User login
RTNI to Safely Reduce Dysglycemia
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
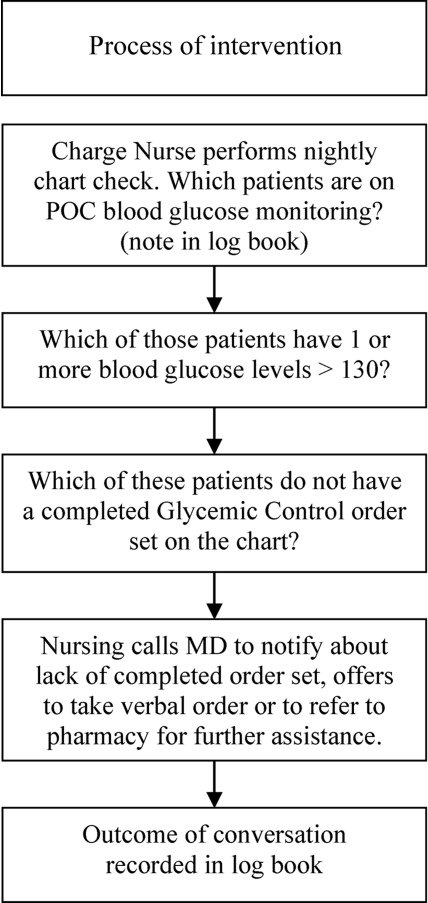
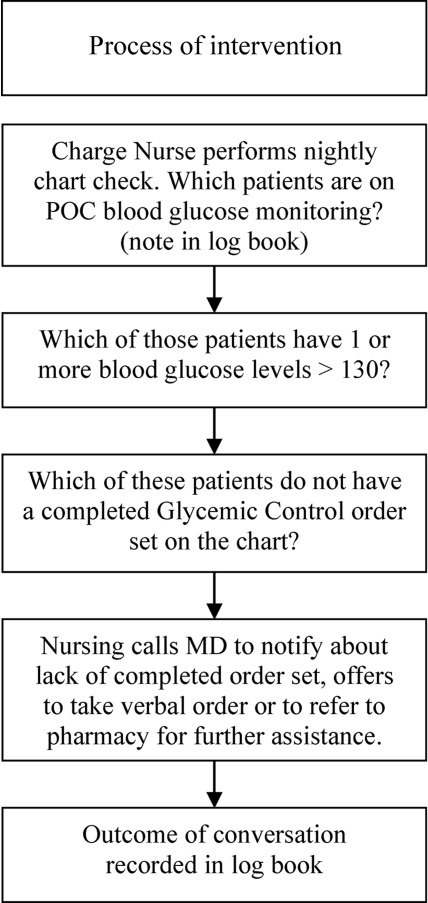
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).
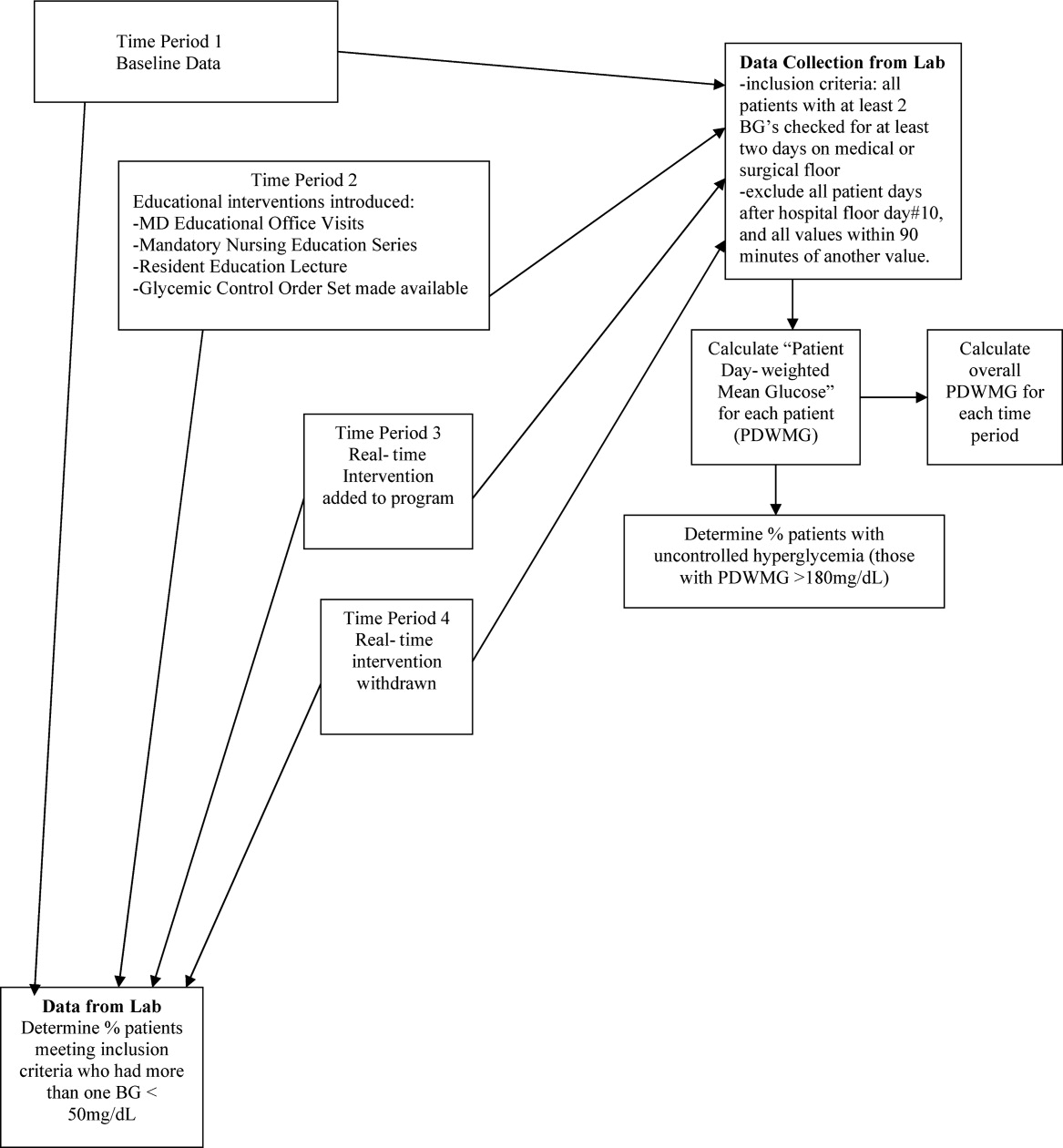
The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).
Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
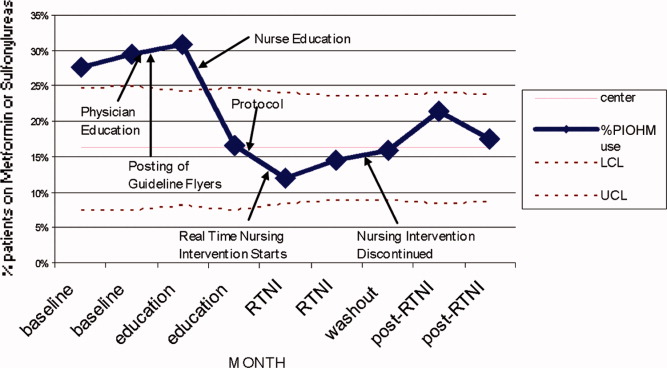
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
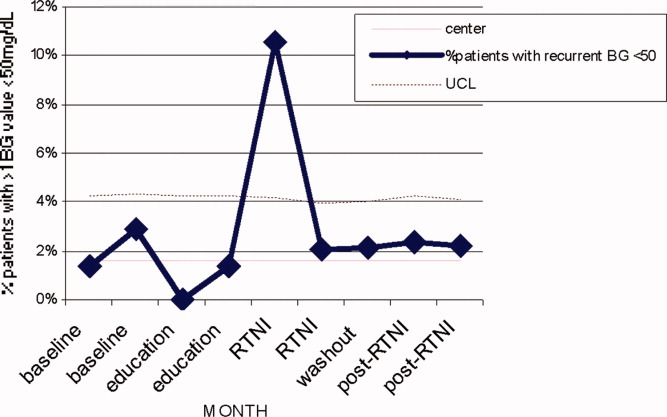
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.

| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).

The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).

Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.



| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).

The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).

Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.



| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
Copyright © 2010 Society of Hospital Medicine
ICU Characteristics in Michigan
Organization of physician services in intensive care units (ICUs) varies widely and influences mortality, morbidity, and costs of care. Intensive care provided by intensivists in a high‐intensity physician staffing model, in which intensivists are the sole attending physicians or consult on all patients, has been associated with desirable outcomes such as decreased length of stay, resource utilization, and mortality.1‐4 As a result, higher intensity ICU models have been recommended by various healthcare agencies, including the National Quality Forum and the Leapfrog Group.5‐7
One national survey indicated that 47% of ICUs surveyed had some intensivist coverage and only 4% of intensive care units met Leapfrog high‐intensity model standards.8 However, only one‐third of ICUs responded to this survey, smaller ICUs were overrepresented, and the survey may not have reflected the influence of newer policy initiatives because it was conducted in 1997. Though the attributes by which intensivists improve patient outcomes is unknown, researchers have suggested it is by having a knowledgeable physician present in the ICU, having a physician communicate with other clinicians and families, and by having a physician who manages the ICU by writing policies and procedures and administrative activities.9
Results have been conflicting as patients managed by intensivists have also been found to have an increased mortality, particularly when managed on an elective consultation basis in an open ICU, where patient orders are written by several physician specialties.10, 11 Alternative ICU staffing models, such as the use of hospitalists, have been utilized to compensate for the intensivist workforce shortage. Hospitalists often provide ICU care, although they are seldom board‐certified in critical care. Hospitalist care has been shown to provide clinical and efficiency benefits such as decreased length of hospital stay.12‐14
Understanding the manner in which critical care is currently delivered, particularly the utilization of intensivist and nonintensivist care providers, can provide insights into subsequent allocation of a limited intensivist workforce as nonintensivist care providers such as hospitalists become more available. To understand how intensivists and other practitioners, such as hospitalists, deliver critical care in Michigan, we performed a cross‐sectional survey of Michigan hospitals participating in the Keystone ICU project, a statewide quality‐improvement initiative.
Methods
The hospitals involved and the methods of Keystone ICU have been published previously.15 The Keystone ICU project is a collaborative quality improvement initiative first organized in October 2003 by the Michigan Health and Hospitals Association (MHA) Keystone Center for Patient Safety and Quality. At its inception, 103 ICUs voluntarily agreed to participate in Keystone ICU and reported data representing 85% of ICU beds in Michigan. Nonparticipating hospitals (n = 37) were smaller, 79% having fewer than 100 beds, many of which did not have ICUs. All ICUs from the 72 hospitals participating in the Keystone ICU project as of July 2005 were asked to complete surveys as part of ongoing data collection.
Keystone ICU sought to improve safety culture, increase adherence to evidence‐based practices among patients receiving mechanical ventilation, and reduce central lineassociated bloodstream infections and ventilator‐associated pneumonia through a number of interventions. Keystone also encouraged teams to standardize their physician staffing, and presented teams with evidence regarding the benefits of ICU physician staffing. Because many of the ICUs were small and believed it was not practical to staff their ICUs with intensivists, Keystone encouraged ICUs to create as many of the attributes of intensivist staffing as possible: having someone present who is knowledgeable, able to manage at the unit level, and who communicates well with clinicians and families.9 As part of this project, we developed a survey to describe the physician staffing in Michigan ICUs. Additional elements of the survey sought to ascertain how medical decision‐making occurred, which decisions were made by what types of clinicians, and who performed various procedures in the ICU.
Survey Development
The survey for this study was developed based on expert opinion and on previous work by the research team (A.D.A., P.J.P., S.A.F.). The survey was pilot tested in a small group of non‐Michigan hospitals and found to be understandable and readable. The survey was then revised and disseminated to all hospitals participating in the Keystone ICU project. Construct validity was determined by review of literature and discussion with the research team (A.D.A., P.J.P., S.A.F., R.C.H.). Content validity was determined by the pilot test, which included interviews with the individuals who pilot‐tested the survey. The survey sought to describe the organization of ICU physician services (including both intensivist and nonintensivist). A copy of the survey is available upon request.
Survey Protocol
Surveys were sent by e‐mail to the official nurse and/or physician project leader at each site in July 2005 from contact information provided by MHA. Another copy of the survey was emailed to ICUs that did not respond to the initial survey after 3 months and, if needed, a third survey was sent at 6 months with a follow‐up telephone call by 1 of the investigators (R.C.H.). The completed surveys were returned to MHA for compilation and analysis. The research project was reviewed by the University of Michigan Institutional Review Board and determined to be exempt from ongoing IRB review per federal exemption category 45 CFR 46.101.(b). The funder was not involved in the design of the study, collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
Statistical Analysis
Survey respondents were first characterized using simple univariable and bivariable methods. When appropriate, groups were compared based on chi‐square, Mann‐Whitney U test, or t test. Additionally, a series of multivariable analyses was performed, which sought to understand structural factors associated with the presence of higher‐intensity models, as well as use of hospitalists or intensivists. Results of the multivariate analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). The critical region was defined as an alpha of 0.05. Statistical analysis was performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
Response Rate
Ninety‐seven responses were received, including at least 1 response from every Keystone ICU hospital located in Michigan. Because our goal was to describe the organization of ICU physician services in non‐Federal hospitals, 1 Michigan VA hospital was eliminated from further consideration. Four hospitals with more than 1 ICU, which delivered care identically in all of their ICUs, provided 1 response and were counted as 1 site. As a result, 96 survey responses representing 115 ICUs in 72 Michigan hospitals were each counted as 1 site in the analysis. This included responses from ICUs not included in earlier analyses, which joined Keystone ICU after earlier work had been underway.15
Baseline Demographics
The mean (standard deviation [SD]) hospital size represented in the survey was 280 (22) beds, with a median of 249 (range, 40‐1031) beds. The mean size (SD) of the ICU was 13.3 (7.0) beds, median 12 beds, range 4 to 42 beds. There were 16 ICUs dedicated exclusively to the care of medicine patients, 14 dedicated surgical units, 8 dedicated cardiac ICUs, and 3 dedicated Neuro ICUs. The remainder had a mixed patient population. Seventy‐one ICUs (74%) cared for medical patients, 69 (72%) cared for surgical patients, 64 (67%) cared for cardiac patients, and 52 (53%) cared for neurological patients.
ICU Staffing Models
To better understand the role of intensivists in critical care delivery in Michigan, we examined differences in sites where patients are managed as closed sites exclusively by intensivists (closed ICU sites) in comparison to ICUs that had multiple attending specialties (open ICU sites). In addition, ICU sites where intensivists made most clinical decisionsa circumstance likely reflecting a high‐intensity staffing model of care5were compared with ICUs sites where decision‐making was made by nonintensivists or was shared (Table 1). Twenty‐four of 96 (25%) ICU sites were closed, and only intensivists served as the attending of record. Hospitals with closed ICUs or in which intensivists made most clinical decisions were larger and had larger ICUs than sites with open ICUs or with nonintensivist decision‐making (P < 0.05). These 24 closed sites represented 17 of 72 hospitals (24%), with the remainder of hospitals (76%) not having closed ICUs. Intensivists participated in rounds in 43 of 72 sites (60%) that were not closed. House officer participation in the care of ICU patients was not related to the presence or absence of intensivists (2 = 0.04; P = 0.847), although the average size of hospitals with house officers was larger than those without house officers (P < 0.0001).
| Closed ICUs (n = 24) [n (%)] | Open ICUs (n = 72) [n (%)] | Intensivist Decision‐making (n = 30) [n (%)] | Shared Decision‐making (n = 31) [n (%)] | Nonintensivist Decision‐making (n = 34) [n (%)] | |
|---|---|---|---|---|---|
| |||||
| ICU beds (mean SD) | 21.8 15.3* | 15.2 13.0* | 21.3 18.7* | 19.2 13.4 | 10.5 5.2* |
| Hospital beds (mean SD) | 489.8 295.3* | 326.3 222.6* | 460.8 222.3* | 408.6 259.7 | 247.8 230.0* |
| Nonintensivist attendings | |||||
| Hospitalist | 34 (47.2) | 9 (30) | 14 (45.1) | 13 (38.2) | |
| Primary care physician | 55 (76.4) | 11 (36.7) | 23 (74.2) | 27 (79.4) | |
| Cardiologist | 54 (75) | 10 (33.3) | 25 (80.6) | 23 (67.6) | |
| Pulmonologist | 34 (47.2) | 9 (30) | 15 (48.3) | 15 (44.1) | |
| Other IM specialist | 48 (66.7) | 11 (36.7) | 25 (80.6) | 17 (50) | |
| Surgeon | 59 (81.9) | 14 (46.7) | 25 (80.6) | 27 (79.4) | |
| Critical care board certification (% of attending physicians) | (n = 28) | (n = 31) | (n = 33) | ||
| 100 | 11 (45.8) | 7 (10.1) | 11 (39.3) | 6 (19.4) | 0 (0) |
| 75 | 3 (12.5) | 6 (8.7) | 7 (25.0) | 2 (6.5) | 0 (0) |
| 50 | 2 (8.3) | 4 (5.8) | 3 (10.7) | 2 (6.5) | 1 (3.0) |
| <50 | 8 (33.3) | 52 (75.4) | 7 (25.0) | 21 (67.7) | 32 (97.0) |
| ICU administration | |||||
| ICU director financial support | 18 (75.0) | 49 (68.1) | 25 (83.3) | 23 (74.2) | 18 (52.9) |
| Meeting with ICU team | 21 (87.5) | 56 (77.8) | 26 (86.7) | 27 (87.1) | 23 (67.7) |
| M&M sessions | 9 (37.5) | 33 (45.8) | 16 (53.3) | 12 (38.7) | 14 (41.2) |
Multivariate analysis determined that the presence of hospitalists serving as attending physicians was strongly associated with an open ICU (OR = 12.2; 95%CI = 2.5‐60.2), as was the absence of intensivists at the site (OR = 12.2; 95%CI = 1.4‐105.8), while ICU and hospital size were not associated. When the analyses were limited to hospitals with intensivists (n = 69), decision‐making by intensivists was not associated with ICU or hospital size (OR = 1.0; 95%CI = 1.0‐1.0); or whether hospitalists acted as attendings (OR = 0.7; 95%CI = 0.2‐2.0).
Board Certification and ICU Administration
Only 18 sites (20%) acknowledged that 100% of their ICU attending physicians were board‐certified in critical care, with nearly two‐thirds of sites having fewer than 50% critical‐care board‐certified attending physicians (Table 1). The medical director of the ICU met for an administrative meeting with the ICU team of nurses, respiratory therapists, and other personnel on a regular (ie, at least quarterly) basis at 77 sites (80%) and held regular morbidity and mortality sessions to discuss ICU care with other physicians who work in the ICU at 43 sites (45%). The majority of sites (n = 67; 70%) provided salary support for the ICU medical director.
Critical‐care board‐certification was more common at sites with closed ICUs and at sites where decision‐making was performed by intensivists (P < 0.001). However, board‐certification was not uniform in closed ICUs (100% certification = 46%, >50% certification = 67%) or in ICUs where intensivists made most decisions (100% certification = 39%, >50% certification = 75%).
Hospitals in which hospitalists served as attending physicians were less likely to have 50% or greater critical‐care board‐certification in their ICU (OR = 0.13; 95%CI = 0.03‐0.50). ICU size, hospital size, and years in practice were not associated with critical‐care board‐certification. Hospital size, ICU size, and the presence of intensivists or hospitalists were not associated with whether the medical director receives support from the hospital.
Physician Extenders
Nineteen sites (20%) reported the utilization of advanced practice nurses; 15 sites (16%) reported use of physician assistants; and 7 sites (7%) reported use of both advance practice nurses and physician assistants to provide intensive care. Physician extenders were not more likely to work in closed ICUs (10/24) than in open ICUs (14/72) (2 = 3.63; P = 0.57).
Of the 27 sites reporting use of advanced practice nurses or physician assistants, the role of physician extenders was described as being similar to physicians in 8 sites (30%), somewhat autonomous but with limitations in 18 (67%), and in a role closer to a ward clerk or assistant in 1 site (4%). The activities of physician extenders included writing orders at 24 of these 27 sites (89%); writing progress notes at 25 sites (92%); communicating with consultants at 24 (89%) and with primary care physicians at 22 sites (82%); and coordinating discharge plans at 20 sites (74%). Physician extenders rounded alone at 16 sites (33%).
Clinical Activities
Intensivists participated in daily rounds at most sites (n = 67; 70%). Nonintensivists served as attending of record in 72 (75%) sites. Nonintensivist physicians participating in daily patient rounds were: surgeons (n = 66; 68% of sites), primary care physicians (n = 61; 64%), nonpulmonary internal medicine specialists (n = 53; 55%), cardiologists (n = 58; 60%), non‐critical‐care pulmonologists (n = 39; 41%), and hospitalists (n = 36; 38%). Intensivists were the primary decision‐makers at 30 sites (31%), nonintensivists at 34 (35%), and decision making was shared at 31 (32%).
At more than one‐half of sites, decisions regarding mechanical ventilation, the use of sedatives or paralytics, and the choice of vasopressor agents were made by intensivists, with other decisionssuch as the decision to call consultants, choice of antibiotics, or family meetingsshared between intensivists and nonintensivists more than 40% of the time (Table 2). During regular working hours, invasive procedures were performed by multiple clinicians, including house officers, intensivists, surgeons, and anesthesiologists and were not the province of any particular type of clinician (Table 3).
| Decision‐making | |||
|---|---|---|---|
| Intensivist n (%) | Nonintensivist n (%) | Shared n (%) | |
| |||
| Ventilator management | 62 (66.7) | 24 (25.8) | 7 (7.5) |
| Choice of ventilator weaning strategies | 64 (68.8) | 24 (25.8) | 5 (5.4) |
| Decision to extubate | 63 (68.5) | 24 (26.1) | 5 (5.4) |
| Choice of sedation or paralytic agents | 56 (65.1) | 24 (27.9) | 6 (7.0) |
| Choice of vasopressor agents | 47 (51.1) | 25 (27.1) | 20 (21.7) |
| Decision to call other consultants (eg, cardiology, infectious diseases) | 19 (20.4) | 31 (33.3) | 43 (46.2) |
| Choices related to more general medical management (eg, antibiotics, diabetes management) | 30 (32.2) | 25 (26.9) | 38 (40.1) |
| Family meetings, code status discussions | 26 (28.6) | 26 (28.6) | 39 (42.8) |
| Procedure | Hospitalist n (%) | Intensivist n (%) | Surgeon n (%) | Anesthesiologist n (%) | House Officer or Other MD n (%) | Other non‐MD n (%) |
|---|---|---|---|---|---|---|
| Arterial line placement | 15 (15.6) | 50 (52.1) | 40 (41.7) | 31 (32.3) | 59 (61.4) | 7 (7.3) |
| Femoral venous line placement | 14 (14.6) | 54 (56.3) | 42 (43.8) | 17 (17.7) | 55 (57.3) | 4 (4.2) |
| Subclavian or internal jugular line placement | 14 (14.6) | 54 (56.2) | 47 (49.0) | 25 (26.0) | 62 (64.6) | 5 (5.2) |
| Pulmonary artery catheterization | 8 (8.3) | 56 (58.3) | 24 (25.0) | 21 (21.9) | 54 (56.2) | 2 (2.1) |
| Intubation | 14 (14.6) | 47 (49.0) | 14 (14.6) | 74 (77.1) | 42 (43.8) | 15 (15.6) |
| Bronchoscopy | 2 (2.1) | 67 (69.8) | 17 (17.7) | 5 (5.2) | 29 (30.2) | 0 (0) |
Regardless of the staffing model employed, the majority of sites (88%) provided care on a call‐based, rather than shift‐based system. Nighttime admissions and cross‐coverage issues were handled by house officers at more than one‐third of sites, with nonintensivist house physicians performing these tasks at 15% of sites (Table 4). Intensivists managed cross‐coverage issues by telephone at 29% of sites, and saw new admissions in person after hours at 8% of sites. Intensivists did not deliver care in scheduled shifts at any of these sites.
| Care Provider | Nighttime Admissions n (%) | Cross‐coverage n (%) |
|---|---|---|
| ||
| Emergency room physician | 13 (13.5) | 8 (8.3) |
| House physician | 15 (15.6) | 17 (17.7) |
| House officer | 42 (43.8) | 37 (38.5) |
| ICU nurse | 5 (5.2) | 10 (10.4) |
| PA or NP | 8 (8.3) | 5 (5.2) |
| Intensivist in person | 8 (8.3) | |
| Intensivist by telephone | 28 (29.2) | |
| Other | 9 (9.4) | 9 (9.4) |
Discussion
As all Keystone ICU participating sites responded to the questionnaire, we believe these results to be representative of critical care practice in the state of Michigan at the present time. Michigan ICU staffing structures are variable. Only a minority (25%) of Michigan Keystone ICU sites operated in an environment where intensivists are the only attending physicians of record. Although intensivists rounded in 60% of sites not utilizing a closed model, 75% of sites had nonintensivist attending physicians, with primary care physicians and hospitalists commonly providing ICU services. The utilization of hospitalists to provide critical care services was found in the absence of intensivists, regardless of hospital or ICU size.
Closed ICUs were seen in larger hospitals and in larger ICUs. This finding is similar to data obtained on a national level.8‐16 A high‐intensity model of care was also uncommon, although decision‐making was at least shared between intensivists and nonintensivists at two‐thirds of sites. These findings are in keeping with the observation that intensivist‐directed care advocated by the Leapfrog Group has not been widely implemented,17 including in Michigan, a regional rollout leader for the Leapfrog Group.
Fewer ICUs reported utilizing a nonintensivist model than was reported in the survey by Angus et al.,8 where approximately one‐half of ICUs delivered care in this manner. This survey was performed in 1997, prior to the launch of the Leapfrog Group effort, and may have reflected a relative over representation of smaller, general ICUs. Our study is the first statewide analysis of critical care practices in the postLeapfrog Group era. Our finding that an array of approaches to critical care delivery existed in Michigan, even when intensivists rounded on patients, is similar to that found among Leapfrog‐compliant hospitals sampled from several regions of the United States.18
Other than intensivists, surgeons, primary care, and hospitalist physicians provided care in Michigan ICUs. The hospitalist movement is relatively new.19 However, in our survey 37.5% of sites had hospitalists serving as attending physicians. Although the closed ICU model was more prevalent in larger ICUs and hospitals, the use of a hospitalist model to staff ICUs was not related to hospital size, but was instead a function of whether or not intensivists were present in a given setting. In lieu of a projected shortage of intensivists, we believe this confirms the crucial role that hospitalists will play in the provision of critical care services in the future.
The attributes of intensivist care that led to improved outcomes in previous studies1‐4 are unknown. To the extent that the involvement of intensivists on an elective rather than mandatory consultative basis may explain the higher mortality found in 1 recent study,1011 we hypothesize that having a knowledgeable physician present who communicates with clinicians and families and manages at the unit level is an important factor leading to improved outcomes. While hospitalists can have these attributes, their knowledge of specific critical care therapies and technologies may vary with the extent of their ICU training and experience. Further research should seek to quantify the attributes by which intensivists are associated with improved outcomes and seek ways to foster those attributes among hospitalists who participate in critical care delivery. Central to this will be ensuring that training programs ensure competency in critical care therapies and technologies among hospitalists and other non‐ICU physicians.
We recognize several limitations in this study. First, the validity of the survey may introduce misclassification of ICU staffing. However, the survey instrument was informed by previously‐validated instruments and experts in ICU physician staffing and hospitalist care. Second, we did not link variation in staffing to outcomes. While such analysis is important, it is beyond the scope of this survey. Third, our study was conducted in 1 state and the results may not be generalizable across the United States. Nevertheless, Michigan is a large state with a diverse array of hospitals, and as our study sample broadly represented this diversity, we believe our results are likely to be generalizable.
In conclusion, few ICUs in Michigan are closed and many utilize nonintensivist critical‐care providers such as hospitalists, primary care providers, and physician extenders to deliver clinical care. Our findings have significant implications for future efforts at a national level that involve the training of hospitalists and their acceptance as critical care practitioners. We suggest future research involving intensive care delivery focus on the feasibility of training sufficient hospitalists to satisfy a growing need for critical care that cannot be filled by intensivists, along with strategic planning to insure the model of care provided is commensurate with the complexity of illness. Although this approach appears to be occurring in Michigan on an ad hoc basis, we believe coordination between larger, intensivist‐run ICUs and smaller, nonintensivist‐run ICUs should be formalized in order to optimize the delivery of intensive care.25
- ,,, et al.;the members of the American College of Critical Care Medicine Task Force on Models for the Definition of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model.Crit Care Med.2001;29;2007–2019.
- ,,, et al.Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of “open” and “closed” formats.JAMA.1996;276:24–31.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- ,,, et al.Effects of an organized critical care service on outcomes and resource utilization: a cohort study.Crit Care Med.1999;27:270–274.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002;288:2151–2162.
- Leapfrog Group. Leapfrog Group Factsheet: ICU physician staffing (IPS). Available at: http://www.leapfroggroup.org/media/file/Leapfrog‐ICU_ Physician_Staffing_Fact_Sheet.pdf. Accessed June 2009.
- National Quality Forum. Safe Practices for Better Healthcare. Available at: http://www.qualityforum.org/pdf/reports/safe_practices.pdf. Accessed June 2009.
- ,,,,,; on behalf of the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS).Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34:1016–1024.
- ,,, et al.Team care: beyond open and closed intensive care units.Curr Opin Crit Care.2006;12:604–608.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–809.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Outcomes of care by hospitalists, general internists and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Descriptive analysis of critical care units in the United States.Crit Care Med.1992;20:846–863.
- .Leapfrog and critical care: evidence‐ and reality‐based intensive care for the 21st century.Am J Med.2004;116:188–193.
- ,,,,.The organization of intensive care unit physician services.Crit Care Med.2007;35:2256–2261.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2:327–330.
- ,,, et al.Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care.Crit Care Med.2003;31:2677–2683.
Organization of physician services in intensive care units (ICUs) varies widely and influences mortality, morbidity, and costs of care. Intensive care provided by intensivists in a high‐intensity physician staffing model, in which intensivists are the sole attending physicians or consult on all patients, has been associated with desirable outcomes such as decreased length of stay, resource utilization, and mortality.1‐4 As a result, higher intensity ICU models have been recommended by various healthcare agencies, including the National Quality Forum and the Leapfrog Group.5‐7
One national survey indicated that 47% of ICUs surveyed had some intensivist coverage and only 4% of intensive care units met Leapfrog high‐intensity model standards.8 However, only one‐third of ICUs responded to this survey, smaller ICUs were overrepresented, and the survey may not have reflected the influence of newer policy initiatives because it was conducted in 1997. Though the attributes by which intensivists improve patient outcomes is unknown, researchers have suggested it is by having a knowledgeable physician present in the ICU, having a physician communicate with other clinicians and families, and by having a physician who manages the ICU by writing policies and procedures and administrative activities.9
Results have been conflicting as patients managed by intensivists have also been found to have an increased mortality, particularly when managed on an elective consultation basis in an open ICU, where patient orders are written by several physician specialties.10, 11 Alternative ICU staffing models, such as the use of hospitalists, have been utilized to compensate for the intensivist workforce shortage. Hospitalists often provide ICU care, although they are seldom board‐certified in critical care. Hospitalist care has been shown to provide clinical and efficiency benefits such as decreased length of hospital stay.12‐14
Understanding the manner in which critical care is currently delivered, particularly the utilization of intensivist and nonintensivist care providers, can provide insights into subsequent allocation of a limited intensivist workforce as nonintensivist care providers such as hospitalists become more available. To understand how intensivists and other practitioners, such as hospitalists, deliver critical care in Michigan, we performed a cross‐sectional survey of Michigan hospitals participating in the Keystone ICU project, a statewide quality‐improvement initiative.
Methods
The hospitals involved and the methods of Keystone ICU have been published previously.15 The Keystone ICU project is a collaborative quality improvement initiative first organized in October 2003 by the Michigan Health and Hospitals Association (MHA) Keystone Center for Patient Safety and Quality. At its inception, 103 ICUs voluntarily agreed to participate in Keystone ICU and reported data representing 85% of ICU beds in Michigan. Nonparticipating hospitals (n = 37) were smaller, 79% having fewer than 100 beds, many of which did not have ICUs. All ICUs from the 72 hospitals participating in the Keystone ICU project as of July 2005 were asked to complete surveys as part of ongoing data collection.
Keystone ICU sought to improve safety culture, increase adherence to evidence‐based practices among patients receiving mechanical ventilation, and reduce central lineassociated bloodstream infections and ventilator‐associated pneumonia through a number of interventions. Keystone also encouraged teams to standardize their physician staffing, and presented teams with evidence regarding the benefits of ICU physician staffing. Because many of the ICUs were small and believed it was not practical to staff their ICUs with intensivists, Keystone encouraged ICUs to create as many of the attributes of intensivist staffing as possible: having someone present who is knowledgeable, able to manage at the unit level, and who communicates well with clinicians and families.9 As part of this project, we developed a survey to describe the physician staffing in Michigan ICUs. Additional elements of the survey sought to ascertain how medical decision‐making occurred, which decisions were made by what types of clinicians, and who performed various procedures in the ICU.
Survey Development
The survey for this study was developed based on expert opinion and on previous work by the research team (A.D.A., P.J.P., S.A.F.). The survey was pilot tested in a small group of non‐Michigan hospitals and found to be understandable and readable. The survey was then revised and disseminated to all hospitals participating in the Keystone ICU project. Construct validity was determined by review of literature and discussion with the research team (A.D.A., P.J.P., S.A.F., R.C.H.). Content validity was determined by the pilot test, which included interviews with the individuals who pilot‐tested the survey. The survey sought to describe the organization of ICU physician services (including both intensivist and nonintensivist). A copy of the survey is available upon request.
Survey Protocol
Surveys were sent by e‐mail to the official nurse and/or physician project leader at each site in July 2005 from contact information provided by MHA. Another copy of the survey was emailed to ICUs that did not respond to the initial survey after 3 months and, if needed, a third survey was sent at 6 months with a follow‐up telephone call by 1 of the investigators (R.C.H.). The completed surveys were returned to MHA for compilation and analysis. The research project was reviewed by the University of Michigan Institutional Review Board and determined to be exempt from ongoing IRB review per federal exemption category 45 CFR 46.101.(b). The funder was not involved in the design of the study, collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
Statistical Analysis
Survey respondents were first characterized using simple univariable and bivariable methods. When appropriate, groups were compared based on chi‐square, Mann‐Whitney U test, or t test. Additionally, a series of multivariable analyses was performed, which sought to understand structural factors associated with the presence of higher‐intensity models, as well as use of hospitalists or intensivists. Results of the multivariate analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). The critical region was defined as an alpha of 0.05. Statistical analysis was performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
Response Rate
Ninety‐seven responses were received, including at least 1 response from every Keystone ICU hospital located in Michigan. Because our goal was to describe the organization of ICU physician services in non‐Federal hospitals, 1 Michigan VA hospital was eliminated from further consideration. Four hospitals with more than 1 ICU, which delivered care identically in all of their ICUs, provided 1 response and were counted as 1 site. As a result, 96 survey responses representing 115 ICUs in 72 Michigan hospitals were each counted as 1 site in the analysis. This included responses from ICUs not included in earlier analyses, which joined Keystone ICU after earlier work had been underway.15
Baseline Demographics
The mean (standard deviation [SD]) hospital size represented in the survey was 280 (22) beds, with a median of 249 (range, 40‐1031) beds. The mean size (SD) of the ICU was 13.3 (7.0) beds, median 12 beds, range 4 to 42 beds. There were 16 ICUs dedicated exclusively to the care of medicine patients, 14 dedicated surgical units, 8 dedicated cardiac ICUs, and 3 dedicated Neuro ICUs. The remainder had a mixed patient population. Seventy‐one ICUs (74%) cared for medical patients, 69 (72%) cared for surgical patients, 64 (67%) cared for cardiac patients, and 52 (53%) cared for neurological patients.
ICU Staffing Models
To better understand the role of intensivists in critical care delivery in Michigan, we examined differences in sites where patients are managed as closed sites exclusively by intensivists (closed ICU sites) in comparison to ICUs that had multiple attending specialties (open ICU sites). In addition, ICU sites where intensivists made most clinical decisionsa circumstance likely reflecting a high‐intensity staffing model of care5were compared with ICUs sites where decision‐making was made by nonintensivists or was shared (Table 1). Twenty‐four of 96 (25%) ICU sites were closed, and only intensivists served as the attending of record. Hospitals with closed ICUs or in which intensivists made most clinical decisions were larger and had larger ICUs than sites with open ICUs or with nonintensivist decision‐making (P < 0.05). These 24 closed sites represented 17 of 72 hospitals (24%), with the remainder of hospitals (76%) not having closed ICUs. Intensivists participated in rounds in 43 of 72 sites (60%) that were not closed. House officer participation in the care of ICU patients was not related to the presence or absence of intensivists (2 = 0.04; P = 0.847), although the average size of hospitals with house officers was larger than those without house officers (P < 0.0001).
| Closed ICUs (n = 24) [n (%)] | Open ICUs (n = 72) [n (%)] | Intensivist Decision‐making (n = 30) [n (%)] | Shared Decision‐making (n = 31) [n (%)] | Nonintensivist Decision‐making (n = 34) [n (%)] | |
|---|---|---|---|---|---|
| |||||
| ICU beds (mean SD) | 21.8 15.3* | 15.2 13.0* | 21.3 18.7* | 19.2 13.4 | 10.5 5.2* |
| Hospital beds (mean SD) | 489.8 295.3* | 326.3 222.6* | 460.8 222.3* | 408.6 259.7 | 247.8 230.0* |
| Nonintensivist attendings | |||||
| Hospitalist | 34 (47.2) | 9 (30) | 14 (45.1) | 13 (38.2) | |
| Primary care physician | 55 (76.4) | 11 (36.7) | 23 (74.2) | 27 (79.4) | |
| Cardiologist | 54 (75) | 10 (33.3) | 25 (80.6) | 23 (67.6) | |
| Pulmonologist | 34 (47.2) | 9 (30) | 15 (48.3) | 15 (44.1) | |
| Other IM specialist | 48 (66.7) | 11 (36.7) | 25 (80.6) | 17 (50) | |
| Surgeon | 59 (81.9) | 14 (46.7) | 25 (80.6) | 27 (79.4) | |
| Critical care board certification (% of attending physicians) | (n = 28) | (n = 31) | (n = 33) | ||
| 100 | 11 (45.8) | 7 (10.1) | 11 (39.3) | 6 (19.4) | 0 (0) |
| 75 | 3 (12.5) | 6 (8.7) | 7 (25.0) | 2 (6.5) | 0 (0) |
| 50 | 2 (8.3) | 4 (5.8) | 3 (10.7) | 2 (6.5) | 1 (3.0) |
| <50 | 8 (33.3) | 52 (75.4) | 7 (25.0) | 21 (67.7) | 32 (97.0) |
| ICU administration | |||||
| ICU director financial support | 18 (75.0) | 49 (68.1) | 25 (83.3) | 23 (74.2) | 18 (52.9) |
| Meeting with ICU team | 21 (87.5) | 56 (77.8) | 26 (86.7) | 27 (87.1) | 23 (67.7) |
| M&M sessions | 9 (37.5) | 33 (45.8) | 16 (53.3) | 12 (38.7) | 14 (41.2) |
Multivariate analysis determined that the presence of hospitalists serving as attending physicians was strongly associated with an open ICU (OR = 12.2; 95%CI = 2.5‐60.2), as was the absence of intensivists at the site (OR = 12.2; 95%CI = 1.4‐105.8), while ICU and hospital size were not associated. When the analyses were limited to hospitals with intensivists (n = 69), decision‐making by intensivists was not associated with ICU or hospital size (OR = 1.0; 95%CI = 1.0‐1.0); or whether hospitalists acted as attendings (OR = 0.7; 95%CI = 0.2‐2.0).
Board Certification and ICU Administration
Only 18 sites (20%) acknowledged that 100% of their ICU attending physicians were board‐certified in critical care, with nearly two‐thirds of sites having fewer than 50% critical‐care board‐certified attending physicians (Table 1). The medical director of the ICU met for an administrative meeting with the ICU team of nurses, respiratory therapists, and other personnel on a regular (ie, at least quarterly) basis at 77 sites (80%) and held regular morbidity and mortality sessions to discuss ICU care with other physicians who work in the ICU at 43 sites (45%). The majority of sites (n = 67; 70%) provided salary support for the ICU medical director.
Critical‐care board‐certification was more common at sites with closed ICUs and at sites where decision‐making was performed by intensivists (P < 0.001). However, board‐certification was not uniform in closed ICUs (100% certification = 46%, >50% certification = 67%) or in ICUs where intensivists made most decisions (100% certification = 39%, >50% certification = 75%).
Hospitals in which hospitalists served as attending physicians were less likely to have 50% or greater critical‐care board‐certification in their ICU (OR = 0.13; 95%CI = 0.03‐0.50). ICU size, hospital size, and years in practice were not associated with critical‐care board‐certification. Hospital size, ICU size, and the presence of intensivists or hospitalists were not associated with whether the medical director receives support from the hospital.
Physician Extenders
Nineteen sites (20%) reported the utilization of advanced practice nurses; 15 sites (16%) reported use of physician assistants; and 7 sites (7%) reported use of both advance practice nurses and physician assistants to provide intensive care. Physician extenders were not more likely to work in closed ICUs (10/24) than in open ICUs (14/72) (2 = 3.63; P = 0.57).
Of the 27 sites reporting use of advanced practice nurses or physician assistants, the role of physician extenders was described as being similar to physicians in 8 sites (30%), somewhat autonomous but with limitations in 18 (67%), and in a role closer to a ward clerk or assistant in 1 site (4%). The activities of physician extenders included writing orders at 24 of these 27 sites (89%); writing progress notes at 25 sites (92%); communicating with consultants at 24 (89%) and with primary care physicians at 22 sites (82%); and coordinating discharge plans at 20 sites (74%). Physician extenders rounded alone at 16 sites (33%).
Clinical Activities
Intensivists participated in daily rounds at most sites (n = 67; 70%). Nonintensivists served as attending of record in 72 (75%) sites. Nonintensivist physicians participating in daily patient rounds were: surgeons (n = 66; 68% of sites), primary care physicians (n = 61; 64%), nonpulmonary internal medicine specialists (n = 53; 55%), cardiologists (n = 58; 60%), non‐critical‐care pulmonologists (n = 39; 41%), and hospitalists (n = 36; 38%). Intensivists were the primary decision‐makers at 30 sites (31%), nonintensivists at 34 (35%), and decision making was shared at 31 (32%).
At more than one‐half of sites, decisions regarding mechanical ventilation, the use of sedatives or paralytics, and the choice of vasopressor agents were made by intensivists, with other decisionssuch as the decision to call consultants, choice of antibiotics, or family meetingsshared between intensivists and nonintensivists more than 40% of the time (Table 2). During regular working hours, invasive procedures were performed by multiple clinicians, including house officers, intensivists, surgeons, and anesthesiologists and were not the province of any particular type of clinician (Table 3).
| Decision‐making | |||
|---|---|---|---|
| Intensivist n (%) | Nonintensivist n (%) | Shared n (%) | |
| |||
| Ventilator management | 62 (66.7) | 24 (25.8) | 7 (7.5) |
| Choice of ventilator weaning strategies | 64 (68.8) | 24 (25.8) | 5 (5.4) |
| Decision to extubate | 63 (68.5) | 24 (26.1) | 5 (5.4) |
| Choice of sedation or paralytic agents | 56 (65.1) | 24 (27.9) | 6 (7.0) |
| Choice of vasopressor agents | 47 (51.1) | 25 (27.1) | 20 (21.7) |
| Decision to call other consultants (eg, cardiology, infectious diseases) | 19 (20.4) | 31 (33.3) | 43 (46.2) |
| Choices related to more general medical management (eg, antibiotics, diabetes management) | 30 (32.2) | 25 (26.9) | 38 (40.1) |
| Family meetings, code status discussions | 26 (28.6) | 26 (28.6) | 39 (42.8) |
| Procedure | Hospitalist n (%) | Intensivist n (%) | Surgeon n (%) | Anesthesiologist n (%) | House Officer or Other MD n (%) | Other non‐MD n (%) |
|---|---|---|---|---|---|---|
| Arterial line placement | 15 (15.6) | 50 (52.1) | 40 (41.7) | 31 (32.3) | 59 (61.4) | 7 (7.3) |
| Femoral venous line placement | 14 (14.6) | 54 (56.3) | 42 (43.8) | 17 (17.7) | 55 (57.3) | 4 (4.2) |
| Subclavian or internal jugular line placement | 14 (14.6) | 54 (56.2) | 47 (49.0) | 25 (26.0) | 62 (64.6) | 5 (5.2) |
| Pulmonary artery catheterization | 8 (8.3) | 56 (58.3) | 24 (25.0) | 21 (21.9) | 54 (56.2) | 2 (2.1) |
| Intubation | 14 (14.6) | 47 (49.0) | 14 (14.6) | 74 (77.1) | 42 (43.8) | 15 (15.6) |
| Bronchoscopy | 2 (2.1) | 67 (69.8) | 17 (17.7) | 5 (5.2) | 29 (30.2) | 0 (0) |
Regardless of the staffing model employed, the majority of sites (88%) provided care on a call‐based, rather than shift‐based system. Nighttime admissions and cross‐coverage issues were handled by house officers at more than one‐third of sites, with nonintensivist house physicians performing these tasks at 15% of sites (Table 4). Intensivists managed cross‐coverage issues by telephone at 29% of sites, and saw new admissions in person after hours at 8% of sites. Intensivists did not deliver care in scheduled shifts at any of these sites.
| Care Provider | Nighttime Admissions n (%) | Cross‐coverage n (%) |
|---|---|---|
| ||
| Emergency room physician | 13 (13.5) | 8 (8.3) |
| House physician | 15 (15.6) | 17 (17.7) |
| House officer | 42 (43.8) | 37 (38.5) |
| ICU nurse | 5 (5.2) | 10 (10.4) |
| PA or NP | 8 (8.3) | 5 (5.2) |
| Intensivist in person | 8 (8.3) | |
| Intensivist by telephone | 28 (29.2) | |
| Other | 9 (9.4) | 9 (9.4) |
Discussion
As all Keystone ICU participating sites responded to the questionnaire, we believe these results to be representative of critical care practice in the state of Michigan at the present time. Michigan ICU staffing structures are variable. Only a minority (25%) of Michigan Keystone ICU sites operated in an environment where intensivists are the only attending physicians of record. Although intensivists rounded in 60% of sites not utilizing a closed model, 75% of sites had nonintensivist attending physicians, with primary care physicians and hospitalists commonly providing ICU services. The utilization of hospitalists to provide critical care services was found in the absence of intensivists, regardless of hospital or ICU size.
Closed ICUs were seen in larger hospitals and in larger ICUs. This finding is similar to data obtained on a national level.8‐16 A high‐intensity model of care was also uncommon, although decision‐making was at least shared between intensivists and nonintensivists at two‐thirds of sites. These findings are in keeping with the observation that intensivist‐directed care advocated by the Leapfrog Group has not been widely implemented,17 including in Michigan, a regional rollout leader for the Leapfrog Group.
Fewer ICUs reported utilizing a nonintensivist model than was reported in the survey by Angus et al.,8 where approximately one‐half of ICUs delivered care in this manner. This survey was performed in 1997, prior to the launch of the Leapfrog Group effort, and may have reflected a relative over representation of smaller, general ICUs. Our study is the first statewide analysis of critical care practices in the postLeapfrog Group era. Our finding that an array of approaches to critical care delivery existed in Michigan, even when intensivists rounded on patients, is similar to that found among Leapfrog‐compliant hospitals sampled from several regions of the United States.18
Other than intensivists, surgeons, primary care, and hospitalist physicians provided care in Michigan ICUs. The hospitalist movement is relatively new.19 However, in our survey 37.5% of sites had hospitalists serving as attending physicians. Although the closed ICU model was more prevalent in larger ICUs and hospitals, the use of a hospitalist model to staff ICUs was not related to hospital size, but was instead a function of whether or not intensivists were present in a given setting. In lieu of a projected shortage of intensivists, we believe this confirms the crucial role that hospitalists will play in the provision of critical care services in the future.
The attributes of intensivist care that led to improved outcomes in previous studies1‐4 are unknown. To the extent that the involvement of intensivists on an elective rather than mandatory consultative basis may explain the higher mortality found in 1 recent study,1011 we hypothesize that having a knowledgeable physician present who communicates with clinicians and families and manages at the unit level is an important factor leading to improved outcomes. While hospitalists can have these attributes, their knowledge of specific critical care therapies and technologies may vary with the extent of their ICU training and experience. Further research should seek to quantify the attributes by which intensivists are associated with improved outcomes and seek ways to foster those attributes among hospitalists who participate in critical care delivery. Central to this will be ensuring that training programs ensure competency in critical care therapies and technologies among hospitalists and other non‐ICU physicians.
We recognize several limitations in this study. First, the validity of the survey may introduce misclassification of ICU staffing. However, the survey instrument was informed by previously‐validated instruments and experts in ICU physician staffing and hospitalist care. Second, we did not link variation in staffing to outcomes. While such analysis is important, it is beyond the scope of this survey. Third, our study was conducted in 1 state and the results may not be generalizable across the United States. Nevertheless, Michigan is a large state with a diverse array of hospitals, and as our study sample broadly represented this diversity, we believe our results are likely to be generalizable.
In conclusion, few ICUs in Michigan are closed and many utilize nonintensivist critical‐care providers such as hospitalists, primary care providers, and physician extenders to deliver clinical care. Our findings have significant implications for future efforts at a national level that involve the training of hospitalists and their acceptance as critical care practitioners. We suggest future research involving intensive care delivery focus on the feasibility of training sufficient hospitalists to satisfy a growing need for critical care that cannot be filled by intensivists, along with strategic planning to insure the model of care provided is commensurate with the complexity of illness. Although this approach appears to be occurring in Michigan on an ad hoc basis, we believe coordination between larger, intensivist‐run ICUs and smaller, nonintensivist‐run ICUs should be formalized in order to optimize the delivery of intensive care.25
Organization of physician services in intensive care units (ICUs) varies widely and influences mortality, morbidity, and costs of care. Intensive care provided by intensivists in a high‐intensity physician staffing model, in which intensivists are the sole attending physicians or consult on all patients, has been associated with desirable outcomes such as decreased length of stay, resource utilization, and mortality.1‐4 As a result, higher intensity ICU models have been recommended by various healthcare agencies, including the National Quality Forum and the Leapfrog Group.5‐7
One national survey indicated that 47% of ICUs surveyed had some intensivist coverage and only 4% of intensive care units met Leapfrog high‐intensity model standards.8 However, only one‐third of ICUs responded to this survey, smaller ICUs were overrepresented, and the survey may not have reflected the influence of newer policy initiatives because it was conducted in 1997. Though the attributes by which intensivists improve patient outcomes is unknown, researchers have suggested it is by having a knowledgeable physician present in the ICU, having a physician communicate with other clinicians and families, and by having a physician who manages the ICU by writing policies and procedures and administrative activities.9
Results have been conflicting as patients managed by intensivists have also been found to have an increased mortality, particularly when managed on an elective consultation basis in an open ICU, where patient orders are written by several physician specialties.10, 11 Alternative ICU staffing models, such as the use of hospitalists, have been utilized to compensate for the intensivist workforce shortage. Hospitalists often provide ICU care, although they are seldom board‐certified in critical care. Hospitalist care has been shown to provide clinical and efficiency benefits such as decreased length of hospital stay.12‐14
Understanding the manner in which critical care is currently delivered, particularly the utilization of intensivist and nonintensivist care providers, can provide insights into subsequent allocation of a limited intensivist workforce as nonintensivist care providers such as hospitalists become more available. To understand how intensivists and other practitioners, such as hospitalists, deliver critical care in Michigan, we performed a cross‐sectional survey of Michigan hospitals participating in the Keystone ICU project, a statewide quality‐improvement initiative.
Methods
The hospitals involved and the methods of Keystone ICU have been published previously.15 The Keystone ICU project is a collaborative quality improvement initiative first organized in October 2003 by the Michigan Health and Hospitals Association (MHA) Keystone Center for Patient Safety and Quality. At its inception, 103 ICUs voluntarily agreed to participate in Keystone ICU and reported data representing 85% of ICU beds in Michigan. Nonparticipating hospitals (n = 37) were smaller, 79% having fewer than 100 beds, many of which did not have ICUs. All ICUs from the 72 hospitals participating in the Keystone ICU project as of July 2005 were asked to complete surveys as part of ongoing data collection.
Keystone ICU sought to improve safety culture, increase adherence to evidence‐based practices among patients receiving mechanical ventilation, and reduce central lineassociated bloodstream infections and ventilator‐associated pneumonia through a number of interventions. Keystone also encouraged teams to standardize their physician staffing, and presented teams with evidence regarding the benefits of ICU physician staffing. Because many of the ICUs were small and believed it was not practical to staff their ICUs with intensivists, Keystone encouraged ICUs to create as many of the attributes of intensivist staffing as possible: having someone present who is knowledgeable, able to manage at the unit level, and who communicates well with clinicians and families.9 As part of this project, we developed a survey to describe the physician staffing in Michigan ICUs. Additional elements of the survey sought to ascertain how medical decision‐making occurred, which decisions were made by what types of clinicians, and who performed various procedures in the ICU.
Survey Development
The survey for this study was developed based on expert opinion and on previous work by the research team (A.D.A., P.J.P., S.A.F.). The survey was pilot tested in a small group of non‐Michigan hospitals and found to be understandable and readable. The survey was then revised and disseminated to all hospitals participating in the Keystone ICU project. Construct validity was determined by review of literature and discussion with the research team (A.D.A., P.J.P., S.A.F., R.C.H.). Content validity was determined by the pilot test, which included interviews with the individuals who pilot‐tested the survey. The survey sought to describe the organization of ICU physician services (including both intensivist and nonintensivist). A copy of the survey is available upon request.
Survey Protocol
Surveys were sent by e‐mail to the official nurse and/or physician project leader at each site in July 2005 from contact information provided by MHA. Another copy of the survey was emailed to ICUs that did not respond to the initial survey after 3 months and, if needed, a third survey was sent at 6 months with a follow‐up telephone call by 1 of the investigators (R.C.H.). The completed surveys were returned to MHA for compilation and analysis. The research project was reviewed by the University of Michigan Institutional Review Board and determined to be exempt from ongoing IRB review per federal exemption category 45 CFR 46.101.(b). The funder was not involved in the design of the study, collection, analysis, and interpretation of the data, or the decision to approve publication of the finished manuscript.
Statistical Analysis
Survey respondents were first characterized using simple univariable and bivariable methods. When appropriate, groups were compared based on chi‐square, Mann‐Whitney U test, or t test. Additionally, a series of multivariable analyses was performed, which sought to understand structural factors associated with the presence of higher‐intensity models, as well as use of hospitalists or intensivists. Results of the multivariate analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). The critical region was defined as an alpha of 0.05. Statistical analysis was performed using SAS (version 9.1; SAS Institute, Inc., Cary, NC).
Results
Response Rate
Ninety‐seven responses were received, including at least 1 response from every Keystone ICU hospital located in Michigan. Because our goal was to describe the organization of ICU physician services in non‐Federal hospitals, 1 Michigan VA hospital was eliminated from further consideration. Four hospitals with more than 1 ICU, which delivered care identically in all of their ICUs, provided 1 response and were counted as 1 site. As a result, 96 survey responses representing 115 ICUs in 72 Michigan hospitals were each counted as 1 site in the analysis. This included responses from ICUs not included in earlier analyses, which joined Keystone ICU after earlier work had been underway.15
Baseline Demographics
The mean (standard deviation [SD]) hospital size represented in the survey was 280 (22) beds, with a median of 249 (range, 40‐1031) beds. The mean size (SD) of the ICU was 13.3 (7.0) beds, median 12 beds, range 4 to 42 beds. There were 16 ICUs dedicated exclusively to the care of medicine patients, 14 dedicated surgical units, 8 dedicated cardiac ICUs, and 3 dedicated Neuro ICUs. The remainder had a mixed patient population. Seventy‐one ICUs (74%) cared for medical patients, 69 (72%) cared for surgical patients, 64 (67%) cared for cardiac patients, and 52 (53%) cared for neurological patients.
ICU Staffing Models
To better understand the role of intensivists in critical care delivery in Michigan, we examined differences in sites where patients are managed as closed sites exclusively by intensivists (closed ICU sites) in comparison to ICUs that had multiple attending specialties (open ICU sites). In addition, ICU sites where intensivists made most clinical decisionsa circumstance likely reflecting a high‐intensity staffing model of care5were compared with ICUs sites where decision‐making was made by nonintensivists or was shared (Table 1). Twenty‐four of 96 (25%) ICU sites were closed, and only intensivists served as the attending of record. Hospitals with closed ICUs or in which intensivists made most clinical decisions were larger and had larger ICUs than sites with open ICUs or with nonintensivist decision‐making (P < 0.05). These 24 closed sites represented 17 of 72 hospitals (24%), with the remainder of hospitals (76%) not having closed ICUs. Intensivists participated in rounds in 43 of 72 sites (60%) that were not closed. House officer participation in the care of ICU patients was not related to the presence or absence of intensivists (2 = 0.04; P = 0.847), although the average size of hospitals with house officers was larger than those without house officers (P < 0.0001).
| Closed ICUs (n = 24) [n (%)] | Open ICUs (n = 72) [n (%)] | Intensivist Decision‐making (n = 30) [n (%)] | Shared Decision‐making (n = 31) [n (%)] | Nonintensivist Decision‐making (n = 34) [n (%)] | |
|---|---|---|---|---|---|
| |||||
| ICU beds (mean SD) | 21.8 15.3* | 15.2 13.0* | 21.3 18.7* | 19.2 13.4 | 10.5 5.2* |
| Hospital beds (mean SD) | 489.8 295.3* | 326.3 222.6* | 460.8 222.3* | 408.6 259.7 | 247.8 230.0* |
| Nonintensivist attendings | |||||
| Hospitalist | 34 (47.2) | 9 (30) | 14 (45.1) | 13 (38.2) | |
| Primary care physician | 55 (76.4) | 11 (36.7) | 23 (74.2) | 27 (79.4) | |
| Cardiologist | 54 (75) | 10 (33.3) | 25 (80.6) | 23 (67.6) | |
| Pulmonologist | 34 (47.2) | 9 (30) | 15 (48.3) | 15 (44.1) | |
| Other IM specialist | 48 (66.7) | 11 (36.7) | 25 (80.6) | 17 (50) | |
| Surgeon | 59 (81.9) | 14 (46.7) | 25 (80.6) | 27 (79.4) | |
| Critical care board certification (% of attending physicians) | (n = 28) | (n = 31) | (n = 33) | ||
| 100 | 11 (45.8) | 7 (10.1) | 11 (39.3) | 6 (19.4) | 0 (0) |
| 75 | 3 (12.5) | 6 (8.7) | 7 (25.0) | 2 (6.5) | 0 (0) |
| 50 | 2 (8.3) | 4 (5.8) | 3 (10.7) | 2 (6.5) | 1 (3.0) |
| <50 | 8 (33.3) | 52 (75.4) | 7 (25.0) | 21 (67.7) | 32 (97.0) |
| ICU administration | |||||
| ICU director financial support | 18 (75.0) | 49 (68.1) | 25 (83.3) | 23 (74.2) | 18 (52.9) |
| Meeting with ICU team | 21 (87.5) | 56 (77.8) | 26 (86.7) | 27 (87.1) | 23 (67.7) |
| M&M sessions | 9 (37.5) | 33 (45.8) | 16 (53.3) | 12 (38.7) | 14 (41.2) |
Multivariate analysis determined that the presence of hospitalists serving as attending physicians was strongly associated with an open ICU (OR = 12.2; 95%CI = 2.5‐60.2), as was the absence of intensivists at the site (OR = 12.2; 95%CI = 1.4‐105.8), while ICU and hospital size were not associated. When the analyses were limited to hospitals with intensivists (n = 69), decision‐making by intensivists was not associated with ICU or hospital size (OR = 1.0; 95%CI = 1.0‐1.0); or whether hospitalists acted as attendings (OR = 0.7; 95%CI = 0.2‐2.0).
Board Certification and ICU Administration
Only 18 sites (20%) acknowledged that 100% of their ICU attending physicians were board‐certified in critical care, with nearly two‐thirds of sites having fewer than 50% critical‐care board‐certified attending physicians (Table 1). The medical director of the ICU met for an administrative meeting with the ICU team of nurses, respiratory therapists, and other personnel on a regular (ie, at least quarterly) basis at 77 sites (80%) and held regular morbidity and mortality sessions to discuss ICU care with other physicians who work in the ICU at 43 sites (45%). The majority of sites (n = 67; 70%) provided salary support for the ICU medical director.
Critical‐care board‐certification was more common at sites with closed ICUs and at sites where decision‐making was performed by intensivists (P < 0.001). However, board‐certification was not uniform in closed ICUs (100% certification = 46%, >50% certification = 67%) or in ICUs where intensivists made most decisions (100% certification = 39%, >50% certification = 75%).
Hospitals in which hospitalists served as attending physicians were less likely to have 50% or greater critical‐care board‐certification in their ICU (OR = 0.13; 95%CI = 0.03‐0.50). ICU size, hospital size, and years in practice were not associated with critical‐care board‐certification. Hospital size, ICU size, and the presence of intensivists or hospitalists were not associated with whether the medical director receives support from the hospital.
Physician Extenders
Nineteen sites (20%) reported the utilization of advanced practice nurses; 15 sites (16%) reported use of physician assistants; and 7 sites (7%) reported use of both advance practice nurses and physician assistants to provide intensive care. Physician extenders were not more likely to work in closed ICUs (10/24) than in open ICUs (14/72) (2 = 3.63; P = 0.57).
Of the 27 sites reporting use of advanced practice nurses or physician assistants, the role of physician extenders was described as being similar to physicians in 8 sites (30%), somewhat autonomous but with limitations in 18 (67%), and in a role closer to a ward clerk or assistant in 1 site (4%). The activities of physician extenders included writing orders at 24 of these 27 sites (89%); writing progress notes at 25 sites (92%); communicating with consultants at 24 (89%) and with primary care physicians at 22 sites (82%); and coordinating discharge plans at 20 sites (74%). Physician extenders rounded alone at 16 sites (33%).
Clinical Activities
Intensivists participated in daily rounds at most sites (n = 67; 70%). Nonintensivists served as attending of record in 72 (75%) sites. Nonintensivist physicians participating in daily patient rounds were: surgeons (n = 66; 68% of sites), primary care physicians (n = 61; 64%), nonpulmonary internal medicine specialists (n = 53; 55%), cardiologists (n = 58; 60%), non‐critical‐care pulmonologists (n = 39; 41%), and hospitalists (n = 36; 38%). Intensivists were the primary decision‐makers at 30 sites (31%), nonintensivists at 34 (35%), and decision making was shared at 31 (32%).
At more than one‐half of sites, decisions regarding mechanical ventilation, the use of sedatives or paralytics, and the choice of vasopressor agents were made by intensivists, with other decisionssuch as the decision to call consultants, choice of antibiotics, or family meetingsshared between intensivists and nonintensivists more than 40% of the time (Table 2). During regular working hours, invasive procedures were performed by multiple clinicians, including house officers, intensivists, surgeons, and anesthesiologists and were not the province of any particular type of clinician (Table 3).
| Decision‐making | |||
|---|---|---|---|
| Intensivist n (%) | Nonintensivist n (%) | Shared n (%) | |
| |||
| Ventilator management | 62 (66.7) | 24 (25.8) | 7 (7.5) |
| Choice of ventilator weaning strategies | 64 (68.8) | 24 (25.8) | 5 (5.4) |
| Decision to extubate | 63 (68.5) | 24 (26.1) | 5 (5.4) |
| Choice of sedation or paralytic agents | 56 (65.1) | 24 (27.9) | 6 (7.0) |
| Choice of vasopressor agents | 47 (51.1) | 25 (27.1) | 20 (21.7) |
| Decision to call other consultants (eg, cardiology, infectious diseases) | 19 (20.4) | 31 (33.3) | 43 (46.2) |
| Choices related to more general medical management (eg, antibiotics, diabetes management) | 30 (32.2) | 25 (26.9) | 38 (40.1) |
| Family meetings, code status discussions | 26 (28.6) | 26 (28.6) | 39 (42.8) |
| Procedure | Hospitalist n (%) | Intensivist n (%) | Surgeon n (%) | Anesthesiologist n (%) | House Officer or Other MD n (%) | Other non‐MD n (%) |
|---|---|---|---|---|---|---|
| Arterial line placement | 15 (15.6) | 50 (52.1) | 40 (41.7) | 31 (32.3) | 59 (61.4) | 7 (7.3) |
| Femoral venous line placement | 14 (14.6) | 54 (56.3) | 42 (43.8) | 17 (17.7) | 55 (57.3) | 4 (4.2) |
| Subclavian or internal jugular line placement | 14 (14.6) | 54 (56.2) | 47 (49.0) | 25 (26.0) | 62 (64.6) | 5 (5.2) |
| Pulmonary artery catheterization | 8 (8.3) | 56 (58.3) | 24 (25.0) | 21 (21.9) | 54 (56.2) | 2 (2.1) |
| Intubation | 14 (14.6) | 47 (49.0) | 14 (14.6) | 74 (77.1) | 42 (43.8) | 15 (15.6) |
| Bronchoscopy | 2 (2.1) | 67 (69.8) | 17 (17.7) | 5 (5.2) | 29 (30.2) | 0 (0) |
Regardless of the staffing model employed, the majority of sites (88%) provided care on a call‐based, rather than shift‐based system. Nighttime admissions and cross‐coverage issues were handled by house officers at more than one‐third of sites, with nonintensivist house physicians performing these tasks at 15% of sites (Table 4). Intensivists managed cross‐coverage issues by telephone at 29% of sites, and saw new admissions in person after hours at 8% of sites. Intensivists did not deliver care in scheduled shifts at any of these sites.
| Care Provider | Nighttime Admissions n (%) | Cross‐coverage n (%) |
|---|---|---|
| ||
| Emergency room physician | 13 (13.5) | 8 (8.3) |
| House physician | 15 (15.6) | 17 (17.7) |
| House officer | 42 (43.8) | 37 (38.5) |
| ICU nurse | 5 (5.2) | 10 (10.4) |
| PA or NP | 8 (8.3) | 5 (5.2) |
| Intensivist in person | 8 (8.3) | |
| Intensivist by telephone | 28 (29.2) | |
| Other | 9 (9.4) | 9 (9.4) |
Discussion
As all Keystone ICU participating sites responded to the questionnaire, we believe these results to be representative of critical care practice in the state of Michigan at the present time. Michigan ICU staffing structures are variable. Only a minority (25%) of Michigan Keystone ICU sites operated in an environment where intensivists are the only attending physicians of record. Although intensivists rounded in 60% of sites not utilizing a closed model, 75% of sites had nonintensivist attending physicians, with primary care physicians and hospitalists commonly providing ICU services. The utilization of hospitalists to provide critical care services was found in the absence of intensivists, regardless of hospital or ICU size.
Closed ICUs were seen in larger hospitals and in larger ICUs. This finding is similar to data obtained on a national level.8‐16 A high‐intensity model of care was also uncommon, although decision‐making was at least shared between intensivists and nonintensivists at two‐thirds of sites. These findings are in keeping with the observation that intensivist‐directed care advocated by the Leapfrog Group has not been widely implemented,17 including in Michigan, a regional rollout leader for the Leapfrog Group.
Fewer ICUs reported utilizing a nonintensivist model than was reported in the survey by Angus et al.,8 where approximately one‐half of ICUs delivered care in this manner. This survey was performed in 1997, prior to the launch of the Leapfrog Group effort, and may have reflected a relative over representation of smaller, general ICUs. Our study is the first statewide analysis of critical care practices in the postLeapfrog Group era. Our finding that an array of approaches to critical care delivery existed in Michigan, even when intensivists rounded on patients, is similar to that found among Leapfrog‐compliant hospitals sampled from several regions of the United States.18
Other than intensivists, surgeons, primary care, and hospitalist physicians provided care in Michigan ICUs. The hospitalist movement is relatively new.19 However, in our survey 37.5% of sites had hospitalists serving as attending physicians. Although the closed ICU model was more prevalent in larger ICUs and hospitals, the use of a hospitalist model to staff ICUs was not related to hospital size, but was instead a function of whether or not intensivists were present in a given setting. In lieu of a projected shortage of intensivists, we believe this confirms the crucial role that hospitalists will play in the provision of critical care services in the future.
The attributes of intensivist care that led to improved outcomes in previous studies1‐4 are unknown. To the extent that the involvement of intensivists on an elective rather than mandatory consultative basis may explain the higher mortality found in 1 recent study,1011 we hypothesize that having a knowledgeable physician present who communicates with clinicians and families and manages at the unit level is an important factor leading to improved outcomes. While hospitalists can have these attributes, their knowledge of specific critical care therapies and technologies may vary with the extent of their ICU training and experience. Further research should seek to quantify the attributes by which intensivists are associated with improved outcomes and seek ways to foster those attributes among hospitalists who participate in critical care delivery. Central to this will be ensuring that training programs ensure competency in critical care therapies and technologies among hospitalists and other non‐ICU physicians.
We recognize several limitations in this study. First, the validity of the survey may introduce misclassification of ICU staffing. However, the survey instrument was informed by previously‐validated instruments and experts in ICU physician staffing and hospitalist care. Second, we did not link variation in staffing to outcomes. While such analysis is important, it is beyond the scope of this survey. Third, our study was conducted in 1 state and the results may not be generalizable across the United States. Nevertheless, Michigan is a large state with a diverse array of hospitals, and as our study sample broadly represented this diversity, we believe our results are likely to be generalizable.
In conclusion, few ICUs in Michigan are closed and many utilize nonintensivist critical‐care providers such as hospitalists, primary care providers, and physician extenders to deliver clinical care. Our findings have significant implications for future efforts at a national level that involve the training of hospitalists and their acceptance as critical care practitioners. We suggest future research involving intensive care delivery focus on the feasibility of training sufficient hospitalists to satisfy a growing need for critical care that cannot be filled by intensivists, along with strategic planning to insure the model of care provided is commensurate with the complexity of illness. Although this approach appears to be occurring in Michigan on an ad hoc basis, we believe coordination between larger, intensivist‐run ICUs and smaller, nonintensivist‐run ICUs should be formalized in order to optimize the delivery of intensive care.25
- ,,, et al.;the members of the American College of Critical Care Medicine Task Force on Models for the Definition of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model.Crit Care Med.2001;29;2007–2019.
- ,,, et al.Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of “open” and “closed” formats.JAMA.1996;276:24–31.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- ,,, et al.Effects of an organized critical care service on outcomes and resource utilization: a cohort study.Crit Care Med.1999;27:270–274.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002;288:2151–2162.
- Leapfrog Group. Leapfrog Group Factsheet: ICU physician staffing (IPS). Available at: http://www.leapfroggroup.org/media/file/Leapfrog‐ICU_ Physician_Staffing_Fact_Sheet.pdf. Accessed June 2009.
- National Quality Forum. Safe Practices for Better Healthcare. Available at: http://www.qualityforum.org/pdf/reports/safe_practices.pdf. Accessed June 2009.
- ,,,,,; on behalf of the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS).Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34:1016–1024.
- ,,, et al.Team care: beyond open and closed intensive care units.Curr Opin Crit Care.2006;12:604–608.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–809.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Outcomes of care by hospitalists, general internists and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Descriptive analysis of critical care units in the United States.Crit Care Med.1992;20:846–863.
- .Leapfrog and critical care: evidence‐ and reality‐based intensive care for the 21st century.Am J Med.2004;116:188–193.
- ,,,,.The organization of intensive care unit physician services.Crit Care Med.2007;35:2256–2261.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2:327–330.
- ,,, et al.Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care.Crit Care Med.2003;31:2677–2683.
- ,,, et al.;the members of the American College of Critical Care Medicine Task Force on Models for the Definition of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model.Crit Care Med.2001;29;2007–2019.
- ,,, et al.Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of “open” and “closed” formats.JAMA.1996;276:24–31.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- ,,, et al.Effects of an organized critical care service on outcomes and resource utilization: a cohort study.Crit Care Med.1999;27:270–274.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002;288:2151–2162.
- Leapfrog Group. Leapfrog Group Factsheet: ICU physician staffing (IPS). Available at: http://www.leapfroggroup.org/media/file/Leapfrog‐ICU_ Physician_Staffing_Fact_Sheet.pdf. Accessed June 2009.
- National Quality Forum. Safe Practices for Better Healthcare. Available at: http://www.qualityforum.org/pdf/reports/safe_practices.pdf. Accessed June 2009.
- ,,,,,; on behalf of the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS).Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations.Crit Care Med.2006;34:1016–1024.
- ,,, et al.Team care: beyond open and closed intensive care units.Curr Opin Crit Care.2006;12:604–608.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–809.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.Outcomes of care by hospitalists, general internists and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Descriptive analysis of critical care units in the United States.Crit Care Med.1992;20:846–863.
- .Leapfrog and critical care: evidence‐ and reality‐based intensive care for the 21st century.Am J Med.2004;116:188–193.
- ,,,,.The organization of intensive care unit physician services.Crit Care Med.2007;35:2256–2261.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2:327–330.
- ,,, et al.Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care.Crit Care Med.2003;31:2677–2683.
Copyright © 2010 Society of Hospital Medicine
Delayed Antimicrobial Administration
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).
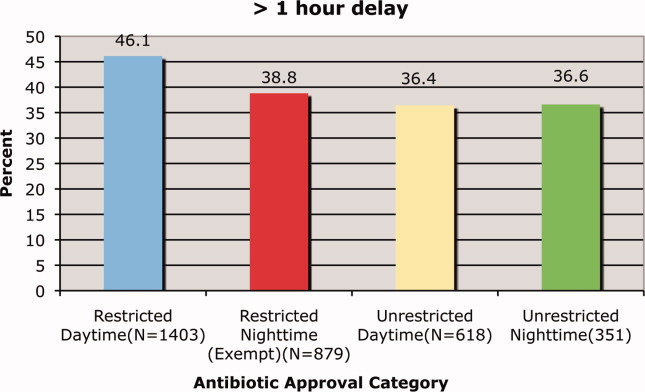
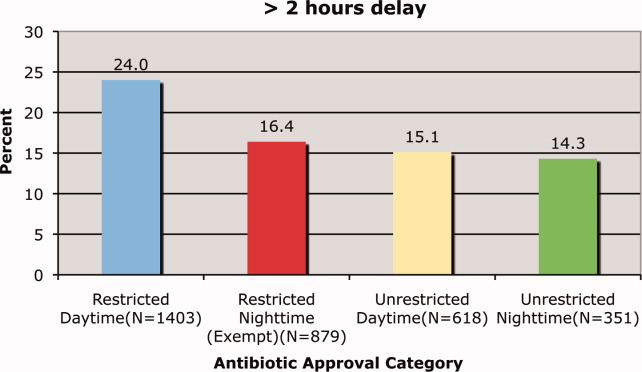
Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).


Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
In‐hospital administration of antimicrobials is often subject to controls and policies designed to limit the indiscriminant use of antimicrobials in situations where they are not warranted, to control costs, and to reduce the potential for the development of resistant microorganismsa major public health threat and patient safety concern. These controls and policies may include strategies such as limiting the choices of antimicrobials on formulary, rotational schedules of available antimicrobials, and antimicrobial approval processes.16 Antimicrobial approval processes commonly require that the bedside clinician obtain permission from a secondary source to administer a particular antimicrobial. This approval process may take the form of submitting written justification forms and/or direct telephone/fax requests to an Infectious Disease specialist, Clinical Pharmacist, or other surrogate prior to release of the antibiotic from the pharmacy. While these approval processes and other strategies have been shown to reduce the development of resistance,7, 8 improve outcomes,8 and provide education and revision of antimicrobial choice that may be more appropriate for the patient and suspected infection,9 they have the potential to delay the administration of the necessary antimicrobial by adding additional steps to the sequence of ordering, obtaining, and administering the medication. While it is certainly desirable to control the indiscriminant use of these medications, delays in antimicrobial administration may, in turn, worsen outcomes, thus counteracting the beneficial effects of control policies. The early and timely administration of appropriate broad spectrum antimicrobials chosen to cover the most expected organisms has been consistently shown to improve outcomes1027 and has been cited as an essential element of the Surviving Sepsis Campaign (
At our institution, the antimicrobial approval process requires written justification forms and/or a call or fax to an Infectious Disease specialist or Clinical Pharmacist prior to the antimicrobial being released from the pharmacy. We hypothesized that this process is associated with significant delays in patients receiving their prescribed antimicrobials when a restricted drug was chosen. The antibiotic approval process at our institution allows 1‐time stat doses of restricted antimicrobials to be ordered without preapproval at night (10 PM to 8 AM), but not during the day. This allowed us to compare the time‐to‐administration of restricted antimicrobials to their unrestricted counterparts and to themselves during this exempt time period.
Methods
Study Design
This was a single‐institution retrospective cohort study. We included all patients admitted to Johns Hopkins Hospital units utilizing the hospital's computerized provider order entry system (CPOE) who had stat orders placed for any of 24 preselected commonly ordered intravenous antimicrobials over a 1‐year period between November 1, 2005 and October 31, 2006. We did not include oral medications that were similarly subject to the approval process since we anticipated that the amount of time required to prepare the drug in the pharmacy and to deliver it to the patient would be systematically different than for intravenous medications. The CPOE system captures time of administration of all drugs (as charted by the nurse) in addition to the time of order entry by the provider. Institutional guidelines dictate that when drugs are ordered stat, they should be administered within 30 minutes of the order.
Classification of Antibiotics
Particular antimicrobials (24 different drugs) were chosen prospectively to include a broad range of commonly ordered drugs available at our hospital in intravenous form (Table 1). For this analysis, we only considered the first dose of the prescribed antimicrobial, since subsequent doses are not generally ordered stat and the timing of administration is clinically less important than the timing of the initial dose. Additionally, for patients who had multiple orders for stat antimicrobials during the course of hospitalization, we only included the first stat order in analysis.
| Restricted Antimicrobials | Unrestricted Antimicrobials |
|---|---|
| Ampicillin/sulbactam | Acyclovir |
| Azithromycin | Amphotericin |
| Cefepime | Ampicillin |
| Ciprofloxacin | Cefazolin |
| Fluconazole | Ceftriaxone |
| Gatifloxacin | Cephalexin |
| Linezolid | Clindamycin |
| Meropenem | Doxycycline |
| Moxifloxacin | Ertapenem |
| Vancomycin | Gentamicin |
| Metronidazole | |
| Oxacillin | |
| Tobramycin | |
| Trimethoprim/sulfamethoxazole |
For the purposes of the analysis, we defined antimicrobials as restricted or unrestricted. At our institution, restricted antimicrobials require either written or telephone approval from an Infectious Disease physician or Clinical Pharmacist. Obtaining written approval involves filling out a form that indicates the choice of agent (including route and dose) for an approved indication (eg, vancomycin for proven methicillin‐resistant Staphylococcus aureus bacteremia) and faxing this form to the hospital pharmacy. In other cases, approval requires a phone conversation with an Infectious Disease clinical fellow or a Clinical Pharmacist. None of these processes can be initiated or executed from within the CPOE system itself.
At our institution, the first dose of any restricted antimicrobial may be administered without going through the approval process if it is prescribed in the overnight hours defined as between 10 PM and 8 AM (Table 2). Retroactive approval is required the following day if the antimicrobial is to be continued.
| Restricted Antimicrobial (eg, Vancomycin) | Unrestricted Antimicrobial (eg, Cefazolin) | |
|---|---|---|
| Daytime order (8 AM to 10 PM) | Approval required | No approval required |
| Nighttime order (10 PM to 8 AM) | Exempted from approval for first dose (approval required during the next daytime period) | No approval required |
Data Acquisition
All antibiotic administration data were extracted electronically from the CPOE system. During the time of the study, not all hospital units utilized this system. Although CPOE was in place for all general medical wards, the intensive care units and many surgical services were not using CPOE at the time of the study. Based on limitations of the data entered into our CPOE system, many relevant clinical variables were not available for this study, such as clinical diagnoses or the level of care (eg, intensive care unit vs step‐down unit, vs regular nursing floor) at the time of the order.
Outcome Measures
We defined time‐to‐administration as the period of time between order entry and administration of the drug to the patient, as charted by the nurse in the CPOE system. However, nursing policy allows nurses to document the time of administration as the exact time of the order provided that the drug is delivered within 1 hour of a stat order, whereas all administration times beyond 1 hour of the order are expected to be documented precisely. This nursing policy eliminated our ability to determine whether antimicrobials were administered within 30 minutes of the order, and limited the value of examining time‐to‐administration as a continuous variable, but allowed us to reliably identify delays of >1 hour or >2 hours presuming that the nurse‐charted administration times were accurate. As our primary outcome, we defined delay in administration as when the antimicrobial was administered >1 hour from time of order. We examined delays of >2 hours as a secondary outcome. Antimicrobials ordered stat but not delivered within 4 hours of the order were excluded from analysis based on the recognition that extended delays might have resulted from changes in clinical circumstances or errors in documentation. Similarly, antimicrobials charted as being delivered prior to the order were excluded.
Statistical Analyses
We used the likelihood‐ratio chi‐square test to determine 2‐sided P values for differences between proportions. Logistic regression models were used to derive odds ratios (ORs) and to adjust for covariables. We adjusted for weekday versus weekend orders, patient characteristics including sex, age, white versus non‐white ethnicity and orders placed on medicine versus non‐medicine (eg, surgical or obstetrical) wards. Analyses were conducted in JMP 5.1 (SAS Institute, Cary, NC).
Results
In total, 3337 orders for stat antimicrobials were written during the study period, of which 86 (2.6%) were excluded based on being outside the specified 4‐hour window. This left a total of 3251 orders in 3251 discrete patients for analysis.
We found that a statistically significantly higher percentage of delays in antimicrobial administration when the antimicrobial was restricted as compared to unrestricted. This was the case for both our primary outcome of a >1 hour delay (Figure 1) and our secondary outcome of a delay of >2 hours (Figure 2). For restricted antimicrobial, delays of >1 hr occurred with 46.1% of orders during the day and with 38.8% of orders at night (when exempt from approval), P < 0.001. For unrestricted antimicrobials, delays of >1 hr occurred in 36.4% and 36.6% of instances, respectively (P = 0.57). The odds ratio for a delay in administration of 1 hour for restricted antimicrobials was 1.49 (95% CI = 1.23‐1.82).


Delays beyond 2 hours occurred 24.0% of the time for restricted antimicrobials during the day versus 16.4% at night. Unrestricted antimicrobials were delayed >2 hrs only 15.1% and 14.3% of the time for day and night periods, respectively (P = 0.35). The odds ratio for a two‐hour or greater delay was 1.78 (95% CI = 1.39‐2.21), P < 0.0001 when the antimicrobial was restricted.
These odds ratios and statistical significance were unchanged by adjustment for primary service (medicine vs. non‐medicine), age, sex, ethnicity or whether the order was placed on a weekend or weekday (data not shown).
Discussion
We found that our institution's antimicrobial approval process was associated with statistically significant delays in the administration of antimicrobials that were ordered stat by the prescribing clinician. These delays were evident both when comparing restricted antimicrobials to unrestricted ones and when these restricted antimicrobials were compared to themselves during the overnight time period when they were temporarily exempt from the approval process. This suggests that the delay is associated with the approval process itself and not the specific drug or the time of day. We also found that over one‐third of all stat antimicrobial orders were not carried out in the within one hour. This rate approached nearly 50% for restricted drugs ordered stat. This high baseline rate for all stat‐ordered antimicrobials underscores system challenges that seem to be exacerbated when restricted antimicrobials are chosen.
We do not know if the delays we observed resulted in patient harm. Indeed it is possible, if not likely, that patient care at our institution is improved by the judicious use of certain antimicrobials, even if the process required to enforce their use may result in delayed antimicrobial administration in some instances. Since we did not collect clinical information on baseline diagnoses or severity of illness, and we did not have information on clinical outcomes, we cannot determine whether the clinical delays we observed might have caused harm. Determining the impact these approval policies have on patient outcomes would require a separate study designed to collect the necessary clinical data to answer that question.
An additional limitation was that we did not ascertain the indications for the antimicrobials to determine whether they truly needed to be given stat. We suspect that antimicrobials are sometimes ordered stat even when the infection being treated is not likely to be serious or life‐threatening. Additionally, since we relied on the nurse‐charted time of administration, it is possible that in some instances there was a charted delay in administration when in reality the patient received the antimicrobial in a timely fashion. In urgent situations, the nurses may be too busy to document that the medication was given until long after the dose is given, and this may result in inaccuracies in the charted administration time. However, this type of documentation error would be expected to affect restricted and unrestricted antimicrobials similarly and would be unlikely to result in a systematic bias.
Because we conducted this study at a single institution, the results may not be applicable to other medical institutions, especially since restriction policies and antimicrobial approval processes vary from hospital to hospital. The burden of delays may be related to the number of restricted antimicrobials on formulary, the types of antimicrobials restricted, the number of steps required to have them released from the pharmacy, whether the approval process is initiated from within the order entry system, and other factors that may streamline or hamper the approval process.
In our institution, there are several steps in the process, any of which might contribute to the delay. Faxed approval sheets may take time to arrive to and be acted upon by the pharmacy, errant pages may delay communication between the provider and the person providing approval, and there may be delays in the final approval being relayed to the pharmacy by the individual providing approval. In fact, an alternative explanation for the observed administration delays is that once ordered in CPOE, the prescribing physicians themselves are slow in initiating the approval process. While this is certainly possible, especially given the stresses surrounding the management of a seriously ill patient on the general ward, this still suggests that having to go through the approval process may impact the process of care.
Other possible explanations for the delays observed when the restrictive antimicrobial policy was in effect may include pharmacy staffing. Since the workload in the pharmacy would be expected to be greater during the day, when more patient care activity is occurring such as clinics and operating rooms, this increased workload may have slowed down the pharmacy filling the orders. However, such human resource‐workload imbalances would also be expected to slow most pharmacy processes and should lead to delays in filling the orders for other medications including the unrestricted antimicrobials. We did not track other non‐antimicrobial medications to examine their patterns of delay. Nursing workload also varies between day and night but the time period where the antimicrobial administration delays occurred is the time when nursing is favorably staffed unlike the night when nurse to patient ratios are low. It is possible that despite better nurse to patient ratios during the day, the workload‐to‐nursing ratio remains high and contributes to delays in administration of otherwise stat‐ordered antimicrobials. Again, it is unclear why this would disproportionately affect the restricted class of antimicrobials.
We do not advocate the abandonment of antimicrobial control policies. The process described here is very institution‐specific and while its benefits are proven, energy should be channeled where appropriate to facilitate this process. These policies are clearly necessary to help reduce costs, limit the unwarranted use of these drugs, and slow the proliferation of ever more resistant strains of microorganisms. However, we do advocate careful consideration of the components of the approval process itself, ensuring that delays in antimicrobial administration are kept to a minimum and are avoided altogether in critically ill patients. One way to accomplish this might be to not require approval for the first administration of a stat antibiotic, but to require approval for subsequent doses. Our institution's overnight exempt period data suggest that this would eliminate the incremental delays incurred by the approval process itself. As important, our results show that even for unrestricted antibiotics, we fall short of achieving recommended best practices, highlighting the challenges inherent to carrying out multi‐step clinical tasks in an efficient fashion.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- .Antimicrobial stewardship.Am J Med.2006;119:S53–S61.
- .Restrictive antibiotic policies are appropriate in intensive care units.Crit Care Med.2003;31:S25–S28.
- ,,, et al.Effects of requiring prior authorization for selected anti‐microbials: expenditures, susceptibilities and clinical outcomes.Clin Infect Dis.1997;25:230–239.
- ,,, et al.Class restriction of cephalosporin use to control total cephaslosporin resistance in nosocomial Klebsiella.JAMA.1998;280:1233–1237.
- ,,, et al.Rotation and restricted use of antibiotics in a medical intensive care unit: impact on the incidence of ventilator associated pneumonia caused by antibiotic resistant Gram‐negative bacteremia.Am J Respir Crit Care Med.2001;63(3 Pt 1):837–843.
- ,,, et al.Impact of rotation of empiric antibiotic schedules on infectious mortality in an intensive care unit.CCM.2001;29:1101–1108.
- ,,, et al.Antimicrobial use control measures to prevent and control antimicrobial resistance in US hospitals.Infect Control Hosp Epidemiol.2006;27:1088–1095.
- ,,,,.Cost‐effectiveness of prospective and continuous parenteral antibiotic control: experience at the Palo Alto Veterans Affairs Medical Center from 1987 to 1989.Am J Med.1991;90:439–444.
- ,.Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital.Clin Infect Dis.1997;24:471–485.
- ,.Antimicrobial stewardship and the role of pharmacokinetics–pharmacodynamics in the modern antibiotic era.Diagn Microbiol Infect Dis.2007;57(3 Suppl.):77S–83S.
- ,,,,,.Improving inpatient antibiotic prescribing: insights from participation in a national collaborative.Jt Comm J Qual Improv.2001;27:387–402.
- ,.Managing antibiotic resistance.N Engl J Med.2000;343:1961–1963.
- ,.Antimicrobial stewardship programs in health care systems.Clin Microbiol Rev.2005;18(4):638–656.
- ,,,,,,,,,,.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005;4:CD003543.
- ,,,,.Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital.Pediatr Infect Dis J.2008;27(2):106–111.
- .The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists.Pharmacotheray.2007;27(9):1306–1312.
- ,,,.Strategies to contain the emergence of antimicrobial resistance: a systematic review of effectiveness and cost‐effectiveness.J Health Serv Res Policy.2002;7(2):111–117.
- ,,,,,,,,,,,,;Infectious Diseases Society of America;Society for Healthcare Epidemiology of America.Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship.Clin Infect Dis.2007;44(2):159–177.
- ,,,, andthe Healthcare Infection Control Practices Advisory Committee. Management of Multi‐Drug Reistsant Organisms in Healthcare Settings: 2006. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf. Accessed May 19,2008.
- ,,.Gram‐negative bacteremia. IV Re‐evaluation of clinical features and treatment in 612 patients.Am J Med.1980;68:344–355.
- ,,,.Gram‐negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients.Am J Med.1980;68:332–343.
- ,,,.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- .Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU‐Acquired Pneumonia Study Group.Intensive Care Med.1996;22:387–394.
- ,,,,.The value of routine microbial investigation in ventilator‐associated pneumonia.Am J Respir Crit Care Med.1997;156:196–200.
- ,,, et al.Impact of BAL data on the therapy and outcome of ventilator‐associated pneumonia.Chest.1997;111:676–685.
- ,.The influence of mini‐BAL cultures on patient outcomes: implications for the antibiotic management of ventilator associated pneumonia.Chest.1998;113:412–420.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
Copyright © 2010 Society of Hospital Medicine
Top 10 Infectious Disease Pitfalls
Hospitalists commonly encounter the challenges of infectious diseases in their hospitalized patients. Choosing the correct antibiotic, interpreting blood cultures, working up causes of fever, treating patients with an allergy to penicillin, and caring for patients with human immunodeficiency virus (HIV) commonly confront the hospitalist. This article presents evidence‐based pearls which will help hospitalists avoid common infectious disease pitfalls and guide their decision about when to consult an infectious diseases specialist.
1. Avoid Spiraling Empiricism and Understand Common Fallacies in Prescribing Empiric Antimicrobial Therapy
The term spiraling empiricism describes the inappropriate treatment, or the unjustifiable escalation of treatment, of suspected but undocumented infectious diseases.1 Initiation of carefully considered empiric broad‐spectrum antibiotic therapy for an acutely ill patient is an entirely appropriate and reasonable strategy. But all too often, practitioners are confronted with clinical dilemmas such as persistent fever or lack of response to therapy. In these circumstances, clinicians are faced with deciding whether to add or change antibiotics to broaden coverage. Changes in empiric therapy should be made sparingly, and only when there is new information or symptoms to justify an addition or change. In order to make an accurate assessment of response, steady‐state levels should be achieved and usually 3 to 5 days should be allowed to pass. Lack of response to broad‐spectrum therapy should trigger further investigation for occult infection or consideration of noninfectious etiologies and not simply the addition of a new antimicrobial agent. If a microbial pathogen is isolated from a blood culture(s) or other relevant source, antimicrobials should be tailored to the narrowest spectrum and least toxic therapy based on the sensitivities of that organism. For critically ill patients or patients who do not appear to be improving, an infectious diseases consultation may be warranted.
2. Know the Important Drug‐Drug Interactions Between Antimicrobials and Commonly‐used Inpatient Medications, Particularly With Those Involving Warfarin
Most antimicrobials (especially antifungals, quinolones, metronidazole, and sulfonamides) can cause unpredictable elevations in the international normalized ratio (INR) concurrent with warfarin administration, either through inhibition of warfarin metabolism or alterations in vitamin Kproducing gut flora. When using antimicrobials in patients on warfarin, the patient's INR should be carefully monitored and adjustment of the warfarin dose may be necessary. Antimicrobials that are inhibitors of cytochrome P‐450 enzymes include ciprofloxacin, levofloxacin, isoniazid, fluconazole, and clarithromycin. In contrast, rifampin is a potent inducer of most known cytochrome P‐450 enzymes and increases the metabolism of many drugs used in patients in the hospital setting, including anticonvulsants, beta‐blockers, calcium channel blockers, and other antibiotics like fluoroquinolones, and sulfonylureas. Moreover, the concurrent oral intake of tablets or solutions (including tube feeds) with a high concentration of trivalent and divalent cations (such as aluminum, magnesium, and, to a lesser extent, calcium, iron, and zinc) impairs gastrointestinal absorption of fluoroquinolones and should be avoided or spaced apart in time. Since fluoroquinolones can potentially prolong the QT interval, careful monitoring is necessary when a patient is prescribed other QT prolonging agents. Finally, many antimicrobials reduce the effectiveness of oral or other systemic hormonal contraceptives and patients should be routinely advised to use nonhormonal methods of birth control during therapy.
3. Positive Blood Cultures for Bacteria or Fungus Should be Repeated Serially Every 24 to 48 Hours Until the Cultures Are Negative
An important step in the management of a positive blood culture for bacteria or yeast is to check follow‐up blood cultures every 24 to 48 hours until the bacteremia or fungemia has cleared. This is particularly true of bacteremia caused by Staphylococcus aureus (S. aureus), Enterococcus species, and fungemia caused by Candida species. The duration of bacteremia or fungemia has a significant impact on the predictive values of further testing for endovascular or deep‐seated sources of infection as well as treatment duration. This is particularly true for the treatment of candidemia in nonneutropenic adults and for bacterial endocarditis, in which the recommended duration of treatment starts from the day of the last positive blood culture.2, 3 In addition to repeat blood cultures, a blood culture positive for S. aureus should always prompt an aggressive workup for a source (including strong consideration of a transesophageal echocardiogram to evaluate for endocarditis). S. aureus bacteremia should never be disregarded as a contaminant, and should prompt strong consideration of removal of all indwelling intravenous lines.4
4. Removal of Indwelling Intravascular Catheters Is Essential in the Management of Patients with Candidemia. In These Patients, Retention of Central Lines Is Significantly Related to Poor Outcomes
In patients with culture‐proven Candida fungemia, all intravascular catheters must be removed if at all possible. In a study by Nguyen et al.,5 the mortality rate for patients with a catheter‐related candidemia in whom catheters were retained was significantly higher than that of patients in whom the catheters were removed (41% vs. 21%, P < 0.001). Likewise, in a separate study, Luzzati et al.6 noted that central line removal independently reduced the high mortality of the disease. This recommendation applies to all Candida species.
5. Although Candida Species Are Frequently Noted to Colonize Sputum and Urine Cultures, Their Recovery From Multiple Sites May Be an Indicator of Occult Candidemia in an Acutely Ill Patient
Candida species uncommonly cause pneumonia or urinary tract infection, so their isolation from cultures of the respiratory and genitourinary tract often represents colonization. However, the presence of Candida species at multiple sites may be an indicator of occult candidemia in a patient with multiple risk factors for candidemia, including intensive care unit (ICU) admission, immunosuppression (particularly neutropenia and recent receipt of corticosteroids), central venous catheterization, total parenteral nutrition, recent broad‐spectrum antibiotics, and recent abdominal or gastrointestinal surgery.7
6. Patients with Asymptomatic Bacteriuria, With or Without Pyuria, Should Not Be Treated with Antibiotics. Pregnant Women and Patients Undergoing a Genitourinary Procedure Are the Exception and Should Be Treated With Antibiotics
Asymptomatic bacteriuria is commonly encountered in the hospital setting, but is usually benign. Bacteriuria is defined as a voided urine specimen with 1 bacterial species isolated in a quantitative count of 105 cfu/mL. Treatment of asymptomatic bacteriuria is only recommended for pregnant women or prior to invasive genitourinary procedures, including transurethral resection of the prostate. Patients with structural or functional abnormalities of the urinary tract may have a high prevalence of bacteriuria. Despite its prevalence, asymptomatic bacteriuria is seldom associated with adverse outcomes. Studies have noted that antimicrobial treatment of asymptomatic bacteriuria does not decrease recurrence. Negative outcomes with antimicrobial treatment do occur, including adverse drug reactions and reinfection with organisms of increasing resistance. Clinical trials in spinal‐cord injury patients, diabetic women, elderly patients living in the community or nursing home, and patients with indwelling urethral catheters have consistently found no benefit with treatment of asymptomatic bacteriuria.8, 9 The presence or absence of pyuria does not differentiate symptomatic from asymptomatic urinary infection. Patients with symptomatic urinary tract infection (fever and/or dysuria) should be treated after urine cultures are obtained. Other causes of pyuria in the absence of an acute urinary tract infection include urethritis, tuberculosis, prostatitis, nephrolithiasis, and malignancy.
7. Evaluate All Patients Who Have a History of Penicillin Allergy and Consider Desensitization for Patients With a History Consistent With Immunoglobulin Emediated Allergy Who Require Treatment With a Beta‐Lactam Antibiotic
Patients commonly claim to have an allergy to penicillin. True penicillin allergy is very serious and can be life‐threatening. Because of this, patients labeled as penicillin allergic are typically not treated with beta‐lactam antibiotics. Instead, they may be prescribed medications which are typically less effective, more toxic, have a broader spectrum, or are more expensive.10, 11 Many patients are inappropriately labeled as having a penicillin allergy. A history of penicillin allergy is reported in approximately 10% of hospitalized patients, but only approximately 10% of those who report a history of penicillin allergy actually have an allergic reaction when treated with penicillin. Exanthems are frequently associated with beta‐lactam use during an episode of infectious mononucleosis but these are not considered an allergic reaction. Such patients are generally able to tolerate beta‐lactams subsequent to this episode. Nonpruritic maculopapular rashes are also reported in 3% to 7% of children taking amoxicillin and are not a contraindication for future beta‐lactam or cephalosporin use.12 All patients who describe an allergy should be questioned in detail about the type of penicillin received, as well as the type, severity, and timing of the reaction. Typical immunoglobulin E (IgE)‐mediated severe reactions to penicillin include urticaria, pruritus, angioedema, bronchospasm, and hypotension. These patients should not be given other agents that share the same beta‐lactam ring, including cephalosporins (risk of cross‐reactivity is greatest with first‐generation and second‐generation cephalosporins). Carbapenems have minimal cross‐reactivity, particularly meropenem.13 Monobactams (eg, aztreonam) do not cross‐react. While skin testing to penicillin can be considered in patients with a history of a severe reaction to penicillin, neither the major nor minor determinants are commercially available at this time. In patients with a history of a possible IgE‐mediated reaction and when there is no suitable alternative antibiotic (usually determined from infectious diseases consultation), desensitization to beta‐lactams or carbapenems can be considered. Desensitization should be reserved only for clinicians experienced with these techniques, preferably in consultation with a specialist in allergy and immunology. Patients who report a non‐IgE‐mediated reaction may be prescribed a cephalosporin if necessary (preferably a third‐generation or fourth‐generation).14
8. An Abrupt Increase in Leukocytosis In a Hospitalized Patient Should Prompt Consideration of Clostridium difficile Infection
In recent years, there has been a marked increase in the incidence and severity of Clostridium difficile (C. difficile) infection (CDI). A new hypervirulent strain, NAP1/BI/027, has emerged and is becoming endemic in the United States, Canada, and Europe. Typically C. difficile causes diarrhea, abdominal pain, and fever. Often patients have received antibiotics in the recent past, placing them at higher risk, but cases can occur sporadically (even in the community setting) or be transmitted nosocomially. Early detection appears to be essential in reducing the serious morbidity and mortality associated with this disease. Observational studies suggested that C. difficile infection is a common cause of unexplained leukocytosis or a sudden worsening of preexisting leukocytosis.15, 16 In a prospective study evaluating 60 patients with unexplained leukocytosis (white blood cell count 15,000/mm3), 58% of patients with leukocytosis in the absence of localizing symptoms and signs of infection were subsequently diagnosed with CDI. The authors believe that the percent may have been as high as 73% when they included patients with a negative toxin assay who rapidly responded to metronidazole therapy.17 White blood cell counts can range from 10,000 to 20,000/mm3 in moderate disease. Counts as high as 40,000/mm3 can occur, especially in patients with severe disease. Although the use of clindamycin and cephalosporins have been classically associated with the subsequent development of CDI, the current widespread use of fluoroquinolones has led to significant fluoroquinolone resistance among strains of C. difficile, especially the hypervirulent NAP1/BI/027 strain.18 The judicious use of antibiotics, especially fluoroquinolones, remains the cornerstone in preventing CDI. Remember that hand washing with soap and water is essential as alcohol‐based hand sanitizers do not eradicate the C. difficile spores. The drug of choice for initial treatment of mild to moderate CDI remains oral metronidazole, and it may be used for a first recurrence of CDI. Increasing data support the use of oral vancomycin for moderately severe to severe CDI or for multiple recurrences.19 Intravenous metronidazole is often added to oral vancomycin in patients with ileus, but it is not reliably effective alone for CDI.
9. Fever Is Common in the First 48 Hours After a Major Surgical Procedure, and Is a Poor Indicator of Infection. The use of Antibiotics in Response to Fever in the Absence of Other Localizing Signs and Symptoms of Infection Should Be Avoided
Early postoperative fever is relatively common but most fevers that develop within the first 48 hours after surgery do not have an infectious etiology.2023 However, fever that begins or persists beyond the fifth postoperative day is much more likely to represent a clinically significant infection. The continued use of antibiotics outside the window for wound prophylaxis (>24 hours) does not decrease the risk of postoperative infection but it does increase the risk of acquiring resistant bacteria and adverse drug reactions, including CDI.
10. Facts All Clinicians Should Know About Patients with HIV Infection
The 2 most common laboratory abnormalities routinely associated with antiretroviral therapy for HIV infection are unconjugated hyperbilirubinemia associated with atazanavir and an elevated mean corpuscular volume (MCV) associated with zidovudine (and, to a lesser extent, stavudine). Immune reconstitution inflammatory syndrome (IRIS) is a condition seen in patients with advanced acquired immune deficiency syndrome (AIDS) who have recently started antiretroviral therapy. As the immune system begins to recover, it may respond to a previously acquired opportunistic infection with an overwhelming inflammatory response that paradoxically makes the symptoms of infection worse. IRIS is associated with a pathological inflammatory response that can have substantial morbidity and mortality.24 For this reason, when considering whether to start or stop continuous or highly active antiretroviral therapy (also known as HAART), an infectious diseases consult is recommended. Pneumocystis jiroveci (PCP) remains a cause of pneumonia in patients with advanced AIDS' though in the era of HAART, its presentation may be more subtle. Finally, the principle of parsimony (Occam's razor) often does not hold in the diagnosis of opportunistic infections in patients with advanced AIDS, as these patients can often present with multiple infections simultaneously.25, 26
Conclusion
Infectious diseases are commonly encountered by physicians who care for hospitalized patients. Early recognition, evaluation, and appropriate treatment and/or referral to an infectious diseases specialist are necessary to moderate the significant morbidity and mortality that are often associated with infectious diseases.
- ,.Observations on spiraling empiricism: its causes, allure, and perils, with particular reference to antibiotic therapy.Am J Med.1989;87(2):201–206.
- ,,, et al.Guidelines for the treatment of candidiasis.Clin Infect Dis.2004;38:161–189.
- ,,, et al.Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation.2005;111:e394–e434.
- ,.Management of methicillin‐resistant Staphylococcus aureus bacteremia.Clin Infect Dis.2008;46:S386–S393.
- ,,, et al.Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study.Arch Intern Med.1995;155(22):2429–2435.
- ,,, et al.Nosocomial candidemia in non‐neutropenic patients at an Italian tertiary care hospital.Eur J Clin Microbiol Infect Dis.2000;19(8):602–607.
- .Candidemia in adults. In: Marr KA, ed.UpToDate.Waltham, MA:UpToDate, Inc.;2008.
- ,,, et al.Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults.Clin Infect Dis.2005;40(5):643–654.
- .Asymptomatic bacteriuria: when to screen and when to treat.Infect Dis Clin North Am.2003;17(2):367–394.
- .Management of patients with a history of allergy to beta‐lactam antibiotics.Am J Med.2008;121(7):572–576.
- ,,.Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic.Clin Infect Dis.2002;35(1):26–31.
- ,,, et al.Adverse effects of orally administered ampicillin.J Pediatr.1973;83:106–108.
- ,,, et al.Brief communication: tolerability of meropenem in patients with IgE‐mediated hypersensitivity to penicillins.Ann Intern Med.2007;146(4):266–269.
- ,,.The rational clinical examination. Is this patient allergic to penicillin? An evidence‐based analysis of the likelihood of penicillin allergy.JAMA.2001;285(19):2498–2505.
- ,,.Leukocytosis in a tertiary care hospital with particular attention to the role of infection caused by Clostridium difficile.Clin Infect Dis.2002;34:1585–1592.
- ,,,.Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea.Am J Gastroenterol.2000;95:3137–3141.
- ,,,.Clostridium difficile infection in patients with unexplained leukocytosis.Am J Med.2003;115:543–546.
- ,.The challenges posed by reemerging Clostridium difficile infection.Clin Infect Dis.2007;45(2):222–227.
- ,,.Treatment of Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S32–S42.
- ,,,,.Diagnostic accuracy of routine postoperative body temperature measurements.Clin Infect Dis.2005;40:1404–1410.
- .Should we measure body temperature for patients who have recently undergone surgery?Clin Infect Dis.2005;40(10):1411–1412.
- ,,,.Evidence for the noninfectious etiology of early postoperative fever.Infect Control.1985;6:273–277.
- .Evaluating postoperative fever: a focused approach.Cleve Clin J Med.2006;73(suppl 1):S62–S66.
- ,,.Immune reconstitution inflammatory syndrome: more answers, more questions.J Antimicrob Chemother.2006;57(2):167–170.
- ,,,,.Clinical problem‐solving. Occam's razor versus Saint's Triad.N Engl J Med.2004;350(6):599–603.
- ,.William of Occam and Occam's razor.Ann Intern Med.2002;136(8):634–635.
Hospitalists commonly encounter the challenges of infectious diseases in their hospitalized patients. Choosing the correct antibiotic, interpreting blood cultures, working up causes of fever, treating patients with an allergy to penicillin, and caring for patients with human immunodeficiency virus (HIV) commonly confront the hospitalist. This article presents evidence‐based pearls which will help hospitalists avoid common infectious disease pitfalls and guide their decision about when to consult an infectious diseases specialist.
1. Avoid Spiraling Empiricism and Understand Common Fallacies in Prescribing Empiric Antimicrobial Therapy
The term spiraling empiricism describes the inappropriate treatment, or the unjustifiable escalation of treatment, of suspected but undocumented infectious diseases.1 Initiation of carefully considered empiric broad‐spectrum antibiotic therapy for an acutely ill patient is an entirely appropriate and reasonable strategy. But all too often, practitioners are confronted with clinical dilemmas such as persistent fever or lack of response to therapy. In these circumstances, clinicians are faced with deciding whether to add or change antibiotics to broaden coverage. Changes in empiric therapy should be made sparingly, and only when there is new information or symptoms to justify an addition or change. In order to make an accurate assessment of response, steady‐state levels should be achieved and usually 3 to 5 days should be allowed to pass. Lack of response to broad‐spectrum therapy should trigger further investigation for occult infection or consideration of noninfectious etiologies and not simply the addition of a new antimicrobial agent. If a microbial pathogen is isolated from a blood culture(s) or other relevant source, antimicrobials should be tailored to the narrowest spectrum and least toxic therapy based on the sensitivities of that organism. For critically ill patients or patients who do not appear to be improving, an infectious diseases consultation may be warranted.
2. Know the Important Drug‐Drug Interactions Between Antimicrobials and Commonly‐used Inpatient Medications, Particularly With Those Involving Warfarin
Most antimicrobials (especially antifungals, quinolones, metronidazole, and sulfonamides) can cause unpredictable elevations in the international normalized ratio (INR) concurrent with warfarin administration, either through inhibition of warfarin metabolism or alterations in vitamin Kproducing gut flora. When using antimicrobials in patients on warfarin, the patient's INR should be carefully monitored and adjustment of the warfarin dose may be necessary. Antimicrobials that are inhibitors of cytochrome P‐450 enzymes include ciprofloxacin, levofloxacin, isoniazid, fluconazole, and clarithromycin. In contrast, rifampin is a potent inducer of most known cytochrome P‐450 enzymes and increases the metabolism of many drugs used in patients in the hospital setting, including anticonvulsants, beta‐blockers, calcium channel blockers, and other antibiotics like fluoroquinolones, and sulfonylureas. Moreover, the concurrent oral intake of tablets or solutions (including tube feeds) with a high concentration of trivalent and divalent cations (such as aluminum, magnesium, and, to a lesser extent, calcium, iron, and zinc) impairs gastrointestinal absorption of fluoroquinolones and should be avoided or spaced apart in time. Since fluoroquinolones can potentially prolong the QT interval, careful monitoring is necessary when a patient is prescribed other QT prolonging agents. Finally, many antimicrobials reduce the effectiveness of oral or other systemic hormonal contraceptives and patients should be routinely advised to use nonhormonal methods of birth control during therapy.
3. Positive Blood Cultures for Bacteria or Fungus Should be Repeated Serially Every 24 to 48 Hours Until the Cultures Are Negative
An important step in the management of a positive blood culture for bacteria or yeast is to check follow‐up blood cultures every 24 to 48 hours until the bacteremia or fungemia has cleared. This is particularly true of bacteremia caused by Staphylococcus aureus (S. aureus), Enterococcus species, and fungemia caused by Candida species. The duration of bacteremia or fungemia has a significant impact on the predictive values of further testing for endovascular or deep‐seated sources of infection as well as treatment duration. This is particularly true for the treatment of candidemia in nonneutropenic adults and for bacterial endocarditis, in which the recommended duration of treatment starts from the day of the last positive blood culture.2, 3 In addition to repeat blood cultures, a blood culture positive for S. aureus should always prompt an aggressive workup for a source (including strong consideration of a transesophageal echocardiogram to evaluate for endocarditis). S. aureus bacteremia should never be disregarded as a contaminant, and should prompt strong consideration of removal of all indwelling intravenous lines.4
4. Removal of Indwelling Intravascular Catheters Is Essential in the Management of Patients with Candidemia. In These Patients, Retention of Central Lines Is Significantly Related to Poor Outcomes
In patients with culture‐proven Candida fungemia, all intravascular catheters must be removed if at all possible. In a study by Nguyen et al.,5 the mortality rate for patients with a catheter‐related candidemia in whom catheters were retained was significantly higher than that of patients in whom the catheters were removed (41% vs. 21%, P < 0.001). Likewise, in a separate study, Luzzati et al.6 noted that central line removal independently reduced the high mortality of the disease. This recommendation applies to all Candida species.
5. Although Candida Species Are Frequently Noted to Colonize Sputum and Urine Cultures, Their Recovery From Multiple Sites May Be an Indicator of Occult Candidemia in an Acutely Ill Patient
Candida species uncommonly cause pneumonia or urinary tract infection, so their isolation from cultures of the respiratory and genitourinary tract often represents colonization. However, the presence of Candida species at multiple sites may be an indicator of occult candidemia in a patient with multiple risk factors for candidemia, including intensive care unit (ICU) admission, immunosuppression (particularly neutropenia and recent receipt of corticosteroids), central venous catheterization, total parenteral nutrition, recent broad‐spectrum antibiotics, and recent abdominal or gastrointestinal surgery.7
6. Patients with Asymptomatic Bacteriuria, With or Without Pyuria, Should Not Be Treated with Antibiotics. Pregnant Women and Patients Undergoing a Genitourinary Procedure Are the Exception and Should Be Treated With Antibiotics
Asymptomatic bacteriuria is commonly encountered in the hospital setting, but is usually benign. Bacteriuria is defined as a voided urine specimen with 1 bacterial species isolated in a quantitative count of 105 cfu/mL. Treatment of asymptomatic bacteriuria is only recommended for pregnant women or prior to invasive genitourinary procedures, including transurethral resection of the prostate. Patients with structural or functional abnormalities of the urinary tract may have a high prevalence of bacteriuria. Despite its prevalence, asymptomatic bacteriuria is seldom associated with adverse outcomes. Studies have noted that antimicrobial treatment of asymptomatic bacteriuria does not decrease recurrence. Negative outcomes with antimicrobial treatment do occur, including adverse drug reactions and reinfection with organisms of increasing resistance. Clinical trials in spinal‐cord injury patients, diabetic women, elderly patients living in the community or nursing home, and patients with indwelling urethral catheters have consistently found no benefit with treatment of asymptomatic bacteriuria.8, 9 The presence or absence of pyuria does not differentiate symptomatic from asymptomatic urinary infection. Patients with symptomatic urinary tract infection (fever and/or dysuria) should be treated after urine cultures are obtained. Other causes of pyuria in the absence of an acute urinary tract infection include urethritis, tuberculosis, prostatitis, nephrolithiasis, and malignancy.
7. Evaluate All Patients Who Have a History of Penicillin Allergy and Consider Desensitization for Patients With a History Consistent With Immunoglobulin Emediated Allergy Who Require Treatment With a Beta‐Lactam Antibiotic
Patients commonly claim to have an allergy to penicillin. True penicillin allergy is very serious and can be life‐threatening. Because of this, patients labeled as penicillin allergic are typically not treated with beta‐lactam antibiotics. Instead, they may be prescribed medications which are typically less effective, more toxic, have a broader spectrum, or are more expensive.10, 11 Many patients are inappropriately labeled as having a penicillin allergy. A history of penicillin allergy is reported in approximately 10% of hospitalized patients, but only approximately 10% of those who report a history of penicillin allergy actually have an allergic reaction when treated with penicillin. Exanthems are frequently associated with beta‐lactam use during an episode of infectious mononucleosis but these are not considered an allergic reaction. Such patients are generally able to tolerate beta‐lactams subsequent to this episode. Nonpruritic maculopapular rashes are also reported in 3% to 7% of children taking amoxicillin and are not a contraindication for future beta‐lactam or cephalosporin use.12 All patients who describe an allergy should be questioned in detail about the type of penicillin received, as well as the type, severity, and timing of the reaction. Typical immunoglobulin E (IgE)‐mediated severe reactions to penicillin include urticaria, pruritus, angioedema, bronchospasm, and hypotension. These patients should not be given other agents that share the same beta‐lactam ring, including cephalosporins (risk of cross‐reactivity is greatest with first‐generation and second‐generation cephalosporins). Carbapenems have minimal cross‐reactivity, particularly meropenem.13 Monobactams (eg, aztreonam) do not cross‐react. While skin testing to penicillin can be considered in patients with a history of a severe reaction to penicillin, neither the major nor minor determinants are commercially available at this time. In patients with a history of a possible IgE‐mediated reaction and when there is no suitable alternative antibiotic (usually determined from infectious diseases consultation), desensitization to beta‐lactams or carbapenems can be considered. Desensitization should be reserved only for clinicians experienced with these techniques, preferably in consultation with a specialist in allergy and immunology. Patients who report a non‐IgE‐mediated reaction may be prescribed a cephalosporin if necessary (preferably a third‐generation or fourth‐generation).14
8. An Abrupt Increase in Leukocytosis In a Hospitalized Patient Should Prompt Consideration of Clostridium difficile Infection
In recent years, there has been a marked increase in the incidence and severity of Clostridium difficile (C. difficile) infection (CDI). A new hypervirulent strain, NAP1/BI/027, has emerged and is becoming endemic in the United States, Canada, and Europe. Typically C. difficile causes diarrhea, abdominal pain, and fever. Often patients have received antibiotics in the recent past, placing them at higher risk, but cases can occur sporadically (even in the community setting) or be transmitted nosocomially. Early detection appears to be essential in reducing the serious morbidity and mortality associated with this disease. Observational studies suggested that C. difficile infection is a common cause of unexplained leukocytosis or a sudden worsening of preexisting leukocytosis.15, 16 In a prospective study evaluating 60 patients with unexplained leukocytosis (white blood cell count 15,000/mm3), 58% of patients with leukocytosis in the absence of localizing symptoms and signs of infection were subsequently diagnosed with CDI. The authors believe that the percent may have been as high as 73% when they included patients with a negative toxin assay who rapidly responded to metronidazole therapy.17 White blood cell counts can range from 10,000 to 20,000/mm3 in moderate disease. Counts as high as 40,000/mm3 can occur, especially in patients with severe disease. Although the use of clindamycin and cephalosporins have been classically associated with the subsequent development of CDI, the current widespread use of fluoroquinolones has led to significant fluoroquinolone resistance among strains of C. difficile, especially the hypervirulent NAP1/BI/027 strain.18 The judicious use of antibiotics, especially fluoroquinolones, remains the cornerstone in preventing CDI. Remember that hand washing with soap and water is essential as alcohol‐based hand sanitizers do not eradicate the C. difficile spores. The drug of choice for initial treatment of mild to moderate CDI remains oral metronidazole, and it may be used for a first recurrence of CDI. Increasing data support the use of oral vancomycin for moderately severe to severe CDI or for multiple recurrences.19 Intravenous metronidazole is often added to oral vancomycin in patients with ileus, but it is not reliably effective alone for CDI.
9. Fever Is Common in the First 48 Hours After a Major Surgical Procedure, and Is a Poor Indicator of Infection. The use of Antibiotics in Response to Fever in the Absence of Other Localizing Signs and Symptoms of Infection Should Be Avoided
Early postoperative fever is relatively common but most fevers that develop within the first 48 hours after surgery do not have an infectious etiology.2023 However, fever that begins or persists beyond the fifth postoperative day is much more likely to represent a clinically significant infection. The continued use of antibiotics outside the window for wound prophylaxis (>24 hours) does not decrease the risk of postoperative infection but it does increase the risk of acquiring resistant bacteria and adverse drug reactions, including CDI.
10. Facts All Clinicians Should Know About Patients with HIV Infection
The 2 most common laboratory abnormalities routinely associated with antiretroviral therapy for HIV infection are unconjugated hyperbilirubinemia associated with atazanavir and an elevated mean corpuscular volume (MCV) associated with zidovudine (and, to a lesser extent, stavudine). Immune reconstitution inflammatory syndrome (IRIS) is a condition seen in patients with advanced acquired immune deficiency syndrome (AIDS) who have recently started antiretroviral therapy. As the immune system begins to recover, it may respond to a previously acquired opportunistic infection with an overwhelming inflammatory response that paradoxically makes the symptoms of infection worse. IRIS is associated with a pathological inflammatory response that can have substantial morbidity and mortality.24 For this reason, when considering whether to start or stop continuous or highly active antiretroviral therapy (also known as HAART), an infectious diseases consult is recommended. Pneumocystis jiroveci (PCP) remains a cause of pneumonia in patients with advanced AIDS' though in the era of HAART, its presentation may be more subtle. Finally, the principle of parsimony (Occam's razor) often does not hold in the diagnosis of opportunistic infections in patients with advanced AIDS, as these patients can often present with multiple infections simultaneously.25, 26
Conclusion
Infectious diseases are commonly encountered by physicians who care for hospitalized patients. Early recognition, evaluation, and appropriate treatment and/or referral to an infectious diseases specialist are necessary to moderate the significant morbidity and mortality that are often associated with infectious diseases.
Hospitalists commonly encounter the challenges of infectious diseases in their hospitalized patients. Choosing the correct antibiotic, interpreting blood cultures, working up causes of fever, treating patients with an allergy to penicillin, and caring for patients with human immunodeficiency virus (HIV) commonly confront the hospitalist. This article presents evidence‐based pearls which will help hospitalists avoid common infectious disease pitfalls and guide their decision about when to consult an infectious diseases specialist.
1. Avoid Spiraling Empiricism and Understand Common Fallacies in Prescribing Empiric Antimicrobial Therapy
The term spiraling empiricism describes the inappropriate treatment, or the unjustifiable escalation of treatment, of suspected but undocumented infectious diseases.1 Initiation of carefully considered empiric broad‐spectrum antibiotic therapy for an acutely ill patient is an entirely appropriate and reasonable strategy. But all too often, practitioners are confronted with clinical dilemmas such as persistent fever or lack of response to therapy. In these circumstances, clinicians are faced with deciding whether to add or change antibiotics to broaden coverage. Changes in empiric therapy should be made sparingly, and only when there is new information or symptoms to justify an addition or change. In order to make an accurate assessment of response, steady‐state levels should be achieved and usually 3 to 5 days should be allowed to pass. Lack of response to broad‐spectrum therapy should trigger further investigation for occult infection or consideration of noninfectious etiologies and not simply the addition of a new antimicrobial agent. If a microbial pathogen is isolated from a blood culture(s) or other relevant source, antimicrobials should be tailored to the narrowest spectrum and least toxic therapy based on the sensitivities of that organism. For critically ill patients or patients who do not appear to be improving, an infectious diseases consultation may be warranted.
2. Know the Important Drug‐Drug Interactions Between Antimicrobials and Commonly‐used Inpatient Medications, Particularly With Those Involving Warfarin
Most antimicrobials (especially antifungals, quinolones, metronidazole, and sulfonamides) can cause unpredictable elevations in the international normalized ratio (INR) concurrent with warfarin administration, either through inhibition of warfarin metabolism or alterations in vitamin Kproducing gut flora. When using antimicrobials in patients on warfarin, the patient's INR should be carefully monitored and adjustment of the warfarin dose may be necessary. Antimicrobials that are inhibitors of cytochrome P‐450 enzymes include ciprofloxacin, levofloxacin, isoniazid, fluconazole, and clarithromycin. In contrast, rifampin is a potent inducer of most known cytochrome P‐450 enzymes and increases the metabolism of many drugs used in patients in the hospital setting, including anticonvulsants, beta‐blockers, calcium channel blockers, and other antibiotics like fluoroquinolones, and sulfonylureas. Moreover, the concurrent oral intake of tablets or solutions (including tube feeds) with a high concentration of trivalent and divalent cations (such as aluminum, magnesium, and, to a lesser extent, calcium, iron, and zinc) impairs gastrointestinal absorption of fluoroquinolones and should be avoided or spaced apart in time. Since fluoroquinolones can potentially prolong the QT interval, careful monitoring is necessary when a patient is prescribed other QT prolonging agents. Finally, many antimicrobials reduce the effectiveness of oral or other systemic hormonal contraceptives and patients should be routinely advised to use nonhormonal methods of birth control during therapy.
3. Positive Blood Cultures for Bacteria or Fungus Should be Repeated Serially Every 24 to 48 Hours Until the Cultures Are Negative
An important step in the management of a positive blood culture for bacteria or yeast is to check follow‐up blood cultures every 24 to 48 hours until the bacteremia or fungemia has cleared. This is particularly true of bacteremia caused by Staphylococcus aureus (S. aureus), Enterococcus species, and fungemia caused by Candida species. The duration of bacteremia or fungemia has a significant impact on the predictive values of further testing for endovascular or deep‐seated sources of infection as well as treatment duration. This is particularly true for the treatment of candidemia in nonneutropenic adults and for bacterial endocarditis, in which the recommended duration of treatment starts from the day of the last positive blood culture.2, 3 In addition to repeat blood cultures, a blood culture positive for S. aureus should always prompt an aggressive workup for a source (including strong consideration of a transesophageal echocardiogram to evaluate for endocarditis). S. aureus bacteremia should never be disregarded as a contaminant, and should prompt strong consideration of removal of all indwelling intravenous lines.4
4. Removal of Indwelling Intravascular Catheters Is Essential in the Management of Patients with Candidemia. In These Patients, Retention of Central Lines Is Significantly Related to Poor Outcomes
In patients with culture‐proven Candida fungemia, all intravascular catheters must be removed if at all possible. In a study by Nguyen et al.,5 the mortality rate for patients with a catheter‐related candidemia in whom catheters were retained was significantly higher than that of patients in whom the catheters were removed (41% vs. 21%, P < 0.001). Likewise, in a separate study, Luzzati et al.6 noted that central line removal independently reduced the high mortality of the disease. This recommendation applies to all Candida species.
5. Although Candida Species Are Frequently Noted to Colonize Sputum and Urine Cultures, Their Recovery From Multiple Sites May Be an Indicator of Occult Candidemia in an Acutely Ill Patient
Candida species uncommonly cause pneumonia or urinary tract infection, so their isolation from cultures of the respiratory and genitourinary tract often represents colonization. However, the presence of Candida species at multiple sites may be an indicator of occult candidemia in a patient with multiple risk factors for candidemia, including intensive care unit (ICU) admission, immunosuppression (particularly neutropenia and recent receipt of corticosteroids), central venous catheterization, total parenteral nutrition, recent broad‐spectrum antibiotics, and recent abdominal or gastrointestinal surgery.7
6. Patients with Asymptomatic Bacteriuria, With or Without Pyuria, Should Not Be Treated with Antibiotics. Pregnant Women and Patients Undergoing a Genitourinary Procedure Are the Exception and Should Be Treated With Antibiotics
Asymptomatic bacteriuria is commonly encountered in the hospital setting, but is usually benign. Bacteriuria is defined as a voided urine specimen with 1 bacterial species isolated in a quantitative count of 105 cfu/mL. Treatment of asymptomatic bacteriuria is only recommended for pregnant women or prior to invasive genitourinary procedures, including transurethral resection of the prostate. Patients with structural or functional abnormalities of the urinary tract may have a high prevalence of bacteriuria. Despite its prevalence, asymptomatic bacteriuria is seldom associated with adverse outcomes. Studies have noted that antimicrobial treatment of asymptomatic bacteriuria does not decrease recurrence. Negative outcomes with antimicrobial treatment do occur, including adverse drug reactions and reinfection with organisms of increasing resistance. Clinical trials in spinal‐cord injury patients, diabetic women, elderly patients living in the community or nursing home, and patients with indwelling urethral catheters have consistently found no benefit with treatment of asymptomatic bacteriuria.8, 9 The presence or absence of pyuria does not differentiate symptomatic from asymptomatic urinary infection. Patients with symptomatic urinary tract infection (fever and/or dysuria) should be treated after urine cultures are obtained. Other causes of pyuria in the absence of an acute urinary tract infection include urethritis, tuberculosis, prostatitis, nephrolithiasis, and malignancy.
7. Evaluate All Patients Who Have a History of Penicillin Allergy and Consider Desensitization for Patients With a History Consistent With Immunoglobulin Emediated Allergy Who Require Treatment With a Beta‐Lactam Antibiotic
Patients commonly claim to have an allergy to penicillin. True penicillin allergy is very serious and can be life‐threatening. Because of this, patients labeled as penicillin allergic are typically not treated with beta‐lactam antibiotics. Instead, they may be prescribed medications which are typically less effective, more toxic, have a broader spectrum, or are more expensive.10, 11 Many patients are inappropriately labeled as having a penicillin allergy. A history of penicillin allergy is reported in approximately 10% of hospitalized patients, but only approximately 10% of those who report a history of penicillin allergy actually have an allergic reaction when treated with penicillin. Exanthems are frequently associated with beta‐lactam use during an episode of infectious mononucleosis but these are not considered an allergic reaction. Such patients are generally able to tolerate beta‐lactams subsequent to this episode. Nonpruritic maculopapular rashes are also reported in 3% to 7% of children taking amoxicillin and are not a contraindication for future beta‐lactam or cephalosporin use.12 All patients who describe an allergy should be questioned in detail about the type of penicillin received, as well as the type, severity, and timing of the reaction. Typical immunoglobulin E (IgE)‐mediated severe reactions to penicillin include urticaria, pruritus, angioedema, bronchospasm, and hypotension. These patients should not be given other agents that share the same beta‐lactam ring, including cephalosporins (risk of cross‐reactivity is greatest with first‐generation and second‐generation cephalosporins). Carbapenems have minimal cross‐reactivity, particularly meropenem.13 Monobactams (eg, aztreonam) do not cross‐react. While skin testing to penicillin can be considered in patients with a history of a severe reaction to penicillin, neither the major nor minor determinants are commercially available at this time. In patients with a history of a possible IgE‐mediated reaction and when there is no suitable alternative antibiotic (usually determined from infectious diseases consultation), desensitization to beta‐lactams or carbapenems can be considered. Desensitization should be reserved only for clinicians experienced with these techniques, preferably in consultation with a specialist in allergy and immunology. Patients who report a non‐IgE‐mediated reaction may be prescribed a cephalosporin if necessary (preferably a third‐generation or fourth‐generation).14
8. An Abrupt Increase in Leukocytosis In a Hospitalized Patient Should Prompt Consideration of Clostridium difficile Infection
In recent years, there has been a marked increase in the incidence and severity of Clostridium difficile (C. difficile) infection (CDI). A new hypervirulent strain, NAP1/BI/027, has emerged and is becoming endemic in the United States, Canada, and Europe. Typically C. difficile causes diarrhea, abdominal pain, and fever. Often patients have received antibiotics in the recent past, placing them at higher risk, but cases can occur sporadically (even in the community setting) or be transmitted nosocomially. Early detection appears to be essential in reducing the serious morbidity and mortality associated with this disease. Observational studies suggested that C. difficile infection is a common cause of unexplained leukocytosis or a sudden worsening of preexisting leukocytosis.15, 16 In a prospective study evaluating 60 patients with unexplained leukocytosis (white blood cell count 15,000/mm3), 58% of patients with leukocytosis in the absence of localizing symptoms and signs of infection were subsequently diagnosed with CDI. The authors believe that the percent may have been as high as 73% when they included patients with a negative toxin assay who rapidly responded to metronidazole therapy.17 White blood cell counts can range from 10,000 to 20,000/mm3 in moderate disease. Counts as high as 40,000/mm3 can occur, especially in patients with severe disease. Although the use of clindamycin and cephalosporins have been classically associated with the subsequent development of CDI, the current widespread use of fluoroquinolones has led to significant fluoroquinolone resistance among strains of C. difficile, especially the hypervirulent NAP1/BI/027 strain.18 The judicious use of antibiotics, especially fluoroquinolones, remains the cornerstone in preventing CDI. Remember that hand washing with soap and water is essential as alcohol‐based hand sanitizers do not eradicate the C. difficile spores. The drug of choice for initial treatment of mild to moderate CDI remains oral metronidazole, and it may be used for a first recurrence of CDI. Increasing data support the use of oral vancomycin for moderately severe to severe CDI or for multiple recurrences.19 Intravenous metronidazole is often added to oral vancomycin in patients with ileus, but it is not reliably effective alone for CDI.
9. Fever Is Common in the First 48 Hours After a Major Surgical Procedure, and Is a Poor Indicator of Infection. The use of Antibiotics in Response to Fever in the Absence of Other Localizing Signs and Symptoms of Infection Should Be Avoided
Early postoperative fever is relatively common but most fevers that develop within the first 48 hours after surgery do not have an infectious etiology.2023 However, fever that begins or persists beyond the fifth postoperative day is much more likely to represent a clinically significant infection. The continued use of antibiotics outside the window for wound prophylaxis (>24 hours) does not decrease the risk of postoperative infection but it does increase the risk of acquiring resistant bacteria and adverse drug reactions, including CDI.
10. Facts All Clinicians Should Know About Patients with HIV Infection
The 2 most common laboratory abnormalities routinely associated with antiretroviral therapy for HIV infection are unconjugated hyperbilirubinemia associated with atazanavir and an elevated mean corpuscular volume (MCV) associated with zidovudine (and, to a lesser extent, stavudine). Immune reconstitution inflammatory syndrome (IRIS) is a condition seen in patients with advanced acquired immune deficiency syndrome (AIDS) who have recently started antiretroviral therapy. As the immune system begins to recover, it may respond to a previously acquired opportunistic infection with an overwhelming inflammatory response that paradoxically makes the symptoms of infection worse. IRIS is associated with a pathological inflammatory response that can have substantial morbidity and mortality.24 For this reason, when considering whether to start or stop continuous or highly active antiretroviral therapy (also known as HAART), an infectious diseases consult is recommended. Pneumocystis jiroveci (PCP) remains a cause of pneumonia in patients with advanced AIDS' though in the era of HAART, its presentation may be more subtle. Finally, the principle of parsimony (Occam's razor) often does not hold in the diagnosis of opportunistic infections in patients with advanced AIDS, as these patients can often present with multiple infections simultaneously.25, 26
Conclusion
Infectious diseases are commonly encountered by physicians who care for hospitalized patients. Early recognition, evaluation, and appropriate treatment and/or referral to an infectious diseases specialist are necessary to moderate the significant morbidity and mortality that are often associated with infectious diseases.
- ,.Observations on spiraling empiricism: its causes, allure, and perils, with particular reference to antibiotic therapy.Am J Med.1989;87(2):201–206.
- ,,, et al.Guidelines for the treatment of candidiasis.Clin Infect Dis.2004;38:161–189.
- ,,, et al.Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation.2005;111:e394–e434.
- ,.Management of methicillin‐resistant Staphylococcus aureus bacteremia.Clin Infect Dis.2008;46:S386–S393.
- ,,, et al.Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study.Arch Intern Med.1995;155(22):2429–2435.
- ,,, et al.Nosocomial candidemia in non‐neutropenic patients at an Italian tertiary care hospital.Eur J Clin Microbiol Infect Dis.2000;19(8):602–607.
- .Candidemia in adults. In: Marr KA, ed.UpToDate.Waltham, MA:UpToDate, Inc.;2008.
- ,,, et al.Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults.Clin Infect Dis.2005;40(5):643–654.
- .Asymptomatic bacteriuria: when to screen and when to treat.Infect Dis Clin North Am.2003;17(2):367–394.
- .Management of patients with a history of allergy to beta‐lactam antibiotics.Am J Med.2008;121(7):572–576.
- ,,.Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic.Clin Infect Dis.2002;35(1):26–31.
- ,,, et al.Adverse effects of orally administered ampicillin.J Pediatr.1973;83:106–108.
- ,,, et al.Brief communication: tolerability of meropenem in patients with IgE‐mediated hypersensitivity to penicillins.Ann Intern Med.2007;146(4):266–269.
- ,,.The rational clinical examination. Is this patient allergic to penicillin? An evidence‐based analysis of the likelihood of penicillin allergy.JAMA.2001;285(19):2498–2505.
- ,,.Leukocytosis in a tertiary care hospital with particular attention to the role of infection caused by Clostridium difficile.Clin Infect Dis.2002;34:1585–1592.
- ,,,.Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea.Am J Gastroenterol.2000;95:3137–3141.
- ,,,.Clostridium difficile infection in patients with unexplained leukocytosis.Am J Med.2003;115:543–546.
- ,.The challenges posed by reemerging Clostridium difficile infection.Clin Infect Dis.2007;45(2):222–227.
- ,,.Treatment of Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S32–S42.
- ,,,,.Diagnostic accuracy of routine postoperative body temperature measurements.Clin Infect Dis.2005;40:1404–1410.
- .Should we measure body temperature for patients who have recently undergone surgery?Clin Infect Dis.2005;40(10):1411–1412.
- ,,,.Evidence for the noninfectious etiology of early postoperative fever.Infect Control.1985;6:273–277.
- .Evaluating postoperative fever: a focused approach.Cleve Clin J Med.2006;73(suppl 1):S62–S66.
- ,,.Immune reconstitution inflammatory syndrome: more answers, more questions.J Antimicrob Chemother.2006;57(2):167–170.
- ,,,,.Clinical problem‐solving. Occam's razor versus Saint's Triad.N Engl J Med.2004;350(6):599–603.
- ,.William of Occam and Occam's razor.Ann Intern Med.2002;136(8):634–635.
- ,.Observations on spiraling empiricism: its causes, allure, and perils, with particular reference to antibiotic therapy.Am J Med.1989;87(2):201–206.
- ,,, et al.Guidelines for the treatment of candidiasis.Clin Infect Dis.2004;38:161–189.
- ,,, et al.Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation.2005;111:e394–e434.
- ,.Management of methicillin‐resistant Staphylococcus aureus bacteremia.Clin Infect Dis.2008;46:S386–S393.
- ,,, et al.Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study.Arch Intern Med.1995;155(22):2429–2435.
- ,,, et al.Nosocomial candidemia in non‐neutropenic patients at an Italian tertiary care hospital.Eur J Clin Microbiol Infect Dis.2000;19(8):602–607.
- .Candidemia in adults. In: Marr KA, ed.UpToDate.Waltham, MA:UpToDate, Inc.;2008.
- ,,, et al.Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults.Clin Infect Dis.2005;40(5):643–654.
- .Asymptomatic bacteriuria: when to screen and when to treat.Infect Dis Clin North Am.2003;17(2):367–394.
- .Management of patients with a history of allergy to beta‐lactam antibiotics.Am J Med.2008;121(7):572–576.
- ,,.Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic.Clin Infect Dis.2002;35(1):26–31.
- ,,, et al.Adverse effects of orally administered ampicillin.J Pediatr.1973;83:106–108.
- ,,, et al.Brief communication: tolerability of meropenem in patients with IgE‐mediated hypersensitivity to penicillins.Ann Intern Med.2007;146(4):266–269.
- ,,.The rational clinical examination. Is this patient allergic to penicillin? An evidence‐based analysis of the likelihood of penicillin allergy.JAMA.2001;285(19):2498–2505.
- ,,.Leukocytosis in a tertiary care hospital with particular attention to the role of infection caused by Clostridium difficile.Clin Infect Dis.2002;34:1585–1592.
- ,,,.Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea.Am J Gastroenterol.2000;95:3137–3141.
- ,,,.Clostridium difficile infection in patients with unexplained leukocytosis.Am J Med.2003;115:543–546.
- ,.The challenges posed by reemerging Clostridium difficile infection.Clin Infect Dis.2007;45(2):222–227.
- ,,.Treatment of Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S32–S42.
- ,,,,.Diagnostic accuracy of routine postoperative body temperature measurements.Clin Infect Dis.2005;40:1404–1410.
- .Should we measure body temperature for patients who have recently undergone surgery?Clin Infect Dis.2005;40(10):1411–1412.
- ,,,.Evidence for the noninfectious etiology of early postoperative fever.Infect Control.1985;6:273–277.
- .Evaluating postoperative fever: a focused approach.Cleve Clin J Med.2006;73(suppl 1):S62–S66.
- ,,.Immune reconstitution inflammatory syndrome: more answers, more questions.J Antimicrob Chemother.2006;57(2):167–170.
- ,,,,.Clinical problem‐solving. Occam's razor versus Saint's Triad.N Engl J Med.2004;350(6):599–603.
- ,.William of Occam and Occam's razor.Ann Intern Med.2002;136(8):634–635.
Hospitalists and Intensivists
A looming gap in the supply of intensivists prompted the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Society of Critical Care Medicine (SCCM) to publish a report in 2000 by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). This study predicted that beginning in 2007 a shortfall would become apparent and steadily increase to 22% by 2020 and to 35% by 2030. Subsequent reports have reiterated those projections, including a report to Congress in 2006 by the U.S. Department of Health and Human Services/Health Resources and Services Administration.14
The concern regarding the shortage of intensivists has been increased by the growing evidence that supports improved critical care outcomesespecially decreased intensive care unit (ICU) and hospital mortalitywith intensivist staffing of ICUs.5, 6 Based on this data and on recommendations from the Society of Critical Care Medicine, the Leapfrog Group made onsite, high‐intensity ICU staffing with intensivists 1 of their 4 leaps.7 A paper by Pronovost et al.8 published in 2001, however, noted that in order for all ICUs in the United States to meet the Leapfrog ICU Physician Staffing (IPS) standard, the number of intensivists would need to increase by a factor of 2.6. Interestingly, a retrospective study published in the Annals of Internal Medicine in June of 2008 by Levy et al.9 suggested that mortality rates may actually be higher in intensivist‐staffed ICUs. An accompanying editorial raised concerns about limitations of the study design, but endorsed Levy's recommendation that more carefully designed, prospective studies were needed; (ie, we still are not certain as to optimal physician staffing for the care of patients requiring the sophisticated treatment available only in an ICU.)10
The health policy challenge, however, remains clear: while there is basic consensus that care of critically ill patients by intensivists improves outcomes, the reality is that the shortage of intensivists in the United States as predicted by the COMPACCS report will only increase, leading some to refer to this as a healthcare crisis. Two major task forces attempted to address this situation, resulting in the publication of the 2004 Framing Options for Critical Care in the United States (FOCCUS) report, The Critical Care Medicine Crisis: A Call for Federal ActionA White Paper from the Critical Care Professional Societies; and the 2007 Prioritizing the Organization and Management of Intensive Care Services in the Unites States (PrOMIS) Conference Report.11, 12 Both reports made specific recommendations including, for example, development of uniform standards for accreditation of institutional critical care capacity, identification and endorsement of core competencies in critical care, investment in health services research, the use of uniform protocols for ICU care, leverage of information technology to promote standardization and improve efficiency, and the development of incentives to attract healthcare professionals to critical care medicine.
A Possible Solution: The Role of Hospitalists
Multiple important efforts are already underway to increase the competency of professionals providing critical care services including the Society of Critical Care's Fundamentals in Critical Care Support (FCCS) program. Additionally, physician assistants and nurse practitioners are playing an increasingly important role as members of critical care services. As another component of this collaborative effort, the PrOMIS Report noted the potential impact of hospitalists in addressing this crisis.
As early as 1999, surveys revealed that as many as 35% of hospitalists were providing critical care services.13 According to the 2005/2006 Society of Hospital Medicine (SHM) National Survey, that number has increased to 75% with a low of 66% in the eastern United States and a high of 84% in the western United States. In community hospitals, 87% of hospitalists care for patients in the ICU, and 30% provide critical care services in academic medical centers.13 While there is some research14, 15 and many anecdotal reports that suggest hospitalists perform well in the ICU, there is, unfortunately, little data addressing outcomes for patients cared for by hospitalists. The results from a prospective, severity‐adjusted study from the Emory University Section of Hospital Medicine and the Division of Pulmonary/Critical Care Medicine examining outcomes for critical care patients cared for by hospitalists with criteria for Pulmonary/Intensivist consults vs. patients cared for by the Pulmonary/Critical Care Medical ICU Service await peer‐review publication.
Despite the lack of outcome data regarding adult hospitalists, it is clear that by default they are already providing a significant proportion of critical care services across the healthcare system, including in tertiary care centers. The two primary models of care include: (1) hospitalists serving as the primary provider without critical care consultant services and (2) comanagement of patients where intensivists and hospitalists collaborate. These collaborative models involve hospitalists actively co‐managing critical care patients along with intensivists or hospitalists managing less critically ill patients with intensivist consultation when indicated. In hospitals lacking intensivists, hospitalists often manage critically ill patients either with intensivist phone consultation, or with the intent to stabilize and transfer. Electronic ICUs are another expanding model of care that provide intensivist support to hospitalists and other primary care providersdecreasing ICU length of stay and severity‐adjusted ICU mortality.16 There are now 40 electronic ICU programs in the United States, and that number continues to grow.
In 2003, there were approximately 10,000 hospitalists in the United States,17 and recent data from an American Hospital Association survey indicates that the number has grown to about 28,000 in 2009. Recent research also documents that hospitalists are soon likely to care for the majority of elderly hospitalized patients in America.18 Aware that the number of intensivists is unlikely to change significantly over the next 25 years the question is no longer if hospitalists should be in the ICU; rather, the question is how to assure quality and improved clinical outcomes through enhanced collaboration between Hospital Medicine and Critical Care Medicine.
Recommendations
There are 3 steps that should be taken urgently to meet this challenge:
-
Per the recommendation of the FOCCUS Report and the PrOMIS Conference Report, uniform protocols for intensive care treatmentmany of which already exist but are not used consistentlyshould be identified and implemented across all ICUs regardless of the level or certification of the provider.
-
Also per the PrOMIS Report, a process for certification of physicians providing critical care services should be established by the appropriate governing bodies, including the Society for Critical Care Medicine, the Society of Hospital Medicine, and the American Thoracic Society, among others. While the PrOMIS Report called for cross‐training of hospital‐based providers to provide intensive care services in lower tier hospitals, a more realistic recommendation given current involvement of hospitalists in the provision of critical care services in secondary and tertiary centers is a competency‐assurance process that includes hospitalists practicing at all levels. This would not be equivalent to board certification, but would be based on a rigorous, comprehensive education and skills training process leading to recognition that would distinguish the recipient as having competencies beyond those obtained in internal medicine residency training. Models for certification could include 4‐month onsite training or a distance learning curriculum with regular blocks of onsite training. Another strategy might be for appropriate governing bodies to establish basic criteria for competency that would then be provided by individual institutions. Emory University, for example, has developed a pilot program incorporating significant components from the European Society for Critical Care Medicine's Syllabus for Competency Based Training in Intensive Care Medicine in Europe.19 Other institutions are also exploring the creation of certification/competency programs. Minimally, and prior to any decision about establishing formal criteria, institutions could identify designated hospitalists within groups who have particular interest and ability in the critical care setting. These providers, based on models already in place at sites across the United States, could, as an example, be required to spend a minimum of 50% of their clinical time in the ICU and to complete 10 to 20 hours of critical care continuing medical education (CME) per year. One strategy to address this issue and develop clear consensus and guidelines would be to convene the often discussed PrOMIS II working group.
-
Per both the FOCCUS Report and the PrOMIS Report as well as a number of other publications,19 health services research in ICU care should be identified, funded, and implemented. A major focus of this effort should be the evaluation of clinical outcomes for ICU patients cared for by hospitalists. This research is needed for at least 2 reasons:
-
As noted, there is little research that has assessed hospitalists' impact on outcomes of ICU patients. Hospitalists are already caring for patients in ICUs across the United States and given the research that has identified the outcomes benefit provided by intensivists, it is important to know objectively if hospitalists have similar levels of performance.
-
An increasing number of hospitals and healthcare systems are now committed to achieving the Leapfrog IPS standard‐a challenge for many because of the difficulty with recruiting intensivists. If new research reveals that hospitalists with board certification in Internal Medicine, and more specifically with additional competency training in critical care, also improve outcomes in the ICU then it may be possible for Leapfrog to revise the criteria for meeting the IPS standard.
Summary
As discussed in a number of publications,20 including an article from the Mayo Clinic in the April 2009 edition of Chest entitled, Physicians Staffing Models and Patient Safety in the ICU,21 along with an accompanying editorial, Should Intensive Care Medicine Itself Be on the Critical List,22 creative and realistic solutions are urgently needed to address the crisis in critical care in the U.S. Collaborative efforts between Critical Care Medicine and Hospital Medicine to meet this challenge benefit all involved:
-
Intensivists will continue to direct tertiary care units and/or co‐manage patients in tertiary and secondary care centers with Hospitalists.
-
Hospitalists will benefit by having the opportunity to secure critical care competency training and by having their appropriate role in the ICU defined.
-
All secondary and tertiary care institutions will have a realistic opportunity to meet Leapfrog IPS criteria and therefore benefit from the potential decreased length of stay (LOS), decreased mortality, and improved quality.
-
Patients benefit by receiving uniform, evidence‐based, protocol‐driven care.
There is now a need and an opportunity for ACCP, SCCM, ATS, and the American Association of Critical Care Nurses (ACCN), to expand the important work they have already begun through the Critical Care Workforce Partnership. The Partnership should join with the SHM to take the lead in supporting and promoting this collaborative relationship between intensivists and hospitalists: aware that in the final analysis, it is the patients we serve who will benefit the most.
- ,,, et al.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284:2762–2770.
- ,,.The critical care professional societies address the critical care crisis in the united states.Chest.2004;125:1512–1513.
- ,,, et al.The critical care crisis in the United States; a report from the profession.Chest.2004;125:1514–1517.
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Report to Congress: the critical care workforce; a study of the supply and demand for critical care physicians. Senate Report 108–181. May2006.
- ,,, et al.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002:288:2151–2162.
- ,,.Do intensivists in ICU improve outcome? Best practice and research.Best Pract Res Clin Anaesthesiol.2005;19:125–135.
- The Leap Frog Group website. Available at: http://www.leapfroggroup.org. Accessed July2009.
- ,,.Impact of critical care physician workforce for intensive care unit physician staffing.Curr Opin Crit Care.2001;7:456–459.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–810.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.The critical care medicine crisis: a call for federal action; a white paper from the critical care professional societies.Chest.2004;125:1518–1521.
- ,,, et al.Prioritizing the organization and management of intensive care services in the Unites States: the PrOMIS conference.Crit Care Med.2007;35:1103–1111.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- .Integrating hospitalists into the pediatric intensive care unit.Crit Care Med.2003;32:813–816.
- ,,, et al.Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system.SD Med2006;59(9):391–393.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- CoBaTrICE Syllabus (Competency‐Based Training in Intensive Care Medicine in Europe), Version 1.0.Brussels:European Society of Intensive Care Medicine;2006.
- .Caring for the critically ill. patient challenges and opportunities.JAMA.2007;298(4):456–458.
- ,.Physician staffing models and patient safety in the ICU.Chest.2009;135:1038–1044.
- ,.Should intensive care medicine itself be on the critical list.Chest.2009;135:892–894.
A looming gap in the supply of intensivists prompted the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Society of Critical Care Medicine (SCCM) to publish a report in 2000 by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). This study predicted that beginning in 2007 a shortfall would become apparent and steadily increase to 22% by 2020 and to 35% by 2030. Subsequent reports have reiterated those projections, including a report to Congress in 2006 by the U.S. Department of Health and Human Services/Health Resources and Services Administration.14
The concern regarding the shortage of intensivists has been increased by the growing evidence that supports improved critical care outcomesespecially decreased intensive care unit (ICU) and hospital mortalitywith intensivist staffing of ICUs.5, 6 Based on this data and on recommendations from the Society of Critical Care Medicine, the Leapfrog Group made onsite, high‐intensity ICU staffing with intensivists 1 of their 4 leaps.7 A paper by Pronovost et al.8 published in 2001, however, noted that in order for all ICUs in the United States to meet the Leapfrog ICU Physician Staffing (IPS) standard, the number of intensivists would need to increase by a factor of 2.6. Interestingly, a retrospective study published in the Annals of Internal Medicine in June of 2008 by Levy et al.9 suggested that mortality rates may actually be higher in intensivist‐staffed ICUs. An accompanying editorial raised concerns about limitations of the study design, but endorsed Levy's recommendation that more carefully designed, prospective studies were needed; (ie, we still are not certain as to optimal physician staffing for the care of patients requiring the sophisticated treatment available only in an ICU.)10
The health policy challenge, however, remains clear: while there is basic consensus that care of critically ill patients by intensivists improves outcomes, the reality is that the shortage of intensivists in the United States as predicted by the COMPACCS report will only increase, leading some to refer to this as a healthcare crisis. Two major task forces attempted to address this situation, resulting in the publication of the 2004 Framing Options for Critical Care in the United States (FOCCUS) report, The Critical Care Medicine Crisis: A Call for Federal ActionA White Paper from the Critical Care Professional Societies; and the 2007 Prioritizing the Organization and Management of Intensive Care Services in the Unites States (PrOMIS) Conference Report.11, 12 Both reports made specific recommendations including, for example, development of uniform standards for accreditation of institutional critical care capacity, identification and endorsement of core competencies in critical care, investment in health services research, the use of uniform protocols for ICU care, leverage of information technology to promote standardization and improve efficiency, and the development of incentives to attract healthcare professionals to critical care medicine.
A Possible Solution: The Role of Hospitalists
Multiple important efforts are already underway to increase the competency of professionals providing critical care services including the Society of Critical Care's Fundamentals in Critical Care Support (FCCS) program. Additionally, physician assistants and nurse practitioners are playing an increasingly important role as members of critical care services. As another component of this collaborative effort, the PrOMIS Report noted the potential impact of hospitalists in addressing this crisis.
As early as 1999, surveys revealed that as many as 35% of hospitalists were providing critical care services.13 According to the 2005/2006 Society of Hospital Medicine (SHM) National Survey, that number has increased to 75% with a low of 66% in the eastern United States and a high of 84% in the western United States. In community hospitals, 87% of hospitalists care for patients in the ICU, and 30% provide critical care services in academic medical centers.13 While there is some research14, 15 and many anecdotal reports that suggest hospitalists perform well in the ICU, there is, unfortunately, little data addressing outcomes for patients cared for by hospitalists. The results from a prospective, severity‐adjusted study from the Emory University Section of Hospital Medicine and the Division of Pulmonary/Critical Care Medicine examining outcomes for critical care patients cared for by hospitalists with criteria for Pulmonary/Intensivist consults vs. patients cared for by the Pulmonary/Critical Care Medical ICU Service await peer‐review publication.
Despite the lack of outcome data regarding adult hospitalists, it is clear that by default they are already providing a significant proportion of critical care services across the healthcare system, including in tertiary care centers. The two primary models of care include: (1) hospitalists serving as the primary provider without critical care consultant services and (2) comanagement of patients where intensivists and hospitalists collaborate. These collaborative models involve hospitalists actively co‐managing critical care patients along with intensivists or hospitalists managing less critically ill patients with intensivist consultation when indicated. In hospitals lacking intensivists, hospitalists often manage critically ill patients either with intensivist phone consultation, or with the intent to stabilize and transfer. Electronic ICUs are another expanding model of care that provide intensivist support to hospitalists and other primary care providersdecreasing ICU length of stay and severity‐adjusted ICU mortality.16 There are now 40 electronic ICU programs in the United States, and that number continues to grow.
In 2003, there were approximately 10,000 hospitalists in the United States,17 and recent data from an American Hospital Association survey indicates that the number has grown to about 28,000 in 2009. Recent research also documents that hospitalists are soon likely to care for the majority of elderly hospitalized patients in America.18 Aware that the number of intensivists is unlikely to change significantly over the next 25 years the question is no longer if hospitalists should be in the ICU; rather, the question is how to assure quality and improved clinical outcomes through enhanced collaboration between Hospital Medicine and Critical Care Medicine.
Recommendations
There are 3 steps that should be taken urgently to meet this challenge:
-
Per the recommendation of the FOCCUS Report and the PrOMIS Conference Report, uniform protocols for intensive care treatmentmany of which already exist but are not used consistentlyshould be identified and implemented across all ICUs regardless of the level or certification of the provider.
-
Also per the PrOMIS Report, a process for certification of physicians providing critical care services should be established by the appropriate governing bodies, including the Society for Critical Care Medicine, the Society of Hospital Medicine, and the American Thoracic Society, among others. While the PrOMIS Report called for cross‐training of hospital‐based providers to provide intensive care services in lower tier hospitals, a more realistic recommendation given current involvement of hospitalists in the provision of critical care services in secondary and tertiary centers is a competency‐assurance process that includes hospitalists practicing at all levels. This would not be equivalent to board certification, but would be based on a rigorous, comprehensive education and skills training process leading to recognition that would distinguish the recipient as having competencies beyond those obtained in internal medicine residency training. Models for certification could include 4‐month onsite training or a distance learning curriculum with regular blocks of onsite training. Another strategy might be for appropriate governing bodies to establish basic criteria for competency that would then be provided by individual institutions. Emory University, for example, has developed a pilot program incorporating significant components from the European Society for Critical Care Medicine's Syllabus for Competency Based Training in Intensive Care Medicine in Europe.19 Other institutions are also exploring the creation of certification/competency programs. Minimally, and prior to any decision about establishing formal criteria, institutions could identify designated hospitalists within groups who have particular interest and ability in the critical care setting. These providers, based on models already in place at sites across the United States, could, as an example, be required to spend a minimum of 50% of their clinical time in the ICU and to complete 10 to 20 hours of critical care continuing medical education (CME) per year. One strategy to address this issue and develop clear consensus and guidelines would be to convene the often discussed PrOMIS II working group.
-
Per both the FOCCUS Report and the PrOMIS Report as well as a number of other publications,19 health services research in ICU care should be identified, funded, and implemented. A major focus of this effort should be the evaluation of clinical outcomes for ICU patients cared for by hospitalists. This research is needed for at least 2 reasons:
-
As noted, there is little research that has assessed hospitalists' impact on outcomes of ICU patients. Hospitalists are already caring for patients in ICUs across the United States and given the research that has identified the outcomes benefit provided by intensivists, it is important to know objectively if hospitalists have similar levels of performance.
-
An increasing number of hospitals and healthcare systems are now committed to achieving the Leapfrog IPS standard‐a challenge for many because of the difficulty with recruiting intensivists. If new research reveals that hospitalists with board certification in Internal Medicine, and more specifically with additional competency training in critical care, also improve outcomes in the ICU then it may be possible for Leapfrog to revise the criteria for meeting the IPS standard.
Summary
As discussed in a number of publications,20 including an article from the Mayo Clinic in the April 2009 edition of Chest entitled, Physicians Staffing Models and Patient Safety in the ICU,21 along with an accompanying editorial, Should Intensive Care Medicine Itself Be on the Critical List,22 creative and realistic solutions are urgently needed to address the crisis in critical care in the U.S. Collaborative efforts between Critical Care Medicine and Hospital Medicine to meet this challenge benefit all involved:
-
Intensivists will continue to direct tertiary care units and/or co‐manage patients in tertiary and secondary care centers with Hospitalists.
-
Hospitalists will benefit by having the opportunity to secure critical care competency training and by having their appropriate role in the ICU defined.
-
All secondary and tertiary care institutions will have a realistic opportunity to meet Leapfrog IPS criteria and therefore benefit from the potential decreased length of stay (LOS), decreased mortality, and improved quality.
-
Patients benefit by receiving uniform, evidence‐based, protocol‐driven care.
There is now a need and an opportunity for ACCP, SCCM, ATS, and the American Association of Critical Care Nurses (ACCN), to expand the important work they have already begun through the Critical Care Workforce Partnership. The Partnership should join with the SHM to take the lead in supporting and promoting this collaborative relationship between intensivists and hospitalists: aware that in the final analysis, it is the patients we serve who will benefit the most.
A looming gap in the supply of intensivists prompted the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the Society of Critical Care Medicine (SCCM) to publish a report in 2000 by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS). This study predicted that beginning in 2007 a shortfall would become apparent and steadily increase to 22% by 2020 and to 35% by 2030. Subsequent reports have reiterated those projections, including a report to Congress in 2006 by the U.S. Department of Health and Human Services/Health Resources and Services Administration.14
The concern regarding the shortage of intensivists has been increased by the growing evidence that supports improved critical care outcomesespecially decreased intensive care unit (ICU) and hospital mortalitywith intensivist staffing of ICUs.5, 6 Based on this data and on recommendations from the Society of Critical Care Medicine, the Leapfrog Group made onsite, high‐intensity ICU staffing with intensivists 1 of their 4 leaps.7 A paper by Pronovost et al.8 published in 2001, however, noted that in order for all ICUs in the United States to meet the Leapfrog ICU Physician Staffing (IPS) standard, the number of intensivists would need to increase by a factor of 2.6. Interestingly, a retrospective study published in the Annals of Internal Medicine in June of 2008 by Levy et al.9 suggested that mortality rates may actually be higher in intensivist‐staffed ICUs. An accompanying editorial raised concerns about limitations of the study design, but endorsed Levy's recommendation that more carefully designed, prospective studies were needed; (ie, we still are not certain as to optimal physician staffing for the care of patients requiring the sophisticated treatment available only in an ICU.)10
The health policy challenge, however, remains clear: while there is basic consensus that care of critically ill patients by intensivists improves outcomes, the reality is that the shortage of intensivists in the United States as predicted by the COMPACCS report will only increase, leading some to refer to this as a healthcare crisis. Two major task forces attempted to address this situation, resulting in the publication of the 2004 Framing Options for Critical Care in the United States (FOCCUS) report, The Critical Care Medicine Crisis: A Call for Federal ActionA White Paper from the Critical Care Professional Societies; and the 2007 Prioritizing the Organization and Management of Intensive Care Services in the Unites States (PrOMIS) Conference Report.11, 12 Both reports made specific recommendations including, for example, development of uniform standards for accreditation of institutional critical care capacity, identification and endorsement of core competencies in critical care, investment in health services research, the use of uniform protocols for ICU care, leverage of information technology to promote standardization and improve efficiency, and the development of incentives to attract healthcare professionals to critical care medicine.
A Possible Solution: The Role of Hospitalists
Multiple important efforts are already underway to increase the competency of professionals providing critical care services including the Society of Critical Care's Fundamentals in Critical Care Support (FCCS) program. Additionally, physician assistants and nurse practitioners are playing an increasingly important role as members of critical care services. As another component of this collaborative effort, the PrOMIS Report noted the potential impact of hospitalists in addressing this crisis.
As early as 1999, surveys revealed that as many as 35% of hospitalists were providing critical care services.13 According to the 2005/2006 Society of Hospital Medicine (SHM) National Survey, that number has increased to 75% with a low of 66% in the eastern United States and a high of 84% in the western United States. In community hospitals, 87% of hospitalists care for patients in the ICU, and 30% provide critical care services in academic medical centers.13 While there is some research14, 15 and many anecdotal reports that suggest hospitalists perform well in the ICU, there is, unfortunately, little data addressing outcomes for patients cared for by hospitalists. The results from a prospective, severity‐adjusted study from the Emory University Section of Hospital Medicine and the Division of Pulmonary/Critical Care Medicine examining outcomes for critical care patients cared for by hospitalists with criteria for Pulmonary/Intensivist consults vs. patients cared for by the Pulmonary/Critical Care Medical ICU Service await peer‐review publication.
Despite the lack of outcome data regarding adult hospitalists, it is clear that by default they are already providing a significant proportion of critical care services across the healthcare system, including in tertiary care centers. The two primary models of care include: (1) hospitalists serving as the primary provider without critical care consultant services and (2) comanagement of patients where intensivists and hospitalists collaborate. These collaborative models involve hospitalists actively co‐managing critical care patients along with intensivists or hospitalists managing less critically ill patients with intensivist consultation when indicated. In hospitals lacking intensivists, hospitalists often manage critically ill patients either with intensivist phone consultation, or with the intent to stabilize and transfer. Electronic ICUs are another expanding model of care that provide intensivist support to hospitalists and other primary care providersdecreasing ICU length of stay and severity‐adjusted ICU mortality.16 There are now 40 electronic ICU programs in the United States, and that number continues to grow.
In 2003, there were approximately 10,000 hospitalists in the United States,17 and recent data from an American Hospital Association survey indicates that the number has grown to about 28,000 in 2009. Recent research also documents that hospitalists are soon likely to care for the majority of elderly hospitalized patients in America.18 Aware that the number of intensivists is unlikely to change significantly over the next 25 years the question is no longer if hospitalists should be in the ICU; rather, the question is how to assure quality and improved clinical outcomes through enhanced collaboration between Hospital Medicine and Critical Care Medicine.
Recommendations
There are 3 steps that should be taken urgently to meet this challenge:
-
Per the recommendation of the FOCCUS Report and the PrOMIS Conference Report, uniform protocols for intensive care treatmentmany of which already exist but are not used consistentlyshould be identified and implemented across all ICUs regardless of the level or certification of the provider.
-
Also per the PrOMIS Report, a process for certification of physicians providing critical care services should be established by the appropriate governing bodies, including the Society for Critical Care Medicine, the Society of Hospital Medicine, and the American Thoracic Society, among others. While the PrOMIS Report called for cross‐training of hospital‐based providers to provide intensive care services in lower tier hospitals, a more realistic recommendation given current involvement of hospitalists in the provision of critical care services in secondary and tertiary centers is a competency‐assurance process that includes hospitalists practicing at all levels. This would not be equivalent to board certification, but would be based on a rigorous, comprehensive education and skills training process leading to recognition that would distinguish the recipient as having competencies beyond those obtained in internal medicine residency training. Models for certification could include 4‐month onsite training or a distance learning curriculum with regular blocks of onsite training. Another strategy might be for appropriate governing bodies to establish basic criteria for competency that would then be provided by individual institutions. Emory University, for example, has developed a pilot program incorporating significant components from the European Society for Critical Care Medicine's Syllabus for Competency Based Training in Intensive Care Medicine in Europe.19 Other institutions are also exploring the creation of certification/competency programs. Minimally, and prior to any decision about establishing formal criteria, institutions could identify designated hospitalists within groups who have particular interest and ability in the critical care setting. These providers, based on models already in place at sites across the United States, could, as an example, be required to spend a minimum of 50% of their clinical time in the ICU and to complete 10 to 20 hours of critical care continuing medical education (CME) per year. One strategy to address this issue and develop clear consensus and guidelines would be to convene the often discussed PrOMIS II working group.
-
Per both the FOCCUS Report and the PrOMIS Report as well as a number of other publications,19 health services research in ICU care should be identified, funded, and implemented. A major focus of this effort should be the evaluation of clinical outcomes for ICU patients cared for by hospitalists. This research is needed for at least 2 reasons:
-
As noted, there is little research that has assessed hospitalists' impact on outcomes of ICU patients. Hospitalists are already caring for patients in ICUs across the United States and given the research that has identified the outcomes benefit provided by intensivists, it is important to know objectively if hospitalists have similar levels of performance.
-
An increasing number of hospitals and healthcare systems are now committed to achieving the Leapfrog IPS standard‐a challenge for many because of the difficulty with recruiting intensivists. If new research reveals that hospitalists with board certification in Internal Medicine, and more specifically with additional competency training in critical care, also improve outcomes in the ICU then it may be possible for Leapfrog to revise the criteria for meeting the IPS standard.
Summary
As discussed in a number of publications,20 including an article from the Mayo Clinic in the April 2009 edition of Chest entitled, Physicians Staffing Models and Patient Safety in the ICU,21 along with an accompanying editorial, Should Intensive Care Medicine Itself Be on the Critical List,22 creative and realistic solutions are urgently needed to address the crisis in critical care in the U.S. Collaborative efforts between Critical Care Medicine and Hospital Medicine to meet this challenge benefit all involved:
-
Intensivists will continue to direct tertiary care units and/or co‐manage patients in tertiary and secondary care centers with Hospitalists.
-
Hospitalists will benefit by having the opportunity to secure critical care competency training and by having their appropriate role in the ICU defined.
-
All secondary and tertiary care institutions will have a realistic opportunity to meet Leapfrog IPS criteria and therefore benefit from the potential decreased length of stay (LOS), decreased mortality, and improved quality.
-
Patients benefit by receiving uniform, evidence‐based, protocol‐driven care.
There is now a need and an opportunity for ACCP, SCCM, ATS, and the American Association of Critical Care Nurses (ACCN), to expand the important work they have already begun through the Critical Care Workforce Partnership. The Partnership should join with the SHM to take the lead in supporting and promoting this collaborative relationship between intensivists and hospitalists: aware that in the final analysis, it is the patients we serve who will benefit the most.
- ,,, et al.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284:2762–2770.
- ,,.The critical care professional societies address the critical care crisis in the united states.Chest.2004;125:1512–1513.
- ,,, et al.The critical care crisis in the United States; a report from the profession.Chest.2004;125:1514–1517.
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Report to Congress: the critical care workforce; a study of the supply and demand for critical care physicians. Senate Report 108–181. May2006.
- ,,, et al.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002:288:2151–2162.
- ,,.Do intensivists in ICU improve outcome? Best practice and research.Best Pract Res Clin Anaesthesiol.2005;19:125–135.
- The Leap Frog Group website. Available at: http://www.leapfroggroup.org. Accessed July2009.
- ,,.Impact of critical care physician workforce for intensive care unit physician staffing.Curr Opin Crit Care.2001;7:456–459.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–810.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.The critical care medicine crisis: a call for federal action; a white paper from the critical care professional societies.Chest.2004;125:1518–1521.
- ,,, et al.Prioritizing the organization and management of intensive care services in the Unites States: the PrOMIS conference.Crit Care Med.2007;35:1103–1111.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- .Integrating hospitalists into the pediatric intensive care unit.Crit Care Med.2003;32:813–816.
- ,,, et al.Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system.SD Med2006;59(9):391–393.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- CoBaTrICE Syllabus (Competency‐Based Training in Intensive Care Medicine in Europe), Version 1.0.Brussels:European Society of Intensive Care Medicine;2006.
- .Caring for the critically ill. patient challenges and opportunities.JAMA.2007;298(4):456–458.
- ,.Physician staffing models and patient safety in the ICU.Chest.2009;135:1038–1044.
- ,.Should intensive care medicine itself be on the critical list.Chest.2009;135:892–894.
- ,,, et al.Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population?JAMA.2000;284:2762–2770.
- ,,.The critical care professional societies address the critical care crisis in the united states.Chest.2004;125:1512–1513.
- ,,, et al.The critical care crisis in the United States; a report from the profession.Chest.2004;125:1514–1517.
- U.S. Department of Health and Human Services, Health Resources and Services Administration. Report to Congress: the critical care workforce; a study of the supply and demand for critical care physicians. Senate Report 108–181. May2006.
- ,,, et al.Physician staffing patterns and clinical outcomes in critically ill patients.JAMA.2002:288:2151–2162.
- ,,.Do intensivists in ICU improve outcome? Best practice and research.Best Pract Res Clin Anaesthesiol.2005;19:125–135.
- The Leap Frog Group website. Available at: http://www.leapfroggroup.org. Accessed July2009.
- ,,.Impact of critical care physician workforce for intensive care unit physician staffing.Curr Opin Crit Care.2001;7:456–459.
- ,,,,,.Association between critical care physician management and patient mortality in the intensive care unit.Ann Intern Med.2008;148:801–810.
- ,.Are intensivists safe?Ann Intern Med.2008;148:877–878.
- ,,,,,.The critical care medicine crisis: a call for federal action; a white paper from the critical care professional societies.Chest.2004;125:1518–1521.
- ,,, et al.Prioritizing the organization and management of intensive care services in the Unites States: the PrOMIS conference.Crit Care Med.2007;35:1103–1111.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130:343–349.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- .Integrating hospitalists into the pediatric intensive care unit.Crit Care Med.2003;32:813–816.
- ,,, et al.Prognostic outcomes after the initiation of an electronic telemedicine intensive care unit (eICU) in a rural health system.SD Med2006;59(9):391–393.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- CoBaTrICE Syllabus (Competency‐Based Training in Intensive Care Medicine in Europe), Version 1.0.Brussels:European Society of Intensive Care Medicine;2006.
- .Caring for the critically ill. patient challenges and opportunities.JAMA.2007;298(4):456–458.
- ,.Physician staffing models and patient safety in the ICU.Chest.2009;135:1038–1044.
- ,.Should intensive care medicine itself be on the critical list.Chest.2009;135:892–894.
Pretibial Myxedema
An 83‐year‐old female reported increased swelling of her legs over the past 2 years. She noted temperature intolerance, low energy, and constipation, but denied any hair loss or nail changes. On exam, she had marked bilateral lower extremity edema that was predominately nonpitting. Overlying the edema there were thickened, well‐defined plaques with a peau d'orange appearance surrounded by brown, thin plaques on the pretibial areas sparing the dorsum of the feet (Figure 1). A punch biopsy was obtained and demonstrated increased deposition of mucin throughout the dermis along with fragmentation and increased numbers of elastic fibers consistent with a diagnosis of pretibial myxedema. Measured thyrotropin level was elevated at 3.9 U/mL (normal, 0.3‐3.8 U/mL), consistent with hypothyroidism. This is a severe example of pretibial myxedema, or infiltrative dermopathy, which can occur, more commonly, in the setting of Graves' disease or, in rare circumstances, hypothyroidism.1 Myxedema results from the accumulation of hyaluronic acid and chondroitin sulfate in the dermis.2 Treatment is difficult and includes topical glucocorticoids under occlusion and, if indicated, thyroid corrective therapy.3

- .Pretibial myxedema.Dermatol Online J.7(1):18.
- ,,.Pretibial myxoedema: a manifestation of lymphoedema?Lancet.1993;341(8842):403–404.
- .Successful treatment of chronic skin diseases with clobetasol propionate and a hydrocolloid occlusive dressing.Acta Derm Venereol.1992;72(1):69–71.
An 83‐year‐old female reported increased swelling of her legs over the past 2 years. She noted temperature intolerance, low energy, and constipation, but denied any hair loss or nail changes. On exam, she had marked bilateral lower extremity edema that was predominately nonpitting. Overlying the edema there were thickened, well‐defined plaques with a peau d'orange appearance surrounded by brown, thin plaques on the pretibial areas sparing the dorsum of the feet (Figure 1). A punch biopsy was obtained and demonstrated increased deposition of mucin throughout the dermis along with fragmentation and increased numbers of elastic fibers consistent with a diagnosis of pretibial myxedema. Measured thyrotropin level was elevated at 3.9 U/mL (normal, 0.3‐3.8 U/mL), consistent with hypothyroidism. This is a severe example of pretibial myxedema, or infiltrative dermopathy, which can occur, more commonly, in the setting of Graves' disease or, in rare circumstances, hypothyroidism.1 Myxedema results from the accumulation of hyaluronic acid and chondroitin sulfate in the dermis.2 Treatment is difficult and includes topical glucocorticoids under occlusion and, if indicated, thyroid corrective therapy.3

An 83‐year‐old female reported increased swelling of her legs over the past 2 years. She noted temperature intolerance, low energy, and constipation, but denied any hair loss or nail changes. On exam, she had marked bilateral lower extremity edema that was predominately nonpitting. Overlying the edema there were thickened, well‐defined plaques with a peau d'orange appearance surrounded by brown, thin plaques on the pretibial areas sparing the dorsum of the feet (Figure 1). A punch biopsy was obtained and demonstrated increased deposition of mucin throughout the dermis along with fragmentation and increased numbers of elastic fibers consistent with a diagnosis of pretibial myxedema. Measured thyrotropin level was elevated at 3.9 U/mL (normal, 0.3‐3.8 U/mL), consistent with hypothyroidism. This is a severe example of pretibial myxedema, or infiltrative dermopathy, which can occur, more commonly, in the setting of Graves' disease or, in rare circumstances, hypothyroidism.1 Myxedema results from the accumulation of hyaluronic acid and chondroitin sulfate in the dermis.2 Treatment is difficult and includes topical glucocorticoids under occlusion and, if indicated, thyroid corrective therapy.3

- .Pretibial myxedema.Dermatol Online J.7(1):18.
- ,,.Pretibial myxoedema: a manifestation of lymphoedema?Lancet.1993;341(8842):403–404.
- .Successful treatment of chronic skin diseases with clobetasol propionate and a hydrocolloid occlusive dressing.Acta Derm Venereol.1992;72(1):69–71.
- .Pretibial myxedema.Dermatol Online J.7(1):18.
- ,,.Pretibial myxoedema: a manifestation of lymphoedema?Lancet.1993;341(8842):403–404.
- .Successful treatment of chronic skin diseases with clobetasol propionate and a hydrocolloid occlusive dressing.Acta Derm Venereol.1992;72(1):69–71.
Early Prediction of Septic Shock
Severe sepsis is responsible for significant morbidity and mortality. In the United States, approximately 750,000 cases occur each year with an estimated mortality of 30% to 50%.1 Early goal‐directed therapy has been shown to decrease mortality in patients with severe sepsis and septic shock.2, 3 As a result, efforts have been focused toward providing early and aggressive intervention once sepsis has been established. In many cases this has been accomplished through the implementation of a protocol with guidelines for fluid management, antibiotic and vasopressor administration, and other interventions.410 Prior studies have demonstrated that care of hospitalized patients before intensive care unit (ICU) admission is often suboptimal,1113 and have suggested that patients with clear indicators of acute deterioration may go unrecognized on the ward. We previously reported the effects of implementing a hospital‐wide protocol for the management of severe sepsis,14 finding that although there was a significant reduction in overall mortality there was no difference for patients who developed severe sepsis on the hospital ward. This finding also suggests that the initial care of patients with severe sepsis on hospital wards may differ in intensity compared to emergency departments and ICUs. Failure on the part of the clinician to recognize the harbingers of impending sepsis before the onset of organ dysfunction or hypotension may contribute to a delay in aggressive therapy.
Previous efforts at early recognition of sepsis have relied on diagnostic studies or specific biomarkers to screen at‐risk patients. These have included such studies as messenger RNA (mRNA) expression,15 C‐reactive protein,16 procalcitonin in newborns,17 immunocompetence measures in burn patients,18 protein C concentration in neutropenic patients,19 and several immune markers (eg, tumor necrosis factor‐alpha, interleukin [IL]‐1 beta, IL‐6, IL‐8, and IL‐10).20 However, these biomarkers have been studied only in specific patient populations, require suspicion on the part of the clinician and the measurement of diagnostic or laboratory values that would otherwise not have been obtained. The ideal tool for predicting the onset of sepsis would be applicable to a broad patient population, not require specific suspicion on the part of the clinician, and use only routinely obtained clinical measurements and laboratory values.
Prediction models and scoring systems that use routine hemodynamic and laboratory values for several endpoints related to sepsis and septic shock have been developed. Many such tools are used to define severity of illness and predict outcome, while others have been developed to predict such events as bacteremia in patients presenting with fever,21 the probability of infection in the critically ill,22 and end‐organ dysfunction in severe sepsis.23 Little work has been done to develop such a model capable of predicting the onset of sepsis,24 and there have been no attempts to deploy a model as a large‐scale screening tool.
Our objective was to develop a simple algorithm that can be used in an automated fashion to screen hospitalized patients for impending septic shock. Such a model would be derived from routine hemodynamic and laboratory values, and take advantage of a computerized medical record system for data collection.
Patients and Methods
Patient Enrollment and Data Collection
This study was conducted at Barnes‐Jewish Hospital, St. Louis, MO, a university‐affiliated, urban teaching hospital. The study was approved by the Washington University (St. Louis, MO) School of Medicine Human Studies Committee. Patients included in the study where those hospitalized during 2005, 2006, and 2007, and who had at least 1 International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD9) discharge diagnosis code for the medical/nonsurgical diagnoses listed in Appendix 1. From this pool of patients, septic shock patients were identified as those who were admitted to the hospital ward and later developed septic shock requiring transfer to an ICU for vasopressor support and hemodynamic monitoring. This was accomplished by using discharge ICD9 codes for acute infection matched to codes for acute organ dysfunction and the need for vasopressors within 24 hours of ICU transfer (Appendix 1). The patients used as controls were then all those remaining in the pool once the septic shock patients were identified and separated.
Case patients were excluded from the analysis if they were transferred to the ICU within 2 hours of hospital admission, as these patients are unlikely to have an adequate amount of pretransfer clinical data available for analysis. Both case and control patients were excluded if they lacked any value for basic, routine laboratory data (serum sodium, chloride, total bicarbonate, urea nitrogen, creatinine, glucose, white blood cell count, neutrophil count, hemoglobin, hematocrit, and platelet count) and certain vital signs (blood pressure, heart rate, temperature). Patient data from 2005 were used in the derivation of the prediction model, and 2006 and 2007 patient data were used to prospectively validate the model. Clinical variables used in the analysis were selected based on both ease of access from the electronic medical record and clinical relevance, and are shown in Table 1.
|
| Age (years) |
| Albumin (g/dL) |
| Arterial blood gas (pH, PaCO2, PaO2) |
| Anion gap |
| Bilirubin (mg/dL) |
| BP, systolic and diastolic (mm of Hg) |
| Blood urea nitrogen (mg/dL) |
| Chloride (mmol/L) |
| Creatinine (mg/dL) |
| Glucose (mg/dL) |
| Hemoglobin (g/dL) |
| International normalized ratio |
| Neutrophil count, absolute (1 103/L) |
| Platelet count (1 103/L) |
| Pulse (beats/minute) |
| Pulse pressure (mm of Hg) |
| Shock index (pulse divided by systolic BP) |
| Sodium (mmol/L) |
| Total bicarbonate (mmol/L) |
| Temperature (degrees Celsius) |
| White blood cell count (1 103/L) |
In performing the Recursive Partitioning And Regression Tree (RPART) analysis to generate a prediction model, data for case patients were extracted in a window from 24 hours to 2 hours before ICU admission. The data collection window excluded the 2 hours prior to ICU transfer in order to minimize the effect of acute hemodynamic or laboratory changes that may have prompted the transfer; the purpose of the model is to identify hemodynamic and laboratory patterns in the several hours before the onset of clinically evident shock, so data from a time during which impending shock was clinically apparent were excluded. For the control patients, data from the first 48 hours of their hospitalization were included in the analysis.
Statistical Analysis
RPART analysis was performed on the 2005 patient data set to generate a prediction algorithm. This method of analysis results in a classification tree that contains a series of binary splits designed to separate patients into mutually exclusive subgroups.25 Each split in the tree is selected based on its ability to produce a partition with the greatest purity. Initially, a large tree that contains splits for all input variables is generated. This initial tree is generally too large to be useful as the final subgroups are too small to make sensible statistical inference.25 A pruning process is then applied to the initial tree with the goal of finding the subtree that is most predictive of the outcome of interest. The analysis was done using the RPART package of the R statistical analysis program, version 2.7.0 (R: A Language and Environment for Statistical Computing, R Development Core Team, Foundation for Statistical Computing, Vienna, Austria). The resulting classification tree was then used as a prediction algorithm and applied in a prospective fashion to the 2006 and 2007 patient data sets.
For the purpose of performing the RPART analysis, each set of case data entered into the analysis consisted of a random extraction of the desired clinical data within the specified extraction window from a single case patient. Thus, if a case patient had more than 1 value available for any variable of interest, 1 value was randomly selected to be entered in combination with the other available clinical data. Furthermore, in order to ensure that the majority of case patient data were included in the analysis, this process was iterated 10 times for each case patient. This resulted in 10 sets of case patient data being entered into the analysis for each case patient in the database, with each set containing a value for all variables of interest randomly extracted from those available for that patient. In addition to ensuring that the majority of case patient data were included, this technique also functionally expands the number of case patients present in the analysis. As there were far more control patients than case patients in the database, this in turn results in a classification tree that does not simply identify controls without regard to the relatively small number of case patients.
Data for the control patients entered into the analysis were extracted in a similar fashion, though only 1 set of data were included in the analysis for each control patient present in the database. As a result, only 1 randomly selected value per variable was included in the analysis.
Results
Patients
During 2005, 562 septic patients and 13,223 control patients were identified. For 2006 and 2007 there were 635 and 667 case patients, and 13,102 and 13,270 control patients, respectively.
Predictors of Sepsis
RPART analysis of the 2005 patient data set demonstrated that the most significant predictors of sepsis in the 24 hours preceding transfer to the medical ICU were the partial pressure of arterial oxygen (PaO2), systolic blood pressure, absolute neutrophil count, blood urea nitrogen (BUN), pH, bicarbonate, chloride, and albumin. This resulted in a simple algorithm with nine classification splits (Figure 1), which was then prospectively applied to the 2006 and 2007 patient data sets. These results are summarized in Table 2.
| Total Number | Number Correctly Classified (%) | Case Identification Time Before ICU Admission (minutes) | PPV (%) | NPV (%) | MCR (%) | |
|---|---|---|---|---|---|---|
| ||||||
| 2005 | 27.9 | 98.1 | 7.8 | |||
| Cases | 562 | 320 (56.9) | ||||
| Controls | 13,223 | 12,394 (93.7) | ||||
| 2006 | 179 230 | 28.7 | 97.7 | 8.4 | ||
| Cases | 635 | 347 (54.7) | ||||
| Controls | 13,102 | 12,241 (93.4) | ||||
| 2007 | 192 210 | 28.3 | 97.6 | 8.8 | ||
| Cases | 667 | 367 (55.0) | ||||
| Controls | 13,270 | 12,341 (93.0) | ||||
The resulting classification model had a low total misclassification rate for the 2005 data. Of the 562 septic patients, 320 (56.9%) were correctly classified, and 12,394 (93.7%) of the control patients were appropriately identified. The number of septic and control patients misclassified was 242 and 829, respectively, yielding a total misclassification rate of 7.8%. When applied to the 2006 patient data set, 347 (54.7%) of the 635 septic shock patients were correctly identified, while 12,241 (93.4%) of the 13,102 control patients were correctly classified. The total misclassification rate for the 2006 patient set was 8.4%. For the 2007 patient data, 367 (55.0%) of the 667 case patients were correctly identified, and 12,341 (93.0%) of the 13,270 control patients were correctly identified. This resulted in a total misclassification rate of 8.8%.
The 2006 and 2007 case patients were identified 179 230 minutes and 192 210 minutes before ICU transfer, respectively (Figure 2). The algorithm demonstrated positive and negative predictive values of 28.7% and 97.7% for the 2006 patient set, respectively, and 28.3% and 97.6% for the 2007 patient set, respectively.
Although the prediction algorithm shown in Figure 1 identified the majority of the case patients with ample time for clinical intervention prior to ICU transfer, the analysis used to derive this model included values for the arterial blood gas (ABG). As this is not a routinely obtained study for hospitalized patients outside of an ICU, it is possible that the performance of this model can in part be attributed to clinical acumen rather than changes in patient physiology. The ABG would likely only be obtained in patients with a more concerning or deteriorating clinical course, and thus more likely to develop shock. To address this possibility, a second analysis was performed that did not include the values for the ABG. The result was an algorithm with 13 classification splits, as shown in Figure 3.
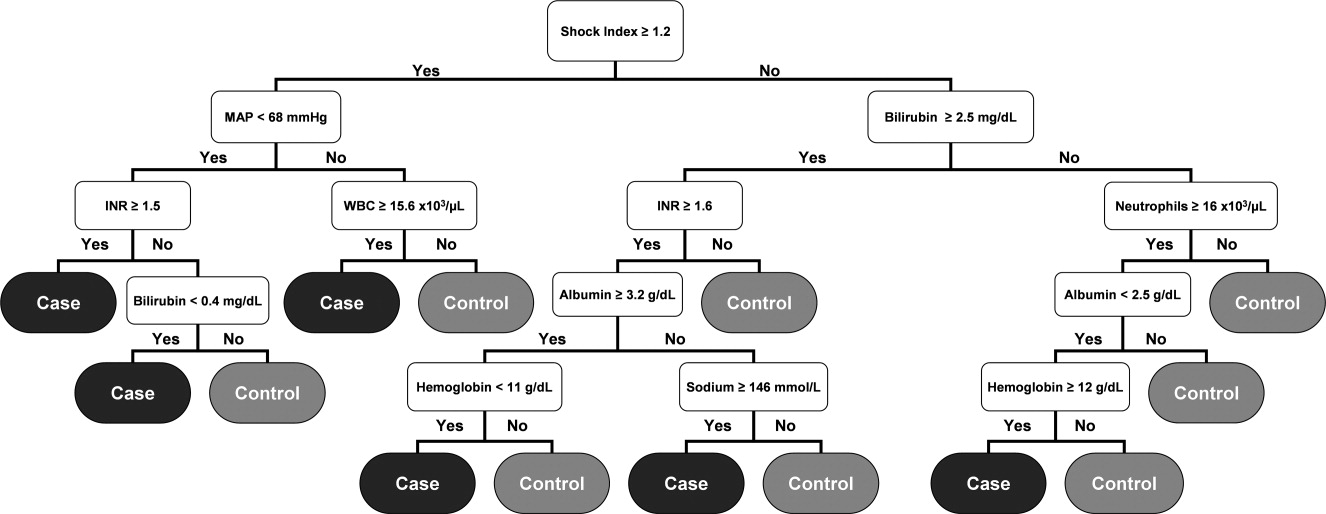
The most predictive clinical variables in this analysis included the shock index (heart rate divided by systolic blood pressure), mean arterial pressure, total bilirubin, international normalized ratio (INR), total white blood cell count, absolute neutrophil count, albumin, hemoglobin, and sodium. This model was again applied to the 2006 and 2007 patient data sets (Table 3).
| Total Number | Number Correctly Classified (%) | Case Identification Time Before ICU Admission (minutes) | PPV (%) | NPV (%) | MCR (%) | |
|---|---|---|---|---|---|---|
| ||||||
| 2005 | 20.5 | 96.7 | 6.7 | |||
| Cases | 562 | 126 (22.4) | ||||
| Controls | 13,223 | 12,735 (96.3) | ||||
| 2006 | 508 536 | 21.4 | 96.1 | 7.0 | ||
| Cases | 635 | 121 (19.1) | ||||
| Controls | 13,102 | 12,657 (96.6) | ||||
| 2007 | 496 512 | 19.5 | 95.8 | 7.1 | ||
| Cases | 667 | 102 (15.3) | ||||
| Controls | 13,270 | 12,850 (96.8) | ||||
The overall misclassification rates for 2006 and 2007 were 7.0% and 7.1%, respectively. The model correctly identified 121 (19.1%) of the 635 cases and 12,657 (96.6%) of the 13,102 control patients from 2006, and 102 (15.3%) of the 667 cases and 12,850 (96.8%) of the 13,270 control patients from 2007. The respective positive and negative predictive values were 21.4% and 96.1% for 2006, respectively, and 19.5% and 95.8% for 2007, respectively.
Although the overall performance of the model derived without the ABG data was not as good, the identification times prior to ICU transfer were significantly improved. For the 2006 data, patients were identified 508 536 minutes before transfer (Figure 4), compared to 179 230 minutes for the model that included the ABG data (P < 0.01). For the 2007 data, patients were identified 496 512 minutes prior to ICU admission (Figure 4), compared to 192 210 minutes for the previous model (P < 0.01).
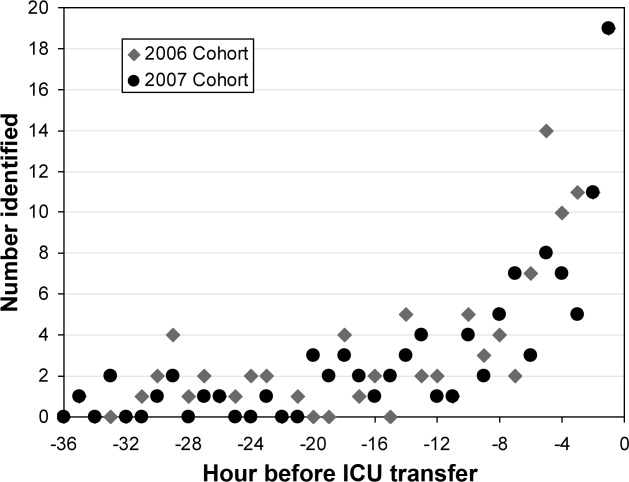
Discussion
We have demonstrated a simple method for generating an algorithm derived from routine laboratory and hemodynamic values that is capable of predicting the onset of sepsis in a significant proportion of non‐ICU patients. Two prediction models were generated, 1 with and 1 without ABG data included in the analysis. In the 2006 and 2007 validation cohorts, the model including these data correctly classified 54.7% and 55.0% of the patients who developed septic shock and 93.4% and 93.0% of control patients, respectively. The second model identified 19.1% and 15.3% of the septic shock patients and 96.6% and 96.8% of the control patients for 2006 and 2007, respectively. The methods used in generating this model are relatively simple and can be executed with the use of an electronic medical record system.
Early, goal‐directed cardiovascular resuscitation and adequate initial antibiotic therapy have been shown to decrease mortality in patients with severe sepsis and septic shock.2, 26 Prior studies employing early, targeted resuscitation strategies have demonstrated decreased use of vasopressors10 and decreased mortality.510 In addition, we previously demonstrated that a standardized order set for the management of severe sepsis in the emergency department that focused on early and aggressive intervention was associated with decreased 28‐day mortality.1 These studies suggest that early, aggressive management of septic shock can improve outcomes. Identification of patients prior to overt clinical deterioration may allow for early intervention aimed at preventing shock or improving its outcome.
The purpose of this method is to develop a model capable of recognizing patterns in clinical data that herald a patient's otherwise unidentified clinical deterioration. It is not intended to replace existing outcome prediction tools or severity of illness scoring systems, where a high degree of accuracy would be required. Rather, it would be best implemented as an automated screening tool incorporated into an electronic medical record system. When a hospitalized patient is identified as a possible septic shock patient by the classification tree, a notification is then issued to the clinicians caring for the patient. The primary goal of this method is to notify clinicians of potential clinical deterioration. Any action taken as a result of this notification is at the discretion of the clinician. This method could be employed for any population of hospitalized patients, though because of variations in clinical practice and patient physiology, different models would need to be generated for differing patient populations.
This method has limitations, the foremost of which is the possible instability of the resulting classification model. This type of analysis results in an algorithm that depends on binary splits to classify patients. In generating the algorithm, the recursive partitioning analysis selects the variables and cutoff values that result in the strongest decision tree with the most pure classifications at the end nodes. These variables and cutoff values may not immediately seem logical from a clinical standpoint, and may vary with changes in practice and even possibly between divisions within a hospital. As a result, the algorithm would likely require intermittent updating to remain effective and a model derived from 1 hospital or patient population would not necessarily be applicable to patients at another institution or from a different population. However, once the method has been developed at an institution, the process of revising the algorithm could be essentially automated and uses few resources.
Another shortcoming of this method is the relatively low sensitivity of the resulting algorithm. In a role as an automated alert system, a low false‐positive rate is particularly desirable to avoid unnecessary frequent distraction of clinicians. The sensitivity of the model can be improved through manipulation of how the analysis is performed, but this would be at the expense of a higher false‐positive rate, which is not acceptable. Finally, prior studies examining treatment for sepsis have demonstrated an advantage to early and aggressive therapy. It is not clear, however, if identifying these patients prior to the onset of clinically evident sepsis would result in improved outcomes. Further work is required to determine if this is the case. We are currently conducting a prospective study that employs the method described here in conjunction with an automated alert system to ascertain if it impacts outcomes on patients admitted to the medicine wards of Barnes‐Jewish Hospital.
In conclusion, the method presented here represents a technique that consumes few resources and is capable of identifying some patients before septic shock becomes clinically evident. When applied in an automated fashion with the capability to alert clinicians caring for a patient, the method demonstrated here may allow for earlier diagnosis and possibly intervention for septic shock patients.
- ,,, et al.Before–after study of a standardized hospital order set for the management of septic shock.Crit Care Med.2006;34(11):2707–2713.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Early goal‐directed therapy in severe sepsis and septic shock revisited; concepts, controversies, and contemporary findings.Chest.2006;130(5):1579–1595.
- .Implementing the severe sepsis care bundles outside the ICU by outreach.Nurs Crit Care.2007;12:225–229.
- ,,, et al.The impact of compliance with 6‐hour and 24‐hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study.Crit Care.2005;9:R764–R770.
- ,,, et al.Translating research to clinical practice, a 1‐year experience with implementing early goal‐directed therapy for septic shock in the emergency department.Chest.2006;129:225–232.
- ,,, et al.Prospective external validation of the clinical effectiveness of an emergency department‐based early goal‐directed therapy protocol for severe sepsis and septic shock.Chest.2007;132:425–432.
- ,,, et al.Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality.Crit Care Med.2007;35:1105–1112.
- ,,, et al.Implementation of an evidence‐based “standard operating procedure” and outcome in septic shock.Crit Care Med.2006;34:943–949.
- ,,, et al.Outcome of septic shock in older adults after implementation of the sepsis “bundle”.J Am Geriatr Soc.2008;56:272–278.
- ,,, et al.Confidential inquiry into quality of care before admission to intensive care.BMJ.1998;316:1853–1858.
- ,,.Unexpected deaths and referrals to intensive care of patients on general wards. Are some cases potentially avoidable?J R Coll Physicians Lond.1999;33(3):255–259.
- ,,, et al.Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units.Crit Care Med.1998;26(6):1020–1024.
- ,,,,,.Hospital‐wide impact of a standardized order set for the management of bacteremic severe sepsis.Crit Care Med.2009;37(3):819–824.
- ,,,,,.The mRNA expression of fatty acid amide hydrolase in human whole blood correlates with sepsis.J Endotoxin Res.2007;13(1):35–38.
- ,,, et al.C‐reactive protein use as an early indicator of infection in patients with systemic inflammatory response syndrome.Intensive Care Med.2004;30(11):2038–2045.
- ,,,,.Procalcitonin as a screening test of late‐onset sepsis in preterm very low birth weight infants.J Perinatol.2005;25(6):397–402.
- ,,.A simple method for predicting severe sepsis in burn patients.Am J Surg.1980;139(4):513–517.
- ,,, et al.Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications.Crit Care Med.2000;28(7):2209–2216.
- ,,,,.Circulating immune parameters predicting the progression from hospital‐acquired pneumonia to septic shock in surgical patients.Crit Care Med.2005;9(6):R662–R669.
- ,,.A simple prediction algorithm for bacteremia in patients with acute febrile illness.Q J Med.2005;98:813–820.
- ,,,.Infection probability score (IPS): a method to help assess the probability of infection in critically ill patients.Crit Care Med.2003;31(11):2579–2584.
- ,.Multivariate regression modeling for the prediction of inflammation, systemic pressure, and end‐organ function in severe sepsis.Shock.1997;8(3):225–231.
- ,,,,,.Abnormal heart rate characteristics preceding neonatal sepsis and sepsis‐like illness.Pediatr Res.2003;53:920–926.
- ,.Statistics for Biology and Health.New York:Springer‐Verlag;1999.
- ,,, et al.Impact of adequate empiric antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
Severe sepsis is responsible for significant morbidity and mortality. In the United States, approximately 750,000 cases occur each year with an estimated mortality of 30% to 50%.1 Early goal‐directed therapy has been shown to decrease mortality in patients with severe sepsis and septic shock.2, 3 As a result, efforts have been focused toward providing early and aggressive intervention once sepsis has been established. In many cases this has been accomplished through the implementation of a protocol with guidelines for fluid management, antibiotic and vasopressor administration, and other interventions.410 Prior studies have demonstrated that care of hospitalized patients before intensive care unit (ICU) admission is often suboptimal,1113 and have suggested that patients with clear indicators of acute deterioration may go unrecognized on the ward. We previously reported the effects of implementing a hospital‐wide protocol for the management of severe sepsis,14 finding that although there was a significant reduction in overall mortality there was no difference for patients who developed severe sepsis on the hospital ward. This finding also suggests that the initial care of patients with severe sepsis on hospital wards may differ in intensity compared to emergency departments and ICUs. Failure on the part of the clinician to recognize the harbingers of impending sepsis before the onset of organ dysfunction or hypotension may contribute to a delay in aggressive therapy.
Previous efforts at early recognition of sepsis have relied on diagnostic studies or specific biomarkers to screen at‐risk patients. These have included such studies as messenger RNA (mRNA) expression,15 C‐reactive protein,16 procalcitonin in newborns,17 immunocompetence measures in burn patients,18 protein C concentration in neutropenic patients,19 and several immune markers (eg, tumor necrosis factor‐alpha, interleukin [IL]‐1 beta, IL‐6, IL‐8, and IL‐10).20 However, these biomarkers have been studied only in specific patient populations, require suspicion on the part of the clinician and the measurement of diagnostic or laboratory values that would otherwise not have been obtained. The ideal tool for predicting the onset of sepsis would be applicable to a broad patient population, not require specific suspicion on the part of the clinician, and use only routinely obtained clinical measurements and laboratory values.
Prediction models and scoring systems that use routine hemodynamic and laboratory values for several endpoints related to sepsis and septic shock have been developed. Many such tools are used to define severity of illness and predict outcome, while others have been developed to predict such events as bacteremia in patients presenting with fever,21 the probability of infection in the critically ill,22 and end‐organ dysfunction in severe sepsis.23 Little work has been done to develop such a model capable of predicting the onset of sepsis,24 and there have been no attempts to deploy a model as a large‐scale screening tool.
Our objective was to develop a simple algorithm that can be used in an automated fashion to screen hospitalized patients for impending septic shock. Such a model would be derived from routine hemodynamic and laboratory values, and take advantage of a computerized medical record system for data collection.
Patients and Methods
Patient Enrollment and Data Collection
This study was conducted at Barnes‐Jewish Hospital, St. Louis, MO, a university‐affiliated, urban teaching hospital. The study was approved by the Washington University (St. Louis, MO) School of Medicine Human Studies Committee. Patients included in the study where those hospitalized during 2005, 2006, and 2007, and who had at least 1 International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD9) discharge diagnosis code for the medical/nonsurgical diagnoses listed in Appendix 1. From this pool of patients, septic shock patients were identified as those who were admitted to the hospital ward and later developed septic shock requiring transfer to an ICU for vasopressor support and hemodynamic monitoring. This was accomplished by using discharge ICD9 codes for acute infection matched to codes for acute organ dysfunction and the need for vasopressors within 24 hours of ICU transfer (Appendix 1). The patients used as controls were then all those remaining in the pool once the septic shock patients were identified and separated.
Case patients were excluded from the analysis if they were transferred to the ICU within 2 hours of hospital admission, as these patients are unlikely to have an adequate amount of pretransfer clinical data available for analysis. Both case and control patients were excluded if they lacked any value for basic, routine laboratory data (serum sodium, chloride, total bicarbonate, urea nitrogen, creatinine, glucose, white blood cell count, neutrophil count, hemoglobin, hematocrit, and platelet count) and certain vital signs (blood pressure, heart rate, temperature). Patient data from 2005 were used in the derivation of the prediction model, and 2006 and 2007 patient data were used to prospectively validate the model. Clinical variables used in the analysis were selected based on both ease of access from the electronic medical record and clinical relevance, and are shown in Table 1.
|
| Age (years) |
| Albumin (g/dL) |
| Arterial blood gas (pH, PaCO2, PaO2) |
| Anion gap |
| Bilirubin (mg/dL) |
| BP, systolic and diastolic (mm of Hg) |
| Blood urea nitrogen (mg/dL) |
| Chloride (mmol/L) |
| Creatinine (mg/dL) |
| Glucose (mg/dL) |
| Hemoglobin (g/dL) |
| International normalized ratio |
| Neutrophil count, absolute (1 103/L) |
| Platelet count (1 103/L) |
| Pulse (beats/minute) |
| Pulse pressure (mm of Hg) |
| Shock index (pulse divided by systolic BP) |
| Sodium (mmol/L) |
| Total bicarbonate (mmol/L) |
| Temperature (degrees Celsius) |
| White blood cell count (1 103/L) |
In performing the Recursive Partitioning And Regression Tree (RPART) analysis to generate a prediction model, data for case patients were extracted in a window from 24 hours to 2 hours before ICU admission. The data collection window excluded the 2 hours prior to ICU transfer in order to minimize the effect of acute hemodynamic or laboratory changes that may have prompted the transfer; the purpose of the model is to identify hemodynamic and laboratory patterns in the several hours before the onset of clinically evident shock, so data from a time during which impending shock was clinically apparent were excluded. For the control patients, data from the first 48 hours of their hospitalization were included in the analysis.
Statistical Analysis
RPART analysis was performed on the 2005 patient data set to generate a prediction algorithm. This method of analysis results in a classification tree that contains a series of binary splits designed to separate patients into mutually exclusive subgroups.25 Each split in the tree is selected based on its ability to produce a partition with the greatest purity. Initially, a large tree that contains splits for all input variables is generated. This initial tree is generally too large to be useful as the final subgroups are too small to make sensible statistical inference.25 A pruning process is then applied to the initial tree with the goal of finding the subtree that is most predictive of the outcome of interest. The analysis was done using the RPART package of the R statistical analysis program, version 2.7.0 (R: A Language and Environment for Statistical Computing, R Development Core Team, Foundation for Statistical Computing, Vienna, Austria). The resulting classification tree was then used as a prediction algorithm and applied in a prospective fashion to the 2006 and 2007 patient data sets.
For the purpose of performing the RPART analysis, each set of case data entered into the analysis consisted of a random extraction of the desired clinical data within the specified extraction window from a single case patient. Thus, if a case patient had more than 1 value available for any variable of interest, 1 value was randomly selected to be entered in combination with the other available clinical data. Furthermore, in order to ensure that the majority of case patient data were included in the analysis, this process was iterated 10 times for each case patient. This resulted in 10 sets of case patient data being entered into the analysis for each case patient in the database, with each set containing a value for all variables of interest randomly extracted from those available for that patient. In addition to ensuring that the majority of case patient data were included, this technique also functionally expands the number of case patients present in the analysis. As there were far more control patients than case patients in the database, this in turn results in a classification tree that does not simply identify controls without regard to the relatively small number of case patients.
Data for the control patients entered into the analysis were extracted in a similar fashion, though only 1 set of data were included in the analysis for each control patient present in the database. As a result, only 1 randomly selected value per variable was included in the analysis.
Results
Patients
During 2005, 562 septic patients and 13,223 control patients were identified. For 2006 and 2007 there were 635 and 667 case patients, and 13,102 and 13,270 control patients, respectively.
Predictors of Sepsis
RPART analysis of the 2005 patient data set demonstrated that the most significant predictors of sepsis in the 24 hours preceding transfer to the medical ICU were the partial pressure of arterial oxygen (PaO2), systolic blood pressure, absolute neutrophil count, blood urea nitrogen (BUN), pH, bicarbonate, chloride, and albumin. This resulted in a simple algorithm with nine classification splits (Figure 1), which was then prospectively applied to the 2006 and 2007 patient data sets. These results are summarized in Table 2.

| Total Number | Number Correctly Classified (%) | Case Identification Time Before ICU Admission (minutes) | PPV (%) | NPV (%) | MCR (%) | |
|---|---|---|---|---|---|---|
| ||||||
| 2005 | 27.9 | 98.1 | 7.8 | |||
| Cases | 562 | 320 (56.9) | ||||
| Controls | 13,223 | 12,394 (93.7) | ||||
| 2006 | 179 230 | 28.7 | 97.7 | 8.4 | ||
| Cases | 635 | 347 (54.7) | ||||
| Controls | 13,102 | 12,241 (93.4) | ||||
| 2007 | 192 210 | 28.3 | 97.6 | 8.8 | ||
| Cases | 667 | 367 (55.0) | ||||
| Controls | 13,270 | 12,341 (93.0) | ||||
The resulting classification model had a low total misclassification rate for the 2005 data. Of the 562 septic patients, 320 (56.9%) were correctly classified, and 12,394 (93.7%) of the control patients were appropriately identified. The number of septic and control patients misclassified was 242 and 829, respectively, yielding a total misclassification rate of 7.8%. When applied to the 2006 patient data set, 347 (54.7%) of the 635 septic shock patients were correctly identified, while 12,241 (93.4%) of the 13,102 control patients were correctly classified. The total misclassification rate for the 2006 patient set was 8.4%. For the 2007 patient data, 367 (55.0%) of the 667 case patients were correctly identified, and 12,341 (93.0%) of the 13,270 control patients were correctly identified. This resulted in a total misclassification rate of 8.8%.
The 2006 and 2007 case patients were identified 179 230 minutes and 192 210 minutes before ICU transfer, respectively (Figure 2). The algorithm demonstrated positive and negative predictive values of 28.7% and 97.7% for the 2006 patient set, respectively, and 28.3% and 97.6% for the 2007 patient set, respectively.

Although the prediction algorithm shown in Figure 1 identified the majority of the case patients with ample time for clinical intervention prior to ICU transfer, the analysis used to derive this model included values for the arterial blood gas (ABG). As this is not a routinely obtained study for hospitalized patients outside of an ICU, it is possible that the performance of this model can in part be attributed to clinical acumen rather than changes in patient physiology. The ABG would likely only be obtained in patients with a more concerning or deteriorating clinical course, and thus more likely to develop shock. To address this possibility, a second analysis was performed that did not include the values for the ABG. The result was an algorithm with 13 classification splits, as shown in Figure 3.

The most predictive clinical variables in this analysis included the shock index (heart rate divided by systolic blood pressure), mean arterial pressure, total bilirubin, international normalized ratio (INR), total white blood cell count, absolute neutrophil count, albumin, hemoglobin, and sodium. This model was again applied to the 2006 and 2007 patient data sets (Table 3).
| Total Number | Number Correctly Classified (%) | Case Identification Time Before ICU Admission (minutes) | PPV (%) | NPV (%) | MCR (%) | |
|---|---|---|---|---|---|---|
| ||||||
| 2005 | 20.5 | 96.7 | 6.7 | |||
| Cases | 562 | 126 (22.4) | ||||
| Controls | 13,223 | 12,735 (96.3) | ||||
| 2006 | 508 536 | 21.4 | 96.1 | 7.0 | ||
| Cases | 635 | 121 (19.1) | ||||
| Controls | 13,102 | 12,657 (96.6) | ||||
| 2007 | 496 512 | 19.5 | 95.8 | 7.1 | ||
| Cases | 667 | 102 (15.3) | ||||
| Controls | 13,270 | 12,850 (96.8) | ||||
The overall misclassification rates for 2006 and 2007 were 7.0% and 7.1%, respectively. The model correctly identified 121 (19.1%) of the 635 cases and 12,657 (96.6%) of the 13,102 control patients from 2006, and 102 (15.3%) of the 667 cases and 12,850 (96.8%) of the 13,270 control patients from 2007. The respective positive and negative predictive values were 21.4% and 96.1% for 2006, respectively, and 19.5% and 95.8% for 2007, respectively.
Although the overall performance of the model derived without the ABG data was not as good, the identification times prior to ICU transfer were significantly improved. For the 2006 data, patients were identified 508 536 minutes before transfer (Figure 4), compared to 179 230 minutes for the model that included the ABG data (P < 0.01). For the 2007 data, patients were identified 496 512 minutes prior to ICU admission (Figure 4), compared to 192 210 minutes for the previous model (P < 0.01).

Discussion
We have demonstrated a simple method for generating an algorithm derived from routine laboratory and hemodynamic values that is capable of predicting the onset of sepsis in a significant proportion of non‐ICU patients. Two prediction models were generated, 1 with and 1 without ABG data included in the analysis. In the 2006 and 2007 validation cohorts, the model including these data correctly classified 54.7% and 55.0% of the patients who developed septic shock and 93.4% and 93.0% of control patients, respectively. The second model identified 19.1% and 15.3% of the septic shock patients and 96.6% and 96.8% of the control patients for 2006 and 2007, respectively. The methods used in generating this model are relatively simple and can be executed with the use of an electronic medical record system.
Early, goal‐directed cardiovascular resuscitation and adequate initial antibiotic therapy have been shown to decrease mortality in patients with severe sepsis and septic shock.2, 26 Prior studies employing early, targeted resuscitation strategies have demonstrated decreased use of vasopressors10 and decreased mortality.510 In addition, we previously demonstrated that a standardized order set for the management of severe sepsis in the emergency department that focused on early and aggressive intervention was associated with decreased 28‐day mortality.1 These studies suggest that early, aggressive management of septic shock can improve outcomes. Identification of patients prior to overt clinical deterioration may allow for early intervention aimed at preventing shock or improving its outcome.
The purpose of this method is to develop a model capable of recognizing patterns in clinical data that herald a patient's otherwise unidentified clinical deterioration. It is not intended to replace existing outcome prediction tools or severity of illness scoring systems, where a high degree of accuracy would be required. Rather, it would be best implemented as an automated screening tool incorporated into an electronic medical record system. When a hospitalized patient is identified as a possible septic shock patient by the classification tree, a notification is then issued to the clinicians caring for the patient. The primary goal of this method is to notify clinicians of potential clinical deterioration. Any action taken as a result of this notification is at the discretion of the clinician. This method could be employed for any population of hospitalized patients, though because of variations in clinical practice and patient physiology, different models would need to be generated for differing patient populations.
This method has limitations, the foremost of which is the possible instability of the resulting classification model. This type of analysis results in an algorithm that depends on binary splits to classify patients. In generating the algorithm, the recursive partitioning analysis selects the variables and cutoff values that result in the strongest decision tree with the most pure classifications at the end nodes. These variables and cutoff values may not immediately seem logical from a clinical standpoint, and may vary with changes in practice and even possibly between divisions within a hospital. As a result, the algorithm would likely require intermittent updating to remain effective and a model derived from 1 hospital or patient population would not necessarily be applicable to patients at another institution or from a different population. However, once the method has been developed at an institution, the process of revising the algorithm could be essentially automated and uses few resources.
Another shortcoming of this method is the relatively low sensitivity of the resulting algorithm. In a role as an automated alert system, a low false‐positive rate is particularly desirable to avoid unnecessary frequent distraction of clinicians. The sensitivity of the model can be improved through manipulation of how the analysis is performed, but this would be at the expense of a higher false‐positive rate, which is not acceptable. Finally, prior studies examining treatment for sepsis have demonstrated an advantage to early and aggressive therapy. It is not clear, however, if identifying these patients prior to the onset of clinically evident sepsis would result in improved outcomes. Further work is required to determine if this is the case. We are currently conducting a prospective study that employs the method described here in conjunction with an automated alert system to ascertain if it impacts outcomes on patients admitted to the medicine wards of Barnes‐Jewish Hospital.
In conclusion, the method presented here represents a technique that consumes few resources and is capable of identifying some patients before septic shock becomes clinically evident. When applied in an automated fashion with the capability to alert clinicians caring for a patient, the method demonstrated here may allow for earlier diagnosis and possibly intervention for septic shock patients.
Severe sepsis is responsible for significant morbidity and mortality. In the United States, approximately 750,000 cases occur each year with an estimated mortality of 30% to 50%.1 Early goal‐directed therapy has been shown to decrease mortality in patients with severe sepsis and septic shock.2, 3 As a result, efforts have been focused toward providing early and aggressive intervention once sepsis has been established. In many cases this has been accomplished through the implementation of a protocol with guidelines for fluid management, antibiotic and vasopressor administration, and other interventions.410 Prior studies have demonstrated that care of hospitalized patients before intensive care unit (ICU) admission is often suboptimal,1113 and have suggested that patients with clear indicators of acute deterioration may go unrecognized on the ward. We previously reported the effects of implementing a hospital‐wide protocol for the management of severe sepsis,14 finding that although there was a significant reduction in overall mortality there was no difference for patients who developed severe sepsis on the hospital ward. This finding also suggests that the initial care of patients with severe sepsis on hospital wards may differ in intensity compared to emergency departments and ICUs. Failure on the part of the clinician to recognize the harbingers of impending sepsis before the onset of organ dysfunction or hypotension may contribute to a delay in aggressive therapy.
Previous efforts at early recognition of sepsis have relied on diagnostic studies or specific biomarkers to screen at‐risk patients. These have included such studies as messenger RNA (mRNA) expression,15 C‐reactive protein,16 procalcitonin in newborns,17 immunocompetence measures in burn patients,18 protein C concentration in neutropenic patients,19 and several immune markers (eg, tumor necrosis factor‐alpha, interleukin [IL]‐1 beta, IL‐6, IL‐8, and IL‐10).20 However, these biomarkers have been studied only in specific patient populations, require suspicion on the part of the clinician and the measurement of diagnostic or laboratory values that would otherwise not have been obtained. The ideal tool for predicting the onset of sepsis would be applicable to a broad patient population, not require specific suspicion on the part of the clinician, and use only routinely obtained clinical measurements and laboratory values.
Prediction models and scoring systems that use routine hemodynamic and laboratory values for several endpoints related to sepsis and septic shock have been developed. Many such tools are used to define severity of illness and predict outcome, while others have been developed to predict such events as bacteremia in patients presenting with fever,21 the probability of infection in the critically ill,22 and end‐organ dysfunction in severe sepsis.23 Little work has been done to develop such a model capable of predicting the onset of sepsis,24 and there have been no attempts to deploy a model as a large‐scale screening tool.
Our objective was to develop a simple algorithm that can be used in an automated fashion to screen hospitalized patients for impending septic shock. Such a model would be derived from routine hemodynamic and laboratory values, and take advantage of a computerized medical record system for data collection.
Patients and Methods
Patient Enrollment and Data Collection
This study was conducted at Barnes‐Jewish Hospital, St. Louis, MO, a university‐affiliated, urban teaching hospital. The study was approved by the Washington University (St. Louis, MO) School of Medicine Human Studies Committee. Patients included in the study where those hospitalized during 2005, 2006, and 2007, and who had at least 1 International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD9) discharge diagnosis code for the medical/nonsurgical diagnoses listed in Appendix 1. From this pool of patients, septic shock patients were identified as those who were admitted to the hospital ward and later developed septic shock requiring transfer to an ICU for vasopressor support and hemodynamic monitoring. This was accomplished by using discharge ICD9 codes for acute infection matched to codes for acute organ dysfunction and the need for vasopressors within 24 hours of ICU transfer (Appendix 1). The patients used as controls were then all those remaining in the pool once the septic shock patients were identified and separated.
Case patients were excluded from the analysis if they were transferred to the ICU within 2 hours of hospital admission, as these patients are unlikely to have an adequate amount of pretransfer clinical data available for analysis. Both case and control patients were excluded if they lacked any value for basic, routine laboratory data (serum sodium, chloride, total bicarbonate, urea nitrogen, creatinine, glucose, white blood cell count, neutrophil count, hemoglobin, hematocrit, and platelet count) and certain vital signs (blood pressure, heart rate, temperature). Patient data from 2005 were used in the derivation of the prediction model, and 2006 and 2007 patient data were used to prospectively validate the model. Clinical variables used in the analysis were selected based on both ease of access from the electronic medical record and clinical relevance, and are shown in Table 1.
|
| Age (years) |
| Albumin (g/dL) |
| Arterial blood gas (pH, PaCO2, PaO2) |
| Anion gap |
| Bilirubin (mg/dL) |
| BP, systolic and diastolic (mm of Hg) |
| Blood urea nitrogen (mg/dL) |
| Chloride (mmol/L) |
| Creatinine (mg/dL) |
| Glucose (mg/dL) |
| Hemoglobin (g/dL) |
| International normalized ratio |
| Neutrophil count, absolute (1 103/L) |
| Platelet count (1 103/L) |
| Pulse (beats/minute) |
| Pulse pressure (mm of Hg) |
| Shock index (pulse divided by systolic BP) |
| Sodium (mmol/L) |
| Total bicarbonate (mmol/L) |
| Temperature (degrees Celsius) |
| White blood cell count (1 103/L) |
In performing the Recursive Partitioning And Regression Tree (RPART) analysis to generate a prediction model, data for case patients were extracted in a window from 24 hours to 2 hours before ICU admission. The data collection window excluded the 2 hours prior to ICU transfer in order to minimize the effect of acute hemodynamic or laboratory changes that may have prompted the transfer; the purpose of the model is to identify hemodynamic and laboratory patterns in the several hours before the onset of clinically evident shock, so data from a time during which impending shock was clinically apparent were excluded. For the control patients, data from the first 48 hours of their hospitalization were included in the analysis.
Statistical Analysis
RPART analysis was performed on the 2005 patient data set to generate a prediction algorithm. This method of analysis results in a classification tree that contains a series of binary splits designed to separate patients into mutually exclusive subgroups.25 Each split in the tree is selected based on its ability to produce a partition with the greatest purity. Initially, a large tree that contains splits for all input variables is generated. This initial tree is generally too large to be useful as the final subgroups are too small to make sensible statistical inference.25 A pruning process is then applied to the initial tree with the goal of finding the subtree that is most predictive of the outcome of interest. The analysis was done using the RPART package of the R statistical analysis program, version 2.7.0 (R: A Language and Environment for Statistical Computing, R Development Core Team, Foundation for Statistical Computing, Vienna, Austria). The resulting classification tree was then used as a prediction algorithm and applied in a prospective fashion to the 2006 and 2007 patient data sets.
For the purpose of performing the RPART analysis, each set of case data entered into the analysis consisted of a random extraction of the desired clinical data within the specified extraction window from a single case patient. Thus, if a case patient had more than 1 value available for any variable of interest, 1 value was randomly selected to be entered in combination with the other available clinical data. Furthermore, in order to ensure that the majority of case patient data were included in the analysis, this process was iterated 10 times for each case patient. This resulted in 10 sets of case patient data being entered into the analysis for each case patient in the database, with each set containing a value for all variables of interest randomly extracted from those available for that patient. In addition to ensuring that the majority of case patient data were included, this technique also functionally expands the number of case patients present in the analysis. As there were far more control patients than case patients in the database, this in turn results in a classification tree that does not simply identify controls without regard to the relatively small number of case patients.
Data for the control patients entered into the analysis were extracted in a similar fashion, though only 1 set of data were included in the analysis for each control patient present in the database. As a result, only 1 randomly selected value per variable was included in the analysis.
Results
Patients
During 2005, 562 septic patients and 13,223 control patients were identified. For 2006 and 2007 there were 635 and 667 case patients, and 13,102 and 13,270 control patients, respectively.
Predictors of Sepsis
RPART analysis of the 2005 patient data set demonstrated that the most significant predictors of sepsis in the 24 hours preceding transfer to the medical ICU were the partial pressure of arterial oxygen (PaO2), systolic blood pressure, absolute neutrophil count, blood urea nitrogen (BUN), pH, bicarbonate, chloride, and albumin. This resulted in a simple algorithm with nine classification splits (Figure 1), which was then prospectively applied to the 2006 and 2007 patient data sets. These results are summarized in Table 2.

| Total Number | Number Correctly Classified (%) | Case Identification Time Before ICU Admission (minutes) | PPV (%) | NPV (%) | MCR (%) | |
|---|---|---|---|---|---|---|
| ||||||
| 2005 | 27.9 | 98.1 | 7.8 | |||
| Cases | 562 | 320 (56.9) | ||||
| Controls | 13,223 | 12,394 (93.7) | ||||
| 2006 | 179 230 | 28.7 | 97.7 | 8.4 | ||
| Cases | 635 | 347 (54.7) | ||||
| Controls | 13,102 | 12,241 (93.4) | ||||
| 2007 | 192 210 | 28.3 | 97.6 | 8.8 | ||
| Cases | 667 | 367 (55.0) | ||||
| Controls | 13,270 | 12,341 (93.0) | ||||
The resulting classification model had a low total misclassification rate for the 2005 data. Of the 562 septic patients, 320 (56.9%) were correctly classified, and 12,394 (93.7%) of the control patients were appropriately identified. The number of septic and control patients misclassified was 242 and 829, respectively, yielding a total misclassification rate of 7.8%. When applied to the 2006 patient data set, 347 (54.7%) of the 635 septic shock patients were correctly identified, while 12,241 (93.4%) of the 13,102 control patients were correctly classified. The total misclassification rate for the 2006 patient set was 8.4%. For the 2007 patient data, 367 (55.0%) of the 667 case patients were correctly identified, and 12,341 (93.0%) of the 13,270 control patients were correctly identified. This resulted in a total misclassification rate of 8.8%.
The 2006 and 2007 case patients were identified 179 230 minutes and 192 210 minutes before ICU transfer, respectively (Figure 2). The algorithm demonstrated positive and negative predictive values of 28.7% and 97.7% for the 2006 patient set, respectively, and 28.3% and 97.6% for the 2007 patient set, respectively.

Although the prediction algorithm shown in Figure 1 identified the majority of the case patients with ample time for clinical intervention prior to ICU transfer, the analysis used to derive this model included values for the arterial blood gas (ABG). As this is not a routinely obtained study for hospitalized patients outside of an ICU, it is possible that the performance of this model can in part be attributed to clinical acumen rather than changes in patient physiology. The ABG would likely only be obtained in patients with a more concerning or deteriorating clinical course, and thus more likely to develop shock. To address this possibility, a second analysis was performed that did not include the values for the ABG. The result was an algorithm with 13 classification splits, as shown in Figure 3.

The most predictive clinical variables in this analysis included the shock index (heart rate divided by systolic blood pressure), mean arterial pressure, total bilirubin, international normalized ratio (INR), total white blood cell count, absolute neutrophil count, albumin, hemoglobin, and sodium. This model was again applied to the 2006 and 2007 patient data sets (Table 3).
| Total Number | Number Correctly Classified (%) | Case Identification Time Before ICU Admission (minutes) | PPV (%) | NPV (%) | MCR (%) | |
|---|---|---|---|---|---|---|
| ||||||
| 2005 | 20.5 | 96.7 | 6.7 | |||
| Cases | 562 | 126 (22.4) | ||||
| Controls | 13,223 | 12,735 (96.3) | ||||
| 2006 | 508 536 | 21.4 | 96.1 | 7.0 | ||
| Cases | 635 | 121 (19.1) | ||||
| Controls | 13,102 | 12,657 (96.6) | ||||
| 2007 | 496 512 | 19.5 | 95.8 | 7.1 | ||
| Cases | 667 | 102 (15.3) | ||||
| Controls | 13,270 | 12,850 (96.8) | ||||
The overall misclassification rates for 2006 and 2007 were 7.0% and 7.1%, respectively. The model correctly identified 121 (19.1%) of the 635 cases and 12,657 (96.6%) of the 13,102 control patients from 2006, and 102 (15.3%) of the 667 cases and 12,850 (96.8%) of the 13,270 control patients from 2007. The respective positive and negative predictive values were 21.4% and 96.1% for 2006, respectively, and 19.5% and 95.8% for 2007, respectively.
Although the overall performance of the model derived without the ABG data was not as good, the identification times prior to ICU transfer were significantly improved. For the 2006 data, patients were identified 508 536 minutes before transfer (Figure 4), compared to 179 230 minutes for the model that included the ABG data (P < 0.01). For the 2007 data, patients were identified 496 512 minutes prior to ICU admission (Figure 4), compared to 192 210 minutes for the previous model (P < 0.01).

Discussion
We have demonstrated a simple method for generating an algorithm derived from routine laboratory and hemodynamic values that is capable of predicting the onset of sepsis in a significant proportion of non‐ICU patients. Two prediction models were generated, 1 with and 1 without ABG data included in the analysis. In the 2006 and 2007 validation cohorts, the model including these data correctly classified 54.7% and 55.0% of the patients who developed septic shock and 93.4% and 93.0% of control patients, respectively. The second model identified 19.1% and 15.3% of the septic shock patients and 96.6% and 96.8% of the control patients for 2006 and 2007, respectively. The methods used in generating this model are relatively simple and can be executed with the use of an electronic medical record system.
Early, goal‐directed cardiovascular resuscitation and adequate initial antibiotic therapy have been shown to decrease mortality in patients with severe sepsis and septic shock.2, 26 Prior studies employing early, targeted resuscitation strategies have demonstrated decreased use of vasopressors10 and decreased mortality.510 In addition, we previously demonstrated that a standardized order set for the management of severe sepsis in the emergency department that focused on early and aggressive intervention was associated with decreased 28‐day mortality.1 These studies suggest that early, aggressive management of septic shock can improve outcomes. Identification of patients prior to overt clinical deterioration may allow for early intervention aimed at preventing shock or improving its outcome.
The purpose of this method is to develop a model capable of recognizing patterns in clinical data that herald a patient's otherwise unidentified clinical deterioration. It is not intended to replace existing outcome prediction tools or severity of illness scoring systems, where a high degree of accuracy would be required. Rather, it would be best implemented as an automated screening tool incorporated into an electronic medical record system. When a hospitalized patient is identified as a possible septic shock patient by the classification tree, a notification is then issued to the clinicians caring for the patient. The primary goal of this method is to notify clinicians of potential clinical deterioration. Any action taken as a result of this notification is at the discretion of the clinician. This method could be employed for any population of hospitalized patients, though because of variations in clinical practice and patient physiology, different models would need to be generated for differing patient populations.
This method has limitations, the foremost of which is the possible instability of the resulting classification model. This type of analysis results in an algorithm that depends on binary splits to classify patients. In generating the algorithm, the recursive partitioning analysis selects the variables and cutoff values that result in the strongest decision tree with the most pure classifications at the end nodes. These variables and cutoff values may not immediately seem logical from a clinical standpoint, and may vary with changes in practice and even possibly between divisions within a hospital. As a result, the algorithm would likely require intermittent updating to remain effective and a model derived from 1 hospital or patient population would not necessarily be applicable to patients at another institution or from a different population. However, once the method has been developed at an institution, the process of revising the algorithm could be essentially automated and uses few resources.
Another shortcoming of this method is the relatively low sensitivity of the resulting algorithm. In a role as an automated alert system, a low false‐positive rate is particularly desirable to avoid unnecessary frequent distraction of clinicians. The sensitivity of the model can be improved through manipulation of how the analysis is performed, but this would be at the expense of a higher false‐positive rate, which is not acceptable. Finally, prior studies examining treatment for sepsis have demonstrated an advantage to early and aggressive therapy. It is not clear, however, if identifying these patients prior to the onset of clinically evident sepsis would result in improved outcomes. Further work is required to determine if this is the case. We are currently conducting a prospective study that employs the method described here in conjunction with an automated alert system to ascertain if it impacts outcomes on patients admitted to the medicine wards of Barnes‐Jewish Hospital.
In conclusion, the method presented here represents a technique that consumes few resources and is capable of identifying some patients before septic shock becomes clinically evident. When applied in an automated fashion with the capability to alert clinicians caring for a patient, the method demonstrated here may allow for earlier diagnosis and possibly intervention for septic shock patients.
- ,,, et al.Before–after study of a standardized hospital order set for the management of septic shock.Crit Care Med.2006;34(11):2707–2713.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Early goal‐directed therapy in severe sepsis and septic shock revisited; concepts, controversies, and contemporary findings.Chest.2006;130(5):1579–1595.
- .Implementing the severe sepsis care bundles outside the ICU by outreach.Nurs Crit Care.2007;12:225–229.
- ,,, et al.The impact of compliance with 6‐hour and 24‐hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study.Crit Care.2005;9:R764–R770.
- ,,, et al.Translating research to clinical practice, a 1‐year experience with implementing early goal‐directed therapy for septic shock in the emergency department.Chest.2006;129:225–232.
- ,,, et al.Prospective external validation of the clinical effectiveness of an emergency department‐based early goal‐directed therapy protocol for severe sepsis and septic shock.Chest.2007;132:425–432.
- ,,, et al.Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality.Crit Care Med.2007;35:1105–1112.
- ,,, et al.Implementation of an evidence‐based “standard operating procedure” and outcome in septic shock.Crit Care Med.2006;34:943–949.
- ,,, et al.Outcome of septic shock in older adults after implementation of the sepsis “bundle”.J Am Geriatr Soc.2008;56:272–278.
- ,,, et al.Confidential inquiry into quality of care before admission to intensive care.BMJ.1998;316:1853–1858.
- ,,.Unexpected deaths and referrals to intensive care of patients on general wards. Are some cases potentially avoidable?J R Coll Physicians Lond.1999;33(3):255–259.
- ,,, et al.Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units.Crit Care Med.1998;26(6):1020–1024.
- ,,,,,.Hospital‐wide impact of a standardized order set for the management of bacteremic severe sepsis.Crit Care Med.2009;37(3):819–824.
- ,,,,,.The mRNA expression of fatty acid amide hydrolase in human whole blood correlates with sepsis.J Endotoxin Res.2007;13(1):35–38.
- ,,, et al.C‐reactive protein use as an early indicator of infection in patients with systemic inflammatory response syndrome.Intensive Care Med.2004;30(11):2038–2045.
- ,,,,.Procalcitonin as a screening test of late‐onset sepsis in preterm very low birth weight infants.J Perinatol.2005;25(6):397–402.
- ,,.A simple method for predicting severe sepsis in burn patients.Am J Surg.1980;139(4):513–517.
- ,,, et al.Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications.Crit Care Med.2000;28(7):2209–2216.
- ,,,,.Circulating immune parameters predicting the progression from hospital‐acquired pneumonia to septic shock in surgical patients.Crit Care Med.2005;9(6):R662–R669.
- ,,.A simple prediction algorithm for bacteremia in patients with acute febrile illness.Q J Med.2005;98:813–820.
- ,,,.Infection probability score (IPS): a method to help assess the probability of infection in critically ill patients.Crit Care Med.2003;31(11):2579–2584.
- ,.Multivariate regression modeling for the prediction of inflammation, systemic pressure, and end‐organ function in severe sepsis.Shock.1997;8(3):225–231.
- ,,,,,.Abnormal heart rate characteristics preceding neonatal sepsis and sepsis‐like illness.Pediatr Res.2003;53:920–926.
- ,.Statistics for Biology and Health.New York:Springer‐Verlag;1999.
- ,,, et al.Impact of adequate empiric antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Before–after study of a standardized hospital order set for the management of septic shock.Crit Care Med.2006;34(11):2707–2713.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Early goal‐directed therapy in severe sepsis and septic shock revisited; concepts, controversies, and contemporary findings.Chest.2006;130(5):1579–1595.
- .Implementing the severe sepsis care bundles outside the ICU by outreach.Nurs Crit Care.2007;12:225–229.
- ,,, et al.The impact of compliance with 6‐hour and 24‐hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study.Crit Care.2005;9:R764–R770.
- ,,, et al.Translating research to clinical practice, a 1‐year experience with implementing early goal‐directed therapy for septic shock in the emergency department.Chest.2006;129:225–232.
- ,,, et al.Prospective external validation of the clinical effectiveness of an emergency department‐based early goal‐directed therapy protocol for severe sepsis and septic shock.Chest.2007;132:425–432.
- ,,, et al.Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality.Crit Care Med.2007;35:1105–1112.
- ,,, et al.Implementation of an evidence‐based “standard operating procedure” and outcome in septic shock.Crit Care Med.2006;34:943–949.
- ,,, et al.Outcome of septic shock in older adults after implementation of the sepsis “bundle”.J Am Geriatr Soc.2008;56:272–278.
- ,,, et al.Confidential inquiry into quality of care before admission to intensive care.BMJ.1998;316:1853–1858.
- ,,.Unexpected deaths and referrals to intensive care of patients on general wards. Are some cases potentially avoidable?J R Coll Physicians Lond.1999;33(3):255–259.
- ,,, et al.Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units.Crit Care Med.1998;26(6):1020–1024.
- ,,,,,.Hospital‐wide impact of a standardized order set for the management of bacteremic severe sepsis.Crit Care Med.2009;37(3):819–824.
- ,,,,,.The mRNA expression of fatty acid amide hydrolase in human whole blood correlates with sepsis.J Endotoxin Res.2007;13(1):35–38.
- ,,, et al.C‐reactive protein use as an early indicator of infection in patients with systemic inflammatory response syndrome.Intensive Care Med.2004;30(11):2038–2045.
- ,,,,.Procalcitonin as a screening test of late‐onset sepsis in preterm very low birth weight infants.J Perinatol.2005;25(6):397–402.
- ,,.A simple method for predicting severe sepsis in burn patients.Am J Surg.1980;139(4):513–517.
- ,,, et al.Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications.Crit Care Med.2000;28(7):2209–2216.
- ,,,,.Circulating immune parameters predicting the progression from hospital‐acquired pneumonia to septic shock in surgical patients.Crit Care Med.2005;9(6):R662–R669.
- ,,.A simple prediction algorithm for bacteremia in patients with acute febrile illness.Q J Med.2005;98:813–820.
- ,,,.Infection probability score (IPS): a method to help assess the probability of infection in critically ill patients.Crit Care Med.2003;31(11):2579–2584.
- ,.Multivariate regression modeling for the prediction of inflammation, systemic pressure, and end‐organ function in severe sepsis.Shock.1997;8(3):225–231.
- ,,,,,.Abnormal heart rate characteristics preceding neonatal sepsis and sepsis‐like illness.Pediatr Res.2003;53:920–926.
- ,.Statistics for Biology and Health.New York:Springer‐Verlag;1999.
- ,,, et al.Impact of adequate empiric antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
Copyright © 2010 Society of Hospital Medicine
In response to: A quality conundrum: Well done but not enough—Quality improvement conundrums: Looking back before moving forward
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
If clinician‐quality improvers are to gain traction as academicians,1 their first objective should be to bring quality improvement (QI) sandly into the world of scientific method. We believe that Dr. Chakraborti's 2 pointsthat the reasons for afferent limb failure need to be more closely investigated, and that lessons learned from 1 hospital's rapid response system (RRS) may not generalize to other hospitalsreflect the immaturity of QI as a science. In clinical science, 3 well‐defined testing phases bring 1 homogeneous, rigorously tested product to market that is monitored in a fourth phase. While Dr. Chakraborti urges us to examine our afferent limb failures more closely, the monitoring and reporting strategies used in the Josie King Patient Safety Program2 resonate with the postmarketing surveillance of Phase IV trials.
Although necessary and valid, we believe that the majority of the QI conundrum of RRS lies in the lack of premarket, stepwise testing of QI products. QI initiatives are often promulgated before an appropriate evidence base has been established. This lack of scientific rigor has resulted in RRS with calling criteria that have poor operating characteristics,3 undetermined methods for achieving afferent success,4 and efferent response arms of varying sizes and compositions.5 Consequently, a heterogeneous group of RRS have produced equivocal outcomes6 and diminished the applicability of lessons learned across institutions.
Indeed, while it is important to ask, What do we do now?, it may be more informative to answer the question, How did we get here?
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
- ,.Clinicians in quality improvement. A new career pathway in academic medicine.JAMA.2009;301(7):766–768.
- Josie King Foundation. Josie King Patient Safety Program. Available at: http://www.josieking.org/page.cfm?pageID=27. Accessed September2009.
- ,,,.Rapid response: a quality improvement conundrum.J Hosp Med.2009;4(4):255–257.
- .The rapid response team paradox: why doesn't anyone call for help?Crit Care Med.2008;36(2):634–636.
- ,,,.Dress for the occasion.Jt Comm J Qual Patient Saf.2009;35(6):295.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2(6):422–432.
Activities of Daily Living
Tears well up in my eyes. Not from pain, but frustration.
I pleaded with my 3‐year‐olds to come to me, so I could help them wash and dress for the day. Ordinarily, I would have come into their room, leaned over their beds, and whispered good morning into their ears.
Four days after surgery, I wasn't able to do that. Not without the crutches. Even with the crutches, I was moving slowly. I scooted up, put a few pillows behind my back, and carefully lifted up my leg. Putting on the knee immobilizer would take too long; I would only be crossing the hall.
Weighing less than 800 g, the immobilizer is an adjustable aluminum frame attached to foam rubber and 4 wide Velcro straps. It is a device of torment. I can never seem to find the proper fit. If it is too tight, my leg hurts and starts turning blue. When it is too loose, it pulls on my incision, multiplying the pain. You put it on while sitting, but you only discover whether it is too tight or too loose when you stand up.
With one foot on the floor at the edge of the bed, I took one crutch in hand, shifted to the left, and grabbed for the other crutch. I squeezed the handgrips of the crutches tightly and pulled myself up.
Put your weight on your hands, NOT your armpits, the instructions said. Easier said than done, because my armpits were now sore too.
Keep your crutches even with each other. I tried to remember to make an equilateral triangle with my good foot and 2 crutches, but the instructions only seemed to account for movement in a straight line.
Keep your elbows slightly bent and close to your sides to help keep the crutches under your arm. I am trying that too.
Lock your elbows. This instruction contradicts the previous one. Which should I follow?
Place your crutches 2 to 3 inches outside of each foot. How do I do this and keep my elbows close to my sides?
Swing your injured leg through first. But not too much, or I'll pole vault across the room.
After 3 steps, my leg started to throb, and my quadriceps went into a spasm. I needed to sit down, immediately.
Non‐weight‐bearing for next 4 weeks, was written on the doctor's note. Four weeks feels like an eternity. If my heel or toe even touched the ground, I felt an immediate throb of pain in my knee.
How do my orthopedic patients do it: those with broken hips and bad or broken knees, with hip replacements, or knee replacements? I was sensitive to their pain and could optimize control. Postoperatively, I could support gastrointestinal and other organ systems. I made sure that the basic weight‐bearing order was correct. I carefully followed the physical and/or occupational therapy recommendation for home, rehabilitation, or a nursing home. I spoke with families. Yet I did not dwell on activities of daily living nor how my patients felt to be dependent on others for simple things they needed. Some patients were non‐weight‐bearing for weeks, some for months, others never walked again. How can one deeply understand it if one has never experienced it?
According to Centers for Disease Control and Prevention (CDC) statistics,1 unintentional injuries are the leading cause of death between the ages of 1 and 44 years. Accidents are again the leading cause of death after age 75 years. More startling is the fact that these numbers have not been significantly reduced since these data were first collected. Nor do these numbers reveal the number of those debilitated, but still living. In my case, I was not playing basketball, water skiing, or rock climbing: I slipped and fell while stepping into a wading pool with my children, severing ligaments, tearing meniscus, and creating a hairline fracture of my tibia.
Expect to move slowly with the crutches. Yes, I am moving very slowly, only 1 to 2 feet with each stride. If I take a longer stride, I lose my balance.
Learn to sit down with the crutches. Learn to stand up. Learn to get into a car.
Learn to go up the stairs. Learn to go down the stairs.
Avoid wet surfaces. Otherwise you'll start skating and reinjure that knee.
It was effortless to think of patients and colleagues of mine. Mr. S., how do you do it? At age 30 years, he was quadriplegic from falling from a tree as a teenager. Mr. D, how about you, in a wheelchair, after being hit by a car and multiple postoperative leg infections and amputations: living in hotels, with estranged family and no social support. How did you do it, while we nagged you about controlling your glucose. Dr. J? The sadness fills my heart. At only 60 years old, my wonderful professor now made rounds in an electric wheelchair, the victim of amyotrophic lateral sclerosis (ALS). My fate, in comparison, is fortunate; my immobility only temporary. I can still think. And talk. And use a phone. And eat, as well as write this essay. And, best of all, read stories to my children. Be careful about this leg, I remind them. Don't come too close!
Maybe what life is trying to tell you is, slow down. In fact, it's forcing you to do that, a colleague and friend said. I had prided myself in thorough and compassionate doctoring. Now, having been a patient on crutches, I have a greater understanding of how limits to mobility can impact daily living on my patients after I discharge them. And the mundane subject of accident prevention has gained a new urgency.
- ,,,.Deaths: final data for 2005.Natl Vital Stat Rep.2008;56(10):1–120.
Tears well up in my eyes. Not from pain, but frustration.
I pleaded with my 3‐year‐olds to come to me, so I could help them wash and dress for the day. Ordinarily, I would have come into their room, leaned over their beds, and whispered good morning into their ears.
Four days after surgery, I wasn't able to do that. Not without the crutches. Even with the crutches, I was moving slowly. I scooted up, put a few pillows behind my back, and carefully lifted up my leg. Putting on the knee immobilizer would take too long; I would only be crossing the hall.
Weighing less than 800 g, the immobilizer is an adjustable aluminum frame attached to foam rubber and 4 wide Velcro straps. It is a device of torment. I can never seem to find the proper fit. If it is too tight, my leg hurts and starts turning blue. When it is too loose, it pulls on my incision, multiplying the pain. You put it on while sitting, but you only discover whether it is too tight or too loose when you stand up.
With one foot on the floor at the edge of the bed, I took one crutch in hand, shifted to the left, and grabbed for the other crutch. I squeezed the handgrips of the crutches tightly and pulled myself up.
Put your weight on your hands, NOT your armpits, the instructions said. Easier said than done, because my armpits were now sore too.
Keep your crutches even with each other. I tried to remember to make an equilateral triangle with my good foot and 2 crutches, but the instructions only seemed to account for movement in a straight line.
Keep your elbows slightly bent and close to your sides to help keep the crutches under your arm. I am trying that too.
Lock your elbows. This instruction contradicts the previous one. Which should I follow?
Place your crutches 2 to 3 inches outside of each foot. How do I do this and keep my elbows close to my sides?
Swing your injured leg through first. But not too much, or I'll pole vault across the room.
After 3 steps, my leg started to throb, and my quadriceps went into a spasm. I needed to sit down, immediately.
Non‐weight‐bearing for next 4 weeks, was written on the doctor's note. Four weeks feels like an eternity. If my heel or toe even touched the ground, I felt an immediate throb of pain in my knee.
How do my orthopedic patients do it: those with broken hips and bad or broken knees, with hip replacements, or knee replacements? I was sensitive to their pain and could optimize control. Postoperatively, I could support gastrointestinal and other organ systems. I made sure that the basic weight‐bearing order was correct. I carefully followed the physical and/or occupational therapy recommendation for home, rehabilitation, or a nursing home. I spoke with families. Yet I did not dwell on activities of daily living nor how my patients felt to be dependent on others for simple things they needed. Some patients were non‐weight‐bearing for weeks, some for months, others never walked again. How can one deeply understand it if one has never experienced it?
According to Centers for Disease Control and Prevention (CDC) statistics,1 unintentional injuries are the leading cause of death between the ages of 1 and 44 years. Accidents are again the leading cause of death after age 75 years. More startling is the fact that these numbers have not been significantly reduced since these data were first collected. Nor do these numbers reveal the number of those debilitated, but still living. In my case, I was not playing basketball, water skiing, or rock climbing: I slipped and fell while stepping into a wading pool with my children, severing ligaments, tearing meniscus, and creating a hairline fracture of my tibia.
Expect to move slowly with the crutches. Yes, I am moving very slowly, only 1 to 2 feet with each stride. If I take a longer stride, I lose my balance.
Learn to sit down with the crutches. Learn to stand up. Learn to get into a car.
Learn to go up the stairs. Learn to go down the stairs.
Avoid wet surfaces. Otherwise you'll start skating and reinjure that knee.
It was effortless to think of patients and colleagues of mine. Mr. S., how do you do it? At age 30 years, he was quadriplegic from falling from a tree as a teenager. Mr. D, how about you, in a wheelchair, after being hit by a car and multiple postoperative leg infections and amputations: living in hotels, with estranged family and no social support. How did you do it, while we nagged you about controlling your glucose. Dr. J? The sadness fills my heart. At only 60 years old, my wonderful professor now made rounds in an electric wheelchair, the victim of amyotrophic lateral sclerosis (ALS). My fate, in comparison, is fortunate; my immobility only temporary. I can still think. And talk. And use a phone. And eat, as well as write this essay. And, best of all, read stories to my children. Be careful about this leg, I remind them. Don't come too close!
Maybe what life is trying to tell you is, slow down. In fact, it's forcing you to do that, a colleague and friend said. I had prided myself in thorough and compassionate doctoring. Now, having been a patient on crutches, I have a greater understanding of how limits to mobility can impact daily living on my patients after I discharge them. And the mundane subject of accident prevention has gained a new urgency.
Tears well up in my eyes. Not from pain, but frustration.
I pleaded with my 3‐year‐olds to come to me, so I could help them wash and dress for the day. Ordinarily, I would have come into their room, leaned over their beds, and whispered good morning into their ears.
Four days after surgery, I wasn't able to do that. Not without the crutches. Even with the crutches, I was moving slowly. I scooted up, put a few pillows behind my back, and carefully lifted up my leg. Putting on the knee immobilizer would take too long; I would only be crossing the hall.
Weighing less than 800 g, the immobilizer is an adjustable aluminum frame attached to foam rubber and 4 wide Velcro straps. It is a device of torment. I can never seem to find the proper fit. If it is too tight, my leg hurts and starts turning blue. When it is too loose, it pulls on my incision, multiplying the pain. You put it on while sitting, but you only discover whether it is too tight or too loose when you stand up.
With one foot on the floor at the edge of the bed, I took one crutch in hand, shifted to the left, and grabbed for the other crutch. I squeezed the handgrips of the crutches tightly and pulled myself up.
Put your weight on your hands, NOT your armpits, the instructions said. Easier said than done, because my armpits were now sore too.
Keep your crutches even with each other. I tried to remember to make an equilateral triangle with my good foot and 2 crutches, but the instructions only seemed to account for movement in a straight line.
Keep your elbows slightly bent and close to your sides to help keep the crutches under your arm. I am trying that too.
Lock your elbows. This instruction contradicts the previous one. Which should I follow?
Place your crutches 2 to 3 inches outside of each foot. How do I do this and keep my elbows close to my sides?
Swing your injured leg through first. But not too much, or I'll pole vault across the room.
After 3 steps, my leg started to throb, and my quadriceps went into a spasm. I needed to sit down, immediately.
Non‐weight‐bearing for next 4 weeks, was written on the doctor's note. Four weeks feels like an eternity. If my heel or toe even touched the ground, I felt an immediate throb of pain in my knee.
How do my orthopedic patients do it: those with broken hips and bad or broken knees, with hip replacements, or knee replacements? I was sensitive to their pain and could optimize control. Postoperatively, I could support gastrointestinal and other organ systems. I made sure that the basic weight‐bearing order was correct. I carefully followed the physical and/or occupational therapy recommendation for home, rehabilitation, or a nursing home. I spoke with families. Yet I did not dwell on activities of daily living nor how my patients felt to be dependent on others for simple things they needed. Some patients were non‐weight‐bearing for weeks, some for months, others never walked again. How can one deeply understand it if one has never experienced it?
According to Centers for Disease Control and Prevention (CDC) statistics,1 unintentional injuries are the leading cause of death between the ages of 1 and 44 years. Accidents are again the leading cause of death after age 75 years. More startling is the fact that these numbers have not been significantly reduced since these data were first collected. Nor do these numbers reveal the number of those debilitated, but still living. In my case, I was not playing basketball, water skiing, or rock climbing: I slipped and fell while stepping into a wading pool with my children, severing ligaments, tearing meniscus, and creating a hairline fracture of my tibia.
Expect to move slowly with the crutches. Yes, I am moving very slowly, only 1 to 2 feet with each stride. If I take a longer stride, I lose my balance.
Learn to sit down with the crutches. Learn to stand up. Learn to get into a car.
Learn to go up the stairs. Learn to go down the stairs.
Avoid wet surfaces. Otherwise you'll start skating and reinjure that knee.
It was effortless to think of patients and colleagues of mine. Mr. S., how do you do it? At age 30 years, he was quadriplegic from falling from a tree as a teenager. Mr. D, how about you, in a wheelchair, after being hit by a car and multiple postoperative leg infections and amputations: living in hotels, with estranged family and no social support. How did you do it, while we nagged you about controlling your glucose. Dr. J? The sadness fills my heart. At only 60 years old, my wonderful professor now made rounds in an electric wheelchair, the victim of amyotrophic lateral sclerosis (ALS). My fate, in comparison, is fortunate; my immobility only temporary. I can still think. And talk. And use a phone. And eat, as well as write this essay. And, best of all, read stories to my children. Be careful about this leg, I remind them. Don't come too close!
Maybe what life is trying to tell you is, slow down. In fact, it's forcing you to do that, a colleague and friend said. I had prided myself in thorough and compassionate doctoring. Now, having been a patient on crutches, I have a greater understanding of how limits to mobility can impact daily living on my patients after I discharge them. And the mundane subject of accident prevention has gained a new urgency.
- ,,,.Deaths: final data for 2005.Natl Vital Stat Rep.2008;56(10):1–120.
- ,,,.Deaths: final data for 2005.Natl Vital Stat Rep.2008;56(10):1–120.
IVIG for Severe Colitis
Clostridium difficile colitis (CDC) is the most common cause of hospital‐acquired diarrhea.1 The incidence of CDC has sharply increased over the past decade despite increasing awareness among health care professionals.24 C. difficile pathogenic strains induce diarrhea through the elaboration and secretion of 2 exotoxins: toxin A and toxin B. Toxin A is an inflammatory toxin, leading to fluid secretion, increased mucosal permeability, and marked enteritis and colitis.5 Toxin B is cytotoxic, leading to cell injury and apoptosis.5 Combined, toxin A and toxin B can cause a wide spectrum of clinical presentations, ranging from mild diarrhea that resolves with the discontinuation of antibiotics to a fulminant colitis requiring surgical intervention.
The severity of clinical manifestations has been shown to be inversely proportional to the host anti‐toxin A antibody level in response to toxin exposure. Kyne et al.6 demonstrated that asymptomatic C. difficile carriers produced significantly higher anti‐toxin A immunoglobulin G (IgG) levels compared to symptomatic patients. Among the latter group, patients with mild disease had a higher antibody level compared to those with severe colitis.6, 7 Additional risk factors predisposing to severe colitis are advanced age, severe underlying illness,8 and immunocompromised state.9
Recently, a new C. difficile strain (BI/NAP1) with a mutated toxin A and toxin B promoter silencer, a binary toxin gene, and fluoroquinolone resistance has been described in Canada and the United States.3, 10 This strain has been associated with an increased incidence of CDC among hospitalized patients, especially the incidence of severe disease requiring colectomy. At the same time, several reports describing metronidazole treatment failure have been published.1114 These recent findings emphasize the importance of finding alternative treatments for CDC.
Intravenous immunoglobulin (IVIG) was used to treat CDC for the first time in 1991.15 Since then, 12 case reports and small case series, along with 1 case‐control study have been published documenting IVIG treatment outcomes.1527 However, only 5 reports to date examined patients with severe CDC.2125 In the present study, we report the largest series of patients with severe CDC treated with IVIG in the literature to our knowledge.
Patients and Methods
Case Series
We used CareScience software (CareScience, Inc., Philadelphia, PA) to retrospectively identify all patients admitted to our institution with a primary or secondary diagnosis of CDC (code 00845) between July 1, 2002 and May 1, 2006. CareScience is commercially available software that tracks all admissions to our institution and allows the performance of patient searches with a wide spectrum of user‐defined search criteria. Using the same software, we further identified those patients who received IVIG during their hospital stay. We then obtained the hospital chart for each patient, from the medical records department and established the study database.
A case was defined as a patient with diarrhea (at least 3 loose stools daily) for at least 2 days who had C. difficile cytotoxin‐positive feces and at least 1 of the following criteria: clinical symptoms (abdominal pain and/or distension and fever); leukemoid reaction (defined as white blood cell count of 20,000 cells/mm3 or above. This cutoff value was chosen as it has been used previously as a prognostic factor)4; radiographic evidence of colitis by computed tomography (CT) of the abdomen; and/or the presence of pseudomembranes on flexible sigmoidoscopy or colonoscopy. We excluded all patients who received IVIG for an indication other than CDC treatment (n = 3). There were no other exclusion criteria.
We used a standardized data collection tool and recorded demographics (age, gender, principal diagnosis), past medical and surgical history, other risk factors for C. difficile infection (previous CDC, antibiotics received during hospital stay, immunosuppressive medications or organ transplantation within the previous 6 weeks, history of malignancy or diabetes mellitus); clinical presentation (abdominal distention, abdominal pain, diarrhea, fever, leukemoid reaction, and hypotension defined as systolic blood pressure <85 mm Hg despite at least 1 L of intravenous normal saline administration and the need for vasopressor use); colonoscopy findings; CT scan and x‐ray findings; laboratory values; date and dose of IVIG infused and other C. difficile pharmacological treatments; and Acute Physiological Assessment and Chronic Health Evaluation (APACHE II) score28 at the first day of IVIG infusion. The primary outcomes were survival at the end of the hospital stay and clinical disease resolution, defined as 2 formed bowel movements or less per day without abdominal pain or distention.
The decision to initiate IVIG therapy and the dose to be used was made by the individual attending physician.
Statistical Analysis
Single (univariate) and multiple (multivariate) logistic regression analysis were applied to identify variables among the ones collected that are independent predictors of CDC mortality. All statistical analyses were completed with the STATA 10 software package (StatCorp LP, College Station, TX).
Review of the Literature
We used PubMed, Web of Science, Scopus, and Excerpta Medica databases to search for any publication in a peer‐reviewed journal on the use of IVIG for the treatment of severe CDC. We used the search words: IVIG or intravenous immunoglobulin and clostridium difficile. Only publications published in English were selected. The date range used was January 1, 1950 to January 7, 2009. We were able to find 5 publications using this search criteria.
Results
Study Population
Of the 1230 patients diagnosed with CDC over the 4‐year study period, 21 patients were treated with IVIG. Table 1 summarizes the patients' characteristics. There were 13 women and 8 men. The mean age was 68 years, with a standard deviation (SD) of 13 years. Sepsis was the primary diagnosis in all patients. Sixteen patients had predisposing risk factors for CDC, including immunosuppression (immunosuppressive medication [n = 2], human immunodeficiency virus [HIV] infection [n = 2]); cancer (n = 3); recent surgery (n = 3); and diabetes mellitus (n = 11). Nine patients had documented previous CDC episodes. The indications for IVIG administration were evidence of pancolitis on abdominal CT scan (n = 12) or severe ileus with cessation of diarrhea, abdominal distention, and requirement for total parenteral nutrition (n = 5), or severe hypotension (n = 4) (defined as systolic blood pressure <85 mm Hg despite at least 1 L of intravenous normal saline administration and the subsequent need for vasopressor use).
| Patient | Age (gender) | Diagnosis | Medical and Surgical History | CDC History | Colonoscopy Findings | Radiographic Findings |
|---|---|---|---|---|---|---|
| ||||||
| A | 40 (female) | CDC with sec. sepsis | Gastric stapling | Yes | * | Diffuse colitis |
| B | 86 (female) | Fulminant CDC | Metastatic ovarian carcinoma | PC | No colonic thickening | |
| C | 72 (male) | Sepsis | Acute pancreatitis with sec. pseudocyst, DM | Yes | PC | Diffuse colitis |
| D | 78 (male) | Discitis | Delayed: normal mucosa | Dilation of small and large bowel | ||
| E | 98 (female) | Urosepsis | Yes | * | No bowel distention | |
| F | 90 (female) | Right lower extremity cellulitis | DM | * | Concentric thickening of rectal wall | |
| G | 64 (male) | Ischemic colitis | DM, recent Hartman pouch closure | Marked inflammation | Diffuse colitis | |
| H | 78 (female) | Toe osteomyelitis and CDC with sec. sepsis | DM | * | Diffuse colitis | |
| I | 35 (female) | Sepsis | Yes | * | Diffuse nonspecific colitis, minimal ascites | |
| J | 47 (female) | Pneumonia and sec. sepsis | * | Diffuse colitis | ||
| K | 56 (female) | Urosepsis | HIV | Yes | * | Colitis involving the right colon |
| L | 76 (male) | CDC with sec. sepsis | Sigmoid bladder fistula repair, DM | * | Diffuse colitis | |
| M | 71 (female) | Pneumonia with sec. sepsis | * | Diffuse colitis | ||
| N | 63 (male) | Urosepsis | DM, lymphoma resection from small intestine | PC | Marked small and large bowel distention | |
| O | 86 (male) | Enterococcus‐induced sepsis | Rheumatoid arthritis on methotrexate | * | Fat stranding suggesting peritonitis | |
| P | 60 (female) | Gastrointestinal bleed and CDC | DM with neuropathy and retinopathies | Yes | * | Thickening of wall of colon in most of the colon |
| Q | 57 (female) | Sepsis | DM | Yes | * | Normal |
| R | 67 (female) | CDC with sec. sepsis | Candidal esophagitis | Yes | * | Large amount of peritoneal fluid, mild small bowel thickening |
| S | 60 (female) | Sepsis sec. to S. aureus and P. aerogenosa | DM, renal transplant (myco, prednisone) | * | Ileus with air fluid level in the small intestine | |
| T | 80 (female) | Sepsis | DM | Yes | * | Thickening of descending colon consistent with colitis |
| U | 72 (male) | CDC, widespread metastatic cancer | DM, metastatic cancer (unknown primary site) | * | Severe colitis up to the splenic flexure | |
Table 2 describes disease severity in these patients. Since CDC starts locally in the colon then secondarily involves multiple organs as part of the systemic inflammatory response syndrome (SIRS), 2 scales were used to characterize the disease in each patient: (1) extent of local colonic inflammation, and (2) severity of systemic involvement. Extensive colonic involvement was evidenced in all patients by pancolitis on abdominal imaging modalities (12 patients), severe ileus requiring total parenteral nutrition (13 patients), or referral for surgical consultation for possible colectomy (12 patients).
| Patient | Complications During the Hospital Stay | APACHE II Score | Monitored Unit | WBC* | K | Alb | Lactate | Cr | Hospital Stay (days) | Surgical Consult/Surgery | TPN for Colitis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| A | Sepsis, DIC, ARDS, intubation | 15 | Yes | 48 | 4 | 1.1 | 2.9 | 1.3 | 26 | No/No | Yes |
| B | Dehydration, weakness | 12 | No | 19 | 3.4 | 3.1 | 1.1 | 16 | No/No | Yes | |
| C | HTP, GI bleed, ischemic colitis, F | 22 | Yes | 21 | 2.7 | 2.5 | 1.3 | ESRD | 34 | Yes/No | Yes |
| D | AD, megacolon, HT, intubation, ARF | 18 | Yes | 25 | 3.4 | 1.4 | 1.1 | 2.6 | 52 | Yes/No | Yes |
| E | Exacerbation of CHF with respiratory distress, Bipap support, confusion | 21 | No | 10 | 2.6 | 2.3 | 1 | 11 | No/No | No | |
| F | Confusion, gout acute attack | 21 | No | 33 | 3.1 | 2.6 | ESRD | 15 | No/No | No | |
| G | Intubation, cardiac arrest, AF with RVR, PNA, ARF, DVT, dysphagia, PEG | 6 | Yes | 15 | 3.1 | 1.7 | 1.9 | 2.2 | 32 | Yes/No | Yes |
| H | Sepsis, intubation, ARF, PEG, toe amputation, PNA, pulmonary edema, TPN, vitamin D deficiency | 34 | Yes | 59 | 2.8 | 1.5 | 4 | 3.8 | 17 | No/No | Yes |
| I | CHF, transient third cranial nerve palsy, DIC | 20 | Yes | 52 | 3.2 | 3.3 | 0.7 | ESRD | 11 | No/No | No |
| J | Aspiration PNA, sepsis, DIC, intubation, MI, F, HT | 32 | Yes | 17 | 2.7 | 1.8 | 14 | ESRD | 21 | Yes/no | No |
| K | Intubation, cardiac arrest, ARF, DIC, AP, F | 30 | Yes | 25 | 2.1 | 2.1 | 13 | 3.6 | 10 | Yes/no | No |
| L | Intubation, ARF, HTP, MI, AD, F | 23 | Yes | 69 | 3.2 | 1.8 | 1.4 | 3.6 | 23 | Yes/no | No |
| M | Intubation, DIC, ARF, GI bleeding, hypothermia, AD | 23 | Yes | 47 | 2.8 | 1.4 | 1.4 | 4.3 | 23 | Yes/no | Yes |
| N | Intubation, HTP, AD, F, ARF with HD, AF, osteomyelitis | 23 | Yes | 46 | 2.9 | 1.5 | 1.5 | 2.7 | 27 | Yes/no | Yes |
| O | Intubation, ARF, 2 cardiac arrests, GI bleed, UTI, rhabdomyolysis, liver shock, AF | 31 | Yes | 49 | 2.5 | 1.1 | 2.3 | 2.9 | 25 | Yes/no | Yes |
| P | Bowel ischemia with bowel resection, ARF, MI, ischemic bowel, fluid overload, respiratory failure | 23 | Yes | 26 | 2.6 | 0.8 | 2.9 | 3.1 | 9 | Yes/Yes | Yes |
| Q | Pulmonary embolism | 39 | Yes | 23 | 2.8 | 1.1 | 8.1 | ESRD | 9 | No/No | Yes |
| R | Fungal peritonitis, aspiration pneumonia, cardiac arrest | 26 | Yes | 30 | 4.1 | 1.1 | 0.9 | ESRD | 36 | No/No | Yes |
| S | Intubation, pneumothorax, CRT, pressor‐dependent shock, ARF | 36 | Yes | 46 | 3.1 | 1.8 | 2.1 | 5.4 | 64 | Yes/Yes | No |
| T | Pressor‐dependent sepsis, pulmonary edema, ARF with HD | 36 | Yes | 35 | 3.3 | 1.3 | 1.8 | 5.5 | 11 | Yes/No | Yes |
| U | Sepsis | 34 | Yes | 58 | 3.1 | 2.2 | 3.3 | 1.6 | 9 | No/No | No |
Of the 21 patients treated with IVIG, 9 did not receive a surgical consultation either because they responded to medical treatment promptly (6 patients), were too unstable for surgery (2 patients), or because the patient/family refused surgery (1 patient). Of the 12 patients who received surgical consultation, 2 underwent surgery. The remainder did not proceed to surgery for the following reasons: they were deemed medically unstable for surgery (6 patients), declined surgery (2 patients), were diagnosed with cancer on colonoscopy (1 patient), or improved with medical treatment (1 patient).
The severity of systemic involvement was measured using the APACHE II score on day 1 of IVIG infusion. The mean APACHE II score was 25. Eighteen patients were in a monitored unit when IVIG was administered. The study group had laboratory results in keeping with those previously used to define severe colitis:4, 9, 19 leukocytosis (defined as white blood cell count higher than 12,000 cells/mL [mean = 36,000 cells/mL]), hypoalbuminemia (mean = 1.78 g/dL, SD = 0.68 g/dL), hypokalemia (mean = 3.02 mg/dL, SD = 0.47 g/dL), and acute renal failure (defined as serum creatinine level >1.5 mg/dL [mean = 2.98 mg/dL, SD = 1.42 g/dL]).
IVIG Use
Table 3 describes the treatment patients received for CDC as well as the total number of antibiotics used throughout the hospital stay. IVIG was used as adjuvant treatment (defined as IVIG administration within 4 days or less after CDC diagnosis) in 8 patients and as second‐line treatment (defined as IVIG administration more than 4 days after CDC diagnosis) in 13 patients. Metronidazole, vancomycin, cholestyramine, and probiotic treatment alone or in different combinations were used for an average of 8 days (SD = 8 days; range, 025 days) before IVIG infusion. The total IVIG dose administered varied depending on the prescribing attending, with a range of 200 mg/kg to 1250 mg/kg and a mode of 250 mg/kg for 1 to 3 days. An average of 5 (SD = 2) different antibiotics that were not active against C. difficile were used per patient without being discontinued after a CDC diagnosis was made. The 3 most common were: cephalosporins (cefazolin, ceftriaxone, cefepime), fluoroquinolones (levofloxacin), and combination antibiotics (piperacillin and tazobactam or ampicillin and sulbactam).
| Patient | Number of Antibiotics | Duration of Treatment Before IVIG (days) | Treatment Before IVIG (days) | Total CDC Treatment (days) | IgG Level | IVIG Dose |
|---|---|---|---|---|---|---|
| ||||||
| A | 5 | 7 | Metro (7), Vanc (7), Choles (2) | Oral and rectal Vanc (26,19), IV Metro (19), Choles (2), Lacto (6) | Low | 300 mg/kg for 1 day |
| B | 1 | 13 | Metro (13), Vanc (13) | Oral Vanc (17) and IV Metro (12) | 300 mg/kg for 1 day | |
| C | 3 | 7 | Metro (7), Vanc (3) | IV Metro (28),Vanc oral and enema (18,3), Lacto (10) | Low | 125 mg/kg for 5 days |
| D | 5 | 25 | Metro (25), Vanc (15) | Oral then IV Metro (10,15), oral Vanc (25), Choles (7) and Lacto (13) | Low | 200 mg/kg for 1 day |
| E | 1 | 4 | Metro (1), Vanc (4), Choles(4) | IV Metro (8), oral Vanc (10) and Choles (4) | 75 mg/kg for 5 days | |
| F | 4 | 2 | Metro (2), Vanc (1) | IV Metro (8) and oral Vanc (9) | 250 mg/kg for 5 days | |
| G | 3 | 17 | Metro (17), Vanc (14) | IV Metro (49) and oral Vanc (61) | 250 mg/kg for 2 days | |
| H | 5 | 1 | Metro (1), Vanc (1) | Oral Metro (18), oral Vanc (22 ), IV Metro (3) | 250 mg/kg for 3 days | |
| I | 6 | 1 | Metro (1) | Oral and IV Metro (7,9), Vanc oral and enema (8,3) | 250 mg/kg for 2 days | |
| J | 8 | 16 | Metro (14), Vanc (2), Lacto (6) | Oral then IV Metro (10,5), oral and rectal Vanc (3,1), Lacto (6) | 300 mg/ kg for 1 day | |
| K | 6 | 7 | Metro (7), Vanc (3) | Oral then IV Metro (10) and oral Vanc (9) | Normal | 400 mg/kg for 2 days |
| L | 4 | 0 | None | IV then oral Metro (7,10), oral Vanc (23), Choles (7), and Lacto (5) | 150 mg/kg for 5 days | |
| M | 6 | 1 | Metro (1) | IV Metro (23) and oral Vanc (9) | 250 mg/kg for 2 days | |
| N | 7 | 1 | Metro (1), Vanc (1) | IV Metro (16), oral Vanc (14), oral Metro (7) | 250 mg /kg for 2 days | |
| O | 6 | 7 | Metro (6), Vanc (4) | IV Metro (6) and oral Vanc (22) | Low | 250 mg/kg for 2 days |
| P | 3 | 6 | Metro (6), Vanc (6) | IV Metro (25) and oral Vanc (17) | 150 mg/kg fro 3 days | |
| Q | 3 | 4 | Metro (4), Vanc (3) | Oral Metro (8),Vanc oral and enema (6,3) | 250 mg/kg for 3 days | |
| R | 5 | 9 | Vanc (9) | Vanc oral and enema (5,4), IV Metro (2) | 250 mg/kg for 1 day | |
| S | 8 | 23 | Metro (23), Vanc (23) | Oral then IV Metro (12,22), oral Vanc (39) | 250 mg/kg for 3 days | |
| T | 4 | 6 | Metro (6), Vanc (1) | Oral Vanc and IV Metro (11) | 250 mg/kg for 1 day | |
| U | 4 | 2 | Metro (2), Vanc (2) | Oral Vanc and IV Metro (6) | 250 mg/kg for 3 days | |
Survival with IVIG Use
Nine patients (43%) survived their illness and were discharged from the hospital. They experienced complete clinical resolution after an average of 10 days from IVIG administration (range, 220 days) (Table 4). The other 12 patients (57%) died during the index hospitalization. The average length of stay was 23 (range, 964) days.0
| Patient | Disposition | Clearance of Clostridium difficile Colitis? | Days to Resolution |
|---|---|---|---|
| |||
| A | Alive | Loose BM persisted but diarrhea resolved 9 days after IVIG. | 9 |
| B | Alive | BM became formed and diarrhea resolved 48 hours after IVIG. | 2 |
| C | Alive | Diarrhea resolved 20 days post‐IVIG infusion. CAT scan: colonic thickening improved. | 20 |
| D | Alive | Diarrhea resolved on discharge. Response to IVIG started next day after administration. | 18 |
| E | Alive | Diarrhea improved next day after IVIG administration and resolved on discharge. | 5 |
| F | Alive | Diarrhea resolved 5 days after IVIG administration. | 5 |
| G | Alive | Diarrhea resolved. C. difficile test became negative. | 13 |
| H | Alive | Diarrhea resolved 2 days before discharge. | 15 |
| I | Alive | Diarrhea slowly improved and resolved 4 days before discharge. | 7 |
| J | Deceased | ||
| K | Deceased | ||
| L | Deceased | ||
| M | Deceased | ||
| N | Deceased | ||
| O | Deceased | ||
| P | Deceased | ||
| Q | Deceased | ||
| R | Deceased | ||
| S | Deceased | ||
| T | Deceased | ||
| U | Deceased | ||
| Study | Number of Patients | Age (SD) (years) | Male | Female | Severity Definition | IVIG Dose | Days to Resolution | Days IVIG Infused | Alive? (%) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| Salcedo et al.22 | 2 | 63, 64 | 1 | 1 | Pancolitis or thumbprinting on CAT scan | 200300 mg/kg once | 12 | 59 | 100 | 1 out of 2 |
| McPherson et al.21 | 8 | 72 (12) | ? | ? | Pancolitis | 200400 mg/kg twice | 226 | 1165 | 75 | 2 out of 6 |
| Juang et al.25 | 18 | 67 (17.4) | 5 | 13 | Modified Rubin et al.9 criteria | 200300 mg/kg once | ? | ? | 83 | ? |
| Hassoun et al.24 | 1 | 72 | 1 | 0 | Pancolitis | 400 mg/kg once | 6 | 15 | 100 | None |
| Chandrasekar et al.23 | 1 | 67 | 0 | 1 | Shock requiring inotropic support and pseudomembranes on colonoscopy | 400 mg/kg for 5 doses | 16 | 35 | 100 | ? |
| This paper | 21 | 68 (16) | 7 | 14 | Pancolitis and APACHE II score | 300 mg/kg once; 250 mg/kg for 5 doses | 220 | 025 | 43 | ? |
To further assess the impact of IVIG on colitis resolution, we investigated all variables in the data set that may have been associated with mortality using univariate Cox regression analysis. Those variables were as follows: APACHE II score on the first day of IVIG infusion, age, sex, previous history of CDC, number of days before IVIG use, peak white blood cell count, serum potassium level and creatinine level, lactate level on first day of IVIG infusion, and number of antibiotics administered that are not active against CDC. Only the APACHE II score (P = 0.006) and lactate level on the day of IVIG infusion (P = 0.004) were (positively) associated with CDC mortality. The positive association between CDC mortality and APACHE II score remained significant (P = 0.04) after adjusting for sex, previous history of CDC, number of days before IVIG use, lactate level on first day of IVIG infusion, and number of antibiotics administered that are not active against CDC using a multivariate Cox regression analysis model. No adjustments were made for age, white blood cell count, potassium level, or creatinine level as those are included within the APACHE II score. The positive association between lactate level on the first day of IVIG infusion and CDC mortality was not statistically significant after adjusting for the factors listed above in the same model (P = 0.13).
Discussion
To our knowledge, the present study is the largest series published in the literature to date on the use of IVIG for severe CDC. It is also the first study to report a high mortality rate compared to the 5 previous smaller studies on this topic. In the first report on IVIG use for CDC, Leung et al.15 used IVIG to treat 5 pediatric patients suffering from chronic relapsing CDC. It was not until 7 years later, in 1997, that the first IVIG use for severe CDC was reported.22 Since then, a total of 13 works have been published on IVIG for CDC treatment, and only in 5 of these was IVIG administered for severe CDC treatment:2125 3 case reports, 1 case series, and 1 case‐control study. Although the 4 uncontrolled reports concluded that IVIG is beneficial for severe CDC, the only controlled study reported no significant difference between cases and controls for all‐cause mortality, length of stay, and colectomy rate.25
The definition of severe CDC varied between reports, making comparison difficult. McPherson et al.21 and Hassoun and Ibrahim24 defined severe disease as one causing pancolitis on CT scan either with or without megacolon. In the study by Juang et al.,25 disease severity was assessed using the modified criteria of Rubin et al.9 Salcedo et al.22 defined severe CDC as one causing pancolitis in one patient and thumbprinting on CT scan in another, whereas Chandrasekar et al.23 defined it as one causing shock requiring inotropic support and presence of pseudomembranes on colonoscopy.
The present report is unique in that it provided 2 scales to characterize disease in each patient. The first scale measured colonic involvement anatomically and physiologically using a combination of computerized axial tomography (CAT) scan findings, presence or absence of ileus or referral for possible colectomy. This is not a prognostic scale, however, since CAT scan findings have been previously shown to be poor predictors of treatment outcome.29 The second scale measured the severity of systemic involvement using a well‐validated and standardized scale, the APACHE II score. We have shown in this report that it is associated with prognosis in the context of IVIG use.
Our study reports a higher mortality rate than previously described, and suggests that risk stratification and patient selection are important before IVIG administration, since not all patients seem to benefit from this treatment as previously suggested by smaller case series. Previously, several physical findings and laboratory values were found to be associated with worse outcome in CDC. These were increasing age, immunosuppression, shock requiring vasopressors, peak white blood cell count, peak serum lactate level, hypoalbuminemia, a fall in serum albumin level of >1.1 g/dL at the onset of CDC symptoms, use of 3 or more antibiotics, comorbid disease, previous history of CDC, acute renal failure and hypotension, underlying altered or depressed mental status, abdominal pain or distention, white blood cell count over 20,000/mm3 or <1500/mm3 and/or a >10% band forms on the white blood cell differential count, and ascites or pneumatosis coli by abdominal imaging.9, 3033 Using the APACHE II scale for the same purpose has the advantage of utilizing a well‐validated and objective scale that is expected to measure the degree of systemic involvement more reliably compared to the clinical and laboratory values above.
Timing of IVIG infusion remains controversial. Due to the lack of randomized controlled trials, the current practice is guided by expert opinion, leading to wide variations between reports. Since the APACHE II score was positively associated with mortality in the setting of IVIG treatment, the same scale could be used to guide decisions regarding timing of IVIG infusion. Our results suggest that IVIG should be preferentially used while the APACHE II score is still relatively low. This association and the specific APACHE II score at which to initiate or hold treatment need to be validated in the setting of a randomized controlled study before being used in clinical practice.
Although the current study was not designed to test this theory, IVIG could be associated conceptually with treatment success for patients with severe disease that is still restricted to the colon (without other organ dysfunction or at least at an early stage of extracolonic organ failure and thus associated with a low APACHE II score) but not for severe colonic disease with secondary multiple organ failure (high APACHE II score). This may be because colonic disease is toxin‐mediated whereas secondary systemic involvement is mediated through toxin‐induced inflammatory mediators (interleukin‐8, macrophage‐inflammatory protein‐2, substance P, tumor necrosis factor‐alpha) released locally in the colon,3436 triggering a SIRS and hematogenous translocation of colonic bacteria,37 both of which are poorly responsive to immunoglobulin infusion. Along the same lines, waiting for failure of conventional therapy before IVIG use might result in IVIG treatment failure because of disease progression and secondary sepsis, at which point no treatment may be effective. No study thus far has addressed this issue specifically.
Overall, the combined cohort of patients with severe CDC treated with IVIG in the literature includes 51 patients (Table 4). The current report contributes 41% of these patients. The patients' average age was 68 years, with a 2 to 1 female‐male ratio. The dose of IVIG used varied largely also, with 400 mg/kg being the mode (range, 75400 mg/kg from 1 to 5 doses). This dose is significantly below the doses used in the treatment of other diseases, like Guillain‐Barr syndrome, myasthenia gravis, Kawasaki disease, autoimmune hemolytic anemia, agammaglobulinemia, and hypogammaglobulinemia, where the usual dose is 400 mg/kg for 5 days. The resolution of diarrhea in these cases occurred after an average of 9 (range, 142) days. The index hospitalization survival rate varied from 43% to 100%. Patients received standard treatment for an average of 13 (range, 065) days before IVIG infusion. Thirty‐two of 51 patients survived their illness (63%). Neither total IgG nor anti‐toxin A IgG levels were measured in any of the reports. Of the 32 patients who had clinical resolution, 3 (10%) experienced symptoms recurrence in a follow‐up period of 1 to 13 months. This number is most probably an underestimation of the true recurrence rate resulting from an incomplete reporting because there was no uniform or active recurrence ascertainment mechanism in any of the studies. The recurrences were at 10, 14, and 30 days posttreatment. Since standard treatment was not discontinued in any of the reports once IVIG was given, the relative contribution and the ideal timing for IVIG infusion are still unclear.
The mechanism of action of IVIG is passive immunization (with anti‐toxin A and anti‐toxin B antibodies present in the pooled immunoglobulin) of a host who is usually unable to mount an adequate protective immune response.15, 22 IVIG is formed from pooling immunoglobulin from several random donors. It has been shown that many such donors express high anti‐toxin A and anti‐toxin B antibody serum titers.38, 39 In addition, high levels of anti‐toxin A and anti‐toxin B antibodies were present in the IVIG preparations and the recipients after infusion.1517, 22 Although constituting only a small fraction of the total IVIG administered, these antitoxin antibodies are believed to neutralize toxin A and B and help the host recover from the disease. In fact, Babcock et al.40 used an experimental hamster model of CDC to demonstrate a mortality reduction from 100% to 55% postinfusion of combined anti‐toxin A and anti‐toxin B antibodies. While some early reports indicated that anti‐toxin B antibodies were the major determinants of protection against colitis,41 later reports correlated disease severity pathologically42 and clinically43, 44 with anti‐toxin A levels. Anti‐toxin B antibodies were later shown to play an adjunctive role in conferring immunity against CDC40, 45, 46 when added to anti‐toxin A antibodies, but not to have any significant role on their own.
However, IVIG has been shown to contain IgG, but not IgA, anti‐toxin A and anti‐toxin B antibodies while it is only the IgA class of anti‐toxin A antibodies, and not the IgG class, that could neutralize toxin A in vitro and in vivo.47, 48 Babcock et al.40 solved this apparent dilemma by showing that a combination of 3 different monoclonal IgG anti‐toxin A antibodies could neutralize toxin A activity in vitro and prevent disease in the hamster model in vivo. Each of the 3 antibodies recognized a different toxin A domain: the first neutralized toxin A enzymatic activity, while the second prevented toxin A binding to its receptor on enterocytes, and the third prevented toxin internalization after binding to the receptor.
Thus, the mechanism of action of IVIG is most likely through the transfer of IgG anti‐toxin A antibodies that gain access to the intestinal lumen presumably secondary to inflammation‐induced mucosal damage and neutralize toxin A. Transfer of yet undetected IgA anti‐toxin A antibodies that prevent toxin A from binding to its receptor is much less likely, although possible.
The present study has limitations. As in all retrospective studies, selection bias was unavoidable. In addition, the decision to initiate IVIG administration was dependent on the attending physician, who also decided the dose, leading to heterogeneity in the total dose of IVIG infused. Such heterogeneity, however, is primarily the result of a lack of a standard dose for IVIG infusion for CDC in the published literature, as reported above. In addition, since IVIG is not yet approved by the U.S. Food and Drug Administration (FDA) for the treatment of severe CDC, standard treatment was not discontinued in any of the reports to date, including ours.
Choosing appropriate controls for patients suffering from severe CDC is challenging. This patient population is usually frail, with severe and multiple underlying diseases. The deteriorating clinical condition (and subsequently the need for multiple antibiotics) may be either the result of or the cause of CDC. Furthermore, IVIG has been in short supply for several years and therefore it has been expensive, making its administration to the number of patients needed to design adequately‐powered controlled studies difficult. These are mainly the reasons no randomized, multicenter, placebo‐controlled trial on IVIG use in severe CDC has been conducted to date.
Conclusions
In the present study, we report the results of IVIG use for the treatment of 21 patients with severe CDC. This is the largest cohort to our knowledge in the literature. Unlike previous studies on the subject, the present report provided 2 scales for disease assessment: the first based on the extent of colonic involvement and the second measuring the severity of systemic involvement using the APACHE II score. The latter was positively associated with mortality in this context. Of the 21 study patients treated with IVIG, only 9 patients (43%) survived their illness. This is the highest reported mortality rate among all studies on this subject so far. Further studies on the ideal timing of IVIG infusion, dose, and patient selection are needed before accepting IVIG as a standard of care for severe CDC treatment. The role of APACHE II score in the decision to use IVIG is promising and should be validated in randomized controlled trials.
Acknowledgements
The authors thank Indrani Mukherjee, MD, for her help and expertise in proofreading the manuscript.
- ,,.Secular trends in hospital‐acquired Clostridium difficile disease in the United States, 1987–2001.J Infect Dis.2004;189:1585–1589.
- ,,, et al.Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications.Ann Surg.2002;235:363–372.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Clostridium difficile‐associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity.CMAJ.2004;171:466–472.
- ,,.Comparison of two toxins produced by Clostridium difficile.Infect Immun.1981;34:1036–1043.
- ,,,.Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A.N Engl J Med.2000;342:390–397.
- ,,,.Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection.Infect Immun.1994;62:384–389.
- ,,.Risk factors for Clostridium difficile carriage and C. difficile‐associated diarrhea in a cohort of hospitalized patients.J Infect Dis.1990;162:678–684.
- ,,.Severe Clostridium difficile colitis.Dis Colon Rectum.1995;38:350–354.
- ,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,.Factors associated with failure of metronidazole in Clostridium difficile‐associated disease.J Clin Gastroenterol.2004;38:414–418.
- ,,.Treatment of Clostridium difficile‐associated disease: old therapies and new strategies.Lancet Infect Dis.2005;5:549–557.
- ,,, et al.Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole.Clin Infect Dis.2005;40:1586–1590.
- ,,, et al.Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada.Clin Infect Dis.2005;40:1591–1597.
- ,,,,,.Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin.J Pediatr.1991;118:633–637.
- ,,,.Gamma globulin administration in relapsing Clostridium difficile‐induced pseudomembranous colitis with a defective antibody response to toxin A.Acta Clin Belg.1995;50:36–39.
- ,,,.Recurrent Clostridium difficile infection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii.Clin Infect Dis.1995;20(suppl 2):S266–S268.
- .Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea.Gut.2002;51:456.
- .Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea.J Antimicrob Chemother.2004;53:882–884.
- ,,.Intravenous immunoglobulin for resistant Clostridium difficile infection.Age Ageing.2006;35:85–86.
- ,,,,.Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea.Dis Colon Rectum.2006;49:640–645.
- ,,, et al.Intravenous immunoglobulin therapy for severe Clostridium difficile colitis.Gut.1997;41:366–370.
- ,,, et. al.Intravenous immunoglobulin therapy for refractory Clostridium difficile toxin colitis in chronic kidney disease: case reports and literature review.NDT Plus.2008;1:20–22.
- ,.Use of intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis.Am J Geriatr Pharmacother.2007;5:48–51.
- ,,, et al.Clinical outcomes of intravenous immune globulin in severe clostridium difficile‐associated diarrhea.Am J Infect Control.2007;35:131–137.
- ,,,.Successful treatment of Clostridium difficile colitis with intravenous immunoglobulin.J Gastrointestin Liver Dis.2008;17:353–359.
- ,,, et al.A durable response to relapsing Clostridium difficile colitis may require combined therapy with high‐dose oral Vancomycin and intravenous immune globulin.Infect Dis Clin Pract.2006;14:217–220.
- ,,,.APACHE II: a severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,,,,.Colonic abnormalities on CT in adult hospitalized patients with Clostridium difficile colitis: prevalence and significance of findings.AJR Am J Roentgenol.2006;186:1393–1400.
- ,,,.Clostridium difficile‐associated diarrhea: predictors of severity in patients presenting to the emergency department.Can J Gastroenterol.2003;17:369–373.
- ,,, et al.Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain.Ann Surg.2007;245:267–272.
- ,, et al.Risk factors for severity and relapse of pseudomembranous colitis in an elderly population.Colorectal Dis.2007;9:173–177.
- ,,,,.Prognostic criteria in Clostridium difficile colitis.Am J Gastroenterol.1996;91:460–464.
- ,,, et al.Clostridium difficile toxin a stimulates macrophage‐inflammatory protein‐2 production in rat intestinal epithelial cells.J Immunol.1998;160:6039–6045.
- ,,, et al.Increased substance P responses in dorsal root ganglia, intestinal macrophages during Clostridium difficile toxin a enteritis in rats.Proc Natl Acad Sci U S A.1997;94:4788–4793.
- ,,,,,.Cytokine response by human monocytes to Clostridium difficile toxin a and toxin B.Infect Immun.1991;59:3659–3666.
- ,,,.Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection.J Med Microbiol.1998;47:591–598.
- ,.Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic‐associated diarrhea.Diagn Microbiol Infect Dis.1994;18:205–209.
- ,,, et al.Serum antibody response to toxins A and B of Clostridium difficile.J Infect Dis.1983;148:93–100.
- ,,, et al.Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile‐induced mortality in hamsters.Infect Immun.2006;74:6339–6347.
- ,,,.Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea.Infection.1985;13:97–101.
- ,,,.Relationship between levels of Clostridium difficile toxin A and toxin B and cecal lesions in gnotobiotic mice.Infect Immun.1989;57:2123–2127.
- ,,,,.Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A.Infect Immun.1991;59:1192–1195.
- ,,.Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile.J Infect Dis.1992;166:1287–1294.
- ,,, et al.Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A‐neutralizing antibodies in mice.Infect Immun.2007;75:2826–2832.
- ,,, et al.Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters.Infect Immun.1999;67:527–538.
- ,,,.Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A‐receptor binding.Gastroenterology.1992;102:35–40.
- ,,,,.Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease.Infect Immun.1995;63:3166–3173.
Clostridium difficile colitis (CDC) is the most common cause of hospital‐acquired diarrhea.1 The incidence of CDC has sharply increased over the past decade despite increasing awareness among health care professionals.24 C. difficile pathogenic strains induce diarrhea through the elaboration and secretion of 2 exotoxins: toxin A and toxin B. Toxin A is an inflammatory toxin, leading to fluid secretion, increased mucosal permeability, and marked enteritis and colitis.5 Toxin B is cytotoxic, leading to cell injury and apoptosis.5 Combined, toxin A and toxin B can cause a wide spectrum of clinical presentations, ranging from mild diarrhea that resolves with the discontinuation of antibiotics to a fulminant colitis requiring surgical intervention.
The severity of clinical manifestations has been shown to be inversely proportional to the host anti‐toxin A antibody level in response to toxin exposure. Kyne et al.6 demonstrated that asymptomatic C. difficile carriers produced significantly higher anti‐toxin A immunoglobulin G (IgG) levels compared to symptomatic patients. Among the latter group, patients with mild disease had a higher antibody level compared to those with severe colitis.6, 7 Additional risk factors predisposing to severe colitis are advanced age, severe underlying illness,8 and immunocompromised state.9
Recently, a new C. difficile strain (BI/NAP1) with a mutated toxin A and toxin B promoter silencer, a binary toxin gene, and fluoroquinolone resistance has been described in Canada and the United States.3, 10 This strain has been associated with an increased incidence of CDC among hospitalized patients, especially the incidence of severe disease requiring colectomy. At the same time, several reports describing metronidazole treatment failure have been published.1114 These recent findings emphasize the importance of finding alternative treatments for CDC.
Intravenous immunoglobulin (IVIG) was used to treat CDC for the first time in 1991.15 Since then, 12 case reports and small case series, along with 1 case‐control study have been published documenting IVIG treatment outcomes.1527 However, only 5 reports to date examined patients with severe CDC.2125 In the present study, we report the largest series of patients with severe CDC treated with IVIG in the literature to our knowledge.
Patients and Methods
Case Series
We used CareScience software (CareScience, Inc., Philadelphia, PA) to retrospectively identify all patients admitted to our institution with a primary or secondary diagnosis of CDC (code 00845) between July 1, 2002 and May 1, 2006. CareScience is commercially available software that tracks all admissions to our institution and allows the performance of patient searches with a wide spectrum of user‐defined search criteria. Using the same software, we further identified those patients who received IVIG during their hospital stay. We then obtained the hospital chart for each patient, from the medical records department and established the study database.
A case was defined as a patient with diarrhea (at least 3 loose stools daily) for at least 2 days who had C. difficile cytotoxin‐positive feces and at least 1 of the following criteria: clinical symptoms (abdominal pain and/or distension and fever); leukemoid reaction (defined as white blood cell count of 20,000 cells/mm3 or above. This cutoff value was chosen as it has been used previously as a prognostic factor)4; radiographic evidence of colitis by computed tomography (CT) of the abdomen; and/or the presence of pseudomembranes on flexible sigmoidoscopy or colonoscopy. We excluded all patients who received IVIG for an indication other than CDC treatment (n = 3). There were no other exclusion criteria.
We used a standardized data collection tool and recorded demographics (age, gender, principal diagnosis), past medical and surgical history, other risk factors for C. difficile infection (previous CDC, antibiotics received during hospital stay, immunosuppressive medications or organ transplantation within the previous 6 weeks, history of malignancy or diabetes mellitus); clinical presentation (abdominal distention, abdominal pain, diarrhea, fever, leukemoid reaction, and hypotension defined as systolic blood pressure <85 mm Hg despite at least 1 L of intravenous normal saline administration and the need for vasopressor use); colonoscopy findings; CT scan and x‐ray findings; laboratory values; date and dose of IVIG infused and other C. difficile pharmacological treatments; and Acute Physiological Assessment and Chronic Health Evaluation (APACHE II) score28 at the first day of IVIG infusion. The primary outcomes were survival at the end of the hospital stay and clinical disease resolution, defined as 2 formed bowel movements or less per day without abdominal pain or distention.
The decision to initiate IVIG therapy and the dose to be used was made by the individual attending physician.
Statistical Analysis
Single (univariate) and multiple (multivariate) logistic regression analysis were applied to identify variables among the ones collected that are independent predictors of CDC mortality. All statistical analyses were completed with the STATA 10 software package (StatCorp LP, College Station, TX).
Review of the Literature
We used PubMed, Web of Science, Scopus, and Excerpta Medica databases to search for any publication in a peer‐reviewed journal on the use of IVIG for the treatment of severe CDC. We used the search words: IVIG or intravenous immunoglobulin and clostridium difficile. Only publications published in English were selected. The date range used was January 1, 1950 to January 7, 2009. We were able to find 5 publications using this search criteria.
Results
Study Population
Of the 1230 patients diagnosed with CDC over the 4‐year study period, 21 patients were treated with IVIG. Table 1 summarizes the patients' characteristics. There were 13 women and 8 men. The mean age was 68 years, with a standard deviation (SD) of 13 years. Sepsis was the primary diagnosis in all patients. Sixteen patients had predisposing risk factors for CDC, including immunosuppression (immunosuppressive medication [n = 2], human immunodeficiency virus [HIV] infection [n = 2]); cancer (n = 3); recent surgery (n = 3); and diabetes mellitus (n = 11). Nine patients had documented previous CDC episodes. The indications for IVIG administration were evidence of pancolitis on abdominal CT scan (n = 12) or severe ileus with cessation of diarrhea, abdominal distention, and requirement for total parenteral nutrition (n = 5), or severe hypotension (n = 4) (defined as systolic blood pressure <85 mm Hg despite at least 1 L of intravenous normal saline administration and the subsequent need for vasopressor use).
| Patient | Age (gender) | Diagnosis | Medical and Surgical History | CDC History | Colonoscopy Findings | Radiographic Findings |
|---|---|---|---|---|---|---|
| ||||||
| A | 40 (female) | CDC with sec. sepsis | Gastric stapling | Yes | * | Diffuse colitis |
| B | 86 (female) | Fulminant CDC | Metastatic ovarian carcinoma | PC | No colonic thickening | |
| C | 72 (male) | Sepsis | Acute pancreatitis with sec. pseudocyst, DM | Yes | PC | Diffuse colitis |
| D | 78 (male) | Discitis | Delayed: normal mucosa | Dilation of small and large bowel | ||
| E | 98 (female) | Urosepsis | Yes | * | No bowel distention | |
| F | 90 (female) | Right lower extremity cellulitis | DM | * | Concentric thickening of rectal wall | |
| G | 64 (male) | Ischemic colitis | DM, recent Hartman pouch closure | Marked inflammation | Diffuse colitis | |
| H | 78 (female) | Toe osteomyelitis and CDC with sec. sepsis | DM | * | Diffuse colitis | |
| I | 35 (female) | Sepsis | Yes | * | Diffuse nonspecific colitis, minimal ascites | |
| J | 47 (female) | Pneumonia and sec. sepsis | * | Diffuse colitis | ||
| K | 56 (female) | Urosepsis | HIV | Yes | * | Colitis involving the right colon |
| L | 76 (male) | CDC with sec. sepsis | Sigmoid bladder fistula repair, DM | * | Diffuse colitis | |
| M | 71 (female) | Pneumonia with sec. sepsis | * | Diffuse colitis | ||
| N | 63 (male) | Urosepsis | DM, lymphoma resection from small intestine | PC | Marked small and large bowel distention | |
| O | 86 (male) | Enterococcus‐induced sepsis | Rheumatoid arthritis on methotrexate | * | Fat stranding suggesting peritonitis | |
| P | 60 (female) | Gastrointestinal bleed and CDC | DM with neuropathy and retinopathies | Yes | * | Thickening of wall of colon in most of the colon |
| Q | 57 (female) | Sepsis | DM | Yes | * | Normal |
| R | 67 (female) | CDC with sec. sepsis | Candidal esophagitis | Yes | * | Large amount of peritoneal fluid, mild small bowel thickening |
| S | 60 (female) | Sepsis sec. to S. aureus and P. aerogenosa | DM, renal transplant (myco, prednisone) | * | Ileus with air fluid level in the small intestine | |
| T | 80 (female) | Sepsis | DM | Yes | * | Thickening of descending colon consistent with colitis |
| U | 72 (male) | CDC, widespread metastatic cancer | DM, metastatic cancer (unknown primary site) | * | Severe colitis up to the splenic flexure | |
Table 2 describes disease severity in these patients. Since CDC starts locally in the colon then secondarily involves multiple organs as part of the systemic inflammatory response syndrome (SIRS), 2 scales were used to characterize the disease in each patient: (1) extent of local colonic inflammation, and (2) severity of systemic involvement. Extensive colonic involvement was evidenced in all patients by pancolitis on abdominal imaging modalities (12 patients), severe ileus requiring total parenteral nutrition (13 patients), or referral for surgical consultation for possible colectomy (12 patients).
| Patient | Complications During the Hospital Stay | APACHE II Score | Monitored Unit | WBC* | K | Alb | Lactate | Cr | Hospital Stay (days) | Surgical Consult/Surgery | TPN for Colitis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| A | Sepsis, DIC, ARDS, intubation | 15 | Yes | 48 | 4 | 1.1 | 2.9 | 1.3 | 26 | No/No | Yes |
| B | Dehydration, weakness | 12 | No | 19 | 3.4 | 3.1 | 1.1 | 16 | No/No | Yes | |
| C | HTP, GI bleed, ischemic colitis, F | 22 | Yes | 21 | 2.7 | 2.5 | 1.3 | ESRD | 34 | Yes/No | Yes |
| D | AD, megacolon, HT, intubation, ARF | 18 | Yes | 25 | 3.4 | 1.4 | 1.1 | 2.6 | 52 | Yes/No | Yes |
| E | Exacerbation of CHF with respiratory distress, Bipap support, confusion | 21 | No | 10 | 2.6 | 2.3 | 1 | 11 | No/No | No | |
| F | Confusion, gout acute attack | 21 | No | 33 | 3.1 | 2.6 | ESRD | 15 | No/No | No | |
| G | Intubation, cardiac arrest, AF with RVR, PNA, ARF, DVT, dysphagia, PEG | 6 | Yes | 15 | 3.1 | 1.7 | 1.9 | 2.2 | 32 | Yes/No | Yes |
| H | Sepsis, intubation, ARF, PEG, toe amputation, PNA, pulmonary edema, TPN, vitamin D deficiency | 34 | Yes | 59 | 2.8 | 1.5 | 4 | 3.8 | 17 | No/No | Yes |
| I | CHF, transient third cranial nerve palsy, DIC | 20 | Yes | 52 | 3.2 | 3.3 | 0.7 | ESRD | 11 | No/No | No |
| J | Aspiration PNA, sepsis, DIC, intubation, MI, F, HT | 32 | Yes | 17 | 2.7 | 1.8 | 14 | ESRD | 21 | Yes/no | No |
| K | Intubation, cardiac arrest, ARF, DIC, AP, F | 30 | Yes | 25 | 2.1 | 2.1 | 13 | 3.6 | 10 | Yes/no | No |
| L | Intubation, ARF, HTP, MI, AD, F | 23 | Yes | 69 | 3.2 | 1.8 | 1.4 | 3.6 | 23 | Yes/no | No |
| M | Intubation, DIC, ARF, GI bleeding, hypothermia, AD | 23 | Yes | 47 | 2.8 | 1.4 | 1.4 | 4.3 | 23 | Yes/no | Yes |
| N | Intubation, HTP, AD, F, ARF with HD, AF, osteomyelitis | 23 | Yes | 46 | 2.9 | 1.5 | 1.5 | 2.7 | 27 | Yes/no | Yes |
| O | Intubation, ARF, 2 cardiac arrests, GI bleed, UTI, rhabdomyolysis, liver shock, AF | 31 | Yes | 49 | 2.5 | 1.1 | 2.3 | 2.9 | 25 | Yes/no | Yes |
| P | Bowel ischemia with bowel resection, ARF, MI, ischemic bowel, fluid overload, respiratory failure | 23 | Yes | 26 | 2.6 | 0.8 | 2.9 | 3.1 | 9 | Yes/Yes | Yes |
| Q | Pulmonary embolism | 39 | Yes | 23 | 2.8 | 1.1 | 8.1 | ESRD | 9 | No/No | Yes |
| R | Fungal peritonitis, aspiration pneumonia, cardiac arrest | 26 | Yes | 30 | 4.1 | 1.1 | 0.9 | ESRD | 36 | No/No | Yes |
| S | Intubation, pneumothorax, CRT, pressor‐dependent shock, ARF | 36 | Yes | 46 | 3.1 | 1.8 | 2.1 | 5.4 | 64 | Yes/Yes | No |
| T | Pressor‐dependent sepsis, pulmonary edema, ARF with HD | 36 | Yes | 35 | 3.3 | 1.3 | 1.8 | 5.5 | 11 | Yes/No | Yes |
| U | Sepsis | 34 | Yes | 58 | 3.1 | 2.2 | 3.3 | 1.6 | 9 | No/No | No |
Of the 21 patients treated with IVIG, 9 did not receive a surgical consultation either because they responded to medical treatment promptly (6 patients), were too unstable for surgery (2 patients), or because the patient/family refused surgery (1 patient). Of the 12 patients who received surgical consultation, 2 underwent surgery. The remainder did not proceed to surgery for the following reasons: they were deemed medically unstable for surgery (6 patients), declined surgery (2 patients), were diagnosed with cancer on colonoscopy (1 patient), or improved with medical treatment (1 patient).
The severity of systemic involvement was measured using the APACHE II score on day 1 of IVIG infusion. The mean APACHE II score was 25. Eighteen patients were in a monitored unit when IVIG was administered. The study group had laboratory results in keeping with those previously used to define severe colitis:4, 9, 19 leukocytosis (defined as white blood cell count higher than 12,000 cells/mL [mean = 36,000 cells/mL]), hypoalbuminemia (mean = 1.78 g/dL, SD = 0.68 g/dL), hypokalemia (mean = 3.02 mg/dL, SD = 0.47 g/dL), and acute renal failure (defined as serum creatinine level >1.5 mg/dL [mean = 2.98 mg/dL, SD = 1.42 g/dL]).
IVIG Use
Table 3 describes the treatment patients received for CDC as well as the total number of antibiotics used throughout the hospital stay. IVIG was used as adjuvant treatment (defined as IVIG administration within 4 days or less after CDC diagnosis) in 8 patients and as second‐line treatment (defined as IVIG administration more than 4 days after CDC diagnosis) in 13 patients. Metronidazole, vancomycin, cholestyramine, and probiotic treatment alone or in different combinations were used for an average of 8 days (SD = 8 days; range, 025 days) before IVIG infusion. The total IVIG dose administered varied depending on the prescribing attending, with a range of 200 mg/kg to 1250 mg/kg and a mode of 250 mg/kg for 1 to 3 days. An average of 5 (SD = 2) different antibiotics that were not active against C. difficile were used per patient without being discontinued after a CDC diagnosis was made. The 3 most common were: cephalosporins (cefazolin, ceftriaxone, cefepime), fluoroquinolones (levofloxacin), and combination antibiotics (piperacillin and tazobactam or ampicillin and sulbactam).
| Patient | Number of Antibiotics | Duration of Treatment Before IVIG (days) | Treatment Before IVIG (days) | Total CDC Treatment (days) | IgG Level | IVIG Dose |
|---|---|---|---|---|---|---|
| ||||||
| A | 5 | 7 | Metro (7), Vanc (7), Choles (2) | Oral and rectal Vanc (26,19), IV Metro (19), Choles (2), Lacto (6) | Low | 300 mg/kg for 1 day |
| B | 1 | 13 | Metro (13), Vanc (13) | Oral Vanc (17) and IV Metro (12) | 300 mg/kg for 1 day | |
| C | 3 | 7 | Metro (7), Vanc (3) | IV Metro (28),Vanc oral and enema (18,3), Lacto (10) | Low | 125 mg/kg for 5 days |
| D | 5 | 25 | Metro (25), Vanc (15) | Oral then IV Metro (10,15), oral Vanc (25), Choles (7) and Lacto (13) | Low | 200 mg/kg for 1 day |
| E | 1 | 4 | Metro (1), Vanc (4), Choles(4) | IV Metro (8), oral Vanc (10) and Choles (4) | 75 mg/kg for 5 days | |
| F | 4 | 2 | Metro (2), Vanc (1) | IV Metro (8) and oral Vanc (9) | 250 mg/kg for 5 days | |
| G | 3 | 17 | Metro (17), Vanc (14) | IV Metro (49) and oral Vanc (61) | 250 mg/kg for 2 days | |
| H | 5 | 1 | Metro (1), Vanc (1) | Oral Metro (18), oral Vanc (22 ), IV Metro (3) | 250 mg/kg for 3 days | |
| I | 6 | 1 | Metro (1) | Oral and IV Metro (7,9), Vanc oral and enema (8,3) | 250 mg/kg for 2 days | |
| J | 8 | 16 | Metro (14), Vanc (2), Lacto (6) | Oral then IV Metro (10,5), oral and rectal Vanc (3,1), Lacto (6) | 300 mg/ kg for 1 day | |
| K | 6 | 7 | Metro (7), Vanc (3) | Oral then IV Metro (10) and oral Vanc (9) | Normal | 400 mg/kg for 2 days |
| L | 4 | 0 | None | IV then oral Metro (7,10), oral Vanc (23), Choles (7), and Lacto (5) | 150 mg/kg for 5 days | |
| M | 6 | 1 | Metro (1) | IV Metro (23) and oral Vanc (9) | 250 mg/kg for 2 days | |
| N | 7 | 1 | Metro (1), Vanc (1) | IV Metro (16), oral Vanc (14), oral Metro (7) | 250 mg /kg for 2 days | |
| O | 6 | 7 | Metro (6), Vanc (4) | IV Metro (6) and oral Vanc (22) | Low | 250 mg/kg for 2 days |
| P | 3 | 6 | Metro (6), Vanc (6) | IV Metro (25) and oral Vanc (17) | 150 mg/kg fro 3 days | |
| Q | 3 | 4 | Metro (4), Vanc (3) | Oral Metro (8),Vanc oral and enema (6,3) | 250 mg/kg for 3 days | |
| R | 5 | 9 | Vanc (9) | Vanc oral and enema (5,4), IV Metro (2) | 250 mg/kg for 1 day | |
| S | 8 | 23 | Metro (23), Vanc (23) | Oral then IV Metro (12,22), oral Vanc (39) | 250 mg/kg for 3 days | |
| T | 4 | 6 | Metro (6), Vanc (1) | Oral Vanc and IV Metro (11) | 250 mg/kg for 1 day | |
| U | 4 | 2 | Metro (2), Vanc (2) | Oral Vanc and IV Metro (6) | 250 mg/kg for 3 days | |
Survival with IVIG Use
Nine patients (43%) survived their illness and were discharged from the hospital. They experienced complete clinical resolution after an average of 10 days from IVIG administration (range, 220 days) (Table 4). The other 12 patients (57%) died during the index hospitalization. The average length of stay was 23 (range, 964) days.0
| Patient | Disposition | Clearance of Clostridium difficile Colitis? | Days to Resolution |
|---|---|---|---|
| |||
| A | Alive | Loose BM persisted but diarrhea resolved 9 days after IVIG. | 9 |
| B | Alive | BM became formed and diarrhea resolved 48 hours after IVIG. | 2 |
| C | Alive | Diarrhea resolved 20 days post‐IVIG infusion. CAT scan: colonic thickening improved. | 20 |
| D | Alive | Diarrhea resolved on discharge. Response to IVIG started next day after administration. | 18 |
| E | Alive | Diarrhea improved next day after IVIG administration and resolved on discharge. | 5 |
| F | Alive | Diarrhea resolved 5 days after IVIG administration. | 5 |
| G | Alive | Diarrhea resolved. C. difficile test became negative. | 13 |
| H | Alive | Diarrhea resolved 2 days before discharge. | 15 |
| I | Alive | Diarrhea slowly improved and resolved 4 days before discharge. | 7 |
| J | Deceased | ||
| K | Deceased | ||
| L | Deceased | ||
| M | Deceased | ||
| N | Deceased | ||
| O | Deceased | ||
| P | Deceased | ||
| Q | Deceased | ||
| R | Deceased | ||
| S | Deceased | ||
| T | Deceased | ||
| U | Deceased | ||
| Study | Number of Patients | Age (SD) (years) | Male | Female | Severity Definition | IVIG Dose | Days to Resolution | Days IVIG Infused | Alive? (%) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| Salcedo et al.22 | 2 | 63, 64 | 1 | 1 | Pancolitis or thumbprinting on CAT scan | 200300 mg/kg once | 12 | 59 | 100 | 1 out of 2 |
| McPherson et al.21 | 8 | 72 (12) | ? | ? | Pancolitis | 200400 mg/kg twice | 226 | 1165 | 75 | 2 out of 6 |
| Juang et al.25 | 18 | 67 (17.4) | 5 | 13 | Modified Rubin et al.9 criteria | 200300 mg/kg once | ? | ? | 83 | ? |
| Hassoun et al.24 | 1 | 72 | 1 | 0 | Pancolitis | 400 mg/kg once | 6 | 15 | 100 | None |
| Chandrasekar et al.23 | 1 | 67 | 0 | 1 | Shock requiring inotropic support and pseudomembranes on colonoscopy | 400 mg/kg for 5 doses | 16 | 35 | 100 | ? |
| This paper | 21 | 68 (16) | 7 | 14 | Pancolitis and APACHE II score | 300 mg/kg once; 250 mg/kg for 5 doses | 220 | 025 | 43 | ? |
To further assess the impact of IVIG on colitis resolution, we investigated all variables in the data set that may have been associated with mortality using univariate Cox regression analysis. Those variables were as follows: APACHE II score on the first day of IVIG infusion, age, sex, previous history of CDC, number of days before IVIG use, peak white blood cell count, serum potassium level and creatinine level, lactate level on first day of IVIG infusion, and number of antibiotics administered that are not active against CDC. Only the APACHE II score (P = 0.006) and lactate level on the day of IVIG infusion (P = 0.004) were (positively) associated with CDC mortality. The positive association between CDC mortality and APACHE II score remained significant (P = 0.04) after adjusting for sex, previous history of CDC, number of days before IVIG use, lactate level on first day of IVIG infusion, and number of antibiotics administered that are not active against CDC using a multivariate Cox regression analysis model. No adjustments were made for age, white blood cell count, potassium level, or creatinine level as those are included within the APACHE II score. The positive association between lactate level on the first day of IVIG infusion and CDC mortality was not statistically significant after adjusting for the factors listed above in the same model (P = 0.13).
Discussion
To our knowledge, the present study is the largest series published in the literature to date on the use of IVIG for severe CDC. It is also the first study to report a high mortality rate compared to the 5 previous smaller studies on this topic. In the first report on IVIG use for CDC, Leung et al.15 used IVIG to treat 5 pediatric patients suffering from chronic relapsing CDC. It was not until 7 years later, in 1997, that the first IVIG use for severe CDC was reported.22 Since then, a total of 13 works have been published on IVIG for CDC treatment, and only in 5 of these was IVIG administered for severe CDC treatment:2125 3 case reports, 1 case series, and 1 case‐control study. Although the 4 uncontrolled reports concluded that IVIG is beneficial for severe CDC, the only controlled study reported no significant difference between cases and controls for all‐cause mortality, length of stay, and colectomy rate.25
The definition of severe CDC varied between reports, making comparison difficult. McPherson et al.21 and Hassoun and Ibrahim24 defined severe disease as one causing pancolitis on CT scan either with or without megacolon. In the study by Juang et al.,25 disease severity was assessed using the modified criteria of Rubin et al.9 Salcedo et al.22 defined severe CDC as one causing pancolitis in one patient and thumbprinting on CT scan in another, whereas Chandrasekar et al.23 defined it as one causing shock requiring inotropic support and presence of pseudomembranes on colonoscopy.
The present report is unique in that it provided 2 scales to characterize disease in each patient. The first scale measured colonic involvement anatomically and physiologically using a combination of computerized axial tomography (CAT) scan findings, presence or absence of ileus or referral for possible colectomy. This is not a prognostic scale, however, since CAT scan findings have been previously shown to be poor predictors of treatment outcome.29 The second scale measured the severity of systemic involvement using a well‐validated and standardized scale, the APACHE II score. We have shown in this report that it is associated with prognosis in the context of IVIG use.
Our study reports a higher mortality rate than previously described, and suggests that risk stratification and patient selection are important before IVIG administration, since not all patients seem to benefit from this treatment as previously suggested by smaller case series. Previously, several physical findings and laboratory values were found to be associated with worse outcome in CDC. These were increasing age, immunosuppression, shock requiring vasopressors, peak white blood cell count, peak serum lactate level, hypoalbuminemia, a fall in serum albumin level of >1.1 g/dL at the onset of CDC symptoms, use of 3 or more antibiotics, comorbid disease, previous history of CDC, acute renal failure and hypotension, underlying altered or depressed mental status, abdominal pain or distention, white blood cell count over 20,000/mm3 or <1500/mm3 and/or a >10% band forms on the white blood cell differential count, and ascites or pneumatosis coli by abdominal imaging.9, 3033 Using the APACHE II scale for the same purpose has the advantage of utilizing a well‐validated and objective scale that is expected to measure the degree of systemic involvement more reliably compared to the clinical and laboratory values above.
Timing of IVIG infusion remains controversial. Due to the lack of randomized controlled trials, the current practice is guided by expert opinion, leading to wide variations between reports. Since the APACHE II score was positively associated with mortality in the setting of IVIG treatment, the same scale could be used to guide decisions regarding timing of IVIG infusion. Our results suggest that IVIG should be preferentially used while the APACHE II score is still relatively low. This association and the specific APACHE II score at which to initiate or hold treatment need to be validated in the setting of a randomized controlled study before being used in clinical practice.
Although the current study was not designed to test this theory, IVIG could be associated conceptually with treatment success for patients with severe disease that is still restricted to the colon (without other organ dysfunction or at least at an early stage of extracolonic organ failure and thus associated with a low APACHE II score) but not for severe colonic disease with secondary multiple organ failure (high APACHE II score). This may be because colonic disease is toxin‐mediated whereas secondary systemic involvement is mediated through toxin‐induced inflammatory mediators (interleukin‐8, macrophage‐inflammatory protein‐2, substance P, tumor necrosis factor‐alpha) released locally in the colon,3436 triggering a SIRS and hematogenous translocation of colonic bacteria,37 both of which are poorly responsive to immunoglobulin infusion. Along the same lines, waiting for failure of conventional therapy before IVIG use might result in IVIG treatment failure because of disease progression and secondary sepsis, at which point no treatment may be effective. No study thus far has addressed this issue specifically.
Overall, the combined cohort of patients with severe CDC treated with IVIG in the literature includes 51 patients (Table 4). The current report contributes 41% of these patients. The patients' average age was 68 years, with a 2 to 1 female‐male ratio. The dose of IVIG used varied largely also, with 400 mg/kg being the mode (range, 75400 mg/kg from 1 to 5 doses). This dose is significantly below the doses used in the treatment of other diseases, like Guillain‐Barr syndrome, myasthenia gravis, Kawasaki disease, autoimmune hemolytic anemia, agammaglobulinemia, and hypogammaglobulinemia, where the usual dose is 400 mg/kg for 5 days. The resolution of diarrhea in these cases occurred after an average of 9 (range, 142) days. The index hospitalization survival rate varied from 43% to 100%. Patients received standard treatment for an average of 13 (range, 065) days before IVIG infusion. Thirty‐two of 51 patients survived their illness (63%). Neither total IgG nor anti‐toxin A IgG levels were measured in any of the reports. Of the 32 patients who had clinical resolution, 3 (10%) experienced symptoms recurrence in a follow‐up period of 1 to 13 months. This number is most probably an underestimation of the true recurrence rate resulting from an incomplete reporting because there was no uniform or active recurrence ascertainment mechanism in any of the studies. The recurrences were at 10, 14, and 30 days posttreatment. Since standard treatment was not discontinued in any of the reports once IVIG was given, the relative contribution and the ideal timing for IVIG infusion are still unclear.
The mechanism of action of IVIG is passive immunization (with anti‐toxin A and anti‐toxin B antibodies present in the pooled immunoglobulin) of a host who is usually unable to mount an adequate protective immune response.15, 22 IVIG is formed from pooling immunoglobulin from several random donors. It has been shown that many such donors express high anti‐toxin A and anti‐toxin B antibody serum titers.38, 39 In addition, high levels of anti‐toxin A and anti‐toxin B antibodies were present in the IVIG preparations and the recipients after infusion.1517, 22 Although constituting only a small fraction of the total IVIG administered, these antitoxin antibodies are believed to neutralize toxin A and B and help the host recover from the disease. In fact, Babcock et al.40 used an experimental hamster model of CDC to demonstrate a mortality reduction from 100% to 55% postinfusion of combined anti‐toxin A and anti‐toxin B antibodies. While some early reports indicated that anti‐toxin B antibodies were the major determinants of protection against colitis,41 later reports correlated disease severity pathologically42 and clinically43, 44 with anti‐toxin A levels. Anti‐toxin B antibodies were later shown to play an adjunctive role in conferring immunity against CDC40, 45, 46 when added to anti‐toxin A antibodies, but not to have any significant role on their own.
However, IVIG has been shown to contain IgG, but not IgA, anti‐toxin A and anti‐toxin B antibodies while it is only the IgA class of anti‐toxin A antibodies, and not the IgG class, that could neutralize toxin A in vitro and in vivo.47, 48 Babcock et al.40 solved this apparent dilemma by showing that a combination of 3 different monoclonal IgG anti‐toxin A antibodies could neutralize toxin A activity in vitro and prevent disease in the hamster model in vivo. Each of the 3 antibodies recognized a different toxin A domain: the first neutralized toxin A enzymatic activity, while the second prevented toxin A binding to its receptor on enterocytes, and the third prevented toxin internalization after binding to the receptor.
Thus, the mechanism of action of IVIG is most likely through the transfer of IgG anti‐toxin A antibodies that gain access to the intestinal lumen presumably secondary to inflammation‐induced mucosal damage and neutralize toxin A. Transfer of yet undetected IgA anti‐toxin A antibodies that prevent toxin A from binding to its receptor is much less likely, although possible.
The present study has limitations. As in all retrospective studies, selection bias was unavoidable. In addition, the decision to initiate IVIG administration was dependent on the attending physician, who also decided the dose, leading to heterogeneity in the total dose of IVIG infused. Such heterogeneity, however, is primarily the result of a lack of a standard dose for IVIG infusion for CDC in the published literature, as reported above. In addition, since IVIG is not yet approved by the U.S. Food and Drug Administration (FDA) for the treatment of severe CDC, standard treatment was not discontinued in any of the reports to date, including ours.
Choosing appropriate controls for patients suffering from severe CDC is challenging. This patient population is usually frail, with severe and multiple underlying diseases. The deteriorating clinical condition (and subsequently the need for multiple antibiotics) may be either the result of or the cause of CDC. Furthermore, IVIG has been in short supply for several years and therefore it has been expensive, making its administration to the number of patients needed to design adequately‐powered controlled studies difficult. These are mainly the reasons no randomized, multicenter, placebo‐controlled trial on IVIG use in severe CDC has been conducted to date.
Conclusions
In the present study, we report the results of IVIG use for the treatment of 21 patients with severe CDC. This is the largest cohort to our knowledge in the literature. Unlike previous studies on the subject, the present report provided 2 scales for disease assessment: the first based on the extent of colonic involvement and the second measuring the severity of systemic involvement using the APACHE II score. The latter was positively associated with mortality in this context. Of the 21 study patients treated with IVIG, only 9 patients (43%) survived their illness. This is the highest reported mortality rate among all studies on this subject so far. Further studies on the ideal timing of IVIG infusion, dose, and patient selection are needed before accepting IVIG as a standard of care for severe CDC treatment. The role of APACHE II score in the decision to use IVIG is promising and should be validated in randomized controlled trials.
Acknowledgements
The authors thank Indrani Mukherjee, MD, for her help and expertise in proofreading the manuscript.
Clostridium difficile colitis (CDC) is the most common cause of hospital‐acquired diarrhea.1 The incidence of CDC has sharply increased over the past decade despite increasing awareness among health care professionals.24 C. difficile pathogenic strains induce diarrhea through the elaboration and secretion of 2 exotoxins: toxin A and toxin B. Toxin A is an inflammatory toxin, leading to fluid secretion, increased mucosal permeability, and marked enteritis and colitis.5 Toxin B is cytotoxic, leading to cell injury and apoptosis.5 Combined, toxin A and toxin B can cause a wide spectrum of clinical presentations, ranging from mild diarrhea that resolves with the discontinuation of antibiotics to a fulminant colitis requiring surgical intervention.
The severity of clinical manifestations has been shown to be inversely proportional to the host anti‐toxin A antibody level in response to toxin exposure. Kyne et al.6 demonstrated that asymptomatic C. difficile carriers produced significantly higher anti‐toxin A immunoglobulin G (IgG) levels compared to symptomatic patients. Among the latter group, patients with mild disease had a higher antibody level compared to those with severe colitis.6, 7 Additional risk factors predisposing to severe colitis are advanced age, severe underlying illness,8 and immunocompromised state.9
Recently, a new C. difficile strain (BI/NAP1) with a mutated toxin A and toxin B promoter silencer, a binary toxin gene, and fluoroquinolone resistance has been described in Canada and the United States.3, 10 This strain has been associated with an increased incidence of CDC among hospitalized patients, especially the incidence of severe disease requiring colectomy. At the same time, several reports describing metronidazole treatment failure have been published.1114 These recent findings emphasize the importance of finding alternative treatments for CDC.
Intravenous immunoglobulin (IVIG) was used to treat CDC for the first time in 1991.15 Since then, 12 case reports and small case series, along with 1 case‐control study have been published documenting IVIG treatment outcomes.1527 However, only 5 reports to date examined patients with severe CDC.2125 In the present study, we report the largest series of patients with severe CDC treated with IVIG in the literature to our knowledge.
Patients and Methods
Case Series
We used CareScience software (CareScience, Inc., Philadelphia, PA) to retrospectively identify all patients admitted to our institution with a primary or secondary diagnosis of CDC (code 00845) between July 1, 2002 and May 1, 2006. CareScience is commercially available software that tracks all admissions to our institution and allows the performance of patient searches with a wide spectrum of user‐defined search criteria. Using the same software, we further identified those patients who received IVIG during their hospital stay. We then obtained the hospital chart for each patient, from the medical records department and established the study database.
A case was defined as a patient with diarrhea (at least 3 loose stools daily) for at least 2 days who had C. difficile cytotoxin‐positive feces and at least 1 of the following criteria: clinical symptoms (abdominal pain and/or distension and fever); leukemoid reaction (defined as white blood cell count of 20,000 cells/mm3 or above. This cutoff value was chosen as it has been used previously as a prognostic factor)4; radiographic evidence of colitis by computed tomography (CT) of the abdomen; and/or the presence of pseudomembranes on flexible sigmoidoscopy or colonoscopy. We excluded all patients who received IVIG for an indication other than CDC treatment (n = 3). There were no other exclusion criteria.
We used a standardized data collection tool and recorded demographics (age, gender, principal diagnosis), past medical and surgical history, other risk factors for C. difficile infection (previous CDC, antibiotics received during hospital stay, immunosuppressive medications or organ transplantation within the previous 6 weeks, history of malignancy or diabetes mellitus); clinical presentation (abdominal distention, abdominal pain, diarrhea, fever, leukemoid reaction, and hypotension defined as systolic blood pressure <85 mm Hg despite at least 1 L of intravenous normal saline administration and the need for vasopressor use); colonoscopy findings; CT scan and x‐ray findings; laboratory values; date and dose of IVIG infused and other C. difficile pharmacological treatments; and Acute Physiological Assessment and Chronic Health Evaluation (APACHE II) score28 at the first day of IVIG infusion. The primary outcomes were survival at the end of the hospital stay and clinical disease resolution, defined as 2 formed bowel movements or less per day without abdominal pain or distention.
The decision to initiate IVIG therapy and the dose to be used was made by the individual attending physician.
Statistical Analysis
Single (univariate) and multiple (multivariate) logistic regression analysis were applied to identify variables among the ones collected that are independent predictors of CDC mortality. All statistical analyses were completed with the STATA 10 software package (StatCorp LP, College Station, TX).
Review of the Literature
We used PubMed, Web of Science, Scopus, and Excerpta Medica databases to search for any publication in a peer‐reviewed journal on the use of IVIG for the treatment of severe CDC. We used the search words: IVIG or intravenous immunoglobulin and clostridium difficile. Only publications published in English were selected. The date range used was January 1, 1950 to January 7, 2009. We were able to find 5 publications using this search criteria.
Results
Study Population
Of the 1230 patients diagnosed with CDC over the 4‐year study period, 21 patients were treated with IVIG. Table 1 summarizes the patients' characteristics. There were 13 women and 8 men. The mean age was 68 years, with a standard deviation (SD) of 13 years. Sepsis was the primary diagnosis in all patients. Sixteen patients had predisposing risk factors for CDC, including immunosuppression (immunosuppressive medication [n = 2], human immunodeficiency virus [HIV] infection [n = 2]); cancer (n = 3); recent surgery (n = 3); and diabetes mellitus (n = 11). Nine patients had documented previous CDC episodes. The indications for IVIG administration were evidence of pancolitis on abdominal CT scan (n = 12) or severe ileus with cessation of diarrhea, abdominal distention, and requirement for total parenteral nutrition (n = 5), or severe hypotension (n = 4) (defined as systolic blood pressure <85 mm Hg despite at least 1 L of intravenous normal saline administration and the subsequent need for vasopressor use).
| Patient | Age (gender) | Diagnosis | Medical and Surgical History | CDC History | Colonoscopy Findings | Radiographic Findings |
|---|---|---|---|---|---|---|
| ||||||
| A | 40 (female) | CDC with sec. sepsis | Gastric stapling | Yes | * | Diffuse colitis |
| B | 86 (female) | Fulminant CDC | Metastatic ovarian carcinoma | PC | No colonic thickening | |
| C | 72 (male) | Sepsis | Acute pancreatitis with sec. pseudocyst, DM | Yes | PC | Diffuse colitis |
| D | 78 (male) | Discitis | Delayed: normal mucosa | Dilation of small and large bowel | ||
| E | 98 (female) | Urosepsis | Yes | * | No bowel distention | |
| F | 90 (female) | Right lower extremity cellulitis | DM | * | Concentric thickening of rectal wall | |
| G | 64 (male) | Ischemic colitis | DM, recent Hartman pouch closure | Marked inflammation | Diffuse colitis | |
| H | 78 (female) | Toe osteomyelitis and CDC with sec. sepsis | DM | * | Diffuse colitis | |
| I | 35 (female) | Sepsis | Yes | * | Diffuse nonspecific colitis, minimal ascites | |
| J | 47 (female) | Pneumonia and sec. sepsis | * | Diffuse colitis | ||
| K | 56 (female) | Urosepsis | HIV | Yes | * | Colitis involving the right colon |
| L | 76 (male) | CDC with sec. sepsis | Sigmoid bladder fistula repair, DM | * | Diffuse colitis | |
| M | 71 (female) | Pneumonia with sec. sepsis | * | Diffuse colitis | ||
| N | 63 (male) | Urosepsis | DM, lymphoma resection from small intestine | PC | Marked small and large bowel distention | |
| O | 86 (male) | Enterococcus‐induced sepsis | Rheumatoid arthritis on methotrexate | * | Fat stranding suggesting peritonitis | |
| P | 60 (female) | Gastrointestinal bleed and CDC | DM with neuropathy and retinopathies | Yes | * | Thickening of wall of colon in most of the colon |
| Q | 57 (female) | Sepsis | DM | Yes | * | Normal |
| R | 67 (female) | CDC with sec. sepsis | Candidal esophagitis | Yes | * | Large amount of peritoneal fluid, mild small bowel thickening |
| S | 60 (female) | Sepsis sec. to S. aureus and P. aerogenosa | DM, renal transplant (myco, prednisone) | * | Ileus with air fluid level in the small intestine | |
| T | 80 (female) | Sepsis | DM | Yes | * | Thickening of descending colon consistent with colitis |
| U | 72 (male) | CDC, widespread metastatic cancer | DM, metastatic cancer (unknown primary site) | * | Severe colitis up to the splenic flexure | |
Table 2 describes disease severity in these patients. Since CDC starts locally in the colon then secondarily involves multiple organs as part of the systemic inflammatory response syndrome (SIRS), 2 scales were used to characterize the disease in each patient: (1) extent of local colonic inflammation, and (2) severity of systemic involvement. Extensive colonic involvement was evidenced in all patients by pancolitis on abdominal imaging modalities (12 patients), severe ileus requiring total parenteral nutrition (13 patients), or referral for surgical consultation for possible colectomy (12 patients).
| Patient | Complications During the Hospital Stay | APACHE II Score | Monitored Unit | WBC* | K | Alb | Lactate | Cr | Hospital Stay (days) | Surgical Consult/Surgery | TPN for Colitis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| A | Sepsis, DIC, ARDS, intubation | 15 | Yes | 48 | 4 | 1.1 | 2.9 | 1.3 | 26 | No/No | Yes |
| B | Dehydration, weakness | 12 | No | 19 | 3.4 | 3.1 | 1.1 | 16 | No/No | Yes | |
| C | HTP, GI bleed, ischemic colitis, F | 22 | Yes | 21 | 2.7 | 2.5 | 1.3 | ESRD | 34 | Yes/No | Yes |
| D | AD, megacolon, HT, intubation, ARF | 18 | Yes | 25 | 3.4 | 1.4 | 1.1 | 2.6 | 52 | Yes/No | Yes |
| E | Exacerbation of CHF with respiratory distress, Bipap support, confusion | 21 | No | 10 | 2.6 | 2.3 | 1 | 11 | No/No | No | |
| F | Confusion, gout acute attack | 21 | No | 33 | 3.1 | 2.6 | ESRD | 15 | No/No | No | |
| G | Intubation, cardiac arrest, AF with RVR, PNA, ARF, DVT, dysphagia, PEG | 6 | Yes | 15 | 3.1 | 1.7 | 1.9 | 2.2 | 32 | Yes/No | Yes |
| H | Sepsis, intubation, ARF, PEG, toe amputation, PNA, pulmonary edema, TPN, vitamin D deficiency | 34 | Yes | 59 | 2.8 | 1.5 | 4 | 3.8 | 17 | No/No | Yes |
| I | CHF, transient third cranial nerve palsy, DIC | 20 | Yes | 52 | 3.2 | 3.3 | 0.7 | ESRD | 11 | No/No | No |
| J | Aspiration PNA, sepsis, DIC, intubation, MI, F, HT | 32 | Yes | 17 | 2.7 | 1.8 | 14 | ESRD | 21 | Yes/no | No |
| K | Intubation, cardiac arrest, ARF, DIC, AP, F | 30 | Yes | 25 | 2.1 | 2.1 | 13 | 3.6 | 10 | Yes/no | No |
| L | Intubation, ARF, HTP, MI, AD, F | 23 | Yes | 69 | 3.2 | 1.8 | 1.4 | 3.6 | 23 | Yes/no | No |
| M | Intubation, DIC, ARF, GI bleeding, hypothermia, AD | 23 | Yes | 47 | 2.8 | 1.4 | 1.4 | 4.3 | 23 | Yes/no | Yes |
| N | Intubation, HTP, AD, F, ARF with HD, AF, osteomyelitis | 23 | Yes | 46 | 2.9 | 1.5 | 1.5 | 2.7 | 27 | Yes/no | Yes |
| O | Intubation, ARF, 2 cardiac arrests, GI bleed, UTI, rhabdomyolysis, liver shock, AF | 31 | Yes | 49 | 2.5 | 1.1 | 2.3 | 2.9 | 25 | Yes/no | Yes |
| P | Bowel ischemia with bowel resection, ARF, MI, ischemic bowel, fluid overload, respiratory failure | 23 | Yes | 26 | 2.6 | 0.8 | 2.9 | 3.1 | 9 | Yes/Yes | Yes |
| Q | Pulmonary embolism | 39 | Yes | 23 | 2.8 | 1.1 | 8.1 | ESRD | 9 | No/No | Yes |
| R | Fungal peritonitis, aspiration pneumonia, cardiac arrest | 26 | Yes | 30 | 4.1 | 1.1 | 0.9 | ESRD | 36 | No/No | Yes |
| S | Intubation, pneumothorax, CRT, pressor‐dependent shock, ARF | 36 | Yes | 46 | 3.1 | 1.8 | 2.1 | 5.4 | 64 | Yes/Yes | No |
| T | Pressor‐dependent sepsis, pulmonary edema, ARF with HD | 36 | Yes | 35 | 3.3 | 1.3 | 1.8 | 5.5 | 11 | Yes/No | Yes |
| U | Sepsis | 34 | Yes | 58 | 3.1 | 2.2 | 3.3 | 1.6 | 9 | No/No | No |
Of the 21 patients treated with IVIG, 9 did not receive a surgical consultation either because they responded to medical treatment promptly (6 patients), were too unstable for surgery (2 patients), or because the patient/family refused surgery (1 patient). Of the 12 patients who received surgical consultation, 2 underwent surgery. The remainder did not proceed to surgery for the following reasons: they were deemed medically unstable for surgery (6 patients), declined surgery (2 patients), were diagnosed with cancer on colonoscopy (1 patient), or improved with medical treatment (1 patient).
The severity of systemic involvement was measured using the APACHE II score on day 1 of IVIG infusion. The mean APACHE II score was 25. Eighteen patients were in a monitored unit when IVIG was administered. The study group had laboratory results in keeping with those previously used to define severe colitis:4, 9, 19 leukocytosis (defined as white blood cell count higher than 12,000 cells/mL [mean = 36,000 cells/mL]), hypoalbuminemia (mean = 1.78 g/dL, SD = 0.68 g/dL), hypokalemia (mean = 3.02 mg/dL, SD = 0.47 g/dL), and acute renal failure (defined as serum creatinine level >1.5 mg/dL [mean = 2.98 mg/dL, SD = 1.42 g/dL]).
IVIG Use
Table 3 describes the treatment patients received for CDC as well as the total number of antibiotics used throughout the hospital stay. IVIG was used as adjuvant treatment (defined as IVIG administration within 4 days or less after CDC diagnosis) in 8 patients and as second‐line treatment (defined as IVIG administration more than 4 days after CDC diagnosis) in 13 patients. Metronidazole, vancomycin, cholestyramine, and probiotic treatment alone or in different combinations were used for an average of 8 days (SD = 8 days; range, 025 days) before IVIG infusion. The total IVIG dose administered varied depending on the prescribing attending, with a range of 200 mg/kg to 1250 mg/kg and a mode of 250 mg/kg for 1 to 3 days. An average of 5 (SD = 2) different antibiotics that were not active against C. difficile were used per patient without being discontinued after a CDC diagnosis was made. The 3 most common were: cephalosporins (cefazolin, ceftriaxone, cefepime), fluoroquinolones (levofloxacin), and combination antibiotics (piperacillin and tazobactam or ampicillin and sulbactam).
| Patient | Number of Antibiotics | Duration of Treatment Before IVIG (days) | Treatment Before IVIG (days) | Total CDC Treatment (days) | IgG Level | IVIG Dose |
|---|---|---|---|---|---|---|
| ||||||
| A | 5 | 7 | Metro (7), Vanc (7), Choles (2) | Oral and rectal Vanc (26,19), IV Metro (19), Choles (2), Lacto (6) | Low | 300 mg/kg for 1 day |
| B | 1 | 13 | Metro (13), Vanc (13) | Oral Vanc (17) and IV Metro (12) | 300 mg/kg for 1 day | |
| C | 3 | 7 | Metro (7), Vanc (3) | IV Metro (28),Vanc oral and enema (18,3), Lacto (10) | Low | 125 mg/kg for 5 days |
| D | 5 | 25 | Metro (25), Vanc (15) | Oral then IV Metro (10,15), oral Vanc (25), Choles (7) and Lacto (13) | Low | 200 mg/kg for 1 day |
| E | 1 | 4 | Metro (1), Vanc (4), Choles(4) | IV Metro (8), oral Vanc (10) and Choles (4) | 75 mg/kg for 5 days | |
| F | 4 | 2 | Metro (2), Vanc (1) | IV Metro (8) and oral Vanc (9) | 250 mg/kg for 5 days | |
| G | 3 | 17 | Metro (17), Vanc (14) | IV Metro (49) and oral Vanc (61) | 250 mg/kg for 2 days | |
| H | 5 | 1 | Metro (1), Vanc (1) | Oral Metro (18), oral Vanc (22 ), IV Metro (3) | 250 mg/kg for 3 days | |
| I | 6 | 1 | Metro (1) | Oral and IV Metro (7,9), Vanc oral and enema (8,3) | 250 mg/kg for 2 days | |
| J | 8 | 16 | Metro (14), Vanc (2), Lacto (6) | Oral then IV Metro (10,5), oral and rectal Vanc (3,1), Lacto (6) | 300 mg/ kg for 1 day | |
| K | 6 | 7 | Metro (7), Vanc (3) | Oral then IV Metro (10) and oral Vanc (9) | Normal | 400 mg/kg for 2 days |
| L | 4 | 0 | None | IV then oral Metro (7,10), oral Vanc (23), Choles (7), and Lacto (5) | 150 mg/kg for 5 days | |
| M | 6 | 1 | Metro (1) | IV Metro (23) and oral Vanc (9) | 250 mg/kg for 2 days | |
| N | 7 | 1 | Metro (1), Vanc (1) | IV Metro (16), oral Vanc (14), oral Metro (7) | 250 mg /kg for 2 days | |
| O | 6 | 7 | Metro (6), Vanc (4) | IV Metro (6) and oral Vanc (22) | Low | 250 mg/kg for 2 days |
| P | 3 | 6 | Metro (6), Vanc (6) | IV Metro (25) and oral Vanc (17) | 150 mg/kg fro 3 days | |
| Q | 3 | 4 | Metro (4), Vanc (3) | Oral Metro (8),Vanc oral and enema (6,3) | 250 mg/kg for 3 days | |
| R | 5 | 9 | Vanc (9) | Vanc oral and enema (5,4), IV Metro (2) | 250 mg/kg for 1 day | |
| S | 8 | 23 | Metro (23), Vanc (23) | Oral then IV Metro (12,22), oral Vanc (39) | 250 mg/kg for 3 days | |
| T | 4 | 6 | Metro (6), Vanc (1) | Oral Vanc and IV Metro (11) | 250 mg/kg for 1 day | |
| U | 4 | 2 | Metro (2), Vanc (2) | Oral Vanc and IV Metro (6) | 250 mg/kg for 3 days | |
Survival with IVIG Use
Nine patients (43%) survived their illness and were discharged from the hospital. They experienced complete clinical resolution after an average of 10 days from IVIG administration (range, 220 days) (Table 4). The other 12 patients (57%) died during the index hospitalization. The average length of stay was 23 (range, 964) days.0
| Patient | Disposition | Clearance of Clostridium difficile Colitis? | Days to Resolution |
|---|---|---|---|
| |||
| A | Alive | Loose BM persisted but diarrhea resolved 9 days after IVIG. | 9 |
| B | Alive | BM became formed and diarrhea resolved 48 hours after IVIG. | 2 |
| C | Alive | Diarrhea resolved 20 days post‐IVIG infusion. CAT scan: colonic thickening improved. | 20 |
| D | Alive | Diarrhea resolved on discharge. Response to IVIG started next day after administration. | 18 |
| E | Alive | Diarrhea improved next day after IVIG administration and resolved on discharge. | 5 |
| F | Alive | Diarrhea resolved 5 days after IVIG administration. | 5 |
| G | Alive | Diarrhea resolved. C. difficile test became negative. | 13 |
| H | Alive | Diarrhea resolved 2 days before discharge. | 15 |
| I | Alive | Diarrhea slowly improved and resolved 4 days before discharge. | 7 |
| J | Deceased | ||
| K | Deceased | ||
| L | Deceased | ||
| M | Deceased | ||
| N | Deceased | ||
| O | Deceased | ||
| P | Deceased | ||
| Q | Deceased | ||
| R | Deceased | ||
| S | Deceased | ||
| T | Deceased | ||
| U | Deceased | ||
| Study | Number of Patients | Age (SD) (years) | Male | Female | Severity Definition | IVIG Dose | Days to Resolution | Days IVIG Infused | Alive? (%) | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| Salcedo et al.22 | 2 | 63, 64 | 1 | 1 | Pancolitis or thumbprinting on CAT scan | 200300 mg/kg once | 12 | 59 | 100 | 1 out of 2 |
| McPherson et al.21 | 8 | 72 (12) | ? | ? | Pancolitis | 200400 mg/kg twice | 226 | 1165 | 75 | 2 out of 6 |
| Juang et al.25 | 18 | 67 (17.4) | 5 | 13 | Modified Rubin et al.9 criteria | 200300 mg/kg once | ? | ? | 83 | ? |
| Hassoun et al.24 | 1 | 72 | 1 | 0 | Pancolitis | 400 mg/kg once | 6 | 15 | 100 | None |
| Chandrasekar et al.23 | 1 | 67 | 0 | 1 | Shock requiring inotropic support and pseudomembranes on colonoscopy | 400 mg/kg for 5 doses | 16 | 35 | 100 | ? |
| This paper | 21 | 68 (16) | 7 | 14 | Pancolitis and APACHE II score | 300 mg/kg once; 250 mg/kg for 5 doses | 220 | 025 | 43 | ? |
To further assess the impact of IVIG on colitis resolution, we investigated all variables in the data set that may have been associated with mortality using univariate Cox regression analysis. Those variables were as follows: APACHE II score on the first day of IVIG infusion, age, sex, previous history of CDC, number of days before IVIG use, peak white blood cell count, serum potassium level and creatinine level, lactate level on first day of IVIG infusion, and number of antibiotics administered that are not active against CDC. Only the APACHE II score (P = 0.006) and lactate level on the day of IVIG infusion (P = 0.004) were (positively) associated with CDC mortality. The positive association between CDC mortality and APACHE II score remained significant (P = 0.04) after adjusting for sex, previous history of CDC, number of days before IVIG use, lactate level on first day of IVIG infusion, and number of antibiotics administered that are not active against CDC using a multivariate Cox regression analysis model. No adjustments were made for age, white blood cell count, potassium level, or creatinine level as those are included within the APACHE II score. The positive association between lactate level on the first day of IVIG infusion and CDC mortality was not statistically significant after adjusting for the factors listed above in the same model (P = 0.13).
Discussion
To our knowledge, the present study is the largest series published in the literature to date on the use of IVIG for severe CDC. It is also the first study to report a high mortality rate compared to the 5 previous smaller studies on this topic. In the first report on IVIG use for CDC, Leung et al.15 used IVIG to treat 5 pediatric patients suffering from chronic relapsing CDC. It was not until 7 years later, in 1997, that the first IVIG use for severe CDC was reported.22 Since then, a total of 13 works have been published on IVIG for CDC treatment, and only in 5 of these was IVIG administered for severe CDC treatment:2125 3 case reports, 1 case series, and 1 case‐control study. Although the 4 uncontrolled reports concluded that IVIG is beneficial for severe CDC, the only controlled study reported no significant difference between cases and controls for all‐cause mortality, length of stay, and colectomy rate.25
The definition of severe CDC varied between reports, making comparison difficult. McPherson et al.21 and Hassoun and Ibrahim24 defined severe disease as one causing pancolitis on CT scan either with or without megacolon. In the study by Juang et al.,25 disease severity was assessed using the modified criteria of Rubin et al.9 Salcedo et al.22 defined severe CDC as one causing pancolitis in one patient and thumbprinting on CT scan in another, whereas Chandrasekar et al.23 defined it as one causing shock requiring inotropic support and presence of pseudomembranes on colonoscopy.
The present report is unique in that it provided 2 scales to characterize disease in each patient. The first scale measured colonic involvement anatomically and physiologically using a combination of computerized axial tomography (CAT) scan findings, presence or absence of ileus or referral for possible colectomy. This is not a prognostic scale, however, since CAT scan findings have been previously shown to be poor predictors of treatment outcome.29 The second scale measured the severity of systemic involvement using a well‐validated and standardized scale, the APACHE II score. We have shown in this report that it is associated with prognosis in the context of IVIG use.
Our study reports a higher mortality rate than previously described, and suggests that risk stratification and patient selection are important before IVIG administration, since not all patients seem to benefit from this treatment as previously suggested by smaller case series. Previously, several physical findings and laboratory values were found to be associated with worse outcome in CDC. These were increasing age, immunosuppression, shock requiring vasopressors, peak white blood cell count, peak serum lactate level, hypoalbuminemia, a fall in serum albumin level of >1.1 g/dL at the onset of CDC symptoms, use of 3 or more antibiotics, comorbid disease, previous history of CDC, acute renal failure and hypotension, underlying altered or depressed mental status, abdominal pain or distention, white blood cell count over 20,000/mm3 or <1500/mm3 and/or a >10% band forms on the white blood cell differential count, and ascites or pneumatosis coli by abdominal imaging.9, 3033 Using the APACHE II scale for the same purpose has the advantage of utilizing a well‐validated and objective scale that is expected to measure the degree of systemic involvement more reliably compared to the clinical and laboratory values above.
Timing of IVIG infusion remains controversial. Due to the lack of randomized controlled trials, the current practice is guided by expert opinion, leading to wide variations between reports. Since the APACHE II score was positively associated with mortality in the setting of IVIG treatment, the same scale could be used to guide decisions regarding timing of IVIG infusion. Our results suggest that IVIG should be preferentially used while the APACHE II score is still relatively low. This association and the specific APACHE II score at which to initiate or hold treatment need to be validated in the setting of a randomized controlled study before being used in clinical practice.
Although the current study was not designed to test this theory, IVIG could be associated conceptually with treatment success for patients with severe disease that is still restricted to the colon (without other organ dysfunction or at least at an early stage of extracolonic organ failure and thus associated with a low APACHE II score) but not for severe colonic disease with secondary multiple organ failure (high APACHE II score). This may be because colonic disease is toxin‐mediated whereas secondary systemic involvement is mediated through toxin‐induced inflammatory mediators (interleukin‐8, macrophage‐inflammatory protein‐2, substance P, tumor necrosis factor‐alpha) released locally in the colon,3436 triggering a SIRS and hematogenous translocation of colonic bacteria,37 both of which are poorly responsive to immunoglobulin infusion. Along the same lines, waiting for failure of conventional therapy before IVIG use might result in IVIG treatment failure because of disease progression and secondary sepsis, at which point no treatment may be effective. No study thus far has addressed this issue specifically.
Overall, the combined cohort of patients with severe CDC treated with IVIG in the literature includes 51 patients (Table 4). The current report contributes 41% of these patients. The patients' average age was 68 years, with a 2 to 1 female‐male ratio. The dose of IVIG used varied largely also, with 400 mg/kg being the mode (range, 75400 mg/kg from 1 to 5 doses). This dose is significantly below the doses used in the treatment of other diseases, like Guillain‐Barr syndrome, myasthenia gravis, Kawasaki disease, autoimmune hemolytic anemia, agammaglobulinemia, and hypogammaglobulinemia, where the usual dose is 400 mg/kg for 5 days. The resolution of diarrhea in these cases occurred after an average of 9 (range, 142) days. The index hospitalization survival rate varied from 43% to 100%. Patients received standard treatment for an average of 13 (range, 065) days before IVIG infusion. Thirty‐two of 51 patients survived their illness (63%). Neither total IgG nor anti‐toxin A IgG levels were measured in any of the reports. Of the 32 patients who had clinical resolution, 3 (10%) experienced symptoms recurrence in a follow‐up period of 1 to 13 months. This number is most probably an underestimation of the true recurrence rate resulting from an incomplete reporting because there was no uniform or active recurrence ascertainment mechanism in any of the studies. The recurrences were at 10, 14, and 30 days posttreatment. Since standard treatment was not discontinued in any of the reports once IVIG was given, the relative contribution and the ideal timing for IVIG infusion are still unclear.
The mechanism of action of IVIG is passive immunization (with anti‐toxin A and anti‐toxin B antibodies present in the pooled immunoglobulin) of a host who is usually unable to mount an adequate protective immune response.15, 22 IVIG is formed from pooling immunoglobulin from several random donors. It has been shown that many such donors express high anti‐toxin A and anti‐toxin B antibody serum titers.38, 39 In addition, high levels of anti‐toxin A and anti‐toxin B antibodies were present in the IVIG preparations and the recipients after infusion.1517, 22 Although constituting only a small fraction of the total IVIG administered, these antitoxin antibodies are believed to neutralize toxin A and B and help the host recover from the disease. In fact, Babcock et al.40 used an experimental hamster model of CDC to demonstrate a mortality reduction from 100% to 55% postinfusion of combined anti‐toxin A and anti‐toxin B antibodies. While some early reports indicated that anti‐toxin B antibodies were the major determinants of protection against colitis,41 later reports correlated disease severity pathologically42 and clinically43, 44 with anti‐toxin A levels. Anti‐toxin B antibodies were later shown to play an adjunctive role in conferring immunity against CDC40, 45, 46 when added to anti‐toxin A antibodies, but not to have any significant role on their own.
However, IVIG has been shown to contain IgG, but not IgA, anti‐toxin A and anti‐toxin B antibodies while it is only the IgA class of anti‐toxin A antibodies, and not the IgG class, that could neutralize toxin A in vitro and in vivo.47, 48 Babcock et al.40 solved this apparent dilemma by showing that a combination of 3 different monoclonal IgG anti‐toxin A antibodies could neutralize toxin A activity in vitro and prevent disease in the hamster model in vivo. Each of the 3 antibodies recognized a different toxin A domain: the first neutralized toxin A enzymatic activity, while the second prevented toxin A binding to its receptor on enterocytes, and the third prevented toxin internalization after binding to the receptor.
Thus, the mechanism of action of IVIG is most likely through the transfer of IgG anti‐toxin A antibodies that gain access to the intestinal lumen presumably secondary to inflammation‐induced mucosal damage and neutralize toxin A. Transfer of yet undetected IgA anti‐toxin A antibodies that prevent toxin A from binding to its receptor is much less likely, although possible.
The present study has limitations. As in all retrospective studies, selection bias was unavoidable. In addition, the decision to initiate IVIG administration was dependent on the attending physician, who also decided the dose, leading to heterogeneity in the total dose of IVIG infused. Such heterogeneity, however, is primarily the result of a lack of a standard dose for IVIG infusion for CDC in the published literature, as reported above. In addition, since IVIG is not yet approved by the U.S. Food and Drug Administration (FDA) for the treatment of severe CDC, standard treatment was not discontinued in any of the reports to date, including ours.
Choosing appropriate controls for patients suffering from severe CDC is challenging. This patient population is usually frail, with severe and multiple underlying diseases. The deteriorating clinical condition (and subsequently the need for multiple antibiotics) may be either the result of or the cause of CDC. Furthermore, IVIG has been in short supply for several years and therefore it has been expensive, making its administration to the number of patients needed to design adequately‐powered controlled studies difficult. These are mainly the reasons no randomized, multicenter, placebo‐controlled trial on IVIG use in severe CDC has been conducted to date.
Conclusions
In the present study, we report the results of IVIG use for the treatment of 21 patients with severe CDC. This is the largest cohort to our knowledge in the literature. Unlike previous studies on the subject, the present report provided 2 scales for disease assessment: the first based on the extent of colonic involvement and the second measuring the severity of systemic involvement using the APACHE II score. The latter was positively associated with mortality in this context. Of the 21 study patients treated with IVIG, only 9 patients (43%) survived their illness. This is the highest reported mortality rate among all studies on this subject so far. Further studies on the ideal timing of IVIG infusion, dose, and patient selection are needed before accepting IVIG as a standard of care for severe CDC treatment. The role of APACHE II score in the decision to use IVIG is promising and should be validated in randomized controlled trials.
Acknowledgements
The authors thank Indrani Mukherjee, MD, for her help and expertise in proofreading the manuscript.
- ,,.Secular trends in hospital‐acquired Clostridium difficile disease in the United States, 1987–2001.J Infect Dis.2004;189:1585–1589.
- ,,, et al.Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications.Ann Surg.2002;235:363–372.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Clostridium difficile‐associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity.CMAJ.2004;171:466–472.
- ,,.Comparison of two toxins produced by Clostridium difficile.Infect Immun.1981;34:1036–1043.
- ,,,.Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A.N Engl J Med.2000;342:390–397.
- ,,,.Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection.Infect Immun.1994;62:384–389.
- ,,.Risk factors for Clostridium difficile carriage and C. difficile‐associated diarrhea in a cohort of hospitalized patients.J Infect Dis.1990;162:678–684.
- ,,.Severe Clostridium difficile colitis.Dis Colon Rectum.1995;38:350–354.
- ,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,.Factors associated with failure of metronidazole in Clostridium difficile‐associated disease.J Clin Gastroenterol.2004;38:414–418.
- ,,.Treatment of Clostridium difficile‐associated disease: old therapies and new strategies.Lancet Infect Dis.2005;5:549–557.
- ,,, et al.Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole.Clin Infect Dis.2005;40:1586–1590.
- ,,, et al.Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada.Clin Infect Dis.2005;40:1591–1597.
- ,,,,,.Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin.J Pediatr.1991;118:633–637.
- ,,,.Gamma globulin administration in relapsing Clostridium difficile‐induced pseudomembranous colitis with a defective antibody response to toxin A.Acta Clin Belg.1995;50:36–39.
- ,,,.Recurrent Clostridium difficile infection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii.Clin Infect Dis.1995;20(suppl 2):S266–S268.
- .Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea.Gut.2002;51:456.
- .Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea.J Antimicrob Chemother.2004;53:882–884.
- ,,.Intravenous immunoglobulin for resistant Clostridium difficile infection.Age Ageing.2006;35:85–86.
- ,,,,.Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea.Dis Colon Rectum.2006;49:640–645.
- ,,, et al.Intravenous immunoglobulin therapy for severe Clostridium difficile colitis.Gut.1997;41:366–370.
- ,,, et. al.Intravenous immunoglobulin therapy for refractory Clostridium difficile toxin colitis in chronic kidney disease: case reports and literature review.NDT Plus.2008;1:20–22.
- ,.Use of intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis.Am J Geriatr Pharmacother.2007;5:48–51.
- ,,, et al.Clinical outcomes of intravenous immune globulin in severe clostridium difficile‐associated diarrhea.Am J Infect Control.2007;35:131–137.
- ,,,.Successful treatment of Clostridium difficile colitis with intravenous immunoglobulin.J Gastrointestin Liver Dis.2008;17:353–359.
- ,,, et al.A durable response to relapsing Clostridium difficile colitis may require combined therapy with high‐dose oral Vancomycin and intravenous immune globulin.Infect Dis Clin Pract.2006;14:217–220.
- ,,,.APACHE II: a severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,,,,.Colonic abnormalities on CT in adult hospitalized patients with Clostridium difficile colitis: prevalence and significance of findings.AJR Am J Roentgenol.2006;186:1393–1400.
- ,,,.Clostridium difficile‐associated diarrhea: predictors of severity in patients presenting to the emergency department.Can J Gastroenterol.2003;17:369–373.
- ,,, et al.Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain.Ann Surg.2007;245:267–272.
- ,, et al.Risk factors for severity and relapse of pseudomembranous colitis in an elderly population.Colorectal Dis.2007;9:173–177.
- ,,,,.Prognostic criteria in Clostridium difficile colitis.Am J Gastroenterol.1996;91:460–464.
- ,,, et al.Clostridium difficile toxin a stimulates macrophage‐inflammatory protein‐2 production in rat intestinal epithelial cells.J Immunol.1998;160:6039–6045.
- ,,, et al.Increased substance P responses in dorsal root ganglia, intestinal macrophages during Clostridium difficile toxin a enteritis in rats.Proc Natl Acad Sci U S A.1997;94:4788–4793.
- ,,,,,.Cytokine response by human monocytes to Clostridium difficile toxin a and toxin B.Infect Immun.1991;59:3659–3666.
- ,,,.Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection.J Med Microbiol.1998;47:591–598.
- ,.Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic‐associated diarrhea.Diagn Microbiol Infect Dis.1994;18:205–209.
- ,,, et al.Serum antibody response to toxins A and B of Clostridium difficile.J Infect Dis.1983;148:93–100.
- ,,, et al.Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile‐induced mortality in hamsters.Infect Immun.2006;74:6339–6347.
- ,,,.Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea.Infection.1985;13:97–101.
- ,,,.Relationship between levels of Clostridium difficile toxin A and toxin B and cecal lesions in gnotobiotic mice.Infect Immun.1989;57:2123–2127.
- ,,,,.Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A.Infect Immun.1991;59:1192–1195.
- ,,.Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile.J Infect Dis.1992;166:1287–1294.
- ,,, et al.Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A‐neutralizing antibodies in mice.Infect Immun.2007;75:2826–2832.
- ,,, et al.Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters.Infect Immun.1999;67:527–538.
- ,,,.Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A‐receptor binding.Gastroenterology.1992;102:35–40.
- ,,,,.Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease.Infect Immun.1995;63:3166–3173.
- ,,.Secular trends in hospital‐acquired Clostridium difficile disease in the United States, 1987–2001.J Infect Dis.2004;189:1585–1589.
- ,,, et al.Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications.Ann Surg.2002;235:363–372.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Clostridium difficile‐associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity.CMAJ.2004;171:466–472.
- ,,.Comparison of two toxins produced by Clostridium difficile.Infect Immun.1981;34:1036–1043.
- ,,,.Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A.N Engl J Med.2000;342:390–397.
- ,,,.Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection.Infect Immun.1994;62:384–389.
- ,,.Risk factors for Clostridium difficile carriage and C. difficile‐associated diarrhea in a cohort of hospitalized patients.J Infect Dis.1990;162:678–684.
- ,,.Severe Clostridium difficile colitis.Dis Colon Rectum.1995;38:350–354.
- ,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,.Factors associated with failure of metronidazole in Clostridium difficile‐associated disease.J Clin Gastroenterol.2004;38:414–418.
- ,,.Treatment of Clostridium difficile‐associated disease: old therapies and new strategies.Lancet Infect Dis.2005;5:549–557.
- ,,, et al.Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole.Clin Infect Dis.2005;40:1586–1590.
- ,,, et al.Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada.Clin Infect Dis.2005;40:1591–1597.
- ,,,,,.Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin.J Pediatr.1991;118:633–637.
- ,,,.Gamma globulin administration in relapsing Clostridium difficile‐induced pseudomembranous colitis with a defective antibody response to toxin A.Acta Clin Belg.1995;50:36–39.
- ,,,.Recurrent Clostridium difficile infection in a patient with selective IgG1 deficiency treated with intravenous immune globulin and Saccharomyces boulardii.Clin Infect Dis.1995;20(suppl 2):S266–S268.
- .Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea.Gut.2002;51:456.
- .Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea.J Antimicrob Chemother.2004;53:882–884.
- ,,.Intravenous immunoglobulin for resistant Clostridium difficile infection.Age Ageing.2006;35:85–86.
- ,,,,.Intravenous immunoglobulin for the treatment of severe, refractory, and recurrent Clostridium difficile diarrhea.Dis Colon Rectum.2006;49:640–645.
- ,,, et al.Intravenous immunoglobulin therapy for severe Clostridium difficile colitis.Gut.1997;41:366–370.
- ,,, et. al.Intravenous immunoglobulin therapy for refractory Clostridium difficile toxin colitis in chronic kidney disease: case reports and literature review.NDT Plus.2008;1:20–22.
- ,.Use of intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis.Am J Geriatr Pharmacother.2007;5:48–51.
- ,,, et al.Clinical outcomes of intravenous immune globulin in severe clostridium difficile‐associated diarrhea.Am J Infect Control.2007;35:131–137.
- ,,,.Successful treatment of Clostridium difficile colitis with intravenous immunoglobulin.J Gastrointestin Liver Dis.2008;17:353–359.
- ,,, et al.A durable response to relapsing Clostridium difficile colitis may require combined therapy with high‐dose oral Vancomycin and intravenous immune globulin.Infect Dis Clin Pract.2006;14:217–220.
- ,,,.APACHE II: a severity of disease classification system.Crit Care Med.1985;13:818–829.
- ,,,,,.Colonic abnormalities on CT in adult hospitalized patients with Clostridium difficile colitis: prevalence and significance of findings.AJR Am J Roentgenol.2006;186:1393–1400.
- ,,,.Clostridium difficile‐associated diarrhea: predictors of severity in patients presenting to the emergency department.Can J Gastroenterol.2003;17:369–373.
- ,,, et al.Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain.Ann Surg.2007;245:267–272.
- ,, et al.Risk factors for severity and relapse of pseudomembranous colitis in an elderly population.Colorectal Dis.2007;9:173–177.
- ,,,,.Prognostic criteria in Clostridium difficile colitis.Am J Gastroenterol.1996;91:460–464.
- ,,, et al.Clostridium difficile toxin a stimulates macrophage‐inflammatory protein‐2 production in rat intestinal epithelial cells.J Immunol.1998;160:6039–6045.
- ,,, et al.Increased substance P responses in dorsal root ganglia, intestinal macrophages during Clostridium difficile toxin a enteritis in rats.Proc Natl Acad Sci U S A.1997;94:4788–4793.
- ,,,,,.Cytokine response by human monocytes to Clostridium difficile toxin a and toxin B.Infect Immun.1991;59:3659–3666.
- ,,,.Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection.J Med Microbiol.1998;47:591–598.
- ,.Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic‐associated diarrhea.Diagn Microbiol Infect Dis.1994;18:205–209.
- ,,, et al.Serum antibody response to toxins A and B of Clostridium difficile.J Infect Dis.1983;148:93–100.
- ,,, et al.Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile‐induced mortality in hamsters.Infect Immun.2006;74:6339–6347.
- ,,,.Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea.Infection.1985;13:97–101.
- ,,,.Relationship between levels of Clostridium difficile toxin A and toxin B and cecal lesions in gnotobiotic mice.Infect Immun.1989;57:2123–2127.
- ,,,,.Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A.Infect Immun.1991;59:1192–1195.
- ,,.Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile.J Infect Dis.1992;166:1287–1294.
- ,,, et al.Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A‐neutralizing antibodies in mice.Infect Immun.2007;75:2826–2832.
- ,,, et al.Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters.Infect Immun.1999;67:527–538.
- ,,,.Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A‐receptor binding.Gastroenterology.1992;102:35–40.
- ,,,,.Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease.Infect Immun.1995;63:3166–3173.
Copyright © 2010 Society of Hospital Medicine