User login
FDA aims for tighter regulation of diagnostic tests
Credit: William Weinert
The US Food and Drug Administration (FDA) is taking steps to ensure better regulation of certain diagnostic tests.
The agency has issued a final guidance on the development, review, and approval of companion diagnostics.
The FDA has also notified Congress of its intention to publish a draft guidance outlining a plan for regulating laboratory-developed tests (LDTs).
The FDA is required to notify Congress before making the draft guidance public. This is mandated by the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA).
Companion diagnostics guidance
A companion diagnostic is a medical device that provides information essential for the safe and effective use of a corresponding drug or biological product. These tests are commonly used to detect certain types of gene-based cancers.
The FDA’s companion diagnostics guidance is intended to help companies identify the need for these tests during the earliest stages of drug development and to plan for the development of a drug and a companion test at the same time.
The ultimate goal of the final guidance is to stimulate early collaborations that will result in faster access to promising new treatments for patients living with serious and life-threatening diseases. This guidance finalizes and takes into consideration public comments on the draft guidance issued in 2011.
LDT oversight
An LDT is a type of in vitro diagnostic test that is designed, manufactured, and used within a single lab. LDTs include some genetic tests and tests used by healthcare professionals to guide patient treatment.
The FDA already oversees direct-to-consumer tests, regardless of whether they are LDTs or traditional diagnostics.
And while the FDA has historically exercised enforcement discretion over LDTs (generally not enforced applicable regulatory requirements), today, these tests may compete with FDA-approved tests without clinical studies to support their use.
The LDT notification to Congress provides the details of a draft guidance in which the FDA would propose to establish an LDT oversight framework. This would include pre-market review for higher-risk LDTs, such as those that have the same intended use as FDA-approved or cleared companion diagnostics currently on the market.
The draft guidance would also propose to phase in enforcement of pre-market review for other high-risk and moderate-risk LDTs over time.
In addition, the FDA intends to propose that it continue to exercise enforcement discretion for low-risk LDTs, LDTs for rare diseases and, under certain circumstances, LDTs for which there is no FDA-approved or cleared test.
“With today’s notification of the agency’s intent to issue the lab-developed test draft guidance, the FDA is seeking a better balanced approach for all diagnostics,” said Jeffrey Shuren, MD, director of the FDA’s Center for Devices and Radiological Health.
“The agency’s oversight would be based on a test’s level of risk to patients, not on whether it is made by a conventional manufacturer or in a single laboratory, while still providing flexibility to encourage innovation that addresses unmet medical needs.”
Finally, the FDA intends to publish a draft guidance outlining how labs can notify the FDA that they are manufacturing and using LDTs, how to provide information about their LDTs, and how they can comply with the medical device reporting requirements.
A provision in FDASIA requires the FDA to provide at least 60 days’ notice to Congress before the agency publishes for public comment any draft guidance on the regulation of LDTs.
As such, the comment period will open at a later date, when the draft guidances are published in the Federal Register and the public is alerted to the start of the comment period. The agency also intends to hold a public meeting during the comment period to collect additional input. ![]()

Credit: William Weinert
The US Food and Drug Administration (FDA) is taking steps to ensure better regulation of certain diagnostic tests.
The agency has issued a final guidance on the development, review, and approval of companion diagnostics.
The FDA has also notified Congress of its intention to publish a draft guidance outlining a plan for regulating laboratory-developed tests (LDTs).
The FDA is required to notify Congress before making the draft guidance public. This is mandated by the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA).
Companion diagnostics guidance
A companion diagnostic is a medical device that provides information essential for the safe and effective use of a corresponding drug or biological product. These tests are commonly used to detect certain types of gene-based cancers.
The FDA’s companion diagnostics guidance is intended to help companies identify the need for these tests during the earliest stages of drug development and to plan for the development of a drug and a companion test at the same time.
The ultimate goal of the final guidance is to stimulate early collaborations that will result in faster access to promising new treatments for patients living with serious and life-threatening diseases. This guidance finalizes and takes into consideration public comments on the draft guidance issued in 2011.
LDT oversight
An LDT is a type of in vitro diagnostic test that is designed, manufactured, and used within a single lab. LDTs include some genetic tests and tests used by healthcare professionals to guide patient treatment.
The FDA already oversees direct-to-consumer tests, regardless of whether they are LDTs or traditional diagnostics.
And while the FDA has historically exercised enforcement discretion over LDTs (generally not enforced applicable regulatory requirements), today, these tests may compete with FDA-approved tests without clinical studies to support their use.
The LDT notification to Congress provides the details of a draft guidance in which the FDA would propose to establish an LDT oversight framework. This would include pre-market review for higher-risk LDTs, such as those that have the same intended use as FDA-approved or cleared companion diagnostics currently on the market.
The draft guidance would also propose to phase in enforcement of pre-market review for other high-risk and moderate-risk LDTs over time.
In addition, the FDA intends to propose that it continue to exercise enforcement discretion for low-risk LDTs, LDTs for rare diseases and, under certain circumstances, LDTs for which there is no FDA-approved or cleared test.
“With today’s notification of the agency’s intent to issue the lab-developed test draft guidance, the FDA is seeking a better balanced approach for all diagnostics,” said Jeffrey Shuren, MD, director of the FDA’s Center for Devices and Radiological Health.
“The agency’s oversight would be based on a test’s level of risk to patients, not on whether it is made by a conventional manufacturer or in a single laboratory, while still providing flexibility to encourage innovation that addresses unmet medical needs.”
Finally, the FDA intends to publish a draft guidance outlining how labs can notify the FDA that they are manufacturing and using LDTs, how to provide information about their LDTs, and how they can comply with the medical device reporting requirements.
A provision in FDASIA requires the FDA to provide at least 60 days’ notice to Congress before the agency publishes for public comment any draft guidance on the regulation of LDTs.
As such, the comment period will open at a later date, when the draft guidances are published in the Federal Register and the public is alerted to the start of the comment period. The agency also intends to hold a public meeting during the comment period to collect additional input. ![]()

Credit: William Weinert
The US Food and Drug Administration (FDA) is taking steps to ensure better regulation of certain diagnostic tests.
The agency has issued a final guidance on the development, review, and approval of companion diagnostics.
The FDA has also notified Congress of its intention to publish a draft guidance outlining a plan for regulating laboratory-developed tests (LDTs).
The FDA is required to notify Congress before making the draft guidance public. This is mandated by the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA).
Companion diagnostics guidance
A companion diagnostic is a medical device that provides information essential for the safe and effective use of a corresponding drug or biological product. These tests are commonly used to detect certain types of gene-based cancers.
The FDA’s companion diagnostics guidance is intended to help companies identify the need for these tests during the earliest stages of drug development and to plan for the development of a drug and a companion test at the same time.
The ultimate goal of the final guidance is to stimulate early collaborations that will result in faster access to promising new treatments for patients living with serious and life-threatening diseases. This guidance finalizes and takes into consideration public comments on the draft guidance issued in 2011.
LDT oversight
An LDT is a type of in vitro diagnostic test that is designed, manufactured, and used within a single lab. LDTs include some genetic tests and tests used by healthcare professionals to guide patient treatment.
The FDA already oversees direct-to-consumer tests, regardless of whether they are LDTs or traditional diagnostics.
And while the FDA has historically exercised enforcement discretion over LDTs (generally not enforced applicable regulatory requirements), today, these tests may compete with FDA-approved tests without clinical studies to support their use.
The LDT notification to Congress provides the details of a draft guidance in which the FDA would propose to establish an LDT oversight framework. This would include pre-market review for higher-risk LDTs, such as those that have the same intended use as FDA-approved or cleared companion diagnostics currently on the market.
The draft guidance would also propose to phase in enforcement of pre-market review for other high-risk and moderate-risk LDTs over time.
In addition, the FDA intends to propose that it continue to exercise enforcement discretion for low-risk LDTs, LDTs for rare diseases and, under certain circumstances, LDTs for which there is no FDA-approved or cleared test.
“With today’s notification of the agency’s intent to issue the lab-developed test draft guidance, the FDA is seeking a better balanced approach for all diagnostics,” said Jeffrey Shuren, MD, director of the FDA’s Center for Devices and Radiological Health.
“The agency’s oversight would be based on a test’s level of risk to patients, not on whether it is made by a conventional manufacturer or in a single laboratory, while still providing flexibility to encourage innovation that addresses unmet medical needs.”
Finally, the FDA intends to publish a draft guidance outlining how labs can notify the FDA that they are manufacturing and using LDTs, how to provide information about their LDTs, and how they can comply with the medical device reporting requirements.
A provision in FDASIA requires the FDA to provide at least 60 days’ notice to Congress before the agency publishes for public comment any draft guidance on the regulation of LDTs.
As such, the comment period will open at a later date, when the draft guidances are published in the Federal Register and the public is alerted to the start of the comment period. The agency also intends to hold a public meeting during the comment period to collect additional input. ![]()
Role of food allergy testing in EOE unclear
The role of food allergy testing in the evaluation and treatment of patients with eosinophilic esophagitis is not yet clear, according to a study by Dr. Seema Sharma Aceves.
The report appears in the August issue of Clinical Gastroenterology and Hepatology (doi: org/10.1016/j.cgh.2013.09.007).
Current data suggest, but do not definitively establish, that testing for food allergies is a reasonable approach for beginning to construct an elimination diet in children with EOE, but the data are inadequate to support that strategy in adults with the disorder, she said.
It is clear that food antigens function as triggers that both induce EOE in the first place and also exacerbate the condition once it is established. And removing food antigens from the diet resolves EOE, improving both endoscopic and histologic features, in more than 60% of adults and children.
But most large studies of food elimination diets have involved only children. "This type of large cohort data does not currently exist for the adult population, and smaller studies have not demonstrated success rates that mirror the pediatric data," Dr. Aceves said.
There are several reasons why an empiric elimination diet, which simply removes the six most allergenic food types from the diet, can actually be superior to testing each patient for the specific food types that trigger his or her EOE and then removing only those items from the diet.
First, simply removing these six food types – dairy, egg, soy, wheat, peanuts/tree nuts, and fish/shellfish – usually induces the same response rate as does the more complicated process of food allergy testing. It also spares patients the anxiety and discomfort of testing.
Second, testing for milk allergy notoriously yields a high rate of false-negative results.
Third, food-specific IgE can be caused by cross-reactivity with environmental allergens. For example, a patient with a respiratory allergy to grass can test positive for food allergy to wheat. In general, EOE patients are highly atopic and tend to be sensitized to multiple food and aeroallergens, she said.
And lastly, food allergy testing may reveal a food trigger but doesn’t address the need to perform endoscopy and biopsy after suspected triggers are eliminated from the diet and after they are eventually reintroduced, said Dr. Aceves of the division of allergy and immunology at Rady Children’s Hospital, San Diego.
An argument in favor of food allergy testing is that patients will not have to avoid so many foods when their own individual triggers are identified. In one study of children, those placed on an empiric elimination diet had to eliminate eight entire food groups, with numerous different foods falling under the general categories of peanuts/tree nuts and fish/shellfish. In contrast, children who eliminated only those items identified on testing had to eliminate an average of three food groups.
Food elimination diets "should be applied judiciously" because there is always the risk that patients will lose their tolerance for a food when it has been avoided for a long period of time. Sometimes patients are sensitized to a food but tolerate it because they have very low but steady exposures that allow the body to adapt to it. When that food is completely eliminated for a period of time and then reintroduced, it can trigger a severe allergic reaction and anaphylaxis.
Before reintroducing an allergenic food that has been eliminated from the diet, gastroenterologists may want to test first for a possible hypersensitivity reaction. Alternatively, the food can be reintroduced in a controlled setting such as an allergist’s office, where the staff can recognize and respond to anaphylaxis, and the necessary medications and equipment are readily available, Dr. Aceves said.
Some diagnostic tools that have recently become available for food allergy testing but have not yet been systematically assessed for identifying food triggers in EOE may eventually prove useful. These include peptide microarrays that gauge the repertoire of IgE in patient serum, component-resolved diagnostic testing that assesses which epitopes within a food antigen are recognized by patient serum, and assays that analyze either the release or the activation of basophils in the periphery.
Finally, the recent finding that food-specific, CD4-positive, IL-5-producing T cells can be found in the peripheral blood is intriguing, Dr. Aceves said. If these cells are found to exist in the esophagus as well, then assays for such peripheral T cells might also function as markers for EOE food triggers.
This work was supported by the National Institute of Allergy and Infectious Diseases. Dr. Aceves reported no financial conflicts of interest.
The role of food allergy testing in the evaluation and treatment of patients with eosinophilic esophagitis is not yet clear, according to a study by Dr. Seema Sharma Aceves.
The report appears in the August issue of Clinical Gastroenterology and Hepatology (doi: org/10.1016/j.cgh.2013.09.007).
Current data suggest, but do not definitively establish, that testing for food allergies is a reasonable approach for beginning to construct an elimination diet in children with EOE, but the data are inadequate to support that strategy in adults with the disorder, she said.
It is clear that food antigens function as triggers that both induce EOE in the first place and also exacerbate the condition once it is established. And removing food antigens from the diet resolves EOE, improving both endoscopic and histologic features, in more than 60% of adults and children.
But most large studies of food elimination diets have involved only children. "This type of large cohort data does not currently exist for the adult population, and smaller studies have not demonstrated success rates that mirror the pediatric data," Dr. Aceves said.
There are several reasons why an empiric elimination diet, which simply removes the six most allergenic food types from the diet, can actually be superior to testing each patient for the specific food types that trigger his or her EOE and then removing only those items from the diet.
First, simply removing these six food types – dairy, egg, soy, wheat, peanuts/tree nuts, and fish/shellfish – usually induces the same response rate as does the more complicated process of food allergy testing. It also spares patients the anxiety and discomfort of testing.
Second, testing for milk allergy notoriously yields a high rate of false-negative results.
Third, food-specific IgE can be caused by cross-reactivity with environmental allergens. For example, a patient with a respiratory allergy to grass can test positive for food allergy to wheat. In general, EOE patients are highly atopic and tend to be sensitized to multiple food and aeroallergens, she said.
And lastly, food allergy testing may reveal a food trigger but doesn’t address the need to perform endoscopy and biopsy after suspected triggers are eliminated from the diet and after they are eventually reintroduced, said Dr. Aceves of the division of allergy and immunology at Rady Children’s Hospital, San Diego.
An argument in favor of food allergy testing is that patients will not have to avoid so many foods when their own individual triggers are identified. In one study of children, those placed on an empiric elimination diet had to eliminate eight entire food groups, with numerous different foods falling under the general categories of peanuts/tree nuts and fish/shellfish. In contrast, children who eliminated only those items identified on testing had to eliminate an average of three food groups.
Food elimination diets "should be applied judiciously" because there is always the risk that patients will lose their tolerance for a food when it has been avoided for a long period of time. Sometimes patients are sensitized to a food but tolerate it because they have very low but steady exposures that allow the body to adapt to it. When that food is completely eliminated for a period of time and then reintroduced, it can trigger a severe allergic reaction and anaphylaxis.
Before reintroducing an allergenic food that has been eliminated from the diet, gastroenterologists may want to test first for a possible hypersensitivity reaction. Alternatively, the food can be reintroduced in a controlled setting such as an allergist’s office, where the staff can recognize and respond to anaphylaxis, and the necessary medications and equipment are readily available, Dr. Aceves said.
Some diagnostic tools that have recently become available for food allergy testing but have not yet been systematically assessed for identifying food triggers in EOE may eventually prove useful. These include peptide microarrays that gauge the repertoire of IgE in patient serum, component-resolved diagnostic testing that assesses which epitopes within a food antigen are recognized by patient serum, and assays that analyze either the release or the activation of basophils in the periphery.
Finally, the recent finding that food-specific, CD4-positive, IL-5-producing T cells can be found in the peripheral blood is intriguing, Dr. Aceves said. If these cells are found to exist in the esophagus as well, then assays for such peripheral T cells might also function as markers for EOE food triggers.
This work was supported by the National Institute of Allergy and Infectious Diseases. Dr. Aceves reported no financial conflicts of interest.
The role of food allergy testing in the evaluation and treatment of patients with eosinophilic esophagitis is not yet clear, according to a study by Dr. Seema Sharma Aceves.
The report appears in the August issue of Clinical Gastroenterology and Hepatology (doi: org/10.1016/j.cgh.2013.09.007).
Current data suggest, but do not definitively establish, that testing for food allergies is a reasonable approach for beginning to construct an elimination diet in children with EOE, but the data are inadequate to support that strategy in adults with the disorder, she said.
It is clear that food antigens function as triggers that both induce EOE in the first place and also exacerbate the condition once it is established. And removing food antigens from the diet resolves EOE, improving both endoscopic and histologic features, in more than 60% of adults and children.
But most large studies of food elimination diets have involved only children. "This type of large cohort data does not currently exist for the adult population, and smaller studies have not demonstrated success rates that mirror the pediatric data," Dr. Aceves said.
There are several reasons why an empiric elimination diet, which simply removes the six most allergenic food types from the diet, can actually be superior to testing each patient for the specific food types that trigger his or her EOE and then removing only those items from the diet.
First, simply removing these six food types – dairy, egg, soy, wheat, peanuts/tree nuts, and fish/shellfish – usually induces the same response rate as does the more complicated process of food allergy testing. It also spares patients the anxiety and discomfort of testing.
Second, testing for milk allergy notoriously yields a high rate of false-negative results.
Third, food-specific IgE can be caused by cross-reactivity with environmental allergens. For example, a patient with a respiratory allergy to grass can test positive for food allergy to wheat. In general, EOE patients are highly atopic and tend to be sensitized to multiple food and aeroallergens, she said.
And lastly, food allergy testing may reveal a food trigger but doesn’t address the need to perform endoscopy and biopsy after suspected triggers are eliminated from the diet and after they are eventually reintroduced, said Dr. Aceves of the division of allergy and immunology at Rady Children’s Hospital, San Diego.
An argument in favor of food allergy testing is that patients will not have to avoid so many foods when their own individual triggers are identified. In one study of children, those placed on an empiric elimination diet had to eliminate eight entire food groups, with numerous different foods falling under the general categories of peanuts/tree nuts and fish/shellfish. In contrast, children who eliminated only those items identified on testing had to eliminate an average of three food groups.
Food elimination diets "should be applied judiciously" because there is always the risk that patients will lose their tolerance for a food when it has been avoided for a long period of time. Sometimes patients are sensitized to a food but tolerate it because they have very low but steady exposures that allow the body to adapt to it. When that food is completely eliminated for a period of time and then reintroduced, it can trigger a severe allergic reaction and anaphylaxis.
Before reintroducing an allergenic food that has been eliminated from the diet, gastroenterologists may want to test first for a possible hypersensitivity reaction. Alternatively, the food can be reintroduced in a controlled setting such as an allergist’s office, where the staff can recognize and respond to anaphylaxis, and the necessary medications and equipment are readily available, Dr. Aceves said.
Some diagnostic tools that have recently become available for food allergy testing but have not yet been systematically assessed for identifying food triggers in EOE may eventually prove useful. These include peptide microarrays that gauge the repertoire of IgE in patient serum, component-resolved diagnostic testing that assesses which epitopes within a food antigen are recognized by patient serum, and assays that analyze either the release or the activation of basophils in the periphery.
Finally, the recent finding that food-specific, CD4-positive, IL-5-producing T cells can be found in the peripheral blood is intriguing, Dr. Aceves said. If these cells are found to exist in the esophagus as well, then assays for such peripheral T cells might also function as markers for EOE food triggers.
This work was supported by the National Institute of Allergy and Infectious Diseases. Dr. Aceves reported no financial conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Myelodysplastic Syndromes
Myelodysplastic syndromes (MDS) are a spectrum of clonal myeloid disorders characterized by ineffective hematopoiesis, cytopenias, qualitative disorders of blood cells, clonal chromosomal abnormalities, and the potential for clonal evolution to acute myeloid leukemia (AML). In this review, we discuss the various pathogenic conditions included in the spectrum of MDS and the associated risk stratification for these conditions. We further discuss the treatment recommendations based on the risk status and the expected prognosis.
To read the full article in PDF:
Myelodysplastic syndromes (MDS) are a spectrum of clonal myeloid disorders characterized by ineffective hematopoiesis, cytopenias, qualitative disorders of blood cells, clonal chromosomal abnormalities, and the potential for clonal evolution to acute myeloid leukemia (AML). In this review, we discuss the various pathogenic conditions included in the spectrum of MDS and the associated risk stratification for these conditions. We further discuss the treatment recommendations based on the risk status and the expected prognosis.
To read the full article in PDF:
Myelodysplastic syndromes (MDS) are a spectrum of clonal myeloid disorders characterized by ineffective hematopoiesis, cytopenias, qualitative disorders of blood cells, clonal chromosomal abnormalities, and the potential for clonal evolution to acute myeloid leukemia (AML). In this review, we discuss the various pathogenic conditions included in the spectrum of MDS and the associated risk stratification for these conditions. We further discuss the treatment recommendations based on the risk status and the expected prognosis.
To read the full article in PDF:
Primary Brain Tumors
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Primary central nervous system tumors are relatively rare, but they can cause significant morbidity. They are also among the most lethal of all neoplasms. Brain tumors are the second most common cause of death due to intracranial disease, second only to stroke. The estimated annual incidence of primary brain tumors is approximately 21 per 100,000 individuals in the United States. The incidence of brain tumors varies by gender, age, race, ethnicity, and geography and has increased over time. Gliomas and germ cell tumors are more common in men, whereas meningiomas are twice as common in women. The only validated environmental risk factor for primary brain tumors is exposure to ionizing radiation.
To read the full article in PDF:
New-onset epilepsy in the elderly: Challenges for the internist
Contrary to the popular belief that epilepsy is mainly a disease of youth, nearly 25% of new-onset seizures occur after age 65.1,2 The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.3 As our population ages, the burden of “elderly-onset” epilepsy will rise.
A seizure diagnosis carries significant implications in older people, who are already vulnerable to cognitive decline, loss of functional independence, driving restrictions, and risk of falls. Newly diagnosed epilepsy further worsens quality of life.4
The causes and clinical manifestations of seizures and epilepsy in the elderly differ from those in younger people.5 Hence, it is often difficult to make a diagnosis with certainty from a wide range of differential diagnoses. Older people are also more likely to have comorbidities, further complicating the situation.
Managing seizures in the elderly is also challenging, as age-associated physiologic changes can affect the pharmacokinetics and pharmacodynamics of antiepileptic drugs. Diagnosing and managing elderly-onset epilepsy can be challenging for a family physician, an internist, a geriatrician, or even a neurologist.
In this review, we emphasize the common causes of new-onset epilepsy in the elderly and the assessment of the clinical clues that are essential for making an accurate diagnosis. We also review the pharmacology of antiepileptic drugs used in old age and highlight the need for psychological support for patients and caregivers.
RISING PREVALENCE IN THE ELDERLY
In US Medicare beneficiaries age 65 and older, the average annual incidence rate of epilepsy in 2001 to 2005 was 10.8 per 1,000.6 A large study in Finland revealed falling incidence rates of epilepsy in childhood and middle age and rising trends in the elderly.7
In the United States, the rates are higher in African Americans (18.7 per 1,000) and lower in Asian Americans and Native Americans (5.5 and 7.7 per 1,000) than in whites (10.2 per 1,000).6 Incidence rates are slightly higher for women than for men and increase with age in both sexes and all racial groups.
Acute symptomatic seizure is also common in older patients. The incidence of acute seizures in patients over age 60 was estimated at 50 to 100 per 100,000 per year in one study.7 The rate was considerably higher in men than in women. The study also found a 3.6% risk of experiencing an acute symptomatic seizure in an 80-year lifespan, which approaches that of developing epilepsy.8 The major causes of acute symptomatic seizure were traumatic brain injury, cerebrovascular disease, drug withdrawal, and central nervous system infection.
CAUSES OF NEW-ONSET EPILEPSY IN THE ELDERLY
The most common causes of new-onset epilepsy in the elderly include cerebrovascular disease, metabolic disturbances, dementia, traumatic brain injury, tumors, and drugs.3,9–11
Cerebrovascular disease
In older adults, acute stroke is the most common cause, accounting for up to half of cases.5,12
Seizures occur in 4.4% to 8.9% of acute cerebrovascular events.13,14 The risk varies by stroke subtype, although all stroke subtypes, including transient ischemic attack, can be associated with seizure.15 For example, although 1% to 2% of patients experienced a seizure within 15 days of a transient ischemic attack or a lacunar infarct, this risk was 16.6% after an embolic stroke.15
Beyond this increased risk of “acute seizure” in the immediate poststroke period (usually defined as 1 week), the risk of epilepsy was also 20 times higher in the first year after a stroke.14 However, seizures tend to occur within the first 48 hours after the onset of ischemic stroke. In subarachnoid hemorrhage, seizures generally occur within hours.16
In a population-based study in Rochester, NY,17 epilepsy developed in two-thirds of patients with seizure related to acute stroke. Two factors that independently predicted the development of epilepsy were early seizure occurrence and recurrence of stroke.
Interestingly, the risk of stroke was three times higher in older patients who had new-onset seizure.18 Therefore, any elderly person with new-onset seizure should be assessed for cerebrovascular risk factors and treated accordingly for stroke prevention.
Metabolic disturbances
Acute metabolic disorders are common in elderly patients because of multiple comorbidities and polypharmacy. Hypoglycemia and hyponatremia need to be particularly considered in this population.19
Other well-documented metabolic causes of acute seizure, including nonketotic hyperglycemia, hypocalcemia, and uremic or hepatic encephalopathy, can all be considerations, albeit less specific to this age group.
Dementia
Primary neurodegenerative disorders associated with cognitive impairment, such as Alzheimer disease, are major risk factors for new-onset epilepsy in older patients.3,5 Seizures occur in about 10% of Alzheimer patients.20 Those who have brief periods of increased confusion may actually be experiencing unrecognized complex partial seizures.21
A case-control study discovered incidence rates of epilepsy almost 10 times higher in patients who had Alzheimer disease or vascular dementia than in nondemented patients.22 A prospective cohort study in patients with mild to moderate Alzheimer disease established that younger age, a greater degree of cognitive impairment, and a history of antipsychotic use were independent risk factors for new-onset seizures in the elderly.23 Preexisting dementia also increases the risk of poststroke epilepsy.24
Traumatic brain injury
The most common cause of brain trauma in the elderly is falls. Subdural hematoma, which can occur in the elderly with trivial trauma or sometimes even without it, needs to be considered. The risk of posttraumatic hemorrhage is especially relevant in patients taking anticoagulants.
Traumatic brain injury has a poorer prognosis in older people than in the young,25 and it accounts for up to 20% of cases of epilepsy in the elderly.26 Although no study has specifically addressed the longitudinal risk of epilepsy after traumatic brain injury in the elderly, a study in children and young adults revealed the risk was highest in the first year, with the increased risk persisting for more than 10 years.27
Brain tumors
Between 10% and 30% of new-onset seizures in the elderly are associated with tumor, typically glioma, meningioma, and brain metastasis.28,29 Seizures are usually associated more with primary than with secondary tumors, and more with low-grade tumors than high-grade ones.30
Drug-induced
Drugs and drug withdrawal can contribute to up to 10% of acute symptomatic seizures in the geriatric population.5,8,29 The elderly are susceptible to drug-induced seizure because of a higher prevalence of polypharmacy, impaired drug clearance, and heightened sensitivity to the proconvulsant side effects of medications.1 A number of commonly used drugs have been implicated,31 including:
Antibiotics such as carbapenems and high-dose penicillin
Antihistamines such as desloratadine (Clarinex)
Pain medications such as tramadol (Ul-tram) and high-dose opiates
Neuromodulators
Antidepressants such as clomipramine (Anafranil), maprotiline (Ludiomil), amoxapine (Asendin), and bupropion (Wellbutrin).32
Seizures also follow alcohol, benzodiazepine, and barbiturate withdrawal.33
Other causes
Paraneoplastic limbic encephalitis is a rare cause of seizures in the elderly.34 It can present with refractory seizures, confusion, and behavioral changes with or without a known concurrent neoplastic disease.
Posterior reversible leukoencephalopathy syndrome, another rare consideration, can particularly affect immunosuppressed elderly patients. This syndrome is characterized clinically by headache, confusion, seizures, vomiting, and visual disturbances with radiographic vasogenic edema.35
CLINICAL PRESENTATION
The signs and symptoms of a seizure may be atypical in the elderly. Seizures more often have a picture of “epileptic amnesia,” with confusion, sleepiness, or clumsiness, rather than motor manifestations such as tonic stiffening or automatism.36,37 Postictal states are also prolonged, particularly if there is underlying brain dysfunction.38 All these features render the clinical seizure manifestations more subtle and, as such, more difficult for the uninitiated caregiver to identify.
Convulsive and nonconvulsive status epilepticus
Status epilepticus is defined as a single generalized seizure lasting more than 5 minutes or a series of seizures lasting longer than 30 minutes without the patient’s regaining consciousness.39 The greatest increase in the incidence of status epilepticus occurs after age 60.40 It is the first seizure in about 30% of new-onset seizures in the elderly.41
Mortality rates increase with age, anoxia, and duration of status epilepticus and are over 50% in patients age 80 and older.40,42
Convulsive status epilepticus is most commonly caused by stroke.40
Absence status epilepticus can occur in elderly patients as a late complication of idiopathic generalized epilepsy related to benzodiazepine withdrawal, alcohol intoxication, or initiation of psychotropic drugs.42
Nonconvulsive status epilepticus manifests as altered mental status, psychosis, lethargy, or coma.42–44 Occasionally, it presents as a more focal cognitive disturbance with aphasia or a neglect syndrome.42,45 Electroencephalographic correlates of nonconvulsive status epilepticus include focal rhythmic discharges, often arising from frontal or temporal lobes, or generalized spike or sharp and slow-wave activity.46 Its management is challenging because of delayed diagnosis or misdiagnosis. The risk of death is higher in patients with severely impaired mental status or acute complications.47
Table 1 lists the typical seizure manifestations peculiar to the elderly.37,48
Differential diagnosis of new-onset epilepsy in the elderly
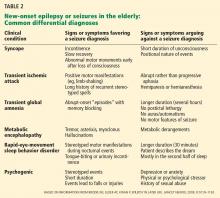
New-onset epilepsy in elderly patients can be confused with syncope, transient ischemic attack, cardiac arrhythmia, metabolic disturbances, transient global amnesia, neurodegenerative disease, rapid-eye-movement sleep behavior disorder, psychogenic disorders, and other conditions (Table 2). If there is a high clinical suspicion of seizure, the patient should undergo electroencephalography (EEG) and be referred to a neurologist or epileptologist.
KEYS TO THE DIAGNOSIS
Clinical history
A reliable history and description of the event from an eyewitness or a video recording of the event are invaluable to the diagnosis of epileptic seizure. Signs and symptoms that suggest the diagnosis include aura, ictal pallor, urinary incontinence, tongue-biting, and motor symptoms, as well as postictal confusion, drowsiness, and speech disturbance.
Electroencephalography
EEG is the most useful diagnostic tool in epilepsy. However, an interictal EEG reading (ie, between epileptic attacks) in an elderly patient has limited utility, showing epileptiform activity in only about one-fourth of patients.49 Nonspecific EEG abnormalities such as intermittent focal slowing are seen in many older people even without seizure.50 Also, normal findings on outpatient EEG do not rule out epilepsy, as EEG is normal in about one-third of patients with epilepsy, irrespective of age.1,49 Activation procedures such as hyperventilation and photic stimulation add little to the diagnosis in the elderly.49
On the other hand, video-EEG monitoring is an excellent tool for evaluating possible epilepsy, as it allows accurate assessment of brain electrical activity during the events in question. Moreover, studies of video-EEG recording of seizures in elderly patients demonstrated epileptiform discharges on EEG in 76% of clinical ictal events.50
Therefore, routine EEG is a useful screening tool, and inpatient video-EEG monitoring is the gold standard to characterize events of concern and distinguish between epileptic and nonepileptic or psychogenic seizures.
Other diagnostic studies
Brain imaging, preferably magnetic resonance imaging with contrast, should be done in every patient with possible epilepsy due to stroke, traumatic brain injury, or other structural brain disease.51
Electrocardiography helps exclude cardiac causes such as arrhythmia.
Blood testing. Metabolically provoked seizure can be distinguished by blood analysis for electrolytes, blood urea nitrogen, creatinine, glucose, calcium, magnesium, liver enzymes, and drug levels (eg, ethanol). A complete blood cell count with differential and platelets should also be done in anticipation of starting antiepileptic drug therapy.
Lumbar puncture for cell count, protein, glucose, stains, and cultures should be performed whenever meningitis or encephalitis is suspected.
A sleep study with concurrent video-EEG monitoring may be required to distinguish epileptic seizures from sleep disorders.
Neuropsychological testing may help account for the degree of cognitive impairment present.
Risk factors for stroke should be assessed in every elderly person who has new-onset seizures, because the risk of stroke is high.17
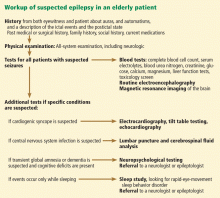
Figure 1 shows the workup for an elderly patient with suspected new-onset epilepsy.
TREATING EPILEPSY IN THE ELDERLY
Therapeutic challenges
Age-associated changes in drug absorption, protein binding, and distribution in body compartments require adjustments in drug selection and dosage. The causes and manifestations of these changes are typically multifactorial, mainly related to altered metabolism, declining plasma albumin concentrations, and increasing competition for protein binding by concomitantly used drugs.
The differences in the pharmacokinetics and pharmacodynamics of antiepileptic drugs depend on the patient’s physical status, relevant comorbidities, and concomitant medications.52 Renal and hepatic function may decline in an elderly patient; accordingly, precaution is needed in the prescribing and dosing of antiepileptic drugs.
Adverse effects from seizure medications are twice as common in elderly patients compared with younger patients. Ataxia, tremor, visual disturbance, and sedation are the most common.1 Antiepileptic drugs are also harmful to bone; induced abnormalities in bone metabolism include hypocalcemia, hypophosphatemia, decreased levels of active vitamin D metabolites, and hyperparathyroidism.53
Elderly patients tend to take multiple drugs, and some drugs can lower the seizure threshold, particularly antidepressants, anti-psychotics, and antibiotics.32 The herbal remedy ginkgo biloba can also precipitate seizure in this population.54
Antiepileptic drugs such as phenobarbital, primidone (Mysoline), phenytoin (Dilantin), and carbamazepine (Tegretol) can be broad-spectrum enzyme-inducers, increasing the metabolism of many drugs, including warfarin (Coumadin), cytotoxic agents, statins, cardiac antiarrhythmics, antihypertensives, corticosteroids, and other immunosuppressants.55 For example, carbamazepine can alter the metabolism of several hepatically metabolized drugs and cause significant hyponatremia. This is problematic in patients already taking sodium-depleting antihypertensives. Age-related cognitive decline can worsen the situation, often leading to misdiagnosis or patient noncompliance.
Table 3 profiles the interactions of commonly used antiepileptic drugs.
The ideal pharmacotherapy
No single drug is ideal for elderly patients with new-onset epilepsy. The choice mostly depends on the type of seizure and the patient’s comorbidities. The ideal antiepileptic drug would have minimal enzyme interaction, little protein binding, linear kinetics, a long half-life, a good safety profile, and a high therapeutic index. The goal of management should be to maintain the patient’s normal lifestyle with complete control of seizures and with minimal side effects.
The only randomized controlled trial in new-onset geriatric epilepsy concluded that gabapentin (Neurontin) and lamotrigine (Lamictal) should be the initial therapy in such patients.56 Trials indicate extended-release carbamazepine or levetiracetam (Keppra) can also be tried.57
The prescribing strategy includes lower initial dose, slower titration, and a lower target dose than for younger patients. Intense monitoring of dosing and drug levels is necessary to avoid toxicity. If the first drug is not tolerated well, another should be substituted. If seizures persist despite increasing dosage, a drug with a different mechanism of action should be tried.58 A patient with drug-resistant epilepsy (failure to respond to two adequate and appropriate antiepileptic drug trials59) should be referred to an epilepsy surgical center for reevaluation and consideration of epilepsy surgery.
Patient and caregiver support is an essential component of management. New-onset epilepsy in the elderly has a significant effect on quality of life, more so if the patient is already cognitively impaired. It erodes self-confidence, survival becomes difficult, and the condition is worse for patients who live alone. Driving restrictions further limit independence and increase isolation. Hence, psychological support programs can significantly boost the self-esteem and morale of such patients and their caregivers.
SPECIAL CONSIDERATION: EPILEPSY IN THE NURSING HOME
Certain points apply to the growing proportion of elderly who reside in nursing homes:
- Several studies in the United States and in Europe60–62 suggest that this subgroup is at higher risk of polypharmacy and more likely to be treated with older antiepileptic drugs.
- Only a minority of these patients (as low as 42% in one study60) received adequate monitoring of antiepileptic drug levels.
- The clinical characteristics and epileptic etiologies of these patients are less well defined.
Together, these observations highlight a particularly vulnerable population, at risk for medication toxicity as well as for undertreatment.
OUR KNOWLEDGE IS STILL GROWING
New-onset epilepsy, although common in the elderly, is difficult to diagnose because of its atypical presentation, concomitant cognitive impairment, and nonspecific abnormalities in routine investigations. But knowledge of its common causes and differential diagnoses makes the task easier. A high suspicion warrants referral to a neurologist or epileptologist.
Challenges to the management of seizures in the elderly include deranged physiologic processes, multiple comorbidities, and polypharmacy. No single drug is ideal for antiepileptic therapy in the elderly; the choice of drug is usually dictated by seizure type, comorbidities, and tolerance level. The treatment regimen in the elderly is more conservative, and the target dosage is lower than for younger adults. Emotional support of patient and caregivers should be an important aspect of management.
Our knowledge about new-onset epilepsy in the elderly is still growing, and future research should explore its diagnosis, treatment strategies, and care-delivery models.
- Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology 2004; 62(suppl 2):S24–S29.
- Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet 1990; 336:1267–1271.
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993; 34:453–468.
- Laccheo I, Ablah E, Heinrichs R, Sadler T, Baade L, Liow K. Assessment of quality of life among the elderly with epilepsy. Epilepsy Behav 2008; 12:257–261.
- Stephen LJ, Brodie MJ. Epilepsy in elderly people. Lancet 2000; 355:1441–1446.
- Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older US Medicare beneficiaries. Neurology 2012; 78:448–453.
- Sillanpää M, Lastunen S, Helenius H, Schmidt D. Regional differences and secular trends in the incidence of epilepsy in Finland: a nationwide 23-year registry study. Epilepsia 2011; 52:1857–1867.
- Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935–1984. Epilepsia 1995; 36:327–333.
- Lühdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia 1986; 27:458–463.
- Granger N, Convers P, Beauchet O, et al. First epileptic seizure in the elderly: electroclinical and etiological data in 341 patients [in French]. Rev Neurol (Paris) 2002; 158:1088–1095.
- Pugh MJ, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC. New-onset epilepsy risk factors in older veterans. J Am Geriatr Soc 2009; 57:237–242.
- Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol 2009; 8:1019–1030.
- Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57:1617–1622.
- Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol 1990; 47:157–160.
- Giroud M, Gras P, Fayolle H, André N, Soichot P, Dumas R. Early seizures after acute stroke: a study of 1,640 cases. Epilepsia 1994; 35:959–964.
- Asconapé JJ, Penry JK. Poststroke seizures in the elderly. Clin Geriatr Med 1991; 7:483–492.
- So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology 1996; 46:350–355.
- Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet 2004; 363:1184–1186.
- Loiseau P. Pathologic processes in the elderly and their association with seizures. In:Rowan AJ, Ramsay RE, editors. Seizures and epilepsy in the elderly. Boston, MA: Butterworth-Heinemann; 1997:63–86.
- Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology 1986; 36:1226–1230.
- Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci 2010; 1184:208–224.
- Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR. Seizures in patients with Alzheimer’s disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 2013; 54:700–707.
- Irizarry MC, Jin S, He F, et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol 2012; 69:368–372.
- Cordonnier C, Hénon H, Derambure P, Pasquier F, Leys D. Influence of pre-existing dementia on the risk of post-stroke epileptic seizures. J Neurol Neurosurg Psychiatry 2005; 76:1649–1653.
- Bruns J, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003; 44(suppl 10):2–10.
- Hiyoshi T, Yagi K. Epilepsy in the elderly. Epilepsia 2000; 41(suppl 9):31–35.
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009; 373:1105–1110.
- Roberts MA, Godfrey JW, Caird FI. Epileptic seizures in the elderly: I. Aetiology and type of seizure. Age Ageing 1982; 11:24–28.
- Loiseau J, Loiseau P, Duché B, Guyot M, Dartigues JF, Aublet B. A survey of epileptic disorders in southwest France: seizures in elderly patients. Ann Neurol 1990; 27:232–237.
- Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer 1998; 34:98–102.
- Franson KL, Hay DP, Neppe V, et al. Drug-induced seizures in the elderly. Causative agents and optimal management. Drugs Aging 1995; 7:38–48.
- Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, Kunkel S. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med 2009; 27:911–915.
- Hauser WA, Ng SK, Brust JC. Alcohol, seizures, and epilepsy. Epilepsia 1988; 29(suppl 2):S66–S78.
- Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014; 13:276–286.
- Ait S, Gilbert T, Cotton F, Bonnefoy M. Cortical blindness and posterior reversible encephalopathy syndrome in an older patient. BMJ Case Rep 2012;pii:bcr0920114782.
- Tinuper P, Provini F, Marini C, et al. Partial epilepsy of long duration: changing semiology with age. Epilepsia 1996; 37:162–164.
- Silveira DC, Jehi L, Chapin J, et al. Seizure semiology and aging. Epilepsy Behav 2011; 20:375–377.
- Theodore WH. The postictal state: effects of age and underlying brain dysfunction. Epilepsy Behav 2010; 19:118–120.
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998; 338:970–976.
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology 1998; 50:735–741.
- Sung CY, Chu NS. Status epilepticus in the elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989; 80:51–56.
- Pro S, Vicenzini E, Randi F, Pulitano P, Mecarelli O. Idiopathic late-onset absence status epilepticus: a case report with an electroclinical 14 years follow-up. Seizure 2011; 20:655–658.
- Martin Y, Artaz MA, Bornand-Rousselot A. Nonconvulsive status epilepticus in the elderly. J Am Geriatr Soc 2004; 52:476–477.
- Fernández-Torre JL, Díaz-Castroverde AG. Non-convulsive status epilepticus in elderly individuals: report of four representative cases. Age Ageing 2004; 33:78–81.
- Chung PW, Seo DW, Kwon JC, Kim H, Na DL. Nonconvulsive status epilepticus presenting as a subacute progressive aphasia. Seizure 2002; 11:449–454.
- Sheth RD, Drazkowski JF, Sirven JI, Gidal BE, Hermann BP. Protracted ictal confusion in elderly patients. Arch Neurol 2006; 63:529–532.
- Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003; 61:1066–1073.
- Kellinghaus C, Loddenkemper T, Dinner DS, Lachhwani D, Lüders HO. Seizure semiology in the elderly: a video analysis. Epilepsia 2004; 45:263–267.
- Drury I, Beydoun A. Interictal epileptiform activity in elderly patients with epilepsy. Electroencephalogr Clin Neurophysiol 1998; 106:369–373.
- McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia 2002; 43:165–169.
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet 2006; 367:1087–1100.
- McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004; 56:163–184.
- Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav 2004; 5(suppl 2):S24–S29.
- Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing 2001; 30:523–525.
- Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006; 61:246–255.
- Rowan AJ, Ramsay RE, Collins JF, et al; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 2005; 64:1868–1673.
- Garnett WR. Optimizing antiepileptic drug therapy in the elderly. Ann Pharmacother 2005; 39:1852–1860.
- Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology 2002; 58(suppl 5):S2–S8.
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51:1069–1077.
- Huying F, Klimpe S, Werhahn KJ. Antiepileptic drug use in nursing home residents: a cross-sectional, regional study. Seizure 2006; 15:194–197.
- Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia 1998; 39:1083–1087.
- Galimberti CA, Magri F, Magnani B, et al. Antiepileptic drug use and epileptic seizures in elderly nursing home residents: a survey in the province of Pavia, Northern Italy. Epilepsy Res 2006; 68:1–8.
Contrary to the popular belief that epilepsy is mainly a disease of youth, nearly 25% of new-onset seizures occur after age 65.1,2 The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.3 As our population ages, the burden of “elderly-onset” epilepsy will rise.
A seizure diagnosis carries significant implications in older people, who are already vulnerable to cognitive decline, loss of functional independence, driving restrictions, and risk of falls. Newly diagnosed epilepsy further worsens quality of life.4
The causes and clinical manifestations of seizures and epilepsy in the elderly differ from those in younger people.5 Hence, it is often difficult to make a diagnosis with certainty from a wide range of differential diagnoses. Older people are also more likely to have comorbidities, further complicating the situation.
Managing seizures in the elderly is also challenging, as age-associated physiologic changes can affect the pharmacokinetics and pharmacodynamics of antiepileptic drugs. Diagnosing and managing elderly-onset epilepsy can be challenging for a family physician, an internist, a geriatrician, or even a neurologist.
In this review, we emphasize the common causes of new-onset epilepsy in the elderly and the assessment of the clinical clues that are essential for making an accurate diagnosis. We also review the pharmacology of antiepileptic drugs used in old age and highlight the need for psychological support for patients and caregivers.
RISING PREVALENCE IN THE ELDERLY
In US Medicare beneficiaries age 65 and older, the average annual incidence rate of epilepsy in 2001 to 2005 was 10.8 per 1,000.6 A large study in Finland revealed falling incidence rates of epilepsy in childhood and middle age and rising trends in the elderly.7
In the United States, the rates are higher in African Americans (18.7 per 1,000) and lower in Asian Americans and Native Americans (5.5 and 7.7 per 1,000) than in whites (10.2 per 1,000).6 Incidence rates are slightly higher for women than for men and increase with age in both sexes and all racial groups.
Acute symptomatic seizure is also common in older patients. The incidence of acute seizures in patients over age 60 was estimated at 50 to 100 per 100,000 per year in one study.7 The rate was considerably higher in men than in women. The study also found a 3.6% risk of experiencing an acute symptomatic seizure in an 80-year lifespan, which approaches that of developing epilepsy.8 The major causes of acute symptomatic seizure were traumatic brain injury, cerebrovascular disease, drug withdrawal, and central nervous system infection.
CAUSES OF NEW-ONSET EPILEPSY IN THE ELDERLY
The most common causes of new-onset epilepsy in the elderly include cerebrovascular disease, metabolic disturbances, dementia, traumatic brain injury, tumors, and drugs.3,9–11
Cerebrovascular disease
In older adults, acute stroke is the most common cause, accounting for up to half of cases.5,12
Seizures occur in 4.4% to 8.9% of acute cerebrovascular events.13,14 The risk varies by stroke subtype, although all stroke subtypes, including transient ischemic attack, can be associated with seizure.15 For example, although 1% to 2% of patients experienced a seizure within 15 days of a transient ischemic attack or a lacunar infarct, this risk was 16.6% after an embolic stroke.15
Beyond this increased risk of “acute seizure” in the immediate poststroke period (usually defined as 1 week), the risk of epilepsy was also 20 times higher in the first year after a stroke.14 However, seizures tend to occur within the first 48 hours after the onset of ischemic stroke. In subarachnoid hemorrhage, seizures generally occur within hours.16
In a population-based study in Rochester, NY,17 epilepsy developed in two-thirds of patients with seizure related to acute stroke. Two factors that independently predicted the development of epilepsy were early seizure occurrence and recurrence of stroke.
Interestingly, the risk of stroke was three times higher in older patients who had new-onset seizure.18 Therefore, any elderly person with new-onset seizure should be assessed for cerebrovascular risk factors and treated accordingly for stroke prevention.
Metabolic disturbances
Acute metabolic disorders are common in elderly patients because of multiple comorbidities and polypharmacy. Hypoglycemia and hyponatremia need to be particularly considered in this population.19
Other well-documented metabolic causes of acute seizure, including nonketotic hyperglycemia, hypocalcemia, and uremic or hepatic encephalopathy, can all be considerations, albeit less specific to this age group.
Dementia
Primary neurodegenerative disorders associated with cognitive impairment, such as Alzheimer disease, are major risk factors for new-onset epilepsy in older patients.3,5 Seizures occur in about 10% of Alzheimer patients.20 Those who have brief periods of increased confusion may actually be experiencing unrecognized complex partial seizures.21
A case-control study discovered incidence rates of epilepsy almost 10 times higher in patients who had Alzheimer disease or vascular dementia than in nondemented patients.22 A prospective cohort study in patients with mild to moderate Alzheimer disease established that younger age, a greater degree of cognitive impairment, and a history of antipsychotic use were independent risk factors for new-onset seizures in the elderly.23 Preexisting dementia also increases the risk of poststroke epilepsy.24
Traumatic brain injury
The most common cause of brain trauma in the elderly is falls. Subdural hematoma, which can occur in the elderly with trivial trauma or sometimes even without it, needs to be considered. The risk of posttraumatic hemorrhage is especially relevant in patients taking anticoagulants.
Traumatic brain injury has a poorer prognosis in older people than in the young,25 and it accounts for up to 20% of cases of epilepsy in the elderly.26 Although no study has specifically addressed the longitudinal risk of epilepsy after traumatic brain injury in the elderly, a study in children and young adults revealed the risk was highest in the first year, with the increased risk persisting for more than 10 years.27
Brain tumors
Between 10% and 30% of new-onset seizures in the elderly are associated with tumor, typically glioma, meningioma, and brain metastasis.28,29 Seizures are usually associated more with primary than with secondary tumors, and more with low-grade tumors than high-grade ones.30
Drug-induced
Drugs and drug withdrawal can contribute to up to 10% of acute symptomatic seizures in the geriatric population.5,8,29 The elderly are susceptible to drug-induced seizure because of a higher prevalence of polypharmacy, impaired drug clearance, and heightened sensitivity to the proconvulsant side effects of medications.1 A number of commonly used drugs have been implicated,31 including:
Antibiotics such as carbapenems and high-dose penicillin
Antihistamines such as desloratadine (Clarinex)
Pain medications such as tramadol (Ul-tram) and high-dose opiates
Neuromodulators
Antidepressants such as clomipramine (Anafranil), maprotiline (Ludiomil), amoxapine (Asendin), and bupropion (Wellbutrin).32
Seizures also follow alcohol, benzodiazepine, and barbiturate withdrawal.33
Other causes
Paraneoplastic limbic encephalitis is a rare cause of seizures in the elderly.34 It can present with refractory seizures, confusion, and behavioral changes with or without a known concurrent neoplastic disease.
Posterior reversible leukoencephalopathy syndrome, another rare consideration, can particularly affect immunosuppressed elderly patients. This syndrome is characterized clinically by headache, confusion, seizures, vomiting, and visual disturbances with radiographic vasogenic edema.35
CLINICAL PRESENTATION
The signs and symptoms of a seizure may be atypical in the elderly. Seizures more often have a picture of “epileptic amnesia,” with confusion, sleepiness, or clumsiness, rather than motor manifestations such as tonic stiffening or automatism.36,37 Postictal states are also prolonged, particularly if there is underlying brain dysfunction.38 All these features render the clinical seizure manifestations more subtle and, as such, more difficult for the uninitiated caregiver to identify.
Convulsive and nonconvulsive status epilepticus
Status epilepticus is defined as a single generalized seizure lasting more than 5 minutes or a series of seizures lasting longer than 30 minutes without the patient’s regaining consciousness.39 The greatest increase in the incidence of status epilepticus occurs after age 60.40 It is the first seizure in about 30% of new-onset seizures in the elderly.41
Mortality rates increase with age, anoxia, and duration of status epilepticus and are over 50% in patients age 80 and older.40,42
Convulsive status epilepticus is most commonly caused by stroke.40
Absence status epilepticus can occur in elderly patients as a late complication of idiopathic generalized epilepsy related to benzodiazepine withdrawal, alcohol intoxication, or initiation of psychotropic drugs.42
Nonconvulsive status epilepticus manifests as altered mental status, psychosis, lethargy, or coma.42–44 Occasionally, it presents as a more focal cognitive disturbance with aphasia or a neglect syndrome.42,45 Electroencephalographic correlates of nonconvulsive status epilepticus include focal rhythmic discharges, often arising from frontal or temporal lobes, or generalized spike or sharp and slow-wave activity.46 Its management is challenging because of delayed diagnosis or misdiagnosis. The risk of death is higher in patients with severely impaired mental status or acute complications.47
Table 1 lists the typical seizure manifestations peculiar to the elderly.37,48
Differential diagnosis of new-onset epilepsy in the elderly
New-onset epilepsy in elderly patients can be confused with syncope, transient ischemic attack, cardiac arrhythmia, metabolic disturbances, transient global amnesia, neurodegenerative disease, rapid-eye-movement sleep behavior disorder, psychogenic disorders, and other conditions (Table 2). If there is a high clinical suspicion of seizure, the patient should undergo electroencephalography (EEG) and be referred to a neurologist or epileptologist.
KEYS TO THE DIAGNOSIS
Clinical history
A reliable history and description of the event from an eyewitness or a video recording of the event are invaluable to the diagnosis of epileptic seizure. Signs and symptoms that suggest the diagnosis include aura, ictal pallor, urinary incontinence, tongue-biting, and motor symptoms, as well as postictal confusion, drowsiness, and speech disturbance.
Electroencephalography
EEG is the most useful diagnostic tool in epilepsy. However, an interictal EEG reading (ie, between epileptic attacks) in an elderly patient has limited utility, showing epileptiform activity in only about one-fourth of patients.49 Nonspecific EEG abnormalities such as intermittent focal slowing are seen in many older people even without seizure.50 Also, normal findings on outpatient EEG do not rule out epilepsy, as EEG is normal in about one-third of patients with epilepsy, irrespective of age.1,49 Activation procedures such as hyperventilation and photic stimulation add little to the diagnosis in the elderly.49
On the other hand, video-EEG monitoring is an excellent tool for evaluating possible epilepsy, as it allows accurate assessment of brain electrical activity during the events in question. Moreover, studies of video-EEG recording of seizures in elderly patients demonstrated epileptiform discharges on EEG in 76% of clinical ictal events.50
Therefore, routine EEG is a useful screening tool, and inpatient video-EEG monitoring is the gold standard to characterize events of concern and distinguish between epileptic and nonepileptic or psychogenic seizures.
Other diagnostic studies
Brain imaging, preferably magnetic resonance imaging with contrast, should be done in every patient with possible epilepsy due to stroke, traumatic brain injury, or other structural brain disease.51
Electrocardiography helps exclude cardiac causes such as arrhythmia.
Blood testing. Metabolically provoked seizure can be distinguished by blood analysis for electrolytes, blood urea nitrogen, creatinine, glucose, calcium, magnesium, liver enzymes, and drug levels (eg, ethanol). A complete blood cell count with differential and platelets should also be done in anticipation of starting antiepileptic drug therapy.
Lumbar puncture for cell count, protein, glucose, stains, and cultures should be performed whenever meningitis or encephalitis is suspected.
A sleep study with concurrent video-EEG monitoring may be required to distinguish epileptic seizures from sleep disorders.
Neuropsychological testing may help account for the degree of cognitive impairment present.
Risk factors for stroke should be assessed in every elderly person who has new-onset seizures, because the risk of stroke is high.17
Figure 1 shows the workup for an elderly patient with suspected new-onset epilepsy.
TREATING EPILEPSY IN THE ELDERLY
Therapeutic challenges
Age-associated changes in drug absorption, protein binding, and distribution in body compartments require adjustments in drug selection and dosage. The causes and manifestations of these changes are typically multifactorial, mainly related to altered metabolism, declining plasma albumin concentrations, and increasing competition for protein binding by concomitantly used drugs.
The differences in the pharmacokinetics and pharmacodynamics of antiepileptic drugs depend on the patient’s physical status, relevant comorbidities, and concomitant medications.52 Renal and hepatic function may decline in an elderly patient; accordingly, precaution is needed in the prescribing and dosing of antiepileptic drugs.
Adverse effects from seizure medications are twice as common in elderly patients compared with younger patients. Ataxia, tremor, visual disturbance, and sedation are the most common.1 Antiepileptic drugs are also harmful to bone; induced abnormalities in bone metabolism include hypocalcemia, hypophosphatemia, decreased levels of active vitamin D metabolites, and hyperparathyroidism.53
Elderly patients tend to take multiple drugs, and some drugs can lower the seizure threshold, particularly antidepressants, anti-psychotics, and antibiotics.32 The herbal remedy ginkgo biloba can also precipitate seizure in this population.54
Antiepileptic drugs such as phenobarbital, primidone (Mysoline), phenytoin (Dilantin), and carbamazepine (Tegretol) can be broad-spectrum enzyme-inducers, increasing the metabolism of many drugs, including warfarin (Coumadin), cytotoxic agents, statins, cardiac antiarrhythmics, antihypertensives, corticosteroids, and other immunosuppressants.55 For example, carbamazepine can alter the metabolism of several hepatically metabolized drugs and cause significant hyponatremia. This is problematic in patients already taking sodium-depleting antihypertensives. Age-related cognitive decline can worsen the situation, often leading to misdiagnosis or patient noncompliance.
Table 3 profiles the interactions of commonly used antiepileptic drugs.
The ideal pharmacotherapy
No single drug is ideal for elderly patients with new-onset epilepsy. The choice mostly depends on the type of seizure and the patient’s comorbidities. The ideal antiepileptic drug would have minimal enzyme interaction, little protein binding, linear kinetics, a long half-life, a good safety profile, and a high therapeutic index. The goal of management should be to maintain the patient’s normal lifestyle with complete control of seizures and with minimal side effects.
The only randomized controlled trial in new-onset geriatric epilepsy concluded that gabapentin (Neurontin) and lamotrigine (Lamictal) should be the initial therapy in such patients.56 Trials indicate extended-release carbamazepine or levetiracetam (Keppra) can also be tried.57
The prescribing strategy includes lower initial dose, slower titration, and a lower target dose than for younger patients. Intense monitoring of dosing and drug levels is necessary to avoid toxicity. If the first drug is not tolerated well, another should be substituted. If seizures persist despite increasing dosage, a drug with a different mechanism of action should be tried.58 A patient with drug-resistant epilepsy (failure to respond to two adequate and appropriate antiepileptic drug trials59) should be referred to an epilepsy surgical center for reevaluation and consideration of epilepsy surgery.
Patient and caregiver support is an essential component of management. New-onset epilepsy in the elderly has a significant effect on quality of life, more so if the patient is already cognitively impaired. It erodes self-confidence, survival becomes difficult, and the condition is worse for patients who live alone. Driving restrictions further limit independence and increase isolation. Hence, psychological support programs can significantly boost the self-esteem and morale of such patients and their caregivers.
SPECIAL CONSIDERATION: EPILEPSY IN THE NURSING HOME
Certain points apply to the growing proportion of elderly who reside in nursing homes:
- Several studies in the United States and in Europe60–62 suggest that this subgroup is at higher risk of polypharmacy and more likely to be treated with older antiepileptic drugs.
- Only a minority of these patients (as low as 42% in one study60) received adequate monitoring of antiepileptic drug levels.
- The clinical characteristics and epileptic etiologies of these patients are less well defined.
Together, these observations highlight a particularly vulnerable population, at risk for medication toxicity as well as for undertreatment.
OUR KNOWLEDGE IS STILL GROWING
New-onset epilepsy, although common in the elderly, is difficult to diagnose because of its atypical presentation, concomitant cognitive impairment, and nonspecific abnormalities in routine investigations. But knowledge of its common causes and differential diagnoses makes the task easier. A high suspicion warrants referral to a neurologist or epileptologist.
Challenges to the management of seizures in the elderly include deranged physiologic processes, multiple comorbidities, and polypharmacy. No single drug is ideal for antiepileptic therapy in the elderly; the choice of drug is usually dictated by seizure type, comorbidities, and tolerance level. The treatment regimen in the elderly is more conservative, and the target dosage is lower than for younger adults. Emotional support of patient and caregivers should be an important aspect of management.
Our knowledge about new-onset epilepsy in the elderly is still growing, and future research should explore its diagnosis, treatment strategies, and care-delivery models.
Contrary to the popular belief that epilepsy is mainly a disease of youth, nearly 25% of new-onset seizures occur after age 65.1,2 The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.3 As our population ages, the burden of “elderly-onset” epilepsy will rise.
A seizure diagnosis carries significant implications in older people, who are already vulnerable to cognitive decline, loss of functional independence, driving restrictions, and risk of falls. Newly diagnosed epilepsy further worsens quality of life.4
The causes and clinical manifestations of seizures and epilepsy in the elderly differ from those in younger people.5 Hence, it is often difficult to make a diagnosis with certainty from a wide range of differential diagnoses. Older people are also more likely to have comorbidities, further complicating the situation.
Managing seizures in the elderly is also challenging, as age-associated physiologic changes can affect the pharmacokinetics and pharmacodynamics of antiepileptic drugs. Diagnosing and managing elderly-onset epilepsy can be challenging for a family physician, an internist, a geriatrician, or even a neurologist.
In this review, we emphasize the common causes of new-onset epilepsy in the elderly and the assessment of the clinical clues that are essential for making an accurate diagnosis. We also review the pharmacology of antiepileptic drugs used in old age and highlight the need for psychological support for patients and caregivers.
RISING PREVALENCE IN THE ELDERLY
In US Medicare beneficiaries age 65 and older, the average annual incidence rate of epilepsy in 2001 to 2005 was 10.8 per 1,000.6 A large study in Finland revealed falling incidence rates of epilepsy in childhood and middle age and rising trends in the elderly.7
In the United States, the rates are higher in African Americans (18.7 per 1,000) and lower in Asian Americans and Native Americans (5.5 and 7.7 per 1,000) than in whites (10.2 per 1,000).6 Incidence rates are slightly higher for women than for men and increase with age in both sexes and all racial groups.
Acute symptomatic seizure is also common in older patients. The incidence of acute seizures in patients over age 60 was estimated at 50 to 100 per 100,000 per year in one study.7 The rate was considerably higher in men than in women. The study also found a 3.6% risk of experiencing an acute symptomatic seizure in an 80-year lifespan, which approaches that of developing epilepsy.8 The major causes of acute symptomatic seizure were traumatic brain injury, cerebrovascular disease, drug withdrawal, and central nervous system infection.
CAUSES OF NEW-ONSET EPILEPSY IN THE ELDERLY
The most common causes of new-onset epilepsy in the elderly include cerebrovascular disease, metabolic disturbances, dementia, traumatic brain injury, tumors, and drugs.3,9–11
Cerebrovascular disease
In older adults, acute stroke is the most common cause, accounting for up to half of cases.5,12
Seizures occur in 4.4% to 8.9% of acute cerebrovascular events.13,14 The risk varies by stroke subtype, although all stroke subtypes, including transient ischemic attack, can be associated with seizure.15 For example, although 1% to 2% of patients experienced a seizure within 15 days of a transient ischemic attack or a lacunar infarct, this risk was 16.6% after an embolic stroke.15
Beyond this increased risk of “acute seizure” in the immediate poststroke period (usually defined as 1 week), the risk of epilepsy was also 20 times higher in the first year after a stroke.14 However, seizures tend to occur within the first 48 hours after the onset of ischemic stroke. In subarachnoid hemorrhage, seizures generally occur within hours.16
In a population-based study in Rochester, NY,17 epilepsy developed in two-thirds of patients with seizure related to acute stroke. Two factors that independently predicted the development of epilepsy were early seizure occurrence and recurrence of stroke.
Interestingly, the risk of stroke was three times higher in older patients who had new-onset seizure.18 Therefore, any elderly person with new-onset seizure should be assessed for cerebrovascular risk factors and treated accordingly for stroke prevention.
Metabolic disturbances
Acute metabolic disorders are common in elderly patients because of multiple comorbidities and polypharmacy. Hypoglycemia and hyponatremia need to be particularly considered in this population.19
Other well-documented metabolic causes of acute seizure, including nonketotic hyperglycemia, hypocalcemia, and uremic or hepatic encephalopathy, can all be considerations, albeit less specific to this age group.
Dementia
Primary neurodegenerative disorders associated with cognitive impairment, such as Alzheimer disease, are major risk factors for new-onset epilepsy in older patients.3,5 Seizures occur in about 10% of Alzheimer patients.20 Those who have brief periods of increased confusion may actually be experiencing unrecognized complex partial seizures.21
A case-control study discovered incidence rates of epilepsy almost 10 times higher in patients who had Alzheimer disease or vascular dementia than in nondemented patients.22 A prospective cohort study in patients with mild to moderate Alzheimer disease established that younger age, a greater degree of cognitive impairment, and a history of antipsychotic use were independent risk factors for new-onset seizures in the elderly.23 Preexisting dementia also increases the risk of poststroke epilepsy.24
Traumatic brain injury
The most common cause of brain trauma in the elderly is falls. Subdural hematoma, which can occur in the elderly with trivial trauma or sometimes even without it, needs to be considered. The risk of posttraumatic hemorrhage is especially relevant in patients taking anticoagulants.
Traumatic brain injury has a poorer prognosis in older people than in the young,25 and it accounts for up to 20% of cases of epilepsy in the elderly.26 Although no study has specifically addressed the longitudinal risk of epilepsy after traumatic brain injury in the elderly, a study in children and young adults revealed the risk was highest in the first year, with the increased risk persisting for more than 10 years.27
Brain tumors
Between 10% and 30% of new-onset seizures in the elderly are associated with tumor, typically glioma, meningioma, and brain metastasis.28,29 Seizures are usually associated more with primary than with secondary tumors, and more with low-grade tumors than high-grade ones.30
Drug-induced
Drugs and drug withdrawal can contribute to up to 10% of acute symptomatic seizures in the geriatric population.5,8,29 The elderly are susceptible to drug-induced seizure because of a higher prevalence of polypharmacy, impaired drug clearance, and heightened sensitivity to the proconvulsant side effects of medications.1 A number of commonly used drugs have been implicated,31 including:
Antibiotics such as carbapenems and high-dose penicillin
Antihistamines such as desloratadine (Clarinex)
Pain medications such as tramadol (Ul-tram) and high-dose opiates
Neuromodulators
Antidepressants such as clomipramine (Anafranil), maprotiline (Ludiomil), amoxapine (Asendin), and bupropion (Wellbutrin).32
Seizures also follow alcohol, benzodiazepine, and barbiturate withdrawal.33
Other causes
Paraneoplastic limbic encephalitis is a rare cause of seizures in the elderly.34 It can present with refractory seizures, confusion, and behavioral changes with or without a known concurrent neoplastic disease.
Posterior reversible leukoencephalopathy syndrome, another rare consideration, can particularly affect immunosuppressed elderly patients. This syndrome is characterized clinically by headache, confusion, seizures, vomiting, and visual disturbances with radiographic vasogenic edema.35
CLINICAL PRESENTATION
The signs and symptoms of a seizure may be atypical in the elderly. Seizures more often have a picture of “epileptic amnesia,” with confusion, sleepiness, or clumsiness, rather than motor manifestations such as tonic stiffening or automatism.36,37 Postictal states are also prolonged, particularly if there is underlying brain dysfunction.38 All these features render the clinical seizure manifestations more subtle and, as such, more difficult for the uninitiated caregiver to identify.
Convulsive and nonconvulsive status epilepticus
Status epilepticus is defined as a single generalized seizure lasting more than 5 minutes or a series of seizures lasting longer than 30 minutes without the patient’s regaining consciousness.39 The greatest increase in the incidence of status epilepticus occurs after age 60.40 It is the first seizure in about 30% of new-onset seizures in the elderly.41
Mortality rates increase with age, anoxia, and duration of status epilepticus and are over 50% in patients age 80 and older.40,42
Convulsive status epilepticus is most commonly caused by stroke.40
Absence status epilepticus can occur in elderly patients as a late complication of idiopathic generalized epilepsy related to benzodiazepine withdrawal, alcohol intoxication, or initiation of psychotropic drugs.42
Nonconvulsive status epilepticus manifests as altered mental status, psychosis, lethargy, or coma.42–44 Occasionally, it presents as a more focal cognitive disturbance with aphasia or a neglect syndrome.42,45 Electroencephalographic correlates of nonconvulsive status epilepticus include focal rhythmic discharges, often arising from frontal or temporal lobes, or generalized spike or sharp and slow-wave activity.46 Its management is challenging because of delayed diagnosis or misdiagnosis. The risk of death is higher in patients with severely impaired mental status or acute complications.47
Table 1 lists the typical seizure manifestations peculiar to the elderly.37,48
Differential diagnosis of new-onset epilepsy in the elderly
New-onset epilepsy in elderly patients can be confused with syncope, transient ischemic attack, cardiac arrhythmia, metabolic disturbances, transient global amnesia, neurodegenerative disease, rapid-eye-movement sleep behavior disorder, psychogenic disorders, and other conditions (Table 2). If there is a high clinical suspicion of seizure, the patient should undergo electroencephalography (EEG) and be referred to a neurologist or epileptologist.
KEYS TO THE DIAGNOSIS
Clinical history
A reliable history and description of the event from an eyewitness or a video recording of the event are invaluable to the diagnosis of epileptic seizure. Signs and symptoms that suggest the diagnosis include aura, ictal pallor, urinary incontinence, tongue-biting, and motor symptoms, as well as postictal confusion, drowsiness, and speech disturbance.
Electroencephalography
EEG is the most useful diagnostic tool in epilepsy. However, an interictal EEG reading (ie, between epileptic attacks) in an elderly patient has limited utility, showing epileptiform activity in only about one-fourth of patients.49 Nonspecific EEG abnormalities such as intermittent focal slowing are seen in many older people even without seizure.50 Also, normal findings on outpatient EEG do not rule out epilepsy, as EEG is normal in about one-third of patients with epilepsy, irrespective of age.1,49 Activation procedures such as hyperventilation and photic stimulation add little to the diagnosis in the elderly.49
On the other hand, video-EEG monitoring is an excellent tool for evaluating possible epilepsy, as it allows accurate assessment of brain electrical activity during the events in question. Moreover, studies of video-EEG recording of seizures in elderly patients demonstrated epileptiform discharges on EEG in 76% of clinical ictal events.50
Therefore, routine EEG is a useful screening tool, and inpatient video-EEG monitoring is the gold standard to characterize events of concern and distinguish between epileptic and nonepileptic or psychogenic seizures.
Other diagnostic studies
Brain imaging, preferably magnetic resonance imaging with contrast, should be done in every patient with possible epilepsy due to stroke, traumatic brain injury, or other structural brain disease.51
Electrocardiography helps exclude cardiac causes such as arrhythmia.
Blood testing. Metabolically provoked seizure can be distinguished by blood analysis for electrolytes, blood urea nitrogen, creatinine, glucose, calcium, magnesium, liver enzymes, and drug levels (eg, ethanol). A complete blood cell count with differential and platelets should also be done in anticipation of starting antiepileptic drug therapy.
Lumbar puncture for cell count, protein, glucose, stains, and cultures should be performed whenever meningitis or encephalitis is suspected.
A sleep study with concurrent video-EEG monitoring may be required to distinguish epileptic seizures from sleep disorders.
Neuropsychological testing may help account for the degree of cognitive impairment present.
Risk factors for stroke should be assessed in every elderly person who has new-onset seizures, because the risk of stroke is high.17
Figure 1 shows the workup for an elderly patient with suspected new-onset epilepsy.
TREATING EPILEPSY IN THE ELDERLY
Therapeutic challenges
Age-associated changes in drug absorption, protein binding, and distribution in body compartments require adjustments in drug selection and dosage. The causes and manifestations of these changes are typically multifactorial, mainly related to altered metabolism, declining plasma albumin concentrations, and increasing competition for protein binding by concomitantly used drugs.
The differences in the pharmacokinetics and pharmacodynamics of antiepileptic drugs depend on the patient’s physical status, relevant comorbidities, and concomitant medications.52 Renal and hepatic function may decline in an elderly patient; accordingly, precaution is needed in the prescribing and dosing of antiepileptic drugs.
Adverse effects from seizure medications are twice as common in elderly patients compared with younger patients. Ataxia, tremor, visual disturbance, and sedation are the most common.1 Antiepileptic drugs are also harmful to bone; induced abnormalities in bone metabolism include hypocalcemia, hypophosphatemia, decreased levels of active vitamin D metabolites, and hyperparathyroidism.53
Elderly patients tend to take multiple drugs, and some drugs can lower the seizure threshold, particularly antidepressants, anti-psychotics, and antibiotics.32 The herbal remedy ginkgo biloba can also precipitate seizure in this population.54
Antiepileptic drugs such as phenobarbital, primidone (Mysoline), phenytoin (Dilantin), and carbamazepine (Tegretol) can be broad-spectrum enzyme-inducers, increasing the metabolism of many drugs, including warfarin (Coumadin), cytotoxic agents, statins, cardiac antiarrhythmics, antihypertensives, corticosteroids, and other immunosuppressants.55 For example, carbamazepine can alter the metabolism of several hepatically metabolized drugs and cause significant hyponatremia. This is problematic in patients already taking sodium-depleting antihypertensives. Age-related cognitive decline can worsen the situation, often leading to misdiagnosis or patient noncompliance.
Table 3 profiles the interactions of commonly used antiepileptic drugs.
The ideal pharmacotherapy
No single drug is ideal for elderly patients with new-onset epilepsy. The choice mostly depends on the type of seizure and the patient’s comorbidities. The ideal antiepileptic drug would have minimal enzyme interaction, little protein binding, linear kinetics, a long half-life, a good safety profile, and a high therapeutic index. The goal of management should be to maintain the patient’s normal lifestyle with complete control of seizures and with minimal side effects.
The only randomized controlled trial in new-onset geriatric epilepsy concluded that gabapentin (Neurontin) and lamotrigine (Lamictal) should be the initial therapy in such patients.56 Trials indicate extended-release carbamazepine or levetiracetam (Keppra) can also be tried.57
The prescribing strategy includes lower initial dose, slower titration, and a lower target dose than for younger patients. Intense monitoring of dosing and drug levels is necessary to avoid toxicity. If the first drug is not tolerated well, another should be substituted. If seizures persist despite increasing dosage, a drug with a different mechanism of action should be tried.58 A patient with drug-resistant epilepsy (failure to respond to two adequate and appropriate antiepileptic drug trials59) should be referred to an epilepsy surgical center for reevaluation and consideration of epilepsy surgery.
Patient and caregiver support is an essential component of management. New-onset epilepsy in the elderly has a significant effect on quality of life, more so if the patient is already cognitively impaired. It erodes self-confidence, survival becomes difficult, and the condition is worse for patients who live alone. Driving restrictions further limit independence and increase isolation. Hence, psychological support programs can significantly boost the self-esteem and morale of such patients and their caregivers.
SPECIAL CONSIDERATION: EPILEPSY IN THE NURSING HOME
Certain points apply to the growing proportion of elderly who reside in nursing homes:
- Several studies in the United States and in Europe60–62 suggest that this subgroup is at higher risk of polypharmacy and more likely to be treated with older antiepileptic drugs.
- Only a minority of these patients (as low as 42% in one study60) received adequate monitoring of antiepileptic drug levels.
- The clinical characteristics and epileptic etiologies of these patients are less well defined.
Together, these observations highlight a particularly vulnerable population, at risk for medication toxicity as well as for undertreatment.
OUR KNOWLEDGE IS STILL GROWING
New-onset epilepsy, although common in the elderly, is difficult to diagnose because of its atypical presentation, concomitant cognitive impairment, and nonspecific abnormalities in routine investigations. But knowledge of its common causes and differential diagnoses makes the task easier. A high suspicion warrants referral to a neurologist or epileptologist.
Challenges to the management of seizures in the elderly include deranged physiologic processes, multiple comorbidities, and polypharmacy. No single drug is ideal for antiepileptic therapy in the elderly; the choice of drug is usually dictated by seizure type, comorbidities, and tolerance level. The treatment regimen in the elderly is more conservative, and the target dosage is lower than for younger adults. Emotional support of patient and caregivers should be an important aspect of management.
Our knowledge about new-onset epilepsy in the elderly is still growing, and future research should explore its diagnosis, treatment strategies, and care-delivery models.
- Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology 2004; 62(suppl 2):S24–S29.
- Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet 1990; 336:1267–1271.
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993; 34:453–468.
- Laccheo I, Ablah E, Heinrichs R, Sadler T, Baade L, Liow K. Assessment of quality of life among the elderly with epilepsy. Epilepsy Behav 2008; 12:257–261.
- Stephen LJ, Brodie MJ. Epilepsy in elderly people. Lancet 2000; 355:1441–1446.
- Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older US Medicare beneficiaries. Neurology 2012; 78:448–453.
- Sillanpää M, Lastunen S, Helenius H, Schmidt D. Regional differences and secular trends in the incidence of epilepsy in Finland: a nationwide 23-year registry study. Epilepsia 2011; 52:1857–1867.
- Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935–1984. Epilepsia 1995; 36:327–333.
- Lühdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia 1986; 27:458–463.
- Granger N, Convers P, Beauchet O, et al. First epileptic seizure in the elderly: electroclinical and etiological data in 341 patients [in French]. Rev Neurol (Paris) 2002; 158:1088–1095.
- Pugh MJ, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC. New-onset epilepsy risk factors in older veterans. J Am Geriatr Soc 2009; 57:237–242.
- Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol 2009; 8:1019–1030.
- Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57:1617–1622.
- Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol 1990; 47:157–160.
- Giroud M, Gras P, Fayolle H, André N, Soichot P, Dumas R. Early seizures after acute stroke: a study of 1,640 cases. Epilepsia 1994; 35:959–964.
- Asconapé JJ, Penry JK. Poststroke seizures in the elderly. Clin Geriatr Med 1991; 7:483–492.
- So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology 1996; 46:350–355.
- Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet 2004; 363:1184–1186.
- Loiseau P. Pathologic processes in the elderly and their association with seizures. In:Rowan AJ, Ramsay RE, editors. Seizures and epilepsy in the elderly. Boston, MA: Butterworth-Heinemann; 1997:63–86.
- Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology 1986; 36:1226–1230.
- Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci 2010; 1184:208–224.
- Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR. Seizures in patients with Alzheimer’s disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 2013; 54:700–707.
- Irizarry MC, Jin S, He F, et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol 2012; 69:368–372.
- Cordonnier C, Hénon H, Derambure P, Pasquier F, Leys D. Influence of pre-existing dementia on the risk of post-stroke epileptic seizures. J Neurol Neurosurg Psychiatry 2005; 76:1649–1653.
- Bruns J, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003; 44(suppl 10):2–10.
- Hiyoshi T, Yagi K. Epilepsy in the elderly. Epilepsia 2000; 41(suppl 9):31–35.
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009; 373:1105–1110.
- Roberts MA, Godfrey JW, Caird FI. Epileptic seizures in the elderly: I. Aetiology and type of seizure. Age Ageing 1982; 11:24–28.
- Loiseau J, Loiseau P, Duché B, Guyot M, Dartigues JF, Aublet B. A survey of epileptic disorders in southwest France: seizures in elderly patients. Ann Neurol 1990; 27:232–237.
- Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer 1998; 34:98–102.
- Franson KL, Hay DP, Neppe V, et al. Drug-induced seizures in the elderly. Causative agents and optimal management. Drugs Aging 1995; 7:38–48.
- Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, Kunkel S. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med 2009; 27:911–915.
- Hauser WA, Ng SK, Brust JC. Alcohol, seizures, and epilepsy. Epilepsia 1988; 29(suppl 2):S66–S78.
- Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014; 13:276–286.
- Ait S, Gilbert T, Cotton F, Bonnefoy M. Cortical blindness and posterior reversible encephalopathy syndrome in an older patient. BMJ Case Rep 2012;pii:bcr0920114782.
- Tinuper P, Provini F, Marini C, et al. Partial epilepsy of long duration: changing semiology with age. Epilepsia 1996; 37:162–164.
- Silveira DC, Jehi L, Chapin J, et al. Seizure semiology and aging. Epilepsy Behav 2011; 20:375–377.
- Theodore WH. The postictal state: effects of age and underlying brain dysfunction. Epilepsy Behav 2010; 19:118–120.
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998; 338:970–976.
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology 1998; 50:735–741.
- Sung CY, Chu NS. Status epilepticus in the elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989; 80:51–56.
- Pro S, Vicenzini E, Randi F, Pulitano P, Mecarelli O. Idiopathic late-onset absence status epilepticus: a case report with an electroclinical 14 years follow-up. Seizure 2011; 20:655–658.
- Martin Y, Artaz MA, Bornand-Rousselot A. Nonconvulsive status epilepticus in the elderly. J Am Geriatr Soc 2004; 52:476–477.
- Fernández-Torre JL, Díaz-Castroverde AG. Non-convulsive status epilepticus in elderly individuals: report of four representative cases. Age Ageing 2004; 33:78–81.
- Chung PW, Seo DW, Kwon JC, Kim H, Na DL. Nonconvulsive status epilepticus presenting as a subacute progressive aphasia. Seizure 2002; 11:449–454.
- Sheth RD, Drazkowski JF, Sirven JI, Gidal BE, Hermann BP. Protracted ictal confusion in elderly patients. Arch Neurol 2006; 63:529–532.
- Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003; 61:1066–1073.
- Kellinghaus C, Loddenkemper T, Dinner DS, Lachhwani D, Lüders HO. Seizure semiology in the elderly: a video analysis. Epilepsia 2004; 45:263–267.
- Drury I, Beydoun A. Interictal epileptiform activity in elderly patients with epilepsy. Electroencephalogr Clin Neurophysiol 1998; 106:369–373.
- McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia 2002; 43:165–169.
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet 2006; 367:1087–1100.
- McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004; 56:163–184.
- Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav 2004; 5(suppl 2):S24–S29.
- Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing 2001; 30:523–525.
- Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006; 61:246–255.
- Rowan AJ, Ramsay RE, Collins JF, et al; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 2005; 64:1868–1673.
- Garnett WR. Optimizing antiepileptic drug therapy in the elderly. Ann Pharmacother 2005; 39:1852–1860.
- Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology 2002; 58(suppl 5):S2–S8.
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51:1069–1077.
- Huying F, Klimpe S, Werhahn KJ. Antiepileptic drug use in nursing home residents: a cross-sectional, regional study. Seizure 2006; 15:194–197.
- Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia 1998; 39:1083–1087.
- Galimberti CA, Magri F, Magnani B, et al. Antiepileptic drug use and epileptic seizures in elderly nursing home residents: a survey in the province of Pavia, Northern Italy. Epilepsy Res 2006; 68:1–8.
- Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology 2004; 62(suppl 2):S24–S29.
- Sander JW, Hart YM, Johnson AL, Shorvon SD. National General Practice Study of Epilepsy: newly diagnosed epileptic seizures in a general population. Lancet 1990; 336:1267–1271.
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993; 34:453–468.
- Laccheo I, Ablah E, Heinrichs R, Sadler T, Baade L, Liow K. Assessment of quality of life among the elderly with epilepsy. Epilepsy Behav 2008; 12:257–261.
- Stephen LJ, Brodie MJ. Epilepsy in elderly people. Lancet 2000; 355:1441–1446.
- Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older US Medicare beneficiaries. Neurology 2012; 78:448–453.
- Sillanpää M, Lastunen S, Helenius H, Schmidt D. Regional differences and secular trends in the incidence of epilepsy in Finland: a nationwide 23-year registry study. Epilepsia 2011; 52:1857–1867.
- Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935–1984. Epilepsia 1995; 36:327–333.
- Lühdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia 1986; 27:458–463.
- Granger N, Convers P, Beauchet O, et al. First epileptic seizure in the elderly: electroclinical and etiological data in 341 patients [in French]. Rev Neurol (Paris) 2002; 158:1088–1095.
- Pugh MJ, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC. New-onset epilepsy risk factors in older veterans. J Am Geriatr Soc 2009; 57:237–242.
- Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol 2009; 8:1019–1030.
- Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57:1617–1622.
- Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol 1990; 47:157–160.
- Giroud M, Gras P, Fayolle H, André N, Soichot P, Dumas R. Early seizures after acute stroke: a study of 1,640 cases. Epilepsia 1994; 35:959–964.
- Asconapé JJ, Penry JK. Poststroke seizures in the elderly. Clin Geriatr Med 1991; 7:483–492.
- So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology 1996; 46:350–355.
- Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet 2004; 363:1184–1186.
- Loiseau P. Pathologic processes in the elderly and their association with seizures. In:Rowan AJ, Ramsay RE, editors. Seizures and epilepsy in the elderly. Boston, MA: Butterworth-Heinemann; 1997:63–86.
- Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology 1986; 36:1226–1230.
- Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci 2010; 1184:208–224.
- Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR. Seizures in patients with Alzheimer’s disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 2013; 54:700–707.
- Irizarry MC, Jin S, He F, et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol 2012; 69:368–372.
- Cordonnier C, Hénon H, Derambure P, Pasquier F, Leys D. Influence of pre-existing dementia on the risk of post-stroke epileptic seizures. J Neurol Neurosurg Psychiatry 2005; 76:1649–1653.
- Bruns J, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003; 44(suppl 10):2–10.
- Hiyoshi T, Yagi K. Epilepsy in the elderly. Epilepsia 2000; 41(suppl 9):31–35.
- Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 2009; 373:1105–1110.
- Roberts MA, Godfrey JW, Caird FI. Epileptic seizures in the elderly: I. Aetiology and type of seizure. Age Ageing 1982; 11:24–28.
- Loiseau J, Loiseau P, Duché B, Guyot M, Dartigues JF, Aublet B. A survey of epileptic disorders in southwest France: seizures in elderly patients. Ann Neurol 1990; 27:232–237.
- Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer 1998; 34:98–102.
- Franson KL, Hay DP, Neppe V, et al. Drug-induced seizures in the elderly. Causative agents and optimal management. Drugs Aging 1995; 7:38–48.
- Starr P, Klein-Schwartz W, Spiller H, Kern P, Ekleberry SE, Kunkel S. Incidence and onset of delayed seizures after overdoses of extended-release bupropion. Am J Emerg Med 2009; 27:911–915.
- Hauser WA, Ng SK, Brust JC. Alcohol, seizures, and epilepsy. Epilepsia 1988; 29(suppl 2):S66–S78.
- Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014; 13:276–286.
- Ait S, Gilbert T, Cotton F, Bonnefoy M. Cortical blindness and posterior reversible encephalopathy syndrome in an older patient. BMJ Case Rep 2012;pii:bcr0920114782.
- Tinuper P, Provini F, Marini C, et al. Partial epilepsy of long duration: changing semiology with age. Epilepsia 1996; 37:162–164.
- Silveira DC, Jehi L, Chapin J, et al. Seizure semiology and aging. Epilepsy Behav 2011; 20:375–377.
- Theodore WH. The postictal state: effects of age and underlying brain dysfunction. Epilepsy Behav 2010; 19:118–120.
- Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med 1998; 338:970–976.
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology 1998; 50:735–741.
- Sung CY, Chu NS. Status epilepticus in the elderly: etiology, seizure type and outcome. Acta Neurol Scand 1989; 80:51–56.
- Pro S, Vicenzini E, Randi F, Pulitano P, Mecarelli O. Idiopathic late-onset absence status epilepticus: a case report with an electroclinical 14 years follow-up. Seizure 2011; 20:655–658.
- Martin Y, Artaz MA, Bornand-Rousselot A. Nonconvulsive status epilepticus in the elderly. J Am Geriatr Soc 2004; 52:476–477.
- Fernández-Torre JL, Díaz-Castroverde AG. Non-convulsive status epilepticus in elderly individuals: report of four representative cases. Age Ageing 2004; 33:78–81.
- Chung PW, Seo DW, Kwon JC, Kim H, Na DL. Nonconvulsive status epilepticus presenting as a subacute progressive aphasia. Seizure 2002; 11:449–454.
- Sheth RD, Drazkowski JF, Sirven JI, Gidal BE, Hermann BP. Protracted ictal confusion in elderly patients. Arch Neurol 2006; 63:529–532.
- Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003; 61:1066–1073.
- Kellinghaus C, Loddenkemper T, Dinner DS, Lachhwani D, Lüders HO. Seizure semiology in the elderly: a video analysis. Epilepsia 2004; 45:263–267.
- Drury I, Beydoun A. Interictal epileptiform activity in elderly patients with epilepsy. Electroencephalogr Clin Neurophysiol 1998; 106:369–373.
- McBride AE, Shih TT, Hirsch LJ. Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia 2002; 43:165–169.
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet 2006; 367:1087–1100.
- McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004; 56:163–184.
- Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy Behav 2004; 5(suppl 2):S24–S29.
- Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing 2001; 30:523–525.
- Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 2006; 61:246–255.
- Rowan AJ, Ramsay RE, Collins JF, et al; VA Cooperative Study 428 Group. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 2005; 64:1868–1673.
- Garnett WR. Optimizing antiepileptic drug therapy in the elderly. Ann Pharmacother 2005; 39:1852–1860.
- Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology 2002; 58(suppl 5):S2–S8.
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51:1069–1077.
- Huying F, Klimpe S, Werhahn KJ. Antiepileptic drug use in nursing home residents: a cross-sectional, regional study. Seizure 2006; 15:194–197.
- Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: effect of age, gender, and comedication on patterns of use. Epilepsia 1998; 39:1083–1087.
- Galimberti CA, Magri F, Magnani B, et al. Antiepileptic drug use and epileptic seizures in elderly nursing home residents: a survey in the province of Pavia, Northern Italy. Epilepsy Res 2006; 68:1–8.
KEY POINTS
- About 25% of new-onset seizures occur after the age of 65.
- Most new-onset cases of epilepsy in the elderly are secondary to cerebrovascular disease, metabolic disturbances, dementia, traumatic brain injury, tumor, or drug therapy.
- The diagnosis is challenging and can be confused with syncope, transient ischemic attack, cardiac arrhythmia, metabolic disturbances, transient global amnesia, neurodegenerative disease, rapid-eye-movement sleep behavior disorder, and psychogenic disorders.
- The clinical presentation of seizures in the elderly differs from that in younger patients.
- A detailed clinical history, blood tests, electrocardiography, magnetic resonance imaging, and EEG can be helpful in diagnosing.
- No single drug is ideal for new-onset epilepsy in the elderly; the choice depends mainly on the type of seizure and the comorbidities present.
Treating epilepsy in the elderly: More art than science
As Drs. ghosh and jehi discuss in this issue of the Journal,1 physicians face many challenges when caring for elderly patients who have epileptic seizures.
Owing to the graying of America and higher rates of incidence and prevalence of epilepsy in older patients than in younger ones, the number of patients with epilepsy will climb steeply in the coming years. Among patients in nursing homes, the numbers are much higher (an incidence of up to 16 per 1,000 per year and a prevalence of 60 per 1,000) than in community-dwelling elderly.2,3
DOES THE PATIENT HAVE EPILEPSY?
The first concern is to make the correct diagnosis. Epilepsy is defined as a condition of the central nervous system predisposing to seizures. Younger patients need to have two unprovoked seizures for epilepsy to be diagnosed. However, a recent modification in the definition allows epilepsy to be diagnosed after a single seizure in a person who has a condition of the central nervous system known to significantly increase the risk of additional seizures.4
CONSIDERATIONS IN TREATMENT
When treating any patient, one size does not fit all, and this is especially true with elderly patients, in whom treatment should be based on health status. Many elderly patients with epilepsy have age-related comorbidities, and one would treat epilepsy differently in patients who are otherwise healthy than in those who are frail or have multiple comorbidities.5 Elderly people who live in their own homes have different needs from those who reside in a nursing home.
These patients have social and psychological problems as well as medical ones. For example, the loss of driving privileges can be a major concern with epilepsy patients; it is often emotionally devastating, in addition to greatly limiting independence.
Comorbidities, seizures, and treatment share a complex and tangled relationship. To decide on the appropriate therapy, a physician needs to evaluate the effects that antiepileptic drugs and central nervous system disorders can have on mood, cognition, and neurologic function. In addition, it is imperative to consider the possible pharmacokinetic and pharmacodynamic interactions of antiepileptic drugs with the many drugs used to treat other conditions.
Should treatment be started?
Antiepileptic drugs can cause side effects, and an elderly person who has had a single seizure may never have another one. On the other hand, given that seizures can pose higher risks to the elderly and lead to injuries that can be more devastating than in the young, preventing recurrent seizures may be very appropriate. Lack of studies of this issue means that there is no evidence to support either decision.
Which antiepileptic drug should be used?
Things to consider when selecting an antiepileptic drug include efficacy, tolerability, pharmacokinetic properties, adverse effects, use of other drugs that interact with these drugs, and compliance.
Pharmacokinetics can be affected by age-related changes in the function of the gastrointestinal tract, kidneys, and liver and in protein binding. However, contrary to common perception, hepatic metabolism in healthy elderly people may not change significantly with advancing age. Ahn et al6 gave radiolabeled phenytoin (Dilantin) intravenously to patients with epilepsy and found that its clearance changed only slightly with age.
Antiepileptic drugs can interact with other drugs, herbal remedies, and food. Physicians need to know about the metabolic pathways of these drugs and other substances to make appropriate decisions about treatment. Interactions between antipsychotic and antiepileptic drugs are particularly worrisome because they involve both pharmacokinetic and pharmacodynamic mechanisms. Certain antiepileptic drugs can also induce (ie, increase) the hepatic metabolism of certain other drugs. Other drugs may lower the threshold for seizures.
Is the patient’s drug level stable?
We assume that if a drug is taken on a regular schedule at the same dose, its serum concentration will remain relatively stable (at a “steady state”). And in younger adults, antiepileptic drug concentrations vary relatively little over time, by about 20% in compliant patients.7 This was assumed to be true for elderly patients as well.
However, Birnbaum et al8 found that phenytoin levels fluctuated as much as two- to threefold in serial measurements in nursing home residents, even though the dose or formulation had not been changed and the patients were not taking any interfering medication. Yet some of the patients had very stable levels. The authors observed similar variations in levels of carbamazepine (Tegretol) and valproic acid (Depakote).9
The reasons for this variability are not known but may involve age-related changes in the gut.
RESEARCH NEEDED
Epilepsy is increasing in elderly people. Yet little basic or clinical research has been done to clarify the mechanisms or to determine the best treatment in terms of quality of life. Lacking appropriate animal models, basic research has been slow. For example, we do not know if the mechanisms leading to seizures after strokes differ from those leading to seizures in people with Alzheimer disease. Thus, it is not possible to choose an antiepileptic drug on the basis of its mechanism of action.
Many elderly patients who have epilepsy also have conditions that may alter the pharmacokinetic and pharmacodynamic properties of antiepileptic drugs, and data from younger people may be misleading.
Given the magnitude of the problem, we need to make a concerted effort to answer these questions with additional research.10 Meanwhile, the treatment of elderly patients with epilepsy is more of an art than a science.
- Ghosh S, Jehi LE. New-onset epilepsy in the elderly: challenges for the internist. Cleve Clin J Med 2014; 81:490–498.
- Garrard J, Cloyd J, Gross C, et al. Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol A Biol Sci Med Sci 2000; 55:M384–M392.
- Garrard J, Harms S, Hardie N, et al. Antiepileptic drug use in nursing home admissions. Ann Neurol 2003; 54:75–85.
- Fisher RS, Leppik I. Debate: when does a seizure imply epilepsy? Epilepsia 2008; 49(suppl 9):7–12.
- Leppik IE. Introduction to the International Geriatric Epilepsy Symposium (IGES). Epilepsy Res 2006; 68(suppl 1):S1–S4.
- Ahn JE, Cloyd JC, Brundage RC, et al. Phenytoin half-life and clearance during maintenance therapy in adults and elderly patients with epilepsy. Neurology 2008; 71:38–43.
- Leppik IE, Cloyd JD, Sawchuk RJ, Pepin SM. Compliance and variability of plasma phenytoin levels in epileptic patients. Ther Drug Mon 1979; 1:475–483.
- Birnbaum A, Hardie NA, Leppik IE, et al. Variability of total phenytoin serum concentrations within elderly nursing home residents. Neurology 2003; 60:555–559.
- Birnbaum AK, Conway JM, Strege MA, Leppik IE. Variability of carbamazepine and valproate concentrations in elderly nursing home residents. Epilepsy Res 2012; 101:22–27.
- Leppik IE, Walczak TS, Birnbaum AK. Challenges of epilepsy in elderly people. Lancet 2012; 380:1128–1130.
As Drs. ghosh and jehi discuss in this issue of the Journal,1 physicians face many challenges when caring for elderly patients who have epileptic seizures.
Owing to the graying of America and higher rates of incidence and prevalence of epilepsy in older patients than in younger ones, the number of patients with epilepsy will climb steeply in the coming years. Among patients in nursing homes, the numbers are much higher (an incidence of up to 16 per 1,000 per year and a prevalence of 60 per 1,000) than in community-dwelling elderly.2,3
DOES THE PATIENT HAVE EPILEPSY?
The first concern is to make the correct diagnosis. Epilepsy is defined as a condition of the central nervous system predisposing to seizures. Younger patients need to have two unprovoked seizures for epilepsy to be diagnosed. However, a recent modification in the definition allows epilepsy to be diagnosed after a single seizure in a person who has a condition of the central nervous system known to significantly increase the risk of additional seizures.4
CONSIDERATIONS IN TREATMENT
When treating any patient, one size does not fit all, and this is especially true with elderly patients, in whom treatment should be based on health status. Many elderly patients with epilepsy have age-related comorbidities, and one would treat epilepsy differently in patients who are otherwise healthy than in those who are frail or have multiple comorbidities.5 Elderly people who live in their own homes have different needs from those who reside in a nursing home.
These patients have social and psychological problems as well as medical ones. For example, the loss of driving privileges can be a major concern with epilepsy patients; it is often emotionally devastating, in addition to greatly limiting independence.
Comorbidities, seizures, and treatment share a complex and tangled relationship. To decide on the appropriate therapy, a physician needs to evaluate the effects that antiepileptic drugs and central nervous system disorders can have on mood, cognition, and neurologic function. In addition, it is imperative to consider the possible pharmacokinetic and pharmacodynamic interactions of antiepileptic drugs with the many drugs used to treat other conditions.
Should treatment be started?
Antiepileptic drugs can cause side effects, and an elderly person who has had a single seizure may never have another one. On the other hand, given that seizures can pose higher risks to the elderly and lead to injuries that can be more devastating than in the young, preventing recurrent seizures may be very appropriate. Lack of studies of this issue means that there is no evidence to support either decision.
Which antiepileptic drug should be used?
Things to consider when selecting an antiepileptic drug include efficacy, tolerability, pharmacokinetic properties, adverse effects, use of other drugs that interact with these drugs, and compliance.
Pharmacokinetics can be affected by age-related changes in the function of the gastrointestinal tract, kidneys, and liver and in protein binding. However, contrary to common perception, hepatic metabolism in healthy elderly people may not change significantly with advancing age. Ahn et al6 gave radiolabeled phenytoin (Dilantin) intravenously to patients with epilepsy and found that its clearance changed only slightly with age.
Antiepileptic drugs can interact with other drugs, herbal remedies, and food. Physicians need to know about the metabolic pathways of these drugs and other substances to make appropriate decisions about treatment. Interactions between antipsychotic and antiepileptic drugs are particularly worrisome because they involve both pharmacokinetic and pharmacodynamic mechanisms. Certain antiepileptic drugs can also induce (ie, increase) the hepatic metabolism of certain other drugs. Other drugs may lower the threshold for seizures.
Is the patient’s drug level stable?
We assume that if a drug is taken on a regular schedule at the same dose, its serum concentration will remain relatively stable (at a “steady state”). And in younger adults, antiepileptic drug concentrations vary relatively little over time, by about 20% in compliant patients.7 This was assumed to be true for elderly patients as well.
However, Birnbaum et al8 found that phenytoin levels fluctuated as much as two- to threefold in serial measurements in nursing home residents, even though the dose or formulation had not been changed and the patients were not taking any interfering medication. Yet some of the patients had very stable levels. The authors observed similar variations in levels of carbamazepine (Tegretol) and valproic acid (Depakote).9
The reasons for this variability are not known but may involve age-related changes in the gut.
RESEARCH NEEDED
Epilepsy is increasing in elderly people. Yet little basic or clinical research has been done to clarify the mechanisms or to determine the best treatment in terms of quality of life. Lacking appropriate animal models, basic research has been slow. For example, we do not know if the mechanisms leading to seizures after strokes differ from those leading to seizures in people with Alzheimer disease. Thus, it is not possible to choose an antiepileptic drug on the basis of its mechanism of action.
Many elderly patients who have epilepsy also have conditions that may alter the pharmacokinetic and pharmacodynamic properties of antiepileptic drugs, and data from younger people may be misleading.
Given the magnitude of the problem, we need to make a concerted effort to answer these questions with additional research.10 Meanwhile, the treatment of elderly patients with epilepsy is more of an art than a science.
As Drs. ghosh and jehi discuss in this issue of the Journal,1 physicians face many challenges when caring for elderly patients who have epileptic seizures.
Owing to the graying of America and higher rates of incidence and prevalence of epilepsy in older patients than in younger ones, the number of patients with epilepsy will climb steeply in the coming years. Among patients in nursing homes, the numbers are much higher (an incidence of up to 16 per 1,000 per year and a prevalence of 60 per 1,000) than in community-dwelling elderly.2,3
DOES THE PATIENT HAVE EPILEPSY?
The first concern is to make the correct diagnosis. Epilepsy is defined as a condition of the central nervous system predisposing to seizures. Younger patients need to have two unprovoked seizures for epilepsy to be diagnosed. However, a recent modification in the definition allows epilepsy to be diagnosed after a single seizure in a person who has a condition of the central nervous system known to significantly increase the risk of additional seizures.4
CONSIDERATIONS IN TREATMENT
When treating any patient, one size does not fit all, and this is especially true with elderly patients, in whom treatment should be based on health status. Many elderly patients with epilepsy have age-related comorbidities, and one would treat epilepsy differently in patients who are otherwise healthy than in those who are frail or have multiple comorbidities.5 Elderly people who live in their own homes have different needs from those who reside in a nursing home.
These patients have social and psychological problems as well as medical ones. For example, the loss of driving privileges can be a major concern with epilepsy patients; it is often emotionally devastating, in addition to greatly limiting independence.
Comorbidities, seizures, and treatment share a complex and tangled relationship. To decide on the appropriate therapy, a physician needs to evaluate the effects that antiepileptic drugs and central nervous system disorders can have on mood, cognition, and neurologic function. In addition, it is imperative to consider the possible pharmacokinetic and pharmacodynamic interactions of antiepileptic drugs with the many drugs used to treat other conditions.
Should treatment be started?
Antiepileptic drugs can cause side effects, and an elderly person who has had a single seizure may never have another one. On the other hand, given that seizures can pose higher risks to the elderly and lead to injuries that can be more devastating than in the young, preventing recurrent seizures may be very appropriate. Lack of studies of this issue means that there is no evidence to support either decision.
Which antiepileptic drug should be used?
Things to consider when selecting an antiepileptic drug include efficacy, tolerability, pharmacokinetic properties, adverse effects, use of other drugs that interact with these drugs, and compliance.
Pharmacokinetics can be affected by age-related changes in the function of the gastrointestinal tract, kidneys, and liver and in protein binding. However, contrary to common perception, hepatic metabolism in healthy elderly people may not change significantly with advancing age. Ahn et al6 gave radiolabeled phenytoin (Dilantin) intravenously to patients with epilepsy and found that its clearance changed only slightly with age.
Antiepileptic drugs can interact with other drugs, herbal remedies, and food. Physicians need to know about the metabolic pathways of these drugs and other substances to make appropriate decisions about treatment. Interactions between antipsychotic and antiepileptic drugs are particularly worrisome because they involve both pharmacokinetic and pharmacodynamic mechanisms. Certain antiepileptic drugs can also induce (ie, increase) the hepatic metabolism of certain other drugs. Other drugs may lower the threshold for seizures.
Is the patient’s drug level stable?
We assume that if a drug is taken on a regular schedule at the same dose, its serum concentration will remain relatively stable (at a “steady state”). And in younger adults, antiepileptic drug concentrations vary relatively little over time, by about 20% in compliant patients.7 This was assumed to be true for elderly patients as well.
However, Birnbaum et al8 found that phenytoin levels fluctuated as much as two- to threefold in serial measurements in nursing home residents, even though the dose or formulation had not been changed and the patients were not taking any interfering medication. Yet some of the patients had very stable levels. The authors observed similar variations in levels of carbamazepine (Tegretol) and valproic acid (Depakote).9
The reasons for this variability are not known but may involve age-related changes in the gut.
RESEARCH NEEDED
Epilepsy is increasing in elderly people. Yet little basic or clinical research has been done to clarify the mechanisms or to determine the best treatment in terms of quality of life. Lacking appropriate animal models, basic research has been slow. For example, we do not know if the mechanisms leading to seizures after strokes differ from those leading to seizures in people with Alzheimer disease. Thus, it is not possible to choose an antiepileptic drug on the basis of its mechanism of action.
Many elderly patients who have epilepsy also have conditions that may alter the pharmacokinetic and pharmacodynamic properties of antiepileptic drugs, and data from younger people may be misleading.
Given the magnitude of the problem, we need to make a concerted effort to answer these questions with additional research.10 Meanwhile, the treatment of elderly patients with epilepsy is more of an art than a science.
- Ghosh S, Jehi LE. New-onset epilepsy in the elderly: challenges for the internist. Cleve Clin J Med 2014; 81:490–498.
- Garrard J, Cloyd J, Gross C, et al. Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol A Biol Sci Med Sci 2000; 55:M384–M392.
- Garrard J, Harms S, Hardie N, et al. Antiepileptic drug use in nursing home admissions. Ann Neurol 2003; 54:75–85.
- Fisher RS, Leppik I. Debate: when does a seizure imply epilepsy? Epilepsia 2008; 49(suppl 9):7–12.
- Leppik IE. Introduction to the International Geriatric Epilepsy Symposium (IGES). Epilepsy Res 2006; 68(suppl 1):S1–S4.
- Ahn JE, Cloyd JC, Brundage RC, et al. Phenytoin half-life and clearance during maintenance therapy in adults and elderly patients with epilepsy. Neurology 2008; 71:38–43.
- Leppik IE, Cloyd JD, Sawchuk RJ, Pepin SM. Compliance and variability of plasma phenytoin levels in epileptic patients. Ther Drug Mon 1979; 1:475–483.
- Birnbaum A, Hardie NA, Leppik IE, et al. Variability of total phenytoin serum concentrations within elderly nursing home residents. Neurology 2003; 60:555–559.
- Birnbaum AK, Conway JM, Strege MA, Leppik IE. Variability of carbamazepine and valproate concentrations in elderly nursing home residents. Epilepsy Res 2012; 101:22–27.
- Leppik IE, Walczak TS, Birnbaum AK. Challenges of epilepsy in elderly people. Lancet 2012; 380:1128–1130.
- Ghosh S, Jehi LE. New-onset epilepsy in the elderly: challenges for the internist. Cleve Clin J Med 2014; 81:490–498.
- Garrard J, Cloyd J, Gross C, et al. Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol A Biol Sci Med Sci 2000; 55:M384–M392.
- Garrard J, Harms S, Hardie N, et al. Antiepileptic drug use in nursing home admissions. Ann Neurol 2003; 54:75–85.
- Fisher RS, Leppik I. Debate: when does a seizure imply epilepsy? Epilepsia 2008; 49(suppl 9):7–12.
- Leppik IE. Introduction to the International Geriatric Epilepsy Symposium (IGES). Epilepsy Res 2006; 68(suppl 1):S1–S4.
- Ahn JE, Cloyd JC, Brundage RC, et al. Phenytoin half-life and clearance during maintenance therapy in adults and elderly patients with epilepsy. Neurology 2008; 71:38–43.
- Leppik IE, Cloyd JD, Sawchuk RJ, Pepin SM. Compliance and variability of plasma phenytoin levels in epileptic patients. Ther Drug Mon 1979; 1:475–483.
- Birnbaum A, Hardie NA, Leppik IE, et al. Variability of total phenytoin serum concentrations within elderly nursing home residents. Neurology 2003; 60:555–559.
- Birnbaum AK, Conway JM, Strege MA, Leppik IE. Variability of carbamazepine and valproate concentrations in elderly nursing home residents. Epilepsy Res 2012; 101:22–27.
- Leppik IE, Walczak TS, Birnbaum AK. Challenges of epilepsy in elderly people. Lancet 2012; 380:1128–1130.
A 78-year-old smoker with an incidental pulmonary mass
When a 78-year-old man underwent magnetic resonance imaging of the lumbar spine because of back pain, the scan revealed a mass in the right lung. He had no respiratory symptoms but had a 40-pack-year smoking history. Physical examination and routine blood tests were unremarkable.
Radiography (Figure 1) showed a large rounded opacity in the right lower lobe. The patient’s age, smoking history, and imaging findings raised concern for lung cancer, so computed tomography (CT) was performed (Figure 2).
DIAGNOSIS: PULMONARY HAMARTOMA
The findings of a well-circumscribed solitary pulmonary nodule or mass containing areas of fat, either as focal islands or more generally distributed, and chondroid “popcorn” calcification are virtually pathognomonic for pulmonary hamartoma.1,2 Unfortunately, although this pattern of calcification is strongly diagnostic, it is present in only a minority of cases of hamartoma.
Pulmonary hamartoma is the most common benign tumor of the lung, accounting for approximately 75% of benign neoplasms and 6% to 8% of all focal lung parenchymal masses.3
Like hamartoma elsewhere in the body, pulmonary hamartoma consists of disorganized overgrowth and aberrant arrangement of normal tissues, including cartilage (which may calcify), smooth muscle, epithelium, and fibrostroma. Pulmonary hamartoma is twice as common in men as in women, and it has a peak incidence in the seventh decade of life.4
Although size ranged from 0.2 to 6 cm in a large case series,4 hamartomas are usually less than 2.5 cm in diameter. As noted in Figure 1, our patient’s lesion was 5 cm.
Pulmonary hamartomas grow slowly and are often asymptomatic, although up to 39% of patients may have symptoms such as cough, dyspnea, and chest tightness.5 The nonspecific nature of these symptoms makes it difficult to be certain that they are caused by the hamartoma; in many cases, they are likely to be coincidental. Lesions tend to occur in the periphery of the lobe and do not favor a particular lobe. Endobronchial lesions can occur but are uncommon.
The internal heterogeneous elements are difficult to see on radiography; CT is usually required to further characterize the lesion and to exclude more sinister differential diagnoses. In some cases the characteristic features of fat and calcification are absent, making a certain diagnosis difficult or impossible radiologically; in such cases, biopsy or resection may be required.
Hamartomas usually do not take up fluorodeoxyglucose avidly on positron-emission tomography CT. However, nuclear medicine studies such as this are superfluous if the classic features are present on CT.
FOLLOW-UP AND TREATMENT
Given the benign nature, slow growth, and usually incidental detection of pulmonary hamartoma in patients without symptoms, no follow-up imaging or treatment is usually required. In the few cases in which symptoms are attributable to the lesion, the lesion can be resected.5 Resection is also an option when the patient is very anxious about the mass, or when imaging studies do not provide a clear diagnosis and tissue needs to be obtained for study.
Because patients often present to different institutions during their lifetime, it is important to counsel them about the natural history of pulmonary hamartomas. Giving them a copy of their imaging may help avoid unnecessary repetition.
- Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000; 20:43–58.
- Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: what does it mean? Ann Thorac Med 2010; 5:67–79.
- Siegelman SS, Khouri NF, Scott WW, et al. Pulmonary hamartoma: CT findings. Radiology 1986; 160:313–317.
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996; 71:14–20.
- Hansen CP, Holtveg H, Francis D, Rasch L, Bertelsen S. Pulmonary hamartoma. J Thorac Cardiovasc Surg 1992; 104:674–678.
When a 78-year-old man underwent magnetic resonance imaging of the lumbar spine because of back pain, the scan revealed a mass in the right lung. He had no respiratory symptoms but had a 40-pack-year smoking history. Physical examination and routine blood tests were unremarkable.
Radiography (Figure 1) showed a large rounded opacity in the right lower lobe. The patient’s age, smoking history, and imaging findings raised concern for lung cancer, so computed tomography (CT) was performed (Figure 2).
DIAGNOSIS: PULMONARY HAMARTOMA
The findings of a well-circumscribed solitary pulmonary nodule or mass containing areas of fat, either as focal islands or more generally distributed, and chondroid “popcorn” calcification are virtually pathognomonic for pulmonary hamartoma.1,2 Unfortunately, although this pattern of calcification is strongly diagnostic, it is present in only a minority of cases of hamartoma.
Pulmonary hamartoma is the most common benign tumor of the lung, accounting for approximately 75% of benign neoplasms and 6% to 8% of all focal lung parenchymal masses.3
Like hamartoma elsewhere in the body, pulmonary hamartoma consists of disorganized overgrowth and aberrant arrangement of normal tissues, including cartilage (which may calcify), smooth muscle, epithelium, and fibrostroma. Pulmonary hamartoma is twice as common in men as in women, and it has a peak incidence in the seventh decade of life.4
Although size ranged from 0.2 to 6 cm in a large case series,4 hamartomas are usually less than 2.5 cm in diameter. As noted in Figure 1, our patient’s lesion was 5 cm.
Pulmonary hamartomas grow slowly and are often asymptomatic, although up to 39% of patients may have symptoms such as cough, dyspnea, and chest tightness.5 The nonspecific nature of these symptoms makes it difficult to be certain that they are caused by the hamartoma; in many cases, they are likely to be coincidental. Lesions tend to occur in the periphery of the lobe and do not favor a particular lobe. Endobronchial lesions can occur but are uncommon.
The internal heterogeneous elements are difficult to see on radiography; CT is usually required to further characterize the lesion and to exclude more sinister differential diagnoses. In some cases the characteristic features of fat and calcification are absent, making a certain diagnosis difficult or impossible radiologically; in such cases, biopsy or resection may be required.
Hamartomas usually do not take up fluorodeoxyglucose avidly on positron-emission tomography CT. However, nuclear medicine studies such as this are superfluous if the classic features are present on CT.
FOLLOW-UP AND TREATMENT
Given the benign nature, slow growth, and usually incidental detection of pulmonary hamartoma in patients without symptoms, no follow-up imaging or treatment is usually required. In the few cases in which symptoms are attributable to the lesion, the lesion can be resected.5 Resection is also an option when the patient is very anxious about the mass, or when imaging studies do not provide a clear diagnosis and tissue needs to be obtained for study.
Because patients often present to different institutions during their lifetime, it is important to counsel them about the natural history of pulmonary hamartomas. Giving them a copy of their imaging may help avoid unnecessary repetition.
When a 78-year-old man underwent magnetic resonance imaging of the lumbar spine because of back pain, the scan revealed a mass in the right lung. He had no respiratory symptoms but had a 40-pack-year smoking history. Physical examination and routine blood tests were unremarkable.
Radiography (Figure 1) showed a large rounded opacity in the right lower lobe. The patient’s age, smoking history, and imaging findings raised concern for lung cancer, so computed tomography (CT) was performed (Figure 2).
DIAGNOSIS: PULMONARY HAMARTOMA
The findings of a well-circumscribed solitary pulmonary nodule or mass containing areas of fat, either as focal islands or more generally distributed, and chondroid “popcorn” calcification are virtually pathognomonic for pulmonary hamartoma.1,2 Unfortunately, although this pattern of calcification is strongly diagnostic, it is present in only a minority of cases of hamartoma.
Pulmonary hamartoma is the most common benign tumor of the lung, accounting for approximately 75% of benign neoplasms and 6% to 8% of all focal lung parenchymal masses.3
Like hamartoma elsewhere in the body, pulmonary hamartoma consists of disorganized overgrowth and aberrant arrangement of normal tissues, including cartilage (which may calcify), smooth muscle, epithelium, and fibrostroma. Pulmonary hamartoma is twice as common in men as in women, and it has a peak incidence in the seventh decade of life.4
Although size ranged from 0.2 to 6 cm in a large case series,4 hamartomas are usually less than 2.5 cm in diameter. As noted in Figure 1, our patient’s lesion was 5 cm.
Pulmonary hamartomas grow slowly and are often asymptomatic, although up to 39% of patients may have symptoms such as cough, dyspnea, and chest tightness.5 The nonspecific nature of these symptoms makes it difficult to be certain that they are caused by the hamartoma; in many cases, they are likely to be coincidental. Lesions tend to occur in the periphery of the lobe and do not favor a particular lobe. Endobronchial lesions can occur but are uncommon.
The internal heterogeneous elements are difficult to see on radiography; CT is usually required to further characterize the lesion and to exclude more sinister differential diagnoses. In some cases the characteristic features of fat and calcification are absent, making a certain diagnosis difficult or impossible radiologically; in such cases, biopsy or resection may be required.
Hamartomas usually do not take up fluorodeoxyglucose avidly on positron-emission tomography CT. However, nuclear medicine studies such as this are superfluous if the classic features are present on CT.
FOLLOW-UP AND TREATMENT
Given the benign nature, slow growth, and usually incidental detection of pulmonary hamartoma in patients without symptoms, no follow-up imaging or treatment is usually required. In the few cases in which symptoms are attributable to the lesion, the lesion can be resected.5 Resection is also an option when the patient is very anxious about the mass, or when imaging studies do not provide a clear diagnosis and tissue needs to be obtained for study.
Because patients often present to different institutions during their lifetime, it is important to counsel them about the natural history of pulmonary hamartomas. Giving them a copy of their imaging may help avoid unnecessary repetition.
- Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000; 20:43–58.
- Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: what does it mean? Ann Thorac Med 2010; 5:67–79.
- Siegelman SS, Khouri NF, Scott WW, et al. Pulmonary hamartoma: CT findings. Radiology 1986; 160:313–317.
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996; 71:14–20.
- Hansen CP, Holtveg H, Francis D, Rasch L, Bertelsen S. Pulmonary hamartoma. J Thorac Cardiovasc Surg 1992; 104:674–678.
- Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000; 20:43–58.
- Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: what does it mean? Ann Thorac Med 2010; 5:67–79.
- Siegelman SS, Khouri NF, Scott WW, et al. Pulmonary hamartoma: CT findings. Radiology 1986; 160:313–317.
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996; 71:14–20.
- Hansen CP, Holtveg H, Francis D, Rasch L, Bertelsen S. Pulmonary hamartoma. J Thorac Cardiovasc Surg 1992; 104:674–678.
Alveolar proteinosis: A slow drowning in mud
A 30-year-old man presented with progressive dyspnea and dry cough, which had developed over the last 6 months. His oxygen saturation was 88% on room air, and he had diffuse bilateral crackles on auscultation. Imaging showed a mixture of diffuse airspace and interstitial abnormalities (Figure 1).
He underwent bronchoscopy. The bronchoalveolar lavage fluid had a turbid appearance that gradually cleared with successive aliquots. Transbronchial biopsy studies confirmed the diagnosis of pulmonary alveolar proteinosis (Figure 2). Sequential whole-lung lavage recovered significant amounts of thick, proteinaceous effluent that slowly cleared. After the procedure, the patient’s symptoms, oxygen saturation, and chest radiographic appearance (Figure 3) improved markedly, with no recurrence at 1 year of follow-up.
ALVEOLAR PROTEINOSIS
Pulmonary alveolar proteinosis is a rare disease characterized by the accumulation of lipoproteinaceous material in the alveolar space secondary to alveolar macrophage dysfunction. The condition can be congenital, secondary, or acquired. Patients typically present with progressive exertional dyspnea, nonproductive cough, variable restrictive ventilatory defects, and diffusion limitation on pulmonary function testing.
Plain chest radiographs usually resemble those seen in pulmonary edema but without features of heart failure, ie, cardiomegaly, Kerley B lines, and effusion.
A “crazy-paving” pattern on computed tomography—a combination of geographic ground-glass appearance and interseptal thickening—suggests alveolar proteinosis, but is not specific for it. Other differential diagnoses for the crazy-paving pattern include Pneumocystis jirovecii infection, invasive mucinous adenocarcinoma, cardiogenic pulmonary edema, alveolar hemorrhage, sarcoidosis, cryptogenic organizing pneumonia, exogenous lipoid pneumonia, drug-induced lung disease, acute radiation pneumonitis, and nonspecific interstitial pneumonia.1
Laboratory testing is not very helpful in the diagnosis, although the serum lactate dehydrogenase level may be mildly elevated. Circulating antibodies to granulocyte macrophage colony-stimulating factor may support the diagnosis, but they are only present in the acquired form. Communication with a research laboratory is usually needed to test for these antibodies.
The bronchoalveolar lavage fluid typically has an opaque, milky, or muddy appearance. The diagnosis is confirmed by demonstration of alveolar filling with material that is periodic acid-Schiff-positive and that is amorphous, eosinophilic, and granular.
Whole-lung lavage2 is the physical removal of surfactant by repeated flooding of the lungs with warmed saline, done under general anesthesia with single-lung ventilation. It remains the standard of care and is indicated in patients with the confirmed diagnosis and one of the following: severe dyspnea, resting hypoxemia (Pao2 < 60 mm Hg at sea level), alveolar-arterial gradient > 40 mm Hg, or a shunt fraction of more than 10%. Successful bronchoscopic lavage has also been reported.3
Other treatments include granulocyte-macrophage colony-stimulating factor, rituximab (Rituxan, an anti-CD20 monoclonal antibody), plasmapheresis, and lung transplantation. Systemic corticosteroids are usually ineffective unless indicated for secondary types of alveolar proteinosis.
Inhalation rather than subcutaneous administration of granulocyte-macrophage colony-stimulating factor seems preferred as it ensures a high concentration in the target organ, avoids systemic complications (injection-site edema, erythema, neutropenia, malaise, and shortness of breath) and achieves lower levels of autoantibodies in bronchoalveolar lavage fluid, which correlates with disease activity.
Data are sparse as to the recurrence of autoimmune pulmonary alveolar proteinosis after whole-lung lavage, yet about 40% of patients require a repeat procedure within 18 months. Recurrence has also been reported after double-lung transplantation.4
Adjuvant therapy with rituximab or, to a lesser extent, with inhaled granulocyte-macrophage colony-stimulating factor has recently been shown to diminish the need for repeated lavage.5 These treatments can also be used when whole-lung lavage cannot be performed or proves to be ineffective.5
Acknowledgment: I would like to thank Dr. Kamelia Velikova for providing the pathology image.
- Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP. ‘Crazy-paving’ pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2003; 23:1509–1519.
- Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest 2009; 136:1678–1681.
- Cheng SL, Chang HT, Lau HP, Lee LN, Yang PC. Pulmonary alveolar proteinosis: treatment by bronchofiberscopic lobar lavage. Chest 2002; 122:1480–1485.
- Parker LA, Novotny DB. Recurrent alveolar proteinosis following double lung transplantation. Chest 1997; 111:1457–1458.
- Leth S, Bendstrup E, Vestergaard H, Hilberg O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology 2013; 18:82–91.
SUGGESTED READING
Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis.Eur Respir Rev 2011; 20:98–107.
Carey B, Trapnell BC. The molecular basis of pulmonary alveolarproteinosis. Clin Immunol 2010; 135:223–235.
Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis.Chron Respir Dis 2006; 3:149–159.
Luisetti M, Kadija Z, Mariani F, Rodi G, Campo I, Trapnell BC.Therapy options in pulmonary alveolar proteinosis. TherAdv Respir Dis 2010; 4:239–248.
A 30-year-old man presented with progressive dyspnea and dry cough, which had developed over the last 6 months. His oxygen saturation was 88% on room air, and he had diffuse bilateral crackles on auscultation. Imaging showed a mixture of diffuse airspace and interstitial abnormalities (Figure 1).

He underwent bronchoscopy. The bronchoalveolar lavage fluid had a turbid appearance that gradually cleared with successive aliquots. Transbronchial biopsy studies confirmed the diagnosis of pulmonary alveolar proteinosis (Figure 2). Sequential whole-lung lavage recovered significant amounts of thick, proteinaceous effluent that slowly cleared. After the procedure, the patient’s symptoms, oxygen saturation, and chest radiographic appearance (Figure 3) improved markedly, with no recurrence at 1 year of follow-up.


ALVEOLAR PROTEINOSIS
Pulmonary alveolar proteinosis is a rare disease characterized by the accumulation of lipoproteinaceous material in the alveolar space secondary to alveolar macrophage dysfunction. The condition can be congenital, secondary, or acquired. Patients typically present with progressive exertional dyspnea, nonproductive cough, variable restrictive ventilatory defects, and diffusion limitation on pulmonary function testing.
Plain chest radiographs usually resemble those seen in pulmonary edema but without features of heart failure, ie, cardiomegaly, Kerley B lines, and effusion.
A “crazy-paving” pattern on computed tomography—a combination of geographic ground-glass appearance and interseptal thickening—suggests alveolar proteinosis, but is not specific for it. Other differential diagnoses for the crazy-paving pattern include Pneumocystis jirovecii infection, invasive mucinous adenocarcinoma, cardiogenic pulmonary edema, alveolar hemorrhage, sarcoidosis, cryptogenic organizing pneumonia, exogenous lipoid pneumonia, drug-induced lung disease, acute radiation pneumonitis, and nonspecific interstitial pneumonia.1
Laboratory testing is not very helpful in the diagnosis, although the serum lactate dehydrogenase level may be mildly elevated. Circulating antibodies to granulocyte macrophage colony-stimulating factor may support the diagnosis, but they are only present in the acquired form. Communication with a research laboratory is usually needed to test for these antibodies.
The bronchoalveolar lavage fluid typically has an opaque, milky, or muddy appearance. The diagnosis is confirmed by demonstration of alveolar filling with material that is periodic acid-Schiff-positive and that is amorphous, eosinophilic, and granular.
Whole-lung lavage2 is the physical removal of surfactant by repeated flooding of the lungs with warmed saline, done under general anesthesia with single-lung ventilation. It remains the standard of care and is indicated in patients with the confirmed diagnosis and one of the following: severe dyspnea, resting hypoxemia (Pao2 < 60 mm Hg at sea level), alveolar-arterial gradient > 40 mm Hg, or a shunt fraction of more than 10%. Successful bronchoscopic lavage has also been reported.3
Other treatments include granulocyte-macrophage colony-stimulating factor, rituximab (Rituxan, an anti-CD20 monoclonal antibody), plasmapheresis, and lung transplantation. Systemic corticosteroids are usually ineffective unless indicated for secondary types of alveolar proteinosis.
Inhalation rather than subcutaneous administration of granulocyte-macrophage colony-stimulating factor seems preferred as it ensures a high concentration in the target organ, avoids systemic complications (injection-site edema, erythema, neutropenia, malaise, and shortness of breath) and achieves lower levels of autoantibodies in bronchoalveolar lavage fluid, which correlates with disease activity.
Data are sparse as to the recurrence of autoimmune pulmonary alveolar proteinosis after whole-lung lavage, yet about 40% of patients require a repeat procedure within 18 months. Recurrence has also been reported after double-lung transplantation.4
Adjuvant therapy with rituximab or, to a lesser extent, with inhaled granulocyte-macrophage colony-stimulating factor has recently been shown to diminish the need for repeated lavage.5 These treatments can also be used when whole-lung lavage cannot be performed or proves to be ineffective.5
Acknowledgment: I would like to thank Dr. Kamelia Velikova for providing the pathology image.
A 30-year-old man presented with progressive dyspnea and dry cough, which had developed over the last 6 months. His oxygen saturation was 88% on room air, and he had diffuse bilateral crackles on auscultation. Imaging showed a mixture of diffuse airspace and interstitial abnormalities (Figure 1).

He underwent bronchoscopy. The bronchoalveolar lavage fluid had a turbid appearance that gradually cleared with successive aliquots. Transbronchial biopsy studies confirmed the diagnosis of pulmonary alveolar proteinosis (Figure 2). Sequential whole-lung lavage recovered significant amounts of thick, proteinaceous effluent that slowly cleared. After the procedure, the patient’s symptoms, oxygen saturation, and chest radiographic appearance (Figure 3) improved markedly, with no recurrence at 1 year of follow-up.


ALVEOLAR PROTEINOSIS
Pulmonary alveolar proteinosis is a rare disease characterized by the accumulation of lipoproteinaceous material in the alveolar space secondary to alveolar macrophage dysfunction. The condition can be congenital, secondary, or acquired. Patients typically present with progressive exertional dyspnea, nonproductive cough, variable restrictive ventilatory defects, and diffusion limitation on pulmonary function testing.
Plain chest radiographs usually resemble those seen in pulmonary edema but without features of heart failure, ie, cardiomegaly, Kerley B lines, and effusion.
A “crazy-paving” pattern on computed tomography—a combination of geographic ground-glass appearance and interseptal thickening—suggests alveolar proteinosis, but is not specific for it. Other differential diagnoses for the crazy-paving pattern include Pneumocystis jirovecii infection, invasive mucinous adenocarcinoma, cardiogenic pulmonary edema, alveolar hemorrhage, sarcoidosis, cryptogenic organizing pneumonia, exogenous lipoid pneumonia, drug-induced lung disease, acute radiation pneumonitis, and nonspecific interstitial pneumonia.1
Laboratory testing is not very helpful in the diagnosis, although the serum lactate dehydrogenase level may be mildly elevated. Circulating antibodies to granulocyte macrophage colony-stimulating factor may support the diagnosis, but they are only present in the acquired form. Communication with a research laboratory is usually needed to test for these antibodies.
The bronchoalveolar lavage fluid typically has an opaque, milky, or muddy appearance. The diagnosis is confirmed by demonstration of alveolar filling with material that is periodic acid-Schiff-positive and that is amorphous, eosinophilic, and granular.
Whole-lung lavage2 is the physical removal of surfactant by repeated flooding of the lungs with warmed saline, done under general anesthesia with single-lung ventilation. It remains the standard of care and is indicated in patients with the confirmed diagnosis and one of the following: severe dyspnea, resting hypoxemia (Pao2 < 60 mm Hg at sea level), alveolar-arterial gradient > 40 mm Hg, or a shunt fraction of more than 10%. Successful bronchoscopic lavage has also been reported.3
Other treatments include granulocyte-macrophage colony-stimulating factor, rituximab (Rituxan, an anti-CD20 monoclonal antibody), plasmapheresis, and lung transplantation. Systemic corticosteroids are usually ineffective unless indicated for secondary types of alveolar proteinosis.
Inhalation rather than subcutaneous administration of granulocyte-macrophage colony-stimulating factor seems preferred as it ensures a high concentration in the target organ, avoids systemic complications (injection-site edema, erythema, neutropenia, malaise, and shortness of breath) and achieves lower levels of autoantibodies in bronchoalveolar lavage fluid, which correlates with disease activity.
Data are sparse as to the recurrence of autoimmune pulmonary alveolar proteinosis after whole-lung lavage, yet about 40% of patients require a repeat procedure within 18 months. Recurrence has also been reported after double-lung transplantation.4
Adjuvant therapy with rituximab or, to a lesser extent, with inhaled granulocyte-macrophage colony-stimulating factor has recently been shown to diminish the need for repeated lavage.5 These treatments can also be used when whole-lung lavage cannot be performed or proves to be ineffective.5
Acknowledgment: I would like to thank Dr. Kamelia Velikova for providing the pathology image.
- Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP. ‘Crazy-paving’ pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2003; 23:1509–1519.
- Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest 2009; 136:1678–1681.
- Cheng SL, Chang HT, Lau HP, Lee LN, Yang PC. Pulmonary alveolar proteinosis: treatment by bronchofiberscopic lobar lavage. Chest 2002; 122:1480–1485.
- Parker LA, Novotny DB. Recurrent alveolar proteinosis following double lung transplantation. Chest 1997; 111:1457–1458.
- Leth S, Bendstrup E, Vestergaard H, Hilberg O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology 2013; 18:82–91.
SUGGESTED READING
Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis.Eur Respir Rev 2011; 20:98–107.
Carey B, Trapnell BC. The molecular basis of pulmonary alveolarproteinosis. Clin Immunol 2010; 135:223–235.
Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis.Chron Respir Dis 2006; 3:149–159.
Luisetti M, Kadija Z, Mariani F, Rodi G, Campo I, Trapnell BC.Therapy options in pulmonary alveolar proteinosis. TherAdv Respir Dis 2010; 4:239–248.
- Rossi SE, Erasmus JJ, Volpacchio M, Franquet T, Castiglioni T, McAdams HP. ‘Crazy-paving’ pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics 2003; 23:1509–1519.
- Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest 2009; 136:1678–1681.
- Cheng SL, Chang HT, Lau HP, Lee LN, Yang PC. Pulmonary alveolar proteinosis: treatment by bronchofiberscopic lobar lavage. Chest 2002; 122:1480–1485.
- Parker LA, Novotny DB. Recurrent alveolar proteinosis following double lung transplantation. Chest 1997; 111:1457–1458.
- Leth S, Bendstrup E, Vestergaard H, Hilberg O. Autoimmune pulmonary alveolar proteinosis: treatment options in year 2013. Respirology 2013; 18:82–91.
SUGGESTED READING
Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis.Eur Respir Rev 2011; 20:98–107.
Carey B, Trapnell BC. The molecular basis of pulmonary alveolarproteinosis. Clin Immunol 2010; 135:223–235.
Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis.Chron Respir Dis 2006; 3:149–159.
Luisetti M, Kadija Z, Mariani F, Rodi G, Campo I, Trapnell BC.Therapy options in pulmonary alveolar proteinosis. TherAdv Respir Dis 2010; 4:239–248.
Do imaging studies have value in a patient with acute, nonspecific low back pain?
A 38-year-old man is evaluated in an urgent care center for back pain. He is a high school mathematics teacher who reports the insidious onset of low back pain 3 weeks ago. Over the last week the pain has become constant, is worsened by movement, and does not respond to naproxen. He has no history of trauma, malignancy, fever, weight loss, or bladder or bowel symptoms. He does not use intravenous drugs. On examination, he appears uncomfortable and stiff, protecting his back against motion. He has intact sensation, strength, and reflexes. The straight-leg-raising maneuver reproduces his lower back pain but does not cause radicular pain. Should I now order an imaging study such as spinal radiography, computed tomography, or magnetic resonance imaging to direct therapy?
IMAGING STUDIES ARE UNLIKELY TO HELP
This man with acute, nonspecific low back pain does not need spinal imaging. Imaging—ie, spine radiography, computed tomography, or magnetic resonance imaging—is unlikely to be helpful in a patient with nonspecific low back pain and may expose him unnecessarily to radiation and the anxiety of findings that are clinically insignificant.
Imaging studies are often ordered inappropriately as part of the evaluation of back pain in patients such as this. In 2008, the total national cost of treating spine (neck and back) problems was estimated to be $86 billion, representing 9% of total health care costs, which is close to the estimated $89 billion per year spent on cancer care.1
Spine imaging should be considered only in patients who have a “red flag” such as advanced age, history of trauma, history of cancer, and prolonged corticosteroid use, all of which have been associated with an increased probability (from 9% to 33%) of either spinal fracture or malignancy.2 Other red flags include duration longer than 6 weeks, fever, weight loss, and progressive neurologic findings on examination. This patient has none of these.
GUIDELINES AND CHOOSING WISELY
High-quality guidelines from different groups recommend against spine imaging in patients with low back pain.3–6 These guidelines vary slightly in their patient populations and definitions of uncomplicated low back pain.
The American College of Radiology4 and the American College of Occupational and Environmental Medicine6 recommend against imaging for patients with both nonspecific and radicular low back pain in the first 6 weeks as long as no red flags are present.
The National Institute for Health and Clinical Excellence3 and, jointly, the American College of Physicians and American Pain Society (ACP/APS)5 recommend against imaging for patients with nonspecific low back pain in both the acute and chronic settings. Nonspecific low back pain is defined as pain without signs of a serious underlying condition (eg, cancer, infection, cauda equina syndrome), spinal stenosis or radiculopathy, or another specific spinal cause (eg, vertebral compression fracture, ankylosing spondylitis).
In addition, imaging in patients with nonspecific low back pain is one of the top five practices that should be questioned by physicians and patients, according to the American Board of Internal Medicine Foundation in its Choosing Wisely campaign (www.choosingwisely.org).
HARMS ASSOCIATED WITH SPINE IMAGING
Several guidelines cite radiation exposure as a potential harmful consequence of spinal imaging by plain radiography and computed tomography. The American College of Radiology guideline4 estimates that the radiation exposure of plain lumbar radiography or lumbar computed tomography ranges between 1 and 10 mSv (3 mSv is the annual amount of ambient radiation in the United States), placing both studies in the medium-range category for relative radiation exposure. The ACP/APS guideline5 states that radiation exposure from imaging is a reason to dissuade clinicians from routine use.
Although lumbar magnetic resonance imaging does not carry the risk of radiation exposure, it may result in harm by detecting clinically insignificant abnormalities in more than 30% of patients.7 These incidental findings increase with age and may lead to additional and possibly unnecessary testing and invasive treatments. The American College of Occupational and Environmental Medicine guideline6 also cites the high prevalence of abnormal findings on plain radiography, magnetic resonance imaging, and other diagnostic tests that are unrelated to symptoms.
CLINICAL BOTTOM LINE
On the basis of current data, the patient described at the beginning of this article should not undergo spine imaging; the results are unlikely to affect his medical management and improve his clinical outcome, and imaging carries a small risk of harm.
A practical approach would be to treat his pain with simple analgesia (a different nonsteroidal anti-inflammatory drug or acetaminophen), address his functional challenges, and reassure him that his chance of having a serious underlying cause of back pain is low (< 1%). He should be told to expect significant improvement in his symptoms within 30 days, be encouraged to stay active, and should be offered patient-focused self-help resources.
The recommendation to conservatively manage patients at low risk without imaging is consistent among all four guidelines. Imaging can be considered for a small subset of patients at high risk with red-flag indications. Potential harms associated with routine imaging of all patients with low back pain include radiation exposure and the high rate of clinically insignificant abnormalities that may lead to unnecessary and invasive interventions that increase expense, patient risk, and anxiety without improving outcomes.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299:656–664. Erratum in: JAMA 2008; 299:2630.
- Downie A, Williams CM, Henschke N, et al. Red flags to screen for malignancy and fracture in patients with low back pain: systematic review. BMJ 2013; 347:f7095.
- National Collaborating Centre for Primary Care. Low back pain. Early management of persistent nonspecific low back pain. London (UK): National Institute for Health and Clinical Excellence (NICE); 2009 May.25p. (Clinical guideline; no. 88) http://guidelines.gov/content.aspx?id=14699&search=low+back+pain. http://guidance.nice.org.uk/CG88. Accessed May 23, 2014
- Davis PC, Wippold FJ, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria® low back pain. Reston, VA: American College of Radiology (ACR); 2011. www.guideline.gov/content.aspx?id=35145. Accessed May 23, 2014.
- Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147:478–491. Erratum in: Ann Intern Med 2008; 148:247–248.
- Low back disorders. In:Hegmann KT, editor. Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine (ACOEM); 2011:333–796. www.guideline.gov/content.aspx?id=38438. Accessed May 23, 2014.
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990; 72:403–408.
A 38-year-old man is evaluated in an urgent care center for back pain. He is a high school mathematics teacher who reports the insidious onset of low back pain 3 weeks ago. Over the last week the pain has become constant, is worsened by movement, and does not respond to naproxen. He has no history of trauma, malignancy, fever, weight loss, or bladder or bowel symptoms. He does not use intravenous drugs. On examination, he appears uncomfortable and stiff, protecting his back against motion. He has intact sensation, strength, and reflexes. The straight-leg-raising maneuver reproduces his lower back pain but does not cause radicular pain. Should I now order an imaging study such as spinal radiography, computed tomography, or magnetic resonance imaging to direct therapy?
IMAGING STUDIES ARE UNLIKELY TO HELP
This man with acute, nonspecific low back pain does not need spinal imaging. Imaging—ie, spine radiography, computed tomography, or magnetic resonance imaging—is unlikely to be helpful in a patient with nonspecific low back pain and may expose him unnecessarily to radiation and the anxiety of findings that are clinically insignificant.
Imaging studies are often ordered inappropriately as part of the evaluation of back pain in patients such as this. In 2008, the total national cost of treating spine (neck and back) problems was estimated to be $86 billion, representing 9% of total health care costs, which is close to the estimated $89 billion per year spent on cancer care.1
Spine imaging should be considered only in patients who have a “red flag” such as advanced age, history of trauma, history of cancer, and prolonged corticosteroid use, all of which have been associated with an increased probability (from 9% to 33%) of either spinal fracture or malignancy.2 Other red flags include duration longer than 6 weeks, fever, weight loss, and progressive neurologic findings on examination. This patient has none of these.
GUIDELINES AND CHOOSING WISELY
High-quality guidelines from different groups recommend against spine imaging in patients with low back pain.3–6 These guidelines vary slightly in their patient populations and definitions of uncomplicated low back pain.
The American College of Radiology4 and the American College of Occupational and Environmental Medicine6 recommend against imaging for patients with both nonspecific and radicular low back pain in the first 6 weeks as long as no red flags are present.
The National Institute for Health and Clinical Excellence3 and, jointly, the American College of Physicians and American Pain Society (ACP/APS)5 recommend against imaging for patients with nonspecific low back pain in both the acute and chronic settings. Nonspecific low back pain is defined as pain without signs of a serious underlying condition (eg, cancer, infection, cauda equina syndrome), spinal stenosis or radiculopathy, or another specific spinal cause (eg, vertebral compression fracture, ankylosing spondylitis).
In addition, imaging in patients with nonspecific low back pain is one of the top five practices that should be questioned by physicians and patients, according to the American Board of Internal Medicine Foundation in its Choosing Wisely campaign (www.choosingwisely.org).
HARMS ASSOCIATED WITH SPINE IMAGING
Several guidelines cite radiation exposure as a potential harmful consequence of spinal imaging by plain radiography and computed tomography. The American College of Radiology guideline4 estimates that the radiation exposure of plain lumbar radiography or lumbar computed tomography ranges between 1 and 10 mSv (3 mSv is the annual amount of ambient radiation in the United States), placing both studies in the medium-range category for relative radiation exposure. The ACP/APS guideline5 states that radiation exposure from imaging is a reason to dissuade clinicians from routine use.
Although lumbar magnetic resonance imaging does not carry the risk of radiation exposure, it may result in harm by detecting clinically insignificant abnormalities in more than 30% of patients.7 These incidental findings increase with age and may lead to additional and possibly unnecessary testing and invasive treatments. The American College of Occupational and Environmental Medicine guideline6 also cites the high prevalence of abnormal findings on plain radiography, magnetic resonance imaging, and other diagnostic tests that are unrelated to symptoms.
CLINICAL BOTTOM LINE
On the basis of current data, the patient described at the beginning of this article should not undergo spine imaging; the results are unlikely to affect his medical management and improve his clinical outcome, and imaging carries a small risk of harm.
A practical approach would be to treat his pain with simple analgesia (a different nonsteroidal anti-inflammatory drug or acetaminophen), address his functional challenges, and reassure him that his chance of having a serious underlying cause of back pain is low (< 1%). He should be told to expect significant improvement in his symptoms within 30 days, be encouraged to stay active, and should be offered patient-focused self-help resources.
The recommendation to conservatively manage patients at low risk without imaging is consistent among all four guidelines. Imaging can be considered for a small subset of patients at high risk with red-flag indications. Potential harms associated with routine imaging of all patients with low back pain include radiation exposure and the high rate of clinically insignificant abnormalities that may lead to unnecessary and invasive interventions that increase expense, patient risk, and anxiety without improving outcomes.
A 38-year-old man is evaluated in an urgent care center for back pain. He is a high school mathematics teacher who reports the insidious onset of low back pain 3 weeks ago. Over the last week the pain has become constant, is worsened by movement, and does not respond to naproxen. He has no history of trauma, malignancy, fever, weight loss, or bladder or bowel symptoms. He does not use intravenous drugs. On examination, he appears uncomfortable and stiff, protecting his back against motion. He has intact sensation, strength, and reflexes. The straight-leg-raising maneuver reproduces his lower back pain but does not cause radicular pain. Should I now order an imaging study such as spinal radiography, computed tomography, or magnetic resonance imaging to direct therapy?
IMAGING STUDIES ARE UNLIKELY TO HELP
This man with acute, nonspecific low back pain does not need spinal imaging. Imaging—ie, spine radiography, computed tomography, or magnetic resonance imaging—is unlikely to be helpful in a patient with nonspecific low back pain and may expose him unnecessarily to radiation and the anxiety of findings that are clinically insignificant.
Imaging studies are often ordered inappropriately as part of the evaluation of back pain in patients such as this. In 2008, the total national cost of treating spine (neck and back) problems was estimated to be $86 billion, representing 9% of total health care costs, which is close to the estimated $89 billion per year spent on cancer care.1
Spine imaging should be considered only in patients who have a “red flag” such as advanced age, history of trauma, history of cancer, and prolonged corticosteroid use, all of which have been associated with an increased probability (from 9% to 33%) of either spinal fracture or malignancy.2 Other red flags include duration longer than 6 weeks, fever, weight loss, and progressive neurologic findings on examination. This patient has none of these.
GUIDELINES AND CHOOSING WISELY
High-quality guidelines from different groups recommend against spine imaging in patients with low back pain.3–6 These guidelines vary slightly in their patient populations and definitions of uncomplicated low back pain.
The American College of Radiology4 and the American College of Occupational and Environmental Medicine6 recommend against imaging for patients with both nonspecific and radicular low back pain in the first 6 weeks as long as no red flags are present.
The National Institute for Health and Clinical Excellence3 and, jointly, the American College of Physicians and American Pain Society (ACP/APS)5 recommend against imaging for patients with nonspecific low back pain in both the acute and chronic settings. Nonspecific low back pain is defined as pain without signs of a serious underlying condition (eg, cancer, infection, cauda equina syndrome), spinal stenosis or radiculopathy, or another specific spinal cause (eg, vertebral compression fracture, ankylosing spondylitis).
In addition, imaging in patients with nonspecific low back pain is one of the top five practices that should be questioned by physicians and patients, according to the American Board of Internal Medicine Foundation in its Choosing Wisely campaign (www.choosingwisely.org).
HARMS ASSOCIATED WITH SPINE IMAGING
Several guidelines cite radiation exposure as a potential harmful consequence of spinal imaging by plain radiography and computed tomography. The American College of Radiology guideline4 estimates that the radiation exposure of plain lumbar radiography or lumbar computed tomography ranges between 1 and 10 mSv (3 mSv is the annual amount of ambient radiation in the United States), placing both studies in the medium-range category for relative radiation exposure. The ACP/APS guideline5 states that radiation exposure from imaging is a reason to dissuade clinicians from routine use.
Although lumbar magnetic resonance imaging does not carry the risk of radiation exposure, it may result in harm by detecting clinically insignificant abnormalities in more than 30% of patients.7 These incidental findings increase with age and may lead to additional and possibly unnecessary testing and invasive treatments. The American College of Occupational and Environmental Medicine guideline6 also cites the high prevalence of abnormal findings on plain radiography, magnetic resonance imaging, and other diagnostic tests that are unrelated to symptoms.
CLINICAL BOTTOM LINE
On the basis of current data, the patient described at the beginning of this article should not undergo spine imaging; the results are unlikely to affect his medical management and improve his clinical outcome, and imaging carries a small risk of harm.
A practical approach would be to treat his pain with simple analgesia (a different nonsteroidal anti-inflammatory drug or acetaminophen), address his functional challenges, and reassure him that his chance of having a serious underlying cause of back pain is low (< 1%). He should be told to expect significant improvement in his symptoms within 30 days, be encouraged to stay active, and should be offered patient-focused self-help resources.
The recommendation to conservatively manage patients at low risk without imaging is consistent among all four guidelines. Imaging can be considered for a small subset of patients at high risk with red-flag indications. Potential harms associated with routine imaging of all patients with low back pain include radiation exposure and the high rate of clinically insignificant abnormalities that may lead to unnecessary and invasive interventions that increase expense, patient risk, and anxiety without improving outcomes.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299:656–664. Erratum in: JAMA 2008; 299:2630.
- Downie A, Williams CM, Henschke N, et al. Red flags to screen for malignancy and fracture in patients with low back pain: systematic review. BMJ 2013; 347:f7095.
- National Collaborating Centre for Primary Care. Low back pain. Early management of persistent nonspecific low back pain. London (UK): National Institute for Health and Clinical Excellence (NICE); 2009 May.25p. (Clinical guideline; no. 88) http://guidelines.gov/content.aspx?id=14699&search=low+back+pain. http://guidance.nice.org.uk/CG88. Accessed May 23, 2014
- Davis PC, Wippold FJ, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria® low back pain. Reston, VA: American College of Radiology (ACR); 2011. www.guideline.gov/content.aspx?id=35145. Accessed May 23, 2014.
- Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147:478–491. Erratum in: Ann Intern Med 2008; 148:247–248.
- Low back disorders. In:Hegmann KT, editor. Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine (ACOEM); 2011:333–796. www.guideline.gov/content.aspx?id=38438. Accessed May 23, 2014.
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990; 72:403–408.
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299:656–664. Erratum in: JAMA 2008; 299:2630.
- Downie A, Williams CM, Henschke N, et al. Red flags to screen for malignancy and fracture in patients with low back pain: systematic review. BMJ 2013; 347:f7095.
- National Collaborating Centre for Primary Care. Low back pain. Early management of persistent nonspecific low back pain. London (UK): National Institute for Health and Clinical Excellence (NICE); 2009 May.25p. (Clinical guideline; no. 88) http://guidelines.gov/content.aspx?id=14699&search=low+back+pain. http://guidance.nice.org.uk/CG88. Accessed May 23, 2014
- Davis PC, Wippold FJ, Cornelius RS, et al; Expert Panel on Neurologic Imaging. ACR appropriateness criteria® low back pain. Reston, VA: American College of Radiology (ACR); 2011. www.guideline.gov/content.aspx?id=35145. Accessed May 23, 2014.
- Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147:478–491. Erratum in: Ann Intern Med 2008; 148:247–248.
- Low back disorders. In:Hegmann KT, editor. Occupational Medicine Practice Guidelines. Evaluation and Management of Common Health Problems and Functional Recovery in Workers. 3rd ed. Elk Grove Village, IL: American College of Occupational and Environmental Medicine (ACOEM); 2011:333–796. www.guideline.gov/content.aspx?id=38438. Accessed May 23, 2014.
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990; 72:403–408.
Better care is the best defense: High-value clinical practice vs defensive medicine
"I view every patient as a potential lawsuit." This statement is jolting. Yet more than 69% of neurosurgeons in a recent study said they agreed or strongly agreed with this survey question.1 What are its implications for patients, for clinical practice, and for the US health care system?
There are many frustrations in the delivery of health care today, for patients as well as for physicians. For physicians, concern about medical liability is a large one, with secondary implications for both health care costs and quality. The Institute of Medicine has estimated that $765 billion—or 30 cents out of every dollar spent on health care—is wasted annually in the United States, adding to the financial burden of health care without benefiting patients.2 A significant portion of this waste, estimated at $210 billion, is related to unnecessary services that are under the control of physicians, including overuse and misuse of diagnostic testing and treatment. This type of care is not only wasteful, but also has the potential to harm.
Factors thought to be responsible for this inappropriate care include the expectations of patients, physician or patient discomfort with uncertainty, and unnecessary and costly consultations. But the factor that physicians cite most often is concern about malpractice suits, raised by 76% of physicians responding to a survey.3
THE DILEMMAS ILLUSTRATED
Here are two cases—to which we will return later—that illustrate the dilemmas faced by physicians deciding how aggressively to pursue a diagnosis:
Patient 1. A 32-year-old woman comes to your office for evaluation of intermittent headaches over the past year. After a detailed history and a normal physical examination, you believe that these are tension headaches. Should you order an imaging study of the brain, just to avoid the risk of a malpractice suit in the unlikely event that this could be the presenting symptom of a brain tumor?
Patient 2. A 60-year-old man presents to the emergency room with pleuritic chest pain. Calculation of pretest probability by modified Wells criteria indicates that pulmonary embolus is unlikely. Because missing the diagnosis can lead to a malpractice suit, should you still order computed tomographic (CT) pulmonary angiography to rule out an embolus?
JUST ONE MORE TEST CAN’T HURT…
Defensive medicine is the ordering or avoiding of tests or procedures primarily out of concern about malpractice liability.4 It increases health care costs, but by how much is unclear.5 It can harm the patient-physician relationship and trust and can also harm patients, especially if overtesting and treatment lead to false-positive results and more tests, which actually can result in liability. And it is not the highest-value care for patients.
Physicians have an ethical duty to do what is best for the individual patient; they also have a responsibility to society to practice effective health care that uses resources responsibly.6 And despite telling ourselves and our patients that one more test will give us confirmation of results and therefore comfort, a recent review found that tests performed based on symptoms with low risk of being caused by serious illness “do little to reassure patients, decrease their anxiety, or resolve their symptoms.”7
MALPRACTICE LIABILITY RISK: PERCEPTION AND REALITY
Physicians often overestimate their risk of liability. Only a small percentage (5%) of claims go to trial, and of those, 90% are won by the physician, according to a 2008 analysis by the Physician Insurers Association of America.8 A study of claims between 2002 and 2005 found that 4.5% of claims resulted in trial verdicts, of which 80% were in favor of the physician, with cases against internists and internal medicine-based subspecialists least likely to result in a trial verdict (2.7%).9
Even so, being sued is extremely stressful and is associated with distinct physical and emotional distress for most physicians.10,11 Charles has found that, “As a group, physicians are acutely sensitive to any suggestions that they have failed to meet the standard of care or are not ‘good’ doctors… This accusation of failure represents a personal assault.”11
Physician concerns about liability are not very different in states with tort reforms such as damage caps compared with those without.5 Some posit that physicians may overestimate the risk of liability as part of the human tendency to overestimate the risk of rare events that are difficult to experience and difficult to control.12
In the study of neurosurgeons cited previously, 72% of respondents said they ordered imaging, 67% did laboratory tests, and 66% referred patients for consults “solely” to “minimize the risk of a lawsuit.”1 The authors of the study maintain that, over time, this affects the standard of care. “While physicians in the past may have used a thorough history and physical to guide imaging, in this study, 72% of neurosurgeons surveyed stated that they order additional imaging studies solely to mitigate liability risk. This suggests that in reality, imaging is becoming a standard part of the initial workup.”1 Unfortunately, this new standard of care is based on false assumptions and is artificially and inappropriately changed. That perception of liability risk deeply influences practice.
DO THE RIGHT THING: AVOIDING UNNECESSARY TESTING
But physicians also acknowledge the need to follow practice guidelines and to avoid unnecessary testing. In one survey,13 79% strongly or moderately agreed with the statement that physicians “should adhere to clinical guidelines that discourage the use of interventions that have a small proven advantage over standard intervention but cost much more”; 89% strongly or moderately agreed that “doctors need to take a more prominent role in limiting use of unnecessary tests”; and 78% said they “should be solely devoted to individual patients’ best interests, even if that is expensive.”13
This may be summarized as, “Provide the clinically appropriate care to the patient based on the best evidence.” But of course, this is easier said than done.
THE ROLE OF EVIDENCE-BASED GUIDELINES
Evidence-based practice guidelines can help support the provision of clinically (and ethically) appropriate care. Medical custom—the care expected of reasonable clinicians under similar circumstances—is generally the legal standard in determining whether a clinician has met a duty of care to a patient in a lawsuit.14 But practice guidelines can provide strong evidence of what constitutes reasonable care and can, over time, help set the standard for quality of care.
Clinical practice guidelines have grown in recent years, especially after the Institute of Medicine embraced them as a means to address variation in practice patterns and quality of care. But guidelines can conflict. Their effective implementation relies on clinical judgment. If a guideline is not appropriate in a particular case, documentation of why the guideline was not followed may prove prudent. Guidelines are not a safe harbor and have and will be used both defensively and offensively. They are not the last word, but rather another type of expert evidence.15 However, they are an important one. At the end of the day, the best care is the best defense.
Guidelines not only educate physicians, they also should be used by physicians to educate patients. In addition to developing guidelines for physicians, professional societies should develop and disseminate public education materials that inform patients and their families and caregivers about clinically appropriate care and the problems resulting from overuse and misuse of care.
GETTING BACK TO BASICS
Kroenke noted that preliminary data suggest that the history typically accounts for 75% or more of the diagnostic yield when evaluating common symptoms, the physical examination 10% to 15%, and testing generally less than 10%.16 Yet health care reimbursement in the United States contains incentives in precisely the reverse order. So, not surprisingly, we keep on testing away. Kroenke says that countering the rush to test will be as challenging and slow as trying to reverse a generation of antibiotic overprescribing.16
Over time, our reliance on technology as a diagnostic tool has increased, with less emphasis on the history and particularly on the physical examination to solve diagnostic puzzles. Yet most diagnostic errors in a study of outpatient primary care visits were related to breakdowns in the clinical interaction, including the taking of the medical history, the performance of the physical examination, and the ordering of tests. Technologies such as the electronic health record, which can assist in the care of patients, are also a potential source of error and shortcuts in care, as when copying and pasting is used inappropriately.17 Recognizing the increasing use of technology in practice and team-based approaches to improving care, Singh et al have called for caution and for more “focus on basic clinical skills and related cognitive processes.”18
The erosion of physical examination skills, discomfort with diagnostic uncertainty, and fear of malpractice litigation have combined to create a perfect storm of technologic overuse and misuse. Unfortunately, this means that our modus operandi is all too frequently built around testing rather than touching.19
At the same time, it is well established that patients often sue because of dissatisfaction, especially with physician communication and interpersonal skills.14 Emphasizing the basic skills that include taking a carefully crafted history, performing a skillful physical examination, and communicating effectively and compassionately with patients at every encounter is probably the most successful strategy for simultaneously avoiding malpractice litigation, reducing overused and misused diagnostic testing, and conserving precious health care resources.
Another part of the strategy should include routinely considering a number of straightforward questions before ordering diagnostic tests, such as “Will the test result change my care of the patient?” and “How does ordering this test compare in value with other management strategies for the patient?”20,21
RETURNING TO THE CASES
Regarding patient 1, the 32-year-old woman with intermittent headaches, the American College of Radiology identified imaging for headache in its list of five areas submitted to the Choosing Wisely campaign in which care may be overused or misused. Specifically, the American College of Radiology says, “Don’t do imaging for uncomplicated headache” in the absence of specific risk factors for structural disease, noting that “incidental findings lead to additional medical procedures and expense that do not improve patient well-being.”22
For patient 2, the 60-year-old man with pleuritic chest pain, both the American College of Physicians and the American College of Radiology strongly recommend against CT pulmonary angiography for patients in whom calculation of pretest probability indicates a low pretest probability of pulmonary embolism.22,23 Patients such as these should undergo D-dimer testing rather than CT pulmonary angiography. In this setting, a negative D-dimer test effectively rules out pulmonary embolism and avoids both the radiation and cost associated with the unnecessary imaging study.
According to the Ethics Manual of the American College of Physicians,6 “physicians have an obligation to promote their patients’ welfare in an increasingly complex health care system. This entails forthrightly helping patients to understand clinical recommendations and make informed choices among all appropriate care options… It also includes stewardship of finite health care resources so that as many health care needs as possible can be met, whether in the physician’s office, in the hospital or long-term care facility, or at home.”6 The basic principles of beneficence and nonmaleficence are aligned with doing the right thing for our patients—ie, providing the appropriate care at the right time and avoiding too much care or too little care. Guided by scientific evidence as well as by guidelines and official recommendations based on such evidence, we are in the best position to provide optimal care for our patients while simultaneously minimizing the risk of malpractice litigation.
As is the case with overprescribing, we must look critically at the inappropriate use of tests and other care applied under the rationale of not wanting to “miss anything”—and the unspoken drivers of financial incentives, new or advanced tests and procedures, and defensive medicine. We know what needs to be done. And nothing short of evidence-based high-value care will do.
Acknowledgment: The authors would like to thank Kathy Wynkoop for editorial assistance.
- Nahed BV, Babu MA, Smith TR, Heary RF. Malpractice liability and defensive medicine: a national survey of neurosurgeons. PLoS One 2012; 7:e39237.
- Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: The National Academies Press, 2010.
- Sirovich BE, Woloshin S, Schwartz LM. Too Little? Too Much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med 2011; 171:1582–1585.
- US Congress Office of Technology Assessment. Defensive Medicine and Medical Malpractice, OTA-H–6O2. Washington, DC: US Government Printing Office, 1994.
- Carrier ER, Reschovsky JD, Katz DA, Mello MM. High physician concern about malpractice risk predicts more aggressive diagnostic testing in office-based practice. Health Aff (Millwood) 2013; 32:1383–1391.
- Snyder LAmerican College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual: sixth edition. Ann Intern Med 2012; 156:73–104.
- Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med 2013; 173:407–416.
- Kane CK. Policy research perspectives: medical liability claim frequency: a 2007–2008 snapshot of physicians. American Medical Association, 2010. Available at www.ama-assn.org. Accessed July 2, 2014.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med 2012; 172:892–894.
- Charles SC, Pyskoty CE, Nelson A. Physicians on trial—self-reported reactions to malpractice trials. West J Med 1988; 148:358–360.
- Charles SC. Coping with a medical malpractice suit. West J Med 2001; 174:55–58.
- Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood) 2010; 29:1585–1592.
- Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US physicians about controlling health care costs. JAMA 2013; 310:380–388.
- Studdert DM, Mello MM, Brennan TA. Medical malpractice. N Engl J Med 2004; 350:283–292.
- Mehlman MJ. Medical practice guidelines as malpractice safe harbors: illusion or deceit? J Law Med Ethics 2012; 40:286–300.
- Kroenke K. Diagnostic testing and the illusory reassurance of normal results: comment on “Reassurance after diagnostic testing with a low pretest probability of serious disease.” JAMA Intern Med 2013; 173:416–417.
- Rattner S, Mathes M, Siegler E. Copy and pasted and misdiagnosed (or cloned notes and blind alleys). ACP Ethics Case Study CME program. Available at https://www.acponline.org/running_practice/ethics/case_studies/. Accessed July 2, 2014.
- Singh H, Giardina TD, Meyer AN, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med 2013; 173:418–425.
- Verghese A, Brady E, Kapur CC, Horwitz RI. The bedside evaluation: ritual and reason. Ann Intern Med 2011; 155:550–553.
- Laine C. High-value testing begins with a few simple questions. Ann Intern Med 2012; 156:162–163.
- Weinberger SE. Providing high-value, cost-conscious care: a critical seventh general competency for physicians. Ann Intern Med 2011; 155:386–388.
- American College of Radiology (ACR). Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/american-college-of-radiology/. Accessed July 2, 2014.
- American College of Physicians (ACP). Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/american-college-of-physicians/. Accessed July 2, 2014.
"I view every patient as a potential lawsuit." This statement is jolting. Yet more than 69% of neurosurgeons in a recent study said they agreed or strongly agreed with this survey question.1 What are its implications for patients, for clinical practice, and for the US health care system?
There are many frustrations in the delivery of health care today, for patients as well as for physicians. For physicians, concern about medical liability is a large one, with secondary implications for both health care costs and quality. The Institute of Medicine has estimated that $765 billion—or 30 cents out of every dollar spent on health care—is wasted annually in the United States, adding to the financial burden of health care without benefiting patients.2 A significant portion of this waste, estimated at $210 billion, is related to unnecessary services that are under the control of physicians, including overuse and misuse of diagnostic testing and treatment. This type of care is not only wasteful, but also has the potential to harm.
Factors thought to be responsible for this inappropriate care include the expectations of patients, physician or patient discomfort with uncertainty, and unnecessary and costly consultations. But the factor that physicians cite most often is concern about malpractice suits, raised by 76% of physicians responding to a survey.3
THE DILEMMAS ILLUSTRATED
Here are two cases—to which we will return later—that illustrate the dilemmas faced by physicians deciding how aggressively to pursue a diagnosis:
Patient 1. A 32-year-old woman comes to your office for evaluation of intermittent headaches over the past year. After a detailed history and a normal physical examination, you believe that these are tension headaches. Should you order an imaging study of the brain, just to avoid the risk of a malpractice suit in the unlikely event that this could be the presenting symptom of a brain tumor?
Patient 2. A 60-year-old man presents to the emergency room with pleuritic chest pain. Calculation of pretest probability by modified Wells criteria indicates that pulmonary embolus is unlikely. Because missing the diagnosis can lead to a malpractice suit, should you still order computed tomographic (CT) pulmonary angiography to rule out an embolus?
JUST ONE MORE TEST CAN’T HURT…
Defensive medicine is the ordering or avoiding of tests or procedures primarily out of concern about malpractice liability.4 It increases health care costs, but by how much is unclear.5 It can harm the patient-physician relationship and trust and can also harm patients, especially if overtesting and treatment lead to false-positive results and more tests, which actually can result in liability. And it is not the highest-value care for patients.
Physicians have an ethical duty to do what is best for the individual patient; they also have a responsibility to society to practice effective health care that uses resources responsibly.6 And despite telling ourselves and our patients that one more test will give us confirmation of results and therefore comfort, a recent review found that tests performed based on symptoms with low risk of being caused by serious illness “do little to reassure patients, decrease their anxiety, or resolve their symptoms.”7
MALPRACTICE LIABILITY RISK: PERCEPTION AND REALITY
Physicians often overestimate their risk of liability. Only a small percentage (5%) of claims go to trial, and of those, 90% are won by the physician, according to a 2008 analysis by the Physician Insurers Association of America.8 A study of claims between 2002 and 2005 found that 4.5% of claims resulted in trial verdicts, of which 80% were in favor of the physician, with cases against internists and internal medicine-based subspecialists least likely to result in a trial verdict (2.7%).9
Even so, being sued is extremely stressful and is associated with distinct physical and emotional distress for most physicians.10,11 Charles has found that, “As a group, physicians are acutely sensitive to any suggestions that they have failed to meet the standard of care or are not ‘good’ doctors… This accusation of failure represents a personal assault.”11
Physician concerns about liability are not very different in states with tort reforms such as damage caps compared with those without.5 Some posit that physicians may overestimate the risk of liability as part of the human tendency to overestimate the risk of rare events that are difficult to experience and difficult to control.12
In the study of neurosurgeons cited previously, 72% of respondents said they ordered imaging, 67% did laboratory tests, and 66% referred patients for consults “solely” to “minimize the risk of a lawsuit.”1 The authors of the study maintain that, over time, this affects the standard of care. “While physicians in the past may have used a thorough history and physical to guide imaging, in this study, 72% of neurosurgeons surveyed stated that they order additional imaging studies solely to mitigate liability risk. This suggests that in reality, imaging is becoming a standard part of the initial workup.”1 Unfortunately, this new standard of care is based on false assumptions and is artificially and inappropriately changed. That perception of liability risk deeply influences practice.
DO THE RIGHT THING: AVOIDING UNNECESSARY TESTING
But physicians also acknowledge the need to follow practice guidelines and to avoid unnecessary testing. In one survey,13 79% strongly or moderately agreed with the statement that physicians “should adhere to clinical guidelines that discourage the use of interventions that have a small proven advantage over standard intervention but cost much more”; 89% strongly or moderately agreed that “doctors need to take a more prominent role in limiting use of unnecessary tests”; and 78% said they “should be solely devoted to individual patients’ best interests, even if that is expensive.”13
This may be summarized as, “Provide the clinically appropriate care to the patient based on the best evidence.” But of course, this is easier said than done.
THE ROLE OF EVIDENCE-BASED GUIDELINES
Evidence-based practice guidelines can help support the provision of clinically (and ethically) appropriate care. Medical custom—the care expected of reasonable clinicians under similar circumstances—is generally the legal standard in determining whether a clinician has met a duty of care to a patient in a lawsuit.14 But practice guidelines can provide strong evidence of what constitutes reasonable care and can, over time, help set the standard for quality of care.
Clinical practice guidelines have grown in recent years, especially after the Institute of Medicine embraced them as a means to address variation in practice patterns and quality of care. But guidelines can conflict. Their effective implementation relies on clinical judgment. If a guideline is not appropriate in a particular case, documentation of why the guideline was not followed may prove prudent. Guidelines are not a safe harbor and have and will be used both defensively and offensively. They are not the last word, but rather another type of expert evidence.15 However, they are an important one. At the end of the day, the best care is the best defense.
Guidelines not only educate physicians, they also should be used by physicians to educate patients. In addition to developing guidelines for physicians, professional societies should develop and disseminate public education materials that inform patients and their families and caregivers about clinically appropriate care and the problems resulting from overuse and misuse of care.
GETTING BACK TO BASICS
Kroenke noted that preliminary data suggest that the history typically accounts for 75% or more of the diagnostic yield when evaluating common symptoms, the physical examination 10% to 15%, and testing generally less than 10%.16 Yet health care reimbursement in the United States contains incentives in precisely the reverse order. So, not surprisingly, we keep on testing away. Kroenke says that countering the rush to test will be as challenging and slow as trying to reverse a generation of antibiotic overprescribing.16
Over time, our reliance on technology as a diagnostic tool has increased, with less emphasis on the history and particularly on the physical examination to solve diagnostic puzzles. Yet most diagnostic errors in a study of outpatient primary care visits were related to breakdowns in the clinical interaction, including the taking of the medical history, the performance of the physical examination, and the ordering of tests. Technologies such as the electronic health record, which can assist in the care of patients, are also a potential source of error and shortcuts in care, as when copying and pasting is used inappropriately.17 Recognizing the increasing use of technology in practice and team-based approaches to improving care, Singh et al have called for caution and for more “focus on basic clinical skills and related cognitive processes.”18
The erosion of physical examination skills, discomfort with diagnostic uncertainty, and fear of malpractice litigation have combined to create a perfect storm of technologic overuse and misuse. Unfortunately, this means that our modus operandi is all too frequently built around testing rather than touching.19
At the same time, it is well established that patients often sue because of dissatisfaction, especially with physician communication and interpersonal skills.14 Emphasizing the basic skills that include taking a carefully crafted history, performing a skillful physical examination, and communicating effectively and compassionately with patients at every encounter is probably the most successful strategy for simultaneously avoiding malpractice litigation, reducing overused and misused diagnostic testing, and conserving precious health care resources.
Another part of the strategy should include routinely considering a number of straightforward questions before ordering diagnostic tests, such as “Will the test result change my care of the patient?” and “How does ordering this test compare in value with other management strategies for the patient?”20,21
RETURNING TO THE CASES
Regarding patient 1, the 32-year-old woman with intermittent headaches, the American College of Radiology identified imaging for headache in its list of five areas submitted to the Choosing Wisely campaign in which care may be overused or misused. Specifically, the American College of Radiology says, “Don’t do imaging for uncomplicated headache” in the absence of specific risk factors for structural disease, noting that “incidental findings lead to additional medical procedures and expense that do not improve patient well-being.”22
For patient 2, the 60-year-old man with pleuritic chest pain, both the American College of Physicians and the American College of Radiology strongly recommend against CT pulmonary angiography for patients in whom calculation of pretest probability indicates a low pretest probability of pulmonary embolism.22,23 Patients such as these should undergo D-dimer testing rather than CT pulmonary angiography. In this setting, a negative D-dimer test effectively rules out pulmonary embolism and avoids both the radiation and cost associated with the unnecessary imaging study.
According to the Ethics Manual of the American College of Physicians,6 “physicians have an obligation to promote their patients’ welfare in an increasingly complex health care system. This entails forthrightly helping patients to understand clinical recommendations and make informed choices among all appropriate care options… It also includes stewardship of finite health care resources so that as many health care needs as possible can be met, whether in the physician’s office, in the hospital or long-term care facility, or at home.”6 The basic principles of beneficence and nonmaleficence are aligned with doing the right thing for our patients—ie, providing the appropriate care at the right time and avoiding too much care or too little care. Guided by scientific evidence as well as by guidelines and official recommendations based on such evidence, we are in the best position to provide optimal care for our patients while simultaneously minimizing the risk of malpractice litigation.
As is the case with overprescribing, we must look critically at the inappropriate use of tests and other care applied under the rationale of not wanting to “miss anything”—and the unspoken drivers of financial incentives, new or advanced tests and procedures, and defensive medicine. We know what needs to be done. And nothing short of evidence-based high-value care will do.
Acknowledgment: The authors would like to thank Kathy Wynkoop for editorial assistance.
"I view every patient as a potential lawsuit." This statement is jolting. Yet more than 69% of neurosurgeons in a recent study said they agreed or strongly agreed with this survey question.1 What are its implications for patients, for clinical practice, and for the US health care system?
There are many frustrations in the delivery of health care today, for patients as well as for physicians. For physicians, concern about medical liability is a large one, with secondary implications for both health care costs and quality. The Institute of Medicine has estimated that $765 billion—or 30 cents out of every dollar spent on health care—is wasted annually in the United States, adding to the financial burden of health care without benefiting patients.2 A significant portion of this waste, estimated at $210 billion, is related to unnecessary services that are under the control of physicians, including overuse and misuse of diagnostic testing and treatment. This type of care is not only wasteful, but also has the potential to harm.
Factors thought to be responsible for this inappropriate care include the expectations of patients, physician or patient discomfort with uncertainty, and unnecessary and costly consultations. But the factor that physicians cite most often is concern about malpractice suits, raised by 76% of physicians responding to a survey.3
THE DILEMMAS ILLUSTRATED
Here are two cases—to which we will return later—that illustrate the dilemmas faced by physicians deciding how aggressively to pursue a diagnosis:
Patient 1. A 32-year-old woman comes to your office for evaluation of intermittent headaches over the past year. After a detailed history and a normal physical examination, you believe that these are tension headaches. Should you order an imaging study of the brain, just to avoid the risk of a malpractice suit in the unlikely event that this could be the presenting symptom of a brain tumor?
Patient 2. A 60-year-old man presents to the emergency room with pleuritic chest pain. Calculation of pretest probability by modified Wells criteria indicates that pulmonary embolus is unlikely. Because missing the diagnosis can lead to a malpractice suit, should you still order computed tomographic (CT) pulmonary angiography to rule out an embolus?
JUST ONE MORE TEST CAN’T HURT…
Defensive medicine is the ordering or avoiding of tests or procedures primarily out of concern about malpractice liability.4 It increases health care costs, but by how much is unclear.5 It can harm the patient-physician relationship and trust and can also harm patients, especially if overtesting and treatment lead to false-positive results and more tests, which actually can result in liability. And it is not the highest-value care for patients.
Physicians have an ethical duty to do what is best for the individual patient; they also have a responsibility to society to practice effective health care that uses resources responsibly.6 And despite telling ourselves and our patients that one more test will give us confirmation of results and therefore comfort, a recent review found that tests performed based on symptoms with low risk of being caused by serious illness “do little to reassure patients, decrease their anxiety, or resolve their symptoms.”7
MALPRACTICE LIABILITY RISK: PERCEPTION AND REALITY
Physicians often overestimate their risk of liability. Only a small percentage (5%) of claims go to trial, and of those, 90% are won by the physician, according to a 2008 analysis by the Physician Insurers Association of America.8 A study of claims between 2002 and 2005 found that 4.5% of claims resulted in trial verdicts, of which 80% were in favor of the physician, with cases against internists and internal medicine-based subspecialists least likely to result in a trial verdict (2.7%).9
Even so, being sued is extremely stressful and is associated with distinct physical and emotional distress for most physicians.10,11 Charles has found that, “As a group, physicians are acutely sensitive to any suggestions that they have failed to meet the standard of care or are not ‘good’ doctors… This accusation of failure represents a personal assault.”11
Physician concerns about liability are not very different in states with tort reforms such as damage caps compared with those without.5 Some posit that physicians may overestimate the risk of liability as part of the human tendency to overestimate the risk of rare events that are difficult to experience and difficult to control.12
In the study of neurosurgeons cited previously, 72% of respondents said they ordered imaging, 67% did laboratory tests, and 66% referred patients for consults “solely” to “minimize the risk of a lawsuit.”1 The authors of the study maintain that, over time, this affects the standard of care. “While physicians in the past may have used a thorough history and physical to guide imaging, in this study, 72% of neurosurgeons surveyed stated that they order additional imaging studies solely to mitigate liability risk. This suggests that in reality, imaging is becoming a standard part of the initial workup.”1 Unfortunately, this new standard of care is based on false assumptions and is artificially and inappropriately changed. That perception of liability risk deeply influences practice.
DO THE RIGHT THING: AVOIDING UNNECESSARY TESTING
But physicians also acknowledge the need to follow practice guidelines and to avoid unnecessary testing. In one survey,13 79% strongly or moderately agreed with the statement that physicians “should adhere to clinical guidelines that discourage the use of interventions that have a small proven advantage over standard intervention but cost much more”; 89% strongly or moderately agreed that “doctors need to take a more prominent role in limiting use of unnecessary tests”; and 78% said they “should be solely devoted to individual patients’ best interests, even if that is expensive.”13
This may be summarized as, “Provide the clinically appropriate care to the patient based on the best evidence.” But of course, this is easier said than done.
THE ROLE OF EVIDENCE-BASED GUIDELINES
Evidence-based practice guidelines can help support the provision of clinically (and ethically) appropriate care. Medical custom—the care expected of reasonable clinicians under similar circumstances—is generally the legal standard in determining whether a clinician has met a duty of care to a patient in a lawsuit.14 But practice guidelines can provide strong evidence of what constitutes reasonable care and can, over time, help set the standard for quality of care.
Clinical practice guidelines have grown in recent years, especially after the Institute of Medicine embraced them as a means to address variation in practice patterns and quality of care. But guidelines can conflict. Their effective implementation relies on clinical judgment. If a guideline is not appropriate in a particular case, documentation of why the guideline was not followed may prove prudent. Guidelines are not a safe harbor and have and will be used both defensively and offensively. They are not the last word, but rather another type of expert evidence.15 However, they are an important one. At the end of the day, the best care is the best defense.
Guidelines not only educate physicians, they also should be used by physicians to educate patients. In addition to developing guidelines for physicians, professional societies should develop and disseminate public education materials that inform patients and their families and caregivers about clinically appropriate care and the problems resulting from overuse and misuse of care.
GETTING BACK TO BASICS
Kroenke noted that preliminary data suggest that the history typically accounts for 75% or more of the diagnostic yield when evaluating common symptoms, the physical examination 10% to 15%, and testing generally less than 10%.16 Yet health care reimbursement in the United States contains incentives in precisely the reverse order. So, not surprisingly, we keep on testing away. Kroenke says that countering the rush to test will be as challenging and slow as trying to reverse a generation of antibiotic overprescribing.16
Over time, our reliance on technology as a diagnostic tool has increased, with less emphasis on the history and particularly on the physical examination to solve diagnostic puzzles. Yet most diagnostic errors in a study of outpatient primary care visits were related to breakdowns in the clinical interaction, including the taking of the medical history, the performance of the physical examination, and the ordering of tests. Technologies such as the electronic health record, which can assist in the care of patients, are also a potential source of error and shortcuts in care, as when copying and pasting is used inappropriately.17 Recognizing the increasing use of technology in practice and team-based approaches to improving care, Singh et al have called for caution and for more “focus on basic clinical skills and related cognitive processes.”18
The erosion of physical examination skills, discomfort with diagnostic uncertainty, and fear of malpractice litigation have combined to create a perfect storm of technologic overuse and misuse. Unfortunately, this means that our modus operandi is all too frequently built around testing rather than touching.19
At the same time, it is well established that patients often sue because of dissatisfaction, especially with physician communication and interpersonal skills.14 Emphasizing the basic skills that include taking a carefully crafted history, performing a skillful physical examination, and communicating effectively and compassionately with patients at every encounter is probably the most successful strategy for simultaneously avoiding malpractice litigation, reducing overused and misused diagnostic testing, and conserving precious health care resources.
Another part of the strategy should include routinely considering a number of straightforward questions before ordering diagnostic tests, such as “Will the test result change my care of the patient?” and “How does ordering this test compare in value with other management strategies for the patient?”20,21
RETURNING TO THE CASES
Regarding patient 1, the 32-year-old woman with intermittent headaches, the American College of Radiology identified imaging for headache in its list of five areas submitted to the Choosing Wisely campaign in which care may be overused or misused. Specifically, the American College of Radiology says, “Don’t do imaging for uncomplicated headache” in the absence of specific risk factors for structural disease, noting that “incidental findings lead to additional medical procedures and expense that do not improve patient well-being.”22
For patient 2, the 60-year-old man with pleuritic chest pain, both the American College of Physicians and the American College of Radiology strongly recommend against CT pulmonary angiography for patients in whom calculation of pretest probability indicates a low pretest probability of pulmonary embolism.22,23 Patients such as these should undergo D-dimer testing rather than CT pulmonary angiography. In this setting, a negative D-dimer test effectively rules out pulmonary embolism and avoids both the radiation and cost associated with the unnecessary imaging study.
According to the Ethics Manual of the American College of Physicians,6 “physicians have an obligation to promote their patients’ welfare in an increasingly complex health care system. This entails forthrightly helping patients to understand clinical recommendations and make informed choices among all appropriate care options… It also includes stewardship of finite health care resources so that as many health care needs as possible can be met, whether in the physician’s office, in the hospital or long-term care facility, or at home.”6 The basic principles of beneficence and nonmaleficence are aligned with doing the right thing for our patients—ie, providing the appropriate care at the right time and avoiding too much care or too little care. Guided by scientific evidence as well as by guidelines and official recommendations based on such evidence, we are in the best position to provide optimal care for our patients while simultaneously minimizing the risk of malpractice litigation.
As is the case with overprescribing, we must look critically at the inappropriate use of tests and other care applied under the rationale of not wanting to “miss anything”—and the unspoken drivers of financial incentives, new or advanced tests and procedures, and defensive medicine. We know what needs to be done. And nothing short of evidence-based high-value care will do.
Acknowledgment: The authors would like to thank Kathy Wynkoop for editorial assistance.
- Nahed BV, Babu MA, Smith TR, Heary RF. Malpractice liability and defensive medicine: a national survey of neurosurgeons. PLoS One 2012; 7:e39237.
- Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: The National Academies Press, 2010.
- Sirovich BE, Woloshin S, Schwartz LM. Too Little? Too Much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med 2011; 171:1582–1585.
- US Congress Office of Technology Assessment. Defensive Medicine and Medical Malpractice, OTA-H–6O2. Washington, DC: US Government Printing Office, 1994.
- Carrier ER, Reschovsky JD, Katz DA, Mello MM. High physician concern about malpractice risk predicts more aggressive diagnostic testing in office-based practice. Health Aff (Millwood) 2013; 32:1383–1391.
- Snyder LAmerican College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual: sixth edition. Ann Intern Med 2012; 156:73–104.
- Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med 2013; 173:407–416.
- Kane CK. Policy research perspectives: medical liability claim frequency: a 2007–2008 snapshot of physicians. American Medical Association, 2010. Available at www.ama-assn.org. Accessed July 2, 2014.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med 2012; 172:892–894.
- Charles SC, Pyskoty CE, Nelson A. Physicians on trial—self-reported reactions to malpractice trials. West J Med 1988; 148:358–360.
- Charles SC. Coping with a medical malpractice suit. West J Med 2001; 174:55–58.
- Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood) 2010; 29:1585–1592.
- Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US physicians about controlling health care costs. JAMA 2013; 310:380–388.
- Studdert DM, Mello MM, Brennan TA. Medical malpractice. N Engl J Med 2004; 350:283–292.
- Mehlman MJ. Medical practice guidelines as malpractice safe harbors: illusion or deceit? J Law Med Ethics 2012; 40:286–300.
- Kroenke K. Diagnostic testing and the illusory reassurance of normal results: comment on “Reassurance after diagnostic testing with a low pretest probability of serious disease.” JAMA Intern Med 2013; 173:416–417.
- Rattner S, Mathes M, Siegler E. Copy and pasted and misdiagnosed (or cloned notes and blind alleys). ACP Ethics Case Study CME program. Available at https://www.acponline.org/running_practice/ethics/case_studies/. Accessed July 2, 2014.
- Singh H, Giardina TD, Meyer AN, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med 2013; 173:418–425.
- Verghese A, Brady E, Kapur CC, Horwitz RI. The bedside evaluation: ritual and reason. Ann Intern Med 2011; 155:550–553.
- Laine C. High-value testing begins with a few simple questions. Ann Intern Med 2012; 156:162–163.
- Weinberger SE. Providing high-value, cost-conscious care: a critical seventh general competency for physicians. Ann Intern Med 2011; 155:386–388.
- American College of Radiology (ACR). Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/american-college-of-radiology/. Accessed July 2, 2014.
- American College of Physicians (ACP). Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/american-college-of-physicians/. Accessed July 2, 2014.
- Nahed BV, Babu MA, Smith TR, Heary RF. Malpractice liability and defensive medicine: a national survey of neurosurgeons. PLoS One 2012; 7:e39237.
- Institute of Medicine. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: The National Academies Press, 2010.
- Sirovich BE, Woloshin S, Schwartz LM. Too Little? Too Much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med 2011; 171:1582–1585.
- US Congress Office of Technology Assessment. Defensive Medicine and Medical Malpractice, OTA-H–6O2. Washington, DC: US Government Printing Office, 1994.
- Carrier ER, Reschovsky JD, Katz DA, Mello MM. High physician concern about malpractice risk predicts more aggressive diagnostic testing in office-based practice. Health Aff (Millwood) 2013; 32:1383–1391.
- Snyder LAmerican College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual: sixth edition. Ann Intern Med 2012; 156:73–104.
- Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med 2013; 173:407–416.
- Kane CK. Policy research perspectives: medical liability claim frequency: a 2007–2008 snapshot of physicians. American Medical Association, 2010. Available at www.ama-assn.org. Accessed July 2, 2014.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med 2012; 172:892–894.
- Charles SC, Pyskoty CE, Nelson A. Physicians on trial—self-reported reactions to malpractice trials. West J Med 1988; 148:358–360.
- Charles SC. Coping with a medical malpractice suit. West J Med 2001; 174:55–58.
- Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood) 2010; 29:1585–1592.
- Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US physicians about controlling health care costs. JAMA 2013; 310:380–388.
- Studdert DM, Mello MM, Brennan TA. Medical malpractice. N Engl J Med 2004; 350:283–292.
- Mehlman MJ. Medical practice guidelines as malpractice safe harbors: illusion or deceit? J Law Med Ethics 2012; 40:286–300.
- Kroenke K. Diagnostic testing and the illusory reassurance of normal results: comment on “Reassurance after diagnostic testing with a low pretest probability of serious disease.” JAMA Intern Med 2013; 173:416–417.
- Rattner S, Mathes M, Siegler E. Copy and pasted and misdiagnosed (or cloned notes and blind alleys). ACP Ethics Case Study CME program. Available at https://www.acponline.org/running_practice/ethics/case_studies/. Accessed July 2, 2014.
- Singh H, Giardina TD, Meyer AN, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med 2013; 173:418–425.
- Verghese A, Brady E, Kapur CC, Horwitz RI. The bedside evaluation: ritual and reason. Ann Intern Med 2011; 155:550–553.
- Laine C. High-value testing begins with a few simple questions. Ann Intern Med 2012; 156:162–163.
- Weinberger SE. Providing high-value, cost-conscious care: a critical seventh general competency for physicians. Ann Intern Med 2011; 155:386–388.
- American College of Radiology (ACR). Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/american-college-of-radiology/. Accessed July 2, 2014.
- American College of Physicians (ACP). Choosing Wisely. Five things physicians and patients should question. http://www.choosingwisely.org/doctor-patient-lists/american-college-of-physicians/. Accessed July 2, 2014.