User login
Dr. Price light on ACA replacement details at Senate hearing
WASHINGTON – Rep. Tom Price (R-Ga.) was light on specifics as to what he would favor in ACA replacement efforts, instead focused on broad goals for reform at a courtesy hearing Jan. 18 before the Senate Committee on Health, Education, Labor & Pensions.
Democratic senators on committee sought firm commitments on many issues – maintaining insurance coverage, women’s access to reproductive health care, coverage of mental health/substance use treatment, drug pricing, and reducing racial disparities – from Dr. Price, President-elect Trump’s nominee to lead the Health & Human Service department and a retired orthopedic surgeon. They also challenged Dr. Price on financial conflicts of interest related to legislation he supported.
Dr. Price consistently avoided committing to specific policies, but insisted that “individuals [should] have the opportunity to gain access to the kind of coverage they desire.”
Senators specifically queried Dr. Price as to whether he would commit to maintaining copay-free insurance coverage of all 18 forms of birth control for women approved by the Food and Drug Administration, as mandated by the ACA.
“Every single American ought to have access to the coverage and care that they desire,” Dr. Price responded.
Similarly, regarding coverage of mental health and substance use disorders, Dr. Price called it an “absolutely an imperative” that “every single American” have access to the care for these health issues.
When pressed by Sen. Maggie Hassan (D-N.H.) to commit to ensuring that there would be no cuts to Medicaid funding for mental health care/substance use disorders, Dr. Price noted that “we will address that need.”
Senators also queried Dr. Price’s commitment to maintaining the HHS Office of Minority Health. Sen. Murray offered a number of statistics demonstrating how minority women in particular have benefited with coverage and access to health care under the ACA.
Dr. Price stopped well short of committing to keeping the office, but instead returned to his desire to pursue policies that ensure “every American has access to the care that they desire.”
Dr. Price did not commit to upholding Mr. Trump’s campaign promise that no dollars would be cut from Medicare; instead, he argued that money spent is the wrong metric to measure health care quality.
Regarding the Center for Medicare & Medicaid Innovation, Dr. Price said that the center has “great promise,” but he “opposed the mandatory nature” of some of its programs, highlighting the comprehensive joint replacement bundle, which he said limits how orthopedic surgeons practice.
Senators also paid special attention to Dr. Price’s potential conflicts of interest. Several pointed to medical industry stock purchases that occurred around the time he introduced legislation that could benefit these companies, including a device manufacturer that would potentially benefit from Dr. Price’s challenging of the comprehensive joint replacement bundle and of pharmaceutical companies that might see benefit from the drug provisions in the 21st Century Cures Act.
He vehemently denied any wrongdoing, noting that he regularly and consistently disclosed all security holdings as required by congressional ethics rules and said he did nothing different from what many people in Congress currently do.
Despite his assurances that his ethics have not been compromised, Sen. Murray called for an ethics probe to address any potential conflicts of interest before his confirmation vote.
In closing the hearing, Chairman Lamar Alexander (R-Tenn.) reiterated his plan for a phased timeline for ACA repeal and replacement, to be completed so that no one would lose coverage. He suggested that while legislative action could be swift, implementation could span years to minimize impact on insurance coverage and access to health care.
Dr. Price’s official confirmation hearing before the Senate Finance Committee is scheduled for Jan. 24.
WASHINGTON – Rep. Tom Price (R-Ga.) was light on specifics as to what he would favor in ACA replacement efforts, instead focused on broad goals for reform at a courtesy hearing Jan. 18 before the Senate Committee on Health, Education, Labor & Pensions.
Democratic senators on committee sought firm commitments on many issues – maintaining insurance coverage, women’s access to reproductive health care, coverage of mental health/substance use treatment, drug pricing, and reducing racial disparities – from Dr. Price, President-elect Trump’s nominee to lead the Health & Human Service department and a retired orthopedic surgeon. They also challenged Dr. Price on financial conflicts of interest related to legislation he supported.
Dr. Price consistently avoided committing to specific policies, but insisted that “individuals [should] have the opportunity to gain access to the kind of coverage they desire.”
Senators specifically queried Dr. Price as to whether he would commit to maintaining copay-free insurance coverage of all 18 forms of birth control for women approved by the Food and Drug Administration, as mandated by the ACA.
“Every single American ought to have access to the coverage and care that they desire,” Dr. Price responded.
Similarly, regarding coverage of mental health and substance use disorders, Dr. Price called it an “absolutely an imperative” that “every single American” have access to the care for these health issues.
When pressed by Sen. Maggie Hassan (D-N.H.) to commit to ensuring that there would be no cuts to Medicaid funding for mental health care/substance use disorders, Dr. Price noted that “we will address that need.”
Senators also queried Dr. Price’s commitment to maintaining the HHS Office of Minority Health. Sen. Murray offered a number of statistics demonstrating how minority women in particular have benefited with coverage and access to health care under the ACA.
Dr. Price stopped well short of committing to keeping the office, but instead returned to his desire to pursue policies that ensure “every American has access to the care that they desire.”
Dr. Price did not commit to upholding Mr. Trump’s campaign promise that no dollars would be cut from Medicare; instead, he argued that money spent is the wrong metric to measure health care quality.
Regarding the Center for Medicare & Medicaid Innovation, Dr. Price said that the center has “great promise,” but he “opposed the mandatory nature” of some of its programs, highlighting the comprehensive joint replacement bundle, which he said limits how orthopedic surgeons practice.
Senators also paid special attention to Dr. Price’s potential conflicts of interest. Several pointed to medical industry stock purchases that occurred around the time he introduced legislation that could benefit these companies, including a device manufacturer that would potentially benefit from Dr. Price’s challenging of the comprehensive joint replacement bundle and of pharmaceutical companies that might see benefit from the drug provisions in the 21st Century Cures Act.
He vehemently denied any wrongdoing, noting that he regularly and consistently disclosed all security holdings as required by congressional ethics rules and said he did nothing different from what many people in Congress currently do.
Despite his assurances that his ethics have not been compromised, Sen. Murray called for an ethics probe to address any potential conflicts of interest before his confirmation vote.
In closing the hearing, Chairman Lamar Alexander (R-Tenn.) reiterated his plan for a phased timeline for ACA repeal and replacement, to be completed so that no one would lose coverage. He suggested that while legislative action could be swift, implementation could span years to minimize impact on insurance coverage and access to health care.
Dr. Price’s official confirmation hearing before the Senate Finance Committee is scheduled for Jan. 24.
WASHINGTON – Rep. Tom Price (R-Ga.) was light on specifics as to what he would favor in ACA replacement efforts, instead focused on broad goals for reform at a courtesy hearing Jan. 18 before the Senate Committee on Health, Education, Labor & Pensions.
Democratic senators on committee sought firm commitments on many issues – maintaining insurance coverage, women’s access to reproductive health care, coverage of mental health/substance use treatment, drug pricing, and reducing racial disparities – from Dr. Price, President-elect Trump’s nominee to lead the Health & Human Service department and a retired orthopedic surgeon. They also challenged Dr. Price on financial conflicts of interest related to legislation he supported.
Dr. Price consistently avoided committing to specific policies, but insisted that “individuals [should] have the opportunity to gain access to the kind of coverage they desire.”
Senators specifically queried Dr. Price as to whether he would commit to maintaining copay-free insurance coverage of all 18 forms of birth control for women approved by the Food and Drug Administration, as mandated by the ACA.
“Every single American ought to have access to the coverage and care that they desire,” Dr. Price responded.
Similarly, regarding coverage of mental health and substance use disorders, Dr. Price called it an “absolutely an imperative” that “every single American” have access to the care for these health issues.
When pressed by Sen. Maggie Hassan (D-N.H.) to commit to ensuring that there would be no cuts to Medicaid funding for mental health care/substance use disorders, Dr. Price noted that “we will address that need.”
Senators also queried Dr. Price’s commitment to maintaining the HHS Office of Minority Health. Sen. Murray offered a number of statistics demonstrating how minority women in particular have benefited with coverage and access to health care under the ACA.
Dr. Price stopped well short of committing to keeping the office, but instead returned to his desire to pursue policies that ensure “every American has access to the care that they desire.”
Dr. Price did not commit to upholding Mr. Trump’s campaign promise that no dollars would be cut from Medicare; instead, he argued that money spent is the wrong metric to measure health care quality.
Regarding the Center for Medicare & Medicaid Innovation, Dr. Price said that the center has “great promise,” but he “opposed the mandatory nature” of some of its programs, highlighting the comprehensive joint replacement bundle, which he said limits how orthopedic surgeons practice.
Senators also paid special attention to Dr. Price’s potential conflicts of interest. Several pointed to medical industry stock purchases that occurred around the time he introduced legislation that could benefit these companies, including a device manufacturer that would potentially benefit from Dr. Price’s challenging of the comprehensive joint replacement bundle and of pharmaceutical companies that might see benefit from the drug provisions in the 21st Century Cures Act.
He vehemently denied any wrongdoing, noting that he regularly and consistently disclosed all security holdings as required by congressional ethics rules and said he did nothing different from what many people in Congress currently do.
Despite his assurances that his ethics have not been compromised, Sen. Murray called for an ethics probe to address any potential conflicts of interest before his confirmation vote.
In closing the hearing, Chairman Lamar Alexander (R-Tenn.) reiterated his plan for a phased timeline for ACA repeal and replacement, to be completed so that no one would lose coverage. He suggested that while legislative action could be swift, implementation could span years to minimize impact on insurance coverage and access to health care.
Dr. Price’s official confirmation hearing before the Senate Finance Committee is scheduled for Jan. 24.
SHM launches Chapter Development Fund to enhance reach, impact of chapters
As hospital medicine continues to experience unparalleled growth, the Society of Hospital Medicine (SHM) seeks to supplement its chapter program via a new $100,000 Chapter Development Fund. The monies will be used to further enhance the reach and impact of SHM’s 50 regional chapters.
Chapters can request up to $5,000 from the fund annually to support projects that promote networking, education, leadership opportunities, and improvements in health care delivery. In addition to growing the chapters, SHM expects that the additional resources will help facilitate relationships with local hospitals and medical schools, and demonstrate the value of membership.
“Chapters that were struggling now feel that they have the support they need to improve, and the ones that have figured out the basics can push their creative limits. That innovation can be passed along and benefit [all chapters],” Dr. Thompson said.
Fund usage already has led to a number of success stories (see Figure 1). During the program’s pilot phase, six chapters – Gulf States, Iowa, Los Angeles, Michigan, New Mexico, and San Francisco – acquired 77 new SHM members through a variety of innovative methods.
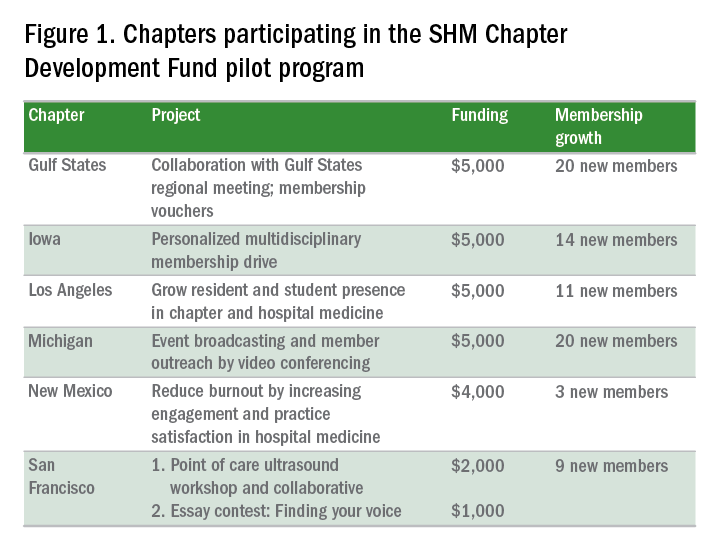
“We were struggling in recruitment, and saw this as an opportunity to attract members,” said chapter leader Venkataraman Palabindala, MD. “We used the funds to create 15 ‘coupons’ for membership. The rest of the money [was used] to start a regional meeting … where chapter leaders were invited to lead talks. [The meeting] really helped us.”
Another example of success comes from the Iowa Chapter, which attracted 14 new members through a multidisciplinary membership drive.
“We … requested funding for a few specific areas. One was marketing, where we had fliers written up to target specific groups, including … students, APPs (advanced practice practitioners), residents, students, and pharmacists, as well as other physicians,” said chapter leader Melinda Johnson, MD, SFHM.
The Iowa Chapter also used funding for SHM-branded “giveaways” (coffee mugs, portable chargers, etc.) to leave behind during meetings with prospective members. Vouchers, offering a 50% discount on a 1-year membership for new members during the pilot program, were especially effective. The combined activities “really increased visibility for SHM within our state and with disciplines besides physicians,” Dr. Johnson said.
Chapters can apply for support on a rolling basis by submitting a proposal to the Chapter Support Committee. For the full details, visit www.hospitalmedicine.org/chapterdevelopment.
When thinking about ideas, Dr. Thompson advises chapters to begin with “a brainstorm of all of the … exciting things that you have wanted to do for your membership. Then think about the ones that are attainable, and map out how to get there. The pilot showed that in a short time, you can reach many people when you plan your project out with timing and specific goals … and let the committee support you.”
In addition to a financial boost, fund recipients enjoy personalized mentorship from the committee, a benefit that both Dr. Johnson and Dr. Palabindala found invaluable. For new and developing chapters, “the support you get, the money, as well as the goal setting and feedback, is amazing,” Dr. Palabindala said.
Chapters, Dr. Johnson said, provide members with networking and leadership opportunities – and ensure that the unique, localized needs of their communities are represented at SHM.
“They become your professional home, providing opportunities,” she said, “that improve personal and professional satisfaction. Anyone is welcome to participate in the conversation.”
For more information on how you can become involved in an SHM chapter, visit www.hospitalmedicine.org/chapters.
Claudia Stahl is a content manager for the Society of Hospital Medicine.
As hospital medicine continues to experience unparalleled growth, the Society of Hospital Medicine (SHM) seeks to supplement its chapter program via a new $100,000 Chapter Development Fund. The monies will be used to further enhance the reach and impact of SHM’s 50 regional chapters.
Chapters can request up to $5,000 from the fund annually to support projects that promote networking, education, leadership opportunities, and improvements in health care delivery. In addition to growing the chapters, SHM expects that the additional resources will help facilitate relationships with local hospitals and medical schools, and demonstrate the value of membership.
“Chapters that were struggling now feel that they have the support they need to improve, and the ones that have figured out the basics can push their creative limits. That innovation can be passed along and benefit [all chapters],” Dr. Thompson said.
Fund usage already has led to a number of success stories (see Figure 1). During the program’s pilot phase, six chapters – Gulf States, Iowa, Los Angeles, Michigan, New Mexico, and San Francisco – acquired 77 new SHM members through a variety of innovative methods.

“We were struggling in recruitment, and saw this as an opportunity to attract members,” said chapter leader Venkataraman Palabindala, MD. “We used the funds to create 15 ‘coupons’ for membership. The rest of the money [was used] to start a regional meeting … where chapter leaders were invited to lead talks. [The meeting] really helped us.”
Another example of success comes from the Iowa Chapter, which attracted 14 new members through a multidisciplinary membership drive.
“We … requested funding for a few specific areas. One was marketing, where we had fliers written up to target specific groups, including … students, APPs (advanced practice practitioners), residents, students, and pharmacists, as well as other physicians,” said chapter leader Melinda Johnson, MD, SFHM.
The Iowa Chapter also used funding for SHM-branded “giveaways” (coffee mugs, portable chargers, etc.) to leave behind during meetings with prospective members. Vouchers, offering a 50% discount on a 1-year membership for new members during the pilot program, were especially effective. The combined activities “really increased visibility for SHM within our state and with disciplines besides physicians,” Dr. Johnson said.
Chapters can apply for support on a rolling basis by submitting a proposal to the Chapter Support Committee. For the full details, visit www.hospitalmedicine.org/chapterdevelopment.
When thinking about ideas, Dr. Thompson advises chapters to begin with “a brainstorm of all of the … exciting things that you have wanted to do for your membership. Then think about the ones that are attainable, and map out how to get there. The pilot showed that in a short time, you can reach many people when you plan your project out with timing and specific goals … and let the committee support you.”
In addition to a financial boost, fund recipients enjoy personalized mentorship from the committee, a benefit that both Dr. Johnson and Dr. Palabindala found invaluable. For new and developing chapters, “the support you get, the money, as well as the goal setting and feedback, is amazing,” Dr. Palabindala said.
Chapters, Dr. Johnson said, provide members with networking and leadership opportunities – and ensure that the unique, localized needs of their communities are represented at SHM.
“They become your professional home, providing opportunities,” she said, “that improve personal and professional satisfaction. Anyone is welcome to participate in the conversation.”
For more information on how you can become involved in an SHM chapter, visit www.hospitalmedicine.org/chapters.
Claudia Stahl is a content manager for the Society of Hospital Medicine.
As hospital medicine continues to experience unparalleled growth, the Society of Hospital Medicine (SHM) seeks to supplement its chapter program via a new $100,000 Chapter Development Fund. The monies will be used to further enhance the reach and impact of SHM’s 50 regional chapters.
Chapters can request up to $5,000 from the fund annually to support projects that promote networking, education, leadership opportunities, and improvements in health care delivery. In addition to growing the chapters, SHM expects that the additional resources will help facilitate relationships with local hospitals and medical schools, and demonstrate the value of membership.
“Chapters that were struggling now feel that they have the support they need to improve, and the ones that have figured out the basics can push their creative limits. That innovation can be passed along and benefit [all chapters],” Dr. Thompson said.
Fund usage already has led to a number of success stories (see Figure 1). During the program’s pilot phase, six chapters – Gulf States, Iowa, Los Angeles, Michigan, New Mexico, and San Francisco – acquired 77 new SHM members through a variety of innovative methods.

“We were struggling in recruitment, and saw this as an opportunity to attract members,” said chapter leader Venkataraman Palabindala, MD. “We used the funds to create 15 ‘coupons’ for membership. The rest of the money [was used] to start a regional meeting … where chapter leaders were invited to lead talks. [The meeting] really helped us.”
Another example of success comes from the Iowa Chapter, which attracted 14 new members through a multidisciplinary membership drive.
“We … requested funding for a few specific areas. One was marketing, where we had fliers written up to target specific groups, including … students, APPs (advanced practice practitioners), residents, students, and pharmacists, as well as other physicians,” said chapter leader Melinda Johnson, MD, SFHM.
The Iowa Chapter also used funding for SHM-branded “giveaways” (coffee mugs, portable chargers, etc.) to leave behind during meetings with prospective members. Vouchers, offering a 50% discount on a 1-year membership for new members during the pilot program, were especially effective. The combined activities “really increased visibility for SHM within our state and with disciplines besides physicians,” Dr. Johnson said.
Chapters can apply for support on a rolling basis by submitting a proposal to the Chapter Support Committee. For the full details, visit www.hospitalmedicine.org/chapterdevelopment.
When thinking about ideas, Dr. Thompson advises chapters to begin with “a brainstorm of all of the … exciting things that you have wanted to do for your membership. Then think about the ones that are attainable, and map out how to get there. The pilot showed that in a short time, you can reach many people when you plan your project out with timing and specific goals … and let the committee support you.”
In addition to a financial boost, fund recipients enjoy personalized mentorship from the committee, a benefit that both Dr. Johnson and Dr. Palabindala found invaluable. For new and developing chapters, “the support you get, the money, as well as the goal setting and feedback, is amazing,” Dr. Palabindala said.
Chapters, Dr. Johnson said, provide members with networking and leadership opportunities – and ensure that the unique, localized needs of their communities are represented at SHM.
“They become your professional home, providing opportunities,” she said, “that improve personal and professional satisfaction. Anyone is welcome to participate in the conversation.”
For more information on how you can become involved in an SHM chapter, visit www.hospitalmedicine.org/chapters.
Claudia Stahl is a content manager for the Society of Hospital Medicine.
EMTALA Part III: Case law
This is the third installment of a three-part series.
Question: Based on case law, which of the following statements is most appropriate regarding EMTALA?
A. Prior to updated regulations, jurisdictions were divided as to whether the Emergency Medical Treatment and Labor Act (EMTALA) covered in-hospital patients.
B. CMS (Centers for Medicare & Medicaid Services) has since clarified that EMTALA does not apply to the in-hospital situation absent bad faith, and all courts now abide by this rule.
C. Stabilization and transfer refer only to moving a patient to another hospital or discharging a patient from the emergency department (ED).
D. Stabilization means a patient may never be transferred while unstable.
E. There is an EMTALA exception for futile treatment in the ED.
Answer: A.
Most cases under EMTALA, the federal anti-dumping statute, deal with the failure to screen or to stabilize patients presenting to the emergency department (ED) of a hospital. For a while, jurisdictions were split as to whether the EMTALA statute was equally applicable to inpatients. A typical case concerned Carol James, a renal-failure patient who complained that she developed an emergency vein-graft malfunction in the hospital but was discharged in violation of EMTALA. The condition was not stabilized before discharge, which caused her to have her hand amputated. However, the Ninth Circuit Court dismissed for failure to state a claim, reasoning that this federal statute could not be and was not based upon a claim of medical malpractice. The Court wrote: “Hospitals are often big buildings or complexes of multiple big buildings. It would make no sense for the authority of physicians proposing to transfer a patient from the eleventh floor of building III to be affected by whether a physician was physically present in the emergency room on the first floor of building I. Congress must have been contemplating patients who were in the emergency room …”1
The defendant hospital moved for summary judgment on the basis that she never presented to the ED for examination in the first place. The Court ruled that EMTALA’s “stabilization before transfer” requirement does not apply to individuals that have been admitted to the hospital for inpatient care, reinforcing the rule that EMTALA’s limited purpose was to eliminate patient dumping and not to federalize medical malpractice. The court also held that she had failed to present any evidence that the hospital had actual knowledge of her emergency medical condition when it discharged her, as she had told the doctor that she was “okay,” “fine,” “comfortable,” and “ready to go home.” Under the statute, a plaintiff must first prove the hospital had actual knowledge of an individual’s unstabilized emergency medical condition to succeed on a claim.
On the other hand, in Thornton v. Southwest Detroit Hospital,3 the Sixth Circuit Court deemed the EMTALA stabilization requirement to extend to the inpatient situation. The patient had been in the hospital for an extended stay for treatment of a stroke before being discharged to home care, where her condition worsened. This was a 1990 case, but even after CMS’ revised rule in 2003 clarifying that EMTALA’s obligation ends upon hospital admission, the same Court chose to ignore the regulation. In Moses v. Providence Hospital and Medical Center,4 a hospitalized psychotic patient was discharged in an arguably unstabilized condition and went on to murder his wife some 10 days later. The Sixth Circuit Court ruled that EMTALA was nonetheless applicable.
Litigation over transfer propriety does not always involve another hospital. For example, in Carlisle v. Frisbie Memorial Hospital,5 an intoxicated and suicidal ED patient refused to see a psychiatrist and was handed over to the police and sent to jail. The court ruled that the hospital was in violation of EMTALA by failing to stabilize the patient prior to “transfer.”
Under EMTALA, an unstable patient may still be transferred so long as certain criteria are met, principally if the original hospital lacks the facilities to provide proper treatment, and the benefits of a transfer outweigh the risks. In Cherukuri v. Shalala,6 Dr. Cherukuri, a surgeon, faced suspension of his license and a civil penalty of $100,000 for allegedly violating the stabilization language of EMTALA. He had transferred two patients with head injuries to the trauma center at St. Mary’s Hospital in Huntington without first stopping intra-abdominal hemorrhage and before receiving express consent from the receiving institution. The patients were victims of an auto accident and were initially brought by ambulance in the early morning hours to Williamson Hospital, a small rural hospital in south Williamson, Kentucky, which had no trauma center and no equipment for neuro-monitoring during anesthesia.
Dr. Cherukuri was the on-call ED surgeon that night. An expert testified that stabilization of internal bleeding required an abdominal operation by the surgeon prior to transfer. Dr. Cherukuri was initially found liable, based on the legal conclusion that he “knew or should have known that the benefits [of transfer] did not outweigh the risks.” However, it was undisputed that the condition of the two patients did not deteriorate during transfer. On appeal, the Sixth Circuit Court reversed, finding that Dr. Cherukuri sufficiently stabilized the two patients to permit transfer and alternatively, that he did not have an anesthesiologist available in order for him to operate.
Probably the most unusual and unexpected challenge to EMTALA faced the Fourth Circuit Court when Baby K, an anencephalic infant, presented repeatedly to a hospital ED with respiratory distress.7 Her physicians and the hospital had petitioned withdrawal of ventilator support, arguing that a requirement to provide respiratory assistance would exceed the prevailing standard of medical care, i.e., provision of warmth, nutrition and hydration, because any treatment of the baby’s condition was futile. The baby’s mother however, insisted that respirator care must be continued, invoking the hospital’s obligations under EMTALA’s stabilization requirement. Citing the statute, the Court ruled that EMTALA required all EDs to provide treatment necessary to prevent the material deterioration of the individual’s condition, and that the statute does not contain a “standard of care” exception. The Court wrote: “We recognize the dilemma facing physicians who are requested to provide treatment they consider morally and ethically inappropriate, but we cannot ignore the plain language of the statute because to do so would transcend our judicial function ...”
References
1. James v. Sunrise Hospital, 86 F.3d 885 (9th Cir. 1996).
2. Dollard v. Allen, 260 F. Supp. 2d 1127 (D. Wyo. 2003).
3. Thornton v. Southwest Detroit Hospital, 895 F.2d 1131 (6th Cir. 1990).
4. Moses v. Providence Hospital and Medical Center, 561 F.3d 573 (6th Cir. 2009).
5. Carlisle v. Frisbie Memorial Hospital, 888 A.2d 405 (N.H. 2005).
6. Cherukuri v. Shalala, 175 F.3d 446 (6th Cir. 1999).
7. In re Baby K, 16 F.3d 590 (4th Cir. 1994).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
This is the third installment of a three-part series.
Question: Based on case law, which of the following statements is most appropriate regarding EMTALA?
A. Prior to updated regulations, jurisdictions were divided as to whether the Emergency Medical Treatment and Labor Act (EMTALA) covered in-hospital patients.
B. CMS (Centers for Medicare & Medicaid Services) has since clarified that EMTALA does not apply to the in-hospital situation absent bad faith, and all courts now abide by this rule.
C. Stabilization and transfer refer only to moving a patient to another hospital or discharging a patient from the emergency department (ED).
D. Stabilization means a patient may never be transferred while unstable.
E. There is an EMTALA exception for futile treatment in the ED.
Answer: A.
Most cases under EMTALA, the federal anti-dumping statute, deal with the failure to screen or to stabilize patients presenting to the emergency department (ED) of a hospital. For a while, jurisdictions were split as to whether the EMTALA statute was equally applicable to inpatients. A typical case concerned Carol James, a renal-failure patient who complained that she developed an emergency vein-graft malfunction in the hospital but was discharged in violation of EMTALA. The condition was not stabilized before discharge, which caused her to have her hand amputated. However, the Ninth Circuit Court dismissed for failure to state a claim, reasoning that this federal statute could not be and was not based upon a claim of medical malpractice. The Court wrote: “Hospitals are often big buildings or complexes of multiple big buildings. It would make no sense for the authority of physicians proposing to transfer a patient from the eleventh floor of building III to be affected by whether a physician was physically present in the emergency room on the first floor of building I. Congress must have been contemplating patients who were in the emergency room …”1
The defendant hospital moved for summary judgment on the basis that she never presented to the ED for examination in the first place. The Court ruled that EMTALA’s “stabilization before transfer” requirement does not apply to individuals that have been admitted to the hospital for inpatient care, reinforcing the rule that EMTALA’s limited purpose was to eliminate patient dumping and not to federalize medical malpractice. The court also held that she had failed to present any evidence that the hospital had actual knowledge of her emergency medical condition when it discharged her, as she had told the doctor that she was “okay,” “fine,” “comfortable,” and “ready to go home.” Under the statute, a plaintiff must first prove the hospital had actual knowledge of an individual’s unstabilized emergency medical condition to succeed on a claim.
On the other hand, in Thornton v. Southwest Detroit Hospital,3 the Sixth Circuit Court deemed the EMTALA stabilization requirement to extend to the inpatient situation. The patient had been in the hospital for an extended stay for treatment of a stroke before being discharged to home care, where her condition worsened. This was a 1990 case, but even after CMS’ revised rule in 2003 clarifying that EMTALA’s obligation ends upon hospital admission, the same Court chose to ignore the regulation. In Moses v. Providence Hospital and Medical Center,4 a hospitalized psychotic patient was discharged in an arguably unstabilized condition and went on to murder his wife some 10 days later. The Sixth Circuit Court ruled that EMTALA was nonetheless applicable.
Litigation over transfer propriety does not always involve another hospital. For example, in Carlisle v. Frisbie Memorial Hospital,5 an intoxicated and suicidal ED patient refused to see a psychiatrist and was handed over to the police and sent to jail. The court ruled that the hospital was in violation of EMTALA by failing to stabilize the patient prior to “transfer.”
Under EMTALA, an unstable patient may still be transferred so long as certain criteria are met, principally if the original hospital lacks the facilities to provide proper treatment, and the benefits of a transfer outweigh the risks. In Cherukuri v. Shalala,6 Dr. Cherukuri, a surgeon, faced suspension of his license and a civil penalty of $100,000 for allegedly violating the stabilization language of EMTALA. He had transferred two patients with head injuries to the trauma center at St. Mary’s Hospital in Huntington without first stopping intra-abdominal hemorrhage and before receiving express consent from the receiving institution. The patients were victims of an auto accident and were initially brought by ambulance in the early morning hours to Williamson Hospital, a small rural hospital in south Williamson, Kentucky, which had no trauma center and no equipment for neuro-monitoring during anesthesia.
Dr. Cherukuri was the on-call ED surgeon that night. An expert testified that stabilization of internal bleeding required an abdominal operation by the surgeon prior to transfer. Dr. Cherukuri was initially found liable, based on the legal conclusion that he “knew or should have known that the benefits [of transfer] did not outweigh the risks.” However, it was undisputed that the condition of the two patients did not deteriorate during transfer. On appeal, the Sixth Circuit Court reversed, finding that Dr. Cherukuri sufficiently stabilized the two patients to permit transfer and alternatively, that he did not have an anesthesiologist available in order for him to operate.
Probably the most unusual and unexpected challenge to EMTALA faced the Fourth Circuit Court when Baby K, an anencephalic infant, presented repeatedly to a hospital ED with respiratory distress.7 Her physicians and the hospital had petitioned withdrawal of ventilator support, arguing that a requirement to provide respiratory assistance would exceed the prevailing standard of medical care, i.e., provision of warmth, nutrition and hydration, because any treatment of the baby’s condition was futile. The baby’s mother however, insisted that respirator care must be continued, invoking the hospital’s obligations under EMTALA’s stabilization requirement. Citing the statute, the Court ruled that EMTALA required all EDs to provide treatment necessary to prevent the material deterioration of the individual’s condition, and that the statute does not contain a “standard of care” exception. The Court wrote: “We recognize the dilemma facing physicians who are requested to provide treatment they consider morally and ethically inappropriate, but we cannot ignore the plain language of the statute because to do so would transcend our judicial function ...”
References
1. James v. Sunrise Hospital, 86 F.3d 885 (9th Cir. 1996).
2. Dollard v. Allen, 260 F. Supp. 2d 1127 (D. Wyo. 2003).
3. Thornton v. Southwest Detroit Hospital, 895 F.2d 1131 (6th Cir. 1990).
4. Moses v. Providence Hospital and Medical Center, 561 F.3d 573 (6th Cir. 2009).
5. Carlisle v. Frisbie Memorial Hospital, 888 A.2d 405 (N.H. 2005).
6. Cherukuri v. Shalala, 175 F.3d 446 (6th Cir. 1999).
7. In re Baby K, 16 F.3d 590 (4th Cir. 1994).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
This is the third installment of a three-part series.
Question: Based on case law, which of the following statements is most appropriate regarding EMTALA?
A. Prior to updated regulations, jurisdictions were divided as to whether the Emergency Medical Treatment and Labor Act (EMTALA) covered in-hospital patients.
B. CMS (Centers for Medicare & Medicaid Services) has since clarified that EMTALA does not apply to the in-hospital situation absent bad faith, and all courts now abide by this rule.
C. Stabilization and transfer refer only to moving a patient to another hospital or discharging a patient from the emergency department (ED).
D. Stabilization means a patient may never be transferred while unstable.
E. There is an EMTALA exception for futile treatment in the ED.
Answer: A.
Most cases under EMTALA, the federal anti-dumping statute, deal with the failure to screen or to stabilize patients presenting to the emergency department (ED) of a hospital. For a while, jurisdictions were split as to whether the EMTALA statute was equally applicable to inpatients. A typical case concerned Carol James, a renal-failure patient who complained that she developed an emergency vein-graft malfunction in the hospital but was discharged in violation of EMTALA. The condition was not stabilized before discharge, which caused her to have her hand amputated. However, the Ninth Circuit Court dismissed for failure to state a claim, reasoning that this federal statute could not be and was not based upon a claim of medical malpractice. The Court wrote: “Hospitals are often big buildings or complexes of multiple big buildings. It would make no sense for the authority of physicians proposing to transfer a patient from the eleventh floor of building III to be affected by whether a physician was physically present in the emergency room on the first floor of building I. Congress must have been contemplating patients who were in the emergency room …”1
The defendant hospital moved for summary judgment on the basis that she never presented to the ED for examination in the first place. The Court ruled that EMTALA’s “stabilization before transfer” requirement does not apply to individuals that have been admitted to the hospital for inpatient care, reinforcing the rule that EMTALA’s limited purpose was to eliminate patient dumping and not to federalize medical malpractice. The court also held that she had failed to present any evidence that the hospital had actual knowledge of her emergency medical condition when it discharged her, as she had told the doctor that she was “okay,” “fine,” “comfortable,” and “ready to go home.” Under the statute, a plaintiff must first prove the hospital had actual knowledge of an individual’s unstabilized emergency medical condition to succeed on a claim.
On the other hand, in Thornton v. Southwest Detroit Hospital,3 the Sixth Circuit Court deemed the EMTALA stabilization requirement to extend to the inpatient situation. The patient had been in the hospital for an extended stay for treatment of a stroke before being discharged to home care, where her condition worsened. This was a 1990 case, but even after CMS’ revised rule in 2003 clarifying that EMTALA’s obligation ends upon hospital admission, the same Court chose to ignore the regulation. In Moses v. Providence Hospital and Medical Center,4 a hospitalized psychotic patient was discharged in an arguably unstabilized condition and went on to murder his wife some 10 days later. The Sixth Circuit Court ruled that EMTALA was nonetheless applicable.
Litigation over transfer propriety does not always involve another hospital. For example, in Carlisle v. Frisbie Memorial Hospital,5 an intoxicated and suicidal ED patient refused to see a psychiatrist and was handed over to the police and sent to jail. The court ruled that the hospital was in violation of EMTALA by failing to stabilize the patient prior to “transfer.”
Under EMTALA, an unstable patient may still be transferred so long as certain criteria are met, principally if the original hospital lacks the facilities to provide proper treatment, and the benefits of a transfer outweigh the risks. In Cherukuri v. Shalala,6 Dr. Cherukuri, a surgeon, faced suspension of his license and a civil penalty of $100,000 for allegedly violating the stabilization language of EMTALA. He had transferred two patients with head injuries to the trauma center at St. Mary’s Hospital in Huntington without first stopping intra-abdominal hemorrhage and before receiving express consent from the receiving institution. The patients were victims of an auto accident and were initially brought by ambulance in the early morning hours to Williamson Hospital, a small rural hospital in south Williamson, Kentucky, which had no trauma center and no equipment for neuro-monitoring during anesthesia.
Dr. Cherukuri was the on-call ED surgeon that night. An expert testified that stabilization of internal bleeding required an abdominal operation by the surgeon prior to transfer. Dr. Cherukuri was initially found liable, based on the legal conclusion that he “knew or should have known that the benefits [of transfer] did not outweigh the risks.” However, it was undisputed that the condition of the two patients did not deteriorate during transfer. On appeal, the Sixth Circuit Court reversed, finding that Dr. Cherukuri sufficiently stabilized the two patients to permit transfer and alternatively, that he did not have an anesthesiologist available in order for him to operate.
Probably the most unusual and unexpected challenge to EMTALA faced the Fourth Circuit Court when Baby K, an anencephalic infant, presented repeatedly to a hospital ED with respiratory distress.7 Her physicians and the hospital had petitioned withdrawal of ventilator support, arguing that a requirement to provide respiratory assistance would exceed the prevailing standard of medical care, i.e., provision of warmth, nutrition and hydration, because any treatment of the baby’s condition was futile. The baby’s mother however, insisted that respirator care must be continued, invoking the hospital’s obligations under EMTALA’s stabilization requirement. Citing the statute, the Court ruled that EMTALA required all EDs to provide treatment necessary to prevent the material deterioration of the individual’s condition, and that the statute does not contain a “standard of care” exception. The Court wrote: “We recognize the dilemma facing physicians who are requested to provide treatment they consider morally and ethically inappropriate, but we cannot ignore the plain language of the statute because to do so would transcend our judicial function ...”
References
1. James v. Sunrise Hospital, 86 F.3d 885 (9th Cir. 1996).
2. Dollard v. Allen, 260 F. Supp. 2d 1127 (D. Wyo. 2003).
3. Thornton v. Southwest Detroit Hospital, 895 F.2d 1131 (6th Cir. 1990).
4. Moses v. Providence Hospital and Medical Center, 561 F.3d 573 (6th Cir. 2009).
5. Carlisle v. Frisbie Memorial Hospital, 888 A.2d 405 (N.H. 2005).
6. Cherukuri v. Shalala, 175 F.3d 446 (6th Cir. 1999).
7. In re Baby K, 16 F.3d 590 (4th Cir. 1994).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
VIDEO: Despite toxicities, ibrutinib is beneficial for treatment-resistant graft-vs.-host disease
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Ibrutinib (420 mg) led to complete responses in one-third of patients with chronic, treatment-resistant graft-vs-host disease.
Major finding: No cardiotoxicities were observed, but 52% of patients had other serious adverse effects, such as sepsis, pyrexia, and pneumonia.
Data source: An open-label phase II study of 42 patients who developed chronic, treatment-resistant graft-vs.-host disease after undergoing allogeneic stem cell transplantation.
Disclosures: Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, reimbursement for travel and expenses, and research funding from Pharmacyclics.
Radiosurgery found not superior to open surgery for temporal lobe epilepsy
HOUSTON – Despite enrollment difficulties that limited the study, a recently completed randomized trial comparing radiosurgery with open lobectomy to treat temporal lobe epilepsy offers some guidance for patients and their physicians.
Radiosurgery’s noninferiority to open lobectomy couldn’t be shown from the ROSE (Radiosurgery or Open Surgery for Epilepsy) trial, but language deficits were similar – and quite small – by 3 years after either procedure. Expected visual field deficits were similar in each procedure as well. However, since the trial didn’t reach its target enrollment, several primary outcome measures could not be fully assessed.
On the face of it, radiosurgery has significant appeal. Although open resective surgery is effective, there’s still some risk of infection and blood loss, and neuropsychological changes as well as other focal neurologic deficits are seen. Still, the study saw many challenges, but the largest, according to the investigators, was in recruitment. “Patients like to choose,” said Nicholas M. Barbaro, MD, chair of the department of neurosurgery at Indiana University, Indianapolis. Dr. Barbaro, one of several ROSE coinvestigators who presented the study findings at the annual meeting of the American Epilepsy Society, noted that if patients felt that lobectomy was the best choice, then there would be no incentive to enter a trial where they might be randomized to radiosurgery. Also, he said, some patients might be reluctant to be irradiated, fearing short-term or long-term toxicity.
Trial hypotheses and protocols
The ROSE trial aimed to show that stereotactic radiosurgery (SRS) would not be inferior to anterior temporal lobectomy (ATL) in achieving a seizure-free state by months 25-36 post procedure. The lag to response after radiosurgery is about 1 year; seizure freedom, defined as 12 consecutive months with no seizures, was assessed from months 25 to 36 of the study for the primary outcome of seizure freedom.
Investigators also hypothesized that fewer SRS patients would have significant reductions in measures of language function; further, they predicted that patients in both treatment arms would experience improvements in quality of life (QOL), and that QOL would improve as seizure freedom increased. Finally, the trial sought to show that SRS was cost effective, compared with ATL, with the marginal cost-utility ratio dropping below $50,000 per quality-adjusted life-year (QALY).
Patients in the ATL arm received a standard “Spencer” ATL, with adequacy of resection assessed by MRI performed 3 months after surgery. An inadequate resection would have been classified as an adverse event, but all ATL patients had an adequate resection by study criteria, and all those whose histopathology was available (n = 20) had some hippocampal sclerosis.
Patients in the SRS arm had the amygdala and anterior 2 cm of the hippocampus, as well as the adjacent parahippocampal gyrus, irradiated. This resulted in a total treatment volume ranging from 5.5 to 7.5 cc. Patients received 4 Gy to the 50% isodose line, and treatment could involve an unlimited number of isocenters. The brain stem could receive no more than 10 Gy and the optic nerve and chiasm no more than 8 Gy. All treatment plans were cleared by the ROSE steering committee. The SRS patients had some variation in dose and volumes treated, but all were within the approved limits of the study.
Trial outcomes
As expected, the surgery arm achieved rapid seizure remission, while the SRS arm saw a steady increase in seizure-free numbers beginning at about 12 months after surgery. During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free. “The null hypothesis of inferiority of SRS was not rejected,” said Mark Quigg, MD, professor of neurology at the University of Virginia, Charlottesville.
Most patients in both groups had no or minimal changes in verbal memory, with no significant differences between the groups at 36 months after treatment.
QOL measures improved rapidly for those who received open surgery, and more slowly for those in the radiosurgery arm, a pattern “consistent with the known association between improved seizure control and quality of life,” said John Langfitt, PhD, a neuropsychologist and professor of neurology and psychiatry at the University of Rochester (N.Y.). However, the study was underpowered to show noninferiority of SRS for QOL measures at 36 months.
“There was a preliminary trend toward reduced health care use over time in the open surgery arm,” said Dr. Langfitt, again noting that the earlier seizure control achieved in surgery reduced health care utilization for that group sooner than for the SRS group. “The power may be limited by sample size and the tendency of utilization to be highly skewed,” he said.
Also as expected, the ATL arm saw early surgery-related adverse events such as scalp wound infections, subdural hematomas, and deep vein thromboses. These were infrequent overall. In contrast, the SRS group saw more cerebral edema–related adverse events during months 9-18, with headaches, new neurologic deficits, and transient seizure exacerbation.
All but three patients received postoperative visual field testing. Of the patients receiving SRS, 34% (10 of 29) had an upper superior quadrant visual field defect, as did 42% (11 of 26) of patients in the ATL arm.
Since the primary treating surgeon and neurologist could not be blinded as to study arm, another neurologist who was blinded was responsible for assessing the outcome measures, and also could identify adverse events. The trial’s steering committee was also blinded to ongoing outcomes.
Pilot study results
A pilot study had previously found that SRS was comparable to the efficacy that had been seen in larger, prospective trials of open surgery, with about two-thirds of patients seizure free at 36 months. Although most patients experienced brief exacerbation of auras or complex partial seizures after radiosurgery, visual field defects were similar to those experienced by patients undergoing standard ATL. Overall, neuropsychological outcomes for those undergoing SRS in the pilot were good, with a low incidence of declines in language and verbal memory function of the dominant hemisphere, and no short-term affective changes were seen. SRS patients who were seizure free after the procedure experienced a significant improvement in QOL.
The promising pilot results contrasted with the limited findings of the ROSE study. In regard to seizure freedom in ROSE, said Dr. Quigg, “The data appear to show that radiosurgery is inferior to ATL, but the low power of the study means that we cannot conclude this with sufficient confidence. Nor can we conclude that the two treatments are noninferior.”
The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Dr. Barbaro reported no other disclosures. Dr. Langfitt reported being a consultant for Monteris. Dr. Quigg reported being an investigator for several antiepileptic drug trials sponsored by pharmaceutical companies.
[email protected]
On Twitter @karioakes
HOUSTON – Despite enrollment difficulties that limited the study, a recently completed randomized trial comparing radiosurgery with open lobectomy to treat temporal lobe epilepsy offers some guidance for patients and their physicians.
Radiosurgery’s noninferiority to open lobectomy couldn’t be shown from the ROSE (Radiosurgery or Open Surgery for Epilepsy) trial, but language deficits were similar – and quite small – by 3 years after either procedure. Expected visual field deficits were similar in each procedure as well. However, since the trial didn’t reach its target enrollment, several primary outcome measures could not be fully assessed.
On the face of it, radiosurgery has significant appeal. Although open resective surgery is effective, there’s still some risk of infection and blood loss, and neuropsychological changes as well as other focal neurologic deficits are seen. Still, the study saw many challenges, but the largest, according to the investigators, was in recruitment. “Patients like to choose,” said Nicholas M. Barbaro, MD, chair of the department of neurosurgery at Indiana University, Indianapolis. Dr. Barbaro, one of several ROSE coinvestigators who presented the study findings at the annual meeting of the American Epilepsy Society, noted that if patients felt that lobectomy was the best choice, then there would be no incentive to enter a trial where they might be randomized to radiosurgery. Also, he said, some patients might be reluctant to be irradiated, fearing short-term or long-term toxicity.
Trial hypotheses and protocols
The ROSE trial aimed to show that stereotactic radiosurgery (SRS) would not be inferior to anterior temporal lobectomy (ATL) in achieving a seizure-free state by months 25-36 post procedure. The lag to response after radiosurgery is about 1 year; seizure freedom, defined as 12 consecutive months with no seizures, was assessed from months 25 to 36 of the study for the primary outcome of seizure freedom.
Investigators also hypothesized that fewer SRS patients would have significant reductions in measures of language function; further, they predicted that patients in both treatment arms would experience improvements in quality of life (QOL), and that QOL would improve as seizure freedom increased. Finally, the trial sought to show that SRS was cost effective, compared with ATL, with the marginal cost-utility ratio dropping below $50,000 per quality-adjusted life-year (QALY).
Patients in the ATL arm received a standard “Spencer” ATL, with adequacy of resection assessed by MRI performed 3 months after surgery. An inadequate resection would have been classified as an adverse event, but all ATL patients had an adequate resection by study criteria, and all those whose histopathology was available (n = 20) had some hippocampal sclerosis.
Patients in the SRS arm had the amygdala and anterior 2 cm of the hippocampus, as well as the adjacent parahippocampal gyrus, irradiated. This resulted in a total treatment volume ranging from 5.5 to 7.5 cc. Patients received 4 Gy to the 50% isodose line, and treatment could involve an unlimited number of isocenters. The brain stem could receive no more than 10 Gy and the optic nerve and chiasm no more than 8 Gy. All treatment plans were cleared by the ROSE steering committee. The SRS patients had some variation in dose and volumes treated, but all were within the approved limits of the study.
Trial outcomes
As expected, the surgery arm achieved rapid seizure remission, while the SRS arm saw a steady increase in seizure-free numbers beginning at about 12 months after surgery. During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free. “The null hypothesis of inferiority of SRS was not rejected,” said Mark Quigg, MD, professor of neurology at the University of Virginia, Charlottesville.
Most patients in both groups had no or minimal changes in verbal memory, with no significant differences between the groups at 36 months after treatment.
QOL measures improved rapidly for those who received open surgery, and more slowly for those in the radiosurgery arm, a pattern “consistent with the known association between improved seizure control and quality of life,” said John Langfitt, PhD, a neuropsychologist and professor of neurology and psychiatry at the University of Rochester (N.Y.). However, the study was underpowered to show noninferiority of SRS for QOL measures at 36 months.
“There was a preliminary trend toward reduced health care use over time in the open surgery arm,” said Dr. Langfitt, again noting that the earlier seizure control achieved in surgery reduced health care utilization for that group sooner than for the SRS group. “The power may be limited by sample size and the tendency of utilization to be highly skewed,” he said.
Also as expected, the ATL arm saw early surgery-related adverse events such as scalp wound infections, subdural hematomas, and deep vein thromboses. These were infrequent overall. In contrast, the SRS group saw more cerebral edema–related adverse events during months 9-18, with headaches, new neurologic deficits, and transient seizure exacerbation.
All but three patients received postoperative visual field testing. Of the patients receiving SRS, 34% (10 of 29) had an upper superior quadrant visual field defect, as did 42% (11 of 26) of patients in the ATL arm.
Since the primary treating surgeon and neurologist could not be blinded as to study arm, another neurologist who was blinded was responsible for assessing the outcome measures, and also could identify adverse events. The trial’s steering committee was also blinded to ongoing outcomes.
Pilot study results
A pilot study had previously found that SRS was comparable to the efficacy that had been seen in larger, prospective trials of open surgery, with about two-thirds of patients seizure free at 36 months. Although most patients experienced brief exacerbation of auras or complex partial seizures after radiosurgery, visual field defects were similar to those experienced by patients undergoing standard ATL. Overall, neuropsychological outcomes for those undergoing SRS in the pilot were good, with a low incidence of declines in language and verbal memory function of the dominant hemisphere, and no short-term affective changes were seen. SRS patients who were seizure free after the procedure experienced a significant improvement in QOL.
The promising pilot results contrasted with the limited findings of the ROSE study. In regard to seizure freedom in ROSE, said Dr. Quigg, “The data appear to show that radiosurgery is inferior to ATL, but the low power of the study means that we cannot conclude this with sufficient confidence. Nor can we conclude that the two treatments are noninferior.”
The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Dr. Barbaro reported no other disclosures. Dr. Langfitt reported being a consultant for Monteris. Dr. Quigg reported being an investigator for several antiepileptic drug trials sponsored by pharmaceutical companies.
[email protected]
On Twitter @karioakes
HOUSTON – Despite enrollment difficulties that limited the study, a recently completed randomized trial comparing radiosurgery with open lobectomy to treat temporal lobe epilepsy offers some guidance for patients and their physicians.
Radiosurgery’s noninferiority to open lobectomy couldn’t be shown from the ROSE (Radiosurgery or Open Surgery for Epilepsy) trial, but language deficits were similar – and quite small – by 3 years after either procedure. Expected visual field deficits were similar in each procedure as well. However, since the trial didn’t reach its target enrollment, several primary outcome measures could not be fully assessed.
On the face of it, radiosurgery has significant appeal. Although open resective surgery is effective, there’s still some risk of infection and blood loss, and neuropsychological changes as well as other focal neurologic deficits are seen. Still, the study saw many challenges, but the largest, according to the investigators, was in recruitment. “Patients like to choose,” said Nicholas M. Barbaro, MD, chair of the department of neurosurgery at Indiana University, Indianapolis. Dr. Barbaro, one of several ROSE coinvestigators who presented the study findings at the annual meeting of the American Epilepsy Society, noted that if patients felt that lobectomy was the best choice, then there would be no incentive to enter a trial where they might be randomized to radiosurgery. Also, he said, some patients might be reluctant to be irradiated, fearing short-term or long-term toxicity.
Trial hypotheses and protocols
The ROSE trial aimed to show that stereotactic radiosurgery (SRS) would not be inferior to anterior temporal lobectomy (ATL) in achieving a seizure-free state by months 25-36 post procedure. The lag to response after radiosurgery is about 1 year; seizure freedom, defined as 12 consecutive months with no seizures, was assessed from months 25 to 36 of the study for the primary outcome of seizure freedom.
Investigators also hypothesized that fewer SRS patients would have significant reductions in measures of language function; further, they predicted that patients in both treatment arms would experience improvements in quality of life (QOL), and that QOL would improve as seizure freedom increased. Finally, the trial sought to show that SRS was cost effective, compared with ATL, with the marginal cost-utility ratio dropping below $50,000 per quality-adjusted life-year (QALY).
Patients in the ATL arm received a standard “Spencer” ATL, with adequacy of resection assessed by MRI performed 3 months after surgery. An inadequate resection would have been classified as an adverse event, but all ATL patients had an adequate resection by study criteria, and all those whose histopathology was available (n = 20) had some hippocampal sclerosis.
Patients in the SRS arm had the amygdala and anterior 2 cm of the hippocampus, as well as the adjacent parahippocampal gyrus, irradiated. This resulted in a total treatment volume ranging from 5.5 to 7.5 cc. Patients received 4 Gy to the 50% isodose line, and treatment could involve an unlimited number of isocenters. The brain stem could receive no more than 10 Gy and the optic nerve and chiasm no more than 8 Gy. All treatment plans were cleared by the ROSE steering committee. The SRS patients had some variation in dose and volumes treated, but all were within the approved limits of the study.
Trial outcomes
As expected, the surgery arm achieved rapid seizure remission, while the SRS arm saw a steady increase in seizure-free numbers beginning at about 12 months after surgery. During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free. “The null hypothesis of inferiority of SRS was not rejected,” said Mark Quigg, MD, professor of neurology at the University of Virginia, Charlottesville.
Most patients in both groups had no or minimal changes in verbal memory, with no significant differences between the groups at 36 months after treatment.
QOL measures improved rapidly for those who received open surgery, and more slowly for those in the radiosurgery arm, a pattern “consistent with the known association between improved seizure control and quality of life,” said John Langfitt, PhD, a neuropsychologist and professor of neurology and psychiatry at the University of Rochester (N.Y.). However, the study was underpowered to show noninferiority of SRS for QOL measures at 36 months.
“There was a preliminary trend toward reduced health care use over time in the open surgery arm,” said Dr. Langfitt, again noting that the earlier seizure control achieved in surgery reduced health care utilization for that group sooner than for the SRS group. “The power may be limited by sample size and the tendency of utilization to be highly skewed,” he said.
Also as expected, the ATL arm saw early surgery-related adverse events such as scalp wound infections, subdural hematomas, and deep vein thromboses. These were infrequent overall. In contrast, the SRS group saw more cerebral edema–related adverse events during months 9-18, with headaches, new neurologic deficits, and transient seizure exacerbation.
All but three patients received postoperative visual field testing. Of the patients receiving SRS, 34% (10 of 29) had an upper superior quadrant visual field defect, as did 42% (11 of 26) of patients in the ATL arm.
Since the primary treating surgeon and neurologist could not be blinded as to study arm, another neurologist who was blinded was responsible for assessing the outcome measures, and also could identify adverse events. The trial’s steering committee was also blinded to ongoing outcomes.
Pilot study results
A pilot study had previously found that SRS was comparable to the efficacy that had been seen in larger, prospective trials of open surgery, with about two-thirds of patients seizure free at 36 months. Although most patients experienced brief exacerbation of auras or complex partial seizures after radiosurgery, visual field defects were similar to those experienced by patients undergoing standard ATL. Overall, neuropsychological outcomes for those undergoing SRS in the pilot were good, with a low incidence of declines in language and verbal memory function of the dominant hemisphere, and no short-term affective changes were seen. SRS patients who were seizure free after the procedure experienced a significant improvement in QOL.
The promising pilot results contrasted with the limited findings of the ROSE study. In regard to seizure freedom in ROSE, said Dr. Quigg, “The data appear to show that radiosurgery is inferior to ATL, but the low power of the study means that we cannot conclude this with sufficient confidence. Nor can we conclude that the two treatments are noninferior.”
The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Dr. Barbaro reported no other disclosures. Dr. Langfitt reported being a consultant for Monteris. Dr. Quigg reported being an investigator for several antiepileptic drug trials sponsored by pharmaceutical companies.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: During study months 25-36, 78% of the ATL arm and 52% of the SRS arm were seizure free.
Data source: Trial of 58 patients with temporal lobe epilepsy randomized to receive ATL or SRS.
Disclosures: The study was partially funded by Elekta, the manufacturer of the Gamma Knife radiosurgery device used in the study. Several of the presenting ROSE steering committee members reported financial relationships with pharmaceutical companies.
Bortezomib bolsters hematologic response in AL amyloidosis
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
AT ASH 2016
Key clinical point: Adding bortezomib (B) to melphalan and dexamethasone (MDex) bolstered hematologic response in immunoglobulin light-chain (AL) amyloidosis.
Major finding: After three treatment cycles, rates of hematologic response were 79% in the BMDex arm and 52% for MDex (P = .002).
Data source: A multicenter randomized trial of 110 patients with newly diagnosed AL amyloidosis.
Disclosures: The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
FDG-PET/CT at maintenance predicts myeloma survival
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
AT ASH 2016
Key clinical point: FDG-PET/CT helped predict survival in newly diagnosed multiple myeloma, regardless of induction regimen.
Major finding: Progression-free survival at 2 years was 84% when patients had less than three focal lesions at the start of maintenance, vs. 47% when they had three or more lesions (hazard ratio, 3.5; P = .01).
Data source: A prospective study of 103 patients with newly diagnosed, transplant-eligible multiple myeloma from a randomized phase III trial.
Disclosures: Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no conflicts of interest.
Low value
The fact that the United States spends more of its gross domestic product on health care (18%) than any other nation is old and depressing news (JAMA. 2012 Apr 11;307[14]:1513-6). There may be some debate about whether the quality of the product we are getting is worth this outsized investment. But it is safe to assume that there must be some wastage in the system. Exactly how much of our health care dollar is going down the drain is unknown. And the thorny question of who is responsible for the leaks has escaped close scrutiny, probably because the answer is guaranteed to result in an uncomfortable and ugly circle of finger pointing. Is it the insurance companies, hospitals, the superspecialists, the drug companies, impatient patients, or those dastardly lawyers? Pediatricians are such small players on the health care stage that our contribution to the wastage must be minimal. Our patients are little people who are generally healthy. Most of us drive midsized cars and live in modest homes. We try to be careful users of the expensive diagnostic and therapeutic tools at our disposal. We don’t deserve a place on the list of likely suspects, do we?
Using a claims-based measure of 20 services that according to evidenced-based guidelines do not improve health, the authors discovered that among the nearly four and half million commercially insured children they studied, 9.6% received at least one of these 20 “low-value” services in 1 year. The ticket for these worthless services was $27 million,of which more than $9 million was out of pocket expenses for families. If extrapolated to all of the commercially insured children in the United States, the total cost of low-value services would be $227 million for 1 year. Regardless of how wasteful cardiologists or plastic surgeons may be, this contribution to the national cost of health care for low-value services by pediatricians cannot be considered chump change.
I urge you to check out the online version of Dr. Chua’s article and then click on Table 1 so you can look at the 20 services that the authors have chosen to label low value. Although I am always leery of accepting a guideline simply because it is has been labeled “evidence-based,” I think you will find that it hard to argue with their choices, such as blood tests in children with a simple febrile seizure, oral antibiotics after tonsillectomy, or neuroimaging in children with headache. How does your practice’s behavior stack up against their list?
The list could be much longer. For example, the authors chose to exclude head imaging ordered for minor head trauma because their claims-based method didn’t provide enough clinical information. I suspect that with an expanded list of clearly low-value services, the annual cost for low-value pediatric services would be a half a billion dollars.
As concerning as the findings in this study may be, it doesn’t answer the question of what we should do to correct the problem. We can dance around the issue by saying that patients and parents are pressuring us to do something even if it’s a low-value service. We can complain that for decades we have been practicing under the dark cloud of a malpractice suit, and that if we don’t turn over every stone in our evaluation of a patient we’re going to trip on one of them and end up in court.
But the bottom line is that we are the ones who are making the choice to order a study or prescribe a medication that is not only of low value, but more than likely worthless and possibly damaging to the patient. With the help of the American Academy of Pediatrics, we need to swallow hard and begin cleaning house, throwing out those low-value services we have gotten in the habit of ordering and prescribing. Education helps, but sometimes we have to do some finger pointing even if the finger points to us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
The fact that the United States spends more of its gross domestic product on health care (18%) than any other nation is old and depressing news (JAMA. 2012 Apr 11;307[14]:1513-6). There may be some debate about whether the quality of the product we are getting is worth this outsized investment. But it is safe to assume that there must be some wastage in the system. Exactly how much of our health care dollar is going down the drain is unknown. And the thorny question of who is responsible for the leaks has escaped close scrutiny, probably because the answer is guaranteed to result in an uncomfortable and ugly circle of finger pointing. Is it the insurance companies, hospitals, the superspecialists, the drug companies, impatient patients, or those dastardly lawyers? Pediatricians are such small players on the health care stage that our contribution to the wastage must be minimal. Our patients are little people who are generally healthy. Most of us drive midsized cars and live in modest homes. We try to be careful users of the expensive diagnostic and therapeutic tools at our disposal. We don’t deserve a place on the list of likely suspects, do we?
Using a claims-based measure of 20 services that according to evidenced-based guidelines do not improve health, the authors discovered that among the nearly four and half million commercially insured children they studied, 9.6% received at least one of these 20 “low-value” services in 1 year. The ticket for these worthless services was $27 million,of which more than $9 million was out of pocket expenses for families. If extrapolated to all of the commercially insured children in the United States, the total cost of low-value services would be $227 million for 1 year. Regardless of how wasteful cardiologists or plastic surgeons may be, this contribution to the national cost of health care for low-value services by pediatricians cannot be considered chump change.
I urge you to check out the online version of Dr. Chua’s article and then click on Table 1 so you can look at the 20 services that the authors have chosen to label low value. Although I am always leery of accepting a guideline simply because it is has been labeled “evidence-based,” I think you will find that it hard to argue with their choices, such as blood tests in children with a simple febrile seizure, oral antibiotics after tonsillectomy, or neuroimaging in children with headache. How does your practice’s behavior stack up against their list?
The list could be much longer. For example, the authors chose to exclude head imaging ordered for minor head trauma because their claims-based method didn’t provide enough clinical information. I suspect that with an expanded list of clearly low-value services, the annual cost for low-value pediatric services would be a half a billion dollars.
As concerning as the findings in this study may be, it doesn’t answer the question of what we should do to correct the problem. We can dance around the issue by saying that patients and parents are pressuring us to do something even if it’s a low-value service. We can complain that for decades we have been practicing under the dark cloud of a malpractice suit, and that if we don’t turn over every stone in our evaluation of a patient we’re going to trip on one of them and end up in court.
But the bottom line is that we are the ones who are making the choice to order a study or prescribe a medication that is not only of low value, but more than likely worthless and possibly damaging to the patient. With the help of the American Academy of Pediatrics, we need to swallow hard and begin cleaning house, throwing out those low-value services we have gotten in the habit of ordering and prescribing. Education helps, but sometimes we have to do some finger pointing even if the finger points to us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
The fact that the United States spends more of its gross domestic product on health care (18%) than any other nation is old and depressing news (JAMA. 2012 Apr 11;307[14]:1513-6). There may be some debate about whether the quality of the product we are getting is worth this outsized investment. But it is safe to assume that there must be some wastage in the system. Exactly how much of our health care dollar is going down the drain is unknown. And the thorny question of who is responsible for the leaks has escaped close scrutiny, probably because the answer is guaranteed to result in an uncomfortable and ugly circle of finger pointing. Is it the insurance companies, hospitals, the superspecialists, the drug companies, impatient patients, or those dastardly lawyers? Pediatricians are such small players on the health care stage that our contribution to the wastage must be minimal. Our patients are little people who are generally healthy. Most of us drive midsized cars and live in modest homes. We try to be careful users of the expensive diagnostic and therapeutic tools at our disposal. We don’t deserve a place on the list of likely suspects, do we?
Using a claims-based measure of 20 services that according to evidenced-based guidelines do not improve health, the authors discovered that among the nearly four and half million commercially insured children they studied, 9.6% received at least one of these 20 “low-value” services in 1 year. The ticket for these worthless services was $27 million,of which more than $9 million was out of pocket expenses for families. If extrapolated to all of the commercially insured children in the United States, the total cost of low-value services would be $227 million for 1 year. Regardless of how wasteful cardiologists or plastic surgeons may be, this contribution to the national cost of health care for low-value services by pediatricians cannot be considered chump change.
I urge you to check out the online version of Dr. Chua’s article and then click on Table 1 so you can look at the 20 services that the authors have chosen to label low value. Although I am always leery of accepting a guideline simply because it is has been labeled “evidence-based,” I think you will find that it hard to argue with their choices, such as blood tests in children with a simple febrile seizure, oral antibiotics after tonsillectomy, or neuroimaging in children with headache. How does your practice’s behavior stack up against their list?
The list could be much longer. For example, the authors chose to exclude head imaging ordered for minor head trauma because their claims-based method didn’t provide enough clinical information. I suspect that with an expanded list of clearly low-value services, the annual cost for low-value pediatric services would be a half a billion dollars.
As concerning as the findings in this study may be, it doesn’t answer the question of what we should do to correct the problem. We can dance around the issue by saying that patients and parents are pressuring us to do something even if it’s a low-value service. We can complain that for decades we have been practicing under the dark cloud of a malpractice suit, and that if we don’t turn over every stone in our evaluation of a patient we’re going to trip on one of them and end up in court.
But the bottom line is that we are the ones who are making the choice to order a study or prescribe a medication that is not only of low value, but more than likely worthless and possibly damaging to the patient. With the help of the American Academy of Pediatrics, we need to swallow hard and begin cleaning house, throwing out those low-value services we have gotten in the habit of ordering and prescribing. Education helps, but sometimes we have to do some finger pointing even if the finger points to us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
‘And a child shall lead them’
With a moistened index finger pointing skyward, I always have tried to remain alert to where the winds of change are blowing. But every now and then I miss a trend in child care, and that is the case with something known as “baby-led weaning.” Influenced by the questionable notion that there is a “natural” way of doing almost everything, the concept hinges on the belief that an infant will “tell” his mother when it is time to stop nursing and begin solids, aka complementary feeding.
At face value, the concept of allowing the baby to lead is a good one simply because of universality of biologic variation. Just as with the question of how much sleep a baby needs, I don’t think anyone (let alone clinicians) can give with assurance an answer that can easily be applied to all infants. There are just too many variables.
For most dyads, breastfeeding is more than just passing calories from one individual to another. Nursing can offer a sense of security and calming both for infants and their mothers. In many cases, the breast unfortunately has become a critical ingredient in the infant’s ritual for falling to sleep. For some mothers, success at breastfeeding becomes an important validation of her feelings of confidence and self-worth that in the past may have been battered by a male-dominated environment. If breastfeeding has been an unpleasant experience, a mother may be more likely to interpret her infant’s behavior as a message that it is time to wean. The bottom line is that a mother’s perception of her baby’s messages about weaning often reflects her own feelings about nursing.
Of course, we clinicians can influence a mother’s perception of her baby’s messages by introducing our own biases about what we believe is the safest, most nutritionally sound way to introduce complementary feeding. And let’s be honest and acknowledge that those are biases mostly unsupported by good scientific study. In many cases, they are more of a reflection of the cultures in which we have grown up.
When asked by parents how they will know when their infant is ready for complementary feeding, I suggest that it’s time when the infant is not only curious about what the adults around him are eating, but obviously is upset that he isn’t being offered a taste. I add that exactly what that food should be is a matter of debate and common sense.
I also encourage parents to allow the child to do as much self-feeding as possible and not worry about the mess. An old shower curtain floor and plenty of sponges and paper towels are a must.
In most cases, I think we can trust babies to take the lead in weaning. But I also believe that as clinicians we must remain alert to the few situations when extended nursing is not in the best interest for the baby who is not growing well or for the mother for whom the nursing is taking an unreasonable toll on her physical and mental health.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
With a moistened index finger pointing skyward, I always have tried to remain alert to where the winds of change are blowing. But every now and then I miss a trend in child care, and that is the case with something known as “baby-led weaning.” Influenced by the questionable notion that there is a “natural” way of doing almost everything, the concept hinges on the belief that an infant will “tell” his mother when it is time to stop nursing and begin solids, aka complementary feeding.
At face value, the concept of allowing the baby to lead is a good one simply because of universality of biologic variation. Just as with the question of how much sleep a baby needs, I don’t think anyone (let alone clinicians) can give with assurance an answer that can easily be applied to all infants. There are just too many variables.
For most dyads, breastfeeding is more than just passing calories from one individual to another. Nursing can offer a sense of security and calming both for infants and their mothers. In many cases, the breast unfortunately has become a critical ingredient in the infant’s ritual for falling to sleep. For some mothers, success at breastfeeding becomes an important validation of her feelings of confidence and self-worth that in the past may have been battered by a male-dominated environment. If breastfeeding has been an unpleasant experience, a mother may be more likely to interpret her infant’s behavior as a message that it is time to wean. The bottom line is that a mother’s perception of her baby’s messages about weaning often reflects her own feelings about nursing.
Of course, we clinicians can influence a mother’s perception of her baby’s messages by introducing our own biases about what we believe is the safest, most nutritionally sound way to introduce complementary feeding. And let’s be honest and acknowledge that those are biases mostly unsupported by good scientific study. In many cases, they are more of a reflection of the cultures in which we have grown up.
When asked by parents how they will know when their infant is ready for complementary feeding, I suggest that it’s time when the infant is not only curious about what the adults around him are eating, but obviously is upset that he isn’t being offered a taste. I add that exactly what that food should be is a matter of debate and common sense.
I also encourage parents to allow the child to do as much self-feeding as possible and not worry about the mess. An old shower curtain floor and plenty of sponges and paper towels are a must.
In most cases, I think we can trust babies to take the lead in weaning. But I also believe that as clinicians we must remain alert to the few situations when extended nursing is not in the best interest for the baby who is not growing well or for the mother for whom the nursing is taking an unreasonable toll on her physical and mental health.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
With a moistened index finger pointing skyward, I always have tried to remain alert to where the winds of change are blowing. But every now and then I miss a trend in child care, and that is the case with something known as “baby-led weaning.” Influenced by the questionable notion that there is a “natural” way of doing almost everything, the concept hinges on the belief that an infant will “tell” his mother when it is time to stop nursing and begin solids, aka complementary feeding.
At face value, the concept of allowing the baby to lead is a good one simply because of universality of biologic variation. Just as with the question of how much sleep a baby needs, I don’t think anyone (let alone clinicians) can give with assurance an answer that can easily be applied to all infants. There are just too many variables.
For most dyads, breastfeeding is more than just passing calories from one individual to another. Nursing can offer a sense of security and calming both for infants and their mothers. In many cases, the breast unfortunately has become a critical ingredient in the infant’s ritual for falling to sleep. For some mothers, success at breastfeeding becomes an important validation of her feelings of confidence and self-worth that in the past may have been battered by a male-dominated environment. If breastfeeding has been an unpleasant experience, a mother may be more likely to interpret her infant’s behavior as a message that it is time to wean. The bottom line is that a mother’s perception of her baby’s messages about weaning often reflects her own feelings about nursing.
Of course, we clinicians can influence a mother’s perception of her baby’s messages by introducing our own biases about what we believe is the safest, most nutritionally sound way to introduce complementary feeding. And let’s be honest and acknowledge that those are biases mostly unsupported by good scientific study. In many cases, they are more of a reflection of the cultures in which we have grown up.
When asked by parents how they will know when their infant is ready for complementary feeding, I suggest that it’s time when the infant is not only curious about what the adults around him are eating, but obviously is upset that he isn’t being offered a taste. I add that exactly what that food should be is a matter of debate and common sense.
I also encourage parents to allow the child to do as much self-feeding as possible and not worry about the mess. An old shower curtain floor and plenty of sponges and paper towels are a must.
In most cases, I think we can trust babies to take the lead in weaning. But I also believe that as clinicians we must remain alert to the few situations when extended nursing is not in the best interest for the baby who is not growing well or for the mother for whom the nursing is taking an unreasonable toll on her physical and mental health.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.” Email him at [email protected].
Chemo-free induction regimen shines in MCL
SAN DIEGO – A chemotherapy-free induction regimen of ibrutinib and rituximab was well tolerated and achieved an overall response rate of 100% among patients with newly diagnosed mantle cell lymphoma (MCL), according to results of a small single-center phase II trial.
A total of 72% of patients had complete responses to induction, while 28% had partial responses, and 100% had complete responses to consolidation, reported Michael Wang, MD, at the annual meeting of the American Society of Hematology. The findings highlight a “window of opportunity” to effectively treat de novo MCL in young, fit patients while potentially sparing them from repeated cycles of intensive chemoimmunotherapy, the investigators noted.
Established treatments for MCL include eight cycles of rituximab–hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with rituximab–methotrexate–Ara-C (cytarabine). Median overall survival with this regimen exceeds 10 years, but during that decade, more than 6% of patients will develop myeloid neoplasms related to treatment, noted Dr. Wang of the University of Texas MD Anderson Cancer Center in Houston.
For the study, patients up to 65 years old with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib (560 mg), plus rituximab (375 mg/m2) administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD, alternating every 28 days with rituximab plus high-dose methotrexate–Ara-C. Complete responders to induction received four cycles of chemoimmunotherapy, while progressors and partial responders received chemoimmunotherapy for two cycles beyond the point of complete remission.
Among 36 evaluable patients, 28% had a partial response and the rest had complete responses to induction. Moreover, all 19 patients who completed consolidation had a complete response. During induction, the most common toxicities were fatigue, diarrhea, rash, and myalgia, nearly all of which were mild or moderate in severity. During consolidation, two patients developed severe neutropenia, one patient developed severe febrile neutropenia, and one developed a severe increase in liver enzymes. There were no treatment-related deaths.
“The toxicity after intensive immune-chemotherapy in shortened cycles [is] much improved compared to historical controls, but longer follow-up is needed,” the researchers wrote. “This unprecedented efficacy and safety may provide a window of opportunity for less chemoimmunotherapy needed for consolidation.”
Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
SAN DIEGO – A chemotherapy-free induction regimen of ibrutinib and rituximab was well tolerated and achieved an overall response rate of 100% among patients with newly diagnosed mantle cell lymphoma (MCL), according to results of a small single-center phase II trial.
A total of 72% of patients had complete responses to induction, while 28% had partial responses, and 100% had complete responses to consolidation, reported Michael Wang, MD, at the annual meeting of the American Society of Hematology. The findings highlight a “window of opportunity” to effectively treat de novo MCL in young, fit patients while potentially sparing them from repeated cycles of intensive chemoimmunotherapy, the investigators noted.
Established treatments for MCL include eight cycles of rituximab–hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with rituximab–methotrexate–Ara-C (cytarabine). Median overall survival with this regimen exceeds 10 years, but during that decade, more than 6% of patients will develop myeloid neoplasms related to treatment, noted Dr. Wang of the University of Texas MD Anderson Cancer Center in Houston.
For the study, patients up to 65 years old with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib (560 mg), plus rituximab (375 mg/m2) administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD, alternating every 28 days with rituximab plus high-dose methotrexate–Ara-C. Complete responders to induction received four cycles of chemoimmunotherapy, while progressors and partial responders received chemoimmunotherapy for two cycles beyond the point of complete remission.
Among 36 evaluable patients, 28% had a partial response and the rest had complete responses to induction. Moreover, all 19 patients who completed consolidation had a complete response. During induction, the most common toxicities were fatigue, diarrhea, rash, and myalgia, nearly all of which were mild or moderate in severity. During consolidation, two patients developed severe neutropenia, one patient developed severe febrile neutropenia, and one developed a severe increase in liver enzymes. There were no treatment-related deaths.
“The toxicity after intensive immune-chemotherapy in shortened cycles [is] much improved compared to historical controls, but longer follow-up is needed,” the researchers wrote. “This unprecedented efficacy and safety may provide a window of opportunity for less chemoimmunotherapy needed for consolidation.”
Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
SAN DIEGO – A chemotherapy-free induction regimen of ibrutinib and rituximab was well tolerated and achieved an overall response rate of 100% among patients with newly diagnosed mantle cell lymphoma (MCL), according to results of a small single-center phase II trial.
A total of 72% of patients had complete responses to induction, while 28% had partial responses, and 100% had complete responses to consolidation, reported Michael Wang, MD, at the annual meeting of the American Society of Hematology. The findings highlight a “window of opportunity” to effectively treat de novo MCL in young, fit patients while potentially sparing them from repeated cycles of intensive chemoimmunotherapy, the investigators noted.
Established treatments for MCL include eight cycles of rituximab–hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with rituximab–methotrexate–Ara-C (cytarabine). Median overall survival with this regimen exceeds 10 years, but during that decade, more than 6% of patients will develop myeloid neoplasms related to treatment, noted Dr. Wang of the University of Texas MD Anderson Cancer Center in Houston.
For the study, patients up to 65 years old with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib (560 mg), plus rituximab (375 mg/m2) administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD, alternating every 28 days with rituximab plus high-dose methotrexate–Ara-C. Complete responders to induction received four cycles of chemoimmunotherapy, while progressors and partial responders received chemoimmunotherapy for two cycles beyond the point of complete remission.
Among 36 evaluable patients, 28% had a partial response and the rest had complete responses to induction. Moreover, all 19 patients who completed consolidation had a complete response. During induction, the most common toxicities were fatigue, diarrhea, rash, and myalgia, nearly all of which were mild or moderate in severity. During consolidation, two patients developed severe neutropenia, one patient developed severe febrile neutropenia, and one developed a severe increase in liver enzymes. There were no treatment-related deaths.
“The toxicity after intensive immune-chemotherapy in shortened cycles [is] much improved compared to historical controls, but longer follow-up is needed,” the researchers wrote. “This unprecedented efficacy and safety may provide a window of opportunity for less chemoimmunotherapy needed for consolidation.”
Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
AT ASH 2016
Key clinical point: Induction with ibrutinib and rituximab achieved a 100% response rate in newly diagnosed mantle cell lymphoma, enabling patients to receive less intensive consolidation.
Major finding: Rates of complete response were 72% for induction and 100% for induction plus consolidation.
Data source: A single-center phase II trial of 36 patients with newly diagnosed, untreated mantle cell lymphoma.
Disclosures: Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.










