User login
Is anticoagulation appropriate for all patients with portal vein thrombosis?
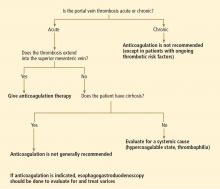
No. in general, the decision to treat portal vein thrombosis with anticoagulant drugs is complex and depends on whether the thrombosis is acute or chronic, and whether the cause is a local factor, cirrhosis of the liver, or a systemic condition (Table 1). A “one-size-fits-all” approach should be avoided (Figure 1).
ACUTE PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
No randomized controlled trial has yet evaluated anticoagulation in acute portal vein thrombosis. But a prospective study published in 2010 showed that the portal vein and its left or right branch were patent in 39% of anticoagulated patients (vs 13% initially), the splenic vein in 80% (vs 57% initially), and the superior mesenteric vein in 73% (vs 42% initially).1 Further, there appears to be a 20% reduction in the overall mortality rate associated with anticoagulation for acute portal vein thrombosis in retrospective studies.2
In the absence of contraindications, anticoagulation with heparin or low-molecular-weight heparin is recommended, with complete bridging to oral anticoagulation with a vitamin K antagonist. Anticoagulation should be continued for at least 3 months, and indefinitely in patients with permanent hypercoaguable risk factors.3
CHRONIC PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
All patients with chronic portal vein thrombosis should undergo esophagogastroduodenoscopy to evaluate for varices. Patients with large varices should be treated orally with a nonselective beta-adrenergic blocker or endoscopically. Though no prospective study has validated this practice, a retrospective analysis showed a decreased risk of first or recurrent bleeding.4
In 2007, a retrospective study showed a lower rate of death in patients with portomesenteric venous thrombosis treated with an oral vitamin K antagonist.5 Patients with chronic portal vein thrombosis with ongoing thrombotic risk factors should be treated with long-term anticoagulation after screening for varices, and if varices are present, primary prophylaxis should be started.3 With this approach, less than 5% of patients died from classic complications of portal vein thrombosis at 5 years of follow-up.4
ACUTE OR CHRONIC PORTAL VEIN THROMBOSIS WITH CIRRHOSIS
Portal vein thrombosis is common in patients with underlying cirrhosis. The risk in patients with cirrhosis significantly increases as liver function worsens. In patients with well-compensated cirrhosis, the risk is less than 1% vs 8% to 25% in those with advanced cirrhosis.6
In patients awaiting liver transplantation, a large retrospective study7 showed that the rate of partial or complete recanalization of the splanchnic veins was significantly higher in those who received anticoagulation (8 of 19) than in those who did not (0 of 10, P = .002). The rate of survival was significantly lower in those who had complete thrombotic obstruction of the portal vein at the time of surgery (P = .04). However, there was no difference in survival rates between those with partial obstruction who received anticoagulation and those with a patent portal vein.7
A later retrospective study8 showed no significant benefit in the rate of transplantation-free survival or survival after liver transplantation in patients with or without chronic portal vein thrombosis.8
Unfortunately, we have no data from prospective controlled trials and only limited data from retrospective studies to make a strong recommendation for or against anticoagulation in either acute and chronic portal vein thrombosis associated with cirrhosis. As such, each case must be evaluated on an individual basis in association with expert consultation.
In our experience, the risk of bleeding in patients with liver cirrhosis is substantial because of the decreased synthesis of coagulation factors and the presence of varices, whereas the efficacy and the benefits of recanalizing the portal vein in asymptomatic patients with liver cirrhosis and portal vein thrombosis are unknown. Therefore, unless the thrombosis extends into the mesenteric vein, thus posing a risk of mesenteric ischemia, we do not generally recommend anticoagulation in asymptomatic portal vein thrombosis in patients with cirrhosis.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51:210–218.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001; 345:1683–1688.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54:691–697.
- John BV, Konjeti VR, Aggarwal A, et al. The impact of portal vein thrombosis (PVT) on cirrhotics awaiting liver transplantation (abstract). Hepatology 2010; 52(suppl1):888A–889A.
No. in general, the decision to treat portal vein thrombosis with anticoagulant drugs is complex and depends on whether the thrombosis is acute or chronic, and whether the cause is a local factor, cirrhosis of the liver, or a systemic condition (Table 1). A “one-size-fits-all” approach should be avoided (Figure 1).
ACUTE PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
No randomized controlled trial has yet evaluated anticoagulation in acute portal vein thrombosis. But a prospective study published in 2010 showed that the portal vein and its left or right branch were patent in 39% of anticoagulated patients (vs 13% initially), the splenic vein in 80% (vs 57% initially), and the superior mesenteric vein in 73% (vs 42% initially).1 Further, there appears to be a 20% reduction in the overall mortality rate associated with anticoagulation for acute portal vein thrombosis in retrospective studies.2
In the absence of contraindications, anticoagulation with heparin or low-molecular-weight heparin is recommended, with complete bridging to oral anticoagulation with a vitamin K antagonist. Anticoagulation should be continued for at least 3 months, and indefinitely in patients with permanent hypercoaguable risk factors.3
CHRONIC PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
All patients with chronic portal vein thrombosis should undergo esophagogastroduodenoscopy to evaluate for varices. Patients with large varices should be treated orally with a nonselective beta-adrenergic blocker or endoscopically. Though no prospective study has validated this practice, a retrospective analysis showed a decreased risk of first or recurrent bleeding.4
In 2007, a retrospective study showed a lower rate of death in patients with portomesenteric venous thrombosis treated with an oral vitamin K antagonist.5 Patients with chronic portal vein thrombosis with ongoing thrombotic risk factors should be treated with long-term anticoagulation after screening for varices, and if varices are present, primary prophylaxis should be started.3 With this approach, less than 5% of patients died from classic complications of portal vein thrombosis at 5 years of follow-up.4
ACUTE OR CHRONIC PORTAL VEIN THROMBOSIS WITH CIRRHOSIS
Portal vein thrombosis is common in patients with underlying cirrhosis. The risk in patients with cirrhosis significantly increases as liver function worsens. In patients with well-compensated cirrhosis, the risk is less than 1% vs 8% to 25% in those with advanced cirrhosis.6
In patients awaiting liver transplantation, a large retrospective study7 showed that the rate of partial or complete recanalization of the splanchnic veins was significantly higher in those who received anticoagulation (8 of 19) than in those who did not (0 of 10, P = .002). The rate of survival was significantly lower in those who had complete thrombotic obstruction of the portal vein at the time of surgery (P = .04). However, there was no difference in survival rates between those with partial obstruction who received anticoagulation and those with a patent portal vein.7
A later retrospective study8 showed no significant benefit in the rate of transplantation-free survival or survival after liver transplantation in patients with or without chronic portal vein thrombosis.8
Unfortunately, we have no data from prospective controlled trials and only limited data from retrospective studies to make a strong recommendation for or against anticoagulation in either acute and chronic portal vein thrombosis associated with cirrhosis. As such, each case must be evaluated on an individual basis in association with expert consultation.
In our experience, the risk of bleeding in patients with liver cirrhosis is substantial because of the decreased synthesis of coagulation factors and the presence of varices, whereas the efficacy and the benefits of recanalizing the portal vein in asymptomatic patients with liver cirrhosis and portal vein thrombosis are unknown. Therefore, unless the thrombosis extends into the mesenteric vein, thus posing a risk of mesenteric ischemia, we do not generally recommend anticoagulation in asymptomatic portal vein thrombosis in patients with cirrhosis.
No. in general, the decision to treat portal vein thrombosis with anticoagulant drugs is complex and depends on whether the thrombosis is acute or chronic, and whether the cause is a local factor, cirrhosis of the liver, or a systemic condition (Table 1). A “one-size-fits-all” approach should be avoided (Figure 1).
ACUTE PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
No randomized controlled trial has yet evaluated anticoagulation in acute portal vein thrombosis. But a prospective study published in 2010 showed that the portal vein and its left or right branch were patent in 39% of anticoagulated patients (vs 13% initially), the splenic vein in 80% (vs 57% initially), and the superior mesenteric vein in 73% (vs 42% initially).1 Further, there appears to be a 20% reduction in the overall mortality rate associated with anticoagulation for acute portal vein thrombosis in retrospective studies.2
In the absence of contraindications, anticoagulation with heparin or low-molecular-weight heparin is recommended, with complete bridging to oral anticoagulation with a vitamin K antagonist. Anticoagulation should be continued for at least 3 months, and indefinitely in patients with permanent hypercoaguable risk factors.3
CHRONIC PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
All patients with chronic portal vein thrombosis should undergo esophagogastroduodenoscopy to evaluate for varices. Patients with large varices should be treated orally with a nonselective beta-adrenergic blocker or endoscopically. Though no prospective study has validated this practice, a retrospective analysis showed a decreased risk of first or recurrent bleeding.4
In 2007, a retrospective study showed a lower rate of death in patients with portomesenteric venous thrombosis treated with an oral vitamin K antagonist.5 Patients with chronic portal vein thrombosis with ongoing thrombotic risk factors should be treated with long-term anticoagulation after screening for varices, and if varices are present, primary prophylaxis should be started.3 With this approach, less than 5% of patients died from classic complications of portal vein thrombosis at 5 years of follow-up.4
ACUTE OR CHRONIC PORTAL VEIN THROMBOSIS WITH CIRRHOSIS
Portal vein thrombosis is common in patients with underlying cirrhosis. The risk in patients with cirrhosis significantly increases as liver function worsens. In patients with well-compensated cirrhosis, the risk is less than 1% vs 8% to 25% in those with advanced cirrhosis.6
In patients awaiting liver transplantation, a large retrospective study7 showed that the rate of partial or complete recanalization of the splanchnic veins was significantly higher in those who received anticoagulation (8 of 19) than in those who did not (0 of 10, P = .002). The rate of survival was significantly lower in those who had complete thrombotic obstruction of the portal vein at the time of surgery (P = .04). However, there was no difference in survival rates between those with partial obstruction who received anticoagulation and those with a patent portal vein.7
A later retrospective study8 showed no significant benefit in the rate of transplantation-free survival or survival after liver transplantation in patients with or without chronic portal vein thrombosis.8
Unfortunately, we have no data from prospective controlled trials and only limited data from retrospective studies to make a strong recommendation for or against anticoagulation in either acute and chronic portal vein thrombosis associated with cirrhosis. As such, each case must be evaluated on an individual basis in association with expert consultation.
In our experience, the risk of bleeding in patients with liver cirrhosis is substantial because of the decreased synthesis of coagulation factors and the presence of varices, whereas the efficacy and the benefits of recanalizing the portal vein in asymptomatic patients with liver cirrhosis and portal vein thrombosis are unknown. Therefore, unless the thrombosis extends into the mesenteric vein, thus posing a risk of mesenteric ischemia, we do not generally recommend anticoagulation in asymptomatic portal vein thrombosis in patients with cirrhosis.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51:210–218.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001; 345:1683–1688.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54:691–697.
- John BV, Konjeti VR, Aggarwal A, et al. The impact of portal vein thrombosis (PVT) on cirrhotics awaiting liver transplantation (abstract). Hepatology 2010; 52(suppl1):888A–889A.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51:210–218.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001; 345:1683–1688.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54:691–697.
- John BV, Konjeti VR, Aggarwal A, et al. The impact of portal vein thrombosis (PVT) on cirrhotics awaiting liver transplantation (abstract). Hepatology 2010; 52(suppl1):888A–889A.
An uncommon syndrome makes us reflect on our approach to diagnosis
In this issue of the Journal, Dr. Soumya Chatterjee and colleagues discuss the antisynthetase syndrome. Although uncommon, this syndrome is important for internists and subspecialists to be aware of. Patients present in several different ways, and potentially life-threatening organ involvement may initially not be recognized or may not be linked with other components of the syndrome, such as involvement of the lungs, muscles, heart, and esophagus and fever.
I am currently on our inpatient rheumatology consultation service, and so I am reminded daily of the challenges hospitalists and subspecialists confront in ordering tests while trying to balance limiting length of stay with cost-efficiency and the desire to obtain a correct diagnosis. And I am repeatedly sensitized to several common test-ordering pitfalls intrinsic to the evaluation of patients with multisystem disease, including myositis. Most have a shared theme—limited time is spent in thoughtful reflection before ordering.
Patients with myositis rarely present with the textbook description of proximal muscle weakness. They describe fatigue, malaise, and sometimes a generalized sense of weakness. It is the probing questioning of their functional capacity and focused examination that reveal that the weakness is characterized by difficulty getting up off the floor, out of a low chair, or off the toilet. Then, with further questioning, some patients note that their fatigue and tiredness may also include getting winded easily with exertion, such as when climbing stairs, thus raising the question of cardiac dysfunction, pulmonary hypertension, or interstitial lung disease.
The responses to those probing questions and the subsequent examination should transform the interpretation of elevated aminotransferase levels (“liver tests”: AST and ALT) from liver disease into suspicion of muscle disease and the appropriate ordering of the creatine kinase level (avoiding liver imaging and hepatology consultation). The carefully repeated and now focused neurologic examination distinguishes the initial “poor cooperation” from the proximal weakness of myopathy. The probing interview leads to the performance of a focused physical examination that frames the appropriate interpretation of the routinely obtained “admission lab studies”!
The thoughtful history and examination are the basic stuff of clinical medicine that can easily be pushed aside by any of us as we deal with the tensions of high-volume, “high-throughput” medical care. It is a low-resistance path from hearing the symptom of fatigue with elevated “liver enzymes” to immediately checking ferritin, ceruloplasmin, and a hepatitis screen in preparation for getting a liver biopsy. It is easy to go through the motions without reflection. Easy, but sometimes wrong. And it is just as easy (but likely to be costly and unhelpful) to identify a patient prematurely with “possible autoimmune disease” and to immediately order a panoply of antinuclear and autoimmune serologies, including the Jo-1 autoantibody test.
As Dr. Chatterjee et al point out, we must continuously reflect on our diagnoses, for even after we navigate the pitfalls and avoid missing the diagnosis of myositis, if we don’t continuously assess all the patient’s symptoms, repeat the examination in a directed manner, and then look for circulating Jo-1 antibody when appropriate, we may well miss the opportunity to recognize that our patient’s ongoing fatigue with exertion is a reflection of the well-described association of myositis with interstitial lung disease (which may warrant a change in therapy), and not steroid myopathy or just poor conditioning.
Alternatively, in evaluating a patient who describes a year of feeling tired, suffering generalized muscle pains with low-grade fevers with temperatures of 99.8°F, and total exhaustion for 3 days after cleaning the oven, testing for antinuclear antibodies, extractable nuclear antigen antibodies, and a “vasculitis panel” in anticipation of a rheumatology consultation is not likely to be useful therapeutically or diagnostically.
Despite the daily pressures, we need to keep ourselves grounded in the fundamentals of clinical care: careful listening, purposeful examination, and directed use of laboratory tests and imaging. The downstream consequences of ordering tests for the sake of efficient throughput are quite real, and thoughtful test ordering is one step toward quality care, as well as cost-effective care.
In future months, the Journal will delve more deeply into test ordering when, in a joint effort with the American College of Physicians, we will be discussing the use and misuse of specific tests.
In this issue of the Journal, Dr. Soumya Chatterjee and colleagues discuss the antisynthetase syndrome. Although uncommon, this syndrome is important for internists and subspecialists to be aware of. Patients present in several different ways, and potentially life-threatening organ involvement may initially not be recognized or may not be linked with other components of the syndrome, such as involvement of the lungs, muscles, heart, and esophagus and fever.
I am currently on our inpatient rheumatology consultation service, and so I am reminded daily of the challenges hospitalists and subspecialists confront in ordering tests while trying to balance limiting length of stay with cost-efficiency and the desire to obtain a correct diagnosis. And I am repeatedly sensitized to several common test-ordering pitfalls intrinsic to the evaluation of patients with multisystem disease, including myositis. Most have a shared theme—limited time is spent in thoughtful reflection before ordering.
Patients with myositis rarely present with the textbook description of proximal muscle weakness. They describe fatigue, malaise, and sometimes a generalized sense of weakness. It is the probing questioning of their functional capacity and focused examination that reveal that the weakness is characterized by difficulty getting up off the floor, out of a low chair, or off the toilet. Then, with further questioning, some patients note that their fatigue and tiredness may also include getting winded easily with exertion, such as when climbing stairs, thus raising the question of cardiac dysfunction, pulmonary hypertension, or interstitial lung disease.
The responses to those probing questions and the subsequent examination should transform the interpretation of elevated aminotransferase levels (“liver tests”: AST and ALT) from liver disease into suspicion of muscle disease and the appropriate ordering of the creatine kinase level (avoiding liver imaging and hepatology consultation). The carefully repeated and now focused neurologic examination distinguishes the initial “poor cooperation” from the proximal weakness of myopathy. The probing interview leads to the performance of a focused physical examination that frames the appropriate interpretation of the routinely obtained “admission lab studies”!
The thoughtful history and examination are the basic stuff of clinical medicine that can easily be pushed aside by any of us as we deal with the tensions of high-volume, “high-throughput” medical care. It is a low-resistance path from hearing the symptom of fatigue with elevated “liver enzymes” to immediately checking ferritin, ceruloplasmin, and a hepatitis screen in preparation for getting a liver biopsy. It is easy to go through the motions without reflection. Easy, but sometimes wrong. And it is just as easy (but likely to be costly and unhelpful) to identify a patient prematurely with “possible autoimmune disease” and to immediately order a panoply of antinuclear and autoimmune serologies, including the Jo-1 autoantibody test.
As Dr. Chatterjee et al point out, we must continuously reflect on our diagnoses, for even after we navigate the pitfalls and avoid missing the diagnosis of myositis, if we don’t continuously assess all the patient’s symptoms, repeat the examination in a directed manner, and then look for circulating Jo-1 antibody when appropriate, we may well miss the opportunity to recognize that our patient’s ongoing fatigue with exertion is a reflection of the well-described association of myositis with interstitial lung disease (which may warrant a change in therapy), and not steroid myopathy or just poor conditioning.
Alternatively, in evaluating a patient who describes a year of feeling tired, suffering generalized muscle pains with low-grade fevers with temperatures of 99.8°F, and total exhaustion for 3 days after cleaning the oven, testing for antinuclear antibodies, extractable nuclear antigen antibodies, and a “vasculitis panel” in anticipation of a rheumatology consultation is not likely to be useful therapeutically or diagnostically.
Despite the daily pressures, we need to keep ourselves grounded in the fundamentals of clinical care: careful listening, purposeful examination, and directed use of laboratory tests and imaging. The downstream consequences of ordering tests for the sake of efficient throughput are quite real, and thoughtful test ordering is one step toward quality care, as well as cost-effective care.
In future months, the Journal will delve more deeply into test ordering when, in a joint effort with the American College of Physicians, we will be discussing the use and misuse of specific tests.
In this issue of the Journal, Dr. Soumya Chatterjee and colleagues discuss the antisynthetase syndrome. Although uncommon, this syndrome is important for internists and subspecialists to be aware of. Patients present in several different ways, and potentially life-threatening organ involvement may initially not be recognized or may not be linked with other components of the syndrome, such as involvement of the lungs, muscles, heart, and esophagus and fever.
I am currently on our inpatient rheumatology consultation service, and so I am reminded daily of the challenges hospitalists and subspecialists confront in ordering tests while trying to balance limiting length of stay with cost-efficiency and the desire to obtain a correct diagnosis. And I am repeatedly sensitized to several common test-ordering pitfalls intrinsic to the evaluation of patients with multisystem disease, including myositis. Most have a shared theme—limited time is spent in thoughtful reflection before ordering.
Patients with myositis rarely present with the textbook description of proximal muscle weakness. They describe fatigue, malaise, and sometimes a generalized sense of weakness. It is the probing questioning of their functional capacity and focused examination that reveal that the weakness is characterized by difficulty getting up off the floor, out of a low chair, or off the toilet. Then, with further questioning, some patients note that their fatigue and tiredness may also include getting winded easily with exertion, such as when climbing stairs, thus raising the question of cardiac dysfunction, pulmonary hypertension, or interstitial lung disease.
The responses to those probing questions and the subsequent examination should transform the interpretation of elevated aminotransferase levels (“liver tests”: AST and ALT) from liver disease into suspicion of muscle disease and the appropriate ordering of the creatine kinase level (avoiding liver imaging and hepatology consultation). The carefully repeated and now focused neurologic examination distinguishes the initial “poor cooperation” from the proximal weakness of myopathy. The probing interview leads to the performance of a focused physical examination that frames the appropriate interpretation of the routinely obtained “admission lab studies”!
The thoughtful history and examination are the basic stuff of clinical medicine that can easily be pushed aside by any of us as we deal with the tensions of high-volume, “high-throughput” medical care. It is a low-resistance path from hearing the symptom of fatigue with elevated “liver enzymes” to immediately checking ferritin, ceruloplasmin, and a hepatitis screen in preparation for getting a liver biopsy. It is easy to go through the motions without reflection. Easy, but sometimes wrong. And it is just as easy (but likely to be costly and unhelpful) to identify a patient prematurely with “possible autoimmune disease” and to immediately order a panoply of antinuclear and autoimmune serologies, including the Jo-1 autoantibody test.
As Dr. Chatterjee et al point out, we must continuously reflect on our diagnoses, for even after we navigate the pitfalls and avoid missing the diagnosis of myositis, if we don’t continuously assess all the patient’s symptoms, repeat the examination in a directed manner, and then look for circulating Jo-1 antibody when appropriate, we may well miss the opportunity to recognize that our patient’s ongoing fatigue with exertion is a reflection of the well-described association of myositis with interstitial lung disease (which may warrant a change in therapy), and not steroid myopathy or just poor conditioning.
Alternatively, in evaluating a patient who describes a year of feeling tired, suffering generalized muscle pains with low-grade fevers with temperatures of 99.8°F, and total exhaustion for 3 days after cleaning the oven, testing for antinuclear antibodies, extractable nuclear antigen antibodies, and a “vasculitis panel” in anticipation of a rheumatology consultation is not likely to be useful therapeutically or diagnostically.
Despite the daily pressures, we need to keep ourselves grounded in the fundamentals of clinical care: careful listening, purposeful examination, and directed use of laboratory tests and imaging. The downstream consequences of ordering tests for the sake of efficient throughput are quite real, and thoughtful test ordering is one step toward quality care, as well as cost-effective care.
In future months, the Journal will delve more deeply into test ordering when, in a joint effort with the American College of Physicians, we will be discussing the use and misuse of specific tests.
Antisynthetase syndrome: Not just an inflammatory myopathy
A 66-year-old man was initially seen in clinic in March 2004 with a 5-month history of polyarthritis (affecting the finger joints, wrists, and knees) and several hours of morning stiffness. He also had significant proximal muscle weakness, progressive exertional dyspnea, and a nonproductive cough. There was no history of fever, chills, rash, dysphagia, or sicca symptoms. Findings on initial tests:
- His creatine kinase level was 700 U/L (reference range 30–220), which later rose to 1,664 U/L.
- He was positive for antinuclear antibody with a 5.7 optical density ratio (normal < 1.5) and for anti-Jo-1 antibody.
- An electromyogram was consistent with a necrotizing myopathy. Left rectus femoris biopsy revealed scattered degenerating and regenerating muscle fibers but no evidence of endomysial inflammation.
- On pulmonary function testing, his forced vital capacity was 80% of predicted, and his carbon monoxide diffusion capacity was 67% of predicted.
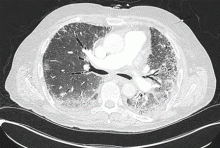
- High-resolution computed tomography revealed evidence of interstitial lung disease, characterized by bilateral patchy ground-glass opacities suggestive of active alveolitis, most extensive at the lung bases.
- Bronchoalveolar lavage indicated alveolitis, and transbronchial biopsy revealed pathologic changes consistent with cryptogenic organizing pneumonia. All cultures were negative.
This constellation of clinical manifestations, including myositis, interstitial lung disease, and polyarthritis, along with positive anti-Jo-1 antibody, confirmed the diagnosis of antisynthetase syndrome.
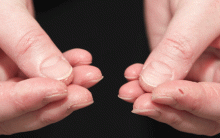
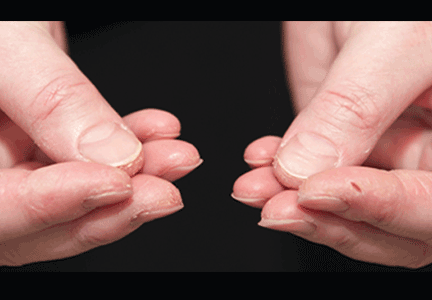
In June 2004, for his interstitial lung disease, he was started on daily oral cyclophosphamide along with high-dose oral prednisone. Three months later the skin of the tips and radial margins of his fingers started thickening and cracking, the appearance of which is classically described as “mechanic’s hands,” a well-described manifestation of antisynthetase syndrome (Figure 1).
Cyclophosphamide was continued for about a year. Subsequently, along with prednisone, he sequentially received various other immunosuppressive medications (methotrexate, tacrolimus, mycophenolate mofetil, and rituximab) over the next few years in an attempt to control his progressive interstitial lung disease. All of these agents were only partially and temporarily effective. Ultimately, despite all of these therapies, as his interstitial lung disease progressed, he needed supplemental oxygen and enrollment in a pulmonary rehabilitation program.
In March 2010, he was admitted with worsening dyspnea and significant peripheral edema and was found to have severe pulmonary arterial hypertension. He was started on bosentan. Eight months later sildenafil was added for progressive pulmonary arterial hypertension. However, his oxygenation status continued to decline.
In July 2011, he presented with chills, increasing shortness of breath, and a mild productive cough. As he was severely hypoxic, he was admitted to the intensive care unit and started on mechanical ventilation and broad-spectrum antibiotics. Despite escalation of oxygen therapy, his respiratory status rapidly deteriorated, and he developed hypotension requiring vasopressors. He ultimately died of cardiac arrest secondary to respiratory failure.
A CONSTELLATION OF MANIFESTATIONS
Antisynthetase syndrome, associated with anti-aminoacyl-transfer RNA (tRNA) synthetase antibodies, is characterized by a constellation of manifestations that include myositis, interstitial lung disease, mechanic’s hands, fever, Raynaud phenomenon, and nonerosive symmetric polyarthritis of the small joints.1
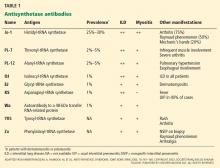
Anti-Jo-1 antibody (anti-histidyl-tRNA synthetase) is the most common of the antibodies and also was the first one to be identified (Table 1). It was named after John P, a patient with polymyositis and interstitial lung disease, in whom it was first detected in 1980.2 The onset of the syndrome associated with anti-Jo-1 antibody is often acute, and the myositis is usually steroid-responsive. However, not uncommonly, severe disease can develop over time, with a tendency to relapse and with a poor long-term prognosis.
RARE BUT UNDERRECOGNIZED
The true population prevalence of antisynthetase syndrome is unknown. Because this syndrome is rare, comprehensive epidemiologic studies are difficult to perform.
In several retrospective studies, the annual incidence of idiopathic inflammatory myopathies has been reported to be 2 to 10 new cases per million adults per year.3 Antisynthetase antibodies are detected in 20% to 40% of such cases.4–6 The disease is two to three times more common in women than in men.7
Early diagnosis is difficult because the clinical presentation is varied and often nonspecific, clinically milder disease may escape detection, and many general practitioners lack familiarity with this syndrome and consequently do not recognize it. Moreover, tests for myositis-specific antibodies (including antisynthetase antibodies) are often not ordered in the evaluation of myositis, and hence the diagnosis of antisynthetase syndrome cannot be substantiated. Furthermore, interstitial lung disease can predominate or can be the sole manifestation in the absence of clinically apparent myositis,8–10 and patients can be misdiagnosed as having idiopathic pulmonary fibrosis when underlying antisynthetase syndrome is not suspected. This distinction may be important because these conditions differ in their pathology and treatment. Histologically, the predominant pattern of lung injury in idiopathic pulmonary fibrosis is “usual interstitial pneumonia” which does not respond to immunosuppressive therapy, and hence lung transplantation is the only therapeutic option. On the other hand, in antisynthetase syndrome, the usual pattern of lung injury is “nonspecific interstitial pneumonia,” in which immunosuppressive therapy has a role.
Anti-Jo-1 antibody is detected in 15% to 25% of patients with polymyositis and in up to 70% of myositis patients with concomitant interstitial lung disease.11 Autoantibodies to seven other, less frequently targeted, aminoacyl tRNA synthetases have also been described in patients with polymyositis and interstitial lung disease (Table 1).11,12 In addition, an autoantibody to a 48-kDa transfer RNA-related protein (Wa) has been described.13 These non-Jo-1 antisynthetase antibodies are detected in only about 3% of myositis patients.14
ROLE OF ANTISYNTHETASE ANTIBODIES
Synthetases play a central role in protein synthesis by catalyzing the acetylation of tRNAs. The propensity of organ involvement in antisynthetase syndrome suggests that tissue-specific changes in muscle or lung lead to the production of unique forms of target autoantigens, the aminoacyl-tRNA synthetases. There is evidence that these enzymes themselves may be involved in recruiting both antigen-presenting and inflammatory cells to the site of muscle or lung injury.15 However, the molecular pathway that initiates and propagates this autoimmune response and the specific role of the antisynthetase antibodies in the pathogenesis of this syndrome are presently unknown.
SIX SALIENT CLINICAL FEATURES
There are six predominant clinical manifestations, which may be present at disease onset or appear later as the disease progresses:
- Fever
- Myositis
- Interstitial lung disease
- Mechanic’s hands
- Raynaud phenomenon
- Inflammatory polyarthritis.
There is considerable clinical heterogeneity, and one or other manifestation can predominate or can be the only expression of the syndrome. Furthermore, in the same patient, the individual features can prevail at different times and may develop years after onset of the disease. Therefore, in addition to patients with myositis, it would be important to suspect antisynthetase syndrome in patients presenting with isolated lung involvement (amyopathic interstitial lung disease), as there are therapeutic implications. Studies have demonstrated the efficacy of immunosuppressive agents in interstitial lung disease associated with antisynthetase syndrome (where the predominant pattern of lung injury is “nonspecific interstitial pneumonitis”), whereas lung transplantation has so far been the only treatment option in idiopathic pulmonary fibrosis.
Fever
About 20% of patients have a fever at disease onset or associated with relapses. Sometimes the fever can persist until treatment of antisynthetase syndrome is started.
Myositis
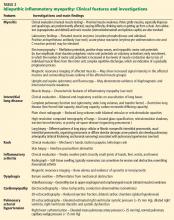
Muscle disease is seen in more than 90% of patients with anti-Jo-1 antisynthetase syndrome. It can be subclinical (in the absence of proximal myopathy), manifested by transient creatine kinase elevation only, which may normalize after therapy is initiated.
However, more commonly, patients develop profound proximal muscle weakness and sometimes muscle pain (Table 2). Weakness of the striated muscles of the upper esophagus, cricopharyngeus, and hypopharynx may cause dysphagia and makes these patients susceptible to aspiration pneumonia. Diaphragmatic and intercostal muscle weakness can contribute to shortness of breath in some patients. Myocarditis has also been reported.
Pulmonary disease
Interstitial lung disease develops in most patients with anti-Jo-1 antisynthetase syndrome, with a reported prevalence of about 90% in one series.16 Patients often present with acute, subacute, or insidious onset of exertional dyspnea. Sometimes there is an intractable nonproductive cough.
At the outset of antisynthetase syndrome, if the patient is profoundly weak because of myopathy or has inflammatory polyarthritis, mobility is significantly compromised, and exertional dyspnea may not be experienced. However, as the interstitial lung disease progresses, shortness of breath becomes overt, more so when the patient’s level of activity improves with treatment of myositis.
Inspiratory crackles on auscultation of the lung bases or changes on chest radiography are relatively insensitive findings and can miss early interstitial lung disease. Therefore, if antisynthetase syndrome is suspected or diagnosed, a baseline pulmonary function test (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease, and the diagnosis can then be confirmed with thoracic high-resolution computed tomography (Figure 2).
Pulmonary hypertension. Recent studies indicate that, similar to patients with other autoimmune rheumatic diseases, pulmonary hypertension can develop in patients with antisynthetase syndrome, with or without concomitant interstitial lung disease.17,18 This complication occurred in the case presented here. It has been found that when pulmonary hypertension coexists with interstitial lung disease, its degree may not correlate with the severity of the latter.17 Additionally, pulmonary hypertension, when present, has been found to contribute independently to prognosis and survival.
Mechanic’s hands
In about 30% of patients, the skin of the tips and margins of the fingers becomes thickened, hyperkeratotic, and fissured, the appearance of which is classically described as mechanic’s hands. It is a common manifestation of antisynthetase syndrome and is particularly prominent on the radial side of the index fingers (Figure 1). Biopsy of affected skin shows an interface psoriasiform dermatitis.19 In addition, some dermatomyositis patients with Gottron papules and a heliotrope rash have antisynthetase antibodies.
Raynaud phenomenon
Raynaud phenomenon develops in about 40% of patients. Some have nailfold capillary abnormalities.20 However, persistent or severe digital ischemia leading to digital ulceration or infarction is uncommon.21
Inflammatory arthritis
Arthralgias and arthritis are common (50%), the most common form being a symmetric polyarthritis of the small joints of the hands and feet. It is typically nonerosive but can sometimes be erosive and destructive.20
Because inflammatory arthritis mimics rheumatoid arthritis, antisynthetase syndrome should be considered in rheumatoid factor-negative patients presenting with polyarthritis.
ASSOCIATION WITH MALIGNANCY
Traditional teaching has been that antisynthetase antibody is protective against an underlying malignancy.22,23 However, several recently published case studies have reported various malignancies occurring within 6 to 12 months of the diagnosis of antisynthetase syndrome.7,24 The debate as to whether these are chance associations or causal (a paraneoplastic phenomenon) has not been resolved at this time.24
It is now recommended that patients with antisynthetase syndrome be screened for malignancies as appropriate for the patient’s age and sex. Screening should include a careful history and physical examination, complete blood cell count, comprehensive metabolic panel, chest radiography, mammography, and a gynecologic examination for women.25 If abnormalities are found, a more thorough evaluation for cancer is appropriate.
DIAGNOSIS
Muscle enzyme levels are often elevated
Muscle enzymes (creatine kinase and aldolase) are often elevated. Serum creatine kinase levels can range between 5 to 50 times the upper limit of normal. In an established case, creatine kinase levels along with careful manual muscle strength testing may help evaluate myositis activity. However, in chronic and advanced disease, creatine kinase may be within the normal range despite active myositis, partly because of extensive loss of muscle mass. In myositis, it may be prudent to check both creatine kinase and aldolase; sometimes only serum aldolase level rises, when immune-mediated injury predominantly affects the early regenerative myocytes.26
Judicious use of autoantibody testing
The characteristic clinical presentation is the initial clue to the diagnosis of antisynthetase syndrome, which is then supported by serologic testing.
Injudicious testing for a long list of antibodies should be avoided, as the cost is considerable and it does not influence further management. However, ordering an anti-Jo-1 antibody test in the correct clinical setting is appropriate, as it has high specificity,27,28 and thus can help establish or refute the clinical suspicion of antisynthetase syndrome.
Screening pulmonary function testing and thoracic high-resolution computed tomography for all patients with polymyositis or dermatomyositis is not considered “standard of care” and will likely not be reimbursed by third-party payers. However, in a patient with symptoms and signs of myositis, the presence of an antisynthetase antibody should prompt screening for occult interstitial lung disease, even in the absence of symptoms. As lung disease ultimately determines the prognosis in antisynthetase syndrome, early diagnosis and management is the key. Therefore, these tests would likely be approved to establish the diagnosis of interstitial lung disease and evaluate its severity.
If a myositis patient is also found to have interstitial lung disease or develops mechanic’s hands, the likely diagnosis is antisynthetase syndrome, which can be confirmed by serologic testing for antisynthetase antibodies. Interstitial lung disease in antisynthetase syndrome is often from “nonspecific interstitial pneumonitis”; therefore, medications tested and proven effective for this condition should be approved and reimbursed by payers.29–32
The coexistence of myositis and interstitial lung disease increases the sensitivity of anti-Jo-1 antibody.11 Thus, the clinician can have more confidence in early recognition and initiation of aggressive but targeted disease-modifying therapy.
Various methods can be used for detecting antisynthetase antibodies, with comparable results.28 Anti-Jo-1 antibody testing costs about $140. If that test is negative and antisynthetase syndrome is still suspected, then testing for the non-Jo-1 antisynthetase antibodies may be justified (Table 1). Though the cost of this panel of autoantibodies is about $300, it helps to confirm the diagnosis, and it influences the choice of second-line immunosuppressive agents such as tacrolimus29 and rituximab32 in patients resistant to conventional immunosuppressive agents such as azathioprine and methotrexate.
Often, anti-Ro52 SS-A antibodies are present concurrently in patients with anti-Jo-1 syndrome.33 In observational studies in patients with anti-Jo-1 antibody-associated interstitial lung disease, coexistence of anti-Ro52 SS-A antibody tended to predict a worse pulmonary outcome than in those with anti-Jo-1 antibody alone.34,35
Electromyography
Electromyography not only helps differentiate between myopathic and neuropathic weakness, but it may also support the diagnosis of “inflammatory” myopathy as suggested by prominent muscle membrane irritability (fibrillations, positive sharp waves) and abnormal motor unit action potentials (spontaneous activity showing small, short, polyphasic potentials and early recruitment). However, the findings can be nonspecific, and may even be normal in 10% to 15% of patients.36 Electromyographic abnormalities are most consistently observed in weak proximal muscles, and electromyography is also helpful in selecting a muscle for biopsy. Although no single electromyographic pattern is considered diagnostic for inflammatory myopathy, abnormalities are present in around 90% of patients (Table 2).3
Magnetic resonance imaging
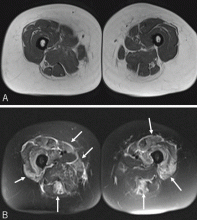
Magnetic resonance imaging may show increased signal intensity in the affected muscles and surrounding tissues (Figure 3).37 Because it lacks sensitivity and specificity, magnetic resonance imaging is not helpful in diagnosing the disease. However, in the correct clinical setting, it may be used to guide muscle biopsy, and it can help in monitoring the disease progress.38
Muscle histopathology
Muscle biopsy, though often helpful, is not always diagnostic, and antisynthetase syndrome should still be suspected in the right clinical context, even in the absence of characteristic pathologic changes.
Biopsy of sites recently studied by electromyography should be avoided, and if the patient has undergone electromyography recently, the contralateral side should be selected for biopsy.
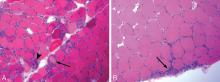
Reports of histopathologic findings in muscle biopsies in patients with antisynthetase syndrome document inflammatory myopathic features (Figure 4). In a series of patients with anti-Jo-1 syndrome, inflammation was noted in all cases, predominantly perimysial in location, with occasional endomysial and perivascular inflammation.39 Many of the inflammatory cells seen were macrophages and lymphocytes, in contrast to the predominantly lymphocytic infiltrates described in classic polymyositis and dermatomyositis. Perifascicular atrophy, similar to what is seen in dermatomyositis, was encountered; however, vascular changes, typical of dermatomyositis, were absent. Occasional degenerating and regenerating muscle fibers were also observed in most cases. Additionally, a characteristic perimysial connective tissue fragmentation was described, a feature less often seen in classic polymyositis and dermatomyositis.39
Pulmonary function testing
If antisynthetase syndrome is suspected or diagnosed, baseline pulmonary function testing (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease (reduced forced vital capacity and carbon monoxide diffusion capacity), and the diagnosis will be substantiated on thoracic high-resolution computed tomography. Respiratory muscle weakness can be detected with upright and supine spirometry.40 Weakness of these muscles contributes to shortness of breath, and patients may need ventilatory support.
Thoracic high-resolution computed tomography
Different patterns of lung injury can be seen in antisynthetase syndrome. Diffuse ground-glass opacification may suggest a nonspecific interstitial pneumonitis pattern, which is the most common form of interstitial lung disease (Figure 2), whereas coarse reticulation or honeycombing correlates with a usual interstitial pneumonitis pattern. Patchy consolidation or air-space disease can occur if cryptogenic organizing pneumonia is the predominant pattern of lung injury.
Swallowing evaluation
A comprehensive swallowing evaluation by a speech therapist may be necessary for evaluation of dysphagia (from oropharyngeal and striated esophageal muscle weakness) and determination of aspiration risk (Table 2).
Lung histopathology
If necessary, a surgical lung biopsy is needed to document the pathologic pattern of injury, including the amount of fibrosis in the lung. Historically, in idiopathic inflammatory myopathy patients in general, this has taken the form of usual interstitial pneumonia, organizing pneumonia, or diffuse alveolar damage.41 With the emergence of the definition of nonspecific interstitial pneumonitis and fibrosis as a documented and accepted pattern, more studies have found this to be the most common pattern of lung injury.16 It is characterized by diffuse involvement of the lung by an interstitial chronic inflammatory infiltrate, a cellular type of nonspecific interstitial pneumonitis that progresses in a uniform pattern to a fibrotic type (Figure 5). This form of fibrosis rarely results in significant remodeling, so-called honeycomb changes. In addition, anti-Jo-1 antibody patients may also have an increased incidence of acute lung injury, including acute diffuse alveolar damage that is often superimposed on the underlying chronic lung disease.42
In patients with pulmonary hypertension, histopathologic studies of the muscular pulmonary arteries often show moderate intimal fibroplasia, suggesting that a pulmonary arteriopathy with intimal thickening and luminal narrowing develops in some of these patients (Figure 5), independent of chronic hypoxic pulmonary vasoconstriction or vascular obstruction due to its entrapment within fibrotic lung tissue.17
TREATMENT
Glucocorticoids are the mainstay
Glucocorticoids are considered the mainstay of treatment. Patients should be advised that long-term use of glucocorticoids is necessary, though the response is variable. It is also important to discuss possible side effects of long-term glucocorticoid use.
Standard practice is to initiate treatment with high doses for the first 4 to 6 weeks to achieve disease control, followed by a slow taper over the next 9 to 12 months to the lowest effective dose to maintain remission. If the patient is profoundly weak, especially with respiratory muscle weakness or significant dysphagia and aspiration risk, hospital admission for intravenous methylprednisolone 1,000 mg daily for 3 to 5 days may be necessary. Otherwise, oral prednisone 1 mg/kg/day would be the usual starting dose.
If the patient’s muscle strength initially improves and then declines weeks to months later despite adequate therapy, glucocorticoid-induced myopathy should be suspected, especially if the muscle enzymes are within the reference range. This is more likely to occur if high-dose prednisone is continued for more than 6 to 8 weeks.
Improvement in muscle strength, which can take several weeks to several months, is a more reliable indicator of response to therapy than the serum creatine kinase level, which may take much longer to normalize. Relying on normalization of the creatine kinase level alone may lead to unnecessary prolongation of high-dose glucocorticoid therapy. It may take several months for the muscle enzymes to normalize, and there is usually a time lag between normalization of muscle enzymes and complete recovery of muscle strength.
Long-term use of high-dose prednisone leads to glucocorticoid-induced osteoporosis. Therefore, patients should receive osteoporosis prophylaxis including antiresorptive therapy with a bisphosphonate. In addition, prophylaxis against Pneumocystis jirovecii is indicated for patients treated with high-dose glucocorticoids.
Additional immunosuppressive agents
Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine and methotrexate are often required, both as glucocorticoid-sparing agents and to achieve adequate disease control.10 Addition of such agents from the outset is particularly necessary in patients with profound muscle weakness or those who have concomitant symptomatic interstitial lung disease.
No randomized controlled trial comparing azathioprine and methotrexate has been conducted to date. Therefore, the choice is based on patient preference, presence of coexisting interstitial lung disease or liver disease, commitment to limit alcohol consumption, and thiopurine methyltransferase status. Most patients need prolonged therapy.
In a randomized clinical trial, concomitant therapy with prednisone and azathioprine resulted in better functional outcomes and a significantly lower prednisone dose requirement for maintenance therapy at 3 years than with prednisone alone.43,44 Although no such randomized study has been conducted using methotrexate, several retrospective studies have demonstrated 70% to 80% response rates, including those for whom monotherapy with glucocorticoids had failed.45,46 The combination of methotrexate and azathioprine may be beneficial in patients who previously had inadequate responses to either of these agents alone.47
For severe pulmonary involvement associated with antisynthetase syndrome, monthly intravenous infusion of cyclophosphamide has been shown to be effective.48,49
Some recent studies established the role of tacrolimus in the treatment of both interstitial lung disease and myositis associated with antisynthetase syndrome.29 Cyclosporine has also been successfully used in a case of interstitial lung disease associated with anti Jo-1 syndrome.30
Rituximab, a monoclonal antibody to Blymphocyte antigen CD20, can also be used successfully in refractory disease,31 including refractory interstitial lung disease.32
In an open-label prospective study, polymyositis refractory to glucocorticoids and multiple conventional immunosuppressive agents responded well to high-dose intravenous immune globulin in the short term.50 However, the antisynthetase antibody status in this cohort was unknown; therefore, no definite conclusion could be drawn about the efficacy of intravenous immune globulin specifically in antisynthetase syndrome.
General measures
In patients with profound muscle weakness, physical therapy and rehabilitation should begin early. The goal is to reduce further muscle wasting from disuse and prevent muscle contractures. Patients with oropharyngeal and esophageal dysmotility should be advised about aspiration precautions and may need a swallow evaluation by a speech therapist; some may need temporary parenteral hyper-alimentation or J-tube insertion.
PROGNOSIS
If skeletal muscle involvement is the sole manifestation of antisynthetase syndrome, patients usually respond to glucocorticoids and immunosuppressive therapy and do fairly well. However, the outcome is not so promising when patients also develop interstitial lung disease, and the severity and type of lung injury usually determine the prognosis. As expected, patients with a progressive course of interstitial lung disease fare poorly, whereas those with a nonprogressive course tend to do relatively better. Older age at onset (> 60 years), presence of a malignancy, and a negative antinuclear antibody test are associated with a poor prognosis.7
Acknowledgment: The authors are grateful to Dr. Stephen Hatem, MD, staff radiologist, musculoskeletal radiology, Cleveland Clinic Imaging Institute, for help in the preparation of the magnetic resonance images. We also thank Dr. Steven Shook, MD, staff neurologist, Cleveland Clinic Neurological Institute, for help in summarizing the EMG findings.
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011; 13:175–181.
- Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 1980; 23:881–888.
- Nagaraju K, Lundberg IE. Inflammatory diseases of muscle and other myopathies. In:Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: Saunders; 2008:1353–1380.
- Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001; 60:116–123.
- Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum 1996; 39:292–296.
- Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996; 39:1507–1518.
- Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ. Clinical heterogeneity and prognostic features of South Australian patients with antisynthetase autoantibodies. Intern Med J 2011; 41:674–679.
- Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 1996; 26:459–467.
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006; 39:233–241.
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008; 63:53–59.
- Targoff IN. Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 2000; 12:475–481.
- Ancuta CM, Ancuta E, Chirieac RM. Aminoacyl-tRNA synthetases in idiopathic inflammatory myopathies: an update on immunopathogenic significance, clinical and therapeutic implications. In:Gran JT, editor. Idiopathic Inflammatory Myopathies - Recent Developments. Rijeka, Croatia: InTech; 2011:77–90.
- Kajihara M, Kuwana M, Tokuda H, et al. Myositis and interstitial lung disease associated with autoantibody to a transfer RNA-related protein Wa. J Rheumatol 2000; 27:2707–2710.
- Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 2006; 54:2004–2009.
- Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Rep 2003; 5:425–430.
- Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 2010; 23:874–880.
- Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res (Hoboken) 2010; 62:425–429.
- Minai OA. Pulmonary hypertension in polymyositis-dermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 2009; 18:1006–1010.
- Bugatti L, De Angelis R, Filosa G, Salaffi F. Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis. Indian J Dermatol Venereol Leprol 2005; 71:137–138.
- Mumm GE, McKown KM, Bell CL. Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 2010; 16:307–312.
- Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 1992; 35:449–456.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991; 70:360–374.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol 2001; 144:825–831.
- Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol 2008; 35:169–171.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010; 22:627–632.
- Casciola-Rosen L, Hall JC, Mammen AL, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012; 30:548–553.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci 2005; 1050:380–388.
- Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity 2005; 38:73–78.
- Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005; 52:2439–2446.
- Jankowska M, Butto B, Debska-Slizien A, Rutkowski B. Beneficial effect of treatment with cyclosporin A in a case of refractory antisynthetase syndrome. Rheumatol Int 2007; 27:775–780.
- Limaye V, Hissaria P, Liew CL, Koszyka B. Efficacy of rituximab in refractory antisynthetase syndrome. Intern Med J 2012; 42:e4–e7.
- Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012; 106:581–587.
- Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ, Van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 1997; 109:32–40.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006; 39:249–253.
- Váncsa A, Csípo I, Németh J, Dévényi K, Gergely L, Dankó K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009; 29:989–994.
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977; 56:255–286.
- Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 1997; 9:475–485.
- O’Connell MJ. Whole-body MR imaging in the diagnosis of polymyositis. Am J Roentgenol 2002; 179:967–971.
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000; 68:472–478.
- Fromageot C, Lofaso F, Annane D, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001; 82:123–128.
- Leslie KO. Historical perspective: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest 2005; 128(suppl 1):513S–519S.
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217.
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 1980; 92:365–369.
- Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum 1981; 24:45–48.
- Metzger AL, Bohan A, Goldberg LS, Bluestone R, Pearson CM. Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 1974; 81:182–189.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med 1993; 94:379–387.
- Villalba L, Hicks JE, Adams EM, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum 1998; 41:392–399.
- Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007; 46:124–130.
- al-Janadi M, Smith CD, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989; 16:1592–1596.
- Cherin P, Pelletier S, Teixeira A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum 2002; 46:467–474.
A 66-year-old man was initially seen in clinic in March 2004 with a 5-month history of polyarthritis (affecting the finger joints, wrists, and knees) and several hours of morning stiffness. He also had significant proximal muscle weakness, progressive exertional dyspnea, and a nonproductive cough. There was no history of fever, chills, rash, dysphagia, or sicca symptoms. Findings on initial tests:
- His creatine kinase level was 700 U/L (reference range 30–220), which later rose to 1,664 U/L.
- He was positive for antinuclear antibody with a 5.7 optical density ratio (normal < 1.5) and for anti-Jo-1 antibody.
- An electromyogram was consistent with a necrotizing myopathy. Left rectus femoris biopsy revealed scattered degenerating and regenerating muscle fibers but no evidence of endomysial inflammation.
- On pulmonary function testing, his forced vital capacity was 80% of predicted, and his carbon monoxide diffusion capacity was 67% of predicted.
- High-resolution computed tomography revealed evidence of interstitial lung disease, characterized by bilateral patchy ground-glass opacities suggestive of active alveolitis, most extensive at the lung bases.
- Bronchoalveolar lavage indicated alveolitis, and transbronchial biopsy revealed pathologic changes consistent with cryptogenic organizing pneumonia. All cultures were negative.
This constellation of clinical manifestations, including myositis, interstitial lung disease, and polyarthritis, along with positive anti-Jo-1 antibody, confirmed the diagnosis of antisynthetase syndrome.
In June 2004, for his interstitial lung disease, he was started on daily oral cyclophosphamide along with high-dose oral prednisone. Three months later the skin of the tips and radial margins of his fingers started thickening and cracking, the appearance of which is classically described as “mechanic’s hands,” a well-described manifestation of antisynthetase syndrome (Figure 1).
Cyclophosphamide was continued for about a year. Subsequently, along with prednisone, he sequentially received various other immunosuppressive medications (methotrexate, tacrolimus, mycophenolate mofetil, and rituximab) over the next few years in an attempt to control his progressive interstitial lung disease. All of these agents were only partially and temporarily effective. Ultimately, despite all of these therapies, as his interstitial lung disease progressed, he needed supplemental oxygen and enrollment in a pulmonary rehabilitation program.
In March 2010, he was admitted with worsening dyspnea and significant peripheral edema and was found to have severe pulmonary arterial hypertension. He was started on bosentan. Eight months later sildenafil was added for progressive pulmonary arterial hypertension. However, his oxygenation status continued to decline.
In July 2011, he presented with chills, increasing shortness of breath, and a mild productive cough. As he was severely hypoxic, he was admitted to the intensive care unit and started on mechanical ventilation and broad-spectrum antibiotics. Despite escalation of oxygen therapy, his respiratory status rapidly deteriorated, and he developed hypotension requiring vasopressors. He ultimately died of cardiac arrest secondary to respiratory failure.
A CONSTELLATION OF MANIFESTATIONS
Antisynthetase syndrome, associated with anti-aminoacyl-transfer RNA (tRNA) synthetase antibodies, is characterized by a constellation of manifestations that include myositis, interstitial lung disease, mechanic’s hands, fever, Raynaud phenomenon, and nonerosive symmetric polyarthritis of the small joints.1
Anti-Jo-1 antibody (anti-histidyl-tRNA synthetase) is the most common of the antibodies and also was the first one to be identified (Table 1). It was named after John P, a patient with polymyositis and interstitial lung disease, in whom it was first detected in 1980.2 The onset of the syndrome associated with anti-Jo-1 antibody is often acute, and the myositis is usually steroid-responsive. However, not uncommonly, severe disease can develop over time, with a tendency to relapse and with a poor long-term prognosis.
RARE BUT UNDERRECOGNIZED
The true population prevalence of antisynthetase syndrome is unknown. Because this syndrome is rare, comprehensive epidemiologic studies are difficult to perform.
In several retrospective studies, the annual incidence of idiopathic inflammatory myopathies has been reported to be 2 to 10 new cases per million adults per year.3 Antisynthetase antibodies are detected in 20% to 40% of such cases.4–6 The disease is two to three times more common in women than in men.7
Early diagnosis is difficult because the clinical presentation is varied and often nonspecific, clinically milder disease may escape detection, and many general practitioners lack familiarity with this syndrome and consequently do not recognize it. Moreover, tests for myositis-specific antibodies (including antisynthetase antibodies) are often not ordered in the evaluation of myositis, and hence the diagnosis of antisynthetase syndrome cannot be substantiated. Furthermore, interstitial lung disease can predominate or can be the sole manifestation in the absence of clinically apparent myositis,8–10 and patients can be misdiagnosed as having idiopathic pulmonary fibrosis when underlying antisynthetase syndrome is not suspected. This distinction may be important because these conditions differ in their pathology and treatment. Histologically, the predominant pattern of lung injury in idiopathic pulmonary fibrosis is “usual interstitial pneumonia” which does not respond to immunosuppressive therapy, and hence lung transplantation is the only therapeutic option. On the other hand, in antisynthetase syndrome, the usual pattern of lung injury is “nonspecific interstitial pneumonia,” in which immunosuppressive therapy has a role.
Anti-Jo-1 antibody is detected in 15% to 25% of patients with polymyositis and in up to 70% of myositis patients with concomitant interstitial lung disease.11 Autoantibodies to seven other, less frequently targeted, aminoacyl tRNA synthetases have also been described in patients with polymyositis and interstitial lung disease (Table 1).11,12 In addition, an autoantibody to a 48-kDa transfer RNA-related protein (Wa) has been described.13 These non-Jo-1 antisynthetase antibodies are detected in only about 3% of myositis patients.14
ROLE OF ANTISYNTHETASE ANTIBODIES
Synthetases play a central role in protein synthesis by catalyzing the acetylation of tRNAs. The propensity of organ involvement in antisynthetase syndrome suggests that tissue-specific changes in muscle or lung lead to the production of unique forms of target autoantigens, the aminoacyl-tRNA synthetases. There is evidence that these enzymes themselves may be involved in recruiting both antigen-presenting and inflammatory cells to the site of muscle or lung injury.15 However, the molecular pathway that initiates and propagates this autoimmune response and the specific role of the antisynthetase antibodies in the pathogenesis of this syndrome are presently unknown.
SIX SALIENT CLINICAL FEATURES
There are six predominant clinical manifestations, which may be present at disease onset or appear later as the disease progresses:
- Fever
- Myositis
- Interstitial lung disease
- Mechanic’s hands
- Raynaud phenomenon
- Inflammatory polyarthritis.
There is considerable clinical heterogeneity, and one or other manifestation can predominate or can be the only expression of the syndrome. Furthermore, in the same patient, the individual features can prevail at different times and may develop years after onset of the disease. Therefore, in addition to patients with myositis, it would be important to suspect antisynthetase syndrome in patients presenting with isolated lung involvement (amyopathic interstitial lung disease), as there are therapeutic implications. Studies have demonstrated the efficacy of immunosuppressive agents in interstitial lung disease associated with antisynthetase syndrome (where the predominant pattern of lung injury is “nonspecific interstitial pneumonitis”), whereas lung transplantation has so far been the only treatment option in idiopathic pulmonary fibrosis.
Fever
About 20% of patients have a fever at disease onset or associated with relapses. Sometimes the fever can persist until treatment of antisynthetase syndrome is started.
Myositis
Muscle disease is seen in more than 90% of patients with anti-Jo-1 antisynthetase syndrome. It can be subclinical (in the absence of proximal myopathy), manifested by transient creatine kinase elevation only, which may normalize after therapy is initiated.
However, more commonly, patients develop profound proximal muscle weakness and sometimes muscle pain (Table 2). Weakness of the striated muscles of the upper esophagus, cricopharyngeus, and hypopharynx may cause dysphagia and makes these patients susceptible to aspiration pneumonia. Diaphragmatic and intercostal muscle weakness can contribute to shortness of breath in some patients. Myocarditis has also been reported.
Pulmonary disease
Interstitial lung disease develops in most patients with anti-Jo-1 antisynthetase syndrome, with a reported prevalence of about 90% in one series.16 Patients often present with acute, subacute, or insidious onset of exertional dyspnea. Sometimes there is an intractable nonproductive cough.
At the outset of antisynthetase syndrome, if the patient is profoundly weak because of myopathy or has inflammatory polyarthritis, mobility is significantly compromised, and exertional dyspnea may not be experienced. However, as the interstitial lung disease progresses, shortness of breath becomes overt, more so when the patient’s level of activity improves with treatment of myositis.
Inspiratory crackles on auscultation of the lung bases or changes on chest radiography are relatively insensitive findings and can miss early interstitial lung disease. Therefore, if antisynthetase syndrome is suspected or diagnosed, a baseline pulmonary function test (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease, and the diagnosis can then be confirmed with thoracic high-resolution computed tomography (Figure 2).
Pulmonary hypertension. Recent studies indicate that, similar to patients with other autoimmune rheumatic diseases, pulmonary hypertension can develop in patients with antisynthetase syndrome, with or without concomitant interstitial lung disease.17,18 This complication occurred in the case presented here. It has been found that when pulmonary hypertension coexists with interstitial lung disease, its degree may not correlate with the severity of the latter.17 Additionally, pulmonary hypertension, when present, has been found to contribute independently to prognosis and survival.
Mechanic’s hands
In about 30% of patients, the skin of the tips and margins of the fingers becomes thickened, hyperkeratotic, and fissured, the appearance of which is classically described as mechanic’s hands. It is a common manifestation of antisynthetase syndrome and is particularly prominent on the radial side of the index fingers (Figure 1). Biopsy of affected skin shows an interface psoriasiform dermatitis.19 In addition, some dermatomyositis patients with Gottron papules and a heliotrope rash have antisynthetase antibodies.
Raynaud phenomenon
Raynaud phenomenon develops in about 40% of patients. Some have nailfold capillary abnormalities.20 However, persistent or severe digital ischemia leading to digital ulceration or infarction is uncommon.21
Inflammatory arthritis
Arthralgias and arthritis are common (50%), the most common form being a symmetric polyarthritis of the small joints of the hands and feet. It is typically nonerosive but can sometimes be erosive and destructive.20
Because inflammatory arthritis mimics rheumatoid arthritis, antisynthetase syndrome should be considered in rheumatoid factor-negative patients presenting with polyarthritis.
ASSOCIATION WITH MALIGNANCY
Traditional teaching has been that antisynthetase antibody is protective against an underlying malignancy.22,23 However, several recently published case studies have reported various malignancies occurring within 6 to 12 months of the diagnosis of antisynthetase syndrome.7,24 The debate as to whether these are chance associations or causal (a paraneoplastic phenomenon) has not been resolved at this time.24
It is now recommended that patients with antisynthetase syndrome be screened for malignancies as appropriate for the patient’s age and sex. Screening should include a careful history and physical examination, complete blood cell count, comprehensive metabolic panel, chest radiography, mammography, and a gynecologic examination for women.25 If abnormalities are found, a more thorough evaluation for cancer is appropriate.
DIAGNOSIS
Muscle enzyme levels are often elevated
Muscle enzymes (creatine kinase and aldolase) are often elevated. Serum creatine kinase levels can range between 5 to 50 times the upper limit of normal. In an established case, creatine kinase levels along with careful manual muscle strength testing may help evaluate myositis activity. However, in chronic and advanced disease, creatine kinase may be within the normal range despite active myositis, partly because of extensive loss of muscle mass. In myositis, it may be prudent to check both creatine kinase and aldolase; sometimes only serum aldolase level rises, when immune-mediated injury predominantly affects the early regenerative myocytes.26
Judicious use of autoantibody testing
The characteristic clinical presentation is the initial clue to the diagnosis of antisynthetase syndrome, which is then supported by serologic testing.
Injudicious testing for a long list of antibodies should be avoided, as the cost is considerable and it does not influence further management. However, ordering an anti-Jo-1 antibody test in the correct clinical setting is appropriate, as it has high specificity,27,28 and thus can help establish or refute the clinical suspicion of antisynthetase syndrome.
Screening pulmonary function testing and thoracic high-resolution computed tomography for all patients with polymyositis or dermatomyositis is not considered “standard of care” and will likely not be reimbursed by third-party payers. However, in a patient with symptoms and signs of myositis, the presence of an antisynthetase antibody should prompt screening for occult interstitial lung disease, even in the absence of symptoms. As lung disease ultimately determines the prognosis in antisynthetase syndrome, early diagnosis and management is the key. Therefore, these tests would likely be approved to establish the diagnosis of interstitial lung disease and evaluate its severity.
If a myositis patient is also found to have interstitial lung disease or develops mechanic’s hands, the likely diagnosis is antisynthetase syndrome, which can be confirmed by serologic testing for antisynthetase antibodies. Interstitial lung disease in antisynthetase syndrome is often from “nonspecific interstitial pneumonitis”; therefore, medications tested and proven effective for this condition should be approved and reimbursed by payers.29–32
The coexistence of myositis and interstitial lung disease increases the sensitivity of anti-Jo-1 antibody.11 Thus, the clinician can have more confidence in early recognition and initiation of aggressive but targeted disease-modifying therapy.
Various methods can be used for detecting antisynthetase antibodies, with comparable results.28 Anti-Jo-1 antibody testing costs about $140. If that test is negative and antisynthetase syndrome is still suspected, then testing for the non-Jo-1 antisynthetase antibodies may be justified (Table 1). Though the cost of this panel of autoantibodies is about $300, it helps to confirm the diagnosis, and it influences the choice of second-line immunosuppressive agents such as tacrolimus29 and rituximab32 in patients resistant to conventional immunosuppressive agents such as azathioprine and methotrexate.
Often, anti-Ro52 SS-A antibodies are present concurrently in patients with anti-Jo-1 syndrome.33 In observational studies in patients with anti-Jo-1 antibody-associated interstitial lung disease, coexistence of anti-Ro52 SS-A antibody tended to predict a worse pulmonary outcome than in those with anti-Jo-1 antibody alone.34,35
Electromyography
Electromyography not only helps differentiate between myopathic and neuropathic weakness, but it may also support the diagnosis of “inflammatory” myopathy as suggested by prominent muscle membrane irritability (fibrillations, positive sharp waves) and abnormal motor unit action potentials (spontaneous activity showing small, short, polyphasic potentials and early recruitment). However, the findings can be nonspecific, and may even be normal in 10% to 15% of patients.36 Electromyographic abnormalities are most consistently observed in weak proximal muscles, and electromyography is also helpful in selecting a muscle for biopsy. Although no single electromyographic pattern is considered diagnostic for inflammatory myopathy, abnormalities are present in around 90% of patients (Table 2).3
Magnetic resonance imaging
Magnetic resonance imaging may show increased signal intensity in the affected muscles and surrounding tissues (Figure 3).37 Because it lacks sensitivity and specificity, magnetic resonance imaging is not helpful in diagnosing the disease. However, in the correct clinical setting, it may be used to guide muscle biopsy, and it can help in monitoring the disease progress.38
Muscle histopathology
Muscle biopsy, though often helpful, is not always diagnostic, and antisynthetase syndrome should still be suspected in the right clinical context, even in the absence of characteristic pathologic changes.
Biopsy of sites recently studied by electromyography should be avoided, and if the patient has undergone electromyography recently, the contralateral side should be selected for biopsy.
Reports of histopathologic findings in muscle biopsies in patients with antisynthetase syndrome document inflammatory myopathic features (Figure 4). In a series of patients with anti-Jo-1 syndrome, inflammation was noted in all cases, predominantly perimysial in location, with occasional endomysial and perivascular inflammation.39 Many of the inflammatory cells seen were macrophages and lymphocytes, in contrast to the predominantly lymphocytic infiltrates described in classic polymyositis and dermatomyositis. Perifascicular atrophy, similar to what is seen in dermatomyositis, was encountered; however, vascular changes, typical of dermatomyositis, were absent. Occasional degenerating and regenerating muscle fibers were also observed in most cases. Additionally, a characteristic perimysial connective tissue fragmentation was described, a feature less often seen in classic polymyositis and dermatomyositis.39
Pulmonary function testing
If antisynthetase syndrome is suspected or diagnosed, baseline pulmonary function testing (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease (reduced forced vital capacity and carbon monoxide diffusion capacity), and the diagnosis will be substantiated on thoracic high-resolution computed tomography. Respiratory muscle weakness can be detected with upright and supine spirometry.40 Weakness of these muscles contributes to shortness of breath, and patients may need ventilatory support.
Thoracic high-resolution computed tomography
Different patterns of lung injury can be seen in antisynthetase syndrome. Diffuse ground-glass opacification may suggest a nonspecific interstitial pneumonitis pattern, which is the most common form of interstitial lung disease (Figure 2), whereas coarse reticulation or honeycombing correlates with a usual interstitial pneumonitis pattern. Patchy consolidation or air-space disease can occur if cryptogenic organizing pneumonia is the predominant pattern of lung injury.
Swallowing evaluation
A comprehensive swallowing evaluation by a speech therapist may be necessary for evaluation of dysphagia (from oropharyngeal and striated esophageal muscle weakness) and determination of aspiration risk (Table 2).
Lung histopathology
If necessary, a surgical lung biopsy is needed to document the pathologic pattern of injury, including the amount of fibrosis in the lung. Historically, in idiopathic inflammatory myopathy patients in general, this has taken the form of usual interstitial pneumonia, organizing pneumonia, or diffuse alveolar damage.41 With the emergence of the definition of nonspecific interstitial pneumonitis and fibrosis as a documented and accepted pattern, more studies have found this to be the most common pattern of lung injury.16 It is characterized by diffuse involvement of the lung by an interstitial chronic inflammatory infiltrate, a cellular type of nonspecific interstitial pneumonitis that progresses in a uniform pattern to a fibrotic type (Figure 5). This form of fibrosis rarely results in significant remodeling, so-called honeycomb changes. In addition, anti-Jo-1 antibody patients may also have an increased incidence of acute lung injury, including acute diffuse alveolar damage that is often superimposed on the underlying chronic lung disease.42
In patients with pulmonary hypertension, histopathologic studies of the muscular pulmonary arteries often show moderate intimal fibroplasia, suggesting that a pulmonary arteriopathy with intimal thickening and luminal narrowing develops in some of these patients (Figure 5), independent of chronic hypoxic pulmonary vasoconstriction or vascular obstruction due to its entrapment within fibrotic lung tissue.17
TREATMENT
Glucocorticoids are the mainstay
Glucocorticoids are considered the mainstay of treatment. Patients should be advised that long-term use of glucocorticoids is necessary, though the response is variable. It is also important to discuss possible side effects of long-term glucocorticoid use.
Standard practice is to initiate treatment with high doses for the first 4 to 6 weeks to achieve disease control, followed by a slow taper over the next 9 to 12 months to the lowest effective dose to maintain remission. If the patient is profoundly weak, especially with respiratory muscle weakness or significant dysphagia and aspiration risk, hospital admission for intravenous methylprednisolone 1,000 mg daily for 3 to 5 days may be necessary. Otherwise, oral prednisone 1 mg/kg/day would be the usual starting dose.
If the patient’s muscle strength initially improves and then declines weeks to months later despite adequate therapy, glucocorticoid-induced myopathy should be suspected, especially if the muscle enzymes are within the reference range. This is more likely to occur if high-dose prednisone is continued for more than 6 to 8 weeks.
Improvement in muscle strength, which can take several weeks to several months, is a more reliable indicator of response to therapy than the serum creatine kinase level, which may take much longer to normalize. Relying on normalization of the creatine kinase level alone may lead to unnecessary prolongation of high-dose glucocorticoid therapy. It may take several months for the muscle enzymes to normalize, and there is usually a time lag between normalization of muscle enzymes and complete recovery of muscle strength.
Long-term use of high-dose prednisone leads to glucocorticoid-induced osteoporosis. Therefore, patients should receive osteoporosis prophylaxis including antiresorptive therapy with a bisphosphonate. In addition, prophylaxis against Pneumocystis jirovecii is indicated for patients treated with high-dose glucocorticoids.
Additional immunosuppressive agents
Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine and methotrexate are often required, both as glucocorticoid-sparing agents and to achieve adequate disease control.10 Addition of such agents from the outset is particularly necessary in patients with profound muscle weakness or those who have concomitant symptomatic interstitial lung disease.
No randomized controlled trial comparing azathioprine and methotrexate has been conducted to date. Therefore, the choice is based on patient preference, presence of coexisting interstitial lung disease or liver disease, commitment to limit alcohol consumption, and thiopurine methyltransferase status. Most patients need prolonged therapy.
In a randomized clinical trial, concomitant therapy with prednisone and azathioprine resulted in better functional outcomes and a significantly lower prednisone dose requirement for maintenance therapy at 3 years than with prednisone alone.43,44 Although no such randomized study has been conducted using methotrexate, several retrospective studies have demonstrated 70% to 80% response rates, including those for whom monotherapy with glucocorticoids had failed.45,46 The combination of methotrexate and azathioprine may be beneficial in patients who previously had inadequate responses to either of these agents alone.47
For severe pulmonary involvement associated with antisynthetase syndrome, monthly intravenous infusion of cyclophosphamide has been shown to be effective.48,49
Some recent studies established the role of tacrolimus in the treatment of both interstitial lung disease and myositis associated with antisynthetase syndrome.29 Cyclosporine has also been successfully used in a case of interstitial lung disease associated with anti Jo-1 syndrome.30
Rituximab, a monoclonal antibody to Blymphocyte antigen CD20, can also be used successfully in refractory disease,31 including refractory interstitial lung disease.32
In an open-label prospective study, polymyositis refractory to glucocorticoids and multiple conventional immunosuppressive agents responded well to high-dose intravenous immune globulin in the short term.50 However, the antisynthetase antibody status in this cohort was unknown; therefore, no definite conclusion could be drawn about the efficacy of intravenous immune globulin specifically in antisynthetase syndrome.
General measures
In patients with profound muscle weakness, physical therapy and rehabilitation should begin early. The goal is to reduce further muscle wasting from disuse and prevent muscle contractures. Patients with oropharyngeal and esophageal dysmotility should be advised about aspiration precautions and may need a swallow evaluation by a speech therapist; some may need temporary parenteral hyper-alimentation or J-tube insertion.
PROGNOSIS
If skeletal muscle involvement is the sole manifestation of antisynthetase syndrome, patients usually respond to glucocorticoids and immunosuppressive therapy and do fairly well. However, the outcome is not so promising when patients also develop interstitial lung disease, and the severity and type of lung injury usually determine the prognosis. As expected, patients with a progressive course of interstitial lung disease fare poorly, whereas those with a nonprogressive course tend to do relatively better. Older age at onset (> 60 years), presence of a malignancy, and a negative antinuclear antibody test are associated with a poor prognosis.7
Acknowledgment: The authors are grateful to Dr. Stephen Hatem, MD, staff radiologist, musculoskeletal radiology, Cleveland Clinic Imaging Institute, for help in the preparation of the magnetic resonance images. We also thank Dr. Steven Shook, MD, staff neurologist, Cleveland Clinic Neurological Institute, for help in summarizing the EMG findings.
A 66-year-old man was initially seen in clinic in March 2004 with a 5-month history of polyarthritis (affecting the finger joints, wrists, and knees) and several hours of morning stiffness. He also had significant proximal muscle weakness, progressive exertional dyspnea, and a nonproductive cough. There was no history of fever, chills, rash, dysphagia, or sicca symptoms. Findings on initial tests:
- His creatine kinase level was 700 U/L (reference range 30–220), which later rose to 1,664 U/L.
- He was positive for antinuclear antibody with a 5.7 optical density ratio (normal < 1.5) and for anti-Jo-1 antibody.
- An electromyogram was consistent with a necrotizing myopathy. Left rectus femoris biopsy revealed scattered degenerating and regenerating muscle fibers but no evidence of endomysial inflammation.
- On pulmonary function testing, his forced vital capacity was 80% of predicted, and his carbon monoxide diffusion capacity was 67% of predicted.
- High-resolution computed tomography revealed evidence of interstitial lung disease, characterized by bilateral patchy ground-glass opacities suggestive of active alveolitis, most extensive at the lung bases.
- Bronchoalveolar lavage indicated alveolitis, and transbronchial biopsy revealed pathologic changes consistent with cryptogenic organizing pneumonia. All cultures were negative.
This constellation of clinical manifestations, including myositis, interstitial lung disease, and polyarthritis, along with positive anti-Jo-1 antibody, confirmed the diagnosis of antisynthetase syndrome.
In June 2004, for his interstitial lung disease, he was started on daily oral cyclophosphamide along with high-dose oral prednisone. Three months later the skin of the tips and radial margins of his fingers started thickening and cracking, the appearance of which is classically described as “mechanic’s hands,” a well-described manifestation of antisynthetase syndrome (Figure 1).
Cyclophosphamide was continued for about a year. Subsequently, along with prednisone, he sequentially received various other immunosuppressive medications (methotrexate, tacrolimus, mycophenolate mofetil, and rituximab) over the next few years in an attempt to control his progressive interstitial lung disease. All of these agents were only partially and temporarily effective. Ultimately, despite all of these therapies, as his interstitial lung disease progressed, he needed supplemental oxygen and enrollment in a pulmonary rehabilitation program.
In March 2010, he was admitted with worsening dyspnea and significant peripheral edema and was found to have severe pulmonary arterial hypertension. He was started on bosentan. Eight months later sildenafil was added for progressive pulmonary arterial hypertension. However, his oxygenation status continued to decline.
In July 2011, he presented with chills, increasing shortness of breath, and a mild productive cough. As he was severely hypoxic, he was admitted to the intensive care unit and started on mechanical ventilation and broad-spectrum antibiotics. Despite escalation of oxygen therapy, his respiratory status rapidly deteriorated, and he developed hypotension requiring vasopressors. He ultimately died of cardiac arrest secondary to respiratory failure.
A CONSTELLATION OF MANIFESTATIONS
Antisynthetase syndrome, associated with anti-aminoacyl-transfer RNA (tRNA) synthetase antibodies, is characterized by a constellation of manifestations that include myositis, interstitial lung disease, mechanic’s hands, fever, Raynaud phenomenon, and nonerosive symmetric polyarthritis of the small joints.1
Anti-Jo-1 antibody (anti-histidyl-tRNA synthetase) is the most common of the antibodies and also was the first one to be identified (Table 1). It was named after John P, a patient with polymyositis and interstitial lung disease, in whom it was first detected in 1980.2 The onset of the syndrome associated with anti-Jo-1 antibody is often acute, and the myositis is usually steroid-responsive. However, not uncommonly, severe disease can develop over time, with a tendency to relapse and with a poor long-term prognosis.
RARE BUT UNDERRECOGNIZED
The true population prevalence of antisynthetase syndrome is unknown. Because this syndrome is rare, comprehensive epidemiologic studies are difficult to perform.
In several retrospective studies, the annual incidence of idiopathic inflammatory myopathies has been reported to be 2 to 10 new cases per million adults per year.3 Antisynthetase antibodies are detected in 20% to 40% of such cases.4–6 The disease is two to three times more common in women than in men.7
Early diagnosis is difficult because the clinical presentation is varied and often nonspecific, clinically milder disease may escape detection, and many general practitioners lack familiarity with this syndrome and consequently do not recognize it. Moreover, tests for myositis-specific antibodies (including antisynthetase antibodies) are often not ordered in the evaluation of myositis, and hence the diagnosis of antisynthetase syndrome cannot be substantiated. Furthermore, interstitial lung disease can predominate or can be the sole manifestation in the absence of clinically apparent myositis,8–10 and patients can be misdiagnosed as having idiopathic pulmonary fibrosis when underlying antisynthetase syndrome is not suspected. This distinction may be important because these conditions differ in their pathology and treatment. Histologically, the predominant pattern of lung injury in idiopathic pulmonary fibrosis is “usual interstitial pneumonia” which does not respond to immunosuppressive therapy, and hence lung transplantation is the only therapeutic option. On the other hand, in antisynthetase syndrome, the usual pattern of lung injury is “nonspecific interstitial pneumonia,” in which immunosuppressive therapy has a role.
Anti-Jo-1 antibody is detected in 15% to 25% of patients with polymyositis and in up to 70% of myositis patients with concomitant interstitial lung disease.11 Autoantibodies to seven other, less frequently targeted, aminoacyl tRNA synthetases have also been described in patients with polymyositis and interstitial lung disease (Table 1).11,12 In addition, an autoantibody to a 48-kDa transfer RNA-related protein (Wa) has been described.13 These non-Jo-1 antisynthetase antibodies are detected in only about 3% of myositis patients.14
ROLE OF ANTISYNTHETASE ANTIBODIES
Synthetases play a central role in protein synthesis by catalyzing the acetylation of tRNAs. The propensity of organ involvement in antisynthetase syndrome suggests that tissue-specific changes in muscle or lung lead to the production of unique forms of target autoantigens, the aminoacyl-tRNA synthetases. There is evidence that these enzymes themselves may be involved in recruiting both antigen-presenting and inflammatory cells to the site of muscle or lung injury.15 However, the molecular pathway that initiates and propagates this autoimmune response and the specific role of the antisynthetase antibodies in the pathogenesis of this syndrome are presently unknown.
SIX SALIENT CLINICAL FEATURES
There are six predominant clinical manifestations, which may be present at disease onset or appear later as the disease progresses:
- Fever
- Myositis
- Interstitial lung disease
- Mechanic’s hands
- Raynaud phenomenon
- Inflammatory polyarthritis.
There is considerable clinical heterogeneity, and one or other manifestation can predominate or can be the only expression of the syndrome. Furthermore, in the same patient, the individual features can prevail at different times and may develop years after onset of the disease. Therefore, in addition to patients with myositis, it would be important to suspect antisynthetase syndrome in patients presenting with isolated lung involvement (amyopathic interstitial lung disease), as there are therapeutic implications. Studies have demonstrated the efficacy of immunosuppressive agents in interstitial lung disease associated with antisynthetase syndrome (where the predominant pattern of lung injury is “nonspecific interstitial pneumonitis”), whereas lung transplantation has so far been the only treatment option in idiopathic pulmonary fibrosis.
Fever
About 20% of patients have a fever at disease onset or associated with relapses. Sometimes the fever can persist until treatment of antisynthetase syndrome is started.
Myositis
Muscle disease is seen in more than 90% of patients with anti-Jo-1 antisynthetase syndrome. It can be subclinical (in the absence of proximal myopathy), manifested by transient creatine kinase elevation only, which may normalize after therapy is initiated.
However, more commonly, patients develop profound proximal muscle weakness and sometimes muscle pain (Table 2). Weakness of the striated muscles of the upper esophagus, cricopharyngeus, and hypopharynx may cause dysphagia and makes these patients susceptible to aspiration pneumonia. Diaphragmatic and intercostal muscle weakness can contribute to shortness of breath in some patients. Myocarditis has also been reported.
Pulmonary disease
Interstitial lung disease develops in most patients with anti-Jo-1 antisynthetase syndrome, with a reported prevalence of about 90% in one series.16 Patients often present with acute, subacute, or insidious onset of exertional dyspnea. Sometimes there is an intractable nonproductive cough.
At the outset of antisynthetase syndrome, if the patient is profoundly weak because of myopathy or has inflammatory polyarthritis, mobility is significantly compromised, and exertional dyspnea may not be experienced. However, as the interstitial lung disease progresses, shortness of breath becomes overt, more so when the patient’s level of activity improves with treatment of myositis.
Inspiratory crackles on auscultation of the lung bases or changes on chest radiography are relatively insensitive findings and can miss early interstitial lung disease. Therefore, if antisynthetase syndrome is suspected or diagnosed, a baseline pulmonary function test (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease, and the diagnosis can then be confirmed with thoracic high-resolution computed tomography (Figure 2).
Pulmonary hypertension. Recent studies indicate that, similar to patients with other autoimmune rheumatic diseases, pulmonary hypertension can develop in patients with antisynthetase syndrome, with or without concomitant interstitial lung disease.17,18 This complication occurred in the case presented here. It has been found that when pulmonary hypertension coexists with interstitial lung disease, its degree may not correlate with the severity of the latter.17 Additionally, pulmonary hypertension, when present, has been found to contribute independently to prognosis and survival.
Mechanic’s hands
In about 30% of patients, the skin of the tips and margins of the fingers becomes thickened, hyperkeratotic, and fissured, the appearance of which is classically described as mechanic’s hands. It is a common manifestation of antisynthetase syndrome and is particularly prominent on the radial side of the index fingers (Figure 1). Biopsy of affected skin shows an interface psoriasiform dermatitis.19 In addition, some dermatomyositis patients with Gottron papules and a heliotrope rash have antisynthetase antibodies.
Raynaud phenomenon
Raynaud phenomenon develops in about 40% of patients. Some have nailfold capillary abnormalities.20 However, persistent or severe digital ischemia leading to digital ulceration or infarction is uncommon.21
Inflammatory arthritis
Arthralgias and arthritis are common (50%), the most common form being a symmetric polyarthritis of the small joints of the hands and feet. It is typically nonerosive but can sometimes be erosive and destructive.20
Because inflammatory arthritis mimics rheumatoid arthritis, antisynthetase syndrome should be considered in rheumatoid factor-negative patients presenting with polyarthritis.
ASSOCIATION WITH MALIGNANCY
Traditional teaching has been that antisynthetase antibody is protective against an underlying malignancy.22,23 However, several recently published case studies have reported various malignancies occurring within 6 to 12 months of the diagnosis of antisynthetase syndrome.7,24 The debate as to whether these are chance associations or causal (a paraneoplastic phenomenon) has not been resolved at this time.24
It is now recommended that patients with antisynthetase syndrome be screened for malignancies as appropriate for the patient’s age and sex. Screening should include a careful history and physical examination, complete blood cell count, comprehensive metabolic panel, chest radiography, mammography, and a gynecologic examination for women.25 If abnormalities are found, a more thorough evaluation for cancer is appropriate.
DIAGNOSIS
Muscle enzyme levels are often elevated
Muscle enzymes (creatine kinase and aldolase) are often elevated. Serum creatine kinase levels can range between 5 to 50 times the upper limit of normal. In an established case, creatine kinase levels along with careful manual muscle strength testing may help evaluate myositis activity. However, in chronic and advanced disease, creatine kinase may be within the normal range despite active myositis, partly because of extensive loss of muscle mass. In myositis, it may be prudent to check both creatine kinase and aldolase; sometimes only serum aldolase level rises, when immune-mediated injury predominantly affects the early regenerative myocytes.26
Judicious use of autoantibody testing
The characteristic clinical presentation is the initial clue to the diagnosis of antisynthetase syndrome, which is then supported by serologic testing.
Injudicious testing for a long list of antibodies should be avoided, as the cost is considerable and it does not influence further management. However, ordering an anti-Jo-1 antibody test in the correct clinical setting is appropriate, as it has high specificity,27,28 and thus can help establish or refute the clinical suspicion of antisynthetase syndrome.
Screening pulmonary function testing and thoracic high-resolution computed tomography for all patients with polymyositis or dermatomyositis is not considered “standard of care” and will likely not be reimbursed by third-party payers. However, in a patient with symptoms and signs of myositis, the presence of an antisynthetase antibody should prompt screening for occult interstitial lung disease, even in the absence of symptoms. As lung disease ultimately determines the prognosis in antisynthetase syndrome, early diagnosis and management is the key. Therefore, these tests would likely be approved to establish the diagnosis of interstitial lung disease and evaluate its severity.
If a myositis patient is also found to have interstitial lung disease or develops mechanic’s hands, the likely diagnosis is antisynthetase syndrome, which can be confirmed by serologic testing for antisynthetase antibodies. Interstitial lung disease in antisynthetase syndrome is often from “nonspecific interstitial pneumonitis”; therefore, medications tested and proven effective for this condition should be approved and reimbursed by payers.29–32
The coexistence of myositis and interstitial lung disease increases the sensitivity of anti-Jo-1 antibody.11 Thus, the clinician can have more confidence in early recognition and initiation of aggressive but targeted disease-modifying therapy.
Various methods can be used for detecting antisynthetase antibodies, with comparable results.28 Anti-Jo-1 antibody testing costs about $140. If that test is negative and antisynthetase syndrome is still suspected, then testing for the non-Jo-1 antisynthetase antibodies may be justified (Table 1). Though the cost of this panel of autoantibodies is about $300, it helps to confirm the diagnosis, and it influences the choice of second-line immunosuppressive agents such as tacrolimus29 and rituximab32 in patients resistant to conventional immunosuppressive agents such as azathioprine and methotrexate.
Often, anti-Ro52 SS-A antibodies are present concurrently in patients with anti-Jo-1 syndrome.33 In observational studies in patients with anti-Jo-1 antibody-associated interstitial lung disease, coexistence of anti-Ro52 SS-A antibody tended to predict a worse pulmonary outcome than in those with anti-Jo-1 antibody alone.34,35
Electromyography
Electromyography not only helps differentiate between myopathic and neuropathic weakness, but it may also support the diagnosis of “inflammatory” myopathy as suggested by prominent muscle membrane irritability (fibrillations, positive sharp waves) and abnormal motor unit action potentials (spontaneous activity showing small, short, polyphasic potentials and early recruitment). However, the findings can be nonspecific, and may even be normal in 10% to 15% of patients.36 Electromyographic abnormalities are most consistently observed in weak proximal muscles, and electromyography is also helpful in selecting a muscle for biopsy. Although no single electromyographic pattern is considered diagnostic for inflammatory myopathy, abnormalities are present in around 90% of patients (Table 2).3
Magnetic resonance imaging
Magnetic resonance imaging may show increased signal intensity in the affected muscles and surrounding tissues (Figure 3).37 Because it lacks sensitivity and specificity, magnetic resonance imaging is not helpful in diagnosing the disease. However, in the correct clinical setting, it may be used to guide muscle biopsy, and it can help in monitoring the disease progress.38
Muscle histopathology
Muscle biopsy, though often helpful, is not always diagnostic, and antisynthetase syndrome should still be suspected in the right clinical context, even in the absence of characteristic pathologic changes.
Biopsy of sites recently studied by electromyography should be avoided, and if the patient has undergone electromyography recently, the contralateral side should be selected for biopsy.
Reports of histopathologic findings in muscle biopsies in patients with antisynthetase syndrome document inflammatory myopathic features (Figure 4). In a series of patients with anti-Jo-1 syndrome, inflammation was noted in all cases, predominantly perimysial in location, with occasional endomysial and perivascular inflammation.39 Many of the inflammatory cells seen were macrophages and lymphocytes, in contrast to the predominantly lymphocytic infiltrates described in classic polymyositis and dermatomyositis. Perifascicular atrophy, similar to what is seen in dermatomyositis, was encountered; however, vascular changes, typical of dermatomyositis, were absent. Occasional degenerating and regenerating muscle fibers were also observed in most cases. Additionally, a characteristic perimysial connective tissue fragmentation was described, a feature less often seen in classic polymyositis and dermatomyositis.39
Pulmonary function testing
If antisynthetase syndrome is suspected or diagnosed, baseline pulmonary function testing (spirometry and carbon monoxide diffusion capacity) is indicated. It will often detect occult interstitial lung disease (reduced forced vital capacity and carbon monoxide diffusion capacity), and the diagnosis will be substantiated on thoracic high-resolution computed tomography. Respiratory muscle weakness can be detected with upright and supine spirometry.40 Weakness of these muscles contributes to shortness of breath, and patients may need ventilatory support.
Thoracic high-resolution computed tomography
Different patterns of lung injury can be seen in antisynthetase syndrome. Diffuse ground-glass opacification may suggest a nonspecific interstitial pneumonitis pattern, which is the most common form of interstitial lung disease (Figure 2), whereas coarse reticulation or honeycombing correlates with a usual interstitial pneumonitis pattern. Patchy consolidation or air-space disease can occur if cryptogenic organizing pneumonia is the predominant pattern of lung injury.
Swallowing evaluation
A comprehensive swallowing evaluation by a speech therapist may be necessary for evaluation of dysphagia (from oropharyngeal and striated esophageal muscle weakness) and determination of aspiration risk (Table 2).
Lung histopathology
If necessary, a surgical lung biopsy is needed to document the pathologic pattern of injury, including the amount of fibrosis in the lung. Historically, in idiopathic inflammatory myopathy patients in general, this has taken the form of usual interstitial pneumonia, organizing pneumonia, or diffuse alveolar damage.41 With the emergence of the definition of nonspecific interstitial pneumonitis and fibrosis as a documented and accepted pattern, more studies have found this to be the most common pattern of lung injury.16 It is characterized by diffuse involvement of the lung by an interstitial chronic inflammatory infiltrate, a cellular type of nonspecific interstitial pneumonitis that progresses in a uniform pattern to a fibrotic type (Figure 5). This form of fibrosis rarely results in significant remodeling, so-called honeycomb changes. In addition, anti-Jo-1 antibody patients may also have an increased incidence of acute lung injury, including acute diffuse alveolar damage that is often superimposed on the underlying chronic lung disease.42
In patients with pulmonary hypertension, histopathologic studies of the muscular pulmonary arteries often show moderate intimal fibroplasia, suggesting that a pulmonary arteriopathy with intimal thickening and luminal narrowing develops in some of these patients (Figure 5), independent of chronic hypoxic pulmonary vasoconstriction or vascular obstruction due to its entrapment within fibrotic lung tissue.17
TREATMENT
Glucocorticoids are the mainstay
Glucocorticoids are considered the mainstay of treatment. Patients should be advised that long-term use of glucocorticoids is necessary, though the response is variable. It is also important to discuss possible side effects of long-term glucocorticoid use.
Standard practice is to initiate treatment with high doses for the first 4 to 6 weeks to achieve disease control, followed by a slow taper over the next 9 to 12 months to the lowest effective dose to maintain remission. If the patient is profoundly weak, especially with respiratory muscle weakness or significant dysphagia and aspiration risk, hospital admission for intravenous methylprednisolone 1,000 mg daily for 3 to 5 days may be necessary. Otherwise, oral prednisone 1 mg/kg/day would be the usual starting dose.
If the patient’s muscle strength initially improves and then declines weeks to months later despite adequate therapy, glucocorticoid-induced myopathy should be suspected, especially if the muscle enzymes are within the reference range. This is more likely to occur if high-dose prednisone is continued for more than 6 to 8 weeks.
Improvement in muscle strength, which can take several weeks to several months, is a more reliable indicator of response to therapy than the serum creatine kinase level, which may take much longer to normalize. Relying on normalization of the creatine kinase level alone may lead to unnecessary prolongation of high-dose glucocorticoid therapy. It may take several months for the muscle enzymes to normalize, and there is usually a time lag between normalization of muscle enzymes and complete recovery of muscle strength.
Long-term use of high-dose prednisone leads to glucocorticoid-induced osteoporosis. Therefore, patients should receive osteoporosis prophylaxis including antiresorptive therapy with a bisphosphonate. In addition, prophylaxis against Pneumocystis jirovecii is indicated for patients treated with high-dose glucocorticoids.
Additional immunosuppressive agents
Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine and methotrexate are often required, both as glucocorticoid-sparing agents and to achieve adequate disease control.10 Addition of such agents from the outset is particularly necessary in patients with profound muscle weakness or those who have concomitant symptomatic interstitial lung disease.
No randomized controlled trial comparing azathioprine and methotrexate has been conducted to date. Therefore, the choice is based on patient preference, presence of coexisting interstitial lung disease or liver disease, commitment to limit alcohol consumption, and thiopurine methyltransferase status. Most patients need prolonged therapy.
In a randomized clinical trial, concomitant therapy with prednisone and azathioprine resulted in better functional outcomes and a significantly lower prednisone dose requirement for maintenance therapy at 3 years than with prednisone alone.43,44 Although no such randomized study has been conducted using methotrexate, several retrospective studies have demonstrated 70% to 80% response rates, including those for whom monotherapy with glucocorticoids had failed.45,46 The combination of methotrexate and azathioprine may be beneficial in patients who previously had inadequate responses to either of these agents alone.47
For severe pulmonary involvement associated with antisynthetase syndrome, monthly intravenous infusion of cyclophosphamide has been shown to be effective.48,49
Some recent studies established the role of tacrolimus in the treatment of both interstitial lung disease and myositis associated with antisynthetase syndrome.29 Cyclosporine has also been successfully used in a case of interstitial lung disease associated with anti Jo-1 syndrome.30
Rituximab, a monoclonal antibody to Blymphocyte antigen CD20, can also be used successfully in refractory disease,31 including refractory interstitial lung disease.32
In an open-label prospective study, polymyositis refractory to glucocorticoids and multiple conventional immunosuppressive agents responded well to high-dose intravenous immune globulin in the short term.50 However, the antisynthetase antibody status in this cohort was unknown; therefore, no definite conclusion could be drawn about the efficacy of intravenous immune globulin specifically in antisynthetase syndrome.
General measures
In patients with profound muscle weakness, physical therapy and rehabilitation should begin early. The goal is to reduce further muscle wasting from disuse and prevent muscle contractures. Patients with oropharyngeal and esophageal dysmotility should be advised about aspiration precautions and may need a swallow evaluation by a speech therapist; some may need temporary parenteral hyper-alimentation or J-tube insertion.
PROGNOSIS
If skeletal muscle involvement is the sole manifestation of antisynthetase syndrome, patients usually respond to glucocorticoids and immunosuppressive therapy and do fairly well. However, the outcome is not so promising when patients also develop interstitial lung disease, and the severity and type of lung injury usually determine the prognosis. As expected, patients with a progressive course of interstitial lung disease fare poorly, whereas those with a nonprogressive course tend to do relatively better. Older age at onset (> 60 years), presence of a malignancy, and a negative antinuclear antibody test are associated with a poor prognosis.7
Acknowledgment: The authors are grateful to Dr. Stephen Hatem, MD, staff radiologist, musculoskeletal radiology, Cleveland Clinic Imaging Institute, for help in the preparation of the magnetic resonance images. We also thank Dr. Steven Shook, MD, staff neurologist, Cleveland Clinic Neurological Institute, for help in summarizing the EMG findings.
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011; 13:175–181.
- Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 1980; 23:881–888.
- Nagaraju K, Lundberg IE. Inflammatory diseases of muscle and other myopathies. In:Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: Saunders; 2008:1353–1380.
- Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001; 60:116–123.
- Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum 1996; 39:292–296.
- Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996; 39:1507–1518.
- Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ. Clinical heterogeneity and prognostic features of South Australian patients with antisynthetase autoantibodies. Intern Med J 2011; 41:674–679.
- Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 1996; 26:459–467.
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006; 39:233–241.
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008; 63:53–59.
- Targoff IN. Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 2000; 12:475–481.
- Ancuta CM, Ancuta E, Chirieac RM. Aminoacyl-tRNA synthetases in idiopathic inflammatory myopathies: an update on immunopathogenic significance, clinical and therapeutic implications. In:Gran JT, editor. Idiopathic Inflammatory Myopathies - Recent Developments. Rijeka, Croatia: InTech; 2011:77–90.
- Kajihara M, Kuwana M, Tokuda H, et al. Myositis and interstitial lung disease associated with autoantibody to a transfer RNA-related protein Wa. J Rheumatol 2000; 27:2707–2710.
- Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 2006; 54:2004–2009.
- Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Rep 2003; 5:425–430.
- Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 2010; 23:874–880.
- Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res (Hoboken) 2010; 62:425–429.
- Minai OA. Pulmonary hypertension in polymyositis-dermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 2009; 18:1006–1010.
- Bugatti L, De Angelis R, Filosa G, Salaffi F. Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis. Indian J Dermatol Venereol Leprol 2005; 71:137–138.
- Mumm GE, McKown KM, Bell CL. Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 2010; 16:307–312.
- Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 1992; 35:449–456.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991; 70:360–374.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol 2001; 144:825–831.
- Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol 2008; 35:169–171.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010; 22:627–632.
- Casciola-Rosen L, Hall JC, Mammen AL, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012; 30:548–553.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci 2005; 1050:380–388.
- Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity 2005; 38:73–78.
- Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005; 52:2439–2446.
- Jankowska M, Butto B, Debska-Slizien A, Rutkowski B. Beneficial effect of treatment with cyclosporin A in a case of refractory antisynthetase syndrome. Rheumatol Int 2007; 27:775–780.
- Limaye V, Hissaria P, Liew CL, Koszyka B. Efficacy of rituximab in refractory antisynthetase syndrome. Intern Med J 2012; 42:e4–e7.
- Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012; 106:581–587.
- Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ, Van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 1997; 109:32–40.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006; 39:249–253.
- Váncsa A, Csípo I, Németh J, Dévényi K, Gergely L, Dankó K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009; 29:989–994.
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977; 56:255–286.
- Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 1997; 9:475–485.
- O’Connell MJ. Whole-body MR imaging in the diagnosis of polymyositis. Am J Roentgenol 2002; 179:967–971.
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000; 68:472–478.
- Fromageot C, Lofaso F, Annane D, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001; 82:123–128.
- Leslie KO. Historical perspective: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest 2005; 128(suppl 1):513S–519S.
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217.
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 1980; 92:365–369.
- Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum 1981; 24:45–48.
- Metzger AL, Bohan A, Goldberg LS, Bluestone R, Pearson CM. Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 1974; 81:182–189.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med 1993; 94:379–387.
- Villalba L, Hicks JE, Adams EM, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum 1998; 41:392–399.
- Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007; 46:124–130.
- al-Janadi M, Smith CD, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989; 16:1592–1596.
- Cherin P, Pelletier S, Teixeira A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum 2002; 46:467–474.
- Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep 2011; 13:175–181.
- Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 1980; 23:881–888.
- Nagaraju K, Lundberg IE. Inflammatory diseases of muscle and other myopathies. In:Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, Sergent JS, editors. Kelley’s Textbook of Rheumatology. Philadelphia, PA: Saunders; 2008:1353–1380.
- Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 2001; 60:116–123.
- Vázquez-Abad D, Rothfield NF. Sensitivity and specificity of anti-Jo-1 antibodies in autoimmune diseases with myositis. Arthritis Rheum 1996; 39:292–296.
- Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996; 39:1507–1518.
- Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ. Clinical heterogeneity and prognostic features of South Australian patients with antisynthetase autoantibodies. Intern Med J 2011; 41:674–679.
- Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 1996; 26:459–467.
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006; 39:233–241.
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008; 63:53–59.
- Targoff IN. Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 2000; 12:475–481.
- Ancuta CM, Ancuta E, Chirieac RM. Aminoacyl-tRNA synthetases in idiopathic inflammatory myopathies: an update on immunopathogenic significance, clinical and therapeutic implications. In:Gran JT, editor. Idiopathic Inflammatory Myopathies - Recent Developments. Rijeka, Croatia: InTech; 2011:77–90.
- Kajihara M, Kuwana M, Tokuda H, et al. Myositis and interstitial lung disease associated with autoantibody to a transfer RNA-related protein Wa. J Rheumatol 2000; 27:2707–2710.
- Yamasaki Y, Yamada H, Nozaki T, et al. Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 2006; 54:2004–2009.
- Ascherman DP. The role of Jo-1 in the immunopathogenesis of polymyositis: current hypotheses. Curr Rheumatol Rep 2003; 5:425–430.
- Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol 2010; 23:874–880.
- Chatterjee S, Farver C. Severe pulmonary hypertension in anti-Jo-1 syndrome. Arthritis Care Res (Hoboken) 2010; 62:425–429.
- Minai OA. Pulmonary hypertension in polymyositis-dermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 2009; 18:1006–1010.
- Bugatti L, De Angelis R, Filosa G, Salaffi F. Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis. Indian J Dermatol Venereol Leprol 2005; 71:137–138.
- Mumm GE, McKown KM, Bell CL. Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 2010; 16:307–312.
- Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M. Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 1992; 35:449–456.
- Love LA, Leff RL, Fraser DD, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991; 70:360–374.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol 2001; 144:825–831.
- Legault D, McDermott J, Crous-Tsanaclis AM, Boire G. Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome. J Rheumatol 2008; 35:169–171.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, Labrador-Horrillo M. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol 2010; 22:627–632.
- Casciola-Rosen L, Hall JC, Mammen AL, Christopher-Stine L, Rosen A. Isolated elevation of aldolase in the serum of myositis patients: a potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012; 30:548–553.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann N Y Acad Sci 2005; 1050:380–388.
- Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity 2005; 38:73–78.
- Wilkes MR, Sereika SM, Fertig N, Lucas MR, Oddis CV. Treatment of antisynthetase-associated interstitial lung disease with tacrolimus. Arthritis Rheum 2005; 52:2439–2446.
- Jankowska M, Butto B, Debska-Slizien A, Rutkowski B. Beneficial effect of treatment with cyclosporin A in a case of refractory antisynthetase syndrome. Rheumatol Int 2007; 27:775–780.
- Limaye V, Hissaria P, Liew CL, Koszyka B. Efficacy of rituximab in refractory antisynthetase syndrome. Intern Med J 2012; 42:e4–e7.
- Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012; 106:581–587.
- Rutjes SA, Vree Egberts WT, Jongen P, Van Den Hoogen F, Pruijn GJ, Van Venrooij WJ. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 1997; 109:32–40.
- La Corte R, Lo Mo Naco A, Locaputo A, Dolzani F, Trotta F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 2006; 39:249–253.
- Váncsa A, Csípo I, Németh J, Dévényi K, Gergely L, Dankó K. Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 2009; 29:989–994.
- Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977; 56:255–286.
- Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 1997; 9:475–485.
- O’Connell MJ. Whole-body MR imaging in the diagnosis of polymyositis. Am J Roentgenol 2002; 179:967–971.
- Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry 2000; 68:472–478.
- Fromageot C, Lofaso F, Annane D, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001; 82:123–128.
- Leslie KO. Historical perspective: a pathologic approach to the classification of idiopathic interstitial pneumonias. Chest 2005; 128(suppl 1):513S–519S.
- Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000; 162:2213–2217.
- Bunch TW, Worthington JW, Combs JJ, Ilstrup DM, Engel AG. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 1980; 92:365–369.
- Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum 1981; 24:45–48.
- Metzger AL, Bohan A, Goldberg LS, Bluestone R, Pearson CM. Polymyositis and dermatomyositis: combined methotrexate and corticosteroid therapy. Ann Intern Med 1974; 81:182–189.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med 1993; 94:379–387.
- Villalba L, Hicks JE, Adams EM, et al. Treatment of refractory myositis: a randomized crossover study of two new cytotoxic regimens. Arthritis Rheum 1998; 41:392–399.
- Yamasaki Y, Yamada H, Yamasaki M, et al. Intravenous cyclophosphamide therapy for progressive interstitial pneumonia in patients with polymyositis/dermatomyositis. Rheumatology (Oxford) 2007; 46:124–130.
- al-Janadi M, Smith CD, Karsh J. Cyclophosphamide treatment of interstitial pulmonary fibrosis in polymyositis/dermatomyositis. J Rheumatol 1989; 16:1592–1596.
- Cherin P, Pelletier S, Teixeira A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum 2002; 46:467–474.
KEY POINTS
- Antisynthetase syndrome can present with a wide variety of clinical manifestations, including myositis and interstitial lung disease.
- The type and severity of interstitial lung disease usually determine the long-term outcome.
- In the appropriate clinical setting, the diagnosis is usually confirmed by the detection of antibodies to various aminoacyl-transfer RNA synthetases, anti-Jo-1 antibody being the most common.
- Although glucocorticoids are considered the mainstay of treatment, additional immunosuppressive agents such as azathioprine or methotrexate are often required as steroid-sparing agents and also to achieve disease control.
- In the case of severe pulmonary involvement, cyclophosphamide is recommended.
Mycoplasma pneumoniae
Mycoplasma pneumoniae is a cell wall–deficient pleomorphic bacterium and well-reported cause of respiratory tract infection in the school-aged child. Symptoms are variable, and clinical presentations run the gamut from upper respiratory (usually self-limited) and lower respiratory tract involvement (pneumonia) to unusual manifestations including nervous system disease (encephalitis, cerebellar ataxia, transverse myelitis), hemolytic anemia, Stevens-Johnson syndrome, and myocarditis/pericarditis.
Pneumonia occurs in 10% of infected school-aged children, and cough can persist for 3-4 weeks; some children wheeze in the setting of Mycoplasma infection. Radiographic patterns of disease are variable; patchy alveolar infiltrates with small pleural effusions are often described. Consolidated pneumonia, large effusions, and hilar adenopathy are uncommonly reported, and severe disease has been described in certain patient populations, including those with sickle cell disease, children with Down syndrome, and those with immunodeficiencies. The acute chest presentation has been associated with M. pneumoniae in children with sickle cell anemia and prolonged hospitalizations (mean, 10 days), and the need for transfusion and mechanical ventilation was noted in 82% and 6%, respectively, in one study (Pediatrics 2003;112(1 Pt 1):87-95). Community clusters of pneumonia are reported in school-aged children, and in Rhode Island, an outbreak was reported in children from four schools; 76 had pneumonia and 3 had encephalitis (J. Infect. Dis. 2008;198:1365-74).
Considering this is a common pathogen, there are a number of questions regarding the scope of disease and impact of treatment that are incompletely answered. The first problem is that it is hard to confirm diagnostically. Culture is technically difficult, the organism takes up to 3 weeks to grow, and the diagnostic test is offered in very few labs. The old-fashioned cold agglutinin test has a low sensitivity and specificity; an increase in titers can be seen during a variety of viral infections. Polymerase chain reaction (PCR) on respiratory secretions is increasingly available; sensitivity and specificity are said to be 80% and 100%, respectively. The organism can persist in the respiratory tract for several weeks though, even after treatment, so PCR can remain positive for 2-3 weeks. This makes it hard to use PCR to confirm M. pneumoniae as the etiologic agent, especially in the setting of unusual clinical presentations. Serologic testing is often ordered and hard to interpret. False positive IgM antibody tests are not uncommon, and IgM antibody can persist for months. Outside of PCR and culture, acute and convalescent specimens can be used diagnostically, and a fourfold IgG antibody rise is consistent with acute infection.
Macrolides are regarded as the preferred treatment for M. pneumoniae pneumonia, but several studies question whether treatment impacts the clinical course. This may be due to the inherent difficulty of confirming M. pneumoniae as the etiologic agent, as most studies used serology to confirm the diagnosis. In countries outside the United States, macrolide resistance is well reported, and this may be underappreciated in the United States. We recently cared for a teenager with Down syndrome with pneumonia caused by M. pneumoniae who had a protracted clinical course. Fever and hypoxemia were persistent over a several-week period despite two courses of azithromycin and exclusion of virus, bacteria, and fungal pathogens. Bronchoalveolar lavage was performed, M. pneumoniae was detected by PCR, and macrolide resistance was confirmed. Levofloxacin was given, and she recovered over the next week.
Macrolide resistance is commonly reported outside the United States; rates in China are reported to be greater than 90%, in Japan 80%, and in Europe, between 15% and 25%. A recent study from Greg Storch and his colleagues (Pediatr. Infect. Dis. J. 2012;31:409-10) documented macrolide resistance in 8% of respiratory samples collected between 2007 and 2010 (49 patients; mean age, 10 years), noting the resistance rate was 3% in 2007-2008 and 15% in 2009-2010. A recently published study of data from Canada reported that 12.1% of M. pneumoniae–positive specimens collected between 2010 and January 2012 carried nucleotide mutations associated with macrolide resistance in the 23S rRNA gene (Emerg. Infect. Dis. 2013 September [doi: 10.3201/eid1909.121466]). Anecdotal studies suggest that patients with macrolide-resistant M. pneumoniae infection clinically improve when given doxycycline (or minocycline) or levofloxacin.
A number of clinical questions regarding M. pneumoniae may be answered more definitively in the future, but we need more easily available diagnostics (PCR is a good start), routinely accessible susceptibility data, and a good randomized controlled study to investigate the question of whether treatment shortens the course of disease.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri-Kansas City. She said she has no conflicts of interest to disclose. E-mail her at [email protected].
Mycoplasma pneumoniae is a cell wall–deficient pleomorphic bacterium and well-reported cause of respiratory tract infection in the school-aged child. Symptoms are variable, and clinical presentations run the gamut from upper respiratory (usually self-limited) and lower respiratory tract involvement (pneumonia) to unusual manifestations including nervous system disease (encephalitis, cerebellar ataxia, transverse myelitis), hemolytic anemia, Stevens-Johnson syndrome, and myocarditis/pericarditis.
Pneumonia occurs in 10% of infected school-aged children, and cough can persist for 3-4 weeks; some children wheeze in the setting of Mycoplasma infection. Radiographic patterns of disease are variable; patchy alveolar infiltrates with small pleural effusions are often described. Consolidated pneumonia, large effusions, and hilar adenopathy are uncommonly reported, and severe disease has been described in certain patient populations, including those with sickle cell disease, children with Down syndrome, and those with immunodeficiencies. The acute chest presentation has been associated with M. pneumoniae in children with sickle cell anemia and prolonged hospitalizations (mean, 10 days), and the need for transfusion and mechanical ventilation was noted in 82% and 6%, respectively, in one study (Pediatrics 2003;112(1 Pt 1):87-95). Community clusters of pneumonia are reported in school-aged children, and in Rhode Island, an outbreak was reported in children from four schools; 76 had pneumonia and 3 had encephalitis (J. Infect. Dis. 2008;198:1365-74).
Considering this is a common pathogen, there are a number of questions regarding the scope of disease and impact of treatment that are incompletely answered. The first problem is that it is hard to confirm diagnostically. Culture is technically difficult, the organism takes up to 3 weeks to grow, and the diagnostic test is offered in very few labs. The old-fashioned cold agglutinin test has a low sensitivity and specificity; an increase in titers can be seen during a variety of viral infections. Polymerase chain reaction (PCR) on respiratory secretions is increasingly available; sensitivity and specificity are said to be 80% and 100%, respectively. The organism can persist in the respiratory tract for several weeks though, even after treatment, so PCR can remain positive for 2-3 weeks. This makes it hard to use PCR to confirm M. pneumoniae as the etiologic agent, especially in the setting of unusual clinical presentations. Serologic testing is often ordered and hard to interpret. False positive IgM antibody tests are not uncommon, and IgM antibody can persist for months. Outside of PCR and culture, acute and convalescent specimens can be used diagnostically, and a fourfold IgG antibody rise is consistent with acute infection.
Macrolides are regarded as the preferred treatment for M. pneumoniae pneumonia, but several studies question whether treatment impacts the clinical course. This may be due to the inherent difficulty of confirming M. pneumoniae as the etiologic agent, as most studies used serology to confirm the diagnosis. In countries outside the United States, macrolide resistance is well reported, and this may be underappreciated in the United States. We recently cared for a teenager with Down syndrome with pneumonia caused by M. pneumoniae who had a protracted clinical course. Fever and hypoxemia were persistent over a several-week period despite two courses of azithromycin and exclusion of virus, bacteria, and fungal pathogens. Bronchoalveolar lavage was performed, M. pneumoniae was detected by PCR, and macrolide resistance was confirmed. Levofloxacin was given, and she recovered over the next week.
Macrolide resistance is commonly reported outside the United States; rates in China are reported to be greater than 90%, in Japan 80%, and in Europe, between 15% and 25%. A recent study from Greg Storch and his colleagues (Pediatr. Infect. Dis. J. 2012;31:409-10) documented macrolide resistance in 8% of respiratory samples collected between 2007 and 2010 (49 patients; mean age, 10 years), noting the resistance rate was 3% in 2007-2008 and 15% in 2009-2010. A recently published study of data from Canada reported that 12.1% of M. pneumoniae–positive specimens collected between 2010 and January 2012 carried nucleotide mutations associated with macrolide resistance in the 23S rRNA gene (Emerg. Infect. Dis. 2013 September [doi: 10.3201/eid1909.121466]). Anecdotal studies suggest that patients with macrolide-resistant M. pneumoniae infection clinically improve when given doxycycline (or minocycline) or levofloxacin.
A number of clinical questions regarding M. pneumoniae may be answered more definitively in the future, but we need more easily available diagnostics (PCR is a good start), routinely accessible susceptibility data, and a good randomized controlled study to investigate the question of whether treatment shortens the course of disease.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri-Kansas City. She said she has no conflicts of interest to disclose. E-mail her at [email protected].
Mycoplasma pneumoniae is a cell wall–deficient pleomorphic bacterium and well-reported cause of respiratory tract infection in the school-aged child. Symptoms are variable, and clinical presentations run the gamut from upper respiratory (usually self-limited) and lower respiratory tract involvement (pneumonia) to unusual manifestations including nervous system disease (encephalitis, cerebellar ataxia, transverse myelitis), hemolytic anemia, Stevens-Johnson syndrome, and myocarditis/pericarditis.
Pneumonia occurs in 10% of infected school-aged children, and cough can persist for 3-4 weeks; some children wheeze in the setting of Mycoplasma infection. Radiographic patterns of disease are variable; patchy alveolar infiltrates with small pleural effusions are often described. Consolidated pneumonia, large effusions, and hilar adenopathy are uncommonly reported, and severe disease has been described in certain patient populations, including those with sickle cell disease, children with Down syndrome, and those with immunodeficiencies. The acute chest presentation has been associated with M. pneumoniae in children with sickle cell anemia and prolonged hospitalizations (mean, 10 days), and the need for transfusion and mechanical ventilation was noted in 82% and 6%, respectively, in one study (Pediatrics 2003;112(1 Pt 1):87-95). Community clusters of pneumonia are reported in school-aged children, and in Rhode Island, an outbreak was reported in children from four schools; 76 had pneumonia and 3 had encephalitis (J. Infect. Dis. 2008;198:1365-74).
Considering this is a common pathogen, there are a number of questions regarding the scope of disease and impact of treatment that are incompletely answered. The first problem is that it is hard to confirm diagnostically. Culture is technically difficult, the organism takes up to 3 weeks to grow, and the diagnostic test is offered in very few labs. The old-fashioned cold agglutinin test has a low sensitivity and specificity; an increase in titers can be seen during a variety of viral infections. Polymerase chain reaction (PCR) on respiratory secretions is increasingly available; sensitivity and specificity are said to be 80% and 100%, respectively. The organism can persist in the respiratory tract for several weeks though, even after treatment, so PCR can remain positive for 2-3 weeks. This makes it hard to use PCR to confirm M. pneumoniae as the etiologic agent, especially in the setting of unusual clinical presentations. Serologic testing is often ordered and hard to interpret. False positive IgM antibody tests are not uncommon, and IgM antibody can persist for months. Outside of PCR and culture, acute and convalescent specimens can be used diagnostically, and a fourfold IgG antibody rise is consistent with acute infection.
Macrolides are regarded as the preferred treatment for M. pneumoniae pneumonia, but several studies question whether treatment impacts the clinical course. This may be due to the inherent difficulty of confirming M. pneumoniae as the etiologic agent, as most studies used serology to confirm the diagnosis. In countries outside the United States, macrolide resistance is well reported, and this may be underappreciated in the United States. We recently cared for a teenager with Down syndrome with pneumonia caused by M. pneumoniae who had a protracted clinical course. Fever and hypoxemia were persistent over a several-week period despite two courses of azithromycin and exclusion of virus, bacteria, and fungal pathogens. Bronchoalveolar lavage was performed, M. pneumoniae was detected by PCR, and macrolide resistance was confirmed. Levofloxacin was given, and she recovered over the next week.
Macrolide resistance is commonly reported outside the United States; rates in China are reported to be greater than 90%, in Japan 80%, and in Europe, between 15% and 25%. A recent study from Greg Storch and his colleagues (Pediatr. Infect. Dis. J. 2012;31:409-10) documented macrolide resistance in 8% of respiratory samples collected between 2007 and 2010 (49 patients; mean age, 10 years), noting the resistance rate was 3% in 2007-2008 and 15% in 2009-2010. A recently published study of data from Canada reported that 12.1% of M. pneumoniae–positive specimens collected between 2010 and January 2012 carried nucleotide mutations associated with macrolide resistance in the 23S rRNA gene (Emerg. Infect. Dis. 2013 September [doi: 10.3201/eid1909.121466]). Anecdotal studies suggest that patients with macrolide-resistant M. pneumoniae infection clinically improve when given doxycycline (or minocycline) or levofloxacin.
A number of clinical questions regarding M. pneumoniae may be answered more definitively in the future, but we need more easily available diagnostics (PCR is a good start), routinely accessible susceptibility data, and a good randomized controlled study to investigate the question of whether treatment shortens the course of disease.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri-Kansas City. She said she has no conflicts of interest to disclose. E-mail her at [email protected].
Managing Bipolar Depression: An Evidence-Based Approach
Bipolar disorder is characterized by the cyclical occurrence of elevated (manic or hypomanic) and depressed mood states. The illness, which includes the bipolar I and bipolar II subtypes, exacts a heavy toll in terms of quality of life, functioning, morbidity, comorbidity, and mortality.1 Depressive episodes and symptoms deserve particular attention: Not only do they dominate the long-term course of the illness; they are associated with similar or greater psychosocial impairment than corresponding levels of manic or hypomanic symptoms.1
Bipolar disorder is characterized by the cyclical occurrence of elevated (manic or hypomanic) and depressed mood states. The illness, which includes the bipolar I and bipolar II subtypes, exacts a heavy toll in terms of quality of life, functioning, morbidity, comorbidity, and mortality.1 Depressive episodes and symptoms deserve particular attention: Not only do they dominate the long-term course of the illness; they are associated with similar or greater psychosocial impairment than corresponding levels of manic or hypomanic symptoms.1
Bipolar disorder is characterized by the cyclical occurrence of elevated (manic or hypomanic) and depressed mood states. The illness, which includes the bipolar I and bipolar II subtypes, exacts a heavy toll in terms of quality of life, functioning, morbidity, comorbidity, and mortality.1 Depressive episodes and symptoms deserve particular attention: Not only do they dominate the long-term course of the illness; they are associated with similar or greater psychosocial impairment than corresponding levels of manic or hypomanic symptoms.1
Tailored therapy needed to conquer IPV
Conducting effective family therapy is never a one-size-fits-all proposition. In our work with families, we must keep in mind that, just as the dynamics in each family are different, so, too, must be our approaches. This is particularly the case for people who are experiencing intimate partner violence, or IPV.
The cases of three patients described below illustrate that point well; I’ve changed the patients’ names to protect their anonymity.
Belkis
She is a hardworking immigrant making minimum wage as a housekeeper. She presents to her psychiatric outpatient appointment with complaints of being sad and anxious. When asked about her husband and their relationship, she says tentatively that they disagree about things. She doesn’t acknowledge any abuse until she is asked directly, then she hangs her head down and looks ashamed. "He tells me I am not a good wife." With encouragement, she admits that she would like to leave him but has nowhere to go and is afraid that he will really hurt her if she tries to leave. "I tried before, and he threatened to kill me if I tried again."
Melanie
She is a well-educated women working as a writer and has a good income. She presents to her psychiatric outpatient appointment with complaints of being sad and anxious. When asked about her husband and their relationship, she says that he is abusive to her. She states that she would like to come into therapy to figure out why she has not been able to leave him. "What ties me to him?" She says her friends tell her she should leave. "There must be a reason I stay; can you help me figure it out? I cannot move to another relationship without understanding what is going on in this one."
Zelda
She is a successful saleswoman and comes into the office with the complaint that she and her husband are having problems. On direct questioning, she affirms that that two do engage in direct fighting that gets physical at times. She says she often initiates the violence. She wants to stay with her husband and says they both want to make a go of things. They want to come into couples therapy and work on improving their relationship. But they fear that if they do go to a therapist together, as soon as she says there is violence in their relationship, she will be refused treatment.
Different cases, different approaches
Belkis benefits from treatment that focuses on support, education about domestic violence, and help with developing a safety plan. She wants to leave but needs the help and structure to do so.
Melanie enters individual therapy, and comes to understand that in her relationship with her husband, she is reenacting the relationship she had as a child with her parents. As a child, she felt like she was there only to help her mother clean and care for her sibling and that her needs and desires did not matter.
She felt that her elder brother was the favorite and that she had to support him as he pursued his studies. In her current relationship, she strives to "matter as a person" and not be seen as someone to do the cooking and the chores. She speaks back to her husband and challenges him when he demeans her. When she understands that the dynamic that binds her to her husband is the same dynamic that she experienced growing up, she feels relieved. "Now I can begin to think about taking care of myself and setting my own goals for my life. Now I can leave my past behind," she said.
Zelda and her husband want couples therapy. Both are committed to the relationship and stopping the violence as well as learning how to solve problems, communicate better, and meet each other’s needs by asking and negotiating. Before entering couples therapy, they agree to stop any violence while in treatment. The therapist teaches them skills to "take a time out" when conflict arises. As I’ve written previously, if patients are unable to discuss the issue calmly, they bring it to therapy (Adv. Psychiatric Treat. 2007;13:376-83).
How common is IPV?
Violence against women was not viewed as a serious issue until the second wave of feminism increased awareness, pushed for legislation, and increased resources.
How should we understand IPV? This phenomenon is often bidirectional, where each partner is both an aggressor and a victim, although women remain much more likely to be injured by partner violence than are men (Am. J. Public Health 2007;97:941-7).
In an outpatient sample of couples seeking marital therapy, 64% of wives and 61% of husbands were classified as aggressive (Violence Vict. 1994;9:107-24). In 272 engaged couples, 44% of women and 31% of men reported physical violence toward their partners (J. Consult. Clin. Psychol. 1989;57:263-8). As illustrated by the three cases above, IPV occurs across a range, from the classic male perpetrator and female victim, to the couple that engages in mutual violence.
Why do women stay?
Researchers such as Virginia Goldner, Ph.D., of the Ackerman Institute for the Family in New York, have contributed significantly to the understanding of why women stay in violent relationships. Dr. Goldner describes a generational imperative that is passed from mothers to daughters (Fam. Process 1990;29:343-64). This often includes the view that the role of women is to preserve the family, regardless of either the personal cost or the presence of abuse or violence.
Daughters raised in a highly patriarchal family might suffer existential neglect and be undervalued except in their capacity as caregiver to others in the family. Therefore, staying in a relationship protects the woman against guilt that she might feel if she gives up her caretaking role. These daughters may grow up with the belief that "being loved" is contingent upon denial of their self, being selfless. They may see the opposite as "being selfish" and not compatible with their self-image. Melanie certainly identified this guilt and had difficulty thinking about meeting her own needs.
Asking women about their mothers and the internalized view of themselves as independent agents can expose this dilemma. These daughters may see their mothers as powerless, devalued, and depressed. Being loyal to their mothers means accepting a subjugated role, while allying with their fathers means betraying their mothers and their own sense of themselves, as a woman.
If you decide to take a couple into therapy, it is important to interview each member of the couple individually before starting couples therapy. The information you glean from these interviews also will help determine when to offer and when not to offer couples therapy.
Factors that should encourage you not to proceed with couples therapy include the uncontrolled, continuous use of alcohol or drugs; fear of serious injury from the patient’s partner; severe violence that has resulted in the victim requiring medical attention; conviction for a violent crime or violation of a restraining order; prior use of a weapon against the partner; prior threat to kill the partner; stalking or other partner-focused obsessional behavior; and bizarre forms of violence, such as sadistic violence.
Here are a few guidelines for assessing intimate partner violence:
• Ask about relationship violence. Consider use of a questionnaire.
• If present, determine severity and ask about fear of partner.
• Identify risk factors for the potentially lethal relationship.
• If substance misuse is present, recommend abstinence and refer for treatment.
• If the couple wishes to stay together and to resolve the intimate partner violence, refer for conjoint treatment with a specialized family therapist.
• Assess and treat common comorbidities such as major depressive disorder and post-traumatic stress disorder.
Belkis, Melanie, and Zelda are three different women in abusive relationships who require three different solutions. Each patient requires a treatment based on their unique history and goals. Make sure that you are a family psychiatrist who understands the differences between your patients – and that you are able to provide a solution tailored to teach patient’s needs.
Elements of a safety plan
Encourage patients who in the midst of intimate partner violence to take the following steps to keep them and their families safe:
• Memorize phone numbers of people to call in emergency.
• Teach older children important phone numbers and when to dial 911.
• Keep information about domestic violence shelters in a safe place where you can get it quickly when you need it.
• Buy a cell phone that the abuser does not know about.
• Try to open your own bank account.
• Stay in touch with friends and neighbors. Do not cut yourself off from people.
• Rehearse your escape plan until you know it by heart.
• Leave a set of car keys, extra money, a change of clothes, and copies of important documents with a trusted friend or relative.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Conducting effective family therapy is never a one-size-fits-all proposition. In our work with families, we must keep in mind that, just as the dynamics in each family are different, so, too, must be our approaches. This is particularly the case for people who are experiencing intimate partner violence, or IPV.
The cases of three patients described below illustrate that point well; I’ve changed the patients’ names to protect their anonymity.
Belkis
She is a hardworking immigrant making minimum wage as a housekeeper. She presents to her psychiatric outpatient appointment with complaints of being sad and anxious. When asked about her husband and their relationship, she says tentatively that they disagree about things. She doesn’t acknowledge any abuse until she is asked directly, then she hangs her head down and looks ashamed. "He tells me I am not a good wife." With encouragement, she admits that she would like to leave him but has nowhere to go and is afraid that he will really hurt her if she tries to leave. "I tried before, and he threatened to kill me if I tried again."
Melanie
She is a well-educated women working as a writer and has a good income. She presents to her psychiatric outpatient appointment with complaints of being sad and anxious. When asked about her husband and their relationship, she says that he is abusive to her. She states that she would like to come into therapy to figure out why she has not been able to leave him. "What ties me to him?" She says her friends tell her she should leave. "There must be a reason I stay; can you help me figure it out? I cannot move to another relationship without understanding what is going on in this one."
Zelda
She is a successful saleswoman and comes into the office with the complaint that she and her husband are having problems. On direct questioning, she affirms that that two do engage in direct fighting that gets physical at times. She says she often initiates the violence. She wants to stay with her husband and says they both want to make a go of things. They want to come into couples therapy and work on improving their relationship. But they fear that if they do go to a therapist together, as soon as she says there is violence in their relationship, she will be refused treatment.
Different cases, different approaches
Belkis benefits from treatment that focuses on support, education about domestic violence, and help with developing a safety plan. She wants to leave but needs the help and structure to do so.
Melanie enters individual therapy, and comes to understand that in her relationship with her husband, she is reenacting the relationship she had as a child with her parents. As a child, she felt like she was there only to help her mother clean and care for her sibling and that her needs and desires did not matter.
She felt that her elder brother was the favorite and that she had to support him as he pursued his studies. In her current relationship, she strives to "matter as a person" and not be seen as someone to do the cooking and the chores. She speaks back to her husband and challenges him when he demeans her. When she understands that the dynamic that binds her to her husband is the same dynamic that she experienced growing up, she feels relieved. "Now I can begin to think about taking care of myself and setting my own goals for my life. Now I can leave my past behind," she said.
Zelda and her husband want couples therapy. Both are committed to the relationship and stopping the violence as well as learning how to solve problems, communicate better, and meet each other’s needs by asking and negotiating. Before entering couples therapy, they agree to stop any violence while in treatment. The therapist teaches them skills to "take a time out" when conflict arises. As I’ve written previously, if patients are unable to discuss the issue calmly, they bring it to therapy (Adv. Psychiatric Treat. 2007;13:376-83).
How common is IPV?
Violence against women was not viewed as a serious issue until the second wave of feminism increased awareness, pushed for legislation, and increased resources.
How should we understand IPV? This phenomenon is often bidirectional, where each partner is both an aggressor and a victim, although women remain much more likely to be injured by partner violence than are men (Am. J. Public Health 2007;97:941-7).
In an outpatient sample of couples seeking marital therapy, 64% of wives and 61% of husbands were classified as aggressive (Violence Vict. 1994;9:107-24). In 272 engaged couples, 44% of women and 31% of men reported physical violence toward their partners (J. Consult. Clin. Psychol. 1989;57:263-8). As illustrated by the three cases above, IPV occurs across a range, from the classic male perpetrator and female victim, to the couple that engages in mutual violence.
Why do women stay?
Researchers such as Virginia Goldner, Ph.D., of the Ackerman Institute for the Family in New York, have contributed significantly to the understanding of why women stay in violent relationships. Dr. Goldner describes a generational imperative that is passed from mothers to daughters (Fam. Process 1990;29:343-64). This often includes the view that the role of women is to preserve the family, regardless of either the personal cost or the presence of abuse or violence.
Daughters raised in a highly patriarchal family might suffer existential neglect and be undervalued except in their capacity as caregiver to others in the family. Therefore, staying in a relationship protects the woman against guilt that she might feel if she gives up her caretaking role. These daughters may grow up with the belief that "being loved" is contingent upon denial of their self, being selfless. They may see the opposite as "being selfish" and not compatible with their self-image. Melanie certainly identified this guilt and had difficulty thinking about meeting her own needs.
Asking women about their mothers and the internalized view of themselves as independent agents can expose this dilemma. These daughters may see their mothers as powerless, devalued, and depressed. Being loyal to their mothers means accepting a subjugated role, while allying with their fathers means betraying their mothers and their own sense of themselves, as a woman.
If you decide to take a couple into therapy, it is important to interview each member of the couple individually before starting couples therapy. The information you glean from these interviews also will help determine when to offer and when not to offer couples therapy.
Factors that should encourage you not to proceed with couples therapy include the uncontrolled, continuous use of alcohol or drugs; fear of serious injury from the patient’s partner; severe violence that has resulted in the victim requiring medical attention; conviction for a violent crime or violation of a restraining order; prior use of a weapon against the partner; prior threat to kill the partner; stalking or other partner-focused obsessional behavior; and bizarre forms of violence, such as sadistic violence.
Here are a few guidelines for assessing intimate partner violence:
• Ask about relationship violence. Consider use of a questionnaire.
• If present, determine severity and ask about fear of partner.
• Identify risk factors for the potentially lethal relationship.
• If substance misuse is present, recommend abstinence and refer for treatment.
• If the couple wishes to stay together and to resolve the intimate partner violence, refer for conjoint treatment with a specialized family therapist.
• Assess and treat common comorbidities such as major depressive disorder and post-traumatic stress disorder.
Belkis, Melanie, and Zelda are three different women in abusive relationships who require three different solutions. Each patient requires a treatment based on their unique history and goals. Make sure that you are a family psychiatrist who understands the differences between your patients – and that you are able to provide a solution tailored to teach patient’s needs.
Elements of a safety plan
Encourage patients who in the midst of intimate partner violence to take the following steps to keep them and their families safe:
• Memorize phone numbers of people to call in emergency.
• Teach older children important phone numbers and when to dial 911.
• Keep information about domestic violence shelters in a safe place where you can get it quickly when you need it.
• Buy a cell phone that the abuser does not know about.
• Try to open your own bank account.
• Stay in touch with friends and neighbors. Do not cut yourself off from people.
• Rehearse your escape plan until you know it by heart.
• Leave a set of car keys, extra money, a change of clothes, and copies of important documents with a trusted friend or relative.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
Conducting effective family therapy is never a one-size-fits-all proposition. In our work with families, we must keep in mind that, just as the dynamics in each family are different, so, too, must be our approaches. This is particularly the case for people who are experiencing intimate partner violence, or IPV.
The cases of three patients described below illustrate that point well; I’ve changed the patients’ names to protect their anonymity.
Belkis
She is a hardworking immigrant making minimum wage as a housekeeper. She presents to her psychiatric outpatient appointment with complaints of being sad and anxious. When asked about her husband and their relationship, she says tentatively that they disagree about things. She doesn’t acknowledge any abuse until she is asked directly, then she hangs her head down and looks ashamed. "He tells me I am not a good wife." With encouragement, she admits that she would like to leave him but has nowhere to go and is afraid that he will really hurt her if she tries to leave. "I tried before, and he threatened to kill me if I tried again."
Melanie
She is a well-educated women working as a writer and has a good income. She presents to her psychiatric outpatient appointment with complaints of being sad and anxious. When asked about her husband and their relationship, she says that he is abusive to her. She states that she would like to come into therapy to figure out why she has not been able to leave him. "What ties me to him?" She says her friends tell her she should leave. "There must be a reason I stay; can you help me figure it out? I cannot move to another relationship without understanding what is going on in this one."
Zelda
She is a successful saleswoman and comes into the office with the complaint that she and her husband are having problems. On direct questioning, she affirms that that two do engage in direct fighting that gets physical at times. She says she often initiates the violence. She wants to stay with her husband and says they both want to make a go of things. They want to come into couples therapy and work on improving their relationship. But they fear that if they do go to a therapist together, as soon as she says there is violence in their relationship, she will be refused treatment.
Different cases, different approaches
Belkis benefits from treatment that focuses on support, education about domestic violence, and help with developing a safety plan. She wants to leave but needs the help and structure to do so.
Melanie enters individual therapy, and comes to understand that in her relationship with her husband, she is reenacting the relationship she had as a child with her parents. As a child, she felt like she was there only to help her mother clean and care for her sibling and that her needs and desires did not matter.
She felt that her elder brother was the favorite and that she had to support him as he pursued his studies. In her current relationship, she strives to "matter as a person" and not be seen as someone to do the cooking and the chores. She speaks back to her husband and challenges him when he demeans her. When she understands that the dynamic that binds her to her husband is the same dynamic that she experienced growing up, she feels relieved. "Now I can begin to think about taking care of myself and setting my own goals for my life. Now I can leave my past behind," she said.
Zelda and her husband want couples therapy. Both are committed to the relationship and stopping the violence as well as learning how to solve problems, communicate better, and meet each other’s needs by asking and negotiating. Before entering couples therapy, they agree to stop any violence while in treatment. The therapist teaches them skills to "take a time out" when conflict arises. As I’ve written previously, if patients are unable to discuss the issue calmly, they bring it to therapy (Adv. Psychiatric Treat. 2007;13:376-83).
How common is IPV?
Violence against women was not viewed as a serious issue until the second wave of feminism increased awareness, pushed for legislation, and increased resources.
How should we understand IPV? This phenomenon is often bidirectional, where each partner is both an aggressor and a victim, although women remain much more likely to be injured by partner violence than are men (Am. J. Public Health 2007;97:941-7).
In an outpatient sample of couples seeking marital therapy, 64% of wives and 61% of husbands were classified as aggressive (Violence Vict. 1994;9:107-24). In 272 engaged couples, 44% of women and 31% of men reported physical violence toward their partners (J. Consult. Clin. Psychol. 1989;57:263-8). As illustrated by the three cases above, IPV occurs across a range, from the classic male perpetrator and female victim, to the couple that engages in mutual violence.
Why do women stay?
Researchers such as Virginia Goldner, Ph.D., of the Ackerman Institute for the Family in New York, have contributed significantly to the understanding of why women stay in violent relationships. Dr. Goldner describes a generational imperative that is passed from mothers to daughters (Fam. Process 1990;29:343-64). This often includes the view that the role of women is to preserve the family, regardless of either the personal cost or the presence of abuse or violence.
Daughters raised in a highly patriarchal family might suffer existential neglect and be undervalued except in their capacity as caregiver to others in the family. Therefore, staying in a relationship protects the woman against guilt that she might feel if she gives up her caretaking role. These daughters may grow up with the belief that "being loved" is contingent upon denial of their self, being selfless. They may see the opposite as "being selfish" and not compatible with their self-image. Melanie certainly identified this guilt and had difficulty thinking about meeting her own needs.
Asking women about their mothers and the internalized view of themselves as independent agents can expose this dilemma. These daughters may see their mothers as powerless, devalued, and depressed. Being loyal to their mothers means accepting a subjugated role, while allying with their fathers means betraying their mothers and their own sense of themselves, as a woman.
If you decide to take a couple into therapy, it is important to interview each member of the couple individually before starting couples therapy. The information you glean from these interviews also will help determine when to offer and when not to offer couples therapy.
Factors that should encourage you not to proceed with couples therapy include the uncontrolled, continuous use of alcohol or drugs; fear of serious injury from the patient’s partner; severe violence that has resulted in the victim requiring medical attention; conviction for a violent crime or violation of a restraining order; prior use of a weapon against the partner; prior threat to kill the partner; stalking or other partner-focused obsessional behavior; and bizarre forms of violence, such as sadistic violence.
Here are a few guidelines for assessing intimate partner violence:
• Ask about relationship violence. Consider use of a questionnaire.
• If present, determine severity and ask about fear of partner.
• Identify risk factors for the potentially lethal relationship.
• If substance misuse is present, recommend abstinence and refer for treatment.
• If the couple wishes to stay together and to resolve the intimate partner violence, refer for conjoint treatment with a specialized family therapist.
• Assess and treat common comorbidities such as major depressive disorder and post-traumatic stress disorder.
Belkis, Melanie, and Zelda are three different women in abusive relationships who require three different solutions. Each patient requires a treatment based on their unique history and goals. Make sure that you are a family psychiatrist who understands the differences between your patients – and that you are able to provide a solution tailored to teach patient’s needs.
Elements of a safety plan
Encourage patients who in the midst of intimate partner violence to take the following steps to keep them and their families safe:
• Memorize phone numbers of people to call in emergency.
• Teach older children important phone numbers and when to dial 911.
• Keep information about domestic violence shelters in a safe place where you can get it quickly when you need it.
• Buy a cell phone that the abuser does not know about.
• Try to open your own bank account.
• Stay in touch with friends and neighbors. Do not cut yourself off from people.
• Rehearse your escape plan until you know it by heart.
• Leave a set of car keys, extra money, a change of clothes, and copies of important documents with a trusted friend or relative.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
New and Noteworthy Information—October 2013
By lowering homocysteine levels, vitamin B supplementation may reduce the risk of stroke significantly for some individuals, according to research published online ahead of print September 18 in Neurology. Investigators analyzed data from 14 randomized controlled trials published before August 2012. The trials included 54,913 participants, and the investigators measured the association between B vitamin supplementation and end point events using a fixed-effects model and χ2 tests. The group observed a reduction in overall stroke events resulting from reduction in homocysteine levels following B vitamin supplementation, but not in subgroups divided according to primary or secondary prevention measures, ischemic versus hemorrhagic stroke, or occurrence of fatal stroke. Vitamin B reduced stroke events in subgroups with three or more years of follow-up and without cereal folate fortification or chronic kidney disease.
The deletion of information from chromosome 22 may be a genetic risk factor for early-onset Parkinson’s disease, researchers reported online ahead of print September 9 in JAMA Neurology. The investigators conducted an observational study of the occurrence of Parkinson’s disease in a cohort of 159 adults with a molecularly confirmed diagnosis of 22q11.2 deletion syndrome. The group examined postmortem brain tissue from patients with 22q11.2 deletion syndrome and a clinical history of Parkinson’s disease for neurodegenerative changes and compared it with tissue from persons with no history of a movement disorder. Adults with 22q11.2 deletion syndrome had a significantly elevated occurrence of Parkinson’s disease, compared with standard population estimates. Individuals with early-onset Parkinson’s disease and classic features of 22q11.2 deletion syndrome should be considered for genetic testing, said the authors.
High levels of lipid-depleted (LD) apolipoproteins are associated with cognitive difficulties but may be mitigated by diet, according to research published in the August issue of JAMA Neurology. Investigators randomized 20 adults with normal cognition (mean age, 69) and 27 adults with amnestic mild cognitive impairment (mean age, 67) to a diet high in saturated fat content and with a high glycemic index or to a diet low in saturated fat content and with a low glycemic index. Baseline levels of LD b-amyloid were greater for adults with mild cognitive impairment, compared with adults with normal cognition. The diet low in saturated fat tended to decrease LD b-amyloid levels, and the diet high in saturated fat increased these fractions.
The parkin protein may trigger the destruction of the bacterium that causes tuberculosis, according to research published online ahead of print September 4 in Nature. Genetic polymorphisms in the PARK2 regulatory region are associated with increased susceptibility to intracellular bacterial pathogens in humans. In mouse and human macrophages infected with Mycobacterium tuberculosis, parkin played a role in fighting the bacteria. Mice genetically engineered to lack parkin died after being infected by M. tuberculosis, while control mice survived the infection. In addition, parkin-deficient mice and flies were more sensitive to intracellular bacterial infections. The study results reveal an unexpected functional link between mitophagy and infectious disease, said the researchers. Strategies under investigation for combating Parkinson’s disease also might help fight tuberculosis, the authors added.
Low cardiovascular fitness early in life may be associated with an increased risk of epilepsy in adulthood, according to research published September 17 in Neurology. Investigators examined a population-based cohort study of approximately 1.2 million Swedish male conscripts born from 1950 to 1987 who were followed for as many as 40 years. Data on cardiovascular fitness were collected during conscription exams, and researchers linked the data with hospital registers to calculate later risk of epilepsy using Cox proportional hazard models. Low and medium cardiovascular fitness (compared with high cardiovascular fitness) at age 18 was associated with increased risk of future epilepsy (hazard ratios 1.79 and 1.36, respectively). The associations changed marginally after adjustment for familial influences and prior severe traumatic brain injury, cerebrovascular disease, or diabetes.
Whole-body MRI may predict cardiac and cerebrovascular events in patients with diabetes, according to a study published online ahead of print September 10 in Radiology. Researchers followed up 65 patients with types 1 and 2 diabetes who underwent a comprehensive, contrast-enhanced whole-body MRI protocol at baseline. Follow-up was performed by phone interview. The primary end point was a major adverse cardiac and cerebrovascular event (MACCE), which was defined as composite cardiac-cerebrovascular death, myocardial infarction, cerebrovascular event, or revascularization. Follow-up was completed in 61 patients. Normal whole-body MRI excluded MACCE during the follow-up period, but detectable ischemic or atherosclerotic changes at whole-body MRI were associated with a cumulative event rate of 20% at three years and 35% at six years. Whole-body MRI summary estimate of disease was strongly predictive for MACCE.
Obese individuals may have an elevated risk of episodic migraine, compared with healthy persons, according to research published online ahead of print September 11 in Neurology. Investigators analyzed data for 3,862 adult participants (including African Americans and Caucasians) in the National Comorbidity Survey Replication. Diagnostic criteria for episodic migraine were based on the International Classification of Headache Disorders. BMI was classified as underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), or obese (≥30 kg/m2). The adjusted odds of episodic migraine were 81% greater in individuals who were obese, compared with those of normal weight. Stratified analyses demonstrated that the odds of episodic migraine were greater in obese, compared with normal-weight individuals, who were younger than 50, Caucasian, or female.
Approximately 15% of all ischemic strokes occur in young adults and adolescents, according to a consensus document developed by an expert panel of the American Academy of Neurology and published September 17 in Neurology. Few public-health and research initiatives have focused on stroke in the young, said the authors. Early diagnosis of ischemic stroke is challenging because of the lack of awareness and the relative infrequency of stroke, compared with stroke mimics. The heterogeneity and relative rarity of the causes of ischemic stroke in the young result in uncertainties about diagnostic evaluation and cause-specific management. For these reasons, it is important to formulate and enact strategies to increase awareness and access to resources for young patients with stroke, their caregivers and families, and health care professionals, said the authors.
Retired National Football League (NFL) players may have an increased prevalence of late-life cognitive impairment indicative of diminished cerebral reserve, according to research published in the September issue of the Journal of the International Neuropsychological Society. After examining informant AD8 inventory data for a sample of 513 retired NFL players, the researchers found that 35.1% of the sample had possible cognitive impairment. When the researchers compared neurocognitive profiles in a subsample of this group to those in a clinical sample of patients with a diagnosis of mild cognitive impairment due to Alzheimer’s disease, they found a highly similar profile of impairments. However, said lead author Christopher Randolph, PhD, “there is essentially no evidence to support the existence of any unique clinical disorder such as CTE.” The findings emphasize the need for larger, controlled studies on this issue, he added.
Treatment with 4 g/day of ascorbic acid may not improve neuropathy in subjects with Charcot-Marie-Tooth disease type 1A, according to research published in the August issue of JAMA Neurology. Researchers randomized 110 patients with Charcot-Marie-Tooth disease type 1A to oral ascorbic acid (87 subjects) or matching placebo (23 individuals). Patients’ mean two-year change in Charcot-Marie-Tooth Neuropathy Score (CMTNS) was −0.21 for the ascorbic acid group and −0.92 for the placebo group. The mean two-year change according to natural history is +1.33. Because the results were well below 50% reduction of CMTNS worsening from natural history, the investigators could not declare the study futile. It is unlikely that the results support undertaking a larger trial of 4 g/day of ascorbic acid, said the researchers.
Pilots with occupational exposure to hypobaria may have significantly greater volume and number of white-matter hyperintensity (WMH) lesions, compared with controls, according to data published August 20 in Neurology. Researchers used a 3-T MRI scanner to collect three-dimensional, T2-weighted, high-resolution imaging data for 102 U-2 pilots and 91 controls matched for age, health, and education levels. The investigators compared whole-brain and regional WMH volume and number between groups using a two-tailed Wilcoxon rank sum test. U-2 pilots had an increase in volume (394%) and number (295%) of WMH. Also, WMH were more uniformly distributed throughout the brain in U-2 pilots, compared with a predominantly frontal distribution in controls. Further studies will be necessary to clarify the pathologic mechanisms responsible for the damage, said the researchers.
Nine independent risk factors that can be traced to adolescence, most of which are modifiable, may account for most cases of young-onset dementia in men, according to a study published online ahead of print August 12 in JAMA Internal Medicine. Investigators analyzed data for 488,484 Swedish men from the Swedish Military Service Conscription Register. Multivariate Cox regression analysis indicated that alcohol intoxication, stroke, antipsychotic use, depression, father’s dementia, intoxication with drugs other than alcohol, low cognitive function at conscription, low height at conscription, and high systolic blood pressure at conscription were significant risk factors for young-onset dementia. The population-attributable risk associated with all nine risk factors was 68%. The study results suggest excellent opportunities for early prevention, according to the researchers.
Researchers observed a novel brain phenomenon in humans and animals in a coma and with an isoelectric (ie, “flat”) EEG, according to research published September 18 in PLOS One. The researchers first detected the state in a human in postanoxic coma who had received medication. They replicated the state by applying high doses of isoflurane in cats. All subjects had an EEG activity of quasi-rhythmic sharp waves that the investigators propose to call Nu-complexes. Simultaneous intracellular recordings in vivo in the cortex and hippocampus, especially in the CA3 region, demonstrated that Nu-complexes arise in the hippocampus and are transmitted to the cortex. The creation of a hippocampal Nu-complex depends on another hippocampal activity (ie, ripple activity), which is not overtly detectable at the cortical level.
—Erik Greb
Senior Associate Editor
By lowering homocysteine levels, vitamin B supplementation may reduce the risk of stroke significantly for some individuals, according to research published online ahead of print September 18 in Neurology. Investigators analyzed data from 14 randomized controlled trials published before August 2012. The trials included 54,913 participants, and the investigators measured the association between B vitamin supplementation and end point events using a fixed-effects model and χ2 tests. The group observed a reduction in overall stroke events resulting from reduction in homocysteine levels following B vitamin supplementation, but not in subgroups divided according to primary or secondary prevention measures, ischemic versus hemorrhagic stroke, or occurrence of fatal stroke. Vitamin B reduced stroke events in subgroups with three or more years of follow-up and without cereal folate fortification or chronic kidney disease.
The deletion of information from chromosome 22 may be a genetic risk factor for early-onset Parkinson’s disease, researchers reported online ahead of print September 9 in JAMA Neurology. The investigators conducted an observational study of the occurrence of Parkinson’s disease in a cohort of 159 adults with a molecularly confirmed diagnosis of 22q11.2 deletion syndrome. The group examined postmortem brain tissue from patients with 22q11.2 deletion syndrome and a clinical history of Parkinson’s disease for neurodegenerative changes and compared it with tissue from persons with no history of a movement disorder. Adults with 22q11.2 deletion syndrome had a significantly elevated occurrence of Parkinson’s disease, compared with standard population estimates. Individuals with early-onset Parkinson’s disease and classic features of 22q11.2 deletion syndrome should be considered for genetic testing, said the authors.
High levels of lipid-depleted (LD) apolipoproteins are associated with cognitive difficulties but may be mitigated by diet, according to research published in the August issue of JAMA Neurology. Investigators randomized 20 adults with normal cognition (mean age, 69) and 27 adults with amnestic mild cognitive impairment (mean age, 67) to a diet high in saturated fat content and with a high glycemic index or to a diet low in saturated fat content and with a low glycemic index. Baseline levels of LD b-amyloid were greater for adults with mild cognitive impairment, compared with adults with normal cognition. The diet low in saturated fat tended to decrease LD b-amyloid levels, and the diet high in saturated fat increased these fractions.
The parkin protein may trigger the destruction of the bacterium that causes tuberculosis, according to research published online ahead of print September 4 in Nature. Genetic polymorphisms in the PARK2 regulatory region are associated with increased susceptibility to intracellular bacterial pathogens in humans. In mouse and human macrophages infected with Mycobacterium tuberculosis, parkin played a role in fighting the bacteria. Mice genetically engineered to lack parkin died after being infected by M. tuberculosis, while control mice survived the infection. In addition, parkin-deficient mice and flies were more sensitive to intracellular bacterial infections. The study results reveal an unexpected functional link between mitophagy and infectious disease, said the researchers. Strategies under investigation for combating Parkinson’s disease also might help fight tuberculosis, the authors added.
Low cardiovascular fitness early in life may be associated with an increased risk of epilepsy in adulthood, according to research published September 17 in Neurology. Investigators examined a population-based cohort study of approximately 1.2 million Swedish male conscripts born from 1950 to 1987 who were followed for as many as 40 years. Data on cardiovascular fitness were collected during conscription exams, and researchers linked the data with hospital registers to calculate later risk of epilepsy using Cox proportional hazard models. Low and medium cardiovascular fitness (compared with high cardiovascular fitness) at age 18 was associated with increased risk of future epilepsy (hazard ratios 1.79 and 1.36, respectively). The associations changed marginally after adjustment for familial influences and prior severe traumatic brain injury, cerebrovascular disease, or diabetes.
Whole-body MRI may predict cardiac and cerebrovascular events in patients with diabetes, according to a study published online ahead of print September 10 in Radiology. Researchers followed up 65 patients with types 1 and 2 diabetes who underwent a comprehensive, contrast-enhanced whole-body MRI protocol at baseline. Follow-up was performed by phone interview. The primary end point was a major adverse cardiac and cerebrovascular event (MACCE), which was defined as composite cardiac-cerebrovascular death, myocardial infarction, cerebrovascular event, or revascularization. Follow-up was completed in 61 patients. Normal whole-body MRI excluded MACCE during the follow-up period, but detectable ischemic or atherosclerotic changes at whole-body MRI were associated with a cumulative event rate of 20% at three years and 35% at six years. Whole-body MRI summary estimate of disease was strongly predictive for MACCE.
Obese individuals may have an elevated risk of episodic migraine, compared with healthy persons, according to research published online ahead of print September 11 in Neurology. Investigators analyzed data for 3,862 adult participants (including African Americans and Caucasians) in the National Comorbidity Survey Replication. Diagnostic criteria for episodic migraine were based on the International Classification of Headache Disorders. BMI was classified as underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), or obese (≥30 kg/m2). The adjusted odds of episodic migraine were 81% greater in individuals who were obese, compared with those of normal weight. Stratified analyses demonstrated that the odds of episodic migraine were greater in obese, compared with normal-weight individuals, who were younger than 50, Caucasian, or female.
Approximately 15% of all ischemic strokes occur in young adults and adolescents, according to a consensus document developed by an expert panel of the American Academy of Neurology and published September 17 in Neurology. Few public-health and research initiatives have focused on stroke in the young, said the authors. Early diagnosis of ischemic stroke is challenging because of the lack of awareness and the relative infrequency of stroke, compared with stroke mimics. The heterogeneity and relative rarity of the causes of ischemic stroke in the young result in uncertainties about diagnostic evaluation and cause-specific management. For these reasons, it is important to formulate and enact strategies to increase awareness and access to resources for young patients with stroke, their caregivers and families, and health care professionals, said the authors.
Retired National Football League (NFL) players may have an increased prevalence of late-life cognitive impairment indicative of diminished cerebral reserve, according to research published in the September issue of the Journal of the International Neuropsychological Society. After examining informant AD8 inventory data for a sample of 513 retired NFL players, the researchers found that 35.1% of the sample had possible cognitive impairment. When the researchers compared neurocognitive profiles in a subsample of this group to those in a clinical sample of patients with a diagnosis of mild cognitive impairment due to Alzheimer’s disease, they found a highly similar profile of impairments. However, said lead author Christopher Randolph, PhD, “there is essentially no evidence to support the existence of any unique clinical disorder such as CTE.” The findings emphasize the need for larger, controlled studies on this issue, he added.
Treatment with 4 g/day of ascorbic acid may not improve neuropathy in subjects with Charcot-Marie-Tooth disease type 1A, according to research published in the August issue of JAMA Neurology. Researchers randomized 110 patients with Charcot-Marie-Tooth disease type 1A to oral ascorbic acid (87 subjects) or matching placebo (23 individuals). Patients’ mean two-year change in Charcot-Marie-Tooth Neuropathy Score (CMTNS) was −0.21 for the ascorbic acid group and −0.92 for the placebo group. The mean two-year change according to natural history is +1.33. Because the results were well below 50% reduction of CMTNS worsening from natural history, the investigators could not declare the study futile. It is unlikely that the results support undertaking a larger trial of 4 g/day of ascorbic acid, said the researchers.
Pilots with occupational exposure to hypobaria may have significantly greater volume and number of white-matter hyperintensity (WMH) lesions, compared with controls, according to data published August 20 in Neurology. Researchers used a 3-T MRI scanner to collect three-dimensional, T2-weighted, high-resolution imaging data for 102 U-2 pilots and 91 controls matched for age, health, and education levels. The investigators compared whole-brain and regional WMH volume and number between groups using a two-tailed Wilcoxon rank sum test. U-2 pilots had an increase in volume (394%) and number (295%) of WMH. Also, WMH were more uniformly distributed throughout the brain in U-2 pilots, compared with a predominantly frontal distribution in controls. Further studies will be necessary to clarify the pathologic mechanisms responsible for the damage, said the researchers.
Nine independent risk factors that can be traced to adolescence, most of which are modifiable, may account for most cases of young-onset dementia in men, according to a study published online ahead of print August 12 in JAMA Internal Medicine. Investigators analyzed data for 488,484 Swedish men from the Swedish Military Service Conscription Register. Multivariate Cox regression analysis indicated that alcohol intoxication, stroke, antipsychotic use, depression, father’s dementia, intoxication with drugs other than alcohol, low cognitive function at conscription, low height at conscription, and high systolic blood pressure at conscription were significant risk factors for young-onset dementia. The population-attributable risk associated with all nine risk factors was 68%. The study results suggest excellent opportunities for early prevention, according to the researchers.
Researchers observed a novel brain phenomenon in humans and animals in a coma and with an isoelectric (ie, “flat”) EEG, according to research published September 18 in PLOS One. The researchers first detected the state in a human in postanoxic coma who had received medication. They replicated the state by applying high doses of isoflurane in cats. All subjects had an EEG activity of quasi-rhythmic sharp waves that the investigators propose to call Nu-complexes. Simultaneous intracellular recordings in vivo in the cortex and hippocampus, especially in the CA3 region, demonstrated that Nu-complexes arise in the hippocampus and are transmitted to the cortex. The creation of a hippocampal Nu-complex depends on another hippocampal activity (ie, ripple activity), which is not overtly detectable at the cortical level.
—Erik Greb
Senior Associate Editor
By lowering homocysteine levels, vitamin B supplementation may reduce the risk of stroke significantly for some individuals, according to research published online ahead of print September 18 in Neurology. Investigators analyzed data from 14 randomized controlled trials published before August 2012. The trials included 54,913 participants, and the investigators measured the association between B vitamin supplementation and end point events using a fixed-effects model and χ2 tests. The group observed a reduction in overall stroke events resulting from reduction in homocysteine levels following B vitamin supplementation, but not in subgroups divided according to primary or secondary prevention measures, ischemic versus hemorrhagic stroke, or occurrence of fatal stroke. Vitamin B reduced stroke events in subgroups with three or more years of follow-up and without cereal folate fortification or chronic kidney disease.
The deletion of information from chromosome 22 may be a genetic risk factor for early-onset Parkinson’s disease, researchers reported online ahead of print September 9 in JAMA Neurology. The investigators conducted an observational study of the occurrence of Parkinson’s disease in a cohort of 159 adults with a molecularly confirmed diagnosis of 22q11.2 deletion syndrome. The group examined postmortem brain tissue from patients with 22q11.2 deletion syndrome and a clinical history of Parkinson’s disease for neurodegenerative changes and compared it with tissue from persons with no history of a movement disorder. Adults with 22q11.2 deletion syndrome had a significantly elevated occurrence of Parkinson’s disease, compared with standard population estimates. Individuals with early-onset Parkinson’s disease and classic features of 22q11.2 deletion syndrome should be considered for genetic testing, said the authors.
High levels of lipid-depleted (LD) apolipoproteins are associated with cognitive difficulties but may be mitigated by diet, according to research published in the August issue of JAMA Neurology. Investigators randomized 20 adults with normal cognition (mean age, 69) and 27 adults with amnestic mild cognitive impairment (mean age, 67) to a diet high in saturated fat content and with a high glycemic index or to a diet low in saturated fat content and with a low glycemic index. Baseline levels of LD b-amyloid were greater for adults with mild cognitive impairment, compared with adults with normal cognition. The diet low in saturated fat tended to decrease LD b-amyloid levels, and the diet high in saturated fat increased these fractions.
The parkin protein may trigger the destruction of the bacterium that causes tuberculosis, according to research published online ahead of print September 4 in Nature. Genetic polymorphisms in the PARK2 regulatory region are associated with increased susceptibility to intracellular bacterial pathogens in humans. In mouse and human macrophages infected with Mycobacterium tuberculosis, parkin played a role in fighting the bacteria. Mice genetically engineered to lack parkin died after being infected by M. tuberculosis, while control mice survived the infection. In addition, parkin-deficient mice and flies were more sensitive to intracellular bacterial infections. The study results reveal an unexpected functional link between mitophagy and infectious disease, said the researchers. Strategies under investigation for combating Parkinson’s disease also might help fight tuberculosis, the authors added.
Low cardiovascular fitness early in life may be associated with an increased risk of epilepsy in adulthood, according to research published September 17 in Neurology. Investigators examined a population-based cohort study of approximately 1.2 million Swedish male conscripts born from 1950 to 1987 who were followed for as many as 40 years. Data on cardiovascular fitness were collected during conscription exams, and researchers linked the data with hospital registers to calculate later risk of epilepsy using Cox proportional hazard models. Low and medium cardiovascular fitness (compared with high cardiovascular fitness) at age 18 was associated with increased risk of future epilepsy (hazard ratios 1.79 and 1.36, respectively). The associations changed marginally after adjustment for familial influences and prior severe traumatic brain injury, cerebrovascular disease, or diabetes.
Whole-body MRI may predict cardiac and cerebrovascular events in patients with diabetes, according to a study published online ahead of print September 10 in Radiology. Researchers followed up 65 patients with types 1 and 2 diabetes who underwent a comprehensive, contrast-enhanced whole-body MRI protocol at baseline. Follow-up was performed by phone interview. The primary end point was a major adverse cardiac and cerebrovascular event (MACCE), which was defined as composite cardiac-cerebrovascular death, myocardial infarction, cerebrovascular event, or revascularization. Follow-up was completed in 61 patients. Normal whole-body MRI excluded MACCE during the follow-up period, but detectable ischemic or atherosclerotic changes at whole-body MRI were associated with a cumulative event rate of 20% at three years and 35% at six years. Whole-body MRI summary estimate of disease was strongly predictive for MACCE.
Obese individuals may have an elevated risk of episodic migraine, compared with healthy persons, according to research published online ahead of print September 11 in Neurology. Investigators analyzed data for 3,862 adult participants (including African Americans and Caucasians) in the National Comorbidity Survey Replication. Diagnostic criteria for episodic migraine were based on the International Classification of Headache Disorders. BMI was classified as underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), or obese (≥30 kg/m2). The adjusted odds of episodic migraine were 81% greater in individuals who were obese, compared with those of normal weight. Stratified analyses demonstrated that the odds of episodic migraine were greater in obese, compared with normal-weight individuals, who were younger than 50, Caucasian, or female.
Approximately 15% of all ischemic strokes occur in young adults and adolescents, according to a consensus document developed by an expert panel of the American Academy of Neurology and published September 17 in Neurology. Few public-health and research initiatives have focused on stroke in the young, said the authors. Early diagnosis of ischemic stroke is challenging because of the lack of awareness and the relative infrequency of stroke, compared with stroke mimics. The heterogeneity and relative rarity of the causes of ischemic stroke in the young result in uncertainties about diagnostic evaluation and cause-specific management. For these reasons, it is important to formulate and enact strategies to increase awareness and access to resources for young patients with stroke, their caregivers and families, and health care professionals, said the authors.
Retired National Football League (NFL) players may have an increased prevalence of late-life cognitive impairment indicative of diminished cerebral reserve, according to research published in the September issue of the Journal of the International Neuropsychological Society. After examining informant AD8 inventory data for a sample of 513 retired NFL players, the researchers found that 35.1% of the sample had possible cognitive impairment. When the researchers compared neurocognitive profiles in a subsample of this group to those in a clinical sample of patients with a diagnosis of mild cognitive impairment due to Alzheimer’s disease, they found a highly similar profile of impairments. However, said lead author Christopher Randolph, PhD, “there is essentially no evidence to support the existence of any unique clinical disorder such as CTE.” The findings emphasize the need for larger, controlled studies on this issue, he added.
Treatment with 4 g/day of ascorbic acid may not improve neuropathy in subjects with Charcot-Marie-Tooth disease type 1A, according to research published in the August issue of JAMA Neurology. Researchers randomized 110 patients with Charcot-Marie-Tooth disease type 1A to oral ascorbic acid (87 subjects) or matching placebo (23 individuals). Patients’ mean two-year change in Charcot-Marie-Tooth Neuropathy Score (CMTNS) was −0.21 for the ascorbic acid group and −0.92 for the placebo group. The mean two-year change according to natural history is +1.33. Because the results were well below 50% reduction of CMTNS worsening from natural history, the investigators could not declare the study futile. It is unlikely that the results support undertaking a larger trial of 4 g/day of ascorbic acid, said the researchers.
Pilots with occupational exposure to hypobaria may have significantly greater volume and number of white-matter hyperintensity (WMH) lesions, compared with controls, according to data published August 20 in Neurology. Researchers used a 3-T MRI scanner to collect three-dimensional, T2-weighted, high-resolution imaging data for 102 U-2 pilots and 91 controls matched for age, health, and education levels. The investigators compared whole-brain and regional WMH volume and number between groups using a two-tailed Wilcoxon rank sum test. U-2 pilots had an increase in volume (394%) and number (295%) of WMH. Also, WMH were more uniformly distributed throughout the brain in U-2 pilots, compared with a predominantly frontal distribution in controls. Further studies will be necessary to clarify the pathologic mechanisms responsible for the damage, said the researchers.
Nine independent risk factors that can be traced to adolescence, most of which are modifiable, may account for most cases of young-onset dementia in men, according to a study published online ahead of print August 12 in JAMA Internal Medicine. Investigators analyzed data for 488,484 Swedish men from the Swedish Military Service Conscription Register. Multivariate Cox regression analysis indicated that alcohol intoxication, stroke, antipsychotic use, depression, father’s dementia, intoxication with drugs other than alcohol, low cognitive function at conscription, low height at conscription, and high systolic blood pressure at conscription were significant risk factors for young-onset dementia. The population-attributable risk associated with all nine risk factors was 68%. The study results suggest excellent opportunities for early prevention, according to the researchers.
Researchers observed a novel brain phenomenon in humans and animals in a coma and with an isoelectric (ie, “flat”) EEG, according to research published September 18 in PLOS One. The researchers first detected the state in a human in postanoxic coma who had received medication. They replicated the state by applying high doses of isoflurane in cats. All subjects had an EEG activity of quasi-rhythmic sharp waves that the investigators propose to call Nu-complexes. Simultaneous intracellular recordings in vivo in the cortex and hippocampus, especially in the CA3 region, demonstrated that Nu-complexes arise in the hippocampus and are transmitted to the cortex. The creation of a hippocampal Nu-complex depends on another hippocampal activity (ie, ripple activity), which is not overtly detectable at the cortical level.
—Erik Greb
Senior Associate Editor
Drug can prevent CMV in HSCT recipients
A new drug can prevent cytomegalovirus (CMV) in patients undergoing hematopoietic stem cell transplant (HSCT), according to a study published in The New England Journal of Medicine.
The drug, called CMX001, is an oral nucleotide analog lipid-conjugate that blocks replication of double-stranded DNA viruses.
HSCT recipients who took CMX001 after engraftment were less likely to develop CMV than HSCT patients who received placebo, researchers found.
“With current agents, between 3% and 5% of allogeneic transplant patients develop CMV disease within 6 months of transplantation, and a small number of them may die of it,” said investigator Francisco Marty, MD, of the Dana-Farber Cancer Institute in Boston.
“There clearly is a need for better treatments with fewer adverse effects. This clinical trial examined whether the disease can be prevented, rather than waiting for blood tests to show that treatment is needed.”
The phase 2 trial involved 230 HSCT recipients treated at 27 centers across the US. The patients were randomized to receive placebo or CMX001 at doses ranging from 40 mg a week to 200 mg twice a week.
Treatment began after engraftment, at about 2 to 3 weeks post-transplant, and continued for 9 to 11 weeks.
The study’s primary efficacy outcome was a “CMV event,” which was defined as CMV that affects the lung, digestive tract, or other organs, or a detectable amount of CMV in the blood at the end of treatment.
CMV events occurred in 25% (43/171) of patients who received CMX001 and 37% (22/59) of patients who received placebo. However, when CMX001 was given at the optimal dose—100 mg twice a week—only 10% of patients had a CMV event.
“The results show the effectiveness of CMX001 in preventing CMV infections in this group of patients,” Dr Marty said. “Because CMX001 is known to be active against other herpes viruses and against adenoviruses that sometimes affect transplant patients, it may be useful as a preventive or treatment agent for those infections as well.”
Most of the side effects associated with CMX001 were gastrointestinal in nature. Diarrhea was common and often serious in patients who received the drug at 200 mg twice a week.
Patients in this dose group were more likely to experience elevated alanine aminotransferase levels as well. But this was not associated with increases in levels of bilirubin or aspartate aminotransferase.
This research was funded by Chimerix, the company developing CMX001.![]()
A new drug can prevent cytomegalovirus (CMV) in patients undergoing hematopoietic stem cell transplant (HSCT), according to a study published in The New England Journal of Medicine.
The drug, called CMX001, is an oral nucleotide analog lipid-conjugate that blocks replication of double-stranded DNA viruses.
HSCT recipients who took CMX001 after engraftment were less likely to develop CMV than HSCT patients who received placebo, researchers found.
“With current agents, between 3% and 5% of allogeneic transplant patients develop CMV disease within 6 months of transplantation, and a small number of them may die of it,” said investigator Francisco Marty, MD, of the Dana-Farber Cancer Institute in Boston.
“There clearly is a need for better treatments with fewer adverse effects. This clinical trial examined whether the disease can be prevented, rather than waiting for blood tests to show that treatment is needed.”
The phase 2 trial involved 230 HSCT recipients treated at 27 centers across the US. The patients were randomized to receive placebo or CMX001 at doses ranging from 40 mg a week to 200 mg twice a week.
Treatment began after engraftment, at about 2 to 3 weeks post-transplant, and continued for 9 to 11 weeks.
The study’s primary efficacy outcome was a “CMV event,” which was defined as CMV that affects the lung, digestive tract, or other organs, or a detectable amount of CMV in the blood at the end of treatment.
CMV events occurred in 25% (43/171) of patients who received CMX001 and 37% (22/59) of patients who received placebo. However, when CMX001 was given at the optimal dose—100 mg twice a week—only 10% of patients had a CMV event.
“The results show the effectiveness of CMX001 in preventing CMV infections in this group of patients,” Dr Marty said. “Because CMX001 is known to be active against other herpes viruses and against adenoviruses that sometimes affect transplant patients, it may be useful as a preventive or treatment agent for those infections as well.”
Most of the side effects associated with CMX001 were gastrointestinal in nature. Diarrhea was common and often serious in patients who received the drug at 200 mg twice a week.
Patients in this dose group were more likely to experience elevated alanine aminotransferase levels as well. But this was not associated with increases in levels of bilirubin or aspartate aminotransferase.
This research was funded by Chimerix, the company developing CMX001.![]()
A new drug can prevent cytomegalovirus (CMV) in patients undergoing hematopoietic stem cell transplant (HSCT), according to a study published in The New England Journal of Medicine.
The drug, called CMX001, is an oral nucleotide analog lipid-conjugate that blocks replication of double-stranded DNA viruses.
HSCT recipients who took CMX001 after engraftment were less likely to develop CMV than HSCT patients who received placebo, researchers found.
“With current agents, between 3% and 5% of allogeneic transplant patients develop CMV disease within 6 months of transplantation, and a small number of them may die of it,” said investigator Francisco Marty, MD, of the Dana-Farber Cancer Institute in Boston.
“There clearly is a need for better treatments with fewer adverse effects. This clinical trial examined whether the disease can be prevented, rather than waiting for blood tests to show that treatment is needed.”
The phase 2 trial involved 230 HSCT recipients treated at 27 centers across the US. The patients were randomized to receive placebo or CMX001 at doses ranging from 40 mg a week to 200 mg twice a week.
Treatment began after engraftment, at about 2 to 3 weeks post-transplant, and continued for 9 to 11 weeks.
The study’s primary efficacy outcome was a “CMV event,” which was defined as CMV that affects the lung, digestive tract, or other organs, or a detectable amount of CMV in the blood at the end of treatment.
CMV events occurred in 25% (43/171) of patients who received CMX001 and 37% (22/59) of patients who received placebo. However, when CMX001 was given at the optimal dose—100 mg twice a week—only 10% of patients had a CMV event.
“The results show the effectiveness of CMX001 in preventing CMV infections in this group of patients,” Dr Marty said. “Because CMX001 is known to be active against other herpes viruses and against adenoviruses that sometimes affect transplant patients, it may be useful as a preventive or treatment agent for those infections as well.”
Most of the side effects associated with CMX001 were gastrointestinal in nature. Diarrhea was common and often serious in patients who received the drug at 200 mg twice a week.
Patients in this dose group were more likely to experience elevated alanine aminotransferase levels as well. But this was not associated with increases in levels of bilirubin or aspartate aminotransferase.
This research was funded by Chimerix, the company developing CMX001.![]()
Autoantibodies play role in myositis classification, treatment
LAS VEGAS – Autoantibodies and autoantibody subsets can be particularly helpful for classifying and treating patients with myositis, including those with myositis-associated interstitial lung disease, according to Dr. Chester V. Oddis.
Many autoantibody subsets are phenotypically and clinically well defined, and have clinical relevance, said Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh.
Serological classification isn’t always accurate or routinely available for all clinicians, but this may change in the near future as improved techniques for autoantibody detection become available, he said at Perspectives in Rheumatic Diseases 2013.
Anti-MDA-5 and interstitial lung disease
One autoantibody that has gained attention in recent years is anti-MDA-5, also known as anti-CADM-140, which is often seen in patients with amyopathic dermatomyositis (ADM).
Patients with ADM represent a subset of dermatomyositis patients who have cutaneous manifestations of dermatomyositis for 6 months or longer and have no clinical evidence of proximal muscle weakness but may have mild serum muscle enzyme abnormalities. More extensive muscle testing in these patients generally demonstrates no or minimal abnormalities. However, these patients should not be considered to have simply a benign cutaneous form of disease; in fact, they have a frequency of malignancy similar to that of patients with classic dermatomyositis (14% in one series of nearly 300 patients, compared with 15% in classic dermatomyositis).
In addition, ADM patients also have a relatively high frequency of lung disease, Dr. Oddis said. In a published review of the literature of nearly 200 patients with ADM, 10% had interstitial lung disease (ILD) – an important point given that the rash of dermatomyositis may be subtle and missed, he noted.
The Asian population seems to be particularly at risk for this complication. Two studies in recent years have demonstrated that Japanese ADM patients with anti-MDA-5 present with rapidly progressive ILD. A 2011 study showed an increased incidence of acute or subacute interstitial pneumonitis in Chinese patients. Other studies have shown similar findings in Korean and other Asian populations, Dr. Oddis noted.
The presence of anti-MDA-5 represents a novel cutaneous phenotype involving palmar papules and cutaneous ulcerations, severe vasculopathy, and rapidly progressive ILD. The target autoantigen in these cases is MDA-5, which is involved in innate immune defense against viruses, he explained, noting that this supports the possibility that a viral trigger plays a role in the disease.
"I think this complication is filtering into the U.S. population, as we have seen it in our myositis cohort," Dr. Oddis said of the anti-MDA-5 association with ADM and severe ILD. He noted that he recently cared for a 70-year-old white male with "double pneumonia" (a finding that "should always raise suspicion of autoimmune ILD") in June of 2012, a rash of dermatomyositis in September of 2012, and vasculitic skin changes in January of 2013. He presented without muscle weakness.
Anti-synthetase syndrome and ILD
Another autoantibody myositis subset involves the anti-synthetases, including PL-7, PL-12, EJ, and Jo-1.
Patients with anti-synthetase syndrome are generally a clinically homogeneous patient population characterized by fever, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. About 30%-40% of myositis patients have ILD, which is a significant contributor to morbidity and mortality.
Anti-Jo-1 is found in 50%-75% of these patients, and the coexistence of Ro52 may portend worse prognosis, Dr. Oddis said.
There are certain clinical features of ILD in polymyositis and dermatomyositis, including progressive dyspnea with or without nonproductive cough, lack of digital clubbing (unlike in idiopathic pulmonary fibrosis), and lack of pleuritis and pleural effusion in most cases (unlike in systemic lupus erythematosus). However, presentation can be variable, with about one-third of patients developing ILD before muscle or skin manifestations are apparent. Some patients present with acute disease (acute respiratory distress syndrome), and others present with subacute disease that is chronic and slowly progressing or asymptomatic.
It is important to understand when making a diagnosis of autoimmune ILD that not all patients will present with the classic anti-synthetase syndrome, Dr. Oddis said.
In some cases, patients will have an "incomplete" clinical syndrome with ILD alone or ILD with only subtle connective tissue disease findings, myositis-specific autoantibodies in the absence of myositis, and/or a negative antinuclear antibody (ANA) test, he explained.
The initial symptoms in patients may vary depending on the anti-synthetase autoantibody that is present. In a University of Pittsburgh study, for example, Jo-1 was found in 60% of cases; non-Jo-1 synthetase positive cases more often experienced Raynaud’s as their initial symptom, less often experienced muscle and joint problems as their initial symptom, and had a longer delay in diagnosis, compared with Jo-1 patients. Survival was also decreased, compared with Jo-1 patients.
As for ANA, about half of patients tested positive, whereas 72% demonstrated positive anti-cytoplasmic staining on indirect immunofluorescence.
The diagnosis of autoimmune ILD can be missed when there is a failure to recognize "incomplete" clinical syndromes, when there is a failure to order or detect myositis-specific autoantibodies – even in patients without myositis – and when a negative ANA is considered to be reassuring, Dr. Oddis said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Autoantibodies and autoantibody subsets can be particularly helpful for classifying and treating patients with myositis, including those with myositis-associated interstitial lung disease, according to Dr. Chester V. Oddis.
Many autoantibody subsets are phenotypically and clinically well defined, and have clinical relevance, said Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh.
Serological classification isn’t always accurate or routinely available for all clinicians, but this may change in the near future as improved techniques for autoantibody detection become available, he said at Perspectives in Rheumatic Diseases 2013.
Anti-MDA-5 and interstitial lung disease
One autoantibody that has gained attention in recent years is anti-MDA-5, also known as anti-CADM-140, which is often seen in patients with amyopathic dermatomyositis (ADM).
Patients with ADM represent a subset of dermatomyositis patients who have cutaneous manifestations of dermatomyositis for 6 months or longer and have no clinical evidence of proximal muscle weakness but may have mild serum muscle enzyme abnormalities. More extensive muscle testing in these patients generally demonstrates no or minimal abnormalities. However, these patients should not be considered to have simply a benign cutaneous form of disease; in fact, they have a frequency of malignancy similar to that of patients with classic dermatomyositis (14% in one series of nearly 300 patients, compared with 15% in classic dermatomyositis).
In addition, ADM patients also have a relatively high frequency of lung disease, Dr. Oddis said. In a published review of the literature of nearly 200 patients with ADM, 10% had interstitial lung disease (ILD) – an important point given that the rash of dermatomyositis may be subtle and missed, he noted.
The Asian population seems to be particularly at risk for this complication. Two studies in recent years have demonstrated that Japanese ADM patients with anti-MDA-5 present with rapidly progressive ILD. A 2011 study showed an increased incidence of acute or subacute interstitial pneumonitis in Chinese patients. Other studies have shown similar findings in Korean and other Asian populations, Dr. Oddis noted.
The presence of anti-MDA-5 represents a novel cutaneous phenotype involving palmar papules and cutaneous ulcerations, severe vasculopathy, and rapidly progressive ILD. The target autoantigen in these cases is MDA-5, which is involved in innate immune defense against viruses, he explained, noting that this supports the possibility that a viral trigger plays a role in the disease.
"I think this complication is filtering into the U.S. population, as we have seen it in our myositis cohort," Dr. Oddis said of the anti-MDA-5 association with ADM and severe ILD. He noted that he recently cared for a 70-year-old white male with "double pneumonia" (a finding that "should always raise suspicion of autoimmune ILD") in June of 2012, a rash of dermatomyositis in September of 2012, and vasculitic skin changes in January of 2013. He presented without muscle weakness.
Anti-synthetase syndrome and ILD
Another autoantibody myositis subset involves the anti-synthetases, including PL-7, PL-12, EJ, and Jo-1.
Patients with anti-synthetase syndrome are generally a clinically homogeneous patient population characterized by fever, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. About 30%-40% of myositis patients have ILD, which is a significant contributor to morbidity and mortality.
Anti-Jo-1 is found in 50%-75% of these patients, and the coexistence of Ro52 may portend worse prognosis, Dr. Oddis said.
There are certain clinical features of ILD in polymyositis and dermatomyositis, including progressive dyspnea with or without nonproductive cough, lack of digital clubbing (unlike in idiopathic pulmonary fibrosis), and lack of pleuritis and pleural effusion in most cases (unlike in systemic lupus erythematosus). However, presentation can be variable, with about one-third of patients developing ILD before muscle or skin manifestations are apparent. Some patients present with acute disease (acute respiratory distress syndrome), and others present with subacute disease that is chronic and slowly progressing or asymptomatic.
It is important to understand when making a diagnosis of autoimmune ILD that not all patients will present with the classic anti-synthetase syndrome, Dr. Oddis said.
In some cases, patients will have an "incomplete" clinical syndrome with ILD alone or ILD with only subtle connective tissue disease findings, myositis-specific autoantibodies in the absence of myositis, and/or a negative antinuclear antibody (ANA) test, he explained.
The initial symptoms in patients may vary depending on the anti-synthetase autoantibody that is present. In a University of Pittsburgh study, for example, Jo-1 was found in 60% of cases; non-Jo-1 synthetase positive cases more often experienced Raynaud’s as their initial symptom, less often experienced muscle and joint problems as their initial symptom, and had a longer delay in diagnosis, compared with Jo-1 patients. Survival was also decreased, compared with Jo-1 patients.
As for ANA, about half of patients tested positive, whereas 72% demonstrated positive anti-cytoplasmic staining on indirect immunofluorescence.
The diagnosis of autoimmune ILD can be missed when there is a failure to recognize "incomplete" clinical syndromes, when there is a failure to order or detect myositis-specific autoantibodies – even in patients without myositis – and when a negative ANA is considered to be reassuring, Dr. Oddis said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Autoantibodies and autoantibody subsets can be particularly helpful for classifying and treating patients with myositis, including those with myositis-associated interstitial lung disease, according to Dr. Chester V. Oddis.
Many autoantibody subsets are phenotypically and clinically well defined, and have clinical relevance, said Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh.
Serological classification isn’t always accurate or routinely available for all clinicians, but this may change in the near future as improved techniques for autoantibody detection become available, he said at Perspectives in Rheumatic Diseases 2013.
Anti-MDA-5 and interstitial lung disease
One autoantibody that has gained attention in recent years is anti-MDA-5, also known as anti-CADM-140, which is often seen in patients with amyopathic dermatomyositis (ADM).
Patients with ADM represent a subset of dermatomyositis patients who have cutaneous manifestations of dermatomyositis for 6 months or longer and have no clinical evidence of proximal muscle weakness but may have mild serum muscle enzyme abnormalities. More extensive muscle testing in these patients generally demonstrates no or minimal abnormalities. However, these patients should not be considered to have simply a benign cutaneous form of disease; in fact, they have a frequency of malignancy similar to that of patients with classic dermatomyositis (14% in one series of nearly 300 patients, compared with 15% in classic dermatomyositis).
In addition, ADM patients also have a relatively high frequency of lung disease, Dr. Oddis said. In a published review of the literature of nearly 200 patients with ADM, 10% had interstitial lung disease (ILD) – an important point given that the rash of dermatomyositis may be subtle and missed, he noted.
The Asian population seems to be particularly at risk for this complication. Two studies in recent years have demonstrated that Japanese ADM patients with anti-MDA-5 present with rapidly progressive ILD. A 2011 study showed an increased incidence of acute or subacute interstitial pneumonitis in Chinese patients. Other studies have shown similar findings in Korean and other Asian populations, Dr. Oddis noted.
The presence of anti-MDA-5 represents a novel cutaneous phenotype involving palmar papules and cutaneous ulcerations, severe vasculopathy, and rapidly progressive ILD. The target autoantigen in these cases is MDA-5, which is involved in innate immune defense against viruses, he explained, noting that this supports the possibility that a viral trigger plays a role in the disease.
"I think this complication is filtering into the U.S. population, as we have seen it in our myositis cohort," Dr. Oddis said of the anti-MDA-5 association with ADM and severe ILD. He noted that he recently cared for a 70-year-old white male with "double pneumonia" (a finding that "should always raise suspicion of autoimmune ILD") in June of 2012, a rash of dermatomyositis in September of 2012, and vasculitic skin changes in January of 2013. He presented without muscle weakness.
Anti-synthetase syndrome and ILD
Another autoantibody myositis subset involves the anti-synthetases, including PL-7, PL-12, EJ, and Jo-1.
Patients with anti-synthetase syndrome are generally a clinically homogeneous patient population characterized by fever, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. About 30%-40% of myositis patients have ILD, which is a significant contributor to morbidity and mortality.
Anti-Jo-1 is found in 50%-75% of these patients, and the coexistence of Ro52 may portend worse prognosis, Dr. Oddis said.
There are certain clinical features of ILD in polymyositis and dermatomyositis, including progressive dyspnea with or without nonproductive cough, lack of digital clubbing (unlike in idiopathic pulmonary fibrosis), and lack of pleuritis and pleural effusion in most cases (unlike in systemic lupus erythematosus). However, presentation can be variable, with about one-third of patients developing ILD before muscle or skin manifestations are apparent. Some patients present with acute disease (acute respiratory distress syndrome), and others present with subacute disease that is chronic and slowly progressing or asymptomatic.
It is important to understand when making a diagnosis of autoimmune ILD that not all patients will present with the classic anti-synthetase syndrome, Dr. Oddis said.
In some cases, patients will have an "incomplete" clinical syndrome with ILD alone or ILD with only subtle connective tissue disease findings, myositis-specific autoantibodies in the absence of myositis, and/or a negative antinuclear antibody (ANA) test, he explained.
The initial symptoms in patients may vary depending on the anti-synthetase autoantibody that is present. In a University of Pittsburgh study, for example, Jo-1 was found in 60% of cases; non-Jo-1 synthetase positive cases more often experienced Raynaud’s as their initial symptom, less often experienced muscle and joint problems as their initial symptom, and had a longer delay in diagnosis, compared with Jo-1 patients. Survival was also decreased, compared with Jo-1 patients.
As for ANA, about half of patients tested positive, whereas 72% demonstrated positive anti-cytoplasmic staining on indirect immunofluorescence.
The diagnosis of autoimmune ILD can be missed when there is a failure to recognize "incomplete" clinical syndromes, when there is a failure to order or detect myositis-specific autoantibodies – even in patients without myositis – and when a negative ANA is considered to be reassuring, Dr. Oddis said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
AT PERSPECTIVES IN RHEUMATIC DISEASES 2013
Doctoring in the information age
Many of my patients come in having already looked up their symptoms. Frequently, this admission is accompanied by a mumbled apology, an acknowledgment that doctors "hate that."
I do not disapprove of patients looking up their symptoms at all. I find that their online forays only enrich the discussion because they are prepared for the meeting and have excellent questions. I appreciate their curiosity; it serves the pedant in me well. Not only do I get to explain what is not clear, I also get to correct misinformation.
It also indicates a sense of ownership, participation rather than passivity. The patients are engaged in coming up with a treatment plan, and hopefully this translates into compliance. Patients have asked about dietary modifications for their gout, pool exercises for their osteoarthritis, or the wisdom of biologic treatments in light of their past breast cancer. I think it provides patients with a sense of some degree, however small, of control over their disease and its treatment.
But not all information is created equal, and I think some information may be more fraught for a nonmedical consumer.
At the moment, there are some electronic health record (EHR) software programs that give patients access to some lab results. This can be quite handy; for example, I’ve been saved the trouble of interrupting a patient visit to track down lab results because the patient could access the results using his smartphone.
But it can be pernicious, too. The fine art of medicine does not always lend itself to discrete labeling. Think of all the slightly elevated creatine kinases, erythrocyte sedimentation rates, and antinuclear antibodies that you encounter daily, as well as the training that you went through to understand what to do with these mildly abnormal labs. For all our focus on evidence-based medicine, there is a great deal of nuance to what we do, and nominal "normal" vs "abnormal" results can be misleading at best and anxiety-provoking at worst.
In addition, we may someday be required to allow patients to see office notes. Already I’ve received a handful of angry phone calls from patients because they got a copy of my note. One patient was mad because he thought I called him anorexic (he calmed down when I explained what anorexia meant). Another patient was angry because I used obesity as a diagnosis (she, on the other hand, did not calm down at all). I had a patient come in for her follow-up visit with a copy of my first note, which she had very heavily annotated with details that were irrelevant in a medical context, demanding that I modify my first note.
You may have heard of the study published last October in the Annals of Internal Medicine (2012;157:461-70), in which 105 doctors in three different health care systems allowed more than 13,000 patients to read their notes for a year. Overall, the results were not bad. Doctors felt that it improved transparency and shared decision making, and patients agreed that it fostered trust.
But a minority of patients did report feeling anxious or offended. One out of three felt that they ought to be able to approve the note. And doctors reported having to modify their note – not discussing alternative diagnoses, for example, or not being as candid about obesity. Which belies the idea that the system "improved transparency," no?
I have an old mentor who used to say that in order for consent to be truly informed, the patient should have also gone to medical school. If we are being frank, we ought to admit that there are limits to what a patient can understand, and full disclosure may not be always be appropriate.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Many of my patients come in having already looked up their symptoms. Frequently, this admission is accompanied by a mumbled apology, an acknowledgment that doctors "hate that."
I do not disapprove of patients looking up their symptoms at all. I find that their online forays only enrich the discussion because they are prepared for the meeting and have excellent questions. I appreciate their curiosity; it serves the pedant in me well. Not only do I get to explain what is not clear, I also get to correct misinformation.
It also indicates a sense of ownership, participation rather than passivity. The patients are engaged in coming up with a treatment plan, and hopefully this translates into compliance. Patients have asked about dietary modifications for their gout, pool exercises for their osteoarthritis, or the wisdom of biologic treatments in light of their past breast cancer. I think it provides patients with a sense of some degree, however small, of control over their disease and its treatment.
But not all information is created equal, and I think some information may be more fraught for a nonmedical consumer.
At the moment, there are some electronic health record (EHR) software programs that give patients access to some lab results. This can be quite handy; for example, I’ve been saved the trouble of interrupting a patient visit to track down lab results because the patient could access the results using his smartphone.
But it can be pernicious, too. The fine art of medicine does not always lend itself to discrete labeling. Think of all the slightly elevated creatine kinases, erythrocyte sedimentation rates, and antinuclear antibodies that you encounter daily, as well as the training that you went through to understand what to do with these mildly abnormal labs. For all our focus on evidence-based medicine, there is a great deal of nuance to what we do, and nominal "normal" vs "abnormal" results can be misleading at best and anxiety-provoking at worst.
In addition, we may someday be required to allow patients to see office notes. Already I’ve received a handful of angry phone calls from patients because they got a copy of my note. One patient was mad because he thought I called him anorexic (he calmed down when I explained what anorexia meant). Another patient was angry because I used obesity as a diagnosis (she, on the other hand, did not calm down at all). I had a patient come in for her follow-up visit with a copy of my first note, which she had very heavily annotated with details that were irrelevant in a medical context, demanding that I modify my first note.
You may have heard of the study published last October in the Annals of Internal Medicine (2012;157:461-70), in which 105 doctors in three different health care systems allowed more than 13,000 patients to read their notes for a year. Overall, the results were not bad. Doctors felt that it improved transparency and shared decision making, and patients agreed that it fostered trust.
But a minority of patients did report feeling anxious or offended. One out of three felt that they ought to be able to approve the note. And doctors reported having to modify their note – not discussing alternative diagnoses, for example, or not being as candid about obesity. Which belies the idea that the system "improved transparency," no?
I have an old mentor who used to say that in order for consent to be truly informed, the patient should have also gone to medical school. If we are being frank, we ought to admit that there are limits to what a patient can understand, and full disclosure may not be always be appropriate.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Many of my patients come in having already looked up their symptoms. Frequently, this admission is accompanied by a mumbled apology, an acknowledgment that doctors "hate that."
I do not disapprove of patients looking up their symptoms at all. I find that their online forays only enrich the discussion because they are prepared for the meeting and have excellent questions. I appreciate their curiosity; it serves the pedant in me well. Not only do I get to explain what is not clear, I also get to correct misinformation.
It also indicates a sense of ownership, participation rather than passivity. The patients are engaged in coming up with a treatment plan, and hopefully this translates into compliance. Patients have asked about dietary modifications for their gout, pool exercises for their osteoarthritis, or the wisdom of biologic treatments in light of their past breast cancer. I think it provides patients with a sense of some degree, however small, of control over their disease and its treatment.
But not all information is created equal, and I think some information may be more fraught for a nonmedical consumer.
At the moment, there are some electronic health record (EHR) software programs that give patients access to some lab results. This can be quite handy; for example, I’ve been saved the trouble of interrupting a patient visit to track down lab results because the patient could access the results using his smartphone.
But it can be pernicious, too. The fine art of medicine does not always lend itself to discrete labeling. Think of all the slightly elevated creatine kinases, erythrocyte sedimentation rates, and antinuclear antibodies that you encounter daily, as well as the training that you went through to understand what to do with these mildly abnormal labs. For all our focus on evidence-based medicine, there is a great deal of nuance to what we do, and nominal "normal" vs "abnormal" results can be misleading at best and anxiety-provoking at worst.
In addition, we may someday be required to allow patients to see office notes. Already I’ve received a handful of angry phone calls from patients because they got a copy of my note. One patient was mad because he thought I called him anorexic (he calmed down when I explained what anorexia meant). Another patient was angry because I used obesity as a diagnosis (she, on the other hand, did not calm down at all). I had a patient come in for her follow-up visit with a copy of my first note, which she had very heavily annotated with details that were irrelevant in a medical context, demanding that I modify my first note.
You may have heard of the study published last October in the Annals of Internal Medicine (2012;157:461-70), in which 105 doctors in three different health care systems allowed more than 13,000 patients to read their notes for a year. Overall, the results were not bad. Doctors felt that it improved transparency and shared decision making, and patients agreed that it fostered trust.
But a minority of patients did report feeling anxious or offended. One out of three felt that they ought to be able to approve the note. And doctors reported having to modify their note – not discussing alternative diagnoses, for example, or not being as candid about obesity. Which belies the idea that the system "improved transparency," no?
I have an old mentor who used to say that in order for consent to be truly informed, the patient should have also gone to medical school. If we are being frank, we ought to admit that there are limits to what a patient can understand, and full disclosure may not be always be appropriate.
Dr. Chan practices rheumatology in Pawtucket, R.I.