User login
When snoring is more than an annoyance
We have all seen cartoons of an unhappy wife awake in bed next to her loudly snoring husband. Casual conversations with friends, particularly female ones, indicate that this is an accurate representation of a common scenario. As I have started to probe more diligently for evidence of obstructive sleep apnea (OSA) in my patients, not just in those who complain of “fatigue” (more patients use this term with me than “sleepiness”), I see a lot of shaking of heads from the wives of men who deny that they snore or have disrupted sleep. I am not implying that this is solely a male disease. Far from it. But as in other medical scenarios, the Y chromosome seems somehow linked to denial or lack of awareness of symptoms. In any event, I was not a bit surprised to read in the review by Dr. Mehra in this issue of the Journal that 17% of adults may have OSA.
As awareness of OSA has grown and testing for it has become easier, multiple reports have documented associated comorbidities: hypertension, restless leg syndrome, gout, and neurocognitive deficits. Home devices to treat OSA have significantly improved. Technological advances have led to the development of small, quiet, smart pumps that provide continuous positive airway pressure (CPAP) via nasal or relatively comfortable full-face masks. Compliance and patient acceptance of CPAP have improved, although patient education and a bit of cajoling in the office are still necessary—less so if the bedroom partner is also present for this discussion.
Perhaps surprising is a growing pool of data showing that CPAP’s benefits extend to more than just reducing sleepiness. It can reduce nocturia, restless leg syndrome, arrhythmias including atrial fibrillation, gastric reflux, and fatal and nonfatal cardiovascular events. Snoring and thus probably sleep-partner satisfaction are also improved.
Several physiologic mechanisms may explain the benefits of CPAP, including reducing hypoxic episodes (explaining its effect on atrial fibrillation), altered atrial natriuretic factor levels (thus reducing nocturia), and changing intrathoracic pressure (thus reducing gastric reflux). It will be interesting to see if there are long-term effects of successfully applied CPAP on neurocognition and progression of neurodegenerative diseases.
While high-decibel snoring and snorting are not present in all patients with OSA, it is quite clear now that they represent far more than an annoyance. We should be vigilant about looking for OSA and strongly encourage a trial of CPAP in appropriately diagnosed patients.
We have all seen cartoons of an unhappy wife awake in bed next to her loudly snoring husband. Casual conversations with friends, particularly female ones, indicate that this is an accurate representation of a common scenario. As I have started to probe more diligently for evidence of obstructive sleep apnea (OSA) in my patients, not just in those who complain of “fatigue” (more patients use this term with me than “sleepiness”), I see a lot of shaking of heads from the wives of men who deny that they snore or have disrupted sleep. I am not implying that this is solely a male disease. Far from it. But as in other medical scenarios, the Y chromosome seems somehow linked to denial or lack of awareness of symptoms. In any event, I was not a bit surprised to read in the review by Dr. Mehra in this issue of the Journal that 17% of adults may have OSA.
As awareness of OSA has grown and testing for it has become easier, multiple reports have documented associated comorbidities: hypertension, restless leg syndrome, gout, and neurocognitive deficits. Home devices to treat OSA have significantly improved. Technological advances have led to the development of small, quiet, smart pumps that provide continuous positive airway pressure (CPAP) via nasal or relatively comfortable full-face masks. Compliance and patient acceptance of CPAP have improved, although patient education and a bit of cajoling in the office are still necessary—less so if the bedroom partner is also present for this discussion.
Perhaps surprising is a growing pool of data showing that CPAP’s benefits extend to more than just reducing sleepiness. It can reduce nocturia, restless leg syndrome, arrhythmias including atrial fibrillation, gastric reflux, and fatal and nonfatal cardiovascular events. Snoring and thus probably sleep-partner satisfaction are also improved.
Several physiologic mechanisms may explain the benefits of CPAP, including reducing hypoxic episodes (explaining its effect on atrial fibrillation), altered atrial natriuretic factor levels (thus reducing nocturia), and changing intrathoracic pressure (thus reducing gastric reflux). It will be interesting to see if there are long-term effects of successfully applied CPAP on neurocognition and progression of neurodegenerative diseases.
While high-decibel snoring and snorting are not present in all patients with OSA, it is quite clear now that they represent far more than an annoyance. We should be vigilant about looking for OSA and strongly encourage a trial of CPAP in appropriately diagnosed patients.
We have all seen cartoons of an unhappy wife awake in bed next to her loudly snoring husband. Casual conversations with friends, particularly female ones, indicate that this is an accurate representation of a common scenario. As I have started to probe more diligently for evidence of obstructive sleep apnea (OSA) in my patients, not just in those who complain of “fatigue” (more patients use this term with me than “sleepiness”), I see a lot of shaking of heads from the wives of men who deny that they snore or have disrupted sleep. I am not implying that this is solely a male disease. Far from it. But as in other medical scenarios, the Y chromosome seems somehow linked to denial or lack of awareness of symptoms. In any event, I was not a bit surprised to read in the review by Dr. Mehra in this issue of the Journal that 17% of adults may have OSA.
As awareness of OSA has grown and testing for it has become easier, multiple reports have documented associated comorbidities: hypertension, restless leg syndrome, gout, and neurocognitive deficits. Home devices to treat OSA have significantly improved. Technological advances have led to the development of small, quiet, smart pumps that provide continuous positive airway pressure (CPAP) via nasal or relatively comfortable full-face masks. Compliance and patient acceptance of CPAP have improved, although patient education and a bit of cajoling in the office are still necessary—less so if the bedroom partner is also present for this discussion.
Perhaps surprising is a growing pool of data showing that CPAP’s benefits extend to more than just reducing sleepiness. It can reduce nocturia, restless leg syndrome, arrhythmias including atrial fibrillation, gastric reflux, and fatal and nonfatal cardiovascular events. Snoring and thus probably sleep-partner satisfaction are also improved.
Several physiologic mechanisms may explain the benefits of CPAP, including reducing hypoxic episodes (explaining its effect on atrial fibrillation), altered atrial natriuretic factor levels (thus reducing nocturia), and changing intrathoracic pressure (thus reducing gastric reflux). It will be interesting to see if there are long-term effects of successfully applied CPAP on neurocognition and progression of neurodegenerative diseases.
While high-decibel snoring and snorting are not present in all patients with OSA, it is quite clear now that they represent far more than an annoyance. We should be vigilant about looking for OSA and strongly encourage a trial of CPAP in appropriately diagnosed patients.
Sleep apnea ABCs: Airway, breathing, circulation
Obstructive sleep apnea (OSA) is common and poorly recognized and, if untreated, leads to serious health consequences. This article discusses the epidemiology of OSA, describes common presenting signs and symptoms, and reviews diagnostic testing and treatment options. Adverse health effects related to untreated sleep apnea are also discussed.
COMMON, POORLY RECOGNIZED, AND COSTLY IF UNTREATED
OSA is very common in the general population and is associated with substantial morbidity and mortality. An estimated 17% of the general adult population has OSA, and the numbers are increasing with the obesity epidemic. Nearly 1 in 15 adults has at least moderate sleep apnea,1,2 and approximately 85% of cases are estimated to be undiagnosed.3 A 1999 study estimated that untreated OSA resulted in approximately $3.4 billion in additional medical costs per year in the United States,4 a figure that is likely to be higher now, given the rising prevalence of OSA. The prevalence of OSA in primary care and subspecialty clinics is even higher than in the community, as more than half of patients who have diabetes or hypertension and 30% to 40% of patients with coronary artery disease are estimated to have OSA.5–7
REPETITIVE UPPER-AIRWAY COLLAPSE
During sleep, parasympathetic activity is enhanced and the muscle tone of the upper airway is decreased, particularly in the pharyngeal dilator muscles. Still, even in the supine position, a healthy person maintains patency of the airway and adequate airflow during sleep.
OSA is characterized by repetitive complete or partial collapse of the upper airway during sleep, resulting in an apneic or hypopneic event, respectively, and often causing snoring from upper-airway tissue vibration.
People who are susceptible to OSA typically have a smaller, more collapsible airway that is often less distensible and has a higher critical closing pressure. Radiographic and physiologic data have shown that the airway dimensions of patients with OSA are smaller than in those without OSA. The shape of the airway of a patient with OSA is often elliptical, given the extrinsic compression of the lateral aspects of the airway by increased size of the parapharyngeal fat pads. OSA episodes are characterized by closure of the upper airway and by progressively increasing respiratory efforts driven by chemoreceptor and mechanoreceptor stimuli, culminating in an arousal from sleep and a reopening of the airway.
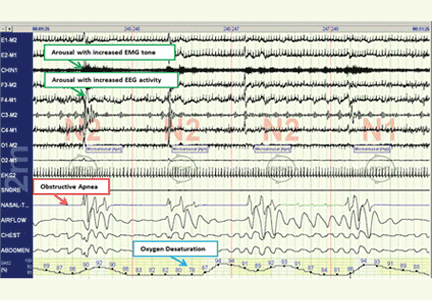
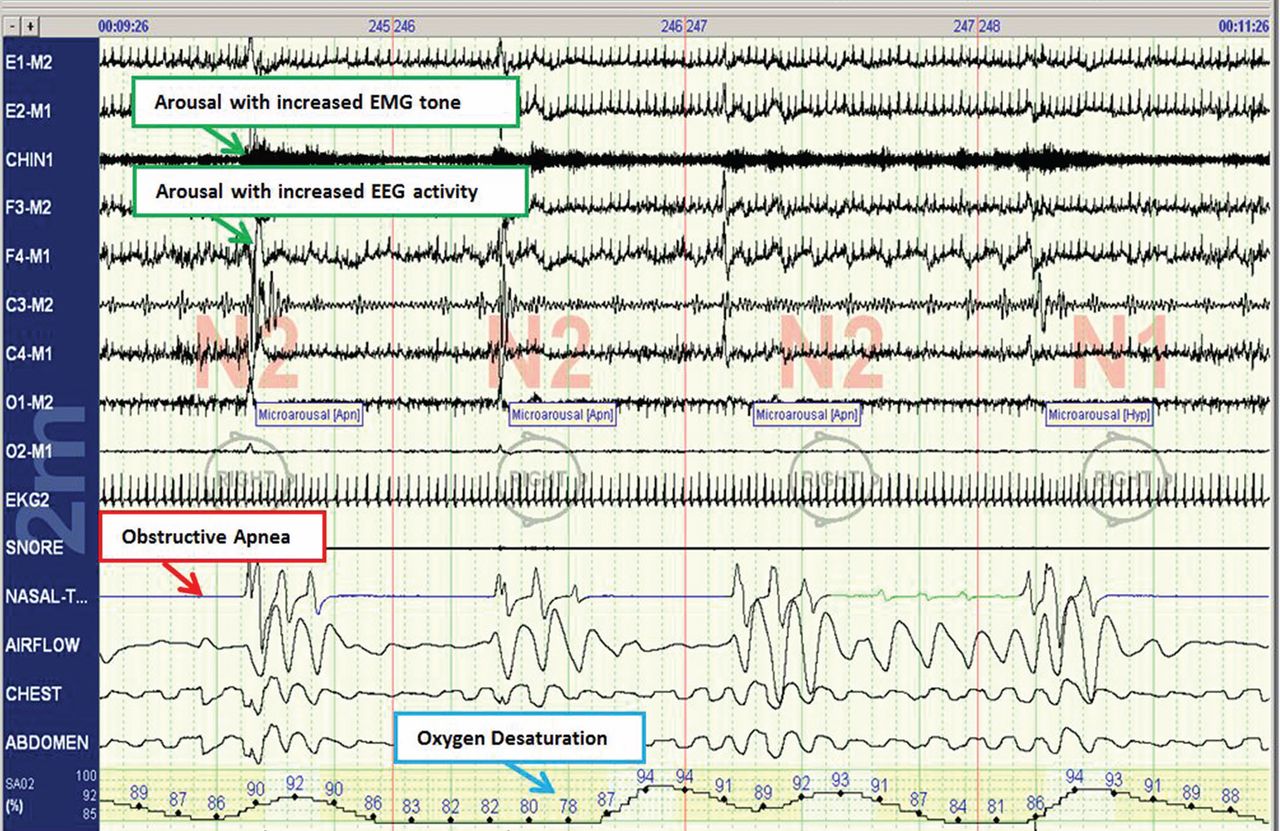
The disease-defining metric used for assessing OSA severity is the apnea-hypopnea index, ie, the number of apneas and hypopneas that occur per hour of sleep.8 An apneic or hypopneic event is identified during polysomnography by the complete cessation of airflow or by a reduction in airflow for 10 seconds or longer (Figure 1).
HEALTH CONSEQUENCES IF UNTREATED
Untreated sleep apnea causes numerous pathophysiologic perturbations, including chronic intermittent hypoxia, ventilatory overshoot hyperoxia, increased sympathetic nervous system activity, intrathoracic pressure swings, hypercapnea, sleep fragmentation, increased arousals, reduced sleep duration, and fragmentation of rapid-eye-movement sleep.
Intermittent hypoxia activates the sympathetic nervous system and causes pulmonary vasoconstriction, with increases in pulmonary arterial pressures and myocardial workload. Sympathetic activation, ascertained by peroneal microneurography, has been shown to be increased not only during sleep but also persisting during wakefulness in patients with untreated OSA vs those without OSA.9 Autonomic nervous system fluctuations accompany apneic episodes, resulting in enhanced parasympathetic tone and sympathetic activation associated with a rise in blood pressure and heart rate that occur after the respiratory event.
Intermediate pathways that link the negative pathophysiologic effects of OSA with adverse health outcomes include increased systemic inflammation, increased oxidative stress, metabolic dysfunction, insulin resistance, hypercoagulability, endothelial dysfunction, and autonomic dysfunction.
As a result, a variety of adverse clinical outcomes are associated with untreated OSA, including systemic hypertension, ischemic heart disease and atherosclerosis, diastolic dysfunction, congestive heart failure, cardiac arrhythmias, stroke, increased risk of death, and sudden death, as well as noncardiovascular outcomes such as gout, neurocognitive deficits, and mood disorders.10
Inflammatory and atherogenic effects
Increased levels of markers of systemic inflammation, prothrombosis, and oxidative stress have been observed in OSA and may be key pathophysiologic links between OSA and cardiovascular sequelae. OSA has been associated with up-regulation of a number of inflammatory mediators: interleukin (IL) 6, soluble IL-6 receptor, IL-8, tumor necrosis factor alpha, and C-reactive protein. Soluble IL-6 levels in particular are higher in people who have sleep-disordered breathing, as reflected by the apnea-hypopnea index independent of obesity, with relationships stronger in the morning than in the evening. This likely reflects the overnight OSA-related physiologic stress.11
Thrombotic potential is also enhanced, with higher levels of plasminogen activator inhibitor 1, fibrinogen, P-selectin, and vascular endothelial growth factor. Some of these factors normally have a diurnal cycle, with higher levels in the morning, but in OSA, increasing OSA severity is associated with increased prothrombotic potential in the morning hours. Of interest, levels of these substances showed a plateau effect, rising in people who had only mildly elevated apnea-hypopnea indices and then leveling off.12 Intermittent hypoxia followed by ventilatory overshoot hyperoxia, characteristic of sleep apnea, provides the ideal environment for augmentation of oxidative stress, with evidence of increased oxidation of serum proteins and lipids. Hypoxia and oxygen-derived free radicals may result in cardiac myocyte injury. Experimental data demonstrate that intermittent hypoxia combined with a high-fat diet results in synergistic acceleration of evidence of atherogenic lesions.
Patients with OSA also have evidence of endothelial dysfunction, insulin resistance, and dyslipidemia, all pathways that can facilitate the progression of atherosclerosis in OSA.13–15
Cardiac arrhythmias
In the Sleep Heart Health Study, a multicenter epidemiologic study designed to examine the relationships of OSA and cardiovascular outcomes, those who had moderate to severe OSA had a risk of ventricular and atrial arrhythmias two to four times higher than those without OSA, even after correction for the confounding influences of obesity and underlying cardiovascular risk.14 These findings were corroborated in subsequent work highlighting monotonic dose-response relationships with increasing OSA severity and increased odds of atrial and ventricular arrhythmia in a cohort of about 3,000 older men.11 Additional compelling evidence of a causal relationship is that the risk of discrete arrhythmic events is markedly increased after a respiratory disturbance in sleep.16
In patients who successfully underwent cardioversion for atrial fibrillation, those who had sleep apnea but were not treated with continuous positive airway pressure (CPAP) had a much higher rate of recurrence of atrial fibrillation during the subsequent year than those with CPAP-treated sleep apnea and than controls never diagnosed with sleep apnea. In the untreated patients with sleep apnea, the mean nocturnal fall in oxygen saturation was significantly greater in those who had recurrence of atrial fibrillation than in those who did not, suggesting hypoxia as an important mechanism contributing to atrial fibrillation.17
Since then, several other retrospective studies have shown similar findings after pulmonary vein antrum isolation and ablation in terms of reduction of atrial fibrillation recurrence with CPAP treatment in OSA.18
Walia et al19 described a patient with moderate sleep apnea who underwent a split-night study. During the baseline part of the study, the patient had about 18 ectopic beats per minute. During the second portion of the study while CPAP was applied, progressively fewer ectopic beats occurred as airway pressure was increased until a normal rhythm without ectopic beats was achieved at the goal treatment CPAP pressure setting.
Cardiovascular disease, stroke, and death
Marin et al20 followed about 1,500 men for 10 years, including some who had severe OSA, some with sleep apnea who were treated with CPAP, and controls. The risk of nonfatal and fatal cardiovascular disease events was nearly three times higher in those with severe disease than in healthy participants. Those treated with CPAP had a risk approximately the same as in the control group.
The Sleep Heart Study followed approximately 6,000 people with untreated sleep apnea for a median of nearly 9 years. It found a significant association between the apnea-hypopnea index and ischemic stroke, especially in men.21 Survival in patients with heart failure is also associated with the degree of OSA; patients with more severe disease (an apnea-hypopnea index ≥ 15) have a nearly three times greater risk of death than those with no disease or only mild disease (apnea-hypopnea index < 15).22
From the standpoint of health care utilization, findings that central sleep apnea predicts an increased risk of hospital readmission in heart failure are of particular interest.23
People with OSA are at increased risk of nocturnal sudden cardiac death.24 Sleep apnea is also associated with an increased overall death rate, and the higher the apnea-hypopnea index, the higher the death rate,25 even after adjusting for age, sex, body mass index, and underlying cardiovascular risk, with findings most pronounced in men under age 70.
Motor vehicle accidents
The need for caution during driving should be discussed with every patient, as motor vehicle accidents are an immediate danger to the patient and others. The association with motor vehicle accidents is independent of sleepiness, and drivers with sleep apnea often do not perceive performance impairment. Young et al26 found that men who snored were 3.4 times as likely to have an accident over a 5-year period, and that men and women with an apnea-hypopnea index greater than 15 were more than 7 times as likely to have multiple accidents over a 5-year period, highlighting the importance of discussing, documenting, and expeditiously diagnosing and treating OSA, particularly in those who report drowsiness while driving.
CLINICAL RISK FACTORS
Risk factors can be divided into nonmodifiable and modifiable ones.
Nonmodifiable factors
Age. Bimodal distributions in OSA prevalence have been observed; ie, that the pediatric population and people who are middle-aged have the highest prevalence of OSA. A linear relationship between sleep apnea prevalence and age until about age 65 was identified in data from the Sleep Heart Health Study.27 After that, the prevalence rates plateau; it is unclear if this is secondary to natural remission of the disease after a certain age or because patients with more severe disease have died by that age (ie, survivorship bias), blunting an increase in prevalence.
Sex. Men develop sleep apnea at a rate three to five times that of women. Several explanations have been proposed to account for this.28,29 Sex hormones are one factor; women with sleep apnea on hormone replacement therapy have a significantly less-severe sleep apnea burden than other postmenopausal women,30 suggesting a positive effect from estrogen. Sex-based differences in fat distribution, length and collapsibility of the upper airway, genioglossal activity, neurochemical control mechanisms, and arousal response may also contribute to prevalence differences between men and women.
As with coronary artery disease, the presentation of sleep apnea may be atypical in women, particularly around menopause. Sleep apnea should be considered in women who have snoring and daytime sleepiness.
Race. Whites, African Americans, and Asians have a similar prevalence of sleep apnea, but groups differ in obesity rates and craniofacial anatomy.31–34 Asians tend to have craniofacial skeletal restriction. African Americans are more likely to have upper-airway soft-tissue risk and to develop more severe OSA. Whites tend to have both craniofacial and soft-tissue risk. For those with craniofacial anatomy predisposing to OSA, even mild obesity can make it manifest.
Syndromes that predispose to OSA can include craniofacial structural abnormalities, connective tissue problems, or alterations in ventilatory control (eg, Marfan, Down, and Pierre Robin syndromes).
Modifiable risk factors
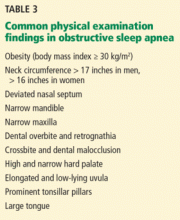
Obesity (body mass index ≥ 30 kg/m2) is a firmly established risk factor, but not all obese patients develop obstructive sleep apnea, and not all people with sleep apnea are obese.
Obesity increases risk by altering the geometry and function of the upper airway, increasing collapsibility. The changes are particularly pronounced in the lateral aspects of the pharynx.35
Obesity also affects respiratory drive, likely in part from leptin resistance. Load compensation is another contributing factor: the increased mass in the thorax and abdomen increases the work of breathing and reduces functional residual capacity, increasing oxygen demands and leading to atelectasis and ventilation-perfusion mismatch.
Although obesity is an important risk factor, it is important to recognize that obesity is not the only one to consider: most people with an apnea-hypopnea index of 5 or greater are not obese. The relationship between body mass index and sleep apnea is weaker in children and in the elderly, probably because other risk factors are more pronounced.36
Craniofacial structural abnormalities such as retrognathia (abnormal posterior position of the mandible) and micrognathia (undersized mandible) can increase the risk of OSA because of a resulting posteriorly displaced genioglossus muscle. Other conditions can alter chemosensitivity, affecting the pH and carbon dioxide level of the blood and therefore affecting ventilatory control mechanisms, making the person more prone to developing sleep apnea. Children and young adults may have tonsillar tissue that obstructs the airway.
The site of obstruction can be behind the palate (retropalatal), behind the tongue (retroglossal), or below the pharynx (hypopharyngeal). This helps explain why positive air way pressure—unlike surgery, which addresses a specific area—is often successful, as it serves to splint or treat all aspects of the airway.
FATIGUE, SLEEPINESS, SNORING, RESTLESS SLEEP
Sleep apnea can result in presentation of multiple signs and symptoms (Table 1).
Daytime sleepiness and fatigue are the most common symptoms. Although nonspecific, they are often quite pronounced. Two short questionnaires—the Epworth Sleepiness Scale37 and the Fatigue Severity Scale—can help distinguish between these two symptoms and assess their impact on a patient’s daily life. In the Epworth Sleepiness Scale, the patient rates his or her chance of dozing on a 4-point scale (0 = would never doze, to 3 = high chance of dozing) in eight situations:
- Sitting and reading
- Watching television
- Sitting inactive in a public place
- As a passenger in a car for an hour without a break
- Lying down to rest in the afternoon
- Sitting and talking to someone
- Sitting quietly after a lunch without alcohol
- In a car while stopped for a few minutes in traffic.
A score of 10 or more is consistent with significant subjective sleepiness.
The Fatigue Severity Scale assesses the impact of fatigue on daily living.
Snoring is a common and specific symptom of sleep apnea; however, not all patients who snore have OSA.
Restlessness during sleep is very common—patients may disturb their bed partner by moving around a lot during sleep or report that the sheets are “all over the place” by morning.
Nocturia can also be a sign of sleep apnea and can contribute to sleep fragmentation. A proposed mechanism of this symptom includes alterations of intrathoracic pressure resulting in atrial stretch, which release atrial natriuretic peptide, leading to nocturia. Treating with CPAP has been found to reduce levels of atrial natriuretic peptide, contributing to better sleep.38
Morning headache may occur and is likely related to increased CO2 levels, which appear to culminate in the morning hours. End-tidal or transcutaneous CO2 monitoring during polysomnography can help elucidate the presence of sleep-related hypoventilation.
Libido is often diminished and can actually be improved with CPAP. This is therefore an important point to discuss with patients, as improved libido can often serve as an incentive for adherence to OSA treatment.
Insomnia exists in about 15% of patients, primarily as a result of sleep apnea-related with treatment.
Sweating, particularly forehead sweating associated with sleep apnea, more commonly occurs in children.
The STOP-BANG questionnaire (Table 2)39 was primarily validated in preoperative anesthesia testing. However, because of its ease of use and favorable performance characteristics, it is increasingly used to predict the likelihood of finding OSA before polysomnography. A score of 3 or more has a sensitivity of 93%.
PHYSICAL EXAMINATION PROVIDES CLUES
Although the physical examination may be normal, certain findings indicate risk (Table 3). Obesity alone is not an accepted indication for polysomnography unless there are concomitant worrisome signs or symptoms. Of note, those who are morbidly obese (BMI > 40 kg/m2) have a prevalence of sleep apnea greater than 70%.
The classification by Friedman et al40 provides an indicator of risk. The patient is examined with the mouth opened wide and the tongue in a neutral natural position. Grades:
- I—Entire uvula and tonsils are visible
- II—Entire uvula is visible, but tonsils are not
- III—Soft palate is visible, but uvula is not
- IV—Only the hard palate is visible.
Especially in children and young adults, enlarged tonsils (or “kissing tonsils”) and a boggy edematous uvula set the stage for obstructive sleep apnea.
DIAGNOSIS REQUIRES SLEEP TESTING
A sleep study is the primary means of diagnosing OSA. Polysomnography includes electrooculography to determine when rapid-eye-movement sleep occurs; electromyography to measure muscle activity in the chin to help determine onset of sleep, with peripheral leads in the leg to measure leg movements; electroencephalography (EEG) to measure neural activity; electrocardiography; pulse oximetry to measure oxygen saturation; measurement of oronasal flow; and measurements of chest wall effort and body position using thoracic and abdominal belts that expand and contract with breathing; and audio recording to detect snoring.
Attended polysomnography requires the constant presence of a trained sleep technologist to monitor for technical issues and patient adherence.
End-tidal CO2 monitoring is a reasonable method to detect sleep-related hypoventilation but is not routinely performed in the United States. Transcutaneous CO2 monitoring is a different way to monitor CO2 used in the setting of positive airway pressure.
Polysomnography in a normal patient shows a regular pattern of increasing and decreasing airflow with inspiration and expiration while stable oxygen saturation is maintained.
In contrast, polysomnography of a patient with sleep apnea shows repetitive periods of no airflow, oxygen desaturation, and often evidence of thoracoabdominal paradox, punctuated by arousals on EEG associated with sympathetic activation (Figure 1). When the patient falls asleep, upper-airway muscle tone is reduced, causing an apneic event with hypoxia and pleural pressure swings. These prompt arousals with sympathetic activation that reestablish upper-airway muscle tone, allowing ventilation and reoxygenation to resume with a return to sleep.
Apnea-hypopnea index indicates severity
Sleep apnea severity is graded using the apnea-hypopnea index, ie, the number of apneic and hypopneic events per hour of sleep (Table 4).41 Events must last at least 10 seconds to be considered, ie, two consecutive missed breaths based on an average normal respiratory rate of about 12 breaths per minute for the typical adult.
The apnea-hypopnea index usually correlates with the severity of oxygen desaturation and with electrocardiographic abnormalities, including tachybradycardia and arrhythmias.
Although history, physical examination, and prediction tools are helpful in determining the likelihood that a patient has OSA, only polysomnography testing can establish the diagnosis. To diagnose OSA, 15 or more obstructive events per hour must be observed by polysomnography, or at least 5 events per hour with one of the following:
- Daytime sleepiness, sleep attacks, unrefreshing sleep, fatigue, or insomnia
- Waking with breath-holding, gasping, or choking
- Observer-reported loud snoring or breathing interruptions.41
Split-night study determines diagnosis and optimum treatment
The split-night study has two parts: the first is diagnostic polysomnography, followed by identification of the positive airway pressure that optimally treats the sleep apnea. The apnea-hypopnea index guides the need for the split-night study, with 40 being the established threshold according to the American Academy of Sleep Medicine.
A home sleep study is appropriate for some patients
Home sleep testing is typically more limited than standard polysomnography; it monitors airflow, effort, and oxygenation. The test is intended for adults with a high pretest probability of moderate to severe obstructive sleep apnea (STOP-BANG score ≥ 3). It is not intended for screening of asymptomatic patients or for those with coexisting sleep disorders (eg, central sleep apnea, sleep hypoventilation, periodic limb movements, insomnia, circadian rhythm disorders, parasomnias, narcolepsy) or medical disorders (eg, moderate to severe heart failure or other cardiac disease, symptomatic neurologic disease, moderate to severe pulmonary disease).42 Since March 2008, the Centers for Medicare and Medicaid Services has covered CPAP for obstructive sleep apnea based on diagnosis by home sleep study testing.43
TREATMENT OF SLEEP APNEA
Basic steps for reducing OSA are:
Weight loss. Even small weight changes can significantly affect the severity of sleep apnea, perhaps even leading to a reassessment of the degree of OSA and CPAP requirements. Longitudinal epidemiologic data demonstrate that a 10% weight loss correlates with a 26% reduction in the apnea-hypopnea index, and conversely, a 10% weight gain is associated with a 32% increase.44
Some studies have found that bariatric surgery cures OSA in 75% to 88% of cases, independent of approach.45,46 However, a trial in 60 obese patients with OSA who were randomized to either a low-calorie diet or bariatric surgery found no statistical difference in the apnea-hypopnea index between the two groups despite greater weight loss in the surgery group.47
Avoiding certain medications. Benzodiazepines, narcotics, and alcohol reduce upper airway muscle tone and should be avoided. No medications are associated with improvement of OSA, although acetazolamide may be used to treat central sleep apnea.
Positional therapy. Sleeping on the back exacerbates the problem. Supine-related OSA occurs as a result of several factors, including gravity, airway anatomy, airway critical closing pressures, and effects on upper-airway dilator muscle function.
Sleep hygiene. General recommendations to engage in behaviors to promote sleep are recommended, including keeping consistent sleep-wake times, not watching television in bed, and avoidance of caffeine intake, particularly within 4 to 6 hours of bedtime.
POSITIVE AIRWAY PRESSURE THERAPY
Nasal CPAP is the treatment of choice and is successful in 95% of patients when used consistently. It is not as costly as surgery, and results in improved long-term survival compared with uvulopalatopharyngoplasty. Another advantage is that the pressure can be retitrated as the patient’s condition changes, for example after a weight change or during pregnancy.
More than 15 randomized controlled trials have examined the effect of sleep apnea treatment with CPAP compared with either sham CPAP or another control. In a meta-analysis, CPAP was found to lead to an average systolic blood pressure reduction of about 2.5 mm Hg and a diastolic blood pressure reduction of 1.8 mm Hg. Although these reductions may seem negligible, benefits may be significant for cardiovascular outcomes.48,49
Challenges to treatment adherence
Adherence is the most commonly discussed problem with CPAP, but long-term adherence rates are comparable to medication compliance—about 60% to 70%. To optimize adherence, communication is important to ensure that problems are identified and addressed as they arise. Showing patients examples of apneic events and oxygen desaturation from their sleep study can enhance their understanding of OSA and its importance. Patients need to understand the serious nature of the disease and that CPAP therapy can significantly improve their quality of life and overall health, particularly from a cardiovascular perspective.
CPAP masks can be uncomfortable, posing a major barrier to compliance. But a number of mask designs are available, such as the nasal mask, the nasal pillow mask, and the oronasal mask. For patients with claustrophobia, the nasal pillow mask is an option, as it does not cover the face.
Some patients note symptoms of nasal congestion, although in many patients CPAP improves it. If congestion is a problem, the use of heated humidification with the machine, intranasal saline or gel, or nasal corticosteroids can help relieve it.
Pressure intolerance is a common problem. For those who feel that the pressure is too high, settings can be adjusted so that the pressure is gradually reduced between inspiration and expiration, ie, the use of expiratory pressure relief or consideration of the use of bilevel positive airway pressure.
Aerophagia (swallowing air) is a less common problem. It can also potentially be relieved with use of bilevel positive airway pressure.
Many patients develop skin irritation, which can be helped with moleskin, available at any pharmacy.
Social stigma can be a problem. Education regarding the importance of the treatment to health is essential.
Machine noise is less of a problem with the new machine models, but if it is a problem, a white-noise device or earplugs may help.
Other measures to improve compliance are keeping the regimen simple and ensuring that family support is adequate.
Medicare requires evidence of use and benefit
Medicare requires that clinical benefit be documented between the 31st and 91st day after initiating CPAP therapy. This requires face-to-face clinical reevaluation by the treating physician to document improved symptoms and objective evidence of adherence to use of the device. The devices can store usage patterns, and Medicare requires at least 4 hours per night on 70% of nights during a consecutive 30-day period in the first 3 months of use.
ALTERNATIVE THERAPIES
Alternative therapies may be options for some patients, in particular those who cannot use CPAP or who get no benefit from it. These include oral appliances for those with mild to moderate OSA50–53 and various surgical procedures, eg, uvulopalatopharyngoplasty,54,55 maxillomanibular advancement,56 tracheostomy (standard treatment before CPAP was identified as an effective treatment),57,58 and adenotonsillectomy (in children).59
Supplemental oxygen is not a first-line treatment for OSA and in general has not been found to be very effective, particularly in terms of intermediate cardiovascular outcomes,60–62 although a subset of patients with high loop gain may benefit from it.63 Loop gain is a measure of the tendency of the ventilatory control system to amplify respiration in response to a change, conferring less stable control of breathing.
Several novel alternative therapies are starting to be used. Although all of them have been shown to improve measures of OSA, none is as effective as CPAP in improving OSA severity. New therapies include the nasal expiratory positive airway pressure device,64 oral pressure therapy,65 and hypoglossal nerve stimulation.66
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25:289–296.
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999; 22:749–755.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109:659–663.
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92:79–84.
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Second Edition: Diagnostic and coding manual. Westchester, IL; American Academy of Sleep Medicine, 2005.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Curr Resp Med Rev 2007; 3:258–269.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166:1725–1731.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Mehra R, Storfer-Isser A, Tracy R, Jenny N, Redline S. Association of sleep disordered breathing and oxidized LDL [abstract]. Am J Respir Crit Care Med 2010; 181:A2474.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54:1797–1804.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107:2589–2594.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445–451.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7:397–398.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182:269–277.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18:534–540.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–1214.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6( 8):e1000132. doi: 10.1371/journal.pmed.1000132.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20:608–613.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239.
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12:481–496.
- Shaher E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167:1186–1192.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167:1181–1185.
- Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949.
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. Erratum in: Am J Respir Crit Care Med 1997; 155:1820.
- Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012; 17:213–222.
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152:1673–1689.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Krieger J, Imbs J-L, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med 1988; 148:1337–1340.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngal Head Neck Surg 2002; 127:13–21.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Centers for Medicare & Medicaid Services (CMS). Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). MLN Matters 2008. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6048.pdf. Accessed June 2, 2014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284:3015–3021.
- Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest 2003; 124:1615–1619.
- Crooks PF. Surgical treatment of morbid obesity. Annu Rev Med 2006; 57:243–264.
- Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308:1142–1149.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50:417–423.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006; 29:240–243.
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath 2006; 10:29–36.
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19:61–66.
- Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 2005; 84:554–558.
- Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89:923–934.
- Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am 2001; 45:759–796.
- Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 2002; 133:1489–1497.
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003; 113:201–204.
- Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. JAMA 1981; 246:347–350.
- Marcus CL, Moore RH, Rosen CL, et al; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368:2366–2376.
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 2013; 36:975–980.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006; 29:564–571.
- Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 1988; 93:471–475.
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respire Physiol Neurobiol 2008; 162:144–151.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34:479–485.
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013; 14:830–837.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139–149.
Obstructive sleep apnea (OSA) is common and poorly recognized and, if untreated, leads to serious health consequences. This article discusses the epidemiology of OSA, describes common presenting signs and symptoms, and reviews diagnostic testing and treatment options. Adverse health effects related to untreated sleep apnea are also discussed.
COMMON, POORLY RECOGNIZED, AND COSTLY IF UNTREATED
OSA is very common in the general population and is associated with substantial morbidity and mortality. An estimated 17% of the general adult population has OSA, and the numbers are increasing with the obesity epidemic. Nearly 1 in 15 adults has at least moderate sleep apnea,1,2 and approximately 85% of cases are estimated to be undiagnosed.3 A 1999 study estimated that untreated OSA resulted in approximately $3.4 billion in additional medical costs per year in the United States,4 a figure that is likely to be higher now, given the rising prevalence of OSA. The prevalence of OSA in primary care and subspecialty clinics is even higher than in the community, as more than half of patients who have diabetes or hypertension and 30% to 40% of patients with coronary artery disease are estimated to have OSA.5–7
REPETITIVE UPPER-AIRWAY COLLAPSE
During sleep, parasympathetic activity is enhanced and the muscle tone of the upper airway is decreased, particularly in the pharyngeal dilator muscles. Still, even in the supine position, a healthy person maintains patency of the airway and adequate airflow during sleep.
OSA is characterized by repetitive complete or partial collapse of the upper airway during sleep, resulting in an apneic or hypopneic event, respectively, and often causing snoring from upper-airway tissue vibration.
People who are susceptible to OSA typically have a smaller, more collapsible airway that is often less distensible and has a higher critical closing pressure. Radiographic and physiologic data have shown that the airway dimensions of patients with OSA are smaller than in those without OSA. The shape of the airway of a patient with OSA is often elliptical, given the extrinsic compression of the lateral aspects of the airway by increased size of the parapharyngeal fat pads. OSA episodes are characterized by closure of the upper airway and by progressively increasing respiratory efforts driven by chemoreceptor and mechanoreceptor stimuli, culminating in an arousal from sleep and a reopening of the airway.
The disease-defining metric used for assessing OSA severity is the apnea-hypopnea index, ie, the number of apneas and hypopneas that occur per hour of sleep.8 An apneic or hypopneic event is identified during polysomnography by the complete cessation of airflow or by a reduction in airflow for 10 seconds or longer (Figure 1).

HEALTH CONSEQUENCES IF UNTREATED
Untreated sleep apnea causes numerous pathophysiologic perturbations, including chronic intermittent hypoxia, ventilatory overshoot hyperoxia, increased sympathetic nervous system activity, intrathoracic pressure swings, hypercapnea, sleep fragmentation, increased arousals, reduced sleep duration, and fragmentation of rapid-eye-movement sleep.
Intermittent hypoxia activates the sympathetic nervous system and causes pulmonary vasoconstriction, with increases in pulmonary arterial pressures and myocardial workload. Sympathetic activation, ascertained by peroneal microneurography, has been shown to be increased not only during sleep but also persisting during wakefulness in patients with untreated OSA vs those without OSA.9 Autonomic nervous system fluctuations accompany apneic episodes, resulting in enhanced parasympathetic tone and sympathetic activation associated with a rise in blood pressure and heart rate that occur after the respiratory event.
Intermediate pathways that link the negative pathophysiologic effects of OSA with adverse health outcomes include increased systemic inflammation, increased oxidative stress, metabolic dysfunction, insulin resistance, hypercoagulability, endothelial dysfunction, and autonomic dysfunction.
As a result, a variety of adverse clinical outcomes are associated with untreated OSA, including systemic hypertension, ischemic heart disease and atherosclerosis, diastolic dysfunction, congestive heart failure, cardiac arrhythmias, stroke, increased risk of death, and sudden death, as well as noncardiovascular outcomes such as gout, neurocognitive deficits, and mood disorders.10
Inflammatory and atherogenic effects
Increased levels of markers of systemic inflammation, prothrombosis, and oxidative stress have been observed in OSA and may be key pathophysiologic links between OSA and cardiovascular sequelae. OSA has been associated with up-regulation of a number of inflammatory mediators: interleukin (IL) 6, soluble IL-6 receptor, IL-8, tumor necrosis factor alpha, and C-reactive protein. Soluble IL-6 levels in particular are higher in people who have sleep-disordered breathing, as reflected by the apnea-hypopnea index independent of obesity, with relationships stronger in the morning than in the evening. This likely reflects the overnight OSA-related physiologic stress.11
Thrombotic potential is also enhanced, with higher levels of plasminogen activator inhibitor 1, fibrinogen, P-selectin, and vascular endothelial growth factor. Some of these factors normally have a diurnal cycle, with higher levels in the morning, but in OSA, increasing OSA severity is associated with increased prothrombotic potential in the morning hours. Of interest, levels of these substances showed a plateau effect, rising in people who had only mildly elevated apnea-hypopnea indices and then leveling off.12 Intermittent hypoxia followed by ventilatory overshoot hyperoxia, characteristic of sleep apnea, provides the ideal environment for augmentation of oxidative stress, with evidence of increased oxidation of serum proteins and lipids. Hypoxia and oxygen-derived free radicals may result in cardiac myocyte injury. Experimental data demonstrate that intermittent hypoxia combined with a high-fat diet results in synergistic acceleration of evidence of atherogenic lesions.
Patients with OSA also have evidence of endothelial dysfunction, insulin resistance, and dyslipidemia, all pathways that can facilitate the progression of atherosclerosis in OSA.13–15
Cardiac arrhythmias
In the Sleep Heart Health Study, a multicenter epidemiologic study designed to examine the relationships of OSA and cardiovascular outcomes, those who had moderate to severe OSA had a risk of ventricular and atrial arrhythmias two to four times higher than those without OSA, even after correction for the confounding influences of obesity and underlying cardiovascular risk.14 These findings were corroborated in subsequent work highlighting monotonic dose-response relationships with increasing OSA severity and increased odds of atrial and ventricular arrhythmia in a cohort of about 3,000 older men.11 Additional compelling evidence of a causal relationship is that the risk of discrete arrhythmic events is markedly increased after a respiratory disturbance in sleep.16
In patients who successfully underwent cardioversion for atrial fibrillation, those who had sleep apnea but were not treated with continuous positive airway pressure (CPAP) had a much higher rate of recurrence of atrial fibrillation during the subsequent year than those with CPAP-treated sleep apnea and than controls never diagnosed with sleep apnea. In the untreated patients with sleep apnea, the mean nocturnal fall in oxygen saturation was significantly greater in those who had recurrence of atrial fibrillation than in those who did not, suggesting hypoxia as an important mechanism contributing to atrial fibrillation.17
Since then, several other retrospective studies have shown similar findings after pulmonary vein antrum isolation and ablation in terms of reduction of atrial fibrillation recurrence with CPAP treatment in OSA.18
Walia et al19 described a patient with moderate sleep apnea who underwent a split-night study. During the baseline part of the study, the patient had about 18 ectopic beats per minute. During the second portion of the study while CPAP was applied, progressively fewer ectopic beats occurred as airway pressure was increased until a normal rhythm without ectopic beats was achieved at the goal treatment CPAP pressure setting.
Cardiovascular disease, stroke, and death
Marin et al20 followed about 1,500 men for 10 years, including some who had severe OSA, some with sleep apnea who were treated with CPAP, and controls. The risk of nonfatal and fatal cardiovascular disease events was nearly three times higher in those with severe disease than in healthy participants. Those treated with CPAP had a risk approximately the same as in the control group.
The Sleep Heart Study followed approximately 6,000 people with untreated sleep apnea for a median of nearly 9 years. It found a significant association between the apnea-hypopnea index and ischemic stroke, especially in men.21 Survival in patients with heart failure is also associated with the degree of OSA; patients with more severe disease (an apnea-hypopnea index ≥ 15) have a nearly three times greater risk of death than those with no disease or only mild disease (apnea-hypopnea index < 15).22
From the standpoint of health care utilization, findings that central sleep apnea predicts an increased risk of hospital readmission in heart failure are of particular interest.23
People with OSA are at increased risk of nocturnal sudden cardiac death.24 Sleep apnea is also associated with an increased overall death rate, and the higher the apnea-hypopnea index, the higher the death rate,25 even after adjusting for age, sex, body mass index, and underlying cardiovascular risk, with findings most pronounced in men under age 70.
Motor vehicle accidents
The need for caution during driving should be discussed with every patient, as motor vehicle accidents are an immediate danger to the patient and others. The association with motor vehicle accidents is independent of sleepiness, and drivers with sleep apnea often do not perceive performance impairment. Young et al26 found that men who snored were 3.4 times as likely to have an accident over a 5-year period, and that men and women with an apnea-hypopnea index greater than 15 were more than 7 times as likely to have multiple accidents over a 5-year period, highlighting the importance of discussing, documenting, and expeditiously diagnosing and treating OSA, particularly in those who report drowsiness while driving.
CLINICAL RISK FACTORS
Risk factors can be divided into nonmodifiable and modifiable ones.
Nonmodifiable factors
Age. Bimodal distributions in OSA prevalence have been observed; ie, that the pediatric population and people who are middle-aged have the highest prevalence of OSA. A linear relationship between sleep apnea prevalence and age until about age 65 was identified in data from the Sleep Heart Health Study.27 After that, the prevalence rates plateau; it is unclear if this is secondary to natural remission of the disease after a certain age or because patients with more severe disease have died by that age (ie, survivorship bias), blunting an increase in prevalence.
Sex. Men develop sleep apnea at a rate three to five times that of women. Several explanations have been proposed to account for this.28,29 Sex hormones are one factor; women with sleep apnea on hormone replacement therapy have a significantly less-severe sleep apnea burden than other postmenopausal women,30 suggesting a positive effect from estrogen. Sex-based differences in fat distribution, length and collapsibility of the upper airway, genioglossal activity, neurochemical control mechanisms, and arousal response may also contribute to prevalence differences between men and women.
As with coronary artery disease, the presentation of sleep apnea may be atypical in women, particularly around menopause. Sleep apnea should be considered in women who have snoring and daytime sleepiness.
Race. Whites, African Americans, and Asians have a similar prevalence of sleep apnea, but groups differ in obesity rates and craniofacial anatomy.31–34 Asians tend to have craniofacial skeletal restriction. African Americans are more likely to have upper-airway soft-tissue risk and to develop more severe OSA. Whites tend to have both craniofacial and soft-tissue risk. For those with craniofacial anatomy predisposing to OSA, even mild obesity can make it manifest.
Syndromes that predispose to OSA can include craniofacial structural abnormalities, connective tissue problems, or alterations in ventilatory control (eg, Marfan, Down, and Pierre Robin syndromes).
Modifiable risk factors
Obesity (body mass index ≥ 30 kg/m2) is a firmly established risk factor, but not all obese patients develop obstructive sleep apnea, and not all people with sleep apnea are obese.
Obesity increases risk by altering the geometry and function of the upper airway, increasing collapsibility. The changes are particularly pronounced in the lateral aspects of the pharynx.35
Obesity also affects respiratory drive, likely in part from leptin resistance. Load compensation is another contributing factor: the increased mass in the thorax and abdomen increases the work of breathing and reduces functional residual capacity, increasing oxygen demands and leading to atelectasis and ventilation-perfusion mismatch.
Although obesity is an important risk factor, it is important to recognize that obesity is not the only one to consider: most people with an apnea-hypopnea index of 5 or greater are not obese. The relationship between body mass index and sleep apnea is weaker in children and in the elderly, probably because other risk factors are more pronounced.36
Craniofacial structural abnormalities such as retrognathia (abnormal posterior position of the mandible) and micrognathia (undersized mandible) can increase the risk of OSA because of a resulting posteriorly displaced genioglossus muscle. Other conditions can alter chemosensitivity, affecting the pH and carbon dioxide level of the blood and therefore affecting ventilatory control mechanisms, making the person more prone to developing sleep apnea. Children and young adults may have tonsillar tissue that obstructs the airway.
The site of obstruction can be behind the palate (retropalatal), behind the tongue (retroglossal), or below the pharynx (hypopharyngeal). This helps explain why positive air way pressure—unlike surgery, which addresses a specific area—is often successful, as it serves to splint or treat all aspects of the airway.
FATIGUE, SLEEPINESS, SNORING, RESTLESS SLEEP
Sleep apnea can result in presentation of multiple signs and symptoms (Table 1).
Daytime sleepiness and fatigue are the most common symptoms. Although nonspecific, they are often quite pronounced. Two short questionnaires—the Epworth Sleepiness Scale37 and the Fatigue Severity Scale—can help distinguish between these two symptoms and assess their impact on a patient’s daily life. In the Epworth Sleepiness Scale, the patient rates his or her chance of dozing on a 4-point scale (0 = would never doze, to 3 = high chance of dozing) in eight situations:
- Sitting and reading
- Watching television
- Sitting inactive in a public place
- As a passenger in a car for an hour without a break
- Lying down to rest in the afternoon
- Sitting and talking to someone
- Sitting quietly after a lunch without alcohol
- In a car while stopped for a few minutes in traffic.
A score of 10 or more is consistent with significant subjective sleepiness.
The Fatigue Severity Scale assesses the impact of fatigue on daily living.
Snoring is a common and specific symptom of sleep apnea; however, not all patients who snore have OSA.
Restlessness during sleep is very common—patients may disturb their bed partner by moving around a lot during sleep or report that the sheets are “all over the place” by morning.
Nocturia can also be a sign of sleep apnea and can contribute to sleep fragmentation. A proposed mechanism of this symptom includes alterations of intrathoracic pressure resulting in atrial stretch, which release atrial natriuretic peptide, leading to nocturia. Treating with CPAP has been found to reduce levels of atrial natriuretic peptide, contributing to better sleep.38
Morning headache may occur and is likely related to increased CO2 levels, which appear to culminate in the morning hours. End-tidal or transcutaneous CO2 monitoring during polysomnography can help elucidate the presence of sleep-related hypoventilation.
Libido is often diminished and can actually be improved with CPAP. This is therefore an important point to discuss with patients, as improved libido can often serve as an incentive for adherence to OSA treatment.
Insomnia exists in about 15% of patients, primarily as a result of sleep apnea-related with treatment.
Sweating, particularly forehead sweating associated with sleep apnea, more commonly occurs in children.
The STOP-BANG questionnaire (Table 2)39 was primarily validated in preoperative anesthesia testing. However, because of its ease of use and favorable performance characteristics, it is increasingly used to predict the likelihood of finding OSA before polysomnography. A score of 3 or more has a sensitivity of 93%.
PHYSICAL EXAMINATION PROVIDES CLUES
Although the physical examination may be normal, certain findings indicate risk (Table 3). Obesity alone is not an accepted indication for polysomnography unless there are concomitant worrisome signs or symptoms. Of note, those who are morbidly obese (BMI > 40 kg/m2) have a prevalence of sleep apnea greater than 70%.
The classification by Friedman et al40 provides an indicator of risk. The patient is examined with the mouth opened wide and the tongue in a neutral natural position. Grades:
- I—Entire uvula and tonsils are visible
- II—Entire uvula is visible, but tonsils are not
- III—Soft palate is visible, but uvula is not
- IV—Only the hard palate is visible.
Especially in children and young adults, enlarged tonsils (or “kissing tonsils”) and a boggy edematous uvula set the stage for obstructive sleep apnea.
DIAGNOSIS REQUIRES SLEEP TESTING
A sleep study is the primary means of diagnosing OSA. Polysomnography includes electrooculography to determine when rapid-eye-movement sleep occurs; electromyography to measure muscle activity in the chin to help determine onset of sleep, with peripheral leads in the leg to measure leg movements; electroencephalography (EEG) to measure neural activity; electrocardiography; pulse oximetry to measure oxygen saturation; measurement of oronasal flow; and measurements of chest wall effort and body position using thoracic and abdominal belts that expand and contract with breathing; and audio recording to detect snoring.
Attended polysomnography requires the constant presence of a trained sleep technologist to monitor for technical issues and patient adherence.
End-tidal CO2 monitoring is a reasonable method to detect sleep-related hypoventilation but is not routinely performed in the United States. Transcutaneous CO2 monitoring is a different way to monitor CO2 used in the setting of positive airway pressure.
Polysomnography in a normal patient shows a regular pattern of increasing and decreasing airflow with inspiration and expiration while stable oxygen saturation is maintained.
In contrast, polysomnography of a patient with sleep apnea shows repetitive periods of no airflow, oxygen desaturation, and often evidence of thoracoabdominal paradox, punctuated by arousals on EEG associated with sympathetic activation (Figure 1). When the patient falls asleep, upper-airway muscle tone is reduced, causing an apneic event with hypoxia and pleural pressure swings. These prompt arousals with sympathetic activation that reestablish upper-airway muscle tone, allowing ventilation and reoxygenation to resume with a return to sleep.
Apnea-hypopnea index indicates severity
Sleep apnea severity is graded using the apnea-hypopnea index, ie, the number of apneic and hypopneic events per hour of sleep (Table 4).41 Events must last at least 10 seconds to be considered, ie, two consecutive missed breaths based on an average normal respiratory rate of about 12 breaths per minute for the typical adult.
The apnea-hypopnea index usually correlates with the severity of oxygen desaturation and with electrocardiographic abnormalities, including tachybradycardia and arrhythmias.
Although history, physical examination, and prediction tools are helpful in determining the likelihood that a patient has OSA, only polysomnography testing can establish the diagnosis. To diagnose OSA, 15 or more obstructive events per hour must be observed by polysomnography, or at least 5 events per hour with one of the following:
- Daytime sleepiness, sleep attacks, unrefreshing sleep, fatigue, or insomnia
- Waking with breath-holding, gasping, or choking
- Observer-reported loud snoring or breathing interruptions.41
Split-night study determines diagnosis and optimum treatment
The split-night study has two parts: the first is diagnostic polysomnography, followed by identification of the positive airway pressure that optimally treats the sleep apnea. The apnea-hypopnea index guides the need for the split-night study, with 40 being the established threshold according to the American Academy of Sleep Medicine.
A home sleep study is appropriate for some patients
Home sleep testing is typically more limited than standard polysomnography; it monitors airflow, effort, and oxygenation. The test is intended for adults with a high pretest probability of moderate to severe obstructive sleep apnea (STOP-BANG score ≥ 3). It is not intended for screening of asymptomatic patients or for those with coexisting sleep disorders (eg, central sleep apnea, sleep hypoventilation, periodic limb movements, insomnia, circadian rhythm disorders, parasomnias, narcolepsy) or medical disorders (eg, moderate to severe heart failure or other cardiac disease, symptomatic neurologic disease, moderate to severe pulmonary disease).42 Since March 2008, the Centers for Medicare and Medicaid Services has covered CPAP for obstructive sleep apnea based on diagnosis by home sleep study testing.43
TREATMENT OF SLEEP APNEA
Basic steps for reducing OSA are:
Weight loss. Even small weight changes can significantly affect the severity of sleep apnea, perhaps even leading to a reassessment of the degree of OSA and CPAP requirements. Longitudinal epidemiologic data demonstrate that a 10% weight loss correlates with a 26% reduction in the apnea-hypopnea index, and conversely, a 10% weight gain is associated with a 32% increase.44
Some studies have found that bariatric surgery cures OSA in 75% to 88% of cases, independent of approach.45,46 However, a trial in 60 obese patients with OSA who were randomized to either a low-calorie diet or bariatric surgery found no statistical difference in the apnea-hypopnea index between the two groups despite greater weight loss in the surgery group.47
Avoiding certain medications. Benzodiazepines, narcotics, and alcohol reduce upper airway muscle tone and should be avoided. No medications are associated with improvement of OSA, although acetazolamide may be used to treat central sleep apnea.
Positional therapy. Sleeping on the back exacerbates the problem. Supine-related OSA occurs as a result of several factors, including gravity, airway anatomy, airway critical closing pressures, and effects on upper-airway dilator muscle function.
Sleep hygiene. General recommendations to engage in behaviors to promote sleep are recommended, including keeping consistent sleep-wake times, not watching television in bed, and avoidance of caffeine intake, particularly within 4 to 6 hours of bedtime.
POSITIVE AIRWAY PRESSURE THERAPY
Nasal CPAP is the treatment of choice and is successful in 95% of patients when used consistently. It is not as costly as surgery, and results in improved long-term survival compared with uvulopalatopharyngoplasty. Another advantage is that the pressure can be retitrated as the patient’s condition changes, for example after a weight change or during pregnancy.
More than 15 randomized controlled trials have examined the effect of sleep apnea treatment with CPAP compared with either sham CPAP or another control. In a meta-analysis, CPAP was found to lead to an average systolic blood pressure reduction of about 2.5 mm Hg and a diastolic blood pressure reduction of 1.8 mm Hg. Although these reductions may seem negligible, benefits may be significant for cardiovascular outcomes.48,49
Challenges to treatment adherence
Adherence is the most commonly discussed problem with CPAP, but long-term adherence rates are comparable to medication compliance—about 60% to 70%. To optimize adherence, communication is important to ensure that problems are identified and addressed as they arise. Showing patients examples of apneic events and oxygen desaturation from their sleep study can enhance their understanding of OSA and its importance. Patients need to understand the serious nature of the disease and that CPAP therapy can significantly improve their quality of life and overall health, particularly from a cardiovascular perspective.
CPAP masks can be uncomfortable, posing a major barrier to compliance. But a number of mask designs are available, such as the nasal mask, the nasal pillow mask, and the oronasal mask. For patients with claustrophobia, the nasal pillow mask is an option, as it does not cover the face.
Some patients note symptoms of nasal congestion, although in many patients CPAP improves it. If congestion is a problem, the use of heated humidification with the machine, intranasal saline or gel, or nasal corticosteroids can help relieve it.
Pressure intolerance is a common problem. For those who feel that the pressure is too high, settings can be adjusted so that the pressure is gradually reduced between inspiration and expiration, ie, the use of expiratory pressure relief or consideration of the use of bilevel positive airway pressure.
Aerophagia (swallowing air) is a less common problem. It can also potentially be relieved with use of bilevel positive airway pressure.
Many patients develop skin irritation, which can be helped with moleskin, available at any pharmacy.
Social stigma can be a problem. Education regarding the importance of the treatment to health is essential.
Machine noise is less of a problem with the new machine models, but if it is a problem, a white-noise device or earplugs may help.
Other measures to improve compliance are keeping the regimen simple and ensuring that family support is adequate.
Medicare requires evidence of use and benefit
Medicare requires that clinical benefit be documented between the 31st and 91st day after initiating CPAP therapy. This requires face-to-face clinical reevaluation by the treating physician to document improved symptoms and objective evidence of adherence to use of the device. The devices can store usage patterns, and Medicare requires at least 4 hours per night on 70% of nights during a consecutive 30-day period in the first 3 months of use.
ALTERNATIVE THERAPIES
Alternative therapies may be options for some patients, in particular those who cannot use CPAP or who get no benefit from it. These include oral appliances for those with mild to moderate OSA50–53 and various surgical procedures, eg, uvulopalatopharyngoplasty,54,55 maxillomanibular advancement,56 tracheostomy (standard treatment before CPAP was identified as an effective treatment),57,58 and adenotonsillectomy (in children).59
Supplemental oxygen is not a first-line treatment for OSA and in general has not been found to be very effective, particularly in terms of intermediate cardiovascular outcomes,60–62 although a subset of patients with high loop gain may benefit from it.63 Loop gain is a measure of the tendency of the ventilatory control system to amplify respiration in response to a change, conferring less stable control of breathing.
Several novel alternative therapies are starting to be used. Although all of them have been shown to improve measures of OSA, none is as effective as CPAP in improving OSA severity. New therapies include the nasal expiratory positive airway pressure device,64 oral pressure therapy,65 and hypoglossal nerve stimulation.66
Obstructive sleep apnea (OSA) is common and poorly recognized and, if untreated, leads to serious health consequences. This article discusses the epidemiology of OSA, describes common presenting signs and symptoms, and reviews diagnostic testing and treatment options. Adverse health effects related to untreated sleep apnea are also discussed.
COMMON, POORLY RECOGNIZED, AND COSTLY IF UNTREATED
OSA is very common in the general population and is associated with substantial morbidity and mortality. An estimated 17% of the general adult population has OSA, and the numbers are increasing with the obesity epidemic. Nearly 1 in 15 adults has at least moderate sleep apnea,1,2 and approximately 85% of cases are estimated to be undiagnosed.3 A 1999 study estimated that untreated OSA resulted in approximately $3.4 billion in additional medical costs per year in the United States,4 a figure that is likely to be higher now, given the rising prevalence of OSA. The prevalence of OSA in primary care and subspecialty clinics is even higher than in the community, as more than half of patients who have diabetes or hypertension and 30% to 40% of patients with coronary artery disease are estimated to have OSA.5–7
REPETITIVE UPPER-AIRWAY COLLAPSE
During sleep, parasympathetic activity is enhanced and the muscle tone of the upper airway is decreased, particularly in the pharyngeal dilator muscles. Still, even in the supine position, a healthy person maintains patency of the airway and adequate airflow during sleep.
OSA is characterized by repetitive complete or partial collapse of the upper airway during sleep, resulting in an apneic or hypopneic event, respectively, and often causing snoring from upper-airway tissue vibration.
People who are susceptible to OSA typically have a smaller, more collapsible airway that is often less distensible and has a higher critical closing pressure. Radiographic and physiologic data have shown that the airway dimensions of patients with OSA are smaller than in those without OSA. The shape of the airway of a patient with OSA is often elliptical, given the extrinsic compression of the lateral aspects of the airway by increased size of the parapharyngeal fat pads. OSA episodes are characterized by closure of the upper airway and by progressively increasing respiratory efforts driven by chemoreceptor and mechanoreceptor stimuli, culminating in an arousal from sleep and a reopening of the airway.
The disease-defining metric used for assessing OSA severity is the apnea-hypopnea index, ie, the number of apneas and hypopneas that occur per hour of sleep.8 An apneic or hypopneic event is identified during polysomnography by the complete cessation of airflow or by a reduction in airflow for 10 seconds or longer (Figure 1).

HEALTH CONSEQUENCES IF UNTREATED
Untreated sleep apnea causes numerous pathophysiologic perturbations, including chronic intermittent hypoxia, ventilatory overshoot hyperoxia, increased sympathetic nervous system activity, intrathoracic pressure swings, hypercapnea, sleep fragmentation, increased arousals, reduced sleep duration, and fragmentation of rapid-eye-movement sleep.
Intermittent hypoxia activates the sympathetic nervous system and causes pulmonary vasoconstriction, with increases in pulmonary arterial pressures and myocardial workload. Sympathetic activation, ascertained by peroneal microneurography, has been shown to be increased not only during sleep but also persisting during wakefulness in patients with untreated OSA vs those without OSA.9 Autonomic nervous system fluctuations accompany apneic episodes, resulting in enhanced parasympathetic tone and sympathetic activation associated with a rise in blood pressure and heart rate that occur after the respiratory event.
Intermediate pathways that link the negative pathophysiologic effects of OSA with adverse health outcomes include increased systemic inflammation, increased oxidative stress, metabolic dysfunction, insulin resistance, hypercoagulability, endothelial dysfunction, and autonomic dysfunction.
As a result, a variety of adverse clinical outcomes are associated with untreated OSA, including systemic hypertension, ischemic heart disease and atherosclerosis, diastolic dysfunction, congestive heart failure, cardiac arrhythmias, stroke, increased risk of death, and sudden death, as well as noncardiovascular outcomes such as gout, neurocognitive deficits, and mood disorders.10
Inflammatory and atherogenic effects
Increased levels of markers of systemic inflammation, prothrombosis, and oxidative stress have been observed in OSA and may be key pathophysiologic links between OSA and cardiovascular sequelae. OSA has been associated with up-regulation of a number of inflammatory mediators: interleukin (IL) 6, soluble IL-6 receptor, IL-8, tumor necrosis factor alpha, and C-reactive protein. Soluble IL-6 levels in particular are higher in people who have sleep-disordered breathing, as reflected by the apnea-hypopnea index independent of obesity, with relationships stronger in the morning than in the evening. This likely reflects the overnight OSA-related physiologic stress.11
Thrombotic potential is also enhanced, with higher levels of plasminogen activator inhibitor 1, fibrinogen, P-selectin, and vascular endothelial growth factor. Some of these factors normally have a diurnal cycle, with higher levels in the morning, but in OSA, increasing OSA severity is associated with increased prothrombotic potential in the morning hours. Of interest, levels of these substances showed a plateau effect, rising in people who had only mildly elevated apnea-hypopnea indices and then leveling off.12 Intermittent hypoxia followed by ventilatory overshoot hyperoxia, characteristic of sleep apnea, provides the ideal environment for augmentation of oxidative stress, with evidence of increased oxidation of serum proteins and lipids. Hypoxia and oxygen-derived free radicals may result in cardiac myocyte injury. Experimental data demonstrate that intermittent hypoxia combined with a high-fat diet results in synergistic acceleration of evidence of atherogenic lesions.
Patients with OSA also have evidence of endothelial dysfunction, insulin resistance, and dyslipidemia, all pathways that can facilitate the progression of atherosclerosis in OSA.13–15
Cardiac arrhythmias
In the Sleep Heart Health Study, a multicenter epidemiologic study designed to examine the relationships of OSA and cardiovascular outcomes, those who had moderate to severe OSA had a risk of ventricular and atrial arrhythmias two to four times higher than those without OSA, even after correction for the confounding influences of obesity and underlying cardiovascular risk.14 These findings were corroborated in subsequent work highlighting monotonic dose-response relationships with increasing OSA severity and increased odds of atrial and ventricular arrhythmia in a cohort of about 3,000 older men.11 Additional compelling evidence of a causal relationship is that the risk of discrete arrhythmic events is markedly increased after a respiratory disturbance in sleep.16
In patients who successfully underwent cardioversion for atrial fibrillation, those who had sleep apnea but were not treated with continuous positive airway pressure (CPAP) had a much higher rate of recurrence of atrial fibrillation during the subsequent year than those with CPAP-treated sleep apnea and than controls never diagnosed with sleep apnea. In the untreated patients with sleep apnea, the mean nocturnal fall in oxygen saturation was significantly greater in those who had recurrence of atrial fibrillation than in those who did not, suggesting hypoxia as an important mechanism contributing to atrial fibrillation.17
Since then, several other retrospective studies have shown similar findings after pulmonary vein antrum isolation and ablation in terms of reduction of atrial fibrillation recurrence with CPAP treatment in OSA.18
Walia et al19 described a patient with moderate sleep apnea who underwent a split-night study. During the baseline part of the study, the patient had about 18 ectopic beats per minute. During the second portion of the study while CPAP was applied, progressively fewer ectopic beats occurred as airway pressure was increased until a normal rhythm without ectopic beats was achieved at the goal treatment CPAP pressure setting.
Cardiovascular disease, stroke, and death
Marin et al20 followed about 1,500 men for 10 years, including some who had severe OSA, some with sleep apnea who were treated with CPAP, and controls. The risk of nonfatal and fatal cardiovascular disease events was nearly three times higher in those with severe disease than in healthy participants. Those treated with CPAP had a risk approximately the same as in the control group.
The Sleep Heart Study followed approximately 6,000 people with untreated sleep apnea for a median of nearly 9 years. It found a significant association between the apnea-hypopnea index and ischemic stroke, especially in men.21 Survival in patients with heart failure is also associated with the degree of OSA; patients with more severe disease (an apnea-hypopnea index ≥ 15) have a nearly three times greater risk of death than those with no disease or only mild disease (apnea-hypopnea index < 15).22
From the standpoint of health care utilization, findings that central sleep apnea predicts an increased risk of hospital readmission in heart failure are of particular interest.23
People with OSA are at increased risk of nocturnal sudden cardiac death.24 Sleep apnea is also associated with an increased overall death rate, and the higher the apnea-hypopnea index, the higher the death rate,25 even after adjusting for age, sex, body mass index, and underlying cardiovascular risk, with findings most pronounced in men under age 70.
Motor vehicle accidents
The need for caution during driving should be discussed with every patient, as motor vehicle accidents are an immediate danger to the patient and others. The association with motor vehicle accidents is independent of sleepiness, and drivers with sleep apnea often do not perceive performance impairment. Young et al26 found that men who snored were 3.4 times as likely to have an accident over a 5-year period, and that men and women with an apnea-hypopnea index greater than 15 were more than 7 times as likely to have multiple accidents over a 5-year period, highlighting the importance of discussing, documenting, and expeditiously diagnosing and treating OSA, particularly in those who report drowsiness while driving.
CLINICAL RISK FACTORS
Risk factors can be divided into nonmodifiable and modifiable ones.
Nonmodifiable factors
Age. Bimodal distributions in OSA prevalence have been observed; ie, that the pediatric population and people who are middle-aged have the highest prevalence of OSA. A linear relationship between sleep apnea prevalence and age until about age 65 was identified in data from the Sleep Heart Health Study.27 After that, the prevalence rates plateau; it is unclear if this is secondary to natural remission of the disease after a certain age or because patients with more severe disease have died by that age (ie, survivorship bias), blunting an increase in prevalence.
Sex. Men develop sleep apnea at a rate three to five times that of women. Several explanations have been proposed to account for this.28,29 Sex hormones are one factor; women with sleep apnea on hormone replacement therapy have a significantly less-severe sleep apnea burden than other postmenopausal women,30 suggesting a positive effect from estrogen. Sex-based differences in fat distribution, length and collapsibility of the upper airway, genioglossal activity, neurochemical control mechanisms, and arousal response may also contribute to prevalence differences between men and women.
As with coronary artery disease, the presentation of sleep apnea may be atypical in women, particularly around menopause. Sleep apnea should be considered in women who have snoring and daytime sleepiness.
Race. Whites, African Americans, and Asians have a similar prevalence of sleep apnea, but groups differ in obesity rates and craniofacial anatomy.31–34 Asians tend to have craniofacial skeletal restriction. African Americans are more likely to have upper-airway soft-tissue risk and to develop more severe OSA. Whites tend to have both craniofacial and soft-tissue risk. For those with craniofacial anatomy predisposing to OSA, even mild obesity can make it manifest.
Syndromes that predispose to OSA can include craniofacial structural abnormalities, connective tissue problems, or alterations in ventilatory control (eg, Marfan, Down, and Pierre Robin syndromes).
Modifiable risk factors
Obesity (body mass index ≥ 30 kg/m2) is a firmly established risk factor, but not all obese patients develop obstructive sleep apnea, and not all people with sleep apnea are obese.
Obesity increases risk by altering the geometry and function of the upper airway, increasing collapsibility. The changes are particularly pronounced in the lateral aspects of the pharynx.35
Obesity also affects respiratory drive, likely in part from leptin resistance. Load compensation is another contributing factor: the increased mass in the thorax and abdomen increases the work of breathing and reduces functional residual capacity, increasing oxygen demands and leading to atelectasis and ventilation-perfusion mismatch.
Although obesity is an important risk factor, it is important to recognize that obesity is not the only one to consider: most people with an apnea-hypopnea index of 5 or greater are not obese. The relationship between body mass index and sleep apnea is weaker in children and in the elderly, probably because other risk factors are more pronounced.36
Craniofacial structural abnormalities such as retrognathia (abnormal posterior position of the mandible) and micrognathia (undersized mandible) can increase the risk of OSA because of a resulting posteriorly displaced genioglossus muscle. Other conditions can alter chemosensitivity, affecting the pH and carbon dioxide level of the blood and therefore affecting ventilatory control mechanisms, making the person more prone to developing sleep apnea. Children and young adults may have tonsillar tissue that obstructs the airway.
The site of obstruction can be behind the palate (retropalatal), behind the tongue (retroglossal), or below the pharynx (hypopharyngeal). This helps explain why positive air way pressure—unlike surgery, which addresses a specific area—is often successful, as it serves to splint or treat all aspects of the airway.
FATIGUE, SLEEPINESS, SNORING, RESTLESS SLEEP
Sleep apnea can result in presentation of multiple signs and symptoms (Table 1).
Daytime sleepiness and fatigue are the most common symptoms. Although nonspecific, they are often quite pronounced. Two short questionnaires—the Epworth Sleepiness Scale37 and the Fatigue Severity Scale—can help distinguish between these two symptoms and assess their impact on a patient’s daily life. In the Epworth Sleepiness Scale, the patient rates his or her chance of dozing on a 4-point scale (0 = would never doze, to 3 = high chance of dozing) in eight situations:
- Sitting and reading
- Watching television
- Sitting inactive in a public place
- As a passenger in a car for an hour without a break
- Lying down to rest in the afternoon
- Sitting and talking to someone
- Sitting quietly after a lunch without alcohol
- In a car while stopped for a few minutes in traffic.
A score of 10 or more is consistent with significant subjective sleepiness.
The Fatigue Severity Scale assesses the impact of fatigue on daily living.
Snoring is a common and specific symptom of sleep apnea; however, not all patients who snore have OSA.
Restlessness during sleep is very common—patients may disturb their bed partner by moving around a lot during sleep or report that the sheets are “all over the place” by morning.
Nocturia can also be a sign of sleep apnea and can contribute to sleep fragmentation. A proposed mechanism of this symptom includes alterations of intrathoracic pressure resulting in atrial stretch, which release atrial natriuretic peptide, leading to nocturia. Treating with CPAP has been found to reduce levels of atrial natriuretic peptide, contributing to better sleep.38
Morning headache may occur and is likely related to increased CO2 levels, which appear to culminate in the morning hours. End-tidal or transcutaneous CO2 monitoring during polysomnography can help elucidate the presence of sleep-related hypoventilation.
Libido is often diminished and can actually be improved with CPAP. This is therefore an important point to discuss with patients, as improved libido can often serve as an incentive for adherence to OSA treatment.
Insomnia exists in about 15% of patients, primarily as a result of sleep apnea-related with treatment.
Sweating, particularly forehead sweating associated with sleep apnea, more commonly occurs in children.
The STOP-BANG questionnaire (Table 2)39 was primarily validated in preoperative anesthesia testing. However, because of its ease of use and favorable performance characteristics, it is increasingly used to predict the likelihood of finding OSA before polysomnography. A score of 3 or more has a sensitivity of 93%.
PHYSICAL EXAMINATION PROVIDES CLUES
Although the physical examination may be normal, certain findings indicate risk (Table 3). Obesity alone is not an accepted indication for polysomnography unless there are concomitant worrisome signs or symptoms. Of note, those who are morbidly obese (BMI > 40 kg/m2) have a prevalence of sleep apnea greater than 70%.
The classification by Friedman et al40 provides an indicator of risk. The patient is examined with the mouth opened wide and the tongue in a neutral natural position. Grades:
- I—Entire uvula and tonsils are visible
- II—Entire uvula is visible, but tonsils are not
- III—Soft palate is visible, but uvula is not
- IV—Only the hard palate is visible.
Especially in children and young adults, enlarged tonsils (or “kissing tonsils”) and a boggy edematous uvula set the stage for obstructive sleep apnea.
DIAGNOSIS REQUIRES SLEEP TESTING
A sleep study is the primary means of diagnosing OSA. Polysomnography includes electrooculography to determine when rapid-eye-movement sleep occurs; electromyography to measure muscle activity in the chin to help determine onset of sleep, with peripheral leads in the leg to measure leg movements; electroencephalography (EEG) to measure neural activity; electrocardiography; pulse oximetry to measure oxygen saturation; measurement of oronasal flow; and measurements of chest wall effort and body position using thoracic and abdominal belts that expand and contract with breathing; and audio recording to detect snoring.
Attended polysomnography requires the constant presence of a trained sleep technologist to monitor for technical issues and patient adherence.
End-tidal CO2 monitoring is a reasonable method to detect sleep-related hypoventilation but is not routinely performed in the United States. Transcutaneous CO2 monitoring is a different way to monitor CO2 used in the setting of positive airway pressure.
Polysomnography in a normal patient shows a regular pattern of increasing and decreasing airflow with inspiration and expiration while stable oxygen saturation is maintained.
In contrast, polysomnography of a patient with sleep apnea shows repetitive periods of no airflow, oxygen desaturation, and often evidence of thoracoabdominal paradox, punctuated by arousals on EEG associated with sympathetic activation (Figure 1). When the patient falls asleep, upper-airway muscle tone is reduced, causing an apneic event with hypoxia and pleural pressure swings. These prompt arousals with sympathetic activation that reestablish upper-airway muscle tone, allowing ventilation and reoxygenation to resume with a return to sleep.
Apnea-hypopnea index indicates severity
Sleep apnea severity is graded using the apnea-hypopnea index, ie, the number of apneic and hypopneic events per hour of sleep (Table 4).41 Events must last at least 10 seconds to be considered, ie, two consecutive missed breaths based on an average normal respiratory rate of about 12 breaths per minute for the typical adult.
The apnea-hypopnea index usually correlates with the severity of oxygen desaturation and with electrocardiographic abnormalities, including tachybradycardia and arrhythmias.
Although history, physical examination, and prediction tools are helpful in determining the likelihood that a patient has OSA, only polysomnography testing can establish the diagnosis. To diagnose OSA, 15 or more obstructive events per hour must be observed by polysomnography, or at least 5 events per hour with one of the following:
- Daytime sleepiness, sleep attacks, unrefreshing sleep, fatigue, or insomnia
- Waking with breath-holding, gasping, or choking
- Observer-reported loud snoring or breathing interruptions.41
Split-night study determines diagnosis and optimum treatment
The split-night study has two parts: the first is diagnostic polysomnography, followed by identification of the positive airway pressure that optimally treats the sleep apnea. The apnea-hypopnea index guides the need for the split-night study, with 40 being the established threshold according to the American Academy of Sleep Medicine.
A home sleep study is appropriate for some patients
Home sleep testing is typically more limited than standard polysomnography; it monitors airflow, effort, and oxygenation. The test is intended for adults with a high pretest probability of moderate to severe obstructive sleep apnea (STOP-BANG score ≥ 3). It is not intended for screening of asymptomatic patients or for those with coexisting sleep disorders (eg, central sleep apnea, sleep hypoventilation, periodic limb movements, insomnia, circadian rhythm disorders, parasomnias, narcolepsy) or medical disorders (eg, moderate to severe heart failure or other cardiac disease, symptomatic neurologic disease, moderate to severe pulmonary disease).42 Since March 2008, the Centers for Medicare and Medicaid Services has covered CPAP for obstructive sleep apnea based on diagnosis by home sleep study testing.43
TREATMENT OF SLEEP APNEA
Basic steps for reducing OSA are:
Weight loss. Even small weight changes can significantly affect the severity of sleep apnea, perhaps even leading to a reassessment of the degree of OSA and CPAP requirements. Longitudinal epidemiologic data demonstrate that a 10% weight loss correlates with a 26% reduction in the apnea-hypopnea index, and conversely, a 10% weight gain is associated with a 32% increase.44
Some studies have found that bariatric surgery cures OSA in 75% to 88% of cases, independent of approach.45,46 However, a trial in 60 obese patients with OSA who were randomized to either a low-calorie diet or bariatric surgery found no statistical difference in the apnea-hypopnea index between the two groups despite greater weight loss in the surgery group.47
Avoiding certain medications. Benzodiazepines, narcotics, and alcohol reduce upper airway muscle tone and should be avoided. No medications are associated with improvement of OSA, although acetazolamide may be used to treat central sleep apnea.
Positional therapy. Sleeping on the back exacerbates the problem. Supine-related OSA occurs as a result of several factors, including gravity, airway anatomy, airway critical closing pressures, and effects on upper-airway dilator muscle function.
Sleep hygiene. General recommendations to engage in behaviors to promote sleep are recommended, including keeping consistent sleep-wake times, not watching television in bed, and avoidance of caffeine intake, particularly within 4 to 6 hours of bedtime.
POSITIVE AIRWAY PRESSURE THERAPY
Nasal CPAP is the treatment of choice and is successful in 95% of patients when used consistently. It is not as costly as surgery, and results in improved long-term survival compared with uvulopalatopharyngoplasty. Another advantage is that the pressure can be retitrated as the patient’s condition changes, for example after a weight change or during pregnancy.
More than 15 randomized controlled trials have examined the effect of sleep apnea treatment with CPAP compared with either sham CPAP or another control. In a meta-analysis, CPAP was found to lead to an average systolic blood pressure reduction of about 2.5 mm Hg and a diastolic blood pressure reduction of 1.8 mm Hg. Although these reductions may seem negligible, benefits may be significant for cardiovascular outcomes.48,49
Challenges to treatment adherence
Adherence is the most commonly discussed problem with CPAP, but long-term adherence rates are comparable to medication compliance—about 60% to 70%. To optimize adherence, communication is important to ensure that problems are identified and addressed as they arise. Showing patients examples of apneic events and oxygen desaturation from their sleep study can enhance their understanding of OSA and its importance. Patients need to understand the serious nature of the disease and that CPAP therapy can significantly improve their quality of life and overall health, particularly from a cardiovascular perspective.
CPAP masks can be uncomfortable, posing a major barrier to compliance. But a number of mask designs are available, such as the nasal mask, the nasal pillow mask, and the oronasal mask. For patients with claustrophobia, the nasal pillow mask is an option, as it does not cover the face.
Some patients note symptoms of nasal congestion, although in many patients CPAP improves it. If congestion is a problem, the use of heated humidification with the machine, intranasal saline or gel, or nasal corticosteroids can help relieve it.
Pressure intolerance is a common problem. For those who feel that the pressure is too high, settings can be adjusted so that the pressure is gradually reduced between inspiration and expiration, ie, the use of expiratory pressure relief or consideration of the use of bilevel positive airway pressure.
Aerophagia (swallowing air) is a less common problem. It can also potentially be relieved with use of bilevel positive airway pressure.
Many patients develop skin irritation, which can be helped with moleskin, available at any pharmacy.
Social stigma can be a problem. Education regarding the importance of the treatment to health is essential.
Machine noise is less of a problem with the new machine models, but if it is a problem, a white-noise device or earplugs may help.
Other measures to improve compliance are keeping the regimen simple and ensuring that family support is adequate.
Medicare requires evidence of use and benefit
Medicare requires that clinical benefit be documented between the 31st and 91st day after initiating CPAP therapy. This requires face-to-face clinical reevaluation by the treating physician to document improved symptoms and objective evidence of adherence to use of the device. The devices can store usage patterns, and Medicare requires at least 4 hours per night on 70% of nights during a consecutive 30-day period in the first 3 months of use.
ALTERNATIVE THERAPIES
Alternative therapies may be options for some patients, in particular those who cannot use CPAP or who get no benefit from it. These include oral appliances for those with mild to moderate OSA50–53 and various surgical procedures, eg, uvulopalatopharyngoplasty,54,55 maxillomanibular advancement,56 tracheostomy (standard treatment before CPAP was identified as an effective treatment),57,58 and adenotonsillectomy (in children).59
Supplemental oxygen is not a first-line treatment for OSA and in general has not been found to be very effective, particularly in terms of intermediate cardiovascular outcomes,60–62 although a subset of patients with high loop gain may benefit from it.63 Loop gain is a measure of the tendency of the ventilatory control system to amplify respiration in response to a change, conferring less stable control of breathing.
Several novel alternative therapies are starting to be used. Although all of them have been shown to improve measures of OSA, none is as effective as CPAP in improving OSA severity. New therapies include the nasal expiratory positive airway pressure device,64 oral pressure therapy,65 and hypoglossal nerve stimulation.66
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25:289–296.
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999; 22:749–755.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109:659–663.
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92:79–84.
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Second Edition: Diagnostic and coding manual. Westchester, IL; American Academy of Sleep Medicine, 2005.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Curr Resp Med Rev 2007; 3:258–269.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166:1725–1731.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Mehra R, Storfer-Isser A, Tracy R, Jenny N, Redline S. Association of sleep disordered breathing and oxidized LDL [abstract]. Am J Respir Crit Care Med 2010; 181:A2474.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54:1797–1804.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107:2589–2594.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445–451.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7:397–398.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182:269–277.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18:534–540.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–1214.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6( 8):e1000132. doi: 10.1371/journal.pmed.1000132.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20:608–613.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239.
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12:481–496.
- Shaher E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167:1186–1192.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167:1181–1185.
- Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949.
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. Erratum in: Am J Respir Crit Care Med 1997; 155:1820.
- Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012; 17:213–222.
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152:1673–1689.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Krieger J, Imbs J-L, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med 1988; 148:1337–1340.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngal Head Neck Surg 2002; 127:13–21.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Centers for Medicare & Medicaid Services (CMS). Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). MLN Matters 2008. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6048.pdf. Accessed June 2, 2014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284:3015–3021.
- Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest 2003; 124:1615–1619.
- Crooks PF. Surgical treatment of morbid obesity. Annu Rev Med 2006; 57:243–264.
- Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308:1142–1149.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50:417–423.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006; 29:240–243.
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath 2006; 10:29–36.
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19:61–66.
- Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 2005; 84:554–558.
- Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89:923–934.
- Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am 2001; 45:759–796.
- Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 2002; 133:1489–1497.
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003; 113:201–204.
- Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. JAMA 1981; 246:347–350.
- Marcus CL, Moore RH, Rosen CL, et al; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368:2366–2376.
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 2013; 36:975–980.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006; 29:564–571.
- Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 1988; 93:471–475.
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respire Physiol Neurobiol 2008; 162:144–151.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34:479–485.
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013; 14:830–837.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139–149.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Kapur VK, Redline S, Nieto FJ, Young TB, Newman AB, Henderson JA; Sleep Heart Health Research Group. The relationship between chronically disrupted sleep and healthcare use. Sleep 2002; 25:289–296.
- Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep 1999; 22:749–755.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109:659–663.
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92:79–84.
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164:2147–2165.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Second Edition: Diagnostic and coding manual. Westchester, IL; American Academy of Sleep Medicine, 2005.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Mehra R. Sleep-disordered breathing and cardiovascular disease: exploring pathophysiology and existing data. Curr Resp Med Rev 2007; 3:258–269.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166:1725–1731.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Mehra R, Storfer-Isser A, Tracy R, Jenny N, Redline S. Association of sleep disordered breathing and oxidized LDL [abstract]. Am J Respir Crit Care Med 2010; 181:A2474.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182:826–833.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54:1797–1804.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107:2589–2594.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol 2010; 3:445–451.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7:397–398.
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010; 182:269–277.
- Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49:1625–1631.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18:534–540.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–1214.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6( 8):e1000132. doi: 10.1371/journal.pmed.1000132.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20:608–613.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165:1217–1239.
- Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12:481–496.
- Shaher E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167:1186–1192.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167:1181–1185.
- Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949.
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162:893–900.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. Erratum in: Am J Respir Crit Care Med 1997; 155:1820.
- Sutherland K, Lee RWW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology 2012; 17:213–222.
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995; 152:1673–1689.
- Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283:1829–1836.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14:540–545.
- Krieger J, Imbs J-L, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med 1988; 148:1337–1340.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngal Head Neck Surg 2002; 127:13–21.
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Report of an American Academy of Sleep Medicine Task Force. Sleep 1999; 22:667–689.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Centers for Medicare & Medicaid Services (CMS). Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). MLN Matters 2008. www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6048.pdf. Accessed June 2, 2014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284:3015–3021.
- Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest 2003; 124:1615–1619.
- Crooks PF. Surgical treatment of morbid obesity. Annu Rev Med 2006; 57:243–264.
- Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 308:1142–1149.
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension 2007; 50:417–423.
- Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 2001; 19:2271–2277.
- Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006; 29:240–243.
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath 2006; 10:29–36.
- Yoshida K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int J Prosthodont 2006; 19:61–66.
- Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res 2005; 84:554–558.
- Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89:923–934.
- Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am 2001; 45:759–796.
- Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc 2002; 133:1489–1497.
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003; 113:201–204.
- Conway WA, Victor LD, Magilligan DJ, Fujita S, Zorick FJ, Roth T. Adverse effects of tracheostomy for sleep apnea. JAMA 1981; 246:347–350.
- Marcus CL, Moore RH, Rosen CL, et al; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013; 368:2366–2376.
- Gottlieb DJ, Craig SE, Lorenzi-Filho G, et al. Sleep apnea cardiovascular clinical trials-current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 2013; 36:975–980.
- Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo-CPAP-controlled study. Sleep 2006; 29:564–571.
- Phillips BA, McConnell JW, Smith MD. The effects of hypoxemia on cardiac output. A dose-response curve. Chest 1988; 93:471–475.
- Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respire Physiol Neurobiol 2008; 162:144–151.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34:479–485.
- Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med 2013; 14:830–837.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370:139–149.
KEY POINTS
- Although obesity and snoring are common features of OSA, they are not always present.
- Home sleep testing is appropriate for those highly likely to have sleep apnea and without other significant sleep or cardiovascular, respiratory, or neurologic disorders.
- Upper-airway surgery has a limited role—it is indicated primarily for those unable to tolerate CPAP.
- Risk of motor vehicle accidents is dramatically increased in untreated sleep apnea; patients should be counseled on the dangers of drowsy driving.
Perioperative beta-blockers in noncardiac surgery: The evidence continues to evolve
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
Prophylactic use of beta-blockers in the perioperative period is highly controversial. Initial studies in the 1990s were favorable, but evidence has been conflicting since then.
The pendulum swung away from routinely recommending beta-blockers after the publication of negative results from several studies, including the Perioperative Ischemic Evaluation (POISE) trial in 2008.1 Highlighting this change in practice, a Canadian study2 found that the use of perioperative beta-blockade increased between 1999 and 2005 but subsequently declined from 2005 to 2010. However, there was no appreciable change in this pattern after the POISE trial or after changes in the American College of Cardiology guidelines in 2002 and 2006.3
In 2008, Harte and Jaffer reviewed the perioperative use of beta-blockers in noncardiac surgery in this journal.4 Since then, a number of meta-analyses and retrospective observational studies have reported variable findings related to specific beta-blockers and specific complications.
In this paper, we review the rationale and recent evidence for and against the perioperative use of beta-blockers as guidance for internists and hospitalists.
POTENTIAL CARDIOPROTECTIVE EFFECTS OF BETA-BLOCKERS
Myocardial infarction and unstable angina are the leading cardiovascular causes of death after surgery.5 These events are multifactorial. Some are caused by the stress of surgery, which precipitates physiologic changes related to inflammatory mediators, sympathetic tone, and oxygen supply and demand; others are caused by acute plaque rupture, thrombosis, and occlusion.6 Most perioperative infarcts are non-Q-wave events7 and occur within the first 2 days after the procedure, when the effects of anesthetics, pain, fluid shifts, and physiologic changes are greatest. Because multiple causes may contribute to perioperative myocardial infarction, a single preventive strategy may not be sufficient.8,9
Beta-blockers do several things that may be beneficial in the perioperative setting. They reduce myocardial oxygen demand by decreasing the force of contraction and by slowing the heart rate, and slowing the heart rate increases diastolic perfusion time.10 They suppress arrhythmias; they limit leukocyte recruitment, the production of free radicals, metalloproteinase activity, monocyte activation, release of growth factors, and inflammatory cytokine response; and they stabilize plaque.11 Their long-term use may also alter intracellular signaling processes, thus improving cell survival by decreasing the expression of receptors for substances that induce apoptosis.12
INITIAL POSITIVE TRIALS
Mangano et al13 began the beta-blocker trend in 1996 with a study in 200 patients known to have coronary artery disease or risk factors for it who were undergoing noncardiac surgery. Patients were randomized to receive either atenolol orally and intravenously, titrated to control the heart rate, or placebo in the immediate perioperative period.
The atenolol group had less perioperative ischemia but no difference in short-term rates of myocardial infarction and death. However, the death rate was lower in the atenolol group at 6 months after discharge and at 2 years, although patients who died in the immediate postoperative period were excluded from the analysis.
Although this finding did not appear to make sense physiologically, we now know that patients may experience myocardial injury without infarction after noncardiac surgery, a phenomenon associated with an increased risk of death in the short term and the long term.14 Preventing these episodes may be the explanation for the improved outcome.
The DECREASE trial15 (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) provided additional support for beta-blocker use. The patients were at high risk, had abnormal dobutamine stress echocardiograms, and were undergoing vascular surgery; 112 patients were randomized to receive either oral bisoprolol (started 1 month before surgery, titrated to control the heart rate, and continued for 1 month after surgery) or placebo.
The study was stopped early because the bisoprolol group reportedly had a 90% lower rate of myocardial infarction and cardiac death 1 month after surgery. However, the study was criticized because the total number of patients enrolled was small and the benefit was much greater than usual for any pharmacologic intervention, thus calling the results into question.
In a follow-up study,16 survivors continued to be followed while receiving bisoprolol or usual care. The incidence of myocardial infarction or cardiac death at 2 years was significantly lower in the group receiving bisoprolol (12% vs 32%, odds ratio [OR] 0.30, P = .025).
Boersma et al,17 in an observational study, analyzed data from all 1,351 patients scheduled for major vascular surgery being considered for enrollment in the DECREASE trial. The DECREASE protocol required patients to undergo dobutamine stress echocardiography if they had one or more risk factors (age 70 or older, angina, prior myocardial infarction, congestive heart failure, treatment for ventricular arrhythmia, treatment for diabetes mellitus, or limited exercise capacity) or if their physician requested it. Twenty-seven percent received beta-blockers.
In multivariate analysis, clinical predictors of adverse outcome were age 70 or older; current or prior history of angina; and prior myocardial infarction, heart failure, or cerebrovascular accident.
In patients who had fewer than three clinical risk factors, beta-blocker use was associated with a lower rate of complications (0.8% vs 2.3%). Dobutamine stress echocardiography had minimal predictive value in this lower-risk group, suggesting that stress testing may not be necessary in this group if beta-blockers are used appropriately. However, in patients who had three or more risk factors, this test did provide additional prognostic information; those without stress-induced ischemia had lower event rates than those with ischemia, and beta-blocker use further reduced those rates, except in patients with extensive ischemia (more than five left ventricular segments involved).
The Revised Cardiac Risk Index. Lee et al18 devised an index to assist in preoperative cardiac risk stratification that was subsequently incorporated into the 2007 American College of Cardiology/American Heart Association preoperative risk guidelines. (It does not, however, address the beta-blocker issue.) It consists of six independent risk-predictors of major cardiac complications derived from 4,315 patients over age 50 undergoing non-cardiac surgery. The risk factors, each of which is given 1 point, are:
- Congestive heart failure based on history or examination
- Renal insufficiency (serum creatinine level > 2 mg/dL)
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- History of transient ischemic attack or stroke
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with 3 or more points are considered to be at high risk, and those with 1 or 2 points are considered to be at intermediate risk. The American College of Cardiology/American Heart Association preoperative cardiac risk algorithm subsequently included five of these six risk factors (the type of surgery was considered separately) and made recommendations concerning noninvasive stress testing and heart rate control.
On the basis of these studies, specialty societies, guideline committees, and hospitals enthusiastically recommended the prophylactic use of beta-blockers to decrease postoperative cardiac complications.
THREE NEGATIVE TRIALS OF METOPROLOL
In 2005 and 2006, two studies in vascular surgery patients and another in patients with diabetes cast doubt on the role of beta-blockers when the results failed to show a benefit. The trials used metoprolol, started shortly before surgery, and with no titration to control the heart rate.
The MaVS study19 (Metoprolol After Vascular Surgery) randomized 496 patients to receive metoprolol or placebo 2 hours before surgery and until hospital discharge or a maximum of 5 days after surgery. The metoprolol dose varied by weight: patients weighing 75 kg or more got 100 mg, those weighing between 40 and 75 kg got 50 mg, and those weighing less than 40 kg got 25 mg. Overall effects at 6 months were not significantly different, but intraoperative bradycardia and hypotension requiring intervention were more frequent in the metoprolol group.
The POBBLE study20 (Perioperative Beta Blockade) randomized 103 patients who had no history of myocardial infarction to receive either metoprolol 50 mg twice daily or placebo from admission to 7 days after surgery. Myocardial ischemia was present in one-third of the patients after surgery. Metoprolol did not reduce the 30-day cardiac mortality rate, but it was associated with a shorter length of stay.
The DIPOM trial21 (Diabetic Postoperative Mortality and Morbidity) randomized 921 diabetic patients to receive long-acting metoprolol succinate controlled-release/extended release (CR/XL) or placebo. Patients in the metoprolol group received a test dose of 50 mg the evening before surgery, another dose 2 hours before surgery (100 mg if the heart rate was more than 65 bpm, or 50 mg if between 55 and 65 bpm), and daily thereafter until discharge or a maximum of 8 days. The dose was not titrated to heart-rate control.
Metoprolol had no statistically significant effect on the composite primary outcome measures of time to death from any cause, acute myocardial infarction, unstable angina, or congestive heart failure or on the secondary outcome measures of time to death from any cause, death from a cardiac cause, and nonfatal cardiac morbidity.
ADDITIONAL POSITIVE STUDIES
Lindenauer et al22 retrospectively evaluated the use of beta-blockers in the first 2 days after surgery in 782,969 patients undergoing non-cardiac surgery. Using propensity score matching and Revised Cardiac Risk Index scores, they found a lower rate of postoperative mortality in patients with three or more risk factors who received a beta-blocker. There was no significant difference in the group with two risk factors, but in the lowest-risk group (with a score of 0 to 1), beta-blockers were not beneficial and may have been associated with harm as evidenced by a higher odds ratio for death, although this was probably artifactual and reflecting database limitations.
Feringa et al,23 in an observational cohort study of 272 patients undergoing vascular surgery, reported that higher doses of beta-blockers and tight heart-rate control were associated with less perioperative myocardial ischemia, lower troponin T levels, and better long-term outcome.
THE POISE TRIAL: MIXED RESULTS
The randomized POISE trial,1 published in 2008, compared the effects of extended-release metoprolol succinate vs placebo on the 30-day risk of major cardiovascular events in 8,351 patients with or at risk of atherosclerotic disease who were undergoing noncardiac surgery. The metoprolol regimen was 100 mg 2 to 4 hours before surgery, another 100 mg by 6 hours after surgery, and then 200 mg 12 hours later and once daily for 30 days.
The incidence of the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days was lower in the metoprolol group than in the placebo group (5.8% vs 6.9%; P = .04), primarily because of fewer nonfatal myocardial infarctions. However, more patients in the metoprolol group died of any cause (3.1% vs 2.3% P = .03) or had a stroke (1.0% vs 0.5% P = .005) than in the placebo group.
The metoprolol group had a higher incidence of clinically significant hypotension, bradycardia, and stroke, which could account for much of the increase in the mortality rate. Sepsis was the major cause of death in this group; hypotension may have increased the risk of infection, and beta-blockers may have potentiated hypotension in patients who were already septic. Also, the bradycardic and negative inotropic effects of the beta-blocker could have masked the physiologic response to systemic infection, thereby delaying recognition and treatment or impeding the normal immune response.
One of the major criticisms of the POISE trial was its aggressive dosing regimen (200 to 400 mg within a 36-hour period) in patients who had not been on beta-blockers before then. Also, the drug was started only a few hours before surgery. In addition, these patients were at higher risk of death and stroke than those in other trials based on a high baseline rate of cerebrovascular disease, and inclusion of urgent and emergency surgical procedures.
STUDIES SINCE POISE
The POISE trial results1 prompted further questioning of the prophylactic perioperative use of beta-blockers. However, proponents of beta-blockers voiced serious criticisms of the trial, particularly the dosing regimen, and continued to believe that these drugs were beneficial if used appropriately.
The DECREASE IV trial. Dunkelgrun et al,24 in a study using bisoprolol started approximately 1 month before surgery and titrated to control the heart rate, reported beneficial results in intermediate-risk patients. In their randomized open-label study with a 2 × 2 factorial design, 1,066 patients at intermediate cardiac risk were assigned to receive bisoprolol, fluvastatin, combination treatment, or control therapy at least 34 days before surgery. Bisoprolol was started at 2.5 mg orally daily and slowly titrated up to a maximum dose of 10 mg to keep the heart rate between 50 and 70 beats per minute. The group of 533 patients randomized to receive bisoprolol had a lower incidence rate of cardiac death and nonfatal myocardial infarction than the control group (2.1% vs 6.0%, HR 0.34, P = .002). A potential limitation of this study was its open-label design, which might have led to treatment bias.
Updated guidelines. Based on the results from POISE and DECREASE IV, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines25 published a focused update on beta-blockers in 2009 as an amendment to their 2007 guidelines on perioperative evaluation and care for noncardiac surgery. The European Society of Cardiology26 released similar but somewhat more liberal guidelines (Table 1).
London et al,27 in an observational study published in 2013, found a lower 30-day overall mortality rate with beta-blockers (relative risk [RR] 0.73, 95% confidence interval [CI] 0.65–0.83, P < .001, number needed to treat [NNT] 241), as well as a lower rate of cardiac morbidity (nonfatal myocardial infarction and cardiac death), but only in nonvascular surgery patients who were on beta-blockers within 7 days of scheduled surgery. Moreover, similar to the findings of Lindenauer et al,22 only patients with a Revised Cardiac Risk Index score of 2 or more benefited from beta-blocker use in terms of a lower risk of death, whereas the lower-risk patients did not:
- Risk score of 0 or 1—no association
- Score of 2—RR 0.63, 95% CI 0.50–0.80, P < .001, NNT 105
- Score of 3—RR 0.54, 95% CI 0.39–0.73, P < .001, NNT 41
- Score of 4 or more—RR 0.40, 95% CI 0.24–0.73, P < .001, NNT 18).
Beta-blocker exposure was associated with a significantly lower rate of cardiac complications (RR 0.67, 95% CI 0.57–0.79, P < .001, NNT 339), also limited to nonvascular surgery patients with a risk score of 2 or 3.
The Danish Nationwide Cohort Study28 examined the effect of beta-blockers on major adverse cardiac events (MACE, ie, myocardial infarction, cerebrovascular accident, and death) in 28,263 patients with ischemic heart disease undergoing noncardiac surgery; 7,990 with heart failure and 20,273 without. Beta-blockers were used in 53% of patients with heart failure and 36% of those without heart failure. Outcomes for all of the beta-blocker recipients:
- MACE—HR 0.90, 95% CI 0.79–1.02
- All-cause mortality—HR 0.95, 95% CI 0.85–1.06.
Outcomes for patients with heart failure if they received beta-blockers:
- MACE—HR 0.75, 95% CI 0.70–0.87
- All-cause mortality—HR 0.80, 95% CI 0.70–0.92.
There was no significant benefit from beta-blockers in patients without heart failure. Outcomes for those patients if they received beta-blockers:
- MACE—HR 1.11, 95% CI 0.92–1.33
- All-cause mortality—HR 1.15, 95% CI 0.98–1.35.
However, in patients without heart failure but with a history of myocardial infarction within the past 2 years, beta-blockers were associated with a lower risk of MACE and all-cause mortality. In patients with neither heart failure nor a recent myocardial infarction, beta-blockers were associated with an increased risk of MACE and all-cause mortality.
This difference in efficacy depending on the presence and timing of a prior myocardial infarction is consistent with the 2012 American College of Cardiology/American Heart Association guidelines for secondary prevention, in which beta-blockers are given a class I recommendation only for patients with a myocardial infarction within the past 3 years.
Meta-analyses and outcomes
A number of meta-analyses have been published over the past 10 years, with conflicting results (Table 2). The divergent findings are primarily due to the different studies included in the analyses as well as the strong influence of the POISE trial.1 The studies varied in terms of the specific beta-blocker used, dose titration and heart rate control, time of initiation of beta-blocker use before surgery, type of surgery, patient characteristics, comorbidities, biomarkers and diagnosis of myocardial infarction, and clinical end points.
In general, these meta-analyses have found that prophylactic perioperative use of beta-blockers decreases ischemia and tends to reduce the risk of nonfatal myocardial infarction. They vary on whether the overall mortality risk is decreased. The meta-analyses that included POISE1 found an increased incidence of stroke, whereas those that excluded POISE found no significant difference, although there appeared to be slightly more strokes in the beta-blocker groups.
The beta-blocker controversy increased even further when Dr. Don Poldermans was fired by Erasmus Medical Center in November 2011 for violations of academic integrity involving his research, including the DECREASE trials. The most recent meta-analysis, by Bouri et al,29 included nine “secure trials” and excluded the DECREASE trials in view of the controversy about their authenticity. The analysis showed an increase in overall mortality as well as stroke, primarily because it was heavily influenced by POISE.1 In contrast, the DECREASE trials had reported a decreased risk of myocardial infarction and death, with no significant increase in stroke. The authors concluded that guideline bodies should “retract their recommendations based on the fictitious data without further delay.”29
Although the design of the DECREASE trials (in which beta-blockers were started well in advance of surgery and doses were titrated to achieve heart rate control) is physiologically more compelling than those of the negative trials, the results have been questioned in light of the integrity issue. However, to date, none of the published DECREASE trials have been retracted.
Two other meta-analyses,30,31 published in 2013, also found a decreased risk of myocardial infarction and increased risk of stroke but no significant difference in short-term all-cause mortality.
ARE ALL BETA-BLOCKERS EQUIVALENT?
In various studies evaluating specific beta-blockers, the more cardioselective agents bisoprolol and atenolol were associated with better outcomes than metoprolol. The affinity ratios for beta-1/beta-2 receptors range from 13.5 for bisoprolol to 4.7 for atenolol and 2.3 for metoprolol.32 Blocking beta-1 receptors blunts tachycardia, whereas blocking beta-2 receptors may block systemic or cerebral vasodilation.
In patients with anemia, beta-blockade in general may be harmful, but beta-2 blockade may be even worse. Beta-blockers were associated with an increased risk of MACE (6.5% vs 3.0%)33 in patients with acute surgical anemia if the hemoglobin concentration decreased to less than 35% of baseline, and increased risks of hospital death (OR 6.65) and multiorgan dysfunction syndrome (OR 4.18) with severe bleeding during aortic surgery.34
In addition, the pathway by which the beta-blocker is metabolized may also affect outcome, with less benefit from beta-blockers metabolized by the CYP2D6 isoenzyme of the cytochrome P450 system. Individual variations in CYP2D6 activity related to genetics or drug interactions may result in insufficient or excessive beta-blockade. Because metoprolol is the most dependent on this system, patients using it may be more susceptible to bradycardia.35
Studies comparing atenolol and metoprolol found that the atenolol groups had fewer myocardial infarctions and deaths36 and lower 30-day and 1-year mortality rates37 than the groups on metoprolol. Studies comparing the three beta-blockers found better outcomes with atenolol and bisoprolol than with metoprolol—fewer strokes,38,39 a lower mortality rate,31 and a better composite outcome39 (Table 3 and Table 4).
START THE BETA-BLOCKER EARLY, TITRATE TO CONTROL THE HEART RATE
A number of studies suggest that how long the beta-blocker is given before surgery may influence the outcome (Table 5). The best results were achieved when beta-blockers were started approximately 1 month before surgery and titrated to control the heart rate.
Because this long lead-in time is not always practical, it is important to determine the shortest time before surgery in which starting beta-blockers may be beneficial and yet safe. Some evidence suggests that results are better when the beta-blocker is started more than 1 week preoperatively compared with less than 1 week, but it is unknown what the minimum or optimal time period should be.
If a beta-blocker is started well in advance of the scheduled surgery, there is adequate time for dose titration and tighter heart rate control. Most of the studies demonstrating beneficial effects of perioperative beta-blockers used dose titration and achieved lower heart rates in the treatment group than in the control group. A criticism of the MaVs,19 POBBLE,20 and DIPOM21 trials was that the patients did not receive adequate beta-blockade. The POISE trial1 used a much higher dose of metoprolol in an attempt to assure beta-blockade without dose titration, and although the regimen decreased nonfatal myocardial infarctions, it increased strokes and the overall mortality rate, probably related to excess bradycardia and hypotension. The target heart rate should probably be between 55 and 70 beats per minute.
RISK OF STROKE
POISE1 was the first trial to note a clinically and statistically significant increase in strokes with perioperative beta-blocker use. Although no other study has shown a similar increased risk, almost all reported a higher number of strokes in the beta-blocker groups, although the absolute numbers and differences were small and not statistically significant. This risk may also vary from one beta-blocker to another (Table 4).
The usual incidence rate of postoperative stroke after noncardiac, noncarotid surgery is well under 1% in patients with no prior history of stroke but increases to approximately 3% in patients with a previous stroke.40 An observational study from the Dutch group reported a very low incidence of stroke overall (0.02%) in 186,779 patients undergoing noncardiac surgery with no significant difference in those on chronic beta-blocker therapy.41 The DECREASE trials, with a total of 3,884 patients, also found no statistically significant increase in stroke with beta-blocker use (0.46% overall vs 0.5% with a beta-blocker),42 which in this case was bisoprolol started well in advance of surgery and titrated to control the heart rate. Although the DECREASE data are under suspicion, they seem reasonable and consistent with those of observational studies.
Proposed mechanisms by which beta-blockers may increase stroke risk include the side effects of hypotension and bradycardia, particularly in the setting of anemia. They may also cause cerebral ischemia by blocking cerebral vasodilation. This effect on cerebral blood flow may be more pronounced with the less cardioselective beta-blockers, which may explain the apparent increased stroke risk associated with metoprolol.
WHAT SHOULD WE DO NOW?
The evidence for the safety and efficacy of beta-blockers in the perioperative setting continues to evolve, and new clinical trials are needed to clarify the ongoing controversy, particularly regarding the risk of stroke.
If patients have other indications for beta-blocker therapy, such as history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation for rate control, they should be receiving them if time permits.
If prophylactic beta-blockers are to be effective in minimizing perioperative complications, it appears that they may need to be more cardioselective, started at least 1 week before surgery, titrated to control heart rate, and used in high-risk patients (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
Ideally, a large randomized controlled trial using a cardioselective beta-blocker started in advance of surgery in patients with a Revised Cardiac Risk Index score greater than 2, undergoing intermediate or high-risk procedures, is needed to fully answer the questions raised by the current data.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
- POISE Study Group; Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371:1839–1847.
- Wijeysundera DN, Mamdani M, Laupacis A, et al. Clinical evidence, practice guidelines, and ß-blocker utilization before major noncardiac surgery. Circ Cardiovasc Qual Outcomes 2012; 5:558–565.
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006; 47:2343–2355.
- Harte B, Jaffer AK. Perioperative beta-blockers in noncardiac surgery: evolution of the evidence. Cleve Clin J Med 2008; 75:513–519.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foëx P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Poldermans D, Boersma E, Bax JJ, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol reduces cardiac death and myocardial infarction in high-risk patients as long as 2 years after successful major vascular surgery. Eur Heart J 2001; 22:1353–1358.
- Boersma E, Poldermans D, Bax JJ, et al; DECREASE Study Group (Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiogrpahy). Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR; POBBLE trial investigators. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al; DIPOM Trial Group. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114(suppl 1):1344–1349.
- Dunkelgrun M, Boersma E, Schouten O, et al; Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV). Ann Surg 2009; 249:921–926.
- American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery; Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2009; 54:e13–e118.
- Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC); Poldermans D, Bax JJ, Boersma E, et al. Guidelines for preoperative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009; 30:2769–2812.
- London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309:1704–1713.
- Andersson C, Mérie C, Jørgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Intern Med 2014; 174:336–344.
- Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart 2014; 100:456–464.
- Guay J, Ochroch EA. Beta-blocking agents for surgery: influence on mortality and major outcomes. A meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:834–844.
- Dai N, Xu D, Zhang J, et al. Different beta-blockers and initiation time in patients undergoing noncardiac surgery: a meta-analysis. Am J Med Sci 2014; 347:235–244.
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005; 144:317–322.
- Beattie WS, Wijeysundera DN, Karkouti K, et al. Acute surgical anemia influences the cardioprotective effects of beta-blockade: a single-center, propensity-matched cohort study. Anesthesiology 2010; 112:25–33.
- Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology 2012; 117:1203–1211.
- Badgett RG, Lawrence VA, Cohn SL. Variations in pharmacology of beta-blockers may contribute to heterogeneous results in trials of perioperative beta-blockade. Anesthesiology 2010; 113:585–592.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- Wallace AW, Au S, Cason BA. Perioperative beta-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114:824–836.
- Mashour GA, Sharifpour M, Freundlich RE, et al. Perioperative metoprolol and risk of stroke after noncardiac surgery. Anesthesiology 2013; 119:1340–1346.
- Ashes C, Judelman S, Wijeysundera DN, et al. Selective beta1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 2013; 119:777–787.
- Selim M. Perioperative stroke. N Engl J Med 2007; 356:706–713.
- van Lier F, Schouten O, van Domburg RT, et al. Effect of chronic beta-blocker use on stroke after noncardiac surgery. Am J Cardiol 2009; 104:429–433.
- van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta-blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol 2010; 105:43–47.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ 2005; 331:313–321.
- McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138:171–179.
- Schouten O, Shaw LJ, Boersma E, et al. A meta-analysis of safety and effectiveness of perioperative beta-blocker use for the prevention of cardiac events in different types of noncardiac surgery. Coron Artery Dis 2006; 17:173–179.
- Wiesbauer F, Schlager O, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity: a systematic review and meta-analysis. Anesth Analg 2007; 104:27–41.
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008; 372:1962–1976.
- Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of preoperative beta-blocker treatment in vascular surgery patients: influence on postoperative outcome. J Am Coll Cardiol 2010; 56:1922–1929.
- Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol 2014; 30:217–223.
KEY POINTS
- If patients have other indications for beta-blocker therapy, such as a history of heart failure, myocardial infarction in the past 3 years, or atrial fibrillation, they should be started on a beta-blocker before surgery if time permits.
- Of the various beta-blockers, the cardioselective ones appear to be preferable in the perioperative setting.
- Beta-blockers may need to be started at least 1 week before surgery, titrated to control the heart rate, and used only in patients at high risk (Revised Cardiac Risk Index score > 2 or 3) undergoing high-risk surgery.
- Further clinical trials are necessary to clarify the ongoing controversy, particularly regarding the risk of stroke, which was increased in the large Perioperative Ischemic Evaluation (POISE) trial.
LISTEN NOW: Clinical Decision-Making Live
This month’s podcast feature follows up a session at SHM’s annual meeting, HM14, on clinical decision making in which Dr. Gupreet Dhaliwal, professor of medicine at the University of California at San Francisco, diagnosed two complex patient cases presented by Dr. Daniel Brotman, director of the hospitalist program at Johns Hopkins Hospital. Dr. Dhaliwal says while rare and challenging cases are appealing, diagnosing common problems presented by many cases is a great way to demonstrate thinking through a diagnosis. He also discusses how cognitive bias can work in a doctor’s favor. Dr. Brotman explains why the teamwork on problem solving that happens at these live sessions is one of their best features.
For more features, visit The Hospitalist's podcast archive.
This month’s podcast feature follows up a session at SHM’s annual meeting, HM14, on clinical decision making in which Dr. Gupreet Dhaliwal, professor of medicine at the University of California at San Francisco, diagnosed two complex patient cases presented by Dr. Daniel Brotman, director of the hospitalist program at Johns Hopkins Hospital. Dr. Dhaliwal says while rare and challenging cases are appealing, diagnosing common problems presented by many cases is a great way to demonstrate thinking through a diagnosis. He also discusses how cognitive bias can work in a doctor’s favor. Dr. Brotman explains why the teamwork on problem solving that happens at these live sessions is one of their best features.
For more features, visit The Hospitalist's podcast archive.
This month’s podcast feature follows up a session at SHM’s annual meeting, HM14, on clinical decision making in which Dr. Gupreet Dhaliwal, professor of medicine at the University of California at San Francisco, diagnosed two complex patient cases presented by Dr. Daniel Brotman, director of the hospitalist program at Johns Hopkins Hospital. Dr. Dhaliwal says while rare and challenging cases are appealing, diagnosing common problems presented by many cases is a great way to demonstrate thinking through a diagnosis. He also discusses how cognitive bias can work in a doctor’s favor. Dr. Brotman explains why the teamwork on problem solving that happens at these live sessions is one of their best features.
For more features, visit The Hospitalist's podcast archive.
Woman With Blue-Gray Palate and Nail Beds
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
A 62-year-old African-American woman presented for evaluation of a bluish discoloration of the hard palate and nail beds, noticeable for several months. In addition, she had complaints of fatigue and arthralgia. She reported that she had been taking hydroxychloroquine 400 mg/d and quinacrine 100 mg/d for several years for the treatment of systemic lupus erythematosus (SLE). Her medical history was also significant for dry mouth syndrome treated with pilocarpine.
The patient’s vital signs included a temperature of 97°F;
respiratory rate, 15 breaths/min; pulse, 72 beats/min; and blood pressure, 130/80 mm Hg. Height was 62 in, weight was 189 lb, and BMI was 34.56. A bluish gray color was noted in the subungual areas of her nails (see Figure 1). There were several circumferential areas of skin hyperpigmentation resulting from healed lupus skin lesions on her arms. Nailfold capillaroscopy revealed several dilated blood vessels. The sclerae appeared dry, but no erythema or inflammation was noted.
Examination of the mouth revealed a bluish discoloration of the hard palate (see Figure 2) and decreased salivary pool. Respiratory, cardiovascular, and abdominal examination findings were normal. Musculoskeletal examination was unremarkable for acute joint tenderness or synovitis. Crepitation and bony changes were noted in the left knee, without effusion or decreased range of motion.
Laboratory studies were ordered, and the results are listed in the table.
DISCUSSION
Hyperpigmentation of the oral mucosa can be associated with a number of conditions, including adrenal insufficiency, Peutz-Jeghers syndrome, hemochromatosis, polyostotic fibrous dysplasia, hyperparathyroidism, neurofibromatosis, and bronchogenic malignancy.1,2 Other causes of oral hyperpigmentation include physiologic pigmentary or postinflammatory changes, oral melanoacanthosis, blue nevus, and melanoma.2,3 While these diagnoses should be considered when encountering a mucosal lesion, they were unlikely in this patient because of the color changes in her nail beds.
Systemic skin and mucous membrane discoloration can also occur with the use of certain drugs and other substances, including chemotherapeutic agents, benzodiazepines, hormones, carotenoids, phenolphthalein, heavy metal salts, and several antimicrobial agents.1 In dark-skinned individuals, hyperpigmentation of the oral mucosa can be caused by a physiologic deposition of melanin.4
Pigmentary Changes
The use of antimalarial drugs, such as quinacrine, chloroquine, and hydroxychloroquine, has long been associated with pigmentary changes to the palatal mucosa and subungual areas.1,3 These drugs can stimulate melanin production and cause hemosiderin deposition, resulting in pigmentary changes.5 Skin discoloration is believed to be the result of the formation of a melanin-drug complex in areas with an elevated affinity for melanin.1 Besides malaria, these drugs are commonly used to treat SLE and discoid lupus erythematosus, rheumatoid arthritis, and other rheumatologic conditions.5
The diagnosis of drug-induced hyperpigmentation is generally clinical, supported by the patient’s history—which often includes the use of antimalarial drugs—and presentation.1 If a clear cause cannot be determined by clinical evaluation, then a biopsy to confirm a drug-induced cause may be necessary.2 A classic study by Tuffanelli et al reported that the onset of hyperpigmentation related to antimalarial drug therapy may not occur until 4 to 70 months after initiation of treatment.6 Once the offending drug is discontinued, pigmentation changes slowly fade but often do not completely resolve,7 and patients should be advised of this.
Ocular Retinopathy
While pigmentary changes associated with antimalarial drugs are benign,3 a rare but serious adverse effect of antimalarials is retinal toxicity. Ocular retinopathy related to chloroquine and hydroxychloroquine therapy has been well documented and may result in irreversible vision loss.8,9 The most recent recommendations from the American Academy of Ophthalmology suggest a baseline eye examination at initiation of antimalarial treatment and annual examinations starting after five years of therapy because the risk for toxicity relates to the cumulative dose.8 More frequent ophthalmologic evaluations are recommended for individuals at higher risk, such as those with preexisting retinal or macular disease.9
Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
A biopsy of the roof of the patient’s mouth confirmed that the palatal hyperpigmentation was caused by her antimalarial medications. Since the patient displayed no evidence of active lupus skin lesions and laboratory results indicated that her SLE was inactive, one of the drugs, quinacrine, was discontinued.
The patient was referred for an ophthalmologic evaluation. No evidence of retinal toxicity was found.
Follow-up evaluations at two months and six months revealed no significant improvement in the discoloration of the patient’s oral mucosa or nail beds. At the six-month visit, her dosage of hydroxychloroquine was reevaluated.
The patient’s hydroxychloroquine dosage was determined based on 7.3 mg/kg/d. In the case of an overweight patient, especially one of shorter-than-average stature, hydroxychloroquine dosing should be based on ideal body weight to minimize the risk for overdosage; in general, a maximum dosage of 6.5 mg/kg/d is recommended.8,9 As a result, the patient’s dosage was decreased to 300 mg/d.
At her nine-month follow-up evaluation, the discoloration to the patient’s oral mucosa had faded but had not resolved completely (see Figure 3). No significant change was noted in the subungual discoloration. The patient had experienced no exacerbations of lupus-related symptoms since her medication adjustments.
CONCLUSION
Although this patient’s hyperpigmentation was benign, staying alert to this potential adverse effect of antimalarial drugs is important in making a diagnosis. As with many skin lesions, if the clinical evaluation does not provide a clear cause, a biopsy may be needed. For anyone taking antimalarial drugs, regular ophthalmologic evaluations are recommended to facilitate early detection of the rare adverse effect of retinal toxicity. Nevertheless, with careful monitoring, antimalarial drugs are safe and effective for the treatment of inflammatory conditions such as SLE and rheumatoid arthritis.
REFERENCES
1. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(2):189-194.
2. Gondak R-O, da Silva-Jorge R, Jorge J, et al. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Pathol Oral Cir Bucal. 2012;17(6):e919-e924.
3. Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;
107:8-12.
4. Kalampalikis A, Goetze S, Elsner P. Isolated hyperpigmentation of the oral mucosa due to hydroxychloroquine. J Dtsch Dermatol Ges. 2012; 10(12):921-922.
5. de Andrade BA, Fonseca FP, Pires FR, et al. Hard palate hyperpigmentation secondary to chronic chloroquine therapy: report of five cases.
J Cutan Pathol. 2013;40(9):833-838.
6. Tuffanelli D, Abraham RK, Dubois EI. Pigmentation from antimalarial therapy: its possible relationship to the ocular lesions. Arch Derm. 1963; 88:419-426.
7. Melikoglu MA, Melikoglu M, Gurbuz U, et al. Hydroxychloroquine-induced hyperpigmentation: a case report. J Clin Pharm Ther. 2008; 33(6):699-701.
8. Marmor MF, Kellner U, Lai YY, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):
415-422.
9. Screening for hydroxychloroquine retinopathy. Position statement, American College of Rheumatology. www.rheumatology.org/Practice/Clinical/Position/Position_Statements/. Accessed July 17, 2014.
Pediatric Hospital Medicine 2014: Behavioral Emergencies: Stay Safe, Stay Calm
Presenters
David Pressel, Jessica Tomaszewski, Emily Fingado, Adam Pressel
Summary
Behavioral emergencies occur when a patient is physically aggressive or potentially harmful to him/herself or others. Behavioral emergencies may be rare, but they are high-risk situations and staff might be untrained and uncomfortable dealing with these events.
Patients with underlying psychiatric or developmental disorders, have ingested substances, or have a medication side effect are at highest risk for becoming violent. Triggers for these events could be due to pain, hunger, isolation, change in routine, or even the hospital’s physical environment. Early warning signs for a behavioral emergency can include verbal threats, yelling, or silence. Physical signs may include pacing, crossed arms, furrowed brow, or throwing.
The first response to a potential behavioral emergency is to try to de-escalate the situation. Speak in a quiet, calm voice; back off and give personal space. Try to reduce a source of discomfort and use distractions or rewards. If de-escalation is not successful and a patient becomes violent, the provider’s first role is to be safe: get away and get help. Hospitals should have (or should develop) a violent patient response team, which may then physically restrain the patient. Medications can be used to treat medical issues, but should not be used solely for chemical restraint.
Once a patient is safely restrained, a number of JCAHO mandated actions must occur. The legal guardian and attending of record must be notified. A debrief must occur regarding the events; this must be documented in the medical record. Finally, a strategy must be formulated to enable the patient to be safely removed from restraints as soon as safe.
The presenters demonstrated various personal safety techniques to escape from a violent patient, as well as the use of physical restraints. Participants engaged in a mock behavioral emergency to experience the chaos of these events.
Hospitalists should ensure that their home institutions have developed policies and procedures, as well as ongoing training to address patient behavioral emergencies. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters
David Pressel, Jessica Tomaszewski, Emily Fingado, Adam Pressel
Summary
Behavioral emergencies occur when a patient is physically aggressive or potentially harmful to him/herself or others. Behavioral emergencies may be rare, but they are high-risk situations and staff might be untrained and uncomfortable dealing with these events.
Patients with underlying psychiatric or developmental disorders, have ingested substances, or have a medication side effect are at highest risk for becoming violent. Triggers for these events could be due to pain, hunger, isolation, change in routine, or even the hospital’s physical environment. Early warning signs for a behavioral emergency can include verbal threats, yelling, or silence. Physical signs may include pacing, crossed arms, furrowed brow, or throwing.
The first response to a potential behavioral emergency is to try to de-escalate the situation. Speak in a quiet, calm voice; back off and give personal space. Try to reduce a source of discomfort and use distractions or rewards. If de-escalation is not successful and a patient becomes violent, the provider’s first role is to be safe: get away and get help. Hospitals should have (or should develop) a violent patient response team, which may then physically restrain the patient. Medications can be used to treat medical issues, but should not be used solely for chemical restraint.
Once a patient is safely restrained, a number of JCAHO mandated actions must occur. The legal guardian and attending of record must be notified. A debrief must occur regarding the events; this must be documented in the medical record. Finally, a strategy must be formulated to enable the patient to be safely removed from restraints as soon as safe.
The presenters demonstrated various personal safety techniques to escape from a violent patient, as well as the use of physical restraints. Participants engaged in a mock behavioral emergency to experience the chaos of these events.
Hospitalists should ensure that their home institutions have developed policies and procedures, as well as ongoing training to address patient behavioral emergencies. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters
David Pressel, Jessica Tomaszewski, Emily Fingado, Adam Pressel
Summary
Behavioral emergencies occur when a patient is physically aggressive or potentially harmful to him/herself or others. Behavioral emergencies may be rare, but they are high-risk situations and staff might be untrained and uncomfortable dealing with these events.
Patients with underlying psychiatric or developmental disorders, have ingested substances, or have a medication side effect are at highest risk for becoming violent. Triggers for these events could be due to pain, hunger, isolation, change in routine, or even the hospital’s physical environment. Early warning signs for a behavioral emergency can include verbal threats, yelling, or silence. Physical signs may include pacing, crossed arms, furrowed brow, or throwing.
The first response to a potential behavioral emergency is to try to de-escalate the situation. Speak in a quiet, calm voice; back off and give personal space. Try to reduce a source of discomfort and use distractions or rewards. If de-escalation is not successful and a patient becomes violent, the provider’s first role is to be safe: get away and get help. Hospitals should have (or should develop) a violent patient response team, which may then physically restrain the patient. Medications can be used to treat medical issues, but should not be used solely for chemical restraint.
Once a patient is safely restrained, a number of JCAHO mandated actions must occur. The legal guardian and attending of record must be notified. A debrief must occur regarding the events; this must be documented in the medical record. Finally, a strategy must be formulated to enable the patient to be safely removed from restraints as soon as safe.
The presenters demonstrated various personal safety techniques to escape from a violent patient, as well as the use of physical restraints. Participants engaged in a mock behavioral emergency to experience the chaos of these events.
Hospitalists should ensure that their home institutions have developed policies and procedures, as well as ongoing training to address patient behavioral emergencies. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Pediatric Hospital Medicine 2014: Co-Management in Pediatric Hospital Medicine
Presenters
Sarah F. Denniston, Jack M. Percelay, David M. Pressel, David I. Rappaport, Elisabeth H. Villavicencio
Summary
Co-management is a growing area of pediatric HM involving both surgical and medical subspecialties. According to SHM, co-management is “shared responsibility, authority, and accountability for the care of a hospitalized patient across clinical specialties.”
Motivation for starting a co-management program may come from administrators due to quality, safety, or nursing concerns; surgeons or subspecialists driven by time or knowledge constraints; or from hospitalists looking to enhance patient safety, clinical skills, and practice development.
Pitfalls for hospitalists include patient “dumping,” care fragmentation, and working outside their scope of practice.
SHM identifies five keys to success for hospitalist co-management programs:
- Identify obstacles and challenges, including the program’s stakeholders, goals, risks and assumptions.
- Clarify roles and responsibilities for areas such as admission and discharge, communication, documentation and delineation of responsibilities. These should be specified in a service agreement.
- Identify champions, ideally to include a surgeon or subspecialist, hospitalist, administrator, and input from a family advisory council.
- Measure performance in areas such as length of stay, resource utilization, quality and safety metrics.
- Address financial issues. Most programs require some financial support to supplement billing revenue.
The AMA ethical guidelines for co-management arrangements state that the highest-quality care, not economic considerations, should be the guiding factor. Additionally, one physician should ultimately be responsible for the patient, there can be no kickbacks, and co-management arrangements need to be disclosed to the patient or family. TH
David Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters
Sarah F. Denniston, Jack M. Percelay, David M. Pressel, David I. Rappaport, Elisabeth H. Villavicencio
Summary
Co-management is a growing area of pediatric HM involving both surgical and medical subspecialties. According to SHM, co-management is “shared responsibility, authority, and accountability for the care of a hospitalized patient across clinical specialties.”
Motivation for starting a co-management program may come from administrators due to quality, safety, or nursing concerns; surgeons or subspecialists driven by time or knowledge constraints; or from hospitalists looking to enhance patient safety, clinical skills, and practice development.
Pitfalls for hospitalists include patient “dumping,” care fragmentation, and working outside their scope of practice.
SHM identifies five keys to success for hospitalist co-management programs:
- Identify obstacles and challenges, including the program’s stakeholders, goals, risks and assumptions.
- Clarify roles and responsibilities for areas such as admission and discharge, communication, documentation and delineation of responsibilities. These should be specified in a service agreement.
- Identify champions, ideally to include a surgeon or subspecialist, hospitalist, administrator, and input from a family advisory council.
- Measure performance in areas such as length of stay, resource utilization, quality and safety metrics.
- Address financial issues. Most programs require some financial support to supplement billing revenue.
The AMA ethical guidelines for co-management arrangements state that the highest-quality care, not economic considerations, should be the guiding factor. Additionally, one physician should ultimately be responsible for the patient, there can be no kickbacks, and co-management arrangements need to be disclosed to the patient or family. TH
David Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters
Sarah F. Denniston, Jack M. Percelay, David M. Pressel, David I. Rappaport, Elisabeth H. Villavicencio
Summary
Co-management is a growing area of pediatric HM involving both surgical and medical subspecialties. According to SHM, co-management is “shared responsibility, authority, and accountability for the care of a hospitalized patient across clinical specialties.”
Motivation for starting a co-management program may come from administrators due to quality, safety, or nursing concerns; surgeons or subspecialists driven by time or knowledge constraints; or from hospitalists looking to enhance patient safety, clinical skills, and practice development.
Pitfalls for hospitalists include patient “dumping,” care fragmentation, and working outside their scope of practice.
SHM identifies five keys to success for hospitalist co-management programs:
- Identify obstacles and challenges, including the program’s stakeholders, goals, risks and assumptions.
- Clarify roles and responsibilities for areas such as admission and discharge, communication, documentation and delineation of responsibilities. These should be specified in a service agreement.
- Identify champions, ideally to include a surgeon or subspecialist, hospitalist, administrator, and input from a family advisory council.
- Measure performance in areas such as length of stay, resource utilization, quality and safety metrics.
- Address financial issues. Most programs require some financial support to supplement billing revenue.
The AMA ethical guidelines for co-management arrangements state that the highest-quality care, not economic considerations, should be the guiding factor. Additionally, one physician should ultimately be responsible for the patient, there can be no kickbacks, and co-management arrangements need to be disclosed to the patient or family. TH
David Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Subacute Thyroiditis
Jerry, a 48-year-old white man, is referred to endocrinology for abnormal results of thyroid tests performed four weeks ago (see table for values). Two months ago, Jerry developed an upper respiratory infection (URI) with fever, odynophagia, and anterior neck discomfort. His symptoms resolved after two weeks; however, he has since developed fatigue and nervousness.
The remaining review of systems is unremarkable. Medical history is negative. Jerry denies any factors that can affect thyroid function: He does not take thyroid medication, OTC thyroid supplements, amiodarone, lithium, or interferon-α, does not have high iodine intake, and has not undergone head/neck irradiation. There is no personal or family history of thyroid disease, organ-specific autoimmune disease (ie, vitiligo, myasthenia gravis, or Sjögren syndrome) or systemic autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis).
Vital signs are stable. On physical examination, his thyroid gland is firm, with slight enlargement of the left lobe and mild tenderness. There are no palpable nodules or cervical adenopathy. The remainder of the exam is unremarkable.
Lab studies (see table) reveal an elevated erythrocyte sedimentation rate (ESR) and suppressed TSH, with normal free thyroxine (T4) and free triiodothyronine (T3) levels. His thyroid peroxidase antibody (Anti-TPO) is negative. Radioactive iodine uptake (RAIU) reveals a low 24-hour uptake of 4% (normal, 5% to 30%).
Jerry is given the presumptive diagnosis of subacute thyroiditis (SAT). He is advised that the condition will progress through multiple phases—from the initial thyrotoxicosis to euthyroidism
to transient hypothyroid—before resolution and is educated on the symptoms and signs to watch for. Since he presented in a euthyroid phase, with only mild anterior neck tenderness, no treatment is indicated. He is instructed to follow up for thyroid function testing in four to six weeks and to call with any symptomatic changes.
Two months later, Jerry returns with complaints of ongoing fatigue, unintentional weight gain, and “mental fog.” Physical exam findings are unremarkable except for a small, firm thyroid gland without the tenderness elicited previously. Labwork reveals an elevated TSH with low free T4 and free T3. He is again counseled regarding the natural history of SAT and reassured that his symptoms will abate as his thyroid hormone levels normalize. He is advised to continue the plan of follow-up testing every four to six weeks.
Approximately eight weeks later, Jerry’s thyroid function studies indicate normal levels, and he is notified of the results. Jerry comments that his symptoms have completely resolved and he is back to feeling like his usual self. He is discharged to follow-up as needed.
What is subacute thyroiditis?
WHAT IS SUBACUTE THYROIDITIS?
Subacute thyroiditis is also known as de Quervain thyroiditis or granulomatous giant cell thyroiditis.1,2 The most common cause of thyroid pain, it is a self-limited inflammatory disorder in which a painful tender goiter is associated with malaise, fever, and transient thyroid dysfunction.2,3 As with other thyroid disorders, SAT occurs most frequently in women ages 40 to 50.2,3 Thought to be of viral origin, it usually occurs after a URI and commonly correlates with the peak incidence of viral infections (spring/fall).2,3
The disruptive process begins with inflammatory destruction of thyroid follicles.2 This causes leakage of stored colloid, which is broken down, releasing unregulated T4 and T3 into the circulation and resulting in a thyrotoxicosis that typically lasts six weeks.1,2,4 Thyroid cells are incapable of producing new thyroid hormone during this time, so as excess circulating hormone is utilized, T4 and T3 levels become normal, then deficient, and the patient transitions through a period of euthyroidism to transient hypothyroidism.1,2,4 As the disruption of thyroid parenchyma abates, recovery ensues. The follicles regenerate, colloid is repleted, and normal thyroid function is restored.1-4
SAT typically lasts four to six months, although painful thyromegaly may persist for one year after resolution of thyroid dysfunction.2 Throughout the course of SAT, thyroid test results can be confusing, and misdiagnosis of hyperthyroidism or hypothyroidism may occur unless each phase of SAT is recognized.
Phases of SAT >>
PRODROME
The precursor URI is followed in days or weeks by the clinical manifestations of SAT. These typically include myalgia, pharyngitis, low-grade fever, and fatigue.2
There may be pain of varying degrees in part or all of one or both lobes; the pain often migrates to the entire gland and may radiate to the angle of the jaw or the ear of the affected side(s). Moving the head, swallowing, or coughing aggravates the pain.2
The hallmark of SAT is a markedly elevated ESR (often > 100 mm/h).1-3 Leukocyte count is normal (50% of cases) or only slightly elevated (50%).2
THYROTOXIC PHASE
Fifty percent of patients have mild to moderate symptoms of hyperthyroidism, including nervousness, weight loss, heat intolerance, or palpitations; hoarseness or dysphagia may be present.2 Signs include tremors or tachycardia. The thyroid gland may reveal slight to moderate unilateral enlargement, usually firm in the involved area, and tenderness may be mild, moderate, or severe.2 Cervical lymphadenopathy is absent.2 Serum T4 and T3 levels are elevated, and TSH is suppressed.1-4
Thyroid antibodies (antithyroid peroxidase antibodies [Anti-TPO or TPOAb] or antithyroglobulin antibodies [Anti-TG or TgAb]) have been found in 42% to 62% of patients with SAT.2 These transitory immunologic markers develop several weeks after the onset and appear to be a physiologic response to the inflammatory insult to the gland.2 In most patients, the antibody titer gradually decreases, then disappears as the disease resolves.2-4
The 24-hour RAIU is low
(< 5%) in the toxic phase of SAT, and thyroid scan will reveal a patchy and irregular distribution of the tracer.2,3 The thyrotoxicosis during this early phase is caused by the inflammatory release of preformed thyroid hormones (not hyperfunctioning in the gland), resulting in a “low-uptake thyrotoxicosis.”2 This differentiates SAT from the elevated uptake seen in Graves disease (> 30% at 24 hours).2
TRANSIENT HYPOTHYROIDISM PHASE
As circulating T4 and T3 are utilized but follicular function remains temporarily impaired, levels decline, resulting in a period of euthyroidism followed by hypothyroidism. TSH levels, previously suppressed in the thyrotoxic phase, now become elevated. This transient hypothyroidism occurs in two-thirds of patients, and the presentation varies from subclinical to pronounced.2
RECOVERY PHASE
After several weeks or months, all thyroid function studies return to normal and complete recovery commonly ensues. SAT rarely recurs, most likely due to immunity to the precipitating virus.1,2,4
Management of SAT >>
MANAGEMENT
Thyroid function should be monitored by testing every two to four weeks, dependent on the severity of the patient’s symptoms and rate of progression.1 Often, no treatment is required.1,2
Symptomatic relief of mild thyroid pain can be achieved with NSAIDs or aspirin (2 to 3 g/d). Severe symptoms can be treated with short-term prednisone, which should be tapered and discontinued.1-3 Steroids suppress the inflammatory response, and the dramatic relief of thyroid pain within 24 hours can be diagnostic of SAT.2
During the thyrotoxic phase, β-blockers (propranolol) can alleviate adrenergic symptoms, with the dose tapered once the patient is euthyroid.1-3 Antithyroid medications that directly inhibit thyroid hormone synthesis (eg, methimazole or propylthiouracil) are ineffective due to the lack of T4 and T3 production in the follicular cells after the inflammatory response.2,3
During the transient hypothyroid phase, thyroid hormone replacement may be indicated if the TSH level is markedly elevated or the phase refractory. However, levothyroxine therapy should be low dose (< 100 μg) and not be considered lifelong.2,3
DIFFERENTIAL DIAGNOSIS
During the prodrome, SAT is often misdiagnosed as pharyngitis. Acute suppurative thyroiditis initially may mimic SAT, but the febrile and leukocytic responses are greater, and localized edema, erythema, and tenderness become more evident as the condition progresses.
Painless or silent thyroiditis is distinguished from SAT by the lack of pain or tenderness and a normal ESR in the presence of a similar pattern of thyroid dysfunction. Graves disease presents with symptoms similar to the thyrotoxic phase of SAT, but T3 is usually disproportionately elevated compared to T4, RAIU is elevated, and thyroid antibodies are prevalent.2
CONCLUSION
Primary care providers may encounter SAT at some point, and a level of clinical suspicion must be maintained. Referral to endocrinology may be warranted in some cases; however, textbook cases can often be followed in primary care. Patient education is the foundation of SAT care. Symptomatic treatments may be employed as needed. Fortunately, for most patients, this self-limited disease state rarely leads to complications.
REFERENCES
1. Cooper DS. The thyroid gland. In: Gardner D, Shobeck D (eds). Greenspan’s Basic and Clinical Endocrinology. 9th ed. China: McGraw-Hill; 2011:163-226.
2. Guimaraes VC. Subacute and Riedel’s thyroiditis. In: Jameson JL, De Groot LJ (eds). Endocrinology Adult and Pediatric. 6th ed. Philadelphia: Saunders; 2010:1595-1600.
3. Jameson JL. Disorders of the thyroid gland. In: Jameson JL (ed). Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010: 62-98.
4. Smallridge RC. Thyroiditis. In: McDermott MT (ed). Endocrine Secrets. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:289-293.
Jerry, a 48-year-old white man, is referred to endocrinology for abnormal results of thyroid tests performed four weeks ago (see table for values). Two months ago, Jerry developed an upper respiratory infection (URI) with fever, odynophagia, and anterior neck discomfort. His symptoms resolved after two weeks; however, he has since developed fatigue and nervousness.
The remaining review of systems is unremarkable. Medical history is negative. Jerry denies any factors that can affect thyroid function: He does not take thyroid medication, OTC thyroid supplements, amiodarone, lithium, or interferon-α, does not have high iodine intake, and has not undergone head/neck irradiation. There is no personal or family history of thyroid disease, organ-specific autoimmune disease (ie, vitiligo, myasthenia gravis, or Sjögren syndrome) or systemic autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis).
Vital signs are stable. On physical examination, his thyroid gland is firm, with slight enlargement of the left lobe and mild tenderness. There are no palpable nodules or cervical adenopathy. The remainder of the exam is unremarkable.
Lab studies (see table) reveal an elevated erythrocyte sedimentation rate (ESR) and suppressed TSH, with normal free thyroxine (T4) and free triiodothyronine (T3) levels. His thyroid peroxidase antibody (Anti-TPO) is negative. Radioactive iodine uptake (RAIU) reveals a low 24-hour uptake of 4% (normal, 5% to 30%).
Jerry is given the presumptive diagnosis of subacute thyroiditis (SAT). He is advised that the condition will progress through multiple phases—from the initial thyrotoxicosis to euthyroidism
to transient hypothyroid—before resolution and is educated on the symptoms and signs to watch for. Since he presented in a euthyroid phase, with only mild anterior neck tenderness, no treatment is indicated. He is instructed to follow up for thyroid function testing in four to six weeks and to call with any symptomatic changes.
Two months later, Jerry returns with complaints of ongoing fatigue, unintentional weight gain, and “mental fog.” Physical exam findings are unremarkable except for a small, firm thyroid gland without the tenderness elicited previously. Labwork reveals an elevated TSH with low free T4 and free T3. He is again counseled regarding the natural history of SAT and reassured that his symptoms will abate as his thyroid hormone levels normalize. He is advised to continue the plan of follow-up testing every four to six weeks.
Approximately eight weeks later, Jerry’s thyroid function studies indicate normal levels, and he is notified of the results. Jerry comments that his symptoms have completely resolved and he is back to feeling like his usual self. He is discharged to follow-up as needed.
What is subacute thyroiditis?
WHAT IS SUBACUTE THYROIDITIS?
Subacute thyroiditis is also known as de Quervain thyroiditis or granulomatous giant cell thyroiditis.1,2 The most common cause of thyroid pain, it is a self-limited inflammatory disorder in which a painful tender goiter is associated with malaise, fever, and transient thyroid dysfunction.2,3 As with other thyroid disorders, SAT occurs most frequently in women ages 40 to 50.2,3 Thought to be of viral origin, it usually occurs after a URI and commonly correlates with the peak incidence of viral infections (spring/fall).2,3
The disruptive process begins with inflammatory destruction of thyroid follicles.2 This causes leakage of stored colloid, which is broken down, releasing unregulated T4 and T3 into the circulation and resulting in a thyrotoxicosis that typically lasts six weeks.1,2,4 Thyroid cells are incapable of producing new thyroid hormone during this time, so as excess circulating hormone is utilized, T4 and T3 levels become normal, then deficient, and the patient transitions through a period of euthyroidism to transient hypothyroidism.1,2,4 As the disruption of thyroid parenchyma abates, recovery ensues. The follicles regenerate, colloid is repleted, and normal thyroid function is restored.1-4
SAT typically lasts four to six months, although painful thyromegaly may persist for one year after resolution of thyroid dysfunction.2 Throughout the course of SAT, thyroid test results can be confusing, and misdiagnosis of hyperthyroidism or hypothyroidism may occur unless each phase of SAT is recognized.
Phases of SAT >>
PRODROME
The precursor URI is followed in days or weeks by the clinical manifestations of SAT. These typically include myalgia, pharyngitis, low-grade fever, and fatigue.2
There may be pain of varying degrees in part or all of one or both lobes; the pain often migrates to the entire gland and may radiate to the angle of the jaw or the ear of the affected side(s). Moving the head, swallowing, or coughing aggravates the pain.2
The hallmark of SAT is a markedly elevated ESR (often > 100 mm/h).1-3 Leukocyte count is normal (50% of cases) or only slightly elevated (50%).2
THYROTOXIC PHASE
Fifty percent of patients have mild to moderate symptoms of hyperthyroidism, including nervousness, weight loss, heat intolerance, or palpitations; hoarseness or dysphagia may be present.2 Signs include tremors or tachycardia. The thyroid gland may reveal slight to moderate unilateral enlargement, usually firm in the involved area, and tenderness may be mild, moderate, or severe.2 Cervical lymphadenopathy is absent.2 Serum T4 and T3 levels are elevated, and TSH is suppressed.1-4
Thyroid antibodies (antithyroid peroxidase antibodies [Anti-TPO or TPOAb] or antithyroglobulin antibodies [Anti-TG or TgAb]) have been found in 42% to 62% of patients with SAT.2 These transitory immunologic markers develop several weeks after the onset and appear to be a physiologic response to the inflammatory insult to the gland.2 In most patients, the antibody titer gradually decreases, then disappears as the disease resolves.2-4
The 24-hour RAIU is low
(< 5%) in the toxic phase of SAT, and thyroid scan will reveal a patchy and irregular distribution of the tracer.2,3 The thyrotoxicosis during this early phase is caused by the inflammatory release of preformed thyroid hormones (not hyperfunctioning in the gland), resulting in a “low-uptake thyrotoxicosis.”2 This differentiates SAT from the elevated uptake seen in Graves disease (> 30% at 24 hours).2
TRANSIENT HYPOTHYROIDISM PHASE
As circulating T4 and T3 are utilized but follicular function remains temporarily impaired, levels decline, resulting in a period of euthyroidism followed by hypothyroidism. TSH levels, previously suppressed in the thyrotoxic phase, now become elevated. This transient hypothyroidism occurs in two-thirds of patients, and the presentation varies from subclinical to pronounced.2
RECOVERY PHASE
After several weeks or months, all thyroid function studies return to normal and complete recovery commonly ensues. SAT rarely recurs, most likely due to immunity to the precipitating virus.1,2,4
Management of SAT >>
MANAGEMENT
Thyroid function should be monitored by testing every two to four weeks, dependent on the severity of the patient’s symptoms and rate of progression.1 Often, no treatment is required.1,2
Symptomatic relief of mild thyroid pain can be achieved with NSAIDs or aspirin (2 to 3 g/d). Severe symptoms can be treated with short-term prednisone, which should be tapered and discontinued.1-3 Steroids suppress the inflammatory response, and the dramatic relief of thyroid pain within 24 hours can be diagnostic of SAT.2
During the thyrotoxic phase, β-blockers (propranolol) can alleviate adrenergic symptoms, with the dose tapered once the patient is euthyroid.1-3 Antithyroid medications that directly inhibit thyroid hormone synthesis (eg, methimazole or propylthiouracil) are ineffective due to the lack of T4 and T3 production in the follicular cells after the inflammatory response.2,3
During the transient hypothyroid phase, thyroid hormone replacement may be indicated if the TSH level is markedly elevated or the phase refractory. However, levothyroxine therapy should be low dose (< 100 μg) and not be considered lifelong.2,3
DIFFERENTIAL DIAGNOSIS
During the prodrome, SAT is often misdiagnosed as pharyngitis. Acute suppurative thyroiditis initially may mimic SAT, but the febrile and leukocytic responses are greater, and localized edema, erythema, and tenderness become more evident as the condition progresses.
Painless or silent thyroiditis is distinguished from SAT by the lack of pain or tenderness and a normal ESR in the presence of a similar pattern of thyroid dysfunction. Graves disease presents with symptoms similar to the thyrotoxic phase of SAT, but T3 is usually disproportionately elevated compared to T4, RAIU is elevated, and thyroid antibodies are prevalent.2
CONCLUSION
Primary care providers may encounter SAT at some point, and a level of clinical suspicion must be maintained. Referral to endocrinology may be warranted in some cases; however, textbook cases can often be followed in primary care. Patient education is the foundation of SAT care. Symptomatic treatments may be employed as needed. Fortunately, for most patients, this self-limited disease state rarely leads to complications.
REFERENCES
1. Cooper DS. The thyroid gland. In: Gardner D, Shobeck D (eds). Greenspan’s Basic and Clinical Endocrinology. 9th ed. China: McGraw-Hill; 2011:163-226.
2. Guimaraes VC. Subacute and Riedel’s thyroiditis. In: Jameson JL, De Groot LJ (eds). Endocrinology Adult and Pediatric. 6th ed. Philadelphia: Saunders; 2010:1595-1600.
3. Jameson JL. Disorders of the thyroid gland. In: Jameson JL (ed). Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010: 62-98.
4. Smallridge RC. Thyroiditis. In: McDermott MT (ed). Endocrine Secrets. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:289-293.
Jerry, a 48-year-old white man, is referred to endocrinology for abnormal results of thyroid tests performed four weeks ago (see table for values). Two months ago, Jerry developed an upper respiratory infection (URI) with fever, odynophagia, and anterior neck discomfort. His symptoms resolved after two weeks; however, he has since developed fatigue and nervousness.
The remaining review of systems is unremarkable. Medical history is negative. Jerry denies any factors that can affect thyroid function: He does not take thyroid medication, OTC thyroid supplements, amiodarone, lithium, or interferon-α, does not have high iodine intake, and has not undergone head/neck irradiation. There is no personal or family history of thyroid disease, organ-specific autoimmune disease (ie, vitiligo, myasthenia gravis, or Sjögren syndrome) or systemic autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus, or progressive systemic sclerosis).
Vital signs are stable. On physical examination, his thyroid gland is firm, with slight enlargement of the left lobe and mild tenderness. There are no palpable nodules or cervical adenopathy. The remainder of the exam is unremarkable.
Lab studies (see table) reveal an elevated erythrocyte sedimentation rate (ESR) and suppressed TSH, with normal free thyroxine (T4) and free triiodothyronine (T3) levels. His thyroid peroxidase antibody (Anti-TPO) is negative. Radioactive iodine uptake (RAIU) reveals a low 24-hour uptake of 4% (normal, 5% to 30%).
Jerry is given the presumptive diagnosis of subacute thyroiditis (SAT). He is advised that the condition will progress through multiple phases—from the initial thyrotoxicosis to euthyroidism
to transient hypothyroid—before resolution and is educated on the symptoms and signs to watch for. Since he presented in a euthyroid phase, with only mild anterior neck tenderness, no treatment is indicated. He is instructed to follow up for thyroid function testing in four to six weeks and to call with any symptomatic changes.
Two months later, Jerry returns with complaints of ongoing fatigue, unintentional weight gain, and “mental fog.” Physical exam findings are unremarkable except for a small, firm thyroid gland without the tenderness elicited previously. Labwork reveals an elevated TSH with low free T4 and free T3. He is again counseled regarding the natural history of SAT and reassured that his symptoms will abate as his thyroid hormone levels normalize. He is advised to continue the plan of follow-up testing every four to six weeks.
Approximately eight weeks later, Jerry’s thyroid function studies indicate normal levels, and he is notified of the results. Jerry comments that his symptoms have completely resolved and he is back to feeling like his usual self. He is discharged to follow-up as needed.
What is subacute thyroiditis?
WHAT IS SUBACUTE THYROIDITIS?
Subacute thyroiditis is also known as de Quervain thyroiditis or granulomatous giant cell thyroiditis.1,2 The most common cause of thyroid pain, it is a self-limited inflammatory disorder in which a painful tender goiter is associated with malaise, fever, and transient thyroid dysfunction.2,3 As with other thyroid disorders, SAT occurs most frequently in women ages 40 to 50.2,3 Thought to be of viral origin, it usually occurs after a URI and commonly correlates with the peak incidence of viral infections (spring/fall).2,3
The disruptive process begins with inflammatory destruction of thyroid follicles.2 This causes leakage of stored colloid, which is broken down, releasing unregulated T4 and T3 into the circulation and resulting in a thyrotoxicosis that typically lasts six weeks.1,2,4 Thyroid cells are incapable of producing new thyroid hormone during this time, so as excess circulating hormone is utilized, T4 and T3 levels become normal, then deficient, and the patient transitions through a period of euthyroidism to transient hypothyroidism.1,2,4 As the disruption of thyroid parenchyma abates, recovery ensues. The follicles regenerate, colloid is repleted, and normal thyroid function is restored.1-4
SAT typically lasts four to six months, although painful thyromegaly may persist for one year after resolution of thyroid dysfunction.2 Throughout the course of SAT, thyroid test results can be confusing, and misdiagnosis of hyperthyroidism or hypothyroidism may occur unless each phase of SAT is recognized.
Phases of SAT >>
PRODROME
The precursor URI is followed in days or weeks by the clinical manifestations of SAT. These typically include myalgia, pharyngitis, low-grade fever, and fatigue.2
There may be pain of varying degrees in part or all of one or both lobes; the pain often migrates to the entire gland and may radiate to the angle of the jaw or the ear of the affected side(s). Moving the head, swallowing, or coughing aggravates the pain.2
The hallmark of SAT is a markedly elevated ESR (often > 100 mm/h).1-3 Leukocyte count is normal (50% of cases) or only slightly elevated (50%).2
THYROTOXIC PHASE
Fifty percent of patients have mild to moderate symptoms of hyperthyroidism, including nervousness, weight loss, heat intolerance, or palpitations; hoarseness or dysphagia may be present.2 Signs include tremors or tachycardia. The thyroid gland may reveal slight to moderate unilateral enlargement, usually firm in the involved area, and tenderness may be mild, moderate, or severe.2 Cervical lymphadenopathy is absent.2 Serum T4 and T3 levels are elevated, and TSH is suppressed.1-4
Thyroid antibodies (antithyroid peroxidase antibodies [Anti-TPO or TPOAb] or antithyroglobulin antibodies [Anti-TG or TgAb]) have been found in 42% to 62% of patients with SAT.2 These transitory immunologic markers develop several weeks after the onset and appear to be a physiologic response to the inflammatory insult to the gland.2 In most patients, the antibody titer gradually decreases, then disappears as the disease resolves.2-4
The 24-hour RAIU is low
(< 5%) in the toxic phase of SAT, and thyroid scan will reveal a patchy and irregular distribution of the tracer.2,3 The thyrotoxicosis during this early phase is caused by the inflammatory release of preformed thyroid hormones (not hyperfunctioning in the gland), resulting in a “low-uptake thyrotoxicosis.”2 This differentiates SAT from the elevated uptake seen in Graves disease (> 30% at 24 hours).2
TRANSIENT HYPOTHYROIDISM PHASE
As circulating T4 and T3 are utilized but follicular function remains temporarily impaired, levels decline, resulting in a period of euthyroidism followed by hypothyroidism. TSH levels, previously suppressed in the thyrotoxic phase, now become elevated. This transient hypothyroidism occurs in two-thirds of patients, and the presentation varies from subclinical to pronounced.2
RECOVERY PHASE
After several weeks or months, all thyroid function studies return to normal and complete recovery commonly ensues. SAT rarely recurs, most likely due to immunity to the precipitating virus.1,2,4
Management of SAT >>
MANAGEMENT
Thyroid function should be monitored by testing every two to four weeks, dependent on the severity of the patient’s symptoms and rate of progression.1 Often, no treatment is required.1,2
Symptomatic relief of mild thyroid pain can be achieved with NSAIDs or aspirin (2 to 3 g/d). Severe symptoms can be treated with short-term prednisone, which should be tapered and discontinued.1-3 Steroids suppress the inflammatory response, and the dramatic relief of thyroid pain within 24 hours can be diagnostic of SAT.2
During the thyrotoxic phase, β-blockers (propranolol) can alleviate adrenergic symptoms, with the dose tapered once the patient is euthyroid.1-3 Antithyroid medications that directly inhibit thyroid hormone synthesis (eg, methimazole or propylthiouracil) are ineffective due to the lack of T4 and T3 production in the follicular cells after the inflammatory response.2,3
During the transient hypothyroid phase, thyroid hormone replacement may be indicated if the TSH level is markedly elevated or the phase refractory. However, levothyroxine therapy should be low dose (< 100 μg) and not be considered lifelong.2,3
DIFFERENTIAL DIAGNOSIS
During the prodrome, SAT is often misdiagnosed as pharyngitis. Acute suppurative thyroiditis initially may mimic SAT, but the febrile and leukocytic responses are greater, and localized edema, erythema, and tenderness become more evident as the condition progresses.
Painless or silent thyroiditis is distinguished from SAT by the lack of pain or tenderness and a normal ESR in the presence of a similar pattern of thyroid dysfunction. Graves disease presents with symptoms similar to the thyrotoxic phase of SAT, but T3 is usually disproportionately elevated compared to T4, RAIU is elevated, and thyroid antibodies are prevalent.2
CONCLUSION
Primary care providers may encounter SAT at some point, and a level of clinical suspicion must be maintained. Referral to endocrinology may be warranted in some cases; however, textbook cases can often be followed in primary care. Patient education is the foundation of SAT care. Symptomatic treatments may be employed as needed. Fortunately, for most patients, this self-limited disease state rarely leads to complications.
REFERENCES
1. Cooper DS. The thyroid gland. In: Gardner D, Shobeck D (eds). Greenspan’s Basic and Clinical Endocrinology. 9th ed. China: McGraw-Hill; 2011:163-226.
2. Guimaraes VC. Subacute and Riedel’s thyroiditis. In: Jameson JL, De Groot LJ (eds). Endocrinology Adult and Pediatric. 6th ed. Philadelphia: Saunders; 2010:1595-1600.
3. Jameson JL. Disorders of the thyroid gland. In: Jameson JL (ed). Harrison’s Endocrinology. 2nd ed. China: McGraw-Hill; 2010: 62-98.
4. Smallridge RC. Thyroiditis. In: McDermott MT (ed). Endocrine Secrets. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:289-293.
Skip the Compression Stockings Following DVT
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
PRACTICE CHANGER
Do not recommend elastic compression stockings to decrease the incidence of postthrombotic syndrome after deep vein thrombosis.1
STRENGTH OF RECOMMENDATION
B: Based on a large randomized controlled trial1
ILLUSTRATIVE CASE
A 56-year-old man presents to your clinic three days after receiving a diagnosis of lower extremity deep vein thrombosis (DVT). He was prescribed warfarin (5 mg/d) with enoxaparin bridging (120 mg/d). He has read about postthrombotic syndrome (PTS) online and is very concerned about this possible adverse effect. He asks about using elastic compression stockings (ECS). What should you tell him?
PTS can be a frustrating, debilitating condition. Its clinical features range from minor limb swelling to severe edema and pain, irreversible skin changes, and leg ulcerations.2 It occurs in 25% to 50% of patients after DVT.3 Because current PTS treatments are not very effective, prevention is essential.4,5
Patients are frequently encouraged to wear ECS after DVT to reduce the incidence of PTS by decreasing venous hypertension and reflux. These stockings are expensive and uncomfortable. Prior research suggested that use of ECS can reduce PTS incidence by half, but the studies were small, single-center, and not placebo-controlled.6,7
On the next page: Study summary >>
STUDY SUMMARY
RCT sets aside a common practice
Kahn et al1 conducted a randomized, placebo-controlled trial of active versus placebo ECS in patients from 24 centers in the United States and Canada who’d had an ultrasound-confirmed proximal DVT (in the popliteal or more proximal deep leg vein) within the previous 14 days. Most patients received standard anticoagulation therapy to treat their DVT (five to 10 days of heparin and three to six months of warfarin). Patients were excluded if they had received thrombolytics, had arterial claudication, had a life expectancy of less than six months, were unable to put on ECS due to physical disabilities or allergy, or were unable to participate in follow-up visits.
Patients were randomly assigned to wear active (30 to 40 mm Hg graduated) ECS or identical-looking placebo ECS (< 5 mm Hg compression at the ankle) for two years. Providers, study personnel and statisticians, and patients were all blinded to treatment allocation. Patients were asked to wear the stocking on the affected leg each day from waking until bedtime.
Follow-up occurred at one, six, 12, 18, and 24 months. The primary outcome was cumulative incidence of PTS diagnosed at six months or later using the Ginsberg criteria of ipsilateral pain and swelling of at least one month’s duration.8 Secondary outcomes included severity of PTS, leg ulcers, recurrence of venous thromboembolism (VTE), death, adverse events, venous valvular reflux, and quality of life (QOL). Outcomes were measured objectively through use of a validated scale (the Villalta scale) for PTS severity and two questionnaires to assess QOL.9-11
There were 409 patients in the ECS group and 394 in the placebo group. Baseline characteristics, including BMI, VTE risk factors, and anticoagulation treatment regimens, were similar between groups. The average age of participants in the study group was 55.4 years and in the placebo group, 54.8 years. Men comprised 62.4% of the active group and 57.9% of the placebo group. Approximately 90% of the participants in both groups were white.
At one month, approximately 95% of participants in both groups used the stockings; at 24 months, that was reduced to a little less than 70%. The percentage of people who used the stockings for at least three days per week was similar in both groups.
The cumulative incidence of PTS during follow-up was 14.2% in the active group and 12.7% in the placebo group (hazard ratio, 1.13). There were no differences in any of the secondary outcomes. Prespecified subgroup analyses found that age, BMI, and severity of DVT had no effect on outcomes. There was a marginal benefit for ECS for women versus men, but this does not likely reflect a true difference because the confidence intervals surrounding the hazard ratios for men and women overlapped and crossed the null value.
On the next page: What's new & challenges to implementation >>
WHAT’S NEW
New evidence contradicts previous studies
Two prior studies showed that using 30 to 40 mm Hg ECS decreased the incidence of PTS after proximal DVT.6,7 However, these were smaller, open-label, single-center studies. This study by Kahn et al1 was the first placebo-controlled, randomized, multicenter study that used validated instruments to measure PTS and QOL. It found no benefit in using ECS, thus contradicting the results of the prior studies.
There are currently no guidelines or consensus statements that recommend for or against the use of ECS after DVT.
CAVEATS
High nonadherence rates might have affected results
In both groups, adherence to the assigned intervention diminished throughout the study (from 95% at one month to slightly less than 70% at two years). Theoretically, this could have affected efficacy outcomes. However, the decrease was similar in both groups and represents what is observed in clinical practice. A prespecified per protocol analysis of patients who wore their ECS more regularly found no benefit.
It is possible that a “placebo effect” could explain the lack of difference between groups. However, the placebo stockings provided virtually no compression, and the two-year cumulative incidence of PTS in both the treatment and placebo groups was similar to that seen in control groups in prior studies.6,7
Finally, the incidence of PTS in this study was much lower than the 25% to 50% incidence reported previously. Kahn et al1 suggested that this was because they used more stringent and standardized criteria for PTS than was used in previous research.
CHALLENGES TO IMPLEMENTATION
There are no barriers to ending this practice
We can identify no challenges to implementation of this recommendation.
On the next page: References >>
REFERENCES
1. Kahn SR, Shapiro S, Wells PS, et al; SOX trial investigators. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880-888.
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
3. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996; 125:1-7.
4. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141: 308-320.
5. Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:
500-509.
6. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:
759-762.
7. Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249-256.
8. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
9. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a.
10. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263.
11. Kahn SR, Lamping DL, Ducruet T, et al; VETO Study Investigators. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006; 59:1049-1056.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(7):388-390.
Man Falls on Buttocks
ANSWER
There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.
ANSWER
There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.
ANSWER
There are degenerative changes present. Bilateral hip prostheses are noted. Within the coccyx, there is bone remodeling and angulation that are likely chronic and related to remote trauma or injury (arrow). Below this, some cortical lucency (circled) is noted, most likely consistent with an acute fracture. The patient was prescribed a nonsteroidal medication and a mild narcotic pain medication.
A 75-year-old man presents to the urgent care center for evaluation of pain in his buttocks after a fall. He states he was walking when his “legs gave out” and he hit the ground. He landed squarely on his buttocks, causing immediate pain. He was eventually able to get up with some assistance. He denies any current weakness or any bowel or bladder complaints. His medical/surgical history is significant for coronary artery disease, hypertension, and bilateral hip replacements. Physical exam reveals an elderly male who is uncomfortable but in no obvious distress. His vital signs are stable. He has moderate point tenderness over his sacrum but is able to move all his extremities well, with normal strength. Radiograph of his sacrum/coccyx is shown. What is your impression?