User login
Prophylaxis proves safer than screen-and-treat method

Photo by Nina Matthews
A new study suggests that treating pregnant women according to the results of malaria tests does not lower the risk of adverse pregnancy outcomes when compared to treating all women prophylactically.
In fact, the screen-and-treat approach, in which women received dihydroartemisinin-piperaquine (DP) only if they tested positive for malaria, was associated with higher fetal loss and more malaria at delivery than the prophylactic approach, in which women just received treatment with sulfadoxine-pyrimethamine (SP).
This is in spite of the fact that the study was conducted in an area of high SP resistance.
Feiko ter Kuile, MD, PhD, of the Liverpool School of Tropical Medicine in the UK, and his colleagues conducted this study and detailed the results in PLOS Medicine.
During pregnancy, undetected infection with malaria parasites can lead to maternal anemia, low birthweight, and fetal loss.
Therefore, in areas where malaria is endemic, the World Health Organization recommends treating pregnant women with SP. However, in some areas, more than 90% of Plasmodium parasites are resistant to SP.
In the current study, researchers compared this standard of care to a screening approach where pregnant women were tested for malaria using rapid diagnostic tests and treated with DP only if they tested positive for the parasite.
The study included 1873 HIV-negative pregnant women treated at 3 sites in Malawi, Africa. All of the women had 3 or 4 scheduled visits in the second and third trimester, 4 to 6 weeks apart.
The women were randomized to receive SP at each visit (n=921) or to be screened for malaria at every visit and treated with DP if they tested positive (n=923).
The prevalence of malaria at delivery was higher in the screening-DP group than in the SP group—48.7% and 40.8%, respectively (relative risk=1.19; P=0.007).
And fetal loss was higher in the screening-DP group than the SP group—2.6% and 1.3%, respectively (relative risk=2.06; P=0.046).
However, the risk of live adverse birth outcomes was similar between the screening-DP and SP groups—29.9% and 28.8%, respectively (relative risk=1.04, P=0.625).
The researchers said these results suggest that intermittent malaria screening and treatment with DP is not a viable strategy to replace intermittent preventive therapy with SP in malaria-endemic areas in sub-Saharan Africa, despite the high levels of resistance to SP. ![]()

Photo by Nina Matthews
A new study suggests that treating pregnant women according to the results of malaria tests does not lower the risk of adverse pregnancy outcomes when compared to treating all women prophylactically.
In fact, the screen-and-treat approach, in which women received dihydroartemisinin-piperaquine (DP) only if they tested positive for malaria, was associated with higher fetal loss and more malaria at delivery than the prophylactic approach, in which women just received treatment with sulfadoxine-pyrimethamine (SP).
This is in spite of the fact that the study was conducted in an area of high SP resistance.
Feiko ter Kuile, MD, PhD, of the Liverpool School of Tropical Medicine in the UK, and his colleagues conducted this study and detailed the results in PLOS Medicine.
During pregnancy, undetected infection with malaria parasites can lead to maternal anemia, low birthweight, and fetal loss.
Therefore, in areas where malaria is endemic, the World Health Organization recommends treating pregnant women with SP. However, in some areas, more than 90% of Plasmodium parasites are resistant to SP.
In the current study, researchers compared this standard of care to a screening approach where pregnant women were tested for malaria using rapid diagnostic tests and treated with DP only if they tested positive for the parasite.
The study included 1873 HIV-negative pregnant women treated at 3 sites in Malawi, Africa. All of the women had 3 or 4 scheduled visits in the second and third trimester, 4 to 6 weeks apart.
The women were randomized to receive SP at each visit (n=921) or to be screened for malaria at every visit and treated with DP if they tested positive (n=923).
The prevalence of malaria at delivery was higher in the screening-DP group than in the SP group—48.7% and 40.8%, respectively (relative risk=1.19; P=0.007).
And fetal loss was higher in the screening-DP group than the SP group—2.6% and 1.3%, respectively (relative risk=2.06; P=0.046).
However, the risk of live adverse birth outcomes was similar between the screening-DP and SP groups—29.9% and 28.8%, respectively (relative risk=1.04, P=0.625).
The researchers said these results suggest that intermittent malaria screening and treatment with DP is not a viable strategy to replace intermittent preventive therapy with SP in malaria-endemic areas in sub-Saharan Africa, despite the high levels of resistance to SP. ![]()

Photo by Nina Matthews
A new study suggests that treating pregnant women according to the results of malaria tests does not lower the risk of adverse pregnancy outcomes when compared to treating all women prophylactically.
In fact, the screen-and-treat approach, in which women received dihydroartemisinin-piperaquine (DP) only if they tested positive for malaria, was associated with higher fetal loss and more malaria at delivery than the prophylactic approach, in which women just received treatment with sulfadoxine-pyrimethamine (SP).
This is in spite of the fact that the study was conducted in an area of high SP resistance.
Feiko ter Kuile, MD, PhD, of the Liverpool School of Tropical Medicine in the UK, and his colleagues conducted this study and detailed the results in PLOS Medicine.
During pregnancy, undetected infection with malaria parasites can lead to maternal anemia, low birthweight, and fetal loss.
Therefore, in areas where malaria is endemic, the World Health Organization recommends treating pregnant women with SP. However, in some areas, more than 90% of Plasmodium parasites are resistant to SP.
In the current study, researchers compared this standard of care to a screening approach where pregnant women were tested for malaria using rapid diagnostic tests and treated with DP only if they tested positive for the parasite.
The study included 1873 HIV-negative pregnant women treated at 3 sites in Malawi, Africa. All of the women had 3 or 4 scheduled visits in the second and third trimester, 4 to 6 weeks apart.
The women were randomized to receive SP at each visit (n=921) or to be screened for malaria at every visit and treated with DP if they tested positive (n=923).
The prevalence of malaria at delivery was higher in the screening-DP group than in the SP group—48.7% and 40.8%, respectively (relative risk=1.19; P=0.007).
And fetal loss was higher in the screening-DP group than the SP group—2.6% and 1.3%, respectively (relative risk=2.06; P=0.046).
However, the risk of live adverse birth outcomes was similar between the screening-DP and SP groups—29.9% and 28.8%, respectively (relative risk=1.04, P=0.625).
The researchers said these results suggest that intermittent malaria screening and treatment with DP is not a viable strategy to replace intermittent preventive therapy with SP in malaria-endemic areas in sub-Saharan Africa, despite the high levels of resistance to SP. ![]()
Exenatide/dapagliflozin combo may be better in stubborn diabetes
The combination of exenatide and dapagliflozin produced better results than did either drug alone in patients with type 2 diabetes whose glucose levels aren’t controlled effectively by metformin.
At 28 weeks, HbA1c levels were lower in the combination group, where a third of patients lost more than 5% of their body weight, and 45% reached HbA1c levels under 7.0%, outpacing those on the solo treatments.
Cristian Guja, MD, of the Carol Davila University of Medicine and Pharmacy in Bucharest, Romania, presented the findings of DURATION-8, a 28-week randomized, double-blinded study at 109 sites in six countries, Sept. 16 at the annual meeting of the European Association for the Study of Diabetes. The study was published simultaneously in the Lancet Diabetes & Endocrinology.
In 2014 and 2015, 695 adults with type 2 diabetes and insufficient glycemic control, defined as HbA1c 8%-12%, were randomly assigned to one of three groups: exenatide plus dapagliflozin (n = 231), exenatide alone (n = 231; n = 1 untreated), or dapagliflozin alone (n = 233). All were given placebo pills or injections for 1 week before randomization.
Patients’ average age was 54-55 years, most were white (82%-85%), with 37%-42% reporting Hispanic heritage. Men and women were nearly equally represented. The average body mass index was 32-33 kg/m2. Patients took basal insulin on a rescue basis as needed and were continued on prescribed blood pressure and cholesterol medications.
At 28 weeks, baseline HbA1c in the combination group fell by 2.0% (9.3% to 7.3%), compared with 1.6% (9.3% to 7.6%) in the exenatide-only group and by 1.4% (9.3% to 7.8%) in the dapagliflozin-only group.
Combination therapy was “significantly superior” to either drug alone for all secondary endpoints, including reduced fasting plasma and postprandial glucose, more patients with an HbA1c less than 7.0%, weight-related measures, and lowered systolic blood pressure (Lancet Diabetes Endocrinol. 2016 Sep 16. doi. org/10.1016/S2213-8587(16)30267-4).
Specifically, the percentage of patients with an HbA1c less than 7.0% was 45% for the combination group, 27% for the exenatide group, and 19% for the dapagliflozin group at 28 weeks. A third of patients taking the combination lost more than 5% of their weight, compared with 14% of those on exenatide alone and 20% of those on dapagliflozin.
The side-effect rate (57%) was highest in the combination group and lower (54% and 52%, respectively) for the exenatide and dapagliflozin groups.
Diarrhea, injection-site nodules, nausea, and urinary tract infection occurred in 5% or more of patients in at least one of the three groups. Across all study groups, 2%-5% of patients discontinued treatment because of side effects; discontinuation was greatest (5%) in the exenatide group. Three patients in the combination group died, as did one patient in each of the solo-treatment groups.
This study “provides high-quality evidence that the combination of exenatide and dapagliflozin is more effective than either drug alone in patients with inadequate response to metformin monotherapy,” Dr. Guja and his colleagues noted.
In a commentary, Michael A. Nauck, MD, and Juris J. Meier, MD, both of St. Josef Hospital in Bochum, Germany, called the percentage of patients (45%) who reached an HbA1c level of less than 7.0% in the combination treatment group “disappointing.” They suggested that GLP-1 receptor agonists (i.e., exenatide) may be more effective when combined with SGLT2 inhibitors (i.e., dapagliflozin) than when used with insulin treatment.
The study was funded by AstraZeneca, maker of both exenatide and dapagliflozin.
The combination of exenatide and dapagliflozin produced better results than did either drug alone in patients with type 2 diabetes whose glucose levels aren’t controlled effectively by metformin.
At 28 weeks, HbA1c levels were lower in the combination group, where a third of patients lost more than 5% of their body weight, and 45% reached HbA1c levels under 7.0%, outpacing those on the solo treatments.
Cristian Guja, MD, of the Carol Davila University of Medicine and Pharmacy in Bucharest, Romania, presented the findings of DURATION-8, a 28-week randomized, double-blinded study at 109 sites in six countries, Sept. 16 at the annual meeting of the European Association for the Study of Diabetes. The study was published simultaneously in the Lancet Diabetes & Endocrinology.
In 2014 and 2015, 695 adults with type 2 diabetes and insufficient glycemic control, defined as HbA1c 8%-12%, were randomly assigned to one of three groups: exenatide plus dapagliflozin (n = 231), exenatide alone (n = 231; n = 1 untreated), or dapagliflozin alone (n = 233). All were given placebo pills or injections for 1 week before randomization.
Patients’ average age was 54-55 years, most were white (82%-85%), with 37%-42% reporting Hispanic heritage. Men and women were nearly equally represented. The average body mass index was 32-33 kg/m2. Patients took basal insulin on a rescue basis as needed and were continued on prescribed blood pressure and cholesterol medications.
At 28 weeks, baseline HbA1c in the combination group fell by 2.0% (9.3% to 7.3%), compared with 1.6% (9.3% to 7.6%) in the exenatide-only group and by 1.4% (9.3% to 7.8%) in the dapagliflozin-only group.
Combination therapy was “significantly superior” to either drug alone for all secondary endpoints, including reduced fasting plasma and postprandial glucose, more patients with an HbA1c less than 7.0%, weight-related measures, and lowered systolic blood pressure (Lancet Diabetes Endocrinol. 2016 Sep 16. doi. org/10.1016/S2213-8587(16)30267-4).
Specifically, the percentage of patients with an HbA1c less than 7.0% was 45% for the combination group, 27% for the exenatide group, and 19% for the dapagliflozin group at 28 weeks. A third of patients taking the combination lost more than 5% of their weight, compared with 14% of those on exenatide alone and 20% of those on dapagliflozin.
The side-effect rate (57%) was highest in the combination group and lower (54% and 52%, respectively) for the exenatide and dapagliflozin groups.
Diarrhea, injection-site nodules, nausea, and urinary tract infection occurred in 5% or more of patients in at least one of the three groups. Across all study groups, 2%-5% of patients discontinued treatment because of side effects; discontinuation was greatest (5%) in the exenatide group. Three patients in the combination group died, as did one patient in each of the solo-treatment groups.
This study “provides high-quality evidence that the combination of exenatide and dapagliflozin is more effective than either drug alone in patients with inadequate response to metformin monotherapy,” Dr. Guja and his colleagues noted.
In a commentary, Michael A. Nauck, MD, and Juris J. Meier, MD, both of St. Josef Hospital in Bochum, Germany, called the percentage of patients (45%) who reached an HbA1c level of less than 7.0% in the combination treatment group “disappointing.” They suggested that GLP-1 receptor agonists (i.e., exenatide) may be more effective when combined with SGLT2 inhibitors (i.e., dapagliflozin) than when used with insulin treatment.
The study was funded by AstraZeneca, maker of both exenatide and dapagliflozin.
The combination of exenatide and dapagliflozin produced better results than did either drug alone in patients with type 2 diabetes whose glucose levels aren’t controlled effectively by metformin.
At 28 weeks, HbA1c levels were lower in the combination group, where a third of patients lost more than 5% of their body weight, and 45% reached HbA1c levels under 7.0%, outpacing those on the solo treatments.
Cristian Guja, MD, of the Carol Davila University of Medicine and Pharmacy in Bucharest, Romania, presented the findings of DURATION-8, a 28-week randomized, double-blinded study at 109 sites in six countries, Sept. 16 at the annual meeting of the European Association for the Study of Diabetes. The study was published simultaneously in the Lancet Diabetes & Endocrinology.
In 2014 and 2015, 695 adults with type 2 diabetes and insufficient glycemic control, defined as HbA1c 8%-12%, were randomly assigned to one of three groups: exenatide plus dapagliflozin (n = 231), exenatide alone (n = 231; n = 1 untreated), or dapagliflozin alone (n = 233). All were given placebo pills or injections for 1 week before randomization.
Patients’ average age was 54-55 years, most were white (82%-85%), with 37%-42% reporting Hispanic heritage. Men and women were nearly equally represented. The average body mass index was 32-33 kg/m2. Patients took basal insulin on a rescue basis as needed and were continued on prescribed blood pressure and cholesterol medications.
At 28 weeks, baseline HbA1c in the combination group fell by 2.0% (9.3% to 7.3%), compared with 1.6% (9.3% to 7.6%) in the exenatide-only group and by 1.4% (9.3% to 7.8%) in the dapagliflozin-only group.
Combination therapy was “significantly superior” to either drug alone for all secondary endpoints, including reduced fasting plasma and postprandial glucose, more patients with an HbA1c less than 7.0%, weight-related measures, and lowered systolic blood pressure (Lancet Diabetes Endocrinol. 2016 Sep 16. doi. org/10.1016/S2213-8587(16)30267-4).
Specifically, the percentage of patients with an HbA1c less than 7.0% was 45% for the combination group, 27% for the exenatide group, and 19% for the dapagliflozin group at 28 weeks. A third of patients taking the combination lost more than 5% of their weight, compared with 14% of those on exenatide alone and 20% of those on dapagliflozin.
The side-effect rate (57%) was highest in the combination group and lower (54% and 52%, respectively) for the exenatide and dapagliflozin groups.
Diarrhea, injection-site nodules, nausea, and urinary tract infection occurred in 5% or more of patients in at least one of the three groups. Across all study groups, 2%-5% of patients discontinued treatment because of side effects; discontinuation was greatest (5%) in the exenatide group. Three patients in the combination group died, as did one patient in each of the solo-treatment groups.
This study “provides high-quality evidence that the combination of exenatide and dapagliflozin is more effective than either drug alone in patients with inadequate response to metformin monotherapy,” Dr. Guja and his colleagues noted.
In a commentary, Michael A. Nauck, MD, and Juris J. Meier, MD, both of St. Josef Hospital in Bochum, Germany, called the percentage of patients (45%) who reached an HbA1c level of less than 7.0% in the combination treatment group “disappointing.” They suggested that GLP-1 receptor agonists (i.e., exenatide) may be more effective when combined with SGLT2 inhibitors (i.e., dapagliflozin) than when used with insulin treatment.
The study was funded by AstraZeneca, maker of both exenatide and dapagliflozin.
FROM EASD 2016
Key clinical point: An exenatide-dapagliflozin combination may be better than either drug alone for patients with type 2 diabetes who have not responded to metformin.
Major finding: At 28 weeks, baseline HbA1c was reduced by 2.0% to 7.3% in the combination group, compared with 1.6% to 7.6% in the exenatide-only group and by 1.4% to 7.8% in the dapagliflozin-only group.
Data source: Double-blind, randomized, active-controlled phase III trial of 685 patients (611 completed) over 28 weeks.
Disclosures: The study was funded by AstraZeneca. Study authors reported grants and similar funding from many pharmaceutical companies including AstraZeneca.
Case-control study points to Zika virus as cause of microcephaly
A new study from Brazil demonstrates that microcephaly is strongly associated with congenital Zika virus infections, offering case-control evidence of a causal relationship.
“This is the first case-control study to examine the association between Zika virus and microcephaly using molecular and serological analysis to identify Zika virus in cases and controls at the time of birth,” Thalia Velho Barreto de Araújo, PhD, of the Federal University of Pernambuco, Recife, Brazil, said in a statement. “Our findings suggest that Zika virus should be officially added to the list of congenital infections alongside toxoplasmosis, syphilis, varicella-zoster, parvovirus B19, rubella, cytomegalovirus, and herpes. However, many questions still remain to be answered including the role of previous dengue infection.”
In April, officials at the Centers for Disease Control and Prevention determined that Zika virus infection is a cause of microcephaly, following a systematic review of the available Zika virus research.
In the current study, the investigators looked for cases of infants born with microcephaly at eight public hospitals in Pernambuco, a state in northeastern Brazil. Thirty-two such cases were included for analysis, along with 62 controls. All infants in the study were born between Jan. 15, 2016 and May 2, 2016 (Lancet Infect Dis. 2016 Sep 15. doi: 10.1016/S1473-3099[16]30318-8).
Zika-specific immunoglobulin M (IgM) and reverse transcription–polymerase chain reaction (RT-PCR) tests were conducted on serum from both microcephaly and control infants, and cerebrospinal fluid samples only from infants with microcephaly. Mothers underwent serum testing for Zika virus and dengue virus via plaque reduction neutralization assay testing. Odds ratios and 95% confidence intervals were then calculated to determine the association between congenital Zika virus and microcephaly.
Of the 30 women who gave birth to infants with microcephaly, 24 (80%) had Zika virus infections, compared with 39 of the 61 women (64%) in the control group (P = .12). Additionally, while 13 of the 32 infants born with microcephaly had Zika virus infections confirmed by laboratory testing, none of the infants in the control group had laboratory-confirmed Zika virus infection.
A total of 7 out 27 infants with microcephaly who underwent CT scans showed signs of brain abnormalities, suggesting that “congenital Zika virus syndrome can be present in neonates with microcephaly and no radiological brain abnormalities,” according to the investigators.
While the study is still ongoing, the investigators called for more research to assess other potential risk factors and to confirm the strength of association in a larger sample size, as well as to gauge the significance and role of previous dengue infections in the mothers.
The study was funded by the Brazilian Ministry of Health, the Pan American Health Organization, and Enhancing Research Activity in Epidemic Situations. The investigators reported having no relevant financial disclosures.
A new study from Brazil demonstrates that microcephaly is strongly associated with congenital Zika virus infections, offering case-control evidence of a causal relationship.
“This is the first case-control study to examine the association between Zika virus and microcephaly using molecular and serological analysis to identify Zika virus in cases and controls at the time of birth,” Thalia Velho Barreto de Araújo, PhD, of the Federal University of Pernambuco, Recife, Brazil, said in a statement. “Our findings suggest that Zika virus should be officially added to the list of congenital infections alongside toxoplasmosis, syphilis, varicella-zoster, parvovirus B19, rubella, cytomegalovirus, and herpes. However, many questions still remain to be answered including the role of previous dengue infection.”
In April, officials at the Centers for Disease Control and Prevention determined that Zika virus infection is a cause of microcephaly, following a systematic review of the available Zika virus research.
In the current study, the investigators looked for cases of infants born with microcephaly at eight public hospitals in Pernambuco, a state in northeastern Brazil. Thirty-two such cases were included for analysis, along with 62 controls. All infants in the study were born between Jan. 15, 2016 and May 2, 2016 (Lancet Infect Dis. 2016 Sep 15. doi: 10.1016/S1473-3099[16]30318-8).
Zika-specific immunoglobulin M (IgM) and reverse transcription–polymerase chain reaction (RT-PCR) tests were conducted on serum from both microcephaly and control infants, and cerebrospinal fluid samples only from infants with microcephaly. Mothers underwent serum testing for Zika virus and dengue virus via plaque reduction neutralization assay testing. Odds ratios and 95% confidence intervals were then calculated to determine the association between congenital Zika virus and microcephaly.
Of the 30 women who gave birth to infants with microcephaly, 24 (80%) had Zika virus infections, compared with 39 of the 61 women (64%) in the control group (P = .12). Additionally, while 13 of the 32 infants born with microcephaly had Zika virus infections confirmed by laboratory testing, none of the infants in the control group had laboratory-confirmed Zika virus infection.
A total of 7 out 27 infants with microcephaly who underwent CT scans showed signs of brain abnormalities, suggesting that “congenital Zika virus syndrome can be present in neonates with microcephaly and no radiological brain abnormalities,” according to the investigators.
While the study is still ongoing, the investigators called for more research to assess other potential risk factors and to confirm the strength of association in a larger sample size, as well as to gauge the significance and role of previous dengue infections in the mothers.
The study was funded by the Brazilian Ministry of Health, the Pan American Health Organization, and Enhancing Research Activity in Epidemic Situations. The investigators reported having no relevant financial disclosures.
A new study from Brazil demonstrates that microcephaly is strongly associated with congenital Zika virus infections, offering case-control evidence of a causal relationship.
“This is the first case-control study to examine the association between Zika virus and microcephaly using molecular and serological analysis to identify Zika virus in cases and controls at the time of birth,” Thalia Velho Barreto de Araújo, PhD, of the Federal University of Pernambuco, Recife, Brazil, said in a statement. “Our findings suggest that Zika virus should be officially added to the list of congenital infections alongside toxoplasmosis, syphilis, varicella-zoster, parvovirus B19, rubella, cytomegalovirus, and herpes. However, many questions still remain to be answered including the role of previous dengue infection.”
In April, officials at the Centers for Disease Control and Prevention determined that Zika virus infection is a cause of microcephaly, following a systematic review of the available Zika virus research.
In the current study, the investigators looked for cases of infants born with microcephaly at eight public hospitals in Pernambuco, a state in northeastern Brazil. Thirty-two such cases were included for analysis, along with 62 controls. All infants in the study were born between Jan. 15, 2016 and May 2, 2016 (Lancet Infect Dis. 2016 Sep 15. doi: 10.1016/S1473-3099[16]30318-8).
Zika-specific immunoglobulin M (IgM) and reverse transcription–polymerase chain reaction (RT-PCR) tests were conducted on serum from both microcephaly and control infants, and cerebrospinal fluid samples only from infants with microcephaly. Mothers underwent serum testing for Zika virus and dengue virus via plaque reduction neutralization assay testing. Odds ratios and 95% confidence intervals were then calculated to determine the association between congenital Zika virus and microcephaly.
Of the 30 women who gave birth to infants with microcephaly, 24 (80%) had Zika virus infections, compared with 39 of the 61 women (64%) in the control group (P = .12). Additionally, while 13 of the 32 infants born with microcephaly had Zika virus infections confirmed by laboratory testing, none of the infants in the control group had laboratory-confirmed Zika virus infection.
A total of 7 out 27 infants with microcephaly who underwent CT scans showed signs of brain abnormalities, suggesting that “congenital Zika virus syndrome can be present in neonates with microcephaly and no radiological brain abnormalities,” according to the investigators.
While the study is still ongoing, the investigators called for more research to assess other potential risk factors and to confirm the strength of association in a larger sample size, as well as to gauge the significance and role of previous dengue infections in the mothers.
The study was funded by the Brazilian Ministry of Health, the Pan American Health Organization, and Enhancing Research Activity in Epidemic Situations. The investigators reported having no relevant financial disclosures.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: The current microcephaly epidemic is a result of congenital Zika virus infection.
Major finding: In total, 41% of infants born with microcephaly had laboratory-confirmed Zika virus infection, compared with none of the infants in the control group.
Data source: Prospective, ongoing case-control study of 32 microcephaly cases and 62 controls at eight hospitals in Brazil between Jan. 15, 2016 and May 2, 2016.
Disclosures: The study was funded by the Brazilian Ministry of Health, the Pan American Health Organization, and Enhancing Research Activity in Epidemic Situations. The investigators reported having no relevant financial disclosures.
Minimal residual disease negativity predicts better survival in MM
Minimal residual disease negativity following treatment for newly diagnosed multiple myeloma is associated with long-term survival, according to findings from a meta-analysis of relevant data.
The findings suggest that assessment for minimal residual disease (MRD) should be included as an end point in clinical trials of multiple myeloma, Nikhil C. Munshi, MD, of Harvard Medical School, Boston, and his colleagues reported online Sept. 15 in JAMA Oncology.
“This large cohort meta-analysis confirms that MRD status has prognostic value and is a valid surrogate marker for both PFS [progression-free survival] and OS [overall survival] in patients with multiple myeloma, including those who had achieved a CR [complete response],” the researchers wrote, noting that all of the studies confirmed the impact of MRD status on outcome, indicating that the predictive value of MRD status was independent of the type of treatment used.
Of 1,273 patients from 14 studies that looked at the impact of MRD status on PFS, 660 were MRD-negative and 613 were MRD-positive. Of 1,100 patients from 12 studies that looked at the impact of MRD on OS, 599 were MRD-negative, and 501 were MRD-positive. MRD-negative vs. -positive status was associated with better PFS and OS (hazard ratio, 0.41 and 0.57, respectively).
“Median PFS was 54 months for MRD-negative patients and 26 months for MRD-positive patients; median OS was 98 and 82 months, respectively,” the researchers wrote (JAMA Oncol. 2016 Sep 15. doi: 10.1001/jamaoncol.2016.3160).
Further, to evaluate the impact of MRD status on PFS and OS in patients who achieved conventional CR, they analyzed data from five studies looking at PFS in 396 MRD-negative and 178 MRD-positive patients, and 6 studies looking at OS in 430 MRD-negative and 186 MRD-positive patients. In patients achieving CR, the presence of MRD predicted shorter PFS (HR, 0.44) and shorter OS (HR, 0.47), they said.
“Median PFS was 56 months for MRD-negative patients and 34 months for MRD-positive patients, and median OS was 112 and 82 months, respectively. The OS rate was higher for MRD-negative patients, compared with MRD-positive patients at 3 years (94% vs. 80%), 5 years (80% vs. 61%), and 7 years (67% vs. 47%),” they wrote.
Although none of the studies included in the analysis compared the effect of two different treatment approaches on MRD status, five did evaluate MRD status before and after autologous stem cell transplantation, and all indicated that the treatment increased the proportion of MRD-negative patients.
Maintenance therapy also appeared, based on some of the studies, to have a beneficial effect on MRD status. In one study, MRD-negative status was maintained in 96% of patients receiving thalidomide maintenance therapy vs. 69% of MRD-negative patients receiving no maintenance therapy.
Minimal residual disease status is already considered an important prognostic factor in patients with multiple myeloma, and testing could be used to monitor response to therapy and guide subsequent treatment decisions, the investigators wrote, noting that “recent development of multiparameter flow cytometry– and next-generation sequencing–based methods has allowed for MRD assessment in larger studies.”
The findings provide quantitative evidence to support the conceptual basis for integrating MRD assessment after initial treatment in clinical trials as a surrogate end point for PFS and/or OS and in clinical practice to aid in prognostication and to guide treatment. However, “integration of MRD testing into standard practice requires optimization and standardization of MRD assessment and standardization of its timing,” they said.
This study was supported by the National Institutes of Health, the Medical Research Council, and Celgene. Dr. Munshi reported consultancy positions and advisory committee memberships with Celgene, Takeda, Janssen, and Merck.
The meta-analysis by Munshi et al. begins to answer questions surrounding MRD, and highlights areas that require further investigation.
For example, the threshold used to define an MRD level that correlates best with clinical benefit remains to be determined.
While some data suggest that “deeper is better,” this remains unclear as deeper levels may be unnecessary – and harmful if achieved with greater toxic effects – in some patients. Alternatively, if deeper is better, perhaps MRD should be considered a continuous variable rather than a dichotomous one with a set cutoff.
Among other factors that require more examination are the role of MRD in patients not receiving autologous stem cell transplantation, whether MRD should only be assessed in those with complete response or those who attain a very good partial response, and whether – given the impact of cytogenetics on MRD – MRD negativity in patients with high-risk cytogenetics should be considered the same as MRD negativity in those with standard-risk cytogenetics.
What trial design or analysis features should be implemented to address this issue? Is stratification based on cytogenetic risk sufficient? What is the appropriate timing of MRD assessment? How will novel agents, such as monoclonal antibodies, affect the MRD assessment? What is the role of imaging? While the analysis is a considerable first step toward addressing these questions regarding MRD and its potential as a surrogate end point, many unanswered questions remain to be resolved.
Nicole J. Gormley, MD, and her colleagues made these comments in an editorial (JAMA Oncol. 2016 Sep 15. doi: 10.1001/jamaoncol.2016.3112) that accompanied the study. Dr. Gormley is with the Division of Hematology Products, U.S. Food and Drug Administration, Silver Spring, Md. She reported having no disclosures.
The meta-analysis by Munshi et al. begins to answer questions surrounding MRD, and highlights areas that require further investigation.
For example, the threshold used to define an MRD level that correlates best with clinical benefit remains to be determined.
While some data suggest that “deeper is better,” this remains unclear as deeper levels may be unnecessary – and harmful if achieved with greater toxic effects – in some patients. Alternatively, if deeper is better, perhaps MRD should be considered a continuous variable rather than a dichotomous one with a set cutoff.
Among other factors that require more examination are the role of MRD in patients not receiving autologous stem cell transplantation, whether MRD should only be assessed in those with complete response or those who attain a very good partial response, and whether – given the impact of cytogenetics on MRD – MRD negativity in patients with high-risk cytogenetics should be considered the same as MRD negativity in those with standard-risk cytogenetics.
What trial design or analysis features should be implemented to address this issue? Is stratification based on cytogenetic risk sufficient? What is the appropriate timing of MRD assessment? How will novel agents, such as monoclonal antibodies, affect the MRD assessment? What is the role of imaging? While the analysis is a considerable first step toward addressing these questions regarding MRD and its potential as a surrogate end point, many unanswered questions remain to be resolved.
Nicole J. Gormley, MD, and her colleagues made these comments in an editorial (JAMA Oncol. 2016 Sep 15. doi: 10.1001/jamaoncol.2016.3112) that accompanied the study. Dr. Gormley is with the Division of Hematology Products, U.S. Food and Drug Administration, Silver Spring, Md. She reported having no disclosures.
The meta-analysis by Munshi et al. begins to answer questions surrounding MRD, and highlights areas that require further investigation.
For example, the threshold used to define an MRD level that correlates best with clinical benefit remains to be determined.
While some data suggest that “deeper is better,” this remains unclear as deeper levels may be unnecessary – and harmful if achieved with greater toxic effects – in some patients. Alternatively, if deeper is better, perhaps MRD should be considered a continuous variable rather than a dichotomous one with a set cutoff.
Among other factors that require more examination are the role of MRD in patients not receiving autologous stem cell transplantation, whether MRD should only be assessed in those with complete response or those who attain a very good partial response, and whether – given the impact of cytogenetics on MRD – MRD negativity in patients with high-risk cytogenetics should be considered the same as MRD negativity in those with standard-risk cytogenetics.
What trial design or analysis features should be implemented to address this issue? Is stratification based on cytogenetic risk sufficient? What is the appropriate timing of MRD assessment? How will novel agents, such as monoclonal antibodies, affect the MRD assessment? What is the role of imaging? While the analysis is a considerable first step toward addressing these questions regarding MRD and its potential as a surrogate end point, many unanswered questions remain to be resolved.
Nicole J. Gormley, MD, and her colleagues made these comments in an editorial (JAMA Oncol. 2016 Sep 15. doi: 10.1001/jamaoncol.2016.3112) that accompanied the study. Dr. Gormley is with the Division of Hematology Products, U.S. Food and Drug Administration, Silver Spring, Md. She reported having no disclosures.
Minimal residual disease negativity following treatment for newly diagnosed multiple myeloma is associated with long-term survival, according to findings from a meta-analysis of relevant data.
The findings suggest that assessment for minimal residual disease (MRD) should be included as an end point in clinical trials of multiple myeloma, Nikhil C. Munshi, MD, of Harvard Medical School, Boston, and his colleagues reported online Sept. 15 in JAMA Oncology.
“This large cohort meta-analysis confirms that MRD status has prognostic value and is a valid surrogate marker for both PFS [progression-free survival] and OS [overall survival] in patients with multiple myeloma, including those who had achieved a CR [complete response],” the researchers wrote, noting that all of the studies confirmed the impact of MRD status on outcome, indicating that the predictive value of MRD status was independent of the type of treatment used.
Of 1,273 patients from 14 studies that looked at the impact of MRD status on PFS, 660 were MRD-negative and 613 were MRD-positive. Of 1,100 patients from 12 studies that looked at the impact of MRD on OS, 599 were MRD-negative, and 501 were MRD-positive. MRD-negative vs. -positive status was associated with better PFS and OS (hazard ratio, 0.41 and 0.57, respectively).
“Median PFS was 54 months for MRD-negative patients and 26 months for MRD-positive patients; median OS was 98 and 82 months, respectively,” the researchers wrote (JAMA Oncol. 2016 Sep 15. doi: 10.1001/jamaoncol.2016.3160).
Further, to evaluate the impact of MRD status on PFS and OS in patients who achieved conventional CR, they analyzed data from five studies looking at PFS in 396 MRD-negative and 178 MRD-positive patients, and 6 studies looking at OS in 430 MRD-negative and 186 MRD-positive patients. In patients achieving CR, the presence of MRD predicted shorter PFS (HR, 0.44) and shorter OS (HR, 0.47), they said.
“Median PFS was 56 months for MRD-negative patients and 34 months for MRD-positive patients, and median OS was 112 and 82 months, respectively. The OS rate was higher for MRD-negative patients, compared with MRD-positive patients at 3 years (94% vs. 80%), 5 years (80% vs. 61%), and 7 years (67% vs. 47%),” they wrote.
Although none of the studies included in the analysis compared the effect of two different treatment approaches on MRD status, five did evaluate MRD status before and after autologous stem cell transplantation, and all indicated that the treatment increased the proportion of MRD-negative patients.
Maintenance therapy also appeared, based on some of the studies, to have a beneficial effect on MRD status. In one study, MRD-negative status was maintained in 96% of patients receiving thalidomide maintenance therapy vs. 69% of MRD-negative patients receiving no maintenance therapy.
Minimal residual disease status is already considered an important prognostic factor in patients with multiple myeloma, and testing could be used to monitor response to therapy and guide subsequent treatment decisions, the investigators wrote, noting that “recent development of multiparameter flow cytometry– and next-generation sequencing–based methods has allowed for MRD assessment in larger studies.”
The findings provide quantitative evidence to support the conceptual basis for integrating MRD assessment after initial treatment in clinical trials as a surrogate end point for PFS and/or OS and in clinical practice to aid in prognostication and to guide treatment. However, “integration of MRD testing into standard practice requires optimization and standardization of MRD assessment and standardization of its timing,” they said.
This study was supported by the National Institutes of Health, the Medical Research Council, and Celgene. Dr. Munshi reported consultancy positions and advisory committee memberships with Celgene, Takeda, Janssen, and Merck.
Minimal residual disease negativity following treatment for newly diagnosed multiple myeloma is associated with long-term survival, according to findings from a meta-analysis of relevant data.
The findings suggest that assessment for minimal residual disease (MRD) should be included as an end point in clinical trials of multiple myeloma, Nikhil C. Munshi, MD, of Harvard Medical School, Boston, and his colleagues reported online Sept. 15 in JAMA Oncology.
“This large cohort meta-analysis confirms that MRD status has prognostic value and is a valid surrogate marker for both PFS [progression-free survival] and OS [overall survival] in patients with multiple myeloma, including those who had achieved a CR [complete response],” the researchers wrote, noting that all of the studies confirmed the impact of MRD status on outcome, indicating that the predictive value of MRD status was independent of the type of treatment used.
Of 1,273 patients from 14 studies that looked at the impact of MRD status on PFS, 660 were MRD-negative and 613 were MRD-positive. Of 1,100 patients from 12 studies that looked at the impact of MRD on OS, 599 were MRD-negative, and 501 were MRD-positive. MRD-negative vs. -positive status was associated with better PFS and OS (hazard ratio, 0.41 and 0.57, respectively).
“Median PFS was 54 months for MRD-negative patients and 26 months for MRD-positive patients; median OS was 98 and 82 months, respectively,” the researchers wrote (JAMA Oncol. 2016 Sep 15. doi: 10.1001/jamaoncol.2016.3160).
Further, to evaluate the impact of MRD status on PFS and OS in patients who achieved conventional CR, they analyzed data from five studies looking at PFS in 396 MRD-negative and 178 MRD-positive patients, and 6 studies looking at OS in 430 MRD-negative and 186 MRD-positive patients. In patients achieving CR, the presence of MRD predicted shorter PFS (HR, 0.44) and shorter OS (HR, 0.47), they said.
“Median PFS was 56 months for MRD-negative patients and 34 months for MRD-positive patients, and median OS was 112 and 82 months, respectively. The OS rate was higher for MRD-negative patients, compared with MRD-positive patients at 3 years (94% vs. 80%), 5 years (80% vs. 61%), and 7 years (67% vs. 47%),” they wrote.
Although none of the studies included in the analysis compared the effect of two different treatment approaches on MRD status, five did evaluate MRD status before and after autologous stem cell transplantation, and all indicated that the treatment increased the proportion of MRD-negative patients.
Maintenance therapy also appeared, based on some of the studies, to have a beneficial effect on MRD status. In one study, MRD-negative status was maintained in 96% of patients receiving thalidomide maintenance therapy vs. 69% of MRD-negative patients receiving no maintenance therapy.
Minimal residual disease status is already considered an important prognostic factor in patients with multiple myeloma, and testing could be used to monitor response to therapy and guide subsequent treatment decisions, the investigators wrote, noting that “recent development of multiparameter flow cytometry– and next-generation sequencing–based methods has allowed for MRD assessment in larger studies.”
The findings provide quantitative evidence to support the conceptual basis for integrating MRD assessment after initial treatment in clinical trials as a surrogate end point for PFS and/or OS and in clinical practice to aid in prognostication and to guide treatment. However, “integration of MRD testing into standard practice requires optimization and standardization of MRD assessment and standardization of its timing,” they said.
This study was supported by the National Institutes of Health, the Medical Research Council, and Celgene. Dr. Munshi reported consultancy positions and advisory committee memberships with Celgene, Takeda, Janssen, and Merck.
FROM JAMA ONCOLOGY
Key clinical point: Minimal residual disease negativity following treatment for newly diagnosed multiple myeloma is associated with long-term survival, according to findings from a meta-analysis of relevant data.
Major finding: MRD-negative vs. -positive status was associated with better PFS and OS (HR, 0.41 and 0.57, respectively).
Data source: A meta-analysis of data from 21 studies.
Disclosures: This study was supported by the National Institutes of Health, the Medical Research Council, and Celgene. Dr. Munshi reported consultancy positions and advisory committee memberships with Celgene, Takeda, Janssen, and Merck.
Number of Zika-infected pregnancies jumps in states/D.C.
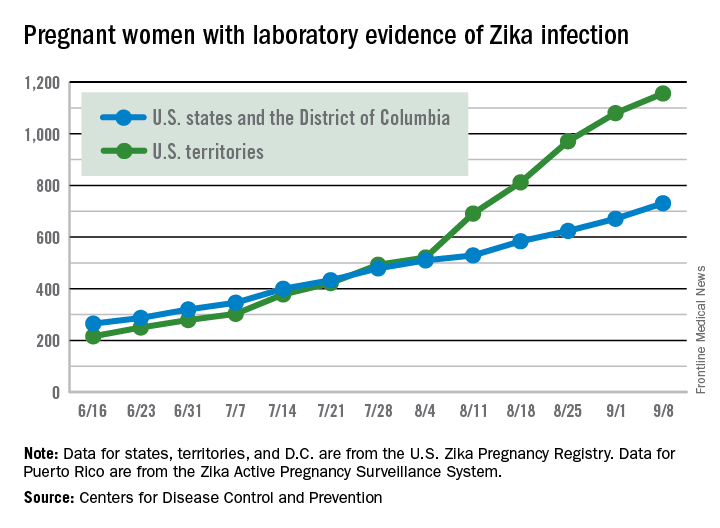
There were 60 more pregnant women in the 50 states and the District of Columbia with laboratory evidence of Zika infection for the week ending Sept. 8, according to the Centers for Disease Control and Prevention.
That is the largest weekly increase yet among that population, and it brings the total number of Zika-infected pregnant women to 731 in the 50 states and D.C. so far in 2016. The U.S. territories reported 76 new cases for the week ending Sept. 8, for a territorial total of 1,156 and a combined U.S. total of 1,887 pregnant women with Zika virus, the CDC reported Sept. 15.
For the second week in a row, a liveborn infant with Zika-related birth defects was born in the 50 states/D.C. The total is now 19 for the year: 18 in the states/D.C. and 1 in the territories. There were no new pregnancy losses with Zika-related birth defects, so the number holds at six for the year: five in the states/D.C. and one in the territories, the CDC said.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 60 more pregnant women in the 50 states and the District of Columbia with laboratory evidence of Zika infection for the week ending Sept. 8, according to the Centers for Disease Control and Prevention.
That is the largest weekly increase yet among that population, and it brings the total number of Zika-infected pregnant women to 731 in the 50 states and D.C. so far in 2016. The U.S. territories reported 76 new cases for the week ending Sept. 8, for a territorial total of 1,156 and a combined U.S. total of 1,887 pregnant women with Zika virus, the CDC reported Sept. 15.
For the second week in a row, a liveborn infant with Zika-related birth defects was born in the 50 states/D.C. The total is now 19 for the year: 18 in the states/D.C. and 1 in the territories. There were no new pregnancy losses with Zika-related birth defects, so the number holds at six for the year: five in the states/D.C. and one in the territories, the CDC said.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 60 more pregnant women in the 50 states and the District of Columbia with laboratory evidence of Zika infection for the week ending Sept. 8, according to the Centers for Disease Control and Prevention.
That is the largest weekly increase yet among that population, and it brings the total number of Zika-infected pregnant women to 731 in the 50 states and D.C. so far in 2016. The U.S. territories reported 76 new cases for the week ending Sept. 8, for a territorial total of 1,156 and a combined U.S. total of 1,887 pregnant women with Zika virus, the CDC reported Sept. 15.
For the second week in a row, a liveborn infant with Zika-related birth defects was born in the 50 states/D.C. The total is now 19 for the year: 18 in the states/D.C. and 1 in the territories. There were no new pregnancy losses with Zika-related birth defects, so the number holds at six for the year: five in the states/D.C. and one in the territories, the CDC said.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Pain starting in knee later arises in other joints
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
People who develop knee pain associated with osteoarthritis often subsequently develop pain in other joints, according to a study of two observational, community-based cohorts that could not discern any pattern of new pain sites.
In the “first investigation of the association of knee pain with pain in multiple other sites,” David T. Felson, MD, of Boston University and his colleagues reported that the regions where pain developed after first appearing in the knee varied from person to person and occurred in both upper and lower extremities, which goes against the hypothesis that adjacent joints are most often affected by knee pain.
The study involved patients from the MOST (Multicenter Osteoarthritis Study) trial, including 281 with knee pain at the index visit (168 unilaterally) and 852 without, as well as patients from OAI (the Osteoarthritis Initiative), including 412 with knee pain at the index visit (241 unilaterally), and 1,941 without. The investigators assessed the patients’ data for 14 total joints outside of the knees: 2 each of feet, ankles, hips, hands, wrists, elbows, and shoulders (Arthritis Rheumatol. 2016 Sep 2. doi: 10.1002/art.39848).
Patients with new-onset knee pain at the index visit reported a mean of 2.3 painful joints outside the knee, compared with a significantly lower number of 1.3 reported by those without knee pain. The mean number of nonknee joints with pain was higher among patients with bilateral knee pain, compared with unilateral knee pain. The percentage of patients who reported pain outside the knee rose with the number of painful knees: 80% for two, 64% for one, and 50% for none.
The patients who developed new unilateral knee pain at the index visit also experienced an increase in prevalent joint pain in multiple joints in upper- and lower-extremity sites. In particular, the investigators noted that ipsilateral prevalent hip joint pain, which they characterized as pain in the groin or front of the thigh, was more than twice as likely to occur among those with new unilateral knee pain at the index visit, but the odds for contralateral hip joint pain did not reach statistical significance. The comparisons were adjusted for age, sex, body mass index, depression at the index visit, study (MOST or OAI), and count of painful upper and lower limb joints at the index visit (excluding knees).
When examining only patients with new-onset joint pain outside of the knee, the odds of patients with new knee pain to later develop new-onset joint pain outside the knee were 30% higher than for those without knee pain. Patients with new knee pain had a mean 2.6 new painful joints out of 12.1 eligible joints, compared with 2.0 new painful joints in those without knee pain out of 12.7 eligible joints. (Joint regions with prevalent symptoms at the index visit were excluded as incident painful sites.) Patients with knee pain also had a consistently higher rate of new-onset pain in nonknee joints when compared with patients without knee pain in at least half of the follow-up visits over the course of the MOST and OAI studies. Sensitivity analyses indicated that the association between knee pain and subsequent pain in other joints was not driven by the inclusion of patients with widespread pain.
“There was no clear-cut predilection for pain in any specific lower-extremity joint region,” the investigators wrote.
The investigators noted that other researchers have suggested that patients with knee pain may be at higher risk for lower-extremity joint pain because of changes to their gait that gradually cause damage to other joints, but evidence in this study doesn’t “necessarily support the argument that in persons with knee pain, aberrant loading by altered movement patterns induces pain in only nearby joints. Our findings suggest that the sites affected are more than just hip and ankle and that there is no special predilection for pain in these locations.”
While the investigators cannot differentiate underlying mechanisms for their study’s finding of multiple co-occurring sites of joint pain in people with new-onset knee pain, they suggested that it “supports either a predilection for osteoarthritic changes at multiple joint sites and/or raises the possibility that nervous system–driven pain sensitization increases the risk not only of widespread pain but even of regional pain. Since symptomatic OA is unusual in some of these painful sites (e.g., elbow, shoulder, ankle), pain sensitization would seem a more likely explanation.”
Some of the study’s limitations described by the investigators included the uncertainty surrounding whether new-onset knee pain was truly new onset or whether it was a reoccurrence, and also the fact that most of the people in the two cohorts had multiple sites of joint pain at both the baseline and the index visit and there were too few people with no sites of pain outside the knee to carry out subanalyses in that group, which “speaks to the high prevalence of multiple joint pains in older adult cohorts.”
The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:People with frequently painful knees often develop pain in joints outside the knee, and the sites vary from person to person.
Major finding: The odds of patients with new knee pain to later develop joint pain outside the knee were 30% higher than for those without knee pain.
Data source: A study of 693 persons with index visit knee pain and 2,793 without it from two community-based cohorts.
Disclosures: The research was supported by grants from the National Institutes of Health. The authors had no disclosures to report.
Telerehabilitation May Be As Effective As In-Person Rehabilitation in MS
LONDON—In patients with multiple sclerosis (MS), telerehabilitation is a convenient and practical method of performing physical therapy with efficacy comparable to that of conventional in-person physical therapy, as measured by objective outcomes of gait and balance. Telerehabilitation should be investigated further and used more extensively as a means of improving function and quality of life in MS, according to researchers who spoke at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS).
MS often results in physical and cognitive disability. Rehabilitation methods that include physical therapy can achieve functional improvement of established physical deficits. Factors such as availability, geographical distance, mobility, transportation, and cost, however, limit access to specialized rehabilitation services. Telecommunication technology opens the possibility of supervising and directing a physical therapy program remotely through audio and visual communication in real time.
Gabriel Pardo, MD, Director of the Oklahoma Medical Research Foundation MS Center of Excellence in Oklahoma City, and colleagues sought to demonstrate the feasibility of a tele-health rehabilitation program in individuals with ambulatory deficits secondary to MS. The researchers also intended to evaluate the efficacy of the tele-health rehabilitation program and compare it with that of conventional physical therapy.
Dr. Pardo and colleagues included 30 individuals in a single-center, prospective, randomized, three-arm, evaluator-blinded study that lasted for eight weeks. About 69% of participants were female, and the population’s mean age was 54.7. Approximately 60% of participants had relapsing-remitting MS, 23% had secondary progressive MS, and 17% had primary progressive MS. The population’s mean Expanded Disability Status Scale (EDSS) score was 4.3.
All participants performed a home-based exercise program (HEP) unsupervised on five days per week for eight weeks. Participants were randomized to three intervention groups. Group 1 underwent HEP alone. Group 2 underwent HEP plus remote physical therapy supervised via audio and visual real-time telecommunication two to three times per week. Group 3 underwent HEP plus in-person physical therapy at the medical facility two to three times per week. The study outcomes were multiple measurements of gait and balance, as well as patient-reported outcomes. Selected outcomes were performed with a computerized system (ie, Neurocom SmartBalance).
Functional gait assessment improved from baseline in all groups. Improvements were no different between the telerehabilitation and the conventional PT groups, but this finding was not statistically significant. Other outcomes that were similar for Groups 2 and 3 were the Timed 25-Foot Walk, stride length, the Berg balance scale, step width, tandem sway, tandem width, limits of stability, and the sensory organization test. One participant dropped out of the study because of an MS relapse.
The researchers observed no problems with adherence in any of the groups. “If we are to demonstrate a more significant intergroup difference, we need [larger] cohorts, and consequently, further research is needed,” Dr. Pardo concluded.
LONDON—In patients with multiple sclerosis (MS), telerehabilitation is a convenient and practical method of performing physical therapy with efficacy comparable to that of conventional in-person physical therapy, as measured by objective outcomes of gait and balance. Telerehabilitation should be investigated further and used more extensively as a means of improving function and quality of life in MS, according to researchers who spoke at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS).
MS often results in physical and cognitive disability. Rehabilitation methods that include physical therapy can achieve functional improvement of established physical deficits. Factors such as availability, geographical distance, mobility, transportation, and cost, however, limit access to specialized rehabilitation services. Telecommunication technology opens the possibility of supervising and directing a physical therapy program remotely through audio and visual communication in real time.
Gabriel Pardo, MD, Director of the Oklahoma Medical Research Foundation MS Center of Excellence in Oklahoma City, and colleagues sought to demonstrate the feasibility of a tele-health rehabilitation program in individuals with ambulatory deficits secondary to MS. The researchers also intended to evaluate the efficacy of the tele-health rehabilitation program and compare it with that of conventional physical therapy.
Dr. Pardo and colleagues included 30 individuals in a single-center, prospective, randomized, three-arm, evaluator-blinded study that lasted for eight weeks. About 69% of participants were female, and the population’s mean age was 54.7. Approximately 60% of participants had relapsing-remitting MS, 23% had secondary progressive MS, and 17% had primary progressive MS. The population’s mean Expanded Disability Status Scale (EDSS) score was 4.3.
All participants performed a home-based exercise program (HEP) unsupervised on five days per week for eight weeks. Participants were randomized to three intervention groups. Group 1 underwent HEP alone. Group 2 underwent HEP plus remote physical therapy supervised via audio and visual real-time telecommunication two to three times per week. Group 3 underwent HEP plus in-person physical therapy at the medical facility two to three times per week. The study outcomes were multiple measurements of gait and balance, as well as patient-reported outcomes. Selected outcomes were performed with a computerized system (ie, Neurocom SmartBalance).
Functional gait assessment improved from baseline in all groups. Improvements were no different between the telerehabilitation and the conventional PT groups, but this finding was not statistically significant. Other outcomes that were similar for Groups 2 and 3 were the Timed 25-Foot Walk, stride length, the Berg balance scale, step width, tandem sway, tandem width, limits of stability, and the sensory organization test. One participant dropped out of the study because of an MS relapse.
The researchers observed no problems with adherence in any of the groups. “If we are to demonstrate a more significant intergroup difference, we need [larger] cohorts, and consequently, further research is needed,” Dr. Pardo concluded.
LONDON—In patients with multiple sclerosis (MS), telerehabilitation is a convenient and practical method of performing physical therapy with efficacy comparable to that of conventional in-person physical therapy, as measured by objective outcomes of gait and balance. Telerehabilitation should be investigated further and used more extensively as a means of improving function and quality of life in MS, according to researchers who spoke at the 32nd Congress of the European Committee for Treatment and Research in MS (ECTRIMS).
MS often results in physical and cognitive disability. Rehabilitation methods that include physical therapy can achieve functional improvement of established physical deficits. Factors such as availability, geographical distance, mobility, transportation, and cost, however, limit access to specialized rehabilitation services. Telecommunication technology opens the possibility of supervising and directing a physical therapy program remotely through audio and visual communication in real time.
Gabriel Pardo, MD, Director of the Oklahoma Medical Research Foundation MS Center of Excellence in Oklahoma City, and colleagues sought to demonstrate the feasibility of a tele-health rehabilitation program in individuals with ambulatory deficits secondary to MS. The researchers also intended to evaluate the efficacy of the tele-health rehabilitation program and compare it with that of conventional physical therapy.
Dr. Pardo and colleagues included 30 individuals in a single-center, prospective, randomized, three-arm, evaluator-blinded study that lasted for eight weeks. About 69% of participants were female, and the population’s mean age was 54.7. Approximately 60% of participants had relapsing-remitting MS, 23% had secondary progressive MS, and 17% had primary progressive MS. The population’s mean Expanded Disability Status Scale (EDSS) score was 4.3.
All participants performed a home-based exercise program (HEP) unsupervised on five days per week for eight weeks. Participants were randomized to three intervention groups. Group 1 underwent HEP alone. Group 2 underwent HEP plus remote physical therapy supervised via audio and visual real-time telecommunication two to three times per week. Group 3 underwent HEP plus in-person physical therapy at the medical facility two to three times per week. The study outcomes were multiple measurements of gait and balance, as well as patient-reported outcomes. Selected outcomes were performed with a computerized system (ie, Neurocom SmartBalance).
Functional gait assessment improved from baseline in all groups. Improvements were no different between the telerehabilitation and the conventional PT groups, but this finding was not statistically significant. Other outcomes that were similar for Groups 2 and 3 were the Timed 25-Foot Walk, stride length, the Berg balance scale, step width, tandem sway, tandem width, limits of stability, and the sensory organization test. One participant dropped out of the study because of an MS relapse.
The researchers observed no problems with adherence in any of the groups. “If we are to demonstrate a more significant intergroup difference, we need [larger] cohorts, and consequently, further research is needed,” Dr. Pardo concluded.
Retinal Measurements Predict 10-Year Disability in MS
LONDON—As has been previously shown with brain atrophy and lesion volume, retinal measures can have predictive value for medium-term disability in multiple sclerosis (MS), according to research presented at the 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). “Our preliminary findings support the utility of optical coherence tomography (OCT) as a tool to predict neurodegeneration and disease progression over time in patients with MS,” said Alissa M. Rothman, MD, a post-doctoral research coordinator at the Johns Hopkins MS Center in Baltimore.
Measures of retinal layer thicknesses obtained with OCT have been shown to correlate with visual function, grey matter volume, and Expanded Disability Status Scale (EDSS) scores in MS. However, the prognostic value of retinal measurements for predicting long-term disability in patients with MS is still being evaluated. In the present study, Dr. Rothman and colleagues sought to determine whether retinal thicknesses as assessed by OCT predicts disability in MS 10 years later.
A total of 89 patients with MS were scanned on Stratus OCT between 2006 and 2007. During 2015 and 2016, these patients underwent formal, blinded EDSS determination. Average peripapillary retinal nerve fiber layer (RNFL) thickness and total macular volume (TMV) were assessed by calculating the mean value of these measures for both eyes in each subject. Patients were categorized by baseline diagnosis as relapsing remitting (RRMS), secondary progressive (SPMS), or primary progressive MS (PPMS). Mixed effects linear regression models were used to investigate whether average TMV and RNFL thicknesses at baseline predict EDSS scores after 10 years.
The final analysis included 75 patients with RRMS, nine patients with SPMS, and five patients with PPMS. Fourteen of the 75 patients with a baseline diagnosis of RRMS transitioned to SPMS during the follow-up period. Baseline analyses revealed that the RRMS cohort was significantly younger than the SPMS and PPMS cohorts (mean differences = 21.5 years and 11.7 years, respectively) and that patients with SPMS patients had a longer disease duration than patients with RRMS and PPMS (mean differences = 14.2 years and 13.2 years, respectively). A history of optic neuritis (ON) was observed in the RRMS and SPMS cohorts (41% and 44%, respectively), but not in the PPMS cohorts (0%). Adjusting for age, sex, and a history of ON, the mean TMV values at baseline predicted EDSS scores after a median follow-up of 9.3 years. On average, a 1 mm3 lower TMV value at baseline predicted a mean decrease of 2 in EDSS at follow-up. Mean baseline RNFL values did not significantly predict EDSS scores.
—Glenn S. Williams
LONDON—As has been previously shown with brain atrophy and lesion volume, retinal measures can have predictive value for medium-term disability in multiple sclerosis (MS), according to research presented at the 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). “Our preliminary findings support the utility of optical coherence tomography (OCT) as a tool to predict neurodegeneration and disease progression over time in patients with MS,” said Alissa M. Rothman, MD, a post-doctoral research coordinator at the Johns Hopkins MS Center in Baltimore.
Measures of retinal layer thicknesses obtained with OCT have been shown to correlate with visual function, grey matter volume, and Expanded Disability Status Scale (EDSS) scores in MS. However, the prognostic value of retinal measurements for predicting long-term disability in patients with MS is still being evaluated. In the present study, Dr. Rothman and colleagues sought to determine whether retinal thicknesses as assessed by OCT predicts disability in MS 10 years later.
A total of 89 patients with MS were scanned on Stratus OCT between 2006 and 2007. During 2015 and 2016, these patients underwent formal, blinded EDSS determination. Average peripapillary retinal nerve fiber layer (RNFL) thickness and total macular volume (TMV) were assessed by calculating the mean value of these measures for both eyes in each subject. Patients were categorized by baseline diagnosis as relapsing remitting (RRMS), secondary progressive (SPMS), or primary progressive MS (PPMS). Mixed effects linear regression models were used to investigate whether average TMV and RNFL thicknesses at baseline predict EDSS scores after 10 years.
The final analysis included 75 patients with RRMS, nine patients with SPMS, and five patients with PPMS. Fourteen of the 75 patients with a baseline diagnosis of RRMS transitioned to SPMS during the follow-up period. Baseline analyses revealed that the RRMS cohort was significantly younger than the SPMS and PPMS cohorts (mean differences = 21.5 years and 11.7 years, respectively) and that patients with SPMS patients had a longer disease duration than patients with RRMS and PPMS (mean differences = 14.2 years and 13.2 years, respectively). A history of optic neuritis (ON) was observed in the RRMS and SPMS cohorts (41% and 44%, respectively), but not in the PPMS cohorts (0%). Adjusting for age, sex, and a history of ON, the mean TMV values at baseline predicted EDSS scores after a median follow-up of 9.3 years. On average, a 1 mm3 lower TMV value at baseline predicted a mean decrease of 2 in EDSS at follow-up. Mean baseline RNFL values did not significantly predict EDSS scores.
—Glenn S. Williams
LONDON—As has been previously shown with brain atrophy and lesion volume, retinal measures can have predictive value for medium-term disability in multiple sclerosis (MS), according to research presented at the 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). “Our preliminary findings support the utility of optical coherence tomography (OCT) as a tool to predict neurodegeneration and disease progression over time in patients with MS,” said Alissa M. Rothman, MD, a post-doctoral research coordinator at the Johns Hopkins MS Center in Baltimore.
Measures of retinal layer thicknesses obtained with OCT have been shown to correlate with visual function, grey matter volume, and Expanded Disability Status Scale (EDSS) scores in MS. However, the prognostic value of retinal measurements for predicting long-term disability in patients with MS is still being evaluated. In the present study, Dr. Rothman and colleagues sought to determine whether retinal thicknesses as assessed by OCT predicts disability in MS 10 years later.
A total of 89 patients with MS were scanned on Stratus OCT between 2006 and 2007. During 2015 and 2016, these patients underwent formal, blinded EDSS determination. Average peripapillary retinal nerve fiber layer (RNFL) thickness and total macular volume (TMV) were assessed by calculating the mean value of these measures for both eyes in each subject. Patients were categorized by baseline diagnosis as relapsing remitting (RRMS), secondary progressive (SPMS), or primary progressive MS (PPMS). Mixed effects linear regression models were used to investigate whether average TMV and RNFL thicknesses at baseline predict EDSS scores after 10 years.
The final analysis included 75 patients with RRMS, nine patients with SPMS, and five patients with PPMS. Fourteen of the 75 patients with a baseline diagnosis of RRMS transitioned to SPMS during the follow-up period. Baseline analyses revealed that the RRMS cohort was significantly younger than the SPMS and PPMS cohorts (mean differences = 21.5 years and 11.7 years, respectively) and that patients with SPMS patients had a longer disease duration than patients with RRMS and PPMS (mean differences = 14.2 years and 13.2 years, respectively). A history of optic neuritis (ON) was observed in the RRMS and SPMS cohorts (41% and 44%, respectively), but not in the PPMS cohorts (0%). Adjusting for age, sex, and a history of ON, the mean TMV values at baseline predicted EDSS scores after a median follow-up of 9.3 years. On average, a 1 mm3 lower TMV value at baseline predicted a mean decrease of 2 in EDSS at follow-up. Mean baseline RNFL values did not significantly predict EDSS scores.
—Glenn S. Williams
VIVA: Assessing aortic repair and more in the latest trials
Along with a strong focus on peripheral arterial disease, VIVA late-breaking clinical trial reports will also address other critical areas of the vascular domain, from the aorta to the carotids.
On Tuesday morning, the results of two trials will be presented, which are investigating endovascular aortic repair. Ali Azizzadeh, MD, Memorial Hermann Heart and Vascular Institute, Houston, will discuss the 3-year results from the Valiant IDE Study, examining endovascular repair in acute, complicated type B aortic dissection.
Then, Zvonimir Krajcer, MD, Texas Heart Institute, Houston, will present the final results from the Prospective LIFE Registry, looking at fast-track endovascular aortic repair.
Also on Tuesday morning, the issue of interventions after the creation of an arteriovenous fistula (AVF) will be addressed by Charmaine Lok, MD, Toronto General Hospital, comparing traditional surgical AVF creation with a new endovascular approach.
Wednesday morning, Ido Weinberg, MD, Massachusetts General Hospital, Boston, will address the issue of carotid stent fractures, demonstrating that they are not associated with restenosis, stroke, myocardial infarction, or death based on the results from the ACT 1 Multicenter Randomized Trial.
Along with a strong focus on peripheral arterial disease, VIVA late-breaking clinical trial reports will also address other critical areas of the vascular domain, from the aorta to the carotids.
On Tuesday morning, the results of two trials will be presented, which are investigating endovascular aortic repair. Ali Azizzadeh, MD, Memorial Hermann Heart and Vascular Institute, Houston, will discuss the 3-year results from the Valiant IDE Study, examining endovascular repair in acute, complicated type B aortic dissection.
Then, Zvonimir Krajcer, MD, Texas Heart Institute, Houston, will present the final results from the Prospective LIFE Registry, looking at fast-track endovascular aortic repair.
Also on Tuesday morning, the issue of interventions after the creation of an arteriovenous fistula (AVF) will be addressed by Charmaine Lok, MD, Toronto General Hospital, comparing traditional surgical AVF creation with a new endovascular approach.
Wednesday morning, Ido Weinberg, MD, Massachusetts General Hospital, Boston, will address the issue of carotid stent fractures, demonstrating that they are not associated with restenosis, stroke, myocardial infarction, or death based on the results from the ACT 1 Multicenter Randomized Trial.
Along with a strong focus on peripheral arterial disease, VIVA late-breaking clinical trial reports will also address other critical areas of the vascular domain, from the aorta to the carotids.
On Tuesday morning, the results of two trials will be presented, which are investigating endovascular aortic repair. Ali Azizzadeh, MD, Memorial Hermann Heart and Vascular Institute, Houston, will discuss the 3-year results from the Valiant IDE Study, examining endovascular repair in acute, complicated type B aortic dissection.
Then, Zvonimir Krajcer, MD, Texas Heart Institute, Houston, will present the final results from the Prospective LIFE Registry, looking at fast-track endovascular aortic repair.
Also on Tuesday morning, the issue of interventions after the creation of an arteriovenous fistula (AVF) will be addressed by Charmaine Lok, MD, Toronto General Hospital, comparing traditional surgical AVF creation with a new endovascular approach.
Wednesday morning, Ido Weinberg, MD, Massachusetts General Hospital, Boston, will address the issue of carotid stent fractures, demonstrating that they are not associated with restenosis, stroke, myocardial infarction, or death based on the results from the ACT 1 Multicenter Randomized Trial.
United States an expensive place for knee, hip replacement
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.
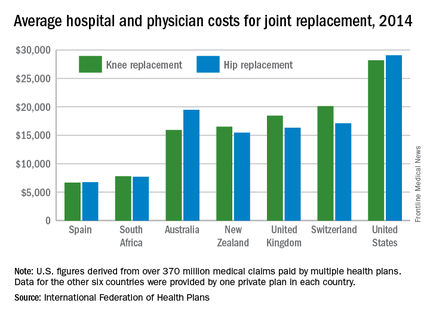
“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.

“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.
Knee and hip replacement surgeries were more expensive in the United States than in a group of six other industrialized countries in 2014, according to a report from the International Federation of Health Plans.
The U.S. average for total hospital and physician costs was $28,184 for knee replacement and $29,067 for hip replacement. Switzerland was the next most expensive country for knee replacements at $20,132, and Australia was second for hip replacements at $19,484. Spain had the lowest average cost for both surgeries: $6,687 for knee replacement and $6,757 for hip replacement, the IFHP reported.

“We look at these numbers every year and it’s always a shocking demonstration of how much procedures and prescription drugs actually cost,” IFHP Chief Executive Tom Sackville said in a written statement. “There is no reason why identical procedures and products should vary in price so much across countries: it illustrates the damaging effects of an inadequately regulated health care market.”
The IFHP consists of 80 member companies in 25 countries. For the survey, costs for each country were submitted by participating member plans. Costs for the United States are derived from over 370 million employer-sponsored medical claims incurred from Jan. 1, 2014, to Dec. 31, 2014, and paid by multiple health plans. Cost data for the other six countries were provided by one private plan in each country.