User login
New DEA CME mandate affects 2 million prescribers
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.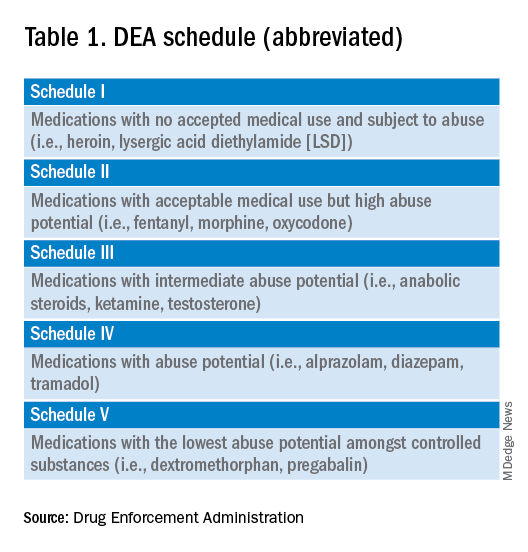
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 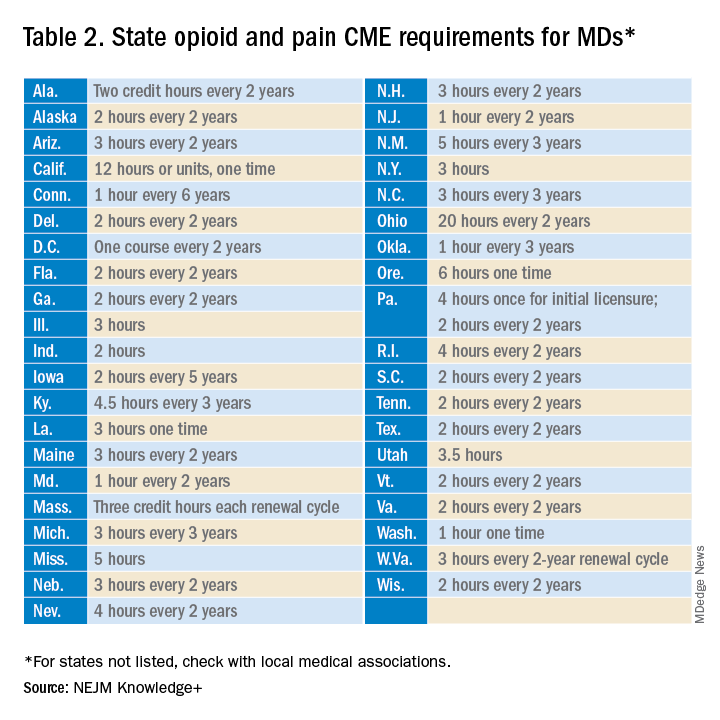
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
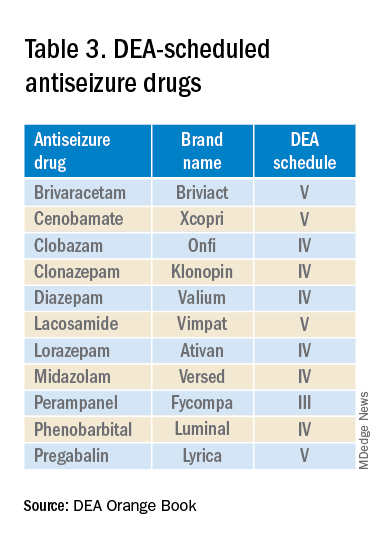
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).
Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
Fade haircut or something else?
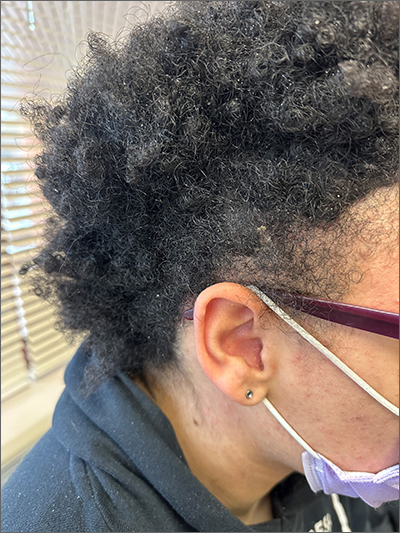
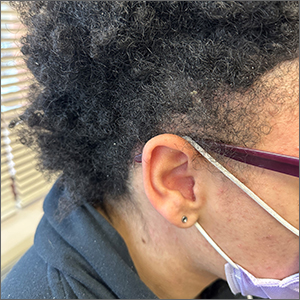
The areas of hair loss and different length hairs in a patient with otherwise normal-density hair was consistent with a diagnosis of trichotillomania. The physician initially thought the patient had a “fade” hairstyle, but then observed him twirling his hair. Further queries confirmed a history of hair pulling (trichotillomania) and ingesting the hair (trichophagia).
In adulthood, trichotillomania affects females about 4 times more than males, although in childhood, the sex distribution appears about equal.1 The typical age of onset is between 10 to 13 years. Several mental health conditions are associated with trichotillomania, such as major depressive disorder, anxiety disorders, and substance use disorder, with trichotillomania generally preceding these comorbid disorders. It is important to rule out obsessive compulsive disorder and consider body dysmorphic disorder when making a diagnosis of trichotillomania.1
It is common to see androgenetic alopecia precipitated by hormone therapy in FTM individuals. This would usually cause thinning of the hair rather than the irregular hair lengths and pattern seen in this patient. Tinea capitis is also part of the differential diagnosis in a case such as this. It can be diagnosed by potassium hydroxide (KOH) preparations in the setting of friable and broken hair shafts (and sometimes erythema and inflammation).
Patients with trichotillomania may have hair with blunt or tapered ends; hair may also look like uneven stubble.2 The scalp is the most commonly involved site (72.8%), followed by eyebrows (50.7%), and the pubic region (50.7%).1 Useful diagnostic clues include an unusual shape of the affected area, accessible location, and a changing pattern from visit to visit.2 If trichotillomania is suspected, it might be useful to ask about hair-playing activities, such as twirling or twisting, or playing with the ends of eyelashes or eyebrows.2
Unwanted medical consequences of trichotillomania include skin damage (if sharp instruments are used for hair pulling), and the formation of gastrointestinal trichobezoars (hairballs) if trichophagia is present. Trichobezoars that cause bowel obstructions may need surgical intervention.1
Behavioral therapy is the first-line treatment for trichotillomania in all age groups.1,3 Treating any coexisting mental health disorders is also essential. There are currently no FDA-approved medications for treatment; however, there is evidence that N-acetylcysteine may be useful in treating adults with trichotillomania and other repetitive skin disorders.3 Antipsychotics and cannabinoid agonists also may be beneficial.1
This patient was continued on his current lithium, naltrexone, and bupropion regimen (rather than adding additional psychiatric medicines) as he was doing better than his previous baseline. He was advised to continue with his cognitive behavioral therapy. His hormone replacement therapy also was continued because it was not thought to be contributory. He was provided with education about symptoms of trichobezoar and red flag symptoms (eg, worsening nausea, vomiting, abdominal pain) that would necessitate emergency follow-up. His GERD symptoms were thought not to be related to his trichophagia. He was scheduled to follow up for routine primary care in 6 months.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Sarasawati Keeni, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Grant JE and Chamberlain SR. Trichotillomania. Am J Psychiatry. 2016;173:868-874. doi: 10.1176/appi.ajp.2016.15111432
2. Sah DE, Koo J, Price VH. Trichotillomania. Dermatol Ther. 2008;21:13-21. doi: 10.1111/j.1529-8019.2008.00165.x
3. Henkel ED, Jaquez SD, Diaz LZ. Pediatric trichotillomania: review of management. Pediatr Dermatol. 2019;36:803-807. doi: 10.1111/pde.13954

The areas of hair loss and different length hairs in a patient with otherwise normal-density hair was consistent with a diagnosis of trichotillomania. The physician initially thought the patient had a “fade” hairstyle, but then observed him twirling his hair. Further queries confirmed a history of hair pulling (trichotillomania) and ingesting the hair (trichophagia).
In adulthood, trichotillomania affects females about 4 times more than males, although in childhood, the sex distribution appears about equal.1 The typical age of onset is between 10 to 13 years. Several mental health conditions are associated with trichotillomania, such as major depressive disorder, anxiety disorders, and substance use disorder, with trichotillomania generally preceding these comorbid disorders. It is important to rule out obsessive compulsive disorder and consider body dysmorphic disorder when making a diagnosis of trichotillomania.1
It is common to see androgenetic alopecia precipitated by hormone therapy in FTM individuals. This would usually cause thinning of the hair rather than the irregular hair lengths and pattern seen in this patient. Tinea capitis is also part of the differential diagnosis in a case such as this. It can be diagnosed by potassium hydroxide (KOH) preparations in the setting of friable and broken hair shafts (and sometimes erythema and inflammation).
Patients with trichotillomania may have hair with blunt or tapered ends; hair may also look like uneven stubble.2 The scalp is the most commonly involved site (72.8%), followed by eyebrows (50.7%), and the pubic region (50.7%).1 Useful diagnostic clues include an unusual shape of the affected area, accessible location, and a changing pattern from visit to visit.2 If trichotillomania is suspected, it might be useful to ask about hair-playing activities, such as twirling or twisting, or playing with the ends of eyelashes or eyebrows.2
Unwanted medical consequences of trichotillomania include skin damage (if sharp instruments are used for hair pulling), and the formation of gastrointestinal trichobezoars (hairballs) if trichophagia is present. Trichobezoars that cause bowel obstructions may need surgical intervention.1
Behavioral therapy is the first-line treatment for trichotillomania in all age groups.1,3 Treating any coexisting mental health disorders is also essential. There are currently no FDA-approved medications for treatment; however, there is evidence that N-acetylcysteine may be useful in treating adults with trichotillomania and other repetitive skin disorders.3 Antipsychotics and cannabinoid agonists also may be beneficial.1
This patient was continued on his current lithium, naltrexone, and bupropion regimen (rather than adding additional psychiatric medicines) as he was doing better than his previous baseline. He was advised to continue with his cognitive behavioral therapy. His hormone replacement therapy also was continued because it was not thought to be contributory. He was provided with education about symptoms of trichobezoar and red flag symptoms (eg, worsening nausea, vomiting, abdominal pain) that would necessitate emergency follow-up. His GERD symptoms were thought not to be related to his trichophagia. He was scheduled to follow up for routine primary care in 6 months.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Sarasawati Keeni, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The areas of hair loss and different length hairs in a patient with otherwise normal-density hair was consistent with a diagnosis of trichotillomania. The physician initially thought the patient had a “fade” hairstyle, but then observed him twirling his hair. Further queries confirmed a history of hair pulling (trichotillomania) and ingesting the hair (trichophagia).
In adulthood, trichotillomania affects females about 4 times more than males, although in childhood, the sex distribution appears about equal.1 The typical age of onset is between 10 to 13 years. Several mental health conditions are associated with trichotillomania, such as major depressive disorder, anxiety disorders, and substance use disorder, with trichotillomania generally preceding these comorbid disorders. It is important to rule out obsessive compulsive disorder and consider body dysmorphic disorder when making a diagnosis of trichotillomania.1
It is common to see androgenetic alopecia precipitated by hormone therapy in FTM individuals. This would usually cause thinning of the hair rather than the irregular hair lengths and pattern seen in this patient. Tinea capitis is also part of the differential diagnosis in a case such as this. It can be diagnosed by potassium hydroxide (KOH) preparations in the setting of friable and broken hair shafts (and sometimes erythema and inflammation).
Patients with trichotillomania may have hair with blunt or tapered ends; hair may also look like uneven stubble.2 The scalp is the most commonly involved site (72.8%), followed by eyebrows (50.7%), and the pubic region (50.7%).1 Useful diagnostic clues include an unusual shape of the affected area, accessible location, and a changing pattern from visit to visit.2 If trichotillomania is suspected, it might be useful to ask about hair-playing activities, such as twirling or twisting, or playing with the ends of eyelashes or eyebrows.2
Unwanted medical consequences of trichotillomania include skin damage (if sharp instruments are used for hair pulling), and the formation of gastrointestinal trichobezoars (hairballs) if trichophagia is present. Trichobezoars that cause bowel obstructions may need surgical intervention.1
Behavioral therapy is the first-line treatment for trichotillomania in all age groups.1,3 Treating any coexisting mental health disorders is also essential. There are currently no FDA-approved medications for treatment; however, there is evidence that N-acetylcysteine may be useful in treating adults with trichotillomania and other repetitive skin disorders.3 Antipsychotics and cannabinoid agonists also may be beneficial.1
This patient was continued on his current lithium, naltrexone, and bupropion regimen (rather than adding additional psychiatric medicines) as he was doing better than his previous baseline. He was advised to continue with his cognitive behavioral therapy. His hormone replacement therapy also was continued because it was not thought to be contributory. He was provided with education about symptoms of trichobezoar and red flag symptoms (eg, worsening nausea, vomiting, abdominal pain) that would necessitate emergency follow-up. His GERD symptoms were thought not to be related to his trichophagia. He was scheduled to follow up for routine primary care in 6 months.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Sarasawati Keeni, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Grant JE and Chamberlain SR. Trichotillomania. Am J Psychiatry. 2016;173:868-874. doi: 10.1176/appi.ajp.2016.15111432
2. Sah DE, Koo J, Price VH. Trichotillomania. Dermatol Ther. 2008;21:13-21. doi: 10.1111/j.1529-8019.2008.00165.x
3. Henkel ED, Jaquez SD, Diaz LZ. Pediatric trichotillomania: review of management. Pediatr Dermatol. 2019;36:803-807. doi: 10.1111/pde.13954
1. Grant JE and Chamberlain SR. Trichotillomania. Am J Psychiatry. 2016;173:868-874. doi: 10.1176/appi.ajp.2016.15111432
2. Sah DE, Koo J, Price VH. Trichotillomania. Dermatol Ther. 2008;21:13-21. doi: 10.1111/j.1529-8019.2008.00165.x
3. Henkel ED, Jaquez SD, Diaz LZ. Pediatric trichotillomania: review of management. Pediatr Dermatol. 2019;36:803-807. doi: 10.1111/pde.13954
PsA Differential Diagnosis
HPV rates skyrocket despite safe, effective vaccine
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
Depression Treatment
Once-weekly basal insulin nears market for type 2 diabetes
SAN DIEGO – results from two new phase 3a studies suggest.
Data from Novo Nordisk’s ONWARDS 1, comparing once-weekly icodec with once-daily glargine, and ONWARDS 3, comparing once-weekly icodec with daily degludec (Tresiba, Novo Nordisk), both in insulin-naive patients with type 2 diabetes, were presented at the annual scientific sessions of the American Diabetes Association.
In both trials, primary endpoints of superiority and noninferiority in A1c reduction were achieved, and in ONWARDS 1, patients spent more time in target blood glucose range.
“I feel that weekly insulins have the potential to become transformational as preferred options for basal insulin replacement in people with type 2 diabetes in need of initiation of insulin therapy,” said Julio Rosenstock, MD, the lead author of ONWARDS 1.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, said: “The data certainly support approval of Icodec.”
Dr. Alexander said that an ideal candidate for once-weekly insulin “is someone who’s already on once-weekly [glucagon-like peptide-1 (GLP-1) agonist]. Then, taking your GLP-1 [agonist] and your basal insulin at the same time once a week makes a lot of sense ... Since they’re taking a weekly injection anyway, it’s relatively easy for a person to remember ‘When I take my weekly GLP-1 [agonist], I’ll take my weekly basal insulin.’ ”
However, he also pointed out: “Payers may say they don’t care about the convenience of once-weekly and they prefer to pay for the cheaper daily basal [insulin] ... I think a lot of people will continue to use [insulin] glargine because it is cheaper than either degludec or icodec.”
The data from ONWARDS 1 was published in the New England Journal of Medicine, and the data from ONWARDS 3 was published in JAMA.
Six ONWARDS trials make up Novo Nordisk’s phase 3a clinical development program comparing the efficacy and safety of once-weekly insulin icodec with once-daily basal insulin comparators.
Previously, findings from ONWARDS 2, in which patients with type 2 diabetes taking basal insulin had improved A1c after being switched to once-weekly icodec or once-daily degludec, were presented at the annual meeting of the European Association for the Study of Diabetes.
Insulin icodec has been submitted for regulatory review in the United States, Canada, Europe, China, Australia, Switzerland, and Brazil, with decisions anticipated starting in the first half of 2024.
Hypoglycemia: Is the slight increase clinically significant?
One concern about the once-weekly insulins is that they might result in higher rates of hypoglycemia because they stay active in the body for so long.
Differences in rates of combined level 2 (clinically significant) and level 3 (severe) hypoglycemia were increased with borderline significance in ONWARDS 1.
In ONWARDS 3 there was a threefold significant difference, but the overall risk was still low, equating to one episode per patient per 3 years, said Ildiko Lingvay, MD, of University of Texas Southwestern Medical Center, Dallas, who is lead author for ONWARDS 1 and a co-author for ONWARDS 3.
“Insulin is insulin. When we use insulin there will always be hypoglycemia. But we only have less than one event per year,” added Dr. Rosenstock, of Velocity Clinical Research at Medical City, Dallas.
Dr. Alexander pointed out that in ONWARDS 3 just under half of both groups were taking a sulfonylurea, although the trial design allowed for cutting the dose in half when the basal insulin was added.
In ONWARDS 1, in contrast, sulfonylureas and glinides were stopped at the time of randomization. “That’s not definitive, but I would argue that’s the explanation, to be proven by formal testing.”
Indeed, an audience member asked about that during the discussion, and Dr. Lingvay said they were still analyzing those data. “We’re working on that. It’s very important.”
Dr. Alexander noted, “I think the message here is don’t continue sulfonylureas or glinides in someone you’re giving insulin to because you’re going to get hypoglycemia.”
Better glycemic control, with fewer injections
ONWARDS 1 was a 78-week, randomized, open-label, treat-to-target trial, with a main 52-week phase and a 26-week extension phase. A total of 984 patients with type 2 diabetes and A1c 7%-11% with no prior insulin treatment were randomized 1:1 to once-weekly icodec or daily insulin glargine. All baseline medications except sulfonylureas and glinides were continued.
The primary endpoint was change in A1c from baseline to week 52, and this dropped from 8.5% to 6.9% with icodec, versus 8.4% to 7.1% with glargine, a significant difference, confirming both noninferiority (P < .001) and superiority (P = .02) of icodec, Dr. Rosenstock said.
The percentage of time in blood glucose range (70-180 mg/dL) was also significantly higher with icodec than glargine (71.9% vs. 66.9%; P < .001), also confirming superiority.
Rates of combined clinically significant or severe hypoglycemia at 83 weeks were 0.30 versus 0.16 events per person-year of exposure at week 83 (P = .043). No new safety signals were identified, and incidences of adverse events were similar in the two groups.
A significantly higher proportion of participants achieved an A1c of less than 7% without clinically significant or severe hypoglycemia with once-weekly basal insulin icodec versus once-daily basal insulin glargine (52.6% vs. 42.6%).
ONWARDS 3 randomized 588 patients each to once-weekly insulin icodec plus once-weekly placebo or once-daily insulin degludec plus once-weekly placebo. The primary endpoint, change in A1c from baseline to week 26, fell from 8.6% to 7.0% with icodec and from 8.5% to 7.2% with degludec, confirming both noninferiority (P < .001) and superiority (P = .002).
There were no significant differences between the two insulins in change in fasting plasma glucose, mean weekly insulin dose, or body weight.
Combined level 2 or 3 hypoglycemia rates were numerically higher in the icodec group than in the degludec group from week 0 to 31 (0.31 vs. 0.15 events per patient-year exposure; P = .11) and statistically higher in the icodec group from week 0 to 26 (0.35 vs. 0.12 events per patient-year exposure; P = .01).
The percentage of patients achieving an A1c of less than 7% without level 2 or 3 hypoglycemia was 52.1% with icodec versus 39.9% with degludec.
Dr. Lingvay and Dr. Rosenstock have reported financial relationships with multiple companies.
A version of this article originally appeared on Medscape.com.
SAN DIEGO – results from two new phase 3a studies suggest.
Data from Novo Nordisk’s ONWARDS 1, comparing once-weekly icodec with once-daily glargine, and ONWARDS 3, comparing once-weekly icodec with daily degludec (Tresiba, Novo Nordisk), both in insulin-naive patients with type 2 diabetes, were presented at the annual scientific sessions of the American Diabetes Association.
In both trials, primary endpoints of superiority and noninferiority in A1c reduction were achieved, and in ONWARDS 1, patients spent more time in target blood glucose range.
“I feel that weekly insulins have the potential to become transformational as preferred options for basal insulin replacement in people with type 2 diabetes in need of initiation of insulin therapy,” said Julio Rosenstock, MD, the lead author of ONWARDS 1.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, said: “The data certainly support approval of Icodec.”
Dr. Alexander said that an ideal candidate for once-weekly insulin “is someone who’s already on once-weekly [glucagon-like peptide-1 (GLP-1) agonist]. Then, taking your GLP-1 [agonist] and your basal insulin at the same time once a week makes a lot of sense ... Since they’re taking a weekly injection anyway, it’s relatively easy for a person to remember ‘When I take my weekly GLP-1 [agonist], I’ll take my weekly basal insulin.’ ”
However, he also pointed out: “Payers may say they don’t care about the convenience of once-weekly and they prefer to pay for the cheaper daily basal [insulin] ... I think a lot of people will continue to use [insulin] glargine because it is cheaper than either degludec or icodec.”
The data from ONWARDS 1 was published in the New England Journal of Medicine, and the data from ONWARDS 3 was published in JAMA.
Six ONWARDS trials make up Novo Nordisk’s phase 3a clinical development program comparing the efficacy and safety of once-weekly insulin icodec with once-daily basal insulin comparators.
Previously, findings from ONWARDS 2, in which patients with type 2 diabetes taking basal insulin had improved A1c after being switched to once-weekly icodec or once-daily degludec, were presented at the annual meeting of the European Association for the Study of Diabetes.
Insulin icodec has been submitted for regulatory review in the United States, Canada, Europe, China, Australia, Switzerland, and Brazil, with decisions anticipated starting in the first half of 2024.
Hypoglycemia: Is the slight increase clinically significant?
One concern about the once-weekly insulins is that they might result in higher rates of hypoglycemia because they stay active in the body for so long.
Differences in rates of combined level 2 (clinically significant) and level 3 (severe) hypoglycemia were increased with borderline significance in ONWARDS 1.
In ONWARDS 3 there was a threefold significant difference, but the overall risk was still low, equating to one episode per patient per 3 years, said Ildiko Lingvay, MD, of University of Texas Southwestern Medical Center, Dallas, who is lead author for ONWARDS 1 and a co-author for ONWARDS 3.
“Insulin is insulin. When we use insulin there will always be hypoglycemia. But we only have less than one event per year,” added Dr. Rosenstock, of Velocity Clinical Research at Medical City, Dallas.
Dr. Alexander pointed out that in ONWARDS 3 just under half of both groups were taking a sulfonylurea, although the trial design allowed for cutting the dose in half when the basal insulin was added.
In ONWARDS 1, in contrast, sulfonylureas and glinides were stopped at the time of randomization. “That’s not definitive, but I would argue that’s the explanation, to be proven by formal testing.”
Indeed, an audience member asked about that during the discussion, and Dr. Lingvay said they were still analyzing those data. “We’re working on that. It’s very important.”
Dr. Alexander noted, “I think the message here is don’t continue sulfonylureas or glinides in someone you’re giving insulin to because you’re going to get hypoglycemia.”
Better glycemic control, with fewer injections
ONWARDS 1 was a 78-week, randomized, open-label, treat-to-target trial, with a main 52-week phase and a 26-week extension phase. A total of 984 patients with type 2 diabetes and A1c 7%-11% with no prior insulin treatment were randomized 1:1 to once-weekly icodec or daily insulin glargine. All baseline medications except sulfonylureas and glinides were continued.
The primary endpoint was change in A1c from baseline to week 52, and this dropped from 8.5% to 6.9% with icodec, versus 8.4% to 7.1% with glargine, a significant difference, confirming both noninferiority (P < .001) and superiority (P = .02) of icodec, Dr. Rosenstock said.
The percentage of time in blood glucose range (70-180 mg/dL) was also significantly higher with icodec than glargine (71.9% vs. 66.9%; P < .001), also confirming superiority.
Rates of combined clinically significant or severe hypoglycemia at 83 weeks were 0.30 versus 0.16 events per person-year of exposure at week 83 (P = .043). No new safety signals were identified, and incidences of adverse events were similar in the two groups.
A significantly higher proportion of participants achieved an A1c of less than 7% without clinically significant or severe hypoglycemia with once-weekly basal insulin icodec versus once-daily basal insulin glargine (52.6% vs. 42.6%).
ONWARDS 3 randomized 588 patients each to once-weekly insulin icodec plus once-weekly placebo or once-daily insulin degludec plus once-weekly placebo. The primary endpoint, change in A1c from baseline to week 26, fell from 8.6% to 7.0% with icodec and from 8.5% to 7.2% with degludec, confirming both noninferiority (P < .001) and superiority (P = .002).
There were no significant differences between the two insulins in change in fasting plasma glucose, mean weekly insulin dose, or body weight.
Combined level 2 or 3 hypoglycemia rates were numerically higher in the icodec group than in the degludec group from week 0 to 31 (0.31 vs. 0.15 events per patient-year exposure; P = .11) and statistically higher in the icodec group from week 0 to 26 (0.35 vs. 0.12 events per patient-year exposure; P = .01).
The percentage of patients achieving an A1c of less than 7% without level 2 or 3 hypoglycemia was 52.1% with icodec versus 39.9% with degludec.
Dr. Lingvay and Dr. Rosenstock have reported financial relationships with multiple companies.
A version of this article originally appeared on Medscape.com.
SAN DIEGO – results from two new phase 3a studies suggest.
Data from Novo Nordisk’s ONWARDS 1, comparing once-weekly icodec with once-daily glargine, and ONWARDS 3, comparing once-weekly icodec with daily degludec (Tresiba, Novo Nordisk), both in insulin-naive patients with type 2 diabetes, were presented at the annual scientific sessions of the American Diabetes Association.
In both trials, primary endpoints of superiority and noninferiority in A1c reduction were achieved, and in ONWARDS 1, patients spent more time in target blood glucose range.
“I feel that weekly insulins have the potential to become transformational as preferred options for basal insulin replacement in people with type 2 diabetes in need of initiation of insulin therapy,” said Julio Rosenstock, MD, the lead author of ONWARDS 1.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, said: “The data certainly support approval of Icodec.”
Dr. Alexander said that an ideal candidate for once-weekly insulin “is someone who’s already on once-weekly [glucagon-like peptide-1 (GLP-1) agonist]. Then, taking your GLP-1 [agonist] and your basal insulin at the same time once a week makes a lot of sense ... Since they’re taking a weekly injection anyway, it’s relatively easy for a person to remember ‘When I take my weekly GLP-1 [agonist], I’ll take my weekly basal insulin.’ ”
However, he also pointed out: “Payers may say they don’t care about the convenience of once-weekly and they prefer to pay for the cheaper daily basal [insulin] ... I think a lot of people will continue to use [insulin] glargine because it is cheaper than either degludec or icodec.”
The data from ONWARDS 1 was published in the New England Journal of Medicine, and the data from ONWARDS 3 was published in JAMA.
Six ONWARDS trials make up Novo Nordisk’s phase 3a clinical development program comparing the efficacy and safety of once-weekly insulin icodec with once-daily basal insulin comparators.
Previously, findings from ONWARDS 2, in which patients with type 2 diabetes taking basal insulin had improved A1c after being switched to once-weekly icodec or once-daily degludec, were presented at the annual meeting of the European Association for the Study of Diabetes.
Insulin icodec has been submitted for regulatory review in the United States, Canada, Europe, China, Australia, Switzerland, and Brazil, with decisions anticipated starting in the first half of 2024.
Hypoglycemia: Is the slight increase clinically significant?
One concern about the once-weekly insulins is that they might result in higher rates of hypoglycemia because they stay active in the body for so long.
Differences in rates of combined level 2 (clinically significant) and level 3 (severe) hypoglycemia were increased with borderline significance in ONWARDS 1.
In ONWARDS 3 there was a threefold significant difference, but the overall risk was still low, equating to one episode per patient per 3 years, said Ildiko Lingvay, MD, of University of Texas Southwestern Medical Center, Dallas, who is lead author for ONWARDS 1 and a co-author for ONWARDS 3.
“Insulin is insulin. When we use insulin there will always be hypoglycemia. But we only have less than one event per year,” added Dr. Rosenstock, of Velocity Clinical Research at Medical City, Dallas.
Dr. Alexander pointed out that in ONWARDS 3 just under half of both groups were taking a sulfonylurea, although the trial design allowed for cutting the dose in half when the basal insulin was added.
In ONWARDS 1, in contrast, sulfonylureas and glinides were stopped at the time of randomization. “That’s not definitive, but I would argue that’s the explanation, to be proven by formal testing.”
Indeed, an audience member asked about that during the discussion, and Dr. Lingvay said they were still analyzing those data. “We’re working on that. It’s very important.”
Dr. Alexander noted, “I think the message here is don’t continue sulfonylureas or glinides in someone you’re giving insulin to because you’re going to get hypoglycemia.”
Better glycemic control, with fewer injections
ONWARDS 1 was a 78-week, randomized, open-label, treat-to-target trial, with a main 52-week phase and a 26-week extension phase. A total of 984 patients with type 2 diabetes and A1c 7%-11% with no prior insulin treatment were randomized 1:1 to once-weekly icodec or daily insulin glargine. All baseline medications except sulfonylureas and glinides were continued.
The primary endpoint was change in A1c from baseline to week 52, and this dropped from 8.5% to 6.9% with icodec, versus 8.4% to 7.1% with glargine, a significant difference, confirming both noninferiority (P < .001) and superiority (P = .02) of icodec, Dr. Rosenstock said.
The percentage of time in blood glucose range (70-180 mg/dL) was also significantly higher with icodec than glargine (71.9% vs. 66.9%; P < .001), also confirming superiority.
Rates of combined clinically significant or severe hypoglycemia at 83 weeks were 0.30 versus 0.16 events per person-year of exposure at week 83 (P = .043). No new safety signals were identified, and incidences of adverse events were similar in the two groups.
A significantly higher proportion of participants achieved an A1c of less than 7% without clinically significant or severe hypoglycemia with once-weekly basal insulin icodec versus once-daily basal insulin glargine (52.6% vs. 42.6%).
ONWARDS 3 randomized 588 patients each to once-weekly insulin icodec plus once-weekly placebo or once-daily insulin degludec plus once-weekly placebo. The primary endpoint, change in A1c from baseline to week 26, fell from 8.6% to 7.0% with icodec and from 8.5% to 7.2% with degludec, confirming both noninferiority (P < .001) and superiority (P = .002).
There were no significant differences between the two insulins in change in fasting plasma glucose, mean weekly insulin dose, or body weight.
Combined level 2 or 3 hypoglycemia rates were numerically higher in the icodec group than in the degludec group from week 0 to 31 (0.31 vs. 0.15 events per patient-year exposure; P = .11) and statistically higher in the icodec group from week 0 to 26 (0.35 vs. 0.12 events per patient-year exposure; P = .01).
The percentage of patients achieving an A1c of less than 7% without level 2 or 3 hypoglycemia was 52.1% with icodec versus 39.9% with degludec.
Dr. Lingvay and Dr. Rosenstock have reported financial relationships with multiple companies.
A version of this article originally appeared on Medscape.com.
AT ADA 2023
White Spots on the Extremities
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
A 52-year-old Black woman presented with self-described whitened spots on the arms and legs of 2 years’ duration. She experienced no improvement with ketoconazole cream and topical calcineurin inhibitors prescribed during a prior dermatology visit at an outside institution. She denied pain or pruritus. A review of systems as well as the patient’s medical history were noncontributory. A prior biopsy at an outside institution revealed an interface dermatitis suggestive of cutaneous lupus erythematosus. The patient noted social drinking and denied tobacco use. She had no known allergies to medications and currently was on tamoxifen for breast cancer following a right mastectomy. Physical examination showed hypopigmented macules and patches on the left upper arm and right proximal leg. The center of the lesions was not erythematous or scaly. Palpation did not reveal enlarged lymph nodes, and laboratory analyses ruled out low levels of red blood cells, white blood cells, or platelets. Punch biopsies from the left arm and right thigh were performed.
No link between heartburn meds and dementia
A new study provides reassurance about the safety of long-term proton pump inhibitor (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
It was published online in Gastroenterology.
The post hoc observational study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston.
The researchers analyzed results from the Aspirin in Reducing Events in the Elderly clinical trial. The randomized trial of aspirin included 18,934 adults aged 65 and older from the United States and Australia. Patients’ use of PPI and H2RA was tracked, along with dementia incidence and cognitive changes.
The results showed that there was no link to new dementia diagnoses in patients who used PPIs (25%) and H2RA (2%) at baseline, versus those who did not use either heartburn medication.
Limitations of prior studies are referenced, including the potential for residual confounding and underestimation of PPI and H2RA use, the lack of data on medication dose and duration, and the absence of apo E4 allele status.
The study was funded by grants from the National Institute on Aging, the National Cancer Institute, and other institutions. Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
A new study provides reassurance about the safety of long-term proton pump inhibitor (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
It was published online in Gastroenterology.
The post hoc observational study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston.
The researchers analyzed results from the Aspirin in Reducing Events in the Elderly clinical trial. The randomized trial of aspirin included 18,934 adults aged 65 and older from the United States and Australia. Patients’ use of PPI and H2RA was tracked, along with dementia incidence and cognitive changes.
The results showed that there was no link to new dementia diagnoses in patients who used PPIs (25%) and H2RA (2%) at baseline, versus those who did not use either heartburn medication.
Limitations of prior studies are referenced, including the potential for residual confounding and underestimation of PPI and H2RA use, the lack of data on medication dose and duration, and the absence of apo E4 allele status.
The study was funded by grants from the National Institute on Aging, the National Cancer Institute, and other institutions. Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
A new study provides reassurance about the safety of long-term proton pump inhibitor (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
It was published online in Gastroenterology.
The post hoc observational study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston.
The researchers analyzed results from the Aspirin in Reducing Events in the Elderly clinical trial. The randomized trial of aspirin included 18,934 adults aged 65 and older from the United States and Australia. Patients’ use of PPI and H2RA was tracked, along with dementia incidence and cognitive changes.
The results showed that there was no link to new dementia diagnoses in patients who used PPIs (25%) and H2RA (2%) at baseline, versus those who did not use either heartburn medication.
Limitations of prior studies are referenced, including the potential for residual confounding and underestimation of PPI and H2RA use, the lack of data on medication dose and duration, and the absence of apo E4 allele status.
The study was funded by grants from the National Institute on Aging, the National Cancer Institute, and other institutions. Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM GASTROENTEROLOGY
ADA: Screen all with type 2 diabetes for fatty liver disease
SAN DIEGO – and provides new recommendations for management in those with the condition or who are at risk for it.
Liver disease affects up to 70% of people with type 2 diabetes and is common in people with prediabetes and in those with type 1 diabetes who also have obesity. Non-alcoholic fatty liver disease (NAFLD) is the most common form of liver disease in people with diabetes. It can lead to cirrhosis and liver cancer and is associated with an increased risk for cardiovascular disease and death. The condition includes non-alcoholic steatohepatitis (NASH).
“The ADA has recognized that this has become a big problem for their patients because NASH is becoming the number one cause of cirrhosis in people with type 2 diabetes and the number one cause of liver transplantation in the United States, so we have to do something about it,” Kenneth Cusi, MD, who presented a summary of the new guidance at the annual scientific sessions of the American Diabetes Association, said in an interview.
The new ADA guidance was published as a mid-year update to the ADA’s Standards of Care in Diabetes–2023 in the section on “Comprehensive Medical Evaluation and Assessment of Comorbidities.”
Asked to comment, Atlanta endocrinologist Scott Isaacs, MD, said, “It is wonderful to see that the ADA has recognized NAFLD ... as the hepatic complication of type 2 diabetes and has updated the Standards of Care reflecting the current knowledge and evidence of this ubiquitous and often silent disease.”
The new ADA guidance aligns with those of other professional societies, including the American Association for the Study of Liver Diseases, the American Gastroenterological Society, and the American Association of Clinical Endocrinology.
Dr. Isaacs, who chaired the AACE guidance writing panel, noted, “The ADA update essentially repeats the same guidance in the AACE and AASLD documents. It is excellent to see this type of alignment of guidance among the major organizations.”
FIB-4: Easy calculation in the EHR
The ADA now advises screening all adults with type 2 diabetes or prediabetes, particularly those with obesity or cardiometabolic risk factors or established cardiovascular disease – even those with normal liver enzyme levels. People with type 1 diabetes who have obesity and/or cardiovascular risk factors are also to be screened for NAFLD.
The recommended screening tool is the fibrosis-4 index (FIB-4), a calculation that includes the patient’s age, liver enzyme levels, and platelet counts. A score of 1.3 or higher is considered high risk for clinically significant fibrosis and above 2.6 is very high-risk.
Dr. Cusi noted, “The reason we advise using the FIB-4 ... instead of liver enzymes as ADA advised in the past, is that now we know that 70% of people with type 2 diabetes have steatosis already and about one in five have fibrosis, but if you go by liver enzymes you will miss most of them. Liver enzymes are ineffective as a screening tool.”
The FIB-4 is “a simple tool we already have in our electronic health records (EHR) but we’re just simply not using it,” noted Dr. Cusi, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville.
Indeed, Dr. Isaacs said, “The FIB-4 is a simple ... great screening test because it is essentially free.” But he cautioned that it has some limitations.
“It is a good test for ruling out advanced liver disease but can have false positives and false negatives. The FIB-4 cutoffs need to be adjusted for persons over 65 years old and [should] not to be used for persons under 30 years old.”
Dr. Isaacs also pointed out that, while the calculation can be done from a website, “even this adds time to a clinician’s busy day. Ideally, the FIB-4 should be automatically calculated in the EHR or on the lab report, similar to the [estimated glomerular filtration rate] calculation [for kidney function] and flagged if greater than 1.3.”
The ADA update also provides guidance on follow-up for patients flagged with the FIB-4, including when referral to a gastroenterologist or hepatologist is appropriate.
Treatment: Lifestyle modification plus GLP-1 agonists or pioglitazone
Lifestyle modification is recommended for all adults with diabetes or prediabetes and NAFLD, particularly those with overweight or obesity.
In addition, the ADA now advises consideration of a using a glucagonlike peptide–1 (GLP-1) agonist with demonstrated benefits in NAFLD as adjunctive therapy to lifestyle interventions for weight loss in those with type 2 diabetes, particularly with overweight/obesity.
And for those with biopsy-proven NASH or who are identified with clinically significant liver fibrosis using non-invasive tests, either a GLP-1 agonist or pioglitazone are the “preferred treatments.”
However, insulin is the preferred treatment for hyperglycemia in adults with type 2 diabetes who have decompensated cirrhosis.
Dr. Isaacs commented, “Pioglitazone has so many benefits and a few known risks ... it is an underused medication. It is very inexpensive. Pioglitazone should be considered as a first line treatment for patients with type 2 diabetes and NAFLD.”
The ADA update also advises statin therapy for people with type 2 diabetes and NAFLD, given their increased cardiovascular risk. However, statins are not recommended for people with decompensated cirrhosis because of limited safety and efficacy data.
Dr. Cusi noted that he has been advocating for fatty liver screening in people with type 2 diabetes for over a decade.
“Doctors have already been adopting it, but ADA as an organization in diabetes care has a big impact. I dreamed many years ago that the day would come when we would screen all people with type 2 diabetes, and that day is today.”
Dr. Cusi is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, BMS, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, Thera Technologies, and MSD. Dr. Isaacs reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – and provides new recommendations for management in those with the condition or who are at risk for it.
Liver disease affects up to 70% of people with type 2 diabetes and is common in people with prediabetes and in those with type 1 diabetes who also have obesity. Non-alcoholic fatty liver disease (NAFLD) is the most common form of liver disease in people with diabetes. It can lead to cirrhosis and liver cancer and is associated with an increased risk for cardiovascular disease and death. The condition includes non-alcoholic steatohepatitis (NASH).
“The ADA has recognized that this has become a big problem for their patients because NASH is becoming the number one cause of cirrhosis in people with type 2 diabetes and the number one cause of liver transplantation in the United States, so we have to do something about it,” Kenneth Cusi, MD, who presented a summary of the new guidance at the annual scientific sessions of the American Diabetes Association, said in an interview.
The new ADA guidance was published as a mid-year update to the ADA’s Standards of Care in Diabetes–2023 in the section on “Comprehensive Medical Evaluation and Assessment of Comorbidities.”
Asked to comment, Atlanta endocrinologist Scott Isaacs, MD, said, “It is wonderful to see that the ADA has recognized NAFLD ... as the hepatic complication of type 2 diabetes and has updated the Standards of Care reflecting the current knowledge and evidence of this ubiquitous and often silent disease.”
The new ADA guidance aligns with those of other professional societies, including the American Association for the Study of Liver Diseases, the American Gastroenterological Society, and the American Association of Clinical Endocrinology.
Dr. Isaacs, who chaired the AACE guidance writing panel, noted, “The ADA update essentially repeats the same guidance in the AACE and AASLD documents. It is excellent to see this type of alignment of guidance among the major organizations.”
FIB-4: Easy calculation in the EHR
The ADA now advises screening all adults with type 2 diabetes or prediabetes, particularly those with obesity or cardiometabolic risk factors or established cardiovascular disease – even those with normal liver enzyme levels. People with type 1 diabetes who have obesity and/or cardiovascular risk factors are also to be screened for NAFLD.
The recommended screening tool is the fibrosis-4 index (FIB-4), a calculation that includes the patient’s age, liver enzyme levels, and platelet counts. A score of 1.3 or higher is considered high risk for clinically significant fibrosis and above 2.6 is very high-risk.
Dr. Cusi noted, “The reason we advise using the FIB-4 ... instead of liver enzymes as ADA advised in the past, is that now we know that 70% of people with type 2 diabetes have steatosis already and about one in five have fibrosis, but if you go by liver enzymes you will miss most of them. Liver enzymes are ineffective as a screening tool.”
The FIB-4 is “a simple tool we already have in our electronic health records (EHR) but we’re just simply not using it,” noted Dr. Cusi, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville.
Indeed, Dr. Isaacs said, “The FIB-4 is a simple ... great screening test because it is essentially free.” But he cautioned that it has some limitations.
“It is a good test for ruling out advanced liver disease but can have false positives and false negatives. The FIB-4 cutoffs need to be adjusted for persons over 65 years old and [should] not to be used for persons under 30 years old.”
Dr. Isaacs also pointed out that, while the calculation can be done from a website, “even this adds time to a clinician’s busy day. Ideally, the FIB-4 should be automatically calculated in the EHR or on the lab report, similar to the [estimated glomerular filtration rate] calculation [for kidney function] and flagged if greater than 1.3.”
The ADA update also provides guidance on follow-up for patients flagged with the FIB-4, including when referral to a gastroenterologist or hepatologist is appropriate.
Treatment: Lifestyle modification plus GLP-1 agonists or pioglitazone
Lifestyle modification is recommended for all adults with diabetes or prediabetes and NAFLD, particularly those with overweight or obesity.
In addition, the ADA now advises consideration of a using a glucagonlike peptide–1 (GLP-1) agonist with demonstrated benefits in NAFLD as adjunctive therapy to lifestyle interventions for weight loss in those with type 2 diabetes, particularly with overweight/obesity.
And for those with biopsy-proven NASH or who are identified with clinically significant liver fibrosis using non-invasive tests, either a GLP-1 agonist or pioglitazone are the “preferred treatments.”
However, insulin is the preferred treatment for hyperglycemia in adults with type 2 diabetes who have decompensated cirrhosis.
Dr. Isaacs commented, “Pioglitazone has so many benefits and a few known risks ... it is an underused medication. It is very inexpensive. Pioglitazone should be considered as a first line treatment for patients with type 2 diabetes and NAFLD.”
The ADA update also advises statin therapy for people with type 2 diabetes and NAFLD, given their increased cardiovascular risk. However, statins are not recommended for people with decompensated cirrhosis because of limited safety and efficacy data.
Dr. Cusi noted that he has been advocating for fatty liver screening in people with type 2 diabetes for over a decade.
“Doctors have already been adopting it, but ADA as an organization in diabetes care has a big impact. I dreamed many years ago that the day would come when we would screen all people with type 2 diabetes, and that day is today.”
Dr. Cusi is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, BMS, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, Thera Technologies, and MSD. Dr. Isaacs reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – and provides new recommendations for management in those with the condition or who are at risk for it.
Liver disease affects up to 70% of people with type 2 diabetes and is common in people with prediabetes and in those with type 1 diabetes who also have obesity. Non-alcoholic fatty liver disease (NAFLD) is the most common form of liver disease in people with diabetes. It can lead to cirrhosis and liver cancer and is associated with an increased risk for cardiovascular disease and death. The condition includes non-alcoholic steatohepatitis (NASH).
“The ADA has recognized that this has become a big problem for their patients because NASH is becoming the number one cause of cirrhosis in people with type 2 diabetes and the number one cause of liver transplantation in the United States, so we have to do something about it,” Kenneth Cusi, MD, who presented a summary of the new guidance at the annual scientific sessions of the American Diabetes Association, said in an interview.
The new ADA guidance was published as a mid-year update to the ADA’s Standards of Care in Diabetes–2023 in the section on “Comprehensive Medical Evaluation and Assessment of Comorbidities.”
Asked to comment, Atlanta endocrinologist Scott Isaacs, MD, said, “It is wonderful to see that the ADA has recognized NAFLD ... as the hepatic complication of type 2 diabetes and has updated the Standards of Care reflecting the current knowledge and evidence of this ubiquitous and often silent disease.”
The new ADA guidance aligns with those of other professional societies, including the American Association for the Study of Liver Diseases, the American Gastroenterological Society, and the American Association of Clinical Endocrinology.
Dr. Isaacs, who chaired the AACE guidance writing panel, noted, “The ADA update essentially repeats the same guidance in the AACE and AASLD documents. It is excellent to see this type of alignment of guidance among the major organizations.”
FIB-4: Easy calculation in the EHR
The ADA now advises screening all adults with type 2 diabetes or prediabetes, particularly those with obesity or cardiometabolic risk factors or established cardiovascular disease – even those with normal liver enzyme levels. People with type 1 diabetes who have obesity and/or cardiovascular risk factors are also to be screened for NAFLD.
The recommended screening tool is the fibrosis-4 index (FIB-4), a calculation that includes the patient’s age, liver enzyme levels, and platelet counts. A score of 1.3 or higher is considered high risk for clinically significant fibrosis and above 2.6 is very high-risk.
Dr. Cusi noted, “The reason we advise using the FIB-4 ... instead of liver enzymes as ADA advised in the past, is that now we know that 70% of people with type 2 diabetes have steatosis already and about one in five have fibrosis, but if you go by liver enzymes you will miss most of them. Liver enzymes are ineffective as a screening tool.”
The FIB-4 is “a simple tool we already have in our electronic health records (EHR) but we’re just simply not using it,” noted Dr. Cusi, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville.
Indeed, Dr. Isaacs said, “The FIB-4 is a simple ... great screening test because it is essentially free.” But he cautioned that it has some limitations.
“It is a good test for ruling out advanced liver disease but can have false positives and false negatives. The FIB-4 cutoffs need to be adjusted for persons over 65 years old and [should] not to be used for persons under 30 years old.”
Dr. Isaacs also pointed out that, while the calculation can be done from a website, “even this adds time to a clinician’s busy day. Ideally, the FIB-4 should be automatically calculated in the EHR or on the lab report, similar to the [estimated glomerular filtration rate] calculation [for kidney function] and flagged if greater than 1.3.”
The ADA update also provides guidance on follow-up for patients flagged with the FIB-4, including when referral to a gastroenterologist or hepatologist is appropriate.
Treatment: Lifestyle modification plus GLP-1 agonists or pioglitazone
Lifestyle modification is recommended for all adults with diabetes or prediabetes and NAFLD, particularly those with overweight or obesity.
In addition, the ADA now advises consideration of a using a glucagonlike peptide–1 (GLP-1) agonist with demonstrated benefits in NAFLD as adjunctive therapy to lifestyle interventions for weight loss in those with type 2 diabetes, particularly with overweight/obesity.
And for those with biopsy-proven NASH or who are identified with clinically significant liver fibrosis using non-invasive tests, either a GLP-1 agonist or pioglitazone are the “preferred treatments.”
However, insulin is the preferred treatment for hyperglycemia in adults with type 2 diabetes who have decompensated cirrhosis.
Dr. Isaacs commented, “Pioglitazone has so many benefits and a few known risks ... it is an underused medication. It is very inexpensive. Pioglitazone should be considered as a first line treatment for patients with type 2 diabetes and NAFLD.”
The ADA update also advises statin therapy for people with type 2 diabetes and NAFLD, given their increased cardiovascular risk. However, statins are not recommended for people with decompensated cirrhosis because of limited safety and efficacy data.
Dr. Cusi noted that he has been advocating for fatty liver screening in people with type 2 diabetes for over a decade.
“Doctors have already been adopting it, but ADA as an organization in diabetes care has a big impact. I dreamed many years ago that the day would come when we would screen all people with type 2 diabetes, and that day is today.”
Dr. Cusi is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, BMS, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, Thera Technologies, and MSD. Dr. Isaacs reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADA 2023
Intermittent fasting, cutting calories give same weight loss
a new study published online in Annals of Internal Medicine has found. The small, unblinded study compared weight loss in 77 participants who either intermittently fasted, adhered to a calorie-restricted diet, or were in a control group with no eating restrictions.
Compared with the control group, absolute weight loss for people in the intermittent fasting group was about 4.6 kg (10 lb), compared with 5.4 kg (12 lb) for those in the calorie-restriction group, after 12 months, with no significant difference between the intervention groups.
Intermittent fasting, or time-restricted eating, relies on the idea that the time you eat is more important for weight loss than what or how much you eat. The term is a catch-all for eating patterns that could include several full days of fasting per week or time-restricted eating during the day.
The effect of having less time to eat is thought to lead to the consumption of fewer calories, thought to be the main reason the approach works. Indeed, this trial found the intermittent fasting group ate 425 fewer calories per day, compared with 405 fewer calories per day in the calorie-restricted group.
“Time-restricted eating is undoubtedly an attractive approach to weight loss in that it does not require the purchase of expensive food products, allows persons to continue consuming familiar foods, and omits complicated calorie tracking,” Shuhao Lin, RD, University of Illinois at Chicago, and colleagues write.
During the trial, participants were in a weight-loss phase for 6 months. The intermittent fasting group could eat anything they wanted to between 12 p.m. and 8 p.m., and didn’t have to count calories. The later time window is on par with the eating pattern of most people in the United States who fast.
The calorie-restriction group had to cut 25% of their daily calorie intake based on their total energy expenditure. They were also told to fill half of every plate with fruits or vegetables, and consume about half their energy as carbohydrates, 30% as fat, and 20% as protein.
The weight-loss phase was followed by a 6-month weight-maintenance phase. During this phase, the window for eating was extended from 10 a.m. to 8 p.m. for the intermittent fasting group, and the calorie-restriction group was told to match their energy needs, which overall, had reduced by about 15%, compared with baseline.
Most participants were women with a mean body weight of about 100 kg (220 pounds) at baseline.
Both the time-restricted eating and calorie-restriction groups regularly met with dietitians, which the authors of an accompanying editorial say could have made the intermittent fasting more effective than in previous trials.
An earlier, shorter trial found about 0.9 kg (2 lb) weight loss after 12 weeks of adhering to a similar eating window, a more modest result, compared with the 4 kg (9 lb) weight loss at 6 months in this trial.
“The difference in outcomes between these two trials is likely attributable to differences in dietary counseling,” write the editorialists, Adam Gilden, MD, and Victoria Catenacci, MD, from University of Colorado at Denver, Aurora.
Previous studies of intermittent fasting have been short and showed similar findings, compared with a calorie-restricted diet.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
a new study published online in Annals of Internal Medicine has found. The small, unblinded study compared weight loss in 77 participants who either intermittently fasted, adhered to a calorie-restricted diet, or were in a control group with no eating restrictions.
Compared with the control group, absolute weight loss for people in the intermittent fasting group was about 4.6 kg (10 lb), compared with 5.4 kg (12 lb) for those in the calorie-restriction group, after 12 months, with no significant difference between the intervention groups.
Intermittent fasting, or time-restricted eating, relies on the idea that the time you eat is more important for weight loss than what or how much you eat. The term is a catch-all for eating patterns that could include several full days of fasting per week or time-restricted eating during the day.
The effect of having less time to eat is thought to lead to the consumption of fewer calories, thought to be the main reason the approach works. Indeed, this trial found the intermittent fasting group ate 425 fewer calories per day, compared with 405 fewer calories per day in the calorie-restricted group.
“Time-restricted eating is undoubtedly an attractive approach to weight loss in that it does not require the purchase of expensive food products, allows persons to continue consuming familiar foods, and omits complicated calorie tracking,” Shuhao Lin, RD, University of Illinois at Chicago, and colleagues write.
During the trial, participants were in a weight-loss phase for 6 months. The intermittent fasting group could eat anything they wanted to between 12 p.m. and 8 p.m., and didn’t have to count calories. The later time window is on par with the eating pattern of most people in the United States who fast.
The calorie-restriction group had to cut 25% of their daily calorie intake based on their total energy expenditure. They were also told to fill half of every plate with fruits or vegetables, and consume about half their energy as carbohydrates, 30% as fat, and 20% as protein.
The weight-loss phase was followed by a 6-month weight-maintenance phase. During this phase, the window for eating was extended from 10 a.m. to 8 p.m. for the intermittent fasting group, and the calorie-restriction group was told to match their energy needs, which overall, had reduced by about 15%, compared with baseline.
Most participants were women with a mean body weight of about 100 kg (220 pounds) at baseline.
Both the time-restricted eating and calorie-restriction groups regularly met with dietitians, which the authors of an accompanying editorial say could have made the intermittent fasting more effective than in previous trials.
An earlier, shorter trial found about 0.9 kg (2 lb) weight loss after 12 weeks of adhering to a similar eating window, a more modest result, compared with the 4 kg (9 lb) weight loss at 6 months in this trial.
“The difference in outcomes between these two trials is likely attributable to differences in dietary counseling,” write the editorialists, Adam Gilden, MD, and Victoria Catenacci, MD, from University of Colorado at Denver, Aurora.
Previous studies of intermittent fasting have been short and showed similar findings, compared with a calorie-restricted diet.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
a new study published online in Annals of Internal Medicine has found. The small, unblinded study compared weight loss in 77 participants who either intermittently fasted, adhered to a calorie-restricted diet, or were in a control group with no eating restrictions.
Compared with the control group, absolute weight loss for people in the intermittent fasting group was about 4.6 kg (10 lb), compared with 5.4 kg (12 lb) for those in the calorie-restriction group, after 12 months, with no significant difference between the intervention groups.
Intermittent fasting, or time-restricted eating, relies on the idea that the time you eat is more important for weight loss than what or how much you eat. The term is a catch-all for eating patterns that could include several full days of fasting per week or time-restricted eating during the day.
The effect of having less time to eat is thought to lead to the consumption of fewer calories, thought to be the main reason the approach works. Indeed, this trial found the intermittent fasting group ate 425 fewer calories per day, compared with 405 fewer calories per day in the calorie-restricted group.
“Time-restricted eating is undoubtedly an attractive approach to weight loss in that it does not require the purchase of expensive food products, allows persons to continue consuming familiar foods, and omits complicated calorie tracking,” Shuhao Lin, RD, University of Illinois at Chicago, and colleagues write.
During the trial, participants were in a weight-loss phase for 6 months. The intermittent fasting group could eat anything they wanted to between 12 p.m. and 8 p.m., and didn’t have to count calories. The later time window is on par with the eating pattern of most people in the United States who fast.
The calorie-restriction group had to cut 25% of their daily calorie intake based on their total energy expenditure. They were also told to fill half of every plate with fruits or vegetables, and consume about half their energy as carbohydrates, 30% as fat, and 20% as protein.
The weight-loss phase was followed by a 6-month weight-maintenance phase. During this phase, the window for eating was extended from 10 a.m. to 8 p.m. for the intermittent fasting group, and the calorie-restriction group was told to match their energy needs, which overall, had reduced by about 15%, compared with baseline.
Most participants were women with a mean body weight of about 100 kg (220 pounds) at baseline.
Both the time-restricted eating and calorie-restriction groups regularly met with dietitians, which the authors of an accompanying editorial say could have made the intermittent fasting more effective than in previous trials.
An earlier, shorter trial found about 0.9 kg (2 lb) weight loss after 12 weeks of adhering to a similar eating window, a more modest result, compared with the 4 kg (9 lb) weight loss at 6 months in this trial.
“The difference in outcomes between these two trials is likely attributable to differences in dietary counseling,” write the editorialists, Adam Gilden, MD, and Victoria Catenacci, MD, from University of Colorado at Denver, Aurora.
Previous studies of intermittent fasting have been short and showed similar findings, compared with a calorie-restricted diet.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE