User login
Point/Counterpoint: Should breast MRI be used routinely in the preoperative evaluation of breast cancer?
Yes: MRI should be considered in the preoperative setting for specific clinical indications.
MRI, like any technology, has its strengths and weaknesses, with high sensitivity but low specificity. Importantly, MRI provides excellent soft tissue contrast with anatomic 3-D detail, and is not impeded by high breast density.
Admittedly, MRI only incrementally increases cancer detection rates in either the ipsilateral or contralateral breasts of all patients, and when used in the preoperative setting does not affect short-term surgical outcomes for all patients. Therefore, MRI should not be used for routine screening or routine preoperative screening.
That said, there are specific clinical situations where preoperative MRI may provide surgeons with valuable information. These include patients who have:
• Invasive lobular carcinoma.
• Neoadjuvant chemotherapy.
• Occult primaries in extremely dense breasts.
Invasive lobular carcinomas are more likely to be multi-centric, multi-focal, and/or bilateral than other breast cancer types, and they are more difficult to diagnose because they infiltrate into tissue, making it extremely difficult to determine the extent of disease. In this setting, MRI can more accurately determine tumor size than mammography. Mammography underestimates the tumor size significantly more frequently than does MRI. In addition, among women with this cancer subtype, MRI can significantly reduce the rate of excision (Breast Cancer Res Treat. 2010;119;415-22).
We know that women at risk for systemic recurrence will not be cured with surgery alone. Neoadjuvant therapies give us the opportunity to refine local therapy options, better understand the patient’s response to therapy and prognosis, and accelerate targeted drug development to improve outcomes. To accomplish all of these goals, we need a noninvasive way to assess tumors before, during, and after neoadjuvant treatment. MRI is unsurpassed for evaluating the extent of tumors, showing in larger tumors, for example, the complexity of tumor and stroma.
MRI is also a biomarker for response to therapy and has been shown to be an independent predictor of event-free survival. In addition, MRI is more accurate than either clinical exam, mammography, or ultrasound for determining residual tumor size following neoadjuvant chemotherapy (Radiology. 2012;263:663-72).
Lastly, for patients with an occult primary (by imaging) breast cancer or primary presentation of axillary node involvement, MRI has been found to have an approximately 90% sensitivity for identifying a primary tumor, and a 95% accuracy at locating the tumor in patients who undergo surgical excision. Mammography cannot distinguish a tumor mass that is dense relative to surrounding tissue. However, MRI can distinguish a tumor which is obscured by dense breast tissue because tumors are visualized on MRI by rapid contrast uptake and washout.
MRI is a catalyst for change, but you have to use it and all technology wisely: At the time of diagnosis for select patients, for screening only those patients with very breast dense tissue and very high risk for developing breast cancer, and, perhaps most importantly, for postcancer surveillance only in women at very high risk of recurrence where standard tools such as mammography are expected to have lower performance (for example, very dense breast tissue). Overuse of MRI will increase false positives, anxiety, and cost. However, used appropriately, MRI can be used to help usher in a change in practice through the evaluation of response to neoadjuvant therapy and novel therapeutic approaches to both invasive and in situ lesions.
With improvements in technology and techniques such as diffusion-weighted imaging, the value of MRI in the preoperative setting can only continue to grow. We can also expect greater performance for presurgical staging with more refined technologies for breast imaging, localization, and biopsy, but the costs have to come down. Breast-dedicated MRI technologies may address this need.
Laura Esserman, MD, is a professor of surgery and radiology at the University of California, San Francisco, and Director of the Carol Franc Buck Breast Care Center at the UCSF Mount Zion campus.
No: MRI leads to unnecessary surgeries and does not improve short-term surgical outcomes.
The key word in this debate is “routinely.” I agree that preoperative MRI may have a role in about 5% of all cases – namely in women with occult primaries and those who undergo neoadjuvant chemotherapy. But for the vast majority of patients, the 95%, I would argue that preoperative MRI has the potential to do more harm than good.
Thirty years of experience providing breast conserving therapies without MRI has taught us several important lessons:
• Selection of patients for breast-conserving therapy is not a big problem.
• The incidences of local recurrence and contralateral breast cancers have decreased over time, antedating the use of MRI.
• Surgical excision of all microscopic subclinical disease is not necessary to achieve good long-term outcomes.
In National Surgical Adjuvant Breast and Bowel Project studies from the 1990s, in the era before the use of aromatase inhibitors or HER-2 blockade, the 10-year incidence of ipsilateral breast tumor recurrence ranged from 3.5% to 6.5% in the breast cancer population at large.
In addition, the incidence of contralateral breast cancers has been declining at a rate of approximately 3% per year, thanks to the use of adjuvant systemic therapies.
We have known since the 1960s, thanks to our colleagues in pathology, that somewhere between 30% and 60% of breast cancers that appear to be localized have microscopic subclinical disease which is treated with breast irradiation. More recently, we have recognized that we can leave behind cancer in the axilla in anywhere from 13% to 27% of patients who are not receiving direct axillary radiation, and see failure rates of 1% or less.
In the context, then, of our current understanding of breast cancer biology, what outcomes could MRI be expected to improve, since the purpose of a test is to improve patient outcomes?
We know that MRI will not improve survival, because 30 years ago randomized trials showed us that survival was equal between breast conservation and mastectomy.
MRI has no apparent effect on reducing local recurrences either, as shown in an analysis of data on 3,180 patients published in 2014 (J Clin Oncol. 2014;32:292).
Regarding contralateral breast cancer, a 2007 study (N Engl J Med. 2007;356:1295-303) showed that among 969 women, MRI found unsuspected cancer in the contralateral breast within 1 year of diagnosis in 3.1%, a finding used to support the argument that all women with breast cancer should have an MRI. But a second study using Surveillance, Epidemiology and End Results (SEER) data on 339,790 women with 2.5 million person-years of follow-up found that the 10-year rate of contralateral cancers was 2%-3% of women with estrogen-receptor–positive tumors, and in 5%-6% of those with estrogen-receptor–negative tumors, strongly suggesting that MRI leads to detection and treatment of disease that would never become clinically evident.
Finally, I would point to evidence that MRI does not improve short-term surgical outcomes, as shown in a meta-analysis my colleagues and I conducted of two randomized controlled trials and seven cohort studies, involving a total of 3,112 patients (Ann Surg. 2013;257:249-55).
We found that after adjustment for age, having an MRI was associated with a three-fold greater chance of having a mastectomy, and MRI did not significantly reduce either the need for re-excision or unexpected conversion to mastectomy. We also performed a subanalysis of infiltrating lobular cancers, and found that there was no statistically significant benefit for MRI in these patients.
The overall rate of mastectomy was 25.5% among patients who had an MRI, vs. 18.2% for those who did not.
So to summarize: MRI finds two to three times more cancer than observed rates of local recurrence, leading to unnecessary mastectomies and does not improve short-term surgical outcomes, and there is no evidence indicating that MRI decreases local recurrence.
Monica Morrow, MD, FACS, is chief of the breast service in the department of surgery and holds the Anne Burnett Windfohr Chair of Clinical Oncology at Memorial Sloan Kettering Cancer Center in New York.
Yes: MRI should be considered in the preoperative setting for specific clinical indications.
MRI, like any technology, has its strengths and weaknesses, with high sensitivity but low specificity. Importantly, MRI provides excellent soft tissue contrast with anatomic 3-D detail, and is not impeded by high breast density.
Admittedly, MRI only incrementally increases cancer detection rates in either the ipsilateral or contralateral breasts of all patients, and when used in the preoperative setting does not affect short-term surgical outcomes for all patients. Therefore, MRI should not be used for routine screening or routine preoperative screening.
That said, there are specific clinical situations where preoperative MRI may provide surgeons with valuable information. These include patients who have:
• Invasive lobular carcinoma.
• Neoadjuvant chemotherapy.
• Occult primaries in extremely dense breasts.
Invasive lobular carcinomas are more likely to be multi-centric, multi-focal, and/or bilateral than other breast cancer types, and they are more difficult to diagnose because they infiltrate into tissue, making it extremely difficult to determine the extent of disease. In this setting, MRI can more accurately determine tumor size than mammography. Mammography underestimates the tumor size significantly more frequently than does MRI. In addition, among women with this cancer subtype, MRI can significantly reduce the rate of excision (Breast Cancer Res Treat. 2010;119;415-22).
We know that women at risk for systemic recurrence will not be cured with surgery alone. Neoadjuvant therapies give us the opportunity to refine local therapy options, better understand the patient’s response to therapy and prognosis, and accelerate targeted drug development to improve outcomes. To accomplish all of these goals, we need a noninvasive way to assess tumors before, during, and after neoadjuvant treatment. MRI is unsurpassed for evaluating the extent of tumors, showing in larger tumors, for example, the complexity of tumor and stroma.
MRI is also a biomarker for response to therapy and has been shown to be an independent predictor of event-free survival. In addition, MRI is more accurate than either clinical exam, mammography, or ultrasound for determining residual tumor size following neoadjuvant chemotherapy (Radiology. 2012;263:663-72).
Lastly, for patients with an occult primary (by imaging) breast cancer or primary presentation of axillary node involvement, MRI has been found to have an approximately 90% sensitivity for identifying a primary tumor, and a 95% accuracy at locating the tumor in patients who undergo surgical excision. Mammography cannot distinguish a tumor mass that is dense relative to surrounding tissue. However, MRI can distinguish a tumor which is obscured by dense breast tissue because tumors are visualized on MRI by rapid contrast uptake and washout.
MRI is a catalyst for change, but you have to use it and all technology wisely: At the time of diagnosis for select patients, for screening only those patients with very breast dense tissue and very high risk for developing breast cancer, and, perhaps most importantly, for postcancer surveillance only in women at very high risk of recurrence where standard tools such as mammography are expected to have lower performance (for example, very dense breast tissue). Overuse of MRI will increase false positives, anxiety, and cost. However, used appropriately, MRI can be used to help usher in a change in practice through the evaluation of response to neoadjuvant therapy and novel therapeutic approaches to both invasive and in situ lesions.
With improvements in technology and techniques such as diffusion-weighted imaging, the value of MRI in the preoperative setting can only continue to grow. We can also expect greater performance for presurgical staging with more refined technologies for breast imaging, localization, and biopsy, but the costs have to come down. Breast-dedicated MRI technologies may address this need.
Laura Esserman, MD, is a professor of surgery and radiology at the University of California, San Francisco, and Director of the Carol Franc Buck Breast Care Center at the UCSF Mount Zion campus.
No: MRI leads to unnecessary surgeries and does not improve short-term surgical outcomes.
The key word in this debate is “routinely.” I agree that preoperative MRI may have a role in about 5% of all cases – namely in women with occult primaries and those who undergo neoadjuvant chemotherapy. But for the vast majority of patients, the 95%, I would argue that preoperative MRI has the potential to do more harm than good.
Thirty years of experience providing breast conserving therapies without MRI has taught us several important lessons:
• Selection of patients for breast-conserving therapy is not a big problem.
• The incidences of local recurrence and contralateral breast cancers have decreased over time, antedating the use of MRI.
• Surgical excision of all microscopic subclinical disease is not necessary to achieve good long-term outcomes.
In National Surgical Adjuvant Breast and Bowel Project studies from the 1990s, in the era before the use of aromatase inhibitors or HER-2 blockade, the 10-year incidence of ipsilateral breast tumor recurrence ranged from 3.5% to 6.5% in the breast cancer population at large.
In addition, the incidence of contralateral breast cancers has been declining at a rate of approximately 3% per year, thanks to the use of adjuvant systemic therapies.
We have known since the 1960s, thanks to our colleagues in pathology, that somewhere between 30% and 60% of breast cancers that appear to be localized have microscopic subclinical disease which is treated with breast irradiation. More recently, we have recognized that we can leave behind cancer in the axilla in anywhere from 13% to 27% of patients who are not receiving direct axillary radiation, and see failure rates of 1% or less.
In the context, then, of our current understanding of breast cancer biology, what outcomes could MRI be expected to improve, since the purpose of a test is to improve patient outcomes?
We know that MRI will not improve survival, because 30 years ago randomized trials showed us that survival was equal between breast conservation and mastectomy.
MRI has no apparent effect on reducing local recurrences either, as shown in an analysis of data on 3,180 patients published in 2014 (J Clin Oncol. 2014;32:292).
Regarding contralateral breast cancer, a 2007 study (N Engl J Med. 2007;356:1295-303) showed that among 969 women, MRI found unsuspected cancer in the contralateral breast within 1 year of diagnosis in 3.1%, a finding used to support the argument that all women with breast cancer should have an MRI. But a second study using Surveillance, Epidemiology and End Results (SEER) data on 339,790 women with 2.5 million person-years of follow-up found that the 10-year rate of contralateral cancers was 2%-3% of women with estrogen-receptor–positive tumors, and in 5%-6% of those with estrogen-receptor–negative tumors, strongly suggesting that MRI leads to detection and treatment of disease that would never become clinically evident.
Finally, I would point to evidence that MRI does not improve short-term surgical outcomes, as shown in a meta-analysis my colleagues and I conducted of two randomized controlled trials and seven cohort studies, involving a total of 3,112 patients (Ann Surg. 2013;257:249-55).
We found that after adjustment for age, having an MRI was associated with a three-fold greater chance of having a mastectomy, and MRI did not significantly reduce either the need for re-excision or unexpected conversion to mastectomy. We also performed a subanalysis of infiltrating lobular cancers, and found that there was no statistically significant benefit for MRI in these patients.
The overall rate of mastectomy was 25.5% among patients who had an MRI, vs. 18.2% for those who did not.
So to summarize: MRI finds two to three times more cancer than observed rates of local recurrence, leading to unnecessary mastectomies and does not improve short-term surgical outcomes, and there is no evidence indicating that MRI decreases local recurrence.
Monica Morrow, MD, FACS, is chief of the breast service in the department of surgery and holds the Anne Burnett Windfohr Chair of Clinical Oncology at Memorial Sloan Kettering Cancer Center in New York.
Yes: MRI should be considered in the preoperative setting for specific clinical indications.
MRI, like any technology, has its strengths and weaknesses, with high sensitivity but low specificity. Importantly, MRI provides excellent soft tissue contrast with anatomic 3-D detail, and is not impeded by high breast density.
Admittedly, MRI only incrementally increases cancer detection rates in either the ipsilateral or contralateral breasts of all patients, and when used in the preoperative setting does not affect short-term surgical outcomes for all patients. Therefore, MRI should not be used for routine screening or routine preoperative screening.
That said, there are specific clinical situations where preoperative MRI may provide surgeons with valuable information. These include patients who have:
• Invasive lobular carcinoma.
• Neoadjuvant chemotherapy.
• Occult primaries in extremely dense breasts.
Invasive lobular carcinomas are more likely to be multi-centric, multi-focal, and/or bilateral than other breast cancer types, and they are more difficult to diagnose because they infiltrate into tissue, making it extremely difficult to determine the extent of disease. In this setting, MRI can more accurately determine tumor size than mammography. Mammography underestimates the tumor size significantly more frequently than does MRI. In addition, among women with this cancer subtype, MRI can significantly reduce the rate of excision (Breast Cancer Res Treat. 2010;119;415-22).
We know that women at risk for systemic recurrence will not be cured with surgery alone. Neoadjuvant therapies give us the opportunity to refine local therapy options, better understand the patient’s response to therapy and prognosis, and accelerate targeted drug development to improve outcomes. To accomplish all of these goals, we need a noninvasive way to assess tumors before, during, and after neoadjuvant treatment. MRI is unsurpassed for evaluating the extent of tumors, showing in larger tumors, for example, the complexity of tumor and stroma.
MRI is also a biomarker for response to therapy and has been shown to be an independent predictor of event-free survival. In addition, MRI is more accurate than either clinical exam, mammography, or ultrasound for determining residual tumor size following neoadjuvant chemotherapy (Radiology. 2012;263:663-72).
Lastly, for patients with an occult primary (by imaging) breast cancer or primary presentation of axillary node involvement, MRI has been found to have an approximately 90% sensitivity for identifying a primary tumor, and a 95% accuracy at locating the tumor in patients who undergo surgical excision. Mammography cannot distinguish a tumor mass that is dense relative to surrounding tissue. However, MRI can distinguish a tumor which is obscured by dense breast tissue because tumors are visualized on MRI by rapid contrast uptake and washout.
MRI is a catalyst for change, but you have to use it and all technology wisely: At the time of diagnosis for select patients, for screening only those patients with very breast dense tissue and very high risk for developing breast cancer, and, perhaps most importantly, for postcancer surveillance only in women at very high risk of recurrence where standard tools such as mammography are expected to have lower performance (for example, very dense breast tissue). Overuse of MRI will increase false positives, anxiety, and cost. However, used appropriately, MRI can be used to help usher in a change in practice through the evaluation of response to neoadjuvant therapy and novel therapeutic approaches to both invasive and in situ lesions.
With improvements in technology and techniques such as diffusion-weighted imaging, the value of MRI in the preoperative setting can only continue to grow. We can also expect greater performance for presurgical staging with more refined technologies for breast imaging, localization, and biopsy, but the costs have to come down. Breast-dedicated MRI technologies may address this need.
Laura Esserman, MD, is a professor of surgery and radiology at the University of California, San Francisco, and Director of the Carol Franc Buck Breast Care Center at the UCSF Mount Zion campus.
No: MRI leads to unnecessary surgeries and does not improve short-term surgical outcomes.
The key word in this debate is “routinely.” I agree that preoperative MRI may have a role in about 5% of all cases – namely in women with occult primaries and those who undergo neoadjuvant chemotherapy. But for the vast majority of patients, the 95%, I would argue that preoperative MRI has the potential to do more harm than good.
Thirty years of experience providing breast conserving therapies without MRI has taught us several important lessons:
• Selection of patients for breast-conserving therapy is not a big problem.
• The incidences of local recurrence and contralateral breast cancers have decreased over time, antedating the use of MRI.
• Surgical excision of all microscopic subclinical disease is not necessary to achieve good long-term outcomes.
In National Surgical Adjuvant Breast and Bowel Project studies from the 1990s, in the era before the use of aromatase inhibitors or HER-2 blockade, the 10-year incidence of ipsilateral breast tumor recurrence ranged from 3.5% to 6.5% in the breast cancer population at large.
In addition, the incidence of contralateral breast cancers has been declining at a rate of approximately 3% per year, thanks to the use of adjuvant systemic therapies.
We have known since the 1960s, thanks to our colleagues in pathology, that somewhere between 30% and 60% of breast cancers that appear to be localized have microscopic subclinical disease which is treated with breast irradiation. More recently, we have recognized that we can leave behind cancer in the axilla in anywhere from 13% to 27% of patients who are not receiving direct axillary radiation, and see failure rates of 1% or less.
In the context, then, of our current understanding of breast cancer biology, what outcomes could MRI be expected to improve, since the purpose of a test is to improve patient outcomes?
We know that MRI will not improve survival, because 30 years ago randomized trials showed us that survival was equal between breast conservation and mastectomy.
MRI has no apparent effect on reducing local recurrences either, as shown in an analysis of data on 3,180 patients published in 2014 (J Clin Oncol. 2014;32:292).
Regarding contralateral breast cancer, a 2007 study (N Engl J Med. 2007;356:1295-303) showed that among 969 women, MRI found unsuspected cancer in the contralateral breast within 1 year of diagnosis in 3.1%, a finding used to support the argument that all women with breast cancer should have an MRI. But a second study using Surveillance, Epidemiology and End Results (SEER) data on 339,790 women with 2.5 million person-years of follow-up found that the 10-year rate of contralateral cancers was 2%-3% of women with estrogen-receptor–positive tumors, and in 5%-6% of those with estrogen-receptor–negative tumors, strongly suggesting that MRI leads to detection and treatment of disease that would never become clinically evident.
Finally, I would point to evidence that MRI does not improve short-term surgical outcomes, as shown in a meta-analysis my colleagues and I conducted of two randomized controlled trials and seven cohort studies, involving a total of 3,112 patients (Ann Surg. 2013;257:249-55).
We found that after adjustment for age, having an MRI was associated with a three-fold greater chance of having a mastectomy, and MRI did not significantly reduce either the need for re-excision or unexpected conversion to mastectomy. We also performed a subanalysis of infiltrating lobular cancers, and found that there was no statistically significant benefit for MRI in these patients.
The overall rate of mastectomy was 25.5% among patients who had an MRI, vs. 18.2% for those who did not.
So to summarize: MRI finds two to three times more cancer than observed rates of local recurrence, leading to unnecessary mastectomies and does not improve short-term surgical outcomes, and there is no evidence indicating that MRI decreases local recurrence.
Monica Morrow, MD, FACS, is chief of the breast service in the department of surgery and holds the Anne Burnett Windfohr Chair of Clinical Oncology at Memorial Sloan Kettering Cancer Center in New York.
House Republicans unveil long-awaited plan to replace health law
Six years after promising a plan to “repeal and replace” the federal health law, House Republicans are finally ready to deliver.
The 37-page white paper, called “A Better Way,” includes virtually every idea on health care proposed by Republicans going back at least 2 decades. It would bring back “high-risk pools” for people with very high medical expenses, end open-ended funding for the Medicaid program, and encourage small businesses to band together to get better bargaining power in “Association Health Plans.”
What the plan does not include, however, is any idea of how much it would cost, or how it would be financed. Also unclear is how many of the 20 million Americans who have gained coverage since the law took effect would be able to remain insured.
“It’s a framework,” a senior House Republican leadership aide said on a conference call with reporters Tuesday, with the specifics to be determined next year by congressional committees, assuming the GOP maintains its majority. He likened the document to the white paper issued just after President Barack Obama’s election by then–Senate Finance Committee Chairman Max Baucus (D-Mont.). That document foreshadowed many of the key elements of the Affordable Care Act.
The plan starts with repeal of the health law and its requirements and taxes, but it would then put back many of its most popular elements: Allowing young adults to stay on their parents’ health plan to age 26; banning insurers from charging people with preexisting health problems higher premiums; and forbidding insurers from dropping coverage if a policyholder gets sick.
It would repeal the current scheme of exchanges where consumers buy insurance and government tax credits to help moderate-income Americans pay their premiums if they don’t have an employer to help. Instead, everyone buying policies in the individual market would receive tax credits. Older people charged more by insurers would receive larger credits, though the House Republicans don’t specify how much.
But the GOP plan also would likely make insurance more expensive for older people by proposing a broader range for premiums based on age. Current premiums can vary only threefold based on age, which is “driving out younger and healthier patients” who can’t afford them, the GOP aide said.
Under the plan, insurance companies could not charge higher rates to people with preexisting conditions so long as they maintain continuous coverage, whether from an employer or in a policy they purchase themselves. The new high-risk pools would be available for those who have a break in coverage, or who fail to purchase during a one-time open enrollment under the plan.
The plan would get rid of most of the coverage requirements under the Medicaid program for the poor, so states could make them more or less generous than they are currently. It would also limit funding. States could opt for either a per-person cap or a block grant to spend much as they wish.
On Medicare, the proposal would encourage the existing movement of patients from the program’s traditional fee-for-service program to managed care plans, and would transition from the existing financing structure based on benefits to a controversial structure called “premium support” that puts cost-controlling responsibilities more on private insurance companies. That change has been pushed by House Speaker Paul Ryan (R-Wisc.) for nearly a decade.
Backers of the existing health law were quick to criticize the GOP outline.
“The proposal introduced by Speaker Ryan is nothing more than vague and recycled ideas to take health insurance away from millions and increase costs for seniors and hardworking families,” said White House Assistant Press Secretary Katie Hill.
Families USA Executive Director Ron Pollack, who pushed hard for passage of the Affordable Care Act, said: “Make no mistake, Ryan’s approach is not a better way forward, but a bitter path backward that returns us to the bad old days when vast swaths of Americans were left to the tender mercies of the insurance industry and could not afford needed care.”
This story appears courtesy of Kaiser Health News, a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Six years after promising a plan to “repeal and replace” the federal health law, House Republicans are finally ready to deliver.
The 37-page white paper, called “A Better Way,” includes virtually every idea on health care proposed by Republicans going back at least 2 decades. It would bring back “high-risk pools” for people with very high medical expenses, end open-ended funding for the Medicaid program, and encourage small businesses to band together to get better bargaining power in “Association Health Plans.”
What the plan does not include, however, is any idea of how much it would cost, or how it would be financed. Also unclear is how many of the 20 million Americans who have gained coverage since the law took effect would be able to remain insured.
“It’s a framework,” a senior House Republican leadership aide said on a conference call with reporters Tuesday, with the specifics to be determined next year by congressional committees, assuming the GOP maintains its majority. He likened the document to the white paper issued just after President Barack Obama’s election by then–Senate Finance Committee Chairman Max Baucus (D-Mont.). That document foreshadowed many of the key elements of the Affordable Care Act.
The plan starts with repeal of the health law and its requirements and taxes, but it would then put back many of its most popular elements: Allowing young adults to stay on their parents’ health plan to age 26; banning insurers from charging people with preexisting health problems higher premiums; and forbidding insurers from dropping coverage if a policyholder gets sick.
It would repeal the current scheme of exchanges where consumers buy insurance and government tax credits to help moderate-income Americans pay their premiums if they don’t have an employer to help. Instead, everyone buying policies in the individual market would receive tax credits. Older people charged more by insurers would receive larger credits, though the House Republicans don’t specify how much.
But the GOP plan also would likely make insurance more expensive for older people by proposing a broader range for premiums based on age. Current premiums can vary only threefold based on age, which is “driving out younger and healthier patients” who can’t afford them, the GOP aide said.
Under the plan, insurance companies could not charge higher rates to people with preexisting conditions so long as they maintain continuous coverage, whether from an employer or in a policy they purchase themselves. The new high-risk pools would be available for those who have a break in coverage, or who fail to purchase during a one-time open enrollment under the plan.
The plan would get rid of most of the coverage requirements under the Medicaid program for the poor, so states could make them more or less generous than they are currently. It would also limit funding. States could opt for either a per-person cap or a block grant to spend much as they wish.
On Medicare, the proposal would encourage the existing movement of patients from the program’s traditional fee-for-service program to managed care plans, and would transition from the existing financing structure based on benefits to a controversial structure called “premium support” that puts cost-controlling responsibilities more on private insurance companies. That change has been pushed by House Speaker Paul Ryan (R-Wisc.) for nearly a decade.
Backers of the existing health law were quick to criticize the GOP outline.
“The proposal introduced by Speaker Ryan is nothing more than vague and recycled ideas to take health insurance away from millions and increase costs for seniors and hardworking families,” said White House Assistant Press Secretary Katie Hill.
Families USA Executive Director Ron Pollack, who pushed hard for passage of the Affordable Care Act, said: “Make no mistake, Ryan’s approach is not a better way forward, but a bitter path backward that returns us to the bad old days when vast swaths of Americans were left to the tender mercies of the insurance industry and could not afford needed care.”
This story appears courtesy of Kaiser Health News, a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Six years after promising a plan to “repeal and replace” the federal health law, House Republicans are finally ready to deliver.
The 37-page white paper, called “A Better Way,” includes virtually every idea on health care proposed by Republicans going back at least 2 decades. It would bring back “high-risk pools” for people with very high medical expenses, end open-ended funding for the Medicaid program, and encourage small businesses to band together to get better bargaining power in “Association Health Plans.”
What the plan does not include, however, is any idea of how much it would cost, or how it would be financed. Also unclear is how many of the 20 million Americans who have gained coverage since the law took effect would be able to remain insured.
“It’s a framework,” a senior House Republican leadership aide said on a conference call with reporters Tuesday, with the specifics to be determined next year by congressional committees, assuming the GOP maintains its majority. He likened the document to the white paper issued just after President Barack Obama’s election by then–Senate Finance Committee Chairman Max Baucus (D-Mont.). That document foreshadowed many of the key elements of the Affordable Care Act.
The plan starts with repeal of the health law and its requirements and taxes, but it would then put back many of its most popular elements: Allowing young adults to stay on their parents’ health plan to age 26; banning insurers from charging people with preexisting health problems higher premiums; and forbidding insurers from dropping coverage if a policyholder gets sick.
It would repeal the current scheme of exchanges where consumers buy insurance and government tax credits to help moderate-income Americans pay their premiums if they don’t have an employer to help. Instead, everyone buying policies in the individual market would receive tax credits. Older people charged more by insurers would receive larger credits, though the House Republicans don’t specify how much.
But the GOP plan also would likely make insurance more expensive for older people by proposing a broader range for premiums based on age. Current premiums can vary only threefold based on age, which is “driving out younger and healthier patients” who can’t afford them, the GOP aide said.
Under the plan, insurance companies could not charge higher rates to people with preexisting conditions so long as they maintain continuous coverage, whether from an employer or in a policy they purchase themselves. The new high-risk pools would be available for those who have a break in coverage, or who fail to purchase during a one-time open enrollment under the plan.
The plan would get rid of most of the coverage requirements under the Medicaid program for the poor, so states could make them more or less generous than they are currently. It would also limit funding. States could opt for either a per-person cap or a block grant to spend much as they wish.
On Medicare, the proposal would encourage the existing movement of patients from the program’s traditional fee-for-service program to managed care plans, and would transition from the existing financing structure based on benefits to a controversial structure called “premium support” that puts cost-controlling responsibilities more on private insurance companies. That change has been pushed by House Speaker Paul Ryan (R-Wisc.) for nearly a decade.
Backers of the existing health law were quick to criticize the GOP outline.
“The proposal introduced by Speaker Ryan is nothing more than vague and recycled ideas to take health insurance away from millions and increase costs for seniors and hardworking families,” said White House Assistant Press Secretary Katie Hill.
Families USA Executive Director Ron Pollack, who pushed hard for passage of the Affordable Care Act, said: “Make no mistake, Ryan’s approach is not a better way forward, but a bitter path backward that returns us to the bad old days when vast swaths of Americans were left to the tender mercies of the insurance industry and could not afford needed care.”
This story appears courtesy of Kaiser Health News, a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
ACIP recommends MenACWY vaccine for HIV-infected persons 2 months and older
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously voted to recommend that all HIV-infected persons aged 2 months and older receive the meningococcal ACWY (MenACWY) vaccine.
Guidance for this recommendation states that persons 2 months and older with HIV who have not been vaccinated previously should receive a two-dose, primary series of MenACWY; and that HIV-infected persons who have been vaccinated previously with one dose of MenACWY should receive a second dose at the earliest opportunity, with an 8-week minimum interval between doses. After that, boosters are to be given at the appropriate intervals.
Committee members voted in favor of immunizing earlier rather than waiting until 11 years of age or older, in part because human complement (hSBA) antibody titers following up to two doses of MenACWY vaccine in HIV-infected children ages 2-10 years is higher than in those ages 11-24 years. Also, the agreed upon recommended policy for earlier immunization is in step with current ACIP recommendations for use of the vaccine in persons with functional/anatomic asplenia or complement component deficiencies.
Despite an overall decline in the risk of meningococcal disease in the United States, there was a 13-fold increased risk in HIV-infected persons aged 25-64 years between 2000 and 2008, according to surveillance data presented to ACIP by Ms. Jessica MacNeil, MPH, an epidemiologist at the National Center for Immunization and Respiratory Diseases at the CDC in Atlanta. A ten-fold increase in risk was recorded in New York City alone in this population between 2000 and 2011.
Although fatality data are mixed, the infections were due primarily to serogroups C, W, and Y, for which the immune response wanes rapidly, according to Ms. MacNeil.
There are no safety or immunogenicity data currently available for the use of serogroup B meningococcal vaccines in HIV-infected persons, she said.
ACIP members also unanimously recommended this vaccine be covered under the Vaccines for Children program.
No information about disclosures was available at press time.
On Twitter @whitneymcknight
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously voted to recommend that all HIV-infected persons aged 2 months and older receive the meningococcal ACWY (MenACWY) vaccine.
Guidance for this recommendation states that persons 2 months and older with HIV who have not been vaccinated previously should receive a two-dose, primary series of MenACWY; and that HIV-infected persons who have been vaccinated previously with one dose of MenACWY should receive a second dose at the earliest opportunity, with an 8-week minimum interval between doses. After that, boosters are to be given at the appropriate intervals.
Committee members voted in favor of immunizing earlier rather than waiting until 11 years of age or older, in part because human complement (hSBA) antibody titers following up to two doses of MenACWY vaccine in HIV-infected children ages 2-10 years is higher than in those ages 11-24 years. Also, the agreed upon recommended policy for earlier immunization is in step with current ACIP recommendations for use of the vaccine in persons with functional/anatomic asplenia or complement component deficiencies.
Despite an overall decline in the risk of meningococcal disease in the United States, there was a 13-fold increased risk in HIV-infected persons aged 25-64 years between 2000 and 2008, according to surveillance data presented to ACIP by Ms. Jessica MacNeil, MPH, an epidemiologist at the National Center for Immunization and Respiratory Diseases at the CDC in Atlanta. A ten-fold increase in risk was recorded in New York City alone in this population between 2000 and 2011.
Although fatality data are mixed, the infections were due primarily to serogroups C, W, and Y, for which the immune response wanes rapidly, according to Ms. MacNeil.
There are no safety or immunogenicity data currently available for the use of serogroup B meningococcal vaccines in HIV-infected persons, she said.
ACIP members also unanimously recommended this vaccine be covered under the Vaccines for Children program.
No information about disclosures was available at press time.
On Twitter @whitneymcknight
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously voted to recommend that all HIV-infected persons aged 2 months and older receive the meningococcal ACWY (MenACWY) vaccine.
Guidance for this recommendation states that persons 2 months and older with HIV who have not been vaccinated previously should receive a two-dose, primary series of MenACWY; and that HIV-infected persons who have been vaccinated previously with one dose of MenACWY should receive a second dose at the earliest opportunity, with an 8-week minimum interval between doses. After that, boosters are to be given at the appropriate intervals.
Committee members voted in favor of immunizing earlier rather than waiting until 11 years of age or older, in part because human complement (hSBA) antibody titers following up to two doses of MenACWY vaccine in HIV-infected children ages 2-10 years is higher than in those ages 11-24 years. Also, the agreed upon recommended policy for earlier immunization is in step with current ACIP recommendations for use of the vaccine in persons with functional/anatomic asplenia or complement component deficiencies.
Despite an overall decline in the risk of meningococcal disease in the United States, there was a 13-fold increased risk in HIV-infected persons aged 25-64 years between 2000 and 2008, according to surveillance data presented to ACIP by Ms. Jessica MacNeil, MPH, an epidemiologist at the National Center for Immunization and Respiratory Diseases at the CDC in Atlanta. A ten-fold increase in risk was recorded in New York City alone in this population between 2000 and 2011.
Although fatality data are mixed, the infections were due primarily to serogroups C, W, and Y, for which the immune response wanes rapidly, according to Ms. MacNeil.
There are no safety or immunogenicity data currently available for the use of serogroup B meningococcal vaccines in HIV-infected persons, she said.
ACIP members also unanimously recommended this vaccine be covered under the Vaccines for Children program.
No information about disclosures was available at press time.
On Twitter @whitneymcknight
FROM AN ACIP MEETING
Medicare Trust Fund projected to run dry in 2028
The Medicare Trust Fund is projected to become insolvent in 2028, 2 years earlier than projected this time last year.
The change is based on two key factors: an expected reduction in payroll taxes and a slowdown in declining rate of inpatient utilization, Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said at a June 22 press conference to review the annual Medicare Trustees report.
“The good news is inpatient utilization is still declining, it’s just declining at a slightly lower rate,” Mr. Slavitt said.
He also highlighted the continuing rising cost of prescription medications, which he called “a major driver” in Medicare spending growth.
“For the second year in a row, we saw spending growth for prescription drugs dramatically outpace cost growth for other Medicare services,” Mr. Slavitt said. “Through 2025, Medicare Part D expenditures per enrollee are estimated to increase nearly 50% higher than the estimated increase in GDP per capita and higher than the combined per enrollee growth rate of Medicare Part A and Medicare Part B combined.”
Overall, total Medicare expenditures “are slightly lower than estimated last year,” Mr. Slavitt said, adding that over the next decade, “Medicare per-enrollee spending is projected to continue to grow slower than historical rates, at 4.3%, lower than the growth in overall per capita national health expenditures.”
The growth rate is not projected to trigger the activation of the Independent Payment Advisory Board, according to the report.
In 2015, Medicare covered 55.3 million people, including 46.3 million aged 65 and older, and 9 million disabled individuals.
The Medicare Trust Fund is projected to become insolvent in 2028, 2 years earlier than projected this time last year.
The change is based on two key factors: an expected reduction in payroll taxes and a slowdown in declining rate of inpatient utilization, Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said at a June 22 press conference to review the annual Medicare Trustees report.
“The good news is inpatient utilization is still declining, it’s just declining at a slightly lower rate,” Mr. Slavitt said.
He also highlighted the continuing rising cost of prescription medications, which he called “a major driver” in Medicare spending growth.
“For the second year in a row, we saw spending growth for prescription drugs dramatically outpace cost growth for other Medicare services,” Mr. Slavitt said. “Through 2025, Medicare Part D expenditures per enrollee are estimated to increase nearly 50% higher than the estimated increase in GDP per capita and higher than the combined per enrollee growth rate of Medicare Part A and Medicare Part B combined.”
Overall, total Medicare expenditures “are slightly lower than estimated last year,” Mr. Slavitt said, adding that over the next decade, “Medicare per-enrollee spending is projected to continue to grow slower than historical rates, at 4.3%, lower than the growth in overall per capita national health expenditures.”
The growth rate is not projected to trigger the activation of the Independent Payment Advisory Board, according to the report.
In 2015, Medicare covered 55.3 million people, including 46.3 million aged 65 and older, and 9 million disabled individuals.
The Medicare Trust Fund is projected to become insolvent in 2028, 2 years earlier than projected this time last year.
The change is based on two key factors: an expected reduction in payroll taxes and a slowdown in declining rate of inpatient utilization, Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said at a June 22 press conference to review the annual Medicare Trustees report.
“The good news is inpatient utilization is still declining, it’s just declining at a slightly lower rate,” Mr. Slavitt said.
He also highlighted the continuing rising cost of prescription medications, which he called “a major driver” in Medicare spending growth.
“For the second year in a row, we saw spending growth for prescription drugs dramatically outpace cost growth for other Medicare services,” Mr. Slavitt said. “Through 2025, Medicare Part D expenditures per enrollee are estimated to increase nearly 50% higher than the estimated increase in GDP per capita and higher than the combined per enrollee growth rate of Medicare Part A and Medicare Part B combined.”
Overall, total Medicare expenditures “are slightly lower than estimated last year,” Mr. Slavitt said, adding that over the next decade, “Medicare per-enrollee spending is projected to continue to grow slower than historical rates, at 4.3%, lower than the growth in overall per capita national health expenditures.”
The growth rate is not projected to trigger the activation of the Independent Payment Advisory Board, according to the report.
In 2015, Medicare covered 55.3 million people, including 46.3 million aged 65 and older, and 9 million disabled individuals.
Dos and don’ts of dealing with disruptive behavior
CHICAGO – Dealing with disruptive behavior by staff and colleagues isn’t just about knowing what to do – it’s also about knowing what not to do.
Often, mishandling disruptive behavior can make matters worse and lead to further conflict among physicians and employees, health law experts warn. At a conference held by the American Bar Association, attorneys offered guidance on the dos and don’ts of disruptive behavior management.
Don’t discipline for the wrong reasons
Know what disruptive behavior is not, advised Margo S. Struthers, a Minneapolis-based health law attorney. Criticism offered in good faith with the aim of improving patient care should not be considered disruptive, she said.
“This is a problem that comes up a lot because, often, there is some element of criticism that is offered by the supposed disruptive physician, which may or may not be justified, may or may have been done in good faith, and may or may not have been in a respectful manner.”
An isolated incident of behavior that is not reflective of a pattern of inappropriate, deep-seated, and habitual behavior should not be construed as disruptive, Ms. Struthers added. In addition, disruptive behavior is not respectful disagreement with leadership, presentation of controversial ideas, or the respectful complaining about processes that endanger patient care.
Do address behavior that is truly disruptive
According to the American Medical Association, disruptive behavior is defined as personal conduct, whether verbal or physical, that negatively affects or that potentially may negatively affect patient care, including conduct that interferes the ability to work with members of the health care team. Such behavior can be passive, such as ignoring calls or frequently missing meetings; passive-aggressive, such as excessive sarcasm or veiled threats; or aggressive, such as yelling or bullying.
Don’t focus solely on the behavior
Most disruptive behavior has a root cause, and efforts should be made to get to the bottom of the conduct, according to Sidney Welch, an Atlanta-based health law attorney.
“Often, there’s an underlying frustration in terms of clinical care or what they’re being told to do or the systems and processes [in play],” she said “Where is the sources of the tension that is creating the behavior?”
Do identify contributing factors
Personality characteristics that could lead to hostile behavior include self-centeredness, immaturity, resentfulness, or a need for power and control. Systemic factors could include increased productivity demands, cost-containment requirements, embedded hierarchies, fear of litigation, ineffective or absent conflict-resolution processes, competition between hospitals and medical staff, new care settings, and marketplace demands. Shortages of staff and high work burdens also could fuel disruptive behavior, Ms. Welch said.
“There are situations where there [is] a psychiatric disorder or a personality disorder that’s the root cause of the disruption,” she said. But “sometimes it’s just a stressful situation. A lot of these cases [in which] the physician is the disruption, we’re seeing them in high stakes emergency departments or situations where decisions have to be made very quickly, or fatigue and external stresses may be the source.”
Don’t apply corrective actions inconsistently
Make expectations clear by having a code of conduct supported by policies that apply to every employee, Ms. Welch noted. A lack of fairness among employees can create greater tension and generate increased conflict during a disruptive situation. Ensure that physicians are not be treated differently than nurses or administrators when addressing complaints, she said.
Do implement a graduated set of responses
A tiered response system (informal, formal, disciplinary, regulatory) helps manage disruptive situations based on the extent of conduct, Ms. Welch said.
“The process and disciplinary process [should] to be multileveled so that people know the rules of the road, and the parameters and the bumpers so to speak, are defined.”
Don’t necessarily involve HR
Be cautious of allowing human resource (HR) departments to direct potential disruptive physician issues, Ms. Struthers said.
“I have some concerns about HR getting involved for a couple of reasons,” she said. “If you get nonphysicians involved, it seems to exacerbate the level of tension.”
In addition, if a hospital has a significant number of both employed and independent physicians, HR can sometimes apply different standards and varied courses of action depending on employment status, she said. Of course, if the alleged disrupter is a nonphysician, HR is generally the only route for remedy within a hospital setting, Ms. Welch noted.
Do address the issue through internal processes
Every medical staff should develop and adopt bylaw provisions or policies for intervening in situations in which a physician’s behavior is identified as disruptive, according to AMA policy. Medical staff bylaw provisions or policies should contain procedural safeguards that protect due process.
For doctors in groups or small practices, employment policies and procedures should address protocols when disruption matters arise, Ms. Struthers said.
“The dynamics in a small clinic is that some doctors may more have power than others, so it may be harder in some contexts to treat everyone the same,” she said in an interview. “But that’s a really important thing to do. Any well-advised clinics would have a code of conduct or conduct policy, really to protect the clinic from employee lawsuits.”
Don’t let policies collect dust
“As we all know, you can write the policies, you can put them on the shelf, but if people aren’t reading them and understanding them and aren’t educated on them, then it really does no good,” Ms. Welch said.
Do ensure everyone knows how disruptive behavior is handled
Make certain that all staff review disruptive behavior policies and are adequately trained in how the process works, Ms. Welch added. Employees should know where to seek help if experiencing a disruption matter. Consider having staff members sign or acknowledge a code of conduct upon credentialing or hiring.
“Obviously, disruptive behavior can impact patient care, and it can come from a lot of differ directions; It’s not just physicians,” Ms. Struthers said. “Hospitals [and practices] like other places of business, need to have comprehensive polices and procedures, and they need to follow them.”
On Twitter @legal_med
CHICAGO – Dealing with disruptive behavior by staff and colleagues isn’t just about knowing what to do – it’s also about knowing what not to do.
Often, mishandling disruptive behavior can make matters worse and lead to further conflict among physicians and employees, health law experts warn. At a conference held by the American Bar Association, attorneys offered guidance on the dos and don’ts of disruptive behavior management.
Don’t discipline for the wrong reasons
Know what disruptive behavior is not, advised Margo S. Struthers, a Minneapolis-based health law attorney. Criticism offered in good faith with the aim of improving patient care should not be considered disruptive, she said.
“This is a problem that comes up a lot because, often, there is some element of criticism that is offered by the supposed disruptive physician, which may or may not be justified, may or may have been done in good faith, and may or may not have been in a respectful manner.”
An isolated incident of behavior that is not reflective of a pattern of inappropriate, deep-seated, and habitual behavior should not be construed as disruptive, Ms. Struthers added. In addition, disruptive behavior is not respectful disagreement with leadership, presentation of controversial ideas, or the respectful complaining about processes that endanger patient care.
Do address behavior that is truly disruptive
According to the American Medical Association, disruptive behavior is defined as personal conduct, whether verbal or physical, that negatively affects or that potentially may negatively affect patient care, including conduct that interferes the ability to work with members of the health care team. Such behavior can be passive, such as ignoring calls or frequently missing meetings; passive-aggressive, such as excessive sarcasm or veiled threats; or aggressive, such as yelling or bullying.
Don’t focus solely on the behavior
Most disruptive behavior has a root cause, and efforts should be made to get to the bottom of the conduct, according to Sidney Welch, an Atlanta-based health law attorney.
“Often, there’s an underlying frustration in terms of clinical care or what they’re being told to do or the systems and processes [in play],” she said “Where is the sources of the tension that is creating the behavior?”
Do identify contributing factors
Personality characteristics that could lead to hostile behavior include self-centeredness, immaturity, resentfulness, or a need for power and control. Systemic factors could include increased productivity demands, cost-containment requirements, embedded hierarchies, fear of litigation, ineffective or absent conflict-resolution processes, competition between hospitals and medical staff, new care settings, and marketplace demands. Shortages of staff and high work burdens also could fuel disruptive behavior, Ms. Welch said.
“There are situations where there [is] a psychiatric disorder or a personality disorder that’s the root cause of the disruption,” she said. But “sometimes it’s just a stressful situation. A lot of these cases [in which] the physician is the disruption, we’re seeing them in high stakes emergency departments or situations where decisions have to be made very quickly, or fatigue and external stresses may be the source.”
Don’t apply corrective actions inconsistently
Make expectations clear by having a code of conduct supported by policies that apply to every employee, Ms. Welch noted. A lack of fairness among employees can create greater tension and generate increased conflict during a disruptive situation. Ensure that physicians are not be treated differently than nurses or administrators when addressing complaints, she said.
Do implement a graduated set of responses
A tiered response system (informal, formal, disciplinary, regulatory) helps manage disruptive situations based on the extent of conduct, Ms. Welch said.
“The process and disciplinary process [should] to be multileveled so that people know the rules of the road, and the parameters and the bumpers so to speak, are defined.”
Don’t necessarily involve HR
Be cautious of allowing human resource (HR) departments to direct potential disruptive physician issues, Ms. Struthers said.
“I have some concerns about HR getting involved for a couple of reasons,” she said. “If you get nonphysicians involved, it seems to exacerbate the level of tension.”
In addition, if a hospital has a significant number of both employed and independent physicians, HR can sometimes apply different standards and varied courses of action depending on employment status, she said. Of course, if the alleged disrupter is a nonphysician, HR is generally the only route for remedy within a hospital setting, Ms. Welch noted.
Do address the issue through internal processes
Every medical staff should develop and adopt bylaw provisions or policies for intervening in situations in which a physician’s behavior is identified as disruptive, according to AMA policy. Medical staff bylaw provisions or policies should contain procedural safeguards that protect due process.
For doctors in groups or small practices, employment policies and procedures should address protocols when disruption matters arise, Ms. Struthers said.
“The dynamics in a small clinic is that some doctors may more have power than others, so it may be harder in some contexts to treat everyone the same,” she said in an interview. “But that’s a really important thing to do. Any well-advised clinics would have a code of conduct or conduct policy, really to protect the clinic from employee lawsuits.”
Don’t let policies collect dust
“As we all know, you can write the policies, you can put them on the shelf, but if people aren’t reading them and understanding them and aren’t educated on them, then it really does no good,” Ms. Welch said.
Do ensure everyone knows how disruptive behavior is handled
Make certain that all staff review disruptive behavior policies and are adequately trained in how the process works, Ms. Welch added. Employees should know where to seek help if experiencing a disruption matter. Consider having staff members sign or acknowledge a code of conduct upon credentialing or hiring.
“Obviously, disruptive behavior can impact patient care, and it can come from a lot of differ directions; It’s not just physicians,” Ms. Struthers said. “Hospitals [and practices] like other places of business, need to have comprehensive polices and procedures, and they need to follow them.”
On Twitter @legal_med
CHICAGO – Dealing with disruptive behavior by staff and colleagues isn’t just about knowing what to do – it’s also about knowing what not to do.
Often, mishandling disruptive behavior can make matters worse and lead to further conflict among physicians and employees, health law experts warn. At a conference held by the American Bar Association, attorneys offered guidance on the dos and don’ts of disruptive behavior management.
Don’t discipline for the wrong reasons
Know what disruptive behavior is not, advised Margo S. Struthers, a Minneapolis-based health law attorney. Criticism offered in good faith with the aim of improving patient care should not be considered disruptive, she said.
“This is a problem that comes up a lot because, often, there is some element of criticism that is offered by the supposed disruptive physician, which may or may not be justified, may or may have been done in good faith, and may or may not have been in a respectful manner.”
An isolated incident of behavior that is not reflective of a pattern of inappropriate, deep-seated, and habitual behavior should not be construed as disruptive, Ms. Struthers added. In addition, disruptive behavior is not respectful disagreement with leadership, presentation of controversial ideas, or the respectful complaining about processes that endanger patient care.
Do address behavior that is truly disruptive
According to the American Medical Association, disruptive behavior is defined as personal conduct, whether verbal or physical, that negatively affects or that potentially may negatively affect patient care, including conduct that interferes the ability to work with members of the health care team. Such behavior can be passive, such as ignoring calls or frequently missing meetings; passive-aggressive, such as excessive sarcasm or veiled threats; or aggressive, such as yelling or bullying.
Don’t focus solely on the behavior
Most disruptive behavior has a root cause, and efforts should be made to get to the bottom of the conduct, according to Sidney Welch, an Atlanta-based health law attorney.
“Often, there’s an underlying frustration in terms of clinical care or what they’re being told to do or the systems and processes [in play],” she said “Where is the sources of the tension that is creating the behavior?”
Do identify contributing factors
Personality characteristics that could lead to hostile behavior include self-centeredness, immaturity, resentfulness, or a need for power and control. Systemic factors could include increased productivity demands, cost-containment requirements, embedded hierarchies, fear of litigation, ineffective or absent conflict-resolution processes, competition between hospitals and medical staff, new care settings, and marketplace demands. Shortages of staff and high work burdens also could fuel disruptive behavior, Ms. Welch said.
“There are situations where there [is] a psychiatric disorder or a personality disorder that’s the root cause of the disruption,” she said. But “sometimes it’s just a stressful situation. A lot of these cases [in which] the physician is the disruption, we’re seeing them in high stakes emergency departments or situations where decisions have to be made very quickly, or fatigue and external stresses may be the source.”
Don’t apply corrective actions inconsistently
Make expectations clear by having a code of conduct supported by policies that apply to every employee, Ms. Welch noted. A lack of fairness among employees can create greater tension and generate increased conflict during a disruptive situation. Ensure that physicians are not be treated differently than nurses or administrators when addressing complaints, she said.
Do implement a graduated set of responses
A tiered response system (informal, formal, disciplinary, regulatory) helps manage disruptive situations based on the extent of conduct, Ms. Welch said.
“The process and disciplinary process [should] to be multileveled so that people know the rules of the road, and the parameters and the bumpers so to speak, are defined.”
Don’t necessarily involve HR
Be cautious of allowing human resource (HR) departments to direct potential disruptive physician issues, Ms. Struthers said.
“I have some concerns about HR getting involved for a couple of reasons,” she said. “If you get nonphysicians involved, it seems to exacerbate the level of tension.”
In addition, if a hospital has a significant number of both employed and independent physicians, HR can sometimes apply different standards and varied courses of action depending on employment status, she said. Of course, if the alleged disrupter is a nonphysician, HR is generally the only route for remedy within a hospital setting, Ms. Welch noted.
Do address the issue through internal processes
Every medical staff should develop and adopt bylaw provisions or policies for intervening in situations in which a physician’s behavior is identified as disruptive, according to AMA policy. Medical staff bylaw provisions or policies should contain procedural safeguards that protect due process.
For doctors in groups or small practices, employment policies and procedures should address protocols when disruption matters arise, Ms. Struthers said.
“The dynamics in a small clinic is that some doctors may more have power than others, so it may be harder in some contexts to treat everyone the same,” she said in an interview. “But that’s a really important thing to do. Any well-advised clinics would have a code of conduct or conduct policy, really to protect the clinic from employee lawsuits.”
Don’t let policies collect dust
“As we all know, you can write the policies, you can put them on the shelf, but if people aren’t reading them and understanding them and aren’t educated on them, then it really does no good,” Ms. Welch said.
Do ensure everyone knows how disruptive behavior is handled
Make certain that all staff review disruptive behavior policies and are adequately trained in how the process works, Ms. Welch added. Employees should know where to seek help if experiencing a disruption matter. Consider having staff members sign or acknowledge a code of conduct upon credentialing or hiring.
“Obviously, disruptive behavior can impact patient care, and it can come from a lot of differ directions; It’s not just physicians,” Ms. Struthers said. “Hospitals [and practices] like other places of business, need to have comprehensive polices and procedures, and they need to follow them.”
On Twitter @legal_med
AT THE PHYSICIANS LEGAL ISSUES CONFERENCE
Sleep Apnea in Later Life More Than Doubles Subsequent Alzheimer’s Risk
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.
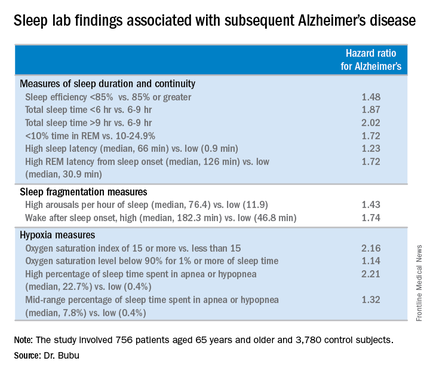
Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.

Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.

Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
AT SLEEP 2016
Sleep apnea in later life more than doubles subsequent Alzheimer’s risk
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.
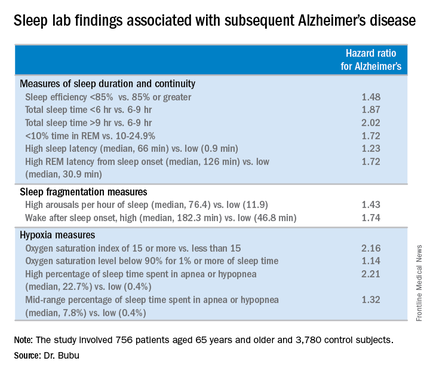
Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.

Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
DENVER – Obstructive sleep apnea diagnosed later in life is associated with an increased likelihood of subsequent Alzheimer’s disease, Dr. Omonigho Bubu reported at the annual meeting of the Associated Professional Sleep Societies.
He presented a retrospective cohort study in which a dose-reponse relationship was apparent. The more severe an individual’s obstructive sleep apnea (OSA) as reflected in a higher apnea-hypopnea index on polysomnography, the greater the risk of later being diagnosed with Alzheimer’s disease, compared with matched controls during up to 13 years of follow-up.
The study also identified several possible contributing factors for the observed OSA/Alzheimer’s relationship. Those OSA patients with more severe sleep fragmentation, nocturnal hypoxia, and abnormal sleep duration were significantly more likely to subsequently develop Alzheimer’s disease than were OSA patients with less severely disrupted sleep measures, added Dr. Bubu of the University of South Florida, Tampa.
The study included 756 patients aged 65 years and older with no history of cognitive decline when diagnosed with OSA by polysomnography at Tampa General Hospital during 2001-2005. They were matched by age, race, sex, body mass index, and zip code to two control groups totaling 3,780 subjects. The controls, drawn from outpatient medical clinics at the hospital, had a variety of medical problems but no sleep disorders or cognitive impairment.
During a mean 10.5-year follow-up period, 513 subjects were diagnosed with Alzheimer’s disease, according to Medicare data. In a Cox proportional hazards analysis adjusted for age, sex, race, body mass index, and education level, OSA was independently associated with a 2.2-fold increased risk. Further adjustment for alcohol intake, smoking, use of sleep medications, and chronic medical conditions didn’t substantially change the results.
However, the investigators were not able to control for apolipoprotein E (APOE)–epsilon 4 allele status, which is a known risk factor for both OSA and Alzheimer’s disease, so it makes one wonder whether the association is “all related to APOE,” said Dr. Richard J. Caselli, professor of neurology at the Mayo Clinic, Scottsdale, Ariz., when asked to comment on the study.
Time to onset of Alzheimer’s disease was shorter in the OSA patients: The mean time to diagnosis was 60.8 months after diagnosis of OSA, compared with 73 and 78 months in members of the two control groups who developed the dementia.
When the risk of developing Alzheimer’s disease was stratified according to baseline OSA severity, a dose-response effect was seen. Mild OSA, defined as 5-14 apnea-hypopnea events per hour of sleep, was associated with a 1.67-fold greater risk than in controls. The moderate OSA group, who had 15-29 events per hour, had a 1.81-fold increased risk. Patients with severe OSA, with 30 or more events per hour, had a 2.63-fold increased risk.

Gender, race, and education modified the relationship between OSA and Alzheimer’s disease, Dr. Bubu said. Women with OSA had a 2.28-fold greater risk of later developing the disease, compared with controls; men had a 1.42-fold increased risk. African-Americans with OSA were at 2.56-fold greater risk than were controls, while Hispanics with OSA were at 1.8-fold increased risk and non-Hispanic whites were at 1.87-fold increased risk. OSA patients with a high school education or less were at 2.73 times greater risk of Alzheimer’s disease than were controls, those with at least some college or technical school were at 1.82-fold risk, and OSA patients who’d been to graduate school had a 1.31-fold increased risk.
“Our results definitely show that OSA precedes the onset of Alzheimer’s disease. But we cannot say that’s causation. That will be left to future research examining the potential mechanisms we’ve identified,” Dr. Bubu said in an interview.
A key missing link in establishing a causal relationship is the lack of data on how many of the older patients diagnosed with OSA accepted treatment for the condition, and what their response rates were. In other words, it remains to be seen whether OSA occurring later in life is a modifiable risk factor for Alzheimer’s disease as opposed to an early expression of the dementing disease process whereby treatment of the sleep disorder doesn’t affect the progressive cognitive decline.
Both short sleep duration of less than 6 hours as well as a mean total sleep time greater than 9 hours in patients with OSA were associated with significantly increased risk of Alzheimer’s disease, compared with a sleep time of 6-9 hours. Patients with a high sleep-onset latency in the sleep lab, a high REM latency from sleep onset, a low percentage of time spent in REM, an oxygen saturation level of less than 90% for at least 1% of sleep time, and/or a high number of arousals per hour of sleep were also at increased risk of subsequent Alzheimer’s disease.
The study was supported by the Byrd Alzheimer’s Institute. Dr. Bubu reported having no financial conflicts.
AT SLEEP 2016
Key clinical point: Patients diagnosed with obstructive sleep apnea after age 65 may be at increased risk of later developing Alzheimer’s disease.
Major finding: A dose-response effect was seen: obstructive sleep apnea classified as mild was associated with a 1.67-fold increased risk of subsequent Alzheimer’s disease, moderate with a 1.81-fold risk, and severe obstructive sleep apnea carried a 2.63-fold increased risk.
Data source: This was a retrospective cohort study including 756 patients age 65 or older with no history of cognitive impairment who were diagnosed with obstructive sleep apnea during 2001-2005 and 3,780 matched controls, all followed for up to 13 years.
Disclosures: The study was supported by the Byrd Alzheimer’s Institute. The presenter reported having no financial conflicts.
Insomnia Is Pervasive in Adult Neurodevelopmental Disorders
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
AT SLEEP 2016
Insomnia is pervasive in adult neurodevelopmental disorders
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
DENVER – Adults with ADHD or autism spectrum disorder experience a high burden of sleep disturbances, especially insomnia, Dr. Anastasios Galanopoulos reported at the annual meeting of the Associated Professional Sleep Societies.
This has previously been established to be the case in pediatric patients with these neurodevelopmental disorders. However, sleep pathology hasn’t previously been well studied in affected adults, according to Dr. Galanopoulos, a consulting psychiatrist at Maudsley Hospital and King’s College London.
He presented a cross-sectional study of insomnia in a clinically representative sample comprising 164 adult patients: 98 with a DSM-5 diagnosis of ADHD, 30 with autism spectrum disorder, and 34 carrying both diagnoses.
Fully 91% of participants fell into the “poor” sleep category on the Pittsburgh Sleep Quality Index. Moreover, 44% of subjects had either moderate or severe clinical insomnia as reflected in a score of 15 or more on the Insomnia Severity Index. The rate of high sleep disruption scores was similar, regardless of whether the diagnosis was ADHD, autism spectrum disorder, or both.
Anxiety but not depression ratings on the Hospital Anxiety and Depression Scale correlated with insomnia scores, regardless of neurodevelopmental diagnosis. Insomnia scores also increased in concert with higher levels of hyperactivity symptoms as scored on the Barkley Adult ADHD Rating Scale. In contrast, inattentiveness scores were unrelated to insomnia.
Hyperactivity scores on the Barkley scale were significantly higher in adults with ADHD than in those with autism spectrum disorder. On the other hand, anxiety scores were higher in adults with autism spectrum disorder.
While the burden of sleep disturbances is similarly high in adults with ADHD and autism spectrum disorder, the underlying mechanism may well be different. For this reason, Dr. Galanopoulos and coinvestigators have begun studies systematically looking at various possible interventions for sleep disorders in adults with neurodevelopmental disorders.
He reported having no financial conflicts regarding this study, which was conducted without commercial support.
AT SLEEP 2016
Key clinical point: Clinically significant insomnia is extremely common in adults with ADHD or autism spectrum disorder.
Major finding: 44% of adults with ADHD, autism spectrum disorder, or both diagnoses had moderate or severe insomnia on the validated Insomnia Severity Index.
Data source: This was a cross-sectional study of insomnia in 164 adults with neurodevelopmental disorders.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted without commercial support.
Autism spectrum disorders in gender-nonconforming youth
Despite the lack of clarity about the exact prevalence in youth, particularly prepubertal youth, it’s clear that an increasing number of children and teenagers are presenting to multidisciplinary clinics for evaluation and management of gender identity issues (Pediatrics. 2012 Mar;129[3]:418-25). Recent literature has indicated that the prevalence of individuals overall identifying as transgender and/or experiencing gender dysphoria is about 0.2%-0.3% of the population (JAMA Pediatr. 2016;170[5]:423-4), while being mindful of the variability in the characteristics of the populations included in certain studies.
Although gender variation, in and of itself, is not a disorder, these youth are a considerably underserved and poorly researched population with specific medical and mental health needs. They are at risk for being victims of abuse and developing a range of mental health struggles, including mood disorders, suicidal thinking, self-harming, and anxiety, possibly because of the stresses they experience socially (family rejection, victimization). In addition to these more discrete psychiatric phenomena, co-occurring autism spectrum disorders (or traits thereof) are increasingly being recognized in transgender youth.
Case summary
Maddie is a 20-year-old college sophomore who presents for an autism diagnostic evaluation after being seen by a mental health colleague for consultation regarding medical treatment associated with female-to-male gender transition. In brief, Maddie reports a longstanding history of feeling “socially overwhelmed.” He is an articulate young adult who shares that he’s frequently uncomfortable in social situations, explaining that he has trouble “reading people,” acknowledging a tendency to become overly focused on the details of others and missing the gestalt of interpersonal interactions. Maddie discloses never having close friendships, and although he identifies as lonely, he doesn’t “understand the appeal of casual social interactions.” Maddie and his mother seek an understanding of such social difficulties, wondering about the possibility of Asperger’s syndrome.
Maddie, whose assigned gender at birth was female, now identifies as male. His mother recalls him to have been a precocious preschooler in terms of language development who had no significant developmental delays and became, per report, increasingly withdrawn in elementary school. Over the years, educators have commented repeatedly on the fact that Maddie has needed to work on his “people skills,” with past providers ascribing such struggles to anxiety and social awkwardness that were thought to inherently accompany gender-variant behaviors and thinking. Maddie socially transitioned from a female to male in grade 11 and began taking prescribed testosterone about 4 years ago.
In the interview, Maddie is a hesitant male-appearing individual whose eye contact and spontaneity are limited. He has a guarded manner, and although he is able to answer questions posed to him, rapport is difficult to establish as he appears interpersonally uncomfortable.
Prior to the visit, Maddie’s mother completed the Social Responsiveness Scales, which revealed elevated scores in the area of Social Communication and Social Motivation. Despite appearing cognitively bright and having an array of academic and creative strengths, Maddie’s troubles forming meaningful relationships are striking. Deficits in social-emotional competence are incommensurate with his suspected cognitive abilities, and although he relates learning social skills from film over the years, he endorses pervasively compromised social-communication aptitudes and enduring functional impairments. “I’m always playing catch-up socially,” he shares, wondering how this will affect him in his nursing career.
Discussion
Maddie and his mother participated in an autism diagnostic evaluation, including the administration of the Autism Diagnostic Interview–Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS). The evaluation revealed a profile of social impairments and inflexible thinking dating back to early childhood that supported a diagnosis of an autism spectrum disorder (ASD). Other diagnoses, including anxiety disorders, a depressive disorder, a social pragmatic communication disorder, and a nonverbal learning disorder, were considered, but ultimately it was concluded that ASD best captured Maddie, who also continued to display features consistent with gender dysphoria.
Over the past 30 years, the relationship between gender-related concerns and ASD has begun to be discussed in the scientific literature, beginning with case reports and more recently with a retrospective chart study that yielded results suggesting that subjects diagnosed with ASD were almost eight times more likely to report gender variance than a nonreferred standardization sample of the Child Behavior Checklist (CBCL) (Transgender Health. 2016 Feb;1[1]:63-8). These results echo those of a prior study showing that 7.8% of individuals presenting for an evaluation in a Dutch gender clinic had an ASD diagnosis (J Autism Dev Disord. 2010 Aug;40[8]:930-6).
With the individual prevalences for ASD and gender dysphoria taken into account, these numbers suggest that the prevalence of ASD in gender-dysphoric youth is about four times higher than that found in the general population (Sex Med Rev. 2016;4:3e14). These data raise questions regarding potential shared etiology and the neurobiology between a disorder of social communication and gender identity–related issues; however, to date, despite several theories attempting to explain the connection (the notion of an “extreme male brain,” role of sex hormones, etc.), the relationship remains puzzling. Some have argued that the rigid thinking that can characterize ASD may lead those individuals who display any gender-variant behaviors to automatically identify as the gender opposite their biologic gender, even though they aren’t necessarily experiencing frank gender dysphoria or a core gender identity that is firmly discordant from their anatomical sex. Thinking about the relationship from another perspective, perhaps those with ASD are less aware of the social constructs of gender and are able to express their gender identity (whether it be crossgender or cisgender) more freely and without the worry of how they may be perceived by others. Undoubtedly, a complex interplay between ASD and gender dysphoria/gender-nonconforming behavior is suspected, and more quality research is needed – research that, at a minimum, is informed by the use of representative sample groups drawn from all developmental periods and well-defined diagnostic constructs.
Clinical pearl
Maddie, a transgender man, received a diagnosis of an autism spectrum disorder as a young adult – a full 16 years after the median age of diagnosis (MMWR Surveill Summ. 2016;65[3]:1-23).
The case illustrates some of the difficulties of attempting to parse out the cause of social impairments in an individual who also may have other phenomena that influence interpersonal functioning (attention-deficit/hyperactivity disorder, anxiety, gender dysphoria, etc.) in a developmental manner. Diagnostic “overshadowing” (mistakenly attributing symptoms to a particular condition and paying little attention to other possibilities) is not uncommon in youth with ASD and co-occurring conditions. Given the overlap between gender-nonconforming youth, individuals with gender dysphoria, and ASD, primary care providers may opt to incorporate mental health professionals familiar with ASD into the assessment and care of these children and adolescents. Getting a complete developmental history and paying attention to the possibility of early red flags for ASD can help to elucidate diagnostic possibilities and inform treatment planning. If a co-occurring diagnosis is confirmed, implementation of evidence-based interventions that are provided through the lens of ASD can help to minimize the risk of developing other co-occurring pathology, build resilience, alleviate social impairment, and foster self-advocacy skills in this very vulnerable population. Regardless of whether gender-variant youth and adolescents have an autistic disorder, mental health providers can have an integral role in assisting these individuals and their families to navigate a complicated system of care, address gender dysphoria, and promote healthy identity development (J Am Acad Child Adolesc Psychiatry. 2016 Jun;55[6]:441-3).
Dr. Dickerson, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, where he is director of the autism diagnostic clinic.
Despite the lack of clarity about the exact prevalence in youth, particularly prepubertal youth, it’s clear that an increasing number of children and teenagers are presenting to multidisciplinary clinics for evaluation and management of gender identity issues (Pediatrics. 2012 Mar;129[3]:418-25). Recent literature has indicated that the prevalence of individuals overall identifying as transgender and/or experiencing gender dysphoria is about 0.2%-0.3% of the population (JAMA Pediatr. 2016;170[5]:423-4), while being mindful of the variability in the characteristics of the populations included in certain studies.
Although gender variation, in and of itself, is not a disorder, these youth are a considerably underserved and poorly researched population with specific medical and mental health needs. They are at risk for being victims of abuse and developing a range of mental health struggles, including mood disorders, suicidal thinking, self-harming, and anxiety, possibly because of the stresses they experience socially (family rejection, victimization). In addition to these more discrete psychiatric phenomena, co-occurring autism spectrum disorders (or traits thereof) are increasingly being recognized in transgender youth.
Case summary
Maddie is a 20-year-old college sophomore who presents for an autism diagnostic evaluation after being seen by a mental health colleague for consultation regarding medical treatment associated with female-to-male gender transition. In brief, Maddie reports a longstanding history of feeling “socially overwhelmed.” He is an articulate young adult who shares that he’s frequently uncomfortable in social situations, explaining that he has trouble “reading people,” acknowledging a tendency to become overly focused on the details of others and missing the gestalt of interpersonal interactions. Maddie discloses never having close friendships, and although he identifies as lonely, he doesn’t “understand the appeal of casual social interactions.” Maddie and his mother seek an understanding of such social difficulties, wondering about the possibility of Asperger’s syndrome.
Maddie, whose assigned gender at birth was female, now identifies as male. His mother recalls him to have been a precocious preschooler in terms of language development who had no significant developmental delays and became, per report, increasingly withdrawn in elementary school. Over the years, educators have commented repeatedly on the fact that Maddie has needed to work on his “people skills,” with past providers ascribing such struggles to anxiety and social awkwardness that were thought to inherently accompany gender-variant behaviors and thinking. Maddie socially transitioned from a female to male in grade 11 and began taking prescribed testosterone about 4 years ago.
In the interview, Maddie is a hesitant male-appearing individual whose eye contact and spontaneity are limited. He has a guarded manner, and although he is able to answer questions posed to him, rapport is difficult to establish as he appears interpersonally uncomfortable.
Prior to the visit, Maddie’s mother completed the Social Responsiveness Scales, which revealed elevated scores in the area of Social Communication and Social Motivation. Despite appearing cognitively bright and having an array of academic and creative strengths, Maddie’s troubles forming meaningful relationships are striking. Deficits in social-emotional competence are incommensurate with his suspected cognitive abilities, and although he relates learning social skills from film over the years, he endorses pervasively compromised social-communication aptitudes and enduring functional impairments. “I’m always playing catch-up socially,” he shares, wondering how this will affect him in his nursing career.
Discussion
Maddie and his mother participated in an autism diagnostic evaluation, including the administration of the Autism Diagnostic Interview–Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS). The evaluation revealed a profile of social impairments and inflexible thinking dating back to early childhood that supported a diagnosis of an autism spectrum disorder (ASD). Other diagnoses, including anxiety disorders, a depressive disorder, a social pragmatic communication disorder, and a nonverbal learning disorder, were considered, but ultimately it was concluded that ASD best captured Maddie, who also continued to display features consistent with gender dysphoria.
Over the past 30 years, the relationship between gender-related concerns and ASD has begun to be discussed in the scientific literature, beginning with case reports and more recently with a retrospective chart study that yielded results suggesting that subjects diagnosed with ASD were almost eight times more likely to report gender variance than a nonreferred standardization sample of the Child Behavior Checklist (CBCL) (Transgender Health. 2016 Feb;1[1]:63-8). These results echo those of a prior study showing that 7.8% of individuals presenting for an evaluation in a Dutch gender clinic had an ASD diagnosis (J Autism Dev Disord. 2010 Aug;40[8]:930-6).
With the individual prevalences for ASD and gender dysphoria taken into account, these numbers suggest that the prevalence of ASD in gender-dysphoric youth is about four times higher than that found in the general population (Sex Med Rev. 2016;4:3e14). These data raise questions regarding potential shared etiology and the neurobiology between a disorder of social communication and gender identity–related issues; however, to date, despite several theories attempting to explain the connection (the notion of an “extreme male brain,” role of sex hormones, etc.), the relationship remains puzzling. Some have argued that the rigid thinking that can characterize ASD may lead those individuals who display any gender-variant behaviors to automatically identify as the gender opposite their biologic gender, even though they aren’t necessarily experiencing frank gender dysphoria or a core gender identity that is firmly discordant from their anatomical sex. Thinking about the relationship from another perspective, perhaps those with ASD are less aware of the social constructs of gender and are able to express their gender identity (whether it be crossgender or cisgender) more freely and without the worry of how they may be perceived by others. Undoubtedly, a complex interplay between ASD and gender dysphoria/gender-nonconforming behavior is suspected, and more quality research is needed – research that, at a minimum, is informed by the use of representative sample groups drawn from all developmental periods and well-defined diagnostic constructs.
Clinical pearl
Maddie, a transgender man, received a diagnosis of an autism spectrum disorder as a young adult – a full 16 years after the median age of diagnosis (MMWR Surveill Summ. 2016;65[3]:1-23).
The case illustrates some of the difficulties of attempting to parse out the cause of social impairments in an individual who also may have other phenomena that influence interpersonal functioning (attention-deficit/hyperactivity disorder, anxiety, gender dysphoria, etc.) in a developmental manner. Diagnostic “overshadowing” (mistakenly attributing symptoms to a particular condition and paying little attention to other possibilities) is not uncommon in youth with ASD and co-occurring conditions. Given the overlap between gender-nonconforming youth, individuals with gender dysphoria, and ASD, primary care providers may opt to incorporate mental health professionals familiar with ASD into the assessment and care of these children and adolescents. Getting a complete developmental history and paying attention to the possibility of early red flags for ASD can help to elucidate diagnostic possibilities and inform treatment planning. If a co-occurring diagnosis is confirmed, implementation of evidence-based interventions that are provided through the lens of ASD can help to minimize the risk of developing other co-occurring pathology, build resilience, alleviate social impairment, and foster self-advocacy skills in this very vulnerable population. Regardless of whether gender-variant youth and adolescents have an autistic disorder, mental health providers can have an integral role in assisting these individuals and their families to navigate a complicated system of care, address gender dysphoria, and promote healthy identity development (J Am Acad Child Adolesc Psychiatry. 2016 Jun;55[6]:441-3).
Dr. Dickerson, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, where he is director of the autism diagnostic clinic.
Despite the lack of clarity about the exact prevalence in youth, particularly prepubertal youth, it’s clear that an increasing number of children and teenagers are presenting to multidisciplinary clinics for evaluation and management of gender identity issues (Pediatrics. 2012 Mar;129[3]:418-25). Recent literature has indicated that the prevalence of individuals overall identifying as transgender and/or experiencing gender dysphoria is about 0.2%-0.3% of the population (JAMA Pediatr. 2016;170[5]:423-4), while being mindful of the variability in the characteristics of the populations included in certain studies.
Although gender variation, in and of itself, is not a disorder, these youth are a considerably underserved and poorly researched population with specific medical and mental health needs. They are at risk for being victims of abuse and developing a range of mental health struggles, including mood disorders, suicidal thinking, self-harming, and anxiety, possibly because of the stresses they experience socially (family rejection, victimization). In addition to these more discrete psychiatric phenomena, co-occurring autism spectrum disorders (or traits thereof) are increasingly being recognized in transgender youth.
Case summary
Maddie is a 20-year-old college sophomore who presents for an autism diagnostic evaluation after being seen by a mental health colleague for consultation regarding medical treatment associated with female-to-male gender transition. In brief, Maddie reports a longstanding history of feeling “socially overwhelmed.” He is an articulate young adult who shares that he’s frequently uncomfortable in social situations, explaining that he has trouble “reading people,” acknowledging a tendency to become overly focused on the details of others and missing the gestalt of interpersonal interactions. Maddie discloses never having close friendships, and although he identifies as lonely, he doesn’t “understand the appeal of casual social interactions.” Maddie and his mother seek an understanding of such social difficulties, wondering about the possibility of Asperger’s syndrome.
Maddie, whose assigned gender at birth was female, now identifies as male. His mother recalls him to have been a precocious preschooler in terms of language development who had no significant developmental delays and became, per report, increasingly withdrawn in elementary school. Over the years, educators have commented repeatedly on the fact that Maddie has needed to work on his “people skills,” with past providers ascribing such struggles to anxiety and social awkwardness that were thought to inherently accompany gender-variant behaviors and thinking. Maddie socially transitioned from a female to male in grade 11 and began taking prescribed testosterone about 4 years ago.
In the interview, Maddie is a hesitant male-appearing individual whose eye contact and spontaneity are limited. He has a guarded manner, and although he is able to answer questions posed to him, rapport is difficult to establish as he appears interpersonally uncomfortable.
Prior to the visit, Maddie’s mother completed the Social Responsiveness Scales, which revealed elevated scores in the area of Social Communication and Social Motivation. Despite appearing cognitively bright and having an array of academic and creative strengths, Maddie’s troubles forming meaningful relationships are striking. Deficits in social-emotional competence are incommensurate with his suspected cognitive abilities, and although he relates learning social skills from film over the years, he endorses pervasively compromised social-communication aptitudes and enduring functional impairments. “I’m always playing catch-up socially,” he shares, wondering how this will affect him in his nursing career.
Discussion
Maddie and his mother participated in an autism diagnostic evaluation, including the administration of the Autism Diagnostic Interview–Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS). The evaluation revealed a profile of social impairments and inflexible thinking dating back to early childhood that supported a diagnosis of an autism spectrum disorder (ASD). Other diagnoses, including anxiety disorders, a depressive disorder, a social pragmatic communication disorder, and a nonverbal learning disorder, were considered, but ultimately it was concluded that ASD best captured Maddie, who also continued to display features consistent with gender dysphoria.
Over the past 30 years, the relationship between gender-related concerns and ASD has begun to be discussed in the scientific literature, beginning with case reports and more recently with a retrospective chart study that yielded results suggesting that subjects diagnosed with ASD were almost eight times more likely to report gender variance than a nonreferred standardization sample of the Child Behavior Checklist (CBCL) (Transgender Health. 2016 Feb;1[1]:63-8). These results echo those of a prior study showing that 7.8% of individuals presenting for an evaluation in a Dutch gender clinic had an ASD diagnosis (J Autism Dev Disord. 2010 Aug;40[8]:930-6).
With the individual prevalences for ASD and gender dysphoria taken into account, these numbers suggest that the prevalence of ASD in gender-dysphoric youth is about four times higher than that found in the general population (Sex Med Rev. 2016;4:3e14). These data raise questions regarding potential shared etiology and the neurobiology between a disorder of social communication and gender identity–related issues; however, to date, despite several theories attempting to explain the connection (the notion of an “extreme male brain,” role of sex hormones, etc.), the relationship remains puzzling. Some have argued that the rigid thinking that can characterize ASD may lead those individuals who display any gender-variant behaviors to automatically identify as the gender opposite their biologic gender, even though they aren’t necessarily experiencing frank gender dysphoria or a core gender identity that is firmly discordant from their anatomical sex. Thinking about the relationship from another perspective, perhaps those with ASD are less aware of the social constructs of gender and are able to express their gender identity (whether it be crossgender or cisgender) more freely and without the worry of how they may be perceived by others. Undoubtedly, a complex interplay between ASD and gender dysphoria/gender-nonconforming behavior is suspected, and more quality research is needed – research that, at a minimum, is informed by the use of representative sample groups drawn from all developmental periods and well-defined diagnostic constructs.
Clinical pearl
Maddie, a transgender man, received a diagnosis of an autism spectrum disorder as a young adult – a full 16 years after the median age of diagnosis (MMWR Surveill Summ. 2016;65[3]:1-23).
The case illustrates some of the difficulties of attempting to parse out the cause of social impairments in an individual who also may have other phenomena that influence interpersonal functioning (attention-deficit/hyperactivity disorder, anxiety, gender dysphoria, etc.) in a developmental manner. Diagnostic “overshadowing” (mistakenly attributing symptoms to a particular condition and paying little attention to other possibilities) is not uncommon in youth with ASD and co-occurring conditions. Given the overlap between gender-nonconforming youth, individuals with gender dysphoria, and ASD, primary care providers may opt to incorporate mental health professionals familiar with ASD into the assessment and care of these children and adolescents. Getting a complete developmental history and paying attention to the possibility of early red flags for ASD can help to elucidate diagnostic possibilities and inform treatment planning. If a co-occurring diagnosis is confirmed, implementation of evidence-based interventions that are provided through the lens of ASD can help to minimize the risk of developing other co-occurring pathology, build resilience, alleviate social impairment, and foster self-advocacy skills in this very vulnerable population. Regardless of whether gender-variant youth and adolescents have an autistic disorder, mental health providers can have an integral role in assisting these individuals and their families to navigate a complicated system of care, address gender dysphoria, and promote healthy identity development (J Am Acad Child Adolesc Psychiatry. 2016 Jun;55[6]:441-3).
Dr. Dickerson, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, where he is director of the autism diagnostic clinic.