home. If you call and there is no answer, it could be that they aren’t home, or they are home and just not answering.”) A biopsy usually is not required, but may provide a diagnosis when scabies is unsuspected.3
The differential for red papules that itch in children includes atopic dermatitis, impetigo, papular urticaria, contact dermatitis, and other infestations, including bites from mosquitoes, fleas, and bed bugs. Atopic dermatitis (AD) can present as eczematous, erythematous papular lesions with oozing in flexural areas similar to areas affected by scabies. However, there are no burrows seen in AD, and the lesions are not in the interdigital web spaces. Impetigo has erythematous vesiculopustular lesions that form honey-colored crusts. Papular urticaria may be a hypersensitivity reaction to another insect, and the urticarial lesions are usually on the exposed parts of extremities. Unlike scabies, there are no burrows and usually no symptomatic family members. Contact dermatitis can present with vesicular, bullous, and sometimes papular erythematous lesions. It occurs after exposure to an allergen and often has well-demarcated borders with geometric shapes.
Treatment
Few randomized control or head-to-head trials exist on scabies treatment.2 First-line treatment is permethrin 5% cream, which is applied to the entire body surface from the neck down in children, but includes the face and scalp in infants.8,9 It is applied at bedtime and is washed off in the morning, and a second application is recommended after 7 days.3,8 It can be used in infants 2 months of age and older, and in pregnant females.3 Household contacts should be treated too, and those who are asymptomatic only require one application of permethrin.3 Permethrin is effective and more cost effective than ivermectin. Oral ivermectin has been used to treat scabies with a dose of 0.2 mg/kg and then repeated 2 weeks later, but the safety in children under 15 kg has not been determined.9 Lindane is an alternative option and is not recommended as first-line therapy because of its toxicity. It only should be used if the patient cannot tolerate or failed the previously mentioned therapies, with particular concern in children less than 50 kg.8,9 Infants and young children should be treated with permethrin and not with lindane.8 Clothes and bed linens can be decontaminated by machine-washing at a hot temperature.3,8 Topical corticosteroids and oral antihistamines can be helpful post-treatment to minimize pruritus and secondary eczematous changes.9
References
1. Int J Dermatol. 1998 Aug;37(8):625-30.
3. Lookingbill and Marks’ Principles of Dermatology, 5th edition (Philadelphia: Elsevier, 2013).
4. BMJ. 2005 Sep 17;331(7517):619-22.
5. Br Med J. 1941 Sep 20;2(4211):405-6.
6. Lancet. 2006 May 27;367(9524):1767-74.
7. Am J Clin Dermatol. 2002;3(1):9-18.
8. MMWR June 5, 2015 / 64(RR3);1-137.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Borok is a medical student at the University of California, Los Angeles. Dr. Eichenfield and Ms. Borok said they had no relevant financial disclosures. Email them at [email protected].
A 3-month-old boy presents to his physician for evaluation of a diffusely itchy rash. The rash started about 1 month ago and has been getting progressively worse; it is now very pruritic. The rash is diffuse, but includes the face, trunk, hands, and feet.
He is otherwise healthy and has no history of eczema or infections.
There are no animals at home. The infant was born at term with an unremarkable perinatal history.
FDA approves ibrutinib to treat rel/ref MZL

Photo courtesy of
Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica®) for the treatment of marginal zone lymphoma (MZL).
The drug is now approved to treat patients with relapsed/refractory MZL who require systemic therapy and have received at least 1 prior anti-CD20-based therapy.
Ibrutinib has accelerated approval for this indication, based on the overall response rate the drug produced in a phase 2 trial.
Continued approval of ibrutinib as a treatment for MZL may be contingent upon verification and description of clinical benefit in a confirmatory trial.
The FDA’s approval of ibrutinib for MZL makes it the first treatment approved specifically for patients with this disease. It also marks the seventh FDA approval and fifth disease indication for ibrutinib since the drug was first approved in 2013.
Ibrutinib is also FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, patients with mantle cell lymphoma who have received at least 1 prior therapy, and patients with Waldenström’s macroglobulinemia. The approval for mantle cell lymphoma is an accelerated approval.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Phase 2 trial
The FDA’s approval of ibrutinib for MZL is based on data from the phase 2, single-arm PCYC-1121 study, in which researchers evaluated the drug in MZL patients who required systemic therapy and had received at least 1 prior anti-CD20-based therapy.
Results from this study were presented at the 2016 ASH Annual Meeting (abstract 1213).
The efficacy analysis included 63 patients with 3 subtypes of MZL: mucosa-associated lymphoid tissue (n=32), nodal (n=17), and splenic (n=14).
The overall response rate was 46%, with a partial response rate of 42.9% and a complete response rate of 3.2%. Responses were observed across all 3 MZL subtypes.
The median time to response was 4.5 months (range, 2.3-16.4 months). And the median duration of response was not reached (range, 16.7 months to not reached).
Overall, the safety data from this study was consistent with the known safety profile of ibrutinib in B-cell malignancies.
The most common adverse events of all grades (occurring in >20% of patients) were thrombocytopenia (49%), fatigue (44%), anemia (43%), diarrhea (43%), bruising (41%), musculoskeletal pain (40%), hemorrhage (30%), rash (29%), nausea (25%), peripheral edema (24%), arthralgia (24%), neutropenia (22%), cough (22%), dyspnea (21%), and upper respiratory tract infection (21%).
The most common (>10%) grade 3 or 4 events were decreases in hemoglobin and neutrophils (13% each) and pneumonia (10%).
The risks associated with ibrutinib as listed in the Warnings and Precautions section of the prescribing information are hemorrhage, infections, cytopenias, atrial fibrillation, hypertension, secondary primary malignancies, tumor lysis syndrome, and embryo fetal toxicities. ![]()

Photo courtesy of
Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica®) for the treatment of marginal zone lymphoma (MZL).
The drug is now approved to treat patients with relapsed/refractory MZL who require systemic therapy and have received at least 1 prior anti-CD20-based therapy.
Ibrutinib has accelerated approval for this indication, based on the overall response rate the drug produced in a phase 2 trial.
Continued approval of ibrutinib as a treatment for MZL may be contingent upon verification and description of clinical benefit in a confirmatory trial.
The FDA’s approval of ibrutinib for MZL makes it the first treatment approved specifically for patients with this disease. It also marks the seventh FDA approval and fifth disease indication for ibrutinib since the drug was first approved in 2013.
Ibrutinib is also FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, patients with mantle cell lymphoma who have received at least 1 prior therapy, and patients with Waldenström’s macroglobulinemia. The approval for mantle cell lymphoma is an accelerated approval.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Phase 2 trial
The FDA’s approval of ibrutinib for MZL is based on data from the phase 2, single-arm PCYC-1121 study, in which researchers evaluated the drug in MZL patients who required systemic therapy and had received at least 1 prior anti-CD20-based therapy.
Results from this study were presented at the 2016 ASH Annual Meeting (abstract 1213).
The efficacy analysis included 63 patients with 3 subtypes of MZL: mucosa-associated lymphoid tissue (n=32), nodal (n=17), and splenic (n=14).
The overall response rate was 46%, with a partial response rate of 42.9% and a complete response rate of 3.2%. Responses were observed across all 3 MZL subtypes.
The median time to response was 4.5 months (range, 2.3-16.4 months). And the median duration of response was not reached (range, 16.7 months to not reached).
Overall, the safety data from this study was consistent with the known safety profile of ibrutinib in B-cell malignancies.
The most common adverse events of all grades (occurring in >20% of patients) were thrombocytopenia (49%), fatigue (44%), anemia (43%), diarrhea (43%), bruising (41%), musculoskeletal pain (40%), hemorrhage (30%), rash (29%), nausea (25%), peripheral edema (24%), arthralgia (24%), neutropenia (22%), cough (22%), dyspnea (21%), and upper respiratory tract infection (21%).
The most common (>10%) grade 3 or 4 events were decreases in hemoglobin and neutrophils (13% each) and pneumonia (10%).
The risks associated with ibrutinib as listed in the Warnings and Precautions section of the prescribing information are hemorrhage, infections, cytopenias, atrial fibrillation, hypertension, secondary primary malignancies, tumor lysis syndrome, and embryo fetal toxicities. ![]()

Photo courtesy of
Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica®) for the treatment of marginal zone lymphoma (MZL).
The drug is now approved to treat patients with relapsed/refractory MZL who require systemic therapy and have received at least 1 prior anti-CD20-based therapy.
Ibrutinib has accelerated approval for this indication, based on the overall response rate the drug produced in a phase 2 trial.
Continued approval of ibrutinib as a treatment for MZL may be contingent upon verification and description of clinical benefit in a confirmatory trial.
The FDA’s approval of ibrutinib for MZL makes it the first treatment approved specifically for patients with this disease. It also marks the seventh FDA approval and fifth disease indication for ibrutinib since the drug was first approved in 2013.
Ibrutinib is also FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, patients with mantle cell lymphoma who have received at least 1 prior therapy, and patients with Waldenström’s macroglobulinemia. The approval for mantle cell lymphoma is an accelerated approval.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Phase 2 trial
The FDA’s approval of ibrutinib for MZL is based on data from the phase 2, single-arm PCYC-1121 study, in which researchers evaluated the drug in MZL patients who required systemic therapy and had received at least 1 prior anti-CD20-based therapy.
Results from this study were presented at the 2016 ASH Annual Meeting (abstract 1213).
The efficacy analysis included 63 patients with 3 subtypes of MZL: mucosa-associated lymphoid tissue (n=32), nodal (n=17), and splenic (n=14).
The overall response rate was 46%, with a partial response rate of 42.9% and a complete response rate of 3.2%. Responses were observed across all 3 MZL subtypes.
The median time to response was 4.5 months (range, 2.3-16.4 months). And the median duration of response was not reached (range, 16.7 months to not reached).
Overall, the safety data from this study was consistent with the known safety profile of ibrutinib in B-cell malignancies.
The most common adverse events of all grades (occurring in >20% of patients) were thrombocytopenia (49%), fatigue (44%), anemia (43%), diarrhea (43%), bruising (41%), musculoskeletal pain (40%), hemorrhage (30%), rash (29%), nausea (25%), peripheral edema (24%), arthralgia (24%), neutropenia (22%), cough (22%), dyspnea (21%), and upper respiratory tract infection (21%).
The most common (>10%) grade 3 or 4 events were decreases in hemoglobin and neutrophils (13% each) and pneumonia (10%).
The risks associated with ibrutinib as listed in the Warnings and Precautions section of the prescribing information are hemorrhage, infections, cytopenias, atrial fibrillation, hypertension, secondary primary malignancies, tumor lysis syndrome, and embryo fetal toxicities. ![]()
PI ties to industry linked to positive trial results

capsules for a clinical trial
Photo by Esther Dyson
Financial ties between principal investigators (PIs) and drug companies are independently associated with positive clinical trial results, according to new research.
The study showed a significant association between positive trial outcomes and PIs having financial ties to the manufacturer of the study drug, even after accounting for the source of research funding.
Researchers reported these findings in The BMJ.
Salomeh Keyhani, MD, of the University of California, San Francisco, and her colleagues conducted this research, analyzing a random sample of 195 drug trials published in 2013.
The team found financial ties between PIs and manufacturers of the study drug for 67.7% of the studies (n=132). In all, 58% of the PIs had financial ties to the manufacturers (231/397).
Types of financial ties included:
- Advisor/consultancy payments (39%)
- Speakers’ fees (20%)
- Unspecified financial ties (20%)
- Honoraria (13%)
- Employee relationships (13%)
- Travel fees (13%)
- Stock ownership (10%)
- Having a patent related to the study drug (5%).
PIs reported financial ties to the drug manufacturer in 76% (103/136) of studies with positive results and 49% (29/59) of studies with negative results (P<0.001).
In a multivariate analysis adjusted for the study’s funding source, a financial tie was significantly associated with a positive trial outcome. The odds ratio was 3.57 (P=0.001).
In a multivariate analysis adjusted for a range of other study-related factors as well, a financial tie remained significantly associated with a positive trial outcome. The odds ratio was 3.37 (P=0.006).
Dr Keyhani and her colleagues stressed that this analysis was observational and cannot be used to draw conclusions about causation.
However, they said, given the importance of industry and academic collaboration in advancing the development of new treatments, “more thought needs to be given to the roles that investigators, policy makers, and journal editors can play in ensuring the credibility of the evidence base.”
Authors of a related editorial said more research is needed to determine how industry funding and financial ties could influence trial results.
The authors—Andreas Lundh, PhD, of the University of Southern Denmark, and Lisa Bero, PhD, of the University of Sydney in Australia—urged trial investigators to share their data and participate in industry-funded trials only if data are made publicly available.
The authors also suggested journals could help by rejecting research by investigators who are unwilling to share their data and by penalizing investigators who fail to disclose financial ties. The role of sponsors, or companies with which investigators have ties, in the research must also be transparent.
In the meantime, trials with industry funding or investigators with financial ties “should be interpreted with caution until all relevant information is fully disclosed and easily accessible,” the authors concluded. ![]()

capsules for a clinical trial
Photo by Esther Dyson
Financial ties between principal investigators (PIs) and drug companies are independently associated with positive clinical trial results, according to new research.
The study showed a significant association between positive trial outcomes and PIs having financial ties to the manufacturer of the study drug, even after accounting for the source of research funding.
Researchers reported these findings in The BMJ.
Salomeh Keyhani, MD, of the University of California, San Francisco, and her colleagues conducted this research, analyzing a random sample of 195 drug trials published in 2013.
The team found financial ties between PIs and manufacturers of the study drug for 67.7% of the studies (n=132). In all, 58% of the PIs had financial ties to the manufacturers (231/397).
Types of financial ties included:
- Advisor/consultancy payments (39%)
- Speakers’ fees (20%)
- Unspecified financial ties (20%)
- Honoraria (13%)
- Employee relationships (13%)
- Travel fees (13%)
- Stock ownership (10%)
- Having a patent related to the study drug (5%).
PIs reported financial ties to the drug manufacturer in 76% (103/136) of studies with positive results and 49% (29/59) of studies with negative results (P<0.001).
In a multivariate analysis adjusted for the study’s funding source, a financial tie was significantly associated with a positive trial outcome. The odds ratio was 3.57 (P=0.001).
In a multivariate analysis adjusted for a range of other study-related factors as well, a financial tie remained significantly associated with a positive trial outcome. The odds ratio was 3.37 (P=0.006).
Dr Keyhani and her colleagues stressed that this analysis was observational and cannot be used to draw conclusions about causation.
However, they said, given the importance of industry and academic collaboration in advancing the development of new treatments, “more thought needs to be given to the roles that investigators, policy makers, and journal editors can play in ensuring the credibility of the evidence base.”
Authors of a related editorial said more research is needed to determine how industry funding and financial ties could influence trial results.
The authors—Andreas Lundh, PhD, of the University of Southern Denmark, and Lisa Bero, PhD, of the University of Sydney in Australia—urged trial investigators to share their data and participate in industry-funded trials only if data are made publicly available.
The authors also suggested journals could help by rejecting research by investigators who are unwilling to share their data and by penalizing investigators who fail to disclose financial ties. The role of sponsors, or companies with which investigators have ties, in the research must also be transparent.
In the meantime, trials with industry funding or investigators with financial ties “should be interpreted with caution until all relevant information is fully disclosed and easily accessible,” the authors concluded. ![]()

capsules for a clinical trial
Photo by Esther Dyson
Financial ties between principal investigators (PIs) and drug companies are independently associated with positive clinical trial results, according to new research.
The study showed a significant association between positive trial outcomes and PIs having financial ties to the manufacturer of the study drug, even after accounting for the source of research funding.
Researchers reported these findings in The BMJ.
Salomeh Keyhani, MD, of the University of California, San Francisco, and her colleagues conducted this research, analyzing a random sample of 195 drug trials published in 2013.
The team found financial ties between PIs and manufacturers of the study drug for 67.7% of the studies (n=132). In all, 58% of the PIs had financial ties to the manufacturers (231/397).
Types of financial ties included:
- Advisor/consultancy payments (39%)
- Speakers’ fees (20%)
- Unspecified financial ties (20%)
- Honoraria (13%)
- Employee relationships (13%)
- Travel fees (13%)
- Stock ownership (10%)
- Having a patent related to the study drug (5%).
PIs reported financial ties to the drug manufacturer in 76% (103/136) of studies with positive results and 49% (29/59) of studies with negative results (P<0.001).
In a multivariate analysis adjusted for the study’s funding source, a financial tie was significantly associated with a positive trial outcome. The odds ratio was 3.57 (P=0.001).
In a multivariate analysis adjusted for a range of other study-related factors as well, a financial tie remained significantly associated with a positive trial outcome. The odds ratio was 3.37 (P=0.006).
Dr Keyhani and her colleagues stressed that this analysis was observational and cannot be used to draw conclusions about causation.
However, they said, given the importance of industry and academic collaboration in advancing the development of new treatments, “more thought needs to be given to the roles that investigators, policy makers, and journal editors can play in ensuring the credibility of the evidence base.”
Authors of a related editorial said more research is needed to determine how industry funding and financial ties could influence trial results.
The authors—Andreas Lundh, PhD, of the University of Southern Denmark, and Lisa Bero, PhD, of the University of Sydney in Australia—urged trial investigators to share their data and participate in industry-funded trials only if data are made publicly available.
The authors also suggested journals could help by rejecting research by investigators who are unwilling to share their data and by penalizing investigators who fail to disclose financial ties. The role of sponsors, or companies with which investigators have ties, in the research must also be transparent.
In the meantime, trials with industry funding or investigators with financial ties “should be interpreted with caution until all relevant information is fully disclosed and easily accessible,” the authors concluded. ![]()
US agencies update regulations on research subjects

tumor in a test tube
Photo by Rhoda Baer
The US Department of Health and Human Services (HHS) and 15 other federal agencies have issued a final rule to update regulations intended to safeguard individuals who participate in research.
Most provisions in the new rule will go into effect in 2018.
The HHS says the new rule strengthens protections for people who volunteer to participate in research, while ensuring that the oversight system does not add inappropriate administrative burdens.
The current regulations, which have been in place since 1991, are often referred to as the “Common Rule.”
In September 2015, HHS and the other Common Rule agencies published a proposed new rule regarding research subjects—a notice of proposed rulemaking (NPRM)—which drew more than 2100 comments.
In response to concerns raised during the review process, the final rule contains a number of significant changes from the NPRM.
Research covered
The final rule does not cover clinical trials that are not federally funded.
The Common Rule has historically applied only to research conducted or supported by a Common Rule department or agency. And, although the NPRM proposed changing this policy, the final rule remains in line with the Common Rule.
Consent
The final rule requires consent forms to provide potential research subjects with a better understanding of a project’s scope so they can make a more fully informed decision about whether to participate.
Consent forms should include a concise explanation—at the beginning of the document—of the information that would be most important to individuals contemplating participation in a particular study, including the purpose of the research, the risks and benefits, and appropriate alternative treatments that might be beneficial to the prospective subject.
The rule also requires that consent forms for certain federally funded clinical trials be posted on a public website.
Institutional review boards
The final rule requires, in many cases, use of a single institutional review board (IRB) for multi-institutional research studies.
However, the final rule has been modified from the NPRM to add substantial increased flexibility in now allowing broad groups of studies (instead of just specific studies) to be removed from this requirement.
Privacy
The final rule does not include the standardized privacy safeguards for identifiable private information and identifiable biospecimens that were proposed in the NPRM.
In most respects, the final rule retains the current approach to privacy standards.
For studies on stored identifiable data or identifiable biospecimens, researchers will have the option of relying on broad consent obtained for future research as an alternative to seeking IRB approval to waive the consent requirement.
As under the current rule, researchers will not have to obtain consent for studies on non-identified stored data or biospecimens.
Exemptions
The final rule establishes new exempt categories of research based on the level of risk they pose to participants.
For example, to reduce unnecessary regulatory burden and allow IRBs to focus their attention on higher-risk studies, there is a new exemption for secondary research involving identifiable private information if the research is regulated by and participants are protected under the HIPAA rules.
Review
The final rule removes the requirement to conduct continuing review of ongoing research studies in certain instances where such review does little to protect subjects.
For more details, see the final rule. ![]()

tumor in a test tube
Photo by Rhoda Baer
The US Department of Health and Human Services (HHS) and 15 other federal agencies have issued a final rule to update regulations intended to safeguard individuals who participate in research.
Most provisions in the new rule will go into effect in 2018.
The HHS says the new rule strengthens protections for people who volunteer to participate in research, while ensuring that the oversight system does not add inappropriate administrative burdens.
The current regulations, which have been in place since 1991, are often referred to as the “Common Rule.”
In September 2015, HHS and the other Common Rule agencies published a proposed new rule regarding research subjects—a notice of proposed rulemaking (NPRM)—which drew more than 2100 comments.
In response to concerns raised during the review process, the final rule contains a number of significant changes from the NPRM.
Research covered
The final rule does not cover clinical trials that are not federally funded.
The Common Rule has historically applied only to research conducted or supported by a Common Rule department or agency. And, although the NPRM proposed changing this policy, the final rule remains in line with the Common Rule.
Consent
The final rule requires consent forms to provide potential research subjects with a better understanding of a project’s scope so they can make a more fully informed decision about whether to participate.
Consent forms should include a concise explanation—at the beginning of the document—of the information that would be most important to individuals contemplating participation in a particular study, including the purpose of the research, the risks and benefits, and appropriate alternative treatments that might be beneficial to the prospective subject.
The rule also requires that consent forms for certain federally funded clinical trials be posted on a public website.
Institutional review boards
The final rule requires, in many cases, use of a single institutional review board (IRB) for multi-institutional research studies.
However, the final rule has been modified from the NPRM to add substantial increased flexibility in now allowing broad groups of studies (instead of just specific studies) to be removed from this requirement.
Privacy
The final rule does not include the standardized privacy safeguards for identifiable private information and identifiable biospecimens that were proposed in the NPRM.
In most respects, the final rule retains the current approach to privacy standards.
For studies on stored identifiable data or identifiable biospecimens, researchers will have the option of relying on broad consent obtained for future research as an alternative to seeking IRB approval to waive the consent requirement.
As under the current rule, researchers will not have to obtain consent for studies on non-identified stored data or biospecimens.
Exemptions
The final rule establishes new exempt categories of research based on the level of risk they pose to participants.
For example, to reduce unnecessary regulatory burden and allow IRBs to focus their attention on higher-risk studies, there is a new exemption for secondary research involving identifiable private information if the research is regulated by and participants are protected under the HIPAA rules.
Review
The final rule removes the requirement to conduct continuing review of ongoing research studies in certain instances where such review does little to protect subjects.
For more details, see the final rule. ![]()

tumor in a test tube
Photo by Rhoda Baer
The US Department of Health and Human Services (HHS) and 15 other federal agencies have issued a final rule to update regulations intended to safeguard individuals who participate in research.
Most provisions in the new rule will go into effect in 2018.
The HHS says the new rule strengthens protections for people who volunteer to participate in research, while ensuring that the oversight system does not add inappropriate administrative burdens.
The current regulations, which have been in place since 1991, are often referred to as the “Common Rule.”
In September 2015, HHS and the other Common Rule agencies published a proposed new rule regarding research subjects—a notice of proposed rulemaking (NPRM)—which drew more than 2100 comments.
In response to concerns raised during the review process, the final rule contains a number of significant changes from the NPRM.
Research covered
The final rule does not cover clinical trials that are not federally funded.
The Common Rule has historically applied only to research conducted or supported by a Common Rule department or agency. And, although the NPRM proposed changing this policy, the final rule remains in line with the Common Rule.
Consent
The final rule requires consent forms to provide potential research subjects with a better understanding of a project’s scope so they can make a more fully informed decision about whether to participate.
Consent forms should include a concise explanation—at the beginning of the document—of the information that would be most important to individuals contemplating participation in a particular study, including the purpose of the research, the risks and benefits, and appropriate alternative treatments that might be beneficial to the prospective subject.
The rule also requires that consent forms for certain federally funded clinical trials be posted on a public website.
Institutional review boards
The final rule requires, in many cases, use of a single institutional review board (IRB) for multi-institutional research studies.
However, the final rule has been modified from the NPRM to add substantial increased flexibility in now allowing broad groups of studies (instead of just specific studies) to be removed from this requirement.
Privacy
The final rule does not include the standardized privacy safeguards for identifiable private information and identifiable biospecimens that were proposed in the NPRM.
In most respects, the final rule retains the current approach to privacy standards.
For studies on stored identifiable data or identifiable biospecimens, researchers will have the option of relying on broad consent obtained for future research as an alternative to seeking IRB approval to waive the consent requirement.
As under the current rule, researchers will not have to obtain consent for studies on non-identified stored data or biospecimens.
Exemptions
The final rule establishes new exempt categories of research based on the level of risk they pose to participants.
For example, to reduce unnecessary regulatory burden and allow IRBs to focus their attention on higher-risk studies, there is a new exemption for secondary research involving identifiable private information if the research is regulated by and participants are protected under the HIPAA rules.
Review
The final rule removes the requirement to conduct continuing review of ongoing research studies in certain instances where such review does little to protect subjects.
For more details, see the final rule. ![]()
Group creates library of SCD-specific iPSCs

Photo from Salk Institute
Researchers have created an induced pluripotent stem cell (iPSC) library intended to aid the study of sickle cell disease (SCD).
The library consists of iPSCs generated from blood samples taken from ethnically diverse SCD patients from around the world.
The researchers say these iPSCs can be used to create disease models, which may allow scientists to better understand how SCD occurs and develop and test new treatments for the disease.
As a complement to the library, the researchers also designed CRISPR/Cas gene editing tools to correct the sickle hemoglobin mutation.
The team described this work in Stem Cell Reports.
“Sickle cell disease affects millions of people worldwide and is an emerging global health burden,” said study author George Murphy, PhD, of the Center for Regenerative Medicine at Boston University School of Medicine in Massachusetts.
“iPSCs have the potential to revolutionize the way we study human development, model life-threatening diseases, and, eventually, treat patients.”
The researchers’ library includes SCD-specific iPSCs from patients of different ethnicities with different β-globin gene haplotypes and fetal hemoglobin levels.
The researchers generated 54 iPSC lines from blood samples collected from individuals of African American, Brazilian, and Saudi Arabian descent. Both genders were represented, as well as a range of ages (3 to 53 years of age).
Most of the cell lines in the library, along with accompanying genetic and hematologic data, are freely available via the WiCell website.
“In addition to the library, we’ve designed and are using gene editing tools to correct the sickle hemoglobin mutation using the stem cell lines,” said Gustavo Mostoslavsky, MD, PhD, also of the Center for Regenerative Medicine at Boston University School of Medicine.
“When coupled with corrected sickle cell disease-specific iPSCs, these tools could one day provide a functional cure for the disorder.” ![]()

Photo from Salk Institute
Researchers have created an induced pluripotent stem cell (iPSC) library intended to aid the study of sickle cell disease (SCD).
The library consists of iPSCs generated from blood samples taken from ethnically diverse SCD patients from around the world.
The researchers say these iPSCs can be used to create disease models, which may allow scientists to better understand how SCD occurs and develop and test new treatments for the disease.
As a complement to the library, the researchers also designed CRISPR/Cas gene editing tools to correct the sickle hemoglobin mutation.
The team described this work in Stem Cell Reports.
“Sickle cell disease affects millions of people worldwide and is an emerging global health burden,” said study author George Murphy, PhD, of the Center for Regenerative Medicine at Boston University School of Medicine in Massachusetts.
“iPSCs have the potential to revolutionize the way we study human development, model life-threatening diseases, and, eventually, treat patients.”
The researchers’ library includes SCD-specific iPSCs from patients of different ethnicities with different β-globin gene haplotypes and fetal hemoglobin levels.
The researchers generated 54 iPSC lines from blood samples collected from individuals of African American, Brazilian, and Saudi Arabian descent. Both genders were represented, as well as a range of ages (3 to 53 years of age).
Most of the cell lines in the library, along with accompanying genetic and hematologic data, are freely available via the WiCell website.
“In addition to the library, we’ve designed and are using gene editing tools to correct the sickle hemoglobin mutation using the stem cell lines,” said Gustavo Mostoslavsky, MD, PhD, also of the Center for Regenerative Medicine at Boston University School of Medicine.
“When coupled with corrected sickle cell disease-specific iPSCs, these tools could one day provide a functional cure for the disorder.” ![]()

Photo from Salk Institute
Researchers have created an induced pluripotent stem cell (iPSC) library intended to aid the study of sickle cell disease (SCD).
The library consists of iPSCs generated from blood samples taken from ethnically diverse SCD patients from around the world.
The researchers say these iPSCs can be used to create disease models, which may allow scientists to better understand how SCD occurs and develop and test new treatments for the disease.
As a complement to the library, the researchers also designed CRISPR/Cas gene editing tools to correct the sickle hemoglobin mutation.
The team described this work in Stem Cell Reports.
“Sickle cell disease affects millions of people worldwide and is an emerging global health burden,” said study author George Murphy, PhD, of the Center for Regenerative Medicine at Boston University School of Medicine in Massachusetts.
“iPSCs have the potential to revolutionize the way we study human development, model life-threatening diseases, and, eventually, treat patients.”
The researchers’ library includes SCD-specific iPSCs from patients of different ethnicities with different β-globin gene haplotypes and fetal hemoglobin levels.
The researchers generated 54 iPSC lines from blood samples collected from individuals of African American, Brazilian, and Saudi Arabian descent. Both genders were represented, as well as a range of ages (3 to 53 years of age).
Most of the cell lines in the library, along with accompanying genetic and hematologic data, are freely available via the WiCell website.
“In addition to the library, we’ve designed and are using gene editing tools to correct the sickle hemoglobin mutation using the stem cell lines,” said Gustavo Mostoslavsky, MD, PhD, also of the Center for Regenerative Medicine at Boston University School of Medicine.
“When coupled with corrected sickle cell disease-specific iPSCs, these tools could one day provide a functional cure for the disorder.” ![]()
The Importance of Subclavian Angiography in the Evaluation of Chest Pain: Coronary-Subclavian Steal Syndrome
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
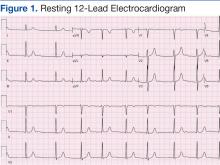
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
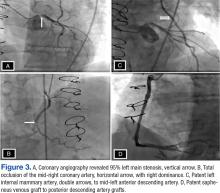
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
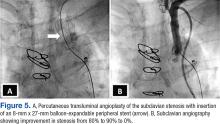
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
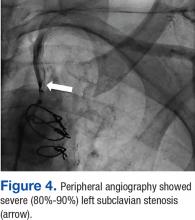
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
How should urine electrolytes be ordered and interpreted in acute kidney injury and electrolyte abnormalities?
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.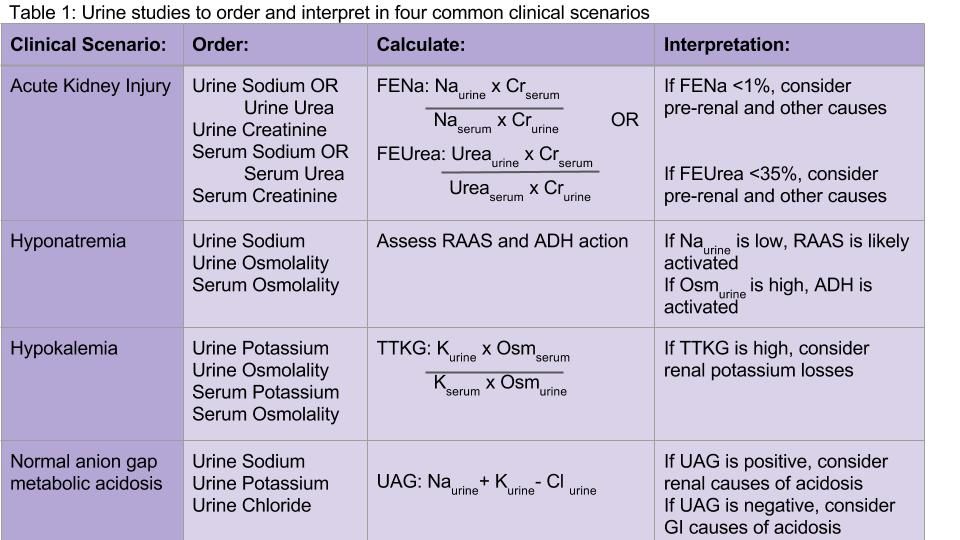
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
Sneak Peek: Journal of Hospital Medicine
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
From the Washington Office: ACS Works to Establish Joint Trauma System in NDAA
I am frequently asked for examples of successes from the College’s advocacy efforts in DC. While many of our successes come in the form of legislation or regulation we are either able to significantly modify into more favorable form or to outright prevent from being enacted, this month’s topic provides an example of how our advocacy efforts are equally successful in obtaining specific provisions in legislation.
Over a year ago, staff of the Division of Advocacy and Health Policy were approached by members and staff of the Military Health System Strategic Partnership American College of Surgeons to assist them in their effort toward the establishment of both a Joint Trauma System (JTS) within the Defense Health Agency (to promote continuous improvement of trauma care provided to members of the Armed Forces) and a Joint Trauma Education and Training Directorate (JTETD) (to ensure military traumatologists maintain readiness with regard to critical surgical skills). I am pleased and proud to report that when the U.S. House of Representatives, on December 2, and the Senate, on December 8, passed the National Defense Authorization Act (NDAA), provisions for both the JTS and the JTETD, in nearly the precise wording as was proposed by ACS, were included in the legislation.
Our success in this effort was strongly supported by both Rep. Joe Heck, DO (R-Nev.), Chairman of the House Armed Services Subcommittee on Military Personnel, and Rep. Brad Wenstrup, DPM (R-Ohio). Rep. Heck, who is a Brigadier General in the Army Reserve, ensured that the language establishing the JTS within the U.S. Department of Defense and the JTETD were included in the House version of the NDAA. Rep. Wenstrup, who also serves in the Army Reserve, was key to securing language providing for review of the military trauma system under the JTS by a “non-government entity with subject matter experts.” This is an activity that the ACS Committee on Trauma Verification, Review, and Consultation Program conducts on a regular basis.
The Joint Trauma System will standardize trauma care in the military by establishing uniform standards for all military medical treatment facilities. The Joint Trauma Education and Training Directorate is charged with ensuring that trauma providers of the U.S. Armed Forces maintain a state of readiness. Under this provision, partnerships will be established with level one trauma centers in civilian academic medical centers and large metropolitan teaching hospitals where combat casualty care teams will embed to provide military surgeons with regular exposure to critically injured patients.
The Senate version of the NDAA did not contain language specifically outlining provisions for either the JTS or the JTETD. Because the House and Senate versions of the NDAA were different, a conference committee from both legislative bodies was appointed to settle the differences between the two versions of the legislation. Over the several months duration of the conference committee process, members of the ACS legislative affairs team met regularly with the offices of several key senators who serve on the Senate Armed Services Committee as well as with committee staff for both the Republican and Democrat members of the committee. During these meetings, we repeatedly “made the case” relative to the critically important nature of these provisions and were able to answer questions and address concerns relative to why ACS felt it was vitally important to include the House language in the final version of the bill. No doubt, these efforts were key in the decision of the Senate negotiators to recede their position and agree to the House language in the final version of the bill relative to these specific provisions.
Prior to the final House vote on the conference committee language of the NDAA, Rep. Wenstrup spoke on the House floor in support of the JTETD.
As I write, the legislation is awaiting signature by President Obama and it is expected he will do so in the coming days.
ACS’ successful efforts toward the establishment of the Joint Trauma System and the Joint Trauma Education and Training Directorate represent a significant achievement toward ensuring that our soldiers, sailors, airmen, Marines and guardsmen continue to receive the best of the best in trauma care while in the service of our nation.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
I am frequently asked for examples of successes from the College’s advocacy efforts in DC. While many of our successes come in the form of legislation or regulation we are either able to significantly modify into more favorable form or to outright prevent from being enacted, this month’s topic provides an example of how our advocacy efforts are equally successful in obtaining specific provisions in legislation.
Over a year ago, staff of the Division of Advocacy and Health Policy were approached by members and staff of the Military Health System Strategic Partnership American College of Surgeons to assist them in their effort toward the establishment of both a Joint Trauma System (JTS) within the Defense Health Agency (to promote continuous improvement of trauma care provided to members of the Armed Forces) and a Joint Trauma Education and Training Directorate (JTETD) (to ensure military traumatologists maintain readiness with regard to critical surgical skills). I am pleased and proud to report that when the U.S. House of Representatives, on December 2, and the Senate, on December 8, passed the National Defense Authorization Act (NDAA), provisions for both the JTS and the JTETD, in nearly the precise wording as was proposed by ACS, were included in the legislation.
Our success in this effort was strongly supported by both Rep. Joe Heck, DO (R-Nev.), Chairman of the House Armed Services Subcommittee on Military Personnel, and Rep. Brad Wenstrup, DPM (R-Ohio). Rep. Heck, who is a Brigadier General in the Army Reserve, ensured that the language establishing the JTS within the U.S. Department of Defense and the JTETD were included in the House version of the NDAA. Rep. Wenstrup, who also serves in the Army Reserve, was key to securing language providing for review of the military trauma system under the JTS by a “non-government entity with subject matter experts.” This is an activity that the ACS Committee on Trauma Verification, Review, and Consultation Program conducts on a regular basis.
The Joint Trauma System will standardize trauma care in the military by establishing uniform standards for all military medical treatment facilities. The Joint Trauma Education and Training Directorate is charged with ensuring that trauma providers of the U.S. Armed Forces maintain a state of readiness. Under this provision, partnerships will be established with level one trauma centers in civilian academic medical centers and large metropolitan teaching hospitals where combat casualty care teams will embed to provide military surgeons with regular exposure to critically injured patients.
The Senate version of the NDAA did not contain language specifically outlining provisions for either the JTS or the JTETD. Because the House and Senate versions of the NDAA were different, a conference committee from both legislative bodies was appointed to settle the differences between the two versions of the legislation. Over the several months duration of the conference committee process, members of the ACS legislative affairs team met regularly with the offices of several key senators who serve on the Senate Armed Services Committee as well as with committee staff for both the Republican and Democrat members of the committee. During these meetings, we repeatedly “made the case” relative to the critically important nature of these provisions and were able to answer questions and address concerns relative to why ACS felt it was vitally important to include the House language in the final version of the bill. No doubt, these efforts were key in the decision of the Senate negotiators to recede their position and agree to the House language in the final version of the bill relative to these specific provisions.
Prior to the final House vote on the conference committee language of the NDAA, Rep. Wenstrup spoke on the House floor in support of the JTETD.
As I write, the legislation is awaiting signature by President Obama and it is expected he will do so in the coming days.
ACS’ successful efforts toward the establishment of the Joint Trauma System and the Joint Trauma Education and Training Directorate represent a significant achievement toward ensuring that our soldiers, sailors, airmen, Marines and guardsmen continue to receive the best of the best in trauma care while in the service of our nation.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
I am frequently asked for examples of successes from the College’s advocacy efforts in DC. While many of our successes come in the form of legislation or regulation we are either able to significantly modify into more favorable form or to outright prevent from being enacted, this month’s topic provides an example of how our advocacy efforts are equally successful in obtaining specific provisions in legislation.
Over a year ago, staff of the Division of Advocacy and Health Policy were approached by members and staff of the Military Health System Strategic Partnership American College of Surgeons to assist them in their effort toward the establishment of both a Joint Trauma System (JTS) within the Defense Health Agency (to promote continuous improvement of trauma care provided to members of the Armed Forces) and a Joint Trauma Education and Training Directorate (JTETD) (to ensure military traumatologists maintain readiness with regard to critical surgical skills). I am pleased and proud to report that when the U.S. House of Representatives, on December 2, and the Senate, on December 8, passed the National Defense Authorization Act (NDAA), provisions for both the JTS and the JTETD, in nearly the precise wording as was proposed by ACS, were included in the legislation.
Our success in this effort was strongly supported by both Rep. Joe Heck, DO (R-Nev.), Chairman of the House Armed Services Subcommittee on Military Personnel, and Rep. Brad Wenstrup, DPM (R-Ohio). Rep. Heck, who is a Brigadier General in the Army Reserve, ensured that the language establishing the JTS within the U.S. Department of Defense and the JTETD were included in the House version of the NDAA. Rep. Wenstrup, who also serves in the Army Reserve, was key to securing language providing for review of the military trauma system under the JTS by a “non-government entity with subject matter experts.” This is an activity that the ACS Committee on Trauma Verification, Review, and Consultation Program conducts on a regular basis.
The Joint Trauma System will standardize trauma care in the military by establishing uniform standards for all military medical treatment facilities. The Joint Trauma Education and Training Directorate is charged with ensuring that trauma providers of the U.S. Armed Forces maintain a state of readiness. Under this provision, partnerships will be established with level one trauma centers in civilian academic medical centers and large metropolitan teaching hospitals where combat casualty care teams will embed to provide military surgeons with regular exposure to critically injured patients.
The Senate version of the NDAA did not contain language specifically outlining provisions for either the JTS or the JTETD. Because the House and Senate versions of the NDAA were different, a conference committee from both legislative bodies was appointed to settle the differences between the two versions of the legislation. Over the several months duration of the conference committee process, members of the ACS legislative affairs team met regularly with the offices of several key senators who serve on the Senate Armed Services Committee as well as with committee staff for both the Republican and Democrat members of the committee. During these meetings, we repeatedly “made the case” relative to the critically important nature of these provisions and were able to answer questions and address concerns relative to why ACS felt it was vitally important to include the House language in the final version of the bill. No doubt, these efforts were key in the decision of the Senate negotiators to recede their position and agree to the House language in the final version of the bill relative to these specific provisions.
Prior to the final House vote on the conference committee language of the NDAA, Rep. Wenstrup spoke on the House floor in support of the JTETD.
As I write, the legislation is awaiting signature by President Obama and it is expected he will do so in the coming days.
ACS’ successful efforts toward the establishment of the Joint Trauma System and the Joint Trauma Education and Training Directorate represent a significant achievement toward ensuring that our soldiers, sailors, airmen, Marines and guardsmen continue to receive the best of the best in trauma care while in the service of our nation.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
From the Editors: One pebble at a time
This is a story about Sarah Prince, FRCS, and thousands of others here and abroad who are surgeons. Only a few of you may have heard of Miss Prince, consultant surgeon from Fort William, Scotland; but she represents to me one of thousands of stories that make surgery such a rich subject that spans more than pure science. Sarah achieved immortality in what she accomplished in 43 short years.
Sarah was trained in the United Kingdom system, attaining specialty training in hepatobiliary disease. While she loved that sort of work she decided, with her internist husband Patrick Byrne, to work in a rural town in northern Scotland. In nine years she built up the hospital there and its training paradigm. She went on to work toward creating a better rural surgical system in Scotland, eventually becoming an expert who spoke all over the world about rural surgery and allocating resources to build surgical capacity in rural areas. She understood the volume debate and the need for rural surgeons to have a connection that was substantive with a larger center in a collaborative way benefiting both locales.
I bring her up because she represents something we all can do. A few surgeons become academic giants known far and wide, but all surgeons have the ability to be local giants, unknown but immortal and essential in their own way. Sarah’s accomplishments confirm that.
Unlike surgery in the United States, the U.K. system is more regimented in many ways and even more political than what the average U.S. surgeon experiences. It is a single-payer system that was there long before Sarah became a surgeon and will be there long after. The fact that the system into which she was born was not of her making did not deter Sarah from taking on that very system to make her corner of the world a better place. I was always surprised when speaking with her that the problems she faced in Scotland were much the same as what I’ve seen in rural surgery in the United States and in other countries. She didn’t bend the whole system but she made a significant dent in how things were done. Isn’t that the challenge for us all?
Recently on the ACS Communities and elsewhere, the debate on single-payer, multitier, and market-driven health care is being argued. In light of the current political environment, the path forward seems bewilderingly tangled. Most surgeons just want to operate. The OR may be the last bastion of control we surgeons have in our professional lives. There may be a barrage of obstacles getting to the OR and hordes of explanations and details postoperatively, but in the OR we still get to do what we think is best at the moment using all those skills we so painfully acquired during a career of learning and practice. To despair is easy until one takes a look at what so many surgeons achieve in their lives.
Like Sarah, most of us try to make the profession a little better. In small town Iowa, that may be getting sonography privileges for FAST exams that improves the lot of trauma patients in that town. In an exburbia hospital, the surgeon may bring new expertise not previously available. It goes on and on with each of us contributing one pebble at a time to a mountain of effort. Any one pebble seems so insignificant in itself and sometimes just placing it on the mountain takes enormous effort, but each is worth the toil to put it there.
Which brings us back to Miss Prince (it is a faux pas to call a consultant surgeon in the U.K. by the honorific doctor). Sarah faced just as many challenges and perhaps more than surgeons elsewhere. Yet she brought her best every day to her hospital until cruel fate delivered her a fatal blow at a young age. Even then facing her imminent death, Sarah made sure that her patients and trainees would be well cared for after her passing. Her indomitable approach to surgical life shows that no matter what the opposition, a surgeon can with grit and wit make life better in his or her town, region, and maybe even the world.
As we face 2017 with all its potential for defeat or victory for our patients, let us remember surgeons like Sarah Prince who made a difference and commit ourselves to the same goal. We can do it one pebble at a time until we’ve created a mountain of accomplishment.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
This is a story about Sarah Prince, FRCS, and thousands of others here and abroad who are surgeons. Only a few of you may have heard of Miss Prince, consultant surgeon from Fort William, Scotland; but she represents to me one of thousands of stories that make surgery such a rich subject that spans more than pure science. Sarah achieved immortality in what she accomplished in 43 short years.
Sarah was trained in the United Kingdom system, attaining specialty training in hepatobiliary disease. While she loved that sort of work she decided, with her internist husband Patrick Byrne, to work in a rural town in northern Scotland. In nine years she built up the hospital there and its training paradigm. She went on to work toward creating a better rural surgical system in Scotland, eventually becoming an expert who spoke all over the world about rural surgery and allocating resources to build surgical capacity in rural areas. She understood the volume debate and the need for rural surgeons to have a connection that was substantive with a larger center in a collaborative way benefiting both locales.
I bring her up because she represents something we all can do. A few surgeons become academic giants known far and wide, but all surgeons have the ability to be local giants, unknown but immortal and essential in their own way. Sarah’s accomplishments confirm that.
Unlike surgery in the United States, the U.K. system is more regimented in many ways and even more political than what the average U.S. surgeon experiences. It is a single-payer system that was there long before Sarah became a surgeon and will be there long after. The fact that the system into which she was born was not of her making did not deter Sarah from taking on that very system to make her corner of the world a better place. I was always surprised when speaking with her that the problems she faced in Scotland were much the same as what I’ve seen in rural surgery in the United States and in other countries. She didn’t bend the whole system but she made a significant dent in how things were done. Isn’t that the challenge for us all?
Recently on the ACS Communities and elsewhere, the debate on single-payer, multitier, and market-driven health care is being argued. In light of the current political environment, the path forward seems bewilderingly tangled. Most surgeons just want to operate. The OR may be the last bastion of control we surgeons have in our professional lives. There may be a barrage of obstacles getting to the OR and hordes of explanations and details postoperatively, but in the OR we still get to do what we think is best at the moment using all those skills we so painfully acquired during a career of learning and practice. To despair is easy until one takes a look at what so many surgeons achieve in their lives.
Like Sarah, most of us try to make the profession a little better. In small town Iowa, that may be getting sonography privileges for FAST exams that improves the lot of trauma patients in that town. In an exburbia hospital, the surgeon may bring new expertise not previously available. It goes on and on with each of us contributing one pebble at a time to a mountain of effort. Any one pebble seems so insignificant in itself and sometimes just placing it on the mountain takes enormous effort, but each is worth the toil to put it there.
Which brings us back to Miss Prince (it is a faux pas to call a consultant surgeon in the U.K. by the honorific doctor). Sarah faced just as many challenges and perhaps more than surgeons elsewhere. Yet she brought her best every day to her hospital until cruel fate delivered her a fatal blow at a young age. Even then facing her imminent death, Sarah made sure that her patients and trainees would be well cared for after her passing. Her indomitable approach to surgical life shows that no matter what the opposition, a surgeon can with grit and wit make life better in his or her town, region, and maybe even the world.
As we face 2017 with all its potential for defeat or victory for our patients, let us remember surgeons like Sarah Prince who made a difference and commit ourselves to the same goal. We can do it one pebble at a time until we’ve created a mountain of accomplishment.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.
This is a story about Sarah Prince, FRCS, and thousands of others here and abroad who are surgeons. Only a few of you may have heard of Miss Prince, consultant surgeon from Fort William, Scotland; but she represents to me one of thousands of stories that make surgery such a rich subject that spans more than pure science. Sarah achieved immortality in what she accomplished in 43 short years.
Sarah was trained in the United Kingdom system, attaining specialty training in hepatobiliary disease. While she loved that sort of work she decided, with her internist husband Patrick Byrne, to work in a rural town in northern Scotland. In nine years she built up the hospital there and its training paradigm. She went on to work toward creating a better rural surgical system in Scotland, eventually becoming an expert who spoke all over the world about rural surgery and allocating resources to build surgical capacity in rural areas. She understood the volume debate and the need for rural surgeons to have a connection that was substantive with a larger center in a collaborative way benefiting both locales.
I bring her up because she represents something we all can do. A few surgeons become academic giants known far and wide, but all surgeons have the ability to be local giants, unknown but immortal and essential in their own way. Sarah’s accomplishments confirm that.
Unlike surgery in the United States, the U.K. system is more regimented in many ways and even more political than what the average U.S. surgeon experiences. It is a single-payer system that was there long before Sarah became a surgeon and will be there long after. The fact that the system into which she was born was not of her making did not deter Sarah from taking on that very system to make her corner of the world a better place. I was always surprised when speaking with her that the problems she faced in Scotland were much the same as what I’ve seen in rural surgery in the United States and in other countries. She didn’t bend the whole system but she made a significant dent in how things were done. Isn’t that the challenge for us all?
Recently on the ACS Communities and elsewhere, the debate on single-payer, multitier, and market-driven health care is being argued. In light of the current political environment, the path forward seems bewilderingly tangled. Most surgeons just want to operate. The OR may be the last bastion of control we surgeons have in our professional lives. There may be a barrage of obstacles getting to the OR and hordes of explanations and details postoperatively, but in the OR we still get to do what we think is best at the moment using all those skills we so painfully acquired during a career of learning and practice. To despair is easy until one takes a look at what so many surgeons achieve in their lives.
Like Sarah, most of us try to make the profession a little better. In small town Iowa, that may be getting sonography privileges for FAST exams that improves the lot of trauma patients in that town. In an exburbia hospital, the surgeon may bring new expertise not previously available. It goes on and on with each of us contributing one pebble at a time to a mountain of effort. Any one pebble seems so insignificant in itself and sometimes just placing it on the mountain takes enormous effort, but each is worth the toil to put it there.
Which brings us back to Miss Prince (it is a faux pas to call a consultant surgeon in the U.K. by the honorific doctor). Sarah faced just as many challenges and perhaps more than surgeons elsewhere. Yet she brought her best every day to her hospital until cruel fate delivered her a fatal blow at a young age. Even then facing her imminent death, Sarah made sure that her patients and trainees would be well cared for after her passing. Her indomitable approach to surgical life shows that no matter what the opposition, a surgeon can with grit and wit make life better in his or her town, region, and maybe even the world.
As we face 2017 with all its potential for defeat or victory for our patients, let us remember surgeons like Sarah Prince who made a difference and commit ourselves to the same goal. We can do it one pebble at a time until we’ve created a mountain of accomplishment.
Dr. Hughes is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, and Co-Editor of ACS Surgery News.