User login
New consensus on managing acetaminophen poisoning
TOPLINE:
An expert panel has updated recommendations for emergency department assessment, management, and treatment of acetaminophen poisoning.
METHODOLOGY:
The United States and Canada have no formal guidelines for managing acetaminophen poisoning, which is characterized by hepatocellular damage and potential liver failure, which can be life-threatening.
The past 25 years has seen the introduction of products that contain greater amounts of acetaminophen, extended-release preparations, and new drugs that combine acetaminophen with opioids or other ingredients.
From the medical literature and current poison control guidelines, the panel used a modified Delphi method to create a decision framework and determine appropriate management, including triage and laboratory evaluation, for acetaminophen poisoning, addressing scenarios such as extended-release and high-risk ingestion, co-ingestion of anticholinergics or opioids, pregnancy, weight greater than 100 kg, and criteria for consultation with a toxicologist.
TAKEAWAY:
The panel emphasized the role of the patient’s history; an inaccurate estimate of the time of ingestion, for example, can lead to the erroneous conclusion that acetylcysteine, a medication used to treat overdose, is not needed or can be discontinued prematurely – a potentially fatal mistake.
The initial dose of acetylcysteine should be administered as soon as the need becomes evident, with the panel recommending at least 300 mg/kg orally or intravenously during the first 20 or 24 hours of treatment.
Management of ingestion of extended-release preparations is the same, with the exception of obtaining a second acetaminophen blood concentration in some cases.
When acetaminophen is co-ingested with anticholinergic or opioid agonist medications, management is the same, except if the first acetaminophen concentration measured at 4-24 hours after ingestion is 10 mcg/mL or less, another measurement and acetylcysteine treatment are not needed.
IN PRACTICE:
“A guideline that provides management guidance could optimize patient outcomes, reduce disruption for patients and caregivers, and reduce costs by shortening the length of hospitalization,” write the authors.
SOURCE:
The study was conducted by Richard C. Dart, MD, PhD, Rocky Mountain Poison and Drug Safety, University of Colorado, Denver, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
The work lacked high-quality data that address clinical decisions needed for managing acetaminophen poisoning. There were only a few well-controlled comparative studies, which focused on specific issues and not on patient management.
DISCLOSURES:
The work was supported by a grant from Johnson & Johnson Consumer Inc. Dr. Dart has reported receiving grants from Johnson & Johnson outside the submitted work. See paper for disclosures of other authors.
A version of this article appeared on Medscape.com.
TOPLINE:
An expert panel has updated recommendations for emergency department assessment, management, and treatment of acetaminophen poisoning.
METHODOLOGY:
The United States and Canada have no formal guidelines for managing acetaminophen poisoning, which is characterized by hepatocellular damage and potential liver failure, which can be life-threatening.
The past 25 years has seen the introduction of products that contain greater amounts of acetaminophen, extended-release preparations, and new drugs that combine acetaminophen with opioids or other ingredients.
From the medical literature and current poison control guidelines, the panel used a modified Delphi method to create a decision framework and determine appropriate management, including triage and laboratory evaluation, for acetaminophen poisoning, addressing scenarios such as extended-release and high-risk ingestion, co-ingestion of anticholinergics or opioids, pregnancy, weight greater than 100 kg, and criteria for consultation with a toxicologist.
TAKEAWAY:
The panel emphasized the role of the patient’s history; an inaccurate estimate of the time of ingestion, for example, can lead to the erroneous conclusion that acetylcysteine, a medication used to treat overdose, is not needed or can be discontinued prematurely – a potentially fatal mistake.
The initial dose of acetylcysteine should be administered as soon as the need becomes evident, with the panel recommending at least 300 mg/kg orally or intravenously during the first 20 or 24 hours of treatment.
Management of ingestion of extended-release preparations is the same, with the exception of obtaining a second acetaminophen blood concentration in some cases.
When acetaminophen is co-ingested with anticholinergic or opioid agonist medications, management is the same, except if the first acetaminophen concentration measured at 4-24 hours after ingestion is 10 mcg/mL or less, another measurement and acetylcysteine treatment are not needed.
IN PRACTICE:
“A guideline that provides management guidance could optimize patient outcomes, reduce disruption for patients and caregivers, and reduce costs by shortening the length of hospitalization,” write the authors.
SOURCE:
The study was conducted by Richard C. Dart, MD, PhD, Rocky Mountain Poison and Drug Safety, University of Colorado, Denver, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
The work lacked high-quality data that address clinical decisions needed for managing acetaminophen poisoning. There were only a few well-controlled comparative studies, which focused on specific issues and not on patient management.
DISCLOSURES:
The work was supported by a grant from Johnson & Johnson Consumer Inc. Dr. Dart has reported receiving grants from Johnson & Johnson outside the submitted work. See paper for disclosures of other authors.
A version of this article appeared on Medscape.com.
TOPLINE:
An expert panel has updated recommendations for emergency department assessment, management, and treatment of acetaminophen poisoning.
METHODOLOGY:
The United States and Canada have no formal guidelines for managing acetaminophen poisoning, which is characterized by hepatocellular damage and potential liver failure, which can be life-threatening.
The past 25 years has seen the introduction of products that contain greater amounts of acetaminophen, extended-release preparations, and new drugs that combine acetaminophen with opioids or other ingredients.
From the medical literature and current poison control guidelines, the panel used a modified Delphi method to create a decision framework and determine appropriate management, including triage and laboratory evaluation, for acetaminophen poisoning, addressing scenarios such as extended-release and high-risk ingestion, co-ingestion of anticholinergics or opioids, pregnancy, weight greater than 100 kg, and criteria for consultation with a toxicologist.
TAKEAWAY:
The panel emphasized the role of the patient’s history; an inaccurate estimate of the time of ingestion, for example, can lead to the erroneous conclusion that acetylcysteine, a medication used to treat overdose, is not needed or can be discontinued prematurely – a potentially fatal mistake.
The initial dose of acetylcysteine should be administered as soon as the need becomes evident, with the panel recommending at least 300 mg/kg orally or intravenously during the first 20 or 24 hours of treatment.
Management of ingestion of extended-release preparations is the same, with the exception of obtaining a second acetaminophen blood concentration in some cases.
When acetaminophen is co-ingested with anticholinergic or opioid agonist medications, management is the same, except if the first acetaminophen concentration measured at 4-24 hours after ingestion is 10 mcg/mL or less, another measurement and acetylcysteine treatment are not needed.
IN PRACTICE:
“A guideline that provides management guidance could optimize patient outcomes, reduce disruption for patients and caregivers, and reduce costs by shortening the length of hospitalization,” write the authors.
SOURCE:
The study was conducted by Richard C. Dart, MD, PhD, Rocky Mountain Poison and Drug Safety, University of Colorado, Denver, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
The work lacked high-quality data that address clinical decisions needed for managing acetaminophen poisoning. There were only a few well-controlled comparative studies, which focused on specific issues and not on patient management.
DISCLOSURES:
The work was supported by a grant from Johnson & Johnson Consumer Inc. Dr. Dart has reported receiving grants from Johnson & Johnson outside the submitted work. See paper for disclosures of other authors.
A version of this article appeared on Medscape.com.
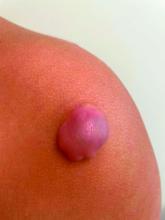
What's the diagnosis?
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
Given the characteristic clinical presentation, the most likely diagnosis is pilomatrixoma.
Pilomatrixomas are benign adnexal tumors that arise from immature matrix cells of the hair follicles located on dermal or subcutaneous tissue.
The cause of pilomatrixoma remains unclear. Recent studies have suggested that the development of pilomatrixoma are related to mutations in the Wnt signaling pathway, where beta-catenin gene (CTNNB1) mutation is the most frequently reported.1-4
Pilomatrixomas are more common in children and often present before 10 years of age.1,2,5 They commonly appear in head and neck, as well as upper extremities, trunk, and lower extremities.2,6
The clinical manifestations of pilomatrixomas are diverse and according to their appearance five classic clinical types are described: mass, pigmented, mixed, ulcerated, and keloid-like.2,3 The mass type is the predominant form, where it generally presents as a hard and freely mobile nodule covered by skin that may present a firm calcified protruding nodule. Other less common types include: lymphangiectasic, anetodermic, perforating, and bullous.2,6,7
Pilomatrixomas are mostly solitary, whereas multiple forms are reported to be associated with familial inheritance or syndromic conditions, such as myotonic dystrophy, Gardner syndrome, Turner’s syndrome, and Rubinstein-Taybi syndrome.2-4 However, children and adolescents occasionally present with multiple pilomatricomas with no associated syndrome.
On physical exam a helpful features for the diagnosis is the “teeter-totter sign,” which can be illustrated by pressing on one edge of the lesion that will cause the opposite edge to protrude from the skin. Another helpful tool is to use a light to transilluminate and the calcification produces a bluish opaque hue,8 as light cannot transmit through the calcification, often differentiating it from epidermal inclusion cysts or other noncalcified lesions.
What is the differential diagnosis?
Because of the diverse clinical presentations, pilomatrixomas are frequently misdiagnosed. The percentage of correct preoperative diagnosis reported is low, varying from 16% to 43% in different series.1,9-11 They most frequently are misdiagnosed as other types of cysts such as epidermal, dermoid, or sebaceous.2,3,5,12,13 Rapidly growing pilomatrixomas can be also be misdiagnosed as malignant soft-tissue tumors, cutaneous lymphoma, or sarcomas.5,13
When presenting with a classic history and physical features, diagnosis is clinical, and no further studies are recommended.14 To improve diagnostic accuracy when encountering unusual subtypes, imaging is recommended, including ultrasound. Ultrasound adds a high positive predictive value (95.56%).2 Generally, on ultrasound a pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing.2 The definitive diagnosis is, however, made by histopathologic examination.
Pilomatrixomas do not spontaneously regress, therefore complete surgical resection is the standard treatment. During the follow-up period, very low recurrence rates have been reported, varying from 1.5% to 2% which generally occurs because of incomplete resection.2,3
Plexiform neurofibromas are usually congenital tumors of peripheral nerve sheath associated with neurofibromatosis type 1, often with a “bag of worms” feel on palpation. Epidermoid cysts generally present as dermal nodules often with a visible puncture, mobile on soft and mobile on palpation. Dermatofibromas present as firm, usually hyperpigmented papule or nodules that are fixed to subcutaneous tissue, thus often “dimpling” when pitched. Dermatofibrosarcoma protuberans is a rare soft-tissue sarcoma which presents as a firm, slow growing indurated plaques growing over months to years.
Conclusion
Pilomatrixomas are a benign adnexal tumor that sometimes can present as atypical forms such as this case. Diagnosis is usually based on clinical diagnosis, and transillumination can be a bedside clue. When the clinical diagnosis remains obscure an ultrasound can be helpful. The main aim of this case is to improve awareness of the variable presentations of pilomatrixomas and the importance of high level of suspicion supported by careful clinical evaluation.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology, University of California, San Diego. Dr. Guelfand is a visiting dermatology resident in the division of pediaric and adolescent dermatology, University of Califonia, San Diego.
References
1. Jones CD et al. Am J Dermatopathol. 2018;40:631-41.
2. Hu JL et al. Arch Craniofac Surg. 2020;21(5):288-93.
3. Adhikari G and Jadhav GS. Cureus. 2022;14(2):22228.
4. Cóbar JP et al. J Surg Case Rep. 2023;2023(4):rjad182.
5. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
6. Kose D et al. J Cancer Res Ther. 2014;10(3):549-51.
7. Sabater-Abad J et al. Dermatol Online J. 2020;26(8):13030/qt4h16s45w.
8. Alkatan HM et al. Int J Surg Case Rep. 2021;84:106068.
9. Pant I et al. Indian J Dermatol. 2010;55:390-2.
10. Kaddu S et al. Am J Dermatopathol. 1996;18(4):333-8
11. Schwarz Y et al. Int J Pediatr Otorhinolaryngol. 2016;85:148-53.
12. Wang YN et al. Chin Med J (Engl). 2021;134(16):2011-2.
13. Yannoutsos A et al. Am J Dermatopathol. 2018;40(9):690-3.
14. Zhao A et al. Ear Nose Throat J. 2021;1455613211044778.
On physical exam there was a well-circumscribed skin-colored nodule measuring 3.1 x 3 cm that was tender on palpation. The nodule was mobile, with a firm, stony feel, and no punctum was visualized. Transillumination revealed a subtle bluish hue within the nodule.
Gout: Suboptimal management a continuing problem
The prevalence of gout is skyrocketing worldwide, and while drugs in the pipeline hold promise for improving the efficacy and safety of treatment, experts warn that “gout remains suboptimally managed.”
“For a really well-understood disease, gout is remarkably undertreated,” said Robert A. Terkeltaub, MD, professor of medicine emeritus at the University of California, San Diego. “This is amazing and depressing because allopurinol has been around for about 60 years or so.”
Randomized, controlled trials show that 80%-90% of patients with gout can be effectively treated to target with existing gout therapies. “Over a year or two, gout flares improve and patients do well,” Dr. Terkeltaub said.
By lowering excessive levels of serum urate, current therapies slow the formation of monosodium urate crystals that precipitate within joints and soft tissues, inducing a highly inflammatory local response with superimposed systemic inflammation. These therapies reduce the frequency of excruciatingly painful gout flares.
“Many patients with gout are not taking urate-lowering therapy at all,” Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and head of crystal-induced arthritis diseases at Brigham and Women’s Hospital, also in Boston, said in an interview.
“Unfortunately, a common problem in gout is treatment inertia,” said Tuhina Neogi, MD, PhD, chief of rheumatology at Boston Medical Center.
On a global scale, only one-third of patients with gout are started on urate-lowering therapy, and more than 50% abandon treatment after 1 year. As a result, the effectiveness of urate-lowering therapies in reality is well below 50%, Dr. Terkeltaub said.
“I think gout has been taken less seriously than it should be for quite some time,” he explained in an interview. Gout’s impact on health and well-being is no trivial matter. A recent study showed that a diagnosis of gout was associated with an increased risk of anxiety and depression, and there is new evidence suggesting that gout flares are associated with an increased risk of cardiovascular events, including fatal myocardial infarction and stroke.
“We need drugs that are not just effective but also safe, and we need to incorporate real-world data into our assessment of treatment effectiveness, especially in the presence of comorbidities,” Dr. Terkeltaub said.
The prevalence of what used to be thought of as the “disease of kings” has increased 100% over the last 30 years, outstripping world population growth and life expectancy. In the United States, an estimated 5% of adults, or 12 million, have gout. Globally, the number affected exceeds 50 million.
The patient demographics associated with gout have also expanded. Once seen primarily in fleshy, middle-aged men of privilege, gout affects more women, more adults at either end of the age spectrum, and more people in Third World countries than ever before.
Management
In the United States, the optimal management of gout remains the subject of debate, with differences in expert opinion reflected in evidence-based clinical guidelines. “We know that the perception of gout is different between primary care physicians, patients, and rheumatologists,” Dr. Terkeltaub said.
The 2017 American College of Physicians guidelines for the management of gout recommend a treat-to-symptom approach to urate-lowering therapy. However, the 2020 American College of Rheumatology guidelines reinforce a standard treat-to-target strategy to a serum urate target of < 6.0 mg/dL.
In their report, the ACR guidelines’ authors stated that the use of urate-lowering therapy for gout has not increased in the last decade. Research shows that adherence to treatment for gout continues to be the lowest among seven common chronic medical conditions, including hypertension and seizure disorders, they said.
Some physicians don’t recommend urate-lowering medication to their patients with gout, and others don’t up-titrate it sufficiently to meet the recommended serum urate target, said Dr. Tedeschi. The latter “can require increasing the dose of allopurinol well beyond the 300 mg that often seems the landing point for many patients with gout,” she pointed out.
In fact, it can take up to 800 mg a day of allopurinol – less in patients with moderate to severe kidney disease – to reduce the symptom burden in gout. And it can take a year or longer of drug testing and titration to reach the optimal serum urate target. Paradoxically, gout flares usually get worse during this time.
“We need to reduce the time it takes to get the patient to the serum urate target, and simplify regimens with once-a-day dosing,” Dr. Terkeltaub said. “We also need greater precision so that we can get a home run, hitting the serum urate target the first or second visit, with minimal dose titration.”
Clinician education is important, but education alone is not enough, Dr. Neogi emphasized. “Just as clinicians treat-to-target in other conditions such as hypertension and diabetes, or titrate warfarin to maintain a certain level of anticoagulation, gout must be monitored and treatments adjusted accordingly,” she said.
Practice changes, such as partnering with nursing or pharmacy, may help facilitate in-clinic dose titration, “much like a warfarin clinic,” Dr. Neogi suggested.
That’s exactly what Dr. Terkeltaub has done. Overwhelmed by the number of gout consults, Dr. Terkeltaub and his team set up a pharmacist-managed, rheumatology-supervised clinic to care for gout patients remotely. The model has been very successful, he said. Nurses and clinical pharmacists educate the patients and manage their lab testing and prescriptions, all according to ACR guidelines.
The treatment of gout has become more complex, with a greater risk of drug complications and interactions, particularly in older patients with comorbid diabetes, chronic kidney disease, and heart disease. Many of the patients he sees are already on “10, 15, or 20 other medications,” Dr. Terkeltaub noted.
The steps involved in the titration of urate-lowering therapy also complicate the treatment of gout, making it impractical for many patients and impossible for others whose access to primary care is limited to one or two visits a year. The process of drug titration, with steadily increasing doses, can make patients anxious about the possibility of being overmedicated. Taking a drug every day, even when joints feel “normal,” can also increase the risk of nonadherence.
“In our conversations with patients with gout, it’s extremely important that we counsel them about the need to take urate-lowering therapy on an ongoing basis to reduce the risk of a gout flare,” said Dr. Tedeschi. “Patients need to have prescription refills available and know to contact the doctor before they run out, so that the chances of having a gout flare are reduced.”
Current drugs
Although urate-lowering drugs form the cornerstone of gout therapy, there are only three oral medications available in the United States currently, and all have significant limitations. “We need more drugs, basically,” Dr. Terkeltaub said.
- Allopurinol (Zyloprim, Aloprim), an inexpensive xanthine oxidase inhibitor (XOI), is still considered a first-line treatment, but is associated with allopurinol hypersensitivity syndrome. In select patients of Asian, African, and Arab descent, this adverse drug reaction can be life-threatening, and is associated with a mortality rate of 20%-25%.
- Febuxostat (Uloric), another XOI, is considered a second-line drug in the treatment of gout, but has carried a boxed warning from the Food and Drug Administration since 2019. It is associated with a significantly increased risk of cardiovascular death.
- Probenecid (Probalan), a uricosuric agent that increases renal uric acid excretion, is associated with an increased risk of drug interactions and kidney stones, and is rarely used.
Drugs in the pipeline
New drugs in the pipeline offer treatment options that are not only effective but also safe. “This will be important in clinical practice, especially for patients in whom existing medications are contraindicated or there is an increased risk of side effects,” Dr. Neogi said.
Most of these investigational drugs are uricosuric agents that increase the renal excretion of uric acid, reducing serum levels. “The pipeline of new drugs is rich,” Dr. Terkeltaub said. “These drugs are very selective and really work well and they appear to be safe.”
AR882, an inhibitor of selective uric acid transporter 1 (URAT1), is shaping up to be one of them. In July, results from a phase 2b study of AR882 were presented at the annual European Congress of Rheumatology in Milan. They showed that in the intent-to-treat population, 73% of patients had serum uric acid levels < 5 mg/dL and 55% had < 4 mg/dL by week 12 of therapy. In the per-protocol analysis, 82% had serum uric acid levels < 5 mg/dL and 63% < 4 mg/dL.
“These efficacy results are not typically what you see with a once-daily oral medication, so it is really exciting,” said Robert Keenan, MD, chief medical officer of Arthrosi Therapeutics, San Diego, who presented the results.
“More efficacious URAT1 inhibitors that are safe and have a reduced pill burden will be useful additions to current urate lowering options,” Dr. Neogi said.
The recent phase 3 DISSOLVE I and II trials of the investigational uricase-based infusion therapy SEL-212 in refractory gout have also demonstrated encouraging results, particularly in older patients. In DISSOLVE I, a response rate of 65% was observed in patients 50 years of age and older at least 80% of the time during month 6 of treatment. In DISSOLVE II, a response rate of 47% was reported in older patients.
SEL-212, which is made up of PEGylated uricase (pegadricase) coadministered with sirolimus (Rapamycin), will be submitted for U.S. regulatory approval in the first half of 2024.
In the management of gout flares, interleukin (IL)-1beta and inflammasome inhibitors, both of which target specific inflammatory pathways, could also provide attractive additions to urate-lowering therapies. Other agents commonly used in the treatment of flares, such as NSAIDs, steroids, and colchicine (Colcrys), are not as specific, and have side effects that often limit their usability, Dr. Neogi said.
In the meantime, new research indicates that an inflammasome inhibitor that has already been approved for use in diabetes may provide distinct benefits for the management of gout. An analysis of data from 15,067 adults with both gout and type 2 diabetes showed that when a sodium-glucose cotransporter 2 (SGLT-2) inhibitor was added to urate-lowering therapy, the symptoms of gout, including flares, were significantly reduced, resulting in fewer emergency department visits and hospitalizations.
“SGLT-2 inhibitors have anti-inflammatory activity that limits the progression of kidney failure, heart failure, and will also lower the serum uric acid,” said Dr. Terkeltaub. “That’s a major development.”
Dr. Neogi disclosed relationships with Novartis, Pfizer/Lilly, and Regeneron, Dr. Terkeltaub reported relationships with Dyve, Fortress, and Atom, and Dr. Tedeschi reported a relationship with Novartis.
This story was updated on August 14, 2023.
The prevalence of gout is skyrocketing worldwide, and while drugs in the pipeline hold promise for improving the efficacy and safety of treatment, experts warn that “gout remains suboptimally managed.”
“For a really well-understood disease, gout is remarkably undertreated,” said Robert A. Terkeltaub, MD, professor of medicine emeritus at the University of California, San Diego. “This is amazing and depressing because allopurinol has been around for about 60 years or so.”
Randomized, controlled trials show that 80%-90% of patients with gout can be effectively treated to target with existing gout therapies. “Over a year or two, gout flares improve and patients do well,” Dr. Terkeltaub said.
By lowering excessive levels of serum urate, current therapies slow the formation of monosodium urate crystals that precipitate within joints and soft tissues, inducing a highly inflammatory local response with superimposed systemic inflammation. These therapies reduce the frequency of excruciatingly painful gout flares.
“Many patients with gout are not taking urate-lowering therapy at all,” Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and head of crystal-induced arthritis diseases at Brigham and Women’s Hospital, also in Boston, said in an interview.
“Unfortunately, a common problem in gout is treatment inertia,” said Tuhina Neogi, MD, PhD, chief of rheumatology at Boston Medical Center.
On a global scale, only one-third of patients with gout are started on urate-lowering therapy, and more than 50% abandon treatment after 1 year. As a result, the effectiveness of urate-lowering therapies in reality is well below 50%, Dr. Terkeltaub said.
“I think gout has been taken less seriously than it should be for quite some time,” he explained in an interview. Gout’s impact on health and well-being is no trivial matter. A recent study showed that a diagnosis of gout was associated with an increased risk of anxiety and depression, and there is new evidence suggesting that gout flares are associated with an increased risk of cardiovascular events, including fatal myocardial infarction and stroke.
“We need drugs that are not just effective but also safe, and we need to incorporate real-world data into our assessment of treatment effectiveness, especially in the presence of comorbidities,” Dr. Terkeltaub said.
The prevalence of what used to be thought of as the “disease of kings” has increased 100% over the last 30 years, outstripping world population growth and life expectancy. In the United States, an estimated 5% of adults, or 12 million, have gout. Globally, the number affected exceeds 50 million.
The patient demographics associated with gout have also expanded. Once seen primarily in fleshy, middle-aged men of privilege, gout affects more women, more adults at either end of the age spectrum, and more people in Third World countries than ever before.
Management
In the United States, the optimal management of gout remains the subject of debate, with differences in expert opinion reflected in evidence-based clinical guidelines. “We know that the perception of gout is different between primary care physicians, patients, and rheumatologists,” Dr. Terkeltaub said.
The 2017 American College of Physicians guidelines for the management of gout recommend a treat-to-symptom approach to urate-lowering therapy. However, the 2020 American College of Rheumatology guidelines reinforce a standard treat-to-target strategy to a serum urate target of < 6.0 mg/dL.
In their report, the ACR guidelines’ authors stated that the use of urate-lowering therapy for gout has not increased in the last decade. Research shows that adherence to treatment for gout continues to be the lowest among seven common chronic medical conditions, including hypertension and seizure disorders, they said.
Some physicians don’t recommend urate-lowering medication to their patients with gout, and others don’t up-titrate it sufficiently to meet the recommended serum urate target, said Dr. Tedeschi. The latter “can require increasing the dose of allopurinol well beyond the 300 mg that often seems the landing point for many patients with gout,” she pointed out.
In fact, it can take up to 800 mg a day of allopurinol – less in patients with moderate to severe kidney disease – to reduce the symptom burden in gout. And it can take a year or longer of drug testing and titration to reach the optimal serum urate target. Paradoxically, gout flares usually get worse during this time.
“We need to reduce the time it takes to get the patient to the serum urate target, and simplify regimens with once-a-day dosing,” Dr. Terkeltaub said. “We also need greater precision so that we can get a home run, hitting the serum urate target the first or second visit, with minimal dose titration.”
Clinician education is important, but education alone is not enough, Dr. Neogi emphasized. “Just as clinicians treat-to-target in other conditions such as hypertension and diabetes, or titrate warfarin to maintain a certain level of anticoagulation, gout must be monitored and treatments adjusted accordingly,” she said.
Practice changes, such as partnering with nursing or pharmacy, may help facilitate in-clinic dose titration, “much like a warfarin clinic,” Dr. Neogi suggested.
That’s exactly what Dr. Terkeltaub has done. Overwhelmed by the number of gout consults, Dr. Terkeltaub and his team set up a pharmacist-managed, rheumatology-supervised clinic to care for gout patients remotely. The model has been very successful, he said. Nurses and clinical pharmacists educate the patients and manage their lab testing and prescriptions, all according to ACR guidelines.
The treatment of gout has become more complex, with a greater risk of drug complications and interactions, particularly in older patients with comorbid diabetes, chronic kidney disease, and heart disease. Many of the patients he sees are already on “10, 15, or 20 other medications,” Dr. Terkeltaub noted.
The steps involved in the titration of urate-lowering therapy also complicate the treatment of gout, making it impractical for many patients and impossible for others whose access to primary care is limited to one or two visits a year. The process of drug titration, with steadily increasing doses, can make patients anxious about the possibility of being overmedicated. Taking a drug every day, even when joints feel “normal,” can also increase the risk of nonadherence.
“In our conversations with patients with gout, it’s extremely important that we counsel them about the need to take urate-lowering therapy on an ongoing basis to reduce the risk of a gout flare,” said Dr. Tedeschi. “Patients need to have prescription refills available and know to contact the doctor before they run out, so that the chances of having a gout flare are reduced.”
Current drugs
Although urate-lowering drugs form the cornerstone of gout therapy, there are only three oral medications available in the United States currently, and all have significant limitations. “We need more drugs, basically,” Dr. Terkeltaub said.
- Allopurinol (Zyloprim, Aloprim), an inexpensive xanthine oxidase inhibitor (XOI), is still considered a first-line treatment, but is associated with allopurinol hypersensitivity syndrome. In select patients of Asian, African, and Arab descent, this adverse drug reaction can be life-threatening, and is associated with a mortality rate of 20%-25%.
- Febuxostat (Uloric), another XOI, is considered a second-line drug in the treatment of gout, but has carried a boxed warning from the Food and Drug Administration since 2019. It is associated with a significantly increased risk of cardiovascular death.
- Probenecid (Probalan), a uricosuric agent that increases renal uric acid excretion, is associated with an increased risk of drug interactions and kidney stones, and is rarely used.
Drugs in the pipeline
New drugs in the pipeline offer treatment options that are not only effective but also safe. “This will be important in clinical practice, especially for patients in whom existing medications are contraindicated or there is an increased risk of side effects,” Dr. Neogi said.
Most of these investigational drugs are uricosuric agents that increase the renal excretion of uric acid, reducing serum levels. “The pipeline of new drugs is rich,” Dr. Terkeltaub said. “These drugs are very selective and really work well and they appear to be safe.”
AR882, an inhibitor of selective uric acid transporter 1 (URAT1), is shaping up to be one of them. In July, results from a phase 2b study of AR882 were presented at the annual European Congress of Rheumatology in Milan. They showed that in the intent-to-treat population, 73% of patients had serum uric acid levels < 5 mg/dL and 55% had < 4 mg/dL by week 12 of therapy. In the per-protocol analysis, 82% had serum uric acid levels < 5 mg/dL and 63% < 4 mg/dL.
“These efficacy results are not typically what you see with a once-daily oral medication, so it is really exciting,” said Robert Keenan, MD, chief medical officer of Arthrosi Therapeutics, San Diego, who presented the results.
“More efficacious URAT1 inhibitors that are safe and have a reduced pill burden will be useful additions to current urate lowering options,” Dr. Neogi said.
The recent phase 3 DISSOLVE I and II trials of the investigational uricase-based infusion therapy SEL-212 in refractory gout have also demonstrated encouraging results, particularly in older patients. In DISSOLVE I, a response rate of 65% was observed in patients 50 years of age and older at least 80% of the time during month 6 of treatment. In DISSOLVE II, a response rate of 47% was reported in older patients.
SEL-212, which is made up of PEGylated uricase (pegadricase) coadministered with sirolimus (Rapamycin), will be submitted for U.S. regulatory approval in the first half of 2024.
In the management of gout flares, interleukin (IL)-1beta and inflammasome inhibitors, both of which target specific inflammatory pathways, could also provide attractive additions to urate-lowering therapies. Other agents commonly used in the treatment of flares, such as NSAIDs, steroids, and colchicine (Colcrys), are not as specific, and have side effects that often limit their usability, Dr. Neogi said.
In the meantime, new research indicates that an inflammasome inhibitor that has already been approved for use in diabetes may provide distinct benefits for the management of gout. An analysis of data from 15,067 adults with both gout and type 2 diabetes showed that when a sodium-glucose cotransporter 2 (SGLT-2) inhibitor was added to urate-lowering therapy, the symptoms of gout, including flares, were significantly reduced, resulting in fewer emergency department visits and hospitalizations.
“SGLT-2 inhibitors have anti-inflammatory activity that limits the progression of kidney failure, heart failure, and will also lower the serum uric acid,” said Dr. Terkeltaub. “That’s a major development.”
Dr. Neogi disclosed relationships with Novartis, Pfizer/Lilly, and Regeneron, Dr. Terkeltaub reported relationships with Dyve, Fortress, and Atom, and Dr. Tedeschi reported a relationship with Novartis.
This story was updated on August 14, 2023.
The prevalence of gout is skyrocketing worldwide, and while drugs in the pipeline hold promise for improving the efficacy and safety of treatment, experts warn that “gout remains suboptimally managed.”
“For a really well-understood disease, gout is remarkably undertreated,” said Robert A. Terkeltaub, MD, professor of medicine emeritus at the University of California, San Diego. “This is amazing and depressing because allopurinol has been around for about 60 years or so.”
Randomized, controlled trials show that 80%-90% of patients with gout can be effectively treated to target with existing gout therapies. “Over a year or two, gout flares improve and patients do well,” Dr. Terkeltaub said.
By lowering excessive levels of serum urate, current therapies slow the formation of monosodium urate crystals that precipitate within joints and soft tissues, inducing a highly inflammatory local response with superimposed systemic inflammation. These therapies reduce the frequency of excruciatingly painful gout flares.
“Many patients with gout are not taking urate-lowering therapy at all,” Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and head of crystal-induced arthritis diseases at Brigham and Women’s Hospital, also in Boston, said in an interview.
“Unfortunately, a common problem in gout is treatment inertia,” said Tuhina Neogi, MD, PhD, chief of rheumatology at Boston Medical Center.
On a global scale, only one-third of patients with gout are started on urate-lowering therapy, and more than 50% abandon treatment after 1 year. As a result, the effectiveness of urate-lowering therapies in reality is well below 50%, Dr. Terkeltaub said.
“I think gout has been taken less seriously than it should be for quite some time,” he explained in an interview. Gout’s impact on health and well-being is no trivial matter. A recent study showed that a diagnosis of gout was associated with an increased risk of anxiety and depression, and there is new evidence suggesting that gout flares are associated with an increased risk of cardiovascular events, including fatal myocardial infarction and stroke.
“We need drugs that are not just effective but also safe, and we need to incorporate real-world data into our assessment of treatment effectiveness, especially in the presence of comorbidities,” Dr. Terkeltaub said.
The prevalence of what used to be thought of as the “disease of kings” has increased 100% over the last 30 years, outstripping world population growth and life expectancy. In the United States, an estimated 5% of adults, or 12 million, have gout. Globally, the number affected exceeds 50 million.
The patient demographics associated with gout have also expanded. Once seen primarily in fleshy, middle-aged men of privilege, gout affects more women, more adults at either end of the age spectrum, and more people in Third World countries than ever before.
Management
In the United States, the optimal management of gout remains the subject of debate, with differences in expert opinion reflected in evidence-based clinical guidelines. “We know that the perception of gout is different between primary care physicians, patients, and rheumatologists,” Dr. Terkeltaub said.
The 2017 American College of Physicians guidelines for the management of gout recommend a treat-to-symptom approach to urate-lowering therapy. However, the 2020 American College of Rheumatology guidelines reinforce a standard treat-to-target strategy to a serum urate target of < 6.0 mg/dL.
In their report, the ACR guidelines’ authors stated that the use of urate-lowering therapy for gout has not increased in the last decade. Research shows that adherence to treatment for gout continues to be the lowest among seven common chronic medical conditions, including hypertension and seizure disorders, they said.
Some physicians don’t recommend urate-lowering medication to their patients with gout, and others don’t up-titrate it sufficiently to meet the recommended serum urate target, said Dr. Tedeschi. The latter “can require increasing the dose of allopurinol well beyond the 300 mg that often seems the landing point for many patients with gout,” she pointed out.
In fact, it can take up to 800 mg a day of allopurinol – less in patients with moderate to severe kidney disease – to reduce the symptom burden in gout. And it can take a year or longer of drug testing and titration to reach the optimal serum urate target. Paradoxically, gout flares usually get worse during this time.
“We need to reduce the time it takes to get the patient to the serum urate target, and simplify regimens with once-a-day dosing,” Dr. Terkeltaub said. “We also need greater precision so that we can get a home run, hitting the serum urate target the first or second visit, with minimal dose titration.”
Clinician education is important, but education alone is not enough, Dr. Neogi emphasized. “Just as clinicians treat-to-target in other conditions such as hypertension and diabetes, or titrate warfarin to maintain a certain level of anticoagulation, gout must be monitored and treatments adjusted accordingly,” she said.
Practice changes, such as partnering with nursing or pharmacy, may help facilitate in-clinic dose titration, “much like a warfarin clinic,” Dr. Neogi suggested.
That’s exactly what Dr. Terkeltaub has done. Overwhelmed by the number of gout consults, Dr. Terkeltaub and his team set up a pharmacist-managed, rheumatology-supervised clinic to care for gout patients remotely. The model has been very successful, he said. Nurses and clinical pharmacists educate the patients and manage their lab testing and prescriptions, all according to ACR guidelines.
The treatment of gout has become more complex, with a greater risk of drug complications and interactions, particularly in older patients with comorbid diabetes, chronic kidney disease, and heart disease. Many of the patients he sees are already on “10, 15, or 20 other medications,” Dr. Terkeltaub noted.
The steps involved in the titration of urate-lowering therapy also complicate the treatment of gout, making it impractical for many patients and impossible for others whose access to primary care is limited to one or two visits a year. The process of drug titration, with steadily increasing doses, can make patients anxious about the possibility of being overmedicated. Taking a drug every day, even when joints feel “normal,” can also increase the risk of nonadherence.
“In our conversations with patients with gout, it’s extremely important that we counsel them about the need to take urate-lowering therapy on an ongoing basis to reduce the risk of a gout flare,” said Dr. Tedeschi. “Patients need to have prescription refills available and know to contact the doctor before they run out, so that the chances of having a gout flare are reduced.”
Current drugs
Although urate-lowering drugs form the cornerstone of gout therapy, there are only three oral medications available in the United States currently, and all have significant limitations. “We need more drugs, basically,” Dr. Terkeltaub said.
- Allopurinol (Zyloprim, Aloprim), an inexpensive xanthine oxidase inhibitor (XOI), is still considered a first-line treatment, but is associated with allopurinol hypersensitivity syndrome. In select patients of Asian, African, and Arab descent, this adverse drug reaction can be life-threatening, and is associated with a mortality rate of 20%-25%.
- Febuxostat (Uloric), another XOI, is considered a second-line drug in the treatment of gout, but has carried a boxed warning from the Food and Drug Administration since 2019. It is associated with a significantly increased risk of cardiovascular death.
- Probenecid (Probalan), a uricosuric agent that increases renal uric acid excretion, is associated with an increased risk of drug interactions and kidney stones, and is rarely used.
Drugs in the pipeline
New drugs in the pipeline offer treatment options that are not only effective but also safe. “This will be important in clinical practice, especially for patients in whom existing medications are contraindicated or there is an increased risk of side effects,” Dr. Neogi said.
Most of these investigational drugs are uricosuric agents that increase the renal excretion of uric acid, reducing serum levels. “The pipeline of new drugs is rich,” Dr. Terkeltaub said. “These drugs are very selective and really work well and they appear to be safe.”
AR882, an inhibitor of selective uric acid transporter 1 (URAT1), is shaping up to be one of them. In July, results from a phase 2b study of AR882 were presented at the annual European Congress of Rheumatology in Milan. They showed that in the intent-to-treat population, 73% of patients had serum uric acid levels < 5 mg/dL and 55% had < 4 mg/dL by week 12 of therapy. In the per-protocol analysis, 82% had serum uric acid levels < 5 mg/dL and 63% < 4 mg/dL.
“These efficacy results are not typically what you see with a once-daily oral medication, so it is really exciting,” said Robert Keenan, MD, chief medical officer of Arthrosi Therapeutics, San Diego, who presented the results.
“More efficacious URAT1 inhibitors that are safe and have a reduced pill burden will be useful additions to current urate lowering options,” Dr. Neogi said.
The recent phase 3 DISSOLVE I and II trials of the investigational uricase-based infusion therapy SEL-212 in refractory gout have also demonstrated encouraging results, particularly in older patients. In DISSOLVE I, a response rate of 65% was observed in patients 50 years of age and older at least 80% of the time during month 6 of treatment. In DISSOLVE II, a response rate of 47% was reported in older patients.
SEL-212, which is made up of PEGylated uricase (pegadricase) coadministered with sirolimus (Rapamycin), will be submitted for U.S. regulatory approval in the first half of 2024.
In the management of gout flares, interleukin (IL)-1beta and inflammasome inhibitors, both of which target specific inflammatory pathways, could also provide attractive additions to urate-lowering therapies. Other agents commonly used in the treatment of flares, such as NSAIDs, steroids, and colchicine (Colcrys), are not as specific, and have side effects that often limit their usability, Dr. Neogi said.
In the meantime, new research indicates that an inflammasome inhibitor that has already been approved for use in diabetes may provide distinct benefits for the management of gout. An analysis of data from 15,067 adults with both gout and type 2 diabetes showed that when a sodium-glucose cotransporter 2 (SGLT-2) inhibitor was added to urate-lowering therapy, the symptoms of gout, including flares, were significantly reduced, resulting in fewer emergency department visits and hospitalizations.
“SGLT-2 inhibitors have anti-inflammatory activity that limits the progression of kidney failure, heart failure, and will also lower the serum uric acid,” said Dr. Terkeltaub. “That’s a major development.”
Dr. Neogi disclosed relationships with Novartis, Pfizer/Lilly, and Regeneron, Dr. Terkeltaub reported relationships with Dyve, Fortress, and Atom, and Dr. Tedeschi reported a relationship with Novartis.
This story was updated on August 14, 2023.
We asked doctors using AI scribes: Just how good are they?
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Andrea Partida, DO, an obstetrician and gynecologist in Enid, Okla., loves her new assistant.
The 15 or 20 minutes she used to spend on documentation for each patient visit is now 3. The 2-3 hours she’d spend charting outside clinic hours is maybe 1.
All that time saved allows her to see two to five more patients a day, provide better care to each patient, and get more involved in hospital leadership at Integris Health, where she works.
“I have a better work-life balance with my family,” Dr. Partida said. “I leave work at work and get home earlier.”
You’ve probably figured out the plot twist: Dr. Partida’s assistant is not a person – it’s artificial intelligence (AI).
Dr. Partida uses IRIS, a tool from OnPoint Healthcare Partners, part of a fast-growing niche of AI medical scribes designed to automate onerous data entry. The evolution of generative AI – specifically, large language models, such as ChatGPT – has led to a rapid explosion of these tools. Other companies in the space include Abridge, Ambience Healthcare, Augmedix, DeepScribe, Nuance (part of Microsoft), and Suki. The newest kid on the block, Amazon Web Services, announced the launch of HealthScribe in July.
These tools – some of which are already on the market, with more on the way – record patient visits and generate notes for treatment and billing. Earlier iterations combine AI with offsite human scribes who provide quality control. But more and more are fully automated, no human required. Some also offer video recording and foreign language translation.
The promise is alluring: Ease your workload and reclaim hours in your day so you can spend more time with patients or try that “work-life balance” thing you’ve heard so much about.
But do these tools fulfill that promise?
According to Dr. Partida and other doctors who spoke with this news organization, the answer is a resounding yes.
A tech solution for a tech problem
“I believe a lot of doctors see patients for free. They get paid to do paperwork,” said Anthony J. Mazzarelli, MD, JD, MBE, co-president and CEO of Cooper University Health Care, in Camden, N.J.
Indeed, for every hour U.S. clinicians spend with their patients, they may spend 2 more hours documenting in electronic health records (EHRs), estimates show. About half of doctors, especially those in primary care, report feeling burned out, and some 42% say they want to quit clinical practice.
Enter AI scribes.
“The holy grail in medicine right now is improving burnout while also maintaining or improving productivity and quality,” said Patricia Garcia, MD, associate clinical information officer for ambulatory care at Stanford (Calif.) Health Care. “These ambient digital scribes have the potential to do just that.”
While anyone can buy these products, their use has been mostly limited to pilot programs and early adopters so far, said Dr. Garcia, who has been helping to pilot Nuance’s digital scribe, DAX, at Stanford.
But that’s expected to change quickly. “I don’t think the time horizon is a decade,” Dr. Garcia said. “I think within a matter of 2 or 3 years, these tools will be pervasive throughout health care.”
Since introducing these tools at Cooper, “our doctors’ paperwork burden is significantly lighter,” said Dr. Mazzarelli, who decides which technologies Cooper should invest in and who monitors their results. In Cooper studies, physicians who used DAX more than half the time spent 43% less time working on notes.
“They spend more time connecting with their patients, talking with them, and looking them in the eye,” Dr. Mazzarelli said. That, in turn, seems to improve patient outcomes, reduce doctor burnout and turnover, and lower costs.
The AI scribes, by virtue of eliminating the distraction of note taking, also allow doctors to give their full attention to the patient. “The patient relationship is the most important aspect of medicine,” said Raul Ayala, MD, MHCM, a family medicine physician at Adventist Health, in Hanford, Calif., who uses Augmedix. The digital scribe “helps us strengthen that relationship.”
What’s it like to use an AI medical scribe?
The scribes feature hardware (typically a smartphone or tablet) and software built on automatic speech recognition, natural language processing, and machine learning. Download an app to your device, and you’re ready to go. Use it to record in-person or telehealth visits.
In the first week, a company may help train you to use the hardware and software. You’ll likely start by using it for a few patient visits per day, ramping up gradually. Dr. Partida said she was comfortable using the system for all her patients in 6 weeks.
Each day, Dr. Partida logs in to a dedicated smartphone or tablet, opens the app, and reviews her schedule, including details she needs to prepare for each patient.
At the start of each patient visit, Dr. Partida taps the app icon to begin recording and lays the device nearby. She can pause as needed. At the end of the visit, she taps the icon again to stop recording.
The AI listens, creates the note, and updates relevant data in the EHR. The note includes patient problems, assessment, treatment plan, patient history, orders, and tasks for staff, along with medications, referrals, and preauthorizations. A human scribe, who is also a physician, reviews the information for accuracy and edits it as needed. By the next morning, the data are ready for Dr. Partida to review.
Fully automated versions can generate notes much faster. Jack Shilling, MD, MBA, an orthopedic surgeon at Cooper University Health Care, in Voorhees, N.J., uses DAX. A new feature called DAX Express – which uses OpenAI’s GPT-4 but no humans – provides him with a draft of his clinical notes in just seconds.
How accurate are AI notes?
The accuracy of those notes remains an open question, Dr. Garcia said – mostly because accuracy can be hard to define.
“If you asked five docs to write a note based on the same patient encounter, you’d get five different notes,” Dr. Garcia said. “That makes it hard to assess these technologies in a scientifically rigorous way.”
Still, the onus is on the physician to review the notes and edit them as needed, Dr. Garcia said. How light or heavy those edits are can depend on your unique preferences.
Dr. Shilling said he may need to lightly edit transcripts of his conversations with patients. “When someone tells me how long their knee hurts, slight variability in their transcribed words is tolerable,” he said. But for some things – such as physical exam notes and x-ray readings – he dictates directly into the device, speaking at a closer range and being less conversational, more exact in his speech.
Should you let patients know they’re being recorded?
The federal Health Insurance Portability and Accountability Act (HIPAA) does not require providers to inform patients that their face-to-face conversations are being recorded, said Daniel Lebovic, JD, corporate legal counsel at Compliancy Group, in Greenlawn, N.Y., a company that helps providers adhere to HIPAA rules.
But make sure you know the laws in your state and the policies at your health care practice. State laws may require providers to inform patients and to get patients’ consent in advance of being recorded.
All the doctors who spoke to this news organization said their patients are informed that they’ll be recorded and that they can opt out if they wish.
How much do AI scribes cost?
As the marketplace for these tools expands, companies are offering more products and services at different price points that target a range of organizations, from large health care systems to small private practices.
Price models vary, said Dr. Garcia. Some are based on the number of users, others on the number of notes, and still others on minutes.
Amazon’s HealthScribe is priced at 10 cents per minute. For 1,000 consultation transcripts per month, with each call averaging 15 minutes, it would take 15,000 minutes at a total cost of $1,500 for the month.
In general, the rapidly growing competition in this space could mean prices become more affordable, Dr. Garcia said. “It’s good that so many are getting into this game, because that means the price will come down and it will be a lot more accessible to everybody.”
A version of this article appeared on Medscape.com.
Generic inhalers for COPD support hold their own
Sometimes we get what we pay for. Other times we pay too much.
That’s the message of a study published in Annals of Internal Medicine, which finds that a generic maintenance inhaler is as effective at managing symptoms of chronic obstructive pulmonary disorder (COPD) as a pricier branded alternative.
In 2019, the Food and Drug Administration approved Wixela Inhub (the combination corticosteroid/long-acting beta2 adrenergic agonist fluticasone-salmeterol; Viatris) as a generic dry powder inhaler for managing symptoms of COPD. This approval was based on evidence of the generic’s effectiveness against asthma, although COPD also was on the product label. The study authors compared Wixela’s effectiveness in controlling symptoms of COPD with that of the brand name inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline), which uses the same active ingredients.
The result: “The generic looks to be as safe and effective as the brand name. I don’t see a clinical reason why one would ever need to get the brand name over the generic version,” said study author William Feldman, MD, DPhil, MPH, a health services researcher and pulmonologist at Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
Same types of patients, different inhalers, same outcomes
Dr. Feldman and colleagues compared the medical records of 10,000 patients with COPD who began using the branded inhaler to the records of another 10,000 patients with COPD who opted for the generic alternative. Participants in the two groups were evenly matched by age, sex, race, and ethnicity, region, severity of COPD, and presence of other comorbidities, according to the researchers. Participants were all older than age 40, and the average age in both groups was 72 years.
The researchers looked for a difference in a first episode of a moderate exacerbation of COPD, defined as requiring a course of prednisone for 5-14 days. They also looked for cases of severe COPD exacerbation requiring hospitalization in the year after people began using either the generic or brand name inhaler. And they looked for differences across 1 year in rates of hospitalization for pneumonia.
For none of those outcomes, however, did the type of inhaler appear to matter. Compared with the brand-name drug, using the generic was associated with nearly identical rates of moderate or severe COPD exacerbation (hazard ratio, 0.97; 95% confidence interval, 0.90-1.04. The same was true for the proportion of people who went to the hospital for pneumonia at least once (HR, 0.99; 95% CI, 0.86-1.15).
“To get through the FDA as an interchangeable generic, the generic firms have to show that their product can be used in just the same way as the brand-name version,” Dr. Feldman said, which may explain why the generic and brand-name versions of the inhaler performed so similarly.
Dr. Feldman cautioned that the price savings for patients who opt for the generic over the branded product are hard to determine, given the vagaries of different insurance plans and potential rebates when using the branded project. As a general matter, having a single generic competitor will not lower costs much, Dr. Feldman noted, pointing to 2017 research from Harvard that found a profusion of generic competitors is needed to significantly lower health care costs.
“I don’t want to in any way underestimate the importance of getting that first generic onto the market, because it sets the stage for future generics,” Dr. Feldman said.
“There are very few generic options for patients with COPD,” said Surya Bhatt, MD, director of the Pulmonary Function and Exercise Physiology Lab at the University of Alabama at Birmingham. Even the rescue inhalers that people with COPD use to manage acute episodes of the condition are usually branded at this time, Dr. Bhatt noted, with few generic options.*
“The results are quite compelling,” said Dr. Bhatt, who was not involved in the research. Although the trial was not randomized, he commended the researchers for stratifying participants in the two groups to be as comparable as possible.
Dr. Bhatt noted that the FDA’s 2019 approval – given that the agency requires bioequivalence studies between branded and generic products – was enough to cause him to begin prescribing the generic inhaler. The fact that this approval was based on asthma but not also COPD is not a concern.
“There are so many similarities between asthma, COPD, and some obstructive lung diseases,” Dr. Bhatt noted.
In his experience, the only time someone with COPD continues using the branded inhaler – now that a potentially cheaper generic is available – is when their insurance plan makes their out-of-pocket cost minimal. Otherwise, brand loyalty does not exist.
“Patients are generally okay with being on a generic for inhalers, just because of the high cost,” Dr. Bhatt said.
The study was primarily supported by the National Heart, Lung, and Blood Institute. Dr. Feldman reported funding from Arnold Ventures, the Commonwealth Fund, and the FDA, and consulting relationships with Alosa Health and Aetion. Dr. Bhatt reported no relevant financial relationships.
*Correction, 8/16/23: An earlier version of this article mischaracterized Dr. Bhatt's comments on the availability of generic options.
A version of this article first appeared on Medscape.com.
Sometimes we get what we pay for. Other times we pay too much.
That’s the message of a study published in Annals of Internal Medicine, which finds that a generic maintenance inhaler is as effective at managing symptoms of chronic obstructive pulmonary disorder (COPD) as a pricier branded alternative.
In 2019, the Food and Drug Administration approved Wixela Inhub (the combination corticosteroid/long-acting beta2 adrenergic agonist fluticasone-salmeterol; Viatris) as a generic dry powder inhaler for managing symptoms of COPD. This approval was based on evidence of the generic’s effectiveness against asthma, although COPD also was on the product label. The study authors compared Wixela’s effectiveness in controlling symptoms of COPD with that of the brand name inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline), which uses the same active ingredients.
The result: “The generic looks to be as safe and effective as the brand name. I don’t see a clinical reason why one would ever need to get the brand name over the generic version,” said study author William Feldman, MD, DPhil, MPH, a health services researcher and pulmonologist at Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
Same types of patients, different inhalers, same outcomes
Dr. Feldman and colleagues compared the medical records of 10,000 patients with COPD who began using the branded inhaler to the records of another 10,000 patients with COPD who opted for the generic alternative. Participants in the two groups were evenly matched by age, sex, race, and ethnicity, region, severity of COPD, and presence of other comorbidities, according to the researchers. Participants were all older than age 40, and the average age in both groups was 72 years.
The researchers looked for a difference in a first episode of a moderate exacerbation of COPD, defined as requiring a course of prednisone for 5-14 days. They also looked for cases of severe COPD exacerbation requiring hospitalization in the year after people began using either the generic or brand name inhaler. And they looked for differences across 1 year in rates of hospitalization for pneumonia.
For none of those outcomes, however, did the type of inhaler appear to matter. Compared with the brand-name drug, using the generic was associated with nearly identical rates of moderate or severe COPD exacerbation (hazard ratio, 0.97; 95% confidence interval, 0.90-1.04. The same was true for the proportion of people who went to the hospital for pneumonia at least once (HR, 0.99; 95% CI, 0.86-1.15).
“To get through the FDA as an interchangeable generic, the generic firms have to show that their product can be used in just the same way as the brand-name version,” Dr. Feldman said, which may explain why the generic and brand-name versions of the inhaler performed so similarly.
Dr. Feldman cautioned that the price savings for patients who opt for the generic over the branded product are hard to determine, given the vagaries of different insurance plans and potential rebates when using the branded project. As a general matter, having a single generic competitor will not lower costs much, Dr. Feldman noted, pointing to 2017 research from Harvard that found a profusion of generic competitors is needed to significantly lower health care costs.
“I don’t want to in any way underestimate the importance of getting that first generic onto the market, because it sets the stage for future generics,” Dr. Feldman said.
“There are very few generic options for patients with COPD,” said Surya Bhatt, MD, director of the Pulmonary Function and Exercise Physiology Lab at the University of Alabama at Birmingham. Even the rescue inhalers that people with COPD use to manage acute episodes of the condition are usually branded at this time, Dr. Bhatt noted, with few generic options.*
“The results are quite compelling,” said Dr. Bhatt, who was not involved in the research. Although the trial was not randomized, he commended the researchers for stratifying participants in the two groups to be as comparable as possible.
Dr. Bhatt noted that the FDA’s 2019 approval – given that the agency requires bioequivalence studies between branded and generic products – was enough to cause him to begin prescribing the generic inhaler. The fact that this approval was based on asthma but not also COPD is not a concern.
“There are so many similarities between asthma, COPD, and some obstructive lung diseases,” Dr. Bhatt noted.
In his experience, the only time someone with COPD continues using the branded inhaler – now that a potentially cheaper generic is available – is when their insurance plan makes their out-of-pocket cost minimal. Otherwise, brand loyalty does not exist.
“Patients are generally okay with being on a generic for inhalers, just because of the high cost,” Dr. Bhatt said.
The study was primarily supported by the National Heart, Lung, and Blood Institute. Dr. Feldman reported funding from Arnold Ventures, the Commonwealth Fund, and the FDA, and consulting relationships with Alosa Health and Aetion. Dr. Bhatt reported no relevant financial relationships.
*Correction, 8/16/23: An earlier version of this article mischaracterized Dr. Bhatt's comments on the availability of generic options.
A version of this article first appeared on Medscape.com.
Sometimes we get what we pay for. Other times we pay too much.
That’s the message of a study published in Annals of Internal Medicine, which finds that a generic maintenance inhaler is as effective at managing symptoms of chronic obstructive pulmonary disorder (COPD) as a pricier branded alternative.
In 2019, the Food and Drug Administration approved Wixela Inhub (the combination corticosteroid/long-acting beta2 adrenergic agonist fluticasone-salmeterol; Viatris) as a generic dry powder inhaler for managing symptoms of COPD. This approval was based on evidence of the generic’s effectiveness against asthma, although COPD also was on the product label. The study authors compared Wixela’s effectiveness in controlling symptoms of COPD with that of the brand name inhaler Advair Diskus (fluticasone-salmeterol; GlaxoSmithKline), which uses the same active ingredients.
The result: “The generic looks to be as safe and effective as the brand name. I don’t see a clinical reason why one would ever need to get the brand name over the generic version,” said study author William Feldman, MD, DPhil, MPH, a health services researcher and pulmonologist at Harvard Medical School and Brigham and Women’s Hospital, both in Boston.
Same types of patients, different inhalers, same outcomes
Dr. Feldman and colleagues compared the medical records of 10,000 patients with COPD who began using the branded inhaler to the records of another 10,000 patients with COPD who opted for the generic alternative. Participants in the two groups were evenly matched by age, sex, race, and ethnicity, region, severity of COPD, and presence of other comorbidities, according to the researchers. Participants were all older than age 40, and the average age in both groups was 72 years.
The researchers looked for a difference in a first episode of a moderate exacerbation of COPD, defined as requiring a course of prednisone for 5-14 days. They also looked for cases of severe COPD exacerbation requiring hospitalization in the year after people began using either the generic or brand name inhaler. And they looked for differences across 1 year in rates of hospitalization for pneumonia.
For none of those outcomes, however, did the type of inhaler appear to matter. Compared with the brand-name drug, using the generic was associated with nearly identical rates of moderate or severe COPD exacerbation (hazard ratio, 0.97; 95% confidence interval, 0.90-1.04. The same was true for the proportion of people who went to the hospital for pneumonia at least once (HR, 0.99; 95% CI, 0.86-1.15).
“To get through the FDA as an interchangeable generic, the generic firms have to show that their product can be used in just the same way as the brand-name version,” Dr. Feldman said, which may explain why the generic and brand-name versions of the inhaler performed so similarly.
Dr. Feldman cautioned that the price savings for patients who opt for the generic over the branded product are hard to determine, given the vagaries of different insurance plans and potential rebates when using the branded project. As a general matter, having a single generic competitor will not lower costs much, Dr. Feldman noted, pointing to 2017 research from Harvard that found a profusion of generic competitors is needed to significantly lower health care costs.
“I don’t want to in any way underestimate the importance of getting that first generic onto the market, because it sets the stage for future generics,” Dr. Feldman said.
“There are very few generic options for patients with COPD,” said Surya Bhatt, MD, director of the Pulmonary Function and Exercise Physiology Lab at the University of Alabama at Birmingham. Even the rescue inhalers that people with COPD use to manage acute episodes of the condition are usually branded at this time, Dr. Bhatt noted, with few generic options.*
“The results are quite compelling,” said Dr. Bhatt, who was not involved in the research. Although the trial was not randomized, he commended the researchers for stratifying participants in the two groups to be as comparable as possible.
Dr. Bhatt noted that the FDA’s 2019 approval – given that the agency requires bioequivalence studies between branded and generic products – was enough to cause him to begin prescribing the generic inhaler. The fact that this approval was based on asthma but not also COPD is not a concern.
“There are so many similarities between asthma, COPD, and some obstructive lung diseases,” Dr. Bhatt noted.
In his experience, the only time someone with COPD continues using the branded inhaler – now that a potentially cheaper generic is available – is when their insurance plan makes their out-of-pocket cost minimal. Otherwise, brand loyalty does not exist.
“Patients are generally okay with being on a generic for inhalers, just because of the high cost,” Dr. Bhatt said.
The study was primarily supported by the National Heart, Lung, and Blood Institute. Dr. Feldman reported funding from Arnold Ventures, the Commonwealth Fund, and the FDA, and consulting relationships with Alosa Health and Aetion. Dr. Bhatt reported no relevant financial relationships.
*Correction, 8/16/23: An earlier version of this article mischaracterized Dr. Bhatt's comments on the availability of generic options.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
COPD plus PRISm may promote frailty progression
Chronic obstructive pulmonary disease and a new phenotype of lung function impairment predicted progression of frailty in older adults, based on data from more than 5,000 individuals.
COPD has been associated with frailty, but longitudinal data on the association of COPD with progression of frailty are limited, as are data on the potential association of preserved ratio impaired spirometry (PRISm) with frailty progression, wrote Di He, BS, of Zhejiang University, China, and colleagues.
PRISm has been defined in recent studies as “proportional impairments in FEV1 and FVC, resulting in the normal ratio of FEV1 and FVC.” Individuals with PRISm may transition to normal spirometry or COPD over time, the researchers wrote.
In a study published in the journal Chest, the researchers reviewed data from 5,901 adults aged 50 years and older who were participating on the English Longitudinal Study of Ageing (ELSA), a prospective cohort study. Of these, 3,765 were included in an additional analysis of the association between transitions from normal spirometry to PRISm and the progression of frailty. The mean age of the participants was 65.5 years; 54.9% were women.
The median follow-up period for analysis with frailty progression was 9.5 years for PRISm and COPD and 5.8 years for PRISm transitions. Lung function data were collected at baseline. Based on spirometry data, participants were divided into three lung function groups – normal spirometry, PRISm, and COPD – and each of these was classified based on severity. Frailty was assessed using the frailty index (FI) during the follow-up period.
with additional annual increases of 0.301 and 0.172, respectively (P < .001 for both).
When stratified by severity, individuals with more severe PRISm and with more COPD had higher baseline FI and faster FI progression, compared with those with mild PRISm and COPD.
PRISm transitions were assessed over a 4-year interval at the start of the ELSA. Individuals with normal spirometry who transitioned to PRISm during the study had accelerated progression of frailty, as did those with COPD who transitioned to PRISm. However, no significant frailty progression occurred in those who changed from PRISm to normal spirometry.
The mechanisms behind the associations of PRISm and COPD with frailty remain unclear, but the results were consistent after controlling for multiple confounders, “suggesting PRISm and COPD had independent pathophysiological mechanisms for frailty,” the researchers write in their discussion. Other recent studies have identified sarcopenia as a complication for individuals with lung function impairment, they noted. “Therefore, another plausible explanation could be that PRISm and COPD caused sarcopenia, which accelerated frailty progression,” they say.
The findings were limited by several factors, including the observational design and the potential underestimation of lung function in participants with reversible airflow obstruction because of the use of prebronchodilator spirometry in the cohort study, the researchers noted.
However, the results were strengthened by the large sample size and high-quality data from the ELSA, as well as by the repeat measures of FI and lung function. The results were consistent after controlling for multiple confounders, and support the need for more research to explore the causality behind the association of PRISm and COPD with frailty, the researchers concluded.
The study was supported by the Zhejiang Provincial Basic Public Welfare Research Project, the Zhoushan Science and Technology Project, and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease and a new phenotype of lung function impairment predicted progression of frailty in older adults, based on data from more than 5,000 individuals.
COPD has been associated with frailty, but longitudinal data on the association of COPD with progression of frailty are limited, as are data on the potential association of preserved ratio impaired spirometry (PRISm) with frailty progression, wrote Di He, BS, of Zhejiang University, China, and colleagues.
PRISm has been defined in recent studies as “proportional impairments in FEV1 and FVC, resulting in the normal ratio of FEV1 and FVC.” Individuals with PRISm may transition to normal spirometry or COPD over time, the researchers wrote.
In a study published in the journal Chest, the researchers reviewed data from 5,901 adults aged 50 years and older who were participating on the English Longitudinal Study of Ageing (ELSA), a prospective cohort study. Of these, 3,765 were included in an additional analysis of the association between transitions from normal spirometry to PRISm and the progression of frailty. The mean age of the participants was 65.5 years; 54.9% were women.
The median follow-up period for analysis with frailty progression was 9.5 years for PRISm and COPD and 5.8 years for PRISm transitions. Lung function data were collected at baseline. Based on spirometry data, participants were divided into three lung function groups – normal spirometry, PRISm, and COPD – and each of these was classified based on severity. Frailty was assessed using the frailty index (FI) during the follow-up period.
with additional annual increases of 0.301 and 0.172, respectively (P < .001 for both).
When stratified by severity, individuals with more severe PRISm and with more COPD had higher baseline FI and faster FI progression, compared with those with mild PRISm and COPD.
PRISm transitions were assessed over a 4-year interval at the start of the ELSA. Individuals with normal spirometry who transitioned to PRISm during the study had accelerated progression of frailty, as did those with COPD who transitioned to PRISm. However, no significant frailty progression occurred in those who changed from PRISm to normal spirometry.
The mechanisms behind the associations of PRISm and COPD with frailty remain unclear, but the results were consistent after controlling for multiple confounders, “suggesting PRISm and COPD had independent pathophysiological mechanisms for frailty,” the researchers write in their discussion. Other recent studies have identified sarcopenia as a complication for individuals with lung function impairment, they noted. “Therefore, another plausible explanation could be that PRISm and COPD caused sarcopenia, which accelerated frailty progression,” they say.
The findings were limited by several factors, including the observational design and the potential underestimation of lung function in participants with reversible airflow obstruction because of the use of prebronchodilator spirometry in the cohort study, the researchers noted.
However, the results were strengthened by the large sample size and high-quality data from the ELSA, as well as by the repeat measures of FI and lung function. The results were consistent after controlling for multiple confounders, and support the need for more research to explore the causality behind the association of PRISm and COPD with frailty, the researchers concluded.
The study was supported by the Zhejiang Provincial Basic Public Welfare Research Project, the Zhoushan Science and Technology Project, and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease and a new phenotype of lung function impairment predicted progression of frailty in older adults, based on data from more than 5,000 individuals.
COPD has been associated with frailty, but longitudinal data on the association of COPD with progression of frailty are limited, as are data on the potential association of preserved ratio impaired spirometry (PRISm) with frailty progression, wrote Di He, BS, of Zhejiang University, China, and colleagues.
PRISm has been defined in recent studies as “proportional impairments in FEV1 and FVC, resulting in the normal ratio of FEV1 and FVC.” Individuals with PRISm may transition to normal spirometry or COPD over time, the researchers wrote.
In a study published in the journal Chest, the researchers reviewed data from 5,901 adults aged 50 years and older who were participating on the English Longitudinal Study of Ageing (ELSA), a prospective cohort study. Of these, 3,765 were included in an additional analysis of the association between transitions from normal spirometry to PRISm and the progression of frailty. The mean age of the participants was 65.5 years; 54.9% were women.
The median follow-up period for analysis with frailty progression was 9.5 years for PRISm and COPD and 5.8 years for PRISm transitions. Lung function data were collected at baseline. Based on spirometry data, participants were divided into three lung function groups – normal spirometry, PRISm, and COPD – and each of these was classified based on severity. Frailty was assessed using the frailty index (FI) during the follow-up period.
with additional annual increases of 0.301 and 0.172, respectively (P < .001 for both).
When stratified by severity, individuals with more severe PRISm and with more COPD had higher baseline FI and faster FI progression, compared with those with mild PRISm and COPD.
PRISm transitions were assessed over a 4-year interval at the start of the ELSA. Individuals with normal spirometry who transitioned to PRISm during the study had accelerated progression of frailty, as did those with COPD who transitioned to PRISm. However, no significant frailty progression occurred in those who changed from PRISm to normal spirometry.
The mechanisms behind the associations of PRISm and COPD with frailty remain unclear, but the results were consistent after controlling for multiple confounders, “suggesting PRISm and COPD had independent pathophysiological mechanisms for frailty,” the researchers write in their discussion. Other recent studies have identified sarcopenia as a complication for individuals with lung function impairment, they noted. “Therefore, another plausible explanation could be that PRISm and COPD caused sarcopenia, which accelerated frailty progression,” they say.
The findings were limited by several factors, including the observational design and the potential underestimation of lung function in participants with reversible airflow obstruction because of the use of prebronchodilator spirometry in the cohort study, the researchers noted.
However, the results were strengthened by the large sample size and high-quality data from the ELSA, as well as by the repeat measures of FI and lung function. The results were consistent after controlling for multiple confounders, and support the need for more research to explore the causality behind the association of PRISm and COPD with frailty, the researchers concluded.
The study was supported by the Zhejiang Provincial Basic Public Welfare Research Project, the Zhoushan Science and Technology Project, and the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province. The researchers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST
Study highlights diagnostic challenges of differentiating lichen sclerosus from vitiligo
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
of cases.
Researchers who tallied symptoms and physical exam findings observed fewer statistically significant differences between LS and vitiligo patients than expected, and LS and vitiligo were sometimes misdiagnosed as each other.
“LS must be treated aggressively to prevent long-term sequelae such as permanent scarring and vulvar squamous cell carcinoma, making an accurate diagnosis crucial,” the authors write in a poster they presented at the annual meeting of the Society for Pediatric Dermatology.
LS is symptomatic and has multiple exam findings, but once treated or quiescent, the discoloration can persist and create diagnostic uncertainty, lead study author Kaiane Habeshian, MD, a pediatric dermatologist at Children’s National Hospital, Washington, told this news organization following the SPD meeting.
The diagnostic uncertainty is especially true in patients with darker skin tones, who may have vitiligoid LS, an LS variant that has overlapping features of both LS and vitiligo.
Vitiligoid LS “presents clinically as a depigmented symmetric white vulvar and perianal white patch, often with minimal signs of inflammation, but is symptomatic and appears consistent with LS on histopathology,” Dr. Habeshian said.
“In our experience, in patients with medium to dark skin tones, there is a variable amount of repigmentation after treating LS,” she added. “After use of high potency topical corticosteroids, some patients almost completely repigment, while others have minimal repigmentation, and this can fluctuate over time, sometimes independent of other signs or symptoms of a flare up. This can lead to diagnostic confusion. For example, if an LS patient is examined after treatment, and their symptoms have resolved, they may subsequently be given a diagnosis of vitiligo.”
In the study, Dr. Habeshian and her coauthors aimed to characterize differences in LS and vitiligo based on history, physical exam, and demographic findings at the time of the initial clinic visit. She and her colleagues extracted and reviewed the medical records of 98 patients with a diagnosis of LS or vitiligo who were seen at a joint pediatric dermatology-gynecology vulvar clinic over 6.8 years. The median and mean age of the study population at diagnosis was about 6 years, with ages ranging from 2 to 20. The team used descriptive statistics and Z tests for data analysis.
The researchers found that pruritus, constipation, and dysuria were the most common symptoms experienced by both LS and vitiligo patients. All were experienced more frequently by LS patients, but only pruritus reached statistical significance (P = .040). Other symptoms experienced only by LS patients included vulvar pain, bleeding, and pain with defecation.
Meanwhile, apart from hypopigmentation and erythema, all physical exam findings were more frequent in LS patients, compared with vitiligo patients, including fissures and purpura/petechiae, but only epidermal atrophy and figure-of-8 distribution of hypopigmentation reached statistical significance (P values of .047 and .036, respectively).
In other findings, LS and vitiligo were misdiagnosed as each other 15 times. Nearly half of the misdiagnoses (46.7%) were made in Black patients, who composed 38.8% of all patients in the study.
“I suspect that some vitiligo cases that were previously ‘misdiagnosed’ as LS were actually LS that just didn’t repigment and then were labeled as vitiligo in the chart,” Dr. Habeshian said.
“And some of those LS cases that previously were misdiagnosed as vitiligo likely had other more subtle LS findings that were missed (shininess and wrinkling of the skin, small fissures, constipation) or that were attributed to comorbid irritant contact dermatitis or another condition,” she said. “It was interesting to see that even in a vulvar dermatology clinic there can be confusion between these diagnoses because the literature on pediatric LS in darker skin tones is so sparse.”
She emphasized that a close exam and detailed history are needed to properly diagnose patients with anogenital skin conditions.
“Don’t forget to ask about constipation and urinary symptoms as well as psychosocial and, in the appropriate patient, sexual and reproductive function,” Dr. Habeshian said. “Based on my experience, pediatric LS is much more common in our community than the literature would suggest. Its psychosocial impact is tremendous but not well documented, particularly in pediatric patients. In my experience, the longer LS is misdiagnosed or mistreated, the more challenging it becomes to manage. You don’t want to miss LS.”
She acknowledged certain limitations of the study, including the fact that photographs were not available for review for many of the earlier years of the clinic. “Therefore, we had to depend on the diagnosis given at the time of the visit,” she said. “This likely accounts in part for the smaller number than expected of significant exam and history findings between LS and vitiligo. We need further studies utilizing a standardized approach to accurate diagnosis.”
Her coauthors were Nikita Menta, Aneka Khilnani, MS, and Tazim Dowlut-McElroy, MD. The researchers reported having no financial disclosures.
FROM SPD 2023
Ancestry may predict bipolar patients’ response to lithium
Lithium remains the first-line treatment for BPD, but clinical improvement occurs in less than one-third of patients, and factors that might affect response, especially genetic factors, have not been well studied, wrote Ana M. Díaz-Zuluaga, MD, of University of Antioquia, Medellín, Colombia, and colleagues.
Previous genetic research identified four linked single nucleotide polymorphisms (SNPs) in a single locus on chromosome 21 that were associated with lithium response, but the study was limited to individuals with European and Asian ancestry, the researchers said.
In a study published in the Journal of Affective Disorders, the researchers identified 172 adults aged 18 and older with a diagnosis of BPD I or II based on the DSM-IV-TR criteria. Participants had been taking lithium continuously for at least 6 months. Lithium response was defined using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with BD, also known as the Alda scale. Total Alda scale scores of 7 or higher indicated a responder phenotype; scores less than 7 were considered nonresponders.
Ancestry was determined using DNA samples and the software Structure Version 2.2, and participants were classified as Amerindian, African, or European.
The overall response rate to lithium was 15.11% (26 of 172 patients). In a univariate analysis, no significant differences emerged between responders and nonresponders in demographics or clinical characteristics. However, patients responsive to lithium were significantly less likely of African ancestry, compared with nonresponders (0.1 vs. 0.2, P = .005) and more likely of European ancestry (0.5 vs. 0.3, P = .024), and had fewer depressive episodes (2 vs. 3.9, P = .002). The difference in responders vs. nonresponders of Amerindian ancestry was not statistically significant (0.4 vs. 0.5, P = .204).
The researchers then used machine learning based on Advanced Recursive Partitioning Approaches (ARPAs) to create classification trees with and without ancestry components for predicting response to lithium. “Variable importance analysis shows that the most important predictor is the probability of Amerindian ancestry component, followed by the Amerindian and European ancestral components individual variances, and then by the African and European ancestry components,” the researchers wrote.
Without the ancestry component, the sensitivity and specificity for predicting a treatment response to lithium were 50% and 94.5% respectively, with an area under the curve of 72.2%.
“However, when ancestral components are included in the model, the sensitivity and specificity are 93 % and 84 %, respectively,” with an AUC of 89.2%, the researchers said.
Clinical predictors of treatment response included disease duration, number of depressive episodes, total number of affective episodes, and number of manic episodes.
The findings were limited by several factors including the cross-sectional design and potential impact of other psychotropic drugs, the researchers noted. A replication of the study in an independent dataset is needed to validate the findings, they said.
However, the study is the first known to explore the effect of ancestry on bipolar patients’ response to lithium, and suggests that ancestry components have potential predictive value in the clinical setting that could support a more personalized approach to treatment, the researchers said.
The study was supported by PRISMA U.T., Colciencias, Invitaci
Lithium remains the first-line treatment for BPD, but clinical improvement occurs in less than one-third of patients, and factors that might affect response, especially genetic factors, have not been well studied, wrote Ana M. Díaz-Zuluaga, MD, of University of Antioquia, Medellín, Colombia, and colleagues.
Previous genetic research identified four linked single nucleotide polymorphisms (SNPs) in a single locus on chromosome 21 that were associated with lithium response, but the study was limited to individuals with European and Asian ancestry, the researchers said.
In a study published in the Journal of Affective Disorders, the researchers identified 172 adults aged 18 and older with a diagnosis of BPD I or II based on the DSM-IV-TR criteria. Participants had been taking lithium continuously for at least 6 months. Lithium response was defined using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with BD, also known as the Alda scale. Total Alda scale scores of 7 or higher indicated a responder phenotype; scores less than 7 were considered nonresponders.
Ancestry was determined using DNA samples and the software Structure Version 2.2, and participants were classified as Amerindian, African, or European.
The overall response rate to lithium was 15.11% (26 of 172 patients). In a univariate analysis, no significant differences emerged between responders and nonresponders in demographics or clinical characteristics. However, patients responsive to lithium were significantly less likely of African ancestry, compared with nonresponders (0.1 vs. 0.2, P = .005) and more likely of European ancestry (0.5 vs. 0.3, P = .024), and had fewer depressive episodes (2 vs. 3.9, P = .002). The difference in responders vs. nonresponders of Amerindian ancestry was not statistically significant (0.4 vs. 0.5, P = .204).
The researchers then used machine learning based on Advanced Recursive Partitioning Approaches (ARPAs) to create classification trees with and without ancestry components for predicting response to lithium. “Variable importance analysis shows that the most important predictor is the probability of Amerindian ancestry component, followed by the Amerindian and European ancestral components individual variances, and then by the African and European ancestry components,” the researchers wrote.
Without the ancestry component, the sensitivity and specificity for predicting a treatment response to lithium were 50% and 94.5% respectively, with an area under the curve of 72.2%.
“However, when ancestral components are included in the model, the sensitivity and specificity are 93 % and 84 %, respectively,” with an AUC of 89.2%, the researchers said.
Clinical predictors of treatment response included disease duration, number of depressive episodes, total number of affective episodes, and number of manic episodes.
The findings were limited by several factors including the cross-sectional design and potential impact of other psychotropic drugs, the researchers noted. A replication of the study in an independent dataset is needed to validate the findings, they said.
However, the study is the first known to explore the effect of ancestry on bipolar patients’ response to lithium, and suggests that ancestry components have potential predictive value in the clinical setting that could support a more personalized approach to treatment, the researchers said.
The study was supported by PRISMA U.T., Colciencias, Invitaci
Lithium remains the first-line treatment for BPD, but clinical improvement occurs in less than one-third of patients, and factors that might affect response, especially genetic factors, have not been well studied, wrote Ana M. Díaz-Zuluaga, MD, of University of Antioquia, Medellín, Colombia, and colleagues.
Previous genetic research identified four linked single nucleotide polymorphisms (SNPs) in a single locus on chromosome 21 that were associated with lithium response, but the study was limited to individuals with European and Asian ancestry, the researchers said.
In a study published in the Journal of Affective Disorders, the researchers identified 172 adults aged 18 and older with a diagnosis of BPD I or II based on the DSM-IV-TR criteria. Participants had been taking lithium continuously for at least 6 months. Lithium response was defined using the Retrospective Criteria of Long-Term Treatment Response in Research Subjects with BD, also known as the Alda scale. Total Alda scale scores of 7 or higher indicated a responder phenotype; scores less than 7 were considered nonresponders.
Ancestry was determined using DNA samples and the software Structure Version 2.2, and participants were classified as Amerindian, African, or European.
The overall response rate to lithium was 15.11% (26 of 172 patients). In a univariate analysis, no significant differences emerged between responders and nonresponders in demographics or clinical characteristics. However, patients responsive to lithium were significantly less likely of African ancestry, compared with nonresponders (0.1 vs. 0.2, P = .005) and more likely of European ancestry (0.5 vs. 0.3, P = .024), and had fewer depressive episodes (2 vs. 3.9, P = .002). The difference in responders vs. nonresponders of Amerindian ancestry was not statistically significant (0.4 vs. 0.5, P = .204).
The researchers then used machine learning based on Advanced Recursive Partitioning Approaches (ARPAs) to create classification trees with and without ancestry components for predicting response to lithium. “Variable importance analysis shows that the most important predictor is the probability of Amerindian ancestry component, followed by the Amerindian and European ancestral components individual variances, and then by the African and European ancestry components,” the researchers wrote.
Without the ancestry component, the sensitivity and specificity for predicting a treatment response to lithium were 50% and 94.5% respectively, with an area under the curve of 72.2%.
“However, when ancestral components are included in the model, the sensitivity and specificity are 93 % and 84 %, respectively,” with an AUC of 89.2%, the researchers said.
Clinical predictors of treatment response included disease duration, number of depressive episodes, total number of affective episodes, and number of manic episodes.
The findings were limited by several factors including the cross-sectional design and potential impact of other psychotropic drugs, the researchers noted. A replication of the study in an independent dataset is needed to validate the findings, they said.
However, the study is the first known to explore the effect of ancestry on bipolar patients’ response to lithium, and suggests that ancestry components have potential predictive value in the clinical setting that could support a more personalized approach to treatment, the researchers said.
The study was supported by PRISMA U.T., Colciencias, Invitaci
FROM THE JOURNAL OF AFFECTIVE DISORDERS
A step forward in diabetic foot disease management
As we navigate the ever-evolving landscape of diabetic foot disease management, The goal is to create a common language of risk that is easily related from clinician to clinician to patient.
Whatever language we use, though, the problem we face is vast:
- Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.
- They are associated with high rates of premature death, with a 5-year mortality rate of 30%. This rate is greater than 70% for those with above-foot amputations, worse than all but the most aggressive cancers.
- The direct costs of treating diabetic foot ulcers in the United States is estimated at $9 billion-$13 billion annually.
- Over 550 million people worldwide have diabetes, with 18.6 million developing foot ulcers annually. Up to 34% of those with diabetes will develop a foot ulcer.
- About 20% of those with a diabetic foot ulcer will undergo amputation, a major cause of which is infection, which affects 50% of foot ulcers.
- Up to 20% of those with a foot ulcer require hospitalization, with 15%-20% undergoing amputation. Inequities exist in diabetes-related foot complications:
- –Rates of major amputation are higher in non-Hispanic Black, Hispanic, and Native American populations, compared with non-Hispanic White populations.
- –Non-Hispanic Black and Hispanic populations present with more advanced ulcers and peripheral artery disease, and are more likely to undergo amputation without revascularization attempt.
The IWGDF, a multidisciplinary team of international experts, has recently updated its guidelines. This team, comprising endocrinologists, internal medicine physicians, physiatrists, podiatrists, and vascular surgeons from across the globe, has worked tirelessly to provide us with a comprehensive guide to managing diabetes-related foot ulcers.
The updated guidelines address five critical clinical questions, each with up to 13 important outcomes. The systematic review that underpins these guidelines identified 149 eligible studies, assessing 28 different systems. This exhaustive research has led to the development of seven key recommendations that address the clinical questions and consider the existence of different clinical settings.
One of the significant updates in the 2023 guidelines is the recommendation of SINBAD – site, ischemia, neuropathy, bacterial infection, area, and depth – as the priority wound classification system for people with diabetes and a foot ulcer. This system is particularly useful for interprofessional communication, describing each composite variable, and conducting clinical audits using the full score. However, the guidelines also recommend the use of other, more specific assessment systems for infection and peripheral artery disease from the Infectious Diseases Society of America/IWGDF when resources and an appropriate level of expertise exist.
The introduction of the Wound, Ischemia and Foot Infection (WIfI) classification system in the guidelines is also a noteworthy development. This system is crucial in assessing perfusion and the likely benefit of revascularization in a person with diabetes and a foot ulcer. By assessing the level of wound ischemia and infection, we can make informed decisions about the need for vascular intervention, which can significantly affect the patient’s outcome. This can be done simply by classifying each of the three categories of wound, ischemia, or foot infection as none, mild, moderate, or severe. By simplifying the very dynamic comorbidities of tissue loss, ischemia, and infection into a usable and predictive scale, it helps us to communicate risk across disciplines. This has been found to be highly predictive of healing, amputation, and mortality.
We use WIfI every day across our system. An example might include a patient we recently treated:
A 76-year-old woman presented with a wound to her left foot. Her past medical history revealed type 2 diabetes, peripheral neuropathy, and documented peripheral artery disease with prior bilateral femoral-popliteal bypass conducted at an external facility. In addition to gangrenous changes to her fourth toe, she displayed erythema and lymphangitic streaking up her dorsal foot. While she was afebrile, her white cell count was 13,000/mcL. Radiographic examinations did not show signs of osteomyelitis. Noninvasive vascular evaluations revealed an ankle brachial index of 0.4 and a toe pressure of 10 mm Hg. An aortogram with a lower-extremity runoff arteriogram confirmed the obstruction of her left femoral-popliteal bypass.
Taking these results into account, her WIfI score was determined as: wound 2 (moderate), ischemia 3 (severe), foot infection 2 (moderate, no sepsis), translating to a clinical stage 4. This denotes a high risk for major amputation.
Following a team discussion, she was taken to the operating room for an initial debridement of her infection which consisted of a partial fourth ray resection to the level of the mid-metatarsal. Following control of the infection, she received a vascular assessment which ultimately constituted a femoral to distal anterior tibial bypass. Following both of these, she was discharged on a negative-pressure wound therapy device, receiving a split-thickness skin graft 4 weeks later.
The guidelines also emphasize the need for specific training, skills, and experience to ensure the accuracy of the recommended systems for characterizing foot ulcers. The person applying these systems should be appropriately trained and, according to their national or regional standards, should have the knowledge, expertise, and skills necessary to manage people with a diabetes-related foot ulcer.
As we continue to navigate the complexities of diabetes-related foot disease, these guidelines serve as a valuable compass, guiding our decisions and actions. They remind us of the importance of continuous learning, collaboration, and the application of evidence-based practice in our work.
I encourage you to delve into these guidelines. Let’s use them to improve our practice, enhance our communication, and, ultimately, provide better care for our patients.
Dr. Armstrong is professor of surgery, director of limb preservation, University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As we navigate the ever-evolving landscape of diabetic foot disease management, The goal is to create a common language of risk that is easily related from clinician to clinician to patient.
Whatever language we use, though, the problem we face is vast:
- Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.
- They are associated with high rates of premature death, with a 5-year mortality rate of 30%. This rate is greater than 70% for those with above-foot amputations, worse than all but the most aggressive cancers.
- The direct costs of treating diabetic foot ulcers in the United States is estimated at $9 billion-$13 billion annually.
- Over 550 million people worldwide have diabetes, with 18.6 million developing foot ulcers annually. Up to 34% of those with diabetes will develop a foot ulcer.
- About 20% of those with a diabetic foot ulcer will undergo amputation, a major cause of which is infection, which affects 50% of foot ulcers.
- Up to 20% of those with a foot ulcer require hospitalization, with 15%-20% undergoing amputation. Inequities exist in diabetes-related foot complications:
- –Rates of major amputation are higher in non-Hispanic Black, Hispanic, and Native American populations, compared with non-Hispanic White populations.
- –Non-Hispanic Black and Hispanic populations present with more advanced ulcers and peripheral artery disease, and are more likely to undergo amputation without revascularization attempt.
The IWGDF, a multidisciplinary team of international experts, has recently updated its guidelines. This team, comprising endocrinologists, internal medicine physicians, physiatrists, podiatrists, and vascular surgeons from across the globe, has worked tirelessly to provide us with a comprehensive guide to managing diabetes-related foot ulcers.
The updated guidelines address five critical clinical questions, each with up to 13 important outcomes. The systematic review that underpins these guidelines identified 149 eligible studies, assessing 28 different systems. This exhaustive research has led to the development of seven key recommendations that address the clinical questions and consider the existence of different clinical settings.
One of the significant updates in the 2023 guidelines is the recommendation of SINBAD – site, ischemia, neuropathy, bacterial infection, area, and depth – as the priority wound classification system for people with diabetes and a foot ulcer. This system is particularly useful for interprofessional communication, describing each composite variable, and conducting clinical audits using the full score. However, the guidelines also recommend the use of other, more specific assessment systems for infection and peripheral artery disease from the Infectious Diseases Society of America/IWGDF when resources and an appropriate level of expertise exist.
The introduction of the Wound, Ischemia and Foot Infection (WIfI) classification system in the guidelines is also a noteworthy development. This system is crucial in assessing perfusion and the likely benefit of revascularization in a person with diabetes and a foot ulcer. By assessing the level of wound ischemia and infection, we can make informed decisions about the need for vascular intervention, which can significantly affect the patient’s outcome. This can be done simply by classifying each of the three categories of wound, ischemia, or foot infection as none, mild, moderate, or severe. By simplifying the very dynamic comorbidities of tissue loss, ischemia, and infection into a usable and predictive scale, it helps us to communicate risk across disciplines. This has been found to be highly predictive of healing, amputation, and mortality.
We use WIfI every day across our system. An example might include a patient we recently treated:
A 76-year-old woman presented with a wound to her left foot. Her past medical history revealed type 2 diabetes, peripheral neuropathy, and documented peripheral artery disease with prior bilateral femoral-popliteal bypass conducted at an external facility. In addition to gangrenous changes to her fourth toe, she displayed erythema and lymphangitic streaking up her dorsal foot. While she was afebrile, her white cell count was 13,000/mcL. Radiographic examinations did not show signs of osteomyelitis. Noninvasive vascular evaluations revealed an ankle brachial index of 0.4 and a toe pressure of 10 mm Hg. An aortogram with a lower-extremity runoff arteriogram confirmed the obstruction of her left femoral-popliteal bypass.
Taking these results into account, her WIfI score was determined as: wound 2 (moderate), ischemia 3 (severe), foot infection 2 (moderate, no sepsis), translating to a clinical stage 4. This denotes a high risk for major amputation.
Following a team discussion, she was taken to the operating room for an initial debridement of her infection which consisted of a partial fourth ray resection to the level of the mid-metatarsal. Following control of the infection, she received a vascular assessment which ultimately constituted a femoral to distal anterior tibial bypass. Following both of these, she was discharged on a negative-pressure wound therapy device, receiving a split-thickness skin graft 4 weeks later.
The guidelines also emphasize the need for specific training, skills, and experience to ensure the accuracy of the recommended systems for characterizing foot ulcers. The person applying these systems should be appropriately trained and, according to their national or regional standards, should have the knowledge, expertise, and skills necessary to manage people with a diabetes-related foot ulcer.
As we continue to navigate the complexities of diabetes-related foot disease, these guidelines serve as a valuable compass, guiding our decisions and actions. They remind us of the importance of continuous learning, collaboration, and the application of evidence-based practice in our work.
I encourage you to delve into these guidelines. Let’s use them to improve our practice, enhance our communication, and, ultimately, provide better care for our patients.
Dr. Armstrong is professor of surgery, director of limb preservation, University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As we navigate the ever-evolving landscape of diabetic foot disease management, The goal is to create a common language of risk that is easily related from clinician to clinician to patient.
Whatever language we use, though, the problem we face is vast:
- Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.
- They are associated with high rates of premature death, with a 5-year mortality rate of 30%. This rate is greater than 70% for those with above-foot amputations, worse than all but the most aggressive cancers.
- The direct costs of treating diabetic foot ulcers in the United States is estimated at $9 billion-$13 billion annually.
- Over 550 million people worldwide have diabetes, with 18.6 million developing foot ulcers annually. Up to 34% of those with diabetes will develop a foot ulcer.
- About 20% of those with a diabetic foot ulcer will undergo amputation, a major cause of which is infection, which affects 50% of foot ulcers.
- Up to 20% of those with a foot ulcer require hospitalization, with 15%-20% undergoing amputation. Inequities exist in diabetes-related foot complications:
- –Rates of major amputation are higher in non-Hispanic Black, Hispanic, and Native American populations, compared with non-Hispanic White populations.
- –Non-Hispanic Black and Hispanic populations present with more advanced ulcers and peripheral artery disease, and are more likely to undergo amputation without revascularization attempt.
The IWGDF, a multidisciplinary team of international experts, has recently updated its guidelines. This team, comprising endocrinologists, internal medicine physicians, physiatrists, podiatrists, and vascular surgeons from across the globe, has worked tirelessly to provide us with a comprehensive guide to managing diabetes-related foot ulcers.
The updated guidelines address five critical clinical questions, each with up to 13 important outcomes. The systematic review that underpins these guidelines identified 149 eligible studies, assessing 28 different systems. This exhaustive research has led to the development of seven key recommendations that address the clinical questions and consider the existence of different clinical settings.
One of the significant updates in the 2023 guidelines is the recommendation of SINBAD – site, ischemia, neuropathy, bacterial infection, area, and depth – as the priority wound classification system for people with diabetes and a foot ulcer. This system is particularly useful for interprofessional communication, describing each composite variable, and conducting clinical audits using the full score. However, the guidelines also recommend the use of other, more specific assessment systems for infection and peripheral artery disease from the Infectious Diseases Society of America/IWGDF when resources and an appropriate level of expertise exist.
The introduction of the Wound, Ischemia and Foot Infection (WIfI) classification system in the guidelines is also a noteworthy development. This system is crucial in assessing perfusion and the likely benefit of revascularization in a person with diabetes and a foot ulcer. By assessing the level of wound ischemia and infection, we can make informed decisions about the need for vascular intervention, which can significantly affect the patient’s outcome. This can be done simply by classifying each of the three categories of wound, ischemia, or foot infection as none, mild, moderate, or severe. By simplifying the very dynamic comorbidities of tissue loss, ischemia, and infection into a usable and predictive scale, it helps us to communicate risk across disciplines. This has been found to be highly predictive of healing, amputation, and mortality.
We use WIfI every day across our system. An example might include a patient we recently treated:
A 76-year-old woman presented with a wound to her left foot. Her past medical history revealed type 2 diabetes, peripheral neuropathy, and documented peripheral artery disease with prior bilateral femoral-popliteal bypass conducted at an external facility. In addition to gangrenous changes to her fourth toe, she displayed erythema and lymphangitic streaking up her dorsal foot. While she was afebrile, her white cell count was 13,000/mcL. Radiographic examinations did not show signs of osteomyelitis. Noninvasive vascular evaluations revealed an ankle brachial index of 0.4 and a toe pressure of 10 mm Hg. An aortogram with a lower-extremity runoff arteriogram confirmed the obstruction of her left femoral-popliteal bypass.
Taking these results into account, her WIfI score was determined as: wound 2 (moderate), ischemia 3 (severe), foot infection 2 (moderate, no sepsis), translating to a clinical stage 4. This denotes a high risk for major amputation.
Following a team discussion, she was taken to the operating room for an initial debridement of her infection which consisted of a partial fourth ray resection to the level of the mid-metatarsal. Following control of the infection, she received a vascular assessment which ultimately constituted a femoral to distal anterior tibial bypass. Following both of these, she was discharged on a negative-pressure wound therapy device, receiving a split-thickness skin graft 4 weeks later.
The guidelines also emphasize the need for specific training, skills, and experience to ensure the accuracy of the recommended systems for characterizing foot ulcers. The person applying these systems should be appropriately trained and, according to their national or regional standards, should have the knowledge, expertise, and skills necessary to manage people with a diabetes-related foot ulcer.
As we continue to navigate the complexities of diabetes-related foot disease, these guidelines serve as a valuable compass, guiding our decisions and actions. They remind us of the importance of continuous learning, collaboration, and the application of evidence-based practice in our work.
I encourage you to delve into these guidelines. Let’s use them to improve our practice, enhance our communication, and, ultimately, provide better care for our patients.
Dr. Armstrong is professor of surgery, director of limb preservation, University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Semaglutide cuts cardiovascular events in landmark trial
, in the pivotal SELECT trial, with more than 17,000 enrolled people with overweight or obesity and established cardiovascular disease (CVD), but no diabetes.
The finding should fuel improved patient access to this glucagon-like peptide-1 (GLP-1) agonist weight-loss agent that has historically been hindered by skepticism among U.S. payers, many of whom have criticized the health benefits and cost effectiveness of this drug in people whose only indication for treatment is overweight or obesity.
According to top-line results from SELECT released by Novo Nordisk on Aug. 8, the people randomly assigned to receive weekly 2.4-mg subcutaneous injections of semaglutide showed a significant 20% reduction in their incidence of the combined endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The announcement added that semaglutide treatment also significantly linked with a drop in the incidence of each of these individual three endpoints; the magnitude of these reductions, however, wasn’t specified, nor was the duration of treatment and follow-up.
The results also showed a level of safety and patient tolerance for weekly 2.4-mg injections of semaglutide that were consistent with prior reports on the agent. Semaglutide as Wegovy received marketing approval from the U.S. Food and Drug Administration in 2021 for weight loss, and in 2017 for glucose control in people with type 2 diabetes, at a weekly maximum dose of 2.0 mg (for which it’s marketed as Ozempic).
SELECT began in 2018 and randomly assigned 17,604 adults aged 45 years and older at more than 800 sites in 41 countries. The company’s announcement noted that the trial had accrued a total of 1,270 study participants with a first MACE event but did not break this total down based on treatment received.
‘A good result for patients’
“The topline results from SELECT are exciting, as preventing heart attacks and stroke with a drug that also lowers weight is very important for many patients, especially if the data also show – as I suspect they will – a meaningful improvement of quality of life for patients due to associated weight loss,” commented Naveed Sattar, PhD, a professor of metabolic medicine at the University of Glasgow who was not involved with the study.
Despite this lack of current clarity over the role that weight loss by itself played in driving the observed result, the SELECT findings seem poised to reset a long-standing prejudice against the medical necessity and safety of weight-loss agents when used for the sole indication of helping people lose weight.
Changing how obesity is regarded
“To date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke, or cardiovascular death,” said Martin Holst Lange, executive vice president for development at Novo Nordisk, in the company’s press release.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4 mg has the potential to change how obesity is regarded and treated.”
Several of the early medical options for aiding weight loss had substantial adverse effects, including increased MACE rates, a history that led to pervasive wariness among physicians over the safety of antiobesity agents and the wisdom of using medically aided weight loss to produce health benefits.
This attitude also helped dampen health insurance coverage of weight-loss treatments. For example, Medicare has a long-standing policy against reimbursing the cost for medications that are used for the indication of weight loss, and a 2003 U.S. law prohibited part D plans from providing this coverage.
Semaglutide belongs to the class of agents that mimic the action of the incretin GLP-1. The introduction of this class of GLP-1 agonists for weight loss began in 2014 with the FDA’s approval of liraglutide (Saxenda), a daily subcutaneous injection that marked the first step toward establishing the class as safe and effective for weight loss and launching a new era in weight-loss treatment.
According to the Novo Nordisk announcement, a full report on results from SELECT will occur “at a scientific meeting later in 2023.”
SELECT is sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Sattar is a consultant to several companies that market GLP-1 receptor agonists, including Novo Nordisk and Lilly, but has had no involvement in SELECT.
A version of this article first appeared on Medscape.com.
, in the pivotal SELECT trial, with more than 17,000 enrolled people with overweight or obesity and established cardiovascular disease (CVD), but no diabetes.
The finding should fuel improved patient access to this glucagon-like peptide-1 (GLP-1) agonist weight-loss agent that has historically been hindered by skepticism among U.S. payers, many of whom have criticized the health benefits and cost effectiveness of this drug in people whose only indication for treatment is overweight or obesity.
According to top-line results from SELECT released by Novo Nordisk on Aug. 8, the people randomly assigned to receive weekly 2.4-mg subcutaneous injections of semaglutide showed a significant 20% reduction in their incidence of the combined endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The announcement added that semaglutide treatment also significantly linked with a drop in the incidence of each of these individual three endpoints; the magnitude of these reductions, however, wasn’t specified, nor was the duration of treatment and follow-up.
The results also showed a level of safety and patient tolerance for weekly 2.4-mg injections of semaglutide that were consistent with prior reports on the agent. Semaglutide as Wegovy received marketing approval from the U.S. Food and Drug Administration in 2021 for weight loss, and in 2017 for glucose control in people with type 2 diabetes, at a weekly maximum dose of 2.0 mg (for which it’s marketed as Ozempic).
SELECT began in 2018 and randomly assigned 17,604 adults aged 45 years and older at more than 800 sites in 41 countries. The company’s announcement noted that the trial had accrued a total of 1,270 study participants with a first MACE event but did not break this total down based on treatment received.
‘A good result for patients’
“The topline results from SELECT are exciting, as preventing heart attacks and stroke with a drug that also lowers weight is very important for many patients, especially if the data also show – as I suspect they will – a meaningful improvement of quality of life for patients due to associated weight loss,” commented Naveed Sattar, PhD, a professor of metabolic medicine at the University of Glasgow who was not involved with the study.
Despite this lack of current clarity over the role that weight loss by itself played in driving the observed result, the SELECT findings seem poised to reset a long-standing prejudice against the medical necessity and safety of weight-loss agents when used for the sole indication of helping people lose weight.
Changing how obesity is regarded
“To date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke, or cardiovascular death,” said Martin Holst Lange, executive vice president for development at Novo Nordisk, in the company’s press release.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4 mg has the potential to change how obesity is regarded and treated.”
Several of the early medical options for aiding weight loss had substantial adverse effects, including increased MACE rates, a history that led to pervasive wariness among physicians over the safety of antiobesity agents and the wisdom of using medically aided weight loss to produce health benefits.
This attitude also helped dampen health insurance coverage of weight-loss treatments. For example, Medicare has a long-standing policy against reimbursing the cost for medications that are used for the indication of weight loss, and a 2003 U.S. law prohibited part D plans from providing this coverage.
Semaglutide belongs to the class of agents that mimic the action of the incretin GLP-1. The introduction of this class of GLP-1 agonists for weight loss began in 2014 with the FDA’s approval of liraglutide (Saxenda), a daily subcutaneous injection that marked the first step toward establishing the class as safe and effective for weight loss and launching a new era in weight-loss treatment.
According to the Novo Nordisk announcement, a full report on results from SELECT will occur “at a scientific meeting later in 2023.”
SELECT is sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Sattar is a consultant to several companies that market GLP-1 receptor agonists, including Novo Nordisk and Lilly, but has had no involvement in SELECT.
A version of this article first appeared on Medscape.com.
, in the pivotal SELECT trial, with more than 17,000 enrolled people with overweight or obesity and established cardiovascular disease (CVD), but no diabetes.
The finding should fuel improved patient access to this glucagon-like peptide-1 (GLP-1) agonist weight-loss agent that has historically been hindered by skepticism among U.S. payers, many of whom have criticized the health benefits and cost effectiveness of this drug in people whose only indication for treatment is overweight or obesity.
According to top-line results from SELECT released by Novo Nordisk on Aug. 8, the people randomly assigned to receive weekly 2.4-mg subcutaneous injections of semaglutide showed a significant 20% reduction in their incidence of the combined endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The announcement added that semaglutide treatment also significantly linked with a drop in the incidence of each of these individual three endpoints; the magnitude of these reductions, however, wasn’t specified, nor was the duration of treatment and follow-up.
The results also showed a level of safety and patient tolerance for weekly 2.4-mg injections of semaglutide that were consistent with prior reports on the agent. Semaglutide as Wegovy received marketing approval from the U.S. Food and Drug Administration in 2021 for weight loss, and in 2017 for glucose control in people with type 2 diabetes, at a weekly maximum dose of 2.0 mg (for which it’s marketed as Ozempic).
SELECT began in 2018 and randomly assigned 17,604 adults aged 45 years and older at more than 800 sites in 41 countries. The company’s announcement noted that the trial had accrued a total of 1,270 study participants with a first MACE event but did not break this total down based on treatment received.
‘A good result for patients’
“The topline results from SELECT are exciting, as preventing heart attacks and stroke with a drug that also lowers weight is very important for many patients, especially if the data also show – as I suspect they will – a meaningful improvement of quality of life for patients due to associated weight loss,” commented Naveed Sattar, PhD, a professor of metabolic medicine at the University of Glasgow who was not involved with the study.
Despite this lack of current clarity over the role that weight loss by itself played in driving the observed result, the SELECT findings seem poised to reset a long-standing prejudice against the medical necessity and safety of weight-loss agents when used for the sole indication of helping people lose weight.
Changing how obesity is regarded
“To date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke, or cardiovascular death,” said Martin Holst Lange, executive vice president for development at Novo Nordisk, in the company’s press release.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4 mg has the potential to change how obesity is regarded and treated.”
Several of the early medical options for aiding weight loss had substantial adverse effects, including increased MACE rates, a history that led to pervasive wariness among physicians over the safety of antiobesity agents and the wisdom of using medically aided weight loss to produce health benefits.
This attitude also helped dampen health insurance coverage of weight-loss treatments. For example, Medicare has a long-standing policy against reimbursing the cost for medications that are used for the indication of weight loss, and a 2003 U.S. law prohibited part D plans from providing this coverage.
Semaglutide belongs to the class of agents that mimic the action of the incretin GLP-1. The introduction of this class of GLP-1 agonists for weight loss began in 2014 with the FDA’s approval of liraglutide (Saxenda), a daily subcutaneous injection that marked the first step toward establishing the class as safe and effective for weight loss and launching a new era in weight-loss treatment.
According to the Novo Nordisk announcement, a full report on results from SELECT will occur “at a scientific meeting later in 2023.”
SELECT is sponsored by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Sattar is a consultant to several companies that market GLP-1 receptor agonists, including Novo Nordisk and Lilly, but has had no involvement in SELECT.
A version of this article first appeared on Medscape.com.