User login
Fatty liver risk rises in years after transplant
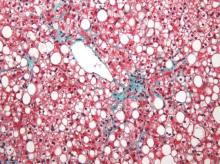
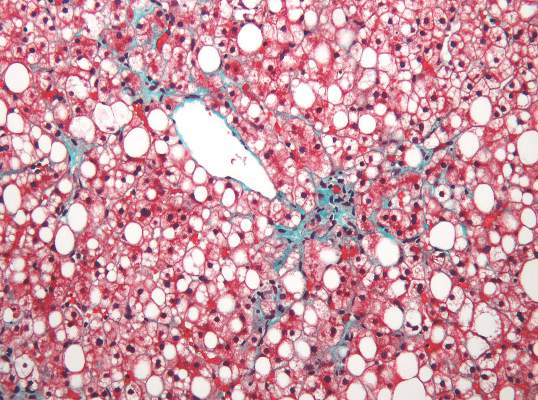
Steatosis may be present in at least half of liver transplant recipients, and the prevalence increases significantly over time, according to data from a retrospective study of 548 adult patients.
Although steatosis is common after transplantation, the prevalence, risk factors, and impact on patient survival has not been well studied, wrote Dr. Irena Hejlova of the Institute for Clinical and Experimental Medicine in Prague, Czech Republic, and her colleagues.
“Our study was the first to document that the prevalence of steatosis in LT [liver transplant] recipients may be far higher than previously reported,” they said.
The researchers reviewed liver biopsies and patient survival data and found steatosis in 309 (56%) of the patients, including 93 (17%) with significant steatosis (defined as greater than 33%). Pretransplant factors associated with significant steatosis included cirrhosis caused by alcohol consumption as well as a high body mass index. Post-transplant risk factors associated with increased risk of significant steatosis included increased body mass index, increased serum triglycerides, alcohol consumption, and type 2 diabetes. However, “Although patients transplanted for alcoholic cirrhosis are at an increased risk, the vast majority of post-transplant steatosis is nonalcohol-related,” the researchers noted.
The overall prevalence of steatosis increased from 30% at 1 year after transplant to 48% at 10 years after transplant. Post-transplant steatosis was not associated with worse patient survival in the short term, but the long-term survival of patients with significant steatosis tended to be worse.
Read the full study here (Liver Transpl. 2016 Apr 5. doi: 10.1002/lt.24393).
Steatosis may be present in at least half of liver transplant recipients, and the prevalence increases significantly over time, according to data from a retrospective study of 548 adult patients.
Although steatosis is common after transplantation, the prevalence, risk factors, and impact on patient survival has not been well studied, wrote Dr. Irena Hejlova of the Institute for Clinical and Experimental Medicine in Prague, Czech Republic, and her colleagues.
“Our study was the first to document that the prevalence of steatosis in LT [liver transplant] recipients may be far higher than previously reported,” they said.
The researchers reviewed liver biopsies and patient survival data and found steatosis in 309 (56%) of the patients, including 93 (17%) with significant steatosis (defined as greater than 33%). Pretransplant factors associated with significant steatosis included cirrhosis caused by alcohol consumption as well as a high body mass index. Post-transplant risk factors associated with increased risk of significant steatosis included increased body mass index, increased serum triglycerides, alcohol consumption, and type 2 diabetes. However, “Although patients transplanted for alcoholic cirrhosis are at an increased risk, the vast majority of post-transplant steatosis is nonalcohol-related,” the researchers noted.
The overall prevalence of steatosis increased from 30% at 1 year after transplant to 48% at 10 years after transplant. Post-transplant steatosis was not associated with worse patient survival in the short term, but the long-term survival of patients with significant steatosis tended to be worse.
Read the full study here (Liver Transpl. 2016 Apr 5. doi: 10.1002/lt.24393).
Steatosis may be present in at least half of liver transplant recipients, and the prevalence increases significantly over time, according to data from a retrospective study of 548 adult patients.
Although steatosis is common after transplantation, the prevalence, risk factors, and impact on patient survival has not been well studied, wrote Dr. Irena Hejlova of the Institute for Clinical and Experimental Medicine in Prague, Czech Republic, and her colleagues.
“Our study was the first to document that the prevalence of steatosis in LT [liver transplant] recipients may be far higher than previously reported,” they said.
The researchers reviewed liver biopsies and patient survival data and found steatosis in 309 (56%) of the patients, including 93 (17%) with significant steatosis (defined as greater than 33%). Pretransplant factors associated with significant steatosis included cirrhosis caused by alcohol consumption as well as a high body mass index. Post-transplant risk factors associated with increased risk of significant steatosis included increased body mass index, increased serum triglycerides, alcohol consumption, and type 2 diabetes. However, “Although patients transplanted for alcoholic cirrhosis are at an increased risk, the vast majority of post-transplant steatosis is nonalcohol-related,” the researchers noted.
The overall prevalence of steatosis increased from 30% at 1 year after transplant to 48% at 10 years after transplant. Post-transplant steatosis was not associated with worse patient survival in the short term, but the long-term survival of patients with significant steatosis tended to be worse.
Read the full study here (Liver Transpl. 2016 Apr 5. doi: 10.1002/lt.24393).
FROM LIVER TRANSPLANTATION
More Evidence of HPV’s Role in Cancer
Some types of human papillomavirus (HPV) that are implicated in a variety of cancers have been suggested as a risk factor for esophageal cancer. However, the frequency of HPV infection in patients with esophageal premalignant lesions or carcinomas varies as widely as 0% to 88% in different studies, say researchers from Affiliated Cancer Hospital of Zhengzhou University, in Zhengzhou, China.
The most common high-risk oncogenic subtypes are HPV-16 and HPV-18. One meta-analysis found HPV-16 in 38% of esophageal cancer cases. But HPV-18 is less defined, the researchers say. They conducted a meta-analysis to determine the prevalence of HPV-18 in China, which has one of the highest rates in the world of esophageal cancer as well as one of the highest rates of HPV prevalence in esophageal squamous cell carcinoma.
Their analysis of 19 studies included 2,556 cases of esophageal cancer. Overall, the prevalence of HPV-18 was > 4%—less than cervical cancer (15.3%), ovarian cancer (12.2%), laryngeal cancer (6.2%), bladder cancer (5.9%), and lung cancer (5.6%). The estimates of HPV prevalence in esophageal cancer varied widely, the researchers found, by geographic region.
Related: Promising Method to Evaluate Response to Treatment
Although their study doesn’t answer the question of etiology of HPV and esophageal cancer, the researchers say it is an important preliminary step toward evaluating the relationship. They add that their findings could also give some indication of the effect of the HPV vaccine against esophageal cancer.
Source:Guo LW, Zhang SK, Liu SZ, et al. Epidemiol Infect. 2016;144(3):469-477.doi: 10.1017/S0950268815001703.
Some types of human papillomavirus (HPV) that are implicated in a variety of cancers have been suggested as a risk factor for esophageal cancer. However, the frequency of HPV infection in patients with esophageal premalignant lesions or carcinomas varies as widely as 0% to 88% in different studies, say researchers from Affiliated Cancer Hospital of Zhengzhou University, in Zhengzhou, China.
The most common high-risk oncogenic subtypes are HPV-16 and HPV-18. One meta-analysis found HPV-16 in 38% of esophageal cancer cases. But HPV-18 is less defined, the researchers say. They conducted a meta-analysis to determine the prevalence of HPV-18 in China, which has one of the highest rates in the world of esophageal cancer as well as one of the highest rates of HPV prevalence in esophageal squamous cell carcinoma.
Their analysis of 19 studies included 2,556 cases of esophageal cancer. Overall, the prevalence of HPV-18 was > 4%—less than cervical cancer (15.3%), ovarian cancer (12.2%), laryngeal cancer (6.2%), bladder cancer (5.9%), and lung cancer (5.6%). The estimates of HPV prevalence in esophageal cancer varied widely, the researchers found, by geographic region.
Related: Promising Method to Evaluate Response to Treatment
Although their study doesn’t answer the question of etiology of HPV and esophageal cancer, the researchers say it is an important preliminary step toward evaluating the relationship. They add that their findings could also give some indication of the effect of the HPV vaccine against esophageal cancer.
Source:Guo LW, Zhang SK, Liu SZ, et al. Epidemiol Infect. 2016;144(3):469-477.doi: 10.1017/S0950268815001703.
Some types of human papillomavirus (HPV) that are implicated in a variety of cancers have been suggested as a risk factor for esophageal cancer. However, the frequency of HPV infection in patients with esophageal premalignant lesions or carcinomas varies as widely as 0% to 88% in different studies, say researchers from Affiliated Cancer Hospital of Zhengzhou University, in Zhengzhou, China.
The most common high-risk oncogenic subtypes are HPV-16 and HPV-18. One meta-analysis found HPV-16 in 38% of esophageal cancer cases. But HPV-18 is less defined, the researchers say. They conducted a meta-analysis to determine the prevalence of HPV-18 in China, which has one of the highest rates in the world of esophageal cancer as well as one of the highest rates of HPV prevalence in esophageal squamous cell carcinoma.
Their analysis of 19 studies included 2,556 cases of esophageal cancer. Overall, the prevalence of HPV-18 was > 4%—less than cervical cancer (15.3%), ovarian cancer (12.2%), laryngeal cancer (6.2%), bladder cancer (5.9%), and lung cancer (5.6%). The estimates of HPV prevalence in esophageal cancer varied widely, the researchers found, by geographic region.
Related: Promising Method to Evaluate Response to Treatment
Although their study doesn’t answer the question of etiology of HPV and esophageal cancer, the researchers say it is an important preliminary step toward evaluating the relationship. They add that their findings could also give some indication of the effect of the HPV vaccine against esophageal cancer.
Source:Guo LW, Zhang SK, Liu SZ, et al. Epidemiol Infect. 2016;144(3):469-477.doi: 10.1017/S0950268815001703.
Robot-assisted laparoscopic myomectomy
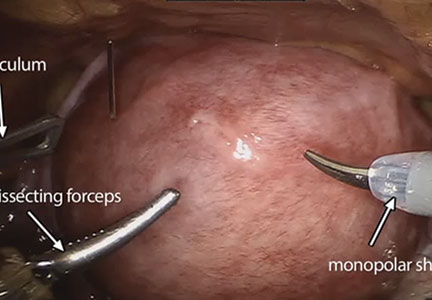
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The management of symptomatic uterine fibroids in the patient desiring conservative surgical therapy can be challenging at times. The advent of robot-assisted laparoscopy has provided surgeons with an enabling tool and patients with the option for a minimally invasive approach to myomectomy.
This month’s video was produced in order to demonstrate a systematic approach to the robot-assisted laparoscopic myomectomy in patients who are candidates. The example case is removal of a 5-cm, intrauterine posterior myoma in a 39-year-old woman (G3P1021) with heavy menstrual bleeding who desires future fertility.
Key objectives of the video include:
- understanding the role of radiologic imaging as part of preoperative surgical planning
- recognizing the key robotic instruments and suture selected to perform the procedure
- discussing robot-specific techniques that facilitate fibroid enucleation and hysterotomy repair.
Also integrated into this video is the application of the ExCITE technique—a manual cold knife tissue extraction technique utilizing an extracorporeal semi-circle “C-incision” approach—for tissue extraction. This technique was featured in an earlier installment of the video channel.1
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
- Truong M, Advincula A. Minimally invasive tissue extraction made simple: the Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
Make the Diagnosis - April 2016
Diagnosis: Lymphomatoid papulosis
Lymphomatoid papulosis is a rare, chronic, and benign papulonodular or papulonecrotic skin disorder. LyP affects people of all ages, and its peak incidence occurs in the 5th decade. It generally has no predilection for a particular sex; however, some have reported a slight predominance in males. Patients of all races may be diagnosed with LyP, although it is less common in black patients. In addition, 10% of LyP cases are associated with anaplastic large cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin’s lymphoma.
Patients typically present with multiple erythematous papules that evolve into red-brown papulopustular or papulovesicular lesions. The papules may be mildly pruritic or asymptomatic and can be few in number to hundreds at presentation. The lesions usually appear in crops that resolve within 2-8 weeks with or without scarring, and can continue this cyclic process for months to years. The arms, legs, and trunk are most commonly affected, although LyP can present anywhere on the body.
The diagnosis of LyP is classically based upon histopathologic examination. Hematoxylin and eosin staining reveals a dense dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils; lymphocytes are CD30+. Vessels in the dermis also appear with fibrin deposition, endothelial edema, and red blood cell extravasation. In addition, LyP can be classified as type A, type B, type C, and/or type D. These subtypes are determined by the size of atypical lymphocytes, type of atypical cells, location and amount of infiltrate, and CD30 and CD8 staining.
The differential diagnosis of LyP includes anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, insect bites, Langerhans cell histiocytosis, leukemia cutis, milia, miliaria, and scabies.
The etiology of LyP is unknown. It is unclear whether the proliferation of T cells is a benign and chronic disorder initiated by a stimulus or an indolent T-cell malignancy that the immune system monitors and only allows for localized, cutaneous involvement.
Mild forms of LyP can often be managed with topical corticosteroids. However, other therapies such as intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate are effective in treating cases of more persistent and widespread disease.
Our patient’s biopsy showed an irregular epidermis with scale, focal ulceration, scattered eosinophils, and dermal lymphocytes and histiocytes present in a perivascular pattern. Many of the lymphoid cells were enlarged, hyperchromatic, and irregular. Immunohistochemical staining was CD30+. These histologic changes were most consistent with lymphomatoid papulosis.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to [email protected].
Diagnosis: Lymphomatoid papulosis
Lymphomatoid papulosis is a rare, chronic, and benign papulonodular or papulonecrotic skin disorder. LyP affects people of all ages, and its peak incidence occurs in the 5th decade. It generally has no predilection for a particular sex; however, some have reported a slight predominance in males. Patients of all races may be diagnosed with LyP, although it is less common in black patients. In addition, 10% of LyP cases are associated with anaplastic large cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin’s lymphoma.
Patients typically present with multiple erythematous papules that evolve into red-brown papulopustular or papulovesicular lesions. The papules may be mildly pruritic or asymptomatic and can be few in number to hundreds at presentation. The lesions usually appear in crops that resolve within 2-8 weeks with or without scarring, and can continue this cyclic process for months to years. The arms, legs, and trunk are most commonly affected, although LyP can present anywhere on the body.
The diagnosis of LyP is classically based upon histopathologic examination. Hematoxylin and eosin staining reveals a dense dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils; lymphocytes are CD30+. Vessels in the dermis also appear with fibrin deposition, endothelial edema, and red blood cell extravasation. In addition, LyP can be classified as type A, type B, type C, and/or type D. These subtypes are determined by the size of atypical lymphocytes, type of atypical cells, location and amount of infiltrate, and CD30 and CD8 staining.
The differential diagnosis of LyP includes anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, insect bites, Langerhans cell histiocytosis, leukemia cutis, milia, miliaria, and scabies.
The etiology of LyP is unknown. It is unclear whether the proliferation of T cells is a benign and chronic disorder initiated by a stimulus or an indolent T-cell malignancy that the immune system monitors and only allows for localized, cutaneous involvement.
Mild forms of LyP can often be managed with topical corticosteroids. However, other therapies such as intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate are effective in treating cases of more persistent and widespread disease.
Our patient’s biopsy showed an irregular epidermis with scale, focal ulceration, scattered eosinophils, and dermal lymphocytes and histiocytes present in a perivascular pattern. Many of the lymphoid cells were enlarged, hyperchromatic, and irregular. Immunohistochemical staining was CD30+. These histologic changes were most consistent with lymphomatoid papulosis.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to [email protected].
Diagnosis: Lymphomatoid papulosis
Lymphomatoid papulosis is a rare, chronic, and benign papulonodular or papulonecrotic skin disorder. LyP affects people of all ages, and its peak incidence occurs in the 5th decade. It generally has no predilection for a particular sex; however, some have reported a slight predominance in males. Patients of all races may be diagnosed with LyP, although it is less common in black patients. In addition, 10% of LyP cases are associated with anaplastic large cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin’s lymphoma.
Patients typically present with multiple erythematous papules that evolve into red-brown papulopustular or papulovesicular lesions. The papules may be mildly pruritic or asymptomatic and can be few in number to hundreds at presentation. The lesions usually appear in crops that resolve within 2-8 weeks with or without scarring, and can continue this cyclic process for months to years. The arms, legs, and trunk are most commonly affected, although LyP can present anywhere on the body.
The diagnosis of LyP is classically based upon histopathologic examination. Hematoxylin and eosin staining reveals a dense dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils; lymphocytes are CD30+. Vessels in the dermis also appear with fibrin deposition, endothelial edema, and red blood cell extravasation. In addition, LyP can be classified as type A, type B, type C, and/or type D. These subtypes are determined by the size of atypical lymphocytes, type of atypical cells, location and amount of infiltrate, and CD30 and CD8 staining.
The differential diagnosis of LyP includes anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, insect bites, Langerhans cell histiocytosis, leukemia cutis, milia, miliaria, and scabies.
The etiology of LyP is unknown. It is unclear whether the proliferation of T cells is a benign and chronic disorder initiated by a stimulus or an indolent T-cell malignancy that the immune system monitors and only allows for localized, cutaneous involvement.
Mild forms of LyP can often be managed with topical corticosteroids. However, other therapies such as intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate are effective in treating cases of more persistent and widespread disease.
Our patient’s biopsy showed an irregular epidermis with scale, focal ulceration, scattered eosinophils, and dermal lymphocytes and histiocytes present in a perivascular pattern. Many of the lymphoid cells were enlarged, hyperchromatic, and irregular. Immunohistochemical staining was CD30+. These histologic changes were most consistent with lymphomatoid papulosis.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to [email protected].
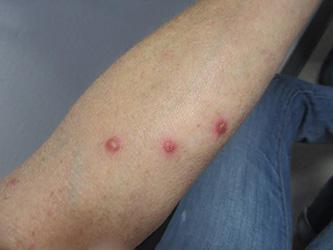
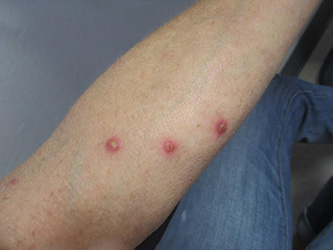
A 61-year-old woman with no significant past medical history presented with a rash on her arms. The lesions were pruritic and showed mild improvement after she took an oral antihistamine. The patient stated that she had similar outbreaks in the past that were treated with minocycline by an outside dermatologist. At that time, one lesion was cultured, showing no evidence of bacteria or herpes. She denied any history of gardening. On physical exam, she had erythematous papules and pustules in a sporotrichoid pattern on the arms, axilla, upper back, and antecubital fossa. A biopsy was performed.
FIRE AND ICE trial called a win for cryoablation of AF
CHICAGO – The largest-ever randomized trial of catheter ablation of atrial fibrillation has ended in a draw between radiofrequency and cryoballoon ablation in safety and efficacy – and that actually represents a win for cryoablation, a simpler and far more easily mastered procedure, Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
“We can teach physicians how to do cryoablation much more easily. That will allow more patients with atrial fibrillation to get access to catheter ablation, which is what we really need,” according to Dr. Kuck, principal investigator in the poetically named FIRE AND ICE trial and head of cardiology at St. Georg Hospital in Hamburg (Germany).
FIRE AND ICE included 769 patients in eight European countries. The participants, all of whom had antiarrhythmic drug–refractory paroxysmal atrial fibrillation (AF), were randomized to radiofrequency ablation – the long-time standard – or to cryoablation, a newer technology. Radiofrequency ablation was guided by three-dimensional electroanatomic mapping, while cryoablation utilized fluoroscopic guidance.
The primary efficacy endpoint was the 1-year rate of clinical failure, defined as an occurrence of AF, atrial flutter, or atrial tachycardia lasting for at least 30 seconds, or repeat ablation or the use of antiarrhythmic drugs following a 90-day postprocedural blanking period. The clinical failure rate was 34.6% in the cryoballoon group and similar at 35.9% in the radiofrequency group.
Serious treatment-related adverse events occurred in 10.2% of the cryoballoon group and 12.8% of the radiofrequency group, a nonsignificant difference. No procedural deaths occurred in the study.
There were, however, several significant procedural differences. Procedure time averaged 124 minutes in the cryoablation group, nearly 20 minutes less than the 142 minutes for radiofrequency ablation. However, the 17-minute fluoroscopy time in the radiofrequency group was 5 minutes shorter than for cryoablation.
Dr. Kuck said the study underestimates the true procedural differences because FIRE AND ICE was carried out by extremely experienced operators. In routine clinical practice involving non-elite operators, it’s not unusual for radiofrequency ablation fluoroscopy times to be two or even three times longer than the 17 minutes seen in the study. Plus, FIRE AND ICE was conducted when the procedure entailed two applications of the cryoballoon. Now only one application is recommended, cutting an additional 12 minutes off the total procedure time, he added.
Radiofrequency ablation takes longer because it entails creating a series of point-to-point lesions in a circle to isolate the pulmonary veins. With cryoablation, the balloon is moved into position, inflated, and a 3-minute-freeze is administered to create a circle of necrotic tissue in a single-step procedure.
Discussant Dr. Hugh G. Calkins praised the FIRE AND ICE investigators’ use of a rigorous definition of recurrence that required as little as a 30-second episode of atrial arrhythmia.
“That’s a very high bar, so I think the results are very impressive,” said Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
He commented that “this study is a clear reminder that 90% success rates just don’t happen in this field,” despite what some practitioners have claimed.
Asked how he predicts the study results will influence the field of AF ablation, Dr. Kuck replied that he foresees much wider adoption of cryoablation and a stronger endorsement of the technology in updated guideline recommendations.
“I personally believe this will be the most important development in our field in the next several years,” he added.
The electrophysiologist noted that even though current guidelines give a class Ia recommendation to catheter ablation of paroxysmal AF that’s refractory to at least one antiarrhythmic drug, at present only 4% of such patients actually undergo the procedure.
“Having just 4% of patients with AF undergo catheter ablation cannot be what we are looking for as physicians,” Dr. Kuck said. “I believe if we want to roll out catheter ablation for AF, we need simple and safe tools. This trial elegantly shows that with a simpler device that allows single-shot isolation of the pulmonary veins, we can get the same safety and efficacy as with radiofrequency ablation. I often tell people that radiofrequency ablation of atrial fibrillation is the most challenging procedure in all cardiology. We do this procedure from the groin in a moving heart. It’s a very complex technology.”
His dream, he continued, is that cryoablation will eventually enable patients with atrial fibrillation to be managed the same way electrophysiologists treat patients with Wolff-Parkinson-White syndrome; with the first episode, the patient goes to the electrophysiology catheterization lab for an ablation procedure.
“I think there’s a great message here: The cryoballoon will move catheter ablation from a niche procedure performed in specialized centers by the few guys in the world who can do it really well out into the broader world. To do that you need a tool that is safe, simple, and can be handled by the average doctor,” Dr. Kuck said.
Discussant Dr. Anthony DeMaria commented that it would be premature at this point to start thinking about cryoablation as a first approach to new-onset AF, given the roughly 35% clinical failure rate at 1 year seen in FIRE AND ICE. That rate doubtless would have been even higher had patients been equipped with implantable loop recorders, added Dr. DeMaria, professor of medicine at the University of California, San Diego.
Dr. Kuck conceded that the high recurrence rate is one of the great unsolved limitations of catheter ablation of AF.
“We don’t know how to get the pulmonary veins permanently isolated,” he said. “We can create acute lesions, but over time what we’ve seen is recovery of tissue and then reconduction by the pulmonary veins. I believe that 20% of the 40% recurrence rate is due to reconduction from the pulmonary veins, and the rest is probably due to triggers coming from other sites.”
The FIRE AND ICE trial was funded in part by Medtronic, which markets the Arctic Front Advance cryoablation catheter used in the study. Dr. Kuck reported serving on a speakers’ bureau for Medtronic and acting as a consultant to Biosense Webster, Edwards, and St. Jude.
Simultaneous with Dr. Kuck’s presentation at ACC 16, the results of FIRE AND ICE were published online (N Engl J Med. 2016 Apr 4. doi: 10.1056/NEJMoa1602014).
CHICAGO – The largest-ever randomized trial of catheter ablation of atrial fibrillation has ended in a draw between radiofrequency and cryoballoon ablation in safety and efficacy – and that actually represents a win for cryoablation, a simpler and far more easily mastered procedure, Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
“We can teach physicians how to do cryoablation much more easily. That will allow more patients with atrial fibrillation to get access to catheter ablation, which is what we really need,” according to Dr. Kuck, principal investigator in the poetically named FIRE AND ICE trial and head of cardiology at St. Georg Hospital in Hamburg (Germany).
FIRE AND ICE included 769 patients in eight European countries. The participants, all of whom had antiarrhythmic drug–refractory paroxysmal atrial fibrillation (AF), were randomized to radiofrequency ablation – the long-time standard – or to cryoablation, a newer technology. Radiofrequency ablation was guided by three-dimensional electroanatomic mapping, while cryoablation utilized fluoroscopic guidance.
The primary efficacy endpoint was the 1-year rate of clinical failure, defined as an occurrence of AF, atrial flutter, or atrial tachycardia lasting for at least 30 seconds, or repeat ablation or the use of antiarrhythmic drugs following a 90-day postprocedural blanking period. The clinical failure rate was 34.6% in the cryoballoon group and similar at 35.9% in the radiofrequency group.
Serious treatment-related adverse events occurred in 10.2% of the cryoballoon group and 12.8% of the radiofrequency group, a nonsignificant difference. No procedural deaths occurred in the study.
There were, however, several significant procedural differences. Procedure time averaged 124 minutes in the cryoablation group, nearly 20 minutes less than the 142 minutes for radiofrequency ablation. However, the 17-minute fluoroscopy time in the radiofrequency group was 5 minutes shorter than for cryoablation.
Dr. Kuck said the study underestimates the true procedural differences because FIRE AND ICE was carried out by extremely experienced operators. In routine clinical practice involving non-elite operators, it’s not unusual for radiofrequency ablation fluoroscopy times to be two or even three times longer than the 17 minutes seen in the study. Plus, FIRE AND ICE was conducted when the procedure entailed two applications of the cryoballoon. Now only one application is recommended, cutting an additional 12 minutes off the total procedure time, he added.
Radiofrequency ablation takes longer because it entails creating a series of point-to-point lesions in a circle to isolate the pulmonary veins. With cryoablation, the balloon is moved into position, inflated, and a 3-minute-freeze is administered to create a circle of necrotic tissue in a single-step procedure.
Discussant Dr. Hugh G. Calkins praised the FIRE AND ICE investigators’ use of a rigorous definition of recurrence that required as little as a 30-second episode of atrial arrhythmia.
“That’s a very high bar, so I think the results are very impressive,” said Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
He commented that “this study is a clear reminder that 90% success rates just don’t happen in this field,” despite what some practitioners have claimed.
Asked how he predicts the study results will influence the field of AF ablation, Dr. Kuck replied that he foresees much wider adoption of cryoablation and a stronger endorsement of the technology in updated guideline recommendations.
“I personally believe this will be the most important development in our field in the next several years,” he added.
The electrophysiologist noted that even though current guidelines give a class Ia recommendation to catheter ablation of paroxysmal AF that’s refractory to at least one antiarrhythmic drug, at present only 4% of such patients actually undergo the procedure.
“Having just 4% of patients with AF undergo catheter ablation cannot be what we are looking for as physicians,” Dr. Kuck said. “I believe if we want to roll out catheter ablation for AF, we need simple and safe tools. This trial elegantly shows that with a simpler device that allows single-shot isolation of the pulmonary veins, we can get the same safety and efficacy as with radiofrequency ablation. I often tell people that radiofrequency ablation of atrial fibrillation is the most challenging procedure in all cardiology. We do this procedure from the groin in a moving heart. It’s a very complex technology.”
His dream, he continued, is that cryoablation will eventually enable patients with atrial fibrillation to be managed the same way electrophysiologists treat patients with Wolff-Parkinson-White syndrome; with the first episode, the patient goes to the electrophysiology catheterization lab for an ablation procedure.
“I think there’s a great message here: The cryoballoon will move catheter ablation from a niche procedure performed in specialized centers by the few guys in the world who can do it really well out into the broader world. To do that you need a tool that is safe, simple, and can be handled by the average doctor,” Dr. Kuck said.
Discussant Dr. Anthony DeMaria commented that it would be premature at this point to start thinking about cryoablation as a first approach to new-onset AF, given the roughly 35% clinical failure rate at 1 year seen in FIRE AND ICE. That rate doubtless would have been even higher had patients been equipped with implantable loop recorders, added Dr. DeMaria, professor of medicine at the University of California, San Diego.
Dr. Kuck conceded that the high recurrence rate is one of the great unsolved limitations of catheter ablation of AF.
“We don’t know how to get the pulmonary veins permanently isolated,” he said. “We can create acute lesions, but over time what we’ve seen is recovery of tissue and then reconduction by the pulmonary veins. I believe that 20% of the 40% recurrence rate is due to reconduction from the pulmonary veins, and the rest is probably due to triggers coming from other sites.”
The FIRE AND ICE trial was funded in part by Medtronic, which markets the Arctic Front Advance cryoablation catheter used in the study. Dr. Kuck reported serving on a speakers’ bureau for Medtronic and acting as a consultant to Biosense Webster, Edwards, and St. Jude.
Simultaneous with Dr. Kuck’s presentation at ACC 16, the results of FIRE AND ICE were published online (N Engl J Med. 2016 Apr 4. doi: 10.1056/NEJMoa1602014).
CHICAGO – The largest-ever randomized trial of catheter ablation of atrial fibrillation has ended in a draw between radiofrequency and cryoballoon ablation in safety and efficacy – and that actually represents a win for cryoablation, a simpler and far more easily mastered procedure, Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
“We can teach physicians how to do cryoablation much more easily. That will allow more patients with atrial fibrillation to get access to catheter ablation, which is what we really need,” according to Dr. Kuck, principal investigator in the poetically named FIRE AND ICE trial and head of cardiology at St. Georg Hospital in Hamburg (Germany).
FIRE AND ICE included 769 patients in eight European countries. The participants, all of whom had antiarrhythmic drug–refractory paroxysmal atrial fibrillation (AF), were randomized to radiofrequency ablation – the long-time standard – or to cryoablation, a newer technology. Radiofrequency ablation was guided by three-dimensional electroanatomic mapping, while cryoablation utilized fluoroscopic guidance.
The primary efficacy endpoint was the 1-year rate of clinical failure, defined as an occurrence of AF, atrial flutter, or atrial tachycardia lasting for at least 30 seconds, or repeat ablation or the use of antiarrhythmic drugs following a 90-day postprocedural blanking period. The clinical failure rate was 34.6% in the cryoballoon group and similar at 35.9% in the radiofrequency group.
Serious treatment-related adverse events occurred in 10.2% of the cryoballoon group and 12.8% of the radiofrequency group, a nonsignificant difference. No procedural deaths occurred in the study.
There were, however, several significant procedural differences. Procedure time averaged 124 minutes in the cryoablation group, nearly 20 minutes less than the 142 minutes for radiofrequency ablation. However, the 17-minute fluoroscopy time in the radiofrequency group was 5 minutes shorter than for cryoablation.
Dr. Kuck said the study underestimates the true procedural differences because FIRE AND ICE was carried out by extremely experienced operators. In routine clinical practice involving non-elite operators, it’s not unusual for radiofrequency ablation fluoroscopy times to be two or even three times longer than the 17 minutes seen in the study. Plus, FIRE AND ICE was conducted when the procedure entailed two applications of the cryoballoon. Now only one application is recommended, cutting an additional 12 minutes off the total procedure time, he added.
Radiofrequency ablation takes longer because it entails creating a series of point-to-point lesions in a circle to isolate the pulmonary veins. With cryoablation, the balloon is moved into position, inflated, and a 3-minute-freeze is administered to create a circle of necrotic tissue in a single-step procedure.
Discussant Dr. Hugh G. Calkins praised the FIRE AND ICE investigators’ use of a rigorous definition of recurrence that required as little as a 30-second episode of atrial arrhythmia.
“That’s a very high bar, so I think the results are very impressive,” said Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
He commented that “this study is a clear reminder that 90% success rates just don’t happen in this field,” despite what some practitioners have claimed.
Asked how he predicts the study results will influence the field of AF ablation, Dr. Kuck replied that he foresees much wider adoption of cryoablation and a stronger endorsement of the technology in updated guideline recommendations.
“I personally believe this will be the most important development in our field in the next several years,” he added.
The electrophysiologist noted that even though current guidelines give a class Ia recommendation to catheter ablation of paroxysmal AF that’s refractory to at least one antiarrhythmic drug, at present only 4% of such patients actually undergo the procedure.
“Having just 4% of patients with AF undergo catheter ablation cannot be what we are looking for as physicians,” Dr. Kuck said. “I believe if we want to roll out catheter ablation for AF, we need simple and safe tools. This trial elegantly shows that with a simpler device that allows single-shot isolation of the pulmonary veins, we can get the same safety and efficacy as with radiofrequency ablation. I often tell people that radiofrequency ablation of atrial fibrillation is the most challenging procedure in all cardiology. We do this procedure from the groin in a moving heart. It’s a very complex technology.”
His dream, he continued, is that cryoablation will eventually enable patients with atrial fibrillation to be managed the same way electrophysiologists treat patients with Wolff-Parkinson-White syndrome; with the first episode, the patient goes to the electrophysiology catheterization lab for an ablation procedure.
“I think there’s a great message here: The cryoballoon will move catheter ablation from a niche procedure performed in specialized centers by the few guys in the world who can do it really well out into the broader world. To do that you need a tool that is safe, simple, and can be handled by the average doctor,” Dr. Kuck said.
Discussant Dr. Anthony DeMaria commented that it would be premature at this point to start thinking about cryoablation as a first approach to new-onset AF, given the roughly 35% clinical failure rate at 1 year seen in FIRE AND ICE. That rate doubtless would have been even higher had patients been equipped with implantable loop recorders, added Dr. DeMaria, professor of medicine at the University of California, San Diego.
Dr. Kuck conceded that the high recurrence rate is one of the great unsolved limitations of catheter ablation of AF.
“We don’t know how to get the pulmonary veins permanently isolated,” he said. “We can create acute lesions, but over time what we’ve seen is recovery of tissue and then reconduction by the pulmonary veins. I believe that 20% of the 40% recurrence rate is due to reconduction from the pulmonary veins, and the rest is probably due to triggers coming from other sites.”
The FIRE AND ICE trial was funded in part by Medtronic, which markets the Arctic Front Advance cryoablation catheter used in the study. Dr. Kuck reported serving on a speakers’ bureau for Medtronic and acting as a consultant to Biosense Webster, Edwards, and St. Jude.
Simultaneous with Dr. Kuck’s presentation at ACC 16, the results of FIRE AND ICE were published online (N Engl J Med. 2016 Apr 4. doi: 10.1056/NEJMoa1602014).
AT ACC 16
Key clinical point: Cryoablation of atrial fibrillation offers significant advantages over radiofrequency ablation.
Major finding: In a rigorous randomized trial, cryoablation of atrial fibrillation had a 34.6% clinical failure rate at 1 year, similar to the 35.9% rate for radiofrequency ablation.
Data source: The FIRE AND ICE trial, which randomized 769 patients with paroxysmal atrial fibrillation in eight European countries.
Disclosures: FIRE AND ICE was funded in part by Medtronic. The presenter reported serving on a speakers’ bureau for the company.
Ob.gyn. residency changes with the times
In 1966, Dr. Charles Hammond was wrapping up a 2-year stint at the National Institutes of Health where he served at the behest of the military draft board. He had graduated from medical school just 5 years prior, and was in the middle of his ob.gyn. residency training at Duke University in Durham, N.C. when he was called to serve.
His experience wasn’t unusual for the time.
“When the draft board called, you went,” he said in an interview.
When he returned, he picked up where he left off. Residencies at that time were an “open-ended thing,” sometimes lasting 5 or 6 years, depending on staffing needs and other considerations.
Dr. Hammond, now an emeritus professor at Duke, regards his public service commission as an opportunity that advanced his academic career – despite the interruption of his residency training.
Such draft-related interruptions ended in the wake of the Vietnam War, of course, but the late 1960s and the 1970s ushered in a whole new era of changes in ob.gyn. residency training programs.
By 1968, residencies lasted 4 years, and fellowships were for 2 years. Ob.gyn. subspecialties hadn’t yet been introduced, explained Dr. Sandra A. Carson, vice president for education at the American College of Obstetricians and Gynecologists.
“That is essentially how things worked for a long time,” she said in an interview.
That’s not to say there weren’t numerous other changes taking place in the specialty. In a series of interviews with physicians and educators who discussed the myriad ways that residency training has evolved over the last 50 years, a number of themes emerged.
Women in medicine
A striking change over the past 5 decades has been the increasing number of women in medicine. Nowhere has that been in greater evidence than in obstetrics and gynecology.
“There were a few – but very few,” Dr. Hammond said of women in medicine in the 1960s.
There was “a philosophy that men did it better,” he said, adding, “That has been nicely shown to be inaccurate.”
Currently, about 80% of first-year ob.gyn. residents are women, compared with 15% in 1975.
“Maybe even 83% now,” Dr. Carson said, noting that even in the early 1980s when she was in training, women were “few and far between.”
According to a 2011 workforce report by Dr. William F. Rayburn, obstetrics and gynecology has the highest percentage of women residents of any medical specialty – 80% in 2009 versus an average of 46% for other specialties combined, and that figure has remained fairly constant.
Dr. Kasandra Scales, a fourth-year resident at the State University of New York, Syracuse, said she is glad to be part of this era of the specialty where women play an integral role in the advancement of women’s health care.
“I believe our voice and unique perspective to relate with common experiences, such as the physical birth of a child or personal choices in contraception... has enhanced our specialty,” she said.
That said, the fact that men are noticeably absent from the pool of ob.gyn. applicants and residents concerns her. “There should be a balance, she said. “I think it is important to have diversity of all types in the healthcare system.”
Dr. Hammond looked back on his days in residency training and recalled pockets of resistance to the increasing number of women in medicine, but the ultimate effect was good for the specialty, he said, explaining that the quality of the resident pool improved steadily, because the number of qualified candidates increased.
“It has been an interesting interval to watch,” he said, specifically mentioning the demands that women faced in terms of family obligations, childbirth, and childrearing.
Restrictions on work hours instituted in residency programs in more recent years may have played an important role in opening the door to more women, he said.
Work hours down, learning curve up
Dr. Carson agreed that work-hour restrictions instituted in 2003 and updated in 2011, which cap the work week at 80 hours and also apply limits on shift hours, likely encouraged more women to enter the field. One constant over the last 50 years is the biological clock, she said, explaining that the pressures and demands of residency before limits were put in place may have steered women away.
Work-hour restrictions provide more flexibility, but they aren’t without controversy.
Dr. Hammond said he sees the value in work hour restrictions, but working long hours as a resident – sometimes as many as 110 hours per week – had its benefits, too.
“I remember one time when I’d been on call for about 2 and a half days, and up and working the whole time,” Dr. Hammond said. “I left the hospital, walked out to a bench, sat down, and fell asleep. I woke up and distinctly remember thinking, ‘Why am I doing this?’ But I did do it, and that fatigue helped me with learning to endure. You learned from it.”
Not only have long hours been viewed as a rite of passage in medicine, he said, but there were concerns initially that the level of education would diminish and that the risk of patient errors would increase as patients were handed off from one shift to the next, he said.
Data on the effects of work-hour rules have been conflicting. In one study, Dr. Roger P. Smith found little overall effect on total technical experience among residents before and after the restrictions were put in place (there was no statistically significant difference in the average of median total cases in the 3 years before and after). Previous studies had documented increased costs and reduced faculty job satisfaction, while still others had shown no significant changes in 30-day readmission rates, in-hospital mortality, patient length of stay, or resident performance, he noted. “What is emerging is that both the great hopes and the great fears surrounding resident work-hour restrictions have not come to pass,” Dr. Smith wrote (Obstet Gynecol. 2010 Jun;115[6]:1166-71).
Dr. Scales, who is currently chair of the Junior Fellow District II Advisory Council for ACOG, comes down on the side of wishing for more hours.
“[The restrictions] do limit the things we can do and the exposure we may otherwise have,” she said, noting that it’s frustrating to have to leave when she’d rather stay and “see a cool case.”
“It’s a nice idea in principle, but the same amount of work has to be done. It’s not real life,” she said of work-hour restrictions. “It’s hard, at least for me, to want to give up my patients. Our job is to take in as much as you can before you leave to go out into the big bad world.”
It may be difficult to determine the actual impact of work hour limits on patient outcomes because the field of obstetrics and gynecology has changed so much over time.
Dr. David Forstein, vice chair of clinical operations in the department of obstetrics and gynecology at the University of South Carolina, Greenville, and a member of the Accreditation Council for Graduate Medical Education’s task force on work hours said that, for one thing, patients are generally sicker now than ever before, due in part to the obesity epidemic.
Further, changing trends mean that residents are getting less exposure to some procedures like operative vaginal deliveries, while also having to learn more ways to perform hysterectomy. Residents aren’t necessarily less prepared. They’re just having to work very hard because of the depth and breadth of the required knowledge has increased, Dr. Forstein said. “There’s a lot more to learn.”
Dr. Carson agreed that the approach to education has changed, and that those changes are largely a reflection of overall shifts in education and technology.
Technology trends
Every physician interviewed for this article cited laparoscopy and robotic surgery as key technological advances. Fifty years ago, the surgical tools were simpler, Dr. Carson said. Now residents must learn four approaches to hysterectomy: vaginal, abdominal, laparoscopic, and robotic-assisted laparoscopic hysterectomy.
From ultrasound and birth control to genetic screening and robotic surgery, the evolution of the field has been astounding during this time period. The effects of the birth control pill on family planning alone forced an expansion of curriculum not only to the physiology of these things, but also to the treatment of women as a whole person and often as part of a family unit, she said.
Many of the technologies have dramatically changed the landscape, both in terms of how learning is accomplished (for example, simulation), and how physicians interact with patients, Dr. Hammond agreed. With ultrasound, for example, there was a sense that part of the physician-patient relationship was lost.
“To a point, some of us old guys felt like they were doing ultrasound assessment of patients rather than the tried-and-true ‘talk to them and examine them’ [approach],” he said. “I guess whichever generation you are in seems to be the right one, but it’s probably somewhere in between.”
Residency in 2016
If Dr. Scales is any indication, concerns about the loss of a personal touch are unfounded. She says that for her, that’s what it’s all about.
“We were exposed to [technology] since we were 5 or 6 – it’s all we know,” she said of herself and her fellow residents. “It’s not a disadvantage. It’s about efficiency.”
“We have to get things done as quickly as possible and technology helps us with that,” said Dr. Scales, the daughter of a teacher and blue collar worker, who spent most of her life “surrounded by the underprivileged.”
She always desired to help lift that population up, and while she didn’t have a draft board directing her toward public service, she had her own calling of sorts. As a premed major in college, she worked with a nonprofit organization, and later she worked with Hurricane Katrina survivors.
“I liked that aspect of medicine. I wanted to be able to identify with people on an individual level,” she said.
Technology, work-hour restrictions, gender distribution – they’re just part of the journey.
“I’m glad I chose ob.gyn.,” she said. “Sometimes you go through ... reflection ... Am I ready? My answer is yes. I’m excited about the next step, I’m comfortable in the skill I learned in my residency program, I’m excited about the work I do every day, and I’m very excited about the next chapters.”
In 1966, Dr. Charles Hammond was wrapping up a 2-year stint at the National Institutes of Health where he served at the behest of the military draft board. He had graduated from medical school just 5 years prior, and was in the middle of his ob.gyn. residency training at Duke University in Durham, N.C. when he was called to serve.
His experience wasn’t unusual for the time.
“When the draft board called, you went,” he said in an interview.
When he returned, he picked up where he left off. Residencies at that time were an “open-ended thing,” sometimes lasting 5 or 6 years, depending on staffing needs and other considerations.
Dr. Hammond, now an emeritus professor at Duke, regards his public service commission as an opportunity that advanced his academic career – despite the interruption of his residency training.
Such draft-related interruptions ended in the wake of the Vietnam War, of course, but the late 1960s and the 1970s ushered in a whole new era of changes in ob.gyn. residency training programs.
By 1968, residencies lasted 4 years, and fellowships were for 2 years. Ob.gyn. subspecialties hadn’t yet been introduced, explained Dr. Sandra A. Carson, vice president for education at the American College of Obstetricians and Gynecologists.
“That is essentially how things worked for a long time,” she said in an interview.
That’s not to say there weren’t numerous other changes taking place in the specialty. In a series of interviews with physicians and educators who discussed the myriad ways that residency training has evolved over the last 50 years, a number of themes emerged.
Women in medicine
A striking change over the past 5 decades has been the increasing number of women in medicine. Nowhere has that been in greater evidence than in obstetrics and gynecology.
“There were a few – but very few,” Dr. Hammond said of women in medicine in the 1960s.
There was “a philosophy that men did it better,” he said, adding, “That has been nicely shown to be inaccurate.”
Currently, about 80% of first-year ob.gyn. residents are women, compared with 15% in 1975.
“Maybe even 83% now,” Dr. Carson said, noting that even in the early 1980s when she was in training, women were “few and far between.”
According to a 2011 workforce report by Dr. William F. Rayburn, obstetrics and gynecology has the highest percentage of women residents of any medical specialty – 80% in 2009 versus an average of 46% for other specialties combined, and that figure has remained fairly constant.
Dr. Kasandra Scales, a fourth-year resident at the State University of New York, Syracuse, said she is glad to be part of this era of the specialty where women play an integral role in the advancement of women’s health care.
“I believe our voice and unique perspective to relate with common experiences, such as the physical birth of a child or personal choices in contraception... has enhanced our specialty,” she said.
That said, the fact that men are noticeably absent from the pool of ob.gyn. applicants and residents concerns her. “There should be a balance, she said. “I think it is important to have diversity of all types in the healthcare system.”
Dr. Hammond looked back on his days in residency training and recalled pockets of resistance to the increasing number of women in medicine, but the ultimate effect was good for the specialty, he said, explaining that the quality of the resident pool improved steadily, because the number of qualified candidates increased.
“It has been an interesting interval to watch,” he said, specifically mentioning the demands that women faced in terms of family obligations, childbirth, and childrearing.
Restrictions on work hours instituted in residency programs in more recent years may have played an important role in opening the door to more women, he said.
Work hours down, learning curve up
Dr. Carson agreed that work-hour restrictions instituted in 2003 and updated in 2011, which cap the work week at 80 hours and also apply limits on shift hours, likely encouraged more women to enter the field. One constant over the last 50 years is the biological clock, she said, explaining that the pressures and demands of residency before limits were put in place may have steered women away.
Work-hour restrictions provide more flexibility, but they aren’t without controversy.
Dr. Hammond said he sees the value in work hour restrictions, but working long hours as a resident – sometimes as many as 110 hours per week – had its benefits, too.
“I remember one time when I’d been on call for about 2 and a half days, and up and working the whole time,” Dr. Hammond said. “I left the hospital, walked out to a bench, sat down, and fell asleep. I woke up and distinctly remember thinking, ‘Why am I doing this?’ But I did do it, and that fatigue helped me with learning to endure. You learned from it.”
Not only have long hours been viewed as a rite of passage in medicine, he said, but there were concerns initially that the level of education would diminish and that the risk of patient errors would increase as patients were handed off from one shift to the next, he said.
Data on the effects of work-hour rules have been conflicting. In one study, Dr. Roger P. Smith found little overall effect on total technical experience among residents before and after the restrictions were put in place (there was no statistically significant difference in the average of median total cases in the 3 years before and after). Previous studies had documented increased costs and reduced faculty job satisfaction, while still others had shown no significant changes in 30-day readmission rates, in-hospital mortality, patient length of stay, or resident performance, he noted. “What is emerging is that both the great hopes and the great fears surrounding resident work-hour restrictions have not come to pass,” Dr. Smith wrote (Obstet Gynecol. 2010 Jun;115[6]:1166-71).
Dr. Scales, who is currently chair of the Junior Fellow District II Advisory Council for ACOG, comes down on the side of wishing for more hours.
“[The restrictions] do limit the things we can do and the exposure we may otherwise have,” she said, noting that it’s frustrating to have to leave when she’d rather stay and “see a cool case.”
“It’s a nice idea in principle, but the same amount of work has to be done. It’s not real life,” she said of work-hour restrictions. “It’s hard, at least for me, to want to give up my patients. Our job is to take in as much as you can before you leave to go out into the big bad world.”
It may be difficult to determine the actual impact of work hour limits on patient outcomes because the field of obstetrics and gynecology has changed so much over time.
Dr. David Forstein, vice chair of clinical operations in the department of obstetrics and gynecology at the University of South Carolina, Greenville, and a member of the Accreditation Council for Graduate Medical Education’s task force on work hours said that, for one thing, patients are generally sicker now than ever before, due in part to the obesity epidemic.
Further, changing trends mean that residents are getting less exposure to some procedures like operative vaginal deliveries, while also having to learn more ways to perform hysterectomy. Residents aren’t necessarily less prepared. They’re just having to work very hard because of the depth and breadth of the required knowledge has increased, Dr. Forstein said. “There’s a lot more to learn.”
Dr. Carson agreed that the approach to education has changed, and that those changes are largely a reflection of overall shifts in education and technology.
Technology trends
Every physician interviewed for this article cited laparoscopy and robotic surgery as key technological advances. Fifty years ago, the surgical tools were simpler, Dr. Carson said. Now residents must learn four approaches to hysterectomy: vaginal, abdominal, laparoscopic, and robotic-assisted laparoscopic hysterectomy.
From ultrasound and birth control to genetic screening and robotic surgery, the evolution of the field has been astounding during this time period. The effects of the birth control pill on family planning alone forced an expansion of curriculum not only to the physiology of these things, but also to the treatment of women as a whole person and often as part of a family unit, she said.
Many of the technologies have dramatically changed the landscape, both in terms of how learning is accomplished (for example, simulation), and how physicians interact with patients, Dr. Hammond agreed. With ultrasound, for example, there was a sense that part of the physician-patient relationship was lost.
“To a point, some of us old guys felt like they were doing ultrasound assessment of patients rather than the tried-and-true ‘talk to them and examine them’ [approach],” he said. “I guess whichever generation you are in seems to be the right one, but it’s probably somewhere in between.”
Residency in 2016
If Dr. Scales is any indication, concerns about the loss of a personal touch are unfounded. She says that for her, that’s what it’s all about.
“We were exposed to [technology] since we were 5 or 6 – it’s all we know,” she said of herself and her fellow residents. “It’s not a disadvantage. It’s about efficiency.”
“We have to get things done as quickly as possible and technology helps us with that,” said Dr. Scales, the daughter of a teacher and blue collar worker, who spent most of her life “surrounded by the underprivileged.”
She always desired to help lift that population up, and while she didn’t have a draft board directing her toward public service, she had her own calling of sorts. As a premed major in college, she worked with a nonprofit organization, and later she worked with Hurricane Katrina survivors.
“I liked that aspect of medicine. I wanted to be able to identify with people on an individual level,” she said.
Technology, work-hour restrictions, gender distribution – they’re just part of the journey.
“I’m glad I chose ob.gyn.,” she said. “Sometimes you go through ... reflection ... Am I ready? My answer is yes. I’m excited about the next step, I’m comfortable in the skill I learned in my residency program, I’m excited about the work I do every day, and I’m very excited about the next chapters.”
In 1966, Dr. Charles Hammond was wrapping up a 2-year stint at the National Institutes of Health where he served at the behest of the military draft board. He had graduated from medical school just 5 years prior, and was in the middle of his ob.gyn. residency training at Duke University in Durham, N.C. when he was called to serve.
His experience wasn’t unusual for the time.
“When the draft board called, you went,” he said in an interview.
When he returned, he picked up where he left off. Residencies at that time were an “open-ended thing,” sometimes lasting 5 or 6 years, depending on staffing needs and other considerations.
Dr. Hammond, now an emeritus professor at Duke, regards his public service commission as an opportunity that advanced his academic career – despite the interruption of his residency training.
Such draft-related interruptions ended in the wake of the Vietnam War, of course, but the late 1960s and the 1970s ushered in a whole new era of changes in ob.gyn. residency training programs.
By 1968, residencies lasted 4 years, and fellowships were for 2 years. Ob.gyn. subspecialties hadn’t yet been introduced, explained Dr. Sandra A. Carson, vice president for education at the American College of Obstetricians and Gynecologists.
“That is essentially how things worked for a long time,” she said in an interview.
That’s not to say there weren’t numerous other changes taking place in the specialty. In a series of interviews with physicians and educators who discussed the myriad ways that residency training has evolved over the last 50 years, a number of themes emerged.
Women in medicine
A striking change over the past 5 decades has been the increasing number of women in medicine. Nowhere has that been in greater evidence than in obstetrics and gynecology.
“There were a few – but very few,” Dr. Hammond said of women in medicine in the 1960s.
There was “a philosophy that men did it better,” he said, adding, “That has been nicely shown to be inaccurate.”
Currently, about 80% of first-year ob.gyn. residents are women, compared with 15% in 1975.
“Maybe even 83% now,” Dr. Carson said, noting that even in the early 1980s when she was in training, women were “few and far between.”
According to a 2011 workforce report by Dr. William F. Rayburn, obstetrics and gynecology has the highest percentage of women residents of any medical specialty – 80% in 2009 versus an average of 46% for other specialties combined, and that figure has remained fairly constant.
Dr. Kasandra Scales, a fourth-year resident at the State University of New York, Syracuse, said she is glad to be part of this era of the specialty where women play an integral role in the advancement of women’s health care.
“I believe our voice and unique perspective to relate with common experiences, such as the physical birth of a child or personal choices in contraception... has enhanced our specialty,” she said.
That said, the fact that men are noticeably absent from the pool of ob.gyn. applicants and residents concerns her. “There should be a balance, she said. “I think it is important to have diversity of all types in the healthcare system.”
Dr. Hammond looked back on his days in residency training and recalled pockets of resistance to the increasing number of women in medicine, but the ultimate effect was good for the specialty, he said, explaining that the quality of the resident pool improved steadily, because the number of qualified candidates increased.
“It has been an interesting interval to watch,” he said, specifically mentioning the demands that women faced in terms of family obligations, childbirth, and childrearing.
Restrictions on work hours instituted in residency programs in more recent years may have played an important role in opening the door to more women, he said.
Work hours down, learning curve up
Dr. Carson agreed that work-hour restrictions instituted in 2003 and updated in 2011, which cap the work week at 80 hours and also apply limits on shift hours, likely encouraged more women to enter the field. One constant over the last 50 years is the biological clock, she said, explaining that the pressures and demands of residency before limits were put in place may have steered women away.
Work-hour restrictions provide more flexibility, but they aren’t without controversy.
Dr. Hammond said he sees the value in work hour restrictions, but working long hours as a resident – sometimes as many as 110 hours per week – had its benefits, too.
“I remember one time when I’d been on call for about 2 and a half days, and up and working the whole time,” Dr. Hammond said. “I left the hospital, walked out to a bench, sat down, and fell asleep. I woke up and distinctly remember thinking, ‘Why am I doing this?’ But I did do it, and that fatigue helped me with learning to endure. You learned from it.”
Not only have long hours been viewed as a rite of passage in medicine, he said, but there were concerns initially that the level of education would diminish and that the risk of patient errors would increase as patients were handed off from one shift to the next, he said.
Data on the effects of work-hour rules have been conflicting. In one study, Dr. Roger P. Smith found little overall effect on total technical experience among residents before and after the restrictions were put in place (there was no statistically significant difference in the average of median total cases in the 3 years before and after). Previous studies had documented increased costs and reduced faculty job satisfaction, while still others had shown no significant changes in 30-day readmission rates, in-hospital mortality, patient length of stay, or resident performance, he noted. “What is emerging is that both the great hopes and the great fears surrounding resident work-hour restrictions have not come to pass,” Dr. Smith wrote (Obstet Gynecol. 2010 Jun;115[6]:1166-71).
Dr. Scales, who is currently chair of the Junior Fellow District II Advisory Council for ACOG, comes down on the side of wishing for more hours.
“[The restrictions] do limit the things we can do and the exposure we may otherwise have,” she said, noting that it’s frustrating to have to leave when she’d rather stay and “see a cool case.”
“It’s a nice idea in principle, but the same amount of work has to be done. It’s not real life,” she said of work-hour restrictions. “It’s hard, at least for me, to want to give up my patients. Our job is to take in as much as you can before you leave to go out into the big bad world.”
It may be difficult to determine the actual impact of work hour limits on patient outcomes because the field of obstetrics and gynecology has changed so much over time.
Dr. David Forstein, vice chair of clinical operations in the department of obstetrics and gynecology at the University of South Carolina, Greenville, and a member of the Accreditation Council for Graduate Medical Education’s task force on work hours said that, for one thing, patients are generally sicker now than ever before, due in part to the obesity epidemic.
Further, changing trends mean that residents are getting less exposure to some procedures like operative vaginal deliveries, while also having to learn more ways to perform hysterectomy. Residents aren’t necessarily less prepared. They’re just having to work very hard because of the depth and breadth of the required knowledge has increased, Dr. Forstein said. “There’s a lot more to learn.”
Dr. Carson agreed that the approach to education has changed, and that those changes are largely a reflection of overall shifts in education and technology.
Technology trends
Every physician interviewed for this article cited laparoscopy and robotic surgery as key technological advances. Fifty years ago, the surgical tools were simpler, Dr. Carson said. Now residents must learn four approaches to hysterectomy: vaginal, abdominal, laparoscopic, and robotic-assisted laparoscopic hysterectomy.
From ultrasound and birth control to genetic screening and robotic surgery, the evolution of the field has been astounding during this time period. The effects of the birth control pill on family planning alone forced an expansion of curriculum not only to the physiology of these things, but also to the treatment of women as a whole person and often as part of a family unit, she said.
Many of the technologies have dramatically changed the landscape, both in terms of how learning is accomplished (for example, simulation), and how physicians interact with patients, Dr. Hammond agreed. With ultrasound, for example, there was a sense that part of the physician-patient relationship was lost.
“To a point, some of us old guys felt like they were doing ultrasound assessment of patients rather than the tried-and-true ‘talk to them and examine them’ [approach],” he said. “I guess whichever generation you are in seems to be the right one, but it’s probably somewhere in between.”
Residency in 2016
If Dr. Scales is any indication, concerns about the loss of a personal touch are unfounded. She says that for her, that’s what it’s all about.
“We were exposed to [technology] since we were 5 or 6 – it’s all we know,” she said of herself and her fellow residents. “It’s not a disadvantage. It’s about efficiency.”
“We have to get things done as quickly as possible and technology helps us with that,” said Dr. Scales, the daughter of a teacher and blue collar worker, who spent most of her life “surrounded by the underprivileged.”
She always desired to help lift that population up, and while she didn’t have a draft board directing her toward public service, she had her own calling of sorts. As a premed major in college, she worked with a nonprofit organization, and later she worked with Hurricane Katrina survivors.
“I liked that aspect of medicine. I wanted to be able to identify with people on an individual level,” she said.
Technology, work-hour restrictions, gender distribution – they’re just part of the journey.
“I’m glad I chose ob.gyn.,” she said. “Sometimes you go through ... reflection ... Am I ready? My answer is yes. I’m excited about the next step, I’m comfortable in the skill I learned in my residency program, I’m excited about the work I do every day, and I’m very excited about the next chapters.”
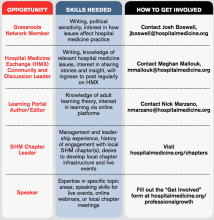
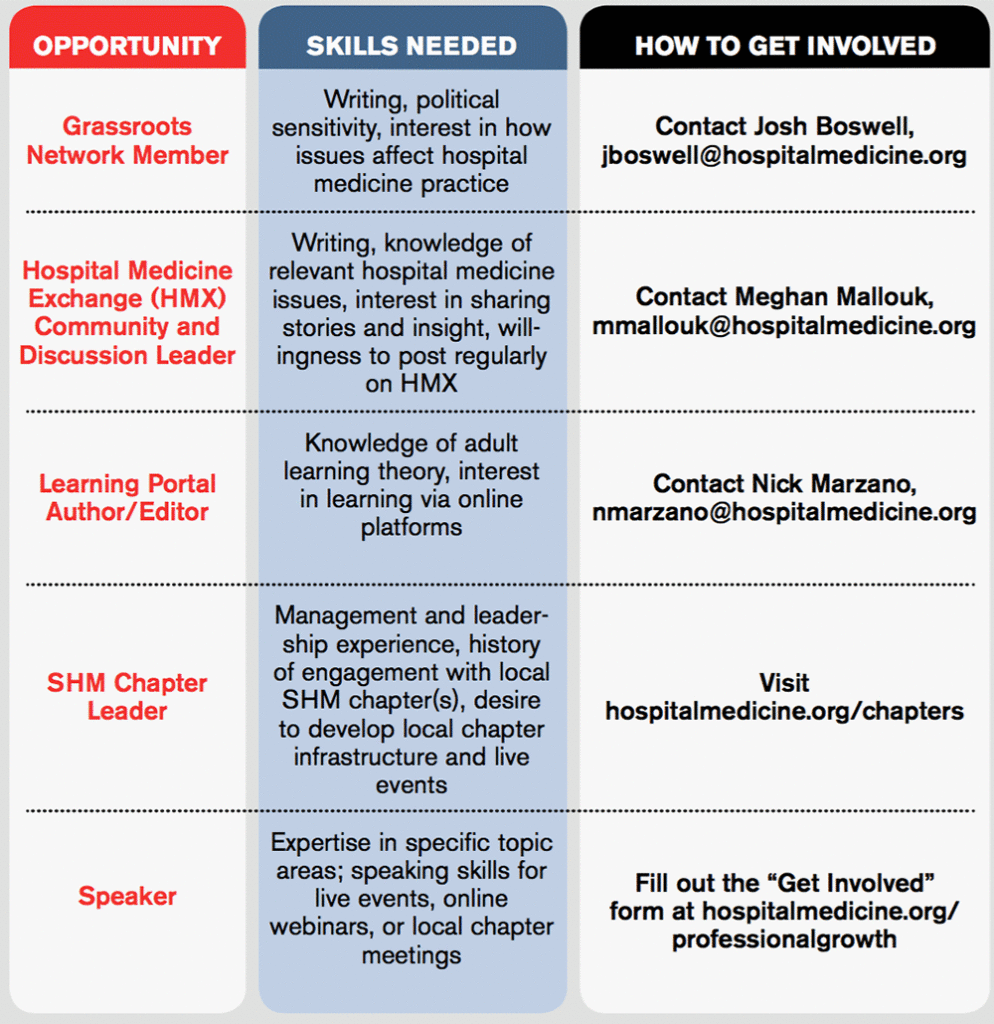
Take Advantage of SHM's Volunteer Experiences
Are you interested in growing professionally and getting involved in work that you are excited about with colleagues across the country? You are in the driver’s seat as a hospitalist. You are in a position to lead, initiate quality improvement, impact patient outcomes, and advocate for your patients and your specialty in healthcare legislation.
SHM offers a wealth of volunteer experiences that will grow your strengths and interests, sharpen your professional acumen, and enhance your profile. New engagement opportunities are added regularly and represent unique ways to make a difference in hospital medicine. Check out some highlights:
These are just a few potential opportunities available from SHM. To learn more and find one that fits your interests, visit hospitalmedicine.org/professionalgrowth.
Are you interested in growing professionally and getting involved in work that you are excited about with colleagues across the country? You are in the driver’s seat as a hospitalist. You are in a position to lead, initiate quality improvement, impact patient outcomes, and advocate for your patients and your specialty in healthcare legislation.
SHM offers a wealth of volunteer experiences that will grow your strengths and interests, sharpen your professional acumen, and enhance your profile. New engagement opportunities are added regularly and represent unique ways to make a difference in hospital medicine. Check out some highlights:
These are just a few potential opportunities available from SHM. To learn more and find one that fits your interests, visit hospitalmedicine.org/professionalgrowth.
Are you interested in growing professionally and getting involved in work that you are excited about with colleagues across the country? You are in the driver’s seat as a hospitalist. You are in a position to lead, initiate quality improvement, impact patient outcomes, and advocate for your patients and your specialty in healthcare legislation.
SHM offers a wealth of volunteer experiences that will grow your strengths and interests, sharpen your professional acumen, and enhance your profile. New engagement opportunities are added regularly and represent unique ways to make a difference in hospital medicine. Check out some highlights:
These are just a few potential opportunities available from SHM. To learn more and find one that fits your interests, visit hospitalmedicine.org/professionalgrowth.
Administrators Share Strategies for High-Performing Hospitalist Groups at HM16
In November, Barbara Weisenbach took a new job as practice manager for the hospitalist group at Northwest Hospital in Seattle. She’s an experienced administrator but as for hospital medicine, not so much. And she is the group’s first full-fledged practice manager—as in, she’s not a physician taking on admin responsibilities and seeing a partial census.
She’s doing a lot of reshaping and a lot of learning, she said, standing outside Room 10 of the San Diego Convention Center, where a daylong pre-course on practice management was being held at SHM’s annual meeting.
“There have been a lot of business things that have been overlooked and not addressed ever before,” she said.
The pre-course, “The Highly Effective Hospital Medicine Group: Using SHM’s Key Characteristics to Drive Performance,” was led by John Nelson, MD, MHM, and Leslie Flores, MHA, SFHM, and offered one useful lesson after another, Weisenbach said.
“One of the most practical portions of the session this morning was about dashboards, which is something I’m currently working on and could definitely use some insight,” Weisenbach said, adding that a list of metrics a dashboard should include and general guidelines on effective dashboards were things she’ll find useful in her own implementation.
The pre-course expanded on the key principles and traits for effective groups, including effective leadership, engaged hospitalists, adequate resources, alignment with the hospital, and care coordination across settings.
HM16 also included two and a half days of practice management sessions. Plus, management themes were woven through workshops and sprinkled into other sessions.
In one session on handling change, presenters used a surfing analogy: Like a surfer’s intensity just before riding a wave, a laser focus is called for when the moment arrives to execute change.
“Get ready for the ride,” said Steve Behnke, MD, president of Columbus, Ohio–based MedOne Hospital Physicians.
He discussed details of introducing the electronic health record system Epic at their group. There was 18 months of planning involving the practice’s whole operational team, then a doubling of the staffing ratios when the system went live, followed by catered lunches to gather feedback and identify problems.
Presenters emphasized the idea of agility in responding to obstacles and realizing that change affects everyone. Successful change, they said, involves seeing the process from all perspectives and leaders should expect resistance.
“Court them. Listen to them. I can’t tell you how many times I’ve done that,” said Dea Robinson, MA, MedOne’s vice president of operations. “Just listening and giving a platform.”
Back at the pre-course, Dr. Nelson, a hospital medicine consultant, talked about the importance of effective leadership.
“An effective group leader is a really key element of a successful group,” said The Hospitalist’s resident practice management columnist. “I’ve worked on-site with many hundreds of hospitalist groups around the country. There’s pretty good correlation between the effectiveness of the leader and the success of the group overall. But a good leader alone is not enough.”
He added that there are too “few leaders to go around.”
A good leader is an active one, he said, adding with funny-because-it’s-true humor that a lot of leaders say their main job is to make the schedule. Good leaders, he said, need to be focused on making the group high-functioning, should be available for administrative work even when not on a clinical shift, and must be able to delegate.
Another critical ingredient for a successful group, he said, is having engaged frontline hospitalists. Reviews need to be meaningful, and meetings should be held regularly with attendance essentially mandatory. Meetings, he said, might need a “tune-up,” with actual voting, written agendas, minutes taken, and group problem-solving above one-way information.
Win Whitcomb, MD, MHM, on care coordination, said the relationship with primary care physicians is crucial though difficult.
“I think we have to go out of our way to build relationships,” he said. “And we don’t have occasion to see them, so we need to figure out a way to get to know our community.”
He suggested:
- Having dedicated transcriptionists for hospitalists,
- Tracking the rate at which discharge summaries are generated within 24 hours,
- Making sure PCPs know how to reach hospitalists, and
- Scheduling events—perhaps an annual event—for meeting PCPs and skill-nursing facility healthcare professionals.
It was clear that, in a field whose dimensions seem to be changing all the time, practice management remained a top interest at HM16. Robert Clothier, RN, a practice manager for the hospitalist group at ThedaCare in Wisconsin, recently switched from managing a cardiology clinic. He said there were huge differences in hospital medicine.
“The profession is growing so fast, and really nobody knows where the end is,” he said. “I can’t even think of anything where you could say, ‘Well, no, they’ll never do that.’ It’s endless. That’s going to be hardest thing. People are going to be pulling on us, and leadership from the hospital is going to be saying, ‘You guys need to do this.’
“So how can I control what we pick, and how can I make sure that we have the resources to do it?” TH
In November, Barbara Weisenbach took a new job as practice manager for the hospitalist group at Northwest Hospital in Seattle. She’s an experienced administrator but as for hospital medicine, not so much. And she is the group’s first full-fledged practice manager—as in, she’s not a physician taking on admin responsibilities and seeing a partial census.
She’s doing a lot of reshaping and a lot of learning, she said, standing outside Room 10 of the San Diego Convention Center, where a daylong pre-course on practice management was being held at SHM’s annual meeting.
“There have been a lot of business things that have been overlooked and not addressed ever before,” she said.
The pre-course, “The Highly Effective Hospital Medicine Group: Using SHM’s Key Characteristics to Drive Performance,” was led by John Nelson, MD, MHM, and Leslie Flores, MHA, SFHM, and offered one useful lesson after another, Weisenbach said.
“One of the most practical portions of the session this morning was about dashboards, which is something I’m currently working on and could definitely use some insight,” Weisenbach said, adding that a list of metrics a dashboard should include and general guidelines on effective dashboards were things she’ll find useful in her own implementation.
The pre-course expanded on the key principles and traits for effective groups, including effective leadership, engaged hospitalists, adequate resources, alignment with the hospital, and care coordination across settings.
HM16 also included two and a half days of practice management sessions. Plus, management themes were woven through workshops and sprinkled into other sessions.
In one session on handling change, presenters used a surfing analogy: Like a surfer’s intensity just before riding a wave, a laser focus is called for when the moment arrives to execute change.
“Get ready for the ride,” said Steve Behnke, MD, president of Columbus, Ohio–based MedOne Hospital Physicians.
He discussed details of introducing the electronic health record system Epic at their group. There was 18 months of planning involving the practice’s whole operational team, then a doubling of the staffing ratios when the system went live, followed by catered lunches to gather feedback and identify problems.
Presenters emphasized the idea of agility in responding to obstacles and realizing that change affects everyone. Successful change, they said, involves seeing the process from all perspectives and leaders should expect resistance.
“Court them. Listen to them. I can’t tell you how many times I’ve done that,” said Dea Robinson, MA, MedOne’s vice president of operations. “Just listening and giving a platform.”
Back at the pre-course, Dr. Nelson, a hospital medicine consultant, talked about the importance of effective leadership.
“An effective group leader is a really key element of a successful group,” said The Hospitalist’s resident practice management columnist. “I’ve worked on-site with many hundreds of hospitalist groups around the country. There’s pretty good correlation between the effectiveness of the leader and the success of the group overall. But a good leader alone is not enough.”
He added that there are too “few leaders to go around.”
A good leader is an active one, he said, adding with funny-because-it’s-true humor that a lot of leaders say their main job is to make the schedule. Good leaders, he said, need to be focused on making the group high-functioning, should be available for administrative work even when not on a clinical shift, and must be able to delegate.
Another critical ingredient for a successful group, he said, is having engaged frontline hospitalists. Reviews need to be meaningful, and meetings should be held regularly with attendance essentially mandatory. Meetings, he said, might need a “tune-up,” with actual voting, written agendas, minutes taken, and group problem-solving above one-way information.
Win Whitcomb, MD, MHM, on care coordination, said the relationship with primary care physicians is crucial though difficult.
“I think we have to go out of our way to build relationships,” he said. “And we don’t have occasion to see them, so we need to figure out a way to get to know our community.”
He suggested:
- Having dedicated transcriptionists for hospitalists,
- Tracking the rate at which discharge summaries are generated within 24 hours,
- Making sure PCPs know how to reach hospitalists, and
- Scheduling events—perhaps an annual event—for meeting PCPs and skill-nursing facility healthcare professionals.
It was clear that, in a field whose dimensions seem to be changing all the time, practice management remained a top interest at HM16. Robert Clothier, RN, a practice manager for the hospitalist group at ThedaCare in Wisconsin, recently switched from managing a cardiology clinic. He said there were huge differences in hospital medicine.
“The profession is growing so fast, and really nobody knows where the end is,” he said. “I can’t even think of anything where you could say, ‘Well, no, they’ll never do that.’ It’s endless. That’s going to be hardest thing. People are going to be pulling on us, and leadership from the hospital is going to be saying, ‘You guys need to do this.’
“So how can I control what we pick, and how can I make sure that we have the resources to do it?” TH
In November, Barbara Weisenbach took a new job as practice manager for the hospitalist group at Northwest Hospital in Seattle. She’s an experienced administrator but as for hospital medicine, not so much. And she is the group’s first full-fledged practice manager—as in, she’s not a physician taking on admin responsibilities and seeing a partial census.
She’s doing a lot of reshaping and a lot of learning, she said, standing outside Room 10 of the San Diego Convention Center, where a daylong pre-course on practice management was being held at SHM’s annual meeting.
“There have been a lot of business things that have been overlooked and not addressed ever before,” she said.
The pre-course, “The Highly Effective Hospital Medicine Group: Using SHM’s Key Characteristics to Drive Performance,” was led by John Nelson, MD, MHM, and Leslie Flores, MHA, SFHM, and offered one useful lesson after another, Weisenbach said.
“One of the most practical portions of the session this morning was about dashboards, which is something I’m currently working on and could definitely use some insight,” Weisenbach said, adding that a list of metrics a dashboard should include and general guidelines on effective dashboards were things she’ll find useful in her own implementation.
The pre-course expanded on the key principles and traits for effective groups, including effective leadership, engaged hospitalists, adequate resources, alignment with the hospital, and care coordination across settings.
HM16 also included two and a half days of practice management sessions. Plus, management themes were woven through workshops and sprinkled into other sessions.
In one session on handling change, presenters used a surfing analogy: Like a surfer’s intensity just before riding a wave, a laser focus is called for when the moment arrives to execute change.
“Get ready for the ride,” said Steve Behnke, MD, president of Columbus, Ohio–based MedOne Hospital Physicians.
He discussed details of introducing the electronic health record system Epic at their group. There was 18 months of planning involving the practice’s whole operational team, then a doubling of the staffing ratios when the system went live, followed by catered lunches to gather feedback and identify problems.
Presenters emphasized the idea of agility in responding to obstacles and realizing that change affects everyone. Successful change, they said, involves seeing the process from all perspectives and leaders should expect resistance.
“Court them. Listen to them. I can’t tell you how many times I’ve done that,” said Dea Robinson, MA, MedOne’s vice president of operations. “Just listening and giving a platform.”
Back at the pre-course, Dr. Nelson, a hospital medicine consultant, talked about the importance of effective leadership.
“An effective group leader is a really key element of a successful group,” said The Hospitalist’s resident practice management columnist. “I’ve worked on-site with many hundreds of hospitalist groups around the country. There’s pretty good correlation between the effectiveness of the leader and the success of the group overall. But a good leader alone is not enough.”
He added that there are too “few leaders to go around.”
A good leader is an active one, he said, adding with funny-because-it’s-true humor that a lot of leaders say their main job is to make the schedule. Good leaders, he said, need to be focused on making the group high-functioning, should be available for administrative work even when not on a clinical shift, and must be able to delegate.
Another critical ingredient for a successful group, he said, is having engaged frontline hospitalists. Reviews need to be meaningful, and meetings should be held regularly with attendance essentially mandatory. Meetings, he said, might need a “tune-up,” with actual voting, written agendas, minutes taken, and group problem-solving above one-way information.
Win Whitcomb, MD, MHM, on care coordination, said the relationship with primary care physicians is crucial though difficult.
“I think we have to go out of our way to build relationships,” he said. “And we don’t have occasion to see them, so we need to figure out a way to get to know our community.”
He suggested:
- Having dedicated transcriptionists for hospitalists,
- Tracking the rate at which discharge summaries are generated within 24 hours,
- Making sure PCPs know how to reach hospitalists, and
- Scheduling events—perhaps an annual event—for meeting PCPs and skill-nursing facility healthcare professionals.
It was clear that, in a field whose dimensions seem to be changing all the time, practice management remained a top interest at HM16. Robert Clothier, RN, a practice manager for the hospitalist group at ThedaCare in Wisconsin, recently switched from managing a cardiology clinic. He said there were huge differences in hospital medicine.
“The profession is growing so fast, and really nobody knows where the end is,” he said. “I can’t even think of anything where you could say, ‘Well, no, they’ll never do that.’ It’s endless. That’s going to be hardest thing. People are going to be pulling on us, and leadership from the hospital is going to be saying, ‘You guys need to do this.’
“So how can I control what we pick, and how can I make sure that we have the resources to do it?” TH
UK Report Shows Prevalence of Antibiotic Resistance in Pediatric Urinary Tract Infection
NEW YORK (Reuters Health) - The prevalence of antibiotic resistance in pediatric urinary tract infection (UTI) has reached such high levels in many countries that existing empiric therapies may no longer be effective, researchers from UK report."
Prevalence of resistance to commonly prescribed antibiotics in primary care in children with urinary tract infections caused by E. coli is high, and there was remarkable variability in E. coli resistance among countries in the study, particularly in countries outside the OECD (Organization for Economic Cooperation and Development), where one possible explanation is the availability of antibiotics over the counter," Ashley Bryce from the University of Bristol in the U.K. and Dr. Céire E. Costelloe from Imperial College London told Reuters Health in a joint email.
"This could render some antibiotics ineffective as first-line treatments for urinary tract infection," they said.
E. coli is responsible for more than 80% of all UTIs and is also the most common cause of bacteremia and foodborne infections and one cause of meningitis in neonates.
Bryce, Dr. Costelloe, and colleagues investigated the prevalence of resistance in community-acquired E. coli UTI to the most commonly prescribed antibiotics given to children in primary care in their systematic review of 58 published reports.
For all antibiotics tested, the prevalence of antibiotic resistance was higher in non-OECD countries than in OECD countries, the team reports in an article online March 15 in The BMJ.
The prevalence of resistance was highest for ampicillin, ranging from 41% in Switzerland to 100% in Ghana and Nigeria.
Resistance to co-trimoxazole and trimethoprim was 30% in OECD countries and 67% in Saudi Arabia, the only non-OECD country for which rates were available.
Pooled prevalences of resistance to ciprofloxacin and ceftazidime were around 2% in OECD countries but over 26% in non-OECD countries.
For all time periods analyzed, the odds of resistance were greater in children exposed to antibiotics than in those who were unexposed.
"The Infectious Diseases Society of America (IDSA) in collaboration with the European Society for Microbiology and Infectious Diseases (ESCMID) recommend that an antibiotic should be selected for first line empirical treatment of urinary tract infection only if the local prevalence of resistance is less than 20%," the researchers note.
"According to these guidelines, our review suggests ampicillin, co-trimoxazole, and trimethoprim are no longer suitable first line treatment options for urinary tract infection in many OECD countries and that as a result many guidelines, such as those published by the National Institute for Health and Care Excellence (NICE), might need updating," they write. "In non-OECD countries, resistance to all first line antibiotics specified for urinary tract infections was in excess of 20%, suggesting that choices of first line treatment might need to be re-evaluated in less well developed countries."
"We are not able to advise clinicians on which antibiotic is best to prescribe as this often depends on the individual case," Bryce and Dr. Costelloe said. "Clinicians should, however, adhere to local or national guidelines wherever possible, which is why it is of great importance that such guidelines are kept up to date and reflect current resistance rates."
"Clinicians may also wish to consider the antibiotic history of the child when they present to primary care with symptoms of an infection, especially in light of the suggestion of our results that previous treatment with an antibiotic is associated with resistance to that same antibiotic, and that this association may be present up to 6 months post treatment," they added.
Dr. Grant Russell from Monash University in Melbourne, Australia, wrote an editorial accompanying the report. He told Reuters Health by email, "I found the extent of the resistance (and the fact that it covered all of the regularly used empiric antibiotics) both concerning and surprising. The fact that choices are diminishing is disturbing, and the fact that the situation is dire in the developing world is deeply troubling."
"We need to do what we can do to prevent bacterial infections, and when treating them to consider that effective antibiotics are a finite resource," he said. "We all have a responsibility in attempting to conserve that resource."
"No new classes of antibiotics have been developed in the last 30 years - this and the dire situation in both the developed and the developing world suggests that the 'global problem' of antibiotic resistance is going to become more and more of an issue in years and decades to come," Dr. Russell concluded.
NEW YORK (Reuters Health) - The prevalence of antibiotic resistance in pediatric urinary tract infection (UTI) has reached such high levels in many countries that existing empiric therapies may no longer be effective, researchers from UK report."
Prevalence of resistance to commonly prescribed antibiotics in primary care in children with urinary tract infections caused by E. coli is high, and there was remarkable variability in E. coli resistance among countries in the study, particularly in countries outside the OECD (Organization for Economic Cooperation and Development), where one possible explanation is the availability of antibiotics over the counter," Ashley Bryce from the University of Bristol in the U.K. and Dr. Céire E. Costelloe from Imperial College London told Reuters Health in a joint email.
"This could render some antibiotics ineffective as first-line treatments for urinary tract infection," they said.
E. coli is responsible for more than 80% of all UTIs and is also the most common cause of bacteremia and foodborne infections and one cause of meningitis in neonates.
Bryce, Dr. Costelloe, and colleagues investigated the prevalence of resistance in community-acquired E. coli UTI to the most commonly prescribed antibiotics given to children in primary care in their systematic review of 58 published reports.
For all antibiotics tested, the prevalence of antibiotic resistance was higher in non-OECD countries than in OECD countries, the team reports in an article online March 15 in The BMJ.
The prevalence of resistance was highest for ampicillin, ranging from 41% in Switzerland to 100% in Ghana and Nigeria.
Resistance to co-trimoxazole and trimethoprim was 30% in OECD countries and 67% in Saudi Arabia, the only non-OECD country for which rates were available.
Pooled prevalences of resistance to ciprofloxacin and ceftazidime were around 2% in OECD countries but over 26% in non-OECD countries.
For all time periods analyzed, the odds of resistance were greater in children exposed to antibiotics than in those who were unexposed.
"The Infectious Diseases Society of America (IDSA) in collaboration with the European Society for Microbiology and Infectious Diseases (ESCMID) recommend that an antibiotic should be selected for first line empirical treatment of urinary tract infection only if the local prevalence of resistance is less than 20%," the researchers note.
"According to these guidelines, our review suggests ampicillin, co-trimoxazole, and trimethoprim are no longer suitable first line treatment options for urinary tract infection in many OECD countries and that as a result many guidelines, such as those published by the National Institute for Health and Care Excellence (NICE), might need updating," they write. "In non-OECD countries, resistance to all first line antibiotics specified for urinary tract infections was in excess of 20%, suggesting that choices of first line treatment might need to be re-evaluated in less well developed countries."
"We are not able to advise clinicians on which antibiotic is best to prescribe as this often depends on the individual case," Bryce and Dr. Costelloe said. "Clinicians should, however, adhere to local or national guidelines wherever possible, which is why it is of great importance that such guidelines are kept up to date and reflect current resistance rates."
"Clinicians may also wish to consider the antibiotic history of the child when they present to primary care with symptoms of an infection, especially in light of the suggestion of our results that previous treatment with an antibiotic is associated with resistance to that same antibiotic, and that this association may be present up to 6 months post treatment," they added.
Dr. Grant Russell from Monash University in Melbourne, Australia, wrote an editorial accompanying the report. He told Reuters Health by email, "I found the extent of the resistance (and the fact that it covered all of the regularly used empiric antibiotics) both concerning and surprising. The fact that choices are diminishing is disturbing, and the fact that the situation is dire in the developing world is deeply troubling."
"We need to do what we can do to prevent bacterial infections, and when treating them to consider that effective antibiotics are a finite resource," he said. "We all have a responsibility in attempting to conserve that resource."
"No new classes of antibiotics have been developed in the last 30 years - this and the dire situation in both the developed and the developing world suggests that the 'global problem' of antibiotic resistance is going to become more and more of an issue in years and decades to come," Dr. Russell concluded.
NEW YORK (Reuters Health) - The prevalence of antibiotic resistance in pediatric urinary tract infection (UTI) has reached such high levels in many countries that existing empiric therapies may no longer be effective, researchers from UK report."
Prevalence of resistance to commonly prescribed antibiotics in primary care in children with urinary tract infections caused by E. coli is high, and there was remarkable variability in E. coli resistance among countries in the study, particularly in countries outside the OECD (Organization for Economic Cooperation and Development), where one possible explanation is the availability of antibiotics over the counter," Ashley Bryce from the University of Bristol in the U.K. and Dr. Céire E. Costelloe from Imperial College London told Reuters Health in a joint email.
"This could render some antibiotics ineffective as first-line treatments for urinary tract infection," they said.
E. coli is responsible for more than 80% of all UTIs and is also the most common cause of bacteremia and foodborne infections and one cause of meningitis in neonates.
Bryce, Dr. Costelloe, and colleagues investigated the prevalence of resistance in community-acquired E. coli UTI to the most commonly prescribed antibiotics given to children in primary care in their systematic review of 58 published reports.
For all antibiotics tested, the prevalence of antibiotic resistance was higher in non-OECD countries than in OECD countries, the team reports in an article online March 15 in The BMJ.
The prevalence of resistance was highest for ampicillin, ranging from 41% in Switzerland to 100% in Ghana and Nigeria.
Resistance to co-trimoxazole and trimethoprim was 30% in OECD countries and 67% in Saudi Arabia, the only non-OECD country for which rates were available.
Pooled prevalences of resistance to ciprofloxacin and ceftazidime were around 2% in OECD countries but over 26% in non-OECD countries.
For all time periods analyzed, the odds of resistance were greater in children exposed to antibiotics than in those who were unexposed.
"The Infectious Diseases Society of America (IDSA) in collaboration with the European Society for Microbiology and Infectious Diseases (ESCMID) recommend that an antibiotic should be selected for first line empirical treatment of urinary tract infection only if the local prevalence of resistance is less than 20%," the researchers note.
"According to these guidelines, our review suggests ampicillin, co-trimoxazole, and trimethoprim are no longer suitable first line treatment options for urinary tract infection in many OECD countries and that as a result many guidelines, such as those published by the National Institute for Health and Care Excellence (NICE), might need updating," they write. "In non-OECD countries, resistance to all first line antibiotics specified for urinary tract infections was in excess of 20%, suggesting that choices of first line treatment might need to be re-evaluated in less well developed countries."
"We are not able to advise clinicians on which antibiotic is best to prescribe as this often depends on the individual case," Bryce and Dr. Costelloe said. "Clinicians should, however, adhere to local or national guidelines wherever possible, which is why it is of great importance that such guidelines are kept up to date and reflect current resistance rates."
"Clinicians may also wish to consider the antibiotic history of the child when they present to primary care with symptoms of an infection, especially in light of the suggestion of our results that previous treatment with an antibiotic is associated with resistance to that same antibiotic, and that this association may be present up to 6 months post treatment," they added.
Dr. Grant Russell from Monash University in Melbourne, Australia, wrote an editorial accompanying the report. He told Reuters Health by email, "I found the extent of the resistance (and the fact that it covered all of the regularly used empiric antibiotics) both concerning and surprising. The fact that choices are diminishing is disturbing, and the fact that the situation is dire in the developing world is deeply troubling."
"We need to do what we can do to prevent bacterial infections, and when treating them to consider that effective antibiotics are a finite resource," he said. "We all have a responsibility in attempting to conserve that resource."
"No new classes of antibiotics have been developed in the last 30 years - this and the dire situation in both the developed and the developing world suggests that the 'global problem' of antibiotic resistance is going to become more and more of an issue in years and decades to come," Dr. Russell concluded.
Expanded UCB product provides clinical benefit

Photo courtesy of NHS
VALENCIA, SPAIN—The expanded umbilical cord blood (UCB) product NiCord can provide clinical benefits in patients with high-risk hematologic malignancies, according to data presented at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation.
NiCord consists of cells from a single UCB unit cultured in nicotinamide—a vitamin B derivative—and cytokines that are typically used for expansion—thrombopoietin, interleukin 6, FLT3 ligand, and stem cell factor.
The data showed that patients transplanted with NiCord had fewer moderate to severe bacterial infections and shorter hospital stays than patients who received standard UCB transplants.
“We saw a significant reduction in serious bacterial infections during the first 100 days in the NiCord group,” said Mitchell Horwitz, MD, of the Duke University School of Medicine in Durham, North Carolina.
“This is encouraging because this type of infection is a major cause of early death following UCB transplantation. We also saw a significant reduction in hospitalization time in the NiCord group, indicating a faster recovery of these patients in comparison to those transplanted with standard umbilical cord blood.”
These results were presented at the meeting as abstract O090. The research was funded by Gamida Cell, the company developing NiCord.
Dr Horwitz and his colleagues analyzed 18 patients with high-risk hematologic malignancies—most with acute leukemia or myelodysplastic syndromes (90%)—who were transplanted with NiCord.
Ten of the patients received NiCord with a second, unmanipulated UCB unit, and 8 patients received NiCord as a single UCB graft.
The researchers compared these patients to 101 patients who received standard single or double UCB transplants at Duke University from January 2005 to March 2015.
Patients in both groups received a total body irradiation-based myeloablative preparative regimen.
The median time to neutrophil engraftment was significantly shorter in the NiCord group than the control group—12.5 days and 27 days, respectively (P<0.001).
All 18 patients in the Nicord group and 100 patients in the control group had at least 1 infection.
Patients in the NiCord group had a significantly lower incidence of grade 2-3 bacterial infections than patients in the control group—22% and 54%, respectively (P=0.015).
However, there was no significant difference between the groups with regard to grade 2-3 viral infections (39% and 35%, respectively, P=0.729), fungal infections (0% and 5%, respectively, P=1.0), or non-microbiologically defined infections (0% and 17%, respectively, P=0.072).
In the first 100 days after transplant, patients in the NiCord group spent significantly more days out of the hospital than patients in the control group. The median number of days for each group was 74 and 53, respectively (P=0.002).
“These results demonstrate that the rapid hematopoietic recovery from NiCord transplantation results in clinical benefit, in comparison to similar site controls,” Dr Horwitz concluded. ![]()

Photo courtesy of NHS
VALENCIA, SPAIN—The expanded umbilical cord blood (UCB) product NiCord can provide clinical benefits in patients with high-risk hematologic malignancies, according to data presented at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation.
NiCord consists of cells from a single UCB unit cultured in nicotinamide—a vitamin B derivative—and cytokines that are typically used for expansion—thrombopoietin, interleukin 6, FLT3 ligand, and stem cell factor.
The data showed that patients transplanted with NiCord had fewer moderate to severe bacterial infections and shorter hospital stays than patients who received standard UCB transplants.
“We saw a significant reduction in serious bacterial infections during the first 100 days in the NiCord group,” said Mitchell Horwitz, MD, of the Duke University School of Medicine in Durham, North Carolina.
“This is encouraging because this type of infection is a major cause of early death following UCB transplantation. We also saw a significant reduction in hospitalization time in the NiCord group, indicating a faster recovery of these patients in comparison to those transplanted with standard umbilical cord blood.”
These results were presented at the meeting as abstract O090. The research was funded by Gamida Cell, the company developing NiCord.
Dr Horwitz and his colleagues analyzed 18 patients with high-risk hematologic malignancies—most with acute leukemia or myelodysplastic syndromes (90%)—who were transplanted with NiCord.
Ten of the patients received NiCord with a second, unmanipulated UCB unit, and 8 patients received NiCord as a single UCB graft.
The researchers compared these patients to 101 patients who received standard single or double UCB transplants at Duke University from January 2005 to March 2015.
Patients in both groups received a total body irradiation-based myeloablative preparative regimen.
The median time to neutrophil engraftment was significantly shorter in the NiCord group than the control group—12.5 days and 27 days, respectively (P<0.001).
All 18 patients in the Nicord group and 100 patients in the control group had at least 1 infection.
Patients in the NiCord group had a significantly lower incidence of grade 2-3 bacterial infections than patients in the control group—22% and 54%, respectively (P=0.015).
However, there was no significant difference between the groups with regard to grade 2-3 viral infections (39% and 35%, respectively, P=0.729), fungal infections (0% and 5%, respectively, P=1.0), or non-microbiologically defined infections (0% and 17%, respectively, P=0.072).
In the first 100 days after transplant, patients in the NiCord group spent significantly more days out of the hospital than patients in the control group. The median number of days for each group was 74 and 53, respectively (P=0.002).
“These results demonstrate that the rapid hematopoietic recovery from NiCord transplantation results in clinical benefit, in comparison to similar site controls,” Dr Horwitz concluded. ![]()

Photo courtesy of NHS
VALENCIA, SPAIN—The expanded umbilical cord blood (UCB) product NiCord can provide clinical benefits in patients with high-risk hematologic malignancies, according to data presented at the 42nd Annual Meeting of the European Society for Blood and Marrow Transplantation.
NiCord consists of cells from a single UCB unit cultured in nicotinamide—a vitamin B derivative—and cytokines that are typically used for expansion—thrombopoietin, interleukin 6, FLT3 ligand, and stem cell factor.
The data showed that patients transplanted with NiCord had fewer moderate to severe bacterial infections and shorter hospital stays than patients who received standard UCB transplants.
“We saw a significant reduction in serious bacterial infections during the first 100 days in the NiCord group,” said Mitchell Horwitz, MD, of the Duke University School of Medicine in Durham, North Carolina.
“This is encouraging because this type of infection is a major cause of early death following UCB transplantation. We also saw a significant reduction in hospitalization time in the NiCord group, indicating a faster recovery of these patients in comparison to those transplanted with standard umbilical cord blood.”
These results were presented at the meeting as abstract O090. The research was funded by Gamida Cell, the company developing NiCord.
Dr Horwitz and his colleagues analyzed 18 patients with high-risk hematologic malignancies—most with acute leukemia or myelodysplastic syndromes (90%)—who were transplanted with NiCord.
Ten of the patients received NiCord with a second, unmanipulated UCB unit, and 8 patients received NiCord as a single UCB graft.
The researchers compared these patients to 101 patients who received standard single or double UCB transplants at Duke University from January 2005 to March 2015.
Patients in both groups received a total body irradiation-based myeloablative preparative regimen.
The median time to neutrophil engraftment was significantly shorter in the NiCord group than the control group—12.5 days and 27 days, respectively (P<0.001).
All 18 patients in the Nicord group and 100 patients in the control group had at least 1 infection.
Patients in the NiCord group had a significantly lower incidence of grade 2-3 bacterial infections than patients in the control group—22% and 54%, respectively (P=0.015).
However, there was no significant difference between the groups with regard to grade 2-3 viral infections (39% and 35%, respectively, P=0.729), fungal infections (0% and 5%, respectively, P=1.0), or non-microbiologically defined infections (0% and 17%, respectively, P=0.072).
In the first 100 days after transplant, patients in the NiCord group spent significantly more days out of the hospital than patients in the control group. The median number of days for each group was 74 and 53, respectively (P=0.002).
“These results demonstrate that the rapid hematopoietic recovery from NiCord transplantation results in clinical benefit, in comparison to similar site controls,” Dr Horwitz concluded. ![]()