User login
Acalabrutinib shows efficacy as monotherapy in untreated CLL
CHICAGO – Acalabrutinib, which has shown efficacy in relapsed chronic lymphocytic leukemia, has now shown efficacy as a monotherapy for patients with previously untreated CLL, based on results from an ongoing phase I-II study presented as a poster at the annual meeting of the American Society of Clinical Oncology.
In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months. CLL has not progressed in any of the patients, and none have experienced Richter’s transformation, Dr. John C. Byrd, the D. Warren Brown Chair of Leukemia Research at The Ohio State University, Columbus, and his colleagues reported. Based on its favorable safety profile and durable response rates, a phase III trialof acalabrutinib therapy has been initiated (NCT02475681).
Oral acalabrutinib was given at doses of 100 mg twice daily to 37 patients or 200 mg daily to 37 other patients. About half of the patients had bulky lymph nodes of at least 5 cm and 38 of 67 patients had an unmutated IGHV gene. Median time on the study was 11 months.
All patients had rapid declines in lymphadenopathy. Both dose schedules were associated with clinical activity, with Bruton’s tyrosine kinase occupancy highest at 98% with twice-daily dosing and 93% with once-daily dosing. Treatment-related lymphocytosis occurred in 39 of 74 patients and resolved in 38 of the 39. In general, lymphocytosis peaked at a median of 1 week and resolved by a median of 7 weeks.
Acalabrutinib was well tolerated with 72 of 74 patients continuing on the drug. Most adverse events were grade 2 or less, and included headache (42%), diarrhea (35%), arthralgia (22%), contusion (18%), nausea (18%) and increased weight (18%). Grade 3-4 adverse events that occurred in at least two patients included syncope (two patients) and hypertension (two patients). There was one grade 3 upper GI bleed from a gastric ulcer and aspirin use, and one grade 5 case of pneumonia. No atrial fibrillation was reported.
Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
On Twitter @maryjodales
CHICAGO – Acalabrutinib, which has shown efficacy in relapsed chronic lymphocytic leukemia, has now shown efficacy as a monotherapy for patients with previously untreated CLL, based on results from an ongoing phase I-II study presented as a poster at the annual meeting of the American Society of Clinical Oncology.
In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months. CLL has not progressed in any of the patients, and none have experienced Richter’s transformation, Dr. John C. Byrd, the D. Warren Brown Chair of Leukemia Research at The Ohio State University, Columbus, and his colleagues reported. Based on its favorable safety profile and durable response rates, a phase III trialof acalabrutinib therapy has been initiated (NCT02475681).
Oral acalabrutinib was given at doses of 100 mg twice daily to 37 patients or 200 mg daily to 37 other patients. About half of the patients had bulky lymph nodes of at least 5 cm and 38 of 67 patients had an unmutated IGHV gene. Median time on the study was 11 months.
All patients had rapid declines in lymphadenopathy. Both dose schedules were associated with clinical activity, with Bruton’s tyrosine kinase occupancy highest at 98% with twice-daily dosing and 93% with once-daily dosing. Treatment-related lymphocytosis occurred in 39 of 74 patients and resolved in 38 of the 39. In general, lymphocytosis peaked at a median of 1 week and resolved by a median of 7 weeks.
Acalabrutinib was well tolerated with 72 of 74 patients continuing on the drug. Most adverse events were grade 2 or less, and included headache (42%), diarrhea (35%), arthralgia (22%), contusion (18%), nausea (18%) and increased weight (18%). Grade 3-4 adverse events that occurred in at least two patients included syncope (two patients) and hypertension (two patients). There was one grade 3 upper GI bleed from a gastric ulcer and aspirin use, and one grade 5 case of pneumonia. No atrial fibrillation was reported.
Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
On Twitter @maryjodales
CHICAGO – Acalabrutinib, which has shown efficacy in relapsed chronic lymphocytic leukemia, has now shown efficacy as a monotherapy for patients with previously untreated CLL, based on results from an ongoing phase I-II study presented as a poster at the annual meeting of the American Society of Clinical Oncology.
In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months. CLL has not progressed in any of the patients, and none have experienced Richter’s transformation, Dr. John C. Byrd, the D. Warren Brown Chair of Leukemia Research at The Ohio State University, Columbus, and his colleagues reported. Based on its favorable safety profile and durable response rates, a phase III trialof acalabrutinib therapy has been initiated (NCT02475681).
Oral acalabrutinib was given at doses of 100 mg twice daily to 37 patients or 200 mg daily to 37 other patients. About half of the patients had bulky lymph nodes of at least 5 cm and 38 of 67 patients had an unmutated IGHV gene. Median time on the study was 11 months.
All patients had rapid declines in lymphadenopathy. Both dose schedules were associated with clinical activity, with Bruton’s tyrosine kinase occupancy highest at 98% with twice-daily dosing and 93% with once-daily dosing. Treatment-related lymphocytosis occurred in 39 of 74 patients and resolved in 38 of the 39. In general, lymphocytosis peaked at a median of 1 week and resolved by a median of 7 weeks.
Acalabrutinib was well tolerated with 72 of 74 patients continuing on the drug. Most adverse events were grade 2 or less, and included headache (42%), diarrhea (35%), arthralgia (22%), contusion (18%), nausea (18%) and increased weight (18%). Grade 3-4 adverse events that occurred in at least two patients included syncope (two patients) and hypertension (two patients). There was one grade 3 upper GI bleed from a gastric ulcer and aspirin use, and one grade 5 case of pneumonia. No atrial fibrillation was reported.
Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
On Twitter @maryjodales
FROM 2016 ASCO ANNUAL MEETING
Key clinical point: Acalabrutinib, which has shown efficacy in relapsed CLL, has now shown efficacy as a monotherapy for patients with previously untreated CLL.
Major finding: In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months.
Data source: An ongoing phase I-II study.
Disclosures: Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
Interim PET-CT can spare HL patients intensive chemo

The use of interim PET-CT scans can spare some advanced Hodgkin lymphoma (HL) patients the toxicity associated with bleomycin, according to researchers.
The team found that patients with negative PET-CT scans after 2 cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) could go on to receive AVD (doxorubicin, vinblastine, and dacarbazine) without experiencing a significant decrease in progression-free survival (PFS) or overall survival (OS).
Peter Johnson, MD, of the University of Southampton in the UK, and his colleagues reported these findings in NEJM.
“The good news is that the majority of people diagnosed with Hodgkin lymphoma can be cured,” Dr Johnson said. “In this trial, more than 95% of patients are alive after 3 years.”
“But we worry about the long-term side effects from the treatments we use. As we’ve done in this trial, personalizing treatment based on how well it works is a major development for patients with Hodgkin lymphoma and sets a new standard of care.”
Patients and treatment
For this study, Dr Johnson and his colleagues enrolled 1214 patients with newly diagnosed, advanced, classic HL. The patients’ median age was 33 (range, 18 to 79), and 54.5% were male. More patients had stage II disease (41.6%) than stage III (30.2%) or IV (28.3%).
A total of 1119 patients underwent a baseline PET-CT scan, received 2 cycles of ABVD, and underwent an interim PET-CT scan.
The patients with negative interim scans were then randomized to continue treatment with ABVD (n=470) or with AVD (n=465) in cycles 3 through 6.
Patients with positive interim scans (n=182) went on to receive BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, n=172), salvage treatments (n=6), or ABVD (n=4).
Results
The study’s primary outcome was the difference in 3-year PFS between the randomized groups of PET-CT-negative patients.
With a median follow-up of 41 months, the 3-year PFS was 85.7% in the ABVD group and 84.4% in the AVD group. The hazard ratio was 1.13 (95% CI, 0.81 to 1.57; P=0.48) in the intention-to-treat analysis and 1.10 (95% CI, 0.79 to 1.53; P=0.58) in the per-protocol analysis.
The absolute difference in 3-year PFS (ABVD minus AVD) was 1.6 percentage points (95% CI, −3.2 to 5.3).
The OS rates were 97.2% in the ABVD group and 97.6% in the AVD group. The hazard ratio in the intention-to-treat analysis was 0.90 (95% CI, 0.47 to 1.74; P=0.76).
Patients in the ABVD group had a significantly higher rate of clinical adverse events than patients in the AVD group—31% and 21%, respectively (P<0.005).
Patients in the ABVD group also had significantly (P<0.05) higher rates of fatigue (3% vs 1%), febrile neutropenia (5% vs 2%), pulmonary/upper respiratory events (3% vs 1%), and dyspnea (2% vs <0.5%). But patients in the AVD group had a significantly higher rate of thrombocytopenia (3% vs 1%).
For patients who had positive interim PET-CT scans, the 3-year PFS was 67.5%, and the OS was 87.8%. Among the 172 patients who went on to receive BEACOPP, 74.4% had negative findings on a third PET-CT scan.
Overall, 62 patients died during the trial—24 from HL. So, for the entire study cohort, the 3-year PFS was 82.6%, and the OS was 95.8%. ![]()

The use of interim PET-CT scans can spare some advanced Hodgkin lymphoma (HL) patients the toxicity associated with bleomycin, according to researchers.
The team found that patients with negative PET-CT scans after 2 cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) could go on to receive AVD (doxorubicin, vinblastine, and dacarbazine) without experiencing a significant decrease in progression-free survival (PFS) or overall survival (OS).
Peter Johnson, MD, of the University of Southampton in the UK, and his colleagues reported these findings in NEJM.
“The good news is that the majority of people diagnosed with Hodgkin lymphoma can be cured,” Dr Johnson said. “In this trial, more than 95% of patients are alive after 3 years.”
“But we worry about the long-term side effects from the treatments we use. As we’ve done in this trial, personalizing treatment based on how well it works is a major development for patients with Hodgkin lymphoma and sets a new standard of care.”
Patients and treatment
For this study, Dr Johnson and his colleagues enrolled 1214 patients with newly diagnosed, advanced, classic HL. The patients’ median age was 33 (range, 18 to 79), and 54.5% were male. More patients had stage II disease (41.6%) than stage III (30.2%) or IV (28.3%).
A total of 1119 patients underwent a baseline PET-CT scan, received 2 cycles of ABVD, and underwent an interim PET-CT scan.
The patients with negative interim scans were then randomized to continue treatment with ABVD (n=470) or with AVD (n=465) in cycles 3 through 6.
Patients with positive interim scans (n=182) went on to receive BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, n=172), salvage treatments (n=6), or ABVD (n=4).
Results
The study’s primary outcome was the difference in 3-year PFS between the randomized groups of PET-CT-negative patients.
With a median follow-up of 41 months, the 3-year PFS was 85.7% in the ABVD group and 84.4% in the AVD group. The hazard ratio was 1.13 (95% CI, 0.81 to 1.57; P=0.48) in the intention-to-treat analysis and 1.10 (95% CI, 0.79 to 1.53; P=0.58) in the per-protocol analysis.
The absolute difference in 3-year PFS (ABVD minus AVD) was 1.6 percentage points (95% CI, −3.2 to 5.3).
The OS rates were 97.2% in the ABVD group and 97.6% in the AVD group. The hazard ratio in the intention-to-treat analysis was 0.90 (95% CI, 0.47 to 1.74; P=0.76).
Patients in the ABVD group had a significantly higher rate of clinical adverse events than patients in the AVD group—31% and 21%, respectively (P<0.005).
Patients in the ABVD group also had significantly (P<0.05) higher rates of fatigue (3% vs 1%), febrile neutropenia (5% vs 2%), pulmonary/upper respiratory events (3% vs 1%), and dyspnea (2% vs <0.5%). But patients in the AVD group had a significantly higher rate of thrombocytopenia (3% vs 1%).
For patients who had positive interim PET-CT scans, the 3-year PFS was 67.5%, and the OS was 87.8%. Among the 172 patients who went on to receive BEACOPP, 74.4% had negative findings on a third PET-CT scan.
Overall, 62 patients died during the trial—24 from HL. So, for the entire study cohort, the 3-year PFS was 82.6%, and the OS was 95.8%. ![]()

The use of interim PET-CT scans can spare some advanced Hodgkin lymphoma (HL) patients the toxicity associated with bleomycin, according to researchers.
The team found that patients with negative PET-CT scans after 2 cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) could go on to receive AVD (doxorubicin, vinblastine, and dacarbazine) without experiencing a significant decrease in progression-free survival (PFS) or overall survival (OS).
Peter Johnson, MD, of the University of Southampton in the UK, and his colleagues reported these findings in NEJM.
“The good news is that the majority of people diagnosed with Hodgkin lymphoma can be cured,” Dr Johnson said. “In this trial, more than 95% of patients are alive after 3 years.”
“But we worry about the long-term side effects from the treatments we use. As we’ve done in this trial, personalizing treatment based on how well it works is a major development for patients with Hodgkin lymphoma and sets a new standard of care.”
Patients and treatment
For this study, Dr Johnson and his colleagues enrolled 1214 patients with newly diagnosed, advanced, classic HL. The patients’ median age was 33 (range, 18 to 79), and 54.5% were male. More patients had stage II disease (41.6%) than stage III (30.2%) or IV (28.3%).
A total of 1119 patients underwent a baseline PET-CT scan, received 2 cycles of ABVD, and underwent an interim PET-CT scan.
The patients with negative interim scans were then randomized to continue treatment with ABVD (n=470) or with AVD (n=465) in cycles 3 through 6.
Patients with positive interim scans (n=182) went on to receive BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, n=172), salvage treatments (n=6), or ABVD (n=4).
Results
The study’s primary outcome was the difference in 3-year PFS between the randomized groups of PET-CT-negative patients.
With a median follow-up of 41 months, the 3-year PFS was 85.7% in the ABVD group and 84.4% in the AVD group. The hazard ratio was 1.13 (95% CI, 0.81 to 1.57; P=0.48) in the intention-to-treat analysis and 1.10 (95% CI, 0.79 to 1.53; P=0.58) in the per-protocol analysis.
The absolute difference in 3-year PFS (ABVD minus AVD) was 1.6 percentage points (95% CI, −3.2 to 5.3).
The OS rates were 97.2% in the ABVD group and 97.6% in the AVD group. The hazard ratio in the intention-to-treat analysis was 0.90 (95% CI, 0.47 to 1.74; P=0.76).
Patients in the ABVD group had a significantly higher rate of clinical adverse events than patients in the AVD group—31% and 21%, respectively (P<0.005).
Patients in the ABVD group also had significantly (P<0.05) higher rates of fatigue (3% vs 1%), febrile neutropenia (5% vs 2%), pulmonary/upper respiratory events (3% vs 1%), and dyspnea (2% vs <0.5%). But patients in the AVD group had a significantly higher rate of thrombocytopenia (3% vs 1%).
For patients who had positive interim PET-CT scans, the 3-year PFS was 67.5%, and the OS was 87.8%. Among the 172 patients who went on to receive BEACOPP, 74.4% had negative findings on a third PET-CT scan.
Overall, 62 patients died during the trial—24 from HL. So, for the entire study cohort, the 3-year PFS was 82.6%, and the OS was 95.8%. ![]()
SHM SPARK Helps Bridge Gap for Hospitalist MOC Exam Prep
SHM SPARK delivers 175 vignette-style, multiple-choice questions that bridge the primary knowledge gaps found within existing MOC exam-preparation products. It offers up to 10.5 AMA PRA Category 1 Credits of CME. Content areas covered include:
- Palliative care, ethics, and decision making
- Patient safety
- Perioperative care and consultative co-management
- Quality, cost, and clinical reasoning
The Hospitalist recently spoke with three SHM SPARK users about its impact on their exam-preparation efforts: Louis O’Boyle, DO, SFHM, CLHM, medical director of Advanced Inpatient Medicine, a hospitalist management company in Lakeville, Pa.; Timothy Crone, MD, SFHM, medical director of Enterprise Intelligence and Analytics and former vice-chairman of the Department of Hospital Medicine at Cleveland Clinic; and Aroop Pal, MD, FHM, hospitalist, associate professor, and program director of transitions of care services at University of Kansas Medical Center.
Question: Why did you choose to purchase SHM SPARK?
Dr. O’Boyle: I was expecting the FPHM exam to be more challenging than the traditional exam, so I wanted to get as much help as possible, particularly in those areas that are less utilized in day-to-day hospitalist practice. This exam covered the 40% or so that is not covered in a typical review course.
Dr. Crone: I was selected to receive SHM SPARK as a test user, but had I not been, I probably would have purchased it as there was not another single tool that addressed the content gap preparing for the exam that SHM SPARK did. On HMX and in conversations with other hospitalists, I was aware of “collections” of tools, like books and websites, that people had put together to review information not covered in MKSAP or other standard test-prep materials. SHM SPARK brought that content together in a single space, which allowed me to take a systematic approach to reviewing the content areas covered as opposed to a potentially incomplete, piecemeal approach.
Dr. Pal: There are no board review products focused on the 40% of the FPHM exam not based on traditional clinical knowledge. Thus, it made sense to give SHM SPARK a try, especially since it was affordable for members.
Q: During your preparation, what was the most useful aspect of SHM SPARK?
Dr. Crone: I find that working through computer-based questions similar in format to the actual exam is most helpful to me. Both in terms of knowledge acquisition and comfort level with the exam itself, the “context-dependent learning” aspect is important for me. SHM SPARK allowed me to work through its content areas in that way and also helped me identify and correct gaps in my knowledge as opposed to guessing what was important and searching for source material on my own.
Dr. Pal: SHM SPARK helped frame how quality and patient safety questions would or could be posed on the FPHM exam. This made it helpful to determine what content is fair game for the exam and the key competencies ABIM was focusing on—especially since traditional board review materials do not cover as much quality-specific content.
Dr. O’Boyle: The most useful aspects were the topics that are not encountered specifically in everyday practice, such as the sections on quality, cost, and clinical reasoning, as well as patient safety.
Q: After taking the exam, in retrospect, how effective was SHM SPARK in preparing you?
Dr. Pal: SHM SPARK was valuable to me and worth the time and effort. The board exam itself is a little bit of a blur; if nothing else, it helped me identify areas that I needed more information on and reinforced some knowledge I had prior to taking the exam.
Dr. O’Boyle: The SHM SPARK review absolutely helped me perform well on the sections that were covered. I think it is almost essential to prepare for the Focused Practice in Hospital Medicine exam.
Dr. Crone: I passed, so I’d label that effective! In some cases, using SHM SPARK, I scored 90% or better on a first pass on questions with no review; that has not been my experience with MKSAP or Med Study questions. The only recommendation I would have is to make some of the questions a bit more rigorous. However, SHM SPARK clearly met a need nothing else did.
Q: If you were to tell a fellow hospitalist one thing about SHM SPARK, what would it be?
Dr. O’Boyle: I encourage everyone to purchase it. It is an excellent resource guide. The way the exam is currently designed, you may need the additional 40% of exam content covered by SHM SPARK in order to pass. By that, I mean that some of the medical questions were so complicated and cumbersome that they were at the specialist level and not at all representative of what a typical hospitalist routinely encounters, in my opinion. Therefore, knowing this portion of the exam content through SHM SPARK made up for the questions that I felt should not have been fair game. I, for one, would likely not have passed without SHM SPARK.
Dr. Crone: It’s worth the time, effort, and cost. Although much or most of the exam content was couched in a clinical scenario, substantive content existed on the MOC exam around these subject areas. To not use some form of structured approach to covering this material would have been a mistake.
Dr. Pal: SHM SPARK is extremely valuable if you plan to take the FPHM exam as it highlights many areas not covered by any other review material. It offers great CME, too! TH
Brett Radler is SHM’s communications specialist.
More Info
For more information about how SHM SPARK can help you master your preparation for the FPHM MOC exam this fall, visit www.hospitalmedicine.org/SPARK.
SHM SPARK delivers 175 vignette-style, multiple-choice questions that bridge the primary knowledge gaps found within existing MOC exam-preparation products. It offers up to 10.5 AMA PRA Category 1 Credits of CME. Content areas covered include:
- Palliative care, ethics, and decision making
- Patient safety
- Perioperative care and consultative co-management
- Quality, cost, and clinical reasoning
The Hospitalist recently spoke with three SHM SPARK users about its impact on their exam-preparation efforts: Louis O’Boyle, DO, SFHM, CLHM, medical director of Advanced Inpatient Medicine, a hospitalist management company in Lakeville, Pa.; Timothy Crone, MD, SFHM, medical director of Enterprise Intelligence and Analytics and former vice-chairman of the Department of Hospital Medicine at Cleveland Clinic; and Aroop Pal, MD, FHM, hospitalist, associate professor, and program director of transitions of care services at University of Kansas Medical Center.
Question: Why did you choose to purchase SHM SPARK?
Dr. O’Boyle: I was expecting the FPHM exam to be more challenging than the traditional exam, so I wanted to get as much help as possible, particularly in those areas that are less utilized in day-to-day hospitalist practice. This exam covered the 40% or so that is not covered in a typical review course.
Dr. Crone: I was selected to receive SHM SPARK as a test user, but had I not been, I probably would have purchased it as there was not another single tool that addressed the content gap preparing for the exam that SHM SPARK did. On HMX and in conversations with other hospitalists, I was aware of “collections” of tools, like books and websites, that people had put together to review information not covered in MKSAP or other standard test-prep materials. SHM SPARK brought that content together in a single space, which allowed me to take a systematic approach to reviewing the content areas covered as opposed to a potentially incomplete, piecemeal approach.
Dr. Pal: There are no board review products focused on the 40% of the FPHM exam not based on traditional clinical knowledge. Thus, it made sense to give SHM SPARK a try, especially since it was affordable for members.
Q: During your preparation, what was the most useful aspect of SHM SPARK?
Dr. Crone: I find that working through computer-based questions similar in format to the actual exam is most helpful to me. Both in terms of knowledge acquisition and comfort level with the exam itself, the “context-dependent learning” aspect is important for me. SHM SPARK allowed me to work through its content areas in that way and also helped me identify and correct gaps in my knowledge as opposed to guessing what was important and searching for source material on my own.
Dr. Pal: SHM SPARK helped frame how quality and patient safety questions would or could be posed on the FPHM exam. This made it helpful to determine what content is fair game for the exam and the key competencies ABIM was focusing on—especially since traditional board review materials do not cover as much quality-specific content.
Dr. O’Boyle: The most useful aspects were the topics that are not encountered specifically in everyday practice, such as the sections on quality, cost, and clinical reasoning, as well as patient safety.
Q: After taking the exam, in retrospect, how effective was SHM SPARK in preparing you?
Dr. Pal: SHM SPARK was valuable to me and worth the time and effort. The board exam itself is a little bit of a blur; if nothing else, it helped me identify areas that I needed more information on and reinforced some knowledge I had prior to taking the exam.
Dr. O’Boyle: The SHM SPARK review absolutely helped me perform well on the sections that were covered. I think it is almost essential to prepare for the Focused Practice in Hospital Medicine exam.
Dr. Crone: I passed, so I’d label that effective! In some cases, using SHM SPARK, I scored 90% or better on a first pass on questions with no review; that has not been my experience with MKSAP or Med Study questions. The only recommendation I would have is to make some of the questions a bit more rigorous. However, SHM SPARK clearly met a need nothing else did.
Q: If you were to tell a fellow hospitalist one thing about SHM SPARK, what would it be?
Dr. O’Boyle: I encourage everyone to purchase it. It is an excellent resource guide. The way the exam is currently designed, you may need the additional 40% of exam content covered by SHM SPARK in order to pass. By that, I mean that some of the medical questions were so complicated and cumbersome that they were at the specialist level and not at all representative of what a typical hospitalist routinely encounters, in my opinion. Therefore, knowing this portion of the exam content through SHM SPARK made up for the questions that I felt should not have been fair game. I, for one, would likely not have passed without SHM SPARK.
Dr. Crone: It’s worth the time, effort, and cost. Although much or most of the exam content was couched in a clinical scenario, substantive content existed on the MOC exam around these subject areas. To not use some form of structured approach to covering this material would have been a mistake.
Dr. Pal: SHM SPARK is extremely valuable if you plan to take the FPHM exam as it highlights many areas not covered by any other review material. It offers great CME, too! TH
Brett Radler is SHM’s communications specialist.
More Info
For more information about how SHM SPARK can help you master your preparation for the FPHM MOC exam this fall, visit www.hospitalmedicine.org/SPARK.
SHM SPARK delivers 175 vignette-style, multiple-choice questions that bridge the primary knowledge gaps found within existing MOC exam-preparation products. It offers up to 10.5 AMA PRA Category 1 Credits of CME. Content areas covered include:
- Palliative care, ethics, and decision making
- Patient safety
- Perioperative care and consultative co-management
- Quality, cost, and clinical reasoning
The Hospitalist recently spoke with three SHM SPARK users about its impact on their exam-preparation efforts: Louis O’Boyle, DO, SFHM, CLHM, medical director of Advanced Inpatient Medicine, a hospitalist management company in Lakeville, Pa.; Timothy Crone, MD, SFHM, medical director of Enterprise Intelligence and Analytics and former vice-chairman of the Department of Hospital Medicine at Cleveland Clinic; and Aroop Pal, MD, FHM, hospitalist, associate professor, and program director of transitions of care services at University of Kansas Medical Center.
Question: Why did you choose to purchase SHM SPARK?
Dr. O’Boyle: I was expecting the FPHM exam to be more challenging than the traditional exam, so I wanted to get as much help as possible, particularly in those areas that are less utilized in day-to-day hospitalist practice. This exam covered the 40% or so that is not covered in a typical review course.
Dr. Crone: I was selected to receive SHM SPARK as a test user, but had I not been, I probably would have purchased it as there was not another single tool that addressed the content gap preparing for the exam that SHM SPARK did. On HMX and in conversations with other hospitalists, I was aware of “collections” of tools, like books and websites, that people had put together to review information not covered in MKSAP or other standard test-prep materials. SHM SPARK brought that content together in a single space, which allowed me to take a systematic approach to reviewing the content areas covered as opposed to a potentially incomplete, piecemeal approach.
Dr. Pal: There are no board review products focused on the 40% of the FPHM exam not based on traditional clinical knowledge. Thus, it made sense to give SHM SPARK a try, especially since it was affordable for members.
Q: During your preparation, what was the most useful aspect of SHM SPARK?
Dr. Crone: I find that working through computer-based questions similar in format to the actual exam is most helpful to me. Both in terms of knowledge acquisition and comfort level with the exam itself, the “context-dependent learning” aspect is important for me. SHM SPARK allowed me to work through its content areas in that way and also helped me identify and correct gaps in my knowledge as opposed to guessing what was important and searching for source material on my own.
Dr. Pal: SHM SPARK helped frame how quality and patient safety questions would or could be posed on the FPHM exam. This made it helpful to determine what content is fair game for the exam and the key competencies ABIM was focusing on—especially since traditional board review materials do not cover as much quality-specific content.
Dr. O’Boyle: The most useful aspects were the topics that are not encountered specifically in everyday practice, such as the sections on quality, cost, and clinical reasoning, as well as patient safety.
Q: After taking the exam, in retrospect, how effective was SHM SPARK in preparing you?
Dr. Pal: SHM SPARK was valuable to me and worth the time and effort. The board exam itself is a little bit of a blur; if nothing else, it helped me identify areas that I needed more information on and reinforced some knowledge I had prior to taking the exam.
Dr. O’Boyle: The SHM SPARK review absolutely helped me perform well on the sections that were covered. I think it is almost essential to prepare for the Focused Practice in Hospital Medicine exam.
Dr. Crone: I passed, so I’d label that effective! In some cases, using SHM SPARK, I scored 90% or better on a first pass on questions with no review; that has not been my experience with MKSAP or Med Study questions. The only recommendation I would have is to make some of the questions a bit more rigorous. However, SHM SPARK clearly met a need nothing else did.
Q: If you were to tell a fellow hospitalist one thing about SHM SPARK, what would it be?
Dr. O’Boyle: I encourage everyone to purchase it. It is an excellent resource guide. The way the exam is currently designed, you may need the additional 40% of exam content covered by SHM SPARK in order to pass. By that, I mean that some of the medical questions were so complicated and cumbersome that they were at the specialist level and not at all representative of what a typical hospitalist routinely encounters, in my opinion. Therefore, knowing this portion of the exam content through SHM SPARK made up for the questions that I felt should not have been fair game. I, for one, would likely not have passed without SHM SPARK.
Dr. Crone: It’s worth the time, effort, and cost. Although much or most of the exam content was couched in a clinical scenario, substantive content existed on the MOC exam around these subject areas. To not use some form of structured approach to covering this material would have been a mistake.
Dr. Pal: SHM SPARK is extremely valuable if you plan to take the FPHM exam as it highlights many areas not covered by any other review material. It offers great CME, too! TH
Brett Radler is SHM’s communications specialist.
More Info
For more information about how SHM SPARK can help you master your preparation for the FPHM MOC exam this fall, visit www.hospitalmedicine.org/SPARK.
Measure Hospitalist Engagement with SHM’s Engagement Benchmarking Service
One of the most important questions for leaders of hospital medicine groups is, “How can I measure the level of engagement of my hospitalists?” Measuring hospitalist engagement can be difficult, and many leaders are not satisfied with the tools they currently have at their disposal.
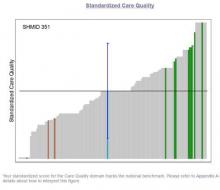
SHM is here to help. SHM developed an Engagement Benchmarking Service to evaluate relationships with leaders, care quality, autonomy, effective motivation, burnout risk, and more. You can see your standardized score in the various domains and where it falls within the national benchmark. This helps you know what is working well and identifies the areas for improvement in your hospital medicine group.
More than 80% of respondents from 2015 indicated that they will utilize the service again and plan to recommend it to a colleague. Help ensure hospitalists are engaged in your HM group by registering now for the next cohort at www.hospitalmedicine.org/pmad3.
One of the most important questions for leaders of hospital medicine groups is, “How can I measure the level of engagement of my hospitalists?” Measuring hospitalist engagement can be difficult, and many leaders are not satisfied with the tools they currently have at their disposal.
SHM is here to help. SHM developed an Engagement Benchmarking Service to evaluate relationships with leaders, care quality, autonomy, effective motivation, burnout risk, and more. You can see your standardized score in the various domains and where it falls within the national benchmark. This helps you know what is working well and identifies the areas for improvement in your hospital medicine group.
More than 80% of respondents from 2015 indicated that they will utilize the service again and plan to recommend it to a colleague. Help ensure hospitalists are engaged in your HM group by registering now for the next cohort at www.hospitalmedicine.org/pmad3.
One of the most important questions for leaders of hospital medicine groups is, “How can I measure the level of engagement of my hospitalists?” Measuring hospitalist engagement can be difficult, and many leaders are not satisfied with the tools they currently have at their disposal.
SHM is here to help. SHM developed an Engagement Benchmarking Service to evaluate relationships with leaders, care quality, autonomy, effective motivation, burnout risk, and more. You can see your standardized score in the various domains and where it falls within the national benchmark. This helps you know what is working well and identifies the areas for improvement in your hospital medicine group.
More than 80% of respondents from 2015 indicated that they will utilize the service again and plan to recommend it to a colleague. Help ensure hospitalists are engaged in your HM group by registering now for the next cohort at www.hospitalmedicine.org/pmad3.
Stopping TKI therapy can be safe, study suggests

COPENHAGEN—Results of a large study suggest that stopping treatment with tyrosine kinase inhibitors (TKIs) can be safe for patients with chronic myeloid leukemia (CML) in deep molecular response (MR4).
Six months after patients stopped receiving a TKI, the relapse-free survival was 62%. At 12 months, it was 56%.
Havinga longer duration of TKI treatment and a longer duration of deep molecular response were both associated with a higher likelihood of relapse-free survival.
These results, from the EURO-SKI trial, were presented at the 21st Congress of the European Hematology Association (abstract S145*) by Johan Richter, MD, PhD, of Skåne University Hospital in Lund, Sweden.
The goal of the EURO-SKI study was to define prognostic markers to increase the proportion of patients in durable deep molecular response after stopping TKI treatment.
The trial included 760 adults with chronic phase CML who were on TKI treatment for at least 3 years. Patients were either on their first TKI or on their second TKI due to toxicity with their first. (None had failed TKI treatment.)
Patients had been in MR4 (BCR/ABL <0.01%) for at least a year, which was confirmed by 3 consecutive polymerase chain reaction (PCR) results during the last 12 months. The final MR4 confirmation was performed in a EUTOS standardized laboratory.
After the final MR4 confirmation, patients stopped TKI treatment. They underwent real-time quantitative PCR (RQ-PCR) every 4 weeks for the first 6 months and every 6 weeks for the next 6 months. In years 2 and 3, they underwent RQ-PCR every third month.
The patients had a median age at diagnosis of 52 (range, 11.2-85.5) and a median age at TKI stop of 60.3 (range, 19.5-89.9). The median duration of TKI therapy was 7.6 years (range, 3.0-14.2), and the median duration of MR4 before TKI stop was 4.7 years (range, 1.0-13.3).
Most patients had received imatinib (n=710) as first-line TKI treatment, though some received nilotinib (n=35) or dasatinib (n=14). The type of first-line TKI was unknown in 1 patient. Second-line TKI treatment included imatinib (n=7), nilotinib (n=47), and dasatinib (n=57).
Relapse, survival, and safety
Six months after stopping TKI treatment, the cumulative incidence of molecular relapse was 37%. It was 43% at 12 months, 47% at 24 months, and 50% at 36 months.
In all, 347 patients had a molecular relapse. Seventy-two patients had BCR/ABL >1%, and 11 lost their complete cytogenetic response. None of the patients progressed to accelerated phase or blast crisis.
Among patients who restarted TKI treatment, the median time to restart was 4.1 months. Fourteen patients restarted treatment without a loss of major molecular response.
Dr Richter noted that the study is still ongoing, but, thus far, more than 80% of patients who restarted TKI therapy have achieved MR4 again.
The molecular relapse-free survival was 62% at 6 months after TKI stop, 56% at 12 months, 52% at 24 months, and 49% at 36 months.
There were 9 on-trial deaths, none of which were related to CML. Five patients died while in remission.
Previous studies revealed a TKI withdrawal syndrome that consists of (mostly transient) musculoskeletal pain or discomfort. In this study, 30.9% of patients (n=235) reported musculoskeletal symptoms, 226 with grade 1-2 events and 9 with grade 3 events.
Prognostic factors
The researchers performed prognostic modeling in 448 patients who previously received imatinib. Univariate analysis revealed no significant association between molecular relapse-free survival at 6 months and age, gender, depth of molecular response, Sokal score, EURO score, EUTOS score, or ELTS score.
However, TKI treatment duration and MR4 duration were both significantly (P<0.001) associated with major molecular response status at 6 months.
The odds ratio for treatment duration was 1.16 (95% CI, 1.08-1.25), which means that an additional year of imatinib treatment increases a patient’s odds of staying in major molecular response at 6 months by 16%.
The odds ratio for MR4 duration was also 1.16 (95% CI, 1.076-1.253), which means that an additional year in MR4 before TKI stop increases a patient’s odds of staying in major molecular response at 6 months by 16%.
Dr Richter noted that treatment duration and MR4 duration were highly correlated, which prevented a significant multiple model including both variables. He said the researchers will conduct further analyses to overcome the correlation between the 2 variables and determine an optimal cutoff for MR4 duration.
The team also plans to collect more data on pretreatment with interferon, as there is reason to suspect it has an influence on major molecular response duration after TKI discontinuation. ![]()
*Data in the abstract differ from data presented at the meeting.

COPENHAGEN—Results of a large study suggest that stopping treatment with tyrosine kinase inhibitors (TKIs) can be safe for patients with chronic myeloid leukemia (CML) in deep molecular response (MR4).
Six months after patients stopped receiving a TKI, the relapse-free survival was 62%. At 12 months, it was 56%.
Havinga longer duration of TKI treatment and a longer duration of deep molecular response were both associated with a higher likelihood of relapse-free survival.
These results, from the EURO-SKI trial, were presented at the 21st Congress of the European Hematology Association (abstract S145*) by Johan Richter, MD, PhD, of Skåne University Hospital in Lund, Sweden.
The goal of the EURO-SKI study was to define prognostic markers to increase the proportion of patients in durable deep molecular response after stopping TKI treatment.
The trial included 760 adults with chronic phase CML who were on TKI treatment for at least 3 years. Patients were either on their first TKI or on their second TKI due to toxicity with their first. (None had failed TKI treatment.)
Patients had been in MR4 (BCR/ABL <0.01%) for at least a year, which was confirmed by 3 consecutive polymerase chain reaction (PCR) results during the last 12 months. The final MR4 confirmation was performed in a EUTOS standardized laboratory.
After the final MR4 confirmation, patients stopped TKI treatment. They underwent real-time quantitative PCR (RQ-PCR) every 4 weeks for the first 6 months and every 6 weeks for the next 6 months. In years 2 and 3, they underwent RQ-PCR every third month.
The patients had a median age at diagnosis of 52 (range, 11.2-85.5) and a median age at TKI stop of 60.3 (range, 19.5-89.9). The median duration of TKI therapy was 7.6 years (range, 3.0-14.2), and the median duration of MR4 before TKI stop was 4.7 years (range, 1.0-13.3).
Most patients had received imatinib (n=710) as first-line TKI treatment, though some received nilotinib (n=35) or dasatinib (n=14). The type of first-line TKI was unknown in 1 patient. Second-line TKI treatment included imatinib (n=7), nilotinib (n=47), and dasatinib (n=57).
Relapse, survival, and safety
Six months after stopping TKI treatment, the cumulative incidence of molecular relapse was 37%. It was 43% at 12 months, 47% at 24 months, and 50% at 36 months.
In all, 347 patients had a molecular relapse. Seventy-two patients had BCR/ABL >1%, and 11 lost their complete cytogenetic response. None of the patients progressed to accelerated phase or blast crisis.
Among patients who restarted TKI treatment, the median time to restart was 4.1 months. Fourteen patients restarted treatment without a loss of major molecular response.
Dr Richter noted that the study is still ongoing, but, thus far, more than 80% of patients who restarted TKI therapy have achieved MR4 again.
The molecular relapse-free survival was 62% at 6 months after TKI stop, 56% at 12 months, 52% at 24 months, and 49% at 36 months.
There were 9 on-trial deaths, none of which were related to CML. Five patients died while in remission.
Previous studies revealed a TKI withdrawal syndrome that consists of (mostly transient) musculoskeletal pain or discomfort. In this study, 30.9% of patients (n=235) reported musculoskeletal symptoms, 226 with grade 1-2 events and 9 with grade 3 events.
Prognostic factors
The researchers performed prognostic modeling in 448 patients who previously received imatinib. Univariate analysis revealed no significant association between molecular relapse-free survival at 6 months and age, gender, depth of molecular response, Sokal score, EURO score, EUTOS score, or ELTS score.
However, TKI treatment duration and MR4 duration were both significantly (P<0.001) associated with major molecular response status at 6 months.
The odds ratio for treatment duration was 1.16 (95% CI, 1.08-1.25), which means that an additional year of imatinib treatment increases a patient’s odds of staying in major molecular response at 6 months by 16%.
The odds ratio for MR4 duration was also 1.16 (95% CI, 1.076-1.253), which means that an additional year in MR4 before TKI stop increases a patient’s odds of staying in major molecular response at 6 months by 16%.
Dr Richter noted that treatment duration and MR4 duration were highly correlated, which prevented a significant multiple model including both variables. He said the researchers will conduct further analyses to overcome the correlation between the 2 variables and determine an optimal cutoff for MR4 duration.
The team also plans to collect more data on pretreatment with interferon, as there is reason to suspect it has an influence on major molecular response duration after TKI discontinuation. ![]()
*Data in the abstract differ from data presented at the meeting.

COPENHAGEN—Results of a large study suggest that stopping treatment with tyrosine kinase inhibitors (TKIs) can be safe for patients with chronic myeloid leukemia (CML) in deep molecular response (MR4).
Six months after patients stopped receiving a TKI, the relapse-free survival was 62%. At 12 months, it was 56%.
Havinga longer duration of TKI treatment and a longer duration of deep molecular response were both associated with a higher likelihood of relapse-free survival.
These results, from the EURO-SKI trial, were presented at the 21st Congress of the European Hematology Association (abstract S145*) by Johan Richter, MD, PhD, of Skåne University Hospital in Lund, Sweden.
The goal of the EURO-SKI study was to define prognostic markers to increase the proportion of patients in durable deep molecular response after stopping TKI treatment.
The trial included 760 adults with chronic phase CML who were on TKI treatment for at least 3 years. Patients were either on their first TKI or on their second TKI due to toxicity with their first. (None had failed TKI treatment.)
Patients had been in MR4 (BCR/ABL <0.01%) for at least a year, which was confirmed by 3 consecutive polymerase chain reaction (PCR) results during the last 12 months. The final MR4 confirmation was performed in a EUTOS standardized laboratory.
After the final MR4 confirmation, patients stopped TKI treatment. They underwent real-time quantitative PCR (RQ-PCR) every 4 weeks for the first 6 months and every 6 weeks for the next 6 months. In years 2 and 3, they underwent RQ-PCR every third month.
The patients had a median age at diagnosis of 52 (range, 11.2-85.5) and a median age at TKI stop of 60.3 (range, 19.5-89.9). The median duration of TKI therapy was 7.6 years (range, 3.0-14.2), and the median duration of MR4 before TKI stop was 4.7 years (range, 1.0-13.3).
Most patients had received imatinib (n=710) as first-line TKI treatment, though some received nilotinib (n=35) or dasatinib (n=14). The type of first-line TKI was unknown in 1 patient. Second-line TKI treatment included imatinib (n=7), nilotinib (n=47), and dasatinib (n=57).
Relapse, survival, and safety
Six months after stopping TKI treatment, the cumulative incidence of molecular relapse was 37%. It was 43% at 12 months, 47% at 24 months, and 50% at 36 months.
In all, 347 patients had a molecular relapse. Seventy-two patients had BCR/ABL >1%, and 11 lost their complete cytogenetic response. None of the patients progressed to accelerated phase or blast crisis.
Among patients who restarted TKI treatment, the median time to restart was 4.1 months. Fourteen patients restarted treatment without a loss of major molecular response.
Dr Richter noted that the study is still ongoing, but, thus far, more than 80% of patients who restarted TKI therapy have achieved MR4 again.
The molecular relapse-free survival was 62% at 6 months after TKI stop, 56% at 12 months, 52% at 24 months, and 49% at 36 months.
There were 9 on-trial deaths, none of which were related to CML. Five patients died while in remission.
Previous studies revealed a TKI withdrawal syndrome that consists of (mostly transient) musculoskeletal pain or discomfort. In this study, 30.9% of patients (n=235) reported musculoskeletal symptoms, 226 with grade 1-2 events and 9 with grade 3 events.
Prognostic factors
The researchers performed prognostic modeling in 448 patients who previously received imatinib. Univariate analysis revealed no significant association between molecular relapse-free survival at 6 months and age, gender, depth of molecular response, Sokal score, EURO score, EUTOS score, or ELTS score.
However, TKI treatment duration and MR4 duration were both significantly (P<0.001) associated with major molecular response status at 6 months.
The odds ratio for treatment duration was 1.16 (95% CI, 1.08-1.25), which means that an additional year of imatinib treatment increases a patient’s odds of staying in major molecular response at 6 months by 16%.
The odds ratio for MR4 duration was also 1.16 (95% CI, 1.076-1.253), which means that an additional year in MR4 before TKI stop increases a patient’s odds of staying in major molecular response at 6 months by 16%.
Dr Richter noted that treatment duration and MR4 duration were highly correlated, which prevented a significant multiple model including both variables. He said the researchers will conduct further analyses to overcome the correlation between the 2 variables and determine an optimal cutoff for MR4 duration.
The team also plans to collect more data on pretreatment with interferon, as there is reason to suspect it has an influence on major molecular response duration after TKI discontinuation. ![]()
*Data in the abstract differ from data presented at the meeting.
Therapy granted fast track designation to treat GVHD

Photo by Chad McNeeley
The US Food and Drug Administration (FDA) has granted fast track designation for ProTmune™, a programmed cellular immunotherapy, to reduce the incidence and severity of acute graft-versus-host disease (GVHD) in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT).
ProTmune is produced by modulating a donor-sourced, mobilized peripheral blood (mPB) graft ex vivo with 2 small molecules (FT1050 and FT4145) to enhance the biological properties and therapeutic function of the graft’s immune cells.
The programmed mPB graft is adoptively transferred and administered to a patient as a one-time intravenous infusion.
ProTmune is being developed by Fate Therapeutics, Inc.
The company is conducting a phase 1/2 trial testing ProTmune for the prevention of acute GVHD and cytomegalovirus infection in adults with hematologic malignancies who are undergoing HSCT.
The trial design consists of an initial 10-subject, phase 1 stage, during which all subjects undergoing allogeneic mPB HSCT will receive ProTmune.
Following an independent data monitoring committee safety review, a 60-subject, randomized, controlled phase 2 stage is expected to begin. In this stage, subjects undergoing allogeneic mPB HSCT will be assigned to receive either ProTmune or a conventional mPB cell graft in a 1:1 ratio.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()

Photo by Chad McNeeley
The US Food and Drug Administration (FDA) has granted fast track designation for ProTmune™, a programmed cellular immunotherapy, to reduce the incidence and severity of acute graft-versus-host disease (GVHD) in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT).
ProTmune is produced by modulating a donor-sourced, mobilized peripheral blood (mPB) graft ex vivo with 2 small molecules (FT1050 and FT4145) to enhance the biological properties and therapeutic function of the graft’s immune cells.
The programmed mPB graft is adoptively transferred and administered to a patient as a one-time intravenous infusion.
ProTmune is being developed by Fate Therapeutics, Inc.
The company is conducting a phase 1/2 trial testing ProTmune for the prevention of acute GVHD and cytomegalovirus infection in adults with hematologic malignancies who are undergoing HSCT.
The trial design consists of an initial 10-subject, phase 1 stage, during which all subjects undergoing allogeneic mPB HSCT will receive ProTmune.
Following an independent data monitoring committee safety review, a 60-subject, randomized, controlled phase 2 stage is expected to begin. In this stage, subjects undergoing allogeneic mPB HSCT will be assigned to receive either ProTmune or a conventional mPB cell graft in a 1:1 ratio.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()

Photo by Chad McNeeley
The US Food and Drug Administration (FDA) has granted fast track designation for ProTmune™, a programmed cellular immunotherapy, to reduce the incidence and severity of acute graft-versus-host disease (GVHD) in patients undergoing allogeneic hematopoietic stem cell transplant (HSCT).
ProTmune is produced by modulating a donor-sourced, mobilized peripheral blood (mPB) graft ex vivo with 2 small molecules (FT1050 and FT4145) to enhance the biological properties and therapeutic function of the graft’s immune cells.
The programmed mPB graft is adoptively transferred and administered to a patient as a one-time intravenous infusion.
ProTmune is being developed by Fate Therapeutics, Inc.
The company is conducting a phase 1/2 trial testing ProTmune for the prevention of acute GVHD and cytomegalovirus infection in adults with hematologic malignancies who are undergoing HSCT.
The trial design consists of an initial 10-subject, phase 1 stage, during which all subjects undergoing allogeneic mPB HSCT will receive ProTmune.
Following an independent data monitoring committee safety review, a 60-subject, randomized, controlled phase 2 stage is expected to begin. In this stage, subjects undergoing allogeneic mPB HSCT will be assigned to receive either ProTmune or a conventional mPB cell graft in a 1:1 ratio.
About fast track designation
The FDA’s fast track program is designed to facilitate and expedite the development and review of new drugs intended to treat serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the drug may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings with the FDA to discuss the drug’s development plan and ensure collection of the appropriate data needed to support drug approval. And the designation allows for more frequent written communication from the FDA about things such as the design of proposed clinical trials and the use of biomarkers. ![]()
A new approach to treat AML?

Preclinical research suggests that activating the STING pathway may be a feasible approach for treating acute myeloid leukemia (AML).
The STING protein has been shown to play a crucial role in the immune system’s ability to “sense” cancer by recognizing and responding to DNA from tumor cells.
In past studies, researchers injected compounds that activate the STING pathway directly into solid tumors in mice, and this produced potent anti-tumor immune responses.
In a new study, researchers injected substances that mimic tumor-cell DNA into the bloodstream and found they could stimulate STING to provoke a life-extending immune response in mice with AML.
“Delivery of these substances into the blood led to massive immune responses,” said study author Justin Kline, MD, of the University of Chicago in Illinois.
“I’ve worked extensively with animal models of this disease, and have never seen responses like this.”
This research, published in Cell Reports, is the first demonstration that activating the STING pathway could be effective in hematologic malignancies.
STING (short for STimulator of INterferon Genes) plays a role in detecting threats, such as viral infections or cancer. STING is activated when DNA turns up in the wrong place, inside the cell but outside the nucleus.
When it encounters such misplaced DNA, STING induces the production of interferon-beta and other chemical signals that recruit certain components of the immune system to manage the threat, such as leukemia-specific killer T cells.
In this study, the researchers found that mice with established AML were rarely able to launch an effective immune response against the disease.
But when the team exposed the mice to DMXAA (5,6-dimethylxanthenone-4-acetic acid), a molecule that activates STING, the immune system responded aggressively, culminating in the activation of highly potent, cancer-cell killing T cells.
This response prolonged survival and, in some cases, cured the mice of their leukemia. About 60% of DMXAA-treated mice survived long-term. They were even able to protect themselves when “re-challenged” with AML cells.
Because of significant differences between mice and humans, DMXAA does not activate the human STING pathway, but researchers have found that several cyclic dinucleotides—signaling molecules produced by bacteria—have a comparable effect in stimulating the STING pathway.
This leads to an immune response that begins with the production of type I interferons and proceeds to later, more powerful stages, ultimately including leukemia-specific T cells.
“Our results provide strong rationale for the clinical translation of STING agonists as immune therapy for leukemia and possibly other hematologic
malignancies,” said study author Emily Curran, MD, of the University of Chicago.
However, Dr Kline noted that this approach is “not without risk.” He said it can induce “a lot of inflammation, fever, even shock.” Such a stimulated immune system can be “too effective,” especially when the therapy is given through the blood stream, rather than injected into a solid tumor.
“I think drug makers will want to focus on intra-tumoral injection studies before they are ready to bet on systemic infusion,” Dr Kline said. “But this is an important first step.” ![]()

Preclinical research suggests that activating the STING pathway may be a feasible approach for treating acute myeloid leukemia (AML).
The STING protein has been shown to play a crucial role in the immune system’s ability to “sense” cancer by recognizing and responding to DNA from tumor cells.
In past studies, researchers injected compounds that activate the STING pathway directly into solid tumors in mice, and this produced potent anti-tumor immune responses.
In a new study, researchers injected substances that mimic tumor-cell DNA into the bloodstream and found they could stimulate STING to provoke a life-extending immune response in mice with AML.
“Delivery of these substances into the blood led to massive immune responses,” said study author Justin Kline, MD, of the University of Chicago in Illinois.
“I’ve worked extensively with animal models of this disease, and have never seen responses like this.”
This research, published in Cell Reports, is the first demonstration that activating the STING pathway could be effective in hematologic malignancies.
STING (short for STimulator of INterferon Genes) plays a role in detecting threats, such as viral infections or cancer. STING is activated when DNA turns up in the wrong place, inside the cell but outside the nucleus.
When it encounters such misplaced DNA, STING induces the production of interferon-beta and other chemical signals that recruit certain components of the immune system to manage the threat, such as leukemia-specific killer T cells.
In this study, the researchers found that mice with established AML were rarely able to launch an effective immune response against the disease.
But when the team exposed the mice to DMXAA (5,6-dimethylxanthenone-4-acetic acid), a molecule that activates STING, the immune system responded aggressively, culminating in the activation of highly potent, cancer-cell killing T cells.
This response prolonged survival and, in some cases, cured the mice of their leukemia. About 60% of DMXAA-treated mice survived long-term. They were even able to protect themselves when “re-challenged” with AML cells.
Because of significant differences between mice and humans, DMXAA does not activate the human STING pathway, but researchers have found that several cyclic dinucleotides—signaling molecules produced by bacteria—have a comparable effect in stimulating the STING pathway.
This leads to an immune response that begins with the production of type I interferons and proceeds to later, more powerful stages, ultimately including leukemia-specific T cells.
“Our results provide strong rationale for the clinical translation of STING agonists as immune therapy for leukemia and possibly other hematologic
malignancies,” said study author Emily Curran, MD, of the University of Chicago.
However, Dr Kline noted that this approach is “not without risk.” He said it can induce “a lot of inflammation, fever, even shock.” Such a stimulated immune system can be “too effective,” especially when the therapy is given through the blood stream, rather than injected into a solid tumor.
“I think drug makers will want to focus on intra-tumoral injection studies before they are ready to bet on systemic infusion,” Dr Kline said. “But this is an important first step.” ![]()

Preclinical research suggests that activating the STING pathway may be a feasible approach for treating acute myeloid leukemia (AML).
The STING protein has been shown to play a crucial role in the immune system’s ability to “sense” cancer by recognizing and responding to DNA from tumor cells.
In past studies, researchers injected compounds that activate the STING pathway directly into solid tumors in mice, and this produced potent anti-tumor immune responses.
In a new study, researchers injected substances that mimic tumor-cell DNA into the bloodstream and found they could stimulate STING to provoke a life-extending immune response in mice with AML.
“Delivery of these substances into the blood led to massive immune responses,” said study author Justin Kline, MD, of the University of Chicago in Illinois.
“I’ve worked extensively with animal models of this disease, and have never seen responses like this.”
This research, published in Cell Reports, is the first demonstration that activating the STING pathway could be effective in hematologic malignancies.
STING (short for STimulator of INterferon Genes) plays a role in detecting threats, such as viral infections or cancer. STING is activated when DNA turns up in the wrong place, inside the cell but outside the nucleus.
When it encounters such misplaced DNA, STING induces the production of interferon-beta and other chemical signals that recruit certain components of the immune system to manage the threat, such as leukemia-specific killer T cells.
In this study, the researchers found that mice with established AML were rarely able to launch an effective immune response against the disease.
But when the team exposed the mice to DMXAA (5,6-dimethylxanthenone-4-acetic acid), a molecule that activates STING, the immune system responded aggressively, culminating in the activation of highly potent, cancer-cell killing T cells.
This response prolonged survival and, in some cases, cured the mice of their leukemia. About 60% of DMXAA-treated mice survived long-term. They were even able to protect themselves when “re-challenged” with AML cells.
Because of significant differences between mice and humans, DMXAA does not activate the human STING pathway, but researchers have found that several cyclic dinucleotides—signaling molecules produced by bacteria—have a comparable effect in stimulating the STING pathway.
This leads to an immune response that begins with the production of type I interferons and proceeds to later, more powerful stages, ultimately including leukemia-specific T cells.
“Our results provide strong rationale for the clinical translation of STING agonists as immune therapy for leukemia and possibly other hematologic
malignancies,” said study author Emily Curran, MD, of the University of Chicago.
However, Dr Kline noted that this approach is “not without risk.” He said it can induce “a lot of inflammation, fever, even shock.” Such a stimulated immune system can be “too effective,” especially when the therapy is given through the blood stream, rather than injected into a solid tumor.
“I think drug makers will want to focus on intra-tumoral injection studies before they are ready to bet on systemic infusion,” Dr Kline said. “But this is an important first step.” ![]()
Visiting Professor in Hospital Medicine
Hospital medicine is an emerging specialty comprised predominantly of early‐career faculty, often less than 5 years postresidency and predominately at instructor or assistant professor level.[1] Effective mentoring has been identified as a critical component of academic success.[2, 3] Published data suggest that most academic hospitalists do not have a mentor, and when they do, the majority of them spend less than 4 hours per year with their mentor.[2] The reasons for this are multifactorial but largely result from the lack of structure, opportunities, and local senior academic hospitalists.[1, 4] Early‐career faculty have difficulty establishing external mentoring relationships, and new models beyond the traditional intrainstitutional dyad are needed.[3, 4] The need for mentors and structured mentorship networks may be particularly high in hospital medicine.[5]
The Visiting Professorship in Hospital Medicine Program was designed to promote cross‐institutional mentorship, share hospitalist innovations, and facilitate academic collaboration between hospitalist groups. We describe the design and early experience with this program across 5 academic hospital medicine programs.
PROGRAM DESIGN
Objectives
The program was designed to promote mentoring relationships between early‐career hospitalist faculty and a visiting professor from another academic hospital medicine group. The program sought to provide immediate career advice during the visits, but also create opportunities for long‐term mentorship and collaboration between institutions. Goals for each visiting professorship included: (1) follow‐up contact between early‐career faculty and visiting professor in the 6 months following the visit, (2) long‐term mentoring relationship with at least 1 early‐career faculty at the visited institution, and (3) identification of opportunities for interinstitutional collaboration to disseminate innovations.
Selection of Sites and Faculty
The first 2 academic medical centers (AMCs) for the visiting professorship exchange designed the program (University of Colorado and University of New Mexico). In subsequent years, each participating AMC was able to solicit additional sites for faculty exchange. This model can expand without requiring ongoing central direction. No criteria were set for selection of AMCs. Visiting professors in hospital medicine were explicitly targeted to be at midcareer stage of late assistant professor or early associate professor and within 1 to 2 years of promotion. It was felt that this group would gain the maximal career benefit from delivering an invited visit to an external AMC, yet have a sufficient track record to deliver effective mentoring advice to early‐career hospitalists. The hospitalist group sending the visiting professor would propose a few candidates, with the innovations they would be able to present, and the hosting site would select 1 for the visit. Early‐career faculty at the hosting institution were generally instructor to early assistant professors.
Visit Itinerary
The visit itinerary was set up as follows:
- Visiting professor delivers a formal 1‐hour presentation to hospitalist faculty, describing an innovation in clinical care, quality improvement, patient safety, or education.
- Individual meetings with 3 to 5 early‐career hospitalists to review faculty portfolios and provide career advice.
- Group lunch between the visiting professor and faculty with similar interests to promote cross‐institutional networking and spark potential collaborations.
- Meeting with hospital medicine program leadership.
- Visiting professor receives exposure to an innovation developed at the hosting institution.
- Dinner with the hosting faculty including the senior hospitalist coordinating the visit.
In advance of the visit, both early‐career faculty and visiting professors receive written materials describing the program, its objectives, and tips to prepare for the visit (see Supporting Information in the online version of this article). The curricula vitae of early‐career faculty at the hosting institution were provided to the visiting professor. Visit costs were covered by the visiting professor's institution. Honoraria were not offered.
Program Evaluation
Within a month of each visit, a paper survey was administered to the visiting professor and the faculty with whom she/he met. In addition to demographic data including gender, self‐reported minority status, academic rank, years at rank, and total years in academic medicine, the survey asked faculty to rate on a 5‐point Likert scale their assessment of the usefulness of the visit to accomplish the 4 core goals of the program: (1) cross‐institutional dissemination of innovations in clinical medicine, education, or research; (2) advancing the respondent's academic career; (3) fostering cross‐institutional mentor‐mentee relationships; and (4) promoting cross‐institutional collaborations. Free‐text responses for overall impression of program and suggestions for improvement were solicited.
At the time of this writing, 1 year has passed from the initial visits for the first 3 visiting professorships. A 1‐year follow‐up survey was administered assessing (1) total number of contacts with the visiting professor in the year following the visit, (2) whether a letter of recommendation resulted from the visit, (3) whether the respondent had seen evidence of spread of innovative ideas as a result of the program, (4) participation in a cross‐institutional collaboration as a result of the program, and (5) assessment of benefit in continuing the program in the next year. The respondents were also asked to rate the global utility of the program to their professional development on a 5‐point scale ranging from not at all useful to very useful (Thinking about what has happened to you since the visit a year ago, please rate the usefulness of the entire program to your professional life: overall usefulness for my professional development.). Domain‐specific utility in improving clinical, research, quality improvement, and administrative skills were also elicited (results not shown). Finally, suggestions to improve the program for the future were solicited. The Colorado Multiple Institutional Review Board determined that the study of this faculty development program did not qualify as human subjects research, and subjects were therefore not asked to provide informed consent for participation.
RESULTS
To date, 5 academic medical centers have participated in the visiting professorship program, with 7 visiting professors interacting with 29 early‐career faculty. Of the 29 early‐career faculty, 72% (21/29) were at the rank of assistant professor, 17% (5/29) instructor, 7% (2/29) residents with plans to hire, and 3% (1/29) associate professor. The median was 2 years in academic medicine and 1 year at current academic rank. Forty‐one percent (12/29) were women and 7% (2/29) identified as ethnic minority. Of the 7 visiting professors, 57% (4/7) were assistant professor and 43% (3/7) were associate professors. The median was 5 years in academic medicine, 29% (2/7) were women, and none identified as ethnic minority.
Immediate postvisit survey response was obtained for all participating faculty. In the immediate postvisit survey, on a 5‐point Likert scale, the 29 early‐career faculty rated the visit: 4.4 for promoting cross‐institutional dissemination of innovations, 4.2 for advancing my academic career, 4.2 for fostering cross‐institutional mentor‐mentee relationships, and 4.4 for promoting cross‐institutional collaborations. Ninety‐three percent (26/28 accounting for 1 nonresponse to this question) reported the visiting professorship had high potential to disseminate innovation (rated greater than 3 on the 5‐point Likert score). Eighty‐three percent (24/29) of the early‐career faculty rated the visit highly useful in advancing their career, 76% (22/29) responded that the visit was highly likely to foster external mentorship relationships, and 90% (26/29) reported the visit highly effective in promoting cross‐institutional collaborations. In the immediate postvisit survey, the 7 visiting professors rated the visit 4.9 for promoting cross‐institutional dissemination of innovations, 4.3 for advancing my academic career, 4.0 for fostering cross‐institutional mentor‐mentee relationships, and 4.3 for promoting cross‐institutional collaborations.
Free‐text comments from both visiting professors and early‐career faculty were generally favorable (Table 1). Some comments offered constructive input on appropriate matching of faculty, previsit preparation, or desire for more time in sessions (Table 1).
| Visiting Professors (n = 7) | Early‐Career Faculty (n = 29) |
|---|---|
| I was very impressed with the degree of organization, preparation, and structure from [host institution]. The project is a great concept and may well lead to similar and even more developed ones in the future. It is very helpful to get the pulse on another program and to hear of some of the same struggles and successes of another hospitalist program. The potential for cross‐site mentor‐mentee relationships and collaborations is a win‐win for both programs. | I really enjoyed my individual meeting with [visiting professor]. She was helpful in reviewing current projects from another perspective and very helpful in making suggestions for future projects. Also enjoyed her Grand Rounds and plan to follow‐up on this issue for possible cross‐institutional collaboration. |
| Overall, this exchange is a great program. It is fun, promotes idea exchange, and is immensely helpful to the visiting professor for promotion. Every meeting I had with faculty at [host institution] was interesting and worthwhile. The primary challenge is maintaining mentorship ties and momentum after the visit. I personally e‐mailed every person I met and received many responses, including several explicit requests for ongoing advising and collaboration. | I think this is a great program. It definitely gives us the opportunity to meet people outside of the [host institution] community and foster relationships, mentorship, and possible collaborations with projects and programs. |
| I liked multidisciplinary rounding. Research club. Meeting with faculty and trying to find common areas of interest. | I think this is a fantastic program so far. [Visiting professor] was very energetic and interested in making the most of the day. She contacted me after the visit and offered to keep in touch in the future. Right now I can see the program as being most useful in establishing new mentor/mentee relationships. |
| Most of the faculty I met with see value in being involved in systems/quality improvement, but most do not express interest in specific projects. Areas needing improvement were identified by everyone I met with so developing projects around these areas should be doable. They might benefit from access to mentoring in quality improvement. | It was fantastic to meet with [visiting professor] and get a sense for his work and also brainstorm about how we might do similar work here in the future (eg, in high‐value care). It was also great to then see him 2 days later at [national conference]. I feel this is a great program to improve our connections cross‐institutionally and hopefully to spark some future collaborations. |
| Very worthwhile. Was really helpful to meet with various faculty and leadership to see similarities and differences between our institutions. Generated several ideas for collaborative activities already. Also really helpful to have a somewhat structured way to share my work at an outside institution, as well as to create opportunities for mentor‐mentee relationships outside my home institution. | Incredibly valuable to promote this kind of cross‐pollination for both collaboration and innovation. |
| Wonderful, inspiring, professionally advantageous. | |
| Good idea. Good way to help midcareer faculty with advancement. Offers promise for collaboration of research/workshops. | |
| Suggestions for Improvement | |
| Please have e‐mails of the folks we meet available immediately after the visit. It is hard to know if anyone felt enough of a connection to want mentorship from me. | I feel like I may be a bit early on to benefit as much as I could have. |
| Develop a mentorship program for quality improvement. As part of this exchange, consider treating visits as similar to a consultation. Have visitor with specific focus that they can offer help with. | Nice to have personal access to accomplished faculty from other institutions. Their perspective and career trajectory don't always align due to differences in institution culture, specifics of promotion process, and so on, but still a useful experience. |
| Share any possible more‐formal topics for discussion with leadership prior to the visit so can prepare ahead of time (eg, gather information they may have questions on). Otherwise it was great! | For early career faculty, more discussions prior in regard to what to expect. |
| A question is who should continue to push? Is it the prospective mentee, the mentee's institution, an so on? | Great idea. Would have loved to be involved in more aspects. More time for discussion would have been good. Did not get to discuss collaboration in person. |
| Great to get to talk to someone from totally different system. Wish we had more time to talk. | |
One‐year follow‐up was obtained for all but 1 early‐career faculty member receiving the follow‐up survey, and all 3 visiting professors. Of the 3 visiting professorships that occurred more than 1 year ago, 16 mentorship contacts occurred in total (phone, e‐mail, or in person) between 13 early‐career faculty and 3 visiting professors in the year after the initial visits (range, 04 contacts). Follow‐up contact occurred for 3 of 4 early‐career faculty from the first visiting professorship, 3 of 5 from the second visiting professorship, and 2 of 4 from the third visiting professorship. One early‐career faculty member from each host academic medical center had 3 or more additional contacts with the visiting professor in the year following the initial visit. Overall, 8/13 (62%) of early‐career faculty had at least 1 follow‐up mentoring discussion. On 1‐year follow‐up, overall utility for professional development was rated an average of 3.5 by early‐career faculty (with a trend of higher ratings of efficacy with increasing number of follow‐up contacts) and 4.7 by visiting professors. Half (8/16) of the involved faculty report having seen evidence of cross‐institutional dissemination of innovation. Ninety‐four percent (15/16) of participants at 1‐year follow‐up felt there was benefit to their institution in continuing the program for the next year.
Objective evidence of cross‐institutional scholarship, assessed by email query of both visiting professors and senior hospitalists coordinating the visits, includes 2 collaborative peer reviewed publications including mentors and mentees participating in the visiting professorship.[6, 7] Joint educational curriculum development on high‐value care between sites is planned. The Visiting Professorship in Hospital Medicine Program has resulted in 1 external letter to support a visiting professor's promotion to date.
DISCUSSION
Hospital Medicine is a young, rapidly growing field, hence the number of experienced academic hospitalist mentors with expertise in successfully navigating an academic career is limited. A national study of hospitalist leaders found that 75% of clinician‐educators and 58% of research faculty feel that lack of mentorship is a major issue.[1] Mentorship for hospitalist clinician‐investigators is often delivered by nonhospitalists.[2, 8] There is little evidence of external mentorship for academic clinician‐educators in hospital medicine.[1] Without explicit programmatic support, many faculty may find this to be a barrier to career advancement. A study of successfully promoted hospitalists identified difficulty identifying external senior hospitalists to write letters in support of promotion as an obstacle.[9] Our study of the Visiting Professorship in Hospital Medicine Program found that early‐career faculty rated the visit as useful in advancing their career and fostered external mentorship relationships. Subsequent experience suggests more than half of the early‐career faculty will maintain contact with the visiting professor over the year following the visit. Visiting professors rate the experience particularly highly in their own career advancement.
The hospitalist movement is built on a foundation of innovation. The focus of each presentation was on an innovation developed by the visiting professor, and each visit showcased an innovation of the visited institution. This is distinct from traditional Hospital Grand Rounds, which more often focus on basic science research or clinical pathophysiology/disease management based on subspecialty topics.[10] The Visiting Professorship in Hospital Medicine Program was judged by participants to be an effective means of spreading innovation.
Insights from experience with the Visiting Professorship in Hospital Medicine Program include the importance of preliminary work prior to each visit. Program directors need to attend closely to the fit between the interests and career path of the visiting professor and those of the early‐career faculty. The innovations being shared should be aligned with organizational interests to maximize the chance of subsequent spread of the innovation and future collaboration. Providing faculty information about the objectives of the program in advance of the visit and arranging an exchange of curricula vitae between the early‐career faculty and the visiting professor allows participants to prepare for the in‐person coaching. Based on comments from participants, prompting contact from the visiting professor after the visit may be helpful to initiate the longitudinal relationship. We also found that early‐career faculty may not be aware of how to effectively use a mentoring relationship with an external faculty member. Training sessions for both mentors and mentees on effective mentorship relationships before visiting professorships might improve early‐career faculty confidence in initiating relationships and maximize value from mentor coaching.
A key issue is finding the right level of career maturity for the visiting professor. Our approach in selecting visiting professors was congruent with utilization of midcareer peer coaches employed by intrainstitutional hospital medicine mentoring programs.[11] The visiting professor should have sufficient experience and accomplishments to be able to effectively counsel junior faculty. However, it is important that the visiting professor also has sufficient time and interest to take on additional mentees and to be a full participant in shared scholarship projects emerging from the experience.
This study represents the experience of 5 mature academic hospitalist groups, and results may not be generalizable to dissimilar institutions or if only the most senior faculty are selected to perform visits. There is an inherent selection bias in the choice of both visiting professor and early‐career faculty. The small sample size of the faculty exposed to this program is a limitation to generalizability of the results of this evaluation. Whether this program will result in greater success in promotion of academic hospitalists cannot be assessed based on the follow‐up available. The Visiting Professorship in Hospital Medicine Program has continued to be sustained with an additional academic medical center enrolled and 2 additional site visits planned. The costs of the program are low, largely air travel and a night of lodging, as well as nominal administrative logistical support. Perceived benefits by participants and academic medical centers make this modest investment worth considering for academic hospitalist groups.
CONCLUSIONS
The Visiting Professorship in Hospital Medicine Program offers structure, opportunities, and access to senior mentors to advance the development of early‐career hospitalists while spreading innovation to distant sites. It is assessed by participants to facilitate external mentoring relationships and has the potential to advance the careers of both early‐career faculty as well as the visiting professors.
Disclosure
Nothing to report.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5–9.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , . Mentoring faculty in academic medicine: a new paradigm? J Gen Intern Med. 2005;20(9):866–870.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4:240–246.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6:1–2.
- , , , . Procedural skills for hospitalists. Hosp Med Clin. 2016;5:114–136.
- , , . Cedecea davisae' s role in a polymicrobial lung infection in a cystic fibrosis patient. Case reports in infectious diseases. Case Rep Infect Dis. 2012;2012:176864.
- , , , . Innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314–318.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6:411–415.
- , , , , . A case study of medical grand rounds: are we using effective methods? Acad Med. 2009;84(8):1144–1151.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6:161–166.
Hospital medicine is an emerging specialty comprised predominantly of early‐career faculty, often less than 5 years postresidency and predominately at instructor or assistant professor level.[1] Effective mentoring has been identified as a critical component of academic success.[2, 3] Published data suggest that most academic hospitalists do not have a mentor, and when they do, the majority of them spend less than 4 hours per year with their mentor.[2] The reasons for this are multifactorial but largely result from the lack of structure, opportunities, and local senior academic hospitalists.[1, 4] Early‐career faculty have difficulty establishing external mentoring relationships, and new models beyond the traditional intrainstitutional dyad are needed.[3, 4] The need for mentors and structured mentorship networks may be particularly high in hospital medicine.[5]
The Visiting Professorship in Hospital Medicine Program was designed to promote cross‐institutional mentorship, share hospitalist innovations, and facilitate academic collaboration between hospitalist groups. We describe the design and early experience with this program across 5 academic hospital medicine programs.
PROGRAM DESIGN
Objectives
The program was designed to promote mentoring relationships between early‐career hospitalist faculty and a visiting professor from another academic hospital medicine group. The program sought to provide immediate career advice during the visits, but also create opportunities for long‐term mentorship and collaboration between institutions. Goals for each visiting professorship included: (1) follow‐up contact between early‐career faculty and visiting professor in the 6 months following the visit, (2) long‐term mentoring relationship with at least 1 early‐career faculty at the visited institution, and (3) identification of opportunities for interinstitutional collaboration to disseminate innovations.
Selection of Sites and Faculty
The first 2 academic medical centers (AMCs) for the visiting professorship exchange designed the program (University of Colorado and University of New Mexico). In subsequent years, each participating AMC was able to solicit additional sites for faculty exchange. This model can expand without requiring ongoing central direction. No criteria were set for selection of AMCs. Visiting professors in hospital medicine were explicitly targeted to be at midcareer stage of late assistant professor or early associate professor and within 1 to 2 years of promotion. It was felt that this group would gain the maximal career benefit from delivering an invited visit to an external AMC, yet have a sufficient track record to deliver effective mentoring advice to early‐career hospitalists. The hospitalist group sending the visiting professor would propose a few candidates, with the innovations they would be able to present, and the hosting site would select 1 for the visit. Early‐career faculty at the hosting institution were generally instructor to early assistant professors.
Visit Itinerary
The visit itinerary was set up as follows:
- Visiting professor delivers a formal 1‐hour presentation to hospitalist faculty, describing an innovation in clinical care, quality improvement, patient safety, or education.
- Individual meetings with 3 to 5 early‐career hospitalists to review faculty portfolios and provide career advice.
- Group lunch between the visiting professor and faculty with similar interests to promote cross‐institutional networking and spark potential collaborations.
- Meeting with hospital medicine program leadership.
- Visiting professor receives exposure to an innovation developed at the hosting institution.
- Dinner with the hosting faculty including the senior hospitalist coordinating the visit.
In advance of the visit, both early‐career faculty and visiting professors receive written materials describing the program, its objectives, and tips to prepare for the visit (see Supporting Information in the online version of this article). The curricula vitae of early‐career faculty at the hosting institution were provided to the visiting professor. Visit costs were covered by the visiting professor's institution. Honoraria were not offered.
Program Evaluation
Within a month of each visit, a paper survey was administered to the visiting professor and the faculty with whom she/he met. In addition to demographic data including gender, self‐reported minority status, academic rank, years at rank, and total years in academic medicine, the survey asked faculty to rate on a 5‐point Likert scale their assessment of the usefulness of the visit to accomplish the 4 core goals of the program: (1) cross‐institutional dissemination of innovations in clinical medicine, education, or research; (2) advancing the respondent's academic career; (3) fostering cross‐institutional mentor‐mentee relationships; and (4) promoting cross‐institutional collaborations. Free‐text responses for overall impression of program and suggestions for improvement were solicited.
At the time of this writing, 1 year has passed from the initial visits for the first 3 visiting professorships. A 1‐year follow‐up survey was administered assessing (1) total number of contacts with the visiting professor in the year following the visit, (2) whether a letter of recommendation resulted from the visit, (3) whether the respondent had seen evidence of spread of innovative ideas as a result of the program, (4) participation in a cross‐institutional collaboration as a result of the program, and (5) assessment of benefit in continuing the program in the next year. The respondents were also asked to rate the global utility of the program to their professional development on a 5‐point scale ranging from not at all useful to very useful (Thinking about what has happened to you since the visit a year ago, please rate the usefulness of the entire program to your professional life: overall usefulness for my professional development.). Domain‐specific utility in improving clinical, research, quality improvement, and administrative skills were also elicited (results not shown). Finally, suggestions to improve the program for the future were solicited. The Colorado Multiple Institutional Review Board determined that the study of this faculty development program did not qualify as human subjects research, and subjects were therefore not asked to provide informed consent for participation.
RESULTS
To date, 5 academic medical centers have participated in the visiting professorship program, with 7 visiting professors interacting with 29 early‐career faculty. Of the 29 early‐career faculty, 72% (21/29) were at the rank of assistant professor, 17% (5/29) instructor, 7% (2/29) residents with plans to hire, and 3% (1/29) associate professor. The median was 2 years in academic medicine and 1 year at current academic rank. Forty‐one percent (12/29) were women and 7% (2/29) identified as ethnic minority. Of the 7 visiting professors, 57% (4/7) were assistant professor and 43% (3/7) were associate professors. The median was 5 years in academic medicine, 29% (2/7) were women, and none identified as ethnic minority.
Immediate postvisit survey response was obtained for all participating faculty. In the immediate postvisit survey, on a 5‐point Likert scale, the 29 early‐career faculty rated the visit: 4.4 for promoting cross‐institutional dissemination of innovations, 4.2 for advancing my academic career, 4.2 for fostering cross‐institutional mentor‐mentee relationships, and 4.4 for promoting cross‐institutional collaborations. Ninety‐three percent (26/28 accounting for 1 nonresponse to this question) reported the visiting professorship had high potential to disseminate innovation (rated greater than 3 on the 5‐point Likert score). Eighty‐three percent (24/29) of the early‐career faculty rated the visit highly useful in advancing their career, 76% (22/29) responded that the visit was highly likely to foster external mentorship relationships, and 90% (26/29) reported the visit highly effective in promoting cross‐institutional collaborations. In the immediate postvisit survey, the 7 visiting professors rated the visit 4.9 for promoting cross‐institutional dissemination of innovations, 4.3 for advancing my academic career, 4.0 for fostering cross‐institutional mentor‐mentee relationships, and 4.3 for promoting cross‐institutional collaborations.
Free‐text comments from both visiting professors and early‐career faculty were generally favorable (Table 1). Some comments offered constructive input on appropriate matching of faculty, previsit preparation, or desire for more time in sessions (Table 1).
| Visiting Professors (n = 7) | Early‐Career Faculty (n = 29) |
|---|---|
| I was very impressed with the degree of organization, preparation, and structure from [host institution]. The project is a great concept and may well lead to similar and even more developed ones in the future. It is very helpful to get the pulse on another program and to hear of some of the same struggles and successes of another hospitalist program. The potential for cross‐site mentor‐mentee relationships and collaborations is a win‐win for both programs. | I really enjoyed my individual meeting with [visiting professor]. She was helpful in reviewing current projects from another perspective and very helpful in making suggestions for future projects. Also enjoyed her Grand Rounds and plan to follow‐up on this issue for possible cross‐institutional collaboration. |
| Overall, this exchange is a great program. It is fun, promotes idea exchange, and is immensely helpful to the visiting professor for promotion. Every meeting I had with faculty at [host institution] was interesting and worthwhile. The primary challenge is maintaining mentorship ties and momentum after the visit. I personally e‐mailed every person I met and received many responses, including several explicit requests for ongoing advising and collaboration. | I think this is a great program. It definitely gives us the opportunity to meet people outside of the [host institution] community and foster relationships, mentorship, and possible collaborations with projects and programs. |
| I liked multidisciplinary rounding. Research club. Meeting with faculty and trying to find common areas of interest. | I think this is a fantastic program so far. [Visiting professor] was very energetic and interested in making the most of the day. She contacted me after the visit and offered to keep in touch in the future. Right now I can see the program as being most useful in establishing new mentor/mentee relationships. |
| Most of the faculty I met with see value in being involved in systems/quality improvement, but most do not express interest in specific projects. Areas needing improvement were identified by everyone I met with so developing projects around these areas should be doable. They might benefit from access to mentoring in quality improvement. | It was fantastic to meet with [visiting professor] and get a sense for his work and also brainstorm about how we might do similar work here in the future (eg, in high‐value care). It was also great to then see him 2 days later at [national conference]. I feel this is a great program to improve our connections cross‐institutionally and hopefully to spark some future collaborations. |
| Very worthwhile. Was really helpful to meet with various faculty and leadership to see similarities and differences between our institutions. Generated several ideas for collaborative activities already. Also really helpful to have a somewhat structured way to share my work at an outside institution, as well as to create opportunities for mentor‐mentee relationships outside my home institution. | Incredibly valuable to promote this kind of cross‐pollination for both collaboration and innovation. |
| Wonderful, inspiring, professionally advantageous. | |
| Good idea. Good way to help midcareer faculty with advancement. Offers promise for collaboration of research/workshops. | |
| Suggestions for Improvement | |
| Please have e‐mails of the folks we meet available immediately after the visit. It is hard to know if anyone felt enough of a connection to want mentorship from me. | I feel like I may be a bit early on to benefit as much as I could have. |
| Develop a mentorship program for quality improvement. As part of this exchange, consider treating visits as similar to a consultation. Have visitor with specific focus that they can offer help with. | Nice to have personal access to accomplished faculty from other institutions. Their perspective and career trajectory don't always align due to differences in institution culture, specifics of promotion process, and so on, but still a useful experience. |
| Share any possible more‐formal topics for discussion with leadership prior to the visit so can prepare ahead of time (eg, gather information they may have questions on). Otherwise it was great! | For early career faculty, more discussions prior in regard to what to expect. |
| A question is who should continue to push? Is it the prospective mentee, the mentee's institution, an so on? | Great idea. Would have loved to be involved in more aspects. More time for discussion would have been good. Did not get to discuss collaboration in person. |
| Great to get to talk to someone from totally different system. Wish we had more time to talk. | |
One‐year follow‐up was obtained for all but 1 early‐career faculty member receiving the follow‐up survey, and all 3 visiting professors. Of the 3 visiting professorships that occurred more than 1 year ago, 16 mentorship contacts occurred in total (phone, e‐mail, or in person) between 13 early‐career faculty and 3 visiting professors in the year after the initial visits (range, 04 contacts). Follow‐up contact occurred for 3 of 4 early‐career faculty from the first visiting professorship, 3 of 5 from the second visiting professorship, and 2 of 4 from the third visiting professorship. One early‐career faculty member from each host academic medical center had 3 or more additional contacts with the visiting professor in the year following the initial visit. Overall, 8/13 (62%) of early‐career faculty had at least 1 follow‐up mentoring discussion. On 1‐year follow‐up, overall utility for professional development was rated an average of 3.5 by early‐career faculty (with a trend of higher ratings of efficacy with increasing number of follow‐up contacts) and 4.7 by visiting professors. Half (8/16) of the involved faculty report having seen evidence of cross‐institutional dissemination of innovation. Ninety‐four percent (15/16) of participants at 1‐year follow‐up felt there was benefit to their institution in continuing the program for the next year.
Objective evidence of cross‐institutional scholarship, assessed by email query of both visiting professors and senior hospitalists coordinating the visits, includes 2 collaborative peer reviewed publications including mentors and mentees participating in the visiting professorship.[6, 7] Joint educational curriculum development on high‐value care between sites is planned. The Visiting Professorship in Hospital Medicine Program has resulted in 1 external letter to support a visiting professor's promotion to date.

DISCUSSION
Hospital Medicine is a young, rapidly growing field, hence the number of experienced academic hospitalist mentors with expertise in successfully navigating an academic career is limited. A national study of hospitalist leaders found that 75% of clinician‐educators and 58% of research faculty feel that lack of mentorship is a major issue.[1] Mentorship for hospitalist clinician‐investigators is often delivered by nonhospitalists.[2, 8] There is little evidence of external mentorship for academic clinician‐educators in hospital medicine.[1] Without explicit programmatic support, many faculty may find this to be a barrier to career advancement. A study of successfully promoted hospitalists identified difficulty identifying external senior hospitalists to write letters in support of promotion as an obstacle.[9] Our study of the Visiting Professorship in Hospital Medicine Program found that early‐career faculty rated the visit as useful in advancing their career and fostered external mentorship relationships. Subsequent experience suggests more than half of the early‐career faculty will maintain contact with the visiting professor over the year following the visit. Visiting professors rate the experience particularly highly in their own career advancement.
The hospitalist movement is built on a foundation of innovation. The focus of each presentation was on an innovation developed by the visiting professor, and each visit showcased an innovation of the visited institution. This is distinct from traditional Hospital Grand Rounds, which more often focus on basic science research or clinical pathophysiology/disease management based on subspecialty topics.[10] The Visiting Professorship in Hospital Medicine Program was judged by participants to be an effective means of spreading innovation.
Insights from experience with the Visiting Professorship in Hospital Medicine Program include the importance of preliminary work prior to each visit. Program directors need to attend closely to the fit between the interests and career path of the visiting professor and those of the early‐career faculty. The innovations being shared should be aligned with organizational interests to maximize the chance of subsequent spread of the innovation and future collaboration. Providing faculty information about the objectives of the program in advance of the visit and arranging an exchange of curricula vitae between the early‐career faculty and the visiting professor allows participants to prepare for the in‐person coaching. Based on comments from participants, prompting contact from the visiting professor after the visit may be helpful to initiate the longitudinal relationship. We also found that early‐career faculty may not be aware of how to effectively use a mentoring relationship with an external faculty member. Training sessions for both mentors and mentees on effective mentorship relationships before visiting professorships might improve early‐career faculty confidence in initiating relationships and maximize value from mentor coaching.
A key issue is finding the right level of career maturity for the visiting professor. Our approach in selecting visiting professors was congruent with utilization of midcareer peer coaches employed by intrainstitutional hospital medicine mentoring programs.[11] The visiting professor should have sufficient experience and accomplishments to be able to effectively counsel junior faculty. However, it is important that the visiting professor also has sufficient time and interest to take on additional mentees and to be a full participant in shared scholarship projects emerging from the experience.
This study represents the experience of 5 mature academic hospitalist groups, and results may not be generalizable to dissimilar institutions or if only the most senior faculty are selected to perform visits. There is an inherent selection bias in the choice of both visiting professor and early‐career faculty. The small sample size of the faculty exposed to this program is a limitation to generalizability of the results of this evaluation. Whether this program will result in greater success in promotion of academic hospitalists cannot be assessed based on the follow‐up available. The Visiting Professorship in Hospital Medicine Program has continued to be sustained with an additional academic medical center enrolled and 2 additional site visits planned. The costs of the program are low, largely air travel and a night of lodging, as well as nominal administrative logistical support. Perceived benefits by participants and academic medical centers make this modest investment worth considering for academic hospitalist groups.
CONCLUSIONS
The Visiting Professorship in Hospital Medicine Program offers structure, opportunities, and access to senior mentors to advance the development of early‐career hospitalists while spreading innovation to distant sites. It is assessed by participants to facilitate external mentoring relationships and has the potential to advance the careers of both early‐career faculty as well as the visiting professors.
Disclosure
Nothing to report.
Hospital medicine is an emerging specialty comprised predominantly of early‐career faculty, often less than 5 years postresidency and predominately at instructor or assistant professor level.[1] Effective mentoring has been identified as a critical component of academic success.[2, 3] Published data suggest that most academic hospitalists do not have a mentor, and when they do, the majority of them spend less than 4 hours per year with their mentor.[2] The reasons for this are multifactorial but largely result from the lack of structure, opportunities, and local senior academic hospitalists.[1, 4] Early‐career faculty have difficulty establishing external mentoring relationships, and new models beyond the traditional intrainstitutional dyad are needed.[3, 4] The need for mentors and structured mentorship networks may be particularly high in hospital medicine.[5]
The Visiting Professorship in Hospital Medicine Program was designed to promote cross‐institutional mentorship, share hospitalist innovations, and facilitate academic collaboration between hospitalist groups. We describe the design and early experience with this program across 5 academic hospital medicine programs.
PROGRAM DESIGN
Objectives
The program was designed to promote mentoring relationships between early‐career hospitalist faculty and a visiting professor from another academic hospital medicine group. The program sought to provide immediate career advice during the visits, but also create opportunities for long‐term mentorship and collaboration between institutions. Goals for each visiting professorship included: (1) follow‐up contact between early‐career faculty and visiting professor in the 6 months following the visit, (2) long‐term mentoring relationship with at least 1 early‐career faculty at the visited institution, and (3) identification of opportunities for interinstitutional collaboration to disseminate innovations.
Selection of Sites and Faculty
The first 2 academic medical centers (AMCs) for the visiting professorship exchange designed the program (University of Colorado and University of New Mexico). In subsequent years, each participating AMC was able to solicit additional sites for faculty exchange. This model can expand without requiring ongoing central direction. No criteria were set for selection of AMCs. Visiting professors in hospital medicine were explicitly targeted to be at midcareer stage of late assistant professor or early associate professor and within 1 to 2 years of promotion. It was felt that this group would gain the maximal career benefit from delivering an invited visit to an external AMC, yet have a sufficient track record to deliver effective mentoring advice to early‐career hospitalists. The hospitalist group sending the visiting professor would propose a few candidates, with the innovations they would be able to present, and the hosting site would select 1 for the visit. Early‐career faculty at the hosting institution were generally instructor to early assistant professors.
Visit Itinerary
The visit itinerary was set up as follows:
- Visiting professor delivers a formal 1‐hour presentation to hospitalist faculty, describing an innovation in clinical care, quality improvement, patient safety, or education.
- Individual meetings with 3 to 5 early‐career hospitalists to review faculty portfolios and provide career advice.
- Group lunch between the visiting professor and faculty with similar interests to promote cross‐institutional networking and spark potential collaborations.
- Meeting with hospital medicine program leadership.
- Visiting professor receives exposure to an innovation developed at the hosting institution.
- Dinner with the hosting faculty including the senior hospitalist coordinating the visit.
In advance of the visit, both early‐career faculty and visiting professors receive written materials describing the program, its objectives, and tips to prepare for the visit (see Supporting Information in the online version of this article). The curricula vitae of early‐career faculty at the hosting institution were provided to the visiting professor. Visit costs were covered by the visiting professor's institution. Honoraria were not offered.
Program Evaluation
Within a month of each visit, a paper survey was administered to the visiting professor and the faculty with whom she/he met. In addition to demographic data including gender, self‐reported minority status, academic rank, years at rank, and total years in academic medicine, the survey asked faculty to rate on a 5‐point Likert scale their assessment of the usefulness of the visit to accomplish the 4 core goals of the program: (1) cross‐institutional dissemination of innovations in clinical medicine, education, or research; (2) advancing the respondent's academic career; (3) fostering cross‐institutional mentor‐mentee relationships; and (4) promoting cross‐institutional collaborations. Free‐text responses for overall impression of program and suggestions for improvement were solicited.
At the time of this writing, 1 year has passed from the initial visits for the first 3 visiting professorships. A 1‐year follow‐up survey was administered assessing (1) total number of contacts with the visiting professor in the year following the visit, (2) whether a letter of recommendation resulted from the visit, (3) whether the respondent had seen evidence of spread of innovative ideas as a result of the program, (4) participation in a cross‐institutional collaboration as a result of the program, and (5) assessment of benefit in continuing the program in the next year. The respondents were also asked to rate the global utility of the program to their professional development on a 5‐point scale ranging from not at all useful to very useful (Thinking about what has happened to you since the visit a year ago, please rate the usefulness of the entire program to your professional life: overall usefulness for my professional development.). Domain‐specific utility in improving clinical, research, quality improvement, and administrative skills were also elicited (results not shown). Finally, suggestions to improve the program for the future were solicited. The Colorado Multiple Institutional Review Board determined that the study of this faculty development program did not qualify as human subjects research, and subjects were therefore not asked to provide informed consent for participation.
RESULTS
To date, 5 academic medical centers have participated in the visiting professorship program, with 7 visiting professors interacting with 29 early‐career faculty. Of the 29 early‐career faculty, 72% (21/29) were at the rank of assistant professor, 17% (5/29) instructor, 7% (2/29) residents with plans to hire, and 3% (1/29) associate professor. The median was 2 years in academic medicine and 1 year at current academic rank. Forty‐one percent (12/29) were women and 7% (2/29) identified as ethnic minority. Of the 7 visiting professors, 57% (4/7) were assistant professor and 43% (3/7) were associate professors. The median was 5 years in academic medicine, 29% (2/7) were women, and none identified as ethnic minority.
Immediate postvisit survey response was obtained for all participating faculty. In the immediate postvisit survey, on a 5‐point Likert scale, the 29 early‐career faculty rated the visit: 4.4 for promoting cross‐institutional dissemination of innovations, 4.2 for advancing my academic career, 4.2 for fostering cross‐institutional mentor‐mentee relationships, and 4.4 for promoting cross‐institutional collaborations. Ninety‐three percent (26/28 accounting for 1 nonresponse to this question) reported the visiting professorship had high potential to disseminate innovation (rated greater than 3 on the 5‐point Likert score). Eighty‐three percent (24/29) of the early‐career faculty rated the visit highly useful in advancing their career, 76% (22/29) responded that the visit was highly likely to foster external mentorship relationships, and 90% (26/29) reported the visit highly effective in promoting cross‐institutional collaborations. In the immediate postvisit survey, the 7 visiting professors rated the visit 4.9 for promoting cross‐institutional dissemination of innovations, 4.3 for advancing my academic career, 4.0 for fostering cross‐institutional mentor‐mentee relationships, and 4.3 for promoting cross‐institutional collaborations.
Free‐text comments from both visiting professors and early‐career faculty were generally favorable (Table 1). Some comments offered constructive input on appropriate matching of faculty, previsit preparation, or desire for more time in sessions (Table 1).
| Visiting Professors (n = 7) | Early‐Career Faculty (n = 29) |
|---|---|
| I was very impressed with the degree of organization, preparation, and structure from [host institution]. The project is a great concept and may well lead to similar and even more developed ones in the future. It is very helpful to get the pulse on another program and to hear of some of the same struggles and successes of another hospitalist program. The potential for cross‐site mentor‐mentee relationships and collaborations is a win‐win for both programs. | I really enjoyed my individual meeting with [visiting professor]. She was helpful in reviewing current projects from another perspective and very helpful in making suggestions for future projects. Also enjoyed her Grand Rounds and plan to follow‐up on this issue for possible cross‐institutional collaboration. |
| Overall, this exchange is a great program. It is fun, promotes idea exchange, and is immensely helpful to the visiting professor for promotion. Every meeting I had with faculty at [host institution] was interesting and worthwhile. The primary challenge is maintaining mentorship ties and momentum after the visit. I personally e‐mailed every person I met and received many responses, including several explicit requests for ongoing advising and collaboration. | I think this is a great program. It definitely gives us the opportunity to meet people outside of the [host institution] community and foster relationships, mentorship, and possible collaborations with projects and programs. |
| I liked multidisciplinary rounding. Research club. Meeting with faculty and trying to find common areas of interest. | I think this is a fantastic program so far. [Visiting professor] was very energetic and interested in making the most of the day. She contacted me after the visit and offered to keep in touch in the future. Right now I can see the program as being most useful in establishing new mentor/mentee relationships. |
| Most of the faculty I met with see value in being involved in systems/quality improvement, but most do not express interest in specific projects. Areas needing improvement were identified by everyone I met with so developing projects around these areas should be doable. They might benefit from access to mentoring in quality improvement. | It was fantastic to meet with [visiting professor] and get a sense for his work and also brainstorm about how we might do similar work here in the future (eg, in high‐value care). It was also great to then see him 2 days later at [national conference]. I feel this is a great program to improve our connections cross‐institutionally and hopefully to spark some future collaborations. |
| Very worthwhile. Was really helpful to meet with various faculty and leadership to see similarities and differences between our institutions. Generated several ideas for collaborative activities already. Also really helpful to have a somewhat structured way to share my work at an outside institution, as well as to create opportunities for mentor‐mentee relationships outside my home institution. | Incredibly valuable to promote this kind of cross‐pollination for both collaboration and innovation. |
| Wonderful, inspiring, professionally advantageous. | |
| Good idea. Good way to help midcareer faculty with advancement. Offers promise for collaboration of research/workshops. | |
| Suggestions for Improvement | |
| Please have e‐mails of the folks we meet available immediately after the visit. It is hard to know if anyone felt enough of a connection to want mentorship from me. | I feel like I may be a bit early on to benefit as much as I could have. |
| Develop a mentorship program for quality improvement. As part of this exchange, consider treating visits as similar to a consultation. Have visitor with specific focus that they can offer help with. | Nice to have personal access to accomplished faculty from other institutions. Their perspective and career trajectory don't always align due to differences in institution culture, specifics of promotion process, and so on, but still a useful experience. |
| Share any possible more‐formal topics for discussion with leadership prior to the visit so can prepare ahead of time (eg, gather information they may have questions on). Otherwise it was great! | For early career faculty, more discussions prior in regard to what to expect. |
| A question is who should continue to push? Is it the prospective mentee, the mentee's institution, an so on? | Great idea. Would have loved to be involved in more aspects. More time for discussion would have been good. Did not get to discuss collaboration in person. |
| Great to get to talk to someone from totally different system. Wish we had more time to talk. | |
One‐year follow‐up was obtained for all but 1 early‐career faculty member receiving the follow‐up survey, and all 3 visiting professors. Of the 3 visiting professorships that occurred more than 1 year ago, 16 mentorship contacts occurred in total (phone, e‐mail, or in person) between 13 early‐career faculty and 3 visiting professors in the year after the initial visits (range, 04 contacts). Follow‐up contact occurred for 3 of 4 early‐career faculty from the first visiting professorship, 3 of 5 from the second visiting professorship, and 2 of 4 from the third visiting professorship. One early‐career faculty member from each host academic medical center had 3 or more additional contacts with the visiting professor in the year following the initial visit. Overall, 8/13 (62%) of early‐career faculty had at least 1 follow‐up mentoring discussion. On 1‐year follow‐up, overall utility for professional development was rated an average of 3.5 by early‐career faculty (with a trend of higher ratings of efficacy with increasing number of follow‐up contacts) and 4.7 by visiting professors. Half (8/16) of the involved faculty report having seen evidence of cross‐institutional dissemination of innovation. Ninety‐four percent (15/16) of participants at 1‐year follow‐up felt there was benefit to their institution in continuing the program for the next year.
Objective evidence of cross‐institutional scholarship, assessed by email query of both visiting professors and senior hospitalists coordinating the visits, includes 2 collaborative peer reviewed publications including mentors and mentees participating in the visiting professorship.[6, 7] Joint educational curriculum development on high‐value care between sites is planned. The Visiting Professorship in Hospital Medicine Program has resulted in 1 external letter to support a visiting professor's promotion to date.

DISCUSSION
Hospital Medicine is a young, rapidly growing field, hence the number of experienced academic hospitalist mentors with expertise in successfully navigating an academic career is limited. A national study of hospitalist leaders found that 75% of clinician‐educators and 58% of research faculty feel that lack of mentorship is a major issue.[1] Mentorship for hospitalist clinician‐investigators is often delivered by nonhospitalists.[2, 8] There is little evidence of external mentorship for academic clinician‐educators in hospital medicine.[1] Without explicit programmatic support, many faculty may find this to be a barrier to career advancement. A study of successfully promoted hospitalists identified difficulty identifying external senior hospitalists to write letters in support of promotion as an obstacle.[9] Our study of the Visiting Professorship in Hospital Medicine Program found that early‐career faculty rated the visit as useful in advancing their career and fostered external mentorship relationships. Subsequent experience suggests more than half of the early‐career faculty will maintain contact with the visiting professor over the year following the visit. Visiting professors rate the experience particularly highly in their own career advancement.
The hospitalist movement is built on a foundation of innovation. The focus of each presentation was on an innovation developed by the visiting professor, and each visit showcased an innovation of the visited institution. This is distinct from traditional Hospital Grand Rounds, which more often focus on basic science research or clinical pathophysiology/disease management based on subspecialty topics.[10] The Visiting Professorship in Hospital Medicine Program was judged by participants to be an effective means of spreading innovation.
Insights from experience with the Visiting Professorship in Hospital Medicine Program include the importance of preliminary work prior to each visit. Program directors need to attend closely to the fit between the interests and career path of the visiting professor and those of the early‐career faculty. The innovations being shared should be aligned with organizational interests to maximize the chance of subsequent spread of the innovation and future collaboration. Providing faculty information about the objectives of the program in advance of the visit and arranging an exchange of curricula vitae between the early‐career faculty and the visiting professor allows participants to prepare for the in‐person coaching. Based on comments from participants, prompting contact from the visiting professor after the visit may be helpful to initiate the longitudinal relationship. We also found that early‐career faculty may not be aware of how to effectively use a mentoring relationship with an external faculty member. Training sessions for both mentors and mentees on effective mentorship relationships before visiting professorships might improve early‐career faculty confidence in initiating relationships and maximize value from mentor coaching.
A key issue is finding the right level of career maturity for the visiting professor. Our approach in selecting visiting professors was congruent with utilization of midcareer peer coaches employed by intrainstitutional hospital medicine mentoring programs.[11] The visiting professor should have sufficient experience and accomplishments to be able to effectively counsel junior faculty. However, it is important that the visiting professor also has sufficient time and interest to take on additional mentees and to be a full participant in shared scholarship projects emerging from the experience.
This study represents the experience of 5 mature academic hospitalist groups, and results may not be generalizable to dissimilar institutions or if only the most senior faculty are selected to perform visits. There is an inherent selection bias in the choice of both visiting professor and early‐career faculty. The small sample size of the faculty exposed to this program is a limitation to generalizability of the results of this evaluation. Whether this program will result in greater success in promotion of academic hospitalists cannot be assessed based on the follow‐up available. The Visiting Professorship in Hospital Medicine Program has continued to be sustained with an additional academic medical center enrolled and 2 additional site visits planned. The costs of the program are low, largely air travel and a night of lodging, as well as nominal administrative logistical support. Perceived benefits by participants and academic medical centers make this modest investment worth considering for academic hospitalist groups.
CONCLUSIONS
The Visiting Professorship in Hospital Medicine Program offers structure, opportunities, and access to senior mentors to advance the development of early‐career hospitalists while spreading innovation to distant sites. It is assessed by participants to facilitate external mentoring relationships and has the potential to advance the careers of both early‐career faculty as well as the visiting professors.
Disclosure
Nothing to report.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5–9.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , . Mentoring faculty in academic medicine: a new paradigm? J Gen Intern Med. 2005;20(9):866–870.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4:240–246.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6:1–2.
- , , , . Procedural skills for hospitalists. Hosp Med Clin. 2016;5:114–136.
- , , . Cedecea davisae' s role in a polymicrobial lung infection in a cystic fibrosis patient. Case reports in infectious diseases. Case Rep Infect Dis. 2012;2012:176864.
- , , , . Innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314–318.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6:411–415.
- , , , , . A case study of medical grand rounds: are we using effective methods? Acad Med. 2009;84(8):1144–1151.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6:161–166.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6:5–9.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , . Mentoring faculty in academic medicine: a new paradigm? J Gen Intern Med. 2005;20(9):866–870.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4:240–246.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6:1–2.
- , , , . Procedural skills for hospitalists. Hosp Med Clin. 2016;5:114–136.
- , , . Cedecea davisae' s role in a polymicrobial lung infection in a cystic fibrosis patient. Case reports in infectious diseases. Case Rep Infect Dis. 2012;2012:176864.
- , , , . Innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3:314–318.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6:411–415.
- , , , , . A case study of medical grand rounds: are we using effective methods? Acad Med. 2009;84(8):1144–1151.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6:161–166.
Building an Academic Pipeline
Now in its 20th year, hospital medicine hasfrom many perspectivesreached full bloom. Hospitalists populate settings as diverse as academic hospitals, community hospitals, and long‐term care facilities. Many traditional academic medical centers have taken notice of hospitalists, not just for their clinical role in an era of restricted duty hours, but also for the value they provide in advancing the academic and teaching mission of the institution.[1, 2]
As a result, hospital medicine has expanded rapidly, from 10,000 hospitalists in the United States in 2004 to 48,000 hospitalists in 2014.[3] Unfortunately, such growth has led to a profession that is bottom heavy, with leaders that spearheaded the movement occupying the upper echelon followed by a conglomeration of junior faculty at the base. For many at the bottom of this pyramid, the path to promotion remains challenging. Core academic metrics such as peer‐reviewed publications, quality improvement activities, superb teaching evaluations, and a national reputation are challenging to achieve for faculty who remain highly clinically occupied.[4] Mentorship has been cited as a key contributor for academic success and career satisfaction, but not enough senior hospitalists exist with the experience, skills, and bandwidth to adequately groom protgs.[5, 6]
The brief article in this month's edition of the Journal of Hospital Medicine is important and timely. Cumbler and colleagues describe the creation of a visiting professor program specifically aimed at cross‐pollinating junior faculty on the precipice of promotion with senior members from other institutions.[7] Using a model of reciprocity, the visiting professor innovation provided a mechanism by which midcareer faculty could travel to another site, exchange ideas, mentor or be mentored, and find partners and like‐minded faculty to grow their work. Starting with just 2 sites, the program quickly expanded to 5, with exchanges of 7 visiting professors. Initial metrics of success appear promising: early career faculty that interacted with the visiting professor provided positive feedback in key domains including mentor‐mentee relationships and advancement of academic careers. We applaud the authors not only for introducing this novel idea, but also for building in evaluative components such as publications and letters for promotion that allow for assessment of success.
Programs such as these help fill key gaps. First, they provide important mechanisms for expanding the network of available mentors for junior faculty. Second, they provide a venue to promote cross‐institutional collaborations, receive feedback, and grow the circle of stakeholders around innovative projects. Third, they help junior faculty establish a national reputation, propelling them toward promotion. Finally, the program does what few others can; it provides a means by which clinically busy junior faculty can get much‐needed validation for their academic efforts.
How may this innovation be expanded to a national scale? As chairs of the Society of Hospital Medicines Academic and Research Committee, we think this a worthy mission. Following a lively discussion at the national meeting, both committees have established a workgroup to support a visiting professor program. The Visiting Professorship in Hospital Medicine Program will follow the model introduced by Cumbler and colleagues by cross‐linking facilities represented within our committees into the existing network of visiting professor sites. New sites will be asked to name a site lead who will be responsible for identifying appropriate faculty members and areas of expertise that would benefit from interinstitutional exchange. The Society of Hospital Medicine's Chapters Committee has joined the dialogue and will help by developing a database of faculty, domains of expertise, and geographic locations to create a veritable
We are a field that began with innovation. Developing and diffusing a junior faculty program to grow future academic leaders is just an extension of this type of thinking and demonstrates how we continuously remodel our specialty to meet our unique needs. Ultimately, we envision the program to be a national model adopted by the Society of Hospital Medicine to help grow not only academic, but also community‐hospitalist superstars who also have great ideas and innovations. Faced with the constant peril of clinical workload, academic burnout, and career success, our field must begin to invest in infrastructure that nurtures our young and provides them with the opportunities needed to shine. The innovation proposed by Cumbler et al. is a superb example of this type of initiative, one we are proud to help diffuse on a national level. Onward!
Disclosure
Nothing to report.
- , , , . Where should hospitalists sit within the academic medical center? J Gen Intern Med. 2008;23(8):1269–1272.
- . The hospitalist movement—time to move on. N Engl J Med. 2007;357(25):2627–2629.
- , . A history of the hospitalist movement. Obstet Gynecol Clin North Am. 2015;42(3):419–432.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , . Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141–148.
- , , , , , . Visiting professorship in hospital medicine: an innovative twist for a growing specialty. J Hosp Med. 2016;11(10):714–718.
Now in its 20th year, hospital medicine hasfrom many perspectivesreached full bloom. Hospitalists populate settings as diverse as academic hospitals, community hospitals, and long‐term care facilities. Many traditional academic medical centers have taken notice of hospitalists, not just for their clinical role in an era of restricted duty hours, but also for the value they provide in advancing the academic and teaching mission of the institution.[1, 2]
As a result, hospital medicine has expanded rapidly, from 10,000 hospitalists in the United States in 2004 to 48,000 hospitalists in 2014.[3] Unfortunately, such growth has led to a profession that is bottom heavy, with leaders that spearheaded the movement occupying the upper echelon followed by a conglomeration of junior faculty at the base. For many at the bottom of this pyramid, the path to promotion remains challenging. Core academic metrics such as peer‐reviewed publications, quality improvement activities, superb teaching evaluations, and a national reputation are challenging to achieve for faculty who remain highly clinically occupied.[4] Mentorship has been cited as a key contributor for academic success and career satisfaction, but not enough senior hospitalists exist with the experience, skills, and bandwidth to adequately groom protgs.[5, 6]
The brief article in this month's edition of the Journal of Hospital Medicine is important and timely. Cumbler and colleagues describe the creation of a visiting professor program specifically aimed at cross‐pollinating junior faculty on the precipice of promotion with senior members from other institutions.[7] Using a model of reciprocity, the visiting professor innovation provided a mechanism by which midcareer faculty could travel to another site, exchange ideas, mentor or be mentored, and find partners and like‐minded faculty to grow their work. Starting with just 2 sites, the program quickly expanded to 5, with exchanges of 7 visiting professors. Initial metrics of success appear promising: early career faculty that interacted with the visiting professor provided positive feedback in key domains including mentor‐mentee relationships and advancement of academic careers. We applaud the authors not only for introducing this novel idea, but also for building in evaluative components such as publications and letters for promotion that allow for assessment of success.
Programs such as these help fill key gaps. First, they provide important mechanisms for expanding the network of available mentors for junior faculty. Second, they provide a venue to promote cross‐institutional collaborations, receive feedback, and grow the circle of stakeholders around innovative projects. Third, they help junior faculty establish a national reputation, propelling them toward promotion. Finally, the program does what few others can; it provides a means by which clinically busy junior faculty can get much‐needed validation for their academic efforts.
How may this innovation be expanded to a national scale? As chairs of the Society of Hospital Medicines Academic and Research Committee, we think this a worthy mission. Following a lively discussion at the national meeting, both committees have established a workgroup to support a visiting professor program. The Visiting Professorship in Hospital Medicine Program will follow the model introduced by Cumbler and colleagues by cross‐linking facilities represented within our committees into the existing network of visiting professor sites. New sites will be asked to name a site lead who will be responsible for identifying appropriate faculty members and areas of expertise that would benefit from interinstitutional exchange. The Society of Hospital Medicine's Chapters Committee has joined the dialogue and will help by developing a database of faculty, domains of expertise, and geographic locations to create a veritable
We are a field that began with innovation. Developing and diffusing a junior faculty program to grow future academic leaders is just an extension of this type of thinking and demonstrates how we continuously remodel our specialty to meet our unique needs. Ultimately, we envision the program to be a national model adopted by the Society of Hospital Medicine to help grow not only academic, but also community‐hospitalist superstars who also have great ideas and innovations. Faced with the constant peril of clinical workload, academic burnout, and career success, our field must begin to invest in infrastructure that nurtures our young and provides them with the opportunities needed to shine. The innovation proposed by Cumbler et al. is a superb example of this type of initiative, one we are proud to help diffuse on a national level. Onward!
Disclosure
Nothing to report.
Now in its 20th year, hospital medicine hasfrom many perspectivesreached full bloom. Hospitalists populate settings as diverse as academic hospitals, community hospitals, and long‐term care facilities. Many traditional academic medical centers have taken notice of hospitalists, not just for their clinical role in an era of restricted duty hours, but also for the value they provide in advancing the academic and teaching mission of the institution.[1, 2]
As a result, hospital medicine has expanded rapidly, from 10,000 hospitalists in the United States in 2004 to 48,000 hospitalists in 2014.[3] Unfortunately, such growth has led to a profession that is bottom heavy, with leaders that spearheaded the movement occupying the upper echelon followed by a conglomeration of junior faculty at the base. For many at the bottom of this pyramid, the path to promotion remains challenging. Core academic metrics such as peer‐reviewed publications, quality improvement activities, superb teaching evaluations, and a national reputation are challenging to achieve for faculty who remain highly clinically occupied.[4] Mentorship has been cited as a key contributor for academic success and career satisfaction, but not enough senior hospitalists exist with the experience, skills, and bandwidth to adequately groom protgs.[5, 6]
The brief article in this month's edition of the Journal of Hospital Medicine is important and timely. Cumbler and colleagues describe the creation of a visiting professor program specifically aimed at cross‐pollinating junior faculty on the precipice of promotion with senior members from other institutions.[7] Using a model of reciprocity, the visiting professor innovation provided a mechanism by which midcareer faculty could travel to another site, exchange ideas, mentor or be mentored, and find partners and like‐minded faculty to grow their work. Starting with just 2 sites, the program quickly expanded to 5, with exchanges of 7 visiting professors. Initial metrics of success appear promising: early career faculty that interacted with the visiting professor provided positive feedback in key domains including mentor‐mentee relationships and advancement of academic careers. We applaud the authors not only for introducing this novel idea, but also for building in evaluative components such as publications and letters for promotion that allow for assessment of success.
Programs such as these help fill key gaps. First, they provide important mechanisms for expanding the network of available mentors for junior faculty. Second, they provide a venue to promote cross‐institutional collaborations, receive feedback, and grow the circle of stakeholders around innovative projects. Third, they help junior faculty establish a national reputation, propelling them toward promotion. Finally, the program does what few others can; it provides a means by which clinically busy junior faculty can get much‐needed validation for their academic efforts.
How may this innovation be expanded to a national scale? As chairs of the Society of Hospital Medicines Academic and Research Committee, we think this a worthy mission. Following a lively discussion at the national meeting, both committees have established a workgroup to support a visiting professor program. The Visiting Professorship in Hospital Medicine Program will follow the model introduced by Cumbler and colleagues by cross‐linking facilities represented within our committees into the existing network of visiting professor sites. New sites will be asked to name a site lead who will be responsible for identifying appropriate faculty members and areas of expertise that would benefit from interinstitutional exchange. The Society of Hospital Medicine's Chapters Committee has joined the dialogue and will help by developing a database of faculty, domains of expertise, and geographic locations to create a veritable
We are a field that began with innovation. Developing and diffusing a junior faculty program to grow future academic leaders is just an extension of this type of thinking and demonstrates how we continuously remodel our specialty to meet our unique needs. Ultimately, we envision the program to be a national model adopted by the Society of Hospital Medicine to help grow not only academic, but also community‐hospitalist superstars who also have great ideas and innovations. Faced with the constant peril of clinical workload, academic burnout, and career success, our field must begin to invest in infrastructure that nurtures our young and provides them with the opportunities needed to shine. The innovation proposed by Cumbler et al. is a superb example of this type of initiative, one we are proud to help diffuse on a national level. Onward!
Disclosure
Nothing to report.
- , , , . Where should hospitalists sit within the academic medical center? J Gen Intern Med. 2008;23(8):1269–1272.
- . The hospitalist movement—time to move on. N Engl J Med. 2007;357(25):2627–2629.
- , . A history of the hospitalist movement. Obstet Gynecol Clin North Am. 2015;42(3):419–432.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , . Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141–148.
- , , , , , . Visiting professorship in hospital medicine: an innovative twist for a growing specialty. J Hosp Med. 2016;11(10):714–718.
- , , , . Where should hospitalists sit within the academic medical center? J Gen Intern Med. 2008;23(8):1269–1272.
- . The hospitalist movement—time to move on. N Engl J Med. 2007;357(25):2627–2629.
- , . A history of the hospitalist movement. Obstet Gynecol Clin North Am. 2015;42(3):419–432.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , . Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141–148.
- , , , , , . Visiting professorship in hospital medicine: an innovative twist for a growing specialty. J Hosp Med. 2016;11(10):714–718.
Ibrutinib at below standard dose may achieve good survival in CLL
Ibrutinib given at doses lower than the standard dose of 420 mg/day was associated with comparable overall survival and progression-free survival as the standard dose in patients with chronic lymphocytic leukemia (CLL), based on the results of a multicenter, retrospective study submitted as a poster at the annual meeting of the American Society of Clinical Oncology.
The initial findings indicate patients who experience toxicity on the 420 mg/day dosage can still do well on a slightly lower dose of ibrutinib, reported Colleen Timlin, Pharm.D., of the Hospital of the University of Pennsylvania, Philadelphia, and her associates.
At a median follow-up of 13.5 months, the median progression-free survival was 37.4 months in the standard dose group and the median progression-free survival had not been reached in the reduced-dose group. The median overall survival had not been reached in either group. The hazard ratio in the reduced-dose group was 1.2 for progression-free survival and 0.6 for overall survival; neither difference was statistically significant. Best overall response rate was 85% in the standard-dose group and 84% in the reduced-dose group.
The study included 197 patients, 37 of whom were shifted to reduced doses of ibrutinib within 3 months of initiating therapy at the recommended 420 mg/day dosage. For reduced-dose patients, the median ibrutinib dose was 4.3 mg/kg per day. The most common reasons for reducing the ibrutinib dose were gastrointestinal toxicity, bleeding, rash, cardiotoxicity, and renal insufficiency, the researchers said.
Most of the patients treated with doses less than 420 mg/day still maintained a dose of greater than 2.5 mg/kg per day, which assured adequate Bruton’s tyrosine kinase occupancy. The 420 mg/day dosage noted in the labeling was established, based on achievement of greater than 90% Bruton’s tyrosine kinase occupancy. Fewer patients achieve greater than 90% occupancy at lower doses, but lower doses may not translate into inferior outcomes.
Weight-based dosing should be considered in future studies and pharmacoeconomic analyses. Comparative analyses of toxicity profiles stratified by ibrutinib dose are underway, according to Dr. Timlin and her colleagues.
Dr. Timlin had no relevant disclosures. Several of her colleagues had multiple financial disclosures.
On Twitter @maryjodales
Ibrutinib given at doses lower than the standard dose of 420 mg/day was associated with comparable overall survival and progression-free survival as the standard dose in patients with chronic lymphocytic leukemia (CLL), based on the results of a multicenter, retrospective study submitted as a poster at the annual meeting of the American Society of Clinical Oncology.
The initial findings indicate patients who experience toxicity on the 420 mg/day dosage can still do well on a slightly lower dose of ibrutinib, reported Colleen Timlin, Pharm.D., of the Hospital of the University of Pennsylvania, Philadelphia, and her associates.
At a median follow-up of 13.5 months, the median progression-free survival was 37.4 months in the standard dose group and the median progression-free survival had not been reached in the reduced-dose group. The median overall survival had not been reached in either group. The hazard ratio in the reduced-dose group was 1.2 for progression-free survival and 0.6 for overall survival; neither difference was statistically significant. Best overall response rate was 85% in the standard-dose group and 84% in the reduced-dose group.
The study included 197 patients, 37 of whom were shifted to reduced doses of ibrutinib within 3 months of initiating therapy at the recommended 420 mg/day dosage. For reduced-dose patients, the median ibrutinib dose was 4.3 mg/kg per day. The most common reasons for reducing the ibrutinib dose were gastrointestinal toxicity, bleeding, rash, cardiotoxicity, and renal insufficiency, the researchers said.
Most of the patients treated with doses less than 420 mg/day still maintained a dose of greater than 2.5 mg/kg per day, which assured adequate Bruton’s tyrosine kinase occupancy. The 420 mg/day dosage noted in the labeling was established, based on achievement of greater than 90% Bruton’s tyrosine kinase occupancy. Fewer patients achieve greater than 90% occupancy at lower doses, but lower doses may not translate into inferior outcomes.
Weight-based dosing should be considered in future studies and pharmacoeconomic analyses. Comparative analyses of toxicity profiles stratified by ibrutinib dose are underway, according to Dr. Timlin and her colleagues.
Dr. Timlin had no relevant disclosures. Several of her colleagues had multiple financial disclosures.
On Twitter @maryjodales
Ibrutinib given at doses lower than the standard dose of 420 mg/day was associated with comparable overall survival and progression-free survival as the standard dose in patients with chronic lymphocytic leukemia (CLL), based on the results of a multicenter, retrospective study submitted as a poster at the annual meeting of the American Society of Clinical Oncology.
The initial findings indicate patients who experience toxicity on the 420 mg/day dosage can still do well on a slightly lower dose of ibrutinib, reported Colleen Timlin, Pharm.D., of the Hospital of the University of Pennsylvania, Philadelphia, and her associates.
At a median follow-up of 13.5 months, the median progression-free survival was 37.4 months in the standard dose group and the median progression-free survival had not been reached in the reduced-dose group. The median overall survival had not been reached in either group. The hazard ratio in the reduced-dose group was 1.2 for progression-free survival and 0.6 for overall survival; neither difference was statistically significant. Best overall response rate was 85% in the standard-dose group and 84% in the reduced-dose group.
The study included 197 patients, 37 of whom were shifted to reduced doses of ibrutinib within 3 months of initiating therapy at the recommended 420 mg/day dosage. For reduced-dose patients, the median ibrutinib dose was 4.3 mg/kg per day. The most common reasons for reducing the ibrutinib dose were gastrointestinal toxicity, bleeding, rash, cardiotoxicity, and renal insufficiency, the researchers said.
Most of the patients treated with doses less than 420 mg/day still maintained a dose of greater than 2.5 mg/kg per day, which assured adequate Bruton’s tyrosine kinase occupancy. The 420 mg/day dosage noted in the labeling was established, based on achievement of greater than 90% Bruton’s tyrosine kinase occupancy. Fewer patients achieve greater than 90% occupancy at lower doses, but lower doses may not translate into inferior outcomes.
Weight-based dosing should be considered in future studies and pharmacoeconomic analyses. Comparative analyses of toxicity profiles stratified by ibrutinib dose are underway, according to Dr. Timlin and her colleagues.
Dr. Timlin had no relevant disclosures. Several of her colleagues had multiple financial disclosures.
On Twitter @maryjodales
Key clinical point: Ibrutinib given at doses lower than the standard dose of 420 mg/day may achieve comparable overall survival and progression-free survival.
Major finding: At a median follow-up of 13.5 months, the median progression-free survival was 37.4 months in the standard dose group, and the median progression-free survival had not been reached in the reduced-dose group.
Data source: A multicenter, retrospective study of 197 patients.
Disclosures: Dr. Timlin had no relevant disclosures. Several of her colleagues had multiple financial disclosures.