User login
MM: Newest IKEMA results back isatuximab
Median follow up was 44 months in the new update, about 2 additional years past the earlier report.
As in the earlier analysis, adding the anti-CD38 antibody to carfilzomib and dexamethasone brought substantial benefits, including a median progression free survival (PFS) of 35.7 months versus 19.2 months with placebo, as well as a higher rates of complete response (CR, 44.1% vs. 28.5%), minimal residual disease (MRD) negativity (33.5% vs. 15.4%), and MRD negativity CR (26.3% vs. 12.2%).
Although overall survival data are not yet mature, the probability of being alive at 42 months was 66.3% with isatuximab add-on versus 54.5% with placebo.
Investigators led by Thomas G. Martin, MD, director of the University of California, San Francisco, myeloma program, noted that median PFS of nearly 3 years “is the longest PFS reported to date with a PI-based regimen in the relapsed MM [multiple myeloma] setting.” The updated results further support the combination “as a standard of care treatment for patients with relapsed MM.”
Overall, the trial adds “another effective triplet in the treatment of patients with” relapsed/refractory MM, Sergio A. Giralt, MD, head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center, New York, said when asked for comment. The study was published May 9 in Blood Cancer Journal.
Safety similar to interim analysis
IKEMA randomized 179 patients to isatuximab add-on and 123 to placebo. Patients had relapsed/refractory MM with one to three prior treatment lines. Isatuximab was dosed at10 mg/kg IV in the open-label trial, weekly in the first cycle then biweekly.
The PFS benefit held across various subgroups, including the elderly and others with poor prognoses.
In their write-up, the investigators acknowledged isatuximab’s rival anti-CD38 antibody, daratumumab (Darzalex), which is also approved in the United States for use in combination with carfilzomib and dexamethasone for relapsed/refractory MM after one to three treatment lines.
“Although inter-trial evaluations should be interpreted with caution,” they noted that PFS in the latest analysis of daratumumab’s CANDOR trial in combination with carfilzomib and dexamethasone was shorter than in IKEMA, 28.6 months versus 15.2 months with placebo.
Like efficacy, safety in latest update of IKEMA was similar to that of the interim analysis. However, while there was no difference in the incidence of all-cause serious treatment-emergent adverse events (TEAEs) in the earlier report, the incidence was higher with isatuximab than placebo in the newest findings (70.1% vs. 59.8%).
The investigators said the difference was likely because patients in the isatuximab arm stayed on treatment longer, a median of 94 weeks versus 61.9 weeks in the placebo arm, making adverse events more likely.
The most common, nonhematologic TEAEs were infusion reactions (45.8% in the isatuximab arm vs. 3.3% in the placebo group), diarrhea (39.5% vs. 32%), hypertension (37.9% vs 35.2%), upper respiratory tract infection (37.3% vs 27%), and fatigue (31.6% vs 20.5%).
Grade 3 or higher pneumonia occurred in 18.6% patients in the isatuximab arm versus 12.3% in the placebo group. The incidence of skin cancer was 6.2% with isatuximab versus 3.3%. The incidence of treatment-emergent fatal events remained similar between study arms, 5.6% with isatuximab versus 4.9% with placebo.
The study was funded by Sanofi, maker of isatuximab. Investigators included two Sanofi employees. Others reported a range of ties to the company, including Dr. Martin, who reported research funding and sitting on a Sanofi steering committee.
Median follow up was 44 months in the new update, about 2 additional years past the earlier report.
As in the earlier analysis, adding the anti-CD38 antibody to carfilzomib and dexamethasone brought substantial benefits, including a median progression free survival (PFS) of 35.7 months versus 19.2 months with placebo, as well as a higher rates of complete response (CR, 44.1% vs. 28.5%), minimal residual disease (MRD) negativity (33.5% vs. 15.4%), and MRD negativity CR (26.3% vs. 12.2%).
Although overall survival data are not yet mature, the probability of being alive at 42 months was 66.3% with isatuximab add-on versus 54.5% with placebo.
Investigators led by Thomas G. Martin, MD, director of the University of California, San Francisco, myeloma program, noted that median PFS of nearly 3 years “is the longest PFS reported to date with a PI-based regimen in the relapsed MM [multiple myeloma] setting.” The updated results further support the combination “as a standard of care treatment for patients with relapsed MM.”
Overall, the trial adds “another effective triplet in the treatment of patients with” relapsed/refractory MM, Sergio A. Giralt, MD, head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center, New York, said when asked for comment. The study was published May 9 in Blood Cancer Journal.
Safety similar to interim analysis
IKEMA randomized 179 patients to isatuximab add-on and 123 to placebo. Patients had relapsed/refractory MM with one to three prior treatment lines. Isatuximab was dosed at10 mg/kg IV in the open-label trial, weekly in the first cycle then biweekly.
The PFS benefit held across various subgroups, including the elderly and others with poor prognoses.
In their write-up, the investigators acknowledged isatuximab’s rival anti-CD38 antibody, daratumumab (Darzalex), which is also approved in the United States for use in combination with carfilzomib and dexamethasone for relapsed/refractory MM after one to three treatment lines.
“Although inter-trial evaluations should be interpreted with caution,” they noted that PFS in the latest analysis of daratumumab’s CANDOR trial in combination with carfilzomib and dexamethasone was shorter than in IKEMA, 28.6 months versus 15.2 months with placebo.
Like efficacy, safety in latest update of IKEMA was similar to that of the interim analysis. However, while there was no difference in the incidence of all-cause serious treatment-emergent adverse events (TEAEs) in the earlier report, the incidence was higher with isatuximab than placebo in the newest findings (70.1% vs. 59.8%).
The investigators said the difference was likely because patients in the isatuximab arm stayed on treatment longer, a median of 94 weeks versus 61.9 weeks in the placebo arm, making adverse events more likely.
The most common, nonhematologic TEAEs were infusion reactions (45.8% in the isatuximab arm vs. 3.3% in the placebo group), diarrhea (39.5% vs. 32%), hypertension (37.9% vs 35.2%), upper respiratory tract infection (37.3% vs 27%), and fatigue (31.6% vs 20.5%).
Grade 3 or higher pneumonia occurred in 18.6% patients in the isatuximab arm versus 12.3% in the placebo group. The incidence of skin cancer was 6.2% with isatuximab versus 3.3%. The incidence of treatment-emergent fatal events remained similar between study arms, 5.6% with isatuximab versus 4.9% with placebo.
The study was funded by Sanofi, maker of isatuximab. Investigators included two Sanofi employees. Others reported a range of ties to the company, including Dr. Martin, who reported research funding and sitting on a Sanofi steering committee.
Median follow up was 44 months in the new update, about 2 additional years past the earlier report.
As in the earlier analysis, adding the anti-CD38 antibody to carfilzomib and dexamethasone brought substantial benefits, including a median progression free survival (PFS) of 35.7 months versus 19.2 months with placebo, as well as a higher rates of complete response (CR, 44.1% vs. 28.5%), minimal residual disease (MRD) negativity (33.5% vs. 15.4%), and MRD negativity CR (26.3% vs. 12.2%).
Although overall survival data are not yet mature, the probability of being alive at 42 months was 66.3% with isatuximab add-on versus 54.5% with placebo.
Investigators led by Thomas G. Martin, MD, director of the University of California, San Francisco, myeloma program, noted that median PFS of nearly 3 years “is the longest PFS reported to date with a PI-based regimen in the relapsed MM [multiple myeloma] setting.” The updated results further support the combination “as a standard of care treatment for patients with relapsed MM.”
Overall, the trial adds “another effective triplet in the treatment of patients with” relapsed/refractory MM, Sergio A. Giralt, MD, head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center, New York, said when asked for comment. The study was published May 9 in Blood Cancer Journal.
Safety similar to interim analysis
IKEMA randomized 179 patients to isatuximab add-on and 123 to placebo. Patients had relapsed/refractory MM with one to three prior treatment lines. Isatuximab was dosed at10 mg/kg IV in the open-label trial, weekly in the first cycle then biweekly.
The PFS benefit held across various subgroups, including the elderly and others with poor prognoses.
In their write-up, the investigators acknowledged isatuximab’s rival anti-CD38 antibody, daratumumab (Darzalex), which is also approved in the United States for use in combination with carfilzomib and dexamethasone for relapsed/refractory MM after one to three treatment lines.
“Although inter-trial evaluations should be interpreted with caution,” they noted that PFS in the latest analysis of daratumumab’s CANDOR trial in combination with carfilzomib and dexamethasone was shorter than in IKEMA, 28.6 months versus 15.2 months with placebo.
Like efficacy, safety in latest update of IKEMA was similar to that of the interim analysis. However, while there was no difference in the incidence of all-cause serious treatment-emergent adverse events (TEAEs) in the earlier report, the incidence was higher with isatuximab than placebo in the newest findings (70.1% vs. 59.8%).
The investigators said the difference was likely because patients in the isatuximab arm stayed on treatment longer, a median of 94 weeks versus 61.9 weeks in the placebo arm, making adverse events more likely.
The most common, nonhematologic TEAEs were infusion reactions (45.8% in the isatuximab arm vs. 3.3% in the placebo group), diarrhea (39.5% vs. 32%), hypertension (37.9% vs 35.2%), upper respiratory tract infection (37.3% vs 27%), and fatigue (31.6% vs 20.5%).
Grade 3 or higher pneumonia occurred in 18.6% patients in the isatuximab arm versus 12.3% in the placebo group. The incidence of skin cancer was 6.2% with isatuximab versus 3.3%. The incidence of treatment-emergent fatal events remained similar between study arms, 5.6% with isatuximab versus 4.9% with placebo.
The study was funded by Sanofi, maker of isatuximab. Investigators included two Sanofi employees. Others reported a range of ties to the company, including Dr. Martin, who reported research funding and sitting on a Sanofi steering committee.
FROM BLOOD CANCER JOURNAL
Enlarging Pigmented Lesion on the Thigh
The Diagnosis: Localized Cutaneous Argyria
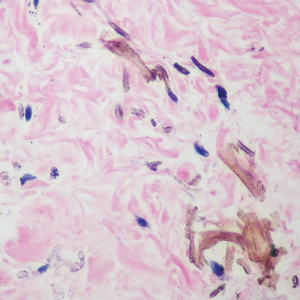
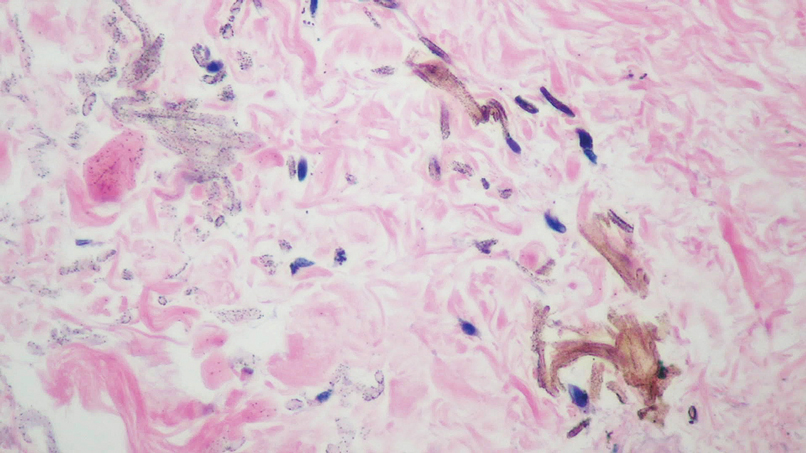
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
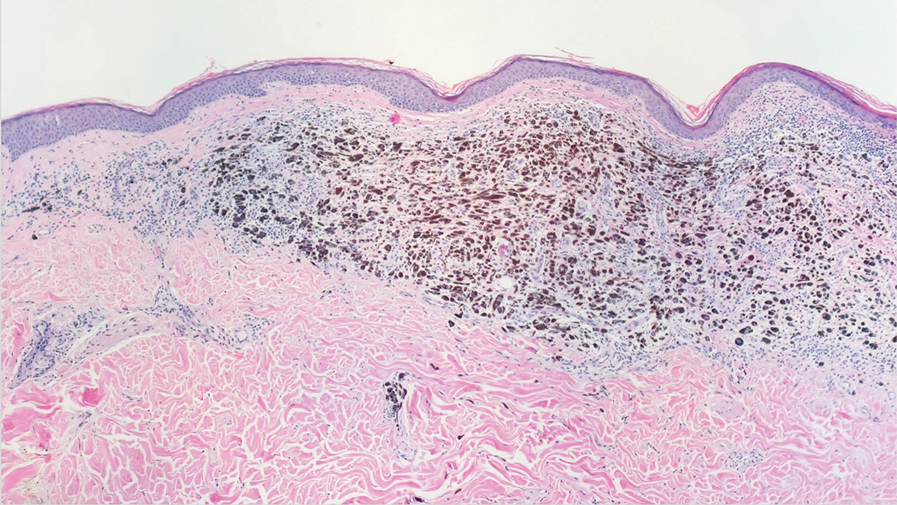
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
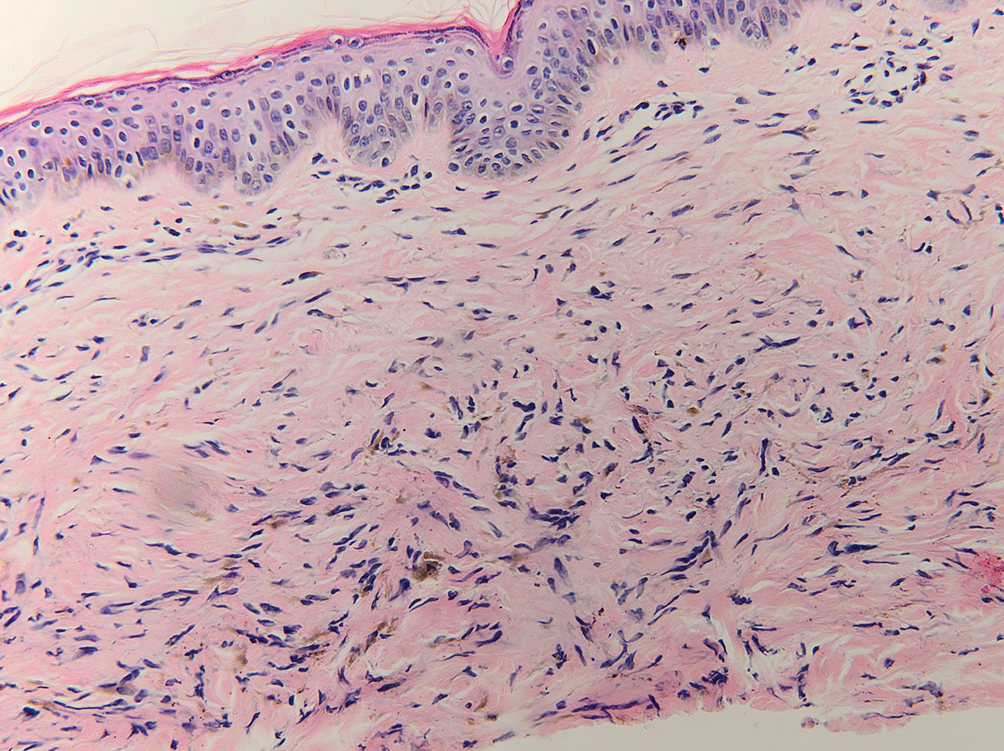
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6
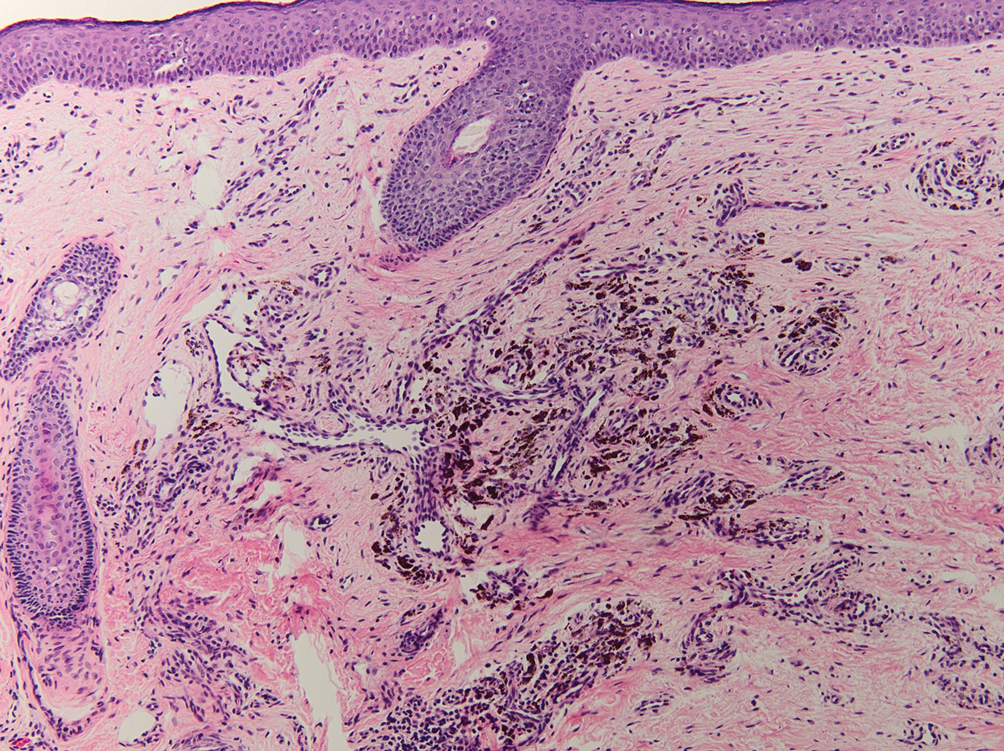
Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7
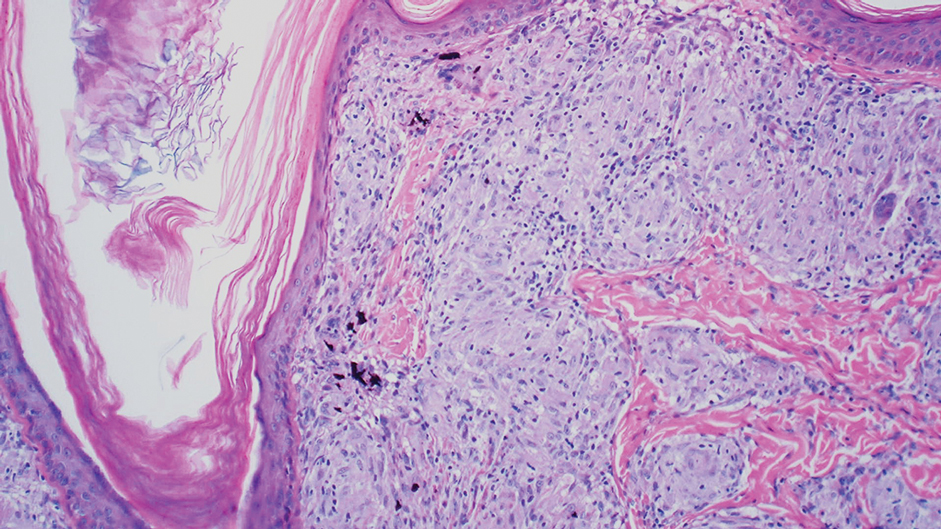
Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8
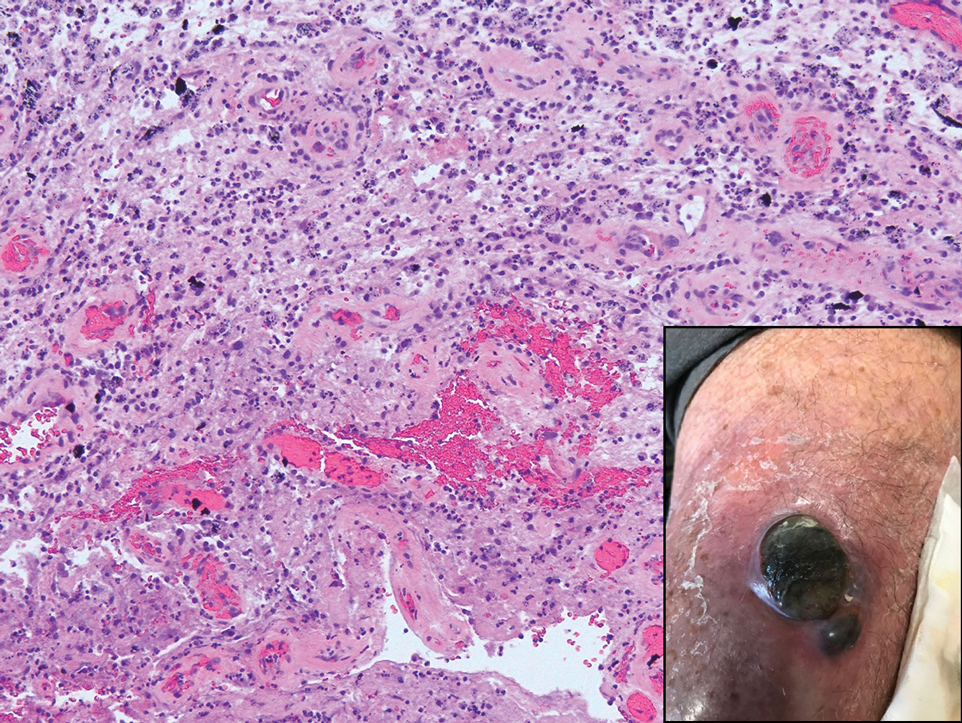
Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3
Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
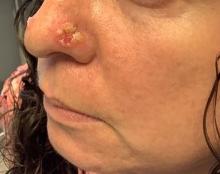
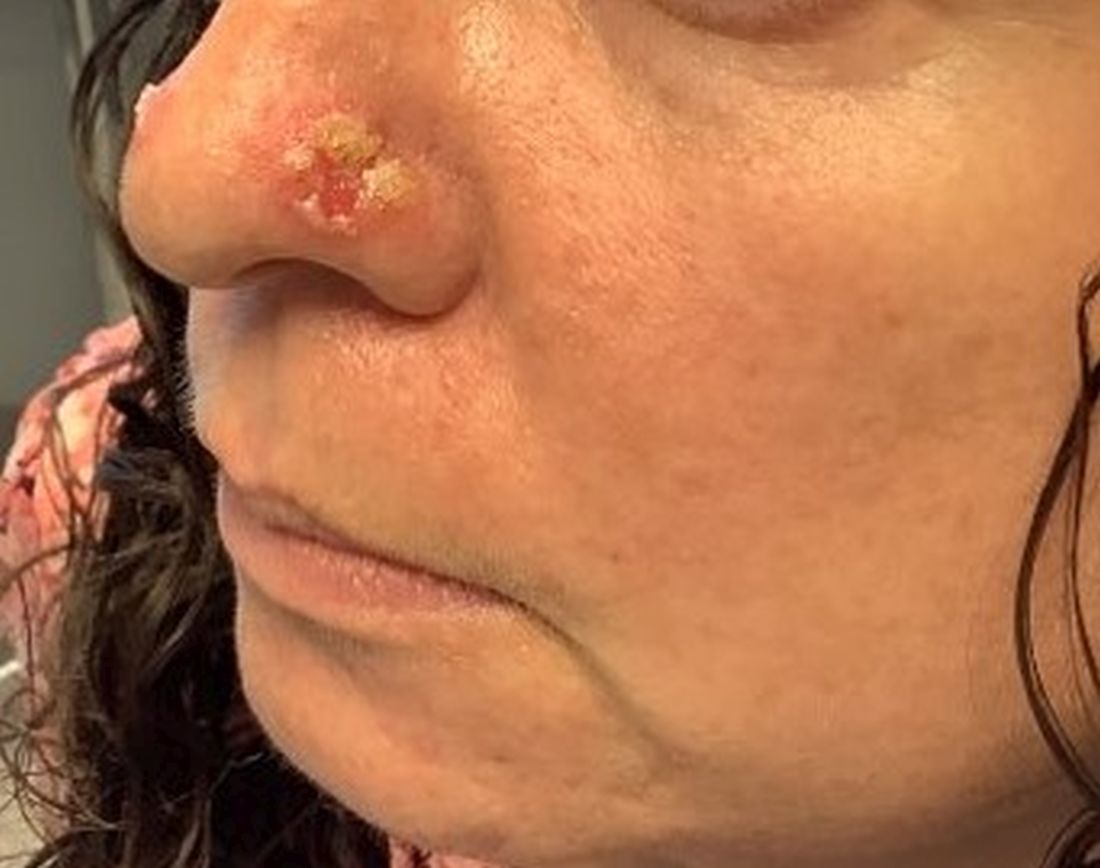
An 80-year-old man presented with a pigmented lesion on the left lateral thigh near the knee that had been gradually enlarging over several weeks (top [inset]). He underwent a left knee replacement surgery for advanced osteoarthritis many months prior that was complicated by postoperative Staphylococcus aureus infection with sinus tract formation that was persistent for 6 months and treated with a topical medication. A pigmented lesion developed near the opening of the sinus tract. His medical history was remarkable for extensive actinic damage as well as multiple actinic keratoses treated with cryotherapy but no history of melanoma. An excisional biopsy was performed (top and bottom).
Serum Ferritin Levels: A Clinical Guide in Patients With Hair Loss
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
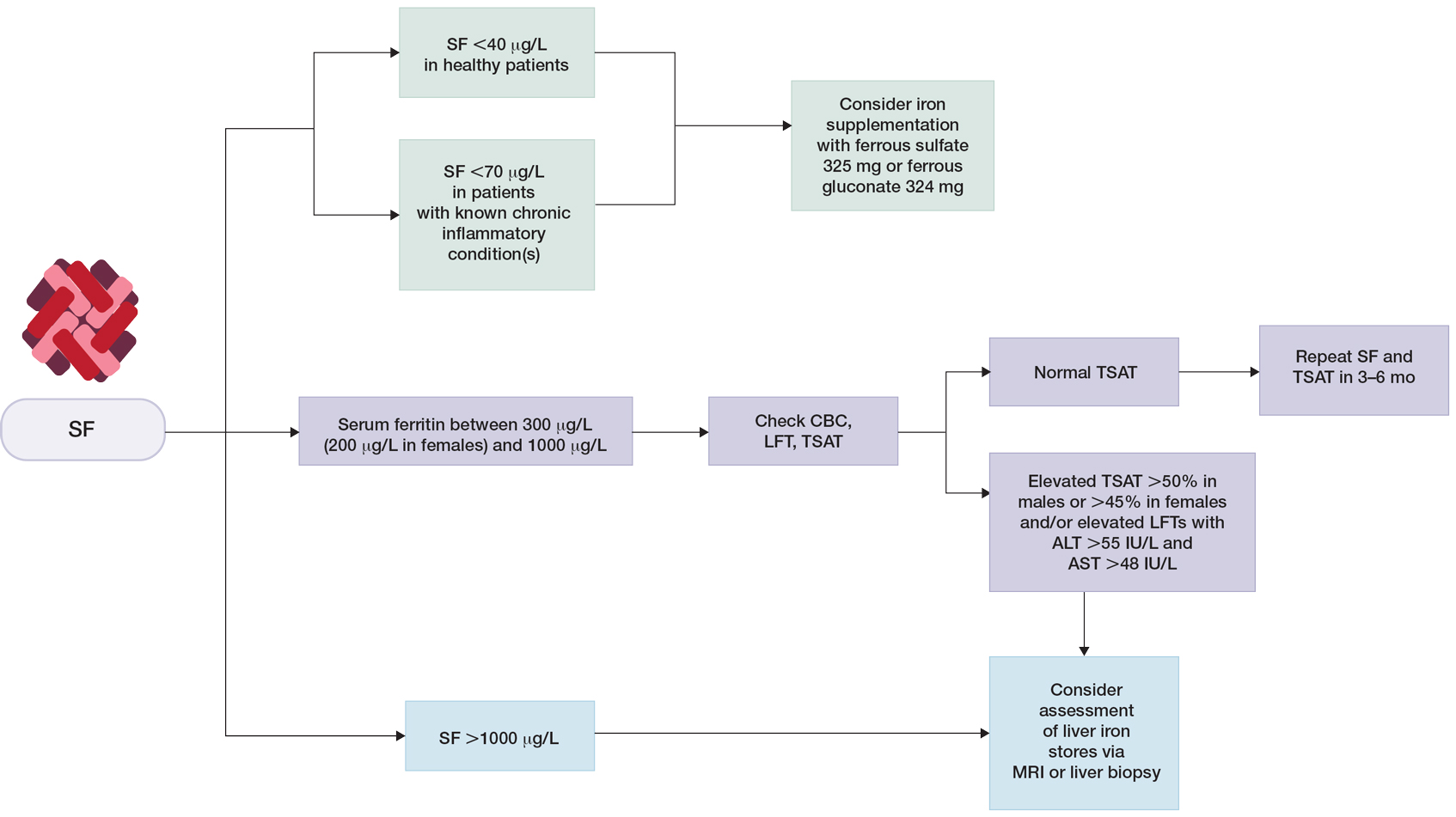
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.
Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Practice Points
- In patients who are otherwise healthy without chronic systemic disease, hepatic disease, or inflammatory disorders, serum ferritin levels directly correlate with body iron status.
- Elevated serum ferritin should be interpreted in the context of other indicators of iron status, including transferrin saturation, complete blood cell count, and/or liver function panel.
- Low serum ferritin is a specific marker for iron deficiency, and iron supplementation should be initiated based on age-, sex-, and condition-specific thresholds.
Minimally Invasive Nail Surgery: Techniques to Improve the Patient Experience
Nail surgical procedures including biopsies, correction of onychocryptosis and other deformities, and excision of tumors are essential for diagnosing and treating nail disorders. Nail surgery often is perceived by dermatologists as a difficult-to-perform, high-risk procedure associated with patient anxiety, pain, and permanent scarring, which may limit implementation. Misconceptions about nail surgical techniques, aftercare, and patient outcomes are prevalent, and a paucity of nail surgery randomized clinical trials hinder formulation of standardized guidelines.1 In a survey-based study of 95 dermatology residency programs (240 total respondents), 58% of residents said they performed 10 or fewer nail procedures, 10% performed more than 10 procedures, 25% only observed nail procedures, 4% were exposed by lecture only, and 1% had no exposure; 30% said they felt incompetent performing nail biopsies.2 In a retrospective study of nail biopsies performed from 2012 to 2017 in the Medicare Provider Utilization and Payment Database, only 0.28% and 1.01% of all general dermatologists and Mohs surgeons, respectively, performed nail biopsies annually.3 A minimally invasive nail surgery technique is essential to alleviating dermatologist and patient apprehension, which may lead to greater adoption and improved outcomes.
Reduce Patient Anxiety During Nail Surgery
The prospect of undergoing nail surgery can be psychologically distressing to patients because the nail unit is highly sensitive, intraoperative and postoperative pain are common concerns, patient education materials generally are scarce and inaccurate,4 and procedures are performed under local anesthesia with the patient fully awake. In a prospective study of 48 patients undergoing nail surgery, the median preoperative Spielberger State-Trait Anxiety Inventory level was 42.00 (IQR, 6.50).5 Patient distress may be minimized by providing verbal and written educational materials, discussing expectations, and preoperatively using fast-acting benzodiazepines when necessary.6 Utilizing a sleep mask,7 stress ball,8 music,9 and/or virtual reality10 also may reduce patient anxiety during nail surgery.
Use Proper Anesthetic Techniques
Proper anesthetic technique is crucial to achieve the optimal patient experience during nail surgery. With a wing block, the anesthetic is injected into 3 points: (1) the proximal nail fold, (2) the medial/lateral fold, and (3) the hyponychium. The wing block is the preferred technique by many nail surgeons because the second and third injections are given in skin that is already anesthetized, reducing patient discomfort to a single pinprick11; additionally, there is lower postoperative paresthesia risk with the wing block compared with other digital nerve blocks.12 Ropivacaine, a fast-acting and long-acting anesthetic, is preferred over lidocaine to minimize immediate postoperative pain. Buffering the anesthetic solution to physiologic pH and slow infiltration can reduce pain during infiltration.12 Distraction12 provided by ethyl chloride refrigerant spray, an air-cooling device,13 or vibration also can reduce pain during anesthesia.
Punch Biopsy and Excision Tips
The punch biopsy is a minimally invasive method for diagnosing various neoplastic and inflammatory nail unit conditions, except for pigmented lesions.12 For polydactylous nail conditions requiring biopsy, a digit on the nondominant hand should be selected if possible. The punch is applied directly to the nail plate and twisted with downward pressure until the bone is reached, with the instrument withdrawn slowly to prevent surrounding nail plate detachment. Hemostasis is easily achieved with direct pressure and/or use of epinephrine or ropivacaine during anesthesia, and a digital tourniquet generally is not required. Applying microporous polysaccharide hemospheres powder14 or kaolin-impregnated gauze15 with direct pressure is helpful in managing continued bleeding following nail surgery. Punching through the proximal nail matrix should be avoided to prevent permanent onychodystrophy.
A tangential matrix shave biopsy requires a more practiced technique and is preferred for sampling longitudinal melanonychia. A partial proximal nail plate avulsion adequately exposes the origin of pigment and avoids complete avulsion, which may cause more onychodystrophy.16 For broad erythronychia, a total nail avulsion may be necessary. For narrow, well-defined erythronychia, a less-invasive approach such as trap-door avulsion, longitudinal nail strip, or lateral nail plate curl, depending on the etiology, often is sufficient. Tissue excision should be tailored to the specific etiology, with localized excision sufficient for glomus tumors; onychopapillomas require tangential excision of the distal matrix, entire nail bed, and hyperkeratotic papule at the hyponychium. Pushing the cuticle with an elevator/spatula instead of making 2 tangential incisions on the proximal nail fold has been suggested to decrease postoperative paronychia risk.12 A Teflon-coated blade is used to achieve a smooth cut with minimal drag, enabling collection of specimens less than 1 mm thick, which provides sufficient nail matrix epithelium and dermis for histologic examination.16 After obtaining the specimen, the avulsed nail plate may be sutured back to the nail bed using a rapidly absorbable suture such as polyglactin 910, serving as a temporary biological dressing and splint for the nail unit during healing.12 In a retrospective study of 30 patients with longitudinal melanonychia undergoing tangential matrix excision, 27% (8/30) developed postoperative onychodystrophy.17 Although this technique carries relatively lower risk of permanent onychodystrophy compared to other methods, it still is important to acknowledge during the preoperative consent process.12
The lateral longitudinal excision is a valuable technique for diagnosing nail unit inflammatory conditions. Classically, a longitudinal sample including the proximal nail fold, complete matrix, lateral plate, lateral nail fold, hyponychium, and distal tip skin is obtained, with a 10% narrowing of the nail plate expected. If the lateral horn of the nail matrix is missed, permanent lateral malalignment and spicule formation are potential risks. To minimize narrowing of the nail plate and postoperative paronychia, a longitudinal nail strip—where the proximal nail fold and matrix are left intact—is an alternative technique.18
Pain Management Approaches
Appropriate postoperative pain management is crucial for optimizing patient outcomes. In a prospective study of 20 patients undergoing nail biopsy, the mean pain score 6 to 12 hours postprocedure was 5.7 on a scale of 0 to 10. Patients with presurgery pain vs those without experienced significantly higher pain levels both during anesthesia and after surgery (both P<.05).19 Therefore, a personalized approach to pain management based on presence of presurgical pain is warranted. In a randomized clinical trial of 16 patients anesthetized with lidocaine 2% and intraoperative infiltration with a combination of ropivacaine 0.5 mL and triamcinolone (10 mg/mL [0.5 mL]) vs lidocaine 2% alone, the intraoperative mixture reduced postoperative pain (mean pain score, 2 of 10 at 48 hours postprocedure vs 7.88 of 10 in the control group [P<.001]).20
A Cochrane review of 4 unpublished dental and orthopedic surgery studies showed that gabapentin is superior to placebo in the treatment of acute postoperative pain. Therefore, a single dose of gabapentin (250 mg) may be considered in patients at risk for high postoperative pain.21 In a randomized double-blind trial of 210 Mohs micrographic surgery patients, those receiving acetaminophen and ibuprofen reported lower pain scores at 2, 4, 8, and 12 hours postprocedure compared with patients taking acetaminophen and codeine or acetaminophen alone.22 However, the role of opioids in pain management following nail surgery has not been adequately studied.
Wound Care
An efficient dressing protects the surgical wound, facilitates healing, and provides comfort. In our experience, an initial layer of petrolatum-impregnated gauze followed by a pressure-padded bandage consisting of folded dry gauze secured in place with longitudinally applied tape to avoid a tourniquet effect is effective for nail surgical wounds. As the last step, self-adherent elastic wrap is applied around the digit and extended proximally to prevent a tourniquet effect.23
Final Thoughts
Due to the intricate anatomy of the nail unit, nail surgeries are inherently more invasive than most dermatologic surgical procedures. It is crucial to adopt a minimally invasive approach to reduce tissue damage and potential complications in both the short-term and long-term. Adopting this approach may substantially improve patient outcomes and enhance diagnostic and treatment efficacy.
- Ricardo JW, Lipner SR. Nail surgery myths and truths. J Drugs Dermatol. 2020;19:230-234.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.E4835. doi:10.1016/j.jaad.2010.05.044
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:E14928. doi:10.1111/dth.14928
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Göktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39. doi:10.1177/1203475415588645
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient—a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097/DSS.0000000000001854
- Jellinek NJ, Vélez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271. doi:10.1016/j.det.2014.12.007
- Baltz JO, Jellinek NJ. Nail surgery: six essential techniques. Dermatol Clin. 2021;39:305-318. doi:10.1016/j.det.2020.12.015
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Ricardo JW, Lipner SR. Microporous polysaccharide hemospheres powder for hemostasis following nail surgery [published online March 26, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.069
- Ricardo JW, Lipner SR. Kaolin-impregnated gauze for hemostasis following nail surgery. J Am Acad Dermatol. 2021;85:E13-E14. doi:10.1016/j.jaad.2020.02.008
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56:803-810. doi:10.1016/j.jaad.2006.12.001
- Richert B, Theunis A, Norrenberg S, et al. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol. 2013;69:96-104. doi:10.1016/j.jaad.2013.01.029
- Godse R, Jariwala N, Rubin AI. How we do it: the longitudinal nail strip biopsy for nail unit inflammatory dermatoses. Dermatol Surg. 2023;49:311-313. doi:10.1097/DSS.0000000000003707
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Di Chiacchio N, Ocampo-Garza J, Villarreal-Villarreal CD, et al. Post-nail procedure analgesia: a randomized control pilot study. J Am Acad Dermatol. 2019;81:860-862. doi:10.1016/j.jaad.2019.05.015
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12, 2010]. Cochrane Database Syst Rev. 2010;2010:CD008183. doi:10.1002/14651858.CD008183.pub2
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013. doi:10.1111/j.1524-4725.2011.02022.x
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442-444. doi:10.1097/DSS.0000000000002371
Nail surgical procedures including biopsies, correction of onychocryptosis and other deformities, and excision of tumors are essential for diagnosing and treating nail disorders. Nail surgery often is perceived by dermatologists as a difficult-to-perform, high-risk procedure associated with patient anxiety, pain, and permanent scarring, which may limit implementation. Misconceptions about nail surgical techniques, aftercare, and patient outcomes are prevalent, and a paucity of nail surgery randomized clinical trials hinder formulation of standardized guidelines.1 In a survey-based study of 95 dermatology residency programs (240 total respondents), 58% of residents said they performed 10 or fewer nail procedures, 10% performed more than 10 procedures, 25% only observed nail procedures, 4% were exposed by lecture only, and 1% had no exposure; 30% said they felt incompetent performing nail biopsies.2 In a retrospective study of nail biopsies performed from 2012 to 2017 in the Medicare Provider Utilization and Payment Database, only 0.28% and 1.01% of all general dermatologists and Mohs surgeons, respectively, performed nail biopsies annually.3 A minimally invasive nail surgery technique is essential to alleviating dermatologist and patient apprehension, which may lead to greater adoption and improved outcomes.
Reduce Patient Anxiety During Nail Surgery
The prospect of undergoing nail surgery can be psychologically distressing to patients because the nail unit is highly sensitive, intraoperative and postoperative pain are common concerns, patient education materials generally are scarce and inaccurate,4 and procedures are performed under local anesthesia with the patient fully awake. In a prospective study of 48 patients undergoing nail surgery, the median preoperative Spielberger State-Trait Anxiety Inventory level was 42.00 (IQR, 6.50).5 Patient distress may be minimized by providing verbal and written educational materials, discussing expectations, and preoperatively using fast-acting benzodiazepines when necessary.6 Utilizing a sleep mask,7 stress ball,8 music,9 and/or virtual reality10 also may reduce patient anxiety during nail surgery.
Use Proper Anesthetic Techniques
Proper anesthetic technique is crucial to achieve the optimal patient experience during nail surgery. With a wing block, the anesthetic is injected into 3 points: (1) the proximal nail fold, (2) the medial/lateral fold, and (3) the hyponychium. The wing block is the preferred technique by many nail surgeons because the second and third injections are given in skin that is already anesthetized, reducing patient discomfort to a single pinprick11; additionally, there is lower postoperative paresthesia risk with the wing block compared with other digital nerve blocks.12 Ropivacaine, a fast-acting and long-acting anesthetic, is preferred over lidocaine to minimize immediate postoperative pain. Buffering the anesthetic solution to physiologic pH and slow infiltration can reduce pain during infiltration.12 Distraction12 provided by ethyl chloride refrigerant spray, an air-cooling device,13 or vibration also can reduce pain during anesthesia.
Punch Biopsy and Excision Tips
The punch biopsy is a minimally invasive method for diagnosing various neoplastic and inflammatory nail unit conditions, except for pigmented lesions.12 For polydactylous nail conditions requiring biopsy, a digit on the nondominant hand should be selected if possible. The punch is applied directly to the nail plate and twisted with downward pressure until the bone is reached, with the instrument withdrawn slowly to prevent surrounding nail plate detachment. Hemostasis is easily achieved with direct pressure and/or use of epinephrine or ropivacaine during anesthesia, and a digital tourniquet generally is not required. Applying microporous polysaccharide hemospheres powder14 or kaolin-impregnated gauze15 with direct pressure is helpful in managing continued bleeding following nail surgery. Punching through the proximal nail matrix should be avoided to prevent permanent onychodystrophy.
A tangential matrix shave biopsy requires a more practiced technique and is preferred for sampling longitudinal melanonychia. A partial proximal nail plate avulsion adequately exposes the origin of pigment and avoids complete avulsion, which may cause more onychodystrophy.16 For broad erythronychia, a total nail avulsion may be necessary. For narrow, well-defined erythronychia, a less-invasive approach such as trap-door avulsion, longitudinal nail strip, or lateral nail plate curl, depending on the etiology, often is sufficient. Tissue excision should be tailored to the specific etiology, with localized excision sufficient for glomus tumors; onychopapillomas require tangential excision of the distal matrix, entire nail bed, and hyperkeratotic papule at the hyponychium. Pushing the cuticle with an elevator/spatula instead of making 2 tangential incisions on the proximal nail fold has been suggested to decrease postoperative paronychia risk.12 A Teflon-coated blade is used to achieve a smooth cut with minimal drag, enabling collection of specimens less than 1 mm thick, which provides sufficient nail matrix epithelium and dermis for histologic examination.16 After obtaining the specimen, the avulsed nail plate may be sutured back to the nail bed using a rapidly absorbable suture such as polyglactin 910, serving as a temporary biological dressing and splint for the nail unit during healing.12 In a retrospective study of 30 patients with longitudinal melanonychia undergoing tangential matrix excision, 27% (8/30) developed postoperative onychodystrophy.17 Although this technique carries relatively lower risk of permanent onychodystrophy compared to other methods, it still is important to acknowledge during the preoperative consent process.12
The lateral longitudinal excision is a valuable technique for diagnosing nail unit inflammatory conditions. Classically, a longitudinal sample including the proximal nail fold, complete matrix, lateral plate, lateral nail fold, hyponychium, and distal tip skin is obtained, with a 10% narrowing of the nail plate expected. If the lateral horn of the nail matrix is missed, permanent lateral malalignment and spicule formation are potential risks. To minimize narrowing of the nail plate and postoperative paronychia, a longitudinal nail strip—where the proximal nail fold and matrix are left intact—is an alternative technique.18
Pain Management Approaches
Appropriate postoperative pain management is crucial for optimizing patient outcomes. In a prospective study of 20 patients undergoing nail biopsy, the mean pain score 6 to 12 hours postprocedure was 5.7 on a scale of 0 to 10. Patients with presurgery pain vs those without experienced significantly higher pain levels both during anesthesia and after surgery (both P<.05).19 Therefore, a personalized approach to pain management based on presence of presurgical pain is warranted. In a randomized clinical trial of 16 patients anesthetized with lidocaine 2% and intraoperative infiltration with a combination of ropivacaine 0.5 mL and triamcinolone (10 mg/mL [0.5 mL]) vs lidocaine 2% alone, the intraoperative mixture reduced postoperative pain (mean pain score, 2 of 10 at 48 hours postprocedure vs 7.88 of 10 in the control group [P<.001]).20
A Cochrane review of 4 unpublished dental and orthopedic surgery studies showed that gabapentin is superior to placebo in the treatment of acute postoperative pain. Therefore, a single dose of gabapentin (250 mg) may be considered in patients at risk for high postoperative pain.21 In a randomized double-blind trial of 210 Mohs micrographic surgery patients, those receiving acetaminophen and ibuprofen reported lower pain scores at 2, 4, 8, and 12 hours postprocedure compared with patients taking acetaminophen and codeine or acetaminophen alone.22 However, the role of opioids in pain management following nail surgery has not been adequately studied.
Wound Care
An efficient dressing protects the surgical wound, facilitates healing, and provides comfort. In our experience, an initial layer of petrolatum-impregnated gauze followed by a pressure-padded bandage consisting of folded dry gauze secured in place with longitudinally applied tape to avoid a tourniquet effect is effective for nail surgical wounds. As the last step, self-adherent elastic wrap is applied around the digit and extended proximally to prevent a tourniquet effect.23
Final Thoughts
Due to the intricate anatomy of the nail unit, nail surgeries are inherently more invasive than most dermatologic surgical procedures. It is crucial to adopt a minimally invasive approach to reduce tissue damage and potential complications in both the short-term and long-term. Adopting this approach may substantially improve patient outcomes and enhance diagnostic and treatment efficacy.
Nail surgical procedures including biopsies, correction of onychocryptosis and other deformities, and excision of tumors are essential for diagnosing and treating nail disorders. Nail surgery often is perceived by dermatologists as a difficult-to-perform, high-risk procedure associated with patient anxiety, pain, and permanent scarring, which may limit implementation. Misconceptions about nail surgical techniques, aftercare, and patient outcomes are prevalent, and a paucity of nail surgery randomized clinical trials hinder formulation of standardized guidelines.1 In a survey-based study of 95 dermatology residency programs (240 total respondents), 58% of residents said they performed 10 or fewer nail procedures, 10% performed more than 10 procedures, 25% only observed nail procedures, 4% were exposed by lecture only, and 1% had no exposure; 30% said they felt incompetent performing nail biopsies.2 In a retrospective study of nail biopsies performed from 2012 to 2017 in the Medicare Provider Utilization and Payment Database, only 0.28% and 1.01% of all general dermatologists and Mohs surgeons, respectively, performed nail biopsies annually.3 A minimally invasive nail surgery technique is essential to alleviating dermatologist and patient apprehension, which may lead to greater adoption and improved outcomes.
Reduce Patient Anxiety During Nail Surgery
The prospect of undergoing nail surgery can be psychologically distressing to patients because the nail unit is highly sensitive, intraoperative and postoperative pain are common concerns, patient education materials generally are scarce and inaccurate,4 and procedures are performed under local anesthesia with the patient fully awake. In a prospective study of 48 patients undergoing nail surgery, the median preoperative Spielberger State-Trait Anxiety Inventory level was 42.00 (IQR, 6.50).5 Patient distress may be minimized by providing verbal and written educational materials, discussing expectations, and preoperatively using fast-acting benzodiazepines when necessary.6 Utilizing a sleep mask,7 stress ball,8 music,9 and/or virtual reality10 also may reduce patient anxiety during nail surgery.
Use Proper Anesthetic Techniques
Proper anesthetic technique is crucial to achieve the optimal patient experience during nail surgery. With a wing block, the anesthetic is injected into 3 points: (1) the proximal nail fold, (2) the medial/lateral fold, and (3) the hyponychium. The wing block is the preferred technique by many nail surgeons because the second and third injections are given in skin that is already anesthetized, reducing patient discomfort to a single pinprick11; additionally, there is lower postoperative paresthesia risk with the wing block compared with other digital nerve blocks.12 Ropivacaine, a fast-acting and long-acting anesthetic, is preferred over lidocaine to minimize immediate postoperative pain. Buffering the anesthetic solution to physiologic pH and slow infiltration can reduce pain during infiltration.12 Distraction12 provided by ethyl chloride refrigerant spray, an air-cooling device,13 or vibration also can reduce pain during anesthesia.
Punch Biopsy and Excision Tips
The punch biopsy is a minimally invasive method for diagnosing various neoplastic and inflammatory nail unit conditions, except for pigmented lesions.12 For polydactylous nail conditions requiring biopsy, a digit on the nondominant hand should be selected if possible. The punch is applied directly to the nail plate and twisted with downward pressure until the bone is reached, with the instrument withdrawn slowly to prevent surrounding nail plate detachment. Hemostasis is easily achieved with direct pressure and/or use of epinephrine or ropivacaine during anesthesia, and a digital tourniquet generally is not required. Applying microporous polysaccharide hemospheres powder14 or kaolin-impregnated gauze15 with direct pressure is helpful in managing continued bleeding following nail surgery. Punching through the proximal nail matrix should be avoided to prevent permanent onychodystrophy.
A tangential matrix shave biopsy requires a more practiced technique and is preferred for sampling longitudinal melanonychia. A partial proximal nail plate avulsion adequately exposes the origin of pigment and avoids complete avulsion, which may cause more onychodystrophy.16 For broad erythronychia, a total nail avulsion may be necessary. For narrow, well-defined erythronychia, a less-invasive approach such as trap-door avulsion, longitudinal nail strip, or lateral nail plate curl, depending on the etiology, often is sufficient. Tissue excision should be tailored to the specific etiology, with localized excision sufficient for glomus tumors; onychopapillomas require tangential excision of the distal matrix, entire nail bed, and hyperkeratotic papule at the hyponychium. Pushing the cuticle with an elevator/spatula instead of making 2 tangential incisions on the proximal nail fold has been suggested to decrease postoperative paronychia risk.12 A Teflon-coated blade is used to achieve a smooth cut with minimal drag, enabling collection of specimens less than 1 mm thick, which provides sufficient nail matrix epithelium and dermis for histologic examination.16 After obtaining the specimen, the avulsed nail plate may be sutured back to the nail bed using a rapidly absorbable suture such as polyglactin 910, serving as a temporary biological dressing and splint for the nail unit during healing.12 In a retrospective study of 30 patients with longitudinal melanonychia undergoing tangential matrix excision, 27% (8/30) developed postoperative onychodystrophy.17 Although this technique carries relatively lower risk of permanent onychodystrophy compared to other methods, it still is important to acknowledge during the preoperative consent process.12
The lateral longitudinal excision is a valuable technique for diagnosing nail unit inflammatory conditions. Classically, a longitudinal sample including the proximal nail fold, complete matrix, lateral plate, lateral nail fold, hyponychium, and distal tip skin is obtained, with a 10% narrowing of the nail plate expected. If the lateral horn of the nail matrix is missed, permanent lateral malalignment and spicule formation are potential risks. To minimize narrowing of the nail plate and postoperative paronychia, a longitudinal nail strip—where the proximal nail fold and matrix are left intact—is an alternative technique.18
Pain Management Approaches
Appropriate postoperative pain management is crucial for optimizing patient outcomes. In a prospective study of 20 patients undergoing nail biopsy, the mean pain score 6 to 12 hours postprocedure was 5.7 on a scale of 0 to 10. Patients with presurgery pain vs those without experienced significantly higher pain levels both during anesthesia and after surgery (both P<.05).19 Therefore, a personalized approach to pain management based on presence of presurgical pain is warranted. In a randomized clinical trial of 16 patients anesthetized with lidocaine 2% and intraoperative infiltration with a combination of ropivacaine 0.5 mL and triamcinolone (10 mg/mL [0.5 mL]) vs lidocaine 2% alone, the intraoperative mixture reduced postoperative pain (mean pain score, 2 of 10 at 48 hours postprocedure vs 7.88 of 10 in the control group [P<.001]).20
A Cochrane review of 4 unpublished dental and orthopedic surgery studies showed that gabapentin is superior to placebo in the treatment of acute postoperative pain. Therefore, a single dose of gabapentin (250 mg) may be considered in patients at risk for high postoperative pain.21 In a randomized double-blind trial of 210 Mohs micrographic surgery patients, those receiving acetaminophen and ibuprofen reported lower pain scores at 2, 4, 8, and 12 hours postprocedure compared with patients taking acetaminophen and codeine or acetaminophen alone.22 However, the role of opioids in pain management following nail surgery has not been adequately studied.
Wound Care
An efficient dressing protects the surgical wound, facilitates healing, and provides comfort. In our experience, an initial layer of petrolatum-impregnated gauze followed by a pressure-padded bandage consisting of folded dry gauze secured in place with longitudinally applied tape to avoid a tourniquet effect is effective for nail surgical wounds. As the last step, self-adherent elastic wrap is applied around the digit and extended proximally to prevent a tourniquet effect.23
Final Thoughts
Due to the intricate anatomy of the nail unit, nail surgeries are inherently more invasive than most dermatologic surgical procedures. It is crucial to adopt a minimally invasive approach to reduce tissue damage and potential complications in both the short-term and long-term. Adopting this approach may substantially improve patient outcomes and enhance diagnostic and treatment efficacy.
- Ricardo JW, Lipner SR. Nail surgery myths and truths. J Drugs Dermatol. 2020;19:230-234.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.E4835. doi:10.1016/j.jaad.2010.05.044
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:E14928. doi:10.1111/dth.14928
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Göktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39. doi:10.1177/1203475415588645
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient—a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097/DSS.0000000000001854
- Jellinek NJ, Vélez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271. doi:10.1016/j.det.2014.12.007
- Baltz JO, Jellinek NJ. Nail surgery: six essential techniques. Dermatol Clin. 2021;39:305-318. doi:10.1016/j.det.2020.12.015
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Ricardo JW, Lipner SR. Microporous polysaccharide hemospheres powder for hemostasis following nail surgery [published online March 26, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.069
- Ricardo JW, Lipner SR. Kaolin-impregnated gauze for hemostasis following nail surgery. J Am Acad Dermatol. 2021;85:E13-E14. doi:10.1016/j.jaad.2020.02.008
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56:803-810. doi:10.1016/j.jaad.2006.12.001
- Richert B, Theunis A, Norrenberg S, et al. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol. 2013;69:96-104. doi:10.1016/j.jaad.2013.01.029
- Godse R, Jariwala N, Rubin AI. How we do it: the longitudinal nail strip biopsy for nail unit inflammatory dermatoses. Dermatol Surg. 2023;49:311-313. doi:10.1097/DSS.0000000000003707
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Di Chiacchio N, Ocampo-Garza J, Villarreal-Villarreal CD, et al. Post-nail procedure analgesia: a randomized control pilot study. J Am Acad Dermatol. 2019;81:860-862. doi:10.1016/j.jaad.2019.05.015
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12, 2010]. Cochrane Database Syst Rev. 2010;2010:CD008183. doi:10.1002/14651858.CD008183.pub2
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013. doi:10.1111/j.1524-4725.2011.02022.x
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442-444. doi:10.1097/DSS.0000000000002371
- Ricardo JW, Lipner SR. Nail surgery myths and truths. J Drugs Dermatol. 2020;19:230-234.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.E4835. doi:10.1016/j.jaad.2010.05.044
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:E14928. doi:10.1111/dth.14928
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Göktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39. doi:10.1177/1203475415588645
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient—a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097/DSS.0000000000001854
- Jellinek NJ, Vélez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271. doi:10.1016/j.det.2014.12.007
- Baltz JO, Jellinek NJ. Nail surgery: six essential techniques. Dermatol Clin. 2021;39:305-318. doi:10.1016/j.det.2020.12.015
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Ricardo JW, Lipner SR. Microporous polysaccharide hemospheres powder for hemostasis following nail surgery [published online March 26, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.069
- Ricardo JW, Lipner SR. Kaolin-impregnated gauze for hemostasis following nail surgery. J Am Acad Dermatol. 2021;85:E13-E14. doi:10.1016/j.jaad.2020.02.008
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56:803-810. doi:10.1016/j.jaad.2006.12.001
- Richert B, Theunis A, Norrenberg S, et al. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol. 2013;69:96-104. doi:10.1016/j.jaad.2013.01.029
- Godse R, Jariwala N, Rubin AI. How we do it: the longitudinal nail strip biopsy for nail unit inflammatory dermatoses. Dermatol Surg. 2023;49:311-313. doi:10.1097/DSS.0000000000003707
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Di Chiacchio N, Ocampo-Garza J, Villarreal-Villarreal CD, et al. Post-nail procedure analgesia: a randomized control pilot study. J Am Acad Dermatol. 2019;81:860-862. doi:10.1016/j.jaad.2019.05.015
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12, 2010]. Cochrane Database Syst Rev. 2010;2010:CD008183. doi:10.1002/14651858.CD008183.pub2
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013. doi:10.1111/j.1524-4725.2011.02022.x
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442-444. doi:10.1097/DSS.0000000000002371
A 45-year-old White woman with no significant medical history presented with a 1-month history of lesions on the nose and right cheek
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
For NSCLC, neoadjuvant, adjuvant, or both?
This transcript has been edited for clarity.
Dr. West: Here at ASCO 2023 [American Society of Clinical Oncology] in Chicago, we’ve seen some blockbuster presentations in thoracic oncology. Many of these have brought up some important questions about the clinical implications that we need to discuss further.
At ASCO, as well as in the couple or 3 months preceding ASCO, we’ve gotten more and more data on perioperative approaches. Of course, over the past couple of years, we’ve had some new options of postoperative immunotherapy for a year, say, after chemotherapy or possibly after chemotherapy.
We have also had very influential data, such as the CheckMate 816 trial that gave three cycles of chemotherapy with nivolumab vs. chemotherapy alone to patients with stage IB to IIIA disease, but largely, nearly two thirds, with IIIA disease. That showed a very clear improvement in the pathologic complete response (pCR) rate with nivolumab added to chemotherapy and also a highly significant improvement in event-free survival and a strong trend toward improved overall survival. This is FDA approved and has been increasingly adopted, I would say, maybe with some variability by geography and center, but really a good amount of enthusiasm.
Now, we have a bunch of trials that give chemotherapy with immunotherapy. We’ve got the AEGEAN trial with durvalumab. We have Neotorch with chemotherapy and toripalimab. At ASCO 2023, we had a highly prominent presentation of KEYNOTE-671, giving four cycles of chemotherapy with pembrolizumab vs. chemotherapy and placebo.
Then there’s the built-in postoperative component of a year of immunotherapy as well, in all these trials. The data for KEYNOTE-671 look quite good. Of course, the other trials also were significant. I would say the comparator now is not nothing or chemotherapy alone anymore; it’s really against what is the best current standard of care.
It certainly adds some cost, it adds some risk for toxicity, and it adds a year of a patient coming into the clinic and getting IV infusions all this time to get a treatment that the patient has already had for four cycles in most of these trials.
If your cancer is resistant, is there going to be an incremental benefit to giving more of it? What are your thoughts about the risk and benefit? Going to a patient in your own clinic, how are you going to counsel your patients? Will anything change after the presentation of all these data and how you approach preoperatively?
Dr. Rotow: I agree. In some sense, it’s an embarrassment of riches, right?
Dr. West: Yes.
Dr. Rotow: We have so many positive studies looking at perioperative immunotherapy for our patients. They all show improved outcomes, but of course, they all compare with the old control arm of chemotherapy alone in some form, and this is no longer a useful control in this space. The open question is, do you use neoadjuvant, do you use adjuvant, or do you use both?
My high-level takeaway from these data is that the neoadjuvant component appears to be important. I think the overall trend, comparing across studies, of course, is that outcomes seem to be better with the neoadjuvant component. You also get the advantage of potential downstaging and potential greater ease of surgical resection. We know they have lower morbidity resection and shorter surgeries. You can comment on that. You also get your pathologic response data.
Dr. West: You get the feedback.
Dr. Rotow: Exactly.
Dr. West: The deliverability is also a big issue. You know you can much more reliably deliver your intended treatment by doing neoadjuvant followed by surgery.
Dr. Rotow: Exactly.
Dr. West: We know there’s major drop-off if patients have surgery, and in the recovery room they hear you got it all, and then they need to come back and maybe get chemotherapy and immunotherapy for a year. They’d ask, “What for? I can’t see anything.”
Dr. Rotow: Exactly. I think there are many advantages to that neoadjuvant component. I think all or many of us now have integrated this into our routine practice. Now the question is, do you need the adjuvant element or not on top? That is challenging because no trial has compared adjuvant to nonadjuvant. I think we all advocate for the need for this trial to answer this in a more randomized, prospective fashion. Of course, that doesn’t help our clinic practice tomorrow when we see a patient.
Dr. West: Or for the next 4 years.
Dr. Rotow: Or for the next 4 years – exactly. There’s going to be the open question of who really needs this? In some sense, we may be guided by the path response during the surgery itself. I think there may be those who claim that if you have a pCR, do you really need additional therapy? We don’t know the answer, but it’s tempting to say we know the outcomes in event-free survival are extremely good with a pCR.
Dr. West: Which is only 20% or 25% of patients, so it’s not most.
Dr. Rotow: It’s not most, but it’s better than the 2% or so with chemotherapy alone. That’s real progress, and it’s nice to have that readout. For that 80% without a pCR, what to do? I suspect there will be variation from provider to provider and from patient to patient, depending on tolerability to prior therapy, the patient’s wishes around the goals of care, and the patient’s risk for autoimmune toxicities.
Maybe there’s a patient with underlying autoimmune disease who’s gotten their neoadjuvant therapy and done well. You don’t want to risk that ongoing risk of exposure. Perhaps a patient with no risk factors who desires very aggressive treatment might be interested in more treatment.
In KEYNOTE-671, I was interested in the PD-L1 subgroups. These did trend the way you expect, with better responses in PD-L1 high, but there were also good outcomes and benefit to immunotherapy with the perioperative strategy in PD-L1–negative patients.
Dr. West: That didn’t really exclude anybody.
Dr. Rotow: It didn’t exclude anybody. In CheckMate 816, everyone benefited, but the benefit was less with those PD-L1–negative patients.
Dr. West: True.
Dr. Rotow: Absent further data to guide me or any prospective data here comparing these strategies, I might lean toward a longer course of immunotherapy in that population in hopes of triggering a response. I suspect that there will be variation from clinician to clinician in that space.
Dr. West: This is a setting where I feel like I have equipoise. I really feel that the incremental benefit is pretty small.
Dr. Rotow: Small. I agree.
Dr. West: It’s, frankly, somewhat dubious. On the other hand, you’re in a situation where if you know that three of four patients will experience a relapse and less-than-amazing outcomes, it’s hard to leave something that’s FDA approved and studied and a well-sanctioned option on the table if this patient may have relapse later.
In the end, I feel like I’d like to offer this and discuss it with all my patients. I think it’s a great place for shared decision-making because if a patient hears about that and decides they’re not interested, I’ll be fine with that. I think that’s a very sensible approach, but I don’t want to make it unilaterally. Other patients may say they want every opportunity, and if it comes back, at least I’ll know I did everything we could.
Dr. Rotow: Exactly. I agree with your statement about equipoise. I truly think that this is present here in the situation, and that there’s room for discussion in both directions with patients.
Now, one caveat I’d like to add to all these data is that the data should not apply to patients with some of our classic nonsmoking-associated driver mutations. This is another piece to the neoadjuvant data that I think is worth commenting on – the need to get appropriate testing before initiation of therapy and the pitfalls of starting this kind of treatment without knowing full biomarker testing. I think that’s something we have to watch for in our clinical practice as well.
Dr. West: Perhaps especially if we’re talking about doing a year of postoperative and someone has an ALK rearrangement or an EGFR mutation and we didn’t know it. That is a group where we’re worried about a rapid transition and potentially prohibitive, even life-threatening, toxicities from not planning in advance for this. This is something you don’t want to give concurrently or one right on top of the other. You don’t want to give immunotherapy and then transition right to targeted therapy. It’s dangerous.
Dr. Rotow: Exactly. The stakes were already high with neoadjuvant alone, but at least you had that gap of the presurgical period, surgical recovery, and then initiation of adjuvant therapy, if needed, or at relapse. With a postoperative long adjuvant period, those stakes are elevated because the immunotherapy exposure continues, so it’s something to be mindful of.
Dr. West: We have a general sense that many, but not all, of the targets that we’re talking about are associated with low benefit from immunotherapy. It’s not that well studied. I think this is another place for individualized discussion of the pros and cons. They were included in the trial, but they probably benefit less.
Dr. Rotow: Exactly. I think with the best established, EGFR and ALK probably are not benefiting much. They were actually included in the trial. Many of the neoadjuvant studies do not allow them to enroll if they’re known. On the other end of that spectrum, I think KRAS is just fine to treat with immunotherapy.
Dr. West: Sure.
Dr. Rotow: It’s an actionable driver. It’s not a traditional nonsmoking-associated driver, and those do just fine.
Dr. West: The studies show that these patients benefit just as much, at least, as the other patients.
Dr. Rotow: Exactly. I would never withhold this form of therapy for a KRAS driver mutation. The others, I think, are still in a gray zone. Depending on the patient demographics and tobacco use, I may elicit more or less caution in that space.
Dr. West: Well, I think we’re going to have much to still tease apart, with room for judgment here without a strong sense of the data telling us exactly what to do.
Dr. Rotow: Exactly.
Dr. West: There’s a large amount of excitement and interest in these new data, but there are still many open questions. I hope we continue to mull it over as we get more data and more insight to shape our plans.
Dr. West is an associate professor at City of Hope Comprehensive Cancer Center in Duarte, Calif., and vice president of network strategy at AccessHope in Los Angeles. Dr. Rotow is the clinical director of the Lowe Center for Thoracic Oncology at the Dana-Farber Cancer Institute in Boston. Dr. West reported conflicts of interest with Ariad/Takeda, Bristol Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly. Dr. Rotow reported conflicts of interest with Genentech, AstraZeneca,Guardant, and Janssen.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. West: Here at ASCO 2023 [American Society of Clinical Oncology] in Chicago, we’ve seen some blockbuster presentations in thoracic oncology. Many of these have brought up some important questions about the clinical implications that we need to discuss further.
At ASCO, as well as in the couple or 3 months preceding ASCO, we’ve gotten more and more data on perioperative approaches. Of course, over the past couple of years, we’ve had some new options of postoperative immunotherapy for a year, say, after chemotherapy or possibly after chemotherapy.
We have also had very influential data, such as the CheckMate 816 trial that gave three cycles of chemotherapy with nivolumab vs. chemotherapy alone to patients with stage IB to IIIA disease, but largely, nearly two thirds, with IIIA disease. That showed a very clear improvement in the pathologic complete response (pCR) rate with nivolumab added to chemotherapy and also a highly significant improvement in event-free survival and a strong trend toward improved overall survival. This is FDA approved and has been increasingly adopted, I would say, maybe with some variability by geography and center, but really a good amount of enthusiasm.
Now, we have a bunch of trials that give chemotherapy with immunotherapy. We’ve got the AEGEAN trial with durvalumab. We have Neotorch with chemotherapy and toripalimab. At ASCO 2023, we had a highly prominent presentation of KEYNOTE-671, giving four cycles of chemotherapy with pembrolizumab vs. chemotherapy and placebo.
Then there’s the built-in postoperative component of a year of immunotherapy as well, in all these trials. The data for KEYNOTE-671 look quite good. Of course, the other trials also were significant. I would say the comparator now is not nothing or chemotherapy alone anymore; it’s really against what is the best current standard of care.
It certainly adds some cost, it adds some risk for toxicity, and it adds a year of a patient coming into the clinic and getting IV infusions all this time to get a treatment that the patient has already had for four cycles in most of these trials.
If your cancer is resistant, is there going to be an incremental benefit to giving more of it? What are your thoughts about the risk and benefit? Going to a patient in your own clinic, how are you going to counsel your patients? Will anything change after the presentation of all these data and how you approach preoperatively?
Dr. Rotow: I agree. In some sense, it’s an embarrassment of riches, right?
Dr. West: Yes.
Dr. Rotow: We have so many positive studies looking at perioperative immunotherapy for our patients. They all show improved outcomes, but of course, they all compare with the old control arm of chemotherapy alone in some form, and this is no longer a useful control in this space. The open question is, do you use neoadjuvant, do you use adjuvant, or do you use both?
My high-level takeaway from these data is that the neoadjuvant component appears to be important. I think the overall trend, comparing across studies, of course, is that outcomes seem to be better with the neoadjuvant component. You also get the advantage of potential downstaging and potential greater ease of surgical resection. We know they have lower morbidity resection and shorter surgeries. You can comment on that. You also get your pathologic response data.
Dr. West: You get the feedback.
Dr. Rotow: Exactly.
Dr. West: The deliverability is also a big issue. You know you can much more reliably deliver your intended treatment by doing neoadjuvant followed by surgery.
Dr. Rotow: Exactly.
Dr. West: We know there’s major drop-off if patients have surgery, and in the recovery room they hear you got it all, and then they need to come back and maybe get chemotherapy and immunotherapy for a year. They’d ask, “What for? I can’t see anything.”
Dr. Rotow: Exactly. I think there are many advantages to that neoadjuvant component. I think all or many of us now have integrated this into our routine practice. Now the question is, do you need the adjuvant element or not on top? That is challenging because no trial has compared adjuvant to nonadjuvant. I think we all advocate for the need for this trial to answer this in a more randomized, prospective fashion. Of course, that doesn’t help our clinic practice tomorrow when we see a patient.
Dr. West: Or for the next 4 years.
Dr. Rotow: Or for the next 4 years – exactly. There’s going to be the open question of who really needs this? In some sense, we may be guided by the path response during the surgery itself. I think there may be those who claim that if you have a pCR, do you really need additional therapy? We don’t know the answer, but it’s tempting to say we know the outcomes in event-free survival are extremely good with a pCR.
Dr. West: Which is only 20% or 25% of patients, so it’s not most.
Dr. Rotow: It’s not most, but it’s better than the 2% or so with chemotherapy alone. That’s real progress, and it’s nice to have that readout. For that 80% without a pCR, what to do? I suspect there will be variation from provider to provider and from patient to patient, depending on tolerability to prior therapy, the patient’s wishes around the goals of care, and the patient’s risk for autoimmune toxicities.
Maybe there’s a patient with underlying autoimmune disease who’s gotten their neoadjuvant therapy and done well. You don’t want to risk that ongoing risk of exposure. Perhaps a patient with no risk factors who desires very aggressive treatment might be interested in more treatment.
In KEYNOTE-671, I was interested in the PD-L1 subgroups. These did trend the way you expect, with better responses in PD-L1 high, but there were also good outcomes and benefit to immunotherapy with the perioperative strategy in PD-L1–negative patients.
Dr. West: That didn’t really exclude anybody.
Dr. Rotow: It didn’t exclude anybody. In CheckMate 816, everyone benefited, but the benefit was less with those PD-L1–negative patients.
Dr. West: True.
Dr. Rotow: Absent further data to guide me or any prospective data here comparing these strategies, I might lean toward a longer course of immunotherapy in that population in hopes of triggering a response. I suspect that there will be variation from clinician to clinician in that space.
Dr. West: This is a setting where I feel like I have equipoise. I really feel that the incremental benefit is pretty small.
Dr. Rotow: Small. I agree.
Dr. West: It’s, frankly, somewhat dubious. On the other hand, you’re in a situation where if you know that three of four patients will experience a relapse and less-than-amazing outcomes, it’s hard to leave something that’s FDA approved and studied and a well-sanctioned option on the table if this patient may have relapse later.
In the end, I feel like I’d like to offer this and discuss it with all my patients. I think it’s a great place for shared decision-making because if a patient hears about that and decides they’re not interested, I’ll be fine with that. I think that’s a very sensible approach, but I don’t want to make it unilaterally. Other patients may say they want every opportunity, and if it comes back, at least I’ll know I did everything we could.
Dr. Rotow: Exactly. I agree with your statement about equipoise. I truly think that this is present here in the situation, and that there’s room for discussion in both directions with patients.
Now, one caveat I’d like to add to all these data is that the data should not apply to patients with some of our classic nonsmoking-associated driver mutations. This is another piece to the neoadjuvant data that I think is worth commenting on – the need to get appropriate testing before initiation of therapy and the pitfalls of starting this kind of treatment without knowing full biomarker testing. I think that’s something we have to watch for in our clinical practice as well.
Dr. West: Perhaps especially if we’re talking about doing a year of postoperative and someone has an ALK rearrangement or an EGFR mutation and we didn’t know it. That is a group where we’re worried about a rapid transition and potentially prohibitive, even life-threatening, toxicities from not planning in advance for this. This is something you don’t want to give concurrently or one right on top of the other. You don’t want to give immunotherapy and then transition right to targeted therapy. It’s dangerous.
Dr. Rotow: Exactly. The stakes were already high with neoadjuvant alone, but at least you had that gap of the presurgical period, surgical recovery, and then initiation of adjuvant therapy, if needed, or at relapse. With a postoperative long adjuvant period, those stakes are elevated because the immunotherapy exposure continues, so it’s something to be mindful of.
Dr. West: We have a general sense that many, but not all, of the targets that we’re talking about are associated with low benefit from immunotherapy. It’s not that well studied. I think this is another place for individualized discussion of the pros and cons. They were included in the trial, but they probably benefit less.
Dr. Rotow: Exactly. I think with the best established, EGFR and ALK probably are not benefiting much. They were actually included in the trial. Many of the neoadjuvant studies do not allow them to enroll if they’re known. On the other end of that spectrum, I think KRAS is just fine to treat with immunotherapy.
Dr. West: Sure.
Dr. Rotow: It’s an actionable driver. It’s not a traditional nonsmoking-associated driver, and those do just fine.
Dr. West: The studies show that these patients benefit just as much, at least, as the other patients.
Dr. Rotow: Exactly. I would never withhold this form of therapy for a KRAS driver mutation. The others, I think, are still in a gray zone. Depending on the patient demographics and tobacco use, I may elicit more or less caution in that space.
Dr. West: Well, I think we’re going to have much to still tease apart, with room for judgment here without a strong sense of the data telling us exactly what to do.
Dr. Rotow: Exactly.
Dr. West: There’s a large amount of excitement and interest in these new data, but there are still many open questions. I hope we continue to mull it over as we get more data and more insight to shape our plans.
Dr. West is an associate professor at City of Hope Comprehensive Cancer Center in Duarte, Calif., and vice president of network strategy at AccessHope in Los Angeles. Dr. Rotow is the clinical director of the Lowe Center for Thoracic Oncology at the Dana-Farber Cancer Institute in Boston. Dr. West reported conflicts of interest with Ariad/Takeda, Bristol Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly. Dr. Rotow reported conflicts of interest with Genentech, AstraZeneca,Guardant, and Janssen.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Dr. West: Here at ASCO 2023 [American Society of Clinical Oncology] in Chicago, we’ve seen some blockbuster presentations in thoracic oncology. Many of these have brought up some important questions about the clinical implications that we need to discuss further.
At ASCO, as well as in the couple or 3 months preceding ASCO, we’ve gotten more and more data on perioperative approaches. Of course, over the past couple of years, we’ve had some new options of postoperative immunotherapy for a year, say, after chemotherapy or possibly after chemotherapy.
We have also had very influential data, such as the CheckMate 816 trial that gave three cycles of chemotherapy with nivolumab vs. chemotherapy alone to patients with stage IB to IIIA disease, but largely, nearly two thirds, with IIIA disease. That showed a very clear improvement in the pathologic complete response (pCR) rate with nivolumab added to chemotherapy and also a highly significant improvement in event-free survival and a strong trend toward improved overall survival. This is FDA approved and has been increasingly adopted, I would say, maybe with some variability by geography and center, but really a good amount of enthusiasm.
Now, we have a bunch of trials that give chemotherapy with immunotherapy. We’ve got the AEGEAN trial with durvalumab. We have Neotorch with chemotherapy and toripalimab. At ASCO 2023, we had a highly prominent presentation of KEYNOTE-671, giving four cycles of chemotherapy with pembrolizumab vs. chemotherapy and placebo.
Then there’s the built-in postoperative component of a year of immunotherapy as well, in all these trials. The data for KEYNOTE-671 look quite good. Of course, the other trials also were significant. I would say the comparator now is not nothing or chemotherapy alone anymore; it’s really against what is the best current standard of care.
It certainly adds some cost, it adds some risk for toxicity, and it adds a year of a patient coming into the clinic and getting IV infusions all this time to get a treatment that the patient has already had for four cycles in most of these trials.
If your cancer is resistant, is there going to be an incremental benefit to giving more of it? What are your thoughts about the risk and benefit? Going to a patient in your own clinic, how are you going to counsel your patients? Will anything change after the presentation of all these data and how you approach preoperatively?
Dr. Rotow: I agree. In some sense, it’s an embarrassment of riches, right?
Dr. West: Yes.
Dr. Rotow: We have so many positive studies looking at perioperative immunotherapy for our patients. They all show improved outcomes, but of course, they all compare with the old control arm of chemotherapy alone in some form, and this is no longer a useful control in this space. The open question is, do you use neoadjuvant, do you use adjuvant, or do you use both?
My high-level takeaway from these data is that the neoadjuvant component appears to be important. I think the overall trend, comparing across studies, of course, is that outcomes seem to be better with the neoadjuvant component. You also get the advantage of potential downstaging and potential greater ease of surgical resection. We know they have lower morbidity resection and shorter surgeries. You can comment on that. You also get your pathologic response data.
Dr. West: You get the feedback.
Dr. Rotow: Exactly.
Dr. West: The deliverability is also a big issue. You know you can much more reliably deliver your intended treatment by doing neoadjuvant followed by surgery.
Dr. Rotow: Exactly.
Dr. West: We know there’s major drop-off if patients have surgery, and in the recovery room they hear you got it all, and then they need to come back and maybe get chemotherapy and immunotherapy for a year. They’d ask, “What for? I can’t see anything.”
Dr. Rotow: Exactly. I think there are many advantages to that neoadjuvant component. I think all or many of us now have integrated this into our routine practice. Now the question is, do you need the adjuvant element or not on top? That is challenging because no trial has compared adjuvant to nonadjuvant. I think we all advocate for the need for this trial to answer this in a more randomized, prospective fashion. Of course, that doesn’t help our clinic practice tomorrow when we see a patient.
Dr. West: Or for the next 4 years.
Dr. Rotow: Or for the next 4 years – exactly. There’s going to be the open question of who really needs this? In some sense, we may be guided by the path response during the surgery itself. I think there may be those who claim that if you have a pCR, do you really need additional therapy? We don’t know the answer, but it’s tempting to say we know the outcomes in event-free survival are extremely good with a pCR.
Dr. West: Which is only 20% or 25% of patients, so it’s not most.
Dr. Rotow: It’s not most, but it’s better than the 2% or so with chemotherapy alone. That’s real progress, and it’s nice to have that readout. For that 80% without a pCR, what to do? I suspect there will be variation from provider to provider and from patient to patient, depending on tolerability to prior therapy, the patient’s wishes around the goals of care, and the patient’s risk for autoimmune toxicities.
Maybe there’s a patient with underlying autoimmune disease who’s gotten their neoadjuvant therapy and done well. You don’t want to risk that ongoing risk of exposure. Perhaps a patient with no risk factors who desires very aggressive treatment might be interested in more treatment.
In KEYNOTE-671, I was interested in the PD-L1 subgroups. These did trend the way you expect, with better responses in PD-L1 high, but there were also good outcomes and benefit to immunotherapy with the perioperative strategy in PD-L1–negative patients.
Dr. West: That didn’t really exclude anybody.
Dr. Rotow: It didn’t exclude anybody. In CheckMate 816, everyone benefited, but the benefit was less with those PD-L1–negative patients.
Dr. West: True.
Dr. Rotow: Absent further data to guide me or any prospective data here comparing these strategies, I might lean toward a longer course of immunotherapy in that population in hopes of triggering a response. I suspect that there will be variation from clinician to clinician in that space.
Dr. West: This is a setting where I feel like I have equipoise. I really feel that the incremental benefit is pretty small.
Dr. Rotow: Small. I agree.
Dr. West: It’s, frankly, somewhat dubious. On the other hand, you’re in a situation where if you know that three of four patients will experience a relapse and less-than-amazing outcomes, it’s hard to leave something that’s FDA approved and studied and a well-sanctioned option on the table if this patient may have relapse later.
In the end, I feel like I’d like to offer this and discuss it with all my patients. I think it’s a great place for shared decision-making because if a patient hears about that and decides they’re not interested, I’ll be fine with that. I think that’s a very sensible approach, but I don’t want to make it unilaterally. Other patients may say they want every opportunity, and if it comes back, at least I’ll know I did everything we could.
Dr. Rotow: Exactly. I agree with your statement about equipoise. I truly think that this is present here in the situation, and that there’s room for discussion in both directions with patients.
Now, one caveat I’d like to add to all these data is that the data should not apply to patients with some of our classic nonsmoking-associated driver mutations. This is another piece to the neoadjuvant data that I think is worth commenting on – the need to get appropriate testing before initiation of therapy and the pitfalls of starting this kind of treatment without knowing full biomarker testing. I think that’s something we have to watch for in our clinical practice as well.
Dr. West: Perhaps especially if we’re talking about doing a year of postoperative and someone has an ALK rearrangement or an EGFR mutation and we didn’t know it. That is a group where we’re worried about a rapid transition and potentially prohibitive, even life-threatening, toxicities from not planning in advance for this. This is something you don’t want to give concurrently or one right on top of the other. You don’t want to give immunotherapy and then transition right to targeted therapy. It’s dangerous.
Dr. Rotow: Exactly. The stakes were already high with neoadjuvant alone, but at least you had that gap of the presurgical period, surgical recovery, and then initiation of adjuvant therapy, if needed, or at relapse. With a postoperative long adjuvant period, those stakes are elevated because the immunotherapy exposure continues, so it’s something to be mindful of.
Dr. West: We have a general sense that many, but not all, of the targets that we’re talking about are associated with low benefit from immunotherapy. It’s not that well studied. I think this is another place for individualized discussion of the pros and cons. They were included in the trial, but they probably benefit less.
Dr. Rotow: Exactly. I think with the best established, EGFR and ALK probably are not benefiting much. They were actually included in the trial. Many of the neoadjuvant studies do not allow them to enroll if they’re known. On the other end of that spectrum, I think KRAS is just fine to treat with immunotherapy.
Dr. West: Sure.
Dr. Rotow: It’s an actionable driver. It’s not a traditional nonsmoking-associated driver, and those do just fine.
Dr. West: The studies show that these patients benefit just as much, at least, as the other patients.
Dr. Rotow: Exactly. I would never withhold this form of therapy for a KRAS driver mutation. The others, I think, are still in a gray zone. Depending on the patient demographics and tobacco use, I may elicit more or less caution in that space.
Dr. West: Well, I think we’re going to have much to still tease apart, with room for judgment here without a strong sense of the data telling us exactly what to do.
Dr. Rotow: Exactly.
Dr. West: There’s a large amount of excitement and interest in these new data, but there are still many open questions. I hope we continue to mull it over as we get more data and more insight to shape our plans.
Dr. West is an associate professor at City of Hope Comprehensive Cancer Center in Duarte, Calif., and vice president of network strategy at AccessHope in Los Angeles. Dr. Rotow is the clinical director of the Lowe Center for Thoracic Oncology at the Dana-Farber Cancer Institute in Boston. Dr. West reported conflicts of interest with Ariad/Takeda, Bristol Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly. Dr. Rotow reported conflicts of interest with Genentech, AstraZeneca,Guardant, and Janssen.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023
Early-life antibiotic use may raise risk of early-onset CRC
TOPLINE:
, particularly in people with a variant in a specific gut microbiota regulatory gene, a new analysis found.
METHODOLOGY:
- Researchers analyzed data from UK Biobank participants who were recruited between 2006 and 2010 and were followed up to February 2022.
- They evaluated associations between early-life factors and early-onset CRC risk overall, focusing on long-term and recurrent antibiotic use.
- The team also estimated associations between long-term and recurrent antibiotic use in early life and CRC risk by polygenic risk score using 127 CRC-related genetic variants, as well as a particular gut microbiota regulatory gene FUT2.
- Associations for early-onset colorectal adenomas, as precursor to CRC, were also evaluated.
- The study included 113,256 participants. There were 165 early-onset CRC cases and 719 early-onset adenoma cases.
TAKEAWAY:
- Early-life, long-term, and recurrent antibiotic use was “nominally” associated with an increased risk of early-onset CRC (odds ratio, 1.48; P = .046) and adenomas (OR, 1.40; P < .001).
- Regarding variants of FUT2, the risk of early-onset CRC appeared to be greater for individuals with the rs281377 TT genotype (OR, 2.74) in comparison with those with the CT and TT genotypes, but none of the estimates reached statistical significance.
- The researchers found a strong positive association between long-term and recurrent antibiotic use and adenomas, largely in patients with rs281377 TT (OR, 1.75) and CT genotypes (OR, 1.51).
- Individuals with a high polygenic risk score were at higher risk of early-onset CRC (OR, 1.72; P = .019), while those with low polygenic risk scores were not at higher risk (OR, 1.05; P = .889). The association between antibiotic use and early-onset CRC risk by family history was also higher (OR, 2.34).
IN PRACTICE:
“Our findings suggested that individuals with genetic risk factors (i.e., family history of CRC) who have experienced early-life antibiotics use on a long-term basis are probably at increased early-onset CRC risk,” the authors concluded. “Given that antibiotics remain valuable in the management of bacterial infections during early life, investigating the pros and cons of early-life antibiotic use is of great significance.”
SOURCE:
The study, led by Fangyuan Jiang, with Zhejiang University, Hangzhou, China, was published online in the International Journal of Cancer.
LIMITATIONS:
The study relied on participants’ recall of early-life antibiotics use, which could introduce recall bias and misclassification of this exposure.
DISCLOSURES:
No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
, particularly in people with a variant in a specific gut microbiota regulatory gene, a new analysis found.
METHODOLOGY:
- Researchers analyzed data from UK Biobank participants who were recruited between 2006 and 2010 and were followed up to February 2022.
- They evaluated associations between early-life factors and early-onset CRC risk overall, focusing on long-term and recurrent antibiotic use.
- The team also estimated associations between long-term and recurrent antibiotic use in early life and CRC risk by polygenic risk score using 127 CRC-related genetic variants, as well as a particular gut microbiota regulatory gene FUT2.
- Associations for early-onset colorectal adenomas, as precursor to CRC, were also evaluated.
- The study included 113,256 participants. There were 165 early-onset CRC cases and 719 early-onset adenoma cases.
TAKEAWAY:
- Early-life, long-term, and recurrent antibiotic use was “nominally” associated with an increased risk of early-onset CRC (odds ratio, 1.48; P = .046) and adenomas (OR, 1.40; P < .001).
- Regarding variants of FUT2, the risk of early-onset CRC appeared to be greater for individuals with the rs281377 TT genotype (OR, 2.74) in comparison with those with the CT and TT genotypes, but none of the estimates reached statistical significance.
- The researchers found a strong positive association between long-term and recurrent antibiotic use and adenomas, largely in patients with rs281377 TT (OR, 1.75) and CT genotypes (OR, 1.51).
- Individuals with a high polygenic risk score were at higher risk of early-onset CRC (OR, 1.72; P = .019), while those with low polygenic risk scores were not at higher risk (OR, 1.05; P = .889). The association between antibiotic use and early-onset CRC risk by family history was also higher (OR, 2.34).
IN PRACTICE:
“Our findings suggested that individuals with genetic risk factors (i.e., family history of CRC) who have experienced early-life antibiotics use on a long-term basis are probably at increased early-onset CRC risk,” the authors concluded. “Given that antibiotics remain valuable in the management of bacterial infections during early life, investigating the pros and cons of early-life antibiotic use is of great significance.”
SOURCE:
The study, led by Fangyuan Jiang, with Zhejiang University, Hangzhou, China, was published online in the International Journal of Cancer.
LIMITATIONS:
The study relied on participants’ recall of early-life antibiotics use, which could introduce recall bias and misclassification of this exposure.
DISCLOSURES:
No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
, particularly in people with a variant in a specific gut microbiota regulatory gene, a new analysis found.
METHODOLOGY:
- Researchers analyzed data from UK Biobank participants who were recruited between 2006 and 2010 and were followed up to February 2022.
- They evaluated associations between early-life factors and early-onset CRC risk overall, focusing on long-term and recurrent antibiotic use.
- The team also estimated associations between long-term and recurrent antibiotic use in early life and CRC risk by polygenic risk score using 127 CRC-related genetic variants, as well as a particular gut microbiota regulatory gene FUT2.
- Associations for early-onset colorectal adenomas, as precursor to CRC, were also evaluated.
- The study included 113,256 participants. There were 165 early-onset CRC cases and 719 early-onset adenoma cases.
TAKEAWAY:
- Early-life, long-term, and recurrent antibiotic use was “nominally” associated with an increased risk of early-onset CRC (odds ratio, 1.48; P = .046) and adenomas (OR, 1.40; P < .001).
- Regarding variants of FUT2, the risk of early-onset CRC appeared to be greater for individuals with the rs281377 TT genotype (OR, 2.74) in comparison with those with the CT and TT genotypes, but none of the estimates reached statistical significance.
- The researchers found a strong positive association between long-term and recurrent antibiotic use and adenomas, largely in patients with rs281377 TT (OR, 1.75) and CT genotypes (OR, 1.51).
- Individuals with a high polygenic risk score were at higher risk of early-onset CRC (OR, 1.72; P = .019), while those with low polygenic risk scores were not at higher risk (OR, 1.05; P = .889). The association between antibiotic use and early-onset CRC risk by family history was also higher (OR, 2.34).
IN PRACTICE:
“Our findings suggested that individuals with genetic risk factors (i.e., family history of CRC) who have experienced early-life antibiotics use on a long-term basis are probably at increased early-onset CRC risk,” the authors concluded. “Given that antibiotics remain valuable in the management of bacterial infections during early life, investigating the pros and cons of early-life antibiotic use is of great significance.”
SOURCE:
The study, led by Fangyuan Jiang, with Zhejiang University, Hangzhou, China, was published online in the International Journal of Cancer.
LIMITATIONS:
The study relied on participants’ recall of early-life antibiotics use, which could introduce recall bias and misclassification of this exposure.
DISCLOSURES:
No conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL JOURNAL OF CANCER
Prior auth is a self-inflicted wound; is there a way out?
A few months ago, a close friend called me in a panic.
Her mother had been on an oral treatment for relapsed multiple myeloma for nearly 1 year. Originally, she had been prescribed the oral pill pomalidomide as part of her regimen, but it had caused cytopenias, constipation, and nausea even at lower doses. Now, she was tolerating her new pill, ixazomib, quite well.
Then, without so much as a letter from her insurance company, the patient’s pharmacy called to tell her that ixazomib was no longer covered without a peer-to-peer conversation given its cost.
The peer to peer left the oncology team and patient even more frustrated. After leaving the team on hold for nearly an hour, the insurance company denied the request for ixazomib. The hematologist conducting the peer-to-peer did not seem to understand why the patient had discontinued her previous medication.
Ultimately, the patient was forced to return to the old, less expensive drug. Since going back to pomalidomide, she has needed multiple dosing changes to manage the intense drug side effects.
I wish that stories like this were uncommon. I am often reminded of the ongoing challenges related to prior authorization when I see colleagues regaling the Twitterverse with their latest horror stories about an insurance company rejecting standard of care treatment or imaging. The data confirm the frequency of these experiences. Among a general cohort of physicians, the American Medical Association has reported that most practices complete 29 prior authorizations per physician every week and spend on average 14.6 hours, or nearly 2 full business days, of staff time each week on these requirements.
Given the growing burden of prior authorization, reform has now become a major policy issue at the federal and state level. Within the past year, organizations like the American Society of Clinical Oncology have released policy positions on prior authorization and are devoting sizable advocacy dollars to fight payers’ ability to enact it. Entire sections of the ASCO education book are devoted to detailing the negative effects of prior authorization, and recent ASCO-commissioned surveys have highlighted alarming statistics about how prior authorization harms patient care.
As a practicing oncologist, I welcome increased attention to the burdens of prior authorization. This attention shows that our professional organizations care about one of the largest contributors to burnout.
But as a researcher who studies systems of care, I worry that focusing our collective ire on health insurance companies is somewhat misplaced or unlikely to succeed.
Why? Because policy statements, Tweets, and legislation ignore the fact that prior authorization is a self-inflicted wound caused by decades of pharmaceutical companies, health systems, oncologists, and guideline bodies failing to factor costs of care into clinical practice.
Making cost-conscious oncology care a priority would be ideal, but it is also a tall order.
But, as costs continue to rise uncontrollably, the prior authorization system has ballooned out of control and reining in this system will be quite a challenge. Perhaps, we can look to my oncology practice at a Veterans Affairs Medical Center as a guide.
A self-inflicted wound
In oncology, most medications and imaging are subject to prior authorization, particularly higher-cost items, and dealing with prior authorization typically requires an army of staff. As enrollment in commercial health plans or Medicare Advantage skyrockets, prior authorization will only become more prevalent in oncology.
Why is this happening?
The lazy answer to this question is because health insurance companies put profits over patients.
However, prior authorization is a symptom, not a disease. And that symptom is exploding cancer care costs. The causes of high costs are varied: Pharmaceutical companies and device manufacturers set ungodly prices for drugs; oncologists and other specialists are often reimbursed for volume of care, not quality; and hospitals and health systems charge incredible amounts for routine labs and imaging, amounts that are orders of magnitude higher than what these services actually cost.
There is no easy solution to fix the drivers of such costs. One approach to rein in drug costs would be to allow Medicare to negotiate drug prices and set site-neutral pricing.
Another would be to incentivize doctors or health systems to reduce the use of high-cost services. But physicians are often unaware of the costs of a given treatment to a system or patient, and practices reimbursed by volume of care typically have little incentive to curb overall spending.
We are then left with payers, the primary means of reimbursing care and, specifically, utilization management, such as prior authorization and restrictive formularies, as the major mechanism to reduce this spending.
Prior authorization is broken
Here’s the rub though: Although payers are incentivized to curb costs through prior authorization, the data that they have to adjudicate therapeutic appropriateness are often terrible.
Administrative claims contain no data on biomarkers or performance status and are notoriously bad at reflecting basic information, such as a patient’s stage of cancer. For instance, the positive predictive value of claims-based algorithms to identify stage IV breast cancer is well below 50%, meaning that a claims algorithm that classifies a patient as stage IV is wrong more often than right.
If payers had better data, they could be more selective in their prior-authorization requirements. In other words, if payers could reliably identify who has metastatic breast cancer, an aromatase inhibitor, a common, well-accepted adjuvant therapy in hormone-positive, late-stage breast cancer, wouldn’t need prior authorization. What we have now is an inefficient system that sets prior authorization as a guardrail for most oncology care and then forces doctors to submit all relevant information about a patient to justify their treatment choices.
Keeping up with oncology treatment advances is also a challenge and requires tremendous expertise. Many insurers delegate their prior-authorization responsibilities to medication management companies, such as Magellan Rx or New Century Health, who maintain proprietary treatment pathways.
These companies anchor their prior authorization requirements to common guidelines. That would be fine if guideline-producing bodies like the National Comprehensive Cancer Network provided more firm recommendations on high-value treatments.
The NCCN recommendations, however, are expert-driven more than evidence or value driven. Often, therapies with less evidence make it into recommended treatment guidelines, and in some cases, the NCCN will equally recommend five options for treating a certain cancer, even when there is an obvious lower-cost option.
The effect of this, however, is that payers may then cover these five options, despite a 40-fold price difference among them, but then lean on requiring prior authorization for everything rather than being selective. Broad rather than targeted use of prior authorization alongside well-known issue like uncertain time lines, huge numbers of forms, and nonexperts doing peer to peers make for a huge mess.
What can we do?
How can we begin to solve the prior authorization crisis? A first step would be for guideline bodies to have more teeth in their recommendations. If NCCN and other guideline bodies, for instance, incorporated cost into their recommendations and designated these as “preferred” regimens, then clinicians could have better direction on therapy selection and payers could align their prior authorization policies with those recommendations. If patients had adverse effects with low-cost drugs, then a preferred alternative could be specified in such guidelines rather than subject patients, like my friend’s mother, to a toxic drug.
Second, payers could tailor the intensity of prior authorization requirements to the type of physician and clinical scenario at hand. Payers have rich data on practice patterns of oncologists. Payers should incentivize oncologists who follow guideline-based, high-value treatment pathways by lowering the need for frequent peer-to-peers or other prior authorization for “good performers.” This strategy, often termed gold carding, would use relief from prior authorization as a carrot.
Similarly, payers could reward practices that implement clinical pathways that enforce high-value care. For example, a practice could develop a treatment pathway that emphasizes access to urgent care to avoid hospitalizations as well as prioritizes access to relatively lower cost but equally effective options for therapy. If a payer reviewed and approved the pathway, perhaps payers could propose relief of future prior authorizations for practices whose oncologist practice on this pathway.
Third, payers could step up the intensity of prior authorization for certain high-cost or low-value treatments and lessen requirements for more routine services. For example, if every initial staging PET-CT required a peer to peer, oncologists would spend most of the day on the phone. Rather, lower-level tasks such as imaging may require a simple electronic EHR message, whereas high-cost items such as indefinite systemic therapy may require more frequent peer to peers.
Fourth, health systems and real-world data companies should devise better data sharing partnerships with payers so that payers could automatically examine attributes that clarify the choice of therapy. For example, if a payer could view that a patient had estrogen receptor/progesterone receptor–positive early-stage breast cancer post surgery, perhaps that payer would not require a prior authorization for an aromatase inhibitor. These real-time data sharing partnerships could reduce friction points in the system.
Finally, researchers and other groups should partner with payers to continually examine the effectiveness of any prior-authorization program. If a prior-authorization policy is no longer effective because evidence changes and evolves, then payers should consider retiring it.
In my primary oncology practice at a VA Medical Center, none of my treatments require an external prior authorization. Why? Because our local practice agreed to an established formulary, and national treatment pathways firmly specify a recommended treatment course.
Do I sometimes go off pathway? Yes, when I feel there’s a compelling reason. But that requires a structured electronic form to a central pharmacy body. I get a response within 24 hours, with no onerous prior authorization form or lengthy peer to peer.
Though there are plenty of unique qualities about the VA, the fact is that health systems and guideline bodies assuming the burden of cost containment could reduce prior-authorization requirements from payers.
Ultimately, the goal should be for oncologists to choose the highest-value treatment possible. Perhaps then, when the end-goal of cost-conscious oncology care with payers maintain an arm’s length from the patient-doctor relationship, we could all stop shouting at the wind about the burden of prior authorization.
Dr. Parikh is a medical oncologist and faculty member at the University of Pennsylvania, Philadelphia, and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care. He reported conflicts of interest with GNS Healthcare, Nanology, Cancer Study Group, Embedded Healthcare, Veterans Affairs, PCF, National Palliative Care Research Center, MUSC, and Flatiron Health.
A version of this article first appeared on Medscape.com.
A few months ago, a close friend called me in a panic.
Her mother had been on an oral treatment for relapsed multiple myeloma for nearly 1 year. Originally, she had been prescribed the oral pill pomalidomide as part of her regimen, but it had caused cytopenias, constipation, and nausea even at lower doses. Now, she was tolerating her new pill, ixazomib, quite well.
Then, without so much as a letter from her insurance company, the patient’s pharmacy called to tell her that ixazomib was no longer covered without a peer-to-peer conversation given its cost.
The peer to peer left the oncology team and patient even more frustrated. After leaving the team on hold for nearly an hour, the insurance company denied the request for ixazomib. The hematologist conducting the peer-to-peer did not seem to understand why the patient had discontinued her previous medication.
Ultimately, the patient was forced to return to the old, less expensive drug. Since going back to pomalidomide, she has needed multiple dosing changes to manage the intense drug side effects.
I wish that stories like this were uncommon. I am often reminded of the ongoing challenges related to prior authorization when I see colleagues regaling the Twitterverse with their latest horror stories about an insurance company rejecting standard of care treatment or imaging. The data confirm the frequency of these experiences. Among a general cohort of physicians, the American Medical Association has reported that most practices complete 29 prior authorizations per physician every week and spend on average 14.6 hours, or nearly 2 full business days, of staff time each week on these requirements.
Given the growing burden of prior authorization, reform has now become a major policy issue at the federal and state level. Within the past year, organizations like the American Society of Clinical Oncology have released policy positions on prior authorization and are devoting sizable advocacy dollars to fight payers’ ability to enact it. Entire sections of the ASCO education book are devoted to detailing the negative effects of prior authorization, and recent ASCO-commissioned surveys have highlighted alarming statistics about how prior authorization harms patient care.
As a practicing oncologist, I welcome increased attention to the burdens of prior authorization. This attention shows that our professional organizations care about one of the largest contributors to burnout.
But as a researcher who studies systems of care, I worry that focusing our collective ire on health insurance companies is somewhat misplaced or unlikely to succeed.
Why? Because policy statements, Tweets, and legislation ignore the fact that prior authorization is a self-inflicted wound caused by decades of pharmaceutical companies, health systems, oncologists, and guideline bodies failing to factor costs of care into clinical practice.
Making cost-conscious oncology care a priority would be ideal, but it is also a tall order.
But, as costs continue to rise uncontrollably, the prior authorization system has ballooned out of control and reining in this system will be quite a challenge. Perhaps, we can look to my oncology practice at a Veterans Affairs Medical Center as a guide.
A self-inflicted wound
In oncology, most medications and imaging are subject to prior authorization, particularly higher-cost items, and dealing with prior authorization typically requires an army of staff. As enrollment in commercial health plans or Medicare Advantage skyrockets, prior authorization will only become more prevalent in oncology.
Why is this happening?
The lazy answer to this question is because health insurance companies put profits over patients.
However, prior authorization is a symptom, not a disease. And that symptom is exploding cancer care costs. The causes of high costs are varied: Pharmaceutical companies and device manufacturers set ungodly prices for drugs; oncologists and other specialists are often reimbursed for volume of care, not quality; and hospitals and health systems charge incredible amounts for routine labs and imaging, amounts that are orders of magnitude higher than what these services actually cost.
There is no easy solution to fix the drivers of such costs. One approach to rein in drug costs would be to allow Medicare to negotiate drug prices and set site-neutral pricing.
Another would be to incentivize doctors or health systems to reduce the use of high-cost services. But physicians are often unaware of the costs of a given treatment to a system or patient, and practices reimbursed by volume of care typically have little incentive to curb overall spending.
We are then left with payers, the primary means of reimbursing care and, specifically, utilization management, such as prior authorization and restrictive formularies, as the major mechanism to reduce this spending.
Prior authorization is broken
Here’s the rub though: Although payers are incentivized to curb costs through prior authorization, the data that they have to adjudicate therapeutic appropriateness are often terrible.
Administrative claims contain no data on biomarkers or performance status and are notoriously bad at reflecting basic information, such as a patient’s stage of cancer. For instance, the positive predictive value of claims-based algorithms to identify stage IV breast cancer is well below 50%, meaning that a claims algorithm that classifies a patient as stage IV is wrong more often than right.
If payers had better data, they could be more selective in their prior-authorization requirements. In other words, if payers could reliably identify who has metastatic breast cancer, an aromatase inhibitor, a common, well-accepted adjuvant therapy in hormone-positive, late-stage breast cancer, wouldn’t need prior authorization. What we have now is an inefficient system that sets prior authorization as a guardrail for most oncology care and then forces doctors to submit all relevant information about a patient to justify their treatment choices.
Keeping up with oncology treatment advances is also a challenge and requires tremendous expertise. Many insurers delegate their prior-authorization responsibilities to medication management companies, such as Magellan Rx or New Century Health, who maintain proprietary treatment pathways.
These companies anchor their prior authorization requirements to common guidelines. That would be fine if guideline-producing bodies like the National Comprehensive Cancer Network provided more firm recommendations on high-value treatments.
The NCCN recommendations, however, are expert-driven more than evidence or value driven. Often, therapies with less evidence make it into recommended treatment guidelines, and in some cases, the NCCN will equally recommend five options for treating a certain cancer, even when there is an obvious lower-cost option.
The effect of this, however, is that payers may then cover these five options, despite a 40-fold price difference among them, but then lean on requiring prior authorization for everything rather than being selective. Broad rather than targeted use of prior authorization alongside well-known issue like uncertain time lines, huge numbers of forms, and nonexperts doing peer to peers make for a huge mess.
What can we do?
How can we begin to solve the prior authorization crisis? A first step would be for guideline bodies to have more teeth in their recommendations. If NCCN and other guideline bodies, for instance, incorporated cost into their recommendations and designated these as “preferred” regimens, then clinicians could have better direction on therapy selection and payers could align their prior authorization policies with those recommendations. If patients had adverse effects with low-cost drugs, then a preferred alternative could be specified in such guidelines rather than subject patients, like my friend’s mother, to a toxic drug.
Second, payers could tailor the intensity of prior authorization requirements to the type of physician and clinical scenario at hand. Payers have rich data on practice patterns of oncologists. Payers should incentivize oncologists who follow guideline-based, high-value treatment pathways by lowering the need for frequent peer-to-peers or other prior authorization for “good performers.” This strategy, often termed gold carding, would use relief from prior authorization as a carrot.
Similarly, payers could reward practices that implement clinical pathways that enforce high-value care. For example, a practice could develop a treatment pathway that emphasizes access to urgent care to avoid hospitalizations as well as prioritizes access to relatively lower cost but equally effective options for therapy. If a payer reviewed and approved the pathway, perhaps payers could propose relief of future prior authorizations for practices whose oncologist practice on this pathway.
Third, payers could step up the intensity of prior authorization for certain high-cost or low-value treatments and lessen requirements for more routine services. For example, if every initial staging PET-CT required a peer to peer, oncologists would spend most of the day on the phone. Rather, lower-level tasks such as imaging may require a simple electronic EHR message, whereas high-cost items such as indefinite systemic therapy may require more frequent peer to peers.
Fourth, health systems and real-world data companies should devise better data sharing partnerships with payers so that payers could automatically examine attributes that clarify the choice of therapy. For example, if a payer could view that a patient had estrogen receptor/progesterone receptor–positive early-stage breast cancer post surgery, perhaps that payer would not require a prior authorization for an aromatase inhibitor. These real-time data sharing partnerships could reduce friction points in the system.
Finally, researchers and other groups should partner with payers to continually examine the effectiveness of any prior-authorization program. If a prior-authorization policy is no longer effective because evidence changes and evolves, then payers should consider retiring it.
In my primary oncology practice at a VA Medical Center, none of my treatments require an external prior authorization. Why? Because our local practice agreed to an established formulary, and national treatment pathways firmly specify a recommended treatment course.
Do I sometimes go off pathway? Yes, when I feel there’s a compelling reason. But that requires a structured electronic form to a central pharmacy body. I get a response within 24 hours, with no onerous prior authorization form or lengthy peer to peer.
Though there are plenty of unique qualities about the VA, the fact is that health systems and guideline bodies assuming the burden of cost containment could reduce prior-authorization requirements from payers.
Ultimately, the goal should be for oncologists to choose the highest-value treatment possible. Perhaps then, when the end-goal of cost-conscious oncology care with payers maintain an arm’s length from the patient-doctor relationship, we could all stop shouting at the wind about the burden of prior authorization.
Dr. Parikh is a medical oncologist and faculty member at the University of Pennsylvania, Philadelphia, and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care. He reported conflicts of interest with GNS Healthcare, Nanology, Cancer Study Group, Embedded Healthcare, Veterans Affairs, PCF, National Palliative Care Research Center, MUSC, and Flatiron Health.
A version of this article first appeared on Medscape.com.
A few months ago, a close friend called me in a panic.
Her mother had been on an oral treatment for relapsed multiple myeloma for nearly 1 year. Originally, she had been prescribed the oral pill pomalidomide as part of her regimen, but it had caused cytopenias, constipation, and nausea even at lower doses. Now, she was tolerating her new pill, ixazomib, quite well.
Then, without so much as a letter from her insurance company, the patient’s pharmacy called to tell her that ixazomib was no longer covered without a peer-to-peer conversation given its cost.
The peer to peer left the oncology team and patient even more frustrated. After leaving the team on hold for nearly an hour, the insurance company denied the request for ixazomib. The hematologist conducting the peer-to-peer did not seem to understand why the patient had discontinued her previous medication.
Ultimately, the patient was forced to return to the old, less expensive drug. Since going back to pomalidomide, she has needed multiple dosing changes to manage the intense drug side effects.
I wish that stories like this were uncommon. I am often reminded of the ongoing challenges related to prior authorization when I see colleagues regaling the Twitterverse with their latest horror stories about an insurance company rejecting standard of care treatment or imaging. The data confirm the frequency of these experiences. Among a general cohort of physicians, the American Medical Association has reported that most practices complete 29 prior authorizations per physician every week and spend on average 14.6 hours, or nearly 2 full business days, of staff time each week on these requirements.
Given the growing burden of prior authorization, reform has now become a major policy issue at the federal and state level. Within the past year, organizations like the American Society of Clinical Oncology have released policy positions on prior authorization and are devoting sizable advocacy dollars to fight payers’ ability to enact it. Entire sections of the ASCO education book are devoted to detailing the negative effects of prior authorization, and recent ASCO-commissioned surveys have highlighted alarming statistics about how prior authorization harms patient care.
As a practicing oncologist, I welcome increased attention to the burdens of prior authorization. This attention shows that our professional organizations care about one of the largest contributors to burnout.
But as a researcher who studies systems of care, I worry that focusing our collective ire on health insurance companies is somewhat misplaced or unlikely to succeed.
Why? Because policy statements, Tweets, and legislation ignore the fact that prior authorization is a self-inflicted wound caused by decades of pharmaceutical companies, health systems, oncologists, and guideline bodies failing to factor costs of care into clinical practice.
Making cost-conscious oncology care a priority would be ideal, but it is also a tall order.
But, as costs continue to rise uncontrollably, the prior authorization system has ballooned out of control and reining in this system will be quite a challenge. Perhaps, we can look to my oncology practice at a Veterans Affairs Medical Center as a guide.
A self-inflicted wound
In oncology, most medications and imaging are subject to prior authorization, particularly higher-cost items, and dealing with prior authorization typically requires an army of staff. As enrollment in commercial health plans or Medicare Advantage skyrockets, prior authorization will only become more prevalent in oncology.
Why is this happening?
The lazy answer to this question is because health insurance companies put profits over patients.
However, prior authorization is a symptom, not a disease. And that symptom is exploding cancer care costs. The causes of high costs are varied: Pharmaceutical companies and device manufacturers set ungodly prices for drugs; oncologists and other specialists are often reimbursed for volume of care, not quality; and hospitals and health systems charge incredible amounts for routine labs and imaging, amounts that are orders of magnitude higher than what these services actually cost.
There is no easy solution to fix the drivers of such costs. One approach to rein in drug costs would be to allow Medicare to negotiate drug prices and set site-neutral pricing.
Another would be to incentivize doctors or health systems to reduce the use of high-cost services. But physicians are often unaware of the costs of a given treatment to a system or patient, and practices reimbursed by volume of care typically have little incentive to curb overall spending.
We are then left with payers, the primary means of reimbursing care and, specifically, utilization management, such as prior authorization and restrictive formularies, as the major mechanism to reduce this spending.
Prior authorization is broken
Here’s the rub though: Although payers are incentivized to curb costs through prior authorization, the data that they have to adjudicate therapeutic appropriateness are often terrible.
Administrative claims contain no data on biomarkers or performance status and are notoriously bad at reflecting basic information, such as a patient’s stage of cancer. For instance, the positive predictive value of claims-based algorithms to identify stage IV breast cancer is well below 50%, meaning that a claims algorithm that classifies a patient as stage IV is wrong more often than right.
If payers had better data, they could be more selective in their prior-authorization requirements. In other words, if payers could reliably identify who has metastatic breast cancer, an aromatase inhibitor, a common, well-accepted adjuvant therapy in hormone-positive, late-stage breast cancer, wouldn’t need prior authorization. What we have now is an inefficient system that sets prior authorization as a guardrail for most oncology care and then forces doctors to submit all relevant information about a patient to justify their treatment choices.
Keeping up with oncology treatment advances is also a challenge and requires tremendous expertise. Many insurers delegate their prior-authorization responsibilities to medication management companies, such as Magellan Rx or New Century Health, who maintain proprietary treatment pathways.
These companies anchor their prior authorization requirements to common guidelines. That would be fine if guideline-producing bodies like the National Comprehensive Cancer Network provided more firm recommendations on high-value treatments.
The NCCN recommendations, however, are expert-driven more than evidence or value driven. Often, therapies with less evidence make it into recommended treatment guidelines, and in some cases, the NCCN will equally recommend five options for treating a certain cancer, even when there is an obvious lower-cost option.
The effect of this, however, is that payers may then cover these five options, despite a 40-fold price difference among them, but then lean on requiring prior authorization for everything rather than being selective. Broad rather than targeted use of prior authorization alongside well-known issue like uncertain time lines, huge numbers of forms, and nonexperts doing peer to peers make for a huge mess.
What can we do?
How can we begin to solve the prior authorization crisis? A first step would be for guideline bodies to have more teeth in their recommendations. If NCCN and other guideline bodies, for instance, incorporated cost into their recommendations and designated these as “preferred” regimens, then clinicians could have better direction on therapy selection and payers could align their prior authorization policies with those recommendations. If patients had adverse effects with low-cost drugs, then a preferred alternative could be specified in such guidelines rather than subject patients, like my friend’s mother, to a toxic drug.
Second, payers could tailor the intensity of prior authorization requirements to the type of physician and clinical scenario at hand. Payers have rich data on practice patterns of oncologists. Payers should incentivize oncologists who follow guideline-based, high-value treatment pathways by lowering the need for frequent peer-to-peers or other prior authorization for “good performers.” This strategy, often termed gold carding, would use relief from prior authorization as a carrot.
Similarly, payers could reward practices that implement clinical pathways that enforce high-value care. For example, a practice could develop a treatment pathway that emphasizes access to urgent care to avoid hospitalizations as well as prioritizes access to relatively lower cost but equally effective options for therapy. If a payer reviewed and approved the pathway, perhaps payers could propose relief of future prior authorizations for practices whose oncologist practice on this pathway.
Third, payers could step up the intensity of prior authorization for certain high-cost or low-value treatments and lessen requirements for more routine services. For example, if every initial staging PET-CT required a peer to peer, oncologists would spend most of the day on the phone. Rather, lower-level tasks such as imaging may require a simple electronic EHR message, whereas high-cost items such as indefinite systemic therapy may require more frequent peer to peers.
Fourth, health systems and real-world data companies should devise better data sharing partnerships with payers so that payers could automatically examine attributes that clarify the choice of therapy. For example, if a payer could view that a patient had estrogen receptor/progesterone receptor–positive early-stage breast cancer post surgery, perhaps that payer would not require a prior authorization for an aromatase inhibitor. These real-time data sharing partnerships could reduce friction points in the system.
Finally, researchers and other groups should partner with payers to continually examine the effectiveness of any prior-authorization program. If a prior-authorization policy is no longer effective because evidence changes and evolves, then payers should consider retiring it.
In my primary oncology practice at a VA Medical Center, none of my treatments require an external prior authorization. Why? Because our local practice agreed to an established formulary, and national treatment pathways firmly specify a recommended treatment course.
Do I sometimes go off pathway? Yes, when I feel there’s a compelling reason. But that requires a structured electronic form to a central pharmacy body. I get a response within 24 hours, with no onerous prior authorization form or lengthy peer to peer.
Though there are plenty of unique qualities about the VA, the fact is that health systems and guideline bodies assuming the burden of cost containment could reduce prior-authorization requirements from payers.
Ultimately, the goal should be for oncologists to choose the highest-value treatment possible. Perhaps then, when the end-goal of cost-conscious oncology care with payers maintain an arm’s length from the patient-doctor relationship, we could all stop shouting at the wind about the burden of prior authorization.
Dr. Parikh is a medical oncologist and faculty member at the University of Pennsylvania, Philadelphia, and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care. He reported conflicts of interest with GNS Healthcare, Nanology, Cancer Study Group, Embedded Healthcare, Veterans Affairs, PCF, National Palliative Care Research Center, MUSC, and Flatiron Health.
A version of this article first appeared on Medscape.com.
Increasing number of children being poisoned by liquid nicotine
Doctors say a 2016 law aimed at lowering the risk contained a big flaw, NBC News reported. The Child Nicotine Poisoning Prevention Act required child-resistant packaging on vaping liquid – but not on the vaping devices themselves.
Contact with the vaping liquid, or liquid nicotine, can cause children to get dizzy, pass out, and suffer drops in blood pressure. A few drops of the liquid can be fatal for a toddler.
Last year, 6,731 cases of vaping-related nicotine exposure were reported, according to Poison Help. “As of June 30, 2023, poison centers have managed 3,863 exposure cases about e-cigarette devices and liquid nicotine,” the organization said.
“Poison centers began receiving calls about e-cigarettes and liquid nicotine products in 2011, which coincides with the initial period where these products reached the U.S. market,” according to Poison Help.
“These products often contain a greater concentration of nicotine, a stimulant, than other nicotine/tobacco products on the market. Some children and toddlers who come in contact with e-cigarette devices or liquid nicotine have become very ill; some even requiring emergency department visits with nausea and vomiting being the most significant symptoms.”
Toxicologist Ryan Marino, MD, told NBC that refillable vapes are designed to hold liquid nicotine in a central reservoir, making them dangerous to children.
“Even vapes that appear more child-resistant – because their nicotine is sealed inside a removable cartridge – present a risk, because the cartridges can be pried open,” NBC said. “And some disposable e-cigarettes, now the top-selling type on the market, allow users to take thousands of ‘puffs’ and contain as much nicotine as multiple packs of cigarettes.”
A spokesperson for the vaping industry said all e-liquid bottles made in this country conform to U.S. law.
“Not only are the caps child-resistant, but the flow of liquid is restricted so that only small amounts can be dispensed,” said April Meyers of the Smoke-Free Alternatives Trade Association, which represents the vaping industry.
A version of this article first appeared on WebMD.com.
Doctors say a 2016 law aimed at lowering the risk contained a big flaw, NBC News reported. The Child Nicotine Poisoning Prevention Act required child-resistant packaging on vaping liquid – but not on the vaping devices themselves.
Contact with the vaping liquid, or liquid nicotine, can cause children to get dizzy, pass out, and suffer drops in blood pressure. A few drops of the liquid can be fatal for a toddler.
Last year, 6,731 cases of vaping-related nicotine exposure were reported, according to Poison Help. “As of June 30, 2023, poison centers have managed 3,863 exposure cases about e-cigarette devices and liquid nicotine,” the organization said.
“Poison centers began receiving calls about e-cigarettes and liquid nicotine products in 2011, which coincides with the initial period where these products reached the U.S. market,” according to Poison Help.
“These products often contain a greater concentration of nicotine, a stimulant, than other nicotine/tobacco products on the market. Some children and toddlers who come in contact with e-cigarette devices or liquid nicotine have become very ill; some even requiring emergency department visits with nausea and vomiting being the most significant symptoms.”
Toxicologist Ryan Marino, MD, told NBC that refillable vapes are designed to hold liquid nicotine in a central reservoir, making them dangerous to children.
“Even vapes that appear more child-resistant – because their nicotine is sealed inside a removable cartridge – present a risk, because the cartridges can be pried open,” NBC said. “And some disposable e-cigarettes, now the top-selling type on the market, allow users to take thousands of ‘puffs’ and contain as much nicotine as multiple packs of cigarettes.”
A spokesperson for the vaping industry said all e-liquid bottles made in this country conform to U.S. law.
“Not only are the caps child-resistant, but the flow of liquid is restricted so that only small amounts can be dispensed,” said April Meyers of the Smoke-Free Alternatives Trade Association, which represents the vaping industry.
A version of this article first appeared on WebMD.com.
Doctors say a 2016 law aimed at lowering the risk contained a big flaw, NBC News reported. The Child Nicotine Poisoning Prevention Act required child-resistant packaging on vaping liquid – but not on the vaping devices themselves.
Contact with the vaping liquid, or liquid nicotine, can cause children to get dizzy, pass out, and suffer drops in blood pressure. A few drops of the liquid can be fatal for a toddler.
Last year, 6,731 cases of vaping-related nicotine exposure were reported, according to Poison Help. “As of June 30, 2023, poison centers have managed 3,863 exposure cases about e-cigarette devices and liquid nicotine,” the organization said.
“Poison centers began receiving calls about e-cigarettes and liquid nicotine products in 2011, which coincides with the initial period where these products reached the U.S. market,” according to Poison Help.
“These products often contain a greater concentration of nicotine, a stimulant, than other nicotine/tobacco products on the market. Some children and toddlers who come in contact with e-cigarette devices or liquid nicotine have become very ill; some even requiring emergency department visits with nausea and vomiting being the most significant symptoms.”
Toxicologist Ryan Marino, MD, told NBC that refillable vapes are designed to hold liquid nicotine in a central reservoir, making them dangerous to children.
“Even vapes that appear more child-resistant – because their nicotine is sealed inside a removable cartridge – present a risk, because the cartridges can be pried open,” NBC said. “And some disposable e-cigarettes, now the top-selling type on the market, allow users to take thousands of ‘puffs’ and contain as much nicotine as multiple packs of cigarettes.”
A spokesperson for the vaping industry said all e-liquid bottles made in this country conform to U.S. law.
“Not only are the caps child-resistant, but the flow of liquid is restricted so that only small amounts can be dispensed,” said April Meyers of the Smoke-Free Alternatives Trade Association, which represents the vaping industry.
A version of this article first appeared on WebMD.com.
Thrombectomy improves outcomes in pediatric stroke
A matched case-control study followed 52 patients in Canada and Australia with acute stroke and assessed functional outcomes at 3 months for those who received thrombectomy, compared with those who did not. Patients receiving the procedure had significantly improved clinical outcomes (odds ratio [OR], 3.76). The procedure is the standard of care for adults with large vessel occlusion (LVO) stroke, but limited data exist for children.
“In the absence of a randomized trial, this case-control study demonstrates better clinical outcomes with thrombectomy than medical management for pediatric patients aged 2 to 18 years with anterior circulation LVO stroke,” the authors concluded. The study was published in JAMA Neurology.
Improved results
Untreated LVO stroke is associated with poor outcomes, indicated in this study with scoring based on the modified Rankin Scale. Based on this scoring, 53.8% of patients who were managed conservatively had poor outcomes (moderate disability or greater) at 3 months, confirming previous findings. The data were drawn from five hospitals in Australia and Canada between January 2011 and April 2022.
Removing blood clots with mechanical thrombectomy resulted in improved outcomes 3 months after stroke for the patients included in the study, compared with the neuroprotective measures of medical therapy alone. The improved outcomes persisted in the final available follow-up (OR, 3.65).
In adults, thrombectomy has previously been demonstrated to be a safe and effective treatment for LVO stroke and is currently the standard of care. This study sought to expand the data for pediatric patients, for whom stroke is rarer and difficult to diagnose.
The authors cautioned, however, that the outcomes are from hospitals with pediatric neurology expertise and should not be generalized to settings without specialists.
Case-control study
While previous population-based studies of children with LVO stroke found that conservative treatment was associated with poor outcomes, these studies may include significant selection bias. The investigators chose to conduct the case-control study as an alternative to a randomized control trial, which would require withholding treatment from some patients and would not be considered ethical.
The study included 26 patients in each cohort, either receiving mechanical thrombectomy or medical treatment alone. The investigators matched patients by site and side of occlusion, age, and sex. Cases that could not be matched by site of occlusion, the primary criterion, were excluded.
With this methodology, the investigators reduced the impact of selection bias with the aim of providing “the next highest level of comparative evidence,” they stated in the study. However, they also noted that, without randomization, there is likely still some selection bias present.
The two cohorts were not significantly different based on factors such as sex or age. All patients in the study presented within 24 hours of symptom onset, with most eligible for thrombectomy by adult standards. There was a difference between the two cohorts in the timing of arrival to a dedicated hospital and imaging. “Our triage, imaging, and decision-making pathways require streamlining,” the authors concluded, regarding the difference.
‘A heterogeneous condition’
In a comment, Ratika Srivastava, MD, a pediatric neurologist at the University of Alberta, Edmonton, said she was glad to see a well-designed study dedicated to pediatric stroke. Neurologists have traditionally extrapolated from research on adult stroke due to the rarity of pediatric stroke and difficulty of diagnosis.
While physicians have previously relied on findings in adults, stroke presents differently in children. “The challenge is that it’s such a heterogeneous condition,” said Dr. Srivastava, who was not involved in the study. In children, stroke may have several different etiologies, such as a lesion in the heart or arterial disease. “Sometimes it’s amenable to taking the clot out and sometimes it’s not. So you have to figure out: Are they a good candidate for thrombectomy?” This study helps demonstrate that thrombectomy is a good option for some children with LVO stroke, she said.
The study was independently supported. Dr. Srivastava reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A matched case-control study followed 52 patients in Canada and Australia with acute stroke and assessed functional outcomes at 3 months for those who received thrombectomy, compared with those who did not. Patients receiving the procedure had significantly improved clinical outcomes (odds ratio [OR], 3.76). The procedure is the standard of care for adults with large vessel occlusion (LVO) stroke, but limited data exist for children.
“In the absence of a randomized trial, this case-control study demonstrates better clinical outcomes with thrombectomy than medical management for pediatric patients aged 2 to 18 years with anterior circulation LVO stroke,” the authors concluded. The study was published in JAMA Neurology.
Improved results
Untreated LVO stroke is associated with poor outcomes, indicated in this study with scoring based on the modified Rankin Scale. Based on this scoring, 53.8% of patients who were managed conservatively had poor outcomes (moderate disability or greater) at 3 months, confirming previous findings. The data were drawn from five hospitals in Australia and Canada between January 2011 and April 2022.
Removing blood clots with mechanical thrombectomy resulted in improved outcomes 3 months after stroke for the patients included in the study, compared with the neuroprotective measures of medical therapy alone. The improved outcomes persisted in the final available follow-up (OR, 3.65).
In adults, thrombectomy has previously been demonstrated to be a safe and effective treatment for LVO stroke and is currently the standard of care. This study sought to expand the data for pediatric patients, for whom stroke is rarer and difficult to diagnose.
The authors cautioned, however, that the outcomes are from hospitals with pediatric neurology expertise and should not be generalized to settings without specialists.
Case-control study
While previous population-based studies of children with LVO stroke found that conservative treatment was associated with poor outcomes, these studies may include significant selection bias. The investigators chose to conduct the case-control study as an alternative to a randomized control trial, which would require withholding treatment from some patients and would not be considered ethical.
The study included 26 patients in each cohort, either receiving mechanical thrombectomy or medical treatment alone. The investigators matched patients by site and side of occlusion, age, and sex. Cases that could not be matched by site of occlusion, the primary criterion, were excluded.
With this methodology, the investigators reduced the impact of selection bias with the aim of providing “the next highest level of comparative evidence,” they stated in the study. However, they also noted that, without randomization, there is likely still some selection bias present.
The two cohorts were not significantly different based on factors such as sex or age. All patients in the study presented within 24 hours of symptom onset, with most eligible for thrombectomy by adult standards. There was a difference between the two cohorts in the timing of arrival to a dedicated hospital and imaging. “Our triage, imaging, and decision-making pathways require streamlining,” the authors concluded, regarding the difference.
‘A heterogeneous condition’
In a comment, Ratika Srivastava, MD, a pediatric neurologist at the University of Alberta, Edmonton, said she was glad to see a well-designed study dedicated to pediatric stroke. Neurologists have traditionally extrapolated from research on adult stroke due to the rarity of pediatric stroke and difficulty of diagnosis.
While physicians have previously relied on findings in adults, stroke presents differently in children. “The challenge is that it’s such a heterogeneous condition,” said Dr. Srivastava, who was not involved in the study. In children, stroke may have several different etiologies, such as a lesion in the heart or arterial disease. “Sometimes it’s amenable to taking the clot out and sometimes it’s not. So you have to figure out: Are they a good candidate for thrombectomy?” This study helps demonstrate that thrombectomy is a good option for some children with LVO stroke, she said.
The study was independently supported. Dr. Srivastava reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A matched case-control study followed 52 patients in Canada and Australia with acute stroke and assessed functional outcomes at 3 months for those who received thrombectomy, compared with those who did not. Patients receiving the procedure had significantly improved clinical outcomes (odds ratio [OR], 3.76). The procedure is the standard of care for adults with large vessel occlusion (LVO) stroke, but limited data exist for children.
“In the absence of a randomized trial, this case-control study demonstrates better clinical outcomes with thrombectomy than medical management for pediatric patients aged 2 to 18 years with anterior circulation LVO stroke,” the authors concluded. The study was published in JAMA Neurology.
Improved results
Untreated LVO stroke is associated with poor outcomes, indicated in this study with scoring based on the modified Rankin Scale. Based on this scoring, 53.8% of patients who were managed conservatively had poor outcomes (moderate disability or greater) at 3 months, confirming previous findings. The data were drawn from five hospitals in Australia and Canada between January 2011 and April 2022.
Removing blood clots with mechanical thrombectomy resulted in improved outcomes 3 months after stroke for the patients included in the study, compared with the neuroprotective measures of medical therapy alone. The improved outcomes persisted in the final available follow-up (OR, 3.65).
In adults, thrombectomy has previously been demonstrated to be a safe and effective treatment for LVO stroke and is currently the standard of care. This study sought to expand the data for pediatric patients, for whom stroke is rarer and difficult to diagnose.
The authors cautioned, however, that the outcomes are from hospitals with pediatric neurology expertise and should not be generalized to settings without specialists.
Case-control study
While previous population-based studies of children with LVO stroke found that conservative treatment was associated with poor outcomes, these studies may include significant selection bias. The investigators chose to conduct the case-control study as an alternative to a randomized control trial, which would require withholding treatment from some patients and would not be considered ethical.
The study included 26 patients in each cohort, either receiving mechanical thrombectomy or medical treatment alone. The investigators matched patients by site and side of occlusion, age, and sex. Cases that could not be matched by site of occlusion, the primary criterion, were excluded.
With this methodology, the investigators reduced the impact of selection bias with the aim of providing “the next highest level of comparative evidence,” they stated in the study. However, they also noted that, without randomization, there is likely still some selection bias present.
The two cohorts were not significantly different based on factors such as sex or age. All patients in the study presented within 24 hours of symptom onset, with most eligible for thrombectomy by adult standards. There was a difference between the two cohorts in the timing of arrival to a dedicated hospital and imaging. “Our triage, imaging, and decision-making pathways require streamlining,” the authors concluded, regarding the difference.
‘A heterogeneous condition’
In a comment, Ratika Srivastava, MD, a pediatric neurologist at the University of Alberta, Edmonton, said she was glad to see a well-designed study dedicated to pediatric stroke. Neurologists have traditionally extrapolated from research on adult stroke due to the rarity of pediatric stroke and difficulty of diagnosis.
While physicians have previously relied on findings in adults, stroke presents differently in children. “The challenge is that it’s such a heterogeneous condition,” said Dr. Srivastava, who was not involved in the study. In children, stroke may have several different etiologies, such as a lesion in the heart or arterial disease. “Sometimes it’s amenable to taking the clot out and sometimes it’s not. So you have to figure out: Are they a good candidate for thrombectomy?” This study helps demonstrate that thrombectomy is a good option for some children with LVO stroke, she said.
The study was independently supported. Dr. Srivastava reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY