User login
Moles: Their Role in Skin Cancer Diagnosis
ANSWER
The correct answer is none of the above (choice “d”). These lesions are all intradermal nevi, which have little, if any, risk for malignant transformation. Deeper nevi are considered quite safe, unless significant change has occurred. Despite the unlikelihood, however, it is risky to declare a 0% chance of skin cancer.
DISCUSSION
Slow growth and increased prominence are not the kinds of changes to look for in skin lesions. Rather, look for marked asymmetry (eg, the growth of a new, darker, macular component) or other change in color or consistency.
Hairs on these lesions are quite normal and are actually reassuring in confirming their benign nature. Skin cancers seldom support hair growth.
Most melanomas don’t come from moles. Instead, they are “de novo” lesions, literally coming from nothing, out of clear skin. It is true that the more moles someone has, the greater his or her risk for skin cancer, though not necessarily in one of the moles. When melanomas do develop from nevi (a collection of a certain type of melanocyte), this usually occurs in superficial types, such as compound or junctional nevi. From an objective standpoint, in this patient’s case, family history means nothing.
What does matter is to pay as much attention to the owner as to the lesion. The more fair-skinned and sun-damaged (freckles, blue eyes, red hair) the patient is, the more worrisome a lesion can be.
This patient had none of those traits, and she will likely have one of her lesions surgically excised to ensure she’s satisfied with the resulting scar. Of course, the tissue sample will be sent for pathologic examination, as any specimen should be.
ANSWER
The correct answer is none of the above (choice “d”). These lesions are all intradermal nevi, which have little, if any, risk for malignant transformation. Deeper nevi are considered quite safe, unless significant change has occurred. Despite the unlikelihood, however, it is risky to declare a 0% chance of skin cancer.
DISCUSSION
Slow growth and increased prominence are not the kinds of changes to look for in skin lesions. Rather, look for marked asymmetry (eg, the growth of a new, darker, macular component) or other change in color or consistency.
Hairs on these lesions are quite normal and are actually reassuring in confirming their benign nature. Skin cancers seldom support hair growth.
Most melanomas don’t come from moles. Instead, they are “de novo” lesions, literally coming from nothing, out of clear skin. It is true that the more moles someone has, the greater his or her risk for skin cancer, though not necessarily in one of the moles. When melanomas do develop from nevi (a collection of a certain type of melanocyte), this usually occurs in superficial types, such as compound or junctional nevi. From an objective standpoint, in this patient’s case, family history means nothing.
What does matter is to pay as much attention to the owner as to the lesion. The more fair-skinned and sun-damaged (freckles, blue eyes, red hair) the patient is, the more worrisome a lesion can be.
This patient had none of those traits, and she will likely have one of her lesions surgically excised to ensure she’s satisfied with the resulting scar. Of course, the tissue sample will be sent for pathologic examination, as any specimen should be.
ANSWER
The correct answer is none of the above (choice “d”). These lesions are all intradermal nevi, which have little, if any, risk for malignant transformation. Deeper nevi are considered quite safe, unless significant change has occurred. Despite the unlikelihood, however, it is risky to declare a 0% chance of skin cancer.
DISCUSSION
Slow growth and increased prominence are not the kinds of changes to look for in skin lesions. Rather, look for marked asymmetry (eg, the growth of a new, darker, macular component) or other change in color or consistency.
Hairs on these lesions are quite normal and are actually reassuring in confirming their benign nature. Skin cancers seldom support hair growth.
Most melanomas don’t come from moles. Instead, they are “de novo” lesions, literally coming from nothing, out of clear skin. It is true that the more moles someone has, the greater his or her risk for skin cancer, though not necessarily in one of the moles. When melanomas do develop from nevi (a collection of a certain type of melanocyte), this usually occurs in superficial types, such as compound or junctional nevi. From an objective standpoint, in this patient’s case, family history means nothing.
What does matter is to pay as much attention to the owner as to the lesion. The more fair-skinned and sun-damaged (freckles, blue eyes, red hair) the patient is, the more worrisome a lesion can be.
This patient had none of those traits, and she will likely have one of her lesions surgically excised to ensure she’s satisfied with the resulting scar. Of course, the tissue sample will be sent for pathologic examination, as any specimen should be.
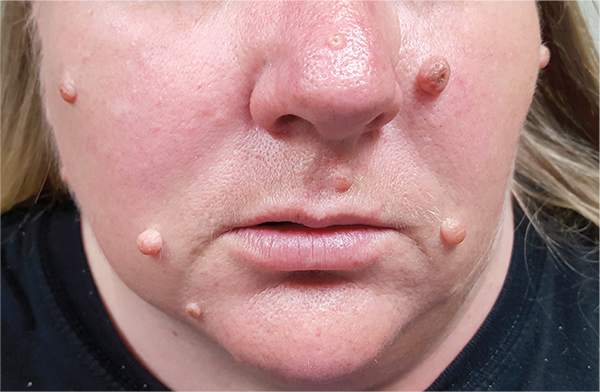
A 39-year-old woman self-refers for evaluation of moles she’s had on her face “all her life.” They have become more prominent with age, and many now have hairs growing in them. They are often traumatized by contact with fingernails or clothing. The patient worries that they might “turn into cancer” the way her grandfather’s moles did. The patient looks her stated age, is moderately overweight, and has more than her share of moles (some of which exceed 6 mm in diameter.) For the most part, they are skin-colored, and several are hair-bearing. Further questioning reveals that her moles manifested during puberty and have not been present “all her life.” Her type II skin is otherwise unremarkable and free of sun damage.
E-cigarettes: How “safe” are they?
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
› Inform patients that e-cigarette vapors contain toxic substances, including the heavy metals lead, cadmium, and nickel. A
› Educate all patients—particularly young people and those who are pregnant or lactating—about the potential health risks of e-cigarettes. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Electronic cigarettes (e-cigarettes) have become increasingly popular over the last decade. Although they are perceived by many to be safer than traditional cigarettes, many of the devices still contain nicotine, and inhaling their vapors exposes users to toxic substances, including lead, cadmium, and nickel—heavy metals that are associated with significant health problems.1 (For more on how e-cigarettes work, see “Cigarettes vs e-cigarettes: How does the experience (and cost) compare?”)
In addition, many people use e-cigarettes as a means to stop smoking, but few who do so achieve abstinence.2,3 They frequently end up utilizing both, increasing their health risks by exposing themselves to the dangers of 2 products instead of one.1
Further complicating the issue is that the manufacture and distribution of e-cigarettes has not been well regulated. Without regulation, there is no way to know with certainty how much nicotine the devices contain and what else is in them.
Things, however, are changing. The Food and Drug Administration (FDA) recently announced that e-cigarettes and other tobacco products like cigars and hookahs will now be regulated in the same way the government regulates tobacco cigarettes and smokeless tobacco.4 The rule will not take effect immediately because companies requested time to comply, but once it is enacted, packaging will be required to list what the products contain, among other changes.
Keeping up on the latest information on e-cigarettes is now—and will continue to be—important as family physicians are increasingly asked about them. What follows is a review of what we know about their potential risks.
A nicotine system developed by a pharmacist
E-cigarettes, or electronic nicotine delivery systems, were patented in 2003 by a Chinese pharmacist.5 Since their introduction to North America and Europe in 2007, the devices have become known by over 400 different brand names.6 Consumption among adults doubled by 2012, and by 2014, about 4% of US adults used e-cigarettes every day or some days.7 Many of them are dual users of tobacco and electronic cigarettes. In fact, Jenkins and colleagues reports in this issue of JFP (see "E-cigarettes: Who's using them and why?") that over half of cigarette smokers (52%) in their study use e-cigarettes, usually to either lower their cigarette consumption or aid in smoking cessation. (Throughout this article, we will use “cigarettes” and “smoking” to refer to the use of traditional tobacco cigarettes.)
In addition to concern over an increase in use among the general population, there is significant concern about the increase in e-cigarette use among US middle and high school students.1,8,9 In 2015, e-cigarettes were the most commonly used smoking product among middle and high school students, with 620,000 middle school students and nearly 2.4 million high school students using the battery-powered devices in the past 30 days.10
Many factors have contributed to the growing popularity of e-cigarettes.
- Perceived safety. With tobacco’s dangers so thoroughly documented, many advertising campaigns tout e-cigarettes as less dangerous than conventional cigarettes in terms of their ability to cause cardiac and lung diseases and low birth weights. This is largely because e-cigarettes do not produce the combustion products of tar, ash, or carbon monoxide. In addition, many consumers are mistakenly less fearful about the nicotine added to many e-cigarettes.
- Expectation that it helps smokers quit. Many smokers view e-cigarettes as an aid to smoking cessation.6 In fact, testimonials of efficacy in tobacco cessation abound in promotional materials and on the Web, and e-cigarettes are recommended by some physicians as a means to quit or lessen smoking of tobacco cigarettes.11
- Wide availability and opportunities for use. The use of electronic nicotine delivery devices is sometimes permitted in places where smoking of conventional cigarettes is banned, although rules vary widely in different parts of the country. In addition, e-cigarettes are readily available for purchase on the Internet without age verification.
- Extensive advertising. There are increasing concerns that advertising campaigns unduly target adolescents, young adults, and women.12-155 In addition to advertising, the media and social influences play significant roles in young people’s experimentation with “vaping,” the term for inhaling electronic cigarette aerosols.14,15
- Regulation, legislation remain controversial. Currently, e-cigarettes are not required to be tested before marketing,16 but that may change with the FDA’s new regulations. The British National Public Health body, Public Health England, has documented public health benefits of e-cigarettes when used as a way to quit smoking, and provides evidence that the devices are less dangerous than traditional cigarettes.17 But this issue and public policy are the subject of ongoing debate. In 2015, the United Kingdom made it illegal to sell e-cigarettes or e-liquids to people younger than 18 years of age and urged child-proof packaging.
What’s “in” an e-cigarette—and are the ingredients toxic?
Because e-cigarettes are relatively new to the global marketplace, little research exists regarding the long-term effects and safety of their use, especially among habitual users.
Vapor/refills. E-liquids may contain a variety of substances because they have been largely unregulated, but they generally include some combination of nicotine, propylene glycol, glycerin, and flavorings. In fact, up to 7000 flavors are available,6 including such kid-friendly flavors as chocolate, cherry crush, and bubble gum.
When the refills do contain nicotine, users generally derive less of the substance from the electronic devices than they do from a conventional cigarette. Researchers found that individual puffs from an e-cigarette contained 0 to 35 µg nicotine per puff.1,18 Assuming an amount at the high end of the spectrum (30 µg nicotine), it would take about 30 puffs of an e-cigarette to derive the same amount of nicotine (1 mg) typically delivered by a conventional cigarette.
The chemical make-up of the vapor and the biologic effects on animal models have been investigated using 42 different liquid refills.19,20 All contained potentially harmful compounds, but the levels were within exposure limits authorized by the FDA. These potentially dangerous chemicals include the known toxins formaldehyde, acrolein, and hydrocarbons.20
An inflammatory response to the inhalation of the vapors was demonstrated in mouse lungs; exposure to e-cigarette aerosols reduced lung glutathione—an important enzyme in maintaining oxidation-reduction balance—to a degree similar to that of cigarette smoke exposure.20 Less of the enzyme facilitates increased pulmonary inflammation.
In addition, human lung cells release pro-inflammatory cytokines when exposed to e-cigarette aerosols.20 Other health risks include:
Harm to indoor air quality/secondhand exposure. Even though e-cigarettes do not emit smoke, bystanders are exposed to the aerosol or vapor exhaled by the user, and researchers have found varying levels of such substances as formaldehyde, acetaldehyde, isoprene, acetic acid, acetone, propanol, propylene glycol, and nicotine in the air. However, it is unclear at this time whether the ultra-fine particles in the e-cigarette vapor have health effects commensurate with the emissions of conventional cigarettes.1,21,22
Cartridge refill ingestion by children. Accidental nicotine poisonings, particularly among children drawn to the colors, flavors, and scents of the e-liquids, have been problematic. In 2014, for example, over 3500 exposures occurred and more than half of those were in children younger than 6 years of age. (Exposure is defined as contact with the substance in some way including ingestion, inhalation, absorption by the skin/eyes, etc; not all exposures are poisonings or overdoses).23 Although incidence has tapered off somewhat, the American Association of Poison Control Centers reports that there were 623 exposures across all age groups between January 1, 2016 and April 30, 2016.23
Environmental impact of discarded e-cigarettes. Discarded e-cigarettes filling our landfills is a new and emerging public health concern. Their batteries, as do all batteries, pollute the land and water and have the potential to leach lead into the environment.24 Similarly, incompletely used liquid cartridges and refills may contain nicotine and heavy metals, which add to these risks.24
Explosions. Fires and explosions have been documented with e-cigarette use, mostly due to malfunctioning lithium-ion batteries.25 Thermal injuries to the face and hands can be significant.
Heavy metals. The presence of lead, cadmium, and nickel in inhaled e-cigarette vapor is another area of significant concern, particularly for younger people who might have long-term exposure.1 All 3 heavy metals are known to be toxic to humans, and safe levels of inhalation have not been established.
Inhalation and/or ingestion of lead, in particular, can cause severe neurologic damage, especially to the developing brains of children.26 Lead also results in hematologic dysfunction. Because of the risks associated with inhalation of this heavy metal, the substance was removed from gasoline years ago.
Inhaled cadmium induces kidney, liver, bone, and respiratory tract pathology27 and can cause organ failure, hypertension, anemias, fractures, osteoporosis, and/or osteomalacia.28 And inhaling nickel produces an inflammatory pulmonary reaction.29
Pregnancy/lactation. Since no clear evidence exists on the safety of e-cigarette use during pregnancy, women should avoid exposure to these vapors during the entire perinatal period. Similarly, the effects of e-cigarettes on infants who are breastfeeding are not established. Pregnant and breastfeeding women should not replace cigarettes with e-cigarettes.30,31 For pregnant women who smoke, the US Preventive Services Task Force (USPSTF) advises using only behavioral methods to stop cigarette use.32 And until more information becomes available, exposing infants and young children to e-cigarette vapor during breastfeeding is not recommended.
On the flip side, without tobacco, tar, ash, or carbon monoxide, e-cigarettes may have some advantages when compared with the use of traditional cigarettes, but that has not been substantiated.
Cigarettes vs e-cigarettes: How does the experience (and cost) compare?
If you were to ask a smoker to describe how cigarette smoking compares to using e-cigarettes, he or she would probably tell you that while the process of drawing on an e-cigarette is similar to that of a conventional cigarette, the experience in terms of reaching that state of relaxation or getting that “smoker’s high” is not.
In fact, a recent national survey of current and former smokers found that more than three-quarters of current smokers (77%) rated e-cigarettes less satisfying than conventional cigarettes and stopped using them.1 “Being less harmful” was the most highly rated reason for continuing to use the devices among people who switched from conventional to e-cigarettes.
How do they work? E-cigarettes do not burn anything and users do not light them. E-cigarettes work in much the same way as a smoke or fog machine. They use battery power (usually a rechargeable lithium battery) to heat a solution—usually containing nicotine, flavorings, and other chemicals—to the point that it turns into vapor. Much of whatever substances are in the vapor enter the bloodstream through the buccal mucosa, rather than the lungs.
Devices typically have an on/off button or switch, an atomizer containing a heating coil, a battery, and an LED light, which is designed to simulate a burning cigarette. A sensor detects when a user takes a drag and activates the atomizer and light. Some of the devices can be charged with a USB cord.
Because e-cigarettes don’t burn anything, they don’t have any smoke. They also don’t have any tar, ash, carbon monoxide, or odor (except perhaps a faint, short-lived scent matching the flavor liquid chosen). But the issues of second-hand exposure and effects on air quality are still being investigated.
With over 500 brands available, devices generally fall into one of 3 categories:2
- Cigalikes: About the same size and shape of a conventional cigarette, these cigarette look-alikes may come pre-filled with about a day’s worth of liquid and then may be discarded, or they may be non-disposable and have a replaceable cartridge.
- eGo’s: Also known as "vape pens," these devices tend to be longer and wider than cigalikes, have a more powerful battery, and usually are refillable or have a replaceable cartridge.
- Mods: Short for “modules,” these “vaporizers” tend to be the largest and most expensive type of e-cigarette. They may be refilled with e-liquid or accept replaceable cartridges and have even more powerful batteries.
What do they cost? A pack of cigarettes (containing 20 cigarettes) costs anywhere from $5 to $14, depending on where one lives.3 The price of e-cigarette devices starts at about $8 and can climb higher than $100. A 5-pack of flavor cartridges or a refill tank of e-liquid (which may last as long as about 150 cigarettes) costs about $10 to $15.4
To put this in perspective, a pack-a-day smoker in New York might spend about $5000 a year on cigarettes ($14 per pack x 365 days in a year), whereas someone who uses an e-cigarette device ($10) plus a refill tank per week ($14 x 52 weeks per year) will spend about $740 a year. (The actual cost will be higher because atomizers or devices as a whole must be replaced periodically, with some lasting only days and others lasting weeks or months, depending largely on how often one uses them. Although the cost of atomizers ranges widely, many can be found for $3-$5.)
Of course, the difference between cigarettes and e-cigarettes will be less dramatic in states where cigarettes are cheaper.
References
1. Pechacek TF, Nayak P, Gregory KR, et al. The potential that electronic delivery systems can be a disruptive technology: results from a national survey. Nicotine Tob Res. 2016. Available at: http://ntr.oxfordjournals.org/content/early/2016/05/03/ntr.ntw102.abstract. Accessed May 13, 2016.
2. Center for Environmental Health. A smoking gun: cancer-causing chemicals in e-cigarettes. Available at: http://www.ceh.org/wp-content/uploads/CEH-2015-report_A-Smoking-Gun_-Cancer-Causing-Chemicals-in-E-Cigarettes_alt.pdf. Accessed May 11, 2016.
3. Holmes H. The price of being an American. What a pack of cigarettes costs, in every state. August 28, 2015. Available at: http://www.theawl.com/2015/08/what-a-pack-of-cigarettes-costs-in-every-state. Accessed May 11, 2016.
4. Blu. How much do e-cigs cost? E-cig & vapor cigarette prices. Available at: http://www.blucigs.com/much-e-cigs-cost/. Accessed May 13, 2016.
Don’t substitute one form of nicotine for another
The USPSTF has not determined the benefit-to-harm ratio of using e-cigarettes as a smoking cessation aid, but recommends prescribing behavioral techniques and/or pharmacologic alternatives instead.32 Because the devices have been promoted as an aid to smoking cessation, intention to quit using tobacco products is a reason often stated for utilizing e-cigarettes.2,33,34 Indeed, use of e-cigarettes is much more likely among those who already utilize tobacco products.35-37
At least one study reports that e-cigarettes have efficacy similar to nicotine patches in achieving smoking abstinence among smokers who want to quit.38 Former smokers who used e-cigarettes to quit smoking reported fewer withdrawal symptoms than those who used nicotine skin patches.39 In addition, former smokers were more likely to endorse e-cigarettes than nicotine patches as a tobacco cigarette cessation aid. Significant reduction in tobacco smoke exposure has been demonstrated in dual users of tobacco and electronic cigarettes;40,41 however, both of these nicotine delivery systems sustain nicotine addiction.
Despite many ongoing studies to determine if e-cigarettes are useful as a smoking cessation aid, the results vary widely and are inconclusive at this time.42
E-cigarettes do not increase long-term tobacco abstinence
Contrary to popular belief, research shows that e-cigarette use among smokers is not associated with long-term tobacco abstinence.1 E-cigarette users, however, may make more attempts to quit smoking compared with smokers not using them.43 In addition, even though there is some evidence that e-cigarettes help smokers reduce the number of cigarettes smoked per day, simply reducing the daily number of cigarettes does not equate with safety.44 Smoking just one to 4 cigarettes per day poses 3 times the risk of myocardial infarction and lung cancer compared with not smoking.44 And since many individuals continue to use traditional and electronic cigarettes, they end up in double jeopardy of toxicity through exposure to the dangers of both.
A gateway to other substances of abuse?
There is also fear that nicotine exposure via e-cigarettes, especially in young people, serves as a “gateway” to tobacco consumption and other substance abuses, and increases the risk for nicotine addiction.34 Such nicotine-induced effects are a result of changes in brain chemistry, and have been documented in humans and animals.34
These concerns about negative health consequences, combined with the fact that e-cigarettes are undocumented as a smoking cessation aid, add urgency to the need for legislative and regulatory actions that hopefully can curb all nicotine exposures, particularly for our nation’s youth. In the meantime, it is important for physicians to advise patients—and the public—about the risks of e-cigarettes and the importance of quitting all forms of nicotine inhalation because nicotine—regardless of how it is delivered—is still an addictive drug.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville School of Medicine, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
1. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972-1986.
2. Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787-1791.
3. Grana R, Popova L, Ling P. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Int Med. 2014;174:812-813.
4. U.S. Food and Drug Administration. Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). Available at: http://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm. Accessed May 12, 2016.
5. Grana R, Benowitz N, Glantz SA. Background paper on E-cigarettes (electronic nicotine delivery systems). Center for Tobacco Control Research and Education, University of California, San Francisco, a WHO Collaborating Center on Tobacco Control. Prepared for World Health Organization Tobacco Free Initiative. December 2013. Available at: http://pvw.escholarship.org/uc/item/13p2b72n. Accessed March 31, 2014.
6. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23:iii3-iii9.
7. Electronic Cigarette Use Among Adults: United States, 2014. NCHStats: A blog of the National Center for Health Statistics. Available at: http://nchstats.com/2015/10/28/electronic-cigarette-use-among-adults-united-states-2014/. Accessed April 22, 2016.
8. Centers for Disease Control and Prevention. E-cigarette use more than doubles among U.S. middle and high school students from 2011-2012. Available at: http://www.cdc.gov/media/releases/2013/p0905-ecigarette-use.html. Accessed April 22, 2016.
9. Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students — United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729-730.
10. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rpt. 2016;65:361-367.
11. Kandra KL, Ranney LM, Lee JG, et al. Physicians’ attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PloS One. 2014;9:e103462.
12. Schraufnagel DE. Electronic cigarettes: vulnerability of youth. Pediatr Allergy Immunol Pulmonol. 2015;28:2-6.
13. White J, Li J, Newcombe R, et al. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56:522-528.
14. Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134:29-36.
15. Huang J, Kornfield R, Szczypka G, et al. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23:iii26-iii30.
16. Rojewski AM, Coleman N, Toll BA. Position Statement: Emerging policy issues regarding electronic nicotine delivery systems: a need for regulation. Society of Behavioral Medicine. 2016. Available at: http://www.sbm.org/UserFiles/file/e-cig-statement_v2_lores.pdf. Accessed April 22, 2016.
17. McNeill A, Brose LS, Calder R, et al. E-cigarettes: an evidence update. A report commissioned by Public Health England. 2015. Available at: https://www.gov.uk/government/publications/e-cigarettes-an-evidence-update. Accessed April 22, 2016.
18. Goniewicz ML, Kuma T, Gawron M, et al. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158-166.
19. Varlet V, Farsalinos K, Augsburger M, et al. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12:4796-4815.
20. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732.
21. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217:628-637.
22. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25-31.
23. The American Association of Poison Control Centers. E-cigarettes and liquid nicotine. Available at: http://www.aapcc.org/alerts/e-cigarettes/. Accessed May 12, 2016.
24. Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57-62.
25. U.S. Fire Administration. Electronic cigarette fires and explosions. October 2014. Available at: https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. Accessed May 17, 2016.
26. Skerfving S, Löfmark L, Lundh T, et al. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology. 2015;49:114-120.
27. Bernhoft RA. Cadmium toxicity and treatment. Scientific World Journal. 2013;394652.
28. Agency for Toxic Substances and Disease Registry. Case studies in environmental medicine (CSEM) Cadmium Toxicity. Available at: http://www.atsdr.cdc.gov/csem/cadmium/docs/cadmium.pdf. Accessed April 22, 2016.
29. Das KK, Buchner V. Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health. 2007;22:157-173.
30. England LJ, Bunnell RE, Pechacek TF, et al. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49:286-293.
31. Suter MA, Mastrobattista J, Sachs M, et al. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth defects research. Birth Defects Res A Clin Mol Teratol. 2015;103:186-195.
32. U.S. Preventive Services Task Force. Draft Recommendation Statement. Tobacco smoking cessation in adults and pregnant women: behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement147/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed March 22, 2016.
33. Peters EN, Harrell PT, Hendricks PS, et al. Electronic cigarettes in adults in outpatient substance use treatment: awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24:233-239.
34. Kandel ER, Kandel DB. A molecular basis for nicotine as a gateway drug. N Engl J Med. 2014;371:932-943.
35. King BA, Patel R, Nguyen KH, et al. Trends in awareness and use of electronic cigarettes among US Adults, 2010-2013. Nicotine Tob Res. 2015;17:219-227.
36. McMillen RC, Gottlieb MA, Shaefer RM, et al. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;1195-1202.
37. Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684-690.
38. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629-1637.
39. Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: a cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300-305.
40. Caponnetto P, Campagna D, Cibella F, et al. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
41. Polosa R, Caponnetto P, Morjaria JB, et al. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786.
42. Malas M, van der Tempel J, Schwartz R, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. 2016. [Epub ahead of print].
43. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
44. Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315-320.
From The Journal of Family Practice | 2016;65(6):380-385.
Cognitive bias and diagnostic error
To the Editor: I appreciated the article on cognitive biases and diagnostic error by Mull et al in the November 2015 issue.1 They presented an excellent description of the pitfalls of diagnosis as reflected in a case of a patient misdiagnosed with heart failure who ultimately died of pulmonary tuberculosis complicated by pulmonary embolism (the latter possibly from using the wrong form of heparin). To the points they raised, I would like to add a few of my own about diagnosis in general and heart failure in particular.
First, any initial diagnosis not confirmed objectively within the first 24 hours should be questioned, and other possibilities should be investigated. I have found this to be essential for every day’s stay in the hospital and for every outpatient visit. The authors mention checklists as part of the solution to the problem of misdiagnosis, and I would suggest that confirmation of initial diagnoses be built into these checklists.
In the case of a presumptive diagnosis of an acute exacerbation of heart failure treated empirically with diuretics, the diagnosis should be confirmed by the next day’s response to the diuretics, ie, increased urine output, a lower respiratory rate, and a fall in the pro-B-type natriuretic peptide level. Moreover, a change in the radiographic appearance should be seen, and respiratory and pulmonary function should improve after the first 24 hours on oxygen supplementation plus diuretics. Daily patient weights are also critical in determining response to a diuretic, and are rarely done accurately. I order weights and review them daily for patients like this.
Second, it is good to look at things yourself, including the patient, medication lists, laboratory values, and radiographic films. The attending physician should look at the radiographs together with a senior radiologist. Seeing no improvement or change on the second hospital day, or seeing signs incompatible with heart failure, one could order computed tomography of the chest and begin to entertain pulmonary diagnoses.
Even vital signs can be questionable. For example, in the case presented here, with a temperature of 99°F, a heart rate of 105, and a pulse oxygenation saturation of 89%, a respiratory rate of 24 seems unbelievably low. In my experience, the respiratory rate is recorded erroneously most of the time unless it is recorded electronically or checked at the bedside by the physician using a timepiece with a sweep second-hand.
Additionally, I have found that ordering several days’ laboratory tests (eg, complete blood cell counts, chemistry panels) in advance, in many cases, risks missing important findings and wastes time, energy, and the patient’s blood. I have learned to evaluate each patient daily and to order the most pertinent laboratory tests. With electronic medical records, I can check laboratory results as soon as they are available.
- Mull N, Reilly JB, Myers JS. An elderly woman with ‘heart failure’: cognitive biases and diagnostic error. Cleve Clin J Med 2015; 82:745–753.
To the Editor: I appreciated the article on cognitive biases and diagnostic error by Mull et al in the November 2015 issue.1 They presented an excellent description of the pitfalls of diagnosis as reflected in a case of a patient misdiagnosed with heart failure who ultimately died of pulmonary tuberculosis complicated by pulmonary embolism (the latter possibly from using the wrong form of heparin). To the points they raised, I would like to add a few of my own about diagnosis in general and heart failure in particular.
First, any initial diagnosis not confirmed objectively within the first 24 hours should be questioned, and other possibilities should be investigated. I have found this to be essential for every day’s stay in the hospital and for every outpatient visit. The authors mention checklists as part of the solution to the problem of misdiagnosis, and I would suggest that confirmation of initial diagnoses be built into these checklists.
In the case of a presumptive diagnosis of an acute exacerbation of heart failure treated empirically with diuretics, the diagnosis should be confirmed by the next day’s response to the diuretics, ie, increased urine output, a lower respiratory rate, and a fall in the pro-B-type natriuretic peptide level. Moreover, a change in the radiographic appearance should be seen, and respiratory and pulmonary function should improve after the first 24 hours on oxygen supplementation plus diuretics. Daily patient weights are also critical in determining response to a diuretic, and are rarely done accurately. I order weights and review them daily for patients like this.
Second, it is good to look at things yourself, including the patient, medication lists, laboratory values, and radiographic films. The attending physician should look at the radiographs together with a senior radiologist. Seeing no improvement or change on the second hospital day, or seeing signs incompatible with heart failure, one could order computed tomography of the chest and begin to entertain pulmonary diagnoses.
Even vital signs can be questionable. For example, in the case presented here, with a temperature of 99°F, a heart rate of 105, and a pulse oxygenation saturation of 89%, a respiratory rate of 24 seems unbelievably low. In my experience, the respiratory rate is recorded erroneously most of the time unless it is recorded electronically or checked at the bedside by the physician using a timepiece with a sweep second-hand.
Additionally, I have found that ordering several days’ laboratory tests (eg, complete blood cell counts, chemistry panels) in advance, in many cases, risks missing important findings and wastes time, energy, and the patient’s blood. I have learned to evaluate each patient daily and to order the most pertinent laboratory tests. With electronic medical records, I can check laboratory results as soon as they are available.
To the Editor: I appreciated the article on cognitive biases and diagnostic error by Mull et al in the November 2015 issue.1 They presented an excellent description of the pitfalls of diagnosis as reflected in a case of a patient misdiagnosed with heart failure who ultimately died of pulmonary tuberculosis complicated by pulmonary embolism (the latter possibly from using the wrong form of heparin). To the points they raised, I would like to add a few of my own about diagnosis in general and heart failure in particular.
First, any initial diagnosis not confirmed objectively within the first 24 hours should be questioned, and other possibilities should be investigated. I have found this to be essential for every day’s stay in the hospital and for every outpatient visit. The authors mention checklists as part of the solution to the problem of misdiagnosis, and I would suggest that confirmation of initial diagnoses be built into these checklists.
In the case of a presumptive diagnosis of an acute exacerbation of heart failure treated empirically with diuretics, the diagnosis should be confirmed by the next day’s response to the diuretics, ie, increased urine output, a lower respiratory rate, and a fall in the pro-B-type natriuretic peptide level. Moreover, a change in the radiographic appearance should be seen, and respiratory and pulmonary function should improve after the first 24 hours on oxygen supplementation plus diuretics. Daily patient weights are also critical in determining response to a diuretic, and are rarely done accurately. I order weights and review them daily for patients like this.
Second, it is good to look at things yourself, including the patient, medication lists, laboratory values, and radiographic films. The attending physician should look at the radiographs together with a senior radiologist. Seeing no improvement or change on the second hospital day, or seeing signs incompatible with heart failure, one could order computed tomography of the chest and begin to entertain pulmonary diagnoses.
Even vital signs can be questionable. For example, in the case presented here, with a temperature of 99°F, a heart rate of 105, and a pulse oxygenation saturation of 89%, a respiratory rate of 24 seems unbelievably low. In my experience, the respiratory rate is recorded erroneously most of the time unless it is recorded electronically or checked at the bedside by the physician using a timepiece with a sweep second-hand.
Additionally, I have found that ordering several days’ laboratory tests (eg, complete blood cell counts, chemistry panels) in advance, in many cases, risks missing important findings and wastes time, energy, and the patient’s blood. I have learned to evaluate each patient daily and to order the most pertinent laboratory tests. With electronic medical records, I can check laboratory results as soon as they are available.
- Mull N, Reilly JB, Myers JS. An elderly woman with ‘heart failure’: cognitive biases and diagnostic error. Cleve Clin J Med 2015; 82:745–753.
- Mull N, Reilly JB, Myers JS. An elderly woman with ‘heart failure’: cognitive biases and diagnostic error. Cleve Clin J Med 2015; 82:745–753.
In reply: Cognitive bias and diagnostic error
In Reply: We thank Dr. Field for his insights and personal observations related to diagnosis and biases that contribute to diagnostic errors.
Dr. Field’s comment about the importance of revisiting one’s initial working diagnosis is consistent with our proposed diagnostic time out. A diagnostic time out can incorporate a short checklist and aid in debiasing clinicians when findings do not fit the case presentation, such as lack of response to diuretic therapy. Being mindful of slowing down and not necessarily rushing to judgment is another important component.1 Of note, the residents in our case did revisit their initial working diagnosis, as suggested by Dr. Field. Questions from learners have great potential to serve as debiasing instruments and should always be encouraged. Those who do not work with students can do the same by speaking with nurses or other members of the healthcare team, who offer observations that busy physicians might miss.
Our case highlights the problem that we lack objective criteria to diagnose symptomatic heart failure. While B-type natriuretic factor (BNP) has a strong negative predictive value, serial BNP measurements have not been established to be helpful in the management of heart failure.2 Although certain findings on chest radiography have strong positive and negative likelihood associations, the role of serial chest radiographs is less clear.3 Thus, heart failure remains a clinical diagnosis in current practice.
As Dr. Field points out, the accuracy and performance characteristics of diagnostic testing, such as the respiratory rate, need to be considered in conjunction with debiasing strategies to achieve higher diagnostic accuracy. Multiple factors can contribute to low-performing or misinterpreted diagnostic tests, and inaccurate vital signs have been shown to be similarly prone to potential error.4
Finally, we wholeheartedly agree with Dr. Field’s comment on unnecessary testing. High-value care is appropriate care. Using Bayesian reasoning to guide testing, monitoring the treatment course appropriately, and eliminating waste is highly likely to improve both value and diagnostic accuracy. Automated, ritual ordering of daily tests can indicate that thinking has been shut off, leaving clinicians susceptible to premature closure of the diagnostic process as well as the potential for “incidentalomas” to distract them from the right diagnosis, all the while leading to low-value care such as wasteful spending, patient dissatisfaction, and hospital-acquired anemia.5 We believe that deciding on a daily basis what the next day’s tests will be can be another powerful debiasing habit, one with benefits beyond diagnosis.
- Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med 2008; 121(suppl):S38–S42.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Philip KE, Pack E, Cambiano V, Rollmann H, Weil S, O’Beirne J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: a cross-sectional study. J Clin Monit Comput 2015; 29:455–460.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med 2013; 8:506–512.
In Reply: We thank Dr. Field for his insights and personal observations related to diagnosis and biases that contribute to diagnostic errors.
Dr. Field’s comment about the importance of revisiting one’s initial working diagnosis is consistent with our proposed diagnostic time out. A diagnostic time out can incorporate a short checklist and aid in debiasing clinicians when findings do not fit the case presentation, such as lack of response to diuretic therapy. Being mindful of slowing down and not necessarily rushing to judgment is another important component.1 Of note, the residents in our case did revisit their initial working diagnosis, as suggested by Dr. Field. Questions from learners have great potential to serve as debiasing instruments and should always be encouraged. Those who do not work with students can do the same by speaking with nurses or other members of the healthcare team, who offer observations that busy physicians might miss.
Our case highlights the problem that we lack objective criteria to diagnose symptomatic heart failure. While B-type natriuretic factor (BNP) has a strong negative predictive value, serial BNP measurements have not been established to be helpful in the management of heart failure.2 Although certain findings on chest radiography have strong positive and negative likelihood associations, the role of serial chest radiographs is less clear.3 Thus, heart failure remains a clinical diagnosis in current practice.
As Dr. Field points out, the accuracy and performance characteristics of diagnostic testing, such as the respiratory rate, need to be considered in conjunction with debiasing strategies to achieve higher diagnostic accuracy. Multiple factors can contribute to low-performing or misinterpreted diagnostic tests, and inaccurate vital signs have been shown to be similarly prone to potential error.4
Finally, we wholeheartedly agree with Dr. Field’s comment on unnecessary testing. High-value care is appropriate care. Using Bayesian reasoning to guide testing, monitoring the treatment course appropriately, and eliminating waste is highly likely to improve both value and diagnostic accuracy. Automated, ritual ordering of daily tests can indicate that thinking has been shut off, leaving clinicians susceptible to premature closure of the diagnostic process as well as the potential for “incidentalomas” to distract them from the right diagnosis, all the while leading to low-value care such as wasteful spending, patient dissatisfaction, and hospital-acquired anemia.5 We believe that deciding on a daily basis what the next day’s tests will be can be another powerful debiasing habit, one with benefits beyond diagnosis.
In Reply: We thank Dr. Field for his insights and personal observations related to diagnosis and biases that contribute to diagnostic errors.
Dr. Field’s comment about the importance of revisiting one’s initial working diagnosis is consistent with our proposed diagnostic time out. A diagnostic time out can incorporate a short checklist and aid in debiasing clinicians when findings do not fit the case presentation, such as lack of response to diuretic therapy. Being mindful of slowing down and not necessarily rushing to judgment is another important component.1 Of note, the residents in our case did revisit their initial working diagnosis, as suggested by Dr. Field. Questions from learners have great potential to serve as debiasing instruments and should always be encouraged. Those who do not work with students can do the same by speaking with nurses or other members of the healthcare team, who offer observations that busy physicians might miss.
Our case highlights the problem that we lack objective criteria to diagnose symptomatic heart failure. While B-type natriuretic factor (BNP) has a strong negative predictive value, serial BNP measurements have not been established to be helpful in the management of heart failure.2 Although certain findings on chest radiography have strong positive and negative likelihood associations, the role of serial chest radiographs is less clear.3 Thus, heart failure remains a clinical diagnosis in current practice.
As Dr. Field points out, the accuracy and performance characteristics of diagnostic testing, such as the respiratory rate, need to be considered in conjunction with debiasing strategies to achieve higher diagnostic accuracy. Multiple factors can contribute to low-performing or misinterpreted diagnostic tests, and inaccurate vital signs have been shown to be similarly prone to potential error.4
Finally, we wholeheartedly agree with Dr. Field’s comment on unnecessary testing. High-value care is appropriate care. Using Bayesian reasoning to guide testing, monitoring the treatment course appropriately, and eliminating waste is highly likely to improve both value and diagnostic accuracy. Automated, ritual ordering of daily tests can indicate that thinking has been shut off, leaving clinicians susceptible to premature closure of the diagnostic process as well as the potential for “incidentalomas” to distract them from the right diagnosis, all the while leading to low-value care such as wasteful spending, patient dissatisfaction, and hospital-acquired anemia.5 We believe that deciding on a daily basis what the next day’s tests will be can be another powerful debiasing habit, one with benefits beyond diagnosis.
- Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med 2008; 121(suppl):S38–S42.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Philip KE, Pack E, Cambiano V, Rollmann H, Weil S, O’Beirne J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: a cross-sectional study. J Clin Monit Comput 2015; 29:455–460.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med 2013; 8:506–512.
- Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med 2008; 121(suppl):S38–S42.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128:e240–e327.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Philip KE, Pack E, Cambiano V, Rollmann H, Weil S, O’Beirne J. The accuracy of respiratory rate assessment by doctors in a London teaching hospital: a cross-sectional study. J Clin Monit Comput 2015; 29:455–460.
- Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med 2013; 8:506–512.
Dietary and medical management of recurrent nephrolithiasis
Nephrolithiasis is common and often recurs. This review focuses on measures to prevent recurrent stone formation. Some measures apply to all patients, and some apply to specific types of stones.
COMMON AND INCREASING
According to data from the 2007–2010 National Health and Nutrition Examination Survey, the prevalence of nephrolithiasis in the United States was 10.6% in men and 7.1% in women. On average, 1 in 11 Americans will develop kidney stones at least once in their lifetime.1
By race and sex, white men have the highest incidence of nephrolithiasis and Asian women have the lowest. It is less common before age 20 and peaks in incidence in the third and fourth decades of life.
The prevalence has steadily increased in the past few decades (Table 1),1,2 but the reasons are not clear. The trend may be due to changes in diet and lifestyle, increasing prevalence of obesity and diabetes, migration from rural to urban areas, and global warming, with higher temperature resulting in dehydration and high urinary concentration of calcium and other stone-forming salts.3 Nephrolithiasis is now recognized as a systemic disorder associated with chronic kidney disease, bone loss and fractures, increased risk of coronary artery disease, hypertension, type 2 diabetes mellitus, and metabolic syndrome (Table 2).4–7
Without medical treatment, the 5-year recurrence rate is high, ranging from 35% to 50% after an initial stone event.8 Annual medical costs of care for kidney stones in the United States exceed $4.5 billion, with additional costs from missed work. Therefore, this condition has a considerable economic and social burden, which underscores the importance of prevention.9
MOST STONES CONTAIN CALCIUM
About 80% of kidney stones in adults contain calcium, and calcium oxalate stones are more common than calcium phosphate stones. Uric acid and struvite stones account for 5% to 15%, and cystine, protease inhibitor, triamterene, 2,8-dihydroxyadenine (2,8-DHA) and xanthine stones each account for less than 1%.10
Stones form when the urinary concentration of stone-forming salts, which is inversely proportional to urine volume, is higher than their saturation point, which is affected by urine pH. Acidic urine (low pH) predisposes to the formation of uric acid and cystine stones, whereas alkaline urine (high pH) favors calcium phosphate stones.
INCREASED FLUID INTAKE FOR ALL
High fluid intake, enough to produce at least 2.5 L of urine per day, should be the initial therapy to prevent stone recurrence.11
Borghi et al12 randomly assigned 199 patients who had a first calcium stone to high oral fluid intake or no intervention and followed them prospectively for 5 years. The recurrence rate was 12% in the treated group and 27% in the control group. Another study, in patients who had undergone shock wave lithotripsy, found a recurrence rate of 8% in those randomized to increase fluid intake to achieve urine output greater than 2.5 L/day, compared with 56% in those assigned to no treatment.13
Certain beverages increase the risk of stones and should be avoided. Sugar-sweetened noncola soda and punch are associated with a 33% higher risk of kidney stones, and cola sodas are associated with a 23% higher risk.14 Prospective studies have shown that the consumption of coffee, beer, wine, and orange juice is associated with a lower likelihood of stone formation.13,15
Table 3 is a brief summary of the dietary and pharmacologic interventions in the management of recurrent nephrolithiasis.
PREVENTING CALCIUM OXALATE STONES
Major urinary risk factors associated with calcium oxalate stones are hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, and low urine volume.16 Preventing calcium stones therefore depends on reducing the urinary concentration of calcium and oxalate, increasing urinary levels of inhibitors such as citrate, and increasing urine volume.
Reducing calcium excretion
Hypercalciuria has been traditionally defined as 24-hour urinary calcium excretion greater than 300 mg/day in men, greater than 250 mg in women, or greater than 4 mg/kg in men or women.17 It is a graded risk factor, and the cut points used in published research and clinical laboratories vary substantially. Some institutions use the same value for hypercalciuria in both sexes, eg, greater than 200 mg/day.18
Excessive sodium intake is the most common cause of hypercalciuria. Systemic conditions such as primary hyperparathyroidism, sarcoidosis, and renal tubular acidosis also cause hypercalciuria but are uncommon.19 Management depends on the underlying cause and includes dietary modifications and pharmacologic therapy.
Dietary modifications have a pivotal role in the management of recurrent stones that are due to hypercalciuria.
Dietary calcium should not be restricted, since calcium reduces the excretion of urinary oxalate by decreasing intestinal absorption of oxalate. Guidelines from the American Urological Association recommend a daily calcium intake of 1,000 to 1,200 mg.11–20 Moreover, restriction of dietary calcium to less than 800 mg/day (the current recommended daily allowance for adults) can lead to negative calcium balance and bone loss.
Sodium intake also influences hypercalciuria. Calcium is reabsorbed passively in the proximal tubule due to the concentration gradient created by active reabsorption of sodium. A high sodium intake causes volume expansion, leading to a decrease in proximal sodium and calcium reabsorption and enhancing calcium excretion. A low-sodium diet (80–100 mmol/day, or 1,800–2,300 mg/day) is recommended. This enhances proximal sodium and passive calcium absorption and leads to a decrease in calcium excretion.21
Dietary protein increases the acid load by production of sulfuric acid and leads to hypercalciuria by its action on bone and kidney. Animal protein has a higher content of sulfur and generates a higher acid load compared with vegetable protein and has been associated with an increased incidence of stone formation, at least in men.20,22 Borghi et al23 reported that the combination of restricted intake of animal protein (52 g/day), restricted salt intake (50 mmol, or 2,900 mg/day of sodium chloride), and normal calcium intake (30 mmol/day, or 1,200 mg/day) was associated with a lower incidence of stone recurrence in men with hypercalciuria compared with traditional low-calcium intake (10 mmol, or 400 mg/day). Patients should therefore be advised to avoid excessive intake of animal protein.
Increasing the dietary intake of fruits and vegetables as in the Dietary Approach to Stop Hypertension (DASH) diet is beneficial and reduces the risk of stone recurrence, mainly by increasing citrate excretion.24
Pharmacologic therapy in hypercalciuria. Thiazide diuretics are the mainstay of pharmacotherapy for preventing recurrent stones in patients with idiopathic hypercalciuria. They reduce the risk of stone recurrence by about 50%, as reported in a recent meta-analysis that looked at five trials comparing thiazide diuretics with placebo.25 They lower calcium excretion by causing volume depletion, thereby increasing proximal sodium and passive calcium reabsorption.
Chlorthalidone and hydrochlorothiazide are the thiazides commonly used to treat hypercalciuria. The dosage is titrated to the urinary calcium excretion, and a common mistake is to use doses that are too low. They are usually started at 25 mg/day, but often require an increase to 50 to 100 mg/day for adequate lowering of urinary calcium.
Care should be taken to avoid hypokalemia. If it occurs, it can be corrected by adding the potassium-sparing diuretic amiloride (5–10 mg/day), which increases calcium reabsorption in collecting ducts or, in patients with hypocitraturia, potassium citrate-potassium bicarbonate. (Sodium salts should be avoided, since they increase renal calcium excretion.)26
Management of hypercalciuria with metabolic causes, which include primary hyperparathyroidism and chronic acidemia. Patients who have hypercalciuria from primary hyperparathyroidism are treated with parathyroidectomy.27 Chronic metabolic acidosis causes hypercalciuria by loss of bone calcium and hypocitraturia by increasing active proximal absorption of citrate. Potassium citrate or potassium bicarbonate is used to prevent stones in such patients; sodium salts should be avoided.28
Reducing oxalate excretion
Hyperoxaluria has traditionally been defined as urinary oxalate excretion of more than 45 mg/day. However, the optimal cutoff point for urinary oxalate excretion is unclear, as is the optimal cutoff for hypercalciuria. The risk of stone formation has been shown to increase with oxalate excretion even above 25 mg/day, which is within the normal limit.18
Idiopathic hyperoxaluria. High dietary oxalate intake, especially when associated with low calcium intake, leads to idiopathic hyperoxaluria. However, the contribution of abnormal endogenous oxalate metabolism is uncertain. Ingested calcium binds to oxalate in the intestinal tract and reduces both the absorption of intestinal oxalate absorption and the excretion of urinary oxalate.29 High dietary oxalate intake has usually been regarded as a major risk factor for kidney stones.
Taylor and Curhan,30 in a prospective study, reported a mild increase in the risk of stones in the highest quintile of dietary oxalate intake compared with the lowest quintile for men (relative risk [RR] 1.22, 95% confidence interval [CI] 1.03–1.45) and older women (RR 1.21, 95% CI 1.01–1.44). They also demonstrated that eating eight or more servings of spinach per month compared with fewer than one serving per month was associated with a similar increase of stone risk in men (RR 1.30, 95% CI 1.08–1.58) and older women (RR 1.34 95% CI 1.1–1.64). In contrast, spinach and dietary oxalate intake did not increase the risk of nephrolithiasis in young women. The authors concluded that the risk associated with oxalate intake was modest, and their data did not support the contention that dietary oxalate is a major risk factor for kidney stones.
Higher oxalate intake increases urinary oxalate excretion and presumably the risk of nephrolithiasis. Limiting dietary oxalate to prevent stones is recommended if habitually high dietary intake of oxalate is identified or follow-up urine measurements show a decrease in oxalate excretion.31 Foods rich in oxalate include spinach, rhubarb, nuts, legumes, cocoa, okra, and chocolate.
The DASH diet, which is high in fruits and vegetables, moderate in low-fat dairy products, and low in animal protein, is an effective dietary alternative and has been associated with a lower risk of calcium oxalate stones.24 Consuming fruits and vegetables increases the excretion of urinary citrate, which is an inhibitor of stone formation. Also, it has been proposed that the DASH diet contains unknown factors that reduce stone risk.
Taylor et al32 prospectively examined the relationship between the DASH diet and the incidence of kidney stones and found that the diet significantly reduced the risk of kidney stones. The relative risks of occurrence of kidney stones in participants in the highest quintile of the DASH score (a measure of adherence to the DASH diet) compared with the lowest quintile were 0.55 (95% CI 0.46–0.65) for men, 0.58 (95% CI 0.49–0.68) for older women, and 0.60 (95% CI 0.52–0.70) for younger women, which the authors characterized as “a marked decrease in kidney stone risk.”
Vitamin C intake should be restricted to 90 mg/day in patients who have a history of calcium oxalate stones. Urivetzky et al33 found that urinary oxalate excretion increased by 6 to 13 mg/day at doses of ascorbic acid greater than 500 mg.
Pyridoxine (vitamin B6), a coenzyme of alanine-glyoxylate aminotransferase (AGT), increases the conversion of glyoxylate to glycine instead of oxalate and is used in the treatment of type 1 primary hyperoxaluria (see below).34 However, its effect in preventing stones in idiopathic hyperoxaluria is not well known, and it has not been studied in a randomized controlled trial. In a prospective study, Curhan et al35 reported that high intake of pyridoxine (> 40 mg/day) was associated with a lower risk of stone formation in women, but no such benefit was found in men.
Enteric hyperoxaluria. About 90% of dietary oxalate binds to calcium in the small intestine and is excreted in the stool. The remaining 10% is absorbed in the colon and is secreted in urine. Hyperoxaluria is frequently seen with fat malabsorption from inflammatory bowel disease, short gut syndrome, and gastric bypass surgery. In these conditions, excess fat binds to dietary calcium, leading to increased absorption of free oxalate in the colon.36
Treatment is directed at decreasing intestinal oxalate absorption and should include high fluid intake and oral calcium supplements. Calcium carbonate or citrate causes precipitation of oxalate in the intestinal lumen and is prescribed as 1 to 4 g in three to four divided doses, always with meals. Calcium citrate is preferred over calcium carbonate in stone-formers because of the benefit of citrate and calcium citrate’s higher solubility and greater effectiveness in the presence of achlorhydria.37 Patients should be advised to avoid foods high in oxalate and fat.
Primary hyperoxaluria is caused by inherited inborn errors of glyoxylate metabolism that cause overproduction of oxalate and urinary oxalate excretion above 135 to 270 mg/day.
Type 1 primary hyperoxaluria is the most common (accounting for 90% of cases) and is caused by reduced activity of hepatic peroxisomal AGT.
Type 2 is from a deficiency of glyoxylate reductase-hydroxypyruvate reductase (GRHPR).
Type 3 is from mutations in the HOGA1 gene, which codes for the liver-specific mitochondrial 4-hydroxy-2-oxoglutarate aldolase enzyme involved in degradation of hydroxyproline to pyruvate and glyoxalate.38
High fluid intake to produce a urinary volume of 3 L/day reduces intratubular oxalate deposition and should be encouraged. Potassium citrate (0.15 mg/kg), oral phosphate supplements (30–40 mg/kg of orthophosphate), and magnesium oxide (500 mg/day/m2) inhibit precipitation of calcium oxalate in the urine.39,40 Pyridoxine, a coenzyme of AGT, increases the conversion of glyoxylate to glycine instead of oxalate and is prescribed at a starting dose of 5 mg/kg (which can be titrated up to 20 mg/kg if there is no response) in patients with type 1 primary hyperoxaluria. About 50% of patients with type 1 respond successfully to pyridoxine, and a 3- to 6-month trial should be given in all patients in this category.34 AGT is present only in hepatocytes, and GRHPR is found in multiple tissues; therefore, combined liver-kidney transplant is the treatment of choice in patients with type 1 primary hyperoxaluria, whereas isolated kidney transplant is recommended in patients with type 2.41
Reducing uric acid excretion
Hyperuricosuria is defined as uric acid excretion of greater than 800 mg/day in men and greater than 750 mg/day in women.
The association of hyperuricosuria with increased risk of calcium oxalate stone formation is controversial. Curhan and Taylor,18 in a cross-sectional study of 3,350 men and women, reported that there was no difference in mean 24-hour uric acid excretion in individuals with and without a history of stones.
The mechanism by which uric acid leads to calcium oxalate stones is not completely known and could be the “salting out” of calcium oxalate from the urine.42
Dietary purine restriction, ie, limiting intake of nondairy animal protein to 0.8 to 1 g/kg/day, is the initial dietary intervention.11 Allopurinol is the alternative approach if the patient is not compliant or if dietary restriction fails.43
In a study by Ettinger et al,44 60 patients with hyperuricosuria and normocalciuria were randomized to receive allopurinol (100 mg three times daily) or a placebo. The allopurinol group had a rate of calculus events of 0.12 per patient per year, compared with 0.26 in the placebo group.
Increasing citrate excretion
Hypocitraturia is a well-known risk factor for the formation of kidney stones. It is usually defined as a citrate excretion of less than 320 mg/day for adults.
Citrate prevents formation of calcium crystals by binding to calcium, thereby lowering the concentration of calcium oxalate below the saturation point.45
Diet therapy. Patients with calcium oxalate stones and hypocitraturia should be encouraged to increase their intake of fruits and vegetables, which enhances urinary citrate excretion, and to limit their intake of nondairy animal protein.11
The use of citrus products in preventing stones in patients with hypocitraturia is controversial, however, and needs to be studied more.
One study46 demonstrated that lemon juice was beneficial in hypocitraturic nephrolithiasis: 4 oz/day of lemon juice concentrate in the form of lemonade was associated with an increase in urinary citrate excretion to 346 mg/day from 142 mg/day in 11 of 12 patients who participated.
Odvina47 compared the effects of orange juice with those of lemonade on the acid-base profile and urinary stone risk under controlled metabolic conditions in 13 volunteers. Orange juice was reported to have greater alkalinizing and citraturic effects and was associated with lower calculated calcium oxalate supersaturation compared with lemonade.
Lemonade therapy may be used as adjunctive treatment in patients who do not comply with or cannot tolerate alkali therapy. However, we advise caution about recommending citrus products, as they can increase oxalate excretion.
Pharmacotherapy includes alkali therapy. Barcelo et al48 compared the effects of potassium citrate and placebo in 57 patients with calcium oxalate stones and hypocitraturia. Patients treated with potassium citrate had a rate of stone formation of 0.1 event per patient per year, compared with 1.1 in the placebo group.
Many forms of alkaline citrate are available. Potassium citrate is preferred over sodium citrate since the latter may increase urine calcium excretion.49 Treatment is usually started at 30 mEq/day and is titrated to a maximal dose of 60 mEq/day for a urinary citrate excretion greater than 500 mg/day.
Common side effects are abdominal bloating and hyperkalemia (especially with renal insufficiency), and in such cases sodium-based alkali, sodium citrate, or sodium bicarbonate can be prescribed.
PREVENTING CALCIUM PHOSPHATE STONES
Risk factors for calcium phosphate stones are similar to those for calcium oxalate stones (other than hyperoxaluria), but calcium phosphate stones are formed in alkaline urine (usually urine pH > 6.0), often the result of distal renal tubular acidosis. Preventive measures are similar to those for calcium oxalate stones.
Alkali therapy should be used with caution because of its effect on urinary pH and the risk of precipitation of calcium phosphate crystals.50 Use of potassium citrate was found to be associated with increases in both urinary citrate excretion and calcium phosphate supersaturation in hypercalciuric stone-forming rats.51 It is therefore challenging to manage patients with calcium phosphate stones and hypocitraturia. Alkali administration in this setting may diminish the formation of new stones by correcting hypocitraturia, but at the same time it may increase the likelihood of calcium phosphate stone formation by increasing the urinary pH. When the urine pH increases to above 6.5 with no significant change in urine citrate or urine calcium excretion, we recommend stopping alkali therapy.
PREVENTING URIC ACID STONES
Clinical conditions associated with uric acid stones include metabolic syndrome, diabetes mellitus, gout, chronic diarrheal illness, and conditions that increase tissue turnover and uric acid production, such as malignancies. Other risk factors for uric acid stone formation are low urine volume, low uric pH, and hyperuricosuria.
Abnormally acidic urine is the most common risk factor. Metabolic syndrome and diabetes mellitus reduce ammonia production, resulting in a lower urinary pH, which predisposes to uric acid stone formation. Chronic diarrhea also acidifies the urine by loss of bicarbonate. Similarly, in gout, the predisposing factor in uric acid stone formation is the persistently acidic urine due to impaired ammonium excretion.52 Uric acid precipitates to form uric acid stones in a low urinary pH even with normal excretion rates of 600 to 800 mg/day and a urinary volume of 1 to 1.5 L.53
Therefore, apart from increasing fluid intake, urinary alkalization is the cornerstone of management of uric acid stones. Potassium citrate is the preferred alkali salt and is started at a dose of 30 mEq/day for a goal urinary pH of 6 to 6.5.47
Patients with hyperuricosuria are also advised to restrict their protein intake to no more than 0.8 to 1 mg/kg/day.
If the above measures fail, patients are treated with a xanthine oxidase inhibitor, ie, allopurinol or febuxostat, even if their uric acid excretion is normal.54
PREVENTING STRUVITE STONES
Struvite stones contain magnesium ammonium phosphate and are due to chronic upper urinary tract infection with urea-splitting bacteria such as Proteus, Klebsiella, Pseudomonas, and enterococci. Urea hydrolysis releases hydroxyl ions, resulting in alkaline urine that promotes struvite stone formation. Early detection and treatment are important, since struvite stones are associated with morbidity and rapid progression.
Medical treatment of struvite stones is usually unsuccessful, and the patient is referred to a urologist for surgical removal of the stones, the gold standard treatment.55 Long-term use of culture-specific antibiotics to prevent new stone growth is not well studied. Medical therapy by itself is preferred in patients who refuse stone removal or cannot tolerate it. Urease inhibitors such as acetohydroxamic acid have been successful in preventing or slowing stone growth, but their use is limited by frequent side effects such as nausea, headache, rash, and thrombophlebitis.56
CYSTINE STONES
Cystine stones occur in people with inherited defects of renal tubular and intestinal transport of cysteine and dibasic amino acids that cause excessive excretion of urinary cystine, ie, 480 to 3,600 mg/day.
Cystine is formed from two cysteine molecules linked by a disulfide bond. The solubility of cystine is pH-dependent, with increased solubility at higher urinary pH. The goal is to maintain a urinary cystine concentration below its solubility level by keeping the cystine concentration below 243 mg/L and the urine cystine supersaturation (the ratio of the urine cysteine concentration to the cysteine solubility in the same sample) less than 0.6.57 Therapy is aimed at increasing daily urinary volume to 3 L and urine alkalization to pH above 7, in order to increase cystine solubility by 300%.58
Overnight dehydration should be prevented, and patients should be encouraged to wake up at least once a night to void and drink additional water. Sodium restriction to 100 mmol/day (2,300 mg/day) and moderate protein restriction to 0.8 to 1 g/kg/day are associated with decreased cystine excretion, but long-term studies demonstrating their benefit in preventing cystine stones are lacking.59
A thiol-containing drug, eg, D-penicillamine (0.5–2 g/day) or tiopronin (400–1,200 mg/day), should be added to the conservative measures if they have not been effective for 3 months or if there is history of noncompliance.60 Thiol-containing drugs have a sulfhydryl group that reduces the disulfide bond, and they form soluble disulfide cysteine-drug complexes with greater ability to solubilize cystine in alkaline urine. They must always be used in conjunction with fluid and alkali therapy.61
Both drugs have severe and common adverse effects including leukopenia, aplastic anemia, fever, rash, arthritis, hepatotoxicity, pyridoxine deficiency, and proteinuria (membranous nephropathy). However, tiopronin seems to have a lesser incidence of side effects.62 Regular monitoring of complete blood cell counts, liver enzymes, and urine protein should be done.
Captopril contains a sulfhydryl group, and the captopril-cysteine disulfide is more soluble than cysteine alone. The amount of captopril that appears in the urine is low, and doses of 150 mg/day are usually required to reduce cysteine excretion, which can lead to hypotension. The efficacy of captopril in treating cystine stones is unproven, and this drug is used only if patients cannot tolerate other thiol-containing drugs.63
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol 2012; 62:160–165.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM Jr, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010; 12:e86–e96.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2011; 79:393–403.
- Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens 2008; 17:304–309.
- Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int J Urol 2005; 12:859–863.
- Ritz E. Metabolic syndrome: an emerging threat to renal function. Clin J Am Soc Nephrol 2007; 2:869–871.
- Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med 1989; 111:1006–1009.
- Saigal CS, Joyce G, Timilsina AR; Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 2005; 68:1808–1814.
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet 2006; 367:333–344.
- Pearle MS, Goldfarb DS, Assimos DG, et al; American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol 2014; 192:316–324.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843.
- Sarica K, Inal Y, Erturhan S, Yagci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res 2006; 34:184–189.
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013; 8:1389–1395.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998; 128:534–540.
- Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med 1980; 69:19–30.
- Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med 2009; 76:583–591.
- Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 2008; 73:489–496.
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115:2598–2608.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int 1982; 22:292–296.
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 1988; 66:140–146.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Noori N, Honarkar E, Goldfarb DS, et al. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: a randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis 2014; 63:456–463.
- Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med 2013; 158:535–543.
- Alon U, Costanzo LS, Chan JC. Additive hypocalciuric effects of amiloride and hydrochlorothiazide in patients treated with calcitriol. Miner Electrolyte Metab 1984; 10:379–386.
- Corbetta S, Baccarelli A, Aroldi A, et al. Risk factors associated to kidney stones in primary hyperparathyroidism. J Endocrinol Invest 2005; 28:122–128.
- Haymann JP. Metabolic disorders: stones as first clinical manifestation of significant diseases. World J Urol 2015; 33:187–192.
- Jaeger P, Portmann L, Jacquet AF, Burckhardt P. Influence of the calcium content of the diet on the incidence of mild hyperoxaluria in idiopathic renal stone formers. Am J Nephrol 1985; 5:40–44.
- Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 2007; 18:2198–2204.
- Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 2010; 78:1178–1185.
- Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 2009; 20:2253–2259.
- Urivetzky M, Kessaris D, Smith AD. Ascorbic acid overdosing: a risk factor for calcium oxalate nephrolithiasis. J Urol 1992; 147:1215–1218.
- Hoyer-Kuhn H, Kohbrok S, Volland R, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol 2014; 9:468–477.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 1999; 10:840–845.
- Parks JH, Worcester EM, O'Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63:255–265.
- Hess B, Jost C, Zipperle L, Takkinen R, Jaeger P. High-calcium intake abolishes hyperoxaluria and reduces urinary crystallization during a 20-fold normal oxalate load in humans. Nephrol Dial Transplant 1998; 13:2241–2247.
- Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int 2009; 75:1264–1271.
- Cochat P, Hulton SA, Acquaviva C, et al; OxalEurope. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 2012; 27:1729–1736.
- Leumann E, Hoppe B, Neuhaus T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol 1993; 7:207–211.
- Bergstralh EJ, Monico CG, Lieske JC, et al; IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant 2010; 10:2493–2501.
- Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol 2003; 10:271–278.
- Coe FL, Parks JH. Hyperuricosuria and calcium nephrolithiasis. Urol Clin North Am 1981; 8:227–244.
- Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386–1389.
- Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol 2009; 11:134–144.
- Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol 1996; 156:907–909.
- Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol 2006; 1:1269–1274.
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 1993; 150:1761–1764.
- Lemann J Jr, Gray RW, Pleuss JA. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 1989; 35:688–695.
- Gault MH, Chafe LL, Morgan JM, et al. Comparison of patients with idiopathic calcium phosphate and calcium oxalate stones. Medicine (Baltimore) 1991; 70:345–359.
- Krieger NS, Asplin JR, Frick KK, et al. Effect of potassium citrate on calcium phosphate stones in a model of hypercalciuria. J Am Soc Nephrol 2015; 26:3001–3008.
- Falls WF Jr. Comparison of urinary acidification and ammonium excretion in normal and gouty subjects. Metabolism 1972; 21:433–445.
- Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med 1992; 327:1141–1152.
- Kenny JE, Goldfarb DS. Update on the pathophysiology and management of uric acid renal stones. Curr Rheumatol Rep 2010; 12:125–129.
- Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS Jr (AUA Nephrolithiasis Guideline Panel). Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol 2005; 173:1991–2000.
- Williams JJ, Rodman JS, Peterson CM. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med 1984; 311:760–764.
- Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL. Clinical use of cystine supersaturation measurements. J Urol 2000; 164:1481–1485.
- Palacın MGP, Nunes V, Gasparini P. Cystinuria. In: Shriver CR, editor. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001:4909–4932.
- Goldfarb DS, Coe FL, Asplin JR. Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int 2006; 69:1041–1047.
- Barbey F, Joly D, Rieu P, Mejean A, Daudon M, Jungers P. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol 2000; 163:1419–1423.
- Asplin DM, Asplin JR. The Interaction of thiol drugs and urine pH in the treatment of cystinuria. J Urol 2013; 189:2147–2151.
- Habib GS, Saliba W, Nashashibi M, Armali Z. Penicillamine and nephrotic syndrome. Eur J Intern Med 2006; 17:343–348.
- Sloand JA, Izzo JL Jr. Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 1987; 147:1409–1412.
Nephrolithiasis is common and often recurs. This review focuses on measures to prevent recurrent stone formation. Some measures apply to all patients, and some apply to specific types of stones.
COMMON AND INCREASING
According to data from the 2007–2010 National Health and Nutrition Examination Survey, the prevalence of nephrolithiasis in the United States was 10.6% in men and 7.1% in women. On average, 1 in 11 Americans will develop kidney stones at least once in their lifetime.1
By race and sex, white men have the highest incidence of nephrolithiasis and Asian women have the lowest. It is less common before age 20 and peaks in incidence in the third and fourth decades of life.
The prevalence has steadily increased in the past few decades (Table 1),1,2 but the reasons are not clear. The trend may be due to changes in diet and lifestyle, increasing prevalence of obesity and diabetes, migration from rural to urban areas, and global warming, with higher temperature resulting in dehydration and high urinary concentration of calcium and other stone-forming salts.3 Nephrolithiasis is now recognized as a systemic disorder associated with chronic kidney disease, bone loss and fractures, increased risk of coronary artery disease, hypertension, type 2 diabetes mellitus, and metabolic syndrome (Table 2).4–7
Without medical treatment, the 5-year recurrence rate is high, ranging from 35% to 50% after an initial stone event.8 Annual medical costs of care for kidney stones in the United States exceed $4.5 billion, with additional costs from missed work. Therefore, this condition has a considerable economic and social burden, which underscores the importance of prevention.9
MOST STONES CONTAIN CALCIUM
About 80% of kidney stones in adults contain calcium, and calcium oxalate stones are more common than calcium phosphate stones. Uric acid and struvite stones account for 5% to 15%, and cystine, protease inhibitor, triamterene, 2,8-dihydroxyadenine (2,8-DHA) and xanthine stones each account for less than 1%.10
Stones form when the urinary concentration of stone-forming salts, which is inversely proportional to urine volume, is higher than their saturation point, which is affected by urine pH. Acidic urine (low pH) predisposes to the formation of uric acid and cystine stones, whereas alkaline urine (high pH) favors calcium phosphate stones.
INCREASED FLUID INTAKE FOR ALL
High fluid intake, enough to produce at least 2.5 L of urine per day, should be the initial therapy to prevent stone recurrence.11
Borghi et al12 randomly assigned 199 patients who had a first calcium stone to high oral fluid intake or no intervention and followed them prospectively for 5 years. The recurrence rate was 12% in the treated group and 27% in the control group. Another study, in patients who had undergone shock wave lithotripsy, found a recurrence rate of 8% in those randomized to increase fluid intake to achieve urine output greater than 2.5 L/day, compared with 56% in those assigned to no treatment.13
Certain beverages increase the risk of stones and should be avoided. Sugar-sweetened noncola soda and punch are associated with a 33% higher risk of kidney stones, and cola sodas are associated with a 23% higher risk.14 Prospective studies have shown that the consumption of coffee, beer, wine, and orange juice is associated with a lower likelihood of stone formation.13,15
Table 3 is a brief summary of the dietary and pharmacologic interventions in the management of recurrent nephrolithiasis.
PREVENTING CALCIUM OXALATE STONES
Major urinary risk factors associated with calcium oxalate stones are hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, and low urine volume.16 Preventing calcium stones therefore depends on reducing the urinary concentration of calcium and oxalate, increasing urinary levels of inhibitors such as citrate, and increasing urine volume.
Reducing calcium excretion
Hypercalciuria has been traditionally defined as 24-hour urinary calcium excretion greater than 300 mg/day in men, greater than 250 mg in women, or greater than 4 mg/kg in men or women.17 It is a graded risk factor, and the cut points used in published research and clinical laboratories vary substantially. Some institutions use the same value for hypercalciuria in both sexes, eg, greater than 200 mg/day.18
Excessive sodium intake is the most common cause of hypercalciuria. Systemic conditions such as primary hyperparathyroidism, sarcoidosis, and renal tubular acidosis also cause hypercalciuria but are uncommon.19 Management depends on the underlying cause and includes dietary modifications and pharmacologic therapy.
Dietary modifications have a pivotal role in the management of recurrent stones that are due to hypercalciuria.
Dietary calcium should not be restricted, since calcium reduces the excretion of urinary oxalate by decreasing intestinal absorption of oxalate. Guidelines from the American Urological Association recommend a daily calcium intake of 1,000 to 1,200 mg.11–20 Moreover, restriction of dietary calcium to less than 800 mg/day (the current recommended daily allowance for adults) can lead to negative calcium balance and bone loss.
Sodium intake also influences hypercalciuria. Calcium is reabsorbed passively in the proximal tubule due to the concentration gradient created by active reabsorption of sodium. A high sodium intake causes volume expansion, leading to a decrease in proximal sodium and calcium reabsorption and enhancing calcium excretion. A low-sodium diet (80–100 mmol/day, or 1,800–2,300 mg/day) is recommended. This enhances proximal sodium and passive calcium absorption and leads to a decrease in calcium excretion.21
Dietary protein increases the acid load by production of sulfuric acid and leads to hypercalciuria by its action on bone and kidney. Animal protein has a higher content of sulfur and generates a higher acid load compared with vegetable protein and has been associated with an increased incidence of stone formation, at least in men.20,22 Borghi et al23 reported that the combination of restricted intake of animal protein (52 g/day), restricted salt intake (50 mmol, or 2,900 mg/day of sodium chloride), and normal calcium intake (30 mmol/day, or 1,200 mg/day) was associated with a lower incidence of stone recurrence in men with hypercalciuria compared with traditional low-calcium intake (10 mmol, or 400 mg/day). Patients should therefore be advised to avoid excessive intake of animal protein.
Increasing the dietary intake of fruits and vegetables as in the Dietary Approach to Stop Hypertension (DASH) diet is beneficial and reduces the risk of stone recurrence, mainly by increasing citrate excretion.24
Pharmacologic therapy in hypercalciuria. Thiazide diuretics are the mainstay of pharmacotherapy for preventing recurrent stones in patients with idiopathic hypercalciuria. They reduce the risk of stone recurrence by about 50%, as reported in a recent meta-analysis that looked at five trials comparing thiazide diuretics with placebo.25 They lower calcium excretion by causing volume depletion, thereby increasing proximal sodium and passive calcium reabsorption.
Chlorthalidone and hydrochlorothiazide are the thiazides commonly used to treat hypercalciuria. The dosage is titrated to the urinary calcium excretion, and a common mistake is to use doses that are too low. They are usually started at 25 mg/day, but often require an increase to 50 to 100 mg/day for adequate lowering of urinary calcium.
Care should be taken to avoid hypokalemia. If it occurs, it can be corrected by adding the potassium-sparing diuretic amiloride (5–10 mg/day), which increases calcium reabsorption in collecting ducts or, in patients with hypocitraturia, potassium citrate-potassium bicarbonate. (Sodium salts should be avoided, since they increase renal calcium excretion.)26
Management of hypercalciuria with metabolic causes, which include primary hyperparathyroidism and chronic acidemia. Patients who have hypercalciuria from primary hyperparathyroidism are treated with parathyroidectomy.27 Chronic metabolic acidosis causes hypercalciuria by loss of bone calcium and hypocitraturia by increasing active proximal absorption of citrate. Potassium citrate or potassium bicarbonate is used to prevent stones in such patients; sodium salts should be avoided.28
Reducing oxalate excretion
Hyperoxaluria has traditionally been defined as urinary oxalate excretion of more than 45 mg/day. However, the optimal cutoff point for urinary oxalate excretion is unclear, as is the optimal cutoff for hypercalciuria. The risk of stone formation has been shown to increase with oxalate excretion even above 25 mg/day, which is within the normal limit.18
Idiopathic hyperoxaluria. High dietary oxalate intake, especially when associated with low calcium intake, leads to idiopathic hyperoxaluria. However, the contribution of abnormal endogenous oxalate metabolism is uncertain. Ingested calcium binds to oxalate in the intestinal tract and reduces both the absorption of intestinal oxalate absorption and the excretion of urinary oxalate.29 High dietary oxalate intake has usually been regarded as a major risk factor for kidney stones.
Taylor and Curhan,30 in a prospective study, reported a mild increase in the risk of stones in the highest quintile of dietary oxalate intake compared with the lowest quintile for men (relative risk [RR] 1.22, 95% confidence interval [CI] 1.03–1.45) and older women (RR 1.21, 95% CI 1.01–1.44). They also demonstrated that eating eight or more servings of spinach per month compared with fewer than one serving per month was associated with a similar increase of stone risk in men (RR 1.30, 95% CI 1.08–1.58) and older women (RR 1.34 95% CI 1.1–1.64). In contrast, spinach and dietary oxalate intake did not increase the risk of nephrolithiasis in young women. The authors concluded that the risk associated with oxalate intake was modest, and their data did not support the contention that dietary oxalate is a major risk factor for kidney stones.
Higher oxalate intake increases urinary oxalate excretion and presumably the risk of nephrolithiasis. Limiting dietary oxalate to prevent stones is recommended if habitually high dietary intake of oxalate is identified or follow-up urine measurements show a decrease in oxalate excretion.31 Foods rich in oxalate include spinach, rhubarb, nuts, legumes, cocoa, okra, and chocolate.
The DASH diet, which is high in fruits and vegetables, moderate in low-fat dairy products, and low in animal protein, is an effective dietary alternative and has been associated with a lower risk of calcium oxalate stones.24 Consuming fruits and vegetables increases the excretion of urinary citrate, which is an inhibitor of stone formation. Also, it has been proposed that the DASH diet contains unknown factors that reduce stone risk.
Taylor et al32 prospectively examined the relationship between the DASH diet and the incidence of kidney stones and found that the diet significantly reduced the risk of kidney stones. The relative risks of occurrence of kidney stones in participants in the highest quintile of the DASH score (a measure of adherence to the DASH diet) compared with the lowest quintile were 0.55 (95% CI 0.46–0.65) for men, 0.58 (95% CI 0.49–0.68) for older women, and 0.60 (95% CI 0.52–0.70) for younger women, which the authors characterized as “a marked decrease in kidney stone risk.”
Vitamin C intake should be restricted to 90 mg/day in patients who have a history of calcium oxalate stones. Urivetzky et al33 found that urinary oxalate excretion increased by 6 to 13 mg/day at doses of ascorbic acid greater than 500 mg.
Pyridoxine (vitamin B6), a coenzyme of alanine-glyoxylate aminotransferase (AGT), increases the conversion of glyoxylate to glycine instead of oxalate and is used in the treatment of type 1 primary hyperoxaluria (see below).34 However, its effect in preventing stones in idiopathic hyperoxaluria is not well known, and it has not been studied in a randomized controlled trial. In a prospective study, Curhan et al35 reported that high intake of pyridoxine (> 40 mg/day) was associated with a lower risk of stone formation in women, but no such benefit was found in men.
Enteric hyperoxaluria. About 90% of dietary oxalate binds to calcium in the small intestine and is excreted in the stool. The remaining 10% is absorbed in the colon and is secreted in urine. Hyperoxaluria is frequently seen with fat malabsorption from inflammatory bowel disease, short gut syndrome, and gastric bypass surgery. In these conditions, excess fat binds to dietary calcium, leading to increased absorption of free oxalate in the colon.36
Treatment is directed at decreasing intestinal oxalate absorption and should include high fluid intake and oral calcium supplements. Calcium carbonate or citrate causes precipitation of oxalate in the intestinal lumen and is prescribed as 1 to 4 g in three to four divided doses, always with meals. Calcium citrate is preferred over calcium carbonate in stone-formers because of the benefit of citrate and calcium citrate’s higher solubility and greater effectiveness in the presence of achlorhydria.37 Patients should be advised to avoid foods high in oxalate and fat.
Primary hyperoxaluria is caused by inherited inborn errors of glyoxylate metabolism that cause overproduction of oxalate and urinary oxalate excretion above 135 to 270 mg/day.
Type 1 primary hyperoxaluria is the most common (accounting for 90% of cases) and is caused by reduced activity of hepatic peroxisomal AGT.
Type 2 is from a deficiency of glyoxylate reductase-hydroxypyruvate reductase (GRHPR).
Type 3 is from mutations in the HOGA1 gene, which codes for the liver-specific mitochondrial 4-hydroxy-2-oxoglutarate aldolase enzyme involved in degradation of hydroxyproline to pyruvate and glyoxalate.38
High fluid intake to produce a urinary volume of 3 L/day reduces intratubular oxalate deposition and should be encouraged. Potassium citrate (0.15 mg/kg), oral phosphate supplements (30–40 mg/kg of orthophosphate), and magnesium oxide (500 mg/day/m2) inhibit precipitation of calcium oxalate in the urine.39,40 Pyridoxine, a coenzyme of AGT, increases the conversion of glyoxylate to glycine instead of oxalate and is prescribed at a starting dose of 5 mg/kg (which can be titrated up to 20 mg/kg if there is no response) in patients with type 1 primary hyperoxaluria. About 50% of patients with type 1 respond successfully to pyridoxine, and a 3- to 6-month trial should be given in all patients in this category.34 AGT is present only in hepatocytes, and GRHPR is found in multiple tissues; therefore, combined liver-kidney transplant is the treatment of choice in patients with type 1 primary hyperoxaluria, whereas isolated kidney transplant is recommended in patients with type 2.41
Reducing uric acid excretion
Hyperuricosuria is defined as uric acid excretion of greater than 800 mg/day in men and greater than 750 mg/day in women.
The association of hyperuricosuria with increased risk of calcium oxalate stone formation is controversial. Curhan and Taylor,18 in a cross-sectional study of 3,350 men and women, reported that there was no difference in mean 24-hour uric acid excretion in individuals with and without a history of stones.
The mechanism by which uric acid leads to calcium oxalate stones is not completely known and could be the “salting out” of calcium oxalate from the urine.42
Dietary purine restriction, ie, limiting intake of nondairy animal protein to 0.8 to 1 g/kg/day, is the initial dietary intervention.11 Allopurinol is the alternative approach if the patient is not compliant or if dietary restriction fails.43
In a study by Ettinger et al,44 60 patients with hyperuricosuria and normocalciuria were randomized to receive allopurinol (100 mg three times daily) or a placebo. The allopurinol group had a rate of calculus events of 0.12 per patient per year, compared with 0.26 in the placebo group.
Increasing citrate excretion
Hypocitraturia is a well-known risk factor for the formation of kidney stones. It is usually defined as a citrate excretion of less than 320 mg/day for adults.
Citrate prevents formation of calcium crystals by binding to calcium, thereby lowering the concentration of calcium oxalate below the saturation point.45
Diet therapy. Patients with calcium oxalate stones and hypocitraturia should be encouraged to increase their intake of fruits and vegetables, which enhances urinary citrate excretion, and to limit their intake of nondairy animal protein.11
The use of citrus products in preventing stones in patients with hypocitraturia is controversial, however, and needs to be studied more.
One study46 demonstrated that lemon juice was beneficial in hypocitraturic nephrolithiasis: 4 oz/day of lemon juice concentrate in the form of lemonade was associated with an increase in urinary citrate excretion to 346 mg/day from 142 mg/day in 11 of 12 patients who participated.
Odvina47 compared the effects of orange juice with those of lemonade on the acid-base profile and urinary stone risk under controlled metabolic conditions in 13 volunteers. Orange juice was reported to have greater alkalinizing and citraturic effects and was associated with lower calculated calcium oxalate supersaturation compared with lemonade.
Lemonade therapy may be used as adjunctive treatment in patients who do not comply with or cannot tolerate alkali therapy. However, we advise caution about recommending citrus products, as they can increase oxalate excretion.
Pharmacotherapy includes alkali therapy. Barcelo et al48 compared the effects of potassium citrate and placebo in 57 patients with calcium oxalate stones and hypocitraturia. Patients treated with potassium citrate had a rate of stone formation of 0.1 event per patient per year, compared with 1.1 in the placebo group.
Many forms of alkaline citrate are available. Potassium citrate is preferred over sodium citrate since the latter may increase urine calcium excretion.49 Treatment is usually started at 30 mEq/day and is titrated to a maximal dose of 60 mEq/day for a urinary citrate excretion greater than 500 mg/day.
Common side effects are abdominal bloating and hyperkalemia (especially with renal insufficiency), and in such cases sodium-based alkali, sodium citrate, or sodium bicarbonate can be prescribed.
PREVENTING CALCIUM PHOSPHATE STONES
Risk factors for calcium phosphate stones are similar to those for calcium oxalate stones (other than hyperoxaluria), but calcium phosphate stones are formed in alkaline urine (usually urine pH > 6.0), often the result of distal renal tubular acidosis. Preventive measures are similar to those for calcium oxalate stones.
Alkali therapy should be used with caution because of its effect on urinary pH and the risk of precipitation of calcium phosphate crystals.50 Use of potassium citrate was found to be associated with increases in both urinary citrate excretion and calcium phosphate supersaturation in hypercalciuric stone-forming rats.51 It is therefore challenging to manage patients with calcium phosphate stones and hypocitraturia. Alkali administration in this setting may diminish the formation of new stones by correcting hypocitraturia, but at the same time it may increase the likelihood of calcium phosphate stone formation by increasing the urinary pH. When the urine pH increases to above 6.5 with no significant change in urine citrate or urine calcium excretion, we recommend stopping alkali therapy.
PREVENTING URIC ACID STONES
Clinical conditions associated with uric acid stones include metabolic syndrome, diabetes mellitus, gout, chronic diarrheal illness, and conditions that increase tissue turnover and uric acid production, such as malignancies. Other risk factors for uric acid stone formation are low urine volume, low uric pH, and hyperuricosuria.
Abnormally acidic urine is the most common risk factor. Metabolic syndrome and diabetes mellitus reduce ammonia production, resulting in a lower urinary pH, which predisposes to uric acid stone formation. Chronic diarrhea also acidifies the urine by loss of bicarbonate. Similarly, in gout, the predisposing factor in uric acid stone formation is the persistently acidic urine due to impaired ammonium excretion.52 Uric acid precipitates to form uric acid stones in a low urinary pH even with normal excretion rates of 600 to 800 mg/day and a urinary volume of 1 to 1.5 L.53
Therefore, apart from increasing fluid intake, urinary alkalization is the cornerstone of management of uric acid stones. Potassium citrate is the preferred alkali salt and is started at a dose of 30 mEq/day for a goal urinary pH of 6 to 6.5.47
Patients with hyperuricosuria are also advised to restrict their protein intake to no more than 0.8 to 1 mg/kg/day.
If the above measures fail, patients are treated with a xanthine oxidase inhibitor, ie, allopurinol or febuxostat, even if their uric acid excretion is normal.54
PREVENTING STRUVITE STONES
Struvite stones contain magnesium ammonium phosphate and are due to chronic upper urinary tract infection with urea-splitting bacteria such as Proteus, Klebsiella, Pseudomonas, and enterococci. Urea hydrolysis releases hydroxyl ions, resulting in alkaline urine that promotes struvite stone formation. Early detection and treatment are important, since struvite stones are associated with morbidity and rapid progression.
Medical treatment of struvite stones is usually unsuccessful, and the patient is referred to a urologist for surgical removal of the stones, the gold standard treatment.55 Long-term use of culture-specific antibiotics to prevent new stone growth is not well studied. Medical therapy by itself is preferred in patients who refuse stone removal or cannot tolerate it. Urease inhibitors such as acetohydroxamic acid have been successful in preventing or slowing stone growth, but their use is limited by frequent side effects such as nausea, headache, rash, and thrombophlebitis.56
CYSTINE STONES
Cystine stones occur in people with inherited defects of renal tubular and intestinal transport of cysteine and dibasic amino acids that cause excessive excretion of urinary cystine, ie, 480 to 3,600 mg/day.
Cystine is formed from two cysteine molecules linked by a disulfide bond. The solubility of cystine is pH-dependent, with increased solubility at higher urinary pH. The goal is to maintain a urinary cystine concentration below its solubility level by keeping the cystine concentration below 243 mg/L and the urine cystine supersaturation (the ratio of the urine cysteine concentration to the cysteine solubility in the same sample) less than 0.6.57 Therapy is aimed at increasing daily urinary volume to 3 L and urine alkalization to pH above 7, in order to increase cystine solubility by 300%.58
Overnight dehydration should be prevented, and patients should be encouraged to wake up at least once a night to void and drink additional water. Sodium restriction to 100 mmol/day (2,300 mg/day) and moderate protein restriction to 0.8 to 1 g/kg/day are associated with decreased cystine excretion, but long-term studies demonstrating their benefit in preventing cystine stones are lacking.59
A thiol-containing drug, eg, D-penicillamine (0.5–2 g/day) or tiopronin (400–1,200 mg/day), should be added to the conservative measures if they have not been effective for 3 months or if there is history of noncompliance.60 Thiol-containing drugs have a sulfhydryl group that reduces the disulfide bond, and they form soluble disulfide cysteine-drug complexes with greater ability to solubilize cystine in alkaline urine. They must always be used in conjunction with fluid and alkali therapy.61
Both drugs have severe and common adverse effects including leukopenia, aplastic anemia, fever, rash, arthritis, hepatotoxicity, pyridoxine deficiency, and proteinuria (membranous nephropathy). However, tiopronin seems to have a lesser incidence of side effects.62 Regular monitoring of complete blood cell counts, liver enzymes, and urine protein should be done.
Captopril contains a sulfhydryl group, and the captopril-cysteine disulfide is more soluble than cysteine alone. The amount of captopril that appears in the urine is low, and doses of 150 mg/day are usually required to reduce cysteine excretion, which can lead to hypotension. The efficacy of captopril in treating cystine stones is unproven, and this drug is used only if patients cannot tolerate other thiol-containing drugs.63
Nephrolithiasis is common and often recurs. This review focuses on measures to prevent recurrent stone formation. Some measures apply to all patients, and some apply to specific types of stones.
COMMON AND INCREASING
According to data from the 2007–2010 National Health and Nutrition Examination Survey, the prevalence of nephrolithiasis in the United States was 10.6% in men and 7.1% in women. On average, 1 in 11 Americans will develop kidney stones at least once in their lifetime.1
By race and sex, white men have the highest incidence of nephrolithiasis and Asian women have the lowest. It is less common before age 20 and peaks in incidence in the third and fourth decades of life.
The prevalence has steadily increased in the past few decades (Table 1),1,2 but the reasons are not clear. The trend may be due to changes in diet and lifestyle, increasing prevalence of obesity and diabetes, migration from rural to urban areas, and global warming, with higher temperature resulting in dehydration and high urinary concentration of calcium and other stone-forming salts.3 Nephrolithiasis is now recognized as a systemic disorder associated with chronic kidney disease, bone loss and fractures, increased risk of coronary artery disease, hypertension, type 2 diabetes mellitus, and metabolic syndrome (Table 2).4–7
Without medical treatment, the 5-year recurrence rate is high, ranging from 35% to 50% after an initial stone event.8 Annual medical costs of care for kidney stones in the United States exceed $4.5 billion, with additional costs from missed work. Therefore, this condition has a considerable economic and social burden, which underscores the importance of prevention.9
MOST STONES CONTAIN CALCIUM
About 80% of kidney stones in adults contain calcium, and calcium oxalate stones are more common than calcium phosphate stones. Uric acid and struvite stones account for 5% to 15%, and cystine, protease inhibitor, triamterene, 2,8-dihydroxyadenine (2,8-DHA) and xanthine stones each account for less than 1%.10
Stones form when the urinary concentration of stone-forming salts, which is inversely proportional to urine volume, is higher than their saturation point, which is affected by urine pH. Acidic urine (low pH) predisposes to the formation of uric acid and cystine stones, whereas alkaline urine (high pH) favors calcium phosphate stones.
INCREASED FLUID INTAKE FOR ALL
High fluid intake, enough to produce at least 2.5 L of urine per day, should be the initial therapy to prevent stone recurrence.11
Borghi et al12 randomly assigned 199 patients who had a first calcium stone to high oral fluid intake or no intervention and followed them prospectively for 5 years. The recurrence rate was 12% in the treated group and 27% in the control group. Another study, in patients who had undergone shock wave lithotripsy, found a recurrence rate of 8% in those randomized to increase fluid intake to achieve urine output greater than 2.5 L/day, compared with 56% in those assigned to no treatment.13
Certain beverages increase the risk of stones and should be avoided. Sugar-sweetened noncola soda and punch are associated with a 33% higher risk of kidney stones, and cola sodas are associated with a 23% higher risk.14 Prospective studies have shown that the consumption of coffee, beer, wine, and orange juice is associated with a lower likelihood of stone formation.13,15
Table 3 is a brief summary of the dietary and pharmacologic interventions in the management of recurrent nephrolithiasis.
PREVENTING CALCIUM OXALATE STONES
Major urinary risk factors associated with calcium oxalate stones are hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, and low urine volume.16 Preventing calcium stones therefore depends on reducing the urinary concentration of calcium and oxalate, increasing urinary levels of inhibitors such as citrate, and increasing urine volume.
Reducing calcium excretion
Hypercalciuria has been traditionally defined as 24-hour urinary calcium excretion greater than 300 mg/day in men, greater than 250 mg in women, or greater than 4 mg/kg in men or women.17 It is a graded risk factor, and the cut points used in published research and clinical laboratories vary substantially. Some institutions use the same value for hypercalciuria in both sexes, eg, greater than 200 mg/day.18
Excessive sodium intake is the most common cause of hypercalciuria. Systemic conditions such as primary hyperparathyroidism, sarcoidosis, and renal tubular acidosis also cause hypercalciuria but are uncommon.19 Management depends on the underlying cause and includes dietary modifications and pharmacologic therapy.
Dietary modifications have a pivotal role in the management of recurrent stones that are due to hypercalciuria.
Dietary calcium should not be restricted, since calcium reduces the excretion of urinary oxalate by decreasing intestinal absorption of oxalate. Guidelines from the American Urological Association recommend a daily calcium intake of 1,000 to 1,200 mg.11–20 Moreover, restriction of dietary calcium to less than 800 mg/day (the current recommended daily allowance for adults) can lead to negative calcium balance and bone loss.
Sodium intake also influences hypercalciuria. Calcium is reabsorbed passively in the proximal tubule due to the concentration gradient created by active reabsorption of sodium. A high sodium intake causes volume expansion, leading to a decrease in proximal sodium and calcium reabsorption and enhancing calcium excretion. A low-sodium diet (80–100 mmol/day, or 1,800–2,300 mg/day) is recommended. This enhances proximal sodium and passive calcium absorption and leads to a decrease in calcium excretion.21
Dietary protein increases the acid load by production of sulfuric acid and leads to hypercalciuria by its action on bone and kidney. Animal protein has a higher content of sulfur and generates a higher acid load compared with vegetable protein and has been associated with an increased incidence of stone formation, at least in men.20,22 Borghi et al23 reported that the combination of restricted intake of animal protein (52 g/day), restricted salt intake (50 mmol, or 2,900 mg/day of sodium chloride), and normal calcium intake (30 mmol/day, or 1,200 mg/day) was associated with a lower incidence of stone recurrence in men with hypercalciuria compared with traditional low-calcium intake (10 mmol, or 400 mg/day). Patients should therefore be advised to avoid excessive intake of animal protein.
Increasing the dietary intake of fruits and vegetables as in the Dietary Approach to Stop Hypertension (DASH) diet is beneficial and reduces the risk of stone recurrence, mainly by increasing citrate excretion.24
Pharmacologic therapy in hypercalciuria. Thiazide diuretics are the mainstay of pharmacotherapy for preventing recurrent stones in patients with idiopathic hypercalciuria. They reduce the risk of stone recurrence by about 50%, as reported in a recent meta-analysis that looked at five trials comparing thiazide diuretics with placebo.25 They lower calcium excretion by causing volume depletion, thereby increasing proximal sodium and passive calcium reabsorption.
Chlorthalidone and hydrochlorothiazide are the thiazides commonly used to treat hypercalciuria. The dosage is titrated to the urinary calcium excretion, and a common mistake is to use doses that are too low. They are usually started at 25 mg/day, but often require an increase to 50 to 100 mg/day for adequate lowering of urinary calcium.
Care should be taken to avoid hypokalemia. If it occurs, it can be corrected by adding the potassium-sparing diuretic amiloride (5–10 mg/day), which increases calcium reabsorption in collecting ducts or, in patients with hypocitraturia, potassium citrate-potassium bicarbonate. (Sodium salts should be avoided, since they increase renal calcium excretion.)26
Management of hypercalciuria with metabolic causes, which include primary hyperparathyroidism and chronic acidemia. Patients who have hypercalciuria from primary hyperparathyroidism are treated with parathyroidectomy.27 Chronic metabolic acidosis causes hypercalciuria by loss of bone calcium and hypocitraturia by increasing active proximal absorption of citrate. Potassium citrate or potassium bicarbonate is used to prevent stones in such patients; sodium salts should be avoided.28
Reducing oxalate excretion
Hyperoxaluria has traditionally been defined as urinary oxalate excretion of more than 45 mg/day. However, the optimal cutoff point for urinary oxalate excretion is unclear, as is the optimal cutoff for hypercalciuria. The risk of stone formation has been shown to increase with oxalate excretion even above 25 mg/day, which is within the normal limit.18
Idiopathic hyperoxaluria. High dietary oxalate intake, especially when associated with low calcium intake, leads to idiopathic hyperoxaluria. However, the contribution of abnormal endogenous oxalate metabolism is uncertain. Ingested calcium binds to oxalate in the intestinal tract and reduces both the absorption of intestinal oxalate absorption and the excretion of urinary oxalate.29 High dietary oxalate intake has usually been regarded as a major risk factor for kidney stones.
Taylor and Curhan,30 in a prospective study, reported a mild increase in the risk of stones in the highest quintile of dietary oxalate intake compared with the lowest quintile for men (relative risk [RR] 1.22, 95% confidence interval [CI] 1.03–1.45) and older women (RR 1.21, 95% CI 1.01–1.44). They also demonstrated that eating eight or more servings of spinach per month compared with fewer than one serving per month was associated with a similar increase of stone risk in men (RR 1.30, 95% CI 1.08–1.58) and older women (RR 1.34 95% CI 1.1–1.64). In contrast, spinach and dietary oxalate intake did not increase the risk of nephrolithiasis in young women. The authors concluded that the risk associated with oxalate intake was modest, and their data did not support the contention that dietary oxalate is a major risk factor for kidney stones.
Higher oxalate intake increases urinary oxalate excretion and presumably the risk of nephrolithiasis. Limiting dietary oxalate to prevent stones is recommended if habitually high dietary intake of oxalate is identified or follow-up urine measurements show a decrease in oxalate excretion.31 Foods rich in oxalate include spinach, rhubarb, nuts, legumes, cocoa, okra, and chocolate.
The DASH diet, which is high in fruits and vegetables, moderate in low-fat dairy products, and low in animal protein, is an effective dietary alternative and has been associated with a lower risk of calcium oxalate stones.24 Consuming fruits and vegetables increases the excretion of urinary citrate, which is an inhibitor of stone formation. Also, it has been proposed that the DASH diet contains unknown factors that reduce stone risk.
Taylor et al32 prospectively examined the relationship between the DASH diet and the incidence of kidney stones and found that the diet significantly reduced the risk of kidney stones. The relative risks of occurrence of kidney stones in participants in the highest quintile of the DASH score (a measure of adherence to the DASH diet) compared with the lowest quintile were 0.55 (95% CI 0.46–0.65) for men, 0.58 (95% CI 0.49–0.68) for older women, and 0.60 (95% CI 0.52–0.70) for younger women, which the authors characterized as “a marked decrease in kidney stone risk.”
Vitamin C intake should be restricted to 90 mg/day in patients who have a history of calcium oxalate stones. Urivetzky et al33 found that urinary oxalate excretion increased by 6 to 13 mg/day at doses of ascorbic acid greater than 500 mg.
Pyridoxine (vitamin B6), a coenzyme of alanine-glyoxylate aminotransferase (AGT), increases the conversion of glyoxylate to glycine instead of oxalate and is used in the treatment of type 1 primary hyperoxaluria (see below).34 However, its effect in preventing stones in idiopathic hyperoxaluria is not well known, and it has not been studied in a randomized controlled trial. In a prospective study, Curhan et al35 reported that high intake of pyridoxine (> 40 mg/day) was associated with a lower risk of stone formation in women, but no such benefit was found in men.
Enteric hyperoxaluria. About 90% of dietary oxalate binds to calcium in the small intestine and is excreted in the stool. The remaining 10% is absorbed in the colon and is secreted in urine. Hyperoxaluria is frequently seen with fat malabsorption from inflammatory bowel disease, short gut syndrome, and gastric bypass surgery. In these conditions, excess fat binds to dietary calcium, leading to increased absorption of free oxalate in the colon.36
Treatment is directed at decreasing intestinal oxalate absorption and should include high fluid intake and oral calcium supplements. Calcium carbonate or citrate causes precipitation of oxalate in the intestinal lumen and is prescribed as 1 to 4 g in three to four divided doses, always with meals. Calcium citrate is preferred over calcium carbonate in stone-formers because of the benefit of citrate and calcium citrate’s higher solubility and greater effectiveness in the presence of achlorhydria.37 Patients should be advised to avoid foods high in oxalate and fat.
Primary hyperoxaluria is caused by inherited inborn errors of glyoxylate metabolism that cause overproduction of oxalate and urinary oxalate excretion above 135 to 270 mg/day.
Type 1 primary hyperoxaluria is the most common (accounting for 90% of cases) and is caused by reduced activity of hepatic peroxisomal AGT.
Type 2 is from a deficiency of glyoxylate reductase-hydroxypyruvate reductase (GRHPR).
Type 3 is from mutations in the HOGA1 gene, which codes for the liver-specific mitochondrial 4-hydroxy-2-oxoglutarate aldolase enzyme involved in degradation of hydroxyproline to pyruvate and glyoxalate.38
High fluid intake to produce a urinary volume of 3 L/day reduces intratubular oxalate deposition and should be encouraged. Potassium citrate (0.15 mg/kg), oral phosphate supplements (30–40 mg/kg of orthophosphate), and magnesium oxide (500 mg/day/m2) inhibit precipitation of calcium oxalate in the urine.39,40 Pyridoxine, a coenzyme of AGT, increases the conversion of glyoxylate to glycine instead of oxalate and is prescribed at a starting dose of 5 mg/kg (which can be titrated up to 20 mg/kg if there is no response) in patients with type 1 primary hyperoxaluria. About 50% of patients with type 1 respond successfully to pyridoxine, and a 3- to 6-month trial should be given in all patients in this category.34 AGT is present only in hepatocytes, and GRHPR is found in multiple tissues; therefore, combined liver-kidney transplant is the treatment of choice in patients with type 1 primary hyperoxaluria, whereas isolated kidney transplant is recommended in patients with type 2.41
Reducing uric acid excretion
Hyperuricosuria is defined as uric acid excretion of greater than 800 mg/day in men and greater than 750 mg/day in women.
The association of hyperuricosuria with increased risk of calcium oxalate stone formation is controversial. Curhan and Taylor,18 in a cross-sectional study of 3,350 men and women, reported that there was no difference in mean 24-hour uric acid excretion in individuals with and without a history of stones.
The mechanism by which uric acid leads to calcium oxalate stones is not completely known and could be the “salting out” of calcium oxalate from the urine.42
Dietary purine restriction, ie, limiting intake of nondairy animal protein to 0.8 to 1 g/kg/day, is the initial dietary intervention.11 Allopurinol is the alternative approach if the patient is not compliant or if dietary restriction fails.43
In a study by Ettinger et al,44 60 patients with hyperuricosuria and normocalciuria were randomized to receive allopurinol (100 mg three times daily) or a placebo. The allopurinol group had a rate of calculus events of 0.12 per patient per year, compared with 0.26 in the placebo group.
Increasing citrate excretion
Hypocitraturia is a well-known risk factor for the formation of kidney stones. It is usually defined as a citrate excretion of less than 320 mg/day for adults.
Citrate prevents formation of calcium crystals by binding to calcium, thereby lowering the concentration of calcium oxalate below the saturation point.45
Diet therapy. Patients with calcium oxalate stones and hypocitraturia should be encouraged to increase their intake of fruits and vegetables, which enhances urinary citrate excretion, and to limit their intake of nondairy animal protein.11
The use of citrus products in preventing stones in patients with hypocitraturia is controversial, however, and needs to be studied more.
One study46 demonstrated that lemon juice was beneficial in hypocitraturic nephrolithiasis: 4 oz/day of lemon juice concentrate in the form of lemonade was associated with an increase in urinary citrate excretion to 346 mg/day from 142 mg/day in 11 of 12 patients who participated.
Odvina47 compared the effects of orange juice with those of lemonade on the acid-base profile and urinary stone risk under controlled metabolic conditions in 13 volunteers. Orange juice was reported to have greater alkalinizing and citraturic effects and was associated with lower calculated calcium oxalate supersaturation compared with lemonade.
Lemonade therapy may be used as adjunctive treatment in patients who do not comply with or cannot tolerate alkali therapy. However, we advise caution about recommending citrus products, as they can increase oxalate excretion.
Pharmacotherapy includes alkali therapy. Barcelo et al48 compared the effects of potassium citrate and placebo in 57 patients with calcium oxalate stones and hypocitraturia. Patients treated with potassium citrate had a rate of stone formation of 0.1 event per patient per year, compared with 1.1 in the placebo group.
Many forms of alkaline citrate are available. Potassium citrate is preferred over sodium citrate since the latter may increase urine calcium excretion.49 Treatment is usually started at 30 mEq/day and is titrated to a maximal dose of 60 mEq/day for a urinary citrate excretion greater than 500 mg/day.
Common side effects are abdominal bloating and hyperkalemia (especially with renal insufficiency), and in such cases sodium-based alkali, sodium citrate, or sodium bicarbonate can be prescribed.
PREVENTING CALCIUM PHOSPHATE STONES
Risk factors for calcium phosphate stones are similar to those for calcium oxalate stones (other than hyperoxaluria), but calcium phosphate stones are formed in alkaline urine (usually urine pH > 6.0), often the result of distal renal tubular acidosis. Preventive measures are similar to those for calcium oxalate stones.
Alkali therapy should be used with caution because of its effect on urinary pH and the risk of precipitation of calcium phosphate crystals.50 Use of potassium citrate was found to be associated with increases in both urinary citrate excretion and calcium phosphate supersaturation in hypercalciuric stone-forming rats.51 It is therefore challenging to manage patients with calcium phosphate stones and hypocitraturia. Alkali administration in this setting may diminish the formation of new stones by correcting hypocitraturia, but at the same time it may increase the likelihood of calcium phosphate stone formation by increasing the urinary pH. When the urine pH increases to above 6.5 with no significant change in urine citrate or urine calcium excretion, we recommend stopping alkali therapy.
PREVENTING URIC ACID STONES
Clinical conditions associated with uric acid stones include metabolic syndrome, diabetes mellitus, gout, chronic diarrheal illness, and conditions that increase tissue turnover and uric acid production, such as malignancies. Other risk factors for uric acid stone formation are low urine volume, low uric pH, and hyperuricosuria.
Abnormally acidic urine is the most common risk factor. Metabolic syndrome and diabetes mellitus reduce ammonia production, resulting in a lower urinary pH, which predisposes to uric acid stone formation. Chronic diarrhea also acidifies the urine by loss of bicarbonate. Similarly, in gout, the predisposing factor in uric acid stone formation is the persistently acidic urine due to impaired ammonium excretion.52 Uric acid precipitates to form uric acid stones in a low urinary pH even with normal excretion rates of 600 to 800 mg/day and a urinary volume of 1 to 1.5 L.53
Therefore, apart from increasing fluid intake, urinary alkalization is the cornerstone of management of uric acid stones. Potassium citrate is the preferred alkali salt and is started at a dose of 30 mEq/day for a goal urinary pH of 6 to 6.5.47
Patients with hyperuricosuria are also advised to restrict their protein intake to no more than 0.8 to 1 mg/kg/day.
If the above measures fail, patients are treated with a xanthine oxidase inhibitor, ie, allopurinol or febuxostat, even if their uric acid excretion is normal.54
PREVENTING STRUVITE STONES
Struvite stones contain magnesium ammonium phosphate and are due to chronic upper urinary tract infection with urea-splitting bacteria such as Proteus, Klebsiella, Pseudomonas, and enterococci. Urea hydrolysis releases hydroxyl ions, resulting in alkaline urine that promotes struvite stone formation. Early detection and treatment are important, since struvite stones are associated with morbidity and rapid progression.
Medical treatment of struvite stones is usually unsuccessful, and the patient is referred to a urologist for surgical removal of the stones, the gold standard treatment.55 Long-term use of culture-specific antibiotics to prevent new stone growth is not well studied. Medical therapy by itself is preferred in patients who refuse stone removal or cannot tolerate it. Urease inhibitors such as acetohydroxamic acid have been successful in preventing or slowing stone growth, but their use is limited by frequent side effects such as nausea, headache, rash, and thrombophlebitis.56
CYSTINE STONES
Cystine stones occur in people with inherited defects of renal tubular and intestinal transport of cysteine and dibasic amino acids that cause excessive excretion of urinary cystine, ie, 480 to 3,600 mg/day.
Cystine is formed from two cysteine molecules linked by a disulfide bond. The solubility of cystine is pH-dependent, with increased solubility at higher urinary pH. The goal is to maintain a urinary cystine concentration below its solubility level by keeping the cystine concentration below 243 mg/L and the urine cystine supersaturation (the ratio of the urine cysteine concentration to the cysteine solubility in the same sample) less than 0.6.57 Therapy is aimed at increasing daily urinary volume to 3 L and urine alkalization to pH above 7, in order to increase cystine solubility by 300%.58
Overnight dehydration should be prevented, and patients should be encouraged to wake up at least once a night to void and drink additional water. Sodium restriction to 100 mmol/day (2,300 mg/day) and moderate protein restriction to 0.8 to 1 g/kg/day are associated with decreased cystine excretion, but long-term studies demonstrating their benefit in preventing cystine stones are lacking.59
A thiol-containing drug, eg, D-penicillamine (0.5–2 g/day) or tiopronin (400–1,200 mg/day), should be added to the conservative measures if they have not been effective for 3 months or if there is history of noncompliance.60 Thiol-containing drugs have a sulfhydryl group that reduces the disulfide bond, and they form soluble disulfide cysteine-drug complexes with greater ability to solubilize cystine in alkaline urine. They must always be used in conjunction with fluid and alkali therapy.61
Both drugs have severe and common adverse effects including leukopenia, aplastic anemia, fever, rash, arthritis, hepatotoxicity, pyridoxine deficiency, and proteinuria (membranous nephropathy). However, tiopronin seems to have a lesser incidence of side effects.62 Regular monitoring of complete blood cell counts, liver enzymes, and urine protein should be done.
Captopril contains a sulfhydryl group, and the captopril-cysteine disulfide is more soluble than cysteine alone. The amount of captopril that appears in the urine is low, and doses of 150 mg/day are usually required to reduce cysteine excretion, which can lead to hypotension. The efficacy of captopril in treating cystine stones is unproven, and this drug is used only if patients cannot tolerate other thiol-containing drugs.63
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol 2012; 62:160–165.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM Jr, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010; 12:e86–e96.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2011; 79:393–403.
- Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens 2008; 17:304–309.
- Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int J Urol 2005; 12:859–863.
- Ritz E. Metabolic syndrome: an emerging threat to renal function. Clin J Am Soc Nephrol 2007; 2:869–871.
- Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med 1989; 111:1006–1009.
- Saigal CS, Joyce G, Timilsina AR; Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 2005; 68:1808–1814.
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet 2006; 367:333–344.
- Pearle MS, Goldfarb DS, Assimos DG, et al; American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol 2014; 192:316–324.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843.
- Sarica K, Inal Y, Erturhan S, Yagci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res 2006; 34:184–189.
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013; 8:1389–1395.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998; 128:534–540.
- Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med 1980; 69:19–30.
- Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med 2009; 76:583–591.
- Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 2008; 73:489–496.
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115:2598–2608.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int 1982; 22:292–296.
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 1988; 66:140–146.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Noori N, Honarkar E, Goldfarb DS, et al. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: a randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis 2014; 63:456–463.
- Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med 2013; 158:535–543.
- Alon U, Costanzo LS, Chan JC. Additive hypocalciuric effects of amiloride and hydrochlorothiazide in patients treated with calcitriol. Miner Electrolyte Metab 1984; 10:379–386.
- Corbetta S, Baccarelli A, Aroldi A, et al. Risk factors associated to kidney stones in primary hyperparathyroidism. J Endocrinol Invest 2005; 28:122–128.
- Haymann JP. Metabolic disorders: stones as first clinical manifestation of significant diseases. World J Urol 2015; 33:187–192.
- Jaeger P, Portmann L, Jacquet AF, Burckhardt P. Influence of the calcium content of the diet on the incidence of mild hyperoxaluria in idiopathic renal stone formers. Am J Nephrol 1985; 5:40–44.
- Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 2007; 18:2198–2204.
- Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 2010; 78:1178–1185.
- Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 2009; 20:2253–2259.
- Urivetzky M, Kessaris D, Smith AD. Ascorbic acid overdosing: a risk factor for calcium oxalate nephrolithiasis. J Urol 1992; 147:1215–1218.
- Hoyer-Kuhn H, Kohbrok S, Volland R, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol 2014; 9:468–477.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 1999; 10:840–845.
- Parks JH, Worcester EM, O'Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63:255–265.
- Hess B, Jost C, Zipperle L, Takkinen R, Jaeger P. High-calcium intake abolishes hyperoxaluria and reduces urinary crystallization during a 20-fold normal oxalate load in humans. Nephrol Dial Transplant 1998; 13:2241–2247.
- Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int 2009; 75:1264–1271.
- Cochat P, Hulton SA, Acquaviva C, et al; OxalEurope. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 2012; 27:1729–1736.
- Leumann E, Hoppe B, Neuhaus T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol 1993; 7:207–211.
- Bergstralh EJ, Monico CG, Lieske JC, et al; IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant 2010; 10:2493–2501.
- Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol 2003; 10:271–278.
- Coe FL, Parks JH. Hyperuricosuria and calcium nephrolithiasis. Urol Clin North Am 1981; 8:227–244.
- Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386–1389.
- Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol 2009; 11:134–144.
- Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol 1996; 156:907–909.
- Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol 2006; 1:1269–1274.
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 1993; 150:1761–1764.
- Lemann J Jr, Gray RW, Pleuss JA. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 1989; 35:688–695.
- Gault MH, Chafe LL, Morgan JM, et al. Comparison of patients with idiopathic calcium phosphate and calcium oxalate stones. Medicine (Baltimore) 1991; 70:345–359.
- Krieger NS, Asplin JR, Frick KK, et al. Effect of potassium citrate on calcium phosphate stones in a model of hypercalciuria. J Am Soc Nephrol 2015; 26:3001–3008.
- Falls WF Jr. Comparison of urinary acidification and ammonium excretion in normal and gouty subjects. Metabolism 1972; 21:433–445.
- Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med 1992; 327:1141–1152.
- Kenny JE, Goldfarb DS. Update on the pathophysiology and management of uric acid renal stones. Curr Rheumatol Rep 2010; 12:125–129.
- Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS Jr (AUA Nephrolithiasis Guideline Panel). Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol 2005; 173:1991–2000.
- Williams JJ, Rodman JS, Peterson CM. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med 1984; 311:760–764.
- Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL. Clinical use of cystine supersaturation measurements. J Urol 2000; 164:1481–1485.
- Palacın MGP, Nunes V, Gasparini P. Cystinuria. In: Shriver CR, editor. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001:4909–4932.
- Goldfarb DS, Coe FL, Asplin JR. Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int 2006; 69:1041–1047.
- Barbey F, Joly D, Rieu P, Mejean A, Daudon M, Jungers P. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol 2000; 163:1419–1423.
- Asplin DM, Asplin JR. The Interaction of thiol drugs and urine pH in the treatment of cystinuria. J Urol 2013; 189:2147–2151.
- Habib GS, Saliba W, Nashashibi M, Armali Z. Penicillamine and nephrotic syndrome. Eur J Intern Med 2006; 17:343–348.
- Sloand JA, Izzo JL Jr. Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 1987; 147:1409–1412.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol 2012; 62:160–165.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM Jr, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003; 63:1817–1823.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010; 12:e86–e96.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2011; 79:393–403.
- Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens 2008; 17:304–309.
- Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int J Urol 2005; 12:859–863.
- Ritz E. Metabolic syndrome: an emerging threat to renal function. Clin J Am Soc Nephrol 2007; 2:869–871.
- Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med 1989; 111:1006–1009.
- Saigal CS, Joyce G, Timilsina AR; Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 2005; 68:1808–1814.
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet 2006; 367:333–344.
- Pearle MS, Goldfarb DS, Assimos DG, et al; American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol 2014; 192:316–324.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996; 155:839–843.
- Sarica K, Inal Y, Erturhan S, Yagci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res 2006; 34:184–189.
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013; 8:1389–1395.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998; 128:534–540.
- Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med 1980; 69:19–30.
- Hall PM. Nephrolithiasis: treatment, causes, and prevention. Cleve Clin J Med 2009; 76:583–591.
- Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 2008; 73:489–496.
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115:2598–2608.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int 1982; 22:292–296.
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 1988; 66:140–146.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Noori N, Honarkar E, Goldfarb DS, et al. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: a randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis 2014; 63:456–463.
- Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med 2013; 158:535–543.
- Alon U, Costanzo LS, Chan JC. Additive hypocalciuric effects of amiloride and hydrochlorothiazide in patients treated with calcitriol. Miner Electrolyte Metab 1984; 10:379–386.
- Corbetta S, Baccarelli A, Aroldi A, et al. Risk factors associated to kidney stones in primary hyperparathyroidism. J Endocrinol Invest 2005; 28:122–128.
- Haymann JP. Metabolic disorders: stones as first clinical manifestation of significant diseases. World J Urol 2015; 33:187–192.
- Jaeger P, Portmann L, Jacquet AF, Burckhardt P. Influence of the calcium content of the diet on the incidence of mild hyperoxaluria in idiopathic renal stone formers. Am J Nephrol 1985; 5:40–44.
- Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 2007; 18:2198–2204.
- Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 2010; 78:1178–1185.
- Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 2009; 20:2253–2259.
- Urivetzky M, Kessaris D, Smith AD. Ascorbic acid overdosing: a risk factor for calcium oxalate nephrolithiasis. J Urol 1992; 147:1215–1218.
- Hoyer-Kuhn H, Kohbrok S, Volland R, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol 2014; 9:468–477.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 1999; 10:840–845.
- Parks JH, Worcester EM, O'Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63:255–265.
- Hess B, Jost C, Zipperle L, Takkinen R, Jaeger P. High-calcium intake abolishes hyperoxaluria and reduces urinary crystallization during a 20-fold normal oxalate load in humans. Nephrol Dial Transplant 1998; 13:2241–2247.
- Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int 2009; 75:1264–1271.
- Cochat P, Hulton SA, Acquaviva C, et al; OxalEurope. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 2012; 27:1729–1736.
- Leumann E, Hoppe B, Neuhaus T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr Nephrol 1993; 7:207–211.
- Bergstralh EJ, Monico CG, Lieske JC, et al; IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant 2010; 10:2493–2501.
- Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol 2003; 10:271–278.
- Coe FL, Parks JH. Hyperuricosuria and calcium nephrolithiasis. Urol Clin North Am 1981; 8:227–244.
- Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 1986; 315:1386–1389.
- Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol 2009; 11:134–144.
- Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol 1996; 156:907–909.
- Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol 2006; 1:1269–1274.
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 1993; 150:1761–1764.
- Lemann J Jr, Gray RW, Pleuss JA. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 1989; 35:688–695.
- Gault MH, Chafe LL, Morgan JM, et al. Comparison of patients with idiopathic calcium phosphate and calcium oxalate stones. Medicine (Baltimore) 1991; 70:345–359.
- Krieger NS, Asplin JR, Frick KK, et al. Effect of potassium citrate on calcium phosphate stones in a model of hypercalciuria. J Am Soc Nephrol 2015; 26:3001–3008.
- Falls WF Jr. Comparison of urinary acidification and ammonium excretion in normal and gouty subjects. Metabolism 1972; 21:433–445.
- Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med 1992; 327:1141–1152.
- Kenny JE, Goldfarb DS. Update on the pathophysiology and management of uric acid renal stones. Curr Rheumatol Rep 2010; 12:125–129.
- Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS Jr (AUA Nephrolithiasis Guideline Panel). Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol 2005; 173:1991–2000.
- Williams JJ, Rodman JS, Peterson CM. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med 1984; 311:760–764.
- Nakagawa Y, Asplin JR, Goldfarb DS, Parks JH, Coe FL. Clinical use of cystine supersaturation measurements. J Urol 2000; 164:1481–1485.
- Palacın MGP, Nunes V, Gasparini P. Cystinuria. In: Shriver CR, editor. The Metabolic and Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001:4909–4932.
- Goldfarb DS, Coe FL, Asplin JR. Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int 2006; 69:1041–1047.
- Barbey F, Joly D, Rieu P, Mejean A, Daudon M, Jungers P. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol 2000; 163:1419–1423.
- Asplin DM, Asplin JR. The Interaction of thiol drugs and urine pH in the treatment of cystinuria. J Urol 2013; 189:2147–2151.
- Habib GS, Saliba W, Nashashibi M, Armali Z. Penicillamine and nephrotic syndrome. Eur J Intern Med 2006; 17:343–348.
- Sloand JA, Izzo JL Jr. Captopril reduces urinary cystine excretion in cystinuria. Arch Intern Med 1987; 147:1409–1412.
KEY POINTS
- Nephrolithiasis is common and widespread, and its incidence and prevalence are increasing.
- Calcium stones are the most common type, and of these, calcium oxalate stones predominate.
- The most common risk factors for recurrent calcium stones are low urinary output, hypercalciuria, hyperoxaluria, hypocitraturia, and hyperuricosuria.
- Less common types of stones are usually associated with genetic abnormalities, infections, or medications.
When does chest CT require contrast enhancement?
Computed tomography (CT) plays an important role in the diagnosis and treatment of many clinical conditions1 involving the chest wall, mediastinum, pleura, pulmonary arteries, and lung parenchyma. The need for enhancement with intravenous (IV) contrast depends on the specific clinical indication (Table 1).
EVALUATION OF SUSPECTED CANCER
CT is commonly used to diagnose, stage, and plan treatment for lung cancer, other primary neoplastic processes involving the chest, and metastatic disease.2 The need for contrast varies on a case-by-case basis, and the benefits of contrast should be weighed against the potential risks in each patient.
When the neoplasm has CT attenuation similar to that of adjacent structures (lymph nodes in the hilum, masses in the mediastinum or chest wall), IV contrast can improve identification of the lesion and delineation of its margins and the relationship with adjacent structures (eg, vascular structures) (Figure 1).
CT without contrast for screening
The diagnostic algorithm for lung cancer screening is evolving. The US Preventive Services Task Force currently recommends low-dose CT without contrast, along with appropriate patient counseling, for patients with a history of smoking and an age range as detailed in the Task Force statement.3
Follow-up of a solitary pulmonary nodule also typically does not require contrast enhancement, though some investigators have reported high sensitivity with dynamic contrast enhancement of pulmonary nodules.4 This represents a rare clinical application of chest CT with and without contrast.
EVALUATION OF THORACIC VASCULAR DISEASE
For the assessment of vascular disease, CT in most cases requires IV contrast to delineate the vessel lumen. Pulmonary embolic disease is the third most common cause of acute cardiovascular disease.5 CT pulmonary angiography is the most common way to assess for pulmonary embolic disease, as it is accurate, fast, and widely available, and can assess alternate pathologies in cases of undifferentiated chest pain. Contrast enhancement of the pulmonary arteries is key, as embolic disease is identified as abnormal filling defects within the pulmonary arteries (Figure 2).
Contrast enhancement is also used to evaluate superior vena cava syndrome. At our institution, the CT protocol includes concomitant injections in the upper-extremity veins, with imaging timed for venous phase enhancement (pulmonary venogram). In cases of suspected arteriovenous malformation, a protocol similar to that used for suspected pulmonary embolus is used (Figure 3), although in some instances, the imaging features of arteriovenous malformation may be detectable without IV contrast.
EVALUATION OF PULMONARY PARENCHYMAL DISEASE
Infection, inflammation, and edema of the lung parenchyma are usually well depicted on CT without contrast enhancement. However, contrast may be helpful if there are concerns about complications such as chest wall involvement, where contrast enhancement may help further delineate the extent of complications.
Assessment of interstitial lung disease does not require use of IV contrast; rather, a tailored protocol with thinner slices and noncontiguous expiratory images can be used to evaluate for air-trapping and dynamic airway compromise (Figure 4). Evaluation of chronic obstructive pulmonary disease also does not require IV contrast.
EVALUATION OF THE PLEURA
In pleural effusion, CT assessment for the presence, location, and extent of the effusion does not require contrast. However, contrast enhancement is used to evaluate suspected or known exudative effusions and empyema.6 It also aids the evaluation of metastatic or primary malignancy of the pleura, particularly in cases of occult disease, as enhancement and thickening of the pleura are of diagnostic interest.
EVALUATION OF AIRWAY DISEASE
Diseases of the large airway, such as stenosis and thickening, and diseases of the small airways, such as bronchiolitis, typically do not require contrast enhancement. At our institution, to assess dynamic airway narrowing, we use a dedicated airway protocol, including inspiratory and expiratory phases and multiplanar reformatted images.
EVALUATION OF STERNAL AND MEDIASTINAL INFECTIONS
Postoperative sternal wound infections are not uncommon and range from cellulitis to frank osteomyelitis. Mediastinitis may likewise be iatrogenic or may spread from the oropharynx. CT with contrast can help to depict infection of the chest wall or mediastinum and in some instances can also delineate the route of spread.7
TYPES OF IV CONTRAST MEDIA
Contrast media used in CT contain iodine, which causes increased absorption and scattering of radiation in body tissues and blood. Other contrast media, such as those used for magnetic resonance imaging or barium enemas, do not contain iodine. This absorption and scattering in turn results in higher CT attenuation values, or “enhancement” on CT images. The extent of enhancement depends on the amount and rate of contrast material administered, as well as on patient factors (eg, tissue vascularity, permeability, interstitial space) and the energy (tube voltage) of the incident x-rays.8
Adverse reactions
Contrast materials are generally safe; however, as with any pharmaceutical, there is the potential for adverse reactions. These reactions are relatively rare and are usually mild but occasionally can be severe.9 Anaphylactoid reactions have an unclear etiology but mimic allergic reactions, and they are more likely to occur in patients with a previous reaction to contrast and in patients with asthma or cardiovascular or renal disease.
Nonanaphylactoid reactions are dependent on contrast osmolality and on the volume and route of injection (unlike anaphylactoid reactions).10 Typical symptoms include warmth, metallic taste, and nausea or vomiting.
Contrast-related nephrotoxicity has been reported,11 although this has been challenged more recently.12 Suspected risk factors for this complication include advanced age, cardiovascular disease, treatment with chemotherapy, elevated serum creatinine level, dehydration, diabetes, use of nonsteroidal anti-inflammatory medications, myeloma,13 renal disease, and kidney transplant.
Detailed protocols for premedication and management of contrast adverse reactions are beyond the scope of this review and the reader is advised to refer to dedicated manuals.10
Acknowledgment: We are grateful for the editorial assistance of Megan M. Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic.
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014; 273(suppl 2):S45–S74.
- American College of Radiology. ACR-SCBT-MR-SPR practice parameter for the performance of thoracic computed tomography (CT). www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Thoracic.pdf. Accessed March 30, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199.
- Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013; 201:971–976.
- Kraus GJ. The split pleura sign. Radiology 2007; 243:297–298.
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30:1335–1352.
- Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008; 36:69–74.
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.1. 2015. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed March 29, 2016.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol 1994; 5:125–137.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014; 273:714–725.
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology 1992; 183:519–521.
Computed tomography (CT) plays an important role in the diagnosis and treatment of many clinical conditions1 involving the chest wall, mediastinum, pleura, pulmonary arteries, and lung parenchyma. The need for enhancement with intravenous (IV) contrast depends on the specific clinical indication (Table 1).
EVALUATION OF SUSPECTED CANCER
CT is commonly used to diagnose, stage, and plan treatment for lung cancer, other primary neoplastic processes involving the chest, and metastatic disease.2 The need for contrast varies on a case-by-case basis, and the benefits of contrast should be weighed against the potential risks in each patient.
When the neoplasm has CT attenuation similar to that of adjacent structures (lymph nodes in the hilum, masses in the mediastinum or chest wall), IV contrast can improve identification of the lesion and delineation of its margins and the relationship with adjacent structures (eg, vascular structures) (Figure 1).

CT without contrast for screening
The diagnostic algorithm for lung cancer screening is evolving. The US Preventive Services Task Force currently recommends low-dose CT without contrast, along with appropriate patient counseling, for patients with a history of smoking and an age range as detailed in the Task Force statement.3
Follow-up of a solitary pulmonary nodule also typically does not require contrast enhancement, though some investigators have reported high sensitivity with dynamic contrast enhancement of pulmonary nodules.4 This represents a rare clinical application of chest CT with and without contrast.
EVALUATION OF THORACIC VASCULAR DISEASE
For the assessment of vascular disease, CT in most cases requires IV contrast to delineate the vessel lumen. Pulmonary embolic disease is the third most common cause of acute cardiovascular disease.5 CT pulmonary angiography is the most common way to assess for pulmonary embolic disease, as it is accurate, fast, and widely available, and can assess alternate pathologies in cases of undifferentiated chest pain. Contrast enhancement of the pulmonary arteries is key, as embolic disease is identified as abnormal filling defects within the pulmonary arteries (Figure 2).

Contrast enhancement is also used to evaluate superior vena cava syndrome. At our institution, the CT protocol includes concomitant injections in the upper-extremity veins, with imaging timed for venous phase enhancement (pulmonary venogram). In cases of suspected arteriovenous malformation, a protocol similar to that used for suspected pulmonary embolus is used (Figure 3), although in some instances, the imaging features of arteriovenous malformation may be detectable without IV contrast.

EVALUATION OF PULMONARY PARENCHYMAL DISEASE
Infection, inflammation, and edema of the lung parenchyma are usually well depicted on CT without contrast enhancement. However, contrast may be helpful if there are concerns about complications such as chest wall involvement, where contrast enhancement may help further delineate the extent of complications.
Assessment of interstitial lung disease does not require use of IV contrast; rather, a tailored protocol with thinner slices and noncontiguous expiratory images can be used to evaluate for air-trapping and dynamic airway compromise (Figure 4). Evaluation of chronic obstructive pulmonary disease also does not require IV contrast.

EVALUATION OF THE PLEURA
In pleural effusion, CT assessment for the presence, location, and extent of the effusion does not require contrast. However, contrast enhancement is used to evaluate suspected or known exudative effusions and empyema.6 It also aids the evaluation of metastatic or primary malignancy of the pleura, particularly in cases of occult disease, as enhancement and thickening of the pleura are of diagnostic interest.
EVALUATION OF AIRWAY DISEASE
Diseases of the large airway, such as stenosis and thickening, and diseases of the small airways, such as bronchiolitis, typically do not require contrast enhancement. At our institution, to assess dynamic airway narrowing, we use a dedicated airway protocol, including inspiratory and expiratory phases and multiplanar reformatted images.
EVALUATION OF STERNAL AND MEDIASTINAL INFECTIONS
Postoperative sternal wound infections are not uncommon and range from cellulitis to frank osteomyelitis. Mediastinitis may likewise be iatrogenic or may spread from the oropharynx. CT with contrast can help to depict infection of the chest wall or mediastinum and in some instances can also delineate the route of spread.7
TYPES OF IV CONTRAST MEDIA
Contrast media used in CT contain iodine, which causes increased absorption and scattering of radiation in body tissues and blood. Other contrast media, such as those used for magnetic resonance imaging or barium enemas, do not contain iodine. This absorption and scattering in turn results in higher CT attenuation values, or “enhancement” on CT images. The extent of enhancement depends on the amount and rate of contrast material administered, as well as on patient factors (eg, tissue vascularity, permeability, interstitial space) and the energy (tube voltage) of the incident x-rays.8
Adverse reactions
Contrast materials are generally safe; however, as with any pharmaceutical, there is the potential for adverse reactions. These reactions are relatively rare and are usually mild but occasionally can be severe.9 Anaphylactoid reactions have an unclear etiology but mimic allergic reactions, and they are more likely to occur in patients with a previous reaction to contrast and in patients with asthma or cardiovascular or renal disease.
Nonanaphylactoid reactions are dependent on contrast osmolality and on the volume and route of injection (unlike anaphylactoid reactions).10 Typical symptoms include warmth, metallic taste, and nausea or vomiting.
Contrast-related nephrotoxicity has been reported,11 although this has been challenged more recently.12 Suspected risk factors for this complication include advanced age, cardiovascular disease, treatment with chemotherapy, elevated serum creatinine level, dehydration, diabetes, use of nonsteroidal anti-inflammatory medications, myeloma,13 renal disease, and kidney transplant.
Detailed protocols for premedication and management of contrast adverse reactions are beyond the scope of this review and the reader is advised to refer to dedicated manuals.10
Acknowledgment: We are grateful for the editorial assistance of Megan M. Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic.
Computed tomography (CT) plays an important role in the diagnosis and treatment of many clinical conditions1 involving the chest wall, mediastinum, pleura, pulmonary arteries, and lung parenchyma. The need for enhancement with intravenous (IV) contrast depends on the specific clinical indication (Table 1).
EVALUATION OF SUSPECTED CANCER
CT is commonly used to diagnose, stage, and plan treatment for lung cancer, other primary neoplastic processes involving the chest, and metastatic disease.2 The need for contrast varies on a case-by-case basis, and the benefits of contrast should be weighed against the potential risks in each patient.
When the neoplasm has CT attenuation similar to that of adjacent structures (lymph nodes in the hilum, masses in the mediastinum or chest wall), IV contrast can improve identification of the lesion and delineation of its margins and the relationship with adjacent structures (eg, vascular structures) (Figure 1).

CT without contrast for screening
The diagnostic algorithm for lung cancer screening is evolving. The US Preventive Services Task Force currently recommends low-dose CT without contrast, along with appropriate patient counseling, for patients with a history of smoking and an age range as detailed in the Task Force statement.3
Follow-up of a solitary pulmonary nodule also typically does not require contrast enhancement, though some investigators have reported high sensitivity with dynamic contrast enhancement of pulmonary nodules.4 This represents a rare clinical application of chest CT with and without contrast.
EVALUATION OF THORACIC VASCULAR DISEASE
For the assessment of vascular disease, CT in most cases requires IV contrast to delineate the vessel lumen. Pulmonary embolic disease is the third most common cause of acute cardiovascular disease.5 CT pulmonary angiography is the most common way to assess for pulmonary embolic disease, as it is accurate, fast, and widely available, and can assess alternate pathologies in cases of undifferentiated chest pain. Contrast enhancement of the pulmonary arteries is key, as embolic disease is identified as abnormal filling defects within the pulmonary arteries (Figure 2).

Contrast enhancement is also used to evaluate superior vena cava syndrome. At our institution, the CT protocol includes concomitant injections in the upper-extremity veins, with imaging timed for venous phase enhancement (pulmonary venogram). In cases of suspected arteriovenous malformation, a protocol similar to that used for suspected pulmonary embolus is used (Figure 3), although in some instances, the imaging features of arteriovenous malformation may be detectable without IV contrast.

EVALUATION OF PULMONARY PARENCHYMAL DISEASE
Infection, inflammation, and edema of the lung parenchyma are usually well depicted on CT without contrast enhancement. However, contrast may be helpful if there are concerns about complications such as chest wall involvement, where contrast enhancement may help further delineate the extent of complications.
Assessment of interstitial lung disease does not require use of IV contrast; rather, a tailored protocol with thinner slices and noncontiguous expiratory images can be used to evaluate for air-trapping and dynamic airway compromise (Figure 4). Evaluation of chronic obstructive pulmonary disease also does not require IV contrast.

EVALUATION OF THE PLEURA
In pleural effusion, CT assessment for the presence, location, and extent of the effusion does not require contrast. However, contrast enhancement is used to evaluate suspected or known exudative effusions and empyema.6 It also aids the evaluation of metastatic or primary malignancy of the pleura, particularly in cases of occult disease, as enhancement and thickening of the pleura are of diagnostic interest.
EVALUATION OF AIRWAY DISEASE
Diseases of the large airway, such as stenosis and thickening, and diseases of the small airways, such as bronchiolitis, typically do not require contrast enhancement. At our institution, to assess dynamic airway narrowing, we use a dedicated airway protocol, including inspiratory and expiratory phases and multiplanar reformatted images.
EVALUATION OF STERNAL AND MEDIASTINAL INFECTIONS
Postoperative sternal wound infections are not uncommon and range from cellulitis to frank osteomyelitis. Mediastinitis may likewise be iatrogenic or may spread from the oropharynx. CT with contrast can help to depict infection of the chest wall or mediastinum and in some instances can also delineate the route of spread.7
TYPES OF IV CONTRAST MEDIA
Contrast media used in CT contain iodine, which causes increased absorption and scattering of radiation in body tissues and blood. Other contrast media, such as those used for magnetic resonance imaging or barium enemas, do not contain iodine. This absorption and scattering in turn results in higher CT attenuation values, or “enhancement” on CT images. The extent of enhancement depends on the amount and rate of contrast material administered, as well as on patient factors (eg, tissue vascularity, permeability, interstitial space) and the energy (tube voltage) of the incident x-rays.8
Adverse reactions
Contrast materials are generally safe; however, as with any pharmaceutical, there is the potential for adverse reactions. These reactions are relatively rare and are usually mild but occasionally can be severe.9 Anaphylactoid reactions have an unclear etiology but mimic allergic reactions, and they are more likely to occur in patients with a previous reaction to contrast and in patients with asthma or cardiovascular or renal disease.
Nonanaphylactoid reactions are dependent on contrast osmolality and on the volume and route of injection (unlike anaphylactoid reactions).10 Typical symptoms include warmth, metallic taste, and nausea or vomiting.
Contrast-related nephrotoxicity has been reported,11 although this has been challenged more recently.12 Suspected risk factors for this complication include advanced age, cardiovascular disease, treatment with chemotherapy, elevated serum creatinine level, dehydration, diabetes, use of nonsteroidal anti-inflammatory medications, myeloma,13 renal disease, and kidney transplant.
Detailed protocols for premedication and management of contrast adverse reactions are beyond the scope of this review and the reader is advised to refer to dedicated manuals.10
Acknowledgment: We are grateful for the editorial assistance of Megan M. Griffiths, scientific writer for the Imaging Institute, Cleveland Clinic.
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014; 273(suppl 2):S45–S74.
- American College of Radiology. ACR-SCBT-MR-SPR practice parameter for the performance of thoracic computed tomography (CT). www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Thoracic.pdf. Accessed March 30, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199.
- Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013; 201:971–976.
- Kraus GJ. The split pleura sign. Radiology 2007; 243:297–298.
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30:1335–1352.
- Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008; 36:69–74.
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.1. 2015. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed March 29, 2016.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol 1994; 5:125–137.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014; 273:714–725.
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology 1992; 183:519–521.
- Rubin GD. Computed tomography: revolutionizing the practice of medicine for 40 years. Radiology 2014; 273(suppl 2):S45–S74.
- American College of Radiology. ACR-SCBT-MR-SPR practice parameter for the performance of thoracic computed tomography (CT). www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Thoracic.pdf. Accessed March 30, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:330–338.
- Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199.
- Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: a prospective randomized study. AJR Am J Roentgenol 2013; 201:971–976.
- Kraus GJ. The split pleura sign. Radiology 2007; 243:297–298.
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256:32–61.
- Capps EF, Kinsella JJ, Gupta M, Bhatki AM, Opatowsky MJ. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30:1335–1352.
- Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008; 36:69–74.
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Version 10.1. 2015. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed March 29, 2016.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol 1994; 5:125–137.
- McDonald RJ, McDonald JS, Carter RE, et al. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology 2014; 273:714–725.
- McCarthy CS, Becker JA. Multiple myeloma and contrast media. Radiology 1992; 183:519–521.
Should I suspect obstructive sleep apnea if a patient has hard-to-control hypertension?
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
Yes. Obstructive sleep apnea is common and is associated with hypertension and resistant hypertension. Physicians taking care of patients who have hard-to-control hypertension should be aware of the possible diagnosis of obstructive sleep apnea and screen them for it. In-laboratory polysomnography or home sleep testing should be offered if appropriate, and if obstructive sleep apnea is detected, it should be treated, as this treatment may help to control blood pressure more effectively.
OBSTRUCTIVE SLEEP APNEA IS COMMON
Obstructive sleep apnea is characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, with partial collapse leading to hypopnea and complete collapse leading to apnea. These episodes result in intermittent hypoxemia, microarousals, sleep fragmentation, daytime sleepiness, and impairment in quality of life.
In tandem with the increasing obesity epidemic, the prevalence of moderate to severe obstructive sleep apnea is 17% in men and 9% in women 50 to 70 years old.1
LINKED TO HYPERTENSION
The respiratory events that occur in obstructive sleep apnea are associated with blood pressure surges during sleep that can cause persistent elevated blood pressure while awake. Obstructive sleep apnea has been independently associated with incident hypertension in large epidemiologic studies, even after correction for confounding factors such as obesity and its surrogate markers.
Moreover, the more severe the obstructive sleep apnea, the greater the risk of incident hypertension.2 And large, long-term observational studies have shown higher incidence rates of hypertension in people with untreated obstructive sleep apnea than in those who underwent treatment for it with continuous positive airway pressure (CPAP).3
Obstructive sleep apnea is also associated with nocturnal nondipping of blood pressure (defined as failure of blood pressure to decline by at least 10% during sleep), which is an independent marker for worse cardiovascular outcomes and hypertension-induced target organ damage.
Obstructive sleep apnea is particularly common in those with drug-resistant hypertension,4 which is defined as a suboptimal control of blood pressure despite the use of multiple antihypertensive medications of different classes, a condition associated with significant rates of cardiovascular morbidity and mortality. Even in patients at high risk of cardiovascular disease, we found that those with severe obstruction of the upper airway during sleep had fourfold higher odds of having resistant elevated blood pressure.5
The seventh Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as one of the causes of secondary hypertension.6 The 2013 European Society of Hypertension/European Society of Cardiology guidelines7 suggested an evaluation of obstructive sleep apnea symptoms for the management of hypertension.
MECHANISMS LINKING OBSTRUCTIVE SLEEP APNEA AND HYPERTENSION
Pathophysiologic mechanisms that may explain the association between obstructive sleep apnea and hypertension include stimulation of sympathetic activity,8 increased arterial stiffness, and endothelial dysfunction driven by apnea-related intermittent hypoxemia.9 Increased systemic inflammation and oxidative stress caused by obstructive sleep apnea are other proposed mechanisms.
Conversely, resistant hypertension may worsen obstructive sleep apnea. Some propose that activation of the renin-angiotensin-aldosterone system can cause parapharyngeal edema and rostral fluid shifts during sleep and thereby increase upper airway obstruction and worsen the severity of obstructive sleep apnea.10
CONSIDER SCREENING
Patients with resistant hypertension and risk factors for obstructive sleep apnea should be screened for it, as it is very common in this population.
A simple screening tool that can be used to detect sleep apnea is the STOP-BANG questionnaire11:
- Snore: Have you been told that you snore loudly?
- Tired: Are you often tired during the day?
- Observed apnea: Do you know if you stop breathing, or has anyone witnessed you stop breathing while sleeping?
- Pressure: Do you have or are you being treated for high blood pressure?
- Body mass index: Is your body mass index greater than 35 kg/m2?
- Age: older than 50?
- Neck circumference: greater than 40 cm?
- Gender: Male?
A score of 3 or more indicates a high risk of obstructive sleep apnea, and further workup for it is appropriate. Some of the other symptoms and signs are listed in Table 1.
SLEEP STUDIES: IN THE LABORATORY OR AT HOME
In-laboratory polysomnography entails electro-oculography, electromyography, electroencephalography, electrocardiography, pulse oximetry, and measurement of oronasal flow and thoracoabdominal movement (using sensors and belts). It should be performed in patients who have significant comorbid conditions.
A home sleep study, which is more limited than polysomnography, is appropriate in those who have a high probability of obstructive sleep apnea and who do not have other sleep disorders or significant cardiovascular, neurologic, or respiratory disorders.
Subsequently, if obstructive sleep apnea is found, a positive airway pressure titration study is performed to determine the optimal pressure requirements.
CPAP IS THE GOLD STANDARD TREATMENT
Behavioral changes are recommended to correct factors that predispose to obstructive sleep apnea or aggravate it. These changes include avoiding alcohol, sleeping on one’s side rather than supine, weight reduction in overweight individuals, and treating nasal congestion. In some situations, oral appliances or surgical options can be considered. However, CPAP is the gold standard therapy and the one most commonly used.
CPAP LOWERS BLOOD PRESSURE
Effective treatment of obstructive sleep apnea, added to an antihypertensive regimen, can further lower the blood pressure more than the antihypertensive medication regimen by itself.
Several meta-analyses have shown modest improvements in blood pressure with CPAP in hypertensive patients. CPAP’s effect on blood pressure seems to be more pronounced in those with resistant hypertension, in whom a meta-analysis of randomized controlled trials demonstrated a mean reduction in systolic blood pressure of 6.74 mm Hg and a mean reduction in diastolic blood pressure of 5.94 mm Hg.12 A recent clinic-based (“real-world”) study revealed lowering of blood pressure in patients with resistant and nonresistant hypertension—approximately 2 to 3 mm Hg after CPAP therapy.13
Furthermore, a randomized controlled trial in Spain showed that the nocturnal nondipping pattern observed in patients with resistant hypertension was reversed with the use of CPAP.14
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307:2169–2176.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10:835–843.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Mancia G, Fagard R, Narkiewicz K, et al; Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep 2002; 25:850–855.
- Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012; 26:281–287.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014; 32:2341–2350.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149:747–755.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310:2407–2415.
Whiplash-shaped acute rash
A previously healthy 32-year-old man presented to the emergency room with a persistent, nonpruritic rash on his trunk, which had suddenly appeared 2 days after he ate Chinese food.
Physical examination revealed multiple crosslinked linear plaques that appeared like scratches over his chest, back, and shoulders (Figures 1 and 2). He had no dermatographism, and his scalp, nails, palms, and soles were not affected. He had no signs of lymphadenopathy or systemic involvement.
Basic blood and urinary laboratory testing, blood cultures, and serologic studies showed normal or negative results.
Given the presentation and results of initial testing, his rash was diagnosed as flagellate erythema, likely due to shiitake mushroom intake. The diagnosis does not require histopathologic confirmation.
The rash resolved spontaneously over the next 2 weeks with use of a topical emollient and without scarring or residual hyperpigmentation.
FLAGELLATE ERYTHEMA
Flagellate erythema is a peculiar cutaneous eruption characterized by the progressive or sudden onset of parallel linear or curvilinear plaques, most commonly on the trunk. The plaques are typically arranged in a scratch pattern resembling marks left by the lashes of a whip.1 In contrast to other itchy dermatoses and neurotic excoriations that may present with self-induced linear marks, flagellate erythema appears spontaneously.
Drug-related causes, disease associations
Originally described in association with bleomycin treatment, flagellate erythema is currently considered a distinct feature of several dermatologic and systemic disorders, and therefore the ability to recognize it is valuable in daily practice.2 In addition to bleomycin analogues and anticancer agents such as peplomycin,1 bendamustine,3 and docetaxel,4 physicians should consider shiitake dermatitis5 and other less commonly reported associations such as dermatomyositis,6 lupus,7 Still disease,8 and parvovirus infection.9
Diagnostic features
The diagnosis of flagellate erythema is mainly based on the morphologic features of the clinical lesions.1 Shiitake dermatitis and flagellate erythema related to rheumatologic disease usually present with more inflammatory and erythematous plaques. Chemotherapy-induced flagellate rash typically has a violaceous or purpuric coloration, which tends to leave noticeable hyperpigmentation for several months.2
Skin biopsy may be necessary to distinguish it from similar-looking dermatoses with different histologic findings, such as dermatographism, phytophotodermatitis, erythema gyratum repens, and factitious dermatoses, which may require specific treatments or be related to important underlying pathology.1,2
Treatment
Treatment includes both specific treatment of the underlying cause and symptomatic care of the skin with topical emollients and, in cases of associated pruritus, oral antihistamines. The patient should also be reassured about the self-healing nature of shiitake dermatitis rash.5
- Yamamoto T, Nishioka K. Flagellate erythema. Int J Dermatol 2006; 45:627–631.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014; 80:149–152.
- Mahmoud BH, Eide MJ. Bendamustine-induced “flagellate dermatitis.” Dermatol Online J 2012; 18:12.
- Tallon B, Lamb S. Flagellate erythema induced by docetaxel. Clin Exp Dermatol 2008; 33:276–277.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol 2012; 67:140–141.
- Jara M, Amérigo J, Duce S, Borbujo J. Dermatomyositis and flagellate erythema. Clin Exp Dermatol 1996; 21:440–441.
- Niiyama S, Katsuoka K. Systemic lupus erythematosus with flagellate erythema. Eur J Dermatol 2012; 22:808–809.
- Ciliberto H, Kumar MG, Musiek A. Flagellate erythema in a patient with fever. JAMA Dermatol 2013; 149:1425–1426.
- Miguélez A, Dueñas J, Hervás D, Hervás JA, Salva F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol 2014; 53:e583–e585.
A previously healthy 32-year-old man presented to the emergency room with a persistent, nonpruritic rash on his trunk, which had suddenly appeared 2 days after he ate Chinese food.
Physical examination revealed multiple crosslinked linear plaques that appeared like scratches over his chest, back, and shoulders (Figures 1 and 2). He had no dermatographism, and his scalp, nails, palms, and soles were not affected. He had no signs of lymphadenopathy or systemic involvement.
Basic blood and urinary laboratory testing, blood cultures, and serologic studies showed normal or negative results.
Given the presentation and results of initial testing, his rash was diagnosed as flagellate erythema, likely due to shiitake mushroom intake. The diagnosis does not require histopathologic confirmation.
The rash resolved spontaneously over the next 2 weeks with use of a topical emollient and without scarring or residual hyperpigmentation.
FLAGELLATE ERYTHEMA
Flagellate erythema is a peculiar cutaneous eruption characterized by the progressive or sudden onset of parallel linear or curvilinear plaques, most commonly on the trunk. The plaques are typically arranged in a scratch pattern resembling marks left by the lashes of a whip.1 In contrast to other itchy dermatoses and neurotic excoriations that may present with self-induced linear marks, flagellate erythema appears spontaneously.
Drug-related causes, disease associations
Originally described in association with bleomycin treatment, flagellate erythema is currently considered a distinct feature of several dermatologic and systemic disorders, and therefore the ability to recognize it is valuable in daily practice.2 In addition to bleomycin analogues and anticancer agents such as peplomycin,1 bendamustine,3 and docetaxel,4 physicians should consider shiitake dermatitis5 and other less commonly reported associations such as dermatomyositis,6 lupus,7 Still disease,8 and parvovirus infection.9
Diagnostic features
The diagnosis of flagellate erythema is mainly based on the morphologic features of the clinical lesions.1 Shiitake dermatitis and flagellate erythema related to rheumatologic disease usually present with more inflammatory and erythematous plaques. Chemotherapy-induced flagellate rash typically has a violaceous or purpuric coloration, which tends to leave noticeable hyperpigmentation for several months.2
Skin biopsy may be necessary to distinguish it from similar-looking dermatoses with different histologic findings, such as dermatographism, phytophotodermatitis, erythema gyratum repens, and factitious dermatoses, which may require specific treatments or be related to important underlying pathology.1,2
Treatment
Treatment includes both specific treatment of the underlying cause and symptomatic care of the skin with topical emollients and, in cases of associated pruritus, oral antihistamines. The patient should also be reassured about the self-healing nature of shiitake dermatitis rash.5
A previously healthy 32-year-old man presented to the emergency room with a persistent, nonpruritic rash on his trunk, which had suddenly appeared 2 days after he ate Chinese food.
Physical examination revealed multiple crosslinked linear plaques that appeared like scratches over his chest, back, and shoulders (Figures 1 and 2). He had no dermatographism, and his scalp, nails, palms, and soles were not affected. He had no signs of lymphadenopathy or systemic involvement.
Basic blood and urinary laboratory testing, blood cultures, and serologic studies showed normal or negative results.
Given the presentation and results of initial testing, his rash was diagnosed as flagellate erythema, likely due to shiitake mushroom intake. The diagnosis does not require histopathologic confirmation.
The rash resolved spontaneously over the next 2 weeks with use of a topical emollient and without scarring or residual hyperpigmentation.
FLAGELLATE ERYTHEMA
Flagellate erythema is a peculiar cutaneous eruption characterized by the progressive or sudden onset of parallel linear or curvilinear plaques, most commonly on the trunk. The plaques are typically arranged in a scratch pattern resembling marks left by the lashes of a whip.1 In contrast to other itchy dermatoses and neurotic excoriations that may present with self-induced linear marks, flagellate erythema appears spontaneously.
Drug-related causes, disease associations
Originally described in association with bleomycin treatment, flagellate erythema is currently considered a distinct feature of several dermatologic and systemic disorders, and therefore the ability to recognize it is valuable in daily practice.2 In addition to bleomycin analogues and anticancer agents such as peplomycin,1 bendamustine,3 and docetaxel,4 physicians should consider shiitake dermatitis5 and other less commonly reported associations such as dermatomyositis,6 lupus,7 Still disease,8 and parvovirus infection.9
Diagnostic features
The diagnosis of flagellate erythema is mainly based on the morphologic features of the clinical lesions.1 Shiitake dermatitis and flagellate erythema related to rheumatologic disease usually present with more inflammatory and erythematous plaques. Chemotherapy-induced flagellate rash typically has a violaceous or purpuric coloration, which tends to leave noticeable hyperpigmentation for several months.2
Skin biopsy may be necessary to distinguish it from similar-looking dermatoses with different histologic findings, such as dermatographism, phytophotodermatitis, erythema gyratum repens, and factitious dermatoses, which may require specific treatments or be related to important underlying pathology.1,2
Treatment
Treatment includes both specific treatment of the underlying cause and symptomatic care of the skin with topical emollients and, in cases of associated pruritus, oral antihistamines. The patient should also be reassured about the self-healing nature of shiitake dermatitis rash.5
- Yamamoto T, Nishioka K. Flagellate erythema. Int J Dermatol 2006; 45:627–631.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014; 80:149–152.
- Mahmoud BH, Eide MJ. Bendamustine-induced “flagellate dermatitis.” Dermatol Online J 2012; 18:12.
- Tallon B, Lamb S. Flagellate erythema induced by docetaxel. Clin Exp Dermatol 2008; 33:276–277.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol 2012; 67:140–141.
- Jara M, Amérigo J, Duce S, Borbujo J. Dermatomyositis and flagellate erythema. Clin Exp Dermatol 1996; 21:440–441.
- Niiyama S, Katsuoka K. Systemic lupus erythematosus with flagellate erythema. Eur J Dermatol 2012; 22:808–809.
- Ciliberto H, Kumar MG, Musiek A. Flagellate erythema in a patient with fever. JAMA Dermatol 2013; 149:1425–1426.
- Miguélez A, Dueñas J, Hervás D, Hervás JA, Salva F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol 2014; 53:e583–e585.
- Yamamoto T, Nishioka K. Flagellate erythema. Int J Dermatol 2006; 45:627–631.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014; 80:149–152.
- Mahmoud BH, Eide MJ. Bendamustine-induced “flagellate dermatitis.” Dermatol Online J 2012; 18:12.
- Tallon B, Lamb S. Flagellate erythema induced by docetaxel. Clin Exp Dermatol 2008; 33:276–277.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol 2012; 67:140–141.
- Jara M, Amérigo J, Duce S, Borbujo J. Dermatomyositis and flagellate erythema. Clin Exp Dermatol 1996; 21:440–441.
- Niiyama S, Katsuoka K. Systemic lupus erythematosus with flagellate erythema. Eur J Dermatol 2012; 22:808–809.
- Ciliberto H, Kumar MG, Musiek A. Flagellate erythema in a patient with fever. JAMA Dermatol 2013; 149:1425–1426.
- Miguélez A, Dueñas J, Hervás D, Hervás JA, Salva F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol 2014; 53:e583–e585.
A guide to managing acute liver failure
When the liver fails, it usually fails gradually. The sudden (acute) onset of liver failure, while less common, demands prompt management, with transfer to an intensive care unit, specific treatment depending on the cause, and consideration of liver transplant, without which the mortality rate is high.
This article reviews the definition, epidemiology, etiology, and management of acute liver failure.
DEFINITIONS
Acute liver failure is defined as a syndrome of acute hepatitis with evidence of abnormal coagulation (eg, an international normalized ratio > 1.5) complicated by the development of mental alteration (encephalopathy) within 26 weeks of the onset of illness in a patient without a history of liver disease.1 In general, patients have no evidence of underlying chronic liver disease, but there are exceptions; patients with Wilson disease, vertically acquired hepatitis B virus infection, or autoimmune hepatitis can present with acute liver failure superimposed on chronic liver disease or even cirrhosis.
The term acute liver failure has replaced older terms such as fulminant hepatic failure, hyperacute liver failure, and subacute liver failure, which were used for prognostic purposes. Patients with hyperacute liver failure (defined as development of encephalopathy within 7 days of onset of illness) generally have a good prognosis with medical management, whereas those with subacute liver failure (defined as development of encephalopathy within 5 to 26 weeks of onset of illness) have a poor prognosis without liver transplant.2,3
NEARLY 2,000 CASES A YEAR
There are nearly 2,000 cases of acute liver failure each year in the United States, and it accounts for 6% of all deaths due to liver disease.4 It is more common in women than in men, and more common in white people than in other races. The peak incidence is at a fairly young age, ie, 35 to 45 years.
CAUSES
The most common cause of acute liver failure in the United States and other Western countries is acetaminophen toxicity, followed by viral hepatitis. In contrast, viral hepatitis is the most common cause in developing countries.5
Acetaminophen toxicity
Patients with acetaminophen-induced liver failure tend to be younger than other patients with acute liver failure.1 Nearly half of them present after intentionally taking a single large dose, while the rest present with unintentional toxicity while taking acetaminophen for pain relief on a long-term basis and ingesting more than the recommended dose.6
After ingestion, 52% to 57% of acetaminophen is converted to glucuronide conjugates, and 30% to 44% is converted to sulfate conjugates. These compounds are nontoxic, water-soluble, and rapidly excreted in the urine.
However, about 5% to 10% of ingested acetaminophen is shunted to the cytochrome P450 system. P450 2E1 is the main isoenzyme involved in acetaminophen metabolism, but 1A2, 3A4, and 2A6 also contribute.7,8 P450 2E1 is the same isoenzyme responsible for ethanol metabolism and is inducible. Thus, regular alcohol consumption can increase P450 2E1 activity, setting the stage under certain circumstances for increased acetaminophen metabolism through this pathway.
Metabolism of acetaminophen through the cytochrome P450 pathway results in production of N-acetyl-p-benzoquinone imine (NAPQI), the compound that damages the liver. NAPQI is rendered nontoxic by binding to glutathione, forming NAPQI-glutathione adducts. Glutathione capacity is limited, however. With too much acetaminophen, glutathione becomes depleted and NAPQI accumulates, binds with proteins to form adducts, and leads to necrosis of hepatocytes (Figure 1).9,10
Acetylcysteine, used in treating acetaminophen toxicity, is a substrate for glutathione synthesis and ultimately increases the amount of glutathione available to bind NAPQI and prevent damage to hepatocytes.11
Acetaminophen is a dose-related toxin. Most ingestions leading to acute liver failure exceed 10 g/day (> 150 mg/kg/day). Moderate chronic ingestion, eg, 4 g/day, usually leads to transient mild elevation of liver enzymes in healthy individuals12 but can in rare cases cause acute liver failure.13
Whitcomb and Block14 retrospectively identified 49 patients who presented with acetaminophen-induced hepatotoxicity in 1987 through 1993; 21 (43%) had been taking acetaminophen for therapeutic purposes. All 49 patients took more than the recommended limit of 4 g/day, many of them while fasting and some while using alcohol. Acute liver failure was seen with ingestion of more than 12 g/day—or more than 10 g/day in alcohol users. The authors attributed the increased risk to activation of cytochrome P450 2E1 by alcohol and depletion of glutathione stores by starvation or alcohol abuse.
Advice to patients taking acetaminophen is given in Table 1.
Other drugs and supplements
A number of other drugs and herbal supplements can also cause acute liver failure (Table 2), the most common being antimicrobial and antiepileptic drugs.15 Of the antimicrobials, antitubercular drugs (especially isoniazid) are believed to be the most common causes, followed by trimethoprim-sulfamethoxazole. Phenytoin is the antiepileptic drug most often implicated in acute liver failure.
Statins can also cause acute liver failure, especially when combined with other hepatotoxic agents.16
The herbal supplements and weight-loss agents Hydroxycut and Herbalife have both been reported to cause acute liver failure, with patients presenting with either the hepatocellular or the cholestatic pattern of liver injury.17 The exact chemical in these supplements that causes liver injury has not yet been determined.
The National Institutes of Health maintains a database of cases of liver failure due to medications and supplements at livertox.nih.gov. The database includes the pattern of hepatic injury, mechanism of injury, management, and outcomes.
Viral hepatitis
Hepatitis B virus is the most common viral cause of acute liver failure and is responsible for about 8% of cases.18
Patients with chronic hepatitis B virus infection—as evidenced by positive hepatitis B surface antigen—can develop acute liver failure if the infection is reactivated by the use of immunosuppressive drugs for solid-organ or bone-marrow transplant or medications such as anti-tumor necrosis agents, rituximab, or chemotherapy. These patients should be treated prophylactically with a nucleoside analogue, which should be continued for 6 months after immunosuppressive therapy is completed.
Hepatitis A virus is responsible for about 4% of cases.18
Hepatitis C virus rarely causes acute liver failure, especially in the absence of hepatitis A and hepatitis B.3,19
Hepatitis E virus, which is endemic in areas of Asia and Africa, can cause liver disease in pregnant women and in young adults who have concomitant liver disease from another cause. It tends to cause acute liver failure more frequently in pregnant women than in the rest of the population and carries a mortality rate of more than 20% in this subgroup.
TT (transfusion-transmitted) virus was reported in the 1990s to cause acute liver failure in about 27% of patients in whom no other cause could be found.20
Other rare viral causes of acute liver failure include Epstein-Barr virus, cytomegalovirus, and herpes simplex virus types 1, 2, and 6.
Other causes
Other causes of acute liver failure include ischemic hepatitis, autoimmune hepatitis, Wilson disease, Budd-Chiari syndrome, and HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome.
MANY PATIENTS NEED LIVER TRANSPLANT
Many patients with acute liver failure ultimately require orthotopic liver transplant,21 especially if they present with severe encephalopathy. Other aspects of treatment vary according to the cause of liver failure (Table 3).
SPECIFIC MANAGEMENT
Management of acetaminophen toxicity
If the time of ingestion is known, checking the acetaminophen level can help determine the cause of acute liver failure and also predict the risk of hepatotoxicity, based on the work of Rumack and Matthew.22 Calculators are available, eg, http://reference.medscape.com/calculator/acetaminophen-toxicity.
If a patient presents with acute liver failure several days after ingesting acetaminophen, the level can be in the nontoxic range, however. In this scenario, measuring acetaminophen-protein adducts can help establish acetaminophen toxicity as the cause, as the adducts last longer in the serum and provide 100% sensitivity and specificity.23 While most laboratories can rapidly measure acetaminophen levels, only a few can measure acetaminophen-protein adducts, and thus this test is not used clinically.
Acetylcysteine is the main drug used for acetaminophen toxicity. Ideally, it should be given within 8 hours of acetaminophen ingestion, but giving it later is also useful.1
Acetylcysteine is available in oral and intravenous forms, the latter for patients who have encephalopathy or cannot tolerate oral intake due to repeated episodes of vomiting.24,25 The oral form is much less costly and is thus preferred over intravenous acetylcysteine in patients who can tolerate oral intake. Intravenous acetylcysteine should be given in a loading dose of 150 mg/kg in 5% dextrose over 15 minutes, followed by a maintenance dose of 50 mg/kg over 4 hours and then 100 mg/kg given over 16 hours.1 No dose adjustment is needed in patients who have renal toxicity (acetaminophen can also be toxic to the kidneys).
Most patients with acetaminophen-induced liver failure survive with medical management alone and do not need a liver transplant.3,26 Cirrhosis does not occur in these patients.
Management of viral acute liver failure
When patients present with acute liver failure, it is necessary to look for a viral cause by serologic testing, including hepatitis A virus IgM antibody, hepatitis B surface antigen, and hepatitis B core IgM antibody.
Hepatitis B can become reactivated in immunocompromised patients, and therefore the hepatitis B virus DNA level should be checked. Detection of hepatitis B virus DNA in a patient previously known to have undetectable hepatitis B virus DNA confirms hepatitis B reactivation.
Patients with hepatitis B-induced acute liver failure should be treated with entecavir or tenofovir. Although this treatment may not change the course of acute liver failure or accelerate the recovery, it can prevent reinfection in the transplanted liver if liver transplant becomes indicated.27–29
Herpes simplex virus should be suspected in patients presenting with anicteric hepatitis with fever. Polymerase chain reaction testing for herpes simplex virus should be done,30 and if positive, patients should be given intravenous acyclovir.31 Despite treatment, herpes simplex virus disease is associated with a very poor prognosis without liver transplant.
Autoimmune hepatitis
The autoantibodies usually seen in autoimmune hepatitis are antinuclear antibody, antismooth muscle antibody, and anti-liver-kidney microsomal antibody, and patients need to be tested for them.
The diagnosis of autoimmune hepatitis can be challenging, as these autoimmune markers can be negative in 5% of patients. Liver biopsy becomes essential to establish the diagnosis in that setting.32
Guidelines advise starting prednisone 40 to 60 mg/day and placing the patient on the liver transplant list.1
Wilson disease
Although it is an uncommon cause of liver failure, Wilson disease needs special attention because it has a poor prognosis. The mortality rate in acute liver failure from Wilson disease reaches 100% without liver transplant.
Wilson disease is caused by a genetic defect that allows copper to accumulate in the liver and other organs. However, diagnosing Wilson disease as the cause of acute liver failure can be challenging because elevated serum and urine copper levels are not specific to Wilson disease and can be seen in patients with acute liver failure from any cause. In addition, the ceruloplasmin level is usually normal or high because it is an acute-phase reactant. Accumulation of copper in the liver parenchyma is usually patchy; therefore, qualitative copper staining on random liver biopsy samples provides low diagnostic yield. Quantitative copper on liver biopsy is the gold standard test to establish the diagnosis, but the test is time-consuming. Kayser-Fleischer rings around the iris are considered pathognomic for Wilson disease when seen with acute liver failure, but they are seen in only about 50% of patients.33
A unique feature of acute Wilson disease is that most patients have very high bilirubin levels and low alkaline phosphatase levels. An alkaline phosphatase-to-bilirubin ratio less than 2 in patients with acute liver failure is highly suggestive of Wilson disease.34
Another clue to the diagnosis is that patients with Wilson disease tend to develop Coombs-negative hemolytic anemia, which leads to a disproportionate elevation in aminotransferase levels, with aspartate aminotransferase being higher than alanine aminotransferase.
Once Wilson disease is suspected, the patient should be listed for liver transplant because death is almost certain without it. For patients awaiting liver transplant, the American Association for the Study of Liver Diseases guidelines recommend certain measures to lower the serum copper level such as albumin dialysis, continuous hemofiltration, plasmapheresis, and plasma exchange,1 but the evidence supporting their use is limited.
NONSPECIFIC MANAGEMENT
Acute liver failure can affect a number of organs and systems in addition to the liver (Figure 2).
General considerations
Because their condition can rapidly deteriorate, patients with acute liver failure are best managed in intensive care.
Patients who present to a center that does not have the facilities for liver transplant should be transferred to a transplant center as soon as possible, preferably by air. If the patient may not be able to protect the airway, endotracheal intubation should be performed before transfer.
The major causes of death in patients with acute liver failure are cerebral edema and infection. Gastrointestinal bleeding was a major cause of death in the past, but with prophylactic use of histamine H2 receptor blockers and proton pump inhibitors, the incidence of gastrointestinal bleeding has been significantly reduced.
Although initially used only in patients with acetaminophen-induced liver failure, acetylcysteine has also shown benefit in patients with acute liver failure from other causes. In patients with grade 1 or 2 encephalopathy on a scale of 0 (minimal) to 4 (comatose), the transplant-free survival rate is higher when acetylcysteine is given compared with placebo, but this benefit does not extend to patients with a higher grade of encephalopathy.35
Cerebral edema and intracranial hypertension
Cerebral edema is the leading cause of death in patients with acute liver failure, and it develops in nearly 40% of patients.36
The mechanism by which cerebral edema develops is not well understood. Some have proposed that ammonia is converted to glutamine, which causes cerebral edema either directly by its osmotic effect37,38 or indirectly by decreasing other osmolytes, thereby promoting water retention.39
Cerebral edema leads to intracranial hypertension, which can ultimately cause cerebral herniation and death. Because of the high mortality rate associated with cerebral edema, invasive devices were extensively used in the past to monitor intracranial pressure. However, in light of known complications of these devices, including bleeding,40 and lack of evidence of long-term benefit in terms of mortality rates, their use has come under debate.
Treatments. Many treatments are available for cerebral edema and intracranial hypertension. The first step is to elevate the head of the bed about 30 degrees. In addition, hyponatremia should be corrected, as it can worsen cerebral edema.41 If patients are intubated, maintaining a hypercapneic state is advisable to decrease the intracranial pressure.
Of the two pharmacologic options, mannitol is more often used.42 It is given as a bolus dose of 0.5 to 1 g/kg intravenously if the serum osmolality is less than 320 mOsm/L.1 Given the risk of fluid overload with mannitol, caution must be exercised in patients with renal dysfunction. The other pharmacologic option is 3% hypertonic saline.
Therapeutic hypothermia is a newer treatment for cerebral edema. Lowering the body temperature to 32 to 33°C (89.6 to 91.4°F) using cooling blankets decreases intracranial pressure and cerebral blood flow and improves the cerebral perfusion pressure.43 With this treatment, patients should be closely monitored for side effects of infection, coagulopathy, and cardiac arrythmias.1
l-ornithine l-aspartate was successfully used to prevent brain edema in rats, but in humans, no benefit was seen compared with placebo.44,45 The underlying basis for this experimental treatment is that supplemental ornithine and aspartate should increase glutamate synthesis, which should increase the activity of enzyme glutamine synthetase in skeletal muscles. With the increase in enzyme activity, conversion of ammonia to glutamine should increase, thereby decreasing ammonia circulation and thus decreasing cerebral edema.
Patients with cerebral edema have a high incidence of seizures, but prophylactic antiseizure medications such as phenytoin have not been proven to be beneficial.46
Infection
Nearly 80% of patients with acute liver failure develop an infectious complication, which can be attributed to a state of immunodeficiency.47
The respiratory and urinary tracts are the most common sources of infection.48 In patients with bacteremia, Enterococcus species and coagulase-negative Staphylococcus species49 are the commonly isolated organisms. Also, in patients with acute liver failure, fungal infections account for 30% of all infections.50
Infected patients often develop worsening of their encephalopathy51 without fever or elevated white blood cell count.49,52 Thus, in any patient in whom encephalopathy is worsening, an evaluation must be done to rule out infection. In these patients, systemic inflammatory response syndrome is an independent risk factor for death.53
Despite the high mortality rate with infection, whether using antibiotics prophylactically in acute liver failure is beneficial is controversial.54,55
Gastrointestinal bleeding
The current prevalence of upper gastrointestinal bleeding in acute liver failure patients is about 1.5%.56 Coagulopathy and endotracheal intubation are the main risk factors for upper gastrointestinal bleeding in these patients.57 The most common source of bleeding is stress ulcers in the stomach. The ulcers develop from a combination of factors, including decreased blood flow to the mucosa causing ischemia and hypoperfusion-reperfusion injury.
Pharmacologic inhibition of gastric acid secretion has been shown to reduce upper gastrointestinal bleeding in acute liver failure. A histamine H2 receptor blocker or proton pump inhibitor should be given to prevent gastrointestinal bleeding in patients with acute liver failure.1,58
EXPERIMENTAL TREATMENTS
Artificial liver support systems
Membranes and dialysate solutions have been developed to remove toxic substances that are normally metabolized by the liver. Two of these—the molecular adsorbent recycling system (MARS) and the extracorporeal liver assist device (ELAD)—were developed in the late 1990s. MARS consisted of a highly permeable hollow fiber membrane mixed with albumin, and ELAD consisted of porcine hepatocytes attached to microcarriers in the extracapillary space of the hollow fiber membrane. Both systems allowed for transfer of water-soluble and protein-bound toxins in the blood across the membrane and into the dialysate.59 The clinical benefit offered by these devices is controversial,60–62 thus limiting their use to experimental purposes only.
Hepatocyte transplant
Use of hepatocyte transplant as a bridge to liver transplant was tested in 1970s, first in rats and later in humans.63 By reducing the blood ammonia level and improving cerebral perfusion pressure and cardiac function, replacement of 1% to 2% of the total liver cell mass by transplanted hepatocytes acts as a bridge to orthotopic liver transplant.64,65
PROGNOSIS
Different criteria have been used to identify patients with poor prognosis who may eventually need to undergo liver transplant.
The King’s College criteria system is the most commonly used for prognosis (Table 4).37,66–69 Its main drawback is that it is applicable only in patients with encephalopathy, and when patients reach this stage, their condition often deteriorates rapidly, and they die while awaiting liver transplant.37,66,67
The Model for End-Stage Liver Disease (MELD) score is an alternative to the King’s College criteria. A high MELD score on admission signifies advanced disease, and patients with a high MELD score tend to have a worse prognosis than those with a low score.68
The Acute Physiology and Chronic Health Evaluation (APACHE) II score can also be used, as it is more sensitive than the King’s College criteria.6
The Clichy criteria66,69 can also be used.
Liver biopsy. In addition to helping establish the cause of acute liver failure, liver biopsy can also be used as a prognostic tool. Hepatocellular necrosis greater than 70% on the biopsy predicts death with a specificity of 90% and a sensitivity of 56%.70
Hypophosphatemia has been reported to indicate recovering liver function in patients with acute liver failure.71 As the liver regenerates, its energy requirement increases. To supply the energy, adenosine triphosphate production increases, and phosphorus shifts from the extracellular to the intracellular compartment to meet the need for extra phosphorus during this process. A serum phosphorus level of 2.9 mg/dL or higher appears to indicate a poor prognosis in patients with acute liver failure, as it signifies that adequate hepatocyte regeneration is not occurring.
- Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41:1179–1197.
- O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993; 342:273–275.
- Ostapowicz G, Fontana RJ, Schiodt FV, et al; US Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947–954.
- Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology 2008; 47:1401–1415.
- Acharya SK, Panda SK, Saxena A, Gupta SD. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol 2000; 15:473–479.
- Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Patten CJ, Thomas PE, Guy RL, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 1993; 6:511–518.
- Chen W, Koenigs LL, Thompson SJ, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 1998; 11:295-301.
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 1973; 187:211–217.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med 2010; 77:19–27.
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest 1983; 71:980–991.
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93.
- Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med 1997; 337:1112–1117.
- Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994; 272:1845–1850.
- Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135:1924–1934 e1–4
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology 2010; 52:2065–2076.
- Stevens T, Qadri A, Zein NN. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann Intern Med 2005; 142:477–478.
- Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: a curable disease by 2024? J Hepatol 2015; 62(suppl 1):S112–S120.
- Schiodt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol 2003; 98:448–453.
- Charlton M, Adjei P, Poterucha J, et al. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology 1998; 28:839–842.
- Bismuth H, Samuel D, Gugenheim J, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med 1987; 107:337–341.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876.
- Davern TJ 2nd, James LP, Hinson JA, et al; Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 2006; 130:687–694.
- Perry HE, Shannon MW. Efficacy of oral versus intravenous N-acetylcysteine in acetaminophen overdose: results of an open-label, clinical trial. J Pediatr 1998; 132:149–152.
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988; 319:1557–1562.
- Makin AJ, Wendon J, Williams R. A 7-year experience of severe acetaminophen-induced hepatotoxicity (1987-1993). Gastroenterology 1995; 109:1907–1916.
- Tsang SW, Chan HL, Leung NW, et al. Lamivudine treatment for fulminant hepatic failure due to acute exacerbation of chronic hepatitis B infection. Aliment Pharmacol Ther 2001; 15:1737–1744.
- Yu JW, Sun LJ, Yan BZ, Kang P, Zhao YH. Lamivudine treatment is associated with improved survival in fulminant hepatitis B. Liver Int 2011; 31:499–506.
- Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 2011; 53:774–780.
- Pinna AD, Rakela J, Demetris AJ, Fung JJ. Five cases of fulminant hepatitis due to herpes simplex virus in adults. Dig Dis Sci 2002; 47:750–754.
- Farr RW, Short S, Weissman D. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: report of two cases and review of the literature. Clin Infect Dis 1997; 24:1191–1194.
- Czaja AJ, Freese DK; American Association for the Study of Liver Disease. Diagnosis and treatment of autoimmune hepatitis. Hepatology 2002; 36:479–497.
- Roberts EA, Schilsky ML. A practice guideline on Wilson disease. Hepatology 2003; 37:1475–1492.
- Berman DH, Leventhal RI, Gavaler JS, Cadoff EM, Van Thiel DH. Clinical differentiation of fulminant Wilsonian hepatitis from other causes of hepatic failure. Gastroenterology 1991; 100:1129–1134.
- Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137:856–864.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445.
- Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999; 29:648–653.
- Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 1992; 15:449–453.
- Haussinger D, Laubenberger J, vom Dahl S, et al. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology 1994; 107:1475–1480.
- Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet 1993; 341:157–158.
- Cordoba J, Gottstein J, Blei AT. Chronic hyponatremia exacerbates ammonia-induced brain edema in rats after portacaval anastomosis. J Hepatol 1998; 29:589–594.
- Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut 1982; 23:625–629.
- Jalan R, SW OD, Deutz NE, Lee A, Hayes PC. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet 1999; 354:1164–1168.
- Rose C, Michalak A, Rao KV, Quack G, Kircheis G, Butterworth RF. L-ornithine-L-aspartate lowers plasma and cerebrospinal fluid ammonia and prevents brain edema in rats with acute liver failure. Hepatology 1999; 30:636–640.
- Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 2009; 136:2159–2168.
- Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure—a controlled clinical trial. J Hepatol 2004; 41:89–96.
- Canalese J, Gove CD, Gimson AE, Wilkinson SP, Wardle EN, Williams R. Reticuloendothelial system and hepatocytic function in fulminant hepatic failure. Gut 1982; 23:265–269.
- Rolando N, Harvey F, Brahm J, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology 1990; 11:49–53.
- Rolando N, Wade JJ, Stangou A, et al. Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg 1996; 2:8–13.
- Rolando N, Harvey F, Brahm J, et al. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol 1991; 12:1–9.
- Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology 2003; 125:755–764.
- Rolando N, Philpott-Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis 1996; 16:389–402.
- Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000; 32:734–739.
- Rolando N, Gimson A, Wade J, Philpott- Howard J, Casewell M, Williams R. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 1993; 17:196–201.
- Karvellas CJ, Cavazos J, Battenhouse H, et al; US Acute Liver Failure Study Group. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol 2014; 12:1942–1949.
- Acharya SK, Dasarathy S, Kumer TL, et al. Fulminant hepatitis in a tropical population: clinical course, cause, and early predictors of outcome. Hepatology 1996; 23:1148–1155.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- MacDougall BR, Williams R. H2-receptor antagonist in the prevention of acute gastrointestinal hemorrhage in fulminant hepatic failure: a controlled trial. Gastroenterology 1978; 74:464–465.
- Stange J, Mitzner SR, Risler T, et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs 1999; 23:319–330.
- Vaid A, Chewich H, Balk EM, Jaber BL. Molecular adsorbent recirculating system as artificial support therapy for liver failure: a meta-analysis. ASAIO J 2012; 58:51–59.
- Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl 2004; 10:1099–1106.
- Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003; 289:217–222.
- Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc 1979; 11:578–584.
- Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology 1988; 8:1006–1009.
- Strom SC, Fisher RA, Thompson MT, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 1997; 63:559–569.
- Pauwels A, Mostefa-Kara N, Florent C, Levy VG. Emergency liver transplantation for acute liver failure. Evaluation of London and Clichy criteria. J Hepatol 1993; 17:124–127.
- Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol 1997; 26:62–68.
- Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology 2007; 45:789–796.
- Bernuau J, Goudeau A, Poynard T, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology 1986; 6:648–651.
- Donaldson BW, Gopinath R, Wanless IR, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology 1993; 18:1370–1376.
- Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology 2002; 36:659–665.
When the liver fails, it usually fails gradually. The sudden (acute) onset of liver failure, while less common, demands prompt management, with transfer to an intensive care unit, specific treatment depending on the cause, and consideration of liver transplant, without which the mortality rate is high.
This article reviews the definition, epidemiology, etiology, and management of acute liver failure.
DEFINITIONS
Acute liver failure is defined as a syndrome of acute hepatitis with evidence of abnormal coagulation (eg, an international normalized ratio > 1.5) complicated by the development of mental alteration (encephalopathy) within 26 weeks of the onset of illness in a patient without a history of liver disease.1 In general, patients have no evidence of underlying chronic liver disease, but there are exceptions; patients with Wilson disease, vertically acquired hepatitis B virus infection, or autoimmune hepatitis can present with acute liver failure superimposed on chronic liver disease or even cirrhosis.
The term acute liver failure has replaced older terms such as fulminant hepatic failure, hyperacute liver failure, and subacute liver failure, which were used for prognostic purposes. Patients with hyperacute liver failure (defined as development of encephalopathy within 7 days of onset of illness) generally have a good prognosis with medical management, whereas those with subacute liver failure (defined as development of encephalopathy within 5 to 26 weeks of onset of illness) have a poor prognosis without liver transplant.2,3
NEARLY 2,000 CASES A YEAR
There are nearly 2,000 cases of acute liver failure each year in the United States, and it accounts for 6% of all deaths due to liver disease.4 It is more common in women than in men, and more common in white people than in other races. The peak incidence is at a fairly young age, ie, 35 to 45 years.
CAUSES
The most common cause of acute liver failure in the United States and other Western countries is acetaminophen toxicity, followed by viral hepatitis. In contrast, viral hepatitis is the most common cause in developing countries.5
Acetaminophen toxicity
Patients with acetaminophen-induced liver failure tend to be younger than other patients with acute liver failure.1 Nearly half of them present after intentionally taking a single large dose, while the rest present with unintentional toxicity while taking acetaminophen for pain relief on a long-term basis and ingesting more than the recommended dose.6
After ingestion, 52% to 57% of acetaminophen is converted to glucuronide conjugates, and 30% to 44% is converted to sulfate conjugates. These compounds are nontoxic, water-soluble, and rapidly excreted in the urine.
However, about 5% to 10% of ingested acetaminophen is shunted to the cytochrome P450 system. P450 2E1 is the main isoenzyme involved in acetaminophen metabolism, but 1A2, 3A4, and 2A6 also contribute.7,8 P450 2E1 is the same isoenzyme responsible for ethanol metabolism and is inducible. Thus, regular alcohol consumption can increase P450 2E1 activity, setting the stage under certain circumstances for increased acetaminophen metabolism through this pathway.
Metabolism of acetaminophen through the cytochrome P450 pathway results in production of N-acetyl-p-benzoquinone imine (NAPQI), the compound that damages the liver. NAPQI is rendered nontoxic by binding to glutathione, forming NAPQI-glutathione adducts. Glutathione capacity is limited, however. With too much acetaminophen, glutathione becomes depleted and NAPQI accumulates, binds with proteins to form adducts, and leads to necrosis of hepatocytes (Figure 1).9,10
Acetylcysteine, used in treating acetaminophen toxicity, is a substrate for glutathione synthesis and ultimately increases the amount of glutathione available to bind NAPQI and prevent damage to hepatocytes.11
Acetaminophen is a dose-related toxin. Most ingestions leading to acute liver failure exceed 10 g/day (> 150 mg/kg/day). Moderate chronic ingestion, eg, 4 g/day, usually leads to transient mild elevation of liver enzymes in healthy individuals12 but can in rare cases cause acute liver failure.13
Whitcomb and Block14 retrospectively identified 49 patients who presented with acetaminophen-induced hepatotoxicity in 1987 through 1993; 21 (43%) had been taking acetaminophen for therapeutic purposes. All 49 patients took more than the recommended limit of 4 g/day, many of them while fasting and some while using alcohol. Acute liver failure was seen with ingestion of more than 12 g/day—or more than 10 g/day in alcohol users. The authors attributed the increased risk to activation of cytochrome P450 2E1 by alcohol and depletion of glutathione stores by starvation or alcohol abuse.
Advice to patients taking acetaminophen is given in Table 1.
Other drugs and supplements
A number of other drugs and herbal supplements can also cause acute liver failure (Table 2), the most common being antimicrobial and antiepileptic drugs.15 Of the antimicrobials, antitubercular drugs (especially isoniazid) are believed to be the most common causes, followed by trimethoprim-sulfamethoxazole. Phenytoin is the antiepileptic drug most often implicated in acute liver failure.
Statins can also cause acute liver failure, especially when combined with other hepatotoxic agents.16
The herbal supplements and weight-loss agents Hydroxycut and Herbalife have both been reported to cause acute liver failure, with patients presenting with either the hepatocellular or the cholestatic pattern of liver injury.17 The exact chemical in these supplements that causes liver injury has not yet been determined.
The National Institutes of Health maintains a database of cases of liver failure due to medications and supplements at livertox.nih.gov. The database includes the pattern of hepatic injury, mechanism of injury, management, and outcomes.
Viral hepatitis
Hepatitis B virus is the most common viral cause of acute liver failure and is responsible for about 8% of cases.18
Patients with chronic hepatitis B virus infection—as evidenced by positive hepatitis B surface antigen—can develop acute liver failure if the infection is reactivated by the use of immunosuppressive drugs for solid-organ or bone-marrow transplant or medications such as anti-tumor necrosis agents, rituximab, or chemotherapy. These patients should be treated prophylactically with a nucleoside analogue, which should be continued for 6 months after immunosuppressive therapy is completed.
Hepatitis A virus is responsible for about 4% of cases.18
Hepatitis C virus rarely causes acute liver failure, especially in the absence of hepatitis A and hepatitis B.3,19
Hepatitis E virus, which is endemic in areas of Asia and Africa, can cause liver disease in pregnant women and in young adults who have concomitant liver disease from another cause. It tends to cause acute liver failure more frequently in pregnant women than in the rest of the population and carries a mortality rate of more than 20% in this subgroup.
TT (transfusion-transmitted) virus was reported in the 1990s to cause acute liver failure in about 27% of patients in whom no other cause could be found.20
Other rare viral causes of acute liver failure include Epstein-Barr virus, cytomegalovirus, and herpes simplex virus types 1, 2, and 6.
Other causes
Other causes of acute liver failure include ischemic hepatitis, autoimmune hepatitis, Wilson disease, Budd-Chiari syndrome, and HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome.
MANY PATIENTS NEED LIVER TRANSPLANT
Many patients with acute liver failure ultimately require orthotopic liver transplant,21 especially if they present with severe encephalopathy. Other aspects of treatment vary according to the cause of liver failure (Table 3).
SPECIFIC MANAGEMENT
Management of acetaminophen toxicity
If the time of ingestion is known, checking the acetaminophen level can help determine the cause of acute liver failure and also predict the risk of hepatotoxicity, based on the work of Rumack and Matthew.22 Calculators are available, eg, http://reference.medscape.com/calculator/acetaminophen-toxicity.
If a patient presents with acute liver failure several days after ingesting acetaminophen, the level can be in the nontoxic range, however. In this scenario, measuring acetaminophen-protein adducts can help establish acetaminophen toxicity as the cause, as the adducts last longer in the serum and provide 100% sensitivity and specificity.23 While most laboratories can rapidly measure acetaminophen levels, only a few can measure acetaminophen-protein adducts, and thus this test is not used clinically.
Acetylcysteine is the main drug used for acetaminophen toxicity. Ideally, it should be given within 8 hours of acetaminophen ingestion, but giving it later is also useful.1
Acetylcysteine is available in oral and intravenous forms, the latter for patients who have encephalopathy or cannot tolerate oral intake due to repeated episodes of vomiting.24,25 The oral form is much less costly and is thus preferred over intravenous acetylcysteine in patients who can tolerate oral intake. Intravenous acetylcysteine should be given in a loading dose of 150 mg/kg in 5% dextrose over 15 minutes, followed by a maintenance dose of 50 mg/kg over 4 hours and then 100 mg/kg given over 16 hours.1 No dose adjustment is needed in patients who have renal toxicity (acetaminophen can also be toxic to the kidneys).
Most patients with acetaminophen-induced liver failure survive with medical management alone and do not need a liver transplant.3,26 Cirrhosis does not occur in these patients.
Management of viral acute liver failure
When patients present with acute liver failure, it is necessary to look for a viral cause by serologic testing, including hepatitis A virus IgM antibody, hepatitis B surface antigen, and hepatitis B core IgM antibody.
Hepatitis B can become reactivated in immunocompromised patients, and therefore the hepatitis B virus DNA level should be checked. Detection of hepatitis B virus DNA in a patient previously known to have undetectable hepatitis B virus DNA confirms hepatitis B reactivation.
Patients with hepatitis B-induced acute liver failure should be treated with entecavir or tenofovir. Although this treatment may not change the course of acute liver failure or accelerate the recovery, it can prevent reinfection in the transplanted liver if liver transplant becomes indicated.27–29
Herpes simplex virus should be suspected in patients presenting with anicteric hepatitis with fever. Polymerase chain reaction testing for herpes simplex virus should be done,30 and if positive, patients should be given intravenous acyclovir.31 Despite treatment, herpes simplex virus disease is associated with a very poor prognosis without liver transplant.
Autoimmune hepatitis
The autoantibodies usually seen in autoimmune hepatitis are antinuclear antibody, antismooth muscle antibody, and anti-liver-kidney microsomal antibody, and patients need to be tested for them.
The diagnosis of autoimmune hepatitis can be challenging, as these autoimmune markers can be negative in 5% of patients. Liver biopsy becomes essential to establish the diagnosis in that setting.32
Guidelines advise starting prednisone 40 to 60 mg/day and placing the patient on the liver transplant list.1
Wilson disease
Although it is an uncommon cause of liver failure, Wilson disease needs special attention because it has a poor prognosis. The mortality rate in acute liver failure from Wilson disease reaches 100% without liver transplant.
Wilson disease is caused by a genetic defect that allows copper to accumulate in the liver and other organs. However, diagnosing Wilson disease as the cause of acute liver failure can be challenging because elevated serum and urine copper levels are not specific to Wilson disease and can be seen in patients with acute liver failure from any cause. In addition, the ceruloplasmin level is usually normal or high because it is an acute-phase reactant. Accumulation of copper in the liver parenchyma is usually patchy; therefore, qualitative copper staining on random liver biopsy samples provides low diagnostic yield. Quantitative copper on liver biopsy is the gold standard test to establish the diagnosis, but the test is time-consuming. Kayser-Fleischer rings around the iris are considered pathognomic for Wilson disease when seen with acute liver failure, but they are seen in only about 50% of patients.33
A unique feature of acute Wilson disease is that most patients have very high bilirubin levels and low alkaline phosphatase levels. An alkaline phosphatase-to-bilirubin ratio less than 2 in patients with acute liver failure is highly suggestive of Wilson disease.34
Another clue to the diagnosis is that patients with Wilson disease tend to develop Coombs-negative hemolytic anemia, which leads to a disproportionate elevation in aminotransferase levels, with aspartate aminotransferase being higher than alanine aminotransferase.
Once Wilson disease is suspected, the patient should be listed for liver transplant because death is almost certain without it. For patients awaiting liver transplant, the American Association for the Study of Liver Diseases guidelines recommend certain measures to lower the serum copper level such as albumin dialysis, continuous hemofiltration, plasmapheresis, and plasma exchange,1 but the evidence supporting their use is limited.
NONSPECIFIC MANAGEMENT
Acute liver failure can affect a number of organs and systems in addition to the liver (Figure 2).
General considerations
Because their condition can rapidly deteriorate, patients with acute liver failure are best managed in intensive care.
Patients who present to a center that does not have the facilities for liver transplant should be transferred to a transplant center as soon as possible, preferably by air. If the patient may not be able to protect the airway, endotracheal intubation should be performed before transfer.
The major causes of death in patients with acute liver failure are cerebral edema and infection. Gastrointestinal bleeding was a major cause of death in the past, but with prophylactic use of histamine H2 receptor blockers and proton pump inhibitors, the incidence of gastrointestinal bleeding has been significantly reduced.
Although initially used only in patients with acetaminophen-induced liver failure, acetylcysteine has also shown benefit in patients with acute liver failure from other causes. In patients with grade 1 or 2 encephalopathy on a scale of 0 (minimal) to 4 (comatose), the transplant-free survival rate is higher when acetylcysteine is given compared with placebo, but this benefit does not extend to patients with a higher grade of encephalopathy.35
Cerebral edema and intracranial hypertension
Cerebral edema is the leading cause of death in patients with acute liver failure, and it develops in nearly 40% of patients.36
The mechanism by which cerebral edema develops is not well understood. Some have proposed that ammonia is converted to glutamine, which causes cerebral edema either directly by its osmotic effect37,38 or indirectly by decreasing other osmolytes, thereby promoting water retention.39
Cerebral edema leads to intracranial hypertension, which can ultimately cause cerebral herniation and death. Because of the high mortality rate associated with cerebral edema, invasive devices were extensively used in the past to monitor intracranial pressure. However, in light of known complications of these devices, including bleeding,40 and lack of evidence of long-term benefit in terms of mortality rates, their use has come under debate.
Treatments. Many treatments are available for cerebral edema and intracranial hypertension. The first step is to elevate the head of the bed about 30 degrees. In addition, hyponatremia should be corrected, as it can worsen cerebral edema.41 If patients are intubated, maintaining a hypercapneic state is advisable to decrease the intracranial pressure.
Of the two pharmacologic options, mannitol is more often used.42 It is given as a bolus dose of 0.5 to 1 g/kg intravenously if the serum osmolality is less than 320 mOsm/L.1 Given the risk of fluid overload with mannitol, caution must be exercised in patients with renal dysfunction. The other pharmacologic option is 3% hypertonic saline.
Therapeutic hypothermia is a newer treatment for cerebral edema. Lowering the body temperature to 32 to 33°C (89.6 to 91.4°F) using cooling blankets decreases intracranial pressure and cerebral blood flow and improves the cerebral perfusion pressure.43 With this treatment, patients should be closely monitored for side effects of infection, coagulopathy, and cardiac arrythmias.1
l-ornithine l-aspartate was successfully used to prevent brain edema in rats, but in humans, no benefit was seen compared with placebo.44,45 The underlying basis for this experimental treatment is that supplemental ornithine and aspartate should increase glutamate synthesis, which should increase the activity of enzyme glutamine synthetase in skeletal muscles. With the increase in enzyme activity, conversion of ammonia to glutamine should increase, thereby decreasing ammonia circulation and thus decreasing cerebral edema.
Patients with cerebral edema have a high incidence of seizures, but prophylactic antiseizure medications such as phenytoin have not been proven to be beneficial.46
Infection
Nearly 80% of patients with acute liver failure develop an infectious complication, which can be attributed to a state of immunodeficiency.47
The respiratory and urinary tracts are the most common sources of infection.48 In patients with bacteremia, Enterococcus species and coagulase-negative Staphylococcus species49 are the commonly isolated organisms. Also, in patients with acute liver failure, fungal infections account for 30% of all infections.50
Infected patients often develop worsening of their encephalopathy51 without fever or elevated white blood cell count.49,52 Thus, in any patient in whom encephalopathy is worsening, an evaluation must be done to rule out infection. In these patients, systemic inflammatory response syndrome is an independent risk factor for death.53
Despite the high mortality rate with infection, whether using antibiotics prophylactically in acute liver failure is beneficial is controversial.54,55
Gastrointestinal bleeding
The current prevalence of upper gastrointestinal bleeding in acute liver failure patients is about 1.5%.56 Coagulopathy and endotracheal intubation are the main risk factors for upper gastrointestinal bleeding in these patients.57 The most common source of bleeding is stress ulcers in the stomach. The ulcers develop from a combination of factors, including decreased blood flow to the mucosa causing ischemia and hypoperfusion-reperfusion injury.
Pharmacologic inhibition of gastric acid secretion has been shown to reduce upper gastrointestinal bleeding in acute liver failure. A histamine H2 receptor blocker or proton pump inhibitor should be given to prevent gastrointestinal bleeding in patients with acute liver failure.1,58
EXPERIMENTAL TREATMENTS
Artificial liver support systems
Membranes and dialysate solutions have been developed to remove toxic substances that are normally metabolized by the liver. Two of these—the molecular adsorbent recycling system (MARS) and the extracorporeal liver assist device (ELAD)—were developed in the late 1990s. MARS consisted of a highly permeable hollow fiber membrane mixed with albumin, and ELAD consisted of porcine hepatocytes attached to microcarriers in the extracapillary space of the hollow fiber membrane. Both systems allowed for transfer of water-soluble and protein-bound toxins in the blood across the membrane and into the dialysate.59 The clinical benefit offered by these devices is controversial,60–62 thus limiting their use to experimental purposes only.
Hepatocyte transplant
Use of hepatocyte transplant as a bridge to liver transplant was tested in 1970s, first in rats and later in humans.63 By reducing the blood ammonia level and improving cerebral perfusion pressure and cardiac function, replacement of 1% to 2% of the total liver cell mass by transplanted hepatocytes acts as a bridge to orthotopic liver transplant.64,65
PROGNOSIS
Different criteria have been used to identify patients with poor prognosis who may eventually need to undergo liver transplant.
The King’s College criteria system is the most commonly used for prognosis (Table 4).37,66–69 Its main drawback is that it is applicable only in patients with encephalopathy, and when patients reach this stage, their condition often deteriorates rapidly, and they die while awaiting liver transplant.37,66,67
The Model for End-Stage Liver Disease (MELD) score is an alternative to the King’s College criteria. A high MELD score on admission signifies advanced disease, and patients with a high MELD score tend to have a worse prognosis than those with a low score.68
The Acute Physiology and Chronic Health Evaluation (APACHE) II score can also be used, as it is more sensitive than the King’s College criteria.6
The Clichy criteria66,69 can also be used.
Liver biopsy. In addition to helping establish the cause of acute liver failure, liver biopsy can also be used as a prognostic tool. Hepatocellular necrosis greater than 70% on the biopsy predicts death with a specificity of 90% and a sensitivity of 56%.70
Hypophosphatemia has been reported to indicate recovering liver function in patients with acute liver failure.71 As the liver regenerates, its energy requirement increases. To supply the energy, adenosine triphosphate production increases, and phosphorus shifts from the extracellular to the intracellular compartment to meet the need for extra phosphorus during this process. A serum phosphorus level of 2.9 mg/dL or higher appears to indicate a poor prognosis in patients with acute liver failure, as it signifies that adequate hepatocyte regeneration is not occurring.
When the liver fails, it usually fails gradually. The sudden (acute) onset of liver failure, while less common, demands prompt management, with transfer to an intensive care unit, specific treatment depending on the cause, and consideration of liver transplant, without which the mortality rate is high.
This article reviews the definition, epidemiology, etiology, and management of acute liver failure.
DEFINITIONS
Acute liver failure is defined as a syndrome of acute hepatitis with evidence of abnormal coagulation (eg, an international normalized ratio > 1.5) complicated by the development of mental alteration (encephalopathy) within 26 weeks of the onset of illness in a patient without a history of liver disease.1 In general, patients have no evidence of underlying chronic liver disease, but there are exceptions; patients with Wilson disease, vertically acquired hepatitis B virus infection, or autoimmune hepatitis can present with acute liver failure superimposed on chronic liver disease or even cirrhosis.
The term acute liver failure has replaced older terms such as fulminant hepatic failure, hyperacute liver failure, and subacute liver failure, which were used for prognostic purposes. Patients with hyperacute liver failure (defined as development of encephalopathy within 7 days of onset of illness) generally have a good prognosis with medical management, whereas those with subacute liver failure (defined as development of encephalopathy within 5 to 26 weeks of onset of illness) have a poor prognosis without liver transplant.2,3
NEARLY 2,000 CASES A YEAR
There are nearly 2,000 cases of acute liver failure each year in the United States, and it accounts for 6% of all deaths due to liver disease.4 It is more common in women than in men, and more common in white people than in other races. The peak incidence is at a fairly young age, ie, 35 to 45 years.
CAUSES
The most common cause of acute liver failure in the United States and other Western countries is acetaminophen toxicity, followed by viral hepatitis. In contrast, viral hepatitis is the most common cause in developing countries.5
Acetaminophen toxicity
Patients with acetaminophen-induced liver failure tend to be younger than other patients with acute liver failure.1 Nearly half of them present after intentionally taking a single large dose, while the rest present with unintentional toxicity while taking acetaminophen for pain relief on a long-term basis and ingesting more than the recommended dose.6
After ingestion, 52% to 57% of acetaminophen is converted to glucuronide conjugates, and 30% to 44% is converted to sulfate conjugates. These compounds are nontoxic, water-soluble, and rapidly excreted in the urine.
However, about 5% to 10% of ingested acetaminophen is shunted to the cytochrome P450 system. P450 2E1 is the main isoenzyme involved in acetaminophen metabolism, but 1A2, 3A4, and 2A6 also contribute.7,8 P450 2E1 is the same isoenzyme responsible for ethanol metabolism and is inducible. Thus, regular alcohol consumption can increase P450 2E1 activity, setting the stage under certain circumstances for increased acetaminophen metabolism through this pathway.
Metabolism of acetaminophen through the cytochrome P450 pathway results in production of N-acetyl-p-benzoquinone imine (NAPQI), the compound that damages the liver. NAPQI is rendered nontoxic by binding to glutathione, forming NAPQI-glutathione adducts. Glutathione capacity is limited, however. With too much acetaminophen, glutathione becomes depleted and NAPQI accumulates, binds with proteins to form adducts, and leads to necrosis of hepatocytes (Figure 1).9,10
Acetylcysteine, used in treating acetaminophen toxicity, is a substrate for glutathione synthesis and ultimately increases the amount of glutathione available to bind NAPQI and prevent damage to hepatocytes.11
Acetaminophen is a dose-related toxin. Most ingestions leading to acute liver failure exceed 10 g/day (> 150 mg/kg/day). Moderate chronic ingestion, eg, 4 g/day, usually leads to transient mild elevation of liver enzymes in healthy individuals12 but can in rare cases cause acute liver failure.13
Whitcomb and Block14 retrospectively identified 49 patients who presented with acetaminophen-induced hepatotoxicity in 1987 through 1993; 21 (43%) had been taking acetaminophen for therapeutic purposes. All 49 patients took more than the recommended limit of 4 g/day, many of them while fasting and some while using alcohol. Acute liver failure was seen with ingestion of more than 12 g/day—or more than 10 g/day in alcohol users. The authors attributed the increased risk to activation of cytochrome P450 2E1 by alcohol and depletion of glutathione stores by starvation or alcohol abuse.
Advice to patients taking acetaminophen is given in Table 1.
Other drugs and supplements
A number of other drugs and herbal supplements can also cause acute liver failure (Table 2), the most common being antimicrobial and antiepileptic drugs.15 Of the antimicrobials, antitubercular drugs (especially isoniazid) are believed to be the most common causes, followed by trimethoprim-sulfamethoxazole. Phenytoin is the antiepileptic drug most often implicated in acute liver failure.
Statins can also cause acute liver failure, especially when combined with other hepatotoxic agents.16
The herbal supplements and weight-loss agents Hydroxycut and Herbalife have both been reported to cause acute liver failure, with patients presenting with either the hepatocellular or the cholestatic pattern of liver injury.17 The exact chemical in these supplements that causes liver injury has not yet been determined.
The National Institutes of Health maintains a database of cases of liver failure due to medications and supplements at livertox.nih.gov. The database includes the pattern of hepatic injury, mechanism of injury, management, and outcomes.
Viral hepatitis
Hepatitis B virus is the most common viral cause of acute liver failure and is responsible for about 8% of cases.18
Patients with chronic hepatitis B virus infection—as evidenced by positive hepatitis B surface antigen—can develop acute liver failure if the infection is reactivated by the use of immunosuppressive drugs for solid-organ or bone-marrow transplant or medications such as anti-tumor necrosis agents, rituximab, or chemotherapy. These patients should be treated prophylactically with a nucleoside analogue, which should be continued for 6 months after immunosuppressive therapy is completed.
Hepatitis A virus is responsible for about 4% of cases.18
Hepatitis C virus rarely causes acute liver failure, especially in the absence of hepatitis A and hepatitis B.3,19
Hepatitis E virus, which is endemic in areas of Asia and Africa, can cause liver disease in pregnant women and in young adults who have concomitant liver disease from another cause. It tends to cause acute liver failure more frequently in pregnant women than in the rest of the population and carries a mortality rate of more than 20% in this subgroup.
TT (transfusion-transmitted) virus was reported in the 1990s to cause acute liver failure in about 27% of patients in whom no other cause could be found.20
Other rare viral causes of acute liver failure include Epstein-Barr virus, cytomegalovirus, and herpes simplex virus types 1, 2, and 6.
Other causes
Other causes of acute liver failure include ischemic hepatitis, autoimmune hepatitis, Wilson disease, Budd-Chiari syndrome, and HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome.
MANY PATIENTS NEED LIVER TRANSPLANT
Many patients with acute liver failure ultimately require orthotopic liver transplant,21 especially if they present with severe encephalopathy. Other aspects of treatment vary according to the cause of liver failure (Table 3).
SPECIFIC MANAGEMENT
Management of acetaminophen toxicity
If the time of ingestion is known, checking the acetaminophen level can help determine the cause of acute liver failure and also predict the risk of hepatotoxicity, based on the work of Rumack and Matthew.22 Calculators are available, eg, http://reference.medscape.com/calculator/acetaminophen-toxicity.
If a patient presents with acute liver failure several days after ingesting acetaminophen, the level can be in the nontoxic range, however. In this scenario, measuring acetaminophen-protein adducts can help establish acetaminophen toxicity as the cause, as the adducts last longer in the serum and provide 100% sensitivity and specificity.23 While most laboratories can rapidly measure acetaminophen levels, only a few can measure acetaminophen-protein adducts, and thus this test is not used clinically.
Acetylcysteine is the main drug used for acetaminophen toxicity. Ideally, it should be given within 8 hours of acetaminophen ingestion, but giving it later is also useful.1
Acetylcysteine is available in oral and intravenous forms, the latter for patients who have encephalopathy or cannot tolerate oral intake due to repeated episodes of vomiting.24,25 The oral form is much less costly and is thus preferred over intravenous acetylcysteine in patients who can tolerate oral intake. Intravenous acetylcysteine should be given in a loading dose of 150 mg/kg in 5% dextrose over 15 minutes, followed by a maintenance dose of 50 mg/kg over 4 hours and then 100 mg/kg given over 16 hours.1 No dose adjustment is needed in patients who have renal toxicity (acetaminophen can also be toxic to the kidneys).
Most patients with acetaminophen-induced liver failure survive with medical management alone and do not need a liver transplant.3,26 Cirrhosis does not occur in these patients.
Management of viral acute liver failure
When patients present with acute liver failure, it is necessary to look for a viral cause by serologic testing, including hepatitis A virus IgM antibody, hepatitis B surface antigen, and hepatitis B core IgM antibody.
Hepatitis B can become reactivated in immunocompromised patients, and therefore the hepatitis B virus DNA level should be checked. Detection of hepatitis B virus DNA in a patient previously known to have undetectable hepatitis B virus DNA confirms hepatitis B reactivation.
Patients with hepatitis B-induced acute liver failure should be treated with entecavir or tenofovir. Although this treatment may not change the course of acute liver failure or accelerate the recovery, it can prevent reinfection in the transplanted liver if liver transplant becomes indicated.27–29
Herpes simplex virus should be suspected in patients presenting with anicteric hepatitis with fever. Polymerase chain reaction testing for herpes simplex virus should be done,30 and if positive, patients should be given intravenous acyclovir.31 Despite treatment, herpes simplex virus disease is associated with a very poor prognosis without liver transplant.
Autoimmune hepatitis
The autoantibodies usually seen in autoimmune hepatitis are antinuclear antibody, antismooth muscle antibody, and anti-liver-kidney microsomal antibody, and patients need to be tested for them.
The diagnosis of autoimmune hepatitis can be challenging, as these autoimmune markers can be negative in 5% of patients. Liver biopsy becomes essential to establish the diagnosis in that setting.32
Guidelines advise starting prednisone 40 to 60 mg/day and placing the patient on the liver transplant list.1
Wilson disease
Although it is an uncommon cause of liver failure, Wilson disease needs special attention because it has a poor prognosis. The mortality rate in acute liver failure from Wilson disease reaches 100% without liver transplant.
Wilson disease is caused by a genetic defect that allows copper to accumulate in the liver and other organs. However, diagnosing Wilson disease as the cause of acute liver failure can be challenging because elevated serum and urine copper levels are not specific to Wilson disease and can be seen in patients with acute liver failure from any cause. In addition, the ceruloplasmin level is usually normal or high because it is an acute-phase reactant. Accumulation of copper in the liver parenchyma is usually patchy; therefore, qualitative copper staining on random liver biopsy samples provides low diagnostic yield. Quantitative copper on liver biopsy is the gold standard test to establish the diagnosis, but the test is time-consuming. Kayser-Fleischer rings around the iris are considered pathognomic for Wilson disease when seen with acute liver failure, but they are seen in only about 50% of patients.33
A unique feature of acute Wilson disease is that most patients have very high bilirubin levels and low alkaline phosphatase levels. An alkaline phosphatase-to-bilirubin ratio less than 2 in patients with acute liver failure is highly suggestive of Wilson disease.34
Another clue to the diagnosis is that patients with Wilson disease tend to develop Coombs-negative hemolytic anemia, which leads to a disproportionate elevation in aminotransferase levels, with aspartate aminotransferase being higher than alanine aminotransferase.
Once Wilson disease is suspected, the patient should be listed for liver transplant because death is almost certain without it. For patients awaiting liver transplant, the American Association for the Study of Liver Diseases guidelines recommend certain measures to lower the serum copper level such as albumin dialysis, continuous hemofiltration, plasmapheresis, and plasma exchange,1 but the evidence supporting their use is limited.
NONSPECIFIC MANAGEMENT
Acute liver failure can affect a number of organs and systems in addition to the liver (Figure 2).
General considerations
Because their condition can rapidly deteriorate, patients with acute liver failure are best managed in intensive care.
Patients who present to a center that does not have the facilities for liver transplant should be transferred to a transplant center as soon as possible, preferably by air. If the patient may not be able to protect the airway, endotracheal intubation should be performed before transfer.
The major causes of death in patients with acute liver failure are cerebral edema and infection. Gastrointestinal bleeding was a major cause of death in the past, but with prophylactic use of histamine H2 receptor blockers and proton pump inhibitors, the incidence of gastrointestinal bleeding has been significantly reduced.
Although initially used only in patients with acetaminophen-induced liver failure, acetylcysteine has also shown benefit in patients with acute liver failure from other causes. In patients with grade 1 or 2 encephalopathy on a scale of 0 (minimal) to 4 (comatose), the transplant-free survival rate is higher when acetylcysteine is given compared with placebo, but this benefit does not extend to patients with a higher grade of encephalopathy.35
Cerebral edema and intracranial hypertension
Cerebral edema is the leading cause of death in patients with acute liver failure, and it develops in nearly 40% of patients.36
The mechanism by which cerebral edema develops is not well understood. Some have proposed that ammonia is converted to glutamine, which causes cerebral edema either directly by its osmotic effect37,38 or indirectly by decreasing other osmolytes, thereby promoting water retention.39
Cerebral edema leads to intracranial hypertension, which can ultimately cause cerebral herniation and death. Because of the high mortality rate associated with cerebral edema, invasive devices were extensively used in the past to monitor intracranial pressure. However, in light of known complications of these devices, including bleeding,40 and lack of evidence of long-term benefit in terms of mortality rates, their use has come under debate.
Treatments. Many treatments are available for cerebral edema and intracranial hypertension. The first step is to elevate the head of the bed about 30 degrees. In addition, hyponatremia should be corrected, as it can worsen cerebral edema.41 If patients are intubated, maintaining a hypercapneic state is advisable to decrease the intracranial pressure.
Of the two pharmacologic options, mannitol is more often used.42 It is given as a bolus dose of 0.5 to 1 g/kg intravenously if the serum osmolality is less than 320 mOsm/L.1 Given the risk of fluid overload with mannitol, caution must be exercised in patients with renal dysfunction. The other pharmacologic option is 3% hypertonic saline.
Therapeutic hypothermia is a newer treatment for cerebral edema. Lowering the body temperature to 32 to 33°C (89.6 to 91.4°F) using cooling blankets decreases intracranial pressure and cerebral blood flow and improves the cerebral perfusion pressure.43 With this treatment, patients should be closely monitored for side effects of infection, coagulopathy, and cardiac arrythmias.1
l-ornithine l-aspartate was successfully used to prevent brain edema in rats, but in humans, no benefit was seen compared with placebo.44,45 The underlying basis for this experimental treatment is that supplemental ornithine and aspartate should increase glutamate synthesis, which should increase the activity of enzyme glutamine synthetase in skeletal muscles. With the increase in enzyme activity, conversion of ammonia to glutamine should increase, thereby decreasing ammonia circulation and thus decreasing cerebral edema.
Patients with cerebral edema have a high incidence of seizures, but prophylactic antiseizure medications such as phenytoin have not been proven to be beneficial.46
Infection
Nearly 80% of patients with acute liver failure develop an infectious complication, which can be attributed to a state of immunodeficiency.47
The respiratory and urinary tracts are the most common sources of infection.48 In patients with bacteremia, Enterococcus species and coagulase-negative Staphylococcus species49 are the commonly isolated organisms. Also, in patients with acute liver failure, fungal infections account for 30% of all infections.50
Infected patients often develop worsening of their encephalopathy51 without fever or elevated white blood cell count.49,52 Thus, in any patient in whom encephalopathy is worsening, an evaluation must be done to rule out infection. In these patients, systemic inflammatory response syndrome is an independent risk factor for death.53
Despite the high mortality rate with infection, whether using antibiotics prophylactically in acute liver failure is beneficial is controversial.54,55
Gastrointestinal bleeding
The current prevalence of upper gastrointestinal bleeding in acute liver failure patients is about 1.5%.56 Coagulopathy and endotracheal intubation are the main risk factors for upper gastrointestinal bleeding in these patients.57 The most common source of bleeding is stress ulcers in the stomach. The ulcers develop from a combination of factors, including decreased blood flow to the mucosa causing ischemia and hypoperfusion-reperfusion injury.
Pharmacologic inhibition of gastric acid secretion has been shown to reduce upper gastrointestinal bleeding in acute liver failure. A histamine H2 receptor blocker or proton pump inhibitor should be given to prevent gastrointestinal bleeding in patients with acute liver failure.1,58
EXPERIMENTAL TREATMENTS
Artificial liver support systems
Membranes and dialysate solutions have been developed to remove toxic substances that are normally metabolized by the liver. Two of these—the molecular adsorbent recycling system (MARS) and the extracorporeal liver assist device (ELAD)—were developed in the late 1990s. MARS consisted of a highly permeable hollow fiber membrane mixed with albumin, and ELAD consisted of porcine hepatocytes attached to microcarriers in the extracapillary space of the hollow fiber membrane. Both systems allowed for transfer of water-soluble and protein-bound toxins in the blood across the membrane and into the dialysate.59 The clinical benefit offered by these devices is controversial,60–62 thus limiting their use to experimental purposes only.
Hepatocyte transplant
Use of hepatocyte transplant as a bridge to liver transplant was tested in 1970s, first in rats and later in humans.63 By reducing the blood ammonia level and improving cerebral perfusion pressure and cardiac function, replacement of 1% to 2% of the total liver cell mass by transplanted hepatocytes acts as a bridge to orthotopic liver transplant.64,65
PROGNOSIS
Different criteria have been used to identify patients with poor prognosis who may eventually need to undergo liver transplant.
The King’s College criteria system is the most commonly used for prognosis (Table 4).37,66–69 Its main drawback is that it is applicable only in patients with encephalopathy, and when patients reach this stage, their condition often deteriorates rapidly, and they die while awaiting liver transplant.37,66,67
The Model for End-Stage Liver Disease (MELD) score is an alternative to the King’s College criteria. A high MELD score on admission signifies advanced disease, and patients with a high MELD score tend to have a worse prognosis than those with a low score.68
The Acute Physiology and Chronic Health Evaluation (APACHE) II score can also be used, as it is more sensitive than the King’s College criteria.6
The Clichy criteria66,69 can also be used.
Liver biopsy. In addition to helping establish the cause of acute liver failure, liver biopsy can also be used as a prognostic tool. Hepatocellular necrosis greater than 70% on the biopsy predicts death with a specificity of 90% and a sensitivity of 56%.70
Hypophosphatemia has been reported to indicate recovering liver function in patients with acute liver failure.71 As the liver regenerates, its energy requirement increases. To supply the energy, adenosine triphosphate production increases, and phosphorus shifts from the extracellular to the intracellular compartment to meet the need for extra phosphorus during this process. A serum phosphorus level of 2.9 mg/dL or higher appears to indicate a poor prognosis in patients with acute liver failure, as it signifies that adequate hepatocyte regeneration is not occurring.
- Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41:1179–1197.
- O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993; 342:273–275.
- Ostapowicz G, Fontana RJ, Schiodt FV, et al; US Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947–954.
- Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology 2008; 47:1401–1415.
- Acharya SK, Panda SK, Saxena A, Gupta SD. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol 2000; 15:473–479.
- Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Patten CJ, Thomas PE, Guy RL, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 1993; 6:511–518.
- Chen W, Koenigs LL, Thompson SJ, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 1998; 11:295-301.
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 1973; 187:211–217.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med 2010; 77:19–27.
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest 1983; 71:980–991.
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93.
- Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med 1997; 337:1112–1117.
- Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994; 272:1845–1850.
- Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135:1924–1934 e1–4
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology 2010; 52:2065–2076.
- Stevens T, Qadri A, Zein NN. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann Intern Med 2005; 142:477–478.
- Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: a curable disease by 2024? J Hepatol 2015; 62(suppl 1):S112–S120.
- Schiodt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol 2003; 98:448–453.
- Charlton M, Adjei P, Poterucha J, et al. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology 1998; 28:839–842.
- Bismuth H, Samuel D, Gugenheim J, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med 1987; 107:337–341.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876.
- Davern TJ 2nd, James LP, Hinson JA, et al; Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 2006; 130:687–694.
- Perry HE, Shannon MW. Efficacy of oral versus intravenous N-acetylcysteine in acetaminophen overdose: results of an open-label, clinical trial. J Pediatr 1998; 132:149–152.
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988; 319:1557–1562.
- Makin AJ, Wendon J, Williams R. A 7-year experience of severe acetaminophen-induced hepatotoxicity (1987-1993). Gastroenterology 1995; 109:1907–1916.
- Tsang SW, Chan HL, Leung NW, et al. Lamivudine treatment for fulminant hepatic failure due to acute exacerbation of chronic hepatitis B infection. Aliment Pharmacol Ther 2001; 15:1737–1744.
- Yu JW, Sun LJ, Yan BZ, Kang P, Zhao YH. Lamivudine treatment is associated with improved survival in fulminant hepatitis B. Liver Int 2011; 31:499–506.
- Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 2011; 53:774–780.
- Pinna AD, Rakela J, Demetris AJ, Fung JJ. Five cases of fulminant hepatitis due to herpes simplex virus in adults. Dig Dis Sci 2002; 47:750–754.
- Farr RW, Short S, Weissman D. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: report of two cases and review of the literature. Clin Infect Dis 1997; 24:1191–1194.
- Czaja AJ, Freese DK; American Association for the Study of Liver Disease. Diagnosis and treatment of autoimmune hepatitis. Hepatology 2002; 36:479–497.
- Roberts EA, Schilsky ML. A practice guideline on Wilson disease. Hepatology 2003; 37:1475–1492.
- Berman DH, Leventhal RI, Gavaler JS, Cadoff EM, Van Thiel DH. Clinical differentiation of fulminant Wilsonian hepatitis from other causes of hepatic failure. Gastroenterology 1991; 100:1129–1134.
- Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137:856–864.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445.
- Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999; 29:648–653.
- Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 1992; 15:449–453.
- Haussinger D, Laubenberger J, vom Dahl S, et al. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology 1994; 107:1475–1480.
- Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet 1993; 341:157–158.
- Cordoba J, Gottstein J, Blei AT. Chronic hyponatremia exacerbates ammonia-induced brain edema in rats after portacaval anastomosis. J Hepatol 1998; 29:589–594.
- Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut 1982; 23:625–629.
- Jalan R, SW OD, Deutz NE, Lee A, Hayes PC. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet 1999; 354:1164–1168.
- Rose C, Michalak A, Rao KV, Quack G, Kircheis G, Butterworth RF. L-ornithine-L-aspartate lowers plasma and cerebrospinal fluid ammonia and prevents brain edema in rats with acute liver failure. Hepatology 1999; 30:636–640.
- Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 2009; 136:2159–2168.
- Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure—a controlled clinical trial. J Hepatol 2004; 41:89–96.
- Canalese J, Gove CD, Gimson AE, Wilkinson SP, Wardle EN, Williams R. Reticuloendothelial system and hepatocytic function in fulminant hepatic failure. Gut 1982; 23:265–269.
- Rolando N, Harvey F, Brahm J, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology 1990; 11:49–53.
- Rolando N, Wade JJ, Stangou A, et al. Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg 1996; 2:8–13.
- Rolando N, Harvey F, Brahm J, et al. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol 1991; 12:1–9.
- Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology 2003; 125:755–764.
- Rolando N, Philpott-Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis 1996; 16:389–402.
- Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000; 32:734–739.
- Rolando N, Gimson A, Wade J, Philpott- Howard J, Casewell M, Williams R. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 1993; 17:196–201.
- Karvellas CJ, Cavazos J, Battenhouse H, et al; US Acute Liver Failure Study Group. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol 2014; 12:1942–1949.
- Acharya SK, Dasarathy S, Kumer TL, et al. Fulminant hepatitis in a tropical population: clinical course, cause, and early predictors of outcome. Hepatology 1996; 23:1148–1155.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- MacDougall BR, Williams R. H2-receptor antagonist in the prevention of acute gastrointestinal hemorrhage in fulminant hepatic failure: a controlled trial. Gastroenterology 1978; 74:464–465.
- Stange J, Mitzner SR, Risler T, et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs 1999; 23:319–330.
- Vaid A, Chewich H, Balk EM, Jaber BL. Molecular adsorbent recirculating system as artificial support therapy for liver failure: a meta-analysis. ASAIO J 2012; 58:51–59.
- Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl 2004; 10:1099–1106.
- Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003; 289:217–222.
- Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc 1979; 11:578–584.
- Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology 1988; 8:1006–1009.
- Strom SC, Fisher RA, Thompson MT, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 1997; 63:559–569.
- Pauwels A, Mostefa-Kara N, Florent C, Levy VG. Emergency liver transplantation for acute liver failure. Evaluation of London and Clichy criteria. J Hepatol 1993; 17:124–127.
- Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol 1997; 26:62–68.
- Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology 2007; 45:789–796.
- Bernuau J, Goudeau A, Poynard T, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology 1986; 6:648–651.
- Donaldson BW, Gopinath R, Wanless IR, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology 1993; 18:1370–1376.
- Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology 2002; 36:659–665.
- Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41:1179–1197.
- O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993; 342:273–275.
- Ostapowicz G, Fontana RJ, Schiodt FV, et al; US Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137:947–954.
- Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology 2008; 47:1401–1415.
- Acharya SK, Panda SK, Saxena A, Gupta SD. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol 2000; 15:473–479.
- Larson AM, Polson J, Fontana RJ, et al; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42:1364–1372.
- Patten CJ, Thomas PE, Guy RL, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 1993; 6:511–518.
- Chen W, Koenigs LL, Thompson SJ, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol 1998; 11:295-301.
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 1973; 187:211–217.
- Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med 2010; 77:19–27.
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest 1983; 71:980–991.
- Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006; 296:87–93.
- Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med 1997; 337:1112–1117.
- Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994; 272:1845–1850.
- Chalasani N, Fontana RJ, Bonkovsky HL, et al; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135:1924–1934 e1–4
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology 2010; 52:2065–2076.
- Stevens T, Qadri A, Zein NN. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann Intern Med 2005; 142:477–478.
- Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: a curable disease by 2024? J Hepatol 2015; 62(suppl 1):S112–S120.
- Schiodt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol 2003; 98:448–453.
- Charlton M, Adjei P, Poterucha J, et al. TT-virus infection in North American blood donors, patients with fulminant hepatic failure, and cryptogenic cirrhosis. Hepatology 1998; 28:839–842.
- Bismuth H, Samuel D, Gugenheim J, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med 1987; 107:337–341.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975; 55:871–876.
- Davern TJ 2nd, James LP, Hinson JA, et al; Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 2006; 130:687–694.
- Perry HE, Shannon MW. Efficacy of oral versus intravenous N-acetylcysteine in acetaminophen overdose: results of an open-label, clinical trial. J Pediatr 1998; 132:149–152.
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988; 319:1557–1562.
- Makin AJ, Wendon J, Williams R. A 7-year experience of severe acetaminophen-induced hepatotoxicity (1987-1993). Gastroenterology 1995; 109:1907–1916.
- Tsang SW, Chan HL, Leung NW, et al. Lamivudine treatment for fulminant hepatic failure due to acute exacerbation of chronic hepatitis B infection. Aliment Pharmacol Ther 2001; 15:1737–1744.
- Yu JW, Sun LJ, Yan BZ, Kang P, Zhao YH. Lamivudine treatment is associated with improved survival in fulminant hepatitis B. Liver Int 2011; 31:499–506.
- Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 2011; 53:774–780.
- Pinna AD, Rakela J, Demetris AJ, Fung JJ. Five cases of fulminant hepatitis due to herpes simplex virus in adults. Dig Dis Sci 2002; 47:750–754.
- Farr RW, Short S, Weissman D. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: report of two cases and review of the literature. Clin Infect Dis 1997; 24:1191–1194.
- Czaja AJ, Freese DK; American Association for the Study of Liver Disease. Diagnosis and treatment of autoimmune hepatitis. Hepatology 2002; 36:479–497.
- Roberts EA, Schilsky ML. A practice guideline on Wilson disease. Hepatology 2003; 37:1475–1492.
- Berman DH, Leventhal RI, Gavaler JS, Cadoff EM, Van Thiel DH. Clinical differentiation of fulminant Wilsonian hepatitis from other causes of hepatic failure. Gastroenterology 1991; 100:1129–1134.
- Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137:856–864.
- O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445.
- Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999; 29:648–653.
- Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 1992; 15:449–453.
- Haussinger D, Laubenberger J, vom Dahl S, et al. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology 1994; 107:1475–1480.
- Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet 1993; 341:157–158.
- Cordoba J, Gottstein J, Blei AT. Chronic hyponatremia exacerbates ammonia-induced brain edema in rats after portacaval anastomosis. J Hepatol 1998; 29:589–594.
- Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut 1982; 23:625–629.
- Jalan R, SW OD, Deutz NE, Lee A, Hayes PC. Moderate hypothermia for uncontrolled intracranial hypertension in acute liver failure. Lancet 1999; 354:1164–1168.
- Rose C, Michalak A, Rao KV, Quack G, Kircheis G, Butterworth RF. L-ornithine-L-aspartate lowers plasma and cerebrospinal fluid ammonia and prevents brain edema in rats with acute liver failure. Hepatology 1999; 30:636–640.
- Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK. Efficacy of L-ornithine L-aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology 2009; 136:2159–2168.
- Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure—a controlled clinical trial. J Hepatol 2004; 41:89–96.
- Canalese J, Gove CD, Gimson AE, Wilkinson SP, Wardle EN, Williams R. Reticuloendothelial system and hepatocytic function in fulminant hepatic failure. Gut 1982; 23:265–269.
- Rolando N, Harvey F, Brahm J, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology 1990; 11:49–53.
- Rolando N, Wade JJ, Stangou A, et al. Prospective study comparing the efficacy of prophylactic parenteral antimicrobials, with or without enteral decontamination, in patients with acute liver failure. Liver Transpl Surg 1996; 2:8–13.
- Rolando N, Harvey F, Brahm J, et al. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol 1991; 12:1–9.
- Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology 2003; 125:755–764.
- Rolando N, Philpott-Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis 1996; 16:389–402.
- Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000; 32:734–739.
- Rolando N, Gimson A, Wade J, Philpott- Howard J, Casewell M, Williams R. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 1993; 17:196–201.
- Karvellas CJ, Cavazos J, Battenhouse H, et al; US Acute Liver Failure Study Group. Effects of antimicrobial prophylaxis and blood stream infections in patients with acute liver failure: a retrospective cohort study. Clin Gastroenterol Hepatol 2014; 12:1942–1949.
- Acharya SK, Dasarathy S, Kumer TL, et al. Fulminant hepatitis in a tropical population: clinical course, cause, and early predictors of outcome. Hepatology 1996; 23:1148–1155.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- MacDougall BR, Williams R. H2-receptor antagonist in the prevention of acute gastrointestinal hemorrhage in fulminant hepatic failure: a controlled trial. Gastroenterology 1978; 74:464–465.
- Stange J, Mitzner SR, Risler T, et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs 1999; 23:319–330.
- Vaid A, Chewich H, Balk EM, Jaber BL. Molecular adsorbent recirculating system as artificial support therapy for liver failure: a meta-analysis. ASAIO J 2012; 58:51–59.
- Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl 2004; 10:1099–1106.
- Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003; 289:217–222.
- Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc 1979; 11:578–584.
- Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology 1988; 8:1006–1009.
- Strom SC, Fisher RA, Thompson MT, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 1997; 63:559–569.
- Pauwels A, Mostefa-Kara N, Florent C, Levy VG. Emergency liver transplantation for acute liver failure. Evaluation of London and Clichy criteria. J Hepatol 1993; 17:124–127.
- Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol 1997; 26:62–68.
- Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology 2007; 45:789–796.
- Bernuau J, Goudeau A, Poynard T, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology 1986; 6:648–651.
- Donaldson BW, Gopinath R, Wanless IR, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology 1993; 18:1370–1376.
- Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology 2002; 36:659–665.
KEY POINTS
- In the United States, the most common cause of acute liver failure is acetaminophen toxicity, followed by viral hepatitis.
- Testing for the cause of acute liver failure needs to start as soon as possible so that specific treatment can be initiated and the patient can be placed on the transplant list if needed.
- Acetylcysteine and either a proton pump inhibitor or a histamine H2 receptor blocker should be given to all patients with acute liver failure. Liver transplant is the cornerstone of therapy in patients not responding to other treatments.
- There are a number of prognostic scores for acute liver failure, but each has limitations.
Multiple linear subcutaneous nodules
A 34-year-old woman sought consultation at our clinic for an asymptomatic swelling on her right foot that had been growing very slowly over the last 15 years. She said she had presented to other healthcare facilities, but no diagnosis had been made and no treatment had been offered.
Examination revealed a linear swelling extending from the lower third to the mid-dorsal surface of the right foot (Figure 1). Palpation revealed multiple, closely set nodules arranged in a linear fashion. This finding along with the history raised the suspicion of neurofibroma and other conditions in the differential diagnosis, eg, pure neuritic Hansen disease, phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The rest of the mucocutaneous examination results were normal. No café-au-lait spots, axillary freckling, or other swelling suggestive of neurofibroma was seen. She had no family history of mucocutaneous disease or other systemic disorder.
Because of the suspicion of neurofibromatosis, slit-lamp examination of the eyes was done to rule out Lisch nodules, a common feature of neurofibromatosis; the results were normal. Plain radiography of the right foot showed only soft-tissue swelling. Magnetic resonance imaging with contrast, done to determine the extent of the lesions, revealed multiple dumbbell-shaped lesions with homogeneous enhancement (Figure 2). Histopathologic study of a biopsy specimen of the lesions showed tumor cells in the dermis. The cells were long, with elongated nuclei with pointed ends, arranged in long and short fascicles—an appearance characteristic of neurofibroma. Areas of hypocellularity and hypercellularity were seen, and on S100 protein immunostaining, the tumor cells showed strong nuclear and cytoplasmic positivity (Figure 3).
The histologic evaluation confirmed neurofibroma. The specific diagnosis of sporadic solitary neurofibroma was made based on the onset of the lesions, the number of lesions (one in this patient), and the absence of features suggestive of neurofibromatosis.
SPORADIC SOLITARY NEUROFIBROMA
Neurofibroma is a common tumor of the peripheral nerve sheath and, when present with features such as café-au-lait spots, axillary freckling, and characteristic bone changes, it is pathognomic of neurofibromatosis type 1.1 But solitary neurofibromas can occur sporadically in the absence of other features of neurofibromatosis.
Sporadic solitary neurofibroma arises from small nerves, is benign in nature, and carries a lower rate of malignant transformation than its counterpart that occurs in the setting of neurofibromatosis.2 Though sporadic solitary neurofibroma can occur in any part of the body, it is commonly seen on the head and neck, and occasionally on the presacral and parasacral space, thigh, intrascrotal area,3 the ankle and foot,4,5 and the subungual region.6 A series of 397 peripheral neural sheath tumors examined over 30 years showed 55 sporadic solitary neurofibromas occurring in the brachial plexus region, 45 in the upper extremities, 10 in the pelvic plexus, and 31 in the lower extremities.7
Management of sporadic solitary neurofibroma depends on the patient’s discomfort. For asymptomatic lesions, serial observation is all that is required. Complete surgical excision including the parent nerve is the treatment for large lesions. More research is needed to define the potential role of drugs such as pirfenidone and tipifarnib.
THE DIFFERENTIAL DIAGNOSIS
Sporadic solitary neurofibroma can masquerade as pure neuritic Hansen disease (leprosy), phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The absence of neural symptoms and no evidence of trophic changes exclude pure neuritic Hansen disease. Phaeohyphomycosis clinically presents as a single cyst that may evolve into pigmented plaques,8 and the diagnosis relies on the presence of fungus in tissue. The absence of cystic changes clinically and fungi histopathologically in this patient did not favor phaeohyphomycosis. Palisaded neutrophilic granulomatous dermatitis is characterized clinically by cordlike skin lesions (the “rope sign”) and is accompanied by extracutaneous, mostly articular features. Histopathologically, it shows intense neutrophilic infiltrate and interstitial histiocytic infiltrate along with collagen degeneration. The absence of extracutaneous and classical histologic features negated this possibility in this patient.
Though sporotrichosis and cutaneous atypical mycobacterial infections may present in linear fashion following the course of the lymphatic vessels, the absence of epidermal changes after a disease course of 15 years and the absence of granulomatous infiltrate in histopathology excluded these possibilities in this patient.
The patient was referred to a plastic surgeon, and the lesions were successfully resected. She did not return for additional review after that.
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014; 13:834–843.
- Pulathan Z, Imamoglu M, Cay A, Guven YK. Intermittent claudication due to right common femoral artery compression by a solitary neurofibroma. Eur J Pediatr 2005; 164:463–465.
- Hosseini MM, Geramizadeh B, Shakeri S, Karimi MH. Intrascrotal solitary neurofibroma: a case report and review of the literature. Urol Ann 2012; 4:119–121.
- Carvajal JA, Cuartas E, Qadir R, Levi AD, Temple HT. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int 2011; 32:163–167.
- Tahririan MA, Hekmatnia A, Ahrar H, Heidarpour M, Hekmatnia F. Solitary giant neurofibroma of thigh. Adv Biomed Res 2014; 3:158.
- Huajun J, Wei Q, Ming L, Chongyang F, Weiguo Z, Decheng L. Solitary subungual neurofibroma in the right first finger. Int J Dermatol 2012; 51:335–338.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102:246–255.
- Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis 2009; 22:559–563.
A 34-year-old woman sought consultation at our clinic for an asymptomatic swelling on her right foot that had been growing very slowly over the last 15 years. She said she had presented to other healthcare facilities, but no diagnosis had been made and no treatment had been offered.
Examination revealed a linear swelling extending from the lower third to the mid-dorsal surface of the right foot (Figure 1). Palpation revealed multiple, closely set nodules arranged in a linear fashion. This finding along with the history raised the suspicion of neurofibroma and other conditions in the differential diagnosis, eg, pure neuritic Hansen disease, phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The rest of the mucocutaneous examination results were normal. No café-au-lait spots, axillary freckling, or other swelling suggestive of neurofibroma was seen. She had no family history of mucocutaneous disease or other systemic disorder.
Because of the suspicion of neurofibromatosis, slit-lamp examination of the eyes was done to rule out Lisch nodules, a common feature of neurofibromatosis; the results were normal. Plain radiography of the right foot showed only soft-tissue swelling. Magnetic resonance imaging with contrast, done to determine the extent of the lesions, revealed multiple dumbbell-shaped lesions with homogeneous enhancement (Figure 2). Histopathologic study of a biopsy specimen of the lesions showed tumor cells in the dermis. The cells were long, with elongated nuclei with pointed ends, arranged in long and short fascicles—an appearance characteristic of neurofibroma. Areas of hypocellularity and hypercellularity were seen, and on S100 protein immunostaining, the tumor cells showed strong nuclear and cytoplasmic positivity (Figure 3).
The histologic evaluation confirmed neurofibroma. The specific diagnosis of sporadic solitary neurofibroma was made based on the onset of the lesions, the number of lesions (one in this patient), and the absence of features suggestive of neurofibromatosis.
SPORADIC SOLITARY NEUROFIBROMA
Neurofibroma is a common tumor of the peripheral nerve sheath and, when present with features such as café-au-lait spots, axillary freckling, and characteristic bone changes, it is pathognomic of neurofibromatosis type 1.1 But solitary neurofibromas can occur sporadically in the absence of other features of neurofibromatosis.
Sporadic solitary neurofibroma arises from small nerves, is benign in nature, and carries a lower rate of malignant transformation than its counterpart that occurs in the setting of neurofibromatosis.2 Though sporadic solitary neurofibroma can occur in any part of the body, it is commonly seen on the head and neck, and occasionally on the presacral and parasacral space, thigh, intrascrotal area,3 the ankle and foot,4,5 and the subungual region.6 A series of 397 peripheral neural sheath tumors examined over 30 years showed 55 sporadic solitary neurofibromas occurring in the brachial plexus region, 45 in the upper extremities, 10 in the pelvic plexus, and 31 in the lower extremities.7
Management of sporadic solitary neurofibroma depends on the patient’s discomfort. For asymptomatic lesions, serial observation is all that is required. Complete surgical excision including the parent nerve is the treatment for large lesions. More research is needed to define the potential role of drugs such as pirfenidone and tipifarnib.
THE DIFFERENTIAL DIAGNOSIS
Sporadic solitary neurofibroma can masquerade as pure neuritic Hansen disease (leprosy), phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The absence of neural symptoms and no evidence of trophic changes exclude pure neuritic Hansen disease. Phaeohyphomycosis clinically presents as a single cyst that may evolve into pigmented plaques,8 and the diagnosis relies on the presence of fungus in tissue. The absence of cystic changes clinically and fungi histopathologically in this patient did not favor phaeohyphomycosis. Palisaded neutrophilic granulomatous dermatitis is characterized clinically by cordlike skin lesions (the “rope sign”) and is accompanied by extracutaneous, mostly articular features. Histopathologically, it shows intense neutrophilic infiltrate and interstitial histiocytic infiltrate along with collagen degeneration. The absence of extracutaneous and classical histologic features negated this possibility in this patient.
Though sporotrichosis and cutaneous atypical mycobacterial infections may present in linear fashion following the course of the lymphatic vessels, the absence of epidermal changes after a disease course of 15 years and the absence of granulomatous infiltrate in histopathology excluded these possibilities in this patient.
The patient was referred to a plastic surgeon, and the lesions were successfully resected. She did not return for additional review after that.
A 34-year-old woman sought consultation at our clinic for an asymptomatic swelling on her right foot that had been growing very slowly over the last 15 years. She said she had presented to other healthcare facilities, but no diagnosis had been made and no treatment had been offered.
Examination revealed a linear swelling extending from the lower third to the mid-dorsal surface of the right foot (Figure 1). Palpation revealed multiple, closely set nodules arranged in a linear fashion. This finding along with the history raised the suspicion of neurofibroma and other conditions in the differential diagnosis, eg, pure neuritic Hansen disease, phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The rest of the mucocutaneous examination results were normal. No café-au-lait spots, axillary freckling, or other swelling suggestive of neurofibroma was seen. She had no family history of mucocutaneous disease or other systemic disorder.
Because of the suspicion of neurofibromatosis, slit-lamp examination of the eyes was done to rule out Lisch nodules, a common feature of neurofibromatosis; the results were normal. Plain radiography of the right foot showed only soft-tissue swelling. Magnetic resonance imaging with contrast, done to determine the extent of the lesions, revealed multiple dumbbell-shaped lesions with homogeneous enhancement (Figure 2). Histopathologic study of a biopsy specimen of the lesions showed tumor cells in the dermis. The cells were long, with elongated nuclei with pointed ends, arranged in long and short fascicles—an appearance characteristic of neurofibroma. Areas of hypocellularity and hypercellularity were seen, and on S100 protein immunostaining, the tumor cells showed strong nuclear and cytoplasmic positivity (Figure 3).
The histologic evaluation confirmed neurofibroma. The specific diagnosis of sporadic solitary neurofibroma was made based on the onset of the lesions, the number of lesions (one in this patient), and the absence of features suggestive of neurofibromatosis.
SPORADIC SOLITARY NEUROFIBROMA
Neurofibroma is a common tumor of the peripheral nerve sheath and, when present with features such as café-au-lait spots, axillary freckling, and characteristic bone changes, it is pathognomic of neurofibromatosis type 1.1 But solitary neurofibromas can occur sporadically in the absence of other features of neurofibromatosis.
Sporadic solitary neurofibroma arises from small nerves, is benign in nature, and carries a lower rate of malignant transformation than its counterpart that occurs in the setting of neurofibromatosis.2 Though sporadic solitary neurofibroma can occur in any part of the body, it is commonly seen on the head and neck, and occasionally on the presacral and parasacral space, thigh, intrascrotal area,3 the ankle and foot,4,5 and the subungual region.6 A series of 397 peripheral neural sheath tumors examined over 30 years showed 55 sporadic solitary neurofibromas occurring in the brachial plexus region, 45 in the upper extremities, 10 in the pelvic plexus, and 31 in the lower extremities.7
Management of sporadic solitary neurofibroma depends on the patient’s discomfort. For asymptomatic lesions, serial observation is all that is required. Complete surgical excision including the parent nerve is the treatment for large lesions. More research is needed to define the potential role of drugs such as pirfenidone and tipifarnib.
THE DIFFERENTIAL DIAGNOSIS
Sporadic solitary neurofibroma can masquerade as pure neuritic Hansen disease (leprosy), phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The absence of neural symptoms and no evidence of trophic changes exclude pure neuritic Hansen disease. Phaeohyphomycosis clinically presents as a single cyst that may evolve into pigmented plaques,8 and the diagnosis relies on the presence of fungus in tissue. The absence of cystic changes clinically and fungi histopathologically in this patient did not favor phaeohyphomycosis. Palisaded neutrophilic granulomatous dermatitis is characterized clinically by cordlike skin lesions (the “rope sign”) and is accompanied by extracutaneous, mostly articular features. Histopathologically, it shows intense neutrophilic infiltrate and interstitial histiocytic infiltrate along with collagen degeneration. The absence of extracutaneous and classical histologic features negated this possibility in this patient.
Though sporotrichosis and cutaneous atypical mycobacterial infections may present in linear fashion following the course of the lymphatic vessels, the absence of epidermal changes after a disease course of 15 years and the absence of granulomatous infiltrate in histopathology excluded these possibilities in this patient.
The patient was referred to a plastic surgeon, and the lesions were successfully resected. She did not return for additional review after that.
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014; 13:834–843.
- Pulathan Z, Imamoglu M, Cay A, Guven YK. Intermittent claudication due to right common femoral artery compression by a solitary neurofibroma. Eur J Pediatr 2005; 164:463–465.
- Hosseini MM, Geramizadeh B, Shakeri S, Karimi MH. Intrascrotal solitary neurofibroma: a case report and review of the literature. Urol Ann 2012; 4:119–121.
- Carvajal JA, Cuartas E, Qadir R, Levi AD, Temple HT. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int 2011; 32:163–167.
- Tahririan MA, Hekmatnia A, Ahrar H, Heidarpour M, Hekmatnia F. Solitary giant neurofibroma of thigh. Adv Biomed Res 2014; 3:158.
- Huajun J, Wei Q, Ming L, Chongyang F, Weiguo Z, Decheng L. Solitary subungual neurofibroma in the right first finger. Int J Dermatol 2012; 51:335–338.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102:246–255.
- Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis 2009; 22:559–563.
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014; 13:834–843.
- Pulathan Z, Imamoglu M, Cay A, Guven YK. Intermittent claudication due to right common femoral artery compression by a solitary neurofibroma. Eur J Pediatr 2005; 164:463–465.
- Hosseini MM, Geramizadeh B, Shakeri S, Karimi MH. Intrascrotal solitary neurofibroma: a case report and review of the literature. Urol Ann 2012; 4:119–121.
- Carvajal JA, Cuartas E, Qadir R, Levi AD, Temple HT. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int 2011; 32:163–167.
- Tahririan MA, Hekmatnia A, Ahrar H, Heidarpour M, Hekmatnia F. Solitary giant neurofibroma of thigh. Adv Biomed Res 2014; 3:158.
- Huajun J, Wei Q, Ming L, Chongyang F, Weiguo Z, Decheng L. Solitary subungual neurofibroma in the right first finger. Int J Dermatol 2012; 51:335–338.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102:246–255.
- Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis 2009; 22:559–563.