User login
Don’t fear POTS: Tips for diagnosis and treatment
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
Supplemental oxygen fails to improve echocardiographic measures in PE patients
compared with ambient oxygen in a pilot study of 70 individuals.
Anticoagulation monotherapy is the standard of care for patients with intermediate-risk pulmonary embolism (PE), but persistent short-term complication rates may approach 10%, wrote Deisy Barrios, MD, of Hospital Ramón y Cajal (IRYCIS), Madrid, and colleagues. Additional strategies are needed, and the use of supplemental oxygen in non-hypoxemic patients with intermediate-risk PE has not been explored, they said.
In a study published in the journal Chest, the researchers recruited 36 women and 34 men who were non-hypoxemic with stable PE and intermediate risk, defined as echocardiographic RV enlargement. The study recruitment ended prematurely because of the COVID-19 pandemic. The mean age of the participants was 67.3 years. Patients were randomized within 24 hours of hospital admission to anticoagulation plus supplemental oxygen or anticoagulation alone. The groups were similar in echocardiographic mean RV end-diameter and RV/LV ratios at baseline.
The intervention patients received supplemental oxygen at a 35% concentration (7 L/min) continuously for 48 hours via a face mask, and through a nasal cannula during meal times.
The primary outcome was normalization of right ventricle size (defined as an RV/LV diameter ratio less than 1.0 from the subcostal or apical view) at 48 hours after randomization. Secondary outcomes included change in the right ventricle/left ventricle diameter as measured at 48 hours and 7 days after randomization compared to baseline.
The proportion of patients with an RV/LV ratio of 1.0 or less at 48 hours was not significantly different between the intervention and control groups (42.4% vs. 21.6%, P = .08). Similarly, the proportion of patients with an RV/LV ratio of 1.0 or less at 7 days was not significantly different between the groups (76% vs. 70%).
The between-group reduction in RV/LV ratio was significantly greater in the supplemental oxygen group vs. the control group from baseline to 48 hours (0.28 vs. 0.12 P = .02).
However, the within-group mean RV/LV ratio was significantly reduced in both the supplemental oxygen group and the control group compared to baseline at 48 hours and at 7 days after randomization.
None of the patients experienced hemodynamic collapse or recurrent venous thromboembolism during the follow-up period.
The findings were limited by several factors including the small sample size and open-label design, and lack of power to detect clinical outcomes, the researchers noted.
However, the results suggest that although supplemental oxygen had no significant impact of RV/LV normalization, “supplemental oxygen might increase the likelihood of reducing echocardiographic RV dilatation,” and the findings warrant a definitive clinical outcomes trial of supplemental oxygen vs. ambient air to improve outcomes in non-hypoxemic patients with intermediate-risk PE, they concluded.
The study was supported by the Instituto de Salud Carlos III. Dr. Barrios had no financial conflicts to disclose.
compared with ambient oxygen in a pilot study of 70 individuals.
Anticoagulation monotherapy is the standard of care for patients with intermediate-risk pulmonary embolism (PE), but persistent short-term complication rates may approach 10%, wrote Deisy Barrios, MD, of Hospital Ramón y Cajal (IRYCIS), Madrid, and colleagues. Additional strategies are needed, and the use of supplemental oxygen in non-hypoxemic patients with intermediate-risk PE has not been explored, they said.
In a study published in the journal Chest, the researchers recruited 36 women and 34 men who were non-hypoxemic with stable PE and intermediate risk, defined as echocardiographic RV enlargement. The study recruitment ended prematurely because of the COVID-19 pandemic. The mean age of the participants was 67.3 years. Patients were randomized within 24 hours of hospital admission to anticoagulation plus supplemental oxygen or anticoagulation alone. The groups were similar in echocardiographic mean RV end-diameter and RV/LV ratios at baseline.
The intervention patients received supplemental oxygen at a 35% concentration (7 L/min) continuously for 48 hours via a face mask, and through a nasal cannula during meal times.
The primary outcome was normalization of right ventricle size (defined as an RV/LV diameter ratio less than 1.0 from the subcostal or apical view) at 48 hours after randomization. Secondary outcomes included change in the right ventricle/left ventricle diameter as measured at 48 hours and 7 days after randomization compared to baseline.
The proportion of patients with an RV/LV ratio of 1.0 or less at 48 hours was not significantly different between the intervention and control groups (42.4% vs. 21.6%, P = .08). Similarly, the proportion of patients with an RV/LV ratio of 1.0 or less at 7 days was not significantly different between the groups (76% vs. 70%).
The between-group reduction in RV/LV ratio was significantly greater in the supplemental oxygen group vs. the control group from baseline to 48 hours (0.28 vs. 0.12 P = .02).
However, the within-group mean RV/LV ratio was significantly reduced in both the supplemental oxygen group and the control group compared to baseline at 48 hours and at 7 days after randomization.
None of the patients experienced hemodynamic collapse or recurrent venous thromboembolism during the follow-up period.
The findings were limited by several factors including the small sample size and open-label design, and lack of power to detect clinical outcomes, the researchers noted.
However, the results suggest that although supplemental oxygen had no significant impact of RV/LV normalization, “supplemental oxygen might increase the likelihood of reducing echocardiographic RV dilatation,” and the findings warrant a definitive clinical outcomes trial of supplemental oxygen vs. ambient air to improve outcomes in non-hypoxemic patients with intermediate-risk PE, they concluded.
The study was supported by the Instituto de Salud Carlos III. Dr. Barrios had no financial conflicts to disclose.
compared with ambient oxygen in a pilot study of 70 individuals.
Anticoagulation monotherapy is the standard of care for patients with intermediate-risk pulmonary embolism (PE), but persistent short-term complication rates may approach 10%, wrote Deisy Barrios, MD, of Hospital Ramón y Cajal (IRYCIS), Madrid, and colleagues. Additional strategies are needed, and the use of supplemental oxygen in non-hypoxemic patients with intermediate-risk PE has not been explored, they said.
In a study published in the journal Chest, the researchers recruited 36 women and 34 men who were non-hypoxemic with stable PE and intermediate risk, defined as echocardiographic RV enlargement. The study recruitment ended prematurely because of the COVID-19 pandemic. The mean age of the participants was 67.3 years. Patients were randomized within 24 hours of hospital admission to anticoagulation plus supplemental oxygen or anticoagulation alone. The groups were similar in echocardiographic mean RV end-diameter and RV/LV ratios at baseline.
The intervention patients received supplemental oxygen at a 35% concentration (7 L/min) continuously for 48 hours via a face mask, and through a nasal cannula during meal times.
The primary outcome was normalization of right ventricle size (defined as an RV/LV diameter ratio less than 1.0 from the subcostal or apical view) at 48 hours after randomization. Secondary outcomes included change in the right ventricle/left ventricle diameter as measured at 48 hours and 7 days after randomization compared to baseline.
The proportion of patients with an RV/LV ratio of 1.0 or less at 48 hours was not significantly different between the intervention and control groups (42.4% vs. 21.6%, P = .08). Similarly, the proportion of patients with an RV/LV ratio of 1.0 or less at 7 days was not significantly different between the groups (76% vs. 70%).
The between-group reduction in RV/LV ratio was significantly greater in the supplemental oxygen group vs. the control group from baseline to 48 hours (0.28 vs. 0.12 P = .02).
However, the within-group mean RV/LV ratio was significantly reduced in both the supplemental oxygen group and the control group compared to baseline at 48 hours and at 7 days after randomization.
None of the patients experienced hemodynamic collapse or recurrent venous thromboembolism during the follow-up period.
The findings were limited by several factors including the small sample size and open-label design, and lack of power to detect clinical outcomes, the researchers noted.
However, the results suggest that although supplemental oxygen had no significant impact of RV/LV normalization, “supplemental oxygen might increase the likelihood of reducing echocardiographic RV dilatation,” and the findings warrant a definitive clinical outcomes trial of supplemental oxygen vs. ambient air to improve outcomes in non-hypoxemic patients with intermediate-risk PE, they concluded.
The study was supported by the Instituto de Salud Carlos III. Dr. Barrios had no financial conflicts to disclose.
FROM THE JOURNAL CHEST
Growing ‘tranq’ threat poses challenges for PCPs
The widening threat of the animal tranquilizer xylazine, otherwise known as tranq, which has been found in illegally manufactured fentanyl, necessitates wider testing, a better understanding of its effects, and more research on treatment options, according to a narrative review published in the Annals of Internal Medicine.
“A lot of doctors and providers are asking about this drug,” said Joseph D’Orazio, MD, an addiction medicine specialist and medical toxicologist at Cooper University Healthcare, Camden, N.J., who led the review.
Xylazine is believed to prolong or intensify the effects of opioids, making it a popular additive to illegally produced opioids, particularly fentanyl, according to the Drug Enforcement Administration. Users end up in a zombie-like state with slowed breathing, and they sometimes develop skin ulcers. Because xylazine is not an opioid, common antidotes such as naloxone are ineffective. The White House has called the fentanyl-xylazine combo an “emerging threat.”
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA administrator Anne Milgram, in a statement on the agency’s website. “DEA has seized xylazine and fentanyl mixtures in 48 of 50 States. The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Dr. D’Orazio paired clinical experience with available research to provide guidance on the care of patients exposed to xylazine.
He and his team issued a call for more research on the drug’s effects, including more details on dependency and withdrawal.
Testing a patient who may have been exposed to xylazine requires forensic lab capabilities, which makes testing complicated and costly. The review found no evidence of the origin of the drug or why it causes open sores.
The review calls for more education of providers, including primary care physicians, on the treatment and care of patients who have used xylazine and fentanyl. The authors also call for expanding standard urine analysis to test for xylazine and for intensifying surveillance of the drug supply and distribution of xylazine test strips.
The authors of an editorial that accompanied the journal article urged the health care community to get ahead of xylazine before the crisis worsens.
“Not testing for xylazine in current unaffected areas and populations may lead to delays in responding if and when the drug becomes prevalent in the drug supply,” the authors wrote.
Xylazine was detected in 90% of street opioid samples tested in Philadelphia in 2021, and a toxic surveillance study of drug paraphernalia in Maryland found xylazine in 80% of samples tested between 2021 and 2022.
Dr. D’Orazio stressed that although Narcan is ineffective in treating xylazine, because the sedative is almost always mixed with fentanyl or another opiate, the opioid antagonist should still be used in emergencies.
Angelique Campen, MD, an emergency medicine physician at Providence St. Joseph Medical Center, Burbank, Calif., said she has seen an increase in patients entering the emergency department under the influence of what seems like fentanyl or heroin, but standard treatments such as Narcan have a limited effect. These patients remain in a prolonged period of sedation.
Recently, she admitted to her hospital’s intensive care unit a patient suspected of a xylazine overdose who was not responding to treatment.
Dr. Campen said that patients are screened for fentanyl, but because no test is available for xylazine, she presumed xylazine was causing the complication.
“It makes perfect medical sense to me that that’s what was going on,” Dr. Campen, who has worked at St. Joseph’s for 25 years, said. “I’m hoping with physicians being more aware of it that we can have that part of our regular urine drug screen.”
Dr. Campen also said she hopes an antidote is soon developed.
“If we can just keep delivering that message, hopefully, [to] more and more people, it will get through to them,” she said. “Every time you’re taking this, even though you may have taken it a week before and been fine, you never know: The next dose you take may be the lethal dose.”
A review author reports being awarded $1,000 to cover travel cost for Best Overall Abstract at the American Society of Addiction Medicine 2023 Annual Meeting. Another author reports receiving payments for training conducted as part of a NJDMAHS training grant to educate on substance use disorders. Dr. D’Orazio reports a $500 honorarium for a one-time lecture on xylazine at Yale; and a $500 honorarium for speaking one to three times per year on various topics regarding opioid use disorder at the Health Federation of Philadelphia. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The widening threat of the animal tranquilizer xylazine, otherwise known as tranq, which has been found in illegally manufactured fentanyl, necessitates wider testing, a better understanding of its effects, and more research on treatment options, according to a narrative review published in the Annals of Internal Medicine.
“A lot of doctors and providers are asking about this drug,” said Joseph D’Orazio, MD, an addiction medicine specialist and medical toxicologist at Cooper University Healthcare, Camden, N.J., who led the review.
Xylazine is believed to prolong or intensify the effects of opioids, making it a popular additive to illegally produced opioids, particularly fentanyl, according to the Drug Enforcement Administration. Users end up in a zombie-like state with slowed breathing, and they sometimes develop skin ulcers. Because xylazine is not an opioid, common antidotes such as naloxone are ineffective. The White House has called the fentanyl-xylazine combo an “emerging threat.”
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA administrator Anne Milgram, in a statement on the agency’s website. “DEA has seized xylazine and fentanyl mixtures in 48 of 50 States. The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Dr. D’Orazio paired clinical experience with available research to provide guidance on the care of patients exposed to xylazine.
He and his team issued a call for more research on the drug’s effects, including more details on dependency and withdrawal.
Testing a patient who may have been exposed to xylazine requires forensic lab capabilities, which makes testing complicated and costly. The review found no evidence of the origin of the drug or why it causes open sores.
The review calls for more education of providers, including primary care physicians, on the treatment and care of patients who have used xylazine and fentanyl. The authors also call for expanding standard urine analysis to test for xylazine and for intensifying surveillance of the drug supply and distribution of xylazine test strips.
The authors of an editorial that accompanied the journal article urged the health care community to get ahead of xylazine before the crisis worsens.
“Not testing for xylazine in current unaffected areas and populations may lead to delays in responding if and when the drug becomes prevalent in the drug supply,” the authors wrote.
Xylazine was detected in 90% of street opioid samples tested in Philadelphia in 2021, and a toxic surveillance study of drug paraphernalia in Maryland found xylazine in 80% of samples tested between 2021 and 2022.
Dr. D’Orazio stressed that although Narcan is ineffective in treating xylazine, because the sedative is almost always mixed with fentanyl or another opiate, the opioid antagonist should still be used in emergencies.
Angelique Campen, MD, an emergency medicine physician at Providence St. Joseph Medical Center, Burbank, Calif., said she has seen an increase in patients entering the emergency department under the influence of what seems like fentanyl or heroin, but standard treatments such as Narcan have a limited effect. These patients remain in a prolonged period of sedation.
Recently, she admitted to her hospital’s intensive care unit a patient suspected of a xylazine overdose who was not responding to treatment.
Dr. Campen said that patients are screened for fentanyl, but because no test is available for xylazine, she presumed xylazine was causing the complication.
“It makes perfect medical sense to me that that’s what was going on,” Dr. Campen, who has worked at St. Joseph’s for 25 years, said. “I’m hoping with physicians being more aware of it that we can have that part of our regular urine drug screen.”
Dr. Campen also said she hopes an antidote is soon developed.
“If we can just keep delivering that message, hopefully, [to] more and more people, it will get through to them,” she said. “Every time you’re taking this, even though you may have taken it a week before and been fine, you never know: The next dose you take may be the lethal dose.”
A review author reports being awarded $1,000 to cover travel cost for Best Overall Abstract at the American Society of Addiction Medicine 2023 Annual Meeting. Another author reports receiving payments for training conducted as part of a NJDMAHS training grant to educate on substance use disorders. Dr. D’Orazio reports a $500 honorarium for a one-time lecture on xylazine at Yale; and a $500 honorarium for speaking one to three times per year on various topics regarding opioid use disorder at the Health Federation of Philadelphia. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
The widening threat of the animal tranquilizer xylazine, otherwise known as tranq, which has been found in illegally manufactured fentanyl, necessitates wider testing, a better understanding of its effects, and more research on treatment options, according to a narrative review published in the Annals of Internal Medicine.
“A lot of doctors and providers are asking about this drug,” said Joseph D’Orazio, MD, an addiction medicine specialist and medical toxicologist at Cooper University Healthcare, Camden, N.J., who led the review.
Xylazine is believed to prolong or intensify the effects of opioids, making it a popular additive to illegally produced opioids, particularly fentanyl, according to the Drug Enforcement Administration. Users end up in a zombie-like state with slowed breathing, and they sometimes develop skin ulcers. Because xylazine is not an opioid, common antidotes such as naloxone are ineffective. The White House has called the fentanyl-xylazine combo an “emerging threat.”
“Xylazine is making the deadliest drug threat our country has ever faced, fentanyl, even deadlier,” said DEA administrator Anne Milgram, in a statement on the agency’s website. “DEA has seized xylazine and fentanyl mixtures in 48 of 50 States. The DEA Laboratory System is reporting that in 2022 approximately 23% of fentanyl powder and 7% of fentanyl pills seized by the DEA contained xylazine.”
Dr. D’Orazio paired clinical experience with available research to provide guidance on the care of patients exposed to xylazine.
He and his team issued a call for more research on the drug’s effects, including more details on dependency and withdrawal.
Testing a patient who may have been exposed to xylazine requires forensic lab capabilities, which makes testing complicated and costly. The review found no evidence of the origin of the drug or why it causes open sores.
The review calls for more education of providers, including primary care physicians, on the treatment and care of patients who have used xylazine and fentanyl. The authors also call for expanding standard urine analysis to test for xylazine and for intensifying surveillance of the drug supply and distribution of xylazine test strips.
The authors of an editorial that accompanied the journal article urged the health care community to get ahead of xylazine before the crisis worsens.
“Not testing for xylazine in current unaffected areas and populations may lead to delays in responding if and when the drug becomes prevalent in the drug supply,” the authors wrote.
Xylazine was detected in 90% of street opioid samples tested in Philadelphia in 2021, and a toxic surveillance study of drug paraphernalia in Maryland found xylazine in 80% of samples tested between 2021 and 2022.
Dr. D’Orazio stressed that although Narcan is ineffective in treating xylazine, because the sedative is almost always mixed with fentanyl or another opiate, the opioid antagonist should still be used in emergencies.
Angelique Campen, MD, an emergency medicine physician at Providence St. Joseph Medical Center, Burbank, Calif., said she has seen an increase in patients entering the emergency department under the influence of what seems like fentanyl or heroin, but standard treatments such as Narcan have a limited effect. These patients remain in a prolonged period of sedation.
Recently, she admitted to her hospital’s intensive care unit a patient suspected of a xylazine overdose who was not responding to treatment.
Dr. Campen said that patients are screened for fentanyl, but because no test is available for xylazine, she presumed xylazine was causing the complication.
“It makes perfect medical sense to me that that’s what was going on,” Dr. Campen, who has worked at St. Joseph’s for 25 years, said. “I’m hoping with physicians being more aware of it that we can have that part of our regular urine drug screen.”
Dr. Campen also said she hopes an antidote is soon developed.
“If we can just keep delivering that message, hopefully, [to] more and more people, it will get through to them,” she said. “Every time you’re taking this, even though you may have taken it a week before and been fine, you never know: The next dose you take may be the lethal dose.”
A review author reports being awarded $1,000 to cover travel cost for Best Overall Abstract at the American Society of Addiction Medicine 2023 Annual Meeting. Another author reports receiving payments for training conducted as part of a NJDMAHS training grant to educate on substance use disorders. Dr. D’Orazio reports a $500 honorarium for a one-time lecture on xylazine at Yale; and a $500 honorarium for speaking one to three times per year on various topics regarding opioid use disorder at the Health Federation of Philadelphia. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
This is how you get patients back for follow-up cancer testing
according to authors of a new study published in the Journal of the American Medical Association.
Results from the clustered, randomized clinical trial indicate that systems-based interventions, such as automating reminders in electronic health records (EHRs), outreach in the form of phone calls or letters, and assistance with barriers to health care, such as housing insecurity, can increase the number of patients who complete appropriate diagnostic follow-up after an abnormal result.
Patients who received an EHR reminder, outreach call or letter, and additional calls to screen for and assist with nine barriers to health care – housing insecurity, food insecurity, paying for basic utilities, family caregiving, legal issues, transportation, financial compensation for treatment, education, and employment – completed follow-up within 120 days of study enrollment at a rate of 31.4%. The follow-up rate was 31% for those who received only an EHR reminder and outreach, 22.7% for those who received only an EHR reminder, and 22.9% for those who received usual care.
“The benefits of cancer screening won’t be fully realized without systems to ensure timely follow-up of abnormal results,” said Anna Tosteson, ScD, director of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H., a coauthor of the study.
Current payment incentives and quality-of-care indicators focus on getting people in for screening but should also address completion of screening – meaning timely and appropriate follow-up of results that could be indicative of cancer, Dr. Tosteson said.
“There’s a disconnect if you have screening rates that are high but once people have an abnormal result, which is potentially one step closer to a cancer diagnosis, there are no systems in place to help clinicians track them,” said study coauthor Jennifer Haas, MD, director of the Center for Primary Care Research at Massachusetts General Hospital in Boston.
In a 2016 study, researchers found that follow-up rates after abnormal cancer screenings varied widely. While 95.6% of patients with abnormal breast cancer screenings underwent timely follow-up testing, only 68.1% of patients with colorectal abnormalities and 44.8% of patients with cervical abnormalities did so.
Researchers for the new study used guideline recommendations and specialist input to create automated EHR algorithms that determined a follow-up period and diagnostic test.
They put the algorithm into practice with 11,980 patients who were part of 44 primary care practices within three health networks between August 2020 and December 2021. All patients had received abnormal test results for colorectal, breast, cervical, or lung cancer in varying risk categories.
All patients received usual care from their providers, which consisted of a “hodgepodge of whatever their clinic usually does,” Dr. Haas said. Without standards and systems in place for follow-up, the burden of testing and tracking patients with abnormal results typically falls on the primary care provider.
The researchers intervened only when patients were overdue for completion of follow-up. They then staggered the interventions sequentially.
All study participants received an automated, algorithm-triggered EHR reminder for follow-up in their patient portal along with routine health maintenance reminders. To view the reminder, patients had to log into their portal. Participants in the outreach and outreach and navigation groups also received a phone call, an EHR message, or a physical letter 2 weeks after receiving an EHR notification if they hadn’t completed follow-up. Research assistants performed the outreach after having been prompted by the algorithm.
After another 4 weeks, those in the EHR, outreach, and navigation group received a call from a patient navigator who helped them address nine barriers to health care, chiefly by providing them with referrals to free resources.
Among patients who received navigation, outcomes were not significantly better than among those who received EHR and outreach, indicating social determinants of health did not significantly affect the population studied or that the modest approach to navigation and the resources provided were insufficient, Dr. Haas said.
The complexity of an automated platform that encompasses many types of cancers, test results, and other data elements could prove difficult to apply in settings with less infrastructure, said Steven Atlas, MD, MPH, director of the Practice-Based Research and Quality Improvement Network in the division of general internal medicine at Mass General.
“I think there’s a role for the federal government to take on these initiatives,” Dr. Atlas said. Government intervention could help create “national IT systems to create standards for creating code for what an abnormal result is and how it should be followed,” he said.
While interventions improved patient follow-up, the overall rates were still low.
“What concerns me is that despite the various interventions implemented to encourage and support patients to return for follow-up testing, over 60% of patients still did not return for the recommended testing,” said Joann G. Elmore, MD, MPH, professor of medicine at the University of California, Los Angeles. Dr. Elmore was not involved with the study.
The research took place during the COVID-19 pandemic, which may have reduced follow-up, the study authors wrote. Still, given that previous research has shown that follow-up tends to be low, the rates highlight “the need to understand factors associated with not completing follow-up that go beyond reminder effort,” they wrote. These include a need for patient education about the meaning of test results and what follow-up procedures involve.
The study was supported by the National Cancer Institute and the American Cancer Society. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to authors of a new study published in the Journal of the American Medical Association.
Results from the clustered, randomized clinical trial indicate that systems-based interventions, such as automating reminders in electronic health records (EHRs), outreach in the form of phone calls or letters, and assistance with barriers to health care, such as housing insecurity, can increase the number of patients who complete appropriate diagnostic follow-up after an abnormal result.
Patients who received an EHR reminder, outreach call or letter, and additional calls to screen for and assist with nine barriers to health care – housing insecurity, food insecurity, paying for basic utilities, family caregiving, legal issues, transportation, financial compensation for treatment, education, and employment – completed follow-up within 120 days of study enrollment at a rate of 31.4%. The follow-up rate was 31% for those who received only an EHR reminder and outreach, 22.7% for those who received only an EHR reminder, and 22.9% for those who received usual care.
“The benefits of cancer screening won’t be fully realized without systems to ensure timely follow-up of abnormal results,” said Anna Tosteson, ScD, director of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H., a coauthor of the study.
Current payment incentives and quality-of-care indicators focus on getting people in for screening but should also address completion of screening – meaning timely and appropriate follow-up of results that could be indicative of cancer, Dr. Tosteson said.
“There’s a disconnect if you have screening rates that are high but once people have an abnormal result, which is potentially one step closer to a cancer diagnosis, there are no systems in place to help clinicians track them,” said study coauthor Jennifer Haas, MD, director of the Center for Primary Care Research at Massachusetts General Hospital in Boston.
In a 2016 study, researchers found that follow-up rates after abnormal cancer screenings varied widely. While 95.6% of patients with abnormal breast cancer screenings underwent timely follow-up testing, only 68.1% of patients with colorectal abnormalities and 44.8% of patients with cervical abnormalities did so.
Researchers for the new study used guideline recommendations and specialist input to create automated EHR algorithms that determined a follow-up period and diagnostic test.
They put the algorithm into practice with 11,980 patients who were part of 44 primary care practices within three health networks between August 2020 and December 2021. All patients had received abnormal test results for colorectal, breast, cervical, or lung cancer in varying risk categories.
All patients received usual care from their providers, which consisted of a “hodgepodge of whatever their clinic usually does,” Dr. Haas said. Without standards and systems in place for follow-up, the burden of testing and tracking patients with abnormal results typically falls on the primary care provider.
The researchers intervened only when patients were overdue for completion of follow-up. They then staggered the interventions sequentially.
All study participants received an automated, algorithm-triggered EHR reminder for follow-up in their patient portal along with routine health maintenance reminders. To view the reminder, patients had to log into their portal. Participants in the outreach and outreach and navigation groups also received a phone call, an EHR message, or a physical letter 2 weeks after receiving an EHR notification if they hadn’t completed follow-up. Research assistants performed the outreach after having been prompted by the algorithm.
After another 4 weeks, those in the EHR, outreach, and navigation group received a call from a patient navigator who helped them address nine barriers to health care, chiefly by providing them with referrals to free resources.
Among patients who received navigation, outcomes were not significantly better than among those who received EHR and outreach, indicating social determinants of health did not significantly affect the population studied or that the modest approach to navigation and the resources provided were insufficient, Dr. Haas said.
The complexity of an automated platform that encompasses many types of cancers, test results, and other data elements could prove difficult to apply in settings with less infrastructure, said Steven Atlas, MD, MPH, director of the Practice-Based Research and Quality Improvement Network in the division of general internal medicine at Mass General.
“I think there’s a role for the federal government to take on these initiatives,” Dr. Atlas said. Government intervention could help create “national IT systems to create standards for creating code for what an abnormal result is and how it should be followed,” he said.
While interventions improved patient follow-up, the overall rates were still low.
“What concerns me is that despite the various interventions implemented to encourage and support patients to return for follow-up testing, over 60% of patients still did not return for the recommended testing,” said Joann G. Elmore, MD, MPH, professor of medicine at the University of California, Los Angeles. Dr. Elmore was not involved with the study.
The research took place during the COVID-19 pandemic, which may have reduced follow-up, the study authors wrote. Still, given that previous research has shown that follow-up tends to be low, the rates highlight “the need to understand factors associated with not completing follow-up that go beyond reminder effort,” they wrote. These include a need for patient education about the meaning of test results and what follow-up procedures involve.
The study was supported by the National Cancer Institute and the American Cancer Society. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to authors of a new study published in the Journal of the American Medical Association.
Results from the clustered, randomized clinical trial indicate that systems-based interventions, such as automating reminders in electronic health records (EHRs), outreach in the form of phone calls or letters, and assistance with barriers to health care, such as housing insecurity, can increase the number of patients who complete appropriate diagnostic follow-up after an abnormal result.
Patients who received an EHR reminder, outreach call or letter, and additional calls to screen for and assist with nine barriers to health care – housing insecurity, food insecurity, paying for basic utilities, family caregiving, legal issues, transportation, financial compensation for treatment, education, and employment – completed follow-up within 120 days of study enrollment at a rate of 31.4%. The follow-up rate was 31% for those who received only an EHR reminder and outreach, 22.7% for those who received only an EHR reminder, and 22.9% for those who received usual care.
“The benefits of cancer screening won’t be fully realized without systems to ensure timely follow-up of abnormal results,” said Anna Tosteson, ScD, director of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H., a coauthor of the study.
Current payment incentives and quality-of-care indicators focus on getting people in for screening but should also address completion of screening – meaning timely and appropriate follow-up of results that could be indicative of cancer, Dr. Tosteson said.
“There’s a disconnect if you have screening rates that are high but once people have an abnormal result, which is potentially one step closer to a cancer diagnosis, there are no systems in place to help clinicians track them,” said study coauthor Jennifer Haas, MD, director of the Center for Primary Care Research at Massachusetts General Hospital in Boston.
In a 2016 study, researchers found that follow-up rates after abnormal cancer screenings varied widely. While 95.6% of patients with abnormal breast cancer screenings underwent timely follow-up testing, only 68.1% of patients with colorectal abnormalities and 44.8% of patients with cervical abnormalities did so.
Researchers for the new study used guideline recommendations and specialist input to create automated EHR algorithms that determined a follow-up period and diagnostic test.
They put the algorithm into practice with 11,980 patients who were part of 44 primary care practices within three health networks between August 2020 and December 2021. All patients had received abnormal test results for colorectal, breast, cervical, or lung cancer in varying risk categories.
All patients received usual care from their providers, which consisted of a “hodgepodge of whatever their clinic usually does,” Dr. Haas said. Without standards and systems in place for follow-up, the burden of testing and tracking patients with abnormal results typically falls on the primary care provider.
The researchers intervened only when patients were overdue for completion of follow-up. They then staggered the interventions sequentially.
All study participants received an automated, algorithm-triggered EHR reminder for follow-up in their patient portal along with routine health maintenance reminders. To view the reminder, patients had to log into their portal. Participants in the outreach and outreach and navigation groups also received a phone call, an EHR message, or a physical letter 2 weeks after receiving an EHR notification if they hadn’t completed follow-up. Research assistants performed the outreach after having been prompted by the algorithm.
After another 4 weeks, those in the EHR, outreach, and navigation group received a call from a patient navigator who helped them address nine barriers to health care, chiefly by providing them with referrals to free resources.
Among patients who received navigation, outcomes were not significantly better than among those who received EHR and outreach, indicating social determinants of health did not significantly affect the population studied or that the modest approach to navigation and the resources provided were insufficient, Dr. Haas said.
The complexity of an automated platform that encompasses many types of cancers, test results, and other data elements could prove difficult to apply in settings with less infrastructure, said Steven Atlas, MD, MPH, director of the Practice-Based Research and Quality Improvement Network in the division of general internal medicine at Mass General.
“I think there’s a role for the federal government to take on these initiatives,” Dr. Atlas said. Government intervention could help create “national IT systems to create standards for creating code for what an abnormal result is and how it should be followed,” he said.
While interventions improved patient follow-up, the overall rates were still low.
“What concerns me is that despite the various interventions implemented to encourage and support patients to return for follow-up testing, over 60% of patients still did not return for the recommended testing,” said Joann G. Elmore, MD, MPH, professor of medicine at the University of California, Los Angeles. Dr. Elmore was not involved with the study.
The research took place during the COVID-19 pandemic, which may have reduced follow-up, the study authors wrote. Still, given that previous research has shown that follow-up tends to be low, the rates highlight “the need to understand factors associated with not completing follow-up that go beyond reminder effort,” they wrote. These include a need for patient education about the meaning of test results and what follow-up procedures involve.
The study was supported by the National Cancer Institute and the American Cancer Society. The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA
Dietary changes to microbiome may improve lung function
HONOLULU – , and the days immediately following the 9/11 attacks.
Among NYC firefighters enrolled in the randomized FIREHOUSE (Food Intake Restriction for Health Outcome Support and Education) study who took part in a microbiome substudy, those who followed a low-calorie, Mediterranean-style diet had higher levels in stools samples at 6 months of Bacteroides ovatus, a bacterial species associated with protection against bowel inflammation.
In contrast, participants who followed a usual-care diet had elevated 6-month levels of a species associated with high-fat diets and inflammation, reported Rachel Lam, a predoctoral fellow in the Nolan Lab at NYU Langone Medical Center, at the annual meeting of the American College of Chest Physicians (CHEST).
“Overall, we found that in our validation cohort, Bacteroides ovatus was increased in the LoCalMed arm after 6 months, and this bacterial species is associated with fewer negative health effects,” she said.
Ms. Lam noted that in a murine model of high-fat diets, mice gavaged with Bacteroides ovatus had reductions in body mass index and decreased serum LDL cholesterol and triglyceride levels.
FIREHOUSE cohort
Senior author Anna Nolan, MD, whose lab members study predictors of lung function loss in a cohort of firefighters who were exposed to the particulate matter clouding the air of lower Manhattan on 9/11 and the ensuing days, told this news organization that the findings, while preliminary, support previous research findings on potential links between intestinal microbiota and lung function.
“It’s interesting that we saw this done in other models, like mouse models and such, where certain bacteria were viewed as healthy for the system, and if they were able to bring that bacteria out in larger amounts they saw anti-inflammatory effects, so we’re hoping to mirror that and also do a mouse model,” she said.
Dr. Nolan’s group has previously shown that markers for the metabolic syndrome, inflammation, and vascular injury detected in serum samples taken within 6 months of 9/11 were predictive for later abnormal lung function. In addition, their group has found that elevated serum levels of an LDL metabolite after intense World Trade Center dust exposure is a risk factor for future impaired lung function as measured by forced expiratory volume in 1 second (FEV1).
In the FIREHOUSE trial, 89 patients were randomly assigned either to a technology-supported educational and behavioral intervention targeting calorie restriction for weight loss while following a low-calorie Mediterranean diet, or to usual care. The usual-care arm included participants who were informed about their weight, BMI, and other standard measures at annual visits and were given general advice about healthy eating, but were not assigned to a specific diet.
Participants in the LoCalMed group had significant decreases in BMI and increases in FEV1, compared with those in the usual-care group. In addition, the LoCalMed group had improved vascular health, better dietary habits, decreases in fats and calories from sweets, and decreases in inflammation as measured by a lower white blood cell count.
Microbiome substudy
At CHEST 2023, Ms. Lam reported on microbiome pilot and validation substudies of FIREHOUSE.
The pilot study included five patients in each arm. The validation sample included 15 participants in the Mediterranean diet group and 16 in the usual-care diet group.
Each participant’s microbiome was assessed with genomic sequencing with sequences aligned to a bacterial database. The number and diversity of bacterial species in each sample were determined with the Chao1 Index and Shannon Index, respectively.
There were no significant differences among the study groups in mean age, exposure at the World Trade Center site, or years of service.
Although bacterial diversity did not differ between the study arms either at baseline or at 6 months, in both groups it significantly decreased over time (P = .02 in the pilot, P < .0001 in the validation arm).
In the pilot study, there was an increase over 6 months in the usual care arm only of Bilophila wadsworthia, a species associated with high-fat diets and inflammation.
In the validation study, patients in the LoCalMed arm had significant reductions in Ruminococcaceae (P = .015) and increases in both Bacteroides ovatus (P = .03) and Alistipes shahii (P = .038), a recently identified species with uncertain protective or pathogenic potential.
In contrast, there were no significant increases in species in the usual-care group, but there were significant declines in several other bacterial species; Ms.Lam, however, did not say whether these changes had clinical significance. “Future studies will assess microbial association with clinical outcomes,” Ms. Lam said.
Confounding factors
Samuel Evans, MD, a pulmonologist at Straub Medical Center in Honolulu who moderated the oral abstract session where the data were presented, commented that the data are interesting but added that associations are difficult to determine given the heterogeneity of exposures that firefighters encounter.
“I think it’s interesting that clearly diet is influencing the type of bacteria in the biome in the gut, and perhaps some are favorable, and some are not favorable,” he told this news organization “We already know that the Mediterranean diet is associated with better health outcomes, so it makes sense, but can we tease out in the microbiome which bacteria are harmful and which are helpful.”
He noted that there are a lot of confounding factors and that “it’s hard to find the right signal when you have so many variables.”
The FIREHOUSE study is supported by the Centers for Disease Control and Prevention’s National Institute of Occupational Safety & Health and the National Heart, Lung, and Blood Institute. Ms. Lam, Dr. Nolan, and Dr. Evans report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – , and the days immediately following the 9/11 attacks.
Among NYC firefighters enrolled in the randomized FIREHOUSE (Food Intake Restriction for Health Outcome Support and Education) study who took part in a microbiome substudy, those who followed a low-calorie, Mediterranean-style diet had higher levels in stools samples at 6 months of Bacteroides ovatus, a bacterial species associated with protection against bowel inflammation.
In contrast, participants who followed a usual-care diet had elevated 6-month levels of a species associated with high-fat diets and inflammation, reported Rachel Lam, a predoctoral fellow in the Nolan Lab at NYU Langone Medical Center, at the annual meeting of the American College of Chest Physicians (CHEST).
“Overall, we found that in our validation cohort, Bacteroides ovatus was increased in the LoCalMed arm after 6 months, and this bacterial species is associated with fewer negative health effects,” she said.
Ms. Lam noted that in a murine model of high-fat diets, mice gavaged with Bacteroides ovatus had reductions in body mass index and decreased serum LDL cholesterol and triglyceride levels.
FIREHOUSE cohort
Senior author Anna Nolan, MD, whose lab members study predictors of lung function loss in a cohort of firefighters who were exposed to the particulate matter clouding the air of lower Manhattan on 9/11 and the ensuing days, told this news organization that the findings, while preliminary, support previous research findings on potential links between intestinal microbiota and lung function.
“It’s interesting that we saw this done in other models, like mouse models and such, where certain bacteria were viewed as healthy for the system, and if they were able to bring that bacteria out in larger amounts they saw anti-inflammatory effects, so we’re hoping to mirror that and also do a mouse model,” she said.
Dr. Nolan’s group has previously shown that markers for the metabolic syndrome, inflammation, and vascular injury detected in serum samples taken within 6 months of 9/11 were predictive for later abnormal lung function. In addition, their group has found that elevated serum levels of an LDL metabolite after intense World Trade Center dust exposure is a risk factor for future impaired lung function as measured by forced expiratory volume in 1 second (FEV1).
In the FIREHOUSE trial, 89 patients were randomly assigned either to a technology-supported educational and behavioral intervention targeting calorie restriction for weight loss while following a low-calorie Mediterranean diet, or to usual care. The usual-care arm included participants who were informed about their weight, BMI, and other standard measures at annual visits and were given general advice about healthy eating, but were not assigned to a specific diet.
Participants in the LoCalMed group had significant decreases in BMI and increases in FEV1, compared with those in the usual-care group. In addition, the LoCalMed group had improved vascular health, better dietary habits, decreases in fats and calories from sweets, and decreases in inflammation as measured by a lower white blood cell count.
Microbiome substudy
At CHEST 2023, Ms. Lam reported on microbiome pilot and validation substudies of FIREHOUSE.
The pilot study included five patients in each arm. The validation sample included 15 participants in the Mediterranean diet group and 16 in the usual-care diet group.
Each participant’s microbiome was assessed with genomic sequencing with sequences aligned to a bacterial database. The number and diversity of bacterial species in each sample were determined with the Chao1 Index and Shannon Index, respectively.
There were no significant differences among the study groups in mean age, exposure at the World Trade Center site, or years of service.
Although bacterial diversity did not differ between the study arms either at baseline or at 6 months, in both groups it significantly decreased over time (P = .02 in the pilot, P < .0001 in the validation arm).
In the pilot study, there was an increase over 6 months in the usual care arm only of Bilophila wadsworthia, a species associated with high-fat diets and inflammation.
In the validation study, patients in the LoCalMed arm had significant reductions in Ruminococcaceae (P = .015) and increases in both Bacteroides ovatus (P = .03) and Alistipes shahii (P = .038), a recently identified species with uncertain protective or pathogenic potential.
In contrast, there were no significant increases in species in the usual-care group, but there were significant declines in several other bacterial species; Ms.Lam, however, did not say whether these changes had clinical significance. “Future studies will assess microbial association with clinical outcomes,” Ms. Lam said.
Confounding factors
Samuel Evans, MD, a pulmonologist at Straub Medical Center in Honolulu who moderated the oral abstract session where the data were presented, commented that the data are interesting but added that associations are difficult to determine given the heterogeneity of exposures that firefighters encounter.
“I think it’s interesting that clearly diet is influencing the type of bacteria in the biome in the gut, and perhaps some are favorable, and some are not favorable,” he told this news organization “We already know that the Mediterranean diet is associated with better health outcomes, so it makes sense, but can we tease out in the microbiome which bacteria are harmful and which are helpful.”
He noted that there are a lot of confounding factors and that “it’s hard to find the right signal when you have so many variables.”
The FIREHOUSE study is supported by the Centers for Disease Control and Prevention’s National Institute of Occupational Safety & Health and the National Heart, Lung, and Blood Institute. Ms. Lam, Dr. Nolan, and Dr. Evans report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – , and the days immediately following the 9/11 attacks.
Among NYC firefighters enrolled in the randomized FIREHOUSE (Food Intake Restriction for Health Outcome Support and Education) study who took part in a microbiome substudy, those who followed a low-calorie, Mediterranean-style diet had higher levels in stools samples at 6 months of Bacteroides ovatus, a bacterial species associated with protection against bowel inflammation.
In contrast, participants who followed a usual-care diet had elevated 6-month levels of a species associated with high-fat diets and inflammation, reported Rachel Lam, a predoctoral fellow in the Nolan Lab at NYU Langone Medical Center, at the annual meeting of the American College of Chest Physicians (CHEST).
“Overall, we found that in our validation cohort, Bacteroides ovatus was increased in the LoCalMed arm after 6 months, and this bacterial species is associated with fewer negative health effects,” she said.
Ms. Lam noted that in a murine model of high-fat diets, mice gavaged with Bacteroides ovatus had reductions in body mass index and decreased serum LDL cholesterol and triglyceride levels.
FIREHOUSE cohort
Senior author Anna Nolan, MD, whose lab members study predictors of lung function loss in a cohort of firefighters who were exposed to the particulate matter clouding the air of lower Manhattan on 9/11 and the ensuing days, told this news organization that the findings, while preliminary, support previous research findings on potential links between intestinal microbiota and lung function.
“It’s interesting that we saw this done in other models, like mouse models and such, where certain bacteria were viewed as healthy for the system, and if they were able to bring that bacteria out in larger amounts they saw anti-inflammatory effects, so we’re hoping to mirror that and also do a mouse model,” she said.
Dr. Nolan’s group has previously shown that markers for the metabolic syndrome, inflammation, and vascular injury detected in serum samples taken within 6 months of 9/11 were predictive for later abnormal lung function. In addition, their group has found that elevated serum levels of an LDL metabolite after intense World Trade Center dust exposure is a risk factor for future impaired lung function as measured by forced expiratory volume in 1 second (FEV1).
In the FIREHOUSE trial, 89 patients were randomly assigned either to a technology-supported educational and behavioral intervention targeting calorie restriction for weight loss while following a low-calorie Mediterranean diet, or to usual care. The usual-care arm included participants who were informed about their weight, BMI, and other standard measures at annual visits and were given general advice about healthy eating, but were not assigned to a specific diet.
Participants in the LoCalMed group had significant decreases in BMI and increases in FEV1, compared with those in the usual-care group. In addition, the LoCalMed group had improved vascular health, better dietary habits, decreases in fats and calories from sweets, and decreases in inflammation as measured by a lower white blood cell count.
Microbiome substudy
At CHEST 2023, Ms. Lam reported on microbiome pilot and validation substudies of FIREHOUSE.
The pilot study included five patients in each arm. The validation sample included 15 participants in the Mediterranean diet group and 16 in the usual-care diet group.
Each participant’s microbiome was assessed with genomic sequencing with sequences aligned to a bacterial database. The number and diversity of bacterial species in each sample were determined with the Chao1 Index and Shannon Index, respectively.
There were no significant differences among the study groups in mean age, exposure at the World Trade Center site, or years of service.
Although bacterial diversity did not differ between the study arms either at baseline or at 6 months, in both groups it significantly decreased over time (P = .02 in the pilot, P < .0001 in the validation arm).
In the pilot study, there was an increase over 6 months in the usual care arm only of Bilophila wadsworthia, a species associated with high-fat diets and inflammation.
In the validation study, patients in the LoCalMed arm had significant reductions in Ruminococcaceae (P = .015) and increases in both Bacteroides ovatus (P = .03) and Alistipes shahii (P = .038), a recently identified species with uncertain protective or pathogenic potential.
In contrast, there were no significant increases in species in the usual-care group, but there were significant declines in several other bacterial species; Ms.Lam, however, did not say whether these changes had clinical significance. “Future studies will assess microbial association with clinical outcomes,” Ms. Lam said.
Confounding factors
Samuel Evans, MD, a pulmonologist at Straub Medical Center in Honolulu who moderated the oral abstract session where the data were presented, commented that the data are interesting but added that associations are difficult to determine given the heterogeneity of exposures that firefighters encounter.
“I think it’s interesting that clearly diet is influencing the type of bacteria in the biome in the gut, and perhaps some are favorable, and some are not favorable,” he told this news organization “We already know that the Mediterranean diet is associated with better health outcomes, so it makes sense, but can we tease out in the microbiome which bacteria are harmful and which are helpful.”
He noted that there are a lot of confounding factors and that “it’s hard to find the right signal when you have so many variables.”
The FIREHOUSE study is supported by the Centers for Disease Control and Prevention’s National Institute of Occupational Safety & Health and the National Heart, Lung, and Blood Institute. Ms. Lam, Dr. Nolan, and Dr. Evans report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT CHEST 2023
Semaglutide win in HFpEF with obesity regardless of ejection fraction: STEP-HFpEF
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.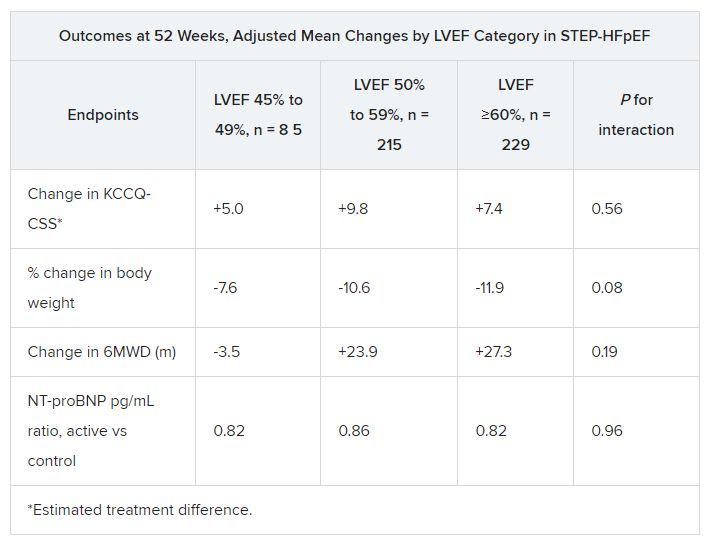
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
AT HFSA 2023
IPF pipeline crowded with new drug candidates
With the emergence of pirfenidone and nintedanib over the past decade or so, pulmonologists now have at their disposal two breakthrough antifibrotic agents for the treatment of idiopathic pulmonary fibrosis.
But these two drugs have a number of shortcomings that a host of investigative agents are aiming to address. For one, while pirfenidone and nintedanib have been shown to slow disease progression and improve symptoms, they don’t stop or reverse the disease. Also, a large number of patients with IPF don’t tolerate these drugs well. And, their high cost is a barrier for many patients.
in terms of therapies or interventions that have better efficacy, better long-term tolerability, and that improve symptoms and quality of life for our patients with IPF disease,” said Joyce Lee, MD, associate professor of medicine–pulmonary at the University of Colorado at Denver, Aurora, and senior medical adviser for research and health care quality for the Pulmonary Fibrosis Foundation.
The National Institutes of Health estimates that more than 30,000 new cases of IPF are diagnosed in the United States annually, and as many as 3 million people have the disease worldwide. The 5-year survival rate is less than 40% after diagnosis. Bloomberg News reported that more than 80 pharmaceutical companies are working on IPF treatments. iHealthcareAnalyst estimates the global market for IPF will reach $10.1 billion by 2029 thanks to rapidly increasing prevalence and incidence with age, premium-priced drugs, and rapid approval of new treatments.
The perils of phase 3 studies
A search on ClinicalTrials.gov turned up 89 investigative IPF treatments in human trials. However, the search for alternatives can be perilous. “In the field, we have gotten used to promising phase 2 studies that failed in the phase 3 stage of development,” Dr. Lee said. “I don’t hold my breath these days just in terms of trying to predict whether or not the efficacy will be present in the phase 3 clinical trial.”
Three notable phase 3 flops include the ISABELA 1 and 2 trials of the autotaxin inhibitor ziritaxestat, which failed to meet their primary endpoint and were halted early (JAMA. 2023;329:1567-78). The phase 3 ZEPHYRUS-1 trial failed to show any benefit of pamrevlumab to improve percent predicted force vital capacity (ppFVC) at week 48, causing discontinuation of a second phase 3 trial. The phase 3 STARSCAPE-OLE study of intravenous recombinant human pentraxin-2 was terminated earlier this year when the sponsor, Hoffmann-LaRoche, decided it was unable to meet its primary objective (NCT04594707).
In the meantime, these six other phase 3 programs in IPF are still in the field:
Anlotinib. A phase 2 and 3 trial in China is evaluating 1-year outcomes of once-daily oral anlotinib for treatment of IPF/progressive fibrosis-interstitial lung disease (PF-ILDS) (NCT05828953). Anlotinib is a tyrosine kinase inhibitor (TKI) that targets four factors: vascular endothelial growth factor receptor (VEGR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit. It’s approved in China as a third-line therapy for non–small cell lung cancer (NSCLC).
BI 101550. Enrollment in the FIBRONEER-IPF trial commenced last fall (NCT05321069), with completion scheduled for late next year. BI 1015550 is an oral phosphodiesterase 4B (PDE4B) inhibitor. FIBRONEER-ILD is a separate phase 3 trial in fibrosing idiopathic lung disease (NCT05321082). In both trials, the primary endpoint is the absolute change from baseline in FVC at week 52.
BMS-986278. Results of a phase 2 trial showed that twice-daily treatment with oral BMS-986278 60 mg over 26 weeks reduced the rate of decline in ppFVC by 69% vs. placebo. The phase 3 ALOFT trial has been approved but hasn’t yet started recruiting patients (NCT06003426). BMS-986278 is a lysophosphatidic acid receptor 1 (LPA1) antagonist.
Lanxoprazole. Commonly used to treat and prevent gastrointestinal problems like stomach ulcers and esophagitis, this oral proton pump inhibitor (PPI) is the focus of a trial in the United Kingdom evaluating if PPIs can slow the progression of IPF (NCT04965298).
N-acetylcysteine (NAC). The PRECISIONS trial is evaluating the effect of NAC plus standard-of-care treatment in IPF patients who have the TOLLIP rs3750910 TT genotype (NCT04300920). Participants receive 600 mg NAC orally or matched placebo three times daily for 24 months. Trial completion is scheduled for 2025.
Treprostinil. Already approved to treat pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease, inhaled Treprostinil is the subject of the TETON 1 and 2 trials evaluating its impact on ppFVC after 52 weeks of treatment (NCT04708782, NCT05255991).
Phase 2 candidates
The primary endpoint in most of the phase 2 trials is change in ppFVC capacity from baseline to week 24. The following investigative therapies are in phase 2 trials:
Bexotegrast (PLN-74809), an oral, small molecule, dual-selective inhibitor of alphav/beta6 and alphav/beta1 (NCT04396756).
BBT-877, described as a potent autotaxin (ATX) inhibitor, demonstrated its ability to inhibit lysophosphatidic acid (LPA) production by as much as 90 percent (NCT05483907).
CC-90001, an oral, once-daily c-Jun N-terminal kinases (JNK) inhibitor. JNKs have been implicated in the underlying mechanisms of fibrosis, including epithelial cell death, inflammation and polarization of profibrotic macrophages, fibroblast activation, and collagen production (NCT03142191).
C21 targets the underlying fibrosis in IPF by stimulating the protective arm of the renin-angiotensin system. It also has an upstream effect by promoting alveolar repair by which it can reduce fibrosis formation, stabilize disease, and increase lung capacity (NCT04533022).
CSL312 (garadacimab) is a humanized anti-FXIIa monoclonal antibody administrated intravenously (NCT05130970).
Cudetaxestat, a noncompetitive autotaxin inhibitor (NCT05373914).
Bersiposocin/DWN12088, an inhibitor of prolyl-tRNA synthetase 1 (PARS1), which is suspected to control the pathologic accumulation of collagen containing high amounts of proline in fibrotic diseases (NCT05389215).
ENV-101, a small-molecule inhibitor of the Hedgehog (Hh) signaling pathway, which plays a key role in IPF. This agent was originally developed to target Hh-driven cancers (NCT04968574).
GKT137831 (setanaxib) inhibits nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) isoforms. (NCT03865927).
HZN-825, a lysophosphatidic acid receptor 1 (LPAR1) antagonist. (NCT05032066)
Ifetroban, a potent and selective thromboxane-prostanoid receptor (TPr) antagonist, which exhibits a high affinity for TPr on platelets, vascular and airway smooth muscle, and fibroblasts, and lacks agonistic activity (NCT05571059).
INS018_055, a small-molecule, oral antifibrotic candidate notable for being the first entirely AI-generated drug to enter phase 2 trials. Trial enrollment started in October (NCT05975983, NCT05983920)
Jaktinib dihydrochloride monohydrate, an oral JAK1, JAK2, and JAK3 inhibitor (NCT04312594).
Leramistat, an anti–tumor necrosis factor (TNF) agent (NCT05951296).
LTP001, an oral, selectively deuterated form of pirfenidone designed to retain the antifibrotic and anti-inflammatory activity of pirfenidone with a differentiated pharmacokinetic profile (NCT05497284, NCT05321420).
ME-015 (suplatast tosilate) aims to stabilize ion channels in the neuronal endings in the lungs that mediate IPF-related cough (NCT05983471).
Nalbuphine, a small-molecule, dual-mechanism treatment for chronic cough in IPF. It acts as both a mu opioid receptor antagonist and a kappa opioid receptor agonist (NCT05964335). The CANAL trial, complete last year, is evaluating an extended-release formulation (NCT04030026).
NP-120 (ifenprodil), a small-molecule N-methyl-D-aspartate (NMDA) receptor antagonist, specifically targets the NMDA-type subunit 2B (GluN2B) (NCT04318704).
Orvepitant, a selective antagonist for the NK₁ receptor, is being evaluated to treat IPF-related cough (NCT05815089).
RXC007 (zelasudil), a Rho-associated coiled-coil–containing protein kinase 2 (ROCK2) selective inhibitor, was granted FDA orphan drug designation in August 2023 (NCT05570058).
Saracatinib, a selective Src kinase inhibitor originally developed for oncological indications (NCT04598919).
SHR-1906, an intravenous treatment, inhibits binding of a target protein to a variety of cytokines and growth factors, affects downstream signaling pathways, and reduces cell proliferation and migration (NCT05722964).
TTI-101, an oral, small-molecule inhibitor of signal transducer and activator of transcription (STAT3), which has been found to accumulate in the lungs of IPF patients (NCT05671835).
VAY736 (lanalumab), a BAFF-R inhibitor (NCT03287414).
Vixarelimab, a human monoclonal oncastatin M receptor beta antibody (NCT05785624).
Some investigative programs, however, didn’t make it out of phase 2. The trial evaluating inhaled GB0139, a selective functional antagonist of G-protein–coupled receptor 84, which plays a key role in fibrosis, failed to meet its primary endpoint (NCT03832946). Likewise, oral GLPG1205 failed to show a significant difference in FVC decline vs. placebo (NCT03725852). The program to develop SAR156597, also known as romilkimab, was halted (NCT02345070). ND-L02-s0201n, an siRNA oligonucleotide drug designed to inhibit heat shock protein 47 (HSP47), which regulates collagen synthesis and secretion that causes fibrosis, didn’t show the expected efficacy (NCT03538301).
Phase 1 trials
No fewer than 27 phase 1 trials are evaluating investigative treatments for IPF, many in the early phase or not yet recruiting. According to GlobalData, phase 1 drugs for IPF have a 66% chance of moving onto phase 2. Among the advanced phase 1 trials that have gained corporate backing are:
9MW3811, an anti–interleukin-11 monoclonal antibody IV injection (NCT05912049).
ANG-3070, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor (PDGFR) alpha and beta (NCT05387785).
C106, an angiotensin II type 2 receptor agonist (NCT05427253).
HuL001, which targets alpha-enolase (NCT04540770).
LTI-03, a Caveolin-1 (Cav1)-related peptide designed to restore Cav1 expression in lung tissue (NCT05954988).
ORIN1001, a first-in-class small molecule that selectively blocks the inositol requiring enzyme 1alphase (IRE1) RNAse and blocks X-box binding protein 1 (XBP1) activation (NCT04643769).
PRS-220 is an orally inhaled anticalin protein targeting connective tissue growth factor (CTGF) (NTC05473533).
TRK-250, a single-strand, long-chain nucleic acid that selectively suppresses expression of transforming growth factor-beta 1 (TGF-beta1) protein (NCT03727802).
“While we have therapies that we’re able to give patients, we need to do more and we need to do better,” Dr. Lee said. “We’re all hopeful the next phase 3 clinical trial will be something that will help change the treatment paradigm for our patients. We’re very patient, and hopefully those that are interested in improving this treatment landscape will continue to persist.”
Dr. Lee disclosed financial relationships with Boehringer Ingelheim, Pliant Therapeutics, Blade Therapeutics, United Therapeutics, Eleven P15. and Avalyn Pharma.
With the emergence of pirfenidone and nintedanib over the past decade or so, pulmonologists now have at their disposal two breakthrough antifibrotic agents for the treatment of idiopathic pulmonary fibrosis.
But these two drugs have a number of shortcomings that a host of investigative agents are aiming to address. For one, while pirfenidone and nintedanib have been shown to slow disease progression and improve symptoms, they don’t stop or reverse the disease. Also, a large number of patients with IPF don’t tolerate these drugs well. And, their high cost is a barrier for many patients.
in terms of therapies or interventions that have better efficacy, better long-term tolerability, and that improve symptoms and quality of life for our patients with IPF disease,” said Joyce Lee, MD, associate professor of medicine–pulmonary at the University of Colorado at Denver, Aurora, and senior medical adviser for research and health care quality for the Pulmonary Fibrosis Foundation.
The National Institutes of Health estimates that more than 30,000 new cases of IPF are diagnosed in the United States annually, and as many as 3 million people have the disease worldwide. The 5-year survival rate is less than 40% after diagnosis. Bloomberg News reported that more than 80 pharmaceutical companies are working on IPF treatments. iHealthcareAnalyst estimates the global market for IPF will reach $10.1 billion by 2029 thanks to rapidly increasing prevalence and incidence with age, premium-priced drugs, and rapid approval of new treatments.
The perils of phase 3 studies
A search on ClinicalTrials.gov turned up 89 investigative IPF treatments in human trials. However, the search for alternatives can be perilous. “In the field, we have gotten used to promising phase 2 studies that failed in the phase 3 stage of development,” Dr. Lee said. “I don’t hold my breath these days just in terms of trying to predict whether or not the efficacy will be present in the phase 3 clinical trial.”
Three notable phase 3 flops include the ISABELA 1 and 2 trials of the autotaxin inhibitor ziritaxestat, which failed to meet their primary endpoint and were halted early (JAMA. 2023;329:1567-78). The phase 3 ZEPHYRUS-1 trial failed to show any benefit of pamrevlumab to improve percent predicted force vital capacity (ppFVC) at week 48, causing discontinuation of a second phase 3 trial. The phase 3 STARSCAPE-OLE study of intravenous recombinant human pentraxin-2 was terminated earlier this year when the sponsor, Hoffmann-LaRoche, decided it was unable to meet its primary objective (NCT04594707).
In the meantime, these six other phase 3 programs in IPF are still in the field:
Anlotinib. A phase 2 and 3 trial in China is evaluating 1-year outcomes of once-daily oral anlotinib for treatment of IPF/progressive fibrosis-interstitial lung disease (PF-ILDS) (NCT05828953). Anlotinib is a tyrosine kinase inhibitor (TKI) that targets four factors: vascular endothelial growth factor receptor (VEGR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit. It’s approved in China as a third-line therapy for non–small cell lung cancer (NSCLC).
BI 101550. Enrollment in the FIBRONEER-IPF trial commenced last fall (NCT05321069), with completion scheduled for late next year. BI 1015550 is an oral phosphodiesterase 4B (PDE4B) inhibitor. FIBRONEER-ILD is a separate phase 3 trial in fibrosing idiopathic lung disease (NCT05321082). In both trials, the primary endpoint is the absolute change from baseline in FVC at week 52.
BMS-986278. Results of a phase 2 trial showed that twice-daily treatment with oral BMS-986278 60 mg over 26 weeks reduced the rate of decline in ppFVC by 69% vs. placebo. The phase 3 ALOFT trial has been approved but hasn’t yet started recruiting patients (NCT06003426). BMS-986278 is a lysophosphatidic acid receptor 1 (LPA1) antagonist.
Lanxoprazole. Commonly used to treat and prevent gastrointestinal problems like stomach ulcers and esophagitis, this oral proton pump inhibitor (PPI) is the focus of a trial in the United Kingdom evaluating if PPIs can slow the progression of IPF (NCT04965298).
N-acetylcysteine (NAC). The PRECISIONS trial is evaluating the effect of NAC plus standard-of-care treatment in IPF patients who have the TOLLIP rs3750910 TT genotype (NCT04300920). Participants receive 600 mg NAC orally or matched placebo three times daily for 24 months. Trial completion is scheduled for 2025.
Treprostinil. Already approved to treat pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease, inhaled Treprostinil is the subject of the TETON 1 and 2 trials evaluating its impact on ppFVC after 52 weeks of treatment (NCT04708782, NCT05255991).
Phase 2 candidates
The primary endpoint in most of the phase 2 trials is change in ppFVC capacity from baseline to week 24. The following investigative therapies are in phase 2 trials:
Bexotegrast (PLN-74809), an oral, small molecule, dual-selective inhibitor of alphav/beta6 and alphav/beta1 (NCT04396756).
BBT-877, described as a potent autotaxin (ATX) inhibitor, demonstrated its ability to inhibit lysophosphatidic acid (LPA) production by as much as 90 percent (NCT05483907).
CC-90001, an oral, once-daily c-Jun N-terminal kinases (JNK) inhibitor. JNKs have been implicated in the underlying mechanisms of fibrosis, including epithelial cell death, inflammation and polarization of profibrotic macrophages, fibroblast activation, and collagen production (NCT03142191).
C21 targets the underlying fibrosis in IPF by stimulating the protective arm of the renin-angiotensin system. It also has an upstream effect by promoting alveolar repair by which it can reduce fibrosis formation, stabilize disease, and increase lung capacity (NCT04533022).
CSL312 (garadacimab) is a humanized anti-FXIIa monoclonal antibody administrated intravenously (NCT05130970).
Cudetaxestat, a noncompetitive autotaxin inhibitor (NCT05373914).
Bersiposocin/DWN12088, an inhibitor of prolyl-tRNA synthetase 1 (PARS1), which is suspected to control the pathologic accumulation of collagen containing high amounts of proline in fibrotic diseases (NCT05389215).
ENV-101, a small-molecule inhibitor of the Hedgehog (Hh) signaling pathway, which plays a key role in IPF. This agent was originally developed to target Hh-driven cancers (NCT04968574).
GKT137831 (setanaxib) inhibits nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) isoforms. (NCT03865927).
HZN-825, a lysophosphatidic acid receptor 1 (LPAR1) antagonist. (NCT05032066)
Ifetroban, a potent and selective thromboxane-prostanoid receptor (TPr) antagonist, which exhibits a high affinity for TPr on platelets, vascular and airway smooth muscle, and fibroblasts, and lacks agonistic activity (NCT05571059).
INS018_055, a small-molecule, oral antifibrotic candidate notable for being the first entirely AI-generated drug to enter phase 2 trials. Trial enrollment started in October (NCT05975983, NCT05983920)
Jaktinib dihydrochloride monohydrate, an oral JAK1, JAK2, and JAK3 inhibitor (NCT04312594).
Leramistat, an anti–tumor necrosis factor (TNF) agent (NCT05951296).
LTP001, an oral, selectively deuterated form of pirfenidone designed to retain the antifibrotic and anti-inflammatory activity of pirfenidone with a differentiated pharmacokinetic profile (NCT05497284, NCT05321420).
ME-015 (suplatast tosilate) aims to stabilize ion channels in the neuronal endings in the lungs that mediate IPF-related cough (NCT05983471).
Nalbuphine, a small-molecule, dual-mechanism treatment for chronic cough in IPF. It acts as both a mu opioid receptor antagonist and a kappa opioid receptor agonist (NCT05964335). The CANAL trial, complete last year, is evaluating an extended-release formulation (NCT04030026).
NP-120 (ifenprodil), a small-molecule N-methyl-D-aspartate (NMDA) receptor antagonist, specifically targets the NMDA-type subunit 2B (GluN2B) (NCT04318704).
Orvepitant, a selective antagonist for the NK₁ receptor, is being evaluated to treat IPF-related cough (NCT05815089).
RXC007 (zelasudil), a Rho-associated coiled-coil–containing protein kinase 2 (ROCK2) selective inhibitor, was granted FDA orphan drug designation in August 2023 (NCT05570058).
Saracatinib, a selective Src kinase inhibitor originally developed for oncological indications (NCT04598919).
SHR-1906, an intravenous treatment, inhibits binding of a target protein to a variety of cytokines and growth factors, affects downstream signaling pathways, and reduces cell proliferation and migration (NCT05722964).
TTI-101, an oral, small-molecule inhibitor of signal transducer and activator of transcription (STAT3), which has been found to accumulate in the lungs of IPF patients (NCT05671835).
VAY736 (lanalumab), a BAFF-R inhibitor (NCT03287414).
Vixarelimab, a human monoclonal oncastatin M receptor beta antibody (NCT05785624).
Some investigative programs, however, didn’t make it out of phase 2. The trial evaluating inhaled GB0139, a selective functional antagonist of G-protein–coupled receptor 84, which plays a key role in fibrosis, failed to meet its primary endpoint (NCT03832946). Likewise, oral GLPG1205 failed to show a significant difference in FVC decline vs. placebo (NCT03725852). The program to develop SAR156597, also known as romilkimab, was halted (NCT02345070). ND-L02-s0201n, an siRNA oligonucleotide drug designed to inhibit heat shock protein 47 (HSP47), which regulates collagen synthesis and secretion that causes fibrosis, didn’t show the expected efficacy (NCT03538301).
Phase 1 trials
No fewer than 27 phase 1 trials are evaluating investigative treatments for IPF, many in the early phase or not yet recruiting. According to GlobalData, phase 1 drugs for IPF have a 66% chance of moving onto phase 2. Among the advanced phase 1 trials that have gained corporate backing are:
9MW3811, an anti–interleukin-11 monoclonal antibody IV injection (NCT05912049).
ANG-3070, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor (PDGFR) alpha and beta (NCT05387785).
C106, an angiotensin II type 2 receptor agonist (NCT05427253).
HuL001, which targets alpha-enolase (NCT04540770).
LTI-03, a Caveolin-1 (Cav1)-related peptide designed to restore Cav1 expression in lung tissue (NCT05954988).
ORIN1001, a first-in-class small molecule that selectively blocks the inositol requiring enzyme 1alphase (IRE1) RNAse and blocks X-box binding protein 1 (XBP1) activation (NCT04643769).
PRS-220 is an orally inhaled anticalin protein targeting connective tissue growth factor (CTGF) (NTC05473533).
TRK-250, a single-strand, long-chain nucleic acid that selectively suppresses expression of transforming growth factor-beta 1 (TGF-beta1) protein (NCT03727802).
“While we have therapies that we’re able to give patients, we need to do more and we need to do better,” Dr. Lee said. “We’re all hopeful the next phase 3 clinical trial will be something that will help change the treatment paradigm for our patients. We’re very patient, and hopefully those that are interested in improving this treatment landscape will continue to persist.”
Dr. Lee disclosed financial relationships with Boehringer Ingelheim, Pliant Therapeutics, Blade Therapeutics, United Therapeutics, Eleven P15. and Avalyn Pharma.
With the emergence of pirfenidone and nintedanib over the past decade or so, pulmonologists now have at their disposal two breakthrough antifibrotic agents for the treatment of idiopathic pulmonary fibrosis.
But these two drugs have a number of shortcomings that a host of investigative agents are aiming to address. For one, while pirfenidone and nintedanib have been shown to slow disease progression and improve symptoms, they don’t stop or reverse the disease. Also, a large number of patients with IPF don’t tolerate these drugs well. And, their high cost is a barrier for many patients.
in terms of therapies or interventions that have better efficacy, better long-term tolerability, and that improve symptoms and quality of life for our patients with IPF disease,” said Joyce Lee, MD, associate professor of medicine–pulmonary at the University of Colorado at Denver, Aurora, and senior medical adviser for research and health care quality for the Pulmonary Fibrosis Foundation.
The National Institutes of Health estimates that more than 30,000 new cases of IPF are diagnosed in the United States annually, and as many as 3 million people have the disease worldwide. The 5-year survival rate is less than 40% after diagnosis. Bloomberg News reported that more than 80 pharmaceutical companies are working on IPF treatments. iHealthcareAnalyst estimates the global market for IPF will reach $10.1 billion by 2029 thanks to rapidly increasing prevalence and incidence with age, premium-priced drugs, and rapid approval of new treatments.
The perils of phase 3 studies
A search on ClinicalTrials.gov turned up 89 investigative IPF treatments in human trials. However, the search for alternatives can be perilous. “In the field, we have gotten used to promising phase 2 studies that failed in the phase 3 stage of development,” Dr. Lee said. “I don’t hold my breath these days just in terms of trying to predict whether or not the efficacy will be present in the phase 3 clinical trial.”
Three notable phase 3 flops include the ISABELA 1 and 2 trials of the autotaxin inhibitor ziritaxestat, which failed to meet their primary endpoint and were halted early (JAMA. 2023;329:1567-78). The phase 3 ZEPHYRUS-1 trial failed to show any benefit of pamrevlumab to improve percent predicted force vital capacity (ppFVC) at week 48, causing discontinuation of a second phase 3 trial. The phase 3 STARSCAPE-OLE study of intravenous recombinant human pentraxin-2 was terminated earlier this year when the sponsor, Hoffmann-LaRoche, decided it was unable to meet its primary objective (NCT04594707).
In the meantime, these six other phase 3 programs in IPF are still in the field:
Anlotinib. A phase 2 and 3 trial in China is evaluating 1-year outcomes of once-daily oral anlotinib for treatment of IPF/progressive fibrosis-interstitial lung disease (PF-ILDS) (NCT05828953). Anlotinib is a tyrosine kinase inhibitor (TKI) that targets four factors: vascular endothelial growth factor receptor (VEGR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptors (PDGFR), and c-kit. It’s approved in China as a third-line therapy for non–small cell lung cancer (NSCLC).
BI 101550. Enrollment in the FIBRONEER-IPF trial commenced last fall (NCT05321069), with completion scheduled for late next year. BI 1015550 is an oral phosphodiesterase 4B (PDE4B) inhibitor. FIBRONEER-ILD is a separate phase 3 trial in fibrosing idiopathic lung disease (NCT05321082). In both trials, the primary endpoint is the absolute change from baseline in FVC at week 52.
BMS-986278. Results of a phase 2 trial showed that twice-daily treatment with oral BMS-986278 60 mg over 26 weeks reduced the rate of decline in ppFVC by 69% vs. placebo. The phase 3 ALOFT trial has been approved but hasn’t yet started recruiting patients (NCT06003426). BMS-986278 is a lysophosphatidic acid receptor 1 (LPA1) antagonist.
Lanxoprazole. Commonly used to treat and prevent gastrointestinal problems like stomach ulcers and esophagitis, this oral proton pump inhibitor (PPI) is the focus of a trial in the United Kingdom evaluating if PPIs can slow the progression of IPF (NCT04965298).
N-acetylcysteine (NAC). The PRECISIONS trial is evaluating the effect of NAC plus standard-of-care treatment in IPF patients who have the TOLLIP rs3750910 TT genotype (NCT04300920). Participants receive 600 mg NAC orally or matched placebo three times daily for 24 months. Trial completion is scheduled for 2025.
Treprostinil. Already approved to treat pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease, inhaled Treprostinil is the subject of the TETON 1 and 2 trials evaluating its impact on ppFVC after 52 weeks of treatment (NCT04708782, NCT05255991).
Phase 2 candidates
The primary endpoint in most of the phase 2 trials is change in ppFVC capacity from baseline to week 24. The following investigative therapies are in phase 2 trials:
Bexotegrast (PLN-74809), an oral, small molecule, dual-selective inhibitor of alphav/beta6 and alphav/beta1 (NCT04396756).
BBT-877, described as a potent autotaxin (ATX) inhibitor, demonstrated its ability to inhibit lysophosphatidic acid (LPA) production by as much as 90 percent (NCT05483907).
CC-90001, an oral, once-daily c-Jun N-terminal kinases (JNK) inhibitor. JNKs have been implicated in the underlying mechanisms of fibrosis, including epithelial cell death, inflammation and polarization of profibrotic macrophages, fibroblast activation, and collagen production (NCT03142191).
C21 targets the underlying fibrosis in IPF by stimulating the protective arm of the renin-angiotensin system. It also has an upstream effect by promoting alveolar repair by which it can reduce fibrosis formation, stabilize disease, and increase lung capacity (NCT04533022).
CSL312 (garadacimab) is a humanized anti-FXIIa monoclonal antibody administrated intravenously (NCT05130970).
Cudetaxestat, a noncompetitive autotaxin inhibitor (NCT05373914).
Bersiposocin/DWN12088, an inhibitor of prolyl-tRNA synthetase 1 (PARS1), which is suspected to control the pathologic accumulation of collagen containing high amounts of proline in fibrotic diseases (NCT05389215).
ENV-101, a small-molecule inhibitor of the Hedgehog (Hh) signaling pathway, which plays a key role in IPF. This agent was originally developed to target Hh-driven cancers (NCT04968574).
GKT137831 (setanaxib) inhibits nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) isoforms. (NCT03865927).
HZN-825, a lysophosphatidic acid receptor 1 (LPAR1) antagonist. (NCT05032066)
Ifetroban, a potent and selective thromboxane-prostanoid receptor (TPr) antagonist, which exhibits a high affinity for TPr on platelets, vascular and airway smooth muscle, and fibroblasts, and lacks agonistic activity (NCT05571059).
INS018_055, a small-molecule, oral antifibrotic candidate notable for being the first entirely AI-generated drug to enter phase 2 trials. Trial enrollment started in October (NCT05975983, NCT05983920)
Jaktinib dihydrochloride monohydrate, an oral JAK1, JAK2, and JAK3 inhibitor (NCT04312594).
Leramistat, an anti–tumor necrosis factor (TNF) agent (NCT05951296).
LTP001, an oral, selectively deuterated form of pirfenidone designed to retain the antifibrotic and anti-inflammatory activity of pirfenidone with a differentiated pharmacokinetic profile (NCT05497284, NCT05321420).
ME-015 (suplatast tosilate) aims to stabilize ion channels in the neuronal endings in the lungs that mediate IPF-related cough (NCT05983471).
Nalbuphine, a small-molecule, dual-mechanism treatment for chronic cough in IPF. It acts as both a mu opioid receptor antagonist and a kappa opioid receptor agonist (NCT05964335). The CANAL trial, complete last year, is evaluating an extended-release formulation (NCT04030026).
NP-120 (ifenprodil), a small-molecule N-methyl-D-aspartate (NMDA) receptor antagonist, specifically targets the NMDA-type subunit 2B (GluN2B) (NCT04318704).
Orvepitant, a selective antagonist for the NK₁ receptor, is being evaluated to treat IPF-related cough (NCT05815089).
RXC007 (zelasudil), a Rho-associated coiled-coil–containing protein kinase 2 (ROCK2) selective inhibitor, was granted FDA orphan drug designation in August 2023 (NCT05570058).
Saracatinib, a selective Src kinase inhibitor originally developed for oncological indications (NCT04598919).
SHR-1906, an intravenous treatment, inhibits binding of a target protein to a variety of cytokines and growth factors, affects downstream signaling pathways, and reduces cell proliferation and migration (NCT05722964).
TTI-101, an oral, small-molecule inhibitor of signal transducer and activator of transcription (STAT3), which has been found to accumulate in the lungs of IPF patients (NCT05671835).
VAY736 (lanalumab), a BAFF-R inhibitor (NCT03287414).
Vixarelimab, a human monoclonal oncastatin M receptor beta antibody (NCT05785624).
Some investigative programs, however, didn’t make it out of phase 2. The trial evaluating inhaled GB0139, a selective functional antagonist of G-protein–coupled receptor 84, which plays a key role in fibrosis, failed to meet its primary endpoint (NCT03832946). Likewise, oral GLPG1205 failed to show a significant difference in FVC decline vs. placebo (NCT03725852). The program to develop SAR156597, also known as romilkimab, was halted (NCT02345070). ND-L02-s0201n, an siRNA oligonucleotide drug designed to inhibit heat shock protein 47 (HSP47), which regulates collagen synthesis and secretion that causes fibrosis, didn’t show the expected efficacy (NCT03538301).
Phase 1 trials
No fewer than 27 phase 1 trials are evaluating investigative treatments for IPF, many in the early phase or not yet recruiting. According to GlobalData, phase 1 drugs for IPF have a 66% chance of moving onto phase 2. Among the advanced phase 1 trials that have gained corporate backing are:
9MW3811, an anti–interleukin-11 monoclonal antibody IV injection (NCT05912049).
ANG-3070, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor (PDGFR) alpha and beta (NCT05387785).
C106, an angiotensin II type 2 receptor agonist (NCT05427253).
HuL001, which targets alpha-enolase (NCT04540770).
LTI-03, a Caveolin-1 (Cav1)-related peptide designed to restore Cav1 expression in lung tissue (NCT05954988).
ORIN1001, a first-in-class small molecule that selectively blocks the inositol requiring enzyme 1alphase (IRE1) RNAse and blocks X-box binding protein 1 (XBP1) activation (NCT04643769).
PRS-220 is an orally inhaled anticalin protein targeting connective tissue growth factor (CTGF) (NTC05473533).
TRK-250, a single-strand, long-chain nucleic acid that selectively suppresses expression of transforming growth factor-beta 1 (TGF-beta1) protein (NCT03727802).
“While we have therapies that we’re able to give patients, we need to do more and we need to do better,” Dr. Lee said. “We’re all hopeful the next phase 3 clinical trial will be something that will help change the treatment paradigm for our patients. We’re very patient, and hopefully those that are interested in improving this treatment landscape will continue to persist.”
Dr. Lee disclosed financial relationships with Boehringer Ingelheim, Pliant Therapeutics, Blade Therapeutics, United Therapeutics, Eleven P15. and Avalyn Pharma.
Short, long-lasting bronchodilators similar for exacerbated COPD
HONOLULU – in safety and efficacy to a short-acting combination of albuterol and ipratropium.
The 2023 Gold Report on prevention, management, and diagnosis of COPD recommended switching to long-acting bronchodilators despite a lack of clinical evidence showing safety in patients hospitalized for COPD exacerbation, according to Rajiv Dhand, MD, who presented the new study at the annual meeting of the American College of Chest Physicians (CHEST).
“We wanted to establish the safety, because long-acting agents are approved only for use in nonhospitalized patients. We established that it was safe and that it was comparably effective, but you could give 30% lower doses. Patients don’t have to be woken up to get the medication, and there’s a better chance that all the doses will be administered to these patients. So I think that it provides convenience with similar efficacy and safety,” said Dr. Dhand, a pulmonologist and professor of medicine at the University of Tennessee, Knoxville.
The researchers randomized 60 patients to receive nebulized albuterol (2.5 mg) and ipratropium (0.5 mg) every 6 hours (short-acting group) or nebulized formoterol (20 mcg) every 12 hours and revefenacin (175 mcg) every 24 hours (long-acting group). The mean age was 63.2 years, 58.3% were male, and 65% were current smokers.
The median decrease between day 1 and day 3 in the Modified Borg Dyspnea score was 4.0 in the long-acting group (P < .001), and 2.0 in the short-acting group, though the latter was not statistically significant (P = .134). Both groups had a decrease in supplemental oxygen requirement, with no difference between the two groups. There was also no difference in the number of respiratory visits for rescue therapy.
Respiratory therapists in the audience welcomed the new evidence. “As a respiratory therapist, I feel that we should move away from giving good short acting [therapies] ... the new guidelines state that we should move away from them, but I think that physicians in general have not gone that way. The way that we’re working, giving short acting every four hours – I don’t see that it’s a benefit to our patients,” said Sharon Armstead, who attended the session and was asked to comment on the study. She is a respiratory therapist at Ascension Health and an instructor at Concordia University, Austin, Texas. Ms. Armstead has asthma, and has first-hand experience as a patient when respiratory therapists are unable to attend to the patient every 4 hours.
She suggested that continued use of short-acting therapies may be due to inertia. “It’s easier [for a physician] to click a button on [a computer screen] than to actually slow down and write the order. If we need a rescue, then we’ll call for a rescue,” Ms. Armstead said.
She anticipates that long-acting therapies will ultimately lead to better outcomes because they will increase the time that respiratory therapists can spend with patients. “That’s what we really want to do. We want to spend time with our patients and stay there and watch our patients. But if you’re just telling us to [administer a therapy] every 4 hours, it’s not really giving the patient what they need.”
Specifically, there were concerns about cardiovascular safety, but the researchers found no between-group differences.
Asked for comment, session co-moderator Brittany Duchene, MD remarked: “It’s super interesting, but I worry about the cost. From a practical perspective, it’s challenging to get those drugs placed on an outpatient basis. They are very expensive, and they’re newer [drugs], but I think overall it’s good to give less,” said Dr. Duchene, a pulmonary critical care physician at Northeastern Vermont Regional Hospital, St. Johnsbury.
A potential concern raised by one audience member is that some patients are used to frequent treatment and may grow anxious with less frequent therapy. “I think we just need some reeducation that this is like a long-acting medicine. It also decreases the burden on our respiratory therapists, which is very good,” said Dr. Duchene.
The study was funded by Mylan/Theravance Biopharma. Dr. Dhand has received research support from Theravance, Mylan, and Viatris. He has received honoraria from Teva and UpToDate. Ms. Armstead and Dr. Duchene have no relevant financial disclosures.
HONOLULU – in safety and efficacy to a short-acting combination of albuterol and ipratropium.
The 2023 Gold Report on prevention, management, and diagnosis of COPD recommended switching to long-acting bronchodilators despite a lack of clinical evidence showing safety in patients hospitalized for COPD exacerbation, according to Rajiv Dhand, MD, who presented the new study at the annual meeting of the American College of Chest Physicians (CHEST).
“We wanted to establish the safety, because long-acting agents are approved only for use in nonhospitalized patients. We established that it was safe and that it was comparably effective, but you could give 30% lower doses. Patients don’t have to be woken up to get the medication, and there’s a better chance that all the doses will be administered to these patients. So I think that it provides convenience with similar efficacy and safety,” said Dr. Dhand, a pulmonologist and professor of medicine at the University of Tennessee, Knoxville.
The researchers randomized 60 patients to receive nebulized albuterol (2.5 mg) and ipratropium (0.5 mg) every 6 hours (short-acting group) or nebulized formoterol (20 mcg) every 12 hours and revefenacin (175 mcg) every 24 hours (long-acting group). The mean age was 63.2 years, 58.3% were male, and 65% were current smokers.
The median decrease between day 1 and day 3 in the Modified Borg Dyspnea score was 4.0 in the long-acting group (P < .001), and 2.0 in the short-acting group, though the latter was not statistically significant (P = .134). Both groups had a decrease in supplemental oxygen requirement, with no difference between the two groups. There was also no difference in the number of respiratory visits for rescue therapy.
Respiratory therapists in the audience welcomed the new evidence. “As a respiratory therapist, I feel that we should move away from giving good short acting [therapies] ... the new guidelines state that we should move away from them, but I think that physicians in general have not gone that way. The way that we’re working, giving short acting every four hours – I don’t see that it’s a benefit to our patients,” said Sharon Armstead, who attended the session and was asked to comment on the study. She is a respiratory therapist at Ascension Health and an instructor at Concordia University, Austin, Texas. Ms. Armstead has asthma, and has first-hand experience as a patient when respiratory therapists are unable to attend to the patient every 4 hours.
She suggested that continued use of short-acting therapies may be due to inertia. “It’s easier [for a physician] to click a button on [a computer screen] than to actually slow down and write the order. If we need a rescue, then we’ll call for a rescue,” Ms. Armstead said.
She anticipates that long-acting therapies will ultimately lead to better outcomes because they will increase the time that respiratory therapists can spend with patients. “That’s what we really want to do. We want to spend time with our patients and stay there and watch our patients. But if you’re just telling us to [administer a therapy] every 4 hours, it’s not really giving the patient what they need.”
Specifically, there were concerns about cardiovascular safety, but the researchers found no between-group differences.
Asked for comment, session co-moderator Brittany Duchene, MD remarked: “It’s super interesting, but I worry about the cost. From a practical perspective, it’s challenging to get those drugs placed on an outpatient basis. They are very expensive, and they’re newer [drugs], but I think overall it’s good to give less,” said Dr. Duchene, a pulmonary critical care physician at Northeastern Vermont Regional Hospital, St. Johnsbury.
A potential concern raised by one audience member is that some patients are used to frequent treatment and may grow anxious with less frequent therapy. “I think we just need some reeducation that this is like a long-acting medicine. It also decreases the burden on our respiratory therapists, which is very good,” said Dr. Duchene.
The study was funded by Mylan/Theravance Biopharma. Dr. Dhand has received research support from Theravance, Mylan, and Viatris. He has received honoraria from Teva and UpToDate. Ms. Armstead and Dr. Duchene have no relevant financial disclosures.
HONOLULU – in safety and efficacy to a short-acting combination of albuterol and ipratropium.
The 2023 Gold Report on prevention, management, and diagnosis of COPD recommended switching to long-acting bronchodilators despite a lack of clinical evidence showing safety in patients hospitalized for COPD exacerbation, according to Rajiv Dhand, MD, who presented the new study at the annual meeting of the American College of Chest Physicians (CHEST).
“We wanted to establish the safety, because long-acting agents are approved only for use in nonhospitalized patients. We established that it was safe and that it was comparably effective, but you could give 30% lower doses. Patients don’t have to be woken up to get the medication, and there’s a better chance that all the doses will be administered to these patients. So I think that it provides convenience with similar efficacy and safety,” said Dr. Dhand, a pulmonologist and professor of medicine at the University of Tennessee, Knoxville.
The researchers randomized 60 patients to receive nebulized albuterol (2.5 mg) and ipratropium (0.5 mg) every 6 hours (short-acting group) or nebulized formoterol (20 mcg) every 12 hours and revefenacin (175 mcg) every 24 hours (long-acting group). The mean age was 63.2 years, 58.3% were male, and 65% were current smokers.
The median decrease between day 1 and day 3 in the Modified Borg Dyspnea score was 4.0 in the long-acting group (P < .001), and 2.0 in the short-acting group, though the latter was not statistically significant (P = .134). Both groups had a decrease in supplemental oxygen requirement, with no difference between the two groups. There was also no difference in the number of respiratory visits for rescue therapy.
Respiratory therapists in the audience welcomed the new evidence. “As a respiratory therapist, I feel that we should move away from giving good short acting [therapies] ... the new guidelines state that we should move away from them, but I think that physicians in general have not gone that way. The way that we’re working, giving short acting every four hours – I don’t see that it’s a benefit to our patients,” said Sharon Armstead, who attended the session and was asked to comment on the study. She is a respiratory therapist at Ascension Health and an instructor at Concordia University, Austin, Texas. Ms. Armstead has asthma, and has first-hand experience as a patient when respiratory therapists are unable to attend to the patient every 4 hours.
She suggested that continued use of short-acting therapies may be due to inertia. “It’s easier [for a physician] to click a button on [a computer screen] than to actually slow down and write the order. If we need a rescue, then we’ll call for a rescue,” Ms. Armstead said.
She anticipates that long-acting therapies will ultimately lead to better outcomes because they will increase the time that respiratory therapists can spend with patients. “That’s what we really want to do. We want to spend time with our patients and stay there and watch our patients. But if you’re just telling us to [administer a therapy] every 4 hours, it’s not really giving the patient what they need.”
Specifically, there were concerns about cardiovascular safety, but the researchers found no between-group differences.
Asked for comment, session co-moderator Brittany Duchene, MD remarked: “It’s super interesting, but I worry about the cost. From a practical perspective, it’s challenging to get those drugs placed on an outpatient basis. They are very expensive, and they’re newer [drugs], but I think overall it’s good to give less,” said Dr. Duchene, a pulmonary critical care physician at Northeastern Vermont Regional Hospital, St. Johnsbury.
A potential concern raised by one audience member is that some patients are used to frequent treatment and may grow anxious with less frequent therapy. “I think we just need some reeducation that this is like a long-acting medicine. It also decreases the burden on our respiratory therapists, which is very good,” said Dr. Duchene.
The study was funded by Mylan/Theravance Biopharma. Dr. Dhand has received research support from Theravance, Mylan, and Viatris. He has received honoraria from Teva and UpToDate. Ms. Armstead and Dr. Duchene have no relevant financial disclosures.
AT CHEST 2023
Take two pills and make a donation
I was a resident, on morning rounds. The attending neurologist was young and ambitious (weren’t we all once?), trying to get the hospital to help him fund a research program in his subspecialty of interest.
One of the patients we saw that morning was a locally known successful businessman who’d been admitted, fortunately not for anything too serious.
My attending took the history, verifying the one I’d presented, and examined the gentleman. He then made some teaching points and explained the care plan to the patient.
Pretty standard up to that point.
After answering questions, however, the attending suddenly went into a sales pitch on his new research program, asking the guy for a financial donation, and giving him the card for the person at his office handling the funding.
I don’t remember anymore if he repeated that with other patients, but even now it still leaves a bad taste in my mouth. As a resident I wasn’t in a position to criticize him, nor did I want to endanger my own standing in the program by talking to someone higher up.
He was, fortunately, the only attending I ever worked with who did that. It still stands out in my mind, perhaps as an example of what not to do, and sometimes I still think about it.
Perhaps I’m naive, but I assumed he was an aberration. Apparently not, as the American College of Physicians recently issued a position paper advising its members not to ask patients for donations to the doctor’s workplace. There’s actually an acronym, GPF (Grateful Patient Fundraising) for this.
I understand a lot of these doctors are in academics and need funding for research and other programs. I know that a lot of good comes from this research, and I fully support it.
But this seems to be a bad way of doing it. Standing at the bedside on that long-ago morning, I remember thinking the patient (who looked kind of surprised) was going to wonder if this was a vague sort of hint: You’ll get better care if you pay up. Or a veiled threat that you may not get decent care if you don’t. I have no idea if he donated.
There must be a better way to get funding than hitting up a patient as part of the care plan. Perhaps discharge materials might include a brochure about how to make a donation, if interested. Or the ubiquitous portal might have a “donate” box in the task bar.
If the patient were to initiate this on his own, I wouldn’t have an issue with it. He gets out of the hospital, is grateful for his care, and calls the physician’s office to say he’d like to make a donation to whatever his program is (or just goes online to do it). That’s fine. I’ve even had the occasional patient call my office to say they’d like to make a donation to my favorite charity, and I give them a list of various neurology research foundations (none of which I’m affiliated with, for the record).
But to actively solicit donations from someone under your care is tasteless and inappropriate. It creates a conflict of interest for both parties.
The patient may believe he’ll get better care, and is obligated to keep giving – or else. The physician may feel like he’s stuck going beyond what’s really needed, ordering unnecessary tests and such to keep the financial VIP happy. And what happens if the big donor patient calls in because he hurt his ankle and needs a Percocet refill that another doctor won’t give him?
The statement by the ACP is appropriate. The only thing that bothers me about it is that it had to be made at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I was a resident, on morning rounds. The attending neurologist was young and ambitious (weren’t we all once?), trying to get the hospital to help him fund a research program in his subspecialty of interest.
One of the patients we saw that morning was a locally known successful businessman who’d been admitted, fortunately not for anything too serious.
My attending took the history, verifying the one I’d presented, and examined the gentleman. He then made some teaching points and explained the care plan to the patient.
Pretty standard up to that point.
After answering questions, however, the attending suddenly went into a sales pitch on his new research program, asking the guy for a financial donation, and giving him the card for the person at his office handling the funding.
I don’t remember anymore if he repeated that with other patients, but even now it still leaves a bad taste in my mouth. As a resident I wasn’t in a position to criticize him, nor did I want to endanger my own standing in the program by talking to someone higher up.
He was, fortunately, the only attending I ever worked with who did that. It still stands out in my mind, perhaps as an example of what not to do, and sometimes I still think about it.
Perhaps I’m naive, but I assumed he was an aberration. Apparently not, as the American College of Physicians recently issued a position paper advising its members not to ask patients for donations to the doctor’s workplace. There’s actually an acronym, GPF (Grateful Patient Fundraising) for this.
I understand a lot of these doctors are in academics and need funding for research and other programs. I know that a lot of good comes from this research, and I fully support it.
But this seems to be a bad way of doing it. Standing at the bedside on that long-ago morning, I remember thinking the patient (who looked kind of surprised) was going to wonder if this was a vague sort of hint: You’ll get better care if you pay up. Or a veiled threat that you may not get decent care if you don’t. I have no idea if he donated.
There must be a better way to get funding than hitting up a patient as part of the care plan. Perhaps discharge materials might include a brochure about how to make a donation, if interested. Or the ubiquitous portal might have a “donate” box in the task bar.
If the patient were to initiate this on his own, I wouldn’t have an issue with it. He gets out of the hospital, is grateful for his care, and calls the physician’s office to say he’d like to make a donation to whatever his program is (or just goes online to do it). That’s fine. I’ve even had the occasional patient call my office to say they’d like to make a donation to my favorite charity, and I give them a list of various neurology research foundations (none of which I’m affiliated with, for the record).
But to actively solicit donations from someone under your care is tasteless and inappropriate. It creates a conflict of interest for both parties.
The patient may believe he’ll get better care, and is obligated to keep giving – or else. The physician may feel like he’s stuck going beyond what’s really needed, ordering unnecessary tests and such to keep the financial VIP happy. And what happens if the big donor patient calls in because he hurt his ankle and needs a Percocet refill that another doctor won’t give him?
The statement by the ACP is appropriate. The only thing that bothers me about it is that it had to be made at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I was a resident, on morning rounds. The attending neurologist was young and ambitious (weren’t we all once?), trying to get the hospital to help him fund a research program in his subspecialty of interest.
One of the patients we saw that morning was a locally known successful businessman who’d been admitted, fortunately not for anything too serious.
My attending took the history, verifying the one I’d presented, and examined the gentleman. He then made some teaching points and explained the care plan to the patient.
Pretty standard up to that point.
After answering questions, however, the attending suddenly went into a sales pitch on his new research program, asking the guy for a financial donation, and giving him the card for the person at his office handling the funding.
I don’t remember anymore if he repeated that with other patients, but even now it still leaves a bad taste in my mouth. As a resident I wasn’t in a position to criticize him, nor did I want to endanger my own standing in the program by talking to someone higher up.
He was, fortunately, the only attending I ever worked with who did that. It still stands out in my mind, perhaps as an example of what not to do, and sometimes I still think about it.
Perhaps I’m naive, but I assumed he was an aberration. Apparently not, as the American College of Physicians recently issued a position paper advising its members not to ask patients for donations to the doctor’s workplace. There’s actually an acronym, GPF (Grateful Patient Fundraising) for this.
I understand a lot of these doctors are in academics and need funding for research and other programs. I know that a lot of good comes from this research, and I fully support it.
But this seems to be a bad way of doing it. Standing at the bedside on that long-ago morning, I remember thinking the patient (who looked kind of surprised) was going to wonder if this was a vague sort of hint: You’ll get better care if you pay up. Or a veiled threat that you may not get decent care if you don’t. I have no idea if he donated.
There must be a better way to get funding than hitting up a patient as part of the care plan. Perhaps discharge materials might include a brochure about how to make a donation, if interested. Or the ubiquitous portal might have a “donate” box in the task bar.
If the patient were to initiate this on his own, I wouldn’t have an issue with it. He gets out of the hospital, is grateful for his care, and calls the physician’s office to say he’d like to make a donation to whatever his program is (or just goes online to do it). That’s fine. I’ve even had the occasional patient call my office to say they’d like to make a donation to my favorite charity, and I give them a list of various neurology research foundations (none of which I’m affiliated with, for the record).
But to actively solicit donations from someone under your care is tasteless and inappropriate. It creates a conflict of interest for both parties.
The patient may believe he’ll get better care, and is obligated to keep giving – or else. The physician may feel like he’s stuck going beyond what’s really needed, ordering unnecessary tests and such to keep the financial VIP happy. And what happens if the big donor patient calls in because he hurt his ankle and needs a Percocet refill that another doctor won’t give him?
The statement by the ACP is appropriate. The only thing that bothers me about it is that it had to be made at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Sleep irregularity
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In discussions between health care providers and patients, the words “regularity” and “irregularity” come up primarily in reference to either constipation or menstrual cycles. However, the participants in a recent panel convened by the National Sleep Foundation think we should also be discussing irregularity when we are discussing sleep with our patients.
The sleep experts on the panel began by considering 40,000 papers that directly or tangentially dealt with the topic of irregular sleep patterns. The reviewers uncovered numerous references to an association between sleep irregularity and a wide variety of adverse health outcomes, including obesity and metabolic disorders, hypertension and other cardiovascular disorders, and elevations in several inflammatory markers. Not surprisingly, the investigators also found an abundance of references supporting an association between irregular sleep and a suite of mental health problems, including depression, mood disorders, lower self esteem, poor academic performance, and deficits in attention. For example, several of the studies the panel reviewed found that in college students, GPA was lower when their sleep pattern was irregular. There were some papers that found no significant association between irregular sleep and other adverse health outcomes, but none of the studies demonstrated an association with better or improved health outcomes.
There is currently no universally accepted definition of an irregular sleep pattern. The experts pointed to some papers that used a standard deviation of 1 hour from the patient’s usual bed time determined by averaging over an interval measured in weeks. You and I shouldn’t be surprised that irregular sleep is unhealthy, but the breadth of the panel’s findings is impressive.
Although it has been long in coming, sleep is finally beginning to get some attention by the media. The focus is usually on the optimal number of hours we need each night. This panel’s findings suggest that total sleep time is only part of the story, and may even be less important than the regularity of our sleep patterns.
For those of us in pediatrics, the place where irregularity raises its ugly head is with teenagers and weekends. Although the numbers are far from clear, the question remains of how effective is catch-up sleep after a week of too-early mornings and too-late bedtimes for the chronically under-slept adolescent.
In some studies in which patients had the demonstrable effects of sleep deprivation (e.g., metabolic and cardiovascular) there was some improvement when weekend sleep was extended by 1 or 2 hours, but none beyond 2 hours.
The panel’s findings, while certainly significant, merely add weight and nuance to the existing evidence of importance of sleep and the damage done by sleep deprivation. As one of the panel members has said, “Sleep is the third pillar of health, equally important as diet and exercise, if not more.” However, this message is not getting out, or at least it is not being heeded. Like obesity, our efforts as advisers to our patients isn’t working. Unfortunately, this is because our advice is often whispered and given halfheartedly.
There was some evidence of improvement as a result of the pandemic, when those fortunate enough to be able to work from home were taking advantage of the flexibility in their schedules and getting more sleep. But health care providers certainly can’t take responsibility for what was an accident of nature.
Those of you who have been reading Letters from Maine for the last 3 decades may tire of my beating the tired horse of sleep deprivation. But I will not be deterred. I see very little evidence among health care professionals in taking the importance of sleep seriously. Sure, they may include it buried in the list of potential contributors to their patient’s complaint, but I see very little effort to move it higher on their list of priorities and almost no movement toward making substantive recommendations and then reinforcing them with follow-up.
Like obesity, sleep deprivation is a societal problem. We can lay some of the blame on Thomas Edison, but Until that time you will continue to read columns like this one when I encounter significant studies on the importance of sleep.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].






