User login
Really? Cancer screening doesn’t save lives?
This transcript from Impact Factor has been edited for clarity.
If you are my age or older, and like me, you are something of a rule follower, then you’re getting screened for various cancers.
Colonoscopies, mammograms, cervical cancer screening, chest CTs for people with a significant smoking history. The tests are done and usually, but not always, they are negative. And if positive, usually, but not always, follow-up tests are negative, and if they aren’t and a new cancer is diagnosed you tell yourself, Well, at least we caught it early. Isn’t it good that I’m a rule follower? My life was just saved.
But it turns out, proving that cancer screening actually saves lives is quite difficult. Is it possible that all this screening is for nothing?
The benefits, risks, or perhaps futility of cancer screening is in the news this week because of this article, appearing in JAMA Internal Medicine.
It’s a meta-analysis of very specific randomized trials of cancer screening modalities and concludes that, with the exception of sigmoidoscopy for colon cancer screening, none of them meaningfully change life expectancy.
Now – a bit of inside baseball here – I almost never choose to discuss meta-analyses on Impact Factor. It’s hard enough to dig deep into the methodology of a single study, but with a meta-analysis, you’re sort of obligated to review all the included studies, and, what’s worse, the studies that were not included but might bear on the central question.
In this case, though, the topic is important enough to think about a bit more, and the conclusions have large enough implications for public health that we should question them a bit.
First, let’s run down the study as presented.
The authors searched for randomized trials of cancer screening modalities. This is important, and I think appropriate. They wanted studies that took some people and assigned them to screening, and some people to no screening – avoiding the confounding that would come from observational data (rule followers like me tend to live longer owing to a variety of healthful behaviors, not just cancer screening).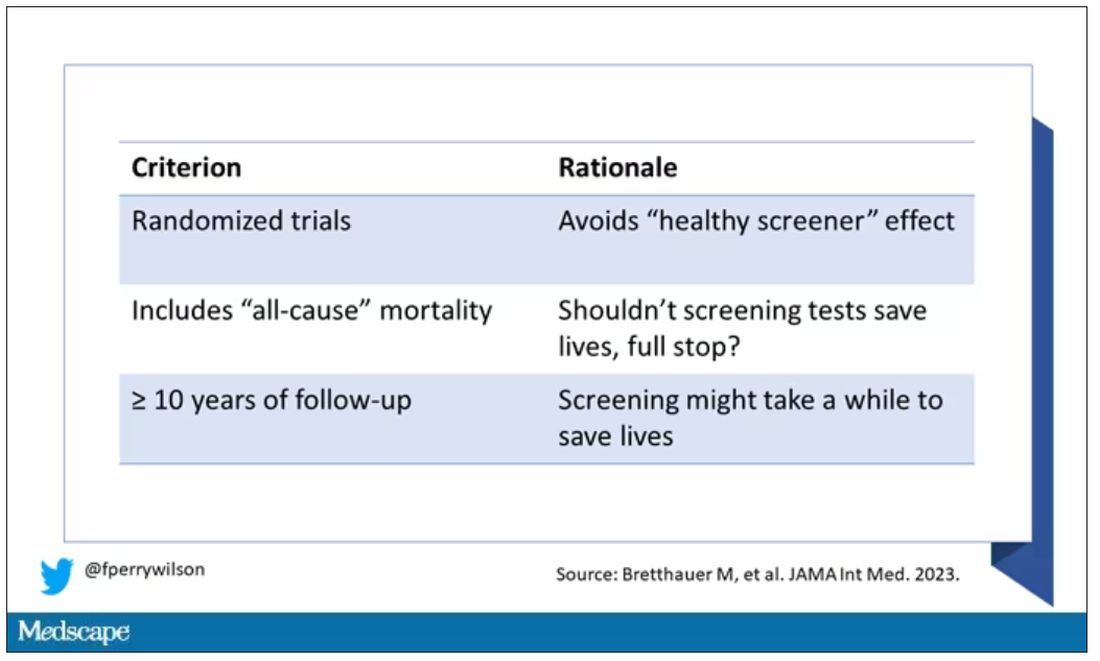
They didn’t stop at just randomized trials, though. They wanted trials that reported on all-cause, not cancer-specific, mortality. We’ll dig into the distinction in a sec. Finally, they wanted trials with at least 10 years of follow-up time.
These are pretty strict criteria – and after applying that filter, we are left with a grand total of 18 studies to analyze. Most were in the colon cancer space; only two studies met criteria for mammography screening.
Right off the bat, this raises concerns to me. In the universe of high-quality studies of cancer screening modalities, this is just the tip of the iceberg. And the results of meta-analyses are always dependent on the included studies – definitionally.
The results as presented are compelling.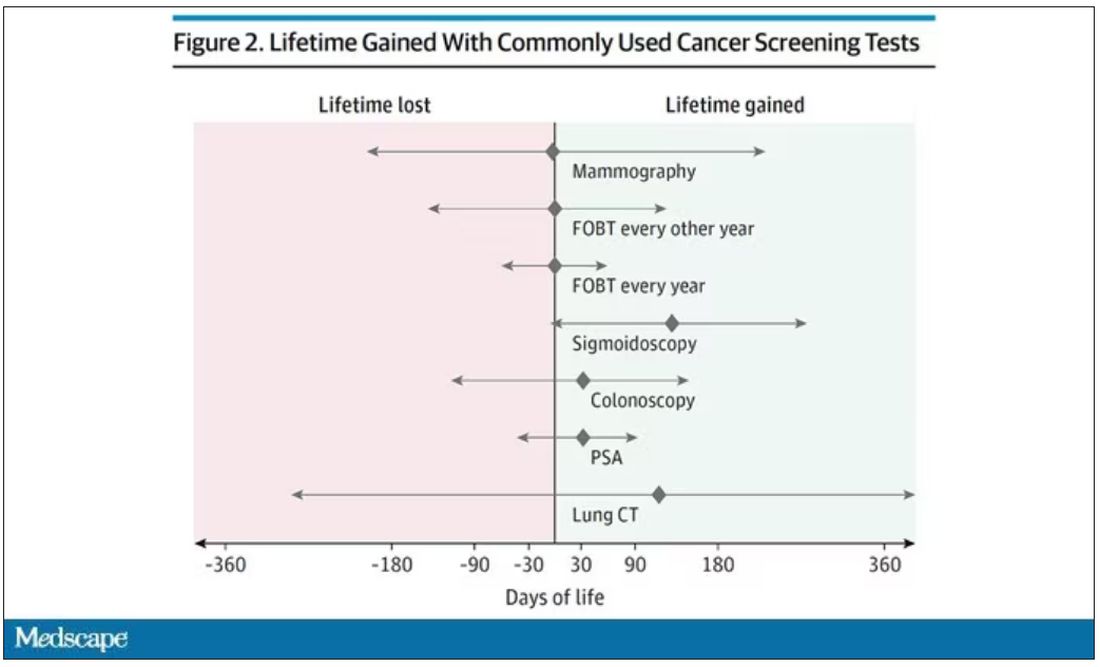
(Side note: Averages are tricky here. It’s not like everyone who gets screened gets 110 extra days. Most people get nothing, and some people – those whose colon cancer was detected early – get a bunch of extra days.)
And a thing about meta-analysis: Meeting the criteria to be included in a meta-analysis does not necessarily mean the study was a good one. For example, one of the two mammography screening studies included is this one, from Miller and colleagues.
On the surface, it looks good – a large randomized trial of mammography screening in Canada, with long-term follow-up including all-cause mortality. Showing, by the way, no effect of screening on either breast cancer–specific or all-cause mortality.
But that study came under a lot of criticism owing to allegations that randomization was broken and women with palpable breast masses were preferentially put into the mammography group, making those outcomes worse.
The authors of the current meta-analysis don’t mention this. Indeed, they state that they don’t perform any assessments of the quality of the included studies.
But I don’t want to criticize all the included studies. Let’s think bigger picture.
Randomized trials of screening for cancers like colon, breast, and lung cancer in smokers have generally shown that those randomized to screening had lower target-cancer–specific mortality. Across all the randomized mammography studies, for example, women randomized to mammography were about 20% less likely to die of breast cancer than were those who were randomized to not be screened – particularly among those above age 50.
But it’s true that all-cause mortality, on the whole, has not differed statistically between those randomized to mammography vs. no mammography. What’s the deal?
Well, the authors of the meta-analysis engage in some zero-sum thinking here. They say that if it is true that screening tests reduce cancer-specific deaths, but all-cause mortality is not different, screening tests must increase mortality due to other causes. How? They cite colonic perforation during colonoscopy as an example of a harm that could lead to earlier death, which makes some sense. For mammogram and other less invasive screening modalities, they suggest that the stress and anxiety associated with screening might increase the risk for death – this is a bit harder for me to defend.
The thing is, statistics really isn’t a zero-sum game. It’s a question of signal vs. noise. Take breast cancer, for example. Without screening, about 3.2% of women in this country would die of breast cancer. With screening, 2.8% would die (that’s a 20% reduction on the relative scale). The truth is, most women don’t die of breast cancer. Most people don’t die of colon cancer. Even most smokers don’t die of lung cancer. Most people die of heart disease. And then cancer – but there are a lot of cancers out there, and only a handful have decent screening tests.
In other words, the screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it will help some small number of those people being screened a lot, potentially saving their lives. If we knew who those people were in advance, it would be great, but then I suppose we wouldn’t need the screening test in the first place.
It’s not fair, then, to say that mammography increases non–breast cancer causes of death. In reality, it’s just that the impact of mammography on all-cause mortality is washed out by the random noise inherent to studying a sample of individuals rather than the entire population.
I’m reminded of that old story about the girl on the beach after a storm, throwing beached starfish back into the water. Someone comes by and says, “Why are you doing that? There are millions of starfish here – it doesn’t matter if you throw a few back.” And she says, “It matters for this one.”
There are other issues with aggregating data like these and concluding that there is no effect on all-cause mortality. For one, it assumes the people randomized to no screening never got screening. Most of these studies lasted 5-10 years, some with longer follow-up, but many people in the no-screening arm may have been screened as recommendations have changed. That would tend to bias the results against screening because the so-called control group, well, isn’t.
It also fails to acknowledge the reality that screening for disease can be thought of as a package deal. Instead of asking whether screening for breast cancer, and colon cancer, and lung cancer individually saves lives, the real relevant question is whether a policy of screening for cancer in general saves lives. And that hasn’t been studied very broadly, except in one trial looking at screening for four cancers. That study is in this meta-analysis and, interestingly, seems to suggest that the policy does extend life – by 123 days. Again, be careful how you think about that average.
I don’t want to be an absolutist here. Whether these screening tests are a good idea or not is actually a moving target. As treatment for cancer gets better, detecting cancer early may not be as important. As new screening modalities emerge, older ones may not be preferable any longer. Better testing, genetic or otherwise, might allow us to tailor screening more narrowly than the population-based approach we have now.
But I worry that a meta-analysis like this, which concludes that screening doesn’t help on the basis of a handful of studies – without acknowledgment of the signal-to-noise problem, without accounting for screening in the control group, without acknowledging that screening should be thought of as a package – will lead some people to make the decision to forgo screening. for, say, 49 out of 50 of them, that may be fine. But for 1 out of 50 or so, well, it matters for that one.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript from Impact Factor has been edited for clarity.
If you are my age or older, and like me, you are something of a rule follower, then you’re getting screened for various cancers.
Colonoscopies, mammograms, cervical cancer screening, chest CTs for people with a significant smoking history. The tests are done and usually, but not always, they are negative. And if positive, usually, but not always, follow-up tests are negative, and if they aren’t and a new cancer is diagnosed you tell yourself, Well, at least we caught it early. Isn’t it good that I’m a rule follower? My life was just saved.
But it turns out, proving that cancer screening actually saves lives is quite difficult. Is it possible that all this screening is for nothing?
The benefits, risks, or perhaps futility of cancer screening is in the news this week because of this article, appearing in JAMA Internal Medicine.
It’s a meta-analysis of very specific randomized trials of cancer screening modalities and concludes that, with the exception of sigmoidoscopy for colon cancer screening, none of them meaningfully change life expectancy.
Now – a bit of inside baseball here – I almost never choose to discuss meta-analyses on Impact Factor. It’s hard enough to dig deep into the methodology of a single study, but with a meta-analysis, you’re sort of obligated to review all the included studies, and, what’s worse, the studies that were not included but might bear on the central question.
In this case, though, the topic is important enough to think about a bit more, and the conclusions have large enough implications for public health that we should question them a bit.
First, let’s run down the study as presented.
The authors searched for randomized trials of cancer screening modalities. This is important, and I think appropriate. They wanted studies that took some people and assigned them to screening, and some people to no screening – avoiding the confounding that would come from observational data (rule followers like me tend to live longer owing to a variety of healthful behaviors, not just cancer screening).
They didn’t stop at just randomized trials, though. They wanted trials that reported on all-cause, not cancer-specific, mortality. We’ll dig into the distinction in a sec. Finally, they wanted trials with at least 10 years of follow-up time.
These are pretty strict criteria – and after applying that filter, we are left with a grand total of 18 studies to analyze. Most were in the colon cancer space; only two studies met criteria for mammography screening.
Right off the bat, this raises concerns to me. In the universe of high-quality studies of cancer screening modalities, this is just the tip of the iceberg. And the results of meta-analyses are always dependent on the included studies – definitionally.
The results as presented are compelling.
(Side note: Averages are tricky here. It’s not like everyone who gets screened gets 110 extra days. Most people get nothing, and some people – those whose colon cancer was detected early – get a bunch of extra days.)
And a thing about meta-analysis: Meeting the criteria to be included in a meta-analysis does not necessarily mean the study was a good one. For example, one of the two mammography screening studies included is this one, from Miller and colleagues.
On the surface, it looks good – a large randomized trial of mammography screening in Canada, with long-term follow-up including all-cause mortality. Showing, by the way, no effect of screening on either breast cancer–specific or all-cause mortality.
But that study came under a lot of criticism owing to allegations that randomization was broken and women with palpable breast masses were preferentially put into the mammography group, making those outcomes worse.
The authors of the current meta-analysis don’t mention this. Indeed, they state that they don’t perform any assessments of the quality of the included studies.
But I don’t want to criticize all the included studies. Let’s think bigger picture.
Randomized trials of screening for cancers like colon, breast, and lung cancer in smokers have generally shown that those randomized to screening had lower target-cancer–specific mortality. Across all the randomized mammography studies, for example, women randomized to mammography were about 20% less likely to die of breast cancer than were those who were randomized to not be screened – particularly among those above age 50.
But it’s true that all-cause mortality, on the whole, has not differed statistically between those randomized to mammography vs. no mammography. What’s the deal?
Well, the authors of the meta-analysis engage in some zero-sum thinking here. They say that if it is true that screening tests reduce cancer-specific deaths, but all-cause mortality is not different, screening tests must increase mortality due to other causes. How? They cite colonic perforation during colonoscopy as an example of a harm that could lead to earlier death, which makes some sense. For mammogram and other less invasive screening modalities, they suggest that the stress and anxiety associated with screening might increase the risk for death – this is a bit harder for me to defend.
The thing is, statistics really isn’t a zero-sum game. It’s a question of signal vs. noise. Take breast cancer, for example. Without screening, about 3.2% of women in this country would die of breast cancer. With screening, 2.8% would die (that’s a 20% reduction on the relative scale). The truth is, most women don’t die of breast cancer. Most people don’t die of colon cancer. Even most smokers don’t die of lung cancer. Most people die of heart disease. And then cancer – but there are a lot of cancers out there, and only a handful have decent screening tests.
In other words, the screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it will help some small number of those people being screened a lot, potentially saving their lives. If we knew who those people were in advance, it would be great, but then I suppose we wouldn’t need the screening test in the first place.
It’s not fair, then, to say that mammography increases non–breast cancer causes of death. In reality, it’s just that the impact of mammography on all-cause mortality is washed out by the random noise inherent to studying a sample of individuals rather than the entire population.
I’m reminded of that old story about the girl on the beach after a storm, throwing beached starfish back into the water. Someone comes by and says, “Why are you doing that? There are millions of starfish here – it doesn’t matter if you throw a few back.” And she says, “It matters for this one.”
There are other issues with aggregating data like these and concluding that there is no effect on all-cause mortality. For one, it assumes the people randomized to no screening never got screening. Most of these studies lasted 5-10 years, some with longer follow-up, but many people in the no-screening arm may have been screened as recommendations have changed. That would tend to bias the results against screening because the so-called control group, well, isn’t.
It also fails to acknowledge the reality that screening for disease can be thought of as a package deal. Instead of asking whether screening for breast cancer, and colon cancer, and lung cancer individually saves lives, the real relevant question is whether a policy of screening for cancer in general saves lives. And that hasn’t been studied very broadly, except in one trial looking at screening for four cancers. That study is in this meta-analysis and, interestingly, seems to suggest that the policy does extend life – by 123 days. Again, be careful how you think about that average.
I don’t want to be an absolutist here. Whether these screening tests are a good idea or not is actually a moving target. As treatment for cancer gets better, detecting cancer early may not be as important. As new screening modalities emerge, older ones may not be preferable any longer. Better testing, genetic or otherwise, might allow us to tailor screening more narrowly than the population-based approach we have now.
But I worry that a meta-analysis like this, which concludes that screening doesn’t help on the basis of a handful of studies – without acknowledgment of the signal-to-noise problem, without accounting for screening in the control group, without acknowledging that screening should be thought of as a package – will lead some people to make the decision to forgo screening. for, say, 49 out of 50 of them, that may be fine. But for 1 out of 50 or so, well, it matters for that one.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript from Impact Factor has been edited for clarity.
If you are my age or older, and like me, you are something of a rule follower, then you’re getting screened for various cancers.
Colonoscopies, mammograms, cervical cancer screening, chest CTs for people with a significant smoking history. The tests are done and usually, but not always, they are negative. And if positive, usually, but not always, follow-up tests are negative, and if they aren’t and a new cancer is diagnosed you tell yourself, Well, at least we caught it early. Isn’t it good that I’m a rule follower? My life was just saved.
But it turns out, proving that cancer screening actually saves lives is quite difficult. Is it possible that all this screening is for nothing?
The benefits, risks, or perhaps futility of cancer screening is in the news this week because of this article, appearing in JAMA Internal Medicine.
It’s a meta-analysis of very specific randomized trials of cancer screening modalities and concludes that, with the exception of sigmoidoscopy for colon cancer screening, none of them meaningfully change life expectancy.
Now – a bit of inside baseball here – I almost never choose to discuss meta-analyses on Impact Factor. It’s hard enough to dig deep into the methodology of a single study, but with a meta-analysis, you’re sort of obligated to review all the included studies, and, what’s worse, the studies that were not included but might bear on the central question.
In this case, though, the topic is important enough to think about a bit more, and the conclusions have large enough implications for public health that we should question them a bit.
First, let’s run down the study as presented.
The authors searched for randomized trials of cancer screening modalities. This is important, and I think appropriate. They wanted studies that took some people and assigned them to screening, and some people to no screening – avoiding the confounding that would come from observational data (rule followers like me tend to live longer owing to a variety of healthful behaviors, not just cancer screening).
They didn’t stop at just randomized trials, though. They wanted trials that reported on all-cause, not cancer-specific, mortality. We’ll dig into the distinction in a sec. Finally, they wanted trials with at least 10 years of follow-up time.
These are pretty strict criteria – and after applying that filter, we are left with a grand total of 18 studies to analyze. Most were in the colon cancer space; only two studies met criteria for mammography screening.
Right off the bat, this raises concerns to me. In the universe of high-quality studies of cancer screening modalities, this is just the tip of the iceberg. And the results of meta-analyses are always dependent on the included studies – definitionally.
The results as presented are compelling.
(Side note: Averages are tricky here. It’s not like everyone who gets screened gets 110 extra days. Most people get nothing, and some people – those whose colon cancer was detected early – get a bunch of extra days.)
And a thing about meta-analysis: Meeting the criteria to be included in a meta-analysis does not necessarily mean the study was a good one. For example, one of the two mammography screening studies included is this one, from Miller and colleagues.
On the surface, it looks good – a large randomized trial of mammography screening in Canada, with long-term follow-up including all-cause mortality. Showing, by the way, no effect of screening on either breast cancer–specific or all-cause mortality.
But that study came under a lot of criticism owing to allegations that randomization was broken and women with palpable breast masses were preferentially put into the mammography group, making those outcomes worse.
The authors of the current meta-analysis don’t mention this. Indeed, they state that they don’t perform any assessments of the quality of the included studies.
But I don’t want to criticize all the included studies. Let’s think bigger picture.
Randomized trials of screening for cancers like colon, breast, and lung cancer in smokers have generally shown that those randomized to screening had lower target-cancer–specific mortality. Across all the randomized mammography studies, for example, women randomized to mammography were about 20% less likely to die of breast cancer than were those who were randomized to not be screened – particularly among those above age 50.
But it’s true that all-cause mortality, on the whole, has not differed statistically between those randomized to mammography vs. no mammography. What’s the deal?
Well, the authors of the meta-analysis engage in some zero-sum thinking here. They say that if it is true that screening tests reduce cancer-specific deaths, but all-cause mortality is not different, screening tests must increase mortality due to other causes. How? They cite colonic perforation during colonoscopy as an example of a harm that could lead to earlier death, which makes some sense. For mammogram and other less invasive screening modalities, they suggest that the stress and anxiety associated with screening might increase the risk for death – this is a bit harder for me to defend.
The thing is, statistics really isn’t a zero-sum game. It’s a question of signal vs. noise. Take breast cancer, for example. Without screening, about 3.2% of women in this country would die of breast cancer. With screening, 2.8% would die (that’s a 20% reduction on the relative scale). The truth is, most women don’t die of breast cancer. Most people don’t die of colon cancer. Even most smokers don’t die of lung cancer. Most people die of heart disease. And then cancer – but there are a lot of cancers out there, and only a handful have decent screening tests.
In other words, the screening tests are unlikely to help most people because most people will not die of the particular type of cancer being screened for. But it will help some small number of those people being screened a lot, potentially saving their lives. If we knew who those people were in advance, it would be great, but then I suppose we wouldn’t need the screening test in the first place.
It’s not fair, then, to say that mammography increases non–breast cancer causes of death. In reality, it’s just that the impact of mammography on all-cause mortality is washed out by the random noise inherent to studying a sample of individuals rather than the entire population.
I’m reminded of that old story about the girl on the beach after a storm, throwing beached starfish back into the water. Someone comes by and says, “Why are you doing that? There are millions of starfish here – it doesn’t matter if you throw a few back.” And she says, “It matters for this one.”
There are other issues with aggregating data like these and concluding that there is no effect on all-cause mortality. For one, it assumes the people randomized to no screening never got screening. Most of these studies lasted 5-10 years, some with longer follow-up, but many people in the no-screening arm may have been screened as recommendations have changed. That would tend to bias the results against screening because the so-called control group, well, isn’t.
It also fails to acknowledge the reality that screening for disease can be thought of as a package deal. Instead of asking whether screening for breast cancer, and colon cancer, and lung cancer individually saves lives, the real relevant question is whether a policy of screening for cancer in general saves lives. And that hasn’t been studied very broadly, except in one trial looking at screening for four cancers. That study is in this meta-analysis and, interestingly, seems to suggest that the policy does extend life – by 123 days. Again, be careful how you think about that average.
I don’t want to be an absolutist here. Whether these screening tests are a good idea or not is actually a moving target. As treatment for cancer gets better, detecting cancer early may not be as important. As new screening modalities emerge, older ones may not be preferable any longer. Better testing, genetic or otherwise, might allow us to tailor screening more narrowly than the population-based approach we have now.
But I worry that a meta-analysis like this, which concludes that screening doesn’t help on the basis of a handful of studies – without acknowledgment of the signal-to-noise problem, without accounting for screening in the control group, without acknowledging that screening should be thought of as a package – will lead some people to make the decision to forgo screening. for, say, 49 out of 50 of them, that may be fine. But for 1 out of 50 or so, well, it matters for that one.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
No link between most cancers and depression/anxiety: Study
from a large, individual participant data meta-analysis.
An exception was for lung and smoking-related cancers, but key covariates appeared to explain the relationship between depression, anxiety, and these cancer types, the investigators reported.
The findings challenge a common theory that depression and anxiety increase cancer risk and should “change current thinking,” they argue.
“Our results may come as a relief to many patients with cancer who believe their diagnosis is attributed to previous anxiety or depression,” first author Lonneke A. van Tuijl, PhD, of the University of Groningen and Utrecht University, the Netherlands, noted in a press release.
Analyses included data from up to nearly 320,000 individuals from the 18 prospective cohorts included in the international Psychosocial Factors and Cancer Incidence (PSY-CA) consortium. The cohorts are from studies conducted in the Netherlands, United Kingdom, Norway, and Canada, and included 25,803 patients with cancer. During follow-up of up to 26 years and more than 3.2 million person-years, depression and anxiety symptoms and diagnoses showed no association with overall breast, prostate, colorectal, and alcohol-related cancers (hazard ratios, 0.98-1.05).
For the specific cancer types, the investigators “found no evidence for an association between depression or anxiety and the incidence of colorectal cancer (HRs, 0.88-1.13), prostate cancer (HRs, 0.97-1.17), or alcohol-related cancers (HRs, 0.97-1.06).”
“For breast cancer, all pooled HRs were consistently negative but mean pooled HRs were close to 1 (HRs, 0.92-0.98) and the upper limit of the 95% confidence intervals all exceeded 1 (with the exception of anxiety symptoms),” they noted.
An increase in risk observed between depression and anxiety symptoms and diagnoses and lung cancer (HRs, 1.12-1.60) and smoking-related cancers (HRs, 1.06-1.60), in minimally adjusted models, was substantially attenuated after adjusting for known risk factors such as smoking, alcohol use, and body mass index (HRs, 1.04-1.08), the investigators reported.
The findings were published online in Cancer.
“Depression and anxiety have long been hypothesized to increase the risk for cancer. It is thought that the increased cancer risk can occur via several pathways, including health behaviors, or by influencing mutation, viral oncogenes, cell proliferation, or DNA repair,” the authors explained, noting that “[c]onclusions drawn in meta-analyses vary greatly, with some supporting an association between depression, anxiety, and cancer incidence and others finding no or a negligible association.”
The current findings “may help health professionals to alleviate feelings of guilt and self-blame in patients with cancer who attribute their diagnosis to previous depression or anxiety,” they said, noting that the findings “also underscore the importance of addressing tobacco smoking and other unhealthy behaviors – including those that may develop as a result of anxiety or depression.”
“However, further research is needed to understand exactly how depression, anxiety, health behaviors, and lung cancer are related,” said Dr. Tuijl.
Dr. Tuijl has received grants and travel support from the Dutch Cancer Society (KWF).
from a large, individual participant data meta-analysis.
An exception was for lung and smoking-related cancers, but key covariates appeared to explain the relationship between depression, anxiety, and these cancer types, the investigators reported.
The findings challenge a common theory that depression and anxiety increase cancer risk and should “change current thinking,” they argue.
“Our results may come as a relief to many patients with cancer who believe their diagnosis is attributed to previous anxiety or depression,” first author Lonneke A. van Tuijl, PhD, of the University of Groningen and Utrecht University, the Netherlands, noted in a press release.
Analyses included data from up to nearly 320,000 individuals from the 18 prospective cohorts included in the international Psychosocial Factors and Cancer Incidence (PSY-CA) consortium. The cohorts are from studies conducted in the Netherlands, United Kingdom, Norway, and Canada, and included 25,803 patients with cancer. During follow-up of up to 26 years and more than 3.2 million person-years, depression and anxiety symptoms and diagnoses showed no association with overall breast, prostate, colorectal, and alcohol-related cancers (hazard ratios, 0.98-1.05).
For the specific cancer types, the investigators “found no evidence for an association between depression or anxiety and the incidence of colorectal cancer (HRs, 0.88-1.13), prostate cancer (HRs, 0.97-1.17), or alcohol-related cancers (HRs, 0.97-1.06).”
“For breast cancer, all pooled HRs were consistently negative but mean pooled HRs were close to 1 (HRs, 0.92-0.98) and the upper limit of the 95% confidence intervals all exceeded 1 (with the exception of anxiety symptoms),” they noted.
An increase in risk observed between depression and anxiety symptoms and diagnoses and lung cancer (HRs, 1.12-1.60) and smoking-related cancers (HRs, 1.06-1.60), in minimally adjusted models, was substantially attenuated after adjusting for known risk factors such as smoking, alcohol use, and body mass index (HRs, 1.04-1.08), the investigators reported.
The findings were published online in Cancer.
“Depression and anxiety have long been hypothesized to increase the risk for cancer. It is thought that the increased cancer risk can occur via several pathways, including health behaviors, or by influencing mutation, viral oncogenes, cell proliferation, or DNA repair,” the authors explained, noting that “[c]onclusions drawn in meta-analyses vary greatly, with some supporting an association between depression, anxiety, and cancer incidence and others finding no or a negligible association.”
The current findings “may help health professionals to alleviate feelings of guilt and self-blame in patients with cancer who attribute their diagnosis to previous depression or anxiety,” they said, noting that the findings “also underscore the importance of addressing tobacco smoking and other unhealthy behaviors – including those that may develop as a result of anxiety or depression.”
“However, further research is needed to understand exactly how depression, anxiety, health behaviors, and lung cancer are related,” said Dr. Tuijl.
Dr. Tuijl has received grants and travel support from the Dutch Cancer Society (KWF).
from a large, individual participant data meta-analysis.
An exception was for lung and smoking-related cancers, but key covariates appeared to explain the relationship between depression, anxiety, and these cancer types, the investigators reported.
The findings challenge a common theory that depression and anxiety increase cancer risk and should “change current thinking,” they argue.
“Our results may come as a relief to many patients with cancer who believe their diagnosis is attributed to previous anxiety or depression,” first author Lonneke A. van Tuijl, PhD, of the University of Groningen and Utrecht University, the Netherlands, noted in a press release.
Analyses included data from up to nearly 320,000 individuals from the 18 prospective cohorts included in the international Psychosocial Factors and Cancer Incidence (PSY-CA) consortium. The cohorts are from studies conducted in the Netherlands, United Kingdom, Norway, and Canada, and included 25,803 patients with cancer. During follow-up of up to 26 years and more than 3.2 million person-years, depression and anxiety symptoms and diagnoses showed no association with overall breast, prostate, colorectal, and alcohol-related cancers (hazard ratios, 0.98-1.05).
For the specific cancer types, the investigators “found no evidence for an association between depression or anxiety and the incidence of colorectal cancer (HRs, 0.88-1.13), prostate cancer (HRs, 0.97-1.17), or alcohol-related cancers (HRs, 0.97-1.06).”
“For breast cancer, all pooled HRs were consistently negative but mean pooled HRs were close to 1 (HRs, 0.92-0.98) and the upper limit of the 95% confidence intervals all exceeded 1 (with the exception of anxiety symptoms),” they noted.
An increase in risk observed between depression and anxiety symptoms and diagnoses and lung cancer (HRs, 1.12-1.60) and smoking-related cancers (HRs, 1.06-1.60), in minimally adjusted models, was substantially attenuated after adjusting for known risk factors such as smoking, alcohol use, and body mass index (HRs, 1.04-1.08), the investigators reported.
The findings were published online in Cancer.
“Depression and anxiety have long been hypothesized to increase the risk for cancer. It is thought that the increased cancer risk can occur via several pathways, including health behaviors, or by influencing mutation, viral oncogenes, cell proliferation, or DNA repair,” the authors explained, noting that “[c]onclusions drawn in meta-analyses vary greatly, with some supporting an association between depression, anxiety, and cancer incidence and others finding no or a negligible association.”
The current findings “may help health professionals to alleviate feelings of guilt and self-blame in patients with cancer who attribute their diagnosis to previous depression or anxiety,” they said, noting that the findings “also underscore the importance of addressing tobacco smoking and other unhealthy behaviors – including those that may develop as a result of anxiety or depression.”
“However, further research is needed to understand exactly how depression, anxiety, health behaviors, and lung cancer are related,” said Dr. Tuijl.
Dr. Tuijl has received grants and travel support from the Dutch Cancer Society (KWF).
FROM CANCER
Even an hour’s walk a week lowers risk in type 2 diabetes
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM DIABETES CARE
When is antibiotic prophylaxis required for dermatologic surgery?
SAN DIEGO – The need for antibiotic prophylaxis in dermatologic surgery depends on the type of procedure, the patient, what infection you’re trying to keep at bay, and the type of wound, according to Tissa Hata, MD, professor of dermatology at the University of California, San Diego.
Among the many studies in the medical literature that have examined the use of antibiotics to prevent surgical site infections, one study published in 2006 has the largest number of patients to date, Dr. Hata said at a conference on superficial anatomy and cutaneous surgery sponsored by UCSD and Scripps Clinic. In the prospective study of wound infections in patients undergoing dermatologic surgery without prophylactic antibiotics, researchers in Australia prospectively examined 5,091 lesions, mostly nonmelanoma skin cancers, in 2,424 patients over the course of 3 years.
By procedure, the infection rate was highest for skin grafts (8.70%) and wedge excision of the lip or ear (8.57%), followed by skin flap repairs (2.94%), curettage (0.73%), and simple excision and closure (0.54%). By anatomic site, groin excisional surgery had the highest infection rate (10%), followed by surgical procedures below the knee (6.92%), while those performed on the face had a low rate (0.81%). “Based on their analysis, they suggest antibiotic prophylaxis for all procedures below the knee and groin, wedge excisions of the lip and ear, and all skin grafts,” Dr. Hata said.
In 2008, an advisory statement published in the Journal of the American Academy of Dermatology expanded the procedure location and techniques requiring antibiotic prophylaxis to include procedures on the nose and the lower extremity (especially the leg), and for patients with extensive inflammatory disease. According to the statement, in patients without a penicillin allergy, the suggested antibiotic prophylaxis regimen for wedge excision of the lip/ear, flaps on the nose, or all skin grafts include 2 g oral cephalexin or dicloxacillin. In patients with penicillin allergy, the recommended prophylaxis regimen for wedge excision of the lip/ear, flaps on the nose, or all skin grafts include 600 mg oral clindamycin or 500 mg oral azithromycin/clarithromycin.
In the statement, for patients with no penicillin allergy, the suggested prophylaxis regimen for lesions in the groin or on the lower extremities include 2 g oral cephalexin, 1 tablet of oral trimethoprim/sulfamethoxazole (TMP-SMX) DS, or 500 mg of levofloxacin. In patients with penicillin allergy, the recommended prophylaxis regimen for lesions on the groin and lower extremities is 1 tablet of TMP-SMX DS or 500 mg of levofloxacin.
In 2020, a meta-analysis of surgical site infections in patients undergoing Mohs surgery of the ear and nose found that there was no difference in infections in those locations whether patients received oral antibiotic prophylaxis or not. “But the researchers did not specify the type of closure, whether it was a graft or a flap closure,” Dr. Hata commented.
Endocarditis prophylaxis
Dr. Hata also discussed antibiotic recommendations for endocarditis prophylaxis, noting that the mortality rate from endocarditis is as high as 76%, and an estimated 40% of affected patients require heart valve replacement within 5-8 years. “But the good news is that fewer than 10 cases have been possibly linked to dermatologic procedures,” she said.
During outpatient dermatologic surgery, the incidence of bacteremia is in the range of 1.9%-3%, similar to the incidence of 2% that occurs spontaneously in healthy adults, according to Dr. Hata. She said that the following activities or procedures pose a much higher risk of bacteremia: mastication (17%-24%), tooth brushing (24%-40%), tooth extraction (60%-90%), and incision and drainage of an abscess (38%).
American Heart Association guidelines from 2007 recommend antibiotic prophylaxis in only the highest-risk categories of patients. These guidelines were updated in 2017 to include patients with transcatheter prosthetic valves and those with prosthetic material in valve repair. “The primary reason for revision of guidelines is that endocarditis is much more likely to result from frequent exposure to random bacteremia associated with daily activity such as brushing our teeth or having a tooth extracted,” Dr. Hata explained. “Prophylaxis may prevent an exceedingly small number of cases. Authors of the guidelines concluded that the risk of antibiotic-associated adverse event exceeds the benefit of prophylactic therapy, and that maintenance of optimal oral health is more important than prophylactic antibiotics.”
The 2017 AHA guidelines recommend antibiotic prophylaxis in patients with the following cardiac conditions: those with a prosthetic cardiac valve including transcatheter-implanted prostheses and homografts; those with previous endocarditis; those with prosthetic material used for heart valve repair, such as annuloplasty rings, chords or clips; cardiac transplantation recipients who develop cardiac valvulopathy; and those with certain types of congenital heart disease, including unrepaired cyanotic CHD, a completely repaired congenital heart defect with a prosthetic material or device during the first 6 months after the procedure, and repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device.
Procedures that may require prophylaxis for endocarditis include all dental procedures that involve manipulation of the gingival tissue or the periapical region of teeth or perforation of the oral mucosa, and respiratory tract procedures that involve incision or biopsy of the respiratory mucosa such as tonsillectomy or adenoidectomy. Antibiotic prophylaxis is not recommended for procedures involving the gastrointestinal tract or the genitourinary tract unless an active infection is present. As for skin procedures, the guidelines recommend antibiotic prophylaxis for patients in the high-risk category who undergo a surgical procedure that involves infected skin, skin structure, or musculoskeletal tissue.
In the 2017 AHA guidelines, patients with no penicillin allergy, the suggested antibiotic prophylaxis regimen for endocarditis in non-oral sites includes 2 g oral cephalexin or dicloxacillin, while in patients with penicillin allergy, the suggested prophylaxis for endocarditis in non-oral sites includes 600 mg oral clindamycin or 500 mg oral azithromycin/clarithromycin. In patients without a penicillin allergy, the suggested prophylaxis for endocarditis in oral sites is 2 g oral amoxicillin, while in those with penicillin allergy, the suggested antibiotic prophylaxis for endocarditis in oral sites is 500 mg azithromycin/clarithromycin or doxycycline 100 mg.
“Antibiotic prophylaxis for endocarditis should be given 30-60 minutes prior to surgery, and a follow-up dose of antibiotics is no longer recommended,” Dr. Hata said. “If you forget [to administer the antibiotics] or the patient forgets, antibiotics may be given up to 2 hours after the procedure.”
Dr. Hata reported having no relevant disclosures.
SAN DIEGO – The need for antibiotic prophylaxis in dermatologic surgery depends on the type of procedure, the patient, what infection you’re trying to keep at bay, and the type of wound, according to Tissa Hata, MD, professor of dermatology at the University of California, San Diego.
Among the many studies in the medical literature that have examined the use of antibiotics to prevent surgical site infections, one study published in 2006 has the largest number of patients to date, Dr. Hata said at a conference on superficial anatomy and cutaneous surgery sponsored by UCSD and Scripps Clinic. In the prospective study of wound infections in patients undergoing dermatologic surgery without prophylactic antibiotics, researchers in Australia prospectively examined 5,091 lesions, mostly nonmelanoma skin cancers, in 2,424 patients over the course of 3 years.
By procedure, the infection rate was highest for skin grafts (8.70%) and wedge excision of the lip or ear (8.57%), followed by skin flap repairs (2.94%), curettage (0.73%), and simple excision and closure (0.54%). By anatomic site, groin excisional surgery had the highest infection rate (10%), followed by surgical procedures below the knee (6.92%), while those performed on the face had a low rate (0.81%). “Based on their analysis, they suggest antibiotic prophylaxis for all procedures below the knee and groin, wedge excisions of the lip and ear, and all skin grafts,” Dr. Hata said.
In 2008, an advisory statement published in the Journal of the American Academy of Dermatology expanded the procedure location and techniques requiring antibiotic prophylaxis to include procedures on the nose and the lower extremity (especially the leg), and for patients with extensive inflammatory disease. According to the statement, in patients without a penicillin allergy, the suggested antibiotic prophylaxis regimen for wedge excision of the lip/ear, flaps on the nose, or all skin grafts include 2 g oral cephalexin or dicloxacillin. In patients with penicillin allergy, the recommended prophylaxis regimen for wedge excision of the lip/ear, flaps on the nose, or all skin grafts include 600 mg oral clindamycin or 500 mg oral azithromycin/clarithromycin.
In the statement, for patients with no penicillin allergy, the suggested prophylaxis regimen for lesions in the groin or on the lower extremities include 2 g oral cephalexin, 1 tablet of oral trimethoprim/sulfamethoxazole (TMP-SMX) DS, or 500 mg of levofloxacin. In patients with penicillin allergy, the recommended prophylaxis regimen for lesions on the groin and lower extremities is 1 tablet of TMP-SMX DS or 500 mg of levofloxacin.
In 2020, a meta-analysis of surgical site infections in patients undergoing Mohs surgery of the ear and nose found that there was no difference in infections in those locations whether patients received oral antibiotic prophylaxis or not. “But the researchers did not specify the type of closure, whether it was a graft or a flap closure,” Dr. Hata commented.
Endocarditis prophylaxis
Dr. Hata also discussed antibiotic recommendations for endocarditis prophylaxis, noting that the mortality rate from endocarditis is as high as 76%, and an estimated 40% of affected patients require heart valve replacement within 5-8 years. “But the good news is that fewer than 10 cases have been possibly linked to dermatologic procedures,” she said.
During outpatient dermatologic surgery, the incidence of bacteremia is in the range of 1.9%-3%, similar to the incidence of 2% that occurs spontaneously in healthy adults, according to Dr. Hata. She said that the following activities or procedures pose a much higher risk of bacteremia: mastication (17%-24%), tooth brushing (24%-40%), tooth extraction (60%-90%), and incision and drainage of an abscess (38%).
American Heart Association guidelines from 2007 recommend antibiotic prophylaxis in only the highest-risk categories of patients. These guidelines were updated in 2017 to include patients with transcatheter prosthetic valves and those with prosthetic material in valve repair. “The primary reason for revision of guidelines is that endocarditis is much more likely to result from frequent exposure to random bacteremia associated with daily activity such as brushing our teeth or having a tooth extracted,” Dr. Hata explained. “Prophylaxis may prevent an exceedingly small number of cases. Authors of the guidelines concluded that the risk of antibiotic-associated adverse event exceeds the benefit of prophylactic therapy, and that maintenance of optimal oral health is more important than prophylactic antibiotics.”
The 2017 AHA guidelines recommend antibiotic prophylaxis in patients with the following cardiac conditions: those with a prosthetic cardiac valve including transcatheter-implanted prostheses and homografts; those with previous endocarditis; those with prosthetic material used for heart valve repair, such as annuloplasty rings, chords or clips; cardiac transplantation recipients who develop cardiac valvulopathy; and those with certain types of congenital heart disease, including unrepaired cyanotic CHD, a completely repaired congenital heart defect with a prosthetic material or device during the first 6 months after the procedure, and repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device.
Procedures that may require prophylaxis for endocarditis include all dental procedures that involve manipulation of the gingival tissue or the periapical region of teeth or perforation of the oral mucosa, and respiratory tract procedures that involve incision or biopsy of the respiratory mucosa such as tonsillectomy or adenoidectomy. Antibiotic prophylaxis is not recommended for procedures involving the gastrointestinal tract or the genitourinary tract unless an active infection is present. As for skin procedures, the guidelines recommend antibiotic prophylaxis for patients in the high-risk category who undergo a surgical procedure that involves infected skin, skin structure, or musculoskeletal tissue.
In the 2017 AHA guidelines, patients with no penicillin allergy, the suggested antibiotic prophylaxis regimen for endocarditis in non-oral sites includes 2 g oral cephalexin or dicloxacillin, while in patients with penicillin allergy, the suggested prophylaxis for endocarditis in non-oral sites includes 600 mg oral clindamycin or 500 mg oral azithromycin/clarithromycin. In patients without a penicillin allergy, the suggested prophylaxis for endocarditis in oral sites is 2 g oral amoxicillin, while in those with penicillin allergy, the suggested antibiotic prophylaxis for endocarditis in oral sites is 500 mg azithromycin/clarithromycin or doxycycline 100 mg.
“Antibiotic prophylaxis for endocarditis should be given 30-60 minutes prior to surgery, and a follow-up dose of antibiotics is no longer recommended,” Dr. Hata said. “If you forget [to administer the antibiotics] or the patient forgets, antibiotics may be given up to 2 hours after the procedure.”
Dr. Hata reported having no relevant disclosures.
SAN DIEGO – The need for antibiotic prophylaxis in dermatologic surgery depends on the type of procedure, the patient, what infection you’re trying to keep at bay, and the type of wound, according to Tissa Hata, MD, professor of dermatology at the University of California, San Diego.
Among the many studies in the medical literature that have examined the use of antibiotics to prevent surgical site infections, one study published in 2006 has the largest number of patients to date, Dr. Hata said at a conference on superficial anatomy and cutaneous surgery sponsored by UCSD and Scripps Clinic. In the prospective study of wound infections in patients undergoing dermatologic surgery without prophylactic antibiotics, researchers in Australia prospectively examined 5,091 lesions, mostly nonmelanoma skin cancers, in 2,424 patients over the course of 3 years.
By procedure, the infection rate was highest for skin grafts (8.70%) and wedge excision of the lip or ear (8.57%), followed by skin flap repairs (2.94%), curettage (0.73%), and simple excision and closure (0.54%). By anatomic site, groin excisional surgery had the highest infection rate (10%), followed by surgical procedures below the knee (6.92%), while those performed on the face had a low rate (0.81%). “Based on their analysis, they suggest antibiotic prophylaxis for all procedures below the knee and groin, wedge excisions of the lip and ear, and all skin grafts,” Dr. Hata said.
In 2008, an advisory statement published in the Journal of the American Academy of Dermatology expanded the procedure location and techniques requiring antibiotic prophylaxis to include procedures on the nose and the lower extremity (especially the leg), and for patients with extensive inflammatory disease. According to the statement, in patients without a penicillin allergy, the suggested antibiotic prophylaxis regimen for wedge excision of the lip/ear, flaps on the nose, or all skin grafts include 2 g oral cephalexin or dicloxacillin. In patients with penicillin allergy, the recommended prophylaxis regimen for wedge excision of the lip/ear, flaps on the nose, or all skin grafts include 600 mg oral clindamycin or 500 mg oral azithromycin/clarithromycin.
In the statement, for patients with no penicillin allergy, the suggested prophylaxis regimen for lesions in the groin or on the lower extremities include 2 g oral cephalexin, 1 tablet of oral trimethoprim/sulfamethoxazole (TMP-SMX) DS, or 500 mg of levofloxacin. In patients with penicillin allergy, the recommended prophylaxis regimen for lesions on the groin and lower extremities is 1 tablet of TMP-SMX DS or 500 mg of levofloxacin.
In 2020, a meta-analysis of surgical site infections in patients undergoing Mohs surgery of the ear and nose found that there was no difference in infections in those locations whether patients received oral antibiotic prophylaxis or not. “But the researchers did not specify the type of closure, whether it was a graft or a flap closure,” Dr. Hata commented.
Endocarditis prophylaxis
Dr. Hata also discussed antibiotic recommendations for endocarditis prophylaxis, noting that the mortality rate from endocarditis is as high as 76%, and an estimated 40% of affected patients require heart valve replacement within 5-8 years. “But the good news is that fewer than 10 cases have been possibly linked to dermatologic procedures,” she said.
During outpatient dermatologic surgery, the incidence of bacteremia is in the range of 1.9%-3%, similar to the incidence of 2% that occurs spontaneously in healthy adults, according to Dr. Hata. She said that the following activities or procedures pose a much higher risk of bacteremia: mastication (17%-24%), tooth brushing (24%-40%), tooth extraction (60%-90%), and incision and drainage of an abscess (38%).
American Heart Association guidelines from 2007 recommend antibiotic prophylaxis in only the highest-risk categories of patients. These guidelines were updated in 2017 to include patients with transcatheter prosthetic valves and those with prosthetic material in valve repair. “The primary reason for revision of guidelines is that endocarditis is much more likely to result from frequent exposure to random bacteremia associated with daily activity such as brushing our teeth or having a tooth extracted,” Dr. Hata explained. “Prophylaxis may prevent an exceedingly small number of cases. Authors of the guidelines concluded that the risk of antibiotic-associated adverse event exceeds the benefit of prophylactic therapy, and that maintenance of optimal oral health is more important than prophylactic antibiotics.”
The 2017 AHA guidelines recommend antibiotic prophylaxis in patients with the following cardiac conditions: those with a prosthetic cardiac valve including transcatheter-implanted prostheses and homografts; those with previous endocarditis; those with prosthetic material used for heart valve repair, such as annuloplasty rings, chords or clips; cardiac transplantation recipients who develop cardiac valvulopathy; and those with certain types of congenital heart disease, including unrepaired cyanotic CHD, a completely repaired congenital heart defect with a prosthetic material or device during the first 6 months after the procedure, and repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device.
Procedures that may require prophylaxis for endocarditis include all dental procedures that involve manipulation of the gingival tissue or the periapical region of teeth or perforation of the oral mucosa, and respiratory tract procedures that involve incision or biopsy of the respiratory mucosa such as tonsillectomy or adenoidectomy. Antibiotic prophylaxis is not recommended for procedures involving the gastrointestinal tract or the genitourinary tract unless an active infection is present. As for skin procedures, the guidelines recommend antibiotic prophylaxis for patients in the high-risk category who undergo a surgical procedure that involves infected skin, skin structure, or musculoskeletal tissue.
In the 2017 AHA guidelines, patients with no penicillin allergy, the suggested antibiotic prophylaxis regimen for endocarditis in non-oral sites includes 2 g oral cephalexin or dicloxacillin, while in patients with penicillin allergy, the suggested prophylaxis for endocarditis in non-oral sites includes 600 mg oral clindamycin or 500 mg oral azithromycin/clarithromycin. In patients without a penicillin allergy, the suggested prophylaxis for endocarditis in oral sites is 2 g oral amoxicillin, while in those with penicillin allergy, the suggested antibiotic prophylaxis for endocarditis in oral sites is 500 mg azithromycin/clarithromycin or doxycycline 100 mg.
“Antibiotic prophylaxis for endocarditis should be given 30-60 minutes prior to surgery, and a follow-up dose of antibiotics is no longer recommended,” Dr. Hata said. “If you forget [to administer the antibiotics] or the patient forgets, antibiotics may be given up to 2 hours after the procedure.”
Dr. Hata reported having no relevant disclosures.
AT A CONFERENCE ON SUPERFICIAL ANATOMY AND CUTANEOUS SURGERY
Interstitial lung disease plus pulmonary hypertension equals poor outcomes in systemic sclerosis
, based on data from more than 3,000 individuals.
Pulmonary complications are now the most common causes of death in adults with systemic sclerosis (SSc), but the impact of patient characteristics and risk factors such as interstitial lung disease (ILD) and pulmonary hypertension (PH) on SSc outcomes remains unclear, wrote Pia Moinzadeh, MD, of University Hospital Cologne (Germany) and colleagues.
Although the role of ILD and PH in different SSc subtypes has been studied, larger studies of the effects of ILD and combining ILD and PH on outcomes are needed, since survival rates can change over time with new classification criteria, diagnostic tools, and improved therapies, they said.
In a study published in the journal Chest, the researchers reviewed data from 3,257 adults aged 18 years and older with SSc over a mean follow-up of 3.45 years. Participants were part of the German Network for Systemic Sclerosis (DNSS) that included 25 clinical centers in Germany. The participants were divided into SSc subsets: 54.2% with limited cutaneous SSc (lcSSc), 31.4% with diffuse cutaneous SSc (dcSSc), and 14.4% SSc overlapping syndromes.
The baseline prevalence of ILD was 34.5%, including 200 patients with ILD-PH and 923 with ILD but without PH. The baseline prevalence of PH without ILD was 4.5%. ILD was defined as SSc associated when other causes were excluded. PH was defined as an increase in mean arterial pressure of at least 25 mm Hg at rest, and also was defined by an estimated right ventricular systolic pressure greater than 35 mm Hg based on echocardiography.
By the end of the study period, 47.6% of SSc patients had ILD, 15.2% had ILD-PH, and 6.5% had pulmonary arterial hypertension (PAH). Of the SSc patients with ILD, 57.3% had dcSSc; the prevalence of PAH was not significantly different between the SSc subtypes. Patients with dcSSc were more likely to develop ILD-PH (52.2%) and ILD without PH (52.1%); patients with lcSSc were more likely to have PAH (64.9%) or no pulmonary involvement (64.1%).
“For all subsets, a significant increase in the frequency of SSc-ILD was observed during follow-ups,” the researchers noted.
Overall survival at 5 years was worst for patients with both ILD and PH (79.1%). Five-year OS for patients with PAH was 85.0%. OS at 5 years was significantly better for patients with ILD without PH (92.8%) and those with no pulmonary involvement (96.4%), compared with the ILD and PH patients (P < 0.001).
In a multivariate analysis, the risk of death was more than five times higher for patients with ILD-PH, compared with the reference group of patients without pulmonary involvement (hazard ratio, 5.3). Factors associated with reduced risk of death included female sex (HR, 0.3), higher body mass index (HR, 0.9), and higher diffusing capacity of the lung for carbon monoxide (HR, 0.98).
The findings were limited by several factors including the incomplete data for patients enrolled early in the registry, lack of complete radiology data, and the inability to determine whether the association between pulmonary involvement and survival was related to ILD or to pulmonary vascular disease, the researchers noted.
However, the results suggest that a combination of ILD and PH is the main predictor of death in patients with SSc and ILD, although the overall survival for SSc patients with and without pulmonary involvement has improved in recent decades thanks to improved therapies, multidisciplinary care, and greater attention to the disease worldwide, they concluded.
The study received no outside funding. Dr. Moinzadeh disclosed lecture fees from Boehringer Ingelheim.
, based on data from more than 3,000 individuals.
Pulmonary complications are now the most common causes of death in adults with systemic sclerosis (SSc), but the impact of patient characteristics and risk factors such as interstitial lung disease (ILD) and pulmonary hypertension (PH) on SSc outcomes remains unclear, wrote Pia Moinzadeh, MD, of University Hospital Cologne (Germany) and colleagues.
Although the role of ILD and PH in different SSc subtypes has been studied, larger studies of the effects of ILD and combining ILD and PH on outcomes are needed, since survival rates can change over time with new classification criteria, diagnostic tools, and improved therapies, they said.
In a study published in the journal Chest, the researchers reviewed data from 3,257 adults aged 18 years and older with SSc over a mean follow-up of 3.45 years. Participants were part of the German Network for Systemic Sclerosis (DNSS) that included 25 clinical centers in Germany. The participants were divided into SSc subsets: 54.2% with limited cutaneous SSc (lcSSc), 31.4% with diffuse cutaneous SSc (dcSSc), and 14.4% SSc overlapping syndromes.
The baseline prevalence of ILD was 34.5%, including 200 patients with ILD-PH and 923 with ILD but without PH. The baseline prevalence of PH without ILD was 4.5%. ILD was defined as SSc associated when other causes were excluded. PH was defined as an increase in mean arterial pressure of at least 25 mm Hg at rest, and also was defined by an estimated right ventricular systolic pressure greater than 35 mm Hg based on echocardiography.
By the end of the study period, 47.6% of SSc patients had ILD, 15.2% had ILD-PH, and 6.5% had pulmonary arterial hypertension (PAH). Of the SSc patients with ILD, 57.3% had dcSSc; the prevalence of PAH was not significantly different between the SSc subtypes. Patients with dcSSc were more likely to develop ILD-PH (52.2%) and ILD without PH (52.1%); patients with lcSSc were more likely to have PAH (64.9%) or no pulmonary involvement (64.1%).
“For all subsets, a significant increase in the frequency of SSc-ILD was observed during follow-ups,” the researchers noted.
Overall survival at 5 years was worst for patients with both ILD and PH (79.1%). Five-year OS for patients with PAH was 85.0%. OS at 5 years was significantly better for patients with ILD without PH (92.8%) and those with no pulmonary involvement (96.4%), compared with the ILD and PH patients (P < 0.001).
In a multivariate analysis, the risk of death was more than five times higher for patients with ILD-PH, compared with the reference group of patients without pulmonary involvement (hazard ratio, 5.3). Factors associated with reduced risk of death included female sex (HR, 0.3), higher body mass index (HR, 0.9), and higher diffusing capacity of the lung for carbon monoxide (HR, 0.98).
The findings were limited by several factors including the incomplete data for patients enrolled early in the registry, lack of complete radiology data, and the inability to determine whether the association between pulmonary involvement and survival was related to ILD or to pulmonary vascular disease, the researchers noted.
However, the results suggest that a combination of ILD and PH is the main predictor of death in patients with SSc and ILD, although the overall survival for SSc patients with and without pulmonary involvement has improved in recent decades thanks to improved therapies, multidisciplinary care, and greater attention to the disease worldwide, they concluded.
The study received no outside funding. Dr. Moinzadeh disclosed lecture fees from Boehringer Ingelheim.
, based on data from more than 3,000 individuals.
Pulmonary complications are now the most common causes of death in adults with systemic sclerosis (SSc), but the impact of patient characteristics and risk factors such as interstitial lung disease (ILD) and pulmonary hypertension (PH) on SSc outcomes remains unclear, wrote Pia Moinzadeh, MD, of University Hospital Cologne (Germany) and colleagues.
Although the role of ILD and PH in different SSc subtypes has been studied, larger studies of the effects of ILD and combining ILD and PH on outcomes are needed, since survival rates can change over time with new classification criteria, diagnostic tools, and improved therapies, they said.
In a study published in the journal Chest, the researchers reviewed data from 3,257 adults aged 18 years and older with SSc over a mean follow-up of 3.45 years. Participants were part of the German Network for Systemic Sclerosis (DNSS) that included 25 clinical centers in Germany. The participants were divided into SSc subsets: 54.2% with limited cutaneous SSc (lcSSc), 31.4% with diffuse cutaneous SSc (dcSSc), and 14.4% SSc overlapping syndromes.
The baseline prevalence of ILD was 34.5%, including 200 patients with ILD-PH and 923 with ILD but without PH. The baseline prevalence of PH without ILD was 4.5%. ILD was defined as SSc associated when other causes were excluded. PH was defined as an increase in mean arterial pressure of at least 25 mm Hg at rest, and also was defined by an estimated right ventricular systolic pressure greater than 35 mm Hg based on echocardiography.
By the end of the study period, 47.6% of SSc patients had ILD, 15.2% had ILD-PH, and 6.5% had pulmonary arterial hypertension (PAH). Of the SSc patients with ILD, 57.3% had dcSSc; the prevalence of PAH was not significantly different between the SSc subtypes. Patients with dcSSc were more likely to develop ILD-PH (52.2%) and ILD without PH (52.1%); patients with lcSSc were more likely to have PAH (64.9%) or no pulmonary involvement (64.1%).
“For all subsets, a significant increase in the frequency of SSc-ILD was observed during follow-ups,” the researchers noted.
Overall survival at 5 years was worst for patients with both ILD and PH (79.1%). Five-year OS for patients with PAH was 85.0%. OS at 5 years was significantly better for patients with ILD without PH (92.8%) and those with no pulmonary involvement (96.4%), compared with the ILD and PH patients (P < 0.001).
In a multivariate analysis, the risk of death was more than five times higher for patients with ILD-PH, compared with the reference group of patients without pulmonary involvement (hazard ratio, 5.3). Factors associated with reduced risk of death included female sex (HR, 0.3), higher body mass index (HR, 0.9), and higher diffusing capacity of the lung for carbon monoxide (HR, 0.98).
The findings were limited by several factors including the incomplete data for patients enrolled early in the registry, lack of complete radiology data, and the inability to determine whether the association between pulmonary involvement and survival was related to ILD or to pulmonary vascular disease, the researchers noted.
However, the results suggest that a combination of ILD and PH is the main predictor of death in patients with SSc and ILD, although the overall survival for SSc patients with and without pulmonary involvement has improved in recent decades thanks to improved therapies, multidisciplinary care, and greater attention to the disease worldwide, they concluded.
The study received no outside funding. Dr. Moinzadeh disclosed lecture fees from Boehringer Ingelheim.
FROM THE JOURNAL CHEST
PTSD: Written exposure therapy matches prolonged exposure therapy
Investigators also found that participants randomly assigned to receive WET were significantly less likely to drop out of treatment than those receiving PE.
Written exposure therapy involves writing about thoughts and feelings during a specific traumatic event during five supervised, 30-minute sessions and discussing the writing process with the therapist supervising the sessions.
In the latter sessions, the participant talks about how the event affected them.
“Clinicians should consider using WET in their practices as some clients would prefer a shorter treatment approach, and it may be the only option for some clients – for instance, those who have limited time for therapy and may not be able to do a longer treatment,” study investigator Denise Sloan, PhD, said in an interview.
She also noted that WET is covered by insurance and that “most providers I know indicate that they list it as CBT [cognitive-behavioral therapy] code to insurance companies.”
Sloan is senior clinician investigator of the National Center for PTSD at VA Boston Healthcare System and professor of psychiatry at Boston University.
The findings were published online in JAMA Psychiatry.
High attrition rates
The disadvantage to the three major types of therapy used most often to treat PTSD in veterans – eye movement desensitization and reprocessing, cognitive processing therapy (CPT), and PE – are the dropout rates, that range from 18% to as high as 50%.
Prior studies have shown that WET is briefer and just as effective as CPT, but investigators noted that it had never been tested against PE in a randomized clinical trial.
To find out how the two types of therapy compare, Dr. Sloan and associates randomized 178 veterans with PTSD from three VA centers – Boston; Charleston, S.C.; and Madison, Wisc. – to receive either WET or PE.
PE consisted of 8-15 90-minute therapy sessions during which participants imagine the most distressing aspect of their traumatic memory, and between sessions, they confront the people, places, or situations they have been avoiding because of the trauma.
The investigators used the Structured Clinical Interview for DSM-5 at baseline to screen participants at high risk for suicide, comorbid substance use disorder, and unstable bipolar disorder, who were excluded from the study.
At baseline, 10, 20, and 30 weeks after the first treatment session, the investigators measured the severity of each patient’s PTSD symptoms with the Clinician-Administered PTSD Scale for DSM-5, which has a range of 0 (no PTSD symptoms) to 80 (most severe PTSD symptoms).
Of the 178 veterans, 134 were men, and their mean age was 45 years. The majority (63%) was White, while 21% were Black.
The researchers found that study participants were not significantly more likely to meet PTSD diagnostic criteria in the WET or PE conditions at any assessment.
WET briefer, better retention
Investigators noted the largest difference in PTSD scores in favor of WET at the 10-month assessment: The mean score for those receiving WET was 27.7, and the mean score for those receiving PE was 30.1 (odds ratio, 0.72; 95% CI, 0.35-1.46).
Among those who finished treatment, the mean number of treatment sessions was 12.5 for PE and 6 for WET.
Participants assigned to receive PE were significantly more likely to drop out of the study prematurely; 32 (35.6%) dropped out, compared with 11 (12.5%) participants assigned to WET.
Notably, of the 32 participants who dropped out of PE, 30 did so by session 7, so the increased dropout in PE was not related to the greater number of sessions, the investigators noted.
They added that findings could have been limited by stressors related to the global COVID-19 pandemic, which was taking place during the treatment, and the fact that all of the participants were veterans, which could limit the generalizability of the findings.
In an editorial, Charles Taylor, PhD, and Murray Stein, MD, MPH, both from the department of psychiatry at the University of California, San Diego, wrote that “WET achieved comparable reductions in PTSD symptoms through fewer sessions, shorter duration sessions, less therapist involvement, and no explicit prescription of homework.
“These findings should galvanize the psychotherapy field to design parsimonious treatments from the start, systematically testing the effects of different dose parameters,” they concluded.
The study was supported by the VA. Dr. Sloan reported receiving royalty payments for the published Written Exposure Therapy manual from the American Psychological Association outside the submitted work.
A version of this article appeared on Medscape.com.
Investigators also found that participants randomly assigned to receive WET were significantly less likely to drop out of treatment than those receiving PE.
Written exposure therapy involves writing about thoughts and feelings during a specific traumatic event during five supervised, 30-minute sessions and discussing the writing process with the therapist supervising the sessions.
In the latter sessions, the participant talks about how the event affected them.
“Clinicians should consider using WET in their practices as some clients would prefer a shorter treatment approach, and it may be the only option for some clients – for instance, those who have limited time for therapy and may not be able to do a longer treatment,” study investigator Denise Sloan, PhD, said in an interview.
She also noted that WET is covered by insurance and that “most providers I know indicate that they list it as CBT [cognitive-behavioral therapy] code to insurance companies.”
Sloan is senior clinician investigator of the National Center for PTSD at VA Boston Healthcare System and professor of psychiatry at Boston University.
The findings were published online in JAMA Psychiatry.
High attrition rates
The disadvantage to the three major types of therapy used most often to treat PTSD in veterans – eye movement desensitization and reprocessing, cognitive processing therapy (CPT), and PE – are the dropout rates, that range from 18% to as high as 50%.
Prior studies have shown that WET is briefer and just as effective as CPT, but investigators noted that it had never been tested against PE in a randomized clinical trial.
To find out how the two types of therapy compare, Dr. Sloan and associates randomized 178 veterans with PTSD from three VA centers – Boston; Charleston, S.C.; and Madison, Wisc. – to receive either WET or PE.
PE consisted of 8-15 90-minute therapy sessions during which participants imagine the most distressing aspect of their traumatic memory, and between sessions, they confront the people, places, or situations they have been avoiding because of the trauma.
The investigators used the Structured Clinical Interview for DSM-5 at baseline to screen participants at high risk for suicide, comorbid substance use disorder, and unstable bipolar disorder, who were excluded from the study.
At baseline, 10, 20, and 30 weeks after the first treatment session, the investigators measured the severity of each patient’s PTSD symptoms with the Clinician-Administered PTSD Scale for DSM-5, which has a range of 0 (no PTSD symptoms) to 80 (most severe PTSD symptoms).
Of the 178 veterans, 134 were men, and their mean age was 45 years. The majority (63%) was White, while 21% were Black.
The researchers found that study participants were not significantly more likely to meet PTSD diagnostic criteria in the WET or PE conditions at any assessment.
WET briefer, better retention
Investigators noted the largest difference in PTSD scores in favor of WET at the 10-month assessment: The mean score for those receiving WET was 27.7, and the mean score for those receiving PE was 30.1 (odds ratio, 0.72; 95% CI, 0.35-1.46).
Among those who finished treatment, the mean number of treatment sessions was 12.5 for PE and 6 for WET.
Participants assigned to receive PE were significantly more likely to drop out of the study prematurely; 32 (35.6%) dropped out, compared with 11 (12.5%) participants assigned to WET.
Notably, of the 32 participants who dropped out of PE, 30 did so by session 7, so the increased dropout in PE was not related to the greater number of sessions, the investigators noted.
They added that findings could have been limited by stressors related to the global COVID-19 pandemic, which was taking place during the treatment, and the fact that all of the participants were veterans, which could limit the generalizability of the findings.
In an editorial, Charles Taylor, PhD, and Murray Stein, MD, MPH, both from the department of psychiatry at the University of California, San Diego, wrote that “WET achieved comparable reductions in PTSD symptoms through fewer sessions, shorter duration sessions, less therapist involvement, and no explicit prescription of homework.
“These findings should galvanize the psychotherapy field to design parsimonious treatments from the start, systematically testing the effects of different dose parameters,” they concluded.
The study was supported by the VA. Dr. Sloan reported receiving royalty payments for the published Written Exposure Therapy manual from the American Psychological Association outside the submitted work.
A version of this article appeared on Medscape.com.
Investigators also found that participants randomly assigned to receive WET were significantly less likely to drop out of treatment than those receiving PE.
Written exposure therapy involves writing about thoughts and feelings during a specific traumatic event during five supervised, 30-minute sessions and discussing the writing process with the therapist supervising the sessions.
In the latter sessions, the participant talks about how the event affected them.
“Clinicians should consider using WET in their practices as some clients would prefer a shorter treatment approach, and it may be the only option for some clients – for instance, those who have limited time for therapy and may not be able to do a longer treatment,” study investigator Denise Sloan, PhD, said in an interview.
She also noted that WET is covered by insurance and that “most providers I know indicate that they list it as CBT [cognitive-behavioral therapy] code to insurance companies.”
Sloan is senior clinician investigator of the National Center for PTSD at VA Boston Healthcare System and professor of psychiatry at Boston University.
The findings were published online in JAMA Psychiatry.
High attrition rates
The disadvantage to the three major types of therapy used most often to treat PTSD in veterans – eye movement desensitization and reprocessing, cognitive processing therapy (CPT), and PE – are the dropout rates, that range from 18% to as high as 50%.
Prior studies have shown that WET is briefer and just as effective as CPT, but investigators noted that it had never been tested against PE in a randomized clinical trial.
To find out how the two types of therapy compare, Dr. Sloan and associates randomized 178 veterans with PTSD from three VA centers – Boston; Charleston, S.C.; and Madison, Wisc. – to receive either WET or PE.
PE consisted of 8-15 90-minute therapy sessions during which participants imagine the most distressing aspect of their traumatic memory, and between sessions, they confront the people, places, or situations they have been avoiding because of the trauma.
The investigators used the Structured Clinical Interview for DSM-5 at baseline to screen participants at high risk for suicide, comorbid substance use disorder, and unstable bipolar disorder, who were excluded from the study.
At baseline, 10, 20, and 30 weeks after the first treatment session, the investigators measured the severity of each patient’s PTSD symptoms with the Clinician-Administered PTSD Scale for DSM-5, which has a range of 0 (no PTSD symptoms) to 80 (most severe PTSD symptoms).
Of the 178 veterans, 134 were men, and their mean age was 45 years. The majority (63%) was White, while 21% were Black.
The researchers found that study participants were not significantly more likely to meet PTSD diagnostic criteria in the WET or PE conditions at any assessment.
WET briefer, better retention
Investigators noted the largest difference in PTSD scores in favor of WET at the 10-month assessment: The mean score for those receiving WET was 27.7, and the mean score for those receiving PE was 30.1 (odds ratio, 0.72; 95% CI, 0.35-1.46).
Among those who finished treatment, the mean number of treatment sessions was 12.5 for PE and 6 for WET.
Participants assigned to receive PE were significantly more likely to drop out of the study prematurely; 32 (35.6%) dropped out, compared with 11 (12.5%) participants assigned to WET.
Notably, of the 32 participants who dropped out of PE, 30 did so by session 7, so the increased dropout in PE was not related to the greater number of sessions, the investigators noted.
They added that findings could have been limited by stressors related to the global COVID-19 pandemic, which was taking place during the treatment, and the fact that all of the participants were veterans, which could limit the generalizability of the findings.
In an editorial, Charles Taylor, PhD, and Murray Stein, MD, MPH, both from the department of psychiatry at the University of California, San Diego, wrote that “WET achieved comparable reductions in PTSD symptoms through fewer sessions, shorter duration sessions, less therapist involvement, and no explicit prescription of homework.
“These findings should galvanize the psychotherapy field to design parsimonious treatments from the start, systematically testing the effects of different dose parameters,” they concluded.
The study was supported by the VA. Dr. Sloan reported receiving royalty payments for the published Written Exposure Therapy manual from the American Psychological Association outside the submitted work.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
CTE common among young athletes in largest brain donor study
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Analysis of brain tissue from athletes who were exposed to RHIs and died before the age of 30 revealed neuropathological evidence of shrinkage of the brain and microscopic changes that indicate a breach of the blood-brain barrier. The case series also identified the first known American female athlete with CTE.
Nearly all of those with CTE had a mild form of the disease and 71% played only at the amateur level in youth, high school, or college sports.
“A lot of people think CTE is a result of high-level, professional play such as football, ice hockey, and boxing, but it can affect amateur athletes and can affect people at a young age,” lead author Ann McKee, MD, professor of neurology and pathology and director of the Chronic Traumatic Encephalopathy Center at Boston University, said in an interview.
The findings were published online in JAMA Neurology.
A rare look
Brain donation at younger ages is rare, so most of what is known about CTE comes from studies in older athletes.
“We’ve always known that young people could develop this disease early after just amateur high school, youth, and college exposure, but this is the largest study of donor brains at this age,” Dr. McKee said.
The case series included 152 brains of athletes who played contact sports, experienced RHIs, and died before age 30. The tissues are part of the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank and were donated between February 2008 and September 2022.
Researchers reviewed the donors’ medical records and conducted retrospective interviews with the donors’ next of kin to assess cognitive symptoms, mood disturbances, and neurobehavioral issues.
Donors died between the ages of 13 and 29 years, 92.8% were male and 73% were White. In 57.2% of the cases, suicide was the cause of death, with no difference between those with or without CTE.
CTE was neuropathologically diagnosed in 41.4% of athletes, using diagnostic criteria developed by the National Institute of Neurological Disorders and Stroke.
More than 95% had mild CTE. Diagnosis was associated with older age (mean difference, 3.92 years; P < .001) and significantly more years of exposure to contact sports (11.6 vs. 8.8 years).
Among those with CTE, 71.4% played amateur sports, including football (60.9%), soccer (17.2%), hockey (7.8%), and wrestling (7%).
The cohort includes the first known American female athlete with CTE. Recruiting female brain donors has always been a challenge, Dr. McKee said. In this study, females comprised about 7% of the entire cohort and tended to be younger and play fewer years of a sport, compared with their male counterparts. All of that could lower their risk for CTE, Dr. McKee said.
“We don’t have enough brain donations to make any comments about differences between the genders, but we’ve always known that women can develop CTE,” she said. “It’s been reported after domestic violence and in an autistic woman who was a headbanger, so it was just a matter of time before we found our first case.”
Early stage of CTE?
Neuropathological analysis revealed neuronal p-tau aggregates in all CTE cases, a hallmark of the disease.
Young athletes with CTE had significantly more ventricular dilatation, suggesting atrophy or shrinkage of the brain, and more cavum septum pellucidum.
“I was surprised that even at this very young age group we could see structural changes to the gross pathology,” Dr. McKee said.
Investigators also found evidence of perivascular macrophages in the deep white matter, a microscopic change that correlated with CTE and years of play and indicates a breach of the blood-brain barrier that could allow pro-inflammatory molecules to enter the brain, setting up a neuroinflammatory response.
“Neuroinflammation is a very early change after repetitive head impacts, as well as in CTE,” Dr. McKee said. “This may be one of the mechanisms by which the inflammation starts, meaning microvascular injury might be an integral part of the pathogenesis of CTE.”
A message for clinicians
All athletes had symptoms of mood and neurobehavioral dysfunction common in people with RHIs. There were no significant differences in those clinical symptoms based on CTE diagnosis, which is likely related to the retrospective nature of the clinical evaluations, Dr. McKee said.
While the study leaves many questions about CTE in younger athletes unanswered, there is a message for clinicians and for patients in the findings, she said.
For clinicians, it’s important to note that “this young population of amateur athletes can be very symptomatic, and in all likelihood, a lot of these symptoms are reversible with proper care and management,” Dr. McKee said.
“For individual athletes, it’s important to note that 58% of this cohort did not have CTE, so just because you have these symptoms is not an indication that you have a neurodegenerative disease,” she added.
The study was funded by Andlinger Foundation, the National Football League, Mac Parkman Foundation, National Operating Committee on Standards for Athletic Equipment, and the Nick and Lynn Buoniconti Foundation, World Wrestling Entertainment, Alzheimer’s Association, National Institutes of Health, Concussion Legacy Foundation, U.S. Department of Defense and the U.S. Department of Veterans Affairs. Dr. McKee is a member of the Mackey-White Health and Safety Committee of the National Football League Players Association and reported receiving grants from the NIH and Department of Veteran Affairs and other funding from the Buoniconti Foundation and Mac Parkman Foundation during the conduct of the study.
A version of this article appeared on Medscape.com.
Advanced HF no obstacle to AFib ablation success: CASTLE-HTx
Catheter ablation had long taken atrial fibrillation (AF) rhythm control to the next level before clinical trials showed it could help keep AF patients with heart failure (HF) alive and out of the hospital.
But those trials didn’t include many patients with AF on top of advanced or even end-stage HF. Lacking much of an evidence base and often viewed as too sick to gain a lot from the procedure, patients with AF and advanced HF aren’t offered ablation very often.
Now a randomized trial suggests that, on the contrary, AF ablation may confer a similar benefit to patients with HF so advanced that they were referred for evaluation at a transplant center.
The study, modestly sized with fewer than 200 such patients and conducted at a single center, assigned half of them to receive ablation and the other half to continued medical management.
Risk for the composite primary endpoint plunged 76% over a median of 18 months for those who underwent ablation. The outcome comprised death from any cause, implantation of a left ventricular assist device (LVAD), or urgent heart transplantation.
The advantage for ablation emerged early enough that the trial, CASTLE-HTx, was halted for benefit only a year after reaching its planned enrollment, observed Christian Sohns, MD, when formally presenting the results in Amsterdam at the annual congress of the European Society of Cardiology.
The difference in the primary endpoint “in this severely sick cohort of advanced, end-stage heart failure patients,” he said, was driven mostly by fewer deaths, especially cardiovascular deaths, in the ablation group.
Ablation’s effect on outcomes was associated, perhaps causally, with significant gains in left ventricular (LV) function and more than triple the reduction in AF burden seen in the control group, noted Dr. Sohns, from the Heart and Diabetes Center North-Rhine Westphalia, Bad Oeynhausen, Germany.
states the CASTLE-HTx primary report, published in the New England Journal of Medicine, with Dr. Sohns as lead author, in tandem with his ESC presentation.
One of the study’s key messages “is that AF ablation is safe and effective in patients with end-stage heart failure” and “should be part of our armamentarium” for treating them, said Philipp Sommer, MD, also with Heart and Diabetes Center North-Rhine Westphalia, at a press conference preceding Dr. Sohns’ presentation of CASTLE-HTx.
The intervention could potentially help such patients survive longer on transplant wait lists and even delay need for the surgery, proposed Dr. Sommer, who is senior author on the trial’s publication.
CASTLE-HTx suggests that patients with advanced HF and even persistent AF, “if they have reasonably small atria, should be actually considered for ablation, as it may prevent the need for heart transplant or LVAD implant,” said invited discussant Finn Gustafsson, MD, PhD, DMSc, after Dr. Sohns’ presentation. “And that, of course, would be a huge achievement.”
The trial “should, if anything, help eradicate the current somewhat nihilistic approach to atrial fibrillation management in patients with advanced heart failure,” said Dr. Gustafsson, medical director of cardiac transplantation and mechanical circulatory support, Rigshopsitalet Copenhagen University Hospital.
Still, he disputed the characterization by the investigators and indeed the published report that the patients, or most of them, had “end-stage heart failure.”
For example, about a third of the trial’s patients started out in NYHA class 2, Dr. Gustafsson noted. Not that they weren’t “high-risk” or their HF wasn’t severe, he offered, but they don’t seem to have been “a truly advanced heart failure population.”
The trial population consisted of “patients referred to an advanced heart failure center, rather than patients with advanced heart failure,” agreed Mandeep R. Mehra, MD, director of the Center for Advanced Heart Disease at Brigham and Woman’s Hospital, Boston.
Also citing a large prevalence of patients in NYHA class-2, Dr. Mehra added that “we almost never see paroxysmal atrial fib in these patients. It’s usually an early-stage phenomenon.” In advanced HF, AF “is usually permanent,” he told this news organization. Yet it was paroxysmal in about 30% of cases.
To its credit, Dr. Mehra observed, the study does assert that advanced HF is no reason, necessarily, to avoid catheter ablation. Nor should an AF patient’s referral to an advanced-HF center “mean that you should rush to an LVAD or transplant” before considering ablation.
The study seems to be saying, “please exhaust all options before you biologically replace the heart or put in an LVAD,” Dr. Mehra said. “Certainly, this paper steers you in that direction.”
The trial entered 194 patients with symptomatic AF and HF of at least NYHA class 2, with impaired functional capacity by the 6-minute walk test, who had been referred to a major center in Germany for a heart-transplantation workup. With all on guideline-directed medical therapy, 97 were randomly assigned open-label to catheter ablation and 97 to continued standard care.
Catheter ablation was actually carried out in 81 patients (84%) who had been assigned to it and in 16 (16%) of those in the control group, the report states.
A total of 8 in the ablation group and 29 in the control arm died, received an LVAD, or went to urgent transplantation, for a hazard ratio of 0.24 (95% confidence interval, 0.11-0.52; P < .001) for the primary endpoint.
Death from any cause apparently played a big role in the risk reduction; its HR was 0.29 (95% CI, 0.12-0.72).
One peculiarity of the data, Dr. Mehra said, is that event curves for the primary endpoint and its individual components “diverge almost from day 1.” That would mean the ablation group right away started having fewer deaths, LVAD placements, or heart transplants than the control group.
“It is surprising to see such a large effect size on endpoints that are very much dependent on operators and diverge within the first day.” Probably, Dr. Mehra said, “it has to do with this being a single-center study that may not be generalizable to other practices.”
CASTLE HTx was supported by a grant from Else Kröner-Fresenius-Stiftung. Dr. Sommer discloses consulting for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Dr. Sohns reported no relevant financial relationships. Dr. Gustafsson discloses receiving honoraria or fees for consulting from Abbott, Alnylam Amgen, Boehringer Ingelheim, Ionis, Novartis, and Pfizer; serving on a speakers bureau for Astra Zeneca and Orion; and receiving grants from Corvia Research. Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board for NuPulseCV, Leviticus, and FineHeart.
A version of this article first appeared on Medscape.com.
Catheter ablation had long taken atrial fibrillation (AF) rhythm control to the next level before clinical trials showed it could help keep AF patients with heart failure (HF) alive and out of the hospital.
But those trials didn’t include many patients with AF on top of advanced or even end-stage HF. Lacking much of an evidence base and often viewed as too sick to gain a lot from the procedure, patients with AF and advanced HF aren’t offered ablation very often.
Now a randomized trial suggests that, on the contrary, AF ablation may confer a similar benefit to patients with HF so advanced that they were referred for evaluation at a transplant center.
The study, modestly sized with fewer than 200 such patients and conducted at a single center, assigned half of them to receive ablation and the other half to continued medical management.
Risk for the composite primary endpoint plunged 76% over a median of 18 months for those who underwent ablation. The outcome comprised death from any cause, implantation of a left ventricular assist device (LVAD), or urgent heart transplantation.
The advantage for ablation emerged early enough that the trial, CASTLE-HTx, was halted for benefit only a year after reaching its planned enrollment, observed Christian Sohns, MD, when formally presenting the results in Amsterdam at the annual congress of the European Society of Cardiology.
The difference in the primary endpoint “in this severely sick cohort of advanced, end-stage heart failure patients,” he said, was driven mostly by fewer deaths, especially cardiovascular deaths, in the ablation group.
Ablation’s effect on outcomes was associated, perhaps causally, with significant gains in left ventricular (LV) function and more than triple the reduction in AF burden seen in the control group, noted Dr. Sohns, from the Heart and Diabetes Center North-Rhine Westphalia, Bad Oeynhausen, Germany.
states the CASTLE-HTx primary report, published in the New England Journal of Medicine, with Dr. Sohns as lead author, in tandem with his ESC presentation.
One of the study’s key messages “is that AF ablation is safe and effective in patients with end-stage heart failure” and “should be part of our armamentarium” for treating them, said Philipp Sommer, MD, also with Heart and Diabetes Center North-Rhine Westphalia, at a press conference preceding Dr. Sohns’ presentation of CASTLE-HTx.
The intervention could potentially help such patients survive longer on transplant wait lists and even delay need for the surgery, proposed Dr. Sommer, who is senior author on the trial’s publication.
CASTLE-HTx suggests that patients with advanced HF and even persistent AF, “if they have reasonably small atria, should be actually considered for ablation, as it may prevent the need for heart transplant or LVAD implant,” said invited discussant Finn Gustafsson, MD, PhD, DMSc, after Dr. Sohns’ presentation. “And that, of course, would be a huge achievement.”
The trial “should, if anything, help eradicate the current somewhat nihilistic approach to atrial fibrillation management in patients with advanced heart failure,” said Dr. Gustafsson, medical director of cardiac transplantation and mechanical circulatory support, Rigshopsitalet Copenhagen University Hospital.
Still, he disputed the characterization by the investigators and indeed the published report that the patients, or most of them, had “end-stage heart failure.”
For example, about a third of the trial’s patients started out in NYHA class 2, Dr. Gustafsson noted. Not that they weren’t “high-risk” or their HF wasn’t severe, he offered, but they don’t seem to have been “a truly advanced heart failure population.”
The trial population consisted of “patients referred to an advanced heart failure center, rather than patients with advanced heart failure,” agreed Mandeep R. Mehra, MD, director of the Center for Advanced Heart Disease at Brigham and Woman’s Hospital, Boston.
Also citing a large prevalence of patients in NYHA class-2, Dr. Mehra added that “we almost never see paroxysmal atrial fib in these patients. It’s usually an early-stage phenomenon.” In advanced HF, AF “is usually permanent,” he told this news organization. Yet it was paroxysmal in about 30% of cases.
To its credit, Dr. Mehra observed, the study does assert that advanced HF is no reason, necessarily, to avoid catheter ablation. Nor should an AF patient’s referral to an advanced-HF center “mean that you should rush to an LVAD or transplant” before considering ablation.
The study seems to be saying, “please exhaust all options before you biologically replace the heart or put in an LVAD,” Dr. Mehra said. “Certainly, this paper steers you in that direction.”
The trial entered 194 patients with symptomatic AF and HF of at least NYHA class 2, with impaired functional capacity by the 6-minute walk test, who had been referred to a major center in Germany for a heart-transplantation workup. With all on guideline-directed medical therapy, 97 were randomly assigned open-label to catheter ablation and 97 to continued standard care.
Catheter ablation was actually carried out in 81 patients (84%) who had been assigned to it and in 16 (16%) of those in the control group, the report states.
A total of 8 in the ablation group and 29 in the control arm died, received an LVAD, or went to urgent transplantation, for a hazard ratio of 0.24 (95% confidence interval, 0.11-0.52; P < .001) for the primary endpoint.
Death from any cause apparently played a big role in the risk reduction; its HR was 0.29 (95% CI, 0.12-0.72).
One peculiarity of the data, Dr. Mehra said, is that event curves for the primary endpoint and its individual components “diverge almost from day 1.” That would mean the ablation group right away started having fewer deaths, LVAD placements, or heart transplants than the control group.
“It is surprising to see such a large effect size on endpoints that are very much dependent on operators and diverge within the first day.” Probably, Dr. Mehra said, “it has to do with this being a single-center study that may not be generalizable to other practices.”
CASTLE HTx was supported by a grant from Else Kröner-Fresenius-Stiftung. Dr. Sommer discloses consulting for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Dr. Sohns reported no relevant financial relationships. Dr. Gustafsson discloses receiving honoraria or fees for consulting from Abbott, Alnylam Amgen, Boehringer Ingelheim, Ionis, Novartis, and Pfizer; serving on a speakers bureau for Astra Zeneca and Orion; and receiving grants from Corvia Research. Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board for NuPulseCV, Leviticus, and FineHeart.
A version of this article first appeared on Medscape.com.
Catheter ablation had long taken atrial fibrillation (AF) rhythm control to the next level before clinical trials showed it could help keep AF patients with heart failure (HF) alive and out of the hospital.
But those trials didn’t include many patients with AF on top of advanced or even end-stage HF. Lacking much of an evidence base and often viewed as too sick to gain a lot from the procedure, patients with AF and advanced HF aren’t offered ablation very often.
Now a randomized trial suggests that, on the contrary, AF ablation may confer a similar benefit to patients with HF so advanced that they were referred for evaluation at a transplant center.
The study, modestly sized with fewer than 200 such patients and conducted at a single center, assigned half of them to receive ablation and the other half to continued medical management.
Risk for the composite primary endpoint plunged 76% over a median of 18 months for those who underwent ablation. The outcome comprised death from any cause, implantation of a left ventricular assist device (LVAD), or urgent heart transplantation.
The advantage for ablation emerged early enough that the trial, CASTLE-HTx, was halted for benefit only a year after reaching its planned enrollment, observed Christian Sohns, MD, when formally presenting the results in Amsterdam at the annual congress of the European Society of Cardiology.
The difference in the primary endpoint “in this severely sick cohort of advanced, end-stage heart failure patients,” he said, was driven mostly by fewer deaths, especially cardiovascular deaths, in the ablation group.
Ablation’s effect on outcomes was associated, perhaps causally, with significant gains in left ventricular (LV) function and more than triple the reduction in AF burden seen in the control group, noted Dr. Sohns, from the Heart and Diabetes Center North-Rhine Westphalia, Bad Oeynhausen, Germany.
states the CASTLE-HTx primary report, published in the New England Journal of Medicine, with Dr. Sohns as lead author, in tandem with his ESC presentation.
One of the study’s key messages “is that AF ablation is safe and effective in patients with end-stage heart failure” and “should be part of our armamentarium” for treating them, said Philipp Sommer, MD, also with Heart and Diabetes Center North-Rhine Westphalia, at a press conference preceding Dr. Sohns’ presentation of CASTLE-HTx.
The intervention could potentially help such patients survive longer on transplant wait lists and even delay need for the surgery, proposed Dr. Sommer, who is senior author on the trial’s publication.
CASTLE-HTx suggests that patients with advanced HF and even persistent AF, “if they have reasonably small atria, should be actually considered for ablation, as it may prevent the need for heart transplant or LVAD implant,” said invited discussant Finn Gustafsson, MD, PhD, DMSc, after Dr. Sohns’ presentation. “And that, of course, would be a huge achievement.”
The trial “should, if anything, help eradicate the current somewhat nihilistic approach to atrial fibrillation management in patients with advanced heart failure,” said Dr. Gustafsson, medical director of cardiac transplantation and mechanical circulatory support, Rigshopsitalet Copenhagen University Hospital.
Still, he disputed the characterization by the investigators and indeed the published report that the patients, or most of them, had “end-stage heart failure.”
For example, about a third of the trial’s patients started out in NYHA class 2, Dr. Gustafsson noted. Not that they weren’t “high-risk” or their HF wasn’t severe, he offered, but they don’t seem to have been “a truly advanced heart failure population.”
The trial population consisted of “patients referred to an advanced heart failure center, rather than patients with advanced heart failure,” agreed Mandeep R. Mehra, MD, director of the Center for Advanced Heart Disease at Brigham and Woman’s Hospital, Boston.
Also citing a large prevalence of patients in NYHA class-2, Dr. Mehra added that “we almost never see paroxysmal atrial fib in these patients. It’s usually an early-stage phenomenon.” In advanced HF, AF “is usually permanent,” he told this news organization. Yet it was paroxysmal in about 30% of cases.
To its credit, Dr. Mehra observed, the study does assert that advanced HF is no reason, necessarily, to avoid catheter ablation. Nor should an AF patient’s referral to an advanced-HF center “mean that you should rush to an LVAD or transplant” before considering ablation.
The study seems to be saying, “please exhaust all options before you biologically replace the heart or put in an LVAD,” Dr. Mehra said. “Certainly, this paper steers you in that direction.”
The trial entered 194 patients with symptomatic AF and HF of at least NYHA class 2, with impaired functional capacity by the 6-minute walk test, who had been referred to a major center in Germany for a heart-transplantation workup. With all on guideline-directed medical therapy, 97 were randomly assigned open-label to catheter ablation and 97 to continued standard care.
Catheter ablation was actually carried out in 81 patients (84%) who had been assigned to it and in 16 (16%) of those in the control group, the report states.
A total of 8 in the ablation group and 29 in the control arm died, received an LVAD, or went to urgent transplantation, for a hazard ratio of 0.24 (95% confidence interval, 0.11-0.52; P < .001) for the primary endpoint.
Death from any cause apparently played a big role in the risk reduction; its HR was 0.29 (95% CI, 0.12-0.72).
One peculiarity of the data, Dr. Mehra said, is that event curves for the primary endpoint and its individual components “diverge almost from day 1.” That would mean the ablation group right away started having fewer deaths, LVAD placements, or heart transplants than the control group.
“It is surprising to see such a large effect size on endpoints that are very much dependent on operators and diverge within the first day.” Probably, Dr. Mehra said, “it has to do with this being a single-center study that may not be generalizable to other practices.”
CASTLE HTx was supported by a grant from Else Kröner-Fresenius-Stiftung. Dr. Sommer discloses consulting for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Dr. Sohns reported no relevant financial relationships. Dr. Gustafsson discloses receiving honoraria or fees for consulting from Abbott, Alnylam Amgen, Boehringer Ingelheim, Ionis, Novartis, and Pfizer; serving on a speakers bureau for Astra Zeneca and Orion; and receiving grants from Corvia Research. Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board for NuPulseCV, Leviticus, and FineHeart.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Slight weight gain, no blood pressure increase with low-dose steroids for RA
Patients taking long-term, low-dose glucocorticoids for rheumatoid arthritis over 2 years had a very modest relative weight gain but no relative increase in blood pressure when compared with patients who did not take the drugs, according to findings from a combined study of randomized, controlled trials (RCTs).
“This pooled analysis of five RCTs in RA found that 2 years of low-dose glucocorticoid treatment [at 7.5 mg/day or less] leads to a modest weight gain of about 1 kg but has no effect on blood pressure,” lead study author Andriko Palmowski, MD, a physician and researcher in rheumatology and clinical immunology at Charité-Universitätsmedizin Berlin, and colleagues wrote in the study, published in Annals of Internal Medicine.
“Many clinicians fear using even low-dose glucocorticoids because of the adverse effects associated with their long-term use at higher doses,” noted Leslie J. Crofford, MD, professor of medicine, pathology, microbiology, and immunology, and director of the division of rheumatology and immunology at Vanderbilt University, Nashville, Tenn.
“Indeed, long-term use of even these low doses increases risk for many significant adverse effects, including osteoporosis and cataracts in observational cohorts,” added Dr. Crofford, who was not involved in the study.
Studies were combined for stronger results
Observational studies are prone to confounding, and the RCTs in the literature have been small, resulting in low statistical power, the authors explained.
To overcome these limitations, Dr. Palmowski and associates combined individual participant data from five RCTs of glucocorticoid treatment for RA in 12 countries in Europe. The 1,112 participants had early and established RA, averaged 61.4 years of age, and 68% were women. The GLORIA trial, an RCT that contributed about 40% of the overall study population, “explicitly included elderly patients and patients with multimorbidity who are often excluded from RA trials,” the authors wrote.
Participants in the intervention group took low-dose glucocorticoids (prednisone equivalent, ≤ 7.5 mg/day; three trials used a dose of 5 mg prednisone equivalent per day); and patients in the control groups took placebo, disease-modifying antirheumatic drugs, or both. The researchers compared change over 2 years in body weight and mean arterial pressure between the groups.
At 2 years, both groups gained weight, but participants who took glucocorticoids gained an average of 1.1 kg (P < .001) more than the controls. Mean arterial pressure increased by around 2 mm Hg in both groups, with a –0.4 mm Hg between-group difference (P = .187).
Daniel G. Arkfeld MD, DDS, professor of clinical medicine in the division of rheumatology at the University of Southern California, Los Angeles, found this “a fascinating analysis” and called the lack of change in blood pressure important.
“Steroids are used less in RA due to perceived side effects. Yet many patients have ongoing synovitis and need steroids to enable them to work and perform other activities,” said Dr. Arkfeld, who also was not involved in the study. “NSAIDs are more of an issue, with up to 10% raising blood pressure. Should we be using more steroids and less NSAIDs?”
Dr. Arkfeld also was concerned that the small 2-year weight gain may become significant over time.
Kim Marie Huffman, MD, PhD, associate professor of medicine at Duke University, Durham, N.C., agreed.
“More investigations and longer (or shorter) time periods may have yielded additional findings,” said Dr. Huffman, who also was not involved in the study. “Efforts should be made to minimize long-term prednisone use to minimize impact on weight gain and resulting consequences.”
Are these results applicable to U.S. patients?
“Low-dose prednisone is commonly used in the U.S.,” Dr. Huffman said. “Extrapolating the results to a U.S. population is probably fine.”
Dr. Arkfeld agreed that the results can be used to treat U.S. patients because of the large number of study participants.
According to Rebecca B. Blank, MD, PhD, rheumatologist and instructor of medicine at NYU Langone Health, New York, this is an important study. But she cautioned that the literature does not contain good data for other potential harmful effects of long-term, low-dose glucocorticoid use. “Therefore, as per both ACR [American College of Rheumatology] and EULAR [European Alliance of Associations for Rheumatology] recommendations, we should still try to limit glucocorticoids to the lowest dose and shortest duration possible in our RA patients,” advised Dr. Blank, who was not an author in the study.
Strengths, weaknesses, and thoughts on further research
“Pooling trials can be tricky, but these investigators used individual-level data, which increases the rigor of the analyses,” Dr. Crofford noted. “There were differences in patient populations and with the glucocorticoid doses and routes of administration. The fact that the patients in each of the studies were randomized is very important in determining if the outcomes can be attributed to the drugs or could be the results of other exposures.”
Dr. Arkfeld would like to know whether early versus late RA patients may have different results because they may have different pathophysiologies.
Dr. Huffman is interested in low-dose glucocorticoids’ impacts on glucose homeostasis, bone density, infection, and other common adverse effects.
In an accompanying editorial, David Fernandez, MD, PhD, of Hospital for Special Surgery, New York, wrote: “These findings provide a more quantifiable assessment of the potential adverse effects of steroid therapy than had existed previously and will be helpful to providers and patients as they decide on the relative risks and benefits of glucocorticoids as part of their therapy plan in rheumatoid arthritis.”
The study received no specific funding. Four of the study’s 13 authors reported financial relationships with pharmaceutical companies. Dr. Fernandez and all outside experts who commented on the study reported no relevant financial relationships.
Patients taking long-term, low-dose glucocorticoids for rheumatoid arthritis over 2 years had a very modest relative weight gain but no relative increase in blood pressure when compared with patients who did not take the drugs, according to findings from a combined study of randomized, controlled trials (RCTs).
“This pooled analysis of five RCTs in RA found that 2 years of low-dose glucocorticoid treatment [at 7.5 mg/day or less] leads to a modest weight gain of about 1 kg but has no effect on blood pressure,” lead study author Andriko Palmowski, MD, a physician and researcher in rheumatology and clinical immunology at Charité-Universitätsmedizin Berlin, and colleagues wrote in the study, published in Annals of Internal Medicine.
“Many clinicians fear using even low-dose glucocorticoids because of the adverse effects associated with their long-term use at higher doses,” noted Leslie J. Crofford, MD, professor of medicine, pathology, microbiology, and immunology, and director of the division of rheumatology and immunology at Vanderbilt University, Nashville, Tenn.
“Indeed, long-term use of even these low doses increases risk for many significant adverse effects, including osteoporosis and cataracts in observational cohorts,” added Dr. Crofford, who was not involved in the study.
Studies were combined for stronger results
Observational studies are prone to confounding, and the RCTs in the literature have been small, resulting in low statistical power, the authors explained.
To overcome these limitations, Dr. Palmowski and associates combined individual participant data from five RCTs of glucocorticoid treatment for RA in 12 countries in Europe. The 1,112 participants had early and established RA, averaged 61.4 years of age, and 68% were women. The GLORIA trial, an RCT that contributed about 40% of the overall study population, “explicitly included elderly patients and patients with multimorbidity who are often excluded from RA trials,” the authors wrote.
Participants in the intervention group took low-dose glucocorticoids (prednisone equivalent, ≤ 7.5 mg/day; three trials used a dose of 5 mg prednisone equivalent per day); and patients in the control groups took placebo, disease-modifying antirheumatic drugs, or both. The researchers compared change over 2 years in body weight and mean arterial pressure between the groups.
At 2 years, both groups gained weight, but participants who took glucocorticoids gained an average of 1.1 kg (P < .001) more than the controls. Mean arterial pressure increased by around 2 mm Hg in both groups, with a –0.4 mm Hg between-group difference (P = .187).
Daniel G. Arkfeld MD, DDS, professor of clinical medicine in the division of rheumatology at the University of Southern California, Los Angeles, found this “a fascinating analysis” and called the lack of change in blood pressure important.
“Steroids are used less in RA due to perceived side effects. Yet many patients have ongoing synovitis and need steroids to enable them to work and perform other activities,” said Dr. Arkfeld, who also was not involved in the study. “NSAIDs are more of an issue, with up to 10% raising blood pressure. Should we be using more steroids and less NSAIDs?”
Dr. Arkfeld also was concerned that the small 2-year weight gain may become significant over time.
Kim Marie Huffman, MD, PhD, associate professor of medicine at Duke University, Durham, N.C., agreed.
“More investigations and longer (or shorter) time periods may have yielded additional findings,” said Dr. Huffman, who also was not involved in the study. “Efforts should be made to minimize long-term prednisone use to minimize impact on weight gain and resulting consequences.”
Are these results applicable to U.S. patients?
“Low-dose prednisone is commonly used in the U.S.,” Dr. Huffman said. “Extrapolating the results to a U.S. population is probably fine.”
Dr. Arkfeld agreed that the results can be used to treat U.S. patients because of the large number of study participants.
According to Rebecca B. Blank, MD, PhD, rheumatologist and instructor of medicine at NYU Langone Health, New York, this is an important study. But she cautioned that the literature does not contain good data for other potential harmful effects of long-term, low-dose glucocorticoid use. “Therefore, as per both ACR [American College of Rheumatology] and EULAR [European Alliance of Associations for Rheumatology] recommendations, we should still try to limit glucocorticoids to the lowest dose and shortest duration possible in our RA patients,” advised Dr. Blank, who was not an author in the study.
Strengths, weaknesses, and thoughts on further research
“Pooling trials can be tricky, but these investigators used individual-level data, which increases the rigor of the analyses,” Dr. Crofford noted. “There were differences in patient populations and with the glucocorticoid doses and routes of administration. The fact that the patients in each of the studies were randomized is very important in determining if the outcomes can be attributed to the drugs or could be the results of other exposures.”
Dr. Arkfeld would like to know whether early versus late RA patients may have different results because they may have different pathophysiologies.
Dr. Huffman is interested in low-dose glucocorticoids’ impacts on glucose homeostasis, bone density, infection, and other common adverse effects.
In an accompanying editorial, David Fernandez, MD, PhD, of Hospital for Special Surgery, New York, wrote: “These findings provide a more quantifiable assessment of the potential adverse effects of steroid therapy than had existed previously and will be helpful to providers and patients as they decide on the relative risks and benefits of glucocorticoids as part of their therapy plan in rheumatoid arthritis.”
The study received no specific funding. Four of the study’s 13 authors reported financial relationships with pharmaceutical companies. Dr. Fernandez and all outside experts who commented on the study reported no relevant financial relationships.
Patients taking long-term, low-dose glucocorticoids for rheumatoid arthritis over 2 years had a very modest relative weight gain but no relative increase in blood pressure when compared with patients who did not take the drugs, according to findings from a combined study of randomized, controlled trials (RCTs).
“This pooled analysis of five RCTs in RA found that 2 years of low-dose glucocorticoid treatment [at 7.5 mg/day or less] leads to a modest weight gain of about 1 kg but has no effect on blood pressure,” lead study author Andriko Palmowski, MD, a physician and researcher in rheumatology and clinical immunology at Charité-Universitätsmedizin Berlin, and colleagues wrote in the study, published in Annals of Internal Medicine.
“Many clinicians fear using even low-dose glucocorticoids because of the adverse effects associated with their long-term use at higher doses,” noted Leslie J. Crofford, MD, professor of medicine, pathology, microbiology, and immunology, and director of the division of rheumatology and immunology at Vanderbilt University, Nashville, Tenn.
“Indeed, long-term use of even these low doses increases risk for many significant adverse effects, including osteoporosis and cataracts in observational cohorts,” added Dr. Crofford, who was not involved in the study.
Studies were combined for stronger results
Observational studies are prone to confounding, and the RCTs in the literature have been small, resulting in low statistical power, the authors explained.
To overcome these limitations, Dr. Palmowski and associates combined individual participant data from five RCTs of glucocorticoid treatment for RA in 12 countries in Europe. The 1,112 participants had early and established RA, averaged 61.4 years of age, and 68% were women. The GLORIA trial, an RCT that contributed about 40% of the overall study population, “explicitly included elderly patients and patients with multimorbidity who are often excluded from RA trials,” the authors wrote.
Participants in the intervention group took low-dose glucocorticoids (prednisone equivalent, ≤ 7.5 mg/day; three trials used a dose of 5 mg prednisone equivalent per day); and patients in the control groups took placebo, disease-modifying antirheumatic drugs, or both. The researchers compared change over 2 years in body weight and mean arterial pressure between the groups.
At 2 years, both groups gained weight, but participants who took glucocorticoids gained an average of 1.1 kg (P < .001) more than the controls. Mean arterial pressure increased by around 2 mm Hg in both groups, with a –0.4 mm Hg between-group difference (P = .187).
Daniel G. Arkfeld MD, DDS, professor of clinical medicine in the division of rheumatology at the University of Southern California, Los Angeles, found this “a fascinating analysis” and called the lack of change in blood pressure important.
“Steroids are used less in RA due to perceived side effects. Yet many patients have ongoing synovitis and need steroids to enable them to work and perform other activities,” said Dr. Arkfeld, who also was not involved in the study. “NSAIDs are more of an issue, with up to 10% raising blood pressure. Should we be using more steroids and less NSAIDs?”
Dr. Arkfeld also was concerned that the small 2-year weight gain may become significant over time.
Kim Marie Huffman, MD, PhD, associate professor of medicine at Duke University, Durham, N.C., agreed.
“More investigations and longer (or shorter) time periods may have yielded additional findings,” said Dr. Huffman, who also was not involved in the study. “Efforts should be made to minimize long-term prednisone use to minimize impact on weight gain and resulting consequences.”
Are these results applicable to U.S. patients?
“Low-dose prednisone is commonly used in the U.S.,” Dr. Huffman said. “Extrapolating the results to a U.S. population is probably fine.”
Dr. Arkfeld agreed that the results can be used to treat U.S. patients because of the large number of study participants.
According to Rebecca B. Blank, MD, PhD, rheumatologist and instructor of medicine at NYU Langone Health, New York, this is an important study. But she cautioned that the literature does not contain good data for other potential harmful effects of long-term, low-dose glucocorticoid use. “Therefore, as per both ACR [American College of Rheumatology] and EULAR [European Alliance of Associations for Rheumatology] recommendations, we should still try to limit glucocorticoids to the lowest dose and shortest duration possible in our RA patients,” advised Dr. Blank, who was not an author in the study.
Strengths, weaknesses, and thoughts on further research
“Pooling trials can be tricky, but these investigators used individual-level data, which increases the rigor of the analyses,” Dr. Crofford noted. “There were differences in patient populations and with the glucocorticoid doses and routes of administration. The fact that the patients in each of the studies were randomized is very important in determining if the outcomes can be attributed to the drugs or could be the results of other exposures.”
Dr. Arkfeld would like to know whether early versus late RA patients may have different results because they may have different pathophysiologies.
Dr. Huffman is interested in low-dose glucocorticoids’ impacts on glucose homeostasis, bone density, infection, and other common adverse effects.
In an accompanying editorial, David Fernandez, MD, PhD, of Hospital for Special Surgery, New York, wrote: “These findings provide a more quantifiable assessment of the potential adverse effects of steroid therapy than had existed previously and will be helpful to providers and patients as they decide on the relative risks and benefits of glucocorticoids as part of their therapy plan in rheumatoid arthritis.”
The study received no specific funding. Four of the study’s 13 authors reported financial relationships with pharmaceutical companies. Dr. Fernandez and all outside experts who commented on the study reported no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE








