User login
What is the best beta-blocker for systolic heart failure?
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3
RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
Three beta-blockers—carvedilol, metoprolol succinate, and bisoprolol—reduce mortality equally (by about 30% over one year) in patients with Class III or IV systolic heart failure. Insufficient evidence exists comparing equipotent doses of these medications head-to-head to recommend any one over the others (strength of recommendation [SOR]: A, systematic review/meta-analysis).
EVIDENCE SUMMARY
A 2013 network meta-analysis compared beta-blockers with placebo or standard treatment by analyzing 21 randomized trials with a total of 23,122 patients.1 Investigators found that beta-blockers as a class significantly reduced mortality after a median of 12 months (odds ratio=0.71, 95% confidence interval [CI], 0.64-0.80; number needed to treat [NNT]=23).
They also compared atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol with each other and found no significant difference in risk of death, sudden cardiac death, death resulting from pump failure, or tolerability.
Three drugs are more effective and tolerable than others
A 2013 stratified subset meta-analysis used data from landmark randomized controlled trials (RCTs) that evaluated beta-blockers vs placebo in patients with systolic heart failure to compare metoprolol succinate (MERIT-HF) vs placebo with bisoprolol (CIBIS-II), carvedilol (COPERNICUS), and nebivolol (SENIORS-SHF) vs placebo (TABLE).2
Three of the drugs—bisoprolol, carvedilol, and metoprolol succinate—showed similar reductions relative to placebo in all-cause mortality, hospitalization for heart failure, and tolerability. Investigators concluded that the 3 drugs have comparable efficacy and tolerability, whereas nebivolol is less effective and tolerable.
Carvedilol vs beta-1-selective beta-blockers
Another 2013 meta-analysis of 8 RCTs with 4563 adult patients 18 years or older with systolic heart failure compared carvedilol with the beta-1-selective beta-blockers atenolol, bisoprolol, nebivolol, and metoprolol.3 Investigators found that carvedilol significantly reduced all-cause mortality (relative risk=0.85; 95% CI, 0.78-0.93; NNT=23) compared with beta-1-selective beta-blockers.
However, 4 trials (including COMET, N=3029) compared carvedilol with short-acting metoprolol tartrate, which may have skewed results in favor of carvedilol. Moreover, 2 trials comparing carvedilol with bisoprolol and 2 trials comparing carvedilol with nebivolol found no significant difference in all-cause mortality.3

RECOMMENDATIONS
The 2010 Heart Failure Society of America Comprehensive Heart Failure Practice Guideline notes that the marked beneficial effects of beta blockade with carvedilol, bisoprolol, and controlled- or extended-release metoprolol have been well-demonstrated in large-scale clinical trials of symptomatic patients with Class II to IV heart failure and reduced left ventricular ejection fraction.4
The 2013 American College of Cardiology Foundation/American Heart Association heart failure guideline recommends the use of one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol, or sustained-release metoprolol succinate) for all patients with current or previous symptoms of heart failure with reduced ejection fraction, unless contraindicated, to reduce morbidity and mortality.5
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
1. Chatterjee S, Biondi-Zoccai G, Abbate A, et al. Benefits of b blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55.
2. Wikstrand J, Wedel H, Castagno D, et al. The large-scale placebo-controlled beta-blocker studies in systolic heart failure revisited: results from CIBIS-II, COPERNICUS and SENIORS-SHF compared with stratified subsets from MERIT-HF. J Intern Med. 2014;275:134-143.
3. DiNicolantonio JJ, Lavie CJ, Fares H, et al. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol). Am J Cardiol. 2013;111:765-769.
4. Heart Failure Society of America. Executive summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2010;16:475-539.
5. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
Evidence-based answers from the Family Physicians Inquiries Network
February 2015 Quiz 1
ANSWER: D
Critique
Cough is considered to be one of the atypical manifestations of reflux disease. The mechanisms include regurgitation with tracheal aspiration of gastric content, but also triggering of reflex bronchial reactivity with small amounts of acid in the distal esophagus in predisposed individuals. However, environmental allergens, postnasal drip, and airway disorders can also trigger chronic cough. Upper endoscopy with esophageal biopsies may demonstrate reflux esophagitis, but this does not establish whether cough is triggered by reflux events. A solid bolus barium swallow is useful in the evaluation of esophageal dysphagia. Impedance planimetry assesses biomechanical properties of the esophageal wall, and does not help assess the role of reflux in chronic cough. Esophageal high-resolution manometry will demonstrate esophageal motor patterns, but does not determine causality of chronic cough. Wireless pH testing off PPI therapy has the potential to determine whether cough events correlate with acidic reflux events. Some investigators have combined this with a cough monitor that can precisely time cough events, allowing for more accurate assessments of the association between cough and reflux events.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
ANSWER: D
Critique
Cough is considered to be one of the atypical manifestations of reflux disease. The mechanisms include regurgitation with tracheal aspiration of gastric content, but also triggering of reflex bronchial reactivity with small amounts of acid in the distal esophagus in predisposed individuals. However, environmental allergens, postnasal drip, and airway disorders can also trigger chronic cough. Upper endoscopy with esophageal biopsies may demonstrate reflux esophagitis, but this does not establish whether cough is triggered by reflux events. A solid bolus barium swallow is useful in the evaluation of esophageal dysphagia. Impedance planimetry assesses biomechanical properties of the esophageal wall, and does not help assess the role of reflux in chronic cough. Esophageal high-resolution manometry will demonstrate esophageal motor patterns, but does not determine causality of chronic cough. Wireless pH testing off PPI therapy has the potential to determine whether cough events correlate with acidic reflux events. Some investigators have combined this with a cough monitor that can precisely time cough events, allowing for more accurate assessments of the association between cough and reflux events.
ANSWER: D
Critique
Cough is considered to be one of the atypical manifestations of reflux disease. The mechanisms include regurgitation with tracheal aspiration of gastric content, but also triggering of reflex bronchial reactivity with small amounts of acid in the distal esophagus in predisposed individuals. However, environmental allergens, postnasal drip, and airway disorders can also trigger chronic cough. Upper endoscopy with esophageal biopsies may demonstrate reflux esophagitis, but this does not establish whether cough is triggered by reflux events. A solid bolus barium swallow is useful in the evaluation of esophageal dysphagia. Impedance planimetry assesses biomechanical properties of the esophageal wall, and does not help assess the role of reflux in chronic cough. Esophageal high-resolution manometry will demonstrate esophageal motor patterns, but does not determine causality of chronic cough. Wireless pH testing off PPI therapy has the potential to determine whether cough events correlate with acidic reflux events. Some investigators have combined this with a cough monitor that can precisely time cough events, allowing for more accurate assessments of the association between cough and reflux events.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
- Sifrim D, Dupont L, Blondeau K, Zhang X, Tack J, Janssens J. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449–54.
- Smith J, Woodcock A, Houghton L. New developments in reflux-associated cough. Lung 2010;188(Suppl1)S81-6.
- Sifrim D, Barnes N. GERD related chronic cough: How to identify patients who will respond to antireflux therapy. J. Clin. Gastroenterol. 2010;44:234-6.
February 2015 Quiz 2
ANSWER: C
Critique
The diagnosis of H. pylori may be made using either invasive or noninvasive methods. Invasive diagnostic methods either detect organisms directly (i.e., histological identification with appropriate stains or bacterial culture) or indirectly (i.e., rapid urease testing of biopsy specimens). Noninvasive methods include serum antibody, stool antigen, and detecting the metabolites of the bacterial enzyme urease (i.e., urea breath testing). It should be noted that serology should not be used to test for eradication as the antibodies may remain elevated for years after successful eradication therapy.
Treatment of H. pylori infection has become problematic recently primarily because of increasing antibiotic resistance. The eradication rate of standard triple therapy consisting of a PPI combined with clarithromycin (500 mg) and amoxicillin (1 g) (or metronidazole (500 mg)), all given b.i.d. for 7-14 days has now declined to unacceptable levels, averaging 70%-80% but reported to be as low as 55%. It is currently recommended that a noninvasive method be used (other than serology) to confirm eradication in patients in whom eradication is deemed necessary.
- Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64.
- De F, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Lerardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointest. Liver Dis. 2010;19:409-14.
- Kearney DJ, Brousal A. Treatment of Helicobacter pylori infection in clinical practice in the United States – Results from 224 patients. Dig. Dis. Sci. 2000;45:265-71.
ANSWER: C
Critique
The diagnosis of H. pylori may be made using either invasive or noninvasive methods. Invasive diagnostic methods either detect organisms directly (i.e., histological identification with appropriate stains or bacterial culture) or indirectly (i.e., rapid urease testing of biopsy specimens). Noninvasive methods include serum antibody, stool antigen, and detecting the metabolites of the bacterial enzyme urease (i.e., urea breath testing). It should be noted that serology should not be used to test for eradication as the antibodies may remain elevated for years after successful eradication therapy.
Treatment of H. pylori infection has become problematic recently primarily because of increasing antibiotic resistance. The eradication rate of standard triple therapy consisting of a PPI combined with clarithromycin (500 mg) and amoxicillin (1 g) (or metronidazole (500 mg)), all given b.i.d. for 7-14 days has now declined to unacceptable levels, averaging 70%-80% but reported to be as low as 55%. It is currently recommended that a noninvasive method be used (other than serology) to confirm eradication in patients in whom eradication is deemed necessary.
ANSWER: C
Critique
The diagnosis of H. pylori may be made using either invasive or noninvasive methods. Invasive diagnostic methods either detect organisms directly (i.e., histological identification with appropriate stains or bacterial culture) or indirectly (i.e., rapid urease testing of biopsy specimens). Noninvasive methods include serum antibody, stool antigen, and detecting the metabolites of the bacterial enzyme urease (i.e., urea breath testing). It should be noted that serology should not be used to test for eradication as the antibodies may remain elevated for years after successful eradication therapy.
Treatment of H. pylori infection has become problematic recently primarily because of increasing antibiotic resistance. The eradication rate of standard triple therapy consisting of a PPI combined with clarithromycin (500 mg) and amoxicillin (1 g) (or metronidazole (500 mg)), all given b.i.d. for 7-14 days has now declined to unacceptable levels, averaging 70%-80% but reported to be as low as 55%. It is currently recommended that a noninvasive method be used (other than serology) to confirm eradication in patients in whom eradication is deemed necessary.
- Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64.
- De F, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Lerardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointest. Liver Dis. 2010;19:409-14.
- Kearney DJ, Brousal A. Treatment of Helicobacter pylori infection in clinical practice in the United States – Results from 224 patients. Dig. Dis. Sci. 2000;45:265-71.
- Malfertheiner P, Megraud F, O’Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64.
- De F, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Lerardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J. Gastrointest. Liver Dis. 2010;19:409-14.
- Kearney DJ, Brousal A. Treatment of Helicobacter pylori infection in clinical practice in the United States – Results from 224 patients. Dig. Dis. Sci. 2000;45:265-71.
Team touts a new, improved hydrogel
Louis Heiser & Robert Ackland
A new hydrogel improves on previous models by enabling the generation of more mature blood vessels, according to research published in ACS Nano.
The hydrogel also overcomes several other issues that have kept previous hydrogels from reaching their potential to treat injuries and forming new vasculature to treat heart attack, stroke, and ischemic tissue diseases.
Like earlier versions, the new hydrogel can be injected in liquid form and turns into a nanofiber-infused gel at the site of the injury. The difference with this hydrogel, according to researchers, is the quality of the blood vessels that are formed.
This hydrogel is made of self-assembling synthetic peptides that form nanofiber scaffolds. And the peptides incorporate a mimic of vascular endothelial growth factor, a signal protein that promotes angiogenesis.
Furthermore, the hydrogel can be easily delivered by syringe, is quickly infiltrated by hematopoietic and mesenchymal cells, and quickly forms a mature vascular network.
“In a lot of the published literature, you see rings that only have the endothelial cell lining, and that indicates a very immature blood vessel,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“These types of vessels usually don’t persist and disappear shortly after they show up. In ours, you see that same endothelial cell layer, but surrounding it is a smooth muscle cell layer that indicates a much more mature vessel that’s likely to persist.”
Furthermore, the scaffolds the hydrogel forms show no signs of fibrous encapsulation. After 3 weeks, they are resorbed into the native tissue.
In previous studies, implanted synthetic materials tended to become encapsulated by fibrous barriers that kept cells and blood vessels from infiltrating the scaffold, Dr Hartgerink said.
“That is an extremely common problem in synthetic materials put into the body,” he explained. “Some avoid this problem, but if the body doesn’t like a material and isn’t able to destroy it, the solution is to wall it off.”
“As soon as that happens, the flow of nutrients across that barrier decreases to almost nothing. So the fact that we’ve developed syringe-directed delivery of a material that doesn’t develop fibrous encapsulation is really important.”
Other negative characteristics of earlier hydrogels—unwanted immune responses, surface degradation preceding their integration into biological systems, and the release of artificial degradation byproducts—have been eliminated as well, Dr Hartgerink said.
“There are a lot of features about this hydrogel that come together to make it a unique system,” he added. “If you look through the literature at what other people have done, each concept that is involved in our system probably exists somewhere already. The difference is that we have all these features in one place working together.” ![]()

Louis Heiser & Robert Ackland
A new hydrogel improves on previous models by enabling the generation of more mature blood vessels, according to research published in ACS Nano.
The hydrogel also overcomes several other issues that have kept previous hydrogels from reaching their potential to treat injuries and forming new vasculature to treat heart attack, stroke, and ischemic tissue diseases.
Like earlier versions, the new hydrogel can be injected in liquid form and turns into a nanofiber-infused gel at the site of the injury. The difference with this hydrogel, according to researchers, is the quality of the blood vessels that are formed.
This hydrogel is made of self-assembling synthetic peptides that form nanofiber scaffolds. And the peptides incorporate a mimic of vascular endothelial growth factor, a signal protein that promotes angiogenesis.
Furthermore, the hydrogel can be easily delivered by syringe, is quickly infiltrated by hematopoietic and mesenchymal cells, and quickly forms a mature vascular network.
“In a lot of the published literature, you see rings that only have the endothelial cell lining, and that indicates a very immature blood vessel,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“These types of vessels usually don’t persist and disappear shortly after they show up. In ours, you see that same endothelial cell layer, but surrounding it is a smooth muscle cell layer that indicates a much more mature vessel that’s likely to persist.”
Furthermore, the scaffolds the hydrogel forms show no signs of fibrous encapsulation. After 3 weeks, they are resorbed into the native tissue.
In previous studies, implanted synthetic materials tended to become encapsulated by fibrous barriers that kept cells and blood vessels from infiltrating the scaffold, Dr Hartgerink said.
“That is an extremely common problem in synthetic materials put into the body,” he explained. “Some avoid this problem, but if the body doesn’t like a material and isn’t able to destroy it, the solution is to wall it off.”
“As soon as that happens, the flow of nutrients across that barrier decreases to almost nothing. So the fact that we’ve developed syringe-directed delivery of a material that doesn’t develop fibrous encapsulation is really important.”
Other negative characteristics of earlier hydrogels—unwanted immune responses, surface degradation preceding their integration into biological systems, and the release of artificial degradation byproducts—have been eliminated as well, Dr Hartgerink said.
“There are a lot of features about this hydrogel that come together to make it a unique system,” he added. “If you look through the literature at what other people have done, each concept that is involved in our system probably exists somewhere already. The difference is that we have all these features in one place working together.” ![]()

Louis Heiser & Robert Ackland
A new hydrogel improves on previous models by enabling the generation of more mature blood vessels, according to research published in ACS Nano.
The hydrogel also overcomes several other issues that have kept previous hydrogels from reaching their potential to treat injuries and forming new vasculature to treat heart attack, stroke, and ischemic tissue diseases.
Like earlier versions, the new hydrogel can be injected in liquid form and turns into a nanofiber-infused gel at the site of the injury. The difference with this hydrogel, according to researchers, is the quality of the blood vessels that are formed.
This hydrogel is made of self-assembling synthetic peptides that form nanofiber scaffolds. And the peptides incorporate a mimic of vascular endothelial growth factor, a signal protein that promotes angiogenesis.
Furthermore, the hydrogel can be easily delivered by syringe, is quickly infiltrated by hematopoietic and mesenchymal cells, and quickly forms a mature vascular network.
“In a lot of the published literature, you see rings that only have the endothelial cell lining, and that indicates a very immature blood vessel,” said study author Jeffrey Hartgerink, PhD, of Rice University in Houston, Texas.
“These types of vessels usually don’t persist and disappear shortly after they show up. In ours, you see that same endothelial cell layer, but surrounding it is a smooth muscle cell layer that indicates a much more mature vessel that’s likely to persist.”
Furthermore, the scaffolds the hydrogel forms show no signs of fibrous encapsulation. After 3 weeks, they are resorbed into the native tissue.
In previous studies, implanted synthetic materials tended to become encapsulated by fibrous barriers that kept cells and blood vessels from infiltrating the scaffold, Dr Hartgerink said.
“That is an extremely common problem in synthetic materials put into the body,” he explained. “Some avoid this problem, but if the body doesn’t like a material and isn’t able to destroy it, the solution is to wall it off.”
“As soon as that happens, the flow of nutrients across that barrier decreases to almost nothing. So the fact that we’ve developed syringe-directed delivery of a material that doesn’t develop fibrous encapsulation is really important.”
Other negative characteristics of earlier hydrogels—unwanted immune responses, surface degradation preceding their integration into biological systems, and the release of artificial degradation byproducts—have been eliminated as well, Dr Hartgerink said.
“There are a lot of features about this hydrogel that come together to make it a unique system,” he added. “If you look through the literature at what other people have done, each concept that is involved in our system probably exists somewhere already. The difference is that we have all these features in one place working together.” ![]()
Would a cholesterol medication have made a difference? … More
Would a cholesterol medication have made a difference?
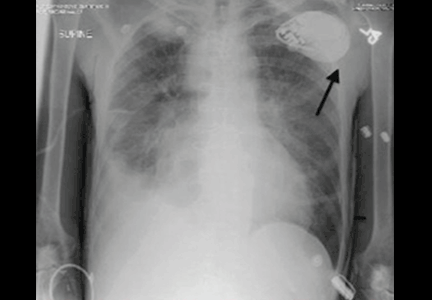
A WOMAN WITH A HISTORY OF HYPERTENSION and hyperlipidemia sought treatment from her family physician (FP) for a protracted, nonproductive cough. The FP diagnosed sinusitis and reactive airway disease and prescribed steroids and antibiotics. The patient returned to the FP 5 more times over the next 9 weeks. The patient’s symptoms waxed and waned, but her cough continued. She reported chest tightness and shortness of breath on exertion. A chest x-ray revealed moderate heart enlargement. An echocardiogram was scheduled.
During the patient’s last visit, her FP noted that she had shortness of breath on exertion, but no chest pain. Three days later she suffered a massive myocardial infarction (MI). Cardiac catheterization found 80% occlusion of the left anterior descending artery. She underwent angioplasty and stent placement; after this procedure her ejection fraction was 25% to 30%. One month later, the patient received a pacemaker/defibrillator. The patient’s cardiac symptoms returned 7 months later, and she underwent another angioplasty. She improved and her last echocardiogram showed near-normal heart function.
PLAINTIFF’S CLAIM Although the patient had persistently elevated cholesterol levels, the FP failed to order repeat cholesterol studies and arrange for drug therapy. If the patient’s hyperlipidemia had been medically managed, her coronary artery disease would not have progressed to unstable angina and MI. The FP also failed to obtain routine electrocardiograms or an urgent cardiac consult after a chest x-ray showed an enlarged heart. The FP also failed to send the patient to an emergency department when she complained of shortness of breath on exertion.
THE DEFENSE An urgent cardiac work-up was not indicated and the patient’s cholesterol levels were only mildly elevated and did not require medical management. Her MI was unavoidable since most infarctions are due to plaque rupture in coronary vessels that aren’t occluded enough to require treatment.
VERDICT $1.6 million Michigan verdict.
COMMENT I think the key issue in this difficult diagnostic case is not the lack of prescribing cholesterol medication, but the repeated office visits with no definite diagnosis. If the physician had escalated the evaluation more quickly, the MI might have been avoided.
Narcotic misstep has tragic consequences
A 47-YEAR-OLD MAN SOUGHT TREATMENT FOR DRUG ADDICTION. His physician prescribed methadone, despite not being licensed to do so. After 4 days of taking methadone, the patient went to the hospital because he felt dizzy and was having difficulty breathing. Two days after being examined and discharged, he died from methadone toxicity.
PLAINTIFF’S CLAIM The toxicity was caused by simultaneous use of methadone and alprazolam, which the patient also had been prescribed. The physician failed to recognize the potential toxicity and should have performed testing that could have revealed the simultaneous use of other drugs. In addition, the physician was not licensed to prescribe methadone.
THE DEFENSE The physician had recommended a licensed, qualified facility that could have treated the plaintiff, but the plaintiff preferred treatment in a setting that allowed him to remain anonymous.
VERDICT $1.15 million New York settlement.
COMMENT Don’t break the law, even if your patient asks you to. Know your state laws regarding narcotic prescribing. These are getting more stringent due to the rapid rise in prescription narcotic overdose deaths in the United States.
Would a cholesterol medication have made a difference?
A WOMAN WITH A HISTORY OF HYPERTENSION and hyperlipidemia sought treatment from her family physician (FP) for a protracted, nonproductive cough. The FP diagnosed sinusitis and reactive airway disease and prescribed steroids and antibiotics. The patient returned to the FP 5 more times over the next 9 weeks. The patient’s symptoms waxed and waned, but her cough continued. She reported chest tightness and shortness of breath on exertion. A chest x-ray revealed moderate heart enlargement. An echocardiogram was scheduled.
During the patient’s last visit, her FP noted that she had shortness of breath on exertion, but no chest pain. Three days later she suffered a massive myocardial infarction (MI). Cardiac catheterization found 80% occlusion of the left anterior descending artery. She underwent angioplasty and stent placement; after this procedure her ejection fraction was 25% to 30%. One month later, the patient received a pacemaker/defibrillator. The patient’s cardiac symptoms returned 7 months later, and she underwent another angioplasty. She improved and her last echocardiogram showed near-normal heart function.
PLAINTIFF’S CLAIM Although the patient had persistently elevated cholesterol levels, the FP failed to order repeat cholesterol studies and arrange for drug therapy. If the patient’s hyperlipidemia had been medically managed, her coronary artery disease would not have progressed to unstable angina and MI. The FP also failed to obtain routine electrocardiograms or an urgent cardiac consult after a chest x-ray showed an enlarged heart. The FP also failed to send the patient to an emergency department when she complained of shortness of breath on exertion.
THE DEFENSE An urgent cardiac work-up was not indicated and the patient’s cholesterol levels were only mildly elevated and did not require medical management. Her MI was unavoidable since most infarctions are due to plaque rupture in coronary vessels that aren’t occluded enough to require treatment.
VERDICT $1.6 million Michigan verdict.
COMMENT I think the key issue in this difficult diagnostic case is not the lack of prescribing cholesterol medication, but the repeated office visits with no definite diagnosis. If the physician had escalated the evaluation more quickly, the MI might have been avoided.
Narcotic misstep has tragic consequences
A 47-YEAR-OLD MAN SOUGHT TREATMENT FOR DRUG ADDICTION. His physician prescribed methadone, despite not being licensed to do so. After 4 days of taking methadone, the patient went to the hospital because he felt dizzy and was having difficulty breathing. Two days after being examined and discharged, he died from methadone toxicity.
PLAINTIFF’S CLAIM The toxicity was caused by simultaneous use of methadone and alprazolam, which the patient also had been prescribed. The physician failed to recognize the potential toxicity and should have performed testing that could have revealed the simultaneous use of other drugs. In addition, the physician was not licensed to prescribe methadone.
THE DEFENSE The physician had recommended a licensed, qualified facility that could have treated the plaintiff, but the plaintiff preferred treatment in a setting that allowed him to remain anonymous.
VERDICT $1.15 million New York settlement.
COMMENT Don’t break the law, even if your patient asks you to. Know your state laws regarding narcotic prescribing. These are getting more stringent due to the rapid rise in prescription narcotic overdose deaths in the United States.
Would a cholesterol medication have made a difference?
A WOMAN WITH A HISTORY OF HYPERTENSION and hyperlipidemia sought treatment from her family physician (FP) for a protracted, nonproductive cough. The FP diagnosed sinusitis and reactive airway disease and prescribed steroids and antibiotics. The patient returned to the FP 5 more times over the next 9 weeks. The patient’s symptoms waxed and waned, but her cough continued. She reported chest tightness and shortness of breath on exertion. A chest x-ray revealed moderate heart enlargement. An echocardiogram was scheduled.
During the patient’s last visit, her FP noted that she had shortness of breath on exertion, but no chest pain. Three days later she suffered a massive myocardial infarction (MI). Cardiac catheterization found 80% occlusion of the left anterior descending artery. She underwent angioplasty and stent placement; after this procedure her ejection fraction was 25% to 30%. One month later, the patient received a pacemaker/defibrillator. The patient’s cardiac symptoms returned 7 months later, and she underwent another angioplasty. She improved and her last echocardiogram showed near-normal heart function.
PLAINTIFF’S CLAIM Although the patient had persistently elevated cholesterol levels, the FP failed to order repeat cholesterol studies and arrange for drug therapy. If the patient’s hyperlipidemia had been medically managed, her coronary artery disease would not have progressed to unstable angina and MI. The FP also failed to obtain routine electrocardiograms or an urgent cardiac consult after a chest x-ray showed an enlarged heart. The FP also failed to send the patient to an emergency department when she complained of shortness of breath on exertion.
THE DEFENSE An urgent cardiac work-up was not indicated and the patient’s cholesterol levels were only mildly elevated and did not require medical management. Her MI was unavoidable since most infarctions are due to plaque rupture in coronary vessels that aren’t occluded enough to require treatment.
VERDICT $1.6 million Michigan verdict.
COMMENT I think the key issue in this difficult diagnostic case is not the lack of prescribing cholesterol medication, but the repeated office visits with no definite diagnosis. If the physician had escalated the evaluation more quickly, the MI might have been avoided.
Narcotic misstep has tragic consequences
A 47-YEAR-OLD MAN SOUGHT TREATMENT FOR DRUG ADDICTION. His physician prescribed methadone, despite not being licensed to do so. After 4 days of taking methadone, the patient went to the hospital because he felt dizzy and was having difficulty breathing. Two days after being examined and discharged, he died from methadone toxicity.
PLAINTIFF’S CLAIM The toxicity was caused by simultaneous use of methadone and alprazolam, which the patient also had been prescribed. The physician failed to recognize the potential toxicity and should have performed testing that could have revealed the simultaneous use of other drugs. In addition, the physician was not licensed to prescribe methadone.
THE DEFENSE The physician had recommended a licensed, qualified facility that could have treated the plaintiff, but the plaintiff preferred treatment in a setting that allowed him to remain anonymous.
VERDICT $1.15 million New York settlement.
COMMENT Don’t break the law, even if your patient asks you to. Know your state laws regarding narcotic prescribing. These are getting more stringent due to the rapid rise in prescription narcotic overdose deaths in the United States.
Malpractice Counsel
Traumatic Back Pain
An 84-year-old man with low-back pain following a motor vehicle crash was brought to the ED by emergency medical services (EMS). He had been the restrained driver, stopped at a traffic light, when he was struck from behind by a second vehicle.
In the ED, the patient only complained of low-back pain. He denied any radiation of pain or lower-extremity numbness or weakness. He also denied any head injury, loss of consciousness, neck pain, or abdominal pain. His past medical history was significant for hypertension, arthritis, and coronary artery disease.
On physical examination, the patient’s vital signs were normal. The head, eyes, ears, nose, and throat (HEENT) examination was also normal; specifically, there was no tenderness to palpation of the cervical spine in the posterior midline. Regarding the cardiopulmonary examination, auscultation of the lungs revealed clear, bilateral breath sounds; the heart examination was normal. The patient had a soft abdomen, without tenderness, guarding, or rebound. His pelvis was stable, but he did exhibit some tenderness on palpation of the lower-thoracic and upper-lumbar spine. The neurological examination revealed normal motor strength and sensation in the lower extremities.
The emergency physician (EP) ordered X-rays of the thoracic and lumbar spine and a urinalysis. The films were interpreted by both the EP and radiologist as normal; the results of the urinalysis were also normal. The patient was diagnosed with a lower back strain secondary to the motor vehicle crash and was discharged home with an analgesic.
The next day, however, the patient began to complain of increased back pain and lower-extremity numbness and weakness. He was brought back to the same hospital ED where he was noted to have severe weakness of both lower extremities and decreased sensation to touch. Additional imaging was performed, which demonstrated a fracture of T11 with spinal cord impingement. He was taken to surgery, but unfortunately the injury was permanent, and the patient was left with lower-extremity paralysis and bowel and bladder incontinence.
The plaintiff sued the EP and the radiologist for not properly interpreting the initial X-rays. The defendants denied liability, asserting the patient’s injury was a result of the collision and that nothing could have prevented it. According to a published account, the jury returned a verdict finding the EP to be 40% at fault and the radiologist 60% at fault.
Discussion
Emergency physicians frequently manage patients experiencing pain or injury following a motor vehicle crash. If the patient is complaining of neck or back pain, the prehospital providers will immobilize the patient with a rigid cervical collar (ie, if neck pain is present) and a long backboard if pain anywhere along the spine is present (ie, cervical, thoracic, or lumbar).
When the initial airway, breathing, circulation, and disability assessment for the trauma patient is performed and found to be normal, a secondary examination should be performed. Trauma patients with back pain should be log-rolled onto their side, with spinal immobilization followed by visual inspection and palpation/percussion of the midline of the thoracic and lumbar spine. The presence of midline tenderness suggests an acute injury and the need to keep the patient immobilized. Patients should be removed off the backboard and onto the gurney mattress while immobilizing the spine. The standard hospital mattress provides acceptable spinal support.1
Historically, plain radiographs of the thoracic and lumbar spine have been the imaging test of choice in the initial evaluation of suspected traumatic spinal column injury. However, similar to cervical spine trauma, computed tomography (CT) is assuming a larger role in the evaluation of patients with suspected thoracic or lumbar spine injury. When thoracic and abdominal CT scans are performed to evaluate for possible chest or abdominal trauma, those images can be reformatted and used to reconstruct images of the thoracic and lumbar spine, significantly reducing radiation exposure.1 While CT is the gold standard imaging study for evaluation of bony or ligamentous injury of the spine, magnetic resonance imaging (MRI) is the study of choice for patients with neurological deficits or suspected spinal cord injury.
This patient had a completely normal neurological examination at initial presentation, so there was no indication for an MRI. The bony injury to T11 must have been very subtle for both the EP and the radiologist to have missed it. Unfortunately, the jury appears to have used the standard of “perfection,” rather than the “reasonable and prudent physician” in judging that the injury should have been detected. This case serves as a reminder that EPs cannot rely on consulting specialists to consistently and reliably provide accurate information. Moreover, this case emphasizes the need to consider CT imaging of the spine in the evaluation of patients with severe back pain of traumatic origin when plain radiographs appear normal.
Hip-Reduction Problem
A 79-year-old man with left hip pain presented to the ED via EMS. The patient stated that when he had bent over to retrieve his dropped glasses, he experienced the immediate onset of left hip pain and fell to the floor. He was unable to get up on his own and called EMS. The patient had undergone total left hip replacement 1 month prior. At presentation, he complained only of severe pain in his left hip; he denied head injury, neck pain or stiffness, chest pain, or abdominal pain. His past medical history was significant for hypertension and type 2 diabetes mellitus. The patient had no known drug allergies.
On physical examination, he was mildly tachycardic. His vital signs were: heart rate, 102 beats/minute; blood pressure, 156/88 mm Hg; respiratory rate, 20 breaths/minutes; and temperature, afebrile. His pulse oximetry was 98% on room air. The HEENT, lung, heart, and abdominal examinations were all normal. Standing at the foot of the bed, the patient had obvious shortening, internal rotation, and adduction of the left leg. The left knee was without tenderness or swelling. The neurovascular examination of the left lower extremity was completely normal.
Plain radiographs of the pelvis and left hip ordered by the EP demonstrated a posterior hip dislocation with intact hardware. The EP consulted the patient’s orthopedic physician, and both agreed the EP should attempt to reduce the dislocation in the ED. Using conscious sedation, the EP was able to reduce the dislocation, but postreduction films demonstrated a new fracture requiring orthopedic surgery. Unfortunately, the patient had a very difficult recovery, ultimately resulting in death.
The patient’s estate sued the EP, stating he should have had the orthopedic physician reduce the dislocation. The defense argued that fracture is a known complication of reduction of a dislocated hip. A defense verdict was returned.
Discussion
Approximately 85% to 90% of hip dislocations are posterior; the remaining 10% are anterior. Posterior hip dislocations are a common complication following total hip-replacement surgery.1 Hip dislocation is a true orthopedic and time-dependent emergency. The longer the hip remains dislocated, the more likely complications are to occur, including osteonecrosis of the femoral head, arthritic degeneration of the hip joint, and long-term neurological sequelae.2 The treatment of posterior hip dislocation (without fracture) is closed reduction as quickly as possible, and preferably within 6 hours.3 As this case demonstrates, minimal forces can result in a hip dislocation following a total hip replacement. In healthy patients, however, significant forces (eg, high-speed motor vehicle crashes) are required to cause posterior hip dislocation.
Patients with a posterior hip dislocation will present in severe pain and an inability to ambulate. In most cases of posterior hip dislocation, the affected lower extremity will be visibly shortened, internally rotated, and adducted. The knee should always be examined for injury, as well as performance of a thorough neurovascular examination of the affected extremity.
Plain X-ray films will usually identify a posterior hip dislocation. On an anteroposterior pelvis X-ray, the femoral head will be seen outside and just superior to the acetabulum. Special attention should be made to the acetabulum to ensure a concomitant acetabular fracture is not missed.
Indications for closed reduction of a posterior hip dislocation include dislocation with or without neurological deficit and no associated fracture, or dislocation with an associated fracture if no neurological deficits are present.2 An open traumatic hip dislocation should only be reduced in the operating room.
It is certainly within the purview of the EP to attempt a closed reduction for a posterior hip dislocation if no contraindications exist. The patient will need to be sedated (ie, procedural sedation, conscious sedation, or moderate sedation) for any chance of success at reduction. While it is beyond the scope of this article to review the various techniques used to reduce a posterior hip dislocation, one of the guiding principles is that after two or three unsuccessful attempts by the EP to reduce the dislocation, no further attempts should be made and orthopedic surgery services should be consulted. This is because the risk of complications increases as the number of failed attempts increase.
It is unclear how many attempts the EP made in this case. Fracture is a known complication when attempting reduction for a hip dislocation, be it an orthopedic surgeon or an EP. It was certainly appropriate for the EP in this case to attempt closed reduction, given the importance of timely reduction.
Reference (Traumatic Back Pain)
- Baron BJ, McSherry KJ, Larson JL, Scalea TM. Spinal and spinal cord trauma In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1709-1730.
(Hip-Reduction Problem)
- Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of proceduralsedation for the reduction of dislocated total hip arthroplasty.West J Emerg Med. 2014:15(1):76-80.
- Davenport M. Joint reduction, hip dislocation, posterior. Medscape Web site. eMedicine.medscape.com/article/109225. Updated February 11, 2014. Accessed January 27, 2015.
- Steele MT, Stubbs AM. Hip and femur injuries. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1848-1856.
Traumatic Back Pain
An 84-year-old man with low-back pain following a motor vehicle crash was brought to the ED by emergency medical services (EMS). He had been the restrained driver, stopped at a traffic light, when he was struck from behind by a second vehicle.
In the ED, the patient only complained of low-back pain. He denied any radiation of pain or lower-extremity numbness or weakness. He also denied any head injury, loss of consciousness, neck pain, or abdominal pain. His past medical history was significant for hypertension, arthritis, and coronary artery disease.
On physical examination, the patient’s vital signs were normal. The head, eyes, ears, nose, and throat (HEENT) examination was also normal; specifically, there was no tenderness to palpation of the cervical spine in the posterior midline. Regarding the cardiopulmonary examination, auscultation of the lungs revealed clear, bilateral breath sounds; the heart examination was normal. The patient had a soft abdomen, without tenderness, guarding, or rebound. His pelvis was stable, but he did exhibit some tenderness on palpation of the lower-thoracic and upper-lumbar spine. The neurological examination revealed normal motor strength and sensation in the lower extremities.
The emergency physician (EP) ordered X-rays of the thoracic and lumbar spine and a urinalysis. The films were interpreted by both the EP and radiologist as normal; the results of the urinalysis were also normal. The patient was diagnosed with a lower back strain secondary to the motor vehicle crash and was discharged home with an analgesic.
The next day, however, the patient began to complain of increased back pain and lower-extremity numbness and weakness. He was brought back to the same hospital ED where he was noted to have severe weakness of both lower extremities and decreased sensation to touch. Additional imaging was performed, which demonstrated a fracture of T11 with spinal cord impingement. He was taken to surgery, but unfortunately the injury was permanent, and the patient was left with lower-extremity paralysis and bowel and bladder incontinence.
The plaintiff sued the EP and the radiologist for not properly interpreting the initial X-rays. The defendants denied liability, asserting the patient’s injury was a result of the collision and that nothing could have prevented it. According to a published account, the jury returned a verdict finding the EP to be 40% at fault and the radiologist 60% at fault.
Discussion
Emergency physicians frequently manage patients experiencing pain or injury following a motor vehicle crash. If the patient is complaining of neck or back pain, the prehospital providers will immobilize the patient with a rigid cervical collar (ie, if neck pain is present) and a long backboard if pain anywhere along the spine is present (ie, cervical, thoracic, or lumbar).
When the initial airway, breathing, circulation, and disability assessment for the trauma patient is performed and found to be normal, a secondary examination should be performed. Trauma patients with back pain should be log-rolled onto their side, with spinal immobilization followed by visual inspection and palpation/percussion of the midline of the thoracic and lumbar spine. The presence of midline tenderness suggests an acute injury and the need to keep the patient immobilized. Patients should be removed off the backboard and onto the gurney mattress while immobilizing the spine. The standard hospital mattress provides acceptable spinal support.1
Historically, plain radiographs of the thoracic and lumbar spine have been the imaging test of choice in the initial evaluation of suspected traumatic spinal column injury. However, similar to cervical spine trauma, computed tomography (CT) is assuming a larger role in the evaluation of patients with suspected thoracic or lumbar spine injury. When thoracic and abdominal CT scans are performed to evaluate for possible chest or abdominal trauma, those images can be reformatted and used to reconstruct images of the thoracic and lumbar spine, significantly reducing radiation exposure.1 While CT is the gold standard imaging study for evaluation of bony or ligamentous injury of the spine, magnetic resonance imaging (MRI) is the study of choice for patients with neurological deficits or suspected spinal cord injury.
This patient had a completely normal neurological examination at initial presentation, so there was no indication for an MRI. The bony injury to T11 must have been very subtle for both the EP and the radiologist to have missed it. Unfortunately, the jury appears to have used the standard of “perfection,” rather than the “reasonable and prudent physician” in judging that the injury should have been detected. This case serves as a reminder that EPs cannot rely on consulting specialists to consistently and reliably provide accurate information. Moreover, this case emphasizes the need to consider CT imaging of the spine in the evaluation of patients with severe back pain of traumatic origin when plain radiographs appear normal.
Hip-Reduction Problem
A 79-year-old man with left hip pain presented to the ED via EMS. The patient stated that when he had bent over to retrieve his dropped glasses, he experienced the immediate onset of left hip pain and fell to the floor. He was unable to get up on his own and called EMS. The patient had undergone total left hip replacement 1 month prior. At presentation, he complained only of severe pain in his left hip; he denied head injury, neck pain or stiffness, chest pain, or abdominal pain. His past medical history was significant for hypertension and type 2 diabetes mellitus. The patient had no known drug allergies.
On physical examination, he was mildly tachycardic. His vital signs were: heart rate, 102 beats/minute; blood pressure, 156/88 mm Hg; respiratory rate, 20 breaths/minutes; and temperature, afebrile. His pulse oximetry was 98% on room air. The HEENT, lung, heart, and abdominal examinations were all normal. Standing at the foot of the bed, the patient had obvious shortening, internal rotation, and adduction of the left leg. The left knee was without tenderness or swelling. The neurovascular examination of the left lower extremity was completely normal.
Plain radiographs of the pelvis and left hip ordered by the EP demonstrated a posterior hip dislocation with intact hardware. The EP consulted the patient’s orthopedic physician, and both agreed the EP should attempt to reduce the dislocation in the ED. Using conscious sedation, the EP was able to reduce the dislocation, but postreduction films demonstrated a new fracture requiring orthopedic surgery. Unfortunately, the patient had a very difficult recovery, ultimately resulting in death.
The patient’s estate sued the EP, stating he should have had the orthopedic physician reduce the dislocation. The defense argued that fracture is a known complication of reduction of a dislocated hip. A defense verdict was returned.
Discussion
Approximately 85% to 90% of hip dislocations are posterior; the remaining 10% are anterior. Posterior hip dislocations are a common complication following total hip-replacement surgery.1 Hip dislocation is a true orthopedic and time-dependent emergency. The longer the hip remains dislocated, the more likely complications are to occur, including osteonecrosis of the femoral head, arthritic degeneration of the hip joint, and long-term neurological sequelae.2 The treatment of posterior hip dislocation (without fracture) is closed reduction as quickly as possible, and preferably within 6 hours.3 As this case demonstrates, minimal forces can result in a hip dislocation following a total hip replacement. In healthy patients, however, significant forces (eg, high-speed motor vehicle crashes) are required to cause posterior hip dislocation.
Patients with a posterior hip dislocation will present in severe pain and an inability to ambulate. In most cases of posterior hip dislocation, the affected lower extremity will be visibly shortened, internally rotated, and adducted. The knee should always be examined for injury, as well as performance of a thorough neurovascular examination of the affected extremity.
Plain X-ray films will usually identify a posterior hip dislocation. On an anteroposterior pelvis X-ray, the femoral head will be seen outside and just superior to the acetabulum. Special attention should be made to the acetabulum to ensure a concomitant acetabular fracture is not missed.
Indications for closed reduction of a posterior hip dislocation include dislocation with or without neurological deficit and no associated fracture, or dislocation with an associated fracture if no neurological deficits are present.2 An open traumatic hip dislocation should only be reduced in the operating room.
It is certainly within the purview of the EP to attempt a closed reduction for a posterior hip dislocation if no contraindications exist. The patient will need to be sedated (ie, procedural sedation, conscious sedation, or moderate sedation) for any chance of success at reduction. While it is beyond the scope of this article to review the various techniques used to reduce a posterior hip dislocation, one of the guiding principles is that after two or three unsuccessful attempts by the EP to reduce the dislocation, no further attempts should be made and orthopedic surgery services should be consulted. This is because the risk of complications increases as the number of failed attempts increase.
It is unclear how many attempts the EP made in this case. Fracture is a known complication when attempting reduction for a hip dislocation, be it an orthopedic surgeon or an EP. It was certainly appropriate for the EP in this case to attempt closed reduction, given the importance of timely reduction.
Traumatic Back Pain
An 84-year-old man with low-back pain following a motor vehicle crash was brought to the ED by emergency medical services (EMS). He had been the restrained driver, stopped at a traffic light, when he was struck from behind by a second vehicle.
In the ED, the patient only complained of low-back pain. He denied any radiation of pain or lower-extremity numbness or weakness. He also denied any head injury, loss of consciousness, neck pain, or abdominal pain. His past medical history was significant for hypertension, arthritis, and coronary artery disease.
On physical examination, the patient’s vital signs were normal. The head, eyes, ears, nose, and throat (HEENT) examination was also normal; specifically, there was no tenderness to palpation of the cervical spine in the posterior midline. Regarding the cardiopulmonary examination, auscultation of the lungs revealed clear, bilateral breath sounds; the heart examination was normal. The patient had a soft abdomen, without tenderness, guarding, or rebound. His pelvis was stable, but he did exhibit some tenderness on palpation of the lower-thoracic and upper-lumbar spine. The neurological examination revealed normal motor strength and sensation in the lower extremities.
The emergency physician (EP) ordered X-rays of the thoracic and lumbar spine and a urinalysis. The films were interpreted by both the EP and radiologist as normal; the results of the urinalysis were also normal. The patient was diagnosed with a lower back strain secondary to the motor vehicle crash and was discharged home with an analgesic.
The next day, however, the patient began to complain of increased back pain and lower-extremity numbness and weakness. He was brought back to the same hospital ED where he was noted to have severe weakness of both lower extremities and decreased sensation to touch. Additional imaging was performed, which demonstrated a fracture of T11 with spinal cord impingement. He was taken to surgery, but unfortunately the injury was permanent, and the patient was left with lower-extremity paralysis and bowel and bladder incontinence.
The plaintiff sued the EP and the radiologist for not properly interpreting the initial X-rays. The defendants denied liability, asserting the patient’s injury was a result of the collision and that nothing could have prevented it. According to a published account, the jury returned a verdict finding the EP to be 40% at fault and the radiologist 60% at fault.
Discussion
Emergency physicians frequently manage patients experiencing pain or injury following a motor vehicle crash. If the patient is complaining of neck or back pain, the prehospital providers will immobilize the patient with a rigid cervical collar (ie, if neck pain is present) and a long backboard if pain anywhere along the spine is present (ie, cervical, thoracic, or lumbar).
When the initial airway, breathing, circulation, and disability assessment for the trauma patient is performed and found to be normal, a secondary examination should be performed. Trauma patients with back pain should be log-rolled onto their side, with spinal immobilization followed by visual inspection and palpation/percussion of the midline of the thoracic and lumbar spine. The presence of midline tenderness suggests an acute injury and the need to keep the patient immobilized. Patients should be removed off the backboard and onto the gurney mattress while immobilizing the spine. The standard hospital mattress provides acceptable spinal support.1
Historically, plain radiographs of the thoracic and lumbar spine have been the imaging test of choice in the initial evaluation of suspected traumatic spinal column injury. However, similar to cervical spine trauma, computed tomography (CT) is assuming a larger role in the evaluation of patients with suspected thoracic or lumbar spine injury. When thoracic and abdominal CT scans are performed to evaluate for possible chest or abdominal trauma, those images can be reformatted and used to reconstruct images of the thoracic and lumbar spine, significantly reducing radiation exposure.1 While CT is the gold standard imaging study for evaluation of bony or ligamentous injury of the spine, magnetic resonance imaging (MRI) is the study of choice for patients with neurological deficits or suspected spinal cord injury.
This patient had a completely normal neurological examination at initial presentation, so there was no indication for an MRI. The bony injury to T11 must have been very subtle for both the EP and the radiologist to have missed it. Unfortunately, the jury appears to have used the standard of “perfection,” rather than the “reasonable and prudent physician” in judging that the injury should have been detected. This case serves as a reminder that EPs cannot rely on consulting specialists to consistently and reliably provide accurate information. Moreover, this case emphasizes the need to consider CT imaging of the spine in the evaluation of patients with severe back pain of traumatic origin when plain radiographs appear normal.
Hip-Reduction Problem
A 79-year-old man with left hip pain presented to the ED via EMS. The patient stated that when he had bent over to retrieve his dropped glasses, he experienced the immediate onset of left hip pain and fell to the floor. He was unable to get up on his own and called EMS. The patient had undergone total left hip replacement 1 month prior. At presentation, he complained only of severe pain in his left hip; he denied head injury, neck pain or stiffness, chest pain, or abdominal pain. His past medical history was significant for hypertension and type 2 diabetes mellitus. The patient had no known drug allergies.
On physical examination, he was mildly tachycardic. His vital signs were: heart rate, 102 beats/minute; blood pressure, 156/88 mm Hg; respiratory rate, 20 breaths/minutes; and temperature, afebrile. His pulse oximetry was 98% on room air. The HEENT, lung, heart, and abdominal examinations were all normal. Standing at the foot of the bed, the patient had obvious shortening, internal rotation, and adduction of the left leg. The left knee was without tenderness or swelling. The neurovascular examination of the left lower extremity was completely normal.
Plain radiographs of the pelvis and left hip ordered by the EP demonstrated a posterior hip dislocation with intact hardware. The EP consulted the patient’s orthopedic physician, and both agreed the EP should attempt to reduce the dislocation in the ED. Using conscious sedation, the EP was able to reduce the dislocation, but postreduction films demonstrated a new fracture requiring orthopedic surgery. Unfortunately, the patient had a very difficult recovery, ultimately resulting in death.
The patient’s estate sued the EP, stating he should have had the orthopedic physician reduce the dislocation. The defense argued that fracture is a known complication of reduction of a dislocated hip. A defense verdict was returned.
Discussion
Approximately 85% to 90% of hip dislocations are posterior; the remaining 10% are anterior. Posterior hip dislocations are a common complication following total hip-replacement surgery.1 Hip dislocation is a true orthopedic and time-dependent emergency. The longer the hip remains dislocated, the more likely complications are to occur, including osteonecrosis of the femoral head, arthritic degeneration of the hip joint, and long-term neurological sequelae.2 The treatment of posterior hip dislocation (without fracture) is closed reduction as quickly as possible, and preferably within 6 hours.3 As this case demonstrates, minimal forces can result in a hip dislocation following a total hip replacement. In healthy patients, however, significant forces (eg, high-speed motor vehicle crashes) are required to cause posterior hip dislocation.
Patients with a posterior hip dislocation will present in severe pain and an inability to ambulate. In most cases of posterior hip dislocation, the affected lower extremity will be visibly shortened, internally rotated, and adducted. The knee should always be examined for injury, as well as performance of a thorough neurovascular examination of the affected extremity.
Plain X-ray films will usually identify a posterior hip dislocation. On an anteroposterior pelvis X-ray, the femoral head will be seen outside and just superior to the acetabulum. Special attention should be made to the acetabulum to ensure a concomitant acetabular fracture is not missed.
Indications for closed reduction of a posterior hip dislocation include dislocation with or without neurological deficit and no associated fracture, or dislocation with an associated fracture if no neurological deficits are present.2 An open traumatic hip dislocation should only be reduced in the operating room.
It is certainly within the purview of the EP to attempt a closed reduction for a posterior hip dislocation if no contraindications exist. The patient will need to be sedated (ie, procedural sedation, conscious sedation, or moderate sedation) for any chance of success at reduction. While it is beyond the scope of this article to review the various techniques used to reduce a posterior hip dislocation, one of the guiding principles is that after two or three unsuccessful attempts by the EP to reduce the dislocation, no further attempts should be made and orthopedic surgery services should be consulted. This is because the risk of complications increases as the number of failed attempts increase.
It is unclear how many attempts the EP made in this case. Fracture is a known complication when attempting reduction for a hip dislocation, be it an orthopedic surgeon or an EP. It was certainly appropriate for the EP in this case to attempt closed reduction, given the importance of timely reduction.
Reference (Traumatic Back Pain)
- Baron BJ, McSherry KJ, Larson JL, Scalea TM. Spinal and spinal cord trauma In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1709-1730.
(Hip-Reduction Problem)
- Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of proceduralsedation for the reduction of dislocated total hip arthroplasty.West J Emerg Med. 2014:15(1):76-80.
- Davenport M. Joint reduction, hip dislocation, posterior. Medscape Web site. eMedicine.medscape.com/article/109225. Updated February 11, 2014. Accessed January 27, 2015.
- Steele MT, Stubbs AM. Hip and femur injuries. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1848-1856.
Reference (Traumatic Back Pain)
- Baron BJ, McSherry KJ, Larson JL, Scalea TM. Spinal and spinal cord trauma In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1709-1730.
(Hip-Reduction Problem)
- Dela Cruz JE, Sullivan DN, Varboncouer E, et al. Comparison of proceduralsedation for the reduction of dislocated total hip arthroplasty.West J Emerg Med. 2014:15(1):76-80.
- Davenport M. Joint reduction, hip dislocation, posterior. Medscape Web site. eMedicine.medscape.com/article/109225. Updated February 11, 2014. Accessed January 27, 2015.
- Steele MT, Stubbs AM. Hip and femur injuries. In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine—A Comprehensive Study Guide. 7th ed. New York: NY: McGraw Hill Medical; 2011:1848-1856.
Transoral fundoplication can be effective against GERD symptoms
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Transoral esophagogastric fundoplication can be an effective treatment for patients seeking to alleviate symptoms associated with gastroesophageal reflux disease, particularly in individuals with persistent regurgitation despite prior treatment with proton pump inhibitor therapy, according to the results of a new study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.009).
“Gastroesophageal reflux disease (GERD) remains one of the most common conditions for which Americans take daily medication, and PPI use has more than doubled in the last decade,” wrote lead authors Dr. John G. Hunter of Oregon Health & Science University in Portland, and Dr. Peter J. Kahrilas of Northwestern University in Chicago, and their associates. “Despite this, up to 40% of proton pump inhibitor (PPI)–dependent GERD patients have troublesome symptoms of GERD, despite PPI therapy.”
In the Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial, investigators screened 696 patients who were experiencing “troublesome regurgitation” despite daily PPI treatment. These subjects were evaluated via three validated GERD-specific symptom scales, and were either on or off PPI use at the time of trial commencement. Post trial, patients were blinded to therapy and were reassessed at intervals of 2, 12, and 26 weeks. All patients underwent 48-hour esophageal pH monitoring and esophagogastroduodenoscopy at 66 months after the trial ended.
Regurgitation severity was based on the Montreal definition, which was used to measure efficacy of treatments given as part of the study. The Montreal definition of reflux is described by the authors as “either mucosal damage or troublesome symptoms attributable to reflux.” Those with “least troublesome” regurgitation while on PPIs “underwent barium swallow, esophagogastroduodenoscopy, 48-hour esophageal pH monitoring (off PPIs), and high-resolution esophageal manometry analyses.”
Eighty-seven subjects with GERD and hiatal hernias of at least 2 centimeters were randomly assigned to groups that underwent transoral fundoplication (TF) followed by placebo treatment after 6 months, while 42 subjects, who made up the control group, underwent a “sham surgery” and began regimens of once- or twice-daily omeprazole medication for 6 months.
Results showed that 67% of patients who received TF treatment experienced elimination of adverse regurgitation vs. 45% of those treated with PPI (P = .023). Control of esophageal pH also improved noticeably in patients who received TF treatment versus those who did not (9.3% vs. 6.3% on average, respectively, P < .001), but not in patients who received the “sham surgery” (8.6% preop vs. 8.9% postop on average). Fewer patients who received TF treatment recorded having “no response” after 3 months compared with those in the control group (11% vs. 36%, respectively, P = .004).
“Transoral fundoplication may fill the ‘therapeutic gap’ that exists between PPI and laparoscopic fundoplication,” wrote the authors. “Considering the virtual absence of dysphagia and bloating after TF, which may be problematic with LINX [LINX Reflux Management System], it would appear that TF is an option for patients with troublesome regurgitation, as well as for patients with troublesome GERD symptoms who wish not to take PPI over a protracted period of time.”
Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Transoral esophagogastric fundoplication (TF) is an effective treatment for gastroesophageal reflux disease symptoms, particularly in patients with persistent regurgitation despite proton pump inhibitor therapy (PPI).
Major finding: Of patients who received TF, 67% experienced elimination of adverse regurgitation, compared with 45% of those treated with PPI (P = .023).
Data source: Randomized EsophyX vs Sham, Placebo-Controlled Transoral Fundoplication (RESPECT) trial.
Disclosures: Several coauthors disclosed ties with the study sponsor EndoGastric Solutions of Redmond, Wash., as well as individual potential conflicts of interest.
Should patients stop taking aspirin for primary prevention?
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
Syncope from a twiddled ICD
A 61-year-old man with ischemic cardiomyopathy underwent implantation of a cardioverter-defibrillator (ICD) in a left subpectoral pocket. Placement was confirmed with chest radiography (Figure 1). Six weeks later, he presented to the emergency department reporting two episodes of syncope within the previous 24 hours.
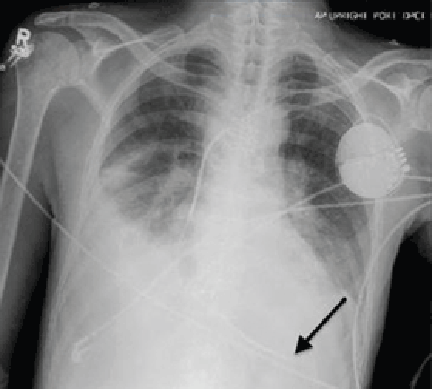
Electrocardiography at the time of presentation showed a normal sinus rhythm with inappropriate ICD discharges (Figure 2). This finding prompted chest radiography, which showed that the ICD had rotated 90 degrees from its original position, and that the lead had completely wrapped around the generator and thus was no longer correctly positioned (Figure 3). The patient admitted to frequently rubbing (ie, twiddling) the area, which led to twisting and dislocation of the device, a complication known as twiddler syndrome.
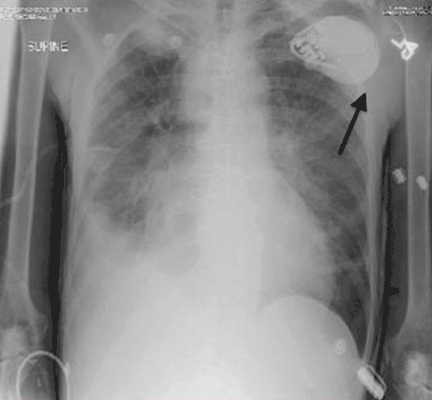
Twiddler syndrome is a rare complication of ICD placement, occurring in only about 0.1% of cases.1 It is usually a result of intentional manipulation (ie, twiddling) of the device by the patient, causing the ICD and the leads to dislodge, break, or retract. It is seen more often in women and in patients with cognitive dysfunction or psychiatric illness, in obese patients, and in patients with laxity of subcutaneous tissues, such as elderly people or people with accelerated weight loss. Placement of the ICD in an inappropriately large pocket predisposes to device rotation.2,3
Twiddler syndrome may cause mild discomfort or may even go unnoticed by the patient.4 In rare cases, it may lead to stimulation of the phrenic nerve, causing diaphragmatic pacing, or to stimulation of the brachial plexus, causing muscle twitching.2,3 However, malfunction of an ICD is life-threatening and requires immediate repair and replacement of the device. Educating the patient about the risks of twiddling the device site and the importance of periodic device checks is imperative to prevent this syndrome and ensure its early identification if it should occur. The patient should understand that dislodging the ICD can make it unable to sense abnormal rhythms and cause the device to deliver inappropriate shocks2,3,5 or stop delivering shocks altogether.
HOW IT IS TREATED
Treatment involves replacing the leads and affixing the device with sutures in the existing pocket or in a new pocket. The subpectoral position is preferred as it is more secure,3 although subcutaneous reimplantation has been successful.1 The device may also be placed in a fabric pouch to help lower the risk of migration or manipulation.3,6
In this case, because of the patient’s slender body habitus (body mass index 13 kg/m2), the ICD was removed from the subpectoral pocket and a new ICD was sutured into a subcutaneous pocket. The single lead was secured to the sternum and fascia using nonabsorbable sutures and a tie-down sleeve, making the device less susceptible to dislodgement by twiddling. Electrocardiography after replacement showed a normal sinus rhythm (Figure 4).
- Constandse J, Smit JJ, Ramdat Misier AR, Elvan A, Delnoy PP. Unusual twiddler syndrome: movement ties the knot. Neth Heart J 2013; 21:253–254.
- Spencker S, Poppelbaum A, Müller D. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008; 129:e24–e26.
- Benezet-Mazuecos J, Benezet J, Ortega-Carnicer J. Pacemaker twiddler syndrome. Eur Heart J 2007; 28:2000.
- Chemello D, Subramanian A, Cameron D. Twiddler syndrome with 180 degrees rotation of an implantable cardioverter defibrillator generator resulting in malfunction of one of the shocking coils. Europace 2009; 11:1259.
- Garweg C, Alzand BS, Willems R. Twiddler syndrome causing an inappropriate implantable cardioverter-defibrillator shock. Eur Heart J 2014; 35:516.
- Parsonnet V, Bernstein AD, Neglia D, Omar A. The usefulness of a stretch-polyester pouch to encase implanted pacemakers and defibrillators. Pacing Clin Electrophysiol 1994; 17:2274–2278.
A 61-year-old man with ischemic cardiomyopathy underwent implantation of a cardioverter-defibrillator (ICD) in a left subpectoral pocket. Placement was confirmed with chest radiography (Figure 1). Six weeks later, he presented to the emergency department reporting two episodes of syncope within the previous 24 hours.

Electrocardiography at the time of presentation showed a normal sinus rhythm with inappropriate ICD discharges (Figure 2). This finding prompted chest radiography, which showed that the ICD had rotated 90 degrees from its original position, and that the lead had completely wrapped around the generator and thus was no longer correctly positioned (Figure 3). The patient admitted to frequently rubbing (ie, twiddling) the area, which led to twisting and dislocation of the device, a complication known as twiddler syndrome.


Twiddler syndrome is a rare complication of ICD placement, occurring in only about 0.1% of cases.1 It is usually a result of intentional manipulation (ie, twiddling) of the device by the patient, causing the ICD and the leads to dislodge, break, or retract. It is seen more often in women and in patients with cognitive dysfunction or psychiatric illness, in obese patients, and in patients with laxity of subcutaneous tissues, such as elderly people or people with accelerated weight loss. Placement of the ICD in an inappropriately large pocket predisposes to device rotation.2,3
Twiddler syndrome may cause mild discomfort or may even go unnoticed by the patient.4 In rare cases, it may lead to stimulation of the phrenic nerve, causing diaphragmatic pacing, or to stimulation of the brachial plexus, causing muscle twitching.2,3 However, malfunction of an ICD is life-threatening and requires immediate repair and replacement of the device. Educating the patient about the risks of twiddling the device site and the importance of periodic device checks is imperative to prevent this syndrome and ensure its early identification if it should occur. The patient should understand that dislodging the ICD can make it unable to sense abnormal rhythms and cause the device to deliver inappropriate shocks2,3,5 or stop delivering shocks altogether.
HOW IT IS TREATED
Treatment involves replacing the leads and affixing the device with sutures in the existing pocket or in a new pocket. The subpectoral position is preferred as it is more secure,3 although subcutaneous reimplantation has been successful.1 The device may also be placed in a fabric pouch to help lower the risk of migration or manipulation.3,6
In this case, because of the patient’s slender body habitus (body mass index 13 kg/m2), the ICD was removed from the subpectoral pocket and a new ICD was sutured into a subcutaneous pocket. The single lead was secured to the sternum and fascia using nonabsorbable sutures and a tie-down sleeve, making the device less susceptible to dislodgement by twiddling. Electrocardiography after replacement showed a normal sinus rhythm (Figure 4).

A 61-year-old man with ischemic cardiomyopathy underwent implantation of a cardioverter-defibrillator (ICD) in a left subpectoral pocket. Placement was confirmed with chest radiography (Figure 1). Six weeks later, he presented to the emergency department reporting two episodes of syncope within the previous 24 hours.

Electrocardiography at the time of presentation showed a normal sinus rhythm with inappropriate ICD discharges (Figure 2). This finding prompted chest radiography, which showed that the ICD had rotated 90 degrees from its original position, and that the lead had completely wrapped around the generator and thus was no longer correctly positioned (Figure 3). The patient admitted to frequently rubbing (ie, twiddling) the area, which led to twisting and dislocation of the device, a complication known as twiddler syndrome.


Twiddler syndrome is a rare complication of ICD placement, occurring in only about 0.1% of cases.1 It is usually a result of intentional manipulation (ie, twiddling) of the device by the patient, causing the ICD and the leads to dislodge, break, or retract. It is seen more often in women and in patients with cognitive dysfunction or psychiatric illness, in obese patients, and in patients with laxity of subcutaneous tissues, such as elderly people or people with accelerated weight loss. Placement of the ICD in an inappropriately large pocket predisposes to device rotation.2,3
Twiddler syndrome may cause mild discomfort or may even go unnoticed by the patient.4 In rare cases, it may lead to stimulation of the phrenic nerve, causing diaphragmatic pacing, or to stimulation of the brachial plexus, causing muscle twitching.2,3 However, malfunction of an ICD is life-threatening and requires immediate repair and replacement of the device. Educating the patient about the risks of twiddling the device site and the importance of periodic device checks is imperative to prevent this syndrome and ensure its early identification if it should occur. The patient should understand that dislodging the ICD can make it unable to sense abnormal rhythms and cause the device to deliver inappropriate shocks2,3,5 or stop delivering shocks altogether.
HOW IT IS TREATED
Treatment involves replacing the leads and affixing the device with sutures in the existing pocket or in a new pocket. The subpectoral position is preferred as it is more secure,3 although subcutaneous reimplantation has been successful.1 The device may also be placed in a fabric pouch to help lower the risk of migration or manipulation.3,6
In this case, because of the patient’s slender body habitus (body mass index 13 kg/m2), the ICD was removed from the subpectoral pocket and a new ICD was sutured into a subcutaneous pocket. The single lead was secured to the sternum and fascia using nonabsorbable sutures and a tie-down sleeve, making the device less susceptible to dislodgement by twiddling. Electrocardiography after replacement showed a normal sinus rhythm (Figure 4).

- Constandse J, Smit JJ, Ramdat Misier AR, Elvan A, Delnoy PP. Unusual twiddler syndrome: movement ties the knot. Neth Heart J 2013; 21:253–254.
- Spencker S, Poppelbaum A, Müller D. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008; 129:e24–e26.
- Benezet-Mazuecos J, Benezet J, Ortega-Carnicer J. Pacemaker twiddler syndrome. Eur Heart J 2007; 28:2000.
- Chemello D, Subramanian A, Cameron D. Twiddler syndrome with 180 degrees rotation of an implantable cardioverter defibrillator generator resulting in malfunction of one of the shocking coils. Europace 2009; 11:1259.
- Garweg C, Alzand BS, Willems R. Twiddler syndrome causing an inappropriate implantable cardioverter-defibrillator shock. Eur Heart J 2014; 35:516.
- Parsonnet V, Bernstein AD, Neglia D, Omar A. The usefulness of a stretch-polyester pouch to encase implanted pacemakers and defibrillators. Pacing Clin Electrophysiol 1994; 17:2274–2278.
- Constandse J, Smit JJ, Ramdat Misier AR, Elvan A, Delnoy PP. Unusual twiddler syndrome: movement ties the knot. Neth Heart J 2013; 21:253–254.
- Spencker S, Poppelbaum A, Müller D. An unusual cause of oversensing leading to inappropriate ICD discharges. Int J Cardiol 2008; 129:e24–e26.
- Benezet-Mazuecos J, Benezet J, Ortega-Carnicer J. Pacemaker twiddler syndrome. Eur Heart J 2007; 28:2000.
- Chemello D, Subramanian A, Cameron D. Twiddler syndrome with 180 degrees rotation of an implantable cardioverter defibrillator generator resulting in malfunction of one of the shocking coils. Europace 2009; 11:1259.
- Garweg C, Alzand BS, Willems R. Twiddler syndrome causing an inappropriate implantable cardioverter-defibrillator shock. Eur Heart J 2014; 35:516.
- Parsonnet V, Bernstein AD, Neglia D, Omar A. The usefulness of a stretch-polyester pouch to encase implanted pacemakers and defibrillators. Pacing Clin Electrophysiol 1994; 17:2274–2278.
The health care ‘iron triangle’ and the Patient Protection and Affordable Care Act
Health care economists have long understood that the Patient Protection and Affordable Care Act (PPACA) could never function as intended. The reasoning behind this bold statement is simple. The PPACA aspires toward an end point that no law, system, or intervention has been able to accomplish: breaking the health care “iron triangle.”
According to the concept of the health care iron triangle, health care is a tightly interlocked, self-reinforcing system of three vertices—access, quality, and cost—and improvement in two vertices necessarily results in a worsening in the third.1 Interventions in health care inherently require trade-offs, which prevent simultaneous improvement in all three components.
The PPACA is explicitly designed to disrupt this paradox, ambitiously aiming to increase access and improve quality while lowering costs.2 Emerging evidence suggests, however, that the practical implementation of the PPACA will trump its intended benefits. Though there are numerous ways in which the PPACA could paradoxically decrease access to care, lower the quality of care, or raise costs, the outcome is almost certain that the PPACA may bend—but will never break—the health care iron triangle.
CONSTRAINING ACCESS
The PPACA seeks to increase health care access through four mechanisms: mandating that virtually all Americans obtain health insurance or pay a tax; expanding Medicaid to individuals earning less than 138% of the federal poverty level; requiring employers who have 50 or more employees to provide adequate health insurance or pay a fine; and preventing insurers from denying coverage based on preexisting medical conditions.3 Of these initiatives, only preexisting coverage requirements are a guaranteed outcome of the PPACA’s efforts to improve access.
Young adults are historically underinsured, for several reasons: they are generally in good health, tolerate greater risk, have higher unemployment levels, and are less likely to be able to afford insurance on an open market.4 With the threat of being denied insurance on the basis of preexisting conditions eliminated, this demographic may elect to pay a penalty and forgo insurance until it is needed. This not only decreases the number of insured Americans, but also deprives insurers of low-cost consumers that subsidize higher users, thus raising premiums and forcing participants out of private markets.
In 2012, the US Supreme Court largely upheld the PPACA, except that states retain jurisdiction over the decision to expand Medicaid. Nearly half of the states will keep their Medicaid programs as they are, for reasons ranging from financial (states bear 10% of the cost of this new population beginning in 2020) to ideological (partisan dislike of the PPACA).5 Irrespective of the rationale for nonexpansion, millions of Americans will not have access to Medicaid as written in the PPACA.
Employers, mindful of the expenses they face as a result of the law, may shield their financial liabilities as health insurance providers. At present, approximately half of all Americans obtain insurance through an employer, though that proportion could diminish if employers reorganize their businesses to avoid PPACA requirements.6 For example, businesses with fewer than 50 employees are exempt from offering insurance and could restrict payroll size to 49 employees or fewer to avoid the $2,000 penalty. Since the employer mandate of the PPACA only applies to full-time employees—defined as those working at least 30 hours a week—larger employers may switch hiring patterns toward more part-time employees. The nonpartisan Congressional Budget Office (CBO) recognizes this phenomenon and projects that the number of total hours worked in the United States will decline between 1.5% and 2% through 2024 as a result of PPACA implementation. Ultimately, the decline in full-time employment resulting from the PPACA will lead to “some people not being employed at all and other people working fewer hours” and will disproportionately impact “lower-wage workers.”7
The CBO analysis predicts that the equivalent of 2 to 2.5 million full-time jobs will be lost as a result of the PPACA’s implementation over the next 10 years. Employers and employees responding to financial disincentives perpetuate a cycle in which increased rates of unemployment and underemployment lead not only to fewer insured Americans, but also to fewer Americans insured by their employers.8
DIMINISHED QUALITY
If the PPACA improves access at constrained cost, quality of care may suffer from the increased strain on the most finite (and most demanded) resource in health care—a provider’s time. Much as a car factory that increases production without appropriate expansion may turn out poorer quality vehicles, tasking a finite number of providers with caring for more patients may lead to poorer patient care. Not only has the PPACA increased the number of patients seeking care, it also has increased the administrative components of practicing medicine. Both outcomes lead to delays in care and increased out-of-pocket expenditures for patients.9
The PPACA also fails to address the mismatch between the supply of physicians and the increased demand for their services. First, the law provides no new funding for training or expanding the physician workforce. Second, the PPACA may expedite the retirement of physicians daunted by changes in the new health care environment, thus decreasing both patient and peer access to those with a career’s worth of knowledge.10 Adding insult to injury, the known shortage of primary care physicians (estimated to exceed 25,000 before the PPACA’s enactment) is predicted to worsen by an estimated 5,000 because of increased demand, further stretching an already thin workforce.11
Patients may also experience a decrease in quality if their access to the best health care is in name only. There is no requirement that providers accept the insurance plans of those who gain coverage through the PPACA.12 This is particularly relevant to the 11 million individuals projected to obtain coverage through Medicaid, as existing Medicaid participants routinely confront access issues when they need to see a specialist or, increasingly, a primary care provider.13
Quality declines if a change in insurance fails to cover existing necessary benefits or provides those benefits at increased cost. Federal taxing of “Cadillac” insurance plans, employers offering relatively less-generous coverage plans, and individuals opting for lower-tiered (eg, “bronze” or “silver”) plans in the health insurance marketplace when previously insured under higher-tiered (“gold” or “platinum”) plans all either diminish quality by decreasing the breadth of coverage or make obtaining coverage more expensive.14,15
RISING COSTS
The PPACA is hardly an unfunded mandate. The federal government estimates spending $1.168 billion over 10 years on the insurance coverage provisions of the Act.13 While Congress’ pay-as-you-go rules require the PPACA to reduce federal expenditures, states (through new Medicaid enrollees) and individuals (through individual mandate penalties and the aforementioned “Cadillac” tax) will confront higher net costs.16–18
Early indicators suggest that implementing the cost-reducing portions of the law may not be as feasible as intended. In a recent pilot of the PPACA’s accountable care organization concept, 32 organizations participated in the Pioneering Accountable Care Organization Model. While the Center for Medicare and Medicaid Services says that 13 of these organizations produced savings of $87.6 million in 2012, overall costs for these participants still increased 0.3% (albeit less than the 0.8% growth observed outside the model).19 Additionally, 7 organizations intend to switch out of the Pioneering model to a program in which they bear less financial responsibility, and 2 will leave the program altogether, suggesting that health systems are hesitant about care-management models that threaten a financial bottom line.
The recent decision to delay the employer mandate by 1 year will result in $12 billion of lost tax revenue and additional charges, largely through the loss of $10 billion in penalties to employers.20 Out-of-pocket spending caps on deductibles and copayments, due to take effect in 2014, were also pushed back 1 year, which will increase costs for some with expensive or chronic illnesses.21 The medical device tax is a similarly unpopular (but revenue-generating) component that could yield to political pressure, further increasing the cost of the PPACA.22 And it remains to be seen whether the Independent Payment Advisory Board, which has theoretical control over expenditures for the sickest patients, will retain the authority to rein in costs.
AS IRONCLAD AS EVER
The PPACA is a game-changing law, one that will revolutionize the practice and delivery of health care. Some argue that its implementation has already succeeded in bending the cost curve (ie, reducing the rate of health care expenditures), though critics counter that the reduction may have been a byproduct of the Great Recession and did not actually lower costs.23 Others contend that the PPACA is responsible for a renewed interest in practice redesign and rethinking of the ways in which medicine is delivered. While interest in reducing costs appears to be at an all-time high, and while such enthusiasm may succeed in reducing per capita costs of care, a long-term absolute reduction in the amount spent on care as a result of these efforts will remain conspicuously absent.
The reality remains that the PPACA is an ambitious law that cannot overcome economic realities. Almost certainly, it will succeed in decreasing the number of uninsured Americans, who have two new avenues to obtain insurance: Medicaid expansion and the health insurance marketplace. Both can absorb applicants who lose employer-subsidized insurance plans. In addition, patients, providers, and politicians will readily reject compromises to quality. While the permutations of potential threats are nearly infinite, any observed decrease in the quality of care resulting from the PPACA will prompt brisk legislative action by lawmakers to rectify perceived deficiencies.
To assuage short-term concerns about access and quality, the path of least resistance will be to delay cost-containing measures and to spend money to remedy perceived deficiencies of the PPACA. Such delays have already occurred—as seen with the spending caps on deductibles and copays—and may potentially be extended to the individual mandate itself. Given lawmakers’ well-documented inability to constrain the powers of the purse, the Achilles’ heel of the PPACA will be a never-ending spiral of rising costs. The health care iron triangle remains as ironclad as ever.
Acknowledgment: The author would like to recognize Devdutta Sangvai, MD, MBA, for his assistance in reviewing this manuscript, as well as his work as associate program director of the Management and Leadership Pathway for Residents training program.
- Kissick WL. The past is prologue, in medicine’s dilemmas: infinite needs versus finite resources. New Haven, CT: Yale University Press; 1994.
- US Department of Health and Human Services. Key features of the Affordable Care Act by year. www.hhs.gov/healthcare/facts/timeline/timeline-text.html. Accessed December 2, 2014.
- US Government Printing Office. Public Law 111-148. The Patient Protection and Affordable Care Act. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf. Accessed December 2, 2014.
- The Commonwealth Fund. Young, uninsured, and in debt: why young adults lack health insurance and how the affordable care act is helping—Findings from the Commonwealth Fund Health Insurance Tracking Survey of Young Adults, 2011. www.commonwealthfund.org/publications/issue-briefs/2012/jun/young-adults-2012. Accessed December 2, 2014.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision, 2014. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Accessed December 2, 2014.
- United States Census Bureau. Employment-based health insurance: 2010. www.census.gov/prod/2013pubs/p70-134.pdf. Accessed December 2, 2014.
- Congressional Budget Office. The budget and economic outlook: 2014 to 2024. www.cbo.gov/sites/default/files/cbofiles/attachments/45010-breakout-AppendixC.pdf. Accessed December 3, 2014.
- Review & outlook: ObamaCare and the ‘29ers.’ The Wall Street Journal. February 26, 2013. http://online.wsj.com/news/articles/SB10001424127887324616604578304072420873666. Accessed December 2, 2014.
- Gold J. Kaiser Health News. New ACA insurance causes headaches in some doctors’ offices. www.kaiserhealthnews.org/stories/2014/february/25/new-aca-insurance-causes-headaches-in-some-doctors-offices.aspx. Accessed December 2, 2014.
- Deloitte Center for Health Solutions. Deloitte 2013 survey of US physicians: physician perspectives about health care reform and the future of the medical profession. http://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-deloitte-2013-physician-survey-10012014.pdf. Accessed December 2, 2014.
- Howard P, Feyman Y. Rhetoric and reality. The Obamacare evaluation project: access to care and the physician shortage. www.manhattan-institute.org/pdf/mpr_15.pdf. Accessed December 2, 2014.
- Ollove M. Kaiser Health News. Are there enough doctors for the newly insured? www.kaiserhealthnews.org/Stories/2014/January/03/doctor-shortage-primary-care-specialist.aspx. Accessed December 2, 2014.
- Congressional Budget Office. Estimates for the insurance coverage provisions of the Affordable Care Act updated for the recent Supreme Court decision. www.cbo.gov/sites/default/files/cbofiles/attachments/43472-07-24-2012-CoverageEstimates.pdf. Accessed December 2, 2014.
- Health Policy Briefs. Excise tax on “Cadillac” plans. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=99. Accessed December 2, 2014.
- McKinsey Center for US Health Care Reform. Exchanges go live: early trends in exchange dynamics. www.mckinsey.com/~/media/McKinsey/dotcom/client_service/Healthcare%20Systems%20and%20Services/PDFs/Exchanges_Go_Live_Early_Trends_in_Exchange_Filings_October_2013_FINAL.ashx. Accessed December 2, 2014.
- Elmendorf DW. Letter to the Honorable Harry Reid. www.cbo.gov/sites/default/files/cbofiles/ftpdocs/113xx/doc11307/reid_letter_hr3590.pdf. Accessed December 2, 2014.
- Deloitte Center for Health Solutions. The fiscal impact to states of the Affordable Care Act: comprehensive analysis. http://www.statecoverage.org/files/DeloitteFisca_ImpacttoStatesACA.pdf. Accessed December 2, 2014.
- Congressional Budget Office. CBO releases updated estimates for the insurance coverage provisions of the Affordable Care Act. www.cbo.gov/publication/43080. Accessed December 2, 2014.
- Centers for Medicare & Medicaid Services. Pioneer accountable care organizations succeed in improving care, lowering costs. www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-07-16.html. Accessed December 2, 2014.
- Congressional Budget Office. Analysis of the administration’s announced delay of certain requirements under the Affordable Care Act. www.cbo.gov/publication/44465. Accessed December 2, 2014.
- Pear R. A limit on consumer costs is delayed in health care law. The New York Times. August 13, 2013. www.nytimes.com/2013/08/13/us/a-limit-on-consumer-costs-is-delayed-in-health-care-law.html?pagewanted=all&_r=0. Accessed December 2, 2014.
- Rubin R, Hunter K. Republicans push medical-device tax in US Senate. Bloomberg. May 13, 2014. www.bloomberg.com/news/2014-05-14/republicans-push-medical-device-tax-repeal-in-u-s-senate.html. Accessed December 2, 2014.
- Blumenthal D, Stremikis K, Cutler D. Health care spending—a giant slain or sleeping? N Engl J Med 2013; 369:2551–2557.
Health care economists have long understood that the Patient Protection and Affordable Care Act (PPACA) could never function as intended. The reasoning behind this bold statement is simple. The PPACA aspires toward an end point that no law, system, or intervention has been able to accomplish: breaking the health care “iron triangle.”
According to the concept of the health care iron triangle, health care is a tightly interlocked, self-reinforcing system of three vertices—access, quality, and cost—and improvement in two vertices necessarily results in a worsening in the third.1 Interventions in health care inherently require trade-offs, which prevent simultaneous improvement in all three components.
The PPACA is explicitly designed to disrupt this paradox, ambitiously aiming to increase access and improve quality while lowering costs.2 Emerging evidence suggests, however, that the practical implementation of the PPACA will trump its intended benefits. Though there are numerous ways in which the PPACA could paradoxically decrease access to care, lower the quality of care, or raise costs, the outcome is almost certain that the PPACA may bend—but will never break—the health care iron triangle.
CONSTRAINING ACCESS
The PPACA seeks to increase health care access through four mechanisms: mandating that virtually all Americans obtain health insurance or pay a tax; expanding Medicaid to individuals earning less than 138% of the federal poverty level; requiring employers who have 50 or more employees to provide adequate health insurance or pay a fine; and preventing insurers from denying coverage based on preexisting medical conditions.3 Of these initiatives, only preexisting coverage requirements are a guaranteed outcome of the PPACA’s efforts to improve access.
Young adults are historically underinsured, for several reasons: they are generally in good health, tolerate greater risk, have higher unemployment levels, and are less likely to be able to afford insurance on an open market.4 With the threat of being denied insurance on the basis of preexisting conditions eliminated, this demographic may elect to pay a penalty and forgo insurance until it is needed. This not only decreases the number of insured Americans, but also deprives insurers of low-cost consumers that subsidize higher users, thus raising premiums and forcing participants out of private markets.
In 2012, the US Supreme Court largely upheld the PPACA, except that states retain jurisdiction over the decision to expand Medicaid. Nearly half of the states will keep their Medicaid programs as they are, for reasons ranging from financial (states bear 10% of the cost of this new population beginning in 2020) to ideological (partisan dislike of the PPACA).5 Irrespective of the rationale for nonexpansion, millions of Americans will not have access to Medicaid as written in the PPACA.
Employers, mindful of the expenses they face as a result of the law, may shield their financial liabilities as health insurance providers. At present, approximately half of all Americans obtain insurance through an employer, though that proportion could diminish if employers reorganize their businesses to avoid PPACA requirements.6 For example, businesses with fewer than 50 employees are exempt from offering insurance and could restrict payroll size to 49 employees or fewer to avoid the $2,000 penalty. Since the employer mandate of the PPACA only applies to full-time employees—defined as those working at least 30 hours a week—larger employers may switch hiring patterns toward more part-time employees. The nonpartisan Congressional Budget Office (CBO) recognizes this phenomenon and projects that the number of total hours worked in the United States will decline between 1.5% and 2% through 2024 as a result of PPACA implementation. Ultimately, the decline in full-time employment resulting from the PPACA will lead to “some people not being employed at all and other people working fewer hours” and will disproportionately impact “lower-wage workers.”7
The CBO analysis predicts that the equivalent of 2 to 2.5 million full-time jobs will be lost as a result of the PPACA’s implementation over the next 10 years. Employers and employees responding to financial disincentives perpetuate a cycle in which increased rates of unemployment and underemployment lead not only to fewer insured Americans, but also to fewer Americans insured by their employers.8
DIMINISHED QUALITY
If the PPACA improves access at constrained cost, quality of care may suffer from the increased strain on the most finite (and most demanded) resource in health care—a provider’s time. Much as a car factory that increases production without appropriate expansion may turn out poorer quality vehicles, tasking a finite number of providers with caring for more patients may lead to poorer patient care. Not only has the PPACA increased the number of patients seeking care, it also has increased the administrative components of practicing medicine. Both outcomes lead to delays in care and increased out-of-pocket expenditures for patients.9
The PPACA also fails to address the mismatch between the supply of physicians and the increased demand for their services. First, the law provides no new funding for training or expanding the physician workforce. Second, the PPACA may expedite the retirement of physicians daunted by changes in the new health care environment, thus decreasing both patient and peer access to those with a career’s worth of knowledge.10 Adding insult to injury, the known shortage of primary care physicians (estimated to exceed 25,000 before the PPACA’s enactment) is predicted to worsen by an estimated 5,000 because of increased demand, further stretching an already thin workforce.11
Patients may also experience a decrease in quality if their access to the best health care is in name only. There is no requirement that providers accept the insurance plans of those who gain coverage through the PPACA.12 This is particularly relevant to the 11 million individuals projected to obtain coverage through Medicaid, as existing Medicaid participants routinely confront access issues when they need to see a specialist or, increasingly, a primary care provider.13
Quality declines if a change in insurance fails to cover existing necessary benefits or provides those benefits at increased cost. Federal taxing of “Cadillac” insurance plans, employers offering relatively less-generous coverage plans, and individuals opting for lower-tiered (eg, “bronze” or “silver”) plans in the health insurance marketplace when previously insured under higher-tiered (“gold” or “platinum”) plans all either diminish quality by decreasing the breadth of coverage or make obtaining coverage more expensive.14,15
RISING COSTS
The PPACA is hardly an unfunded mandate. The federal government estimates spending $1.168 billion over 10 years on the insurance coverage provisions of the Act.13 While Congress’ pay-as-you-go rules require the PPACA to reduce federal expenditures, states (through new Medicaid enrollees) and individuals (through individual mandate penalties and the aforementioned “Cadillac” tax) will confront higher net costs.16–18
Early indicators suggest that implementing the cost-reducing portions of the law may not be as feasible as intended. In a recent pilot of the PPACA’s accountable care organization concept, 32 organizations participated in the Pioneering Accountable Care Organization Model. While the Center for Medicare and Medicaid Services says that 13 of these organizations produced savings of $87.6 million in 2012, overall costs for these participants still increased 0.3% (albeit less than the 0.8% growth observed outside the model).19 Additionally, 7 organizations intend to switch out of the Pioneering model to a program in which they bear less financial responsibility, and 2 will leave the program altogether, suggesting that health systems are hesitant about care-management models that threaten a financial bottom line.
The recent decision to delay the employer mandate by 1 year will result in $12 billion of lost tax revenue and additional charges, largely through the loss of $10 billion in penalties to employers.20 Out-of-pocket spending caps on deductibles and copayments, due to take effect in 2014, were also pushed back 1 year, which will increase costs for some with expensive or chronic illnesses.21 The medical device tax is a similarly unpopular (but revenue-generating) component that could yield to political pressure, further increasing the cost of the PPACA.22 And it remains to be seen whether the Independent Payment Advisory Board, which has theoretical control over expenditures for the sickest patients, will retain the authority to rein in costs.
AS IRONCLAD AS EVER
The PPACA is a game-changing law, one that will revolutionize the practice and delivery of health care. Some argue that its implementation has already succeeded in bending the cost curve (ie, reducing the rate of health care expenditures), though critics counter that the reduction may have been a byproduct of the Great Recession and did not actually lower costs.23 Others contend that the PPACA is responsible for a renewed interest in practice redesign and rethinking of the ways in which medicine is delivered. While interest in reducing costs appears to be at an all-time high, and while such enthusiasm may succeed in reducing per capita costs of care, a long-term absolute reduction in the amount spent on care as a result of these efforts will remain conspicuously absent.
The reality remains that the PPACA is an ambitious law that cannot overcome economic realities. Almost certainly, it will succeed in decreasing the number of uninsured Americans, who have two new avenues to obtain insurance: Medicaid expansion and the health insurance marketplace. Both can absorb applicants who lose employer-subsidized insurance plans. In addition, patients, providers, and politicians will readily reject compromises to quality. While the permutations of potential threats are nearly infinite, any observed decrease in the quality of care resulting from the PPACA will prompt brisk legislative action by lawmakers to rectify perceived deficiencies.
To assuage short-term concerns about access and quality, the path of least resistance will be to delay cost-containing measures and to spend money to remedy perceived deficiencies of the PPACA. Such delays have already occurred—as seen with the spending caps on deductibles and copays—and may potentially be extended to the individual mandate itself. Given lawmakers’ well-documented inability to constrain the powers of the purse, the Achilles’ heel of the PPACA will be a never-ending spiral of rising costs. The health care iron triangle remains as ironclad as ever.
Acknowledgment: The author would like to recognize Devdutta Sangvai, MD, MBA, for his assistance in reviewing this manuscript, as well as his work as associate program director of the Management and Leadership Pathway for Residents training program.
Health care economists have long understood that the Patient Protection and Affordable Care Act (PPACA) could never function as intended. The reasoning behind this bold statement is simple. The PPACA aspires toward an end point that no law, system, or intervention has been able to accomplish: breaking the health care “iron triangle.”
According to the concept of the health care iron triangle, health care is a tightly interlocked, self-reinforcing system of three vertices—access, quality, and cost—and improvement in two vertices necessarily results in a worsening in the third.1 Interventions in health care inherently require trade-offs, which prevent simultaneous improvement in all three components.
The PPACA is explicitly designed to disrupt this paradox, ambitiously aiming to increase access and improve quality while lowering costs.2 Emerging evidence suggests, however, that the practical implementation of the PPACA will trump its intended benefits. Though there are numerous ways in which the PPACA could paradoxically decrease access to care, lower the quality of care, or raise costs, the outcome is almost certain that the PPACA may bend—but will never break—the health care iron triangle.
CONSTRAINING ACCESS
The PPACA seeks to increase health care access through four mechanisms: mandating that virtually all Americans obtain health insurance or pay a tax; expanding Medicaid to individuals earning less than 138% of the federal poverty level; requiring employers who have 50 or more employees to provide adequate health insurance or pay a fine; and preventing insurers from denying coverage based on preexisting medical conditions.3 Of these initiatives, only preexisting coverage requirements are a guaranteed outcome of the PPACA’s efforts to improve access.
Young adults are historically underinsured, for several reasons: they are generally in good health, tolerate greater risk, have higher unemployment levels, and are less likely to be able to afford insurance on an open market.4 With the threat of being denied insurance on the basis of preexisting conditions eliminated, this demographic may elect to pay a penalty and forgo insurance until it is needed. This not only decreases the number of insured Americans, but also deprives insurers of low-cost consumers that subsidize higher users, thus raising premiums and forcing participants out of private markets.
In 2012, the US Supreme Court largely upheld the PPACA, except that states retain jurisdiction over the decision to expand Medicaid. Nearly half of the states will keep their Medicaid programs as they are, for reasons ranging from financial (states bear 10% of the cost of this new population beginning in 2020) to ideological (partisan dislike of the PPACA).5 Irrespective of the rationale for nonexpansion, millions of Americans will not have access to Medicaid as written in the PPACA.
Employers, mindful of the expenses they face as a result of the law, may shield their financial liabilities as health insurance providers. At present, approximately half of all Americans obtain insurance through an employer, though that proportion could diminish if employers reorganize their businesses to avoid PPACA requirements.6 For example, businesses with fewer than 50 employees are exempt from offering insurance and could restrict payroll size to 49 employees or fewer to avoid the $2,000 penalty. Since the employer mandate of the PPACA only applies to full-time employees—defined as those working at least 30 hours a week—larger employers may switch hiring patterns toward more part-time employees. The nonpartisan Congressional Budget Office (CBO) recognizes this phenomenon and projects that the number of total hours worked in the United States will decline between 1.5% and 2% through 2024 as a result of PPACA implementation. Ultimately, the decline in full-time employment resulting from the PPACA will lead to “some people not being employed at all and other people working fewer hours” and will disproportionately impact “lower-wage workers.”7
The CBO analysis predicts that the equivalent of 2 to 2.5 million full-time jobs will be lost as a result of the PPACA’s implementation over the next 10 years. Employers and employees responding to financial disincentives perpetuate a cycle in which increased rates of unemployment and underemployment lead not only to fewer insured Americans, but also to fewer Americans insured by their employers.8
DIMINISHED QUALITY
If the PPACA improves access at constrained cost, quality of care may suffer from the increased strain on the most finite (and most demanded) resource in health care—a provider’s time. Much as a car factory that increases production without appropriate expansion may turn out poorer quality vehicles, tasking a finite number of providers with caring for more patients may lead to poorer patient care. Not only has the PPACA increased the number of patients seeking care, it also has increased the administrative components of practicing medicine. Both outcomes lead to delays in care and increased out-of-pocket expenditures for patients.9
The PPACA also fails to address the mismatch between the supply of physicians and the increased demand for their services. First, the law provides no new funding for training or expanding the physician workforce. Second, the PPACA may expedite the retirement of physicians daunted by changes in the new health care environment, thus decreasing both patient and peer access to those with a career’s worth of knowledge.10 Adding insult to injury, the known shortage of primary care physicians (estimated to exceed 25,000 before the PPACA’s enactment) is predicted to worsen by an estimated 5,000 because of increased demand, further stretching an already thin workforce.11
Patients may also experience a decrease in quality if their access to the best health care is in name only. There is no requirement that providers accept the insurance plans of those who gain coverage through the PPACA.12 This is particularly relevant to the 11 million individuals projected to obtain coverage through Medicaid, as existing Medicaid participants routinely confront access issues when they need to see a specialist or, increasingly, a primary care provider.13
Quality declines if a change in insurance fails to cover existing necessary benefits or provides those benefits at increased cost. Federal taxing of “Cadillac” insurance plans, employers offering relatively less-generous coverage plans, and individuals opting for lower-tiered (eg, “bronze” or “silver”) plans in the health insurance marketplace when previously insured under higher-tiered (“gold” or “platinum”) plans all either diminish quality by decreasing the breadth of coverage or make obtaining coverage more expensive.14,15
RISING COSTS
The PPACA is hardly an unfunded mandate. The federal government estimates spending $1.168 billion over 10 years on the insurance coverage provisions of the Act.13 While Congress’ pay-as-you-go rules require the PPACA to reduce federal expenditures, states (through new Medicaid enrollees) and individuals (through individual mandate penalties and the aforementioned “Cadillac” tax) will confront higher net costs.16–18
Early indicators suggest that implementing the cost-reducing portions of the law may not be as feasible as intended. In a recent pilot of the PPACA’s accountable care organization concept, 32 organizations participated in the Pioneering Accountable Care Organization Model. While the Center for Medicare and Medicaid Services says that 13 of these organizations produced savings of $87.6 million in 2012, overall costs for these participants still increased 0.3% (albeit less than the 0.8% growth observed outside the model).19 Additionally, 7 organizations intend to switch out of the Pioneering model to a program in which they bear less financial responsibility, and 2 will leave the program altogether, suggesting that health systems are hesitant about care-management models that threaten a financial bottom line.
The recent decision to delay the employer mandate by 1 year will result in $12 billion of lost tax revenue and additional charges, largely through the loss of $10 billion in penalties to employers.20 Out-of-pocket spending caps on deductibles and copayments, due to take effect in 2014, were also pushed back 1 year, which will increase costs for some with expensive or chronic illnesses.21 The medical device tax is a similarly unpopular (but revenue-generating) component that could yield to political pressure, further increasing the cost of the PPACA.22 And it remains to be seen whether the Independent Payment Advisory Board, which has theoretical control over expenditures for the sickest patients, will retain the authority to rein in costs.
AS IRONCLAD AS EVER
The PPACA is a game-changing law, one that will revolutionize the practice and delivery of health care. Some argue that its implementation has already succeeded in bending the cost curve (ie, reducing the rate of health care expenditures), though critics counter that the reduction may have been a byproduct of the Great Recession and did not actually lower costs.23 Others contend that the PPACA is responsible for a renewed interest in practice redesign and rethinking of the ways in which medicine is delivered. While interest in reducing costs appears to be at an all-time high, and while such enthusiasm may succeed in reducing per capita costs of care, a long-term absolute reduction in the amount spent on care as a result of these efforts will remain conspicuously absent.
The reality remains that the PPACA is an ambitious law that cannot overcome economic realities. Almost certainly, it will succeed in decreasing the number of uninsured Americans, who have two new avenues to obtain insurance: Medicaid expansion and the health insurance marketplace. Both can absorb applicants who lose employer-subsidized insurance plans. In addition, patients, providers, and politicians will readily reject compromises to quality. While the permutations of potential threats are nearly infinite, any observed decrease in the quality of care resulting from the PPACA will prompt brisk legislative action by lawmakers to rectify perceived deficiencies.
To assuage short-term concerns about access and quality, the path of least resistance will be to delay cost-containing measures and to spend money to remedy perceived deficiencies of the PPACA. Such delays have already occurred—as seen with the spending caps on deductibles and copays—and may potentially be extended to the individual mandate itself. Given lawmakers’ well-documented inability to constrain the powers of the purse, the Achilles’ heel of the PPACA will be a never-ending spiral of rising costs. The health care iron triangle remains as ironclad as ever.
Acknowledgment: The author would like to recognize Devdutta Sangvai, MD, MBA, for his assistance in reviewing this manuscript, as well as his work as associate program director of the Management and Leadership Pathway for Residents training program.
- Kissick WL. The past is prologue, in medicine’s dilemmas: infinite needs versus finite resources. New Haven, CT: Yale University Press; 1994.
- US Department of Health and Human Services. Key features of the Affordable Care Act by year. www.hhs.gov/healthcare/facts/timeline/timeline-text.html. Accessed December 2, 2014.
- US Government Printing Office. Public Law 111-148. The Patient Protection and Affordable Care Act. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf. Accessed December 2, 2014.
- The Commonwealth Fund. Young, uninsured, and in debt: why young adults lack health insurance and how the affordable care act is helping—Findings from the Commonwealth Fund Health Insurance Tracking Survey of Young Adults, 2011. www.commonwealthfund.org/publications/issue-briefs/2012/jun/young-adults-2012. Accessed December 2, 2014.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision, 2014. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Accessed December 2, 2014.
- United States Census Bureau. Employment-based health insurance: 2010. www.census.gov/prod/2013pubs/p70-134.pdf. Accessed December 2, 2014.
- Congressional Budget Office. The budget and economic outlook: 2014 to 2024. www.cbo.gov/sites/default/files/cbofiles/attachments/45010-breakout-AppendixC.pdf. Accessed December 3, 2014.
- Review & outlook: ObamaCare and the ‘29ers.’ The Wall Street Journal. February 26, 2013. http://online.wsj.com/news/articles/SB10001424127887324616604578304072420873666. Accessed December 2, 2014.
- Gold J. Kaiser Health News. New ACA insurance causes headaches in some doctors’ offices. www.kaiserhealthnews.org/stories/2014/february/25/new-aca-insurance-causes-headaches-in-some-doctors-offices.aspx. Accessed December 2, 2014.
- Deloitte Center for Health Solutions. Deloitte 2013 survey of US physicians: physician perspectives about health care reform and the future of the medical profession. http://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-deloitte-2013-physician-survey-10012014.pdf. Accessed December 2, 2014.
- Howard P, Feyman Y. Rhetoric and reality. The Obamacare evaluation project: access to care and the physician shortage. www.manhattan-institute.org/pdf/mpr_15.pdf. Accessed December 2, 2014.
- Ollove M. Kaiser Health News. Are there enough doctors for the newly insured? www.kaiserhealthnews.org/Stories/2014/January/03/doctor-shortage-primary-care-specialist.aspx. Accessed December 2, 2014.
- Congressional Budget Office. Estimates for the insurance coverage provisions of the Affordable Care Act updated for the recent Supreme Court decision. www.cbo.gov/sites/default/files/cbofiles/attachments/43472-07-24-2012-CoverageEstimates.pdf. Accessed December 2, 2014.
- Health Policy Briefs. Excise tax on “Cadillac” plans. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=99. Accessed December 2, 2014.
- McKinsey Center for US Health Care Reform. Exchanges go live: early trends in exchange dynamics. www.mckinsey.com/~/media/McKinsey/dotcom/client_service/Healthcare%20Systems%20and%20Services/PDFs/Exchanges_Go_Live_Early_Trends_in_Exchange_Filings_October_2013_FINAL.ashx. Accessed December 2, 2014.
- Elmendorf DW. Letter to the Honorable Harry Reid. www.cbo.gov/sites/default/files/cbofiles/ftpdocs/113xx/doc11307/reid_letter_hr3590.pdf. Accessed December 2, 2014.
- Deloitte Center for Health Solutions. The fiscal impact to states of the Affordable Care Act: comprehensive analysis. http://www.statecoverage.org/files/DeloitteFisca_ImpacttoStatesACA.pdf. Accessed December 2, 2014.
- Congressional Budget Office. CBO releases updated estimates for the insurance coverage provisions of the Affordable Care Act. www.cbo.gov/publication/43080. Accessed December 2, 2014.
- Centers for Medicare & Medicaid Services. Pioneer accountable care organizations succeed in improving care, lowering costs. www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-07-16.html. Accessed December 2, 2014.
- Congressional Budget Office. Analysis of the administration’s announced delay of certain requirements under the Affordable Care Act. www.cbo.gov/publication/44465. Accessed December 2, 2014.
- Pear R. A limit on consumer costs is delayed in health care law. The New York Times. August 13, 2013. www.nytimes.com/2013/08/13/us/a-limit-on-consumer-costs-is-delayed-in-health-care-law.html?pagewanted=all&_r=0. Accessed December 2, 2014.
- Rubin R, Hunter K. Republicans push medical-device tax in US Senate. Bloomberg. May 13, 2014. www.bloomberg.com/news/2014-05-14/republicans-push-medical-device-tax-repeal-in-u-s-senate.html. Accessed December 2, 2014.
- Blumenthal D, Stremikis K, Cutler D. Health care spending—a giant slain or sleeping? N Engl J Med 2013; 369:2551–2557.
- Kissick WL. The past is prologue, in medicine’s dilemmas: infinite needs versus finite resources. New Haven, CT: Yale University Press; 1994.
- US Department of Health and Human Services. Key features of the Affordable Care Act by year. www.hhs.gov/healthcare/facts/timeline/timeline-text.html. Accessed December 2, 2014.
- US Government Printing Office. Public Law 111-148. The Patient Protection and Affordable Care Act. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf. Accessed December 2, 2014.
- The Commonwealth Fund. Young, uninsured, and in debt: why young adults lack health insurance and how the affordable care act is helping—Findings from the Commonwealth Fund Health Insurance Tracking Survey of Young Adults, 2011. www.commonwealthfund.org/publications/issue-briefs/2012/jun/young-adults-2012. Accessed December 2, 2014.
- The Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision, 2014. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Accessed December 2, 2014.
- United States Census Bureau. Employment-based health insurance: 2010. www.census.gov/prod/2013pubs/p70-134.pdf. Accessed December 2, 2014.
- Congressional Budget Office. The budget and economic outlook: 2014 to 2024. www.cbo.gov/sites/default/files/cbofiles/attachments/45010-breakout-AppendixC.pdf. Accessed December 3, 2014.
- Review & outlook: ObamaCare and the ‘29ers.’ The Wall Street Journal. February 26, 2013. http://online.wsj.com/news/articles/SB10001424127887324616604578304072420873666. Accessed December 2, 2014.
- Gold J. Kaiser Health News. New ACA insurance causes headaches in some doctors’ offices. www.kaiserhealthnews.org/stories/2014/february/25/new-aca-insurance-causes-headaches-in-some-doctors-offices.aspx. Accessed December 2, 2014.
- Deloitte Center for Health Solutions. Deloitte 2013 survey of US physicians: physician perspectives about health care reform and the future of the medical profession. http://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-deloitte-2013-physician-survey-10012014.pdf. Accessed December 2, 2014.
- Howard P, Feyman Y. Rhetoric and reality. The Obamacare evaluation project: access to care and the physician shortage. www.manhattan-institute.org/pdf/mpr_15.pdf. Accessed December 2, 2014.
- Ollove M. Kaiser Health News. Are there enough doctors for the newly insured? www.kaiserhealthnews.org/Stories/2014/January/03/doctor-shortage-primary-care-specialist.aspx. Accessed December 2, 2014.
- Congressional Budget Office. Estimates for the insurance coverage provisions of the Affordable Care Act updated for the recent Supreme Court decision. www.cbo.gov/sites/default/files/cbofiles/attachments/43472-07-24-2012-CoverageEstimates.pdf. Accessed December 2, 2014.
- Health Policy Briefs. Excise tax on “Cadillac” plans. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=99. Accessed December 2, 2014.
- McKinsey Center for US Health Care Reform. Exchanges go live: early trends in exchange dynamics. www.mckinsey.com/~/media/McKinsey/dotcom/client_service/Healthcare%20Systems%20and%20Services/PDFs/Exchanges_Go_Live_Early_Trends_in_Exchange_Filings_October_2013_FINAL.ashx. Accessed December 2, 2014.
- Elmendorf DW. Letter to the Honorable Harry Reid. www.cbo.gov/sites/default/files/cbofiles/ftpdocs/113xx/doc11307/reid_letter_hr3590.pdf. Accessed December 2, 2014.
- Deloitte Center for Health Solutions. The fiscal impact to states of the Affordable Care Act: comprehensive analysis. http://www.statecoverage.org/files/DeloitteFisca_ImpacttoStatesACA.pdf. Accessed December 2, 2014.
- Congressional Budget Office. CBO releases updated estimates for the insurance coverage provisions of the Affordable Care Act. www.cbo.gov/publication/43080. Accessed December 2, 2014.
- Centers for Medicare & Medicaid Services. Pioneer accountable care organizations succeed in improving care, lowering costs. www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-07-16.html. Accessed December 2, 2014.
- Congressional Budget Office. Analysis of the administration’s announced delay of certain requirements under the Affordable Care Act. www.cbo.gov/publication/44465. Accessed December 2, 2014.
- Pear R. A limit on consumer costs is delayed in health care law. The New York Times. August 13, 2013. www.nytimes.com/2013/08/13/us/a-limit-on-consumer-costs-is-delayed-in-health-care-law.html?pagewanted=all&_r=0. Accessed December 2, 2014.
- Rubin R, Hunter K. Republicans push medical-device tax in US Senate. Bloomberg. May 13, 2014. www.bloomberg.com/news/2014-05-14/republicans-push-medical-device-tax-repeal-in-u-s-senate.html. Accessed December 2, 2014.
- Blumenthal D, Stremikis K, Cutler D. Health care spending—a giant slain or sleeping? N Engl J Med 2013; 369:2551–2557.