User login
Study aims to determine prognostic factors for subset of thyroid cancer patients
CORONADO, CALIF. – In patients with radioactive iodine–refractory differentiated thyroid cancer, those with target lesions less than 1.5 cm in size appeared to derive less benefit from sorafenib in terms of progression-free survival, results from an international study showed.
In addition, papillary histology was a positive predictive factor and a predictive factor for benefit from sorafenib.
“Patients with radioactive iodine–refractory differentiated thyroid cancer have a poor prognosis, and there is a lack of effective treatments,” Dr. Martin Schlumberger said at the annual meeting of the American Thyroid Association. “The median survival for this subset is estimated to be 2.5-5 years.”
Sorafenib was approved by the Food and Drug Administration in November 2013 for the treatment of radioactive iodine–refractory differentiated thyroid cancer based on results from the randomized, controlled, double-blind phase III DECISION trial (Lancet 2014;384:319-28). Investigators found that the use of sorafenib extended median progression-free survival by 5 months, compared with placebo (10.8 vs 5.8 months; P < .0001). The purpose of the current analysis was to determine which demographic baseline or disease-related characteristics are prognostic for better outcomes in this patient population. To do so, Dr. Schlumberger of the department of nuclear medicine and endocrine oncology at Gustave Roussy, Villejuif, France, and his associates performed multivariate Cox proportional hazards models adjusted for treatment effect.
He reported findings from 417 patients. Of these, 210 were randomized to receive placebo and 207 were randomized to receive sorafenib. Variables found to be prognostic factors for progression-free survival in placebo patients, and in all patients when adjusted for sorafenib treatment, included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia vs. Europe and North America. Subgroup analyses of patients in the sorafenib arm revealed that the following baseline or disease-related variables were predictive of progression-free survival: papillary histology, tumor size of at least 1.5 cm, and having only lung metastases.
In a post-hoc exploratory analysis of progression-free survival by thyroid cancer symptoms among all 417 patients at study entry, the researchers found that both symptomatic and asymptomatic patients had improved progression-free survival following treatment with sorafenib.
On the basis of these findings, radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm “appear to have a good prognosis and may be candidates for a ‘watch and wait’ approach before initiating treatment with sorafenib,” Dr. Schlumberger concluded.
Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
On Twitter @dougbrunk
CORONADO, CALIF. – In patients with radioactive iodine–refractory differentiated thyroid cancer, those with target lesions less than 1.5 cm in size appeared to derive less benefit from sorafenib in terms of progression-free survival, results from an international study showed.
In addition, papillary histology was a positive predictive factor and a predictive factor for benefit from sorafenib.
“Patients with radioactive iodine–refractory differentiated thyroid cancer have a poor prognosis, and there is a lack of effective treatments,” Dr. Martin Schlumberger said at the annual meeting of the American Thyroid Association. “The median survival for this subset is estimated to be 2.5-5 years.”
Sorafenib was approved by the Food and Drug Administration in November 2013 for the treatment of radioactive iodine–refractory differentiated thyroid cancer based on results from the randomized, controlled, double-blind phase III DECISION trial (Lancet 2014;384:319-28). Investigators found that the use of sorafenib extended median progression-free survival by 5 months, compared with placebo (10.8 vs 5.8 months; P < .0001). The purpose of the current analysis was to determine which demographic baseline or disease-related characteristics are prognostic for better outcomes in this patient population. To do so, Dr. Schlumberger of the department of nuclear medicine and endocrine oncology at Gustave Roussy, Villejuif, France, and his associates performed multivariate Cox proportional hazards models adjusted for treatment effect.
He reported findings from 417 patients. Of these, 210 were randomized to receive placebo and 207 were randomized to receive sorafenib. Variables found to be prognostic factors for progression-free survival in placebo patients, and in all patients when adjusted for sorafenib treatment, included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia vs. Europe and North America. Subgroup analyses of patients in the sorafenib arm revealed that the following baseline or disease-related variables were predictive of progression-free survival: papillary histology, tumor size of at least 1.5 cm, and having only lung metastases.
In a post-hoc exploratory analysis of progression-free survival by thyroid cancer symptoms among all 417 patients at study entry, the researchers found that both symptomatic and asymptomatic patients had improved progression-free survival following treatment with sorafenib.
On the basis of these findings, radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm “appear to have a good prognosis and may be candidates for a ‘watch and wait’ approach before initiating treatment with sorafenib,” Dr. Schlumberger concluded.
Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
On Twitter @dougbrunk
CORONADO, CALIF. – In patients with radioactive iodine–refractory differentiated thyroid cancer, those with target lesions less than 1.5 cm in size appeared to derive less benefit from sorafenib in terms of progression-free survival, results from an international study showed.
In addition, papillary histology was a positive predictive factor and a predictive factor for benefit from sorafenib.
“Patients with radioactive iodine–refractory differentiated thyroid cancer have a poor prognosis, and there is a lack of effective treatments,” Dr. Martin Schlumberger said at the annual meeting of the American Thyroid Association. “The median survival for this subset is estimated to be 2.5-5 years.”
Sorafenib was approved by the Food and Drug Administration in November 2013 for the treatment of radioactive iodine–refractory differentiated thyroid cancer based on results from the randomized, controlled, double-blind phase III DECISION trial (Lancet 2014;384:319-28). Investigators found that the use of sorafenib extended median progression-free survival by 5 months, compared with placebo (10.8 vs 5.8 months; P < .0001). The purpose of the current analysis was to determine which demographic baseline or disease-related characteristics are prognostic for better outcomes in this patient population. To do so, Dr. Schlumberger of the department of nuclear medicine and endocrine oncology at Gustave Roussy, Villejuif, France, and his associates performed multivariate Cox proportional hazards models adjusted for treatment effect.
He reported findings from 417 patients. Of these, 210 were randomized to receive placebo and 207 were randomized to receive sorafenib. Variables found to be prognostic factors for progression-free survival in placebo patients, and in all patients when adjusted for sorafenib treatment, included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia vs. Europe and North America. Subgroup analyses of patients in the sorafenib arm revealed that the following baseline or disease-related variables were predictive of progression-free survival: papillary histology, tumor size of at least 1.5 cm, and having only lung metastases.
In a post-hoc exploratory analysis of progression-free survival by thyroid cancer symptoms among all 417 patients at study entry, the researchers found that both symptomatic and asymptomatic patients had improved progression-free survival following treatment with sorafenib.
On the basis of these findings, radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm “appear to have a good prognosis and may be candidates for a ‘watch and wait’ approach before initiating treatment with sorafenib,” Dr. Schlumberger concluded.
Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm may be candidates for a “watch and wait” approach before initiating treatment with sorafenib.
Major finding: Baseline or disease-related variables found to be prognostic factors for progression-free survival in placebo patients and in all patients when adjusted for sorafenib treatment included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia versus Europe and North America.
Data source: An analysis of 417 patients from the randomized, controlled, double-blind, phase III DECISION trial.
Disclosures: Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
Product News: 01 2015
Onexton
Valeant Pharmaceuticals International, Inc, an-nounces US Food and Drug Administration approval of Onexton Gel (clindamycin phosphate 1.2% and benzoyl peroxide 3.75%) for the once-daily treatment of comedonal and inflammatory acne in patients 12 years and older. This dual-action topical therapy has a favorable cutaneous tolerability profile and contains no surfactants, alcohol, or preservatives. Onexton is expected to launch in early 2015. For more information, visit www.valeant.com.
Physical Eye UV Defense Sunscreen
SkinCeuticals presents Physical Eye UV Defense Sunscreen that provides broad-spectrum SPF 50 protection without migrating into or irritating the eyes. Physical Eye UV Defense unifies natural skin tone around the eye and provides a translucent universal tint. It should be applied around the entire eye area and is optimized for application under makeup. It also can be used following hyaluronic acid filler and botulinum toxin injections. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
Refining Mineral Mask
Revision Skincare introduces the limited edition Refining Mineral Mask, a warming mask to reduce the appearance of pores and leave skin looking refined. It contains kaolin to purify the complexion, pumpkin enzymes to gently exfoliate skin, zeolite to provide an extra boost of radiance with its warming effect, and vitamin E microspheres to condition skin. Revision Skincare products are available exclusively through dermatologists, plastic surgeons, and medical spas. For more information, visit www.revisionskincare.com.
Resveratrol B E
SkinCeuticals launches Resveratrol B E, an intensive antioxidant night concentrate that boosts the skin’s endogenous antioxidant defense system, which loses efficiency with age and accumulated damage. It works by neutralizing age-accelerating internal free radicals, strength-ening functionality to resist new damage, and promoting skin’s natural repair to diminish the signs of accumulated damage. Resveratrol B E corrects signs of photodamage, loss of firmness and radiance, poor elasticity, and fine lines and wrinkles. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Onexton
Valeant Pharmaceuticals International, Inc, an-nounces US Food and Drug Administration approval of Onexton Gel (clindamycin phosphate 1.2% and benzoyl peroxide 3.75%) for the once-daily treatment of comedonal and inflammatory acne in patients 12 years and older. This dual-action topical therapy has a favorable cutaneous tolerability profile and contains no surfactants, alcohol, or preservatives. Onexton is expected to launch in early 2015. For more information, visit www.valeant.com.
Physical Eye UV Defense Sunscreen
SkinCeuticals presents Physical Eye UV Defense Sunscreen that provides broad-spectrum SPF 50 protection without migrating into or irritating the eyes. Physical Eye UV Defense unifies natural skin tone around the eye and provides a translucent universal tint. It should be applied around the entire eye area and is optimized for application under makeup. It also can be used following hyaluronic acid filler and botulinum toxin injections. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
Refining Mineral Mask
Revision Skincare introduces the limited edition Refining Mineral Mask, a warming mask to reduce the appearance of pores and leave skin looking refined. It contains kaolin to purify the complexion, pumpkin enzymes to gently exfoliate skin, zeolite to provide an extra boost of radiance with its warming effect, and vitamin E microspheres to condition skin. Revision Skincare products are available exclusively through dermatologists, plastic surgeons, and medical spas. For more information, visit www.revisionskincare.com.
Resveratrol B E
SkinCeuticals launches Resveratrol B E, an intensive antioxidant night concentrate that boosts the skin’s endogenous antioxidant defense system, which loses efficiency with age and accumulated damage. It works by neutralizing age-accelerating internal free radicals, strength-ening functionality to resist new damage, and promoting skin’s natural repair to diminish the signs of accumulated damage. Resveratrol B E corrects signs of photodamage, loss of firmness and radiance, poor elasticity, and fine lines and wrinkles. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Onexton
Valeant Pharmaceuticals International, Inc, an-nounces US Food and Drug Administration approval of Onexton Gel (clindamycin phosphate 1.2% and benzoyl peroxide 3.75%) for the once-daily treatment of comedonal and inflammatory acne in patients 12 years and older. This dual-action topical therapy has a favorable cutaneous tolerability profile and contains no surfactants, alcohol, or preservatives. Onexton is expected to launch in early 2015. For more information, visit www.valeant.com.
Physical Eye UV Defense Sunscreen
SkinCeuticals presents Physical Eye UV Defense Sunscreen that provides broad-spectrum SPF 50 protection without migrating into or irritating the eyes. Physical Eye UV Defense unifies natural skin tone around the eye and provides a translucent universal tint. It should be applied around the entire eye area and is optimized for application under makeup. It also can be used following hyaluronic acid filler and botulinum toxin injections. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
Refining Mineral Mask
Revision Skincare introduces the limited edition Refining Mineral Mask, a warming mask to reduce the appearance of pores and leave skin looking refined. It contains kaolin to purify the complexion, pumpkin enzymes to gently exfoliate skin, zeolite to provide an extra boost of radiance with its warming effect, and vitamin E microspheres to condition skin. Revision Skincare products are available exclusively through dermatologists, plastic surgeons, and medical spas. For more information, visit www.revisionskincare.com.
Resveratrol B E
SkinCeuticals launches Resveratrol B E, an intensive antioxidant night concentrate that boosts the skin’s endogenous antioxidant defense system, which loses efficiency with age and accumulated damage. It works by neutralizing age-accelerating internal free radicals, strength-ening functionality to resist new damage, and promoting skin’s natural repair to diminish the signs of accumulated damage. Resveratrol B E corrects signs of photodamage, loss of firmness and radiance, poor elasticity, and fine lines and wrinkles. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Reduced Degree of Irritation During a Second Cycle of Ingenol Mebutate Gel 0.015% for the Treatment of Actinic Keratosis
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
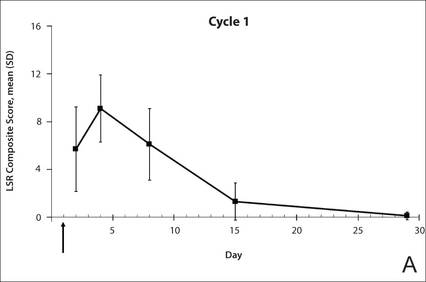
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
|

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
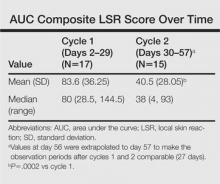
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
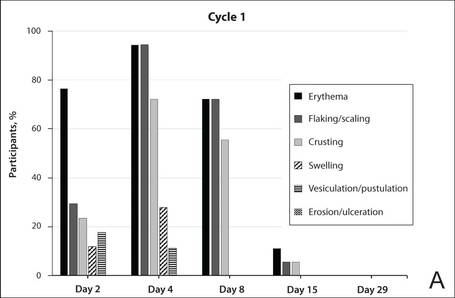
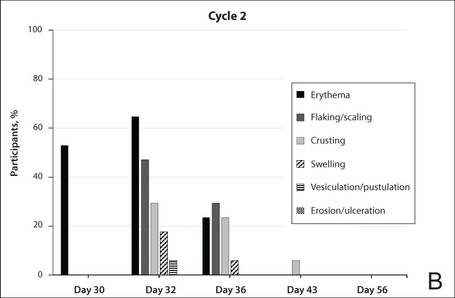
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
|
|
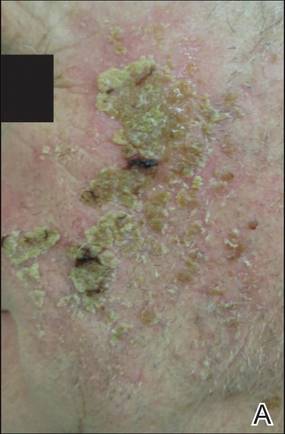
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
|

|
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.

|

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.

|

|
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes

|

|
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.

|

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.

|

|
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes

|

|
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Practice Points
- Reapplication of ingenol mebutate gel 0.015% to the same treatment area on the face or scalp produced a less intense inflammatory reaction than the first treatment course.
- Ingenol mebutate may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over 2 treatment cycles.
- Almost all patients were either clear or almost clear of actinic keratosis lesions by 4 weeks following the second application of ingenol mebutate.
Survey reveals cancer survivors’ unmet needs

patient and her father
Credit: Rhoda Baer
New research shows that, even decades after being cured, many cancer survivors face challenges resulting from their disease and its treatment.
A survey of more than 1500 cancer survivors revealed 16 themes of challenges or unmet needs, such as physical dysfunction, financial problems, a lack of education about cancer survival, and anxiety about cancer recurrence.
Mary Ann Burg, PhD, of the University of Central Florida in Orlando, and her colleagues reported these findings in Cancer.
To assess the unmet needs of cancer survivors, the researchers evaluated responses from an American Cancer Society survey in which subjects responded to the open-ended question, “Please tell us about any needs you have now as a cancer survivor that are not being met to your satisfaction.”
There were a total of 1514 respondents who were 2, 5, or 10 years from cancer diagnosis. They were 24 to 97 years of age, 65.4% were female, and 24.8% were racial/ethnic minorities (black and Hispanic/Latino).
“This study was unique in that it gave a very large sample of cancer survivors a real voice to express their needs and concerns,” Dr Burg said.
The researchers found that the number and type of challenges/unmet needs were not associated with a subject’s time since cancer treatment, although older cancer survivors tended to report fewer unmet needs than younger survivors.
Sixteen themes of challenges/unmet needs emerged from respondents’ answers, with physical issues being the most common. About 38% of respondents reported physical issues, such as pain, symptoms, and sexual dysfunction.
About 20% reported financial problems, such as issues with insurance and the affordability of needed services and products. About 20% also said they had needs related to unanswered questions and a lack of knowledge about what to expect as a cancer survivor, including guidance on follow-up care and cancer risks, causes, and prevention.
About 16% of respondents cited issues relating to personal control (a lack of physical and social autonomy). And about 16% described flaws and constraints in the healthcare system that affected early detection, diagnosis, treatment, follow-up care, and continuity of care.
About 14% of respondents reported a lack of resources (such as supplies, equipment, and medications), and about 14% cited emotional and mental health issues (such as fear of cancer recurrence, depression, and anxiety).
About 13% of respondents said they lacked social support (such as access to support groups), and 10% reported issues relating to societal perceptions of cancer survivors (such as discrimination and misinformation).
About 9% of respondents expressed the need to talk about or explain the cancer experience with their physician, friends, and family. And about 9% cited a lack of trust in healthcare providers.
Other themes included the wish for more effective cancer treatments (3.5%), body image issues such as feeling unattractive or losing trust in the body (3.5%), issues with the “survivor” identity (3.1%), trouble obtaining or maintaining appropriate employment (2.3%), and existential issues, such as finding meaning in the cancer experience (0.6%).
“Overall, we found that cancer survivors are often caught off guard by the lingering problems they experience after cancer treatment,” Dr Burg said. “In the wake of cancer, many survivors feel they have lost a sense of personal control, have reduced quality of life, and are frustrated that these problems are not sufficiently addressed within the medical care system.”
She added that this study points to several areas in which we might work to improve the situation, including raising public awareness of cancer survivors’ problems, promoting honest professional communication about the side effects of cancer and its treatment, and coordinating medical care resources to help survivors and their families cope with lingering challenges. ![]()

patient and her father
Credit: Rhoda Baer
New research shows that, even decades after being cured, many cancer survivors face challenges resulting from their disease and its treatment.
A survey of more than 1500 cancer survivors revealed 16 themes of challenges or unmet needs, such as physical dysfunction, financial problems, a lack of education about cancer survival, and anxiety about cancer recurrence.
Mary Ann Burg, PhD, of the University of Central Florida in Orlando, and her colleagues reported these findings in Cancer.
To assess the unmet needs of cancer survivors, the researchers evaluated responses from an American Cancer Society survey in which subjects responded to the open-ended question, “Please tell us about any needs you have now as a cancer survivor that are not being met to your satisfaction.”
There were a total of 1514 respondents who were 2, 5, or 10 years from cancer diagnosis. They were 24 to 97 years of age, 65.4% were female, and 24.8% were racial/ethnic minorities (black and Hispanic/Latino).
“This study was unique in that it gave a very large sample of cancer survivors a real voice to express their needs and concerns,” Dr Burg said.
The researchers found that the number and type of challenges/unmet needs were not associated with a subject’s time since cancer treatment, although older cancer survivors tended to report fewer unmet needs than younger survivors.
Sixteen themes of challenges/unmet needs emerged from respondents’ answers, with physical issues being the most common. About 38% of respondents reported physical issues, such as pain, symptoms, and sexual dysfunction.
About 20% reported financial problems, such as issues with insurance and the affordability of needed services and products. About 20% also said they had needs related to unanswered questions and a lack of knowledge about what to expect as a cancer survivor, including guidance on follow-up care and cancer risks, causes, and prevention.
About 16% of respondents cited issues relating to personal control (a lack of physical and social autonomy). And about 16% described flaws and constraints in the healthcare system that affected early detection, diagnosis, treatment, follow-up care, and continuity of care.
About 14% of respondents reported a lack of resources (such as supplies, equipment, and medications), and about 14% cited emotional and mental health issues (such as fear of cancer recurrence, depression, and anxiety).
About 13% of respondents said they lacked social support (such as access to support groups), and 10% reported issues relating to societal perceptions of cancer survivors (such as discrimination and misinformation).
About 9% of respondents expressed the need to talk about or explain the cancer experience with their physician, friends, and family. And about 9% cited a lack of trust in healthcare providers.
Other themes included the wish for more effective cancer treatments (3.5%), body image issues such as feeling unattractive or losing trust in the body (3.5%), issues with the “survivor” identity (3.1%), trouble obtaining or maintaining appropriate employment (2.3%), and existential issues, such as finding meaning in the cancer experience (0.6%).
“Overall, we found that cancer survivors are often caught off guard by the lingering problems they experience after cancer treatment,” Dr Burg said. “In the wake of cancer, many survivors feel they have lost a sense of personal control, have reduced quality of life, and are frustrated that these problems are not sufficiently addressed within the medical care system.”
She added that this study points to several areas in which we might work to improve the situation, including raising public awareness of cancer survivors’ problems, promoting honest professional communication about the side effects of cancer and its treatment, and coordinating medical care resources to help survivors and their families cope with lingering challenges. ![]()

patient and her father
Credit: Rhoda Baer
New research shows that, even decades after being cured, many cancer survivors face challenges resulting from their disease and its treatment.
A survey of more than 1500 cancer survivors revealed 16 themes of challenges or unmet needs, such as physical dysfunction, financial problems, a lack of education about cancer survival, and anxiety about cancer recurrence.
Mary Ann Burg, PhD, of the University of Central Florida in Orlando, and her colleagues reported these findings in Cancer.
To assess the unmet needs of cancer survivors, the researchers evaluated responses from an American Cancer Society survey in which subjects responded to the open-ended question, “Please tell us about any needs you have now as a cancer survivor that are not being met to your satisfaction.”
There were a total of 1514 respondents who were 2, 5, or 10 years from cancer diagnosis. They were 24 to 97 years of age, 65.4% were female, and 24.8% were racial/ethnic minorities (black and Hispanic/Latino).
“This study was unique in that it gave a very large sample of cancer survivors a real voice to express their needs and concerns,” Dr Burg said.
The researchers found that the number and type of challenges/unmet needs were not associated with a subject’s time since cancer treatment, although older cancer survivors tended to report fewer unmet needs than younger survivors.
Sixteen themes of challenges/unmet needs emerged from respondents’ answers, with physical issues being the most common. About 38% of respondents reported physical issues, such as pain, symptoms, and sexual dysfunction.
About 20% reported financial problems, such as issues with insurance and the affordability of needed services and products. About 20% also said they had needs related to unanswered questions and a lack of knowledge about what to expect as a cancer survivor, including guidance on follow-up care and cancer risks, causes, and prevention.
About 16% of respondents cited issues relating to personal control (a lack of physical and social autonomy). And about 16% described flaws and constraints in the healthcare system that affected early detection, diagnosis, treatment, follow-up care, and continuity of care.
About 14% of respondents reported a lack of resources (such as supplies, equipment, and medications), and about 14% cited emotional and mental health issues (such as fear of cancer recurrence, depression, and anxiety).
About 13% of respondents said they lacked social support (such as access to support groups), and 10% reported issues relating to societal perceptions of cancer survivors (such as discrimination and misinformation).
About 9% of respondents expressed the need to talk about or explain the cancer experience with their physician, friends, and family. And about 9% cited a lack of trust in healthcare providers.
Other themes included the wish for more effective cancer treatments (3.5%), body image issues such as feeling unattractive or losing trust in the body (3.5%), issues with the “survivor” identity (3.1%), trouble obtaining or maintaining appropriate employment (2.3%), and existential issues, such as finding meaning in the cancer experience (0.6%).
“Overall, we found that cancer survivors are often caught off guard by the lingering problems they experience after cancer treatment,” Dr Burg said. “In the wake of cancer, many survivors feel they have lost a sense of personal control, have reduced quality of life, and are frustrated that these problems are not sufficiently addressed within the medical care system.”
She added that this study points to several areas in which we might work to improve the situation, including raising public awareness of cancer survivors’ problems, promoting honest professional communication about the side effects of cancer and its treatment, and coordinating medical care resources to help survivors and their families cope with lingering challenges. ![]()
‘Mother of bone marrow transplantation’ dies

husband, E. Donnall Thomas,
at a 2005 reunion of transplant
patients in Seattle
Photo by Jim Linna
Dorothy “Dottie” Thomas, the wife and research partner of 1990 Nobel laureate E. Donnall “Don” Thomas, MD, passed away on January 9 at the age of 92.
Don, pioneer of the bone marrow transplant (BMT), preceded Dottie in passing away himself on October 20, 2012, also at the age of 92.
The Thomases formed the core of a team that proved BMT could cure leukemias and other hematologic malignancies, work that spanned several decades.
Dottie may have gotten the name “the mother of bone marrow transplantation,” from the late George Santos, MD, a BMT expert at Johns Hopkins University School of Medicine in Baltimore, Maryland, and a colleague.
“If Dr Thomas is the father of bone marrow transplantation, then Dottie Thomas is the mother,” he once said.
“Dottie’s life had a profound impact, not just on those who knew her personally, but also countless patients,” said Gary Gilliland, MD, PhD, president and director of the Fred Hutchinson Cancer Research Center in Seattle, Washington, who became friends with the Thomases when he and Don served on the advisory board of the José Carreras Leukemia Foundation.
“She and Don were amazing together in both what they accomplished and the way they cared for each other. They were so sweet together. Now, their legacy continues through the many whose lives have been saved by bone marrow transplant and those who will be saved in the future. Dottie truly helped change the future of medicine. All of us at Fred Hutch are part of her legacy.”
A romantic partnership becomes a professional one
A snowball to the face during a rare Texas snowfall in 1940 precipitated a partnership in love and work between Don and Dottie that spanned 70 years.
“I was a senior at the University of Texas when she was a freshman,” Don told The Seattle Times in a 1999 interview. “I was waiting tables at the girls dormitory, which is how I got my food.”
“It snowed in Texas, which is very unusual. And I came out of the dormitory after we’d finished serving breakfast, and there was about 6 inches of snow. This girl whacked me in the face with a snowball. She still claims she was throwing it at another fellow and hit me by mistake. One thing led to another, and we seemed to hit it off.”
The couple married in December 1942. Dottie was a journalism major in college when, in March 1943, Don was admitted to Harvard University Medical School under a US Army program. Dottie got a job as a secretary with the Navy while Don attended medical school.
“Dottie and I talked it over, and we decided that if we were going to spend time together, which it turned out we liked to do, that she probably ought to change her profession,” Don told The Seattle Times. “She’d taken a lot of science in her time in school, much more than most journalists. She liked science.”
So Dottie left her Navy job and enrolled in the medical technology training program at New England Deaconess Hospital.
“Because Dottie was a hematology technician, we used to look at smears and bone marrow together when we were students,” Don said.
She worked as a medical technician for some doctors in Boston until Don had his own laboratory. Then, she began to work with him. She worked part-time when their children were small, but, otherwise, she was in the lab full-time with her husband.
“Dottie was there at Don’s side through every part of developing marrow transplantation as a science,” said Fred Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center.
“Besides raising 3 children together, Dottie was Don’s partner in every aspect of his professional life, from working in the laboratory to editing manuscripts and administering his research program.”
Dottie’s journalism training was a big asset to the team, according to Don.
“In the laboratory days, my friends pointed out that Dottie, who had the library experience, would go to the library and look up all the background information for a study that we were going to do, and then she would go into the laboratory and do the work and get the data, and then, with her writing skills, she’d write the paper and complete the bibliography,” Don recalled. “All I would do is sign the letter to the editor.”
The couple moved to Seattle in 1963. Don joined the Fred Hutchinson Cancer Research Center in 1975, the year its doors opened in Seattle’s First Hill neighborhood. For the next 15 years, Dottie served as the chief administrator for the Clinical Research Division. Don stepped down from the clinical leadership position in 1990 and retired from the center in 2002.
The Thomases are survived by 2 sons and a daughter, 8 grandchildren, and 2 great-grandchildren.
The family requests that people who wish to honor Dottie do so by contributing to Dottie’s Bridge, an endowment to assist young researchers. ![]()

husband, E. Donnall Thomas,
at a 2005 reunion of transplant
patients in Seattle
Photo by Jim Linna
Dorothy “Dottie” Thomas, the wife and research partner of 1990 Nobel laureate E. Donnall “Don” Thomas, MD, passed away on January 9 at the age of 92.
Don, pioneer of the bone marrow transplant (BMT), preceded Dottie in passing away himself on October 20, 2012, also at the age of 92.
The Thomases formed the core of a team that proved BMT could cure leukemias and other hematologic malignancies, work that spanned several decades.
Dottie may have gotten the name “the mother of bone marrow transplantation,” from the late George Santos, MD, a BMT expert at Johns Hopkins University School of Medicine in Baltimore, Maryland, and a colleague.
“If Dr Thomas is the father of bone marrow transplantation, then Dottie Thomas is the mother,” he once said.
“Dottie’s life had a profound impact, not just on those who knew her personally, but also countless patients,” said Gary Gilliland, MD, PhD, president and director of the Fred Hutchinson Cancer Research Center in Seattle, Washington, who became friends with the Thomases when he and Don served on the advisory board of the José Carreras Leukemia Foundation.
“She and Don were amazing together in both what they accomplished and the way they cared for each other. They were so sweet together. Now, their legacy continues through the many whose lives have been saved by bone marrow transplant and those who will be saved in the future. Dottie truly helped change the future of medicine. All of us at Fred Hutch are part of her legacy.”
A romantic partnership becomes a professional one
A snowball to the face during a rare Texas snowfall in 1940 precipitated a partnership in love and work between Don and Dottie that spanned 70 years.
“I was a senior at the University of Texas when she was a freshman,” Don told The Seattle Times in a 1999 interview. “I was waiting tables at the girls dormitory, which is how I got my food.”
“It snowed in Texas, which is very unusual. And I came out of the dormitory after we’d finished serving breakfast, and there was about 6 inches of snow. This girl whacked me in the face with a snowball. She still claims she was throwing it at another fellow and hit me by mistake. One thing led to another, and we seemed to hit it off.”
The couple married in December 1942. Dottie was a journalism major in college when, in March 1943, Don was admitted to Harvard University Medical School under a US Army program. Dottie got a job as a secretary with the Navy while Don attended medical school.
“Dottie and I talked it over, and we decided that if we were going to spend time together, which it turned out we liked to do, that she probably ought to change her profession,” Don told The Seattle Times. “She’d taken a lot of science in her time in school, much more than most journalists. She liked science.”
So Dottie left her Navy job and enrolled in the medical technology training program at New England Deaconess Hospital.
“Because Dottie was a hematology technician, we used to look at smears and bone marrow together when we were students,” Don said.
She worked as a medical technician for some doctors in Boston until Don had his own laboratory. Then, she began to work with him. She worked part-time when their children were small, but, otherwise, she was in the lab full-time with her husband.
“Dottie was there at Don’s side through every part of developing marrow transplantation as a science,” said Fred Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center.
“Besides raising 3 children together, Dottie was Don’s partner in every aspect of his professional life, from working in the laboratory to editing manuscripts and administering his research program.”
Dottie’s journalism training was a big asset to the team, according to Don.
“In the laboratory days, my friends pointed out that Dottie, who had the library experience, would go to the library and look up all the background information for a study that we were going to do, and then she would go into the laboratory and do the work and get the data, and then, with her writing skills, she’d write the paper and complete the bibliography,” Don recalled. “All I would do is sign the letter to the editor.”
The couple moved to Seattle in 1963. Don joined the Fred Hutchinson Cancer Research Center in 1975, the year its doors opened in Seattle’s First Hill neighborhood. For the next 15 years, Dottie served as the chief administrator for the Clinical Research Division. Don stepped down from the clinical leadership position in 1990 and retired from the center in 2002.
The Thomases are survived by 2 sons and a daughter, 8 grandchildren, and 2 great-grandchildren.
The family requests that people who wish to honor Dottie do so by contributing to Dottie’s Bridge, an endowment to assist young researchers. ![]()

husband, E. Donnall Thomas,
at a 2005 reunion of transplant
patients in Seattle
Photo by Jim Linna
Dorothy “Dottie” Thomas, the wife and research partner of 1990 Nobel laureate E. Donnall “Don” Thomas, MD, passed away on January 9 at the age of 92.
Don, pioneer of the bone marrow transplant (BMT), preceded Dottie in passing away himself on October 20, 2012, also at the age of 92.
The Thomases formed the core of a team that proved BMT could cure leukemias and other hematologic malignancies, work that spanned several decades.
Dottie may have gotten the name “the mother of bone marrow transplantation,” from the late George Santos, MD, a BMT expert at Johns Hopkins University School of Medicine in Baltimore, Maryland, and a colleague.
“If Dr Thomas is the father of bone marrow transplantation, then Dottie Thomas is the mother,” he once said.
“Dottie’s life had a profound impact, not just on those who knew her personally, but also countless patients,” said Gary Gilliland, MD, PhD, president and director of the Fred Hutchinson Cancer Research Center in Seattle, Washington, who became friends with the Thomases when he and Don served on the advisory board of the José Carreras Leukemia Foundation.
“She and Don were amazing together in both what they accomplished and the way they cared for each other. They were so sweet together. Now, their legacy continues through the many whose lives have been saved by bone marrow transplant and those who will be saved in the future. Dottie truly helped change the future of medicine. All of us at Fred Hutch are part of her legacy.”
A romantic partnership becomes a professional one
A snowball to the face during a rare Texas snowfall in 1940 precipitated a partnership in love and work between Don and Dottie that spanned 70 years.
“I was a senior at the University of Texas when she was a freshman,” Don told The Seattle Times in a 1999 interview. “I was waiting tables at the girls dormitory, which is how I got my food.”
“It snowed in Texas, which is very unusual. And I came out of the dormitory after we’d finished serving breakfast, and there was about 6 inches of snow. This girl whacked me in the face with a snowball. She still claims she was throwing it at another fellow and hit me by mistake. One thing led to another, and we seemed to hit it off.”
The couple married in December 1942. Dottie was a journalism major in college when, in March 1943, Don was admitted to Harvard University Medical School under a US Army program. Dottie got a job as a secretary with the Navy while Don attended medical school.
“Dottie and I talked it over, and we decided that if we were going to spend time together, which it turned out we liked to do, that she probably ought to change her profession,” Don told The Seattle Times. “She’d taken a lot of science in her time in school, much more than most journalists. She liked science.”
So Dottie left her Navy job and enrolled in the medical technology training program at New England Deaconess Hospital.
“Because Dottie was a hematology technician, we used to look at smears and bone marrow together when we were students,” Don said.
She worked as a medical technician for some doctors in Boston until Don had his own laboratory. Then, she began to work with him. She worked part-time when their children were small, but, otherwise, she was in the lab full-time with her husband.
“Dottie was there at Don’s side through every part of developing marrow transplantation as a science,” said Fred Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center.
“Besides raising 3 children together, Dottie was Don’s partner in every aspect of his professional life, from working in the laboratory to editing manuscripts and administering his research program.”
Dottie’s journalism training was a big asset to the team, according to Don.
“In the laboratory days, my friends pointed out that Dottie, who had the library experience, would go to the library and look up all the background information for a study that we were going to do, and then she would go into the laboratory and do the work and get the data, and then, with her writing skills, she’d write the paper and complete the bibliography,” Don recalled. “All I would do is sign the letter to the editor.”
The couple moved to Seattle in 1963. Don joined the Fred Hutchinson Cancer Research Center in 1975, the year its doors opened in Seattle’s First Hill neighborhood. For the next 15 years, Dottie served as the chief administrator for the Clinical Research Division. Don stepped down from the clinical leadership position in 1990 and retired from the center in 2002.
The Thomases are survived by 2 sons and a daughter, 8 grandchildren, and 2 great-grandchildren.
The family requests that people who wish to honor Dottie do so by contributing to Dottie’s Bridge, an endowment to assist young researchers. ![]()
Bedside Swallow Examination Review
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.
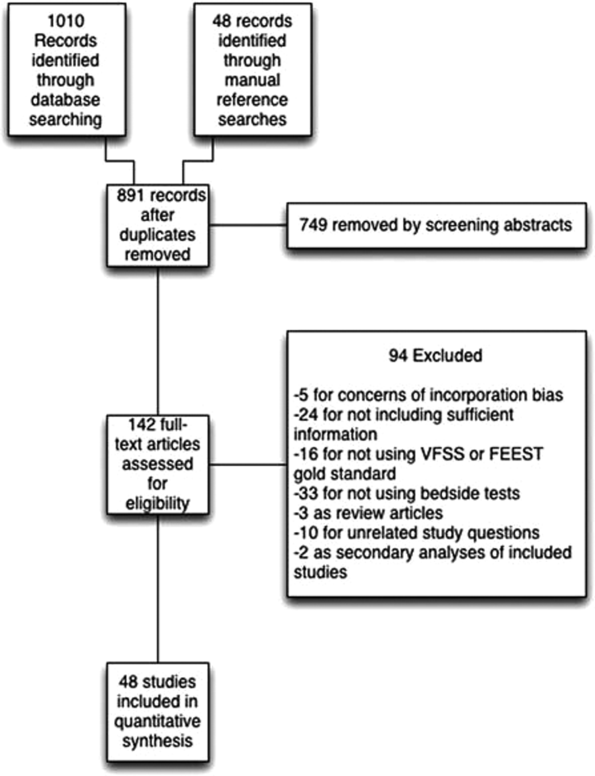
Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.
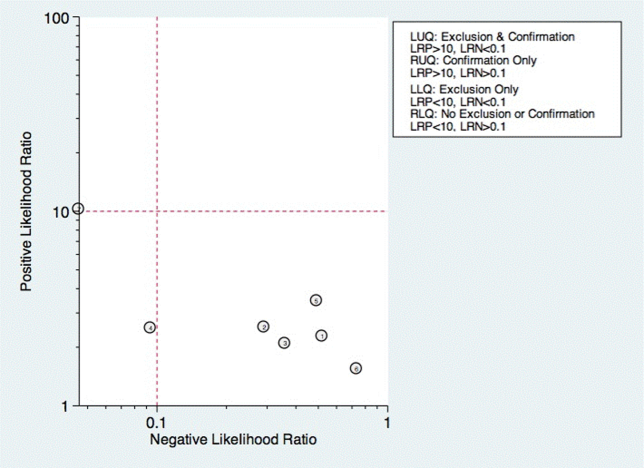
Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.
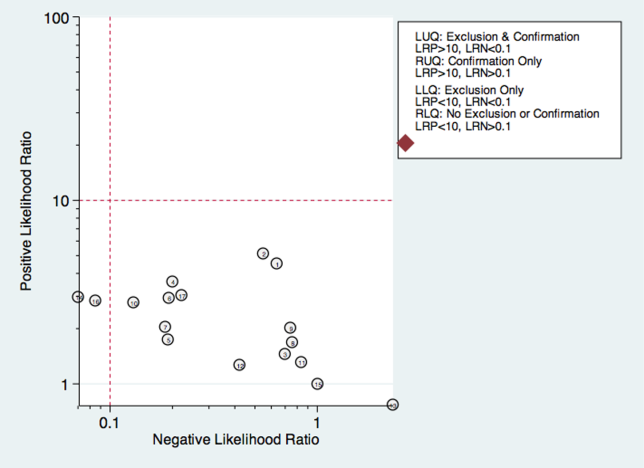
Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]
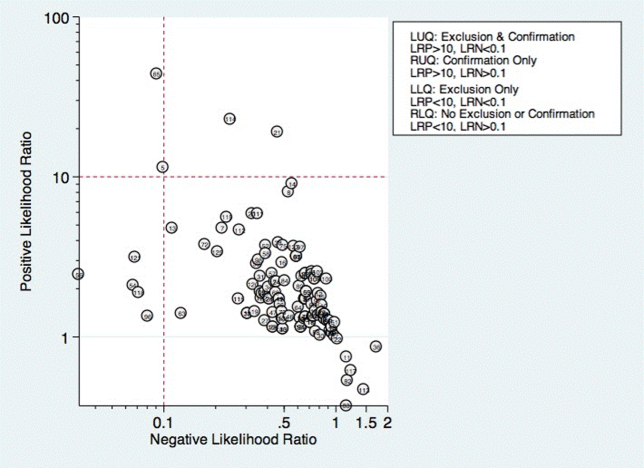
McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.

Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.

Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.

Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]

McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
Dysphagia is a serious medical condition that can lead to aspiration pneumonia, malnutrition, and dehydration.[1] Dysphagia is the result of a variety of medical etiologies, including stroke, traumatic brain injury, progressive neurologic conditions, head and neck cancers, and general deconditioning. Prevalence estimates for dysphagia vary depending upon the etiology and patient age, but estimates as high as 38% for lifetime prevalence have been reported in those over age 65 years.[2]
To avoid adverse health outcomes, early detection of dysphagia is essential. In hospitalized patients, early detection has been associated with reduced risk of pneumonia, decreased length of hospital stay, and improved cost‐effectiveness resulting from a reduction in hospital days due to fewer cases of aspiration pneumonia.[3, 4, 5] Stroke guidelines in the United States recommend screening for dysphagia for all patients admitted with stroke.[6] Consequently, the majority of screening procedures have been designed for and tested in this population.[7, 8, 9, 10]
The videofluoroscopic swallow study (VFSS) is a commonly accepted, reference standard, instrumental evaluation technique for dysphagia, as it provides the most comprehensive information regarding anatomic and physiologic function for swallowing diagnosis and treatment. Flexible endoscopic evaluation of swallowing (FEES) is also available, as are several less commonly used techniques (scintigraphy, manometry, and ultrasound). Due to availability, patient compliance, and expertise needed, it is not possible to perform instrumental examination on every patient with suspected dysphagia. Therefore, a number of minimally invasive bedside screening procedures for dysphagia have been developed.
The value of any diagnostic screening test centers on performance characteristics, which under ideal circumstances include a positive result for all those who have dysphagia (sensitivity) and negative result for all those who do not have dysphagia (specificity). Such an ideal screening procedure would reduce unnecessary referrals and testing, thus resulting in cost savings, more effective utilization of speech‐language pathology consultation services, and less unnecessary radiation exposure. In addition, an effective screen would detect all those at risk for aspiration pneumonia in need of intervention. However, most available bedside screening tools are lacking in some or all of these desirable attributes.[11, 12] We undertook a systematic review and meta‐analysis of bedside procedures to screen for dysphagia.
METHODS
Data Sources and Searches
We conducted a comprehensive search of 7 databases, including MEDLINE, Embase, and Scopus, from each database's earliest inception through June 9, 2014 for English‐language articles and abstracts. The search strategy was designed and conducted by an experienced librarian with input from 1 researcher (J.C.O.). Controlled vocabulary supplemented with keywords was used to search for comparative studies of bedside screening tests for predicting dysphagia (see Supporting Information, Appendix 1, in the online version of this article for the full strategy).
All abstracts were screened, and potentially relevant articles were identified for full‐text review. Those references were manually inspected to identify all relevant studies.
Study Selection
A study was eligible for inclusion if it tested a diagnostic swallow study of any variety against an acceptable reference standard (VFSS or flexible endoscopic evaluation of swallowing with sensory testing [FEEST]).
Data Extraction and Quality Assessment
The primary outcome of the study was aspiration, as predicted by a bedside exam, compared to gold‐standard visualization of aspirated material entering below the vocal cords. From each study, data were abstracted based on the type of diagnostic method and reference standard study population and inclusion/exclusion characteristics, design, and prediction of aspiration. Prediction of aspiration was compared against the reference standard to yield true positives, true negatives, false positives, and false negatives. Additional potential confounding variables were abstracted using a standard form based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis[13] (see Supporting Information, Appendix 2, in the online version of this article for the full abstraction template).
Data Synthesis and Analysis
Sensitivity and specificity for each test that identified the presence of dysphagia was calculated for each study. These were used to generate positive and negative likelihood ratios (LRs), which were plotted on a likelihood matrix, a graphic depiction of the logarithm of the +LR on the ordinate versus the logarithm of the LR on the abscissa, dividing the graphic into quadrants such that the right upper quadrant is tests that can be used for confirmation, right lower quadrant neither confirmation nor exclusion, left lower quadrant exclusion only, and left upper quadrant an ideal test with both exclusionary and confirmatory properties.[14] A good screening test would thus be on the left half of the graphic to effectively rule out dysphagia, and the ideal test with both good sensitivity and specificity would be found in the left upper quadrant. Graphics were constructed using the Stata MIDAS package (Stata Corp., College Station, TX).[15]
RESULTS
We identified 891 distinct articles. Of these, 749 were excluded based on abstract review. After reviewing the remaining 142 full‐text articles, 48 articles were determined to meet inclusion criteria, which included 10,437 observations across 7414 patients (Figure 1). We initially intended to conduct a meta‐analysis on each type, but heterogeneity in design and statistical heterogeneity in aggregate measures precluded pooling of results.

Characteristics of Included Studies
Of the 48 included studies, the majority (n=42) were prospective observational studies,[7, 8, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53] whereas 2 were randomized trials,[9, 54] 2 studies were double‐blind observational,[9, 16] 1 was a case‐control design,[55] and 1 was a retrospective case series.[56] The majority of studies were exclusively inpatient,[7, 8, 9, 14, 17, 18, 19, 21, 22, 24, 25, 26, 31, 32, 33, 35, 36, 38, 41, 43, 44, 45, 46, 47, 49, 51, 52, 53, 55, 57] with 5 in mixed in and outpatient populations,[20, 27, 40, 55, 58] 2 in outpatient populations,[23, 41] and the remainder not reporting the setting from which they drew their study populations.
The indications for swallow evaluations fit broadly into 4 categories: stroke,[7, 8, 9, 14, 21, 22, 24, 25, 26, 31, 33, 34, 35, 38, 40, 41, 42, 43, 45, 48, 52, 56, 58] other neurologic disorders,[17, 18, 23, 28, 39, 47] all causes,[16, 20, 27, 29, 30, 36, 37, 44, 46, 49, 51, 52, 53, 54, 58] and postsurgical.[19, 32, 34] Most used VFSS as a reference standard,[7, 8, 9, 14, 16, 17, 18, 19, 21, 22, 23, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 50, 51, 52, 53, 54, 56, 57, 58] with 8 using FEEST,[20, 24, 31, 32, 33, 35, 49, 55] and 1 accepting either videofluoroscopic evaluation of swallow or FEEST.[48]
Studies were placed into 1 or more of the following 4 categories: subjective bedside examination,[8, 9, 18, 19, 31, 34, 48] questionnaire‐based tools,[17, 23, 46, 53] protocolized multi‐item evaluations,[20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] and single‐item exam maneuvers, symptoms, or signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 56, 58, 59] The characteristics of all studies are detailed in Table 1.
| Study | Location | Design | Mean Age (SD) | Reason(s) for Dysphagia | Indx Test | Description | Reference Standard | Sample Size, No. of Patients | Sample Size, No. of Observations |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Splaingard et al., 198844 | Milwaukee, WI, USA | Prospective observational study | NR | Multiple | Clinical bedside swallow exam | Combination of scored comprehensive physical exam, history, and observed swallow. | VFSS | 107 | 107 |
| DePippo et al., 199243 | White Plains, NY, USA | Prospective observational study | 71 (10) | Stroke | WST | Observation of swallow. | VFSS | 44 | |
| Horner et al., 199356 | Durham, NC, USA | Retrospective case series | 64* | Stroke | Clinical bedside swallow evaluation | VFSS | 38 | 114 | |
| Kidd et al., 199342 | Belfast, UK | Prospective observational study | 72 (10) | Stroke | Bedside 50‐mL swallow evaluation | Patient swallows 50 mL of water in 5‐mL aliquots, with therapist assessing for choking, coughing, or change in vocal quality after each swallow. | VFSS | 60 | 240 |
| Collins and Bakheit, 199741 | Southampton, UK | Prospective observational study | 65* | Stroke | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 54 | 54 |
| Daniels et al., 199740 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside examination | 6 individual bedside assessments (dysphonia, dysphagia, cough before/after swallow, gag reflex and voice change) examined as predictors for aspiration risk. | VFSS | 59 | 354 |
| Mari et al., 199739 | Ancona, Italy | Prospective observational study | 60 (16) | Mixed neurologic diseases | Combined history and exam | Assessed symptoms of dysphagia, cough, and 3‐oz water swallow. | VFSS | 93 | 372 |
| Daniels et al., 19987 | New Orleans, LA, USA | Prospective observational study | 66 (11) | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 55 | 330 |
| Smithard et al., 19988 | Ashford, UK | Prospective observational study | 79* | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 83 | 249 |
| Addington et al., 199938 | Kansas City, MO, USA | Prospective observational study | 80* | Stroke | NR | Reflex cough. | VFSS | 40 | 40 |
| Logemann et al., 199937 | Evanston, IL, USA | Prospective observational study | 65 | Multiple | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 200 | 1400 |
| Smith et al., 20009 | Manchester, UK | Double blind observational | 69 | Stroke | Clinical bedside swallow evaluation, pulse oximetry evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. Also evaluated if patient desaturates by at least 2% during evaluation. | VFSS | 53 | 53 |
| Warms et al., 200036 | Melbourne, Australia | Prospective observational study | 67 | Multiple | Wet voice | Voice was recorded and analyzed with Sony digital audio tape during videofluoroscopy. | VFSS | 23 | 708 |
| Lim et al., 200135 | Singapore, Singapore | Prospective observational study | NR | Stroke | Water swallow test, desaturation during swallow | 50‐mL swallow done in 5‐mL aliquots with assessment of phonation/choking afterward; desaturation >2% during swallow, | FEEST | 50 | 100 |
| McCullough et al., 200134 | Nashville, TN, USA | Prospective observational study | 60 (10) | Stroke | Clinical bedside swallow evaluation | 15‐item physical exam with observed swallow. | VFSS | 2040 | 60 |
| Rosen et al., 2001[74] | Newark, NJ, USA | Prospective observational study | 60 | Head and Neck cancer | Wet voice | Observation of swallow. | VFSS | 26 | 26 |
| Leder and Espinosa, 200233 | New Haven, CT, USA | Prospective observational study | 70* | Stroke | Clinical exam | Checklist evaluation of cough and voice change after swallow, volitional cough, dysphonia, dysarthria, and abnormal gag. | FEEST | 49 | 49 |
| Belafsky et al., 200332 | San Francisco, CA, USA | Prospective observational study | 65 (11) | Post‐tracheostomy patients | Modified Evans Blue Dye Test | 3 boluses of dye‐impregnated ice are given to patient. Tracheal secretions are suctioned, and evaluated for the presence of dye. | FEES | 30 | 30 |
| Chong et al., 200331 | Jalan Tan Tock Seng, Singapore | Prospective observational study | 75 (7) | Stroke | Water swallow test, desaturation during, clinical exam | Subjective exam, drinking 50 mL of water in 10‐mL aliquots, and evaluating for desaturation >2% during FEES. | FEEST | 50 | 150 |
| Tohara et al., 200330 | Tokyo, Japan | Prospective observational study | 63 (17) | Multiple | Food/water swallow tests, and a combination of the 2 | Protocolized observation of sequential food and water swallows with scored outcomes. | VFSS | 63 | 63 |
| Rosenbek et al., 200414 | Gainesville, FL, USA | Prospective observational study | 68* | Stroke | Clinical bedside swallow evaluation | Describes 5 parameters of voice quality and 15 physical examination maneuvers used. | VFSS | 60 | 1200 |
| Ryu et al., 200429 | Seoul, South Korea | Prospective observational study | 64 (14) | Multiple | Voice analysis parameters | Analysis of the/a/vowel sound with Visi‐Pitch II 3300. | VFSS | 93 | 372 |
| Shaw et al., 200428 | Sheffield, UK | Prospective observational study | 71 | Neurologic disease | Bronchial auscultation | Auscultation over the right main bronchus during trial feeding to listen for sounds of aspiration. | VFSS | 105 | 105 |
| Wu et al., 200427 | Taipei, Taiwan | Prospective observational study | 72 (11) | Multiple | 100‐mL swallow test | Patient lifts a glass of 100 mL of water and drinks as quickly as possible, and is assessed for signs of choking, coughing, or wet voice, and is timed for speed of drinking. | VFSS | 54 | 54 |
| Nishiwaki et al., 200526 | Shizuoaka, Japan | Prospective observational study | 70* | Stroke | Clinical bedside swallow evaluation | Describes sensitivity and specificity of several component physical exam maneuvers comprising the bedside exam. | VFSS | 31 | 248 |
| Wang et al., 200554 | Taipei, Taiwan | Prospective double‐blind study | 41* | Multiple | Desaturation | Desaturation of at least 2% during videofluoroscopic study. | VFSS | 60 | 60 |
| Ramsey et al., 200625 | Kent, UK | Prospective observational study | 71 (10) | Stroke | BSA | Assessment of lip seal, tongue movement, voice quality, cough, and observed 5‐mL swallow. | VFSS | 54 | 54 |
| Trapl et al., 200724 | Krems, Austria | Prospective observational study | 76 (2) | Stroke | Gugging Swallow Screen | Progressive observed swallow trials with saliva, then with 350 mL liquid, then dry bread. | FEEST | 49 | 49 |
| Suiter and Leder, 200849 | Several centers across the USA | Prospective observational study | 68.3 | Multiple | 3‐oz water swallow test | Observation of swallow. | FEEST | 3000 | 3000 |
| Wagasugi et al., 200850 | Tokyo, Japan | Prospective observational study | NR | Multiple | Cough test | Acoustic analysis of cough. | VFSS | 204 | 204 |
| Baylow et al., 200945 | New York, NY, USA | Prospective observational study | NR | Stroke | Northwestern Dysphagia Check Sheet | 28‐item screening procedure including history, observed swallow, and physical exam. | VFSS | 15 | 30 |
| Cox et al., 200923 | Leiden, the Netherlands | Prospective observational study | 68 (8) | Inclusion body myositis | Dysphagia questionnaire | Questionnaire assessing symptoms of dysphagia. | VFSS | 57 | 57 |
| Kagaya et al., 201051 | Tokyo, Japan | Prospective observational study | NR | Multiple | Simple Swallow Provocation Test | Injection of 1‐2 mL of water through nasal tube directed at the suprapharynx. | VFSS | 46 | 46 |
| Martino et al., 200957 | Toronto, Canada | Randomized trial | 69 (14) | Stroke | Toronto Bedside Swallow Screening Test | 4‐item physical assessment including Kidd water swallow test, pharyngeal sensation, tongue movement, and dysphonia (before and after water swallow). | VFSS | 59 | 59 |
| Santamato et al., 200955 | Bari, Italy | Case control | NR | Multiple | Acoustic analysis, postswallow apnea | Acoustic analysis of cough. | VFSS | 15 | 15 |
| Smith Hammond et al., 200948 | Durham, NC, USA | Prospective observational study | 67.7 (1.2) | Multiple | Cough, expiratory phase peak flow | Acoustic analysis of cough. | VFSS or FEES | 96 | 288 |
| Leigh et al., 201022 | Seoul, South Korea | Prospective observational study | NR | Stroke | Clinical bedside swallow evaluation | Not described. | VFSS | 167 | 167 |
| Pitts et al., 201047 | Gainesville, FL, USA | Prospective observational study | NR | Parkinson | Cough compression phase duration | Acoustic analysis of cough. | VFSS | 58 | 232 |
| Cohen and Manor, 201146 | Tel Aviv, Israel | Prospective observational Study | NR | Multiple | Swallow Disturbance Questionnaire | 15‐item questionnaire. | FEES | 100 | 100 |
| Edmiaston et al., 201121 | St. Louis, MO, USA | Prospective observational study | 63* | Stroke | SWALLOW‐3D Acute Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Mandysova et al., 201120 | Pardubice, Czech Republic | Prospective observational study | 69 (13) | Multiple | Brief Bedside Dysphagia Screening Test | 8‐item physician exam including ability to clench teeth; symmetry/strength of tongue, facial, and shoulder muscles; dysarthria; and choking, coughing, or dripping of food after taking thick liquid. | FEES | 87 | 87 |
| Steele et al., 201158 | Toronto, Canada | Double blind observational | 67 | Stroke | 4‐item bedside exam | Tongue lateralization, cough, throat clear, and voice quality. | VFSS | 400 | 40 |
| Yamamoto et al., 201117 | Kodaira, Japan | Prospective observational study | 67 (9) | Parkinson's Disease | Swallowing Disturbance Questionnaire | 15‐item questionnaire. | VFSS | 61 | 61 |
| Bhama et al., 201219 | Pittsburgh, PA, USA | Prospective observational study | 57 (14) | Post‐lung transplant | Clinical bedside swallow evaluation | Not described. | VFSS | 128 | 128 |
| Shem et al., 201218 | San Jose, CA, USA | Prospective observational study | 42 (17) | Spinal cord injuries resulting in tetraplegia | Clinical bedside swallow evaluation | After eating/drinking, patient is evaluated for signs of aspiration including coughing, choking, or "wet voice." Procedure is repeated with several consistencies. | VFSS | 26 | 26 |
| Steele et al., 201316 | Toronto, Canada | Prospective observational study | 67 (14) | Multiple | Dual‐axis accelerometry | Computed accelerometry of swallow. | VFSS | 37 | 37 |
| Edmiaston et al., 201452 | St. Louis, MO, USA | Prospective observational study | 63 (15) | Stroke | Barnes Jewish Stroke Dysphagia Screen | 5‐item screen including mental status; asymmetry or weakness of face, tongue, or palate; and subjective signs of aspiration when drinking 3 oz water. | VFSS | 225 | 225 |
| Rofes et al., 201453 | Barcelona, Spain | Prospective observational study | 74 (12) | Mixed | EAT‐10 questionnaire and variable viscosity swallow test | Symptom‐based questionnaire (EAT‐10) and repeated observations and measurements of swallow with different thickness liquids. | VFS | 134 | 134 |
Subjective Clinical Exam
Seven studies reported the sensitivity and specificity of subjective assessments of nurses and speech‐language pathologists in observing swallowing and predicting aspiration.[8, 9, 18, 19, 31, 34, 48] The overall distribution of studies is summarized in the likelihood matrix in Figure 2. Two studies, Chong et al.[31] and Shem et al.,[18] were on the left side of the matrix, indicating a sensitive rule‐out test. However, both were small studies, and only Chong et al. reported reasonable sensitivity with incorporation bias from knowledge of a desaturation study outcome. Overall, subjective exams did not appear reliable in ruling out dysphagia.

Questionnaire‐Based Tools
Only 4 studies used questionnaire‐based tools filled out by the patient, asking about subjective assessment of dysphagia symptoms and frequency.[17, 23, 46, 53] Yamamoto et al. reported results of using the swallow dysphagia questionnaire in patients with Parkinson's disease.[17] Rofes et al. looked at the Eating Assessment Tool (EAT‐10) questionnaire among all referred patients and a small population of healthy volunteers.[53] Each was administered the questionnaire before undergoing a videofluoroscopic study. Overall, sensitivity and specificity were 77.8% and 84.6%, respectively. Cox et al. studied a different questionnaire in a group of patients with inclusion body myositis, finding 70% sensitivity and 44% specificity.[23] Cohen and Manor examined the swallow dysphagia questionnaire across several different causes of dysphagia, finding at optimum, the test is 78% specific and 73% sensitive.[46] Rofes et al. had an 86% sensitivity and 68% specificity for the EAT‐10 tool.[53]
Multi‐Item Exam Protocols
Sixteen studies reported multistep protocols for determining a patient's risk for aspiration.[9, 20, 21, 22, 25, 30, 33, 34, 37, 39, 44, 45, 52, 53, 57, 58] Each involved a combination of physical exam maneuvers and history elements, detailed in Table 1. This is shown in the likelihood matrix in Figure 3. Only 2 of these studies were in the left lower quadrant, Edmiaston et al. 201121 and 2014.[52] Both studies were restricted to stroke populations, but found reasonable sensitivity and specificity in identifying dysphagia.

Individual Exam Maneuvers
Thirty studies reported the diagnostic performance of individual exam maneuvers and signs.[7, 9, 14, 16, 24, 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 51, 54, 56, 58] Each is depicted in Figure 4 as a likelihood matrix demonstrating the +LR and LR for individual maneuvers as seen in the figure; most fall into the right lower quadrant, where they are not diagnostically useful tests. Studies in the left lower quadrant demonstrating the ability to exclude aspiration desirable in a screening test were dysphonia in McCullough et al.,[34] dual‐axis accelerometry in Steele et al.,[16] and the water swallow test in DePippo et al.[43] and Suiter and Leder.[49]

McCullough et al. found dysphonia to be the most discriminatory sign or symptom assessed, with an area under the curve (AUC) of 0.818. Dysphonia was judged by a sustained/a/and had 100% sensitivity but only 27% specificity. Wet voice within the same study was slightly less informative, with an AUC of 0.77 (sensitivity 50% and specificity 84%).[34]
Kidd et al. verified the diagnosis of stroke, and then assessed several neurologic parameters, including speech, muscle strength, and sensation. Pharyngeal sensation was assessed by touching each side of the pharyngeal wall and asking patients if they felt sensation that differed from each side. Patient report of abnormal sensation during this maneuver was 80% sensitive and 86% specific as a predictor of aspiration on VFSS.[42]
Steele et al. described the technique of dual axis accelerometry, where an accelerometer was placed at the midline of the neck over the cricoid cartilage during VFSS. The movement of the cricoid cartilage was captured for analysis in a computer algorithm to identify abnormal pharyngeal swallow behavior. Sensitivity was 100%, and specificity was 54%. Although the study was small (n=40), this novel method demonstrated good discrimination.[58]
DePippo et al. evaluated a 3‐oz water swallow in stroke patients. This protocol called for patients to drink the bolus of water without interruption, and be observed for 1 minute after for cough or wet‐hoarse voice. Presence of either sign was considered abnormal. Overall, sensitivity was 94% and specificity 30% looking for the presence of either sign.[43] Suiter and Leder used a similar protocol, with sensitivity of 97% and specificity of 49%.[49]
DISCUSSION
Our results show that most bedside swallow examinations lack the sensitivity to be used as a screening test for dysphagia across all patient populations examined. This is unfortunate as the ability to determine which patients require formal speech language pathology consultation or imaging as part of their diagnostic evaluation early in the hospital stay would lead to improved allocation of resources, cost reductions, and earlier implementation of effective therapy approaches. Furthermore, although radiation doses received during VFSS are not high when compared with other radiologic exams like computed tomography scans,[60] increasing awareness about the long‐term malignancy risks associated with medical imaging makes it desirable to reduce any test involving ionizing radiation.
There were several categories of screening procedures identified during this review process. Those classified as subjective bedside exams and protocolized multi‐item evaluations were found to have high heterogeneity in their sensitivity and specificity, though a few exam protocols did have a reasonable sensitivity and specificity.[21, 31, 52] The following individual exam maneuvers were found to demonstrate high sensitivity and an ability to exclude aspiration: a test for dysphonia through production of a sustained/a/34 and use of dual‐axis accelerometry.[16] Two other tests, the 3‐oz water swallow test[43] and testing of abnormal pharyngeal sensation,[42] were each found effective in a single study, with conflicting results from other studies.
Our results extend the findings from previous systematic reviews on this subject, most of which focused only on stroke patients.[5, 12, 61, 62] Martino and colleagues[5] conducted a review focused on screening for adults poststroke. From 13 identified articles, it was concluded that evidence to support inclusion or exclusion of screening was poor. Daniels et al. conducted a systematic review of swallowing screening tools specific to patients with acute or chronic stroke.[12] Based on 16 articles, the authors concluded that a combination of swallowing and nonswallowing features may be necessary for development of a valid screening tool. The generalizability of these reviews is limited given that all were conducted in patients poststroke, and therefore results and recommendations may not be generalizable to other patients.
Wilkinson et al.[62] conducted a recent systematic review that focused on screening techniques for inpatients 65 years or older that excluded patients with stroke or Parkinson's disease. The purpose of that review was to examine sensitivity and specificity of bedside screening tests as well as ability to accurately predict pneumonia. The authors concluded that existing evidence is not sufficient to recommend the use of bedside tests in a general older population.[62]
Specific screening tools identified by Martino and colleagues[5] to have good predictive value in detecting aspiration as a diagnostic marker of dysphagia were an abnormal test of pharyngeal sensation[42] and the 50‐mL water swallow test. Daniels et al. identified a water swallow test as an important component of a screen.[7] These results were consistent with those of this review in that the abnormal test of pharyngeal sensation[42] was identified for high levels of sensitivity. However, the 3‐oz water swallow test,[43, 49] rather than the 50‐mL water swallow test,[42] was identified in this review as the version of the water swallow test with the best predictive value in ruling out aspiration. Results of our review identified 2 additional individual items, dual‐axis accelerometry[16] and dysphonia,[34] that may be important to include in a comprehensive screening tool. In the absence of better tools, the 3 oz swallow test, properly executed, seems to be the best currently available tool validated in more than 1 study.
Several studies in the current review included an assessment of oral tongue movement that is not described thoroughly and varies between studies. Tongue movement as an individual item on a screening protocol was not found to yield high sensitivity or specificity. However, tongue movement or range of motion is only 1 aspect of oral tongue function; pressures produced by the tongue reflecting strength also may be important and warrant evaluation. Multiple studies have shown patients with dysphagia resulting from a variety of etiologies to produce lower than normal maximum isometric lingual pressures,[63, 64, 65, 66, 67, 68] or pressures produced when the tongue is pushed as hard as possible against the hard palate. Tongue strengthening protocols that result in higher maximum isometric lingual pressures have been shown to carry over to positive changes in swallow function.[69, 70, 71, 72, 73] Inclusion of tongue pressure measurement in a comprehensive screening tool may help to improve predictive capabilities.
We believe our results have implications for practicing clinicians, and serve as a call to action for development of an easy‐to‐perform, accurate tool for dysphagia screening. Future prospective studies should focus on practical tools that can be deployed at the bedside, and correlate the results with not only gold‐standard VFSS and FEES, but with clinical outcomes such as pneumonia and aspiration events leading to prolonged length of stay.
There were several limitations to this review. High levels of heterogeneity were reported in the screening tests present in the literature, precluding meaningful meta‐analysis. In addition, the majority of studies included were in poststroke adults, which limits the generalizability of results.
In conclusion, no screening protocol has been shown to provide adequate predictive value for presence of aspiration. Several individual exam maneuvers demonstrate high sensitivity; however, the most effective combination of screening protocol components is unknown. There is a need for future research focused on the development of a comprehensive screening tool that can be applied across patient populations for accurate detection of dysphagia as well as prediction of other adverse health outcomes, including pneumonia.
Acknowledgements
The authors thank Drs. Byun‐Mo Oh and Catrionia Steele for providing additional information in response to requests for unpublished information.
Disclosures: Nasia Safdar MD, is supported by a National Institutes of Health R03 GEMSSTAR award and a VA MERIT award. The authors report no conflicts of interest.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
- , , , et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66.
- , , , Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–865.
- , , Formal dysphagia screening protocols prevent pneumonia. Stroke. 2006;37(3):765.
- , , Swallow management in patients on an acute stroke pathway: quality is cost effective. Arch Phys Med Rehabil. 1995;76(12):1130–1133.
- , , Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15(1):19–30.
- , , , et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(3):870–947.
- , , , , , Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19.
- , , , et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27(2):99–106.
- , , , The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29(6):495–499.
- , , , Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–364.
- , Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16(1):7–18.
- , , Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43(3):892–897.
- , , , , Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269, W64.
- , , Is the information about a test important? Applying the methods of evidence‐based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–450.
- MIDAS: Stata module for meta‐analytical integration of diagnostic test accuracy studies. Statistical Software Components. S456880, Boston College Department of Economics, 2009.
- , , Noninvasive detection of thin‐liquid aspiration using dual‐axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112.
- , , , , Validation of the Japanese translation of the Swallowing Disturbance Questionnaire in Parkinson's disease patients. Qual Life Res. 2012;21(7):1299–1303.
- , , , , , Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R. 2012;4(4):283–289.
- , et al. 723 Aspiration after Lung Transplantation: Incidence, Risk Factors, and Accuracy of the Bedside Swallow Evaluation. The J Heart Lung Transplant. 2012;31(4 suppl 1):S247–S248.
- , , , Cerny M. Development of the brief bedside dysphagia screening test in the Czech Republic. Nurs Health Sci. 2011;13(4):388–395.
- , , SWALLOW‐3D, a simple 2‐minute bedside screening test, detects dysphagia in acute stroke patients with high sensitivity when validated against video‐fluoroscopy. Stroke. 2011;42(3):e352.
- , , , , Bedside screening and subacute reassessment of post‐stroke dysphagia: a prospective study. Int J Stroke. 2010;5:200.
- , , , , , Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–2013.
- , , , et al. Dysphagia bedside screening for acute‐stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948–2952.
- , , Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37(12):2984–2988.
- , , , , , Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37(4):247–251.
- , , , Evaluating swallowing dysfunction using a 100‐ml water swallowing test. Dysphagia. 2004;19(1):43–47.
- , , , et al. Bronchial auscultation: an effective adjunct to speech and language therapy bedside assessment when detecting dysphagia and aspiration? Dysphagia. 2004;19(4):211–218.
- , , Prediction of laryngeal aspiration using voice analysis. Am J Phys Med Rehabil. 2004;83(10):753–757.
- , , , , Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18(2):126–134.
- , , , , Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Ann Acad Med Singapore. 2003;32(6):790–794.
- , , , The accuracy of the modified Evan's blue dye test in predicting aspiration. Laryngoscope. 2003;113(11):1969–1972.
- , Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17(3):214–218.
- , , Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1‐2):55–72.
- , , , et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6.
- , “Wet Voice” as a predictor of penetration and aspiration in oropharyngeal dysphagia. Dysphagia. 2000;15(2):84–88.
- , , A screening procedure for oropharyngeal dysphagia. Dysphagia. 1999;14(1):44–51.
- , , , Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999;80(2):150–154.
- , , , , , Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63(4):456–460.
- , , , Clinical assessment of swallowing and prediction of dysphagia severity. Am J Speech Lang Pathol. 1997;6(4):17–24.
- , Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke. 1997;28(9):1773–1775.
- , , , Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med. 1993;86(12):825–829.
- , , Validation of the 3‐oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49(12):1259–1261.
- , , , Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–640.
- , , , Accuracy of clinical judgment of the chin‐down posture for dysphagia during the clinical/bedside assessment as corroborated by videofluoroscopy in adults with acute stroke. Dysphagia. 2009;24(4):423–433.
- , Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121(7):1383–1387.
- , , , , , . Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431.
- , , , et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777.
- , Clinical utility of the 3‐ounce water swallow test. Dysphagia. 2008;23(3):244–250.
- , , , et al. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23(4):364–370.
- , , , , , Simple swallowing provocation test has limited applicability as a screening tool for detecting aspiration, silent aspiration, or penetration. Dysphagia. 2010;25(1):6–10.
- , , , A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23 (4):712–716.
- , , , Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–1265.
- , , , Pulse oximetry does not reliably detect aspiration on videofluoroscopic swallowing study. Arch Phys Med Rehabil. 2005;86(4):730–734.
- , , , et al. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. J Rehabil Med. 2009;41(8):639–645.
- , , Aspiration in bilateral stroke patients: a validation study. Neurology. 1993;43(2):430–433.
- , , , et al. The Toronto Bedside Swallowing Screening Test (TOR‐BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–561.
- , , , et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228–242.
- , , , et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976.
- , , , et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28(1):77–85.
- Detection of eating difficulties after stroke: a systematic review. Int Nurs Rev. 2006;53(2):143–149.
- , , Aspiration in older patients without stroke: A systematic review of bedside diagnostic tests and predictors of pneumonia. Eur Geriatr Med. 2012;3(3):145–152.
- , , A tongue force measurement system for the assessment of oral‐phase swallowing disorders. Arch Phys Med Rehabil. 1991;72(1):38–42.
- , , Strength, Endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43(1):256–267.
- , , , et al. Effects of radiotherapy with or without chemotherapy on tongue strength and swallowing in patients with oral cancer. Head Neck. 2007;29(7):632–637.
- , , , , Tongue pressure against hard palate during swallowing in post‐stroke patients. Gerodontology. 2005;22(4):227–233.
- , Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):148–156.
- , , , et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–397.
- , , , Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55(4):199–205.
- , , , , , The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. S2005;53(9):1483–1489.
- , , , et al. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88(2):150–158.
- , , , , , Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope. 2008;118(1):39–43.
- , , , Effects of directional exercise on lingual strength. J Speech Lang Hear Res. 2009;52(4):1034–1047.
- , , et al. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Archives of Otolaryngology – Head 127(8):975–979.
Biopsies don’t promote cancer spread, group finds
A study of more than 2000 patients refutes the idea that biopsies cause cancer to spread.
In a study published in Gut, researchers showed that patients who received a biopsy had better overall survival and similar cancer-free survival rates as patients who did not have a biopsy.
The team studied pancreatic cancer but said their findings likely apply to other cancers because the diagnostic technique used in this study—fine needle aspiration—is commonly used across tumor types.
“This study shows that physicians and patients should feel reassured that a biopsy is very safe,” said study author Michael Wallace, MD, of the Mayo Clinic in Jacksonville, Florida.
“We do millions of biopsies of cancer a year in the US, but one or two case studies have led to this common myth that biopsies spread cancer.”
This is the second study Dr Wallace and his team have conducted to examine the risk of biopsy.
In a 2013 study published in Endoscopy, the researchers examined outcomes in 256 pancreatic cancer patients treated at the Mayo Clinic in Jacksonville. The team found no difference in cancer recurrence between 208 patients who had ultrasound-guided fine needle aspiration (EUS-FNA) and the 48 patients who did not have a biopsy.
In the current study, the researchers examined 11 years (1998-2009) of Medicare data on patients with non-metastatic pancreatic cancer who underwent surgery. The team examined overall survival and pancreatic cancer-specific survival in 498 patients who had EUS-FNA and in 1536 patients who did not have a biopsy.
During a mean follow-up time of 21 months, 285 patients (57%) in the EUS-FNA group and 1167 patients (76%) in the non-EUS-FNA group died. Pancreatic cancer was identified as the cause of death for 251 patients (50%) in the EUS-FNA group and 980 patients (64%) in the non-EUS-FNA group.
The median overall survival in the EUS-FNA group was 22 months, compared to 15 months in the non-EUS-FNA group. Multivariate analysis showed that receipt of EUS-FNA had a borderline significant association with improved overall survival (hazard ratio=0.84, P=0.03).
The median cancer-specific survival was 24 months in the EUS-FNA group and 18 months in the non-EUS-FNA group. Multivariate analysis revealed no significant difference between the two groups (hazard ratio=0.87, P=0.12).
“[Biopsies provide] very valuable information that allow us to tailor treatment,” Dr Wallace noted. “In some cases, we can offer chemotherapy and radiation before surgery for a better outcome, and, in other cases, we can avoid surgery and other therapy altogether.” ![]()

A study of more than 2000 patients refutes the idea that biopsies cause cancer to spread.
In a study published in Gut, researchers showed that patients who received a biopsy had better overall survival and similar cancer-free survival rates as patients who did not have a biopsy.
The team studied pancreatic cancer but said their findings likely apply to other cancers because the diagnostic technique used in this study—fine needle aspiration—is commonly used across tumor types.
“This study shows that physicians and patients should feel reassured that a biopsy is very safe,” said study author Michael Wallace, MD, of the Mayo Clinic in Jacksonville, Florida.
“We do millions of biopsies of cancer a year in the US, but one or two case studies have led to this common myth that biopsies spread cancer.”
This is the second study Dr Wallace and his team have conducted to examine the risk of biopsy.
In a 2013 study published in Endoscopy, the researchers examined outcomes in 256 pancreatic cancer patients treated at the Mayo Clinic in Jacksonville. The team found no difference in cancer recurrence between 208 patients who had ultrasound-guided fine needle aspiration (EUS-FNA) and the 48 patients who did not have a biopsy.
In the current study, the researchers examined 11 years (1998-2009) of Medicare data on patients with non-metastatic pancreatic cancer who underwent surgery. The team examined overall survival and pancreatic cancer-specific survival in 498 patients who had EUS-FNA and in 1536 patients who did not have a biopsy.
During a mean follow-up time of 21 months, 285 patients (57%) in the EUS-FNA group and 1167 patients (76%) in the non-EUS-FNA group died. Pancreatic cancer was identified as the cause of death for 251 patients (50%) in the EUS-FNA group and 980 patients (64%) in the non-EUS-FNA group.
The median overall survival in the EUS-FNA group was 22 months, compared to 15 months in the non-EUS-FNA group. Multivariate analysis showed that receipt of EUS-FNA had a borderline significant association with improved overall survival (hazard ratio=0.84, P=0.03).
The median cancer-specific survival was 24 months in the EUS-FNA group and 18 months in the non-EUS-FNA group. Multivariate analysis revealed no significant difference between the two groups (hazard ratio=0.87, P=0.12).
“[Biopsies provide] very valuable information that allow us to tailor treatment,” Dr Wallace noted. “In some cases, we can offer chemotherapy and radiation before surgery for a better outcome, and, in other cases, we can avoid surgery and other therapy altogether.” ![]()

A study of more than 2000 patients refutes the idea that biopsies cause cancer to spread.
In a study published in Gut, researchers showed that patients who received a biopsy had better overall survival and similar cancer-free survival rates as patients who did not have a biopsy.
The team studied pancreatic cancer but said their findings likely apply to other cancers because the diagnostic technique used in this study—fine needle aspiration—is commonly used across tumor types.
“This study shows that physicians and patients should feel reassured that a biopsy is very safe,” said study author Michael Wallace, MD, of the Mayo Clinic in Jacksonville, Florida.
“We do millions of biopsies of cancer a year in the US, but one or two case studies have led to this common myth that biopsies spread cancer.”
This is the second study Dr Wallace and his team have conducted to examine the risk of biopsy.
In a 2013 study published in Endoscopy, the researchers examined outcomes in 256 pancreatic cancer patients treated at the Mayo Clinic in Jacksonville. The team found no difference in cancer recurrence between 208 patients who had ultrasound-guided fine needle aspiration (EUS-FNA) and the 48 patients who did not have a biopsy.
In the current study, the researchers examined 11 years (1998-2009) of Medicare data on patients with non-metastatic pancreatic cancer who underwent surgery. The team examined overall survival and pancreatic cancer-specific survival in 498 patients who had EUS-FNA and in 1536 patients who did not have a biopsy.
During a mean follow-up time of 21 months, 285 patients (57%) in the EUS-FNA group and 1167 patients (76%) in the non-EUS-FNA group died. Pancreatic cancer was identified as the cause of death for 251 patients (50%) in the EUS-FNA group and 980 patients (64%) in the non-EUS-FNA group.
The median overall survival in the EUS-FNA group was 22 months, compared to 15 months in the non-EUS-FNA group. Multivariate analysis showed that receipt of EUS-FNA had a borderline significant association with improved overall survival (hazard ratio=0.84, P=0.03).
The median cancer-specific survival was 24 months in the EUS-FNA group and 18 months in the non-EUS-FNA group. Multivariate analysis revealed no significant difference between the two groups (hazard ratio=0.87, P=0.12).
“[Biopsies provide] very valuable information that allow us to tailor treatment,” Dr Wallace noted. “In some cases, we can offer chemotherapy and radiation before surgery for a better outcome, and, in other cases, we can avoid surgery and other therapy altogether.” ![]()
Drug reverses anticoagulation activity of rivaroxaban

Credit: Kevin MacKenzie
An intravenous (IV) bolus of the factor Xa inhibitor antidote andexanet alfa can significantly and immediately reverse the steady-state anticoagulation activity of rivaroxaban in healthy subjects, according to initial results of the phase 3 ANNEXA-R study.
Portola Pharmaceuticals, the company developing andexanet alfa, recently announced these results from the first part of the study.
The company expects to present the full data set on March 16 at the American College of Cardiology’s 64th Annual Scientific Session & Expo in San Diego.
The second part of the ANNEXA-R study, in which researchers are evaluating a bolus plus a continuous infusion of andexanet alfa to sustain reversal, is ongoing.
Portola is developing andexanet alfa as a universal antidote for patients treated with oral and injectable factor Xa inhibitors who are experiencing a major bleeding episode or who require emergency surgery.
Andexanet alfa acts as a factor Xa decoy that targets and sequesters both oral and injectable factor Xa inhibitors in the blood. Once bound, the factor Xa inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
ANNEXA-R details
This randomized, double-blind, placebo-controlled study is an evaluation of andexanet alfa in reversing rivaroxaban-induced anticoagulation in healthy volunteers ages 50 to 75 years.
In the first part of the study, 41 subjects received rivaroxaban at 20 mg once daily for 4 days. Then, they were randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg IV bolus (n=27) or to placebo (n=14).
Results showed that andexanet alfa significantly and immediately reversed the anticoagulation activity of rivaroxaban. Furthermore, andexanet alfa appeared to be well tolerated.
For the second part of the ANNEXA-R study, researchers plan to enroll about 40 healthy volunteers and give them rivaroxaban at 20 mg once daily for 4 days.
Then, subjects will be randomized to receive either placebo or andexanet alfa administered as an 800 mg IV bolus, followed by a continuous infusion of 8 mg/min for 120 minutes. Data from this part of the study are expected in mid-2015.
Andexanet alfa development
Andexanet alfa is the only compound being studied as a reversal agent for factor Xa inhibitors that directly and specifically corrects anti-factor Xa activity.
Portola is evaluating andexanet alfa in randomized, placebo-controlled phase 3 ANNEXA registration studies using pharmacodynamic endpoints agreed to with the US Food and Drug Administration (FDA), such as anti-factor Xa inhibitor units, to demonstrate efficacy.
Researchers recently reported statistically significant results from the first part of the phase 3 ANNEXA-A study, in which researchers evaluated andexanet alfa administered as a single IV bolus dose with the direct factor Xa inhibitor apixaban.
The second part of the study is ongoing. It’s an evaluation of an IV bolus plus a continuous infusion of andexanet alfa to sustain the reversal of anticoagulation activity.
“The statistically significant phase 3 ANNEXA-R study data, together with results presented previously with apixaban, provide compelling evidence that this ground-breaking agent could serve as a universal antidote for factor Xa inhibitor anticoagulants,” said John T. Curnutte, MD, PhD, executive vice president, research and development for Portola.
“Andexanet alfa is unique among the other reversal agents in development in that it has been the only agent to immediately and significantly reverse all of the key pharmacodynamic measurements of coagulation that have been agreed to with the FDA for accelerated approval. These include anti-factor Xa levels, thrombin generation, and unbound anticoagulant (free-fraction). This has been demonstrated with all of the factor Xa inhibitors studied to date—apixaban, rivaroxaban, edoxaban, and enoxaparin.” ![]()

Credit: Kevin MacKenzie
An intravenous (IV) bolus of the factor Xa inhibitor antidote andexanet alfa can significantly and immediately reverse the steady-state anticoagulation activity of rivaroxaban in healthy subjects, according to initial results of the phase 3 ANNEXA-R study.
Portola Pharmaceuticals, the company developing andexanet alfa, recently announced these results from the first part of the study.
The company expects to present the full data set on March 16 at the American College of Cardiology’s 64th Annual Scientific Session & Expo in San Diego.
The second part of the ANNEXA-R study, in which researchers are evaluating a bolus plus a continuous infusion of andexanet alfa to sustain reversal, is ongoing.
Portola is developing andexanet alfa as a universal antidote for patients treated with oral and injectable factor Xa inhibitors who are experiencing a major bleeding episode or who require emergency surgery.
Andexanet alfa acts as a factor Xa decoy that targets and sequesters both oral and injectable factor Xa inhibitors in the blood. Once bound, the factor Xa inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
ANNEXA-R details
This randomized, double-blind, placebo-controlled study is an evaluation of andexanet alfa in reversing rivaroxaban-induced anticoagulation in healthy volunteers ages 50 to 75 years.
In the first part of the study, 41 subjects received rivaroxaban at 20 mg once daily for 4 days. Then, they were randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg IV bolus (n=27) or to placebo (n=14).
Results showed that andexanet alfa significantly and immediately reversed the anticoagulation activity of rivaroxaban. Furthermore, andexanet alfa appeared to be well tolerated.
For the second part of the ANNEXA-R study, researchers plan to enroll about 40 healthy volunteers and give them rivaroxaban at 20 mg once daily for 4 days.
Then, subjects will be randomized to receive either placebo or andexanet alfa administered as an 800 mg IV bolus, followed by a continuous infusion of 8 mg/min for 120 minutes. Data from this part of the study are expected in mid-2015.
Andexanet alfa development
Andexanet alfa is the only compound being studied as a reversal agent for factor Xa inhibitors that directly and specifically corrects anti-factor Xa activity.
Portola is evaluating andexanet alfa in randomized, placebo-controlled phase 3 ANNEXA registration studies using pharmacodynamic endpoints agreed to with the US Food and Drug Administration (FDA), such as anti-factor Xa inhibitor units, to demonstrate efficacy.
Researchers recently reported statistically significant results from the first part of the phase 3 ANNEXA-A study, in which researchers evaluated andexanet alfa administered as a single IV bolus dose with the direct factor Xa inhibitor apixaban.
The second part of the study is ongoing. It’s an evaluation of an IV bolus plus a continuous infusion of andexanet alfa to sustain the reversal of anticoagulation activity.
“The statistically significant phase 3 ANNEXA-R study data, together with results presented previously with apixaban, provide compelling evidence that this ground-breaking agent could serve as a universal antidote for factor Xa inhibitor anticoagulants,” said John T. Curnutte, MD, PhD, executive vice president, research and development for Portola.
“Andexanet alfa is unique among the other reversal agents in development in that it has been the only agent to immediately and significantly reverse all of the key pharmacodynamic measurements of coagulation that have been agreed to with the FDA for accelerated approval. These include anti-factor Xa levels, thrombin generation, and unbound anticoagulant (free-fraction). This has been demonstrated with all of the factor Xa inhibitors studied to date—apixaban, rivaroxaban, edoxaban, and enoxaparin.” ![]()

Credit: Kevin MacKenzie
An intravenous (IV) bolus of the factor Xa inhibitor antidote andexanet alfa can significantly and immediately reverse the steady-state anticoagulation activity of rivaroxaban in healthy subjects, according to initial results of the phase 3 ANNEXA-R study.
Portola Pharmaceuticals, the company developing andexanet alfa, recently announced these results from the first part of the study.
The company expects to present the full data set on March 16 at the American College of Cardiology’s 64th Annual Scientific Session & Expo in San Diego.
The second part of the ANNEXA-R study, in which researchers are evaluating a bolus plus a continuous infusion of andexanet alfa to sustain reversal, is ongoing.
Portola is developing andexanet alfa as a universal antidote for patients treated with oral and injectable factor Xa inhibitors who are experiencing a major bleeding episode or who require emergency surgery.
Andexanet alfa acts as a factor Xa decoy that targets and sequesters both oral and injectable factor Xa inhibitors in the blood. Once bound, the factor Xa inhibitors are unable to bind to and inhibit native factor Xa, thus allowing for the restoration of normal hemostatic processes.
ANNEXA-R details
This randomized, double-blind, placebo-controlled study is an evaluation of andexanet alfa in reversing rivaroxaban-induced anticoagulation in healthy volunteers ages 50 to 75 years.
In the first part of the study, 41 subjects received rivaroxaban at 20 mg once daily for 4 days. Then, they were randomized in a 2:1 ratio to receive either andexanet alfa administered as an 800 mg IV bolus (n=27) or to placebo (n=14).
Results showed that andexanet alfa significantly and immediately reversed the anticoagulation activity of rivaroxaban. Furthermore, andexanet alfa appeared to be well tolerated.
For the second part of the ANNEXA-R study, researchers plan to enroll about 40 healthy volunteers and give them rivaroxaban at 20 mg once daily for 4 days.
Then, subjects will be randomized to receive either placebo or andexanet alfa administered as an 800 mg IV bolus, followed by a continuous infusion of 8 mg/min for 120 minutes. Data from this part of the study are expected in mid-2015.
Andexanet alfa development
Andexanet alfa is the only compound being studied as a reversal agent for factor Xa inhibitors that directly and specifically corrects anti-factor Xa activity.
Portola is evaluating andexanet alfa in randomized, placebo-controlled phase 3 ANNEXA registration studies using pharmacodynamic endpoints agreed to with the US Food and Drug Administration (FDA), such as anti-factor Xa inhibitor units, to demonstrate efficacy.
Researchers recently reported statistically significant results from the first part of the phase 3 ANNEXA-A study, in which researchers evaluated andexanet alfa administered as a single IV bolus dose with the direct factor Xa inhibitor apixaban.
The second part of the study is ongoing. It’s an evaluation of an IV bolus plus a continuous infusion of andexanet alfa to sustain the reversal of anticoagulation activity.
“The statistically significant phase 3 ANNEXA-R study data, together with results presented previously with apixaban, provide compelling evidence that this ground-breaking agent could serve as a universal antidote for factor Xa inhibitor anticoagulants,” said John T. Curnutte, MD, PhD, executive vice president, research and development for Portola.
“Andexanet alfa is unique among the other reversal agents in development in that it has been the only agent to immediately and significantly reverse all of the key pharmacodynamic measurements of coagulation that have been agreed to with the FDA for accelerated approval. These include anti-factor Xa levels, thrombin generation, and unbound anticoagulant (free-fraction). This has been demonstrated with all of the factor Xa inhibitors studied to date—apixaban, rivaroxaban, edoxaban, and enoxaparin.” ![]()
Localized Argyria With Pseudo-ochronosis
Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.

A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.
Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.
Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.
Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6
- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.


A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.

Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.

Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.

Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6

Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.


A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.

Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.

Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.

Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6

- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
Edoxaban approved for atrial fib, DVT, and PE indications
Edoxaban, a selective factor Xa-inhibitor, has been approved by the Food and Drug Administration for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, with a statement in the boxed warning that it should not be used in patients with normal renal function.
The warning reflects the results of a subgroup analysis in the pivotal trial, which found that the 60-mg dose was superior to warfarin in terms of reducing the stroke risk in mildly renally impaired patients, but was worse in patients with normal renal function. This was the main focus of a meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Panel meeting in October, in which the panel voted 9-1 to recommend approval of edoxaban for this indication, but had mixed opinions on whether approval should be limited to patients with mild to moderate renal impairment.
The approved prescribing information recommends that a patient’s creatinine clearance should be checked before edoxaban is prescribed. “Patients with creatinine clearance greater than 95 mL/min have an increased risk of stroke, compared to similar patients given warfarin,” and should be treated with another anticoagulant, the FDA said in the Jan. 9 statement announcing the approval. The recommended dose for those with a creatinine clearance between 50 mL/min and 95 mL/min is 60 mg once a day; for those with a creatinine clearance of 15-50 mL/min, the recommended dose is 30 mg once a day, according to the prescribing information.
Edoxaban, the fourth novel oral anticoagulant drug approved by the FDA, will be marketed as Savaysa by Daiichi Sankyo. It was also approved to treat deep vein thrombosis and pulmonary embolism following 5-10 days of initial therapy with a parenteral anticoagulant. The recommended dose for this indication is 60 mg once a day. For patients with a creatinine clearance of 15-50 mL/min, or who weigh up to 60 kg (about 132 pounds), or who are taking “certain P-glycoprotein inhibitors,” the 30-mg/day dose is recommended.
Approval for the nonvalvular AF indication was based on ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) study, comparing once-daily edoxaban (60 mg and 30 mg) to warfarin in 21,015 patients with nonvalvular AF, at a moderate to high risk of thromboembolic events (N. Engl. J. Med. 2013;369:2093-104). Over a median of almost 3 years, both doses were noninferior to warfarin in the primary efficacy endpoint, the occurrence of first stroke or of a systemic embolic event. Overall, major bleeding events were significantly lower among those on the 60-mg and 30-mg doses, compared with those on warfarin. However, the rate of ischemic stroke was higher relative to warfarin in patients with a creatinine clearance over 95 mL/min.
About half of the edoxaban dose is eliminated by the kidneys, and patients with a creatinine clearance above 95 mL/min have lower plasma edoxaban levels, according to a statement in the clinical trials section of the prescribing information, which adds: “Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Savaysa, compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.”
Approval of the DVT and PE indication was based on the Hokusai-VTE study of about 8,200 people comparing edoxaban to warfarin, which found that the edoxaban 60 mg once a day was noninferior to warfarin in the rate of symptomatic venous thromboembolism (3.2% vs. 3.5% in those on warfarin). The rate of major or clinically relevant nonmajor bleeding events was 8.5% among those on edoxaban vs. 10.3% in those on warfarin (N. Engl. J. Med. 2013;369:1406-15).
Bleeding and anemia were the most common adverse events among patients with nonvalvular atrial fibrillation in clinical trials, and “as with other FDA-approved anticlotting drugs, bleeding, including life-threatening bleeding, is the most serious risk with Savaysa,” the FDA statement said. Among those treated for DVT and PE, the most common adverse events were bleeding, rash, abnormal liver function tests, and anemia.
Savaysa is the fourth novel oral anticoagulant to be cleared by the FDA, after dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Serious adverse events associated with edoxaban should be reported to the FDA’s MedWatch program or at 800-332-1088.
A precedent does not come readily to mind where one restricts the use of a drug to patients with mild to moderately impaired renal function to optimize the benefit-risk balance. Typically, one avoids the drug or reduces the dose in such subgroups. For this reason, I think the drug is likely going to be a “nonstarter” for many clinicians.
During the FDA Advisory Committee panel meeting on edoxaban, the emphasis was on excluding patients with a creatinine clearance greater than 80 mL/min (representing about 37% of patients in the pivotal trial). The FDA approval used a cut-off of 95 mL/min (representing about 22% of patients in the pivotal trial). Even though the latter increases the eligible pool of patients for edoxaban, the major challenge is going to be clinical acceptability and marketability, especially given no unique advantage of this drug over other approved novel anticoagulants.
The CrCl cut off of 95mL/min applies to nonvalvular atrial fibrillation indication, and not to the venous thromboembolism (DVT/PE) indication. However, the latter indication requires 5-10 days of parenteral anticoagulant therapy, which puts it at a disadvantage compared with rivaroxaban or apixaban.
It is interesting to note that active pathological bleeding qualifies as a contraindication, but use in patients with normal renal function does not. There is a twofold increased risk of overall stroke or systemic embolism (primary endpoint) and ischemic stroke in patients with normal renal function (CrCl > 95 mL/min) along with an attenuation of the hemorrhagic risk advantage in this subgroup. Even though adjudicated major bleeding is still favorable for edoxaban in this subgroup, in my opinion the ischemic stroke risk outweighs the major bleeding advantage.
Sanjay Kaul, M.D., is professor of medicine at the University of California, Los Angeles. He was a member of the FDA’s Cardiovascular and Renal Drugs Advisory Committee that reviewed edoxaban at a meeting on Oct. 30, 2014.
A precedent does not come readily to mind where one restricts the use of a drug to patients with mild to moderately impaired renal function to optimize the benefit-risk balance. Typically, one avoids the drug or reduces the dose in such subgroups. For this reason, I think the drug is likely going to be a “nonstarter” for many clinicians.
During the FDA Advisory Committee panel meeting on edoxaban, the emphasis was on excluding patients with a creatinine clearance greater than 80 mL/min (representing about 37% of patients in the pivotal trial). The FDA approval used a cut-off of 95 mL/min (representing about 22% of patients in the pivotal trial). Even though the latter increases the eligible pool of patients for edoxaban, the major challenge is going to be clinical acceptability and marketability, especially given no unique advantage of this drug over other approved novel anticoagulants.
The CrCl cut off of 95mL/min applies to nonvalvular atrial fibrillation indication, and not to the venous thromboembolism (DVT/PE) indication. However, the latter indication requires 5-10 days of parenteral anticoagulant therapy, which puts it at a disadvantage compared with rivaroxaban or apixaban.
It is interesting to note that active pathological bleeding qualifies as a contraindication, but use in patients with normal renal function does not. There is a twofold increased risk of overall stroke or systemic embolism (primary endpoint) and ischemic stroke in patients with normal renal function (CrCl > 95 mL/min) along with an attenuation of the hemorrhagic risk advantage in this subgroup. Even though adjudicated major bleeding is still favorable for edoxaban in this subgroup, in my opinion the ischemic stroke risk outweighs the major bleeding advantage.
Sanjay Kaul, M.D., is professor of medicine at the University of California, Los Angeles. He was a member of the FDA’s Cardiovascular and Renal Drugs Advisory Committee that reviewed edoxaban at a meeting on Oct. 30, 2014.
A precedent does not come readily to mind where one restricts the use of a drug to patients with mild to moderately impaired renal function to optimize the benefit-risk balance. Typically, one avoids the drug or reduces the dose in such subgroups. For this reason, I think the drug is likely going to be a “nonstarter” for many clinicians.
During the FDA Advisory Committee panel meeting on edoxaban, the emphasis was on excluding patients with a creatinine clearance greater than 80 mL/min (representing about 37% of patients in the pivotal trial). The FDA approval used a cut-off of 95 mL/min (representing about 22% of patients in the pivotal trial). Even though the latter increases the eligible pool of patients for edoxaban, the major challenge is going to be clinical acceptability and marketability, especially given no unique advantage of this drug over other approved novel anticoagulants.
The CrCl cut off of 95mL/min applies to nonvalvular atrial fibrillation indication, and not to the venous thromboembolism (DVT/PE) indication. However, the latter indication requires 5-10 days of parenteral anticoagulant therapy, which puts it at a disadvantage compared with rivaroxaban or apixaban.
It is interesting to note that active pathological bleeding qualifies as a contraindication, but use in patients with normal renal function does not. There is a twofold increased risk of overall stroke or systemic embolism (primary endpoint) and ischemic stroke in patients with normal renal function (CrCl > 95 mL/min) along with an attenuation of the hemorrhagic risk advantage in this subgroup. Even though adjudicated major bleeding is still favorable for edoxaban in this subgroup, in my opinion the ischemic stroke risk outweighs the major bleeding advantage.
Sanjay Kaul, M.D., is professor of medicine at the University of California, Los Angeles. He was a member of the FDA’s Cardiovascular and Renal Drugs Advisory Committee that reviewed edoxaban at a meeting on Oct. 30, 2014.
Edoxaban, a selective factor Xa-inhibitor, has been approved by the Food and Drug Administration for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, with a statement in the boxed warning that it should not be used in patients with normal renal function.
The warning reflects the results of a subgroup analysis in the pivotal trial, which found that the 60-mg dose was superior to warfarin in terms of reducing the stroke risk in mildly renally impaired patients, but was worse in patients with normal renal function. This was the main focus of a meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Panel meeting in October, in which the panel voted 9-1 to recommend approval of edoxaban for this indication, but had mixed opinions on whether approval should be limited to patients with mild to moderate renal impairment.
The approved prescribing information recommends that a patient’s creatinine clearance should be checked before edoxaban is prescribed. “Patients with creatinine clearance greater than 95 mL/min have an increased risk of stroke, compared to similar patients given warfarin,” and should be treated with another anticoagulant, the FDA said in the Jan. 9 statement announcing the approval. The recommended dose for those with a creatinine clearance between 50 mL/min and 95 mL/min is 60 mg once a day; for those with a creatinine clearance of 15-50 mL/min, the recommended dose is 30 mg once a day, according to the prescribing information.
Edoxaban, the fourth novel oral anticoagulant drug approved by the FDA, will be marketed as Savaysa by Daiichi Sankyo. It was also approved to treat deep vein thrombosis and pulmonary embolism following 5-10 days of initial therapy with a parenteral anticoagulant. The recommended dose for this indication is 60 mg once a day. For patients with a creatinine clearance of 15-50 mL/min, or who weigh up to 60 kg (about 132 pounds), or who are taking “certain P-glycoprotein inhibitors,” the 30-mg/day dose is recommended.
Approval for the nonvalvular AF indication was based on ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) study, comparing once-daily edoxaban (60 mg and 30 mg) to warfarin in 21,015 patients with nonvalvular AF, at a moderate to high risk of thromboembolic events (N. Engl. J. Med. 2013;369:2093-104). Over a median of almost 3 years, both doses were noninferior to warfarin in the primary efficacy endpoint, the occurrence of first stroke or of a systemic embolic event. Overall, major bleeding events were significantly lower among those on the 60-mg and 30-mg doses, compared with those on warfarin. However, the rate of ischemic stroke was higher relative to warfarin in patients with a creatinine clearance over 95 mL/min.
About half of the edoxaban dose is eliminated by the kidneys, and patients with a creatinine clearance above 95 mL/min have lower plasma edoxaban levels, according to a statement in the clinical trials section of the prescribing information, which adds: “Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Savaysa, compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.”
Approval of the DVT and PE indication was based on the Hokusai-VTE study of about 8,200 people comparing edoxaban to warfarin, which found that the edoxaban 60 mg once a day was noninferior to warfarin in the rate of symptomatic venous thromboembolism (3.2% vs. 3.5% in those on warfarin). The rate of major or clinically relevant nonmajor bleeding events was 8.5% among those on edoxaban vs. 10.3% in those on warfarin (N. Engl. J. Med. 2013;369:1406-15).
Bleeding and anemia were the most common adverse events among patients with nonvalvular atrial fibrillation in clinical trials, and “as with other FDA-approved anticlotting drugs, bleeding, including life-threatening bleeding, is the most serious risk with Savaysa,” the FDA statement said. Among those treated for DVT and PE, the most common adverse events were bleeding, rash, abnormal liver function tests, and anemia.
Savaysa is the fourth novel oral anticoagulant to be cleared by the FDA, after dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Serious adverse events associated with edoxaban should be reported to the FDA’s MedWatch program or at 800-332-1088.
Edoxaban, a selective factor Xa-inhibitor, has been approved by the Food and Drug Administration for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, with a statement in the boxed warning that it should not be used in patients with normal renal function.
The warning reflects the results of a subgroup analysis in the pivotal trial, which found that the 60-mg dose was superior to warfarin in terms of reducing the stroke risk in mildly renally impaired patients, but was worse in patients with normal renal function. This was the main focus of a meeting of the FDA’s Cardiovascular and Renal Drugs Advisory Panel meeting in October, in which the panel voted 9-1 to recommend approval of edoxaban for this indication, but had mixed opinions on whether approval should be limited to patients with mild to moderate renal impairment.
The approved prescribing information recommends that a patient’s creatinine clearance should be checked before edoxaban is prescribed. “Patients with creatinine clearance greater than 95 mL/min have an increased risk of stroke, compared to similar patients given warfarin,” and should be treated with another anticoagulant, the FDA said in the Jan. 9 statement announcing the approval. The recommended dose for those with a creatinine clearance between 50 mL/min and 95 mL/min is 60 mg once a day; for those with a creatinine clearance of 15-50 mL/min, the recommended dose is 30 mg once a day, according to the prescribing information.
Edoxaban, the fourth novel oral anticoagulant drug approved by the FDA, will be marketed as Savaysa by Daiichi Sankyo. It was also approved to treat deep vein thrombosis and pulmonary embolism following 5-10 days of initial therapy with a parenteral anticoagulant. The recommended dose for this indication is 60 mg once a day. For patients with a creatinine clearance of 15-50 mL/min, or who weigh up to 60 kg (about 132 pounds), or who are taking “certain P-glycoprotein inhibitors,” the 30-mg/day dose is recommended.
Approval for the nonvalvular AF indication was based on ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) study, comparing once-daily edoxaban (60 mg and 30 mg) to warfarin in 21,015 patients with nonvalvular AF, at a moderate to high risk of thromboembolic events (N. Engl. J. Med. 2013;369:2093-104). Over a median of almost 3 years, both doses were noninferior to warfarin in the primary efficacy endpoint, the occurrence of first stroke or of a systemic embolic event. Overall, major bleeding events were significantly lower among those on the 60-mg and 30-mg doses, compared with those on warfarin. However, the rate of ischemic stroke was higher relative to warfarin in patients with a creatinine clearance over 95 mL/min.
About half of the edoxaban dose is eliminated by the kidneys, and patients with a creatinine clearance above 95 mL/min have lower plasma edoxaban levels, according to a statement in the clinical trials section of the prescribing information, which adds: “Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of Savaysa, compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.”
Approval of the DVT and PE indication was based on the Hokusai-VTE study of about 8,200 people comparing edoxaban to warfarin, which found that the edoxaban 60 mg once a day was noninferior to warfarin in the rate of symptomatic venous thromboembolism (3.2% vs. 3.5% in those on warfarin). The rate of major or clinically relevant nonmajor bleeding events was 8.5% among those on edoxaban vs. 10.3% in those on warfarin (N. Engl. J. Med. 2013;369:1406-15).
Bleeding and anemia were the most common adverse events among patients with nonvalvular atrial fibrillation in clinical trials, and “as with other FDA-approved anticlotting drugs, bleeding, including life-threatening bleeding, is the most serious risk with Savaysa,” the FDA statement said. Among those treated for DVT and PE, the most common adverse events were bleeding, rash, abnormal liver function tests, and anemia.
Savaysa is the fourth novel oral anticoagulant to be cleared by the FDA, after dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Serious adverse events associated with edoxaban should be reported to the FDA’s MedWatch program or at 800-332-1088.