User login
Commentary: Updates in mantle cell lymphoma, September 2023
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by t(11;14) and cyclin D1 overexpression. It is also known to be clinically heterogenous, with disease presentations ranging from indolent to aggressive. Baseline risk can be determined on the basis of a combination of clinical and pathologic features. A key prognostic tool, for example, is the Mantle Cell Lymphoma International Prognostic Index-Combined (MIPI-c), which integrates the standard MIPI clinical factors (age, performance status, lactate dehydrogenase, and leukocyte count) with estimates of proliferation (Ki-67).1 Other features, including the presence of TP53 alterations, have also been associated with poor outcomes, even with intensive therapy.2
Recently, a study aimed to further refine prognostication in MCL in order to identify high-risk patients that may be more likely to benefit from novel treatment strategies (Scheubeck et al). This retrospective study included 684 patients with MCL from the MCL-Younger and MCL-Elderly trials with evaluable data for Ki-67 or p53 expression (a surrogate for TP53 alterations). Patients were classified as having high-risk disease on the basis of a high-risk MIPI-c or p53 expression > 50% or as having low-risk disease on the basis of low, low-intermediate, or high-intermediate MIPI-c and p53 expression ≤ 50%. Patients with high-risk disease had significantly shorter median failure-free survival (1.1 vs 5.6 years; P < .0001) and overall survival (2.2 vs 13.2 years; P < .0001) compared with those with low-risk disease. The differences were confirmed in two validation cohorts from the Italian MCL0208 and Nordic-MCL4 trials. These data highlight the poor outcomes of conventional therapy in patients with high-risk MCL. Evaluation of novel approaches should be considered in these patients.
Bruton tyrosine kinase (BTK) inhibitors have been promising options for patients with MCL, including those with high-risk features. Acalabrutinib is a second-generation covalent BTK inhibitor that is approved by the US Food and Drug Administration for patients who have received at least one prior line of therapy. The final results of the single-arm, phase 2 ACE-LY-004 study recently demonstrated long-term safety and efficacy in patients with relapsed/refractory MCL (Le Gouill et al). The overall and complete response rates were 81.5% (95% CI 73.5%-87.9%) and 47.6% (95% CI 38.5%-56.7%), respectively. After a 38.1-month median follow-up, the median duration of response and progression-free survival were 28.6 months (95% CI 17.5-39.1) and 22.0 months (95% CI 16.6-33.3), respectively. Responses were also seen in patients with high-risk features, including blastoid morphology, high-risk MIPI score, and high Ki-67. No new safety signals were observed. This study confirms the role of BTK inhibitors in MCL and providers longer-term estimates of response. Evaluation of BTK inhibitors in earlier lines of therapy and in combination with other agents are ongoing.
Although the majority of patients with MCL will have favorable responses to initial therapy, those with high-risk features, particularly TP53 aberrations, have poor outcomes with standard approaches. Despite a growing number of treatment options in the relapsed setting, such as targeted therapies and chimeric antigen receptor (CAR) T-cell therapy, relapses remain common. Allogenic stem cell transplantation can be associated with prolonged response for patients with relapsed MCL, though it has the potential for significant treatment-associated toxicity.
Recently, prolonged follow-up of a retrospective cohort of patients with MCL, including a subset with TP53 aberrations, was reported (Lew et al). Thirty-six patients with MCL were included, including 13 with TP53-mutated disease. A subset of patients (61%) received an allogeneic transplant in first remission. The estimated overall survival rates after allogenic transplant were 56% (95% CI 36%-72%) at 10 years for the overall cohort and 59% (95% CI 21%-75%) at 4 years for patients with TP53-mutated disease at median follow-ups of 10.8 and 4.2 years, respectively. No relapses were observed in the TP53-mutated subset beyond 6 months after transplantation. These data suggest a potentially curative option for patients with high-risk MCL. Given the availability of CAR T-cell therapy, the optimal timing of allogenic stem cell transplant has become less clear for patients with TP53-mutant disease. Although this study was small and retrospective, these data are encouraging for patients with high-risk disease.
Additional References
1. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. doi: 10.1200/JCO.2015.63.8387
2. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-1910. doi: 10.1182/blood-2017-04-77973
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by t(11;14) and cyclin D1 overexpression. It is also known to be clinically heterogenous, with disease presentations ranging from indolent to aggressive. Baseline risk can be determined on the basis of a combination of clinical and pathologic features. A key prognostic tool, for example, is the Mantle Cell Lymphoma International Prognostic Index-Combined (MIPI-c), which integrates the standard MIPI clinical factors (age, performance status, lactate dehydrogenase, and leukocyte count) with estimates of proliferation (Ki-67).1 Other features, including the presence of TP53 alterations, have also been associated with poor outcomes, even with intensive therapy.2
Recently, a study aimed to further refine prognostication in MCL in order to identify high-risk patients that may be more likely to benefit from novel treatment strategies (Scheubeck et al). This retrospective study included 684 patients with MCL from the MCL-Younger and MCL-Elderly trials with evaluable data for Ki-67 or p53 expression (a surrogate for TP53 alterations). Patients were classified as having high-risk disease on the basis of a high-risk MIPI-c or p53 expression > 50% or as having low-risk disease on the basis of low, low-intermediate, or high-intermediate MIPI-c and p53 expression ≤ 50%. Patients with high-risk disease had significantly shorter median failure-free survival (1.1 vs 5.6 years; P < .0001) and overall survival (2.2 vs 13.2 years; P < .0001) compared with those with low-risk disease. The differences were confirmed in two validation cohorts from the Italian MCL0208 and Nordic-MCL4 trials. These data highlight the poor outcomes of conventional therapy in patients with high-risk MCL. Evaluation of novel approaches should be considered in these patients.
Bruton tyrosine kinase (BTK) inhibitors have been promising options for patients with MCL, including those with high-risk features. Acalabrutinib is a second-generation covalent BTK inhibitor that is approved by the US Food and Drug Administration for patients who have received at least one prior line of therapy. The final results of the single-arm, phase 2 ACE-LY-004 study recently demonstrated long-term safety and efficacy in patients with relapsed/refractory MCL (Le Gouill et al). The overall and complete response rates were 81.5% (95% CI 73.5%-87.9%) and 47.6% (95% CI 38.5%-56.7%), respectively. After a 38.1-month median follow-up, the median duration of response and progression-free survival were 28.6 months (95% CI 17.5-39.1) and 22.0 months (95% CI 16.6-33.3), respectively. Responses were also seen in patients with high-risk features, including blastoid morphology, high-risk MIPI score, and high Ki-67. No new safety signals were observed. This study confirms the role of BTK inhibitors in MCL and providers longer-term estimates of response. Evaluation of BTK inhibitors in earlier lines of therapy and in combination with other agents are ongoing.
Although the majority of patients with MCL will have favorable responses to initial therapy, those with high-risk features, particularly TP53 aberrations, have poor outcomes with standard approaches. Despite a growing number of treatment options in the relapsed setting, such as targeted therapies and chimeric antigen receptor (CAR) T-cell therapy, relapses remain common. Allogenic stem cell transplantation can be associated with prolonged response for patients with relapsed MCL, though it has the potential for significant treatment-associated toxicity.
Recently, prolonged follow-up of a retrospective cohort of patients with MCL, including a subset with TP53 aberrations, was reported (Lew et al). Thirty-six patients with MCL were included, including 13 with TP53-mutated disease. A subset of patients (61%) received an allogeneic transplant in first remission. The estimated overall survival rates after allogenic transplant were 56% (95% CI 36%-72%) at 10 years for the overall cohort and 59% (95% CI 21%-75%) at 4 years for patients with TP53-mutated disease at median follow-ups of 10.8 and 4.2 years, respectively. No relapses were observed in the TP53-mutated subset beyond 6 months after transplantation. These data suggest a potentially curative option for patients with high-risk MCL. Given the availability of CAR T-cell therapy, the optimal timing of allogenic stem cell transplant has become less clear for patients with TP53-mutant disease. Although this study was small and retrospective, these data are encouraging for patients with high-risk disease.
Additional References
1. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. doi: 10.1200/JCO.2015.63.8387
2. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-1910. doi: 10.1182/blood-2017-04-77973
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by t(11;14) and cyclin D1 overexpression. It is also known to be clinically heterogenous, with disease presentations ranging from indolent to aggressive. Baseline risk can be determined on the basis of a combination of clinical and pathologic features. A key prognostic tool, for example, is the Mantle Cell Lymphoma International Prognostic Index-Combined (MIPI-c), which integrates the standard MIPI clinical factors (age, performance status, lactate dehydrogenase, and leukocyte count) with estimates of proliferation (Ki-67).1 Other features, including the presence of TP53 alterations, have also been associated with poor outcomes, even with intensive therapy.2
Recently, a study aimed to further refine prognostication in MCL in order to identify high-risk patients that may be more likely to benefit from novel treatment strategies (Scheubeck et al). This retrospective study included 684 patients with MCL from the MCL-Younger and MCL-Elderly trials with evaluable data for Ki-67 or p53 expression (a surrogate for TP53 alterations). Patients were classified as having high-risk disease on the basis of a high-risk MIPI-c or p53 expression > 50% or as having low-risk disease on the basis of low, low-intermediate, or high-intermediate MIPI-c and p53 expression ≤ 50%. Patients with high-risk disease had significantly shorter median failure-free survival (1.1 vs 5.6 years; P < .0001) and overall survival (2.2 vs 13.2 years; P < .0001) compared with those with low-risk disease. The differences were confirmed in two validation cohorts from the Italian MCL0208 and Nordic-MCL4 trials. These data highlight the poor outcomes of conventional therapy in patients with high-risk MCL. Evaluation of novel approaches should be considered in these patients.
Bruton tyrosine kinase (BTK) inhibitors have been promising options for patients with MCL, including those with high-risk features. Acalabrutinib is a second-generation covalent BTK inhibitor that is approved by the US Food and Drug Administration for patients who have received at least one prior line of therapy. The final results of the single-arm, phase 2 ACE-LY-004 study recently demonstrated long-term safety and efficacy in patients with relapsed/refractory MCL (Le Gouill et al). The overall and complete response rates were 81.5% (95% CI 73.5%-87.9%) and 47.6% (95% CI 38.5%-56.7%), respectively. After a 38.1-month median follow-up, the median duration of response and progression-free survival were 28.6 months (95% CI 17.5-39.1) and 22.0 months (95% CI 16.6-33.3), respectively. Responses were also seen in patients with high-risk features, including blastoid morphology, high-risk MIPI score, and high Ki-67. No new safety signals were observed. This study confirms the role of BTK inhibitors in MCL and providers longer-term estimates of response. Evaluation of BTK inhibitors in earlier lines of therapy and in combination with other agents are ongoing.
Although the majority of patients with MCL will have favorable responses to initial therapy, those with high-risk features, particularly TP53 aberrations, have poor outcomes with standard approaches. Despite a growing number of treatment options in the relapsed setting, such as targeted therapies and chimeric antigen receptor (CAR) T-cell therapy, relapses remain common. Allogenic stem cell transplantation can be associated with prolonged response for patients with relapsed MCL, though it has the potential for significant treatment-associated toxicity.
Recently, prolonged follow-up of a retrospective cohort of patients with MCL, including a subset with TP53 aberrations, was reported (Lew et al). Thirty-six patients with MCL were included, including 13 with TP53-mutated disease. A subset of patients (61%) received an allogeneic transplant in first remission. The estimated overall survival rates after allogenic transplant were 56% (95% CI 36%-72%) at 10 years for the overall cohort and 59% (95% CI 21%-75%) at 4 years for patients with TP53-mutated disease at median follow-ups of 10.8 and 4.2 years, respectively. No relapses were observed in the TP53-mutated subset beyond 6 months after transplantation. These data suggest a potentially curative option for patients with high-risk MCL. Given the availability of CAR T-cell therapy, the optimal timing of allogenic stem cell transplant has become less clear for patients with TP53-mutant disease. Although this study was small and retrospective, these data are encouraging for patients with high-risk disease.
Additional References
1. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. doi: 10.1200/JCO.2015.63.8387
2. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-1910. doi: 10.1182/blood-2017-04-77973
Commentary: Cardiovascular risk, anti-drug antibodies, and prednisolone in RA, September 2023
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
ACR: Rheumatologists help reduce ED, hospitalization costs
Rheumatology care can save health systems more than $2,700 per patient per year, according to a new report from the American College of Rheumatology.
In a white paper and corresponding position statement, the organization outlined how rheumatology care delivers financial benefits for health systems.
The work also highlighted prior research on the positive outcomes associated with rheumatology care, including a decline in hip and knee replacements for patients with rheumatoid arthritis after the introduction of biologics, while the total number of hip and knee replacements for patients with osteoarthritis increased, as well as lower 30-day readmission rates among patients with systemic lupus erythematosus with access to a rheumatology clinic post discharge.
“Many rheumatologists can attest to the value they bring to the care team at a health care system,” said Christina Downey, MD, an assistant professor of medicine at Loma Linda (Calif.) University, in a press release. She is the lead author of the white paper and chair of the ACR’s Government Affairs Committee. “Our goal with the paper and position statement is to emphasize what that value looks like from a preventive and financial perspective. A rheumatologist on the care team benefits patients, practices, and the economy.”
The analysis used adjusted claims insurance data to compare markets with a high vs. low supply of rheumatologists. A high supply was defined as at least 1.5 rheumatologists per 100,000 population, whereas a low supply was less than this amount. On average, markets with a high supply of rheumatologists had lower emergency department (ED) and hospitalization costs per patient per year.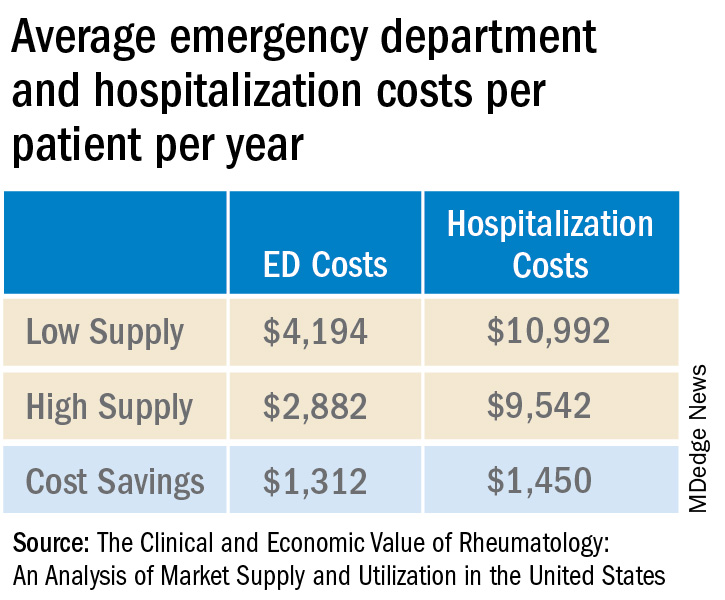
Added together, high-supply rheumatology markets save on average $2,762 in ED visit and hospitalization costs per patient per year.
Dr. Downey and colleagues also tallied the direct and downstream billings associated with rheumatologists, including office visits, consultations, lab testing, and radiology services. The average revenue generated per rheumatologist was $3.5 million per year.
“Emphasizing the impact rheumatologists have on the entire medical community is more important than ever, especially as we contend with an impending rheumatology workforce shortage coupled with an expected increase in patient demand for rheumatologic care,” Dr. Downey said. “This paper supports our recruitment and sustainability efforts for the specialty by spotlighting the significant contributions we make every day and every year to patient outcomes, hospitals, and other health care practices.”
A version of this article first appeared on Medscape.com.
Rheumatology care can save health systems more than $2,700 per patient per year, according to a new report from the American College of Rheumatology.
In a white paper and corresponding position statement, the organization outlined how rheumatology care delivers financial benefits for health systems.
The work also highlighted prior research on the positive outcomes associated with rheumatology care, including a decline in hip and knee replacements for patients with rheumatoid arthritis after the introduction of biologics, while the total number of hip and knee replacements for patients with osteoarthritis increased, as well as lower 30-day readmission rates among patients with systemic lupus erythematosus with access to a rheumatology clinic post discharge.
“Many rheumatologists can attest to the value they bring to the care team at a health care system,” said Christina Downey, MD, an assistant professor of medicine at Loma Linda (Calif.) University, in a press release. She is the lead author of the white paper and chair of the ACR’s Government Affairs Committee. “Our goal with the paper and position statement is to emphasize what that value looks like from a preventive and financial perspective. A rheumatologist on the care team benefits patients, practices, and the economy.”
The analysis used adjusted claims insurance data to compare markets with a high vs. low supply of rheumatologists. A high supply was defined as at least 1.5 rheumatologists per 100,000 population, whereas a low supply was less than this amount. On average, markets with a high supply of rheumatologists had lower emergency department (ED) and hospitalization costs per patient per year.
Added together, high-supply rheumatology markets save on average $2,762 in ED visit and hospitalization costs per patient per year.
Dr. Downey and colleagues also tallied the direct and downstream billings associated with rheumatologists, including office visits, consultations, lab testing, and radiology services. The average revenue generated per rheumatologist was $3.5 million per year.
“Emphasizing the impact rheumatologists have on the entire medical community is more important than ever, especially as we contend with an impending rheumatology workforce shortage coupled with an expected increase in patient demand for rheumatologic care,” Dr. Downey said. “This paper supports our recruitment and sustainability efforts for the specialty by spotlighting the significant contributions we make every day and every year to patient outcomes, hospitals, and other health care practices.”
A version of this article first appeared on Medscape.com.
Rheumatology care can save health systems more than $2,700 per patient per year, according to a new report from the American College of Rheumatology.
In a white paper and corresponding position statement, the organization outlined how rheumatology care delivers financial benefits for health systems.
The work also highlighted prior research on the positive outcomes associated with rheumatology care, including a decline in hip and knee replacements for patients with rheumatoid arthritis after the introduction of biologics, while the total number of hip and knee replacements for patients with osteoarthritis increased, as well as lower 30-day readmission rates among patients with systemic lupus erythematosus with access to a rheumatology clinic post discharge.
“Many rheumatologists can attest to the value they bring to the care team at a health care system,” said Christina Downey, MD, an assistant professor of medicine at Loma Linda (Calif.) University, in a press release. She is the lead author of the white paper and chair of the ACR’s Government Affairs Committee. “Our goal with the paper and position statement is to emphasize what that value looks like from a preventive and financial perspective. A rheumatologist on the care team benefits patients, practices, and the economy.”
The analysis used adjusted claims insurance data to compare markets with a high vs. low supply of rheumatologists. A high supply was defined as at least 1.5 rheumatologists per 100,000 population, whereas a low supply was less than this amount. On average, markets with a high supply of rheumatologists had lower emergency department (ED) and hospitalization costs per patient per year.
Added together, high-supply rheumatology markets save on average $2,762 in ED visit and hospitalization costs per patient per year.
Dr. Downey and colleagues also tallied the direct and downstream billings associated with rheumatologists, including office visits, consultations, lab testing, and radiology services. The average revenue generated per rheumatologist was $3.5 million per year.
“Emphasizing the impact rheumatologists have on the entire medical community is more important than ever, especially as we contend with an impending rheumatology workforce shortage coupled with an expected increase in patient demand for rheumatologic care,” Dr. Downey said. “This paper supports our recruitment and sustainability efforts for the specialty by spotlighting the significant contributions we make every day and every year to patient outcomes, hospitals, and other health care practices.”
A version of this article first appeared on Medscape.com.
Commentary: Alcohol, PPI use, BMI, and lymph node dissection in BC, September 2023
The use of proton pump inhibitors (PPI) can affect the bioavailability and effectiveness of concomitant medications, including cancer therapies. A retrospective study by Lee and colleagues aimed to identify the clinical outcomes of patients with hormone receptor–positive (HR+) and human epidermal growth factor receptor 2–negative advanced or metastatic BC who were concomitantly using PPI and palbociclib. The study included 1310 patients, of which 344 received concomitant PPI plus palbociclib and 966 patients received palbociclib alone. Results showed that patients who received concomitant PPI plus palbociclib had significantly shorter progression-free survival (hazard ratio 1.76; 95% CI 1.46-2.13) and overall survival (hazard ratio 2.72; 95% CI 2.07-3.53) rates compared with those who received palbociclib alone. These results suggest that the concomitant use of PPI with palbociclib may alter the therapeutic efficacy of the drug. More research studies are needed to confirm these findings.
Pfeiler and colleagues examined the association of BMI with side effects, treatment discontinuation, and efficacy of palbociclib. This study looked at 5698 patients with early-stage HR+ BC who received palbociclib plus endocrine therapy as part of a preplanned analysis of the PALLAS trial. Results showed that in women who received adjuvant palbociclib, higher BMI was associated with a significantly lower rate of neutropenia (odds ratio for a 1-unit change in BMI 0.93; 95% CI 0.92-0.95) and a lower rate of treatment discontinuation (adjusted hazard ratio for a 10-unit change in BMI 0.75; 95% CI 0.67-0.83) compared with normal-weight patients. No effect of BMI on palbociclib efficacy was observed at 31 months of follow-up. Further studies are needed to validate these findings in different cohorts.
In cases of early-stage breast cancer (clinical T1, T2) where patients undergo upfront breast-conserving therapy and sentinel lymph node biopsy (SLNB), completion of axillary lymph node dissection (CLND) is often omitted if only one or two positive sentinel lymph nodes are detected. A study by Zaveri and colleagues looked at outcomes among 548 patients with cT1-2 N0 BC who were treated with upfront mastectomy and had one or two positive lymph nodes on SLNB. The 5-year cumulative incidence rate of overall locoregional recurrence was comparable between patients who underwent vs those who did not undergo CLND (1.8% vs 1.3%; P = .93); receipt of post-mastectomy radiation therapy did not affect the locoregional recurrence rate in both categories of patients who underwent SLNB alone and SLNB with CLND (P = .1638). These results suggest that CLND may not necessarily improve outcomes in this patient population. Larger prospective studies are needed to confirm these findings.
The use of proton pump inhibitors (PPI) can affect the bioavailability and effectiveness of concomitant medications, including cancer therapies. A retrospective study by Lee and colleagues aimed to identify the clinical outcomes of patients with hormone receptor–positive (HR+) and human epidermal growth factor receptor 2–negative advanced or metastatic BC who were concomitantly using PPI and palbociclib. The study included 1310 patients, of which 344 received concomitant PPI plus palbociclib and 966 patients received palbociclib alone. Results showed that patients who received concomitant PPI plus palbociclib had significantly shorter progression-free survival (hazard ratio 1.76; 95% CI 1.46-2.13) and overall survival (hazard ratio 2.72; 95% CI 2.07-3.53) rates compared with those who received palbociclib alone. These results suggest that the concomitant use of PPI with palbociclib may alter the therapeutic efficacy of the drug. More research studies are needed to confirm these findings.
Pfeiler and colleagues examined the association of BMI with side effects, treatment discontinuation, and efficacy of palbociclib. This study looked at 5698 patients with early-stage HR+ BC who received palbociclib plus endocrine therapy as part of a preplanned analysis of the PALLAS trial. Results showed that in women who received adjuvant palbociclib, higher BMI was associated with a significantly lower rate of neutropenia (odds ratio for a 1-unit change in BMI 0.93; 95% CI 0.92-0.95) and a lower rate of treatment discontinuation (adjusted hazard ratio for a 10-unit change in BMI 0.75; 95% CI 0.67-0.83) compared with normal-weight patients. No effect of BMI on palbociclib efficacy was observed at 31 months of follow-up. Further studies are needed to validate these findings in different cohorts.
In cases of early-stage breast cancer (clinical T1, T2) where patients undergo upfront breast-conserving therapy and sentinel lymph node biopsy (SLNB), completion of axillary lymph node dissection (CLND) is often omitted if only one or two positive sentinel lymph nodes are detected. A study by Zaveri and colleagues looked at outcomes among 548 patients with cT1-2 N0 BC who were treated with upfront mastectomy and had one or two positive lymph nodes on SLNB. The 5-year cumulative incidence rate of overall locoregional recurrence was comparable between patients who underwent vs those who did not undergo CLND (1.8% vs 1.3%; P = .93); receipt of post-mastectomy radiation therapy did not affect the locoregional recurrence rate in both categories of patients who underwent SLNB alone and SLNB with CLND (P = .1638). These results suggest that CLND may not necessarily improve outcomes in this patient population. Larger prospective studies are needed to confirm these findings.
The use of proton pump inhibitors (PPI) can affect the bioavailability and effectiveness of concomitant medications, including cancer therapies. A retrospective study by Lee and colleagues aimed to identify the clinical outcomes of patients with hormone receptor–positive (HR+) and human epidermal growth factor receptor 2–negative advanced or metastatic BC who were concomitantly using PPI and palbociclib. The study included 1310 patients, of which 344 received concomitant PPI plus palbociclib and 966 patients received palbociclib alone. Results showed that patients who received concomitant PPI plus palbociclib had significantly shorter progression-free survival (hazard ratio 1.76; 95% CI 1.46-2.13) and overall survival (hazard ratio 2.72; 95% CI 2.07-3.53) rates compared with those who received palbociclib alone. These results suggest that the concomitant use of PPI with palbociclib may alter the therapeutic efficacy of the drug. More research studies are needed to confirm these findings.
Pfeiler and colleagues examined the association of BMI with side effects, treatment discontinuation, and efficacy of palbociclib. This study looked at 5698 patients with early-stage HR+ BC who received palbociclib plus endocrine therapy as part of a preplanned analysis of the PALLAS trial. Results showed that in women who received adjuvant palbociclib, higher BMI was associated with a significantly lower rate of neutropenia (odds ratio for a 1-unit change in BMI 0.93; 95% CI 0.92-0.95) and a lower rate of treatment discontinuation (adjusted hazard ratio for a 10-unit change in BMI 0.75; 95% CI 0.67-0.83) compared with normal-weight patients. No effect of BMI on palbociclib efficacy was observed at 31 months of follow-up. Further studies are needed to validate these findings in different cohorts.
In cases of early-stage breast cancer (clinical T1, T2) where patients undergo upfront breast-conserving therapy and sentinel lymph node biopsy (SLNB), completion of axillary lymph node dissection (CLND) is often omitted if only one or two positive sentinel lymph nodes are detected. A study by Zaveri and colleagues looked at outcomes among 548 patients with cT1-2 N0 BC who were treated with upfront mastectomy and had one or two positive lymph nodes on SLNB. The 5-year cumulative incidence rate of overall locoregional recurrence was comparable between patients who underwent vs those who did not undergo CLND (1.8% vs 1.3%; P = .93); receipt of post-mastectomy radiation therapy did not affect the locoregional recurrence rate in both categories of patients who underwent SLNB alone and SLNB with CLND (P = .1638). These results suggest that CLND may not necessarily improve outcomes in this patient population. Larger prospective studies are needed to confirm these findings.
Ruxolitinib for vitiligo: Experts share experiences from first year
.
The Food and Drug Administration approved the cream formulation of ruxolitinib (Opzelura), a JAK inhibitor, for repigmentation of nonsegmental vitiligo in July 2022 for people aged 12 years and older.
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that he likes to use ruxolitinib cream in combination with other treatments.
“In the real world with vitiligo patients, we’re oftentimes doing combinatorial therapy anyway. So phototherapy, specifically, narrow-band UVB, is something that we have a lot of clinical evidence for over the years, and it’s a modality that can combine with topical steroids and topical calcineurin inhibitors.”
He said trials to study combinations will yield better guidance on optimal use of ruxolitinib cream. “In general, vitiligo patients can really benefit from phototherapy,” he said in an interview. (Labeling recommends against combination with other JAK inhibitors, biologics, or potent immunosuppressants, such as azathioprine or cyclosporine.)
This first year has shown that ruxolitinib is an effective option, but counseling patients to expect slow improvement is important so that patients stick with it, he noted.
Documenting what treatments patients with vitiligo have used before is important, he said, as is counseling patients that ruxolitinib is approved only for use on up to 10% of a person’s body surface area. (Product labeling recommends that a thin layer be applied twice a day to affected areas up to 10% of body surface area.)
Ruxolitinib has brought a “louder voice” to vitiligo and has opened up options for patients with the disease, Dr. Chovatiya said. “Having the ability to topically treat people who have very extensive disease really gives us a lot more flexibility than we have had before.”
Good experiences with payers at safety-net hospital
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, said that real-world experience with topical ruxolitinib will be more evident after its been on the market for 18-24 months.
Dr. Heath said she, too, encourages use of narrow-band UVB phototherapy in conjunction with the treatment.
From an insurance reimbursement standpoint, she said that she is glad that there have been fewer hurdles in getting ruxolitinib to patients than she has experienced with other medications.
In her safety-net hospital, she told this news organization, she sees patients with many types of insurance, but most have Medicaid. “So, I’m always expecting the step therapies, denials, pushbacks, etc.,” she said. But the path has been smoother for ruxolitinib coverage, she noted.
Her colleagues are committed to documenting everything the patient has tried, she added, and that helps with prior authorization.
Dr. Heath said that pointing out to insurers that ruxolitinib is the only approved treatment for repigmentation helps facilitate coverage.
“The science is advancing, and I’m happy to be practicing during a time when we actually have something approved for vitiligo,” she said. But she pointed out that phototherapy often is not covered for vitiligo, “which is horrible, when it is readily approved for psoriasis and atopic dermatitis.”
To document progress, Dr. Heath said that she always takes photographs of her patients with vitiligo because “the pictures remind us how far we have come.”
Data spotlight success in adolescents
Data from two trials give a clinical picture of the drug’s safety and efficacy in younger patients.
Adolescents had particularly good results in the first year with ruxolitinib, according to pooled phase 3 data from TRuE-V1 and TRuE-V2, this news organization reported.
The findings, presented at the 25th World Congress of Dermatology in Singapore, indicate that more than half of the participants achieved at least a 50% improvement from baseline in the total Vitiligo Area Scoring Index (T-VASI50) at 52 weeks.
The percentages of young patients aged 12-17 years taking twice-daily ruxolitinib who achieved T-VASI 50 at weeks 12, 24, and 52 were 11.5%, 26.9%, and 57.7%, respectively. The corresponding percentages for all in the study population were 10.7%, 22.7%, and 44.4%, respectively.
At the meeting, the presenter, Julien Seneschal, MD, PhD, professor of dermatology and head of the vitiligo and pigmentary disorders clinic at the University of Bordeaux, France, said, “This suggests that younger patients can respond better to the treatment.” He noted, however, that there were few adolescents in the studies.
New excitement in the field
Daniel Gutierrez, MD, assistant professor of dermatology at New York University, said the treatment has brought new excitement to the field.
“Patients with vitiligo are very motivated to treat their disease,” he said, because it typically is on the face and other highly visual areas, which can affect their overall perception of self.
Previously, he noted in an interview, the only FDA-approved treatment was monobenzone, but that was for depigmentation rather than repigmentation.
Otherwise, treatments were being used off label, and patients were receiving compounded formulations that often weren’t covered by insurance and often had shorter shelf life.
He said that he still occasionally gets denials from payers who consider vitiligo a cosmetic condition.
“I’ve had more luck with insurance, at least in the New York State area.” He added that sometimes payers require use of a topical calcineurin inhibitor for about 12 weeks before they will cover ruxolitinib.
Dr. Gutierrez also recommends using phototherapy with topical ruxolitinib “because they work on slightly different pathways.”
When he starts patients on a new therapy such as ruxolitinib, he asks them to come back in 3 months, and often by then, progress is evident. Facial areas show the most response, he said, while hands and feet are less likely to show significant improvement.
He said that it’s important for physicians and patients to know that improvements can take weeks or months to be noticeable. “I tell patients not to give up,” he added.
Showing the patients pictures from the current appointment and comparing them with pictures from previous appointments can help them better understand their progress, he said.
Lead investigator adds observations
David Rosmarin, MD, chair of the department of dermatology at Indiana University, Indianapolis, was the lead investigator of the pivotal TruE-V1 and TruE-V2 trials for vitiligo. In that role, he has been treating vitiligo patients with topical ruxolitinib since 2015.
In an interview, he said that many patients “don’t hit their optimal results at 3 months, 6 months, even the year mark. With continued use, many can see continued benefit.”
Other patients, he said, don’t respond within the first 6 months but with continued use may eventually respond, he said.
“Unfortunately, we have no way of knowing, based on clinical characteristics or baseline demographics, whether a patient will be a delayed responder or not or an early responder,” Dr. Rosmarin added.
He provided several observations about people who have stopped taking the medication.
“When people stop,” he said, “some maintain their response, but some start to depigment again. Again, we have no way of predicting who will be in which category.”
He said that once patients have hit their desired response, he usually advises them to taper down to maybe twice a week or to stop treatment, but if they see any recurrence, they should start reusing the medicine.
“We have some patients who have gone 6 or 7 years now before they had a recurrence, but others may start to depigment again in 2 to 3 months,” Dr. Rosmarin said.
As for phototherapy, he said, the combination with topical ruxolitinib is being studied.
“We think the combination is synergistic and better than either alone, but we’re still waiting for data to prove that,” he said.
In his practice, he offers patients the option either to use just ruxolitinib cream or the combination early on. Many patients, because of convenience, say they’ll first try the cream to see if that works.
“The challenge with light [therapy] is that it can be very inconvenient,” he said. Patients have to live close to a phototherapy unit to receive therapy 2-3 times a week or have a phototherapy product in their home.
Next in the pipeline
Experts say the progress doesn’t stop with ruxolitinib cream. Current trials of several medications show there’s more to come for patients with vitiligo.
Dr. Chovatiya said that next up may be oral ritlecitinib (Litfulo), a JAK inhibitor that was approved for severe alopecia areata in June for people aged 12 years and older. Phase 2 results have been published for its use with vitiligo.
“This would be an oral medication that may be able to help people with much more extensive disease as far as vitiligo goes,” he said, adding that he expects approval for a vitiligo indication within a few years.
He pointed out that longer-term safety data will be available because it is already on the market for alopecia.
Upadacitinib (Rinvoq), an oral JAK inhibitor, is approved for atopic dermatitis but is being studied for vitiligo as well, he noted. “I’m very excited to see what that holds for patients as well,” Dr. Chovatiya said.
Dr. Gutierrez said that he is excited about oral JAK inhibitors but sees potential in finding new ways to transplant melanocytes into areas where there are none.
The pigmentation field has seen new energy since last year’s approval, he said, particularly among people of color.
“We have new options for vitiligo that were lacking compared with other conditions, such as atopic dermatitis and psoriasis,” he said. “Hopefully, there will be more promising breakthroughs.”
Dr. Rosmarin is the chief investigator for the pivotal trials that led to FDA approval of ruxolitinib. He disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio. Dr. Chovatiya disclosed ties with AbbVie, Arcutis, Arena, Argenx, Beiersdorf, Bristol-Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, LEO Pharma, L’Oréal, National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB. Dr. Heath and Dr. Gutierrez report no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The Food and Drug Administration approved the cream formulation of ruxolitinib (Opzelura), a JAK inhibitor, for repigmentation of nonsegmental vitiligo in July 2022 for people aged 12 years and older.
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that he likes to use ruxolitinib cream in combination with other treatments.
“In the real world with vitiligo patients, we’re oftentimes doing combinatorial therapy anyway. So phototherapy, specifically, narrow-band UVB, is something that we have a lot of clinical evidence for over the years, and it’s a modality that can combine with topical steroids and topical calcineurin inhibitors.”
He said trials to study combinations will yield better guidance on optimal use of ruxolitinib cream. “In general, vitiligo patients can really benefit from phototherapy,” he said in an interview. (Labeling recommends against combination with other JAK inhibitors, biologics, or potent immunosuppressants, such as azathioprine or cyclosporine.)
This first year has shown that ruxolitinib is an effective option, but counseling patients to expect slow improvement is important so that patients stick with it, he noted.
Documenting what treatments patients with vitiligo have used before is important, he said, as is counseling patients that ruxolitinib is approved only for use on up to 10% of a person’s body surface area. (Product labeling recommends that a thin layer be applied twice a day to affected areas up to 10% of body surface area.)
Ruxolitinib has brought a “louder voice” to vitiligo and has opened up options for patients with the disease, Dr. Chovatiya said. “Having the ability to topically treat people who have very extensive disease really gives us a lot more flexibility than we have had before.”
Good experiences with payers at safety-net hospital
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, said that real-world experience with topical ruxolitinib will be more evident after its been on the market for 18-24 months.
Dr. Heath said she, too, encourages use of narrow-band UVB phototherapy in conjunction with the treatment.
From an insurance reimbursement standpoint, she said that she is glad that there have been fewer hurdles in getting ruxolitinib to patients than she has experienced with other medications.
In her safety-net hospital, she told this news organization, she sees patients with many types of insurance, but most have Medicaid. “So, I’m always expecting the step therapies, denials, pushbacks, etc.,” she said. But the path has been smoother for ruxolitinib coverage, she noted.
Her colleagues are committed to documenting everything the patient has tried, she added, and that helps with prior authorization.
Dr. Heath said that pointing out to insurers that ruxolitinib is the only approved treatment for repigmentation helps facilitate coverage.
“The science is advancing, and I’m happy to be practicing during a time when we actually have something approved for vitiligo,” she said. But she pointed out that phototherapy often is not covered for vitiligo, “which is horrible, when it is readily approved for psoriasis and atopic dermatitis.”
To document progress, Dr. Heath said that she always takes photographs of her patients with vitiligo because “the pictures remind us how far we have come.”
Data spotlight success in adolescents
Data from two trials give a clinical picture of the drug’s safety and efficacy in younger patients.
Adolescents had particularly good results in the first year with ruxolitinib, according to pooled phase 3 data from TRuE-V1 and TRuE-V2, this news organization reported.
The findings, presented at the 25th World Congress of Dermatology in Singapore, indicate that more than half of the participants achieved at least a 50% improvement from baseline in the total Vitiligo Area Scoring Index (T-VASI50) at 52 weeks.
The percentages of young patients aged 12-17 years taking twice-daily ruxolitinib who achieved T-VASI 50 at weeks 12, 24, and 52 were 11.5%, 26.9%, and 57.7%, respectively. The corresponding percentages for all in the study population were 10.7%, 22.7%, and 44.4%, respectively.
At the meeting, the presenter, Julien Seneschal, MD, PhD, professor of dermatology and head of the vitiligo and pigmentary disorders clinic at the University of Bordeaux, France, said, “This suggests that younger patients can respond better to the treatment.” He noted, however, that there were few adolescents in the studies.
New excitement in the field
Daniel Gutierrez, MD, assistant professor of dermatology at New York University, said the treatment has brought new excitement to the field.
“Patients with vitiligo are very motivated to treat their disease,” he said, because it typically is on the face and other highly visual areas, which can affect their overall perception of self.
Previously, he noted in an interview, the only FDA-approved treatment was monobenzone, but that was for depigmentation rather than repigmentation.
Otherwise, treatments were being used off label, and patients were receiving compounded formulations that often weren’t covered by insurance and often had shorter shelf life.
He said that he still occasionally gets denials from payers who consider vitiligo a cosmetic condition.
“I’ve had more luck with insurance, at least in the New York State area.” He added that sometimes payers require use of a topical calcineurin inhibitor for about 12 weeks before they will cover ruxolitinib.
Dr. Gutierrez also recommends using phototherapy with topical ruxolitinib “because they work on slightly different pathways.”
When he starts patients on a new therapy such as ruxolitinib, he asks them to come back in 3 months, and often by then, progress is evident. Facial areas show the most response, he said, while hands and feet are less likely to show significant improvement.
He said that it’s important for physicians and patients to know that improvements can take weeks or months to be noticeable. “I tell patients not to give up,” he added.
Showing the patients pictures from the current appointment and comparing them with pictures from previous appointments can help them better understand their progress, he said.
Lead investigator adds observations
David Rosmarin, MD, chair of the department of dermatology at Indiana University, Indianapolis, was the lead investigator of the pivotal TruE-V1 and TruE-V2 trials for vitiligo. In that role, he has been treating vitiligo patients with topical ruxolitinib since 2015.
In an interview, he said that many patients “don’t hit their optimal results at 3 months, 6 months, even the year mark. With continued use, many can see continued benefit.”
Other patients, he said, don’t respond within the first 6 months but with continued use may eventually respond, he said.
“Unfortunately, we have no way of knowing, based on clinical characteristics or baseline demographics, whether a patient will be a delayed responder or not or an early responder,” Dr. Rosmarin added.
He provided several observations about people who have stopped taking the medication.
“When people stop,” he said, “some maintain their response, but some start to depigment again. Again, we have no way of predicting who will be in which category.”
He said that once patients have hit their desired response, he usually advises them to taper down to maybe twice a week or to stop treatment, but if they see any recurrence, they should start reusing the medicine.
“We have some patients who have gone 6 or 7 years now before they had a recurrence, but others may start to depigment again in 2 to 3 months,” Dr. Rosmarin said.
As for phototherapy, he said, the combination with topical ruxolitinib is being studied.
“We think the combination is synergistic and better than either alone, but we’re still waiting for data to prove that,” he said.
In his practice, he offers patients the option either to use just ruxolitinib cream or the combination early on. Many patients, because of convenience, say they’ll first try the cream to see if that works.
“The challenge with light [therapy] is that it can be very inconvenient,” he said. Patients have to live close to a phototherapy unit to receive therapy 2-3 times a week or have a phototherapy product in their home.
Next in the pipeline
Experts say the progress doesn’t stop with ruxolitinib cream. Current trials of several medications show there’s more to come for patients with vitiligo.
Dr. Chovatiya said that next up may be oral ritlecitinib (Litfulo), a JAK inhibitor that was approved for severe alopecia areata in June for people aged 12 years and older. Phase 2 results have been published for its use with vitiligo.
“This would be an oral medication that may be able to help people with much more extensive disease as far as vitiligo goes,” he said, adding that he expects approval for a vitiligo indication within a few years.
He pointed out that longer-term safety data will be available because it is already on the market for alopecia.
Upadacitinib (Rinvoq), an oral JAK inhibitor, is approved for atopic dermatitis but is being studied for vitiligo as well, he noted. “I’m very excited to see what that holds for patients as well,” Dr. Chovatiya said.
Dr. Gutierrez said that he is excited about oral JAK inhibitors but sees potential in finding new ways to transplant melanocytes into areas where there are none.
The pigmentation field has seen new energy since last year’s approval, he said, particularly among people of color.
“We have new options for vitiligo that were lacking compared with other conditions, such as atopic dermatitis and psoriasis,” he said. “Hopefully, there will be more promising breakthroughs.”
Dr. Rosmarin is the chief investigator for the pivotal trials that led to FDA approval of ruxolitinib. He disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio. Dr. Chovatiya disclosed ties with AbbVie, Arcutis, Arena, Argenx, Beiersdorf, Bristol-Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, LEO Pharma, L’Oréal, National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB. Dr. Heath and Dr. Gutierrez report no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The Food and Drug Administration approved the cream formulation of ruxolitinib (Opzelura), a JAK inhibitor, for repigmentation of nonsegmental vitiligo in July 2022 for people aged 12 years and older.
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that he likes to use ruxolitinib cream in combination with other treatments.
“In the real world with vitiligo patients, we’re oftentimes doing combinatorial therapy anyway. So phototherapy, specifically, narrow-band UVB, is something that we have a lot of clinical evidence for over the years, and it’s a modality that can combine with topical steroids and topical calcineurin inhibitors.”
He said trials to study combinations will yield better guidance on optimal use of ruxolitinib cream. “In general, vitiligo patients can really benefit from phototherapy,” he said in an interview. (Labeling recommends against combination with other JAK inhibitors, biologics, or potent immunosuppressants, such as azathioprine or cyclosporine.)
This first year has shown that ruxolitinib is an effective option, but counseling patients to expect slow improvement is important so that patients stick with it, he noted.
Documenting what treatments patients with vitiligo have used before is important, he said, as is counseling patients that ruxolitinib is approved only for use on up to 10% of a person’s body surface area. (Product labeling recommends that a thin layer be applied twice a day to affected areas up to 10% of body surface area.)
Ruxolitinib has brought a “louder voice” to vitiligo and has opened up options for patients with the disease, Dr. Chovatiya said. “Having the ability to topically treat people who have very extensive disease really gives us a lot more flexibility than we have had before.”
Good experiences with payers at safety-net hospital
Candrice R. Heath, MD, assistant professor of dermatology at Temple University, Philadelphia, said that real-world experience with topical ruxolitinib will be more evident after its been on the market for 18-24 months.
Dr. Heath said she, too, encourages use of narrow-band UVB phototherapy in conjunction with the treatment.
From an insurance reimbursement standpoint, she said that she is glad that there have been fewer hurdles in getting ruxolitinib to patients than she has experienced with other medications.
In her safety-net hospital, she told this news organization, she sees patients with many types of insurance, but most have Medicaid. “So, I’m always expecting the step therapies, denials, pushbacks, etc.,” she said. But the path has been smoother for ruxolitinib coverage, she noted.
Her colleagues are committed to documenting everything the patient has tried, she added, and that helps with prior authorization.
Dr. Heath said that pointing out to insurers that ruxolitinib is the only approved treatment for repigmentation helps facilitate coverage.
“The science is advancing, and I’m happy to be practicing during a time when we actually have something approved for vitiligo,” she said. But she pointed out that phototherapy often is not covered for vitiligo, “which is horrible, when it is readily approved for psoriasis and atopic dermatitis.”
To document progress, Dr. Heath said that she always takes photographs of her patients with vitiligo because “the pictures remind us how far we have come.”
Data spotlight success in adolescents
Data from two trials give a clinical picture of the drug’s safety and efficacy in younger patients.
Adolescents had particularly good results in the first year with ruxolitinib, according to pooled phase 3 data from TRuE-V1 and TRuE-V2, this news organization reported.
The findings, presented at the 25th World Congress of Dermatology in Singapore, indicate that more than half of the participants achieved at least a 50% improvement from baseline in the total Vitiligo Area Scoring Index (T-VASI50) at 52 weeks.
The percentages of young patients aged 12-17 years taking twice-daily ruxolitinib who achieved T-VASI 50 at weeks 12, 24, and 52 were 11.5%, 26.9%, and 57.7%, respectively. The corresponding percentages for all in the study population were 10.7%, 22.7%, and 44.4%, respectively.
At the meeting, the presenter, Julien Seneschal, MD, PhD, professor of dermatology and head of the vitiligo and pigmentary disorders clinic at the University of Bordeaux, France, said, “This suggests that younger patients can respond better to the treatment.” He noted, however, that there were few adolescents in the studies.
New excitement in the field
Daniel Gutierrez, MD, assistant professor of dermatology at New York University, said the treatment has brought new excitement to the field.
“Patients with vitiligo are very motivated to treat their disease,” he said, because it typically is on the face and other highly visual areas, which can affect their overall perception of self.
Previously, he noted in an interview, the only FDA-approved treatment was monobenzone, but that was for depigmentation rather than repigmentation.
Otherwise, treatments were being used off label, and patients were receiving compounded formulations that often weren’t covered by insurance and often had shorter shelf life.
He said that he still occasionally gets denials from payers who consider vitiligo a cosmetic condition.
“I’ve had more luck with insurance, at least in the New York State area.” He added that sometimes payers require use of a topical calcineurin inhibitor for about 12 weeks before they will cover ruxolitinib.
Dr. Gutierrez also recommends using phototherapy with topical ruxolitinib “because they work on slightly different pathways.”
When he starts patients on a new therapy such as ruxolitinib, he asks them to come back in 3 months, and often by then, progress is evident. Facial areas show the most response, he said, while hands and feet are less likely to show significant improvement.
He said that it’s important for physicians and patients to know that improvements can take weeks or months to be noticeable. “I tell patients not to give up,” he added.
Showing the patients pictures from the current appointment and comparing them with pictures from previous appointments can help them better understand their progress, he said.
Lead investigator adds observations
David Rosmarin, MD, chair of the department of dermatology at Indiana University, Indianapolis, was the lead investigator of the pivotal TruE-V1 and TruE-V2 trials for vitiligo. In that role, he has been treating vitiligo patients with topical ruxolitinib since 2015.
In an interview, he said that many patients “don’t hit their optimal results at 3 months, 6 months, even the year mark. With continued use, many can see continued benefit.”
Other patients, he said, don’t respond within the first 6 months but with continued use may eventually respond, he said.
“Unfortunately, we have no way of knowing, based on clinical characteristics or baseline demographics, whether a patient will be a delayed responder or not or an early responder,” Dr. Rosmarin added.
He provided several observations about people who have stopped taking the medication.
“When people stop,” he said, “some maintain their response, but some start to depigment again. Again, we have no way of predicting who will be in which category.”
He said that once patients have hit their desired response, he usually advises them to taper down to maybe twice a week or to stop treatment, but if they see any recurrence, they should start reusing the medicine.
“We have some patients who have gone 6 or 7 years now before they had a recurrence, but others may start to depigment again in 2 to 3 months,” Dr. Rosmarin said.
As for phototherapy, he said, the combination with topical ruxolitinib is being studied.
“We think the combination is synergistic and better than either alone, but we’re still waiting for data to prove that,” he said.
In his practice, he offers patients the option either to use just ruxolitinib cream or the combination early on. Many patients, because of convenience, say they’ll first try the cream to see if that works.
“The challenge with light [therapy] is that it can be very inconvenient,” he said. Patients have to live close to a phototherapy unit to receive therapy 2-3 times a week or have a phototherapy product in their home.
Next in the pipeline
Experts say the progress doesn’t stop with ruxolitinib cream. Current trials of several medications show there’s more to come for patients with vitiligo.
Dr. Chovatiya said that next up may be oral ritlecitinib (Litfulo), a JAK inhibitor that was approved for severe alopecia areata in June for people aged 12 years and older. Phase 2 results have been published for its use with vitiligo.
“This would be an oral medication that may be able to help people with much more extensive disease as far as vitiligo goes,” he said, adding that he expects approval for a vitiligo indication within a few years.
He pointed out that longer-term safety data will be available because it is already on the market for alopecia.
Upadacitinib (Rinvoq), an oral JAK inhibitor, is approved for atopic dermatitis but is being studied for vitiligo as well, he noted. “I’m very excited to see what that holds for patients as well,” Dr. Chovatiya said.
Dr. Gutierrez said that he is excited about oral JAK inhibitors but sees potential in finding new ways to transplant melanocytes into areas where there are none.
The pigmentation field has seen new energy since last year’s approval, he said, particularly among people of color.
“We have new options for vitiligo that were lacking compared with other conditions, such as atopic dermatitis and psoriasis,” he said. “Hopefully, there will be more promising breakthroughs.”
Dr. Rosmarin is the chief investigator for the pivotal trials that led to FDA approval of ruxolitinib. He disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Galderma, Incyte, Janssen, Kyowa Kirin, Lilly, Merck, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio. Dr. Chovatiya disclosed ties with AbbVie, Arcutis, Arena, Argenx, Beiersdorf, Bristol-Myers Squibb, Dermavant, Eli Lilly, EPI Health, Incyte, LEO Pharma, L’Oréal, National Eczema Association, Pfizer, Regeneron, Sanofi, and UCB. Dr. Heath and Dr. Gutierrez report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Experts debate low-carb diets for people with diabetes
It’s an ongoing debate in the diabetes world: Is it ideal to consume a very-low-carbohydrate diet, or is it better to go with moderate amounts of healthful carbs?
At the annual scientific sessions of the American Diabetes Association, Carol F. Kirkpatrick, PhD, RDN, spoke first, arguing in favor of diets consisting of moderate, high-quality carbohydrates.
Dina Hafez Griauzde, MD, countered that very-low-carbohydrate diets are more beneficial for people with diabetes, primarily type 2 diabetes.
Both speakers based their arguments on published evidence but agreed in the end that discussion with patients about individual dietary preferences should play a major role in the ultimate decision.
Moderate-carbohydrate eating is best
Dr. Kirkpatrick began by explaining that definitions of “low carb” vary in the literature, which makes comparisons between studies difficult. On the basis of a 2019 review that she coauthored, “moderate” carbohydrate consumption was defined as a diet in which 26%-44% of total daily calories are from carbohydrates. “Low” carbohydrate consumption was defined as a diet in which 10%-25% of calories were from carbohydrates. Consuming less than 10% was defined as a very-low-carbohydrate diet (i.e., a ketogenic diet).
Across studies, she noted, the literature shows that within the first 6 months weight loss is typically greater with carbohydrate-restricted diets than with higher-carbohydrate diets, but that by 1 year and beyond weight loss is similar.
“That can be partly due to the difficulty in people maintaining that very severe dietary restriction, although ... we can all acknowledge that it’s difficult for patients to adhere to any dietary pattern, so for sure by 12 months, the difference in the weight loss is gone between the two,” said Dr. Kirkpatrick, of Midwest Biomedical Research, Pocatello, Idaho.
In a recent meta-analysis of 35 trials that examined the dose-dependent effects of carbohydrate restriction for patients with type 2 diabetes, there was a significant decrease in weight as carbohydrates were reduced. But by 12 months (17 trials), the greatest weight reduction was seen at 35% carbohydrate intake.
“It may just be that people were able to adhere to that moderate intake better,” she explained.
Regarding lipids, in her 2019 review and in several meta-analyses since, the effects on low-density lipoprotein cholesterol (LDL-C) varied. For some patients, adhering to a low-carb diet led to reductions in LDL-C, especially if the participants also lost weight, whereas in other patients, a low-carb diet led to an increase in LDL-C.
Either way, a high intake of saturated fatty acids is key to an increase in LDL-C, Dr. Kirkpatrick noted. “So, it’s important that, if a patient chooses to follow a very low carbohydrate diet or any kind of dietary pattern that restricts carbohydrate, that they replace the carbohydrate with unsaturated fat and not saturated fatty acid foods to avoid that increase in LDL-C.”
Generally, the evidence also shows that carbohydrate restriction typically leads to lower triglyceride levels and higher high-density lipoprotein (HDL) cholesterol levels. However, the same meta-analysis showed that the greatest reduction in LDL-C occurred at about 40% carbohydrate consumption.
Another recent meta-analysis showed that LDL-C rose significantly by an average 12.4 mg/dL with very-low-carb (3%-30%) diets, but only slightly, by 0.4 mg/dL, with moderate carb (40%-45%) intake.
Consuming very-low-carb diets did lead to greater reductions in triglycerides, compared with consuming moderate carb diets (23.9 mg/dL vs. 8.9 mg/dL).
“However, in terms of cardiovascular health, we are not entirely sure what that means. ... We have to look at the overall results in the presence of both triglyceride lowering as well as LDL cholesterol,” Dr. Kirkpatrick noted.
Carbohydrate restriction did consistently lead to lower hemoglobin A1c levels by an average of 0.4, 0.6, and 1.0 percentage points at 6 months for diets of 40%, 30%, and 15% carbohydrate, respectively. However, by 12 months, the effect had waned to 0.15, 0.2, and 0.4 A1c percentage points.
“Again, carbohydrate restriction, especially severe, is difficult for people to adhere to, and moderate carbohydrate intake would allow our patients to consume an appropriate amount of carbohydrate and still achieve improved glycemic control,” Dr. Kirkpatrick said.
Two large randomized controlled trials – PREDIMED and CORDIOPREV – examined the effects of the Mediterranean diet on cardiovascular disease prevention. Both showed a decrease in cardiovascular events with the Mediterranean diet, which involves consuming moderate amounts of carbohydrates.
“The Mediterranean dietary pattern has the strongest evidence for benefit, and it’s moderate in carbohydrates,” she concluded.
Very-low-carbohydrate eating is best
Dr. Griauzde was a last-minute replacement speaker for William S. Yancy Jr, MD, of Duke University, Durham, N.C., and presented his slides. She argued that consuming a very low carb diet improves glycemia and that it does not increase but possibly lowers cardiovascular risk.
She began by noting that prior to the discovery of insulin very-low-carb diets had been consumed for over a century to prolong life for people with type 1 diabetes.
“We have long recognized the deleterious role of carbohydrate in type 1 diabetes management, and we have increasingly recognized that role in the management of type 2 diabetes,” said Dr. Griauzde of the University of Michigan in Ann Arbor.
In a small study that compared maintaining a very-low-carb diet for 2 weeks with maintaining a high-carb diet for 2 weeks, total glucose areas under the curve were substantially lower (P < .05) during the low-carb phase, while A1c levels dropped from 7.3% to 6.8% (P = .006).
“We don’t see those outcomes with meds,” Dr. Griauzde noted, adding, “A diet very low in carbohydrates is one of the most potent tools we have to help our patients achieve glycemic control.”
Dr. Griauzde said that the carbohydrate-insulin model provides an explanation for why dietary carbohydrates are particularly obesogenic and metabolically harmful. That model contrasts with the energy balance model, which suggests that all calories are equal.
The rationale of the carbohydrate-insulin model is that dietary carbohydrate – either sugar or starch – raises serum glucose and insulin levels. A carbohydrate-restricted diet therefore reduces the dietary contribution to serum glucose, which then results in lower insulin levels. Insulin is a potent stimulator of lipogenesis (fat storage), and it is a potent inhibitor of lipolysis (the burning of fat). By lowering insulin levels, stored body fat is burned, serum ketone levels increase, and body weight is lowered.
This model suggests that, when insulin levels are chronically high because of excess carbohydrate consumption, circulating fuels are lowered, which leads to an increase in hunger and to overeating. This was demonstrated in a study that compared different levels of isocaloric glycemic index diets in 12 teenage boys with overweight or obesity. The higher-carbohydrate meals led to higher glucose and insulin levels and more food consumption.
In a systematic review of 13 trials of restricted-carbohydrate diets (< 45% carbohydrates) for adults with diabetes, the degree of improvement in A1c level correlated with the degree of carbohydrate restriction over 2-26 weeks (P = .013).
And in a network meta-analysis of 56 trials that compared nine diets among a total of 4,937 participants with type 2 diabetes, one conclusion was that “for reducing A1c, the low-carbohydrate diet was ranked as the best dietary approach (SUCRA: 84%), followed by the Mediterranean diet (80%), and Paleolithic diet (76%), compared with a control diet.”
Regarding the criticism that very-low-carbohydrate diets are high in saturated fat and therefore raise the risk of cardiovascular disease, Dr. Griauzde pointed to another meta-analysis of 21 prospective studies with more than 300,000 participants with 5-25 years of follow-up. In that analysis, the intake of saturated fat was not associated with an increased risk of cardiovascular disease or stroke.
Furthermore, a 12-week randomized controlled trial that involved 40 adults with overweight also suggested that a very-low-carb diet may be superior to a low-fat diet in improving aspects of the metabolic syndrome, including body mass index, lipid levels, and insulin sensitivity. Small LDL particles, which are more atherogenic than larger LDL particles, also decreased despite a threefold increase in saturated fat intake.
Rebuttals: Overall diet, patient preference matter
During the rebuttals, Dr. Kirkpatrick pointed out that large LDL particles are also atherogenic. In addition, she noted that the studies that showed that saturated fat isn’t associated with cardiovascular disease didn’t consider the macronutrients that replaced the saturated fat.
“It really is about increasing consumption of foods that we know are associated with cardiovascular benefit, including plant-based foods that are high quality and not refined carbohydrates ... and healthy protein sources. ... Hopefully we can step away from just looking at macronutrients and look at the total amount of food that people are choosing to eat.”
Importantly, Dr. Kirkpatrick said, patients need to be asked about their current dietary patterns and preferences. “Interventions should be patient centered and sensitive to cultural differences. Personalized lifestyle interventions increase the likelihood of success.”
Dr. Griauzde pointed out that newer antiobesity drugs can be added to any diet to decrease appetite and enhance adherence.
Dr. Griauzde also observed, “We can label a very-low-carbohydrate diet ‘extreme,’ but maybe, from the patient’s perspective, it’s extreme to take 200 units of insulin a day. If you can give them the opportunity to discontinue use of the insulin by following a very-low-carbohydrate dietary pattern, that is the opportunity that our patients deserve to have.”
But overall, she agreed with Dr. Kirkpatrick about individualizing any dietary approach: “We will never know from any of the trials that have been done or that will be done in the future what diet is best for an individual patient. ... Our job is to help our patients find the dietary approach that works best for them.”
Dr. Kirkpatrick is a clinical scientist with Midwest Biomedical Research, which has received funding from various food and pharmaceutical companies. She has not received any direct funding. Dr. Yancy is a consultant for The Simply Good Foods Co. Dr. Griauzde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s an ongoing debate in the diabetes world: Is it ideal to consume a very-low-carbohydrate diet, or is it better to go with moderate amounts of healthful carbs?
At the annual scientific sessions of the American Diabetes Association, Carol F. Kirkpatrick, PhD, RDN, spoke first, arguing in favor of diets consisting of moderate, high-quality carbohydrates.
Dina Hafez Griauzde, MD, countered that very-low-carbohydrate diets are more beneficial for people with diabetes, primarily type 2 diabetes.
Both speakers based their arguments on published evidence but agreed in the end that discussion with patients about individual dietary preferences should play a major role in the ultimate decision.
Moderate-carbohydrate eating is best
Dr. Kirkpatrick began by explaining that definitions of “low carb” vary in the literature, which makes comparisons between studies difficult. On the basis of a 2019 review that she coauthored, “moderate” carbohydrate consumption was defined as a diet in which 26%-44% of total daily calories are from carbohydrates. “Low” carbohydrate consumption was defined as a diet in which 10%-25% of calories were from carbohydrates. Consuming less than 10% was defined as a very-low-carbohydrate diet (i.e., a ketogenic diet).
Across studies, she noted, the literature shows that within the first 6 months weight loss is typically greater with carbohydrate-restricted diets than with higher-carbohydrate diets, but that by 1 year and beyond weight loss is similar.
“That can be partly due to the difficulty in people maintaining that very severe dietary restriction, although ... we can all acknowledge that it’s difficult for patients to adhere to any dietary pattern, so for sure by 12 months, the difference in the weight loss is gone between the two,” said Dr. Kirkpatrick, of Midwest Biomedical Research, Pocatello, Idaho.
In a recent meta-analysis of 35 trials that examined the dose-dependent effects of carbohydrate restriction for patients with type 2 diabetes, there was a significant decrease in weight as carbohydrates were reduced. But by 12 months (17 trials), the greatest weight reduction was seen at 35% carbohydrate intake.
“It may just be that people were able to adhere to that moderate intake better,” she explained.
Regarding lipids, in her 2019 review and in several meta-analyses since, the effects on low-density lipoprotein cholesterol (LDL-C) varied. For some patients, adhering to a low-carb diet led to reductions in LDL-C, especially if the participants also lost weight, whereas in other patients, a low-carb diet led to an increase in LDL-C.
Either way, a high intake of saturated fatty acids is key to an increase in LDL-C, Dr. Kirkpatrick noted. “So, it’s important that, if a patient chooses to follow a very low carbohydrate diet or any kind of dietary pattern that restricts carbohydrate, that they replace the carbohydrate with unsaturated fat and not saturated fatty acid foods to avoid that increase in LDL-C.”
Generally, the evidence also shows that carbohydrate restriction typically leads to lower triglyceride levels and higher high-density lipoprotein (HDL) cholesterol levels. However, the same meta-analysis showed that the greatest reduction in LDL-C occurred at about 40% carbohydrate consumption.
Another recent meta-analysis showed that LDL-C rose significantly by an average 12.4 mg/dL with very-low-carb (3%-30%) diets, but only slightly, by 0.4 mg/dL, with moderate carb (40%-45%) intake.
Consuming very-low-carb diets did lead to greater reductions in triglycerides, compared with consuming moderate carb diets (23.9 mg/dL vs. 8.9 mg/dL).
“However, in terms of cardiovascular health, we are not entirely sure what that means. ... We have to look at the overall results in the presence of both triglyceride lowering as well as LDL cholesterol,” Dr. Kirkpatrick noted.
Carbohydrate restriction did consistently lead to lower hemoglobin A1c levels by an average of 0.4, 0.6, and 1.0 percentage points at 6 months for diets of 40%, 30%, and 15% carbohydrate, respectively. However, by 12 months, the effect had waned to 0.15, 0.2, and 0.4 A1c percentage points.
“Again, carbohydrate restriction, especially severe, is difficult for people to adhere to, and moderate carbohydrate intake would allow our patients to consume an appropriate amount of carbohydrate and still achieve improved glycemic control,” Dr. Kirkpatrick said.
Two large randomized controlled trials – PREDIMED and CORDIOPREV – examined the effects of the Mediterranean diet on cardiovascular disease prevention. Both showed a decrease in cardiovascular events with the Mediterranean diet, which involves consuming moderate amounts of carbohydrates.
“The Mediterranean dietary pattern has the strongest evidence for benefit, and it’s moderate in carbohydrates,” she concluded.
Very-low-carbohydrate eating is best
Dr. Griauzde was a last-minute replacement speaker for William S. Yancy Jr, MD, of Duke University, Durham, N.C., and presented his slides. She argued that consuming a very low carb diet improves glycemia and that it does not increase but possibly lowers cardiovascular risk.
She began by noting that prior to the discovery of insulin very-low-carb diets had been consumed for over a century to prolong life for people with type 1 diabetes.
“We have long recognized the deleterious role of carbohydrate in type 1 diabetes management, and we have increasingly recognized that role in the management of type 2 diabetes,” said Dr. Griauzde of the University of Michigan in Ann Arbor.
In a small study that compared maintaining a very-low-carb diet for 2 weeks with maintaining a high-carb diet for 2 weeks, total glucose areas under the curve were substantially lower (P < .05) during the low-carb phase, while A1c levels dropped from 7.3% to 6.8% (P = .006).
“We don’t see those outcomes with meds,” Dr. Griauzde noted, adding, “A diet very low in carbohydrates is one of the most potent tools we have to help our patients achieve glycemic control.”
Dr. Griauzde said that the carbohydrate-insulin model provides an explanation for why dietary carbohydrates are particularly obesogenic and metabolically harmful. That model contrasts with the energy balance model, which suggests that all calories are equal.
The rationale of the carbohydrate-insulin model is that dietary carbohydrate – either sugar or starch – raises serum glucose and insulin levels. A carbohydrate-restricted diet therefore reduces the dietary contribution to serum glucose, which then results in lower insulin levels. Insulin is a potent stimulator of lipogenesis (fat storage), and it is a potent inhibitor of lipolysis (the burning of fat). By lowering insulin levels, stored body fat is burned, serum ketone levels increase, and body weight is lowered.
This model suggests that, when insulin levels are chronically high because of excess carbohydrate consumption, circulating fuels are lowered, which leads to an increase in hunger and to overeating. This was demonstrated in a study that compared different levels of isocaloric glycemic index diets in 12 teenage boys with overweight or obesity. The higher-carbohydrate meals led to higher glucose and insulin levels and more food consumption.
In a systematic review of 13 trials of restricted-carbohydrate diets (< 45% carbohydrates) for adults with diabetes, the degree of improvement in A1c level correlated with the degree of carbohydrate restriction over 2-26 weeks (P = .013).
And in a network meta-analysis of 56 trials that compared nine diets among a total of 4,937 participants with type 2 diabetes, one conclusion was that “for reducing A1c, the low-carbohydrate diet was ranked as the best dietary approach (SUCRA: 84%), followed by the Mediterranean diet (80%), and Paleolithic diet (76%), compared with a control diet.”
Regarding the criticism that very-low-carbohydrate diets are high in saturated fat and therefore raise the risk of cardiovascular disease, Dr. Griauzde pointed to another meta-analysis of 21 prospective studies with more than 300,000 participants with 5-25 years of follow-up. In that analysis, the intake of saturated fat was not associated with an increased risk of cardiovascular disease or stroke.
Furthermore, a 12-week randomized controlled trial that involved 40 adults with overweight also suggested that a very-low-carb diet may be superior to a low-fat diet in improving aspects of the metabolic syndrome, including body mass index, lipid levels, and insulin sensitivity. Small LDL particles, which are more atherogenic than larger LDL particles, also decreased despite a threefold increase in saturated fat intake.
Rebuttals: Overall diet, patient preference matter
During the rebuttals, Dr. Kirkpatrick pointed out that large LDL particles are also atherogenic. In addition, she noted that the studies that showed that saturated fat isn’t associated with cardiovascular disease didn’t consider the macronutrients that replaced the saturated fat.
“It really is about increasing consumption of foods that we know are associated with cardiovascular benefit, including plant-based foods that are high quality and not refined carbohydrates ... and healthy protein sources. ... Hopefully we can step away from just looking at macronutrients and look at the total amount of food that people are choosing to eat.”
Importantly, Dr. Kirkpatrick said, patients need to be asked about their current dietary patterns and preferences. “Interventions should be patient centered and sensitive to cultural differences. Personalized lifestyle interventions increase the likelihood of success.”
Dr. Griauzde pointed out that newer antiobesity drugs can be added to any diet to decrease appetite and enhance adherence.
Dr. Griauzde also observed, “We can label a very-low-carbohydrate diet ‘extreme,’ but maybe, from the patient’s perspective, it’s extreme to take 200 units of insulin a day. If you can give them the opportunity to discontinue use of the insulin by following a very-low-carbohydrate dietary pattern, that is the opportunity that our patients deserve to have.”
But overall, she agreed with Dr. Kirkpatrick about individualizing any dietary approach: “We will never know from any of the trials that have been done or that will be done in the future what diet is best for an individual patient. ... Our job is to help our patients find the dietary approach that works best for them.”
Dr. Kirkpatrick is a clinical scientist with Midwest Biomedical Research, which has received funding from various food and pharmaceutical companies. She has not received any direct funding. Dr. Yancy is a consultant for The Simply Good Foods Co. Dr. Griauzde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s an ongoing debate in the diabetes world: Is it ideal to consume a very-low-carbohydrate diet, or is it better to go with moderate amounts of healthful carbs?
At the annual scientific sessions of the American Diabetes Association, Carol F. Kirkpatrick, PhD, RDN, spoke first, arguing in favor of diets consisting of moderate, high-quality carbohydrates.
Dina Hafez Griauzde, MD, countered that very-low-carbohydrate diets are more beneficial for people with diabetes, primarily type 2 diabetes.
Both speakers based their arguments on published evidence but agreed in the end that discussion with patients about individual dietary preferences should play a major role in the ultimate decision.
Moderate-carbohydrate eating is best
Dr. Kirkpatrick began by explaining that definitions of “low carb” vary in the literature, which makes comparisons between studies difficult. On the basis of a 2019 review that she coauthored, “moderate” carbohydrate consumption was defined as a diet in which 26%-44% of total daily calories are from carbohydrates. “Low” carbohydrate consumption was defined as a diet in which 10%-25% of calories were from carbohydrates. Consuming less than 10% was defined as a very-low-carbohydrate diet (i.e., a ketogenic diet).
Across studies, she noted, the literature shows that within the first 6 months weight loss is typically greater with carbohydrate-restricted diets than with higher-carbohydrate diets, but that by 1 year and beyond weight loss is similar.
“That can be partly due to the difficulty in people maintaining that very severe dietary restriction, although ... we can all acknowledge that it’s difficult for patients to adhere to any dietary pattern, so for sure by 12 months, the difference in the weight loss is gone between the two,” said Dr. Kirkpatrick, of Midwest Biomedical Research, Pocatello, Idaho.
In a recent meta-analysis of 35 trials that examined the dose-dependent effects of carbohydrate restriction for patients with type 2 diabetes, there was a significant decrease in weight as carbohydrates were reduced. But by 12 months (17 trials), the greatest weight reduction was seen at 35% carbohydrate intake.
“It may just be that people were able to adhere to that moderate intake better,” she explained.
Regarding lipids, in her 2019 review and in several meta-analyses since, the effects on low-density lipoprotein cholesterol (LDL-C) varied. For some patients, adhering to a low-carb diet led to reductions in LDL-C, especially if the participants also lost weight, whereas in other patients, a low-carb diet led to an increase in LDL-C.
Either way, a high intake of saturated fatty acids is key to an increase in LDL-C, Dr. Kirkpatrick noted. “So, it’s important that, if a patient chooses to follow a very low carbohydrate diet or any kind of dietary pattern that restricts carbohydrate, that they replace the carbohydrate with unsaturated fat and not saturated fatty acid foods to avoid that increase in LDL-C.”
Generally, the evidence also shows that carbohydrate restriction typically leads to lower triglyceride levels and higher high-density lipoprotein (HDL) cholesterol levels. However, the same meta-analysis showed that the greatest reduction in LDL-C occurred at about 40% carbohydrate consumption.
Another recent meta-analysis showed that LDL-C rose significantly by an average 12.4 mg/dL with very-low-carb (3%-30%) diets, but only slightly, by 0.4 mg/dL, with moderate carb (40%-45%) intake.
Consuming very-low-carb diets did lead to greater reductions in triglycerides, compared with consuming moderate carb diets (23.9 mg/dL vs. 8.9 mg/dL).
“However, in terms of cardiovascular health, we are not entirely sure what that means. ... We have to look at the overall results in the presence of both triglyceride lowering as well as LDL cholesterol,” Dr. Kirkpatrick noted.
Carbohydrate restriction did consistently lead to lower hemoglobin A1c levels by an average of 0.4, 0.6, and 1.0 percentage points at 6 months for diets of 40%, 30%, and 15% carbohydrate, respectively. However, by 12 months, the effect had waned to 0.15, 0.2, and 0.4 A1c percentage points.
“Again, carbohydrate restriction, especially severe, is difficult for people to adhere to, and moderate carbohydrate intake would allow our patients to consume an appropriate amount of carbohydrate and still achieve improved glycemic control,” Dr. Kirkpatrick said.
Two large randomized controlled trials – PREDIMED and CORDIOPREV – examined the effects of the Mediterranean diet on cardiovascular disease prevention. Both showed a decrease in cardiovascular events with the Mediterranean diet, which involves consuming moderate amounts of carbohydrates.
“The Mediterranean dietary pattern has the strongest evidence for benefit, and it’s moderate in carbohydrates,” she concluded.
Very-low-carbohydrate eating is best
Dr. Griauzde was a last-minute replacement speaker for William S. Yancy Jr, MD, of Duke University, Durham, N.C., and presented his slides. She argued that consuming a very low carb diet improves glycemia and that it does not increase but possibly lowers cardiovascular risk.
She began by noting that prior to the discovery of insulin very-low-carb diets had been consumed for over a century to prolong life for people with type 1 diabetes.
“We have long recognized the deleterious role of carbohydrate in type 1 diabetes management, and we have increasingly recognized that role in the management of type 2 diabetes,” said Dr. Griauzde of the University of Michigan in Ann Arbor.
In a small study that compared maintaining a very-low-carb diet for 2 weeks with maintaining a high-carb diet for 2 weeks, total glucose areas under the curve were substantially lower (P < .05) during the low-carb phase, while A1c levels dropped from 7.3% to 6.8% (P = .006).
“We don’t see those outcomes with meds,” Dr. Griauzde noted, adding, “A diet very low in carbohydrates is one of the most potent tools we have to help our patients achieve glycemic control.”
Dr. Griauzde said that the carbohydrate-insulin model provides an explanation for why dietary carbohydrates are particularly obesogenic and metabolically harmful. That model contrasts with the energy balance model, which suggests that all calories are equal.
The rationale of the carbohydrate-insulin model is that dietary carbohydrate – either sugar or starch – raises serum glucose and insulin levels. A carbohydrate-restricted diet therefore reduces the dietary contribution to serum glucose, which then results in lower insulin levels. Insulin is a potent stimulator of lipogenesis (fat storage), and it is a potent inhibitor of lipolysis (the burning of fat). By lowering insulin levels, stored body fat is burned, serum ketone levels increase, and body weight is lowered.
This model suggests that, when insulin levels are chronically high because of excess carbohydrate consumption, circulating fuels are lowered, which leads to an increase in hunger and to overeating. This was demonstrated in a study that compared different levels of isocaloric glycemic index diets in 12 teenage boys with overweight or obesity. The higher-carbohydrate meals led to higher glucose and insulin levels and more food consumption.
In a systematic review of 13 trials of restricted-carbohydrate diets (< 45% carbohydrates) for adults with diabetes, the degree of improvement in A1c level correlated with the degree of carbohydrate restriction over 2-26 weeks (P = .013).
And in a network meta-analysis of 56 trials that compared nine diets among a total of 4,937 participants with type 2 diabetes, one conclusion was that “for reducing A1c, the low-carbohydrate diet was ranked as the best dietary approach (SUCRA: 84%), followed by the Mediterranean diet (80%), and Paleolithic diet (76%), compared with a control diet.”
Regarding the criticism that very-low-carbohydrate diets are high in saturated fat and therefore raise the risk of cardiovascular disease, Dr. Griauzde pointed to another meta-analysis of 21 prospective studies with more than 300,000 participants with 5-25 years of follow-up. In that analysis, the intake of saturated fat was not associated with an increased risk of cardiovascular disease or stroke.
Furthermore, a 12-week randomized controlled trial that involved 40 adults with overweight also suggested that a very-low-carb diet may be superior to a low-fat diet in improving aspects of the metabolic syndrome, including body mass index, lipid levels, and insulin sensitivity. Small LDL particles, which are more atherogenic than larger LDL particles, also decreased despite a threefold increase in saturated fat intake.
Rebuttals: Overall diet, patient preference matter
During the rebuttals, Dr. Kirkpatrick pointed out that large LDL particles are also atherogenic. In addition, she noted that the studies that showed that saturated fat isn’t associated with cardiovascular disease didn’t consider the macronutrients that replaced the saturated fat.
“It really is about increasing consumption of foods that we know are associated with cardiovascular benefit, including plant-based foods that are high quality and not refined carbohydrates ... and healthy protein sources. ... Hopefully we can step away from just looking at macronutrients and look at the total amount of food that people are choosing to eat.”
Importantly, Dr. Kirkpatrick said, patients need to be asked about their current dietary patterns and preferences. “Interventions should be patient centered and sensitive to cultural differences. Personalized lifestyle interventions increase the likelihood of success.”
Dr. Griauzde pointed out that newer antiobesity drugs can be added to any diet to decrease appetite and enhance adherence.
Dr. Griauzde also observed, “We can label a very-low-carbohydrate diet ‘extreme,’ but maybe, from the patient’s perspective, it’s extreme to take 200 units of insulin a day. If you can give them the opportunity to discontinue use of the insulin by following a very-low-carbohydrate dietary pattern, that is the opportunity that our patients deserve to have.”
But overall, she agreed with Dr. Kirkpatrick about individualizing any dietary approach: “We will never know from any of the trials that have been done or that will be done in the future what diet is best for an individual patient. ... Our job is to help our patients find the dietary approach that works best for them.”
Dr. Kirkpatrick is a clinical scientist with Midwest Biomedical Research, which has received funding from various food and pharmaceutical companies. She has not received any direct funding. Dr. Yancy is a consultant for The Simply Good Foods Co. Dr. Griauzde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADA 2023
Weight loss linked to mortality risk in older women
Weight loss of at least 5% over a 3-year period was associated with significantly increased mortality in women at age 90, 95, and 100 years compared with those whose weight remained stable, based on data from more than 50,000 individuals.
Previous studies of later-life weight changes and mortality have yielded inconsistent results driven by considerations of weight loss intentionality, and data on older adults in particular are limited, wrote Aladdin H. Shadyab, PhD, of the University of California, San Diego, and colleagues.
In a study published in the Journals of Gerontology: Medical Sciences, the researchers reviewed data from the Women’s Health Initiative, a prospective study of factors affecting chronic disease development in postmenopausal women. The study population included 54,437 women who entered the WHI between 1993 and 1998 at ages 50-79 years. The mean baseline age was 69.8 years; 89.5% of the participants were White, 5.7% were Black, 2.7% were Asian, 2.5% were Hispanic/Latino, and the remaining 1.0% were multiracial, American Indian/Alaskan Native, Native Hawaiian/Other Pacific Islander, or unknown.
The primary outcomes were the associations of short-term (3-year) and long-term (10-year) weight changes with survival to ages 90, 95, and 100 years.
A total of 30,647 women survived to at least 90 years (56.3%).
Overall, women with a short-term weight loss of 5% or more of body weight were 33% less likely to survive to age 90 years, 35% less likely to survive to age 95 years, and 38% less likely to survive to age 100 years than were those whose weight remained stable (odds ratios, 0.67, 0.65, and 0.62, respectively).
The associations were stronger in cases of unintentional short-term weight loss. Intentional weight loss from baseline to year 3 was associated with 17% lower odds of survival to age 90 compared to stable weight (OR, 0.83), but unintentional weight loss was associated with 51% lower odds of survival to age 90 (OR, 0.49).
Similarly, women with 10-year weight loss of at least 5% were 40% less likely to survive to 90 years and 49% less likely to survive to 95 years (OR, 0.60 and OR, 0.51, respectively). The sample size was too small to assess the relation of 10-year weight loss with survival to 100 years, and intentionality was not assessed for 10-year weight changes.
By contrast, weight gain of at least 5% had no significant effect on survival to ages 90, 95, or 100 years, but stable weight over time increased the odds of living to ages 90 to 100 years by 1.2-fold to 2-fold compared to either intentional or unintentional weight loss of at least 5%.
The trends in results were similar across body weight categories (normal weight, overweight, and obese as defined by body mass index). Baseline age and smoking status had no significant effect on the results.
Some of the proportion of self-reported intentional weight loss in the study population may have been unintentional, the researchers wrote in their discussion.
“It is important to note that perceived intentionality of weight loss may be influenced by the many societal pressures to lose weight, especially among women, and therefore overestimate the behavioral changes underlying experienced weight loss in older adults,” they said.
The findings were limited by several factors including the potential for inaccurate self-reported weight loss intention, and the likelihood that the mean older age of the population at baseline (older than 60 years) meant that they were more likely to live longer regardless of weight changes, the researchers noted. Other limitations included the primarily White study population, and other residual confounding factors such as ill health that might drive weight loss, the researchers noted.
However, the results were strengthened by the large sample size and long follow-up period, and suggest that “blanket recommendations for weight loss in older women are unlikely to lead to better survival at advanced ages,” they concluded.
Data support weight monitoring
The investigators acknowledged that their data do not affect clinical recommendations for moderate weight loss in older women to improve health outcomes, especially in those with overweight or obesity, but instead “support close monitoring of the amount and speed of weight loss, particularly when unintentional, as an indicator of underlying poor health and predictor of decreased lifespan in older women.”
Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, agreed with this conclusion. The current study suggests that when older women lose a significant amount of weight unintentionally, it could be a sign of failing health, he said.
Weight gain or loss in old age is very different from weight issues in younger people, where clinicians may be encouraging weight loss to improve health outcomes, Dr. Skolnik said in an interview.
A key take-home message for clinicians, in addition to monitoring weight in older patients, is to emphasize nutrition for individuals in their 80s, 90s, and beyond, he said.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shadyab had no financial conflicts to disclose. Dr. Skolnik had no financial conflicts to disclose and serves on the editorial advisory board of Family Practice News.
Weight loss of at least 5% over a 3-year period was associated with significantly increased mortality in women at age 90, 95, and 100 years compared with those whose weight remained stable, based on data from more than 50,000 individuals.
Previous studies of later-life weight changes and mortality have yielded inconsistent results driven by considerations of weight loss intentionality, and data on older adults in particular are limited, wrote Aladdin H. Shadyab, PhD, of the University of California, San Diego, and colleagues.
In a study published in the Journals of Gerontology: Medical Sciences, the researchers reviewed data from the Women’s Health Initiative, a prospective study of factors affecting chronic disease development in postmenopausal women. The study population included 54,437 women who entered the WHI between 1993 and 1998 at ages 50-79 years. The mean baseline age was 69.8 years; 89.5% of the participants were White, 5.7% were Black, 2.7% were Asian, 2.5% were Hispanic/Latino, and the remaining 1.0% were multiracial, American Indian/Alaskan Native, Native Hawaiian/Other Pacific Islander, or unknown.
The primary outcomes were the associations of short-term (3-year) and long-term (10-year) weight changes with survival to ages 90, 95, and 100 years.
A total of 30,647 women survived to at least 90 years (56.3%).
Overall, women with a short-term weight loss of 5% or more of body weight were 33% less likely to survive to age 90 years, 35% less likely to survive to age 95 years, and 38% less likely to survive to age 100 years than were those whose weight remained stable (odds ratios, 0.67, 0.65, and 0.62, respectively).
The associations were stronger in cases of unintentional short-term weight loss. Intentional weight loss from baseline to year 3 was associated with 17% lower odds of survival to age 90 compared to stable weight (OR, 0.83), but unintentional weight loss was associated with 51% lower odds of survival to age 90 (OR, 0.49).
Similarly, women with 10-year weight loss of at least 5% were 40% less likely to survive to 90 years and 49% less likely to survive to 95 years (OR, 0.60 and OR, 0.51, respectively). The sample size was too small to assess the relation of 10-year weight loss with survival to 100 years, and intentionality was not assessed for 10-year weight changes.
By contrast, weight gain of at least 5% had no significant effect on survival to ages 90, 95, or 100 years, but stable weight over time increased the odds of living to ages 90 to 100 years by 1.2-fold to 2-fold compared to either intentional or unintentional weight loss of at least 5%.
The trends in results were similar across body weight categories (normal weight, overweight, and obese as defined by body mass index). Baseline age and smoking status had no significant effect on the results.
Some of the proportion of self-reported intentional weight loss in the study population may have been unintentional, the researchers wrote in their discussion.
“It is important to note that perceived intentionality of weight loss may be influenced by the many societal pressures to lose weight, especially among women, and therefore overestimate the behavioral changes underlying experienced weight loss in older adults,” they said.
The findings were limited by several factors including the potential for inaccurate self-reported weight loss intention, and the likelihood that the mean older age of the population at baseline (older than 60 years) meant that they were more likely to live longer regardless of weight changes, the researchers noted. Other limitations included the primarily White study population, and other residual confounding factors such as ill health that might drive weight loss, the researchers noted.
However, the results were strengthened by the large sample size and long follow-up period, and suggest that “blanket recommendations for weight loss in older women are unlikely to lead to better survival at advanced ages,” they concluded.
Data support weight monitoring
The investigators acknowledged that their data do not affect clinical recommendations for moderate weight loss in older women to improve health outcomes, especially in those with overweight or obesity, but instead “support close monitoring of the amount and speed of weight loss, particularly when unintentional, as an indicator of underlying poor health and predictor of decreased lifespan in older women.”
Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, agreed with this conclusion. The current study suggests that when older women lose a significant amount of weight unintentionally, it could be a sign of failing health, he said.
Weight gain or loss in old age is very different from weight issues in younger people, where clinicians may be encouraging weight loss to improve health outcomes, Dr. Skolnik said in an interview.
A key take-home message for clinicians, in addition to monitoring weight in older patients, is to emphasize nutrition for individuals in their 80s, 90s, and beyond, he said.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shadyab had no financial conflicts to disclose. Dr. Skolnik had no financial conflicts to disclose and serves on the editorial advisory board of Family Practice News.
Weight loss of at least 5% over a 3-year period was associated with significantly increased mortality in women at age 90, 95, and 100 years compared with those whose weight remained stable, based on data from more than 50,000 individuals.
Previous studies of later-life weight changes and mortality have yielded inconsistent results driven by considerations of weight loss intentionality, and data on older adults in particular are limited, wrote Aladdin H. Shadyab, PhD, of the University of California, San Diego, and colleagues.
In a study published in the Journals of Gerontology: Medical Sciences, the researchers reviewed data from the Women’s Health Initiative, a prospective study of factors affecting chronic disease development in postmenopausal women. The study population included 54,437 women who entered the WHI between 1993 and 1998 at ages 50-79 years. The mean baseline age was 69.8 years; 89.5% of the participants were White, 5.7% were Black, 2.7% were Asian, 2.5% were Hispanic/Latino, and the remaining 1.0% were multiracial, American Indian/Alaskan Native, Native Hawaiian/Other Pacific Islander, or unknown.
The primary outcomes were the associations of short-term (3-year) and long-term (10-year) weight changes with survival to ages 90, 95, and 100 years.
A total of 30,647 women survived to at least 90 years (56.3%).
Overall, women with a short-term weight loss of 5% or more of body weight were 33% less likely to survive to age 90 years, 35% less likely to survive to age 95 years, and 38% less likely to survive to age 100 years than were those whose weight remained stable (odds ratios, 0.67, 0.65, and 0.62, respectively).
The associations were stronger in cases of unintentional short-term weight loss. Intentional weight loss from baseline to year 3 was associated with 17% lower odds of survival to age 90 compared to stable weight (OR, 0.83), but unintentional weight loss was associated with 51% lower odds of survival to age 90 (OR, 0.49).
Similarly, women with 10-year weight loss of at least 5% were 40% less likely to survive to 90 years and 49% less likely to survive to 95 years (OR, 0.60 and OR, 0.51, respectively). The sample size was too small to assess the relation of 10-year weight loss with survival to 100 years, and intentionality was not assessed for 10-year weight changes.
By contrast, weight gain of at least 5% had no significant effect on survival to ages 90, 95, or 100 years, but stable weight over time increased the odds of living to ages 90 to 100 years by 1.2-fold to 2-fold compared to either intentional or unintentional weight loss of at least 5%.
The trends in results were similar across body weight categories (normal weight, overweight, and obese as defined by body mass index). Baseline age and smoking status had no significant effect on the results.
Some of the proportion of self-reported intentional weight loss in the study population may have been unintentional, the researchers wrote in their discussion.
“It is important to note that perceived intentionality of weight loss may be influenced by the many societal pressures to lose weight, especially among women, and therefore overestimate the behavioral changes underlying experienced weight loss in older adults,” they said.
The findings were limited by several factors including the potential for inaccurate self-reported weight loss intention, and the likelihood that the mean older age of the population at baseline (older than 60 years) meant that they were more likely to live longer regardless of weight changes, the researchers noted. Other limitations included the primarily White study population, and other residual confounding factors such as ill health that might drive weight loss, the researchers noted.
However, the results were strengthened by the large sample size and long follow-up period, and suggest that “blanket recommendations for weight loss in older women are unlikely to lead to better survival at advanced ages,” they concluded.
Data support weight monitoring
The investigators acknowledged that their data do not affect clinical recommendations for moderate weight loss in older women to improve health outcomes, especially in those with overweight or obesity, but instead “support close monitoring of the amount and speed of weight loss, particularly when unintentional, as an indicator of underlying poor health and predictor of decreased lifespan in older women.”
Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, agreed with this conclusion. The current study suggests that when older women lose a significant amount of weight unintentionally, it could be a sign of failing health, he said.
Weight gain or loss in old age is very different from weight issues in younger people, where clinicians may be encouraging weight loss to improve health outcomes, Dr. Skolnik said in an interview.
A key take-home message for clinicians, in addition to monitoring weight in older patients, is to emphasize nutrition for individuals in their 80s, 90s, and beyond, he said.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shadyab had no financial conflicts to disclose. Dr. Skolnik had no financial conflicts to disclose and serves on the editorial advisory board of Family Practice News.
FROM THE JOURNALS OF GERONTOLOGY: MEDICAL SCIENCES
Diabetes drug class appears to reduce recurrent gout flares
The glucose-lowering drug class sodium-glucose cotransporter 2 (SGLT2) inhibitors appear to reduce the risk for recurrent gout flares in people with gout and type 2 diabetes, and to lessen excess mortality in those individuals, compared with those who initiated other types of glucose-lowering medications, new data suggest.
Among nearly 6,000 adults with both type 2 diabetes and gout from a U.K. primary care database, initiation of SGLT2 inhibitor treatment was associated with 19% fewer recurrent gout flares and 29% lower mortality.
Moreover, unlike other urate-lowering therapies, there were no apparent transient increases in the risk of gout flares after initiating therapy, Jie Wei, PhD, of Health Management Center, Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in JAMA Network Open.
These results are important because current management of gout is suboptimal. Many patients either don’t receive adequate urate-lowering therapies such as allopurinol or stop taking them, Dr. Wei and colleagues said.
In addition to lowering glucose, SGLT2 inhibitors also reduce the risk for major adverse cardiovascular events and all-cause mortality in people regardless of their diabetes status. Previous studies have also found that SGLT2 inhibitors reduce the risk for developing gout and of gout flares.
Asked to comment, gout specialist John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, said in an interview: “I think it’s a well-done paper, with a large dataset. I think it just reinforces the findings from the other papers. Mostly anything that lowers uric acid levels is going to lower recurrent gout attacks, so it all makes sense.”
However, while Dr. FitzGerald thinks the drug class is a good option for people with diabetes or cardiorenal indications for them who also have gout, he doesn’t envision it as first-line for most other patients with gout. “The current treatments are very effective. Allopurinol brings down uric acid levels by 5-7 points. There are patients who fail allopurinol, but those are less than 5%.”
The most common reason patients stop taking allopurinol is the frequent initial gout flare. But that’s preventable, Dr. FitzGerald said, either by titrating up slowly, or by adding colchicine along with it. “By going slowly, you can avoid that flare risk. I think that’s what’s going on with the SGLT2 inhibitor. It’s not a dramatic urate-lowering drug, but it is clinically meaningful. I think that’s what this paper is showing.”
But, he noted, “I think there are so many reasons to start the SGLT2 inhibitors that if somebody also has gout, all the better. And, if somebody is on the margin with diabetes and gout control and can’t go with allopurinol, it would be great to add for both conditions.”
Less gout recurrence, lower mortality
The retrospective study was conducted from Jan. 1, 2013, to March 31, 2022. Among 5,931 patients with both type 2 diabetes and gout, 1,548 (26.1%) initiated an SGLT2 inhibitor (dapagliflozin, empagliflozin, or canagliflozin), while 4,383 (73.9%) initiated treatment with other active comparators, mostly (92.6%) dipeptidyl peptidase–4 inhibitors.
Gout flares were identified in the charts for a total of 86% of the participants. The weighted incidence rates for the first recurrent flare were 32.4 versus 41.2 per 1,000 person-years in the SGLT2 inhibitor versus comparator groups, with a weighted absolute rate difference of –8.8/1,000 and weighted hazard ratio of 0.81, a significant difference.
All-cause mortality was 18.8 versus 24.9 per 1,000 person-years, respectively, giving an HR of 0.71 at 5-year follow-up.
Dr. FitzGerald, who chaired the American College of Rheumatology’s 2020 gout guidelines, said he anticipates that the SGLT2 inhibitors will be mentioned in the next update to the ACR’s now “living” guidelines, although he was not speaking on the organization’s behalf.
“We talk about losartan in the current [ACR guidelines], about its specific uric acid–lowering effect. Drugs can make uric acid worse or better. For example, thiazides make it higher. I think the SGLT2 [inhibitors] are important, but I don’t think they’re huge. The study is great, and I think the drugs are great, but I don’t think they will change the way gout is managed.”
This work was supported by grants from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and from the Natural Science Foundation of Hunan Province. Dr. Wei reported receiving grant funding from Xiangya Hospital Central South University Project Program of National Clinical Research Center for Geriatric Disorders and the Science and Technology Department of Hunan Province, the Natural Science Foundation of Hunan Province, during the conduct of the study. Dr. FitzGerald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The glucose-lowering drug class sodium-glucose cotransporter 2 (SGLT2) inhibitors appear to reduce the risk for recurrent gout flares in people with gout and type 2 diabetes, and to lessen excess mortality in those individuals, compared with those who initiated other types of glucose-lowering medications, new data suggest.
Among nearly 6,000 adults with both type 2 diabetes and gout from a U.K. primary care database, initiation of SGLT2 inhibitor treatment was associated with 19% fewer recurrent gout flares and 29% lower mortality.
Moreover, unlike other urate-lowering therapies, there were no apparent transient increases in the risk of gout flares after initiating therapy, Jie Wei, PhD, of Health Management Center, Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in JAMA Network Open.
These results are important because current management of gout is suboptimal. Many patients either don’t receive adequate urate-lowering therapies such as allopurinol or stop taking them, Dr. Wei and colleagues said.
In addition to lowering glucose, SGLT2 inhibitors also reduce the risk for major adverse cardiovascular events and all-cause mortality in people regardless of their diabetes status. Previous studies have also found that SGLT2 inhibitors reduce the risk for developing gout and of gout flares.
Asked to comment, gout specialist John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, said in an interview: “I think it’s a well-done paper, with a large dataset. I think it just reinforces the findings from the other papers. Mostly anything that lowers uric acid levels is going to lower recurrent gout attacks, so it all makes sense.”
However, while Dr. FitzGerald thinks the drug class is a good option for people with diabetes or cardiorenal indications for them who also have gout, he doesn’t envision it as first-line for most other patients with gout. “The current treatments are very effective. Allopurinol brings down uric acid levels by 5-7 points. There are patients who fail allopurinol, but those are less than 5%.”
The most common reason patients stop taking allopurinol is the frequent initial gout flare. But that’s preventable, Dr. FitzGerald said, either by titrating up slowly, or by adding colchicine along with it. “By going slowly, you can avoid that flare risk. I think that’s what’s going on with the SGLT2 inhibitor. It’s not a dramatic urate-lowering drug, but it is clinically meaningful. I think that’s what this paper is showing.”
But, he noted, “I think there are so many reasons to start the SGLT2 inhibitors that if somebody also has gout, all the better. And, if somebody is on the margin with diabetes and gout control and can’t go with allopurinol, it would be great to add for both conditions.”
Less gout recurrence, lower mortality
The retrospective study was conducted from Jan. 1, 2013, to March 31, 2022. Among 5,931 patients with both type 2 diabetes and gout, 1,548 (26.1%) initiated an SGLT2 inhibitor (dapagliflozin, empagliflozin, or canagliflozin), while 4,383 (73.9%) initiated treatment with other active comparators, mostly (92.6%) dipeptidyl peptidase–4 inhibitors.
Gout flares were identified in the charts for a total of 86% of the participants. The weighted incidence rates for the first recurrent flare were 32.4 versus 41.2 per 1,000 person-years in the SGLT2 inhibitor versus comparator groups, with a weighted absolute rate difference of –8.8/1,000 and weighted hazard ratio of 0.81, a significant difference.
All-cause mortality was 18.8 versus 24.9 per 1,000 person-years, respectively, giving an HR of 0.71 at 5-year follow-up.
Dr. FitzGerald, who chaired the American College of Rheumatology’s 2020 gout guidelines, said he anticipates that the SGLT2 inhibitors will be mentioned in the next update to the ACR’s now “living” guidelines, although he was not speaking on the organization’s behalf.
“We talk about losartan in the current [ACR guidelines], about its specific uric acid–lowering effect. Drugs can make uric acid worse or better. For example, thiazides make it higher. I think the SGLT2 [inhibitors] are important, but I don’t think they’re huge. The study is great, and I think the drugs are great, but I don’t think they will change the way gout is managed.”
This work was supported by grants from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and from the Natural Science Foundation of Hunan Province. Dr. Wei reported receiving grant funding from Xiangya Hospital Central South University Project Program of National Clinical Research Center for Geriatric Disorders and the Science and Technology Department of Hunan Province, the Natural Science Foundation of Hunan Province, during the conduct of the study. Dr. FitzGerald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The glucose-lowering drug class sodium-glucose cotransporter 2 (SGLT2) inhibitors appear to reduce the risk for recurrent gout flares in people with gout and type 2 diabetes, and to lessen excess mortality in those individuals, compared with those who initiated other types of glucose-lowering medications, new data suggest.
Among nearly 6,000 adults with both type 2 diabetes and gout from a U.K. primary care database, initiation of SGLT2 inhibitor treatment was associated with 19% fewer recurrent gout flares and 29% lower mortality.
Moreover, unlike other urate-lowering therapies, there were no apparent transient increases in the risk of gout flares after initiating therapy, Jie Wei, PhD, of Health Management Center, Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in JAMA Network Open.
These results are important because current management of gout is suboptimal. Many patients either don’t receive adequate urate-lowering therapies such as allopurinol or stop taking them, Dr. Wei and colleagues said.
In addition to lowering glucose, SGLT2 inhibitors also reduce the risk for major adverse cardiovascular events and all-cause mortality in people regardless of their diabetes status. Previous studies have also found that SGLT2 inhibitors reduce the risk for developing gout and of gout flares.
Asked to comment, gout specialist John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, said in an interview: “I think it’s a well-done paper, with a large dataset. I think it just reinforces the findings from the other papers. Mostly anything that lowers uric acid levels is going to lower recurrent gout attacks, so it all makes sense.”
However, while Dr. FitzGerald thinks the drug class is a good option for people with diabetes or cardiorenal indications for them who also have gout, he doesn’t envision it as first-line for most other patients with gout. “The current treatments are very effective. Allopurinol brings down uric acid levels by 5-7 points. There are patients who fail allopurinol, but those are less than 5%.”
The most common reason patients stop taking allopurinol is the frequent initial gout flare. But that’s preventable, Dr. FitzGerald said, either by titrating up slowly, or by adding colchicine along with it. “By going slowly, you can avoid that flare risk. I think that’s what’s going on with the SGLT2 inhibitor. It’s not a dramatic urate-lowering drug, but it is clinically meaningful. I think that’s what this paper is showing.”
But, he noted, “I think there are so many reasons to start the SGLT2 inhibitors that if somebody also has gout, all the better. And, if somebody is on the margin with diabetes and gout control and can’t go with allopurinol, it would be great to add for both conditions.”
Less gout recurrence, lower mortality
The retrospective study was conducted from Jan. 1, 2013, to March 31, 2022. Among 5,931 patients with both type 2 diabetes and gout, 1,548 (26.1%) initiated an SGLT2 inhibitor (dapagliflozin, empagliflozin, or canagliflozin), while 4,383 (73.9%) initiated treatment with other active comparators, mostly (92.6%) dipeptidyl peptidase–4 inhibitors.
Gout flares were identified in the charts for a total of 86% of the participants. The weighted incidence rates for the first recurrent flare were 32.4 versus 41.2 per 1,000 person-years in the SGLT2 inhibitor versus comparator groups, with a weighted absolute rate difference of –8.8/1,000 and weighted hazard ratio of 0.81, a significant difference.
All-cause mortality was 18.8 versus 24.9 per 1,000 person-years, respectively, giving an HR of 0.71 at 5-year follow-up.
Dr. FitzGerald, who chaired the American College of Rheumatology’s 2020 gout guidelines, said he anticipates that the SGLT2 inhibitors will be mentioned in the next update to the ACR’s now “living” guidelines, although he was not speaking on the organization’s behalf.
“We talk about losartan in the current [ACR guidelines], about its specific uric acid–lowering effect. Drugs can make uric acid worse or better. For example, thiazides make it higher. I think the SGLT2 [inhibitors] are important, but I don’t think they’re huge. The study is great, and I think the drugs are great, but I don’t think they will change the way gout is managed.”
This work was supported by grants from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and from the Natural Science Foundation of Hunan Province. Dr. Wei reported receiving grant funding from Xiangya Hospital Central South University Project Program of National Clinical Research Center for Geriatric Disorders and the Science and Technology Department of Hunan Province, the Natural Science Foundation of Hunan Province, during the conduct of the study. Dr. FitzGerald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
NPs, PAs, and physicians hope to join doctors’ union in rare alliance
Advanced practice providers (APPs) such as nurse practitioners (NPs) and physician assistants (PAs) have long been at odds with doctor groups over scope of practice issues. But in a rare alliance, in late September. If successful, the Allina group will join the Doctors Council SEIU, Local 10MD.
The Allina health care providers share concerns about their working conditions, such as understaffing and inadequate resources, limited decision-making authority, and health systems valuing productivity and profit over patient care.
Although doctors and APPs have said that they generally work well together, the relationship has been strained in recent years as APPs argue for greater scope of practice. Meanwhile, physician groups, such as the American Medical Association, believe that APPs need more oversight.
An Allina union organizer, Britta V. Kasmarik, CNP, acknowledges the tension between physicians and APPs. But she said in an interview that the union effort helped bond this group of health care providers. “We share common goals of providing high-quality care for patients in a safe way, and we see the same things day in and day out with our patients.”
Matt Hoffman, MD, a primary care physician at Allina, told this news organization that APPs in his specialty perform the same job as doctors “and the working conditions are really identical. In our view, that means we should be unionizing together.”
The decision to hold a union vote follows similar action by nearly 150 Allina Mercy Hospital physicians in March. Allina Health appealed the vote.
In response to a New York Times investigation, the Minnesota Attorney General’s office began reviewing reports of aggressive billing practices and denied care at Allina Health.
The Allina Health system, which reports $4 billion in annual revenue, cut off nonemergency services to patients, including children, if their medical debt exceeded $4,500, according to the New York Times article. For Allina’s physicians and APPs, that meant leaving patients’ illnesses untreated.
Less than a week after the attorney general announced its investigation, the health system ended this practice.
In a prepared statement to this news organization, Allina Health said that its providers are “critical members of our teams. … We deeply value and share their commitment to providing high-quality care to our patients.”
The health system said it planned to make operational improvements, implement new communication tools, and provide additional well-being resources and enhanced employee benefits “to improve the provider experience.” In addition, it hoped to continue to “foster a culture of collaboration with all our employees.”
Having a union will allow health care providers to advocate for their patients and give health care providers more decision-making power instead of corporate leaders maintaining full authority, Ms. Kasmarik told this news organization.
Union organizers are also concerned with changes to the daily practice of medicine. “We don’t want to be spending our time doing paperwork and calling insurance companies and filling out forms,” said Dr. Hoffman. “We want to be in the exam room with a patient.”
The Allina providers organized after multiple requests to corporate managers failed to address their concerns. Their demands include increased staffing and help with nonclinical work so that clinicians can spend more time with their patients.
“What I’m really excited about is that we will be able to work with the other unionized groups to make change ... by being involved in health care policy at a state or national level,” Dr. Hoffman said. For example, that involvement might include challenging insurance company decisions.
Doctors Council bills itself as the largest union for attending physicians in the country, with 3,500 members, according to Joe Crane, national organizing director.
Despite an increase in union efforts since the pandemic, health care workers – particularly doctors – have been slow to join unions. Mr. Crane estimated that only about 3% of U.S. physicians are currently union members. He cited union campaigns in Massachusetts, New York, and Washington, DC. For comparison, a minority of advanced practice registered nurses (APRNs) (9%) report union membership, according to Medscape’s APRN compensation report last year.
Dr. Hoffman is confident the Allina health care providers will have enough votes to win the election to join the union. “We should have done this years ago.”
A version of this article appeared on Medscape.com.
Advanced practice providers (APPs) such as nurse practitioners (NPs) and physician assistants (PAs) have long been at odds with doctor groups over scope of practice issues. But in a rare alliance, in late September. If successful, the Allina group will join the Doctors Council SEIU, Local 10MD.
The Allina health care providers share concerns about their working conditions, such as understaffing and inadequate resources, limited decision-making authority, and health systems valuing productivity and profit over patient care.
Although doctors and APPs have said that they generally work well together, the relationship has been strained in recent years as APPs argue for greater scope of practice. Meanwhile, physician groups, such as the American Medical Association, believe that APPs need more oversight.
An Allina union organizer, Britta V. Kasmarik, CNP, acknowledges the tension between physicians and APPs. But she said in an interview that the union effort helped bond this group of health care providers. “We share common goals of providing high-quality care for patients in a safe way, and we see the same things day in and day out with our patients.”
Matt Hoffman, MD, a primary care physician at Allina, told this news organization that APPs in his specialty perform the same job as doctors “and the working conditions are really identical. In our view, that means we should be unionizing together.”
The decision to hold a union vote follows similar action by nearly 150 Allina Mercy Hospital physicians in March. Allina Health appealed the vote.
In response to a New York Times investigation, the Minnesota Attorney General’s office began reviewing reports of aggressive billing practices and denied care at Allina Health.
The Allina Health system, which reports $4 billion in annual revenue, cut off nonemergency services to patients, including children, if their medical debt exceeded $4,500, according to the New York Times article. For Allina’s physicians and APPs, that meant leaving patients’ illnesses untreated.
Less than a week after the attorney general announced its investigation, the health system ended this practice.
In a prepared statement to this news organization, Allina Health said that its providers are “critical members of our teams. … We deeply value and share their commitment to providing high-quality care to our patients.”
The health system said it planned to make operational improvements, implement new communication tools, and provide additional well-being resources and enhanced employee benefits “to improve the provider experience.” In addition, it hoped to continue to “foster a culture of collaboration with all our employees.”
Having a union will allow health care providers to advocate for their patients and give health care providers more decision-making power instead of corporate leaders maintaining full authority, Ms. Kasmarik told this news organization.
Union organizers are also concerned with changes to the daily practice of medicine. “We don’t want to be spending our time doing paperwork and calling insurance companies and filling out forms,” said Dr. Hoffman. “We want to be in the exam room with a patient.”
The Allina providers organized after multiple requests to corporate managers failed to address their concerns. Their demands include increased staffing and help with nonclinical work so that clinicians can spend more time with their patients.
“What I’m really excited about is that we will be able to work with the other unionized groups to make change ... by being involved in health care policy at a state or national level,” Dr. Hoffman said. For example, that involvement might include challenging insurance company decisions.
Doctors Council bills itself as the largest union for attending physicians in the country, with 3,500 members, according to Joe Crane, national organizing director.
Despite an increase in union efforts since the pandemic, health care workers – particularly doctors – have been slow to join unions. Mr. Crane estimated that only about 3% of U.S. physicians are currently union members. He cited union campaigns in Massachusetts, New York, and Washington, DC. For comparison, a minority of advanced practice registered nurses (APRNs) (9%) report union membership, according to Medscape’s APRN compensation report last year.
Dr. Hoffman is confident the Allina health care providers will have enough votes to win the election to join the union. “We should have done this years ago.”
A version of this article appeared on Medscape.com.
Advanced practice providers (APPs) such as nurse practitioners (NPs) and physician assistants (PAs) have long been at odds with doctor groups over scope of practice issues. But in a rare alliance, in late September. If successful, the Allina group will join the Doctors Council SEIU, Local 10MD.
The Allina health care providers share concerns about their working conditions, such as understaffing and inadequate resources, limited decision-making authority, and health systems valuing productivity and profit over patient care.
Although doctors and APPs have said that they generally work well together, the relationship has been strained in recent years as APPs argue for greater scope of practice. Meanwhile, physician groups, such as the American Medical Association, believe that APPs need more oversight.
An Allina union organizer, Britta V. Kasmarik, CNP, acknowledges the tension between physicians and APPs. But she said in an interview that the union effort helped bond this group of health care providers. “We share common goals of providing high-quality care for patients in a safe way, and we see the same things day in and day out with our patients.”
Matt Hoffman, MD, a primary care physician at Allina, told this news organization that APPs in his specialty perform the same job as doctors “and the working conditions are really identical. In our view, that means we should be unionizing together.”
The decision to hold a union vote follows similar action by nearly 150 Allina Mercy Hospital physicians in March. Allina Health appealed the vote.
In response to a New York Times investigation, the Minnesota Attorney General’s office began reviewing reports of aggressive billing practices and denied care at Allina Health.
The Allina Health system, which reports $4 billion in annual revenue, cut off nonemergency services to patients, including children, if their medical debt exceeded $4,500, according to the New York Times article. For Allina’s physicians and APPs, that meant leaving patients’ illnesses untreated.
Less than a week after the attorney general announced its investigation, the health system ended this practice.
In a prepared statement to this news organization, Allina Health said that its providers are “critical members of our teams. … We deeply value and share their commitment to providing high-quality care to our patients.”
The health system said it planned to make operational improvements, implement new communication tools, and provide additional well-being resources and enhanced employee benefits “to improve the provider experience.” In addition, it hoped to continue to “foster a culture of collaboration with all our employees.”
Having a union will allow health care providers to advocate for their patients and give health care providers more decision-making power instead of corporate leaders maintaining full authority, Ms. Kasmarik told this news organization.
Union organizers are also concerned with changes to the daily practice of medicine. “We don’t want to be spending our time doing paperwork and calling insurance companies and filling out forms,” said Dr. Hoffman. “We want to be in the exam room with a patient.”
The Allina providers organized after multiple requests to corporate managers failed to address their concerns. Their demands include increased staffing and help with nonclinical work so that clinicians can spend more time with their patients.
“What I’m really excited about is that we will be able to work with the other unionized groups to make change ... by being involved in health care policy at a state or national level,” Dr. Hoffman said. For example, that involvement might include challenging insurance company decisions.
Doctors Council bills itself as the largest union for attending physicians in the country, with 3,500 members, according to Joe Crane, national organizing director.
Despite an increase in union efforts since the pandemic, health care workers – particularly doctors – have been slow to join unions. Mr. Crane estimated that only about 3% of U.S. physicians are currently union members. He cited union campaigns in Massachusetts, New York, and Washington, DC. For comparison, a minority of advanced practice registered nurses (APRNs) (9%) report union membership, according to Medscape’s APRN compensation report last year.
Dr. Hoffman is confident the Allina health care providers will have enough votes to win the election to join the union. “We should have done this years ago.”
A version of this article appeared on Medscape.com.
These four GI conditions may predict Parkinson’s disease
Early detection of these conditions might help identify patients at risk for PD, potentially prompting preventive strategies, the researchers suggest.
The results of previous experimental studies by the team supported the Braak hypothesis, which states that idiopathic PD originates in the gut in a subset of patients. However, no previous study had investigated a broad range of gastrointestinal symptoms and syndromes that might occur prior to a PD diagnosis.
Given their preclinical work, the authors were not surprised to find that certain GI syndromes were specifically associated with PD, even when compared with Alzheimer’s disease (AD) and cerebrovascular disease (CVD), principal author Pankaj Jay Pasricha, MBBS, MD, of Mayo Clinic Arizona, Scottsdale, said in an interview. However, they were “impressed by the strength of the associations.”
“Experts have known for a very long time that constipation is a potential risk factor for PD, so this study adds to the list of GI conditions that could potentially be risk factors,” he said.
The study was published online in Gut.
Studies converge
To determine the incidence of GI syndromes and interventions preceding PD, the investigators performed a combined case-control and cohort study using a U.S.-based nationwide medical record network.
First, they compared 24,624 individuals with new-onset idiopathic PD with the same number of matched negative controls (NCs), as well as 19,046 people with AD and 23,942 with CVD to investigate the presence of preexisting GI conditions, which the researchers referred to as “exposures.” Overall, the mean age was about 70, and about half of those studied were women.
Eighteen conditions covering the entire GI tract were investigated. These included achalasia, dysphagia, gastroesophageal reflux disease, gastroparesis, functional dyspepsia, paralytic ileus, diarrhea, irritable bowel syndrome (IBS) with and without diarrhea, intestinal pseudo-obstruction, fecal incontinence, Crohn’s disease, ulcerative colitis, and microscopic colitis, as well as appendectomy and vagotomy.
All GI syndromes were significantly increased in the PD group, compared with NCs (odds ratio > 1). However, only preexisting dysphagia (OR, 3.58), gastroparesis (OR, 4.64), functional dyspepsia (OR, 3.39), intestinal pseudo-obstruction (OR, 3.01), diarrhea (OR, 2.85), constipation (OR, 3.32), IBS with constipation (OR, 4.11), IBS with diarrhea (OR, 4.31), IBS without diarrhea (OR, 3.53), and fecal incontinence (OR, 3.76) produced ORs that were numerically greater than the upper limit of the negative exposures.
In addition, only gastroparesis, dysphagia, IBS with constipation, IBS without diarrhea, and constipation were specific for PD, compared with the AD and CVD groups (OR > 1). After correction for false discovery rate, though, gastroparesis and constipation did not remain significantly different, compared with the AD and CVD groups.
Other preexisting GI conditions not only were significantly associated with PD but also showed strong associations with the AD and CVD groups.
To validate the case-control analyses, the team set up a complementary cohort study. Eighteen cohorts – each diagnosed with one of the GI conditions in the case-control analysis – were compared with their respective NC cohorts for the prospective risk of developing PD, AD, or CVD within 5 years.
Gastroparesis, dysphagia, IBS without diarrhea, and constipation showed specific associations with PD versus NCs, AD, and CVD in the cohort analysis. Their relative risks versus NCs were 2.43, 2.27, 1.17, and 2.38, respectively.
Functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not PD specific, but IBS with constipation and intestinal pseudo-obstruction showed PD specificity in both the case-control (OR, 4.11) and cohort analyses (RR, 1.84).
Appendectomy decreased the risk for PD in the cohort analysis (RR, 0.48), but neither inflammatory bowel disease nor vagotomy was associated with PD.
“This study is the first to establish substantial observational evidence that the clinical diagnosis of not only constipation but also dysphagia, gastroparesis, and IBS without diarrhea might specifically predict the development of PD, whereas other exposures were less specific,” the researchers wrote.
However, Dr. Pasricha said, “there is no need for alarm.” Clinicians should reassure patients that “the overall risk for developing PD is low. The overwhelming majority of patients with these GI conditions will never develop PD.”
His team will be doing experimental work on the biological mechanisms that might explain the current study’s findings. “In addition, the U.S. National Institutes of Health has issued a call for proposals to perform research in patients that could help understand these associations better,” he said.
Body or brain?
The Parkinson’s Foundation’s National Medical Advisor, Michael S. Okun, MD, called the study “fascinating.”
The findings “confirm many other studies showing that GI symptoms can precede a Parkinson’s disease diagnosis,” he said in an interview.
Although the study was designed to test the Braak hypothesis, “the dataset really cannot confirm or refute Braak pathology, which can only be accomplished with comparison to postmortem samples,” he added.
“The raging debate in the field of body-first versus brain-first Parkinson’s may be somewhat artificial, especially if we consider that Parkinson’s is not one disease,” Dr. Okun noted. “It will take clinical data, pathology, and the collaboration of many researchers to solve the puzzle.”
“The Foundation continues to monitor all the advancements in the ‘gut’ Parkinson field,” he said. “We do not recommend at this time changing the approach to clinical care based on this data.”
No funding or competing interests were declared. Dr. Okun declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
Early detection of these conditions might help identify patients at risk for PD, potentially prompting preventive strategies, the researchers suggest.
The results of previous experimental studies by the team supported the Braak hypothesis, which states that idiopathic PD originates in the gut in a subset of patients. However, no previous study had investigated a broad range of gastrointestinal symptoms and syndromes that might occur prior to a PD diagnosis.
Given their preclinical work, the authors were not surprised to find that certain GI syndromes were specifically associated with PD, even when compared with Alzheimer’s disease (AD) and cerebrovascular disease (CVD), principal author Pankaj Jay Pasricha, MBBS, MD, of Mayo Clinic Arizona, Scottsdale, said in an interview. However, they were “impressed by the strength of the associations.”
“Experts have known for a very long time that constipation is a potential risk factor for PD, so this study adds to the list of GI conditions that could potentially be risk factors,” he said.
The study was published online in Gut.
Studies converge
To determine the incidence of GI syndromes and interventions preceding PD, the investigators performed a combined case-control and cohort study using a U.S.-based nationwide medical record network.
First, they compared 24,624 individuals with new-onset idiopathic PD with the same number of matched negative controls (NCs), as well as 19,046 people with AD and 23,942 with CVD to investigate the presence of preexisting GI conditions, which the researchers referred to as “exposures.” Overall, the mean age was about 70, and about half of those studied were women.
Eighteen conditions covering the entire GI tract were investigated. These included achalasia, dysphagia, gastroesophageal reflux disease, gastroparesis, functional dyspepsia, paralytic ileus, diarrhea, irritable bowel syndrome (IBS) with and without diarrhea, intestinal pseudo-obstruction, fecal incontinence, Crohn’s disease, ulcerative colitis, and microscopic colitis, as well as appendectomy and vagotomy.
All GI syndromes were significantly increased in the PD group, compared with NCs (odds ratio > 1). However, only preexisting dysphagia (OR, 3.58), gastroparesis (OR, 4.64), functional dyspepsia (OR, 3.39), intestinal pseudo-obstruction (OR, 3.01), diarrhea (OR, 2.85), constipation (OR, 3.32), IBS with constipation (OR, 4.11), IBS with diarrhea (OR, 4.31), IBS without diarrhea (OR, 3.53), and fecal incontinence (OR, 3.76) produced ORs that were numerically greater than the upper limit of the negative exposures.
In addition, only gastroparesis, dysphagia, IBS with constipation, IBS without diarrhea, and constipation were specific for PD, compared with the AD and CVD groups (OR > 1). After correction for false discovery rate, though, gastroparesis and constipation did not remain significantly different, compared with the AD and CVD groups.
Other preexisting GI conditions not only were significantly associated with PD but also showed strong associations with the AD and CVD groups.
To validate the case-control analyses, the team set up a complementary cohort study. Eighteen cohorts – each diagnosed with one of the GI conditions in the case-control analysis – were compared with their respective NC cohorts for the prospective risk of developing PD, AD, or CVD within 5 years.
Gastroparesis, dysphagia, IBS without diarrhea, and constipation showed specific associations with PD versus NCs, AD, and CVD in the cohort analysis. Their relative risks versus NCs were 2.43, 2.27, 1.17, and 2.38, respectively.
Functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not PD specific, but IBS with constipation and intestinal pseudo-obstruction showed PD specificity in both the case-control (OR, 4.11) and cohort analyses (RR, 1.84).
Appendectomy decreased the risk for PD in the cohort analysis (RR, 0.48), but neither inflammatory bowel disease nor vagotomy was associated with PD.
“This study is the first to establish substantial observational evidence that the clinical diagnosis of not only constipation but also dysphagia, gastroparesis, and IBS without diarrhea might specifically predict the development of PD, whereas other exposures were less specific,” the researchers wrote.
However, Dr. Pasricha said, “there is no need for alarm.” Clinicians should reassure patients that “the overall risk for developing PD is low. The overwhelming majority of patients with these GI conditions will never develop PD.”
His team will be doing experimental work on the biological mechanisms that might explain the current study’s findings. “In addition, the U.S. National Institutes of Health has issued a call for proposals to perform research in patients that could help understand these associations better,” he said.
Body or brain?
The Parkinson’s Foundation’s National Medical Advisor, Michael S. Okun, MD, called the study “fascinating.”
The findings “confirm many other studies showing that GI symptoms can precede a Parkinson’s disease diagnosis,” he said in an interview.
Although the study was designed to test the Braak hypothesis, “the dataset really cannot confirm or refute Braak pathology, which can only be accomplished with comparison to postmortem samples,” he added.
“The raging debate in the field of body-first versus brain-first Parkinson’s may be somewhat artificial, especially if we consider that Parkinson’s is not one disease,” Dr. Okun noted. “It will take clinical data, pathology, and the collaboration of many researchers to solve the puzzle.”
“The Foundation continues to monitor all the advancements in the ‘gut’ Parkinson field,” he said. “We do not recommend at this time changing the approach to clinical care based on this data.”
No funding or competing interests were declared. Dr. Okun declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
Early detection of these conditions might help identify patients at risk for PD, potentially prompting preventive strategies, the researchers suggest.
The results of previous experimental studies by the team supported the Braak hypothesis, which states that idiopathic PD originates in the gut in a subset of patients. However, no previous study had investigated a broad range of gastrointestinal symptoms and syndromes that might occur prior to a PD diagnosis.
Given their preclinical work, the authors were not surprised to find that certain GI syndromes were specifically associated with PD, even when compared with Alzheimer’s disease (AD) and cerebrovascular disease (CVD), principal author Pankaj Jay Pasricha, MBBS, MD, of Mayo Clinic Arizona, Scottsdale, said in an interview. However, they were “impressed by the strength of the associations.”
“Experts have known for a very long time that constipation is a potential risk factor for PD, so this study adds to the list of GI conditions that could potentially be risk factors,” he said.
The study was published online in Gut.
Studies converge
To determine the incidence of GI syndromes and interventions preceding PD, the investigators performed a combined case-control and cohort study using a U.S.-based nationwide medical record network.
First, they compared 24,624 individuals with new-onset idiopathic PD with the same number of matched negative controls (NCs), as well as 19,046 people with AD and 23,942 with CVD to investigate the presence of preexisting GI conditions, which the researchers referred to as “exposures.” Overall, the mean age was about 70, and about half of those studied were women.
Eighteen conditions covering the entire GI tract were investigated. These included achalasia, dysphagia, gastroesophageal reflux disease, gastroparesis, functional dyspepsia, paralytic ileus, diarrhea, irritable bowel syndrome (IBS) with and without diarrhea, intestinal pseudo-obstruction, fecal incontinence, Crohn’s disease, ulcerative colitis, and microscopic colitis, as well as appendectomy and vagotomy.
All GI syndromes were significantly increased in the PD group, compared with NCs (odds ratio > 1). However, only preexisting dysphagia (OR, 3.58), gastroparesis (OR, 4.64), functional dyspepsia (OR, 3.39), intestinal pseudo-obstruction (OR, 3.01), diarrhea (OR, 2.85), constipation (OR, 3.32), IBS with constipation (OR, 4.11), IBS with diarrhea (OR, 4.31), IBS without diarrhea (OR, 3.53), and fecal incontinence (OR, 3.76) produced ORs that were numerically greater than the upper limit of the negative exposures.
In addition, only gastroparesis, dysphagia, IBS with constipation, IBS without diarrhea, and constipation were specific for PD, compared with the AD and CVD groups (OR > 1). After correction for false discovery rate, though, gastroparesis and constipation did not remain significantly different, compared with the AD and CVD groups.
Other preexisting GI conditions not only were significantly associated with PD but also showed strong associations with the AD and CVD groups.
To validate the case-control analyses, the team set up a complementary cohort study. Eighteen cohorts – each diagnosed with one of the GI conditions in the case-control analysis – were compared with their respective NC cohorts for the prospective risk of developing PD, AD, or CVD within 5 years.
Gastroparesis, dysphagia, IBS without diarrhea, and constipation showed specific associations with PD versus NCs, AD, and CVD in the cohort analysis. Their relative risks versus NCs were 2.43, 2.27, 1.17, and 2.38, respectively.
Functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not PD specific, but IBS with constipation and intestinal pseudo-obstruction showed PD specificity in both the case-control (OR, 4.11) and cohort analyses (RR, 1.84).
Appendectomy decreased the risk for PD in the cohort analysis (RR, 0.48), but neither inflammatory bowel disease nor vagotomy was associated with PD.
“This study is the first to establish substantial observational evidence that the clinical diagnosis of not only constipation but also dysphagia, gastroparesis, and IBS without diarrhea might specifically predict the development of PD, whereas other exposures were less specific,” the researchers wrote.
However, Dr. Pasricha said, “there is no need for alarm.” Clinicians should reassure patients that “the overall risk for developing PD is low. The overwhelming majority of patients with these GI conditions will never develop PD.”
His team will be doing experimental work on the biological mechanisms that might explain the current study’s findings. “In addition, the U.S. National Institutes of Health has issued a call for proposals to perform research in patients that could help understand these associations better,” he said.
Body or brain?
The Parkinson’s Foundation’s National Medical Advisor, Michael S. Okun, MD, called the study “fascinating.”
The findings “confirm many other studies showing that GI symptoms can precede a Parkinson’s disease diagnosis,” he said in an interview.
Although the study was designed to test the Braak hypothesis, “the dataset really cannot confirm or refute Braak pathology, which can only be accomplished with comparison to postmortem samples,” he added.
“The raging debate in the field of body-first versus brain-first Parkinson’s may be somewhat artificial, especially if we consider that Parkinson’s is not one disease,” Dr. Okun noted. “It will take clinical data, pathology, and the collaboration of many researchers to solve the puzzle.”
“The Foundation continues to monitor all the advancements in the ‘gut’ Parkinson field,” he said. “We do not recommend at this time changing the approach to clinical care based on this data.”
No funding or competing interests were declared. Dr. Okun declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM GUT







