User login
When Should Harm-Reduction Strategies Be Used for Inpatients with Opioid Misuse?
Case
A 33-year-old male with a history of opioid overdose and opioid use disorder is admitted with IV heroin use complicated by injection site cellulitis. He is started on antibiotics with improvement in his cellulitis; however, his hospitalization is complicated by acute opioid withdrawal. Despite his history of opioid overdose and opioid use disorder, he has never seen a substance use disorder specialist nor received any education or treatment for his addiction. He reports that he will stop using illicit drugs but declines any further addiction treatment.
What strategies can be employed to reduce his risk of future harm from opioid misuse?
Background
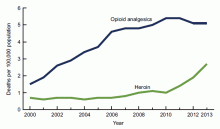
Over the past decade, the U.S. has experienced a rapid increase in the rates of opioid prescriptions and opioid misuse.1 Consequently, the number of ED visits and hospitalizations for opioid-related complications has also increased.2 Many complications result from the practice of injection drug use (IDU), which predisposes individuals to serious blood-borne viral infections such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV) as well as bacterial infections such as infective endocarditis. In addition, individuals who misuse opioids are at risk of death related to opioid overdose. In 2013, there were more than 24,000 deaths in the U.S. due to opioid overdose (see Figure 1).3
In response to the opioid epidemic, there have been a number of local, state, and federal public health initiatives to monitor and secure the opioid drug supply, improve treatment resources, and promulgate harm-reduction interventions. At a more individual level, hospitalists have an important role to play in combating the opioid epidemic. As frontline providers, hospitalists have access to hospitalized individuals with opioid misuse who may not otherwise be exposed to the healthcare system. Therefore, inpatient hospitalizations serve as a unique and important opportunity to engage individuals in the management of their addiction.
There are a number of interventions that hospitalists and substance use disorder specialists can pursue. Psychiatric evaluation and initiation of medication-assisted treatment often aim to aid patients in abstaining from further opioid misuse. However, many individuals with opioid use disorder are not ready for treatment or experience relapses of opioid misuse despite treatment. Given this, a secondary goal is to reduce any harm that may result from opioid misuse. This is done through the implementation of harm-reduction strategies. These strategies include teaching safe injection practices, facilitating the use of syringe exchange programs, and providing opioid overdose education and naloxone distribution.
Overview of Data
Safe Injection Education. People who inject drugs are at risk for viral, bacterial, and fungal infections. These infections are often the result of nonsterile injection and may be minimized by the utilization of safe injection practices. In order to educate people who inject drugs on safe injection practices, the hospitalist must first understand the process involved in injecting drugs. In Table 1, the process of injecting heroin is outlined (of note, other illicit drugs can be injected, and processes may vary).4
As evidenced by Table 1, the process of sterile injection can be complicated, especially for an individual who may be withdrawing from opioids. Table 1 is also optimistic in that it recommends new and sterile products be used with every injection. If new and sterile equipment is not available, another option is to clean the equipment after every use, which can be done by using bleach and water. This may mitigate the risk of viral, bacterial, and fungal infections. However, the risk is still present, so users should not share or use another individual’s equipment even if it has been cleaned. Due to the risk of viral, bacterial, and fungal infections, all hospitalized individuals who inject drugs should receive education on safe injection practices.
Syringe Exchange Programs. IDU accounts for up to 15% of all new HIV infections and is the primary risk factor for the transmission of HCV.5 These infections occur when people inject using equipment contaminated with blood that contains HIV and/or HCV. Given this, if people who inject drugs could access and consistently use sterile syringes and other injection paraphernalia, the risk of transmitting blood-borne infections would be dramatically reduced. This is the concept behind syringe exchange programs (also known as needle exchange programs), which serve to increase access to sterile syringes while removing contaminated or used syringes from the community.
There is compelling evidence that syringe exchange programs decrease the rate of HIV transmission and likely reduce the rate of HCV transmission as well.6 In addition, syringe exchange programs often provide other beneficial services, such as counseling, testing, and prevention efforts for HIV, HCV, and sexually transmitted infections; distribution of condoms; and referrals to treatment services for substance use disorder.5
Unfortunately, in the U.S., restrictive state laws and lack of funding limit the number of established syringe exchange programs. According to the North American Syringe Exchange Network, there are only 226 programs in 33 states and the District of Columbia. Hospitalists and social workers should be aware of available local resources, including syringe exchange programs, and distribute this information to hospitalized individuals who inject drugs.
Opioid Overdose Education and Naloxone Distribution. Syringe exchange programs and safe injection education aim to reduce harm by decreasing the transmission of infections; however, they do not address the problem of deaths related to opioid overdose. The primary harm-reduction strategy used to address deaths related to opioid overdose in the U.S is opioid overdose education and naloxone distribution (OEND). Naloxone is an opioid antagonist that reverses the respiratory depression and decreased consciousness caused by opioids. The OEND strategy involves educating first responders— including individuals and friends and family of individuals who use opioids—to recognize the signs of an opioid overdose, seek help, provide rescue breathing, administer naloxone, and stay with the individual until emergency medical services arrive.7 This strategy has been observed to decrease rates of death related to opioid overdose.7
Given the evolving opioid epidemic and effectiveness of the OEND strategy, it is not surprising that the number of local opioid overdose prevention programs adopting OEND has risen dramatically. As of 2014, there were 140 organizations, with 644 local sites providing naloxone in 29 states and the District of Columbia. These organizations have distributed 152,000 naloxone kits and have reported more than 26,000 reversals.8 Certainly, OEND has prevented morbidity and mortality in some of these patients.
The adoption of OEND can be performed by individual prescribers as well. Naloxone is U.S. FDA-approved for the treatment of opioid overdose, and thus the liability to prescribers is similar to that of other FDA-approved drugs. However, the distribution of naloxone to third parties, such as friends and family of individuals with opioid misuse, is more complex and regulated by state laws. Many states have created liability protection for naloxone prescription to third parties. Individual state laws and additional information can be found at prescribetoprevent.org.
Hospitalists should provide opioid overdose education to all individuals with opioid misuse and friends and family of individuals with opioid misuse. In addition, hospitalists should prescribe naloxone to individuals with opioid misuse and, in states where the law allows, distribute naloxone to friends and family of individuals with opioid misuse as well.
Controversies. In general, opioid use disorder treatment providers; public health officials; and local, state, and federal government agencies have increasingly embraced harm-reduction strategies. However, some feel that harm-reduction strategies are misguided or even detrimental due to concern that they implicitly condone or enable the use of illicit substances. There have been a number of studies to evaluate the potential unintended consequences of harm-reduction strategies, and overwhelmingly, these have been either neutral or have shown the benefit of harm-reduction interventions. At this point, there is no good evidence to prevent the widespread adoption of harm-reduction strategies for hospitalists.
Back to the Case
The case involves an individual who has already had at least two complications of his IV heroin use, including cellulitis and opioid overdose. Ideally, this individual would be willing to see an addiction specialist and start medication-assisted treatment. Unfortunately, he is unwilling to be further evaluated by a specialist at this time. Regardless, he remains at risk of future complications, and it is the hospitalist’s responsibility to intervene with a goal of reducing future harm that may result from his IV heroin use.
The hospitalist in this case advises the patient to abstain from heroin and IDU, encourages him to seek treatment for his opioid use disorder, and gives him resources for linkage to care if he becomes interested. In addition, the hospitalist educates the patient on safe injection practices and provides a list of local syringe exchange programs to decrease future risk of viral, bacterial, and fungal infections. Furthermore, the hospitalist provides opioid overdose education and distributes naloxone to the patient, along with friends and family of the patient, to reduce the risk of death related to opioid overdose.
Bottom Line
Hospitalists should utilize harm-reduction interventions in individuals hospitalized with opioid misuse. TH
Dr. Theisen-Toupal is a hospitalist at the Veterans Affairs Medical Center and assistant professor of medicine at the George Washington University School of Medicine & Health Sciences, both in Washington, D.C.
References
- Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6043a4.htm. Published November 4, 2011.
- Drug abuse warning network, 2011: national estimates of drug-related emergency department visits. Substance Abuse and Mental Health Services Administration website. Available at: http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm#5. Accessed July 29, 2015.
- Hedergaard H, Chen LH, Warner M. Drug-poisoning deaths involving heroin: United States, 2000–2013. National Center for Health Statistics Data Brief. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/nchs/data/databriefs/db190.htm. Published March 2015.
- Getting off right: a safety manual for injection drug users. Harm Reduction Coalition website. Available at: http://harmreduction.org/wp-content/uploads/2011/12/getting-off-right.pdf.
- Syringe exchange programs—United States, 2008. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5945a4.htm/Syringe-Exchange-Programs-United-States-2008. Published November 19, 2010.
- Wodak A, Conney A. Effectiveness of sterile needle and syringe programming in reducing HIV/AIDS among injecting drug users. World Health Organization website. Available at: http://apps.who.int/iris/bitstream/10665/43107/1/9241591641.pdf. Published 2004.
- Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6423a2.htm. Published June 19, 2015.
Case
A 33-year-old male with a history of opioid overdose and opioid use disorder is admitted with IV heroin use complicated by injection site cellulitis. He is started on antibiotics with improvement in his cellulitis; however, his hospitalization is complicated by acute opioid withdrawal. Despite his history of opioid overdose and opioid use disorder, he has never seen a substance use disorder specialist nor received any education or treatment for his addiction. He reports that he will stop using illicit drugs but declines any further addiction treatment.
What strategies can be employed to reduce his risk of future harm from opioid misuse?
Background
Over the past decade, the U.S. has experienced a rapid increase in the rates of opioid prescriptions and opioid misuse.1 Consequently, the number of ED visits and hospitalizations for opioid-related complications has also increased.2 Many complications result from the practice of injection drug use (IDU), which predisposes individuals to serious blood-borne viral infections such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV) as well as bacterial infections such as infective endocarditis. In addition, individuals who misuse opioids are at risk of death related to opioid overdose. In 2013, there were more than 24,000 deaths in the U.S. due to opioid overdose (see Figure 1).3
In response to the opioid epidemic, there have been a number of local, state, and federal public health initiatives to monitor and secure the opioid drug supply, improve treatment resources, and promulgate harm-reduction interventions. At a more individual level, hospitalists have an important role to play in combating the opioid epidemic. As frontline providers, hospitalists have access to hospitalized individuals with opioid misuse who may not otherwise be exposed to the healthcare system. Therefore, inpatient hospitalizations serve as a unique and important opportunity to engage individuals in the management of their addiction.
There are a number of interventions that hospitalists and substance use disorder specialists can pursue. Psychiatric evaluation and initiation of medication-assisted treatment often aim to aid patients in abstaining from further opioid misuse. However, many individuals with opioid use disorder are not ready for treatment or experience relapses of opioid misuse despite treatment. Given this, a secondary goal is to reduce any harm that may result from opioid misuse. This is done through the implementation of harm-reduction strategies. These strategies include teaching safe injection practices, facilitating the use of syringe exchange programs, and providing opioid overdose education and naloxone distribution.
Overview of Data
Safe Injection Education. People who inject drugs are at risk for viral, bacterial, and fungal infections. These infections are often the result of nonsterile injection and may be minimized by the utilization of safe injection practices. In order to educate people who inject drugs on safe injection practices, the hospitalist must first understand the process involved in injecting drugs. In Table 1, the process of injecting heroin is outlined (of note, other illicit drugs can be injected, and processes may vary).4
As evidenced by Table 1, the process of sterile injection can be complicated, especially for an individual who may be withdrawing from opioids. Table 1 is also optimistic in that it recommends new and sterile products be used with every injection. If new and sterile equipment is not available, another option is to clean the equipment after every use, which can be done by using bleach and water. This may mitigate the risk of viral, bacterial, and fungal infections. However, the risk is still present, so users should not share or use another individual’s equipment even if it has been cleaned. Due to the risk of viral, bacterial, and fungal infections, all hospitalized individuals who inject drugs should receive education on safe injection practices.
Syringe Exchange Programs. IDU accounts for up to 15% of all new HIV infections and is the primary risk factor for the transmission of HCV.5 These infections occur when people inject using equipment contaminated with blood that contains HIV and/or HCV. Given this, if people who inject drugs could access and consistently use sterile syringes and other injection paraphernalia, the risk of transmitting blood-borne infections would be dramatically reduced. This is the concept behind syringe exchange programs (also known as needle exchange programs), which serve to increase access to sterile syringes while removing contaminated or used syringes from the community.
There is compelling evidence that syringe exchange programs decrease the rate of HIV transmission and likely reduce the rate of HCV transmission as well.6 In addition, syringe exchange programs often provide other beneficial services, such as counseling, testing, and prevention efforts for HIV, HCV, and sexually transmitted infections; distribution of condoms; and referrals to treatment services for substance use disorder.5
Unfortunately, in the U.S., restrictive state laws and lack of funding limit the number of established syringe exchange programs. According to the North American Syringe Exchange Network, there are only 226 programs in 33 states and the District of Columbia. Hospitalists and social workers should be aware of available local resources, including syringe exchange programs, and distribute this information to hospitalized individuals who inject drugs.
Opioid Overdose Education and Naloxone Distribution. Syringe exchange programs and safe injection education aim to reduce harm by decreasing the transmission of infections; however, they do not address the problem of deaths related to opioid overdose. The primary harm-reduction strategy used to address deaths related to opioid overdose in the U.S is opioid overdose education and naloxone distribution (OEND). Naloxone is an opioid antagonist that reverses the respiratory depression and decreased consciousness caused by opioids. The OEND strategy involves educating first responders— including individuals and friends and family of individuals who use opioids—to recognize the signs of an opioid overdose, seek help, provide rescue breathing, administer naloxone, and stay with the individual until emergency medical services arrive.7 This strategy has been observed to decrease rates of death related to opioid overdose.7
Given the evolving opioid epidemic and effectiveness of the OEND strategy, it is not surprising that the number of local opioid overdose prevention programs adopting OEND has risen dramatically. As of 2014, there were 140 organizations, with 644 local sites providing naloxone in 29 states and the District of Columbia. These organizations have distributed 152,000 naloxone kits and have reported more than 26,000 reversals.8 Certainly, OEND has prevented morbidity and mortality in some of these patients.
The adoption of OEND can be performed by individual prescribers as well. Naloxone is U.S. FDA-approved for the treatment of opioid overdose, and thus the liability to prescribers is similar to that of other FDA-approved drugs. However, the distribution of naloxone to third parties, such as friends and family of individuals with opioid misuse, is more complex and regulated by state laws. Many states have created liability protection for naloxone prescription to third parties. Individual state laws and additional information can be found at prescribetoprevent.org.
Hospitalists should provide opioid overdose education to all individuals with opioid misuse and friends and family of individuals with opioid misuse. In addition, hospitalists should prescribe naloxone to individuals with opioid misuse and, in states where the law allows, distribute naloxone to friends and family of individuals with opioid misuse as well.
Controversies. In general, opioid use disorder treatment providers; public health officials; and local, state, and federal government agencies have increasingly embraced harm-reduction strategies. However, some feel that harm-reduction strategies are misguided or even detrimental due to concern that they implicitly condone or enable the use of illicit substances. There have been a number of studies to evaluate the potential unintended consequences of harm-reduction strategies, and overwhelmingly, these have been either neutral or have shown the benefit of harm-reduction interventions. At this point, there is no good evidence to prevent the widespread adoption of harm-reduction strategies for hospitalists.
Back to the Case
The case involves an individual who has already had at least two complications of his IV heroin use, including cellulitis and opioid overdose. Ideally, this individual would be willing to see an addiction specialist and start medication-assisted treatment. Unfortunately, he is unwilling to be further evaluated by a specialist at this time. Regardless, he remains at risk of future complications, and it is the hospitalist’s responsibility to intervene with a goal of reducing future harm that may result from his IV heroin use.
The hospitalist in this case advises the patient to abstain from heroin and IDU, encourages him to seek treatment for his opioid use disorder, and gives him resources for linkage to care if he becomes interested. In addition, the hospitalist educates the patient on safe injection practices and provides a list of local syringe exchange programs to decrease future risk of viral, bacterial, and fungal infections. Furthermore, the hospitalist provides opioid overdose education and distributes naloxone to the patient, along with friends and family of the patient, to reduce the risk of death related to opioid overdose.
Bottom Line
Hospitalists should utilize harm-reduction interventions in individuals hospitalized with opioid misuse. TH
Dr. Theisen-Toupal is a hospitalist at the Veterans Affairs Medical Center and assistant professor of medicine at the George Washington University School of Medicine & Health Sciences, both in Washington, D.C.
References
- Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6043a4.htm. Published November 4, 2011.
- Drug abuse warning network, 2011: national estimates of drug-related emergency department visits. Substance Abuse and Mental Health Services Administration website. Available at: http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm#5. Accessed July 29, 2015.
- Hedergaard H, Chen LH, Warner M. Drug-poisoning deaths involving heroin: United States, 2000–2013. National Center for Health Statistics Data Brief. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/nchs/data/databriefs/db190.htm. Published March 2015.
- Getting off right: a safety manual for injection drug users. Harm Reduction Coalition website. Available at: http://harmreduction.org/wp-content/uploads/2011/12/getting-off-right.pdf.
- Syringe exchange programs—United States, 2008. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5945a4.htm/Syringe-Exchange-Programs-United-States-2008. Published November 19, 2010.
- Wodak A, Conney A. Effectiveness of sterile needle and syringe programming in reducing HIV/AIDS among injecting drug users. World Health Organization website. Available at: http://apps.who.int/iris/bitstream/10665/43107/1/9241591641.pdf. Published 2004.
- Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6423a2.htm. Published June 19, 2015.
Case
A 33-year-old male with a history of opioid overdose and opioid use disorder is admitted with IV heroin use complicated by injection site cellulitis. He is started on antibiotics with improvement in his cellulitis; however, his hospitalization is complicated by acute opioid withdrawal. Despite his history of opioid overdose and opioid use disorder, he has never seen a substance use disorder specialist nor received any education or treatment for his addiction. He reports that he will stop using illicit drugs but declines any further addiction treatment.
What strategies can be employed to reduce his risk of future harm from opioid misuse?
Background
Over the past decade, the U.S. has experienced a rapid increase in the rates of opioid prescriptions and opioid misuse.1 Consequently, the number of ED visits and hospitalizations for opioid-related complications has also increased.2 Many complications result from the practice of injection drug use (IDU), which predisposes individuals to serious blood-borne viral infections such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV) as well as bacterial infections such as infective endocarditis. In addition, individuals who misuse opioids are at risk of death related to opioid overdose. In 2013, there were more than 24,000 deaths in the U.S. due to opioid overdose (see Figure 1).3
In response to the opioid epidemic, there have been a number of local, state, and federal public health initiatives to monitor and secure the opioid drug supply, improve treatment resources, and promulgate harm-reduction interventions. At a more individual level, hospitalists have an important role to play in combating the opioid epidemic. As frontline providers, hospitalists have access to hospitalized individuals with opioid misuse who may not otherwise be exposed to the healthcare system. Therefore, inpatient hospitalizations serve as a unique and important opportunity to engage individuals in the management of their addiction.
There are a number of interventions that hospitalists and substance use disorder specialists can pursue. Psychiatric evaluation and initiation of medication-assisted treatment often aim to aid patients in abstaining from further opioid misuse. However, many individuals with opioid use disorder are not ready for treatment or experience relapses of opioid misuse despite treatment. Given this, a secondary goal is to reduce any harm that may result from opioid misuse. This is done through the implementation of harm-reduction strategies. These strategies include teaching safe injection practices, facilitating the use of syringe exchange programs, and providing opioid overdose education and naloxone distribution.
Overview of Data
Safe Injection Education. People who inject drugs are at risk for viral, bacterial, and fungal infections. These infections are often the result of nonsterile injection and may be minimized by the utilization of safe injection practices. In order to educate people who inject drugs on safe injection practices, the hospitalist must first understand the process involved in injecting drugs. In Table 1, the process of injecting heroin is outlined (of note, other illicit drugs can be injected, and processes may vary).4
As evidenced by Table 1, the process of sterile injection can be complicated, especially for an individual who may be withdrawing from opioids. Table 1 is also optimistic in that it recommends new and sterile products be used with every injection. If new and sterile equipment is not available, another option is to clean the equipment after every use, which can be done by using bleach and water. This may mitigate the risk of viral, bacterial, and fungal infections. However, the risk is still present, so users should not share or use another individual’s equipment even if it has been cleaned. Due to the risk of viral, bacterial, and fungal infections, all hospitalized individuals who inject drugs should receive education on safe injection practices.
Syringe Exchange Programs. IDU accounts for up to 15% of all new HIV infections and is the primary risk factor for the transmission of HCV.5 These infections occur when people inject using equipment contaminated with blood that contains HIV and/or HCV. Given this, if people who inject drugs could access and consistently use sterile syringes and other injection paraphernalia, the risk of transmitting blood-borne infections would be dramatically reduced. This is the concept behind syringe exchange programs (also known as needle exchange programs), which serve to increase access to sterile syringes while removing contaminated or used syringes from the community.
There is compelling evidence that syringe exchange programs decrease the rate of HIV transmission and likely reduce the rate of HCV transmission as well.6 In addition, syringe exchange programs often provide other beneficial services, such as counseling, testing, and prevention efforts for HIV, HCV, and sexually transmitted infections; distribution of condoms; and referrals to treatment services for substance use disorder.5
Unfortunately, in the U.S., restrictive state laws and lack of funding limit the number of established syringe exchange programs. According to the North American Syringe Exchange Network, there are only 226 programs in 33 states and the District of Columbia. Hospitalists and social workers should be aware of available local resources, including syringe exchange programs, and distribute this information to hospitalized individuals who inject drugs.
Opioid Overdose Education and Naloxone Distribution. Syringe exchange programs and safe injection education aim to reduce harm by decreasing the transmission of infections; however, they do not address the problem of deaths related to opioid overdose. The primary harm-reduction strategy used to address deaths related to opioid overdose in the U.S is opioid overdose education and naloxone distribution (OEND). Naloxone is an opioid antagonist that reverses the respiratory depression and decreased consciousness caused by opioids. The OEND strategy involves educating first responders— including individuals and friends and family of individuals who use opioids—to recognize the signs of an opioid overdose, seek help, provide rescue breathing, administer naloxone, and stay with the individual until emergency medical services arrive.7 This strategy has been observed to decrease rates of death related to opioid overdose.7
Given the evolving opioid epidemic and effectiveness of the OEND strategy, it is not surprising that the number of local opioid overdose prevention programs adopting OEND has risen dramatically. As of 2014, there were 140 organizations, with 644 local sites providing naloxone in 29 states and the District of Columbia. These organizations have distributed 152,000 naloxone kits and have reported more than 26,000 reversals.8 Certainly, OEND has prevented morbidity and mortality in some of these patients.
The adoption of OEND can be performed by individual prescribers as well. Naloxone is U.S. FDA-approved for the treatment of opioid overdose, and thus the liability to prescribers is similar to that of other FDA-approved drugs. However, the distribution of naloxone to third parties, such as friends and family of individuals with opioid misuse, is more complex and regulated by state laws. Many states have created liability protection for naloxone prescription to third parties. Individual state laws and additional information can be found at prescribetoprevent.org.
Hospitalists should provide opioid overdose education to all individuals with opioid misuse and friends and family of individuals with opioid misuse. In addition, hospitalists should prescribe naloxone to individuals with opioid misuse and, in states where the law allows, distribute naloxone to friends and family of individuals with opioid misuse as well.
Controversies. In general, opioid use disorder treatment providers; public health officials; and local, state, and federal government agencies have increasingly embraced harm-reduction strategies. However, some feel that harm-reduction strategies are misguided or even detrimental due to concern that they implicitly condone or enable the use of illicit substances. There have been a number of studies to evaluate the potential unintended consequences of harm-reduction strategies, and overwhelmingly, these have been either neutral or have shown the benefit of harm-reduction interventions. At this point, there is no good evidence to prevent the widespread adoption of harm-reduction strategies for hospitalists.
Back to the Case
The case involves an individual who has already had at least two complications of his IV heroin use, including cellulitis and opioid overdose. Ideally, this individual would be willing to see an addiction specialist and start medication-assisted treatment. Unfortunately, he is unwilling to be further evaluated by a specialist at this time. Regardless, he remains at risk of future complications, and it is the hospitalist’s responsibility to intervene with a goal of reducing future harm that may result from his IV heroin use.
The hospitalist in this case advises the patient to abstain from heroin and IDU, encourages him to seek treatment for his opioid use disorder, and gives him resources for linkage to care if he becomes interested. In addition, the hospitalist educates the patient on safe injection practices and provides a list of local syringe exchange programs to decrease future risk of viral, bacterial, and fungal infections. Furthermore, the hospitalist provides opioid overdose education and distributes naloxone to the patient, along with friends and family of the patient, to reduce the risk of death related to opioid overdose.
Bottom Line
Hospitalists should utilize harm-reduction interventions in individuals hospitalized with opioid misuse. TH
Dr. Theisen-Toupal is a hospitalist at the Veterans Affairs Medical Center and assistant professor of medicine at the George Washington University School of Medicine & Health Sciences, both in Washington, D.C.
References
- Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6043a4.htm. Published November 4, 2011.
- Drug abuse warning network, 2011: national estimates of drug-related emergency department visits. Substance Abuse and Mental Health Services Administration website. Available at: http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm#5. Accessed July 29, 2015.
- Hedergaard H, Chen LH, Warner M. Drug-poisoning deaths involving heroin: United States, 2000–2013. National Center for Health Statistics Data Brief. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/nchs/data/databriefs/db190.htm. Published March 2015.
- Getting off right: a safety manual for injection drug users. Harm Reduction Coalition website. Available at: http://harmreduction.org/wp-content/uploads/2011/12/getting-off-right.pdf.
- Syringe exchange programs—United States, 2008. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5945a4.htm/Syringe-Exchange-Programs-United-States-2008. Published November 19, 2010.
- Wodak A, Conney A. Effectiveness of sterile needle and syringe programming in reducing HIV/AIDS among injecting drug users. World Health Organization website. Available at: http://apps.who.int/iris/bitstream/10665/43107/1/9241591641.pdf. Published 2004.
- Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention website. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6423a2.htm. Published June 19, 2015.
HM16 Q&A: What Problem Do You Hope Health IT Solves?
With the rolling out of the Health IT track on the second full day of HM16, The Hospitalist asked: What problem do you hope health IT solves or helps you solve over the next five years?
Farhanaz Chowdhury, MD, hospitalist, HSHS St. Elizabeth’s Hospital, Belleville, Ill.
“I think that hospital health systems are very primitive. When they make that software, physicians should be more involved so that in everyday life, what we see when we are facing all those problems, you have an algorithm if you want to do something. It should pop up so that you don’t have to write it down and scroll all over.”
Michael Lintner, MD, hospitalist, Aspen Valley Hospital, Colo.
“I think probably the main thing would be work flow, facilitating work flow. I think today hospitalists are just getting more and more and more work. Patient loads are getting increasingly bigger. I think with IT, [we need] systems that facilitate and help with the work flow and help the hospitalist’s day go smoother because there are so many things that we do.”
Miguel Lizardo, MD, hospitalist, University of Massachusetts Memorial Medical Center, Worcester
“It takes a lot of time to interact with the EMRs and all the technology that we have to use. If they can find a way that we can use it in a more user-friendly [way] so that it takes not a long time, that would be great. At least the EMRs that I’ve been in contact with are too cumbersome, too many clicks to get where you want, a bunch of steps to document what you need to. You really are away from the patient and spending a lot of time trying to document.”
Sandeep Palikhel, PA-C, Baylor University Medical Center, Waco, Tex.
“Definitely accuracy. In Texas, where I practice, we get a lot of transfers from rural areas because we are a Level 1 trauma hospital. We get these discharge summaries or progress notes from other hospitals that are handwritten. A lot of information gets missed whenever we’re reading it because it’s not legible, first thing, and it’s not as detail-oriented as the EHRs would be. So that definitely helps. Even going through a medication list, it helps so much to go through an EHR versus going through a handwritten medication list. That’s what I mean by accuracy.”
With the rolling out of the Health IT track on the second full day of HM16, The Hospitalist asked: What problem do you hope health IT solves or helps you solve over the next five years?
Farhanaz Chowdhury, MD, hospitalist, HSHS St. Elizabeth’s Hospital, Belleville, Ill.
“I think that hospital health systems are very primitive. When they make that software, physicians should be more involved so that in everyday life, what we see when we are facing all those problems, you have an algorithm if you want to do something. It should pop up so that you don’t have to write it down and scroll all over.”
Michael Lintner, MD, hospitalist, Aspen Valley Hospital, Colo.
“I think probably the main thing would be work flow, facilitating work flow. I think today hospitalists are just getting more and more and more work. Patient loads are getting increasingly bigger. I think with IT, [we need] systems that facilitate and help with the work flow and help the hospitalist’s day go smoother because there are so many things that we do.”
Miguel Lizardo, MD, hospitalist, University of Massachusetts Memorial Medical Center, Worcester
“It takes a lot of time to interact with the EMRs and all the technology that we have to use. If they can find a way that we can use it in a more user-friendly [way] so that it takes not a long time, that would be great. At least the EMRs that I’ve been in contact with are too cumbersome, too many clicks to get where you want, a bunch of steps to document what you need to. You really are away from the patient and spending a lot of time trying to document.”
Sandeep Palikhel, PA-C, Baylor University Medical Center, Waco, Tex.
“Definitely accuracy. In Texas, where I practice, we get a lot of transfers from rural areas because we are a Level 1 trauma hospital. We get these discharge summaries or progress notes from other hospitals that are handwritten. A lot of information gets missed whenever we’re reading it because it’s not legible, first thing, and it’s not as detail-oriented as the EHRs would be. So that definitely helps. Even going through a medication list, it helps so much to go through an EHR versus going through a handwritten medication list. That’s what I mean by accuracy.”
With the rolling out of the Health IT track on the second full day of HM16, The Hospitalist asked: What problem do you hope health IT solves or helps you solve over the next five years?
Farhanaz Chowdhury, MD, hospitalist, HSHS St. Elizabeth’s Hospital, Belleville, Ill.
“I think that hospital health systems are very primitive. When they make that software, physicians should be more involved so that in everyday life, what we see when we are facing all those problems, you have an algorithm if you want to do something. It should pop up so that you don’t have to write it down and scroll all over.”
Michael Lintner, MD, hospitalist, Aspen Valley Hospital, Colo.
“I think probably the main thing would be work flow, facilitating work flow. I think today hospitalists are just getting more and more and more work. Patient loads are getting increasingly bigger. I think with IT, [we need] systems that facilitate and help with the work flow and help the hospitalist’s day go smoother because there are so many things that we do.”
Miguel Lizardo, MD, hospitalist, University of Massachusetts Memorial Medical Center, Worcester
“It takes a lot of time to interact with the EMRs and all the technology that we have to use. If they can find a way that we can use it in a more user-friendly [way] so that it takes not a long time, that would be great. At least the EMRs that I’ve been in contact with are too cumbersome, too many clicks to get where you want, a bunch of steps to document what you need to. You really are away from the patient and spending a lot of time trying to document.”
Sandeep Palikhel, PA-C, Baylor University Medical Center, Waco, Tex.
“Definitely accuracy. In Texas, where I practice, we get a lot of transfers from rural areas because we are a Level 1 trauma hospital. We get these discharge summaries or progress notes from other hospitals that are handwritten. A lot of information gets missed whenever we’re reading it because it’s not legible, first thing, and it’s not as detail-oriented as the EHRs would be. So that definitely helps. Even going through a medication list, it helps so much to go through an EHR versus going through a handwritten medication list. That’s what I mean by accuracy.”
New Findings Show: Factors Contributing to the Prevalence in readmission for Bariatric Surgery Patients
NEW YORK (Reuters Health) - About one in 20 bariatric surgery patients are readmitted to the hospital within 30 days of having the procedure, according to new findings.
Readmissions are increasingly being used as a quality metric for surgical procedures, Dr. John Morton of Stanford University in California and colleagues note in their report, published online March 19 in the American Journal of Surgery.
"While (the Centers for Medicare and Medicaid Services) has not addressed bariatric surgery readmissions to date, other payors have made readmissions a priority," they add. "Data regarding bariatric surgery readmissions are critical to help better understand and drive quality improvement in this area.
"To investigate the prevalence, causes and risk factors for readmission following bariatric surgery, the researchers looked at data from the 2012 American College of Surgeons National Surgical Quality Improvement Program Public Use File dataset on nearly 18,300 bariatric patients, of whom 55% had laparoscopic Roux-en-Y gastric bypass (LRYGB), 10% had laparoscopic adjustable gastric banding (LAGB), and 35% had laparoscopic sleeve gastrectomy (LSG).
There were 955 readmissions (5.22%), most commonly for gastrointestinal causes (45%), dietary reasons (34%) and bleeding (7%). Readmission rates were nearly 7% for LRYGB; just under 2% for LAGB; and 4% for LSG.
The patients who were readmitted had a significantly longer average operating time (132 vs. 115 minutes) and length of stay (2.76 days vs. 2.23). Forty percent had a complication, versus 4% of patients who were not readmitted. Patients who were readmitted were also more likely to have a body mass index above 50, preoperative diabetes, chronic obstructive pulmonary disease, and hypertension.
Factors independently associated with readmission included African-American race (odds ratio, 1.53), complication (OR, 11.3) and resident involvement (OR, 0.53).
"Other studies have also demonstrated similar predictors of readmission and have also demonstrated that length of stay may also play a role in readmission rates," Dr. Morton and his team state. "This study helps demonstrate that bariatric surgery readmissions are prevalent and potentially preventable."
NEW YORK (Reuters Health) - About one in 20 bariatric surgery patients are readmitted to the hospital within 30 days of having the procedure, according to new findings.
Readmissions are increasingly being used as a quality metric for surgical procedures, Dr. John Morton of Stanford University in California and colleagues note in their report, published online March 19 in the American Journal of Surgery.
"While (the Centers for Medicare and Medicaid Services) has not addressed bariatric surgery readmissions to date, other payors have made readmissions a priority," they add. "Data regarding bariatric surgery readmissions are critical to help better understand and drive quality improvement in this area.
"To investigate the prevalence, causes and risk factors for readmission following bariatric surgery, the researchers looked at data from the 2012 American College of Surgeons National Surgical Quality Improvement Program Public Use File dataset on nearly 18,300 bariatric patients, of whom 55% had laparoscopic Roux-en-Y gastric bypass (LRYGB), 10% had laparoscopic adjustable gastric banding (LAGB), and 35% had laparoscopic sleeve gastrectomy (LSG).
There were 955 readmissions (5.22%), most commonly for gastrointestinal causes (45%), dietary reasons (34%) and bleeding (7%). Readmission rates were nearly 7% for LRYGB; just under 2% for LAGB; and 4% for LSG.
The patients who were readmitted had a significantly longer average operating time (132 vs. 115 minutes) and length of stay (2.76 days vs. 2.23). Forty percent had a complication, versus 4% of patients who were not readmitted. Patients who were readmitted were also more likely to have a body mass index above 50, preoperative diabetes, chronic obstructive pulmonary disease, and hypertension.
Factors independently associated with readmission included African-American race (odds ratio, 1.53), complication (OR, 11.3) and resident involvement (OR, 0.53).
"Other studies have also demonstrated similar predictors of readmission and have also demonstrated that length of stay may also play a role in readmission rates," Dr. Morton and his team state. "This study helps demonstrate that bariatric surgery readmissions are prevalent and potentially preventable."
NEW YORK (Reuters Health) - About one in 20 bariatric surgery patients are readmitted to the hospital within 30 days of having the procedure, according to new findings.
Readmissions are increasingly being used as a quality metric for surgical procedures, Dr. John Morton of Stanford University in California and colleagues note in their report, published online March 19 in the American Journal of Surgery.
"While (the Centers for Medicare and Medicaid Services) has not addressed bariatric surgery readmissions to date, other payors have made readmissions a priority," they add. "Data regarding bariatric surgery readmissions are critical to help better understand and drive quality improvement in this area.
"To investigate the prevalence, causes and risk factors for readmission following bariatric surgery, the researchers looked at data from the 2012 American College of Surgeons National Surgical Quality Improvement Program Public Use File dataset on nearly 18,300 bariatric patients, of whom 55% had laparoscopic Roux-en-Y gastric bypass (LRYGB), 10% had laparoscopic adjustable gastric banding (LAGB), and 35% had laparoscopic sleeve gastrectomy (LSG).
There were 955 readmissions (5.22%), most commonly for gastrointestinal causes (45%), dietary reasons (34%) and bleeding (7%). Readmission rates were nearly 7% for LRYGB; just under 2% for LAGB; and 4% for LSG.
The patients who were readmitted had a significantly longer average operating time (132 vs. 115 minutes) and length of stay (2.76 days vs. 2.23). Forty percent had a complication, versus 4% of patients who were not readmitted. Patients who were readmitted were also more likely to have a body mass index above 50, preoperative diabetes, chronic obstructive pulmonary disease, and hypertension.
Factors independently associated with readmission included African-American race (odds ratio, 1.53), complication (OR, 11.3) and resident involvement (OR, 0.53).
"Other studies have also demonstrated similar predictors of readmission and have also demonstrated that length of stay may also play a role in readmission rates," Dr. Morton and his team state. "This study helps demonstrate that bariatric surgery readmissions are prevalent and potentially preventable."
EMA recommends orphan designation for cancer vaccine

The European Medicines Agency (EMA) has recommended orphan designation for the WT1 cancer vaccine galinpepimut-S as a treatment for patients
with acute myeloid leukemia (AML) and patients with malignant pleural mesothelioma (MPM).
The EMA’s opinion has been forwarded to the European Commission (EC), which makes the final decision.
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the European Union. The product must provide significant benefit to those affected by the condition.
Orphan designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is approved for use.
About the vaccine
The WT1 vaccine consists of 4 modified peptide chains that induce an innate immune response (CD4+/CD8+ T cells) against the WT1 antigen. The vaccine is administered in combination with an adjuvant and an immune modulator to improve the immune response to the target.
Based on the vaccine’s mechanism and the accumulating evidence of activity in mid-stage trials, researchers believe the WT1 vaccine may have the potential to complement currently available therapies by destroying residual tumor cells of cancers in remission and providing ongoing immune surveillance for recurrent tumors.
The WT1 vaccine could potentially target more than 20 cancers that overexpress WT1, many of which are associated with relapse rates of up to 80% or more, as seen in patients with AML and MPM.
The vaccine is being developed by SELLAS Life Sciences Group. The company said that, in a phase 1 study, AML patients treated with the vaccine had a median overall survival of more than 3 years.
In a phase 2 trial of the vaccine, adult AML patients had a median overall survival of around 4 years. Data from the phase 2 trial are scheduled to be presented at the 2016 ASCO Annual Meeting.
SELLAS said it expects to begin a phase 3 trial of the vaccine in AML patients later this year. ![]()

The European Medicines Agency (EMA) has recommended orphan designation for the WT1 cancer vaccine galinpepimut-S as a treatment for patients
with acute myeloid leukemia (AML) and patients with malignant pleural mesothelioma (MPM).
The EMA’s opinion has been forwarded to the European Commission (EC), which makes the final decision.
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the European Union. The product must provide significant benefit to those affected by the condition.
Orphan designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is approved for use.
About the vaccine
The WT1 vaccine consists of 4 modified peptide chains that induce an innate immune response (CD4+/CD8+ T cells) against the WT1 antigen. The vaccine is administered in combination with an adjuvant and an immune modulator to improve the immune response to the target.
Based on the vaccine’s mechanism and the accumulating evidence of activity in mid-stage trials, researchers believe the WT1 vaccine may have the potential to complement currently available therapies by destroying residual tumor cells of cancers in remission and providing ongoing immune surveillance for recurrent tumors.
The WT1 vaccine could potentially target more than 20 cancers that overexpress WT1, many of which are associated with relapse rates of up to 80% or more, as seen in patients with AML and MPM.
The vaccine is being developed by SELLAS Life Sciences Group. The company said that, in a phase 1 study, AML patients treated with the vaccine had a median overall survival of more than 3 years.
In a phase 2 trial of the vaccine, adult AML patients had a median overall survival of around 4 years. Data from the phase 2 trial are scheduled to be presented at the 2016 ASCO Annual Meeting.
SELLAS said it expects to begin a phase 3 trial of the vaccine in AML patients later this year. ![]()

The European Medicines Agency (EMA) has recommended orphan designation for the WT1 cancer vaccine galinpepimut-S as a treatment for patients
with acute myeloid leukemia (AML) and patients with malignant pleural mesothelioma (MPM).
The EMA’s opinion has been forwarded to the European Commission (EC), which makes the final decision.
The EC grants orphan designation to products intended to treat, prevent, or diagnose a life-threatening condition affecting up to 5 in 10,000 people in the European Union. The product must provide significant benefit to those affected by the condition.
Orphan designation from the EC provides companies with certain development incentives, including protocol assistance, a type of scientific advice specific for orphan drugs, and 10 years of market exclusivity once the drug is approved for use.
About the vaccine
The WT1 vaccine consists of 4 modified peptide chains that induce an innate immune response (CD4+/CD8+ T cells) against the WT1 antigen. The vaccine is administered in combination with an adjuvant and an immune modulator to improve the immune response to the target.
Based on the vaccine’s mechanism and the accumulating evidence of activity in mid-stage trials, researchers believe the WT1 vaccine may have the potential to complement currently available therapies by destroying residual tumor cells of cancers in remission and providing ongoing immune surveillance for recurrent tumors.
The WT1 vaccine could potentially target more than 20 cancers that overexpress WT1, many of which are associated with relapse rates of up to 80% or more, as seen in patients with AML and MPM.
The vaccine is being developed by SELLAS Life Sciences Group. The company said that, in a phase 1 study, AML patients treated with the vaccine had a median overall survival of more than 3 years.
In a phase 2 trial of the vaccine, adult AML patients had a median overall survival of around 4 years. Data from the phase 2 trial are scheduled to be presented at the 2016 ASCO Annual Meeting.
SELLAS said it expects to begin a phase 3 trial of the vaccine in AML patients later this year. ![]()
Haplo-HSCT approach appears safe, effective for nonmalignant disorders

Image courtesy of NIAID
VALENCIA, SPAIN—Interim results of a phase 1/2 trial suggest the adjunct T-cell therapy BPX-501 can safely accelerate immune recovery after haploidentical hematopoietic stem cell transplant (haplo-HSCT) in pediatric patients with nonmalignant disorders.
Twenty-four such patients received BPX-501 after haplo-HSCT on this trial.
At a median follow-up of 7 months, all 24 were still alive and disease-free.
In addition, the incidence of graft-versus-host disease (GVHD) was considered “very low.”
Pietro Merli, MD, of Bambino Gesù Children’s Hospital in Rome, Italy, presented these results during the Presidential Symposium of the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT) as abstract O007.*
The trial, known as BP-004, was sponsored by Bellicum Pharmaceuticals, the company developing BPX-501.
About BPX-501
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate cells in the event of toxicity.
The goal is to allow physicians to more safely perform haplo-HSCTs by giving patients BPX-501 to speed immune reconstitution and provide control over viral infections. But the technology is designed to provide a safety net to eliminate BPX-501 alloreactive T cells if severe GVHD occurs.
The CaspaCIDe switch consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway. The idea is that, if a patient develops severe GVHD, he can receive an infusion with the small molecule rimiducid. And this will trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About BP-004
In late 2014, Bellicum initiated BP-004, a phase 1/2 trial in children with leukemias, lymphomas, or orphan inherited blood disorders. The trial is being conducted in European and US pediatric transplant centers and is set to enroll up to 90 patients.
At the EBMT meeting, investigators reported results in 41 patients treated on this trial.
Dr Merli presented data on the 24 patients with nonmalignant disorders, including Fanconi anemia (n=5), beta-thalassemia major (n=5), severe combined immunodeficiency (n=5), Wiskott-Aldrich syndrome (n=4), Diamond-Blackfan anemia (n=1), hemophagocytic lymphohistiocytosis (n=1), immune deficiency due to mutation of XIAP gene (n=1), osteopetrosis (n=1), and sickle cell disease (n=1).
All of these patients received a T-cell-depleted haplo-HSCT without post-transplant GVHD prophylaxis.
The patients received BPX-501 within 14 ± 4 days after haplo-HSCT. The phase 1 portion of the trial consisted of a classical 3+3 design, with 3 cohorts receiving escalating doses of BPX-501 cells—2.5 x 105, 5 x 105, and 1 x 106 cells/kg.
In the phase 2 portion, patients received 1 X 106 BPX-501 cells/kg. Rimiducid was only to be used in the event of uncontrollable GVHD.
Results
The median time to platelet recovery was 10 days (range, 7-16), and the median time to neutrophil recovery was 15 days (range, 10-33).
At a median follow-up of 220 days (range, 61-486), there were no reports of transplant-related mortality.
All 24 patients were still alive and disease-free. And none of the patients developed post-transplant lymphoproliferative disorder.
The cumulative incidence of skin-only acute GVHD was 16.6% (n=4), and the cumulative incidence of mild chronic GVHD was 5% (n=1).
This trial also included 17 patients with acute leukemias. Results in these patients were presented at the EBMT meeting as abstract WP16. ![]()
*Information in the abstract differs from that presented at the meeting.

Image courtesy of NIAID
VALENCIA, SPAIN—Interim results of a phase 1/2 trial suggest the adjunct T-cell therapy BPX-501 can safely accelerate immune recovery after haploidentical hematopoietic stem cell transplant (haplo-HSCT) in pediatric patients with nonmalignant disorders.
Twenty-four such patients received BPX-501 after haplo-HSCT on this trial.
At a median follow-up of 7 months, all 24 were still alive and disease-free.
In addition, the incidence of graft-versus-host disease (GVHD) was considered “very low.”
Pietro Merli, MD, of Bambino Gesù Children’s Hospital in Rome, Italy, presented these results during the Presidential Symposium of the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT) as abstract O007.*
The trial, known as BP-004, was sponsored by Bellicum Pharmaceuticals, the company developing BPX-501.
About BPX-501
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate cells in the event of toxicity.
The goal is to allow physicians to more safely perform haplo-HSCTs by giving patients BPX-501 to speed immune reconstitution and provide control over viral infections. But the technology is designed to provide a safety net to eliminate BPX-501 alloreactive T cells if severe GVHD occurs.
The CaspaCIDe switch consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway. The idea is that, if a patient develops severe GVHD, he can receive an infusion with the small molecule rimiducid. And this will trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About BP-004
In late 2014, Bellicum initiated BP-004, a phase 1/2 trial in children with leukemias, lymphomas, or orphan inherited blood disorders. The trial is being conducted in European and US pediatric transplant centers and is set to enroll up to 90 patients.
At the EBMT meeting, investigators reported results in 41 patients treated on this trial.
Dr Merli presented data on the 24 patients with nonmalignant disorders, including Fanconi anemia (n=5), beta-thalassemia major (n=5), severe combined immunodeficiency (n=5), Wiskott-Aldrich syndrome (n=4), Diamond-Blackfan anemia (n=1), hemophagocytic lymphohistiocytosis (n=1), immune deficiency due to mutation of XIAP gene (n=1), osteopetrosis (n=1), and sickle cell disease (n=1).
All of these patients received a T-cell-depleted haplo-HSCT without post-transplant GVHD prophylaxis.
The patients received BPX-501 within 14 ± 4 days after haplo-HSCT. The phase 1 portion of the trial consisted of a classical 3+3 design, with 3 cohorts receiving escalating doses of BPX-501 cells—2.5 x 105, 5 x 105, and 1 x 106 cells/kg.
In the phase 2 portion, patients received 1 X 106 BPX-501 cells/kg. Rimiducid was only to be used in the event of uncontrollable GVHD.
Results
The median time to platelet recovery was 10 days (range, 7-16), and the median time to neutrophil recovery was 15 days (range, 10-33).
At a median follow-up of 220 days (range, 61-486), there were no reports of transplant-related mortality.
All 24 patients were still alive and disease-free. And none of the patients developed post-transplant lymphoproliferative disorder.
The cumulative incidence of skin-only acute GVHD was 16.6% (n=4), and the cumulative incidence of mild chronic GVHD was 5% (n=1).
This trial also included 17 patients with acute leukemias. Results in these patients were presented at the EBMT meeting as abstract WP16. ![]()
*Information in the abstract differs from that presented at the meeting.

Image courtesy of NIAID
VALENCIA, SPAIN—Interim results of a phase 1/2 trial suggest the adjunct T-cell therapy BPX-501 can safely accelerate immune recovery after haploidentical hematopoietic stem cell transplant (haplo-HSCT) in pediatric patients with nonmalignant disorders.
Twenty-four such patients received BPX-501 after haplo-HSCT on this trial.
At a median follow-up of 7 months, all 24 were still alive and disease-free.
In addition, the incidence of graft-versus-host disease (GVHD) was considered “very low.”
Pietro Merli, MD, of Bambino Gesù Children’s Hospital in Rome, Italy, presented these results during the Presidential Symposium of the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT) as abstract O007.*
The trial, known as BP-004, was sponsored by Bellicum Pharmaceuticals, the company developing BPX-501.
About BPX-501
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate cells in the event of toxicity.
The goal is to allow physicians to more safely perform haplo-HSCTs by giving patients BPX-501 to speed immune reconstitution and provide control over viral infections. But the technology is designed to provide a safety net to eliminate BPX-501 alloreactive T cells if severe GVHD occurs.
The CaspaCIDe switch consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway. The idea is that, if a patient develops severe GVHD, he can receive an infusion with the small molecule rimiducid. And this will trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About BP-004
In late 2014, Bellicum initiated BP-004, a phase 1/2 trial in children with leukemias, lymphomas, or orphan inherited blood disorders. The trial is being conducted in European and US pediatric transplant centers and is set to enroll up to 90 patients.
At the EBMT meeting, investigators reported results in 41 patients treated on this trial.
Dr Merli presented data on the 24 patients with nonmalignant disorders, including Fanconi anemia (n=5), beta-thalassemia major (n=5), severe combined immunodeficiency (n=5), Wiskott-Aldrich syndrome (n=4), Diamond-Blackfan anemia (n=1), hemophagocytic lymphohistiocytosis (n=1), immune deficiency due to mutation of XIAP gene (n=1), osteopetrosis (n=1), and sickle cell disease (n=1).
All of these patients received a T-cell-depleted haplo-HSCT without post-transplant GVHD prophylaxis.
The patients received BPX-501 within 14 ± 4 days after haplo-HSCT. The phase 1 portion of the trial consisted of a classical 3+3 design, with 3 cohorts receiving escalating doses of BPX-501 cells—2.5 x 105, 5 x 105, and 1 x 106 cells/kg.
In the phase 2 portion, patients received 1 X 106 BPX-501 cells/kg. Rimiducid was only to be used in the event of uncontrollable GVHD.
Results
The median time to platelet recovery was 10 days (range, 7-16), and the median time to neutrophil recovery was 15 days (range, 10-33).
At a median follow-up of 220 days (range, 61-486), there were no reports of transplant-related mortality.
All 24 patients were still alive and disease-free. And none of the patients developed post-transplant lymphoproliferative disorder.
The cumulative incidence of skin-only acute GVHD was 16.6% (n=4), and the cumulative incidence of mild chronic GVHD was 5% (n=1).
This trial also included 17 patients with acute leukemias. Results in these patients were presented at the EBMT meeting as abstract WP16. ![]()
*Information in the abstract differs from that presented at the meeting.
Therapy may improve haplo-HSCT in leukemia patients

Photo by Bill Branson
VALENCIA, SPAIN—The adjunct T-cell therapy BPX-501 can make haploidentical hematopoietic stem cell transplant (haplo-HSCT) an “attractive option” for pediatric patients with acute leukemia, according to a presentation at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT).
Acute leukemia patients who received BPX-501 after haplo-HSCT in a phase 1/2 trial tended to have favorable outcomes.
At a median follow-up of 7 months, 16 of the 17 patients were alive and disease-free.
There were several cases of graft-versus-host disease (GVHD), but nearly all of these resolved.
Franco Locatelli, MD, PhD, of Bambino Gesù Children’s Hospital in Rome, Italy, presented these results at the EBMT meeting as abstract WP16.*
The trial, known as BP-004, was sponsored by Bellicum Pharmaceuticals, the company developing BPX-501.
About BPX-501
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate cells in the event of toxicity.
The goal is to allow physicians to more safely perform haplo-HSCTs by giving patients BPX-501 to speed immune reconstitution and provide control over viral infections. But the technology is designed to provide a safety net to eliminate BPX-501 alloreactive T cells if severe GVHD occurs.
The CaspaCIDe switch consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway. The idea is that, if a patient develops severe GVHD, he can receive an infusion with the small molecule rimiducid. And this will trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About BP-004
In late 2014, Bellicum initiated BP-004, a phase 1/2 trial in children with leukemias, lymphomas, or orphan inherited blood disorders. The trial is being conducted in European and US pediatric transplant centers and is set to enroll up to 90 patients.
At the EBMT meeting, researchers reported results in 41 patients treated on this trial.
Dr Locatelli presented data on 17 patients with acute leukemias—13 with acute lymphoblastic leukemia and 4 with acute myeloid leukemia. Their median age at HSCT was 6.5 years (range, 0.9-16.1)
All of these patients received a T-cell-depleted haplo-HSCT without post-transplant GVHD prophylaxis. All were in complete remission at the time of transplant.
The patients received BPX-501 within 14 ± 4 days after haplo-HSCT. The phase 1 portion of the trial consisted of a classical 3+3 design, with 3 cohorts receiving escalating doses of BPX-501 cells—2.5 x 105, 5 x 105, and 1 x 106 cells/kg.
In the phase 2 portion, patients received 1 X 106 BPX-501 cells/kg. Rimiducid was only used in the event of uncontrollable GVHD.
Results
The median follow-up was 7 months (range, 1-15.6). The median time to platelet recovery was 11 days (range, 9-13), and the median time to neutrophil recovery was 17 days (range, 10-22).
Three patients developed skin-only acute GVHD, were treated with topical steroids, and the GVHD resolved. Two patients developed acute grade 3 GVHD, were treated with systemic steroids, and the GVHD resolved.
Two patients developed mild chronic GVHD, received systemic steroids, and the GVHD resolved. And 1 patient developed severe chronic GVHD, received systemic steroids and rimiducid, and the GVHD improved.
One patient relapsed. The estimated 1-year disease-free survival was 92.9%. Dr Locatelli noted that, although the follow-up is still limited, these results compare favorably to results in historical controls.
“These interim results continue to be very encouraging and indicate that a haploidentical transplant, with the addition of BPX-501-modified donor T cells, can be an attractive option for children in need of a transplant,” he said.
“Future studies will address the role of repeated infusions or higher numbers of BPX-501 cells in malignant patients with resistant disease.”
The BP-004 trial also included 24 patients with nonmalignant disorders. Results in these patients were presented at the EBMT meeting as abstract O007. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo by Bill Branson
VALENCIA, SPAIN—The adjunct T-cell therapy BPX-501 can make haploidentical hematopoietic stem cell transplant (haplo-HSCT) an “attractive option” for pediatric patients with acute leukemia, according to a presentation at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT).
Acute leukemia patients who received BPX-501 after haplo-HSCT in a phase 1/2 trial tended to have favorable outcomes.
At a median follow-up of 7 months, 16 of the 17 patients were alive and disease-free.
There were several cases of graft-versus-host disease (GVHD), but nearly all of these resolved.
Franco Locatelli, MD, PhD, of Bambino Gesù Children’s Hospital in Rome, Italy, presented these results at the EBMT meeting as abstract WP16.*
The trial, known as BP-004, was sponsored by Bellicum Pharmaceuticals, the company developing BPX-501.
About BPX-501
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate cells in the event of toxicity.
The goal is to allow physicians to more safely perform haplo-HSCTs by giving patients BPX-501 to speed immune reconstitution and provide control over viral infections. But the technology is designed to provide a safety net to eliminate BPX-501 alloreactive T cells if severe GVHD occurs.
The CaspaCIDe switch consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway. The idea is that, if a patient develops severe GVHD, he can receive an infusion with the small molecule rimiducid. And this will trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About BP-004
In late 2014, Bellicum initiated BP-004, a phase 1/2 trial in children with leukemias, lymphomas, or orphan inherited blood disorders. The trial is being conducted in European and US pediatric transplant centers and is set to enroll up to 90 patients.
At the EBMT meeting, researchers reported results in 41 patients treated on this trial.
Dr Locatelli presented data on 17 patients with acute leukemias—13 with acute lymphoblastic leukemia and 4 with acute myeloid leukemia. Their median age at HSCT was 6.5 years (range, 0.9-16.1)
All of these patients received a T-cell-depleted haplo-HSCT without post-transplant GVHD prophylaxis. All were in complete remission at the time of transplant.
The patients received BPX-501 within 14 ± 4 days after haplo-HSCT. The phase 1 portion of the trial consisted of a classical 3+3 design, with 3 cohorts receiving escalating doses of BPX-501 cells—2.5 x 105, 5 x 105, and 1 x 106 cells/kg.
In the phase 2 portion, patients received 1 X 106 BPX-501 cells/kg. Rimiducid was only used in the event of uncontrollable GVHD.
Results
The median follow-up was 7 months (range, 1-15.6). The median time to platelet recovery was 11 days (range, 9-13), and the median time to neutrophil recovery was 17 days (range, 10-22).
Three patients developed skin-only acute GVHD, were treated with topical steroids, and the GVHD resolved. Two patients developed acute grade 3 GVHD, were treated with systemic steroids, and the GVHD resolved.
Two patients developed mild chronic GVHD, received systemic steroids, and the GVHD resolved. And 1 patient developed severe chronic GVHD, received systemic steroids and rimiducid, and the GVHD improved.
One patient relapsed. The estimated 1-year disease-free survival was 92.9%. Dr Locatelli noted that, although the follow-up is still limited, these results compare favorably to results in historical controls.
“These interim results continue to be very encouraging and indicate that a haploidentical transplant, with the addition of BPX-501-modified donor T cells, can be an attractive option for children in need of a transplant,” he said.
“Future studies will address the role of repeated infusions or higher numbers of BPX-501 cells in malignant patients with resistant disease.”
The BP-004 trial also included 24 patients with nonmalignant disorders. Results in these patients were presented at the EBMT meeting as abstract O007. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo by Bill Branson
VALENCIA, SPAIN—The adjunct T-cell therapy BPX-501 can make haploidentical hematopoietic stem cell transplant (haplo-HSCT) an “attractive option” for pediatric patients with acute leukemia, according to a presentation at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT).
Acute leukemia patients who received BPX-501 after haplo-HSCT in a phase 1/2 trial tended to have favorable outcomes.
At a median follow-up of 7 months, 16 of the 17 patients were alive and disease-free.
There were several cases of graft-versus-host disease (GVHD), but nearly all of these resolved.
Franco Locatelli, MD, PhD, of Bambino Gesù Children’s Hospital in Rome, Italy, presented these results at the EBMT meeting as abstract WP16.*
The trial, known as BP-004, was sponsored by Bellicum Pharmaceuticals, the company developing BPX-501.
About BPX-501
BPX-501 consists of genetically modified donor T cells incorporating the CaspaCIDe safety switch, which is designed to eliminate cells in the event of toxicity.
The goal is to allow physicians to more safely perform haplo-HSCTs by giving patients BPX-501 to speed immune reconstitution and provide control over viral infections. But the technology is designed to provide a safety net to eliminate BPX-501 alloreactive T cells if severe GVHD occurs.
The CaspaCIDe switch consists of the CID-binding domain coupled to the signaling domain of caspase-9, an enzyme that is part of the apoptotic pathway. The idea is that, if a patient develops severe GVHD, he can receive an infusion with the small molecule rimiducid. And this will trigger activation of the domain of caspase-9, which leads to selective apoptosis of the CaspaCIDe-containing cells.
About BP-004
In late 2014, Bellicum initiated BP-004, a phase 1/2 trial in children with leukemias, lymphomas, or orphan inherited blood disorders. The trial is being conducted in European and US pediatric transplant centers and is set to enroll up to 90 patients.
At the EBMT meeting, researchers reported results in 41 patients treated on this trial.
Dr Locatelli presented data on 17 patients with acute leukemias—13 with acute lymphoblastic leukemia and 4 with acute myeloid leukemia. Their median age at HSCT was 6.5 years (range, 0.9-16.1)
All of these patients received a T-cell-depleted haplo-HSCT without post-transplant GVHD prophylaxis. All were in complete remission at the time of transplant.
The patients received BPX-501 within 14 ± 4 days after haplo-HSCT. The phase 1 portion of the trial consisted of a classical 3+3 design, with 3 cohorts receiving escalating doses of BPX-501 cells—2.5 x 105, 5 x 105, and 1 x 106 cells/kg.
In the phase 2 portion, patients received 1 X 106 BPX-501 cells/kg. Rimiducid was only used in the event of uncontrollable GVHD.
Results
The median follow-up was 7 months (range, 1-15.6). The median time to platelet recovery was 11 days (range, 9-13), and the median time to neutrophil recovery was 17 days (range, 10-22).
Three patients developed skin-only acute GVHD, were treated with topical steroids, and the GVHD resolved. Two patients developed acute grade 3 GVHD, were treated with systemic steroids, and the GVHD resolved.
Two patients developed mild chronic GVHD, received systemic steroids, and the GVHD resolved. And 1 patient developed severe chronic GVHD, received systemic steroids and rimiducid, and the GVHD improved.
One patient relapsed. The estimated 1-year disease-free survival was 92.9%. Dr Locatelli noted that, although the follow-up is still limited, these results compare favorably to results in historical controls.
“These interim results continue to be very encouraging and indicate that a haploidentical transplant, with the addition of BPX-501-modified donor T cells, can be an attractive option for children in need of a transplant,” he said.
“Future studies will address the role of repeated infusions or higher numbers of BPX-501 cells in malignant patients with resistant disease.”
The BP-004 trial also included 24 patients with nonmalignant disorders. Results in these patients were presented at the EBMT meeting as abstract O007. ![]()
*Information in the abstract differs from that presented at the meeting.
Interdisciplinary Rounds
Care of hospitalized patients requires effective teamwork within groups composed of physicians (eg, residents, hospitalists, specialists), advanced practice providers, nurses, patient‐care technicians, pharmacists, social workers, and therapists. Sadly, hospital‐based team members often fail to communicate. For example, 2 studies found that nurses and physicians communicated with one another on only 50% to 60% of their patients' hospital days, resulting in a lack of a mutual understanding of the plan of care.[1, 2]
Failure to communicate effectively may be because the hospital setting poses important challenges to teamwork, including the use of large teams with membership that changes frequently because of the need to provide care around the clock. Furthermore, individual team members often have high workloads, care for multiple patients simultaneously, and are seldom in the same place at the same time.
Interdisciplinary rounds (IDR) are a microsystem‐level solution with the goal to share information, achieve mutual understanding, and collaboratively revise the plan of care within care teams. Though common, IDR look very different across hospitals, making studies that evaluate novel strategies to improve IDR and measure their impact of great interest to hospital medicine.
In this issue of the Journal of Hospital Medicine, Bhamidipati and colleagues present a systematic review of published studies evaluating the effect of IDR on patient outcomes.[3] The systematic review included 22 studies, including 12 experimental/quasiexperimental and 10 observational studies. Overall, 13 studies were of low to medium quality, and 9 were high quality. Importantly, relatively few studies reported the degree to which IDR were implemented as planned. The investigators found evidence that IDR had a positive effect on length of stay (LOS) and staff satisfaction, but little evidence to support an effect on patient safety or satisfaction. Furthermore, the investigators found significant variability in IDR design and team composition. Some of this variation is to be expected, as IDR, like other interventions to improve quality and safety of patient care in complex settings, should be implemented with an expectation that the team may need to make adaptations based on local contextual factors such as workload (eg, daily census), environment (eg, open vs closed intensive care unit), local politics (eg, uniquely strong support for/against the intervention), and prior experience (eg, prior failed, similar interventions).[4, 5] Moreover, objectives for IDR may differ across settings. Some hospitals may have room (and a need) to improve LOS, whereas others may prioritize improving patient safety or patient experience metrics.
Bhamidipati and colleagues explain that their review did not reveal a causal pathway between IDR design and outcomes. We believe this lack of association is because most of the included studies did not propose a causal pathway between the IDR components implemented and the outcomes assessed. That is, few studies referred to conceptual models that explain how components of the IDR intervention might influence downstream patient outcomes.
IDR have the potential to influence a number of patient outcomes, including those reflecting efficiency (eg, length of stay), patient safety (eg, adverse events), and patient centeredness (eg, patient satisfaction). However, these outcomes are influenced by many factors, including patient characteristics and other efforts to improve care. As explained by the investigators, the results of many of the included studies may have been confounded due to relatively weak study designs and statistical analyses. Importantly, few of the studies included in this review report the more proximal measure of teamwork. If we hypothesize that IDR improve patient outcomes, they do so by improving teamwork. After all, the purpose of IDR is to assemble team members so they can communicate about and coordinate care. Measuring teamwork behaviors is difficult, especially on medical services. Measuring teamwork climate, the measurable aspects of team culture, is relatively easy. A recent systematic review of teamwork climate assessments in internal medicine identified the Safety Attitudes Questionnaire and the Team Climate Inventory as having substantial validity evidence and association with improved patient outcomes.[6]
Bhamidipati and colleagues proposed a definition for IDR and taxonomy for IDR design and reporting based on their systematic review. Although very useful, the IDR definition may be too limiting as evidenced by the fact that very few studies would be included in a systematic review using this definition as the inclusion criteria. Their proposed taxonomy should serve as a useful framework for future research efforts and appropriately recommends reporting of site characteristics, components of IDR design, and outcomes.
The systematic review by Bhamidipati et al. must also be interpreted in conjunction with another recently published systematic review by Pannick and colleagues assessing the effect of interdisciplinary team care interventions on general medical wards.[7] Contrary to the findings of the Bhamidipati et al. study, Pannick and colleagues found that most interdisciplinary team care interventions had no effect on LOS, but that half of the studies found an improvement in complications of care. Importantly, Pannick and colleagues included only experimental and quasiexperimental studies in their systematic review (ie, no observational studies).
There is clearly more work to be done in researching IDR and other interventions to improve teamwork in general medical settings. Larger studies are needed to provide sufficient power to detect improvement in outcomes. Future studies need to report the degree to which interventions are implemented as planned and need to use stronger study designs (eg, cluster randomized control or interrupted time series) to avoid the influence of confounders. Qualitative methods should be used to assess the influence of contextual factors on the success of interventions.[4] Most importantly, future studies should be based on conceptual models that explain how components of the intervention influence proximal measures of teamwork and downstream patient outcomes.
In the meantime, what is a hospital leader to do? We believe efforts to improve IDR are warranted, but that IDR program leaders need to first specify their primary objective(s). For example, in some hospitals, there may be little room to further reduce LOS, so another goalreducing preventable readmissions or reducing adverse eventsmight be specified as the key performance indicator. This crucial first step of creating a shared goal informs the design, implementation, and evaluation of IDR. We also believe that geographic localization of physicians to specific units is foundational to improving IDR. Physicians cannot feasibly attend IDR if their patients are spread across multiple units (or buildings). Finally, hospital leaders also need to view IDR as part of a larger set of interventions to improve teamwork. Leaders need to assess the adequacy of staffing levels, workflow, and team composition.[8] Unit‐based interdisciplinary leadership models should be used to help link efforts at various levels within a larger system.[9] These models designate a unit medical director and nurse manager who are jointly responsible for unit performance.
In conclusion, IDR play an important role in improving patient outcomes, but only do so by improving teamwork. In redesigning IDR, leaders need to be thoughtful about what outcomes IDR can affect, how IDR affect them, and how IDR fit into larger‐scale efforts to improve performance.
Disclosure
Nothing to report.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 1: Research Findings. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Structure and outcomes of inter‐disciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomny. J Hosp Med. 2016;11:513–523.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- . Improvement interventions are social treatments, not pills. Ann Intern Med. 2014;161(7):526–527.
- , , , et al. Teamwork assessment in internal medicine: a systematic review of validity evidence and outcomes. J Gen Intern Med. 2014;29(6):894–910.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , . Improving the quality and safety of care on the medical ward: A review and synthesis of the evidence base. Eur J Intern Med. 2014;25(10):874–887.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
Care of hospitalized patients requires effective teamwork within groups composed of physicians (eg, residents, hospitalists, specialists), advanced practice providers, nurses, patient‐care technicians, pharmacists, social workers, and therapists. Sadly, hospital‐based team members often fail to communicate. For example, 2 studies found that nurses and physicians communicated with one another on only 50% to 60% of their patients' hospital days, resulting in a lack of a mutual understanding of the plan of care.[1, 2]
Failure to communicate effectively may be because the hospital setting poses important challenges to teamwork, including the use of large teams with membership that changes frequently because of the need to provide care around the clock. Furthermore, individual team members often have high workloads, care for multiple patients simultaneously, and are seldom in the same place at the same time.
Interdisciplinary rounds (IDR) are a microsystem‐level solution with the goal to share information, achieve mutual understanding, and collaboratively revise the plan of care within care teams. Though common, IDR look very different across hospitals, making studies that evaluate novel strategies to improve IDR and measure their impact of great interest to hospital medicine.
In this issue of the Journal of Hospital Medicine, Bhamidipati and colleagues present a systematic review of published studies evaluating the effect of IDR on patient outcomes.[3] The systematic review included 22 studies, including 12 experimental/quasiexperimental and 10 observational studies. Overall, 13 studies were of low to medium quality, and 9 were high quality. Importantly, relatively few studies reported the degree to which IDR were implemented as planned. The investigators found evidence that IDR had a positive effect on length of stay (LOS) and staff satisfaction, but little evidence to support an effect on patient safety or satisfaction. Furthermore, the investigators found significant variability in IDR design and team composition. Some of this variation is to be expected, as IDR, like other interventions to improve quality and safety of patient care in complex settings, should be implemented with an expectation that the team may need to make adaptations based on local contextual factors such as workload (eg, daily census), environment (eg, open vs closed intensive care unit), local politics (eg, uniquely strong support for/against the intervention), and prior experience (eg, prior failed, similar interventions).[4, 5] Moreover, objectives for IDR may differ across settings. Some hospitals may have room (and a need) to improve LOS, whereas others may prioritize improving patient safety or patient experience metrics.
Bhamidipati and colleagues explain that their review did not reveal a causal pathway between IDR design and outcomes. We believe this lack of association is because most of the included studies did not propose a causal pathway between the IDR components implemented and the outcomes assessed. That is, few studies referred to conceptual models that explain how components of the IDR intervention might influence downstream patient outcomes.
IDR have the potential to influence a number of patient outcomes, including those reflecting efficiency (eg, length of stay), patient safety (eg, adverse events), and patient centeredness (eg, patient satisfaction). However, these outcomes are influenced by many factors, including patient characteristics and other efforts to improve care. As explained by the investigators, the results of many of the included studies may have been confounded due to relatively weak study designs and statistical analyses. Importantly, few of the studies included in this review report the more proximal measure of teamwork. If we hypothesize that IDR improve patient outcomes, they do so by improving teamwork. After all, the purpose of IDR is to assemble team members so they can communicate about and coordinate care. Measuring teamwork behaviors is difficult, especially on medical services. Measuring teamwork climate, the measurable aspects of team culture, is relatively easy. A recent systematic review of teamwork climate assessments in internal medicine identified the Safety Attitudes Questionnaire and the Team Climate Inventory as having substantial validity evidence and association with improved patient outcomes.[6]
Bhamidipati and colleagues proposed a definition for IDR and taxonomy for IDR design and reporting based on their systematic review. Although very useful, the IDR definition may be too limiting as evidenced by the fact that very few studies would be included in a systematic review using this definition as the inclusion criteria. Their proposed taxonomy should serve as a useful framework for future research efforts and appropriately recommends reporting of site characteristics, components of IDR design, and outcomes.
The systematic review by Bhamidipati et al. must also be interpreted in conjunction with another recently published systematic review by Pannick and colleagues assessing the effect of interdisciplinary team care interventions on general medical wards.[7] Contrary to the findings of the Bhamidipati et al. study, Pannick and colleagues found that most interdisciplinary team care interventions had no effect on LOS, but that half of the studies found an improvement in complications of care. Importantly, Pannick and colleagues included only experimental and quasiexperimental studies in their systematic review (ie, no observational studies).
There is clearly more work to be done in researching IDR and other interventions to improve teamwork in general medical settings. Larger studies are needed to provide sufficient power to detect improvement in outcomes. Future studies need to report the degree to which interventions are implemented as planned and need to use stronger study designs (eg, cluster randomized control or interrupted time series) to avoid the influence of confounders. Qualitative methods should be used to assess the influence of contextual factors on the success of interventions.[4] Most importantly, future studies should be based on conceptual models that explain how components of the intervention influence proximal measures of teamwork and downstream patient outcomes.
In the meantime, what is a hospital leader to do? We believe efforts to improve IDR are warranted, but that IDR program leaders need to first specify their primary objective(s). For example, in some hospitals, there may be little room to further reduce LOS, so another goalreducing preventable readmissions or reducing adverse eventsmight be specified as the key performance indicator. This crucial first step of creating a shared goal informs the design, implementation, and evaluation of IDR. We also believe that geographic localization of physicians to specific units is foundational to improving IDR. Physicians cannot feasibly attend IDR if their patients are spread across multiple units (or buildings). Finally, hospital leaders also need to view IDR as part of a larger set of interventions to improve teamwork. Leaders need to assess the adequacy of staffing levels, workflow, and team composition.[8] Unit‐based interdisciplinary leadership models should be used to help link efforts at various levels within a larger system.[9] These models designate a unit medical director and nurse manager who are jointly responsible for unit performance.
In conclusion, IDR play an important role in improving patient outcomes, but only do so by improving teamwork. In redesigning IDR, leaders need to be thoughtful about what outcomes IDR can affect, how IDR affect them, and how IDR fit into larger‐scale efforts to improve performance.
Disclosure
Nothing to report.
Care of hospitalized patients requires effective teamwork within groups composed of physicians (eg, residents, hospitalists, specialists), advanced practice providers, nurses, patient‐care technicians, pharmacists, social workers, and therapists. Sadly, hospital‐based team members often fail to communicate. For example, 2 studies found that nurses and physicians communicated with one another on only 50% to 60% of their patients' hospital days, resulting in a lack of a mutual understanding of the plan of care.[1, 2]
Failure to communicate effectively may be because the hospital setting poses important challenges to teamwork, including the use of large teams with membership that changes frequently because of the need to provide care around the clock. Furthermore, individual team members often have high workloads, care for multiple patients simultaneously, and are seldom in the same place at the same time.
Interdisciplinary rounds (IDR) are a microsystem‐level solution with the goal to share information, achieve mutual understanding, and collaboratively revise the plan of care within care teams. Though common, IDR look very different across hospitals, making studies that evaluate novel strategies to improve IDR and measure their impact of great interest to hospital medicine.
In this issue of the Journal of Hospital Medicine, Bhamidipati and colleagues present a systematic review of published studies evaluating the effect of IDR on patient outcomes.[3] The systematic review included 22 studies, including 12 experimental/quasiexperimental and 10 observational studies. Overall, 13 studies were of low to medium quality, and 9 were high quality. Importantly, relatively few studies reported the degree to which IDR were implemented as planned. The investigators found evidence that IDR had a positive effect on length of stay (LOS) and staff satisfaction, but little evidence to support an effect on patient safety or satisfaction. Furthermore, the investigators found significant variability in IDR design and team composition. Some of this variation is to be expected, as IDR, like other interventions to improve quality and safety of patient care in complex settings, should be implemented with an expectation that the team may need to make adaptations based on local contextual factors such as workload (eg, daily census), environment (eg, open vs closed intensive care unit), local politics (eg, uniquely strong support for/against the intervention), and prior experience (eg, prior failed, similar interventions).[4, 5] Moreover, objectives for IDR may differ across settings. Some hospitals may have room (and a need) to improve LOS, whereas others may prioritize improving patient safety or patient experience metrics.
Bhamidipati and colleagues explain that their review did not reveal a causal pathway between IDR design and outcomes. We believe this lack of association is because most of the included studies did not propose a causal pathway between the IDR components implemented and the outcomes assessed. That is, few studies referred to conceptual models that explain how components of the IDR intervention might influence downstream patient outcomes.
IDR have the potential to influence a number of patient outcomes, including those reflecting efficiency (eg, length of stay), patient safety (eg, adverse events), and patient centeredness (eg, patient satisfaction). However, these outcomes are influenced by many factors, including patient characteristics and other efforts to improve care. As explained by the investigators, the results of many of the included studies may have been confounded due to relatively weak study designs and statistical analyses. Importantly, few of the studies included in this review report the more proximal measure of teamwork. If we hypothesize that IDR improve patient outcomes, they do so by improving teamwork. After all, the purpose of IDR is to assemble team members so they can communicate about and coordinate care. Measuring teamwork behaviors is difficult, especially on medical services. Measuring teamwork climate, the measurable aspects of team culture, is relatively easy. A recent systematic review of teamwork climate assessments in internal medicine identified the Safety Attitudes Questionnaire and the Team Climate Inventory as having substantial validity evidence and association with improved patient outcomes.[6]
Bhamidipati and colleagues proposed a definition for IDR and taxonomy for IDR design and reporting based on their systematic review. Although very useful, the IDR definition may be too limiting as evidenced by the fact that very few studies would be included in a systematic review using this definition as the inclusion criteria. Their proposed taxonomy should serve as a useful framework for future research efforts and appropriately recommends reporting of site characteristics, components of IDR design, and outcomes.
The systematic review by Bhamidipati et al. must also be interpreted in conjunction with another recently published systematic review by Pannick and colleagues assessing the effect of interdisciplinary team care interventions on general medical wards.[7] Contrary to the findings of the Bhamidipati et al. study, Pannick and colleagues found that most interdisciplinary team care interventions had no effect on LOS, but that half of the studies found an improvement in complications of care. Importantly, Pannick and colleagues included only experimental and quasiexperimental studies in their systematic review (ie, no observational studies).
There is clearly more work to be done in researching IDR and other interventions to improve teamwork in general medical settings. Larger studies are needed to provide sufficient power to detect improvement in outcomes. Future studies need to report the degree to which interventions are implemented as planned and need to use stronger study designs (eg, cluster randomized control or interrupted time series) to avoid the influence of confounders. Qualitative methods should be used to assess the influence of contextual factors on the success of interventions.[4] Most importantly, future studies should be based on conceptual models that explain how components of the intervention influence proximal measures of teamwork and downstream patient outcomes.
In the meantime, what is a hospital leader to do? We believe efforts to improve IDR are warranted, but that IDR program leaders need to first specify their primary objective(s). For example, in some hospitals, there may be little room to further reduce LOS, so another goalreducing preventable readmissions or reducing adverse eventsmight be specified as the key performance indicator. This crucial first step of creating a shared goal informs the design, implementation, and evaluation of IDR. We also believe that geographic localization of physicians to specific units is foundational to improving IDR. Physicians cannot feasibly attend IDR if their patients are spread across multiple units (or buildings). Finally, hospital leaders also need to view IDR as part of a larger set of interventions to improve teamwork. Leaders need to assess the adequacy of staffing levels, workflow, and team composition.[8] Unit‐based interdisciplinary leadership models should be used to help link efforts at various levels within a larger system.[9] These models designate a unit medical director and nurse manager who are jointly responsible for unit performance.
In conclusion, IDR play an important role in improving patient outcomes, but only do so by improving teamwork. In redesigning IDR, leaders need to be thoughtful about what outcomes IDR can affect, how IDR affect them, and how IDR fit into larger‐scale efforts to improve performance.
Disclosure
Nothing to report.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 1: Research Findings. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Structure and outcomes of inter‐disciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomny. J Hosp Med. 2016;11:513–523.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- . Improvement interventions are social treatments, not pills. Ann Intern Med. 2014;161(7):526–527.
- , , , et al. Teamwork assessment in internal medicine: a systematic review of validity evidence and outcomes. J Gen Intern Med. 2014;29(6):894–910.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , . Improving the quality and safety of care on the medical ward: A review and synthesis of the evidence base. Eur J Intern Med. 2014;25(10):874–887.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
- , , , , , . Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 1: Research Findings. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. Patterns of nurse‐physician communication and agreement on the plan of care. Qual Saf Health Care. 2010;19(3):195–199.
- , , , , , . Structure and outcomes of inter‐disciplinary rounds in hospitalized medicine patients: a systematic review and suggested taxonomny. J Hosp Med. 2016;11:513–523.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- . Improvement interventions are social treatments, not pills. Ann Intern Med. 2014;161(7):526–527.
- , , , et al. Teamwork assessment in internal medicine: a systematic review of validity evidence and outcomes. J Gen Intern Med. 2014;29(6):894–910.
- , , , et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288–1298.
- , , , . Improving the quality and safety of care on the medical ward: A review and synthesis of the evidence base. Eur J Intern Med. 2014;25(10):874–887.
- , , , , , . Unit‐based interprofessional leadership models in six US hospitals. J Hosp Med. 2014;9(8):545–550.
Medicare Insurance Reduces Cost
Up to 10% of the acutely ill patients in hospitals today are not admitted, but cared for under outpatient observation.[1, 2] Hospitals use observation services to replace inpatient services for patients who do not meet inpatient illness standards. Use of this technique for Medicare beneficiaries has grown in recent years, and future policy changes may further increase the percentage of hospital stays classified as observation.[3, 4]
Increased use of observation services has cost‐sharing implications for Medicare beneficiaries. Because hospital observation stays are paid through Medicare's outpatient (Part B) rather than inpatient (Part A) benefit, beneficiaries do not have an out‐of‐pocket maximum per hospital stay.[3] Although prior analyses documented mean out‐of‐pocket costs of $400 to $600 per stay, out‐of‐pocket costs may exceed the Part A deductible and leave beneficiaries responsible for hospital costs that may have been reimbursed by Part A in an inpatient stay.[5, 6, 7] Approximately 6% to 10% of Medicare observation stays result in out‐of‐pocket costs exceeding the Part A deductible nationally, and recent work in this journal documented that 26.6% of beneficiaries with repeat observation stays within a 60‐day period may pay more than the 1‐time deductible‐based payment that beneficiaries are responsible for under Medicare Part A.[5, 6, 7]
However, prior analyses of beneficiary out‐of‐pocket costs did not account for supplemental insurance payments. Approximately 80% to 90% of fee‐for‐service Medicare beneficiaries who use either a private (Medigap or employer based) or state‐based (Medicaid or other plans) supplemental insurance plan.[8, 9, 10] The effect of supplemental insurance on out‐of‐pocket costs has been documented for inpatient stays, yet has not been explored for observation stays.[9] We sought to describe Medicare beneficiaries' out‐of‐pocket costs by accounting for payments from all insurers.
METHODS
We obtained payment data from 2 affiliated hospitals for all Medicare observation hospital stays between April 2013 and March 2014. Stays insured by Medicare Advantage plans (Part C) were excluded, and charges from skilled nursing facility (SNF) stays were not available. Although the exact origin of each stay was not available, the majority of stays at these hospitals came from emergency visits, direct placement from outpatient providers, and postoutpatient procedural monitoring. The dataset included charges, insurance adjustments and payments from all sources, diagnoses, and demographics. Insurance adjustments represented the reductions applied to total stay charges by each insurer in congruence with their contract with the health system. Our dataset included charges for all hospital materials and services usually billed under Part A, including medications. Out‐of‐pocket costs were calculated by subtracting insurance adjustments and payments from the total charges for each stay. To identify potential cost‐shifting from Medicare to beneficiaries, we compared out‐of‐pocket costs to the Part A deductible ($1184 for stays in 2013 and $1216 for 2014). Household income data were estimated using zip code and Internal Revenue Service data for 2013.[11]
The University of California Los Angeles institutional review board approved this study. Statistical significance was calculated using a 2‐tailed unpaired t test for means and 2 for percentages.
RESULTS
There were 2029 total observation stays during the study period, representing 5.0% of all discharges from both hospitals. Medicare beneficiaries accounted for 722 of those observation stays. Among the 498 finalized Medicare observation stays, the median patient age was 73 years, and median household income was $50,591. The median length of stay was 25 hours, with 1.8% of stays lasting longer than 2 midnights. Seventy percent of beneficiaries had private supplemental insurance, whereas 6% had state‐based supplemental plans. Table 1 presents detailed costs. Out‐of‐pocket costs ranged from $0 to $16,196. Stays without supplemental insurance had mean and median out of pocket costs of $537 and $286, respectively. The mean out‐of‐pocket costs for stays with private supplemental insurance decreased to $45 (P 0.01) and $168 (P = 0.21) for stays with state‐based supplemental insurance, with a median below $1 for both. On average, beneficiaries without supplemental insurance were responsible for $654 less than the Part A deductible. Thirteen beneficiaries had multiple finalized stays within 60 days, with a mean out‐of‐pocket cost of $119, median of $20, and 1 stay produced an out‐of‐pocket cost exceeding the Part A deductible. An additional 224 Medicare stays not finalized because of missing supplemental payments had a mean out‐of‐pocket cost of $125 (P 0.01) and median of $5 after applying supplemental insurer adjustments (not shown in Table 1).
| Medicare Stays, N = 498 | |||
|---|---|---|---|
| Mean (SD) | Median (IQR) | Range | |
| |||
| Payments by Medicare | $2,533 (2,883) | $1521 (1,898) | $4$29,633 |
| Payments by supplemental insurers | |||
| Private insurers (N = 351) | $454 (384) | $324 (332) | $0$2,590 |
| State‐based insurers (N = 29) | $222(402) | $43 (102) | $2$1,229 |
| Beneficiary out‐of‐pocket costs | |||
| Without supplemental insurer (N = 118) | $537 (1,557) | $286 (440) | $0$16,196* |
| With private supplemental insurer (N = 351) | $45 (414) | $0.39 (16) | $0$7,670* |
| With state‐based supplemental insurer (N = 29) | $168 (412) | $0.15 (27) | $01,870* |
A minority of observation stays produced out‐of‐pocket costs exceeding the Part A deductible. Those percentages were 7.6% for stays without a supplemental insurer, 3.5% (P 0.01) for stays with a state‐based supplemental insurer, and 0.3% (P 0.01) for stays with a private supplemental insurer. Of the 224 nonfinalized Medicare stays, 1.3% (P 0.01) exceeded the Part A deductible after supplemental insurer adjustments.
DISCUSSION
This study demonstrates that supplemental insurance can dramatically reduce Medicare beneficiaries' out‐of‐pocket costs in observation services. Mean out‐of‐pocket costs of $45 and $168 for stays with private and state‐based supplemental insurer plans are significantly lower than prior estimates calculated without supplemental insurance information, as are the percentages of stays with out‐of‐pocket costs exceeding the Part A deductible, at 0.3% and 3.5%, respectively.[5, 6, 7] Because the majority of Medicare beneficiaries use supplemental insurance, excessive out‐of‐pockets in observation services may occur less frequently than previously reported.[9, 10] Clinicians concerned about excessive out‐of‐pocket costs for Medicare beneficiaries can be reassured they are usually modest for beneficiaries with supplemental insurance.
This study's mean out‐of‐pocket cost and percentage of stays with out‐of‐pocket costs exceeding the Part A deductible for beneficiaries without supplemental insurance are similar to results from prior national analyses performed without supplemental insurer information.[5, 6, 7] But this study was limited by a small sample size from 2 affiliated hospitals, with few repeat observation stays within a 60‐day period. In addition, posthospitalization SNF fees were not included, which traditionally have been a significant source of out‐of‐pocket costs in observation services.[3, 7] Populations with supplemental insurance treated elsewhere may incur hospital out‐of‐pocket costs differing from these results due to dissimilarities in the presence and quality of supplemental insurance.
However, most Medigap plans are federally regulated to cover the majority of out‐of‐pockets unpaid by Medicare.[12] Medicaid plans usually place limits on out‐of‐pocket costs, and any other state or employer‐based supplemental plans will also reduce out‐of‐pocket costs.[13] Thus, it is likely accurate to assume mean observation services out‐of‐pocket costs for hospital fees are lower than previously reported by national analyses performed without supplemental insurance information. Attempts at estimating beneficiary out‐of‐pocket costs in the future should account for supplemental insurance adjustments and payments.
Acknowledgements
The authors thank Andrew Kaufman for his work in obtaining these data.
Disclosures: Dr. Doyle's time was supported by a National Research Service Award from the National Institutes of Health and administered through the University of California Los Angeles. Drs. Ettner and Nuckols received no support for this work. There are no conflicts of interest to report.
- , , , et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173(21):1991–1998.
- , , . Final report observation status related to hospital records. Available at: https://www.hcup‐us.ahrq.gov/reports/methods/FinalReportonObservationStatus_v2Final.pdf. Published September 27, 2002.
- . The two‐midnight rule. Health Policy Briefs. Health Affairs website. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=133. Published January 22, 2015.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , , , . Patient financial responsibility for observation care. J Hosp Med. 2015;10(11):718–723.
- , , L. . Observation status: Financial Implications for Medicare Beneficiaries. AARP Public Policy Institute. Available at: http://www.aarp.org/content/dam/aarp/ppi/2015/Hosp Obs Financial Impact Paper.pdf. Published April 2015.
- . Hospitals' use of observation stays and short inpatient stays for Medicare beneficiaries. Department of Health and Human Services. Office of Inspector General. Available at: https://oig.hhs.gov/oei/reports/oei‐02‐12‐00040.pdf. Published July 29, 2013.
- . Trends in Medicare supplemental insurance and prescription drug benefits, 1996–2001: Data update. Available at: http://www.kff.org/medicare/upload/Trends‐in‐Medicare‐Supplemental‐Insurance‐and‐Prescription‐Drug‐Benefits‐1996–2001Data‐Update.pdf.
- . Medicare beneficiaries' out‐of‐pocket spending for health care. AARP Public Policy Institute. Available at: http://www.aarp.org/content/dam/aarp/research/public_policy_institute/health/medicare‐beneficiaries‐out‐of‐pocket‐spending‐AARP‐ppi‐health.pdf. Published May 2012.
- , , , et al. A primer on Medicare: key facts about the Medicare program and the people it covers. What types of supplemental insurance do beneficiaries have? Kaiser Family Foundation website. Available at: http://kff.org/report‐section/a‐primer‐on‐medicare‐what‐types‐of‐supplemental‐insurance‐do‐beneficiaries‐have. Published March 20, 2015.
- SOI tax stats—individual income tax statistics—ZIP code data (SOI). Available at: https://www.irs.gov/uac/SOI‐Tax‐Stats‐Individual‐Income‐Tax‐Statistics‐ZIP‐Code‐Data‐(SOI). Accessed January 1, 2016.
- How to compare Medigap policies. Medicare.gov website. Available at: https://www.medicare.gov/supplement‐other‐insurance/compare‐medigap/compare‐medigap.html.
- Cost sharing out of pocket costs. Medicaid.gov website. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/by‐topics/cost‐sharing/cost‐sharing‐out‐of‐pocket‐costs.html. Accessed January 26, 2016.
- , , . Balancing margin and mission: hospitals alter billing and collection practices for uninsured patients. Issue Brief Cent Stud Health Syst Change. 2005;(99):1–4.
Up to 10% of the acutely ill patients in hospitals today are not admitted, but cared for under outpatient observation.[1, 2] Hospitals use observation services to replace inpatient services for patients who do not meet inpatient illness standards. Use of this technique for Medicare beneficiaries has grown in recent years, and future policy changes may further increase the percentage of hospital stays classified as observation.[3, 4]
Increased use of observation services has cost‐sharing implications for Medicare beneficiaries. Because hospital observation stays are paid through Medicare's outpatient (Part B) rather than inpatient (Part A) benefit, beneficiaries do not have an out‐of‐pocket maximum per hospital stay.[3] Although prior analyses documented mean out‐of‐pocket costs of $400 to $600 per stay, out‐of‐pocket costs may exceed the Part A deductible and leave beneficiaries responsible for hospital costs that may have been reimbursed by Part A in an inpatient stay.[5, 6, 7] Approximately 6% to 10% of Medicare observation stays result in out‐of‐pocket costs exceeding the Part A deductible nationally, and recent work in this journal documented that 26.6% of beneficiaries with repeat observation stays within a 60‐day period may pay more than the 1‐time deductible‐based payment that beneficiaries are responsible for under Medicare Part A.[5, 6, 7]
However, prior analyses of beneficiary out‐of‐pocket costs did not account for supplemental insurance payments. Approximately 80% to 90% of fee‐for‐service Medicare beneficiaries who use either a private (Medigap or employer based) or state‐based (Medicaid or other plans) supplemental insurance plan.[8, 9, 10] The effect of supplemental insurance on out‐of‐pocket costs has been documented for inpatient stays, yet has not been explored for observation stays.[9] We sought to describe Medicare beneficiaries' out‐of‐pocket costs by accounting for payments from all insurers.
METHODS
We obtained payment data from 2 affiliated hospitals for all Medicare observation hospital stays between April 2013 and March 2014. Stays insured by Medicare Advantage plans (Part C) were excluded, and charges from skilled nursing facility (SNF) stays were not available. Although the exact origin of each stay was not available, the majority of stays at these hospitals came from emergency visits, direct placement from outpatient providers, and postoutpatient procedural monitoring. The dataset included charges, insurance adjustments and payments from all sources, diagnoses, and demographics. Insurance adjustments represented the reductions applied to total stay charges by each insurer in congruence with their contract with the health system. Our dataset included charges for all hospital materials and services usually billed under Part A, including medications. Out‐of‐pocket costs were calculated by subtracting insurance adjustments and payments from the total charges for each stay. To identify potential cost‐shifting from Medicare to beneficiaries, we compared out‐of‐pocket costs to the Part A deductible ($1184 for stays in 2013 and $1216 for 2014). Household income data were estimated using zip code and Internal Revenue Service data for 2013.[11]
The University of California Los Angeles institutional review board approved this study. Statistical significance was calculated using a 2‐tailed unpaired t test for means and 2 for percentages.
RESULTS
There were 2029 total observation stays during the study period, representing 5.0% of all discharges from both hospitals. Medicare beneficiaries accounted for 722 of those observation stays. Among the 498 finalized Medicare observation stays, the median patient age was 73 years, and median household income was $50,591. The median length of stay was 25 hours, with 1.8% of stays lasting longer than 2 midnights. Seventy percent of beneficiaries had private supplemental insurance, whereas 6% had state‐based supplemental plans. Table 1 presents detailed costs. Out‐of‐pocket costs ranged from $0 to $16,196. Stays without supplemental insurance had mean and median out of pocket costs of $537 and $286, respectively. The mean out‐of‐pocket costs for stays with private supplemental insurance decreased to $45 (P 0.01) and $168 (P = 0.21) for stays with state‐based supplemental insurance, with a median below $1 for both. On average, beneficiaries without supplemental insurance were responsible for $654 less than the Part A deductible. Thirteen beneficiaries had multiple finalized stays within 60 days, with a mean out‐of‐pocket cost of $119, median of $20, and 1 stay produced an out‐of‐pocket cost exceeding the Part A deductible. An additional 224 Medicare stays not finalized because of missing supplemental payments had a mean out‐of‐pocket cost of $125 (P 0.01) and median of $5 after applying supplemental insurer adjustments (not shown in Table 1).
| Medicare Stays, N = 498 | |||
|---|---|---|---|
| Mean (SD) | Median (IQR) | Range | |
| |||
| Payments by Medicare | $2,533 (2,883) | $1521 (1,898) | $4$29,633 |
| Payments by supplemental insurers | |||
| Private insurers (N = 351) | $454 (384) | $324 (332) | $0$2,590 |
| State‐based insurers (N = 29) | $222(402) | $43 (102) | $2$1,229 |
| Beneficiary out‐of‐pocket costs | |||
| Without supplemental insurer (N = 118) | $537 (1,557) | $286 (440) | $0$16,196* |
| With private supplemental insurer (N = 351) | $45 (414) | $0.39 (16) | $0$7,670* |
| With state‐based supplemental insurer (N = 29) | $168 (412) | $0.15 (27) | $01,870* |
A minority of observation stays produced out‐of‐pocket costs exceeding the Part A deductible. Those percentages were 7.6% for stays without a supplemental insurer, 3.5% (P 0.01) for stays with a state‐based supplemental insurer, and 0.3% (P 0.01) for stays with a private supplemental insurer. Of the 224 nonfinalized Medicare stays, 1.3% (P 0.01) exceeded the Part A deductible after supplemental insurer adjustments.
DISCUSSION
This study demonstrates that supplemental insurance can dramatically reduce Medicare beneficiaries' out‐of‐pocket costs in observation services. Mean out‐of‐pocket costs of $45 and $168 for stays with private and state‐based supplemental insurer plans are significantly lower than prior estimates calculated without supplemental insurance information, as are the percentages of stays with out‐of‐pocket costs exceeding the Part A deductible, at 0.3% and 3.5%, respectively.[5, 6, 7] Because the majority of Medicare beneficiaries use supplemental insurance, excessive out‐of‐pockets in observation services may occur less frequently than previously reported.[9, 10] Clinicians concerned about excessive out‐of‐pocket costs for Medicare beneficiaries can be reassured they are usually modest for beneficiaries with supplemental insurance.
This study's mean out‐of‐pocket cost and percentage of stays with out‐of‐pocket costs exceeding the Part A deductible for beneficiaries without supplemental insurance are similar to results from prior national analyses performed without supplemental insurer information.[5, 6, 7] But this study was limited by a small sample size from 2 affiliated hospitals, with few repeat observation stays within a 60‐day period. In addition, posthospitalization SNF fees were not included, which traditionally have been a significant source of out‐of‐pocket costs in observation services.[3, 7] Populations with supplemental insurance treated elsewhere may incur hospital out‐of‐pocket costs differing from these results due to dissimilarities in the presence and quality of supplemental insurance.
However, most Medigap plans are federally regulated to cover the majority of out‐of‐pockets unpaid by Medicare.[12] Medicaid plans usually place limits on out‐of‐pocket costs, and any other state or employer‐based supplemental plans will also reduce out‐of‐pocket costs.[13] Thus, it is likely accurate to assume mean observation services out‐of‐pocket costs for hospital fees are lower than previously reported by national analyses performed without supplemental insurance information. Attempts at estimating beneficiary out‐of‐pocket costs in the future should account for supplemental insurance adjustments and payments.
Acknowledgements
The authors thank Andrew Kaufman for his work in obtaining these data.
Disclosures: Dr. Doyle's time was supported by a National Research Service Award from the National Institutes of Health and administered through the University of California Los Angeles. Drs. Ettner and Nuckols received no support for this work. There are no conflicts of interest to report.
Up to 10% of the acutely ill patients in hospitals today are not admitted, but cared for under outpatient observation.[1, 2] Hospitals use observation services to replace inpatient services for patients who do not meet inpatient illness standards. Use of this technique for Medicare beneficiaries has grown in recent years, and future policy changes may further increase the percentage of hospital stays classified as observation.[3, 4]
Increased use of observation services has cost‐sharing implications for Medicare beneficiaries. Because hospital observation stays are paid through Medicare's outpatient (Part B) rather than inpatient (Part A) benefit, beneficiaries do not have an out‐of‐pocket maximum per hospital stay.[3] Although prior analyses documented mean out‐of‐pocket costs of $400 to $600 per stay, out‐of‐pocket costs may exceed the Part A deductible and leave beneficiaries responsible for hospital costs that may have been reimbursed by Part A in an inpatient stay.[5, 6, 7] Approximately 6% to 10% of Medicare observation stays result in out‐of‐pocket costs exceeding the Part A deductible nationally, and recent work in this journal documented that 26.6% of beneficiaries with repeat observation stays within a 60‐day period may pay more than the 1‐time deductible‐based payment that beneficiaries are responsible for under Medicare Part A.[5, 6, 7]
However, prior analyses of beneficiary out‐of‐pocket costs did not account for supplemental insurance payments. Approximately 80% to 90% of fee‐for‐service Medicare beneficiaries who use either a private (Medigap or employer based) or state‐based (Medicaid or other plans) supplemental insurance plan.[8, 9, 10] The effect of supplemental insurance on out‐of‐pocket costs has been documented for inpatient stays, yet has not been explored for observation stays.[9] We sought to describe Medicare beneficiaries' out‐of‐pocket costs by accounting for payments from all insurers.
METHODS
We obtained payment data from 2 affiliated hospitals for all Medicare observation hospital stays between April 2013 and March 2014. Stays insured by Medicare Advantage plans (Part C) were excluded, and charges from skilled nursing facility (SNF) stays were not available. Although the exact origin of each stay was not available, the majority of stays at these hospitals came from emergency visits, direct placement from outpatient providers, and postoutpatient procedural monitoring. The dataset included charges, insurance adjustments and payments from all sources, diagnoses, and demographics. Insurance adjustments represented the reductions applied to total stay charges by each insurer in congruence with their contract with the health system. Our dataset included charges for all hospital materials and services usually billed under Part A, including medications. Out‐of‐pocket costs were calculated by subtracting insurance adjustments and payments from the total charges for each stay. To identify potential cost‐shifting from Medicare to beneficiaries, we compared out‐of‐pocket costs to the Part A deductible ($1184 for stays in 2013 and $1216 for 2014). Household income data were estimated using zip code and Internal Revenue Service data for 2013.[11]
The University of California Los Angeles institutional review board approved this study. Statistical significance was calculated using a 2‐tailed unpaired t test for means and 2 for percentages.
RESULTS
There were 2029 total observation stays during the study period, representing 5.0% of all discharges from both hospitals. Medicare beneficiaries accounted for 722 of those observation stays. Among the 498 finalized Medicare observation stays, the median patient age was 73 years, and median household income was $50,591. The median length of stay was 25 hours, with 1.8% of stays lasting longer than 2 midnights. Seventy percent of beneficiaries had private supplemental insurance, whereas 6% had state‐based supplemental plans. Table 1 presents detailed costs. Out‐of‐pocket costs ranged from $0 to $16,196. Stays without supplemental insurance had mean and median out of pocket costs of $537 and $286, respectively. The mean out‐of‐pocket costs for stays with private supplemental insurance decreased to $45 (P 0.01) and $168 (P = 0.21) for stays with state‐based supplemental insurance, with a median below $1 for both. On average, beneficiaries without supplemental insurance were responsible for $654 less than the Part A deductible. Thirteen beneficiaries had multiple finalized stays within 60 days, with a mean out‐of‐pocket cost of $119, median of $20, and 1 stay produced an out‐of‐pocket cost exceeding the Part A deductible. An additional 224 Medicare stays not finalized because of missing supplemental payments had a mean out‐of‐pocket cost of $125 (P 0.01) and median of $5 after applying supplemental insurer adjustments (not shown in Table 1).
| Medicare Stays, N = 498 | |||
|---|---|---|---|
| Mean (SD) | Median (IQR) | Range | |
| |||
| Payments by Medicare | $2,533 (2,883) | $1521 (1,898) | $4$29,633 |
| Payments by supplemental insurers | |||
| Private insurers (N = 351) | $454 (384) | $324 (332) | $0$2,590 |
| State‐based insurers (N = 29) | $222(402) | $43 (102) | $2$1,229 |
| Beneficiary out‐of‐pocket costs | |||
| Without supplemental insurer (N = 118) | $537 (1,557) | $286 (440) | $0$16,196* |
| With private supplemental insurer (N = 351) | $45 (414) | $0.39 (16) | $0$7,670* |
| With state‐based supplemental insurer (N = 29) | $168 (412) | $0.15 (27) | $01,870* |
A minority of observation stays produced out‐of‐pocket costs exceeding the Part A deductible. Those percentages were 7.6% for stays without a supplemental insurer, 3.5% (P 0.01) for stays with a state‐based supplemental insurer, and 0.3% (P 0.01) for stays with a private supplemental insurer. Of the 224 nonfinalized Medicare stays, 1.3% (P 0.01) exceeded the Part A deductible after supplemental insurer adjustments.
DISCUSSION
This study demonstrates that supplemental insurance can dramatically reduce Medicare beneficiaries' out‐of‐pocket costs in observation services. Mean out‐of‐pocket costs of $45 and $168 for stays with private and state‐based supplemental insurer plans are significantly lower than prior estimates calculated without supplemental insurance information, as are the percentages of stays with out‐of‐pocket costs exceeding the Part A deductible, at 0.3% and 3.5%, respectively.[5, 6, 7] Because the majority of Medicare beneficiaries use supplemental insurance, excessive out‐of‐pockets in observation services may occur less frequently than previously reported.[9, 10] Clinicians concerned about excessive out‐of‐pocket costs for Medicare beneficiaries can be reassured they are usually modest for beneficiaries with supplemental insurance.
This study's mean out‐of‐pocket cost and percentage of stays with out‐of‐pocket costs exceeding the Part A deductible for beneficiaries without supplemental insurance are similar to results from prior national analyses performed without supplemental insurer information.[5, 6, 7] But this study was limited by a small sample size from 2 affiliated hospitals, with few repeat observation stays within a 60‐day period. In addition, posthospitalization SNF fees were not included, which traditionally have been a significant source of out‐of‐pocket costs in observation services.[3, 7] Populations with supplemental insurance treated elsewhere may incur hospital out‐of‐pocket costs differing from these results due to dissimilarities in the presence and quality of supplemental insurance.
However, most Medigap plans are federally regulated to cover the majority of out‐of‐pockets unpaid by Medicare.[12] Medicaid plans usually place limits on out‐of‐pocket costs, and any other state or employer‐based supplemental plans will also reduce out‐of‐pocket costs.[13] Thus, it is likely accurate to assume mean observation services out‐of‐pocket costs for hospital fees are lower than previously reported by national analyses performed without supplemental insurance information. Attempts at estimating beneficiary out‐of‐pocket costs in the future should account for supplemental insurance adjustments and payments.
Acknowledgements
The authors thank Andrew Kaufman for his work in obtaining these data.
Disclosures: Dr. Doyle's time was supported by a National Research Service Award from the National Institutes of Health and administered through the University of California Los Angeles. Drs. Ettner and Nuckols received no support for this work. There are no conflicts of interest to report.
- , , , et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173(21):1991–1998.
- , , . Final report observation status related to hospital records. Available at: https://www.hcup‐us.ahrq.gov/reports/methods/FinalReportonObservationStatus_v2Final.pdf. Published September 27, 2002.
- . The two‐midnight rule. Health Policy Briefs. Health Affairs website. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=133. Published January 22, 2015.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , , , . Patient financial responsibility for observation care. J Hosp Med. 2015;10(11):718–723.
- , , L. . Observation status: Financial Implications for Medicare Beneficiaries. AARP Public Policy Institute. Available at: http://www.aarp.org/content/dam/aarp/ppi/2015/Hosp Obs Financial Impact Paper.pdf. Published April 2015.
- . Hospitals' use of observation stays and short inpatient stays for Medicare beneficiaries. Department of Health and Human Services. Office of Inspector General. Available at: https://oig.hhs.gov/oei/reports/oei‐02‐12‐00040.pdf. Published July 29, 2013.
- . Trends in Medicare supplemental insurance and prescription drug benefits, 1996–2001: Data update. Available at: http://www.kff.org/medicare/upload/Trends‐in‐Medicare‐Supplemental‐Insurance‐and‐Prescription‐Drug‐Benefits‐1996–2001Data‐Update.pdf.
- . Medicare beneficiaries' out‐of‐pocket spending for health care. AARP Public Policy Institute. Available at: http://www.aarp.org/content/dam/aarp/research/public_policy_institute/health/medicare‐beneficiaries‐out‐of‐pocket‐spending‐AARP‐ppi‐health.pdf. Published May 2012.
- , , , et al. A primer on Medicare: key facts about the Medicare program and the people it covers. What types of supplemental insurance do beneficiaries have? Kaiser Family Foundation website. Available at: http://kff.org/report‐section/a‐primer‐on‐medicare‐what‐types‐of‐supplemental‐insurance‐do‐beneficiaries‐have. Published March 20, 2015.
- SOI tax stats—individual income tax statistics—ZIP code data (SOI). Available at: https://www.irs.gov/uac/SOI‐Tax‐Stats‐Individual‐Income‐Tax‐Statistics‐ZIP‐Code‐Data‐(SOI). Accessed January 1, 2016.
- How to compare Medigap policies. Medicare.gov website. Available at: https://www.medicare.gov/supplement‐other‐insurance/compare‐medigap/compare‐medigap.html.
- Cost sharing out of pocket costs. Medicaid.gov website. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/by‐topics/cost‐sharing/cost‐sharing‐out‐of‐pocket‐costs.html. Accessed January 26, 2016.
- , , . Balancing margin and mission: hospitals alter billing and collection practices for uninsured patients. Issue Brief Cent Stud Health Syst Change. 2005;(99):1–4.
- , , , et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173(21):1991–1998.
- , , . Final report observation status related to hospital records. Available at: https://www.hcup‐us.ahrq.gov/reports/methods/FinalReportonObservationStatus_v2Final.pdf. Published September 27, 2002.
- . The two‐midnight rule. Health Policy Briefs. Health Affairs website. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=133. Published January 22, 2015.
- , , . Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251–1259.
- , , , , . Patient financial responsibility for observation care. J Hosp Med. 2015;10(11):718–723.
- , , L. . Observation status: Financial Implications for Medicare Beneficiaries. AARP Public Policy Institute. Available at: http://www.aarp.org/content/dam/aarp/ppi/2015/Hosp Obs Financial Impact Paper.pdf. Published April 2015.
- . Hospitals' use of observation stays and short inpatient stays for Medicare beneficiaries. Department of Health and Human Services. Office of Inspector General. Available at: https://oig.hhs.gov/oei/reports/oei‐02‐12‐00040.pdf. Published July 29, 2013.
- . Trends in Medicare supplemental insurance and prescription drug benefits, 1996–2001: Data update. Available at: http://www.kff.org/medicare/upload/Trends‐in‐Medicare‐Supplemental‐Insurance‐and‐Prescription‐Drug‐Benefits‐1996–2001Data‐Update.pdf.
- . Medicare beneficiaries' out‐of‐pocket spending for health care. AARP Public Policy Institute. Available at: http://www.aarp.org/content/dam/aarp/research/public_policy_institute/health/medicare‐beneficiaries‐out‐of‐pocket‐spending‐AARP‐ppi‐health.pdf. Published May 2012.
- , , , et al. A primer on Medicare: key facts about the Medicare program and the people it covers. What types of supplemental insurance do beneficiaries have? Kaiser Family Foundation website. Available at: http://kff.org/report‐section/a‐primer‐on‐medicare‐what‐types‐of‐supplemental‐insurance‐do‐beneficiaries‐have. Published March 20, 2015.
- SOI tax stats—individual income tax statistics—ZIP code data (SOI). Available at: https://www.irs.gov/uac/SOI‐Tax‐Stats‐Individual‐Income‐Tax‐Statistics‐ZIP‐Code‐Data‐(SOI). Accessed January 1, 2016.
- How to compare Medigap policies. Medicare.gov website. Available at: https://www.medicare.gov/supplement‐other‐insurance/compare‐medigap/compare‐medigap.html.
- Cost sharing out of pocket costs. Medicaid.gov website. Available at: https://www.medicaid.gov/medicaid‐chip‐program‐information/by‐topics/cost‐sharing/cost‐sharing‐out‐of‐pocket‐costs.html. Accessed January 26, 2016.
- , , . Balancing margin and mission: hospitals alter billing and collection practices for uninsured patients. Issue Brief Cent Stud Health Syst Change. 2005;(99):1–4.
Long‐term Antipsychotics in Elders
Delirium, a clinical syndrome characterized by inattention and acute cognitive dysfunction, is very common in older hospitalized patients, with a reported incidence of 18% to 35% at time of admission and overall occurrence rates of 29% to 64%.[1] Previous studies have reported that a diagnosis of delirium is not benign and is associated with other adverse outcomes including prolonged hospitalization, institutionalization, increased cost, and mortality. These outcomes occurred independent of age, prior cognitive functioning, and comorbidities.[2] Guidelines recommend that management of inpatient delirium should be focused on addressing the underlying etiology and managed with nonpharmacological interventions whenever possible.[3, 4, 5] However, implementing these recommendations can prove to be very challenging in hospital settings. Providers frequently have to resort to medical therapies, including antipsychotics (APs). Although these medications are commonly used to treat delirium in elderly patients, there is limited evidence to support their efficacy, and there are currently no proven pharmacological alternatives to these medications.[6] Furthermore, previous studies have demonstrated an increased risk of stroke, infection, cognitive impairment, and mortality in elders with dementia who receive long‐term AP therapy.[7, 8, 9] Yet as many as 48% of hospitalized elders who were newly started on APs had these drugs continued at time of discharge.[10]
There have been few studies describing the long‐term outcomes of elderly patient who are started on APs in the hospital. Most information on outcomes comes from patients with dementia. Therefore, we studied the 1‐year outcomes of a cohort of patients with and without dementia who were started on APs in the hospital and then discharged on these medications. In this cohort, we aimed to describe the number of readmissions, reasons for readmissions, duration of AP therapy, use of other sedating medications such as anxiolytics, hypnotics, and antihistamines as well as the incidence of readmission and death 1 year after the index hospital discharge.
METHODS
We previously described a retrospective cohort of 300 elders (65 years old) admitted to a tertiary care hospital between October 1, 2012 and September 31, 2013 who were newly prescribed APs while hospitalized.[10] Of patients alive at the time of discharge (260), 56% (146 patients) were discharged on APs. Two investigators extracted these 148 patient charts independently to identify and quantify the number of readmissions to the index hospital. We then limited the sample to only the first readmission per patient following the index admission and extracted this readmission for each patient. We first determined if APs were present on the admission medication reconciliation. If APs were not present on admission, we examined whether they were resumed during the hospitalization using the electronic medication administration summary. If they were present on admission, we looked to see if they were discontinued during the readmission and if additional new APs were started during the hospitalizations. We documented the circumstances around APs use and identified patients who died during their hospitalizations. We identified delirium using the same terms that were described in our prior study on the same cohort of patients.[10] We determined if patients were delirious using a predetermined algorithm (Figure 1). Briefly, we first determined delirium was documented. We then examined whether there was a Confusion Assessment Method (CAM) instrument included in the record. If a CAM instrument was not documented, we then looked for documentation using specific terms (eg, disorientation, confusions). We identified patients with dementia by determining whether dementia was documented along with other admission medical comorbidities. If it was not, we determined whether dementia was newly diagnosed during the hospital stay using progress notes or consultation notes. We did not objectively define criteria for diagnosis of dementia. We used the National Death Index (NDI) to determine mortality for all patients 1 year after discharge from the index hospitalization. The NDI is a national database of death records maintained by the National Center for Health Statistics. It has shown consistently high sensitivity and specificity for detection of death.[11]
We used descriptive statistics (means, standard deviations, range, and percents as appropriate to the scale of measurement) to describe the patient sample. We then used multiple logistic regression to identify significant predictors of death within 1 year of discharge.[12] Univariate analysis was used to select candidates for the logistic model (t tests for continuous factors and 2 for discrete factors). All factors with a significance level 0.2 on univariate analysis were included in the logistic regression, in addition to age and sex (regardless of significance). A maximum likelihood procedure was used to calculate the regression coefficients for the logistic model. The likelihood ratio criterion was used to determine the significance of individual factors in the regression model.[13] Factors with a significance level of 0.15 or less were retained in the final model, in addition to age and sex.
RESULTS
The 260 patients discharged alive from their index admissions had a 1‐year mortality rate of 29% (75/260). Of the 146/260 patients discharged on APs, 60 (41%) patients experienced at least 1 readmission (mean = 2 readmissions per patient; range, 18, with 111 total readmissions for 60 patients) within 1 year from discharge (Figure 2). Most common diagnoses at the time of readmissions were related neurological and psychiatric disorders (14%), cardiovascular and circulation disorders (13%), renal injury and electrolyte disorders (11%), and infections (6%). Among patients with at least 1 readmission, the mean age was 81.3 (range, 65.599.7), 60% were male, and 45% were admitted from a skilled nursing facility or rehabilitation facility (Table 1). Median time to readmission was 43.5 days (range, 1343 days), and 79% were readmitted to a medical service. The remaining 20% were admitted to a surgical service. Inpatient mortality during first readmissions was 8% (5/60). At the time of first readmission, 39/60 (65%) of patients were still on the same APs on which they had been discharged, and the APs were continued during the hospitalization in 79% of the patients (61% quetiapine, 19% olanzapine, and 13% risperidone). About half of patients whose APs were discontinued prior to readmission received a new AP during their hospital stays (9/20; 45%). One patient had been started on quetiapine in the outpatient setting. No patients were found to have new benzodiazepines, nonbenzodiazepine hypnotic, or antihistamines on their admission medication list.
| Variables | Value* |
|---|---|
| |
| Age, mean (range), yr | 81.3 (65.599.7) |
| Gender, no. (%) | |
| Male | 36 (60) |
| Female | 24 (40) |
| Admitted from, no. (%) | |
| Home | 33 (55) |
| Rehabilitation facilities | 5 (8) |
| SNF | 22 (37) |
| Services, no. (%) | |
| Medicine | 48 (80) |
| Surgery | 12 (20) |
| Types of APs continued on readmission (from index admission), no. (%) | |
| Quetiapine | 19 (61) |
| Olanzapine | 6 (19) |
| Risperidone | 4 (13) |
| Haloperidol | 2 (7) |
| Types of APs started during readmission, no. (%) | |
| Quetiapine | 7 (39) |
| Risperidone | 2 (11) |
| Haloperidol | 16 (89) |
| Indications for AP use, no. (%) | |
| Delirium | 14 (77) |
| Undocumented | 3 (17) |
| Other | 1 (6) |
| ECG, no. (%) | |
| Prior to APs administration | 17 (94) |
| After APs administration | 4 (22) |
| QTc prolongation >500 ms, no. (%) | |
| Prior to APs administration | 3 (18) |
| After APs administration∥ | 2 (50) |
| Discharge destination, no. (%) | |
| Home | 23 (38) |
| Rehabilitation facilities | 4 (7) |
| SNF | 28 (47) |
| Death | 5 (8) |
Eighteen patients received 1 or more new APs during the readmission hospitalizations. These included haloperidol (89%) and quetiapine (39%). Delirium was the main reported indication for starting APs (78%), but in 17% of cases no indication was documented. An electrocardiogram (ECG) was performed in 94% prior to APs administration and for 22% after APs administration. Corrected QT interval (QTc) of >500 ms was present in 18% of patients in pretreatment ECG and 50% of patients in post‐AP ECG. Of patients who survived readmission, 58% (32/55) were discharged to postacute facilities. Of the 39 patients who were on the same APs from index admission, 27 (69%) patients were eventually discharged on the same APs or new APs started during the readmission.
In the multivariable model (Table 2), predictors of death at 1 year included discharge to postacute facilities after index admission (odds ratio [OR]: 2.28; 95% confidence interval [CI]: 1.10‐4.73, P = 0.03) and QTc prolongation >500 ms during index admission (OR: 3.41; 95% CI: 1.34‐8.67, P = 0.01). Age and gender were not associated with 1‐year mortality.
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| |||
| Age | 1.03 | 0.991.06 | 0.13 |
| Male sex | 0.87 | 0.501.52 | 0.63 |
| Risperdal | 3.53 | 0.6419.40 | 0.15 |
| QTc prolongation after AP administration* | 3.41 | 1.348.67 | 0.01 |
| Presence of geriatric psychiatry consult | 0.30 | 0.091.04 | 0.06 |
| Discharged to postacute facilities vs home | 2.28 | 1.104.73 | 0.03 |
DISCUSSION
In a cohort of elderly patients who were discharged on APs, nearly one‐third (29%) died within 1 year of the hospitalization in which APs were initiated. Nearly half of the survivors from the index admission (41%) experienced at least 1 admission within 1 year from discharge. Of readmitted patients, two‐thirds were taking the same APs that had been started during the index hospitalization. Half of the patients not on APs on readmission were started on an AP during the hospitalization, most often because they became delirious on return to the acute care setting. Compared to patients discharged home after an index admission, patients who were discharged to postacute facilities were almost 4 times as likely to die during the year subsequent to the admission. These data suggest that once patients are started on APs, most are continued on them until the next admission or are restarted during that readmission. Moreover, hospitalized elders who require an AP are at high risk for mortality in the coming year.
Prior studies have reported that patients with delirium have elevated 1‐year mortality rates.[14, 15, 16, 17, 18, 19] A secondary analysis of the Delirium Prevention Trial, which included 437 hospitalized older patients, revealed a 1‐year mortality rate of 20% in those who were never delirious during hospitalization, compared to 26% to 38% in patients with delirium.[19] Additionally, 1‐year mortality in hospitalized older patients with delirium (36%) was shown to be higher than patients with dementia (29%) or depression (26%).[17] Unlike these studies, not all of the patients in our study had documented delirium, but all received an AP. Still, it is notable that the 1‐year mortality rate for delirium in general is similar to what we found in this study.
The literature has also reported that long‐term AP use is associated with excess mortality in elder patients, especially those with dementia.[20, 21, 22] In a retrospective cohort study, older patients with dementia who were taking antipsychotics had significantly higher 1‐year mortality rates (23%29%) than patients not taking antipsychotic medications (15%). In a large Canadian propensity score‐matched cohort study that included over 13,000 demented older adults, the mortality was higher in the community‐dwelling elders who received atypical APs compared to no APs, with a difference of 1.1% in 180‐day mortality rate after initiation of APs.[21] The absolute mortality rate was 2.6% higher in patients who received typical compared to atypical APs. Unlike these studies, not every patient in our cohort had a diagnosis of dementia, but again, mortality rates in these studies appear similar to our cohort.
In contrast, other observational studies have not found an increased risk associated with receipt of APs. For example, a prospective study that enrolled approximately 950 patients with probable dementia showed that AP use was not associated with time to death after adjustment for comorbidities, demographic and cognitive variables.[23] These conflicting results highlight the difficulties of attributing outcomes in high‐risk populations. Although the excess mortality observed in patients taking APs may be related to the risks of APs, it is quite possible that patients who require APs (most often for delirium or agitated dementia) are at higher risk of death. This confounding by indication may be nearly impossible to adjust for retrospectively, even using techniques such as propensity matching.
Our report adds to the literature; we know of no studies to date describing a cohort of patients, most with delirium, who were started on APs in the hospital. We also attempted to identify the reasons that patients were started on APs, which have been infrequently reported. As noted above, our 1‐year mortality rate of 29% among older patients prescribed APs in the hospital was quite similar to mortality rates both for patients with delirium who were not necessarily treated with APs and patients with dementia who were treated with APs. This finding further supports the argument that risk factors for mortality, including dementia, delirium, and AP use are very difficult to tease apart. It is possible that the reasons that APs are prescribed (agitated delirium or dementia) have as much to do with the excess mortality reported in observational studies of APs as the use of APs themselves.
The high rate of continued AP use we observed (two‐thirds of readmitted patients) may reflect limited pharmacological alternatives to these medications with little evidence to support treating the symptoms of delirium with other drug classes, along with suboptimal environmental and behavioral modifications in postacute facilities and hospitals. This is unfortunate given that delirium is often preventable. Systematic implementation of well‐documented strategies to decrease delirium in hospitals and postacute facilities would likely reduce the prescription of APs and has the potential to slow the decline in this vulnerable population. A meta‐analysis incorporating both randomized and nonrandomized trials of medical and surgical patients showed that multicomponent nonpharmacologic interventions decreased delirium by 50%.[24] Thus, simple interventions such as reorientation, early mobilization, optimizing vision and hearing, sleepwake cycle preservation, and hydration might avoid roughly 1 million cases of delirium in hospitalized older adults annually.[24] The Hospital Elder Life Program and Acute Care for Elders units are examples of programs that have been shown to decrease the incidence of delirium.[25, 26]
Despite vigorous efforts to prevent delirium, a subgroup of patients still will become delirious. These patients are at high risk for death. Our mortality prediction model revealed that patients who were discharged to postacute facilities were 4 times more likely to die during the subsequent year compared to patients who were discharged home. Patients discharged to postacute facilities are likely to have a higher burden of disease, greater functional and cognitive impairment, and more frailty than those who are able to return to the community. Very ill and/or frail patients receiving APs in the hospital and requiring APs on discharge to postacute care facilities have limited survival and may benefit from expedited palliative care interventions to clarify prognosis and goals, and relieve suffering. At a minimum, our study identifies a need for further study to identify this very high‐risk group of elders. It is notable that 50% of patients were found to have a post‐treatment ECG with a QTc of >500 ms, a finding that has not been previously described. This would put these patients at higher risk of mortality, and as such we suggest that current guidelines should continue to emphasize the importance of post‐treatment ECGs and set clear criteria for discontinuation in elderly patients.
Our study is limited by its retrospective, single‐center design and small sample size, therefore limiting the interpretation and generalizability of the results to other hospitals. Quetiapine was the most common AP medication used in our hospital; therefore, our findings cannot be generalized to hospitals that utilize other AP agents. Future studies should examine antipsychotic use across hospitals to determine variation in prescribing patterns and outcomes. Nevertheless, the care of these patients were transitioned to a large number of geriatricians and primary care and nursing home physicians after discharge, and the reflected practice patterns extended beyond our hospital. Additionally, we were unable to determine when and why APs were discontinued or started in the outpatient setting. We were only able to detect readmissions to the 3 hospitals within our health system and therefore may have missed some readmissions to other institutions, although the majority of patients in our region tend to return to the same hospital. For patients who were not readmitted, we were also unable to identify whether they remained on the APs initiated during their index hospitalizations. Any retrospective study is limited by the difficulty of distinguishing delirium from the behavioral and psychiatric symptoms of dementia, but we identified delirium using standard terms described in previous literature.[10] We were unable to determine the types of delirium (hyperactive vs hypoactive) given that the documentations on behavioral symptoms were largely missing from the charts. The number of patients with preexisting diagnosis of dementia was likely underestimated, as we were only able to verify the diagnosis from the medical history. Additionally, the retrospective design based on chart review limited the factors that we could detect and grade accurately for inclusion in our mortality prediction model. Of note, our model did not contain objective measures of cognition, agitation, function, and markers for frailty such as walking speed, weak grip strength, weight loss, and low physical activity.
CONCLUSION
Initiating an AP (eg, haloperidol, quetiapine, olanzapine, and risperidone) in the hospital is likely to result in long‐term use of these medications despite the fact that AP use has been associated with multiple risks including falls, fractures, stroke, cardiovascular disease, and increased mortality in those with underlying dementia.[27] When possible, behavioral interventions to prevent delirium and slow the trajectory of decline should be implemented to reduce AP use. If patients with delirium are started on antipsychotics, it is important to monitor for prolonged QTc given the associated risk of mortality. In a subgroup of patients at high risk for death in the upcoming year, occurrence of delirium or use of APs during a hospitalization should both be considered triggers for early advance care planning and possibly palliative care and end‐of‐life discussions, with an emphasis on quality of life.
Disclosures: The research was supported by the Department of Medicine, Baystate Medical Center/Tufts University School of Medicine. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Loh had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the analysis. Drs. Loh, Brennan, Lindenauer, and Lagu conceived of the study. Drs. Loh, Ramdass, and Ms. Garb acquired the data. Ms Garb analyzed and interpreted the data. Drs. Loh, Ramdass, and Thim drafted the manuscript. Drs. Brennan, Lindenauer, and Lagu and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Lagu has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services, for her work on a project to help health systems achieve disability competence. Dr. Brennan is supported by a Geriatric Work Force Enhancement Grant from the US Department of Health and Human Services award number 1 U1QHP287020100. The authors report no conflicts of interest.
- , , . Delirium in elderly people. Lancet. 2014;383:911–922.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156:848–856, W296.
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63:142–150.
- Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1–20.
- , ; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med (Lond). 2006;6:303–308.
- , , . Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21.
- , , , et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72:438–445.
- , . Safety and efficacy of antipsychotic drugs for the behavioral and psychological symptoms of dementia. Indian J Psychiatry. 2009;51(suppl 1):S87–S92.
- , , , . Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. J Clin Psychopharmacol. 2014;34:109–123.
- , , , , , . From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9:802–804.
- , , . Comparison of National Death Index and World Wide Web Death Searches. Am J Epidemiol. 2000;152:107–111.
- . Analysis of Binary Data. London, United Kingdom: Methuen; 1970:76–99.
- . Statistical Methods for Survival Data Analysis. New York, NY: John Wiley 1992:233–236.
- , , , . The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014;43:127–132.
- , , , et al. Risk factors for delirium and inpatient mortality with delirium. J Postgrad Med. 2013;59:263–270.
- , , , , , . Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014;14:129.
- , , , , , . One‐year mortality of elderly inpatients with delirium, dementia, or depression seen by a consultation‐liaison service. Psychosomatics. 2012;53:433–438.
- , , , . Excess mortality in general hospital patients with delirium: a 5‐year follow‐up of 519 patients seen in psychiatric consultation. J Psychosom Res. 1994;38:339–346.
- , , , et al. Older adults discharged from the hospital with delirium: 1‐year outcomes. J Am Geriatr Soc. 2006;54:1245–1250.
- , , , et al. The dementia antipsychotic withdrawal trial (DART‐AD): long‐term follow‐up of a randomised placebo‐controlled trial. Lancet Neurol. 2009;8:151–157.
- , , , et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786.
- , , , et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341.
- , , , et al. The long‐term effects of conventional and atypical antipsychotics in patients with probable Alzheimer's disease. Am J Psychiatry. 2013;170:1051–1058.
- , , , et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta‐analysis. JAMA Intern Med. 2015;175:512–520.
- , , , , . Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59:359–365.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60:2237–2245.
- , . Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81:617–622.
Delirium, a clinical syndrome characterized by inattention and acute cognitive dysfunction, is very common in older hospitalized patients, with a reported incidence of 18% to 35% at time of admission and overall occurrence rates of 29% to 64%.[1] Previous studies have reported that a diagnosis of delirium is not benign and is associated with other adverse outcomes including prolonged hospitalization, institutionalization, increased cost, and mortality. These outcomes occurred independent of age, prior cognitive functioning, and comorbidities.[2] Guidelines recommend that management of inpatient delirium should be focused on addressing the underlying etiology and managed with nonpharmacological interventions whenever possible.[3, 4, 5] However, implementing these recommendations can prove to be very challenging in hospital settings. Providers frequently have to resort to medical therapies, including antipsychotics (APs). Although these medications are commonly used to treat delirium in elderly patients, there is limited evidence to support their efficacy, and there are currently no proven pharmacological alternatives to these medications.[6] Furthermore, previous studies have demonstrated an increased risk of stroke, infection, cognitive impairment, and mortality in elders with dementia who receive long‐term AP therapy.[7, 8, 9] Yet as many as 48% of hospitalized elders who were newly started on APs had these drugs continued at time of discharge.[10]
There have been few studies describing the long‐term outcomes of elderly patient who are started on APs in the hospital. Most information on outcomes comes from patients with dementia. Therefore, we studied the 1‐year outcomes of a cohort of patients with and without dementia who were started on APs in the hospital and then discharged on these medications. In this cohort, we aimed to describe the number of readmissions, reasons for readmissions, duration of AP therapy, use of other sedating medications such as anxiolytics, hypnotics, and antihistamines as well as the incidence of readmission and death 1 year after the index hospital discharge.
METHODS
We previously described a retrospective cohort of 300 elders (65 years old) admitted to a tertiary care hospital between October 1, 2012 and September 31, 2013 who were newly prescribed APs while hospitalized.[10] Of patients alive at the time of discharge (260), 56% (146 patients) were discharged on APs. Two investigators extracted these 148 patient charts independently to identify and quantify the number of readmissions to the index hospital. We then limited the sample to only the first readmission per patient following the index admission and extracted this readmission for each patient. We first determined if APs were present on the admission medication reconciliation. If APs were not present on admission, we examined whether they were resumed during the hospitalization using the electronic medication administration summary. If they were present on admission, we looked to see if they were discontinued during the readmission and if additional new APs were started during the hospitalizations. We documented the circumstances around APs use and identified patients who died during their hospitalizations. We identified delirium using the same terms that were described in our prior study on the same cohort of patients.[10] We determined if patients were delirious using a predetermined algorithm (Figure 1). Briefly, we first determined delirium was documented. We then examined whether there was a Confusion Assessment Method (CAM) instrument included in the record. If a CAM instrument was not documented, we then looked for documentation using specific terms (eg, disorientation, confusions). We identified patients with dementia by determining whether dementia was documented along with other admission medical comorbidities. If it was not, we determined whether dementia was newly diagnosed during the hospital stay using progress notes or consultation notes. We did not objectively define criteria for diagnosis of dementia. We used the National Death Index (NDI) to determine mortality for all patients 1 year after discharge from the index hospitalization. The NDI is a national database of death records maintained by the National Center for Health Statistics. It has shown consistently high sensitivity and specificity for detection of death.[11]

We used descriptive statistics (means, standard deviations, range, and percents as appropriate to the scale of measurement) to describe the patient sample. We then used multiple logistic regression to identify significant predictors of death within 1 year of discharge.[12] Univariate analysis was used to select candidates for the logistic model (t tests for continuous factors and 2 for discrete factors). All factors with a significance level 0.2 on univariate analysis were included in the logistic regression, in addition to age and sex (regardless of significance). A maximum likelihood procedure was used to calculate the regression coefficients for the logistic model. The likelihood ratio criterion was used to determine the significance of individual factors in the regression model.[13] Factors with a significance level of 0.15 or less were retained in the final model, in addition to age and sex.
RESULTS
The 260 patients discharged alive from their index admissions had a 1‐year mortality rate of 29% (75/260). Of the 146/260 patients discharged on APs, 60 (41%) patients experienced at least 1 readmission (mean = 2 readmissions per patient; range, 18, with 111 total readmissions for 60 patients) within 1 year from discharge (Figure 2). Most common diagnoses at the time of readmissions were related neurological and psychiatric disorders (14%), cardiovascular and circulation disorders (13%), renal injury and electrolyte disorders (11%), and infections (6%). Among patients with at least 1 readmission, the mean age was 81.3 (range, 65.599.7), 60% were male, and 45% were admitted from a skilled nursing facility or rehabilitation facility (Table 1). Median time to readmission was 43.5 days (range, 1343 days), and 79% were readmitted to a medical service. The remaining 20% were admitted to a surgical service. Inpatient mortality during first readmissions was 8% (5/60). At the time of first readmission, 39/60 (65%) of patients were still on the same APs on which they had been discharged, and the APs were continued during the hospitalization in 79% of the patients (61% quetiapine, 19% olanzapine, and 13% risperidone). About half of patients whose APs were discontinued prior to readmission received a new AP during their hospital stays (9/20; 45%). One patient had been started on quetiapine in the outpatient setting. No patients were found to have new benzodiazepines, nonbenzodiazepine hypnotic, or antihistamines on their admission medication list.
| Variables | Value* |
|---|---|
| |
| Age, mean (range), yr | 81.3 (65.599.7) |
| Gender, no. (%) | |
| Male | 36 (60) |
| Female | 24 (40) |
| Admitted from, no. (%) | |
| Home | 33 (55) |
| Rehabilitation facilities | 5 (8) |
| SNF | 22 (37) |
| Services, no. (%) | |
| Medicine | 48 (80) |
| Surgery | 12 (20) |
| Types of APs continued on readmission (from index admission), no. (%) | |
| Quetiapine | 19 (61) |
| Olanzapine | 6 (19) |
| Risperidone | 4 (13) |
| Haloperidol | 2 (7) |
| Types of APs started during readmission, no. (%) | |
| Quetiapine | 7 (39) |
| Risperidone | 2 (11) |
| Haloperidol | 16 (89) |
| Indications for AP use, no. (%) | |
| Delirium | 14 (77) |
| Undocumented | 3 (17) |
| Other | 1 (6) |
| ECG, no. (%) | |
| Prior to APs administration | 17 (94) |
| After APs administration | 4 (22) |
| QTc prolongation >500 ms, no. (%) | |
| Prior to APs administration | 3 (18) |
| After APs administration∥ | 2 (50) |
| Discharge destination, no. (%) | |
| Home | 23 (38) |
| Rehabilitation facilities | 4 (7) |
| SNF | 28 (47) |
| Death | 5 (8) |

Eighteen patients received 1 or more new APs during the readmission hospitalizations. These included haloperidol (89%) and quetiapine (39%). Delirium was the main reported indication for starting APs (78%), but in 17% of cases no indication was documented. An electrocardiogram (ECG) was performed in 94% prior to APs administration and for 22% after APs administration. Corrected QT interval (QTc) of >500 ms was present in 18% of patients in pretreatment ECG and 50% of patients in post‐AP ECG. Of patients who survived readmission, 58% (32/55) were discharged to postacute facilities. Of the 39 patients who were on the same APs from index admission, 27 (69%) patients were eventually discharged on the same APs or new APs started during the readmission.
In the multivariable model (Table 2), predictors of death at 1 year included discharge to postacute facilities after index admission (odds ratio [OR]: 2.28; 95% confidence interval [CI]: 1.10‐4.73, P = 0.03) and QTc prolongation >500 ms during index admission (OR: 3.41; 95% CI: 1.34‐8.67, P = 0.01). Age and gender were not associated with 1‐year mortality.
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| |||
| Age | 1.03 | 0.991.06 | 0.13 |
| Male sex | 0.87 | 0.501.52 | 0.63 |
| Risperdal | 3.53 | 0.6419.40 | 0.15 |
| QTc prolongation after AP administration* | 3.41 | 1.348.67 | 0.01 |
| Presence of geriatric psychiatry consult | 0.30 | 0.091.04 | 0.06 |
| Discharged to postacute facilities vs home | 2.28 | 1.104.73 | 0.03 |
DISCUSSION
In a cohort of elderly patients who were discharged on APs, nearly one‐third (29%) died within 1 year of the hospitalization in which APs were initiated. Nearly half of the survivors from the index admission (41%) experienced at least 1 admission within 1 year from discharge. Of readmitted patients, two‐thirds were taking the same APs that had been started during the index hospitalization. Half of the patients not on APs on readmission were started on an AP during the hospitalization, most often because they became delirious on return to the acute care setting. Compared to patients discharged home after an index admission, patients who were discharged to postacute facilities were almost 4 times as likely to die during the year subsequent to the admission. These data suggest that once patients are started on APs, most are continued on them until the next admission or are restarted during that readmission. Moreover, hospitalized elders who require an AP are at high risk for mortality in the coming year.
Prior studies have reported that patients with delirium have elevated 1‐year mortality rates.[14, 15, 16, 17, 18, 19] A secondary analysis of the Delirium Prevention Trial, which included 437 hospitalized older patients, revealed a 1‐year mortality rate of 20% in those who were never delirious during hospitalization, compared to 26% to 38% in patients with delirium.[19] Additionally, 1‐year mortality in hospitalized older patients with delirium (36%) was shown to be higher than patients with dementia (29%) or depression (26%).[17] Unlike these studies, not all of the patients in our study had documented delirium, but all received an AP. Still, it is notable that the 1‐year mortality rate for delirium in general is similar to what we found in this study.
The literature has also reported that long‐term AP use is associated with excess mortality in elder patients, especially those with dementia.[20, 21, 22] In a retrospective cohort study, older patients with dementia who were taking antipsychotics had significantly higher 1‐year mortality rates (23%29%) than patients not taking antipsychotic medications (15%). In a large Canadian propensity score‐matched cohort study that included over 13,000 demented older adults, the mortality was higher in the community‐dwelling elders who received atypical APs compared to no APs, with a difference of 1.1% in 180‐day mortality rate after initiation of APs.[21] The absolute mortality rate was 2.6% higher in patients who received typical compared to atypical APs. Unlike these studies, not every patient in our cohort had a diagnosis of dementia, but again, mortality rates in these studies appear similar to our cohort.
In contrast, other observational studies have not found an increased risk associated with receipt of APs. For example, a prospective study that enrolled approximately 950 patients with probable dementia showed that AP use was not associated with time to death after adjustment for comorbidities, demographic and cognitive variables.[23] These conflicting results highlight the difficulties of attributing outcomes in high‐risk populations. Although the excess mortality observed in patients taking APs may be related to the risks of APs, it is quite possible that patients who require APs (most often for delirium or agitated dementia) are at higher risk of death. This confounding by indication may be nearly impossible to adjust for retrospectively, even using techniques such as propensity matching.
Our report adds to the literature; we know of no studies to date describing a cohort of patients, most with delirium, who were started on APs in the hospital. We also attempted to identify the reasons that patients were started on APs, which have been infrequently reported. As noted above, our 1‐year mortality rate of 29% among older patients prescribed APs in the hospital was quite similar to mortality rates both for patients with delirium who were not necessarily treated with APs and patients with dementia who were treated with APs. This finding further supports the argument that risk factors for mortality, including dementia, delirium, and AP use are very difficult to tease apart. It is possible that the reasons that APs are prescribed (agitated delirium or dementia) have as much to do with the excess mortality reported in observational studies of APs as the use of APs themselves.
The high rate of continued AP use we observed (two‐thirds of readmitted patients) may reflect limited pharmacological alternatives to these medications with little evidence to support treating the symptoms of delirium with other drug classes, along with suboptimal environmental and behavioral modifications in postacute facilities and hospitals. This is unfortunate given that delirium is often preventable. Systematic implementation of well‐documented strategies to decrease delirium in hospitals and postacute facilities would likely reduce the prescription of APs and has the potential to slow the decline in this vulnerable population. A meta‐analysis incorporating both randomized and nonrandomized trials of medical and surgical patients showed that multicomponent nonpharmacologic interventions decreased delirium by 50%.[24] Thus, simple interventions such as reorientation, early mobilization, optimizing vision and hearing, sleepwake cycle preservation, and hydration might avoid roughly 1 million cases of delirium in hospitalized older adults annually.[24] The Hospital Elder Life Program and Acute Care for Elders units are examples of programs that have been shown to decrease the incidence of delirium.[25, 26]
Despite vigorous efforts to prevent delirium, a subgroup of patients still will become delirious. These patients are at high risk for death. Our mortality prediction model revealed that patients who were discharged to postacute facilities were 4 times more likely to die during the subsequent year compared to patients who were discharged home. Patients discharged to postacute facilities are likely to have a higher burden of disease, greater functional and cognitive impairment, and more frailty than those who are able to return to the community. Very ill and/or frail patients receiving APs in the hospital and requiring APs on discharge to postacute care facilities have limited survival and may benefit from expedited palliative care interventions to clarify prognosis and goals, and relieve suffering. At a minimum, our study identifies a need for further study to identify this very high‐risk group of elders. It is notable that 50% of patients were found to have a post‐treatment ECG with a QTc of >500 ms, a finding that has not been previously described. This would put these patients at higher risk of mortality, and as such we suggest that current guidelines should continue to emphasize the importance of post‐treatment ECGs and set clear criteria for discontinuation in elderly patients.
Our study is limited by its retrospective, single‐center design and small sample size, therefore limiting the interpretation and generalizability of the results to other hospitals. Quetiapine was the most common AP medication used in our hospital; therefore, our findings cannot be generalized to hospitals that utilize other AP agents. Future studies should examine antipsychotic use across hospitals to determine variation in prescribing patterns and outcomes. Nevertheless, the care of these patients were transitioned to a large number of geriatricians and primary care and nursing home physicians after discharge, and the reflected practice patterns extended beyond our hospital. Additionally, we were unable to determine when and why APs were discontinued or started in the outpatient setting. We were only able to detect readmissions to the 3 hospitals within our health system and therefore may have missed some readmissions to other institutions, although the majority of patients in our region tend to return to the same hospital. For patients who were not readmitted, we were also unable to identify whether they remained on the APs initiated during their index hospitalizations. Any retrospective study is limited by the difficulty of distinguishing delirium from the behavioral and psychiatric symptoms of dementia, but we identified delirium using standard terms described in previous literature.[10] We were unable to determine the types of delirium (hyperactive vs hypoactive) given that the documentations on behavioral symptoms were largely missing from the charts. The number of patients with preexisting diagnosis of dementia was likely underestimated, as we were only able to verify the diagnosis from the medical history. Additionally, the retrospective design based on chart review limited the factors that we could detect and grade accurately for inclusion in our mortality prediction model. Of note, our model did not contain objective measures of cognition, agitation, function, and markers for frailty such as walking speed, weak grip strength, weight loss, and low physical activity.
CONCLUSION
Initiating an AP (eg, haloperidol, quetiapine, olanzapine, and risperidone) in the hospital is likely to result in long‐term use of these medications despite the fact that AP use has been associated with multiple risks including falls, fractures, stroke, cardiovascular disease, and increased mortality in those with underlying dementia.[27] When possible, behavioral interventions to prevent delirium and slow the trajectory of decline should be implemented to reduce AP use. If patients with delirium are started on antipsychotics, it is important to monitor for prolonged QTc given the associated risk of mortality. In a subgroup of patients at high risk for death in the upcoming year, occurrence of delirium or use of APs during a hospitalization should both be considered triggers for early advance care planning and possibly palliative care and end‐of‐life discussions, with an emphasis on quality of life.
Disclosures: The research was supported by the Department of Medicine, Baystate Medical Center/Tufts University School of Medicine. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Loh had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the analysis. Drs. Loh, Brennan, Lindenauer, and Lagu conceived of the study. Drs. Loh, Ramdass, and Ms. Garb acquired the data. Ms Garb analyzed and interpreted the data. Drs. Loh, Ramdass, and Thim drafted the manuscript. Drs. Brennan, Lindenauer, and Lagu and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Lagu has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services, for her work on a project to help health systems achieve disability competence. Dr. Brennan is supported by a Geriatric Work Force Enhancement Grant from the US Department of Health and Human Services award number 1 U1QHP287020100. The authors report no conflicts of interest.
Delirium, a clinical syndrome characterized by inattention and acute cognitive dysfunction, is very common in older hospitalized patients, with a reported incidence of 18% to 35% at time of admission and overall occurrence rates of 29% to 64%.[1] Previous studies have reported that a diagnosis of delirium is not benign and is associated with other adverse outcomes including prolonged hospitalization, institutionalization, increased cost, and mortality. These outcomes occurred independent of age, prior cognitive functioning, and comorbidities.[2] Guidelines recommend that management of inpatient delirium should be focused on addressing the underlying etiology and managed with nonpharmacological interventions whenever possible.[3, 4, 5] However, implementing these recommendations can prove to be very challenging in hospital settings. Providers frequently have to resort to medical therapies, including antipsychotics (APs). Although these medications are commonly used to treat delirium in elderly patients, there is limited evidence to support their efficacy, and there are currently no proven pharmacological alternatives to these medications.[6] Furthermore, previous studies have demonstrated an increased risk of stroke, infection, cognitive impairment, and mortality in elders with dementia who receive long‐term AP therapy.[7, 8, 9] Yet as many as 48% of hospitalized elders who were newly started on APs had these drugs continued at time of discharge.[10]
There have been few studies describing the long‐term outcomes of elderly patient who are started on APs in the hospital. Most information on outcomes comes from patients with dementia. Therefore, we studied the 1‐year outcomes of a cohort of patients with and without dementia who were started on APs in the hospital and then discharged on these medications. In this cohort, we aimed to describe the number of readmissions, reasons for readmissions, duration of AP therapy, use of other sedating medications such as anxiolytics, hypnotics, and antihistamines as well as the incidence of readmission and death 1 year after the index hospital discharge.
METHODS
We previously described a retrospective cohort of 300 elders (65 years old) admitted to a tertiary care hospital between October 1, 2012 and September 31, 2013 who were newly prescribed APs while hospitalized.[10] Of patients alive at the time of discharge (260), 56% (146 patients) were discharged on APs. Two investigators extracted these 148 patient charts independently to identify and quantify the number of readmissions to the index hospital. We then limited the sample to only the first readmission per patient following the index admission and extracted this readmission for each patient. We first determined if APs were present on the admission medication reconciliation. If APs were not present on admission, we examined whether they were resumed during the hospitalization using the electronic medication administration summary. If they were present on admission, we looked to see if they were discontinued during the readmission and if additional new APs were started during the hospitalizations. We documented the circumstances around APs use and identified patients who died during their hospitalizations. We identified delirium using the same terms that were described in our prior study on the same cohort of patients.[10] We determined if patients were delirious using a predetermined algorithm (Figure 1). Briefly, we first determined delirium was documented. We then examined whether there was a Confusion Assessment Method (CAM) instrument included in the record. If a CAM instrument was not documented, we then looked for documentation using specific terms (eg, disorientation, confusions). We identified patients with dementia by determining whether dementia was documented along with other admission medical comorbidities. If it was not, we determined whether dementia was newly diagnosed during the hospital stay using progress notes or consultation notes. We did not objectively define criteria for diagnosis of dementia. We used the National Death Index (NDI) to determine mortality for all patients 1 year after discharge from the index hospitalization. The NDI is a national database of death records maintained by the National Center for Health Statistics. It has shown consistently high sensitivity and specificity for detection of death.[11]

We used descriptive statistics (means, standard deviations, range, and percents as appropriate to the scale of measurement) to describe the patient sample. We then used multiple logistic regression to identify significant predictors of death within 1 year of discharge.[12] Univariate analysis was used to select candidates for the logistic model (t tests for continuous factors and 2 for discrete factors). All factors with a significance level 0.2 on univariate analysis were included in the logistic regression, in addition to age and sex (regardless of significance). A maximum likelihood procedure was used to calculate the regression coefficients for the logistic model. The likelihood ratio criterion was used to determine the significance of individual factors in the regression model.[13] Factors with a significance level of 0.15 or less were retained in the final model, in addition to age and sex.
RESULTS
The 260 patients discharged alive from their index admissions had a 1‐year mortality rate of 29% (75/260). Of the 146/260 patients discharged on APs, 60 (41%) patients experienced at least 1 readmission (mean = 2 readmissions per patient; range, 18, with 111 total readmissions for 60 patients) within 1 year from discharge (Figure 2). Most common diagnoses at the time of readmissions were related neurological and psychiatric disorders (14%), cardiovascular and circulation disorders (13%), renal injury and electrolyte disorders (11%), and infections (6%). Among patients with at least 1 readmission, the mean age was 81.3 (range, 65.599.7), 60% were male, and 45% were admitted from a skilled nursing facility or rehabilitation facility (Table 1). Median time to readmission was 43.5 days (range, 1343 days), and 79% were readmitted to a medical service. The remaining 20% were admitted to a surgical service. Inpatient mortality during first readmissions was 8% (5/60). At the time of first readmission, 39/60 (65%) of patients were still on the same APs on which they had been discharged, and the APs were continued during the hospitalization in 79% of the patients (61% quetiapine, 19% olanzapine, and 13% risperidone). About half of patients whose APs were discontinued prior to readmission received a new AP during their hospital stays (9/20; 45%). One patient had been started on quetiapine in the outpatient setting. No patients were found to have new benzodiazepines, nonbenzodiazepine hypnotic, or antihistamines on their admission medication list.
| Variables | Value* |
|---|---|
| |
| Age, mean (range), yr | 81.3 (65.599.7) |
| Gender, no. (%) | |
| Male | 36 (60) |
| Female | 24 (40) |
| Admitted from, no. (%) | |
| Home | 33 (55) |
| Rehabilitation facilities | 5 (8) |
| SNF | 22 (37) |
| Services, no. (%) | |
| Medicine | 48 (80) |
| Surgery | 12 (20) |
| Types of APs continued on readmission (from index admission), no. (%) | |
| Quetiapine | 19 (61) |
| Olanzapine | 6 (19) |
| Risperidone | 4 (13) |
| Haloperidol | 2 (7) |
| Types of APs started during readmission, no. (%) | |
| Quetiapine | 7 (39) |
| Risperidone | 2 (11) |
| Haloperidol | 16 (89) |
| Indications for AP use, no. (%) | |
| Delirium | 14 (77) |
| Undocumented | 3 (17) |
| Other | 1 (6) |
| ECG, no. (%) | |
| Prior to APs administration | 17 (94) |
| After APs administration | 4 (22) |
| QTc prolongation >500 ms, no. (%) | |
| Prior to APs administration | 3 (18) |
| After APs administration∥ | 2 (50) |
| Discharge destination, no. (%) | |
| Home | 23 (38) |
| Rehabilitation facilities | 4 (7) |
| SNF | 28 (47) |
| Death | 5 (8) |

Eighteen patients received 1 or more new APs during the readmission hospitalizations. These included haloperidol (89%) and quetiapine (39%). Delirium was the main reported indication for starting APs (78%), but in 17% of cases no indication was documented. An electrocardiogram (ECG) was performed in 94% prior to APs administration and for 22% after APs administration. Corrected QT interval (QTc) of >500 ms was present in 18% of patients in pretreatment ECG and 50% of patients in post‐AP ECG. Of patients who survived readmission, 58% (32/55) were discharged to postacute facilities. Of the 39 patients who were on the same APs from index admission, 27 (69%) patients were eventually discharged on the same APs or new APs started during the readmission.
In the multivariable model (Table 2), predictors of death at 1 year included discharge to postacute facilities after index admission (odds ratio [OR]: 2.28; 95% confidence interval [CI]: 1.10‐4.73, P = 0.03) and QTc prolongation >500 ms during index admission (OR: 3.41; 95% CI: 1.34‐8.67, P = 0.01). Age and gender were not associated with 1‐year mortality.
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| |||
| Age | 1.03 | 0.991.06 | 0.13 |
| Male sex | 0.87 | 0.501.52 | 0.63 |
| Risperdal | 3.53 | 0.6419.40 | 0.15 |
| QTc prolongation after AP administration* | 3.41 | 1.348.67 | 0.01 |
| Presence of geriatric psychiatry consult | 0.30 | 0.091.04 | 0.06 |
| Discharged to postacute facilities vs home | 2.28 | 1.104.73 | 0.03 |
DISCUSSION
In a cohort of elderly patients who were discharged on APs, nearly one‐third (29%) died within 1 year of the hospitalization in which APs were initiated. Nearly half of the survivors from the index admission (41%) experienced at least 1 admission within 1 year from discharge. Of readmitted patients, two‐thirds were taking the same APs that had been started during the index hospitalization. Half of the patients not on APs on readmission were started on an AP during the hospitalization, most often because they became delirious on return to the acute care setting. Compared to patients discharged home after an index admission, patients who were discharged to postacute facilities were almost 4 times as likely to die during the year subsequent to the admission. These data suggest that once patients are started on APs, most are continued on them until the next admission or are restarted during that readmission. Moreover, hospitalized elders who require an AP are at high risk for mortality in the coming year.
Prior studies have reported that patients with delirium have elevated 1‐year mortality rates.[14, 15, 16, 17, 18, 19] A secondary analysis of the Delirium Prevention Trial, which included 437 hospitalized older patients, revealed a 1‐year mortality rate of 20% in those who were never delirious during hospitalization, compared to 26% to 38% in patients with delirium.[19] Additionally, 1‐year mortality in hospitalized older patients with delirium (36%) was shown to be higher than patients with dementia (29%) or depression (26%).[17] Unlike these studies, not all of the patients in our study had documented delirium, but all received an AP. Still, it is notable that the 1‐year mortality rate for delirium in general is similar to what we found in this study.
The literature has also reported that long‐term AP use is associated with excess mortality in elder patients, especially those with dementia.[20, 21, 22] In a retrospective cohort study, older patients with dementia who were taking antipsychotics had significantly higher 1‐year mortality rates (23%29%) than patients not taking antipsychotic medications (15%). In a large Canadian propensity score‐matched cohort study that included over 13,000 demented older adults, the mortality was higher in the community‐dwelling elders who received atypical APs compared to no APs, with a difference of 1.1% in 180‐day mortality rate after initiation of APs.[21] The absolute mortality rate was 2.6% higher in patients who received typical compared to atypical APs. Unlike these studies, not every patient in our cohort had a diagnosis of dementia, but again, mortality rates in these studies appear similar to our cohort.
In contrast, other observational studies have not found an increased risk associated with receipt of APs. For example, a prospective study that enrolled approximately 950 patients with probable dementia showed that AP use was not associated with time to death after adjustment for comorbidities, demographic and cognitive variables.[23] These conflicting results highlight the difficulties of attributing outcomes in high‐risk populations. Although the excess mortality observed in patients taking APs may be related to the risks of APs, it is quite possible that patients who require APs (most often for delirium or agitated dementia) are at higher risk of death. This confounding by indication may be nearly impossible to adjust for retrospectively, even using techniques such as propensity matching.
Our report adds to the literature; we know of no studies to date describing a cohort of patients, most with delirium, who were started on APs in the hospital. We also attempted to identify the reasons that patients were started on APs, which have been infrequently reported. As noted above, our 1‐year mortality rate of 29% among older patients prescribed APs in the hospital was quite similar to mortality rates both for patients with delirium who were not necessarily treated with APs and patients with dementia who were treated with APs. This finding further supports the argument that risk factors for mortality, including dementia, delirium, and AP use are very difficult to tease apart. It is possible that the reasons that APs are prescribed (agitated delirium or dementia) have as much to do with the excess mortality reported in observational studies of APs as the use of APs themselves.
The high rate of continued AP use we observed (two‐thirds of readmitted patients) may reflect limited pharmacological alternatives to these medications with little evidence to support treating the symptoms of delirium with other drug classes, along with suboptimal environmental and behavioral modifications in postacute facilities and hospitals. This is unfortunate given that delirium is often preventable. Systematic implementation of well‐documented strategies to decrease delirium in hospitals and postacute facilities would likely reduce the prescription of APs and has the potential to slow the decline in this vulnerable population. A meta‐analysis incorporating both randomized and nonrandomized trials of medical and surgical patients showed that multicomponent nonpharmacologic interventions decreased delirium by 50%.[24] Thus, simple interventions such as reorientation, early mobilization, optimizing vision and hearing, sleepwake cycle preservation, and hydration might avoid roughly 1 million cases of delirium in hospitalized older adults annually.[24] The Hospital Elder Life Program and Acute Care for Elders units are examples of programs that have been shown to decrease the incidence of delirium.[25, 26]
Despite vigorous efforts to prevent delirium, a subgroup of patients still will become delirious. These patients are at high risk for death. Our mortality prediction model revealed that patients who were discharged to postacute facilities were 4 times more likely to die during the subsequent year compared to patients who were discharged home. Patients discharged to postacute facilities are likely to have a higher burden of disease, greater functional and cognitive impairment, and more frailty than those who are able to return to the community. Very ill and/or frail patients receiving APs in the hospital and requiring APs on discharge to postacute care facilities have limited survival and may benefit from expedited palliative care interventions to clarify prognosis and goals, and relieve suffering. At a minimum, our study identifies a need for further study to identify this very high‐risk group of elders. It is notable that 50% of patients were found to have a post‐treatment ECG with a QTc of >500 ms, a finding that has not been previously described. This would put these patients at higher risk of mortality, and as such we suggest that current guidelines should continue to emphasize the importance of post‐treatment ECGs and set clear criteria for discontinuation in elderly patients.
Our study is limited by its retrospective, single‐center design and small sample size, therefore limiting the interpretation and generalizability of the results to other hospitals. Quetiapine was the most common AP medication used in our hospital; therefore, our findings cannot be generalized to hospitals that utilize other AP agents. Future studies should examine antipsychotic use across hospitals to determine variation in prescribing patterns and outcomes. Nevertheless, the care of these patients were transitioned to a large number of geriatricians and primary care and nursing home physicians after discharge, and the reflected practice patterns extended beyond our hospital. Additionally, we were unable to determine when and why APs were discontinued or started in the outpatient setting. We were only able to detect readmissions to the 3 hospitals within our health system and therefore may have missed some readmissions to other institutions, although the majority of patients in our region tend to return to the same hospital. For patients who were not readmitted, we were also unable to identify whether they remained on the APs initiated during their index hospitalizations. Any retrospective study is limited by the difficulty of distinguishing delirium from the behavioral and psychiatric symptoms of dementia, but we identified delirium using standard terms described in previous literature.[10] We were unable to determine the types of delirium (hyperactive vs hypoactive) given that the documentations on behavioral symptoms were largely missing from the charts. The number of patients with preexisting diagnosis of dementia was likely underestimated, as we were only able to verify the diagnosis from the medical history. Additionally, the retrospective design based on chart review limited the factors that we could detect and grade accurately for inclusion in our mortality prediction model. Of note, our model did not contain objective measures of cognition, agitation, function, and markers for frailty such as walking speed, weak grip strength, weight loss, and low physical activity.
CONCLUSION
Initiating an AP (eg, haloperidol, quetiapine, olanzapine, and risperidone) in the hospital is likely to result in long‐term use of these medications despite the fact that AP use has been associated with multiple risks including falls, fractures, stroke, cardiovascular disease, and increased mortality in those with underlying dementia.[27] When possible, behavioral interventions to prevent delirium and slow the trajectory of decline should be implemented to reduce AP use. If patients with delirium are started on antipsychotics, it is important to monitor for prolonged QTc given the associated risk of mortality. In a subgroup of patients at high risk for death in the upcoming year, occurrence of delirium or use of APs during a hospitalization should both be considered triggers for early advance care planning and possibly palliative care and end‐of‐life discussions, with an emphasis on quality of life.
Disclosures: The research was supported by the Department of Medicine, Baystate Medical Center/Tufts University School of Medicine. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Loh had full access to all of the data in the study. They take responsibility for the integrity of the data and the accuracy of the analysis. Drs. Loh, Brennan, Lindenauer, and Lagu conceived of the study. Drs. Loh, Ramdass, and Ms. Garb acquired the data. Ms Garb analyzed and interpreted the data. Drs. Loh, Ramdass, and Thim drafted the manuscript. Drs. Brennan, Lindenauer, and Lagu and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Lagu has received consulting fees from the Institute for Healthcare Improvement, under contract to the Centers for Medicare and Medicaid Services, for her work on a project to help health systems achieve disability competence. Dr. Brennan is supported by a Geriatric Work Force Enhancement Grant from the US Department of Health and Human Services award number 1 U1QHP287020100. The authors report no conflicts of interest.
- , , . Delirium in elderly people. Lancet. 2014;383:911–922.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156:848–856, W296.
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63:142–150.
- Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1–20.
- , ; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med (Lond). 2006;6:303–308.
- , , . Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21.
- , , , et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72:438–445.
- , . Safety and efficacy of antipsychotic drugs for the behavioral and psychological symptoms of dementia. Indian J Psychiatry. 2009;51(suppl 1):S87–S92.
- , , , . Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. J Clin Psychopharmacol. 2014;34:109–123.
- , , , , , . From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9:802–804.
- , , . Comparison of National Death Index and World Wide Web Death Searches. Am J Epidemiol. 2000;152:107–111.
- . Analysis of Binary Data. London, United Kingdom: Methuen; 1970:76–99.
- . Statistical Methods for Survival Data Analysis. New York, NY: John Wiley 1992:233–236.
- , , , . The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014;43:127–132.
- , , , et al. Risk factors for delirium and inpatient mortality with delirium. J Postgrad Med. 2013;59:263–270.
- , , , , , . Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014;14:129.
- , , , , , . One‐year mortality of elderly inpatients with delirium, dementia, or depression seen by a consultation‐liaison service. Psychosomatics. 2012;53:433–438.
- , , , . Excess mortality in general hospital patients with delirium: a 5‐year follow‐up of 519 patients seen in psychiatric consultation. J Psychosom Res. 1994;38:339–346.
- , , , et al. Older adults discharged from the hospital with delirium: 1‐year outcomes. J Am Geriatr Soc. 2006;54:1245–1250.
- , , , et al. The dementia antipsychotic withdrawal trial (DART‐AD): long‐term follow‐up of a randomised placebo‐controlled trial. Lancet Neurol. 2009;8:151–157.
- , , , et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786.
- , , , et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341.
- , , , et al. The long‐term effects of conventional and atypical antipsychotics in patients with probable Alzheimer's disease. Am J Psychiatry. 2013;170:1051–1058.
- , , , et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta‐analysis. JAMA Intern Med. 2015;175:512–520.
- , , , , . Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59:359–365.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60:2237–2245.
- , . Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81:617–622.
- , , . Delirium in elderly people. Lancet. 2014;383:911–922.
- , , , et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156:848–856, W296.
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63:142–150.
- Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156:1–20.
- , ; Guideline Development Group. The prevention, diagnosis and management of delirium in older people: concise guidelines. Clin Med (Lond). 2006;6:303–308.
- , , . Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21.
- , , , et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72:438–445.
- , . Safety and efficacy of antipsychotic drugs for the behavioral and psychological symptoms of dementia. Indian J Psychiatry. 2009;51(suppl 1):S87–S92.
- , , , . Use and safety of antipsychotics in behavioral disorders in elderly people with dementia. J Clin Psychopharmacol. 2014;34:109–123.
- , , , , , . From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9:802–804.
- , , . Comparison of National Death Index and World Wide Web Death Searches. Am J Epidemiol. 2000;152:107–111.
- . Analysis of Binary Data. London, United Kingdom: Methuen; 1970:76–99.
- . Statistical Methods for Survival Data Analysis. New York, NY: John Wiley 1992:233–236.
- , , , . The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014;43:127–132.
- , , , et al. Risk factors for delirium and inpatient mortality with delirium. J Postgrad Med. 2013;59:263–270.
- , , , , , . Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014;14:129.
- , , , , , . One‐year mortality of elderly inpatients with delirium, dementia, or depression seen by a consultation‐liaison service. Psychosomatics. 2012;53:433–438.
- , , , . Excess mortality in general hospital patients with delirium: a 5‐year follow‐up of 519 patients seen in psychiatric consultation. J Psychosom Res. 1994;38:339–346.
- , , , et al. Older adults discharged from the hospital with delirium: 1‐year outcomes. J Am Geriatr Soc. 2006;54:1245–1250.
- , , , et al. The dementia antipsychotic withdrawal trial (DART‐AD): long‐term follow‐up of a randomised placebo‐controlled trial. Lancet Neurol. 2009;8:151–157.
- , , , et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786.
- , , , et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341.
- , , , et al. The long‐term effects of conventional and atypical antipsychotics in patients with probable Alzheimer's disease. Am J Psychiatry. 2013;170:1051–1058.
- , , , et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta‐analysis. JAMA Intern Med. 2015;175:512–520.
- , , , , . Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59:359–365.
- , , , et al. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta‐analysis. J Am Geriatr Soc. 2012;60:2237–2245.
- , . Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81:617–622.
Inflectra becomes first FDA-approved biosimilar for inflammatory diseases
A biosimilar version of the anti–tumor necrosis factor–alpha agent Remicade has been approved by the Food and Drug Administration, making it the first biosimilar drug approved by the agency for inflammatory diseases and just the second biosimilar it has approved.
The agency said in its April 5 announcement that the biosimilar drug, to be marketed as Inflectra, will have the same indications as Remicade: moderately to severely active Crohn’s disease in patients aged 6 years and older who have had an inadequate response to conventional therapy; moderately to severely active ulcerative colitis that has inadequately responded to conventional therapy; moderately to severely active rheumatoid arthritis in combination with methotrexate; active ankylosing spondylitis; active psoriatic arthritis; and chronic, severe plaque psoriasis.
The drug, given the generic name of infliximab-dyyb under the agency’s nomenclature for biosimilar products, earned its approval as a biosimilar by showing it has no clinically meaningful differences in terms of safety and effectiveness from Remicade. According to FDA regulations, biosimilar products can have only minor differences in clinically inactive components and must have the same mechanism(s) of action (to the extent that it is known) and route(s) of administration, dosage form(s), and strength(s) as the reference product; and can be approved only for the indication(s) and condition(s) of use that have been approved for the reference product.
Inflectra’s approval is only as a biosimilar, not as an interchangeable product. The agency has yet to define the regulatory requirements for interchangeability that are necessary to meet the requirements of the Biologics Price Competition and Innovation Act of 2009. That Act states that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” A statement about implementation of the Act on the FDA website explains that for interchangeability, “a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical result as the reference product in any given patient and, for a biological product that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.”
Like Remicade, Inflectra will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections (tuberculosis, bacterial sepsis, invasive fungal infections, and others), lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Inflectra is manufactured by Celltrion, based in South Korea, for Illinois-based Hospira. Inflectra’s label can be found here.
A biosimilar version of the anti–tumor necrosis factor–alpha agent Remicade has been approved by the Food and Drug Administration, making it the first biosimilar drug approved by the agency for inflammatory diseases and just the second biosimilar it has approved.
The agency said in its April 5 announcement that the biosimilar drug, to be marketed as Inflectra, will have the same indications as Remicade: moderately to severely active Crohn’s disease in patients aged 6 years and older who have had an inadequate response to conventional therapy; moderately to severely active ulcerative colitis that has inadequately responded to conventional therapy; moderately to severely active rheumatoid arthritis in combination with methotrexate; active ankylosing spondylitis; active psoriatic arthritis; and chronic, severe plaque psoriasis.
The drug, given the generic name of infliximab-dyyb under the agency’s nomenclature for biosimilar products, earned its approval as a biosimilar by showing it has no clinically meaningful differences in terms of safety and effectiveness from Remicade. According to FDA regulations, biosimilar products can have only minor differences in clinically inactive components and must have the same mechanism(s) of action (to the extent that it is known) and route(s) of administration, dosage form(s), and strength(s) as the reference product; and can be approved only for the indication(s) and condition(s) of use that have been approved for the reference product.
Inflectra’s approval is only as a biosimilar, not as an interchangeable product. The agency has yet to define the regulatory requirements for interchangeability that are necessary to meet the requirements of the Biologics Price Competition and Innovation Act of 2009. That Act states that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” A statement about implementation of the Act on the FDA website explains that for interchangeability, “a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical result as the reference product in any given patient and, for a biological product that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.”
Like Remicade, Inflectra will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections (tuberculosis, bacterial sepsis, invasive fungal infections, and others), lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Inflectra is manufactured by Celltrion, based in South Korea, for Illinois-based Hospira. Inflectra’s label can be found here.
A biosimilar version of the anti–tumor necrosis factor–alpha agent Remicade has been approved by the Food and Drug Administration, making it the first biosimilar drug approved by the agency for inflammatory diseases and just the second biosimilar it has approved.
The agency said in its April 5 announcement that the biosimilar drug, to be marketed as Inflectra, will have the same indications as Remicade: moderately to severely active Crohn’s disease in patients aged 6 years and older who have had an inadequate response to conventional therapy; moderately to severely active ulcerative colitis that has inadequately responded to conventional therapy; moderately to severely active rheumatoid arthritis in combination with methotrexate; active ankylosing spondylitis; active psoriatic arthritis; and chronic, severe plaque psoriasis.
The drug, given the generic name of infliximab-dyyb under the agency’s nomenclature for biosimilar products, earned its approval as a biosimilar by showing it has no clinically meaningful differences in terms of safety and effectiveness from Remicade. According to FDA regulations, biosimilar products can have only minor differences in clinically inactive components and must have the same mechanism(s) of action (to the extent that it is known) and route(s) of administration, dosage form(s), and strength(s) as the reference product; and can be approved only for the indication(s) and condition(s) of use that have been approved for the reference product.
Inflectra’s approval is only as a biosimilar, not as an interchangeable product. The agency has yet to define the regulatory requirements for interchangeability that are necessary to meet the requirements of the Biologics Price Competition and Innovation Act of 2009. That Act states that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” A statement about implementation of the Act on the FDA website explains that for interchangeability, “a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical result as the reference product in any given patient and, for a biological product that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.”
Like Remicade, Inflectra will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections (tuberculosis, bacterial sepsis, invasive fungal infections, and others), lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Inflectra is manufactured by Celltrion, based in South Korea, for Illinois-based Hospira. Inflectra’s label can be found here.