User login
Taking a look at neurologist burnout
There’s a lot in the news these days about doctor burnout. More specifically, neurologist burnout.
In a 2012 survey study, about 53% of neurologists reported burnout, which was third among all specialties surveyed, behind emergency medicine physicians and general internists. Neurologists also reported the fourth lowest job satisfaction with work-life balance, with about 41% satisfied that work leaves enough time for personal or family life. Neurology was the only one out of five specialties with the highest rates of burnout that was also among the five specialties with the lowest work-life balance.
Granted, the term “burnout” can mean a lot, but these days seems to refer to the fall of the American physician: Overworked, with rising costs, and falling reimbursements, sandwiched between patients who want to be cured immediately and those who want to sue us, and even on a good day facing a litany of terrible diseases.
Heck, I’d be burned out, too. Maybe I am.
Some say this is from the worries of solo practice, since we’re usually more pressed for time and money. I disagree, as I’ve seen it on both sides.
Recently, I saw my own internist. Six months ago she closed her own solo practice to join a large, hospital-owned group. She looked exhausted, worse than I’d ever seen her. She told me that she now gets a secure paycheck, but her stress level is worse. The hospital sets her schedule, tells her how much time she can spend with each patient, gives her quotas she has to meet, and has supplied an electronic health record (EHR) system that’s less than user friendly. (Personally, all of the ones I’ve tried are terrible.) When she goes home, she told me that now after dinner she still has to log on and do 2-3 more hours of charting just to catch up.
The grass is always greener. In her, I see a doctor who doesn’t have to watch each penny and worry about whether she’ll get a paycheck next week. In me, she looks at someone who’s free to pick their vacation days and isn’t chained to a quota system and a burdensome EHR.
Who’s right? I suppose it depends on what your life preferences are. Are we both burned out? We probably are, but in different ways.
But why the high rate of burnout for neurologists? Likely because of the issues I mentioned above. For myself, I’ve seen my salary drop 50% since its highest point in 2005. We’re faced with rising costs (like many other businesses). Unlike other professions, however, we don’t have much control over our reimbursement. Peculiar to medicine is the simple fact that what we charge has no bearing on what we get paid. Those rates are set by factors over which we have no control. Worse, they’re often set by politicians and insurance executives, who see us as the enemy.
There’s also the way reimbursements are set-up: they still favor docs who do a lot of procedures. While neurologists have a few, most of our job is thinking. And that’s not compensated nearly as well as jabbing needles and scalpels in people.
Then you get beyond financial issues. Many of us go through the day feeling like we have a target on our backs, in fear of patients becoming plaintiffs. What else? The nature of our field is such that we deal with diseases that are often challenging to diagnose and sometimes difficult, if not impossible, to treat. Yet, we still have to put on our best show and attitude for those afflicted. Part of why they come to us is to have questions answered and be given any glimmer of hope we can find.
In spite of this, the majority of us go on. Even burned out, we came here to help others. It’s part of what makes us tick and drives us to look in the mirror and head to the office. I wouldn’t trade what I do for anything. But I wish I could do it in a less adversarial world where I’m forced to choose between freedom and a (even temporary) sense of security.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
There’s a lot in the news these days about doctor burnout. More specifically, neurologist burnout.
In a 2012 survey study, about 53% of neurologists reported burnout, which was third among all specialties surveyed, behind emergency medicine physicians and general internists. Neurologists also reported the fourth lowest job satisfaction with work-life balance, with about 41% satisfied that work leaves enough time for personal or family life. Neurology was the only one out of five specialties with the highest rates of burnout that was also among the five specialties with the lowest work-life balance.
Granted, the term “burnout” can mean a lot, but these days seems to refer to the fall of the American physician: Overworked, with rising costs, and falling reimbursements, sandwiched between patients who want to be cured immediately and those who want to sue us, and even on a good day facing a litany of terrible diseases.
Heck, I’d be burned out, too. Maybe I am.
Some say this is from the worries of solo practice, since we’re usually more pressed for time and money. I disagree, as I’ve seen it on both sides.
Recently, I saw my own internist. Six months ago she closed her own solo practice to join a large, hospital-owned group. She looked exhausted, worse than I’d ever seen her. She told me that she now gets a secure paycheck, but her stress level is worse. The hospital sets her schedule, tells her how much time she can spend with each patient, gives her quotas she has to meet, and has supplied an electronic health record (EHR) system that’s less than user friendly. (Personally, all of the ones I’ve tried are terrible.) When she goes home, she told me that now after dinner she still has to log on and do 2-3 more hours of charting just to catch up.
The grass is always greener. In her, I see a doctor who doesn’t have to watch each penny and worry about whether she’ll get a paycheck next week. In me, she looks at someone who’s free to pick their vacation days and isn’t chained to a quota system and a burdensome EHR.
Who’s right? I suppose it depends on what your life preferences are. Are we both burned out? We probably are, but in different ways.
But why the high rate of burnout for neurologists? Likely because of the issues I mentioned above. For myself, I’ve seen my salary drop 50% since its highest point in 2005. We’re faced with rising costs (like many other businesses). Unlike other professions, however, we don’t have much control over our reimbursement. Peculiar to medicine is the simple fact that what we charge has no bearing on what we get paid. Those rates are set by factors over which we have no control. Worse, they’re often set by politicians and insurance executives, who see us as the enemy.
There’s also the way reimbursements are set-up: they still favor docs who do a lot of procedures. While neurologists have a few, most of our job is thinking. And that’s not compensated nearly as well as jabbing needles and scalpels in people.
Then you get beyond financial issues. Many of us go through the day feeling like we have a target on our backs, in fear of patients becoming plaintiffs. What else? The nature of our field is such that we deal with diseases that are often challenging to diagnose and sometimes difficult, if not impossible, to treat. Yet, we still have to put on our best show and attitude for those afflicted. Part of why they come to us is to have questions answered and be given any glimmer of hope we can find.
In spite of this, the majority of us go on. Even burned out, we came here to help others. It’s part of what makes us tick and drives us to look in the mirror and head to the office. I wouldn’t trade what I do for anything. But I wish I could do it in a less adversarial world where I’m forced to choose between freedom and a (even temporary) sense of security.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
There’s a lot in the news these days about doctor burnout. More specifically, neurologist burnout.
In a 2012 survey study, about 53% of neurologists reported burnout, which was third among all specialties surveyed, behind emergency medicine physicians and general internists. Neurologists also reported the fourth lowest job satisfaction with work-life balance, with about 41% satisfied that work leaves enough time for personal or family life. Neurology was the only one out of five specialties with the highest rates of burnout that was also among the five specialties with the lowest work-life balance.
Granted, the term “burnout” can mean a lot, but these days seems to refer to the fall of the American physician: Overworked, with rising costs, and falling reimbursements, sandwiched between patients who want to be cured immediately and those who want to sue us, and even on a good day facing a litany of terrible diseases.
Heck, I’d be burned out, too. Maybe I am.
Some say this is from the worries of solo practice, since we’re usually more pressed for time and money. I disagree, as I’ve seen it on both sides.
Recently, I saw my own internist. Six months ago she closed her own solo practice to join a large, hospital-owned group. She looked exhausted, worse than I’d ever seen her. She told me that she now gets a secure paycheck, but her stress level is worse. The hospital sets her schedule, tells her how much time she can spend with each patient, gives her quotas she has to meet, and has supplied an electronic health record (EHR) system that’s less than user friendly. (Personally, all of the ones I’ve tried are terrible.) When she goes home, she told me that now after dinner she still has to log on and do 2-3 more hours of charting just to catch up.
The grass is always greener. In her, I see a doctor who doesn’t have to watch each penny and worry about whether she’ll get a paycheck next week. In me, she looks at someone who’s free to pick their vacation days and isn’t chained to a quota system and a burdensome EHR.
Who’s right? I suppose it depends on what your life preferences are. Are we both burned out? We probably are, but in different ways.
But why the high rate of burnout for neurologists? Likely because of the issues I mentioned above. For myself, I’ve seen my salary drop 50% since its highest point in 2005. We’re faced with rising costs (like many other businesses). Unlike other professions, however, we don’t have much control over our reimbursement. Peculiar to medicine is the simple fact that what we charge has no bearing on what we get paid. Those rates are set by factors over which we have no control. Worse, they’re often set by politicians and insurance executives, who see us as the enemy.
There’s also the way reimbursements are set-up: they still favor docs who do a lot of procedures. While neurologists have a few, most of our job is thinking. And that’s not compensated nearly as well as jabbing needles and scalpels in people.
Then you get beyond financial issues. Many of us go through the day feeling like we have a target on our backs, in fear of patients becoming plaintiffs. What else? The nature of our field is such that we deal with diseases that are often challenging to diagnose and sometimes difficult, if not impossible, to treat. Yet, we still have to put on our best show and attitude for those afflicted. Part of why they come to us is to have questions answered and be given any glimmer of hope we can find.
In spite of this, the majority of us go on. Even burned out, we came here to help others. It’s part of what makes us tick and drives us to look in the mirror and head to the office. I wouldn’t trade what I do for anything. But I wish I could do it in a less adversarial world where I’m forced to choose between freedom and a (even temporary) sense of security.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Cutaneous Side Effects of Chemotherapy in Pediatric Oncology Patients
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
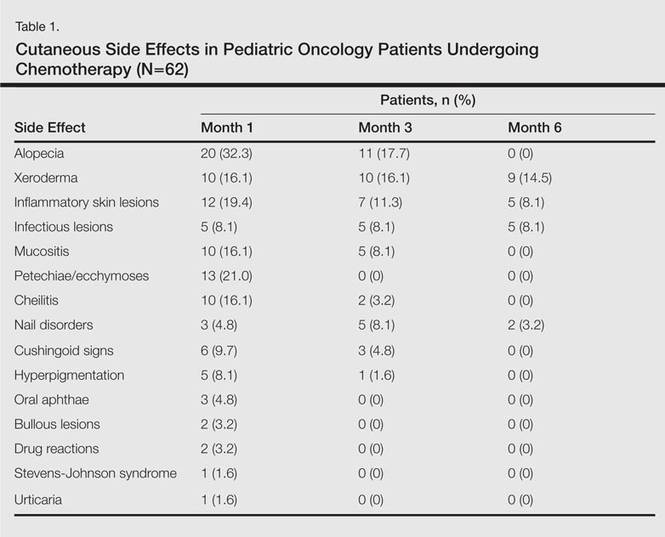
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.
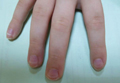
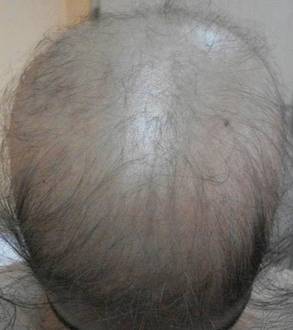
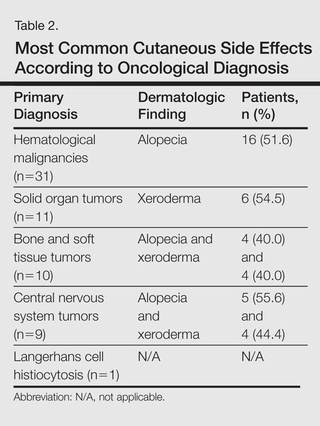
The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).
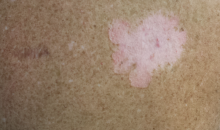
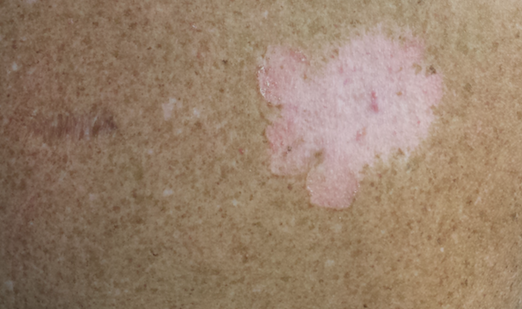
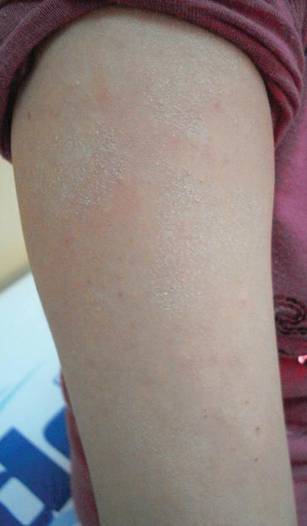
The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).
Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
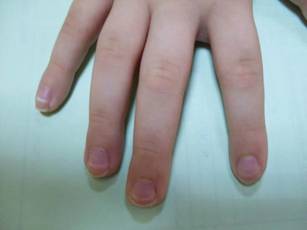
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.
Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Practice Points
- Chemotherapeutic agents can cause a variety of cutaneous side effects.
- Pediatric oncology patients should be examined regularly for cutaneous side effects of chemotherapeutics.
FDA approves oral anticoagulant for NVAF, VTE

Credit: FDA
The US Food and Drug Administration (FDA) has approved the oral, direct factor Xa inhibitor edoxaban (Savaysa) for use in two patient populations.
The anticoagulant is now approved to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) and to treat venous thromboembolism (VTE) in patients who have already received parenteral anticoagulation for 5 to 10 days.
The drug has been approved with a Boxed Warning.
The warning states that edoxaban is less effective in NVAF patients with a creatinine clearance greater than 95 mL/min. Patients with creatinine clearance above this limit have an increased risk of stroke if they receive edoxaban (compared to the risk with warfarin), so these patients should not receive edoxaban.
The warning also states that premature discontinuation of edoxaban increases the risk of stroke. Furthermore, spinal or epidural hematomas may occur in patients on edoxaban who are receiving anesthesia injected around the spine or undergoing spinal puncture.
Edoxaban for VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with deep vein thrombosis and 3319 with pulmonary embolism. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic VTE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
Edoxaban proved superior when it came to the primary safety outcome. Clinically relevant bleeding occurred in 8.5% of edoxaban-treated patients and 10.3% of warfarin-treated patients (P=0.004 for superiority).
In the edoxaban arm, there were 2 fatal bleeds and 13 non-fatal bleeds in a critical site. With warfarin, there were 10 fatal bleeds and 25 non-fatal bleeds in a critical site.
Edoxaban in NVAF
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin for the prevention of stroke or systemic embolic events (SEE) in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or SEE was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
Annualized rates for the secondary composite endpoint of stroke, SEE, and cardiovascular death were 4.43% with warfarin, 3.85% with edoxaban at 60 mg (P=0.005), and 4.23% with edoxaban at 30 mg (P=0.32).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001).
Edoxaban is under development by Daiichi Sankyo Co., Ltd. ![]()

Credit: FDA
The US Food and Drug Administration (FDA) has approved the oral, direct factor Xa inhibitor edoxaban (Savaysa) for use in two patient populations.
The anticoagulant is now approved to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) and to treat venous thromboembolism (VTE) in patients who have already received parenteral anticoagulation for 5 to 10 days.
The drug has been approved with a Boxed Warning.
The warning states that edoxaban is less effective in NVAF patients with a creatinine clearance greater than 95 mL/min. Patients with creatinine clearance above this limit have an increased risk of stroke if they receive edoxaban (compared to the risk with warfarin), so these patients should not receive edoxaban.
The warning also states that premature discontinuation of edoxaban increases the risk of stroke. Furthermore, spinal or epidural hematomas may occur in patients on edoxaban who are receiving anesthesia injected around the spine or undergoing spinal puncture.
Edoxaban for VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with deep vein thrombosis and 3319 with pulmonary embolism. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic VTE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
Edoxaban proved superior when it came to the primary safety outcome. Clinically relevant bleeding occurred in 8.5% of edoxaban-treated patients and 10.3% of warfarin-treated patients (P=0.004 for superiority).
In the edoxaban arm, there were 2 fatal bleeds and 13 non-fatal bleeds in a critical site. With warfarin, there were 10 fatal bleeds and 25 non-fatal bleeds in a critical site.
Edoxaban in NVAF
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin for the prevention of stroke or systemic embolic events (SEE) in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or SEE was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
Annualized rates for the secondary composite endpoint of stroke, SEE, and cardiovascular death were 4.43% with warfarin, 3.85% with edoxaban at 60 mg (P=0.005), and 4.23% with edoxaban at 30 mg (P=0.32).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001).
Edoxaban is under development by Daiichi Sankyo Co., Ltd. ![]()

Credit: FDA
The US Food and Drug Administration (FDA) has approved the oral, direct factor Xa inhibitor edoxaban (Savaysa) for use in two patient populations.
The anticoagulant is now approved to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) and to treat venous thromboembolism (VTE) in patients who have already received parenteral anticoagulation for 5 to 10 days.
The drug has been approved with a Boxed Warning.
The warning states that edoxaban is less effective in NVAF patients with a creatinine clearance greater than 95 mL/min. Patients with creatinine clearance above this limit have an increased risk of stroke if they receive edoxaban (compared to the risk with warfarin), so these patients should not receive edoxaban.
The warning also states that premature discontinuation of edoxaban increases the risk of stroke. Furthermore, spinal or epidural hematomas may occur in patients on edoxaban who are receiving anesthesia injected around the spine or undergoing spinal puncture.
Edoxaban for VTE
In the Hokusai-VTE trial, researchers evaluated edoxaban in 4921 patients with deep vein thrombosis and 3319 with pulmonary embolism. Patients received initial treatment with low-molecular-weight heparin and were then randomized to receive edoxaban or warfarin daily for 3 to 12 months.
Overall, edoxaban proved as effective as warfarin. Recurrent, symptomatic VTE occurred in 3.2% and 3.5% of patients, respectively (P<0.001 for non-inferiority).
Edoxaban proved superior when it came to the primary safety outcome. Clinically relevant bleeding occurred in 8.5% of edoxaban-treated patients and 10.3% of warfarin-treated patients (P=0.004 for superiority).
In the edoxaban arm, there were 2 fatal bleeds and 13 non-fatal bleeds in a critical site. With warfarin, there were 10 fatal bleeds and 25 non-fatal bleeds in a critical site.
Edoxaban in NVAF
In the ENGAGE AF-TIMI 48 trial, researchers compared edoxaban and warfarin for the prevention of stroke or systemic embolic events (SEE) in patients with NVAF.
The trial included 21,105 patients who were randomized to receive warfarin (n=7036), edoxaban at 60 mg (n=7035), or edoxaban at 30 mg (n=7034).
Edoxaban was at least non-inferior to warfarin with regard to efficacy. The annual incidence of stroke or SEE was 1.50% with warfarin, 1.18% with edoxaban at 60 mg (P<0.001 for non-inferiority), and 1.61% with edoxaban at 30 mg (P=0.005 for non-inferiority).
Annualized rates for the secondary composite endpoint of stroke, SEE, and cardiovascular death were 4.43% with warfarin, 3.85% with edoxaban at 60 mg (P=0.005), and 4.23% with edoxaban at 30 mg (P=0.32).
In addition, edoxaban was associated with a significantly lower rate of major and fatal bleeding. The annual incidence of major bleeding was 3.43% with warfarin, 2.75% with edoxaban at 60 mg (P<0.001), and 1.61% with edoxaban at 30 mg (P<0.001).
Fatal bleeds occurred at an annual rate of 0.38% with warfarin, 0.21% with edoxaban at 60 mg (P=0.006), and 0.13% with edoxaban at 30 mg (P<0.001).
Edoxaban is under development by Daiichi Sankyo Co., Ltd. ![]()
Product gets fast track designation for CTCL

mycosis fungoides
The US Food and Drug Administration (FDA) has granted fast track designation to SGX301 as a first-line treatment for cutaneous T-cell lymphoma (CTCL).
SGX301 is a photodynamic therapy utilizing safe, visible light for activation. The active ingredient in SGX301 is synthetic hypericin, a photosensitizer that is applied to skin lesions and then activated by fluorescent light 16 to 24 hours later.
Combined with photoactivation, hypericin has demonstrated significant antiproliferative effects on activated, normal human lymphoid cells and inhibited the growth of malignant T cells isolated from CTCL patients. Topical hypericin has also proven safe in a phase 1 study of healthy volunteers.
In a phase 2 trial of patients with CTCL (mycosis fungoides only) or psoriasis, topical hypericin conferred a significant improvement over placebo. Among CTCL patients, the treatment prompted a response rate of 58.3%, compared to an 8.3% response rate for placebo (P≤0.04).
Topical hypericin was also well tolerated in this trial. There were no deaths or serious adverse events related to the treatment. However, there were reports of mild to moderate burning, itching, erythema, and pruritus at the application site.
A phase 3 trial of SGX301 is set to begin in the first half of this year. In addition to its new fast track status, SGX301 also has orphan designation from the FDA.
About fast track designation
The FDA grants fast track designation to a drug that is intended to treat a serious or life-threatening condition and that demonstrates the potential to address an unmet medical need for the condition.
Fast track designation is designed to facilitate the development and expedite the review of new drugs. For instance, Soligenix, Inc., the company developing SGX301, is eligible to submit a new drug application (NDA) for SGX301 on a rolling basis, allowing the FDA to review sections of the NDA prior to receiving the complete submission.
Additionally, NDAs for fast track development programs ordinarily will be eligible for priority review, which imparts an abbreviated review time of approximately 6 months. ![]()

mycosis fungoides
The US Food and Drug Administration (FDA) has granted fast track designation to SGX301 as a first-line treatment for cutaneous T-cell lymphoma (CTCL).
SGX301 is a photodynamic therapy utilizing safe, visible light for activation. The active ingredient in SGX301 is synthetic hypericin, a photosensitizer that is applied to skin lesions and then activated by fluorescent light 16 to 24 hours later.
Combined with photoactivation, hypericin has demonstrated significant antiproliferative effects on activated, normal human lymphoid cells and inhibited the growth of malignant T cells isolated from CTCL patients. Topical hypericin has also proven safe in a phase 1 study of healthy volunteers.
In a phase 2 trial of patients with CTCL (mycosis fungoides only) or psoriasis, topical hypericin conferred a significant improvement over placebo. Among CTCL patients, the treatment prompted a response rate of 58.3%, compared to an 8.3% response rate for placebo (P≤0.04).
Topical hypericin was also well tolerated in this trial. There were no deaths or serious adverse events related to the treatment. However, there were reports of mild to moderate burning, itching, erythema, and pruritus at the application site.
A phase 3 trial of SGX301 is set to begin in the first half of this year. In addition to its new fast track status, SGX301 also has orphan designation from the FDA.
About fast track designation
The FDA grants fast track designation to a drug that is intended to treat a serious or life-threatening condition and that demonstrates the potential to address an unmet medical need for the condition.
Fast track designation is designed to facilitate the development and expedite the review of new drugs. For instance, Soligenix, Inc., the company developing SGX301, is eligible to submit a new drug application (NDA) for SGX301 on a rolling basis, allowing the FDA to review sections of the NDA prior to receiving the complete submission.
Additionally, NDAs for fast track development programs ordinarily will be eligible for priority review, which imparts an abbreviated review time of approximately 6 months. ![]()

mycosis fungoides
The US Food and Drug Administration (FDA) has granted fast track designation to SGX301 as a first-line treatment for cutaneous T-cell lymphoma (CTCL).
SGX301 is a photodynamic therapy utilizing safe, visible light for activation. The active ingredient in SGX301 is synthetic hypericin, a photosensitizer that is applied to skin lesions and then activated by fluorescent light 16 to 24 hours later.
Combined with photoactivation, hypericin has demonstrated significant antiproliferative effects on activated, normal human lymphoid cells and inhibited the growth of malignant T cells isolated from CTCL patients. Topical hypericin has also proven safe in a phase 1 study of healthy volunteers.
In a phase 2 trial of patients with CTCL (mycosis fungoides only) or psoriasis, topical hypericin conferred a significant improvement over placebo. Among CTCL patients, the treatment prompted a response rate of 58.3%, compared to an 8.3% response rate for placebo (P≤0.04).
Topical hypericin was also well tolerated in this trial. There were no deaths or serious adverse events related to the treatment. However, there were reports of mild to moderate burning, itching, erythema, and pruritus at the application site.
A phase 3 trial of SGX301 is set to begin in the first half of this year. In addition to its new fast track status, SGX301 also has orphan designation from the FDA.
About fast track designation
The FDA grants fast track designation to a drug that is intended to treat a serious or life-threatening condition and that demonstrates the potential to address an unmet medical need for the condition.
Fast track designation is designed to facilitate the development and expedite the review of new drugs. For instance, Soligenix, Inc., the company developing SGX301, is eligible to submit a new drug application (NDA) for SGX301 on a rolling basis, allowing the FDA to review sections of the NDA prior to receiving the complete submission.
Additionally, NDAs for fast track development programs ordinarily will be eligible for priority review, which imparts an abbreviated review time of approximately 6 months. ![]()
Drug granted orphan designation for MM

The US Food and Drug Administration (FDA) has granted selinexor (KPT-330) orphan drug designation to treat multiple myeloma (MM).
Selinexor already has orphan designation from the FDA to treat acute myeloid leukemia (AML) and diffuse large B-cell lymphoma (DLBCL).
The drug has also received orphan designation from the European Medicines Agency (EMA) to treat MM, AML, DLBCL, and chronic lymphocytic leukemia/small lymphocytic lymphoma, including Richter’s transformation.
“Orphan drug designation by the FDA for multiple myeloma is another significant milestone in the selinexor development program,” said Sharon Shacham, PhD, President and Chief Scientific Officer of Karyopharm Therapeutics, Inc., the company developing selinexor.
In the US, orphan designation qualifies a company for certain benefits, including an accelerated approval process, 7 years of market exclusivity following the drug’s approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
About selinexor
Selinexor (KPT-330) is a first-in-class, oral, selective inhibitor of nuclear export compound. The drug functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
Selinexor combos in MM
In a poster presented at the 2014 ASH Annual Meeting (4773), researchers reported results observed with selinexor plus dexamethasone in preclinical models and in patients with heavily pretreated, refractory MM.
The study included 9 evaluable patients who received selinexor at 45 mg/m2 twice weekly and dexamethasone at 20 mg twice weekly. The combination prompted an overall response rate of 67%, with one stringent complete response (11%) and 5 partial responses (56%), as well as a clinical benefit rate of 89%.
The combination demonstrated a reduction in nausea grades and very little weight loss compared with selinexor alone. The most common grade 1/2 adverse events were nausea, fatigue, anorexia, and vomiting.
The combination was also associated with an increase in time on study relative to selinexor alone. Sixty-six percent of patients remained on study for at least 16 weeks, including one patient for 28 weeks and one for 43 weeks as of December 1, 2014.
During the dose-evaluation part of the study, the 60 mg/m2 selinexor dose was deemed intolerable in this heavily pretreated patient population. So 45 mg/m2 is the recommended future study dose.
In another poster presented at the 2014 ASH Annual Meeting (3443), researchers described the activity of selinexor in combination with carfilzomib. This preclinical study revealed a novel, intracellular, membrane-embedded mechanism of caspase activation.
The results suggested a model of synergy wherein the selinexor-carfilzomib combination promotes caspase activation, likely by induced proximity, cleavage of other caspases, and subsequent apoptosis as well as autophagy. ![]()

The US Food and Drug Administration (FDA) has granted selinexor (KPT-330) orphan drug designation to treat multiple myeloma (MM).
Selinexor already has orphan designation from the FDA to treat acute myeloid leukemia (AML) and diffuse large B-cell lymphoma (DLBCL).
The drug has also received orphan designation from the European Medicines Agency (EMA) to treat MM, AML, DLBCL, and chronic lymphocytic leukemia/small lymphocytic lymphoma, including Richter’s transformation.
“Orphan drug designation by the FDA for multiple myeloma is another significant milestone in the selinexor development program,” said Sharon Shacham, PhD, President and Chief Scientific Officer of Karyopharm Therapeutics, Inc., the company developing selinexor.
In the US, orphan designation qualifies a company for certain benefits, including an accelerated approval process, 7 years of market exclusivity following the drug’s approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
About selinexor
Selinexor (KPT-330) is a first-in-class, oral, selective inhibitor of nuclear export compound. The drug functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
Selinexor combos in MM
In a poster presented at the 2014 ASH Annual Meeting (4773), researchers reported results observed with selinexor plus dexamethasone in preclinical models and in patients with heavily pretreated, refractory MM.
The study included 9 evaluable patients who received selinexor at 45 mg/m2 twice weekly and dexamethasone at 20 mg twice weekly. The combination prompted an overall response rate of 67%, with one stringent complete response (11%) and 5 partial responses (56%), as well as a clinical benefit rate of 89%.
The combination demonstrated a reduction in nausea grades and very little weight loss compared with selinexor alone. The most common grade 1/2 adverse events were nausea, fatigue, anorexia, and vomiting.
The combination was also associated with an increase in time on study relative to selinexor alone. Sixty-six percent of patients remained on study for at least 16 weeks, including one patient for 28 weeks and one for 43 weeks as of December 1, 2014.
During the dose-evaluation part of the study, the 60 mg/m2 selinexor dose was deemed intolerable in this heavily pretreated patient population. So 45 mg/m2 is the recommended future study dose.
In another poster presented at the 2014 ASH Annual Meeting (3443), researchers described the activity of selinexor in combination with carfilzomib. This preclinical study revealed a novel, intracellular, membrane-embedded mechanism of caspase activation.
The results suggested a model of synergy wherein the selinexor-carfilzomib combination promotes caspase activation, likely by induced proximity, cleavage of other caspases, and subsequent apoptosis as well as autophagy. ![]()

The US Food and Drug Administration (FDA) has granted selinexor (KPT-330) orphan drug designation to treat multiple myeloma (MM).
Selinexor already has orphan designation from the FDA to treat acute myeloid leukemia (AML) and diffuse large B-cell lymphoma (DLBCL).
The drug has also received orphan designation from the European Medicines Agency (EMA) to treat MM, AML, DLBCL, and chronic lymphocytic leukemia/small lymphocytic lymphoma, including Richter’s transformation.
“Orphan drug designation by the FDA for multiple myeloma is another significant milestone in the selinexor development program,” said Sharon Shacham, PhD, President and Chief Scientific Officer of Karyopharm Therapeutics, Inc., the company developing selinexor.
In the US, orphan designation qualifies a company for certain benefits, including an accelerated approval process, 7 years of market exclusivity following the drug’s approval, tax credits on US clinical trials, eligibility for orphan drug grants, and a waiver of certain administrative fees.
About selinexor
Selinexor (KPT-330) is a first-in-class, oral, selective inhibitor of nuclear export compound. The drug functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
Selinexor combos in MM
In a poster presented at the 2014 ASH Annual Meeting (4773), researchers reported results observed with selinexor plus dexamethasone in preclinical models and in patients with heavily pretreated, refractory MM.
The study included 9 evaluable patients who received selinexor at 45 mg/m2 twice weekly and dexamethasone at 20 mg twice weekly. The combination prompted an overall response rate of 67%, with one stringent complete response (11%) and 5 partial responses (56%), as well as a clinical benefit rate of 89%.
The combination demonstrated a reduction in nausea grades and very little weight loss compared with selinexor alone. The most common grade 1/2 adverse events were nausea, fatigue, anorexia, and vomiting.
The combination was also associated with an increase in time on study relative to selinexor alone. Sixty-six percent of patients remained on study for at least 16 weeks, including one patient for 28 weeks and one for 43 weeks as of December 1, 2014.
During the dose-evaluation part of the study, the 60 mg/m2 selinexor dose was deemed intolerable in this heavily pretreated patient population. So 45 mg/m2 is the recommended future study dose.
In another poster presented at the 2014 ASH Annual Meeting (3443), researchers described the activity of selinexor in combination with carfilzomib. This preclinical study revealed a novel, intracellular, membrane-embedded mechanism of caspase activation.
The results suggested a model of synergy wherein the selinexor-carfilzomib combination promotes caspase activation, likely by induced proximity, cleavage of other caspases, and subsequent apoptosis as well as autophagy. ![]()
Study raises questions about exchange transfusion

Credit: Vera Kratochvil
Results of a new study indicate that current guidelines for exchange transfusions in infants can successfully prevent kernicterus, a rare and life-threatening type of cerebral palsy triggered by escalating bilirubin that injures the brain.
However, the research also showed that only infants whose levels of bilirubin were well above the level for exchange transfusion actually developed kernicterus. And those infants all had additional risk factors for brain damage.
This suggests perhaps the threshold for exchange transfusion could safely be raised for infants with high bilirubin levels who have no other risk factors for brain injury, according to Yvonne W. Wu, MD, of the University of California, San Francisco (UCSF).
Dr Wu and her colleagues evaluated the health records of two groups of infants selected from 525,409 births. The children had been born at 15 hospitals within the Kaiser Permanente Northern California region from 1995 through 2011.
One group comprised 1833 newborns with levels of bilirubin above the level at which the American Academy of Pediatrics (AAP) recommends exchange transfusions.
The second group was made up of 104,716 randomly sampled newborns, born at at least 35 weeks’ gestation with lower levels of bilirubin. The two groups were followed for an average of 7 and 6 years, respectively.
The researchers confirmed 3 cases of kernicterus based on the brain MRIs of children with cerebral palsy. All 3 cases had occurred in newborns with the highest levels of bilirubin. But further study revealed that each child had 2 or more risk factors for brain damage.
”We found that cerebral palsy consistent with kernicterus did not occur in a single infant with high bilirubin without the presence of additional risk factors for neurotoxicity, such as prematurity, sepsis, and the hereditary blood disorder G6PD deficiency,” said Michael W. Kuzniewicz, MD, of UCSF. “This was the case even in infants with very high bilirubin.”
In 2004, the AAP published a guideline for treating infants whose bilirubin remained high despite phototherapy. It recommended exchange transfusions based on the level of bilirubin, the age of the infant, and other risk factors for brain damage.
“Our study was the first to evaluate how well the exchange transfusion guidelines predicted risk of cerebral palsy and kernicterus in babies with jaundice,” said Thomas B. Newman, MD, of UCSF.
“It was reassuring that brain injury due to high bilirubin was rare and that only those infants whose levels were well above exchange transfusion guidelines developed kernicterus.”
“Based on our study, the current guidelines for when to perform exchange transfusions have been quite successful in preventing kernicterus,” Dr Wu added. “However, our study also raises the question whether the threshold for exchange transfusion could be higher for infants with high bilirubin levels who are otherwise healthy and who have no other risk factors for brain injury.”
This is especially important, she noted, because exchange transfusions pose risks such as blood clot formation, blood pressure instability, bleeding, and changes in blood chemistry.
Dr Wu and her colleagues described this research in JAMA Pediatrics. ![]()

Credit: Vera Kratochvil
Results of a new study indicate that current guidelines for exchange transfusions in infants can successfully prevent kernicterus, a rare and life-threatening type of cerebral palsy triggered by escalating bilirubin that injures the brain.
However, the research also showed that only infants whose levels of bilirubin were well above the level for exchange transfusion actually developed kernicterus. And those infants all had additional risk factors for brain damage.
This suggests perhaps the threshold for exchange transfusion could safely be raised for infants with high bilirubin levels who have no other risk factors for brain injury, according to Yvonne W. Wu, MD, of the University of California, San Francisco (UCSF).
Dr Wu and her colleagues evaluated the health records of two groups of infants selected from 525,409 births. The children had been born at 15 hospitals within the Kaiser Permanente Northern California region from 1995 through 2011.
One group comprised 1833 newborns with levels of bilirubin above the level at which the American Academy of Pediatrics (AAP) recommends exchange transfusions.
The second group was made up of 104,716 randomly sampled newborns, born at at least 35 weeks’ gestation with lower levels of bilirubin. The two groups were followed for an average of 7 and 6 years, respectively.
The researchers confirmed 3 cases of kernicterus based on the brain MRIs of children with cerebral palsy. All 3 cases had occurred in newborns with the highest levels of bilirubin. But further study revealed that each child had 2 or more risk factors for brain damage.
”We found that cerebral palsy consistent with kernicterus did not occur in a single infant with high bilirubin without the presence of additional risk factors for neurotoxicity, such as prematurity, sepsis, and the hereditary blood disorder G6PD deficiency,” said Michael W. Kuzniewicz, MD, of UCSF. “This was the case even in infants with very high bilirubin.”
In 2004, the AAP published a guideline for treating infants whose bilirubin remained high despite phototherapy. It recommended exchange transfusions based on the level of bilirubin, the age of the infant, and other risk factors for brain damage.
“Our study was the first to evaluate how well the exchange transfusion guidelines predicted risk of cerebral palsy and kernicterus in babies with jaundice,” said Thomas B. Newman, MD, of UCSF.
“It was reassuring that brain injury due to high bilirubin was rare and that only those infants whose levels were well above exchange transfusion guidelines developed kernicterus.”
“Based on our study, the current guidelines for when to perform exchange transfusions have been quite successful in preventing kernicterus,” Dr Wu added. “However, our study also raises the question whether the threshold for exchange transfusion could be higher for infants with high bilirubin levels who are otherwise healthy and who have no other risk factors for brain injury.”
This is especially important, she noted, because exchange transfusions pose risks such as blood clot formation, blood pressure instability, bleeding, and changes in blood chemistry.
Dr Wu and her colleagues described this research in JAMA Pediatrics. ![]()

Credit: Vera Kratochvil
Results of a new study indicate that current guidelines for exchange transfusions in infants can successfully prevent kernicterus, a rare and life-threatening type of cerebral palsy triggered by escalating bilirubin that injures the brain.
However, the research also showed that only infants whose levels of bilirubin were well above the level for exchange transfusion actually developed kernicterus. And those infants all had additional risk factors for brain damage.
This suggests perhaps the threshold for exchange transfusion could safely be raised for infants with high bilirubin levels who have no other risk factors for brain injury, according to Yvonne W. Wu, MD, of the University of California, San Francisco (UCSF).
Dr Wu and her colleagues evaluated the health records of two groups of infants selected from 525,409 births. The children had been born at 15 hospitals within the Kaiser Permanente Northern California region from 1995 through 2011.
One group comprised 1833 newborns with levels of bilirubin above the level at which the American Academy of Pediatrics (AAP) recommends exchange transfusions.
The second group was made up of 104,716 randomly sampled newborns, born at at least 35 weeks’ gestation with lower levels of bilirubin. The two groups were followed for an average of 7 and 6 years, respectively.
The researchers confirmed 3 cases of kernicterus based on the brain MRIs of children with cerebral palsy. All 3 cases had occurred in newborns with the highest levels of bilirubin. But further study revealed that each child had 2 or more risk factors for brain damage.
”We found that cerebral palsy consistent with kernicterus did not occur in a single infant with high bilirubin without the presence of additional risk factors for neurotoxicity, such as prematurity, sepsis, and the hereditary blood disorder G6PD deficiency,” said Michael W. Kuzniewicz, MD, of UCSF. “This was the case even in infants with very high bilirubin.”
In 2004, the AAP published a guideline for treating infants whose bilirubin remained high despite phototherapy. It recommended exchange transfusions based on the level of bilirubin, the age of the infant, and other risk factors for brain damage.
“Our study was the first to evaluate how well the exchange transfusion guidelines predicted risk of cerebral palsy and kernicterus in babies with jaundice,” said Thomas B. Newman, MD, of UCSF.
“It was reassuring that brain injury due to high bilirubin was rare and that only those infants whose levels were well above exchange transfusion guidelines developed kernicterus.”
“Based on our study, the current guidelines for when to perform exchange transfusions have been quite successful in preventing kernicterus,” Dr Wu added. “However, our study also raises the question whether the threshold for exchange transfusion could be higher for infants with high bilirubin levels who are otherwise healthy and who have no other risk factors for brain injury.”
This is especially important, she noted, because exchange transfusions pose risks such as blood clot formation, blood pressure instability, bleeding, and changes in blood chemistry.
Dr Wu and her colleagues described this research in JAMA Pediatrics. ![]()
Excessive Sun Damage and a “Fungal Infection”?
A scaly lesion manifested on this 55-year-old man’s back several years ago. Over time, the lesion has grown, despite the application of several different OTC creams, including hydrocortisone and various antifungal creams. Previously asymptomatic, the lesion is now beginning to itch and occasionally bleed.
Additional history-taking reveals that for the first 10 years of his working life, the patient was a concrete finisher, working 12-hour shifts in the sun without a shirt, six days a week for months at a time. Aside from a 40-pack-year history of smoking, his health is “decent.”
EXAMINATION
The lesion is an 8 x 10–cm pink, scaly plaque with thready, well-defined scaly margins. Tiny focal erosions are seen in the peripheral leading edge. Closer inspection of the lesion’s center reveals multiple telangiectasias and a complete lack of hairs or hair follicles. Elsewhere on sun-exposed areas of the patient’s truncal skin, there are thousands of solar lentigines. Multiple small actinic keratoses are noted on his face as well.
What is the diagnosis?
DISCUSSION
Prior to consulting dermatology, this patient had talked extensively with his primary care provider (PCP) about the large lesion on his back. The PCP was quite sure the cause was a fungal organism and assured the patient that the prescribed antifungal cream would almost certainly cure it. This is a scenario in which almost every PCP will participate at some point early in his/her career. When treatment fails, the patient is then referred to dermatology—where a totally unexpected diagnosis is made.
This case represents the natural history of superficial basal cell carcinoma (SBCC), the appearance of which is at odds with any other kind of skin cancer, including the other types of basal cell carcinoma. SBCC is especially common on the shoulders and trunks of patients with a history of excessive sun exposure. The mid-back location on this patient is quite typical, as is the annular, scaly morphology.
The cancer—which originates in a row of cells at the base of the epidermis—effectively destroys surface structures such as skin lines and hair follicles as it spreads laterally. Left in place long enough, SBCCs can eventually become focally invasive and can even spread into regional lymph nodes. Metastasis from them is extremely rare but has been reported.
Treatment is therefore necessary and can range from surgical excision of smaller lesions to electrodessication and curettage to nonsurgical options, such as the application of imiquimod creams or 5-fluorouracil cream, or even radiation therapy. This man was treated with thrice-weekly application of imiquimod 3.75% cream for at least two months, which will cause irritation and scant drainage but will likely result in a cure.
One of the main benefits of his presentation to dermatology was the opportunity to encourage and facilitate yearly skin check-ups—important, given his risk for other sun-caused skin cancers (especially melanoma).
A major learning point to be gained from this case: Just because a lesion is annular and scaly doesn’t mean it’s fungal. The fungi have to have a source (animal, child, soil), and it helps a lot if the affected area is damp and warm (eg, groin or under a bulletproof vest). Immunosuppression (from corticosteroid use, especially) helps as well.
Besides fungal infection, the differential for this type of lesion includes Bowen’s disease (intraepidermal squamous cell carcinoma), psoriasis, and eczema. The fact that the lesion was fixed (did not come and go), grew steadily, and was on the directly sun-exposed skin of a person with a history of heavy sun exposure all pointed to the diagnosis.
TAKE-HOME LEARNING POINTS
• Superficial BCC (SBCC) has also been called “field fire” skin cancer because of the prominent, well-defined scaly border that expands peripherally and leaves a “scorched earth” look behind.
• SBCCs are common on skin directly exposed to the sun, especially backs and arms.
• SBCCs do not resemble other types of BCC at all and are therefore often mistaken for “fungal infection.”
• Pay as much attention to the patient as to the lesion. There was abundant evidence of excessive sun damage on this patient’s trunk, making him an ideal patient for skin cancer.
A scaly lesion manifested on this 55-year-old man’s back several years ago. Over time, the lesion has grown, despite the application of several different OTC creams, including hydrocortisone and various antifungal creams. Previously asymptomatic, the lesion is now beginning to itch and occasionally bleed.
Additional history-taking reveals that for the first 10 years of his working life, the patient was a concrete finisher, working 12-hour shifts in the sun without a shirt, six days a week for months at a time. Aside from a 40-pack-year history of smoking, his health is “decent.”
EXAMINATION
The lesion is an 8 x 10–cm pink, scaly plaque with thready, well-defined scaly margins. Tiny focal erosions are seen in the peripheral leading edge. Closer inspection of the lesion’s center reveals multiple telangiectasias and a complete lack of hairs or hair follicles. Elsewhere on sun-exposed areas of the patient’s truncal skin, there are thousands of solar lentigines. Multiple small actinic keratoses are noted on his face as well.
What is the diagnosis?
DISCUSSION
Prior to consulting dermatology, this patient had talked extensively with his primary care provider (PCP) about the large lesion on his back. The PCP was quite sure the cause was a fungal organism and assured the patient that the prescribed antifungal cream would almost certainly cure it. This is a scenario in which almost every PCP will participate at some point early in his/her career. When treatment fails, the patient is then referred to dermatology—where a totally unexpected diagnosis is made.
This case represents the natural history of superficial basal cell carcinoma (SBCC), the appearance of which is at odds with any other kind of skin cancer, including the other types of basal cell carcinoma. SBCC is especially common on the shoulders and trunks of patients with a history of excessive sun exposure. The mid-back location on this patient is quite typical, as is the annular, scaly morphology.
The cancer—which originates in a row of cells at the base of the epidermis—effectively destroys surface structures such as skin lines and hair follicles as it spreads laterally. Left in place long enough, SBCCs can eventually become focally invasive and can even spread into regional lymph nodes. Metastasis from them is extremely rare but has been reported.
Treatment is therefore necessary and can range from surgical excision of smaller lesions to electrodessication and curettage to nonsurgical options, such as the application of imiquimod creams or 5-fluorouracil cream, or even radiation therapy. This man was treated with thrice-weekly application of imiquimod 3.75% cream for at least two months, which will cause irritation and scant drainage but will likely result in a cure.
One of the main benefits of his presentation to dermatology was the opportunity to encourage and facilitate yearly skin check-ups—important, given his risk for other sun-caused skin cancers (especially melanoma).
A major learning point to be gained from this case: Just because a lesion is annular and scaly doesn’t mean it’s fungal. The fungi have to have a source (animal, child, soil), and it helps a lot if the affected area is damp and warm (eg, groin or under a bulletproof vest). Immunosuppression (from corticosteroid use, especially) helps as well.
Besides fungal infection, the differential for this type of lesion includes Bowen’s disease (intraepidermal squamous cell carcinoma), psoriasis, and eczema. The fact that the lesion was fixed (did not come and go), grew steadily, and was on the directly sun-exposed skin of a person with a history of heavy sun exposure all pointed to the diagnosis.
TAKE-HOME LEARNING POINTS
• Superficial BCC (SBCC) has also been called “field fire” skin cancer because of the prominent, well-defined scaly border that expands peripherally and leaves a “scorched earth” look behind.
• SBCCs are common on skin directly exposed to the sun, especially backs and arms.
• SBCCs do not resemble other types of BCC at all and are therefore often mistaken for “fungal infection.”
• Pay as much attention to the patient as to the lesion. There was abundant evidence of excessive sun damage on this patient’s trunk, making him an ideal patient for skin cancer.
A scaly lesion manifested on this 55-year-old man’s back several years ago. Over time, the lesion has grown, despite the application of several different OTC creams, including hydrocortisone and various antifungal creams. Previously asymptomatic, the lesion is now beginning to itch and occasionally bleed.
Additional history-taking reveals that for the first 10 years of his working life, the patient was a concrete finisher, working 12-hour shifts in the sun without a shirt, six days a week for months at a time. Aside from a 40-pack-year history of smoking, his health is “decent.”
EXAMINATION
The lesion is an 8 x 10–cm pink, scaly plaque with thready, well-defined scaly margins. Tiny focal erosions are seen in the peripheral leading edge. Closer inspection of the lesion’s center reveals multiple telangiectasias and a complete lack of hairs or hair follicles. Elsewhere on sun-exposed areas of the patient’s truncal skin, there are thousands of solar lentigines. Multiple small actinic keratoses are noted on his face as well.
What is the diagnosis?
DISCUSSION
Prior to consulting dermatology, this patient had talked extensively with his primary care provider (PCP) about the large lesion on his back. The PCP was quite sure the cause was a fungal organism and assured the patient that the prescribed antifungal cream would almost certainly cure it. This is a scenario in which almost every PCP will participate at some point early in his/her career. When treatment fails, the patient is then referred to dermatology—where a totally unexpected diagnosis is made.
This case represents the natural history of superficial basal cell carcinoma (SBCC), the appearance of which is at odds with any other kind of skin cancer, including the other types of basal cell carcinoma. SBCC is especially common on the shoulders and trunks of patients with a history of excessive sun exposure. The mid-back location on this patient is quite typical, as is the annular, scaly morphology.
The cancer—which originates in a row of cells at the base of the epidermis—effectively destroys surface structures such as skin lines and hair follicles as it spreads laterally. Left in place long enough, SBCCs can eventually become focally invasive and can even spread into regional lymph nodes. Metastasis from them is extremely rare but has been reported.
Treatment is therefore necessary and can range from surgical excision of smaller lesions to electrodessication and curettage to nonsurgical options, such as the application of imiquimod creams or 5-fluorouracil cream, or even radiation therapy. This man was treated with thrice-weekly application of imiquimod 3.75% cream for at least two months, which will cause irritation and scant drainage but will likely result in a cure.
One of the main benefits of his presentation to dermatology was the opportunity to encourage and facilitate yearly skin check-ups—important, given his risk for other sun-caused skin cancers (especially melanoma).
A major learning point to be gained from this case: Just because a lesion is annular and scaly doesn’t mean it’s fungal. The fungi have to have a source (animal, child, soil), and it helps a lot if the affected area is damp and warm (eg, groin or under a bulletproof vest). Immunosuppression (from corticosteroid use, especially) helps as well.
Besides fungal infection, the differential for this type of lesion includes Bowen’s disease (intraepidermal squamous cell carcinoma), psoriasis, and eczema. The fact that the lesion was fixed (did not come and go), grew steadily, and was on the directly sun-exposed skin of a person with a history of heavy sun exposure all pointed to the diagnosis.
TAKE-HOME LEARNING POINTS
• Superficial BCC (SBCC) has also been called “field fire” skin cancer because of the prominent, well-defined scaly border that expands peripherally and leaves a “scorched earth” look behind.
• SBCCs are common on skin directly exposed to the sun, especially backs and arms.
• SBCCs do not resemble other types of BCC at all and are therefore often mistaken for “fungal infection.”
• Pay as much attention to the patient as to the lesion. There was abundant evidence of excessive sun damage on this patient’s trunk, making him an ideal patient for skin cancer.
FDA panel backs approval of filgrastim biosimilar
SILVER SPRING, MD. – With the unanimous support of a Food and Drug Administration advisory panel and a favorable review by the agency, a “biosimilar” version of filgrastim, the recombinant granulocyte colony-stimulating factor marketed as Neupogen, will likely become the first biosimilar product to be approved in the United States.
At a meeting on Jan. 7, the FDA’s Oncologic Drugs Advisory Committee voted 14-0 that the filgrastim biosimilar should be approved for the five indications approved for Neupogen in the United States, based on “the totality of the evidence,” which includes pharmacokinetic, pharmacodynamic, immunogenicity, and clinical data. With little debate, they agreed that other than minor differences in clinically inactive components, the biosimilar, currently referred to as “EP2006,” was “highly similar” to the reference product, and that there were “no clinically meaningful differences” between EP2006 and Neupogen – the components of the definition of biosimilarity.
The meeting was considered historic, since this is the first biosimilar to be reviewed by an FDA advisory panel, and if approved, will be the first biosimilar to become available as a result of the Biologics Price Competition and Innovation Act of 2009, which was passed as part of the Affordable Care Act, creating “an abbreviated licensure pathway for biological products shown to be ‘biosimilar’ or ‘interchangeable’ with an FDA-licensed biological product,” according to the FDA.
Neupogen was approved by the FDA in 1991 and is marketed by Amgen. If approved, EP2006 would be the first biosimilar product to be approved in the United States, and, like generic drugs, is expected to provide a cheaper version of Neupogen. Sandoz, the generic pharmaceuticals arm of Novartis, plans to market the biosimilar as Zarxio in the United States. The filgrastim biosimilar was approved in 2009 in the European Union, where it is marketed as Zarzio, and it is now approved in more than 60 countries, with more than 7.5 million days of exposure, according to Sandoz.
Between 2009 and 2012, the use of filgrastim increased by 30% in Europe, and the biosimilar is now the most commonly prescribed version of filgrastim in Europe. The cost of filgrastim dropped substantially after approval because of the competition, company officials said.
EP2006 will be available in prefilled syringes containing 300 mcg/0.5 mL or 480 mcg/0.8 mL administered subcutaneously or intravenously, the same strengths as U.S.-approved Neupogen. For approval, Sandoz submitted eight studies, including two studies comparing EP2006 to U.S.-approved Neupogen conducted specifically for the U.S. approval, one of 28 healthy volunteers and another of 218 women with breast cancer, treated with myelosuppressive chemotherapy, evaluating the pharmacokinetics (PK), pharmacodynamics (PD), safety, and immunogenicity (the breast cancer study also evaluated safety and efficacy). The remaining studies included studies in healthy volunteers and breast cancer patients, comparing the biosimilar to European-approved Neupogen, or single-arm studies.
At the meeting, Sigrid Balser, Ph.D., of the global clinical development department at Sandoz, said that in the U.S. study of healthy volunteers, there was a “high degree of similarity” between EP2006 and Neupogen in terms of the PD and PK results, and the absolute neutrophil count (ANC) and CD34+ cell responses. In the U.S. study of breast cancer patients, EP2006 and Neupogen were equivalent in terms of efficacy over the first chemotherapy cycle and had a similar safety profile over six chemotherapy cycles. There were no signs of immunogenicity associated with the biosimilar in the U.S. or European studies in patients with breast cancer or healthy volunteers.
Outside of the United States, more than 3,800 patients have been treated with Zarzio for various indications, including chemotherapy-induced neutropenia, hematopoietic stem cell mobilization, and severe chronic neutropenia, and have been monitored as part of postmarketing trials or routine pharmacovigilance. To date, there have been no signals of any differences in the safety profile of the filgrastim biosimilar, compared with Neupogen;no cases of immunogenicity; and safety and effectiveness has been “confirmed in clinical practice,” Dr. Balser added.
Based on the data, the FDA reviewers concluded that EP2006 is “highly similar to U.S.-licensed Neupogen, and that there are no clinically meaningful difference between the two products,” said Dr. Albert Deisseroth, medical officer and team leader in the division of hematology products, in the FDA’s office of hematology and oncology products.
“Clinically, they appear to really function pretty equally in terms of what you’re asking them to do,” said the panel chair, Dr. Deborah Armstrong, professor of oncology at Johns Hopkins University, Baltimore. The extensive use of the filgrastim biosimilar in other parts of the world provided evidence of “robust safety and efficacy,” which contributed to her support for approval, she added.
Cost is not allowed to be discussed during FDA panel meetings, and Sandoz did not specify any possible price of the biosimilar, but this issue was the “elephant in the room,” as one panelist pointed out. However, Dr. Louis Weiner, chairman of the department of oncology and director of the Lombardi Comprehensive Cancer Center at Georgetown University in Washington, who spoke on behalf of Sandoz at the meeting, said that the availability of the filgrastim biosimilar has “enormous promise in reducing cost and expanding access” to this treatment. Although it has “unquestioned clinical value,” he said that granulocyte colony-stimulating factor therapy is underused.
The five indications approved for Neupogen are to decrease the incidence of infection‚ as manifested by febrile neutropenia in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever; for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of adults with acute myeloid leukemia; to reduce the duration of neutropenia and neutropenia-related clinical sequelae; for the mobilization of hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis; and for chronic administration to reduce the incidence and duration of sequelae of neutropenia in symptomatic patients with congenital‚ cyclic‚ or idiopathic neutropenia.
The FDA usually follows the recommendations of its advisory panels. Panelists were cleared of potential conflicts of interest related to the topic of the meeting. In some cases, a panelist may be given a waiver but not at this meeting. Dr. Weiner’s disclosure included being a consultant to Sandoz.
SILVER SPRING, MD. – With the unanimous support of a Food and Drug Administration advisory panel and a favorable review by the agency, a “biosimilar” version of filgrastim, the recombinant granulocyte colony-stimulating factor marketed as Neupogen, will likely become the first biosimilar product to be approved in the United States.
At a meeting on Jan. 7, the FDA’s Oncologic Drugs Advisory Committee voted 14-0 that the filgrastim biosimilar should be approved for the five indications approved for Neupogen in the United States, based on “the totality of the evidence,” which includes pharmacokinetic, pharmacodynamic, immunogenicity, and clinical data. With little debate, they agreed that other than minor differences in clinically inactive components, the biosimilar, currently referred to as “EP2006,” was “highly similar” to the reference product, and that there were “no clinically meaningful differences” between EP2006 and Neupogen – the components of the definition of biosimilarity.
The meeting was considered historic, since this is the first biosimilar to be reviewed by an FDA advisory panel, and if approved, will be the first biosimilar to become available as a result of the Biologics Price Competition and Innovation Act of 2009, which was passed as part of the Affordable Care Act, creating “an abbreviated licensure pathway for biological products shown to be ‘biosimilar’ or ‘interchangeable’ with an FDA-licensed biological product,” according to the FDA.
Neupogen was approved by the FDA in 1991 and is marketed by Amgen. If approved, EP2006 would be the first biosimilar product to be approved in the United States, and, like generic drugs, is expected to provide a cheaper version of Neupogen. Sandoz, the generic pharmaceuticals arm of Novartis, plans to market the biosimilar as Zarxio in the United States. The filgrastim biosimilar was approved in 2009 in the European Union, where it is marketed as Zarzio, and it is now approved in more than 60 countries, with more than 7.5 million days of exposure, according to Sandoz.
Between 2009 and 2012, the use of filgrastim increased by 30% in Europe, and the biosimilar is now the most commonly prescribed version of filgrastim in Europe. The cost of filgrastim dropped substantially after approval because of the competition, company officials said.
EP2006 will be available in prefilled syringes containing 300 mcg/0.5 mL or 480 mcg/0.8 mL administered subcutaneously or intravenously, the same strengths as U.S.-approved Neupogen. For approval, Sandoz submitted eight studies, including two studies comparing EP2006 to U.S.-approved Neupogen conducted specifically for the U.S. approval, one of 28 healthy volunteers and another of 218 women with breast cancer, treated with myelosuppressive chemotherapy, evaluating the pharmacokinetics (PK), pharmacodynamics (PD), safety, and immunogenicity (the breast cancer study also evaluated safety and efficacy). The remaining studies included studies in healthy volunteers and breast cancer patients, comparing the biosimilar to European-approved Neupogen, or single-arm studies.
At the meeting, Sigrid Balser, Ph.D., of the global clinical development department at Sandoz, said that in the U.S. study of healthy volunteers, there was a “high degree of similarity” between EP2006 and Neupogen in terms of the PD and PK results, and the absolute neutrophil count (ANC) and CD34+ cell responses. In the U.S. study of breast cancer patients, EP2006 and Neupogen were equivalent in terms of efficacy over the first chemotherapy cycle and had a similar safety profile over six chemotherapy cycles. There were no signs of immunogenicity associated with the biosimilar in the U.S. or European studies in patients with breast cancer or healthy volunteers.
Outside of the United States, more than 3,800 patients have been treated with Zarzio for various indications, including chemotherapy-induced neutropenia, hematopoietic stem cell mobilization, and severe chronic neutropenia, and have been monitored as part of postmarketing trials or routine pharmacovigilance. To date, there have been no signals of any differences in the safety profile of the filgrastim biosimilar, compared with Neupogen;no cases of immunogenicity; and safety and effectiveness has been “confirmed in clinical practice,” Dr. Balser added.
Based on the data, the FDA reviewers concluded that EP2006 is “highly similar to U.S.-licensed Neupogen, and that there are no clinically meaningful difference between the two products,” said Dr. Albert Deisseroth, medical officer and team leader in the division of hematology products, in the FDA’s office of hematology and oncology products.
“Clinically, they appear to really function pretty equally in terms of what you’re asking them to do,” said the panel chair, Dr. Deborah Armstrong, professor of oncology at Johns Hopkins University, Baltimore. The extensive use of the filgrastim biosimilar in other parts of the world provided evidence of “robust safety and efficacy,” which contributed to her support for approval, she added.
Cost is not allowed to be discussed during FDA panel meetings, and Sandoz did not specify any possible price of the biosimilar, but this issue was the “elephant in the room,” as one panelist pointed out. However, Dr. Louis Weiner, chairman of the department of oncology and director of the Lombardi Comprehensive Cancer Center at Georgetown University in Washington, who spoke on behalf of Sandoz at the meeting, said that the availability of the filgrastim biosimilar has “enormous promise in reducing cost and expanding access” to this treatment. Although it has “unquestioned clinical value,” he said that granulocyte colony-stimulating factor therapy is underused.
The five indications approved for Neupogen are to decrease the incidence of infection‚ as manifested by febrile neutropenia in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever; for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of adults with acute myeloid leukemia; to reduce the duration of neutropenia and neutropenia-related clinical sequelae; for the mobilization of hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis; and for chronic administration to reduce the incidence and duration of sequelae of neutropenia in symptomatic patients with congenital‚ cyclic‚ or idiopathic neutropenia.
The FDA usually follows the recommendations of its advisory panels. Panelists were cleared of potential conflicts of interest related to the topic of the meeting. In some cases, a panelist may be given a waiver but not at this meeting. Dr. Weiner’s disclosure included being a consultant to Sandoz.
SILVER SPRING, MD. – With the unanimous support of a Food and Drug Administration advisory panel and a favorable review by the agency, a “biosimilar” version of filgrastim, the recombinant granulocyte colony-stimulating factor marketed as Neupogen, will likely become the first biosimilar product to be approved in the United States.
At a meeting on Jan. 7, the FDA’s Oncologic Drugs Advisory Committee voted 14-0 that the filgrastim biosimilar should be approved for the five indications approved for Neupogen in the United States, based on “the totality of the evidence,” which includes pharmacokinetic, pharmacodynamic, immunogenicity, and clinical data. With little debate, they agreed that other than minor differences in clinically inactive components, the biosimilar, currently referred to as “EP2006,” was “highly similar” to the reference product, and that there were “no clinically meaningful differences” between EP2006 and Neupogen – the components of the definition of biosimilarity.
The meeting was considered historic, since this is the first biosimilar to be reviewed by an FDA advisory panel, and if approved, will be the first biosimilar to become available as a result of the Biologics Price Competition and Innovation Act of 2009, which was passed as part of the Affordable Care Act, creating “an abbreviated licensure pathway for biological products shown to be ‘biosimilar’ or ‘interchangeable’ with an FDA-licensed biological product,” according to the FDA.
Neupogen was approved by the FDA in 1991 and is marketed by Amgen. If approved, EP2006 would be the first biosimilar product to be approved in the United States, and, like generic drugs, is expected to provide a cheaper version of Neupogen. Sandoz, the generic pharmaceuticals arm of Novartis, plans to market the biosimilar as Zarxio in the United States. The filgrastim biosimilar was approved in 2009 in the European Union, where it is marketed as Zarzio, and it is now approved in more than 60 countries, with more than 7.5 million days of exposure, according to Sandoz.
Between 2009 and 2012, the use of filgrastim increased by 30% in Europe, and the biosimilar is now the most commonly prescribed version of filgrastim in Europe. The cost of filgrastim dropped substantially after approval because of the competition, company officials said.
EP2006 will be available in prefilled syringes containing 300 mcg/0.5 mL or 480 mcg/0.8 mL administered subcutaneously or intravenously, the same strengths as U.S.-approved Neupogen. For approval, Sandoz submitted eight studies, including two studies comparing EP2006 to U.S.-approved Neupogen conducted specifically for the U.S. approval, one of 28 healthy volunteers and another of 218 women with breast cancer, treated with myelosuppressive chemotherapy, evaluating the pharmacokinetics (PK), pharmacodynamics (PD), safety, and immunogenicity (the breast cancer study also evaluated safety and efficacy). The remaining studies included studies in healthy volunteers and breast cancer patients, comparing the biosimilar to European-approved Neupogen, or single-arm studies.
At the meeting, Sigrid Balser, Ph.D., of the global clinical development department at Sandoz, said that in the U.S. study of healthy volunteers, there was a “high degree of similarity” between EP2006 and Neupogen in terms of the PD and PK results, and the absolute neutrophil count (ANC) and CD34+ cell responses. In the U.S. study of breast cancer patients, EP2006 and Neupogen were equivalent in terms of efficacy over the first chemotherapy cycle and had a similar safety profile over six chemotherapy cycles. There were no signs of immunogenicity associated with the biosimilar in the U.S. or European studies in patients with breast cancer or healthy volunteers.
Outside of the United States, more than 3,800 patients have been treated with Zarzio for various indications, including chemotherapy-induced neutropenia, hematopoietic stem cell mobilization, and severe chronic neutropenia, and have been monitored as part of postmarketing trials or routine pharmacovigilance. To date, there have been no signals of any differences in the safety profile of the filgrastim biosimilar, compared with Neupogen;no cases of immunogenicity; and safety and effectiveness has been “confirmed in clinical practice,” Dr. Balser added.
Based on the data, the FDA reviewers concluded that EP2006 is “highly similar to U.S.-licensed Neupogen, and that there are no clinically meaningful difference between the two products,” said Dr. Albert Deisseroth, medical officer and team leader in the division of hematology products, in the FDA’s office of hematology and oncology products.
“Clinically, they appear to really function pretty equally in terms of what you’re asking them to do,” said the panel chair, Dr. Deborah Armstrong, professor of oncology at Johns Hopkins University, Baltimore. The extensive use of the filgrastim biosimilar in other parts of the world provided evidence of “robust safety and efficacy,” which contributed to her support for approval, she added.
Cost is not allowed to be discussed during FDA panel meetings, and Sandoz did not specify any possible price of the biosimilar, but this issue was the “elephant in the room,” as one panelist pointed out. However, Dr. Louis Weiner, chairman of the department of oncology and director of the Lombardi Comprehensive Cancer Center at Georgetown University in Washington, who spoke on behalf of Sandoz at the meeting, said that the availability of the filgrastim biosimilar has “enormous promise in reducing cost and expanding access” to this treatment. Although it has “unquestioned clinical value,” he said that granulocyte colony-stimulating factor therapy is underused.
The five indications approved for Neupogen are to decrease the incidence of infection‚ as manifested by febrile neutropenia in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever; for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of adults with acute myeloid leukemia; to reduce the duration of neutropenia and neutropenia-related clinical sequelae; for the mobilization of hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis; and for chronic administration to reduce the incidence and duration of sequelae of neutropenia in symptomatic patients with congenital‚ cyclic‚ or idiopathic neutropenia.
The FDA usually follows the recommendations of its advisory panels. Panelists were cleared of potential conflicts of interest related to the topic of the meeting. In some cases, a panelist may be given a waiver but not at this meeting. Dr. Weiner’s disclosure included being a consultant to Sandoz.
AT AN FDA ADVISORY COMMITTEE MEETING
Surgical vs. endovascular repair of popliteal artery aneurysm
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Surgical repair remains the optimal method to treat a popliteal artery aneurysm.
Popliteal artery aneurysm, or PAA, is the most common peripheral aneurysm, but data on this disease are nonetheless limited. A report from the VASCUNET collaboration of registries showed that 1,471 repairs were performed among a population of 58 million people in eight countries, for a rate of 9.6 per million. Most (72%) were elective, and 78% were open repairs (Eur. J. Vasc. Endovasc. Surg. 2014;47:164-71).
Although the endovascular approach has been increasingly used since 2000, outcomes have varied considerably across studies.
A review of 163 relevant studies from more than 1,600 that have been published since 1994 showed extensive heterogeneity with respect to the inclusion of symptomatic vs. asymptomatic patients, emergent vs. elective cases, poor runoff vs. good runoff, types of stents used, and types of conduits used for open repair. This renders the validity of the meta-analysis of these studies uncertain.
However, based on the few studies with complete data concerning mortality, major adverse events, primary and secondary patency, and limb salvage – with separate analysis for elective and emergency repairs – it appears that the availability of the great saphenous vein (GSV) is an important determinant when deciding whether to perform an open repair, that the posterior approach is preferred (except in cases of aneurysms extending to the adductor canal or trifurcation vessels), and that elective open repair is associated with significantly better outcomes than endovascular repair on a number of measures.
For example, no difference was seen in mortality in 23 selected studies, but the 3-year primary patency was significantly better with open repair (85% vs. 58%), while the 3-year rate of major adverse events, including mortality, major amputation, graft thrombosis, and reintervention was lower (20% vs. 38%).
These findings were confirmed in a study of 149 elective repairs. In that series, major adverse events were significantly more common in endovascular vs. open cases (hazard ratio, 2.1), and poor runoff was associated with a higher risk of major adverse events regardless of the technique used (J. Vasc. Surg. 2014 60:631-8.e2).
A recent decision analysis model applied to patients with asymptomatic PAA also demonstrated that elective open repair with a GSV bypass is the preferred treatment for all outcomes, with stenting recommended in high-risk patient or those without a suitable vein (J. Vasc. Surg. 2014;59:651-62).
One concern with endovascular repair is the risk of stent fracture, particularly in younger more active patients as data suggest that the more active the individual, the greater the risk of stent fracture. In one study, the frequency of stent fracture in younger patients was 17%. This suggests that stenting is probably not the best technique to be used in active individuals.
The current data suggest that the best outcomes are achieved with elective open repair using the great saphenous vein. However, patency rates above 80% at 2 years have been reported recently for elective endovascular repair associated with dual antiplatelet therapy, thus it is possible that new stent grafts and best medical therapy could improve the results of endovascular repair.
Open repair also appears to be best in most emergent cases. In one meta-analysis of 11 studies involving 223 patients, graft thrombosis occurred in 8% of open cases vs. 53% of endovascular cases, patency at 6 months was 82% vs. 68%, and reintervention rates were 25% vs. 43% in open vs. endovascular cases, respectively.
Thrombolysis was associated with a significant improvement in 1-year primary graft patency rates compared with surgery alone, but this did not affect the amputation rate, and endovascular repair does not appear to improve the severe prognosis of acute ischemia in patients with PAA.
Despite the deceiving results of endovascular repair in those with acute ischemia, this technique could, however, be very useful in other emergent situations. For example, very old patients presenting with a ruptured PAA and a good runoff could benefit from an endovascular repair.
In summary, no level 1 evidence regarding open vs. endovascular repair for popliteal artery aneurysms can be obtained; most studies are retrospective and lack important characteristics. Based on the data that do exist, however, open repair with a vein bypass appears to be the best technique for most patients with PAA. Stenting should be reserved for high-risk and elderly asymptomatic patients. As for those with acute limb ischemia, no strong recommendation can be made based on the available data.
Dr. Jean-Baptiste Ricco is professor and chief of vascular surgery at the University of Poitiers, France. He reported having no disclosures. This and the accompanying perspective by Dr. Marone were based upon their live debate at the 2014 Vascular Annual Meeting.
Endovascular repair of PAA is an effective and durable treatment.
Outcomes following endovascular repair of PAA are at least equivalent to those following open repair with respect to patency and limb salvage in elective cases.
In the Swedish Vascular Registry – the largest report of open repair, representing 717 limbs treated with a mean follow-up of 7.2 years – the primary patency rate at 1 year for cases performed with a medial approach was 90% with vein conduit, and 72% with prosthetic conduit. For cases involving a posterior approach, the rates were 85% with vein conduit, and 81% with prosthetic conduit. The amputation rate was 9.6% at 1 year and 11% at last follow-up.
Furthermore, open surgical procedures are associated with a high wound complication rate. The average across studies is 7%, and was as high as 28% in some series. Open procedures are also associated with variable graft patency, continued aneurysm expansion (which occurred in a third of limbs treated with a medial approach in one series), and a significant amputation rate.
Data regarding endovascular PAA repair are encouraging. In the only prospective randomized trial to date, no significant difference was seen at 46 months with respect to primary patency (100% with open repair vs. 93.3% with endovascular repair), or limb salvage (100% for both).
Hospital length of stay, however, was significantly shorter with endovascular repair (7.7 days vs. 4.3 days), as was operative time (155 minutes vs. 75 minutes).
An update to that 2005 study (J. Vasc. Surg. 2005;42:185-93) showed no difference in patency at 72 months.
While there is a paucity of level 1 evidence (only 15 patients were included in each arm of that study), prospective cohort studies and institutional reviews also demonstrate the value of endovascular repair. Secondary patency at 1 and 3 years were 87% and 85%, respectively, in a 2013 study (Ann. Vasc. Surg. 2013;27:259-65), and the 1- and 2-year amputation rates were 2% and 3%, respectively.
In another series, 1-year primary patency was 92.9% with endovascular repair, compared with 83.3% with open repair, and 3-year patency was 63.7% vs. 77.8%. The differences were not statistically significant.
Length of stay was 3.9 days vs. 9.5 days. Eight wound infections and 2 hematomas occurred in the open repair patients, and two patients experienced enlargement requiring decompression.
The University of Pittsburgh experience with 50 endovascular repairs and 111 open repairs performed between 2004 and 2010 showed that morbidity was 14% vs. 32% for endovascular vs. open repair, and mortality was 2% vs. 3.6%, respectively, at 29-month follow-up. Wound infection rates were 2% and 16.2%, respectively, length of stay was 1 vs. 4 days, and reintervention and thrombosis rates did not differ significantly (12.2% vs. 10.8%, and 8 vs. 12 patients).
No significant differences were seen in aneurysm growth, primary assisted patency at 3 years, secondary patency at 3 years, or amputation rates at 1 year or 3 years.
A 2013 update also showed no differences in these outcomes.
In summary, endovascular repair of PAA is acceptable, with outcomes comparable to or better than open repair in elective cases. Long-term durability has been demonstrated, limb preservation is equivalent to open repair, and thrombotic complications are rare and can be treated successfully with re-intervention.
Furthermore, endovascular repair can be performed without the need for general anesthesia, lower morbidity can be expected perioperatively, hospital length of stay is shorter, and patients have a quicker return of functional status.
Dr. Luke Marone is a vascular surgeon at the University of Pittsburgh School of Medicine. He disclosed he is a consultant for Abiomed and Abbott.
Drug reverses dabigatran’s effects in elderly/impaired

Credit: CDC
SAN FRANCISCO—An investigational, humanized antibody fragment known as idarucizumab can reverse the anticoagulation effects of dabigatran, new research suggests.
In a small study, idarucizumab led to sustained reversal of dabigatran’s effects in healthy subjects, elderly volunteers, and participants with mild to moderate renal impairment.
Furthermore, dabigatran anticoagulation could be re-established 24 hours after the subjects had received idarucizumab.
Joachim Stangier, PhD, of Boehringer Ingelheim Pharma GmbH & Co KG in Biberach, Germany, presented these results at the 2014 ASH Annual Meeting as abstract 344*. Boehringer Ingelheim is the company developing idarucizumab.
Dr Stangier said idarucizumab binds dabigatran with high affinity, off-target binding is not expected, and no procoagulant effects have been observed with the drug.
In a previous study of healthy volunteers, idarucizumab provided immediate, complete, and sustained reversal of dabigatran’s anticoagulant effect. The effect was sustained when idarucizumab was given at 2 g and 4 g doses.
For the current study, Dr Stangier and his colleagues wanted to evaluate whether and to what extent doses of up to 5 g of idarucizumab would reverse the anticoagulant effects of dabigatran in healthy subjects, elderly participants, and renally impaired volunteers. The team also wanted to determine the effects of dabigatran given after subjects had received idarucizumab.
The study included 46 male and female subjects who were 45 to 80 years of age. Healthy subjects received dabigatran etexilate at 220 mg twice daily, and subjects with mild to moderate renal impairment received 150 mg twice daily, both over 4 days.
Subjects then received idarucizumab at 1 g, 2.5 g, 5 g, or 5 g given as 2 x 2.5 g 1 hour apart. They received these doses as 5-minute intravenous infusions 2 hours after their last dose of dabigatran.
The researchers found that dabigatran-prolonged clotting times—thrombin time, diluted thrombin time, ecarin clotting time, and activated partial thromboplastin time—were reversed to baseline immediately after the end of idarucizumab infusion.
And the team observed sustained reversal of dabigatran’s anticoagulant effects in all subjects.
The researchers then readministered dabigatran to healthy subjects 24 hours after idarucizumab treatment and to subjects who received placebo instead of idarucizumab.
Dabigatran-mediated anticoagulation was similar in subjects who received idarucizumab and those who received placebo. And anticoagulation was restored to levels comparable to initial levels.
Dr Stangier said there were no clinically relevant adverse events (AEs) related to idarucizumab, and there were no relevant changes in any of the investigated safety parameters. There were no AEs indicative of immunogenic reactions.
AEs and local tolerability reactions were similar for placebo and idarucizumab. And there was no relationship between the frequency of AEs and drug dose, gender, or renal function.
The researchers did observe a dose-dependent, transient increase in urine protein and low-weight proteins, but values returned to the normal range within 4 hours to 24 hours.
Based on these results, Dr Stangier said idarucizumab fulfills the requirements for a fast-acting and specific antidote to dabigatran with a favorable safety profile. Additional clinical testing of the antidote is ongoing. ![]()
*Information in the abstract differs from that presented.

Credit: CDC
SAN FRANCISCO—An investigational, humanized antibody fragment known as idarucizumab can reverse the anticoagulation effects of dabigatran, new research suggests.
In a small study, idarucizumab led to sustained reversal of dabigatran’s effects in healthy subjects, elderly volunteers, and participants with mild to moderate renal impairment.
Furthermore, dabigatran anticoagulation could be re-established 24 hours after the subjects had received idarucizumab.
Joachim Stangier, PhD, of Boehringer Ingelheim Pharma GmbH & Co KG in Biberach, Germany, presented these results at the 2014 ASH Annual Meeting as abstract 344*. Boehringer Ingelheim is the company developing idarucizumab.
Dr Stangier said idarucizumab binds dabigatran with high affinity, off-target binding is not expected, and no procoagulant effects have been observed with the drug.
In a previous study of healthy volunteers, idarucizumab provided immediate, complete, and sustained reversal of dabigatran’s anticoagulant effect. The effect was sustained when idarucizumab was given at 2 g and 4 g doses.
For the current study, Dr Stangier and his colleagues wanted to evaluate whether and to what extent doses of up to 5 g of idarucizumab would reverse the anticoagulant effects of dabigatran in healthy subjects, elderly participants, and renally impaired volunteers. The team also wanted to determine the effects of dabigatran given after subjects had received idarucizumab.
The study included 46 male and female subjects who were 45 to 80 years of age. Healthy subjects received dabigatran etexilate at 220 mg twice daily, and subjects with mild to moderate renal impairment received 150 mg twice daily, both over 4 days.
Subjects then received idarucizumab at 1 g, 2.5 g, 5 g, or 5 g given as 2 x 2.5 g 1 hour apart. They received these doses as 5-minute intravenous infusions 2 hours after their last dose of dabigatran.
The researchers found that dabigatran-prolonged clotting times—thrombin time, diluted thrombin time, ecarin clotting time, and activated partial thromboplastin time—were reversed to baseline immediately after the end of idarucizumab infusion.
And the team observed sustained reversal of dabigatran’s anticoagulant effects in all subjects.
The researchers then readministered dabigatran to healthy subjects 24 hours after idarucizumab treatment and to subjects who received placebo instead of idarucizumab.
Dabigatran-mediated anticoagulation was similar in subjects who received idarucizumab and those who received placebo. And anticoagulation was restored to levels comparable to initial levels.
Dr Stangier said there were no clinically relevant adverse events (AEs) related to idarucizumab, and there were no relevant changes in any of the investigated safety parameters. There were no AEs indicative of immunogenic reactions.
AEs and local tolerability reactions were similar for placebo and idarucizumab. And there was no relationship between the frequency of AEs and drug dose, gender, or renal function.
The researchers did observe a dose-dependent, transient increase in urine protein and low-weight proteins, but values returned to the normal range within 4 hours to 24 hours.
Based on these results, Dr Stangier said idarucizumab fulfills the requirements for a fast-acting and specific antidote to dabigatran with a favorable safety profile. Additional clinical testing of the antidote is ongoing. ![]()
*Information in the abstract differs from that presented.

Credit: CDC
SAN FRANCISCO—An investigational, humanized antibody fragment known as idarucizumab can reverse the anticoagulation effects of dabigatran, new research suggests.
In a small study, idarucizumab led to sustained reversal of dabigatran’s effects in healthy subjects, elderly volunteers, and participants with mild to moderate renal impairment.
Furthermore, dabigatran anticoagulation could be re-established 24 hours after the subjects had received idarucizumab.
Joachim Stangier, PhD, of Boehringer Ingelheim Pharma GmbH & Co KG in Biberach, Germany, presented these results at the 2014 ASH Annual Meeting as abstract 344*. Boehringer Ingelheim is the company developing idarucizumab.
Dr Stangier said idarucizumab binds dabigatran with high affinity, off-target binding is not expected, and no procoagulant effects have been observed with the drug.
In a previous study of healthy volunteers, idarucizumab provided immediate, complete, and sustained reversal of dabigatran’s anticoagulant effect. The effect was sustained when idarucizumab was given at 2 g and 4 g doses.
For the current study, Dr Stangier and his colleagues wanted to evaluate whether and to what extent doses of up to 5 g of idarucizumab would reverse the anticoagulant effects of dabigatran in healthy subjects, elderly participants, and renally impaired volunteers. The team also wanted to determine the effects of dabigatran given after subjects had received idarucizumab.
The study included 46 male and female subjects who were 45 to 80 years of age. Healthy subjects received dabigatran etexilate at 220 mg twice daily, and subjects with mild to moderate renal impairment received 150 mg twice daily, both over 4 days.
Subjects then received idarucizumab at 1 g, 2.5 g, 5 g, or 5 g given as 2 x 2.5 g 1 hour apart. They received these doses as 5-minute intravenous infusions 2 hours after their last dose of dabigatran.
The researchers found that dabigatran-prolonged clotting times—thrombin time, diluted thrombin time, ecarin clotting time, and activated partial thromboplastin time—were reversed to baseline immediately after the end of idarucizumab infusion.
And the team observed sustained reversal of dabigatran’s anticoagulant effects in all subjects.
The researchers then readministered dabigatran to healthy subjects 24 hours after idarucizumab treatment and to subjects who received placebo instead of idarucizumab.
Dabigatran-mediated anticoagulation was similar in subjects who received idarucizumab and those who received placebo. And anticoagulation was restored to levels comparable to initial levels.
Dr Stangier said there were no clinically relevant adverse events (AEs) related to idarucizumab, and there were no relevant changes in any of the investigated safety parameters. There were no AEs indicative of immunogenic reactions.
AEs and local tolerability reactions were similar for placebo and idarucizumab. And there was no relationship between the frequency of AEs and drug dose, gender, or renal function.
The researchers did observe a dose-dependent, transient increase in urine protein and low-weight proteins, but values returned to the normal range within 4 hours to 24 hours.
Based on these results, Dr Stangier said idarucizumab fulfills the requirements for a fast-acting and specific antidote to dabigatran with a favorable safety profile. Additional clinical testing of the antidote is ongoing. ![]()
*Information in the abstract differs from that presented.