User login
Artemisinin-resistant malaria found across Myanmar
Photo by James Gathany
Resistance to the antimalarial drug artemisinin is present in Myanmar and has reached within 25 km of the Indian border, according to research published in The Lancet Infectious Diseases.
Researchers believe the spread of artemisinin-resistant malaria parasites into neighboring India would pose a serious threat to the global control and eradication of malaria.
And if drug resistance continues to spread, millions of lives could be at risk.
Kyaw Myo Tun, MD, of the Myanmar Oxford Clinical Research Unit in Yangon, Myanmar, and colleagues uncovered artemisinin resistance by analyzing parasite samples collected at 55 malaria treatment centers across Myanmar.
The group set out to determine if the samples carried mutations in specific regions of the parasite’s kelch gene (K13)—a known genetic marker of artemisinin resistance. And they confirmed the existance of resistant parasites in Homalin, in the Sagaing Region, which is located only 25 km from the Indian border.
“Myanmar is considered the frontline in the battle against artemisinin resistance, as it forms a gateway for resistance to spread to the rest of the world,” said Charles Woodrow, MD, of the University of Oxford in the UK.
“With artemisinins, we are in the unusual position of having molecular markers for resistance before resistance has spread globally. The more we understand about the current situation in the border regions, the better prepared we are to adapt and implement strategies to overcome the spread of further drug resistance.”
The researchers obtained the DNA sequences of 940 samples of Plasmodium falciparum malaria parasites from across Myanmar and neighboring border regions in Thailand and Bangladesh between 2013 and 2014. Of those 940 samples, 371 (39%) carried a resistance-conferring K13 mutation.
“We were able to gather patient samples rapidly across Myanmar, sometimes using discarded malaria blood diagnostic tests, and then test these immediately for the K13 marker, and so generate real-time information on the spread of resistance” said Mallika Imwong, PhD, of Mahidol University in Bangkok, Thailand.
Using this information, the researchers developed maps to display the predicted extent of artemisinin resistance determined by the prevalence of K13 mutations. The maps suggested the overall prevalence of K13 mutations was greater than 10% in large areas of the east and north of Myanmar, including areas close to the border with India.
“The identification of the K13 markers of resistance has transformed our ability to monitor the spread and emergence of artemisinin resistance,” said Philippe Guerin, MD, of the Worldwide Antimalarial Resistance Network in Oxford, UK.
“However, this study highlights that the pace at which artemisinin resistance is spreading or emerging is alarming. We need a more vigorous international effort to address this issue in border regions.” ![]()

Photo by James Gathany
Resistance to the antimalarial drug artemisinin is present in Myanmar and has reached within 25 km of the Indian border, according to research published in The Lancet Infectious Diseases.
Researchers believe the spread of artemisinin-resistant malaria parasites into neighboring India would pose a serious threat to the global control and eradication of malaria.
And if drug resistance continues to spread, millions of lives could be at risk.
Kyaw Myo Tun, MD, of the Myanmar Oxford Clinical Research Unit in Yangon, Myanmar, and colleagues uncovered artemisinin resistance by analyzing parasite samples collected at 55 malaria treatment centers across Myanmar.
The group set out to determine if the samples carried mutations in specific regions of the parasite’s kelch gene (K13)—a known genetic marker of artemisinin resistance. And they confirmed the existance of resistant parasites in Homalin, in the Sagaing Region, which is located only 25 km from the Indian border.
“Myanmar is considered the frontline in the battle against artemisinin resistance, as it forms a gateway for resistance to spread to the rest of the world,” said Charles Woodrow, MD, of the University of Oxford in the UK.
“With artemisinins, we are in the unusual position of having molecular markers for resistance before resistance has spread globally. The more we understand about the current situation in the border regions, the better prepared we are to adapt and implement strategies to overcome the spread of further drug resistance.”
The researchers obtained the DNA sequences of 940 samples of Plasmodium falciparum malaria parasites from across Myanmar and neighboring border regions in Thailand and Bangladesh between 2013 and 2014. Of those 940 samples, 371 (39%) carried a resistance-conferring K13 mutation.
“We were able to gather patient samples rapidly across Myanmar, sometimes using discarded malaria blood diagnostic tests, and then test these immediately for the K13 marker, and so generate real-time information on the spread of resistance” said Mallika Imwong, PhD, of Mahidol University in Bangkok, Thailand.
Using this information, the researchers developed maps to display the predicted extent of artemisinin resistance determined by the prevalence of K13 mutations. The maps suggested the overall prevalence of K13 mutations was greater than 10% in large areas of the east and north of Myanmar, including areas close to the border with India.
“The identification of the K13 markers of resistance has transformed our ability to monitor the spread and emergence of artemisinin resistance,” said Philippe Guerin, MD, of the Worldwide Antimalarial Resistance Network in Oxford, UK.
“However, this study highlights that the pace at which artemisinin resistance is spreading or emerging is alarming. We need a more vigorous international effort to address this issue in border regions.” ![]()

Photo by James Gathany
Resistance to the antimalarial drug artemisinin is present in Myanmar and has reached within 25 km of the Indian border, according to research published in The Lancet Infectious Diseases.
Researchers believe the spread of artemisinin-resistant malaria parasites into neighboring India would pose a serious threat to the global control and eradication of malaria.
And if drug resistance continues to spread, millions of lives could be at risk.
Kyaw Myo Tun, MD, of the Myanmar Oxford Clinical Research Unit in Yangon, Myanmar, and colleagues uncovered artemisinin resistance by analyzing parasite samples collected at 55 malaria treatment centers across Myanmar.
The group set out to determine if the samples carried mutations in specific regions of the parasite’s kelch gene (K13)—a known genetic marker of artemisinin resistance. And they confirmed the existance of resistant parasites in Homalin, in the Sagaing Region, which is located only 25 km from the Indian border.
“Myanmar is considered the frontline in the battle against artemisinin resistance, as it forms a gateway for resistance to spread to the rest of the world,” said Charles Woodrow, MD, of the University of Oxford in the UK.
“With artemisinins, we are in the unusual position of having molecular markers for resistance before resistance has spread globally. The more we understand about the current situation in the border regions, the better prepared we are to adapt and implement strategies to overcome the spread of further drug resistance.”
The researchers obtained the DNA sequences of 940 samples of Plasmodium falciparum malaria parasites from across Myanmar and neighboring border regions in Thailand and Bangladesh between 2013 and 2014. Of those 940 samples, 371 (39%) carried a resistance-conferring K13 mutation.
“We were able to gather patient samples rapidly across Myanmar, sometimes using discarded malaria blood diagnostic tests, and then test these immediately for the K13 marker, and so generate real-time information on the spread of resistance” said Mallika Imwong, PhD, of Mahidol University in Bangkok, Thailand.
Using this information, the researchers developed maps to display the predicted extent of artemisinin resistance determined by the prevalence of K13 mutations. The maps suggested the overall prevalence of K13 mutations was greater than 10% in large areas of the east and north of Myanmar, including areas close to the border with India.
“The identification of the K13 markers of resistance has transformed our ability to monitor the spread and emergence of artemisinin resistance,” said Philippe Guerin, MD, of the Worldwide Antimalarial Resistance Network in Oxford, UK.
“However, this study highlights that the pace at which artemisinin resistance is spreading or emerging is alarming. We need a more vigorous international effort to address this issue in border regions.” ![]()
Psychotic symptoms in children and adolescents
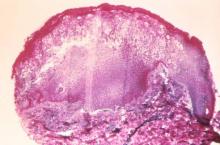
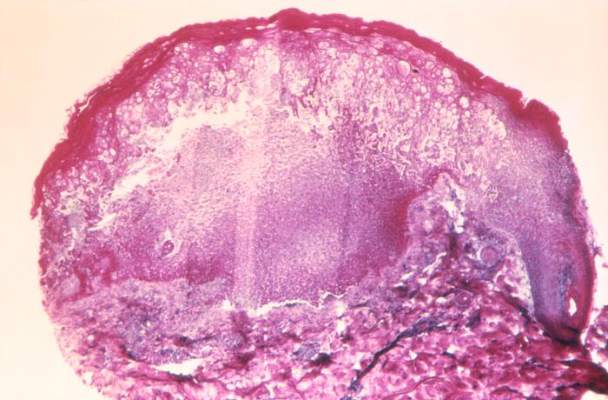
Some of the more disturbing behavioral symptoms to present are psychotic symptoms such as auditory or visual hallucination, delusions such as paranoia, or grossly disorganized thought content. Similar to the worry many families will have that a headache is the result of a brain tumor, concern that the psychotic symptoms represent the onset of schizophrenia often creates considerable alarm for families and primary care clinicians alike. In most cases, however, further evaluation suggests causes of psychotic or psychotic-like symptoms other than primary thought disorders.
Case Summary
Ella is an 8-year-old girl who has lived with her adoptive parents for 5 years. She was removed from the care of her birth parents by child protective services because of a history of abuse and neglect. Ella has struggled for many years with a variety of emotional-behavioral problems including inattention, frequent and intense angry outbursts, anxiety, and mood instability. She currently takes a long-acting methylphenidate preparation. Her parents present to her pediatrician because Ella is now reporting that she is seeing “shadows” in her room at night that frighten her. She also has lately stated that she hears a “mean voice” in her head that tells her that she is a bad person. The parents are not aware of specific psychiatric diagnoses in the birth parents, but state that they did have a history of “mental health problems” and were homeless at times. The parents are worried that these symptoms might be early signs of schizophrenia.
Discussion
Accumulating data demonstrates that while psychotic symptoms are relatively common in children and adolescents, childhood-onset schizophrenia actually is quite rare. Estimates of psychotic symptoms in otherwise healthy children have been as high as 5%, with a recent study of adolescents reporting that 15% of the sample reported hearing a voice that commented on what the person was thinking or feeling (Schizophr. Bull. 2014;40:868-77). At the same time, the incidence of childhood-onset schizophrenia is thought to be less than 0.04% based on data from a group at the National Institute of Mental Health (Child Adolesc. Psychiatr. Clin. N. Am. 2013;22:539-55). This group has been actively evaluating and recruiting children with early onset psychosis and finds that more than 90% of their referrals end up with a diagnosis other than schizophrenia.
The differential diagnosis for psychosis is extensive. In terms of nonpsychiatric diagnoses (what in the past were referred to as “organic” causes), possible etiologies include CNS tumors, encephalitis, metabolic disorders, and various genetic conditions, among others. Some medications, such as corticosteroids, stimulants, and anticholinergic medications, also can result in psychotic symptoms, especially at higher doses. While the acute presence of psychotic symptoms in an otherwise healthy child should certainly prompt suspicion of a possible delirium or other nonpsychiatric condition, it is important to note that some of the above etiologies can be associated with other types of behavioral disturbances; thus, the presence of earlier behavioral problems does not rule out the possibility that one of these nonpsychiatric causes is present.
Clinical tip: From our experience at a busy outpatient child psychiatry clinic, it is often not clear whose job it is to rule out nonpsychiatric causes of behavior problems. There is a risk that the psychiatrist assumes that the pediatrician has done this work-up while the pediatrician assumes that this component is part of a psychiatric evaluation. Communication about this role is important. If a third specialist is needed, such as a pediatric neurologist or geneticist, then it is important to clarify who will initiate that consultation as well.
The differential for psychotic symptoms also includes a number of psychiatric conditions other than schizophrenia, such as bipolar or unipolar depression, obsessive-compulsive disorder, posttraumatic stress disorder, autism, or an eating disorder. Substance use, particularly cannabis, also needs to be strongly considered. A child psychiatrist or other mental health professional can be very helpful here to help decipher what are sometimes subtle differences in the nature and content of the psychotic symptoms between various diagnoses. Receptive and expressive language disorders also can be present in many youth who experience psychotic symptoms.
The decision of if and when to begin treatment with an antipsychotic medication can be a difficult one and should be made very thoughtfully and with the help of consultation. The concern that a longer duration of untreated psychosis may be related to a more protracted course needs to be weighed against other data suggesting that using as little medication as possible may predict higher levels of future functioning (JAMA Psychiatry 2013;70:913-20). It is important to note that there are many nonpharmacological interventions that also can be helpful, including individual and family psychotherapy, family education, school modifications, and other social supports.
Case follow-up
Ella was referred to a child psychologist who performed an evaluation and thought that the patient’s symptoms were most representative of posttraumatic stress disorder. She began treatment with trauma-focused cognitive-behavioral therapy (TF-CBT) which led to a reduction in both her anxiety and psychotic-sounding symptoms.
Dr. Rettew is an associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Dr. Rettew said he has no relevant financial disclosures. Follow him on Twitter @pedipsych. E-mail him at [email protected].
Some of the more disturbing behavioral symptoms to present are psychotic symptoms such as auditory or visual hallucination, delusions such as paranoia, or grossly disorganized thought content. Similar to the worry many families will have that a headache is the result of a brain tumor, concern that the psychotic symptoms represent the onset of schizophrenia often creates considerable alarm for families and primary care clinicians alike. In most cases, however, further evaluation suggests causes of psychotic or psychotic-like symptoms other than primary thought disorders.
Case Summary
Ella is an 8-year-old girl who has lived with her adoptive parents for 5 years. She was removed from the care of her birth parents by child protective services because of a history of abuse and neglect. Ella has struggled for many years with a variety of emotional-behavioral problems including inattention, frequent and intense angry outbursts, anxiety, and mood instability. She currently takes a long-acting methylphenidate preparation. Her parents present to her pediatrician because Ella is now reporting that she is seeing “shadows” in her room at night that frighten her. She also has lately stated that she hears a “mean voice” in her head that tells her that she is a bad person. The parents are not aware of specific psychiatric diagnoses in the birth parents, but state that they did have a history of “mental health problems” and were homeless at times. The parents are worried that these symptoms might be early signs of schizophrenia.
Discussion
Accumulating data demonstrates that while psychotic symptoms are relatively common in children and adolescents, childhood-onset schizophrenia actually is quite rare. Estimates of psychotic symptoms in otherwise healthy children have been as high as 5%, with a recent study of adolescents reporting that 15% of the sample reported hearing a voice that commented on what the person was thinking or feeling (Schizophr. Bull. 2014;40:868-77). At the same time, the incidence of childhood-onset schizophrenia is thought to be less than 0.04% based on data from a group at the National Institute of Mental Health (Child Adolesc. Psychiatr. Clin. N. Am. 2013;22:539-55). This group has been actively evaluating and recruiting children with early onset psychosis and finds that more than 90% of their referrals end up with a diagnosis other than schizophrenia.
The differential diagnosis for psychosis is extensive. In terms of nonpsychiatric diagnoses (what in the past were referred to as “organic” causes), possible etiologies include CNS tumors, encephalitis, metabolic disorders, and various genetic conditions, among others. Some medications, such as corticosteroids, stimulants, and anticholinergic medications, also can result in psychotic symptoms, especially at higher doses. While the acute presence of psychotic symptoms in an otherwise healthy child should certainly prompt suspicion of a possible delirium or other nonpsychiatric condition, it is important to note that some of the above etiologies can be associated with other types of behavioral disturbances; thus, the presence of earlier behavioral problems does not rule out the possibility that one of these nonpsychiatric causes is present.
Clinical tip: From our experience at a busy outpatient child psychiatry clinic, it is often not clear whose job it is to rule out nonpsychiatric causes of behavior problems. There is a risk that the psychiatrist assumes that the pediatrician has done this work-up while the pediatrician assumes that this component is part of a psychiatric evaluation. Communication about this role is important. If a third specialist is needed, such as a pediatric neurologist or geneticist, then it is important to clarify who will initiate that consultation as well.
The differential for psychotic symptoms also includes a number of psychiatric conditions other than schizophrenia, such as bipolar or unipolar depression, obsessive-compulsive disorder, posttraumatic stress disorder, autism, or an eating disorder. Substance use, particularly cannabis, also needs to be strongly considered. A child psychiatrist or other mental health professional can be very helpful here to help decipher what are sometimes subtle differences in the nature and content of the psychotic symptoms between various diagnoses. Receptive and expressive language disorders also can be present in many youth who experience psychotic symptoms.
The decision of if and when to begin treatment with an antipsychotic medication can be a difficult one and should be made very thoughtfully and with the help of consultation. The concern that a longer duration of untreated psychosis may be related to a more protracted course needs to be weighed against other data suggesting that using as little medication as possible may predict higher levels of future functioning (JAMA Psychiatry 2013;70:913-20). It is important to note that there are many nonpharmacological interventions that also can be helpful, including individual and family psychotherapy, family education, school modifications, and other social supports.
Case follow-up
Ella was referred to a child psychologist who performed an evaluation and thought that the patient’s symptoms were most representative of posttraumatic stress disorder. She began treatment with trauma-focused cognitive-behavioral therapy (TF-CBT) which led to a reduction in both her anxiety and psychotic-sounding symptoms.
Dr. Rettew is an associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Dr. Rettew said he has no relevant financial disclosures. Follow him on Twitter @pedipsych. E-mail him at [email protected].
Some of the more disturbing behavioral symptoms to present are psychotic symptoms such as auditory or visual hallucination, delusions such as paranoia, or grossly disorganized thought content. Similar to the worry many families will have that a headache is the result of a brain tumor, concern that the psychotic symptoms represent the onset of schizophrenia often creates considerable alarm for families and primary care clinicians alike. In most cases, however, further evaluation suggests causes of psychotic or psychotic-like symptoms other than primary thought disorders.
Case Summary
Ella is an 8-year-old girl who has lived with her adoptive parents for 5 years. She was removed from the care of her birth parents by child protective services because of a history of abuse and neglect. Ella has struggled for many years with a variety of emotional-behavioral problems including inattention, frequent and intense angry outbursts, anxiety, and mood instability. She currently takes a long-acting methylphenidate preparation. Her parents present to her pediatrician because Ella is now reporting that she is seeing “shadows” in her room at night that frighten her. She also has lately stated that she hears a “mean voice” in her head that tells her that she is a bad person. The parents are not aware of specific psychiatric diagnoses in the birth parents, but state that they did have a history of “mental health problems” and were homeless at times. The parents are worried that these symptoms might be early signs of schizophrenia.
Discussion
Accumulating data demonstrates that while psychotic symptoms are relatively common in children and adolescents, childhood-onset schizophrenia actually is quite rare. Estimates of psychotic symptoms in otherwise healthy children have been as high as 5%, with a recent study of adolescents reporting that 15% of the sample reported hearing a voice that commented on what the person was thinking or feeling (Schizophr. Bull. 2014;40:868-77). At the same time, the incidence of childhood-onset schizophrenia is thought to be less than 0.04% based on data from a group at the National Institute of Mental Health (Child Adolesc. Psychiatr. Clin. N. Am. 2013;22:539-55). This group has been actively evaluating and recruiting children with early onset psychosis and finds that more than 90% of their referrals end up with a diagnosis other than schizophrenia.
The differential diagnosis for psychosis is extensive. In terms of nonpsychiatric diagnoses (what in the past were referred to as “organic” causes), possible etiologies include CNS tumors, encephalitis, metabolic disorders, and various genetic conditions, among others. Some medications, such as corticosteroids, stimulants, and anticholinergic medications, also can result in psychotic symptoms, especially at higher doses. While the acute presence of psychotic symptoms in an otherwise healthy child should certainly prompt suspicion of a possible delirium or other nonpsychiatric condition, it is important to note that some of the above etiologies can be associated with other types of behavioral disturbances; thus, the presence of earlier behavioral problems does not rule out the possibility that one of these nonpsychiatric causes is present.
Clinical tip: From our experience at a busy outpatient child psychiatry clinic, it is often not clear whose job it is to rule out nonpsychiatric causes of behavior problems. There is a risk that the psychiatrist assumes that the pediatrician has done this work-up while the pediatrician assumes that this component is part of a psychiatric evaluation. Communication about this role is important. If a third specialist is needed, such as a pediatric neurologist or geneticist, then it is important to clarify who will initiate that consultation as well.
The differential for psychotic symptoms also includes a number of psychiatric conditions other than schizophrenia, such as bipolar or unipolar depression, obsessive-compulsive disorder, posttraumatic stress disorder, autism, or an eating disorder. Substance use, particularly cannabis, also needs to be strongly considered. A child psychiatrist or other mental health professional can be very helpful here to help decipher what are sometimes subtle differences in the nature and content of the psychotic symptoms between various diagnoses. Receptive and expressive language disorders also can be present in many youth who experience psychotic symptoms.
The decision of if and when to begin treatment with an antipsychotic medication can be a difficult one and should be made very thoughtfully and with the help of consultation. The concern that a longer duration of untreated psychosis may be related to a more protracted course needs to be weighed against other data suggesting that using as little medication as possible may predict higher levels of future functioning (JAMA Psychiatry 2013;70:913-20). It is important to note that there are many nonpharmacological interventions that also can be helpful, including individual and family psychotherapy, family education, school modifications, and other social supports.
Case follow-up
Ella was referred to a child psychologist who performed an evaluation and thought that the patient’s symptoms were most representative of posttraumatic stress disorder. She began treatment with trauma-focused cognitive-behavioral therapy (TF-CBT) which led to a reduction in both her anxiety and psychotic-sounding symptoms.
Dr. Rettew is an associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Dr. Rettew said he has no relevant financial disclosures. Follow him on Twitter @pedipsych. E-mail him at [email protected].
EC expands indication for lenalidomide in MM

Photo courtesy of Celgene
The European Commission (EC) has expanded the marketing authorization for lenalidomide (Revlimid), just 2 days after the US Food and Drug Administration did the same.
Lenalidomide is now approved in the European Union (EU) to treat adults with previously untreated multiple myeloma (MM) who are not eligible for hematopoietic stem cell transplant. These patients can receive the drug continuously until
disease progression.
Lenalidomide was already approved in the EU for use in combination with dexamethasone to treat adults with MM who have received at least 1 prior therapy.
Lenalidomide is also approved in the EU to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion when other therapeutic options are insufficient or inadequate.
“Having a new treatment option now available for patients newly diagnosed with multiple myeloma is a real step forward,” said Thierry Facon, MD, of CHRU Lille in France.
“Treating patients continuously until disease progression is supported by several clinical studies and will have an important impact on how we manage the disease over the long-term.”
The EC’s decision to extend the approved use of lenalidomide was based on the results of 2 studies: MM-015 and MM-020, also known as FIRST.
The FIRST trial
In the phase 3 FIRST trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%), and abdominal pain (20.5%, 14.4%, 11.1%).
The incidence of invasive second primary malignancies was 3% in patients taking continuous Rd, 6% in patients taking Rd18, and 5% in those taking MPT. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
The MM-015 trial
In the phase 3 MM-015 study, researchers enrolled 459 patients who were 65 or older and newly diagnosed with MM.
The team compared melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance (MPR-R) with melphalan-prednisone-lenalidomide (MPR) or melphalan-prednisone (MP) followed by placebo maintenance.
Patients who received MPR-R or MPR had significantly better response rates than patients who received MP, at 77%, 68%, and 50%, respectively (P<0.001 and P=0.002, respectively, for the comparison with MP).
And the median progression-free survival was significantly longer with MPR-R (31 months) than with MPR (14 months, HR=0.49, P<0.001) or MP (13 months, HR=0.40, P<0.001).
During induction, the most frequent adverse events were hematologic. Grade 4 neutropenia occurred in 35% of patients in the MPR-R arm, 32% in the MPR arm, and 8% in the MP arm. The 3-year rate of second primary malignancies was 7%, 7%, and 3%, respectively. ![]()

Photo courtesy of Celgene
The European Commission (EC) has expanded the marketing authorization for lenalidomide (Revlimid), just 2 days after the US Food and Drug Administration did the same.
Lenalidomide is now approved in the European Union (EU) to treat adults with previously untreated multiple myeloma (MM) who are not eligible for hematopoietic stem cell transplant. These patients can receive the drug continuously until
disease progression.
Lenalidomide was already approved in the EU for use in combination with dexamethasone to treat adults with MM who have received at least 1 prior therapy.
Lenalidomide is also approved in the EU to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion when other therapeutic options are insufficient or inadequate.
“Having a new treatment option now available for patients newly diagnosed with multiple myeloma is a real step forward,” said Thierry Facon, MD, of CHRU Lille in France.
“Treating patients continuously until disease progression is supported by several clinical studies and will have an important impact on how we manage the disease over the long-term.”
The EC’s decision to extend the approved use of lenalidomide was based on the results of 2 studies: MM-015 and MM-020, also known as FIRST.
The FIRST trial
In the phase 3 FIRST trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%), and abdominal pain (20.5%, 14.4%, 11.1%).
The incidence of invasive second primary malignancies was 3% in patients taking continuous Rd, 6% in patients taking Rd18, and 5% in those taking MPT. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
The MM-015 trial
In the phase 3 MM-015 study, researchers enrolled 459 patients who were 65 or older and newly diagnosed with MM.
The team compared melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance (MPR-R) with melphalan-prednisone-lenalidomide (MPR) or melphalan-prednisone (MP) followed by placebo maintenance.
Patients who received MPR-R or MPR had significantly better response rates than patients who received MP, at 77%, 68%, and 50%, respectively (P<0.001 and P=0.002, respectively, for the comparison with MP).
And the median progression-free survival was significantly longer with MPR-R (31 months) than with MPR (14 months, HR=0.49, P<0.001) or MP (13 months, HR=0.40, P<0.001).
During induction, the most frequent adverse events were hematologic. Grade 4 neutropenia occurred in 35% of patients in the MPR-R arm, 32% in the MPR arm, and 8% in the MP arm. The 3-year rate of second primary malignancies was 7%, 7%, and 3%, respectively. ![]()

Photo courtesy of Celgene
The European Commission (EC) has expanded the marketing authorization for lenalidomide (Revlimid), just 2 days after the US Food and Drug Administration did the same.
Lenalidomide is now approved in the European Union (EU) to treat adults with previously untreated multiple myeloma (MM) who are not eligible for hematopoietic stem cell transplant. These patients can receive the drug continuously until
disease progression.
Lenalidomide was already approved in the EU for use in combination with dexamethasone to treat adults with MM who have received at least 1 prior therapy.
Lenalidomide is also approved in the EU to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion when other therapeutic options are insufficient or inadequate.
“Having a new treatment option now available for patients newly diagnosed with multiple myeloma is a real step forward,” said Thierry Facon, MD, of CHRU Lille in France.
“Treating patients continuously until disease progression is supported by several clinical studies and will have an important impact on how we manage the disease over the long-term.”
The EC’s decision to extend the approved use of lenalidomide was based on the results of 2 studies: MM-015 and MM-020, also known as FIRST.
The FIRST trial
In the phase 3 FIRST trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%), and abdominal pain (20.5%, 14.4%, 11.1%).
The incidence of invasive second primary malignancies was 3% in patients taking continuous Rd, 6% in patients taking Rd18, and 5% in those taking MPT. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
The MM-015 trial
In the phase 3 MM-015 study, researchers enrolled 459 patients who were 65 or older and newly diagnosed with MM.
The team compared melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance (MPR-R) with melphalan-prednisone-lenalidomide (MPR) or melphalan-prednisone (MP) followed by placebo maintenance.
Patients who received MPR-R or MPR had significantly better response rates than patients who received MP, at 77%, 68%, and 50%, respectively (P<0.001 and P=0.002, respectively, for the comparison with MP).
And the median progression-free survival was significantly longer with MPR-R (31 months) than with MPR (14 months, HR=0.49, P<0.001) or MP (13 months, HR=0.40, P<0.001).
During induction, the most frequent adverse events were hematologic. Grade 4 neutropenia occurred in 35% of patients in the MPR-R arm, 32% in the MPR arm, and 8% in the MP arm. The 3-year rate of second primary malignancies was 7%, 7%, and 3%, respectively. ![]()
March 2015 Quiz 2
ANSWER: B
Critique
This patient has evidence of an acute colonic pseudo-obstruction (known as Ogilvie’s syndrome). This is seen most commonly after non-GI related surgeries such as cardiac or orthopedic surgeries. The exact etiology is uncertain but increased inhibitory sympathetic and/or decreased stimulatory, parasympathetic innervations of the distal colon have been incriminated. The most appropriate first step in management of this patient is a thorough clinical evaluation to ensure there is no evidence of peritonitis to suggest a perforation complication. The next step is to exclude an obstruction and the CT had no clear evidence of obstruction. One could consider a water soluble enema to exclude obstruction but should avoid the use of barium in the event of a perforation. The next steps include restricting all possible culprit medications such as opiates and anticholinergics, encouraging ambulation (although often clinical circumstances limit ambulation), and correcting any potential electrolyte abnormalities.
Placement of a nasogastric tube to low intermittent suction, keeping the patient NPO (nothing by mouth), and placing a rectal tube to gravity are practical measure that can facilitate decompression. If the patient cannot ambulate, some clinicians also advocate rotating the patient into the right lateral decubitus position for several hours, alternating with the supine position, to facilitate gas evacuation. Such conservative measures are appropriate if there is no evidence of clinical toxicity or progression of the condition. Cecal diameters may be monitored on plain abdominal x-rays. If there is no clinical response to the above measures, then further treatments may be considered. Use of an acetylcholinesterase inhibitor has been shown to be beneficial in a placebo-controlled trial. If use of an acetylcholinesterase inhibitor is unsuccessful, colonic decompression can be considered though this typically provides only transient benefit. Finally, an emergent cecostomy can be considered if colonic decompression is unsuccessful.
References
1. De Giorgio R., Cogliandro R.F., Barbara G., Corinaldesi R., Stanghellini V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol. Clin. North Am. 2011;40:787-807.
2. Ponec R.J., Saunders M.D., KImmey M.B. Neostigmine for the treatment of acute colonic pseudo-obstruction. N. Engl. J. Med. 1999;341:137-41.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
ANSWER: B
Critique
This patient has evidence of an acute colonic pseudo-obstruction (known as Ogilvie’s syndrome). This is seen most commonly after non-GI related surgeries such as cardiac or orthopedic surgeries. The exact etiology is uncertain but increased inhibitory sympathetic and/or decreased stimulatory, parasympathetic innervations of the distal colon have been incriminated. The most appropriate first step in management of this patient is a thorough clinical evaluation to ensure there is no evidence of peritonitis to suggest a perforation complication. The next step is to exclude an obstruction and the CT had no clear evidence of obstruction. One could consider a water soluble enema to exclude obstruction but should avoid the use of barium in the event of a perforation. The next steps include restricting all possible culprit medications such as opiates and anticholinergics, encouraging ambulation (although often clinical circumstances limit ambulation), and correcting any potential electrolyte abnormalities.
Placement of a nasogastric tube to low intermittent suction, keeping the patient NPO (nothing by mouth), and placing a rectal tube to gravity are practical measure that can facilitate decompression. If the patient cannot ambulate, some clinicians also advocate rotating the patient into the right lateral decubitus position for several hours, alternating with the supine position, to facilitate gas evacuation. Such conservative measures are appropriate if there is no evidence of clinical toxicity or progression of the condition. Cecal diameters may be monitored on plain abdominal x-rays. If there is no clinical response to the above measures, then further treatments may be considered. Use of an acetylcholinesterase inhibitor has been shown to be beneficial in a placebo-controlled trial. If use of an acetylcholinesterase inhibitor is unsuccessful, colonic decompression can be considered though this typically provides only transient benefit. Finally, an emergent cecostomy can be considered if colonic decompression is unsuccessful.
References
1. De Giorgio R., Cogliandro R.F., Barbara G., Corinaldesi R., Stanghellini V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol. Clin. North Am. 2011;40:787-807.
2. Ponec R.J., Saunders M.D., KImmey M.B. Neostigmine for the treatment of acute colonic pseudo-obstruction. N. Engl. J. Med. 1999;341:137-41.
ANSWER: B
Critique
This patient has evidence of an acute colonic pseudo-obstruction (known as Ogilvie’s syndrome). This is seen most commonly after non-GI related surgeries such as cardiac or orthopedic surgeries. The exact etiology is uncertain but increased inhibitory sympathetic and/or decreased stimulatory, parasympathetic innervations of the distal colon have been incriminated. The most appropriate first step in management of this patient is a thorough clinical evaluation to ensure there is no evidence of peritonitis to suggest a perforation complication. The next step is to exclude an obstruction and the CT had no clear evidence of obstruction. One could consider a water soluble enema to exclude obstruction but should avoid the use of barium in the event of a perforation. The next steps include restricting all possible culprit medications such as opiates and anticholinergics, encouraging ambulation (although often clinical circumstances limit ambulation), and correcting any potential electrolyte abnormalities.
Placement of a nasogastric tube to low intermittent suction, keeping the patient NPO (nothing by mouth), and placing a rectal tube to gravity are practical measure that can facilitate decompression. If the patient cannot ambulate, some clinicians also advocate rotating the patient into the right lateral decubitus position for several hours, alternating with the supine position, to facilitate gas evacuation. Such conservative measures are appropriate if there is no evidence of clinical toxicity or progression of the condition. Cecal diameters may be monitored on plain abdominal x-rays. If there is no clinical response to the above measures, then further treatments may be considered. Use of an acetylcholinesterase inhibitor has been shown to be beneficial in a placebo-controlled trial. If use of an acetylcholinesterase inhibitor is unsuccessful, colonic decompression can be considered though this typically provides only transient benefit. Finally, an emergent cecostomy can be considered if colonic decompression is unsuccessful.
References
1. De Giorgio R., Cogliandro R.F., Barbara G., Corinaldesi R., Stanghellini V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol. Clin. North Am. 2011;40:787-807.
2. Ponec R.J., Saunders M.D., KImmey M.B. Neostigmine for the treatment of acute colonic pseudo-obstruction. N. Engl. J. Med. 1999;341:137-41.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
March 2015 Quiz 1
ANSWER: D
Critique
The patient is most likely to have Zollinger-Ellison syndrome (ZES), a condition caused by a gastrinoma. In 25% of cases, ZES is associated with multiple endocrine neoplasia type 1 (MEN-1). Clinical features of MEN-1 include gastrinoma or other islet cell tumor, hyperparathyroidism, and anterior pituitary tumors. ZES should be especially considered in a patient with multiple, refractory, or recurrent peptic ulcer disease, especially if accompanied by diarrhea or hypercalcemia. Diarrhea is often a predominant symptom and is caused by the large volume of acid that inactivates pancreatic lipase and damages the absorptive mucosa of the proximal gut. Tests to diagnose ZES include serum gastrin radioimmunoassay, secretin stimulation test, somatostatin receptor scintigraphy, and endoscopic ultrasound. Almost all gastrinomas contain somatostatin receptors on the gastrin cells and somatostatin scintigraphy using [111In-DPTA-Dphe1]-octreotide is considered the initial localization study of choice. It has 71% sensitivity and 86% specificity for primary tumors and 92% sensitivity for detection of metastatic disease.
References
1. Murugesan S.V., Varro A., Pritchard D.M. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol. Ther. 2009;29:1055-68.
2. Jensen R.T., Niederle B., Mitry E., et al. Frascati Consensus Conference; European Neuroendocrine Tumor Society. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173-82.
3. Hung, P.D., Schubert, M.L., Mihas, A.A. Zollinger-Ellison Syndrome. Current Treatment Options in Gastroenterology 2003;6:163-70.
4. Gibril F., Reynolds J.C., Doppman J.L., et al. Somatostatin receptor scintigraphy: Its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas - A prospective study. Ann. Intern. Med. 1996;125:26-34.
5. Hung, P.D., Schubert, M.L., Mihas, A.A. Zollinger-Ellison Syndrome. Current Treatment Options in Gastroenterology 2003;6:163-70.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
ANSWER: D
Critique
The patient is most likely to have Zollinger-Ellison syndrome (ZES), a condition caused by a gastrinoma. In 25% of cases, ZES is associated with multiple endocrine neoplasia type 1 (MEN-1). Clinical features of MEN-1 include gastrinoma or other islet cell tumor, hyperparathyroidism, and anterior pituitary tumors. ZES should be especially considered in a patient with multiple, refractory, or recurrent peptic ulcer disease, especially if accompanied by diarrhea or hypercalcemia. Diarrhea is often a predominant symptom and is caused by the large volume of acid that inactivates pancreatic lipase and damages the absorptive mucosa of the proximal gut. Tests to diagnose ZES include serum gastrin radioimmunoassay, secretin stimulation test, somatostatin receptor scintigraphy, and endoscopic ultrasound. Almost all gastrinomas contain somatostatin receptors on the gastrin cells and somatostatin scintigraphy using [111In-DPTA-Dphe1]-octreotide is considered the initial localization study of choice. It has 71% sensitivity and 86% specificity for primary tumors and 92% sensitivity for detection of metastatic disease.
References
1. Murugesan S.V., Varro A., Pritchard D.M. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol. Ther. 2009;29:1055-68.
2. Jensen R.T., Niederle B., Mitry E., et al. Frascati Consensus Conference; European Neuroendocrine Tumor Society. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173-82.
3. Hung, P.D., Schubert, M.L., Mihas, A.A. Zollinger-Ellison Syndrome. Current Treatment Options in Gastroenterology 2003;6:163-70.
4. Gibril F., Reynolds J.C., Doppman J.L., et al. Somatostatin receptor scintigraphy: Its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas - A prospective study. Ann. Intern. Med. 1996;125:26-34.
5. Hung, P.D., Schubert, M.L., Mihas, A.A. Zollinger-Ellison Syndrome. Current Treatment Options in Gastroenterology 2003;6:163-70.
ANSWER: D
Critique
The patient is most likely to have Zollinger-Ellison syndrome (ZES), a condition caused by a gastrinoma. In 25% of cases, ZES is associated with multiple endocrine neoplasia type 1 (MEN-1). Clinical features of MEN-1 include gastrinoma or other islet cell tumor, hyperparathyroidism, and anterior pituitary tumors. ZES should be especially considered in a patient with multiple, refractory, or recurrent peptic ulcer disease, especially if accompanied by diarrhea or hypercalcemia. Diarrhea is often a predominant symptom and is caused by the large volume of acid that inactivates pancreatic lipase and damages the absorptive mucosa of the proximal gut. Tests to diagnose ZES include serum gastrin radioimmunoassay, secretin stimulation test, somatostatin receptor scintigraphy, and endoscopic ultrasound. Almost all gastrinomas contain somatostatin receptors on the gastrin cells and somatostatin scintigraphy using [111In-DPTA-Dphe1]-octreotide is considered the initial localization study of choice. It has 71% sensitivity and 86% specificity for primary tumors and 92% sensitivity for detection of metastatic disease.
References
1. Murugesan S.V., Varro A., Pritchard D.M. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger-Ellison syndrome rather than a more common benign cause. Aliment Pharmacol. Ther. 2009;29:1055-68.
2. Jensen R.T., Niederle B., Mitry E., et al. Frascati Consensus Conference; European Neuroendocrine Tumor Society. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173-82.
3. Hung, P.D., Schubert, M.L., Mihas, A.A. Zollinger-Ellison Syndrome. Current Treatment Options in Gastroenterology 2003;6:163-70.
4. Gibril F., Reynolds J.C., Doppman J.L., et al. Somatostatin receptor scintigraphy: Its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas - A prospective study. Ann. Intern. Med. 1996;125:26-34.
5. Hung, P.D., Schubert, M.L., Mihas, A.A. Zollinger-Ellison Syndrome. Current Treatment Options in Gastroenterology 2003;6:163-70.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
Factor XI inhibitor trims DVTs after knee replacement surgery
SAN FRANCISCO – Reducing factor XI levels with the experimental antisense oligonucleotide FXI-ASO lowered venous thromboembolism rates after total knee arthroplasty without increasing bleeding in a phase II study.
Venous thromboembolism (VTE) rates were 30% among controls (21/69) on enoxaparin (Lovenox) 40 mg, compared with 27% for patients (36/134) given FXI-ASO 200 mg and 4% for those (3/71) given FXI-ASO 300 mg. Low-dose FXI-ASO was noninferior to enoxaparin (P = .59), while the high-dose regimen was superior (P < .001).
A 4% VTE rate “has never ever been seen before in patients undergoing knee surgery,” Dr. Harry Büller said during the late-breaking abstract session at the annual meeting of the American Society of Hematology.
The strategy of targeting factor XI is based on the understanding that patients with factor XI deficiency (plasma levels < 20% of normal) have a reduced risk of deep vein thrombosis (DVT). Experimental data in mice and primates also suggest that reducing factor XI attenuates thrombosis without excess bleeding.
Among the 300 patients in the open-label study, major or clinically relevant bleeding occurred in 3% of both FXI-ASO groups and 8% of the enoxaparin group (P = .09).
The findings provide the first evidence in humans that the factor XI intrinsic pathway is one of the drivers of postoperative thrombosis and support the concept that thrombosis and hemostasis can be dissociated, said Dr. Büller of the Academic Medical Center, Amsterdam.
“FXI-ASO is a promising new investigational antithrombotic agent and I believe you are witnessing the birth of a new class of antithrombotic agents,” he concluded.
During a press conference, Dr. Büller confided to reporters that he felt like a boy in a candy store, finally able to reveal the superb study findings.
Dr. Robert Flaumenhaft of Harvard Medical School, Boston, was far less effusive in an editorial that accompanied the simultaneous publication of the study in the New England Journal of Medicine.
“Do these finding prove that reduction in factor XI levels inhibits thrombosis without affecting bleeding? The conservative answer is no,” he wrote.
Dr. Flaumenhaft observed that the incidence of clinically relevant bleeding is relatively low after knee arthroplasty, even when patients receive anticoagulants, and that this safety outcome did not differ significantly between the enoxaparin and 300-mg FXI-ASO groups.
“These results also do not make a compelling case for the clinical use of the factor XI antisense oligonucleotide over anticoagulants that are currently used for prophylaxis in patients undergoing knee arthroplasty,” he wrote.
Central to this argument are issues of convenience and questions regarding reversibility. Treatment began 36 days before surgery and was associated with a high incidence of adverse events at the injection site and factor XI levels remained about 60% lower 70 days after initiation of therapy.
The half-life of FXI-ASO is about 22 days, “which in the classical setting in terms of bleeding could be seen as something of a disadvantage,” Dr. Büller told reporters. “But if we do the next study and it shows to be safe, it turns into an advantage” … because there is the possibility of giving FXI-ASO once every 3 weeks.
Dr. Flaumenhaft closed the editorial by acknowledging that the study challenges “the current paradigm” regarding the primary mechanism responsible for fibrin formation during thrombosis. “The striking observation that reducing factor XI levels prevents thrombosis after knee arthroplasty provides the best clinical evidence to date that the intrinsic pathway is essential for thrombus formation,” he wrote.
The study was conducted at 19 centers in five countries and randomly assigned 300 patients scheduled for elective primary unilateral total-knee arthroplasty to daily enoxaparin 40 mg or three doses of FXI-ASO. The protocol was amended early on to exclude a 100-mg FXI-ASO dose.
FXI-ASO 200 mg or 300 mg was given subcutaneously beginning 36 days before surgery on days 1, 3, 5, 8, 15, 22, and 29, and 6 hours postoperatively, with a final dose on day 39.
Enoxaparin 40 mg was given subcutaneously once daily, beginning the evening before or 6-8 hours after surgery, according to investigator preference, and was continued for at least 8 days postoperatively.
The primary efficacy point was a composite of asymptomatic DVT, detected by venography, and confirmed symptomatic VTE.
At baseline, the average factor XI level was 1.23 units/mL in the enoxaparin group, 1.20 U/mL in the 200-mg group, and 1.16 U/mL in the 300-mg group.
In patients with an average factor XI level of 0.2 U/mL or less, the incidence of the primary efficacy outcome was 5%.
Isis Pharmaceuticals funded the study. Dr. Büller disclosed ties with Isis, Daiichi-Sankyo, Bayer Healthcare, Pfizer, and Bristol-Myers Squibb. Dr. Flaumenhaft reported having no disclosures.
SAN FRANCISCO – Reducing factor XI levels with the experimental antisense oligonucleotide FXI-ASO lowered venous thromboembolism rates after total knee arthroplasty without increasing bleeding in a phase II study.
Venous thromboembolism (VTE) rates were 30% among controls (21/69) on enoxaparin (Lovenox) 40 mg, compared with 27% for patients (36/134) given FXI-ASO 200 mg and 4% for those (3/71) given FXI-ASO 300 mg. Low-dose FXI-ASO was noninferior to enoxaparin (P = .59), while the high-dose regimen was superior (P < .001).
A 4% VTE rate “has never ever been seen before in patients undergoing knee surgery,” Dr. Harry Büller said during the late-breaking abstract session at the annual meeting of the American Society of Hematology.
The strategy of targeting factor XI is based on the understanding that patients with factor XI deficiency (plasma levels < 20% of normal) have a reduced risk of deep vein thrombosis (DVT). Experimental data in mice and primates also suggest that reducing factor XI attenuates thrombosis without excess bleeding.
Among the 300 patients in the open-label study, major or clinically relevant bleeding occurred in 3% of both FXI-ASO groups and 8% of the enoxaparin group (P = .09).
The findings provide the first evidence in humans that the factor XI intrinsic pathway is one of the drivers of postoperative thrombosis and support the concept that thrombosis and hemostasis can be dissociated, said Dr. Büller of the Academic Medical Center, Amsterdam.
“FXI-ASO is a promising new investigational antithrombotic agent and I believe you are witnessing the birth of a new class of antithrombotic agents,” he concluded.
During a press conference, Dr. Büller confided to reporters that he felt like a boy in a candy store, finally able to reveal the superb study findings.
Dr. Robert Flaumenhaft of Harvard Medical School, Boston, was far less effusive in an editorial that accompanied the simultaneous publication of the study in the New England Journal of Medicine.
“Do these finding prove that reduction in factor XI levels inhibits thrombosis without affecting bleeding? The conservative answer is no,” he wrote.
Dr. Flaumenhaft observed that the incidence of clinically relevant bleeding is relatively low after knee arthroplasty, even when patients receive anticoagulants, and that this safety outcome did not differ significantly between the enoxaparin and 300-mg FXI-ASO groups.
“These results also do not make a compelling case for the clinical use of the factor XI antisense oligonucleotide over anticoagulants that are currently used for prophylaxis in patients undergoing knee arthroplasty,” he wrote.
Central to this argument are issues of convenience and questions regarding reversibility. Treatment began 36 days before surgery and was associated with a high incidence of adverse events at the injection site and factor XI levels remained about 60% lower 70 days after initiation of therapy.
The half-life of FXI-ASO is about 22 days, “which in the classical setting in terms of bleeding could be seen as something of a disadvantage,” Dr. Büller told reporters. “But if we do the next study and it shows to be safe, it turns into an advantage” … because there is the possibility of giving FXI-ASO once every 3 weeks.
Dr. Flaumenhaft closed the editorial by acknowledging that the study challenges “the current paradigm” regarding the primary mechanism responsible for fibrin formation during thrombosis. “The striking observation that reducing factor XI levels prevents thrombosis after knee arthroplasty provides the best clinical evidence to date that the intrinsic pathway is essential for thrombus formation,” he wrote.
The study was conducted at 19 centers in five countries and randomly assigned 300 patients scheduled for elective primary unilateral total-knee arthroplasty to daily enoxaparin 40 mg or three doses of FXI-ASO. The protocol was amended early on to exclude a 100-mg FXI-ASO dose.
FXI-ASO 200 mg or 300 mg was given subcutaneously beginning 36 days before surgery on days 1, 3, 5, 8, 15, 22, and 29, and 6 hours postoperatively, with a final dose on day 39.
Enoxaparin 40 mg was given subcutaneously once daily, beginning the evening before or 6-8 hours after surgery, according to investigator preference, and was continued for at least 8 days postoperatively.
The primary efficacy point was a composite of asymptomatic DVT, detected by venography, and confirmed symptomatic VTE.
At baseline, the average factor XI level was 1.23 units/mL in the enoxaparin group, 1.20 U/mL in the 200-mg group, and 1.16 U/mL in the 300-mg group.
In patients with an average factor XI level of 0.2 U/mL or less, the incidence of the primary efficacy outcome was 5%.
Isis Pharmaceuticals funded the study. Dr. Büller disclosed ties with Isis, Daiichi-Sankyo, Bayer Healthcare, Pfizer, and Bristol-Myers Squibb. Dr. Flaumenhaft reported having no disclosures.
SAN FRANCISCO – Reducing factor XI levels with the experimental antisense oligonucleotide FXI-ASO lowered venous thromboembolism rates after total knee arthroplasty without increasing bleeding in a phase II study.
Venous thromboembolism (VTE) rates were 30% among controls (21/69) on enoxaparin (Lovenox) 40 mg, compared with 27% for patients (36/134) given FXI-ASO 200 mg and 4% for those (3/71) given FXI-ASO 300 mg. Low-dose FXI-ASO was noninferior to enoxaparin (P = .59), while the high-dose regimen was superior (P < .001).
A 4% VTE rate “has never ever been seen before in patients undergoing knee surgery,” Dr. Harry Büller said during the late-breaking abstract session at the annual meeting of the American Society of Hematology.
The strategy of targeting factor XI is based on the understanding that patients with factor XI deficiency (plasma levels < 20% of normal) have a reduced risk of deep vein thrombosis (DVT). Experimental data in mice and primates also suggest that reducing factor XI attenuates thrombosis without excess bleeding.
Among the 300 patients in the open-label study, major or clinically relevant bleeding occurred in 3% of both FXI-ASO groups and 8% of the enoxaparin group (P = .09).
The findings provide the first evidence in humans that the factor XI intrinsic pathway is one of the drivers of postoperative thrombosis and support the concept that thrombosis and hemostasis can be dissociated, said Dr. Büller of the Academic Medical Center, Amsterdam.
“FXI-ASO is a promising new investigational antithrombotic agent and I believe you are witnessing the birth of a new class of antithrombotic agents,” he concluded.
During a press conference, Dr. Büller confided to reporters that he felt like a boy in a candy store, finally able to reveal the superb study findings.
Dr. Robert Flaumenhaft of Harvard Medical School, Boston, was far less effusive in an editorial that accompanied the simultaneous publication of the study in the New England Journal of Medicine.
“Do these finding prove that reduction in factor XI levels inhibits thrombosis without affecting bleeding? The conservative answer is no,” he wrote.
Dr. Flaumenhaft observed that the incidence of clinically relevant bleeding is relatively low after knee arthroplasty, even when patients receive anticoagulants, and that this safety outcome did not differ significantly between the enoxaparin and 300-mg FXI-ASO groups.
“These results also do not make a compelling case for the clinical use of the factor XI antisense oligonucleotide over anticoagulants that are currently used for prophylaxis in patients undergoing knee arthroplasty,” he wrote.
Central to this argument are issues of convenience and questions regarding reversibility. Treatment began 36 days before surgery and was associated with a high incidence of adverse events at the injection site and factor XI levels remained about 60% lower 70 days after initiation of therapy.
The half-life of FXI-ASO is about 22 days, “which in the classical setting in terms of bleeding could be seen as something of a disadvantage,” Dr. Büller told reporters. “But if we do the next study and it shows to be safe, it turns into an advantage” … because there is the possibility of giving FXI-ASO once every 3 weeks.
Dr. Flaumenhaft closed the editorial by acknowledging that the study challenges “the current paradigm” regarding the primary mechanism responsible for fibrin formation during thrombosis. “The striking observation that reducing factor XI levels prevents thrombosis after knee arthroplasty provides the best clinical evidence to date that the intrinsic pathway is essential for thrombus formation,” he wrote.
The study was conducted at 19 centers in five countries and randomly assigned 300 patients scheduled for elective primary unilateral total-knee arthroplasty to daily enoxaparin 40 mg or three doses of FXI-ASO. The protocol was amended early on to exclude a 100-mg FXI-ASO dose.
FXI-ASO 200 mg or 300 mg was given subcutaneously beginning 36 days before surgery on days 1, 3, 5, 8, 15, 22, and 29, and 6 hours postoperatively, with a final dose on day 39.
Enoxaparin 40 mg was given subcutaneously once daily, beginning the evening before or 6-8 hours after surgery, according to investigator preference, and was continued for at least 8 days postoperatively.
The primary efficacy point was a composite of asymptomatic DVT, detected by venography, and confirmed symptomatic VTE.
At baseline, the average factor XI level was 1.23 units/mL in the enoxaparin group, 1.20 U/mL in the 200-mg group, and 1.16 U/mL in the 300-mg group.
In patients with an average factor XI level of 0.2 U/mL or less, the incidence of the primary efficacy outcome was 5%.
Isis Pharmaceuticals funded the study. Dr. Büller disclosed ties with Isis, Daiichi-Sankyo, Bayer Healthcare, Pfizer, and Bristol-Myers Squibb. Dr. Flaumenhaft reported having no disclosures.
AT ASH 2014
Key clinical point: Reducing factor XI levels with FXI-ASO was effective in preventing VTE in patients undergoing knee arthroplasty and appeared safe with respect to bleeding risk.
Major finding: The primary VTE endpoint occurred in 4% of patients on FXI-ASO 300 mg, 27% on FXI-ASO 200 mg, and 30% on enoxaparin.
Data source: Open-label, parallel-group phase II study of 300 patients undergoing primary unilateral total-knee arthroplasty.
Disclosures: Isis Pharmaceuticals funded the study. Dr. Büller disclosed ties with Isis, Daiichi-Sankyo, Bayer Healthcare, Pfizer, and Bristol-Myers Squibb. Dr. Flaumenhaft reported having no disclosures.
Postexposure smallpox vaccination not recommended for immunodeficient patients
Persons exposed to smallpox should be vaccinated with a replication-competent vaccine, unless they are severely immunodeficient, according to a guideline from the Centers for Disease Control and Prevention.
Severely immunodeficient persons won’t benefit from a smallpox vaccination because there will likely be a poor immune response and heightened risk of negative events. These include bone marrow transplant recipients within 4 months of transplantation, people infected with HIV with CD4 cell counts <50 cells/mm3, persons with severe combined immunodeficiency, complete DiGeorge syndrome patients, and people with other severely immunocompromised states requiring isolation.
“If antivirals are not immediately available, it is reasonable to consider the use of Imvamune in the setting of a smallpox virus exposure in persons with severe immunodeficiency,” the CDC added.
Find the full guideline in the MMWR (February 20, 2015 / 64(RR02);1-26).
Persons exposed to smallpox should be vaccinated with a replication-competent vaccine, unless they are severely immunodeficient, according to a guideline from the Centers for Disease Control and Prevention.
Severely immunodeficient persons won’t benefit from a smallpox vaccination because there will likely be a poor immune response and heightened risk of negative events. These include bone marrow transplant recipients within 4 months of transplantation, people infected with HIV with CD4 cell counts <50 cells/mm3, persons with severe combined immunodeficiency, complete DiGeorge syndrome patients, and people with other severely immunocompromised states requiring isolation.
“If antivirals are not immediately available, it is reasonable to consider the use of Imvamune in the setting of a smallpox virus exposure in persons with severe immunodeficiency,” the CDC added.
Find the full guideline in the MMWR (February 20, 2015 / 64(RR02);1-26).
Persons exposed to smallpox should be vaccinated with a replication-competent vaccine, unless they are severely immunodeficient, according to a guideline from the Centers for Disease Control and Prevention.
Severely immunodeficient persons won’t benefit from a smallpox vaccination because there will likely be a poor immune response and heightened risk of negative events. These include bone marrow transplant recipients within 4 months of transplantation, people infected with HIV with CD4 cell counts <50 cells/mm3, persons with severe combined immunodeficiency, complete DiGeorge syndrome patients, and people with other severely immunocompromised states requiring isolation.
“If antivirals are not immediately available, it is reasonable to consider the use of Imvamune in the setting of a smallpox virus exposure in persons with severe immunodeficiency,” the CDC added.
Find the full guideline in the MMWR (February 20, 2015 / 64(RR02);1-26).
Bleeding complications following femoral angiographic access
Bleeding complications following angiographic interventions have recently become an increasing cause of medical malpractice litigation. The reason for this increase is likely a result of several factors including the controversy surrounding the multiple approaches that are currently employed for the treatment of this type of bleeding, as well as the significant morbidity and mortality associated with these treatment paradigms. Allegations in these lawsuits usually include failure to diagnose, failure to treat, failure to transfuse, negligence in the use of an endovascular approach, and negligence in open surgical treatment. Physical examination, close observation, and an aggressive approach to intervention are necessary if litigation is to be avoided in this patient population.
Case 1
The patient underwent cardiac catheterization with placement of a coronary stent. The patient was placed on Integrilin. Following the catheterization, the patient had multiple episodes of hypotension. Each episode responded to fluid boluses and transfusion. No surgical consultation was obtained. The patient’s hemoglobin never fell below 8 grams. The patient was transfused approximately 8 units of blood. The patient’s Integrilin was discontinued. However, the patient progressed to multisystem organ failure and died. Autopsy revealed a massive hematoma of the retroperitoneum and a patent coronary stent. A medical malpractice suit was filed. The case was settled.
Case 2
A patient underwent cardiac catheterization and subsequently developed severe bleeding. She received approximately 10 units of blood prior to vascular surgical consultation. By the time the vascular surgeon saw the patient, the patient was intubated and on vasopressors. The vascular surgeon stated that the patient was not a candidate for surgery. The patient subsequently died. A lawsuit was filed against both the cardiologist and the vascular surgeon. This case was settled by both physicians.
Case 3
A cardiologist calls a vascular surgeon who is at home at 10 p.m. to let her know that he has a patient with a retroperitoneal hematoma following a cardiac cath. He informs the surgeon that the patient is stable. He “just wants her to be aware in case the patient’s condition deteriorates.”
During the night the patient has repeated hypotensive episodes which the cardiologist manages with transfusions. At 5 a.m. the patient arrests and is resuscitated. The surgeon is called, and she takes the patient to surgery but the patient succumbs. The surgeon is sued for not coming in to see the patient and being more involved during the night.
The jury finds in favor of the surgeon. However, the trial took 2 weeks during which time the surgeon had to attend the deliberations and so she was unable work.
Discussion
Unfortunately, the above examples represent the all too often complication of postprocedure bleeding following femoral access.
In case 1, early exploration would more likely than not, and within a reasonable degree of medical certainty, prevented the patient’s death. The presumption is that the patient died from the untreated complication of postcatheterization hemorrhage. The defendant’s claim that the patient’s hemoglobin was never lower than 8 mg did not serve as an adequate defense. Even if the patient was found at autopsy to have an occluded stent which caused an acute MI, the plaintiff’s attorney would likely argue successfully that if the bleeding had been appropriately treated, the stent would have remained patent. The cardiologist was found culpable for not consulting a vascular surgeon.
In the second example, the plaintiff’s expert explained to the jury that without surgery, the patient would continue to bleed and certainly die. He went on to opine that the only possible chance of the patient surviving was with surgical intervention, and this chance was denied to the patient by the vascular surgeon who refused to operate. In these types of cases, the surgeon incorrectly believes that he/she can avoid liability and involvement in the case by not operating on the patient. However, as this case demonstrated, the surgeon is still likely to be named as a defendant in the law suit.
The third case illustrates two common pitfalls for the vascular surgeon and perhaps represents the most dangerous situation for the vascular surgeon. The first pitfall occurs when the vascular surgeon relies on telephone information without examining the patient. Secondly, as in this case, a formal consult was never initiated by the cardiologist. The surgeon incorrectly assumed that because no formal consult was placed she had no liability. However, according to the plaintiff’s expert, since she had been informed about the patient she should have come in to see the patient.
Had she done so, he alleged she would have realized that the patient required an intervention either with a covered stent or a surgical repair of the bleeding external iliac artery. Clearly, the fact that no formal consult was placed in the chart did not prevent the vascular surgeon from being a named defendant in this case.
Several steps should be taken if a physician is to minimize the risk of being named as a defendant in a lawsuit involving postangiographic intervention bleeding. First, if the physician is a not a surgeon, surgical consultation should be obtained as soon as postprocedure bleeding is suspected. Second, surgeons should pursue an aggressive approach to the treatment of this complication. Whereas it may be reasonable to treat a single episode of hypotension with fluid or blood transfusion, unless there are mitigating circumstances, any patient who develops a second episode of hypotension in the face of ongoing bleeding should undergo intervention. Furthermore, there must be clear documentation as to why a particular approach (endovascular versus open repair) was chosen. A medical physician’s inability to perform an open approach or a surgeon’s inability to perform an endovascular approach are not sustainable defenses.
Surgeons who are consulted late in the patient’s clinical course, prior to intervention, should document the patient’s poor prognosis and make it clear that no matter what is done the patient is unlikely to survive.
However, in most situations, the defense that a patient with ongoing bleeding was too unstable to treat is likely to fail.
Access site bleeding following percutaneous interventions is often a readily treatable complication. A low threshold for intervention, cooperation among vascular specialists, and, as always, clear documentation will go a long way to keep physicians working and out of the courtroom.
Dr. Brown is associate editor of Vascular Specialist. Dr. Samson is the medical editor, Vascular Specialist. The opinions expressed by the authors neither imply nor establish a standard of care.
Bleeding complications following angiographic interventions have recently become an increasing cause of medical malpractice litigation. The reason for this increase is likely a result of several factors including the controversy surrounding the multiple approaches that are currently employed for the treatment of this type of bleeding, as well as the significant morbidity and mortality associated with these treatment paradigms. Allegations in these lawsuits usually include failure to diagnose, failure to treat, failure to transfuse, negligence in the use of an endovascular approach, and negligence in open surgical treatment. Physical examination, close observation, and an aggressive approach to intervention are necessary if litigation is to be avoided in this patient population.
Case 1
The patient underwent cardiac catheterization with placement of a coronary stent. The patient was placed on Integrilin. Following the catheterization, the patient had multiple episodes of hypotension. Each episode responded to fluid boluses and transfusion. No surgical consultation was obtained. The patient’s hemoglobin never fell below 8 grams. The patient was transfused approximately 8 units of blood. The patient’s Integrilin was discontinued. However, the patient progressed to multisystem organ failure and died. Autopsy revealed a massive hematoma of the retroperitoneum and a patent coronary stent. A medical malpractice suit was filed. The case was settled.
Case 2
A patient underwent cardiac catheterization and subsequently developed severe bleeding. She received approximately 10 units of blood prior to vascular surgical consultation. By the time the vascular surgeon saw the patient, the patient was intubated and on vasopressors. The vascular surgeon stated that the patient was not a candidate for surgery. The patient subsequently died. A lawsuit was filed against both the cardiologist and the vascular surgeon. This case was settled by both physicians.
Case 3
A cardiologist calls a vascular surgeon who is at home at 10 p.m. to let her know that he has a patient with a retroperitoneal hematoma following a cardiac cath. He informs the surgeon that the patient is stable. He “just wants her to be aware in case the patient’s condition deteriorates.”
During the night the patient has repeated hypotensive episodes which the cardiologist manages with transfusions. At 5 a.m. the patient arrests and is resuscitated. The surgeon is called, and she takes the patient to surgery but the patient succumbs. The surgeon is sued for not coming in to see the patient and being more involved during the night.
The jury finds in favor of the surgeon. However, the trial took 2 weeks during which time the surgeon had to attend the deliberations and so she was unable work.
Discussion
Unfortunately, the above examples represent the all too often complication of postprocedure bleeding following femoral access.
In case 1, early exploration would more likely than not, and within a reasonable degree of medical certainty, prevented the patient’s death. The presumption is that the patient died from the untreated complication of postcatheterization hemorrhage. The defendant’s claim that the patient’s hemoglobin was never lower than 8 mg did not serve as an adequate defense. Even if the patient was found at autopsy to have an occluded stent which caused an acute MI, the plaintiff’s attorney would likely argue successfully that if the bleeding had been appropriately treated, the stent would have remained patent. The cardiologist was found culpable for not consulting a vascular surgeon.
In the second example, the plaintiff’s expert explained to the jury that without surgery, the patient would continue to bleed and certainly die. He went on to opine that the only possible chance of the patient surviving was with surgical intervention, and this chance was denied to the patient by the vascular surgeon who refused to operate. In these types of cases, the surgeon incorrectly believes that he/she can avoid liability and involvement in the case by not operating on the patient. However, as this case demonstrated, the surgeon is still likely to be named as a defendant in the law suit.
The third case illustrates two common pitfalls for the vascular surgeon and perhaps represents the most dangerous situation for the vascular surgeon. The first pitfall occurs when the vascular surgeon relies on telephone information without examining the patient. Secondly, as in this case, a formal consult was never initiated by the cardiologist. The surgeon incorrectly assumed that because no formal consult was placed she had no liability. However, according to the plaintiff’s expert, since she had been informed about the patient she should have come in to see the patient.
Had she done so, he alleged she would have realized that the patient required an intervention either with a covered stent or a surgical repair of the bleeding external iliac artery. Clearly, the fact that no formal consult was placed in the chart did not prevent the vascular surgeon from being a named defendant in this case.
Several steps should be taken if a physician is to minimize the risk of being named as a defendant in a lawsuit involving postangiographic intervention bleeding. First, if the physician is a not a surgeon, surgical consultation should be obtained as soon as postprocedure bleeding is suspected. Second, surgeons should pursue an aggressive approach to the treatment of this complication. Whereas it may be reasonable to treat a single episode of hypotension with fluid or blood transfusion, unless there are mitigating circumstances, any patient who develops a second episode of hypotension in the face of ongoing bleeding should undergo intervention. Furthermore, there must be clear documentation as to why a particular approach (endovascular versus open repair) was chosen. A medical physician’s inability to perform an open approach or a surgeon’s inability to perform an endovascular approach are not sustainable defenses.
Surgeons who are consulted late in the patient’s clinical course, prior to intervention, should document the patient’s poor prognosis and make it clear that no matter what is done the patient is unlikely to survive.
However, in most situations, the defense that a patient with ongoing bleeding was too unstable to treat is likely to fail.
Access site bleeding following percutaneous interventions is often a readily treatable complication. A low threshold for intervention, cooperation among vascular specialists, and, as always, clear documentation will go a long way to keep physicians working and out of the courtroom.
Dr. Brown is associate editor of Vascular Specialist. Dr. Samson is the medical editor, Vascular Specialist. The opinions expressed by the authors neither imply nor establish a standard of care.
Bleeding complications following angiographic interventions have recently become an increasing cause of medical malpractice litigation. The reason for this increase is likely a result of several factors including the controversy surrounding the multiple approaches that are currently employed for the treatment of this type of bleeding, as well as the significant morbidity and mortality associated with these treatment paradigms. Allegations in these lawsuits usually include failure to diagnose, failure to treat, failure to transfuse, negligence in the use of an endovascular approach, and negligence in open surgical treatment. Physical examination, close observation, and an aggressive approach to intervention are necessary if litigation is to be avoided in this patient population.
Case 1
The patient underwent cardiac catheterization with placement of a coronary stent. The patient was placed on Integrilin. Following the catheterization, the patient had multiple episodes of hypotension. Each episode responded to fluid boluses and transfusion. No surgical consultation was obtained. The patient’s hemoglobin never fell below 8 grams. The patient was transfused approximately 8 units of blood. The patient’s Integrilin was discontinued. However, the patient progressed to multisystem organ failure and died. Autopsy revealed a massive hematoma of the retroperitoneum and a patent coronary stent. A medical malpractice suit was filed. The case was settled.
Case 2
A patient underwent cardiac catheterization and subsequently developed severe bleeding. She received approximately 10 units of blood prior to vascular surgical consultation. By the time the vascular surgeon saw the patient, the patient was intubated and on vasopressors. The vascular surgeon stated that the patient was not a candidate for surgery. The patient subsequently died. A lawsuit was filed against both the cardiologist and the vascular surgeon. This case was settled by both physicians.
Case 3
A cardiologist calls a vascular surgeon who is at home at 10 p.m. to let her know that he has a patient with a retroperitoneal hematoma following a cardiac cath. He informs the surgeon that the patient is stable. He “just wants her to be aware in case the patient’s condition deteriorates.”
During the night the patient has repeated hypotensive episodes which the cardiologist manages with transfusions. At 5 a.m. the patient arrests and is resuscitated. The surgeon is called, and she takes the patient to surgery but the patient succumbs. The surgeon is sued for not coming in to see the patient and being more involved during the night.
The jury finds in favor of the surgeon. However, the trial took 2 weeks during which time the surgeon had to attend the deliberations and so she was unable work.
Discussion
Unfortunately, the above examples represent the all too often complication of postprocedure bleeding following femoral access.
In case 1, early exploration would more likely than not, and within a reasonable degree of medical certainty, prevented the patient’s death. The presumption is that the patient died from the untreated complication of postcatheterization hemorrhage. The defendant’s claim that the patient’s hemoglobin was never lower than 8 mg did not serve as an adequate defense. Even if the patient was found at autopsy to have an occluded stent which caused an acute MI, the plaintiff’s attorney would likely argue successfully that if the bleeding had been appropriately treated, the stent would have remained patent. The cardiologist was found culpable for not consulting a vascular surgeon.
In the second example, the plaintiff’s expert explained to the jury that without surgery, the patient would continue to bleed and certainly die. He went on to opine that the only possible chance of the patient surviving was with surgical intervention, and this chance was denied to the patient by the vascular surgeon who refused to operate. In these types of cases, the surgeon incorrectly believes that he/she can avoid liability and involvement in the case by not operating on the patient. However, as this case demonstrated, the surgeon is still likely to be named as a defendant in the law suit.
The third case illustrates two common pitfalls for the vascular surgeon and perhaps represents the most dangerous situation for the vascular surgeon. The first pitfall occurs when the vascular surgeon relies on telephone information without examining the patient. Secondly, as in this case, a formal consult was never initiated by the cardiologist. The surgeon incorrectly assumed that because no formal consult was placed she had no liability. However, according to the plaintiff’s expert, since she had been informed about the patient she should have come in to see the patient.
Had she done so, he alleged she would have realized that the patient required an intervention either with a covered stent or a surgical repair of the bleeding external iliac artery. Clearly, the fact that no formal consult was placed in the chart did not prevent the vascular surgeon from being a named defendant in this case.
Several steps should be taken if a physician is to minimize the risk of being named as a defendant in a lawsuit involving postangiographic intervention bleeding. First, if the physician is a not a surgeon, surgical consultation should be obtained as soon as postprocedure bleeding is suspected. Second, surgeons should pursue an aggressive approach to the treatment of this complication. Whereas it may be reasonable to treat a single episode of hypotension with fluid or blood transfusion, unless there are mitigating circumstances, any patient who develops a second episode of hypotension in the face of ongoing bleeding should undergo intervention. Furthermore, there must be clear documentation as to why a particular approach (endovascular versus open repair) was chosen. A medical physician’s inability to perform an open approach or a surgeon’s inability to perform an endovascular approach are not sustainable defenses.
Surgeons who are consulted late in the patient’s clinical course, prior to intervention, should document the patient’s poor prognosis and make it clear that no matter what is done the patient is unlikely to survive.
However, in most situations, the defense that a patient with ongoing bleeding was too unstable to treat is likely to fail.
Access site bleeding following percutaneous interventions is often a readily treatable complication. A low threshold for intervention, cooperation among vascular specialists, and, as always, clear documentation will go a long way to keep physicians working and out of the courtroom.
Dr. Brown is associate editor of Vascular Specialist. Dr. Samson is the medical editor, Vascular Specialist. The opinions expressed by the authors neither imply nor establish a standard of care.
POINT/COUNTERPOINT: Asymptomatic carotid stenosis: medical treatment, CEA, or CAS?
POINT: Medical treatment ends need for CEA or CAS.
By Anne L. Abbott, M.D.
The medical profession and the wider community must be congratulated upon their sustained efforts over recent decades that have seen an 80% fall in the average annual risk of stroke associated with moderate and severe (50%-99%) asymptomatic carotid stenosis (ACS).1-6 This has been achieved by better medical treatment which consists of encouraging healthy lifestyle habits and appropriate use of medication. This major impact results from the combined effect of addressing all vascular risk factors in individual patients and efforts to use the best medical treatment available at the time. Rates are now so low (around 0.5% per year for ipsilateral stroke) that procedures, such as carotid endarterectomy (CEA), are now more likely to harm than help patients.7
Even if procedures were always completely risk-free, improved medical treatment may mean we have now reached the point where carotid procedures for ACS are safe but essentially ineffective for reducing stroke risk. The latest measurements of stroke risk using medical treatment alone indicate that only about 2.5% of patients with 50%-99% ACS will have a an ipsilateral stroke due to the carotid lesion during their remaining lifetime if they are receiving pretty good quality, current medical treatment alone. This is because the average age of identifying patients with ACS in past studies was about 70 years and the average survival following diagnosis was 10 years.1 Further, only about half the strokes occurring in the distribution of an internal carotid artery with >60% proximal stenosis are due to the carotid lesion.8
Guidelines recommendations for CEA for 50%-99% or 60%-99% ACS rely on marginal, 20- to 30-year-old differences in stroke rates between patients given medical treatment alone versus those given additional CEA in best practice settings.9-11 Such recommendations are not relevant to current clinical practice largely because the medical treatment used in these studies is obsolete. Multiple independent observations regarding the improved stroke prevention efficacy of medical treatment,1-6,12 and the additional observations below, provide ample evidence that current medical treatment alone is the only routine-practice (nontrial) approach we should use for patients with 50%-99% ACS and any future role of carotid procedures in these patients could only apply to very small minority subgroups:
i. Current optimal medical treatment for patients with ACS has not been defined nor its impact measured. This means that it is likely we can lower the risk of stroke and other vascular complications in patients with ACS stenosis even further than has been achieved in the most recent studies. The definition of current optimal medical treatment will vary from patient to patient depending on which vascular risk factors they have and what has been shown effective in modifying these to reduce the risk of any complications of vascular disease.
ii. The 30-day peri-operative risk of stroke or death (and other significant complications) remains above 0% in the most recent results of trials and registries13-15 and is usually not measured in routine practice. Latest measurements of average annual ipsilateral stroke risk with medical treatment alone are about 2-3 times lower than for patients who had CEA or CAS in the Asymp tomatic Carotid Atherosclerosis Study (ACAS)10 or the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).7, 13
iii. Patients with 50%-99% ACS receiving current optimal medical treatment and with a sufficiently high average annual risk of ipsilateral stroke, indicating they may benefit from CEA, have not been identified. This rate would need to be in excess of at least 2.5%-3.0%, using results from ACAS,10 to expect any surgical benefit in routine practice. Studies of baseline degree of ACS within the 50%-99% range,10,11,16 plaque echolucency17 and most studies of detecting asymptomatic stenosis progression18-21 show that these parameters (used separately) confer a relative risk of stroke of only about 2.0-2.5. Therefore, a higher degree of baseline stenosis within the 50%-99% range, the detection of predominantly echolucent carotid plaques or asymptomatic progression are too weak on their own to identity patients likely to benefit from an additional carotid procedure. Combinations of risk markers are required for sufficient risk discrimination. For instance, results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study (largest study so far of medically managed patients with moderate or severe ACS) showed that a combination of clinical features, baseline degree of stenosis and standardized ultrasonic plaque characteristics can achieve average annual ipsilateral stroke risk stratification ranging from <1.0% to 10%.16 However, like all stroke risk stratification studies performed so far, this study was performed before the era of current medical treatment and the results have not been independently tested.
iv. Even if patients with sufficiently higher than average annual risk of ipsilateral stroke are one day reliably identified, randomized trials of an additional carotid procedure will be required to determine if, and to what extent, that procedure is likely to reduce ipsilateral stroke risk in routine practice.
v. The available evidence from randomized trials and registries indicates that CAS causes about twice as many strokes or deaths as surgery (just like it does for symptomatic carotid stenosis). Therefore, currently CAS cannot be recommended.7 In conclusion, the available evidence clearly indicates that current medical treatment alone now offers the best chance of reducing the risk of ipsilateral stroke in patients with 50%-99% ACS. There is no current evidence of benefit from CEA or CAS in these patients overall, or in any particular subgroups. However, there is much evidence regarding procedural risk and unaffordable cost. Risk of ipsilateral stroke is now so low without carotid procedures it is time to shift from the historic approach of identifying ACS primarily to administer CEA. Rather, it is time to properly recognize that carotid stenosis is a risk factor of all complications of vascular disease, more than it is for ipsilateral stroke.22
The priority is to define current optimal medical treatment as best we can, recognising that patients with ACS are a risk-heterogenous population. Then we need quality independent measurements of its impact on risk of all vascular disease complications using quality prospective cohort studies. Risk stratification models should be used to identify those who may benefit from trials of more intensive medical treatment, motivational strategies, plus/minus the safest carotid procedures. If identifying patients with ACS for CEA in routine practice is to be feasible, this needs to be done within well organized environments that support patients with a wide range of stenosis severity with the primary aim of implementing current optimal medical treatment to prevent all vascular complications. Finally, it must be accepted that as medical treatment and its implementation continue to improve, the added value of carotid procedures, including for symptomatic carotid stenosis, will continue to recede until we can say, ‘good job - it is finally fixed and it is time to move on to other major health issues.’
Dr. Abbott is a neurologist and an associate professor at Monash University, Melbourne, Australia.
References
1. Stroke. 2009;40:e573-583
2. Eur J Vasc Endovasc Surg. 2009;37:625-632
3. Nat Rev Cardiol. 2011;9:116-124
4. Stroke. 2013;44
5. Management of asymptomatic carotid stenosis: Technology assessment report. 2012:83
6. Annals of Internal Medicine. 2013;158:676-685
7. Stroke. 2013;44:1186-1190
8. N Engl J Med. 2000;342:1693-1700
9. N Engl J Med. 1993;328:221-227.
10.JAMA. 1995;273:1421-1428
11. Lancet. 2004;363:1491-1502
12. Arch Neurol. 2010;67:180-186
13. N Engl J Med. 2010;363:11-23
14. J Vasc Surg. 2009;49:71-79
15. J Vasc Surg. 2011;53:307-315
16. J Vasc Surg. 2010;52:1486-1496 e1481-1485
17. Gupta A, Kesavabhotla K, Barbadaran H, Kamel H, Panda A, Giambrone A, et al. Plaque echolucency and stroke risk in asymptoamtioc carotid stenosis: A systematic review and meta-analysis. Stroke. 2014 in press.
18. J Vasc Surg. 1999;29:208-214; discussion 214-206
19. J Vasc Surg. 2013;58:128-135 e121
20 Stroke. 2013;44:792-794
21. J Vasc Surg. 2014;59:956-967
22. N Engl J Med. 1986;315:860-865.
COUNTERPOINT: Medical therapy alone is not always enough.
By Mark F. Conrad, M.D., M.MSc., and Richard P. Cambria M.D.
Stroke remains the 3rd leading cause of death in the United States.1 It is estimated that 10% to 20% of ischemic strokes can be attributed to an ipsilateral, typically high-grade carotid stenosis, thus, asymptomatic carotid bifurcation stenosis remains a potentially significant public health problem.2 Epidemiologic studies indicate that 5% to 6% of the population >65 years of age will harbor an asymptomatic and potentially surgically significant carotid stenosis.1 The modern literature linking the degree of stenosis of the internal carotid artery and risk of ipsilateral stroke dates to the natural history studies of Chambers and Norris published in the New England Journal of Medicine in 1986.3 These investigators demonstrated a significant correlation of stroke risk with a >70% ipsilateral carotid artery stenosis and progression under observation.
Carotid endarterectomy (CEA) has been the standard of care for the prevention of stroke in patients with severe (>70%) asymptomatic carotid artery stenosis (ACS) for five decades. This is supported by level 1 evidence from multiple randomized trials4, 5 and consensus guideline recommendations.2, 6 Yet the use of CEA in patients with ACS has been recently challenged by a widely publicized review article7 whose author has embarked upon an anti-CEA crusade with the zealous fervor of one who worships at the altar of the statin. In this review, 11 prospective studies of medical therapy of patients with ACS (defined by the author as >50% stenosis) were stratified by date of publication such that the four series published from 2000-2007 had a lower raw data stroke rate with medical management (.6- 1.3%) than the 1.5% stroke rate reported after CEA in the ACAS study.4,7 The authors concluded that medical management alone is “at least 3-8” times more cost effective than CEA despite a complete lack of cost data in the cited articles. In an effort to debunk this revisionist history, we will begin by addressing the four aforementioned trials and finish with a discussion of the recent literature including an observational study from our own institution.
The first study of the four followed the asymptomatic contralateral carotid artery of all patients enrolled in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for 5 years to determine the risk of stroke in medically managed patients.8 There were 2,377 patients of whom 216 (9%) had a stenosis >60% but only 113 (4.7%) had a stenosis of 75-99%. The 5-year risk of ipsilateral stroke in the 75-99% asymptomatic stenosis cohort was 18.5% or 3.7% per year (this did not include other neurologic events such as TIA or amaurosis fugax).8 However, in the meta-analysis, no stroke rate was included in the raw data column (which was the basis of the final calculated overall stroke risk) and the final estimate quoted used the 60%-99% cohort such that the annual stroke rate was reported as 3.2%.7
The Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischemic Events Study (ACSRS) was a multicenter study that followed 1,115 patients with ACS >50% by duplex scanning for 6-84 months (mean 37.1) with the goal of stratifying patients into cohorts of high and low risk for future neurologic events.9 They concluded that the annual stroke rate in high-risk patients was 4.3% versus 0.7% in low-risk patients. There were 453 patients with 70%-99% stenosis by NASCET criteria with a raw stroke rate of 5.7% (1.9%/ year over an average 3-year follow-up) and a 5-year ipsilateral event rate of about 18%.9 However, when the patients with 50-69% were added, the raw stroke rate decreased to 1.3%.7
The Asymptomatic Stenosis Embolus Detection (ASED) study was a prospective trial that tested the theory that transcranial Doppler embolic signal detection would identify increased risk of ipsilateral neurologic events in patients with >60% ACS.10 Of the 240 arteries studied, 115 (48%) had a stenosis of 70%-99% but 10 of these patients were censored because they underwent CEA during the follow-up period. Their outcomes were not further stratified by degree of stenosis but the average ipsilateral carotid event rate was 3.1% per year with a 1% stroke risk per year.7, 10
The final, and most damning study in the meta-analysis, included a cohort of patients from the Second Manifestations of Arterial disease (SMART) study which is a registry of patients from the Netherlands with risk factors for, or symptoms of, arterial disease.
This study attempted to determine the risk of new vascular event in patients with ACS but 996 (27% of the registry) patients with a history of cerebrovascular disease (undefined) were excluded from analysis.11 They identified 221 patients (8% of 2684 eligible patients) with ACS >50% and reported an ipsilateral stroke event rate of .6%/year.7
However, in the 147 patients with a 70-99% stenosis, the hazard ratio for ischemic stroke was 1.7 but it was not possible to separate the patients with a moderate (50-69%) stenosis from the outcomes of those with more severe (>70%) disease.11
The major flaw with this study is that the authors excluded the 996 patients with a history of cerebrovascular disease who were at highest risk of having an ACS and subsequently suffering a stroke.
The flaw with combining the results of the above studies to conclude that medical therapy is the best way to prevent stroke in patients with severe ACS is that only half (828/1,754, 47%) of the patients included had a severe (70-99%) stenosis that would warrant CEA in the United States.6, 7
The majority of the patients studied would have been treated with best medical therapy and serial Duplex scanning. Indeed, when the moderate patients were excluded, the yearly stroke rates for patients with actual severe stenosis ranged from 2.0%-3.7%; substantially higher than the 1.5% stroke rate associated with CEA.8, 9
Randomized prospective trials such as the asymptomatic carotid artery stenosis (ACAS) study published in 1995 and the more recently updated asymptomatic carotid surgery trial (ACST) indicated a quite similar annual stroke risk in the 2% range for patients treated with medical therapy as opposed to those randomized to carotid endarterectomy (this study was excluded from the meta-analysis because patients with a remote (>6 months before entry) history of neurologic events were included).4,12 In addition, the 10-year follow-up data in the ACST trial demonstrated a sustained benefit for endarterectomy over optimal medical therapy.5 It is important to emphasize that in this trial some 80% of patients were on optimal medical therapy (aspirin plus statin agents) in the later years of the trial.
These long-term data indicate that while the protective effect of endarterectomy was more pronounced in those not on appropriate lipid-lowering therapy (the 10-year advantage of endarterectomy over medical therapy in the prevention of stroke was 5.8% in patients taking a statin and 6.2% in those who were not) the protective effect of endarterectomy over optimal medical therapy was statistically significant (P=0.002).5
This is of course relevant because clinicians in the modern era have typically used data from ACST to counsel patients about the stroke risk of asymptomatic high-grade carotid stenosis.
Finally, we followed at a cohort of 115 patients with severe (>70%) ACS who did not undergo CEA for a variety of reasons.
The average follow-up was 27 months and 86% of patients were on statin therapy.
The 5-year ipsilateral ischemic event rate was 30%, and 48% of these were strokes. When stratified by degree of stenosis, the patients with a 90%-99% stenosis had an ipsilateral neurologic event rate of 55%. This is a single center experience but the stroke rate of 3% per year is consistent with contemporary series and reiterates the reality of this risk for medically treated patients.
The question remains. When the efficacy of a CEA for the prevention of stroke in patients with ACS is supported by level 1 evidence from multiple prospective, randomized trials, why would anyone who treats patients with carotid disease allow a meta-analysis of natural history studies (that redefines a severe stenosis as >50%) convince them otherwise?
We believe that the answer lies in the unsubstantiated conclusion that “current medical intervention was estimated at least 3 to 8 times more cost effective” than CEA.7
As health care in the United States continues to evolve, we are faced with the issue of how best to spend limited resource dollars.
Is it practical or cost efficient to perform preventative surgery?
In England, an annual stroke rate of 2%-3% in a small subset of the population may be acceptable but we are not ready to concede that in the United States.
For now, we will follow the SVS practice guidelines for the management of ACS as they are evidenced based and definitive.
CEA in conjunction with medical therapy remains the best way to prevent stroke in patients with severe (>70%) ACS.
Dr. Conrad is an assistant professor of surgery, Harvard Medical School, Boston. Dr. Cambria is The Robert R. Linton MD Professor of Vascular and Endovascular Surgery, Harvard Medical School, Boston.
References
1.Neurology. 2006;67:1390-1395
2.Circulation. 2012;125:188-197
3.N Engl J of Med. 1986;315:860-865
4.JAMA. 1995;273:1421-1428
5.Lancet. 2010;376:1074-1084
6.Journal of Vascular Surgery. 2011;54:e1-31
7.Stroke. 2009;40:e573-583
8.N Engl J Med. 2000;342:1693-1700
9. Eur J Vasc Endovasc Surg. 2005;30:275-284
10.Stroke. 2005;36:1128-1133
11. Stroke. 2007;38:1470-1475
12. Lancet. 2004;363:1491-1502
POINT: Medical treatment ends need for CEA or CAS.
By Anne L. Abbott, M.D.
The medical profession and the wider community must be congratulated upon their sustained efforts over recent decades that have seen an 80% fall in the average annual risk of stroke associated with moderate and severe (50%-99%) asymptomatic carotid stenosis (ACS).1-6 This has been achieved by better medical treatment which consists of encouraging healthy lifestyle habits and appropriate use of medication. This major impact results from the combined effect of addressing all vascular risk factors in individual patients and efforts to use the best medical treatment available at the time. Rates are now so low (around 0.5% per year for ipsilateral stroke) that procedures, such as carotid endarterectomy (CEA), are now more likely to harm than help patients.7
Even if procedures were always completely risk-free, improved medical treatment may mean we have now reached the point where carotid procedures for ACS are safe but essentially ineffective for reducing stroke risk. The latest measurements of stroke risk using medical treatment alone indicate that only about 2.5% of patients with 50%-99% ACS will have a an ipsilateral stroke due to the carotid lesion during their remaining lifetime if they are receiving pretty good quality, current medical treatment alone. This is because the average age of identifying patients with ACS in past studies was about 70 years and the average survival following diagnosis was 10 years.1 Further, only about half the strokes occurring in the distribution of an internal carotid artery with >60% proximal stenosis are due to the carotid lesion.8
Guidelines recommendations for CEA for 50%-99% or 60%-99% ACS rely on marginal, 20- to 30-year-old differences in stroke rates between patients given medical treatment alone versus those given additional CEA in best practice settings.9-11 Such recommendations are not relevant to current clinical practice largely because the medical treatment used in these studies is obsolete. Multiple independent observations regarding the improved stroke prevention efficacy of medical treatment,1-6,12 and the additional observations below, provide ample evidence that current medical treatment alone is the only routine-practice (nontrial) approach we should use for patients with 50%-99% ACS and any future role of carotid procedures in these patients could only apply to very small minority subgroups:
i. Current optimal medical treatment for patients with ACS has not been defined nor its impact measured. This means that it is likely we can lower the risk of stroke and other vascular complications in patients with ACS stenosis even further than has been achieved in the most recent studies. The definition of current optimal medical treatment will vary from patient to patient depending on which vascular risk factors they have and what has been shown effective in modifying these to reduce the risk of any complications of vascular disease.
ii. The 30-day peri-operative risk of stroke or death (and other significant complications) remains above 0% in the most recent results of trials and registries13-15 and is usually not measured in routine practice. Latest measurements of average annual ipsilateral stroke risk with medical treatment alone are about 2-3 times lower than for patients who had CEA or CAS in the Asymp tomatic Carotid Atherosclerosis Study (ACAS)10 or the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).7, 13
iii. Patients with 50%-99% ACS receiving current optimal medical treatment and with a sufficiently high average annual risk of ipsilateral stroke, indicating they may benefit from CEA, have not been identified. This rate would need to be in excess of at least 2.5%-3.0%, using results from ACAS,10 to expect any surgical benefit in routine practice. Studies of baseline degree of ACS within the 50%-99% range,10,11,16 plaque echolucency17 and most studies of detecting asymptomatic stenosis progression18-21 show that these parameters (used separately) confer a relative risk of stroke of only about 2.0-2.5. Therefore, a higher degree of baseline stenosis within the 50%-99% range, the detection of predominantly echolucent carotid plaques or asymptomatic progression are too weak on their own to identity patients likely to benefit from an additional carotid procedure. Combinations of risk markers are required for sufficient risk discrimination. For instance, results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study (largest study so far of medically managed patients with moderate or severe ACS) showed that a combination of clinical features, baseline degree of stenosis and standardized ultrasonic plaque characteristics can achieve average annual ipsilateral stroke risk stratification ranging from <1.0% to 10%.16 However, like all stroke risk stratification studies performed so far, this study was performed before the era of current medical treatment and the results have not been independently tested.
iv. Even if patients with sufficiently higher than average annual risk of ipsilateral stroke are one day reliably identified, randomized trials of an additional carotid procedure will be required to determine if, and to what extent, that procedure is likely to reduce ipsilateral stroke risk in routine practice.
v. The available evidence from randomized trials and registries indicates that CAS causes about twice as many strokes or deaths as surgery (just like it does for symptomatic carotid stenosis). Therefore, currently CAS cannot be recommended.7 In conclusion, the available evidence clearly indicates that current medical treatment alone now offers the best chance of reducing the risk of ipsilateral stroke in patients with 50%-99% ACS. There is no current evidence of benefit from CEA or CAS in these patients overall, or in any particular subgroups. However, there is much evidence regarding procedural risk and unaffordable cost. Risk of ipsilateral stroke is now so low without carotid procedures it is time to shift from the historic approach of identifying ACS primarily to administer CEA. Rather, it is time to properly recognize that carotid stenosis is a risk factor of all complications of vascular disease, more than it is for ipsilateral stroke.22
The priority is to define current optimal medical treatment as best we can, recognising that patients with ACS are a risk-heterogenous population. Then we need quality independent measurements of its impact on risk of all vascular disease complications using quality prospective cohort studies. Risk stratification models should be used to identify those who may benefit from trials of more intensive medical treatment, motivational strategies, plus/minus the safest carotid procedures. If identifying patients with ACS for CEA in routine practice is to be feasible, this needs to be done within well organized environments that support patients with a wide range of stenosis severity with the primary aim of implementing current optimal medical treatment to prevent all vascular complications. Finally, it must be accepted that as medical treatment and its implementation continue to improve, the added value of carotid procedures, including for symptomatic carotid stenosis, will continue to recede until we can say, ‘good job - it is finally fixed and it is time to move on to other major health issues.’
Dr. Abbott is a neurologist and an associate professor at Monash University, Melbourne, Australia.
References
1. Stroke. 2009;40:e573-583
2. Eur J Vasc Endovasc Surg. 2009;37:625-632
3. Nat Rev Cardiol. 2011;9:116-124
4. Stroke. 2013;44
5. Management of asymptomatic carotid stenosis: Technology assessment report. 2012:83
6. Annals of Internal Medicine. 2013;158:676-685
7. Stroke. 2013;44:1186-1190
8. N Engl J Med. 2000;342:1693-1700
9. N Engl J Med. 1993;328:221-227.
10.JAMA. 1995;273:1421-1428
11. Lancet. 2004;363:1491-1502
12. Arch Neurol. 2010;67:180-186
13. N Engl J Med. 2010;363:11-23
14. J Vasc Surg. 2009;49:71-79
15. J Vasc Surg. 2011;53:307-315
16. J Vasc Surg. 2010;52:1486-1496 e1481-1485
17. Gupta A, Kesavabhotla K, Barbadaran H, Kamel H, Panda A, Giambrone A, et al. Plaque echolucency and stroke risk in asymptoamtioc carotid stenosis: A systematic review and meta-analysis. Stroke. 2014 in press.
18. J Vasc Surg. 1999;29:208-214; discussion 214-206
19. J Vasc Surg. 2013;58:128-135 e121
20 Stroke. 2013;44:792-794
21. J Vasc Surg. 2014;59:956-967
22. N Engl J Med. 1986;315:860-865.
COUNTERPOINT: Medical therapy alone is not always enough.
By Mark F. Conrad, M.D., M.MSc., and Richard P. Cambria M.D.
Stroke remains the 3rd leading cause of death in the United States.1 It is estimated that 10% to 20% of ischemic strokes can be attributed to an ipsilateral, typically high-grade carotid stenosis, thus, asymptomatic carotid bifurcation stenosis remains a potentially significant public health problem.2 Epidemiologic studies indicate that 5% to 6% of the population >65 years of age will harbor an asymptomatic and potentially surgically significant carotid stenosis.1 The modern literature linking the degree of stenosis of the internal carotid artery and risk of ipsilateral stroke dates to the natural history studies of Chambers and Norris published in the New England Journal of Medicine in 1986.3 These investigators demonstrated a significant correlation of stroke risk with a >70% ipsilateral carotid artery stenosis and progression under observation.
Carotid endarterectomy (CEA) has been the standard of care for the prevention of stroke in patients with severe (>70%) asymptomatic carotid artery stenosis (ACS) for five decades. This is supported by level 1 evidence from multiple randomized trials4, 5 and consensus guideline recommendations.2, 6 Yet the use of CEA in patients with ACS has been recently challenged by a widely publicized review article7 whose author has embarked upon an anti-CEA crusade with the zealous fervor of one who worships at the altar of the statin. In this review, 11 prospective studies of medical therapy of patients with ACS (defined by the author as >50% stenosis) were stratified by date of publication such that the four series published from 2000-2007 had a lower raw data stroke rate with medical management (.6- 1.3%) than the 1.5% stroke rate reported after CEA in the ACAS study.4,7 The authors concluded that medical management alone is “at least 3-8” times more cost effective than CEA despite a complete lack of cost data in the cited articles. In an effort to debunk this revisionist history, we will begin by addressing the four aforementioned trials and finish with a discussion of the recent literature including an observational study from our own institution.
The first study of the four followed the asymptomatic contralateral carotid artery of all patients enrolled in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for 5 years to determine the risk of stroke in medically managed patients.8 There were 2,377 patients of whom 216 (9%) had a stenosis >60% but only 113 (4.7%) had a stenosis of 75-99%. The 5-year risk of ipsilateral stroke in the 75-99% asymptomatic stenosis cohort was 18.5% or 3.7% per year (this did not include other neurologic events such as TIA or amaurosis fugax).8 However, in the meta-analysis, no stroke rate was included in the raw data column (which was the basis of the final calculated overall stroke risk) and the final estimate quoted used the 60%-99% cohort such that the annual stroke rate was reported as 3.2%.7
The Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischemic Events Study (ACSRS) was a multicenter study that followed 1,115 patients with ACS >50% by duplex scanning for 6-84 months (mean 37.1) with the goal of stratifying patients into cohorts of high and low risk for future neurologic events.9 They concluded that the annual stroke rate in high-risk patients was 4.3% versus 0.7% in low-risk patients. There were 453 patients with 70%-99% stenosis by NASCET criteria with a raw stroke rate of 5.7% (1.9%/ year over an average 3-year follow-up) and a 5-year ipsilateral event rate of about 18%.9 However, when the patients with 50-69% were added, the raw stroke rate decreased to 1.3%.7
The Asymptomatic Stenosis Embolus Detection (ASED) study was a prospective trial that tested the theory that transcranial Doppler embolic signal detection would identify increased risk of ipsilateral neurologic events in patients with >60% ACS.10 Of the 240 arteries studied, 115 (48%) had a stenosis of 70%-99% but 10 of these patients were censored because they underwent CEA during the follow-up period. Their outcomes were not further stratified by degree of stenosis but the average ipsilateral carotid event rate was 3.1% per year with a 1% stroke risk per year.7, 10
The final, and most damning study in the meta-analysis, included a cohort of patients from the Second Manifestations of Arterial disease (SMART) study which is a registry of patients from the Netherlands with risk factors for, or symptoms of, arterial disease.
This study attempted to determine the risk of new vascular event in patients with ACS but 996 (27% of the registry) patients with a history of cerebrovascular disease (undefined) were excluded from analysis.11 They identified 221 patients (8% of 2684 eligible patients) with ACS >50% and reported an ipsilateral stroke event rate of .6%/year.7
However, in the 147 patients with a 70-99% stenosis, the hazard ratio for ischemic stroke was 1.7 but it was not possible to separate the patients with a moderate (50-69%) stenosis from the outcomes of those with more severe (>70%) disease.11
The major flaw with this study is that the authors excluded the 996 patients with a history of cerebrovascular disease who were at highest risk of having an ACS and subsequently suffering a stroke.
The flaw with combining the results of the above studies to conclude that medical therapy is the best way to prevent stroke in patients with severe ACS is that only half (828/1,754, 47%) of the patients included had a severe (70-99%) stenosis that would warrant CEA in the United States.6, 7
The majority of the patients studied would have been treated with best medical therapy and serial Duplex scanning. Indeed, when the moderate patients were excluded, the yearly stroke rates for patients with actual severe stenosis ranged from 2.0%-3.7%; substantially higher than the 1.5% stroke rate associated with CEA.8, 9
Randomized prospective trials such as the asymptomatic carotid artery stenosis (ACAS) study published in 1995 and the more recently updated asymptomatic carotid surgery trial (ACST) indicated a quite similar annual stroke risk in the 2% range for patients treated with medical therapy as opposed to those randomized to carotid endarterectomy (this study was excluded from the meta-analysis because patients with a remote (>6 months before entry) history of neurologic events were included).4,12 In addition, the 10-year follow-up data in the ACST trial demonstrated a sustained benefit for endarterectomy over optimal medical therapy.5 It is important to emphasize that in this trial some 80% of patients were on optimal medical therapy (aspirin plus statin agents) in the later years of the trial.
These long-term data indicate that while the protective effect of endarterectomy was more pronounced in those not on appropriate lipid-lowering therapy (the 10-year advantage of endarterectomy over medical therapy in the prevention of stroke was 5.8% in patients taking a statin and 6.2% in those who were not) the protective effect of endarterectomy over optimal medical therapy was statistically significant (P=0.002).5
This is of course relevant because clinicians in the modern era have typically used data from ACST to counsel patients about the stroke risk of asymptomatic high-grade carotid stenosis.
Finally, we followed at a cohort of 115 patients with severe (>70%) ACS who did not undergo CEA for a variety of reasons.
The average follow-up was 27 months and 86% of patients were on statin therapy.
The 5-year ipsilateral ischemic event rate was 30%, and 48% of these were strokes. When stratified by degree of stenosis, the patients with a 90%-99% stenosis had an ipsilateral neurologic event rate of 55%. This is a single center experience but the stroke rate of 3% per year is consistent with contemporary series and reiterates the reality of this risk for medically treated patients.
The question remains. When the efficacy of a CEA for the prevention of stroke in patients with ACS is supported by level 1 evidence from multiple prospective, randomized trials, why would anyone who treats patients with carotid disease allow a meta-analysis of natural history studies (that redefines a severe stenosis as >50%) convince them otherwise?
We believe that the answer lies in the unsubstantiated conclusion that “current medical intervention was estimated at least 3 to 8 times more cost effective” than CEA.7
As health care in the United States continues to evolve, we are faced with the issue of how best to spend limited resource dollars.
Is it practical or cost efficient to perform preventative surgery?
In England, an annual stroke rate of 2%-3% in a small subset of the population may be acceptable but we are not ready to concede that in the United States.
For now, we will follow the SVS practice guidelines for the management of ACS as they are evidenced based and definitive.
CEA in conjunction with medical therapy remains the best way to prevent stroke in patients with severe (>70%) ACS.
Dr. Conrad is an assistant professor of surgery, Harvard Medical School, Boston. Dr. Cambria is The Robert R. Linton MD Professor of Vascular and Endovascular Surgery, Harvard Medical School, Boston.
References
1.Neurology. 2006;67:1390-1395
2.Circulation. 2012;125:188-197
3.N Engl J of Med. 1986;315:860-865
4.JAMA. 1995;273:1421-1428
5.Lancet. 2010;376:1074-1084
6.Journal of Vascular Surgery. 2011;54:e1-31
7.Stroke. 2009;40:e573-583
8.N Engl J Med. 2000;342:1693-1700
9. Eur J Vasc Endovasc Surg. 2005;30:275-284
10.Stroke. 2005;36:1128-1133
11. Stroke. 2007;38:1470-1475
12. Lancet. 2004;363:1491-1502
POINT: Medical treatment ends need for CEA or CAS.
By Anne L. Abbott, M.D.
The medical profession and the wider community must be congratulated upon their sustained efforts over recent decades that have seen an 80% fall in the average annual risk of stroke associated with moderate and severe (50%-99%) asymptomatic carotid stenosis (ACS).1-6 This has been achieved by better medical treatment which consists of encouraging healthy lifestyle habits and appropriate use of medication. This major impact results from the combined effect of addressing all vascular risk factors in individual patients and efforts to use the best medical treatment available at the time. Rates are now so low (around 0.5% per year for ipsilateral stroke) that procedures, such as carotid endarterectomy (CEA), are now more likely to harm than help patients.7
Even if procedures were always completely risk-free, improved medical treatment may mean we have now reached the point where carotid procedures for ACS are safe but essentially ineffective for reducing stroke risk. The latest measurements of stroke risk using medical treatment alone indicate that only about 2.5% of patients with 50%-99% ACS will have a an ipsilateral stroke due to the carotid lesion during their remaining lifetime if they are receiving pretty good quality, current medical treatment alone. This is because the average age of identifying patients with ACS in past studies was about 70 years and the average survival following diagnosis was 10 years.1 Further, only about half the strokes occurring in the distribution of an internal carotid artery with >60% proximal stenosis are due to the carotid lesion.8
Guidelines recommendations for CEA for 50%-99% or 60%-99% ACS rely on marginal, 20- to 30-year-old differences in stroke rates between patients given medical treatment alone versus those given additional CEA in best practice settings.9-11 Such recommendations are not relevant to current clinical practice largely because the medical treatment used in these studies is obsolete. Multiple independent observations regarding the improved stroke prevention efficacy of medical treatment,1-6,12 and the additional observations below, provide ample evidence that current medical treatment alone is the only routine-practice (nontrial) approach we should use for patients with 50%-99% ACS and any future role of carotid procedures in these patients could only apply to very small minority subgroups:
i. Current optimal medical treatment for patients with ACS has not been defined nor its impact measured. This means that it is likely we can lower the risk of stroke and other vascular complications in patients with ACS stenosis even further than has been achieved in the most recent studies. The definition of current optimal medical treatment will vary from patient to patient depending on which vascular risk factors they have and what has been shown effective in modifying these to reduce the risk of any complications of vascular disease.
ii. The 30-day peri-operative risk of stroke or death (and other significant complications) remains above 0% in the most recent results of trials and registries13-15 and is usually not measured in routine practice. Latest measurements of average annual ipsilateral stroke risk with medical treatment alone are about 2-3 times lower than for patients who had CEA or CAS in the Asymp tomatic Carotid Atherosclerosis Study (ACAS)10 or the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).7, 13
iii. Patients with 50%-99% ACS receiving current optimal medical treatment and with a sufficiently high average annual risk of ipsilateral stroke, indicating they may benefit from CEA, have not been identified. This rate would need to be in excess of at least 2.5%-3.0%, using results from ACAS,10 to expect any surgical benefit in routine practice. Studies of baseline degree of ACS within the 50%-99% range,10,11,16 plaque echolucency17 and most studies of detecting asymptomatic stenosis progression18-21 show that these parameters (used separately) confer a relative risk of stroke of only about 2.0-2.5. Therefore, a higher degree of baseline stenosis within the 50%-99% range, the detection of predominantly echolucent carotid plaques or asymptomatic progression are too weak on their own to identity patients likely to benefit from an additional carotid procedure. Combinations of risk markers are required for sufficient risk discrimination. For instance, results from the Asymptomatic Carotid Stenosis and Risk of Stroke Study (largest study so far of medically managed patients with moderate or severe ACS) showed that a combination of clinical features, baseline degree of stenosis and standardized ultrasonic plaque characteristics can achieve average annual ipsilateral stroke risk stratification ranging from <1.0% to 10%.16 However, like all stroke risk stratification studies performed so far, this study was performed before the era of current medical treatment and the results have not been independently tested.
iv. Even if patients with sufficiently higher than average annual risk of ipsilateral stroke are one day reliably identified, randomized trials of an additional carotid procedure will be required to determine if, and to what extent, that procedure is likely to reduce ipsilateral stroke risk in routine practice.
v. The available evidence from randomized trials and registries indicates that CAS causes about twice as many strokes or deaths as surgery (just like it does for symptomatic carotid stenosis). Therefore, currently CAS cannot be recommended.7 In conclusion, the available evidence clearly indicates that current medical treatment alone now offers the best chance of reducing the risk of ipsilateral stroke in patients with 50%-99% ACS. There is no current evidence of benefit from CEA or CAS in these patients overall, or in any particular subgroups. However, there is much evidence regarding procedural risk and unaffordable cost. Risk of ipsilateral stroke is now so low without carotid procedures it is time to shift from the historic approach of identifying ACS primarily to administer CEA. Rather, it is time to properly recognize that carotid stenosis is a risk factor of all complications of vascular disease, more than it is for ipsilateral stroke.22
The priority is to define current optimal medical treatment as best we can, recognising that patients with ACS are a risk-heterogenous population. Then we need quality independent measurements of its impact on risk of all vascular disease complications using quality prospective cohort studies. Risk stratification models should be used to identify those who may benefit from trials of more intensive medical treatment, motivational strategies, plus/minus the safest carotid procedures. If identifying patients with ACS for CEA in routine practice is to be feasible, this needs to be done within well organized environments that support patients with a wide range of stenosis severity with the primary aim of implementing current optimal medical treatment to prevent all vascular complications. Finally, it must be accepted that as medical treatment and its implementation continue to improve, the added value of carotid procedures, including for symptomatic carotid stenosis, will continue to recede until we can say, ‘good job - it is finally fixed and it is time to move on to other major health issues.’
Dr. Abbott is a neurologist and an associate professor at Monash University, Melbourne, Australia.
References
1. Stroke. 2009;40:e573-583
2. Eur J Vasc Endovasc Surg. 2009;37:625-632
3. Nat Rev Cardiol. 2011;9:116-124
4. Stroke. 2013;44
5. Management of asymptomatic carotid stenosis: Technology assessment report. 2012:83
6. Annals of Internal Medicine. 2013;158:676-685
7. Stroke. 2013;44:1186-1190
8. N Engl J Med. 2000;342:1693-1700
9. N Engl J Med. 1993;328:221-227.
10.JAMA. 1995;273:1421-1428
11. Lancet. 2004;363:1491-1502
12. Arch Neurol. 2010;67:180-186
13. N Engl J Med. 2010;363:11-23
14. J Vasc Surg. 2009;49:71-79
15. J Vasc Surg. 2011;53:307-315
16. J Vasc Surg. 2010;52:1486-1496 e1481-1485
17. Gupta A, Kesavabhotla K, Barbadaran H, Kamel H, Panda A, Giambrone A, et al. Plaque echolucency and stroke risk in asymptoamtioc carotid stenosis: A systematic review and meta-analysis. Stroke. 2014 in press.
18. J Vasc Surg. 1999;29:208-214; discussion 214-206
19. J Vasc Surg. 2013;58:128-135 e121
20 Stroke. 2013;44:792-794
21. J Vasc Surg. 2014;59:956-967
22. N Engl J Med. 1986;315:860-865.
COUNTERPOINT: Medical therapy alone is not always enough.
By Mark F. Conrad, M.D., M.MSc., and Richard P. Cambria M.D.
Stroke remains the 3rd leading cause of death in the United States.1 It is estimated that 10% to 20% of ischemic strokes can be attributed to an ipsilateral, typically high-grade carotid stenosis, thus, asymptomatic carotid bifurcation stenosis remains a potentially significant public health problem.2 Epidemiologic studies indicate that 5% to 6% of the population >65 years of age will harbor an asymptomatic and potentially surgically significant carotid stenosis.1 The modern literature linking the degree of stenosis of the internal carotid artery and risk of ipsilateral stroke dates to the natural history studies of Chambers and Norris published in the New England Journal of Medicine in 1986.3 These investigators demonstrated a significant correlation of stroke risk with a >70% ipsilateral carotid artery stenosis and progression under observation.
Carotid endarterectomy (CEA) has been the standard of care for the prevention of stroke in patients with severe (>70%) asymptomatic carotid artery stenosis (ACS) for five decades. This is supported by level 1 evidence from multiple randomized trials4, 5 and consensus guideline recommendations.2, 6 Yet the use of CEA in patients with ACS has been recently challenged by a widely publicized review article7 whose author has embarked upon an anti-CEA crusade with the zealous fervor of one who worships at the altar of the statin. In this review, 11 prospective studies of medical therapy of patients with ACS (defined by the author as >50% stenosis) were stratified by date of publication such that the four series published from 2000-2007 had a lower raw data stroke rate with medical management (.6- 1.3%) than the 1.5% stroke rate reported after CEA in the ACAS study.4,7 The authors concluded that medical management alone is “at least 3-8” times more cost effective than CEA despite a complete lack of cost data in the cited articles. In an effort to debunk this revisionist history, we will begin by addressing the four aforementioned trials and finish with a discussion of the recent literature including an observational study from our own institution.
The first study of the four followed the asymptomatic contralateral carotid artery of all patients enrolled in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for 5 years to determine the risk of stroke in medically managed patients.8 There were 2,377 patients of whom 216 (9%) had a stenosis >60% but only 113 (4.7%) had a stenosis of 75-99%. The 5-year risk of ipsilateral stroke in the 75-99% asymptomatic stenosis cohort was 18.5% or 3.7% per year (this did not include other neurologic events such as TIA or amaurosis fugax).8 However, in the meta-analysis, no stroke rate was included in the raw data column (which was the basis of the final calculated overall stroke risk) and the final estimate quoted used the 60%-99% cohort such that the annual stroke rate was reported as 3.2%.7
The Asymptomatic Carotid Stenosis and Risk of Ipsilateral Hemispheric Ischemic Events Study (ACSRS) was a multicenter study that followed 1,115 patients with ACS >50% by duplex scanning for 6-84 months (mean 37.1) with the goal of stratifying patients into cohorts of high and low risk for future neurologic events.9 They concluded that the annual stroke rate in high-risk patients was 4.3% versus 0.7% in low-risk patients. There were 453 patients with 70%-99% stenosis by NASCET criteria with a raw stroke rate of 5.7% (1.9%/ year over an average 3-year follow-up) and a 5-year ipsilateral event rate of about 18%.9 However, when the patients with 50-69% were added, the raw stroke rate decreased to 1.3%.7
The Asymptomatic Stenosis Embolus Detection (ASED) study was a prospective trial that tested the theory that transcranial Doppler embolic signal detection would identify increased risk of ipsilateral neurologic events in patients with >60% ACS.10 Of the 240 arteries studied, 115 (48%) had a stenosis of 70%-99% but 10 of these patients were censored because they underwent CEA during the follow-up period. Their outcomes were not further stratified by degree of stenosis but the average ipsilateral carotid event rate was 3.1% per year with a 1% stroke risk per year.7, 10
The final, and most damning study in the meta-analysis, included a cohort of patients from the Second Manifestations of Arterial disease (SMART) study which is a registry of patients from the Netherlands with risk factors for, or symptoms of, arterial disease.
This study attempted to determine the risk of new vascular event in patients with ACS but 996 (27% of the registry) patients with a history of cerebrovascular disease (undefined) were excluded from analysis.11 They identified 221 patients (8% of 2684 eligible patients) with ACS >50% and reported an ipsilateral stroke event rate of .6%/year.7
However, in the 147 patients with a 70-99% stenosis, the hazard ratio for ischemic stroke was 1.7 but it was not possible to separate the patients with a moderate (50-69%) stenosis from the outcomes of those with more severe (>70%) disease.11
The major flaw with this study is that the authors excluded the 996 patients with a history of cerebrovascular disease who were at highest risk of having an ACS and subsequently suffering a stroke.
The flaw with combining the results of the above studies to conclude that medical therapy is the best way to prevent stroke in patients with severe ACS is that only half (828/1,754, 47%) of the patients included had a severe (70-99%) stenosis that would warrant CEA in the United States.6, 7
The majority of the patients studied would have been treated with best medical therapy and serial Duplex scanning. Indeed, when the moderate patients were excluded, the yearly stroke rates for patients with actual severe stenosis ranged from 2.0%-3.7%; substantially higher than the 1.5% stroke rate associated with CEA.8, 9
Randomized prospective trials such as the asymptomatic carotid artery stenosis (ACAS) study published in 1995 and the more recently updated asymptomatic carotid surgery trial (ACST) indicated a quite similar annual stroke risk in the 2% range for patients treated with medical therapy as opposed to those randomized to carotid endarterectomy (this study was excluded from the meta-analysis because patients with a remote (>6 months before entry) history of neurologic events were included).4,12 In addition, the 10-year follow-up data in the ACST trial demonstrated a sustained benefit for endarterectomy over optimal medical therapy.5 It is important to emphasize that in this trial some 80% of patients were on optimal medical therapy (aspirin plus statin agents) in the later years of the trial.
These long-term data indicate that while the protective effect of endarterectomy was more pronounced in those not on appropriate lipid-lowering therapy (the 10-year advantage of endarterectomy over medical therapy in the prevention of stroke was 5.8% in patients taking a statin and 6.2% in those who were not) the protective effect of endarterectomy over optimal medical therapy was statistically significant (P=0.002).5
This is of course relevant because clinicians in the modern era have typically used data from ACST to counsel patients about the stroke risk of asymptomatic high-grade carotid stenosis.
Finally, we followed at a cohort of 115 patients with severe (>70%) ACS who did not undergo CEA for a variety of reasons.
The average follow-up was 27 months and 86% of patients were on statin therapy.
The 5-year ipsilateral ischemic event rate was 30%, and 48% of these were strokes. When stratified by degree of stenosis, the patients with a 90%-99% stenosis had an ipsilateral neurologic event rate of 55%. This is a single center experience but the stroke rate of 3% per year is consistent with contemporary series and reiterates the reality of this risk for medically treated patients.
The question remains. When the efficacy of a CEA for the prevention of stroke in patients with ACS is supported by level 1 evidence from multiple prospective, randomized trials, why would anyone who treats patients with carotid disease allow a meta-analysis of natural history studies (that redefines a severe stenosis as >50%) convince them otherwise?
We believe that the answer lies in the unsubstantiated conclusion that “current medical intervention was estimated at least 3 to 8 times more cost effective” than CEA.7
As health care in the United States continues to evolve, we are faced with the issue of how best to spend limited resource dollars.
Is it practical or cost efficient to perform preventative surgery?
In England, an annual stroke rate of 2%-3% in a small subset of the population may be acceptable but we are not ready to concede that in the United States.
For now, we will follow the SVS practice guidelines for the management of ACS as they are evidenced based and definitive.
CEA in conjunction with medical therapy remains the best way to prevent stroke in patients with severe (>70%) ACS.
Dr. Conrad is an assistant professor of surgery, Harvard Medical School, Boston. Dr. Cambria is The Robert R. Linton MD Professor of Vascular and Endovascular Surgery, Harvard Medical School, Boston.
References
1.Neurology. 2006;67:1390-1395
2.Circulation. 2012;125:188-197
3.N Engl J of Med. 1986;315:860-865
4.JAMA. 1995;273:1421-1428
5.Lancet. 2010;376:1074-1084
6.Journal of Vascular Surgery. 2011;54:e1-31
7.Stroke. 2009;40:e573-583
8.N Engl J Med. 2000;342:1693-1700
9. Eur J Vasc Endovasc Surg. 2005;30:275-284
10.Stroke. 2005;36:1128-1133
11. Stroke. 2007;38:1470-1475
12. Lancet. 2004;363:1491-1502
Responding to the NYT on the PAD controversy
On January 30, the New York Times published “Medicare Payments Surge for Stents to Unblock Blood Vessels in Limbs,” which questioned the medical necessity of many in-office interventions for peripheral artery disease and the motives of some doctors who perform them. (If you haven’t already, I encourage you to read the article at http://vsweb.org/NYTstents.)
Times reporters Julie Creswell and Reed Abelson analyzed Medicare payments, finding that the top 10 billing cardiologists made about half their Medicare reimbursements from office-based peripheral arterial procedures, with the implication that some doctors were taking advantage of an unregulated site-of-service.
The problem, though, goes beyond a handful of top billers. According to a citation from the Advisory Board Company, while the “number of procedures to open blockages in heart vessels fell by about 30 percent from 2005 to 2013…[o]ver the same time, the number of similar procedures for vessels outside the heart soared by almost 70 percent.”
The article struck a nerve. For several days, it was one of the most emailed articles on the New York Times website, and hundreds of SVS members throughout the world emailed us. Members were particularly outraged at the financial abuses reported. Many mentioned that they themselves had witnessed inappropriate care for asymptomatic PAD patients.
It was clear from the emails I received that our members want SVS to be on the right side of this issue, advocating for appropriate treatment of patients with PAD, and it makes me proud to be part of this community.
Over the past year, SVS has taken steps to address the issue of appropriateness.
Last June at our Vascular Annual Meeting, SVS held the Crawford Forum to discuss how we as a society can develop mechanisms to support appropriate vascular care and ethical decision-making. If you missed it, you can watch the entire symposium online at http://vsweb.org/Crawford.
Our Clinical Practice Council has seized upon appropriate care in office-based facilities as its current project and is working on constructive suggestions to ensure that site-of-service is not only convenient to patients but also provides high-quality, appropriate care.
Last month, we published in the Journal for Vascular Surgery “Practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication,” which emphasize inexpensive physiologic testing in diagnosing PAD and conservative measures such as risk-factor modification and exercise in treating asymptomatic patients and claudicants. You can review the guidelines at http://vsweb.org/LEguidelines.
In addition, our recommendations for Choosing Wisely, a program of the American Board of Internal Medicine Foundation to curb unnecessary tests and treatment, warns against stents and other non-surgical and surgical interventions in asymptomatic patients and claudicants until conservative treatments have been tried. SVS is also partnering with Consumer Reports to educate patients about this issue this spring.
Finally, SVS has partnered with several international vascular societies on the Global Vascular Guidelines for critical limb ischemia and recently agreed to participate in a multi-societal collaboration to develop guidelines for the management of PAD.
There is more work to do. Next month’s Vascular Specialist will include some of the feedback we received from our members as well as suggestions on how SVS can develop criteria for appropriateness. To share your thoughts, please email me at [email protected].
What follows is the text of the Lawrence letter to the New York Times:
To the Editor:
Most of my colleagues in vascular medicine want to provide the best treatment for their patients, but those who don’t should be exposed, as was done in “Medicare Bills Rise for Stents Put Into Limbs” (front page, Jan. 30). This article shows how inappropriate care can harm a patient and greatly increase the cost of health care, while grossly enhancing the income of those who overuse procedures.
Office-based procedures are not inherently bad, if standards for appropriate care are followed. The Society for Vascular Surgery recently held a national symposium to discuss ways to discourage inappropriate use of vascular procedures, and recently, we published evidence-based practice guidelines to encourage appropriate care of peripheral arterial disease.
These guidelines emphasize conservative measures as the first line of treatment for patients without symptoms or with vascular pain only when walking, reserving interventions and surgery for those with more severe problems. They also recommend inexpensive, noninvasive tests to determine whether the pain is truly vascular. These tests should be used in every patient.
Practice guidelines set standards for our members, but all physicians treating patients with vascular disease should also use them. Vascular specialists need an in-depth understanding of vascular disease, as well as technical skill, but they also need the ethics to treat patients like a brother or a sister, not the source of payment for a new car.
PETER F. LAWRENCE, Los Angeles
The writer, chief of vascular surgery at U.C.L.A., is president of the Society for Vascular Surgery.
On January 30, the New York Times published “Medicare Payments Surge for Stents to Unblock Blood Vessels in Limbs,” which questioned the medical necessity of many in-office interventions for peripheral artery disease and the motives of some doctors who perform them. (If you haven’t already, I encourage you to read the article at http://vsweb.org/NYTstents.)
Times reporters Julie Creswell and Reed Abelson analyzed Medicare payments, finding that the top 10 billing cardiologists made about half their Medicare reimbursements from office-based peripheral arterial procedures, with the implication that some doctors were taking advantage of an unregulated site-of-service.
The problem, though, goes beyond a handful of top billers. According to a citation from the Advisory Board Company, while the “number of procedures to open blockages in heart vessels fell by about 30 percent from 2005 to 2013…[o]ver the same time, the number of similar procedures for vessels outside the heart soared by almost 70 percent.”
The article struck a nerve. For several days, it was one of the most emailed articles on the New York Times website, and hundreds of SVS members throughout the world emailed us. Members were particularly outraged at the financial abuses reported. Many mentioned that they themselves had witnessed inappropriate care for asymptomatic PAD patients.
It was clear from the emails I received that our members want SVS to be on the right side of this issue, advocating for appropriate treatment of patients with PAD, and it makes me proud to be part of this community.
Over the past year, SVS has taken steps to address the issue of appropriateness.
Last June at our Vascular Annual Meeting, SVS held the Crawford Forum to discuss how we as a society can develop mechanisms to support appropriate vascular care and ethical decision-making. If you missed it, you can watch the entire symposium online at http://vsweb.org/Crawford.
Our Clinical Practice Council has seized upon appropriate care in office-based facilities as its current project and is working on constructive suggestions to ensure that site-of-service is not only convenient to patients but also provides high-quality, appropriate care.
Last month, we published in the Journal for Vascular Surgery “Practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication,” which emphasize inexpensive physiologic testing in diagnosing PAD and conservative measures such as risk-factor modification and exercise in treating asymptomatic patients and claudicants. You can review the guidelines at http://vsweb.org/LEguidelines.
In addition, our recommendations for Choosing Wisely, a program of the American Board of Internal Medicine Foundation to curb unnecessary tests and treatment, warns against stents and other non-surgical and surgical interventions in asymptomatic patients and claudicants until conservative treatments have been tried. SVS is also partnering with Consumer Reports to educate patients about this issue this spring.
Finally, SVS has partnered with several international vascular societies on the Global Vascular Guidelines for critical limb ischemia and recently agreed to participate in a multi-societal collaboration to develop guidelines for the management of PAD.
There is more work to do. Next month’s Vascular Specialist will include some of the feedback we received from our members as well as suggestions on how SVS can develop criteria for appropriateness. To share your thoughts, please email me at [email protected].
What follows is the text of the Lawrence letter to the New York Times:
To the Editor:
Most of my colleagues in vascular medicine want to provide the best treatment for their patients, but those who don’t should be exposed, as was done in “Medicare Bills Rise for Stents Put Into Limbs” (front page, Jan. 30). This article shows how inappropriate care can harm a patient and greatly increase the cost of health care, while grossly enhancing the income of those who overuse procedures.
Office-based procedures are not inherently bad, if standards for appropriate care are followed. The Society for Vascular Surgery recently held a national symposium to discuss ways to discourage inappropriate use of vascular procedures, and recently, we published evidence-based practice guidelines to encourage appropriate care of peripheral arterial disease.
These guidelines emphasize conservative measures as the first line of treatment for patients without symptoms or with vascular pain only when walking, reserving interventions and surgery for those with more severe problems. They also recommend inexpensive, noninvasive tests to determine whether the pain is truly vascular. These tests should be used in every patient.
Practice guidelines set standards for our members, but all physicians treating patients with vascular disease should also use them. Vascular specialists need an in-depth understanding of vascular disease, as well as technical skill, but they also need the ethics to treat patients like a brother or a sister, not the source of payment for a new car.
PETER F. LAWRENCE, Los Angeles
The writer, chief of vascular surgery at U.C.L.A., is president of the Society for Vascular Surgery.
On January 30, the New York Times published “Medicare Payments Surge for Stents to Unblock Blood Vessels in Limbs,” which questioned the medical necessity of many in-office interventions for peripheral artery disease and the motives of some doctors who perform them. (If you haven’t already, I encourage you to read the article at http://vsweb.org/NYTstents.)
Times reporters Julie Creswell and Reed Abelson analyzed Medicare payments, finding that the top 10 billing cardiologists made about half their Medicare reimbursements from office-based peripheral arterial procedures, with the implication that some doctors were taking advantage of an unregulated site-of-service.
The problem, though, goes beyond a handful of top billers. According to a citation from the Advisory Board Company, while the “number of procedures to open blockages in heart vessels fell by about 30 percent from 2005 to 2013…[o]ver the same time, the number of similar procedures for vessels outside the heart soared by almost 70 percent.”
The article struck a nerve. For several days, it was one of the most emailed articles on the New York Times website, and hundreds of SVS members throughout the world emailed us. Members were particularly outraged at the financial abuses reported. Many mentioned that they themselves had witnessed inappropriate care for asymptomatic PAD patients.
It was clear from the emails I received that our members want SVS to be on the right side of this issue, advocating for appropriate treatment of patients with PAD, and it makes me proud to be part of this community.
Over the past year, SVS has taken steps to address the issue of appropriateness.
Last June at our Vascular Annual Meeting, SVS held the Crawford Forum to discuss how we as a society can develop mechanisms to support appropriate vascular care and ethical decision-making. If you missed it, you can watch the entire symposium online at http://vsweb.org/Crawford.
Our Clinical Practice Council has seized upon appropriate care in office-based facilities as its current project and is working on constructive suggestions to ensure that site-of-service is not only convenient to patients but also provides high-quality, appropriate care.
Last month, we published in the Journal for Vascular Surgery “Practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication,” which emphasize inexpensive physiologic testing in diagnosing PAD and conservative measures such as risk-factor modification and exercise in treating asymptomatic patients and claudicants. You can review the guidelines at http://vsweb.org/LEguidelines.
In addition, our recommendations for Choosing Wisely, a program of the American Board of Internal Medicine Foundation to curb unnecessary tests and treatment, warns against stents and other non-surgical and surgical interventions in asymptomatic patients and claudicants until conservative treatments have been tried. SVS is also partnering with Consumer Reports to educate patients about this issue this spring.
Finally, SVS has partnered with several international vascular societies on the Global Vascular Guidelines for critical limb ischemia and recently agreed to participate in a multi-societal collaboration to develop guidelines for the management of PAD.
There is more work to do. Next month’s Vascular Specialist will include some of the feedback we received from our members as well as suggestions on how SVS can develop criteria for appropriateness. To share your thoughts, please email me at [email protected].
What follows is the text of the Lawrence letter to the New York Times:
To the Editor:
Most of my colleagues in vascular medicine want to provide the best treatment for their patients, but those who don’t should be exposed, as was done in “Medicare Bills Rise for Stents Put Into Limbs” (front page, Jan. 30). This article shows how inappropriate care can harm a patient and greatly increase the cost of health care, while grossly enhancing the income of those who overuse procedures.
Office-based procedures are not inherently bad, if standards for appropriate care are followed. The Society for Vascular Surgery recently held a national symposium to discuss ways to discourage inappropriate use of vascular procedures, and recently, we published evidence-based practice guidelines to encourage appropriate care of peripheral arterial disease.
These guidelines emphasize conservative measures as the first line of treatment for patients without symptoms or with vascular pain only when walking, reserving interventions and surgery for those with more severe problems. They also recommend inexpensive, noninvasive tests to determine whether the pain is truly vascular. These tests should be used in every patient.
Practice guidelines set standards for our members, but all physicians treating patients with vascular disease should also use them. Vascular specialists need an in-depth understanding of vascular disease, as well as technical skill, but they also need the ethics to treat patients like a brother or a sister, not the source of payment for a new car.
PETER F. LAWRENCE, Los Angeles
The writer, chief of vascular surgery at U.C.L.A., is president of the Society for Vascular Surgery.