User login
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6
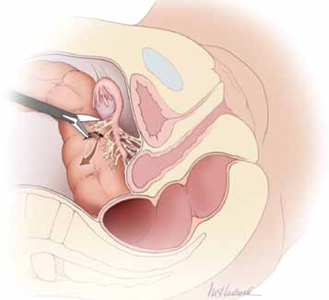
FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.
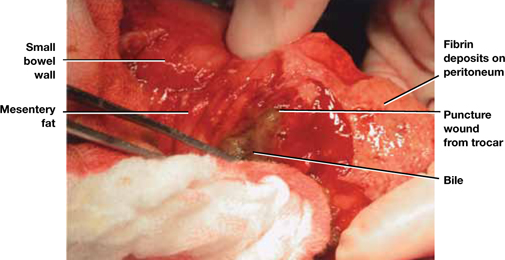
FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6

FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.

FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6

FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.

FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
Antibiotics fail to head off sepsis … Failure to address persistent symptoms proves disastrous… more
Antibiotics fail to head off sepsis
SHORTNESS OF BREATH AND RIGHT-SIDED CHEST PAIN prompted a 45-year-old woman to go to the emergency department (ED) early one morning. She had a history of chronic lung problems with multiple diagnoses of pneumonia, pneumothorax, blebs, and bronchiectasis. The ED doctor diagnosed community-acquired pneumonia and admitted her for intravenous antibiotic treatment.
Late that afternoon the patient’s condition deteriorated rapidly. She was transferred to the intensive care unit, where she died of septic shock caused by Pseudomonas aeruginosa 22 hours after she had arrived at the ED.
PLAINTIFF’S CLAIM The patient should have received broader-spectrum antibiotics.
THE DEFENSE The hospitalist who treated the woman as an inpatient claimed that the treatment she received was appropriate and that she probably would have died even if other antibiotics had been prescribed. The hospitalist also claimed that the nursing staff failed to notify her of the patient’s low blood pressure readings until 10 hours after the initial evaluation. A nurse denied this claim, asserting that the hospitalist had been paged several times during the day. The discharge summary and nursing notes on the patient were missing.
VERDICT $5.28 million arbitration award.
COMMENT It surprises me how often key portions of medical records go missing! Here, the absence of a discharge summary and nursing notes may well have contributed to a $5 million award.
Change, and not for the better
AN ATYPICAL MOLE ON THE LEFT CALF was brought to the attention of a primary care physician by a 36-year-old man during a full physical. The mole was 1 3 1 cm; the patient reported that it had been changing. The mole’s appearance didn’t worry the physician, who described it in his notes as either a hemangioma or dermatofibroma. The doctor advised the patient to return in 6 months if he wanted the mole removed for cosmetic reasons.
Over the next 5 months, the patient noticed further changes in the mole and called the doctor’s office. He was seen by a colleague of his physician, who immediately sent the patient for a biopsy and surgical consultation. The mole was removed and diagnosed as an ulcerating melanoma with downward growth.
Shortly thereafter, the patient underwent wide excision and lymph node dissection, which showed clear margins and no lymph node involvement. Twenty months later, a mass was found in the patient’s liver. Biopsy diagnosed metastatic spread of the melanoma. The patient died 2 months later.
PLAINTIFF’S CLAIM The patient should have had a biopsy and received a surgical referral at the time of the physical examination when he first reported the mole.
THE DEFENSE Waiting for 6 months was appropriate because the mole didn’t look like a melanoma when the patient first called it to the physician’s attention. The melanoma had already metastasized at the time of the physical examination and the diagnostic delay didn’t affect the outcome.
VERDICT $1 million Massachusetts settlement.
COMMENT A changing mole should always raise concern. Biopsy, excision, or a referral could have avoided a million-dollar settlement.
Failure to address persistent symptoms proves disastrous
PAIN IN THE BACK AND CHEST along with respiratory difficulty prompted a 49-year-old man to visit his physician. The physician told him to go to a hospital. The doctor who examined the patient at the hospital diagnosed muscle strain and prescribed muscle relaxants.
The following day, the patient returned to his physician complaining of continuing symptoms. The doctor sent him home. He died the next day of an aortic rupture caused by an undiagnosed dissection.
PLAINTIFF’S CLAIM The 2 physicians should have diagnosed the dissection, which would have permitted treatment and prevented death. The patient had been treated previously at the hospital, and his records should have raised suspicion of an aortic aneurysm. The hospital physician was a new hire and hadn’t received proper training in the hospital’s electronic records system. He should have ordered a computed tomography scan or cardiology consult. The patient’s physician failed to address the ongoing symptoms. He should have hospitalized the patient at the time of the second visit.
THE DEFENSE The hospital physician claimed he had intended to contact the cardiologist who had treated the patient, but the patient couldn’t remember the cardiologist’s name. The patient’s symptoms didn’t suggest an aortic dissection, and the dissection occurred after the patient was discharged from the hospital.
VERDICT $3.4 million New York verdict against the hospital physician only.
COMMENT Although the hoofbeats are usually horses, always remember the zebras (or should it be lions?), particularly when a patient returns repeatedly with ongoing symptoms.
Controlled substances out of control
A WOMAN WITH CHRONIC MIGRAINES, anxiety problems, and nausea also had cardiomyopathy and chronic atrial fibrillation, which could be triggered by pain from her other ailments. She came under the care of a physician who prescribed a number of drugs, including meperidine, hydrocodone, tizanidine, diazepam, promethazine, alprazolam, and oxcarbazepine. The doctor prescribed injectable forms of certain medications after the patient told him her next-door neighbor was a nurse and could help administer the drugs.
Four years after coming under the doctor’s care, the patient signed a Controlled Substance Agreement specifying that the physician would discontinue her as a patient if she got controlled substances from another doctor. (Evidence was later found that the patient was receiving prescriptions from other physicians.)
While under treatment by her doctor, the patient was hospitalized a number of times for medication overdoses. The record from one hospitalization reported that she had made angry, profanity-laced requests for meperidine and promethazine.
About 2 years after signing the Controlled Substance Agreement, the patient received prescriptions from her doctor for 210 doses of meperidine, 100 doses of promethazine, and 60 pills each of diazepam, alprazolam, and acetaminophen and hydrocodone. She filled the prescriptions at 2 pharmacies without objections from the pharmacists. She died of an accidental drug overdose the following month.
Postmortem blood testing showed high levels of meperidine and promethazine. The patient had apparently taken the equivalent of 11 “shots” of meperidine (5 times the maximum prescribed amount), probably by injecting herself through a peripherally inserted central catheter rather than by intramuscular injection, as prescribed.
PLAINTIFF’S CLAIM The patient’s doctor was negligent in prescribing large amounts of controlled substances when he should have known that she was a drug seeker with a drug abuse problem. The pharmacies were negligent for filling the prescriptions without question.
THE DEFENSE The patient was solely responsible for her own death because she gave herself a large overdose.
VERDICT $500,000 Alabama verdict. The case against the pharmacies was dismissed.
COMMENT Increasingly it is expected that physicians (and pharmacists) perform due diligence when prescribing opioids, including taking reasonable precautions against the drug-seeking patient.
Antibiotics fail to head off sepsis
SHORTNESS OF BREATH AND RIGHT-SIDED CHEST PAIN prompted a 45-year-old woman to go to the emergency department (ED) early one morning. She had a history of chronic lung problems with multiple diagnoses of pneumonia, pneumothorax, blebs, and bronchiectasis. The ED doctor diagnosed community-acquired pneumonia and admitted her for intravenous antibiotic treatment.
Late that afternoon the patient’s condition deteriorated rapidly. She was transferred to the intensive care unit, where she died of septic shock caused by Pseudomonas aeruginosa 22 hours after she had arrived at the ED.
PLAINTIFF’S CLAIM The patient should have received broader-spectrum antibiotics.
THE DEFENSE The hospitalist who treated the woman as an inpatient claimed that the treatment she received was appropriate and that she probably would have died even if other antibiotics had been prescribed. The hospitalist also claimed that the nursing staff failed to notify her of the patient’s low blood pressure readings until 10 hours after the initial evaluation. A nurse denied this claim, asserting that the hospitalist had been paged several times during the day. The discharge summary and nursing notes on the patient were missing.
VERDICT $5.28 million arbitration award.
COMMENT It surprises me how often key portions of medical records go missing! Here, the absence of a discharge summary and nursing notes may well have contributed to a $5 million award.
Change, and not for the better
AN ATYPICAL MOLE ON THE LEFT CALF was brought to the attention of a primary care physician by a 36-year-old man during a full physical. The mole was 1 3 1 cm; the patient reported that it had been changing. The mole’s appearance didn’t worry the physician, who described it in his notes as either a hemangioma or dermatofibroma. The doctor advised the patient to return in 6 months if he wanted the mole removed for cosmetic reasons.
Over the next 5 months, the patient noticed further changes in the mole and called the doctor’s office. He was seen by a colleague of his physician, who immediately sent the patient for a biopsy and surgical consultation. The mole was removed and diagnosed as an ulcerating melanoma with downward growth.
Shortly thereafter, the patient underwent wide excision and lymph node dissection, which showed clear margins and no lymph node involvement. Twenty months later, a mass was found in the patient’s liver. Biopsy diagnosed metastatic spread of the melanoma. The patient died 2 months later.
PLAINTIFF’S CLAIM The patient should have had a biopsy and received a surgical referral at the time of the physical examination when he first reported the mole.
THE DEFENSE Waiting for 6 months was appropriate because the mole didn’t look like a melanoma when the patient first called it to the physician’s attention. The melanoma had already metastasized at the time of the physical examination and the diagnostic delay didn’t affect the outcome.
VERDICT $1 million Massachusetts settlement.
COMMENT A changing mole should always raise concern. Biopsy, excision, or a referral could have avoided a million-dollar settlement.
Failure to address persistent symptoms proves disastrous
PAIN IN THE BACK AND CHEST along with respiratory difficulty prompted a 49-year-old man to visit his physician. The physician told him to go to a hospital. The doctor who examined the patient at the hospital diagnosed muscle strain and prescribed muscle relaxants.
The following day, the patient returned to his physician complaining of continuing symptoms. The doctor sent him home. He died the next day of an aortic rupture caused by an undiagnosed dissection.
PLAINTIFF’S CLAIM The 2 physicians should have diagnosed the dissection, which would have permitted treatment and prevented death. The patient had been treated previously at the hospital, and his records should have raised suspicion of an aortic aneurysm. The hospital physician was a new hire and hadn’t received proper training in the hospital’s electronic records system. He should have ordered a computed tomography scan or cardiology consult. The patient’s physician failed to address the ongoing symptoms. He should have hospitalized the patient at the time of the second visit.
THE DEFENSE The hospital physician claimed he had intended to contact the cardiologist who had treated the patient, but the patient couldn’t remember the cardiologist’s name. The patient’s symptoms didn’t suggest an aortic dissection, and the dissection occurred after the patient was discharged from the hospital.
VERDICT $3.4 million New York verdict against the hospital physician only.
COMMENT Although the hoofbeats are usually horses, always remember the zebras (or should it be lions?), particularly when a patient returns repeatedly with ongoing symptoms.
Controlled substances out of control
A WOMAN WITH CHRONIC MIGRAINES, anxiety problems, and nausea also had cardiomyopathy and chronic atrial fibrillation, which could be triggered by pain from her other ailments. She came under the care of a physician who prescribed a number of drugs, including meperidine, hydrocodone, tizanidine, diazepam, promethazine, alprazolam, and oxcarbazepine. The doctor prescribed injectable forms of certain medications after the patient told him her next-door neighbor was a nurse and could help administer the drugs.
Four years after coming under the doctor’s care, the patient signed a Controlled Substance Agreement specifying that the physician would discontinue her as a patient if she got controlled substances from another doctor. (Evidence was later found that the patient was receiving prescriptions from other physicians.)
While under treatment by her doctor, the patient was hospitalized a number of times for medication overdoses. The record from one hospitalization reported that she had made angry, profanity-laced requests for meperidine and promethazine.
About 2 years after signing the Controlled Substance Agreement, the patient received prescriptions from her doctor for 210 doses of meperidine, 100 doses of promethazine, and 60 pills each of diazepam, alprazolam, and acetaminophen and hydrocodone. She filled the prescriptions at 2 pharmacies without objections from the pharmacists. She died of an accidental drug overdose the following month.
Postmortem blood testing showed high levels of meperidine and promethazine. The patient had apparently taken the equivalent of 11 “shots” of meperidine (5 times the maximum prescribed amount), probably by injecting herself through a peripherally inserted central catheter rather than by intramuscular injection, as prescribed.
PLAINTIFF’S CLAIM The patient’s doctor was negligent in prescribing large amounts of controlled substances when he should have known that she was a drug seeker with a drug abuse problem. The pharmacies were negligent for filling the prescriptions without question.
THE DEFENSE The patient was solely responsible for her own death because she gave herself a large overdose.
VERDICT $500,000 Alabama verdict. The case against the pharmacies was dismissed.
COMMENT Increasingly it is expected that physicians (and pharmacists) perform due diligence when prescribing opioids, including taking reasonable precautions against the drug-seeking patient.
Antibiotics fail to head off sepsis
SHORTNESS OF BREATH AND RIGHT-SIDED CHEST PAIN prompted a 45-year-old woman to go to the emergency department (ED) early one morning. She had a history of chronic lung problems with multiple diagnoses of pneumonia, pneumothorax, blebs, and bronchiectasis. The ED doctor diagnosed community-acquired pneumonia and admitted her for intravenous antibiotic treatment.
Late that afternoon the patient’s condition deteriorated rapidly. She was transferred to the intensive care unit, where she died of septic shock caused by Pseudomonas aeruginosa 22 hours after she had arrived at the ED.
PLAINTIFF’S CLAIM The patient should have received broader-spectrum antibiotics.
THE DEFENSE The hospitalist who treated the woman as an inpatient claimed that the treatment she received was appropriate and that she probably would have died even if other antibiotics had been prescribed. The hospitalist also claimed that the nursing staff failed to notify her of the patient’s low blood pressure readings until 10 hours after the initial evaluation. A nurse denied this claim, asserting that the hospitalist had been paged several times during the day. The discharge summary and nursing notes on the patient were missing.
VERDICT $5.28 million arbitration award.
COMMENT It surprises me how often key portions of medical records go missing! Here, the absence of a discharge summary and nursing notes may well have contributed to a $5 million award.
Change, and not for the better
AN ATYPICAL MOLE ON THE LEFT CALF was brought to the attention of a primary care physician by a 36-year-old man during a full physical. The mole was 1 3 1 cm; the patient reported that it had been changing. The mole’s appearance didn’t worry the physician, who described it in his notes as either a hemangioma or dermatofibroma. The doctor advised the patient to return in 6 months if he wanted the mole removed for cosmetic reasons.
Over the next 5 months, the patient noticed further changes in the mole and called the doctor’s office. He was seen by a colleague of his physician, who immediately sent the patient for a biopsy and surgical consultation. The mole was removed and diagnosed as an ulcerating melanoma with downward growth.
Shortly thereafter, the patient underwent wide excision and lymph node dissection, which showed clear margins and no lymph node involvement. Twenty months later, a mass was found in the patient’s liver. Biopsy diagnosed metastatic spread of the melanoma. The patient died 2 months later.
PLAINTIFF’S CLAIM The patient should have had a biopsy and received a surgical referral at the time of the physical examination when he first reported the mole.
THE DEFENSE Waiting for 6 months was appropriate because the mole didn’t look like a melanoma when the patient first called it to the physician’s attention. The melanoma had already metastasized at the time of the physical examination and the diagnostic delay didn’t affect the outcome.
VERDICT $1 million Massachusetts settlement.
COMMENT A changing mole should always raise concern. Biopsy, excision, or a referral could have avoided a million-dollar settlement.
Failure to address persistent symptoms proves disastrous
PAIN IN THE BACK AND CHEST along with respiratory difficulty prompted a 49-year-old man to visit his physician. The physician told him to go to a hospital. The doctor who examined the patient at the hospital diagnosed muscle strain and prescribed muscle relaxants.
The following day, the patient returned to his physician complaining of continuing symptoms. The doctor sent him home. He died the next day of an aortic rupture caused by an undiagnosed dissection.
PLAINTIFF’S CLAIM The 2 physicians should have diagnosed the dissection, which would have permitted treatment and prevented death. The patient had been treated previously at the hospital, and his records should have raised suspicion of an aortic aneurysm. The hospital physician was a new hire and hadn’t received proper training in the hospital’s electronic records system. He should have ordered a computed tomography scan or cardiology consult. The patient’s physician failed to address the ongoing symptoms. He should have hospitalized the patient at the time of the second visit.
THE DEFENSE The hospital physician claimed he had intended to contact the cardiologist who had treated the patient, but the patient couldn’t remember the cardiologist’s name. The patient’s symptoms didn’t suggest an aortic dissection, and the dissection occurred after the patient was discharged from the hospital.
VERDICT $3.4 million New York verdict against the hospital physician only.
COMMENT Although the hoofbeats are usually horses, always remember the zebras (or should it be lions?), particularly when a patient returns repeatedly with ongoing symptoms.
Controlled substances out of control
A WOMAN WITH CHRONIC MIGRAINES, anxiety problems, and nausea also had cardiomyopathy and chronic atrial fibrillation, which could be triggered by pain from her other ailments. She came under the care of a physician who prescribed a number of drugs, including meperidine, hydrocodone, tizanidine, diazepam, promethazine, alprazolam, and oxcarbazepine. The doctor prescribed injectable forms of certain medications after the patient told him her next-door neighbor was a nurse and could help administer the drugs.
Four years after coming under the doctor’s care, the patient signed a Controlled Substance Agreement specifying that the physician would discontinue her as a patient if she got controlled substances from another doctor. (Evidence was later found that the patient was receiving prescriptions from other physicians.)
While under treatment by her doctor, the patient was hospitalized a number of times for medication overdoses. The record from one hospitalization reported that she had made angry, profanity-laced requests for meperidine and promethazine.
About 2 years after signing the Controlled Substance Agreement, the patient received prescriptions from her doctor for 210 doses of meperidine, 100 doses of promethazine, and 60 pills each of diazepam, alprazolam, and acetaminophen and hydrocodone. She filled the prescriptions at 2 pharmacies without objections from the pharmacists. She died of an accidental drug overdose the following month.
Postmortem blood testing showed high levels of meperidine and promethazine. The patient had apparently taken the equivalent of 11 “shots” of meperidine (5 times the maximum prescribed amount), probably by injecting herself through a peripherally inserted central catheter rather than by intramuscular injection, as prescribed.
PLAINTIFF’S CLAIM The patient’s doctor was negligent in prescribing large amounts of controlled substances when he should have known that she was a drug seeker with a drug abuse problem. The pharmacies were negligent for filling the prescriptions without question.
THE DEFENSE The patient was solely responsible for her own death because she gave herself a large overdose.
VERDICT $500,000 Alabama verdict. The case against the pharmacies was dismissed.
COMMENT Increasingly it is expected that physicians (and pharmacists) perform due diligence when prescribing opioids, including taking reasonable precautions against the drug-seeking patient.
Battling influenza: Changes for the 2012-2013 season
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
By all measures, last year’s flu season was a mild one that peaked in early March. The number of cases reported, the total number of pneumonia/influenza deaths, and the number of pediatric deaths were all comparatively low.1 The main circulating strains were the same as the previous 2 years, leaving much of the population protected by immunity gained from natural infections. And there was a good match between the vaccine and the circulating strains, leading to higher vaccine effectiveness.
So what’s new—and what remains the same—for the 2012-2013 season? Annual flu vaccination continues to be recommended for everyone ≥6 months of age, starting as soon as the vaccine is available and continuing through the influenza season. This season’s vaccine contains 2 new strains in addition to the A/California/7/2009 (H1N1) strain that has been in the vaccine the past 2 seasons and was used for a monovalent vaccine in 2009. The new strains are A/Victoria/361/2011 (H3N2) and B/Wisconsin/1/2010.2
Each year, influenza experts decide which strains to include in the vaccine based on the ones currently circulating and the ones expected to circulate into the next season. The degree to which vaccine strains match circulating strains will determine the effectiveness of the vaccine.
A variety of products
The number of influenza vaccine products has increased, and they differ in the ages for which they are approved.2 All trivalent inactivated vaccine (TIV) products are given intramuscularly, with the exception of a relatively new product, Fluzone Intradermal (Sanofi Pasteur). There is also FluMist (MedImmune), a live-attenuated influenza vaccine (LAIV), administered as a nasal spray. And, for adults ≥65 years, the product Fluzone High-Dose contains 4 times the antigen content of other vaccines for this age group. The Centers for Disease Control and Prevention (CDC) does not express a preference for any one of the TIV products over the others.
A new algorithm for children <9 years
To mount an adequate immune response in children <9 years, 2 doses of vaccine are needed in the first year of receiving vaccine. The time between doses should be at least 4 weeks; longer intervals do not appear to matter. This leads to a complex algorithm when taking into consideration vaccines given in past years. To simplify the issue, the CDC has approved an algorithm for this season that involves only 2 questions (FIGURE 1).
However, strict adherence to this algorithm will result in some children receiving 2 doses when one would suffice. If a child has received at least 2 doses of seasonal influenza vaccine in any prior season, and at least one dose of A/California/7/2009 (H1N1), then just a single dose of vaccine is needed this year. The A/California antigen could have been in the monovalent product in 2009 or the regular trivalent products in the past 2 seasons. If any doubt or confusion exists, administer 2 doses, 4 weeks apart. There is no harm in receiving 2 doses if only one is needed.
FIGURE 1
The CDC’s new dosing algorithm for children 6 months–8 years receiving influenza vaccine in the 2012-2013 flu season2
*Doses should be administered at least 4 weeks apart.
†For simplicity, this algorithm takes into consideration only doses of seasonal influenza vaccine received since July 1, 2010. However, if a child 6 months through 8 years of age is known to have received at least 2 seasonal influenza vaccines during any prior season, and at least one dose of a 2009 (H1N1)-containing vaccine-—ie, either 2010-2011 or 2011-2012 seasonal vaccine or the monovalent 2009 (H1N1) vaccine—then the child needs only one dose for 2012-2013.
Egg allergy precautions
While all influenza vaccines are manufactured by injecting virus into chicken eggs, the amount of egg protein in the vaccine is extremely low. Those who react to egg products only with hives can receive TIV (not LAIV) under the care of a clinician who is familiar with egg allergy manifestations. (Patients should be observed for 30 minutes after vaccine administration.) Those who have more severe reactions to eggs (FIGURE 2) require assessment by a physician with allergy expertise before receiving an influenza vaccine. All facilities that administer vaccines should be equipped to respond to anaphylaxis, and all providers who administer vaccines should be adequately trained in anaphylaxis management. The CDC made these recommendations last year, and there was no increase in egg allergy adverse events following vaccination reported during the 2011-2012 flu season.3
FIGURE 2
ACIP 2012-2013 recommendations regarding influenza vaccination for those with egg allergy2
TIV, trivalent inactivated vaccine.
*Individuals with egg allergy may tolerate egg in baked products (eg, bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy.
Febrile seizures and vaccine safety
Last flu season, vaccine safety surveillance detected an increased risk for febrile seizures among children 6 months to 4 years during the 24 hours after receiving TIV, when it was given at the same time as 13-valent pneumococcal conjugate vaccine (PCV13).3 This increased risk was <1 per 1000 children vaccinated, and the Advisory Committee on Immunization Practices (ACIP) did not consider it significant enough to warrant any changes in TIV or PCV13 recommendations.
No other safety concerns arose for influenza vaccines last year. The vaccine safety monitoring system looks specifically at Guillain-Barré syndrome (GBS), and it detected no increased risk for GBS related to influenza vaccine.3
Newer quadrivalent vaccines
Historically, influenza vaccines have contained 3 antigens: 2 type A and 1 type B. A newly approved quadrivalent LAIV (FluMist Quadrivalent, MedImmune) contains 2 antigenically different B strains. The inclusion of 2 B strains is expected to increase the likelihood of the vaccine matching the circulating B influenza strains and thereby increase vaccine effectiveness. This new product will probably not be available this coming flu season, but will be marketed for 2013-2014. Other, inactivated, quadrivalent vaccines are also in development and should be available in future flu seasons.
Improving influenza vaccine coverage
In 2011, only 36.3% of people ≥6 months of age had received influenza vaccination by the first week in November (36.7% of children 6 months to 17 years and 36.2% of adults ≥18 years).4 The Community Preventive Services Task Force (a nonfederal group whose members are appointed by the director of the CDC) recommends a number of evidence-based interventions to increase vaccine rates, including patient reminder and recall systems, provider quality assessment and feedback, and standing orders.5 Increased coverage is important if we are to lower the annual morbidity and mortality associated with influenza.
The role of antivirals
All influenza A and B strains currently circulating have low or zero rates of resistance to the neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza). The circulating A strains continue to have high levels of resistance to the adamantanes (amantadine and rimantadine). Therefore, use only the neuraminidase inhibitors to treat influenza, and for pre- and post exposure chemoprevention.1
Those who should receive treatment include anyone with suspected or confirmed influenza who is hospitalized or who meets specific criteria (TABLE). Details regarding influenza antivirals, doses, and duration of treatment—as well as indications for chemoprevention—are on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm#indications).
Table
Indications for treatment of influenza with antivirals
|
| BMI, body mass index; HIV, human immunodeficiency virus. Source: CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1-24. |
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
1. Finelli L. Influenza surveillance season summary. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/02-influenza-finelli.pdf. Accessed July 15, 2012.
2. CDC. Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61:613–618.
3. Shimabukuro T. Update on influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices (ACIP); June 20, 2012; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed July 15, 2012.
4. CDC. National mid-season flu vaccination coverage. Available at: http://www.cdc.gov/flu/professionals/vaccination/national-flu-survey.htm. Accessed July 15, 2012.
5. Community Preventive Services Task Force. Vaccines to prevent diseases: universally recommended vaccines. Available at: http://www.thecommunityguide.org/vaccines/universally/index.html. Accessed July 15, 2012.
Hypothyroidism management: Is an annual check of TSH level always necessary?
Purpose We conducted this study to identify clinical predictors of normal thyroid-stimulating hormone (TSH) values over a one-year interval in patients treated for hypothyroidism.
Methods We retrospectively reviewed cases of patients treated for hypothyroidism by the Mayo Clinic Department of Family Medicine in 2006. For patients with a normal TSH value during the initial study period in 2006, we assessed the number who then had therapeutic and nontherapeutic TSH values 10 to 14 months later, and evaluated whether body mass index (BMI), age, sex, or dosage of levothyroxine replacement had predictive value of a normal TSH level.
Results The percentage of normal repeat TSH values significantly declined with increasing medication dosage (P=.01). Of those patients whose maintenance dosage was <75 mcg/d, 90.8% had normal repeat TSH values, compared with just 77.5% of those requiring ≥125 mcg/d, who had significantly lower odds of normal repeat TSH (odds ratio, 0.31; 95% confidence interval, 0.13–0.76; P=.01).
Conclusions Age, sex, and BMI were not predictive of stable TSH values in patients treated for hypothyroidism. The dosage of thyroid hormone replacement was predictive of normal TSH values, with dosages ≥125 mcg/d having significantly decreased odds of a normal repeat TSH on follow-up.
Once the level of thyroid-stimulating hormone (TSH) has been normalized in a patient treated for hypothyroidism, the American Association of Clinical Endocrinologists recommends yearly monitoring.1 Annual testing has become the default frequency of surveillance for many primary care providers, despite a relative paucity of data to support the recommendation as customary practice.
Factors that can warrant close monitoring of TSH levels. Elderly patients often require lower average doses of levothyroxine replacement of 1 mcg/kg of body weight.2 Factors that can influence the stability of the TSH level include age, lean body mass changes, pregnancy,3 and malabsorptive states. Concomitant medications, such as antacids, calcium,4,5 and selective serotonin reuptake inhibitors,6 may also affect TSH levels. Various formulations of levothyroxine from different manufacturers may have different bioequivalence; thus a change in brands of medication could affect TSH levels.7
Rationale for monitoring TSH levels. Subtherapeutic replacement may not alleviate potential secondary effects of hypothyroidism, including hyperlipidemia, or cardiovascular and neuropsychiatric effects,8 whereas supratherapeutic replacement is associated with an increased risk of atrial fibrillation9 and decreased bone mass in postmenopausal women.8,10 Due to the potential hazards of excess thyroxine replacement, as well as the desire to avoid inadequate replacement, it is necessary to monitor the patient’s response to replacement. But can the frequency of monitoring vary?
Less frequent monitoring may be acceptable for some. One retrospective study has suggested that an 18-month interval may be more appropriate for patients younger than 60 years taking a levothyroxine dose of 100 to 150 mcg/d.11 Total health care expenditures for chronic care patients could possibly be reduced by decreasing testing frequency. The objective of this retrospective study was to identify predictors of stable TSH values over one year in patients treated for hypothyroidism, which might allow for a longer monitoring interval.
Methods
Patient selection
We reviewed the electronic medical records of patients with hypothyroidism treated by the Mayo Clinic Department of Family Medicine from January 1, 2006 through January 1, 2007, and identified a random sample of 780 patients with a documented TSH value in the normal range (0.3-5.0 mIU/L) and no other exclusionary criteria (see below). We reviewed laboratory results to determine if repeat TSH assay(s) were performed during the subsequent 10 to 14 months. We chose this period in an attempt to approximate the one-year interval of monitoring used in standard practice to follow patients with hypothyroidism not requiring dosage changes.
Out of the 780 patients, we identified 452 who had repeat TSH measurements performed 10 to 14 months after documentation of a normal TSH value. We recorded and analyzed demographic data obtained at the time of the first TSH measurement, including age, sex, body mass index (BMI), and thyroxine dose, to determine if there were any identifiable characteristics predicting normal TSH values on repeat screening.
Exclusion criteria. We excluded 328 patients who had normal TSH levels recorded in 2006 but did not undergo a repeat TSH measurement in the subsequent 10 to 14 months or had a repeat TSH measurement done sooner than 10 months that necessitated a change in levothyroxine dosage. We also excluded individuals who were younger than 18 years at the time of the initial 2006 TSH value; who were pregnant during the study period; who had a history of thyroid cancer (due to different recommendations for TSH goals); or who were taking amiodarone or lithium during the study period.
Outcome variables included TSH levels in the therapeutic range vs outside of the therapeutic range. This study was approved under the Mayo Clinic IRB protocol #09-008343.
Statistical analysis
We used a Student’s t-test to identify any association between age or BMI and a normal repeat TSH value, and a chi-square calculation to test for an association between medication dosage or sex and TSH level at follow-up. We used multiple logistic regression analysis to estimate adjusted odds ratios (ORs) for each independent variable.
Results
Three hundred eighty-six (85.4%) patients were women and 66 (14.6%) were men. Three hundred ten patients were taking levothyroxine at <125 mcg/d, and 142 patients were taking dosages ≥125 mcg/d. At approximately one year, 85.6% of all patients had a normal repeat TSH level.
Results of the 2-way tests are shown in TABLE 1. We found no mean differences in age, sex, or BMI between patients with normal repeat TSH levels and those with abnormal levels at follow-up. However, the percentage of normal repeat TSH values was inversely proportional to medication dosage (P=.01). Of patients whose dosage was <75 mcg/d, 90.8% had normal repeat TSH values, compared with 77.5% of patients taking ≥125 mcg/d. Percentages of low, normal, and high TSH values for each dosage range are shown in TABLE 2.
These findings were confirmed with multiple logistic regression analysis (TABLE 3). Age at index, BMI at index, and sex were not significantly associated with normal TSH at follow-up. However, patients with dosages ≥125 mcg/d had significantly lower odds of normal repeat TSH (OR=0.31, 95% confidence interval [CI]=0.13-0.76, P=.01).
Table 1
Normal TSH levels at one year were more often associated with levothyroxine dosages <125 mcg/d
| TSH normal* | TSH not normal | N | P value | |
|---|---|---|---|---|
| Age at index, mean | 54.7 | 54.9 | 452 | .91 |
| BMI at index, mean | 28.9 | 29.0 | 452 | .93 |
| Sex, % | .57 | |||
| Women | 85.2 | 14.8 | 386 | |
| Men | 87.9 | 12.1 | 66 | |
| Total | 85.6 | 14.4 | 352 | |
| Dosage, mcg/d | .01 | |||
| 0–74.9 | 90.8 | 9.2 | 76 | |
| 75–99.9 | 89.6 | 10.4 | 106 | |
| 100–124.9 | 88.3 | 11.7 | 128 | |
| ≥125 | 77.5 | 22.5 | 142 | |
| Total | 85.6 | 14.4 | 452 | |
| BMI, body mass index; TSH, thyroid-stimulating hormone. *Normal range=0.3-5.0 mIU/L. | ||||
Table 2
Levothyroxine dosages and associated TSH levels* at one year follow-up
| Dosage, mcg/d | Low TSH, n (%) | Normal TSH, n (%) | High TSH, n (%) | Total, n |
|---|---|---|---|---|
| 0-74.9 | 0 | 69 (90.8%) | 7 (9.2%) | 76 |
| 75-99.9 | 5 (4.7%) | 95 (89.6%) | 6 (5.7%) | 106 |
| 100-124.9 | 9 (7.0%) | 113 (88.3%) | 6 (4.7%) | 128 |
| ≥125 | 22 (15.5%) | 110 (77.5%) | 10 (7.0%) | 142 |
| Total | 36 (8.0%) | 387 (85.6%) | 29 (6.4%) | 452 |
| TSH, thyroid-stimulating hormone. *Low TSH=<0.3 mIU/L; normal TSH=0.3-5.0 mIU/L; high TSH=>5.0 mIU/L. | ||||
Table 3
Patients with dosages ≥125 mcg/d had significantly lower odds of normal repeat TSH* (N=452)
| Variable | Odds ratio | Confidence interval | P value |
|---|---|---|---|
| Age at index | 0.99 | 0.98-1.01 | .46 |
| BMI at index | 1.02 | 0.98-1.05 | .41 |
| Men (vs women) | 1.53 | 0.68-3.48 | .31 |
| Dosage, mcg/d | |||
| 0–74.9 | 1 | ||
| 75–99.9 | 0.87 | 0.32-2.37 | .79 |
| 100–124.9 | 0.76 | 0.29-1.97 | .58 |
| ≥125 | 0.31 | 0.13-0.76 | .01 |
| BMI, body mass index; TSH, thyroid-stimulating hormone. *Using multiple logistic regression analysis. | |||
DISCUSSION
Our retrospective study showed that patients taking <125 mcg/d levothyroxine were likely to have a normal TSH value at one year. Thus we propose that TSH values may be measured less frequently in this population. Given that the fee for testing TSH averages $50, there is potential for savings in costs, as well as in patient and provider time. A prospective, randomized controlled trial of less frequent TSH measurements in asymptomatic patients treated for hypothyroidism with replacement levothyroxine <125 mcg/d would yield more definitive conclusions.
Our study also revealed that patients requiring ≥125 mcg/d levothyroxine were less likely than patients requiring lower dosages to have a normal repeat TSH value at one year. The reasons for this are unclear. Although excess body weight may be a reason for patients to be on higher replacement dosages, we did not find BMI to be predictive of continued normal TSH values after one year. Other potential reasons for patients to require higher dosages of thyroid replacement include noncompliance, drug interference, and malabsorption.12 A change in any of these factors could presumably lead to a change in thyroid replacement needs and a change in TSH upon recheck one year later. Based on this finding, we suggest that TSH levels for patients requiring ≥125 mcg/d levothyroxine be repeated at maximum intervals of 12 months.
Limitations of this study
Study limitations were primarily a consequence of the retrospective design. Selection bias may have occurred by capturing patients with higher compliance. Patients who had repeat testing approximately one year later may reflect a population with more monitoring and better medication adherence. Interestingly, our retrospective review revealed that a large number of patients in our study did not have an annual TSH measurement; out of the 780 patients initially identified with a normal TSH value in 2006, 177 (23%) had repeat TSH values obtained more than 14 months later.
Other limitations of this study include an inability to control for, or detect, any changes in levothyroxine brand between the 2 data points. Because different levothyroxine formulations may differ in bioequivalence, a change in brands between measured values has the potential to affect outcomes.7 Additionally, the retrospective design could not address whether patients had any concurrent symptoms of over- or undertreated hypothyroidism or had sustained a serious, recent health status change that may have affected their TSH levels. Another limitation was the possible inclusion of women started on levothyroxine replacement for postpartum thyroiditis who may have subsequently recovered full thyroid function but continued on levothyroxine treatment.
Finally, this study design did not control for concurrent medication use other than lithium and amiodarone, thereby potentially overlooking substances such as antacids and calcium that could reduce levothyroxine absorption. However, as a practical matter, use of over-the-counter supplements and substitution in levothyroxine brand are often not reported by patients.
CORRESPONDENCE Jennifer Pecina, MD, 200 First Street SW, Rochester, MN 55905; [email protected]
1. Garber JR, Hennessey JV, Liebermann JA, 3rd, et al. Clinical update. Managing the challenges of hypothyroidism. J Fam Pract. 2006;55(suppl):S1-S8.
2. Laurberg P, Andersen S, Bulow Pedersen I, et al. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging. 2005;22:23-38.
3. Mandel SJ, Larsen PR, Seely EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91-96.
4. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822-2825.
5. Mersebach H, Rasmussen AK, Kirkegaard L, et al. Intestinal adsorption of levothyroxine by antacids and laxatives: case stories and in vitro experiments. Pharmacol Toxicol. 1999;84:107-109.
6. McCowen KC, Garber JR, Spark R. Elevated serum thyrotropin in thyroxine-treated patients with hypothyroidism given sertraline. N Engl J Med. 1997;337:1010-1011.
7. American Thyroid Association. The ATA unveils new data to FDA on bioequivalence of levothyroxine. October 4, 2006. Available at: http://www.thyroid.org/the-ata-unveils-new-data-to-fda-on-bioequivalence-of-levothyroxine. Accessed February 19, 2011.
8. Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457-469.
9. Jayaprasad N, Johnson F. Atrial fibrillation and hyperthyroidism. Indian Pacing Electrophysiol J. 2005;5:305-311.
10. Schneider DL, Barrett-Connor EL, Morton DJ. Thyroid hormone use and bone mineral density in elderly women. Effects of estrogen. JAMA. 1994;271:1245-1249.
11. Viswanath AK, Avenell A, Philip S, et al. Is annual surveillance of all treated hypothyroid patients necessary? BMC Endocr Disord. 2007;7:4.-
12. Narayanaswamy AKP, Pereira O, Copland S, et al. High levothyroxine requirement: more than just compliance. Endocr Abstr. 2009;19:389.-Available at: http://www.endocrine-abstracts.org/ea/0019/ea0019p389.htm. Accessed February 19, 2011.
Purpose We conducted this study to identify clinical predictors of normal thyroid-stimulating hormone (TSH) values over a one-year interval in patients treated for hypothyroidism.
Methods We retrospectively reviewed cases of patients treated for hypothyroidism by the Mayo Clinic Department of Family Medicine in 2006. For patients with a normal TSH value during the initial study period in 2006, we assessed the number who then had therapeutic and nontherapeutic TSH values 10 to 14 months later, and evaluated whether body mass index (BMI), age, sex, or dosage of levothyroxine replacement had predictive value of a normal TSH level.
Results The percentage of normal repeat TSH values significantly declined with increasing medication dosage (P=.01). Of those patients whose maintenance dosage was <75 mcg/d, 90.8% had normal repeat TSH values, compared with just 77.5% of those requiring ≥125 mcg/d, who had significantly lower odds of normal repeat TSH (odds ratio, 0.31; 95% confidence interval, 0.13–0.76; P=.01).
Conclusions Age, sex, and BMI were not predictive of stable TSH values in patients treated for hypothyroidism. The dosage of thyroid hormone replacement was predictive of normal TSH values, with dosages ≥125 mcg/d having significantly decreased odds of a normal repeat TSH on follow-up.
Once the level of thyroid-stimulating hormone (TSH) has been normalized in a patient treated for hypothyroidism, the American Association of Clinical Endocrinologists recommends yearly monitoring.1 Annual testing has become the default frequency of surveillance for many primary care providers, despite a relative paucity of data to support the recommendation as customary practice.
Factors that can warrant close monitoring of TSH levels. Elderly patients often require lower average doses of levothyroxine replacement of 1 mcg/kg of body weight.2 Factors that can influence the stability of the TSH level include age, lean body mass changes, pregnancy,3 and malabsorptive states. Concomitant medications, such as antacids, calcium,4,5 and selective serotonin reuptake inhibitors,6 may also affect TSH levels. Various formulations of levothyroxine from different manufacturers may have different bioequivalence; thus a change in brands of medication could affect TSH levels.7
Rationale for monitoring TSH levels. Subtherapeutic replacement may not alleviate potential secondary effects of hypothyroidism, including hyperlipidemia, or cardiovascular and neuropsychiatric effects,8 whereas supratherapeutic replacement is associated with an increased risk of atrial fibrillation9 and decreased bone mass in postmenopausal women.8,10 Due to the potential hazards of excess thyroxine replacement, as well as the desire to avoid inadequate replacement, it is necessary to monitor the patient’s response to replacement. But can the frequency of monitoring vary?
Less frequent monitoring may be acceptable for some. One retrospective study has suggested that an 18-month interval may be more appropriate for patients younger than 60 years taking a levothyroxine dose of 100 to 150 mcg/d.11 Total health care expenditures for chronic care patients could possibly be reduced by decreasing testing frequency. The objective of this retrospective study was to identify predictors of stable TSH values over one year in patients treated for hypothyroidism, which might allow for a longer monitoring interval.
Methods
Patient selection
We reviewed the electronic medical records of patients with hypothyroidism treated by the Mayo Clinic Department of Family Medicine from January 1, 2006 through January 1, 2007, and identified a random sample of 780 patients with a documented TSH value in the normal range (0.3-5.0 mIU/L) and no other exclusionary criteria (see below). We reviewed laboratory results to determine if repeat TSH assay(s) were performed during the subsequent 10 to 14 months. We chose this period in an attempt to approximate the one-year interval of monitoring used in standard practice to follow patients with hypothyroidism not requiring dosage changes.
Out of the 780 patients, we identified 452 who had repeat TSH measurements performed 10 to 14 months after documentation of a normal TSH value. We recorded and analyzed demographic data obtained at the time of the first TSH measurement, including age, sex, body mass index (BMI), and thyroxine dose, to determine if there were any identifiable characteristics predicting normal TSH values on repeat screening.
Exclusion criteria. We excluded 328 patients who had normal TSH levels recorded in 2006 but did not undergo a repeat TSH measurement in the subsequent 10 to 14 months or had a repeat TSH measurement done sooner than 10 months that necessitated a change in levothyroxine dosage. We also excluded individuals who were younger than 18 years at the time of the initial 2006 TSH value; who were pregnant during the study period; who had a history of thyroid cancer (due to different recommendations for TSH goals); or who were taking amiodarone or lithium during the study period.
Outcome variables included TSH levels in the therapeutic range vs outside of the therapeutic range. This study was approved under the Mayo Clinic IRB protocol #09-008343.
Statistical analysis
We used a Student’s t-test to identify any association between age or BMI and a normal repeat TSH value, and a chi-square calculation to test for an association between medication dosage or sex and TSH level at follow-up. We used multiple logistic regression analysis to estimate adjusted odds ratios (ORs) for each independent variable.
Results
Three hundred eighty-six (85.4%) patients were women and 66 (14.6%) were men. Three hundred ten patients were taking levothyroxine at <125 mcg/d, and 142 patients were taking dosages ≥125 mcg/d. At approximately one year, 85.6% of all patients had a normal repeat TSH level.
Results of the 2-way tests are shown in TABLE 1. We found no mean differences in age, sex, or BMI between patients with normal repeat TSH levels and those with abnormal levels at follow-up. However, the percentage of normal repeat TSH values was inversely proportional to medication dosage (P=.01). Of patients whose dosage was <75 mcg/d, 90.8% had normal repeat TSH values, compared with 77.5% of patients taking ≥125 mcg/d. Percentages of low, normal, and high TSH values for each dosage range are shown in TABLE 2.
These findings were confirmed with multiple logistic regression analysis (TABLE 3). Age at index, BMI at index, and sex were not significantly associated with normal TSH at follow-up. However, patients with dosages ≥125 mcg/d had significantly lower odds of normal repeat TSH (OR=0.31, 95% confidence interval [CI]=0.13-0.76, P=.01).
Table 1
Normal TSH levels at one year were more often associated with levothyroxine dosages <125 mcg/d
| TSH normal* | TSH not normal | N | P value | |
|---|---|---|---|---|
| Age at index, mean | 54.7 | 54.9 | 452 | .91 |
| BMI at index, mean | 28.9 | 29.0 | 452 | .93 |
| Sex, % | .57 | |||
| Women | 85.2 | 14.8 | 386 | |
| Men | 87.9 | 12.1 | 66 | |
| Total | 85.6 | 14.4 | 352 | |
| Dosage, mcg/d | .01 | |||
| 0–74.9 | 90.8 | 9.2 | 76 | |
| 75–99.9 | 89.6 | 10.4 | 106 | |
| 100–124.9 | 88.3 | 11.7 | 128 | |
| ≥125 | 77.5 | 22.5 | 142 | |
| Total | 85.6 | 14.4 | 452 | |
| BMI, body mass index; TSH, thyroid-stimulating hormone. *Normal range=0.3-5.0 mIU/L. | ||||
Table 2
Levothyroxine dosages and associated TSH levels* at one year follow-up
| Dosage, mcg/d | Low TSH, n (%) | Normal TSH, n (%) | High TSH, n (%) | Total, n |
|---|---|---|---|---|
| 0-74.9 | 0 | 69 (90.8%) | 7 (9.2%) | 76 |
| 75-99.9 | 5 (4.7%) | 95 (89.6%) | 6 (5.7%) | 106 |
| 100-124.9 | 9 (7.0%) | 113 (88.3%) | 6 (4.7%) | 128 |
| ≥125 | 22 (15.5%) | 110 (77.5%) | 10 (7.0%) | 142 |
| Total | 36 (8.0%) | 387 (85.6%) | 29 (6.4%) | 452 |
| TSH, thyroid-stimulating hormone. *Low TSH=<0.3 mIU/L; normal TSH=0.3-5.0 mIU/L; high TSH=>5.0 mIU/L. | ||||
Table 3
Patients with dosages ≥125 mcg/d had significantly lower odds of normal repeat TSH* (N=452)
| Variable | Odds ratio | Confidence interval | P value |
|---|---|---|---|
| Age at index | 0.99 | 0.98-1.01 | .46 |
| BMI at index | 1.02 | 0.98-1.05 | .41 |
| Men (vs women) | 1.53 | 0.68-3.48 | .31 |
| Dosage, mcg/d | |||
| 0–74.9 | 1 | ||
| 75–99.9 | 0.87 | 0.32-2.37 | .79 |
| 100–124.9 | 0.76 | 0.29-1.97 | .58 |
| ≥125 | 0.31 | 0.13-0.76 | .01 |
| BMI, body mass index; TSH, thyroid-stimulating hormone. *Using multiple logistic regression analysis. | |||
DISCUSSION
Our retrospective study showed that patients taking <125 mcg/d levothyroxine were likely to have a normal TSH value at one year. Thus we propose that TSH values may be measured less frequently in this population. Given that the fee for testing TSH averages $50, there is potential for savings in costs, as well as in patient and provider time. A prospective, randomized controlled trial of less frequent TSH measurements in asymptomatic patients treated for hypothyroidism with replacement levothyroxine <125 mcg/d would yield more definitive conclusions.
Our study also revealed that patients requiring ≥125 mcg/d levothyroxine were less likely than patients requiring lower dosages to have a normal repeat TSH value at one year. The reasons for this are unclear. Although excess body weight may be a reason for patients to be on higher replacement dosages, we did not find BMI to be predictive of continued normal TSH values after one year. Other potential reasons for patients to require higher dosages of thyroid replacement include noncompliance, drug interference, and malabsorption.12 A change in any of these factors could presumably lead to a change in thyroid replacement needs and a change in TSH upon recheck one year later. Based on this finding, we suggest that TSH levels for patients requiring ≥125 mcg/d levothyroxine be repeated at maximum intervals of 12 months.
Limitations of this study
Study limitations were primarily a consequence of the retrospective design. Selection bias may have occurred by capturing patients with higher compliance. Patients who had repeat testing approximately one year later may reflect a population with more monitoring and better medication adherence. Interestingly, our retrospective review revealed that a large number of patients in our study did not have an annual TSH measurement; out of the 780 patients initially identified with a normal TSH value in 2006, 177 (23%) had repeat TSH values obtained more than 14 months later.
Other limitations of this study include an inability to control for, or detect, any changes in levothyroxine brand between the 2 data points. Because different levothyroxine formulations may differ in bioequivalence, a change in brands between measured values has the potential to affect outcomes.7 Additionally, the retrospective design could not address whether patients had any concurrent symptoms of over- or undertreated hypothyroidism or had sustained a serious, recent health status change that may have affected their TSH levels. Another limitation was the possible inclusion of women started on levothyroxine replacement for postpartum thyroiditis who may have subsequently recovered full thyroid function but continued on levothyroxine treatment.
Finally, this study design did not control for concurrent medication use other than lithium and amiodarone, thereby potentially overlooking substances such as antacids and calcium that could reduce levothyroxine absorption. However, as a practical matter, use of over-the-counter supplements and substitution in levothyroxine brand are often not reported by patients.
CORRESPONDENCE Jennifer Pecina, MD, 200 First Street SW, Rochester, MN 55905; [email protected]
Purpose We conducted this study to identify clinical predictors of normal thyroid-stimulating hormone (TSH) values over a one-year interval in patients treated for hypothyroidism.
Methods We retrospectively reviewed cases of patients treated for hypothyroidism by the Mayo Clinic Department of Family Medicine in 2006. For patients with a normal TSH value during the initial study period in 2006, we assessed the number who then had therapeutic and nontherapeutic TSH values 10 to 14 months later, and evaluated whether body mass index (BMI), age, sex, or dosage of levothyroxine replacement had predictive value of a normal TSH level.
Results The percentage of normal repeat TSH values significantly declined with increasing medication dosage (P=.01). Of those patients whose maintenance dosage was <75 mcg/d, 90.8% had normal repeat TSH values, compared with just 77.5% of those requiring ≥125 mcg/d, who had significantly lower odds of normal repeat TSH (odds ratio, 0.31; 95% confidence interval, 0.13–0.76; P=.01).
Conclusions Age, sex, and BMI were not predictive of stable TSH values in patients treated for hypothyroidism. The dosage of thyroid hormone replacement was predictive of normal TSH values, with dosages ≥125 mcg/d having significantly decreased odds of a normal repeat TSH on follow-up.
Once the level of thyroid-stimulating hormone (TSH) has been normalized in a patient treated for hypothyroidism, the American Association of Clinical Endocrinologists recommends yearly monitoring.1 Annual testing has become the default frequency of surveillance for many primary care providers, despite a relative paucity of data to support the recommendation as customary practice.
Factors that can warrant close monitoring of TSH levels. Elderly patients often require lower average doses of levothyroxine replacement of 1 mcg/kg of body weight.2 Factors that can influence the stability of the TSH level include age, lean body mass changes, pregnancy,3 and malabsorptive states. Concomitant medications, such as antacids, calcium,4,5 and selective serotonin reuptake inhibitors,6 may also affect TSH levels. Various formulations of levothyroxine from different manufacturers may have different bioequivalence; thus a change in brands of medication could affect TSH levels.7
Rationale for monitoring TSH levels. Subtherapeutic replacement may not alleviate potential secondary effects of hypothyroidism, including hyperlipidemia, or cardiovascular and neuropsychiatric effects,8 whereas supratherapeutic replacement is associated with an increased risk of atrial fibrillation9 and decreased bone mass in postmenopausal women.8,10 Due to the potential hazards of excess thyroxine replacement, as well as the desire to avoid inadequate replacement, it is necessary to monitor the patient’s response to replacement. But can the frequency of monitoring vary?
Less frequent monitoring may be acceptable for some. One retrospective study has suggested that an 18-month interval may be more appropriate for patients younger than 60 years taking a levothyroxine dose of 100 to 150 mcg/d.11 Total health care expenditures for chronic care patients could possibly be reduced by decreasing testing frequency. The objective of this retrospective study was to identify predictors of stable TSH values over one year in patients treated for hypothyroidism, which might allow for a longer monitoring interval.
Methods
Patient selection
We reviewed the electronic medical records of patients with hypothyroidism treated by the Mayo Clinic Department of Family Medicine from January 1, 2006 through January 1, 2007, and identified a random sample of 780 patients with a documented TSH value in the normal range (0.3-5.0 mIU/L) and no other exclusionary criteria (see below). We reviewed laboratory results to determine if repeat TSH assay(s) were performed during the subsequent 10 to 14 months. We chose this period in an attempt to approximate the one-year interval of monitoring used in standard practice to follow patients with hypothyroidism not requiring dosage changes.
Out of the 780 patients, we identified 452 who had repeat TSH measurements performed 10 to 14 months after documentation of a normal TSH value. We recorded and analyzed demographic data obtained at the time of the first TSH measurement, including age, sex, body mass index (BMI), and thyroxine dose, to determine if there were any identifiable characteristics predicting normal TSH values on repeat screening.
Exclusion criteria. We excluded 328 patients who had normal TSH levels recorded in 2006 but did not undergo a repeat TSH measurement in the subsequent 10 to 14 months or had a repeat TSH measurement done sooner than 10 months that necessitated a change in levothyroxine dosage. We also excluded individuals who were younger than 18 years at the time of the initial 2006 TSH value; who were pregnant during the study period; who had a history of thyroid cancer (due to different recommendations for TSH goals); or who were taking amiodarone or lithium during the study period.
Outcome variables included TSH levels in the therapeutic range vs outside of the therapeutic range. This study was approved under the Mayo Clinic IRB protocol #09-008343.
Statistical analysis
We used a Student’s t-test to identify any association between age or BMI and a normal repeat TSH value, and a chi-square calculation to test for an association between medication dosage or sex and TSH level at follow-up. We used multiple logistic regression analysis to estimate adjusted odds ratios (ORs) for each independent variable.
Results
Three hundred eighty-six (85.4%) patients were women and 66 (14.6%) were men. Three hundred ten patients were taking levothyroxine at <125 mcg/d, and 142 patients were taking dosages ≥125 mcg/d. At approximately one year, 85.6% of all patients had a normal repeat TSH level.
Results of the 2-way tests are shown in TABLE 1. We found no mean differences in age, sex, or BMI between patients with normal repeat TSH levels and those with abnormal levels at follow-up. However, the percentage of normal repeat TSH values was inversely proportional to medication dosage (P=.01). Of patients whose dosage was <75 mcg/d, 90.8% had normal repeat TSH values, compared with 77.5% of patients taking ≥125 mcg/d. Percentages of low, normal, and high TSH values for each dosage range are shown in TABLE 2.
These findings were confirmed with multiple logistic regression analysis (TABLE 3). Age at index, BMI at index, and sex were not significantly associated with normal TSH at follow-up. However, patients with dosages ≥125 mcg/d had significantly lower odds of normal repeat TSH (OR=0.31, 95% confidence interval [CI]=0.13-0.76, P=.01).
Table 1
Normal TSH levels at one year were more often associated with levothyroxine dosages <125 mcg/d
| TSH normal* | TSH not normal | N | P value | |
|---|---|---|---|---|
| Age at index, mean | 54.7 | 54.9 | 452 | .91 |
| BMI at index, mean | 28.9 | 29.0 | 452 | .93 |
| Sex, % | .57 | |||
| Women | 85.2 | 14.8 | 386 | |
| Men | 87.9 | 12.1 | 66 | |
| Total | 85.6 | 14.4 | 352 | |
| Dosage, mcg/d | .01 | |||
| 0–74.9 | 90.8 | 9.2 | 76 | |
| 75–99.9 | 89.6 | 10.4 | 106 | |
| 100–124.9 | 88.3 | 11.7 | 128 | |
| ≥125 | 77.5 | 22.5 | 142 | |
| Total | 85.6 | 14.4 | 452 | |
| BMI, body mass index; TSH, thyroid-stimulating hormone. *Normal range=0.3-5.0 mIU/L. | ||||
Table 2
Levothyroxine dosages and associated TSH levels* at one year follow-up
| Dosage, mcg/d | Low TSH, n (%) | Normal TSH, n (%) | High TSH, n (%) | Total, n |
|---|---|---|---|---|
| 0-74.9 | 0 | 69 (90.8%) | 7 (9.2%) | 76 |
| 75-99.9 | 5 (4.7%) | 95 (89.6%) | 6 (5.7%) | 106 |
| 100-124.9 | 9 (7.0%) | 113 (88.3%) | 6 (4.7%) | 128 |
| ≥125 | 22 (15.5%) | 110 (77.5%) | 10 (7.0%) | 142 |
| Total | 36 (8.0%) | 387 (85.6%) | 29 (6.4%) | 452 |
| TSH, thyroid-stimulating hormone. *Low TSH=<0.3 mIU/L; normal TSH=0.3-5.0 mIU/L; high TSH=>5.0 mIU/L. | ||||
Table 3
Patients with dosages ≥125 mcg/d had significantly lower odds of normal repeat TSH* (N=452)
| Variable | Odds ratio | Confidence interval | P value |
|---|---|---|---|
| Age at index | 0.99 | 0.98-1.01 | .46 |
| BMI at index | 1.02 | 0.98-1.05 | .41 |
| Men (vs women) | 1.53 | 0.68-3.48 | .31 |
| Dosage, mcg/d | |||
| 0–74.9 | 1 | ||
| 75–99.9 | 0.87 | 0.32-2.37 | .79 |
| 100–124.9 | 0.76 | 0.29-1.97 | .58 |
| ≥125 | 0.31 | 0.13-0.76 | .01 |
| BMI, body mass index; TSH, thyroid-stimulating hormone. *Using multiple logistic regression analysis. | |||
DISCUSSION
Our retrospective study showed that patients taking <125 mcg/d levothyroxine were likely to have a normal TSH value at one year. Thus we propose that TSH values may be measured less frequently in this population. Given that the fee for testing TSH averages $50, there is potential for savings in costs, as well as in patient and provider time. A prospective, randomized controlled trial of less frequent TSH measurements in asymptomatic patients treated for hypothyroidism with replacement levothyroxine <125 mcg/d would yield more definitive conclusions.
Our study also revealed that patients requiring ≥125 mcg/d levothyroxine were less likely than patients requiring lower dosages to have a normal repeat TSH value at one year. The reasons for this are unclear. Although excess body weight may be a reason for patients to be on higher replacement dosages, we did not find BMI to be predictive of continued normal TSH values after one year. Other potential reasons for patients to require higher dosages of thyroid replacement include noncompliance, drug interference, and malabsorption.12 A change in any of these factors could presumably lead to a change in thyroid replacement needs and a change in TSH upon recheck one year later. Based on this finding, we suggest that TSH levels for patients requiring ≥125 mcg/d levothyroxine be repeated at maximum intervals of 12 months.
Limitations of this study
Study limitations were primarily a consequence of the retrospective design. Selection bias may have occurred by capturing patients with higher compliance. Patients who had repeat testing approximately one year later may reflect a population with more monitoring and better medication adherence. Interestingly, our retrospective review revealed that a large number of patients in our study did not have an annual TSH measurement; out of the 780 patients initially identified with a normal TSH value in 2006, 177 (23%) had repeat TSH values obtained more than 14 months later.
Other limitations of this study include an inability to control for, or detect, any changes in levothyroxine brand between the 2 data points. Because different levothyroxine formulations may differ in bioequivalence, a change in brands between measured values has the potential to affect outcomes.7 Additionally, the retrospective design could not address whether patients had any concurrent symptoms of over- or undertreated hypothyroidism or had sustained a serious, recent health status change that may have affected their TSH levels. Another limitation was the possible inclusion of women started on levothyroxine replacement for postpartum thyroiditis who may have subsequently recovered full thyroid function but continued on levothyroxine treatment.
Finally, this study design did not control for concurrent medication use other than lithium and amiodarone, thereby potentially overlooking substances such as antacids and calcium that could reduce levothyroxine absorption. However, as a practical matter, use of over-the-counter supplements and substitution in levothyroxine brand are often not reported by patients.
CORRESPONDENCE Jennifer Pecina, MD, 200 First Street SW, Rochester, MN 55905; [email protected]
1. Garber JR, Hennessey JV, Liebermann JA, 3rd, et al. Clinical update. Managing the challenges of hypothyroidism. J Fam Pract. 2006;55(suppl):S1-S8.
2. Laurberg P, Andersen S, Bulow Pedersen I, et al. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging. 2005;22:23-38.
3. Mandel SJ, Larsen PR, Seely EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91-96.
4. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822-2825.
5. Mersebach H, Rasmussen AK, Kirkegaard L, et al. Intestinal adsorption of levothyroxine by antacids and laxatives: case stories and in vitro experiments. Pharmacol Toxicol. 1999;84:107-109.
6. McCowen KC, Garber JR, Spark R. Elevated serum thyrotropin in thyroxine-treated patients with hypothyroidism given sertraline. N Engl J Med. 1997;337:1010-1011.
7. American Thyroid Association. The ATA unveils new data to FDA on bioequivalence of levothyroxine. October 4, 2006. Available at: http://www.thyroid.org/the-ata-unveils-new-data-to-fda-on-bioequivalence-of-levothyroxine. Accessed February 19, 2011.
8. Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457-469.
9. Jayaprasad N, Johnson F. Atrial fibrillation and hyperthyroidism. Indian Pacing Electrophysiol J. 2005;5:305-311.
10. Schneider DL, Barrett-Connor EL, Morton DJ. Thyroid hormone use and bone mineral density in elderly women. Effects of estrogen. JAMA. 1994;271:1245-1249.
11. Viswanath AK, Avenell A, Philip S, et al. Is annual surveillance of all treated hypothyroid patients necessary? BMC Endocr Disord. 2007;7:4.-
12. Narayanaswamy AKP, Pereira O, Copland S, et al. High levothyroxine requirement: more than just compliance. Endocr Abstr. 2009;19:389.-Available at: http://www.endocrine-abstracts.org/ea/0019/ea0019p389.htm. Accessed February 19, 2011.
1. Garber JR, Hennessey JV, Liebermann JA, 3rd, et al. Clinical update. Managing the challenges of hypothyroidism. J Fam Pract. 2006;55(suppl):S1-S8.
2. Laurberg P, Andersen S, Bulow Pedersen I, et al. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging. 2005;22:23-38.
3. Mandel SJ, Larsen PR, Seely EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91-96.
4. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822-2825.
5. Mersebach H, Rasmussen AK, Kirkegaard L, et al. Intestinal adsorption of levothyroxine by antacids and laxatives: case stories and in vitro experiments. Pharmacol Toxicol. 1999;84:107-109.
6. McCowen KC, Garber JR, Spark R. Elevated serum thyrotropin in thyroxine-treated patients with hypothyroidism given sertraline. N Engl J Med. 1997;337:1010-1011.
7. American Thyroid Association. The ATA unveils new data to FDA on bioequivalence of levothyroxine. October 4, 2006. Available at: http://www.thyroid.org/the-ata-unveils-new-data-to-fda-on-bioequivalence-of-levothyroxine. Accessed February 19, 2011.
8. Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457-469.
9. Jayaprasad N, Johnson F. Atrial fibrillation and hyperthyroidism. Indian Pacing Electrophysiol J. 2005;5:305-311.
10. Schneider DL, Barrett-Connor EL, Morton DJ. Thyroid hormone use and bone mineral density in elderly women. Effects of estrogen. JAMA. 1994;271:1245-1249.
11. Viswanath AK, Avenell A, Philip S, et al. Is annual surveillance of all treated hypothyroid patients necessary? BMC Endocr Disord. 2007;7:4.-
12. Narayanaswamy AKP, Pereira O, Copland S, et al. High levothyroxine requirement: more than just compliance. Endocr Abstr. 2009;19:389.-Available at: http://www.endocrine-abstracts.org/ea/0019/ea0019p389.htm. Accessed February 19, 2011.
Diagnosing and treating opioid dependence
• Ask all patients about the inappropriate use of substances, including prescription opioids. A
• Recommend pharmacotherapy for patients entering treatment for opioid dependence. A
• Warn patients who are opioid dependent about the risk of accidental fatal overdose, particularly with relapse. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Sam M, age 48, is in your office for the first time in more than 2 years. He has gained a considerable amount of weight and appears a bit sluggish, and you wonder whether he’s depressed. While taking a history, Sam reminds you that he was laid off 16 months ago and had been caring for his wife, who sustained a debilitating back injury. When you saw her recently, she told you she’s back to work and pain-free. So you’re taken aback when Sam asks you to refill his wife’s oxycodone prescription for lingering pain that often keeps her up at night.
If Sam were your patient, would you suspect opioid dependence?
Dependence on opioid analgesics and the adverse consequences associated with it have steadily increased during the past decade. Consider the following:
- Between 2004 and 2008, the number of emergency department visits related to nonmedical prescription opioid use more than doubled, rising by 111%.1
- The increasing prevalence of opioid abuse has led to a recent spike in unintentional deaths,2 with the number of lives lost to opioid analgesic overdose now exceeding that of heroin or cocaine.3
- More than 75% of opioids used for nonmedical purposes were prescribed for someone else.4
The course of opioid use is highly variable. Some people start with a legitimate medical prescription for an opioid analgesic, then continue taking it after the pain subsides. Others experiment briefly with nonmedical prescription opioids or use them intermittently without adverse effect. Some progress from prescription opioids to heroin, despite its dangers.5 Still others have a catastrophic outcome, such as an overdose or severe accident, the first time they use opioids.6 Rapid progression from misuse of opioids to dependence is most likely in vulnerable populations, such as those with concurrent mental illness, other substance use disorders, or increased sensitivity to pain.7
Understanding the terms. Before we continue, a word about terminology is in order. “Misuse” generally refers to the use of a medication in a manner (ie, purpose, dose, or frequency) other than its intended use, while “drug addiction” is the repeated use of a drug despite resulting harm. Here we will use “opioid dependence” to mean a pattern of increasing use characterized by significant impairment and distress and an inability to stop, and “opioid withdrawal” to reflect a constellation of symptoms, such as insomnia, nausea, diarrhea, and muscle aches, that can follow physiological dependence (though not necessarily opioid dependence). Our definitions of these terms are consistent with those of the American Psychiatric Association (APA).8 Worth noting, however, is the fact that as the APA prepares for the publication of the 5th edition of its Diagnostic and Statistical Manual of Mental Disorders, its Substance Disorder Work Group has proposed replacing the term “opioid dependence” with “opioid use disorder” to reduce the confusion associated with these definitions.9
Assessing illicit opioid use: Start with a targeted question
Most patients who are opioid dependent do not seek treatment for it,10 and are typically free of medical sequelae associated with drug addiction when they see family practitioners. The absence of self-reporting and obvious physical signs and symptoms, coupled with the increase in illicit use of prescription opioids, underscores the need for family physicians to identify patients who are abusing opioids and ensure that they get the help they need.
Screening tools. There are a number of screening tools you can use for this purpose—eg, CAGE-Adapted to Include Drugs (CAGE-AID) and Drug Abuse Screening Test (DAST)11,12—but they have not been found to be significantly better than a careful substance abuse history.13
Straightforward questions. You can start by asking, “Do you take any medications for pain?” If the answer is Yes, get the name of the drug and inquire about the frequency of use and the route, the amount typically taken, and the duration of the current use pattern. Ask specifically about opioids when taking a substance abuse history. After a question about alcohol use, you can say, “Do you use any other drugs in a serious way? Marijuana? Opioids like Percocet, Vicodin, or Oxycontin?” Although it can be very difficult to detect opioid dependence if the patient is not forthcoming, other likely indicators of drug-seeking behavior should trigger additional questions. (See “Opioid dependence: Red flags to keep in mind”.14-16)
“Brief” protocols. Recent studies of Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs have found that the simple, time-limited interventions they offer (visit http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf to learn more) lead to a reduction in self-reported illicit opioid use.17,18 Family physicians can readily incorporate SBIRT protocols into routine practice, as an evidence-based and often reimbursable approach to substance abuse.17
Suspect opioid dependence in a patient who:
- describes pain resulting from back or orthopedic injuries without corresponding documentation or imaging
- requests a specific opioid for pain management
- shows little interest in a physical exam, diagnostic testing, or nonpharmacological remedies
- talks about changes in work or relationship status
- ceases to participate in activities or hobbies that previously occupied a considerable amount of his or her time. This may signal social isolation or indicate that the patient is spending a great deal of time in pursuit of opioids.
Additional steps before initiating treatment
After screening and diagnostic evaluation provide evidence that a patient is opioid dependent, you can take several steps to guide him or her to the appropriate treatment.
A thorough biopsychosocial assessment covering co-occurring psychiatric illnesses, pain, psychosocial stressors contributing to opioid use, and infectious disease screening is required to gain a clear picture of the patient’s situation. In every case, acute emergencies such as suicidal ideation require immediate intervention, which may involve hospitalization.19
Assess the patient’s desire for help. After the initial assessment, it is often helpful to categorize the patient’s “stage of change” (precontemplation, contemplation, preparation, action, or maintenance),20 and to tailor your next step accordingly. A patient who denies that opioid use is a problem or is clearly ambivalent about seeking treatment may require a conversation that uses principles of motivational interviewing—a collaborative approach that aims to evoke and strengthen personal motivation for change.21 Consider a question that encourages him or her to express reasons for change, such as: “How would you like your current situation to be different?” As almost everyone abusing opioids has thoughts about stopping, such a question may help the patient focus on specific changes.
CASE When you question Sam about his interest in oxycodone, he breaks down. He’s been unable to find work or to lose the excess weight he gained during the many months he cared for his wife. He tells you that soon after his wife stopped taking the pain pills, he started taking them. At first, he took one occasionally. Then he started taking the opioids every day, and finally, whenever he awakened at night. Now, Sam says, he has no more pills, and he’s nauseous, depressed, and unable to sleep—and looking to you for help.
Sam fits the criteria for opioid withdrawal as a result of physiological dependence; further questioning reveals that he also suffers from opioid dependence, and that he is receptive to treatment.
Recommending treatment and following up
Several options are available for patients who, like Sam, have signs and symptoms of opioid withdrawal as a result of physiological dependence. You can provide a referral to a physician specializing in addiction, recommend detoxification and/or treatment in an inpatient facility, or initiate pharmacological treatment and provide a referral to a behavioral therapist. Whatever the initial approach, most patients will ultimately be treated as outpatients, with a combination of pharmacotherapy and behavioral therapy—often, with monitoring and oversight by a primary care physician. Which approach to pursue should be guided by evidence-based recommendations (TABLE)17,22-27 and jointly decided by physician and patient.
TABLE
Treating opioid dependence: Key clinical recommendations
| Recommendation | Evidence (SOR) | Comments |
|---|---|---|
| Screen all patients for substance use, including opioids. Brief interventions and referral to treatment when appropriate may reduce opioid use17,22 | Consistent findings from RCTs; evidence-based guideline (A) | SBIRT reduces self-reported opioid use; efforts to replicate such reports with objective evidence (eg, toxicology screens) are underway |
| Recommend maintenance medication (ie, buprenorphine, naltrexone, methadone) for all patients entering treatment for opioid dependence with physiological dependence; methadone is the safest for pregnant women23-25 | Consistent findings from RCTs; evidence-based guideline (A) | Methadone is the gold standard for pregnant women; further studies are needed to determine the safety of in utero exposure to buprenorphine and naltrexone |
| Keep patients on maintenance medication for ≥3 months; higher relapse rates are noted when medication is discontinued in <3 months23,24 | Consistent findings from RCTs (A) | Relapse rates are higher when maintenance medication is discontinued in <3 months |
| Caution patients with opioid dependence of the risk for accidental overdose and death with relapse and take action—eg, offering naloxone rescue kits to patients and families, as appropriate26 | Consistent findings from RCTs and prospective cohort studies; evidence-based guideline (A) | |
| Take steps to prevent diversion and accidental ingestion of agonist therapies, using tools such as frequent toxicology screens, random pill counts, and designated pharmacies, and monitoring adherence to psychosocial treatment26,27 | Practice guideline (consensus) (C) | |
| RCTs, randomized clinical trials; SBIRT, Screening, Brief Intervention, and Referral to Treatment; SOR, strength of recommendation. | ||
Medication plays a key role in recovery
Recommend medication-assisted treatment, either with an agonist (buprenorphine or methadone) or an antagonist (naltrexone), for every patient with physiological opioid dependence. The goals of pharmacotherapy are to prevent or reduce withdrawal symptoms and craving, avoid relapse, and restore to a normal state any physiological functions (eg, sleep, bowel movements) that have been disrupted by opioid use.28 When continued for ≥3 months, medication has been shown to improve outcomes.23,24,29 In one recent study, 49% of opioid-dependent participants who were still taking buprenorphine-naloxone at 12 weeks had successful outcomes (minimal or no opioid use), vs 7% of those undergoing a brief buprenorphine-naloxone taper.24
There are risks associated with medication-assisted therapy, however. The ones of greatest concern are a potential increase in drug-drug interactions, the risk of diversion (a concern with both buprenorphine and methadone), and the potential for accidental overdose.2,30
Buprenorphine, a partial mu-opioid receptor agonist, is a Schedule III controlled substance and can be dispensed by a pharmacy, making inpatient opioid detoxification unnecessary for many opioid-dependent patients. Physicians who wish to prescribe buprenorphine for the treatment of opioid dependence must complete an 8-hour course, offered by the American Medical Association and the APA, among other medical groups, and obtain a Drug Enforcement Administration code (“X”) license. 31
Buprenorphine has a high affinity for, and a slow dissociation from, mu-opioid receptors, resulting in the displacement of other opioids from the mu receptor and less severe withdrawal.32 As a partial agonist, buprenorphine attenuates opioid withdrawal symptoms with a ceiling, or near maximal, effect at 16 mg, thereby lowering the risk for overdose.33 A sublingual formulation that combines buprenorphine with naloxone, an opioid antagonist that exerts its full effect when injected but is minimally absorbed sublingually, reduces the potential for abuse of buprenorphine without interfering with its effectiveness.34
Compared with methadone, buprenorphine is less likely to interact with antiretroviral medications or to cause QTc prolongation, erectile dysfunction, or cognitive or psychomotor impairment.31,35-37 Limitations include the ceiling effect, which can be a problem for cases in which more agonist is needed; cost (approximately $12/d), and the lack of approval by the US Food and Drug Administration (FDA) for use during pregnancy.
Buprenorphine maintenance involves 3 phases: induction, stabilization, and maintenance.38 Induction takes place in a clinician’s office at the time the patient experiences opioid withdrawal symptoms, typically 6 to 48 hours after taking the last opioid. Extended treatment improves clinical outcomes,23,24 and longer-term maintenance (of indefinite duration) is frequently required.
Naltrexone is a mu-receptor antagonist, and therefore does not cause physical dependence or have agonist effects such as euphoria and sedation. As a result, it has no diversion value and may appeal to those who view opioid-agonist pharmacotherapy as simply trading one drug for another.39 Naltrexone is not a controlled substance and is not subject to the regulatory requirements that buprenorphine and methadone face.
Although agonists can be started in the first day or 2 after a patient decides to stop using opioids, patients must be opioid-free for ≥7 days before starting naltrexone. That’s because its antagonist properties will precipitate withdrawal if another opioid is present on the opioid receptors. During the 7-day “washout” period, you can treat opioid withdrawal symptoms with medications such as clonidine and dicyclomine, but such symptoms make patients especially vulnerable to relapse while waiting to start naltrexone.
Oral naltrexone’s effectiveness as a treatment for opioid dependence has been limited by poor adherence. But a long-acting intramuscular form of the drug, approved by the FDA in 2010 and requiring once-a-month injection, mitigates this concern.40,41
Methadone is a full mu-opioid agonist, administered daily at specialized clinics, as a maintenance therapy for opioid dependence. Although office-based physicians can prescribe methadone for pain, the drug can only be used for opioid dependence under the auspices of state- and federally regulated opioid treatment programs (http://findtreatment.samhsa.gov/TreatmentLocator/faces/quickSearch.jspx; a mobile phone application is also available at http://www.samhsa.gov/mobile/treatmentlocator.aspx).
Methadone, a Schedule III controlled substance with a half-life averaging 24 to 36 hours, requires daily dosing.42 Its slow metabolism and long half-life increase the risk for overdose.
Methadone is best for patients who are highly dependent on opioids and likely to benefit from a structured treatment environment with daily supervision (although patients who are doing well may earn take-home privileges so they don’t have to come to the clinic every day).43 New patients should receive an initial dose of 30 mg or less, and a maximum first-day dose of 40 mg.44
Methadone remains the standard of care for pregnant women being treated for opioid dependence, while studies of the effects of buprenorphine and naltrexone on a developing fetus continue. Although methadone’s efficacy, particularly in lower doses, is similar to that of buprenorphine,45 its adverse effect profile is worse. Adverse effects include drug-drug interactions, the potential for respiratory depression (especially when combined with alcohol or sedatives), QTc prolongation (which requires monitoring by electrocardiogram), sedation, and weight gain, and should be considered before selecting methadone as a maintenance pharmacotherapy.30,37,46 And, because relapse rates within 12 months of tapering off methadone have been reported to exceed 80%,47 both the clinician and the patient need to consider the likelihood of long-term, even lifelong, maintenance before initiating treatment.
Behavioral interventions are a vital part of the picture
Studies evaluating the extent to which various types and amounts of counseling improve outcomes compared with pharmacotherapy alone have had conflicting results.24,48 Nonetheless, most clinicians consider counseling to be a critical component of treatment for opioid dependence and recommend, at a minimum, either individual or group counseling (various modalities have been shown to be effective) and regular attendance at a self-help group like Narcotics Anonymous. Contingency management, a type of therapy that uses prizes as incentives for desired behaviors; and family therapy, individual counseling, and community-based programs have all been found to improve outcomes.6,49
CASE You refer Sam to an addiction psychiatrist, who stabilizes him on 16 mg buprenorphine/naloxone daily as part of an outpatient treatment program. Sam is enrolled in a weekly buprenorphine stabilization group, where he gives a urine sample each week. He also begins seeing a social worker weekly for counseling and attends Narcotics Anonymous meetings 2 to 3 times a week. At a follow-up appointment with you 6 months later, he reports that he has been abstinent from oxycodone for 6 months, his sleep is improved, and he feels better about his chances of finding another job.
Your role in safeguarding the patient
With the rising prevalence of opioid overdose, patient education aimed at crisis prevention is crucial, as well. Warn patients of the risk of accidental overdose, often associated with relapse, stressing the importance of continuing treatment and taking their maintenance medication exactly as prescribed.
There are other steps you can take to safeguard patients—eg, providing naloxone rescue kits to patients and their families when appropriate. You can also institute diversion and overdose prevention measures for patients taking buprenorphine or methadone—providing a lock box for take-home medication, implementing treatment contracts, and using a designated pharmacy to dispense buprenorphine, for example.26,27,50
Regular monitoring, urine drug screens (see TABLE W1), and random pill counts, in which patients are typically given 24 hours to bring in their prescribed medication so it can be counted, can also help keep patients on track. Treatment for concurrent psychiatric disorders—depression, anxiety, and personality disorders are common among patients with opioid dependence—is likely to improve the outcome of treatment, as well.
TABLE W1
Pharmacokinetics of common opioids: Time detectable in urine*
| Drug (half-life) | Time detectable in urine | Comment |
|---|---|---|
| Codeine (2.5-3 h) | 48 h | Pharmacogenetic-dependent effects may affect detection |
| Fentanyl Transdermal (17 h) Submucosal (7 h) | Not usually detected in urine (lack of metabolites) | Excretion of transdermal fentanyl can last days |
| Hydromorphone IR (2.3 h) ER (18.6 h) | 2-4 d | Significant interpatient variability |
| Methadone (8-59 h) | 3 d | |
| Morphine (1.5-2 h) | 48-72 h | 90% eliminated within 24 h |
| Oxycodone IR (3.2 h) ER (4.5 h) | Often not detected in urine | High-fat meals may increase serum concentrations of ER formulation |
| Propoxyphene Parent drug (6-12 h) Metabolite (30-36 h) | 6-48 h | |
| ER, extended release; IR, immediate release. *Previously appeared in: McBane S, Weige N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633. Sources: Clinical Pharmacology [online]. Tampa, FL: Gold Standard Inc; 2010. Available at: http://cp.gsm.com. Accessed March 5, 2010; Drug Facts and Comparisons [online]. 2010. Available at: http://www.factsandcomparisons.com/. Accessed March 5, 2010. | ||
CORRESPONDENCE Kevin P. Hill, MD, MHS, McLean Hospital, 115 Mill Street, Belmont, MA 02478; [email protected]
1. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59:705-709.
2. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321.
3. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1-8.4.
4. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2010. NSDUH Series H-38A, HHS publication SMA 10-4856. Available at: http://www.samhsa.gov/data/NSDUH/2k9NSDUH/2k9Results.htm. Accessed August 22, 2012.
5. Hser YI, Huang D, Brecht ML, et al. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13-21.
6. Veilleux JC, Colvin PJ, Anderson J, et al. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2011;30:155-166.
7. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2011;35:232-247.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
9. American Psychiatric Association. R 19 opioid use disorder. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=460. Updated April 30, 2012. Accessed June 20, 2012.
10. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2009. NSDUH Series H-36, HHS publication SMA 09-4434. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed August 22, 2012.
11. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
12. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
13. US Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. January 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/druguse/drugrs.htm. Accessed May 7, 2012.
14. Gourlay D, Caplan Y, Heit H. Urine Drug Testing in Clinical Practice: Dispelling the Myths and Designing Strategies. San Francisco, Calif: California Academy of Family Physicians; 2006.
15. Jackman R, Purvis J, Mallett B. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155-1162.
16. McBane S, Weigle N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633.
17. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
18. The InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33:1374-1381.
19. Borges G, Walters EE, Kessler RC. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am J Epidemiol. 2000;151:781-789.
20. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
21. Smedslund G, Berg RC, Hammerstrom KT, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063.-
22. Gryczynski J, Mitchell SG, Peterson TR, et al. The relationship between services delivered and substance use outcomes in New Mexico’s Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152-157.
23. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003-2011.
24. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238-1246.
25. Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491-503.
26. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
27. Zacny J, Bigelow G, Compton P, et al. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215-232.
28. Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205-230.
29. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209.-
30. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
31. Office of National Drug Control Policy Reauthorization Act of 2006 (ONDCPRA), HR 6344, 109th Cong, 2nd Sess (2006).
32. Lewis JW, Walter D. Buprenorphine—background to its development as a treatment for opiate dependence. In: Blaine JD, ed. Buprenorphine: An Alternative Treatment for Opioid Dependence. Rockville, Md: National Institute on Drug Abuse; 1992:5-11. NIDA Research Monograph, No. 121. Available at: http://archives.drugabuse.gov/pdf/monographs/121.pdf. Accessed August 22, 2012.
33. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75-78.
35. Hallinan R, Byrne A, Agho K, et al. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684-692.
36. Rapeli P, Fabritius C, Alho H, et al. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5.-
37. Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469-2475.
38. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, Md: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS publication SMA 04-3939.
39. Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303-2305.
40. Hulse GK, Morris N, Arnold-Reed D, et al. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66:1108-1115.
41. US Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. October 12, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229109.htm. Accessed September 11, 2012.
42. Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol Ther. 1972;13 (5 pt 1):633-637.
43. Stitzer M, Bigelow G, Lawrence C, et al. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9-14.
44. Code of Federal Regulations. Title 42.8.12. Federal Opioid Treatment Standards. October 2010.
45. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290-1297.
46. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387-395.
47. Ball JC, Lange WR, Myers CP, et al. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29:214-226.
48. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365-374.
49. Defulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48-54.
50. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377-384.
• Ask all patients about the inappropriate use of substances, including prescription opioids. A
• Recommend pharmacotherapy for patients entering treatment for opioid dependence. A
• Warn patients who are opioid dependent about the risk of accidental fatal overdose, particularly with relapse. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Sam M, age 48, is in your office for the first time in more than 2 years. He has gained a considerable amount of weight and appears a bit sluggish, and you wonder whether he’s depressed. While taking a history, Sam reminds you that he was laid off 16 months ago and had been caring for his wife, who sustained a debilitating back injury. When you saw her recently, she told you she’s back to work and pain-free. So you’re taken aback when Sam asks you to refill his wife’s oxycodone prescription for lingering pain that often keeps her up at night.
If Sam were your patient, would you suspect opioid dependence?
Dependence on opioid analgesics and the adverse consequences associated with it have steadily increased during the past decade. Consider the following:
- Between 2004 and 2008, the number of emergency department visits related to nonmedical prescription opioid use more than doubled, rising by 111%.1
- The increasing prevalence of opioid abuse has led to a recent spike in unintentional deaths,2 with the number of lives lost to opioid analgesic overdose now exceeding that of heroin or cocaine.3
- More than 75% of opioids used for nonmedical purposes were prescribed for someone else.4
The course of opioid use is highly variable. Some people start with a legitimate medical prescription for an opioid analgesic, then continue taking it after the pain subsides. Others experiment briefly with nonmedical prescription opioids or use them intermittently without adverse effect. Some progress from prescription opioids to heroin, despite its dangers.5 Still others have a catastrophic outcome, such as an overdose or severe accident, the first time they use opioids.6 Rapid progression from misuse of opioids to dependence is most likely in vulnerable populations, such as those with concurrent mental illness, other substance use disorders, or increased sensitivity to pain.7
Understanding the terms. Before we continue, a word about terminology is in order. “Misuse” generally refers to the use of a medication in a manner (ie, purpose, dose, or frequency) other than its intended use, while “drug addiction” is the repeated use of a drug despite resulting harm. Here we will use “opioid dependence” to mean a pattern of increasing use characterized by significant impairment and distress and an inability to stop, and “opioid withdrawal” to reflect a constellation of symptoms, such as insomnia, nausea, diarrhea, and muscle aches, that can follow physiological dependence (though not necessarily opioid dependence). Our definitions of these terms are consistent with those of the American Psychiatric Association (APA).8 Worth noting, however, is the fact that as the APA prepares for the publication of the 5th edition of its Diagnostic and Statistical Manual of Mental Disorders, its Substance Disorder Work Group has proposed replacing the term “opioid dependence” with “opioid use disorder” to reduce the confusion associated with these definitions.9
Assessing illicit opioid use: Start with a targeted question
Most patients who are opioid dependent do not seek treatment for it,10 and are typically free of medical sequelae associated with drug addiction when they see family practitioners. The absence of self-reporting and obvious physical signs and symptoms, coupled with the increase in illicit use of prescription opioids, underscores the need for family physicians to identify patients who are abusing opioids and ensure that they get the help they need.
Screening tools. There are a number of screening tools you can use for this purpose—eg, CAGE-Adapted to Include Drugs (CAGE-AID) and Drug Abuse Screening Test (DAST)11,12—but they have not been found to be significantly better than a careful substance abuse history.13
Straightforward questions. You can start by asking, “Do you take any medications for pain?” If the answer is Yes, get the name of the drug and inquire about the frequency of use and the route, the amount typically taken, and the duration of the current use pattern. Ask specifically about opioids when taking a substance abuse history. After a question about alcohol use, you can say, “Do you use any other drugs in a serious way? Marijuana? Opioids like Percocet, Vicodin, or Oxycontin?” Although it can be very difficult to detect opioid dependence if the patient is not forthcoming, other likely indicators of drug-seeking behavior should trigger additional questions. (See “Opioid dependence: Red flags to keep in mind”.14-16)
“Brief” protocols. Recent studies of Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs have found that the simple, time-limited interventions they offer (visit http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf to learn more) lead to a reduction in self-reported illicit opioid use.17,18 Family physicians can readily incorporate SBIRT protocols into routine practice, as an evidence-based and often reimbursable approach to substance abuse.17
Suspect opioid dependence in a patient who:
- describes pain resulting from back or orthopedic injuries without corresponding documentation or imaging
- requests a specific opioid for pain management
- shows little interest in a physical exam, diagnostic testing, or nonpharmacological remedies
- talks about changes in work or relationship status
- ceases to participate in activities or hobbies that previously occupied a considerable amount of his or her time. This may signal social isolation or indicate that the patient is spending a great deal of time in pursuit of opioids.
Additional steps before initiating treatment
After screening and diagnostic evaluation provide evidence that a patient is opioid dependent, you can take several steps to guide him or her to the appropriate treatment.
A thorough biopsychosocial assessment covering co-occurring psychiatric illnesses, pain, psychosocial stressors contributing to opioid use, and infectious disease screening is required to gain a clear picture of the patient’s situation. In every case, acute emergencies such as suicidal ideation require immediate intervention, which may involve hospitalization.19
Assess the patient’s desire for help. After the initial assessment, it is often helpful to categorize the patient’s “stage of change” (precontemplation, contemplation, preparation, action, or maintenance),20 and to tailor your next step accordingly. A patient who denies that opioid use is a problem or is clearly ambivalent about seeking treatment may require a conversation that uses principles of motivational interviewing—a collaborative approach that aims to evoke and strengthen personal motivation for change.21 Consider a question that encourages him or her to express reasons for change, such as: “How would you like your current situation to be different?” As almost everyone abusing opioids has thoughts about stopping, such a question may help the patient focus on specific changes.
CASE When you question Sam about his interest in oxycodone, he breaks down. He’s been unable to find work or to lose the excess weight he gained during the many months he cared for his wife. He tells you that soon after his wife stopped taking the pain pills, he started taking them. At first, he took one occasionally. Then he started taking the opioids every day, and finally, whenever he awakened at night. Now, Sam says, he has no more pills, and he’s nauseous, depressed, and unable to sleep—and looking to you for help.
Sam fits the criteria for opioid withdrawal as a result of physiological dependence; further questioning reveals that he also suffers from opioid dependence, and that he is receptive to treatment.
Recommending treatment and following up
Several options are available for patients who, like Sam, have signs and symptoms of opioid withdrawal as a result of physiological dependence. You can provide a referral to a physician specializing in addiction, recommend detoxification and/or treatment in an inpatient facility, or initiate pharmacological treatment and provide a referral to a behavioral therapist. Whatever the initial approach, most patients will ultimately be treated as outpatients, with a combination of pharmacotherapy and behavioral therapy—often, with monitoring and oversight by a primary care physician. Which approach to pursue should be guided by evidence-based recommendations (TABLE)17,22-27 and jointly decided by physician and patient.
TABLE
Treating opioid dependence: Key clinical recommendations
| Recommendation | Evidence (SOR) | Comments |
|---|---|---|
| Screen all patients for substance use, including opioids. Brief interventions and referral to treatment when appropriate may reduce opioid use17,22 | Consistent findings from RCTs; evidence-based guideline (A) | SBIRT reduces self-reported opioid use; efforts to replicate such reports with objective evidence (eg, toxicology screens) are underway |
| Recommend maintenance medication (ie, buprenorphine, naltrexone, methadone) for all patients entering treatment for opioid dependence with physiological dependence; methadone is the safest for pregnant women23-25 | Consistent findings from RCTs; evidence-based guideline (A) | Methadone is the gold standard for pregnant women; further studies are needed to determine the safety of in utero exposure to buprenorphine and naltrexone |
| Keep patients on maintenance medication for ≥3 months; higher relapse rates are noted when medication is discontinued in <3 months23,24 | Consistent findings from RCTs (A) | Relapse rates are higher when maintenance medication is discontinued in <3 months |
| Caution patients with opioid dependence of the risk for accidental overdose and death with relapse and take action—eg, offering naloxone rescue kits to patients and families, as appropriate26 | Consistent findings from RCTs and prospective cohort studies; evidence-based guideline (A) | |
| Take steps to prevent diversion and accidental ingestion of agonist therapies, using tools such as frequent toxicology screens, random pill counts, and designated pharmacies, and monitoring adherence to psychosocial treatment26,27 | Practice guideline (consensus) (C) | |
| RCTs, randomized clinical trials; SBIRT, Screening, Brief Intervention, and Referral to Treatment; SOR, strength of recommendation. | ||
Medication plays a key role in recovery
Recommend medication-assisted treatment, either with an agonist (buprenorphine or methadone) or an antagonist (naltrexone), for every patient with physiological opioid dependence. The goals of pharmacotherapy are to prevent or reduce withdrawal symptoms and craving, avoid relapse, and restore to a normal state any physiological functions (eg, sleep, bowel movements) that have been disrupted by opioid use.28 When continued for ≥3 months, medication has been shown to improve outcomes.23,24,29 In one recent study, 49% of opioid-dependent participants who were still taking buprenorphine-naloxone at 12 weeks had successful outcomes (minimal or no opioid use), vs 7% of those undergoing a brief buprenorphine-naloxone taper.24
There are risks associated with medication-assisted therapy, however. The ones of greatest concern are a potential increase in drug-drug interactions, the risk of diversion (a concern with both buprenorphine and methadone), and the potential for accidental overdose.2,30
Buprenorphine, a partial mu-opioid receptor agonist, is a Schedule III controlled substance and can be dispensed by a pharmacy, making inpatient opioid detoxification unnecessary for many opioid-dependent patients. Physicians who wish to prescribe buprenorphine for the treatment of opioid dependence must complete an 8-hour course, offered by the American Medical Association and the APA, among other medical groups, and obtain a Drug Enforcement Administration code (“X”) license. 31
Buprenorphine has a high affinity for, and a slow dissociation from, mu-opioid receptors, resulting in the displacement of other opioids from the mu receptor and less severe withdrawal.32 As a partial agonist, buprenorphine attenuates opioid withdrawal symptoms with a ceiling, or near maximal, effect at 16 mg, thereby lowering the risk for overdose.33 A sublingual formulation that combines buprenorphine with naloxone, an opioid antagonist that exerts its full effect when injected but is minimally absorbed sublingually, reduces the potential for abuse of buprenorphine without interfering with its effectiveness.34
Compared with methadone, buprenorphine is less likely to interact with antiretroviral medications or to cause QTc prolongation, erectile dysfunction, or cognitive or psychomotor impairment.31,35-37 Limitations include the ceiling effect, which can be a problem for cases in which more agonist is needed; cost (approximately $12/d), and the lack of approval by the US Food and Drug Administration (FDA) for use during pregnancy.
Buprenorphine maintenance involves 3 phases: induction, stabilization, and maintenance.38 Induction takes place in a clinician’s office at the time the patient experiences opioid withdrawal symptoms, typically 6 to 48 hours after taking the last opioid. Extended treatment improves clinical outcomes,23,24 and longer-term maintenance (of indefinite duration) is frequently required.
Naltrexone is a mu-receptor antagonist, and therefore does not cause physical dependence or have agonist effects such as euphoria and sedation. As a result, it has no diversion value and may appeal to those who view opioid-agonist pharmacotherapy as simply trading one drug for another.39 Naltrexone is not a controlled substance and is not subject to the regulatory requirements that buprenorphine and methadone face.
Although agonists can be started in the first day or 2 after a patient decides to stop using opioids, patients must be opioid-free for ≥7 days before starting naltrexone. That’s because its antagonist properties will precipitate withdrawal if another opioid is present on the opioid receptors. During the 7-day “washout” period, you can treat opioid withdrawal symptoms with medications such as clonidine and dicyclomine, but such symptoms make patients especially vulnerable to relapse while waiting to start naltrexone.
Oral naltrexone’s effectiveness as a treatment for opioid dependence has been limited by poor adherence. But a long-acting intramuscular form of the drug, approved by the FDA in 2010 and requiring once-a-month injection, mitigates this concern.40,41
Methadone is a full mu-opioid agonist, administered daily at specialized clinics, as a maintenance therapy for opioid dependence. Although office-based physicians can prescribe methadone for pain, the drug can only be used for opioid dependence under the auspices of state- and federally regulated opioid treatment programs (http://findtreatment.samhsa.gov/TreatmentLocator/faces/quickSearch.jspx; a mobile phone application is also available at http://www.samhsa.gov/mobile/treatmentlocator.aspx).
Methadone, a Schedule III controlled substance with a half-life averaging 24 to 36 hours, requires daily dosing.42 Its slow metabolism and long half-life increase the risk for overdose.
Methadone is best for patients who are highly dependent on opioids and likely to benefit from a structured treatment environment with daily supervision (although patients who are doing well may earn take-home privileges so they don’t have to come to the clinic every day).43 New patients should receive an initial dose of 30 mg or less, and a maximum first-day dose of 40 mg.44
Methadone remains the standard of care for pregnant women being treated for opioid dependence, while studies of the effects of buprenorphine and naltrexone on a developing fetus continue. Although methadone’s efficacy, particularly in lower doses, is similar to that of buprenorphine,45 its adverse effect profile is worse. Adverse effects include drug-drug interactions, the potential for respiratory depression (especially when combined with alcohol or sedatives), QTc prolongation (which requires monitoring by electrocardiogram), sedation, and weight gain, and should be considered before selecting methadone as a maintenance pharmacotherapy.30,37,46 And, because relapse rates within 12 months of tapering off methadone have been reported to exceed 80%,47 both the clinician and the patient need to consider the likelihood of long-term, even lifelong, maintenance before initiating treatment.
Behavioral interventions are a vital part of the picture
Studies evaluating the extent to which various types and amounts of counseling improve outcomes compared with pharmacotherapy alone have had conflicting results.24,48 Nonetheless, most clinicians consider counseling to be a critical component of treatment for opioid dependence and recommend, at a minimum, either individual or group counseling (various modalities have been shown to be effective) and regular attendance at a self-help group like Narcotics Anonymous. Contingency management, a type of therapy that uses prizes as incentives for desired behaviors; and family therapy, individual counseling, and community-based programs have all been found to improve outcomes.6,49
CASE You refer Sam to an addiction psychiatrist, who stabilizes him on 16 mg buprenorphine/naloxone daily as part of an outpatient treatment program. Sam is enrolled in a weekly buprenorphine stabilization group, where he gives a urine sample each week. He also begins seeing a social worker weekly for counseling and attends Narcotics Anonymous meetings 2 to 3 times a week. At a follow-up appointment with you 6 months later, he reports that he has been abstinent from oxycodone for 6 months, his sleep is improved, and he feels better about his chances of finding another job.
Your role in safeguarding the patient
With the rising prevalence of opioid overdose, patient education aimed at crisis prevention is crucial, as well. Warn patients of the risk of accidental overdose, often associated with relapse, stressing the importance of continuing treatment and taking their maintenance medication exactly as prescribed.
There are other steps you can take to safeguard patients—eg, providing naloxone rescue kits to patients and their families when appropriate. You can also institute diversion and overdose prevention measures for patients taking buprenorphine or methadone—providing a lock box for take-home medication, implementing treatment contracts, and using a designated pharmacy to dispense buprenorphine, for example.26,27,50
Regular monitoring, urine drug screens (see TABLE W1), and random pill counts, in which patients are typically given 24 hours to bring in their prescribed medication so it can be counted, can also help keep patients on track. Treatment for concurrent psychiatric disorders—depression, anxiety, and personality disorders are common among patients with opioid dependence—is likely to improve the outcome of treatment, as well.
TABLE W1
Pharmacokinetics of common opioids: Time detectable in urine*
| Drug (half-life) | Time detectable in urine | Comment |
|---|---|---|
| Codeine (2.5-3 h) | 48 h | Pharmacogenetic-dependent effects may affect detection |
| Fentanyl Transdermal (17 h) Submucosal (7 h) | Not usually detected in urine (lack of metabolites) | Excretion of transdermal fentanyl can last days |
| Hydromorphone IR (2.3 h) ER (18.6 h) | 2-4 d | Significant interpatient variability |
| Methadone (8-59 h) | 3 d | |
| Morphine (1.5-2 h) | 48-72 h | 90% eliminated within 24 h |
| Oxycodone IR (3.2 h) ER (4.5 h) | Often not detected in urine | High-fat meals may increase serum concentrations of ER formulation |
| Propoxyphene Parent drug (6-12 h) Metabolite (30-36 h) | 6-48 h | |
| ER, extended release; IR, immediate release. *Previously appeared in: McBane S, Weige N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633. Sources: Clinical Pharmacology [online]. Tampa, FL: Gold Standard Inc; 2010. Available at: http://cp.gsm.com. Accessed March 5, 2010; Drug Facts and Comparisons [online]. 2010. Available at: http://www.factsandcomparisons.com/. Accessed March 5, 2010. | ||
CORRESPONDENCE Kevin P. Hill, MD, MHS, McLean Hospital, 115 Mill Street, Belmont, MA 02478; [email protected]
• Ask all patients about the inappropriate use of substances, including prescription opioids. A
• Recommend pharmacotherapy for patients entering treatment for opioid dependence. A
• Warn patients who are opioid dependent about the risk of accidental fatal overdose, particularly with relapse. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Sam M, age 48, is in your office for the first time in more than 2 years. He has gained a considerable amount of weight and appears a bit sluggish, and you wonder whether he’s depressed. While taking a history, Sam reminds you that he was laid off 16 months ago and had been caring for his wife, who sustained a debilitating back injury. When you saw her recently, she told you she’s back to work and pain-free. So you’re taken aback when Sam asks you to refill his wife’s oxycodone prescription for lingering pain that often keeps her up at night.
If Sam were your patient, would you suspect opioid dependence?
Dependence on opioid analgesics and the adverse consequences associated with it have steadily increased during the past decade. Consider the following:
- Between 2004 and 2008, the number of emergency department visits related to nonmedical prescription opioid use more than doubled, rising by 111%.1
- The increasing prevalence of opioid abuse has led to a recent spike in unintentional deaths,2 with the number of lives lost to opioid analgesic overdose now exceeding that of heroin or cocaine.3
- More than 75% of opioids used for nonmedical purposes were prescribed for someone else.4
The course of opioid use is highly variable. Some people start with a legitimate medical prescription for an opioid analgesic, then continue taking it after the pain subsides. Others experiment briefly with nonmedical prescription opioids or use them intermittently without adverse effect. Some progress from prescription opioids to heroin, despite its dangers.5 Still others have a catastrophic outcome, such as an overdose or severe accident, the first time they use opioids.6 Rapid progression from misuse of opioids to dependence is most likely in vulnerable populations, such as those with concurrent mental illness, other substance use disorders, or increased sensitivity to pain.7
Understanding the terms. Before we continue, a word about terminology is in order. “Misuse” generally refers to the use of a medication in a manner (ie, purpose, dose, or frequency) other than its intended use, while “drug addiction” is the repeated use of a drug despite resulting harm. Here we will use “opioid dependence” to mean a pattern of increasing use characterized by significant impairment and distress and an inability to stop, and “opioid withdrawal” to reflect a constellation of symptoms, such as insomnia, nausea, diarrhea, and muscle aches, that can follow physiological dependence (though not necessarily opioid dependence). Our definitions of these terms are consistent with those of the American Psychiatric Association (APA).8 Worth noting, however, is the fact that as the APA prepares for the publication of the 5th edition of its Diagnostic and Statistical Manual of Mental Disorders, its Substance Disorder Work Group has proposed replacing the term “opioid dependence” with “opioid use disorder” to reduce the confusion associated with these definitions.9
Assessing illicit opioid use: Start with a targeted question
Most patients who are opioid dependent do not seek treatment for it,10 and are typically free of medical sequelae associated with drug addiction when they see family practitioners. The absence of self-reporting and obvious physical signs and symptoms, coupled with the increase in illicit use of prescription opioids, underscores the need for family physicians to identify patients who are abusing opioids and ensure that they get the help they need.
Screening tools. There are a number of screening tools you can use for this purpose—eg, CAGE-Adapted to Include Drugs (CAGE-AID) and Drug Abuse Screening Test (DAST)11,12—but they have not been found to be significantly better than a careful substance abuse history.13
Straightforward questions. You can start by asking, “Do you take any medications for pain?” If the answer is Yes, get the name of the drug and inquire about the frequency of use and the route, the amount typically taken, and the duration of the current use pattern. Ask specifically about opioids when taking a substance abuse history. After a question about alcohol use, you can say, “Do you use any other drugs in a serious way? Marijuana? Opioids like Percocet, Vicodin, or Oxycontin?” Although it can be very difficult to detect opioid dependence if the patient is not forthcoming, other likely indicators of drug-seeking behavior should trigger additional questions. (See “Opioid dependence: Red flags to keep in mind”.14-16)
“Brief” protocols. Recent studies of Screening, Brief Intervention, and Referral to Treatment (SBIRT) programs have found that the simple, time-limited interventions they offer (visit http://www.samhsa.gov/prevention/sbirt/SBIRTwhitepaper.pdf to learn more) lead to a reduction in self-reported illicit opioid use.17,18 Family physicians can readily incorporate SBIRT protocols into routine practice, as an evidence-based and often reimbursable approach to substance abuse.17
Suspect opioid dependence in a patient who:
- describes pain resulting from back or orthopedic injuries without corresponding documentation or imaging
- requests a specific opioid for pain management
- shows little interest in a physical exam, diagnostic testing, or nonpharmacological remedies
- talks about changes in work or relationship status
- ceases to participate in activities or hobbies that previously occupied a considerable amount of his or her time. This may signal social isolation or indicate that the patient is spending a great deal of time in pursuit of opioids.
Additional steps before initiating treatment
After screening and diagnostic evaluation provide evidence that a patient is opioid dependent, you can take several steps to guide him or her to the appropriate treatment.
A thorough biopsychosocial assessment covering co-occurring psychiatric illnesses, pain, psychosocial stressors contributing to opioid use, and infectious disease screening is required to gain a clear picture of the patient’s situation. In every case, acute emergencies such as suicidal ideation require immediate intervention, which may involve hospitalization.19
Assess the patient’s desire for help. After the initial assessment, it is often helpful to categorize the patient’s “stage of change” (precontemplation, contemplation, preparation, action, or maintenance),20 and to tailor your next step accordingly. A patient who denies that opioid use is a problem or is clearly ambivalent about seeking treatment may require a conversation that uses principles of motivational interviewing—a collaborative approach that aims to evoke and strengthen personal motivation for change.21 Consider a question that encourages him or her to express reasons for change, such as: “How would you like your current situation to be different?” As almost everyone abusing opioids has thoughts about stopping, such a question may help the patient focus on specific changes.
CASE When you question Sam about his interest in oxycodone, he breaks down. He’s been unable to find work or to lose the excess weight he gained during the many months he cared for his wife. He tells you that soon after his wife stopped taking the pain pills, he started taking them. At first, he took one occasionally. Then he started taking the opioids every day, and finally, whenever he awakened at night. Now, Sam says, he has no more pills, and he’s nauseous, depressed, and unable to sleep—and looking to you for help.
Sam fits the criteria for opioid withdrawal as a result of physiological dependence; further questioning reveals that he also suffers from opioid dependence, and that he is receptive to treatment.
Recommending treatment and following up
Several options are available for patients who, like Sam, have signs and symptoms of opioid withdrawal as a result of physiological dependence. You can provide a referral to a physician specializing in addiction, recommend detoxification and/or treatment in an inpatient facility, or initiate pharmacological treatment and provide a referral to a behavioral therapist. Whatever the initial approach, most patients will ultimately be treated as outpatients, with a combination of pharmacotherapy and behavioral therapy—often, with monitoring and oversight by a primary care physician. Which approach to pursue should be guided by evidence-based recommendations (TABLE)17,22-27 and jointly decided by physician and patient.
TABLE
Treating opioid dependence: Key clinical recommendations
| Recommendation | Evidence (SOR) | Comments |
|---|---|---|
| Screen all patients for substance use, including opioids. Brief interventions and referral to treatment when appropriate may reduce opioid use17,22 | Consistent findings from RCTs; evidence-based guideline (A) | SBIRT reduces self-reported opioid use; efforts to replicate such reports with objective evidence (eg, toxicology screens) are underway |
| Recommend maintenance medication (ie, buprenorphine, naltrexone, methadone) for all patients entering treatment for opioid dependence with physiological dependence; methadone is the safest for pregnant women23-25 | Consistent findings from RCTs; evidence-based guideline (A) | Methadone is the gold standard for pregnant women; further studies are needed to determine the safety of in utero exposure to buprenorphine and naltrexone |
| Keep patients on maintenance medication for ≥3 months; higher relapse rates are noted when medication is discontinued in <3 months23,24 | Consistent findings from RCTs (A) | Relapse rates are higher when maintenance medication is discontinued in <3 months |
| Caution patients with opioid dependence of the risk for accidental overdose and death with relapse and take action—eg, offering naloxone rescue kits to patients and families, as appropriate26 | Consistent findings from RCTs and prospective cohort studies; evidence-based guideline (A) | |
| Take steps to prevent diversion and accidental ingestion of agonist therapies, using tools such as frequent toxicology screens, random pill counts, and designated pharmacies, and monitoring adherence to psychosocial treatment26,27 | Practice guideline (consensus) (C) | |
| RCTs, randomized clinical trials; SBIRT, Screening, Brief Intervention, and Referral to Treatment; SOR, strength of recommendation. | ||
Medication plays a key role in recovery
Recommend medication-assisted treatment, either with an agonist (buprenorphine or methadone) or an antagonist (naltrexone), for every patient with physiological opioid dependence. The goals of pharmacotherapy are to prevent or reduce withdrawal symptoms and craving, avoid relapse, and restore to a normal state any physiological functions (eg, sleep, bowel movements) that have been disrupted by opioid use.28 When continued for ≥3 months, medication has been shown to improve outcomes.23,24,29 In one recent study, 49% of opioid-dependent participants who were still taking buprenorphine-naloxone at 12 weeks had successful outcomes (minimal or no opioid use), vs 7% of those undergoing a brief buprenorphine-naloxone taper.24
There are risks associated with medication-assisted therapy, however. The ones of greatest concern are a potential increase in drug-drug interactions, the risk of diversion (a concern with both buprenorphine and methadone), and the potential for accidental overdose.2,30
Buprenorphine, a partial mu-opioid receptor agonist, is a Schedule III controlled substance and can be dispensed by a pharmacy, making inpatient opioid detoxification unnecessary for many opioid-dependent patients. Physicians who wish to prescribe buprenorphine for the treatment of opioid dependence must complete an 8-hour course, offered by the American Medical Association and the APA, among other medical groups, and obtain a Drug Enforcement Administration code (“X”) license. 31
Buprenorphine has a high affinity for, and a slow dissociation from, mu-opioid receptors, resulting in the displacement of other opioids from the mu receptor and less severe withdrawal.32 As a partial agonist, buprenorphine attenuates opioid withdrawal symptoms with a ceiling, or near maximal, effect at 16 mg, thereby lowering the risk for overdose.33 A sublingual formulation that combines buprenorphine with naloxone, an opioid antagonist that exerts its full effect when injected but is minimally absorbed sublingually, reduces the potential for abuse of buprenorphine without interfering with its effectiveness.34
Compared with methadone, buprenorphine is less likely to interact with antiretroviral medications or to cause QTc prolongation, erectile dysfunction, or cognitive or psychomotor impairment.31,35-37 Limitations include the ceiling effect, which can be a problem for cases in which more agonist is needed; cost (approximately $12/d), and the lack of approval by the US Food and Drug Administration (FDA) for use during pregnancy.
Buprenorphine maintenance involves 3 phases: induction, stabilization, and maintenance.38 Induction takes place in a clinician’s office at the time the patient experiences opioid withdrawal symptoms, typically 6 to 48 hours after taking the last opioid. Extended treatment improves clinical outcomes,23,24 and longer-term maintenance (of indefinite duration) is frequently required.
Naltrexone is a mu-receptor antagonist, and therefore does not cause physical dependence or have agonist effects such as euphoria and sedation. As a result, it has no diversion value and may appeal to those who view opioid-agonist pharmacotherapy as simply trading one drug for another.39 Naltrexone is not a controlled substance and is not subject to the regulatory requirements that buprenorphine and methadone face.
Although agonists can be started in the first day or 2 after a patient decides to stop using opioids, patients must be opioid-free for ≥7 days before starting naltrexone. That’s because its antagonist properties will precipitate withdrawal if another opioid is present on the opioid receptors. During the 7-day “washout” period, you can treat opioid withdrawal symptoms with medications such as clonidine and dicyclomine, but such symptoms make patients especially vulnerable to relapse while waiting to start naltrexone.
Oral naltrexone’s effectiveness as a treatment for opioid dependence has been limited by poor adherence. But a long-acting intramuscular form of the drug, approved by the FDA in 2010 and requiring once-a-month injection, mitigates this concern.40,41
Methadone is a full mu-opioid agonist, administered daily at specialized clinics, as a maintenance therapy for opioid dependence. Although office-based physicians can prescribe methadone for pain, the drug can only be used for opioid dependence under the auspices of state- and federally regulated opioid treatment programs (http://findtreatment.samhsa.gov/TreatmentLocator/faces/quickSearch.jspx; a mobile phone application is also available at http://www.samhsa.gov/mobile/treatmentlocator.aspx).
Methadone, a Schedule III controlled substance with a half-life averaging 24 to 36 hours, requires daily dosing.42 Its slow metabolism and long half-life increase the risk for overdose.
Methadone is best for patients who are highly dependent on opioids and likely to benefit from a structured treatment environment with daily supervision (although patients who are doing well may earn take-home privileges so they don’t have to come to the clinic every day).43 New patients should receive an initial dose of 30 mg or less, and a maximum first-day dose of 40 mg.44
Methadone remains the standard of care for pregnant women being treated for opioid dependence, while studies of the effects of buprenorphine and naltrexone on a developing fetus continue. Although methadone’s efficacy, particularly in lower doses, is similar to that of buprenorphine,45 its adverse effect profile is worse. Adverse effects include drug-drug interactions, the potential for respiratory depression (especially when combined with alcohol or sedatives), QTc prolongation (which requires monitoring by electrocardiogram), sedation, and weight gain, and should be considered before selecting methadone as a maintenance pharmacotherapy.30,37,46 And, because relapse rates within 12 months of tapering off methadone have been reported to exceed 80%,47 both the clinician and the patient need to consider the likelihood of long-term, even lifelong, maintenance before initiating treatment.
Behavioral interventions are a vital part of the picture
Studies evaluating the extent to which various types and amounts of counseling improve outcomes compared with pharmacotherapy alone have had conflicting results.24,48 Nonetheless, most clinicians consider counseling to be a critical component of treatment for opioid dependence and recommend, at a minimum, either individual or group counseling (various modalities have been shown to be effective) and regular attendance at a self-help group like Narcotics Anonymous. Contingency management, a type of therapy that uses prizes as incentives for desired behaviors; and family therapy, individual counseling, and community-based programs have all been found to improve outcomes.6,49
CASE You refer Sam to an addiction psychiatrist, who stabilizes him on 16 mg buprenorphine/naloxone daily as part of an outpatient treatment program. Sam is enrolled in a weekly buprenorphine stabilization group, where he gives a urine sample each week. He also begins seeing a social worker weekly for counseling and attends Narcotics Anonymous meetings 2 to 3 times a week. At a follow-up appointment with you 6 months later, he reports that he has been abstinent from oxycodone for 6 months, his sleep is improved, and he feels better about his chances of finding another job.
Your role in safeguarding the patient
With the rising prevalence of opioid overdose, patient education aimed at crisis prevention is crucial, as well. Warn patients of the risk of accidental overdose, often associated with relapse, stressing the importance of continuing treatment and taking their maintenance medication exactly as prescribed.
There are other steps you can take to safeguard patients—eg, providing naloxone rescue kits to patients and their families when appropriate. You can also institute diversion and overdose prevention measures for patients taking buprenorphine or methadone—providing a lock box for take-home medication, implementing treatment contracts, and using a designated pharmacy to dispense buprenorphine, for example.26,27,50
Regular monitoring, urine drug screens (see TABLE W1), and random pill counts, in which patients are typically given 24 hours to bring in their prescribed medication so it can be counted, can also help keep patients on track. Treatment for concurrent psychiatric disorders—depression, anxiety, and personality disorders are common among patients with opioid dependence—is likely to improve the outcome of treatment, as well.
TABLE W1
Pharmacokinetics of common opioids: Time detectable in urine*
| Drug (half-life) | Time detectable in urine | Comment |
|---|---|---|
| Codeine (2.5-3 h) | 48 h | Pharmacogenetic-dependent effects may affect detection |
| Fentanyl Transdermal (17 h) Submucosal (7 h) | Not usually detected in urine (lack of metabolites) | Excretion of transdermal fentanyl can last days |
| Hydromorphone IR (2.3 h) ER (18.6 h) | 2-4 d | Significant interpatient variability |
| Methadone (8-59 h) | 3 d | |
| Morphine (1.5-2 h) | 48-72 h | 90% eliminated within 24 h |
| Oxycodone IR (3.2 h) ER (4.5 h) | Often not detected in urine | High-fat meals may increase serum concentrations of ER formulation |
| Propoxyphene Parent drug (6-12 h) Metabolite (30-36 h) | 6-48 h | |
| ER, extended release; IR, immediate release. *Previously appeared in: McBane S, Weige N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633. Sources: Clinical Pharmacology [online]. Tampa, FL: Gold Standard Inc; 2010. Available at: http://cp.gsm.com. Accessed March 5, 2010; Drug Facts and Comparisons [online]. 2010. Available at: http://www.factsandcomparisons.com/. Accessed March 5, 2010. | ||
CORRESPONDENCE Kevin P. Hill, MD, MHS, McLean Hospital, 115 Mill Street, Belmont, MA 02478; [email protected]
1. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59:705-709.
2. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321.
3. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1-8.4.
4. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2010. NSDUH Series H-38A, HHS publication SMA 10-4856. Available at: http://www.samhsa.gov/data/NSDUH/2k9NSDUH/2k9Results.htm. Accessed August 22, 2012.
5. Hser YI, Huang D, Brecht ML, et al. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13-21.
6. Veilleux JC, Colvin PJ, Anderson J, et al. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2011;30:155-166.
7. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2011;35:232-247.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
9. American Psychiatric Association. R 19 opioid use disorder. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=460. Updated April 30, 2012. Accessed June 20, 2012.
10. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2009. NSDUH Series H-36, HHS publication SMA 09-4434. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed August 22, 2012.
11. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
12. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
13. US Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. January 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/druguse/drugrs.htm. Accessed May 7, 2012.
14. Gourlay D, Caplan Y, Heit H. Urine Drug Testing in Clinical Practice: Dispelling the Myths and Designing Strategies. San Francisco, Calif: California Academy of Family Physicians; 2006.
15. Jackman R, Purvis J, Mallett B. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155-1162.
16. McBane S, Weigle N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633.
17. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
18. The InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33:1374-1381.
19. Borges G, Walters EE, Kessler RC. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am J Epidemiol. 2000;151:781-789.
20. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
21. Smedslund G, Berg RC, Hammerstrom KT, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063.-
22. Gryczynski J, Mitchell SG, Peterson TR, et al. The relationship between services delivered and substance use outcomes in New Mexico’s Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152-157.
23. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003-2011.
24. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238-1246.
25. Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491-503.
26. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
27. Zacny J, Bigelow G, Compton P, et al. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215-232.
28. Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205-230.
29. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209.-
30. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
31. Office of National Drug Control Policy Reauthorization Act of 2006 (ONDCPRA), HR 6344, 109th Cong, 2nd Sess (2006).
32. Lewis JW, Walter D. Buprenorphine—background to its development as a treatment for opiate dependence. In: Blaine JD, ed. Buprenorphine: An Alternative Treatment for Opioid Dependence. Rockville, Md: National Institute on Drug Abuse; 1992:5-11. NIDA Research Monograph, No. 121. Available at: http://archives.drugabuse.gov/pdf/monographs/121.pdf. Accessed August 22, 2012.
33. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75-78.
35. Hallinan R, Byrne A, Agho K, et al. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684-692.
36. Rapeli P, Fabritius C, Alho H, et al. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5.-
37. Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469-2475.
38. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, Md: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS publication SMA 04-3939.
39. Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303-2305.
40. Hulse GK, Morris N, Arnold-Reed D, et al. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66:1108-1115.
41. US Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. October 12, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229109.htm. Accessed September 11, 2012.
42. Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol Ther. 1972;13 (5 pt 1):633-637.
43. Stitzer M, Bigelow G, Lawrence C, et al. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9-14.
44. Code of Federal Regulations. Title 42.8.12. Federal Opioid Treatment Standards. October 2010.
45. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290-1297.
46. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387-395.
47. Ball JC, Lange WR, Myers CP, et al. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29:214-226.
48. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365-374.
49. Defulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48-54.
50. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377-384.
1. Centers for Disease Control and Prevention (CDC). Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morb Mortal Wkly Rep. 2010;59:705-709.
2. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321.
3. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS Data Brief. 2009;(22):1-8.4.
4. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2010. NSDUH Series H-38A, HHS publication SMA 10-4856. Available at: http://www.samhsa.gov/data/NSDUH/2k9NSDUH/2k9Results.htm. Accessed August 22, 2012.
5. Hser YI, Huang D, Brecht ML, et al. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13-21.
6. Veilleux JC, Colvin PJ, Anderson J, et al. A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2011;30:155-166.
7. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2011;35:232-247.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev (DSM-IV-TR). Arlington, Va: American Psychiatric Association; 2000.
9. American Psychiatric Association. R 19 opioid use disorder. http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=460. Updated April 30, 2012. Accessed June 20, 2012.
10. Substance Abuse and Mental Health Services Administration. Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, Md: SAMHSA, Office of Applied Studies; 2009. NSDUH Series H-36, HHS publication SMA 09-4434. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed August 22, 2012.
11. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
12. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
13. US Preventive Services Task Force. Screening for Illicit Drug Use: U.S. Preventive Services Task Force Recommendation Statement. January 2008. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/druguse/drugrs.htm. Accessed May 7, 2012.
14. Gourlay D, Caplan Y, Heit H. Urine Drug Testing in Clinical Practice: Dispelling the Myths and Designing Strategies. San Francisco, Calif: California Academy of Family Physicians; 2006.
15. Jackman R, Purvis J, Mallett B. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78:1155-1162.
16. McBane S, Weigle N. Is it time to drug test your chronic pain patient? J Fam Pract. 2010;59:628-633.
17. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
18. The InSight Project Research Group. SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res. 2009;33:1374-1381.
19. Borges G, Walters EE, Kessler RC. Associations of substance use, abuse, and dependence with subsequent suicidal behavior. Am J Epidemiol. 2000;151:781-789.
20. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390-395.
21. Smedslund G, Berg RC, Hammerstrom KT, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev. 2011;(5):CD008063.-
22. Gryczynski J, Mitchell SG, Peterson TR, et al. The relationship between services delivered and substance use outcomes in New Mexico’s Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152-157.
23. Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300:2003-2011.
24. Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238-1246.
25. Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491-503.
26. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
27. Zacny J, Bigelow G, Compton P, et al. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215-232.
28. Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205-230.
29. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209.-
30. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
31. Office of National Drug Control Policy Reauthorization Act of 2006 (ONDCPRA), HR 6344, 109th Cong, 2nd Sess (2006).
32. Lewis JW, Walter D. Buprenorphine—background to its development as a treatment for opiate dependence. In: Blaine JD, ed. Buprenorphine: An Alternative Treatment for Opioid Dependence. Rockville, Md: National Institute on Drug Abuse; 1992:5-11. NIDA Research Monograph, No. 121. Available at: http://archives.drugabuse.gov/pdf/monographs/121.pdf. Accessed August 22, 2012.
33. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88:75-78.
35. Hallinan R, Byrne A, Agho K, et al. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684-692.
36. Rapeli P, Fabritius C, Alho H, et al. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5.-
37. Wedam EF, Bigelow GE, Johnson RE, et al. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469-2475.
38. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, Md: Substance Abuse and Mental Health Services Administration; 2004. Treatment Improvement Protocol (TIP) Series 40. DHHS publication SMA 04-3939.
39. Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303-2305.
40. Hulse GK, Morris N, Arnold-Reed D, et al. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch Gen Psychiatry. 2009;66:1108-1115.
41. US Food and Drug Administration. FDA approves injectable drug to treat opioid-dependent patients. October 12, 2010. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm229109.htm. Accessed September 11, 2012.
42. Inturrisi CE, Verebely K. The levels of methadone in the plasma in methadone maintenance. Clin Pharmacol Ther. 1972;13 (5 pt 1):633-637.
43. Stitzer M, Bigelow G, Lawrence C, et al. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9-14.
44. Code of Federal Regulations. Title 42.8.12. Federal Opioid Treatment Standards. October 2010.
45. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290-1297.
46. Krantz MJ, Martin J, Stimmel B, et al. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387-395.
47. Ball JC, Lange WR, Myers CP, et al. Reducing the risk of AIDS through methadone maintenance treatment. J Health Soc Behav. 1988;29:214-226.
48. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365-374.
49. Defulio A, Everly JJ, Leoutsakos JM, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: a randomized controlled trial. Drug Alcohol Depend. 2012;120:48-54.
50. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep. 2009;11:377-384.
Rhabdomyolysis after spin class?
Primary care physicians frequently encourage patients to lead a more active, healthy lifestyle. The rise in popularity of endurance events, yoga, and organized gym-based fitness classes has, no doubt, improved the health of those who participate. But what happens when an individual moves too quickly from a sedentary existence to a more physically active one?
In this article we describe 2 clinical cases of rhabdomyolysis that occurred after healthy individuals participated for the first time in a class involving high-intensity stationary cycling, known as “spinning.” This exercise activity originated in California around 1989 when a competitive cyclist introduced variable resistance and speed training to stationary cycle workouts.1 Over the last 10 years, spinning has gained a worldwide following as a means of building cardiovascular endurance while achieving a significant calorie burn.
CASE 1: Lack of conditioning, improper hydration spell trouble
A previously healthy 38-year-old white man presented with left lower extremity pain and dark urine. Three days earlier, he had participated in a spin class for the first time. Despite a sedentary lifestyle, he had no difficulty completing the session and felt fine during the class. He did feel mildly fatigued afterward. The next day, he played 18 holes of golf in hot, humid weather. He admitted to poor fluid intake, stating he “drank a few beers” during the round. The next day, he began noticing discomfort and swelling in his left knee, which progressed to his anterior thigh. That evening, he became concerned because of a dark red tint to his urine. He was not taking any medications.
The physical exam was unremarkable except for a moderately swollen, tender knee with reduced range of motion. An x-ray of the knee showed a moderate suprapatellar effusion, but no fracture or dislocation. Urinalysis was remarkable for blood and myoglobin. The CPK value was 149,985 U/L (normal, 24-170 U/L), AST was 2234 U/L (normal, 9-25 U/L), ALT was 570 U/L (normal, 7-30 U/L), and BMI was 26.6 kg/m2. Renal function was normal, as evidenced by a BUN of 17 mg/dL and a creatinine level of 1.0 mg/dL. He was afebrile and his WBC count was 9.6 x 103/mm3.
We hospitalized the patient with a diagnosis of rhabdomyolysis and started him on aggressive intravenous (IV) hydration. The patient’s CPK and transaminase levels started trending down the next day, urine output (UOP) remained at goal, and renal function remained stable. Pain and swelling diminished over the next 3 days. He was discharged home on Day 4. At discharge, his CPK level was 26,180 U/L, BUN 10 mg/dL, and creatinine 0.8 mg/dL. At 1 month follow-up, his CPK was within normal limits.
CASE 2: Even those who exercise regularly can overdo it
A previously healthy 26-year-old white woman sought care at our clinic complaining of bilateral leg pain and dark urine. Despite being overweight, she regularly engaged in moderate exercise, and 2 days prior had participated in her first spin class. She felt some discomfort 30 minutes into the class, and the next day noted discomfort in her anterior thighs, which progressively worsened. Two days after the workout, her pain was worse and her urine became reddish-brown. She was not taking any medications.
The physical exam was unremarkable except for antalgic gait and tenderness of the anterior thighs, which were also moderately firm and warm to the touch. Urinalysis showed a large blood concentration and was positive for myoglobin. ALT was 366 U/L, AST was 1383 U/L, CPK was 86,592 U/L, and BMI was 33.36 kg/m2. A BUN level of 11 mg/dL and creatinine level of 0.8 mg/dL suggested normal renal function. Her WBC count was 12.2 x 103/mm3.
We hospitalized the patient for a presumptive diagnosis of rhabdomyolysis, and initiated aggressive IV hydration to achieve a UOP of at least 200 mL/h. CPK levels and renal and liver function were closely monitored. On hospital Day 2, the patient’s thighs were tender and tight, so we consulted orthopedics about possible compartment syndrome. The consultant believed that intervention was unwarranted.
By Day 3, the swelling and pain began to resolve. UOP remained at target, and CPK and transaminase levels continued to trend down. Renal function remained stable. The patient was discharged home on Day 4 with a CPK of 11,388 U/L, BUN of 8 mg/dL, and creatinine of 0.7 mg/dL. At her 2-week follow-up, CPK was down to 772 U/L, and transaminases were within normal limits.
Discussion
Rhabdomyolysis occurs as a result of damage to the striated muscle cell membranes. Such injury releases into the systemic circulation calcium, potassium, phosphate, urate myoglobin, CPK, aldolase, lactate dehydrogenase, AST, and ALT. In the presence of excess calcium, further muscle fiber necrosis occurs and can lead to acute renal failure.2,3 Serum haptoglobin binding capacity becomes overly saturated. This results in free myoglobin, causing renal tubular obstruction. Myoglobin then dissociates into ferrihemate and globulin. Ferrihemate further exacerbates failure of the renal tubular transport system, eventually resulting in cell death and renal failure.2
Military trainees and casual athletes comprise many of the cases of exercise- induced rhabdomyolysis.4-6 People who exercise regularly are less likely to develop the condition than their more sedentary counterparts. As with our cases, a sudden increase in the intensity and duration of vigorous exercise, without proper training, may increase the likelihood of rhabdomyolysis.6
Other potential underlying causes. In addition to exercise and dehydration as depicted in our cases, rhabdomyolysis can result from burns, shock, acidosis, infections, crush trauma, immobility, malignancy, medications, toxins, abuse of drugs, or pre-existing illness such as sickle cell trait or other metabolic conditions.7,8
Clinical presentation varies. Regardless of the cause, patients typically present with muscle pain, weakness and cramping, and discolored urine.4,8 However, many patients will have dark urine associated with other symptoms, such as general malaise, visceral pain, swelling, muscle stiffness and tightness, fever, tachycardia, nausea, and vomiting.2,3 A careful history may help elucidate the cause.
Laboratory clues. Diagnostic guidelines commonly specify a serum CPK level 5 times the upper limit of normal as an indication of rhabdomyolysis, specifically in the exertional variety.9 Typically the level of this is around 1000 U/L.3 However, there is no agreement on what CPK level is diagnostic of rhabdomyolysis. Suggestions range from 1000 to 20,000 U/L.3,8 A CPK level in excess of 5000 U/L increases the risk for acute renal failure and renal cell death.3,10 In athletes, an elevated CPK after working out is not uncommon and may be much higher than in other individuals.6,8 Endurance exercises such as marathon running or cycling have been noted to elevate CPK for up to 2 hours postexercise.8
Myoglobin becomes detectable in urine when it exceeds 1.5 mg/dL.10 Urine becomes tea-colored or reddish-brown when myoglobin is >100 mg/dL.10
Complications from rhabdomyolysis include compartment syndrome, hyperkalemia, disseminated intravascular coagulation, coagulopathies, and acute renal failure.
Treatment for rhabdomyolysis consists of aggressive IV hydration with normal saline (with variable rate) or crystalloids to maintain a UOP of 200 to 300 mL/h.2,3,11 Avoid fluid overload in the elderly and those with renal or cardiac disease.2 As CPK and myoglobin continue to trend down, it’s important to adjust IV fluids and electrolyte replacement.2,11 Using bicarbonate to alkalinize the urine is controversial, with no studies showing any benefit.3,10 In severe situations, consider a nephrology consult for hemodialysis to bring down CPK, which may be secondary to renal failure and hyperkalemia.2,10 However, renal failure is less likely to occur in physically active, healthy athletes.
Advice after recovery. After an episode of acute rhabdomyolysis, conditioned athletes can return to physical training with resolution of their symptoms or a CPK level from 1000 to 5000 U/L, usually within a week.6 A more judicious approach may be needed for less fit individuals. Regardless of their fitness level, advise patients to avoid diuretics and alcohol before exercise, remain hydrated during and after exercise, and avoid overheating to decrease the likelihood of developing rhabdomyolysis.4 However, in patients with sickle cell trait, exertional sickling can occur with intensity of exercise without overheating.7
In the case of our male patient, poor physical conditioning and intensive, prolonged exercise followed by poor hydration and the diuretic effect of alcohol created the perfect storm for the development of rhabdomyolysis. On the other hand, our female patient routinely exercised, but still pushed herself beyond her limit and went too far too fast. Although BMI may play a role in the development of rhabdomyolysis, it does not appear to be as significant a factor as hydration status and overall physical conditioning.
Our patients’ prompt attention to the need for medical help and the recognition of the problem by their clinicians contributed to good outcomes in both cases.
CORRESPONDENCE Jacqueline DuBose, MD, Department of Family Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA 30912; [email protected]
1. Metzker G. The man who put a new spin on stationary bikes. Los Angeles Times. April 17, 2000. Available at: http://articles.latimes.com/2000/apr/17/health/he-20459. Accessed February 7, 2012.
2. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907-912.
3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-756.
4. Sayers SP, Clarkson PM. Excercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59-60.
5. Alpers JP, Jones LK. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487-491.
6. Eichner ER. Exertional rhabdomyolysis. Curr Sports Med Rep. 2008;7:3-4.
7. Eichner ER. Pearls and pitfalls: exertional sickling. Curr Sports Med Rep. 2010;9:3-4.
8. Clarkson PM, Eichner ER. Exertional rhabdomyolysis: does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57-60.
9. Capaccchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158-169.
11. Young IM, Thomson K. Spinning-induced rhabdomyolysis: a case report. Eur J Emerg Med. 2004;11:358-359.
Primary care physicians frequently encourage patients to lead a more active, healthy lifestyle. The rise in popularity of endurance events, yoga, and organized gym-based fitness classes has, no doubt, improved the health of those who participate. But what happens when an individual moves too quickly from a sedentary existence to a more physically active one?
In this article we describe 2 clinical cases of rhabdomyolysis that occurred after healthy individuals participated for the first time in a class involving high-intensity stationary cycling, known as “spinning.” This exercise activity originated in California around 1989 when a competitive cyclist introduced variable resistance and speed training to stationary cycle workouts.1 Over the last 10 years, spinning has gained a worldwide following as a means of building cardiovascular endurance while achieving a significant calorie burn.
CASE 1: Lack of conditioning, improper hydration spell trouble
A previously healthy 38-year-old white man presented with left lower extremity pain and dark urine. Three days earlier, he had participated in a spin class for the first time. Despite a sedentary lifestyle, he had no difficulty completing the session and felt fine during the class. He did feel mildly fatigued afterward. The next day, he played 18 holes of golf in hot, humid weather. He admitted to poor fluid intake, stating he “drank a few beers” during the round. The next day, he began noticing discomfort and swelling in his left knee, which progressed to his anterior thigh. That evening, he became concerned because of a dark red tint to his urine. He was not taking any medications.
The physical exam was unremarkable except for a moderately swollen, tender knee with reduced range of motion. An x-ray of the knee showed a moderate suprapatellar effusion, but no fracture or dislocation. Urinalysis was remarkable for blood and myoglobin. The CPK value was 149,985 U/L (normal, 24-170 U/L), AST was 2234 U/L (normal, 9-25 U/L), ALT was 570 U/L (normal, 7-30 U/L), and BMI was 26.6 kg/m2. Renal function was normal, as evidenced by a BUN of 17 mg/dL and a creatinine level of 1.0 mg/dL. He was afebrile and his WBC count was 9.6 x 103/mm3.
We hospitalized the patient with a diagnosis of rhabdomyolysis and started him on aggressive intravenous (IV) hydration. The patient’s CPK and transaminase levels started trending down the next day, urine output (UOP) remained at goal, and renal function remained stable. Pain and swelling diminished over the next 3 days. He was discharged home on Day 4. At discharge, his CPK level was 26,180 U/L, BUN 10 mg/dL, and creatinine 0.8 mg/dL. At 1 month follow-up, his CPK was within normal limits.
CASE 2: Even those who exercise regularly can overdo it
A previously healthy 26-year-old white woman sought care at our clinic complaining of bilateral leg pain and dark urine. Despite being overweight, she regularly engaged in moderate exercise, and 2 days prior had participated in her first spin class. She felt some discomfort 30 minutes into the class, and the next day noted discomfort in her anterior thighs, which progressively worsened. Two days after the workout, her pain was worse and her urine became reddish-brown. She was not taking any medications.
The physical exam was unremarkable except for antalgic gait and tenderness of the anterior thighs, which were also moderately firm and warm to the touch. Urinalysis showed a large blood concentration and was positive for myoglobin. ALT was 366 U/L, AST was 1383 U/L, CPK was 86,592 U/L, and BMI was 33.36 kg/m2. A BUN level of 11 mg/dL and creatinine level of 0.8 mg/dL suggested normal renal function. Her WBC count was 12.2 x 103/mm3.
We hospitalized the patient for a presumptive diagnosis of rhabdomyolysis, and initiated aggressive IV hydration to achieve a UOP of at least 200 mL/h. CPK levels and renal and liver function were closely monitored. On hospital Day 2, the patient’s thighs were tender and tight, so we consulted orthopedics about possible compartment syndrome. The consultant believed that intervention was unwarranted.
By Day 3, the swelling and pain began to resolve. UOP remained at target, and CPK and transaminase levels continued to trend down. Renal function remained stable. The patient was discharged home on Day 4 with a CPK of 11,388 U/L, BUN of 8 mg/dL, and creatinine of 0.7 mg/dL. At her 2-week follow-up, CPK was down to 772 U/L, and transaminases were within normal limits.
Discussion
Rhabdomyolysis occurs as a result of damage to the striated muscle cell membranes. Such injury releases into the systemic circulation calcium, potassium, phosphate, urate myoglobin, CPK, aldolase, lactate dehydrogenase, AST, and ALT. In the presence of excess calcium, further muscle fiber necrosis occurs and can lead to acute renal failure.2,3 Serum haptoglobin binding capacity becomes overly saturated. This results in free myoglobin, causing renal tubular obstruction. Myoglobin then dissociates into ferrihemate and globulin. Ferrihemate further exacerbates failure of the renal tubular transport system, eventually resulting in cell death and renal failure.2
Military trainees and casual athletes comprise many of the cases of exercise- induced rhabdomyolysis.4-6 People who exercise regularly are less likely to develop the condition than their more sedentary counterparts. As with our cases, a sudden increase in the intensity and duration of vigorous exercise, without proper training, may increase the likelihood of rhabdomyolysis.6
Other potential underlying causes. In addition to exercise and dehydration as depicted in our cases, rhabdomyolysis can result from burns, shock, acidosis, infections, crush trauma, immobility, malignancy, medications, toxins, abuse of drugs, or pre-existing illness such as sickle cell trait or other metabolic conditions.7,8
Clinical presentation varies. Regardless of the cause, patients typically present with muscle pain, weakness and cramping, and discolored urine.4,8 However, many patients will have dark urine associated with other symptoms, such as general malaise, visceral pain, swelling, muscle stiffness and tightness, fever, tachycardia, nausea, and vomiting.2,3 A careful history may help elucidate the cause.
Laboratory clues. Diagnostic guidelines commonly specify a serum CPK level 5 times the upper limit of normal as an indication of rhabdomyolysis, specifically in the exertional variety.9 Typically the level of this is around 1000 U/L.3 However, there is no agreement on what CPK level is diagnostic of rhabdomyolysis. Suggestions range from 1000 to 20,000 U/L.3,8 A CPK level in excess of 5000 U/L increases the risk for acute renal failure and renal cell death.3,10 In athletes, an elevated CPK after working out is not uncommon and may be much higher than in other individuals.6,8 Endurance exercises such as marathon running or cycling have been noted to elevate CPK for up to 2 hours postexercise.8
Myoglobin becomes detectable in urine when it exceeds 1.5 mg/dL.10 Urine becomes tea-colored or reddish-brown when myoglobin is >100 mg/dL.10
Complications from rhabdomyolysis include compartment syndrome, hyperkalemia, disseminated intravascular coagulation, coagulopathies, and acute renal failure.
Treatment for rhabdomyolysis consists of aggressive IV hydration with normal saline (with variable rate) or crystalloids to maintain a UOP of 200 to 300 mL/h.2,3,11 Avoid fluid overload in the elderly and those with renal or cardiac disease.2 As CPK and myoglobin continue to trend down, it’s important to adjust IV fluids and electrolyte replacement.2,11 Using bicarbonate to alkalinize the urine is controversial, with no studies showing any benefit.3,10 In severe situations, consider a nephrology consult for hemodialysis to bring down CPK, which may be secondary to renal failure and hyperkalemia.2,10 However, renal failure is less likely to occur in physically active, healthy athletes.
Advice after recovery. After an episode of acute rhabdomyolysis, conditioned athletes can return to physical training with resolution of their symptoms or a CPK level from 1000 to 5000 U/L, usually within a week.6 A more judicious approach may be needed for less fit individuals. Regardless of their fitness level, advise patients to avoid diuretics and alcohol before exercise, remain hydrated during and after exercise, and avoid overheating to decrease the likelihood of developing rhabdomyolysis.4 However, in patients with sickle cell trait, exertional sickling can occur with intensity of exercise without overheating.7
In the case of our male patient, poor physical conditioning and intensive, prolonged exercise followed by poor hydration and the diuretic effect of alcohol created the perfect storm for the development of rhabdomyolysis. On the other hand, our female patient routinely exercised, but still pushed herself beyond her limit and went too far too fast. Although BMI may play a role in the development of rhabdomyolysis, it does not appear to be as significant a factor as hydration status and overall physical conditioning.
Our patients’ prompt attention to the need for medical help and the recognition of the problem by their clinicians contributed to good outcomes in both cases.
CORRESPONDENCE Jacqueline DuBose, MD, Department of Family Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA 30912; [email protected]
Primary care physicians frequently encourage patients to lead a more active, healthy lifestyle. The rise in popularity of endurance events, yoga, and organized gym-based fitness classes has, no doubt, improved the health of those who participate. But what happens when an individual moves too quickly from a sedentary existence to a more physically active one?
In this article we describe 2 clinical cases of rhabdomyolysis that occurred after healthy individuals participated for the first time in a class involving high-intensity stationary cycling, known as “spinning.” This exercise activity originated in California around 1989 when a competitive cyclist introduced variable resistance and speed training to stationary cycle workouts.1 Over the last 10 years, spinning has gained a worldwide following as a means of building cardiovascular endurance while achieving a significant calorie burn.
CASE 1: Lack of conditioning, improper hydration spell trouble
A previously healthy 38-year-old white man presented with left lower extremity pain and dark urine. Three days earlier, he had participated in a spin class for the first time. Despite a sedentary lifestyle, he had no difficulty completing the session and felt fine during the class. He did feel mildly fatigued afterward. The next day, he played 18 holes of golf in hot, humid weather. He admitted to poor fluid intake, stating he “drank a few beers” during the round. The next day, he began noticing discomfort and swelling in his left knee, which progressed to his anterior thigh. That evening, he became concerned because of a dark red tint to his urine. He was not taking any medications.
The physical exam was unremarkable except for a moderately swollen, tender knee with reduced range of motion. An x-ray of the knee showed a moderate suprapatellar effusion, but no fracture or dislocation. Urinalysis was remarkable for blood and myoglobin. The CPK value was 149,985 U/L (normal, 24-170 U/L), AST was 2234 U/L (normal, 9-25 U/L), ALT was 570 U/L (normal, 7-30 U/L), and BMI was 26.6 kg/m2. Renal function was normal, as evidenced by a BUN of 17 mg/dL and a creatinine level of 1.0 mg/dL. He was afebrile and his WBC count was 9.6 x 103/mm3.
We hospitalized the patient with a diagnosis of rhabdomyolysis and started him on aggressive intravenous (IV) hydration. The patient’s CPK and transaminase levels started trending down the next day, urine output (UOP) remained at goal, and renal function remained stable. Pain and swelling diminished over the next 3 days. He was discharged home on Day 4. At discharge, his CPK level was 26,180 U/L, BUN 10 mg/dL, and creatinine 0.8 mg/dL. At 1 month follow-up, his CPK was within normal limits.
CASE 2: Even those who exercise regularly can overdo it
A previously healthy 26-year-old white woman sought care at our clinic complaining of bilateral leg pain and dark urine. Despite being overweight, she regularly engaged in moderate exercise, and 2 days prior had participated in her first spin class. She felt some discomfort 30 minutes into the class, and the next day noted discomfort in her anterior thighs, which progressively worsened. Two days after the workout, her pain was worse and her urine became reddish-brown. She was not taking any medications.
The physical exam was unremarkable except for antalgic gait and tenderness of the anterior thighs, which were also moderately firm and warm to the touch. Urinalysis showed a large blood concentration and was positive for myoglobin. ALT was 366 U/L, AST was 1383 U/L, CPK was 86,592 U/L, and BMI was 33.36 kg/m2. A BUN level of 11 mg/dL and creatinine level of 0.8 mg/dL suggested normal renal function. Her WBC count was 12.2 x 103/mm3.
We hospitalized the patient for a presumptive diagnosis of rhabdomyolysis, and initiated aggressive IV hydration to achieve a UOP of at least 200 mL/h. CPK levels and renal and liver function were closely monitored. On hospital Day 2, the patient’s thighs were tender and tight, so we consulted orthopedics about possible compartment syndrome. The consultant believed that intervention was unwarranted.
By Day 3, the swelling and pain began to resolve. UOP remained at target, and CPK and transaminase levels continued to trend down. Renal function remained stable. The patient was discharged home on Day 4 with a CPK of 11,388 U/L, BUN of 8 mg/dL, and creatinine of 0.7 mg/dL. At her 2-week follow-up, CPK was down to 772 U/L, and transaminases were within normal limits.
Discussion
Rhabdomyolysis occurs as a result of damage to the striated muscle cell membranes. Such injury releases into the systemic circulation calcium, potassium, phosphate, urate myoglobin, CPK, aldolase, lactate dehydrogenase, AST, and ALT. In the presence of excess calcium, further muscle fiber necrosis occurs and can lead to acute renal failure.2,3 Serum haptoglobin binding capacity becomes overly saturated. This results in free myoglobin, causing renal tubular obstruction. Myoglobin then dissociates into ferrihemate and globulin. Ferrihemate further exacerbates failure of the renal tubular transport system, eventually resulting in cell death and renal failure.2
Military trainees and casual athletes comprise many of the cases of exercise- induced rhabdomyolysis.4-6 People who exercise regularly are less likely to develop the condition than their more sedentary counterparts. As with our cases, a sudden increase in the intensity and duration of vigorous exercise, without proper training, may increase the likelihood of rhabdomyolysis.6
Other potential underlying causes. In addition to exercise and dehydration as depicted in our cases, rhabdomyolysis can result from burns, shock, acidosis, infections, crush trauma, immobility, malignancy, medications, toxins, abuse of drugs, or pre-existing illness such as sickle cell trait or other metabolic conditions.7,8
Clinical presentation varies. Regardless of the cause, patients typically present with muscle pain, weakness and cramping, and discolored urine.4,8 However, many patients will have dark urine associated with other symptoms, such as general malaise, visceral pain, swelling, muscle stiffness and tightness, fever, tachycardia, nausea, and vomiting.2,3 A careful history may help elucidate the cause.
Laboratory clues. Diagnostic guidelines commonly specify a serum CPK level 5 times the upper limit of normal as an indication of rhabdomyolysis, specifically in the exertional variety.9 Typically the level of this is around 1000 U/L.3 However, there is no agreement on what CPK level is diagnostic of rhabdomyolysis. Suggestions range from 1000 to 20,000 U/L.3,8 A CPK level in excess of 5000 U/L increases the risk for acute renal failure and renal cell death.3,10 In athletes, an elevated CPK after working out is not uncommon and may be much higher than in other individuals.6,8 Endurance exercises such as marathon running or cycling have been noted to elevate CPK for up to 2 hours postexercise.8
Myoglobin becomes detectable in urine when it exceeds 1.5 mg/dL.10 Urine becomes tea-colored or reddish-brown when myoglobin is >100 mg/dL.10
Complications from rhabdomyolysis include compartment syndrome, hyperkalemia, disseminated intravascular coagulation, coagulopathies, and acute renal failure.
Treatment for rhabdomyolysis consists of aggressive IV hydration with normal saline (with variable rate) or crystalloids to maintain a UOP of 200 to 300 mL/h.2,3,11 Avoid fluid overload in the elderly and those with renal or cardiac disease.2 As CPK and myoglobin continue to trend down, it’s important to adjust IV fluids and electrolyte replacement.2,11 Using bicarbonate to alkalinize the urine is controversial, with no studies showing any benefit.3,10 In severe situations, consider a nephrology consult for hemodialysis to bring down CPK, which may be secondary to renal failure and hyperkalemia.2,10 However, renal failure is less likely to occur in physically active, healthy athletes.
Advice after recovery. After an episode of acute rhabdomyolysis, conditioned athletes can return to physical training with resolution of their symptoms or a CPK level from 1000 to 5000 U/L, usually within a week.6 A more judicious approach may be needed for less fit individuals. Regardless of their fitness level, advise patients to avoid diuretics and alcohol before exercise, remain hydrated during and after exercise, and avoid overheating to decrease the likelihood of developing rhabdomyolysis.4 However, in patients with sickle cell trait, exertional sickling can occur with intensity of exercise without overheating.7
In the case of our male patient, poor physical conditioning and intensive, prolonged exercise followed by poor hydration and the diuretic effect of alcohol created the perfect storm for the development of rhabdomyolysis. On the other hand, our female patient routinely exercised, but still pushed herself beyond her limit and went too far too fast. Although BMI may play a role in the development of rhabdomyolysis, it does not appear to be as significant a factor as hydration status and overall physical conditioning.
Our patients’ prompt attention to the need for medical help and the recognition of the problem by their clinicians contributed to good outcomes in both cases.
CORRESPONDENCE Jacqueline DuBose, MD, Department of Family Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA 30912; [email protected]
1. Metzker G. The man who put a new spin on stationary bikes. Los Angeles Times. April 17, 2000. Available at: http://articles.latimes.com/2000/apr/17/health/he-20459. Accessed February 7, 2012.
2. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907-912.
3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-756.
4. Sayers SP, Clarkson PM. Excercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59-60.
5. Alpers JP, Jones LK. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487-491.
6. Eichner ER. Exertional rhabdomyolysis. Curr Sports Med Rep. 2008;7:3-4.
7. Eichner ER. Pearls and pitfalls: exertional sickling. Curr Sports Med Rep. 2010;9:3-4.
8. Clarkson PM, Eichner ER. Exertional rhabdomyolysis: does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57-60.
9. Capaccchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158-169.
11. Young IM, Thomson K. Spinning-induced rhabdomyolysis: a case report. Eur J Emerg Med. 2004;11:358-359.
1. Metzker G. The man who put a new spin on stationary bikes. Los Angeles Times. April 17, 2000. Available at: http://articles.latimes.com/2000/apr/17/health/he-20459. Accessed February 7, 2012.
2. Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65:907-912.
3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48:749-756.
4. Sayers SP, Clarkson PM. Excercise-induced rhabdomyolysis. Curr Sports Med Rep. 2002;1:59-60.
5. Alpers JP, Jones LK. Natural history of exertional rhabdomyolysis: a population-based analysis. Muscle Nerve. 2010;42:487-491.
6. Eichner ER. Exertional rhabdomyolysis. Curr Sports Med Rep. 2008;7:3-4.
7. Eichner ER. Pearls and pitfalls: exertional sickling. Curr Sports Med Rep. 2010;9:3-4.
8. Clarkson PM, Eichner ER. Exertional rhabdomyolysis: does elevated blood creatine kinase foretell renal failure? Curr Sports Med Rep. 2006;5:57-60.
9. Capaccchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth Analg. 2009;109:1065-1069.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9:158-169.
11. Young IM, Thomson K. Spinning-induced rhabdomyolysis: a case report. Eur J Emerg Med. 2004;11:358-359.
Which psychotropics carry the greatest risk of QTc prolongation?
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
Can combining triptans with SSRIs or SNRIs cause serotonin syndrome?
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In 2006, the FDA issued a warning of the risk of potentially fatal serotonin syndrome when 5-hydroxytryptamine receptor agonist antimigraine medications (triptans) and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRI) are coprescribed.1 As a result, most drug interaction programs trigger a serotonin syndrome warning when triptans are prescribed with an SSRI or SNRI.2 However, many patients with depression or anxiety also suffer from migraines and require treatment with both triptans and an SSRI or SNRI.3,4 Kalaydjian et al4 found the incidence of major depression and generalized anxiety disorder were approximately 3 times greater in patients with migraines than in those without migraines. Should we avoid coprescribing triptans and SSRIs or SNRIs?
What is serotonin syndrome?
Serotonin syndrome is an adverse drug reaction that results from excessive serotonin stimulation. There are 2 sets of validated diagnostic criteria: the Sternbach Criteria and the Hunter Serotonin Toxicity Criteria; the latter is considered more stringent.3,5-7 Symptoms of serotonin syndrome include mental status changes, autonomic hyperactivity, and neuromuscular changes such as muscle rigidity.5-7 Typical manifestations of serotonin syndrome on physical exam include spontaneous and/or inducible clonus, agitation, diaphoresis, tremor, hyperreflexia, hypertonia, and temperature >38°C.6 In severe cases, serotonin syndrome can lead to seizures, coma, and death. Management includes supportive treatment, discontinuing the offending agents, controlling agitation with medications such as benzodiazepines, and possibly administering cyproheptadine, a 5HT2A antagonist.8 Most cases resolve within 24 hours of discontinuing the offending agents or appropriate treatment.5
What did the FDA say?
The 2006 FDA warning initially was based on 27 reports of serotonin syndrome in patients receiving triptans and SSRIs or SNRIs; this was later expanded to include 29 patients.1,9 No patients died but 13 required hospitalization and 2 had life-threatening symptoms. However, most cases lacked data necessary to diagnose serotonin syndrome.9 Further, reviews of the available clinical information have suggested that in some cases, clinicians did not rule out other disorders as required by diagnostic criteria, while others were viral in nature or resolved despite ongoing treatment with the presumed offending agents.9-11
Some clinicians met the FDA’s assessment with skepticism. Only 10 of the 29 cases met the Sternbach criteria of serotonin syndrome and none met the more rigorous Hunter criteria. Additionally, the theoretical basis has been questioned.9-11 Available evidence indicates that serotonin syndrome requires activation of 5HT2A receptors and a possible limited role of 5HT1A.9-12 However, triptans are agonists at the 5HT1B/1D/1F receptor subtypes, with weak affinity for 5HT1A receptors and no activity at the 5HT2 receptors.13,14 Additionally, triptan medications are used as needed, not as standing treatments, with parameters limiting the maximum dose, dosing interval, and frequency of use. In clinical practice, it appears that these dosing guidelines are being followed: Tepper et al15 found the typical female patient experiences 1 to 2 migraines per month; on average, patients use 1.2 to 1.8 triptan tablets per month.
Our opinion
We believe it is reasonable to coprescribe SSRIs or SNRIs with triptans because:
- data indicate that many patients are treated with a combination of triptans and SSRIs or SNRIs but the number of reported cases of serotonin syndrome is extremely limited
- the nature of serotonin syndrome cases reported in the literature is questionable
- the interaction is biologically implausible
- triptans remain in the body for a limited time
- triptans are used infrequently.5-11
This view is supported by the most recent American Headache Society position paper,11 which states that inadequate data are available to assess the risk but current evidence does not support limiting use of triptans with SSRIs and SNRIs.
How we deal with the warning in clinical practice. In practice we are alerted to this interaction by notification in our e-prescribing systems, by pharmacists calling with concerns about dispensing an SSRI or SNRI for a patient already receiving a triptan, and during patient visits that involve prescribing an SSRI or SNRI.
Although it is relatively easy to override a drug interaction warning in our e-prescribing system, we discuss the issue with pharmacists and patients. We provide information about the signs and symptoms of serotonin syndrome and its potential dangerousness. We note that serotonin syndrome is a theoretical concern, but highly unlikely with this combination of medications because of their pharmacologic properties. We explain the parameters of triptan use, recommend that our patients use triptans for migraines when needed, and reassure patients we are available to answer questions. When a patient uses triptans more than twice monthly, we consider discussing this usage with the patient and the treating physician.
Related Resource
- Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012. www.headachejournal.org/SpringboardWebApp/userfiles/headache/file/sclar.pdf.
Drug Brand Name
- Cyproheptadine • Perinctin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
1. U.S. Food and Drug Administration. Public health advisory—combined use of 5-hydroxytryptamine receptor agonists (triptans), selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome. http://1.usa.gov/U0A0V4. Published July 19, 2006. Accessed September 18, 2012.
2. Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? What does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551.
3. Tepper SJ. Serotonin syndrome: SSRIs SNRIs, triptans, and current clinical practice. Headache. 2012;52(2):195-197.
4. Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med. 2008;70(7):773-780.
5. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
6. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713.
7. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
8. Ables AZ, Nagubilli R. Prevention recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139-1142.
9. Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48.-
10. Gillman PK. Triptans serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264-272.
11. Evans RW, Tepper SJ, Shapiro RE, et al. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099.
12. Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115(1-2):1-4.
13. Pediatric & Neonatal Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc.; 2011.
14. Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203.
15. Tepper S, Allen C, Sanders D, et al. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48.
Management of dermatological toxicities in patients receiving EGFR inhibitors
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Hypertension in cancer patients
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.
Hypertension is the force of blood pushing against the walls of the arteries. It is measured as systolic pressure when the heart beats and pumps blood and as diastolic pressure in the arteries when the heart rests between beats. There are 4 stages in blood pressure classification—normal, prehypertension, stage 1, and stage 2. Hypertension affects approximately 50 million people in the United States and 1 billion people worldwide. People who are normotensive at age 55 years have a 90% chance of developing hypertension in their lifetime. Starting with a blood pressure of 115/75 mmHg, the risk of cardiovascular death doubles with each 20/10 mmHg increment...
*For PDFs of the full article and related Commentary, click on the links to the left of this introduction.