User login
Sore throat • vaginal discharge • labial ulcer • Dx?
THE CASE
The mother of a 13-year-old girl brought her daughter to our family medicine clinic for follow-up after being seen in the emergency department (ED) 3 days earlier. The girl had presented to the ED with a one-day history of back, chest, and vaginal pain. She was diagnosed with a urinary tract infection and treated empirically with phenazopyridine and cephalexin pending a urine culture.
During the follow-up appointment, the patient complained of worsening vaginal pain and increased vaginal discharge, but reported resolution of her back and chest pain. She also said that a week earlier, she’d had a fever that reached 104° F and a sore throat. She denied urinary frequency/urgency, sexual activity, or sexual abuse. The result of the urine culture performed in the ED was <10,000 col/mL (normal urogenital flora).
A genitourinary (GU) exam revealed erythematous patches with small amounts of crusting at the inner labia bilaterally. The labia were also swollen and diffusely tender to palpation. The patient had a white/gray discharge, but no vesicles or papules. The physician was unable to place a speculum due to pain.
The differential diagnosis at the time included candidal vaginitis and cellulitis. Since the patient’s skin was non-erythematous and she had vaginal discharge, she was treated for presumed severe candidal vaginitis with fluconazole and clotrimazole 1% cream. (The antibiotics were stopped because the patient reported worsening symptoms after they were prescribed in the ED.) The patient was told to return to the ED if she experienced signs and symptoms such as worsening vaginal pain or discharge, fever, or chills. A repeat urine culture was performed and the results came back normal.
Worsening symptoms. Six days later, the patient returned to the ED with urinary hesitation and persistent dysuria; she was admitted for pain control. She also complained of worsening labial swelling and increased vaginal discharge despite adherence to the fluconazole and clotrimazole cream regimen, which were discontinued on admission to the ED. She continued to deny being sexually active or abused.
A GU exam showed a 1-cm shallow ulcer on the right labium and a copious amount of foul-smelling white discharge. An Ob/Gyn resident and attending physician examined the patient; their differential diagnosis at this point included herpes simplex virus (HSV), Epstein-Barr virus (EBV), gonorrhea/chlamydia, and trauma. The patient was given topical lidocaine for pain control and started on acyclovir for presumed HSV while awaiting the HSV test results. A pelvic ultrasound and laboratory work-up were ordered at this time as well.
THE DIAGNOSIS
The pelvic ultrasound showed that the uterus was a normal size and that there was no gross mass or significant pelvic fluid. The patient’s right ovary measured 2.8 × 1.6 cm; the left ovary was not seen.
The patient’s laboratory work-up included an unremarkable comprehensive metabolic panel. A complete blood count was within normal limits, except for the patient’s monocyte level, which was at 12.9% (reference range: 0%-12%). The patient had a negative urinary human chorionic gonadotropin test, and was negative for HSV, chlamydia, gonorrhea, and trichomoniasis. A rapid plasma reagin test and human immunodeficiency virus antibody (1+2) tests were nonreactive. A wet prep was negative. A mononuclear spot test (monospot), however, was positive.
Results from the monospot testing took several days to return. By the time the results arrived, the patient had been transferred to a local children’s hospital for assessment in their pediatric urology department, as she was experiencing urinary hesitation and required catheterization. The diagnosis of infectious mononucleosis presenting with genital ulcer was made. EBV cultures were never obtained, but seemed to be the likely cause of the patient’s infectious mononucleosis given her clinical symptoms and lab results.
DISCUSSION
Approximately 95% of adults worldwide are infected with EBV.1 While the infection is often asymptomatic, some patients will develop infectious mononucleosis.1 EBV is the most common cause of infectious mononucleosis, mainly affecting teenagers and young adults (especially college students). At least 25% of teenagers and young adults who become infected with EBV will develop infectious mononucleosis.2
Typical symptoms of infectious mononucleosis include extreme fatigue, fever, sore throat, and head and body aches.2 In this case, the patient did have a fever and sore throat one week prior to presentation at our clinic, but she never complained of fatigue.
The association between mononucleosis and genital ulcers is not well known,3,4 and the exact method by which EBV causes genital ulcers is unclear.5 One review found that only 13 instances of genital ulceration in females attributable to EBV infection had been reported.5 When ulceration does occur, the majority of cases have involved young females who presented with only mild symptoms of mononucleosis.3,6 EBV has been found to present in the cervix, which suggests direct inoculation.3,6
Our patient remained catheterized for 2 days while in the children’s hospital. Her ulcer started to heal and she was sent home in stable condition. No additional follow-up was required and the ulcer did not recur.
THE TAKEAWAY
Include infectious mononucleosis in the differential for patients presenting with vaginal ulcers—especially those who deny sexual activity. Including testing for EBV and mononucleosis antibodies in the work-up can aid in the diagnosis. Cases such as this one are also a good reminder of the need to question young people while their parents/guardians are not in the examroom to foster an open and honest patient-physician relationship.
1. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372-376.
2. Centers for Disease Control and Prevention. About infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/about-mono.html. Accessed April 26, 2016.
3. Lorenzo CV, Robertson WS. Genital ulcerations as presenting symptom of infectious mononucleosis. J Am Board Fam Pract. 2005;18:67-68.
4. Sisson BA, Glick L. Genital ulceration as a presenting manifestation of infectious mononucleosis. J Pediatr Adolesc Gynecol. 1998;11:185-187.
5. Barnes CJ, Alió AB, Cunningham BB, et al. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130-134.
6. Wilson RW. Genital ulcers and mononucleosis. Pediatr Infect Dis J. 1993;12:418.
THE CASE
The mother of a 13-year-old girl brought her daughter to our family medicine clinic for follow-up after being seen in the emergency department (ED) 3 days earlier. The girl had presented to the ED with a one-day history of back, chest, and vaginal pain. She was diagnosed with a urinary tract infection and treated empirically with phenazopyridine and cephalexin pending a urine culture.
During the follow-up appointment, the patient complained of worsening vaginal pain and increased vaginal discharge, but reported resolution of her back and chest pain. She also said that a week earlier, she’d had a fever that reached 104° F and a sore throat. She denied urinary frequency/urgency, sexual activity, or sexual abuse. The result of the urine culture performed in the ED was <10,000 col/mL (normal urogenital flora).
A genitourinary (GU) exam revealed erythematous patches with small amounts of crusting at the inner labia bilaterally. The labia were also swollen and diffusely tender to palpation. The patient had a white/gray discharge, but no vesicles or papules. The physician was unable to place a speculum due to pain.
The differential diagnosis at the time included candidal vaginitis and cellulitis. Since the patient’s skin was non-erythematous and she had vaginal discharge, she was treated for presumed severe candidal vaginitis with fluconazole and clotrimazole 1% cream. (The antibiotics were stopped because the patient reported worsening symptoms after they were prescribed in the ED.) The patient was told to return to the ED if she experienced signs and symptoms such as worsening vaginal pain or discharge, fever, or chills. A repeat urine culture was performed and the results came back normal.
Worsening symptoms. Six days later, the patient returned to the ED with urinary hesitation and persistent dysuria; she was admitted for pain control. She also complained of worsening labial swelling and increased vaginal discharge despite adherence to the fluconazole and clotrimazole cream regimen, which were discontinued on admission to the ED. She continued to deny being sexually active or abused.
A GU exam showed a 1-cm shallow ulcer on the right labium and a copious amount of foul-smelling white discharge. An Ob/Gyn resident and attending physician examined the patient; their differential diagnosis at this point included herpes simplex virus (HSV), Epstein-Barr virus (EBV), gonorrhea/chlamydia, and trauma. The patient was given topical lidocaine for pain control and started on acyclovir for presumed HSV while awaiting the HSV test results. A pelvic ultrasound and laboratory work-up were ordered at this time as well.
THE DIAGNOSIS
The pelvic ultrasound showed that the uterus was a normal size and that there was no gross mass or significant pelvic fluid. The patient’s right ovary measured 2.8 × 1.6 cm; the left ovary was not seen.
The patient’s laboratory work-up included an unremarkable comprehensive metabolic panel. A complete blood count was within normal limits, except for the patient’s monocyte level, which was at 12.9% (reference range: 0%-12%). The patient had a negative urinary human chorionic gonadotropin test, and was negative for HSV, chlamydia, gonorrhea, and trichomoniasis. A rapid plasma reagin test and human immunodeficiency virus antibody (1+2) tests were nonreactive. A wet prep was negative. A mononuclear spot test (monospot), however, was positive.
Results from the monospot testing took several days to return. By the time the results arrived, the patient had been transferred to a local children’s hospital for assessment in their pediatric urology department, as she was experiencing urinary hesitation and required catheterization. The diagnosis of infectious mononucleosis presenting with genital ulcer was made. EBV cultures were never obtained, but seemed to be the likely cause of the patient’s infectious mononucleosis given her clinical symptoms and lab results.
DISCUSSION
Approximately 95% of adults worldwide are infected with EBV.1 While the infection is often asymptomatic, some patients will develop infectious mononucleosis.1 EBV is the most common cause of infectious mononucleosis, mainly affecting teenagers and young adults (especially college students). At least 25% of teenagers and young adults who become infected with EBV will develop infectious mononucleosis.2
Typical symptoms of infectious mononucleosis include extreme fatigue, fever, sore throat, and head and body aches.2 In this case, the patient did have a fever and sore throat one week prior to presentation at our clinic, but she never complained of fatigue.
The association between mononucleosis and genital ulcers is not well known,3,4 and the exact method by which EBV causes genital ulcers is unclear.5 One review found that only 13 instances of genital ulceration in females attributable to EBV infection had been reported.5 When ulceration does occur, the majority of cases have involved young females who presented with only mild symptoms of mononucleosis.3,6 EBV has been found to present in the cervix, which suggests direct inoculation.3,6
Our patient remained catheterized for 2 days while in the children’s hospital. Her ulcer started to heal and she was sent home in stable condition. No additional follow-up was required and the ulcer did not recur.
THE TAKEAWAY
Include infectious mononucleosis in the differential for patients presenting with vaginal ulcers—especially those who deny sexual activity. Including testing for EBV and mononucleosis antibodies in the work-up can aid in the diagnosis. Cases such as this one are also a good reminder of the need to question young people while their parents/guardians are not in the examroom to foster an open and honest patient-physician relationship.
THE CASE
The mother of a 13-year-old girl brought her daughter to our family medicine clinic for follow-up after being seen in the emergency department (ED) 3 days earlier. The girl had presented to the ED with a one-day history of back, chest, and vaginal pain. She was diagnosed with a urinary tract infection and treated empirically with phenazopyridine and cephalexin pending a urine culture.
During the follow-up appointment, the patient complained of worsening vaginal pain and increased vaginal discharge, but reported resolution of her back and chest pain. She also said that a week earlier, she’d had a fever that reached 104° F and a sore throat. She denied urinary frequency/urgency, sexual activity, or sexual abuse. The result of the urine culture performed in the ED was <10,000 col/mL (normal urogenital flora).
A genitourinary (GU) exam revealed erythematous patches with small amounts of crusting at the inner labia bilaterally. The labia were also swollen and diffusely tender to palpation. The patient had a white/gray discharge, but no vesicles or papules. The physician was unable to place a speculum due to pain.
The differential diagnosis at the time included candidal vaginitis and cellulitis. Since the patient’s skin was non-erythematous and she had vaginal discharge, she was treated for presumed severe candidal vaginitis with fluconazole and clotrimazole 1% cream. (The antibiotics were stopped because the patient reported worsening symptoms after they were prescribed in the ED.) The patient was told to return to the ED if she experienced signs and symptoms such as worsening vaginal pain or discharge, fever, or chills. A repeat urine culture was performed and the results came back normal.
Worsening symptoms. Six days later, the patient returned to the ED with urinary hesitation and persistent dysuria; she was admitted for pain control. She also complained of worsening labial swelling and increased vaginal discharge despite adherence to the fluconazole and clotrimazole cream regimen, which were discontinued on admission to the ED. She continued to deny being sexually active or abused.
A GU exam showed a 1-cm shallow ulcer on the right labium and a copious amount of foul-smelling white discharge. An Ob/Gyn resident and attending physician examined the patient; their differential diagnosis at this point included herpes simplex virus (HSV), Epstein-Barr virus (EBV), gonorrhea/chlamydia, and trauma. The patient was given topical lidocaine for pain control and started on acyclovir for presumed HSV while awaiting the HSV test results. A pelvic ultrasound and laboratory work-up were ordered at this time as well.
THE DIAGNOSIS
The pelvic ultrasound showed that the uterus was a normal size and that there was no gross mass or significant pelvic fluid. The patient’s right ovary measured 2.8 × 1.6 cm; the left ovary was not seen.
The patient’s laboratory work-up included an unremarkable comprehensive metabolic panel. A complete blood count was within normal limits, except for the patient’s monocyte level, which was at 12.9% (reference range: 0%-12%). The patient had a negative urinary human chorionic gonadotropin test, and was negative for HSV, chlamydia, gonorrhea, and trichomoniasis. A rapid plasma reagin test and human immunodeficiency virus antibody (1+2) tests were nonreactive. A wet prep was negative. A mononuclear spot test (monospot), however, was positive.
Results from the monospot testing took several days to return. By the time the results arrived, the patient had been transferred to a local children’s hospital for assessment in their pediatric urology department, as she was experiencing urinary hesitation and required catheterization. The diagnosis of infectious mononucleosis presenting with genital ulcer was made. EBV cultures were never obtained, but seemed to be the likely cause of the patient’s infectious mononucleosis given her clinical symptoms and lab results.
DISCUSSION
Approximately 95% of adults worldwide are infected with EBV.1 While the infection is often asymptomatic, some patients will develop infectious mononucleosis.1 EBV is the most common cause of infectious mononucleosis, mainly affecting teenagers and young adults (especially college students). At least 25% of teenagers and young adults who become infected with EBV will develop infectious mononucleosis.2
Typical symptoms of infectious mononucleosis include extreme fatigue, fever, sore throat, and head and body aches.2 In this case, the patient did have a fever and sore throat one week prior to presentation at our clinic, but she never complained of fatigue.
The association between mononucleosis and genital ulcers is not well known,3,4 and the exact method by which EBV causes genital ulcers is unclear.5 One review found that only 13 instances of genital ulceration in females attributable to EBV infection had been reported.5 When ulceration does occur, the majority of cases have involved young females who presented with only mild symptoms of mononucleosis.3,6 EBV has been found to present in the cervix, which suggests direct inoculation.3,6
Our patient remained catheterized for 2 days while in the children’s hospital. Her ulcer started to heal and she was sent home in stable condition. No additional follow-up was required and the ulcer did not recur.
THE TAKEAWAY
Include infectious mononucleosis in the differential for patients presenting with vaginal ulcers—especially those who deny sexual activity. Including testing for EBV and mononucleosis antibodies in the work-up can aid in the diagnosis. Cases such as this one are also a good reminder of the need to question young people while their parents/guardians are not in the examroom to foster an open and honest patient-physician relationship.
1. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372-376.
2. Centers for Disease Control and Prevention. About infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/about-mono.html. Accessed April 26, 2016.
3. Lorenzo CV, Robertson WS. Genital ulcerations as presenting symptom of infectious mononucleosis. J Am Board Fam Pract. 2005;18:67-68.
4. Sisson BA, Glick L. Genital ulceration as a presenting manifestation of infectious mononucleosis. J Pediatr Adolesc Gynecol. 1998;11:185-187.
5. Barnes CJ, Alió AB, Cunningham BB, et al. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130-134.
6. Wilson RW. Genital ulcers and mononucleosis. Pediatr Infect Dis J. 1993;12:418.
1. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372-376.
2. Centers for Disease Control and Prevention. About infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/about-mono.html. Accessed April 26, 2016.
3. Lorenzo CV, Robertson WS. Genital ulcerations as presenting symptom of infectious mononucleosis. J Am Board Fam Pract. 2005;18:67-68.
4. Sisson BA, Glick L. Genital ulceration as a presenting manifestation of infectious mononucleosis. J Pediatr Adolesc Gynecol. 1998;11:185-187.
5. Barnes CJ, Alió AB, Cunningham BB, et al. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130-134.
6. Wilson RW. Genital ulcers and mononucleosis. Pediatr Infect Dis J. 1993;12:418.
Large plaques on a baby boy
A 25-year-old G2P1 mother gave birth to a boy at 40 and 6/7 weeks by vaginal delivery. Labor was induced because of oligohydramnios complicated by chorioamnionitis. The mother was treated with vancomycin and gentamicin. Prenatal lab work and delivery were otherwise unremarkable.
The delivering physician (CG) noted that the neonate had numerous brown, red, and black plaques distributed over his abdomen, lower back, groin, and thighs (FIGURE). Some plaques were hypertrichotic and other areas, apart from the plaques, were thinly desquamated. Apgar scores were 8 and 9 and the remainder of the exam, including the neurologic exam, was normal. The Dermatology Service (JK) was consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Giant congenital nevus
Congenital melanocytic nevi (CMN) are pigmented lesions that are present at birth and created by the abnormal migration of neural crest cells during embryogenesis.1 Nevi are categorized by size as small (<1.5 cm), medium (1.5-20 cm), large (>20 cm), and giant (>40 cm).2 Congenital nevi tend to start out flat, with uniform pigmentation, but can become more variegated in texture and color as normal growth and development continue. Giant congenital nevi are likely to thicken, darken, and enlarge as the patient grows. Some nevi may develop very coarse or dark hair.
CMN can cover any part of the body and occur independent of skin color and other ethnic factors.3 Giant congenital nevi are rare, with an incidence of approximately one in 50,000 live births and with males and females equally affected.3,4 The condition is diagnosed at birth, based on the appearance of the lesions.
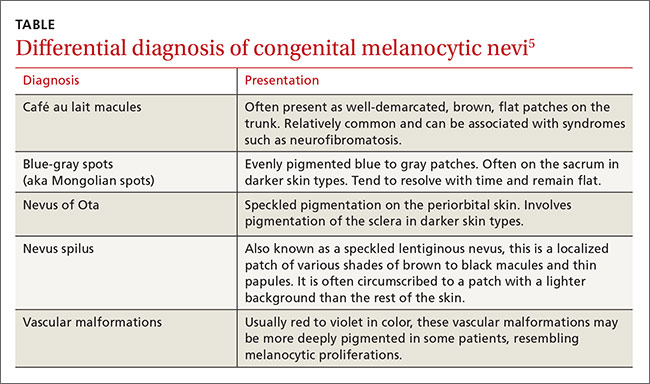
The differential diagnosis for CMN includes café au lait macules, blue-gray spots (aka Mongolian spots), nevus of Ota, nevus spilus, and vascular malformations (TABLE).5 CMN may present in almost any location and may be brown, black, pink, or purple in color. Café au lait macules, blue-gray spots, nevus of Ota, nevus spilus, and vascular malformations have individual location and color characteristics that set them apart clinically.
Monitor patients for melanoma, CNS complications
Patients with CMN are at increased risk of neurocutaneous melanosis (NCM) and cutaneous melanoma.
Neurocutaneous melanosis, a complication of giant congenital nevi, is a melanocyte proliferation in the central nervous system (CNS). Between 6% and 11% of patients with giant congenital nevi develop symptomatic NCM in childhood. Thus, any CNS symptoms should be fully evaluated.4,6 NCM can result in seizures, cranial nerve palsy, hydrocephalus, and leptomeningeal melanoma.
Besides giant congenital nevi, risk factors for NCM include male sex, large numbers of satellite nevi, and the presence of nevi over the posterior midline or head and neck.7 The prognosis is poor for patients who develop neurologic symptoms. NCM is associated with other malignancies, including rhadomyosarcoma, liposarcoma, and malignant peripheral nerve sheath tumors.4
Magnetic resonance imaging (MRI) is helpful to exclude NCM. Ideally, an MRI should be ordered before 4 months of age, at which time myelination begins to make the identification of melanin deposits in the CNS more challenging.7 Not all patients with imaging findings that are consistent with NCM will develop symptoms.8
Melanoma. By age 10, up to 8% of patients with giant congenital nevi will develop melanoma within the nevi; most of these cases occur during the first 2 years of life.7,9 Patients with NCM are at even greater risk: their rate of malignant melanoma is between 40% and 60%.6 As a result, patients should be monitored closely for any signs of the disease. Total body photography, serial clinical photos, and patient self-exam are helpful to detect changes and de novo lesions. New lesions or ulcerations superimposed on existing nevi may indicate malignancy.7 Sun protection is critical to reduce the risk of melanogenesis.
Should patients pursue surgery? It’s debatable
Options for patients with large and giant CMN include early curettage (prior to 2 weeks of life), local excision (often with tissue expansion), dermabrasion, and laser therapy.2 There is considerable debate about surgery. Advocates of surgery cite psychosocial relief as a major treatment benefit and speculate about prevention of melanoma. Opponents worry that excessive surgical intervention may cause melanogenesis in a scar or deep in an area of treatment. And, while smaller congenital nevi are easier to surgically remove, they have a low associated risk of developing melanoma and are typically monitored clinically.
Children with congenital nevi will need support
Several nonprofit organizations offer resources for children with congenital nevi and their families. Nevus Outreach (www.nevus.org) is an organization devoted to improving awareness and providing support for people with CMN and NCM. The group maintains a registry of patients with large nevi in an effort to help researchers improve treatment and identify a cure.
For children with congenital nevi and other skin conditions, the American Academy of Dermatology offers its “Camp Discovery” at locations across the country (https://www.aad.org/public/kids/camp-discovery). Camp Discovery provides full scholarships and includes transportation to each of the individual camps for attendees.
Our patient underwent an MRI on his fifth day of life. The results were normal and he hadn’t developed any neurologic symptoms at 4 months of age. The child sees his family physician for routine well-child visits and a dermatologist annually. The dermatologist is carefully monitoring the nevi, which continue to grow.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Suite 340, Augusta, ME 04330; [email protected].
1. Sarnat HB, Flores-Sarnat L. Embryology of the neural crest: its inductive role in the neurocutaneous syndromes. J Child Neurol. 2005:20:637-643.
2. Gosain AK, Santoro TD, Larson DL, et al. Giant congenital nevi: a 20-year experience and an algorithm for their management. Plast Reconstr Surg. 2001;108:622-636.
3. National Organization for Rare Disorders. Giant congenital melanocytic nevus. National Organization for Rare Disorders Web site. Available at: http://rarediseases.org/rare-diseases/giant-congenital-melanocytic-nevus. Accessed April 29, 2016.
4. Vourc’h-Jourdain M, Martin L, Barbarot S; aRED. Large congenital melanocytic nevi: therapeutic management and melanoma risk: a systematic review. J Am Acad Dermatol. 2013;68:493-498.e1-e14.
5. Jackson SM, Nesbitt LT. Differential Diagnosis for the Dermatologist. 2nd ed. Berlin: Springer; 2012.
6. Jain P, Kannan L, Kumar A, et al. Symptomatic neurocutaneous melanosis in a child. JAMA Neurol. 2013;70:516.
7. Kinsler VA, Chong WK, Aylett SE, et al. Complications of congenital melanocytic naevi in children: analysis of 16 years’ experience and clinical practice. Br J Dermatol. 2008;159:907-914.
8. Agero AL, B envenuto-Andrade C, Dusza SW, et al. Asymptomatic neurocutaneous melanocytosis in patients with large congenital melanocytic nevi: a study of cases from an Internet-based registry. J Am Acad Dermatol. 2005;53:959-965.
9. Zayour M, Lazova R. Congenital melanocytic nevi. Clin Lab Med. 2011;31:267-280.
A 25-year-old G2P1 mother gave birth to a boy at 40 and 6/7 weeks by vaginal delivery. Labor was induced because of oligohydramnios complicated by chorioamnionitis. The mother was treated with vancomycin and gentamicin. Prenatal lab work and delivery were otherwise unremarkable.
The delivering physician (CG) noted that the neonate had numerous brown, red, and black plaques distributed over his abdomen, lower back, groin, and thighs (FIGURE). Some plaques were hypertrichotic and other areas, apart from the plaques, were thinly desquamated. Apgar scores were 8 and 9 and the remainder of the exam, including the neurologic exam, was normal. The Dermatology Service (JK) was consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Giant congenital nevus
Congenital melanocytic nevi (CMN) are pigmented lesions that are present at birth and created by the abnormal migration of neural crest cells during embryogenesis.1 Nevi are categorized by size as small (<1.5 cm), medium (1.5-20 cm), large (>20 cm), and giant (>40 cm).2 Congenital nevi tend to start out flat, with uniform pigmentation, but can become more variegated in texture and color as normal growth and development continue. Giant congenital nevi are likely to thicken, darken, and enlarge as the patient grows. Some nevi may develop very coarse or dark hair.
CMN can cover any part of the body and occur independent of skin color and other ethnic factors.3 Giant congenital nevi are rare, with an incidence of approximately one in 50,000 live births and with males and females equally affected.3,4 The condition is diagnosed at birth, based on the appearance of the lesions.
The differential diagnosis for CMN includes café au lait macules, blue-gray spots (aka Mongolian spots), nevus of Ota, nevus spilus, and vascular malformations (TABLE).5 CMN may present in almost any location and may be brown, black, pink, or purple in color. Café au lait macules, blue-gray spots, nevus of Ota, nevus spilus, and vascular malformations have individual location and color characteristics that set them apart clinically.

Monitor patients for melanoma, CNS complications
Patients with CMN are at increased risk of neurocutaneous melanosis (NCM) and cutaneous melanoma.
Neurocutaneous melanosis, a complication of giant congenital nevi, is a melanocyte proliferation in the central nervous system (CNS). Between 6% and 11% of patients with giant congenital nevi develop symptomatic NCM in childhood. Thus, any CNS symptoms should be fully evaluated.4,6 NCM can result in seizures, cranial nerve palsy, hydrocephalus, and leptomeningeal melanoma.
Besides giant congenital nevi, risk factors for NCM include male sex, large numbers of satellite nevi, and the presence of nevi over the posterior midline or head and neck.7 The prognosis is poor for patients who develop neurologic symptoms. NCM is associated with other malignancies, including rhadomyosarcoma, liposarcoma, and malignant peripheral nerve sheath tumors.4
Magnetic resonance imaging (MRI) is helpful to exclude NCM. Ideally, an MRI should be ordered before 4 months of age, at which time myelination begins to make the identification of melanin deposits in the CNS more challenging.7 Not all patients with imaging findings that are consistent with NCM will develop symptoms.8
Melanoma. By age 10, up to 8% of patients with giant congenital nevi will develop melanoma within the nevi; most of these cases occur during the first 2 years of life.7,9 Patients with NCM are at even greater risk: their rate of malignant melanoma is between 40% and 60%.6 As a result, patients should be monitored closely for any signs of the disease. Total body photography, serial clinical photos, and patient self-exam are helpful to detect changes and de novo lesions. New lesions or ulcerations superimposed on existing nevi may indicate malignancy.7 Sun protection is critical to reduce the risk of melanogenesis.
Should patients pursue surgery? It’s debatable
Options for patients with large and giant CMN include early curettage (prior to 2 weeks of life), local excision (often with tissue expansion), dermabrasion, and laser therapy.2 There is considerable debate about surgery. Advocates of surgery cite psychosocial relief as a major treatment benefit and speculate about prevention of melanoma. Opponents worry that excessive surgical intervention may cause melanogenesis in a scar or deep in an area of treatment. And, while smaller congenital nevi are easier to surgically remove, they have a low associated risk of developing melanoma and are typically monitored clinically.
Children with congenital nevi will need support
Several nonprofit organizations offer resources for children with congenital nevi and their families. Nevus Outreach (www.nevus.org) is an organization devoted to improving awareness and providing support for people with CMN and NCM. The group maintains a registry of patients with large nevi in an effort to help researchers improve treatment and identify a cure.
For children with congenital nevi and other skin conditions, the American Academy of Dermatology offers its “Camp Discovery” at locations across the country (https://www.aad.org/public/kids/camp-discovery). Camp Discovery provides full scholarships and includes transportation to each of the individual camps for attendees.
Our patient underwent an MRI on his fifth day of life. The results were normal and he hadn’t developed any neurologic symptoms at 4 months of age. The child sees his family physician for routine well-child visits and a dermatologist annually. The dermatologist is carefully monitoring the nevi, which continue to grow.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Suite 340, Augusta, ME 04330; [email protected].
A 25-year-old G2P1 mother gave birth to a boy at 40 and 6/7 weeks by vaginal delivery. Labor was induced because of oligohydramnios complicated by chorioamnionitis. The mother was treated with vancomycin and gentamicin. Prenatal lab work and delivery were otherwise unremarkable.
The delivering physician (CG) noted that the neonate had numerous brown, red, and black plaques distributed over his abdomen, lower back, groin, and thighs (FIGURE). Some plaques were hypertrichotic and other areas, apart from the plaques, were thinly desquamated. Apgar scores were 8 and 9 and the remainder of the exam, including the neurologic exam, was normal. The Dermatology Service (JK) was consulted.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Giant congenital nevus
Congenital melanocytic nevi (CMN) are pigmented lesions that are present at birth and created by the abnormal migration of neural crest cells during embryogenesis.1 Nevi are categorized by size as small (<1.5 cm), medium (1.5-20 cm), large (>20 cm), and giant (>40 cm).2 Congenital nevi tend to start out flat, with uniform pigmentation, but can become more variegated in texture and color as normal growth and development continue. Giant congenital nevi are likely to thicken, darken, and enlarge as the patient grows. Some nevi may develop very coarse or dark hair.
CMN can cover any part of the body and occur independent of skin color and other ethnic factors.3 Giant congenital nevi are rare, with an incidence of approximately one in 50,000 live births and with males and females equally affected.3,4 The condition is diagnosed at birth, based on the appearance of the lesions.
The differential diagnosis for CMN includes café au lait macules, blue-gray spots (aka Mongolian spots), nevus of Ota, nevus spilus, and vascular malformations (TABLE).5 CMN may present in almost any location and may be brown, black, pink, or purple in color. Café au lait macules, blue-gray spots, nevus of Ota, nevus spilus, and vascular malformations have individual location and color characteristics that set them apart clinically.

Monitor patients for melanoma, CNS complications
Patients with CMN are at increased risk of neurocutaneous melanosis (NCM) and cutaneous melanoma.
Neurocutaneous melanosis, a complication of giant congenital nevi, is a melanocyte proliferation in the central nervous system (CNS). Between 6% and 11% of patients with giant congenital nevi develop symptomatic NCM in childhood. Thus, any CNS symptoms should be fully evaluated.4,6 NCM can result in seizures, cranial nerve palsy, hydrocephalus, and leptomeningeal melanoma.
Besides giant congenital nevi, risk factors for NCM include male sex, large numbers of satellite nevi, and the presence of nevi over the posterior midline or head and neck.7 The prognosis is poor for patients who develop neurologic symptoms. NCM is associated with other malignancies, including rhadomyosarcoma, liposarcoma, and malignant peripheral nerve sheath tumors.4
Magnetic resonance imaging (MRI) is helpful to exclude NCM. Ideally, an MRI should be ordered before 4 months of age, at which time myelination begins to make the identification of melanin deposits in the CNS more challenging.7 Not all patients with imaging findings that are consistent with NCM will develop symptoms.8
Melanoma. By age 10, up to 8% of patients with giant congenital nevi will develop melanoma within the nevi; most of these cases occur during the first 2 years of life.7,9 Patients with NCM are at even greater risk: their rate of malignant melanoma is between 40% and 60%.6 As a result, patients should be monitored closely for any signs of the disease. Total body photography, serial clinical photos, and patient self-exam are helpful to detect changes and de novo lesions. New lesions or ulcerations superimposed on existing nevi may indicate malignancy.7 Sun protection is critical to reduce the risk of melanogenesis.
Should patients pursue surgery? It’s debatable
Options for patients with large and giant CMN include early curettage (prior to 2 weeks of life), local excision (often with tissue expansion), dermabrasion, and laser therapy.2 There is considerable debate about surgery. Advocates of surgery cite psychosocial relief as a major treatment benefit and speculate about prevention of melanoma. Opponents worry that excessive surgical intervention may cause melanogenesis in a scar or deep in an area of treatment. And, while smaller congenital nevi are easier to surgically remove, they have a low associated risk of developing melanoma and are typically monitored clinically.
Children with congenital nevi will need support
Several nonprofit organizations offer resources for children with congenital nevi and their families. Nevus Outreach (www.nevus.org) is an organization devoted to improving awareness and providing support for people with CMN and NCM. The group maintains a registry of patients with large nevi in an effort to help researchers improve treatment and identify a cure.
For children with congenital nevi and other skin conditions, the American Academy of Dermatology offers its “Camp Discovery” at locations across the country (https://www.aad.org/public/kids/camp-discovery). Camp Discovery provides full scholarships and includes transportation to each of the individual camps for attendees.
Our patient underwent an MRI on his fifth day of life. The results were normal and he hadn’t developed any neurologic symptoms at 4 months of age. The child sees his family physician for routine well-child visits and a dermatologist annually. The dermatologist is carefully monitoring the nevi, which continue to grow.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Suite 340, Augusta, ME 04330; [email protected].
1. Sarnat HB, Flores-Sarnat L. Embryology of the neural crest: its inductive role in the neurocutaneous syndromes. J Child Neurol. 2005:20:637-643.
2. Gosain AK, Santoro TD, Larson DL, et al. Giant congenital nevi: a 20-year experience and an algorithm for their management. Plast Reconstr Surg. 2001;108:622-636.
3. National Organization for Rare Disorders. Giant congenital melanocytic nevus. National Organization for Rare Disorders Web site. Available at: http://rarediseases.org/rare-diseases/giant-congenital-melanocytic-nevus. Accessed April 29, 2016.
4. Vourc’h-Jourdain M, Martin L, Barbarot S; aRED. Large congenital melanocytic nevi: therapeutic management and melanoma risk: a systematic review. J Am Acad Dermatol. 2013;68:493-498.e1-e14.
5. Jackson SM, Nesbitt LT. Differential Diagnosis for the Dermatologist. 2nd ed. Berlin: Springer; 2012.
6. Jain P, Kannan L, Kumar A, et al. Symptomatic neurocutaneous melanosis in a child. JAMA Neurol. 2013;70:516.
7. Kinsler VA, Chong WK, Aylett SE, et al. Complications of congenital melanocytic naevi in children: analysis of 16 years’ experience and clinical practice. Br J Dermatol. 2008;159:907-914.
8. Agero AL, B envenuto-Andrade C, Dusza SW, et al. Asymptomatic neurocutaneous melanocytosis in patients with large congenital melanocytic nevi: a study of cases from an Internet-based registry. J Am Acad Dermatol. 2005;53:959-965.
9. Zayour M, Lazova R. Congenital melanocytic nevi. Clin Lab Med. 2011;31:267-280.
1. Sarnat HB, Flores-Sarnat L. Embryology of the neural crest: its inductive role in the neurocutaneous syndromes. J Child Neurol. 2005:20:637-643.
2. Gosain AK, Santoro TD, Larson DL, et al. Giant congenital nevi: a 20-year experience and an algorithm for their management. Plast Reconstr Surg. 2001;108:622-636.
3. National Organization for Rare Disorders. Giant congenital melanocytic nevus. National Organization for Rare Disorders Web site. Available at: http://rarediseases.org/rare-diseases/giant-congenital-melanocytic-nevus. Accessed April 29, 2016.
4. Vourc’h-Jourdain M, Martin L, Barbarot S; aRED. Large congenital melanocytic nevi: therapeutic management and melanoma risk: a systematic review. J Am Acad Dermatol. 2013;68:493-498.e1-e14.
5. Jackson SM, Nesbitt LT. Differential Diagnosis for the Dermatologist. 2nd ed. Berlin: Springer; 2012.
6. Jain P, Kannan L, Kumar A, et al. Symptomatic neurocutaneous melanosis in a child. JAMA Neurol. 2013;70:516.
7. Kinsler VA, Chong WK, Aylett SE, et al. Complications of congenital melanocytic naevi in children: analysis of 16 years’ experience and clinical practice. Br J Dermatol. 2008;159:907-914.
8. Agero AL, B envenuto-Andrade C, Dusza SW, et al. Asymptomatic neurocutaneous melanocytosis in patients with large congenital melanocytic nevi: a study of cases from an Internet-based registry. J Am Acad Dermatol. 2005;53:959-965.
9. Zayour M, Lazova R. Congenital melanocytic nevi. Clin Lab Med. 2011;31:267-280.
What do we really know about e-cigarettes?
It’s been about 2 years since I had my first e-cigarette discussion with a patient. He was a smoker in his 30s and, since we routinely screen for tobacco use in our practice, I asked him if he was interested in quitting. He said he was cutting down by using e-cigarettes, but had not yet stopped smoking.
According to the 2 articles on e-cigarettes in this issue—one original research study about the prevalence of e-cigarette use in rural Illinois and one review of the safety of e-cigarettes—my experience with this patient is typical of e-cigarette users. Many are “dual users” who turn to e-cigarettes to try to cut down on their tobacco use.
As these 2 articles discuss, we still have a great deal to learn about the potential harms and benefits of e-cigarettes. What chemicals are people taking into their bodies and how dangerous are they? And even if they pose health risks, do e-cigarettes have value as smoking cessation aids if they are less harmful than tobacco?
One could simply take a “just say No” approach, as does my wife who says, “Any chemical you inhale into your lungs can’t be good for you!” Or, one can assume the more moderate lesser-of-two-evils stance of the British health system, which posits that there may be some benefit to e-cigarettes if they help people cut down or stop using tobacco products.
In writing this editorial, I conducted a quick literature search that yielded only 5 legitimate randomized trials of e-cigarettes to reduce or eliminate tobacco use, and the results were underwhelming. At best, e-cigarettes appear to be as effective as other forms of nicotine replacement, such as patches, which do not have chemical additives.
Fortunately, researchers are taking e-cigarettes seriously, and research is ongoing. Using the search term “e-cigarette” yielded 2058 references, indicating a respectable amount of e-cigarette research conducted over the past 6 years. Most of the research so far has been about the chemical constituents of the vapor people inhale or about use patterns. There is still a lack of definitive research on whether e-cigarettes are an effective smoking cessation method or a “gateway” to the use of tobacco and other substances of abuse.
Or perhaps they are both.
Hopefully, in 5 years we will know a great deal more, but until we do, I am happy to see that the US Food and Drug Administration has decided to regulate e-cigarettes like tobacco.
It’s been about 2 years since I had my first e-cigarette discussion with a patient. He was a smoker in his 30s and, since we routinely screen for tobacco use in our practice, I asked him if he was interested in quitting. He said he was cutting down by using e-cigarettes, but had not yet stopped smoking.
According to the 2 articles on e-cigarettes in this issue—one original research study about the prevalence of e-cigarette use in rural Illinois and one review of the safety of e-cigarettes—my experience with this patient is typical of e-cigarette users. Many are “dual users” who turn to e-cigarettes to try to cut down on their tobacco use.
As these 2 articles discuss, we still have a great deal to learn about the potential harms and benefits of e-cigarettes. What chemicals are people taking into their bodies and how dangerous are they? And even if they pose health risks, do e-cigarettes have value as smoking cessation aids if they are less harmful than tobacco?
One could simply take a “just say No” approach, as does my wife who says, “Any chemical you inhale into your lungs can’t be good for you!” Or, one can assume the more moderate lesser-of-two-evils stance of the British health system, which posits that there may be some benefit to e-cigarettes if they help people cut down or stop using tobacco products.
In writing this editorial, I conducted a quick literature search that yielded only 5 legitimate randomized trials of e-cigarettes to reduce or eliminate tobacco use, and the results were underwhelming. At best, e-cigarettes appear to be as effective as other forms of nicotine replacement, such as patches, which do not have chemical additives.
Fortunately, researchers are taking e-cigarettes seriously, and research is ongoing. Using the search term “e-cigarette” yielded 2058 references, indicating a respectable amount of e-cigarette research conducted over the past 6 years. Most of the research so far has been about the chemical constituents of the vapor people inhale or about use patterns. There is still a lack of definitive research on whether e-cigarettes are an effective smoking cessation method or a “gateway” to the use of tobacco and other substances of abuse.
Or perhaps they are both.
Hopefully, in 5 years we will know a great deal more, but until we do, I am happy to see that the US Food and Drug Administration has decided to regulate e-cigarettes like tobacco.
It’s been about 2 years since I had my first e-cigarette discussion with a patient. He was a smoker in his 30s and, since we routinely screen for tobacco use in our practice, I asked him if he was interested in quitting. He said he was cutting down by using e-cigarettes, but had not yet stopped smoking.
According to the 2 articles on e-cigarettes in this issue—one original research study about the prevalence of e-cigarette use in rural Illinois and one review of the safety of e-cigarettes—my experience with this patient is typical of e-cigarette users. Many are “dual users” who turn to e-cigarettes to try to cut down on their tobacco use.
As these 2 articles discuss, we still have a great deal to learn about the potential harms and benefits of e-cigarettes. What chemicals are people taking into their bodies and how dangerous are they? And even if they pose health risks, do e-cigarettes have value as smoking cessation aids if they are less harmful than tobacco?
One could simply take a “just say No” approach, as does my wife who says, “Any chemical you inhale into your lungs can’t be good for you!” Or, one can assume the more moderate lesser-of-two-evils stance of the British health system, which posits that there may be some benefit to e-cigarettes if they help people cut down or stop using tobacco products.
In writing this editorial, I conducted a quick literature search that yielded only 5 legitimate randomized trials of e-cigarettes to reduce or eliminate tobacco use, and the results were underwhelming. At best, e-cigarettes appear to be as effective as other forms of nicotine replacement, such as patches, which do not have chemical additives.
Fortunately, researchers are taking e-cigarettes seriously, and research is ongoing. Using the search term “e-cigarette” yielded 2058 references, indicating a respectable amount of e-cigarette research conducted over the past 6 years. Most of the research so far has been about the chemical constituents of the vapor people inhale or about use patterns. There is still a lack of definitive research on whether e-cigarettes are an effective smoking cessation method or a “gateway” to the use of tobacco and other substances of abuse.
Or perhaps they are both.
Hopefully, in 5 years we will know a great deal more, but until we do, I am happy to see that the US Food and Drug Administration has decided to regulate e-cigarettes like tobacco.
More isn’t better with acute low back pain treatment
Consider treating patients with acute low back pain with naproxen only, as adding cyclobenzaprine or oxycodone/acetaminophen to scheduled naproxen does not improve functional assessment at 7 days or 3 months and increases adverse effects.
Strength of recommendation
B: Based on a high-quality, randomized controlled trial (RCT).1
Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314:1572-1580.
Illustrative Case
A 46-year-old man presents to the emergency department (ED) with low back pain (LBP) after helping a friend move a couch 3 days earlier. He denies any direct trauma to his back and describes the pain as a spasm in his lumbar spinal region with no radicular symptoms. The pain worsens with prolonged standing and any position changes. He has tried acetaminophen with no benefit. You diagnose a lumbar muscular strain. What medications should you prescribe to help relieve his LBP and improve his overall function?
Acute LBP prompts close to 2.7 million ED visits annually in the United States.2 It leads to persistent subjective impairment and continued analgesic usage at 7 days (impairment 70%, analgesic use 69%) and at 3 months (48% and 46%, respectively) after ED discharge.3 Systematic reviews show that monotherapy with nonsteroidal anti-inflammatory drugs (NSAIDs) or muscle relaxers is better than placebo for relieving pain.4,5 A secondary analysis of patients (N=715) from a prospective cohort study showed that patients prescribed opiates for LBP had worse functioning at 6 months than those not prescribed opiates.6
Monotherapy or combination therapy for LBP? That is the question
Because medications used for LBP have different mechanisms of action, clinicians frequently combine them in an attempt to improve symptoms and function.2 Current evidence evaluating combination therapy demonstrates mixed results. A large RCT (N=867) showed that the combination of cyclobenzaprine and ibuprofen led to lower subjective pain intensity, but did not result in self-reported pain improvement (based on answers to the Patient Global Impression of Change and the Oswestry Disability Index) than cyclobenzaprine alone. However, a small RCT (N=40) combining naproxen with cyclobenzaprine demonstrated improved LBP and spasm compared to naproxen alone.7,8
This study sought to determine the benefit of treating acute LBP with cyclobenzaprine or oxycodone/acetaminophen in combination with an NSAID compared to treatment with an NSAID alone.
Study Summary
Adding second pain reliever to the NSAID provided no significant benefit
This double-blinded RCT enrolled 323 adult patients presenting to an ED with ≤2 weeks of nontraumatic, nonradicular LBP, which was defined as pain between the lower border of the scapulae and the upper gluteal folds.1 Participants had a score of >5 on the Roland-Morris Disability Questionnaire (RMDQ), which measures functional impairment due to LBP (range: 0-24). Patients were excluded if they had radicular pain radiating below the gluteal folds, direct trauma to the back within the previous month, pain duration >2 weeks, or a recent history of >1 LBP episode per month. Patients with current or past chronic opioid use were also excluded.
All participants received 10 days’ worth of naproxen (500 mg twice daily). They were then randomized to receive either: oxycodone 5 mg/acetaminophen 325 mg; cyclobenzaprine 5 mg; or placebo, with instructions to take one to 2 tablets prn every 8 hours for 10 days. They were told that if one tablet afforded sufficient relief, there was no need to take the second one, but if the first tablet did not provide relief within 30 minutes, they should take the second one. All patients also received a 10-minute educational session emphasizing the role of exercise, stretching, physical/massage therapy, and other non-pharmacologic interventions.
The primary outcome was change in the RMDQ between ED discharge and a phone call 7 days later, with a 5-point improvement in the RMDQ considered clinically significant. Secondary outcomes at 7 days and 3 months after ED discharge included subjective description of worst pain, frequency of LBP pain, frequency of analgesic use, satisfaction with treatment, median number of days to return to work and usual activities, need for follow-up health care visits, and opioid use. Investigators also asked about any adverse effects at 7 days and 3 months.
At 7 days, patients randomized to naproxen plus placebo improved on reported RMDQ scores by a mean of 9.8 points, naproxen plus cyclobenzaprine by 10.1 points, and naproxen plus oxycodone/acetaminophen by 11.1 points. Between group differences in mean RMDQ changes showed no statistically significant differences with placebo vs cyclobenzaprine (0.3 points; P=.77), placebo vs oxycodone/acetaminophen (1.3 points; P=.28), and cyclobenzaprine vs oxycodone/acetaminophen (0.9 points; P=.45).
Secondary outcomes. At 7 days, there was no significant difference between study groups in subjective pain assessment, frequency of LBP, or use of as-needed medications in the prior 24 hours. There was also no difference in the median number of days to return to work or need for follow-up health care visits. In patients who took more than one dose of the study medication, those who took oxycodone/acetaminophen were more likely to describe their worst pain in the last 24 hours as mild/none when compared to those taking placebo (number needed to treat [NNT]=6). About 72% of all subjects reported that they would choose the same treatment option again, with no difference between groups. At 3 months, no difference existed between groups in subjective pain assessment, frequency of LBP, use of as-needed medications, or opioid use during the previous 72 hours.
Adverse effects, including drowsiness, dizziness, stomach irritation, and nausea or vomiting, were more common in the oxycodone/acetaminophen and cyclobenzaprine treatment groups with a number needed to harm (NNH) of 5.3 and 7.8, respectively.
What’s New
A second pain reliever adds nothing—except adverse effects
This RCT found that adding cyclobenzaprine or oxycodone/acetaminophen to naproxen for the treatment of nontraumatic, nonradicular acute LBP did not significantly improve functional assessment based on RMDQ scores or pain measures at 7 days or 3 months after the initial ED visit. It did, however, increase adverse effects.
Caveats
Researchers studied a specific subset of patients
This study was performed in a single-site urban ED and included a very specific subset of LBP patients, which limits the generalizability of the results. However, patients often present to their primary care physician with similar LBP complaints, and the results of the study should reasonably apply to other settings.
The findings may not generalize to all NSAIDs, but there is no evidence to suggest that other NSAIDs would behave differently when combined with cyclobenzaprine or oxycodone/acetaminophen. In this intention-to-treat analysis, only about one-third of patients used the as-needed medication more than once daily; about another third of patients used the as-needed medication intermittently or never.
Challenges to Implementation
Patients may expect more than an NSAID for their back pain
Patients expect to receive prescriptions, and physicians are inclined to write them if they believe they will help their patients. The evidence, however, does not show a benefit to these prescription-only medications for low back pain.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314:1572-1580.
2. Friedman BW, Chilstrom M, Bijur PE, et al. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine (Phila Pa 1976). 2010;35:E1406-E1411.
3. Friedman BW, O’Mahony S, Mulvey L, et al. One-week and 3-month outcomes after an emergency department visit for undifferentiated musculoskeletal low back pain. Ann Emerg Med. 2012;59:128-133.
4. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
5. van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976). 2003;28:1978-1992.
6. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
7. Childers MK, Borenstein D, Brown RL, et al. Low-dose cyclobenzaprine versus combination therapy with ibuprofen for acute neck or back pain with muscle spasm: a randomized trial. Curr Med Res Opin. 2005;21:1485-1493.
8. Borenstein DG, Lacks S, Wiesel SW. Cyclobenzaprine and naproxen versus naproxen alone in the treatment of acute low back pain and muscle spasm. Clin Ther. 1990;12:125-131.
Consider treating patients with acute low back pain with naproxen only, as adding cyclobenzaprine or oxycodone/acetaminophen to scheduled naproxen does not improve functional assessment at 7 days or 3 months and increases adverse effects.
Strength of recommendation
B: Based on a high-quality, randomized controlled trial (RCT).1
Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314:1572-1580.
Illustrative Case
A 46-year-old man presents to the emergency department (ED) with low back pain (LBP) after helping a friend move a couch 3 days earlier. He denies any direct trauma to his back and describes the pain as a spasm in his lumbar spinal region with no radicular symptoms. The pain worsens with prolonged standing and any position changes. He has tried acetaminophen with no benefit. You diagnose a lumbar muscular strain. What medications should you prescribe to help relieve his LBP and improve his overall function?
Acute LBP prompts close to 2.7 million ED visits annually in the United States.2 It leads to persistent subjective impairment and continued analgesic usage at 7 days (impairment 70%, analgesic use 69%) and at 3 months (48% and 46%, respectively) after ED discharge.3 Systematic reviews show that monotherapy with nonsteroidal anti-inflammatory drugs (NSAIDs) or muscle relaxers is better than placebo for relieving pain.4,5 A secondary analysis of patients (N=715) from a prospective cohort study showed that patients prescribed opiates for LBP had worse functioning at 6 months than those not prescribed opiates.6
Monotherapy or combination therapy for LBP? That is the question
Because medications used for LBP have different mechanisms of action, clinicians frequently combine them in an attempt to improve symptoms and function.2 Current evidence evaluating combination therapy demonstrates mixed results. A large RCT (N=867) showed that the combination of cyclobenzaprine and ibuprofen led to lower subjective pain intensity, but did not result in self-reported pain improvement (based on answers to the Patient Global Impression of Change and the Oswestry Disability Index) than cyclobenzaprine alone. However, a small RCT (N=40) combining naproxen with cyclobenzaprine demonstrated improved LBP and spasm compared to naproxen alone.7,8
This study sought to determine the benefit of treating acute LBP with cyclobenzaprine or oxycodone/acetaminophen in combination with an NSAID compared to treatment with an NSAID alone.
Study Summary
Adding second pain reliever to the NSAID provided no significant benefit
This double-blinded RCT enrolled 323 adult patients presenting to an ED with ≤2 weeks of nontraumatic, nonradicular LBP, which was defined as pain between the lower border of the scapulae and the upper gluteal folds.1 Participants had a score of >5 on the Roland-Morris Disability Questionnaire (RMDQ), which measures functional impairment due to LBP (range: 0-24). Patients were excluded if they had radicular pain radiating below the gluteal folds, direct trauma to the back within the previous month, pain duration >2 weeks, or a recent history of >1 LBP episode per month. Patients with current or past chronic opioid use were also excluded.
All participants received 10 days’ worth of naproxen (500 mg twice daily). They were then randomized to receive either: oxycodone 5 mg/acetaminophen 325 mg; cyclobenzaprine 5 mg; or placebo, with instructions to take one to 2 tablets prn every 8 hours for 10 days. They were told that if one tablet afforded sufficient relief, there was no need to take the second one, but if the first tablet did not provide relief within 30 minutes, they should take the second one. All patients also received a 10-minute educational session emphasizing the role of exercise, stretching, physical/massage therapy, and other non-pharmacologic interventions.
The primary outcome was change in the RMDQ between ED discharge and a phone call 7 days later, with a 5-point improvement in the RMDQ considered clinically significant. Secondary outcomes at 7 days and 3 months after ED discharge included subjective description of worst pain, frequency of LBP pain, frequency of analgesic use, satisfaction with treatment, median number of days to return to work and usual activities, need for follow-up health care visits, and opioid use. Investigators also asked about any adverse effects at 7 days and 3 months.
At 7 days, patients randomized to naproxen plus placebo improved on reported RMDQ scores by a mean of 9.8 points, naproxen plus cyclobenzaprine by 10.1 points, and naproxen plus oxycodone/acetaminophen by 11.1 points. Between group differences in mean RMDQ changes showed no statistically significant differences with placebo vs cyclobenzaprine (0.3 points; P=.77), placebo vs oxycodone/acetaminophen (1.3 points; P=.28), and cyclobenzaprine vs oxycodone/acetaminophen (0.9 points; P=.45).
Secondary outcomes. At 7 days, there was no significant difference between study groups in subjective pain assessment, frequency of LBP, or use of as-needed medications in the prior 24 hours. There was also no difference in the median number of days to return to work or need for follow-up health care visits. In patients who took more than one dose of the study medication, those who took oxycodone/acetaminophen were more likely to describe their worst pain in the last 24 hours as mild/none when compared to those taking placebo (number needed to treat [NNT]=6). About 72% of all subjects reported that they would choose the same treatment option again, with no difference between groups. At 3 months, no difference existed between groups in subjective pain assessment, frequency of LBP, use of as-needed medications, or opioid use during the previous 72 hours.
Adverse effects, including drowsiness, dizziness, stomach irritation, and nausea or vomiting, were more common in the oxycodone/acetaminophen and cyclobenzaprine treatment groups with a number needed to harm (NNH) of 5.3 and 7.8, respectively.
What’s New
A second pain reliever adds nothing—except adverse effects
This RCT found that adding cyclobenzaprine or oxycodone/acetaminophen to naproxen for the treatment of nontraumatic, nonradicular acute LBP did not significantly improve functional assessment based on RMDQ scores or pain measures at 7 days or 3 months after the initial ED visit. It did, however, increase adverse effects.
Caveats
Researchers studied a specific subset of patients
This study was performed in a single-site urban ED and included a very specific subset of LBP patients, which limits the generalizability of the results. However, patients often present to their primary care physician with similar LBP complaints, and the results of the study should reasonably apply to other settings.
The findings may not generalize to all NSAIDs, but there is no evidence to suggest that other NSAIDs would behave differently when combined with cyclobenzaprine or oxycodone/acetaminophen. In this intention-to-treat analysis, only about one-third of patients used the as-needed medication more than once daily; about another third of patients used the as-needed medication intermittently or never.
Challenges to Implementation
Patients may expect more than an NSAID for their back pain
Patients expect to receive prescriptions, and physicians are inclined to write them if they believe they will help their patients. The evidence, however, does not show a benefit to these prescription-only medications for low back pain.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Consider treating patients with acute low back pain with naproxen only, as adding cyclobenzaprine or oxycodone/acetaminophen to scheduled naproxen does not improve functional assessment at 7 days or 3 months and increases adverse effects.
Strength of recommendation
B: Based on a high-quality, randomized controlled trial (RCT).1
Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314:1572-1580.
Illustrative Case
A 46-year-old man presents to the emergency department (ED) with low back pain (LBP) after helping a friend move a couch 3 days earlier. He denies any direct trauma to his back and describes the pain as a spasm in his lumbar spinal region with no radicular symptoms. The pain worsens with prolonged standing and any position changes. He has tried acetaminophen with no benefit. You diagnose a lumbar muscular strain. What medications should you prescribe to help relieve his LBP and improve his overall function?
Acute LBP prompts close to 2.7 million ED visits annually in the United States.2 It leads to persistent subjective impairment and continued analgesic usage at 7 days (impairment 70%, analgesic use 69%) and at 3 months (48% and 46%, respectively) after ED discharge.3 Systematic reviews show that monotherapy with nonsteroidal anti-inflammatory drugs (NSAIDs) or muscle relaxers is better than placebo for relieving pain.4,5 A secondary analysis of patients (N=715) from a prospective cohort study showed that patients prescribed opiates for LBP had worse functioning at 6 months than those not prescribed opiates.6
Monotherapy or combination therapy for LBP? That is the question
Because medications used for LBP have different mechanisms of action, clinicians frequently combine them in an attempt to improve symptoms and function.2 Current evidence evaluating combination therapy demonstrates mixed results. A large RCT (N=867) showed that the combination of cyclobenzaprine and ibuprofen led to lower subjective pain intensity, but did not result in self-reported pain improvement (based on answers to the Patient Global Impression of Change and the Oswestry Disability Index) than cyclobenzaprine alone. However, a small RCT (N=40) combining naproxen with cyclobenzaprine demonstrated improved LBP and spasm compared to naproxen alone.7,8
This study sought to determine the benefit of treating acute LBP with cyclobenzaprine or oxycodone/acetaminophen in combination with an NSAID compared to treatment with an NSAID alone.
Study Summary
Adding second pain reliever to the NSAID provided no significant benefit
This double-blinded RCT enrolled 323 adult patients presenting to an ED with ≤2 weeks of nontraumatic, nonradicular LBP, which was defined as pain between the lower border of the scapulae and the upper gluteal folds.1 Participants had a score of >5 on the Roland-Morris Disability Questionnaire (RMDQ), which measures functional impairment due to LBP (range: 0-24). Patients were excluded if they had radicular pain radiating below the gluteal folds, direct trauma to the back within the previous month, pain duration >2 weeks, or a recent history of >1 LBP episode per month. Patients with current or past chronic opioid use were also excluded.
All participants received 10 days’ worth of naproxen (500 mg twice daily). They were then randomized to receive either: oxycodone 5 mg/acetaminophen 325 mg; cyclobenzaprine 5 mg; or placebo, with instructions to take one to 2 tablets prn every 8 hours for 10 days. They were told that if one tablet afforded sufficient relief, there was no need to take the second one, but if the first tablet did not provide relief within 30 minutes, they should take the second one. All patients also received a 10-minute educational session emphasizing the role of exercise, stretching, physical/massage therapy, and other non-pharmacologic interventions.
The primary outcome was change in the RMDQ between ED discharge and a phone call 7 days later, with a 5-point improvement in the RMDQ considered clinically significant. Secondary outcomes at 7 days and 3 months after ED discharge included subjective description of worst pain, frequency of LBP pain, frequency of analgesic use, satisfaction with treatment, median number of days to return to work and usual activities, need for follow-up health care visits, and opioid use. Investigators also asked about any adverse effects at 7 days and 3 months.
At 7 days, patients randomized to naproxen plus placebo improved on reported RMDQ scores by a mean of 9.8 points, naproxen plus cyclobenzaprine by 10.1 points, and naproxen plus oxycodone/acetaminophen by 11.1 points. Between group differences in mean RMDQ changes showed no statistically significant differences with placebo vs cyclobenzaprine (0.3 points; P=.77), placebo vs oxycodone/acetaminophen (1.3 points; P=.28), and cyclobenzaprine vs oxycodone/acetaminophen (0.9 points; P=.45).
Secondary outcomes. At 7 days, there was no significant difference between study groups in subjective pain assessment, frequency of LBP, or use of as-needed medications in the prior 24 hours. There was also no difference in the median number of days to return to work or need for follow-up health care visits. In patients who took more than one dose of the study medication, those who took oxycodone/acetaminophen were more likely to describe their worst pain in the last 24 hours as mild/none when compared to those taking placebo (number needed to treat [NNT]=6). About 72% of all subjects reported that they would choose the same treatment option again, with no difference between groups. At 3 months, no difference existed between groups in subjective pain assessment, frequency of LBP, use of as-needed medications, or opioid use during the previous 72 hours.
Adverse effects, including drowsiness, dizziness, stomach irritation, and nausea or vomiting, were more common in the oxycodone/acetaminophen and cyclobenzaprine treatment groups with a number needed to harm (NNH) of 5.3 and 7.8, respectively.
What’s New
A second pain reliever adds nothing—except adverse effects
This RCT found that adding cyclobenzaprine or oxycodone/acetaminophen to naproxen for the treatment of nontraumatic, nonradicular acute LBP did not significantly improve functional assessment based on RMDQ scores or pain measures at 7 days or 3 months after the initial ED visit. It did, however, increase adverse effects.
Caveats
Researchers studied a specific subset of patients
This study was performed in a single-site urban ED and included a very specific subset of LBP patients, which limits the generalizability of the results. However, patients often present to their primary care physician with similar LBP complaints, and the results of the study should reasonably apply to other settings.
The findings may not generalize to all NSAIDs, but there is no evidence to suggest that other NSAIDs would behave differently when combined with cyclobenzaprine or oxycodone/acetaminophen. In this intention-to-treat analysis, only about one-third of patients used the as-needed medication more than once daily; about another third of patients used the as-needed medication intermittently or never.
Challenges to Implementation
Patients may expect more than an NSAID for their back pain
Patients expect to receive prescriptions, and physicians are inclined to write them if they believe they will help their patients. The evidence, however, does not show a benefit to these prescription-only medications for low back pain.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314:1572-1580.
2. Friedman BW, Chilstrom M, Bijur PE, et al. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine (Phila Pa 1976). 2010;35:E1406-E1411.
3. Friedman BW, O’Mahony S, Mulvey L, et al. One-week and 3-month outcomes after an emergency department visit for undifferentiated musculoskeletal low back pain. Ann Emerg Med. 2012;59:128-133.
4. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
5. van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976). 2003;28:1978-1992.
6. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
7. Childers MK, Borenstein D, Brown RL, et al. Low-dose cyclobenzaprine versus combination therapy with ibuprofen for acute neck or back pain with muscle spasm: a randomized trial. Curr Med Res Opin. 2005;21:1485-1493.
8. Borenstein DG, Lacks S, Wiesel SW. Cyclobenzaprine and naproxen versus naproxen alone in the treatment of acute low back pain and muscle spasm. Clin Ther. 1990;12:125-131.
1. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314:1572-1580.
2. Friedman BW, Chilstrom M, Bijur PE, et al. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine (Phila Pa 1976). 2010;35:E1406-E1411.
3. Friedman BW, O’Mahony S, Mulvey L, et al. One-week and 3-month outcomes after an emergency department visit for undifferentiated musculoskeletal low back pain. Ann Emerg Med. 2012;59:128-133.
4. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
5. van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine (Phila Pa 1976). 2003;28:1978-1992.
6. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
7. Childers MK, Borenstein D, Brown RL, et al. Low-dose cyclobenzaprine versus combination therapy with ibuprofen for acute neck or back pain with muscle spasm: a randomized trial. Curr Med Res Opin. 2005;21:1485-1493.
8. Borenstein DG, Lacks S, Wiesel SW. Cyclobenzaprine and naproxen versus naproxen alone in the treatment of acute low back pain and muscle spasm. Clin Ther. 1990;12:125-131.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
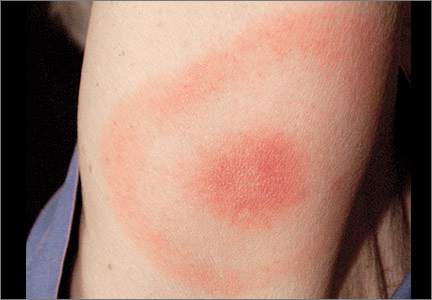
Beyond the bull's eye: Recognizing Lyme disease
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
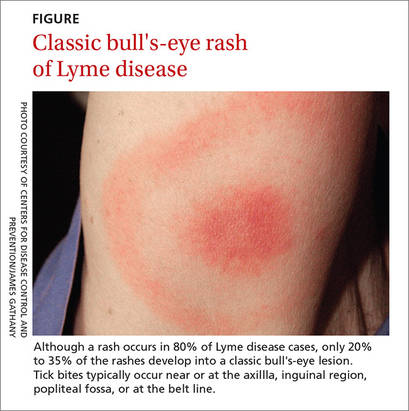
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
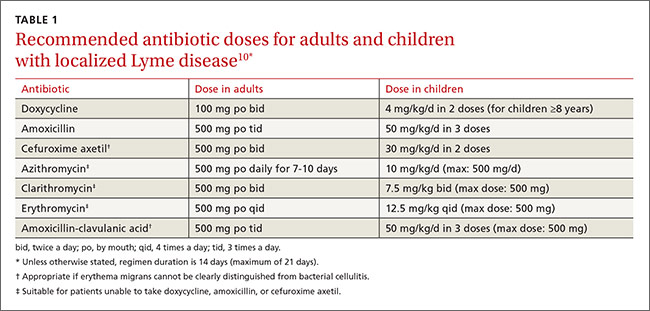
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
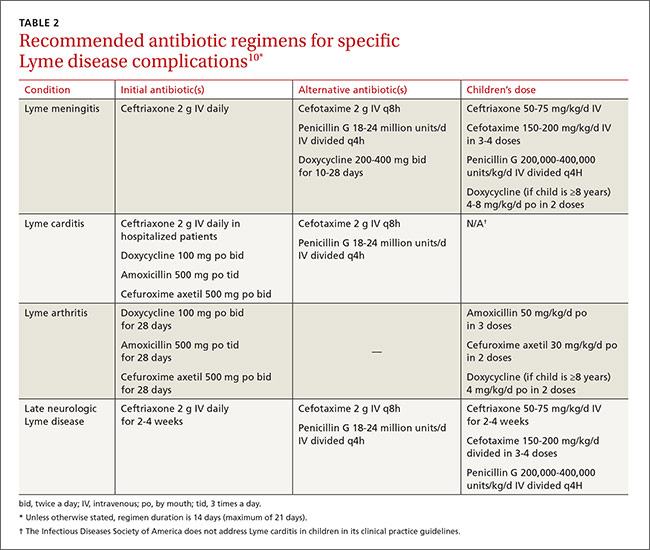
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.

Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17

Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18

Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.

Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17

Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18

Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
From The Journal of Family Practice | 2016;65(6):373-379.
E-cigarettes: Who’s using them and why?
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; [email protected].
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; [email protected].
ABSTRACT
Background Electronic cigarettes (e-cigarettes) are often marketed as safe and effective aids for quitting cigarette smoking, but concerns remain that use of e-cigarettes might actually reduce the number of quit attempts. To address these issues, we characterized the utilization and demographic correlates of dual use of e-cigarettes and traditional cigarettes (referred to here as simply “cigarettes”) among smokers in a rural population of Illinois.
Methods The majority of survey participants were recruited from the 2014 Illinois State Fair and from another event—the Springfield Mile (a motorcycle racing event)—in Springfield, Ill. Survey questions explored participant demographics and cigarette and e-cigarette use history.
Results Of 201 total cigarette smokers, 79 smoked only tobacco cigarettes (smokers), while 122 also used e-cigarettes (dual users). Dual users did not differ significantly from smokers in gender, age, income, or education. Compared to smokers, dual users were more likely to smoke within 30 minutes of awakening (odds ratio [OR]=3.3; 95% confidence interval [CI], 1.8-6.3), but did not smoke more cigarettes per day or perceive a greater likelihood of quit success. Non-white dual users smoked fewer cigarettes per day than smokers. In addition, 79.5% of all dual users reported that they were using e-cigarettes to quit smoking or reduce the number of cigarettes smoked, and white respondents were 6 times more likely than non-whites to use e-cigarettes for ‘trying to quit smoking’ (OR=6.0; 95% CI, 1.1-32.9). Males and respondents with lower income were less likely to say they were using e-cigarettes to reduce the number of cigarettes smoked than females or participants with higher income (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1; 95% CI, 0.0-0.5, respectively).
Conclusions E-cigarettes may significantly alter the landscape of nicotine physical dependence, and local influences likely are associated with use patterns. Future research should continue to examine whether dual use of traditional and electronic cigarettes impacts smoking cessation, and clinicians should be aware that local norms may create differences from national level data.
Approximately 21% of US adults use tobacco products at least occasionally.1 Although smoking prevalence has declined in recent years (from 21% in 2005 to 18% in 2013), it remains high among certain groups (eg, males and those with a high school education or less).2 As we know, the health burden of smoking—as a cause of death from cancer, pulmonary disease, and heart disease—is substantial,3,4 and rural areas experience a significantly higher prevalence of smoking compared to urban areas.2,5,6
However, it is unknown if the context and habits surrounding tobacco use in rural and/or Midwestern areas are similar to those of urban or nationally-representative populations. For example, while many urban residents may encounter a multitude of media messages encouraging smoking cessation resulting in less community acceptance of smoking, rural residents may be exposed to substantially fewer messages (eg, no city bus signs, billboards, subway posters, etc.) and the community may be more accommodating and tolerant of smoking.
Do e-cigarettes increase cigarette smoking?
Public health professionals are concerned about the increased use of e-cigarettes, particularly among young people, and whether this use increases the likelihood that individuals will start smoking tobacco cigarettes.7(Throughout this paper, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.) A recent study found that adolescents who used electronic nicotine delivery systems were twice as likely as non-users to have tried cigarettes in the past year.8
An onslaught of advertising. There are also concerns that e-cigarettes may serve to ‘renormalize’ nicotine addiction, in part through large-scale advertising, which was seen by nearly 70% of the participants in the 2014 National Youth Tobacco Survey.9 Largely as a result of that advertising, e-cigarette sales exceed $1.7 billion in the United States alone.10 With 15% of all US adults having ever tried electronic nicotine delivery systems and more than half (52%) of smokers having done so, questions regarding their health impact cannot be taken lightly.11
Do e-cigarettes help people quit smoking? E-cigarettes are often marketed as a safe and effective means for quitting cigarette smoking.12-14 (See "E-cigarettes: How "safe" are they?") Nearly two-thirds of physicians report being asked about e-cigarettes by their patients and approximately one-third of physicians recommend using them as a smoking cessation aid.15
Claims regarding the usefulness of e-cigarettes in smoking cessation, however, have not been substantiated by high-quality randomized controlled trials (RCTs). In fact, no RCTs have shown them to be safer or more effective than cessation treatments currently approved by the US Food and Drug Administration.16,17
Two studies reflect the conflicting data that are currently available. One small study found intensive e-cigarette users were 6 times more likely than non-users/triers to report successful smoking cessation.18 However, researchers surveying callers of a cigarette quit line found that smokers who used e-cigarettes (dual users) were less likely to quit smoking than non-users.19
The lack of good-quality data substantiates the concern that dual use might discourage quitting by normalizing cigarette use and reducing perceptions of harm.20,21 Dual use may also hamper smoking cessation efforts by increasing nicotine physical dependence and associated withdrawal symptoms when trying to quit.22 And finally, dual use may expose users to more carcinogens and toxins than those who use only one product, and the average number of cigarettes smoked per day may be significantly higher among dual users.23
Unique demographic factors at work? Finally, the social and community context within which smoking occurs, and the prevalence of smoking-associated demographic risk factors, may vary significantly between rural and urban areas and between seemingly similar rural areas.24-27 Few studies have examined differences in e-cigarette use between rural and urban areas. Those that have are contradictory, reporting that rural residents use e-cigarettes both more and less than their urban peers,28,29 but many of these studies were conducted outside the United States, where the context and norms associated with smoking and e-cigarette use likely vary.
For these reasons, we sought to examine e-cigarette use among residents of Illinois, the nation’s fifth largest state and one with a rural population exceeding 1.5 million.30 We compared dual users of e-cigarettes and cigarettes to smokers of cigarettes only in terms of demographic characteristics, nicotine physical dependence, and smoking cessation beliefs, and explored dual smokers' reasons for using both types of cigarettes.
MATERIALS AND METHODS
A survey was fielded during August and September 2014 in Springfield, Ill. To obtain responses, a booth was set up at both the Illinois State Fair and the Springfield Mile (a motorcycle racing event), and participants were recruited via direct solicitation by project staff. This was supplemented by an email invitation to all employees of the Southern Illinois University School of Medicine. The 2 venues and the email strategy were chosen because they draw from a large area of central and southern Illinois and were convenient to the location of the study team. Individuals were eligible to participate if they were ≥18 years of age and used any tobacco product or e-cigarettes. Survey elements were derived from 2 national surveys of health and behavior—the Minnesota Adult Tobacco Survey 201031 and the Brief Smoking Consequences Questionnaire-Adult.32
Survey questions assessed cigarette use, nicotine physical dependence, social norms, perceived risks and benefits, and smoking cessation beliefs and behaviors. Questions were slightly reworded to address not only the use of traditional cigarettes, but the use of e-cigarettes, as well. Ultimately, each participant answered a similarly-worded set of questions for both regular and e-cigarettes. Dual use of cigarettes and e-cigarettes was also assessed. Participants self-reported all data and survey responses on an electronic tablet and received a $10 (cash or gift card) incentive. This project was reviewed and approved by the Springfield Committee for Research Involving Human Subjects.
Stratification of results. Race was dichotomized into white and non-white. Education was stratified into 3 categories: up to and including high school graduation, some college but not a Bachelor’s degree, and Bachelor’s degree and above. Income was divided as being ≤$20,000 or >$20,000, and age was split into 2 groups by the median value. Analyses included descriptions of participant demographics, dual use status, measures of nicotine physical dependence, quit attempts, and e-cigarette use motivations. Bivariate relationships between dual use status and demographic characteristics, nicotine physical dependence, and smoking cessation beliefs were analyzed by chi-square (categorical variables) and ANOVA (continuous/Likert variables).
Multivariable logistic regression modeling of the demographic variables and dual use status (cigarette smoker only vs dual user) was performed to predict 3 factors: number of cigarettes smoked per day (≤10 vs 11+); time to first cigarette (≤30 vs 31+ minutes from waking); and perceived likelihood of quit attempt success (very/somewhat likely vs very/somewhat unlikely). Multivariable models examining the reasons for dual use included the demographic, nicotine physical dependence, and cessation belief items described previously.
RESULTS
Of 309 total survey participants (Fair=288; Race=12; Email=9), there were 235 current cigarette smokers consisting of 79 who smoked only cigarettes (smokers); 122 who used both cigarettes and e-cigarettes (dual users); and 34 former e-cigarette users. Only smokers and dual users were included in this analysis (N=201, although for the purposes of TABLE 1, N=200 or 199 because at least one participant did not provide answers to all of the questions). Approximately 51% of the smokers were male, 78% were white, 12% were 4-year college graduates, and 57% reported incomes >$20,000. The mean age was 37.7 years (SD=14.4); 50% of respondents were <35 years of age. Dual users did not vary significantly from smokers in terms of gender, age, education, or income (all P>.05). However, a greater proportion of whites vs non-whites were dual users (54.9% vs 42.3%; P=.035).
Click here to see an enlarged version of the table.
No big quit differences. Bivariate analyses revealed that dual users were no more likely than smokers to have attempted to quit smoking within the past year (X2=2.3; P=.14), consider quitting in the next one or 6 months (X2=1.1; P=.34), or differ in perceived likelihood of cessation success (X2=0.0; P=1.00). The proportion of dual users who smoked 11+ cigarettes per day did not differ from that of cigarette smokers for the group as a whole or when the group was stratified by gender, income, education, or age. However, among non-whites, dual users smoked fewer cigarettes than cigarette smokers (TABLE 1).
Predicting physical dependence. Significant differences also were observed regarding the timing of the first cigarette of the day, with dual users approximately 3 times more likely than smokers to smoke within 30 minutes of awakening (80% vs 54.4%; OR=3.3; 95% CI, 1.8-6.3), and this difference was upheld among males, females, whites, those with an income >$20,000, those with a high school education or less and those with some college education, and age >34 years. There was no association, however, between dual use and perceived likelihood of quit success.
We then performed multivariable logistic modeling on dual users to determine which variables might predict 3 measures of physical dependence: number of cigarettes smoked per day (≤10 vs 11+), time between waking and smoking the first cigarette of the day (≤30 vs 31+ minutes), and perceived likelihood of cessation success (TABLE 2). Male gender (OR=3.4; 95% CI, 1.8-6.5) and white race (OR=4.4; 95% CI, 1.9-10.1) were significant for predicting smoking 11+ cigarettes a day, while dual use status was insignificant (P=.104). Regarding time to first cigarette, only dual use was significant (OR=3.1; 95% CI, 1.6-5.9), with dual users approximately 3 times more likely than smokers to have their first cigarette within 30 minutes of waking. No variables were significant in predicting perceived likelihood of quit success.
Reasons for dual use. We examined reasons for dual use with the question: Do you use e-cigarettes to reduce your regular tobacco use? Here, 79.5% of smokers reported using e-cigarettes to quit smoking or reduce the number of cigarettes smoked.
A multivariable polynomial logistic regression that included only dual users was performed to examine which variables might predict use for tobacco cessation (“trying to quit smoking”) vs reduction in smoking intensity (“trying to reduce the number of regular cigarettes I smoke per day”) vs no change (“use the same amount of tobacco as always”) (TABLE 2). Whites were approximately 6 times more likely than non-whites to indicate they engage in dual use to try to quit smoking (OR=6.0; 95% CI, 1.1-32.9). Males and people with lower incomes were much less likely to indicate they engaged in dual use to try to reduce the number of regular cigarettes smoked than females or those with higher incomes (OR=0.2; 95% CI, 0.1-0.8 and OR=0.1, 95% CI, 0.0-0.5, respectively). No other demographic variables or measures of nicotine physical dependence were significantly different between dual users and smokers.
Click here to see an enlarged version of the table.
DISCUSSION
E-cigarettes are used by approximately half of smokers (52%), which is much higher than that reported by Delnevo, et al, in their analysis of the National Health Interview Study.33 There, prevalence of dual use of both cigarettes and e-cigarettes ranged from 3.4% to 12.7%. This substantial difference raises important questions regarding study population characterization. Were participants in our study representative of central Illinois, state fair attendees, or the agricultural profession? Further work to identify this group with an increased propensity for dual use will assist clinicians in developing appropriate intervention strategies.
Dual use in our study did not vary by many customary demographic variables. Nor was it associated with different rates of past or future quit attempts or perceived ability to successfully quit if quitting was attempted. These factors—high rates of dual use and insignificant effect on quit attempts—may have implications for local physicians counseling patients who smoke.
In our study, the majority of smokers already use e-cigarettes, and this does not seem to increase their ability/likelihood to quit smoking. Further, dual use did not seem to be associated with overall cigarette consumption; males and white participants smoked more cigarettes than females and non-whites. But dual use was associated with a measure of increased nicotine physical dependence (earlier first cigarette of the day). As a result, physicians may want to think twice before recommending e-cigarette use as a means of smoking cessation.
In addition to the high prevalence of e-cigarette use among smokers, a number of other interesting findings surfaced that run counter to some of the current literature. First, dual users are no more likely than smokers to have tried to quit in the past or to try to quit in the future.21,22,34 It could be that for the relatively small geographical area from which our participants were recruited (central Illinois; ~77% of participants from Sangamon County alone), the local context and culture of smoking differs from that associated with participants in other studies, who were mostly recruited from national and regional online surveys. However, there is no a priori reason to suspect Sangamon County is especially different, as it is quite similar to Illinois as a whole by many measures (eg, percentage rural: 14.1% vs 11.5%; percentage black (only): 12.4% vs 14.7%; education to at least a Bachelor’s degree: 33.0% vs 31.9%; and median household income: $55,565 vs $57,166).30
While we found that dual users did have one measure of increased nicotine physical dependence, the total number of cigarettes consumed per day was not significantly different from that of smokers.23-25 This is contrary to another study of nicotine physical dependence, but, unlike that study, we did not assess length of time of concurrent use.35 There is much uncertainty surrounding the issue of nicotine physical dependence and e-cigarette use, largely because the level of nicotine delivered by various e-products varies significantly.36
Cross-sectional nature, small sample size limit utility of data
There are significant limitations to this study, including the cross-sectional nature of the data, the small sample size, the use of self-report, and the limited scope of recruitment. The relatively small sample size limits our ability to observe small differences and effect sizes. However, small differences often lack practical significance. Finally, participation was limited to those attending a state fair or a local sporting event and those employed by a local medical school. Thus, the results may not be generalizable to populations outside central Illinois. On the other hand, the very low income sample recruited from the Midwestern US, which is underrepresented in prior e-cigarette research, might represent some of the strengths of this work.
Future investigations. Future studies should more closely examine e-cigarette use prevalence on smaller geographic scales and especially in rural areas where there is a paucity of research. As the majority of our respondents came from a single county in central Illinois, one has to ask the questions, “Is this a ‘hot spot’ for e-cigarette use?" And "Do other rural areas experience similar use?” It may be important to know if national surveys are sensitive enough to observe significant local variations. Research also should examine how e-cigarette use and the influence of local culture vary across wider areas.
Several specific areas of study would help to inform policy and intervention development. For example, is tobacco cigarette quit success impacted by concurrent e-cigarette use? While our study showed no difference in past or possible future quit attempts among dual users as compared with smokers, we did not assess actual quit success, and multiple participants in our study anecdotally described using e-cigarettes to successfully quit smoking.
In the end, the rapid increase in the use of e-cigarettes has the potential to significantly alter the landscape of nicotine physical dependence, and local culture and other influences are likely associated with use patterns.
CORRESPONDENCE
Wiley D. Jenkins, PhD, MPH, Science Director, Population Health Science Program, Southern Illinois University School of Medicine, 201 E. Madison St., Springfield, IL 62794-9664; [email protected].
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
1. Agaku IT, King BA, Husten CG, et al; Centers for Disease Control and Prevention (CDC). Tobacco product use among adults—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:542-547.
2. Jamal A, Agaku IT, O’Connor E, et al. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108-1112.
3. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med. 2015;175:1574-1576.
4. US Department of Health and Human Services. Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Available at: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed January 22, 2014.
5. Gamm LD, Hutchison LL, Dabney BJ, et al, eds. (2003). Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center.
6. Doescher MP, Jackson JE, Jerant A, et al. Prevalence and trends in smoking: a national rural study. J Rural Health. 2006;22:112-118.
7. Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011-2013. Nicotine Tob Res. 2015;17:228-235.
8. Cardenas VM, Evans VL, Balamurugan A, et al. Use of electronic nicotine delivery systems and recent initiation of smoking among US youth. Int J Public Health. 2016;61:237-241.
9. Auf R, Trepka MJ, Cano MA, et al. Electronic cigarettes: the renormalisation of nicotine use. BMJ. 2016;352:i425.
10. CNBC. E-cigarette sales are smoking hot, set to hit $1.7 billion. Available at: http://www.cnbc.com/id/100991511. Accessed April 5, 2016.
11. Weaver SR, Majeed BA, Pechacek TF, et al. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61:177-188.
12. Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24:341-347.
13. Paek HJ, Kim S, Hove T, et al. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19:545-560.
14. Kim AE, Arnold KY, Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am J Prev Med. 2014;46:409-412.
15. Steinberg MB, Giovenco DP, Delnevo CD. Patient-physician communication regarding electronic cigarettes. Prev Med Rep. 2015;2:96-98.
16. Gualano MR, Passi S, Bert F, et al. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf). 2015:37:488-497.
17. U.S. National Institutes of Health. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=%22electronic+cigarette%22&Search=Search. Accessed July 10, 2015.
18. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127-133.
19. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
20. Center for Disease Control and Prevention. Press Release February 28,2013. Available at: http://www.cdc.gov/media/releases/2013/p0228_electronic_cigarettes.html. Accessed July 8, 2015.
21. Pisinger C. Why public health people are more worried than excited over e-cigarettes. BMC Med. 2014;12:226.
22. Post A, Gilljam H, Rosendahl I, et al. Symptoms of nicotine dependence in a cohort of Swedish youths: a comparison between smokers, smokeless tobacco users and dual tobacco users. Addiction. 2010;105:740-746.
23. Mazurek JM, Syamlal G, King BA, et al; Division of Respiratory Disease Studies, National Institute for Occupational Safety and Health, CDC. Smokeless tobacco use among working adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2014;63:477-482.
24. Hutcheson TD, Greiner KA, Ellerbeck EF, et al. Understanding smoking cessation in rural communities. J Rural Health. 2008;24:116-124.
25. McMillen R, Breen J, Cosby AG. Rural-urban differences in the social climate surrounding environmental tobacco smoke: a report from the 2002 Social Climate Survey of Tobacco Control. J Rural Health. 2004;20:7-16.
26. Butler KM, Rayens MK, Adkins S, et al. Culturally-specific smoking cessation outreach in a rural community. Public Health Nurs. 2014;31:44-54.
27. Butler KM, Hedgecock S, Record RA, et al. An evidence-based cessation strategy using rural smokers’ experiences with tobacco. Nurs Clin North Am. 2012;47:31-43.
28. Hamilton HA, Ferrence R, Boak A, et al. Ever use of nicotine and nonnicotine electronic cigarettes among high school students in Ontario, Canada. Nicotine Tob Res. 2015;17:1212-1218.
29. Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879-e885.
30. US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed March 13, 2016.
31. Minnesota Adult Tobacco Survey. Tobacco use in Minnesota: 1999-2014. Available at: http://www.mnadulttobaccosurvey.org/. Accessed April 27, 2016.
32. Rash CJ, Copeland AL. The Brief Smoking Consequences Questionnaire-Adult (BSCQ-A): development of a short form of the SCQ-A. Nicotine Tob Res. 2008;10:1633-1643.
33. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18:715-719.
34. Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14-19.
35. Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68-75.
36. Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119.
Disinfection Caps Reduce CLABSI, BCC in Hematology-Oncology Patients
Clinical question: Does the use of disinfection caps on catheter hubs on central venous catheters (CVCs) reduce central-line-associated bloodstream infection (CLABSI) and blood culture contamination (BCC) in hematology-oncology patients?
Background: CVCs have facilitated the administration of chemotherapy, blood products, and fluids in cancer patients; however, their use has also brought about risk of infections. Use of an antiseptic barrier cap may result in decreased rates of CLABSI and BCC.
Study design: Multiphase prospective study
Setting: Memorial Sloan Kettering Cancer Center, New York City.
Synopsis: Disinfection caps on CVCs were sequentially introduced on high-risk units (HRUs) followed by hospital-wide implementation. The primary outcome was hospital-wide and unit-specific rates of hospital-acquired (HA) CLABSI. In Phase 1 and 2, the CDC guidelines for catheter maintenance were followed. In Phase 3, the intervention was implemented in the HRUs. In Phase 4, the intervention extended hospital-wide. HA-CLABSI declined significantly compared to baseline only in HRUs. A possible explanation is that reduction in CLABSI on general wards was not apparent due to the short follow-up period as opposed to the longer follow-up period for the HRUs. The secondary outcome was that the rates of BCC declined significantly in Phase 3 and 4 when compared to Phase 1 and 2. As for limitations, the study is not a randomized controlled trial; variable follow-up periods may have contributed to different outcomes observed on the different units.
Bottom line: Implementation of disinfection caps significantly reduces rates of CLABSI in HRUs and reduces BCCs in both HRUs and general oncology units, with substantial clinical and cost-savings implications.
Citation: Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36(12):1401-1408.
Short Take
High Workload among Attending Physicians Has Negative Outcomes
Retrospective study found associations between higher attending physician workload and lower teaching evaluation scores from residents as well as increased risks to patient safety.
Citation: Wingo MT, Halvorsen AJ, Beckman TJ, Johnson MG, Reed DA. Associations between attending physician workload, teaching effectiveness, and patient safety. J Hosp Med. 2016;11(3):169-173.
Clinical question: Does the use of disinfection caps on catheter hubs on central venous catheters (CVCs) reduce central-line-associated bloodstream infection (CLABSI) and blood culture contamination (BCC) in hematology-oncology patients?
Background: CVCs have facilitated the administration of chemotherapy, blood products, and fluids in cancer patients; however, their use has also brought about risk of infections. Use of an antiseptic barrier cap may result in decreased rates of CLABSI and BCC.
Study design: Multiphase prospective study
Setting: Memorial Sloan Kettering Cancer Center, New York City.
Synopsis: Disinfection caps on CVCs were sequentially introduced on high-risk units (HRUs) followed by hospital-wide implementation. The primary outcome was hospital-wide and unit-specific rates of hospital-acquired (HA) CLABSI. In Phase 1 and 2, the CDC guidelines for catheter maintenance were followed. In Phase 3, the intervention was implemented in the HRUs. In Phase 4, the intervention extended hospital-wide. HA-CLABSI declined significantly compared to baseline only in HRUs. A possible explanation is that reduction in CLABSI on general wards was not apparent due to the short follow-up period as opposed to the longer follow-up period for the HRUs. The secondary outcome was that the rates of BCC declined significantly in Phase 3 and 4 when compared to Phase 1 and 2. As for limitations, the study is not a randomized controlled trial; variable follow-up periods may have contributed to different outcomes observed on the different units.
Bottom line: Implementation of disinfection caps significantly reduces rates of CLABSI in HRUs and reduces BCCs in both HRUs and general oncology units, with substantial clinical and cost-savings implications.
Citation: Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36(12):1401-1408.
Short Take
High Workload among Attending Physicians Has Negative Outcomes
Retrospective study found associations between higher attending physician workload and lower teaching evaluation scores from residents as well as increased risks to patient safety.
Citation: Wingo MT, Halvorsen AJ, Beckman TJ, Johnson MG, Reed DA. Associations between attending physician workload, teaching effectiveness, and patient safety. J Hosp Med. 2016;11(3):169-173.
Clinical question: Does the use of disinfection caps on catheter hubs on central venous catheters (CVCs) reduce central-line-associated bloodstream infection (CLABSI) and blood culture contamination (BCC) in hematology-oncology patients?
Background: CVCs have facilitated the administration of chemotherapy, blood products, and fluids in cancer patients; however, their use has also brought about risk of infections. Use of an antiseptic barrier cap may result in decreased rates of CLABSI and BCC.
Study design: Multiphase prospective study
Setting: Memorial Sloan Kettering Cancer Center, New York City.
Synopsis: Disinfection caps on CVCs were sequentially introduced on high-risk units (HRUs) followed by hospital-wide implementation. The primary outcome was hospital-wide and unit-specific rates of hospital-acquired (HA) CLABSI. In Phase 1 and 2, the CDC guidelines for catheter maintenance were followed. In Phase 3, the intervention was implemented in the HRUs. In Phase 4, the intervention extended hospital-wide. HA-CLABSI declined significantly compared to baseline only in HRUs. A possible explanation is that reduction in CLABSI on general wards was not apparent due to the short follow-up period as opposed to the longer follow-up period for the HRUs. The secondary outcome was that the rates of BCC declined significantly in Phase 3 and 4 when compared to Phase 1 and 2. As for limitations, the study is not a randomized controlled trial; variable follow-up periods may have contributed to different outcomes observed on the different units.
Bottom line: Implementation of disinfection caps significantly reduces rates of CLABSI in HRUs and reduces BCCs in both HRUs and general oncology units, with substantial clinical and cost-savings implications.
Citation: Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36(12):1401-1408.
Short Take
High Workload among Attending Physicians Has Negative Outcomes
Retrospective study found associations between higher attending physician workload and lower teaching evaluation scores from residents as well as increased risks to patient safety.
Citation: Wingo MT, Halvorsen AJ, Beckman TJ, Johnson MG, Reed DA. Associations between attending physician workload, teaching effectiveness, and patient safety. J Hosp Med. 2016;11(3):169-173.
Isopropyl Alcohol Nasal Inhalation Effective Treatment for ED Nausea
Clinical question: Does inhaled isopropyl alcohol alleviate nausea as compared to inhaled saline solution among patients presenting to the ED with a chief complaint of nausea?
Background: Nausea and vomiting account for 4.8 million ED visits each year; however, antiemetics have not shown superiority compared to placebo. Isopropyl alcohol nasal inhalation is more effective than saline solution in treating postoperative nausea and vomiting; however, there have been no investigations of this therapy in the ED setting.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Emergency department at the San Antonio Military Medical Center, Texas.
Synopsis: Investigators randomized a convenience sample of 80 patients in the ED presenting with nausea or vomiting to either inhaled isopropyl alcohol (37) or saline solution (43). Subjects would nasally inhale at 0, 2, and 4 minutes. Nausea outcomes were self-rated on a scale of 0–10, with 0 being no nausea and 10 being worst nausea imaginable. Responses were taken at 0, 2, 4, 6, and 10 minutes postintervention. Primary outcome was the score at 10 minutes postintervention. The minimally significant difference was two points.
Patients in the intervention arm reported lower scores during every study period than the patients in the placebo arm. Median nausea scores at 10 minutes postintervention were lower by three in the intervention arm compared to placebo arm (P<0.001). Limitations include the short (10-minute) evaluation period, which limits identification of any adverse events; limited information on duration of symptom relief and whether the isopropyl alcohol effect persisted; possible selection bias due to utilizing a convenience sample; and use of a subjective scale for the primary outcome.
Bottom line: Isopropyl alcohol inhalation is effective in reducing nausea 10 minutes after intervention as compared with placebo in the ED setting.
Citation: Beadle KL, Helbling AR, Love SL, April MD, Hunter CJ. Isopropyl alcohol nasal inhalation for nausea in the emergency department: a randomized controlled trial [published online ahead of print November 21, 2015]. Ann Emerg Med. doi:10.1016/j.annemergmed.2015.09.031.
Clinical question: Does inhaled isopropyl alcohol alleviate nausea as compared to inhaled saline solution among patients presenting to the ED with a chief complaint of nausea?
Background: Nausea and vomiting account for 4.8 million ED visits each year; however, antiemetics have not shown superiority compared to placebo. Isopropyl alcohol nasal inhalation is more effective than saline solution in treating postoperative nausea and vomiting; however, there have been no investigations of this therapy in the ED setting.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Emergency department at the San Antonio Military Medical Center, Texas.
Synopsis: Investigators randomized a convenience sample of 80 patients in the ED presenting with nausea or vomiting to either inhaled isopropyl alcohol (37) or saline solution (43). Subjects would nasally inhale at 0, 2, and 4 minutes. Nausea outcomes were self-rated on a scale of 0–10, with 0 being no nausea and 10 being worst nausea imaginable. Responses were taken at 0, 2, 4, 6, and 10 minutes postintervention. Primary outcome was the score at 10 minutes postintervention. The minimally significant difference was two points.
Patients in the intervention arm reported lower scores during every study period than the patients in the placebo arm. Median nausea scores at 10 minutes postintervention were lower by three in the intervention arm compared to placebo arm (P<0.001). Limitations include the short (10-minute) evaluation period, which limits identification of any adverse events; limited information on duration of symptom relief and whether the isopropyl alcohol effect persisted; possible selection bias due to utilizing a convenience sample; and use of a subjective scale for the primary outcome.
Bottom line: Isopropyl alcohol inhalation is effective in reducing nausea 10 minutes after intervention as compared with placebo in the ED setting.
Citation: Beadle KL, Helbling AR, Love SL, April MD, Hunter CJ. Isopropyl alcohol nasal inhalation for nausea in the emergency department: a randomized controlled trial [published online ahead of print November 21, 2015]. Ann Emerg Med. doi:10.1016/j.annemergmed.2015.09.031.
Clinical question: Does inhaled isopropyl alcohol alleviate nausea as compared to inhaled saline solution among patients presenting to the ED with a chief complaint of nausea?
Background: Nausea and vomiting account for 4.8 million ED visits each year; however, antiemetics have not shown superiority compared to placebo. Isopropyl alcohol nasal inhalation is more effective than saline solution in treating postoperative nausea and vomiting; however, there have been no investigations of this therapy in the ED setting.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Emergency department at the San Antonio Military Medical Center, Texas.
Synopsis: Investigators randomized a convenience sample of 80 patients in the ED presenting with nausea or vomiting to either inhaled isopropyl alcohol (37) or saline solution (43). Subjects would nasally inhale at 0, 2, and 4 minutes. Nausea outcomes were self-rated on a scale of 0–10, with 0 being no nausea and 10 being worst nausea imaginable. Responses were taken at 0, 2, 4, 6, and 10 minutes postintervention. Primary outcome was the score at 10 minutes postintervention. The minimally significant difference was two points.
Patients in the intervention arm reported lower scores during every study period than the patients in the placebo arm. Median nausea scores at 10 minutes postintervention were lower by three in the intervention arm compared to placebo arm (P<0.001). Limitations include the short (10-minute) evaluation period, which limits identification of any adverse events; limited information on duration of symptom relief and whether the isopropyl alcohol effect persisted; possible selection bias due to utilizing a convenience sample; and use of a subjective scale for the primary outcome.
Bottom line: Isopropyl alcohol inhalation is effective in reducing nausea 10 minutes after intervention as compared with placebo in the ED setting.
Citation: Beadle KL, Helbling AR, Love SL, April MD, Hunter CJ. Isopropyl alcohol nasal inhalation for nausea in the emergency department: a randomized controlled trial [published online ahead of print November 21, 2015]. Ann Emerg Med. doi:10.1016/j.annemergmed.2015.09.031.
CHMP advises against approving MM drug

multiple myeloma
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has advised the European Commission not to approve ixazomib (Ninlaro), an oral proteasome inhibitor, as a treatment for patients with relapsed and/or refractory multiple myeloma (MM).
Takeda Pharmaceutical Company Limited, the company developing ixazomib, said it intends to appeal this opinion and request a re-examination by the CHMP.
“We are disappointed by the CHMP’s opinion,” said Christophe Bianchi, MD, president of Takeda Oncology. “With the support of European key medical experts, we will continue our efforts working closely with the CHMP to make Ninlaro—the first oral proteasome inhibitor—available for patients in Europe.”
“We stand behind the TOURMALINE-MM1 trial data, which were recently published in the New England Journal of Medicine and demonstrated a significant extension in progression-free survival for Ninlaro plus lenalidomide and dexamethasone versus placebo plus lenalidomide and dexamethasone and a favorable benefit-risk profile.”
TOURMALINE-MM1
The trial enrolled 722 patients with relapsed (77%), refractory (11%), relapsed and refractory (12%), or primary refractory (6%) MM.
The patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362). Baseline patient characteristics were similar between the treatment arms.
The study’s primary endpoint was progression-free survival, which was significantly longer in the IRd arm than the Rd arm. The median progression-free survival was 20.6 months and 14.7 months, respectively. The hazard ratio was 0.74 (P=0.01).
At a median follow-up of about 23 months, the median overall survival had not been reached in either treatment arm.
Adverse events (AEs) occurred in 98% of patients in the IRd arm and 99% in the Rd arm. Grade 3 or higher AEs occurred in 74% and 69% of patients, respectively; serious AEs occurred in 47% and 49%, respectively; and on-study deaths occurred in 4% and 6%, respectively.
Grade 3 and 4 thrombocytopenia, rash, and gastrointestinal AEs were more frequent in the IRd arm than the Rd arm.
The incidence of peripheral neuropathy was similar in the 2 arms, as was the percentage patients who developed new primary malignant tumors. ![]()

multiple myeloma
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has advised the European Commission not to approve ixazomib (Ninlaro), an oral proteasome inhibitor, as a treatment for patients with relapsed and/or refractory multiple myeloma (MM).
Takeda Pharmaceutical Company Limited, the company developing ixazomib, said it intends to appeal this opinion and request a re-examination by the CHMP.
“We are disappointed by the CHMP’s opinion,” said Christophe Bianchi, MD, president of Takeda Oncology. “With the support of European key medical experts, we will continue our efforts working closely with the CHMP to make Ninlaro—the first oral proteasome inhibitor—available for patients in Europe.”
“We stand behind the TOURMALINE-MM1 trial data, which were recently published in the New England Journal of Medicine and demonstrated a significant extension in progression-free survival for Ninlaro plus lenalidomide and dexamethasone versus placebo plus lenalidomide and dexamethasone and a favorable benefit-risk profile.”
TOURMALINE-MM1
The trial enrolled 722 patients with relapsed (77%), refractory (11%), relapsed and refractory (12%), or primary refractory (6%) MM.
The patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362). Baseline patient characteristics were similar between the treatment arms.
The study’s primary endpoint was progression-free survival, which was significantly longer in the IRd arm than the Rd arm. The median progression-free survival was 20.6 months and 14.7 months, respectively. The hazard ratio was 0.74 (P=0.01).
At a median follow-up of about 23 months, the median overall survival had not been reached in either treatment arm.
Adverse events (AEs) occurred in 98% of patients in the IRd arm and 99% in the Rd arm. Grade 3 or higher AEs occurred in 74% and 69% of patients, respectively; serious AEs occurred in 47% and 49%, respectively; and on-study deaths occurred in 4% and 6%, respectively.
Grade 3 and 4 thrombocytopenia, rash, and gastrointestinal AEs were more frequent in the IRd arm than the Rd arm.
The incidence of peripheral neuropathy was similar in the 2 arms, as was the percentage patients who developed new primary malignant tumors. ![]()

multiple myeloma
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has advised the European Commission not to approve ixazomib (Ninlaro), an oral proteasome inhibitor, as a treatment for patients with relapsed and/or refractory multiple myeloma (MM).
Takeda Pharmaceutical Company Limited, the company developing ixazomib, said it intends to appeal this opinion and request a re-examination by the CHMP.
“We are disappointed by the CHMP’s opinion,” said Christophe Bianchi, MD, president of Takeda Oncology. “With the support of European key medical experts, we will continue our efforts working closely with the CHMP to make Ninlaro—the first oral proteasome inhibitor—available for patients in Europe.”
“We stand behind the TOURMALINE-MM1 trial data, which were recently published in the New England Journal of Medicine and demonstrated a significant extension in progression-free survival for Ninlaro plus lenalidomide and dexamethasone versus placebo plus lenalidomide and dexamethasone and a favorable benefit-risk profile.”
TOURMALINE-MM1
The trial enrolled 722 patients with relapsed (77%), refractory (11%), relapsed and refractory (12%), or primary refractory (6%) MM.
The patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362). Baseline patient characteristics were similar between the treatment arms.
The study’s primary endpoint was progression-free survival, which was significantly longer in the IRd arm than the Rd arm. The median progression-free survival was 20.6 months and 14.7 months, respectively. The hazard ratio was 0.74 (P=0.01).
At a median follow-up of about 23 months, the median overall survival had not been reached in either treatment arm.
Adverse events (AEs) occurred in 98% of patients in the IRd arm and 99% in the Rd arm. Grade 3 or higher AEs occurred in 74% and 69% of patients, respectively; serious AEs occurred in 47% and 49%, respectively; and on-study deaths occurred in 4% and 6%, respectively.
Grade 3 and 4 thrombocytopenia, rash, and gastrointestinal AEs were more frequent in the IRd arm than the Rd arm.
The incidence of peripheral neuropathy was similar in the 2 arms, as was the percentage patients who developed new primary malignant tumors. ![]()
Public Opinion about Healthcare Reform Becomes More Positive
Reference
1. Jacobs LR, Mettler S. Liking health reform but turned off by toxic politics [published online ahead of print April 2016]. Health Aff. doi:10.1377/hlthaff.2015.1313.
Reference
1. Jacobs LR, Mettler S. Liking health reform but turned off by toxic politics [published online ahead of print April 2016]. Health Aff. doi:10.1377/hlthaff.2015.1313.
Reference
1. Jacobs LR, Mettler S. Liking health reform but turned off by toxic politics [published online ahead of print April 2016]. Health Aff. doi:10.1377/hlthaff.2015.1313.