User login
What would Charles Darwin say about collaborating with and training other specialties?
Vascular surgery has evolved from a limited subspecialty of general and thoracic surgery into a complex and well-defined specialty. The introduction of endovascular treatments and their adoption and embrace by vascular surgeons has made our specialty exciting and attractive.
However, the increasing importance of these endovascular treatments also poses some dangers to vascular surgery. By using these endovascular techniques, other specialists have the tools to treat vascular lesions in vascular patients who previously could be treated by vascular surgeons only.
Some of these other specialists have contributed to the development of endovascular treatment techniques and therefore have a legitimate claim to use these techniques on vascular patients. This certainly applies to interventional radiologists (IRs) and to some extent to interventional cardiologists (ICs).
More recently, cardiac surgeons, whose practices have been diminished by the development of better coronary stents and now transcatheter valves, are venturing more into the treatment of noncardiac vascular lesions and are trying, somewhat belatedly, to become expert in endovascular skills and methods and are using them to treat a panoply of noncardiac vascular lesions. To facilitate this, combined training programs have been proposed.
What is the impact of all this multispecialty outreach, and what is its effect on vascular patients and vascular surgeons? To the extent that different specialists can learn from each other’s skills and techniques and can cross train each other, it is probably a good thing for doctors and patients.
However, this multispecialty interest in noncardiac vascular lesions and patients has some serious potential downsides. When specialists expand their scope of practice to new areas as an add-on to their primary practice, they run the risk of becoming dabblers.
They may be able to use techniques without the appropriate knowledge base to know when the techniques should be used. As a result, they may do more harm than good, and health care costs will rise. In addition, the pool of patients justifiably needing treatment is limited. Thus, if more specialists consider themselves capable of treating a given lesion, it will surely increase the number of unnecessary procedures and complications. All these effects will be bad for patients and the health care system.
In 1996, in an SVS presidential address titled "Charles Darwin and Vascular Surgery" ( J. Vasc. Surg. 1997;25:8-18), I predicted the increasing importance of endovascular treatments and how they would replace most open surgical procedures. My predictions then, thought by most to be too high, have proven actually to be too low.
In that presidential address, I advised vascular surgeons to become endo-competent to avoid extinction. Thankfully they have done so. Because of this, our specialty survives in the endovascular era. I also advocated that vascular surgeons work together with other specialists (IRs and ICs) in vascular centers for the betterment of patient care.
With a few exceptions this has not happened for many reasons which can best be summarized as due to human nature with its tendencies to tribalism, self-interest, and competition for patients and dollars.
Undoubtedly these tendencies will continue to have negative consequences on the care of vascular patients. Nevertheless, vascular surgery, IR, and IC currently exist in the United States in a state of stable equilibrium in the provision of noncardiac vascular care. What will happen in the future remains uncertain.
What does all this have to do with Charles Darwin? Darwin in his classic book, The Origin of Species, hypothesized that there is a relationship between extinct and contemporary species of plants and animals, that there is a competitive struggle for life between species, and that preservation of favored species occurs through a process of evolution and natural selection.
In a Utopian world in which food and resources are unlimited, all species will flourish and prosper. However, the reality is that food and resources are not unlimited. So, in the struggle for life, there is intense competition between species for the available food, resources, and space. The species that are best evolved and adapted to win this competition will flourish and survive. The species that are least well adapted will wither and become extinct.
There are many analogies between medical specialties and species. Patients for a medical specialty are analogous to food for a species. Other resources and space for a species are analogous to access to patients, the workplace, and its resources for a specialty.
For vascular surgeons to prosper and flourish, they must have access to vascular disease patients and the resources to care for them optimally. These resources include the necessary skills. They also include the facilities such as noninvasive laboratories, operating rooms, angiography suites, and postprocedural care areas – with all the necessary permanent and disposable instrumentation and equipment to care for vascular patients and their lesions.
In the Darwinian sense, vascular surgeons are competing with IRs and ICs for scarce food and resources. The likelihood is that we will soon be competing with cardiac surgeons whose food source (patients needing open procedures) is going away.
Vascular surgeons have certain assets such as specialized training and a focus on noncardiac vascular disease, knowledge of its medical treatment and natural history, and the ability to do open vascular operations when needed.
However, these may not be enough to ensure our specialty’s survival, since the other specialties interested in the same patients and lesions as we do also have assets that may counteract their intrinsic liability of not being focused primarily on these patients or lesions.
So what about the often-heard recommendation that we collaborate with these other specialties interested in caring for vascular patients – that we cooperate and help them in their training and practice on their vascular disease patients? In an ideal Utopian world, we should do so, and all interested specialties should work together harmoniously as I suggested in 1996.
But here is what Charles Darwin, if he were alive today, would likely say about this. The real world is not such a Kumbaya place. Experience has shown over and over that human nature with its affinity for competition, tribalism, and self-interest works against Kumbaya. These traits, especially self-interest, which accounts for problems in our politics, our legal profession, and Wall Street, also causes problems for our specialty.
Darwin would also say that our patients, a target of opportunity for other specialties, are limited. So are the resources to take care of them optimally, like dollars, vascular laboratories, angiography suites, hybrid operating rooms, hospital beds, etc.
So regarding our relationship with other specialties interested in caring for vascular patients, especially cardiac surgery, Darwin would say: Vascular surgeons and cardiac surgeons are closely related specialties (species) that are competing for limited resources and space. Vascular surgery adapted more quickly than cardiac surgery to the endovascular revolution and will likely survive and prosper. However cardiac surgeons are aggressive and talented and have open skills.
They can learn endovascular techniques as we did. We should not train them and give away our current competitive advantage.
Vascular surgery must maintain and enhance its niche, possibly by increasing its recognition as a separate specialty. Lastly Darwin would say: Vascular surgery should never forget that it is in a struggle to survive. It should do everything it can to maintain and enhance its competitive edge.
Dr. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or publisher.
Vascular surgery has evolved from a limited subspecialty of general and thoracic surgery into a complex and well-defined specialty. The introduction of endovascular treatments and their adoption and embrace by vascular surgeons has made our specialty exciting and attractive.
However, the increasing importance of these endovascular treatments also poses some dangers to vascular surgery. By using these endovascular techniques, other specialists have the tools to treat vascular lesions in vascular patients who previously could be treated by vascular surgeons only.
Some of these other specialists have contributed to the development of endovascular treatment techniques and therefore have a legitimate claim to use these techniques on vascular patients. This certainly applies to interventional radiologists (IRs) and to some extent to interventional cardiologists (ICs).
More recently, cardiac surgeons, whose practices have been diminished by the development of better coronary stents and now transcatheter valves, are venturing more into the treatment of noncardiac vascular lesions and are trying, somewhat belatedly, to become expert in endovascular skills and methods and are using them to treat a panoply of noncardiac vascular lesions. To facilitate this, combined training programs have been proposed.
What is the impact of all this multispecialty outreach, and what is its effect on vascular patients and vascular surgeons? To the extent that different specialists can learn from each other’s skills and techniques and can cross train each other, it is probably a good thing for doctors and patients.
However, this multispecialty interest in noncardiac vascular lesions and patients has some serious potential downsides. When specialists expand their scope of practice to new areas as an add-on to their primary practice, they run the risk of becoming dabblers.
They may be able to use techniques without the appropriate knowledge base to know when the techniques should be used. As a result, they may do more harm than good, and health care costs will rise. In addition, the pool of patients justifiably needing treatment is limited. Thus, if more specialists consider themselves capable of treating a given lesion, it will surely increase the number of unnecessary procedures and complications. All these effects will be bad for patients and the health care system.
In 1996, in an SVS presidential address titled "Charles Darwin and Vascular Surgery" ( J. Vasc. Surg. 1997;25:8-18), I predicted the increasing importance of endovascular treatments and how they would replace most open surgical procedures. My predictions then, thought by most to be too high, have proven actually to be too low.
In that presidential address, I advised vascular surgeons to become endo-competent to avoid extinction. Thankfully they have done so. Because of this, our specialty survives in the endovascular era. I also advocated that vascular surgeons work together with other specialists (IRs and ICs) in vascular centers for the betterment of patient care.
With a few exceptions this has not happened for many reasons which can best be summarized as due to human nature with its tendencies to tribalism, self-interest, and competition for patients and dollars.
Undoubtedly these tendencies will continue to have negative consequences on the care of vascular patients. Nevertheless, vascular surgery, IR, and IC currently exist in the United States in a state of stable equilibrium in the provision of noncardiac vascular care. What will happen in the future remains uncertain.
What does all this have to do with Charles Darwin? Darwin in his classic book, The Origin of Species, hypothesized that there is a relationship between extinct and contemporary species of plants and animals, that there is a competitive struggle for life between species, and that preservation of favored species occurs through a process of evolution and natural selection.
In a Utopian world in which food and resources are unlimited, all species will flourish and prosper. However, the reality is that food and resources are not unlimited. So, in the struggle for life, there is intense competition between species for the available food, resources, and space. The species that are best evolved and adapted to win this competition will flourish and survive. The species that are least well adapted will wither and become extinct.
There are many analogies between medical specialties and species. Patients for a medical specialty are analogous to food for a species. Other resources and space for a species are analogous to access to patients, the workplace, and its resources for a specialty.
For vascular surgeons to prosper and flourish, they must have access to vascular disease patients and the resources to care for them optimally. These resources include the necessary skills. They also include the facilities such as noninvasive laboratories, operating rooms, angiography suites, and postprocedural care areas – with all the necessary permanent and disposable instrumentation and equipment to care for vascular patients and their lesions.
In the Darwinian sense, vascular surgeons are competing with IRs and ICs for scarce food and resources. The likelihood is that we will soon be competing with cardiac surgeons whose food source (patients needing open procedures) is going away.
Vascular surgeons have certain assets such as specialized training and a focus on noncardiac vascular disease, knowledge of its medical treatment and natural history, and the ability to do open vascular operations when needed.
However, these may not be enough to ensure our specialty’s survival, since the other specialties interested in the same patients and lesions as we do also have assets that may counteract their intrinsic liability of not being focused primarily on these patients or lesions.
So what about the often-heard recommendation that we collaborate with these other specialties interested in caring for vascular patients – that we cooperate and help them in their training and practice on their vascular disease patients? In an ideal Utopian world, we should do so, and all interested specialties should work together harmoniously as I suggested in 1996.
But here is what Charles Darwin, if he were alive today, would likely say about this. The real world is not such a Kumbaya place. Experience has shown over and over that human nature with its affinity for competition, tribalism, and self-interest works against Kumbaya. These traits, especially self-interest, which accounts for problems in our politics, our legal profession, and Wall Street, also causes problems for our specialty.
Darwin would also say that our patients, a target of opportunity for other specialties, are limited. So are the resources to take care of them optimally, like dollars, vascular laboratories, angiography suites, hybrid operating rooms, hospital beds, etc.
So regarding our relationship with other specialties interested in caring for vascular patients, especially cardiac surgery, Darwin would say: Vascular surgeons and cardiac surgeons are closely related specialties (species) that are competing for limited resources and space. Vascular surgery adapted more quickly than cardiac surgery to the endovascular revolution and will likely survive and prosper. However cardiac surgeons are aggressive and talented and have open skills.
They can learn endovascular techniques as we did. We should not train them and give away our current competitive advantage.
Vascular surgery must maintain and enhance its niche, possibly by increasing its recognition as a separate specialty. Lastly Darwin would say: Vascular surgery should never forget that it is in a struggle to survive. It should do everything it can to maintain and enhance its competitive edge.
Dr. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or publisher.
Vascular surgery has evolved from a limited subspecialty of general and thoracic surgery into a complex and well-defined specialty. The introduction of endovascular treatments and their adoption and embrace by vascular surgeons has made our specialty exciting and attractive.
However, the increasing importance of these endovascular treatments also poses some dangers to vascular surgery. By using these endovascular techniques, other specialists have the tools to treat vascular lesions in vascular patients who previously could be treated by vascular surgeons only.
Some of these other specialists have contributed to the development of endovascular treatment techniques and therefore have a legitimate claim to use these techniques on vascular patients. This certainly applies to interventional radiologists (IRs) and to some extent to interventional cardiologists (ICs).
More recently, cardiac surgeons, whose practices have been diminished by the development of better coronary stents and now transcatheter valves, are venturing more into the treatment of noncardiac vascular lesions and are trying, somewhat belatedly, to become expert in endovascular skills and methods and are using them to treat a panoply of noncardiac vascular lesions. To facilitate this, combined training programs have been proposed.
What is the impact of all this multispecialty outreach, and what is its effect on vascular patients and vascular surgeons? To the extent that different specialists can learn from each other’s skills and techniques and can cross train each other, it is probably a good thing for doctors and patients.
However, this multispecialty interest in noncardiac vascular lesions and patients has some serious potential downsides. When specialists expand their scope of practice to new areas as an add-on to their primary practice, they run the risk of becoming dabblers.
They may be able to use techniques without the appropriate knowledge base to know when the techniques should be used. As a result, they may do more harm than good, and health care costs will rise. In addition, the pool of patients justifiably needing treatment is limited. Thus, if more specialists consider themselves capable of treating a given lesion, it will surely increase the number of unnecessary procedures and complications. All these effects will be bad for patients and the health care system.
In 1996, in an SVS presidential address titled "Charles Darwin and Vascular Surgery" ( J. Vasc. Surg. 1997;25:8-18), I predicted the increasing importance of endovascular treatments and how they would replace most open surgical procedures. My predictions then, thought by most to be too high, have proven actually to be too low.
In that presidential address, I advised vascular surgeons to become endo-competent to avoid extinction. Thankfully they have done so. Because of this, our specialty survives in the endovascular era. I also advocated that vascular surgeons work together with other specialists (IRs and ICs) in vascular centers for the betterment of patient care.
With a few exceptions this has not happened for many reasons which can best be summarized as due to human nature with its tendencies to tribalism, self-interest, and competition for patients and dollars.
Undoubtedly these tendencies will continue to have negative consequences on the care of vascular patients. Nevertheless, vascular surgery, IR, and IC currently exist in the United States in a state of stable equilibrium in the provision of noncardiac vascular care. What will happen in the future remains uncertain.
What does all this have to do with Charles Darwin? Darwin in his classic book, The Origin of Species, hypothesized that there is a relationship between extinct and contemporary species of plants and animals, that there is a competitive struggle for life between species, and that preservation of favored species occurs through a process of evolution and natural selection.
In a Utopian world in which food and resources are unlimited, all species will flourish and prosper. However, the reality is that food and resources are not unlimited. So, in the struggle for life, there is intense competition between species for the available food, resources, and space. The species that are best evolved and adapted to win this competition will flourish and survive. The species that are least well adapted will wither and become extinct.
There are many analogies between medical specialties and species. Patients for a medical specialty are analogous to food for a species. Other resources and space for a species are analogous to access to patients, the workplace, and its resources for a specialty.
For vascular surgeons to prosper and flourish, they must have access to vascular disease patients and the resources to care for them optimally. These resources include the necessary skills. They also include the facilities such as noninvasive laboratories, operating rooms, angiography suites, and postprocedural care areas – with all the necessary permanent and disposable instrumentation and equipment to care for vascular patients and their lesions.
In the Darwinian sense, vascular surgeons are competing with IRs and ICs for scarce food and resources. The likelihood is that we will soon be competing with cardiac surgeons whose food source (patients needing open procedures) is going away.
Vascular surgeons have certain assets such as specialized training and a focus on noncardiac vascular disease, knowledge of its medical treatment and natural history, and the ability to do open vascular operations when needed.
However, these may not be enough to ensure our specialty’s survival, since the other specialties interested in the same patients and lesions as we do also have assets that may counteract their intrinsic liability of not being focused primarily on these patients or lesions.
So what about the often-heard recommendation that we collaborate with these other specialties interested in caring for vascular patients – that we cooperate and help them in their training and practice on their vascular disease patients? In an ideal Utopian world, we should do so, and all interested specialties should work together harmoniously as I suggested in 1996.
But here is what Charles Darwin, if he were alive today, would likely say about this. The real world is not such a Kumbaya place. Experience has shown over and over that human nature with its affinity for competition, tribalism, and self-interest works against Kumbaya. These traits, especially self-interest, which accounts for problems in our politics, our legal profession, and Wall Street, also causes problems for our specialty.
Darwin would also say that our patients, a target of opportunity for other specialties, are limited. So are the resources to take care of them optimally, like dollars, vascular laboratories, angiography suites, hybrid operating rooms, hospital beds, etc.
So regarding our relationship with other specialties interested in caring for vascular patients, especially cardiac surgery, Darwin would say: Vascular surgeons and cardiac surgeons are closely related specialties (species) that are competing for limited resources and space. Vascular surgery adapted more quickly than cardiac surgery to the endovascular revolution and will likely survive and prosper. However cardiac surgeons are aggressive and talented and have open skills.
They can learn endovascular techniques as we did. We should not train them and give away our current competitive advantage.
Vascular surgery must maintain and enhance its niche, possibly by increasing its recognition as a separate specialty. Lastly Darwin would say: Vascular surgery should never forget that it is in a struggle to survive. It should do everything it can to maintain and enhance its competitive edge.
Dr. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or publisher.
Success of Recent VRIC
More than 100 investigators, students, and trainees with an interest in translational research attended the Vascular Research Initiatives Conference (VRIC) in Toronto. Held one day before the American Heart Association’s Arteriosclerosis, Thrombosis and Vascular Biology Meeting, VRIC fosters interaction among top scientists of diverse disciplines who are investigating peripheral vascular disease and its treatments.
"Everyone made outstanding presentations of cutting-edge research findings," said Dr. John Curci, course director. "Two themes showing promise in translational research are the use of stem cells to alter vascular disease progression and the evaluation of microRNA in diseased tissue and circulation, which may lead to better understanding of disease pathology as well as novel diagnostic testing strategies."
For the first time, a scientific poster session was added at VRIC, and according to Dr. Curci, it stimulated significant discussion. Additional opportunities for interaction occurred during the group luncheon and postmeeting gathering.
"VRIC’s unique value as a meeting stems from its primary focus on translational research," Dr. Curci said. "Almost by definition, translational research requires a community of interactive and collaborative scientists to identify and develop promising therapeutic and diagnostic technologies. The VRIC meeting has evolved to put that collaborative interaction as a central feature."
VRIC is also dedicated to stimulating and encouraging interest in research among aspiring academic vascular surgeons. Each year, the SVS Foundation supports travel scholarships for the top-scoring abstracts submitted by trainees to attend VRIC. The travel scholarship includes complimentary registration to VRIC and the ATVB meeting, along with a $1,000 award for conference travel. Recipients of this year’s VRIC trainee travel scholarship are:
• Dr. M. Freeman, University of Tennessee Medical Center - Knoxville
Title: Androgen Deficiency Influences Matrix Metalloproteinase Expression and Intimal Hyperplasia Development after Vascular Injury
• Moritz Lindquist Liljeqvist, Karolinska Institutet - Sweden
Title: Finite Element Models With Patient Specific Wall Strength Estimations Improve Growth Predictions of Abdominal Aortic Aneurysms
• Dr. Andrea Obi, University of Michigan
Title: Endothelial Dysfunction Potentiates Deep Venous Thrombosis in a Mouse Model of Sepsis
• Dr. Jonathan R. Thompson, University of Nebraska Medical Center
Title: Mortality Rates of Patients With Peripheral Arterial Disease Are Predicated by The Respiratory Activities of The Gastrocnemius Mitochondrial Complexes I and IV
The next VRIC will be held May 6, 2015, in San Francisco.
More than 100 investigators, students, and trainees with an interest in translational research attended the Vascular Research Initiatives Conference (VRIC) in Toronto. Held one day before the American Heart Association’s Arteriosclerosis, Thrombosis and Vascular Biology Meeting, VRIC fosters interaction among top scientists of diverse disciplines who are investigating peripheral vascular disease and its treatments.
"Everyone made outstanding presentations of cutting-edge research findings," said Dr. John Curci, course director. "Two themes showing promise in translational research are the use of stem cells to alter vascular disease progression and the evaluation of microRNA in diseased tissue and circulation, which may lead to better understanding of disease pathology as well as novel diagnostic testing strategies."
For the first time, a scientific poster session was added at VRIC, and according to Dr. Curci, it stimulated significant discussion. Additional opportunities for interaction occurred during the group luncheon and postmeeting gathering.
"VRIC’s unique value as a meeting stems from its primary focus on translational research," Dr. Curci said. "Almost by definition, translational research requires a community of interactive and collaborative scientists to identify and develop promising therapeutic and diagnostic technologies. The VRIC meeting has evolved to put that collaborative interaction as a central feature."
VRIC is also dedicated to stimulating and encouraging interest in research among aspiring academic vascular surgeons. Each year, the SVS Foundation supports travel scholarships for the top-scoring abstracts submitted by trainees to attend VRIC. The travel scholarship includes complimentary registration to VRIC and the ATVB meeting, along with a $1,000 award for conference travel. Recipients of this year’s VRIC trainee travel scholarship are:
• Dr. M. Freeman, University of Tennessee Medical Center - Knoxville
Title: Androgen Deficiency Influences Matrix Metalloproteinase Expression and Intimal Hyperplasia Development after Vascular Injury
• Moritz Lindquist Liljeqvist, Karolinska Institutet - Sweden
Title: Finite Element Models With Patient Specific Wall Strength Estimations Improve Growth Predictions of Abdominal Aortic Aneurysms
• Dr. Andrea Obi, University of Michigan
Title: Endothelial Dysfunction Potentiates Deep Venous Thrombosis in a Mouse Model of Sepsis
• Dr. Jonathan R. Thompson, University of Nebraska Medical Center
Title: Mortality Rates of Patients With Peripheral Arterial Disease Are Predicated by The Respiratory Activities of The Gastrocnemius Mitochondrial Complexes I and IV
The next VRIC will be held May 6, 2015, in San Francisco.
More than 100 investigators, students, and trainees with an interest in translational research attended the Vascular Research Initiatives Conference (VRIC) in Toronto. Held one day before the American Heart Association’s Arteriosclerosis, Thrombosis and Vascular Biology Meeting, VRIC fosters interaction among top scientists of diverse disciplines who are investigating peripheral vascular disease and its treatments.
"Everyone made outstanding presentations of cutting-edge research findings," said Dr. John Curci, course director. "Two themes showing promise in translational research are the use of stem cells to alter vascular disease progression and the evaluation of microRNA in diseased tissue and circulation, which may lead to better understanding of disease pathology as well as novel diagnostic testing strategies."
For the first time, a scientific poster session was added at VRIC, and according to Dr. Curci, it stimulated significant discussion. Additional opportunities for interaction occurred during the group luncheon and postmeeting gathering.
"VRIC’s unique value as a meeting stems from its primary focus on translational research," Dr. Curci said. "Almost by definition, translational research requires a community of interactive and collaborative scientists to identify and develop promising therapeutic and diagnostic technologies. The VRIC meeting has evolved to put that collaborative interaction as a central feature."
VRIC is also dedicated to stimulating and encouraging interest in research among aspiring academic vascular surgeons. Each year, the SVS Foundation supports travel scholarships for the top-scoring abstracts submitted by trainees to attend VRIC. The travel scholarship includes complimentary registration to VRIC and the ATVB meeting, along with a $1,000 award for conference travel. Recipients of this year’s VRIC trainee travel scholarship are:
• Dr. M. Freeman, University of Tennessee Medical Center - Knoxville
Title: Androgen Deficiency Influences Matrix Metalloproteinase Expression and Intimal Hyperplasia Development after Vascular Injury
• Moritz Lindquist Liljeqvist, Karolinska Institutet - Sweden
Title: Finite Element Models With Patient Specific Wall Strength Estimations Improve Growth Predictions of Abdominal Aortic Aneurysms
• Dr. Andrea Obi, University of Michigan
Title: Endothelial Dysfunction Potentiates Deep Venous Thrombosis in a Mouse Model of Sepsis
• Dr. Jonathan R. Thompson, University of Nebraska Medical Center
Title: Mortality Rates of Patients With Peripheral Arterial Disease Are Predicated by The Respiratory Activities of The Gastrocnemius Mitochondrial Complexes I and IV
The next VRIC will be held May 6, 2015, in San Francisco.
Approach can reduce drug-induced TLS

Photo courtesy of EHA
MILAN—The BCL-2 inhibitor ABT-199 may be a feasible treatment option for patients with chronic lymphocyctic leukemia/small lymphocytic lymphoma (CLL/SLL), according to research presented at the 19th Congress of the European Hematology Association (EHA).
Previous results showed that ABT-199 can elicit responses in patients with CLL/SLL, but it can also induce tumor lysis syndrome (TLS).
In fact, 2 TLS-related deaths prompted the temporary suspension of enrollment in trials of ABT-199.
But now, researchers have reported that a modified dosing schedule, prophylaxis, and patient monitoring can reduce, and perhaps even eliminate, the risk of TLS.
And ABT-199 can produce solid responses, even in high-risk CLL/SLL patients.
John Seymour, MBBS, PhD, of the Peter MacCallum Cancer Center in Victoria, Australia, and his colleagues presented results observed with a TLS prophylactic regimen at the EHA Congress as abstract P868.
Dr Seymour also presented data from a phase 1 study of ABT-199 monotherapy as abstract S702. Both studies were supported by AbbVie and Genentech, the companies developing ABT-199.
Assessing the risk of TLS
To identify pre-treatment risk factors for TLS and appropriate prophylactic measures, Dr Seymour and his colleagues analyzed 77 CLL/SLL patients who were treated with ABT-199 prior to the identification of TLS (from June 2011 to March 2013).
Twenty-four of these patients had values meeting Cairo-Bishop criteria for TLS, and medical adjudication suggested 19 (25%) of them had TLS.
Comparing these patients to those who did not develop TLS, the researchers found that patients were at low risk of developing TLS if they had a nodal mass measuring less than 5 cm and an absolute lymphocyte count (ALC) less than 25,000.
Patients were at medium risk of TLS if they had a nodal mass of 5 cm to 9 cm or an ALC of at least 25,000. And patients were at high risk of TLS if they had a nodal mass of 10 cm or greater or a nodal mass of 5 cm to 9 cm and an ALC of 25,000 or greater.
Dose modification
The researchers also found that TLS events tended to occur within 24 hours of the first dose of ABT-199. And the initial exposure (median Cmax value) for patients who had a TLS incident was higher than patients without TLS (0.49 μg/mL vs 0.23 μg/mL).
However, simulations suggested that, at a 20 mg starting dose, 98% of patients will achieve initial peak exposures similar to patients without TLS (Cmax below 0.23 ug/mL).
So the researchers modified the dosing schedule of ABT-199 in subsequently treated patients. The patients received a 20 mg starting dose, then 50 mg for the rest of week 1, 100 mg in week 2, 200 mg in week 3, and 400 mg in week 4.
However, if 1 or more electrolytes met Cairo-Bishop criteria and/or there was a 30% or greater decrease in ALC with the first dose, patients received the drug at 20 mg in week 1, 50 mg in week 2, 100 mg in week 3, 200 mg in week 4, and 400 mg in week 5.
Prophylactic measures
Dr Seymour and his colleagues also recommended several other steps to minimize the risk of TLS. They said their findings support hospitalizing and monitoring patients for the first dose of 20 mg and 50 mg, regardless of their risk of TLS.
High-risk patients should be hospitalized for all subsequent dose escalations until they are re-categorized to medium- or low-risk groups. The researchers also said hospitalization should be considered at subsequent dose escalation for medium-risk patients with creatinine clearance of 80 mL/min or less.
All patients should receive oral hydration prior to receiving ABT-199, and hospitalized patients should receive intravenous hydration (150-200 cc/hour, as tolerated).
All patients should receive a uric-acid-reducing agent at least 72 hours before their first dose of ABT-199. Rasburicase is strongly suggested for high-risk patients with high baseline uric acid and for patients who develop rapid rises in uric acid values.
The researchers also recommended laboratory assessment at 8 hours and 24 hours in an outpatient setting and at 4, 8, 12, and 24 hours in hospitalized patients.
Approach reduces TLS risk
Lastly, Dr Seymour and his colleagues analyzed the effect of the modified dosing schedule and the aforementioned prophylactic measures on a cohort of 58 CLL/SLL patients treated with ABT-199.
The TLS risk stratification was similar in this cohort and the pre-modification cohort of 77 patients. There was, however, a higher proportion of patients in the post-modification cohort who fell into the high-risk category.
According to Cairo-Bishop criteria, 3 patients (3.9%) had clinical TLS in the pre-modification cohort and 16 (20.8%) had laboratory TLS. In the post-modification cohort, none of the patients had clinical TLS, and 8 (13.8%) had laboratory TLS.
According to the Howard definition of TLS, 3 patients (3.9%) in the pre-modification cohort had clinical TLS, and 7 (9.1%) had laboratory TLS. But none of the patients in the post-modification cohort had clinical or laboratory TLS.
Phase 1 trial of ABT-199 monotherapy
In another presentation at the EHA Congress, Dr Seymour reported results from a phase 1 study of ABT-199 monotherapy in 105 patients with high-risk CLL/SLL.
Following the identification of TLS, patients received treatment according to the modified schedule, as well as TLS prophylaxis.
In all, 7 patients developed TLS. One of these patients died, and 1 required dialysis. As of April 9, 2014, there were no cases of TLS among the 49 patients who received ABT-199 according to the modified dosing schedule, as well as TLS prophylaxis.
Other common treatment-emergent adverse events included diarrhea (40%), neutropenia (36%), and nausea (35%). Grade 3/4 neutropenia occurred in 33% of patients, and febrile neutropenia occurred in 4%.
Thirty-seven patients discontinued treatment—22 due to progressive disease, 12 due to adverse events, and 3 for other reasons (1 required warfarin, and 2 proceeded to transplant).
Seventy-eight patients were evaluable for treatment response. Nineteen of these patients had del (17p), 41 were fludarabine-refractory, and 24 had unmutated IGHV.
The response rate was 77% overall, 79% among patients with del (17p), 76% in those who were fludarabine-refractory, and 75% in those with unmutated IGHV. The complete response rates were 23% 26%, 22%, and 29%, respectively.
As of April 9, the median progression-free survival was about 18 months. The median progression-free survival had not been reached for patients treated at or above 400 mg. ![]()

Photo courtesy of EHA
MILAN—The BCL-2 inhibitor ABT-199 may be a feasible treatment option for patients with chronic lymphocyctic leukemia/small lymphocytic lymphoma (CLL/SLL), according to research presented at the 19th Congress of the European Hematology Association (EHA).
Previous results showed that ABT-199 can elicit responses in patients with CLL/SLL, but it can also induce tumor lysis syndrome (TLS).
In fact, 2 TLS-related deaths prompted the temporary suspension of enrollment in trials of ABT-199.
But now, researchers have reported that a modified dosing schedule, prophylaxis, and patient monitoring can reduce, and perhaps even eliminate, the risk of TLS.
And ABT-199 can produce solid responses, even in high-risk CLL/SLL patients.
John Seymour, MBBS, PhD, of the Peter MacCallum Cancer Center in Victoria, Australia, and his colleagues presented results observed with a TLS prophylactic regimen at the EHA Congress as abstract P868.
Dr Seymour also presented data from a phase 1 study of ABT-199 monotherapy as abstract S702. Both studies were supported by AbbVie and Genentech, the companies developing ABT-199.
Assessing the risk of TLS
To identify pre-treatment risk factors for TLS and appropriate prophylactic measures, Dr Seymour and his colleagues analyzed 77 CLL/SLL patients who were treated with ABT-199 prior to the identification of TLS (from June 2011 to March 2013).
Twenty-four of these patients had values meeting Cairo-Bishop criteria for TLS, and medical adjudication suggested 19 (25%) of them had TLS.
Comparing these patients to those who did not develop TLS, the researchers found that patients were at low risk of developing TLS if they had a nodal mass measuring less than 5 cm and an absolute lymphocyte count (ALC) less than 25,000.
Patients were at medium risk of TLS if they had a nodal mass of 5 cm to 9 cm or an ALC of at least 25,000. And patients were at high risk of TLS if they had a nodal mass of 10 cm or greater or a nodal mass of 5 cm to 9 cm and an ALC of 25,000 or greater.
Dose modification
The researchers also found that TLS events tended to occur within 24 hours of the first dose of ABT-199. And the initial exposure (median Cmax value) for patients who had a TLS incident was higher than patients without TLS (0.49 μg/mL vs 0.23 μg/mL).
However, simulations suggested that, at a 20 mg starting dose, 98% of patients will achieve initial peak exposures similar to patients without TLS (Cmax below 0.23 ug/mL).
So the researchers modified the dosing schedule of ABT-199 in subsequently treated patients. The patients received a 20 mg starting dose, then 50 mg for the rest of week 1, 100 mg in week 2, 200 mg in week 3, and 400 mg in week 4.
However, if 1 or more electrolytes met Cairo-Bishop criteria and/or there was a 30% or greater decrease in ALC with the first dose, patients received the drug at 20 mg in week 1, 50 mg in week 2, 100 mg in week 3, 200 mg in week 4, and 400 mg in week 5.
Prophylactic measures
Dr Seymour and his colleagues also recommended several other steps to minimize the risk of TLS. They said their findings support hospitalizing and monitoring patients for the first dose of 20 mg and 50 mg, regardless of their risk of TLS.
High-risk patients should be hospitalized for all subsequent dose escalations until they are re-categorized to medium- or low-risk groups. The researchers also said hospitalization should be considered at subsequent dose escalation for medium-risk patients with creatinine clearance of 80 mL/min or less.
All patients should receive oral hydration prior to receiving ABT-199, and hospitalized patients should receive intravenous hydration (150-200 cc/hour, as tolerated).
All patients should receive a uric-acid-reducing agent at least 72 hours before their first dose of ABT-199. Rasburicase is strongly suggested for high-risk patients with high baseline uric acid and for patients who develop rapid rises in uric acid values.
The researchers also recommended laboratory assessment at 8 hours and 24 hours in an outpatient setting and at 4, 8, 12, and 24 hours in hospitalized patients.
Approach reduces TLS risk
Lastly, Dr Seymour and his colleagues analyzed the effect of the modified dosing schedule and the aforementioned prophylactic measures on a cohort of 58 CLL/SLL patients treated with ABT-199.
The TLS risk stratification was similar in this cohort and the pre-modification cohort of 77 patients. There was, however, a higher proportion of patients in the post-modification cohort who fell into the high-risk category.
According to Cairo-Bishop criteria, 3 patients (3.9%) had clinical TLS in the pre-modification cohort and 16 (20.8%) had laboratory TLS. In the post-modification cohort, none of the patients had clinical TLS, and 8 (13.8%) had laboratory TLS.
According to the Howard definition of TLS, 3 patients (3.9%) in the pre-modification cohort had clinical TLS, and 7 (9.1%) had laboratory TLS. But none of the patients in the post-modification cohort had clinical or laboratory TLS.
Phase 1 trial of ABT-199 monotherapy
In another presentation at the EHA Congress, Dr Seymour reported results from a phase 1 study of ABT-199 monotherapy in 105 patients with high-risk CLL/SLL.
Following the identification of TLS, patients received treatment according to the modified schedule, as well as TLS prophylaxis.
In all, 7 patients developed TLS. One of these patients died, and 1 required dialysis. As of April 9, 2014, there were no cases of TLS among the 49 patients who received ABT-199 according to the modified dosing schedule, as well as TLS prophylaxis.
Other common treatment-emergent adverse events included diarrhea (40%), neutropenia (36%), and nausea (35%). Grade 3/4 neutropenia occurred in 33% of patients, and febrile neutropenia occurred in 4%.
Thirty-seven patients discontinued treatment—22 due to progressive disease, 12 due to adverse events, and 3 for other reasons (1 required warfarin, and 2 proceeded to transplant).
Seventy-eight patients were evaluable for treatment response. Nineteen of these patients had del (17p), 41 were fludarabine-refractory, and 24 had unmutated IGHV.
The response rate was 77% overall, 79% among patients with del (17p), 76% in those who were fludarabine-refractory, and 75% in those with unmutated IGHV. The complete response rates were 23% 26%, 22%, and 29%, respectively.
As of April 9, the median progression-free survival was about 18 months. The median progression-free survival had not been reached for patients treated at or above 400 mg. ![]()

Photo courtesy of EHA
MILAN—The BCL-2 inhibitor ABT-199 may be a feasible treatment option for patients with chronic lymphocyctic leukemia/small lymphocytic lymphoma (CLL/SLL), according to research presented at the 19th Congress of the European Hematology Association (EHA).
Previous results showed that ABT-199 can elicit responses in patients with CLL/SLL, but it can also induce tumor lysis syndrome (TLS).
In fact, 2 TLS-related deaths prompted the temporary suspension of enrollment in trials of ABT-199.
But now, researchers have reported that a modified dosing schedule, prophylaxis, and patient monitoring can reduce, and perhaps even eliminate, the risk of TLS.
And ABT-199 can produce solid responses, even in high-risk CLL/SLL patients.
John Seymour, MBBS, PhD, of the Peter MacCallum Cancer Center in Victoria, Australia, and his colleagues presented results observed with a TLS prophylactic regimen at the EHA Congress as abstract P868.
Dr Seymour also presented data from a phase 1 study of ABT-199 monotherapy as abstract S702. Both studies were supported by AbbVie and Genentech, the companies developing ABT-199.
Assessing the risk of TLS
To identify pre-treatment risk factors for TLS and appropriate prophylactic measures, Dr Seymour and his colleagues analyzed 77 CLL/SLL patients who were treated with ABT-199 prior to the identification of TLS (from June 2011 to March 2013).
Twenty-four of these patients had values meeting Cairo-Bishop criteria for TLS, and medical adjudication suggested 19 (25%) of them had TLS.
Comparing these patients to those who did not develop TLS, the researchers found that patients were at low risk of developing TLS if they had a nodal mass measuring less than 5 cm and an absolute lymphocyte count (ALC) less than 25,000.
Patients were at medium risk of TLS if they had a nodal mass of 5 cm to 9 cm or an ALC of at least 25,000. And patients were at high risk of TLS if they had a nodal mass of 10 cm or greater or a nodal mass of 5 cm to 9 cm and an ALC of 25,000 or greater.
Dose modification
The researchers also found that TLS events tended to occur within 24 hours of the first dose of ABT-199. And the initial exposure (median Cmax value) for patients who had a TLS incident was higher than patients without TLS (0.49 μg/mL vs 0.23 μg/mL).
However, simulations suggested that, at a 20 mg starting dose, 98% of patients will achieve initial peak exposures similar to patients without TLS (Cmax below 0.23 ug/mL).
So the researchers modified the dosing schedule of ABT-199 in subsequently treated patients. The patients received a 20 mg starting dose, then 50 mg for the rest of week 1, 100 mg in week 2, 200 mg in week 3, and 400 mg in week 4.
However, if 1 or more electrolytes met Cairo-Bishop criteria and/or there was a 30% or greater decrease in ALC with the first dose, patients received the drug at 20 mg in week 1, 50 mg in week 2, 100 mg in week 3, 200 mg in week 4, and 400 mg in week 5.
Prophylactic measures
Dr Seymour and his colleagues also recommended several other steps to minimize the risk of TLS. They said their findings support hospitalizing and monitoring patients for the first dose of 20 mg and 50 mg, regardless of their risk of TLS.
High-risk patients should be hospitalized for all subsequent dose escalations until they are re-categorized to medium- or low-risk groups. The researchers also said hospitalization should be considered at subsequent dose escalation for medium-risk patients with creatinine clearance of 80 mL/min or less.
All patients should receive oral hydration prior to receiving ABT-199, and hospitalized patients should receive intravenous hydration (150-200 cc/hour, as tolerated).
All patients should receive a uric-acid-reducing agent at least 72 hours before their first dose of ABT-199. Rasburicase is strongly suggested for high-risk patients with high baseline uric acid and for patients who develop rapid rises in uric acid values.
The researchers also recommended laboratory assessment at 8 hours and 24 hours in an outpatient setting and at 4, 8, 12, and 24 hours in hospitalized patients.
Approach reduces TLS risk
Lastly, Dr Seymour and his colleagues analyzed the effect of the modified dosing schedule and the aforementioned prophylactic measures on a cohort of 58 CLL/SLL patients treated with ABT-199.
The TLS risk stratification was similar in this cohort and the pre-modification cohort of 77 patients. There was, however, a higher proportion of patients in the post-modification cohort who fell into the high-risk category.
According to Cairo-Bishop criteria, 3 patients (3.9%) had clinical TLS in the pre-modification cohort and 16 (20.8%) had laboratory TLS. In the post-modification cohort, none of the patients had clinical TLS, and 8 (13.8%) had laboratory TLS.
According to the Howard definition of TLS, 3 patients (3.9%) in the pre-modification cohort had clinical TLS, and 7 (9.1%) had laboratory TLS. But none of the patients in the post-modification cohort had clinical or laboratory TLS.
Phase 1 trial of ABT-199 monotherapy
In another presentation at the EHA Congress, Dr Seymour reported results from a phase 1 study of ABT-199 monotherapy in 105 patients with high-risk CLL/SLL.
Following the identification of TLS, patients received treatment according to the modified schedule, as well as TLS prophylaxis.
In all, 7 patients developed TLS. One of these patients died, and 1 required dialysis. As of April 9, 2014, there were no cases of TLS among the 49 patients who received ABT-199 according to the modified dosing schedule, as well as TLS prophylaxis.
Other common treatment-emergent adverse events included diarrhea (40%), neutropenia (36%), and nausea (35%). Grade 3/4 neutropenia occurred in 33% of patients, and febrile neutropenia occurred in 4%.
Thirty-seven patients discontinued treatment—22 due to progressive disease, 12 due to adverse events, and 3 for other reasons (1 required warfarin, and 2 proceeded to transplant).
Seventy-eight patients were evaluable for treatment response. Nineteen of these patients had del (17p), 41 were fludarabine-refractory, and 24 had unmutated IGHV.
The response rate was 77% overall, 79% among patients with del (17p), 76% in those who were fludarabine-refractory, and 75% in those with unmutated IGHV. The complete response rates were 23% 26%, 22%, and 29%, respectively.
As of April 9, the median progression-free survival was about 18 months. The median progression-free survival had not been reached for patients treated at or above 400 mg. ![]()
Gut bacteria may help predict survival after allo-SCT

The diversity of gut microbiota in patients receiving allogeneic stem cell transplants (allo-SCTs) may be an important predictor of their survival, according to a study published in Blood.
Previous studies have suggested the intensive treatment given to allo-SCT recipients can destroy a significant portion of their gut microbiota and reduce its overall diversity.
And disturbances of the gut microbiota have been associated with complications such as bloodstream infections and graft-vs-host disease.
“While the link between gut microbiota and complications in allogeneic SCT has been previously established, until this point, it has remained unclear whether the gut bacteria of transplant recipients could predict their survival,” said study author Ying Taur, MD, of Memorial Sloan-Kettering Cancer Center in New York.
“This study sought to further explore the potential connection between transplantation, gut bacteria, and overall survival.”
To that end, the researchers collected fecal specimens from 80 patients undergoing allo-SCT and sequenced each sample’s bacterial DNA. Specimens were collected within 7 days of engraftment, the point at which the researchers speculated that microbiota diversity would be greatest following pre-transplant conditioning.
The team compared patient outcomes based on diversity of microbiota in their specimens, grouping subjects into high-, intermediate-, and low-microbiota-diversity categories.
At engraftment, 34 patients (42.5%) had low diversity, 20 (25%) had intermediate diversity, and 26 (32.5%) had high diversity. The analysis continued for up to 3 years or until death or last follow-up.
Following their analyses, the researchers found a strong connection between post-transplant gut microbiota diversity and outcomes, observing overall survival rates of 36%, 60%, and 67% among the low-, intermediate-, and high-diversity groups, respectively.
Furthermore, diversity was particularly associated with transplant-related outcomes. Patients with low microbiota diversity were approximately 5 times more likely to die of transplant-related causes within the follow-up period than those with more diverse gut bacteria.
“These results further underscore the significance of the gut microbiota in allogeneic stem cell transplant,” Dr Taur said. “A major question is whether we can improve outcomes by preserving diversity within the gut microbiota.”
“One possible strategy is to find ways to perform transplants in a manner that minimizes damage to the gut microbiota. Another approach would be to replenish the gut with beneficial microbes that are lost after this procedure is performed. We hope that this study will inspire additional research that will further examine the role and importance of the gut microbiota to stem cell transplant outcome.” ![]()

The diversity of gut microbiota in patients receiving allogeneic stem cell transplants (allo-SCTs) may be an important predictor of their survival, according to a study published in Blood.
Previous studies have suggested the intensive treatment given to allo-SCT recipients can destroy a significant portion of their gut microbiota and reduce its overall diversity.
And disturbances of the gut microbiota have been associated with complications such as bloodstream infections and graft-vs-host disease.
“While the link between gut microbiota and complications in allogeneic SCT has been previously established, until this point, it has remained unclear whether the gut bacteria of transplant recipients could predict their survival,” said study author Ying Taur, MD, of Memorial Sloan-Kettering Cancer Center in New York.
“This study sought to further explore the potential connection between transplantation, gut bacteria, and overall survival.”
To that end, the researchers collected fecal specimens from 80 patients undergoing allo-SCT and sequenced each sample’s bacterial DNA. Specimens were collected within 7 days of engraftment, the point at which the researchers speculated that microbiota diversity would be greatest following pre-transplant conditioning.
The team compared patient outcomes based on diversity of microbiota in their specimens, grouping subjects into high-, intermediate-, and low-microbiota-diversity categories.
At engraftment, 34 patients (42.5%) had low diversity, 20 (25%) had intermediate diversity, and 26 (32.5%) had high diversity. The analysis continued for up to 3 years or until death or last follow-up.
Following their analyses, the researchers found a strong connection between post-transplant gut microbiota diversity and outcomes, observing overall survival rates of 36%, 60%, and 67% among the low-, intermediate-, and high-diversity groups, respectively.
Furthermore, diversity was particularly associated with transplant-related outcomes. Patients with low microbiota diversity were approximately 5 times more likely to die of transplant-related causes within the follow-up period than those with more diverse gut bacteria.
“These results further underscore the significance of the gut microbiota in allogeneic stem cell transplant,” Dr Taur said. “A major question is whether we can improve outcomes by preserving diversity within the gut microbiota.”
“One possible strategy is to find ways to perform transplants in a manner that minimizes damage to the gut microbiota. Another approach would be to replenish the gut with beneficial microbes that are lost after this procedure is performed. We hope that this study will inspire additional research that will further examine the role and importance of the gut microbiota to stem cell transplant outcome.” ![]()

The diversity of gut microbiota in patients receiving allogeneic stem cell transplants (allo-SCTs) may be an important predictor of their survival, according to a study published in Blood.
Previous studies have suggested the intensive treatment given to allo-SCT recipients can destroy a significant portion of their gut microbiota and reduce its overall diversity.
And disturbances of the gut microbiota have been associated with complications such as bloodstream infections and graft-vs-host disease.
“While the link between gut microbiota and complications in allogeneic SCT has been previously established, until this point, it has remained unclear whether the gut bacteria of transplant recipients could predict their survival,” said study author Ying Taur, MD, of Memorial Sloan-Kettering Cancer Center in New York.
“This study sought to further explore the potential connection between transplantation, gut bacteria, and overall survival.”
To that end, the researchers collected fecal specimens from 80 patients undergoing allo-SCT and sequenced each sample’s bacterial DNA. Specimens were collected within 7 days of engraftment, the point at which the researchers speculated that microbiota diversity would be greatest following pre-transplant conditioning.
The team compared patient outcomes based on diversity of microbiota in their specimens, grouping subjects into high-, intermediate-, and low-microbiota-diversity categories.
At engraftment, 34 patients (42.5%) had low diversity, 20 (25%) had intermediate diversity, and 26 (32.5%) had high diversity. The analysis continued for up to 3 years or until death or last follow-up.
Following their analyses, the researchers found a strong connection between post-transplant gut microbiota diversity and outcomes, observing overall survival rates of 36%, 60%, and 67% among the low-, intermediate-, and high-diversity groups, respectively.
Furthermore, diversity was particularly associated with transplant-related outcomes. Patients with low microbiota diversity were approximately 5 times more likely to die of transplant-related causes within the follow-up period than those with more diverse gut bacteria.
“These results further underscore the significance of the gut microbiota in allogeneic stem cell transplant,” Dr Taur said. “A major question is whether we can improve outcomes by preserving diversity within the gut microbiota.”
“One possible strategy is to find ways to perform transplants in a manner that minimizes damage to the gut microbiota. Another approach would be to replenish the gut with beneficial microbes that are lost after this procedure is performed. We hope that this study will inspire additional research that will further examine the role and importance of the gut microbiota to stem cell transplant outcome.” ![]()
CNS involvement predicts relapse but not survival in ARL, study shows
CHICAGO—Investigators have found evidence to suggest that identifying central nervous system (CNS) involvement at diagnosis does not impact overall survival for patients with AIDS-related lymphoma (ARL).
The research showed that ARL patients with CNS involvement at diagnosis were nearly 3 times as likely as their peers to have CNS relapse during cancer treatment.
But there was no significant difference between the 2 groups with regard to survival.
This may be due to the low overall incidence of CNS relapse, the use of insufficient treatments, and/or inadequate methods for identifying patients with CNS involvement, according to the investigators.
Stefan K. Barta, MD, of the Fox Chase Cancer Center in Philadelphia, Pennsylvania, and his colleagues conducted this research and presented the results at the 2014 ASCO Annual Meeting (abstract 8570).
In 2013, Dr Barta led the assembly of a database containing medical data from more than 1500 patients newly diagnosed with ARL who participated in clinical trials in Europe and the US from 1990 through 2010.
In the new study, he and his colleagues used the database to identify 880 patients with ARL whose data included complete information on CNS involvement at diagnosis and CNS relapse.
The team set out to find associations between CNS relapse and patient characteristics, including age, sex, CD4 count, lymphoma subtype, treatment history with combination antiretroviral therapies (cART), rituximab use, and the type of initial chemotherapy.
All of the patients had received either intrathecal therapy for CNS involvement or intrathecal prophylaxis with single-agent or 3-agent regimens. Sixty-nine percent of patients (n=607) had received cART, and 31% (n=276) had received rituximab-containing induction chemoimmunotherapy.
CNS involvement was identified in 13% of patients at diagnosis, including 27% of patients with Burkitt lymphoma or Burkitt-like lymphoma and 6% of patients with diffuse large B-cell lymphoma.
There was no difference in the prevalence of baseline CNS involvement between patients treated before and after the introduction of cART (13% each).
In all, 5.3% of patients experienced CNS relapse at a median of 4.2 months after diagnosis (range, 0.3-19.3 months). This included 12% of patients diagnosed with CNS involvement at baseline and 4% of patients who were not.
The median overall survival after CNS relapse was 1.6 months (range, 0-86.4 months). There was no significant difference in overall survival between patients with CNS involvement at diagnosis and those without it. The hazard ratio was 0.85 (P=0.32).
Multivariate analysis showed the only baseline characteristic significantly associated with the frequency of CNS relapse was CNS involvement, with a hazard ratio of 2.9 (P=0.01). None of the treatments had a significant impact on CNS relapse.
Dr Barta said these results suggest that current treatments are insufficient, and the approaches used to identify CNS involvement may be missing many patients who are at risk of CNS relapse.
“A lot of patients who relapsed probably had undetected CNS involvement at diagnosis,” he said. “We want to figure out if there are better strategies to identify patients at risk.” ![]()
CHICAGO—Investigators have found evidence to suggest that identifying central nervous system (CNS) involvement at diagnosis does not impact overall survival for patients with AIDS-related lymphoma (ARL).
The research showed that ARL patients with CNS involvement at diagnosis were nearly 3 times as likely as their peers to have CNS relapse during cancer treatment.
But there was no significant difference between the 2 groups with regard to survival.
This may be due to the low overall incidence of CNS relapse, the use of insufficient treatments, and/or inadequate methods for identifying patients with CNS involvement, according to the investigators.
Stefan K. Barta, MD, of the Fox Chase Cancer Center in Philadelphia, Pennsylvania, and his colleagues conducted this research and presented the results at the 2014 ASCO Annual Meeting (abstract 8570).
In 2013, Dr Barta led the assembly of a database containing medical data from more than 1500 patients newly diagnosed with ARL who participated in clinical trials in Europe and the US from 1990 through 2010.
In the new study, he and his colleagues used the database to identify 880 patients with ARL whose data included complete information on CNS involvement at diagnosis and CNS relapse.
The team set out to find associations between CNS relapse and patient characteristics, including age, sex, CD4 count, lymphoma subtype, treatment history with combination antiretroviral therapies (cART), rituximab use, and the type of initial chemotherapy.
All of the patients had received either intrathecal therapy for CNS involvement or intrathecal prophylaxis with single-agent or 3-agent regimens. Sixty-nine percent of patients (n=607) had received cART, and 31% (n=276) had received rituximab-containing induction chemoimmunotherapy.
CNS involvement was identified in 13% of patients at diagnosis, including 27% of patients with Burkitt lymphoma or Burkitt-like lymphoma and 6% of patients with diffuse large B-cell lymphoma.
There was no difference in the prevalence of baseline CNS involvement between patients treated before and after the introduction of cART (13% each).
In all, 5.3% of patients experienced CNS relapse at a median of 4.2 months after diagnosis (range, 0.3-19.3 months). This included 12% of patients diagnosed with CNS involvement at baseline and 4% of patients who were not.
The median overall survival after CNS relapse was 1.6 months (range, 0-86.4 months). There was no significant difference in overall survival between patients with CNS involvement at diagnosis and those without it. The hazard ratio was 0.85 (P=0.32).
Multivariate analysis showed the only baseline characteristic significantly associated with the frequency of CNS relapse was CNS involvement, with a hazard ratio of 2.9 (P=0.01). None of the treatments had a significant impact on CNS relapse.
Dr Barta said these results suggest that current treatments are insufficient, and the approaches used to identify CNS involvement may be missing many patients who are at risk of CNS relapse.
“A lot of patients who relapsed probably had undetected CNS involvement at diagnosis,” he said. “We want to figure out if there are better strategies to identify patients at risk.” ![]()
CHICAGO—Investigators have found evidence to suggest that identifying central nervous system (CNS) involvement at diagnosis does not impact overall survival for patients with AIDS-related lymphoma (ARL).
The research showed that ARL patients with CNS involvement at diagnosis were nearly 3 times as likely as their peers to have CNS relapse during cancer treatment.
But there was no significant difference between the 2 groups with regard to survival.
This may be due to the low overall incidence of CNS relapse, the use of insufficient treatments, and/or inadequate methods for identifying patients with CNS involvement, according to the investigators.
Stefan K. Barta, MD, of the Fox Chase Cancer Center in Philadelphia, Pennsylvania, and his colleagues conducted this research and presented the results at the 2014 ASCO Annual Meeting (abstract 8570).
In 2013, Dr Barta led the assembly of a database containing medical data from more than 1500 patients newly diagnosed with ARL who participated in clinical trials in Europe and the US from 1990 through 2010.
In the new study, he and his colleagues used the database to identify 880 patients with ARL whose data included complete information on CNS involvement at diagnosis and CNS relapse.
The team set out to find associations between CNS relapse and patient characteristics, including age, sex, CD4 count, lymphoma subtype, treatment history with combination antiretroviral therapies (cART), rituximab use, and the type of initial chemotherapy.
All of the patients had received either intrathecal therapy for CNS involvement or intrathecal prophylaxis with single-agent or 3-agent regimens. Sixty-nine percent of patients (n=607) had received cART, and 31% (n=276) had received rituximab-containing induction chemoimmunotherapy.
CNS involvement was identified in 13% of patients at diagnosis, including 27% of patients with Burkitt lymphoma or Burkitt-like lymphoma and 6% of patients with diffuse large B-cell lymphoma.
There was no difference in the prevalence of baseline CNS involvement between patients treated before and after the introduction of cART (13% each).
In all, 5.3% of patients experienced CNS relapse at a median of 4.2 months after diagnosis (range, 0.3-19.3 months). This included 12% of patients diagnosed with CNS involvement at baseline and 4% of patients who were not.
The median overall survival after CNS relapse was 1.6 months (range, 0-86.4 months). There was no significant difference in overall survival between patients with CNS involvement at diagnosis and those without it. The hazard ratio was 0.85 (P=0.32).
Multivariate analysis showed the only baseline characteristic significantly associated with the frequency of CNS relapse was CNS involvement, with a hazard ratio of 2.9 (P=0.01). None of the treatments had a significant impact on CNS relapse.
Dr Barta said these results suggest that current treatments are insufficient, and the approaches used to identify CNS involvement may be missing many patients who are at risk of CNS relapse.
“A lot of patients who relapsed probably had undetected CNS involvement at diagnosis,” he said. “We want to figure out if there are better strategies to identify patients at risk.” ![]()
How baseline death risk may impact transfusion outcome

Credit: UAB Hospital
A new study suggests the risks and benefits of red blood cell (RBC) transfusions can vary considerably for patients with trauma and major bleeding, depending on the patients’ risk of death at baseline.
Patients with the highest predicted risk of death on arrival at a trauma center received the greatest benefit from RBC transfusions.
But among patients with the lowest predicted risk of death at baseline, transfusion was associated with a higher risk of death post-treatment.
Pablo Perel, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK, and his colleagues reported these findings in PLOS Medicine.
To conduct this study, the team used data from the CRASH-2 trial, which assessed the effect of tranexamic acid in trauma patients. The trial included 20,127 patients with significant bleeding who were treated at 274 hospitals in 40 countries.
Dr Perel and his colleagues used that data to evaluate the association between receiving an RBC transfusion and death from all causes at 28 days post-trauma. The findings were stratified by predicted risk of death based on clinical observations on arrival at the trauma center.
The researchers found that patients with the greatest predicted risk of dying—greater than 50%—had a smaller chance of death from all causes if they were transfused than if they were not. The odds ratio (OR) was 0.59.
For patients whose predicted risk of death ranged from 21% to 50%, there was no significant difference in their chance of dying whether they were transfused or not. The OR was 0.92.
But for patients with a lower risk of death at baseline, transfusion was associated with an increased risk of death.
Patients with a 6% to 20% risk of death at baseline had an OR of 2.31 if they received a transfusion. And for patients whose initial risk of death was below 6%, the OR for death associated with transfusion was 5.40.
In absolute figures, compared to no transfusion, RBC transfusion was associated with 5.1 more deaths per 100 patients in the group with the lowest predicted risk of death but with 11.9 fewer deaths per 100 patients in the group with the highest predicted risk of death.
The researchers noted that, although these data suggest RBC transfusion could be harmful for patients whose predicted risk of death is low, this study was observational. So the team cannot confirm a causal link, and a randomized trial investigating the association is warranted. ![]()

Credit: UAB Hospital
A new study suggests the risks and benefits of red blood cell (RBC) transfusions can vary considerably for patients with trauma and major bleeding, depending on the patients’ risk of death at baseline.
Patients with the highest predicted risk of death on arrival at a trauma center received the greatest benefit from RBC transfusions.
But among patients with the lowest predicted risk of death at baseline, transfusion was associated with a higher risk of death post-treatment.
Pablo Perel, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK, and his colleagues reported these findings in PLOS Medicine.
To conduct this study, the team used data from the CRASH-2 trial, which assessed the effect of tranexamic acid in trauma patients. The trial included 20,127 patients with significant bleeding who were treated at 274 hospitals in 40 countries.
Dr Perel and his colleagues used that data to evaluate the association between receiving an RBC transfusion and death from all causes at 28 days post-trauma. The findings were stratified by predicted risk of death based on clinical observations on arrival at the trauma center.
The researchers found that patients with the greatest predicted risk of dying—greater than 50%—had a smaller chance of death from all causes if they were transfused than if they were not. The odds ratio (OR) was 0.59.
For patients whose predicted risk of death ranged from 21% to 50%, there was no significant difference in their chance of dying whether they were transfused or not. The OR was 0.92.
But for patients with a lower risk of death at baseline, transfusion was associated with an increased risk of death.
Patients with a 6% to 20% risk of death at baseline had an OR of 2.31 if they received a transfusion. And for patients whose initial risk of death was below 6%, the OR for death associated with transfusion was 5.40.
In absolute figures, compared to no transfusion, RBC transfusion was associated with 5.1 more deaths per 100 patients in the group with the lowest predicted risk of death but with 11.9 fewer deaths per 100 patients in the group with the highest predicted risk of death.
The researchers noted that, although these data suggest RBC transfusion could be harmful for patients whose predicted risk of death is low, this study was observational. So the team cannot confirm a causal link, and a randomized trial investigating the association is warranted. ![]()

Credit: UAB Hospital
A new study suggests the risks and benefits of red blood cell (RBC) transfusions can vary considerably for patients with trauma and major bleeding, depending on the patients’ risk of death at baseline.
Patients with the highest predicted risk of death on arrival at a trauma center received the greatest benefit from RBC transfusions.
But among patients with the lowest predicted risk of death at baseline, transfusion was associated with a higher risk of death post-treatment.
Pablo Perel, MD, PhD, of the London School of Hygiene & Tropical Medicine in the UK, and his colleagues reported these findings in PLOS Medicine.
To conduct this study, the team used data from the CRASH-2 trial, which assessed the effect of tranexamic acid in trauma patients. The trial included 20,127 patients with significant bleeding who were treated at 274 hospitals in 40 countries.
Dr Perel and his colleagues used that data to evaluate the association between receiving an RBC transfusion and death from all causes at 28 days post-trauma. The findings were stratified by predicted risk of death based on clinical observations on arrival at the trauma center.
The researchers found that patients with the greatest predicted risk of dying—greater than 50%—had a smaller chance of death from all causes if they were transfused than if they were not. The odds ratio (OR) was 0.59.
For patients whose predicted risk of death ranged from 21% to 50%, there was no significant difference in their chance of dying whether they were transfused or not. The OR was 0.92.
But for patients with a lower risk of death at baseline, transfusion was associated with an increased risk of death.
Patients with a 6% to 20% risk of death at baseline had an OR of 2.31 if they received a transfusion. And for patients whose initial risk of death was below 6%, the OR for death associated with transfusion was 5.40.
In absolute figures, compared to no transfusion, RBC transfusion was associated with 5.1 more deaths per 100 patients in the group with the lowest predicted risk of death but with 11.9 fewer deaths per 100 patients in the group with the highest predicted risk of death.
The researchers noted that, although these data suggest RBC transfusion could be harmful for patients whose predicted risk of death is low, this study was observational. So the team cannot confirm a causal link, and a randomized trial investigating the association is warranted. ![]()
Radiographs Predict Pneumonia Severity
The 2011 Pediatric Infectious Diseases Society and Infectious Diseases Society of America (PIDS/IDSA) guidelines for management of pediatric community‐acquired pneumonia (CAP) recommend that admission chest radiographs be obtained in all children hospitalized with CAP to document the presence and extent of infiltrates and to identify complications.[1] Findings from chest radiographs may also provide clues to etiology and assist with predicting disease outcomes. In adults with CAP, clinical prediction tools use radiographic findings to inform triage decisions, guide management strategies, and predict outcomes.[2, 3, 4, 5, 6, 7] Whether or not radiographic findings could have similar utility among children with CAP is unknown.
Several retrospective studies have examined the ability of chest radiographs to predict pediatric pneumonia disease severity.[8, 9, 10, 11, 12] However, these studies used several different measures of severe pneumonia and/or were limited to young children <5 years of age, leading to inconsistent findings. These studies also rarely considered very severe disease (eg, need for invasive mechanical ventilation) or longitudinal outcome measures such as hospital length of stay. Finally, all of these prior studies were conducted outside of the United States, and most were single‐center investigations, potentially limiting generalizability. We sought to examine associations between admission chest radiographic findings and subsequent hospital care processes and clinical outcomes, including length of stay and resource utilization measures, among children hospitalized with CAP at 4 children's hospitals in the United States.
METHODS
Design and Setting
This study was nested within a multicenter retrospective cohort designed to validate International Classification of Diseases, 9th Revision, Clinical Modification (ICD9‐CM) diagnostic codes for pediatric CAP hospitalizations.[13] The Pediatric Health Information System database (Children's Hospital Association, Overland Park, KS) was used to identify children from 4 freestanding pediatric hospitals (Monroe Carell, Jr. Children's Hospital at Vanderbilt, Nashville, Tennessee; Children's Mercy Hospitals & Clinics, Kansas City, Missouri; Seattle Children's Hospital, Seattle, Washington; and Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio). The institutional review boards at each participating institution approved the study. The validation study included a 25% random sampling of children 60 days to 18 years of age (n=998) who were hospitalized between January 1, 2010 and December 31, 2010 with at least 1 ICD9‐CM discharge code indicating pneumonia. The diagnosis of CAP was confirmed by medical record review.
Study Population
This study was limited to children from the validation study who met criteria for clinical and radiographic CAP, defined as: (1) abnormal temperature or white blood cell count, (2) signs and symptoms of acute respiratory illness (eg, cough, tachypnea), and (3) chest radiograph indicating pneumonia within 48 hours of admission. Children with atelectasis as the only abnormal radiographic finding and those with complex chronic conditions (eg, cystic fibrosis, malignancy) were excluded using a previously described algorithm.[14]
Outcomes
Several measures of disease severity were assessed. Dichotomous outcomes included supplemental oxygen use, need for intensive care unit (ICU) admission, and need for invasive mechanical ventilation. Continuous outcomes included hospital length of stay, and for those requiring supplemental oxygen, duration of oxygen supplementation, measured in hours.
Exposure
To categorize infiltrate patterns and the presence and size of pleural effusions, we reviewed the final report from admission chest radiographs to obtain the final clinical interpretation performed by the attending pediatric radiologist. Infiltrate patterns were classified as single lobar (reference), unilateral multilobar, bilateral multilobar, or interstitial. Children with both lobar and interstitial infiltrates, and those with mention of atelectasis, were classified according to the type of lobar infiltrate. Those with atelectasis only were excluded. Pleural effusions were classified as absent, small, or moderate/large.
Analysis
Descriptive statistics were summarized using frequencies and percentages for categorical variables and median and interquartile range (IQR) values for continuous variables. Our primary exposures were infiltrate pattern and presence and size of pleural effusion on admission chest radiograph. Associations between radiographic findings and disease outcomes were analyzed using logistic and linear regression for dichotomous and continuous variables, respectively. Continuous outcomes were log‐transformed and normality assumptions verified prior to model development.
Due to the large number of covariates relative to outcome events, we used propensity score methods to adjust for potential confounding. The propensity score estimates the likelihood of a given exposure (ie, infiltrate pattern) conditional on a set of covariates. In this way, the propensity score summarizes potential confounding effects from a large number of covariates into a single variable. Including the propensity score as a covariate in multivariable regression improves model efficiency and helps protect against overfitting.[15] Covariates included in the estimation of the propensity score included age, sex, race/ethnicity, payer, hospital, asthma history, hospital transfer, recent hospitalization (within 30 days), recent emergency department or clinic visit (within 2 weeks), recent antibiotics for acute illness (within 5 days), illness duration prior to admission, tachypnea and/or increased work of breathing (retractions, nasal flaring, or grunting) at presentation, receipt of albuterol and/or corticosteroids during the first 2 calendar days of hospitalization, and concurrent diagnosis of bronchiolitis. All analyses included the estimated propensity score, infiltrate pattern, and pleural effusion (absent, small, or moderate/large).
RESULTS
Study Population
The median age of the 406 children with clinical and radiographic CAP was 3 years (IQR, 16 years) (Table 1). Single lobar infiltrate was the most common radiographic pattern (61%). Children with interstitial infiltrates (10%) were younger than those with lobar infiltrates of any type (median age 1 vs 3 years, P=0.02). A concomitant diagnosis of bronchiolitis was assigned to 34% of children with interstitial infiltrates but only 17% of those with lobar infiltrate patterns (range, 11%20%, P=0.03). Pleural effusion was present in 21% of children and was more common among those with lobar infiltrates, particularly multilobar disease. Only 1 child with interstitial infiltrate had a pleural effusion. Overall, 63% of children required supplemental oxygen, 8% required ICU admission, and 3% required invasive mechanical ventilation. Median length of stay was 51.5 hours (IQR, 3991) and median oxygen duration was 31.5 hours [IQR, 1365]. There were no deaths.
| Characteristic | Infiltrate Patterna | P Valueb | |||
|---|---|---|---|---|---|
| Single Lobar | Multilobar, Unilateral | Multilobar, Bilateral | Interstitial | ||
| |||||
| No. | 247 (60.8) | 54 (13.3) | 64 (15.8) | 41 (10.1) | |
| Median age, y | 3 [16] | 3 [17] | 3 [15] | 1 [03] | 0.02 |
| Male sex | 124 (50.2) | 32 (59.3) | 41 (64.1) | 30 (73.2) | 0.02 |
| Race | |||||
| Non‐Hispanic white | 133 (53.8) | 36 (66.7) | 37 (57.8) | 17 (41.5) | 0.69 |
| Non‐Hispanic black | 40 (16.2) | 6 (11.1) | 9 (14.1) | 8 (19.5) | |
| Hispanic | 25 (10.1) | 4 (7.4) | 5 (7.8) | 7 (17.1) | |
| Other | 49 (19.9) | 8 (14.8) | 13 (20.4) | 9 (22) | |
| Insurance | |||||
| Public | 130 (52.6) | 26 (48.1) | 33 (51.6) | 25 (61) | 0.90 |
| Private | 116 (47) | 28 (51.9) | 31 (48.4) | 16 (39) | |
| Concurrent diagnosis | |||||
| Asthma | 80 (32.4) | 16 (29.6) | 17 (26.6) | 12 (29.3) | 0.82 |
| Bronchiolitis | 43 (17.4) | 6 (11.1) | 13 (20.3) | 14 (34.1) | 0.03 |
| Effusion | |||||
| None | 201 (81.4) | 31 (57.4) | 48 (75) | 40 (97.6) | <.01 |
| Small | 34 (13.8) | 20 (37) | 11 (17.2) | 0 | |
| Moderate/large | 12 (4.9) | 3 (5.6) | 5 (7.8) | 1 (2.4) | |
Outcomes According to Radiographic Infiltrate Pattern
Compared to children with single lobar infiltrates, the odds of ICU admission was significantly increased for those with either unilateral or bilateral multilobar infiltrates (unilateral, adjusted odds ratio [aOR]: 8.0, 95% confidence interval [CI]: 2.922.2; bilateral, aOR: 6.6, 95% CI: 2.14.5) (Figure 1, Table 2). Patients with bilateral multilobar infiltrates also had higher odds for supplemental oxygen use (aOR: 2.7, 95% CI: 1.25.8) and need for invasive mechanical ventilation (aOR: 3.0, 95% CI: 1.27.9). There were no differences in duration of oxygen supplementation or hospital length of stay for children with single versus multilobar infiltrates.
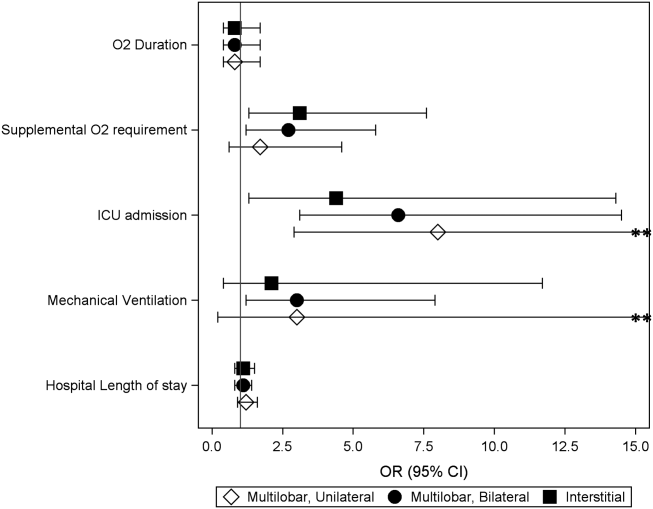
| Outcome | Infiltrate Patterna | P Valueb | |||
|---|---|---|---|---|---|
| Single Lobar, n=247 | Multilobar, Unilateral, n=54 | Multilobar, Bilateral, n=64 | Interstitial, n=41 | ||
| |||||
| Supplemental O2 requirement | 143 (57.9) | 34 (63) | 46 (71.9) | 31 (75.6) | 0.05 |
| ICU admission | 10 (4) | 9 (16.7) | 9 (14.1) | 4 (9.8) | <0.01 |
| Mechanical ventilation | 5 (2) | 4 (7.4) | 4 (6.3) | 1 (2.4) | 0.13 |
| Hospital length of stay, h | 47 [3779] | 63 [45114] | 56.5 [39.5101] | 62 [3993] | <0.01 |
| O2 duration, h | 27 [1059] | 38 [1777] | 38 [2381] | 34.5 [1765] | 0.18 |
Compared to those with single lobar infiltrates, children with interstitial infiltrates had higher odds of need for supplemental oxygen (aOR: 3.1, 95% CI: 1.37.6) and ICU admission (aOR: 4.4, 95% CI: 1.314.3) but not invasive mechanical ventilation. There were also no differences in duration of oxygen supplementation or hospital length of stay.
Outcomes According to Presence and Size of Pleural Effusion
Compared to those without pleural effusion, children with moderate to large effusion had a higher odds of ICU admission (aOR: 3.2, 95% CI: 1.18.9) and invasive mechanical ventilation (aOR: 14.8, 95% CI: 9.822.4), and also had a longer duration of oxygen supplementation (aOR: 3.0, 95% CI: 1.46.5) and hospital length of stay (aOR: 2.6, 95% CI: 1.9‐3.6) (Table 3, Figure 2). The presence of a small pleural effusion was not associated with increased need for supplemental oxygen, ICU admission, or mechanical ventilation compared to those without effusion. However, small effusion was associated with a longer duration of oxygen supplementation (aOR: 1.7, 95% CI: 12.7) and hospital length of stay (aOR: 1.6, 95% CI: 1.3‐1.9).
| Outcome | Pleural Effusion | P Valuea | ||
|---|---|---|---|---|
| None, n=320 | Small, n=65 | Moderate/Large, n=21 | ||
| ||||
| Supplemental O2 requirement | 200 (62.5) | 40 (61.5) | 14 (66.7) | 0.91 |
| ICU admission | 22 (6.9) | 6 (9.2) | 4 (19) | 0.12 |
| Mechanical ventilation | 5 (1.6) | 5 (7.7) | 4 (19) | <0.01 |
| Hospital length of stay, h | 48 [37.576] | 72 [45142] | 160 [82191] | <0.01 |
| Oxygen duration, h | 31 [1157] | 38.5 [1887] | 111 [27154] | <0.01 |
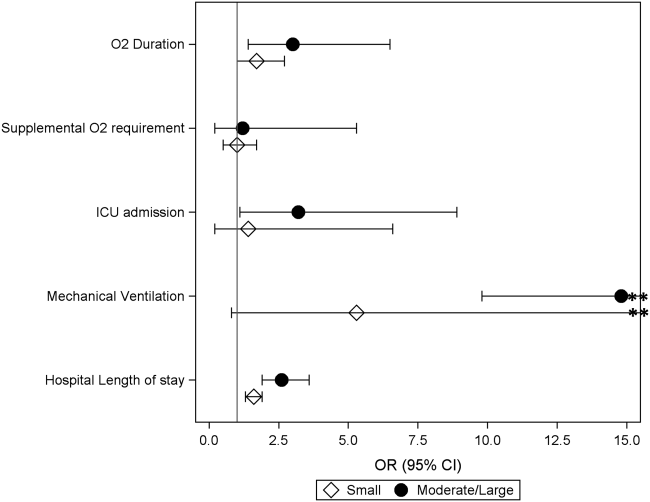
DISCUSSION
We evaluated the association between admission chest radiographic findings and subsequent clinical outcomes and hospital care processes for children hospitalized with CAP at 4 children's hospitals in the United States. We conclude that radiographic findings are associated with important inpatient outcomes. Similar to data from adults, findings of moderate to large pleural effusions and bilateral multilobar infiltrates had the strongest associations with severe disease. Such information, in combination with other prognostic factors, may help clinicians identify high‐risk patients and support management decisions, while also helping to inform families about the expected hospital course.
Previous pediatric studies examining the association between radiographic findings and outcomes have produced inconsistent results.[8, 9, 10, 11, 12] All but 1 of these studies documented 1 radiographic characteristics associated with pneumonia disease severity.[11] Further, although most contrasted lobar/alveolar and interstitial infiltrates, only Patria et al. distinguished among lobar infiltrate patterns (eg, single lobar vs multilobar).[12] Similar to our findings, that study demonstrated increased disease severity among children with bilateral multifocal lobar infiltrates. Of the studies that considered the presence of pleural effusion, only 1 demonstrated this finding to be associated with more severe disease.[9] However, none of these prior studies examined size of the pleural effusion.
In our study, the strongest association with severe pneumonia outcomes was among children with moderate to large pleural effusion. Significant pleural effusions are much more commonly due to infection with bacterial pathogens, particularly Streptococcus pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes, and may also indicate infection with more virulent and/or difficult to treat strains.[16, 17, 18, 19] Surgical intervention is also often required. As such, children with significant pleural effusions are often more ill on presentation and may have a prolonged period of recovery.[20, 21, 22]
Similarly, multilobar infiltrates, particularly bilateral, were associated with increased disease severity in terms of need for supplemental oxygen, ICU admission, and need for invasive mechanical ventilation. Although this finding may be expected, it is interesting to note that the duration of supplemental oxygen and hospital length of stay were similar to those with single lobar disease. One potential explanation is that, although children with multilobar disease are more severe at presentation, rates of recovery are similar to those with less extensive radiographic findings, owing to rapidly effective antimicrobials for uncomplicated bacterial pneumonia. This hypothesis also agrees with the 2011 PIDS/IDSA guidelines, which state that children receiving adequate therapy typically show signs of improvement within 48 to 72 hours regardless of initial severity.[1]
Interstitial infiltrate was also associated with increased severity at presentation but similar length of stay and duration of oxygen requirement compared with single lobar disease. We note that these children were substantially younger than those presenting with any pattern of lobar disease (median age, 1 vs 3 years), were more likely to have a concurrent diagnosis of bronchiolitis (34% vs 17%), and only 1 child with interstitial infiltrates had a documented pleural effusion (vs 23% of children with lobar infiltrates). Primary viral pneumonia is considered more likely to produce interstitial infiltrates on chest radiograph compared to bacterial disease, and although detailed etiologic data are unavailable for this study, our findings above strongly support this assertion.[23, 24]
The 2011 PIDS/IDSA guidelines recommend admission chest radiographs for all children hospitalized with pneumonia to assess extent of disease and identify complications that may requiring additional evaluation or surgical intervention.[1] Our findings highlight additional potential benefits of admission radiographs in terms of disease prognosis and management decisions. In the initial evaluation of a sick child with pneumonia, clinicians are often presented with a number of potential prognostic factors that may influence disease outcomes. However, it is sometimes difficult for providers to consider all available information and/or the relative importance of a single factor, resulting in inaccurate risk perceptions and management decisions that may contribute to poor outcomes.[25] Similar to adults, the development of clinical prediction rules, which incorporate a variety of important predictors including admission radiographic findings, likely would improve risk assessments and potentially outcomes for children with pneumonia. Such prognostic information is also helpful for clinicians who may use these data to inform and prepare families regarding the expected course of hospitalization.
Our study has several limitations. This study was retrospective and only included a sample of pneumonia hospitalizations during the study period, which may raise confounding concerns and potential for selection bias. However, detailed medical record reviews using standardized case definitions for radiographic CAP were used, and a large sample of children was randomly selected from each institution. In addition, a large number of potential confounders were selected a priori and included in multivariable analyses; propensity score adjustment was used to reduce model complexity and avoid overfitting. Radiographic findings were based on clinical interpretation by pediatric radiologists independent of a study protocol. Prior studies have demonstrated good agreement for identification of alveolar/lobar infiltrates and pleural effusion by trained radiologists, although agreement for interstitial infiltrate is poor.[26, 27] This limitation could result in either over‐ or underestimation of the prevalence of interstitial infiltrates likely resulting in a nondifferential bias toward the null. Microbiologic information, which may inform radiographic findings and disease severity, was also not available. However, because pneumonia etiology is frequently unknown in the clinical setting, our study reflects typical practice. We also did not include children from community or nonteaching hospitals. Thus, although findings may have relevance to community or nonteaching hospitals, our results cannot be generalized.
CONCLUSION
Our study demonstrates that among children hospitalized with CAP, admission chest radiographic findings are associated with important clinical outcomes and hospital care processes, highlighting additional benefits of the 2011 PIDS/IDSA guidelines' recommendation for admission chest radiographs for all children hospitalized with pneumonia. These data, in conjunction with other important prognostic information, may help clinicians more rapidly identify children at increased risk for severe illness, and could also offer guidance regarding disease management strategies and facilitate shared decision making with families. Thus, routine admission chest radiography in this population represents a valuable tool that contributes to improved quality of care.
Disclosures
Dr. Williams is supported by funds from the National Institutes of HealthNational Institute of Allergy and Infectious Diseases (K23AI104779). The authors report no conflicts of interest.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia. Clin Infect Dis. 2008;47(3):375–384.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. Risk stratification of early admission to the intensive care unit of patients with no major criteria of severe community‐acquired pneumonia: development of an international prediction rule. Crit Care. 2009;13(2):R54.
- , , , et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community‐acquired pneumonia? Arch Intern Med. 1996;156(19):2206–2212.
- , , , , , . Safety and efficacy of CURB65‐guided antibiotic therapy in community‐acquired pneumonia. J Antimicrob Chemother. 2011;66(2):416–423.
- , , . Severity of childhood community‐acquired pneumonia and chest radiographic findings. Pediatr Pulmonol. 2009;44(3):249–252.
- , , , et al. Can chest x‐ray predict pneumonia severity? Pediatr Pulmonol. 2004;38(6):465–469.
- , , , . Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92(5):394–398.
- , , . Role of chest X‐ray in predicting outcome of acute severe pneumonia. Indian Pediatr. 2008;45(11):893–898.
- , , , , , . Association between radiological findings and severity of community‐acquired pneumonia in children. Ital J Pediatr. 2013;39:56.
- , , , et al. Identifying pediatric community‐acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatrics. 2013;167(9):851–858.
- , , , , , . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99.
- , . Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–333.
- , , , . Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States. Clin Infect Dis. 2010;50(6):805–813.
- , , , et al. Epidemiology and clinical characteristics of community‐acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4):701–707.
- , , , et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289–294.
- , . Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980. Clin Pediatr (Phila). 1983;22(6):414–419.
- , , , et al. Risk factors of progressive community‐acquired pneumonia in hospitalized children: a prospective study [published online ahead of print August 28, 2013]. J Microbiol Immunol Infect. doi: 10.1016/j.jmii.2013.06.009.
- , , , , . Community‐acquired lobar pneumonia in children in the era of universal 7‐valent pneumococcal vaccination: a review of clinical presentations and antimicrobial treatment from a Canadian pediatric hospital. BMC Pediatr. 2012;12:133.
- , , , et al. Clinical characteristics and outcome of complicated pneumococcal pneumonia in a pediatric population. Pediatr Pulmonol. 2006;41(8):726–734.
- , , , , , . Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57(5):438–441.
- , , , et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1–ii23.
- , , , et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010–1016.
- , , , et al. Variability in the interpretation of chest radiographs for the diagnosis of pneumonia in children. J Hosp Med. 2012;7(4):294–298.
- , , , et al. Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT Investigators. Chest. 1996;110(2):343–350.
The 2011 Pediatric Infectious Diseases Society and Infectious Diseases Society of America (PIDS/IDSA) guidelines for management of pediatric community‐acquired pneumonia (CAP) recommend that admission chest radiographs be obtained in all children hospitalized with CAP to document the presence and extent of infiltrates and to identify complications.[1] Findings from chest radiographs may also provide clues to etiology and assist with predicting disease outcomes. In adults with CAP, clinical prediction tools use radiographic findings to inform triage decisions, guide management strategies, and predict outcomes.[2, 3, 4, 5, 6, 7] Whether or not radiographic findings could have similar utility among children with CAP is unknown.
Several retrospective studies have examined the ability of chest radiographs to predict pediatric pneumonia disease severity.[8, 9, 10, 11, 12] However, these studies used several different measures of severe pneumonia and/or were limited to young children <5 years of age, leading to inconsistent findings. These studies also rarely considered very severe disease (eg, need for invasive mechanical ventilation) or longitudinal outcome measures such as hospital length of stay. Finally, all of these prior studies were conducted outside of the United States, and most were single‐center investigations, potentially limiting generalizability. We sought to examine associations between admission chest radiographic findings and subsequent hospital care processes and clinical outcomes, including length of stay and resource utilization measures, among children hospitalized with CAP at 4 children's hospitals in the United States.
METHODS
Design and Setting
This study was nested within a multicenter retrospective cohort designed to validate International Classification of Diseases, 9th Revision, Clinical Modification (ICD9‐CM) diagnostic codes for pediatric CAP hospitalizations.[13] The Pediatric Health Information System database (Children's Hospital Association, Overland Park, KS) was used to identify children from 4 freestanding pediatric hospitals (Monroe Carell, Jr. Children's Hospital at Vanderbilt, Nashville, Tennessee; Children's Mercy Hospitals & Clinics, Kansas City, Missouri; Seattle Children's Hospital, Seattle, Washington; and Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio). The institutional review boards at each participating institution approved the study. The validation study included a 25% random sampling of children 60 days to 18 years of age (n=998) who were hospitalized between January 1, 2010 and December 31, 2010 with at least 1 ICD9‐CM discharge code indicating pneumonia. The diagnosis of CAP was confirmed by medical record review.
Study Population
This study was limited to children from the validation study who met criteria for clinical and radiographic CAP, defined as: (1) abnormal temperature or white blood cell count, (2) signs and symptoms of acute respiratory illness (eg, cough, tachypnea), and (3) chest radiograph indicating pneumonia within 48 hours of admission. Children with atelectasis as the only abnormal radiographic finding and those with complex chronic conditions (eg, cystic fibrosis, malignancy) were excluded using a previously described algorithm.[14]
Outcomes
Several measures of disease severity were assessed. Dichotomous outcomes included supplemental oxygen use, need for intensive care unit (ICU) admission, and need for invasive mechanical ventilation. Continuous outcomes included hospital length of stay, and for those requiring supplemental oxygen, duration of oxygen supplementation, measured in hours.
Exposure
To categorize infiltrate patterns and the presence and size of pleural effusions, we reviewed the final report from admission chest radiographs to obtain the final clinical interpretation performed by the attending pediatric radiologist. Infiltrate patterns were classified as single lobar (reference), unilateral multilobar, bilateral multilobar, or interstitial. Children with both lobar and interstitial infiltrates, and those with mention of atelectasis, were classified according to the type of lobar infiltrate. Those with atelectasis only were excluded. Pleural effusions were classified as absent, small, or moderate/large.
Analysis
Descriptive statistics were summarized using frequencies and percentages for categorical variables and median and interquartile range (IQR) values for continuous variables. Our primary exposures were infiltrate pattern and presence and size of pleural effusion on admission chest radiograph. Associations between radiographic findings and disease outcomes were analyzed using logistic and linear regression for dichotomous and continuous variables, respectively. Continuous outcomes were log‐transformed and normality assumptions verified prior to model development.
Due to the large number of covariates relative to outcome events, we used propensity score methods to adjust for potential confounding. The propensity score estimates the likelihood of a given exposure (ie, infiltrate pattern) conditional on a set of covariates. In this way, the propensity score summarizes potential confounding effects from a large number of covariates into a single variable. Including the propensity score as a covariate in multivariable regression improves model efficiency and helps protect against overfitting.[15] Covariates included in the estimation of the propensity score included age, sex, race/ethnicity, payer, hospital, asthma history, hospital transfer, recent hospitalization (within 30 days), recent emergency department or clinic visit (within 2 weeks), recent antibiotics for acute illness (within 5 days), illness duration prior to admission, tachypnea and/or increased work of breathing (retractions, nasal flaring, or grunting) at presentation, receipt of albuterol and/or corticosteroids during the first 2 calendar days of hospitalization, and concurrent diagnosis of bronchiolitis. All analyses included the estimated propensity score, infiltrate pattern, and pleural effusion (absent, small, or moderate/large).
RESULTS
Study Population
The median age of the 406 children with clinical and radiographic CAP was 3 years (IQR, 16 years) (Table 1). Single lobar infiltrate was the most common radiographic pattern (61%). Children with interstitial infiltrates (10%) were younger than those with lobar infiltrates of any type (median age 1 vs 3 years, P=0.02). A concomitant diagnosis of bronchiolitis was assigned to 34% of children with interstitial infiltrates but only 17% of those with lobar infiltrate patterns (range, 11%20%, P=0.03). Pleural effusion was present in 21% of children and was more common among those with lobar infiltrates, particularly multilobar disease. Only 1 child with interstitial infiltrate had a pleural effusion. Overall, 63% of children required supplemental oxygen, 8% required ICU admission, and 3% required invasive mechanical ventilation. Median length of stay was 51.5 hours (IQR, 3991) and median oxygen duration was 31.5 hours [IQR, 1365]. There were no deaths.
| Characteristic | Infiltrate Patterna | P Valueb | |||
|---|---|---|---|---|---|
| Single Lobar | Multilobar, Unilateral | Multilobar, Bilateral | Interstitial | ||
| |||||
| No. | 247 (60.8) | 54 (13.3) | 64 (15.8) | 41 (10.1) | |
| Median age, y | 3 [16] | 3 [17] | 3 [15] | 1 [03] | 0.02 |
| Male sex | 124 (50.2) | 32 (59.3) | 41 (64.1) | 30 (73.2) | 0.02 |
| Race | |||||
| Non‐Hispanic white | 133 (53.8) | 36 (66.7) | 37 (57.8) | 17 (41.5) | 0.69 |
| Non‐Hispanic black | 40 (16.2) | 6 (11.1) | 9 (14.1) | 8 (19.5) | |
| Hispanic | 25 (10.1) | 4 (7.4) | 5 (7.8) | 7 (17.1) | |
| Other | 49 (19.9) | 8 (14.8) | 13 (20.4) | 9 (22) | |
| Insurance | |||||
| Public | 130 (52.6) | 26 (48.1) | 33 (51.6) | 25 (61) | 0.90 |
| Private | 116 (47) | 28 (51.9) | 31 (48.4) | 16 (39) | |
| Concurrent diagnosis | |||||
| Asthma | 80 (32.4) | 16 (29.6) | 17 (26.6) | 12 (29.3) | 0.82 |
| Bronchiolitis | 43 (17.4) | 6 (11.1) | 13 (20.3) | 14 (34.1) | 0.03 |
| Effusion | |||||
| None | 201 (81.4) | 31 (57.4) | 48 (75) | 40 (97.6) | <.01 |
| Small | 34 (13.8) | 20 (37) | 11 (17.2) | 0 | |
| Moderate/large | 12 (4.9) | 3 (5.6) | 5 (7.8) | 1 (2.4) | |
Outcomes According to Radiographic Infiltrate Pattern
Compared to children with single lobar infiltrates, the odds of ICU admission was significantly increased for those with either unilateral or bilateral multilobar infiltrates (unilateral, adjusted odds ratio [aOR]: 8.0, 95% confidence interval [CI]: 2.922.2; bilateral, aOR: 6.6, 95% CI: 2.14.5) (Figure 1, Table 2). Patients with bilateral multilobar infiltrates also had higher odds for supplemental oxygen use (aOR: 2.7, 95% CI: 1.25.8) and need for invasive mechanical ventilation (aOR: 3.0, 95% CI: 1.27.9). There were no differences in duration of oxygen supplementation or hospital length of stay for children with single versus multilobar infiltrates.

| Outcome | Infiltrate Patterna | P Valueb | |||
|---|---|---|---|---|---|
| Single Lobar, n=247 | Multilobar, Unilateral, n=54 | Multilobar, Bilateral, n=64 | Interstitial, n=41 | ||
| |||||
| Supplemental O2 requirement | 143 (57.9) | 34 (63) | 46 (71.9) | 31 (75.6) | 0.05 |
| ICU admission | 10 (4) | 9 (16.7) | 9 (14.1) | 4 (9.8) | <0.01 |
| Mechanical ventilation | 5 (2) | 4 (7.4) | 4 (6.3) | 1 (2.4) | 0.13 |
| Hospital length of stay, h | 47 [3779] | 63 [45114] | 56.5 [39.5101] | 62 [3993] | <0.01 |
| O2 duration, h | 27 [1059] | 38 [1777] | 38 [2381] | 34.5 [1765] | 0.18 |
Compared to those with single lobar infiltrates, children with interstitial infiltrates had higher odds of need for supplemental oxygen (aOR: 3.1, 95% CI: 1.37.6) and ICU admission (aOR: 4.4, 95% CI: 1.314.3) but not invasive mechanical ventilation. There were also no differences in duration of oxygen supplementation or hospital length of stay.
Outcomes According to Presence and Size of Pleural Effusion
Compared to those without pleural effusion, children with moderate to large effusion had a higher odds of ICU admission (aOR: 3.2, 95% CI: 1.18.9) and invasive mechanical ventilation (aOR: 14.8, 95% CI: 9.822.4), and also had a longer duration of oxygen supplementation (aOR: 3.0, 95% CI: 1.46.5) and hospital length of stay (aOR: 2.6, 95% CI: 1.9‐3.6) (Table 3, Figure 2). The presence of a small pleural effusion was not associated with increased need for supplemental oxygen, ICU admission, or mechanical ventilation compared to those without effusion. However, small effusion was associated with a longer duration of oxygen supplementation (aOR: 1.7, 95% CI: 12.7) and hospital length of stay (aOR: 1.6, 95% CI: 1.3‐1.9).
| Outcome | Pleural Effusion | P Valuea | ||
|---|---|---|---|---|
| None, n=320 | Small, n=65 | Moderate/Large, n=21 | ||
| ||||
| Supplemental O2 requirement | 200 (62.5) | 40 (61.5) | 14 (66.7) | 0.91 |
| ICU admission | 22 (6.9) | 6 (9.2) | 4 (19) | 0.12 |
| Mechanical ventilation | 5 (1.6) | 5 (7.7) | 4 (19) | <0.01 |
| Hospital length of stay, h | 48 [37.576] | 72 [45142] | 160 [82191] | <0.01 |
| Oxygen duration, h | 31 [1157] | 38.5 [1887] | 111 [27154] | <0.01 |

DISCUSSION
We evaluated the association between admission chest radiographic findings and subsequent clinical outcomes and hospital care processes for children hospitalized with CAP at 4 children's hospitals in the United States. We conclude that radiographic findings are associated with important inpatient outcomes. Similar to data from adults, findings of moderate to large pleural effusions and bilateral multilobar infiltrates had the strongest associations with severe disease. Such information, in combination with other prognostic factors, may help clinicians identify high‐risk patients and support management decisions, while also helping to inform families about the expected hospital course.
Previous pediatric studies examining the association between radiographic findings and outcomes have produced inconsistent results.[8, 9, 10, 11, 12] All but 1 of these studies documented 1 radiographic characteristics associated with pneumonia disease severity.[11] Further, although most contrasted lobar/alveolar and interstitial infiltrates, only Patria et al. distinguished among lobar infiltrate patterns (eg, single lobar vs multilobar).[12] Similar to our findings, that study demonstrated increased disease severity among children with bilateral multifocal lobar infiltrates. Of the studies that considered the presence of pleural effusion, only 1 demonstrated this finding to be associated with more severe disease.[9] However, none of these prior studies examined size of the pleural effusion.
In our study, the strongest association with severe pneumonia outcomes was among children with moderate to large pleural effusion. Significant pleural effusions are much more commonly due to infection with bacterial pathogens, particularly Streptococcus pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes, and may also indicate infection with more virulent and/or difficult to treat strains.[16, 17, 18, 19] Surgical intervention is also often required. As such, children with significant pleural effusions are often more ill on presentation and may have a prolonged period of recovery.[20, 21, 22]
Similarly, multilobar infiltrates, particularly bilateral, were associated with increased disease severity in terms of need for supplemental oxygen, ICU admission, and need for invasive mechanical ventilation. Although this finding may be expected, it is interesting to note that the duration of supplemental oxygen and hospital length of stay were similar to those with single lobar disease. One potential explanation is that, although children with multilobar disease are more severe at presentation, rates of recovery are similar to those with less extensive radiographic findings, owing to rapidly effective antimicrobials for uncomplicated bacterial pneumonia. This hypothesis also agrees with the 2011 PIDS/IDSA guidelines, which state that children receiving adequate therapy typically show signs of improvement within 48 to 72 hours regardless of initial severity.[1]
Interstitial infiltrate was also associated with increased severity at presentation but similar length of stay and duration of oxygen requirement compared with single lobar disease. We note that these children were substantially younger than those presenting with any pattern of lobar disease (median age, 1 vs 3 years), were more likely to have a concurrent diagnosis of bronchiolitis (34% vs 17%), and only 1 child with interstitial infiltrates had a documented pleural effusion (vs 23% of children with lobar infiltrates). Primary viral pneumonia is considered more likely to produce interstitial infiltrates on chest radiograph compared to bacterial disease, and although detailed etiologic data are unavailable for this study, our findings above strongly support this assertion.[23, 24]
The 2011 PIDS/IDSA guidelines recommend admission chest radiographs for all children hospitalized with pneumonia to assess extent of disease and identify complications that may requiring additional evaluation or surgical intervention.[1] Our findings highlight additional potential benefits of admission radiographs in terms of disease prognosis and management decisions. In the initial evaluation of a sick child with pneumonia, clinicians are often presented with a number of potential prognostic factors that may influence disease outcomes. However, it is sometimes difficult for providers to consider all available information and/or the relative importance of a single factor, resulting in inaccurate risk perceptions and management decisions that may contribute to poor outcomes.[25] Similar to adults, the development of clinical prediction rules, which incorporate a variety of important predictors including admission radiographic findings, likely would improve risk assessments and potentially outcomes for children with pneumonia. Such prognostic information is also helpful for clinicians who may use these data to inform and prepare families regarding the expected course of hospitalization.
Our study has several limitations. This study was retrospective and only included a sample of pneumonia hospitalizations during the study period, which may raise confounding concerns and potential for selection bias. However, detailed medical record reviews using standardized case definitions for radiographic CAP were used, and a large sample of children was randomly selected from each institution. In addition, a large number of potential confounders were selected a priori and included in multivariable analyses; propensity score adjustment was used to reduce model complexity and avoid overfitting. Radiographic findings were based on clinical interpretation by pediatric radiologists independent of a study protocol. Prior studies have demonstrated good agreement for identification of alveolar/lobar infiltrates and pleural effusion by trained radiologists, although agreement for interstitial infiltrate is poor.[26, 27] This limitation could result in either over‐ or underestimation of the prevalence of interstitial infiltrates likely resulting in a nondifferential bias toward the null. Microbiologic information, which may inform radiographic findings and disease severity, was also not available. However, because pneumonia etiology is frequently unknown in the clinical setting, our study reflects typical practice. We also did not include children from community or nonteaching hospitals. Thus, although findings may have relevance to community or nonteaching hospitals, our results cannot be generalized.
CONCLUSION
Our study demonstrates that among children hospitalized with CAP, admission chest radiographic findings are associated with important clinical outcomes and hospital care processes, highlighting additional benefits of the 2011 PIDS/IDSA guidelines' recommendation for admission chest radiographs for all children hospitalized with pneumonia. These data, in conjunction with other important prognostic information, may help clinicians more rapidly identify children at increased risk for severe illness, and could also offer guidance regarding disease management strategies and facilitate shared decision making with families. Thus, routine admission chest radiography in this population represents a valuable tool that contributes to improved quality of care.
Disclosures
Dr. Williams is supported by funds from the National Institutes of HealthNational Institute of Allergy and Infectious Diseases (K23AI104779). The authors report no conflicts of interest.
The 2011 Pediatric Infectious Diseases Society and Infectious Diseases Society of America (PIDS/IDSA) guidelines for management of pediatric community‐acquired pneumonia (CAP) recommend that admission chest radiographs be obtained in all children hospitalized with CAP to document the presence and extent of infiltrates and to identify complications.[1] Findings from chest radiographs may also provide clues to etiology and assist with predicting disease outcomes. In adults with CAP, clinical prediction tools use radiographic findings to inform triage decisions, guide management strategies, and predict outcomes.[2, 3, 4, 5, 6, 7] Whether or not radiographic findings could have similar utility among children with CAP is unknown.
Several retrospective studies have examined the ability of chest radiographs to predict pediatric pneumonia disease severity.[8, 9, 10, 11, 12] However, these studies used several different measures of severe pneumonia and/or were limited to young children <5 years of age, leading to inconsistent findings. These studies also rarely considered very severe disease (eg, need for invasive mechanical ventilation) or longitudinal outcome measures such as hospital length of stay. Finally, all of these prior studies were conducted outside of the United States, and most were single‐center investigations, potentially limiting generalizability. We sought to examine associations between admission chest radiographic findings and subsequent hospital care processes and clinical outcomes, including length of stay and resource utilization measures, among children hospitalized with CAP at 4 children's hospitals in the United States.
METHODS
Design and Setting
This study was nested within a multicenter retrospective cohort designed to validate International Classification of Diseases, 9th Revision, Clinical Modification (ICD9‐CM) diagnostic codes for pediatric CAP hospitalizations.[13] The Pediatric Health Information System database (Children's Hospital Association, Overland Park, KS) was used to identify children from 4 freestanding pediatric hospitals (Monroe Carell, Jr. Children's Hospital at Vanderbilt, Nashville, Tennessee; Children's Mercy Hospitals & Clinics, Kansas City, Missouri; Seattle Children's Hospital, Seattle, Washington; and Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio). The institutional review boards at each participating institution approved the study. The validation study included a 25% random sampling of children 60 days to 18 years of age (n=998) who were hospitalized between January 1, 2010 and December 31, 2010 with at least 1 ICD9‐CM discharge code indicating pneumonia. The diagnosis of CAP was confirmed by medical record review.
Study Population
This study was limited to children from the validation study who met criteria for clinical and radiographic CAP, defined as: (1) abnormal temperature or white blood cell count, (2) signs and symptoms of acute respiratory illness (eg, cough, tachypnea), and (3) chest radiograph indicating pneumonia within 48 hours of admission. Children with atelectasis as the only abnormal radiographic finding and those with complex chronic conditions (eg, cystic fibrosis, malignancy) were excluded using a previously described algorithm.[14]
Outcomes
Several measures of disease severity were assessed. Dichotomous outcomes included supplemental oxygen use, need for intensive care unit (ICU) admission, and need for invasive mechanical ventilation. Continuous outcomes included hospital length of stay, and for those requiring supplemental oxygen, duration of oxygen supplementation, measured in hours.
Exposure
To categorize infiltrate patterns and the presence and size of pleural effusions, we reviewed the final report from admission chest radiographs to obtain the final clinical interpretation performed by the attending pediatric radiologist. Infiltrate patterns were classified as single lobar (reference), unilateral multilobar, bilateral multilobar, or interstitial. Children with both lobar and interstitial infiltrates, and those with mention of atelectasis, were classified according to the type of lobar infiltrate. Those with atelectasis only were excluded. Pleural effusions were classified as absent, small, or moderate/large.
Analysis
Descriptive statistics were summarized using frequencies and percentages for categorical variables and median and interquartile range (IQR) values for continuous variables. Our primary exposures were infiltrate pattern and presence and size of pleural effusion on admission chest radiograph. Associations between radiographic findings and disease outcomes were analyzed using logistic and linear regression for dichotomous and continuous variables, respectively. Continuous outcomes were log‐transformed and normality assumptions verified prior to model development.
Due to the large number of covariates relative to outcome events, we used propensity score methods to adjust for potential confounding. The propensity score estimates the likelihood of a given exposure (ie, infiltrate pattern) conditional on a set of covariates. In this way, the propensity score summarizes potential confounding effects from a large number of covariates into a single variable. Including the propensity score as a covariate in multivariable regression improves model efficiency and helps protect against overfitting.[15] Covariates included in the estimation of the propensity score included age, sex, race/ethnicity, payer, hospital, asthma history, hospital transfer, recent hospitalization (within 30 days), recent emergency department or clinic visit (within 2 weeks), recent antibiotics for acute illness (within 5 days), illness duration prior to admission, tachypnea and/or increased work of breathing (retractions, nasal flaring, or grunting) at presentation, receipt of albuterol and/or corticosteroids during the first 2 calendar days of hospitalization, and concurrent diagnosis of bronchiolitis. All analyses included the estimated propensity score, infiltrate pattern, and pleural effusion (absent, small, or moderate/large).
RESULTS
Study Population
The median age of the 406 children with clinical and radiographic CAP was 3 years (IQR, 16 years) (Table 1). Single lobar infiltrate was the most common radiographic pattern (61%). Children with interstitial infiltrates (10%) were younger than those with lobar infiltrates of any type (median age 1 vs 3 years, P=0.02). A concomitant diagnosis of bronchiolitis was assigned to 34% of children with interstitial infiltrates but only 17% of those with lobar infiltrate patterns (range, 11%20%, P=0.03). Pleural effusion was present in 21% of children and was more common among those with lobar infiltrates, particularly multilobar disease. Only 1 child with interstitial infiltrate had a pleural effusion. Overall, 63% of children required supplemental oxygen, 8% required ICU admission, and 3% required invasive mechanical ventilation. Median length of stay was 51.5 hours (IQR, 3991) and median oxygen duration was 31.5 hours [IQR, 1365]. There were no deaths.
| Characteristic | Infiltrate Patterna | P Valueb | |||
|---|---|---|---|---|---|
| Single Lobar | Multilobar, Unilateral | Multilobar, Bilateral | Interstitial | ||
| |||||
| No. | 247 (60.8) | 54 (13.3) | 64 (15.8) | 41 (10.1) | |
| Median age, y | 3 [16] | 3 [17] | 3 [15] | 1 [03] | 0.02 |
| Male sex | 124 (50.2) | 32 (59.3) | 41 (64.1) | 30 (73.2) | 0.02 |
| Race | |||||
| Non‐Hispanic white | 133 (53.8) | 36 (66.7) | 37 (57.8) | 17 (41.5) | 0.69 |
| Non‐Hispanic black | 40 (16.2) | 6 (11.1) | 9 (14.1) | 8 (19.5) | |
| Hispanic | 25 (10.1) | 4 (7.4) | 5 (7.8) | 7 (17.1) | |
| Other | 49 (19.9) | 8 (14.8) | 13 (20.4) | 9 (22) | |
| Insurance | |||||
| Public | 130 (52.6) | 26 (48.1) | 33 (51.6) | 25 (61) | 0.90 |
| Private | 116 (47) | 28 (51.9) | 31 (48.4) | 16 (39) | |
| Concurrent diagnosis | |||||
| Asthma | 80 (32.4) | 16 (29.6) | 17 (26.6) | 12 (29.3) | 0.82 |
| Bronchiolitis | 43 (17.4) | 6 (11.1) | 13 (20.3) | 14 (34.1) | 0.03 |
| Effusion | |||||
| None | 201 (81.4) | 31 (57.4) | 48 (75) | 40 (97.6) | <.01 |
| Small | 34 (13.8) | 20 (37) | 11 (17.2) | 0 | |
| Moderate/large | 12 (4.9) | 3 (5.6) | 5 (7.8) | 1 (2.4) | |
Outcomes According to Radiographic Infiltrate Pattern
Compared to children with single lobar infiltrates, the odds of ICU admission was significantly increased for those with either unilateral or bilateral multilobar infiltrates (unilateral, adjusted odds ratio [aOR]: 8.0, 95% confidence interval [CI]: 2.922.2; bilateral, aOR: 6.6, 95% CI: 2.14.5) (Figure 1, Table 2). Patients with bilateral multilobar infiltrates also had higher odds for supplemental oxygen use (aOR: 2.7, 95% CI: 1.25.8) and need for invasive mechanical ventilation (aOR: 3.0, 95% CI: 1.27.9). There were no differences in duration of oxygen supplementation or hospital length of stay for children with single versus multilobar infiltrates.

| Outcome | Infiltrate Patterna | P Valueb | |||
|---|---|---|---|---|---|
| Single Lobar, n=247 | Multilobar, Unilateral, n=54 | Multilobar, Bilateral, n=64 | Interstitial, n=41 | ||
| |||||
| Supplemental O2 requirement | 143 (57.9) | 34 (63) | 46 (71.9) | 31 (75.6) | 0.05 |
| ICU admission | 10 (4) | 9 (16.7) | 9 (14.1) | 4 (9.8) | <0.01 |
| Mechanical ventilation | 5 (2) | 4 (7.4) | 4 (6.3) | 1 (2.4) | 0.13 |
| Hospital length of stay, h | 47 [3779] | 63 [45114] | 56.5 [39.5101] | 62 [3993] | <0.01 |
| O2 duration, h | 27 [1059] | 38 [1777] | 38 [2381] | 34.5 [1765] | 0.18 |
Compared to those with single lobar infiltrates, children with interstitial infiltrates had higher odds of need for supplemental oxygen (aOR: 3.1, 95% CI: 1.37.6) and ICU admission (aOR: 4.4, 95% CI: 1.314.3) but not invasive mechanical ventilation. There were also no differences in duration of oxygen supplementation or hospital length of stay.
Outcomes According to Presence and Size of Pleural Effusion
Compared to those without pleural effusion, children with moderate to large effusion had a higher odds of ICU admission (aOR: 3.2, 95% CI: 1.18.9) and invasive mechanical ventilation (aOR: 14.8, 95% CI: 9.822.4), and also had a longer duration of oxygen supplementation (aOR: 3.0, 95% CI: 1.46.5) and hospital length of stay (aOR: 2.6, 95% CI: 1.9‐3.6) (Table 3, Figure 2). The presence of a small pleural effusion was not associated with increased need for supplemental oxygen, ICU admission, or mechanical ventilation compared to those without effusion. However, small effusion was associated with a longer duration of oxygen supplementation (aOR: 1.7, 95% CI: 12.7) and hospital length of stay (aOR: 1.6, 95% CI: 1.3‐1.9).
| Outcome | Pleural Effusion | P Valuea | ||
|---|---|---|---|---|
| None, n=320 | Small, n=65 | Moderate/Large, n=21 | ||
| ||||
| Supplemental O2 requirement | 200 (62.5) | 40 (61.5) | 14 (66.7) | 0.91 |
| ICU admission | 22 (6.9) | 6 (9.2) | 4 (19) | 0.12 |
| Mechanical ventilation | 5 (1.6) | 5 (7.7) | 4 (19) | <0.01 |
| Hospital length of stay, h | 48 [37.576] | 72 [45142] | 160 [82191] | <0.01 |
| Oxygen duration, h | 31 [1157] | 38.5 [1887] | 111 [27154] | <0.01 |

DISCUSSION
We evaluated the association between admission chest radiographic findings and subsequent clinical outcomes and hospital care processes for children hospitalized with CAP at 4 children's hospitals in the United States. We conclude that radiographic findings are associated with important inpatient outcomes. Similar to data from adults, findings of moderate to large pleural effusions and bilateral multilobar infiltrates had the strongest associations with severe disease. Such information, in combination with other prognostic factors, may help clinicians identify high‐risk patients and support management decisions, while also helping to inform families about the expected hospital course.
Previous pediatric studies examining the association between radiographic findings and outcomes have produced inconsistent results.[8, 9, 10, 11, 12] All but 1 of these studies documented 1 radiographic characteristics associated with pneumonia disease severity.[11] Further, although most contrasted lobar/alveolar and interstitial infiltrates, only Patria et al. distinguished among lobar infiltrate patterns (eg, single lobar vs multilobar).[12] Similar to our findings, that study demonstrated increased disease severity among children with bilateral multifocal lobar infiltrates. Of the studies that considered the presence of pleural effusion, only 1 demonstrated this finding to be associated with more severe disease.[9] However, none of these prior studies examined size of the pleural effusion.
In our study, the strongest association with severe pneumonia outcomes was among children with moderate to large pleural effusion. Significant pleural effusions are much more commonly due to infection with bacterial pathogens, particularly Streptococcus pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes, and may also indicate infection with more virulent and/or difficult to treat strains.[16, 17, 18, 19] Surgical intervention is also often required. As such, children with significant pleural effusions are often more ill on presentation and may have a prolonged period of recovery.[20, 21, 22]
Similarly, multilobar infiltrates, particularly bilateral, were associated with increased disease severity in terms of need for supplemental oxygen, ICU admission, and need for invasive mechanical ventilation. Although this finding may be expected, it is interesting to note that the duration of supplemental oxygen and hospital length of stay were similar to those with single lobar disease. One potential explanation is that, although children with multilobar disease are more severe at presentation, rates of recovery are similar to those with less extensive radiographic findings, owing to rapidly effective antimicrobials for uncomplicated bacterial pneumonia. This hypothesis also agrees with the 2011 PIDS/IDSA guidelines, which state that children receiving adequate therapy typically show signs of improvement within 48 to 72 hours regardless of initial severity.[1]
Interstitial infiltrate was also associated with increased severity at presentation but similar length of stay and duration of oxygen requirement compared with single lobar disease. We note that these children were substantially younger than those presenting with any pattern of lobar disease (median age, 1 vs 3 years), were more likely to have a concurrent diagnosis of bronchiolitis (34% vs 17%), and only 1 child with interstitial infiltrates had a documented pleural effusion (vs 23% of children with lobar infiltrates). Primary viral pneumonia is considered more likely to produce interstitial infiltrates on chest radiograph compared to bacterial disease, and although detailed etiologic data are unavailable for this study, our findings above strongly support this assertion.[23, 24]
The 2011 PIDS/IDSA guidelines recommend admission chest radiographs for all children hospitalized with pneumonia to assess extent of disease and identify complications that may requiring additional evaluation or surgical intervention.[1] Our findings highlight additional potential benefits of admission radiographs in terms of disease prognosis and management decisions. In the initial evaluation of a sick child with pneumonia, clinicians are often presented with a number of potential prognostic factors that may influence disease outcomes. However, it is sometimes difficult for providers to consider all available information and/or the relative importance of a single factor, resulting in inaccurate risk perceptions and management decisions that may contribute to poor outcomes.[25] Similar to adults, the development of clinical prediction rules, which incorporate a variety of important predictors including admission radiographic findings, likely would improve risk assessments and potentially outcomes for children with pneumonia. Such prognostic information is also helpful for clinicians who may use these data to inform and prepare families regarding the expected course of hospitalization.
Our study has several limitations. This study was retrospective and only included a sample of pneumonia hospitalizations during the study period, which may raise confounding concerns and potential for selection bias. However, detailed medical record reviews using standardized case definitions for radiographic CAP were used, and a large sample of children was randomly selected from each institution. In addition, a large number of potential confounders were selected a priori and included in multivariable analyses; propensity score adjustment was used to reduce model complexity and avoid overfitting. Radiographic findings were based on clinical interpretation by pediatric radiologists independent of a study protocol. Prior studies have demonstrated good agreement for identification of alveolar/lobar infiltrates and pleural effusion by trained radiologists, although agreement for interstitial infiltrate is poor.[26, 27] This limitation could result in either over‐ or underestimation of the prevalence of interstitial infiltrates likely resulting in a nondifferential bias toward the null. Microbiologic information, which may inform radiographic findings and disease severity, was also not available. However, because pneumonia etiology is frequently unknown in the clinical setting, our study reflects typical practice. We also did not include children from community or nonteaching hospitals. Thus, although findings may have relevance to community or nonteaching hospitals, our results cannot be generalized.
CONCLUSION
Our study demonstrates that among children hospitalized with CAP, admission chest radiographic findings are associated with important clinical outcomes and hospital care processes, highlighting additional benefits of the 2011 PIDS/IDSA guidelines' recommendation for admission chest radiographs for all children hospitalized with pneumonia. These data, in conjunction with other important prognostic information, may help clinicians more rapidly identify children at increased risk for severe illness, and could also offer guidance regarding disease management strategies and facilitate shared decision making with families. Thus, routine admission chest radiography in this population represents a valuable tool that contributes to improved quality of care.
Disclosures
Dr. Williams is supported by funds from the National Institutes of HealthNational Institute of Allergy and Infectious Diseases (K23AI104779). The authors report no conflicts of interest.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia. Clin Infect Dis. 2008;47(3):375–384.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. Risk stratification of early admission to the intensive care unit of patients with no major criteria of severe community‐acquired pneumonia: development of an international prediction rule. Crit Care. 2009;13(2):R54.
- , , , et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community‐acquired pneumonia? Arch Intern Med. 1996;156(19):2206–2212.
- , , , , , . Safety and efficacy of CURB65‐guided antibiotic therapy in community‐acquired pneumonia. J Antimicrob Chemother. 2011;66(2):416–423.
- , , . Severity of childhood community‐acquired pneumonia and chest radiographic findings. Pediatr Pulmonol. 2009;44(3):249–252.
- , , , et al. Can chest x‐ray predict pneumonia severity? Pediatr Pulmonol. 2004;38(6):465–469.
- , , , . Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92(5):394–398.
- , , . Role of chest X‐ray in predicting outcome of acute severe pneumonia. Indian Pediatr. 2008;45(11):893–898.
- , , , , , . Association between radiological findings and severity of community‐acquired pneumonia in children. Ital J Pediatr. 2013;39:56.
- , , , et al. Identifying pediatric community‐acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatrics. 2013;167(9):851–858.
- , , , , , . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99.
- , . Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–333.
- , , , . Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States. Clin Infect Dis. 2010;50(6):805–813.
- , , , et al. Epidemiology and clinical characteristics of community‐acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4):701–707.
- , , , et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289–294.
- , . Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980. Clin Pediatr (Phila). 1983;22(6):414–419.
- , , , et al. Risk factors of progressive community‐acquired pneumonia in hospitalized children: a prospective study [published online ahead of print August 28, 2013]. J Microbiol Immunol Infect. doi: 10.1016/j.jmii.2013.06.009.
- , , , , . Community‐acquired lobar pneumonia in children in the era of universal 7‐valent pneumococcal vaccination: a review of clinical presentations and antimicrobial treatment from a Canadian pediatric hospital. BMC Pediatr. 2012;12:133.
- , , , et al. Clinical characteristics and outcome of complicated pneumococcal pneumonia in a pediatric population. Pediatr Pulmonol. 2006;41(8):726–734.
- , , , , , . Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57(5):438–441.
- , , , et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1–ii23.
- , , , et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010–1016.
- , , , et al. Variability in the interpretation of chest radiographs for the diagnosis of pneumonia in children. J Hosp Med. 2012;7(4):294–298.
- , , , et al. Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT Investigators. Chest. 1996;110(2):343–350.
- , , , et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia. Clin Infect Dis. 2008;47(3):375–384.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. Risk stratification of early admission to the intensive care unit of patients with no major criteria of severe community‐acquired pneumonia: development of an international prediction rule. Crit Care. 2009;13(2):R54.
- , , , et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community‐acquired pneumonia? Arch Intern Med. 1996;156(19):2206–2212.
- , , , , , . Safety and efficacy of CURB65‐guided antibiotic therapy in community‐acquired pneumonia. J Antimicrob Chemother. 2011;66(2):416–423.
- , , . Severity of childhood community‐acquired pneumonia and chest radiographic findings. Pediatr Pulmonol. 2009;44(3):249–252.
- , , , et al. Can chest x‐ray predict pneumonia severity? Pediatr Pulmonol. 2004;38(6):465–469.
- , , , . Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92(5):394–398.
- , , . Role of chest X‐ray in predicting outcome of acute severe pneumonia. Indian Pediatr. 2008;45(11):893–898.
- , , , , , . Association between radiological findings and severity of community‐acquired pneumonia in children. Ital J Pediatr. 2013;39:56.
- , , , et al. Identifying pediatric community‐acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatrics. 2013;167(9):851–858.
- , , , , , . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99.
- , . Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–333.
- , , , . Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States. Clin Infect Dis. 2010;50(6):805–813.
- , , , et al. Epidemiology and clinical characteristics of community‐acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4):701–707.
- , , , et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289–294.
- , . Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980. Clin Pediatr (Phila). 1983;22(6):414–419.
- , , , et al. Risk factors of progressive community‐acquired pneumonia in hospitalized children: a prospective study [published online ahead of print August 28, 2013]. J Microbiol Immunol Infect. doi: 10.1016/j.jmii.2013.06.009.
- , , , , . Community‐acquired lobar pneumonia in children in the era of universal 7‐valent pneumococcal vaccination: a review of clinical presentations and antimicrobial treatment from a Canadian pediatric hospital. BMC Pediatr. 2012;12:133.
- , , , et al. Clinical characteristics and outcome of complicated pneumococcal pneumonia in a pediatric population. Pediatr Pulmonol. 2006;41(8):726–734.
- , , , , , . Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57(5):438–441.
- , , , et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(suppl 2):ii1–ii23.
- , , , et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010–1016.
- , , , et al. Variability in the interpretation of chest radiographs for the diagnosis of pneumonia in children. J Hosp Med. 2012;7(4):294–298.
- , , , et al. Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT Investigators. Chest. 1996;110(2):343–350.
© 2014 Society of Hospital Medicine
FDA approves vorapaxar, a new oral antiplatelet
The novel antiplatelet drug vorapaxar has been approved for reducing the risk of myocardial infarction, stroke, cardiovascular death, and need for coronary revascularization procedures in patients with a previous MI or peripheral arterial disease, the Food and Drug Administration announced on May 8.
Vorapaxar is an antagonist of protease-activated receptor-1 (PAR-1), which inhibits the action of thrombin on the platelet, and is the first drug in this class to be approved. Merck Sharp & Dohme will market the drug as Zontivity.
It comes in a tablet formulation.
At a meeting in January, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-1 to recommend approval of vorapaxar.
"In patients who have had a heart attack or who have peripheral arterial disease, this drug will lower the risk of heart attack, stroke, and cardiovascular death," Dr. Ellis F. Unger, director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research, reported in the FDA statement.
"In the study that supported the drug’s approval, Zontivity lowered this risk from 9.5% to 7.9% over a 3-year period – about 0.5% per year," he noted.
The FDA’s Cardiovascular and Renal Drugs Advisory Committee recommend approval of the novel antiplatelet drug for reducing atherothrombotic events in patients with a history of MI – the indication proposed by Merck. The recommended dose is one 2.5-mg tablet per day.
Because of the increased risk of bleeding, including life-threatening and fatal bleeding, the drug’s prescribing information includes a boxed warning about this risk and a medication guide informing patients about how to use the drug. A history of stroke, or transient ischemic attack, and a history of intracranial bleeding are contraindications.
The indication also includes the statement that treatment with vorapaxar has been shown to reduce the rate of the combined endpoint of cardiovascular death, MI, stroke, and urgent coronary revascularization.
The company conducted two phase III studies in two different groups of patients. In the TRACER (The Thrombin Receptor Antagonist for Clinical Event Reduction in ACS) study, which compared vorapaxar with placebo, added to standard therapy, as acute therapy (2.5 mg per day after a 40-mg loading dose, or placebo) in almost 13,000 hospitalized patients randomized within 24 hours of presenting with ACS (with non-ST elevation), it reduced the risk of atherothrombotic events (N. Engl. J. Med. 2012;366:20-33).
The study, however, was terminated early after an increased risk of major bleeding, including ICH, was detected and the company stopped the treatment in patients with a history of stroke or new stroke in the other phase III trial, the TRA 2P–TIMI 50 (Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Events) study. The company dropped plans to pursue the acute ACS indication.
The approved indication is based on the results of the TRA 2P–TIMI 50 study, which randomized 26,449 patients to placebo or 2.5 mg of vorapaxar a day, added to standard therapy (including other antiplatelet agents) in 26,449 outpatients with a previous MI, previous ischemic stroke, or peripheral arterial disease (N. Engl. J. Med. 2012;366:1404-13). (Almost 80% of the patients in the study who met the criteria in the proposed indication were on dual antiplatelet therapy with aspirin and a thienopyridine.)
In the overall cohort, including those who had to stop treatment early, compared with placebo, the risk of the primary composite endpoint of cardiovascular death, MI, stroke, or urgent coronary revascularization was reduced by 12%, and the risk of a secondary efficacy endpoint (CV death, MI, stroke) was reduced by 13% over 3 years among those treated with vorapaxar – effects that were statistically significant, Merck said.
The risk of severe or moderate bleeding was increased by 51% among those on vorapaxar over placebo.
The ICH rate over 3 years was 1% among those on vorapaxar, compared with 0.6% among those on placebo, but the absolute risk was higher in those with a history of stroke, according to Merck.
There is "clearly an unmet need" in this group of patients with a history of MI, "and a lack of therapies that have been shown to really be beneficial long term in this population," said the panel chair, Dr. Philip Sager, chair of the scientific programs committee, Cardiac Safety Research Consortium, San Francisco. "While there certainly is some bleeding risk, the benefits on the primary endpoint and secondary endpoint clearly outweigh those risks, and the benefit-risk relationship is positive," he added.
The panelist who voted against approval, Dr. Mori Krantz, director of the Colorado Prevention Center, Denver Health Medical Center, agreed that the primary efficacy endpoint had been met but was concerned about the size of the benefit and the large number of people needed to treat to benefit one patient.
"Although the intracranial hemorrhages and the major bleeds were potentially manageable, I worry about the amplification of that signal," because triple antiplatelet therapy is "unprecedented," he added.
Other safety concerns cited by panelists included a lack of an antidote and the lack of an effect in people weighing less than 60 kg (132 lb), an unresolved issue.
This is the first antiplatelet agent with a specific PAD indication. We now have multiple such agents to consider in our vascular patients. Time will tell which should be used in the management of the asymptomatic patient or those who have had either surgical or endovascular interventions. Currently, the other new agent, ticagrelor, is under investigation in the EUCLID study (Astra Zeneca). Hopefully there will ultimately be comparative studies of all antiplatelet agents. However, in my current practice I will prescribe clopidogrel for patients who do not have a contraindication and who can afford it. Otherwise aspirin will suffice in most patients. I reserve dual-antiplatelet agents for patients who demonstrate high risk or who have a history of graft or endovascular procedure thrombosis.
Dr. Russell Samson is the medical editor of Vascular Specialist.
This is the first antiplatelet agent with a specific PAD indication. We now have multiple such agents to consider in our vascular patients. Time will tell which should be used in the management of the asymptomatic patient or those who have had either surgical or endovascular interventions. Currently, the other new agent, ticagrelor, is under investigation in the EUCLID study (Astra Zeneca). Hopefully there will ultimately be comparative studies of all antiplatelet agents. However, in my current practice I will prescribe clopidogrel for patients who do not have a contraindication and who can afford it. Otherwise aspirin will suffice in most patients. I reserve dual-antiplatelet agents for patients who demonstrate high risk or who have a history of graft or endovascular procedure thrombosis.
Dr. Russell Samson is the medical editor of Vascular Specialist.
This is the first antiplatelet agent with a specific PAD indication. We now have multiple such agents to consider in our vascular patients. Time will tell which should be used in the management of the asymptomatic patient or those who have had either surgical or endovascular interventions. Currently, the other new agent, ticagrelor, is under investigation in the EUCLID study (Astra Zeneca). Hopefully there will ultimately be comparative studies of all antiplatelet agents. However, in my current practice I will prescribe clopidogrel for patients who do not have a contraindication and who can afford it. Otherwise aspirin will suffice in most patients. I reserve dual-antiplatelet agents for patients who demonstrate high risk or who have a history of graft or endovascular procedure thrombosis.
Dr. Russell Samson is the medical editor of Vascular Specialist.
The novel antiplatelet drug vorapaxar has been approved for reducing the risk of myocardial infarction, stroke, cardiovascular death, and need for coronary revascularization procedures in patients with a previous MI or peripheral arterial disease, the Food and Drug Administration announced on May 8.
Vorapaxar is an antagonist of protease-activated receptor-1 (PAR-1), which inhibits the action of thrombin on the platelet, and is the first drug in this class to be approved. Merck Sharp & Dohme will market the drug as Zontivity.
It comes in a tablet formulation.
At a meeting in January, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-1 to recommend approval of vorapaxar.
"In patients who have had a heart attack or who have peripheral arterial disease, this drug will lower the risk of heart attack, stroke, and cardiovascular death," Dr. Ellis F. Unger, director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research, reported in the FDA statement.
"In the study that supported the drug’s approval, Zontivity lowered this risk from 9.5% to 7.9% over a 3-year period – about 0.5% per year," he noted.
The FDA’s Cardiovascular and Renal Drugs Advisory Committee recommend approval of the novel antiplatelet drug for reducing atherothrombotic events in patients with a history of MI – the indication proposed by Merck. The recommended dose is one 2.5-mg tablet per day.
Because of the increased risk of bleeding, including life-threatening and fatal bleeding, the drug’s prescribing information includes a boxed warning about this risk and a medication guide informing patients about how to use the drug. A history of stroke, or transient ischemic attack, and a history of intracranial bleeding are contraindications.
The indication also includes the statement that treatment with vorapaxar has been shown to reduce the rate of the combined endpoint of cardiovascular death, MI, stroke, and urgent coronary revascularization.
The company conducted two phase III studies in two different groups of patients. In the TRACER (The Thrombin Receptor Antagonist for Clinical Event Reduction in ACS) study, which compared vorapaxar with placebo, added to standard therapy, as acute therapy (2.5 mg per day after a 40-mg loading dose, or placebo) in almost 13,000 hospitalized patients randomized within 24 hours of presenting with ACS (with non-ST elevation), it reduced the risk of atherothrombotic events (N. Engl. J. Med. 2012;366:20-33).
The study, however, was terminated early after an increased risk of major bleeding, including ICH, was detected and the company stopped the treatment in patients with a history of stroke or new stroke in the other phase III trial, the TRA 2P–TIMI 50 (Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Events) study. The company dropped plans to pursue the acute ACS indication.
The approved indication is based on the results of the TRA 2P–TIMI 50 study, which randomized 26,449 patients to placebo or 2.5 mg of vorapaxar a day, added to standard therapy (including other antiplatelet agents) in 26,449 outpatients with a previous MI, previous ischemic stroke, or peripheral arterial disease (N. Engl. J. Med. 2012;366:1404-13). (Almost 80% of the patients in the study who met the criteria in the proposed indication were on dual antiplatelet therapy with aspirin and a thienopyridine.)
In the overall cohort, including those who had to stop treatment early, compared with placebo, the risk of the primary composite endpoint of cardiovascular death, MI, stroke, or urgent coronary revascularization was reduced by 12%, and the risk of a secondary efficacy endpoint (CV death, MI, stroke) was reduced by 13% over 3 years among those treated with vorapaxar – effects that were statistically significant, Merck said.
The risk of severe or moderate bleeding was increased by 51% among those on vorapaxar over placebo.
The ICH rate over 3 years was 1% among those on vorapaxar, compared with 0.6% among those on placebo, but the absolute risk was higher in those with a history of stroke, according to Merck.
There is "clearly an unmet need" in this group of patients with a history of MI, "and a lack of therapies that have been shown to really be beneficial long term in this population," said the panel chair, Dr. Philip Sager, chair of the scientific programs committee, Cardiac Safety Research Consortium, San Francisco. "While there certainly is some bleeding risk, the benefits on the primary endpoint and secondary endpoint clearly outweigh those risks, and the benefit-risk relationship is positive," he added.
The panelist who voted against approval, Dr. Mori Krantz, director of the Colorado Prevention Center, Denver Health Medical Center, agreed that the primary efficacy endpoint had been met but was concerned about the size of the benefit and the large number of people needed to treat to benefit one patient.
"Although the intracranial hemorrhages and the major bleeds were potentially manageable, I worry about the amplification of that signal," because triple antiplatelet therapy is "unprecedented," he added.
Other safety concerns cited by panelists included a lack of an antidote and the lack of an effect in people weighing less than 60 kg (132 lb), an unresolved issue.
The novel antiplatelet drug vorapaxar has been approved for reducing the risk of myocardial infarction, stroke, cardiovascular death, and need for coronary revascularization procedures in patients with a previous MI or peripheral arterial disease, the Food and Drug Administration announced on May 8.
Vorapaxar is an antagonist of protease-activated receptor-1 (PAR-1), which inhibits the action of thrombin on the platelet, and is the first drug in this class to be approved. Merck Sharp & Dohme will market the drug as Zontivity.
It comes in a tablet formulation.
At a meeting in January, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-1 to recommend approval of vorapaxar.
"In patients who have had a heart attack or who have peripheral arterial disease, this drug will lower the risk of heart attack, stroke, and cardiovascular death," Dr. Ellis F. Unger, director of the Office of Drug Evaluation I in the FDA’s Center for Drug Evaluation and Research, reported in the FDA statement.
"In the study that supported the drug’s approval, Zontivity lowered this risk from 9.5% to 7.9% over a 3-year period – about 0.5% per year," he noted.
The FDA’s Cardiovascular and Renal Drugs Advisory Committee recommend approval of the novel antiplatelet drug for reducing atherothrombotic events in patients with a history of MI – the indication proposed by Merck. The recommended dose is one 2.5-mg tablet per day.
Because of the increased risk of bleeding, including life-threatening and fatal bleeding, the drug’s prescribing information includes a boxed warning about this risk and a medication guide informing patients about how to use the drug. A history of stroke, or transient ischemic attack, and a history of intracranial bleeding are contraindications.
The indication also includes the statement that treatment with vorapaxar has been shown to reduce the rate of the combined endpoint of cardiovascular death, MI, stroke, and urgent coronary revascularization.
The company conducted two phase III studies in two different groups of patients. In the TRACER (The Thrombin Receptor Antagonist for Clinical Event Reduction in ACS) study, which compared vorapaxar with placebo, added to standard therapy, as acute therapy (2.5 mg per day after a 40-mg loading dose, or placebo) in almost 13,000 hospitalized patients randomized within 24 hours of presenting with ACS (with non-ST elevation), it reduced the risk of atherothrombotic events (N. Engl. J. Med. 2012;366:20-33).
The study, however, was terminated early after an increased risk of major bleeding, including ICH, was detected and the company stopped the treatment in patients with a history of stroke or new stroke in the other phase III trial, the TRA 2P–TIMI 50 (Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Events) study. The company dropped plans to pursue the acute ACS indication.
The approved indication is based on the results of the TRA 2P–TIMI 50 study, which randomized 26,449 patients to placebo or 2.5 mg of vorapaxar a day, added to standard therapy (including other antiplatelet agents) in 26,449 outpatients with a previous MI, previous ischemic stroke, or peripheral arterial disease (N. Engl. J. Med. 2012;366:1404-13). (Almost 80% of the patients in the study who met the criteria in the proposed indication were on dual antiplatelet therapy with aspirin and a thienopyridine.)
In the overall cohort, including those who had to stop treatment early, compared with placebo, the risk of the primary composite endpoint of cardiovascular death, MI, stroke, or urgent coronary revascularization was reduced by 12%, and the risk of a secondary efficacy endpoint (CV death, MI, stroke) was reduced by 13% over 3 years among those treated with vorapaxar – effects that were statistically significant, Merck said.
The risk of severe or moderate bleeding was increased by 51% among those on vorapaxar over placebo.
The ICH rate over 3 years was 1% among those on vorapaxar, compared with 0.6% among those on placebo, but the absolute risk was higher in those with a history of stroke, according to Merck.
There is "clearly an unmet need" in this group of patients with a history of MI, "and a lack of therapies that have been shown to really be beneficial long term in this population," said the panel chair, Dr. Philip Sager, chair of the scientific programs committee, Cardiac Safety Research Consortium, San Francisco. "While there certainly is some bleeding risk, the benefits on the primary endpoint and secondary endpoint clearly outweigh those risks, and the benefit-risk relationship is positive," he added.
The panelist who voted against approval, Dr. Mori Krantz, director of the Colorado Prevention Center, Denver Health Medical Center, agreed that the primary efficacy endpoint had been met but was concerned about the size of the benefit and the large number of people needed to treat to benefit one patient.
"Although the intracranial hemorrhages and the major bleeds were potentially manageable, I worry about the amplification of that signal," because triple antiplatelet therapy is "unprecedented," he added.
Other safety concerns cited by panelists included a lack of an antidote and the lack of an effect in people weighing less than 60 kg (132 lb), an unresolved issue.
Thrombolysis may offer benefit in stable pulmonary embolism
Thrombolytic therapy decreased all-cause mortality in patients with hemodynamically stable pulmonary embolism associated with right ventricular dysfunction – those at "intermediate risk," according to a meta-analysis published online June 17 in JAMA.
The investigators described their study of 16 randomized, controlled clinical trials involving 2,115 patients as "the first analysis of thrombolysis in PE that has sufficient statistical power to detect associations with a meaningful mortality reduction." If their findings are confirmed in future randomized clinical trials, "there may be a shift in the treatment of selected patients with intermediate-risk PE using thrombolytics."
However, "the optimism regarding this clinical advantage must be tempered by [our] finding of significantly increased risk of major bleeding and intracranial hemorrhage associated with thrombolytic therapy, particularly for patients older than 65 years," said Dr. Saurav Chatterjee of the division of cardiology, St. Luke’s-Roosevelt Hospital Center of the Mount Sinai Health System, New York, and his associates (JAMA 2014;311:2414-21).
The study population included 1,499 patients who had hemodynamically stable PE associated with right ventricular dysfunction, the largest subset of patients seen in clinical practice and the group for whom the risks and benefits of thrombolysis are the most unclear.
After a mean follow-up of 82 days, overall mortality was 2.17% in patients who received thrombolysis, compared with 3.89% in those who received anticoagulation. In addition, the risk of recurrent PE was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
However, the rate of major bleeding was 9.24% for thrombolytic therapy, compared with 3.42% for anticoagulation. And the rate of intracranial hemorrhage was 1.46% for thrombolysis, compared with 0.19% for anticoagulation, the investigators said.
The bleeding risk was especially high in patients aged 65 years and older. Attenuation of this risk in younger patients suggests that they may be considered stronger candidates for thrombolytic therapy, Dr. Chatterjee and his associates said.
Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.

|
|
Dr. Chatterjee and his associates calculated the net clinical benefit of thrombolysis, and their result "suggests evidence of modest efficacy in intermediate-risk PE," said Dr. Joshua A. Beckman.
But their findings do not yet add up to a change in the standard of care. Each clinician must decide on an individualized basis which of these patients should receive thrombolytic therapy, based on clinical presentation, comorbid conditions, and both the physician’s and the patient’s tolerance of risk.
Dr. Beckman is in the cardiovascular division at Brigham and Women’s Hospital, Boston. He reported being a board member for Vascular Interventional Advances; receiving grant funding from Bristol-Myers Squibb; and consulting for AstraZeneca, Boston Scientific, Ferring, Merck, and Novartis. These remarks were taken from his editorial accompanying Dr. Chatterjee’s report (JAMA 2014;311:2385-6).

|
|
Dr. Chatterjee and his associates calculated the net clinical benefit of thrombolysis, and their result "suggests evidence of modest efficacy in intermediate-risk PE," said Dr. Joshua A. Beckman.
But their findings do not yet add up to a change in the standard of care. Each clinician must decide on an individualized basis which of these patients should receive thrombolytic therapy, based on clinical presentation, comorbid conditions, and both the physician’s and the patient’s tolerance of risk.
Dr. Beckman is in the cardiovascular division at Brigham and Women’s Hospital, Boston. He reported being a board member for Vascular Interventional Advances; receiving grant funding from Bristol-Myers Squibb; and consulting for AstraZeneca, Boston Scientific, Ferring, Merck, and Novartis. These remarks were taken from his editorial accompanying Dr. Chatterjee’s report (JAMA 2014;311:2385-6).

|
|
Dr. Chatterjee and his associates calculated the net clinical benefit of thrombolysis, and their result "suggests evidence of modest efficacy in intermediate-risk PE," said Dr. Joshua A. Beckman.
But their findings do not yet add up to a change in the standard of care. Each clinician must decide on an individualized basis which of these patients should receive thrombolytic therapy, based on clinical presentation, comorbid conditions, and both the physician’s and the patient’s tolerance of risk.
Dr. Beckman is in the cardiovascular division at Brigham and Women’s Hospital, Boston. He reported being a board member for Vascular Interventional Advances; receiving grant funding from Bristol-Myers Squibb; and consulting for AstraZeneca, Boston Scientific, Ferring, Merck, and Novartis. These remarks were taken from his editorial accompanying Dr. Chatterjee’s report (JAMA 2014;311:2385-6).
Thrombolytic therapy decreased all-cause mortality in patients with hemodynamically stable pulmonary embolism associated with right ventricular dysfunction – those at "intermediate risk," according to a meta-analysis published online June 17 in JAMA.
The investigators described their study of 16 randomized, controlled clinical trials involving 2,115 patients as "the first analysis of thrombolysis in PE that has sufficient statistical power to detect associations with a meaningful mortality reduction." If their findings are confirmed in future randomized clinical trials, "there may be a shift in the treatment of selected patients with intermediate-risk PE using thrombolytics."
However, "the optimism regarding this clinical advantage must be tempered by [our] finding of significantly increased risk of major bleeding and intracranial hemorrhage associated with thrombolytic therapy, particularly for patients older than 65 years," said Dr. Saurav Chatterjee of the division of cardiology, St. Luke’s-Roosevelt Hospital Center of the Mount Sinai Health System, New York, and his associates (JAMA 2014;311:2414-21).
The study population included 1,499 patients who had hemodynamically stable PE associated with right ventricular dysfunction, the largest subset of patients seen in clinical practice and the group for whom the risks and benefits of thrombolysis are the most unclear.
After a mean follow-up of 82 days, overall mortality was 2.17% in patients who received thrombolysis, compared with 3.89% in those who received anticoagulation. In addition, the risk of recurrent PE was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
However, the rate of major bleeding was 9.24% for thrombolytic therapy, compared with 3.42% for anticoagulation. And the rate of intracranial hemorrhage was 1.46% for thrombolysis, compared with 0.19% for anticoagulation, the investigators said.
The bleeding risk was especially high in patients aged 65 years and older. Attenuation of this risk in younger patients suggests that they may be considered stronger candidates for thrombolytic therapy, Dr. Chatterjee and his associates said.
Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.
Thrombolytic therapy decreased all-cause mortality in patients with hemodynamically stable pulmonary embolism associated with right ventricular dysfunction – those at "intermediate risk," according to a meta-analysis published online June 17 in JAMA.
The investigators described their study of 16 randomized, controlled clinical trials involving 2,115 patients as "the first analysis of thrombolysis in PE that has sufficient statistical power to detect associations with a meaningful mortality reduction." If their findings are confirmed in future randomized clinical trials, "there may be a shift in the treatment of selected patients with intermediate-risk PE using thrombolytics."
However, "the optimism regarding this clinical advantage must be tempered by [our] finding of significantly increased risk of major bleeding and intracranial hemorrhage associated with thrombolytic therapy, particularly for patients older than 65 years," said Dr. Saurav Chatterjee of the division of cardiology, St. Luke’s-Roosevelt Hospital Center of the Mount Sinai Health System, New York, and his associates (JAMA 2014;311:2414-21).
The study population included 1,499 patients who had hemodynamically stable PE associated with right ventricular dysfunction, the largest subset of patients seen in clinical practice and the group for whom the risks and benefits of thrombolysis are the most unclear.
After a mean follow-up of 82 days, overall mortality was 2.17% in patients who received thrombolysis, compared with 3.89% in those who received anticoagulation. In addition, the risk of recurrent PE was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
However, the rate of major bleeding was 9.24% for thrombolytic therapy, compared with 3.42% for anticoagulation. And the rate of intracranial hemorrhage was 1.46% for thrombolysis, compared with 0.19% for anticoagulation, the investigators said.
The bleeding risk was especially high in patients aged 65 years and older. Attenuation of this risk in younger patients suggests that they may be considered stronger candidates for thrombolytic therapy, Dr. Chatterjee and his associates said.
Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.
FROM JAMA
Key clinical point: Thrombolysis may be a therapeutic alternative to anticoagulation in some patients with stable, intermediate-risk pulmonary embolism.
Major finding: Mortality was 2.17% in PE patients who received thrombolysis, compared with 3.89% in those who received anticoagulation; the risk of recurrent PE also was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
Data source: A meta-analysis of 16 randomized, controlled trials involving 2,115 patients with PE, including 1,499 with intermediate-risk PE, who were followed for a mean of 82 days.
Disclosures: Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.
Residents, postop complications linked
BOSTON – Resident participation in emergency general surgery cases was independently associated with a host of complications – pulmonary embolism, surgical site infections, and unplanned reoperation in a secondary analysis of the American College of Surgeons prospective National Surgical Quality Improvement database.
Adequate exposure of residents to emergency general surgery is crucial for surgical training, but academic operating teams should be mindful of this association, Dr. George Kasotakis said at the annual meeting of the American Surgical Association.
Three papers have shown resident participation modestly increases complications in elective surgery, but their impact is not well understood for emergency general surgery, where patient physiology is typically deranged and timely surgery is imperative.
Dr. Kasotakis and his colleagues at Boston University identified 141,010 patients who underwent emergency general surgery procedures in the 2005-2010 American College of Surgeons prospective National Surgical Quality Improvement database. Because of the nonrandom assignment of more complex cases to resident participation, patients were matched 1:1 based on age; gender; use of alcohol, tobacco, and steroids; inpatient status; obesity; diabetes; renal failure; cardiopulmonary disease; and expected probability for morbidity and mortality. Regression models were fitted for each outcome and adjusted for the same risk factors and operative time.
The most common procedures were appendectomy (40%), exploratory laparotomy (8.75%), bowel resection (9.2%), cholecystectomy (6%), and lysis of adhesions (6%).
Thirty-day mortality was similar with and without residents (3.25% vs. 2.96%; P = .082), but hospital length of stay was longer by about a half a day with residents (4.97 days vs. 4.59 days; P = .019), said Dr. Kasotakis, an acute care surgeon and intensivist.
Resident participation added about 20 minutes to operative (75 minutes vs. 59 minutes; P less than .001) and anesthesia (122 minutes vs. 100 minutes; P less than .001) times.
Intraoperative transfusions were more common with residents (3.43% vs. 2.55%; P less than .001), perhaps because of longer operating room times, and, as a result, fewer postoperative transfusions were needed (1.12% vs. 1.28% P = .031), he said. Unplanned reoperations, however, were more common with residents, as well (4.22% vs. 3.80%; P = .002).
Postsurgical superficial wound infections (3.5% vs. 2.78%; P less than .001) and organ space surgical site infections (2.27% vs. 1.77%; P less than .001) were more common in the resident group, while wound dehiscence was not (0.63% vs. 0.69%; P = .266), Dr. Kasotakis noted.
Pulmonary complications were significantly more common in the resident group including postoperative pneumonia (1.85% vs. 1.67%; P = .04), reintubation (1.64% vs. 1.15%; P less than .001), and mechanical ventilation for more than 48 hours (2.87% vs. 2.06%; P less than .001).
The same was true for deep vein thrombosis (DVT) (0.80% vs. 0.62%; P = .002) and pulmonary embolism (PE) (0.43% vs. 0.28%; P less than .001).
Urinary tract infections (UTI) were higher with resident participation (1.45% vs. 1.14%; P less than .001), as was sepsis (2.42% vs. 2.13%; P = .005), likely because of the increase in surgical infections, Dr. Kasotakis said.
Thankfully, significant cardiac complications and septic shock were not more common with residents, he said.
Adjusted analyses
After adjustment for operative duration, case complexity and pre-existing comorbidities, residents did not increase length of stay (odds ratio, 0.07; P = .242) or septic events (OR, 1.07; P = .155), but their participation was still independently associated with about 20% more superficial surgical site infections (odds ratio, 1.23; P less than .001), organ space infections (OR, 1.21; P less than .001), UTIs (OR, 1.23; P = .001), and intraoperative transfusions (OR, 1.20; P = .001), he said.
Also, about 8% more patients required a return trip to the operating room when residents participated (OR, 1.08; P = .041).
"These outcomes can perhaps be attributed to their underdeveloped surgical skills," Dr. Kasotakis said.
The incidence of DVT and PE were also higher by about 25% (OR, 1.25; P = .011) and 40% (OR, 1.42; P = .005), respectively, perhaps because of delayed DVT prophylaxis initiation because of concerns of hemostasis or missed doses due to additional return trips to the emergency department, he suggested.
Interestingly, reintubation and prolonged mechanical ventilation rates were increased by about 40% (OR, 1.38; OR, 1.43; both P less than .001), perhaps because of prolonged operative times or greater resuscitation requirements, he added.
Dr. Kasotakis was quick to point out that this was a secondary analysis of a data set not originally intended to assess the effect of trainee participation, that no information was available on the degree of resident involvement during surgery or in perioperative care, and that participating institutions were skewed toward tertiary centers, which typically receive more complex cases.
"Staff surgeons should supervise as needed and minimize unnecessary [emergency department] time. And residents, for their part, should be well prepared for emergency procedures through simulation training and aim to maximize their operating room efficiency," he suggested.
The results sparked a flurry of rebuttals led off by discussant Dr. Julie Ann Sosa, Duke University, Durham, N.C.,who said they conflict with other analyses showing little to no impact from residents in elective cases.
"If not interpreted with care, policy makers, payers, and the public could construe that surgical care at academic health centers is compromised by trainees, which could have unfortunate ramifications for everyone in the room as well as the trainees and the patients," she said.
Dr. Sosa expressed concern about drawing causal inferences from an observational study in the setting of possible selection bias and said attempts to match for case complexity using CPT codes do not necessarily account for say, "the difference between a routine appendectomy that takes 15-30 minutes and a complex one that takes 3 hours for a perforation."
Some attendees questioned why the authors didn’t match the institutions in the analysis and chose to ascribe all of the outcomes to residents, with a round of applause following the suggestion that the paper should be titled "Academic centers increase emergency surgery complications." Other attendees questioned whether the poor outcomes reflect resident training and supervision.
Dr. George Velmahos, Massachusetts General Hospital, Boston, questioned whether hospitals have a medical/legal responsibility to inform patients that a resident is in the operating room and may impact outcomes.
Dr. Kasotakis said that institutions may want to add a clause to consent paperwork stating that residents and trainees will be participating.
The complete manuscript of this study and its presentation at the American Surgical Association’s 134th Annual Meeting is anticipated to be published in the Annals of Surgery, pending editorial review.
Dr. Kasotakis reported no conflicts.

|
| Dr. Sapan S. Desai |
While it is tempting to value the very high sample sizes reported in studies that utilize administrative database sources such as NSQIP, it is with great caution that we should interpret the results of these studies. Databases are a valuable source of determining incidence, costs, and even some correlations among various factors. Correlations, however, do not imply causation. For instance, one could say that there is a high correlation between the number of taxis on the street after 3 a.m. and the number of crimes, but that does not mean that taxi drivers transform into criminals during the hour of the wolf.
Databases are a blunt tool for identifying trends in data over large samples and over many years. However, as noted in the study presented by Dr. Kasotakis, the NSQIP does not track the extent of resident participation in operative cases. In fact, the exact variable that is tracked is entitled "ATTEND" in position 16 of the NSQIP and is as follows: 1) Attending & Resident in OR, 2) Attending Alone, and 3) Attending Not Present, but Available. It does not characterize the amount of the case completed by the resident, nor does it state the level of training of the resident. It does not mention whether medical students, visitors, nurse trainees, PAs, new scrub techs, or other personnel are present. In fact, there is no way to know who did what during the case, which is a requirement in order to be able to reliably and accurately determine that the resident is the culprit for longer case times and more intraoperative transfusions. This specific variable has insufficient granularity: the conclusion that "[adding] a clause to consent paperwork stating that residents and trainees will be participating in the case" due to the "underdeveloped surgical skills" of trainees, among other factors, is invalid.
As Dr. Sosa indicated, it is dangerous to imply that these correlations somehow imply causation, as this may lead to adverse impacts on training and negatively impact patient care far more than a possible 20 minute increase in the duration of an operation. The sole utility of this paper is that it is hypothesis generating; only a properly designed prospective study can truly study the positive and negative impacts on patient care due to resident education in the operating room. The question is, do we really want to know the answer to this question and will it truly affect what we do now?
Sapan S. Desai, M.D., is the resident medical editor for Vascular Specialist.

|
| Dr. Sapan S. Desai |
While it is tempting to value the very high sample sizes reported in studies that utilize administrative database sources such as NSQIP, it is with great caution that we should interpret the results of these studies. Databases are a valuable source of determining incidence, costs, and even some correlations among various factors. Correlations, however, do not imply causation. For instance, one could say that there is a high correlation between the number of taxis on the street after 3 a.m. and the number of crimes, but that does not mean that taxi drivers transform into criminals during the hour of the wolf.
Databases are a blunt tool for identifying trends in data over large samples and over many years. However, as noted in the study presented by Dr. Kasotakis, the NSQIP does not track the extent of resident participation in operative cases. In fact, the exact variable that is tracked is entitled "ATTEND" in position 16 of the NSQIP and is as follows: 1) Attending & Resident in OR, 2) Attending Alone, and 3) Attending Not Present, but Available. It does not characterize the amount of the case completed by the resident, nor does it state the level of training of the resident. It does not mention whether medical students, visitors, nurse trainees, PAs, new scrub techs, or other personnel are present. In fact, there is no way to know who did what during the case, which is a requirement in order to be able to reliably and accurately determine that the resident is the culprit for longer case times and more intraoperative transfusions. This specific variable has insufficient granularity: the conclusion that "[adding] a clause to consent paperwork stating that residents and trainees will be participating in the case" due to the "underdeveloped surgical skills" of trainees, among other factors, is invalid.
As Dr. Sosa indicated, it is dangerous to imply that these correlations somehow imply causation, as this may lead to adverse impacts on training and negatively impact patient care far more than a possible 20 minute increase in the duration of an operation. The sole utility of this paper is that it is hypothesis generating; only a properly designed prospective study can truly study the positive and negative impacts on patient care due to resident education in the operating room. The question is, do we really want to know the answer to this question and will it truly affect what we do now?
Sapan S. Desai, M.D., is the resident medical editor for Vascular Specialist.

|
| Dr. Sapan S. Desai |
While it is tempting to value the very high sample sizes reported in studies that utilize administrative database sources such as NSQIP, it is with great caution that we should interpret the results of these studies. Databases are a valuable source of determining incidence, costs, and even some correlations among various factors. Correlations, however, do not imply causation. For instance, one could say that there is a high correlation between the number of taxis on the street after 3 a.m. and the number of crimes, but that does not mean that taxi drivers transform into criminals during the hour of the wolf.
Databases are a blunt tool for identifying trends in data over large samples and over many years. However, as noted in the study presented by Dr. Kasotakis, the NSQIP does not track the extent of resident participation in operative cases. In fact, the exact variable that is tracked is entitled "ATTEND" in position 16 of the NSQIP and is as follows: 1) Attending & Resident in OR, 2) Attending Alone, and 3) Attending Not Present, but Available. It does not characterize the amount of the case completed by the resident, nor does it state the level of training of the resident. It does not mention whether medical students, visitors, nurse trainees, PAs, new scrub techs, or other personnel are present. In fact, there is no way to know who did what during the case, which is a requirement in order to be able to reliably and accurately determine that the resident is the culprit for longer case times and more intraoperative transfusions. This specific variable has insufficient granularity: the conclusion that "[adding] a clause to consent paperwork stating that residents and trainees will be participating in the case" due to the "underdeveloped surgical skills" of trainees, among other factors, is invalid.
As Dr. Sosa indicated, it is dangerous to imply that these correlations somehow imply causation, as this may lead to adverse impacts on training and negatively impact patient care far more than a possible 20 minute increase in the duration of an operation. The sole utility of this paper is that it is hypothesis generating; only a properly designed prospective study can truly study the positive and negative impacts on patient care due to resident education in the operating room. The question is, do we really want to know the answer to this question and will it truly affect what we do now?
Sapan S. Desai, M.D., is the resident medical editor for Vascular Specialist.
BOSTON – Resident participation in emergency general surgery cases was independently associated with a host of complications – pulmonary embolism, surgical site infections, and unplanned reoperation in a secondary analysis of the American College of Surgeons prospective National Surgical Quality Improvement database.
Adequate exposure of residents to emergency general surgery is crucial for surgical training, but academic operating teams should be mindful of this association, Dr. George Kasotakis said at the annual meeting of the American Surgical Association.
Three papers have shown resident participation modestly increases complications in elective surgery, but their impact is not well understood for emergency general surgery, where patient physiology is typically deranged and timely surgery is imperative.
Dr. Kasotakis and his colleagues at Boston University identified 141,010 patients who underwent emergency general surgery procedures in the 2005-2010 American College of Surgeons prospective National Surgical Quality Improvement database. Because of the nonrandom assignment of more complex cases to resident participation, patients were matched 1:1 based on age; gender; use of alcohol, tobacco, and steroids; inpatient status; obesity; diabetes; renal failure; cardiopulmonary disease; and expected probability for morbidity and mortality. Regression models were fitted for each outcome and adjusted for the same risk factors and operative time.
The most common procedures were appendectomy (40%), exploratory laparotomy (8.75%), bowel resection (9.2%), cholecystectomy (6%), and lysis of adhesions (6%).
Thirty-day mortality was similar with and without residents (3.25% vs. 2.96%; P = .082), but hospital length of stay was longer by about a half a day with residents (4.97 days vs. 4.59 days; P = .019), said Dr. Kasotakis, an acute care surgeon and intensivist.
Resident participation added about 20 minutes to operative (75 minutes vs. 59 minutes; P less than .001) and anesthesia (122 minutes vs. 100 minutes; P less than .001) times.
Intraoperative transfusions were more common with residents (3.43% vs. 2.55%; P less than .001), perhaps because of longer operating room times, and, as a result, fewer postoperative transfusions were needed (1.12% vs. 1.28% P = .031), he said. Unplanned reoperations, however, were more common with residents, as well (4.22% vs. 3.80%; P = .002).
Postsurgical superficial wound infections (3.5% vs. 2.78%; P less than .001) and organ space surgical site infections (2.27% vs. 1.77%; P less than .001) were more common in the resident group, while wound dehiscence was not (0.63% vs. 0.69%; P = .266), Dr. Kasotakis noted.
Pulmonary complications were significantly more common in the resident group including postoperative pneumonia (1.85% vs. 1.67%; P = .04), reintubation (1.64% vs. 1.15%; P less than .001), and mechanical ventilation for more than 48 hours (2.87% vs. 2.06%; P less than .001).
The same was true for deep vein thrombosis (DVT) (0.80% vs. 0.62%; P = .002) and pulmonary embolism (PE) (0.43% vs. 0.28%; P less than .001).
Urinary tract infections (UTI) were higher with resident participation (1.45% vs. 1.14%; P less than .001), as was sepsis (2.42% vs. 2.13%; P = .005), likely because of the increase in surgical infections, Dr. Kasotakis said.
Thankfully, significant cardiac complications and septic shock were not more common with residents, he said.
Adjusted analyses
After adjustment for operative duration, case complexity and pre-existing comorbidities, residents did not increase length of stay (odds ratio, 0.07; P = .242) or septic events (OR, 1.07; P = .155), but their participation was still independently associated with about 20% more superficial surgical site infections (odds ratio, 1.23; P less than .001), organ space infections (OR, 1.21; P less than .001), UTIs (OR, 1.23; P = .001), and intraoperative transfusions (OR, 1.20; P = .001), he said.
Also, about 8% more patients required a return trip to the operating room when residents participated (OR, 1.08; P = .041).
"These outcomes can perhaps be attributed to their underdeveloped surgical skills," Dr. Kasotakis said.
The incidence of DVT and PE were also higher by about 25% (OR, 1.25; P = .011) and 40% (OR, 1.42; P = .005), respectively, perhaps because of delayed DVT prophylaxis initiation because of concerns of hemostasis or missed doses due to additional return trips to the emergency department, he suggested.
Interestingly, reintubation and prolonged mechanical ventilation rates were increased by about 40% (OR, 1.38; OR, 1.43; both P less than .001), perhaps because of prolonged operative times or greater resuscitation requirements, he added.
Dr. Kasotakis was quick to point out that this was a secondary analysis of a data set not originally intended to assess the effect of trainee participation, that no information was available on the degree of resident involvement during surgery or in perioperative care, and that participating institutions were skewed toward tertiary centers, which typically receive more complex cases.
"Staff surgeons should supervise as needed and minimize unnecessary [emergency department] time. And residents, for their part, should be well prepared for emergency procedures through simulation training and aim to maximize their operating room efficiency," he suggested.
The results sparked a flurry of rebuttals led off by discussant Dr. Julie Ann Sosa, Duke University, Durham, N.C.,who said they conflict with other analyses showing little to no impact from residents in elective cases.
"If not interpreted with care, policy makers, payers, and the public could construe that surgical care at academic health centers is compromised by trainees, which could have unfortunate ramifications for everyone in the room as well as the trainees and the patients," she said.
Dr. Sosa expressed concern about drawing causal inferences from an observational study in the setting of possible selection bias and said attempts to match for case complexity using CPT codes do not necessarily account for say, "the difference between a routine appendectomy that takes 15-30 minutes and a complex one that takes 3 hours for a perforation."
Some attendees questioned why the authors didn’t match the institutions in the analysis and chose to ascribe all of the outcomes to residents, with a round of applause following the suggestion that the paper should be titled "Academic centers increase emergency surgery complications." Other attendees questioned whether the poor outcomes reflect resident training and supervision.
Dr. George Velmahos, Massachusetts General Hospital, Boston, questioned whether hospitals have a medical/legal responsibility to inform patients that a resident is in the operating room and may impact outcomes.
Dr. Kasotakis said that institutions may want to add a clause to consent paperwork stating that residents and trainees will be participating.
The complete manuscript of this study and its presentation at the American Surgical Association’s 134th Annual Meeting is anticipated to be published in the Annals of Surgery, pending editorial review.
Dr. Kasotakis reported no conflicts.
BOSTON – Resident participation in emergency general surgery cases was independently associated with a host of complications – pulmonary embolism, surgical site infections, and unplanned reoperation in a secondary analysis of the American College of Surgeons prospective National Surgical Quality Improvement database.
Adequate exposure of residents to emergency general surgery is crucial for surgical training, but academic operating teams should be mindful of this association, Dr. George Kasotakis said at the annual meeting of the American Surgical Association.
Three papers have shown resident participation modestly increases complications in elective surgery, but their impact is not well understood for emergency general surgery, where patient physiology is typically deranged and timely surgery is imperative.
Dr. Kasotakis and his colleagues at Boston University identified 141,010 patients who underwent emergency general surgery procedures in the 2005-2010 American College of Surgeons prospective National Surgical Quality Improvement database. Because of the nonrandom assignment of more complex cases to resident participation, patients were matched 1:1 based on age; gender; use of alcohol, tobacco, and steroids; inpatient status; obesity; diabetes; renal failure; cardiopulmonary disease; and expected probability for morbidity and mortality. Regression models were fitted for each outcome and adjusted for the same risk factors and operative time.
The most common procedures were appendectomy (40%), exploratory laparotomy (8.75%), bowel resection (9.2%), cholecystectomy (6%), and lysis of adhesions (6%).
Thirty-day mortality was similar with and without residents (3.25% vs. 2.96%; P = .082), but hospital length of stay was longer by about a half a day with residents (4.97 days vs. 4.59 days; P = .019), said Dr. Kasotakis, an acute care surgeon and intensivist.
Resident participation added about 20 minutes to operative (75 minutes vs. 59 minutes; P less than .001) and anesthesia (122 minutes vs. 100 minutes; P less than .001) times.
Intraoperative transfusions were more common with residents (3.43% vs. 2.55%; P less than .001), perhaps because of longer operating room times, and, as a result, fewer postoperative transfusions were needed (1.12% vs. 1.28% P = .031), he said. Unplanned reoperations, however, were more common with residents, as well (4.22% vs. 3.80%; P = .002).
Postsurgical superficial wound infections (3.5% vs. 2.78%; P less than .001) and organ space surgical site infections (2.27% vs. 1.77%; P less than .001) were more common in the resident group, while wound dehiscence was not (0.63% vs. 0.69%; P = .266), Dr. Kasotakis noted.
Pulmonary complications were significantly more common in the resident group including postoperative pneumonia (1.85% vs. 1.67%; P = .04), reintubation (1.64% vs. 1.15%; P less than .001), and mechanical ventilation for more than 48 hours (2.87% vs. 2.06%; P less than .001).
The same was true for deep vein thrombosis (DVT) (0.80% vs. 0.62%; P = .002) and pulmonary embolism (PE) (0.43% vs. 0.28%; P less than .001).
Urinary tract infections (UTI) were higher with resident participation (1.45% vs. 1.14%; P less than .001), as was sepsis (2.42% vs. 2.13%; P = .005), likely because of the increase in surgical infections, Dr. Kasotakis said.
Thankfully, significant cardiac complications and septic shock were not more common with residents, he said.
Adjusted analyses
After adjustment for operative duration, case complexity and pre-existing comorbidities, residents did not increase length of stay (odds ratio, 0.07; P = .242) or septic events (OR, 1.07; P = .155), but their participation was still independently associated with about 20% more superficial surgical site infections (odds ratio, 1.23; P less than .001), organ space infections (OR, 1.21; P less than .001), UTIs (OR, 1.23; P = .001), and intraoperative transfusions (OR, 1.20; P = .001), he said.
Also, about 8% more patients required a return trip to the operating room when residents participated (OR, 1.08; P = .041).
"These outcomes can perhaps be attributed to their underdeveloped surgical skills," Dr. Kasotakis said.
The incidence of DVT and PE were also higher by about 25% (OR, 1.25; P = .011) and 40% (OR, 1.42; P = .005), respectively, perhaps because of delayed DVT prophylaxis initiation because of concerns of hemostasis or missed doses due to additional return trips to the emergency department, he suggested.
Interestingly, reintubation and prolonged mechanical ventilation rates were increased by about 40% (OR, 1.38; OR, 1.43; both P less than .001), perhaps because of prolonged operative times or greater resuscitation requirements, he added.
Dr. Kasotakis was quick to point out that this was a secondary analysis of a data set not originally intended to assess the effect of trainee participation, that no information was available on the degree of resident involvement during surgery or in perioperative care, and that participating institutions were skewed toward tertiary centers, which typically receive more complex cases.
"Staff surgeons should supervise as needed and minimize unnecessary [emergency department] time. And residents, for their part, should be well prepared for emergency procedures through simulation training and aim to maximize their operating room efficiency," he suggested.
The results sparked a flurry of rebuttals led off by discussant Dr. Julie Ann Sosa, Duke University, Durham, N.C.,who said they conflict with other analyses showing little to no impact from residents in elective cases.
"If not interpreted with care, policy makers, payers, and the public could construe that surgical care at academic health centers is compromised by trainees, which could have unfortunate ramifications for everyone in the room as well as the trainees and the patients," she said.
Dr. Sosa expressed concern about drawing causal inferences from an observational study in the setting of possible selection bias and said attempts to match for case complexity using CPT codes do not necessarily account for say, "the difference between a routine appendectomy that takes 15-30 minutes and a complex one that takes 3 hours for a perforation."
Some attendees questioned why the authors didn’t match the institutions in the analysis and chose to ascribe all of the outcomes to residents, with a round of applause following the suggestion that the paper should be titled "Academic centers increase emergency surgery complications." Other attendees questioned whether the poor outcomes reflect resident training and supervision.
Dr. George Velmahos, Massachusetts General Hospital, Boston, questioned whether hospitals have a medical/legal responsibility to inform patients that a resident is in the operating room and may impact outcomes.
Dr. Kasotakis said that institutions may want to add a clause to consent paperwork stating that residents and trainees will be participating.
The complete manuscript of this study and its presentation at the American Surgical Association’s 134th Annual Meeting is anticipated to be published in the Annals of Surgery, pending editorial review.
Dr. Kasotakis reported no conflicts.
Major finding: Rates were significantly higher with resident participation than without for DVT (0.80% vs. 0.62%; P = .002) and PE (0.43% vs. 0.28%; P less than .001).
Data source: A secondary analysis of 141,010 emergency general surgery cases in the prospective ACS NSQIP database.
Disclosures: Dr. Kasotakis and his coauthors reported no conflicting interests.