User login
Commentary: Advances in HER2 advanced breast cancer, July 2023
The neoadjuvant setting provides a favorable environment to study de-escalation approaches as treatment response (via pathologic complete response [pCR] assessment) can be used as a surrogate marker for outcome. Studies have shown the effect of HER2-enriched subtype and high ERBB2 expression on pCR rates after receipt of a chemotherapy-free, dual HER2-targeted regimen.2 The prospective, multicenter, neoadjuvant phase 2 WSG-TP-II trial randomly assigned 207 patients with HR+/HER2+ early breast cancer to 12 weeks of endocrine therapy (ET)–trastuzumab-pertuzumab vs paclitaxel-trastuzumab-pertuzumab. The pCR rate was inferior in the ET arm compared with the paclitaxel arm (23.7% vs 56.4%; odds ratio 0.24; 95% CI 0.12-0.46; P < .001). In addition, an immunohistochemistry ERBB2 score of 3 or higher and ERBB2-enriched subtype were predictors of higher pCR rates in both arms (Gluz et al). This study not only supports a deescalated chemotherapy neoadjuvant strategy of paclitaxel + dual HER2 blockade but also suggests that a portion of patients may potentially be spared chemotherapy with very good results. The role of biomarkers is integral to patient selection for these approaches, and the evaluation of response in real-time will allow for the tailoring of therapy to achieve the best outcome.
Systemic staging for locally advanced breast cancer (LABC) is important for informing prognosis as well as aiding in development of an appropriate treatment plan for patients. The PETABC study included 369 patients with LABC (TNM stage III or IIB [T3N0]) with random assignment to 18F-labeled fluorodeoxyglucose PET-CT or conventional staging (bone scan, CT of chest/abdomen/pelvis), and was designed to assess the rate of upstaging with each imaging modality and effect on treatment (Dayes et al). In the PET-CT group, 23% (N = 43) of patients were upstaged to stage IV compared with 11% (N = 21) in the conventional-staging group (absolute difference 12.3%; 95% CI 3.9-19.9; P = .002). Fewer patients in the PET-CT group received combined modality treatment vs those patients in the conventional staging group (81% vs 89.2%; P = .03). These results support the consideration of PET-CT as a staging tool for LABC, and this is reflected in various clinical guidelines. Furthermore, the evolving role of other imaging techniques such as 18F-fluoroestradiol (18F-FES) PET-CT in detection of metastatic lesions related to estrogen receptor–positive breast cancer3 will continue to advance the field of imaging.
Additional References
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489-498. doi: 10.1016/S1470-2045(21)00034-6. Erratum in: Lancet Oncol. 2021;22(5):e184. doi: 10.1016/S1470-2045(21)00194-7
- Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112:46-54. doi: 10.1093/jnci/djz042
- Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-331. doi: 10.2967/jnumed.120.247882
The neoadjuvant setting provides a favorable environment to study de-escalation approaches as treatment response (via pathologic complete response [pCR] assessment) can be used as a surrogate marker for outcome. Studies have shown the effect of HER2-enriched subtype and high ERBB2 expression on pCR rates after receipt of a chemotherapy-free, dual HER2-targeted regimen.2 The prospective, multicenter, neoadjuvant phase 2 WSG-TP-II trial randomly assigned 207 patients with HR+/HER2+ early breast cancer to 12 weeks of endocrine therapy (ET)–trastuzumab-pertuzumab vs paclitaxel-trastuzumab-pertuzumab. The pCR rate was inferior in the ET arm compared with the paclitaxel arm (23.7% vs 56.4%; odds ratio 0.24; 95% CI 0.12-0.46; P < .001). In addition, an immunohistochemistry ERBB2 score of 3 or higher and ERBB2-enriched subtype were predictors of higher pCR rates in both arms (Gluz et al). This study not only supports a deescalated chemotherapy neoadjuvant strategy of paclitaxel + dual HER2 blockade but also suggests that a portion of patients may potentially be spared chemotherapy with very good results. The role of biomarkers is integral to patient selection for these approaches, and the evaluation of response in real-time will allow for the tailoring of therapy to achieve the best outcome.
Systemic staging for locally advanced breast cancer (LABC) is important for informing prognosis as well as aiding in development of an appropriate treatment plan for patients. The PETABC study included 369 patients with LABC (TNM stage III or IIB [T3N0]) with random assignment to 18F-labeled fluorodeoxyglucose PET-CT or conventional staging (bone scan, CT of chest/abdomen/pelvis), and was designed to assess the rate of upstaging with each imaging modality and effect on treatment (Dayes et al). In the PET-CT group, 23% (N = 43) of patients were upstaged to stage IV compared with 11% (N = 21) in the conventional-staging group (absolute difference 12.3%; 95% CI 3.9-19.9; P = .002). Fewer patients in the PET-CT group received combined modality treatment vs those patients in the conventional staging group (81% vs 89.2%; P = .03). These results support the consideration of PET-CT as a staging tool for LABC, and this is reflected in various clinical guidelines. Furthermore, the evolving role of other imaging techniques such as 18F-fluoroestradiol (18F-FES) PET-CT in detection of metastatic lesions related to estrogen receptor–positive breast cancer3 will continue to advance the field of imaging.
Additional References
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489-498. doi: 10.1016/S1470-2045(21)00034-6. Erratum in: Lancet Oncol. 2021;22(5):e184. doi: 10.1016/S1470-2045(21)00194-7
- Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112:46-54. doi: 10.1093/jnci/djz042
- Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-331. doi: 10.2967/jnumed.120.247882
The neoadjuvant setting provides a favorable environment to study de-escalation approaches as treatment response (via pathologic complete response [pCR] assessment) can be used as a surrogate marker for outcome. Studies have shown the effect of HER2-enriched subtype and high ERBB2 expression on pCR rates after receipt of a chemotherapy-free, dual HER2-targeted regimen.2 The prospective, multicenter, neoadjuvant phase 2 WSG-TP-II trial randomly assigned 207 patients with HR+/HER2+ early breast cancer to 12 weeks of endocrine therapy (ET)–trastuzumab-pertuzumab vs paclitaxel-trastuzumab-pertuzumab. The pCR rate was inferior in the ET arm compared with the paclitaxel arm (23.7% vs 56.4%; odds ratio 0.24; 95% CI 0.12-0.46; P < .001). In addition, an immunohistochemistry ERBB2 score of 3 or higher and ERBB2-enriched subtype were predictors of higher pCR rates in both arms (Gluz et al). This study not only supports a deescalated chemotherapy neoadjuvant strategy of paclitaxel + dual HER2 blockade but also suggests that a portion of patients may potentially be spared chemotherapy with very good results. The role of biomarkers is integral to patient selection for these approaches, and the evaluation of response in real-time will allow for the tailoring of therapy to achieve the best outcome.
Systemic staging for locally advanced breast cancer (LABC) is important for informing prognosis as well as aiding in development of an appropriate treatment plan for patients. The PETABC study included 369 patients with LABC (TNM stage III or IIB [T3N0]) with random assignment to 18F-labeled fluorodeoxyglucose PET-CT or conventional staging (bone scan, CT of chest/abdomen/pelvis), and was designed to assess the rate of upstaging with each imaging modality and effect on treatment (Dayes et al). In the PET-CT group, 23% (N = 43) of patients were upstaged to stage IV compared with 11% (N = 21) in the conventional-staging group (absolute difference 12.3%; 95% CI 3.9-19.9; P = .002). Fewer patients in the PET-CT group received combined modality treatment vs those patients in the conventional staging group (81% vs 89.2%; P = .03). These results support the consideration of PET-CT as a staging tool for LABC, and this is reflected in various clinical guidelines. Furthermore, the evolving role of other imaging techniques such as 18F-fluoroestradiol (18F-FES) PET-CT in detection of metastatic lesions related to estrogen receptor–positive breast cancer3 will continue to advance the field of imaging.
Additional References
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489-498. doi: 10.1016/S1470-2045(21)00034-6. Erratum in: Lancet Oncol. 2021;22(5):e184. doi: 10.1016/S1470-2045(21)00194-7
- Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112:46-54. doi: 10.1093/jnci/djz042
- Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-331. doi: 10.2967/jnumed.120.247882
The most important question in medicine
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Today I am going to tell you the single best question you can ask any doctor, the one that has saved my butt countless times throughout my career, the one that every attending physician should be asking every intern and resident when they present a new case. That question: “What else could this be?”
I know, I know – “When you hear hoofbeats, think horses, not zebras.” I get it. But sometimes we get so good at our jobs, so good at recognizing horses, that we stop asking ourselves about zebras at all. You see this in a phenomenon known as “anchoring bias” where physicians, when presented with a diagnosis, tend to latch on to that diagnosis based on the first piece of information given, paying attention to data that support it and ignoring data that point in other directions.
That special question: “What else could this be?”, breaks through that barrier. It forces you, the medical team, everyone, to go through the exercise of real, old-fashioned differential diagnosis. And I promise that if you do this enough, at some point it will save someone’s life.
Though the concept of anchoring bias in medicine is broadly understood, it hasn’t been broadly studied until now, with this study appearing in JAMA Internal Medicine.
Here’s the setup.
The authors hypothesized that there would be substantial anchoring bias when patients with heart failure presented to the emergency department with shortness of breath if the triage “visit reason” section mentioned HF. We’re talking about the subtle difference between the following:
- Visit reason: Shortness of breath
- Visit reason: Shortness of breath/HF
People with HF can be short of breath for lots of reasons. HF exacerbation comes immediately to mind and it should. But there are obviously lots of answers to that “What else could this be?” question: pneumonia, pneumothorax, heart attack, COPD, and, of course, pulmonary embolism (PE).
The authors leveraged the nationwide VA database, allowing them to examine data from over 100,000 patients presenting to various VA EDs with shortness of breath. They then looked for particular tests – D-dimer, CT chest with contrast, V/Q scan, lower-extremity Doppler — that would suggest that the doctor was thinking about PE. The question, then, is whether mentioning HF in that little “visit reason” section would influence the likelihood of testing for PE.
I know what you’re thinking: Not everyone who is short of breath needs an evaluation for PE. And the authors did a nice job accounting for a variety of factors that might predict a PE workup: malignancy, recent surgery, elevated heart rate, low oxygen saturation, etc. Of course, some of those same factors might predict whether that triage nurse will write HF in the visit reason section. All of these things need to be accounted for statistically, and were, but – the unofficial Impact Factor motto reminds us that “there are always more confounders.”
But let’s dig into the results. I’m going to give you the raw numbers first. There were 4,392 people with HF whose visit reason section, in addition to noting shortness of breath, explicitly mentioned HF. Of those, 360 had PE testing and two had a PE diagnosed during that ED visit. So that’s around an 8% testing rate and a 0.5% hit rate for testing. But 43 people, presumably not tested in the ED, had a PE diagnosed within the next 30 days. Assuming that those PEs were present at the ED visit, that means the ED missed 95% of the PEs in the group with that HF label attached to them.
Let’s do the same thing for those whose visit reason just said “shortness of breath.”
Of the 103,627 people in that category, 13,886 were tested for PE and 231 of those tested positive. So that is an overall testing rate of around 13% and a hit rate of 1.7%. And 1,081 of these people had a PE diagnosed within 30 days. Assuming that those PEs were actually present at the ED visit, the docs missed 79% of them.
There’s one other thing to notice from the data: The overall PE rate (diagnosed by 30 days) was basically the same in both groups. That HF label does not really flag a group at lower risk for PE.
Yes, there are a lot of assumptions here, including that all PEs that were actually there in the ED got caught within 30 days, but the numbers do paint a picture. In this unadjusted analysis, it seems that the HF label leads to less testing and more missed PEs. Classic anchoring bias.
The adjusted analysis, accounting for all those PE risk factors, really didn’t change these results. You get nearly the same numbers and thus nearly the same conclusions.
Now, the main missing piece of this puzzle is in the mind of the clinician. We don’t know whether they didn’t consider PE or whether they considered PE but thought it unlikely. And in the end, it’s clear that the vast majority of people in this study did not have PE (though I suspect not all had a simple HF exacerbation). But this type of analysis is useful not only for the empiric evidence of the clinical impact of anchoring bias but because of the fact that it reminds us all to ask that all-important question: What else could this be?
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Today I am going to tell you the single best question you can ask any doctor, the one that has saved my butt countless times throughout my career, the one that every attending physician should be asking every intern and resident when they present a new case. That question: “What else could this be?”
I know, I know – “When you hear hoofbeats, think horses, not zebras.” I get it. But sometimes we get so good at our jobs, so good at recognizing horses, that we stop asking ourselves about zebras at all. You see this in a phenomenon known as “anchoring bias” where physicians, when presented with a diagnosis, tend to latch on to that diagnosis based on the first piece of information given, paying attention to data that support it and ignoring data that point in other directions.
That special question: “What else could this be?”, breaks through that barrier. It forces you, the medical team, everyone, to go through the exercise of real, old-fashioned differential diagnosis. And I promise that if you do this enough, at some point it will save someone’s life.
Though the concept of anchoring bias in medicine is broadly understood, it hasn’t been broadly studied until now, with this study appearing in JAMA Internal Medicine.
Here’s the setup.
The authors hypothesized that there would be substantial anchoring bias when patients with heart failure presented to the emergency department with shortness of breath if the triage “visit reason” section mentioned HF. We’re talking about the subtle difference between the following:
- Visit reason: Shortness of breath
- Visit reason: Shortness of breath/HF
People with HF can be short of breath for lots of reasons. HF exacerbation comes immediately to mind and it should. But there are obviously lots of answers to that “What else could this be?” question: pneumonia, pneumothorax, heart attack, COPD, and, of course, pulmonary embolism (PE).
The authors leveraged the nationwide VA database, allowing them to examine data from over 100,000 patients presenting to various VA EDs with shortness of breath. They then looked for particular tests – D-dimer, CT chest with contrast, V/Q scan, lower-extremity Doppler — that would suggest that the doctor was thinking about PE. The question, then, is whether mentioning HF in that little “visit reason” section would influence the likelihood of testing for PE.
I know what you’re thinking: Not everyone who is short of breath needs an evaluation for PE. And the authors did a nice job accounting for a variety of factors that might predict a PE workup: malignancy, recent surgery, elevated heart rate, low oxygen saturation, etc. Of course, some of those same factors might predict whether that triage nurse will write HF in the visit reason section. All of these things need to be accounted for statistically, and were, but – the unofficial Impact Factor motto reminds us that “there are always more confounders.”
But let’s dig into the results. I’m going to give you the raw numbers first. There were 4,392 people with HF whose visit reason section, in addition to noting shortness of breath, explicitly mentioned HF. Of those, 360 had PE testing and two had a PE diagnosed during that ED visit. So that’s around an 8% testing rate and a 0.5% hit rate for testing. But 43 people, presumably not tested in the ED, had a PE diagnosed within the next 30 days. Assuming that those PEs were present at the ED visit, that means the ED missed 95% of the PEs in the group with that HF label attached to them.
Let’s do the same thing for those whose visit reason just said “shortness of breath.”
Of the 103,627 people in that category, 13,886 were tested for PE and 231 of those tested positive. So that is an overall testing rate of around 13% and a hit rate of 1.7%. And 1,081 of these people had a PE diagnosed within 30 days. Assuming that those PEs were actually present at the ED visit, the docs missed 79% of them.
There’s one other thing to notice from the data: The overall PE rate (diagnosed by 30 days) was basically the same in both groups. That HF label does not really flag a group at lower risk for PE.
Yes, there are a lot of assumptions here, including that all PEs that were actually there in the ED got caught within 30 days, but the numbers do paint a picture. In this unadjusted analysis, it seems that the HF label leads to less testing and more missed PEs. Classic anchoring bias.
The adjusted analysis, accounting for all those PE risk factors, really didn’t change these results. You get nearly the same numbers and thus nearly the same conclusions.
Now, the main missing piece of this puzzle is in the mind of the clinician. We don’t know whether they didn’t consider PE or whether they considered PE but thought it unlikely. And in the end, it’s clear that the vast majority of people in this study did not have PE (though I suspect not all had a simple HF exacerbation). But this type of analysis is useful not only for the empiric evidence of the clinical impact of anchoring bias but because of the fact that it reminds us all to ask that all-important question: What else could this be?
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Today I am going to tell you the single best question you can ask any doctor, the one that has saved my butt countless times throughout my career, the one that every attending physician should be asking every intern and resident when they present a new case. That question: “What else could this be?”
I know, I know – “When you hear hoofbeats, think horses, not zebras.” I get it. But sometimes we get so good at our jobs, so good at recognizing horses, that we stop asking ourselves about zebras at all. You see this in a phenomenon known as “anchoring bias” where physicians, when presented with a diagnosis, tend to latch on to that diagnosis based on the first piece of information given, paying attention to data that support it and ignoring data that point in other directions.
That special question: “What else could this be?”, breaks through that barrier. It forces you, the medical team, everyone, to go through the exercise of real, old-fashioned differential diagnosis. And I promise that if you do this enough, at some point it will save someone’s life.
Though the concept of anchoring bias in medicine is broadly understood, it hasn’t been broadly studied until now, with this study appearing in JAMA Internal Medicine.
Here’s the setup.
The authors hypothesized that there would be substantial anchoring bias when patients with heart failure presented to the emergency department with shortness of breath if the triage “visit reason” section mentioned HF. We’re talking about the subtle difference between the following:
- Visit reason: Shortness of breath
- Visit reason: Shortness of breath/HF
People with HF can be short of breath for lots of reasons. HF exacerbation comes immediately to mind and it should. But there are obviously lots of answers to that “What else could this be?” question: pneumonia, pneumothorax, heart attack, COPD, and, of course, pulmonary embolism (PE).
The authors leveraged the nationwide VA database, allowing them to examine data from over 100,000 patients presenting to various VA EDs with shortness of breath. They then looked for particular tests – D-dimer, CT chest with contrast, V/Q scan, lower-extremity Doppler — that would suggest that the doctor was thinking about PE. The question, then, is whether mentioning HF in that little “visit reason” section would influence the likelihood of testing for PE.
I know what you’re thinking: Not everyone who is short of breath needs an evaluation for PE. And the authors did a nice job accounting for a variety of factors that might predict a PE workup: malignancy, recent surgery, elevated heart rate, low oxygen saturation, etc. Of course, some of those same factors might predict whether that triage nurse will write HF in the visit reason section. All of these things need to be accounted for statistically, and were, but – the unofficial Impact Factor motto reminds us that “there are always more confounders.”
But let’s dig into the results. I’m going to give you the raw numbers first. There were 4,392 people with HF whose visit reason section, in addition to noting shortness of breath, explicitly mentioned HF. Of those, 360 had PE testing and two had a PE diagnosed during that ED visit. So that’s around an 8% testing rate and a 0.5% hit rate for testing. But 43 people, presumably not tested in the ED, had a PE diagnosed within the next 30 days. Assuming that those PEs were present at the ED visit, that means the ED missed 95% of the PEs in the group with that HF label attached to them.
Let’s do the same thing for those whose visit reason just said “shortness of breath.”
Of the 103,627 people in that category, 13,886 were tested for PE and 231 of those tested positive. So that is an overall testing rate of around 13% and a hit rate of 1.7%. And 1,081 of these people had a PE diagnosed within 30 days. Assuming that those PEs were actually present at the ED visit, the docs missed 79% of them.
There’s one other thing to notice from the data: The overall PE rate (diagnosed by 30 days) was basically the same in both groups. That HF label does not really flag a group at lower risk for PE.
Yes, there are a lot of assumptions here, including that all PEs that were actually there in the ED got caught within 30 days, but the numbers do paint a picture. In this unadjusted analysis, it seems that the HF label leads to less testing and more missed PEs. Classic anchoring bias.
The adjusted analysis, accounting for all those PE risk factors, really didn’t change these results. You get nearly the same numbers and thus nearly the same conclusions.
Now, the main missing piece of this puzzle is in the mind of the clinician. We don’t know whether they didn’t consider PE or whether they considered PE but thought it unlikely. And in the end, it’s clear that the vast majority of people in this study did not have PE (though I suspect not all had a simple HF exacerbation). But this type of analysis is useful not only for the empiric evidence of the clinical impact of anchoring bias but because of the fact that it reminds us all to ask that all-important question: What else could this be?
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Advances in endohepatology
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
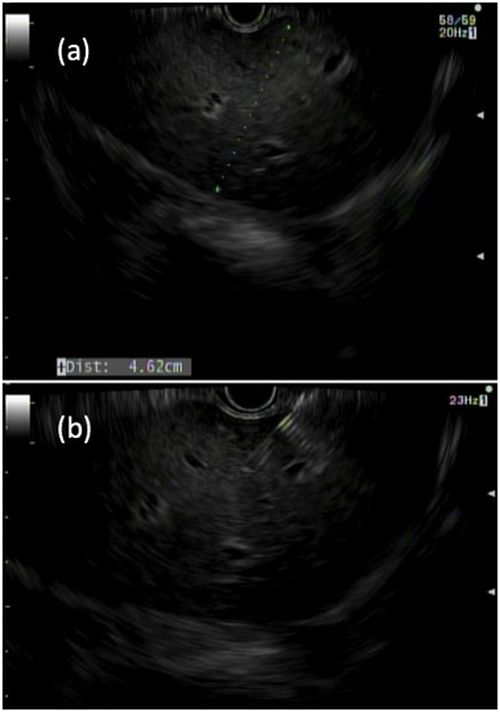
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2
While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5
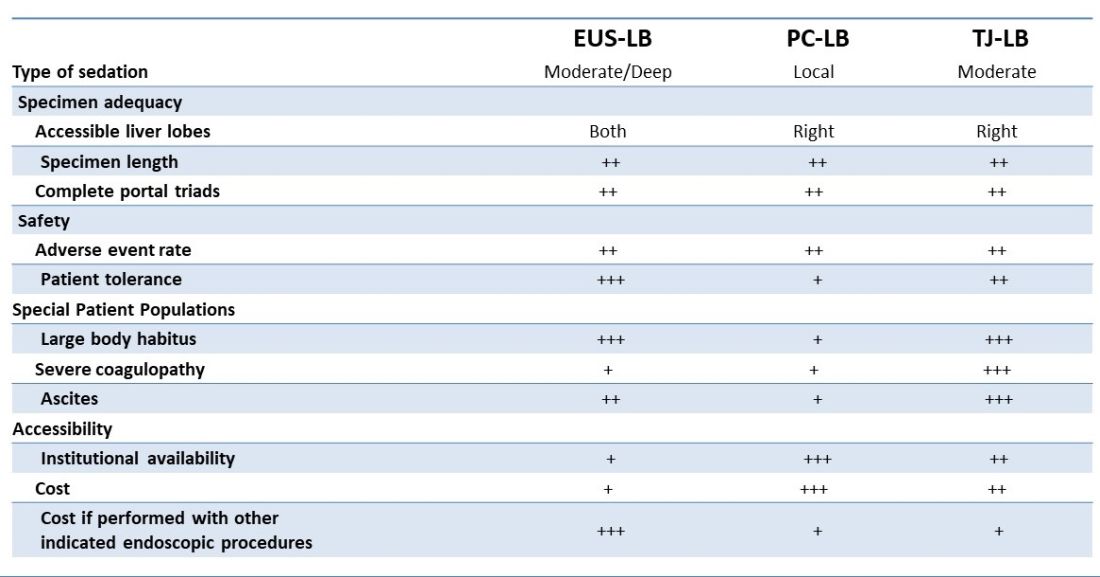
Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Introduction
Historically, the role of endoscopy in hepatology has been limited to intraluminal and bile duct interventions, primarily for the management of varices and biliary strictures. Recently, endoscopic ultrasound (EUS) has broadened the range of endoscopic treatment by enabling transluminal access to the liver parenchyma and associated vasculature. In this review, we will address recent advances in the expanding field of endohepatology.
Endoscopic-ultrasound guided liver biopsy
Liver biopsies are a critical tool in the diagnostic evaluation and management of patients with liver disease. Conventional approaches for obtaining liver tissue have been most commonly through the percutaneous or vascular approaches. In 2007, the first EUS-guided liver biopsy (EUS-LB) was described.1 EUS-LB is performed by advancing a line-array echoendoscope to the duodenal bulb to access the right lobe of the liver or proximal stomach to sample the left lobe. Doppler is first used to identify a pathway with few intervening vessels. Then a 19G or 20G needle is passed and slowly withdrawn to capture tissue (Figure 1). Careful evaluation with Doppler ultrasound to evaluate for bleeding is recommended after EUS-LB and if persistent, a small amount of clot may be reinjected as a blood or “Chang” patch akin to technique to control oozing postlumbar puncture.2

While large prospective studies are needed to compare the methods, it appears that specimen adequacy acquired via EUS-LB are comparable to percutaneous and transjugular approaches.3-5 Utilization of specific needle types and suction may optimize samples. Namely, 19G needles may provide better samples than smaller sizes and contemporary fine-needle biopsy needles with Franseen tips are superior to conventional spring-loaded cutting needles and fork tip needles.6-8 The use of dry suction has been shown to increase the yield of tissue, but at the expense of increased bloodiness. Wet suction, which involves the presence of fluid, rather than air, in the needle lumen to lubricate and improve transmission of negative pressure to the needle tip, is the preferred technique for EUS-LB given improvement in the likelihood of intact liver biopsy cores and increased specimen adequacy.9
There are several advantages to EUS-LB (Table 1). When compared with percutaneous liver biopsy (PC-LB) and transjugular liver biopsy (TJ-LB), EUS-LB is uniquely able to access both liver lobes in a single setting, which minimizes sampling error.3 EUS-LB may also have an advantage in sampling focal liver lesions given the close proximity of the transducer to the liver.10 Another advantage over PC-LB is that EUS-LB can be performed in patients with a large body habitus. Additionally, EUS-LB is better tolerated than PC-LB, with less postprocedure pain and shorter postprocedure monitoring time.4,5

Rates of adverse events appear to be similar between the three methods. Similar to PC-LB, EUS-LB requires capsular puncture, which can lead to intraperitoneal hemorrhage. Therefore, TJ-LB is preferred in patients with significant coagulopathy. While small ascites is not an absolute contraindication for EUS-LB, large ascites can obscure a safe window from the proximal stomach or duodenum to the liver, and thus TJLB is also preferred in these patients.11 Given its relative novelty and logistic challenges, other disadvantages of EUS-LB include limited provider availability and increased cost, especially compared with PC-LB. The most significant limitation is that it requires moderate or deep sedation, as opposed to local anesthetics. However, if there is another indication for endoscopy (that is, variceal screening), then “one-stop shop” procedures including EUS-LB may be more convenient and cost-effective than traditional methods. Nevertheless, rigorous comparative studies are needed.
EUS-guided portal pressure gradient measurement
The presence of clinically significant portal hypertension (CSPH), defined as hepatic venous pressure gradient (HVPG) greater than or equal to 10 is a potent predictor of decompensation. There is growing evidence to support the use of beta-blockers to mitigate this risk.12 Therefore, early identification of patients with CSPH has important diagnostic and therapeutic implications. The current gold standard for diagnosing CSPH is with wedged HVPG measurements performed by interventional radiology.
Since its introduction in 2016, EUS-guided portal pressure gradient measurement (EUS-PPG) has emerged as an alternative to wedged HVPG.13,14 Using a linear echoendoscope, the portal vein is directly accessed with a 25G fine-needle aspiration needle, and three direct measurements are taken using a compact manometer to determine the mean pressure. The hepatic vein, or less commonly the inferior vena cava, pressure is also measured. The direct measurement of portal pressure provides a significant advantage of EUS-PPG over HVPG in patients with presinusoidal and prehepatic portal hypertension. Wedged HVPG, which utilizes the difference between the wedged and free hepatic venous pressure to indirectly estimate the portal venous pressure gradient, yields erroneously low gradients in patients with noncirrhotic portal hypertension.15 An additional advantage of EUS-PPG is that it obviates the need for a central venous line placement, which is associated with thrombosis and, in rare cases, air embolus.16
Observational studies indicate that EUS-PPG has a high degree of consistency with HVPG measurements and a strong correlation between other clinical findings of portal hyper-tension including esophageal varices and thrombocytopenia.13,14 Nevertheless, EUS-PPG is performed under moderate or deep sedation which may impact HVPG measurements.17 In addition, the real-world application of EUS-PPG measurement on clinical care is undefined, but it is the topic of an ongoing clinical trial (ClinicalTrials.gov – NCT05357599).
EUS-guided interventions of gastric varices
Compared with esophageal varices, current approaches to the treatment and prophylaxis of gastric varices are more controversial.18 The most common approach to bleeding gastric varices in the United States is the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Nevertheless, in addition to risks associated with central venous line placement, 5%-35% of individuals develop hepatic encephalopathy after TIPS and ischemic acute liver failure can occur in rare situations.19 Cyanoacrylate (CYA) glue injection is the recommended first-line endoscopic therapy for the treatment of bleeding gastric varices, but use has not been widely adopted in the United States because of a lack of an approved Food and Drug Administration CYA formulation, limited expertise, and risk of serious complications. In particular systemic embolization may result in pulmonary or cerebral infarct.12,18 EUS-guided interventions have been developed to mitigate these safety concerns. EUS-guided coil embolization can be performed, either alone or in combination with CYA injection.20 In the latter approach it acts as a scaffold to prevent migration of the glue bolus. Doppler assessment enables direct visualization of the gastric varix for identification of feeder vessels, more controlled deployment of hemostatic agents, and real-time confirmation of varix obliteration. Fluoroscopy can be used as an adjunct.
EUS-guided interventions in the management of gastric varices appear to be effective and superior to CYA injection under direct endoscopic visualization with improved likelihood of obliteration and lower rebleeding rates, without increase in adverse events.21 Additionally, EUS-guided combination therapy improves technical outcomes and reduces adverse events relative to EUS-guided coil or EUS-guided glue injection therapy alone.21-23 Nevertheless, large-scale prospective trials are needed to determine whether EUS-guided interventions should be considered over TIPS. The role of EUS-guided interventions as primary prophylaxis to prevent bleeding from large gastric varices also requires additional study.24
Future directions
with the goal of optimizing care and increasing efficiency. In addition to new endoscopic procedures to optimize liver biopsy, portal pressure measurement, and gastric variceal treatment, there are a number of emerging technologies including EUS-guided liver elastography, portal venous sampling, liver tumor chemoembolization, and intrahepatic portosystemic shunts.25 However, the practice of endohepatology faces a number of challenges before widespread adoption, including limited provider expertise and institutional availability. Additionally, more robust, multicenter outcomes and cost-effective analyses comparing these novel procedures with traditional approaches are needed to define their clinical impact.
Dr. Bui is a fellow in gastroenterology in the division of gastroenterology and hepatology, University of Southern California, Los Angeles. Dr. Buxbaum is associate professor of medicine (clinical scholar) in the division of gastroenterology and hepatology, University of Southern California. Dr. Buxbaum is a consultant for Cook Medical, Boston Scientific, and Olympus. Dr. Bui has no disclosures.
References
1. Mathew A. Am J Gastroenterol. 2007;102(10):2354-5.
2. Sowa P et al. VideoGIE. 2021;6(11):487-8.
3. Pineda JJ et al. Gastrointest Endosc. 2016;83(2):360-5.
4. Ali AH et al. J Ultrasound. 2020;23(2):157-67.
5. Shuja A et al. Dig Liver Dis. 2019;51(6):826-30.
6. Schulman AR et al. Gastrointest Endosc. 2017;85(2):419-26.
7. DeWitt J et al. Endosc Int Open. 2015;3(5):E471-8.
8. Aggarwal SN et al. Gastrointest Endosc. 2021;93(5):1133-8.
9. Mok SRS et al. Gastrointest Endosc. 2018;88(6):919-25.
10. Lee YN et al. J Gastroenterol Hepatol. 2015;30(7):1161-6.
11. Kalambokis G et al. J Hepatol. 2007;47(2):284-94.
12. de Franchis R et al. J Hepatol. 2022;76(4):959-74.
13. Choi AY et al. J Gastroenterol Hepatol. 2022;37(7):1373-9.
14. Zhang W et al. Gastrointest Endosc. 2021;93(3):565-72.
15. Seijo S et al. Dig Liver Dis. 2012;44(10):855-60.
16. Vesely TM. J Vasc Interv Radiol. 2001;12(11):1291-5.
17. Reverter E et al. Liver Int. 2014;34(1):16-25.
18. Henry Z et al. Clin Gastroenterol Hepatol. 2021;19(6):1098-107.e1091.
19. Ripamonti R et al. Semin Intervent Radiol. 2006;23(2):165-76.
20. Rengstorff DS and Binmoeller KF. Gastrointest Endosc. 2004;59(4):553-8.
21. Mohan BP et al. Endoscopy. 2020;52(4):259-67.
22. Robles-Medranda C et al. Endoscopy. 2020;52(4):268-75.
23. McCarty TR et al. Endosc Ultrasound. 2020;9(1):6-15.
24. Kouanda A et al. Gastrointest Endosc. 2021;94(2):291-6.
25. Bazarbashi AN et al. 2022;24(1):98-107.
Triple-agonist retatrutide hits new weight loss highs
SAN DIEGO – New designer molecules that target weight loss via multiple mechanisms continue to raise the bar of how many pounds people with overweight or obesity can lose.
Retatrutide (Eli Lilly), an investigational agent that combines agonism to three key hormones that influence eating and metabolism into a single molecule, safely produced weight loss at levels never seen before in a pair of phase 2 studies that together randomized more than 600 people with overweight or obesity, with or without type 2 diabetes.
Among 338 randomized people with overweight or obesity and no type 2 diabetes,
Among 281 randomized people with overweight or obesity and type 2 diabetes, the same dose of retatrutide produced a nearly 17% cut in weight from baseline after 36 weeks of treatment.
Never before seen weight loss
“I have never seen weight loss at this level” after nearly 1 year of treatment, Ania M. Jastreboff, MD, PhD, who led the obesity study, said during a press briefing at the annual scientific sessions of the American Diabetes Association.
The average weight loss by study participants taking high-dose retatrutide in the two studies “is really impressive, way beyond my wildest dreams,” said Carel le Roux, MBChB, PhD, an obesity and diabetes researcher at University College Dublin, Ireland, who was not involved with the retatrutide studies.
And Robert A. Gabbay, MD, chief scientific and medical officer of the ADA, called the results “stunning,” and added, “we are now witnessing the first triple-hormone combination being highly effective for not only weight loss but liver disease and diabetes.”
A prespecified subgroup analysis of the obesity study showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those with at least 10% of their liver volume as fat at study entry); that figure increased to about 90% of people on these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the meeting.
Adding glucagon agonism ups liver-fat clearance
“When you add glucagon activity,” one of the three agonist actions of retatrutide, “liver-fat clearance goes up tremendously,” said Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
“To my knowledge, no mono-agonist of the glucagon-like peptide-1 (GLP-1) receptor [such as semaglutide or liraglutide] produces more than 50% clearance of liver fat,” added Dr. Kaplan.
The separate, randomized study of people with type 2 diabetes showed that in addition to producing an unprecedented average level of weight loss at the highest retatrutide dose, the agent also produced an average reduction from baseline levels of A1c of about 2 percentage points, an efficacy roughly comparable to maximum doses of the most potent GLP-1 mono-agonist semaglutide (Ozempic, Novo Nordisk), as well as by tirzepatide (Mounjaro, Eli Lilly), a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who presented the results from the type 2 diabetes study of retatrutide.
For the obesity study, people with a body mass index of 27-50 kg/m2 and no diabetes were randomized to placebo or any of four retatrutide target dosages using specified dose-escalation protocols. Participants were an average of 48 years old, and by design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-related pattern. (Weight loss averaged about 2% among the 70 controls who received placebo.)
Twenty-six percent without diabetes lost at least 30% of body weight
Every person who escalated to receive the 8-mg or 12-mg weekly dose of retatrutide lost at least 5% of their bodyweight after 48 weeks, 83% of those taking the 12-mg dose lost at least 15%, 63% of those on the 12-mg dose lost at least 20%, and 26% of those on the highest dose lost at least 30% of their starting bodyweight, reported Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn.
The highest dose was also associated with an average 40% relative reduction in triglyceride levels from baseline and an average 22% relative drop in LDL cholesterol levels.
The results were simultaneously published online in the New England Journal of Medicine.
The incidence of serious adverse events with retatrutide was low, similar to the rate in those who received placebo, and showed no dose relationship.
The most common adverse events were gastrointestinal, in as many as 16% of those on the highest dose; these were mild to moderate in severity and usually occurred during dose escalation. In general, adverse events were comparable to what is seen with a GLP-1 agonist or the dual agonist tirzepatide, Dr. Jastreboff said.
A1c normalization in 26% at the highest dose
A similar safety pattern occurred in the study of people with type 2 diabetes, which randomized people with an average A1c of 8.3% and an average BMI of 35.0 kg/m2. After 36 weeks of treatment, the 12-mg weekly dose of retatrutide led to normalization of A1c < 5.7% in 27% of people and A1c ≤ 6.5% in 77%.
“The number of people we were able to revert to a normal A1c was impressive,” said Dr. Rosenstock. These results were simultaneously published online in The Lancet.
The additional findings on liver-fat mobilization in people without diabetes enrolled in the obesity study are notable because no agent currently has labeling from the Food and Drug Administration for the indication of reducing excess liver fat, said Dr. Kaplan.
The researchers measured liver fat at baseline and then during treatment using MRI.
“With the level of fat clearance from the liver that we see with retatrutide it is highly likely that we’ll also see improvements in liver fibrosis” in retatrutide-treated patients, Dr. Kaplan predicted.
Next up for retatrutide is testing in pivotal trials, including the TRIUMPH-3 trial that will enroll about 1,800 people with severe obesity and cardiovascular disease, with findings expected toward the end of 2025.
The retatrutide studies are sponsored by Eli Lilly. Dr. Jastreboff, Dr. Rosenstock, Dr. Kaplan, and Dr. Le Roux have reported financial relationships with Eli Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO – New designer molecules that target weight loss via multiple mechanisms continue to raise the bar of how many pounds people with overweight or obesity can lose.
Retatrutide (Eli Lilly), an investigational agent that combines agonism to three key hormones that influence eating and metabolism into a single molecule, safely produced weight loss at levels never seen before in a pair of phase 2 studies that together randomized more than 600 people with overweight or obesity, with or without type 2 diabetes.
Among 338 randomized people with overweight or obesity and no type 2 diabetes,
Among 281 randomized people with overweight or obesity and type 2 diabetes, the same dose of retatrutide produced a nearly 17% cut in weight from baseline after 36 weeks of treatment.
Never before seen weight loss
“I have never seen weight loss at this level” after nearly 1 year of treatment, Ania M. Jastreboff, MD, PhD, who led the obesity study, said during a press briefing at the annual scientific sessions of the American Diabetes Association.
The average weight loss by study participants taking high-dose retatrutide in the two studies “is really impressive, way beyond my wildest dreams,” said Carel le Roux, MBChB, PhD, an obesity and diabetes researcher at University College Dublin, Ireland, who was not involved with the retatrutide studies.
And Robert A. Gabbay, MD, chief scientific and medical officer of the ADA, called the results “stunning,” and added, “we are now witnessing the first triple-hormone combination being highly effective for not only weight loss but liver disease and diabetes.”
A prespecified subgroup analysis of the obesity study showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those with at least 10% of their liver volume as fat at study entry); that figure increased to about 90% of people on these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the meeting.
Adding glucagon agonism ups liver-fat clearance
“When you add glucagon activity,” one of the three agonist actions of retatrutide, “liver-fat clearance goes up tremendously,” said Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
“To my knowledge, no mono-agonist of the glucagon-like peptide-1 (GLP-1) receptor [such as semaglutide or liraglutide] produces more than 50% clearance of liver fat,” added Dr. Kaplan.
The separate, randomized study of people with type 2 diabetes showed that in addition to producing an unprecedented average level of weight loss at the highest retatrutide dose, the agent also produced an average reduction from baseline levels of A1c of about 2 percentage points, an efficacy roughly comparable to maximum doses of the most potent GLP-1 mono-agonist semaglutide (Ozempic, Novo Nordisk), as well as by tirzepatide (Mounjaro, Eli Lilly), a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who presented the results from the type 2 diabetes study of retatrutide.
For the obesity study, people with a body mass index of 27-50 kg/m2 and no diabetes were randomized to placebo or any of four retatrutide target dosages using specified dose-escalation protocols. Participants were an average of 48 years old, and by design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-related pattern. (Weight loss averaged about 2% among the 70 controls who received placebo.)
Twenty-six percent without diabetes lost at least 30% of body weight
Every person who escalated to receive the 8-mg or 12-mg weekly dose of retatrutide lost at least 5% of their bodyweight after 48 weeks, 83% of those taking the 12-mg dose lost at least 15%, 63% of those on the 12-mg dose lost at least 20%, and 26% of those on the highest dose lost at least 30% of their starting bodyweight, reported Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn.
The highest dose was also associated with an average 40% relative reduction in triglyceride levels from baseline and an average 22% relative drop in LDL cholesterol levels.
The results were simultaneously published online in the New England Journal of Medicine.
The incidence of serious adverse events with retatrutide was low, similar to the rate in those who received placebo, and showed no dose relationship.
The most common adverse events were gastrointestinal, in as many as 16% of those on the highest dose; these were mild to moderate in severity and usually occurred during dose escalation. In general, adverse events were comparable to what is seen with a GLP-1 agonist or the dual agonist tirzepatide, Dr. Jastreboff said.
A1c normalization in 26% at the highest dose
A similar safety pattern occurred in the study of people with type 2 diabetes, which randomized people with an average A1c of 8.3% and an average BMI of 35.0 kg/m2. After 36 weeks of treatment, the 12-mg weekly dose of retatrutide led to normalization of A1c < 5.7% in 27% of people and A1c ≤ 6.5% in 77%.
“The number of people we were able to revert to a normal A1c was impressive,” said Dr. Rosenstock. These results were simultaneously published online in The Lancet.
The additional findings on liver-fat mobilization in people without diabetes enrolled in the obesity study are notable because no agent currently has labeling from the Food and Drug Administration for the indication of reducing excess liver fat, said Dr. Kaplan.
The researchers measured liver fat at baseline and then during treatment using MRI.
“With the level of fat clearance from the liver that we see with retatrutide it is highly likely that we’ll also see improvements in liver fibrosis” in retatrutide-treated patients, Dr. Kaplan predicted.
Next up for retatrutide is testing in pivotal trials, including the TRIUMPH-3 trial that will enroll about 1,800 people with severe obesity and cardiovascular disease, with findings expected toward the end of 2025.
The retatrutide studies are sponsored by Eli Lilly. Dr. Jastreboff, Dr. Rosenstock, Dr. Kaplan, and Dr. Le Roux have reported financial relationships with Eli Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
SAN DIEGO – New designer molecules that target weight loss via multiple mechanisms continue to raise the bar of how many pounds people with overweight or obesity can lose.
Retatrutide (Eli Lilly), an investigational agent that combines agonism to three key hormones that influence eating and metabolism into a single molecule, safely produced weight loss at levels never seen before in a pair of phase 2 studies that together randomized more than 600 people with overweight or obesity, with or without type 2 diabetes.
Among 338 randomized people with overweight or obesity and no type 2 diabetes,
Among 281 randomized people with overweight or obesity and type 2 diabetes, the same dose of retatrutide produced a nearly 17% cut in weight from baseline after 36 weeks of treatment.
Never before seen weight loss
“I have never seen weight loss at this level” after nearly 1 year of treatment, Ania M. Jastreboff, MD, PhD, who led the obesity study, said during a press briefing at the annual scientific sessions of the American Diabetes Association.
The average weight loss by study participants taking high-dose retatrutide in the two studies “is really impressive, way beyond my wildest dreams,” said Carel le Roux, MBChB, PhD, an obesity and diabetes researcher at University College Dublin, Ireland, who was not involved with the retatrutide studies.
And Robert A. Gabbay, MD, chief scientific and medical officer of the ADA, called the results “stunning,” and added, “we are now witnessing the first triple-hormone combination being highly effective for not only weight loss but liver disease and diabetes.”
A prespecified subgroup analysis of the obesity study showed that at both 8-mg and 12-mg weekly doses, 24 weeks of retatrutide produced complete resolution of excess liver fat (hepatic steatosis) in about 80% of the people eligible for the analysis (those with at least 10% of their liver volume as fat at study entry); that figure increased to about 90% of people on these doses after 48 weeks, Lee M. Kaplan, MD, reported during a separate presentation at the meeting.
Adding glucagon agonism ups liver-fat clearance
“When you add glucagon activity,” one of the three agonist actions of retatrutide, “liver-fat clearance goes up tremendously,” said Dr. Kaplan, director of the Obesity, Metabolism and Nutrition Institute at Massachusetts General Hospital in Boston.
“To my knowledge, no mono-agonist of the glucagon-like peptide-1 (GLP-1) receptor [such as semaglutide or liraglutide] produces more than 50% clearance of liver fat,” added Dr. Kaplan.
The separate, randomized study of people with type 2 diabetes showed that in addition to producing an unprecedented average level of weight loss at the highest retatrutide dose, the agent also produced an average reduction from baseline levels of A1c of about 2 percentage points, an efficacy roughly comparable to maximum doses of the most potent GLP-1 mono-agonist semaglutide (Ozempic, Novo Nordisk), as well as by tirzepatide (Mounjaro, Eli Lilly), a dual agonist for the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors.
“No other medication has shown an average 17% reduction from baseline bodyweight after 36 weeks in people with type 2 diabetes,” said Julio Rosenstock, MD, director of the Dallas Diabetes Research Center at Medical City, Texas, who presented the results from the type 2 diabetes study of retatrutide.
For the obesity study, people with a body mass index of 27-50 kg/m2 and no diabetes were randomized to placebo or any of four retatrutide target dosages using specified dose-escalation protocols. Participants were an average of 48 years old, and by design, 52% were men. (The study sought to enroll roughly equal numbers of men and women.) Average BMI at study entry was 37 kg/m2.
Weight loss levels after 24 and 48 weeks of retatrutide treatment followed a clear dose-related pattern. (Weight loss averaged about 2% among the 70 controls who received placebo.)
Twenty-six percent without diabetes lost at least 30% of body weight
Every person who escalated to receive the 8-mg or 12-mg weekly dose of retatrutide lost at least 5% of their bodyweight after 48 weeks, 83% of those taking the 12-mg dose lost at least 15%, 63% of those on the 12-mg dose lost at least 20%, and 26% of those on the highest dose lost at least 30% of their starting bodyweight, reported Dr. Jastreboff, director of the Yale Obesity Research Center of Yale University in New Haven, Conn.
The highest dose was also associated with an average 40% relative reduction in triglyceride levels from baseline and an average 22% relative drop in LDL cholesterol levels.
The results were simultaneously published online in the New England Journal of Medicine.
The incidence of serious adverse events with retatrutide was low, similar to the rate in those who received placebo, and showed no dose relationship.
The most common adverse events were gastrointestinal, in as many as 16% of those on the highest dose; these were mild to moderate in severity and usually occurred during dose escalation. In general, adverse events were comparable to what is seen with a GLP-1 agonist or the dual agonist tirzepatide, Dr. Jastreboff said.
A1c normalization in 26% at the highest dose
A similar safety pattern occurred in the study of people with type 2 diabetes, which randomized people with an average A1c of 8.3% and an average BMI of 35.0 kg/m2. After 36 weeks of treatment, the 12-mg weekly dose of retatrutide led to normalization of A1c < 5.7% in 27% of people and A1c ≤ 6.5% in 77%.
“The number of people we were able to revert to a normal A1c was impressive,” said Dr. Rosenstock. These results were simultaneously published online in The Lancet.
The additional findings on liver-fat mobilization in people without diabetes enrolled in the obesity study are notable because no agent currently has labeling from the Food and Drug Administration for the indication of reducing excess liver fat, said Dr. Kaplan.
The researchers measured liver fat at baseline and then during treatment using MRI.
“With the level of fat clearance from the liver that we see with retatrutide it is highly likely that we’ll also see improvements in liver fibrosis” in retatrutide-treated patients, Dr. Kaplan predicted.
Next up for retatrutide is testing in pivotal trials, including the TRIUMPH-3 trial that will enroll about 1,800 people with severe obesity and cardiovascular disease, with findings expected toward the end of 2025.
The retatrutide studies are sponsored by Eli Lilly. Dr. Jastreboff, Dr. Rosenstock, Dr. Kaplan, and Dr. Le Roux have reported financial relationships with Eli Lilly as well as other companies.
A version of this article first appeared on Medscape.com.
AT ADA 2023
Treating Veterans With Small-Cell Lung Cancer
Small-cell lung cancer (SCLC) occurs almost exclusively in cigarette smokers. Veterans are particularly vulnerable to SCLC because of their prevalent smoking history and exposures to carcinogens, including Agent Orange.
SCLC is characterized by the early development of widespread metastases, including liver, bone, and brain.
Unlike, non–-small cell lung cancer, which has seen great improvement in survival from the introduction of immunotherapy and targeted agents, there has been relatively little improvement in SCLC.
Patients generally are classified into limited- and extensive-stage disease, but platinum-based chemotherapy is almost always the standard first-line treatment. Unfortunately, most patients relapse within a year.
In this ReCAP, Dr Shadia Jalal, of Indiana University Melvin and Bren Simon Comprehensive Cancer Center, discusses second-line treatment options for SCLC patients who relapse after chemotherapy. She also discusses four subtypes of SCLC categorized on the basis of specific transcription regulators, which may offer the potential of targeted therapies for this patient population.
--
Shadia Jalal, MD, Associate Professor of Medicine, Physician, Indiana University Melvin and Bren Simon Comprehensive Cancer Center, Indianapolis, Indiana
Shadia Jalal, MD, has disclosed no relevant financial relationships.
Small-cell lung cancer (SCLC) occurs almost exclusively in cigarette smokers. Veterans are particularly vulnerable to SCLC because of their prevalent smoking history and exposures to carcinogens, including Agent Orange.
SCLC is characterized by the early development of widespread metastases, including liver, bone, and brain.
Unlike, non–-small cell lung cancer, which has seen great improvement in survival from the introduction of immunotherapy and targeted agents, there has been relatively little improvement in SCLC.
Patients generally are classified into limited- and extensive-stage disease, but platinum-based chemotherapy is almost always the standard first-line treatment. Unfortunately, most patients relapse within a year.
In this ReCAP, Dr Shadia Jalal, of Indiana University Melvin and Bren Simon Comprehensive Cancer Center, discusses second-line treatment options for SCLC patients who relapse after chemotherapy. She also discusses four subtypes of SCLC categorized on the basis of specific transcription regulators, which may offer the potential of targeted therapies for this patient population.
--
Shadia Jalal, MD, Associate Professor of Medicine, Physician, Indiana University Melvin and Bren Simon Comprehensive Cancer Center, Indianapolis, Indiana
Shadia Jalal, MD, has disclosed no relevant financial relationships.
Small-cell lung cancer (SCLC) occurs almost exclusively in cigarette smokers. Veterans are particularly vulnerable to SCLC because of their prevalent smoking history and exposures to carcinogens, including Agent Orange.
SCLC is characterized by the early development of widespread metastases, including liver, bone, and brain.
Unlike, non–-small cell lung cancer, which has seen great improvement in survival from the introduction of immunotherapy and targeted agents, there has been relatively little improvement in SCLC.
Patients generally are classified into limited- and extensive-stage disease, but platinum-based chemotherapy is almost always the standard first-line treatment. Unfortunately, most patients relapse within a year.
In this ReCAP, Dr Shadia Jalal, of Indiana University Melvin and Bren Simon Comprehensive Cancer Center, discusses second-line treatment options for SCLC patients who relapse after chemotherapy. She also discusses four subtypes of SCLC categorized on the basis of specific transcription regulators, which may offer the potential of targeted therapies for this patient population.
--
Shadia Jalal, MD, Associate Professor of Medicine, Physician, Indiana University Melvin and Bren Simon Comprehensive Cancer Center, Indianapolis, Indiana
Shadia Jalal, MD, has disclosed no relevant financial relationships.

Vaginal microbiota transfer may affect neurodevelopment in cesarean infants
Previous studies have shown that gut microbiota in infancy could affect neurodevelopment, and infants delivered by cesarean are not exposed to potentially helpful microbes acquired by infants during vaginal delivery, wrote Lepeng Zhou, MD, of Southern Medical University, Guangdong, China, and colleagues.
“Infants delivered by C-section start life with very different bacteria than those born vaginally,” corresponding author Jose Clemente, PhD, of Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Because this is the first time the newborn is exposed to microbes, we and others have hypothesized for some time that this ‘first encounter’ might be significant to shape the development of the baby,” he said.
“A few years ago, we demonstrated that it is possible to change the microbiome of C-section–delivered infants using an intervention that makes their microbiome more similar to that of a vaginally-delivered infant,” Dr. Clemente told this news organization. “In this study just published, we show that this procedure not only changes the microbiome of C-section infants, but it also modifies a health outcome (in this case, neurodevelopment). This is highly significant because it opens the way to reduce the risk that C-section infants have for certain conditions through a very simple microbial intervention,” he said.
‘Significantly higher’ ASQ-3 scores
In the current study, published in Cell Host & Microbe, the researchers examined the impact of vaginal microbiota transfer (VMT) on the neurodevelopment of cesarean-delivered infants. They randomized 35 women scheduled for cesarean delivery with a single infant to VMT and 41 to a control intervention of saline gauze for their infants immediately after delivery.
The primary outcome of infant neurodevelopment was assessed using the Ages and Stages Questionnaire (ASQ-3) score at 6 months. The researchers also collected fecal samples and assessed safety outcomes for the infants at 3, 7, 30, and 42 days after birth. The final analysis comprised 32 infants in the VMT group and 36 in the control group. The mean age of the mothers was 32 years; the mean gestational age of the infants was 39 weeks, but the difference was significant and slightly less in the VMT group compared with the controls (38.38 weeks vs. 39.13 weeks, P = .007). A group of 33 vaginally-delivered infants (VD) underwent ASQ-3 testing to serve as a reference group.
At 6 months, ASQ-3 scores were significantly higher (10.09%, P = .014) with VMT compared with controls, and the difference remained significant after adjustment for multiple factors including gestational age.
ASQ-3 total scores at 6 months were not significantly different between the VMT group and the VD reference group (mean difference of 8.84 VMT to VD, P = .346); scores between these groups also were similar at 3 months (mean difference of –1.48 VMT to VD, P = .900) and no significant differences appeared in ASQ-3 subdomains between these groups at either time period.
An examination of gut metabolites in stool showed significant differences in fecal metabolites and metabolic function, signs of gut microbiota maturation, the researchers noted.
“Interestingly, all the genera and metabolites that exhibited positive correlations with neurodevelopmental scores were upregulated in the VMT group, whereas the only negative correlation of Klebsiella was downregulated, indicating that VMT may impact neurodevelopment through the modulation of specific gut microbial genera and metabolites,” the researchers wrote.
No serious adverse events occurred in either group during the study period. Nine adverse events were reported; 4 in the VMT group and 5 in the control group. The most common AEs were mild skin disorders, including papules, pustules, and erythema.
The findings were limited by several factors including the potential for transfer not only of vaginal microbiota, but also vaginal metabolites, mycobiome, and virome, which blurs the potential mechanism of VMT, the researchers noted. Other limitations were the relatively short study period, small sample size, and cervical HPV screening within the past 5 years, not during pregnancy, they wrote.
However, the results suggest that VMT is safe, and may help improve the fecal microbiome in cesarean-delivered infants, and the long-term effects merit further studies in larger populations, they concluded.
Limitations and outlook
Dr. Clemente said in an interview that the researchers were “hopeful that the study would demonstrate a health benefit, as it does with some limitations.” The current study findings confirm some previous results showing that modification of the microbiomes of C-section infants is possible through a transfer of maternal vaginal microbes, he said.
“There is also an important aspect that was confirmed here: The lack of serious adverse events associated with the procedure, and the fact that transferring vaginal microbes did not increase the risk of adverse events compared to the control group or to vaginally-delivered infants. This is fundamental to establish that using rigorous exclusion criteria we can perform this procedure safely for infants and mothers,” he added.
“We are at very early stages yet to talk about clinical implications,” said Dr. Clemente. “This is one of the first studies to demonstrate a benefit to the transfer of microbes from mothers to infants, and as such it opens the way for future trials that confirm these findings. The clinical application is still in the future, but this is an important first step towards that goal.”
Interest in restoring gut microbiota to potentially benefit infants persists, but a recent study published in Frontiers and Cellular and Infection Microbiology contradicted the potential association between maternal vaginal microbiome and an infant’s gut microbiome based on an analysis of infant stool.
“There are many reasons why different studies might reach different conclusions: The experimental procedures, the analytical methods, the cohort under study,” Dr. Clemente said when asked to comment on the Frontiers study. “Further studies are needed to establish whether this procedure is equally effective under all conditions and whether health benefits are generalizable or specific to particular populations.”
Several research gaps remain, Dr. Clemente said. “First, neurodevelopment was measured through a questionnaire that captures various aspects such as communication, motor skills, or problem solving. While this is a standard way to establish that an infant is in the correct neurodevelopmental pathway, it is not a ‘hard’ measure of cellular or biochemical processes being impacted by the intervention. Some of our results suggest that there is a change in the metabolome of this infants, particularly an enrichment in GABA, a neurotransmitter, but the exact mechanisms by which the intervention is resulting in a health benefit still remains to be explored,” he said.
“We have an ongoing study here at Mount Sinai to test whether this microbial intervention can be effective in lowering the risk of developing food allergies in newborns who are at high risk, so that is another important future question: What other conditions could benefit from this approach,” said Dr. Clemente.
A third research goal, he added, is “determining what microbes precisely are responsible for the health benefits; this study uses a full microbial community to colonize infants. We show that this is effective and, importantly, that there were no significant adverse events in the treated infants,” he noted. “However, identifying what specific microbes are beneficial would further lower the risk of any potential side effects, while facilitating the development of drugs based on defined microbial consortia,” he said.
Safety and efficacy support further studies
“It is widely accepted that the gut microbiome of neonates varies based on mode of delivery,” Anna K. Knight, PhD, assistant professor of gynecology and obstetrics at Emory University, Atlanta, said in an interview.
“C-sections have been associated with increased risk of asthma and metabolic disease, and have been associated with differences in the development of the immune system,” said Dr. Knight, who was not involved in the study. “There have been small pilot studies examining the use of vaginal microbiome transplants to shift the gut microbiome of neonates born by C-section to be more like the gut microbiome of neonates born via vaginal delivery, but the safety and efficacy of this treatment has not been well established. This study examines both, while also evaluating potential changes in the metabolome and neurodevelopmental trajectories.”
The current study confirmed the impact of the neonatal gut microbe on neurodevelopmental outcomes during a sensitive period, said Dr. Knight. “The fact that these differences persisted at 6 months suggests that even if the microbiome composition between vaginally-delivered and preterm infants converged at 1-2 years old, there may be lasting impacts of mode of delivery,” she said.
“The results of this study suggest that vaginal microbiome transplant may be a safe and effective way to mitigate the negative impacts of C-section delivery on the neonatal gut microbiome, and may be protective for neurodevelopment,” she added.
Regarding the Frontiers in Medicine study, Dr. Knight noted that it examined a very different population, with Zhou and colleagues focusing on Chinese infants, while Dos Santos and colleagues focused on Canadian infants.
“There was also a substantial difference in sample size between the two studies, with Dos Santos and colleagues examining > 500 more infants,” she said. “Additionally, the two studies differed in the sequencing technology used, sample collection methods, and antibiotic exposure, which can all impact microbiome study results.”
Since the current study showed efficacy and safety of VMT in a small clinical trial, larger trials with more diverse participants are needed to further examine the impact of VMT, said Dr. Knight. “The risks of vaginal microbiome transplant in mothers with infections should also be considered, and the mechanisms by which the neonatal gut microbiome impacts neurodevelopment need further investigation,” she said.
The study was funded by the National Key R&D Program of China, the Canadian Institute of Health Research, the National Natural Science Foundation of China, the Clinical Research Startup Program of Southern Medical University, China, and the Top Talent Program of Foshan Women and Children Hospital, China. The researchers and Dr. Knight had no financial conflicts to disclose.
Previous studies have shown that gut microbiota in infancy could affect neurodevelopment, and infants delivered by cesarean are not exposed to potentially helpful microbes acquired by infants during vaginal delivery, wrote Lepeng Zhou, MD, of Southern Medical University, Guangdong, China, and colleagues.
“Infants delivered by C-section start life with very different bacteria than those born vaginally,” corresponding author Jose Clemente, PhD, of Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Because this is the first time the newborn is exposed to microbes, we and others have hypothesized for some time that this ‘first encounter’ might be significant to shape the development of the baby,” he said.
“A few years ago, we demonstrated that it is possible to change the microbiome of C-section–delivered infants using an intervention that makes their microbiome more similar to that of a vaginally-delivered infant,” Dr. Clemente told this news organization. “In this study just published, we show that this procedure not only changes the microbiome of C-section infants, but it also modifies a health outcome (in this case, neurodevelopment). This is highly significant because it opens the way to reduce the risk that C-section infants have for certain conditions through a very simple microbial intervention,” he said.
‘Significantly higher’ ASQ-3 scores
In the current study, published in Cell Host & Microbe, the researchers examined the impact of vaginal microbiota transfer (VMT) on the neurodevelopment of cesarean-delivered infants. They randomized 35 women scheduled for cesarean delivery with a single infant to VMT and 41 to a control intervention of saline gauze for their infants immediately after delivery.
The primary outcome of infant neurodevelopment was assessed using the Ages and Stages Questionnaire (ASQ-3) score at 6 months. The researchers also collected fecal samples and assessed safety outcomes for the infants at 3, 7, 30, and 42 days after birth. The final analysis comprised 32 infants in the VMT group and 36 in the control group. The mean age of the mothers was 32 years; the mean gestational age of the infants was 39 weeks, but the difference was significant and slightly less in the VMT group compared with the controls (38.38 weeks vs. 39.13 weeks, P = .007). A group of 33 vaginally-delivered infants (VD) underwent ASQ-3 testing to serve as a reference group.
At 6 months, ASQ-3 scores were significantly higher (10.09%, P = .014) with VMT compared with controls, and the difference remained significant after adjustment for multiple factors including gestational age.
ASQ-3 total scores at 6 months were not significantly different between the VMT group and the VD reference group (mean difference of 8.84 VMT to VD, P = .346); scores between these groups also were similar at 3 months (mean difference of –1.48 VMT to VD, P = .900) and no significant differences appeared in ASQ-3 subdomains between these groups at either time period.
An examination of gut metabolites in stool showed significant differences in fecal metabolites and metabolic function, signs of gut microbiota maturation, the researchers noted.
“Interestingly, all the genera and metabolites that exhibited positive correlations with neurodevelopmental scores were upregulated in the VMT group, whereas the only negative correlation of Klebsiella was downregulated, indicating that VMT may impact neurodevelopment through the modulation of specific gut microbial genera and metabolites,” the researchers wrote.
No serious adverse events occurred in either group during the study period. Nine adverse events were reported; 4 in the VMT group and 5 in the control group. The most common AEs were mild skin disorders, including papules, pustules, and erythema.
The findings were limited by several factors including the potential for transfer not only of vaginal microbiota, but also vaginal metabolites, mycobiome, and virome, which blurs the potential mechanism of VMT, the researchers noted. Other limitations were the relatively short study period, small sample size, and cervical HPV screening within the past 5 years, not during pregnancy, they wrote.
However, the results suggest that VMT is safe, and may help improve the fecal microbiome in cesarean-delivered infants, and the long-term effects merit further studies in larger populations, they concluded.
Limitations and outlook
Dr. Clemente said in an interview that the researchers were “hopeful that the study would demonstrate a health benefit, as it does with some limitations.” The current study findings confirm some previous results showing that modification of the microbiomes of C-section infants is possible through a transfer of maternal vaginal microbes, he said.
“There is also an important aspect that was confirmed here: The lack of serious adverse events associated with the procedure, and the fact that transferring vaginal microbes did not increase the risk of adverse events compared to the control group or to vaginally-delivered infants. This is fundamental to establish that using rigorous exclusion criteria we can perform this procedure safely for infants and mothers,” he added.
“We are at very early stages yet to talk about clinical implications,” said Dr. Clemente. “This is one of the first studies to demonstrate a benefit to the transfer of microbes from mothers to infants, and as such it opens the way for future trials that confirm these findings. The clinical application is still in the future, but this is an important first step towards that goal.”
Interest in restoring gut microbiota to potentially benefit infants persists, but a recent study published in Frontiers and Cellular and Infection Microbiology contradicted the potential association between maternal vaginal microbiome and an infant’s gut microbiome based on an analysis of infant stool.
“There are many reasons why different studies might reach different conclusions: The experimental procedures, the analytical methods, the cohort under study,” Dr. Clemente said when asked to comment on the Frontiers study. “Further studies are needed to establish whether this procedure is equally effective under all conditions and whether health benefits are generalizable or specific to particular populations.”
Several research gaps remain, Dr. Clemente said. “First, neurodevelopment was measured through a questionnaire that captures various aspects such as communication, motor skills, or problem solving. While this is a standard way to establish that an infant is in the correct neurodevelopmental pathway, it is not a ‘hard’ measure of cellular or biochemical processes being impacted by the intervention. Some of our results suggest that there is a change in the metabolome of this infants, particularly an enrichment in GABA, a neurotransmitter, but the exact mechanisms by which the intervention is resulting in a health benefit still remains to be explored,” he said.
“We have an ongoing study here at Mount Sinai to test whether this microbial intervention can be effective in lowering the risk of developing food allergies in newborns who are at high risk, so that is another important future question: What other conditions could benefit from this approach,” said Dr. Clemente.
A third research goal, he added, is “determining what microbes precisely are responsible for the health benefits; this study uses a full microbial community to colonize infants. We show that this is effective and, importantly, that there were no significant adverse events in the treated infants,” he noted. “However, identifying what specific microbes are beneficial would further lower the risk of any potential side effects, while facilitating the development of drugs based on defined microbial consortia,” he said.
Safety and efficacy support further studies
“It is widely accepted that the gut microbiome of neonates varies based on mode of delivery,” Anna K. Knight, PhD, assistant professor of gynecology and obstetrics at Emory University, Atlanta, said in an interview.
“C-sections have been associated with increased risk of asthma and metabolic disease, and have been associated with differences in the development of the immune system,” said Dr. Knight, who was not involved in the study. “There have been small pilot studies examining the use of vaginal microbiome transplants to shift the gut microbiome of neonates born by C-section to be more like the gut microbiome of neonates born via vaginal delivery, but the safety and efficacy of this treatment has not been well established. This study examines both, while also evaluating potential changes in the metabolome and neurodevelopmental trajectories.”
The current study confirmed the impact of the neonatal gut microbe on neurodevelopmental outcomes during a sensitive period, said Dr. Knight. “The fact that these differences persisted at 6 months suggests that even if the microbiome composition between vaginally-delivered and preterm infants converged at 1-2 years old, there may be lasting impacts of mode of delivery,” she said.
“The results of this study suggest that vaginal microbiome transplant may be a safe and effective way to mitigate the negative impacts of C-section delivery on the neonatal gut microbiome, and may be protective for neurodevelopment,” she added.
Regarding the Frontiers in Medicine study, Dr. Knight noted that it examined a very different population, with Zhou and colleagues focusing on Chinese infants, while Dos Santos and colleagues focused on Canadian infants.
“There was also a substantial difference in sample size between the two studies, with Dos Santos and colleagues examining > 500 more infants,” she said. “Additionally, the two studies differed in the sequencing technology used, sample collection methods, and antibiotic exposure, which can all impact microbiome study results.”
Since the current study showed efficacy and safety of VMT in a small clinical trial, larger trials with more diverse participants are needed to further examine the impact of VMT, said Dr. Knight. “The risks of vaginal microbiome transplant in mothers with infections should also be considered, and the mechanisms by which the neonatal gut microbiome impacts neurodevelopment need further investigation,” she said.
The study was funded by the National Key R&D Program of China, the Canadian Institute of Health Research, the National Natural Science Foundation of China, the Clinical Research Startup Program of Southern Medical University, China, and the Top Talent Program of Foshan Women and Children Hospital, China. The researchers and Dr. Knight had no financial conflicts to disclose.
Previous studies have shown that gut microbiota in infancy could affect neurodevelopment, and infants delivered by cesarean are not exposed to potentially helpful microbes acquired by infants during vaginal delivery, wrote Lepeng Zhou, MD, of Southern Medical University, Guangdong, China, and colleagues.
“Infants delivered by C-section start life with very different bacteria than those born vaginally,” corresponding author Jose Clemente, PhD, of Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Because this is the first time the newborn is exposed to microbes, we and others have hypothesized for some time that this ‘first encounter’ might be significant to shape the development of the baby,” he said.
“A few years ago, we demonstrated that it is possible to change the microbiome of C-section–delivered infants using an intervention that makes their microbiome more similar to that of a vaginally-delivered infant,” Dr. Clemente told this news organization. “In this study just published, we show that this procedure not only changes the microbiome of C-section infants, but it also modifies a health outcome (in this case, neurodevelopment). This is highly significant because it opens the way to reduce the risk that C-section infants have for certain conditions through a very simple microbial intervention,” he said.
‘Significantly higher’ ASQ-3 scores
In the current study, published in Cell Host & Microbe, the researchers examined the impact of vaginal microbiota transfer (VMT) on the neurodevelopment of cesarean-delivered infants. They randomized 35 women scheduled for cesarean delivery with a single infant to VMT and 41 to a control intervention of saline gauze for their infants immediately after delivery.
The primary outcome of infant neurodevelopment was assessed using the Ages and Stages Questionnaire (ASQ-3) score at 6 months. The researchers also collected fecal samples and assessed safety outcomes for the infants at 3, 7, 30, and 42 days after birth. The final analysis comprised 32 infants in the VMT group and 36 in the control group. The mean age of the mothers was 32 years; the mean gestational age of the infants was 39 weeks, but the difference was significant and slightly less in the VMT group compared with the controls (38.38 weeks vs. 39.13 weeks, P = .007). A group of 33 vaginally-delivered infants (VD) underwent ASQ-3 testing to serve as a reference group.
At 6 months, ASQ-3 scores were significantly higher (10.09%, P = .014) with VMT compared with controls, and the difference remained significant after adjustment for multiple factors including gestational age.
ASQ-3 total scores at 6 months were not significantly different between the VMT group and the VD reference group (mean difference of 8.84 VMT to VD, P = .346); scores between these groups also were similar at 3 months (mean difference of –1.48 VMT to VD, P = .900) and no significant differences appeared in ASQ-3 subdomains between these groups at either time period.
An examination of gut metabolites in stool showed significant differences in fecal metabolites and metabolic function, signs of gut microbiota maturation, the researchers noted.
“Interestingly, all the genera and metabolites that exhibited positive correlations with neurodevelopmental scores were upregulated in the VMT group, whereas the only negative correlation of Klebsiella was downregulated, indicating that VMT may impact neurodevelopment through the modulation of specific gut microbial genera and metabolites,” the researchers wrote.
No serious adverse events occurred in either group during the study period. Nine adverse events were reported; 4 in the VMT group and 5 in the control group. The most common AEs were mild skin disorders, including papules, pustules, and erythema.
The findings were limited by several factors including the potential for transfer not only of vaginal microbiota, but also vaginal metabolites, mycobiome, and virome, which blurs the potential mechanism of VMT, the researchers noted. Other limitations were the relatively short study period, small sample size, and cervical HPV screening within the past 5 years, not during pregnancy, they wrote.
However, the results suggest that VMT is safe, and may help improve the fecal microbiome in cesarean-delivered infants, and the long-term effects merit further studies in larger populations, they concluded.
Limitations and outlook
Dr. Clemente said in an interview that the researchers were “hopeful that the study would demonstrate a health benefit, as it does with some limitations.” The current study findings confirm some previous results showing that modification of the microbiomes of C-section infants is possible through a transfer of maternal vaginal microbes, he said.
“There is also an important aspect that was confirmed here: The lack of serious adverse events associated with the procedure, and the fact that transferring vaginal microbes did not increase the risk of adverse events compared to the control group or to vaginally-delivered infants. This is fundamental to establish that using rigorous exclusion criteria we can perform this procedure safely for infants and mothers,” he added.
“We are at very early stages yet to talk about clinical implications,” said Dr. Clemente. “This is one of the first studies to demonstrate a benefit to the transfer of microbes from mothers to infants, and as such it opens the way for future trials that confirm these findings. The clinical application is still in the future, but this is an important first step towards that goal.”
Interest in restoring gut microbiota to potentially benefit infants persists, but a recent study published in Frontiers and Cellular and Infection Microbiology contradicted the potential association between maternal vaginal microbiome and an infant’s gut microbiome based on an analysis of infant stool.
“There are many reasons why different studies might reach different conclusions: The experimental procedures, the analytical methods, the cohort under study,” Dr. Clemente said when asked to comment on the Frontiers study. “Further studies are needed to establish whether this procedure is equally effective under all conditions and whether health benefits are generalizable or specific to particular populations.”
Several research gaps remain, Dr. Clemente said. “First, neurodevelopment was measured through a questionnaire that captures various aspects such as communication, motor skills, or problem solving. While this is a standard way to establish that an infant is in the correct neurodevelopmental pathway, it is not a ‘hard’ measure of cellular or biochemical processes being impacted by the intervention. Some of our results suggest that there is a change in the metabolome of this infants, particularly an enrichment in GABA, a neurotransmitter, but the exact mechanisms by which the intervention is resulting in a health benefit still remains to be explored,” he said.
“We have an ongoing study here at Mount Sinai to test whether this microbial intervention can be effective in lowering the risk of developing food allergies in newborns who are at high risk, so that is another important future question: What other conditions could benefit from this approach,” said Dr. Clemente.
A third research goal, he added, is “determining what microbes precisely are responsible for the health benefits; this study uses a full microbial community to colonize infants. We show that this is effective and, importantly, that there were no significant adverse events in the treated infants,” he noted. “However, identifying what specific microbes are beneficial would further lower the risk of any potential side effects, while facilitating the development of drugs based on defined microbial consortia,” he said.
Safety and efficacy support further studies
“It is widely accepted that the gut microbiome of neonates varies based on mode of delivery,” Anna K. Knight, PhD, assistant professor of gynecology and obstetrics at Emory University, Atlanta, said in an interview.
“C-sections have been associated with increased risk of asthma and metabolic disease, and have been associated with differences in the development of the immune system,” said Dr. Knight, who was not involved in the study. “There have been small pilot studies examining the use of vaginal microbiome transplants to shift the gut microbiome of neonates born by C-section to be more like the gut microbiome of neonates born via vaginal delivery, but the safety and efficacy of this treatment has not been well established. This study examines both, while also evaluating potential changes in the metabolome and neurodevelopmental trajectories.”
The current study confirmed the impact of the neonatal gut microbe on neurodevelopmental outcomes during a sensitive period, said Dr. Knight. “The fact that these differences persisted at 6 months suggests that even if the microbiome composition between vaginally-delivered and preterm infants converged at 1-2 years old, there may be lasting impacts of mode of delivery,” she said.
“The results of this study suggest that vaginal microbiome transplant may be a safe and effective way to mitigate the negative impacts of C-section delivery on the neonatal gut microbiome, and may be protective for neurodevelopment,” she added.
Regarding the Frontiers in Medicine study, Dr. Knight noted that it examined a very different population, with Zhou and colleagues focusing on Chinese infants, while Dos Santos and colleagues focused on Canadian infants.
“There was also a substantial difference in sample size between the two studies, with Dos Santos and colleagues examining > 500 more infants,” she said. “Additionally, the two studies differed in the sequencing technology used, sample collection methods, and antibiotic exposure, which can all impact microbiome study results.”
Since the current study showed efficacy and safety of VMT in a small clinical trial, larger trials with more diverse participants are needed to further examine the impact of VMT, said Dr. Knight. “The risks of vaginal microbiome transplant in mothers with infections should also be considered, and the mechanisms by which the neonatal gut microbiome impacts neurodevelopment need further investigation,” she said.
The study was funded by the National Key R&D Program of China, the Canadian Institute of Health Research, the National Natural Science Foundation of China, the Clinical Research Startup Program of Southern Medical University, China, and the Top Talent Program of Foshan Women and Children Hospital, China. The researchers and Dr. Knight had no financial conflicts to disclose.
FROM CELL HOST & MICROBE
Malpractice lawsuits over denied abortion care may be on the horizon
Some experts predict those providers could soon face a new legal threat: medical malpractice lawsuits alleging they harmed patients by failing to provide timely, necessary abortion care.
“We will absolutely see medical malpractice cases emerge,” said Diana Nordlund, an emergency physician in Grand Rapids, Mich., and former malpractice defense attorney, who chairs the Medical-Legal Committee of the American College of Emergency Physicians. When physicians decide not to provide treatments widely accepted as the standard of care because of these new laws, “that’s perceived as substandard care and there is increased civil liability.”
To some physicians and malpractice attorneys, the question is when – not if – a pregnant patient will die from lack of care and set the stage for a big-dollar wrongful death claim. Abortion rights supporters said such a case could pressure doctors and hospitals to provide appropriate abortion care, counterbalancing their fears of running afoul of state abortion bans, many of which call for criminal prosecution and revocation of medical licenses as punishment for violations.
“If we want to encourage proper care, there has to be some sort of counter-risk to physicians and hospitals for refusing to provide care that should be legal,” said Greer Donley, an associate professor at the University of Pittsburgh school of law who studies the impact of abortion bans. “But most rational people would be more afraid of going to jail.”
Some supporters of abortion bans said they would welcome malpractice lawsuits. Providers are refusing to use the exceptions in some state laws that allow them to perform abortions to save a patient’s life or health, they said.
“It could help achieve our goal if it clarifies that the law did not contradict standard medical practice,” said John Seago, president of Texas Right to Life, referring to the state’s abortion ban.
A new KFF poll found that 59% of ob.gyns. practicing in states with gestational limits on abortion, and 61% of those in states with bans, are somewhat or very concerned about their legal risk when making decisions about the necessity of an abortion.
Some attorneys are exploring lawsuits on behalf of women who they said have been harmed by a state abortion ban. An attorney for Mylissa Farmer, a Missouri woman who was refused an abortion at two hospitals in August after her water broke about 18 weeks into her pregnancy, said she may sue for malpractice. Missouri’s abortion ban, which took effect last year, makes an exception for medical emergencies.
The federal government recently found that the two hospitals violated a federal emergency care law in denying Ms. Farmer an abortion, which experts said could strengthen a malpractice claim. One of the hospitals, Freeman Health System in Joplin, Mo., did not respond to a request for comment. The other, the University of Kansas Health System in Kansas City, said the care provided “was reviewed by the hospital and found to be in accordance with hospital policy,” according to a spokesperson, Jill Chadwick.
Ms. Farmer “experienced permanent physical and emotional damage,” said Michelle Banker, one of her lawyers at the National Women’s Law Center, who added that Ms. Farmer and her attorneys are “considering all our legal options.”
News reports and medical studies show that some women with pregnancy complications have suffered serious health consequences when doctors and hospitals did not provide once-routine abortion care.
Last month, researchers released a study identifying dozens of cases in 14 states in which physicians said deficiencies in care due to abortion restrictions led to preventable complications and hospitalizations, with some patients nearly dying.
“The patients were sent home and told to come back when they had signs of infection,” said Daniel Grossman, an ob.gyn. at the University of California, San Francisco, who led the study. “Many developed serious infections. And it’s clear many of these cases were very emotionally traumatic.”
He said though the researchers did not track patient outcomes, the lack of timely abortion care in such cases could result in severe health harms including loss of fertility, stroke, or heart attack.
“It’s just a matter of time before there will be a death that comes to light,” Dr. Grossman said.
Still, considering the conflict for doctors between medical ethics and personal risk, some stakeholders said patients may be reluctant to sue doctors and juries may balk at finding them liable.
“It’s a terrible position that providers are being put into, and I don’t think juries will blame the doctor unless it’s a super clear case,” said Morgan Murphy, a malpractice plaintiff’s attorney in Missouri.
She said her firm will not pursue malpractice cases based on abortion denials except in “pretty extreme” situations, such as when a patient dies. “Unless a mother is on her deathbed, it’s pretty hard to fault a provider who thinks if they provide treatment they’re going to be criminally liable or will lose their medical license.”
Another hurdle for malpractice cases is that state abortion bans could undermine the argument that abortion is the legal “standard of care,” meaning that it is a widely accepted and prescribed treatment for pregnancy complications such as miscarriage and for fatal fetal abnormalities.
“I absolutely see a breach of the standard of care in these cases,” said Maria A. Phillis, an ob.gyn. and former lawyer in Cleveland. “But if someone goes to trial in a malpractice case, it will come down to a battle of medical experts about whether it’s no longer the standard of care, and the jury would have to decide.”
An additional justification for physicians not to provide abortions is that medical liability insurers generally do not cover damages from criminal acts, which “puts the finger on the scales even more to not do anything,” Dr. Phillis said.
Stuart Grossman, a prominent malpractice plaintiff’s attorney in Florida, said he would be eager to take an abortion-denial case in which the woman suffered serious health or emotional injuries.
Unlike other states with abortion bans, Florida does not cap damage amounts for pain and suffering in malpractice cases, making it more financially viable to sue there.
Mr. Grossman cited the case of Deborah Dorbert, a Florida woman who reportedly was denied an abortion despite being told by her physicians at 24 weeks of pregnancy that her fetus, with no kidneys and underdeveloped lungs, had a fatal condition called Potter syndrome.
Her doctors and the hospital refused to end the pregnancy even though the state’s abortion ban has an exception for fatal fetal abnormalities. Months later, her baby died in his parents’ arms shortly after birth.
“You can see how she’s been devastated mentally,” Mr. Grossman said. “She has a wrongful death case that I’d take in a minute.” He said the couple could file a malpractice suit for Ms. Dorbert’s physical and emotional damages and a separate malpractice and wrongful death suit for the couple’s suffering over the infant’s death.
Failing to counsel patients about their options and connect them with providers willing to terminate a pregnancy is also possible grounds for a malpractice suit, attorneys said. Katie Watson, an associate professor at Northwestern University, Chicago’s school of medicine who has studied state abortion bans, said counseling and referral are not prohibited under these laws and that physicians have an ethical obligation to offer those services.
“I think breaching the obligation for counseling would make a strong malpractice lawsuit,” she said.
Nancy Davis said she received no counseling or referral assistance last July after her doctors at Woman’s Hospital in Baton Rouge, La., told her 10 weeks into her pregnancy that her fetus would not survive because it was missing the top of its skull, a fatal condition called acrania. She said they recommended that she terminate the pregnancy and she agreed.
Ms. Davis said her doctors then told her a hospital executive had denied permission for the procedure because of Louisiana’s abortion ban, even though the law has an exception for fatal fetal abnormalities. A hospital spokesperson declined to comment.
Ms. Davis, who has three children, contacted Planned Parenthood of Greater New York, which arranged for child care and a flight to New York. She had an abortion performed there in September.
“The whole situation has been mentally and physically draining, and my family and I are receiving counseling,” Ms. Davis said. “I’m still very angry at the hospital and the doctors. I feel like I’m owed compensation for the trauma and the heartbreak.”
She sought the counsel of Benjamin Crump, a prominent attorney known for pursuing high-profile cases like wrongful death lawsuits on behalf of the families of Trayvon Martin and George Floyd.
But Mr. Crump said that after studying Ms. Davis’ legal options, he decided a judge would likely dismiss a malpractice suit and that Ms. Davis could end up paying the defendants’ legal fees and costs.
“The doctor’s lawyers will say, ‘You can’t expect my client to break the law and go to prison for up to 25 years,’ ” Mr. Crump said. “Unless you change the law, there is no option for her to receive compensation.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
Some experts predict those providers could soon face a new legal threat: medical malpractice lawsuits alleging they harmed patients by failing to provide timely, necessary abortion care.
“We will absolutely see medical malpractice cases emerge,” said Diana Nordlund, an emergency physician in Grand Rapids, Mich., and former malpractice defense attorney, who chairs the Medical-Legal Committee of the American College of Emergency Physicians. When physicians decide not to provide treatments widely accepted as the standard of care because of these new laws, “that’s perceived as substandard care and there is increased civil liability.”
To some physicians and malpractice attorneys, the question is when – not if – a pregnant patient will die from lack of care and set the stage for a big-dollar wrongful death claim. Abortion rights supporters said such a case could pressure doctors and hospitals to provide appropriate abortion care, counterbalancing their fears of running afoul of state abortion bans, many of which call for criminal prosecution and revocation of medical licenses as punishment for violations.
“If we want to encourage proper care, there has to be some sort of counter-risk to physicians and hospitals for refusing to provide care that should be legal,” said Greer Donley, an associate professor at the University of Pittsburgh school of law who studies the impact of abortion bans. “But most rational people would be more afraid of going to jail.”
Some supporters of abortion bans said they would welcome malpractice lawsuits. Providers are refusing to use the exceptions in some state laws that allow them to perform abortions to save a patient’s life or health, they said.
“It could help achieve our goal if it clarifies that the law did not contradict standard medical practice,” said John Seago, president of Texas Right to Life, referring to the state’s abortion ban.
A new KFF poll found that 59% of ob.gyns. practicing in states with gestational limits on abortion, and 61% of those in states with bans, are somewhat or very concerned about their legal risk when making decisions about the necessity of an abortion.
Some attorneys are exploring lawsuits on behalf of women who they said have been harmed by a state abortion ban. An attorney for Mylissa Farmer, a Missouri woman who was refused an abortion at two hospitals in August after her water broke about 18 weeks into her pregnancy, said she may sue for malpractice. Missouri’s abortion ban, which took effect last year, makes an exception for medical emergencies.
The federal government recently found that the two hospitals violated a federal emergency care law in denying Ms. Farmer an abortion, which experts said could strengthen a malpractice claim. One of the hospitals, Freeman Health System in Joplin, Mo., did not respond to a request for comment. The other, the University of Kansas Health System in Kansas City, said the care provided “was reviewed by the hospital and found to be in accordance with hospital policy,” according to a spokesperson, Jill Chadwick.
Ms. Farmer “experienced permanent physical and emotional damage,” said Michelle Banker, one of her lawyers at the National Women’s Law Center, who added that Ms. Farmer and her attorneys are “considering all our legal options.”
News reports and medical studies show that some women with pregnancy complications have suffered serious health consequences when doctors and hospitals did not provide once-routine abortion care.
Last month, researchers released a study identifying dozens of cases in 14 states in which physicians said deficiencies in care due to abortion restrictions led to preventable complications and hospitalizations, with some patients nearly dying.
“The patients were sent home and told to come back when they had signs of infection,” said Daniel Grossman, an ob.gyn. at the University of California, San Francisco, who led the study. “Many developed serious infections. And it’s clear many of these cases were very emotionally traumatic.”
He said though the researchers did not track patient outcomes, the lack of timely abortion care in such cases could result in severe health harms including loss of fertility, stroke, or heart attack.
“It’s just a matter of time before there will be a death that comes to light,” Dr. Grossman said.
Still, considering the conflict for doctors between medical ethics and personal risk, some stakeholders said patients may be reluctant to sue doctors and juries may balk at finding them liable.
“It’s a terrible position that providers are being put into, and I don’t think juries will blame the doctor unless it’s a super clear case,” said Morgan Murphy, a malpractice plaintiff’s attorney in Missouri.
She said her firm will not pursue malpractice cases based on abortion denials except in “pretty extreme” situations, such as when a patient dies. “Unless a mother is on her deathbed, it’s pretty hard to fault a provider who thinks if they provide treatment they’re going to be criminally liable or will lose their medical license.”
Another hurdle for malpractice cases is that state abortion bans could undermine the argument that abortion is the legal “standard of care,” meaning that it is a widely accepted and prescribed treatment for pregnancy complications such as miscarriage and for fatal fetal abnormalities.
“I absolutely see a breach of the standard of care in these cases,” said Maria A. Phillis, an ob.gyn. and former lawyer in Cleveland. “But if someone goes to trial in a malpractice case, it will come down to a battle of medical experts about whether it’s no longer the standard of care, and the jury would have to decide.”
An additional justification for physicians not to provide abortions is that medical liability insurers generally do not cover damages from criminal acts, which “puts the finger on the scales even more to not do anything,” Dr. Phillis said.
Stuart Grossman, a prominent malpractice plaintiff’s attorney in Florida, said he would be eager to take an abortion-denial case in which the woman suffered serious health or emotional injuries.
Unlike other states with abortion bans, Florida does not cap damage amounts for pain and suffering in malpractice cases, making it more financially viable to sue there.
Mr. Grossman cited the case of Deborah Dorbert, a Florida woman who reportedly was denied an abortion despite being told by her physicians at 24 weeks of pregnancy that her fetus, with no kidneys and underdeveloped lungs, had a fatal condition called Potter syndrome.
Her doctors and the hospital refused to end the pregnancy even though the state’s abortion ban has an exception for fatal fetal abnormalities. Months later, her baby died in his parents’ arms shortly after birth.
“You can see how she’s been devastated mentally,” Mr. Grossman said. “She has a wrongful death case that I’d take in a minute.” He said the couple could file a malpractice suit for Ms. Dorbert’s physical and emotional damages and a separate malpractice and wrongful death suit for the couple’s suffering over the infant’s death.
Failing to counsel patients about their options and connect them with providers willing to terminate a pregnancy is also possible grounds for a malpractice suit, attorneys said. Katie Watson, an associate professor at Northwestern University, Chicago’s school of medicine who has studied state abortion bans, said counseling and referral are not prohibited under these laws and that physicians have an ethical obligation to offer those services.
“I think breaching the obligation for counseling would make a strong malpractice lawsuit,” she said.
Nancy Davis said she received no counseling or referral assistance last July after her doctors at Woman’s Hospital in Baton Rouge, La., told her 10 weeks into her pregnancy that her fetus would not survive because it was missing the top of its skull, a fatal condition called acrania. She said they recommended that she terminate the pregnancy and she agreed.
Ms. Davis said her doctors then told her a hospital executive had denied permission for the procedure because of Louisiana’s abortion ban, even though the law has an exception for fatal fetal abnormalities. A hospital spokesperson declined to comment.
Ms. Davis, who has three children, contacted Planned Parenthood of Greater New York, which arranged for child care and a flight to New York. She had an abortion performed there in September.
“The whole situation has been mentally and physically draining, and my family and I are receiving counseling,” Ms. Davis said. “I’m still very angry at the hospital and the doctors. I feel like I’m owed compensation for the trauma and the heartbreak.”
She sought the counsel of Benjamin Crump, a prominent attorney known for pursuing high-profile cases like wrongful death lawsuits on behalf of the families of Trayvon Martin and George Floyd.
But Mr. Crump said that after studying Ms. Davis’ legal options, he decided a judge would likely dismiss a malpractice suit and that Ms. Davis could end up paying the defendants’ legal fees and costs.
“The doctor’s lawyers will say, ‘You can’t expect my client to break the law and go to prison for up to 25 years,’ ” Mr. Crump said. “Unless you change the law, there is no option for her to receive compensation.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
Some experts predict those providers could soon face a new legal threat: medical malpractice lawsuits alleging they harmed patients by failing to provide timely, necessary abortion care.
“We will absolutely see medical malpractice cases emerge,” said Diana Nordlund, an emergency physician in Grand Rapids, Mich., and former malpractice defense attorney, who chairs the Medical-Legal Committee of the American College of Emergency Physicians. When physicians decide not to provide treatments widely accepted as the standard of care because of these new laws, “that’s perceived as substandard care and there is increased civil liability.”
To some physicians and malpractice attorneys, the question is when – not if – a pregnant patient will die from lack of care and set the stage for a big-dollar wrongful death claim. Abortion rights supporters said such a case could pressure doctors and hospitals to provide appropriate abortion care, counterbalancing their fears of running afoul of state abortion bans, many of which call for criminal prosecution and revocation of medical licenses as punishment for violations.
“If we want to encourage proper care, there has to be some sort of counter-risk to physicians and hospitals for refusing to provide care that should be legal,” said Greer Donley, an associate professor at the University of Pittsburgh school of law who studies the impact of abortion bans. “But most rational people would be more afraid of going to jail.”
Some supporters of abortion bans said they would welcome malpractice lawsuits. Providers are refusing to use the exceptions in some state laws that allow them to perform abortions to save a patient’s life or health, they said.
“It could help achieve our goal if it clarifies that the law did not contradict standard medical practice,” said John Seago, president of Texas Right to Life, referring to the state’s abortion ban.
A new KFF poll found that 59% of ob.gyns. practicing in states with gestational limits on abortion, and 61% of those in states with bans, are somewhat or very concerned about their legal risk when making decisions about the necessity of an abortion.
Some attorneys are exploring lawsuits on behalf of women who they said have been harmed by a state abortion ban. An attorney for Mylissa Farmer, a Missouri woman who was refused an abortion at two hospitals in August after her water broke about 18 weeks into her pregnancy, said she may sue for malpractice. Missouri’s abortion ban, which took effect last year, makes an exception for medical emergencies.
The federal government recently found that the two hospitals violated a federal emergency care law in denying Ms. Farmer an abortion, which experts said could strengthen a malpractice claim. One of the hospitals, Freeman Health System in Joplin, Mo., did not respond to a request for comment. The other, the University of Kansas Health System in Kansas City, said the care provided “was reviewed by the hospital and found to be in accordance with hospital policy,” according to a spokesperson, Jill Chadwick.
Ms. Farmer “experienced permanent physical and emotional damage,” said Michelle Banker, one of her lawyers at the National Women’s Law Center, who added that Ms. Farmer and her attorneys are “considering all our legal options.”
News reports and medical studies show that some women with pregnancy complications have suffered serious health consequences when doctors and hospitals did not provide once-routine abortion care.
Last month, researchers released a study identifying dozens of cases in 14 states in which physicians said deficiencies in care due to abortion restrictions led to preventable complications and hospitalizations, with some patients nearly dying.
“The patients were sent home and told to come back when they had signs of infection,” said Daniel Grossman, an ob.gyn. at the University of California, San Francisco, who led the study. “Many developed serious infections. And it’s clear many of these cases were very emotionally traumatic.”
He said though the researchers did not track patient outcomes, the lack of timely abortion care in such cases could result in severe health harms including loss of fertility, stroke, or heart attack.
“It’s just a matter of time before there will be a death that comes to light,” Dr. Grossman said.
Still, considering the conflict for doctors between medical ethics and personal risk, some stakeholders said patients may be reluctant to sue doctors and juries may balk at finding them liable.
“It’s a terrible position that providers are being put into, and I don’t think juries will blame the doctor unless it’s a super clear case,” said Morgan Murphy, a malpractice plaintiff’s attorney in Missouri.
She said her firm will not pursue malpractice cases based on abortion denials except in “pretty extreme” situations, such as when a patient dies. “Unless a mother is on her deathbed, it’s pretty hard to fault a provider who thinks if they provide treatment they’re going to be criminally liable or will lose their medical license.”
Another hurdle for malpractice cases is that state abortion bans could undermine the argument that abortion is the legal “standard of care,” meaning that it is a widely accepted and prescribed treatment for pregnancy complications such as miscarriage and for fatal fetal abnormalities.
“I absolutely see a breach of the standard of care in these cases,” said Maria A. Phillis, an ob.gyn. and former lawyer in Cleveland. “But if someone goes to trial in a malpractice case, it will come down to a battle of medical experts about whether it’s no longer the standard of care, and the jury would have to decide.”
An additional justification for physicians not to provide abortions is that medical liability insurers generally do not cover damages from criminal acts, which “puts the finger on the scales even more to not do anything,” Dr. Phillis said.
Stuart Grossman, a prominent malpractice plaintiff’s attorney in Florida, said he would be eager to take an abortion-denial case in which the woman suffered serious health or emotional injuries.
Unlike other states with abortion bans, Florida does not cap damage amounts for pain and suffering in malpractice cases, making it more financially viable to sue there.
Mr. Grossman cited the case of Deborah Dorbert, a Florida woman who reportedly was denied an abortion despite being told by her physicians at 24 weeks of pregnancy that her fetus, with no kidneys and underdeveloped lungs, had a fatal condition called Potter syndrome.
Her doctors and the hospital refused to end the pregnancy even though the state’s abortion ban has an exception for fatal fetal abnormalities. Months later, her baby died in his parents’ arms shortly after birth.
“You can see how she’s been devastated mentally,” Mr. Grossman said. “She has a wrongful death case that I’d take in a minute.” He said the couple could file a malpractice suit for Ms. Dorbert’s physical and emotional damages and a separate malpractice and wrongful death suit for the couple’s suffering over the infant’s death.
Failing to counsel patients about their options and connect them with providers willing to terminate a pregnancy is also possible grounds for a malpractice suit, attorneys said. Katie Watson, an associate professor at Northwestern University, Chicago’s school of medicine who has studied state abortion bans, said counseling and referral are not prohibited under these laws and that physicians have an ethical obligation to offer those services.
“I think breaching the obligation for counseling would make a strong malpractice lawsuit,” she said.
Nancy Davis said she received no counseling or referral assistance last July after her doctors at Woman’s Hospital in Baton Rouge, La., told her 10 weeks into her pregnancy that her fetus would not survive because it was missing the top of its skull, a fatal condition called acrania. She said they recommended that she terminate the pregnancy and she agreed.
Ms. Davis said her doctors then told her a hospital executive had denied permission for the procedure because of Louisiana’s abortion ban, even though the law has an exception for fatal fetal abnormalities. A hospital spokesperson declined to comment.
Ms. Davis, who has three children, contacted Planned Parenthood of Greater New York, which arranged for child care and a flight to New York. She had an abortion performed there in September.
“The whole situation has been mentally and physically draining, and my family and I are receiving counseling,” Ms. Davis said. “I’m still very angry at the hospital and the doctors. I feel like I’m owed compensation for the trauma and the heartbreak.”
She sought the counsel of Benjamin Crump, a prominent attorney known for pursuing high-profile cases like wrongful death lawsuits on behalf of the families of Trayvon Martin and George Floyd.
But Mr. Crump said that after studying Ms. Davis’ legal options, he decided a judge would likely dismiss a malpractice suit and that Ms. Davis could end up paying the defendants’ legal fees and costs.
“The doctor’s lawyers will say, ‘You can’t expect my client to break the law and go to prison for up to 25 years,’ ” Mr. Crump said. “Unless you change the law, there is no option for her to receive compensation.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – an independent source of health policy research, polling, and journalism. Learn more about KFF.
Progress in Management of Advanced Acute Lymphocytic Leukemia in Children
Incidence peaks in children aged 1-4 years, decreasing thereafter. Cases are highest among Native American/Alaskan Native and Hispanic children, and higher in White than Black children.4 ALL is seen more in patients with certain inherited conditions, including Down syndrome, ataxia telangiectasia, neurofibromatosis type 1, and Bloom syndrome.1
Treatment advances have improved remission rates and outcomes for patients. However, relapse is still a leading cause of death for patients of all ages.6 Prompt diagnosis and care are important to optimize outcomes, as treatment delay is associated with poorer survival.7
Pathophysiology
In ALL, abnormal, immature lymphocytes and progenitor B cells/T cells proliferate uncontrollably and eventually replace healthy cells in bone marrow and the lymphatic system. The loss of healthy cells leads to classic symptoms of cytopenia, splenomegaly, and hepatomegaly.1 B cells and T cells are descended from lymphoid stem cells (and are transformed by germline or somatic mutation into pathogenic cells, leading to symptom development and bone marrow dysfunction. Most pediatric patients have extensive bone marrow involvement at diagnosis, with > 25% blast cells in marrow (defined as M3 disease).4
Presentation
Patients usually present with signs and symptoms that are related to disease-associated anemia, thrombocytopenia, or neutropenia; these signs and symptoms may include fatigue or weakness, pale skin, bleeding or bruising easily, fever or infection, joint or extremity pain, B-cell symptoms such as night sweats or unintentional weight loss, and splenomegaly or hepatomegaly. Central nervous system (CNS) symptoms can include stroke-like symptoms due to leukemic cell invasion of CNS vasculature or neuropathies related to increased intracranial pressure. Sometimes, children may present with no symptoms other than joint or extremity pain.1,3,8
Classification
ALL is classified by whether it derives from B-cell or T-cell progenitor cells and, within these, by typical genetic alterations (Table 1).3,9-15 Some cytogenetics are associated with risk assessment as well. Well-identified B-ALL subtypes include Philadelphia (Ph) chromosome-positive, hyper- and hypodiploidy, and KMT2A rearranged, while newer classifications include Ph-like ALL and B-lymphoblastic leukemia with iAMP21. Provisional T-ALL subtypes include early T-cell precursor lymphoblastic leukemia and natural killer cell lymphoblastic leukemia.3
B-cell lineage is present in 88% of pediatric and 75%-80% of adult disease. T-ALL is found in about 12% of pediatric patients and 25% of adults.3,8 Familial syndromes associated with ALL are present in about 4% of pediatric patients, including autosomal dominant germline mutations in RUNX1 (T-cell ALL), ETV6 (B-ALL), PAX5 (B-ALL), IKZF1 (B-ALL and T-ALL), and TP53 (low-hypodiploid ALL).3 If a known-familial genotype is identified, families should be referred for genetic counseling and further testing if needed. If germline mutation is suspected, early identification is important; hereditary ALL can influence treatment choice and use of allogeneic transplantation or radiation.3
A third classification crucial to guiding treatment is Ph-positive vs Ph-negative or Ph-like, the latter strongly associated with abnormal B-cell development due to deletions in related genes.3,16 About 3% to 5% of pediatric patients and 25% of adults have Ph-positive ALL.17 The remission failure rate among pediatric patients treated with chemotherapy was 11% in one study, vs 2%-3% among patients with Ph-negative ALL.10
Diagnosis and Risk Stratification
Diagnosis is based on presentation and molecular features, requiring demonstration of ≥ 20% lymphoblasts in bone marrow biopsy or aspirate or ≥ 1,000 circulating lymphoblasts/mL in peripheral blood. Testing can include immunophenotyping using flow cytometry, molecular characterization of baseline leukemic clone, morphology using hematoxylin and eosin staining and Wright/Giemsa staining, and karyotyping.1,3 CNS involvement is assessed using a lumbar spinal tap.1
Risk stratification is based on molecular features (eg, high- and low-risk mutations, Table 1),3,9-15 which are assessed using fluorescence in-situ hybridization, broad-panel next-generation sequencing, and reverse-transcriptase polymerase chain reaction of bone marrow or peripheral blood.3 Other risk factors include age, CNS involvement, white blood cell (WBC) count, and response to initial induction or consolidation therapy.3
Pediatric patients are assigned standard or high risk based on factors identified by the Children’s Oncology Group and National Comprehensive Cancer Network (NCCN). Patients
aged 1 to < 10 years with WBC < 50 × 109/L are considered standard risk, and all others are considered high risk. Patients with ALL before age 1 have very high risk. All pediatric patients with T-ALL are considered high risk.3 Ph-positive, Ph-like, hypoploidy, failure to achieve remission with induction, and extramedullary disease are high-risk factors as well, whereas hyperploidy and certain mutations convey low risk.3
Newer treatment strategies for initial ALL diagnosis include targeted therapies. One goal of targeted therapy is avoidance of long-term toxicity, leading to improved survival outcomes. Well-studied targeted therapies include the tyrosine kinase inhibitors used in first-line and subsequent treatment of Ph-positive ALL.3
Treatment Options in Relapsed/Refractory ALL
The initial treatment goal is complete remission (CR) defined as minimal residual disease (MRD) < 0.01% on flow cytometry (Table 2).3 Prognosis is dependent on time and location of relapse. Early relapse (< 18 months from diagnosis) predicts poor survival. Relapse in bone marrow is associated with poorer prognosis than relapse in CNS.11-18 Where possible, consolidation with allogeneic hematopoietic cell transplantation improves survival for patients with early relapse.6 Three approaches have advanced treatment options for relapsed/refractory (R/R) B-ALL, all based around common cell markers seen in B-ALL.
The CD22-directed antibody-drug conjugate inotuzumab ozogamicin is approved for adults with R/R B-ALL. In clinical trials, a higher percentage of patients had results below the MRD threshold, and longer progression-free survival and OS compared with standard care.19,20
Blinatumomab is a bispecific T-cell engager that binds to CD19 on the surface of B-ALL cells and to CD3 on T cells to trigger apoptosis.21 It was first approved for R/R ALL in adults or children, and is also now approved for treatment in remission with MRD ≥ 0.1%. Patients must demonstrate CD19-positive disease to qualify.15-22 For R/R ALL, blinatumomab improves OS and CR rates compared with standard chemotherapy.23
The use of CAR T-cell therapies has expanded greatly with increasing knowledge about their efficacy and safety. In R/R ALL, tisagenlecleucel (tisa-gen) is approved for treatment of patients aged ≤ 25 years, and brexucabtagene autoleucel (brexucel) is approved for treatment of adults.3,24,25 Patients undergoing the CAR T-cell process have apheresis to collect T cells, which are then manufactured before being reinfused into the patient. Depending on local capabilities, the time between T-cell harvest and reinfusion can extend to weeks.3,26,27 Cytoreduction with CAR T-cell therapy can allow previously ineligible patients (due to bulky disease) to undergo transplant. Patients treated in key clinical trials with tisa-gen or brexu-cel achieved high overall remission rates and improved event-free survival and OS rates compared with historical experience.25,28,29 Important toxicities with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, which can develop rapidly. NCCN recommends hospitalizing patients at the first sign of either adverse event. Patients can be managed with tocilizumab or steroids for low-grade CRS or steroids for neurotoxicity. The Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and NCCN have guidelines on management of toxicities related to CAR T-cell therapy as well as management of symptoms and other adverse effects of CRS.5,23,24
Programs also incorporate telemedicine for symptom monitoring and follow-up.32-34 Centers providing CAR T-cell therapy must have a certified Risk Evaluation and Mitigation Strategy (REMS), which ensures adherence to specific guidelines for administration, adverse event management, and patient education.35,36 Overcoming technical, social, and financial barriers to CAR T-cell therapy is an ongoing challenge of great interest.37
R/R T-Cell Precursor ALL
Patients with R/R T-ALL have poor prognosis, partly due to limited treatment options. Nelarabine, a nucleoside analog, is the only approved treatment for R/R T-ALL, but has increasingly been used in first-line therapy added to multiagent chemotherapy as a consolidation and maintenance approach to pediatric disease.3,38,39 Four-year DSF in pediatric patients with newly diagnosed T-ALL undergoing treatment incorporating nelarabine was 88.9%.39 Treatment is associated with grade ≥ 3 neurotoxicity in > 10% of patients, and can include CNS toxicity as well as neuropathy.3
In a recently completed phase 2 trial (NCT03384654), daratumumab was added to standard chemotherapy (vincristine, prednisone, PEG-asparaginase, doxorubicin) for R/R T-ALL in pediatric (ages 1-17 years) and young adult patients (age ≥ 18 years).40 Among 24 pediatric patients, CR was 41.7% and overall response rate (ORR; ORR = CR + CRi) was 83% after 1 cycle of treatment. Ten (41.7%) pediatric patients achieved MRD-negative status as well. ORR was 60% in the 5 older patients. All pediatric patients had at least 1 grade ≥ 3 toxicity, but none of the adverse events led to discontinuation.40
Success in achieving MRD-negative responses in patients treated for R/R ALL has increased interest in using targeted therapies for newly diagnosed patients. Recommended treatment approaches are summarized in Table 3.3
Long-Term Follow-Up and Survivorship
A study of > 500 pediatric patients followed for an average 23 years reassuringly found low prevalence of adverse outcomes related to disease or treatment. Major adverse outcomes such as death due to late relapse; secondary malignancy; or development of osteoporosis, cataracts, and diminished functional status were infrequent.41 Most prevalent were growth effects (short stature or growth hormone insufficiency), likely related to certain treatment approaches.41 Guidelines for long-term follow-up of pediatric patients are available from the Children’s Oncology Group.42
A 2017 systematic review concluded that the quality of life for survivors is diminished upon treatment, and persistently over time for some patients.43 In contrast, a 2022 comparison of long-term survivors (median 20.5 years since diagnosis) of pediatric ALL with healthy controls found that survivors had better quality of life in some domains, including general health, vitality, and mental health.44 Smaller percentages of survivors rated themselves happiest about sleep quality, absence of pain, and physical abilities.44
As therapy patterns and options evolve, continued follow-up is important to ensure patients derive optimal benefit from treatment and post-treatment life.
- Puckett Y, Chan O. Acute lymphocytic leukemia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated June 27, 2022. Accessed April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Cancer facts & figures 2023. American Cancer Society. 2023. Accessed April 10, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia. Version 1.2022. April 4, 2022. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Childhood acute lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 16, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities. Version 1.2023. March 10, 2023. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- DuVall AS, Sheade J, Anderson D, et al. Updates in the management of relapsed and refractory acute lymphoplastic leukemia: an urgent plea for new treatments is being answered! JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
- Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204-209. doi:10.1016/j.leukres.2013.11.014
- Acute adult lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 24, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al. Toward a NOTCH1/FBXW7/RAS/PTEN–based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2013;31(34):4333-4342. doi:10.1200/JCO.2012.48.5292
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966-1973. doi:10.1200/JCO.2011.39.7661
- Gao C, Liu SG, Zhang RD, et al. NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol. 2014;166(2):221-228. doi:10.1111/bjh.12866
- Yang YL, Hsiao CC, Chen HY, et al. Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(6):846-851. doi:10.1002/pbc.24021
- Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816-3823. doi:10.1200/JCO.2010.28.3390
- Bandapalli OR, Zimmermann M, Kox C, et al. NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica. 2013;98(6):928-936. doi:10.3324/haematol.2012.073585
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(37):59260-59272. doi:10.18632/oncotarget.10610
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. doi:10.1016/S1470-2045(08)70339-5
- Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi:10.1200/JCO.2010.30.1325
- Nguyen K, Devidas M, Cheng SC, et al.; Children’s Oncology Group. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142-2150. doi:10.1038/leu.2008.251
- Besponsa. Prescribing information. Wyeth Pharmaceuticals Inc; 2017. BESPONSA® (inotuzumab ozogamicin) Dosing & Administration |Safety Info (pfizerpro.com)
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. doi:10.1056/NEJMoa1509277
- Lv M, Liu Y, Liu W, Xing Y, Zhang S. Immunotherapy for pediatric acute lymphoblastic leukemia: recent advances and future perspectives. Front Immunol. 2022;13:921894. doi:10.3389/fimmu.2022.921894
- Blincyto. Prescribing information. Amgen; 2022. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/NEJMoa1609783
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
- Bhaskar ST, Dholaria BR, Singsayadeth S, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3(suppl 1):39-45. doi:10.1002/jha2.335
- Granroth G, Rosenthal A, McCallen M, et al. Supportive care for patients with lymphoma
undergoing CAR-T-cell therapy: the advanced practice provider’s perspective. Curr Oncol Rep. 2022;24(12):1863-1872. doi:10.1007/s11912-022-01330-z - Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphocytic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO.22.00642
- Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi:10.1186/s13045-022-01379-0
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/JCO.21.01992
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transpl. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
- LeBar K, Murawski S, Umayam S, Quinn V. The role of advanced practice providers and telemedicine in reinventing care: the transition of a CAR T-cell transplantation program to the outpatient setting. J Adv Pract Oncol. 2020;11(7):757-763. doi:10.6004/jadpro.2020.11.7.8
- Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy for aggressive B-cell lymphomas and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
- Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; 2022. https://www.fda.gov/media/107296/download
- Tecartus. Prescribing information. Kite Pharma, Inc; 2021. https://www.fda.gov/media/140409/download
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18(12):800-807. doi:10.1200/OP.22.00315
- Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135(3):159-166. doi:10.1182/blood.2019001557
- Summers RJ, Teachey DT. SOHO state of the art updates and next questions: novel approaches to pediatric T-cell ALL and T-lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(10):718-725. doi:10.1016/j.clml.2022.07.010
- Hogan LE, Bhatla T, Teachey DT, et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16 suppl):Abstract 10001. doi:10.1200/JCO.2022.40.16_suppl.10001
- Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Oncol. 2014;15(8):841-851. doi:10.1016/S1470-2045(14)70265-7
- Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group. October 2018. Accessed April 10, 2023. http://www.survivorshipguidelines.org
- Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26489
- Chantziara S, Musoro J, Rowsell AC, et al; European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QLG) and Children’s Leukemia Group (CLG). Quality of life of long-term childhood acute lymphoblastic leukemia survivors: comparison with healthy controls. Psychooncology. 2022;31(12):2159-2168. doi:10.1002/pon.6060
Incidence peaks in children aged 1-4 years, decreasing thereafter. Cases are highest among Native American/Alaskan Native and Hispanic children, and higher in White than Black children.4 ALL is seen more in patients with certain inherited conditions, including Down syndrome, ataxia telangiectasia, neurofibromatosis type 1, and Bloom syndrome.1
Treatment advances have improved remission rates and outcomes for patients. However, relapse is still a leading cause of death for patients of all ages.6 Prompt diagnosis and care are important to optimize outcomes, as treatment delay is associated with poorer survival.7
Pathophysiology
In ALL, abnormal, immature lymphocytes and progenitor B cells/T cells proliferate uncontrollably and eventually replace healthy cells in bone marrow and the lymphatic system. The loss of healthy cells leads to classic symptoms of cytopenia, splenomegaly, and hepatomegaly.1 B cells and T cells are descended from lymphoid stem cells (and are transformed by germline or somatic mutation into pathogenic cells, leading to symptom development and bone marrow dysfunction. Most pediatric patients have extensive bone marrow involvement at diagnosis, with > 25% blast cells in marrow (defined as M3 disease).4
Presentation
Patients usually present with signs and symptoms that are related to disease-associated anemia, thrombocytopenia, or neutropenia; these signs and symptoms may include fatigue or weakness, pale skin, bleeding or bruising easily, fever or infection, joint or extremity pain, B-cell symptoms such as night sweats or unintentional weight loss, and splenomegaly or hepatomegaly. Central nervous system (CNS) symptoms can include stroke-like symptoms due to leukemic cell invasion of CNS vasculature or neuropathies related to increased intracranial pressure. Sometimes, children may present with no symptoms other than joint or extremity pain.1,3,8
Classification
ALL is classified by whether it derives from B-cell or T-cell progenitor cells and, within these, by typical genetic alterations (Table 1).3,9-15 Some cytogenetics are associated with risk assessment as well. Well-identified B-ALL subtypes include Philadelphia (Ph) chromosome-positive, hyper- and hypodiploidy, and KMT2A rearranged, while newer classifications include Ph-like ALL and B-lymphoblastic leukemia with iAMP21. Provisional T-ALL subtypes include early T-cell precursor lymphoblastic leukemia and natural killer cell lymphoblastic leukemia.3
B-cell lineage is present in 88% of pediatric and 75%-80% of adult disease. T-ALL is found in about 12% of pediatric patients and 25% of adults.3,8 Familial syndromes associated with ALL are present in about 4% of pediatric patients, including autosomal dominant germline mutations in RUNX1 (T-cell ALL), ETV6 (B-ALL), PAX5 (B-ALL), IKZF1 (B-ALL and T-ALL), and TP53 (low-hypodiploid ALL).3 If a known-familial genotype is identified, families should be referred for genetic counseling and further testing if needed. If germline mutation is suspected, early identification is important; hereditary ALL can influence treatment choice and use of allogeneic transplantation or radiation.3
A third classification crucial to guiding treatment is Ph-positive vs Ph-negative or Ph-like, the latter strongly associated with abnormal B-cell development due to deletions in related genes.3,16 About 3% to 5% of pediatric patients and 25% of adults have Ph-positive ALL.17 The remission failure rate among pediatric patients treated with chemotherapy was 11% in one study, vs 2%-3% among patients with Ph-negative ALL.10
Diagnosis and Risk Stratification
Diagnosis is based on presentation and molecular features, requiring demonstration of ≥ 20% lymphoblasts in bone marrow biopsy or aspirate or ≥ 1,000 circulating lymphoblasts/mL in peripheral blood. Testing can include immunophenotyping using flow cytometry, molecular characterization of baseline leukemic clone, morphology using hematoxylin and eosin staining and Wright/Giemsa staining, and karyotyping.1,3 CNS involvement is assessed using a lumbar spinal tap.1
Risk stratification is based on molecular features (eg, high- and low-risk mutations, Table 1),3,9-15 which are assessed using fluorescence in-situ hybridization, broad-panel next-generation sequencing, and reverse-transcriptase polymerase chain reaction of bone marrow or peripheral blood.3 Other risk factors include age, CNS involvement, white blood cell (WBC) count, and response to initial induction or consolidation therapy.3
Pediatric patients are assigned standard or high risk based on factors identified by the Children’s Oncology Group and National Comprehensive Cancer Network (NCCN). Patients
aged 1 to < 10 years with WBC < 50 × 109/L are considered standard risk, and all others are considered high risk. Patients with ALL before age 1 have very high risk. All pediatric patients with T-ALL are considered high risk.3 Ph-positive, Ph-like, hypoploidy, failure to achieve remission with induction, and extramedullary disease are high-risk factors as well, whereas hyperploidy and certain mutations convey low risk.3
Newer treatment strategies for initial ALL diagnosis include targeted therapies. One goal of targeted therapy is avoidance of long-term toxicity, leading to improved survival outcomes. Well-studied targeted therapies include the tyrosine kinase inhibitors used in first-line and subsequent treatment of Ph-positive ALL.3
Treatment Options in Relapsed/Refractory ALL
The initial treatment goal is complete remission (CR) defined as minimal residual disease (MRD) < 0.01% on flow cytometry (Table 2).3 Prognosis is dependent on time and location of relapse. Early relapse (< 18 months from diagnosis) predicts poor survival. Relapse in bone marrow is associated with poorer prognosis than relapse in CNS.11-18 Where possible, consolidation with allogeneic hematopoietic cell transplantation improves survival for patients with early relapse.6 Three approaches have advanced treatment options for relapsed/refractory (R/R) B-ALL, all based around common cell markers seen in B-ALL.
The CD22-directed antibody-drug conjugate inotuzumab ozogamicin is approved for adults with R/R B-ALL. In clinical trials, a higher percentage of patients had results below the MRD threshold, and longer progression-free survival and OS compared with standard care.19,20
Blinatumomab is a bispecific T-cell engager that binds to CD19 on the surface of B-ALL cells and to CD3 on T cells to trigger apoptosis.21 It was first approved for R/R ALL in adults or children, and is also now approved for treatment in remission with MRD ≥ 0.1%. Patients must demonstrate CD19-positive disease to qualify.15-22 For R/R ALL, blinatumomab improves OS and CR rates compared with standard chemotherapy.23
The use of CAR T-cell therapies has expanded greatly with increasing knowledge about their efficacy and safety. In R/R ALL, tisagenlecleucel (tisa-gen) is approved for treatment of patients aged ≤ 25 years, and brexucabtagene autoleucel (brexucel) is approved for treatment of adults.3,24,25 Patients undergoing the CAR T-cell process have apheresis to collect T cells, which are then manufactured before being reinfused into the patient. Depending on local capabilities, the time between T-cell harvest and reinfusion can extend to weeks.3,26,27 Cytoreduction with CAR T-cell therapy can allow previously ineligible patients (due to bulky disease) to undergo transplant. Patients treated in key clinical trials with tisa-gen or brexu-cel achieved high overall remission rates and improved event-free survival and OS rates compared with historical experience.25,28,29 Important toxicities with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, which can develop rapidly. NCCN recommends hospitalizing patients at the first sign of either adverse event. Patients can be managed with tocilizumab or steroids for low-grade CRS or steroids for neurotoxicity. The Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and NCCN have guidelines on management of toxicities related to CAR T-cell therapy as well as management of symptoms and other adverse effects of CRS.5,23,24
Programs also incorporate telemedicine for symptom monitoring and follow-up.32-34 Centers providing CAR T-cell therapy must have a certified Risk Evaluation and Mitigation Strategy (REMS), which ensures adherence to specific guidelines for administration, adverse event management, and patient education.35,36 Overcoming technical, social, and financial barriers to CAR T-cell therapy is an ongoing challenge of great interest.37
R/R T-Cell Precursor ALL
Patients with R/R T-ALL have poor prognosis, partly due to limited treatment options. Nelarabine, a nucleoside analog, is the only approved treatment for R/R T-ALL, but has increasingly been used in first-line therapy added to multiagent chemotherapy as a consolidation and maintenance approach to pediatric disease.3,38,39 Four-year DSF in pediatric patients with newly diagnosed T-ALL undergoing treatment incorporating nelarabine was 88.9%.39 Treatment is associated with grade ≥ 3 neurotoxicity in > 10% of patients, and can include CNS toxicity as well as neuropathy.3
In a recently completed phase 2 trial (NCT03384654), daratumumab was added to standard chemotherapy (vincristine, prednisone, PEG-asparaginase, doxorubicin) for R/R T-ALL in pediatric (ages 1-17 years) and young adult patients (age ≥ 18 years).40 Among 24 pediatric patients, CR was 41.7% and overall response rate (ORR; ORR = CR + CRi) was 83% after 1 cycle of treatment. Ten (41.7%) pediatric patients achieved MRD-negative status as well. ORR was 60% in the 5 older patients. All pediatric patients had at least 1 grade ≥ 3 toxicity, but none of the adverse events led to discontinuation.40
Success in achieving MRD-negative responses in patients treated for R/R ALL has increased interest in using targeted therapies for newly diagnosed patients. Recommended treatment approaches are summarized in Table 3.3
Long-Term Follow-Up and Survivorship
A study of > 500 pediatric patients followed for an average 23 years reassuringly found low prevalence of adverse outcomes related to disease or treatment. Major adverse outcomes such as death due to late relapse; secondary malignancy; or development of osteoporosis, cataracts, and diminished functional status were infrequent.41 Most prevalent were growth effects (short stature or growth hormone insufficiency), likely related to certain treatment approaches.41 Guidelines for long-term follow-up of pediatric patients are available from the Children’s Oncology Group.42
A 2017 systematic review concluded that the quality of life for survivors is diminished upon treatment, and persistently over time for some patients.43 In contrast, a 2022 comparison of long-term survivors (median 20.5 years since diagnosis) of pediatric ALL with healthy controls found that survivors had better quality of life in some domains, including general health, vitality, and mental health.44 Smaller percentages of survivors rated themselves happiest about sleep quality, absence of pain, and physical abilities.44
As therapy patterns and options evolve, continued follow-up is important to ensure patients derive optimal benefit from treatment and post-treatment life.
Incidence peaks in children aged 1-4 years, decreasing thereafter. Cases are highest among Native American/Alaskan Native and Hispanic children, and higher in White than Black children.4 ALL is seen more in patients with certain inherited conditions, including Down syndrome, ataxia telangiectasia, neurofibromatosis type 1, and Bloom syndrome.1
Treatment advances have improved remission rates and outcomes for patients. However, relapse is still a leading cause of death for patients of all ages.6 Prompt diagnosis and care are important to optimize outcomes, as treatment delay is associated with poorer survival.7
Pathophysiology
In ALL, abnormal, immature lymphocytes and progenitor B cells/T cells proliferate uncontrollably and eventually replace healthy cells in bone marrow and the lymphatic system. The loss of healthy cells leads to classic symptoms of cytopenia, splenomegaly, and hepatomegaly.1 B cells and T cells are descended from lymphoid stem cells (and are transformed by germline or somatic mutation into pathogenic cells, leading to symptom development and bone marrow dysfunction. Most pediatric patients have extensive bone marrow involvement at diagnosis, with > 25% blast cells in marrow (defined as M3 disease).4
Presentation
Patients usually present with signs and symptoms that are related to disease-associated anemia, thrombocytopenia, or neutropenia; these signs and symptoms may include fatigue or weakness, pale skin, bleeding or bruising easily, fever or infection, joint or extremity pain, B-cell symptoms such as night sweats or unintentional weight loss, and splenomegaly or hepatomegaly. Central nervous system (CNS) symptoms can include stroke-like symptoms due to leukemic cell invasion of CNS vasculature or neuropathies related to increased intracranial pressure. Sometimes, children may present with no symptoms other than joint or extremity pain.1,3,8
Classification
ALL is classified by whether it derives from B-cell or T-cell progenitor cells and, within these, by typical genetic alterations (Table 1).3,9-15 Some cytogenetics are associated with risk assessment as well. Well-identified B-ALL subtypes include Philadelphia (Ph) chromosome-positive, hyper- and hypodiploidy, and KMT2A rearranged, while newer classifications include Ph-like ALL and B-lymphoblastic leukemia with iAMP21. Provisional T-ALL subtypes include early T-cell precursor lymphoblastic leukemia and natural killer cell lymphoblastic leukemia.3
B-cell lineage is present in 88% of pediatric and 75%-80% of adult disease. T-ALL is found in about 12% of pediatric patients and 25% of adults.3,8 Familial syndromes associated with ALL are present in about 4% of pediatric patients, including autosomal dominant germline mutations in RUNX1 (T-cell ALL), ETV6 (B-ALL), PAX5 (B-ALL), IKZF1 (B-ALL and T-ALL), and TP53 (low-hypodiploid ALL).3 If a known-familial genotype is identified, families should be referred for genetic counseling and further testing if needed. If germline mutation is suspected, early identification is important; hereditary ALL can influence treatment choice and use of allogeneic transplantation or radiation.3
A third classification crucial to guiding treatment is Ph-positive vs Ph-negative or Ph-like, the latter strongly associated with abnormal B-cell development due to deletions in related genes.3,16 About 3% to 5% of pediatric patients and 25% of adults have Ph-positive ALL.17 The remission failure rate among pediatric patients treated with chemotherapy was 11% in one study, vs 2%-3% among patients with Ph-negative ALL.10
Diagnosis and Risk Stratification
Diagnosis is based on presentation and molecular features, requiring demonstration of ≥ 20% lymphoblasts in bone marrow biopsy or aspirate or ≥ 1,000 circulating lymphoblasts/mL in peripheral blood. Testing can include immunophenotyping using flow cytometry, molecular characterization of baseline leukemic clone, morphology using hematoxylin and eosin staining and Wright/Giemsa staining, and karyotyping.1,3 CNS involvement is assessed using a lumbar spinal tap.1
Risk stratification is based on molecular features (eg, high- and low-risk mutations, Table 1),3,9-15 which are assessed using fluorescence in-situ hybridization, broad-panel next-generation sequencing, and reverse-transcriptase polymerase chain reaction of bone marrow or peripheral blood.3 Other risk factors include age, CNS involvement, white blood cell (WBC) count, and response to initial induction or consolidation therapy.3
Pediatric patients are assigned standard or high risk based on factors identified by the Children’s Oncology Group and National Comprehensive Cancer Network (NCCN). Patients
aged 1 to < 10 years with WBC < 50 × 109/L are considered standard risk, and all others are considered high risk. Patients with ALL before age 1 have very high risk. All pediatric patients with T-ALL are considered high risk.3 Ph-positive, Ph-like, hypoploidy, failure to achieve remission with induction, and extramedullary disease are high-risk factors as well, whereas hyperploidy and certain mutations convey low risk.3
Newer treatment strategies for initial ALL diagnosis include targeted therapies. One goal of targeted therapy is avoidance of long-term toxicity, leading to improved survival outcomes. Well-studied targeted therapies include the tyrosine kinase inhibitors used in first-line and subsequent treatment of Ph-positive ALL.3
Treatment Options in Relapsed/Refractory ALL
The initial treatment goal is complete remission (CR) defined as minimal residual disease (MRD) < 0.01% on flow cytometry (Table 2).3 Prognosis is dependent on time and location of relapse. Early relapse (< 18 months from diagnosis) predicts poor survival. Relapse in bone marrow is associated with poorer prognosis than relapse in CNS.11-18 Where possible, consolidation with allogeneic hematopoietic cell transplantation improves survival for patients with early relapse.6 Three approaches have advanced treatment options for relapsed/refractory (R/R) B-ALL, all based around common cell markers seen in B-ALL.
The CD22-directed antibody-drug conjugate inotuzumab ozogamicin is approved for adults with R/R B-ALL. In clinical trials, a higher percentage of patients had results below the MRD threshold, and longer progression-free survival and OS compared with standard care.19,20
Blinatumomab is a bispecific T-cell engager that binds to CD19 on the surface of B-ALL cells and to CD3 on T cells to trigger apoptosis.21 It was first approved for R/R ALL in adults or children, and is also now approved for treatment in remission with MRD ≥ 0.1%. Patients must demonstrate CD19-positive disease to qualify.15-22 For R/R ALL, blinatumomab improves OS and CR rates compared with standard chemotherapy.23
The use of CAR T-cell therapies has expanded greatly with increasing knowledge about their efficacy and safety. In R/R ALL, tisagenlecleucel (tisa-gen) is approved for treatment of patients aged ≤ 25 years, and brexucabtagene autoleucel (brexucel) is approved for treatment of adults.3,24,25 Patients undergoing the CAR T-cell process have apheresis to collect T cells, which are then manufactured before being reinfused into the patient. Depending on local capabilities, the time between T-cell harvest and reinfusion can extend to weeks.3,26,27 Cytoreduction with CAR T-cell therapy can allow previously ineligible patients (due to bulky disease) to undergo transplant. Patients treated in key clinical trials with tisa-gen or brexu-cel achieved high overall remission rates and improved event-free survival and OS rates compared with historical experience.25,28,29 Important toxicities with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, which can develop rapidly. NCCN recommends hospitalizing patients at the first sign of either adverse event. Patients can be managed with tocilizumab or steroids for low-grade CRS or steroids for neurotoxicity. The Society for Immunotherapy of Cancer, American Society of Clinical Oncology, and NCCN have guidelines on management of toxicities related to CAR T-cell therapy as well as management of symptoms and other adverse effects of CRS.5,23,24
Programs also incorporate telemedicine for symptom monitoring and follow-up.32-34 Centers providing CAR T-cell therapy must have a certified Risk Evaluation and Mitigation Strategy (REMS), which ensures adherence to specific guidelines for administration, adverse event management, and patient education.35,36 Overcoming technical, social, and financial barriers to CAR T-cell therapy is an ongoing challenge of great interest.37
R/R T-Cell Precursor ALL
Patients with R/R T-ALL have poor prognosis, partly due to limited treatment options. Nelarabine, a nucleoside analog, is the only approved treatment for R/R T-ALL, but has increasingly been used in first-line therapy added to multiagent chemotherapy as a consolidation and maintenance approach to pediatric disease.3,38,39 Four-year DSF in pediatric patients with newly diagnosed T-ALL undergoing treatment incorporating nelarabine was 88.9%.39 Treatment is associated with grade ≥ 3 neurotoxicity in > 10% of patients, and can include CNS toxicity as well as neuropathy.3
In a recently completed phase 2 trial (NCT03384654), daratumumab was added to standard chemotherapy (vincristine, prednisone, PEG-asparaginase, doxorubicin) for R/R T-ALL in pediatric (ages 1-17 years) and young adult patients (age ≥ 18 years).40 Among 24 pediatric patients, CR was 41.7% and overall response rate (ORR; ORR = CR + CRi) was 83% after 1 cycle of treatment. Ten (41.7%) pediatric patients achieved MRD-negative status as well. ORR was 60% in the 5 older patients. All pediatric patients had at least 1 grade ≥ 3 toxicity, but none of the adverse events led to discontinuation.40
Success in achieving MRD-negative responses in patients treated for R/R ALL has increased interest in using targeted therapies for newly diagnosed patients. Recommended treatment approaches are summarized in Table 3.3
Long-Term Follow-Up and Survivorship
A study of > 500 pediatric patients followed for an average 23 years reassuringly found low prevalence of adverse outcomes related to disease or treatment. Major adverse outcomes such as death due to late relapse; secondary malignancy; or development of osteoporosis, cataracts, and diminished functional status were infrequent.41 Most prevalent were growth effects (short stature or growth hormone insufficiency), likely related to certain treatment approaches.41 Guidelines for long-term follow-up of pediatric patients are available from the Children’s Oncology Group.42
A 2017 systematic review concluded that the quality of life for survivors is diminished upon treatment, and persistently over time for some patients.43 In contrast, a 2022 comparison of long-term survivors (median 20.5 years since diagnosis) of pediatric ALL with healthy controls found that survivors had better quality of life in some domains, including general health, vitality, and mental health.44 Smaller percentages of survivors rated themselves happiest about sleep quality, absence of pain, and physical abilities.44
As therapy patterns and options evolve, continued follow-up is important to ensure patients derive optimal benefit from treatment and post-treatment life.
- Puckett Y, Chan O. Acute lymphocytic leukemia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated June 27, 2022. Accessed April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Cancer facts & figures 2023. American Cancer Society. 2023. Accessed April 10, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia. Version 1.2022. April 4, 2022. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Childhood acute lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 16, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities. Version 1.2023. March 10, 2023. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- DuVall AS, Sheade J, Anderson D, et al. Updates in the management of relapsed and refractory acute lymphoplastic leukemia: an urgent plea for new treatments is being answered! JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
- Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204-209. doi:10.1016/j.leukres.2013.11.014
- Acute adult lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 24, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al. Toward a NOTCH1/FBXW7/RAS/PTEN–based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2013;31(34):4333-4342. doi:10.1200/JCO.2012.48.5292
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966-1973. doi:10.1200/JCO.2011.39.7661
- Gao C, Liu SG, Zhang RD, et al. NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol. 2014;166(2):221-228. doi:10.1111/bjh.12866
- Yang YL, Hsiao CC, Chen HY, et al. Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(6):846-851. doi:10.1002/pbc.24021
- Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816-3823. doi:10.1200/JCO.2010.28.3390
- Bandapalli OR, Zimmermann M, Kox C, et al. NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica. 2013;98(6):928-936. doi:10.3324/haematol.2012.073585
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(37):59260-59272. doi:10.18632/oncotarget.10610
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. doi:10.1016/S1470-2045(08)70339-5
- Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi:10.1200/JCO.2010.30.1325
- Nguyen K, Devidas M, Cheng SC, et al.; Children’s Oncology Group. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142-2150. doi:10.1038/leu.2008.251
- Besponsa. Prescribing information. Wyeth Pharmaceuticals Inc; 2017. BESPONSA® (inotuzumab ozogamicin) Dosing & Administration |Safety Info (pfizerpro.com)
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. doi:10.1056/NEJMoa1509277
- Lv M, Liu Y, Liu W, Xing Y, Zhang S. Immunotherapy for pediatric acute lymphoblastic leukemia: recent advances and future perspectives. Front Immunol. 2022;13:921894. doi:10.3389/fimmu.2022.921894
- Blincyto. Prescribing information. Amgen; 2022. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/NEJMoa1609783
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
- Bhaskar ST, Dholaria BR, Singsayadeth S, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3(suppl 1):39-45. doi:10.1002/jha2.335
- Granroth G, Rosenthal A, McCallen M, et al. Supportive care for patients with lymphoma
undergoing CAR-T-cell therapy: the advanced practice provider’s perspective. Curr Oncol Rep. 2022;24(12):1863-1872. doi:10.1007/s11912-022-01330-z - Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphocytic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO.22.00642
- Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi:10.1186/s13045-022-01379-0
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/JCO.21.01992
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transpl. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
- LeBar K, Murawski S, Umayam S, Quinn V. The role of advanced practice providers and telemedicine in reinventing care: the transition of a CAR T-cell transplantation program to the outpatient setting. J Adv Pract Oncol. 2020;11(7):757-763. doi:10.6004/jadpro.2020.11.7.8
- Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy for aggressive B-cell lymphomas and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
- Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; 2022. https://www.fda.gov/media/107296/download
- Tecartus. Prescribing information. Kite Pharma, Inc; 2021. https://www.fda.gov/media/140409/download
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18(12):800-807. doi:10.1200/OP.22.00315
- Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135(3):159-166. doi:10.1182/blood.2019001557
- Summers RJ, Teachey DT. SOHO state of the art updates and next questions: novel approaches to pediatric T-cell ALL and T-lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(10):718-725. doi:10.1016/j.clml.2022.07.010
- Hogan LE, Bhatla T, Teachey DT, et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16 suppl):Abstract 10001. doi:10.1200/JCO.2022.40.16_suppl.10001
- Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Oncol. 2014;15(8):841-851. doi:10.1016/S1470-2045(14)70265-7
- Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group. October 2018. Accessed April 10, 2023. http://www.survivorshipguidelines.org
- Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26489
- Chantziara S, Musoro J, Rowsell AC, et al; European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QLG) and Children’s Leukemia Group (CLG). Quality of life of long-term childhood acute lymphoblastic leukemia survivors: comparison with healthy controls. Psychooncology. 2022;31(12):2159-2168. doi:10.1002/pon.6060
- Puckett Y, Chan O. Acute lymphocytic leukemia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated June 27, 2022. Accessed April 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459149/
- Cancer facts & figures 2023. American Cancer Society. 2023. Accessed April 10, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: acute lymphoblastic leukemia. Version 1.2022. April 4, 2022. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- Childhood acute lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 16, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities. Version 1.2023. March 10, 2023. Accessed April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
- DuVall AS, Sheade J, Anderson D, et al. Updates in the management of relapsed and refractory acute lymphoplastic leukemia: an urgent plea for new treatments is being answered! JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
- Baker JM, To T, Beyene J, Zagorski B, Greenberg ML, Sung L. Influence of length of time to diagnosis and treatment on the survival of children with acute lymphoblastic leukemia: a population-based study. Leuk Res. 2014;38(2):204-209. doi:10.1016/j.leukres.2013.11.014
- Acute adult lymphoblastic leukemia (PDQ)—Health Professional Version. National Cancer Institute. Updated February 24, 2023. Accessed April 10, 2023. https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al. Toward a NOTCH1/FBXW7/RAS/PTEN–based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2013;31(34):4333-4342. doi:10.1200/JCO.2012.48.5292
- Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30(16):1966-1973. doi:10.1200/JCO.2011.39.7661
- Gao C, Liu SG, Zhang RD, et al. NOTCH1 mutations are associated with favourable long-term prognosis in paediatric T-cell acute lymphoblastic leukaemia: a retrospective study of patients treated on BCH-2003 and CCLG-2008 protocol in China. Br J Haematol. 2014;166(2):221-228. doi:10.1111/bjh.12866
- Yang YL, Hsiao CC, Chen HY, et al. Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(6):846-851. doi:10.1002/pbc.24021
- Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816-3823. doi:10.1200/JCO.2010.28.3390
- Bandapalli OR, Zimmermann M, Kox C, et al. NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica. 2013;98(6):928-936. doi:10.3324/haematol.2012.073585
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(37):59260-59272. doi:10.18632/oncotarget.10610
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125-134. doi:10.1016/S1470-2045(08)70339-5
- Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi:10.1200/JCO.2010.30.1325
- Nguyen K, Devidas M, Cheng SC, et al.; Children’s Oncology Group. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22(12):2142-2150. doi:10.1038/leu.2008.251
- Besponsa. Prescribing information. Wyeth Pharmaceuticals Inc; 2017. BESPONSA® (inotuzumab ozogamicin) Dosing & Administration |Safety Info (pfizerpro.com)
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753. doi:10.1056/NEJMoa1509277
- Lv M, Liu Y, Liu W, Xing Y, Zhang S. Immunotherapy for pediatric acute lymphoblastic leukemia: recent advances and future perspectives. Front Immunol. 2022;13:921894. doi:10.3389/fimmu.2022.921894
- Blincyto. Prescribing information. Amgen; 2022. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/NEJMoa1609783
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
- Bhaskar ST, Dholaria BR, Singsayadeth S, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3(suppl 1):39-45. doi:10.1002/jha2.335
- Granroth G, Rosenthal A, McCallen M, et al. Supportive care for patients with lymphoma
undergoing CAR-T-cell therapy: the advanced practice provider’s perspective. Curr Oncol Rep. 2022;24(12):1863-1872. doi:10.1007/s11912-022-01330-z - Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphocytic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO.22.00642
- Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi:10.1186/s13045-022-01379-0
- Maus MV, Alexander S, Bishop MR, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511. doi:10.1136/jitc-2020-001511
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978-3992. doi:10.1200/JCO.21.01992
- Borogovac A, Keruakous A, Bycko M, et al. Safety and feasibility of outpatient chimeric antigen receptor (CAR) T-cell therapy: experience from a tertiary care center. Bone Marrow Transpl. 2022;57(6):1025-1027. doi:10.1038/s41409-022-01664-z
- LeBar K, Murawski S, Umayam S, Quinn V. The role of advanced practice providers and telemedicine in reinventing care: the transition of a CAR T-cell transplantation program to the outpatient setting. J Adv Pract Oncol. 2020;11(7):757-763. doi:10.6004/jadpro.2020.11.7.8
- Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy for aggressive B-cell lymphomas and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):e002056. doi:10.1136/jitc-2020-002056
- Kymriah. Prescribing information. Novartis Pharmaceuticals Corporation; 2022. https://www.fda.gov/media/107296/download
- Tecartus. Prescribing information. Kite Pharma, Inc; 2021. https://www.fda.gov/media/140409/download
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 2022;18(12):800-807. doi:10.1200/OP.22.00315
- Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135(3):159-166. doi:10.1182/blood.2019001557
- Summers RJ, Teachey DT. SOHO state of the art updates and next questions: novel approaches to pediatric T-cell ALL and T-lymphoblastic lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(10):718-725. doi:10.1016/j.clml.2022.07.010
- Hogan LE, Bhatla T, Teachey DT, et al. Efficacy and safety of daratumumab (DARA) in pediatric and young adult patients (pts) with relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL): results from the phase 2 DELPHINUS study. J Clin Oncol. 2022;40(16 suppl):Abstract 10001. doi:10.1200/JCO.2022.40.16_suppl.10001
- Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study. Lancet Oncol. 2014;15(8):841-851. doi:10.1016/S1470-2045(14)70265-7
- Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 5.0. Children’s Oncology Group. October 2018. Accessed April 10, 2023. http://www.survivorshipguidelines.org
- Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26489
- Chantziara S, Musoro J, Rowsell AC, et al; European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life (QLG) and Children’s Leukemia Group (CLG). Quality of life of long-term childhood acute lymphoblastic leukemia survivors: comparison with healthy controls. Psychooncology. 2022;31(12):2159-2168. doi:10.1002/pon.6060
Treatment Needs of Older Adults With Newly Diagnosed Acute Myeloid Leukemia
Within the last 40 years, younger fit patients have benefited from intensive chemotherapy regimens for acute myeloid leukemia (AML) with improved survival, and the possibility of long-term disease-free survival (DFS) (“cure”).1 Older patients are often considered too unfit for standard curative treatment with intensive induction chemotherapy followed by consolidation chemotherapy, allogeneic hematopoietic cell transplantation (allo-HCT), or both.2-4 Higher induction mortality and poor overall survival (OS) are associated with worse performance status, organ impairment, significant comorbidities, and declining cognitive function, all of which are more common with advancing age. Although the suggested criteria for determining unfitness have not been validated (Table 1), they can provide guidance in clinical practice.2-5
The National Comprehensive Cancer Network (NCCN) panel recommends the consideration of a patient’s performance status and comorbid conditions in addition to their age to determine a patient’s fitness for intensive induction therapy.6 Adverse disease features should also be considered, because disease biology may make intensive chemotherapy futile or inappropriate. For example, the mutational driver tumor protein p53 (TP53) appears at a higher frequency in older adults than younger adults and is associated with dismal outcomes even with intensive chemotherapy. Likewise, the spliceosome and chromatin modifier gene mutations are more common in older patients with AML and confer a worse OS with intensive therapy.6,7 Older unfit patients faced a difficult decision: proceed with intensive therapy with some possibility of long-term survival but risk of early mortality and significant toxicity, or opt for supportive care and palliative chemotherapy, such as the hypomethylating agents (HMAs) or low-dose cytarabine, with much shorter survival.
Guidelines for Treating Older Unfit Patients
Evidence-based guidelines for managing older adults with newly diagnosed AML were developed by the American Society of Hematology in 2020; however, these guidelines were released prior to the results of several clinical trials involving older patients with AML (Table 2).8 In 2022, the European LeukemiaNet (ELN) recommendations were updated to include new therapeutic agents that target specific mutations in genes such as tyrosine kinase 3 (FLT3), isocitrate dehydrogenase 1 (IDH1), isocitrate dehydrogenase 2 (IDH2), and B-cell lymphoma 2 (BCL2). Given the important effects of genetic aberrations on disease phenotype, treatment options, and outcomes, screening for genetic aberrations at diagnosis is now essential.9
The potential for clonal evolution leading to new actionable targets that were not present at diagnosis highlights the importance of reevaluation of genetic aberrations throughout clinical progression. Actionable targets can include mutations in IDH1/IDH2, FLT3-internal tandem duplication or FLT3 tyrosine kinase domain.9
Treatment Landscape
Since 2018, several therapeutic agents have been added to the treatment armamentarium that can induce longer-term complete remission (CR) for older unfit patients with newly diagnosed AML (Table 2).4
Management of Primary AML With Less Intensive Induction Therapy
VIALE-A established a new standard of care for older unfit patients by demonstrating the benefit of adding the BCL2 inhibitor venetoclax (VEN) to azacitidine (AZA).2 VIALE-A demonstrated that the rate of CR plus CR with partial hematologic recovery (CRi) was 65% for VEN plus AZA and 18% for AZA. Most remissions in the AZA/VEN arm occurred rapidly in the first 2 cycles. The median survival improved from 9.6 months with AZA to 14.7 months with AZA/VEN. An improvement in survival with VEN and low-dose cytarabine also emerged in a 6-month post hoc analysis of the VIALE-C trial.10 Various other trials examining targeted therapies on specific mutations have provided mixed results in the front-line setting.13,14,18 It is important to note that a recent systematic review found that 12% to 25% of patients who were unfit for intensive therapy were successfully bridged to HCT.19
Management of Postremission Response
Patients with a longer duration of first remission have demonstrated better survival outcomes.15 Two trials have examined postremission therapy in the setting of prior intensive therapy. HOVON97 enrolled older patients who achieved CR/CRi after 2 cycles of intensive therapy to receive either AZA postremission or no further treatment. The proportion of patients with DFS at 12 months was greater in the AZA maintenance group than in the observation group (64% vs 42%), but significant DFS improvement did not translate into improved OS.20 QUAZAR AML-001 demonstrated that OS was longer for older patients receiving maintenance therapy with CC-486 (a non-bioequivalent oral formulation of AZA) vs placebo (24.7 vs 14.8 months).15 CC-486 was FDA-approved for maintenance therapy after intensive induction with or without consolidation in patients who are not candidates for allo-HCT. However, limited evidence exists specifically for postremission therapy in unfit patients who have received less intensive therapy. Continuation of the lower intensive therapy is recommended until disease progression.6 No data are available to support the use of oral AZA therapy alone for maintenance of remission following HMA/VEN-induced remissions.
Management of Relapsed and Refractory AML
Nearly 50% of patients with AML experience relapse and up to 40% may be refractory.19 Importantly, patients who were considered fit for intensive therapy may not remain so with relapsed or refractory AML (r/rAML), so patients should be evaluated for fitness for an intensive salvage regimen. Similar to assessing fitness for induction therapy, no standard definition of fitness exists for r/rAML.19
Disease control is the goal for patients with r/rAML who are unfit for intensive salvage therapy; however, treatment options remain limited and prognosis is poor.19 Depending on the patient’s cytogenetic profile, management can include HMA with or without VEN, glasdegib with LDAC, gilteritinib, ivosidenib or enasidenib, or gemtuzumab ozogamicin.9 Only a few studies have been published involving the r/rAML population not eligible for intensive salvage regimen, and guidelines are needed for this population.19 Thus, the ELN recommends that clinical trial enrollment be considered for patients with r/rAML.9
Management of Secondary AML or High-risk AML
Compared with de novo AML, both secondary AML (sAML) and therapy-related AML (tAML) have been associated with inferior outcomes. Factors that influence poor outcomes can include older age, comorbidities, persistent malignant disease or relapse of primary malignancy, treatment-induced depletion of hematopoietic reserves and/or prolonged myelosuppression, and genetic abnormalities, such as TP53 mutations.21
CPX-351 is a dual drug that contains cytarabine and daunorubicin.9,22 An open-label study (NCT01696084) compared CPX-351 with conventional cytarabine and daunorubicin (induction and consolidation therapy) in older patients (aged 60-75 years) with newly diagnosed high-risk/sAML who were considered fit for intensive therapy. The OS for CPX-351 was longer (9.56 vs 5.95 months) and the safety profiles were similar between the treatment groups.23 Patients achieving CR/CRi received up to 2 cycles of consolidation with CPX-351. An exploratory analysis of this subgroup revealed median OS was longer with CPX-351 consolidation (25.43 vs 8.53 months).22 Patients with TP53 mutations had poor treatment outcomes regardless of treatment arm, whereas patients with sAML-type mutations including spliceosome and chromatin modifier genes had longer OS with CPX-351 therapy.24 The 5-year results of this trial indicate that the survival benefit of CPX-351 was maintained.25 However, data from a retrospective review involving 136 patients with either sAML or AML with myelodysplasia-related changes revealed no difference in survival outcomes between patients treated with either HMA/VEN or CPX-351.26
Case Study: Elderly Woman With Newly Diagnosed AML
In 2018, Ms. W, age 69 years, was diagnosed with seropositive, non-erosive rheumatoid arthritis; she began methotrexate 17.5 mg per week split dosing in conjunction with oral folic acid 2 mg/d with varying doses based on symptoms. Her comorbidities included recurrent episodes of diverticulitis, hypertension, hypothyroidism, obstructive sleep apnea, and gastrointestinal reflux disease. On February 4, 2021, her methotrexate was increased to 20 mg and required intermittent prednisone tapers for flares. In November 2021, a blood test revealed she had a decreased white blood cell (WBC) count at 1.8 K/μL, and her methotrexate dose was decreased to 15 mg weekly. Despite the dose reduction, she had grade 3 neutropenia and anemia (WBC: 0.7 K/μL; HGB:10.5 g/dL) with a normal platelet count (PLT: 165,000/μL). Methotrexate was discontinued and leucovorin was initiated. She then had only modest improvement in her lab values and peripheral blood blasts.
On March 17, 2022, she underwent a bone marrow biopsy and aspirate, which resulted in a diagnosis of AML. She had 55% blasts in a 90% cellular bone marrow with mild reticulin fibrosis and numerous circulating blasts. She was classified as having AML without maturation (FAB AML-M1). Flow cytometry detected a phenotypically abnormal population with CD45 expression and side scatter/forward scatter features of small-to-medium sized blasts, accounting for 23% of total cells. The chromosome analysis demonstrated a normal female karyotype in all 19 available metaphases. Polymerase chain reaction analysis was negative for FLT3-ITD, FLT3-TKD, and NPM1 mutations and positive for an IDH1 R132C missense mutation. The myeloid gene panel identified only a single pathogenic variant, IDH1 R132C (variant allele frequency [VAF] 21.2%), and a variant of unknown significance DNMT3A A575P (VAF 25.7%).
Noting that she does not have favorable risk features, we discussed treatment options. Although she is a candidate for curative therapy, the patient was not interested in pursuing allo-HCT. Her history of diverticulitis is concerning for tolerating intensive chemotherapy. In addition, her immunosuppressive therapy increases her risk for opportunistic infections. Based on the available data from the AGILE and VIALE studies and associated potential adverse reactions, she opted for starting treatment with AZA and IVO.
On March 31, 2022, she began receiving AZA 75 mg/m2 intravenous (IV) once daily days 1-7 and oral IVO 500 mg once daily continuously. She has received 12 cycles and has not needed transfusion. She has not had febrile neutropenia or symptoms of differentiation syndrome. On March 24, 2023, she underwent laparoscopic cholecystectomy, because an ultrasound revealed cholelithiasis, abnormal gallbladder wall thickening, and pericholecystic fluid. She was discharged home the following day and is continuing with AZA/ivosidenib.
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023;108(2):306-307. doi:10.3324/haematol.2022.280803
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. doi:10.1182/blood.2019001239
- Huerga-Domínguez S, Villar S, Prósper F, Alfonso-Piérola A. Updates on the management of acute myeloid leukemia. Cancers (Basel). 2022;14(19):4756. doi:10.3390/cancers14194756
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi:10.6004/jnccn.2019.0028
- Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852-1858. doi:10.1038/s41591-020-1089-8
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. doi:10.1182/blood.2022016867
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. doi:10.1200/JCO.2015.64.0060
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(17):1845-1857. doi:10.1182/blood.2021014586
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740. doi:10.1056/NEJMoa1902688
- Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi:10.1158/1078-0432.CCR-21-3467
- Russell-Smith TA, Gurskyte L, Muresan B, et al. Efficacy of non-intensive therapies approved for relapsed/refractory acute myeloid leukemia: a systematic literature review. Future Oncol. 2022;18(16):2029-2039. doi:10.2217/fon-2021-1355
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649. doi:10.1200/JCO.2014.60.0890
- Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631-640. doi:10.1080/1042819.2019.1688320
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. doi:10.1200/JCO.2017.77.6112
- Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(suppl 1):15. doi:10.1182/blood-2019-124500
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi:10.1016/S2352-3026(21)00134-4
- Alharthy H, Alkaabba F, Williams M, et al. Outcomes of newly diagnosed therapy-related AML and AML with myelodysplasia-related changes treated with 7+3, hypomethylating agents with or without venetoclax and CPX-351: a retrospective cohort study. Blood. 2022;140(suppl 1):9025-9026. doi:10.1182/blood-2022-170688
Within the last 40 years, younger fit patients have benefited from intensive chemotherapy regimens for acute myeloid leukemia (AML) with improved survival, and the possibility of long-term disease-free survival (DFS) (“cure”).1 Older patients are often considered too unfit for standard curative treatment with intensive induction chemotherapy followed by consolidation chemotherapy, allogeneic hematopoietic cell transplantation (allo-HCT), or both.2-4 Higher induction mortality and poor overall survival (OS) are associated with worse performance status, organ impairment, significant comorbidities, and declining cognitive function, all of which are more common with advancing age. Although the suggested criteria for determining unfitness have not been validated (Table 1), they can provide guidance in clinical practice.2-5
The National Comprehensive Cancer Network (NCCN) panel recommends the consideration of a patient’s performance status and comorbid conditions in addition to their age to determine a patient’s fitness for intensive induction therapy.6 Adverse disease features should also be considered, because disease biology may make intensive chemotherapy futile or inappropriate. For example, the mutational driver tumor protein p53 (TP53) appears at a higher frequency in older adults than younger adults and is associated with dismal outcomes even with intensive chemotherapy. Likewise, the spliceosome and chromatin modifier gene mutations are more common in older patients with AML and confer a worse OS with intensive therapy.6,7 Older unfit patients faced a difficult decision: proceed with intensive therapy with some possibility of long-term survival but risk of early mortality and significant toxicity, or opt for supportive care and palliative chemotherapy, such as the hypomethylating agents (HMAs) or low-dose cytarabine, with much shorter survival.
Guidelines for Treating Older Unfit Patients
Evidence-based guidelines for managing older adults with newly diagnosed AML were developed by the American Society of Hematology in 2020; however, these guidelines were released prior to the results of several clinical trials involving older patients with AML (Table 2).8 In 2022, the European LeukemiaNet (ELN) recommendations were updated to include new therapeutic agents that target specific mutations in genes such as tyrosine kinase 3 (FLT3), isocitrate dehydrogenase 1 (IDH1), isocitrate dehydrogenase 2 (IDH2), and B-cell lymphoma 2 (BCL2). Given the important effects of genetic aberrations on disease phenotype, treatment options, and outcomes, screening for genetic aberrations at diagnosis is now essential.9
The potential for clonal evolution leading to new actionable targets that were not present at diagnosis highlights the importance of reevaluation of genetic aberrations throughout clinical progression. Actionable targets can include mutations in IDH1/IDH2, FLT3-internal tandem duplication or FLT3 tyrosine kinase domain.9
Treatment Landscape
Since 2018, several therapeutic agents have been added to the treatment armamentarium that can induce longer-term complete remission (CR) for older unfit patients with newly diagnosed AML (Table 2).4
Management of Primary AML With Less Intensive Induction Therapy
VIALE-A established a new standard of care for older unfit patients by demonstrating the benefit of adding the BCL2 inhibitor venetoclax (VEN) to azacitidine (AZA).2 VIALE-A demonstrated that the rate of CR plus CR with partial hematologic recovery (CRi) was 65% for VEN plus AZA and 18% for AZA. Most remissions in the AZA/VEN arm occurred rapidly in the first 2 cycles. The median survival improved from 9.6 months with AZA to 14.7 months with AZA/VEN. An improvement in survival with VEN and low-dose cytarabine also emerged in a 6-month post hoc analysis of the VIALE-C trial.10 Various other trials examining targeted therapies on specific mutations have provided mixed results in the front-line setting.13,14,18 It is important to note that a recent systematic review found that 12% to 25% of patients who were unfit for intensive therapy were successfully bridged to HCT.19
Management of Postremission Response
Patients with a longer duration of first remission have demonstrated better survival outcomes.15 Two trials have examined postremission therapy in the setting of prior intensive therapy. HOVON97 enrolled older patients who achieved CR/CRi after 2 cycles of intensive therapy to receive either AZA postremission or no further treatment. The proportion of patients with DFS at 12 months was greater in the AZA maintenance group than in the observation group (64% vs 42%), but significant DFS improvement did not translate into improved OS.20 QUAZAR AML-001 demonstrated that OS was longer for older patients receiving maintenance therapy with CC-486 (a non-bioequivalent oral formulation of AZA) vs placebo (24.7 vs 14.8 months).15 CC-486 was FDA-approved for maintenance therapy after intensive induction with or without consolidation in patients who are not candidates for allo-HCT. However, limited evidence exists specifically for postremission therapy in unfit patients who have received less intensive therapy. Continuation of the lower intensive therapy is recommended until disease progression.6 No data are available to support the use of oral AZA therapy alone for maintenance of remission following HMA/VEN-induced remissions.
Management of Relapsed and Refractory AML
Nearly 50% of patients with AML experience relapse and up to 40% may be refractory.19 Importantly, patients who were considered fit for intensive therapy may not remain so with relapsed or refractory AML (r/rAML), so patients should be evaluated for fitness for an intensive salvage regimen. Similar to assessing fitness for induction therapy, no standard definition of fitness exists for r/rAML.19
Disease control is the goal for patients with r/rAML who are unfit for intensive salvage therapy; however, treatment options remain limited and prognosis is poor.19 Depending on the patient’s cytogenetic profile, management can include HMA with or without VEN, glasdegib with LDAC, gilteritinib, ivosidenib or enasidenib, or gemtuzumab ozogamicin.9 Only a few studies have been published involving the r/rAML population not eligible for intensive salvage regimen, and guidelines are needed for this population.19 Thus, the ELN recommends that clinical trial enrollment be considered for patients with r/rAML.9
Management of Secondary AML or High-risk AML
Compared with de novo AML, both secondary AML (sAML) and therapy-related AML (tAML) have been associated with inferior outcomes. Factors that influence poor outcomes can include older age, comorbidities, persistent malignant disease or relapse of primary malignancy, treatment-induced depletion of hematopoietic reserves and/or prolonged myelosuppression, and genetic abnormalities, such as TP53 mutations.21
CPX-351 is a dual drug that contains cytarabine and daunorubicin.9,22 An open-label study (NCT01696084) compared CPX-351 with conventional cytarabine and daunorubicin (induction and consolidation therapy) in older patients (aged 60-75 years) with newly diagnosed high-risk/sAML who were considered fit for intensive therapy. The OS for CPX-351 was longer (9.56 vs 5.95 months) and the safety profiles were similar between the treatment groups.23 Patients achieving CR/CRi received up to 2 cycles of consolidation with CPX-351. An exploratory analysis of this subgroup revealed median OS was longer with CPX-351 consolidation (25.43 vs 8.53 months).22 Patients with TP53 mutations had poor treatment outcomes regardless of treatment arm, whereas patients with sAML-type mutations including spliceosome and chromatin modifier genes had longer OS with CPX-351 therapy.24 The 5-year results of this trial indicate that the survival benefit of CPX-351 was maintained.25 However, data from a retrospective review involving 136 patients with either sAML or AML with myelodysplasia-related changes revealed no difference in survival outcomes between patients treated with either HMA/VEN or CPX-351.26
Case Study: Elderly Woman With Newly Diagnosed AML
In 2018, Ms. W, age 69 years, was diagnosed with seropositive, non-erosive rheumatoid arthritis; she began methotrexate 17.5 mg per week split dosing in conjunction with oral folic acid 2 mg/d with varying doses based on symptoms. Her comorbidities included recurrent episodes of diverticulitis, hypertension, hypothyroidism, obstructive sleep apnea, and gastrointestinal reflux disease. On February 4, 2021, her methotrexate was increased to 20 mg and required intermittent prednisone tapers for flares. In November 2021, a blood test revealed she had a decreased white blood cell (WBC) count at 1.8 K/μL, and her methotrexate dose was decreased to 15 mg weekly. Despite the dose reduction, she had grade 3 neutropenia and anemia (WBC: 0.7 K/μL; HGB:10.5 g/dL) with a normal platelet count (PLT: 165,000/μL). Methotrexate was discontinued and leucovorin was initiated. She then had only modest improvement in her lab values and peripheral blood blasts.
On March 17, 2022, she underwent a bone marrow biopsy and aspirate, which resulted in a diagnosis of AML. She had 55% blasts in a 90% cellular bone marrow with mild reticulin fibrosis and numerous circulating blasts. She was classified as having AML without maturation (FAB AML-M1). Flow cytometry detected a phenotypically abnormal population with CD45 expression and side scatter/forward scatter features of small-to-medium sized blasts, accounting for 23% of total cells. The chromosome analysis demonstrated a normal female karyotype in all 19 available metaphases. Polymerase chain reaction analysis was negative for FLT3-ITD, FLT3-TKD, and NPM1 mutations and positive for an IDH1 R132C missense mutation. The myeloid gene panel identified only a single pathogenic variant, IDH1 R132C (variant allele frequency [VAF] 21.2%), and a variant of unknown significance DNMT3A A575P (VAF 25.7%).
Noting that she does not have favorable risk features, we discussed treatment options. Although she is a candidate for curative therapy, the patient was not interested in pursuing allo-HCT. Her history of diverticulitis is concerning for tolerating intensive chemotherapy. In addition, her immunosuppressive therapy increases her risk for opportunistic infections. Based on the available data from the AGILE and VIALE studies and associated potential adverse reactions, she opted for starting treatment with AZA and IVO.
On March 31, 2022, she began receiving AZA 75 mg/m2 intravenous (IV) once daily days 1-7 and oral IVO 500 mg once daily continuously. She has received 12 cycles and has not needed transfusion. She has not had febrile neutropenia or symptoms of differentiation syndrome. On March 24, 2023, she underwent laparoscopic cholecystectomy, because an ultrasound revealed cholelithiasis, abnormal gallbladder wall thickening, and pericholecystic fluid. She was discharged home the following day and is continuing with AZA/ivosidenib.
Within the last 40 years, younger fit patients have benefited from intensive chemotherapy regimens for acute myeloid leukemia (AML) with improved survival, and the possibility of long-term disease-free survival (DFS) (“cure”).1 Older patients are often considered too unfit for standard curative treatment with intensive induction chemotherapy followed by consolidation chemotherapy, allogeneic hematopoietic cell transplantation (allo-HCT), or both.2-4 Higher induction mortality and poor overall survival (OS) are associated with worse performance status, organ impairment, significant comorbidities, and declining cognitive function, all of which are more common with advancing age. Although the suggested criteria for determining unfitness have not been validated (Table 1), they can provide guidance in clinical practice.2-5
The National Comprehensive Cancer Network (NCCN) panel recommends the consideration of a patient’s performance status and comorbid conditions in addition to their age to determine a patient’s fitness for intensive induction therapy.6 Adverse disease features should also be considered, because disease biology may make intensive chemotherapy futile or inappropriate. For example, the mutational driver tumor protein p53 (TP53) appears at a higher frequency in older adults than younger adults and is associated with dismal outcomes even with intensive chemotherapy. Likewise, the spliceosome and chromatin modifier gene mutations are more common in older patients with AML and confer a worse OS with intensive therapy.6,7 Older unfit patients faced a difficult decision: proceed with intensive therapy with some possibility of long-term survival but risk of early mortality and significant toxicity, or opt for supportive care and palliative chemotherapy, such as the hypomethylating agents (HMAs) or low-dose cytarabine, with much shorter survival.
Guidelines for Treating Older Unfit Patients
Evidence-based guidelines for managing older adults with newly diagnosed AML were developed by the American Society of Hematology in 2020; however, these guidelines were released prior to the results of several clinical trials involving older patients with AML (Table 2).8 In 2022, the European LeukemiaNet (ELN) recommendations were updated to include new therapeutic agents that target specific mutations in genes such as tyrosine kinase 3 (FLT3), isocitrate dehydrogenase 1 (IDH1), isocitrate dehydrogenase 2 (IDH2), and B-cell lymphoma 2 (BCL2). Given the important effects of genetic aberrations on disease phenotype, treatment options, and outcomes, screening for genetic aberrations at diagnosis is now essential.9
The potential for clonal evolution leading to new actionable targets that were not present at diagnosis highlights the importance of reevaluation of genetic aberrations throughout clinical progression. Actionable targets can include mutations in IDH1/IDH2, FLT3-internal tandem duplication or FLT3 tyrosine kinase domain.9
Treatment Landscape
Since 2018, several therapeutic agents have been added to the treatment armamentarium that can induce longer-term complete remission (CR) for older unfit patients with newly diagnosed AML (Table 2).4
Management of Primary AML With Less Intensive Induction Therapy
VIALE-A established a new standard of care for older unfit patients by demonstrating the benefit of adding the BCL2 inhibitor venetoclax (VEN) to azacitidine (AZA).2 VIALE-A demonstrated that the rate of CR plus CR with partial hematologic recovery (CRi) was 65% for VEN plus AZA and 18% for AZA. Most remissions in the AZA/VEN arm occurred rapidly in the first 2 cycles. The median survival improved from 9.6 months with AZA to 14.7 months with AZA/VEN. An improvement in survival with VEN and low-dose cytarabine also emerged in a 6-month post hoc analysis of the VIALE-C trial.10 Various other trials examining targeted therapies on specific mutations have provided mixed results in the front-line setting.13,14,18 It is important to note that a recent systematic review found that 12% to 25% of patients who were unfit for intensive therapy were successfully bridged to HCT.19
Management of Postremission Response
Patients with a longer duration of first remission have demonstrated better survival outcomes.15 Two trials have examined postremission therapy in the setting of prior intensive therapy. HOVON97 enrolled older patients who achieved CR/CRi after 2 cycles of intensive therapy to receive either AZA postremission or no further treatment. The proportion of patients with DFS at 12 months was greater in the AZA maintenance group than in the observation group (64% vs 42%), but significant DFS improvement did not translate into improved OS.20 QUAZAR AML-001 demonstrated that OS was longer for older patients receiving maintenance therapy with CC-486 (a non-bioequivalent oral formulation of AZA) vs placebo (24.7 vs 14.8 months).15 CC-486 was FDA-approved for maintenance therapy after intensive induction with or without consolidation in patients who are not candidates for allo-HCT. However, limited evidence exists specifically for postremission therapy in unfit patients who have received less intensive therapy. Continuation of the lower intensive therapy is recommended until disease progression.6 No data are available to support the use of oral AZA therapy alone for maintenance of remission following HMA/VEN-induced remissions.
Management of Relapsed and Refractory AML
Nearly 50% of patients with AML experience relapse and up to 40% may be refractory.19 Importantly, patients who were considered fit for intensive therapy may not remain so with relapsed or refractory AML (r/rAML), so patients should be evaluated for fitness for an intensive salvage regimen. Similar to assessing fitness for induction therapy, no standard definition of fitness exists for r/rAML.19
Disease control is the goal for patients with r/rAML who are unfit for intensive salvage therapy; however, treatment options remain limited and prognosis is poor.19 Depending on the patient’s cytogenetic profile, management can include HMA with or without VEN, glasdegib with LDAC, gilteritinib, ivosidenib or enasidenib, or gemtuzumab ozogamicin.9 Only a few studies have been published involving the r/rAML population not eligible for intensive salvage regimen, and guidelines are needed for this population.19 Thus, the ELN recommends that clinical trial enrollment be considered for patients with r/rAML.9
Management of Secondary AML or High-risk AML
Compared with de novo AML, both secondary AML (sAML) and therapy-related AML (tAML) have been associated with inferior outcomes. Factors that influence poor outcomes can include older age, comorbidities, persistent malignant disease or relapse of primary malignancy, treatment-induced depletion of hematopoietic reserves and/or prolonged myelosuppression, and genetic abnormalities, such as TP53 mutations.21
CPX-351 is a dual drug that contains cytarabine and daunorubicin.9,22 An open-label study (NCT01696084) compared CPX-351 with conventional cytarabine and daunorubicin (induction and consolidation therapy) in older patients (aged 60-75 years) with newly diagnosed high-risk/sAML who were considered fit for intensive therapy. The OS for CPX-351 was longer (9.56 vs 5.95 months) and the safety profiles were similar between the treatment groups.23 Patients achieving CR/CRi received up to 2 cycles of consolidation with CPX-351. An exploratory analysis of this subgroup revealed median OS was longer with CPX-351 consolidation (25.43 vs 8.53 months).22 Patients with TP53 mutations had poor treatment outcomes regardless of treatment arm, whereas patients with sAML-type mutations including spliceosome and chromatin modifier genes had longer OS with CPX-351 therapy.24 The 5-year results of this trial indicate that the survival benefit of CPX-351 was maintained.25 However, data from a retrospective review involving 136 patients with either sAML or AML with myelodysplasia-related changes revealed no difference in survival outcomes between patients treated with either HMA/VEN or CPX-351.26
Case Study: Elderly Woman With Newly Diagnosed AML
In 2018, Ms. W, age 69 years, was diagnosed with seropositive, non-erosive rheumatoid arthritis; she began methotrexate 17.5 mg per week split dosing in conjunction with oral folic acid 2 mg/d with varying doses based on symptoms. Her comorbidities included recurrent episodes of diverticulitis, hypertension, hypothyroidism, obstructive sleep apnea, and gastrointestinal reflux disease. On February 4, 2021, her methotrexate was increased to 20 mg and required intermittent prednisone tapers for flares. In November 2021, a blood test revealed she had a decreased white blood cell (WBC) count at 1.8 K/μL, and her methotrexate dose was decreased to 15 mg weekly. Despite the dose reduction, she had grade 3 neutropenia and anemia (WBC: 0.7 K/μL; HGB:10.5 g/dL) with a normal platelet count (PLT: 165,000/μL). Methotrexate was discontinued and leucovorin was initiated. She then had only modest improvement in her lab values and peripheral blood blasts.
On March 17, 2022, she underwent a bone marrow biopsy and aspirate, which resulted in a diagnosis of AML. She had 55% blasts in a 90% cellular bone marrow with mild reticulin fibrosis and numerous circulating blasts. She was classified as having AML without maturation (FAB AML-M1). Flow cytometry detected a phenotypically abnormal population with CD45 expression and side scatter/forward scatter features of small-to-medium sized blasts, accounting for 23% of total cells. The chromosome analysis demonstrated a normal female karyotype in all 19 available metaphases. Polymerase chain reaction analysis was negative for FLT3-ITD, FLT3-TKD, and NPM1 mutations and positive for an IDH1 R132C missense mutation. The myeloid gene panel identified only a single pathogenic variant, IDH1 R132C (variant allele frequency [VAF] 21.2%), and a variant of unknown significance DNMT3A A575P (VAF 25.7%).
Noting that she does not have favorable risk features, we discussed treatment options. Although she is a candidate for curative therapy, the patient was not interested in pursuing allo-HCT. Her history of diverticulitis is concerning for tolerating intensive chemotherapy. In addition, her immunosuppressive therapy increases her risk for opportunistic infections. Based on the available data from the AGILE and VIALE studies and associated potential adverse reactions, she opted for starting treatment with AZA and IVO.
On March 31, 2022, she began receiving AZA 75 mg/m2 intravenous (IV) once daily days 1-7 and oral IVO 500 mg once daily continuously. She has received 12 cycles and has not needed transfusion. She has not had febrile neutropenia or symptoms of differentiation syndrome. On March 24, 2023, she underwent laparoscopic cholecystectomy, because an ultrasound revealed cholelithiasis, abnormal gallbladder wall thickening, and pericholecystic fluid. She was discharged home the following day and is continuing with AZA/ivosidenib.
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023;108(2):306-307. doi:10.3324/haematol.2022.280803
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. doi:10.1182/blood.2019001239
- Huerga-Domínguez S, Villar S, Prósper F, Alfonso-Piérola A. Updates on the management of acute myeloid leukemia. Cancers (Basel). 2022;14(19):4756. doi:10.3390/cancers14194756
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi:10.6004/jnccn.2019.0028
- Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852-1858. doi:10.1038/s41591-020-1089-8
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. doi:10.1182/blood.2022016867
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. doi:10.1200/JCO.2015.64.0060
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(17):1845-1857. doi:10.1182/blood.2021014586
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740. doi:10.1056/NEJMoa1902688
- Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi:10.1158/1078-0432.CCR-21-3467
- Russell-Smith TA, Gurskyte L, Muresan B, et al. Efficacy of non-intensive therapies approved for relapsed/refractory acute myeloid leukemia: a systematic literature review. Future Oncol. 2022;18(16):2029-2039. doi:10.2217/fon-2021-1355
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649. doi:10.1200/JCO.2014.60.0890
- Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631-640. doi:10.1080/1042819.2019.1688320
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. doi:10.1200/JCO.2017.77.6112
- Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(suppl 1):15. doi:10.1182/blood-2019-124500
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi:10.1016/S2352-3026(21)00134-4
- Alharthy H, Alkaabba F, Williams M, et al. Outcomes of newly diagnosed therapy-related AML and AML with myelodysplasia-related changes treated with 7+3, hypomethylating agents with or without venetoclax and CPX-351: a retrospective cohort study. Blood. 2022;140(suppl 1):9025-9026. doi:10.1182/blood-2022-170688
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023;108(2):306-307. doi:10.3324/haematol.2022.280803
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. doi:10.1182/blood.2019001239
- Huerga-Domínguez S, Villar S, Prósper F, Alfonso-Piérola A. Updates on the management of acute myeloid leukemia. Cancers (Basel). 2022;14(19):4756. doi:10.3390/cancers14194756
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi:10.6004/jnccn.2019.0028
- Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852-1858. doi:10.1038/s41591-020-1089-8
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. doi:10.1182/blood.2022016867
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. doi:10.1200/JCO.2015.64.0060
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(17):1845-1857. doi:10.1182/blood.2021014586
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740. doi:10.1056/NEJMoa1902688
- Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi:10.1158/1078-0432.CCR-21-3467
- Russell-Smith TA, Gurskyte L, Muresan B, et al. Efficacy of non-intensive therapies approved for relapsed/refractory acute myeloid leukemia: a systematic literature review. Future Oncol. 2022;18(16):2029-2039. doi:10.2217/fon-2021-1355
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649. doi:10.1200/JCO.2014.60.0890
- Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631-640. doi:10.1080/1042819.2019.1688320
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. doi:10.1200/JCO.2017.77.6112
- Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(suppl 1):15. doi:10.1182/blood-2019-124500
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi:10.1016/S2352-3026(21)00134-4
- Alharthy H, Alkaabba F, Williams M, et al. Outcomes of newly diagnosed therapy-related AML and AML with myelodysplasia-related changes treated with 7+3, hypomethylating agents with or without venetoclax and CPX-351: a retrospective cohort study. Blood. 2022;140(suppl 1):9025-9026. doi:10.1182/blood-2022-170688
Advances in Management of Relapsed/Refractory Hairy Cell Leukemia
Pathophysiology
HCL develops from activated, mature memory B-cells that, in most cases, have the acquired mutation in BRAF V600E, which is present in 80% to 90% of patients with classic HCL.1,3,5 BRAF is an integral part of the RAS-BRAF-MEK-ERK cellular pathway that transmits growth factor signals from the cell surface to the nucleus to regulate cell growth and proliferation.6 Mutated BRAF V600E continuously activates BRAF kinase and downstream signaling, resulting in enhanced HCL cell survival and unchecked proliferation.3
Variant HCL (HCLv) is a separate, more virulent disease that lacks BRAF V600E mutation and CD25 expression on flow cytometry.1,7-9 Patients with HCLv have a worse prognosis and poor responses to front-line purine analogs, and a higher proportion of these patients carry the unmutated immunoglobulin heavy chain variable (IGHV) gene (54% vs 17% in HCL).1,10,11 About 30% to 50% have wild-type BRAF and activating mutations in MAP2K1, which encodes aberrant MEK downstream of BRAF.10,12
Most patients with HCL have somatic mutations in the IGHV gene.3,13,14 Patients with unmutated IGHV4-34 and wildtype BRAF have an aggressive form of the disease, even if the HCL cells express CD25 as in classic HCL.1,15 HCL in patients with unmutated IGHV is often refractory to purine analogs and these patients have poor prognosis and rapid progression.16 Other identified mutations include CDKN1B in HCL and MAP2K1 and CCNC3 in HCLv.2
Signs and Symptoms
In many cases, HCL is asymptomatic, and diagnosed when pancytopenia, monocytopenia, and leukopenia are discovered on unrelated blood work.2,3,11 Monocytopenia is a specific presentation of HCL, but not HCLv.11 Typical systemic symptoms include unexplained weight loss and extreme fatigue (80%).1,3 Other symptoms can include fever, recurrent infections, night sweats, splenomegaly and related pain or abdominal fullness, hepatomegaly, and bleeding or bruising due to thrombocytopenia.1,3 Splenomegaly is associated with advanced disease.11
Up to 30% of patients may present with autoimmune disorders such as vasculitis or psoriasis. Although skin involvement is rare with HCL, 10% to 12% of patients will have dermatologic symptoms either due to recurrent infection or autoimmune reactions.1,2 Skin reactions include localized or generalized maculopapular rash, pyoderma gangrenosum (which may be severe), and recurrent bacterial or viral skin infections.17
Diagnosis
After complete history and physical examination, a diagnosis of HCL is usually made based on flow cytometry for immunophenotyping and molecular testing for BRAF V600E (Table 1).2,17
Disease-related fibrosis may impede bone marrow aspiration, and trephine biopsy should be done to make the diagnosis.11 On morphologic examination, HCL cells are small- to medium-sized, with round, oval, or indented, well-defined nuclei. Cytoplasm is pale blue, and cells have small cytoplasmic projections (Figure 1).2,18
On flow cytometry, HCL is positive for B-cell antigens (CD19, CD20, CD22), as well as antigens specific to the disease (CD11c, CD25, CD103, CD123), and by immunohistochemistry (IHC) for cyclin D1 and annexin-A1. CD20, CD123, and CD200 are bright in HCL. The presence of T-cell marker CD103 on B-cells indicates HCL.1-3 HCLv, in contrast, is positive for CD11c and CD103, but usually negative for CD25, CD123, and annexin-A1.2,19
BRAF V600E mutation can be identified using droplet digital polymerase chain reaction (PCR), next-generation molecular sequencing, or IHC with a VE1 stain.3,11 IHC for CD20, annexin-1, and VE1 establish the diagnosis, but also are useful in determining the extent to which leukemic cells have infiltrated bone marrow.11
Differential diagnosis of HCL includes HCL variants, splenic marginal zone lymphoma, and splenic diffuse red pulp small B-cell lymphoma.7,11
Indications for Treatment and Criteria for Response
Over time, about 90% of patients with HCL will require treatment. However, not all such patients will require urgent or immediate treatment, and some can be managed with observation and close monitoring.1,11 The indications for initiating treatment generally are systemic symptoms and significant pancytopenia (Table 2).2,11
The optimal response with treatment of HCL is complete response (CR) without minimal residual disease (MRD-free), which minimizes the risk for relapse.1,11 Hematologic and molecular response is assessed using peripheral blood samples; physical examination, ultrasound, computed tomography, or magnetic resonance imaging is used to determine response in lymph nodes, spleen, or liver.1 MRD-free is defined by the absence of HCL cells by the chosen method (IHC, flow cytometry, or PCR).20 Bone marrow aspirate flow cytometry is the most sensitive standard test for MRD detection.1 Table 3 summarizes response criteria for HCL.2,11
Initial Treatment of HCL
The purine nucleoside analogs (PNAs) cladribine (± rituximab) and pentostatin are widely recommended for initial treatment.1,2,11 As monotherapy, cladribine and pentostatin are considered similarly effective, with CR in 70% to 90% of patients and durations of response > 10 years.1 Adding the anti-CD20 monoclonal antibody rituximab in 8 weekly doses starting the first day of front-line cladribine (CDAR) improves remission, MRD-free rates, and duration of response (94% MRD-free at 96 months), with minimal added toxicity.21 Rituximab is often added 4 weeks after cladribine, which offers more convenience, an equally high CR rate of 100%, and a 76% MRD-free rate at 3 months.11 Bone marrow biopsy should be delayed for 4 to 6 months to allow a full response to develop with cladribine.1,11
Daily (intravenous or subcutaneous) and weekly cladribine are equally safe and effective.2,11 Pentostatin is administered intravenously every 2 weeks for 3 to 6 months, allowing time for hematologic recovery between doses.1,11 Patient factors to consider when choosing treatment include baseline neutropenia, patient preference, and comorbidities.
Toxicities of PNAs include neutropenia and fever, which typically occur during the first month of treatment and are more frequent in patients with baseline severe neutropenia; T-cell recovery may take years.1 CDAR is associated with higher transient thrombocytopenia, but faster platelet and neutrophil recovery at 4 weeks than cladribine alone.21 Both therapies are immunosuppressive. Patients should be evaluated for existing infections and watched for new infections during treatment. Control of active infection prior to treatment initiation is required.11,23
Patients with confirmed BRAF V600E mutation are candidates for vemurafenib if they are unable to tolerate a PNA, have an active infection, or would like effective vaccinations.2,23-25
Treatment at Relapse
At suspected HCL relapse, patients should be evaluated to determine whether cytopenia is due to recurrent disease or lingering effects from prior treatment. Use of successive flow cytometry over time can clarify whether symptoms are related to disease and need interventional treatment, or will resolve with additional time.1
Patients who have an HCL relapse after initial therapy with cladribine or pentostatin may be candidates for re-treatment with the same or alternate PNA plus rituximab.2 Rituximab
monotherapy has been used for patients unable to tolerate PNA but yields CR rates as low as 13%.26 Repeated courses of PNA therapy yield lower rates and durations of response with each course.1,2
For patients with primary refractory disease (less than CR with initial therapy) or relapse within 2 years of initial therapy, treatment with the BRAF V600E inhibitor vemurafenib off-label, with or without rituximab, is an option.2,5 In HCL, vemurafenib for patients with relapsed or refractory disease achieved CR in 35% and 42% in 2 small trials (N = 54). Relapse-free survival among people with CR was 19 months in 1 of the trials.27 Vemurafenib plus rituximab achieved CR in 87% of patients with relapsed or refractory HCL, and an MRD-free CR rate of 57%. Among patients with CR, 85% were relapse-free at a median follow-up of 34 months.5 Treatment with vemurafenib is not myelotoxic—an advantage for HCL patients. Adverse effects with vemurafenib are often manageable with dose reductions, if needed. A specific concern with vemurafenib is the potential development of secondary skin cancers.5,27,28
Novel Targeted Options and Recommended Use
Promising alternatives for patients with relapsed or refractory HCL include combined BRAF and MEK inhibitors and the Bruton tyrosine kinase (BTK) inhibitor ibrutinib. The concept of BRAF/MEK inhibition was validated in studies with BRAF-mutated melanoma, in which dabrafenib plus trametinib (the MEK inhibitor) improved overall survival (OS) with less toxicity and better quality of life than vemurafenib.1,29 In a phase 2 trial in HCL, dabrafenib monotherapy demonstrated an overall response rate (ORR) of 80%, including 30% CR.30 In a subsequent phase 2 trial, dabrafenib combined with trametinib was evaluated in refractory or late relapsed HCL. Among 55 enrolled patients, objective response rate was 89%, including 65.5% CR. Nine of 36 patients with CR were MRD-free. Among responding patients, duration of response was 97.7% at 24 months.31 The most common grade ≥ 3 toxicities were hyperglycemia, pyrexia, neutropenia, and pneumonia. Secondary skin cancers were seen in about 5% of patients.31
BRAF/MEK inhibitor combinations in HCL offer effective therapy with less myelosuppression than PNAs, making them useful for patients with or at risk for infection.23 Their use in HCL is off-label, as they currently are approved for treatment of BRAF-mutated melanoma and some other tumors.32 A study of encorafenib (a BRAF inhibitor) combined with binimetinib (a MEK inhibitor) is ongoing (Table 4).32
Ibrutinib interrupts B-cell receptor signaling to stop tumor cell growth. In a phase 2 trial, patients with relapsed or refractory HCL or HCLv were treated with once-daily oral ibrutinib. Best ORR was 54% (19% CR; 3% MRD-free). Despite the low CR rate, 3-year progression-free survival with ibrutinib was 73% and OS was 85%. Treatment was well-tolerated; cytopenia (including 22% grade ≥ 3 thrombocytopenia and neutropenia) and diarrhea were frequent toxicities.33
Moxetumomab pasudotox is a novel CD22-targeted antibody fused with protein toxin that interrupts protein synthesis in tumor cells.1 As treatment, it was studied in a phase 3 trial of relapsed HCL in heavily pretreated patients, and achieved a CR rate of 41%, including 36% durable CR.34 Although FDA-approved for relapsed or refractory HCL, the drug is being discontinued due to business decisions, not safety or efficacy concerns.2 It is notable that many types of B-cell lymphoma also express CD22.35
Enrollment in a clinical trial to study possible treatment advances is recommended by the National Comprehensive Cancer Network (NCCN) at first and subsequent relapses of HCL for appropriate patients.2 Figure 2 summarizes an approach to treatment choice and sequencing for patients with HCL.
Supportive Care
Patients being treated for HCL should have supportive care to manage adverse effects of their disease. Such care includes prophylaxis against herpes virus if CD4+ T cells < 200 cells/μL and other prophylactic vaccinations to hepatitis B virus, COVID-19 and Influenza. Patients with neutropeni may require broad-spectrum antibacterial prophylaxis or neutrophil growth factors if neutropenic fever develops. Blood product support is recommended if needed.2 Assessment of anti-COVID-19 antibodies is recommended to optimize immunity, particularly prior to beginning anti-CD20 antibody therapy like rituximab.23
Unmet Needs
Despite improvements in response and survival with newer therapies, not all patients with HCL benefit from these advances. Unmet needs are finding optimal treatment for patients with HCLv, despite some success with MEK inhibitors, and for patients with BRAF mutations other than V600E, who have few options beyond PNAs and rituximab.
- Kreitman RJ, Arons E. Diagnosis and treatment of hairy cell leukemia as the COVID-19 pandemic continues. Blood Rev. 2022;51:100888. doi:10.1016/j.blre.2021.100888
- National Comprehensive Cancer Network. NCCN clinical practice guideline in oncology: hairy cell leukemia. Version 1.2023. Published August 30, 2022. Accessed March 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf
- Janus A, Robak T. Hairy cell leukemia. In: Li W, ed. Leukemia [Internet]. Brisbane: Exon Publications; 2022:chap3. Accessed February 16, 2023. doi:10.36255/exon-publications-leukemia-hairy-cell-leukemia
- Tadmor T, Polliack A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract Res Clin Haematol. 2015;28(4):175-179. doi:10.1016/j.beha.2015.10.014
- Tiacci E, De Carolis L, Simonetti E, et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N Engl J Med. 2021;384(19):1810-1823. doi:10.1056/NEJMoa20312986
- Falini B, Martelli MP, Tiacci E. BRAF V600E mutation in hairy cell leukemia: from bench to bedside. Blood. 2016;128(15):1918-1927. doi:10.1182/blood-2016-07-418434
- Matutes E. Diagnostic and therapeutic challenges in hairy cell leukemia-variant: where are we in 2021? Expert Rev Hematol. 2021;14(4):355-363. doi:10.1080/17474086.2021.1908121
- Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4(6):547-559. doi:10.1016/0145-2126(80)90066-1
- Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012;119(14):3330-3332. doi:10.1182/blood-2011-09-379339
- Durham BH, Getta B, Dietrich S, et al. Genomic analysis of hairy cell leukemia identifies novel recurrent genetic alterations. Blood. 2017;130(14):1644-1648. doi:10.1182/blood-2017-01-76510711
- Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with hairy cell leukemia. Blood. 2017;129(5):553-560. doi:10.1182/blood-2016-01-689422
- Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet. 2014;46(1):8-10. doi:10.1038/ng.2828
- Arons E, Sunshine J, Suntum T, Kreitman RJ. Somatic hypermutation and VH gene usage in hairy cell leukaemia. Br J Haematol. 2006;133(5):504-512. doi:10.1111/j.1365-2141.2006.06066.x
- Arons E, Roth L, Sapolsky J, Suntum T, Stetler-Stevenson M, Kreitman RJ. Evidence of canonical somatic hypermutation in hairy cell leukemia. Blood. 2011;117(18):4844-4851. doi:10.1182/blood-2010-11-316737
- Arons E, Suntum T, Stetler-Stevenson M, Kreitman RJ. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood. 2009;114(21):4687-4695. doi:10.1182/blood-2009-01-201731
- Forconi F, Sozzi E, Cencini E, et al. Hairy cell leukemias with unmutated IGHV genes define the minor subset refractory to single-agent cladribine and with more aggressive behavior. Blood. 2009;114(21):4696-4702. doi:10.1182/blood-2009-03-212449
- Robak E, Jesionek-Kupnicka D, Robak T. Skin changes in hairy cell leukemia. Ann Hematol. 2021;100(3):615-625. doi:10.1007/s00277-020-04349-z
- Bouroncle BA. Thirty-five years in the progress of hairy cell leukemia. Leuk Lymphoma. 1994;14(suppl 1):1-12. https://pubmed.ncbi.nlm.nih.gov/7820038/
- Falini B, Tiacci E, Liso A, et al. Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet. 2004;363(9424): 1869-1870. doi:10.1016/S0140-6736(04)16356-3
- Robak T, Robak P. Measurable residual disease in hairy cell leukemia: technical considerations and clinical significance. Front Oncol. 2022;12:976374. doi:10.3389/fonc.2022.976374
- Chihara D, Arons E, Stetler-Stevenson M, et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020;38(14):1527-1538. doi:10.1200/JCO.19.02250
- Chihara D, Kantarjian H, O’Brien S, et al. Long-term durable remission by cladribine followed by rituximab in patients with hairy cell leukaemia: update of a phase II trial. Br J Haematol. 2016;174(5):760-766. doi:10.1111/bjh.14129
- Grever M, Andritsos L, Banerji V, et al. Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia. 2021;35(7):1864-1872. doi:10.1038/s41375-021-01257-7
- Konrat J, Rösler W, Roiss M, et al. BRAF inhibitor treatment of classical hairy cell leukemia allows successful vaccination against SARS-CoV-2. Ann Hematol. 2023;102(2):403-406. doi:10.1007/s00277-022-05026-z
- Park JH, Shukla M, Salcedo JM, et al. First-line chemo-free therapy with the BRAF inhibitor vemurafenib combined with obinutuzumab is effective in patients with HCL. Blood. 2019;134(suppl 1):Abstract 3998. https://doi.org/10.1182/blood-2019-124478
- Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102(3):810-813. doi:10.1182/blood-2003-01-0014
- Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373(18):1733-1747. doi:10.1056/NEJMoa1506583
- Maitre E, Paillassa J, Troussard X. Novel targeted treatments in hairy cell leukemia and other hairy cell-like disorders. Front Oncol. 2022;12:1068981. doi:10.3389/fonc.2022.1068981
- Grob JJ, Amonkar MM, Karaszewska B, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015;16(13):1389-1398. doi:10.1016/S1470-2045(15)00087-X
- Tiacci E, De Carolis L, Simonetti E, et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: a pilot phase-2 clinical trial. Leukemia. 2021;35(11):3314-3318. doi:10.1038/s41375-021-01210-8
- Kreitman RJ, Moreau P, Ravandi F, et al. Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia. Blood. 2023;141(9):996-1006. doi:10.1182/blood.2021013658
- Adashek JJ, Menta AK, Reddy NK, Desai AP, Roszik J, Subbiah V. Tissue agnostic activity of BRAF plus MEK inhibitor in BRAF V600E-mutated tumors. Mol Cancer Ther. 2022;21(6):871-878. doi:10.1158/1535-7163.MCT-21-0950
- Rogers KA, Andritsos LA, Wei L, et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood. 2021;137(25):3473-3483. doi:10.1182/blood.2020009688
- Kreitman RJ, Dearden C, Zinzani PL, et al; Study 1053 investigators. Moxetumomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): long-term follow-up from the pivotal trial. J Hematol Oncol. 2021;14(1):35. doi:10.1186/s13045-020-01004-y
- Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26(25):3704-3713. doi:10.1038/sj.onc.1210370
Pathophysiology
HCL develops from activated, mature memory B-cells that, in most cases, have the acquired mutation in BRAF V600E, which is present in 80% to 90% of patients with classic HCL.1,3,5 BRAF is an integral part of the RAS-BRAF-MEK-ERK cellular pathway that transmits growth factor signals from the cell surface to the nucleus to regulate cell growth and proliferation.6 Mutated BRAF V600E continuously activates BRAF kinase and downstream signaling, resulting in enhanced HCL cell survival and unchecked proliferation.3
Variant HCL (HCLv) is a separate, more virulent disease that lacks BRAF V600E mutation and CD25 expression on flow cytometry.1,7-9 Patients with HCLv have a worse prognosis and poor responses to front-line purine analogs, and a higher proportion of these patients carry the unmutated immunoglobulin heavy chain variable (IGHV) gene (54% vs 17% in HCL).1,10,11 About 30% to 50% have wild-type BRAF and activating mutations in MAP2K1, which encodes aberrant MEK downstream of BRAF.10,12
Most patients with HCL have somatic mutations in the IGHV gene.3,13,14 Patients with unmutated IGHV4-34 and wildtype BRAF have an aggressive form of the disease, even if the HCL cells express CD25 as in classic HCL.1,15 HCL in patients with unmutated IGHV is often refractory to purine analogs and these patients have poor prognosis and rapid progression.16 Other identified mutations include CDKN1B in HCL and MAP2K1 and CCNC3 in HCLv.2
Signs and Symptoms
In many cases, HCL is asymptomatic, and diagnosed when pancytopenia, monocytopenia, and leukopenia are discovered on unrelated blood work.2,3,11 Monocytopenia is a specific presentation of HCL, but not HCLv.11 Typical systemic symptoms include unexplained weight loss and extreme fatigue (80%).1,3 Other symptoms can include fever, recurrent infections, night sweats, splenomegaly and related pain or abdominal fullness, hepatomegaly, and bleeding or bruising due to thrombocytopenia.1,3 Splenomegaly is associated with advanced disease.11
Up to 30% of patients may present with autoimmune disorders such as vasculitis or psoriasis. Although skin involvement is rare with HCL, 10% to 12% of patients will have dermatologic symptoms either due to recurrent infection or autoimmune reactions.1,2 Skin reactions include localized or generalized maculopapular rash, pyoderma gangrenosum (which may be severe), and recurrent bacterial or viral skin infections.17
Diagnosis
After complete history and physical examination, a diagnosis of HCL is usually made based on flow cytometry for immunophenotyping and molecular testing for BRAF V600E (Table 1).2,17
Disease-related fibrosis may impede bone marrow aspiration, and trephine biopsy should be done to make the diagnosis.11 On morphologic examination, HCL cells are small- to medium-sized, with round, oval, or indented, well-defined nuclei. Cytoplasm is pale blue, and cells have small cytoplasmic projections (Figure 1).2,18
On flow cytometry, HCL is positive for B-cell antigens (CD19, CD20, CD22), as well as antigens specific to the disease (CD11c, CD25, CD103, CD123), and by immunohistochemistry (IHC) for cyclin D1 and annexin-A1. CD20, CD123, and CD200 are bright in HCL. The presence of T-cell marker CD103 on B-cells indicates HCL.1-3 HCLv, in contrast, is positive for CD11c and CD103, but usually negative for CD25, CD123, and annexin-A1.2,19
BRAF V600E mutation can be identified using droplet digital polymerase chain reaction (PCR), next-generation molecular sequencing, or IHC with a VE1 stain.3,11 IHC for CD20, annexin-1, and VE1 establish the diagnosis, but also are useful in determining the extent to which leukemic cells have infiltrated bone marrow.11
Differential diagnosis of HCL includes HCL variants, splenic marginal zone lymphoma, and splenic diffuse red pulp small B-cell lymphoma.7,11
Indications for Treatment and Criteria for Response
Over time, about 90% of patients with HCL will require treatment. However, not all such patients will require urgent or immediate treatment, and some can be managed with observation and close monitoring.1,11 The indications for initiating treatment generally are systemic symptoms and significant pancytopenia (Table 2).2,11
The optimal response with treatment of HCL is complete response (CR) without minimal residual disease (MRD-free), which minimizes the risk for relapse.1,11 Hematologic and molecular response is assessed using peripheral blood samples; physical examination, ultrasound, computed tomography, or magnetic resonance imaging is used to determine response in lymph nodes, spleen, or liver.1 MRD-free is defined by the absence of HCL cells by the chosen method (IHC, flow cytometry, or PCR).20 Bone marrow aspirate flow cytometry is the most sensitive standard test for MRD detection.1 Table 3 summarizes response criteria for HCL.2,11
Initial Treatment of HCL
The purine nucleoside analogs (PNAs) cladribine (± rituximab) and pentostatin are widely recommended for initial treatment.1,2,11 As monotherapy, cladribine and pentostatin are considered similarly effective, with CR in 70% to 90% of patients and durations of response > 10 years.1 Adding the anti-CD20 monoclonal antibody rituximab in 8 weekly doses starting the first day of front-line cladribine (CDAR) improves remission, MRD-free rates, and duration of response (94% MRD-free at 96 months), with minimal added toxicity.21 Rituximab is often added 4 weeks after cladribine, which offers more convenience, an equally high CR rate of 100%, and a 76% MRD-free rate at 3 months.11 Bone marrow biopsy should be delayed for 4 to 6 months to allow a full response to develop with cladribine.1,11
Daily (intravenous or subcutaneous) and weekly cladribine are equally safe and effective.2,11 Pentostatin is administered intravenously every 2 weeks for 3 to 6 months, allowing time for hematologic recovery between doses.1,11 Patient factors to consider when choosing treatment include baseline neutropenia, patient preference, and comorbidities.
Toxicities of PNAs include neutropenia and fever, which typically occur during the first month of treatment and are more frequent in patients with baseline severe neutropenia; T-cell recovery may take years.1 CDAR is associated with higher transient thrombocytopenia, but faster platelet and neutrophil recovery at 4 weeks than cladribine alone.21 Both therapies are immunosuppressive. Patients should be evaluated for existing infections and watched for new infections during treatment. Control of active infection prior to treatment initiation is required.11,23
Patients with confirmed BRAF V600E mutation are candidates for vemurafenib if they are unable to tolerate a PNA, have an active infection, or would like effective vaccinations.2,23-25
Treatment at Relapse
At suspected HCL relapse, patients should be evaluated to determine whether cytopenia is due to recurrent disease or lingering effects from prior treatment. Use of successive flow cytometry over time can clarify whether symptoms are related to disease and need interventional treatment, or will resolve with additional time.1
Patients who have an HCL relapse after initial therapy with cladribine or pentostatin may be candidates for re-treatment with the same or alternate PNA plus rituximab.2 Rituximab
monotherapy has been used for patients unable to tolerate PNA but yields CR rates as low as 13%.26 Repeated courses of PNA therapy yield lower rates and durations of response with each course.1,2
For patients with primary refractory disease (less than CR with initial therapy) or relapse within 2 years of initial therapy, treatment with the BRAF V600E inhibitor vemurafenib off-label, with or without rituximab, is an option.2,5 In HCL, vemurafenib for patients with relapsed or refractory disease achieved CR in 35% and 42% in 2 small trials (N = 54). Relapse-free survival among people with CR was 19 months in 1 of the trials.27 Vemurafenib plus rituximab achieved CR in 87% of patients with relapsed or refractory HCL, and an MRD-free CR rate of 57%. Among patients with CR, 85% were relapse-free at a median follow-up of 34 months.5 Treatment with vemurafenib is not myelotoxic—an advantage for HCL patients. Adverse effects with vemurafenib are often manageable with dose reductions, if needed. A specific concern with vemurafenib is the potential development of secondary skin cancers.5,27,28
Novel Targeted Options and Recommended Use
Promising alternatives for patients with relapsed or refractory HCL include combined BRAF and MEK inhibitors and the Bruton tyrosine kinase (BTK) inhibitor ibrutinib. The concept of BRAF/MEK inhibition was validated in studies with BRAF-mutated melanoma, in which dabrafenib plus trametinib (the MEK inhibitor) improved overall survival (OS) with less toxicity and better quality of life than vemurafenib.1,29 In a phase 2 trial in HCL, dabrafenib monotherapy demonstrated an overall response rate (ORR) of 80%, including 30% CR.30 In a subsequent phase 2 trial, dabrafenib combined with trametinib was evaluated in refractory or late relapsed HCL. Among 55 enrolled patients, objective response rate was 89%, including 65.5% CR. Nine of 36 patients with CR were MRD-free. Among responding patients, duration of response was 97.7% at 24 months.31 The most common grade ≥ 3 toxicities were hyperglycemia, pyrexia, neutropenia, and pneumonia. Secondary skin cancers were seen in about 5% of patients.31
BRAF/MEK inhibitor combinations in HCL offer effective therapy with less myelosuppression than PNAs, making them useful for patients with or at risk for infection.23 Their use in HCL is off-label, as they currently are approved for treatment of BRAF-mutated melanoma and some other tumors.32 A study of encorafenib (a BRAF inhibitor) combined with binimetinib (a MEK inhibitor) is ongoing (Table 4).32
Ibrutinib interrupts B-cell receptor signaling to stop tumor cell growth. In a phase 2 trial, patients with relapsed or refractory HCL or HCLv were treated with once-daily oral ibrutinib. Best ORR was 54% (19% CR; 3% MRD-free). Despite the low CR rate, 3-year progression-free survival with ibrutinib was 73% and OS was 85%. Treatment was well-tolerated; cytopenia (including 22% grade ≥ 3 thrombocytopenia and neutropenia) and diarrhea were frequent toxicities.33
Moxetumomab pasudotox is a novel CD22-targeted antibody fused with protein toxin that interrupts protein synthesis in tumor cells.1 As treatment, it was studied in a phase 3 trial of relapsed HCL in heavily pretreated patients, and achieved a CR rate of 41%, including 36% durable CR.34 Although FDA-approved for relapsed or refractory HCL, the drug is being discontinued due to business decisions, not safety or efficacy concerns.2 It is notable that many types of B-cell lymphoma also express CD22.35
Enrollment in a clinical trial to study possible treatment advances is recommended by the National Comprehensive Cancer Network (NCCN) at first and subsequent relapses of HCL for appropriate patients.2 Figure 2 summarizes an approach to treatment choice and sequencing for patients with HCL.
Supportive Care
Patients being treated for HCL should have supportive care to manage adverse effects of their disease. Such care includes prophylaxis against herpes virus if CD4+ T cells < 200 cells/μL and other prophylactic vaccinations to hepatitis B virus, COVID-19 and Influenza. Patients with neutropeni may require broad-spectrum antibacterial prophylaxis or neutrophil growth factors if neutropenic fever develops. Blood product support is recommended if needed.2 Assessment of anti-COVID-19 antibodies is recommended to optimize immunity, particularly prior to beginning anti-CD20 antibody therapy like rituximab.23
Unmet Needs
Despite improvements in response and survival with newer therapies, not all patients with HCL benefit from these advances. Unmet needs are finding optimal treatment for patients with HCLv, despite some success with MEK inhibitors, and for patients with BRAF mutations other than V600E, who have few options beyond PNAs and rituximab.
Pathophysiology
HCL develops from activated, mature memory B-cells that, in most cases, have the acquired mutation in BRAF V600E, which is present in 80% to 90% of patients with classic HCL.1,3,5 BRAF is an integral part of the RAS-BRAF-MEK-ERK cellular pathway that transmits growth factor signals from the cell surface to the nucleus to regulate cell growth and proliferation.6 Mutated BRAF V600E continuously activates BRAF kinase and downstream signaling, resulting in enhanced HCL cell survival and unchecked proliferation.3
Variant HCL (HCLv) is a separate, more virulent disease that lacks BRAF V600E mutation and CD25 expression on flow cytometry.1,7-9 Patients with HCLv have a worse prognosis and poor responses to front-line purine analogs, and a higher proportion of these patients carry the unmutated immunoglobulin heavy chain variable (IGHV) gene (54% vs 17% in HCL).1,10,11 About 30% to 50% have wild-type BRAF and activating mutations in MAP2K1, which encodes aberrant MEK downstream of BRAF.10,12
Most patients with HCL have somatic mutations in the IGHV gene.3,13,14 Patients with unmutated IGHV4-34 and wildtype BRAF have an aggressive form of the disease, even if the HCL cells express CD25 as in classic HCL.1,15 HCL in patients with unmutated IGHV is often refractory to purine analogs and these patients have poor prognosis and rapid progression.16 Other identified mutations include CDKN1B in HCL and MAP2K1 and CCNC3 in HCLv.2
Signs and Symptoms
In many cases, HCL is asymptomatic, and diagnosed when pancytopenia, monocytopenia, and leukopenia are discovered on unrelated blood work.2,3,11 Monocytopenia is a specific presentation of HCL, but not HCLv.11 Typical systemic symptoms include unexplained weight loss and extreme fatigue (80%).1,3 Other symptoms can include fever, recurrent infections, night sweats, splenomegaly and related pain or abdominal fullness, hepatomegaly, and bleeding or bruising due to thrombocytopenia.1,3 Splenomegaly is associated with advanced disease.11
Up to 30% of patients may present with autoimmune disorders such as vasculitis or psoriasis. Although skin involvement is rare with HCL, 10% to 12% of patients will have dermatologic symptoms either due to recurrent infection or autoimmune reactions.1,2 Skin reactions include localized or generalized maculopapular rash, pyoderma gangrenosum (which may be severe), and recurrent bacterial or viral skin infections.17
Diagnosis
After complete history and physical examination, a diagnosis of HCL is usually made based on flow cytometry for immunophenotyping and molecular testing for BRAF V600E (Table 1).2,17
Disease-related fibrosis may impede bone marrow aspiration, and trephine biopsy should be done to make the diagnosis.11 On morphologic examination, HCL cells are small- to medium-sized, with round, oval, or indented, well-defined nuclei. Cytoplasm is pale blue, and cells have small cytoplasmic projections (Figure 1).2,18
On flow cytometry, HCL is positive for B-cell antigens (CD19, CD20, CD22), as well as antigens specific to the disease (CD11c, CD25, CD103, CD123), and by immunohistochemistry (IHC) for cyclin D1 and annexin-A1. CD20, CD123, and CD200 are bright in HCL. The presence of T-cell marker CD103 on B-cells indicates HCL.1-3 HCLv, in contrast, is positive for CD11c and CD103, but usually negative for CD25, CD123, and annexin-A1.2,19
BRAF V600E mutation can be identified using droplet digital polymerase chain reaction (PCR), next-generation molecular sequencing, or IHC with a VE1 stain.3,11 IHC for CD20, annexin-1, and VE1 establish the diagnosis, but also are useful in determining the extent to which leukemic cells have infiltrated bone marrow.11
Differential diagnosis of HCL includes HCL variants, splenic marginal zone lymphoma, and splenic diffuse red pulp small B-cell lymphoma.7,11
Indications for Treatment and Criteria for Response
Over time, about 90% of patients with HCL will require treatment. However, not all such patients will require urgent or immediate treatment, and some can be managed with observation and close monitoring.1,11 The indications for initiating treatment generally are systemic symptoms and significant pancytopenia (Table 2).2,11
The optimal response with treatment of HCL is complete response (CR) without minimal residual disease (MRD-free), which minimizes the risk for relapse.1,11 Hematologic and molecular response is assessed using peripheral blood samples; physical examination, ultrasound, computed tomography, or magnetic resonance imaging is used to determine response in lymph nodes, spleen, or liver.1 MRD-free is defined by the absence of HCL cells by the chosen method (IHC, flow cytometry, or PCR).20 Bone marrow aspirate flow cytometry is the most sensitive standard test for MRD detection.1 Table 3 summarizes response criteria for HCL.2,11
Initial Treatment of HCL
The purine nucleoside analogs (PNAs) cladribine (± rituximab) and pentostatin are widely recommended for initial treatment.1,2,11 As monotherapy, cladribine and pentostatin are considered similarly effective, with CR in 70% to 90% of patients and durations of response > 10 years.1 Adding the anti-CD20 monoclonal antibody rituximab in 8 weekly doses starting the first day of front-line cladribine (CDAR) improves remission, MRD-free rates, and duration of response (94% MRD-free at 96 months), with minimal added toxicity.21 Rituximab is often added 4 weeks after cladribine, which offers more convenience, an equally high CR rate of 100%, and a 76% MRD-free rate at 3 months.11 Bone marrow biopsy should be delayed for 4 to 6 months to allow a full response to develop with cladribine.1,11
Daily (intravenous or subcutaneous) and weekly cladribine are equally safe and effective.2,11 Pentostatin is administered intravenously every 2 weeks for 3 to 6 months, allowing time for hematologic recovery between doses.1,11 Patient factors to consider when choosing treatment include baseline neutropenia, patient preference, and comorbidities.
Toxicities of PNAs include neutropenia and fever, which typically occur during the first month of treatment and are more frequent in patients with baseline severe neutropenia; T-cell recovery may take years.1 CDAR is associated with higher transient thrombocytopenia, but faster platelet and neutrophil recovery at 4 weeks than cladribine alone.21 Both therapies are immunosuppressive. Patients should be evaluated for existing infections and watched for new infections during treatment. Control of active infection prior to treatment initiation is required.11,23
Patients with confirmed BRAF V600E mutation are candidates for vemurafenib if they are unable to tolerate a PNA, have an active infection, or would like effective vaccinations.2,23-25
Treatment at Relapse
At suspected HCL relapse, patients should be evaluated to determine whether cytopenia is due to recurrent disease or lingering effects from prior treatment. Use of successive flow cytometry over time can clarify whether symptoms are related to disease and need interventional treatment, or will resolve with additional time.1
Patients who have an HCL relapse after initial therapy with cladribine or pentostatin may be candidates for re-treatment with the same or alternate PNA plus rituximab.2 Rituximab
monotherapy has been used for patients unable to tolerate PNA but yields CR rates as low as 13%.26 Repeated courses of PNA therapy yield lower rates and durations of response with each course.1,2
For patients with primary refractory disease (less than CR with initial therapy) or relapse within 2 years of initial therapy, treatment with the BRAF V600E inhibitor vemurafenib off-label, with or without rituximab, is an option.2,5 In HCL, vemurafenib for patients with relapsed or refractory disease achieved CR in 35% and 42% in 2 small trials (N = 54). Relapse-free survival among people with CR was 19 months in 1 of the trials.27 Vemurafenib plus rituximab achieved CR in 87% of patients with relapsed or refractory HCL, and an MRD-free CR rate of 57%. Among patients with CR, 85% were relapse-free at a median follow-up of 34 months.5 Treatment with vemurafenib is not myelotoxic—an advantage for HCL patients. Adverse effects with vemurafenib are often manageable with dose reductions, if needed. A specific concern with vemurafenib is the potential development of secondary skin cancers.5,27,28
Novel Targeted Options and Recommended Use
Promising alternatives for patients with relapsed or refractory HCL include combined BRAF and MEK inhibitors and the Bruton tyrosine kinase (BTK) inhibitor ibrutinib. The concept of BRAF/MEK inhibition was validated in studies with BRAF-mutated melanoma, in which dabrafenib plus trametinib (the MEK inhibitor) improved overall survival (OS) with less toxicity and better quality of life than vemurafenib.1,29 In a phase 2 trial in HCL, dabrafenib monotherapy demonstrated an overall response rate (ORR) of 80%, including 30% CR.30 In a subsequent phase 2 trial, dabrafenib combined with trametinib was evaluated in refractory or late relapsed HCL. Among 55 enrolled patients, objective response rate was 89%, including 65.5% CR. Nine of 36 patients with CR were MRD-free. Among responding patients, duration of response was 97.7% at 24 months.31 The most common grade ≥ 3 toxicities were hyperglycemia, pyrexia, neutropenia, and pneumonia. Secondary skin cancers were seen in about 5% of patients.31
BRAF/MEK inhibitor combinations in HCL offer effective therapy with less myelosuppression than PNAs, making them useful for patients with or at risk for infection.23 Their use in HCL is off-label, as they currently are approved for treatment of BRAF-mutated melanoma and some other tumors.32 A study of encorafenib (a BRAF inhibitor) combined with binimetinib (a MEK inhibitor) is ongoing (Table 4).32
Ibrutinib interrupts B-cell receptor signaling to stop tumor cell growth. In a phase 2 trial, patients with relapsed or refractory HCL or HCLv were treated with once-daily oral ibrutinib. Best ORR was 54% (19% CR; 3% MRD-free). Despite the low CR rate, 3-year progression-free survival with ibrutinib was 73% and OS was 85%. Treatment was well-tolerated; cytopenia (including 22% grade ≥ 3 thrombocytopenia and neutropenia) and diarrhea were frequent toxicities.33
Moxetumomab pasudotox is a novel CD22-targeted antibody fused with protein toxin that interrupts protein synthesis in tumor cells.1 As treatment, it was studied in a phase 3 trial of relapsed HCL in heavily pretreated patients, and achieved a CR rate of 41%, including 36% durable CR.34 Although FDA-approved for relapsed or refractory HCL, the drug is being discontinued due to business decisions, not safety or efficacy concerns.2 It is notable that many types of B-cell lymphoma also express CD22.35
Enrollment in a clinical trial to study possible treatment advances is recommended by the National Comprehensive Cancer Network (NCCN) at first and subsequent relapses of HCL for appropriate patients.2 Figure 2 summarizes an approach to treatment choice and sequencing for patients with HCL.
Supportive Care
Patients being treated for HCL should have supportive care to manage adverse effects of their disease. Such care includes prophylaxis against herpes virus if CD4+ T cells < 200 cells/μL and other prophylactic vaccinations to hepatitis B virus, COVID-19 and Influenza. Patients with neutropeni may require broad-spectrum antibacterial prophylaxis or neutrophil growth factors if neutropenic fever develops. Blood product support is recommended if needed.2 Assessment of anti-COVID-19 antibodies is recommended to optimize immunity, particularly prior to beginning anti-CD20 antibody therapy like rituximab.23
Unmet Needs
Despite improvements in response and survival with newer therapies, not all patients with HCL benefit from these advances. Unmet needs are finding optimal treatment for patients with HCLv, despite some success with MEK inhibitors, and for patients with BRAF mutations other than V600E, who have few options beyond PNAs and rituximab.
- Kreitman RJ, Arons E. Diagnosis and treatment of hairy cell leukemia as the COVID-19 pandemic continues. Blood Rev. 2022;51:100888. doi:10.1016/j.blre.2021.100888
- National Comprehensive Cancer Network. NCCN clinical practice guideline in oncology: hairy cell leukemia. Version 1.2023. Published August 30, 2022. Accessed March 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf
- Janus A, Robak T. Hairy cell leukemia. In: Li W, ed. Leukemia [Internet]. Brisbane: Exon Publications; 2022:chap3. Accessed February 16, 2023. doi:10.36255/exon-publications-leukemia-hairy-cell-leukemia
- Tadmor T, Polliack A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract Res Clin Haematol. 2015;28(4):175-179. doi:10.1016/j.beha.2015.10.014
- Tiacci E, De Carolis L, Simonetti E, et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N Engl J Med. 2021;384(19):1810-1823. doi:10.1056/NEJMoa20312986
- Falini B, Martelli MP, Tiacci E. BRAF V600E mutation in hairy cell leukemia: from bench to bedside. Blood. 2016;128(15):1918-1927. doi:10.1182/blood-2016-07-418434
- Matutes E. Diagnostic and therapeutic challenges in hairy cell leukemia-variant: where are we in 2021? Expert Rev Hematol. 2021;14(4):355-363. doi:10.1080/17474086.2021.1908121
- Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4(6):547-559. doi:10.1016/0145-2126(80)90066-1
- Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012;119(14):3330-3332. doi:10.1182/blood-2011-09-379339
- Durham BH, Getta B, Dietrich S, et al. Genomic analysis of hairy cell leukemia identifies novel recurrent genetic alterations. Blood. 2017;130(14):1644-1648. doi:10.1182/blood-2017-01-76510711
- Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with hairy cell leukemia. Blood. 2017;129(5):553-560. doi:10.1182/blood-2016-01-689422
- Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet. 2014;46(1):8-10. doi:10.1038/ng.2828
- Arons E, Sunshine J, Suntum T, Kreitman RJ. Somatic hypermutation and VH gene usage in hairy cell leukaemia. Br J Haematol. 2006;133(5):504-512. doi:10.1111/j.1365-2141.2006.06066.x
- Arons E, Roth L, Sapolsky J, Suntum T, Stetler-Stevenson M, Kreitman RJ. Evidence of canonical somatic hypermutation in hairy cell leukemia. Blood. 2011;117(18):4844-4851. doi:10.1182/blood-2010-11-316737
- Arons E, Suntum T, Stetler-Stevenson M, Kreitman RJ. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood. 2009;114(21):4687-4695. doi:10.1182/blood-2009-01-201731
- Forconi F, Sozzi E, Cencini E, et al. Hairy cell leukemias with unmutated IGHV genes define the minor subset refractory to single-agent cladribine and with more aggressive behavior. Blood. 2009;114(21):4696-4702. doi:10.1182/blood-2009-03-212449
- Robak E, Jesionek-Kupnicka D, Robak T. Skin changes in hairy cell leukemia. Ann Hematol. 2021;100(3):615-625. doi:10.1007/s00277-020-04349-z
- Bouroncle BA. Thirty-five years in the progress of hairy cell leukemia. Leuk Lymphoma. 1994;14(suppl 1):1-12. https://pubmed.ncbi.nlm.nih.gov/7820038/
- Falini B, Tiacci E, Liso A, et al. Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet. 2004;363(9424): 1869-1870. doi:10.1016/S0140-6736(04)16356-3
- Robak T, Robak P. Measurable residual disease in hairy cell leukemia: technical considerations and clinical significance. Front Oncol. 2022;12:976374. doi:10.3389/fonc.2022.976374
- Chihara D, Arons E, Stetler-Stevenson M, et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020;38(14):1527-1538. doi:10.1200/JCO.19.02250
- Chihara D, Kantarjian H, O’Brien S, et al. Long-term durable remission by cladribine followed by rituximab in patients with hairy cell leukaemia: update of a phase II trial. Br J Haematol. 2016;174(5):760-766. doi:10.1111/bjh.14129
- Grever M, Andritsos L, Banerji V, et al. Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia. 2021;35(7):1864-1872. doi:10.1038/s41375-021-01257-7
- Konrat J, Rösler W, Roiss M, et al. BRAF inhibitor treatment of classical hairy cell leukemia allows successful vaccination against SARS-CoV-2. Ann Hematol. 2023;102(2):403-406. doi:10.1007/s00277-022-05026-z
- Park JH, Shukla M, Salcedo JM, et al. First-line chemo-free therapy with the BRAF inhibitor vemurafenib combined with obinutuzumab is effective in patients with HCL. Blood. 2019;134(suppl 1):Abstract 3998. https://doi.org/10.1182/blood-2019-124478
- Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102(3):810-813. doi:10.1182/blood-2003-01-0014
- Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373(18):1733-1747. doi:10.1056/NEJMoa1506583
- Maitre E, Paillassa J, Troussard X. Novel targeted treatments in hairy cell leukemia and other hairy cell-like disorders. Front Oncol. 2022;12:1068981. doi:10.3389/fonc.2022.1068981
- Grob JJ, Amonkar MM, Karaszewska B, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015;16(13):1389-1398. doi:10.1016/S1470-2045(15)00087-X
- Tiacci E, De Carolis L, Simonetti E, et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: a pilot phase-2 clinical trial. Leukemia. 2021;35(11):3314-3318. doi:10.1038/s41375-021-01210-8
- Kreitman RJ, Moreau P, Ravandi F, et al. Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia. Blood. 2023;141(9):996-1006. doi:10.1182/blood.2021013658
- Adashek JJ, Menta AK, Reddy NK, Desai AP, Roszik J, Subbiah V. Tissue agnostic activity of BRAF plus MEK inhibitor in BRAF V600E-mutated tumors. Mol Cancer Ther. 2022;21(6):871-878. doi:10.1158/1535-7163.MCT-21-0950
- Rogers KA, Andritsos LA, Wei L, et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood. 2021;137(25):3473-3483. doi:10.1182/blood.2020009688
- Kreitman RJ, Dearden C, Zinzani PL, et al; Study 1053 investigators. Moxetumomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): long-term follow-up from the pivotal trial. J Hematol Oncol. 2021;14(1):35. doi:10.1186/s13045-020-01004-y
- Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26(25):3704-3713. doi:10.1038/sj.onc.1210370
- Kreitman RJ, Arons E. Diagnosis and treatment of hairy cell leukemia as the COVID-19 pandemic continues. Blood Rev. 2022;51:100888. doi:10.1016/j.blre.2021.100888
- National Comprehensive Cancer Network. NCCN clinical practice guideline in oncology: hairy cell leukemia. Version 1.2023. Published August 30, 2022. Accessed March 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf
- Janus A, Robak T. Hairy cell leukemia. In: Li W, ed. Leukemia [Internet]. Brisbane: Exon Publications; 2022:chap3. Accessed February 16, 2023. doi:10.36255/exon-publications-leukemia-hairy-cell-leukemia
- Tadmor T, Polliack A. Epidemiology and environmental risk in hairy cell leukemia. Best Pract Res Clin Haematol. 2015;28(4):175-179. doi:10.1016/j.beha.2015.10.014
- Tiacci E, De Carolis L, Simonetti E, et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N Engl J Med. 2021;384(19):1810-1823. doi:10.1056/NEJMoa20312986
- Falini B, Martelli MP, Tiacci E. BRAF V600E mutation in hairy cell leukemia: from bench to bedside. Blood. 2016;128(15):1918-1927. doi:10.1182/blood-2016-07-418434
- Matutes E. Diagnostic and therapeutic challenges in hairy cell leukemia-variant: where are we in 2021? Expert Rev Hematol. 2021;14(4):355-363. doi:10.1080/17474086.2021.1908121
- Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4(6):547-559. doi:10.1016/0145-2126(80)90066-1
- Xi L, Arons E, Navarro W, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012;119(14):3330-3332. doi:10.1182/blood-2011-09-379339
- Durham BH, Getta B, Dietrich S, et al. Genomic analysis of hairy cell leukemia identifies novel recurrent genetic alterations. Blood. 2017;130(14):1644-1648. doi:10.1182/blood-2017-01-76510711
- Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with hairy cell leukemia. Blood. 2017;129(5):553-560. doi:10.1182/blood-2016-01-689422
- Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet. 2014;46(1):8-10. doi:10.1038/ng.2828
- Arons E, Sunshine J, Suntum T, Kreitman RJ. Somatic hypermutation and VH gene usage in hairy cell leukaemia. Br J Haematol. 2006;133(5):504-512. doi:10.1111/j.1365-2141.2006.06066.x
- Arons E, Roth L, Sapolsky J, Suntum T, Stetler-Stevenson M, Kreitman RJ. Evidence of canonical somatic hypermutation in hairy cell leukemia. Blood. 2011;117(18):4844-4851. doi:10.1182/blood-2010-11-316737
- Arons E, Suntum T, Stetler-Stevenson M, Kreitman RJ. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood. 2009;114(21):4687-4695. doi:10.1182/blood-2009-01-201731
- Forconi F, Sozzi E, Cencini E, et al. Hairy cell leukemias with unmutated IGHV genes define the minor subset refractory to single-agent cladribine and with more aggressive behavior. Blood. 2009;114(21):4696-4702. doi:10.1182/blood-2009-03-212449
- Robak E, Jesionek-Kupnicka D, Robak T. Skin changes in hairy cell leukemia. Ann Hematol. 2021;100(3):615-625. doi:10.1007/s00277-020-04349-z
- Bouroncle BA. Thirty-five years in the progress of hairy cell leukemia. Leuk Lymphoma. 1994;14(suppl 1):1-12. https://pubmed.ncbi.nlm.nih.gov/7820038/
- Falini B, Tiacci E, Liso A, et al. Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet. 2004;363(9424): 1869-1870. doi:10.1016/S0140-6736(04)16356-3
- Robak T, Robak P. Measurable residual disease in hairy cell leukemia: technical considerations and clinical significance. Front Oncol. 2022;12:976374. doi:10.3389/fonc.2022.976374
- Chihara D, Arons E, Stetler-Stevenson M, et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020;38(14):1527-1538. doi:10.1200/JCO.19.02250
- Chihara D, Kantarjian H, O’Brien S, et al. Long-term durable remission by cladribine followed by rituximab in patients with hairy cell leukaemia: update of a phase II trial. Br J Haematol. 2016;174(5):760-766. doi:10.1111/bjh.14129
- Grever M, Andritsos L, Banerji V, et al. Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia. 2021;35(7):1864-1872. doi:10.1038/s41375-021-01257-7
- Konrat J, Rösler W, Roiss M, et al. BRAF inhibitor treatment of classical hairy cell leukemia allows successful vaccination against SARS-CoV-2. Ann Hematol. 2023;102(2):403-406. doi:10.1007/s00277-022-05026-z
- Park JH, Shukla M, Salcedo JM, et al. First-line chemo-free therapy with the BRAF inhibitor vemurafenib combined with obinutuzumab is effective in patients with HCL. Blood. 2019;134(suppl 1):Abstract 3998. https://doi.org/10.1182/blood-2019-124478
- Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102(3):810-813. doi:10.1182/blood-2003-01-0014
- Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373(18):1733-1747. doi:10.1056/NEJMoa1506583
- Maitre E, Paillassa J, Troussard X. Novel targeted treatments in hairy cell leukemia and other hairy cell-like disorders. Front Oncol. 2022;12:1068981. doi:10.3389/fonc.2022.1068981
- Grob JJ, Amonkar MM, Karaszewska B, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015;16(13):1389-1398. doi:10.1016/S1470-2045(15)00087-X
- Tiacci E, De Carolis L, Simonetti E, et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: a pilot phase-2 clinical trial. Leukemia. 2021;35(11):3314-3318. doi:10.1038/s41375-021-01210-8
- Kreitman RJ, Moreau P, Ravandi F, et al. Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia. Blood. 2023;141(9):996-1006. doi:10.1182/blood.2021013658
- Adashek JJ, Menta AK, Reddy NK, Desai AP, Roszik J, Subbiah V. Tissue agnostic activity of BRAF plus MEK inhibitor in BRAF V600E-mutated tumors. Mol Cancer Ther. 2022;21(6):871-878. doi:10.1158/1535-7163.MCT-21-0950
- Rogers KA, Andritsos LA, Wei L, et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood. 2021;137(25):3473-3483. doi:10.1182/blood.2020009688
- Kreitman RJ, Dearden C, Zinzani PL, et al; Study 1053 investigators. Moxetumomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): long-term follow-up from the pivotal trial. J Hematol Oncol. 2021;14(1):35. doi:10.1186/s13045-020-01004-y
- Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26(25):3704-3713. doi:10.1038/sj.onc.1210370