User login
A nondrug approach to dementia
› Attempt nonpharmacologic treatment for dementia behavioral problems before moving on to medications, which are of questionable efficacy for symptoms other than aggression and psychosis. A
› Obtain informed consent from patients and/or their caregivers if you plan to use antipsychotic medications because their use increases morbidity and mortality in the elderly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Ms. M, 86 years old, lives with her daughter, son-in-law, and granddaughter. For several years she has been forgetful, but she has never had a formal work-up for dementia. Her daughter finally brings her to their primary care physician because she was refusing to take showers, was increasingly irritable, and had tried to hit her daughter’s husband.
In the office, however, Ms. M is calm and pleasant. The family says that most nights Ms. M gets up and wanders around the house. She denies feeling depressed or anxious, but her Folstein Mini-Mental State Exam score is 22/30, indicating moderate dementia. (For more on assessment, see “Tools for assessing patients with dementia—and their caregivers” on page 552.)
The physician offers a trial of risperidone 0.25 mg at bedtime to assist with sleep and behavior.
Was this prescription a wise decision? What other questions should this physician have asked?
Understanding the behavioral symptoms
Noncognitive symptoms of dementia, sometimes referred to as behavioral and psychological symptoms, are common, affecting almost 90% of patients with dementia,1-3 which itself can be classified as early, intermediate, and late.
In early dementia, sociability is usually not affected, but patients may repeat questions, misplace items, use poor judgment, and begin to have difficulty with more complex daily tasks like finances and driving.
In intermediate dementia, basic activities of daily living become impaired and normal social and environmental cues may not register.
In late dementia, patients become entirely dependent on others; they may lose the ability to speak, walk, and eventually, eat. Long- and short-term memory is lost.
Behavioral symptoms most often occur when the condition enters the intermediate phase, but they may occur at any time during the course of the disease.4 Behaviors may include refusal of care, yelling, aggressive behavior, agitation, restlessness, reversal of the normal sleep-wake cycle, wandering, hoarding, sexual disinhibition, culturally inappropriate behaviors, hallucinations, delusions, anxiety, depression, apathy, and psychosis.2,5
Behavioral disturbances often overwhelm families, and lack of treatment increases patient morbidity, may result in physical harm, and almost always precipitates institutionalization.2 Dementia-related behavioral disturbances also increase the risk of caregiver burnout and depression.2
These symptoms are difficult to treat with medications or nonpharmacologic therapy and strong evidence for most therapies is lacking. Physicians have historically prescribed either typical or atypical antipsychotics in an attempt to control these behaviors. In fact, medication is often still considered first-line therapy.6,7
CASE Ms. M’s daughter calls the clinic 2 weeks after the initial visit to tell the physician that her mother has been sleeping much better, but had a fall and was admitted to the hospital for a hip fracture. That’s not surprising; typical and atypical antipsychotics increase the risk of falls in the elderly.8
The risks associated with the use of antipsychotics
In 2005, the US Food and Drug Administration (FDA) issued a black box warning for atypical antipsychotics because they were found to increase mortality in the elderly. The increased mortality is due to cardiac events or infection.9,10 In 2008, the FDA warning was added to typical antipsychotics, as well.11,12 Both typical and atypical antipsychotics have been found to increase the risk of falls and strokes in the elderly,8,13 and their efficacy in treating the behavioral and psychological symptoms of dementia has recently been questioned.13-16
Trazodone and medications approved for the specific treatment of cognitive decline, such as donepezil or memantine, are also prescribed for behavioral disturbances, but evidence to support their efficacy is limited.14,17-20 More recently, a meta-analysis of selective serotonin reuptake inhibitors (SSRIs) suggests that they may be effective for treating agitation associated with dementia.21 However, SSRIs may also contribute to falls and to hyponatremia in the elderly.22,23
Pharmacologic Tx is not your only option
Considering the questionable safety and efficacy of pharmacologic treatment, physicians should consider nondrug therapies first, or at least concurrently with medication.2,15,16,24
But before you get started, be sure to look for and treat medical conditions that cause or contribute to behavioral disturbances, including infection, pain, and adverse effects of medication.6,7,25 Similarly, it is essential that unmet needs, such as hunger, thirst, or desire for attention or socialization, be addressed.6,7,26 Also, discuss disturbing environmental factors, including loud noises, poorly lit quarters, and strong smells, with patients and their caregivers.5-7 In complex situations, you may need to seek assistance from a geriatrician, neurologist, geropsychiatrist, or psychologist, although their availability may be limited.6
CASE Ms. M becomes markedly delirious while in the hospital after hip surgery, and a geriatrics consultation is requested. This is not surprising, given that underlying dementia increases a patient’s risk of delirium in the hospital.27 The geriatrician recommends several measures to reduce the likelihood of delirium—providing good pain control, minimizing night time wake-ups, minimizing Foley catheter use, Hep-locking the IV to encourage mobility, and having staff reorient her frequently by referring to a large print clock and calendar on the wall.
Specific interventions
Most specific nonpharmacologic therapies have not been robustly studied in randomized controlled trials. But a series of smaller studies have been evaluated in systematic reviews. The level of evidence for each intervention is summarized in TABLE 1.28-40
As you review the options that follow, keep 2 things in mind: (1) It is important to set realistic expectations when considering these approaches (as well as pharmacologic ones). Reducing the frequency or severity of problematic behaviors may be more reasonable than their total elimination.6,25 (2) Consider targeting specific symptoms when treating behavioral Behavioral symptoms most often occur when dementia enters the intermediate phase, but they may occur at any time during the course of the disease. disturbances.2,24,41 Such targeting allows physicians and families to better evaluate the effectiveness of interventions because it helps to focus the discussion of the patient’s progress at follow-up visits.
Massage/touch therapy. A 2006 Cochrane review concluded that improvement in nutritional intake and hand massage, when combined with positive encouragement during a meal, may produce a short-term positive effect on agitation.29 Similarly, a meta-analysis of randomized controlled and randomized crossover studies found a statistically significant improvement in agitation with hand massage, although this finding was based on the same single study referenced in the 2006 review.30 Opinions differ among 5 high-quality guidelines included in the systematic review by Azermai et al regarding the value of massage, with 2 of the 5 practice guidelines recommending its use.28
Aromatherapy. Several trials suggest that aroma therapy may reduce agitated behaviors. Lemon balm and lavender oils have been the most commonly studied agents. Two systematic reviews cite the same 2002 randomized controlled trial, which found a reduction in behavioral problems in people who received arm massage with lemon balm compared with those who received arm massage with an odorless cream.30,31 A systematic review by Holt et al also cites a study that found lavender oil placed in a sachet on each side of the pillow for at least one hour during sleep seemed to reduce problem behaviors.31 Several evidence-based guidelines have concluded that aromatherapy may be helpful, and 2 of the 5 practice guidelines reviewed by Azermai et al recommend it.28
Exercise has been shown to benefit patients of all ages, even those with terminal diseases.42 Some studies have indicated a positive effect of physical activities on behaviors ranging from wandering to aggression and agitation. Activities have included group gentle stretches, indoor exercises, and a volunteer-led walking program that encouraged hand holding and singing.34 However, a 2008 Cochrane review concluded that the effect of exercise on behavioral disturbances in dementia has not been adequately studied.35
Music therapy. Numerous types of music therapy have been studied, including listening to music picked out by a patient’s family based on known patient preference, classical music, pleasant sounds such as ocean waves, and even stories and comforting prayer recorded by family members. While most of these smaller studies yielded positive results,34 a 2003 Cochrane review concluded there is not enough evidence to recommend for or against music therapy.43 A more recent meta-analysis suggests that music may be effective for agitation.30 A systematic review of quality guidelines also indicates that most of these guidelines rate the evidence as moderate to high in favor of music and 3 of 5 practice guidelines recommend it.28
Nonphysical barriers have long been used as a creative nonrestraining method of preventing wandering. They include such tricks as camouflaging exits by painting them to look like bookcases, painting a black square in front of an elevator to make it look like a hole, and placing a thin Velcro strip across doorways. Although it would appear from a limited number of small studies and anecdotal evidence that nonphysical barriers work, a Cochrane review concluded that they have not been studied enough to perform a meta-analysis.36
Cognitive stimulation typically consists of activities such as reviewing current events, promoting sensory awareness, drawing, associating words, discussion of hobbies, and planning daily activities. This type of therapy has been shown to improve cognition in patients with dementia, as well as well-being and quality of life. It does not improve behavioral problems, per se.37
Reminiscence therapy is a popular modality that involves stimulating memories of the past by looking at personal photos and newspaper clippings and discussing the past. It is well received by patients and caregivers. It has been shown to improve mood in elderly patients without dementia, but studies of reminiscence therapy have been too dissimilar to draw conclusions regarding its effect on behavioral disturbances in patients with dementia.38
Other therapies that are common in dementia care, such as respite care and specialized dementia units, have simply not been studied well enough to provide any conclusions as to their effectiveness.39,40
CASE When Ms. M is discharged from the hospital, her family enrolls her in an adult day care program, where Ms. M will be able to participate in social activities, exercise, and communal meals. Her daughter asks the family physician what other steps they can take in the home to make things easier on her mother. And as an aside, the daughter admits that while she is glad that she and her family can “be there” for her mother, there have been times when she has simply not felt up to the task.
Help family members care for the patient—and themselves
A recent meta-analysis suggests that caregiver interventions have a positive effect on behavioral problems in patients with dementia.32 Successful programs are tailored to the individual needs of the patient and caregiver and delivered over multiple sessions. Unfortunately, the aforementioned meta-analysis did not provide evidenced-based interventions for specific problems.32 With this in mind, the following are some practical caregiver “do’s and don’ts” that are based on reviews and consensus guidelines.
Don’t take it personally. It is extremely important to help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder.25 Caregivers also need to appreciate that hallucinations are normal in these patients and do not require medications if they don’t disturb the patient or place the patient or anyone else at risk.
Don’t try to reason with the patient; redirect him or her instead. Clinicians should offer caregivers suggestions for reassuring, redirecting, or distracting agitated patients rather than trying to reason with them. Encourage caregivers to develop and maintain routines and consistency.6,25 Using a calm, low tone of voice, giving very simple instructions, and leaving and then reattempting care that is refused the first time may also be effective.5 Some experts have suggested techniques such as giving positive rewards for desired behaviors and not rewarding negative behaviors.6,26
Do create a safe environment. Recommend that caregivers create a safe environment. Make sure that they lock up all guns. Also, encourage them to use locks, alarms, or ID bracelets when patients are prone to wandering.25
Do consider a caregiver support program. Caregivers can make a big difference in the lives of patients with dementia, Help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder. but only if they have support, as well.
A recent meta-analysis concluded that active involvement of caregivers in making choices about treatments distinguishes effective from ineffective support programs, decreases the odds of institutionalization, and may lengthen time to institutionalization.33 To ease caregiver strain and depression, encourage them to make use of resources such as nursing home respite care and community agencies that include the Alzheimer’s Association (http://www.alz.org).6,44,45
CASE Ms. M’s daughter joins a local support group for families of patients with dementia, where she learns redirection techniques to try when her mother refuses care. The exercise and daytime social stimulation that Ms. M receives through the adult day care program helps her to sleep at night. When Ms. M refuses to take a shower—a challenge the family had before her hospitalization—the daughter does not argue with her. Instead, she returns 10 to 20 minutes later and asks again, or tries a bedside sponge bath with a lavender soap that Ms. M seems to like.
Ms. M’s nighttime wandering is markedly reduced and the family no longer uses any antipsychotic medications. The family physician counsels them, however, about the progressive nature of the disease and encourages them to set up periodic follow-up visits, so that he can see how everyone—patient and caregivers alike—are doing.
Welcoming the reprieves, recognizing the realities
The behavioral and psychological symptoms of dementia are the most challenging aspect of dementia care. Unacceptable behaviors sometimes persist even when aggressively addressing modifiable factors and attempting behavioral interventions (TABLE 2).2,5-7,15,16,24-26,32,41,44-46 Patients with behavioral disturbances frequently require a pharmacologic agent or transfer to a different care setting.
But clinicians need to use psychotropic medications with informed patient and/or caregiver consent.7 On a case-by-case basis, a trial of antipsychotics is often justified, despite the black box warning. A family may choose to try an antipsychotic despite the risk to help manage the patient at home in the hope of delaying or preventing institutionalization.
However, even with good home support, in conjunction with nonpharmacologic and/or pharmacologic therapies, most patients with dementia will eventually require institutionalization.47 Because patients and families often rely on family physicians to guide them through these difficult challenges and decisions, you’ll need to remain well versed on the available On a case-by- case basis, a trial of antipsychotics is often justified, despite the black box warning. treatments for the psychological and behavioral symptoms of dementia, as well as the resources available in your community.
CORRESPONDENCE
Jaqueline Raetz, MD, 331 NE Thornton Place, Seattle, WA 98125; [email protected]
1. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
2. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S293-S301.
3. Hort J, O’Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol. 2010;17:1236-1248.
4. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
5. Omelan C. Approach to managing behavioural disturbances in dementia. Can Fam Physician. 2006;52:191-199.
6. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
7. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
8. Hill KD, Wee R. Psychotropic drug-induced falls in older people: a review of interventions aimed at reducing the problem. Drugs Aging. 2012;29:15-30.
9. US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed September 16, 2013.
10. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
11. US Food and Drug Administration. Antipsychotics, conventional and atypical. June 16, 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed September 16, 2013.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335-2341.
13. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
14. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596-608.
15. Lonergan E, Luxenberg J, Colford JM. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
16. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
17. Martinón-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;(4):CD004990.
18. Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD001190.
19. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154.
20. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
21. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2): CD008191.
22. Sterke CS, Ziere G, van Beeck EF, et al. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br J Clin Pharmacol. 2012;73:812-820.
23. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622.
24. Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer’s disease management guideline: update 2008. Alzheimers Dement. 2011;7:e51-e59.
25. Rayner A, O’Brien J, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647-652.
26. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182-2188.
27. Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204-212.
28. Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Aging Res Rev. 2012;11:78-86.
29. Viggo Hansen N, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989.
30. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512-520.
31. Thorgrimsen LM, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150.
32. Brodaty H, Arasaratnam C. Review of meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-953.
33. Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: A meta-analysis. J Am Geriatr Soc. 2008;56:1116-1128.
34. Opie J, Rosewarne R, O’Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Aust N Z J Psychiatry. 1999;33:789-799.
35. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489.
36. Price JD, Hermans DG, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev. 2000;(4):CD001932.
37. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562.
38. Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120.
39. Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2004;(2):CD004396.
40. Lai CK, Yeung JH, Mok V, et al. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev. 2009;(4):CD006470.
41. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1-e26.
42. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421-430.
43. Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477.
44. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020-2029.
45. Bass DM, Clark PA, Looman WJ, et al. The Cleveland Alzheimer’s managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73-85.
46. Gitlin LN, Winter L, Dennis MP, et al.. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983-991.
47. Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519-525.
› Attempt nonpharmacologic treatment for dementia behavioral problems before moving on to medications, which are of questionable efficacy for symptoms other than aggression and psychosis. A
› Obtain informed consent from patients and/or their caregivers if you plan to use antipsychotic medications because their use increases morbidity and mortality in the elderly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Ms. M, 86 years old, lives with her daughter, son-in-law, and granddaughter. For several years she has been forgetful, but she has never had a formal work-up for dementia. Her daughter finally brings her to their primary care physician because she was refusing to take showers, was increasingly irritable, and had tried to hit her daughter’s husband.
In the office, however, Ms. M is calm and pleasant. The family says that most nights Ms. M gets up and wanders around the house. She denies feeling depressed or anxious, but her Folstein Mini-Mental State Exam score is 22/30, indicating moderate dementia. (For more on assessment, see “Tools for assessing patients with dementia—and their caregivers” on page 552.)
The physician offers a trial of risperidone 0.25 mg at bedtime to assist with sleep and behavior.
Was this prescription a wise decision? What other questions should this physician have asked?
Understanding the behavioral symptoms
Noncognitive symptoms of dementia, sometimes referred to as behavioral and psychological symptoms, are common, affecting almost 90% of patients with dementia,1-3 which itself can be classified as early, intermediate, and late.
In early dementia, sociability is usually not affected, but patients may repeat questions, misplace items, use poor judgment, and begin to have difficulty with more complex daily tasks like finances and driving.
In intermediate dementia, basic activities of daily living become impaired and normal social and environmental cues may not register.
In late dementia, patients become entirely dependent on others; they may lose the ability to speak, walk, and eventually, eat. Long- and short-term memory is lost.
Behavioral symptoms most often occur when the condition enters the intermediate phase, but they may occur at any time during the course of the disease.4 Behaviors may include refusal of care, yelling, aggressive behavior, agitation, restlessness, reversal of the normal sleep-wake cycle, wandering, hoarding, sexual disinhibition, culturally inappropriate behaviors, hallucinations, delusions, anxiety, depression, apathy, and psychosis.2,5
Behavioral disturbances often overwhelm families, and lack of treatment increases patient morbidity, may result in physical harm, and almost always precipitates institutionalization.2 Dementia-related behavioral disturbances also increase the risk of caregiver burnout and depression.2
These symptoms are difficult to treat with medications or nonpharmacologic therapy and strong evidence for most therapies is lacking. Physicians have historically prescribed either typical or atypical antipsychotics in an attempt to control these behaviors. In fact, medication is often still considered first-line therapy.6,7
CASE Ms. M’s daughter calls the clinic 2 weeks after the initial visit to tell the physician that her mother has been sleeping much better, but had a fall and was admitted to the hospital for a hip fracture. That’s not surprising; typical and atypical antipsychotics increase the risk of falls in the elderly.8
The risks associated with the use of antipsychotics
In 2005, the US Food and Drug Administration (FDA) issued a black box warning for atypical antipsychotics because they were found to increase mortality in the elderly. The increased mortality is due to cardiac events or infection.9,10 In 2008, the FDA warning was added to typical antipsychotics, as well.11,12 Both typical and atypical antipsychotics have been found to increase the risk of falls and strokes in the elderly,8,13 and their efficacy in treating the behavioral and psychological symptoms of dementia has recently been questioned.13-16
Trazodone and medications approved for the specific treatment of cognitive decline, such as donepezil or memantine, are also prescribed for behavioral disturbances, but evidence to support their efficacy is limited.14,17-20 More recently, a meta-analysis of selective serotonin reuptake inhibitors (SSRIs) suggests that they may be effective for treating agitation associated with dementia.21 However, SSRIs may also contribute to falls and to hyponatremia in the elderly.22,23
Pharmacologic Tx is not your only option
Considering the questionable safety and efficacy of pharmacologic treatment, physicians should consider nondrug therapies first, or at least concurrently with medication.2,15,16,24
But before you get started, be sure to look for and treat medical conditions that cause or contribute to behavioral disturbances, including infection, pain, and adverse effects of medication.6,7,25 Similarly, it is essential that unmet needs, such as hunger, thirst, or desire for attention or socialization, be addressed.6,7,26 Also, discuss disturbing environmental factors, including loud noises, poorly lit quarters, and strong smells, with patients and their caregivers.5-7 In complex situations, you may need to seek assistance from a geriatrician, neurologist, geropsychiatrist, or psychologist, although their availability may be limited.6
CASE Ms. M becomes markedly delirious while in the hospital after hip surgery, and a geriatrics consultation is requested. This is not surprising, given that underlying dementia increases a patient’s risk of delirium in the hospital.27 The geriatrician recommends several measures to reduce the likelihood of delirium—providing good pain control, minimizing night time wake-ups, minimizing Foley catheter use, Hep-locking the IV to encourage mobility, and having staff reorient her frequently by referring to a large print clock and calendar on the wall.
Specific interventions
Most specific nonpharmacologic therapies have not been robustly studied in randomized controlled trials. But a series of smaller studies have been evaluated in systematic reviews. The level of evidence for each intervention is summarized in TABLE 1.28-40
As you review the options that follow, keep 2 things in mind: (1) It is important to set realistic expectations when considering these approaches (as well as pharmacologic ones). Reducing the frequency or severity of problematic behaviors may be more reasonable than their total elimination.6,25 (2) Consider targeting specific symptoms when treating behavioral Behavioral symptoms most often occur when dementia enters the intermediate phase, but they may occur at any time during the course of the disease. disturbances.2,24,41 Such targeting allows physicians and families to better evaluate the effectiveness of interventions because it helps to focus the discussion of the patient’s progress at follow-up visits.
Massage/touch therapy. A 2006 Cochrane review concluded that improvement in nutritional intake and hand massage, when combined with positive encouragement during a meal, may produce a short-term positive effect on agitation.29 Similarly, a meta-analysis of randomized controlled and randomized crossover studies found a statistically significant improvement in agitation with hand massage, although this finding was based on the same single study referenced in the 2006 review.30 Opinions differ among 5 high-quality guidelines included in the systematic review by Azermai et al regarding the value of massage, with 2 of the 5 practice guidelines recommending its use.28
Aromatherapy. Several trials suggest that aroma therapy may reduce agitated behaviors. Lemon balm and lavender oils have been the most commonly studied agents. Two systematic reviews cite the same 2002 randomized controlled trial, which found a reduction in behavioral problems in people who received arm massage with lemon balm compared with those who received arm massage with an odorless cream.30,31 A systematic review by Holt et al also cites a study that found lavender oil placed in a sachet on each side of the pillow for at least one hour during sleep seemed to reduce problem behaviors.31 Several evidence-based guidelines have concluded that aromatherapy may be helpful, and 2 of the 5 practice guidelines reviewed by Azermai et al recommend it.28
Exercise has been shown to benefit patients of all ages, even those with terminal diseases.42 Some studies have indicated a positive effect of physical activities on behaviors ranging from wandering to aggression and agitation. Activities have included group gentle stretches, indoor exercises, and a volunteer-led walking program that encouraged hand holding and singing.34 However, a 2008 Cochrane review concluded that the effect of exercise on behavioral disturbances in dementia has not been adequately studied.35
Music therapy. Numerous types of music therapy have been studied, including listening to music picked out by a patient’s family based on known patient preference, classical music, pleasant sounds such as ocean waves, and even stories and comforting prayer recorded by family members. While most of these smaller studies yielded positive results,34 a 2003 Cochrane review concluded there is not enough evidence to recommend for or against music therapy.43 A more recent meta-analysis suggests that music may be effective for agitation.30 A systematic review of quality guidelines also indicates that most of these guidelines rate the evidence as moderate to high in favor of music and 3 of 5 practice guidelines recommend it.28
Nonphysical barriers have long been used as a creative nonrestraining method of preventing wandering. They include such tricks as camouflaging exits by painting them to look like bookcases, painting a black square in front of an elevator to make it look like a hole, and placing a thin Velcro strip across doorways. Although it would appear from a limited number of small studies and anecdotal evidence that nonphysical barriers work, a Cochrane review concluded that they have not been studied enough to perform a meta-analysis.36
Cognitive stimulation typically consists of activities such as reviewing current events, promoting sensory awareness, drawing, associating words, discussion of hobbies, and planning daily activities. This type of therapy has been shown to improve cognition in patients with dementia, as well as well-being and quality of life. It does not improve behavioral problems, per se.37
Reminiscence therapy is a popular modality that involves stimulating memories of the past by looking at personal photos and newspaper clippings and discussing the past. It is well received by patients and caregivers. It has been shown to improve mood in elderly patients without dementia, but studies of reminiscence therapy have been too dissimilar to draw conclusions regarding its effect on behavioral disturbances in patients with dementia.38
Other therapies that are common in dementia care, such as respite care and specialized dementia units, have simply not been studied well enough to provide any conclusions as to their effectiveness.39,40
CASE When Ms. M is discharged from the hospital, her family enrolls her in an adult day care program, where Ms. M will be able to participate in social activities, exercise, and communal meals. Her daughter asks the family physician what other steps they can take in the home to make things easier on her mother. And as an aside, the daughter admits that while she is glad that she and her family can “be there” for her mother, there have been times when she has simply not felt up to the task.
Help family members care for the patient—and themselves
A recent meta-analysis suggests that caregiver interventions have a positive effect on behavioral problems in patients with dementia.32 Successful programs are tailored to the individual needs of the patient and caregiver and delivered over multiple sessions. Unfortunately, the aforementioned meta-analysis did not provide evidenced-based interventions for specific problems.32 With this in mind, the following are some practical caregiver “do’s and don’ts” that are based on reviews and consensus guidelines.
Don’t take it personally. It is extremely important to help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder.25 Caregivers also need to appreciate that hallucinations are normal in these patients and do not require medications if they don’t disturb the patient or place the patient or anyone else at risk.
Don’t try to reason with the patient; redirect him or her instead. Clinicians should offer caregivers suggestions for reassuring, redirecting, or distracting agitated patients rather than trying to reason with them. Encourage caregivers to develop and maintain routines and consistency.6,25 Using a calm, low tone of voice, giving very simple instructions, and leaving and then reattempting care that is refused the first time may also be effective.5 Some experts have suggested techniques such as giving positive rewards for desired behaviors and not rewarding negative behaviors.6,26
Do create a safe environment. Recommend that caregivers create a safe environment. Make sure that they lock up all guns. Also, encourage them to use locks, alarms, or ID bracelets when patients are prone to wandering.25
Do consider a caregiver support program. Caregivers can make a big difference in the lives of patients with dementia, Help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder. but only if they have support, as well.
A recent meta-analysis concluded that active involvement of caregivers in making choices about treatments distinguishes effective from ineffective support programs, decreases the odds of institutionalization, and may lengthen time to institutionalization.33 To ease caregiver strain and depression, encourage them to make use of resources such as nursing home respite care and community agencies that include the Alzheimer’s Association (http://www.alz.org).6,44,45
CASE Ms. M’s daughter joins a local support group for families of patients with dementia, where she learns redirection techniques to try when her mother refuses care. The exercise and daytime social stimulation that Ms. M receives through the adult day care program helps her to sleep at night. When Ms. M refuses to take a shower—a challenge the family had before her hospitalization—the daughter does not argue with her. Instead, she returns 10 to 20 minutes later and asks again, or tries a bedside sponge bath with a lavender soap that Ms. M seems to like.
Ms. M’s nighttime wandering is markedly reduced and the family no longer uses any antipsychotic medications. The family physician counsels them, however, about the progressive nature of the disease and encourages them to set up periodic follow-up visits, so that he can see how everyone—patient and caregivers alike—are doing.
Welcoming the reprieves, recognizing the realities
The behavioral and psychological symptoms of dementia are the most challenging aspect of dementia care. Unacceptable behaviors sometimes persist even when aggressively addressing modifiable factors and attempting behavioral interventions (TABLE 2).2,5-7,15,16,24-26,32,41,44-46 Patients with behavioral disturbances frequently require a pharmacologic agent or transfer to a different care setting.
But clinicians need to use psychotropic medications with informed patient and/or caregiver consent.7 On a case-by-case basis, a trial of antipsychotics is often justified, despite the black box warning. A family may choose to try an antipsychotic despite the risk to help manage the patient at home in the hope of delaying or preventing institutionalization.
However, even with good home support, in conjunction with nonpharmacologic and/or pharmacologic therapies, most patients with dementia will eventually require institutionalization.47 Because patients and families often rely on family physicians to guide them through these difficult challenges and decisions, you’ll need to remain well versed on the available On a case-by- case basis, a trial of antipsychotics is often justified, despite the black box warning. treatments for the psychological and behavioral symptoms of dementia, as well as the resources available in your community.
CORRESPONDENCE
Jaqueline Raetz, MD, 331 NE Thornton Place, Seattle, WA 98125; [email protected]
› Attempt nonpharmacologic treatment for dementia behavioral problems before moving on to medications, which are of questionable efficacy for symptoms other than aggression and psychosis. A
› Obtain informed consent from patients and/or their caregivers if you plan to use antipsychotic medications because their use increases morbidity and mortality in the elderly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Ms. M, 86 years old, lives with her daughter, son-in-law, and granddaughter. For several years she has been forgetful, but she has never had a formal work-up for dementia. Her daughter finally brings her to their primary care physician because she was refusing to take showers, was increasingly irritable, and had tried to hit her daughter’s husband.
In the office, however, Ms. M is calm and pleasant. The family says that most nights Ms. M gets up and wanders around the house. She denies feeling depressed or anxious, but her Folstein Mini-Mental State Exam score is 22/30, indicating moderate dementia. (For more on assessment, see “Tools for assessing patients with dementia—and their caregivers” on page 552.)
The physician offers a trial of risperidone 0.25 mg at bedtime to assist with sleep and behavior.
Was this prescription a wise decision? What other questions should this physician have asked?
Understanding the behavioral symptoms
Noncognitive symptoms of dementia, sometimes referred to as behavioral and psychological symptoms, are common, affecting almost 90% of patients with dementia,1-3 which itself can be classified as early, intermediate, and late.
In early dementia, sociability is usually not affected, but patients may repeat questions, misplace items, use poor judgment, and begin to have difficulty with more complex daily tasks like finances and driving.
In intermediate dementia, basic activities of daily living become impaired and normal social and environmental cues may not register.
In late dementia, patients become entirely dependent on others; they may lose the ability to speak, walk, and eventually, eat. Long- and short-term memory is lost.
Behavioral symptoms most often occur when the condition enters the intermediate phase, but they may occur at any time during the course of the disease.4 Behaviors may include refusal of care, yelling, aggressive behavior, agitation, restlessness, reversal of the normal sleep-wake cycle, wandering, hoarding, sexual disinhibition, culturally inappropriate behaviors, hallucinations, delusions, anxiety, depression, apathy, and psychosis.2,5
Behavioral disturbances often overwhelm families, and lack of treatment increases patient morbidity, may result in physical harm, and almost always precipitates institutionalization.2 Dementia-related behavioral disturbances also increase the risk of caregiver burnout and depression.2
These symptoms are difficult to treat with medications or nonpharmacologic therapy and strong evidence for most therapies is lacking. Physicians have historically prescribed either typical or atypical antipsychotics in an attempt to control these behaviors. In fact, medication is often still considered first-line therapy.6,7
CASE Ms. M’s daughter calls the clinic 2 weeks after the initial visit to tell the physician that her mother has been sleeping much better, but had a fall and was admitted to the hospital for a hip fracture. That’s not surprising; typical and atypical antipsychotics increase the risk of falls in the elderly.8
The risks associated with the use of antipsychotics
In 2005, the US Food and Drug Administration (FDA) issued a black box warning for atypical antipsychotics because they were found to increase mortality in the elderly. The increased mortality is due to cardiac events or infection.9,10 In 2008, the FDA warning was added to typical antipsychotics, as well.11,12 Both typical and atypical antipsychotics have been found to increase the risk of falls and strokes in the elderly,8,13 and their efficacy in treating the behavioral and psychological symptoms of dementia has recently been questioned.13-16
Trazodone and medications approved for the specific treatment of cognitive decline, such as donepezil or memantine, are also prescribed for behavioral disturbances, but evidence to support their efficacy is limited.14,17-20 More recently, a meta-analysis of selective serotonin reuptake inhibitors (SSRIs) suggests that they may be effective for treating agitation associated with dementia.21 However, SSRIs may also contribute to falls and to hyponatremia in the elderly.22,23
Pharmacologic Tx is not your only option
Considering the questionable safety and efficacy of pharmacologic treatment, physicians should consider nondrug therapies first, or at least concurrently with medication.2,15,16,24
But before you get started, be sure to look for and treat medical conditions that cause or contribute to behavioral disturbances, including infection, pain, and adverse effects of medication.6,7,25 Similarly, it is essential that unmet needs, such as hunger, thirst, or desire for attention or socialization, be addressed.6,7,26 Also, discuss disturbing environmental factors, including loud noises, poorly lit quarters, and strong smells, with patients and their caregivers.5-7 In complex situations, you may need to seek assistance from a geriatrician, neurologist, geropsychiatrist, or psychologist, although their availability may be limited.6
CASE Ms. M becomes markedly delirious while in the hospital after hip surgery, and a geriatrics consultation is requested. This is not surprising, given that underlying dementia increases a patient’s risk of delirium in the hospital.27 The geriatrician recommends several measures to reduce the likelihood of delirium—providing good pain control, minimizing night time wake-ups, minimizing Foley catheter use, Hep-locking the IV to encourage mobility, and having staff reorient her frequently by referring to a large print clock and calendar on the wall.
Specific interventions
Most specific nonpharmacologic therapies have not been robustly studied in randomized controlled trials. But a series of smaller studies have been evaluated in systematic reviews. The level of evidence for each intervention is summarized in TABLE 1.28-40
As you review the options that follow, keep 2 things in mind: (1) It is important to set realistic expectations when considering these approaches (as well as pharmacologic ones). Reducing the frequency or severity of problematic behaviors may be more reasonable than their total elimination.6,25 (2) Consider targeting specific symptoms when treating behavioral Behavioral symptoms most often occur when dementia enters the intermediate phase, but they may occur at any time during the course of the disease. disturbances.2,24,41 Such targeting allows physicians and families to better evaluate the effectiveness of interventions because it helps to focus the discussion of the patient’s progress at follow-up visits.
Massage/touch therapy. A 2006 Cochrane review concluded that improvement in nutritional intake and hand massage, when combined with positive encouragement during a meal, may produce a short-term positive effect on agitation.29 Similarly, a meta-analysis of randomized controlled and randomized crossover studies found a statistically significant improvement in agitation with hand massage, although this finding was based on the same single study referenced in the 2006 review.30 Opinions differ among 5 high-quality guidelines included in the systematic review by Azermai et al regarding the value of massage, with 2 of the 5 practice guidelines recommending its use.28
Aromatherapy. Several trials suggest that aroma therapy may reduce agitated behaviors. Lemon balm and lavender oils have been the most commonly studied agents. Two systematic reviews cite the same 2002 randomized controlled trial, which found a reduction in behavioral problems in people who received arm massage with lemon balm compared with those who received arm massage with an odorless cream.30,31 A systematic review by Holt et al also cites a study that found lavender oil placed in a sachet on each side of the pillow for at least one hour during sleep seemed to reduce problem behaviors.31 Several evidence-based guidelines have concluded that aromatherapy may be helpful, and 2 of the 5 practice guidelines reviewed by Azermai et al recommend it.28
Exercise has been shown to benefit patients of all ages, even those with terminal diseases.42 Some studies have indicated a positive effect of physical activities on behaviors ranging from wandering to aggression and agitation. Activities have included group gentle stretches, indoor exercises, and a volunteer-led walking program that encouraged hand holding and singing.34 However, a 2008 Cochrane review concluded that the effect of exercise on behavioral disturbances in dementia has not been adequately studied.35
Music therapy. Numerous types of music therapy have been studied, including listening to music picked out by a patient’s family based on known patient preference, classical music, pleasant sounds such as ocean waves, and even stories and comforting prayer recorded by family members. While most of these smaller studies yielded positive results,34 a 2003 Cochrane review concluded there is not enough evidence to recommend for or against music therapy.43 A more recent meta-analysis suggests that music may be effective for agitation.30 A systematic review of quality guidelines also indicates that most of these guidelines rate the evidence as moderate to high in favor of music and 3 of 5 practice guidelines recommend it.28
Nonphysical barriers have long been used as a creative nonrestraining method of preventing wandering. They include such tricks as camouflaging exits by painting them to look like bookcases, painting a black square in front of an elevator to make it look like a hole, and placing a thin Velcro strip across doorways. Although it would appear from a limited number of small studies and anecdotal evidence that nonphysical barriers work, a Cochrane review concluded that they have not been studied enough to perform a meta-analysis.36
Cognitive stimulation typically consists of activities such as reviewing current events, promoting sensory awareness, drawing, associating words, discussion of hobbies, and planning daily activities. This type of therapy has been shown to improve cognition in patients with dementia, as well as well-being and quality of life. It does not improve behavioral problems, per se.37
Reminiscence therapy is a popular modality that involves stimulating memories of the past by looking at personal photos and newspaper clippings and discussing the past. It is well received by patients and caregivers. It has been shown to improve mood in elderly patients without dementia, but studies of reminiscence therapy have been too dissimilar to draw conclusions regarding its effect on behavioral disturbances in patients with dementia.38
Other therapies that are common in dementia care, such as respite care and specialized dementia units, have simply not been studied well enough to provide any conclusions as to their effectiveness.39,40
CASE When Ms. M is discharged from the hospital, her family enrolls her in an adult day care program, where Ms. M will be able to participate in social activities, exercise, and communal meals. Her daughter asks the family physician what other steps they can take in the home to make things easier on her mother. And as an aside, the daughter admits that while she is glad that she and her family can “be there” for her mother, there have been times when she has simply not felt up to the task.
Help family members care for the patient—and themselves
A recent meta-analysis suggests that caregiver interventions have a positive effect on behavioral problems in patients with dementia.32 Successful programs are tailored to the individual needs of the patient and caregiver and delivered over multiple sessions. Unfortunately, the aforementioned meta-analysis did not provide evidenced-based interventions for specific problems.32 With this in mind, the following are some practical caregiver “do’s and don’ts” that are based on reviews and consensus guidelines.
Don’t take it personally. It is extremely important to help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder.25 Caregivers also need to appreciate that hallucinations are normal in these patients and do not require medications if they don’t disturb the patient or place the patient or anyone else at risk.
Don’t try to reason with the patient; redirect him or her instead. Clinicians should offer caregivers suggestions for reassuring, redirecting, or distracting agitated patients rather than trying to reason with them. Encourage caregivers to develop and maintain routines and consistency.6,25 Using a calm, low tone of voice, giving very simple instructions, and leaving and then reattempting care that is refused the first time may also be effective.5 Some experts have suggested techniques such as giving positive rewards for desired behaviors and not rewarding negative behaviors.6,26
Do create a safe environment. Recommend that caregivers create a safe environment. Make sure that they lock up all guns. Also, encourage them to use locks, alarms, or ID bracelets when patients are prone to wandering.25
Do consider a caregiver support program. Caregivers can make a big difference in the lives of patients with dementia, Help caregivers understand that the disturbing behaviors of patients with dementia lack intentionality and are part of the normal progression of the disorder. but only if they have support, as well.
A recent meta-analysis concluded that active involvement of caregivers in making choices about treatments distinguishes effective from ineffective support programs, decreases the odds of institutionalization, and may lengthen time to institutionalization.33 To ease caregiver strain and depression, encourage them to make use of resources such as nursing home respite care and community agencies that include the Alzheimer’s Association (http://www.alz.org).6,44,45
CASE Ms. M’s daughter joins a local support group for families of patients with dementia, where she learns redirection techniques to try when her mother refuses care. The exercise and daytime social stimulation that Ms. M receives through the adult day care program helps her to sleep at night. When Ms. M refuses to take a shower—a challenge the family had before her hospitalization—the daughter does not argue with her. Instead, she returns 10 to 20 minutes later and asks again, or tries a bedside sponge bath with a lavender soap that Ms. M seems to like.
Ms. M’s nighttime wandering is markedly reduced and the family no longer uses any antipsychotic medications. The family physician counsels them, however, about the progressive nature of the disease and encourages them to set up periodic follow-up visits, so that he can see how everyone—patient and caregivers alike—are doing.
Welcoming the reprieves, recognizing the realities
The behavioral and psychological symptoms of dementia are the most challenging aspect of dementia care. Unacceptable behaviors sometimes persist even when aggressively addressing modifiable factors and attempting behavioral interventions (TABLE 2).2,5-7,15,16,24-26,32,41,44-46 Patients with behavioral disturbances frequently require a pharmacologic agent or transfer to a different care setting.
But clinicians need to use psychotropic medications with informed patient and/or caregiver consent.7 On a case-by-case basis, a trial of antipsychotics is often justified, despite the black box warning. A family may choose to try an antipsychotic despite the risk to help manage the patient at home in the hope of delaying or preventing institutionalization.
However, even with good home support, in conjunction with nonpharmacologic and/or pharmacologic therapies, most patients with dementia will eventually require institutionalization.47 Because patients and families often rely on family physicians to guide them through these difficult challenges and decisions, you’ll need to remain well versed on the available On a case-by- case basis, a trial of antipsychotics is often justified, despite the black box warning. treatments for the psychological and behavioral symptoms of dementia, as well as the resources available in your community.
CORRESPONDENCE
Jaqueline Raetz, MD, 331 NE Thornton Place, Seattle, WA 98125; [email protected]
1. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
2. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S293-S301.
3. Hort J, O’Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol. 2010;17:1236-1248.
4. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
5. Omelan C. Approach to managing behavioural disturbances in dementia. Can Fam Physician. 2006;52:191-199.
6. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
7. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
8. Hill KD, Wee R. Psychotropic drug-induced falls in older people: a review of interventions aimed at reducing the problem. Drugs Aging. 2012;29:15-30.
9. US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed September 16, 2013.
10. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
11. US Food and Drug Administration. Antipsychotics, conventional and atypical. June 16, 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed September 16, 2013.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335-2341.
13. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
14. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596-608.
15. Lonergan E, Luxenberg J, Colford JM. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
16. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
17. Martinón-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;(4):CD004990.
18. Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD001190.
19. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154.
20. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
21. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2): CD008191.
22. Sterke CS, Ziere G, van Beeck EF, et al. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br J Clin Pharmacol. 2012;73:812-820.
23. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622.
24. Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer’s disease management guideline: update 2008. Alzheimers Dement. 2011;7:e51-e59.
25. Rayner A, O’Brien J, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647-652.
26. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182-2188.
27. Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204-212.
28. Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Aging Res Rev. 2012;11:78-86.
29. Viggo Hansen N, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989.
30. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512-520.
31. Thorgrimsen LM, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150.
32. Brodaty H, Arasaratnam C. Review of meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-953.
33. Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: A meta-analysis. J Am Geriatr Soc. 2008;56:1116-1128.
34. Opie J, Rosewarne R, O’Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Aust N Z J Psychiatry. 1999;33:789-799.
35. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489.
36. Price JD, Hermans DG, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev. 2000;(4):CD001932.
37. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562.
38. Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120.
39. Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2004;(2):CD004396.
40. Lai CK, Yeung JH, Mok V, et al. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev. 2009;(4):CD006470.
41. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1-e26.
42. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421-430.
43. Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477.
44. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020-2029.
45. Bass DM, Clark PA, Looman WJ, et al. The Cleveland Alzheimer’s managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73-85.
46. Gitlin LN, Winter L, Dennis MP, et al.. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983-991.
47. Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519-525.
1. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130-135.
2. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):S293-S301.
3. Hort J, O’Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. Eur J Neurol. 2010;17:1236-1248.
4. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475-1483.
5. Omelan C. Approach to managing behavioural disturbances in dementia. Can Fam Physician. 2006;52:191-199.
6. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
7. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
8. Hill KD, Wee R. Psychotropic drug-induced falls in older people: a review of interventions aimed at reducing the problem. Drugs Aging. 2012;29:15-30.
9. US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. April 11, 2005. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. Accessed September 16, 2013.
10. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934-1943.
11. US Food and Drug Administration. Antipsychotics, conventional and atypical. June 16, 2008. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm110212.htm. Accessed September 16, 2013.
12. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335-2341.
13. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
14. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596-608.
15. Lonergan E, Luxenberg J, Colford JM. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002;(2):CD002852.
16. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
17. Martinón-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev. 2004;(4):CD004990.
18. Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD001190.
19. McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154.
20. Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379-397.
21. Seitz DP, Adunuri N, Gill SS, et al. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev. 2011;(2): CD008191.
22. Sterke CS, Ziere G, van Beeck EF, et al. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br J Clin Pharmacol. 2012;73:812-820.
23. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006;40:1618-1622.
24. Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer’s disease management guideline: update 2008. Alzheimers Dement. 2011;7:e51-e59.
25. Rayner A, O’Brien J, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Physician. 2006;73:647-652.
26. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166:2182-2188.
27. Elie M, Cole MG, Primeau FJ, et al. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204-212.
28. Azermai M, Petrovic M, Elseviers MM, et al. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Aging Res Rev. 2012;11:78-86.
29. Viggo Hansen N, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev. 2006;(4):CD004989.
30. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512-520.
31. Thorgrimsen LM, Spector A, Wiles A, et al. Aroma therapy for dementia. Cochrane Database Syst Rev. 2003;(3):CD003150.
32. Brodaty H, Arasaratnam C. Review of meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-953.
33. Spijker A, Vernooij-Dassen M, Vasse E, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: A meta-analysis. J Am Geriatr Soc. 2008;56:1116-1128.
34. Opie J, Rosewarne R, O’Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Aust N Z J Psychiatry. 1999;33:789-799.
35. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489.
36. Price JD, Hermans DG, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev. 2000;(4):CD001932.
37. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;(2):CD005562.
38. Woods B, Spector A, Jones C, et al. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120.
39. Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev. 2004;(2):CD004396.
40. Lai CK, Yeung JH, Mok V, et al. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev. 2009;(4):CD006470.
41. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1-e26.
42. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: a phase II study. J Pain Symptom Manage. 2006;31:421-430.
43. Vink AC, Birks JS, Bruinsma MS, et al. Music therapy for people with dementia. Cochrane Database Syst Rev. 2004;(3):CD003477.
44. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020-2029.
45. Bass DM, Clark PA, Looman WJ, et al. The Cleveland Alzheimer’s managed care demonstration: outcomes after 12 months of implementation. Gerontologist. 2003;43:73-85.
46. Gitlin LN, Winter L, Dennis MP, et al.. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA. 2010;304:983-991.
47. Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519-525.
Woman, 45, With Red, Scaly Nipple
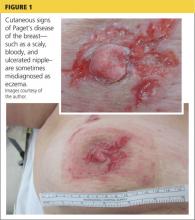
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
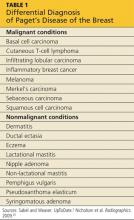
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
A 45-year-old woman noticed some redness and scaling around her right nipple. She applied peroxide and OTC antibiotic ointment for approximately seven months with mixed results. She sought medical attention when pain developed in the breast, along with some bloody discharge from the nipple (see Figure 1). Around that time, she also noticed three small nodules in the upper outer portion of the breast.
A mammogram and ultrasound revealed a 1.7 × 2.0–cm spiculated mass in the axillary tail, as well as two smaller breast lesions. A PET/CT scan ordered subsequently revealed intense uptake in the periareolar region and a suspicious axillary node. By then, the biopsy results had confirmed invasive ductal carcinoma, later determined to be Paget’s disease of the breast (PDB).
The patient’s previous medical history was significant for cystic breasts (never biopsied), chronic back pain, anxiety, and obesity. She was perimenopausal with irregular periods, the last one about 10 months ago. Her obstetric history included two pregnancies resulting in live births and no history of abortion; her menarche occurred at age 14 and her first pregnancy at 27. Family history was significant for leukemia in her maternal grandmother and niece. She did not use tobacco, alcohol, or illicit drugs. She lived at home with her husband and two daughters, who were all very supportive.
The patient elected to undergo a right modified radical mastectomy (MRM) and prophylactic left total mastectomy. MRM was performed on the right breast because sentinel lymph node identification was unsuccessful. This may have been due to involvement of the right subareolar plexus. Five of eight lymph nodes later tested positive for malignancy. The surgery was completed by placement of bilateral tissue expanders for eventual breast reconstruction.
Chemotherapy was started six weeks after surgery and included 15 weeks (five cycles) of docetaxel, carboplatin, and trastuzumab (a combination known as TCH), followed by 51 weeks (17 cycles) of trastuzumab, along with daily tamoxifen. The TCH regimen was followed by four weekly cycles of external beam radiation therapy (EBRT). Adverse effects of treatment have included chest wall dermatitis, right upper extremity lymphedema, nausea/vomiting, dyspnea, peripheral neuropathy, alopecia, and fatigue.
Discussion
Nearly 150 years ago, James Paget recognized a connection between skin changes around the nipple and deeper lesions in the breast.1 The disease that Paget identified is defined as the presence of intraepithelial adenocarcinoma cells (ie, Paget’s cells) within the epidermis of the nipple, with or without an underlying carcinoma.
An underlying breast cancer is present 85% to 95% of the time but is palpable in only approximately 50% of cases (see Figure 2). However, 25% of the time there is neither a palpable mass nor a mammographic abnormality. In these cases particularly, timely diagnosis depends on recognition of suspicious nipple changes, followed by a prompt and thorough diagnostic workup. Unfortunately, the accurate diagnosis of Paget’s disease still takes an average of several months.2
Paget’s disease is rare; it represents only 1% to 3% of new cases of female breast cancer, or about 2,250 cases a year.2-4 (The number of Paget’s disease cases per year was calculated by the author, based on the reported incidence of all breast cancers.) It is even more rare among men. For both genders, the peak age for this disease is between 50 and 60.2
Paget’s disease is an important entity for primary care PAs and NPs because it presents an opportunity to make a timely and life-changing diagnosis, and because it provides an elegant model for understanding current diagnostic and therapeutic approaches to breast cancer.
Clinical Presentation and Pathophysiology
The hallmark of PDB is scaly, vesicular, or ulcerated changes that begin in the nipple and spread to the areola. These changes are most often unilateral and may occur with pruritus, burning pain, and/or oozing from the nipple.5 This presentation is often mistaken for common skin conditions, such as eczema. Like eczema, changes in PDB may improve spontaneously and fluctuate over time, which is confusing for both the patient and clinician. A clinical pearl is that eczema is more likely to spread from the areola to the nipple, and will usually respond to topical corticosteroids. By contrast, changes in PDB tend to spread from the nipple to the areola, and corticosteroids do not provide a sustained response. Of note, Paget’s lesions may heal spontaneously even as the underlying malignancy progresses.6
PDB is unique because the underlying lesion and skin changes are not just coincidental. The cutaneous changes and the malignancy that lies beneath have a causal, not merely co-occurring, relationship. Paget himself believed that the nipple changes were both a precursor, and a promoter, of the underlying cancer.1 This transformation theory states that normal nipple epidermis turns into Paget’s cells spontaneously, before there is any underlying disease. This theory is supported by the fact that, occasionally (though rarely), no underlying breast cancer is ever found. Also, the concomitant tumor may be some distance (> 2 cm) from the nipple-areolar complex (NAC), suggesting a synchronous but causally unrelated lesion.6-8
Modern immunochemistry has turned PDB inside out. Today, PDB is believed to begin within the breast and then to spread “upward” to the NAC, called the epidermotrophic theory. This theory is supported by the fact that Paget’s cells share several molecular markers with their respective parenchymal tumors. Some researchers now propose that there is a single Paget’s progenitor cell with a motility factor that allows it to traverse the ductal system, resulting in nipple and skin changes that have come to be recognized as PDB.6-8
The invasive cancers that are associated with PDB are most likely to be both estrogen- and progesterone-receptor–negative and of a high histologic grade.3,7 Estrogen- and progesterone-sensitive tumors respond to hormonal manipulation therapy. Tumors that are receptor-negative and that have a more aggressive grade are more difficult to treat.
Differential Diagnosis
PDB may be confused with the early stages of inflammatory breast cancer (IBC), an aggressive malignant disease (see Table 1). Both conditions may present with erythema and skin thickening and may be mistaken for mastitis. However, IBC spreads rapidly through the entire breast, and clinical features may include tenderness, a feeling of heat or heaviness, breast enlargement, and significant lymphadenopathy. Current recommendations call for a biopsy of any area of breast inflammation that does not respond to antibiotics within seven days.9
PDB is not the only cutaneous manifestation of breast cancer. Others include carcinoma erysipeloides (inflammatory changes that resemble cellulitis), carcinoma telangiectaticum (vascularized plaques), and/or inflammatory papules or nodules appearing on the breast, back, neck, or scalp. Each of these non–Paget’s conditions involves lymphatic (versus ductal) spread and signifies advanced malignancy.10
Diagnosis and Staging
After biopsy of the nipple lesion(s), diagnosis proceeds to the assessment of the breast itself and ultimately to cancer staging. PDB may occur (in order of incidence):
• In conjunction with an invasive cancer
• With underlying ductal carcinoma in situ (DCIS)
• Without any underlying disease.7
Mammography is used to determine the extent and location of the underlying lesion(s), which is more likely to be peripheral and/or multicentric. However, in some cases, there are no mammographic changes, which is now recognized as an indication for performing a breast MRI.11 Once the lesion is located, direct or image-guided biopsy confirms whether it is invasive cancer or DCIS. Palpable masses that occur with PDB are usually invasive and signal advanced disease.2,6,12,13 Sentinel lymph node biopsy (SLNB), which is usually performed at the time of surgery, plays a critical role in cancer staging and treatment planning. SLNB reliably diagnoses axillary metastasis in approximately 98% of patients.14
Like other breast cancers, PDB is also categorized by the expression of molecular markers, including HER2 (human epidermal growth factor receptor 2). Cancer cells in which HER2 gene is overexpressed tend to proliferate more rapidly than others. HER-status can also provide a clue as to which chemotherapy agents are likely to be most effective.2
Treatment and Management
The primary treatment for breast cancer is surgery, which serves both diagnostic and therapeutic purposes. To be effective, surgical treatment of PDB requires excision of the NAC, also called central lumpectomy. This may be sufficient treatment in those rare cases in which the disease is confined to the NAC.11,12
For underlying tumors, partial mastectomy is an option when the tumor is small (< 2 cm) and located close enough to the NAC to achieve negative margins, while leaving a cosmetically acceptable breast. Partial mastectomy is usually followed by whole breast irradiation. A few centers offer intraoperative radiation therapy (IORT)—performed before the surgeon closes the incision—for patients who wish to avoid or limit the duration of postoperative radiation treatment.15-17
Complete mastectomy (including excision of the NAC) should be considered when:
• The distance between the NAC and the underlying tumor is significant
• Multicentric disease and/or diffuse calcifications exist
• Achieving negative margins would remove too much tissue to leave a cosmetically acceptable breast.
Evaluation of the axillary nodes is the same in PDB as with other breast cancers. Patients with disease localized to the NAC and no underlying carcinoma may choose to forego lymph node biopsy. The same is true for patients who have PDB with a single underlying DCIS. However, lymph node biopsy is always recommended in cases of multicentric DCIS or invasive disease, or if a mastectomy is planned.18,19
Sentinel node biopsy results determine whether the mastectomy should be simple (excision of the breast alone) or modified radical (breast and axillary nodes). Today, complete radical mastectomy (excision of the breast, axillary nodes, and pectoral muscle) is reserved for cases in which disease invades the chest wall.18,19
The use of adjuvant (postoperative) therapy in patients with DCIS (whether or not related to PDB) is still debated. For patients with invasive cancers, both radiation therapy and chemotherapy are usually indicated. The decision to use neoadjuvant (preoperative) chemotherapy is made on a case-by-case basis. All decisions are based on the nature of the underlying cancer, regardless of whether the diagnosis is PDB.
Because PDB is categorized as invasive in at least 85% of cases, and because all invasive breast cancers carry about twice the risk for newly diagnosed contralateral disease, systematic follow-up is extremely important for patients with PDB. A clinical exam and updated history should be performed every four to six months during the first two years and at least annually after that. Screening recommendations, including a yearly mammogram, remain the same for asymptomatic patients. Patients with new or recurring symptoms—because they are at high risk for cancer recurrence—or who are undergoing treatment may have additional testing, including assessing for tumor markers, ultrasound, or MRI.2
PDB is treated with the same chemotherapy regimens as other breast cancers. In the early stages, chemotherapy reduces the risk for recurrence. In advanced breast cancer, the goal of chemotherapy is to reduce tumor size and achieve local control.
Prognosis
Patients with negative lymph node biopsy results have survival rates of 85% and 79% at five and 10 years, respectively. Patients with positive node results face survival rates of 32% at five years and 28% at 10 years. As with other cancers, anything that contributes to disease progression (including delayed diagnosis or treatment) decreases the patient’s survival rate.2,3 The overall prognosis for PDB is based on the nature of the underlying breast cancer, including its stage and other predictive factors—not on the fact that it is PDB.
Patient Outcome
Nearing the end of her treatment with trastuzumab, the patient became concerned about new-onset vaginal and left pelvic pain, along with some lower back discomfort. She mentioned these symptoms to her oncologist immediately. A transvaginal ultrasound could not rule out an ovarian neoplasm.
The patient elected to undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO). This option allowed for removal of a mass discovered during the procedure, minimized the risk for subsequent endometrial cancer, and reduced the chance of recurrence of the patient’s estrogen/progesterone receptor–positive breast cancer. The mass itself turned out to be a benign pedunculated fibroid tumor.
The patient was relieved and continues to recover well. A follow-up PET/CT scan is scheduled for three months from now.
Conclusion
PDB is a complex disease that challenges our current understanding of breast cancer and its diagnosis and treatment. It depends uniquely upon ductal (versus blood or lymphatic) spread. Little did Paget and his contemporaries realize they had opened up such a porthole into modern histology. Nor did they appreciate the fact that they had identified an insidious breast cancer that declares itself through the skin.
Today, it is understood that by the time nipple changes of PDB appear, an underlying breast cancer most likely exists. In at least 25% of cases, there is neither a palpable mass nor a positive mammogram finding. For this reason, clinicians must maintain a high level of clinical suspicion and a low threshold for biopsy when there are skin changes at the nipple. This is especially true because the underlying lesions are more likely to be invasive cancers.
Surgical treatment will often mean complete mastectomy, whether simple, modified radical, or radical. This choice will be driven by the extent and location of the underlying disease. There is a role for partial mastectomy followed by radiation therapy in those rare cases in which PDB is confined to the NAC with no underlying tumor. Partial mastectomy is also a consideration when the underlying tumor is small and/or located close to the NAC. Patients with PDB may consider whole-breast or NAC reconstruction once radiation therapy and/or chemotherapy are completed.
PDB remains a poignant reminder for all clinicians of the importance of a thorough clinical exam and a well-focused history in all patients at risk for breast cancer. Moreover, it is an enduring example of the fact that common symptoms sometimes do signify something uncommon and potentially life- changing.
References
1. Paget J. On disease of the mammary areola preceding cancer of the mammary gland. In: Paget S, ed. Selected Essays and Addresses by Sir James Paget. London: Longmans, Green and Co.; 1902:145-148.
2. Sabel MS, Weaver DL. Paget disease of the breast. In: UpToDate. Chagpar AE, Hayes DF, Pierce LJ, eds. www.uptodate.com/contents/paget-disease-of-the-breast. Updated November 27, 2012. Accessed September 9, 2013.
3. Ortiz-Pagan S, Cunto-Amesty G, Narayan S. Effect of Paget’s disease on survival in breast cancer. Arch Surg. 2001;146:1267-1270.
4. American Cancer Society. Cancer facts & figures 2012. www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012. Accessed September 9, 2013.
5. Ashikari R, Park K, Huvos AG, Urban JA. Paget’s disease of the breast. Cancer. 1970;3:680-685.
6. Sakorafas GH, Blanchard K, Sarr MG, Farley DR. Paget’s disease of the breast. Cancer Treatment Rev. 2001;27:9-18.
7. Chen C-Y, Sun L-M, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer. 2006;107:1448-1458.
8. Paone JF, Baker R. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
9. Nelson JA, Patel D, Mancuso P. Inflammatory breast cancer. ADVANCE for NPs and PAs. 2011;2(10):25-28.
10. Ngan V. Skin metastasis. DermNet NZ. New Zealand Dermatological Society. http://dermnetnz.org/lesions/metastasis.html. Accessed September 9, 2013.
11. Amano G, Yajima M, Moroboshi Y, et al. MRI accurately depicts underlying DCIS in a patient with Paget’s disease of the breast without palpable mass and mammography findings. Jpn J Clin Oncol. 2005;35:149-153.
12. Burrell HC, Evans AJ. Radiological assessment of the breast: what the surgical oncologist needs to know. Eur J Surg Oncol. 2001;27:689-691.
13. Muttarak M, Siriya B, Kongmebhol P. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2001;7:e16, 1-7.
14. Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg. 2006;192:481-483.
15. Pezzi CM, Kukora JS, Audet IM. Breast conservation surgery using nipple-areolar resection for central breast cancers. Arch Surg. 2004;139:32-37.
16. Polgar C, Zsolt O, Tibor K, Janos F. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:1904-1905.
17. Marshall JK, Griffith KA, Haffty BG, Solin LJ. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
18. Vasquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Sem Oncol. 2007;34:234-240.
19. Mamounas EP. Continuing evolution in breast cancer surgical management. J Clin Oncol. 2005;23:1603-1606.
20. Nicholson BT, Harvey JA, Cohen MA. Nipple-areolar complex: normal anatomy and benign and malignant processes. Radiographics. 2009;29:509-523.
Man Has “a Couple of Blocks Somewhere”
ANSWER
The correct interpretation includes sinus bradycardia with first-degree block, a single premature atrial beat with aberrancy, right bundle branch block, left anterior fascicular block (bifascicular block), left ventricular hypertrophy, and T-wave inversions in the inferior leads.
In sinus bradycardia, there is a P wave for every QRS complex with a rate less than 60 beats/min. First-degree block is evidenced by a PR interval ≥ 200 ms.
A single premature atrial contraction is seen as the ninth beat on the ECG. Aberrancy refers to the appearance of the QRS complex; the impulse arises above the AV node but propagates down the AV node and His-Purkinje system to the ventricles before the conduction system is fully repolarized. This results in a QRS complex with intrinsic conduction similar to a normally conducted beat, which then becomes wide and uncharacteristic. Additionally, it resets the sinus node, resulting in a pause before the next normally conducted P wave.
A right bundle branch block is evidenced by the presence of normal conduction with a QRS duration > 120 ms, a terminal R wave in lead V1 (R, rR’, rsR’, or qR), and slurred S waves in leads I and V6.
The presence of left anterior fascicular block is confirmed by the left-axis deviation (–59° in this ECG), a qR complex in leads I and aVL, and an rS pattern in leads II, III, and aVF. The presence of both a right bundle and left anterior fascicular block constitutes bifascicular block. (This is a conduction problem and does not refer to blockage in the arteries, as the patient believed!)
Criteria for left ventricular hypertrophy are met when the sum of the S wave in V1 and the R wave in either V5 or V6 is ≥ 35 mm and the R wave in aVL is ≥ 11 mm.
Two other things to note in this ECG are the presence of T-wave inversions in the inferior leads (II, III, aVF) which are of unclear reason; and the presence of biphasic P waves that do not meet criteria for either right or left atrial hypertrophy.
ANSWER
The correct interpretation includes sinus bradycardia with first-degree block, a single premature atrial beat with aberrancy, right bundle branch block, left anterior fascicular block (bifascicular block), left ventricular hypertrophy, and T-wave inversions in the inferior leads.
In sinus bradycardia, there is a P wave for every QRS complex with a rate less than 60 beats/min. First-degree block is evidenced by a PR interval ≥ 200 ms.
A single premature atrial contraction is seen as the ninth beat on the ECG. Aberrancy refers to the appearance of the QRS complex; the impulse arises above the AV node but propagates down the AV node and His-Purkinje system to the ventricles before the conduction system is fully repolarized. This results in a QRS complex with intrinsic conduction similar to a normally conducted beat, which then becomes wide and uncharacteristic. Additionally, it resets the sinus node, resulting in a pause before the next normally conducted P wave.
A right bundle branch block is evidenced by the presence of normal conduction with a QRS duration > 120 ms, a terminal R wave in lead V1 (R, rR’, rsR’, or qR), and slurred S waves in leads I and V6.
The presence of left anterior fascicular block is confirmed by the left-axis deviation (–59° in this ECG), a qR complex in leads I and aVL, and an rS pattern in leads II, III, and aVF. The presence of both a right bundle and left anterior fascicular block constitutes bifascicular block. (This is a conduction problem and does not refer to blockage in the arteries, as the patient believed!)
Criteria for left ventricular hypertrophy are met when the sum of the S wave in V1 and the R wave in either V5 or V6 is ≥ 35 mm and the R wave in aVL is ≥ 11 mm.
Two other things to note in this ECG are the presence of T-wave inversions in the inferior leads (II, III, aVF) which are of unclear reason; and the presence of biphasic P waves that do not meet criteria for either right or left atrial hypertrophy.
ANSWER
The correct interpretation includes sinus bradycardia with first-degree block, a single premature atrial beat with aberrancy, right bundle branch block, left anterior fascicular block (bifascicular block), left ventricular hypertrophy, and T-wave inversions in the inferior leads.
In sinus bradycardia, there is a P wave for every QRS complex with a rate less than 60 beats/min. First-degree block is evidenced by a PR interval ≥ 200 ms.
A single premature atrial contraction is seen as the ninth beat on the ECG. Aberrancy refers to the appearance of the QRS complex; the impulse arises above the AV node but propagates down the AV node and His-Purkinje system to the ventricles before the conduction system is fully repolarized. This results in a QRS complex with intrinsic conduction similar to a normally conducted beat, which then becomes wide and uncharacteristic. Additionally, it resets the sinus node, resulting in a pause before the next normally conducted P wave.
A right bundle branch block is evidenced by the presence of normal conduction with a QRS duration > 120 ms, a terminal R wave in lead V1 (R, rR’, rsR’, or qR), and slurred S waves in leads I and V6.
The presence of left anterior fascicular block is confirmed by the left-axis deviation (–59° in this ECG), a qR complex in leads I and aVL, and an rS pattern in leads II, III, and aVF. The presence of both a right bundle and left anterior fascicular block constitutes bifascicular block. (This is a conduction problem and does not refer to blockage in the arteries, as the patient believed!)
Criteria for left ventricular hypertrophy are met when the sum of the S wave in V1 and the R wave in either V5 or V6 is ≥ 35 mm and the R wave in aVL is ≥ 11 mm.
Two other things to note in this ECG are the presence of T-wave inversions in the inferior leads (II, III, aVF) which are of unclear reason; and the presence of biphasic P waves that do not meet criteria for either right or left atrial hypertrophy.
A 69-year-old man presents for a routine appointment. His cardiac history is remarkable for systemic hypertension, nonischemic cardiomyopathy, pulmonary hypertension, and dyspnea on exertion. He says a previous provider told him his ECG had “a couple of blocks,” which he believes are “somewhere in the arteries.” He regularly feels lightheaded if he rises from a lying or sitting position too quickly, but he has never lost consciousness. He denies any history of chest pain or symptoms suggestive of angina. Reviewing his prior cardiac workup, you find an echocardiogram that shows moderate left ventricular enlargement with a left ventricular ejection fraction estimated at 35% to 40%; a severely enlarged left atrium; and mild thickening of the mitral leaflets as well as mild mitral regurgitation. A report from an old ECG (conducted at an outside institution) reveals sinus bradycardia, bifascicular block, and left ventricular hypertrophy. Cardiac catheterization performed two years ago showed diffuse disease with no lesions > 30%. Pulmonary function testing showed mild airflow obstruction, no restrictive component, and mildly decreased diffusing capacity. Medical history is positive for two benign colonic polyps that were removed during his last colonoscopy and a remote history of malaria while traveling in Africa 10 years ago. He also has a history of depression, which has been well controlled by medication. His list of medications includes carvedilol, fluoxetine, melatonin, and lisinopril. He is allergic to atenolol and metoprolol. His family history is remarkable for type 1 diabetes (mother). He is a retired baggage handler for a major airline at a nearby international airport. Since high school, he has smoked between one-half and one pack of cigarettes per day. He typically consumes a 12-pack of beer on the weekends. The review of systems is remarkable for corrective lenses, occasional headaches, and dyspnea. The patient denies any other symptoms. Physical examination reveals a blood pressure of 138/80 mm Hg; pulse, 64 beats/min; respiratory rate, 14 breaths/min-1; and O2 saturation, 97%. His height is 188 cm; weight, 107 kg; and BMI, 30. The lungs are clear to auscultation and percussion. The cardiac exam reveals no jugular venous distention, a normal rate and rhythm with occasional skipped beats, and a soft, grade II/VI blowing systolic murmur best heard at the left lower sternal border. The point of maximum impulse is palpable in the left anterior axillary line. The abdomen is soft and nontender, with no organomegaly. The genitourinary exam is normal. Peripheral pulses are 2+ bilaterally in both upper and lower extremities. There is no peripheral edema, and the neurologic exam is grossly intact. The patient is sent for a chest x-ray and an ECG. The latter reveals the following: a ventricular rate of 59 beats/min; PR interval, 284 ms; QRS duration, 130 ms; QT/QTc interval, 472/467 ms; P axis, 70°; R axis, –59°; and T axis, –29°. What is your interpretation of this ECG?
Lifelong Problem Has Caused Embarrassment
ANSWER
The correct answer is discoid lupus (choice “d”); see discussion for further details.
Sarcoidosis (choice “a”) is a worthy item in this differential, since it can be chronic, often affects the face (especially in African-Americans), and frequently defies ready diagnosis. But the biopsy was totally inconsistent with this diagnosis; in a case of sarcoidosis, it instead would have shown noncaseating granulomas, which are characteristic of the condition.
Lichen planus (choice “b”) is likewise worth consideration, since it can present in a similar fashion (although the chronicity of this patient’s lesions would have been atypical). Moreover, lichen planus is almost invariably symptomatic (itch). Biopsy would have shown obliteration of the dermoepidermal junction by an intense lymphocytic infiltrate—findings totally at odds with what was seen.
Polymorphous light eruption (PMLE; choice “c”) is the name given to a variety of photosensitivities that, true to the term polymorphous (or polymorphic), can present in numerous ways—although the lesions on any given patient tend to be monomorphic. These can take the form of vesicles, papules, and even erythema multiforme–like targetoid lesions, most commonly (as expected) on sun-exposed skin. Curiously, though, PMLE seldom affects the face or hands. It can manifest early in a patient’s life, but it would have been “seasonal,” disappearing in winter, and would have revealed a totally different picture on biopsy.
DISCUSSION
This patient suffered needlessly for more than half his life for lack of one simple thing: a correct diagnosis. Truth be known, the patient and his family probably bear some responsibility—but at some point, one of his many providers should have either obtained a punch biopsy or sent him to someone who would do so.
Instead, as is often the case, the emphasis was on treatment: trying one thing after another. The lack of success with these endeavors speaks loudly for the need for a definitive diagnosis. This could only be established one way: with a biopsy.
All the items mentioned in the above differential were legitimately considered. So was the possibility of infection, especially atypical types such as mycobacterial, deep fungal, or those involving other unusual organisms (eg, Nocardia, Actinomycetes). As in this case, tissue can be collected and submitted for culture, but the usual formalin preservative will kill any organism, necessitating prompt processing in saline.
Discoid lupus erythematosus (DLE) can be purely cutaneous (as seen here) or can be a manifestation of more serious systemic lupus. In any case, it is an autoimmune process, made worse by the sun, and can be chronic (though this case is exceptional in that regard).
This patient’s chance of developing systemic lupus erythematosus is slight, at most, since his antinuclear antibody test was negative. But his lifetime risk for another autoimmune disease is high.
TREATMENT
DLE is usually treated successfully with a combination of sun avoidance and a course of oral hydroxychloroquine (200 mg QD to bid, depending on the patient’s body habitus and the severity of the disease). Given the advanced state of this patient’s condition, he received the more frequent dosage, which should yield positive results. However, he will likely be on this regimen for some time.
ANSWER
The correct answer is discoid lupus (choice “d”); see discussion for further details.
Sarcoidosis (choice “a”) is a worthy item in this differential, since it can be chronic, often affects the face (especially in African-Americans), and frequently defies ready diagnosis. But the biopsy was totally inconsistent with this diagnosis; in a case of sarcoidosis, it instead would have shown noncaseating granulomas, which are characteristic of the condition.
Lichen planus (choice “b”) is likewise worth consideration, since it can present in a similar fashion (although the chronicity of this patient’s lesions would have been atypical). Moreover, lichen planus is almost invariably symptomatic (itch). Biopsy would have shown obliteration of the dermoepidermal junction by an intense lymphocytic infiltrate—findings totally at odds with what was seen.
Polymorphous light eruption (PMLE; choice “c”) is the name given to a variety of photosensitivities that, true to the term polymorphous (or polymorphic), can present in numerous ways—although the lesions on any given patient tend to be monomorphic. These can take the form of vesicles, papules, and even erythema multiforme–like targetoid lesions, most commonly (as expected) on sun-exposed skin. Curiously, though, PMLE seldom affects the face or hands. It can manifest early in a patient’s life, but it would have been “seasonal,” disappearing in winter, and would have revealed a totally different picture on biopsy.
DISCUSSION
This patient suffered needlessly for more than half his life for lack of one simple thing: a correct diagnosis. Truth be known, the patient and his family probably bear some responsibility—but at some point, one of his many providers should have either obtained a punch biopsy or sent him to someone who would do so.
Instead, as is often the case, the emphasis was on treatment: trying one thing after another. The lack of success with these endeavors speaks loudly for the need for a definitive diagnosis. This could only be established one way: with a biopsy.
All the items mentioned in the above differential were legitimately considered. So was the possibility of infection, especially atypical types such as mycobacterial, deep fungal, or those involving other unusual organisms (eg, Nocardia, Actinomycetes). As in this case, tissue can be collected and submitted for culture, but the usual formalin preservative will kill any organism, necessitating prompt processing in saline.
Discoid lupus erythematosus (DLE) can be purely cutaneous (as seen here) or can be a manifestation of more serious systemic lupus. In any case, it is an autoimmune process, made worse by the sun, and can be chronic (though this case is exceptional in that regard).
This patient’s chance of developing systemic lupus erythematosus is slight, at most, since his antinuclear antibody test was negative. But his lifetime risk for another autoimmune disease is high.
TREATMENT
DLE is usually treated successfully with a combination of sun avoidance and a course of oral hydroxychloroquine (200 mg QD to bid, depending on the patient’s body habitus and the severity of the disease). Given the advanced state of this patient’s condition, he received the more frequent dosage, which should yield positive results. However, he will likely be on this regimen for some time.
ANSWER
The correct answer is discoid lupus (choice “d”); see discussion for further details.
Sarcoidosis (choice “a”) is a worthy item in this differential, since it can be chronic, often affects the face (especially in African-Americans), and frequently defies ready diagnosis. But the biopsy was totally inconsistent with this diagnosis; in a case of sarcoidosis, it instead would have shown noncaseating granulomas, which are characteristic of the condition.
Lichen planus (choice “b”) is likewise worth consideration, since it can present in a similar fashion (although the chronicity of this patient’s lesions would have been atypical). Moreover, lichen planus is almost invariably symptomatic (itch). Biopsy would have shown obliteration of the dermoepidermal junction by an intense lymphocytic infiltrate—findings totally at odds with what was seen.
Polymorphous light eruption (PMLE; choice “c”) is the name given to a variety of photosensitivities that, true to the term polymorphous (or polymorphic), can present in numerous ways—although the lesions on any given patient tend to be monomorphic. These can take the form of vesicles, papules, and even erythema multiforme–like targetoid lesions, most commonly (as expected) on sun-exposed skin. Curiously, though, PMLE seldom affects the face or hands. It can manifest early in a patient’s life, but it would have been “seasonal,” disappearing in winter, and would have revealed a totally different picture on biopsy.
DISCUSSION
This patient suffered needlessly for more than half his life for lack of one simple thing: a correct diagnosis. Truth be known, the patient and his family probably bear some responsibility—but at some point, one of his many providers should have either obtained a punch biopsy or sent him to someone who would do so.
Instead, as is often the case, the emphasis was on treatment: trying one thing after another. The lack of success with these endeavors speaks loudly for the need for a definitive diagnosis. This could only be established one way: with a biopsy.
All the items mentioned in the above differential were legitimately considered. So was the possibility of infection, especially atypical types such as mycobacterial, deep fungal, or those involving other unusual organisms (eg, Nocardia, Actinomycetes). As in this case, tissue can be collected and submitted for culture, but the usual formalin preservative will kill any organism, necessitating prompt processing in saline.
Discoid lupus erythematosus (DLE) can be purely cutaneous (as seen here) or can be a manifestation of more serious systemic lupus. In any case, it is an autoimmune process, made worse by the sun, and can be chronic (though this case is exceptional in that regard).
This patient’s chance of developing systemic lupus erythematosus is slight, at most, since his antinuclear antibody test was negative. But his lifetime risk for another autoimmune disease is high.
TREATMENT
DLE is usually treated successfully with a combination of sun avoidance and a course of oral hydroxychloroquine (200 mg QD to bid, depending on the patient’s body habitus and the severity of the disease). Given the advanced state of this patient’s condition, he received the more frequent dosage, which should yield positive results. However, he will likely be on this regimen for some time.

Since the fourth grade, this 22-year-old African-American man has had facial lesions that, although constantly present, worsen in the summer. The problem is so severe that it has greatly affected his quality of life: In school and in his neighborhood, he has been subjected to rumors that his condition might be contagious. Despite numerous treatment attempts—including topical and oral anti-acne medications, topical and oral antifungal medications, and oral antibiotics—the condition has persisted. Once, at his mother’s urging, the patient even sought the assistance of a faith healer at a religious revival. Initially seen by a primary care provider at a free clinic, he was then referred to a dermatology clinician at the same facility. History taking reveals that the lesions are asymptomatic and appear in additional locations (eg, arms, ears, and neck). The patient denies any family history of similar problems, as well as persistent fever, cough, or shortness of breath. Lab studies obtained by his primary care provider, including a complete blood count and chem screen, were within normal limits. Most of the lesions on the patient’s face are round areas of slightly erythematous erosion covered by eschar. They range in size from 3 mm to more than 1 cm. Focal postinflammatory hyperpigmentation is noted (a common finding in those with type V/VI skin). Focal hyperpigmentation is also seen on both ears, in some cases with scaling and faint erosion on the surface. A KOH prep is performed with scale collected from perilesional skin; results are negative for fungal elements. There are no palpable nodes in the adjacent nodal locations. Two punch biopsies are done, with samples taken from the active margins of the lesions. One is submitted for routine H&E (hematoxylin and eosin) handling and the other in saline for bacterial, acid-fast bacilli (AFB), and fungal cultures. The biopsy shows hyperkeratosis, follicular plugging, and epidermal atrophy. Marked vacuolar degeneration of the dermoepidermal junction is also noted, along with mucin deposition. Stains for bacteria, AFB, and fungi yield negative results.
Woman Assaulted on Street
The chest radiograph demonstrates a massive amount of soft tissue and subcutaneous emphysema extending from the neck down through the chest and into the lower chest/upper abdomen. In addition, there are several fractured posterior ribs bilaterally. No large pneumothorax or pneumomediastinum is noted.
Because of the extent and mechanism of injury, CT of the chest, abdomen, and pelvis had already been ordered. Arrangements were also made for the patient to be admitted to the ICU for closer observation.
The chest radiograph demonstrates a massive amount of soft tissue and subcutaneous emphysema extending from the neck down through the chest and into the lower chest/upper abdomen. In addition, there are several fractured posterior ribs bilaterally. No large pneumothorax or pneumomediastinum is noted.
Because of the extent and mechanism of injury, CT of the chest, abdomen, and pelvis had already been ordered. Arrangements were also made for the patient to be admitted to the ICU for closer observation.
The chest radiograph demonstrates a massive amount of soft tissue and subcutaneous emphysema extending from the neck down through the chest and into the lower chest/upper abdomen. In addition, there are several fractured posterior ribs bilaterally. No large pneumothorax or pneumomediastinum is noted.
Because of the extent and mechanism of injury, CT of the chest, abdomen, and pelvis had already been ordered. Arrangements were also made for the patient to be admitted to the ICU for closer observation.
A 40-year-old woman is brought in by EMS for evaluation of injuries secondary to being assaulted. She was out walking late last night when she was approached by two men who pushed her to the ground and began punching and kicking her repeatedly on her face, chest, and back. She is primarily complaining of chest wall and back pain. Her medical history is significant for hypertension, diet-controlled diabetes, and “some sort of heart problem” for which she takes medication. Surgical history is significant for hysterectomy, cholecystectomy, and appendectomy. She smokes more than a pack of cigarettes per day and consumes at least a six-pack of beer daily. Initial exam shows an anxious female who appears somewhat uncomfortable but is in no obvious distress. Her vital signs are as follows: blood pressure, 130/88 mm Hg; pulse, 120 beats/min; respiratory rate, 22 breaths/min; and O2 saturation, 100% on room air. Physical exam reveals extensive facial/periorbital swelling, as well as swelling in the neck. Some splinting is noted. There is extensive crepitus noted within the soft tissue of the face, neck, and chest wall. Also, there is moderate tenderness bilaterally over the ribs. Chest radiograph is obtained (shown). What is your impression?
Observation Status Not So Well-Defined in Hospitals
New research suggests use of the designation "observation status" for admitted hospital patients varies in clinical practice, despite rigid criteria the Centers for Medicare & Medicaid Services (CMS) uses to define the term.
CMS defines observation status as "well-defined sets of specific, clinically appropriate services." In most cases, the status applies to inpatient stays of less than 24 hours. Longer than 48 hours is dubbed "rare and exceptional" by the federal agency.
But in the report, “Hospitalized But Not Admitted: Characteristics of Patients With ‘Observation Status’ at an Academic Medical Center," lead author and hospitalist Ann Sheehy, MD, MS, of Wisconsin School of Medicine and Public Health in Madison found that patients' mean length of stay (LOS) in observation was 33.3 hours, but it was longer than 48 hours in 16.5% of cases. Dr. Sheehy adds that 1,141 distinct observation diagnosis codes were used for observation stays during the study period, which ran from July 1, 2010, to Dec. 31, 2011.
"What CMS has as a definition for observation status is clearly not what's happening in clinical practice, based on the length of stay and the wide variety of diagnosis codes," Dr. Sheehy says. "We had over 1,000 diagnosis codes for something CMS says is well-defined."
The issue is of particular note to hospital medicine groups as observation status disproportionately affects the general-medicine population, Dr. Sheehy says. Just over 52% of all observation stays in the study were adult general-medicine patients.
The paper adds that while the cost per encounter for observation care was less than that for inpatient care, the average reimbursement for observation care failed to cover it. The net loss per encounter for an observation stay was $331, compared with a net gain of $2,163 for an inpatient stay.
"We don't want to have hospitals operating on a huge profit margin," Dr. Sheehy says, but "you can't have hospitals delivering care at a loss consistently and have them stay solvent. It's just not going to work."
Visit our website for more information on observation status rules.
New research suggests use of the designation "observation status" for admitted hospital patients varies in clinical practice, despite rigid criteria the Centers for Medicare & Medicaid Services (CMS) uses to define the term.
CMS defines observation status as "well-defined sets of specific, clinically appropriate services." In most cases, the status applies to inpatient stays of less than 24 hours. Longer than 48 hours is dubbed "rare and exceptional" by the federal agency.
But in the report, “Hospitalized But Not Admitted: Characteristics of Patients With ‘Observation Status’ at an Academic Medical Center," lead author and hospitalist Ann Sheehy, MD, MS, of Wisconsin School of Medicine and Public Health in Madison found that patients' mean length of stay (LOS) in observation was 33.3 hours, but it was longer than 48 hours in 16.5% of cases. Dr. Sheehy adds that 1,141 distinct observation diagnosis codes were used for observation stays during the study period, which ran from July 1, 2010, to Dec. 31, 2011.
"What CMS has as a definition for observation status is clearly not what's happening in clinical practice, based on the length of stay and the wide variety of diagnosis codes," Dr. Sheehy says. "We had over 1,000 diagnosis codes for something CMS says is well-defined."
The issue is of particular note to hospital medicine groups as observation status disproportionately affects the general-medicine population, Dr. Sheehy says. Just over 52% of all observation stays in the study were adult general-medicine patients.
The paper adds that while the cost per encounter for observation care was less than that for inpatient care, the average reimbursement for observation care failed to cover it. The net loss per encounter for an observation stay was $331, compared with a net gain of $2,163 for an inpatient stay.
"We don't want to have hospitals operating on a huge profit margin," Dr. Sheehy says, but "you can't have hospitals delivering care at a loss consistently and have them stay solvent. It's just not going to work."
Visit our website for more information on observation status rules.
New research suggests use of the designation "observation status" for admitted hospital patients varies in clinical practice, despite rigid criteria the Centers for Medicare & Medicaid Services (CMS) uses to define the term.
CMS defines observation status as "well-defined sets of specific, clinically appropriate services." In most cases, the status applies to inpatient stays of less than 24 hours. Longer than 48 hours is dubbed "rare and exceptional" by the federal agency.
But in the report, “Hospitalized But Not Admitted: Characteristics of Patients With ‘Observation Status’ at an Academic Medical Center," lead author and hospitalist Ann Sheehy, MD, MS, of Wisconsin School of Medicine and Public Health in Madison found that patients' mean length of stay (LOS) in observation was 33.3 hours, but it was longer than 48 hours in 16.5% of cases. Dr. Sheehy adds that 1,141 distinct observation diagnosis codes were used for observation stays during the study period, which ran from July 1, 2010, to Dec. 31, 2011.
"What CMS has as a definition for observation status is clearly not what's happening in clinical practice, based on the length of stay and the wide variety of diagnosis codes," Dr. Sheehy says. "We had over 1,000 diagnosis codes for something CMS says is well-defined."
The issue is of particular note to hospital medicine groups as observation status disproportionately affects the general-medicine population, Dr. Sheehy says. Just over 52% of all observation stays in the study were adult general-medicine patients.
The paper adds that while the cost per encounter for observation care was less than that for inpatient care, the average reimbursement for observation care failed to cover it. The net loss per encounter for an observation stay was $331, compared with a net gain of $2,163 for an inpatient stay.
"We don't want to have hospitals operating on a huge profit margin," Dr. Sheehy says, but "you can't have hospitals delivering care at a loss consistently and have them stay solvent. It's just not going to work."
Visit our website for more information on observation status rules.
CMS Allows Residents, Advanced Practitioners to Admit Inpatients
A federal rule that some hospitalists feared would bar nonstaff physicians from writing admission orders for hospital inpatients has been clarified to extend those privileges to resident physicians and advanced practitioners.
On Aug. 19, the Centers for Medicare & Medicaid Services (CMS) published its fiscal 2014 hospital Inpatient Prospective Payment System (IPPS) Final Rule, which is effective Oct. 1. Although this document impacts a number of important areas for hospitals, including the use of inpatient admission and observation status, hospitalists also were left with the impression that resident physicians and advanced practitioners (NPs and PAs) were being barred from writing admission orders.
Medical residents, NPs, and PAs do not have admitting privileges in most hospitals, and their inability to write admission orders would pose significant logistical and financial hurdles for many hospitals and physician groups, including hospitalists.
In most academic centers, residents have the opportunity and responsibility to evaluate patients at the time of hospitalization, write initial hospitalization orders, and then discuss patients with the attending physician. The "staffing" of patients typically occurs either later the same day or the following morning. The provision requiring an attending physician knowledgeable of the case to furnish the admission order, and not allowing delegation of this order to their residents, could fundamentally change the way our physicians are trained.
Many hospitals rely on NPs and PAs for the care of hospitalized patients under the supervision of a staff physician. These nonphysician providers often care for hospitalized patients, admit patients, and provide overnight coverage. Requiring admission orders to be placed by physicians with admitting privileges would require a greater presence of staff physicians, which would increase costs.
Some of these concerns were voiced Aug. 15 on CMS' Special Open Door Forum on CMS Rule 1599-F. While clarification was not offered, it was indicated that CMS did not intend to place such limitations on residents, NPs, and PAs. On Sept. 5, CMS released clarifications to some of these provisions in the IPPS Final Rule, and noted that admission orders may come from a physician or other practitioner.
This clarification suggests that when a resident, NP, or PA writes admission orders, they are doing so at the direction of a physician with admitting privileges. This may occur in the form of a verbal order, which requires documentation of the name of the admitting physician, as well as the date and time of the verbal order. This verbal order must then be countersigned by a qualified physician prior to patient discharge.
Failures to obtain appropriate orders co-signed by an appropriate physician are likely at high risk for Medicare payment denial. This clarification appears to address previously held concerns about this new rule.
A federal rule that some hospitalists feared would bar nonstaff physicians from writing admission orders for hospital inpatients has been clarified to extend those privileges to resident physicians and advanced practitioners.
On Aug. 19, the Centers for Medicare & Medicaid Services (CMS) published its fiscal 2014 hospital Inpatient Prospective Payment System (IPPS) Final Rule, which is effective Oct. 1. Although this document impacts a number of important areas for hospitals, including the use of inpatient admission and observation status, hospitalists also were left with the impression that resident physicians and advanced practitioners (NPs and PAs) were being barred from writing admission orders.
Medical residents, NPs, and PAs do not have admitting privileges in most hospitals, and their inability to write admission orders would pose significant logistical and financial hurdles for many hospitals and physician groups, including hospitalists.
In most academic centers, residents have the opportunity and responsibility to evaluate patients at the time of hospitalization, write initial hospitalization orders, and then discuss patients with the attending physician. The "staffing" of patients typically occurs either later the same day or the following morning. The provision requiring an attending physician knowledgeable of the case to furnish the admission order, and not allowing delegation of this order to their residents, could fundamentally change the way our physicians are trained.
Many hospitals rely on NPs and PAs for the care of hospitalized patients under the supervision of a staff physician. These nonphysician providers often care for hospitalized patients, admit patients, and provide overnight coverage. Requiring admission orders to be placed by physicians with admitting privileges would require a greater presence of staff physicians, which would increase costs.
Some of these concerns were voiced Aug. 15 on CMS' Special Open Door Forum on CMS Rule 1599-F. While clarification was not offered, it was indicated that CMS did not intend to place such limitations on residents, NPs, and PAs. On Sept. 5, CMS released clarifications to some of these provisions in the IPPS Final Rule, and noted that admission orders may come from a physician or other practitioner.
This clarification suggests that when a resident, NP, or PA writes admission orders, they are doing so at the direction of a physician with admitting privileges. This may occur in the form of a verbal order, which requires documentation of the name of the admitting physician, as well as the date and time of the verbal order. This verbal order must then be countersigned by a qualified physician prior to patient discharge.
Failures to obtain appropriate orders co-signed by an appropriate physician are likely at high risk for Medicare payment denial. This clarification appears to address previously held concerns about this new rule.
A federal rule that some hospitalists feared would bar nonstaff physicians from writing admission orders for hospital inpatients has been clarified to extend those privileges to resident physicians and advanced practitioners.
On Aug. 19, the Centers for Medicare & Medicaid Services (CMS) published its fiscal 2014 hospital Inpatient Prospective Payment System (IPPS) Final Rule, which is effective Oct. 1. Although this document impacts a number of important areas for hospitals, including the use of inpatient admission and observation status, hospitalists also were left with the impression that resident physicians and advanced practitioners (NPs and PAs) were being barred from writing admission orders.
Medical residents, NPs, and PAs do not have admitting privileges in most hospitals, and their inability to write admission orders would pose significant logistical and financial hurdles for many hospitals and physician groups, including hospitalists.
In most academic centers, residents have the opportunity and responsibility to evaluate patients at the time of hospitalization, write initial hospitalization orders, and then discuss patients with the attending physician. The "staffing" of patients typically occurs either later the same day or the following morning. The provision requiring an attending physician knowledgeable of the case to furnish the admission order, and not allowing delegation of this order to their residents, could fundamentally change the way our physicians are trained.
Many hospitals rely on NPs and PAs for the care of hospitalized patients under the supervision of a staff physician. These nonphysician providers often care for hospitalized patients, admit patients, and provide overnight coverage. Requiring admission orders to be placed by physicians with admitting privileges would require a greater presence of staff physicians, which would increase costs.
Some of these concerns were voiced Aug. 15 on CMS' Special Open Door Forum on CMS Rule 1599-F. While clarification was not offered, it was indicated that CMS did not intend to place such limitations on residents, NPs, and PAs. On Sept. 5, CMS released clarifications to some of these provisions in the IPPS Final Rule, and noted that admission orders may come from a physician or other practitioner.
This clarification suggests that when a resident, NP, or PA writes admission orders, they are doing so at the direction of a physician with admitting privileges. This may occur in the form of a verbal order, which requires documentation of the name of the admitting physician, as well as the date and time of the verbal order. This verbal order must then be countersigned by a qualified physician prior to patient discharge.
Failures to obtain appropriate orders co-signed by an appropriate physician are likely at high risk for Medicare payment denial. This clarification appears to address previously held concerns about this new rule.
Cranial radiotherapy for acute lymphoblastic leukemia linked to impaired neurocognition
Reduced white matter and impaired neuropsychological function were seen 20-30 years later in childhood acute lymphoblastic leukemia and lymphoma survivors who received cranial radiotherapy.
Survivors treated with chemotherapy alone appeared to have milder impairments, with measures that were within one standard deviation of the measures seen in healthy controls, reported Ilse Schuitema of the University of Leiden and her associates.
They also found that a young age at the time of cranial radiotherapy and the radiation dosage were associated with poorer results on the MRI measure used to evaluate the white matter's microstructure. Decreases in this measure were associated with neuropsychological dysfunction. The study was published in the Sept. 20 issue of the Journal of Clinical Oncology (2013;31:3378-88).
The finding "warrants a recommendation to use CRT [cranial radiotherapy] only as a last resort," since chemotherapy is just as effective, based on survival and recurrence rates in patients with acute lymphoblastic leukemia (ALL). Further, indications of accelerated aging seen in survivors given cranial radiotherapy may support screening for early-onset dementia as well as recommending lifestyle modifications such as not smoking and getting regular physical exercise to slow the progress of dementia.
Ms. Schuitema of the department of clinical child and adolescent studies, faculty of social sciences at Leiden (the Netherlands) University, and her co-investigators, followed up with 93 survivors of ALL or lymphoma who had been treated between 1978 and 1990 with cranial radiotherapy or CNS-directed chemotherapy, and compared them with 49 healthy controls. The mean age of patients given chemotherapy only was 27 years and the mean age of those treated with radiotherapy was 31 years.
Testing included magnetic resonance diffusion tensor imaging and neuropsychological tests. Differences in fractional anisotropy on the MRI test were used to analyze white matter microstructure, and whole-brain, voxel-based analysis was performed.
Survivors treated with chemotherapy only had reductions in the MRI measure and neuropsychological performance, but the measures were no more than one standard deviation below the mean values of controls.
When compared with controls, survivors who had cranial radiotherapy had significant reductions in white matter integrity and lower neurocognitive function, with effects that included lower IQ, poorer visuomotor accuracy, and poorer work flow during sustained attention.
Further, there was a "steep decline," in the MRI measure within the frontal and parietal white matter, which "is a strong indication of accelerated aging" the researchers wrote. Some of the anatomical findings among those treated with CRT were similar to those found in people with Alzheimer's disease, suggesting that "the irradiated survivors could be at increased risk of developing early-onset dementia."
The study results highlight the importance of long-term follow-up of children who receive neurotoxic treatments and increase support "for the concept of accelerated aging after CRT implicates screening for early-onset dementia,"the researchers said.
The tests used in the study included the computerized Amsterdam Neuropsychological Tasks (ANT) program, used to assess executive functions, and the four subtest short-form of the Wechsler Adult Intelligence Scale Revised (WAIS-R III).
Ms. Schuitema's coauthors are from the University of Leiden; University Hospitals Leuven, Belgium; and Academic Medical Center, Amsterdam. The authors had no disclosures. The study was funded by grants, including one from the Dutch Cancer Society.
This is one of the first studies to look at the long-term effects of treatment in childhood ALL survivors. These findings raise a significant concern about the long-term CNS and neurocognitive integrity of children who have been treated and are currently being treated with cranial radiotherapy. A significant number of children continue to receive radiation doses that are similar to or higher than the doses that were used to treat the patients in this study. Additional confirmatory studies are needed as is research that may bring about changes in treatment and long-term outcomes for the children we are treating today.
F. Daniel Armstrong, Ph.D., is director of the Mailman Center for Child Development, and professor of pediatrics & psychology at the University of Miami. He made his remarks in an editorial that accompanied the study (J. Clin. Oncol 2013;31:3309-11). He had no relevant disclosures.
This is one of the first studies to look at the long-term effects of treatment in childhood ALL survivors. These findings raise a significant concern about the long-term CNS and neurocognitive integrity of children who have been treated and are currently being treated with cranial radiotherapy. A significant number of children continue to receive radiation doses that are similar to or higher than the doses that were used to treat the patients in this study. Additional confirmatory studies are needed as is research that may bring about changes in treatment and long-term outcomes for the children we are treating today.
F. Daniel Armstrong, Ph.D., is director of the Mailman Center for Child Development, and professor of pediatrics & psychology at the University of Miami. He made his remarks in an editorial that accompanied the study (J. Clin. Oncol 2013;31:3309-11). He had no relevant disclosures.
This is one of the first studies to look at the long-term effects of treatment in childhood ALL survivors. These findings raise a significant concern about the long-term CNS and neurocognitive integrity of children who have been treated and are currently being treated with cranial radiotherapy. A significant number of children continue to receive radiation doses that are similar to or higher than the doses that were used to treat the patients in this study. Additional confirmatory studies are needed as is research that may bring about changes in treatment and long-term outcomes for the children we are treating today.
F. Daniel Armstrong, Ph.D., is director of the Mailman Center for Child Development, and professor of pediatrics & psychology at the University of Miami. He made his remarks in an editorial that accompanied the study (J. Clin. Oncol 2013;31:3309-11). He had no relevant disclosures.
Reduced white matter and impaired neuropsychological function were seen 20-30 years later in childhood acute lymphoblastic leukemia and lymphoma survivors who received cranial radiotherapy.
Survivors treated with chemotherapy alone appeared to have milder impairments, with measures that were within one standard deviation of the measures seen in healthy controls, reported Ilse Schuitema of the University of Leiden and her associates.
They also found that a young age at the time of cranial radiotherapy and the radiation dosage were associated with poorer results on the MRI measure used to evaluate the white matter's microstructure. Decreases in this measure were associated with neuropsychological dysfunction. The study was published in the Sept. 20 issue of the Journal of Clinical Oncology (2013;31:3378-88).
The finding "warrants a recommendation to use CRT [cranial radiotherapy] only as a last resort," since chemotherapy is just as effective, based on survival and recurrence rates in patients with acute lymphoblastic leukemia (ALL). Further, indications of accelerated aging seen in survivors given cranial radiotherapy may support screening for early-onset dementia as well as recommending lifestyle modifications such as not smoking and getting regular physical exercise to slow the progress of dementia.
Ms. Schuitema of the department of clinical child and adolescent studies, faculty of social sciences at Leiden (the Netherlands) University, and her co-investigators, followed up with 93 survivors of ALL or lymphoma who had been treated between 1978 and 1990 with cranial radiotherapy or CNS-directed chemotherapy, and compared them with 49 healthy controls. The mean age of patients given chemotherapy only was 27 years and the mean age of those treated with radiotherapy was 31 years.
Testing included magnetic resonance diffusion tensor imaging and neuropsychological tests. Differences in fractional anisotropy on the MRI test were used to analyze white matter microstructure, and whole-brain, voxel-based analysis was performed.
Survivors treated with chemotherapy only had reductions in the MRI measure and neuropsychological performance, but the measures were no more than one standard deviation below the mean values of controls.
When compared with controls, survivors who had cranial radiotherapy had significant reductions in white matter integrity and lower neurocognitive function, with effects that included lower IQ, poorer visuomotor accuracy, and poorer work flow during sustained attention.
Further, there was a "steep decline," in the MRI measure within the frontal and parietal white matter, which "is a strong indication of accelerated aging" the researchers wrote. Some of the anatomical findings among those treated with CRT were similar to those found in people with Alzheimer's disease, suggesting that "the irradiated survivors could be at increased risk of developing early-onset dementia."
The study results highlight the importance of long-term follow-up of children who receive neurotoxic treatments and increase support "for the concept of accelerated aging after CRT implicates screening for early-onset dementia,"the researchers said.
The tests used in the study included the computerized Amsterdam Neuropsychological Tasks (ANT) program, used to assess executive functions, and the four subtest short-form of the Wechsler Adult Intelligence Scale Revised (WAIS-R III).
Ms. Schuitema's coauthors are from the University of Leiden; University Hospitals Leuven, Belgium; and Academic Medical Center, Amsterdam. The authors had no disclosures. The study was funded by grants, including one from the Dutch Cancer Society.
Reduced white matter and impaired neuropsychological function were seen 20-30 years later in childhood acute lymphoblastic leukemia and lymphoma survivors who received cranial radiotherapy.
Survivors treated with chemotherapy alone appeared to have milder impairments, with measures that were within one standard deviation of the measures seen in healthy controls, reported Ilse Schuitema of the University of Leiden and her associates.
They also found that a young age at the time of cranial radiotherapy and the radiation dosage were associated with poorer results on the MRI measure used to evaluate the white matter's microstructure. Decreases in this measure were associated with neuropsychological dysfunction. The study was published in the Sept. 20 issue of the Journal of Clinical Oncology (2013;31:3378-88).
The finding "warrants a recommendation to use CRT [cranial radiotherapy] only as a last resort," since chemotherapy is just as effective, based on survival and recurrence rates in patients with acute lymphoblastic leukemia (ALL). Further, indications of accelerated aging seen in survivors given cranial radiotherapy may support screening for early-onset dementia as well as recommending lifestyle modifications such as not smoking and getting regular physical exercise to slow the progress of dementia.
Ms. Schuitema of the department of clinical child and adolescent studies, faculty of social sciences at Leiden (the Netherlands) University, and her co-investigators, followed up with 93 survivors of ALL or lymphoma who had been treated between 1978 and 1990 with cranial radiotherapy or CNS-directed chemotherapy, and compared them with 49 healthy controls. The mean age of patients given chemotherapy only was 27 years and the mean age of those treated with radiotherapy was 31 years.
Testing included magnetic resonance diffusion tensor imaging and neuropsychological tests. Differences in fractional anisotropy on the MRI test were used to analyze white matter microstructure, and whole-brain, voxel-based analysis was performed.
Survivors treated with chemotherapy only had reductions in the MRI measure and neuropsychological performance, but the measures were no more than one standard deviation below the mean values of controls.
When compared with controls, survivors who had cranial radiotherapy had significant reductions in white matter integrity and lower neurocognitive function, with effects that included lower IQ, poorer visuomotor accuracy, and poorer work flow during sustained attention.
Further, there was a "steep decline," in the MRI measure within the frontal and parietal white matter, which "is a strong indication of accelerated aging" the researchers wrote. Some of the anatomical findings among those treated with CRT were similar to those found in people with Alzheimer's disease, suggesting that "the irradiated survivors could be at increased risk of developing early-onset dementia."
The study results highlight the importance of long-term follow-up of children who receive neurotoxic treatments and increase support "for the concept of accelerated aging after CRT implicates screening for early-onset dementia,"the researchers said.
The tests used in the study included the computerized Amsterdam Neuropsychological Tasks (ANT) program, used to assess executive functions, and the four subtest short-form of the Wechsler Adult Intelligence Scale Revised (WAIS-R III).
Ms. Schuitema's coauthors are from the University of Leiden; University Hospitals Leuven, Belgium; and Academic Medical Center, Amsterdam. The authors had no disclosures. The study was funded by grants, including one from the Dutch Cancer Society.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Major finding: Compared with controls, survivors who had cranial radiotherapy had significant reductions in white matter integrity and lower neurocognitive function, with effects that included lower IQ, poorer visuomotor accuracy, and poorer work flow during sustained attention.
Data source: Magnetic resonance imaging and neuropsychological test results in 93 survivors of childhood ALL or lymphoma and 49 healthy controls.
Disclosures: The study was supported with grants, including one from the Dutch Cancer Society. The authors had no disclosures.
Ten tips for chronic venous ulcers
The underlying pathophysiology of chronic venous insufficiency is complex and involves many factors. Studies have shown that average venous ulcers may need 6-12 months for complete healing with an anticipated recurrence rate exceeding 2/3 cases in 5 years. These numbers reflect the magnitude of the problem and mandate deploying all efforts to stop progression of the disease. Our group has found the following 10 tips have significantly improved our healing rates.
1. First, rule out any associated arterial, immunologic, endocrine, or other systemic causes for leg/foot ulceration.
2. Be aggressive to stop progression of the disease (fight CEAP 6): any local tenderness at the site of discolored skin at the gaiter area for venous ulcers should initiate a prompt reflux study to evaluate for incompetent perforators.
3. Venous ulcers are associated with an incompetent perforator within 2 cm of the ulcer area.
4. Recurrent venous ulcers at the same location may be associated with venous outflow obstruction, (May-Thurner syndrome is an underestimated pathology) which affects mainly the left leg.
5. When performing iliac vein venograms, make liberal use of intravascular ultrasound.
6. Exudative venous ulcers need multilayer compression dressings and appropriate antibiotics if infection exists.
7. Pentoxifylline (Trental) 800 mg, 3 times daily.
8. Frequent debridement and frequent objective evaluation for ulcer area with each office visit.
9. Bi-layered living cell treatment (Apligraf?) to promote healing.
10. Office/clinic visit every 3 months after complete healing (CEAP 5) and further testing as needed.
Dr. Mousa is an associate professor at the Department of Surgery, West Virginia University, Morgantown.
The underlying pathophysiology of chronic venous insufficiency is complex and involves many factors. Studies have shown that average venous ulcers may need 6-12 months for complete healing with an anticipated recurrence rate exceeding 2/3 cases in 5 years. These numbers reflect the magnitude of the problem and mandate deploying all efforts to stop progression of the disease. Our group has found the following 10 tips have significantly improved our healing rates.
1. First, rule out any associated arterial, immunologic, endocrine, or other systemic causes for leg/foot ulceration.
2. Be aggressive to stop progression of the disease (fight CEAP 6): any local tenderness at the site of discolored skin at the gaiter area for venous ulcers should initiate a prompt reflux study to evaluate for incompetent perforators.
3. Venous ulcers are associated with an incompetent perforator within 2 cm of the ulcer area.
4. Recurrent venous ulcers at the same location may be associated with venous outflow obstruction, (May-Thurner syndrome is an underestimated pathology) which affects mainly the left leg.
5. When performing iliac vein venograms, make liberal use of intravascular ultrasound.
6. Exudative venous ulcers need multilayer compression dressings and appropriate antibiotics if infection exists.
7. Pentoxifylline (Trental) 800 mg, 3 times daily.
8. Frequent debridement and frequent objective evaluation for ulcer area with each office visit.
9. Bi-layered living cell treatment (Apligraf?) to promote healing.
10. Office/clinic visit every 3 months after complete healing (CEAP 5) and further testing as needed.
Dr. Mousa is an associate professor at the Department of Surgery, West Virginia University, Morgantown.
The underlying pathophysiology of chronic venous insufficiency is complex and involves many factors. Studies have shown that average venous ulcers may need 6-12 months for complete healing with an anticipated recurrence rate exceeding 2/3 cases in 5 years. These numbers reflect the magnitude of the problem and mandate deploying all efforts to stop progression of the disease. Our group has found the following 10 tips have significantly improved our healing rates.
1. First, rule out any associated arterial, immunologic, endocrine, or other systemic causes for leg/foot ulceration.
2. Be aggressive to stop progression of the disease (fight CEAP 6): any local tenderness at the site of discolored skin at the gaiter area for venous ulcers should initiate a prompt reflux study to evaluate for incompetent perforators.
3. Venous ulcers are associated with an incompetent perforator within 2 cm of the ulcer area.
4. Recurrent venous ulcers at the same location may be associated with venous outflow obstruction, (May-Thurner syndrome is an underestimated pathology) which affects mainly the left leg.
5. When performing iliac vein venograms, make liberal use of intravascular ultrasound.
6. Exudative venous ulcers need multilayer compression dressings and appropriate antibiotics if infection exists.
7. Pentoxifylline (Trental) 800 mg, 3 times daily.
8. Frequent debridement and frequent objective evaluation for ulcer area with each office visit.
9. Bi-layered living cell treatment (Apligraf?) to promote healing.
10. Office/clinic visit every 3 months after complete healing (CEAP 5) and further testing as needed.
Dr. Mousa is an associate professor at the Department of Surgery, West Virginia University, Morgantown.
Peripheral neuropathy linked to obstructive sleep apnea?
CASE A 57-year-old white woman presented with symptoms of bilateral “stocking-like numbness” and the sensation of “wearing socks for a few weeks” but denied any injury, previous chemotherapy, or diabetes. Her medical history was positive for untreated obstructive sleep apnea (OSA), obesity (body mass index, 36 kg/m2), osteoarthritis in various joints, impaired fasting glucose with normal glycosylated hemoglobin (HbA1c), hypertension, gastroesophageal reflux disease, hypothyroidism, hypercholesterolemia, and osteoporosis.
Our initial examination revealed decreased sensation to light palpation and pin prick over the distal portion of her lower extremities in a stocking-like fashion. Proprioception was decreased at the distal joint of the big toe. Her deep tendon reflex pattern was symmetric with 2+ at the knees, ankles, and toes. The rest of her lower extremity exam was within normal limits and there were no obvious vascular abnormalities.
Given the suspicion of peripheral neuropathy, the patient underwent laboratory tests and a nerve conduction study. Vitamin B12, vitamin B1, methylmalonic acid (MMA), thyroid function, thyroid peroxidase (TPO), serum protein electrophoresis (SPEP), rapid plasma reagin (RPR), sedimentation rate, vitamin D, complete blood count, and chemistry profile 24 were all negative. The antinuclear antibody test revealed a homogenous 1:80 titer with a negative nuclear deoxyribonucleic acid. Her fasting glucose had been elevated between 107 to 117 mg/dL in the last 5 years but HbA1c was normal (5.8%). The patient had not been diagnosed with diabetes and her latest glucose values had been stable.
However, electromyography and a nerve conduction study were abnormal, with electrophysiological evidence of mild axonal polyneuropathy. During the month prior to her presentation, she had developed burning pain in addition to the numbness/stocking sensation. Pregabalin, gabapentin, duloxetine, celecoxib, hydrocodone, methadone, and other medications were ineffective. Eventually the foot pain became so severe—she described it as “walking on tacks”—that she was unable to walk.
Our team decided to do a nerve block to relieve the pain. Initially she underwent right and later left peroneal and posterior tibial nerve blocks, which gave her immediate relief that lasted about 2 months.
Relief from the pain, but what about the OSA symptoms?
In the meantime, our patient developed increasing OSA symptoms, including snoring, nonrestorative sleep, daytime somnolence, and fatigue. (To learn more about OSA, see “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565.)
Her history of mild-to-moderate OSA dated back 2 years, and included an apnea-hypopnea index (AHI) of 20 events per hour and 133 episodes of oxygen desaturation with a low O2 desaturation of 83%. The patient had never been treated, however, because she felt that she couldn’t tolerate the continuous positive airway pressure (CPAP) mask.
The patient finally agreed to a CPAP titration study. Her AHI improved from 20 to <2 events per hour; the oxygen desaturation dropped from 133 to 104 episodes; and the lowest O2 desaturation went from 83% to 85%.
When we initially started CPAP, our patient did not tolerate it very well. However, after consulting with our sleep clinic, she was placed on bilevel positive airway pressure, which she did tolerate. Surprisingly, she also noticed immediate improvement of the neuropathic foot pain; after a few weeks it resolved completely.
Still no foot pain…We continue to follow the patient’s progress and, after 3 years, she remains free of foot pain. Her initial numbness remains, however. She has not developed diabetes, with similar fasting sugar levels and an HbA1c of 5.4%. She is not taking any medication for neuropathic pain, but remains on methadone for unrelated severe intractable osteoarthritic pain of the lumbar spine, bilateral knee joints, and left hip.
The link between sleep apnea and neuropathy
Our case report suggests that clinicians should consider OSA as a cause of neuropathic pain. A recent review of the literature supports the relationship between the 2 conditions.
The prevalence of neuropathy in the general population is 2.4%, rising to 8% with advancing age.1 Many different types of peripheral neuropathy have been described; they have different symptoms and characteristics, depending on the specific part of the nervous system that is affected.2
The literature reveals a strong association between OSA and peripheral neuropathy and sight-threatening retinopathy.3 One study found that nearly 60% of patients with diabetes and OSA also have peripheral neuropathy.4 Another report found that OSA is an independent risk factor for axonal damage of peripheral nerves.5 Furthermore, a case-control study revealed that the impaired neural function is at least partly reversible with treatment for sleep apnea.4 Finally, Tahrani et al6 have found that “neuropathy prevalence was higher in patients with OSA than those without” (60% vs 27%; P<.001), which supports our case finding.
The specific mechanism linking OSA and neuropathy remains elusive, but the evidence suggests that peripheral nervous tissue is affected by chronic endoneural hypoxia in this patient population.7 In patients with OSA, 2 types of nerve dysfunction are apparent: ischemia-related axonal degeneration and resistance to ischemic nerve failure.8
An approach worth considering. While nerve blocks did provide some relief for our patient, they are not a long-term solution. To our knowledge, this case report is the first one published in the United States describing resolution of neuropathic pain by treatment of OSA. This approach is certainly worth considering in patients who have not responded to more traditional therapy.
Correspondence
Fong Wong, DDS, MS, Associate Professor, Department of Restorative Dental Sciences, College of Dentistry, University of Florida, 1395 Center Drive, PO Box 100435, Gainesville, FL 32610; [email protected]
1. Martyn C, Hughes R. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
2. NINDS peripheral neuropathy information page. Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/peripheralneuropathy.htm. Last updated September 19, 2012. Accessed September 16, 2013.
3. Waller E, Bendel R, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83:1251-1261.
4. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295-297.
5. Ludemann P, Dziewas R, Soros P, et al. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685-687.
6. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441.
7. Pfeiffer G, Kunze K, Bruch M, et al. Polyneuropathy associated with chronic hypoxaemia: prevalence in patients with chronic obstructive pulmonary disease. J Neurol. 1990;237:230-233.
8. Mayer P, Dematteis M, Pepin J, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213-219.
CASE A 57-year-old white woman presented with symptoms of bilateral “stocking-like numbness” and the sensation of “wearing socks for a few weeks” but denied any injury, previous chemotherapy, or diabetes. Her medical history was positive for untreated obstructive sleep apnea (OSA), obesity (body mass index, 36 kg/m2), osteoarthritis in various joints, impaired fasting glucose with normal glycosylated hemoglobin (HbA1c), hypertension, gastroesophageal reflux disease, hypothyroidism, hypercholesterolemia, and osteoporosis.
Our initial examination revealed decreased sensation to light palpation and pin prick over the distal portion of her lower extremities in a stocking-like fashion. Proprioception was decreased at the distal joint of the big toe. Her deep tendon reflex pattern was symmetric with 2+ at the knees, ankles, and toes. The rest of her lower extremity exam was within normal limits and there were no obvious vascular abnormalities.
Given the suspicion of peripheral neuropathy, the patient underwent laboratory tests and a nerve conduction study. Vitamin B12, vitamin B1, methylmalonic acid (MMA), thyroid function, thyroid peroxidase (TPO), serum protein electrophoresis (SPEP), rapid plasma reagin (RPR), sedimentation rate, vitamin D, complete blood count, and chemistry profile 24 were all negative. The antinuclear antibody test revealed a homogenous 1:80 titer with a negative nuclear deoxyribonucleic acid. Her fasting glucose had been elevated between 107 to 117 mg/dL in the last 5 years but HbA1c was normal (5.8%). The patient had not been diagnosed with diabetes and her latest glucose values had been stable.
However, electromyography and a nerve conduction study were abnormal, with electrophysiological evidence of mild axonal polyneuropathy. During the month prior to her presentation, she had developed burning pain in addition to the numbness/stocking sensation. Pregabalin, gabapentin, duloxetine, celecoxib, hydrocodone, methadone, and other medications were ineffective. Eventually the foot pain became so severe—she described it as “walking on tacks”—that she was unable to walk.
Our team decided to do a nerve block to relieve the pain. Initially she underwent right and later left peroneal and posterior tibial nerve blocks, which gave her immediate relief that lasted about 2 months.
Relief from the pain, but what about the OSA symptoms?
In the meantime, our patient developed increasing OSA symptoms, including snoring, nonrestorative sleep, daytime somnolence, and fatigue. (To learn more about OSA, see “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565.)
Her history of mild-to-moderate OSA dated back 2 years, and included an apnea-hypopnea index (AHI) of 20 events per hour and 133 episodes of oxygen desaturation with a low O2 desaturation of 83%. The patient had never been treated, however, because she felt that she couldn’t tolerate the continuous positive airway pressure (CPAP) mask.
The patient finally agreed to a CPAP titration study. Her AHI improved from 20 to <2 events per hour; the oxygen desaturation dropped from 133 to 104 episodes; and the lowest O2 desaturation went from 83% to 85%.
When we initially started CPAP, our patient did not tolerate it very well. However, after consulting with our sleep clinic, she was placed on bilevel positive airway pressure, which she did tolerate. Surprisingly, she also noticed immediate improvement of the neuropathic foot pain; after a few weeks it resolved completely.
Still no foot pain…We continue to follow the patient’s progress and, after 3 years, she remains free of foot pain. Her initial numbness remains, however. She has not developed diabetes, with similar fasting sugar levels and an HbA1c of 5.4%. She is not taking any medication for neuropathic pain, but remains on methadone for unrelated severe intractable osteoarthritic pain of the lumbar spine, bilateral knee joints, and left hip.
The link between sleep apnea and neuropathy
Our case report suggests that clinicians should consider OSA as a cause of neuropathic pain. A recent review of the literature supports the relationship between the 2 conditions.
The prevalence of neuropathy in the general population is 2.4%, rising to 8% with advancing age.1 Many different types of peripheral neuropathy have been described; they have different symptoms and characteristics, depending on the specific part of the nervous system that is affected.2
The literature reveals a strong association between OSA and peripheral neuropathy and sight-threatening retinopathy.3 One study found that nearly 60% of patients with diabetes and OSA also have peripheral neuropathy.4 Another report found that OSA is an independent risk factor for axonal damage of peripheral nerves.5 Furthermore, a case-control study revealed that the impaired neural function is at least partly reversible with treatment for sleep apnea.4 Finally, Tahrani et al6 have found that “neuropathy prevalence was higher in patients with OSA than those without” (60% vs 27%; P<.001), which supports our case finding.
The specific mechanism linking OSA and neuropathy remains elusive, but the evidence suggests that peripheral nervous tissue is affected by chronic endoneural hypoxia in this patient population.7 In patients with OSA, 2 types of nerve dysfunction are apparent: ischemia-related axonal degeneration and resistance to ischemic nerve failure.8
An approach worth considering. While nerve blocks did provide some relief for our patient, they are not a long-term solution. To our knowledge, this case report is the first one published in the United States describing resolution of neuropathic pain by treatment of OSA. This approach is certainly worth considering in patients who have not responded to more traditional therapy.
Correspondence
Fong Wong, DDS, MS, Associate Professor, Department of Restorative Dental Sciences, College of Dentistry, University of Florida, 1395 Center Drive, PO Box 100435, Gainesville, FL 32610; [email protected]
CASE A 57-year-old white woman presented with symptoms of bilateral “stocking-like numbness” and the sensation of “wearing socks for a few weeks” but denied any injury, previous chemotherapy, or diabetes. Her medical history was positive for untreated obstructive sleep apnea (OSA), obesity (body mass index, 36 kg/m2), osteoarthritis in various joints, impaired fasting glucose with normal glycosylated hemoglobin (HbA1c), hypertension, gastroesophageal reflux disease, hypothyroidism, hypercholesterolemia, and osteoporosis.
Our initial examination revealed decreased sensation to light palpation and pin prick over the distal portion of her lower extremities in a stocking-like fashion. Proprioception was decreased at the distal joint of the big toe. Her deep tendon reflex pattern was symmetric with 2+ at the knees, ankles, and toes. The rest of her lower extremity exam was within normal limits and there were no obvious vascular abnormalities.
Given the suspicion of peripheral neuropathy, the patient underwent laboratory tests and a nerve conduction study. Vitamin B12, vitamin B1, methylmalonic acid (MMA), thyroid function, thyroid peroxidase (TPO), serum protein electrophoresis (SPEP), rapid plasma reagin (RPR), sedimentation rate, vitamin D, complete blood count, and chemistry profile 24 were all negative. The antinuclear antibody test revealed a homogenous 1:80 titer with a negative nuclear deoxyribonucleic acid. Her fasting glucose had been elevated between 107 to 117 mg/dL in the last 5 years but HbA1c was normal (5.8%). The patient had not been diagnosed with diabetes and her latest glucose values had been stable.
However, electromyography and a nerve conduction study were abnormal, with electrophysiological evidence of mild axonal polyneuropathy. During the month prior to her presentation, she had developed burning pain in addition to the numbness/stocking sensation. Pregabalin, gabapentin, duloxetine, celecoxib, hydrocodone, methadone, and other medications were ineffective. Eventually the foot pain became so severe—she described it as “walking on tacks”—that she was unable to walk.
Our team decided to do a nerve block to relieve the pain. Initially she underwent right and later left peroneal and posterior tibial nerve blocks, which gave her immediate relief that lasted about 2 months.
Relief from the pain, but what about the OSA symptoms?
In the meantime, our patient developed increasing OSA symptoms, including snoring, nonrestorative sleep, daytime somnolence, and fatigue. (To learn more about OSA, see “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565.)
Her history of mild-to-moderate OSA dated back 2 years, and included an apnea-hypopnea index (AHI) of 20 events per hour and 133 episodes of oxygen desaturation with a low O2 desaturation of 83%. The patient had never been treated, however, because she felt that she couldn’t tolerate the continuous positive airway pressure (CPAP) mask.
The patient finally agreed to a CPAP titration study. Her AHI improved from 20 to <2 events per hour; the oxygen desaturation dropped from 133 to 104 episodes; and the lowest O2 desaturation went from 83% to 85%.
When we initially started CPAP, our patient did not tolerate it very well. However, after consulting with our sleep clinic, she was placed on bilevel positive airway pressure, which she did tolerate. Surprisingly, she also noticed immediate improvement of the neuropathic foot pain; after a few weeks it resolved completely.
Still no foot pain…We continue to follow the patient’s progress and, after 3 years, she remains free of foot pain. Her initial numbness remains, however. She has not developed diabetes, with similar fasting sugar levels and an HbA1c of 5.4%. She is not taking any medication for neuropathic pain, but remains on methadone for unrelated severe intractable osteoarthritic pain of the lumbar spine, bilateral knee joints, and left hip.
The link between sleep apnea and neuropathy
Our case report suggests that clinicians should consider OSA as a cause of neuropathic pain. A recent review of the literature supports the relationship between the 2 conditions.
The prevalence of neuropathy in the general population is 2.4%, rising to 8% with advancing age.1 Many different types of peripheral neuropathy have been described; they have different symptoms and characteristics, depending on the specific part of the nervous system that is affected.2
The literature reveals a strong association between OSA and peripheral neuropathy and sight-threatening retinopathy.3 One study found that nearly 60% of patients with diabetes and OSA also have peripheral neuropathy.4 Another report found that OSA is an independent risk factor for axonal damage of peripheral nerves.5 Furthermore, a case-control study revealed that the impaired neural function is at least partly reversible with treatment for sleep apnea.4 Finally, Tahrani et al6 have found that “neuropathy prevalence was higher in patients with OSA than those without” (60% vs 27%; P<.001), which supports our case finding.
The specific mechanism linking OSA and neuropathy remains elusive, but the evidence suggests that peripheral nervous tissue is affected by chronic endoneural hypoxia in this patient population.7 In patients with OSA, 2 types of nerve dysfunction are apparent: ischemia-related axonal degeneration and resistance to ischemic nerve failure.8
An approach worth considering. While nerve blocks did provide some relief for our patient, they are not a long-term solution. To our knowledge, this case report is the first one published in the United States describing resolution of neuropathic pain by treatment of OSA. This approach is certainly worth considering in patients who have not responded to more traditional therapy.
Correspondence
Fong Wong, DDS, MS, Associate Professor, Department of Restorative Dental Sciences, College of Dentistry, University of Florida, 1395 Center Drive, PO Box 100435, Gainesville, FL 32610; [email protected]
1. Martyn C, Hughes R. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
2. NINDS peripheral neuropathy information page. Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/peripheralneuropathy.htm. Last updated September 19, 2012. Accessed September 16, 2013.
3. Waller E, Bendel R, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83:1251-1261.
4. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295-297.
5. Ludemann P, Dziewas R, Soros P, et al. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685-687.
6. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441.
7. Pfeiffer G, Kunze K, Bruch M, et al. Polyneuropathy associated with chronic hypoxaemia: prevalence in patients with chronic obstructive pulmonary disease. J Neurol. 1990;237:230-233.
8. Mayer P, Dematteis M, Pepin J, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213-219.
1. Martyn C, Hughes R. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318.
2. NINDS peripheral neuropathy information page. Available at: http://www.ninds.nih.gov/disorders/peripheralneuropathy/peripheralneuropathy.htm. Last updated September 19, 2012. Accessed September 16, 2013.
3. Waller E, Bendel R, Kaplan J. Sleep disorders and the eye. Mayo Clin Proc. 2008;83:1251-1261.
4. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J Neurol Neurosurg Psychiatry. 2007;78:295-297.
5. Ludemann P, Dziewas R, Soros P, et al. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry. 2001;70:685-687.
6. Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434-441.
7. Pfeiffer G, Kunze K, Bruch M, et al. Polyneuropathy associated with chronic hypoxaemia: prevalence in patients with chronic obstructive pulmonary disease. J Neurol. 1990;237:230-233.
8. Mayer P, Dematteis M, Pepin J, et al. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:213-219.